See discussions, stats, and author profiles for this publication at: http://www researchgate net/publication/262375810
Stage-Gate Process for the Development of Medical Devices
ARTICLE in JOURNAL OF MEDICAL DEVICES · JUNE 2009
Impact Factor: 0 42 DOI: 10 1115/1 3148836
CITATIONS
5 AUTHORS, INCLUDING:

Lauren Aquino Shluzas
Stanford University
13 PUBLICATIONS 23 CITATIONS
SEE PROFILE

Marie-Elisabeth Lucienne Paté-Cornell Stanford University
90 PUBLICATIONS 1,711 CITATIONS
SEE PROFILE
JanB.Pietzsch
DepartmentofManagementScienceand Engineering, StanfordUniversity, 380PanamaWay, Stanford,CA94305-4026; WingTechInc., 9916NewhallRoad, Potomac,MD20854
LaurenA.Shluzas DepartmentofManagementScienceand Engineering, andDepartmentofMechanicalEngineering, StanfordUniversity, 380PanamaWay, Stanford,CA94305-4026
M.ElisabethPaté-Cornell DepartmentofManagementScienceand Engineering, StanfordUniversity, 380PanamaWay, Stanford,CA94305-4026
PaulG.Yock
DepartmentofBioengineering, StanfordUniversity, JamesH.ClarkCenter, 318CampusDrive, E-100, Stanford,CA94305-5428
JohnH.Linehan
DepartmentofBioengineering, StanfordUniversity, JamesH.ClarkCenter, 318CampusDrive, E-100, Stanford,CA94305-5428; ClinicalandTranslationalSciencesInstitute, NorthwesternUniversity, 750NorthLakeShoreDrive, Chicago,IL60611
Stage-GateProcessforthe DevelopmentofMedicalDevices
Themedicaldevicedevelopmentprocesshasbecomeincreasinglycomplexinrecent years.Theadventofnewtechnologyconcepts,stricterregulatoryrequirements,andthe everincreasingimportanceofreimbursementdecisionsforsuccessfuldevicecommercializationrequirecarefulplanningandstrategy-setting,coordinateddecisions,andconsistent,rigorousbusinessprocesses.Thedesignandimplementationofsuchprocesses,often capturedindevelopmentmodelsandaccompanyingstandardoperatingprocedures,have becomeakeydeterminantofthesuccessofdevicecommercialization.Whilevarious modelsmayexistinthedeviceindustry,nocomprehensivedevelopmentmodelhasbeen published.Thispaperreviewsexistingmodelrepresentationsandpresentsanewcomprehensivedevelopmentmodelthatcapturesallaspectsofdevicedevelopmentandcommercializationfromearly-conceptselectiontopostmarketsurveillance.Thismodelwas constructedbasedonbest-practiceanalysisandin-depthinterviewswithmorethan80 seasonedexpertsactivelyinvolvedinthedevelopment,commercialization,andregulation ofmedicaldevices.Thestage-gateprocessincludesthefollowingfivephases:(1)initiation-opportunityandriskanalysis,(2)formulation-conceptandfeasibility,(3)design anddevelopment-verificationandvalidation,(4)finalvalidation-productlaunchpreparation,and(5)productlaunchandpostlaunchassessment.Thestudyresultssuggestthat stage-gateprocessesarethepredominantdevelopmentmodelusedinthemedicaldevice industryandthatregulatoryrequirementssuchasthefoodanddrugadminstration (FDA’s)QualitySystemsRegulationplayasubstantiveroleinshapingactivitiesand decisionsintheprocess.Theresultsalsounderlinethesignificantdifferencesbetween medicaldeviceinnovationanddrugdiscoveryanddevelopment,andunderscorecurrent challengesassociatedwiththesuccessfuldevelopmentoftheincreasingnumberofcombinationproducts.
DOI:10.1115/1.3148836
1Introduction
Medicaldevices1 contributesignificantlytothecontinuousimprovementofhealthcare.Withproductlifecyclesthataresometimesasshortas18months,patientsbenefitfromacontinuous streamofinnovationthathingesheavilyonsuccessfulneedsassessmentandtheexperienceandskillsofengineersandother professionalsinvolvedintheinnovationprocess.Yet,bringinga newproductsuccessfullyfromthebenchtothebedsideishighly complexanddependsheavilyontheimplementationofrigorous processes.Theseprocessesneedtoallowdeveloperstooptimally
1Inthecontextofthisstudy,medicaldevicesaredefinedastechnologiesusedin thediagnosis,cure,mitigation,treatment,orpreventionofdiseasesorconditionsthat donotachievetheirprimarytreatmenteffectbypharmacological,immunological,or metabolicmeans.
ManuscriptreceivedMay12,2008;finalmanuscriptreceivedMay3,2009;publishedonlineJune17,2009.ReviewconductedbyPaulA.Iaizzo.
phasedevelopment,testing,andotheractivities,andtosuccessfullyexecuteonthemanifoldrequirementsofthirdparties,includingregulatorsandpayers.Theseadditionalrequirementsset medicaldevicedevelopmentapartfromthedevelopmentofother products.
Theobjectiveoftheempiricalstudypresentedhereistogivea detailedoverviewandmodelrepresentationofthemedicaldevice developmentprocessanditsvariousactivitiesanddecisions.The motivationforthisworkistwofold.First,athoroughunderstandingofthemultiplestreamsofactivitiesandresponsibilitiesin devicedevelopmentcanhelpengineersandotherprofessionalsto executethebench-to-bedsideprocessofproductdevelopment mosteffectively.Byunderstandingtheintricaciesandchallenges ofdevelopmentandcommercialization,thedevelopmentteamcan betteranticipateexternalrequirementsandtheirimplicationsfor decision-making.Thiscancontributesignificantlytosuccessful productinnovation,especiallyinlightofthemanystartupcompaniesthatprovideinnovationinthemedicaldeviceindustry.Second,theongoingeffortsbyregulatorsandpolicymakerstodesign

theleastburdensomeapproachestomedicaldeviceregulationcan benefitfromathoroughunderstandingoftheinventionanddecisionprocessesinmedicaldevicedevelopment.
Thearticleisstructuredasfollows.Section2reviewsexisting developmentprocessrepresentations.Themethodologyemployed fortheinterviewandmodelbuildingprocessisthenintroduced. Section4presentsthedevelopmentmodelanddetailedinformationaboutthevariousactivitiesanddecisionsinvolvedinthe process.Finally,thefindingsoftheempiricalstudyarediscussed andputinperspective.
2Background
2.1ProductInnovationandDevelopmentProcessModels. Productinnovationandthesuccessfulmanagementofnewproductdevelopmenthasbeenthefocusofbothacademicresearchand managerialconcernformorethan2decades 1,2 .Attheheartof theseeffortsisthedevelopmentofanunderstandingofthefactors responsiblefornewproductsuccess,andthedesiretodrivenew productsfromideatomarketfasterandwithfewermistakes.This alsoinvolvesresearchontheoptimalalignmentoftheNewProductDevelopment NPD effortwiththestrategicobjectivesand competenciesofthefirm 3 ,andmorerecently,questionsofoptimalproductportfoliosforinnovatingcompanies 4
Oneofthemostnotablecontributionsofthelast2decadeshas beenthedevelopmentandimplementationofstage-gateprocesses forproductdevelopment 5 .Thesemodelsarebothconceptual andoperationaltoolstomoveanewproductfromideatolaunch, andrecognizethatproductinnovationisaprocessthatcanbe managed 5 .Astage-gatesystemdividestheinnovationprocess intoapredeterminedsetofstages,separatedbygatescharacterizedbyasetofcriteriatobemetbeforetheproductcanadvance intheprocess.Eachstage,inturn,iscomposedofanumberof prescribedactivitiesthatareinmanycasesrelatedandthatcan occurinparallel.Typicalstagesinanewproductdevelopment processinvolvepreliminaryassessment,detailedinvestigation, actualdevelopment,testingandvalidation,andfullproduction andmarketlaunch compareRef. 5 .Therigorofastage-gate processfacilitatesformaldescriptionsofvariousactivitiesand decisions.Thesedescriptionsareoftensummarizedinstandard operatingproceduresandcanbeusefultodefinebest-practice approachestodevelopment.
Whileapplicationsofformaldevelopmentprocessesandsimilarmodelshavebeendescribedforanumberofindustries,includingtheautomotiveindustry,nocomprehensiveprocessmodelfor newproductdevelopmenthasbeenpublishedforthemedicaldeviceindustry.RochfordandRudelius 6 outlinedanumberof stagesandsuccessesforthedevelopmentofmedicalproducts,but donotprovideacomprehensiveprocessdescriptionorsummary ofactivitiesanddecisions.Kaplanetal. 7 describedseveralof thekeyactivitiesofmedicaldevicedevelopmentfromprototype toregulatoryapproval,butdonotprovideamodelofthedevice developmentprocess.
2.2ExistingProcessRepresentationsAssociatedWith MedicalDeviceDevelopment. Anumberofgraphicalrepresentationshavebeenpublishedthatdelineatevariousaspectsofthe medicaldevicedevelopmentprocess.Theyrangefromrepresen-
tationsoftheproductdefinitionandqualityfunctiondeployment QFD processestomodelsofdesigncontrol,andrepresentations ofregulatoryandreimbursementroutes.Severalofthesemodels arebrieflypresentedSecs.2.2.1–2.2.5asanoverviewoftheavailableliteratureandprovidesomeperspectiveonthesubsequent modelpresentedhere.Additionalbackgroundonmedicaldevice developmentcanbefoundinpertinenttextbooks,includingFries 8 ,KingandFries 9 ,Whitmore 10 ,andKucklick 11
2.2.1DesignInputandProductDefinitionProcessRepresentation.Fries 12 graphicallysummarizedwhathecallsthe productdefinitionprocess,includingcustomerneeds,company needs,companycompetency,andvendor/alliancecompetency. Theprocessrepresentationshowstheprogressionfromspecificationstoavailabletechnologiesandsubsequentopportunitiesto defineapplications,platforms,andenhancementsbasedonthe developedtechnologyconcept Fig.1
2.2.2QFDMatrices.Inthecontextofdesigndevelopment andrefinement,QFDgainedattentioninthe1990sinsomesegmentsofthemedicaldeviceindustry.Itisanoverallmethodology thatbeginswiththedesignprocessandattemptstomapthe customer-definedexpectationanddefinitionofqualityintothe processesandparametersthatwillfulfillthem.QFDisclosely relatedto“houseofquality”methods.Afullunderstandingofthe variouscustomerneeds,constraints,andtechnicalpossibilities, helpsamanufacturerinplanningprocessesandproductsthatare efficientandhavehighcustomerappeal.
QFDoftentakestheformofacomprehensivematrixthatincludes,amongothers,acustomerinformationportion “voiceof thecustomer” andatechnicalinformationportion.Accordingto Fries 12 ,acommonresultofQFDintroductionisthatbuilding thematrixbecomesthemainobjectiveoftheprocess,withpositiveresultssuchasamorecomprehensiveunderstandingofpatientneeds.This,inturn,directlysupportsthedevelopment process.
ThedetailedconstructionofQFDmatrices,includingassessmentofquantitativedataandrelationships,isnotdiscussedhere. Note,however,thatQFDmatricesandtheirrepresentationscanbe understoodaspartialdevelopmentmodels.
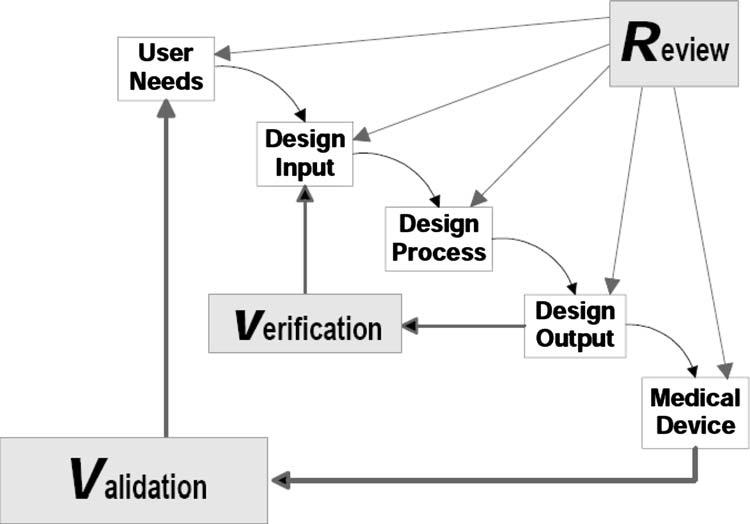
Fig.2Applicationofdesigncontrolstowaterfalldesignprocess †13‡ Fig.1Theproductdefinitionprocess „basedonRef. †12‡…

Fig.3Overviewofadesignchangelifecycle „basedonRef. †14‡…
2.2.3DesignControlProcessRepresentations.Thedesign processisoftengraphicallydepictedintermsofrelativelysimple waterfalldiagrams.Thesediagramslinkuserneedsthroughdesigninput,designprocess,anddesignoutputtothecompleted medicaldevices.Figure2showsawaterfalldiagramthatalso includestheiterativedesigncontrolactivitiesofReview,Verification,andValidation.Whilethismodelprovidesanunderstanding oftheinteractionamongvariousdevelopmentstages,itlacks somedetails,suchasprocessrequirements,health-economics,and reimbursementplanning,whicharecriticaltothesuccessfulcommercializationofanewmedicaltechnology.
Figure3,basedonRef. 14 ,isalinearrepresentationofthe devicedevelopmentphase,withfocusonthedesignchangelifecycle.Itshowsthefourmilestones“StarttoPlan,”“StarttoDesign,”“EndDesign,”and“CommenceBuilding,”withindividual tasksandactivitiesbetweenthesemilestones,andadescriptionof variousdesigncontroloutputsthroughoutthephases.
Stark 15 presentedaschematicoverviewofclinicalresearch activitiesintheproductdevelopmentcycle seeFig.4 .Therepresentationlistsfiveproductdevelopmentphases Concept— Prototype—Prepilot—Pilot—Production ,andforeachphase, showsdesigncontrol,productdevelopment,andclinicalresearch activitiesnexttoeachother,includingsomeofthemajorrelationshipsamongthem.
Riskmanagement,i.e.,theanticipationandreductionofthe chancesoffailureandtheirconsequences,isacriticalelementof thedesigncontrolactivitiesthathasreceivedsignificantattention fromregulatorsandmanufacturersinrecentyears.Figure5 shows,insimplifiedform,theriskmanagementprocessformedicaldevicesaccordingtoANSI/AAMI/ISO14971:2000.
TheFDA 16 haspublishedamorecomprehensiveschematic representationoftheintegrationofriskassessmentintotheformal designcontrolactivities Fig.6 .Thisdescriptionemphasizes variousmethods,includingfaulttreeanalysis FTA andfailure modeandeffectsanalysis FMEA .Italsoputsverificationand validationactivities2 inperspective.Acomprehensiveoverviewof thesemethodscanbefoundinRef. 17
2Verification:Establishingconformanceofdesignoutputstodesigninput.Validation:Establishingconformancethatfinalproductmeetsuserneeds.
2.2.4DevelopmentCycleRepresentations.Severaldevelopmentcyclemodelsexist.OneofthemostprominentdevicelifecyclerepresentationshasbeencreatedattheFDAbyFeigal Fig.7 .Thedevelopmentaspectiscoveredinthefirstpartofthe cyclicmodel designthroughclinicalsciences .Themodelemphasizestheroleofcustomerfeedbackandlearningfromone generationofadevicetothenext,andshowstheintertwinedflow ofinformation.Comparablemodelrepresentationscanbefound intheliterature,amongthemarepresentationbyCaliffetal. 19 thatemphasizestheclinicalandqualityaspectsinthetherapeutic developmentcycle.Themeasuresofqualityandclinicaloutcomes areparticularlyrelevantforallhealth-economicandoutcomesorientedconsiderations.
2.2.5RegulatoryandReimbursementRepresentations.Severalmodelrepresentationsofthedevicedevelopmentandcommercializationprocessexisttodescribeitsregulatoryandreimbursementaspects.Theseaspectsarehighlyrelevantfor developmentbecausetheycanhavesignificantimplicationsfor thedesignandtestingofanewtechnology.
Torepresentthedifferentregulatorypathwaysformedicaldevices,severalflowchartshavebeencreated.AcomprehensiverepresentationoftheUnitedStatesregulatoryprocesspresentedby Helmus 20 isshownineditedform Fig.8 .Thefigureoutlines thedecisionprocessthatdetermineswhetheradevicecanbe broughttomarketviathe510 k premarketnotificationpath,or thealternativepremarketapproval PMA path.Notethatexempt devices classI arenotshowninthisrepresentation,andneither aretheproductdevelopmentprotocol PDP orhumanitariandeviceexemption HDE paths.AdditionalbackgroundandadetailedreviewofUnitedStatesmedicaldeviceregulationcanbe foundinRef. 21 .AdetailedoverviewoftheEuropeandevice regulationisgiveninRef. 22
3Methodology
Thepurposeofthepresentstudywastoconstructacomprehensiverepresentationofthemedicaldevicedevelopmentprocessby conductingafieldstudy betweenOctober2006andSeptember 2007 basedonin-depthinterviewsofexpertsactivelyinvolved withthedevelopment,regulation,anduseofmedicaldevices.
JUNE2009,Vol.3 /021004-3

Amongtheintervieweeswereseniorprofessionalstaffatthe FDA,andexecutivesandotherexpertsinmedicaldevicecompanies.Withinthecohortofinterviewees,allmajorgroupsinvolved withproductdevelopment,startingwithengineeringandstrategic planningtomarketingandsales,clinical,andmanufacturing,were included.ThecompaniesrangedinsizefromstartupstoearlystagetomajormedicalmanufacturerssuchasMedtronicInc.,Biomet,BostonScientific,andEdwardsLifesciences.Thecases studiedrangedfromimagingandsurgicaldevices,toimplantable electrophysiologicalandmechanicaldevices,todrugdelivery technologiesandinvitrodiagnosticmultivariateindexassays IVDMIA .
Forthisempiricalstudy,thedatacollectedinitiallywereusedto generatehypothesesaboutthedevicedevelopmentprocess.The hypotheseswerepresentedtosubsequentintervieweesanditerativelyrevisedandimproved.Inadditiontoprimaryknowledge generationthroughinterviews,informationavailablefromtheliteratureandothersourceswasusedtobuildabodyofknowledge. Thisresearchapproach,knownasgrounded-theorybuilding,or inductivetheorizing,waschosenasawellestablishedresearch methodtodescribeandunderstandcomplexphenomena processes fromanempiricalassessmentofthediversecharacteristics oftheinvestigatedphenomenon 23
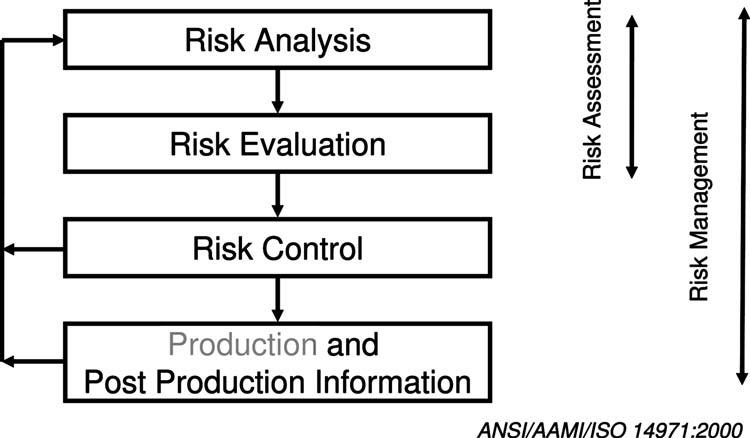
Fig.5SchematicrepresentationoftheriskmanagementprocessaccordingtoANSI/AAMI/ISO14971:2000
Theselected86intervieweescomprisedofindividualsinvolved atvariousstagesofthedevelopmentprocess,fromresearchand inventiontoearly-conceptdefinition,actualdevelopment,regulatoryapproval,andpostmarketfeedback.Inordertoensurethe highestpossiblevalidityandgeneralizabilityoftheresults,intervieweeswerechosenfromvariousbackgrounds,technologyareas,companies,andregions.Inaddition,weinterviewedsenior FDAleaders,professionalstaff,andspecializedconsultants regulatoryaffairs,reimbursement,clinicaltrialsplanning ofthemedicaldeviceindustry.
Theprotocolforthemodelconstructionprocessentailedseveralproceduralstepsasfollows:
• Reviewofstandardoperatingprocedures(SOPs) offour devicecompanies large,midsize,andstartupcompaniesincluded
• Identificationofdifferentfunctionalgroupsinvolvedindevicedevelopmentprocess.Toensureidentificationandinterviewinclusionoftherightconstituenciesinvolvedinmedicaldevicedevelopment,alistoutliningfunctionalpositions andvariousjobresponsibilitieswascreated.
•Inthe firstroundofinterviews,arepresentativesampleof theoutlinedfunctionalgroupswasinterviewed teninterviewstotal,withR&Dreceivingparticularattention .All intervieweeswereaskedtosharetheirperspectiveonthe developmentprocess,andtooutlinetheirindividualactivitiesanddecisionsinthedevelopmentprocess “bench-tobedside”
•BasedonthefirstinterviewsandreviewedSOPs,an initial draftdevelopmentmodel wasconstructed.Thismodeloutlinedthedifferentphasesinthedevelopmentprocess,and theactivitiesperformedineachfunctionduringthese phases,includingthedecisionstakenatvariousproject stages.
•Ina secondroundofinterviews 50interviews ,theinitial processrepresentationwaspresentedtointerviewees,and feedbackwasobtained.Basedonthisfeedback,someadditionsandchangesweremadeandcontinuouslyintegrated.
021004-4/ Vol.3,JUNE2009 TransactionsoftheASME

Inaddition,thevariousactivitiesanddecisionsidentifiedin themodelwerediscussedingreaterdetailwitheachintervieweetoensureaccurateandcomprehensivedescriptionof theseactivitiesanddecisionsaspartofthemodelpresented here.Adistinctdeclineofproposalsforchangeinthemodel overthecourseofthesecondroundofinterviewsshowed convergence,andledustoconcludethatthemodelpresentedinthispaperwasacceptedbyintervieweesasan accuraterepresentationofthedevelopmentprocess.
•A thirdroundofinterviews involvedtheremaining26intervieweesandfocusedonvariousspecificaspectsofthemedicalcommercializationprocessbasedontheconstructed model.Aspartoftheinterviewprocess,intervieweeswere askedaboutthespecificaccuracyofourmodel.Noadditionalchangesoradjustmentsweresuggestedbythe interviewees.
4Results
4.1ConstructedDevelopmentModel. AsoutlinedinSec.3 ofthispaper,constructionofthedevicedevelopmentmodelrelied significantlyoninsightsacquiredduringthesequenceofin-depth
interviews.Themodelispresentedinlinear-formasastage-gated process,accordingtothefeedbackandinformationobtainedinthe fieldstudy.Inreality,manyofthedefinedprocesseswithinthe modelarelikelytobeiterative.Thelinearityofthemodelisthus asimplifiedrepresentationoftheactualprocess.Toillustratethis point,anumberoftypicaliterativeloopsarepresentedafterintroductionofthelinearmodel.
4.1.1LinearModelofDeviceDevelopment.Thelinearmodel isshowninFig.9.Throughoutthefivephasesofdevelopment, activitiesareshowninconjunctionwiththevariousfunctional groupsresponsibleforthem.
Thelinearmedicaldevicedevelopmentmodelidentifiesfive majorphases,separatedbyfourdecisiongates.Predevelopment activitiesoccurpriortoGateI,developmentactivitiesoccurbetweenGatesIandIII,andproductlaunchandpostmarketassessmentoccurafterGateIV.Inthemodel,themajorfunctional groupsareidentifiedinboxesontheleftsideofthechart.Major decisionsareshown inparallelograms atthebottomofeach phase.Upperlevelactivitiesforeachfunctionalareaarehighlightedinboxeswithineachphase.Thehorizontalprogression representsageneralizedtimeline.Themajormilestones/gatescan


occuratdifferenttimesinthedevelopmentprocessdependingon thetypeofdevice.Thefivemajorphasesanddecisiongatesincludethefollowing:
•Phase1/Gate1:.initiation,opportunity,andriskanalysis
•Phase2/Gate2:formulation,concept,andfeasibility
•Phase3/Gate3:designanddevelopment,andverification andvalidation
•Phase4/Gate4:finalvalidation,andproductlaunchpreparation
•Phase5:productlaunchandpostlaunchassessment
Itshouldbenotedthatalthougheachdevelopmentphaseis presentedinadiscretemanner,theiterativeprocessofdevice developmentdoesnotalwaysfollowthelinearidealizedmodel, butratherinvolvesfuzzyboundariesbetweendecisiongates.Becauseofiterationsintheprocess,somepartsofadevelopment projectmayalreadybeinamoreadvancedphase,whilecertain activitiesofapreviousphaseneedtoberepeatedatthesametime. ThemodeldescribesaprocessthatismostapplicabletoPMAand 510 k devicesthatrequiresomeformofclinicaldata,andto devicesthatarelargelymechanicalinnature.Themodelcanbe simplifiedfor510 k devicesthatdonotrequireanyclinicaldata regulatoryclearance.
4.1.2DescriptionofDevelopmentPhases.Inwhatfollows, eachofthefivedevelopmentphasescapturedinthediagramis describedingreaterdetailbasedonthecomprehensiveinterview results.Inaddition,predevelopmentactivitiesthatneedtobe completedbeforetheformaldevelopmentprocessisinitiatedare presented.Foreachphase,majorresponsibilitiesofselectedfunctionalgroupsarediscussed.DeliverablesforeachphaseanddecisionsfacedatthefourdecisiongatesarecollectivelysummarizedinTable1.Theprocesscantakeanywherefrom15months toseveralyears,dependingonthetypeandcomplexityofthe technology,andthequalityofprocessexecution.Clinicaltesting ofsomeheartvalves,forexample,cantakeseveralyears.Similarly,technologiesthatareusedinasmallpopulationonly,canbe delayedbecauseofinsufficientenrollmentintrials.
4.1.2.1Predevelopmentactivities(phase0).Inordertocreate anewmedicaldevice,inventorsanddevicecompaniesmustfocus ontherightclinicalneed.Tothateffect,awiderangeofclinical needsarefirstidentifiedthroughdirectobservation,byspeaking withphysicians,patients,andotherhealthcareproviders,or throughpersonalexperiences,andbyareviewoftherelevant clinicalliterature.Throughaneeds-findingfunnelingprocess Fig. 10 ,inventorstakeafirstpassatnarrowingalargelistofclinical needsbasedontheestimatedmarketsizeandclinicalimpactassociatedwitheach.Tofurthernarrowthelistdown,inventors usuallyexaminepriorartrelatedtoeachneedtodetermineifthere arebarrierstofurtherdevelopmentfromanintellectualproperty perspective.Thisrequirestheveryimportantstepofdefiningpreliminaryproductconcepts.Apreliminarymarketanalysisissubsequentlyperformedtoensurethatthereisasufficientmarket opportunityforeachneed.ThisanalysisisexpandedinPhaseI. Inventorsmustalsodetermineiftheyareinapositiontoefficientlyfollowupinordertoseizeanexistingmarketopportunity. Oncethelistofclinicalneedshasbeensufficientlynarrowed, eachremainingneedtendstogetfurthervalidatedintermsof regulatoryconsiderations,reimbursementstrategies,intellectual property,andbusinessdevelopmentobjectives.Intermsofcompanydevelopment,theprocessistofurthercheckthattheproduct fitsthecompanystrategyandthatthecompanyhasthecapability ofsuccessfullycommercializingtheproduct.
4.1.2.2PhaseI:Initiation-opportunity,andriskanalysis ThepredevelopmentactivitieswithinPhaseImarkthebeginning ofthemedicaldevicedevelopmentprocess,asdefinedhere.In someinstances,thisphaseisalsoreferredtoas“technology phase.”Itischaracterizedbytheearlyevaluationofprojects aimedataddressingclinicalneedsasdescribedinPhase0.Verificationofthepreviouslyidentifiedclinicalneedmightinvolve talkingtophysicians,patients,andhands-ontechnologyusers, suchasnurses,operatingroomandlabtechnicians,andobserving themintheclinicalsetting.Thisphasealsoinvolvesareviewof theexistingmedicaldevicesandproceduresthatarebeingusedto

treatthecondition.Next,apreliminarymarketanalysis,financial review,andcompetitiveproductassessmentareoftenperformed. AreviewoftheexistingIPlandscapewithinaspecificmarketor pathologyareaisalsoconducted,aswellasanearly-stagetech-
nologyriskassessment.TheIPreviewincludesevaluationofthe technologyconceptsthathavebeenidentifiedinPhase0andI. Potentialregulatorypathsandtheirassociatedrisksarestudied andinitialreimbursementstrategiesassessed.

Understandingthemarketforaproposedmedicaldeviceand thelikelyfinancialreturnontheinvestmentarecriticalcomponentsofthepredevelopmentphase.Notethateachoftheactivities listedinthedevelopmentmodelcanincludeseveralsubactivities thatarenotexplicitlyshownonthediagram.Also,manydependenciescanexistbetweenthelistedactivities.“FinancialReview,”forexample,dependsheavilyontheresultsofotheractivitiessuchasmarketanalysis,competitiveassessment,technical feasibility,etc.
Marketanalysis/competitiveassessment
Marketanalysisinvolvesneedsassessmentandvalidation,demographicsanalysis,aSWOT strengths,weaknesses,opportunities,threats analysistoexamineaproduct’sstrengths,weaknesses,opportunities,andthreats;marketresearchaimedat identifyingthemarketsizeandgrowthpotential;andacompetitiveoutlookanalysis.Productpositioningandlaunchstrategyare alsokeyelementstomedicaldevicepredevelopmentactivities. Analystsexaminethecurrentproductmixanddeterminehowthe

newdeviceshouldbeideallypricedandpositionedtomakethe greatestmarketimpact,withoutcannibalizingexistingsales 12 Marketingandpromotionallaunchactivitiesarealsoexaminedat thattime.
Financialreview
Oncethemarketforaproposedmedicaldevicehasbeenidentified,itisnecessarytoperformathoroughfinancialreview.Financialanalysisentailscalculatingsalesprojectionsandprojected grossmarginsforaparticulardeviceoraproposedmarket 12 .In addition,afinancialanalysisoftenincludesaproposedroyalty breakoutforphysicianswhocontributedeithertotheprimarydeviceconceptortointellectualpropertytowardthedevelopmentof theproposeddevice.Afinancialanalysismayalsoincludean assessmentofwhatwouldhappenifthedeviceisnotintroduced tothemarketatthepresenttime,orifitisintroducedatalater time i.e.,theprosandconstohavingafirst-moveradvantage Projectedinventoryanalysesareoftenperformedinordertoassessthenumberofunitsthatwouldneedtobebuiltinorderto satisfymarketdemands.
Legal/IPanalysis
TheinitialreviewoftheIPlandscapeduringthepredevelopmentphaseinvolvesconductingapreliminarysearchofpatents withinandoutsideoftheUnitedStates,includingbothapplicationsandissuedpatents.DependingontheexistingIPlandscape, ariskassessmentismadetodeterminewhetherornotpursuing devicedevelopmentwouldbeaviableinvestment 12 .ThepreliminaryIPreviewcanalsobeusedtoestablishdesignboundaries.InordertoavoidIPviolations,licensingagreementsmaybe consideredatthistimeifexistingIPwasgeneratedfromindependentinventors asopposedtomajorbusinesscompetitors .
Regulatoryandclinicalpath
Theearly-stageregulatoryreviewinvolvesidentifyingthepreferreddomesticregulatorypath,basedonthetypeofproposed deviceanditsmarketentrypoint i.e.,asabreakthroughproduct, lineextension,additiontoproductfamily,etc. .Aninternational regulatoryassessmentcouldbeperformedtodeterminetheinformationneededtoobtainaconformiteeeuropeenne CE mark for marketingofadeviceintheEuropeanUnion orotherforeign regulatoryapprovalsfortheproposedproduct.Aregulatoryrisk assessmentandpreliminaryclinicalplanassesseswhethertheapprovalprocesswillrequireclinicaltrials 12 .Theearlyregulatoryreviewisparticularlyimportantforadevicemanufacturer, becauseinvestmentinaprojectwithhighregulatoryriskscould resultinanetlossforthecompanyifthedeviceisnotapproved foruseorifitrequiresunanticipatedadditionalclinicalstudies priortolaunch.
Reimbursementstrategy
Inordertocommercializeamedicaldevice,companiesoften developanearly-stagereimbursementstrategy.Itiscriticalfor companiestodeterminewhatwillberequiredtosecureapayment strategy,andhowlongitmighttaketoimplementsuchastrategy: “Understandingpotentialreimbursementratesisalsoimperative foreffectivelypricinganewproduct.Particularlyinmedicaldevices,wherethelifecyclefromconcepttolaunchisrelatively short,entrepreneursneedtodevelopplansandprocessestoobtain reimbursementcoveragepriortomarketentry.Thisisimportant tofacilitatemarketacceptanceandhelpgenerateincreaseddemand,” 24
PhaseI:Deliverablesanddecisiongate
ThemajordeliverablesofphaseIandthekeydecisionstobe madeatthedecisiongatearesummarizedinTable1.AkeydeliverableofPhaseIisabusinessplanthatisbuiltoninformation collectedduringthisphase.Theelementsofthebusinessplan includemarketassessment,unmetneeds,productdescription,financialplan P&L ,R&Dplanincludingresourceplans,regulatoryandclinicalplan,marketingplan,manufacturingplan,distri-
butionplan,reimbursementplan,salesanddistributionplan, supplychainstrategyandplan,andariskanalysisforthevarious factorsbeyondpuredesignrisk.Thebusinessplanisusuallywrittenbeforemajorinvestmentsaremade.Theinformationcontained intheplaniscriticaltoreceivingprojectapprovalfrommanagementandresourcesfortheproject,orforasmallcompany,capital frominvestors.
4.1.2.3PhaseII:Formulation-conceptandfeasibility.Ifthe projectisacceptedbyuppermanagement,developmentproceeds toPhaseII.Conceptformulationandfeasibilityassessmentoccur duringthisstage.Across-functionalprojectteamisselected often referredtoas“projectcoreteam” ,andageneralprojectplanand timelineiscreated 12 .Theteamusuallyincludesleadmembers fromR&D,qualityassurance,manufacturing,marketing/sales, regulatory,clinical,andlegalroles.Creationofadesignplanisa formalrequirement per21CFR820.30 andsignalsthebeginningofformaldesigncontrols.TheR&Dcoreteammemberoften leadsdevicedevelopmentduringPhaseIIandassumestheroleof projectmanager.Thereareexceptions,inwhichcaseaseparate projectmanagerwilloverseetimelinesandgeneralprojectplans. Theteamleaderisresponsibleforinitiatingandmanagingthe designhistoryfile DHF ,orarecordindicatingthatthedevice wasdevelopedinaccordancewiththeapproveddesignplan 25,26 .ThedesignplanisgovernedbyFDA’sQualitySystems Regulationandneedstobedefinedbyseniormanagement. Lackofadequaterecordsisafrequentcauseofauditfailureand canjeopardizetheprojecttimelineandabilitytosupportregulatoryfilings.Duringtheearlydevelopmentphase,theteam’smarketingassociateandR&Dengineeroftenmeetwithpotentialusers physicians,nurses,technicians,patients,amongothers to obtaincustomerinput.Usersstatetheirneeds,requirements,and earlydesigninputs per21CFRPart820.30 .Userinput,atthis stage,mayrangefromanapkinsketchtoacompletepatentproposalthatcanbecommercialized.Italsoiscommonfornew deviceideastobegeneratedfromconsideringexistingproduct complaints,whichareeithercommunicateddirectlyfromphysiciansorreviewedfromtheFDA’sMAUDEcomplaintdatabase. Userinputenablesdevicedesignerstogainabetterunderstanding ofthecompetitivelandscapeinaparticularmedicalspecialtyarea, andmaymotivateordissuadeadevelopmentteamfrompursuing aproposeddevice.
Voiceofcustomer/customerdesigninput
Bothduringthisearlystageofdevelopmentandthroughoutthe developmentcycle,marketingisthefunctionalarearesponsible forlisteningtothevoiceofthecustomer VOC andensuringthat userneedsaremet.Designinputsmightincludeitemssuchasthe intendeduseofthedevice,testingrequirements,biocompatibility requirements,functionalrequirements,andphysicalrequirements i.e.,size,material,packaging,sterilization,environmentalcompatibility,andappearance 26 .Marketingalsoparticipatesin early-stageprototypeevaluationsbycustomerphysicians,inconjunctionwiththeR&Dgroup.
Concepts/prototypeanalysis
DuringPhaseII,R&Disresponsibleforgeneratingmoreelaborateconcepts,basedonthedesigninputsreceivedfromateam’s marketingassociateand/orpotentialdeviceusers.Brainstorming sessionsareoftenheldduringthisstageofdevelopmentwith membersofR&D,marketing,andphysicianconsultants.Prototypedevelopmentanddesignanalysisisoftenahighlyiterative process,wherebydevicedesignsarefrequentlychangedandrefinedbeforethefinaldesignisestablished.Athree-dimensional solidmodelofaproposeddeviceisoftendevelopedinorderto conductcomputationalanalysesandconstructphysicalprototypes.Computationalanalyses,suchasfiniteelementanalysis FEA orcomputationalfluiddynamics CFD ,maybeconducted tounderstandthetheoreticalbehaviorofaproposeddevice. Physicalprototypes,suchasmock-upsandstereolithographyprototypes SLAs ,areoftenevaluatedinbench,cadaver,andearly
animalstudies,totestthephysicalperformanceofadevice.Continuousinteractionamongengineersandfuturedeviceusersis criticalduringprototypedevelopment,inordertoensurethatthe newdevicewillsatisfyend-userrequirements.Likewise,asadevicebecomesmorerefinedthroughmultipleprototypeiterations andfeasibilitystudies,theIPlandscapeiscontinuallyreviewed, andpotentiallegalandliabilityrisksareassessed.Throughout PhaseII,R&Dwilloftenholddesignreviews per21CFR 820.30 withallcross-functionalteammembersinordertosystematicallyassessadevice’sdesignprogress.Itshouldbenoted thatmanycompaniesbeginprototypingeffortsasearlyasPhaseI toinformtheirfinancialplanningandsupportdefinitionoftheir productdevelopmentstrategy.
Designriskanalysis/riskmanagement
Riskmanagementisacriticalcomponentoftheanalysis,prototype,anddesigndevelopmentphases.TheFDAexpectscompaniestohaveacompleteriskmanagementplanandsystemin place,whichconsistsofthetwoaspectsofriskanalysis identificationandquantificationofrisks andriskmanagement mitigationoftheidentifiedrisks .Severalyearsago,theindustrysupportedthecreationofISO14971,whichcoversriskmanagement formedicaldevicesandoutlinesspecificmethodstoidentifyand addressrisk.Thisstandardisnowcitedinmanyotherstandards andhasbeenrecognizedandadoptedinmanycountries.
AnumberoftoolsexistthatarecommonlyemployedtoconductriskanalysisandmanagementduringPhaseII:DesignFailureModes,Effects,andCriticalityAnalysis dFMECAor dFMEA isusedtorecognizeandevaluatethepotentialfailureof aproductorprocessanditseffects 26 .dFMEAisalsousedto identifyandprioritizeactionsthatcouldmitigatethechanceofa failure.FTAisanothercommonriskassessmenttool.FTAuses logicblockdiagramsthatdisplaythestateofasystem topevent intermsofthestatesofitscomponents basicevents .Itcanbe usedtocapturefailuresresultingfrombothhumanerrorsand hardwarefailures.Risksaremitigatedtoanacceptablelevel— generallyassafeorsaferthanexistingdevicesusedforthesame purposes.Actualmitigationofrisksneedstobeverifiedaspartof thedesignprocess.Intheremainderofthispaper,theterm“risk analysis”isinseveralinstancesusedasashortformforrisk analysisandriskmanagement.Itexplicitlyincludesriskmitigationefforts.
Designformanufacturing
Initialdesignformanufacturing dFM effortsbeginsinPhase II,inparallelwithdeviceconceptandprototypedevelopment 12 .dFMinvolvesdevelopinganinitialplanforhowfixtureswill bemade,howtoolingwillbedeveloped,whatmachinesshouldbe usedforthedevice’smanufacturingprocess,andwhetherornot moldsneedtobecreatedinordertobuildprototypesforverificationandvalidationtesting.Fordevicesthatbuildonexistingdevicesonthemarket,asignificantportionofmanufacturingwork canbeperformedatthisearlydevelopmentstage.However,for breakthroughproducts,manufacturinglargelysupportsR&DprototypeeffortsduringPhaseII,whilecontributingsuggestionsasto howproposeddevicescanbemanufacturedforhigh-volumeproduction.
Regulatoryandreimbursementstrategies
Theinitialregulatoryandreimbursementstrategiesthatwere initiatedinPhaseIarefurtherdevelopedduringtheconceptand feasibilitystagesofdevelopment.Differentoptionsareexamined regardingwhichregulatorypathtopursue fortheUnitedStates market,forexample,510 k versusPMA;forEurope,different routestoCEmarking;etc. andwhetherornotclinicalstudieswill berequired.Thereimbursementteamassesseswhetherornotexistingreimbursementcodes fortheUnitedStatesmarket,e.g., CPT3 andDRG,4 forinternationalmarkets,thecodesusedinthe
3CPT®:CurrentProceduralTerminology
respectivecountries canbeappliedtoaproposeddevice,andif so,theamountthatinsurersandusersmightbewillingtopayfor theproposedtechnology 27 .Reimbursementinformationfeeds directlyintoearly-stagedevicedevelopmentsinceitestablishesa pricepointthatdesignandmanufacturingeffortsshouldtargetin ordertobuilddevicescapableofgeneratingsufficientprofitmargins.
PhaseII:Deliverablesanddecisiongate
ThemajordeliverablesofPhaseIIandkeydecisionstobe madeatthedecisiongatearesummarizedinTable1.
4.1.2.4PhaseIII:DesignandDevelopment-Verification& Validation.Afteradeviceconcepthasbeenformulatedandseveralprototypingroundshaveoccurredtoassessfeasibility,the developmentproceedsintoPhaseIII.Duringthatphase,averificationandvalidationtestmatrixiscreatedbycross-functional teammembers.Thematrixisintendedtooutlinetheverification andvalidation V&V teststhatoccurbothbeforeandafterdesign freeze PhasesIIIandIV ,aswellastoprovideafoundationfor formalvalidationtestinginPhaseIV.V&Vtestingisconducted primarilybyresearchanddevelopment,testengineering,and qualityengineering.Marketingoftenparticipatesinvalidation testing,suchasphysicianprototypeevaluations.Verificationand validationstudiesincludingtheirmethodsofdocumentationare subjecttodesigncontrols.Withoutproperdocumentation,the studiesmaynotbeusablefromaregulatorystandpointsinceall V&Vstudiesmustbereproducible.
Verificationtesting
Designverificationcharacterizesadevicethroughfeasibility studiesandverificationtesting,andensuresthatdevicedevelopmentcomplieswiththequalitysystem QS regulation,21Code ofFederalRegulationPart820.Designverificationalsoinvolves testingandinspectingadevicetoensurethatdesignoutputssatisfydesigninputs.Testingexamplesincludeanalytical,preliminaryperformance,biocompatibility,anddurability/longevitytests. Anexampleofdesigninspectionincludesperformingtolerance stack-upsonprintsanddrawings.Verificationalsoinvolvesa widerangeoffeasibilitystudies,includingbioburden,exposure/ environmental,sterilization,cleaning,andpackaging/shiptesting 26
Validationtesting
CustomerprototypeevaluationscontinueinPhaseIIItoassess finalprototypes,priortodesignfreeze.Thesetypesofvalidation activitiesensurethatthenewdevicesuccessfullymeetsuserneeds andrequirements 26 .DesignvalidationinPhaseIIIinvolves simulatedusetests,whichmayrequiretheuseofanatomymodels and/orcadavers.Designvalidationcanalsorequirestudiestoinvestigateuserinterfacesandhumanfactors.Validationcouldalso includetestssuchasmechanicaltesting,clinicalevaluationsby physicianusergroups,biocompatibilitystudies,matingpartfunctionaltests,exposure/environmentaltesting,orpackaging/ shipmenttestingandsterility.Humanfactorsareanimportant considerationinvalidationtesting,andhaverecentlyreceivedincreasingscrutinybyregulatoryagencies.
Riskmanagementandprocessvalidation
RiskmanagementinPhaseIIIinvolvescollaborationamongall membersofthecross-functionalteam,withparticularemphasis onthequality,manufacturing,andR&Dfunctionalareas.Design controldeliverables,suchasthedFMECAareupdatedduringthis phase.ThedevelopmentteaminitiatestheprocessFMECA pFMEAorpFMECA toensurethesuccessofthemanufacturing processandtheproductionofsafeandeffectivemedicaldevices 26 .AprocessvalidationplanisalsocreatedinPhaseIIIto ensurethatamanufacturingfacilityisincompliancewithGMPs GoodManufacturingPractices;partoftheFDA’sQualitySys-
4DRG:DiagnosisRelatedGroups
temsRegulation .Aprocessplaninvolvesaprotocolforaninstallationqualification IQ ,operationalqualification OQ ,performancequalification PQ ,andproductperformancequalification PPQ 28 .AfteradesignisfrozenattheendofPhaseIII,a processvalidationplanisoftenexecutedbytheteam’smanufacturingengineerandqualityengineerinPhaseIV.ProcessexcellencetoolssuchasLeanSixSigmaarecommonlyemployedin thisphaseandthesubsequentphaseofthedevelopmentprocess.
Regulatoryandclinical
DuringPhaseIII,severaladditionalactivitiesareconductedby membersoftheregulatoryandclinicaldepartments.Regulatory activitiesincludesubmittingdesignandtestdatatotheFDAfor reviewandregulatoryapproval.FDAsubmissionisamajormilestoneinthedevicedevelopmentprocess.Preparingforasubmissionrequiresastrongcollaborationamongseveralcrossfunctionalareas,suchasclinical,R&D,quality,andregulatory. Theregulatorygroupofaproductdevelopmentteamgenerally includesaRegulatoryAffairsassociatewhohandlessubmissions andmarketclearance,andaRegulatoryComplianceassociate whoadministersthequalitysystem.Ateam’sregulatorygroup overseesthecompletionofrequirementsneededforinternational productuse,suchastheEuropeanCEmarkmentionedearlier.Ifa devicerequiresclinicaltrialsforregulatorysubmission,theteam’s regulatorygroupsubmitsaninvestigationaldeviceexemption IDE toallowthedevicetobeusedinaclinicalstudy 25 Regulatoryassociateswillalsooverseetheclinicaltrials,analyze results,andsubmitdatarequiredforregulatorysubmission.Clinicaltrialsareofsubstantialimportanceforthesuccessfulcommercializationofmedicaldevices,andthusneedtobecarefully plannedandconducted.Adetaileddiscussionofthevariousconsiderationsforclinicaltrialplanningisbeyondthescopeofthis paper.Additionalbackgroundandinformationonthistopiccanbe foundinpertinenttextbooks 29–31
Patentreviewandreimbursementupdate
ThepatentreviewprocessiscontinuedinPhaseIII sometimes alsoearlier,inPhaseII toensurethatinitiallyfiledIPissufficient toprotectthedevelopedtechnology,andtoperformasecond checkonpossibleconflictsthatcouldlimitthecompany’sfreedomtooperate 32
Thedevice’sreimbursementstrategyisfurtherestablishedin PhaseIII,asreimbursementcodesandpartnumbersareassigned tothenewdevice.
PhaseIII:Deliverablesanddecisiongate
ThemajordeliverablesofphaseIIIandkeydecisionstobe madeatthedecisiongatearesummarizedinTable1.
4.1.2.5PhaseIV:Finalvalidation-productlaunch preparation.PhaseIVofthemedicaldevicedevelopmentischaracterizedbythecreationofformaldesignprints,finalproduct verificationandvalidation,saleslaunchpreparation,andregulatoryapproval.
Finaldesigndrawingsandspecifications
Onceadesignisfrozen,formalmanufacturingprintsaregeneratedforthenewdevice,consistingofbothcomponentand assembly-leveldrawings.Finalprintsmustconformtogeometric dimensioningandtolerancing GD&T standardstoensurethat designrequirementsareeffectivelycommunicatedtosuppliers andmanufacturers.Thesameholdsforfinalspecificationsofelectroniccomponents.Tolerancestack-upsarealsoconductedonthe finaldesigntoensurethattherearenomatingpartinterferences withinadevice,orbetweenadeviceandanotherinstrumentwith whichthedeviceinteracts 12 .Materialspecifications,packaging drawings,andmarkingandlabelingspecificationsarealsofinalizedduringPhaseIV.
Designandprocessvalidation
Closureofrecommendedriskmitigatingactionitemsperthe company’sriskmanagementsystem compareearliercomment
aboutISO14971 occursduringthefinalverificationandvalidationphaseofdevicedevelopment.Theseaccompanyingdesign controldeliverablesare“living”documents,whichremainactive throughoutthelifeofthedevice.Aprocessvalidation/ qualificationplan,whichincludesanIQ,OQ,PQ,andPPQ,is executedbyaqualityengineerandamanufacturingengineerin PhaseIV.TheIQdocumentsthatacorrectmanufacturinginstrumentwasreceivedandinstalledproperly,anOQteststhatan instrumentmeetsspecificationsintheuserenvironment,andaPQ teststhatthesystemperformstheselectedapplicationcorrectly.A PPQdemonstratesthatthe“processhasnotadverselyaffectedthe finishedproductandthattheproductmeetsitspredetermined specificationsandqualityattributes” 33 .InPhaseIV,manufacturingeffortsareoftenscaled-upinpreparationforhigh-volume development,althoughnotallproductioneffortsrequirehighvolumescale-up.Statisticalprocesscontrol SPC standardsare established 26 duringthisphaseofdevelopment,andanumber ofspecificindicesarecommonlyusedinthiscontext.Thesocalled“Cp”isanindicatorofprocesscapability 12 .“Cpk,”the processcapabilityindex,involvestheadjustmentofCpforthe effectofnoncentereddistributions.Thatis,Cpkmeasures“how closeaprocessisrunningtoitsspecificationlimits,relativetothe naturalvariabilityoftheprocess” 34
Saleslaunchpreparation
Severalsalesandmarketingactivitiesmusttakeplacepriorto thelaunchofanewmedicaldevice.Acriticaldecisionthatmust bemadeattheoutsetofthisprocessisthechoiceoftheappropriatedistributionchannels,andwhetherinternalorexternalsales representativeswillbeused.Ateam’smarketingassociatemust prepareandequipsalesrepresentativeswithitemssuchasasurgicaltechniqueguidethatdefineshowthedeviceisused,videos illustratingproductuse,andsampleproductkitsforphysicians. Thedeviceisoftenadvertisedinmedicaljournalsandthenshowcasedatalargemeetingormedicalconferenceforphysiciancustomers.Salesreptrainingsitesareestablished,andatrainingprotocoliscreatedforbothsalesrepsandphysicians.Specific hospitals,oftenreferredtoaslimitedmarketrelease LMR sites, areidentifiedforinitialproductrelease.Productbrandingoccurs inPhaseIV,asmarketingworkswiththelegaldepartmentto conducttrademarksearchesinanefforttodetermineanappropriateproductname.Also,anartistormarketingcommunications firmisoftenhiredtocreateanewlogoforthedevice.Finally, marketingmustcommunicatewithmanufacturingandoperations toensurethatproductinventory/launchquantitieswillbeavailabletofulfillprojectedsalesforecasts.
FDAapproval,qualitysystems,andreimbursement
AkeyrequirementthatmustbefulfilledbeforeamedicaldevicecanbelaunchedintheUnitedStatesmarketisregulatory approval/clearancebytheFDA.Thisrequirementincludesthedevelopmentandimplementationofaworkingqualitysystem.Also, acompany’sreimbursementstrategyforthenewdevicemustbe finalizedinPhaseIV.Clinicalvalidationcontinuesbeforeand afterFDAapprovalhasbeengrantedinordertocontinuemonitoringdeviceperformanceandpossiblyexpandadevice’sindicationsforuse.
Fornewmedicaldevices,establishingaqualitysystemisa largecriticaltask.Iftheproductisalineextensiontoanexisting deviceorproductfamily,thenaqualitysystemmayalreadybein placeandworkingwell.Thequalitysystemstartswithdefining, documentingandformallyapprovingandreleasing 90% ofthe businessdocument/systems,bothproductspecific productspec andadministrative purchasingcontrols .Thisisaresourceintensive,timeconsumingtaskonascalesimilartothedevelopment process.Itisnormallydoneinparallelwiththeproductdevelopment,andcannotbedoneretrospectively.
PhaseIV:Deliverablesanddecisiongate ThemajordeliverablesofPhaseIVandkeydecisionstobe madeatthedecisiongatearesummarizedinTable1.
4.1.2.6PhaseV:Productlaunchandpost-launchassessment FinalproductlaunchandpostmarketsurveillanceoccurinPhase Vofthemedicaldevicedevelopmentcycle.“Centersofexcellence”arehospitals,labs,orphysician’sofficeusedforinitial productrelease.Theseincludehospitalsandmedicalcenters staffedwithphysicianswhohavereceivedprelaunchtrainingin theuseofthenewdevice.Thesesitescanbeidenticalwiththe previouslymentionedLMRsites,butdonothavetobe.Oncea devicehasprovensuccessfulinalimitednumberofmedicalfacilities,itwillbemarketedanddistributedforwidespreadclinical use.Apeer-to-peerphysicianeducationmodelisoftenusedto seedmarketingandpromoterapidproductadoption.Thatis,physicianswhohelpedtodevelopaproductandwhoparticipatedin validationtestingareofteninvolvedwithregionalandlocaltrainingsessions i.e.,cadaver,bioskills,ordidactic .Clinicaltrialsare frequentlyperformedbyselectphysiciansfollowingproduct launch.Thesetrialsaidingainingreimbursementsupportand additionalmarketingliterature,aswellasexpandingfurtherindicationsforuse,whichrequiresFDAapproval.
Post-launchR&D
Followingproductlaunch,R&Deffortsaretransferredtoengineersresponsibleformanagingdesignchanges,oftenreferredto asprocessengineeringgroups.Thisfunctionalareaisresponsible forthecontinualimprovementsandchangesmadetoeitherthe productitselfortheprocessesusedtocreatetheproductthroughoutthelifeofthedevice.Whenaproductcomplaintisreceived fromthefield,oneofseveralcoursesofactionmaybetaken:for instance,alabelchange 35 occurswhenanaspectofthedevice hasfailedthatwasnotproperlyindicatedontheinstructionsfor use IFU ;acustomer/feasibilitychangeismadetosatisfythe requestsofaspecificphysiciancustomer;andatotalproductredesignmayoccurwhenthereisamajorflawinthedesignand/or whentheproducthasbeenrecalled 36 fromthemarketbythe FDA.5 Designcontroldeliverablesarerequiredthroughoutthelife ofamedicaldevice.TheDevicemasterrecordisreleasedaspart ofthedesigntransferprocess,andinit,furtherdesignchangesare managed.Inaddition,companiesneedtoimplementapostmarket surveillancesystemthatiscompliantwiththeQualitySystems Regulation.Surveillanceandreportingofproductperformancein thefieldhasrecentlyreceivedincreasingattentionbytheFDA, partlyasaresultofhighlypublicizedproductrecalls.
4.1.2.7Iterationsinthedevelopmentprocess.Atypicalpatternofmedicaldeviceinnovationisitsiterativenature.Inmany cases,technologiesaredevelopedinseveralgenerations,withthe nextgenerationofadeviceincorporatingnewtechnologicalinsightsandexperiencesgainedduringfielduseoftheprevious generationofadevice.Inmanytechnologies,lifecyclesarethus veryshort.Similarly,thedevelopmentprocessofaspecificgenerationofatechnologycanbehighlyiterative,withvariousactivitiesanddecisionsinthelinearmodelrevisited.Suchiterative loopsincludedesignimprovementsbasedonclinicianfeedback, redesignsafterfailedverificationorvalidationtesting,ordesign improvementsbasedonunexpectedresultsofbenchandanimal testing so-calledpreclinicaltestingloops .Therefore,aspointed outearlier,thelinearstage-gatemodelshouldnotbemisunderstoodasaccuratelydescribingwhatinrealitycanbeahighly iterativeprocess.
Figure11showsatypicalexampleofsuchiteration,inwhich unsuccessfulverificationandvalidationtestingleadstoredesign andretestingoftheproduct.Notethatacriticaldecisioninthe
5Notethatrecallsdonotnecessarilyneedtoresultinawithdrawalandredesignof aproduct.Inmanycases,recallsarehandledwithfieldcorrectionsornoticesto users.

Fig.11Designiterationbasedonfailedverificationand validation
designprocessis“designfreeze,”afterwhichnoelementofthe product’sdesignisallowedtobechangedanymore.Alatedesign freezemayreducethelikelihoodofasubsequentiteration,but alsocanleadtoasubstantialdelayinbringingtheproducttothe market.Findingtherightbalancebetweenthetwoalternativescan beamajorchallengeforthedevelopmentteam.Asimilarchallengeistheappropriatedeterminationofthevalueandbenefitof iterations.Itisobviousthatiterationsofhigh-costactivities,such asclinicaltrials,shouldbeavoidedwheneverpossible.
5Discussion
Severalactivitieswithinthedevicedevelopmentprocesshave beenrepresentedasschematicmodelsinthepast,amongthemthe qualityfunctiondeployment,riskmanagement,andregulatoryapprovalprocesses.Similarly,anumberofoverarchingmodelsof thedevelopmentandcommercializationprocesshavebeenpublishedinthepast,forexample,theFDA’swaterfallmodelorthe representationofthecommercializationprocessofPMAdevices. Noneoftheserepresentations,however,documentcomprehensivelythemedicaldevicedevelopmentprocess.
Thefeedbackandresponsesobtainedfromintervieweesduring themodelbuildingprocessledtoarelativelyrapidconvergence towardthefinallinearstage-gatestructurepresentedhere.Inconjunctionwiththediverseempiricalsample,thissuggeststhatthe constructedmodelappliestoabroadrangeofmedicaltechnologiesandinnovationsettings.
Thestage-gateprocessiscomprisedoffivephasesfrom initiation/opportunityandriskanalysistopostlaunchandpostlaunchsurveillance.Someminorprocessdifferencesexistfordevicesrequiringclinicalstudies theso-calledIDEprocessfor PMAdevicesand510 k devicesthatrequireclinicaldata ,and fornon-IDEdevices thetechnologiesnotsubjecttoclinical evaluationsduringdevelopment .Themodelpresentedinthispaperembodiesthemorecompleteprocessbyincludingclinical studies.
TheapparentuniformityofthedevelopmentprocessacrossdevicesandtypesofcompaniescanbeexplainedbythepromulgationoftheverydetailedandcomprehensiveQualitySystems Regulation QSR-21CFR820 bytheFDA.TheQSRprescribes theelementsofthedesignprocess,includingdefinitionofdesign input,designoutput,specificationdevelopment,testing,risk analysis,processqualification,etc.Areviewofthedocument showsthatsignificantaspectsofthedevelopmentprocessaregovernedby,oratleastsubjectto,regulatoryrequirements.Regulatoryrequirementssubstantivelyimpactthemannerinwhichmedicaldevicesaredevelopedandbroughttomarketand,by impactingthetimetoFDAapproval,largelydeterminewhenthe devicecanbroughttotheclinic.Bystandardizingthedevelopmentprocess,thepublicisassuredthatcriticalelementsofgood designpracticesarenotomitted.Butstandardizationdoesnotalwayspermitstreamliningdevelopmentalprocesseswhereitwould makesense.
021004-12/ Vol.3,JUNE2009 TransactionsoftheASME
Itshouldbeappreciatedthatnonadherencetoregulatoryrequirements andthus,nonadherencetoasystematicdevelopment processastheoneoutlinedinthisstudy canleadtosignificant delaysoreventhediscontinuationofdevicedevelopment.As such,knowledgeabouttheregulatoryrequirementsandarigorous qualitysystemanddocumentationprocessareessentialsuccess factorsofanydevicecompany.Inthepublicdiscussion,their impactmightsometimesbeunderestimated,asthefocusofdiscussionisoftenlimitedtotheregulatorypathwaystoapprovaland theassociateddatarequirements.Early,innovation-orientedtechnologyassessmentduringdevelopment see,forexample,Ref. 37 thattakestheserequirementsandtheexpectedperformance ofthetechnologyintoaccount,maypresentanopportunityfor bothindustryandregulatorsinfurtherstreamliningtheinnovation process.
Despitethebenefitsassociatedwitharigorousprocessand clearlydefinedprocedures,innovationoftenoccursinanonlinear, lessstructuredway,sometimesunderunexpectedcircumstances. Inimplementingdevelopmentprocessesinorganizations,itis thereforenecessarytostrikeabalancebetweensufficientprocess rigorandenoughroomforflexibilityandcreativity.Itcanbe arguedthatevolutionaryproductdevelopmentbenefitsfromstructure,whilerevolutionaryproductdevelopmentiscatalyzedbya lessrigorousprocessenvironment.Strikingtheappropriatebalancepresentsacontinuedchallengeforanyorganizationinventinganddevelopingtechnologies,andwilloftenrequireadjustmentofgeneralprocessstructurestotheindividualrequirements ofanorganization.Complexityinprocessmanagementisadded wheninnovationisnotapurelyinternalprocesswithinanorganization,butrequiresoutsidecollaborations,in-licensingandintegrationofnewtechnologies,processes,orcompetences.Thisis oftenthecaseinmedicaldevicedevelopment.
Drug-devicedifferencesandtheirimplications
Severalimportantdifferencesexistbetweenmedicaldevices andpharmaceuticalsingeneral,andtheirdevelopmentprocesses, inparticular, compare,forexample,Refs. 38,39 .Thesedifferencesneedtobeunderstoodtoappreciatetheinherentlydifferent developmentprocessesandregulatoryrequirementsbetweendevicesanddrugs.Drug-devicedifferencesarealsoimportantto understandsincetheyhelpelucidatethechallengesassociated withthedevelopmentofcombinationproducts,whichincreasinglyblurthedistinctionsbetweenmedicaldevicesandpharmaceuticals.
Discernabledifferencesbetween“classic”devicesanddrugs notconsideringcellsandbiomaterials includethefollowing:
•Devicestendtobemoreheterogeneous seebelow asa groupthandrugs.
•Devicesusuallyhavealocalizedtreatmenteffect,asopposedtotheoftensystemicapplicationfoundindrugs.
•Devicesareengineering-basedphysicalobjects,whiledrugs arechemistry-basedcompounds.
•Devicestendtorequiresignificantuserinteraction,while pharmaceuticalsdonot.
•Devicesareinvented,oftenwiththeinvolvementofphysicianusers;pharmaceuticalsarediscoveredinlaboratorybasedresearchprocesses
Thephrase“deviceheterogeneity”referstothewidedifferencesinmodeofoperation,andthewidevarietyoftechnologies withattendantindication-specificdesignfeatures rangingfrom tonguedepressorsandimplantablejointstoimplantabledefibrillatorsandartificialcorneas .Amongotherfactors,heterogeneity leadstoamuchwidervarietyinthewaydevicesaretested e.g., widedifferencesinthetypesofbenchstudiesperformed and regulated.Whileallnewdrugsaresubjecttoasingleapproval process,medicaldevicescanbeapprovedthroughvariousregulatorypathwayswithdifferingrequirementsthatinfluencethedurationandcomplexityofthedevelopmentprocess.Amajorreason
forthedifferentrisk-basedregulatorypathwaysfordevicesisthe engineering-basednatureofdevices,whichmakestheriskprofile moreeasilyunderstoodandquantifiable.Thisisasubstantivedifferencetodrugs,whichrequirecomprehensivetoxicology,pharmacokineticandpharmacodynamictestsfortheirapproval.
Oneoftheresultsofthedifferencesintheregulatoryapproval processesfordevicesanddrugsaretheusuallyshorterlifecycles formedicaldevices.Oncethebasicsafetyandeffectivenessprofileofadevicehasbeenevaluated,testingandapprovalofnew generationsofthedevicecanleveragealreadyexistinginformationaboutthedevice.Shorterlifecyclesindevicesarealsoevidenceoftheimportantroleofuserfeedbackindeviceinnovation. Integrationofuserfeedbackcansignificantlyincreasetheusabilityandeffectivenessofadevice,andisamajorimpetusdriving productinnovationforthebenefitofpatients.
Thefundamentaldifferencesbetweenthescience-baseddrug discoveryprocessandtheengineering-based,hands-ondeviceinventionanddevelopmentprocessalsoleadtovastlydifferent capitalrequirements.Thesedifferencesareevidentinthestructuraldifferencesbetweenthemedicaldeviceandpharmaceutical industry.Whilethemedicaldeviceindustryismuchmorefragmented,withmanysmalltomidsizecompaniescontributingto innovation,thepharmaceuticalindustryisdominatedbyasmall numberoflargecompaniesthathavethefinancialresourcesto supportthemuchmorecostlypharmaceuticaldevelopmentprocess.
Thedifferencesbetweendrugsanddevicesalsoresultindifferentqualitysystemrequirements,whichleadtosubstantialdifferencesinthewaytestingandmanufacturingprocessesaredesignedandmanaged.Forexample,shelf-liferequirementsaspart ofthevalidationprocessplayamuchmoresignificantrolein pharmaceuticals.Similarly,batchtestingisastandardrequirement indrugmanufacturingandisnotrequiredinmedicaldevices.
Combinationproductdevelopment
Drug-devicecombinationssuchasthedrug-elutingstentsas wellasothercombinationproductsthatinvolvecombinationsof deviceswithbiomaterialsorcellsbegintoplayanincreasingly importantroleinhealthcareinnovation.Inlightofthedevice developmentmodelpresentedhereandthediscusseddifferences betweendeviceandpharmaceuticals,someofthechallengesand opportunitiesofcombinationproductdevelopmentandtheirresultingimplicationsarebrieflydiscussedhere.
Ontheregulatoryside,FDAhasrespondedtotheincreasing numberofcombinationproductswiththecreationofitsOfficeof CombinationProducts OCP in2002.OCPhasthecoordinating responsibilityofassessingcombinationproductsthroughouttheir productlifecycles 40 .Theprimaryregulatoryresponsibilities, however,remainwithoneoftheproductcenters CenterforDrug EvaluationandResearch CDER ,theCenterforBiologicsEvaluationandResearch CBER ,ortheCenterforDevicesandRadiologicalHealth CDRH ,basedontheproduct’sprimarymodeof action.Thismeansthattheprincipalregulatorypathwaysofthe respectivecentersare—untilnow—stillguidingthereviewand approvalprocess 41 .AcombinationproductassignedtotheOfficeofDeviceEvaluation ODE ,forexample,issubjecttothe regulatoryrequirementsoutlinedearlier.
Thenatureofcertainactivitiesoutlinedinthestage-gatedevelopmentmodelareinfluencedbydifferencesinpreclinical bench andanimal testing 42 .Fordrug-coateddevices,forexample, fatigueandcorrosiontestingofdrugcoatingsrequiresnewbenchtoptestmethodsemployedtoensurethatdrugcoatingsdonot crackor“flake-off”duringextendedproductuse 43 .Also,in termsoftestingforshelf-lifeandstability,combinationproducts presentnewchallengesforpreclinicaltesting.Thetestingoftraditional“bare”medicaldevicesoftenusestheeffectsofacceleratedagingtosimulatetheeffectsofreal-timeaging.Yet,when drugcompoundsbecomepartofaproduct,theeffectsofdrug compoundstabilitymustbecarefullyexamined,intermsofdrug elution,impuritiesandparticulates 43,44
JUNE2009,Vol.3 /021004-13
Verificationandvalidationactivities,outlinedinPhasesIIIand IVofthelinearstage-gatemodel,alsohavetobeadaptedin certaintypesofcombinationproducts.Asmentionedearlier,the currentgoodmanufacturingpractices cGMPs fordrugsanddevicesaredifferent,hencefollowingdifferentregulationsandapplicablestandards.ThecGMPsforfinishedpharmaceuticalsor drugproductsarecodifiedin21CFRParts210and211.Devices mustbemanufacturedinaccordancewiththeQSregulation,per 21CFRPart820.Whenmarketedseparatelyorwhenmanufacturedseparatelyasconstituentpartsofacombinationthatwill laterbecombined,eachpartofacombinationproductremains subjectonlytoitsgoverningcGMPregulations 21CFR3.2 However,ifcombinationproductsareproducedasasingleentity orarecopackaged,bothsetsofcGMPsareapplicableduringand afterjoiningtheconstituentpartstogether.Therefore,companies arechallengedtoensurethatcombinationproductscomplywith oneorbothmanufacturingstandards,asneeded 45 .
Unlikenoncombinationmedicaldevices,whichcanundergo processchangesratherquickly,combinationproductsrequire manufacturerstocarefullystudyadrug’soutputandstability wheneveraproduct’smanufacturingprocessismodified.Inaddition,manufacturersofcombinationproducts“mustperformmore productandprocesscharacterizationearlierinthedevelopment cyclethantheywouldwithtraditionaldevices,” 46 .Intermsof productcharacterization,traditionalmedicaldevicesareoften manufacturedfromwellcharacterizedrawmaterials;butcombinationproductsinvolvetheuseofnoveldrugexcipients,which mustbefullycharacterizedearlyinthedevelopmentprocess.
Combinationproductdevelopmentislargelyinfluencedand challengedbythedifferencesininnovationcyclesbetweenpharmaceuticalsandmedicaldevices.Pharmaceuticalinnovation cyclestendtooccurevery10–20years 47 ,whereasdevicescan becomeobsoletewithinaperiodofmonths.Therefore,inorderto producenewproductsonamorefrequentbasis,combination productmanufacturersmaychoosetoinnovatethroughdevelopingnewdevicedeliverysystemsthatarecompatiblewithexisting drugtherapies e.g.,newgenerationsofadrug-elutingstent However,robustmaterialtestingandacompleteevaluationof device/pharmaceuticalinteractionsmustoccurforeverydevice iteration 46 ,againrequiringadjustmentsinthelinearstage-gate developmentmodel.
Thedevelopmentofcombinationproductsisdistinctiveinthat itrequiresintegratingtheknowledgebaseofseveraldisciplines i.e.,biology,chemistry,andengineering ,eachofwhichrelyon specificterminologyanddesignmethodologies.Consequently, companiesdevelopingcombinationproductsmustestablisha teamstructurethatisdifferentfromthatofeithertraditionalpharmaceuticalormedicaldevicedevelopment 45 .Forexample,in additiontothefunctionalareaspresentedinthelinearstage-gate modelofdevicedevelopment,combinationproductdevelopment requiresanadditionalR&Danalyticaltestinggroup.Merging teamsofindividualsfrompharmaceuticalanddevicedevelopment,ingeneral,willrequireincreasedcross-functionalcoordination 46 .
Basedonthesedifferencesandadditionalchallenges,itshould beevidentthatthedevelopmentprocessofcombinationproducts ismorecomplexthantheclassicmedicaldevicedevelopment process.Someofthemostnotableadjustmentsthatneedtobe madeifthelinearstage-gateprocessmodelisusedincombination productdevelopmentincludethefollowing: 1 Drug-dosingand coatingsneedtobeestablishedpriororduringPhaseIofthe developmentmodel; 2 anadditionalR&Danalyticaltesting groupneedstobeadded; 3 newpreclinicaltestingmodalities needtobeconsideredforPhaseII; 4 forspecificproducts,both thedeviceandpharmaceuticalqualitysystemsneedtogovern activitiesinPhasesIIIandIVoftheprocess;and 5 supplier qualityisrequiredasaseparategroupincombinationproduct development,distinctfromthemanufacturingandoperations groupsinthetraditionaldevicedevelopmentprocess.
6Conclusions
Thelinearstage-gatemodelgivesacomprehensivedescription ofthevariousactivitiesanddecisionsassociatedwiththedevelopmentofmedicaldevices.Agoodunderstandingofthisprocess canbenefitallstakeholdersinthebench-to-bedsideprocessof devicecommercialization:investors,whoneedtoallocatetheir resourcesinthemostefficientway,andwhoneedtounderstand thefundingrequirementsofdifferenttypesofdevelopment projects;engineersandresearcherswhoaimatimprovingthedesignandbenefitofatechnology;andregulatorswhoneedtoensurethesafetyandeffectivenessofnewproductsinthemost efficientway.
Theprocessmappingpresentsseveralopportunitiesforthedevelopmentofquantitativemodelstosupportdecision-makingat variouslevels.First,informationcanbeusedtocreateearly-stage technologyassessmentandriskmanagementmodelstoinform engineeringandfundingdecisions.Thesemodelscansubsequentlybeintegratedintomorecomprehensivelifecycleriskmanagementmodelsthatallowforcontinuousupdatingoffailurerisk andtheexpectedperformanceofnewtechnologies,information thatcanbeveryusefultobothindustryandregulators.
Asdiscussedinthispaper,themedicaldevicedevelopment processdifferssubstantiallyfromthedevelopmentprocessfor pharmaceuticalproducts.Anappreciationofthesedifferencesis essentialtotheappropriatedesignoffuturedevelopmentmodels andregulatoryrequirementsforvarioustypesofcombination productsthatcrossthelinebetweendevices,pharmaceuticals,and biomaterials.
Acknowledgment
Thispaperwaswrittenbasedontheresearchperformedbythe authorsaspartofastudy“MedicalDeviceDevelopmentModels,” fundedbytheInstituteofHealthTechnologyStudies InHealth TheauthorsgratefullyacknowledgethefinancialsupportbyInHealth.
References
1 BoozAllenHamilton,1982, NewProductManagementforthe1980s,Booz, Allen&Hamilton,NewYork.
2 Cooper,R.G.,andKleinschmidt,E.J.,1986,“AnInvestigationIntotheNew ProductProcess:Steps,Deficiencies,andImpact,”J.Prod.InnovationManage., 3 2 ,pp.71–85.
3 Tzokas,N.,Hultink,E.J.,andHart,S.,2004,“NavigatingtheNewProduct DevelopmentProcess,”Ind.Mark.Manage., 33,pp.619–626.
4 Killen,C.P.,Hunt,R.A.,andKleinschmidt,E.J.,2007,“ManagingtheNew ProductDevelopmentProjectPortfolio:AReviewoftheLiteratureandEmpiricalEvidence,” ManagementofConvergingTechnologies,PICMET‘07, Portland,OR,Sept.2007,T.A.Anderson,ed.,PortlandInternationalCenter forManagementofEngineeringandTechnology,Portland,OR,pp.1864–1874.
5 Cooper,R.G.,1990,“Stage-GateSystems:ANewToolforManagingNew Products,”Bus.Horiz., 33 3 ,pp.44–54.
6 Rochford,L.,andRudelius,W.,1997,“NewProductDevelopmentProcess— StagesandSuccessesintheMedicalProductsIndustry,”Ind.Mark.Manage., 26,pp.67–84.
7 Kaplan,A.V.,Baim,D.S.,Smith,J.J.,Feigal,D.A.,Simons,M.,Jefferys, D.,Fogarty,T.J.,Kuntz,R.E.,andLeon,M.B.,2004,“MedicalDevice Development—FromPrototypetoRegulatoryApproval,”Circulation, 109, pp.3068–3072.
8 Fries,R.C.,ed.,2000, HandbookofMedicalDeviceDesign,CRC,Boca Raton,FL.
9 King,P.H.,andFries,R.C.,2003, DesignofBiomedicalDevicesandSystems, CRC,BocaRaton,FL.
10 Whitmore,E.,2004, DevelopmentofFDA-RegulatedMedicalProducts:PrescriptionDrugs,Biologics,andMedicalDevices,ASQQuality,Milwaukee, WI.
11 Kucklick,T.R.,2005, TheMedicalDeviceR&DHandbook,CRC,BocaRaton,FL.
12 Fries,R.C.,2006, ReliableDesignofMedicalDevices,2nded.,CRC,Boca Raton.FL.
13 1997,FDACDRH1997,DesignControlGuidanceforMedicalDeviceManufacturers.
14 Worona,T.,2006,“AProductDesignApproachtoDevelopingDesignControls,”MDDI,Nov.
15 Stark,N.,2001,“IntegratingClinicalResearchIntotheProductDevelopment Cycle,”MDDI,May.
021004-14/ Vol.3,JUNE2009 TransactionsoftheASME
16 FDA,1996,FDAODEGuidancefortheContentofPremarketSubmissionfor MedicalDevicesContainingSoftware DraftVersion1.3 ,Rockville,MD, Aug.,pp.12.
17 Dhillon,D.S.,2000, MedicalDeviceReliabilityandAssociatedAreas,CRC, BocaRaton,FL.
18 FDA,2005,TotalProductLifecycle,FDA-CDRHPresentationbyCDRHDirectorDr.DavidFeigal,http://www.fda.gov/cdrh/strategic/presentations/ tplc.html.
19 Califf,R.M.,Peterson,E.D.,Gibbons,R.J.,Garson,A.,Jr.,Brindis,R.G., Beller,G.A.,andSmith,S.C.,Jr.,2002,“IntegratingQualityIntotheCycleof TherapeuticDevelopment,”J.Am.Coll.Cardiol., 40,pp.1895–1901.
20 Helmus,M.N.,2003, BiomaterialsintheDesignandReliabilityofMedical Devices,Plenum,NewYork.
21 Pietzsch,J.B.,Aquino,L.M.,Yock,P.G.,Paté-Cornell,M.E.,andLinehan, J.H.,2007,“ReviewofU.S.MedicalDeviceRegulation,”ASMEJ.Med. Devices, 1 4 ,pp.283–292.
22 Chai,J.Y.,2000,“MedicalDeviceRegulationintheUnitedStatesandthe EuropeanUnion:AComparativeStudy,”FoodDrugLawJ., 55,pp.57–80.
23 Strauss,R.L.,andCorbin,J.,1998, BasicsofQualitativeResearch:TechniquesandProceduresforDevelopingGroundedTheory,SagePublications Inc.,ThousandOaks,CA.
24 StanfordBiodesign,2007,BiodesignInnovationSourcebook,Case:OIT-60.
25 FDA,2006,IDEOverview,FDACDRHWebPublication,http://www.fda.gov/ cdrh/devadvice/ide/index.shtml.
26 Teixeira,M.B.,andBradley,R.,2003, DesignControlsfortheMedicalDeviceIndustry,MarcelDekker,NewYork.
27 CentersforMedicareandMedicaidServies CMS ,2008,Innovator’sGuide toNavigatingCMS,Version1.0,August25,2008,CentersforMedicareand MedicaidServices,Baltimore,MD.
28 FDA,2006,PremarketNotification510 k ,FDACDRHWebPublication, http://www.fda.gov/cdrh/devadvice/314.html.
29 Meinert,C.L.,andTonascia,S.,1986, ClinicalTrials:Design,Conduct,and Analysis,OxfordUniversity,NewYork.
30 Chow,S.-C.,andLiu,J.,2003, DesignandAnalysisofClinicalTrials:ConceptsandMethodologies,Wiley-IEEE,NewYork.
31 Abdel-aleem,S.,2009, Design,Execution,andManagementofMedicalDeviceClinicalTrials,Wiley,NewYork.
32 USPTO,2006,35U.S.C.271InfringementofPatent,AppendixLhttp:// www.uspto.gov/web/offices/pac/mpep/documents/appxl_35_U_S_C_271.htm.
33 FDA,2006b,ProcessValidation,FDACDRHWebPublication,:http:// www.fda.gov/cdrh/qsr/04valid.html.
34 Justiniano,J.M.,andGopalaswamy,V.,2004, SixSigmaforMedicalDevice Design,CRC,BocaRaton,FL.
35 FDA,2006c,21CFR801MedicalDeviceLabeling,FDACDRHWebPublication,http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/ CFRSearch.cfm?CFRPart 801.
36 FDA,2006d,FDARecallPolicies,FDAWebPublication,http:// www.cfsan.fda.gov/~lrd/recall2.html.
37 Pietzsch,J.B.,Paté-Cornell,M.E.,2008,“EarlyTechnologyAssessmentof NewMedicalDevices,”Int.J.Technol.AssessHealthCare, 24 1 ,pp.37–45.
38 Gelijns,A.C.,1989,“TechnologicalInnovation:ComparingDevelopmentof Drugs,Devices,andProceduresinMedicine,”BackgroundPaper,Instituteof Medicine IOM
39 Ehreth,J.E.,2005,“IndustryChallengesforOutcomesResearch,”Italian JournalofPublicHealth, 2 2 ,pp.74–75.
40 Foote,S.,andBerlin,R.,2005,“CanRegulationBeasInnovativeasScience andTechnology?TheFDA’sRegulationofCombinationProducts,”Minnesota JournalofLaw,Science&Technology MJLST , 6 2 ,pp.619–644.
41 Lavender,M.,2005, RegulatingInnovativeMedicine:FittingSquarePegsin RoundHoles,DukeLaw&TechnologyReview,Durham,NC,Vol.1.
42 Robinson,R.,2004,“NonclinicalDevelopmentActivitiesforMedicalDevices,”RegulatoryAffairsFocusMagazine,http://www.raps.org.
43 Boam,A.,2003,“Drug-ElutingStents:AnFDACaseStudy,”RegulatoryAffairsFocusMagazine,pp.18–23.
44 Muni,N.,andGross,T.,2004,“ProblemsWithDrug-ElutingCoronary Stents—TheFDAPerspective,”N.Engl.J.Med., 351 16 ,pp.1593–1595.
45 Portnoy,S.,andKoepke,S.,2005,“RegulatoryStrategy:PreclinicalTestingof CombinationProducts,”MedicalDevice&DiagnosticIndustry,pp.152–163.
46 Cramer,C.,andRastogi,S.,2007,“CombinationMedicalProducts:CapitalizingonConvergence,”MDDI,http://www.devicelink.com/mddi/archive/07/ 01/009.html.
47 Bauer,H.H.,andFischer,M.,2000,“ProductLifeCyclePatternsforPharmaceuticalsandTheirImpactonR&DProfitabilityofLateMoverProducts,” Int.Bus.Rev., 9 6 ,pp.703–725.
