10 Workshop
12 Dolomite and Phanerozoic global geochemical models
25

27 2016 Spring Education Week

10 Workshop
12 Dolomite and Phanerozoic global geochemical models
25

27 2016 Spring Education Week
ConocoPhillips Auditorium, Calgary Alberta
Geothermal Energy Workshop
September 29
Canada has enormous geothermal energy potential, but to date this clean and renewable energy resource has not been exploited. This meeting will examine current geothermal research across the country and highlight some of the geoscience needed to enable successful development.


September 30
Industrial carbon management is a new business opportunity for Energy geoscientists and engineers that results from the need for a global energy system transformation. Canada is a global front -runner in many aspects of Carbon Capture, Storage and Utilization (CCSU). This meeting will provide an introduction to CCSU activities, success and challenges with a Canadian emphasis.
Joint field trip
October 1
The joint conference field trip will visit classic outcrop localities and spectacular overviews in the Bow River Corridor with stops in Canmore, Banff and the Lake Louise area. The purpose of the trip is to illustrate and discuss how the stratigraphic and structural history of the Western Canada Sedimentary Basin controls both CO2 storage and geothermal energy extraction potentials with a focus on, but not limited to the Paleozoic successions that are magnificently exposed in the mountains.

#110, 333 – 5th Avenue SW
Calgary, Alberta, Canada T2P 3B6
Tel: 403-264-5610
Web: www.cspg.org
Please visit our website for all tickets sales and event/course registrations Office hours: Monday to Friday, 8:00am to 4:00pm
The CSPG Office is Closed the 1st and 3rd Friday of every month.
Membership Inquiries
Tel: 403-513-1234 Email: membership@cspg.org
Advertising Inquiries: Kristy Casebeer
Tel: 403-513-1233 Email: kristy.casebeer@cspg.org
Sponsorship Opportunities: Lis Bjeld
Tel: 403-513-1235 Email: lis.bjeld@cspg.org
Conference Inquiries: Candace Jones
Tel: 403-513-1227 Email: candace.jones@cspg.org
CSPG Foundation: Kasandra Amaro
Tel: 403-513-1234 Email: kasandra.amaro@cspg.org
Accounting Inquiries: Eric Tang
Tel: 403-513-1232 Email: eric.tang@cspg.org
Executive Director: Lis Bjeld
Tel: 403-513-1235, Email: lis.bjeld@cspg.org
Jason Frank Co-Editor | jfrank@atha.com
Travis Hobbs, Co-Editor | travis.hobbs@encana.com
Please submit RESERVOIR articles to the CSPG office. Submission deadline is the 23rd day of the month, two months prior to issue date. (e.g., January 23 for the March issue).
To publish an article, the CSPG requires digital copies of the document. Text should be in Microsoft Word format and illustrations should be in TIFF format at 300 dpi., at final size.
Emma MacPherson, Communications Coordinator
Canadian Society of Petroleum Geologists
Tel: 403-513-1230, emma.macpherson@cspg.org
The RESERVOIR is published 11 times per year by the Canadian Society of Petroleum Geologists. This includes a combined issue for the months of July and August. The purpose of the RESERVOIR is to publicize the Society’s many activities and to promote the geosciences. We look for both technical and non-technical material to publish. The contents of this publication may not be reproduced either in part or in full without the consent of the publisher. Additional copies of the RESERVOIR are available at the CSPG office.
No official endorsement or sponsorship by the CSPG is implied for any advertisement, insert, or article that appears in the Reservoir unless otherwise noted. All submitted materials are reviewed by the editor. We reserve the right to edit all submissions, including letters to the Editor. Submissions must include your name, address, and membership number (if applicable).The material contained in this publication is intended for informational use only.
While reasonable care has been taken, authors and the CSPG make no guarantees that any of the equations, schematics, or devices discussed will perform as expected or that they will give the desired results. Some information




PRESIDENT
Greg Lynch • Shell Canada Ltd. president@cspg.org Tel: 403.384.7704
PRESIDENT ELECT
Mark Cooper • Sherwood Geoconsulting Ltd. presidentelect@cspg.org
PAST PRESIDENT
Tony Cadrin pastpresident@cspg.org
FINANCE DIRECTOR
Scott Leroux • Long Run Exploration directorfinance@cspg.org Tel: 403.766.5862
FINANCE DIRECTOR ELECT
Shelley Leggitt • NAL Resources Ltd. directorfinanceelect@cspg.org
DIRECTOR
Mark Caplan conferences@cspg.org
DIRECTOR
Jen Russel-Houston • Osum Oil Sands Corp. Jrussel-houston@osumcorp.com Tel: 403.270.4768
DIRECTOR
Eric Street • Jupiter Resources estreet@jupiterresources.com Tel: 587.747.2631
DIRECTOR
John Cody • Statoil Canada Ltd. industryrelations@cspg.org
DIRECTOR
Ryan Lemiski ypg@cspg.org
EXECUTIVE DIRECTOR
Lis Bjeld • CSPG lis.bjeld@cspg.org Tel: 403.513.1235
CSPG Members are justifiably proud of our Science and that pride energizes the many scientific and educational outreach programs of the CSPG. Some of our most passionate and dedicated volunteers work in our outreach and educational programs. When you ask them what motivates their commitment they commonly say that they want young people to be aware of the importance of petroleum and sedimentary basin Geoscience in our lives and society. They also want these same young people to be aware of Geoscience career opportunities to ensure that the “best and brightest” young minds know that they too might enjoy the kind of careers that so many of us have had. Just how important this is, was brought home to me during my most recent wilderness first-aid course. The instructor, an intelligent, fit and energetic person with a love of the outdoors had never taught geologists previously. He was amazed to know that there was a profession that combined a keen interest in the natural world, high-technology, intellectual stimulation and above average compensation. These were some of the work and job characteristics that had drawn him into wilderness first-aid. He expressed, with some regret, that he had not known that petroleum geoscience was a career option. He had been born, raised and educated in Calgary, but somehow our entire discipline and profession had been transparent to him when career choices were being made.
If we want to ensure the vitality and continuity of Petroleum Geoscience in Canadian culture it will be largely up to us to ensure that young people are informed of the opportunities and encouraged to follow career paths similar to the ones that we have chosen. This becomes even more important during “tough times”, when the career prospects are reduced and future of the profession is questioned by many. So how can we try to ensure that the “best and brightest” join our ranks in challenging times like today? I propose the following formula:
• Continue to hire, encourage and help recent graduates and current students find employment.
• Acknowledge and support excellent students.
• Participate in CSPG local and national outreach programs to encourage young people to enter our scientific discipline and profession.
Dr.Jim Christopher,one of our most distinguished and awarded members and the former Head of the Saskatchewan Geological Survey told me

of his early career experience during the downturn of the 1960’s. The industry was hurting, closing its offices in Regina, Virden, Edmonton and Fort St. John and consolidating staff in Calgary. The Provincial Surveys were sure that similar job losses were only a matter of time. Then came the fateful day when the Deputy Minister summoned all the petroleum and sedimentary basin staff together, for what all thought would be the announced job cuts. Instead they were given a rousing and motivational talk about how this was the perfect time to lay the groundwork for the anticipated resurgence of industrial activity as they were all assured of their continued employment. It was an act of faith, but it paid off and it was the right strategy for the long term. It is a challenging proposition to retain and hire young people in the midst of a significant downturn like the current one, but our industry will be back and those who plan now will lay a stronger foundation for future growth.
If you can’t hire, encourage. Finding a job is a job in itself. It can also be a lonely job. Job hunters appreciate human contact, personal advice and encouragement. Be honest, let people know that it might take hundreds of applications. Tell people about the various niches where employment can be found. If you hear about positions share the information. The larger the pool of applicants the better the choice for those hiring. When a recent graduate phones invite them to meet with you and buy them a coffee. Encourage them and help them the best you can. Try to keep in touch because when the turn-around comes it might be very much to your company’s benefit to know where the energetic and bright candidates are. It is still a big industry and the inevitable consolidation of assets that is coming will provide some opportunities, as will the turn-around, when it comes.
The excellent students that are in system now need to be encouraged to stick with it. Elsewhere in this issue you will find the record of recent presentations of the 2016 Andrew D. Baillie Awards to Mr. Bram Komaromi and Mr. Dillon Newitt by CSPG Foundation Trustee Ms. Kathleen Shannon. Both recipients are students at the University of Calgary and both are supervised by Per Kent Pedersen (pg 24). I encourage you to read about this award and (... Continued on page 6)
CORPORATE SPONSORS
SAMARIUM
CSPG Foundation
TITANIUM
geoLOGIC systems ltd.
AGAT Laboratories
Tourmaline Oil Corp.
PLATINUM
Alberta Energy Regulator
GOLD
Imperial Oil Resources
Loring Tarcore Labs Ltd.
Progress Energy Ltd.
SILVER
Chinook Consulting
MEG Energy Corp.
Shell Canada Energy
BRONZE
Schlumberger
Canada Brokerlink Inc.
Pulse Seismic Inc
MJ Systems
Nexen ULC
Repsol Oil and Gas Canada Inc.
Belloy Petroleum Consulting
Weatherford Canada Partnership
Husky Energy Inc.
CSEG Foundation
IHS Global Canada Limited
Birchcliff Energy Ltd.
Pro Geo Consultants
GLJ Petroleum Consultants Ltd.
(...
its past recipients on the CSPG website. The description of the award ceremony appears elsewhere in this this Issue. The abstracts of the two Baillie Award recipients can be found on the GeoConvention 2016 website:
http://www.geoconvention.com/ uploads/2016abstracts/178_GC2016_Natural_ Fracture_Networks_of_the_Turonian_SWS. pdf (talk), and http://www.geoconvention. com/uploads/2016abstracts/111_GC2016_ Contrasting_Reservoir_Properties.pdf (poster),
Both the CSPG and the CSPG Foundation awards and programs continue to encourage and recognize current students. You too can be part of this encouragement. Volunteer for either an awards or student outreach committee with the CSPG. If you can’t give the gift of your time then consider seriously providing a tax-deductible contribution to the CSPG Foundation to strengthen the endowment and ensure the future support for student chapters and the activities and awards that encourage and recognize individual and class achievements. Every bit will help and our efforts to strengthen the CSPG Foundation endowment currently will help to ensure a continuity of resources to support and encourage talent in the future.
Everyone’s situation is unique, but everyone can find a way to give-back to the profession through the CSPG and its Foundation. Each of us has to look at ourselves and determine how we can best help. If you have helped before now is the time to lever that experience and continue your support. If you haven’t contributed before but now find yourself with more free time explore the CSPG portfolios and events and see what might best suit you while helping the CSPG and its programs.
When you decide to become either a CSPG volunteer or a CSPG Foundation donor you make a specific commitment of your time and resources. When you decide to stand on the sidelines you make a life-long commitment. I hope that all of you will join us for the short-term.
Donating to the CSPG Foundation is quick and easy. Go to www.cspg.org/foundation and click the red Donate Now button. Should it be your wish to make a major gift or directed bequest please contact either Brett Norris (brettn@ trans-globe.com), Chairperson or Kirk Osadetz (kirk.osadetz@cmcghg.com), Secretary, of the CSPG Foundation.

As of June 1, 2016 Have you met MEERL? MEERL has a strong foundation, works hard to deliver, and believes in the wise management of energy, mineral, and water resources. MEERL is your path to fast-track your career. Welcome to MEERL – the new Master’s in Earth and Energy Resources Leadership Program at Queen’s. Learn more queensu.ca/earthenergyleadership MEERL ad.indd 1 5/26/2016 7:39:07 PM RESERVOIR ISSUE 06 • JUNE 2016 6
SPEAKER
Paul Durkin11:30 am
Wednesday, September 14, 2016
Marriott Hotel | Kensington Ballroom Calgary, Alberta
Please note: The cut-off date for ticket sales is 1:00 pm, four business days before event [Wednesday, September 08, 2016].
CSPG Member Ticket Price: $39.50 + GST. Non-Member Ticket Price: $47.50 + GST.
Each CSPG Technical Luncheon is 1 APEGA PDH credit. Tickets may be purchased online at https://www.cspg.org
Over the last decade, a series of insightful studies have highlighted fluvial meanderbelt features in strata of the Cretaceous McMurray Formation. High-quality 3-D seismic and image-log data reveal immense point bars, while detrital zircon studies have linked these features to a continental scale drainage system. These observations have prompted further investigation into meanderbelt deposits, aimed at better understanding complex facies distributions, stratigraphic architecture and paleoenvironmental interpretations of bitumen-bearing units. This study utilizes reservoir analogues from the modern Lower Mississippi River, and a Horseshoe Canyon Formation outcrop at the Hoodoos Recreation Area to inform characterization of the McMurray Formation at Surmont, Alberta.
A series of previously understated features of fluvial meander belts are considered in all datasets, including intra-point bar erosion surfaces, mid-channel bars, deposits associated with protracted abandonment processes, and downstream bar translation including counter-point bar development. Anchored in
3-D seismic data, the paleochannel evolution of a McMurray Formation meander belt is characterized and quantified (e.g., metrics for bar preservation and trajectory). This analysis also considers evidence for the tidal backwater zone, including changes in meander-belt width/thickness ratio and channel sinuosity.
Through the re-evaluation of fluvial meanderbelt models, the stratigraphic expressions of geomorphic elements that have been previously understated in rock record interpretations are deduced. The research provides revised perspectives of stratigraphic and paleoenvironmental interpretations for the McMurray Formation, which are used to account for complex facies distributions and internal stratigraphic architecture patterns in oil sands reservoirs.
Dr. Paul Durkin completed his B.Sc. at McMaster University, before moving to the University of Calgary to undertake a Ph.D. with Dr. Stephen Hubbard. His thesis focused on fluvial meanderbelt processes, including point bar evolution, sedimentology and stratigraphic architecture, as well as geocellular modelling. Paul has lead many field trips to his Ph.D. field sites in southern Alberta for industry and academic groups, and is currently a post-doctoral researcher at the University of Calgary.



SPEAKER
Andres Altosaar
Suncor Energy Inc.
12:00 noon
Wednesday, September 14, 2016
ConocoPhillips Auditorium
Gulf Canada Square 401 - 9th Ave SW. Calgary, AB
ABSTRACT
Located 60 km northeast of the city of Fort McMurray, Alberta, Canada, the Firebag Basin is host to bitumen saturated siliciclastics of the Aptian McMurray Formation. These deposits form a portion of Suncor Energy’s resource at the Firebag Steam-Assisted Gravity Drainage (SAGD) Project.
Although rooted in Precambrian basement rocks, the Firebag Basin is most prominently expressed at the sub-Cretaceous unconformity (SCU). Negative relief on this surface is upwards of 50m, and defines a nearly equidimensional rhombic basin with sharp margins. The overall surface area of the basin is upwards of 45 km2, and its shape is reflected in all underlying geologic surfaces.
Regional potential field data sets highlight a major 100 km long, NNW-SSE linear discontinuity transecting the Firebag SAGD Project that is interpreted to be a fault at the level of the Precambrian basement. Higher resolution aeromagnetic data coupled with 3D seismic suggest the fault is strike-slip in nature, locally segmented and contains both paired bend and bypass structures.
The structural history of the Firebag Basin is subdivided into four distinct phases: 1Paleoproterozoic (Taltson-Thelon orogeny),
2- Early-Middle Devonian (Antler orogeny and extension associated with uplift of the Peace River-Athabasca Arch), 3- Middle Jurassic to Early Cretaceous (Columbian orogeny), 4- Early Cretaceous (Columbianearly Laramide orogenies).
Paleozoic compressional events are interpreted to have preferentially activated this regional strike-slip fault in a dextral sense, promoting both extensional and contractional regimes within the study area. The Firebag Basin formed in the extensional zone developed around the northern releasing bend. The corresponding contractional zone formed around the southern restraining bend is defined by basement uplift and erosion. Mesozoic basement fault activation is interpreted to have significantly accelerated and focused the episodic dissolution of Middle Devonian evaporite.
The Firebag Basin is described here as a composite pull-apart/karst basin that owes its origin to sub-regionally unique extensional responses to regional compressional tectonics, and non-unique multi-phase evaporite dissolution-collapse. The second half of the study focuses on the stratigraphic architecture of the basin fill, and highlights a unique piece of sand-rich McMurray Formation stratigraphy (supra-lower McMurray, sub-middle McMurray) that forms the primary reservoir at the Firebag SAGD Project.
The creation and detailed application of a sedimentologic facies scheme to the McMurray Formation has led to a fourfold subdivision of its stratigraphy, and demonstrates a strong relationship to the structural history of the Firebag composite pull-apart/karst basin. The Aptian McMurray Formation unconformably overlies Devonian strata and consists of four distinct units that highlight the episodic nature of early McMurray Formation depositional systems and how they form in response to the structural history of the basin and higher-order, regional geologic controls. In ascending order, they are lower McMurray (lM), a currently unassigned fluvial McMurray unit, middle McMurray (mM), and upper McMurray (uM).
The fluvial McMurray unit (fM) is the primary reservoir unit for the Firebag SAGD project, producing predominantly
from the Firebag Basin. It is also a unique piece of McMurray stratigraphy in the sense that it is well-developed (50+ m thick and high net-to-gross) within the basin, and is poorly-developed to absent on the outside. It is positioned unconformably between the industry standard lower and middle McMurray units. From a regional perspective, the fM unit appears to be confined to karstderived basins in the easternmost areas of the Athabasca oil sands region.
Within the Firebag Basin, collapsed sands, carbonaceous mudstones, and coals of the lower McMurray are unconformably overlain by in-situ stacked, m-scale, fM cut-and-fill sands. In turn, the fM succession is sharply and erosionally overlain by thick and laterally extensive point bar deposits of the tidallyinfluenced mM. Outside of the basin, mM inclined heterolithic strata unconformably overlie Devonian carbonates, lM, or thin and discontinuous fM deposits.
Basic sedimentological and stratigraphic observations highlight the fM/mM boundary as one that reflects a change from locally sourced sediment deposited over rugged karst topography, to regionally sourced sediment deposited by a north-flowing, continental-scale drainage system.
Andres Altosaar is a senior geologist who specializes in structural controls on stratigraphic successions. He has worked with Suncor Energy for over 10 years, the last 5 of which were on the Firebag SAGD Project.
BASS Division talks are free. Please bring your lunch. For further information about the Division, to join our mailing list, receive a list of upcoming talks, or if you wish to present a talk or lead a field trip, please contact either Steve Donaldson at 403-808-8641, or Mark Caplan at 403-975-7701, or visit our web page on the CSPG website at http://www.cspg.org.


of CSPG workshops and field trip on GeoThermal Energy & Carbon Capture Storage and Utilization held Sept. 29th – Oct. 1st, 2016)
Carbon Dioxide Capture Storage and Utilization (CCSU) and the future of Upstream Petroleum Industry Greenhouse Gas emissions management, Sept 29th, Calgary
CONVENERS
Kirk Osadetz (CMC Research Institutes Inc.) and Luc Rock (Shell Canada Ltd.)
Industrial Greenhouse Gas emissions (GHG = CO 2 + CH 4) management presents both an opportunity and a challenge to Canada’s upstream petroleum industry. GHG management will provide for continued sustainable and socially responsible growth of the energy supply and the upstream petroleum industry. It will be part of how business is done in the near-future. The challenge lies in employing existing and discovering new technologies that enable industrial GHG management in a way that creates corporate value and meets environmental and other regulatory goals.
The CSPG is convening a one-day workshop (Sept. 29/16), in tandem with a geothermal energy workshop (Sept. 30/16) and joint field trip (Oct. 1/16) in Calgary and its environs to inform Geological, Geophysical and Engineering professionals and technicians about the current state of, and future plans for, Industrial GHG management from both a Canadian and a global perspective. Your participation will equip you and your company with the tools to plan a sustainable pathway to future business success. We invite you to consider participating in both workshops and the workshop as both the CCSU and Geothermal themes commonly have some overlap and similar stratigraphic interests, such as the basal Cambrian clastic succession.
Key themes addressed at the meeting will include:
• CO2 capture technologies including operating pilots and possible future technologies.
• CO2 storage and monitoring


in geological repositories.
• CO2 utilization in industrial processes and tertiary petroleum recovery.
• Knowledge, barriers and opportunities identified by both studies and existing CO2 management projects.
• Upstream petroleum industry CH4 emissions issues and their impacts.
• Near-term CO2 management initiatives and long-term plans for a future energy supply mix.
Canada is a global leader in the field of Carbon Dioxide Capture, Storage and
Utilization (CCSU). The stratigraphic history of the Western Canada Sedimentary Basin is uniquely endowed with the fossil energy resources that motivate the need for industrial carbon management, and also provides one of the world’s best opportunities for carbon dioxide storage in geological media. It also includes possible novel storage opportunities for carbon dioxide as a solid gas hydrate in the Athabasca region. Canada is home to two of the world’s leading CCSU projects including Shell’s fully integrated Quest CCS Project (capture, transport, storage, monitoring), and
Shell Quest CO2 Injection wellsite, Photo Courtesy of Shell Canadathe combined capture, enhanced recovery and storage project at Boundary Dam, Weyburn and Aquistore in Saskatchewan. These are among the earliest, largest and most successful industrial CCSU projects globally. Other previous, current and planned projects will also be discussed and analyzed.
Without capture there is no need for storage, and the prospects and timelines for the capture technologies will be reviewed and projected. Safe storage depends on an effective repository and monitoring. GHG management projects such as CMC Research Institutes’ Field Research Station
CONVENERS
Canada has enormous geothermal energy resources, and Canadians have the skills and knowledge to realise its full potential. While Canada is a world leader in the areas of science and technology required for a successful geothermal energy industry, compared to the rest of the world, we lag far behind in geothermal energy development. Geoscientist can reduce barriers that have so far limited geothermal development in the country. The challenge is adapting existing expertise to develop new economic opportunities for a renewable resources industry.
The CSPG is convening a one-day Geothermal workshop (Sept. 30/16), in tandem with a Carbon Capture Storage and Utilization workshop (Sept. 29/16) and joint field trip (Oct. 1/16) in Calgary. The Geothermal meeting will provide an overview of current geothermal research being conducted across the country, from high temperature volcanic systems of BC, to hot sedimentary basins of Alberta, and Enhanced Geothermal Energy research in sedimentary basins of eastern Canada. As well, the current status of direct geothermal use and geoexchange systems across Canada will be examined.
Why attend? This forum will provide the most up to date overview of geothermal energy potential in Canada and will include
that study subsurface project conformance and storage complex containment have applications beyond CCSU including steam chamber cap-rock integrity, subsurface disposal, induced hydraulic fracturing and groundwater and environmental protection.
Canadians and Canada’s upstream petroleum industry are up to the challenge of improving industrial environmental performance while maintaining economic competitiveness and exploring new opportunities. The bilateral success of Canada’s joint rehabilitation of the Great Lakes with United States cooperation
provides a model that illustrates the benefits a combination of environmental stewardship and sustainable business activities can provide using GHG emission management strategies and facilities.
This workshop provides you and your company with the opportunity to learn about GHG management, understand the progress to date, the challenges of the present, and the science, technology and skills required for a sustainable future. That future begins September 29th, 2016 in Calgary - plan to be a part of it.
presentations from leading geothermal energy researchers from universities across the country. Attendees will be able to learn what current research is being conducted on key geoscience issues, and where the major resource potential lies.
We stand at the doorstep of a renewable energy industry in Canada based on the foundation of knowledge and expertise developed by the petroleum industry over the last century. Come and be part of this exciting new opportunity.

 Larsen River hot spring in SE Yukon. Lush and colorful microbial maps growing in the steamy outflow waters.
Photo by Steve Grasby
Grayling River hot spring in NE BC. Large travertine mound growing in the steaming thermal waters.
Photo by Steve Grasby
Stephen Grasby (Geological Survey of Canada) and Jasmin Raymond (INRS)
Larsen River hot spring in SE Yukon. Lush and colorful microbial maps growing in the steamy outflow waters.
Photo by Steve Grasby
Grayling River hot spring in NE BC. Large travertine mound growing in the steaming thermal waters.
Photo by Steve Grasby
Stephen Grasby (Geological Survey of Canada) and Jasmin Raymond (INRS)
Introduction
This review is based upon a presentation concerning the role dolomite has played in global geochemical modeling given during the CSPG “inaugural Mountjoy Conference” held at the Banff Centre (August 23-28, 2015). It is an overview of attempts at understanding and modeling the “solid Earth-ocean-atmosphere system” (Holland, 1972), and how the mineral dolomite (CaMg(CO3) 2) has been incorporated. More specifically, this historical overview is focused primarily on an evaluation of these models with respect to current understanding of the origin of sedimentary dolomite.
About 85 percent of the surface area of the Earth is mantled with sediment or sedimentary rocks (http://www.britannica. com/science/sedimentary-rock). Across continental areas the sedimentary cover is slightly less than two kilometers. Carbonates form about 35% of the total mass of all preserved Phanerozoic strata (Gregor, 1985; Given and Wilkinson, 1987). On a worldwide basis, limestone has an estimated total preserved volume of 171*106 cubic kilometers, as compared to dolomite with an estimated total preserved volume of 71*106 cubic kilometers (Mackenzie and Morse, 1992). Dolomite is about 15% of the total mass of all sedimentary strata and clearly must be part of any consideration of the “solid Earth-ocean-atmosphere system”.
Dolomite is well appreciated by industry workers as an important petroleum reservoir facies, as well as an important diagenetic mineral that affects porosity development within both carbonate and siliciclastic reservoirs. Here, we look at changes in the manner in which dolomite has been incorporated into successive global geochemical models. These changes are evaluated in terms of what is now known about the process of penecontemporaneous, or pre-burial, dolomitization and observations concerning sedimentary dolomite in the rock record.
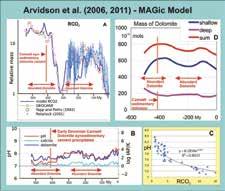
The estimated proportion of dolomite, relative to limestone, in the preserved global sedimentary record varied considerably
through time (Given and Wilkinson, 1987; Sun, 1994; Fig. 1). Sun (1994), following Sibley (1991), suggested that there may be a link between times when dolomite was relatively dominant, such as during the early Paleozoic and the Early and Middle Mesozoic, due to the dominance of low amplitude sea-level fluctuations and the absence of continental glaciation. Conversely, he argued that the Mississippian to Early Permian and the Late Cenozoic time intervals, which were characterized by active continental glaciation, have much lower proportions of dolomite in their shelf carbonate successions (Fig. 1), because of much greater amplitude sealevel fluctuations during glaciations. It does appear to be true that low amplitude peritidal successions of the lower-middle Paleozoic and Mesozoic are much more likely to be largely dolomitized than the greater amplitude, dominantly subtidal and limestone-dominated depositional cycles of the late Paleozoic and Late Cenozoic (Sun, 1994; Fig. 1).
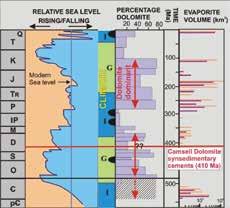
(I) conditions with lower dolomite abundances (after figure 2 in Sun (1994) with the permission of the SEPM Society for Sedimentary Geology).
An estimate of 25% dolomite in Lower Devonian shelf carbonate appears low compared to the 50% dolomite content of most other lower Paleozoic shelf
carbonates (Fig. 1). However, the sources compiled in this comprehensive summary of secular variations in dolomite abundance (Fig. 1) do not provide detailed information on a number of significant Phanerozoic time intervals, including the Lower Devonian (Sun, 1994).
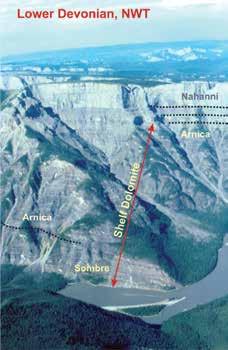
Data from the very thick and geographically extensive Lower Devonian shelf carbonate succession of the northern Canadian mainland have not been included in previous compilations. However, regional stratigraphic work has shown that the majority of these carbonates are composed of peritidal dolomite (e.g. Morrow and Cook, 1987; Morrow, 1991; Meijer-Drees, 1993; Fig. 2). An average worldwide estimate of at least 50% dolomite for Lower Devonian shelf carbonate seems much more realistic (Fig. 1, 2).
Dolomitization and dolomite precipitation is occurring at the present time in modern platform carbonate settings, such as
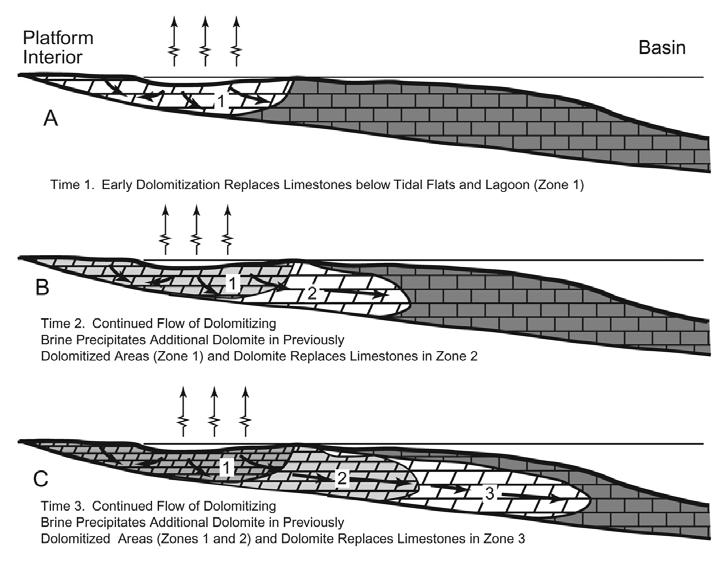
Figure 1. Dolomite abundance and global eustasy. Time intervals, such as the early Paleozoic and the Mesozoic, which saw major evaporite accumulations, are dominated by shallow water peritidal shelf carbonate successions deposited during global greenhouse (G) conditions, and generally correspond to much higher dolomite percentages. Carbonate shelves were dominantly subtidal during icehouse Figure 2.The upper part of the 2500 metre-thick Lower Devonian southern Mackenzie Mountain succession of peritidal carbonates is exposed at First Canyon of the South Nahanni River (NWT).The 1500 metres of strata exposed here include about 1250 metres of dolostone (shelf dolomite)across the Bahama Banks (Whitaker et al., 1994). Computer-based simulations of shallow reflux dolomitization of carbonate platforms by normal salinity, to mesohaline seawater have demonstrated that significant synsedimentary gravity driven reflux dolomitization of shelf lime sediments should occur within 105 My (Jones and Xiao, 2005). This type of shallow gravity-driven reflux dolomitization by mesohaline brines across weakly-rimmed carbonate shelves of slightly restricted seawater circulation and interchange with the off-platform deeper marine is a modern paradigm for many examples of inferred penecontemporaneous dolomitization (e.g. Saller and Henderson, 1998; Fig. 3).
The thermodynamic potential for dolomite precipitation is probably at a maximum in normal seawater undergoing continuous evaporation at a point somewhere below saturation with gypsum, but before gypsum precipitation begins. Dolomite saturation in modern seawater of average composition at 25ºC in equilibrium with the atmosphere (Nordstrom et al., 1979) is about 3.1 using Pitzer-based calculations (PHREEQE 3Parkhurst and Appelo, 2013). Evaporation of this seawater to the point of gypsum saturation will increase the saturation (measured as SI = log(IAP/Ksp), where SI is saturation index, IAP is dolomite ion
activity product, and Ksp is the dolomite equilibrium constant) of dolomite to a potential maximum saturation Index (SI) of slightly greater than 4.6. This potential maximum is not achieved because of some CaCO3 precipitation during evaporation, but it is likely that the saturation of dolomite will be somewhat higher than in normal seawater, as the precipitation of CaCO3 will not proceed to equilibrium and will remain oversaturated, as it is in unevaporated normal seawater (e.g. Nordstrom et al., 1979).
Continued evaporation and the subsequent precipitation of gypsum greatly reduce the saturation of dolomite and other carbonate minerals because of the near complete removal of calcium from evaporative brines. Higher average temperatures, such as occurred in the geologic past, increase the calculated mineral saturation indexes (SI) by a few percent. Dolomitization and dolomite precipitation are clearly thermodynamically enhanced in slightly, to moderately evaporated, mesohaline seawater, as envisaged by Saller and Henderson (1998). Arvidson and Mackenzie (1997, 1999) provide direct experimental data that validate the perception that the degree of supersaturation of dolomite in seawater is a basic driving force for precipitation. Secular changes in the
chemistry of seawater would have affected the rate of dolomitization and dolomite precipitation through the Phanerozoic and ideally should be incorporated as part of global geochemical modeling.
Early Ruminations on the Geochemical History of Seawater and the Rock Cycle
Rubey (1951), following his presidential address to the Geological Society of America, began modern speculations concerning the paleohistory of seawater and the atmosphere and their interaction with sediments and the lithosphere. He recognized that “information” concerning the “palaeochemistry of sea water and atmosphere” may “lie in a comparison of the composition of rocks that have been weathered and of sediments that have been deposited during the geologic past”.
Rubey (1951) recognized that CO 2 must have been continuously contributed to the earth’s atmosphere throughout Phanerozoic time to compensate for its continuous removal via deposition of “calcite” in sedimentary carbonates. He recognized that surface weathering alone was insufficient to return CO 2 in amounts necessary to maintain marine carbonate sedimentation. He deduced that in about one million years the world’s oceans would become sufficiently alkaline to precipitate the mineral brucite (Mg(OH) 2) in place of carbonates if there were no contributor of CO 2, apart from that supplied by continental weathering and riverine input, to the world’s oceans! “Milk of Magnesia™” would be freely available at cliffside outcops. A glaring omission in Rubey (1951) is any mention of the mineral dolomite and its role as a sink for magnesium in seawater, which would lower the saturation of brucite in seawater. Nonetheless, his main point is still valid in that unless CO 2 was continuously contributed to the atmosphere, brucite precipitation would eventually occur in increasingly alkaline seawater.
This paper preceded the spectacular revolution in earth sciences brought about by the recognition of crustal plate tectonics as a dominant process (Hess, 1962). Rubey (1951) suggested that carbon released by metamorphism and igneous melting, such as from hot springs around batholiths and from volcanism, might account for at least part of the discrepancy between the CO 2 removed by sedimentation and that supplied to the atmosphere, in order to maintain
deposition of carbonates. The subsequent recognition of sea floor spreading led to the realization that subduction of tectonic plates is, and was, the main driver of decarbonation of sediments and a major source for atmospheric CO 2, and for carbonate species dissolved in seawater (Barnes, Irwin and White, 1978; Holland, 1972).
Another early key publication, Urey (1952), recognized the importance of reactions involving rock weathering and marine sedimentation for global geochemistry. One representative reaction is simply the equilibrium between calcite, the most common sedimentary carbonate, and its representative silicate counterpart, wollastonite;
CO 2 + CaSiO3 CaCO3 + SiO 2 and the equivalent for magnesium-bearing minerals,
CO 2 + MgSiO3 MgCO3 + SiO 2
in which magnesite reacts with silicate to form enstatite and CO 2 vapor during metamorphism and vice versa during subaerial weathering.
A combined Urey-type reaction may also be written specifically for dolomite, the overwhelmingly common magnesiumbearing sedimentary carbonate mineral,
2CO 2 + MgSiO3 + CaSiO3 CaMg(CO3) 2 + 2SiO 2


In these expressions, Urey (1952), and even earlier, Ebelman (1845), recognized that carbonic acid-mediated weathering of silicate minerals leads ultimately to marine carbonate deposition, as calcite and dolomite. The back reaction returns CO 2 to the atmosphere via decarbonation during high temperature metamorphic and diagenetic processes, and by volcanism. This silicate- carbonate rock cycle is a major long term control on atmospheric CO 2.
The other major control on atmospheric CO 2 is the organic carbon cycle reaction, CO 2 + H 2O CH2O + O 2 (Berner, 1991). This reaction represents the burial of organic material as CH2O following photosynthesis (CO 2 + H 2O) and the subsequent return of CO 2 to the atmosphere after the oxidation of organic material during deep burial or uplift.
The years before quantitative computerbased modeling saw progress in the understanding of aqueous geochemistry and of controls on the chemical composition of the world’s oceans. Holland (1972) emphasized that the overall similarity of sedimentary rocks through geologic time indicates that seawater chemistry has been very conservative. The concept of “residence time” (i.e. average time of residence) for elements in seawater (e.g. 1 My for Ca in seawater) was highlighted by Holland (1972) with regard to sources, such as contribution rate from river inflow versus removal rate by carbonate mineral sedimentation. Dolomite precipitation was cited as a control on the concentration of the dominant dissolved carbonate species
(bicarbonate (HCO3-)) in seawater. Holland (1972) suggested dolomite precipitation may have helped to preclude deposition of non-calcium bearing, or soda, carbonates in marine evaporites. He also noted that the presence of “dolomite as a primary mineral in marine carbonate sections demands that the ratio of the magnesium concentration to the calcium concentration in seawater has never been less than unity”. This at least approximately true, although dolomite may be thermodynamically stable at solution Mg:Ca ratios of less than one at earth surface temperatures (Carpenter, 1980; Morrow, 1994; Machel, 2004). Holland’s (1972) inferences represent the first recognition of the importance of dolomite for what may be termed global geochemical modeling.
Garrels and Mackenzie (1972), documented several other key concepts, such as quantitative fluxes and residence times of 11 major rock-forming elements as ions and gases between global reservoirs (biosphere, atmosphere, ocean, post-Paleozoic and pre-Paleozoic sedimentary rocks). Theirs was the first attempt at a large scale quantitative global geochemical modeling prior to what might be termed “the age of computerization”.
These, and other earlier papers, provided the necessary context for the subsequent development of quantitative computerized models for the “solid Earth-oceanatmosphere system” following rapid developments in mainframe and personal computing from the 1970’s onward.









The “BLAG” model (Berner et al., 1983) emerged as the first computer based global geochemical model (Table 1). It provided a point of departure for subsequent computer-based modeling attempts, and was almost invariably cited in later publications (Table 1).
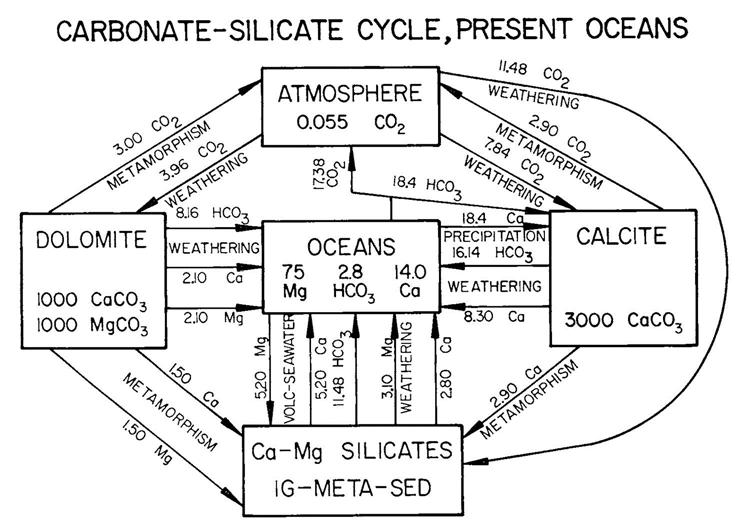
The BLAG model (Berner et al., 1983) incorporates major seawater ions (Ca2+, Mg 2+, HCO3-, SO 42-) and atmospheric CO 2 through the long term silicate-carbonate rock cycle (Fig. 4) from the Late Cretaceous to the present, a time of 100 My. The model achieved mass balance at discrete time steps by means of fluxes of ions, or elements and CO 2 between five reservoirs (oceans, atmosphere CO 2, calcite, dolomite, and Ca-Mg silicates in a combined igneous, metamorphic and sedimentary reservoir) by means of a set of differential equations and of parameters, such as rates of weathering area change, sea floor spreading, and metamorphic-magmatic decarbonation (Fig. 4). The longer term cycle of buried sedimentary organic material and its oxidation was assumed to be in equilibrium over the last 100 My (Berner et al., 1983).
The BLAG model methodology resembles that of the earlier, more publicized, “Club of Rome” model for growth of the global
economy (Meadows et al., 1972), which tracked the limits to growth of the world’s economy based on equations relating supplies of food, energy and minerals against a backdrop of environmental degradation, pollution and population growth. In BLAG, atmospheric CO2 is linked to rates of sea floor spreading and subaerial rock weathering (Berner et al., 1983), a feature retained in subsequent global geochemical models.
Dolomite was recognized as a major carbonate rock component in BLAG (Fig. 4) but acted merely as a contributor of ions to rock silicates during metamorphism and magmatism, and to atmospheric CO2 during metamorphic-igneous decarbonation. Dolomite was not thought to have accumulated during the 100 My time span of the model (Table 1).This misperception caused the size of the dolomite reservoir at the -100 My initial model time to be unrealistically large (Kasting, 1984; Table 1). However, a noteworthy conclusion of Berner et al. (1983) was their inference that atmospheric PCO2 (partial pressure of CO2 in the atmosphere) for Late Cretaceous time was many times greater than that of the present day (Table 1). Most subsequent simulations have reaffirmed this perception (Table 1).
Kasting (1984) critiqued the BLAG model and deduced that there was a strong possibility that the size of the dolomite
reservoir in Late Cretaceous time was approximately equal to that of the present day, with the corollary that dolomite must have been precipitated in past oceans, even if dolomite precipitation in modern oceans was unknown at the time of publication of the BLAG Model.
The increased interest in modern levels of atmospheric carbon dioxide led Berner and his collaborators to focus their attention on only those aspects that are most relevant to the determination of past levels of atmospheric PCO2. The succeeding GEOCARB models (GEOCARB I (Berner (1991), II (Berner (1994), III (Berner and Kothavala, 2001) and GEOCARBSULF (Berner, 2006)) reflect this purpose in that only the flux in carbon was modeled, with the later addition of sulfur in the GEOCARBSULF model (Table 1). The focus of the GEOCARB series of models was the partitioning of carbon between only three global reservoirs, a carbonate rock reservoir, an organic matter reservoir, and a combined single ocean and atmosphere reservoir in a “collapsed” system of rocks, gas and organic matter (Arvidson et al., 2013). However, the calculated history of changes in Phanerozoic atmospheric PCO2 was remarkably similar for all GEOCARB models as well as with that calculated by the later MAGic simulations (Table 1; Fig. 5A).
Atmospheric PCO 2 values in all these model simulations were very high from early to middle Paleozoic, low in late Paleozoic time and high in Mesozoic time, declining to modern day levels through Cenozoic time (Table 1; Fig 5A). The low RCO 2 values for the late Paleozoic in all models (Table 1; Fig. 5A) have been attributed largely to more rapid removal of CO 2 from the atmosphere during the rise of large vascular plants (e.g. trees) in the Late Devonian and Carboniferous (Berner and Kothavala, 2001). This is an effect analogous to the oil industry “carbon sequestration” technology of the present day used to artificially lower modern day atmospheric PCO 2. High GEOCARB model values of atmospheric PCO 2 during the early Paleozoic were attributed to the near-absence of vascular plants and lower solar luminosity, whereas calculated high Mesozoic PCO 2 values were attributed largely to a large increase in the rates of rock weathering due to the rise of gymnosperms and angiosperms (Berner, 1994; Berner and Kovathala, 2001).
TABLE 1
Recently, Arvidson and his collaborators have extended global geochemical modeling with their MAGic model (Arvidson et al., 2006; 2011; 2013; Table 1). This model incorporates most aspects of the previous GEOCARB models, as well as those of the earlier BLAG Model, but also includes a more comprehensive set of model reservoirs and steady state fluxes of a large suite of elements, ions and gases, reminiscent of the previously mentioned, pre-computer age model of Garrels and Mackenzie (1972).
An important feature of MAGic is its incorporation of the Pitzer (1973) method for the accurate calculation of ion activities in seawater. This permitted calculation of the seawater saturations of minerals, such as calcite and dolomite, and consequently of more accurate values for atmospheric PCO 2 (Fig. 5A, B). The MAGic model is much more complex than preceding models, and, as such, will not be described in any detail, except with regard to its treatment of dolomite and dolomitization.
Dolomite in the 2006 MAGic model was partitioned between a shelf dolomite of sedimentary origin, and a burial dolomite formed by the reaction of magnesiumbearing clays during high temperature burial diagenesis. Shelf dolomite accumulation was modeled as a direct precipitate (4HCO3- + Ca2+ + Mg2+ CaMg(CO3)2 + H2CO3 , and as Ca2+ + Mg2+ + 2HCO3- + SO4 2CaMg(CO3)2 + H2SO4) from seawater of a single bulk composition only, and not as a replacement, or “dolomitization”, of calcite, or of aragonite (2CaCO3 + Mg2+ CaMg(CO3)2 + Ca2+ (Carpenter, 1980)), which is more familiar to carbonate sedimentologists. Dolomite precipitation rates in MAGic were governed by the degree of dolomite oversaturation (Arvidson et al., 2006; Arvidson and Mackenzie, 1999). MAGic simulations were constrained to agree with major seawater ion ratios, such as Mg:Ca (Hardie, 1996; Lowenstein et al., 2001; Fig. 6).
Subsequently, the rate of sedimentary dolomite accumulation was varied to assess the effect this would have on alkalinity and the Mg:Ca ratio of seawater (Arvidson et al., 2011). This was in recognition of the possible kinetic factors that influence the rate of dolomite precipitation (e.g. Machel, 2004). Large arbitrary reductions in the depositional flux of dolomite were found to be coupled with unacceptable
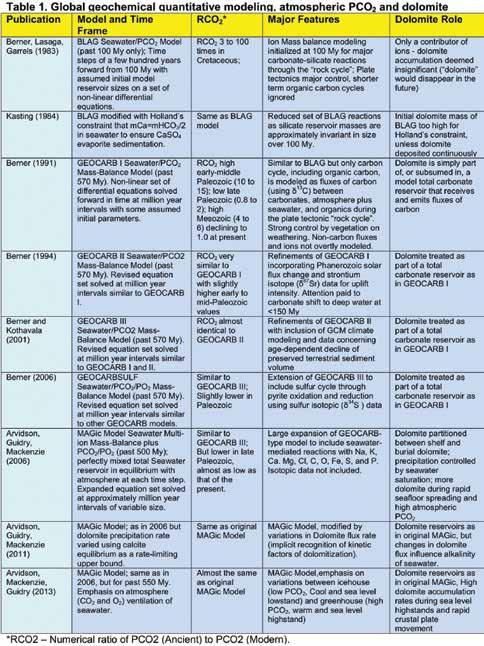
variations in global seawater acidity and alkalinity. Nonetheless, Arvidson et al. (2011) concluded that moderate variations in the depositional flux of dolomite may have exerted a strong control on seawater alkalinity, on Mg:Ca ratios, and on pH throughout much of the Phanerozoic. They concluded that the depositional flux of dolomite was greatest during sea level highstands, during times of high atmospheric PCO2, and, paradoxically, when seawater pH and dolomite saturation were low, such as during the early Paleozoic and early to midMesozoic (Fig. 5A, B). Much lower dolomite depositional flux was estimated for the late Paleozoic and the later Cenozoic when sea-level lowstands and glacial “icehouse” conditions prevailed (e.g. Fig. 1) and the seawater saturation of dolomite was high compared to that during the early Paleozoic
and Mesozoic (Fig. 5B).
B) and CO2 (in A) values at 10 My intervals show strong correlation; D) MAGic model
variation in dolomite mass between the shallow marine (blue) versus deep burial dolomite reservoir (magenta). Double ended red arrows in A, B and D indicate time intervals characterized by abundant dolomite (Sun, 1994). The time of Camsell Early Devonian dolospar precipitation is shown on A, B and D (figures 5A and B are after figure 2A and E of Arvidson et al., 2011 with permission of Aquatic Geochemistry; figure 5D is after figure 13B of Arvidson et al. 2006 with permission of the American Journal of Science).
The estimation of the proportion of CO 2 partitioned between dolomite, limestone, seawater and the atmosphere at each MAGic model time step is not materially affected by ignoring the in-situ process of CaCO3 dolomitization, and modeled values for atmospheric PCO 2 (Fig. 5A) are unaffected. This is because the total mass of CO 2 sequestered by direct precipitation of calcite (or aragonite) and dolomite is little changed by the process of dolomitization because of the nearly complete retention of CO32- in the solid phases.
Arvidson et al. (2006) argued that times of relative dolomite abundance coincided with times of more rapid seafloor spreading and higher atmospheric PCO2 values for the Phanerozoic. Earlier, Mackenzie and Morse (1972) had also suggested that the environmental conditions for early dolomitization, and calcite oöid and cement formation were best met during extended times of high sea level during more rapid midocean ridge accretion when atmospheric CO2 levels were high and, somewhat paradoxically, when the saturation state of seawater with respect to carbonate minerals was relatively low. During times of low sea levels, early dolomitization was less favored, and aragonite was more abundant because of low atmospheric CO2 levels, high Mg:Ca ratios, and higher carbonate mineral saturations. These perceptions are largely consistent with the variations in the percentage of shelf dolomite through the Phanerozoic, as tabulated by Sun (1994; Fig. 1, 5A).
As might be expected, there is a close correlation between calculated pH and atmospheric PCO2 in the Arvidson et al. (2006; 2011) models, with high levels of atmospheric PCO2 strongly correlated with low seawater pH values (Fig. 5A, B, C), analogous to modern day ocean acidification due to our fossil fuel usage and rapidly rising atmospheric CO2 Saturation levels of dolomite and calcite also exhibit a strong positive correlation with
seawater pH in MAGic in that their saturation levels are more elevated during times with more alkaline, higher pH, seawater (Fig. 5B, C). In all iterations of the MAGic model, seawater pH is lower than that of modern seawater, particularly for the entire early Paleozoic (e.g. 5B). Regardless of the inner workings of the MAGic model, there is a first order correlation between calculated pH values and all carbonate mineral saturations (Fig. 5B), as well as between calculated pH and atmospheric PCO2 values (Fig 5D). In other words, times of elevated atmospheric PCO2 correspond to low pH and low dolomite saturations, relative to modern values (Fig. 5).
This is perhaps paradoxical in that we might expect that times of low atmospheric PCO 2 and high seawater pH, Mg:Ca ratios and dolomite saturations would be accompanied by more rapid dolomite precipitational flux and dolomitization. The high MAGic model dolomite depositional fluxes during times of high atmospheric PCO 2, low seawater pH, Mg:Ca ratios, and dolomite saturations can be explained qualitatively as a consequence of the dominance of shallow shelf depositional depositional environments during these times and their relative absence during times of low atmospheric PCO 2 (e.g. Sun, 1994). Certainly there is a good correlation between the times of more abundant dolomite (Fig. 1) and times of high atmospheric PCO 2 calculated in the MAGic and GEOCARB models (Fig. 5A).
There is, however, little consistency between the known variations in the percentage of shelf dolomite (Sun, 1999; Fig. 1) and the secular variations in masses of dolomite calculated in MAGic (Fig. 5D). Also, there is some inconsistency between times of enhanced dolomitization and empirically determined seawater Mg:Ca ratios for Phanerozoic seawater based on the mineralogy of CaCO3 in marine paleofauna (Ries et al., 2010). Dolomite is abundant during the early Paleozoic when Mg:Ca seawater ratios were low, and lowmagnesian calcite was dominant, but also during the Permian and Triassic with higher Mg:Ca ratios and aragonite/hi-magnesian calcite as dominant CaCO3 polymorphs (Fig. 6).
This touches on another known kinetic factor concerning dolomitization in that the rate of dolomitization is considerably more rapid for aragonite than for calcite (Katz and Matthews, 1977). Dolomitization
occurs rapidly in modern day sabkhas along the Trucial Coast of the Persian Gulf, where aragonitic sediments are being dolomitized within about 1000 years (Patterson and Kinsman, 1982).
Paradoxically, geochemical factors that favor rapid dolomitization of CaCO3, or precipitation of dolomite from seawater, such as high Mg:Ca ratios, high dolomite seawater saturation, precursor CaCO3 as aragonite, and elevated pH, are more typical during times of relatively low abundance of sedimentary dolomite, such as the Late Devonian to earliest Permian time interval and the Late Cretaceous to Holocene time interval (Fig. 1, 5B, 6).
Given this information a reasonable conclusion might be that the Sun’s (1994) emphasis on the relatively greater time spent by lime sediments in contact with continuously refluxing seawater across the wide shallow shelves dominated by low amplitude peritidal carbonate successions of the lower Paleozoic and Mesozoic was a dominant factor. This is in contrast to the higher amplitude, subtidally-dominated successions of the Permo-Carboniferous and Cenozoic in which supratidal deposits are almost absent, and which, by implication, were not affected by significant reflux of seawater during their deposition (Sun, 1994).
Observational data supporting an interpretation of direct precipitation of dolomite from ancient seawaters would be relevant concerning the saturation of dolomite in seawater from which dolomite can be documented to have precipitated. In this context, observations linking medium and coarse crystalline dolomite cements to precipitation from seawater during deposition, but before burial, would be relevant. The precipitation of these dolomite cements, or dolospars, would then be a direct reflection of the degree to which dolomite was oversaturated in paleoseawater.
One of the first suggestions that some sedimentary dolomite might have been precipitated directly from ancient seawater was for the finely preserved dolomitic ooids and cement in the late Proterozoic Beck Spring Dolomite (Tucker, 1982). Doubt was
(Continued on page 18...)
cast on this interpretation by Zempolich and Baker (1993) who demonstrated that preservation of fine detail of aragonitic oöids occurred during experimental high temperature dolomitization. The distinction between cement and replacement is important, because open space cementation proceeds in oversaturated diagenetic fluids, whereas replacement implies dissolution of the host and conservation of material through concomitant precipitation in a system that is partly closed to surrounding fluids (Zempolich and Baker, 1993), such as in partly lithified carbonate sediment with intergranular porosity-occupying seawater.
Obviously, the magnesium required for in-situ dolomitization of slightly lithified aragonitic or calcitic lime mud sediment in natural settings, such as sabkhas, must be supplied externally from seawater. However, dolomitization of aragonite and calcite in high temperature laboratory experiments proceeds through several intermediate magnesian calcite phases, which in turn recrystallize to dolomite (Nordeng and Sibley, 1994). Katz and Matthews (1977) found that the intermediate phases in similar high temperature experiments formed within restricted reaction zones surrounding the dissolving grains, and not in full equilibrium with the bulk solution. This is undoubtedly similar to the much slower process of in-situ dolomitization of lime muds in natural settings at earth surface temperatures (e.g. Patterson and Kinsman, 1982). During such in-situ dolomitization, additional magnesium is supplied incrementally from the bulk solution during the dissolution and precipitation of intermediate phases, the dissolution of which causes dolomite oversaturation within the local reaction zone.
In contrast to the in-situ multi-stage dolomitization of lime muds, the precipitation of coarsely crystalline dolospar in open megascopic pore spaces, such as within vugs, in partly to completely lithified lime sediments, is directly dependent upon the degree of dolomite oversaturation in the bulk solution. However, most dolospar cements that infill vugs were precipitated, or are regarded as having been precipitated, in burial settings from solutions other than contemporary seawater (e.g. Machel, 2004).
If instances of dolospar can be shown to have precipitated from contemporary seawater, they would provide direct
evaporites, nonskeletal carbonates, and seawater Mg/Ca ratios. Curve is seawater Mg/Ca. Closed circles are from fluid inclusions. Open circles are Mg/Ca ranges estimated from fossils. Star is modern seawater (Mg/Ca=5.2). Horizontal line divides the calcite (Mg/Ca<2) and aragonite/high Mg calcite (Mg/Ca>2) nucleation fields, with times of primarily aragonitic (“A”) and calcitic (“C”) precipitates. Three intervals of aragonite + high Mg calcite “Icehouse” precipitation alternate with two intervals of calcite “Greenhouse” precipitation. Vertical red line is time of Camsell synsedimentary dolospar precipitation. Red bar indicates the Mg/Ca of 410 My Devonian seawater from Demicco et al. (2006). Paradoxically, times of lower Mg/Ca ratios, like the Early Devonian were times of abundant sedimentary dolomite accumulation (after figure 1 in Ries, 2010 with permission of the journal “Biogeosciences”).
evidence concerning the saturation state of dolomite in seawater of that time, unlike dolomudstones or dololaminites, which are less indicative of bulk seawater dolomite saturation state. However, there are very few published examples of Phanerozoic dolospars within dolomitized stratigraphic successions that can be linked to precipitation from contemporaneous seawater.
Studies of the dolomites of the Cenozoicaged carbonate successions of the Caribbean Islands have indicated that both the finely
crystalline replacement synsedimentary dolomite and the coarser, post-lithification, but pre-burial (i.e. never buried), dolospar cements that are widespread across these islands were all precipitated from contemporaneous seawater, or slightly modified contemporaneous marine fluids (Budd, 1997; Jones, 2004; 2005). This is not surprising because of the high pH value of about 8.3 and high seawater dolomite saturation index of about 3.2, as calculated in MAGic, from the beginning of the Miocene time to the Holocene (Fig. 5B; Budd, 1997). The relatively low percentage
of sedimentary dolomite in carbonates of these ages can then be attributed to Sun’s (1994) explanation that the high amplitude, subtidally-dominated successions of the Cenozoic, in which supratidal deposits are almost absent, were not affected by significant reflux of seawater during their deposition, and hence were relatively undolomitized.
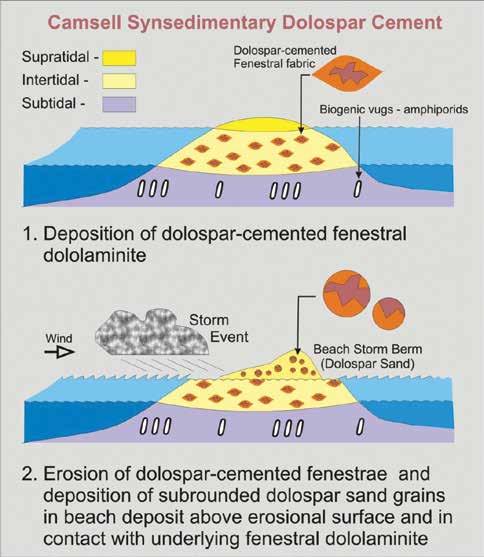
The only other published example (Morrow, 1990) for Phanerozoic strata in which open space-filling dolospar has been linked to the depositional setting of the host strata is in the 410 My Lower Devonian Camsell Formation of the Northwest Territories of Canada (Morrow and Cook, 1987; Fig. 7A, B). In this peritidal succession of silty and sandy dolostone, which borders the evaporitic Root Basin (Fig. 7a), there are sandstones composed almost entirely of eroded grains of coarse dolospar cements (Fig. 7C, D, E, F, G). These yellowish-brown dolospar sand beds are discontinuous, and commonly overlie yellow-weathering dolomudstones and dololaminites containing abundant dolospar-cemented fenestrae that are oriented subparallel to bedding, forming a laminoid fenestral fabric (Tebbutt et al., 1965). The lower contacts of these dolospar sandstone beds overlying fenestral dolomudstone are locally erosional (Fig. 7C, D, E). Millimeter to centimeter-sized irregular birdseye, or fenestrae, such as in the Camsell laminoid fenestral fabrics, originate as voids in intertidal to supratidal environments (Shinn, 1983).
The most probable interpretation consistent with these observations is that the dolospar sand beds represent erosion of underlying weakly lithified fenestral dolomudstones. Dolospar sand sediment was mobilized as storms swept over depositional islands, or carbonate mud mounds (Fig. 8), of the Camsell island belt that bordered Root Basin in Early Devonian time (Morrow and Labonte, 1988). The dolospar forming the sand grains are petrographically identical to the dolospar in the fenestrae of the underlying fenestral dolomudstones and individual dolospar crystals have been truncated by erosion along the edges of grains (Fig. 7D, E, F, G; Fig. 8). There is no indication that the fenestral fabric dolospar, or the dolospar forming the dolomite sand grains, ever had calcite or aragonitic precursors, and delicate cathodoluminescent zonations, indicative of primary precipitation, are well preserved (Morrow, 1990; Fig. 7F, G).
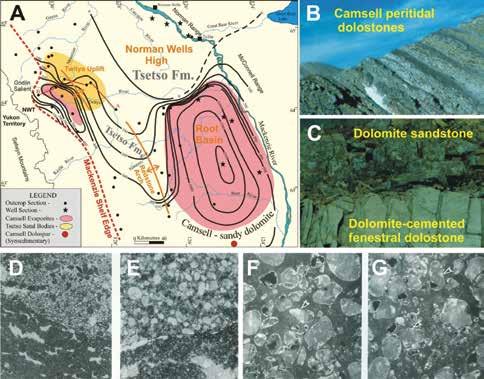
Redeposition of fenestral dolospar as dolospar sands and subrounded dolospar lithoclasts is evidence that dolospar cementation of fenestrae occurred during deposition and directly from contemporary Early Devonian seawater (Morrow, 1990). A corollary of this perception is that Early Devonian seawater must have been greatly oversaturated with dolomite to facilitate synsedimentary dolospar cementation of fenestral voids. However, the Camsell was deposited at about 410 My (Fig. 1) when the MAGic geochemical modeling indicated that seawater had a low pH value of 7.27 and a low dolomite saturation index value of 1.8 (Fig. 5B). These values are fairly representative of the early Paleozoic as a whole, and are much lower than those of the Lare Cenozoic (Fig. 5B).
In other words, the calculated saturation of dolomite in early Paleozoic seas was 10 (3.2-1.8) , or about 25 times less than that of seawater of the late Cenozoic (Fig. 5B). Similarly, early Paleozoic seas were calculated to be 10 (8.3-7.27) , or 10.7 times more acid than late Cenozoic seas (Fig. 5B). We are presented with a paradox in that the relatively acid early Paleozoic seawater of relatively
low dolomite saturation was somehow as conducive to the direct, open space precipitation of dolospar, as was the greatly dolomite oversaturated late Cenozoic (Miocene to Holocene) seawater. This inconsistency is even more apparent in that early Paleozoic seawater was characterized by much lower Mg:Ca ratios than that of the late Cenozoic (Fig. 6). High Mg:Ca solution ratios have been suggested by many workers as a kinetic factor that strongly promotes the rate of dolomitization (e.g. Patterson and Kinsman, 1982; Machel, 2004). This places the burden of explaining the high percentage of dolomite in lower Paleozoic carbonate successions on the prevalence of extensive shallow shelf, dominantly peritidal environments during early Paleozoic time.
These considerations raise a question concerning the accuracy of global geochemical modeling with respect to calculated pH and dolomite saturation (and for other mineral saturations) in Phanerozoic seawater. Are there factors, or approximations, in these models, that have compromised their results?
for the Phanerozoic As mentioned, there is a close linkage between modeled estimates of Phanerozoic atmospheric PCO 2 values and the degree of calculated dolomite oversaturation for seawater in MAGic (Fig. 5A, B). This raises the possibility that there may be large inaccuracies in model calculated atmospheric PCO 2 values with a consequent impact on modeled mineral saturations.
One feature present throughout the history of global geochemical modeling is that atmospheric PCO 2 is consistently estimated to have been very high during the early Paleozoic and Mesozoic (Table 1; Fig. 5A). Independent support is cited from Retallack (2001) and Yapp and Poths (1992) for these modeled high PCO 2 values (Fig. 5A).
The data of Retallack (2001) are restricted to the Mesozoic and Cenozoic and is based on the stomatal density of fossil leaves as an indirect proxy for atmospheric PCO 2. Agreement of Magic and GEOCARB modeled atmospheric PCO 2 values with the data of Retallack (2001) certainly supports the very high model calculated PCO 2 values of up to 8 times modern values, or about 2500 ppm CO 2 (Fig. 5A). However, Royer et al. (2004) in a summary of other published stomatal data found that they indicated a much lower estimate of atmospheric PCO 2 of about 1500 ppm for this time interval (Fig. 1 in Royer et al., 2004). This trend of progressively lower estimates of atmospheric PCO 2 for the Mesozoic and Cenozoic time interval has continued with Franks et al. (2014) who estimated that most of the Phanerozoic, from the Devonian to the present, based upon a reevaluation of fossil stomatal data, was characterized by atmospheric PCO 2 that at most, was only 2 to 3 times that of the present, or less than 1000 ppm.
It is also of interest to note that the early Paleozoic estimate for atmospheric PCO 2 of Yapp and Poths (1992), which is cited in Arvidson et al. (2006) as validation of their very high calculated PCO 2 (Fig. 5A), is inferred from isotopic data of goethite of that age. However, estimates of PCO 2 inferred from isotopic analyses of pedogenic minerals tend to be much higher than those estimated from stomatal data across the Mesozoic and Cenozoic time interval
are contemporaneously dolomitized by reflux and tidal pumping of slightly evaporated mesohaline seawater. Simultaneously fenestral voids are occluded with coarse dolospar cements precipitated from seawater. 2. Storm events rip up the intertidal fenestral dolomites, and eroded fenestrae form poorly to moderately sorted subrounded sandstones as beach deposits which unconformably overlie the eroded intertidal fenestral dolostone beds (Morrow, 1990).
where both types of data are abundant (Royer et al. 2004, 2006). This casts doubt on the accuracy of the estimated very high early Paleozoic PCO 2 value (Fig. 5A) of Yapp and Poths (1992).
Finally, it is worth noting that Rothman (2001), in an elegant analysis restricted to the carbon and strontium isotopic record of the Phanerozoic, concluded that PCO 2 in the early Paleozoic averaged only about 2.5 times that of the present in a stark contrast to estimates from the GEOCARB and MAGic model series.
It is abundantly clear that the issue of estimating the PCO 2 of ancient atmospheres through the means of proxy data of all types, is very contentious, and the accuracy
of such estimates varies widely. The fact that model estimates of carbonate mineral saturations depend strongly on calculated PCO 2 values means that calibration by empirical data concerning ancient PCO 2 is critical, but unfortunately these data are not sufficiently accurate for reliable validation of modeled pH and mineral saturations.
Dolomite has received increasing consideration in global geochemical modeling of the “solid Earth-oceanatmosphere system”. Earlier models (BLAG, GEOCARB), focused on changes in atmospheric PCO 2, included dolomite simply as part of a carbonate reservoir that passively contributed ions through weathering. The MAGic model, the
successor to the various iterations of the GEOCARB model, represents a major advance in that dolomite, along with other minerals, are represented by their precisely calculated saturations in paleoseawater. Precipitation and dissolution of dolomite itself is recognized as an important aspect of the “solid Earth-ocean-atmosphere system”.
Nonetheless, the MAGic model simulations with regard to atmospheric PCO 2 through the Phanerozoic remained largely unchanged from those of the GEOCARB model series. A consequence of this is that paleoseawater pH is calculated to be highly acidic throughout most of the Phanerozoic relative to modern seawater.This suppresses the modeled saturations for dolomite and for other carbonate minerals, particularly during the early Paleozoic and Mesozoic. This is not consistent with observed examples of penecontemporaneous open space precipitation of coarse dolospar in early Paleozoic strata.
The ongoing controversy concerning empirical evidence for levels of atmospheric PCO 2 from proxy data of leaf stomata and pedogenic mineral isotopic data indicates that the extremely high the early Paleozoic and Mesozoic atmospheric PCO 2 of the GEOCARB and MAGic models may not be correct. Lower PCO 2 for these time intervals with correspondingly higher seawater pH values and dolomite saturations would be far more consistent with the high percentage of dolomite in strata of these times.
Arvidson, R. S. and Mackenzie, F. T. 1997. Tentative Kinetic Model for Dolomite Precipitation Rate and Its Application to Dolomite Distribution: Aquatic Geochemistry, v. 2, p. 273--298.
Arvidson, R. S., Mackenzie, F. T. 1999. The dolomite problem: control of precipitation kinetics by temperature and saturation state: American Journal of Science, v. 299, p. 257–288.
Arvidson, R. S., Guidry, M., Mackenzie, F. T. 2006. MAGic: a Phanerozoic model for the geochemical cycling of major rock-forming components: American Journal of Science, v. 306, p. 135–190.
Arvidson, R. S., Guidry, M. W., Mackenzie, F.
T. 2011. Dolomite controls on Phanerozoic seawater chemistry: Aquatic Geochemistry, v. 17, p. 735–747.
Barnes, I., Irwin, W. P. and White, D. E. 1978. Global distribution of carbon dioxide discharges and major zones of seismicity: U.S. Geololgical Survey Water Resources Investigation, Open·file report 78-39, 12 p.
Berner, R. A., 1991. A model for atmospheric CO2 over Phanerozoic time: American Journal of Science, v. 291, p. 339–376.
Berner, R. A., 1994. GEOCARB: II. A revised model of atmospheric CO2 over Phanerozoic time: American Journal of Science, v. 294, p. 56–91.
Berner, R. A. and Kothavala, Z. 2001. Geocarb III: a revised model of atmospheric CO2 over Phanerozoic time: American Journal of Science, v.301, p. 182–204.
Berner, R. A. 2006. GEOCARBSULF: A combined model for Phanerozoic atmospheric O 2 and CO 2: Geochimica et Cosmochimica Acta, v. 70, p. 5653–5664.
Berner, R. A., Lasaga, A. C., and Garrels, R. M., 1983. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years: American Journal of Science, v. 283, p. 641–683.
Budd, D. A. 1997. Cenozoic dolomites of carbonate islands: their attributes and origin; Earth-Science Reviews, v. 42, p. 1–47.
Carpenter, A. B. 1980. The chemistry of dolomite formation I: the stability of dolomite: in Concepts and Models of Dolomitization (D.H. Zenger, J.B. Dunham and R.G. Ethington, eds.): Society of Economic Paleontolologists, Mineralogists Spec. Publ. 28, p. 111-121.
Demicco, R.V., Lowenstein, T. K., Hardie, L. A. and Spencer, R. J. 2005. Model of seawater composition for the Phanerozoic: Geology; v. 33, p. 877–880.
Ebelmen, J. J. 1845. Sur les produits de la decomposition des especes minerales de la famille des silicates: Annales des Mines, v. 7, p. 3–66.
Franks, P.J., Royer, D.L., Beerling, D.J., Van de Water, P.K., Cantrill, D.J., Barbour, M.M.
and Berry, J.A. 2014. New constraints on atmospheric CO 2 concentration for the Phanerozoic: Geophysical Research Letters, v. 41, p. 4685-3694.
Garrels, R. M., and Mackenzie, F. T. 1972. A quantitative model for the sedimentary rock cycle: Marine Chemistry, v. 1, p. 27–41.
Given, R.K. and Wilkinson, B.H. 1987. Dolomite abundance and stratigraphic age, constraints on rates and mechanisms of Phanerozoic dolostone formation: Journal of Sedimentary Petrology, v. 57, p. 10681078.
Gregor, C. B. 1985. The mass-age distribution of Phanerozoic sediments: In Geochronology and the Geologic Record (ed. N. J. Snelling): Geological Society of London Memoir No. 10, p. 284-289.
Hardie, L.A. 1996. Secular variation in seawater chemistry; an explanation for the coupled secular variation in the mineralogies of marine limestones and potash evaporites over the past 600 m.y.; Geology, v. 24, p. 279–283.
Hess, H. H. 1962. History of Ocean Basins: In Petrologic Studies: A Volume to Honor A. F. Buddington (A. E. J. Engel, Harold L. James, and B. F. Leonard (eds.)): Boulder, CO: Geological Society of America. pp. 599–620.
Holland, H. D. 1972. The geologic history of seawater - an attempt to solve the problem: Geochim. et Cosmochim. Acta, v. 36, p. 637651.
Jones, B. 2004. Petrography and significance of zoned dolomite cements from the Cayman Formation (Miocene) of Cayman Brac, British West Indies: Journal of Sedimentary Research, v. 74, p. 95-109.
Jones, B. 2005. Dolomite crystal architecture: genetic implications for the origin of the Tertiary dolostones of the Cayman Islands: Journal of Sedimentary Research, v. 75, p. 177-189.
Jones, G. D. and Xiao,Y. 2005. Dolomitization, anhydrite cementation, and porosity evolution in a reflux system: Insights from reactive transport models: AAPG Bulletin, v. 89, p. 577-601.
Katz, A. and Matthews, A. 1977. The
dolomitization of CaCO3, an experimental study at 252–295°C: Geochimica et Cosmochimica Acta; v. 41, p. 297–308.
Lowenstein, T. K., Timofeeff, M. N., Brennan, S. T., Hardie, L. A., and Demicco, R. V. 2001. Oscillations in Phanerozoic seawater chemistry: evidence from fluid inclusions; Science, v. 294, p. 1086–1088.
Machel, H. G. 2004. Concepts and models of dolomitization: a critical reappraisal: In The Geometry and Petrogenesis of Dolomite Hydrocarbon Reservoirs (Braithwaite, C.J.R., Rizzi, G. and Darke, G. eds.); Geological Society, London, Special Publications, v. 235, p. 7-63.
Mackenzie, F. T. and Morse, J. W. 1992. Sedimentary carbonates through Phanerozoic time; Geochimica et Cosmochimica Acta: v. 56, p. 3281-3295.
Meijer-Drees, N. C. 1975. Geology of the lower Paleozoic formations in the subsurface of the Fort Simpson area, District of Mackenzie, Northwest Territories: Geological Survey of Canada, Paper 74-40.
Meijer-Drees, N. C. 1993. The Devonian succession in the subsurface of the Great Slave and Great Bear Plains, Northwest Territories: Geological Survey of Canada, Bulletin 393, 222 p.
Meadows, D. H., Meadows, D. L. and Randers, J. 1972. The Limits to Growth: A
Report for the Club of Rome’s Project on the Predicament of Mankind: New York, Universe Books.
Morrow, D. W. 1990. Pore-filling dolomite in the Camsell Formation: possible Devonian synsedimentary dolomite cementation: Sedimentology, v.37, p. 763-773.
Morrow, D. W. 1991: The Silurian-Devonian sequence of the northern part of the Mackenzie Shelf, Northwest Territories: Geological Survey of Canada, Bulletin 413, 121p.
Morrow, D. W., Gorham, B. L. and Wong, J. N. Y. 1994. Dolomite-calcite equilibrium at 220 to 240°C at saturation vapour pressure: Experimental data: Geochimica et Cosmochimica Acta, v. 58, p. 169-177.
Morrow, D. W. and Cook, D. G. 1987: The Prairie Creek Embayment and Lower Paleozoic Strata of the Southern Mackenzie Mountains: Geological Survey of Canada, Memoir 412, 195p.
Morrow, D. W. and Labonte, M. 1988. The Lower Devonian Corridor Member, Northwest Territories, Canada: An example of deposition on a tidal flat island complex: In Second International Symposium on the Devonian System (McMillan, N.J., Embry, A.F. and D.J. Glass (eds.)); Canadian Society of Petroleum Geologists, Memoir 14, VII, p. 495 515.
Nordeng, S. H. and Sibley, D. F. 1994. Dolomite stoichiometry and Ostwald’s Step Rule: Geochimica et Cosmochimica Acta, v. 58, p. 191-196.
Nordstrom, D. K., Plummer, L. N., Wigley, T. M .L., Wolery, T. J., Ball, J. W. , Jenne, E. A., Bassett, R. L., Crerar, D. A., Florence, T. M., Fritz, B., Hofman, M., Holdren Jr, G. R., Lafon, G. M., Mattigod, S. V., McDuff, R. E., Morel, F., Reddy, M. M., Sposito, G. and Thrailkill, J. 1979. A comparison of computerized chemical models for equilibrium calculations in aqueous systems: In Chemical Modelling in Aqueous Systems (Ed. E.A. Jenne), ACS Symposium, Ser., 93, p. 857–892.
Parkhurst, D. L. and Appelo, C. A. J. 2013. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. U.S. Geological Survey Techniques and Methods, book 6.
Patterson, R. J. and Kinsman, D. J. J. 1982. Formation of diagenetic dolomite in coastal sabkha along Arabian (Persian) Gulf: AAPG Bulletin, v. 66, no. 1, p. 28 43.
Pitzer, K. S. 1973. Thermodynamics of electrolytes - I.Theoretical basis and general equations; Journal of Physical Chemistry, v. 77, p. 268–277.
Retallack, G. J. 2001. A 300-million-year record of atmospheric carbon dioxide from

fossil plant cuticles: Nature 411, p. 287–290.
Ries, J. B. 2010. Review: geological and experimental evidence for secular variation in seawater Mg/Ca (calcite-aragonite seas) and its effects on marine biological calcification: Biogeosciences, v. 7, p. 2795–2849.
Rothman, D. H. 2002. Atmospheric carbon dioxide levels for the last 500 million years: Proceedings of the National Academy of Sciences USA: v. 99, p. 4167–4171.
Royer, D. L., Berner, R.A., Montañez, I.P., Tabor, N.J. and Beerling,D.J. 2004. CO2 as a primary driver of Phanerozoic climate: GSA Today, v. 14, no. 3, p.4-10.
Saller, A. H. and Henderson, N. 1998. Distribution of porosity and permeability in platform dolomites: Insight from the Permian of west Texas: Reply: AAPG Bulletin, v. 85, p. 1528–1550.
Shinn, E. A. 1983. Birdseyes, fenestrae, shrinkage pores, and loferites:A reevaluation: Journal of Sedimentary Petrology, v. 53, p. 619-628.
Sibley, D. F. 1991. Secular changes in the amount and texture of dolomite: Geology, v. 19, p. 151-154.
Sun, S.Q. 1994. A reappraisal of dolomite abundance and occurrence in the Phanerozoic; Journal of Sedimentary Research, v. A64, p. 396-404.
Tebbutt, G.E., Conley, C.D. and Boyd, D.W. 1965. Lithogenesis of a carbonate rock fabric: Contributions to Geology, v. 4, p. 1-13.
Tucker, M.E. 1982. Precambrian dolomites: petrographic and isotopic evidence that they differ from Phanerozoic dolomites; Geology, v. 10, p. 7-12.
Urey, H. C. 1952. The Planets, Their Origin and Development: New Haven, Yale Univ. Press, 245 p.
Whitaker, F. F., Smart P. L., Vahrenkamp, V. C., Nicholson H. and Wogelius, R. A. 1994. Dolomitization by near-normal seawater? field evidence from the Bahamas: In Dolomites: (B. Purser, M. Tucker, and D. Zenger, eds.): International Association of Sedimentologists Special Publication 21, p. 530–532.
Yapp, C.J. and Poths, H., 1992. Ancient atmospheric CO2 pressures inferred from natural goethites: Nature 355, p. 342–344.
Zempolich, W. G. and Baker, P. A. 1993. Experimental and natural mimetic dolomitization of aragonite ooids: Journal of Sedimentary Petrology, v. 63, p. 596-606.

The CSPG Foundation is pleased to announce the recipients of the 2016 Andrew D. Baillie Award . The award is given for the best student geological poster presentation and the best student geological oral presentation at the annual GeoConvention .
The Baillie award commemorates Dr. Andrew Dollar “Andy” Baillie (1912-2001) whose geological career spanned five decades. His petroleum career began with the British American Oil Company (the precursor to Gulf Canada) in 1953. After retiring from Gulf, Andy spent more than 20 years pursuing his passion for teaching geology in a variety of ways. His technical skills and desire to share his knowledge made him an invaluable asset to the geological community.
In 1997, to celebrate his lifetime of geological achievements, the CSPG awarded Andrew Baillie the Stanley Slipper Gold Medal for “life-long accomplishments and outstanding contributions to petroleum geology in Canada and abroad”. He was an inspiration to literally hundreds of geologists who
came under his influence, however fleetingly, during his life. Andy was an active member of the CSPG and had a particular interest in the CSPG Educational Trust Fund. The award in his name was established in 1992 by the CSPG Executive as a technical recognition award. The idea of an accompanying cash prize was instituted by a donation from Andy himself. His family ensured this tradition will continue with a commemorative gift to the CSPG Educational Trust Fund following his passing. The award recognizes excellence in technical presentations by students and encourages a level of technical prowess worthy of Dr. Andrew D. Baillie.
The recipient of this year’s best student geological poster presentation is Dillon Newitt . The GeoConvention 2016 award committee selected his presentation entitled “ Contrasting Reservoir Properties for Albian Wilrich and Falher Shoreface Sandstones, Spirit River Formation, West-Central Alberta ”, supervised by Per Kent Pedersen at the University of Calgary, to be the best of an excellent group of student posters at the 2016 convention.

The recipient of the best student geological oral presentation is Bram Komaromi His presentation, also supervised by Per Kent Pedersen at the University of Calgary is entitled, “ Natural Fracture Networks of the Turonian Second White Specks Formation, Highwood River, Southwestern Alberta ”.
The 2016 Baillie Awards were presented at the May 4th CSPG Industry Speaker Luncheon by CSPG Foundation Trustee Kathleen Shannon.


On Monday, May 2, 2016 CSPG members gathered at the Fairmont Palliser to celebrate the efforts of our many dedicated volunteers and to kick off Spring Education Week. President Greg Lynch invited the 2015 Service and Volunteer Award recipients to this new event in order to thank and honour each of them for their dedication to CSPG through their volunteer time. In attendance were registrants from the Spring Education week who enjoyed mingling with CSPG volunteers.
In the past CSPG has recognized both Technical and Service awards at the same function which tended to make for a long evening. This year we decided to split the awards up enabling a shorter event and more attention to the recipients. We held this event and the Industry Speaker Luncheon/ Technical Awards presentation in
conjunction with this year’s Spring Education Week and the changes were well-received by all in attendance.
The awards recognized were as follows:
• President’s Award – Paul MacKay
• H.M. Hunter Award – Astrid Arts and Kevin Root
• Tracks Award – Samantha Etherington, Andrew Fox, Raymond Geuder and Ian Kirkland
• Partner Tracks Award – AER Core Research Centre
• Service and Volunteers Awards –recipients listed at www.cpsg.org/awards
Citations for all awards can be read at www. cspg.org/awards.

The 2016 Industry Speaker Luncheon, held on May 4th, 2016, featured Richard (Dick) Walls speaking on “Today’s Challenges for the Entrepreneurial Geologist”. Dick delivered the most inspiring and factual talk portraying his experiences in the oil and gas industry as it moves through its cycles. Dick pointed out how to create opportunities within each cycle.
CSPG showcased this inaugural Industry Speaker Luncheon to feature the CSPG’s most prestigious technical award – the STANLEY SLIPPER GOLD MEDAL.

CSPG is proud to present this year’s recipient – RICHARD (DICK) WALLS. His citation can be read online at www.cspg. org/awards
The 2015 CSPG Technical Awards were presented to:
• Link Award: Lindsay Dunn
• Medal of Merit: Jen Russel-Houston and Ken Gray
CSPG received positive feedback with respect to holding the Technical Awards at a luncheon and reserving the Service and Volunteer Awards for an evening President’s Reception. CSPG will repeat these two events next year and offer them in conjunction with the Spring Education Week which will once again be in May 2017; just prior to GeoConvention.

In 2015 a total of 12 theses were submitted - seven M.Sc. and five Ph.D. thesesrepresenting seven universities across Canada.
The 2015 recipient of the award for the Best Ph.D. is Duncan A. MacKay for his thesis entitled
“A Subsurface, Sedimentological Analysis of the Tide-Dominated Deposits in the Bluesky Formation (Early Cretaceous), Peace River Area, West-Central Alberta”

His thesis was supervised by Prof. Bob Dalrymple at Queen’s University. Duncan’s thesis was a very thorough and innovative subsurface study of the tide-dominated Bluesky Formation in a 16 township block in the Peace River area of West-central Alberta. The Bluesky in this area is part of the Peace River oil sands which is operated by Shell Canada (who helped support Duncan’s thesis) and as such is intensely drilled. Thirty-five cores and two-hundred and fifteen well logs were used in this study. The thesis is based on three papers which examines sedimentary characteristics of individual mudstone layers and their depositional conditions, lithofacies analysis of both mudstones and sandstones and the paleogeographic evolution and sequence stratigraphic architecture of the Gething to Bluesky interval. All three papers have either been or are in the process of being published.
Chapter 2 is a very detailed study of the mudstone layers within the Bluesky. Duncan divides the mudstones into four types based on the a sedimentary response to a distinct set of depositional processes at its relation to suspended sediment concentration (SSC), cohesive forces and shear forces acting on the mud during deposition and current speed. This is then used to interpret the
Bluesky as a tide dominated depositional system based on recent flumes studies and observations in modern coastal marine settings that high density mud suspensions and variable flow velocities are good indicators of strong tidal influences. Results of this chapter have already been published in the Journal of Sedimentary Research (v.81 p.901-920).
Duncan takes a novel approach in Chapter 3 to interpret the facies and depositional environment of the Bluesky. By independently analyzing the characteristics of sandstoneland mudstones layers then combining the sandstone and mudstone assemblages into a matrix, Duncan has generated nine potential facies which are based on peak flow conditions and turbidity levels. These in turn define five facies successions each representing the deposits of a particular morphological element within the system. They are compound dunes, delta-front settings, tidal sand ridges, tidal sand bars and tidal flats - the major building blocks of all tide-dominated estuaries and deltas. Duncan has divided the Bluesky into the “lower Bluesky unit’ which consists of tide-dominated deltaic deposits and the “upper Bluesky Unit” which is composed of tide-dominated estuarine deposits based on this facies classification combined with mud-layer characteristics. The approach taken by Duncan in this chapter is a valuable technique in interpreting and unravelling other complex tidal successions.
Chapter 4 builds on the work of Chapter 3 and looks at the paleogeographic and sequence-stratigraphic evolution of the Bluesky-Gething interval. Using three hierarchical levels of description and interpretation Duncan unravels the complex evolution of this interval from the northward draining river-dominated Gething to the locally incised valley fill succession of the Bluesky which is tidally-dominated and not wave-dominated as has been documented elsewhere. The upper and lower Bluesky are each interpreted to be separate incisedvalleys. The lower Bluesky is interpreted to be deltaic due to the abundance of fluid-mud deposits, the presence of mouth bars and a general seaward-fining of grain size. The upper Bluesky is interpreted to be estuarine because of the absence of mudstone layers, the relatively coarse grain size and presence of tidal sand ridges. The overall succession is in a transgressive system with low
accommodation which limited preservation.
The detailed and innovative approach taken by Duncan in his thesis to expand our knowledge of mudstones combined with the excellent presentation and relevancy the CSPG membership were judged by the committee to represent the best PhD award for 2015. For those who are interested in the Bluesky or tidally-dominated deposits are encouraged to take a look at his work.
Colleen E. Kurcinka is the recipient of the Best M.Sc. for her thesis entitled
“Sedimentology and Facies Architecture of the Tide-Influenced, River-Dominated Delta-Mouth Bars in the Lower Lajas Formation (Jurassic), Argentina”

Her thesis was completed at Queen’s University under the supervision of Bob Dalrymple.
The main goal of Colleen’s thesis was to increase our understanding of tidedominated to tide-influenced deltas. Colleen’s thesis stands out because it is well-written, easy to read and contains exceptional photos from the superb outcrops. Colleen does an outstanding job of integrating the observations from outcrops into a detailed comprehensive interpretation of the Lower Lajas Formation. Colleen did extensive field work logging 23 sections along a superbly exposed 6-km long exposed cliff section of the Lajas Formation in the Neuquen Basin in westcentral Argentina. The measured sections ranged in thickness from 10 to 200m in height. Panoramic photos were used to
help fill in the gaps between the measured sections and correlations were done by walking along individual layers.
16 facies and sub-facies were recognized which were grouped into seven broad facies associations. The facies associations (distal delta front to prodelta, river-dominated mouth bars/delta front, tide-influenced mouth bars, fluvial or distributary channels, interdistributary bay, shell-bed flooding surface and siliclastic flooding surfaces) are used to divide the Lower Lajas into two sequences. The lower sequence (Sequence 1) is a series of four distinct sandstone tongues
composed of multiple mouth bars. This sequence is interpreted to be the highstand system tract. Sequence 1 is interpreted to be a river-dominated suite with prodelta to distal delta front, the river-dominated mouth bars and terminal distributary channels. Sequence 2 contains a series of stacked channels and is more complex and contains a complete relative sea-level cycle. It is composed of both river-dominated and tide influenced mouth bars distributary channels and interdistributary bays.
A detailed discussion comparing the indicators of river-dominated to tide
With GeoConvention usually in May of each year, CSPG held its inaugural Spring Education Week May 2-6, 2016. It was a busy week with three short courses and three social events happening. The collaboration between CSPG volunteer recognition, technical award recognition and the technical content that our short courses provided was a natural fit.
The courses that we ran during the week were as follows:
• Introduction to Petroleum Engineering and Geosciences (joint with SPE Canada)
• SAGD Reservoir Engineering for Geoscientists & NonReservoir Engineers
• Exploration Using Integrated Petrophysics (joint with CWLS for In Transition members)
A big thank you goes to our instructors: Art Irwin, Saad Ibrahim, Roger Hough, Hussain Sheikha and Taras Dziuba.
dominated mouth bars is discussed in Chapter 5. Based on Colleen’s observations she has re-interpreted the Lower Lajas to be primarily river-dominated as opposed to the previous tidally-dominated interpretation based on the overwhelming presence of heterolithic river-flood event beds. In the river-flood deposits there is no evidence of tidal action. Only minor amount of tidal indicators are present in the interflood beds which suggests that only in times when river discharge is low does the tidal limit move landward. Colleen’s thesis would be of interest to CSPG members who work on similar depositional settings.
We also held three social events:
• President’s Reception (Service and Volunteer Award presentations)
• Long Time Members Reception
• Industry Speaker Luncheon (Technical Award presentations)
Next year’s Spring Education Week will be held May 8-12, 2017 (the week before GeoConvention) in Calgary. If you have any suggestions for short course and/or field seminar topics please send an email to membership@cspg.org to tell us what you would like to see.
More and more professionals are actively pursuing mentoring to advance their careers. Whether you are participating as the mentee or mentor, these types of partnerships can benefit your career and develop your skills. Once CSPG has enough interest, we will contact applicants for further information, make matches and email details to participants.

To sign up for the program please visit www.cspg.org/ geomatch and make your selection (please ensure you are signed in to the website), or send an email to membership@cspg.org This program is only open to CSPG members in good standing.
In the second quarter of 2016, a substantial recovery in crude prices has taken place, with both Brent and WTI prices rising from the mid-30s to the low 50s. It’s difficult to attribute this streak to any single reason, but we can certainly identify some of the contributing factors.
First, US Dollar weakness has boosted nearly all commodity prices worldwide, including energy prices. While it has recovered from its early-May low, the US Dollar has now fallen nearly 5 percent, on average, against a basket of other global currencies since January. For energy benchmarks denominated in non-USD currencies, price recoveries have tended to be slightly less pronounced due to the absence of similar currency tailwinds.
On the supply side, there have been significant disruptions around the world. Here in Alberta, the May wildfires in the Fort McMurray region led to a drop in crude production of over 1 MMbopd. In Nigeria, output is at the lowest level in decades due to terrorism and militancy in the Niger Delta, and is only operating at an estimated 50% of capability. Libya has experienced attacks at loading terminals, driving exports down, with production around a quarter of what it was just a few years ago. In Venezuela, pressure from the economic crisis has contributed to a modest drop in crude output as well, with some fearing the worst: if the crisis turns into a full meltdown, production could drop from nearly 3 MMbopd to almost zero. Despite the rocky quarter, supply disruptions appear to be easing somewhat, indicating we may be due for a minor near term price correction, but the significance of these disruptions in carrying crude prices up from last quarter’s lows is clear.
On the demand side, appetite from China and India has been healthier than many expected over the last quarter. Global refinery capacity is anticipated to exceed 100 MMbopd within a few months, pushing feedstock demand higher, although ample stockpiles around the world are expected to be able to fulfill this demand without much trouble in the near term. US crude stocks have been falling, but gasoline and distillate stocks have been rising, leaving question marks about whether spot price gains can continue at their recent pace.
US crude production is expected to average 8.6 MMbopd in 2016, according to the EIA. This would represent a drop of 0.8 MMbopd from average 2015 output. In fact, estimated May 2016 output, 8.7 MMbopd, is now a full million barrels per day below last April’s 9.7 MMbopd. It remains to be seen how shale producers will respond to the substantial price recovery we’ve had since February and whether some of the drilled but uncompleted wells (DUCs) will begin to be brought on-stream.
In Canada, Loonie appreciation has continued, though more slowly than last quarter, resulting in a slightly tempered local crude price recovery when compared to the US. Light, sweet crude in Alberta has traded between 2 and 5 USD/bbl below WTI. Edmonton condensate has averaged a couple dollars below WTI, and the WCS discount to WTI has been relatively stable, and slightly tighter than historical norms, at 12-14 USD/bbl.
Despite some rather extreme volatility in Canadian natural gas prices, recent price movement in North American natural gas prices suggests the past quarter will be remembered as a positive one on the gas price front. Henry Hub prices have improved from well under 2.00 USD/MMBtu to over 2.50 USD/MMBtu on the back of lower than expected recent supply builds and warm weather driving cooling demand. AECO prices in Alberta for July delivery are back above 1.75 CAD/MMBtu at the time of writing, which, while not exactly exciting, are welcome when compared to the sub-1.00 to 1.50 CAD/MMBtu range they occupied for most of the past few months. Alreadyweak AECO prices were exacerbated in May by oil sands outages due to the recent Alberta wildfires. The differential between AECO and Henry Hub has also finally begun to narrow, thanks in part to the NEB’s recommendation to approve the NOVA system expansion in northern Alberta.
Outside North America, natural gas prices have continued their decline since last quarter, but are showing signs of life with a bounce off recent lows. UK NBP gas prices are down significantly from last quarter, but have shown strength in the past month on slightly healthier demand, relative currency valuations and higher global energy prices in general. Japanese spot LNG prices spent some time at 4.30 USD/MMBtu in May, which is down a few dollars from last quarter and down from over 16 USD/MMBtu in 2014. Prices are back up to around 5.00 USD/MMBtu on hotter temperatures and an upswing in near term power demand around the world, but the IEA’s recent outlook suggests the current global LNG glut is likely to last until at least 2019. While international gas prices remain substantially higher than those in North America, it is certainly a more challenging environment for LNG export economics than it was a few years ago.
Finally, Figure 1 shows the ebbs and flows of the daily WTI forward curves plotted against the historical prompt month WTI prices. While there has been a huge amount of movement in both the prompt month prices and forward curves, one thing that becomes apparent in recent years is a tendency of the longer end of the forward curves to exhibit some mean reversion. While forward curves are not true price forecasts, it certainly seems like those willing to hedge (or speculate) more than a couple years out are more comfortable with prices below 100 USD/bbl and above 40 USD/bbl.
To register please go to www.cspg.org
Member Rate: $40+gst | Non-member rate: $50+gst | Student & In-Transition rate: $25+gst Join us on race day on an out-and-back course along the beautiful Bow River pathway. If you are looking for a competitive race or just want to have fun, come join us!




CORPORATE SUPPORTERS
Paradigm Geosciences Ltd.
CMC Research Institutes, Inc.
Crescent Point Energy Trust
ITG Investment Research
Bannatyne Wealth Advisory Group
Canadian Global Exploration Forum
EV Cam Canada Inc.
Halliburton
RPS Energy Canada Ltd.
Sproule Associates Limited
Cabra Consulting Ltd.
McDaniel & Associates Consultants Ltd.
ConocoPhillips
Mount Royal University
Richardson GMP
Surge Energy Inc.
Valeura Energy
Compass Directional Services
Integrated Sustainability Consultants Ltd.
TAQA North Ltd.
RIGSAT Communications
Baker Hughes Calgary
Roke Technologies Ltd.
Signature Seismic Processing Inc.
CSPG is a not-for-profit corporation registered under the NFP Act. Our mission is: To advance the professions of the energy geosciences - as it applies to geology; foster the scientific, technical learning and professional development of its members; and promote the awareness of the profession to industry and the public.
Directors hold office for two years; commencing January 12 th, 2017. Nomination forms are available on www.cspg.org/Society/Governance
Nominations for Directors close September 15 th, 2016.
CSPG is calling for nominations for the Executive Committee:
President Elect – who will continue to serve a total of three (3) years comprised of the first year as President Elect, the second year as President and the third year as Past President.
Finance Director Elect – who shall serve a two (2) year term comprised of the first year as Finance Director Elect and the second year as Finance Director
Four Directors At Large – each year CSPG elects Directors to fill portfolios. For 2017, CSPG is interested in attracting new Directors to cover such portfolios as Education, Publications, Outreach, & Young Geoscience Professionals. Please express your interest on the Nomination form.
Please note that only FULL Members of CSPG can be nominated
The Nomination Process:
CSPG Nominating Committee recruits for potential directors. You may submit your nomination for their consideration. Alternatively, if you support your nomination with 25 Full Member signatures, you can stand for election.
The Nomination form and Nomination Signature form must be submitted by September 15th, 2016 to Lis.Bjeld@cspg.org to the attention of Past President.


Each year the CSPG awards the Medal of Merit to authors of the best peer-reviewed paper published during the previous year on a subject related to the petroleum geology of Canada. A sterling silver medal is presented to each of the authors. The list of previous winners can be seen under awards on the CSPG website.
The Medal of Merit Committee searches the literature for potential candidates and selects the best paper, favouring well written and illustrated papers with novel ideas that have relevance to Canadian petroleum geology and/or the broader practice of petroleum geology. The committee will be evaluating papers published during the calendar year 2015 for the 2016 award. In addition to papers from academic journals, papers which form part of a special publication are also eligible. If you know of a 2015 peer-reviewed paper that the committee should consider, please submit the details by July 31, 2016 to:
Ross Kukulski, Chair Medal of Merit Committee
Ross.kukulski@chevron.com
(403) 234-5450


October 11‐13 | Banff, AB


Our understanding of Clastic Sedimentology has made huge leaps over recent decades. Clastic sediments make up almost 50% of the Earth’s surface, and the study of recent and ancient clastic rocks allows us to recognize th e processes that shape them. The goal of the conference is to showcase the latest thinking and ideas relating to these sediments.
Land Ho!
Andrew Mial & Steve Hasiotis
Beach and Beyond
ChrisFielding&KerrieBann
Gateways to the Sea
MurrayGingras&ShahinDashtgard
INDIVIDUAL RATES
Early Bird Registration
Member Registration
Non-Member Registration
Early Bird Professor & Student
Regular - Professer & Student
Speaker Registration
GROUP RATES
Bulk Registration Package (3 Registrations)
Bulk Registration Package (5 Registrations)
Slope and Basin
Steve Hubbard & Bill Arnott
Mud Matters
GuyPlint&RogerSlatt
Location: Banff Conference Centre | Banff, AB