Introduction to Pharmaceutical Waters for U.S. Products
continued
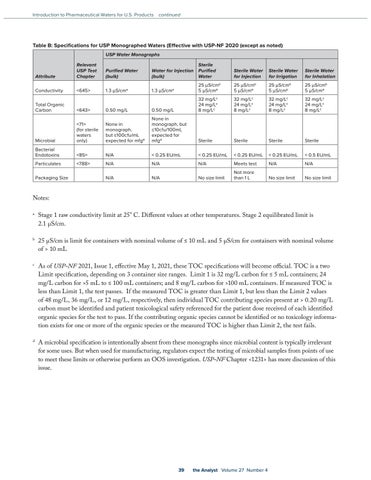
Table B: Specifications for USP Monographed Waters (Effective with USP-NF 2020 (except as noted) USP Water Monographs
Attribute
Relevant USP Test Chapter
Purified Water (bulk)
Water for Injection (bulk)
Sterile Purified Water
Sterile Water for Injection
Sterile Water for Irrigation
Sterile Water for Inhalation
Conductivity
<645>
1.3 µS/cma
1.3 µS/cma
25 µS/cmb 5 µS/cmb
25 µS/cmb 5 µS/cmb
25 µS/cmb 5 µS/cmb
25 µS/cmb 5 µS/cmb
32 mg/Lc 24 mg/Lc 8 mg/Lc
32 mg/Lc 24 mg/Lc 8 mg/Lc
32 mg/Lc 24 mg/Lc 8 mg/Lc
Total Organic Carbon
<643>
0.50 mg/L
0.50 mg/L
32 mg/Lc 24 mg/Lc 8 mg/Lc
Microbial
<71> (for sterile waters only)
None in monograph, but ≤100cfu/mL expected for mfgd
None in monograph, but ≤10cfu/100mL expected for mfgd
Sterile
Sterile
Sterile
Sterile
Bacterial Endotoxins
<85>
N/A
< 0.25 EU/mL
< 0.25 EU/mL
< 0.25 EU/mL
< 0.25 EU/mL
< 0.5 EU/mL
Particulates
<788>
N/A
N/A
N/A
Meets test
N/A
N/A
No size limit
Not more than 1 L
No size limit
No size limit
Packaging Size
N/A
N/A
Notes: a
Stage 1 raw conductivity limit at 25° C. Different values at other temperatures. Stage 2 equilibrated limit is 2.1 µS/cm.
b
25 µS/cm is limit for containers with nominal volume of ≤ 10 mL and 5 µS/cm for containers with nominal volume of > 10 mL
c
As of USP-NF 2021, Issue 1, effective May 1, 2021, these TOC specifications will become official. TOC is a two Limit specification, depending on 3 container size ranges. Limit 1 is 32 mg/L carbon for ≤ 5 mL containers; 24 mg/L carbon for >5 mL to ≤ 100 mL containers; and 8 mg/L carbon for >100 mL containers. If measured TOC is less than Limit 1, the test passes. If the measured TOC is greater than Limit 1, but less than the Limit 2 values of 48 mg/L, 36 mg/L, or 12 mg/L, respectively, then individual TOC contributing species present at > 0.20 mg/L carbon must be identified and patient toxicological safety referenced for the patient dose received of each identified organic species for the test to pass. If the contributing organic species cannot be identified or no toxicology information exists for one or more of the organic species or the measured TOC is higher than Limit 2, the test fails.
d
A microbial specification is intentionally absent from these monographs since microbial content is typically irrelevant for some uses. But when used for manufacturing, regulators expect the testing of microbial samples from points of use to meet these limits or otherwise perform an OOS investigation. USP-NF Chapter <1231> has more discussion of this issue.
39
the Analyst Volume 27 Number 4