Microbial Levels Control with Monochloramines in Building and Municipal Water Systems

Can Bioaugmentation Reduce Hydrogen Sulfide Production in Municipal Wastewater Collection Systems?
Validation of a Portable qPCR Method for the Detection of Legionella
Continuous Maintenance: An Overview of Updates to Legionella Regulations
What is the Impact of Temperature, pH, Time, and Chemical Selection on Membrane Cleaning?
Published by Volume 30 Number 3 1300 Piccard Drive, Suite LL 14 • Rockville, MD 20850 Summer 2023 the ANALYST The Voice of the Water Treatment Industry Volume 30 Number 3 Summer 2023

Cover

Summer 2023
Volume 30
Number 3
8 Microbial Levels Control with Monochloramines in Building and Municipal Water Systems
Alberto Comazzi, Ph.D., Sanipur US LLC
Scientific literature is available that addresses the efficacy of different drinking water disinfectants in preventing and remediating Legionella but not much addressing the efficacy against pathogens. Despite more recent use of monochloramine as a supplemental disinfectant, enough evidence exists in peer-reviewed literature to confirm that it the best option for Legionella. This article reviews the data that is available about the efficacy of monochloramine against other pathogens.
18 Can Bioaugmentation Reduce Hydrogen Sulfide Production in Municipal Wastewater Collection Systems?
Jennifer Cray, Megan Duersteler, Josiah Menako, Dan Romanek, Sona Son, and Mike King, Microbial Discovery Group
Hydrogen sulfide is a highly toxic colorless gas with a pungent unpleasant odor that is a common occurrence in municipal collection systems. Humans can detect H2S odor in the air at concentrations as small as 0.01 to 1.5 parts per million, but exposure to higher concentrations can have serious health effects. Bioaugmentation is the process of enhancing the microbial community that naturally exists in an environment through the addition of bacterial species and/or nutrients that support microbial growth. Bacillus-based bioaugmentation products work to reduce H2S in wastewater systems by removing food sources and habitats. A Bacillus-based bioaugmentation treatment was used to treat 20 different municipal collection systems and was able to effectively reduce H2S in 90% of these systems.
24 Validation of a Portable qPCR Method for the Detection of Legionella
Jeremy Duguay, Harley King, Ph.D., Neil Sharma, Ph.D., and Jordan Schmidt, Ph.D., LuminUltra Technologies Ltd.
The ability to detect Legionella rapidly and reliably is critical to effectively manage risk in water distribution systems and cooling water circuits. The requirement for quick results is often hindered by the need to send collected samples to off-site laboratory facilities, with results taking days to weeks. Quantitative polymerase chain reaction is an established detection and screening method that can be designed to target DNA unique to Legionella to quantitatively measure the bacterial population present within a water system in just hours. This study presents the validation of a qPCR method for the detection of bacteria belonging to the genus Legionella or Legionella pneumophila.
36 Continuous Maintenance: An Overview of Updates to Legionella Regulations
Adam Green, J.D., and Matthew Kim, J.D., Baker, Donelson, Bearman, Caldwell, and Berkowitz PC
Robert J. Cunningham, P.E. International Water Consultants Inc.
John A. Mullen, International Association of Plumbing & Mechanical Officials
Existing Legionella regulations, standards, and guidelines have evolved during the last few years. Most are strictly voluntary and few jurisdictions have codified them into law. This article provides a broad status update and general summary of changes that owners and operators of large buildings and healthcare facilities, as well as service providers to those facilities, should know.
52 What is the Impact of Temperature, pH, Time, and Chemical Selection on Membrane Cleaning?
Joshua Utter, American Water Chemicals
As the demand for clean water grows, membrane-based technologies provide an energy-efficient means for water desalination, with polyamide thin-film composite membranes being the current standard for reverse osmosis. Despite advancements in membrane technology, membrane fouling is a major obstacle in optimizing RO system design and operation. Membrane cleaning is a common solution for recovering lost membrane performance. The effectiveness of cleaning improves significantly when the clean in place system is tailored to the specific fouling problem.
4 Calendar of Events
5 President’s Message
7 Message From the President-Elect
63 Membership Benefits
64 Discovering AWT
67 Making a Splash
68 CWT Spotlight
69 Tales From the Waterside
72 T.U.T.O.R.
80 What’s (Water) on Your Mind?
84 Capital Eyes
87 Advertising Index
3 the ANALYST Volume 30 Number 3
Electron micrograph of amorphous silica scale, fungal hyphae, and calcium carbonate fouling found on an RO membrane autopsy.
Photo courtesy of American Water Chemicals, Inc.
Calendar of Events
1300 Piccard Drive, Suite LL 14
Rockville, MD 20850
(301) 740-1421 • (301) 990-9771 (fax) www.awt.org
2023 AWT Board of Directors
President
Stephen C. Hallier, CWT
President-Elect
Noah Baskin
Secretary
John D. Caloritis, CWT
Treasurer
Kyle J. Rossi, CWT
Immediate Past President
Fred Shurtz
Directors
Craig Bodenmiller, CWT
Tammy Faber, MBA
Michelle Lunn
Michael Bourgeois, CWT
Ex-Officio Supplier Representative
Pam Simmons
Past Presidents
Jack Altschuler
John Baum, CWT
R. Trace Blackmore, CWT,
LEED AP
Michael Bourgeois, CWT
D.C. “Chuck” Brandvold, CWT
Thomas Brandvold, CWT
Brent W. Chettle, CWT
Dennis Clayton
Bernadette Combs, CWT,
LEED AP
Matt Copthorne, CWT
James R. Datesh
John E. Davies, CWT
Jay Farmerie, CWT
Gary Glenna
Charles D. Hamrick Jr., CWT
Joseph M. Hannigan Jr., CWT
Matt Jensen, CWT
Staff
Executive Director
Denise Jackson
Deputy Executive Director
Sara L. Wood, MBA, CAE
Member Services Director
Angela Pike
Vice President, Meetings
Tina Schneider
Meeting Specialist
Caroline Bentley
Mark R. Juhl
Brian Jutzi, CWT
Bruce T. Ketrick Jr., CWT
Bruce T. Ketrick Sr., CWT
Ron Knestaut
Robert D. Lee, CWT
Mark T. Lewis, CWT
Steven MacCarthy, CWT
Anthony J. McNamara, CWT
James Mulloy
Alfred Nickels
Scott W. Olson, CWT
William E. Pearson II, CWT
William C. Smith
Marc Vermeulen, CWT
David Wagenfuhr
Casey Walton, B.Ch.E, CWT
Larry A. Webb
Association Events
2023 Business Owner’s Meeting
October 3, 2023
Amway Grand Hotel
Grand Rapids, Michigan
2023 Annual Convention and Exposition
October 4–7, 2023
DeVos Place Convention Center and Amway Grand Hotel Grand Rapids, Michigan
2024 Technical Training Seminar (West)
March 6–9, 2024
Embassy Suites by Hilton Dallas Frisco Hotel
Dallas, TX
2024 Technical Training Seminar (East)
April 17–20, 2024
Cleveland Marriott Downtown at Key Tower
Cleveland, Ohio
2024 Annual Convention and Exposition
September 10–13, 2024
Louisville Convention Center and Omni Louisville
Louisville, Kentucky
2025 Technical Training Seminars (West)
February 25–28, 2025
Doubletree Mission Valley
San Diego, California
2025 Annual Convention and Exposition
November 12–15, 2025
The Broadmoor Hotel
Colorado Spring, Colorado
2026 Annual Convention and Exposition
September 16–19, 2026
Oklahoma Convention Center and Omni Hotel
Oklahoma City, Oklahoma
2027 Annual Convention and Exposition
September 8–11, 2027
Cleveland Convention Center
Cleveland, Ohio
Meeting Planner, Technical Training Seminars
Tim Foley
Exhibits and Sponsorship Manager
Barbara Bienkowski, CEM Emeritus
Marketing Manager
Mary Claire Gordon
Editorial Services Manager
Heather Rigby
Production Manager
Tiffany Ward
Director of Accounting Services
Dawn Rosenfeld
The Analyst Staff
Publisher
Denise Jackson
Managing Editor
Heather Rigby
Production Manager
Tiffany Ward
Technical Editor
Michael Henley
(303) 324-9507, Email: mdhenleywater@gmail.com
Advertising Sales Manager
Carol Nettles
carol@adboomadvertising.com
Also, please note that the following AWT committees meet on a monthly basis. All times shown are Eastern Time. To become active in one of these committees, please contact us at (301) 740-1421.
Second Tuesday of each month, 11:00 am—Legislative/Regulatory Committee
Second Tuesday of each month, 2:30 pm—Convention Committee
Second Wednesday of each month, 11:00 am—Business Resources Committee
Second Friday of each month, Noon—Pretreatment Subcommittee
Second Friday of each month, 10:00 am—Special Projects Subcommittee
Second Friday of each month, 11:00 am—Cooling Subcommittee
Third Monday of each month, 10:00 am—Certification Committee
Third Monday of each month, 3:30 pm—Young Professionals Task Force
Third Tuesday of each month, 3:00 pm—Education Committee
Third Friday of each month, Noon—Boiler Subcommittee
Third Friday of every other month, 10:00 am—Technical Committee
Third Friday of each month, (call for meeting dates), 11:00 am—Wastewater Subcommittee
Other Industry Events
WEFTEC, Annual Technical Exhibition and Conference, September 30 – October 4, 2023, Chicago, Illinois Ultrapure Micro, Annual Conference, October 10–12, 2023, Austin, Texas IWC, Annual Conference, November 12–16, 2023, San Antonio, Texas RETA, Annual Convention, November 13–16, 2023, Jacksonville, Florida Cooling Technology Institute, February 4 –8, 2024, Houston, Texas
the U.S. (4 issues). Please add $25 for Canada and Mexico. International subscriptions are $200 in U.S. funds.
4 the ANALYST Volume 30 Number 3
The Analyst is published quarterly as the official publication of the Association of Water Technologies. Copyright 2023 by the Association of Water Technologies. Materials may not be reproduced without written permission. Contents of the articles are the sole opinions of the author and do not necessarily express the policies and opinions of the publisher, editor or AWT. Authors are responsible for ensuring that the articles are properly released for classification and proprietary information. All advertising will be subject to publisher’s approval, and advertisers will agree to indemnify and relieve publisher of loss or claims resulting from advertising contents. Editorial material in the Analyst may be reproduced in whole or part with prior written permission. Request permission by writing to: Managing Editor, the Analyst, 1300 Piccard Drive, Suite LL 14, Rockville, MD 20850, USA. Annual subscription rate is $100 per year in
President’s Message
As we move into the summer months, I am reminded of the importance of in-person connections, of the value of being together, in one place at the same time. I value your communication with me, whether via email, phone call, or a Zoom meeting. Please continue to share your thoughts on what AWT is doing well, how we can improve, and what we can do to better serve water treatment professionals globally.
But nothing is better than face-to-face conversation. That is why I am so very excited about the upcoming AWT Annual Convention & Exposition in October in Grand Rapids, Michigan. We have developed a great program for you, with opportunities to learn from others in our business and those who have succeeded elsewhere. It’s an outstanding agenda. What I most enjoy are the informal exchanges on the golf course, over a meal, on a tour, or at a reception. I find that’s when I learn the most.
As always, the exhibit hall will be filled with suppliers and vendors who are there to share the latest technologies and approaches as we work together to improve the field. And, as in past years, the silent auction will benefit our charity partner, Pure Water for the World.
At the AWT Annual Awards Reception and Dinner, I will be proud to present and honor the winners of the Ray Baum Memorial Water Technologist of the Year, Supplier of the Year, and Rising Star Awards.

I look forward to welcoming everyone to the great state of Michigan. We’ve visited Grand Rapids before, and I know you will enjoy the attractions, the food, and the beer. Grand Rapids is the nation’s Craft Beer Capital, so come thirsty.
By Steve Hallier, CWT
Volunteer with AWT
Volunteers are the engine that keeps AWT going. There are projects, committees and subcommittees, task forces and interest groups for all of us. Your service and support for these initiatives are vital to our ongoing success. Take it from me, you will get more from volunteering at AWT than you give. You will learn from colleagues, make new business connections, and grow your network. Volunteering takes time, but it is time well spent. If you are thinking about volunteering and wondering where to get started, reach out to the AWT staff, or visit our website at www.awt.org.
I find it hard to believe, but my term as president will end at the conclusion of the Annual Convention. Serving AWT as a volunteer, a Board Member and as your President has been a truly rewarding experience. So, as I depart, I want to encourage anyone who might be interested to consider serving on the AWT Board. It’s an opportunity to enhance your leadership and business skills. Board service has helped me to stay on top of trends in the profession. I’ve gained a network of peer advisors who have provided me with fresh perspectives and new ideas to help my business succeed. Most importantly, however, Board service has given me the opportunity to help shape the future of AWT. The members of the Board and volunteers have helped shape our updated strategic plan, and I am excited about where we are headed.
Finally, board service is fun. Yes, it is serious work that everyone takes to heart, but it’s also inspiring and energizing to sit around a table with nine smart, highperforming businesspeople, all united in our goal to further the mission of AWT.
Please stay in touch. I can be reached at president@awt.org.
5 the ANALYST Volume 30 Number 3







Manage all aspects of your water treatment service and consulting. By integrating your equipment with software and technology, you have the ability to capture important information about critical parameters, systems and installations. Our team of industry professionals is here to help you save time and improve efficiency in the field. TESTING PRODUCTS & SUPPLIES FEED & CONTROL EQUIPMENT DATA & REPORTING SOFTWARE Where Water and Technology Meet® Now Part of the AquaPhoenix Family Contact our team to integrate your systems today! 866-632-1291 | www.aquaphoenixsci.com
Message From the President-Elect
By Noah Baskin
I am so excited that we are getting close to that time of year again. I hope that you will be joining us for the 2023 AWT Annual Convention & Exposition, October 3–6, 2023 in Grand Rapids, Michigan. This is going to be a great time for us all to get together in person and get re-energized.
Keynote Speaker
Our 2023 Convention Keynote Address—on Thursday, October 5—will be delivered by author, podcaster, and business leader, Jason Jaggard. We are excited to have Jason join us.
Jason is the founder and CEO of Novus Global and the co-founder of The Meta Performance Institute—a non-traditional incubator for world-class coaching, leadership, and management. His work has been featured in multiple media outlets including Forbes, Entrepreneur, Fast Company, Market Watch, and more. As the executive producer and primary host for the award-winning Beyond High Performance podcast, Jason interviews world-class coaches, New York Times bestselling authors,
business leaders, professional athletes, activists, and award-winning entertainers. He is the author of Spark: Transform Your World One Risk at a Time and the forthcoming Beyond High Performance book. In fact, the first 600 member companies to register will receive a complimentary edition of Jason’s new book!
All of us are entrepreneurs and business leaders. Jason will provide insights, advice, and counsel to help our companies succeed. He’s had countless conversations with successful executives and others focused on performing at the highest levels in their fields, so his presentation will be compelling.
At the close of the Annual Convention, I will assume my role as AWT president. I look forward to my tenure with both excitement and humility. It will be my honor and privilege to serve you and our industry in the best way I can while building on the hard work that Steve Hallier and his predecessors have done.
Between now and then, please give me your advice. AWT exists to serve its members, so I welcome your ideas and suggestions. I look forward to hearing from you. I can be reached at nbaskin@towerwater.com.

7 the ANALYST Volume 30 Number 3
Microbial Levels Control with Monochloramines in Building and Municipal Water Systems

8 the ANALYST Volume 30 Number 3
Alberto Comazzi, Ph.D. Sanipur US LLC
Introduction
Legionellae, a gram-negative bacteria genus comprising over 60 known species (1), are ubiquitous in natural and artificial water environments worldwide, and survive in a range of environmental conditions (2). Among these species, a significant number are able to cause disease (generally known as Legionellosis), with a range of different implications: from acute, self-limiting, influenza-like illness without pneumonia (Pontiac Fever) to severe pneumonia that, if untreated, can be fatal (3).
For these reasons, it is of great importance to monitor its presence and to contrast any proliferation in humanrelated water distribution systems. Known risk factors that determine individual infection susceptibility include increasing age, male gender, smoking, chronic lung diseases, and any condition associated with immunodeficiency.
Public water supplies (PWS) usually carry out two disinfection steps in the treatment plants. The first step is with chlorine or chlorine dioxide, and the second is with either chlorine or monochloramine. The aim of disinfection is to kill harmful waterborne pathogens, but sometimes bacteria, such as Legionella , can survive this two-step disinfection process and enter the water distribution system. Generally, Legionella is below the detectable level in the source water supply. However, it does not colonize well in cold water and, therefore, does not constitute a health threat.
That said, it should be noted that the conditions favoring Legionella growth, and the way the infection is transmitted, dramatically increase the risk of acquiring Legionaries’ disease from building water systems. The perfect environments for the proliferation of this opportunistic waterborne pathogen are stagnant water and warm temperatures. This is why Legionella becomes a health risk once the bacteria enter a building’s complex plumbing system and start to colonize it. This is particularly true in domestic hot water systems, where temperatures are ideal for colonization and the formation of complex biofilms.
A Legionella infection can be transmitted when a person breathes water droplets that contain the germs. This is why hundreds of thousands of buildings, such as healthcare facilities, nursing homes, apartment complexes, hotels, and casinos, are at risk. Legionella’s fatality rate is about 10% in the overall population, but the rate goes up to approximately 30% in healthcare reported cases (4). In 2017, the Centers for Medicare & Medicaid Services (CMS) published a letter mandating that all healthcare facilities must implement a water management plan (WMP) in order to mitigate the risk of healthcare acquired Legionella. Just recently, in January 2022, the joint commission published a notice saying that they will start to audit healthcare facilities to verify if the WMPs are being implemented properly.
Even if efforts are being implemented in order to prevent the number of Legionella infections, the number of Legionella cases rose by almost 10 times from 2000 to 2018. The increase in Legionella cases is due to more testing and increased complexity of building water systems, as illustrated in Figure 1.
Although most of the available literature, guidelines, and standards (ASHRAE Standard 188, ASHRAE Guideline 12, ASHRAE Standard 514, CDC toolkit, CMS memo ASSE Standard 12080, and AIHA guideline) are mainly focused on Legionella , there are other pathogens and fungi that are naturally present in water that are a threat to public health. Some of these pathogens include Pseudomonas aeruginosa , Acinetobacter
9 the ANALYST Volume 30 Number 3 Microbial Levels Control with Monochloramines in Building and Municipal Water Systems
0 2000 4000 6000 8000 10000 2000 2002 2004 2006 2008 2010 2012 2014 2016 2018 N u m b e r o f C a s e s Year
Figure 1: Increase in Legionella cases from 2000 to 2018 (CDC)
baumannii, Stenotrophomonas maltophilia , Fusarium spp., Aspergillus spp., and non-tuberculosis mycobacteria (NTMs). The typical infections caused by these pathogens are reported in Table A.
Table A: Type of Infections Caused by Different Pathogens (CDC)
Pathogen Infection
Pseudomonas aeruginosa Blood (wounds) and lungs (pneumonia)
Acinetobacter baumannii Blood (wounds), urinary tract, and lungs (pneumonia)
Stenotrophomonas maltophilia Blood (wounds), urinary tract, and lungs (pneumonia)
Fusarium spp. Nails, eyes, and bloodstream
Aspergillus spp. Allergic reactions, lung infections, and infections in other organs
Non-tuberculosis mycobacteria Skin and soft tissue, bloodstream, lymph nodes, and lungs (pneumonia).
As a part of Legionella control strategies, the water management plan may suggest feeding a disinfectant to drinking water to establish a chemical residual in the building water system. The disinfectants that are fed onsite in buildings are also known as “supplemental” disinfectants; they include chlorine, monochloramine, chlorine dioxide, and copper-silver ions. Chlorine, monochloramine, and chlorine dioxide are listed as disinfectants under the Safe Drinking Water Act, which is enforced by the U.S. Environmental Protection Agency (EPA). The implementation of this type of technology is not always indicated in the WMP but is the only category that ensures long-term protection for the building occupants.
Chlorine is the disinfectant that has been used in building plumbing systems for the longest time, whereas chlorine dioxide has only been available in the marketplace since the mid-1990s. Monochloramine has been used as a drinking water disinfectant by PWS since 1940, but its use as a supplemental disinfectant did not start until the late 2000s.
There is extensive peer-reviewed scientific literature available that addresses the efficacy of different drinking water disinfectants in preventing and remediating Legionella in buildings but not as much addressing the efficacy in inactivating
different pathogens. Despite the more recent use of monochloramine as a supplemental disinfectant, there is enough evidence published in the peer-reviewed literature to confirm that it is the best option for Legionella control in building water systems (5, 6).
It is also documented that buildings whose water is supplied by a PWS that uses monochloramine as a secondary disinfectant experienced a reduction in Legionella contamination and in hospital-acquired Legionnaires’ disease incidence, in comparison with buildings fed by chlorinated water (7-9). The main reason monochloramine is more efficient than other alternatives to remediate and prevent Legionella in building water systems is its stability. Monochloramine is a weaker oxidizer that does not react with organics and does not decay as fast as other oxidants. Thanks to its stability, monochloramine can achieve a consistent residual in the entire plumbing system, resulting in a greater exposure between the disinfectant and the pathogens.
The goal of this article is to review the data that is available about the efficacy of monochloramine against pathogens other than Legionella. The data presented in this article comes from peer-reviewed literature, and five different case studies. All the data is from real scale drinking water applications in different environments, in particular:
Case Study 1: Monochloramine efficacy against Pseudomonas in a 400-bed hospital.
Case Study 2: Monochloramine efficacy against Pseudomonas in a dental clinic with 20 dental chairs.
Case Study 3: Monochloramine efficacy against Pseudomonas in a commercial building.
Case Study 4: Monochloramine efficacy against Pseudomonas in a municipal application.
Case Study 5: Monochloramine efficacy against different pathogens in a 997-bed hospital.
Peer-reviewed paper: Monochloramine efficacy against different pathogens and impact on water quality in a healthcare facility with 317 beds (10).
Monochloramine Generator
Monochloramine was fed to drinking water (either cold or hot) using the same technology in all the studies. A monochloramine generating system A was used on each site. The monochloramine generator produces monochloramine
10 the ANALYST Volume 30 Number 3 Microbial Levels Control with Monochloramines in Building and Municipal Water Systems continued
onsite by combining ammonium ions—supplied as ammonium chloride—with sodium hypochlorite. The generator feeds monochloramine based on water flow and the electronic controller maintains targeted residual in drinking water between 1.5 and 3 milligrams per liter (mg/L). The system monitors and limits the formation of free ammonia by measuring the redox (ORP) potential and adjusting the precursors feed rates.
The chemical precursors are certified to NSF/ANSI Standard 60 as drinking water additives and the monochloramine generator is certified to NSF/ANSI Standard 61 as a drinking water component.
Case Study #1
A 400-bed hospital, located in northern Italy, experienced Pseudomonas aeruginosa colonization in the domestic cold-water system at levels of 160 colony forming units per liter (CFU/L).
In order to eradicate Pseudomonas from the building plumbing system, the hospital water management team decided to implement an aggressive flushing protocol at all distal outlets. While the flushing protocol was being implemented and documented, the levels of incoming disinfectant from the municipality were too low to reduce the microbial contamination. Average incoming free chlorine levels varied from non-detect to 0.2 mg/L. In general, regular flushing can help to control the microbial population in building water systems, but it is proven that regular flushing is not effective over the long term.
Because of the low incoming free chlorine levels, flushing increased the Pseudomonas aeruginosa levels in drinking water. The levels went up to 4,000 CFU/L, possibly because more nutrients were brought into the building water system by the flushing protocol or the incoming water was already colonized.
The building water management team decided to implement monochloramine supplemental disinfection on the domestic cold-water systems. Monochloramine levels in drinking water were maintained between 2 and 3 mg/L. The water management team decided to test for Pseudomonas aeruginosa after one week from the beginning of the treatment and all the samples showed no detectable level. The water management team
decided to continue the treatment and all the samples that were collected periodically from the beginning of the treatment through the time of this writing (approximately one year) were always non-detectable (Figure 2).
Case Study #2
A dental clinic, located in northern Italy, with 20 dental chairs experienced Pseudomonas aeruginosa colonization. The facility performed periodic testing and Pseudomonas aeruginosa levels varied from 400 to 5,000 CFU/L. Heterotopic plate counts (HPC) were in the order of 102 to 103 CFU/mL. The facility occasionally tested for positive Legionella pneumophila, as well, but always at low levels (100 CFU/L).
The facility decided to implement shock hyperchlorination followed by regular flushing. These control measures were successful in reducing the microbial colonization in the building, but they didn’t prove to be effective long-term, and the Pseudomonas aeruginosa levels went back up to pre-treatment levels.
The facility decided to implement monochloramine supplemental disinfection on the domestic cold-water system. Monochloramine levels in drinking water were maintained between 2 mg/L and 3 mg/L.
The first round of water sampling was performed after two weeks, and all the samples showed no detectable levels of Pseudomonas aeruginosa and Legionella pneumophila. The facility continued to feed monochloramine to the building water system, and periodic testing showed complete microbial control from the beginning of the treatment up to the time of this writing (two years). This is shown in Figure 3.
11 the ANALYST Volume 30 Number 3 Microbial Levels Control with Monochloramines in Building and Municipal Water Systems continued
0 2000 4000 6000 8000 10000 2000 2002 2004 2006 2008 2010 2012 2014 2016 2018 N u m b e r o f C a s e s Year
Figure 2: Case Study #1 Results
Case Study #3
The domestic plumbing system of a five-story commercial facility, located in central Italy, was affected by Pseudomonas aeruginosa colonization. The drinking water in the facility feeds service areas, restrooms, a restaurant, and two coffee shops.
Periodical sampling showed Pseudomonas aeruginosa levels in the ≈ 500 CFU/L range. The facility implemented shock treatment with peracetic acid, but this control measure was found to be ineffective in lowering the microbial population.
The facility water management team decided to implement continuous supplemental disinfection of the domestic coldwater system with monochloramine. The monochloramine levels were maintained at 2 mg/L. Water samples were pulled after one month from the beginning of the treatment and showed no detectable levels of Pseudomonas aeruginosa. More rounds of samples were pulled after three months and six months showing the same results. Pseudomonas aeruginosa was never detected after the beginning of the treatment (shown in Figure 4).
Case Study #4
Case Study 4 examines a small public water utility, located in northern Italy, that delivers water to approximately 100 buildings. This system includes residential housing, commercial buildings, and hospitals, used regular free chlorine as a primary and secondary disinfectant. The average levels of free chlorine in the water that was being delivered to the distribution system were in the 0.15 to 0.20 mg/L range.
Even though the free chlorine was maintained at the targeted levels, the public water utility showed Pseudomonas aeruginosa colonization in the entire distribution system at levels between 160 CFU/L and 200 CFU/L. Because of the nature of a PWS and the impossibility of disrupting the water supply to the buildings, remedial shock treatment was not a feasible option.
The public water utility decided to change the secondary disinfectant from regular free chlorine to monochloramine. The PWS obtained the permit from the local authority having jurisdiction to change the secondary disinfectant. Before monochloramine was being fed to the distribution system, the public water utility performed an outreach plan to inform the population about the change in the water disinfectant.
Monochloramine was being fed at 2 mg/L for two weeks. After five days from the beginning of the application, the monochloramine concentration was uniform in the entire distribution system. After two weeks, the levels of Pseudomonas aeruginosa were all non-detectable and HPC levels were 102 CFU/mL. To decrease the HPC levels in drinking water, the public water utility increased the monochloramine residual to 3 mg/L for two additional weeks. After this time, the HPC counts showed all non-detects and the public water utility decreased the monochloramine levels to 2 mg/L. After more than one year, monochloramine shows full control of the microbial population.
Case Study #5
In 2011, a new 997-bed healthcare facility was commissioned in northern Italy; it opened to the public one year later. The building consists of seven five-story towers and a three-story main building. The PWS uses regular chlorine as a secondary disinfectant
12 the ANALYST Volume 30 Number 3 Microbial Levels Control with Monochloramines in Building and Municipal Water Systems continued
0 1000 2000 3000 4000 5000 6000 1 2 3 4 5 6 7 8 9 C F U / L Monochloramine treatment ND No control measures 400 - 5,000 CFU/L 2 weeks Monochloramine treatment ND 2 years
Figure 3: Case Study #2 Results
0 100 200 300 400 500 600 1 2 3 4 5 C F U / L No control measures 500 CFU/L 1 month Monochloramine treatment ND Peracetic acid 500 CFU/L 3 months 6 months
Figure 4: Case Study #3 Results
but the levels of free chlorine in the incoming water were always below the necessary level for waterborne pathogen control.
Since the commissioning phase of the building the water management team decided to include proactive testing for Legionella in the water management plan. The hospital has been collecting an average of 200 samples/year with monthly or bimonthly testing frequencies. According to the WMP, 70% of the samples were collected from representative points of the domestic hot water system (storage tank, DHW supply, distal outlets and DHW return). With little to no disinfectant coming from the PWS, in the first pre-opening round of samples, 18% of the samples were positive for Legionella, and in the second round of pre-opening sampling, 50% of the samples were positive for Legionella . Given the size of the domestic hot water system and the presence of thermostatic mixing valves at the point of use, the water management team decided that heat and flush would have been inadequate for controlling Legionella and decided to feed monochloramine to the domestic hot water system instead. Starting in 2016, the hospital started to sample for Pseudomonas aeruginosa , Acinetobacter baumannii , Stenotrophomonas maltophilia , Fusarium spp. and Aspergillus spp.
Before the building was opened to the public, the water management team decided to perform a shock monochloramine treatment with concentrations at distal outlets of 7 to 10 mg/L. The maximum regulated disinfectant level (MRDL) for monochloramine in drinking water is 4 mg/L. The monochloramine shock treatment proved to be effective in controlling Legionella and all the samples pulled in the third pre-opening round showed non-detectable Legionella levels.
After the building opened to the public, monochloramine levels were maintained between 2.50 mg/L and 3.00 mg/L during the entire seven-year study (2013 to 2019). The average monochloramine and free ammonia levels maintained in the domestic hot water system during the study are reported in Table B.
Table B: Average Monochloramine and Free Ammonia
The hospital continued to perform testing at regular intervals on both the domestic cold and domestic hot water samples for the entire duration of the study.
The Legionella sampling results are reported in Table C.
Table C: Legionella Sample Results
Monochloramine proved to be a great Legionella control strategy in the domestic hot water system during the duration of the entire study. Out of a total of 1,345 water samples collected from the domestic hot water system during the seven-year period, only 16 samples (1.2 %) were positive for Legionella and only five samples (0.4 %) had concentrations greater than 0.1 CFU/mL.
The sample results of other different microorganisms are reported in Table D.
13 the ANALYST Volume 30 Number 3 Microbial Levels Control with Monochloramines in Building and Municipal Water Systems continued
Concentrations Year Monochloramine (mg/L) Free ammonia (mg/L) 2013 2.57 ± 0.35 0.39 ± 0.13 2014 2.50 ± 0.43 0.43 ± 0.08 2015 2.58 ± 0.62 0.34 ± 0.11 2016 2.66 ± 0.21 0.35 ± 0.14 2017 2.56 ± 0.17 0.35 ± 0.11 2018 2.51 ± 0.27 0.37 ± 0.10 2019 2.51 ± 0.25 0.40 ± 0.06
Year Number of samples Positive samples (%) Positive samples > 0.1 CFU/mL (%) 2013 148 HW = 138 0 (0.0 %) 0 (0.0 %) CW = 9 0 (0.0 %) 0 (0.0 %) 2014 183 HW = 161 0 (0.0 %) 0 (0.0 %) CW = 21 0 (0.0 %) 0 (0.0 %) 2015 191 HW = 171 0 (0.0 %) 0 (0.0 %) CW = 20 0 (0.0 %) 0 (0.0 %) 2016 191 HW = 169 2 (1.2 %) 1 (0.6 %) CW = 22 0 (0.0 %) 0 (0.0 %) 2017 259 HW = 232 4 (1.7 %) 1 (0.4 %) CW = 27 0 (0.0 %) 0 (0.0 %) 2018 300 HW = 264 5 (1.9 %) 3 (1.1 %) CW = 36 1 (2.8 %) 1 (2.8 %) 2019 255 HW = 210 3 (1.4 %) 0 (0.0 %) CW = 45 0 (0.0 %) 0 (0.0 %) Note: HW = hot water; CW = cold water.



Improve oxidizing biocide effectiveness against biofilm bacteria WITHOUT high corrosion rates MIC
BCP®6010 BCP® 6010 Cleans, Protects & Boosts Dosing higher levels of an oxidizing biocide to control biofilm bacteria can increase general corrosion rates for many metals. Add AMSA, Inc.’s BCP® 6010 to your oxidant treatment program and reverse the higher corrosion trend! BCP® 6010 is a continuous feed chemical specifically designed to be incorporated into oxidizing biocide cooling water programs to: • Keep the systems CLEAN • PROTECT system surfaces • BOOST biocide effectiveness and corrosion inhibition AMSA, Inc.® 4714 S. Garfield Rd. • Auburn, Michigan, USA • 48611 Tel: (989) 662-0377 Fax: (989) 662-6461 sales@amsainc.com www.amsainc.com ® Call us to learn how BCP® 6010 can help your oxidizing biocide keep your system in control without high corrosion rates.
(Microbially Influenced Corrosion): Pitting on metal surfaces caused by biofilm.
Table D: Other Waterborne Pathogen Sampling Results
The authors of this paper from the U.S. Environmental Protection Agency (EPA) and the Ohio-EPA performed microbiological and water chemistry monitoring for several months prior to the installation of the system and for almost one year after the installation of the monochloramine system. As reported by the authors, the three main goals of the study were:
Better understand the effectiveness of monochloramine disinfection in reducing opportunistic pathogens (Legionella pneumophila, Pseudomonas spp., and NTMs).
Monitor for evidence of nitrification (e.g., nitrate, nitrite).
Monitor for changes in other important drinking water quality parameters (e.g., total chlorine, monochloramine, pH, temperature, DBPs, lead, copper, and other metals).
Based on the results reported in Table D, it did not appear that the hospital had an active colonization of all the different microorganisms that were tested. The number of positive tests was always below 10% with the only exception for the cold-water sampling of Pseudomonas aeruginosa in 2017 (3/27, 11.1 %). Monochloramine demonstrated complete microbial control of different waterborne pathogens as the overall rate of positivity remained low and showed little to no variations. Monochloramine did not promote the growth of certain pathogens.
Peer-Reviewed Paper
A medium-sized (317-bed) healthcare facility, located in Ohio, United States, performed sampling of hot and cold water for Legionella between 2006 and 2013. In 2013, the hospital observed positive Legionella pneumophila samples in the domestic hot water system, even while the average incoming level of free chlorine was 0.80 mg/L with peaks at levels higher than 1.00 mg/L.
More culture results from 2014 confirmed that the hot water system was positive for L. pneumophila serogroup 1 at 71% of distal points. The hospital staff implemented a flushing program of superheated water once every two weeks. While effective at first, analysis indicated that Legionella reappeared shortly after treatment. The hospital then decided to use monochloramine as a supplemental disinfectant and to feed it in the domestic hot water system.
The average monochloramine level in the domestic hot water system during the study was 2.01 ± 0.66 mg/L and they were reliably within the initial target dose range of 2 to 3.50 mg/L, whereas the free ammonia remained in the 0.00 to 0.50 mg/L range. The microbiological results of the study are reported in Table E.
The authors of the article confirmed the efficacy of monochloramine in reducing Legionella colonization in building water systems and proved that the disinfectant was also effective in reducing the percentage of positivity of Pseudomonas aeruginosa and non-tuberculosis mycobacteria.
The authors also reported that the monochloramine application did not have any noticeable impact on water quality and water chemistry. No nitrification was observed as the levels of nitrites and nitrates remained constant during the entire study. The corrosion rates of the plumbing system did not change from pre- to posttreatment, and the levels of copper and lead in drinking water remained constant. The levels of disinfection by
15 the ANALYST Volume 30 Number 3 Microbial Levels Control with Monochloramines in Building and Municipal Water Systems continued
Year Number of Samples P. aeruginosa A. baumannii S. maltophilia Fusarium spp. Aspergillus spp. 2016 135 HW = 124 1 (0.8 %) 0 (0.0 %) 4 (3.2 %) 0 (0.0 %) 1 (0.8 %) CW = 11 0 (0.0 %) 0 (0.0 %) 0 (0.0 %) 0 (0.0 %) 0 (0.0 %) 2017 223 HW = 196 4 (2 %) 0 (0.0 %) 10 (5.1 %) 2 (1.0 %) 4 (2.0 %) CW = 27 3 (11.1 %) 0 (0.0 %) 2 (7.4 %) 0 (0.0 %) 0 (0.0 %) 2018 268 HW = 236 5 (2.1 %) 1 (0.4 %) 1 (0.4 %) 2 (0.8 %) 3 (1.3 %) CW = 32 0 (0.0 %) 0 (0.0 %) 0 (0.0 %) 0 (0.0 %) 0 (0.0 %) 2019 255 HW = 210 8 (3.8 %) 0 (0.0 %) 8 (3.8 %) 1 (0.5 %) 5 (2.4 %) CW = 45 4 (8.9 %) 0 (0.0 %) 2 (4.4 %) 0 (0.0 %) 3 (6.7 %) Note: HW = hot water; CW = cold water.
Pathogen % Positivity pre-treatment % Positivity post-treatment Legionella pneumophila 68 % 6 % Pseudomonas aeruginosa 42 % 1.1 % Non-tuberculosis mycobacteria 61 % 14 %
Table E: Waterborne Pathogen Sampling Results (Reference 10)
products (THMs and HAA5) did not vary between before and after monochloramine was applied to the domestic hot water system.
N-Nitrosodimethylamine (NDMA) was also never detected during the entire duration of the study.
Conclusions
Because of the impact that Legionella has on the healthcare sector, there is data available in the literature addressing the efficacy of monochloramine against Legionella in building water systems. Limited data is available in the literature addressing the efficacy of monochloramine and other disinfectants against different waterborne pathogens that pose a threat to public health. Most of the literature that is available addressing this issue comes from municipal applications. The data from PWS does not necessarily give an understating of the behavior of disinfectants in building water systems, since public water utilities and buildings’ domestic plumbing systems are completely different environments.
The results from this literature review from case studies to a peer-reviewed paper demonstrated that monochloramine is not only effective in remediating and controlling Legionella , but it also reduces the colonization of other waterborne pathogens.
The data reported in the peer-reviewed paper also demonstrated that monochloramine does not have any impact on water quality, with no unintended consequences after the application of the disinfectant.
References
1. Parte, A.C., Sardà Carbasse, J., Meier-Kolthoff, J.P., Reimer, L.C. and Göker, M. (2020). “List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSM,” International Journal of Systematic and Evolutionary Microbiology, 70, pp. 5607-5612; DOI: 10.1099/ijsem.0.004332.
2. Fliermans, C.B.; Soracco, R.J.; Popes, D.H. (1984). “A Note on the Temperature Tolerance of Legionella,” Journal of Applied Bacteriology, 56, pp. 349-350.
3. Castillo, N.E.; Rajasekaran, A.; Ali, S.K. (2016). “Legionnaires’ Disease: A Review,” Infectious Diseases in Clinical Practice 24(5), pp. 248-253.
4. Cunha, B.A.; Burillo, A.; Bouza, E. (2016). “Legionnaires’ Disease,” The Lancet, 387, pp. 387, 376-385.
5. National Academies of Sciences, (2019). “Management of Legionella in Water Systems,” Engineering, and Medicine, The National Academies Press, Washington, D.C., accessible at doi.org/10.17226/25474
6. EPA (September 21, 2016). “Technologies for Legionella Control in Premise Plumbing Systems,” Scientific Literature Review, pdf file, EPA 810-R-16-001, U.S. Environmental Protection Agency, Washington, D.C.
7. Kool, J.L.; Carpenter, J.C.; Fields, B.S. (1999). “Effect of Monochloramine Disinfection of Municipal Drinking Water on Risk of Nosocomial 659 Legionnaires’ Disease,” The Lancet, 353, pp. 272-277.
8. Heffelfinger, J.D.; Kool, J.L.; Fridkin, S.; Fraser V.J., Hageman, J.; Carpenter, J.; Whitney, C.G. (2003). “Risk of Hospital-Acquired Legionnaires’ Disease in Cities Using Monochloramine Versus other Water Disinfectants,” Infection Control & Hospital Epidemiology, 24, pp. 569-574.
9. Flannery, B.; Gelling, L.B.; Vugia, D.J.; Weintraub, J.M.; Salerno, J.J.; Conroy, M.J.; Stevens, V.A.; Rose, C.E.; Moore, M.R.; Fields, B.S.; Besser, R.E. (2006). “Reducing Legionella Colonization in Water Systems with Monochloramine,” Emerging Infectious Diseases 12, pp. 588-596.
10. Lytle, D.A.; Pfaller, S.; Muhlen, C.; Struewing, I.; Triantafyllidou, S.; White, C.; Hayes, S.; King, D.; Lu, J. (2021). “A Comprehensive Evaluation of Monochloramine Disinfection on Water Quality, Legionella, and other Important Microorganisms in a Hospital,” Water Research,189, 116656.
Other Sources
1. ASHRAE (2021). Standard 188-2021: Legionellosis: Risk Management for Building Water Systems, American Society of Heating, Refrigerating and Air-Conditioning Engineers, Atlanta, Georgia.
2. ASHRAE (2020). Guideline 12-2020: Managing the Risk of Legionellosis Associated with Building Water Systems, American Society of Heating, Refrigerating and Air-Conditioning Engineers, Atlanta, Georgia.
3. ASHRAE (2019). Proposed standard: New Building Water Management Standard ASHRAE/NSF 514, approval pending at press time, American Society of Heating, Refrigerating and Air-Conditioning Engineers, Atlanta, Georgia.
4. CDC (n.d.) Toolkit for Controlling Legionella in Common Sources of Exposure, Centers for Disease Control and Prevention, Atlanta, Georgia.
5. ASSE (2020). ASSE/IAPMO/ANSI 12080: Professional Qualifications Standard for Legionella Water Safety and Management Personnel, ASSE International, Mokena, Illinois.
6. AIHA (May 2022). AIHA Guideline: Recognition, Evaluation, and Control of Legionella in Building Water Systems, 2nd ed., American Industrial Hygiene Association, Falls Church, Virginia.
Endnote
A. The monochloramine generation system mentioned in the article that is named SANIKILL and is made by Sanipur SPA, which is based in Brescia, Italy.
Alberto Comazzi, Ph.D., is the Vice President of Sanipur US, based in Philadelphia, Pennsylvania. Dr. Comazzi holds a doctorate in industrial chemistry from the University of Milan (Italy). His research during his academic career focused on the study of the efficacy, stability, and interaction among different chlorine-based water disinfectants. Along with his main research project, Dr. Comazzi collaborated with different industries and universities on several catalysis, and oil and gas research projects. He has presented at more than 20 international conferences and expositions. Dr. Comazzi is a member of the ASHRAE SSPC-188 committee, ASHRAE SPC-514 committee, and the AWWA premise plumbing committee. He is certified to ASSE/IAPMO/ANSI 12080 Legionella Water Safety and Management Personnel.

This paper was presented at the 2022 Association of Water Technologies Annual Conference & Exposition, which was conducted Sept. 21-24, 2022, in Vancouver, British Columbia, Canada.
Keywords: BACTERIA, BIOCIDES, CHLORINE, LEGIONELLA, MONOCHLORAMINE, PSEUDOMONAS.
16 the ANALYST Volume 30 Number 3 Microbial Levels Control with Monochloramines in Building and Municipal Water Systems continued

Have you completed the journey? LOOK TO FRENCH CREEK FOR : French Creek moved the industry towards the digital age in 1989. INAUGURAL AWT INNOVATION AWARD HONOREE FOR WaterCycle® A PRODUCT THAT MOVED THE TECHNOLOGY NEEDLE Incorporating French Creek calculations into your controllers with our UNIX libraries Engineering tools for Scale & Corrosion Prediction & Control Calling French Creek calculations from your applications with our Windows libraries

18 the ANALYST Volume 30 Number 3
Bioaugmentation Reduce Hydrogen Sulfide Production in Municipal Wastewater Collection Systems?
Can
Jennifer Cray, Megan Duersteler, Josiah Menako, Dan Romanek, Sona Son, and Mike King, Microbial Discovery Group
Hydrogen sulfide (H 2 S) is a highly toxic, colorless gas with a pungent, unpleasant odor that is a common occurrence in municipal collection systems. Humans can detect H 2 S odor in the air at concentrations as small as 0.01 to 1.5 parts per million (ppm), but exposure to higher concentrations can have serious health effects. At 100 ppm, eye and respiratory irritation can occur within less than an hour of exposure, and at 700 ppm or greater the gas is deadly within minutes (1). In addition to the health hazards for workers, H 2 S production in collection systems has other costly side effects. Costs range from fines due to odor regulations, to frequent repairs or total replacement of concrete and metal infrastructure due to extensive corrosion (2).
There are multiple methods for controlling H 2 S in wastewater systems. Scrubbers and filters can be installed to physically remove H 2 S gas before it is released from the system. In addition to being a large capital expense, scrubbers and filters do not stop the H 2 S from being generated in the system. Chemical treatments such as precipitants, oxidizers, and nitrates are also common in the industry. Precipitants and oxidizers work to chemically convert existing H 2 S into less harmful compounds (3). Nitrates replace sulfate in bacterial respiration which prevents H 2 S from forming (3). These options can work to minimize the problems associated with H 2 S, but all require large capital expenditures and/or high volumes of chemicals and few are actually addressing the source of the problem.
Lift stations and force mains suffer from high levels of H 2 S because of sulfate-reducing bacteria (SRB) that thrive in those environments. Municipal wastewater treatment systems have abundant food for SRB in the form of volatile fatty acids (VFA). High amounts of sulfate are also found in this environment which SRB utilize in anaerobic bacterial respiration. Additionally, the built-up fats, oils, and grease (FOG) common in municipal wastewater systems provide the anaerobic or anoxic biofilms where SRB prefer to live (4).
SRB use sulfate, which originates from cleaning product residue and breakdown of amino acids, to facilitate cellular respiration and produce energy, and this process creates a byproduct of H 2 S (4, 5). This H 2 S exists in a dissolved form in the water until physical agitation or chemical conditions cause it to be released into the air
(6). Sulfur oxidizing bacteria (SOB) can then use H 2 S to create sulfuric acid, which is what causes corrosion in areas with high H 2 S concentrations (4). The problems associated with high H 2 S are most effectively mitigated by treatments that prevent SRB from thriving in collection systems.
Bioaugmentation is the process of enhancing the microbial community that naturally exists in an environment through the addition of bacterial species and/or nutrients that support microbial growth. Bacillusbased bioaugmentation products work to reduce H 2 S in wastewater systems by removing SRB’s food sources and habitats. VFA are one of the primary food sources for SRB, but Bacillus are able to consume them as well (7). Bacillus can grow rapidly in wastewater systems and, therefore, need to consume a lot of food, including VFA, which limits the amount available for SRB (Figure 1). Bacillus also break down FOG to obtain nutrients, thus disrupting SRB’s preferred anaerobic habitat (Figure 2). As they grow, Bacillus use sulfate to build amino acids and proteins to incorporate into new cells, which reduces the sulfate available for SRB (Figure 3). Through the utilization of VFA and sulfates, and the consumption of FOG, Bacillus can reduce SRB activity in collection systems, thus reducing H 2 S production, and therefore alleviating the associated problems.
A Bacillus-based bioaugmentation treatment A was used to treat 20 different municipal collection systems and was able to effectively reduce H 2S in 90% of these systems.

19 the ANALYST Volume 30 Number 3 Can Bioaugmentation Reduce Hydrogen Sulfide Production in Municipal Wastewater Collection Systems?
Figure 1: In a laboratory study, a minimal growth media was spiked with 100 ppm of volatile fatty acids (VFA). When Bacillus was grown in media with and without VFA, there was a 2.5-fold increase in Bacillus growth within 24 hours when additional VFA were available. This data shows that Bacillus consumes VFA as a carbon source to support growth.




20 the ANALYST Volume 30 Number 3 Can Bioaugmentation Reduce Hydrogen Sulfide Production in Municipal Wastewater Collection Systems? continued
Figure 2: A municipal lift station with fats, oils, and grease (FOG) coverage over most of the surface of the water (left) was treated with the bioaugmentation product. After treatment most of the surface of the water was cleared of FOG (right).
Figure 3: Bacillus use sulfate in wastewater to synthesize amino acids that are incorporated into proteins and used to facilitate growth of new bacterial cells. As the population thrives, more sulfate is used. This limits the amount of sulfate available in the environment for use by SRB in cellular respiration which produces H2S as a byproduct.
Figure 4: H2S levels before and after the bioaugmentation treatment within 20 municipal wastewater systems. Length of treatment varied from 2-12 weeks. A reduction in H2S was observed in 18 of 20 applications.
Figure 5: Example of H2S OdaLog data from two municipal systems given the bioaugmentation treatment. The start of treatment and when H2S reduction was first observed is represented by the green lines on each graph. The graph on the left shows that H2S levels decrease 10 days after treatment begins. The graph on the right shows that H2S levels decrease three days after treatment begins.
Methods
The goal of this study was to analyze the efficacy of the bioaugmentation treatment on H 2 S reduction in municipal wastewater collection systems. Twenty applications were included in this study with average pre-treatment H 2 S levels ranging from less than 10 ppm to greater than 600 ppm. Locations of systems were spread across the United States, covering 11 different states. The size of the systems varied with an average daily flow of 628,000 gallons per day (gpd), and a range of 10,000 to 3,000,000 gpd.
Each location treated underwent an initial survey. The layout of the system was investigated to fully understand the flow and choose appropriate H 2 S monitoring and product dosing locations. Ideally, monitoring was conducted at the site with the most severe level of H 2 S within the system and dosing was set up upstream of the monitoring location. In all systems, H 2 S data was obtained through constant gas monitoring using portable AcruLog or Odalog monitors deployed into lift stations to collect H 2 S levels. Baseline data was collected in each system in order to assess the severity of the problem prior to treatment.
The bioaugmentation treatment was applied via a peristaltic pump that was set to dispense multiple times per day during periods of lowest flow. Treatment dosages and frequency of dosing varied in each application based on flow and severity of H 2 S. The duration of each treatment monitoring period was 2–12 weeks.
Results
The bioaugmentation treatment resulted in a significant (p-value <0.05) decrease in average H 2 S levels of the 20 systems analyzed. Treatment successfully lowered H2S levels in 90% of the systems (Figure 4). In the 18 systems where bioaugmentation was able to reduce H 2 S levels, there was an average of 68% (SD = 18%) reduction in H 2 S after treatment. Measurable H 2 S reductions can be seen as early as the first week of the bioaugmentation treatment (Figure 5).
Conclusions
Bioaugmentation can be an effective strategy to treat wastewater systems with H 2 S challenges. Each of the problems that H 2 S can cause, including unsafe working conditions, equipment corrosion, and odor complaints
can be improved by tackling H 2 S at its source. Many chemical and mechanical treatments only work to remove H 2 S post-production or stop its release from a system instead of preventing its formation.
Our study demonstrated that bioaugmentation worked in most applications to treat H 2 S, but for treatment to be effective it is important to understand the system and dose correctly. Dosing needs to occur in an appropriate location so that the bacteria can reach the problem areas. Bioaugmentation products should be dosed at periods of low flow and upstream of the locations with high H 2 S levels, so the bacteria have the time and opportunity to be effective. It is also important to know the daily flow so that dosage can be calculated correctly. Missing the critical information described here prior to treatment contributed to the lack of response in Systems 19 and 20 in this study where treatment was unsuccessful.
Bioaugmentation can treat and prevent future H 2 S problems in wastewater systems. With the right treatment plan and product, bioaugmentation can reduce or eliminate odor complaints, equipment replacement due to corrosion, and the need for other chemical or mechanical H2S treatments, which will result in ROI for a wastewater treatment facility.
References
1. Occupational Safety and Health Administration (n.d.). Hydrogen Sulfide. United States Department of Labor. Retrieved April 25, 2022, from www.osha.gov/hydrogen-sulfide/hazards
2. Sutherland-Stacey, L.; Corrie, S.; Neethling, A.; Johnson, I.; Gutierrez, O.; Dexter, R.; Hamilton, G. (February 2008). “In Situ Continuous Measurement of Dissolved Sulfide in Sewer Systems,” Water Science & Technology 57(3), pp. 375-381.
3. Yuan, Z.; Ganigue, R.; Jiang, G.; Liu, Y.; Chen, J. (2015). “Sewer Corrosion and Odour Research Linkage Project,” The University of Queensland: Queensland, Australia.
4. Meyer, D.D.; de Andrade, P.A.M.; Durrer, A.; Andreote, F.D.; Corção, G.; Brandelli, A. (2016). “Bacterial Communities Involved in Sulfur Transformations in Wastewater Treatment Plants,” Applied Microbiology and Biotechnology 100(23), pp. 10125-10135.
5. Sekyiamah, K.; Kim, H.; McConnell, L.L.; Torrents, A.; Ramirez, M. (2008). “Identification of Seasonal Variations in Volatile Sulfur Compound Formation and Release from the Secondary Treatment System at a Large Wastewater Treatment Plant,” Water Environment Research 80(12), pp. 2261-2267.
6. Churchill, P.; Elmer, D. (1999). “Hydrogen Sulfide Odor Control in Wastewater Collection Systems,” Journal of the New England Water Environment Association 33(1), pp. 57-63.
7. Sharma, K.R.; Yuan, Z.; De Haas, D.; Hamilton, G.; Corrie, S.; Keller, J. (2008). Dynamics and Dynamic Modelling of H2S Production in Sewer Systems,” Water Research 42(10-11), pp. 2527-2538.
Endnote
A The bioaugmentation treatment used to obtain the results discussed in this study is Biotifx®, which is a Bacillus-based product that is made by Microbial Discovery Group.
21 the ANALYST Volume 30 Number 3 Can Bioaugmentation Reduce Hydrogen Sulfide Production in Municipal Wastewater Collection Systems? continued
Jennifer Cray is a microbiologist with a strong background in biology and biochemistry. Ms. Cray has nine years of industry experience in developing Bacillus-based microbial products for wastewater treatment, IIC, and animal agriculture. Her research on Bacillus-based bioaugmentation and its impact on reducing sludge in wastewater lagoons has been published in Water and Waste Digest.
Megan Duersteler joined MDG in 2017, and currently is an R&D group leader. She is an expert in microbiology, molecular biology, and immunology. Ms. Duersteler has 15 years of industry experience in developing Bacillus-based microbial products for wastewater and animal agriculture. She has published multiple articles in the animal agriculture sector.


Josiah Menako’s career began at MDG as an undergraduate intern in 2015. He then went on to become a microbiologist and then a technical support specialist. He currently serves as the company’s wastewater technical service lead; he has experience in domestic and international in-person wastewater support. Mr. Manako’s expertise has led him to conduct hundreds of training sessions for wastewater professionals across the United States and globally. His commitment to continuous learning keeps him current with advancements in the field, ensuring innovative solutions for evolving wastewater needs.
Dan Romanek holds an M.S. degree in biology from Write State University. He has more than 12 years of experience and has become an expert in wastewater management, specializing in biotechnology, chemistry, and equipment. Mr. Romanek has served various industries, including municipalities, food and beverage manufacturing, petrochemicals, pulp and paper, plastics, and mining. He joined MDG in 2015.


Sona Son is the COO of MDG. She has more than 15 years of experience in biotechnology. She holds a B.S. from the University of Wisconsin – Madison and an MBA from the Kellogg School of Management at Northwestern University. She joined MDG in 2008 and oversees all technical and production operations.

Mike King is the founder and CEO of MDG, a biotechnology company he founded in 2007. Dr. King obtained his B.S. and M.S from Oklahoma State University and his Ph.D. from the University of Illinois UrbanaChampaign. He has a passion for the isolation of novel organisms to find environmental and health solutions to feed, clean and save the world. His commitment to novel bacteria strain isolation extends to various applications, including human, animals, aquaculture, biocontrol, wastewater treatment, and bioremediation applications.
This paper was presented at the 2022 Association of Water Technologies Annual Conference & Exposition, which was conducted Sept. 21-24, 2022, in Vancouver, British Columbia, Canada.
Keywords: BIOTREATMENT, CORROSION, HYDROGEN SULFIDE, ODOR, WASTEWATER

22 the ANALYST Volume 30 Number 3 Can Bioaugmentation Reduce Hydrogen Sulfide Production in Municipal Wastewater Collection Systems? continued




Validation of a Portable qPCR Method for the Detection of Legionella
 Jeremy Duguay, Harley King, Ph.D., Neil Sharma, Ph.D., and Jordan Schmidt, Ph.D., LuminUltra Technologies Ltd.
Jeremy Duguay, Harley King, Ph.D., Neil Sharma, Ph.D., and Jordan Schmidt, Ph.D., LuminUltra Technologies Ltd.
24 the ANALYST Volume 30 Number 3
Background
The ability to detect Legionella rapidly and reliably is critically important to effectively manage risk in water distribution systems and cooling water circuits. The requirement for quick results is often hindered by the need to send collected samples to off-site laboratory facilities where results can take several days or even weeks. Although the gold standard and widely accepted regulatory method of detecting Legionella species remains culture-based, quantitative polymerase chain reaction (qPCR) is an established detection and screening method that can be designed to target DNA unique to Legionella to quantitatively measure the bacterial population present within a water system in hours, rather than the typically required days.
qPCR methods are now being routinely used to monitor for Legionella in water sources, but the available Legionella methods are not all the same. When dealing with organisms dangerous to public health, the consequences of false positive or negative results can be extremely serious, which necessitates robust and continual assessment and validation of any detection method, and the availability of validation data to an informed user-group able to adequately assess methodology is extremely important.
In this study, we present the validation of a qPCR method for the detection of bacteria belonging to the genus Legionella or Legionella pneumophila (serogroups 1-15) in accordance with International Standards Organization (ISO) Technical Specification 12869:2019 (1). The results of this study are presented with special emphasis on end-user assessment of qPCR assays designed for the detection of Legionella and methods of validation applicable to any qPCR-based detection method. These methods include the determination and verification of a linear regression curve used to convert raw qPCR results into a concentration of genome units (GU) of Legionella , inclusivity and exclusivity of the designed qPCR primers, limit of detection (LOD) and limit of quantification (LOQ). In addition, realworld applications of the technology will be presented, including a third-party validation of the method on blind samples used for CDC-ELITE proficiency testing as well as an assessment of the technology in response to typical cooling water biocides.
Introduction:
Legionella bacteria are responsible for the group of diseases referred to as Legionellosis, which includes the more severe pneumonia Legionnaire’s Disease and the milder, non-pneumonia Pontiac Fever. There were approximately 10,000 cases of Legionnaire’s Disease in the United States reported in 2018, but this number is thought to be as much as 10 times higher due to underreporting of the disease (2, 3). Legionella are among the top annual causes of waterborne disease outbreaks in the United States and contribute to large annual healthcare costs (>$400 million) (4). There are many suspected reasons for the steady climb of Legionellosis cases globally, including environmental changes, increased urbanization, an aging population, and green building initiatives that reduce water flow (3).
While Legionella bacteria are naturally found in the environment in low levels, they become a public health risk primarily through the built environment route where optimal growth conditions can lead to their exponential growth to dangerous levels (5). There are several key elements of the built environment that contribute to the growth explosion, including optimal water temperatures from 20 to 45 °C, low flow and water stagnation within pipes that produce the ideal structural scaffolding for surface deposition, and the formation of biofilm.
While the underlying causes for the increases in Legionella cases are varied and complex and may take time to understand completely, there are many organizations and government initiatives underway to help reduce the impact of the disease on the broader population. This includes the publication of guidelines like ASHRAE 188:2021 (6) and the CDC’s toolkit for Developing a Water Management Program to Reduce Legionella Growth and Spread in Buildings (7). The central tenant to reduce the risk posed by Legionella and ultimately reduce the spread of disease is the development of a good water management plan (WMP) that documents the water system, identifies risks presented to the occupants, and monitors that risk continuously.
The best way to ensure your WMP is effective is to verify your control methods through testing for Legionella. Traditionally, the culture method has been considered the gold standard method for detection of viable Legionella , but the method has limitations. The main drawbacks
25 the ANALYST Volume 30 Number 3
a
Validation of
Portable qPCR Method for the Detection of Legionella
being the inability to account for all Legionella species in one growth media/method and the slow time to results are not unique to Legionella monitoring but present with cultural methods in general (8, 9). In addition, the culture method does not capture the viable but not-culturable (VBNC) fraction (10) and impacts from variable sample handling and shipment conditions can lead to variable and reduced Legionella quantification.
For these reasons and others, alternative and rapid methods for the detection of Legionella have been gaining popularity in practical application (11). One such method is the qPCR test, which has been used to detect Legionella in potable and non-potable water (12). In general, qPCR presents many benefits compared to the traditional culture method, including the ability to detect the viable but not culturable fraction and all Legionella species in a very rapid time to result (13).
Detecting and quantifying Legionella spp. or Legionella pneumophila using qPCR is done through the following methodological approach:
1. Isolation and concentration of waterborne bacteria through filtration of water samples.
2. The extraction (and purification) of DNA from the filter.
3. The amplification, detection, and quantification of unique DNA sequences that belong to either the Legionella genus or Legionella pneumophila , depending on the assay you are using.
There are several commercially available test kits and equipment packages for qPCR detection of Legionella One way to be certain that the selected kit is robust, accurate and reliable for the intended job is to assess its performance against standard methods and to validate new methods against rigorous and established performance criteria to determine reliable performance under normal and expected working conditions. One such technical standard is ISO/TS 12869: “Water Quality: Detection and Quantification of Legionella spp. and/or Legionella pneumophila by concentration and genic amplification by quantitative Polymerase Chain Reaction (qPCR).” This technical standard is intended for validating laboratory-based qPCR and presents important criteria for evaluating Legionella qPCR methods.
In this article, we highlight the ISO-12869 technical standard, taking particular care to highlight the performance criteria: Why they are important and what they mean? We will present results of a secondary validation of a qPCR method and present the results of the verification criteria set forth by ISO-12869:2019. In addition, we will present several real-world applications of the technology for rapid detection and quantification of Legionella and discuss the potential applications of portable qPCR to improve control through regular process performance monitoring.
Materials and Methods
qPCR Method
The qPCR method that was validated according to the ISO 12869 technical performance criteria is designed to be a portable method used for onsite detection or in a laboratory. The equipment required to run the protocol fits in a Pelican-style carrying case and includes all necessary pipettes, a centrifuge, and a thermocycler. The reagents and consumables required are all included in DNA purification kits and assay kits for either Legionella spp or Legionella pneumophila (serogroups 1 to 15 inclusive).
Figures
Step 1: Sample Filtration and Concentration. The sample is collected, mixed well and then passed through a filtration process that retains intact cells on the filter while extracellular DNA and other dissolved impurities pass through. Increasing the volume of sample processed improves the accuracy and limit of detection for the method.
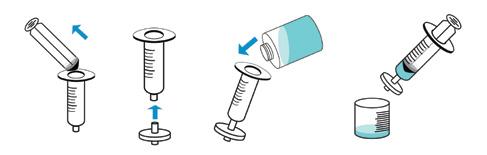

26 the ANALYST Volume 30 Number 3 Validation of a Portable qPCR Method for the Detection of Legionella continue
1 through 5 illustrate the qPCR workflow.
Figure 1: Sample filtration and concentration.
Figure 2: Washing step.
Step 2: Sample Wash. The filtration step captures intact cells on the filter, which then gets washed with a buffer to remove potential inhibitors that may also accumulate on the filter.
master mix components. Assay tubes are then placed in the thermocycler. The cycling parameters are selected based on the assay run and the run is started. All data collection and interpretation were done through the instrument software.
Assay Design
Step 3: DNA Extraction. A lysis solution is backwashed through the filter and aspirated back through the filter a total of five times for complete sample extraction.


The NCBI GenBank was used to design and assess the primers and probes that select for L. spp. and L. pneumophila (serogroups 1-15) specifically, while excluding other genera. An internal positive control (IPC) was also designed and added to the assay to amplify simultaneously along with the L. spp. or L. pneumophila DNA and ensure that inhibition did not impact amplification. Lack of amplification or limited amplification of the IPC is indicative of qPCR inhibitors that have not been removed by the DNA extraction and purification steps. In this case, further purification is required, either by dilution, or an alternative DNA preparation procedure to remove the inhibitors.
ISO/TS 12869 Validation
Step 4: Purification and Elution. A portion of the lysed sample is then added to a gel resin column to further purify the extracted DNA from the lysate solution. The resin helps remove impurities that may impact the qPCR and allows the DNA to pass through into a collection tube after centrifugation for two minutes to fully elute the DNA.

ISO/TS-12869: 2019 Annex G states that method verification, referred to secondary validation, is applicable for any implementation of a third party validated method in a laboratory that has already undergone a primary validation according to Clause 9 in the standard. Since the underlying method presented here has already undergone a primary validation at the 3rd party lab, and the same method was implemented in the laboratory in its entirety, the following secondary verification criteria apply:
Calibration Curve: at least five ranges under intermediate reproducibility conditions
Verification of the LOQ: to verify the first point LOQ of five calibration ranges analysed under intermediate reproducibility conditions
Step 5: Amplification, Detection and Quantification.
For each set of samples run, it’s recommended that a positive control and negative control (no template control) are run. The master mix is supplied in freezedried tubes (specific for either L. spp. or L. pneumophila) of which 20 microliters (µ L) of positive control, 20 µ L of extracted sample DNA or 20 µ L of nuclease free water (negative control) are added to rehydrate the
Verification of the LOD: to verify on a single target concentration (LOD), at the rate of 10 replicates on 2 d (intermediate reproducibility)
Robustness
Recovery
CDC-ELITE Lab Blind Proficiency Sample Testing
A laboratory participating in the Centers for Disease Control and Prevention (CDC) Environmental
27 the ANALYST Volume 30 Number 3 Validation of a Portable qPCR Method for the Detection of Legionella continued
Figure 3: DNA extraction.
Figure 4: Sample purification and elution.
Figure 5: Sample detection and quantification.
Legionella Isolation Techniques Evaluation (ELITE) program was selected to test the qPCR method alongside their culture method for the ability to correctly detect Legionella species and pneumophila samples. The complete qPCR workflow was followed (including DNA extraction combined with Legionella species and Legionella pneumophila qPCR assays) and all samples (six in total) used for the proficiency testing program were subjected to qPCR. The qPCR results were then compared to the true sample identities revealed through the program to assess the methods ability to correctly identify positive and negative samples.
Biocide Evaluation Study
A third-party lab was selected to compare different oxidizing biocides typically used in industrial water treatment programs, determine any impact on the qPCR method, and compare the results to the labs culture method. The water matrix used in this study was chlorinefree tap water adjusted to pH 7.0 to 7.5, filter sterilized and pre-warmed to 35 ± 1°C. The contaminating organisms were L. pneumophila SG 1 ATCC 33152, L. pneumophila SG 2-15 QL 324277-6, and L. micdadei QL 14522-1A. All organisms used in this study were retrieved from frozen stock culture stored at -70°C and propagated on Buffered Charcoal Yeast Extract (BCYE) agar containing cysteine at 35 ± 1°C for three to five days prior to preparing inoculum. A fresh culture suspension of each organism was prepared in sterile 0.45% saline and adjusted to approximately 1.0 McFarland.
A 1-mL volume of each organism suspension was combined to create the Legionella spiking cocktail suspension. The prepared and prewarmed tap water matrix was inoculated with 1 mL of the organism cocktail, mixed thoroughly, and divided into four 100-mL portions in sterile Erlenmeyer flasks. Two of the portions were treated with only one of two oxidizing biocides, chlorine, or bromine (as hypobromous acid, HOBr). One 100-mL portion of inoculated water was heat treated in a pot of boiling water, while the last portion remained untreated by chemicals or heat and served as a live culture control. All individual 100-mL test portion flasks had the opening sealed with Parafilm prior to incubation at 35 ± 1°C.
After several trial rounds to optimize the dosage and contact time, decontamination by chemical means was
conducted with 20-parts per million (ppm) chlorine and 5-ppm bromine. Each biocide was added to the appropriate 100-milliliter (mL) portion of contaminated water and maintained on a platform shaker set to 160 revolutions per minute (rpm) at 35 ± 1°C for 20 to 24 hours. During this round of testing, the heat-treated culture suspension was placed into a pot of boiling water for 90 minutes before moving to 35 ± 1°C for 20 to 24 hours. The broth control flask representing only live cells was inoculated and moved directly to 35 ± 1°C for 20 to 24 hours. Each 100-mL portion was further divided into two 50-mL portions. One 50-mL portion was tested using the Legionella species and L. pneumophila assays following the manufacturer’s Field Test Kit instructions (ver. 1.01), while the other portion was filter concentrated (0.2 micrograms [µm]) and washed in 5 mL of sterile, diluted ¼-strength Ringer’s solution. A 1-mL aliquot was plated onto BCYE agar plates and incubated at 35 ± 1°C for 7–10 days in a humidified atmosphere. The broth control filtrate was serially diluted, plated onto BCYE and incubated as previously described. qPCR analysis was conducted using the computer software interface and the Gene Count Q-16 instrument.
For the culture analysis, the agar plates were incubated inverted at 35 ± 1°C for 7–10 days in a humidified atmosphere and examined using illuminated magnification (10x). Suspect colonies were isolated onto BCYE and sheep blood agar. Isolates demonstrating growth typical of Legionella were identified using the Bruker MALDI Biotyper and a commercially available Legionella slide agglutination tests if necessary.
Results
Inclusivity and Exclusivity
For the design of a qPCR assay, it is very important that the primer and probe set show a high level of specificity such that undesired nucleotide sequences are not amplified, and desired nucleotide sequences are not missed. ISO 12869 defines lists that cover inclusivity and exclusivity guidelines for both L. pneumophila and L. spp. qPCR assays.
For an L. spp. assay, ISO 12869 requires that 40 species/ serogroups are measured to validate for inclusivity. All 40 species/serogroups were measured using the L. spp. assay during the primary third-party validation of this assay.
28 the ANALYST Volume 30 Number 3 Validation of a Portable qPCR Method for the Detection of Legionella continued
Exclusivity testing includes a broad range of genera that are often cohabitants in natural and engineered freshwater systems alongside Legionella, including Enterococcus, Escherichia, Pseudomonas, Shigella, Staphylococcus, and Yersinia, among others. During the primary validation, 119 different species/sub-species were tested with concentrations of at least 10,000 genome units per well (GU/well) and proven to not be detected by the L. spp. primer and probe set.
Internal Positive Control Design
When working with real-world samples taken from cooling towers or drinking water distribution systems, there can be molecules present that might interfere with qPCR measurements. Ideally, all these inhibitors will be removed during the DNA extraction and purification steps, but in the case that there is an excessive number of inhibitors or types of inhibitors that the DNA purification method is not prepared to handle, it is useful to have an internal positive control (IPC) that will identify the presence of these inhibitors. If a measurement is performed, and the IPC is not amplified to a sufficient degree, this means that inhibitors are affecting the results, and additional steps, such as dilution, should be used to purify the sample.
The IPC used for the assay contains the same nucleotide sequence as the targeted Legionella genes so that it is co-amplified using the same primers alongside the bacterial DNA. A probe with a different fluorescence tag is used to measure the fluorescence of the IPC separately from that of the targeted Legionella DNA. During the third-party validation, experiments were performed with different amounts of the IPC to select a suitable amount per assay to indicate the presence of inhibitors, while not affecting the measurement of Legionella bacteria.
Creation and Verification of Linear Regression Curve for Calibration
To convert raw qPCR results (cycle threshold [Ct]) into a quantitative measure of concentration, calibration curves were generated by testing known DNA concentrations of L. spp. and L. pneumophila. Serial dilutions across a broad range of concentrations that will be encountered in the real application of qPCR testing were constructed. For the Legionella species assay, two dilution series were performed across a 5-log 10 range, with the first curve
ranging from 25 to 200,000 GU while the second curve ranged from 100 to 600,000 GU. The linear regression analyses, which plotted the obtained Ct values against log10 GU produced linear plots with correlation coefficients of R2 = 0.9961 and 0.9949 for the first and second curve, respectively. The slope was -3.250 for the first curve and -3.394 for the second curve.
From these standard curves, an average, standard deviation, and accuracy of linearity were calculated for each concentration to verify the linear regression. ISO 12869 stipulates that Elin must be below 0.15 log10 for all concentrations tested to validate the standard curve. All concentrations of L. spp. from both curves were analyzed and had an accuracy of linearity better than 0.15 log10 , which validates the quality of DNA analysis procedure. The performance and accuracy data are presented in Table A and Table B.
lin < 0.15 in all cases, so the linear regression performance is validated.
so the
29 the ANALYST Volume 30 Number 3 Validation of a Portable qPCR Method for the Detection of Legionella continued
Theoretical Concentration (GU) GU 200,000 15,000 3,000 250 25 (log) GU (x) 5.30 4.18 3.48 2.40 1.40 Measured Concentration (log GU) 1 5.40 4.33 3.46 2.48 1.49 2 5.23 4.16 3.63 2.33 1.53 Sum 10.63 8.49 7.10 4.82 3.03 Average (X) 5.31 4.24 3.55 2.41 1.51 Bias (X-x) 0.01 0.07 0.07 0.01 0.11 Standard Deviation 0.12 0.12 0.12 0.11 0.03 Accuracy of Linearity (Elin) 0.12 0.14 0.14 0.11 0.12
Table A: Verification of the Linear Regression Performance for Legionella Species (Curve 1)
E
Theoretical Concentration (GU) GU 600,000 60,000 6,000 600 100 (log) GU (x) 5.78 4.78 3.78 2.78 2.00 Measured Concentration (log GU) 1 5.79 4.82 3.80 2.67 1.93 2 5.65 4.92 3.64 2.78 1.97 Sum 11.44 9.74 7.44 5.46 3.90 Average (X) 5.72 4.87 3.72 2.73 1.95 Bias (X-x) -0.06 0.09 -0.06 -0.05 -0.05 Standard Deviation 0.09 0.07 0.12 0.08 0.03 Accuracy of Linearity (Elin) 0.11 0.11 0.13 0.09 0.05 Elin
cases,
linear regression
Table B: Verification of the Linear Regression Performance for Legionella Species (Curve 2)
< 0.15 in all
performance is validated.
The calibration curve was repeated for the Legionella pneumophila assay over similar ranges and in both cases, all concentrations of L. pneumophila from both curves were analyzed and had an accuracy of linearity better than 0.15 log10 , which validates linearity of the curves according to the requirement imposed by ISO-12869. This is shown in Table C.
Calculation of Amplification Efficiency (e)
Efficiency assesses the yield of the melting and annealing cycles of the PCR reaction and is calculated by the slope value from the linear regression curve using the formula in Equation 1.
e
= (10 -1/a–1) x 100
Eq.
1
In an ideal situation, this will be exactly equal to 100%, which represents a doubling of DNA at each cycle, but this is unrealistic in the real world, where several factors impact this efficiency. For the Legionella species assay, e was calculated to be 103.07% for the first curve and 97.06% for the second curve. For Legionella pneumophila, the efficiencies were 92.3% and 92.8%, respectively. In both cases, the requirements according to ISO 12869 are achieved, which stipulates that efficiency must be between 75% and 125%.
Verification of Limit of Quantification
LOQ is defined as the lowest possible measurable GU with acceptable accuracy. The accuracy at the quantification limit (ELQ ) should be better than or equal to 0.15 log10. The validated LOQ should be used as the first point of the calibration range. For the
Legionella species assay, ten samples were prepared with a concentration of 12 GU and measured to verify the LOQ. See Table D for this data.
The LOQ was also determined for the Legionella pneumophila assay. This data is presented in Table E. Five samples were tested in duplicate at the low level of detection of 19 GU and all were detected with an ELOQ < 0.15 fulfilling the requirements of the ISO/TS 12869.
30 the ANALYST Volume 30 Number 3 Validation of a Portable qPCR Method for the Detection of Legionella continued
Theoretical DNA Copies (GU) (log) Theoretical DNA Copies (GU) Measured Ct Values Average Ct Value Measured Conc. (GU) Linearity Accuracy 217,000 5.34 23.15 23.57 23.36 1.98E+05 0.09 21,700 4.34 27.07 26.44 26.76 2.15E+04 0.13 2,170 3.34 29.77 30.17 29.97 2.62E+03 0.12 217 2.34 33.7 33.83 33.77 2.19E+02 0.03 19 1.28 37.62 37.7 37.66 1.72E+01 0.05 Theoretical DNA Copies (GU) (log) Theoretical DNA Copies (GU) Measured Ct Values Average Ct Values Measured Conc. (GU) Linearity Accuracy 217,000 5.34 23.57 23.7 23.64 2.63E+05 0.09 21,700 4.34 27.84 27.36 27.60 1.95E+04 0.11 2,170 3.34 31.04 31.26 31.15 1.90E+03 0.07 217 2.34 34.94 34.49 34.72 1.83E+02 0.12 19 1.28 37.7 37.96 37.83 2.36E+01 0.11
Table C: Verification of the Linear Regression Performance for Legionella pneumophila (Curves 1 and 2)
) Theoretical Value (x) GU (log) GU 12 1.079 Sample Raw Ct (log) GU 1 37.18 0.96 2 37.06 0.99 3 37.22 0.94 4 37.04 1.00 5 36.74 1.09 6 37.37 0.90 7 36.9 1.04 8 36.52 1.16 9 37.27 0.93 10 38.29 0.88 Average (X) 0.989 Bias (X-x) -0.0899 Standard Deviation 0.086 Accuracy of LOQ (E LOG) 0.12 E LOG < 0.15 so the LOQ is validated.
Table D: Verification of Limit of Quantification (LOQ for L. spp.
Verification of Limit of Detection
The LOD is defined as the lowest number of GU that can be detected as a positive result in 90% of samples. For the Legionella species assay, 10 samples were prepared at a level of 5 GU/well and 90% of samples were positively detected, so the LOD is 5 GU/reaction.
For the Legionella pneumophila assay, a total of 10 points of 10 GU per reaction (GU/rxn) (in duplicate) were analyzed. Legionella pneumophila was detected in 100% of these points. So, the limit of detection was verified to be 10 GU/reaction.
Average Recovery
ISO 12869 describes a protocol to quantify and validate the recovery method by testing artificially spiked samples at a minimum of two different concentrations created by dilution from a mother suspension. The recovery is calculated as a function of the value (in log10 GU) measured for the spiked sample, the concentration of the mother suspension, the dilution factor between the mother suspension and the spiked sample, and the volume used to create the spiked sample. The concentration of the mother suspension is also measured by qPCR in this step and is used as the reference value in the calculation. Five separate dilution samples were created and measured for both the 1,000,000 GU and 5,000 GU levels. The average recovery for the 1,000,000 GU level was -0.26 (log10), while the average recovery for the 5,000 GU level was -0.28 (log10). The ISO standard requires that the average recovery be between -0.6 log10 and +0.3 log10 , and therefore the recovery is validated as being within the acceptable range. This is shown in Tables F and G.
Average recovery was also tested for the Legionella pneumophila assay (see Table H). Following the requirements given in Section 9.6 of ISO/TS 12869, the recovery study was carried out on sterile water samples artificially contaminated with dilutions of mother suspensions made by using a L. pneumophila strain (WDCM 00107). Two different spiked levels were tested using Reference Material. For the recovery determination, some samples have been repeated using a dilution of them due to inhibition (Sample 2, Sample 3, and Sample 4). Recovery must be calculated by logarithm difference, and it shall have a value between -0.6log 10 and +0.3log 10 . In this case, a value of -0.48 log 10 has been achieved, meeting the ISO/TS-12869 requirement.
31 the ANALYST Volume 30 Number 3 Validation of a Portable qPCR Method for the Detection of Legionella continued
Sample Tested Level Ct Quantity (GU/Rxn) (log) Quantity (GU) Average (log) Quantity LQ1 19 38.08 1.80E+0.1 1.255 1.099 39.23 8.73E+00 0.941 LQ2 38.56 1.33E+01 1.124 1.253 37.62 2.41E+01 1.382 LQ3 38.3 1.57E+01 1.196 1.218 38.14 1.74+01 1.241 LQ4 39.12 9.36E+00 0.971 1.052 38.53 1.36E+01 1.134 LQ5 38.0 1.90E+01 1.279 1.319 37.7 2.29E+01 1.360 Average 1.188 Bias -0.905 Standard Deviation 0.11 LOQ Accuracy 0.14
Table E: Verification of Limit of Quantification (LOQ for L. pneumophila)
Sample Spiked Amount (log) Recovered Amount (log) (log) Difference % Recovery 1 6.00 5.82 -0.18 66.0% 2 6.00 5.70 -0.30 50.0% 3 6.00 5.70 -0.30 50.0% 4 6.00 5.50 -0.50 32.0% 5 6.00 5.99 -0.01 99.0% Average 6.00 5.74 -0.26 59.4%
Table F: Average Recovery for Legionella species Assay (1 x 10 6 GU/L)
Sample Spiked Amount (log) Recovered Amount (log) (log) Difference % Recovery 1 3.70 3.50 -0.20 63.0% 2 3.70 3.59 -0.11 78.0% 3 3.70 3.44 -0.26 55.0% 4 3.70 3.36 -0.34 46.0% 5 3.70 3.21 -0.49 32.0% Average 3.70 3.42 -0.28 54.8%
Table G: Average Recovery for Legionella Species Assay (5 x 103 GU/L)
Validation of a Portable qPCR Method for the Detection of Legionella continued
n.d. = not detected
Robustness
Robustness is defined as recovery across different sample matrices, including cooling water and drinking water. This was calculated in a similar manner as described previously in the recovery section by spiking live L. pneumophila into the sample matrix to be processed at the intended level of 1,000 and 100,000 GU. For the Legionella species assay, the average recovery for tap water was between -0.36 to -0.39 log10 , while the average for cooling water was -0.54 to -0.55 log 10 . For the L. pn. assay, the average recovery was -0.45 log 10 for tap water and -0.27 log 10 for cooling water. For both assays, these levels are within the required range of ISO 12869 (-0.6 log 10 and +0.3 log 10), so the robustness of the assay recovery in different sample matrices has been validated.
CDC ELITE blind sample testing:
Results from the third-party lab contracted to run the qPCR method on their CDC-ELITE proficiency testing are reported in Tables I and J. The culture results
were submitted to the CDC-ELITE program and subsequently labs were informed of the identity of the samples and how their results matched those expected. For this set of samples, the Legionella spp. and Legionella pneumophila qPCR assays exactly matched the expected results for each of the six samples, verifying the qPCR method and the culture method for correct identification down to the species level. A detailed breakdown shows the sample identifies, qPCR results (Table I) and the culture results (Table J).
32 the ANALYST Volume 30 Number 3
Sample N1 (Ct value) N2 (Ct value) Average (Ct value) Avg. Conc. (GU/rxn) Dilution Factor Avg. GU/L Avg. GU/L (log) Spiked Mother Suspension (log) Yield (log) 1 23.47 23.47 23.47 1.80E+05 1 9.02E+06 6.96 6.74 0.22 2 n.d. n.d. 1 6.74 2.1 n.d. 33.88 33.88 2.55E+02 10 1.27E+05 5.11 6.74 -1.63 3 n.d. n.d. 1 6.74 3.1 28.91 28.52 28.72 6.61E+03 10 3.30E+06 6.52 6.74 -0.22 4 n.d. n.d. 1 6.74 4.1 29.41 29.05 29.23 4.78E+03 10 2.39E+06 6.38 6.74 -0.36 5 n.d. 37.68 37.68 2.32E+01 1 1.16E+03 3.06 3.53 -0.46 6 37.66 37.43 37.55 2.53E+01 1 1.26E+03 3.10 3.53 -0.42 Average -0.48 Standard Deviation 0.62
Table H: Average Recovery for Two Different Concentrations of Legionella pneumophila Reference Material
Sample Target Result Target Result Identification 1 Legionella spp. Detected L. pneumophila Undetected Legionella feeleii 2 Legionella spp. Undetected L. pneumophila Undetected N/A 3 Legionella spp. Undetected L. pneumophila Undetected N/A 4 Legionella spp. Detected L. pneumophila Detected L. pneumophila SG 1 5 Legionella spp. Undetected L. pneumophila Undetected N/A 6 Legionella spp. Detected L. pneumophila Detected L. pneumophila SG 6
Table I: qPCR Results for CDC-ELITE Blinded Samples
Sample Culture Result Identification 1 Positive Legionella feeleii 2 None Detected N/A 3 None Detected N/A 4 Positive L. pneumophila SG 1 5 None Detected N/A 6 Positive L. pneumophjila SG 6
Table J: Legionella Culture Results and Sample Identification
Biocide Evaluation:
Fresh culture suspensions were treated with biocides, heat treated or received no treatment (broth control) as described in the materials and methods section, and then subjected to the subject qPCR method and culture testing. Results are presented in Table K and show that oxidizing biocides at recommended concentrations and approximately 24 hours of contact time, show no detectable signal for qPCR and no growth on culture plates.
Discussion
qPCR is rapidly becoming an important tool for environmental monitoring of pathogens, whether they are waterborne like Legionella, or spread through other means like SARS-CoV-2. To confidently implement a new technology like qPCR, it is important to ensure it works as intended in the application. To that end, we subjected a novel qPCR method with assays for Legionella species or Legionella pneumophila (serogroups 1-15) to a secondary validation at a third-party lab, in adherence with ISO/ TS-12869:2019 performance criteria. The results showed that both the Legionella species assay and the Legionella pneumophila assay were verified for linear regression performance, amplification efficiency, recovery, and robustness. The LOQ for the Legionella species assay was verified to be 12 GU/rxn, while the Legionella pneumophila assay was 19 GU/rxn. For the LOD, the Legionella species assay was verified at 5 GU/rxn while the Legionella pneumophila assay was verified at 10 GU/rxn.
The verification of robustness and recovery show that the method can be used successfully with different sample matrices where Legionella would require monitoring, including cooling water and potable water sources like drinking water, premise plumbing, and recreational waters like that of hot tubs, pools, saunas, and water fountains. The limits of detection ensure that Legionella threats can be accurately detected in these sources within recommended ranges, and the internal positive control
will provide effective quality assurance that the results are not impacted by inhibition. If there is inhibition of the internal positive control, the assay can be further diluted and assessed through alternative means if necessary to obtain an interference-free result.
A primary validation of the qPCR method used in this study confirmed the inclusivity of the species assay to detect the 40 different species of Legionella specified in ISO/TS-12869 while not cross reacting with 119 co-inhabitant and competing bacteria that would be commonly found with Legionella. This specificity is highly advantageous and highlights an advantage of qPCR relative to culture methods. The advantages of qPCR for negative screening of samples have been published and is one application where the technology can provide immediate benefit in screening environmental samples for Legionella risk (13). The real-world test of CDC-ELITE proficiency testing samples showed the validated qPCR method was able to accurately differentiate which samples had contamination with Legionella and correctly identified the Legionella pneumophila samples within those samples.
Non-pneumophila positive samples were negative on the pneumophila assay but were detected by the species assay, and no false positives were reported. These results highlight the broad-based ability of the species assay to identify other Legionella species and highlights the pneumophila assay’s exclusive detection of Legionella pneumophila serogroups 1-15. While a small sample size, this is a promising result for the technologies ability to accurately discriminate between negative and positive Legionella samples and identify other species, in addition to Legionella pneumophila
qPCR methods have limitations, much like all testing methods, and one criticism often levelled at the technology is the inability for some methods to
33 the ANALYST Volume 30 Number 3 Validation of a Portable qPCR Method for the Detection of Legionella continued
Sample Concentration Target Result Concentration (GU/mL) Target Result Concentration Culture (CFU/mL) Chlorine 20 ppm Legionella spp. Undetected < LOD L. pneumophilia Undetected < LOD < 0.1 Bromine 5 ppm Legionella spp. Undetected < LOD L. pneumophila Undetected < LOD <0.1 Heat Killed Legionella spp. Undetected < LOD L. pneumophila Detected 5.63E+05 < 0.1 Broth Control Legionella spp. Detected 1.95E+05 L. pneumophila Detected 3.54E+06 4.10E+03
Table K: qPCR and Culture Test Results From Biocide Evaluation Study
discriminate whether the DNA amplified is from living or dead cells. While this is somewhat true, the devil is in the details, so it’s important to assess a method’s response to different types of treatment and develop thresholds and action limits based on trends over time rather than static test results. The validated method uses selective filtration and washing to ensure intact cells are captured on the filter membrane and extracellular DNA passes through and does not get extracted in the method. To show this, we contracted a third-party lab to conduct a trial with contaminated samples treated with oxidizing biocides and showed the results were < LOD for bromine and chlorine treated samples after 24 hours of exposure time.
Since oxidizing biocides are the most widely used in potable water and industrial water treatment, this shows that the method will accurately portray the status of a systems risk when these biocides are applied at routine concentrations. Further study is required to compare the response of qPCR methods relative to culture under varying biocide conditions and different water conditions holistically along with other physical and chemical parameters. This will help to put the qPCR data into better context relative to the culture data, so that thresholds can be set and point in time results can be interpreted effectively. That said, a sound strategy for operational qPCR monitoring is to determine a baseline for the system in question with qPCR data collected alongside culture results to determine appropriate qPCR thresholds. If routine testing shows increasing trends from the baseline, then there is active growth in the system that needs to be investigated and addressed (14).
Taken together, the data contained within this article demonstrates the ability of the L. spp. and L. pneumophila assays to accurately differentiate between the different Legionella species and serogroups as defined by ISO 12869 and verifies the methods calibration curve, LOQ, LOD, robustness, and average recovery. The combination of this validated assay, along with field and lab instrumentation and purification kits allows users to monitor concentrations of L. spp. and L. pneumophila in a quick and convenient manner. The ability to assess risk by quantifying the Gus present in a system by qPCR allows the proper treatment, maintenance, or follow-up testing to take place sooner rather than later to help reduce risk.
Acknowledgements
The authors wish to thank ieLabs for the ISO-12869 secondary validation and QLabs for the CDC-ELITE proficiency sample testing and the biocide evaluation study.
References
1. ISO (April 2019). ISO/TS 12869: “Water Quality: Detection and Quantification of Legionella spp. and/or Legionella pneumophila by concentration and genic amplification by quantitative Polymerase Chain Reaction (qPCR),” International Standards Organization, Geneva, Switzerland.
2. Shah, P.; Barskey, A,; Binder, A,; Edens, C.; Lee, S.; Smith, J.; Schrag, S.; Whitney, C.; Cooley, L (2016). “Legionnaires’ Disease Surveillance Summary Report, United States, 2010–2015,” Centers for Disease Control and Prevention, Atlanta, Georgia.
3. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division on Earth and Life Studies; Board on Population Health and Public Health Practice; Board on Life Sciences; Water Science and Technology Board; Committee on Management of Legionella in Water Systems (2019). Management of Legionella in Water Systems, National Academies Press, Washington, D.C, PMID: 32200596.
4. Collier, S.A.; Deng, L.; Adam, E.A.; Benedict, K.M.; Beshearse, E.M.; Blackstock, A.J.; Bruce, B.B.; Derado, G.; Edens, C.; Fullerton, K.E.; Gargano, J.W.; Geissler, A.L.; Hall, A.J.; Havelaar, A.H.; Hill, V.R.; Hoekstra, R.M.; Reddy, S.C.; Scallan, E.; Stokes, E.K.; Yoder, J.S.; Beach, M.J. (January 2021). “Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States,” Emerging Infectious Diseases 27(1), pp.140-149, accessible at doi: 10.3201/eid2701.190676.
5. Craun, G.F.; Brunkard, J.M.; Yoder, J.S.; Roberts, V.A.; Carpenter, J.; Wade, T.; Calderon, R.L.; Roberts, J.M., et al. (2010). “Causes of Outbreaks Associated with Drinking Water in the United States from 1971 to 2006,” . Clinical Microbiology Reviews, 23, pp. 507–528.
6. ASHRAE (2021). ASHRAE Standard 188-2021: Legionellosis: Risk Management for Building Water Systems (ANSI Approved) STANDARD by ASHRAE. American Society of Heating, Refrigerating and Air-Conditioning Engineers, Atlanta, Georgia.
7. CDC (n.d.) Toolkit for Controlling Legionella in Common Sources of Exposure, Centers for Disease Control and Prevention, Atlanta, Georgia.
8. Boulanger, C.A.; Edelstein, P.H. (1995). “Precision and Accuracy of Recovery of Legionella pneumophila from Seeded Tap Water by Filtration and Centrifugation,” Applied and Environmental Microbiology, 61, pp. 1805–1809.
9. Sloan, W.T.; Quince, C.; Curtis, T.P. (2008). “The Uncountables,” Accessing Uncultivated Microorganisms, ASM Press, Washington, D.C.
10. Oliver, J.D. (February 2005). “The Viable but Nonculturable State in Bacteria,” Journal of Microbiology 43(S) (special issue), pp. 93-100.
11. Wang, H.; Bédard, E.; Prévost, M.; Camper, A.K.; Hill, V.R.; Pruden, A. (2017). “Methodological Approaches for Monitoring Opportunistic Pathogens in Premise Plumbing: A Review,” Water Research, 117, pp. 68-86, accessible at doi: 10.1016/j. watres.2017.03.046. Epub 2017 Mar 25.
12. Collins, S.; Jorgensen, F.; Willis, C.; Walker, J. (2015), “Real-Time PCR to Supplement Gold Standard Culture-Based Detection of Legionella in Environmental Samples,” Journal of Applied Microbiology, 119, pp. 1158-1169, accessible at doi.org/10.1111/jam.12911
13. Collins, S.; Stevenson, D.; Walker, J.; Bennett, A. (2017). “Evaluation of Legionella Real-Time PCR against Traditional Culture for Routine and Public Health Testing of Water Samples,” Journal of Applied Microbiology 122(6), pp. 1692-1703, accessible at doi: 10.1111/jam.13461. Epub 2017 May 10. PMID: 28376265.
14. Young, C.; Smith, D.; Wafer, T.; Crook, B. (2021). “Rapid Testing and Interventions to Control Legionella Proliferation Following a Legionnaires’ Disease Outbreak Associated with Cooling Towers,” Microorganisms 9(3), p. 615, doi:10.3390/microorganisms9030615
Jeremy Duguay, MSc., is a molecular biologist with more than 15 years of experience in biotech research, development, and technology integration. He is an applications scientist with LuminUltra and has expertise in environmental and pathogen monitoring technologies. Mr.

34 the ANALYST Volume 30 Number 3 Validation of a Portable qPCR Method for the Detection of Legionella continued
Duguay is a member of AWT, an ASSE 12080 certified Legionella Water Safety and Management specialist and sits as a member of the Standards Council of Canada ISO/TC 147/SC4 (Microbiological Methods) mirror committee. He lives in Fredericton, New Brunswick, Canada, where he enjoys spending time with his family in the great outdoors.
Harley King, PhD, is a scientist at LuminUltra. He designs new qPCR assays for customers using robotics and high-throughput technologies. He has a PhD from the University of Maryland. He loves backpacking the Appalachian Trail with his four sons and theater with his wife.

Neil Sharma is currently VP corporate development at LuminUltra Technologies. He has over 15 years of experience in the development and commercialization of molecular diagnostics devices, assays, and reagents for applied and clinical microbiology applications. He currently lives just outside of Washington, DC.

Jordan Schmidt, PhD, is the senior director of technology and innovation at LuminUltra Technologies. He holds a doctorate in civil engineering from Dalhousie University in Halifax, Canada. Originally trained in biological wastewater treatment, Dr. Schmidt has years of experience working on the monitoring of microorganisms in industrial water systems.

This paper was presented at the 2022 Association of Water Technologies Annual Conference & Exposition, which was conducted Sept. 21-24, 2022, in Vancouver, British Columbia, Canada.


Keywords: BUILDING WATER, COOLING TOWERS, DRINKING WATER, LEGIONELLA, MICROBIALS, MONITORING

35 the ANALYST Volume 30 Number 3 Validation of a Portable qPCR Method for the Detection of Legionella continued
WATER TESTING SHOULD BE SIMPLE That’s why our self-filling ampoules are PREMIXED, PREMEASURED and PRECISE We provide test kits for Low Range Dissolved Oxygen, Ammonia, Chlorine, DEHA, Filming Amines, Hardness, Hydrazine, Iron, Molybdate, Ozone, PAA, Phenol, Phosphate, Sulfide and more. +1.540.788.9026 | www.chemetrics.com
Continuous Maintenance: An Overview of Updates to Legionella Regulations

Adam Green, J.D., and Matthew Kim, J.D., Baker, Donelson, Bearman, Caldwell, and Berkowitz PC
Robert J. Cunningham, P.E., International Water Consultants Inc.
John A. Mullen, International Association of Plumbing & Mechanical Officials
36 the
30
3
ANALYST Volume
Number
Abstract
Previously existing Legionella regulations, standards, and guidelines have evolved during the last few years and new ones continue to be introduced. The majority of the new texts are still strictly voluntary, and, at the time of this writing, very few jurisdictions have codified them into law. In some cases, particularly in the healthcare arena, the authorities having jurisdiction are enforcing specific provisions of these documents with significant liability to health care organizations and the facilities that they operate.
The intent of the authors is to provide a broad status update for the owners and operators of large buildings and healthcare facilities that are potentially impacted, as well as for those providers of services and products that do business with these facilities. This publication provides a general summary of relevant regulations, standards, and guidelines, and notable changes of which the reader should be aware.
Introduction
The dedicated group of thought leaders who labored for a decade to develop American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) 188-2015 prophesied that the Standard would remain under “continuous maintenance” (1). This remains true as of 2022. As Legionella science has evolved, so too have the corresponding guidelines and regulations. As new information is gathered from outbreaks that have evolved into case studies and technology continues to change, industry practices and standards are in a constant state of improvement and revision.
Navigating the morass of nuanced Legionella regulations and authorities can be foreboding. Those who are tasked with adherence to or applying these regulations are frequently intimidated and confused by the underlying complexity of the issues and the sheer number of entities who have offered guidance on the issue. In addition to ASHRAE, other respected entities continue to contribute to the evolution of the available knowledge base relating to Legionellosis prevention. These entities include, but are not limited to, the Centers for Disease Control and Prevention (CDC), the Cooling Technology Institute (CTI), Association of Water Technology (AWT), the American Industrial Hygiene Association
(AIHA), the International Association of Plumbing and Mechanical Officials (IAPMO), the American Society of Plumbing Engineers (ASPE), and the Joint Commission and the Centers for Medicare & Medicaid Services (Joint Commission).
Those who are charged with adhering to or applying appropriate protocols have frequently asked the recurring question: Why isn’t there one set of house rules? While it would certainly be simpler to have one guiding text from one rulemaking entity, Legionella risk minimization has proven to be an inherently complex, highly nuanced, and multi-disciplinary endeavor. Experience dictates that Legionella risk minimization frequently involves the disciplines of chemistry, engineering, metallurgy, plumbing, and microbiology. The solutions often reside where those disciplines intersect. There are trade-offs with this approach. The great benefit is that common blind spots can be eliminated as specialists in each discipline bring unique perspectives and expertise to bear.
The most obvious downsides are complexity and expense. The reference materials are numerous, varied, and frequently complicated. The employment of multiple disciplines is expensive. In addition, the concurrent existence of multiple authorities to address the same issue can create gaps, whether real or perceived, where litigation thrives. Nonetheless, it is difficult to advocate that any of the contributing disciplines or their unique perspectives are not necessary to Legionella risk minimization. Indeed, the opposite is true.
Experience dictates that Legionella risk minimization is an endeavor much more akin to quilt making than blanket making. This quilt cannot be made with a single bolt of cloth. Each square is an important and necessary piece of the ultimate goal and solution. Legionella science and industry response is continuously evolving. Someday, part of that evolution may include the consolidation of the many guidance documents and references into an integrated reference document which would fully embody one set of “House Rules.”
Until that time, this publication contains a general overview and brief summary of selected regulations, standards, and guidelines relating to Legionella in building water systems. This document is not intended to be a fully comprehensive review of all existing
37 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations
regulations, guidelines, standards, or similar texts relating to Legionella.
What’s New?
ASHRAE Standard 188-2015
In 2015, ASHRAE Standard 188-2015 was released as a voluntary consensus standard that established minimum Legionellosis risk management requirements for building water systems in human-occupied commercial, institutional, multi-unit residential, and industrial buildings. ASHRAE Standard 188-2015 is intended for use by owners and managers of humanoccupied buildings and for those involved in the design, construction, installation, commissioning, operation, maintenance, and service of centralized building water systems and components. The standard dictates that the compliance determinations and the associated building surveys shall be physically or electronically on site for review by the authority having jurisdiction (AHJ). This standard does not use or require compliance, training, or certification in any additional hazard analysis, risk assessment, or risk management methodologies.
Updated ASHRAE Standard 188-2021
The 2021 update was made primarily to ensure consistency between ASHRAE Standard 188-2021 and ASHRAE Guideline 12-2020. The updated ASHRAE Standard 188-2021 recites that certain revisions replace permissive language with enforceable language that will “facilitate adoption of the standard for code and regulatory purposes.” Accordingly, the definition of the word “shall” is now expressly included in the definitions section of the standard (2). The update includes additional subtle changes, including more detailed definitions of the terms “nonpotable” and “potable water system.” ASHRAE Standard 188-2021 also references the recently updated Cooling Technology Institute Guideline CTI-159 entitled, “Practices to Reduce the Risk of Legionellosis from Evaporative Heat Rejection Equipment Systems” (Guideline CTI-159). Lastly, the changes within ASHRAE Standard 188-2021 include changes to Section 8.4 governing commissioning. As it relates to procedures for flushing and disinfection, the references to the requirements of American Water Works Association (AWWA) Standard C-652, which prescribes methods for disinfecting water storage tanks, have been removed as the standard is no longer deemed applicable to building water systems.
ASHRAE Guideline 12-2020
While the 2021 changes within ASHRAE Standard 188-2021 are subtle, the revisions and additions to ASHRAE Guideline 12-2020 are substantial.
ASHRAE Guideline 12-2020 provides in-depth and informed guidance useful to the implementation of ASHRAE Standard 188-2021. These extensive updates are summarized in this section.
Section 5: Potable Water Systems (3)
Section 5 of ASHRAE Guideline 12-2020 details relevant methods and considerations relating to potable water systems for the control and treatment of Legionella The possibility of Legionella growth within cold and hot potable water systems can be significant, based on the characteristics of those particular systems. A report from the U.S. Center for Disease Control (CDC) states that 66% of all waterborne disease outbreaks associated with potable water reported from 2011 to 2012 were attributed to Legionella (4).
Accordingly, ASHRAE Guideline 12-2020 is largely dedicated toward addressing the options available to operators in mitigating Legionella growth and spread. Some methods suggested for limiting Legionella growth include keeping the system clean and free of sediment, controlling hot-water and cold-water temperatures, minimizing water age (the residence time of the water in the system) and maintaining a disinfectant residual where water temperatures cannot be reliably maintained at target levels throughout the system or where stagnant or low-flow conditions may exist.
Considerations from a system design/engineering, installation, and commissioning vantage include a focus on detailed process flow diagrams that are recommended. Considerations include building use, location, and occupant profile (at-risk individuals). In addition, pipe system design is crucial and should include considerations of insulation, pipe sizing, presence of dead legs, purge and drain valves/recirculation, water pressure, and sampling points. Detailed information regarding plumbing component design, selection, and installation can be found under Section 5.2.2 of ASHRAE Guideline 12-2020.
Legionella control measures as it relates to potable water systems can include temperature control, supplemental
38 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued
disinfection/treatment, filtration, flushing, recirculation, cleaning, and maintenance. For temperature control, storage water heaters and hot-water storage tanks should deliver water consistently at or above 140°F, unless other compensating control measures are employed. Additionally, measures to prevent scalding should be taken.
While a disinfectant residual throughout the system may be helpful, potable water may still be colonized by Legionella and a control measure in the form of a supplemental disinfection may be needed. It is noted in the update that the inappropriate selection or improper application of disinfectants and treatments may not only be ineffective but harmful to building occupants and damaging to the metallurgy of the building water system.
ASHRAE Guideline 12-2020 update emphasizes the need to consult available scientific and technical evidence when it comes to any application of chemical treatments. In the event of any remedial treatment, ASHRAE Guideline 12-2020 warns that recolonization of Legionella may still occur, and that the system should be flushed following chemical remedial treatment.
Section 6: Ornamental Water Features (5)
Section 6 of ASHRAE Guideline 12-2020 addresses the concerns associated with ornamental or decorative water features and Legionella growth. Ornamental or decorative water features are human-made fountains, waterfalls, cascades, sprays, and the like that use water for architectural, decorative, or aesthetic effects. The design characteristics of water features may influence the colonization, survival, and growth of Legionella. For instance, small water features colonized with Legionella can disseminate contaminated aerosols (water droplets and mists) into the air.
The design and operation of water features will likely vary greatly in size, configuration, and complexity. Most will involve water either being sprayed into the air or cascading over a medium. These features may have a single water pump or an automatic water treatment system consisting of one or more water pumps, water filters, valves, specialty nozzles and lighting. Water features may have limited hours of operation that would allow the water to be especially susceptible to microbial growth due to factors such as water age, heat gain, and loss of biocide residual.
There are various sources of heat in ornamental features that have the potential to increase water temperatures into the range that has been shown to support Legionella growth (77 to 113°F). Potential heat sources include solar heat gain, submerged incandescent lighting, lights over a large surface of the water wall, and submerged circulation pumps. Any increase in water temperature may increase the rate at which disinfectant residual is consumed. If not drained and flushed after each use, water should be circulated at least daily to maintain distribution of water treatment chemicals during idle periods.
As noted previously, water features may produce aerosols with droplets of various sizes, including sizes small enough to be inhaled deeply into the lungs. Cases of Legionella have been linked to fountains that appeared to release little or no aerosols. Indoor water features may release aerosols in a confined space that may provide more risk of a viable exposure pathway than those outdoor water features that may be more readily diluted by fresh air and dissipated by wind.
Sources of contamination can come from a wide variety of sources, including, but not limited to, materials scrubbed from the air, organic and inorganic materials falling into the feature, and corrosion products and other materials from the water feature structure. Water evaporation may operate to concentrate these contaminants. If not adequately treated, microbial growth, including the presence of biofilms, can occur. In addition, exposure of water-wetted surfaces to light can support algae growth. Algae growth coupled with biofilms can support Legionella growth in water feature pools, basins, and on surfaces that may be wet but not submerged.
In an effort to address the underlying conditions that contribute to Legionella growth, feature design and engineering are prioritized. Some but not all factors to consider include overall design, layout, piping configuration, pumps, water filters, lighting, location and component locations, accessibility for maintenance, and other design elements. For more details, consider Section 6.2 of ASHRAE Guideline 12-2020.
In addition to the foregoing, proper operation and maintenance of water features are essential to managing the potential for Legionella growth and transmission.
39 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued
A water management program, such as that contained in ASHRAE Standard 188-2021, Legionellosis: Risk Management for Building Water Systems, should be implemented. Multiple control measures should be in place including operation to avoid elevated water temperature and to control water age, cleaning, adding biocides to suppress microbial growth in the water feature. Additional recommended control measures can be found in Section 6.3 of ASHRAE Guideline 12-2020.
Section 7: Heated Whirlpools/Hot Tubs (6) Section 7 of ASHRAE Guideline 12-2020 addresses Legionella-related concerns associated with heated whirlpool spas and hot tubs. Whirlpool spas are recreational baths or pools holding more than one person and filled with warm turbulent water. Hot tubs are traditionally deeper hot-water baths and are typically made of wood. The concern with both whirlpool spas and hot tubs revolves around the water temperature range being squarely within the range most conducive for Legionella growth. These water temperatures, along with factors such as water aeration and a high ratio of surface area of the bather’s body to water volume, may accelerate the consumption of the biocide. In addition, the whirlpool and hot tub operation continuously creates bubbles that are released as aerosols into the airspace that may contain microorganisms.
To combat Legionella growth in whirlpools and hot tubs, a systems approach to design must be considered. Plumbing should be as simple as possible. There should be no cross connections with other water systems and no stagnant flow areas should exist.
Proper warning signs and procedures should be implemented alerting users of maximum load capacities and bather health restrictions. Routine maintenance and record keeping (including microbial testing) is crucial along with proper training of maintenance staff.
Filter operation is crucial, and cleaning of spa filters should be more intensive than the maintenance required for pools due to the higher ratio of bathers to water volume. In addition, daily back flushing procedures should be completed. Filters may collect sediment providing for potential for biofilm and bacteria growth. Hence, the proper level of disinfectant should be circulated across the filter.
Biocides should be applied consistent with the instructions on the product label. Alternative biocides for spas are available, including ultraviolet (UV) light, and ozone. In the event of a high bacterial count, the spa should be removed from service and shock disinfection with a biocide (chlorine) should be completed. In the event of an outbreak or illness, Legionella testing and all other appropriate procedures should be conducted. The local public health agency may be involved as the circumstances require.
Section 8: Open-Circuit Towers, Closed-Circuit Cooling Towers and Evaporative Condensers (7) Section 8 of ASHRAE Guideline 12-2020 addresses concerns associated with open-circuit cooling towers, closed-circuit cooling towers, and evaporative condensers (collectively known as “evaporative heat rejection equipment”). The growth and transmission of Legionella in evaporative heat rejection systems is linked specifically to the quality of the supply water and circulating water. Specifically, issues may stem from contamination with bacteria and/or solids, system design and maintenance practices, intermittent operation without draining the cooling towers and interruption or loss of water treatment.
Design guidelines should focus on facilitating proper system maintenance and water treatment. In this context, it is important to maximize mass transport and minimize accumulation of water-based solids, airborne contaminants, and biomatter. The siting of the towers is another important consideration. The locations should minimize exit air and drift from being drawn into building air intakes and to minimize exit air and drift human receptors and exposure pathways, where possible. In addition, sufficient space should be provided to enable access for maintenance and inspection.
There are various operational control methods to consider when discussing evaporative heat rejection equipment. As noted, temperature settings are important when controlling the growth and dissemination of Legionella bacteria. The operating temperature of these systems are within the optimal range to promote Legionella growth and are dependent upon the heat source and the cooling water flow. With evaporative heat rejection equipment, the water temperature of the system should be below the range favorable to Legionella and other microorganism growth. Chemical water treatment to control scale,
40 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued
corrosion, sediment, and bacteria may be employed to combat Legionella growth.
Nonchemical and physical water treatment methods may also be used either in conjunction with chemical water treatments or as alternatives. Considerations include supply water quality and water disruptions, the use and design of cooling tower drift eliminators and louvers, and the avoidance of non-circulating lengths of pipe or dead legs. More information concerning active steps to control Legionella growth as it related to evaporative heat rejection equipment can be found under Section 8.2 of ASHRAE Guideline 12-2020.
Section 9: Direct Evaporative Air Coolers, Misters (Atomizers), Air Washers, and Humidifiers (8)
Section 9 of ASHRAE Guideline 12-2020 addresses considerations related to direct evaporative air coolers, misters, air washers, and humidifiers. These systems primarily cool and humidify air by direct contact with the water, either by wet surface materials or with a series of sprays. These devices control temperature and humidity levels for commercial, industrial, residential, and agricultural applications. Given that the design of these systems induce evaporation of water by generating small droplets or aerosols, cases of Legionnaires’ disease have been correspondingly linked. Therefore, care should be taken with all types of evaporative air cooling and humidification equipment. Considerations should include regular cleaning and maintenance, avoidance of dead-end or (dead leg) piping and water stagnation, and placement away from airborne and waterborne sources of organic contamination.
Section 10: Indirect Evaporative Air Coolers (9)
Section 10 of ASHRAE Guideline 12-2020 highlights that as of September 2016, the CDC has not published a link between Indirect Evaporative Air Coolers (IEC) and cases of Legionnaires’ disease.
Section 11: Cooling Coils and Condensate Collection (10)
Section 11 of ASHRAE Guideline 12-2020 discusses the relevant considerations for air-handling equipment and its connection to Legionella growth. Generally, ASHRAE Guideline 12-2020 cautions against the accumulation of dirt, debris, corrosion products, biomass, and stagnant water from coil condensate on surfaces
that, when combined with the normal air velocities present from systems of this type, could disseminate Legionella and cause Legionellosis. Treatment options are summarized in this section and, in part, suggest that UV disinfection can be an effective method for maintaining coils free of organic growth. Notably, UV disinfection alone is not a recommended substitute for best practices for coil and condensate pan maintenance. Biocide treatment can be used for purposes of slime control in drain pans and the prevention of drain clogging.
Section 12: Other Building Water Systems Where Legionella May Grow (11)
Section 12 of ASHRAE Guideline 12-2020 cautions that, in the absence of control, Legionella can grow in almost any system or equipment containing nonsterile water at temperatures conducive to Legionella growth. ASHRAE Guideline 12-2020 recommends operators or individuals at risk of being exposed should evaluate all building systems and equipment that contain or use water with respect to susceptibility to Legionella growth and transmission.
CDC Toolkit Update
Following the release of ASHRAE Standard 188 in 2015, the CDC released a 2016 toolkit to assist building owners and managers in interpreting and understanding the standard. The original toolkit was designed to provide practical insights and guidance to develop, implement and evaluate an ASHRAE compliant water management plan. The bottom-line considerations in the 2016 toolkit included:
1. identifying building water systems for which Legionella control measures are needed;
2. assessing the level of risk of hazardous conditions in water systems;
3. applying control measures to reduce the hazardous conditions to prevent Legionella growth and spread; and
4. the defining factors in ensuring that the program is running effectively as designed (12).
In June 2021, the CDC released an updated version of the toolkit. The most significant and new piece of information from the updated toolkit discusses next steps for a healthcare facility in the event a patient is diagnosed with Legionnaires’ disease or when environmental triggers occur that place the facility at risk for Legionnaires’ disease. In the event of a full public health investigation into the source of the infection, this CDC toolkit provides key elements to consider (12).
41 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued


The toolkit document is a user-friendly distillation of the actions in the most recent updates to ASHRAE Standard 188-2021 and ASHRAE Guideline 12-2020. The toolkit includes an interactive checklist to assist the end user in implementing the water management program as defined in ASHRAE Standard 188-2021.
2021 CDC Legionella Control Toolkit
In January 2021, the CDC published an additional standalone toolkit entitled, Toolkit for Controlling Legionella in Common Sources of Exposure. This toolkit provides further guidance to help develop, implement, and evaluate a Legionella water management program for a building based on ASHRAE Standard 188-2021.
Significantly, a substantial portion of the 2021 toolkit is dedicated to routine testing. Therein, this term is expressly defined as “testing for Legionella to establish a baseline measurement for performance indicators or for validating a water management program or corrective action” (13). The inclusion of routine testing and its definition are significant. For many years, “[most] professional and government agencies that have issued
Legionella position statements and guidelines, [did] not recommend testing for Legionella bacteria on a routine basis” (14). In keeping with this position, the CDC had famously recommended testing only following an outbreak, which it defines as two or more confirmed cases of Legionnaire’s disease associated with a particular location (15).
While the 2021 toolkit does not prescribe or require routine testing, it does direct the reader to “consider testing for Legionella ” in accordance with the guidance in the toolkit and notes that routine testing “may be particularly beneficial for certain types of facilities” and purposes (16). For instance, it is noted that routine testing may be useful for validating a water management program, confirming the success or failure of remedial treatment, investigating potential sources of environmental exposure for persons with disease and establishing general baselines for performance indicators.
This is a substantial departure from the previous position that recommended only non-routine Legionella testing for the purpose of investigating potential
Providing water treatment solutions, not just chemicals

When it comes to water treatment, each business has unique needs and challenges. Our in-house technical professionals utilize our global network to devise innovative solutions and procure the most effective product for each situation. brenntag.com
Our broad product portfolio and services grant you peace of mind, so you can focus on your core business.
44 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued
sources of environmental exposure for persons with disease. Detailed considerations are included in this toolkit for users interested in information related to testing methods, evaluation of test results, and suggested responses (16). The recommendations include information pertinent to considerations relating to “routine testing for Legionella ,” when monitoring should be done, and how much water should be collected (250 milliliters [mL]) for routine monitoring. Each section of this toolkit provides detailed information and recommendations to address questions and considerations related to design, operation, maintenance and control limits, and remediation of various water systems. Much of this toolkit draws from the guidance provided from the ASHRAE Guideline 12-2020, which has been dissected in detail above.
The 2019 Legionella Document
This document serves as an update and revision to the 2003 document released by the Association of Water Technologies (AWT). In the 2019 document, the AWT Legionella Task Force compiles information and data from a wide array of various authorities, including the CDC, OSHA (Occupational Safety and Health Administration), WHO (World Health Organization), EPA (U.S. Environmental Protection Agency), state public health authorities, technical trade organizations and recognized Legionella experts and commercial entities (17). While this update relies on ASHRAE Standard 188-2015 and predates the updated information found within ASHRAE Guideline 12-2020, the information is useful for those interested in a comprehensive reference from various authorities involved in the issuance of regulation of Legionella control and management.
Joint Commission
The Joint Commission is proposing a new water management program standard for the Hospital Accreditation Program (HAP), Critical Access Hospital (CAH), and Nursing Care Center (NCC) accreditation programs and has requested comments regarding the proposed changes. The proposed changes intend to strengthen the Joint Commission’s requirements regarding water management programs and focus on the implementation of comprehensive water management programs. The proposed changes will eliminate standard, EC.02.05.01 EP 14, regarding minimization of
pathogenic biological agents in evaporative cooling water systems, domestic hot and cold-water systems and other aerosolizing water systems. The changes introduce a new standard, EC.02.05.02, which will require hospitals, critical access hospitals and nursing care centers to have a water management program that identifies an individual or team responsible for the program, basic diagrams of water systems, a water risk management plan, a plan for addressing stagnant water events and water monitoring protocols with control measures.
Revisions to CMS Memorandum
While the development and release of ASHRAE Standard 188-2015 was significant, it remained a voluntary consensus standard. In June 2017, the CMS removed the voluntary nature of ASHRAE Standard 188-2015 as it related to Medicare-certified healthcare facilities. Specifically, the CMS issued a memorandum entitled, “Requirement to Reduce Legionella Risk in Healthcare Facility Water Systems to Prevent Cases and Outbreaks of Legionnaires’ Disease.” Therein, the CMS indicated that it expects Medicare certified healthcare facilities to implement water management plans consistent with ASHRAE Standard 188-2015 and the CDC toolkit.
These requirements are based on pertinent CMS regulatory authorities such as 42 CFR §482.42 (providing that hospitals must have active programs for the prevention and control of infections and diseases), 42 CFR §483.80 (requiring hospitals to establish and maintain and infection prevention and control program), and 42 CFR §485.635(a)(3)(vi) (requiring critical access hospitals to have a system for identifying, reporting and controlling infections and diseases in patients and personnel) (18). The CMS Memorandum subjected healthcare facilities to audits and those unable to comply were at the risk of citation for non-compliance with the CMS Conditions of Participation.
At the time of this writing, the latest revision to the CMS Memorandum was published on July 6, 2018. This revision does not impose any new requirements but was released to clarify existing expectations. The revised version has been re-organized and now states the expectations for Healthcare Facilities and Surveyors separately. While the prior iterations indicated that water management plans considering ASHRAE Standard
45 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued
188-2015 and the CDC toolkit were expected, the updated version clarifies in plain language that “facilities must have water management plans (19). Healthcare facilities are now not only expected to implement a water management program but to also develop programs tailored to the specific needs of each facility.
The most significant change in the revision relates to environmental testing for Legionella. In prior iterations, the CMS Memorandum directed healthcare facilities to implement a water management program that includes “environmental testing for pathogens” (20). The 2018 version contains the following conspicuous language: “Note: CMS does not require water cultures for Legionella or other opportunistic water-borne pathogens. Testing protocols are at the discretion of the provider ” (20). In related fashion, the current version indicates that long-term care surveyors will expect that a water management plan (including a facility risk assessment and testing protocols) is available for review but “will not cite the facility based on the specific risk assessment or testing protocols in use” (20).
CTI Guideline 159
In January 2020, the CTI published its Legionellosis Guideline (159) entitled, “Practices to Reduce the Risk of Legionellosis from Evaporative Heat Rejection Equipment Systems” (“Guideline CTI-159”). Guideline CTI-159 serves as an update to the oft-cited 2008 CTI guideline entitled, “Best Practices for Control of Legionella.” Guideline CTI-159 discusses practices that reduce the risk of exposure to Legionella from evaporative heat rejection systems, specifically evaporative condensers, closed-circuit cooling towers, and opencircuit cooling towers.
The update provides helpful information for evaporative heat rejection equipment associated with building water systems to support the development of water management programs as outlined under ASHRAE Standard 188. Additionally, Guideline CTI-159 provides helpful information for evaporative heat rejection equipment in industrial process and power plant systems, programs that are subject to different criteria, operating characteristics, and regulations. This update to WTB-148 specifies biocide treatment procedures, sets Legionella testing method and laboratory requirements, and defines roles, responsibilities, and record-keeping
requirements. These updates are outlined briefly in the remainder of this section. .
Appendix E: Routine Treatment Procedures (21)
The use of halogen or oxidizing biocides such as chlorine or bromine compounds is a generally accepted control method for Legionella in evaporative heat rejection equipment. Certain general principles should be maintained when using these biocides. Operators can use halogen/oxidizing biocides continuously, intermittently, or periodically. Each approach has its own set of specific considerations, which are outlined in this section. It is important to note that non-chemical and physical water treatment methods and equipment may be used in addition to or as an alternative to such chemical programs. These methods should not take precedence over other key Legionella control methods such as eliminating stagnant water zones, maintaining system cleanliness, drift eliminators, and the elimination of controllable sources of nutrients in the cooling water system.
Section 7.3.4: Legionella Testing Methods (21)
Legionella testing can be valuable as a validation tool for a water management program. There are three basic methods for detecting Legionella in environmental samples, each with its own set of advantages and limitations.
Molecular methods detect DNA and RNA of microbial cells. This method is quantitative Polymerase Chain Reaction (q-PCR). The limitations of this method include a failure to discriminate between live bacteria and viable-but-not-culturable (VBNC) bacteria. The presence of VBNC bacteria may result in a positive bias for the q-PCR test as compared to the laboratory culture. There are two commercially available field q-PCR test devices that provide test results within the hour.
Serological methods use antibodies that are tagged with fluorescent labels that can be visualized using special microscopy. These tests are rapid and effective, but interpretation of results is often subjective. These tests are known as the urinary antigen test (UAT), which detects molecules of Legionella bacterium in urine. Limited information is provided with these tests and accuracy concerns persist (Legionella may be present but below the limit of detection or other bacteria may render a false positive for Legionella).
46 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued
Culture methods are intensive, providing capacity to detect various species, serogroups, and strains of Legionella. Sensitivity and specificity of culture methods may vary among laboratories and care must be used in the collection, handling, and shipping of samples to labs. Testing under this method should be performed by an accredited environmental microbiology laboratory that has been certified through the CDC’s Environmental Legionella Isolation Technique Evaluation (ELITE) Program.
Section 8: Roles, Responsibilities & Recordkeeping Requirements (21)
Proper management and maintenance of evaporative cooling water systems contribute to efficient operation. Case studies have established that weaknesses in several key management areas may contribute to outbreaks of Legionnaires’ disease. All personnel responsible for operation of the system should be made aware of the potential for the proliferation of Legionella through training and constant documentation. Records of efforts related to Legionella control should be maintained for a minimum of one year. These records include evidence of precautionary measures, treatments, monitoring results and remedial work. Government record keeping requirements should be followed in conjunction with the records retention policy that is to be developed and followed under this guidance.
2020 AIHA Framework
The 2020 document from the American Industrial Hygiene Association (AIHA) entitled, “Technical Framework: Legionella,” is characterized as an update to the 2015 document, “Recognition, Evaluation, and Control of Legionella in Building Water Systems.”
The 2015 document is a guidance document intended to help building managers “anticipate, evaluate, and control Legionella in buildings” (22). The 2020 update covers the same subject matter as ASHRAE Guideline 12-2020, focusing on identifying sites of Legionella amplification and exposure pathways of various types of building water systems. Much of the testing information provided within the 2015 AIHA guidance document tracks the CDC language as discussed above in Section V of this article.
The 2020 iteration is crafted as a “technical framework” that defines the core knowledge and skills required by
an individual for effective performance in a specific practice or expertise. The “technical framework” is less focused on being a source of comprehensive information but, instead, is a resource for individuals to expand their knowledge and aid in the development of a new training program within specifically defined roles (23). The 2020 update identifies three primary roles in evaluating Legionella risk in building water systems. They include the Program Professional, Responder Professional and Competent Technician (23). Each role requires a unique set of specific “domains and tasks” that must be performed at the respective requisite knowledge levels. A summary of the responsibilities of each role is defined in this section.
Program Professional (23): The Program Professional’s role is to anticipate, recognize, evaluate, and control Legionella exposure in water systems and develop water management programs to minimize the risk of Legionnaires’ disease, and prevent Legionella amplification.
Responder Professional (23): The Responder
Professional is integral in the event of an outbreak or investigation of a case of Legionnaires’ disease. They must be able to coordinate, communicate, investigate, develop, and validate mitigation efforts to prevent additional cases of disease. Specifically, they must develop a testing plan and develop/oversee the implementation of controls to mitigate exposure and remediate amplification sources.
Competent Technician (23): The Competent Technician must be able to record observations and safely collect reliable measurements, samples, and supporting data on the building water systems under the guidance or direction of a Program Professional. Their tasks will be focused on areas relating to routine or investigative risk assessments that have been designed by a Program Professional or Responder Professional.
Future Documents
The following manuals and guidance documents are in production or expected to be released, following a public review. These may supplement and/or be expanded upon by the updates presented in this article.
47 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued
ASPE—Engineering Methodologies to Reduce the Risk of Legionella

The American Society of Plumbing Engineers (ASPE) is a voting member of ASHRAE Standard 188-2021 and ASHRAE Guideline 12-2020. Both documents offer considerable information regarding Legionella mitigation and are referenced throughout the publication. However, neither ASHRAE document provides the practical and actionable information that is sorely needed by plumbing design professionals. For that reason, ASPE has developed the “Engineering Methodologies to Reduce the Risk of Legionella in Premise Plumbing Systems” design guide. One of the primary goals of the working group was to seek input from a diverse audience to provide a technically defensible document for use by plumbing engineers.

AWWA/IAPMO—Manual of Recommended Practices for the Safe Closure and Reopening of Buildings
This manual contains recommended best practices, which are intended to provide expert guidance on building water system safety. It provides effective risk management practices for preparing water systems when
buildings must be closed or put into low use modes, intermittent operation of building water systems during periods of no or low use and evaluating and preparing water systems for reopening. While this document is developed as a guidance document, it is written with enforceable language, so building and health departments can more easily appropriate and codify these requirements.
IAPMO—Manual of Recommended Construction Practices for Potable Water


The International Association of Plumbing and Mechanical Officials (IAPMO) is currently working with its volunteers to produce actionable guidance for the construction uses of potable water. Effective water management during construction can reduce the risk of contamination of the building’s water systems, leading to reduced risk of infections for workers on-site and future occupants. The manual aims to outline a process for contractors installing a building water system and includes resources to help turn over a safe potable water system, post construction.
Self-Brushing
Oxidizer + pH Panels
Our OXIPANEL™ Series provides real-time oxidizer + pH & temperature measurement while continuously brushing the sensor head for wildly accurate readings in the most challenging applications.



48 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued
F-Cl ClO2 Br
Learn More
IAPMO—Water Demand Calculator (WDC®) Innovation Task Group
The IAPMO Innovation task group will survey manufacturers and building owners to develop statistical data for the frequency of use from different types of fixtures during peak occupancy, to capture probability of use. The group is currently aggregating data of fixture counts and types, sorted by building category and by specified fixture flow rates. The WDC innovation task group is actively creating a broader network of experts to develop water solutions for all. This work has an incredible potential to make positive impacts on public health and safety, by reducing water pipe size and leading to potential improvements of water quality and conditions.
Conclusion
Since the release of ASHRAE Standard 188 in 2015, more code modifications, standards, and guidelines have been released to address the problem of Legionella in plumbing systems. Due to the fragmented nature of the plumbing industry in the United States, these documents have come from a wide variety of organizations and entities. A concern, naturally, is that guidance may conflict. However, almost all (if not all) of these documents have been created with public review and response periods allowing for collaboration. If all stakeholders remain engaged, conflicting advice can hopefully be avoided. Water systems are complex (especially considering hydraulics, water chemistry/ quality, microbiology, etc.). To address Legionella holistically, a wide variety of expertise should be considered.
Additionally, Legionella guidance and standards are utilized in different ways by different entities. Scientists and epidemiologists may prefer the latitude of solutions afforded by the general nature of a guidance document. Installers, inspectors and design professionals may prefer a more prescriptive approach with specific, practical and actionable steps. Due to the varied and complex nature of Legionella-related challenges in building water systems, it is difficult for any one document to comprehensively capture all of the requisite guidance. A comprehensive approach with varied industry organizations developing guidance specific to their needs with the concurrent solicitation of public comment to maintain coordination, will hopefully result in an effective minimization of Legionellosis.
References/Notes
1. ASHRAE (2021). ANSI/ASHRAE Standard 188-2021, “Risk Management for Building Water Systems,” ASHRAE, Atlanta, Georgia.
2. In the article, “Shall” is defined as “is/are required to and must.”
3. ASHRAE (2020). ANSI/ASHRAE Guideline 12-2020, “Managing the Risk of Legionellosis Associated with Building Water Systems,” ASHRAE, Atlanta, Georgia.
4. Beer, K.D.; Gargano, J.W.; Roberts, V.A.; Hill, V.R.; Garrison, L.E.; Kutty, P.K.; Hillborn, E.D.; Wade, T.J.; Fulleraton, K.E.; Yoder. J.S. (2015). “Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water– United States— 2011-2012,” Morbidity and Mortality Weekly Report 64(31), pp. 842-48.
5. ASHRAE (2020). ANSI/ASHRAE Guideline 12-2020, Section 6, “Managing the Risk of Legionellosis Associated with Building Water Systems,” ASHRAE, Atlanta, Georgia.
6. Id at Section 7.
7. Id at Section 8.
8. Id at Section 9.
9. Id at Section 10.
10. Id at Section 11.
11. Id at Section 12.
12. CDC (2021). “Developing a Water Management Program to Reduce Legionella Growth & Spread in Buildings: A Practical Guide to Implementing Industry Standards,” N. Messonnier, MD, CAPT USPHS, P. Breysse, Ph.D., CDC, Atlanta, Georgia, available at www.cdc.gov/Legionella/wmp/control-toolkit/index.html
13. CDC (2021). “Toolkit for Controlling Legionella in Common Sources of Exposure,” CDC, Atlanta, Georgia.
14. CTI (July 2008). “Legionellosis, Guideline: Best Practices for Control of Legionella 3,” Cooling Technology Institute, Houston, Texas.
15. WHO (2007). “Legionella and the Prevention of Legionellosis,” 29, World Health Organization, Geneva, Switzerland.
16. CDC (2021). “Toolkit for Controlling Legionella in Common Sources of Exposure,” CDC, Atlanta, Georgia.
17. AWT (2019). Legionella 2019: A Position Statement and Guidance Document, Association of Water Technologies (2019).
18. 42 CFR §482.42 (“The hospital must provide a sanitary environment to avoid sources and transmission of infections and communicable diseases. There must be an active program for the prevention, control, and investigation of infections and communicable diseases.”); 42 CFR §483.80 (for skilled nursing facilities and nursing facilities: “The facility must establish and maintain an infection prevention and control program designed to provide a safe, sanitary, and comfortable environment and to help prevent the development and transmission of communicable diseases and infections.”); 42 CFR §485.635(a)(3)(vi) (for critical access hospitals (CAHs): CAH policies must include: “A system for identifying, reporting, investigating and controlling infections and communicable diseases of patients and personnel.”).
19. CMS (2017). Memo dated June 9, 2017, Centers for Medicare and Medicaid Services, Baltimore, Maryland.
20. CMS (2018). CMS Memo dated June 6th, 2018, available at: www.cms.gov/ Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/ Downloads/Survey-and-Cert-Letter-17-30.pdf
21. CTI (2020). CDL-159 (20), “Legionellosis Guideline: Practices to Reduce the Risk of Legionellosis from Evaporative Heat Rejection Equipment Systems,” Cooling Technology Institute, Houston, Texas.
22. AIHA (2015). “Recognition, Evaluation, and Control of Legionella in Building Water Systems,” American Industrial Hygiene Association, Falls Church, Virginia.
23. AIHA (2020). “Technical Framework: Legionella,” American Industrial Hygiene Association, Falls Church, Virginia.
Disclaimer
The studies and conclusions reported in this paper are the results of the authors’ own work. AWT has not investigated, and AWT expressly disclaims any duty to investigate, any product, service process, procedure,
49 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued
design, or the like that may be described herein. The appearance of any technical data, editorial material, or advertisement in this publication does not constitute endorsement, warranty, or guarantee by AWT of any product, service process, procedure, design, or the like. AWT does not warranty that the information in this publication is free of errors, and AWT does not necessarily agree with any statement or opinion in this publication. The user assumes the entire risk of the use of any information in this publication.
This publication contains a general overview and brief summary of selected regulations, standards and guidelines relating to Legionella in building water systems as of Spring 2022. This document is not intended to be a fully comprehensive review of all existing regulations, guidelines, standards or similar texts relating to Legionella. In addition, some of the documents addressed in this publication are copyright protected and available for purchase. Accordingly, the summaries or synopses contained herein are not a reproduction of any protected texts. This document is not a substitute for competent counsel or site-specific guidance from a Certified Water Technologist, plumbing professional or mechanical official. The facts of each case must be assessed for variables including the applicable regulations, codes, standards and laws of the forum state and other considerations including the evolving state of Legionella science and authority.
Copyright 2022. All rights reserved.
Adam Green is the chairman of Baker Donelson’s Global Water Technology and Water Treatment Group where he serves as the go-to subject matter resource for Legionella and water treatment related matters. Over the past 20 years, he has successfully consulted and defended clients in Legionellosisrelated wrongful death and personal injury lawsuits across a wide range of industries and premises across the country. Mr. Green has defended Legionellosis outbreaks both before and after the creation of ASHRAE 188, the CMS Memorandum, and related documents. His cases also include high value property damage claims arising from catastrophic system failures incident to a myriad of operational, design, maintenance, and treatment related issues. He is a frequent author and speaker at the Association of Water Technologies, Cooling Technology Institute, International Association of Defense Counsel, and others.

Matthew Kim is a corporate transactional associate at Baker Donelson. He is a graduate of both the University of Oregon and Vanderbilt University School of Law. His professional focus is on assisting business clientele in a variety of industries.


Robert J. Cunningham is the president of International Water Consultants, Inc. He is a native of Western Pennsylvania and he has been working in all fields of water treatment since 1964 when he joined Calgon Corp., after earning a degree in chemistry from the University of Pittsburgh. Mr. Cunningham has worked extensively worldwide with all types of boiler, cooling, and wastewater treatment systems across all of the major industries employing these technologies. He provides litigation support on a variety of issues, including corrosion, deposition, and microbial damage, as well as the control of waterborne pathogens, such as Legionella. He and his wife Linda live in Grass Valley, California. Mr. Cunningham can be contacted at rjc5225@gmail.com

John Mullen is a fourth-generation plumber, who has engaged in a multitude of complex plumbing projects from the position of an apprentice to serving as a company executive, where he has experience overseeing operations for large union plumbing and mechanical operations. Mr. Mullen also has worked in compliance and safety in sectors such as healthcare, hospitality, and data centers, among others. Currently, he is the director of Technical Services at the IAPMO.
This paper was presented at the 2022 Association of Water Technologies Annual Conference & Exposition, which was conducted Sept. 21-24, 2022, in Vancouver, British Columbia, Canada.
Keywords: COOLING TOWERS, COOLING WATER, GUIDELINES, LEGIONELLA, MAINTENANCE, REGULATIONS, TESTING
50 the ANALYST Volume 30 Number 3 Continuous Maintenance: An Overview of Updates to Legionella Regulations continued
Gain More Control Of Your Control
Water Treatment Process Water Treatment Process
MicroVision EX keeps your process running efficiently, reducing downtime, reducing scale deposition, and increasing water efficiency protecting expensive equipment and water resources* Optimize chemical utilization by controlling conductivity, pH, ORP, while also metering inhibitor and biocide chemicals to prevent corrosion, scale and biological growth.

Cooling Tower Controllers and Communications


Save Money
• Receive notications of system upsets and make remote changes to parameters without making an unscheduled emergency visit

• Toroidal probe = No annual replacement needed, eliminates expensive service calls
Save Time
• Troubleshoot the system remotely so you can have necessary parts with you to x the issue the rst time
• Toroidal probe = No exposed electrode, no calibration needed, no guessing if the readings are correct
with
Diaphragm Metering Pumps



• Accurate dosing of liquid chemicals
• Auto-degassing liquid end


• Reliable long-life cycle
• Quick prime models
Peristaltic Pumps


• Self-priming
• Inherently degassing

• Reliable metering performance
• Rugged, sealed all metal gear train
 NSF61
Up to 4.2 GPH
NSF61
Up to 4.2 GPH
www.pulsafeeder.com
Up to 25 GPH
* Only when programmed correctly and paired with appropriate chemistry can you get performance benets
NSF61
What is the Impact of Temperature, pH, Time, and Chemical Selection on Membrane Cleaning?
 Joshua Utter American Water Chemicals
Joshua Utter American Water Chemicals
52 the ANALYST Volume 30 Number 3
Introduction
As the global demand for clean water continues to grow, membrane-based technologies can provide an energy-efficient means for water desalination, with polyamide thin-film composite (TFC) membranes being the current industry standard for reverse osmosis (RO). Despite ongoing improvements in membrane technology improving permeability, membrane fouling continues to be a major obstacle in optimizing RO system design and operation.
Continuous operation of an RO system inevitably leads to build up of various materials from the feed water. These built-up foulant layers decrease membrane efficiency manifesting as loss in salt rejection, increased differential pressure, and/or loss in membrane permeability. These foulant layers vary in composition from case to case. The different foulants that appear often can be prevented. Unfortunately, predicting the foulants that have caused membrane performance loss is an uncertain science. Therefore, the selection of the appropriate corrective action can be especially difficult.
Membrane cleaning is a common solution for recovering lost membrane performance. The effectiveness of cleaning improves significantly when the clean in place (CIP) system is tailored to the specific fouling problem. In some cases, incorrect chemical selection can make a situation worse. For these reasons, the type of foulants on the membrane surface should be determined before cleaning. This

can be accomplished by a membrane autopsy. Many chemical suppliers and membrane manufacturers provide “autopsy” services to establish the cause of membrane efficiency losses and failures. From this information, a chemical supplier can provide suggested cleaning protocols.
Cleaning Study Example
An industrial wastewater reclamation system from a particle board manufacturing plant was experiencing rapid fouling, requiring frequent CIP procedures to maintain continuous operation. The cleaning frequency before performing a membrane autopsy reached to a frequency of once every three days. This cleaning frequency was maintained for the entirety of the three months the system was installed.
Onsite cleaning onsite was performed at pH 12, 45° C with a 2% mixture of Treatment Product A A for one hour, followed by low pH cleaning with 2% citric acid at pH 3.5 for one hour. In discussions with the end user, the plant raised concerns of calcium fluoride formation, as happened during system commissioning, this was a potential scaling concern. Additionally, concerns were raised that tannins, resins, and other foulants may be contributing to the fouling issue.
Two parallel elements were removed from the system, one for membrane autopsy and another for cleaning study. The received membrane elements were Hydranautics PRO XT2 hot water elements. Upon receipt, the elements were stained dark brown, lending credence to the concerns of tannins. Due to equipment constraints, the autopsied element was performance tested at brackish water conditions that were determined using a conversion sheet provided by the membrane manufacturer. Initial performance testing of the element found both permeability and membrane salt rejection significantly below specification (Table A).
53 the ANALYST Volume 30 Number 3 What is the Impact of Temperature, pH, Time, and Chemical Selection on
Membrane Cleaning?
Figure 1: Fouled membrane surface. Inset image demonstrating surface close up and the foulant that could be scraped off by spatula.
Table A: Initial Performance of Autopsied Membrane Element
After performance testing, the membrane was unraveled, and the fouled membrane inspected. All the membrane leaves and feed spacers were covered in a thick layer of brown foulant (Figure 1). The foulant surface density was ~535 micrograms per square centimeter (µg/cm 2) when dehydrated, which was considered to be considerable fouling. Loss on ignition testing determined the collected foulant consisted of ~96% organic content and ~4% inorganic content.
Analysis of the membrane surface by SEM/EDS/ SEI/PEDB found the membrane surface was fouled by an apparently uniform layer of organic based matter (Figures 2, 3). No calcium fluoride was detected.


Cleaning Visualization
Coupons were collected from the membrane surface for flat sheet cell testing and cleaning. Cell testing is performed in order to determine the performance of the membrane. Samples of the membrane were soaked in deionized (DI) water for 24 hours to standardize the samples moisture and starting condition. The coupons are then mounted to Sterlitech CF042A acrylic crossflow cells. The membrane coupons were cleaned using a crossflow of 0.8 gallons per minute (gpm), alternating every hour between soaking and circulation. After cleaning, the cells were flushed with DI water. Every hour the cells were photographed to track the membrane performance. Various cleaning conditions were tested,
54 the ANALYST Volume 30 Number 3 What is the Impact of Temperature, pH, Time, and Chemical Selection on Membrane Cleaning? continued
Figure 2: Scanning electron micrographs of fouled membrane surface.
Figure 3: Superimposed Elemental Imaging of fouled membrane surface displaying EDS data.
Parameter Nominal Specification (Normalized to Brackish Conditions) Minimum Specification (Normalized to Brackish Conditions) Wet Test Result (Normalized to 25°C) %Difference From Nominal Specification %Difference From Minimum Specification Permeate Flow (gpd) 4,542 3,860 682.4 -84.97% -82.32% Recovery (%) 15.0% 12.7% 2.3% -84.97% -82.32% Flux (gfd) 13.36 11.35 2.01 -84.97% -82.32% Permeability (gfd/psi) 0.065 0.056 0.010 -85.09% -82.46% Salt rejection 99.78% 99.76% 96.48% -3.31% -3.29% Salt rejection normalized to manufacturer specified flux - - 99.47% -0.31% -0.29% ΔP – Spec Test Condition Average Flow* 1.2* 1.2* - -ΔP – Measured Average Flow 1.2* - 2.6 +111.89% -
based on Reynolds number (function of feed spacer height, temperature, flow velocity) and friction coefficient for a clean new membrane.
*Estimated
starting with new 24-hour-soaked DI coupons each time to determine the most effective cleaning protocol.
Due to the frequent cleaning performed onsite, concerns of membrane damage from frequent onsite CIPs was expected. Cleaning onsite was performed at pH 12, 45°C with 2% Product A for one hour followed by low pH cleaning with 2% citric acid at pH 3.5 for one hour. The cleaning frequency reached once every three days. These cleaning conditions exceed the manufacturer stated limits for temperature–pH time (1).
The cleaning chemical used onsite has a specified use concentration of 2%, with a natural pH of 11 at this concentration. Removal of biofilms and organic foulants is optimized above pH 12 (2), but these conditions are typically considered extreme, possibly leading to membrane performance loss if cleaning is frequently performed at these conditions.
For this reason, a study was performed at pH 11 with 2% Product A. Three separate trials were performed, testing 25°C, 35°C, and 45°C. Each trial was performed for more than six hours, monitoring visible foulant removal every hour (refer to Appendix A: Impact of Temperature). At pH 11, total membrane foulant removal was achieved only by cleaning at 45°C for six hours. Cleaning beyond six hours was not investigated in this case due to constraints in CIP.
The next aspect investigated was the impact of varying pH at the same temperature conditions. For each temperature, cleaning was performed at pH 11, 12, and 12.5. The results for 35°C are presented in Appendix A: Impact of pH. At 35°C cleaning at pH 12 was able to achieve significant removal of membrane foulants after six hours, though residual foulants remained. At pH 12.5, typically considered a very aggressive condition) complete foulant removal was achieved after only three hours. For a control, a cleaning with sodium hydroxide alone was performed. At 45°C and pH 12.5 for six hours, no significant foulant removal with caustic was observed.
After reviewing the results, cleaning at pH 12.5, 35°C was selected for further study. Once a cleaning protocol was determined, additional coupons were performance tested using the test conditions set by the manufacturer.
Salt rejection and flux measurements were compared with the manufacturer’s specifications and the initial full element performance tests. Cell tests were performed before and after cleaning. Refer to Tables B and C.
The cleaning completely recovered permeability, though salt rejection could not be improved. After cleaning, a cleaned membrane coupon was dye tested to determine the extent of any underlying membrane surface damage (refer to Figure 4). No notable dye passage was observed after dye testing, indicating the lost salt rejection was due to loss of membrane selectivity as opposed to perforations in the membrane layer. This loss in selectivity was attributed to the frequent cleaning protocols. In discussions with the end user, the observed salt rejection exceeded requirements.

Full Element Cleaning Study
Based on the results from flat sheet cell test cleaning studies, a full element cleaning study was performed on the second membrane. Due to equipment constraints, the membrane element was performance tested at 225 psi using 1,500 ppm sodium chloride (NaCl) during this study. The element was performance tested after each cleaning step. The test results were compared to the quality control wet test results provided by the manufacturer and normalized to the same conditions. The initial performance of the element showed extremely poor
55 the ANALYST Volume 30 Number 3 What is the Impact of Temperature, pH, Time, and Chemical Selection on Membrane Cleaning? continued
Figure 4: Dye test of cleaned membrane coupon demonstrating no notable dye passage.
membrane permeability, and salt rejection. Refer to Table D. The membrane as received weighed 40 pounds (lb).

The first cleaning step was a high-pH cleaning with 2% Product A adjusted up to pH 12.5. The cleaning was performed at 35°C for six hours. Cleaning was performed with one hour circulation cycles followed by one hour soaks. For the first 30 minutes of cleaning, flow was held at 40gpm as differential pressure across the element reached 10 psi. After 30 minutes, differential pressure sufficiently dropped such that a more optimal cleaning flow of 45gpm could be achieved. Over the course of the cleaning, average temperature was 34°C, and the average pH was 12.4.
The membrane performance after cleaning increased significantly but was still below minimum specification. A second high-pH cleaning at the same conditions but with a 50-gpm crossflow was performed (the maximum cross flow the plant could achieve). This cleaning returned permeability to the manufacturer quality control (QC) test result. The marginal increase in permeability after the second high pH cleaning step may indicate that the full six hours of additional cleaning were not necessary. Differential pressure dropped from 2.6 psi to 1.6 psi after the first cleaning, and then down to 1.4 psi after the second (refer to Table E).
After high pH cleaning the membrane was cleaned with 2% of Product BC at pH 1.8, 31°C for two hours. After cleaning, membrane permeability increased further, while salt rejection decreased further. (Refer to Table E). After cleaning, the membrane weighed 36 lbs, indicating that 4 lbs of membrane foulant was removed by this protocol.
Overall, the cleaning study demonstrated that cleaning with 2% Product A at pH 12.5 at 35°C followed by cleaning with Product B would recover membrane permeability, though salt rejection could not be recovered. The further improvement in permeability observed after low pH cleaning likely indicates that additional foulants (possibly low pH soluble scales) were removed from the membrane surface. The marginal reduction in salt rejection could indicate removal of these foulants exposed to underlying surface damage (Refer to Table E).
Discussion
Factors that Need Optimization
As demonstrated in the cleaning visualization study, pH, temperature, time, and cleaning chemical selection all have significant impact on cleaning effectiveness. Determining the optimal cleaning conditions requires a clear understanding of the fouling that has occurred. For ease of explanation when discussing with operators, this author uses the following relationship as illustrated in Equation 1:
More aggressive pH and temperatures require less time to clean foulants from a membrane; unfortunately, membranes cannot tolerate pH and temperature extremes for very long without loss in efficiency. It is therefore necessary to balance and optimize these parameters.
Importance of Time
For many, water treatment processes time and labor are some of the most expensive costs for operation. Long down times can be ill afforded in many processes and allocating labor for cleaning is often a challenge. For these reasons, many attempt to reduce the cleaning duration. The ideal goal of any cleaning is to reach a clean slate, returning permeability and salt rejection to day-one values. As can be seen in the cleaning visualization, required cleaning time can vary drastically with the cleaning conditions. Without adjusting the other parameters when shortening the cleaning time, partially fouled membranes can remain after a cleaning.
As demonstrated in Figure 5, shortening a cleaning by only one hour can have significant impact on cleaning efficacy. Residual foulant layers will increase required driving pressure to generate the same permeate flow. This can affect flux balance in the system, and effectively drive the clean areas at higher flux. For biofilms, leaving behind residual films allows more rapid colonization, any surviving biological populations can develop tolerance to the stressor (3). For scales, residual deposits can act as nucleation sites allowing for more rapid scale formation (4). These mechanisms can lead to a feedback loop. Reduced cleaning time due to expense leading to more frequent CIPs, and therefore greater time pressure and expense.
56 the ANALYST Volume 30 Number 3 What is the Impact of Temperature, pH, Time, and Chemical Selection on Membrane Cleaning? continued
Eq. 1
What is the Impact of Temperature, pH, Time, and Chemical Selection on Membrane Cleaning? continued
*Estimated based on Reynolds number (function of feed spacer height, temperature, flow velocity) and friction coefficient for a clean new membrane.
57 the ANALYST Volume 30 Number 3
Manufacturer’s Specifications (nominal) 800 psi Manufacturer’s Specifications (minimum) 800 psi Full Element Wet Test Results Normalized Test Conditions (225 psi) % Salt Rejection 99.70% 99.60% 96.48% Permeability (gfd/psi) 0.049 0.042 0.010
Table B: Flat Sheet Cleaning Study Results (1)
Initial Flat Sheet Wet Test Results High pH: 2% Product A at pH 12.3 33ºC For 6 hours Low pH: 2% Product B at pH 1.80 25ºC For 2 hours %Diff. Final Vs. Spec (nominal) %Change from initial Final Salt Rejection Normalized for Flux %Diff. Flux Normalized Rejection vs. Spec Salt Rejection (%) 98.79% 97.05% 98.24% -1.46% -0.56% 98.45% -1.46% Permeability (gfd/psi) 0.016 0.050 0.043 -11.70% 167.66% N/A N/A
Table C: Flat Sheet Cleaning Study Results (2)
Parameter Nominal Specification (Normalized to Brackish Conditions) Minimum Specification (Normalized to Brackish Conditions) Manufacturer Wet Test Results Initial Wet Test Result (Normalized to 25°C) % Difference from Manufacturer’s Test Permeate flow (GPD) 4,542 3,860 3,975 609 -85.13% Recovery (%) 15.0% 12.7% 13.1% 2.0% -85.13% Flux (gfd) 13.36 11.35 11.69 1.79 -85.13% Permeability (gfd/psi) 0.065 0.056 0.057 0.009 -85.28% Salt rejection 99.78% 99.76% 99.83% 97.38% -2.24% Salt rejection normalized to manufacturer specified flux - - - - -0.07% ΔP – Spec Test Condition Avg. Flow* 1.2 1.2 - -ΔP – Measured Avg. Flow 1.3 - 1.2 2.5 -
Table D: Initial Performance of Cleaning Study Membrane
*Estimated based on Reynolds number (function of feed spacer height, temperature, flow velocity) and friction coefficient for a clean new membrane.
Manufacturer Wet Test Results (Normalized to Brackish Conditions) AWC Initial Wet Test Result (Normalized to 25°C) 2% PRODUCT A pH 12.4 34°C 6h 45 gpm cross flow 2% PRODUCT A pH 12.4 35°C 6h 50 gpm cross flow 2% PRODUCT B pH 1.8 31℃ 2h 45 gpm crossflow % Diff. From QC Wet Test % Diff. from Initial Permeate Flow (GPD) 3,975 574 3,495 3,929 4,047 1.81% 604% Recovery (%) 13.1% 1.9% 11.5% 13.0% 13.4% 1.81% 604% Flux (GFD) 11.7 1.69 10.28 11.56 11.90 1.81% 604% Permeability (gfd/psi) 0.057 0.008 0.050 0.056 0.058 1.17% 607% Salt rejection (%) (NaCl) 99.83% 97.53% 94.83% 96.94% 96.74% -3.10% -0.81% Flux normalized salt rejection 99.85% 99.7% 96.02% 97.35% 97.09% -2.76% -2.60% Expected ΔP* (PSI) N/A 1.3 1.30 1.2 1.2 -Measured ΔP (PSI) Normalized to 25°C 1.2 2.6 1.6 1.4 1.4 - -
Table E: Cleaning Study Performance Results Between Individual Cleaning Steps
As demonstrated in the cleaning study in this article, membrane elements can lose selectivity from exposure to cleaning extremes. For optimum membrane performance, the benefits in reduced cleaning time should be weighed against the long-term lifespan of the membrane.
Temperature is a measure of the average kinetic energy of molecules within a given sample. As temperature increases the speed of molecules in a sample increases. Increasing molecular speed increases collisional frequency, and collision energy. All chemical reactions have a certain activation energy, increasing the temperature (and therefore average collision energy) increases the chances that any interaction will result in a reaction.
For the purposes of cleaning, increasing temperature decreases the duration of any cleaning. Unfortunately, it also increases the chances that membrane damage can occur. Benefits in raising temperature need to be weighed against long-term lifespan of the membrane.
Importance of pH
Importance of Temperature
Temperature of cleaning solutions has significant impact on cleaning efficacy and the aggressiveness of the cleaning. Temperature directly impacts rate of reaction, for most reactions increased temperature results in greater reaction rates. For many salts/compounds, solubility increases with temperature.

The pH of the cleaning solution directly impacts cleaning efficacy just as temperature. For removal of most inorganic scales, lower pH improves performance, while for biofilm and organics removal a high pH gives the same result. The foulants present within a membrane system will dictate which extreme pH must be shifted towards to achieve foulant removal. For many systems, both high-pH and low-pH cleanings are required.


58 the ANALYST Volume 30 Number 3 What is the Impact of Temperature, pH, Time, and Chemical Selection on Membrane Cleaning? continued
Figure 5: Demonstration of foulant coverage differences from early stoppage of CIP procedure.
and SaniWare Treatment and control The patented SANIKILL technology now combined with SaniWare water quality monitoring panels! Expand your OPPORTUNITIES! Visit www.sanipur.com - sales@sanipur.com - 484-351-8702
SANIKILL
An aqueous solution’s pH is a measure of the concentration of hydronium ions in solution. With every unit of decrease in pH, the hydronium concentration increases 10-fold. Above pH 7, its best understood as an increase in hydroxide concentration, again 10-fold for every pH unit. Increasing the concentration of acid and base in solution increases the collision frequency of acid and base in solution by 10-fold. For this reason, adjusting pH typically has a greater impact on cleaning than increasing temperature.
Cleaning Chemical Selection
Selecting the appropriate cleaning chemical is a separate but equally (if not more) important aspect. Some foulants require specific chemicals for effective, efficient removal. For example, silica scale can be cleaned at extremely high pH, (pH >12.5) but these conditions exceed many manufacturers’ cleaning thresholds. Alternatively, the use of a fluorine-based, low-pH cleaning treatment can efficiently remove amorphous silica scales at relatively moderate acidic pH conditions.
Proprietary chemicals are typically blends of chemicals determined by the manufacturer to be most effective at removing specific scales/foulants. The use of premium cleaning products effectively saves individual users the research and development cost for determining optimal cleaning solution cocktails.
The cleaning study discussed in this article is an example of cleaning chemical selection providing significant improvements. Cleaning at extremely harsh conditions with sodium hydroxide was unable to provide any significant improvement in foulant removal (refer to Appendix C), while cleaning with Product A was able to effectively remove the heavy organic fouling.
Conclusions
With growing populations and increasingly tighter regulations, the demand for and cost of treated water continues to rise. RO systems are often being called upon to tackle increasingly challenging water sources, while existing systems are looking to increase recovery to meet these rising demands. These water utilities and industrial plants can ill afford down time for routine CIP due to the timeconsuming nature, and the expense of cleaning chemicals.
For this reason, optimization of the cleaning protocol should be performed to reduce the required time/labor
expenditure. Insufficient cleaning protocols can lead to losses in system efficiency over time as durable membrane foulants build up. If an effective optimized CIP protocol is not implemented early enough, this can cause eventual system failure or the need for membrane replacement.
Optimizing a cleaning protocol requires understanding the membrane foulants to correctly determine the ideal chemical, pH, temperature, and duration for the CIP protocol. All of these factors together need to be weighed against the CIP system’s design constraints. Understanding the interactions between pH, temperature, time, and chemical selection allows a plant to appropriately address this balancing act.
References
1. Nitto Hydranautics (2020). Foulants and Cleaning Procedures for composite polyamide RO/NF Membrane Elements, Nitto Hydranautics, Oceanside, California, accessible at www.membranes.com/wp-content/uploads/Documents/TSB/TSB107.pdf
2. DuPont. (2022). FilmTecTM Reverse Osmosis Membranes Technical Manual, 12th ed. DuPont Water Solutions, Wilmington, Delaware, accessible at www.dupont.com/ content/dam/dupont/amer/us/en/water-solutions/public/documents/en/ RO-NF-FilmTec-Manual-45-D01504-en.pdf
3. Bryers, J.D. (May 2008). “Medical Biofilms,” Biotechnology and Bioengineering 100(1), pp. 1-18, accessible at doi: 10.1002/bit.21838. PMID: 18366134; PMCID: PMC2706312.
4. De Yoreo, J.J.; Vekilov, P.G. (2003). “Principles of Crystal Nucleation and Growth,” Reviews in Mineralogy and Geochemistry 54(1), pp. 57-93, accessible at doi: www.doi.org/10.2113/0540057
Endnotes
A. Product A mentioned in the text is AWC C-227, which is made by American Water Chemicals, which is based in Plant City, Florida.
B. SEM = scanning electron microscopy; EDS = energy dispersive spectroscopy; SEI = secondary electron images; PED = precession electron diffraction
C. Product B mentioned in the text is AWC C-234, which is made by American Water Chemicals, which is based in Plant City, Florida.
Joshua Utter has been with American Water Chemicals since 2014. He provides technical support such as membrane cleaning studies and autopsies and on-site support to customers during membrane cleaning. He also assists customers with troubleshooting, data normalization, and other necessary technical tasks for proper monitoring of plant performance.

This paper was presented at the 2022 Association of Water Technologies Annual Conference & Exposition, which was conducted Sept. 21-24, 2022, in Vancouver, British Columbia, Canada.
Keywords: BIOFOULING, CLEANING, CIP, FOULING, MEMBRANES, OPTIMIZATION, REVERSE OSMOSIS, SCALING
59 the ANALYST Volume 30 Number 3 What is the Impact of Temperature, pH, Time, and Chemical Selection on Membrane Cleaning? continued
Article Appendices: Cleaning Visualization Images






Appendix A: Impact of Temperature

Appendix B: Impact of pH
Appendix C: Impact of Chemical Selection

60 the ANALYST Volume 30 Number 3 What is the Impact of Temperature, pH, Time, and Chemical Selection on Membrane Cleaning? continued



C M Y CM MY CY CMY K Analyst-Summer-2023-IntuitionSecurityAd-Publish.pdf 1 6/8/23 10:38 AM



Talk with one of our Certified Water Technologists Today! 800.296.9102 | www.qualichemwt.com AN ISO 9001:2015 COMPANY SUPPLIER OF THE YEAR
Blender Matters There is a world of difference between a one-size-fits-all toll blender and an experienced, custom water treatment blender like QualiChem. We formulate, manufacture, and ship products to private label water treatment partners internationally. Providing Industry-Recognized Benefits And Services – No Direct Sales – Precision Manufacturing – Formulatory Expertise – Application Support – Custom GHS- Compliant Labels and SDSs A Trusted Business Partner With The Expertise To Help Your Business Grow. Please visit us at the AWT 2023 Annual Convention & Exposition – Booth #231.
The
Membership Benefits
AWT Troubleshooting Guides
Have you ever been stuck in the field with a question? Reluctant to call someone about something you should know? The AWT Troubleshooting Guides are the resources you’ve been looking for! As an exclusive member benefit, the troubleshooting guides are there to help you work through scenarios with the best information available from experts in the industry.
Find them at www.awt.org/members-section/troubleshooting-guides
Examples of troubleshooting guides available include:


Algae in a Cooling System
Closed Loop Filtration System
Flow Switch
High Bacteria Counts in Cooling Towers
Low Conductivity in a Cooling Tower
and more!
63 the ANALYST Volume 30 Number 3
Discovering AWT
French Creek Software, Inc.
1220 Valley Forge Road, Suite 21
Valley Forge, PA 19460
(610) 935-8337
www.frenchcreeksoftware.com
Video Server: www.vimeo.com/frenchcreeksoftware
Company History: Rob and Janet Ferguson founded French Creek Software in 1989 to take control of their destiny and escape the world of corporate takeovers and frequent relocations. Mr. Ferguson was a pioneer in water treatment “digitalization,” having developed many of the initial computer programs and modeling techniques while with companies like Nalco, Calgon, and Chemlink. Their initial software offering made the sophisticated speciation engine derived indices and inhibitor optimization algorithms available for operation on a PC rather than the main frames. The French Creek 3-D profiles arose from the philosophy epitomized by the company motto: P-Chem for Fun and Profit. French Creek introduced and test marketed its initial application at the AWT Fall Meeting in 1989. French Creek considers itself to be a company homegrown at AWT.
Current Business: French Creek provides off-theshelf and branded, private-label software tools for scale, corrosion, and inhibitor modeling with editions directed towards the salesman or field engineer, product management, and R&D. Cooling water and oil field production chemistry modeling and inhibitor optimization are their top business areas; other applications are RO, mining, and municipal water. The company offers AWT members software packages that
can help them compete for water treatment business normally considered a territory held by large corporate water businesses.

French Creek began working with inhibitor synthesizers during their first months of operation. Three polymer companies, in particular, were early adopters of the French Creek approach and incorporated it into their new inhibitor screening and modeling stages, and implemented French Creek dosage optimization in their marketing approaches.
French Creek purchased a building for their corporate headquarters at the edge of Valley Forge Park in 2015 and converted the lower level to a scale and corrosion laboratory.
Business Locations: French Creek provides global sales and technical support from its Valley Forge, Pennsylvania offices.

Recognition and Involvement: The company joined AWT in 1989, and in the years since has received these AWT honors: Supplier of the Year (2012), Ray Baum Water Technologist of the Year (2017), and they were the first ever AWT Innovation Award honoree (2020).
Top Executives: Rob Ferguson, president; Janet Ferguson, vice president.

64 the ANALYST Volume 30 Number 3
French Creek helped move the industry from slide rules to sophisticated indices using computer technology.
French Creek’s offices at the edge of Valley Forge, Pennsylvania.
Myron L Company
2450 Impala Drive
Carlsbad, CA 92010 (760) 438-2021
www.myronl.com
Company History: Founded in 1957 by Myron L. Robinson as a research and development company, Myron L® Company is a privately owned and operated California corporation. In its early years, Myron L developed and patented a variety of specialty items for university studies and projects, for the space industry, and for industrial use. Examples of specialty products included an irradiation chamber, a spectrophotometer, plasto-met fittings, and gas detection instrumentation.

Current Business: Myron L’s present product line focuses on monitoring technologies, including handheld and in-line conductivity, resistivity, total dissolved solids (TDS), salinity, pH, oxygen reduction potential (ORP), dissolved oxygen, free-chlorine equivalent (FCE), and nitrate instruments. Calibration solutions, buffers, and related accessories are also available. Myron
L instruments are used by professionals worldwide in a variety of applications, including water treatment, metal finishing, agriculture, aquaculture, printing, reverse osmosis (RO) and deionized (DI) water treatment, hemodialysis, electronics manufacturing, and environmental studies/protection. Markets served by the company include municipal, commercial, and industrial water quality testing and control.
Business Locations: Through its network of sales agents and websites, the company serves water professionals worldwide.
Recognition and Involvement: Myron L is a longtime member of the AWT. Kathryn Robinson, the vice president of sales and marketing, was one of the first women to become a Certified Water Technologist (CWT), earning that certification in 1998.
Top Executives: Gary Robinson, president; Jerry Adams, vice president; Kathryn Robinson, vice president of sales and marketing; and Dan Robinson, North American sales manager.
65 the ANALYST Volume 30 Number 3
Discovering AWT continued
Myron L’s 53,000 square foot (4,900 square meter) facility is located approximately 30 miles north of San Diego in Carlsbad, California.
Seko Dosing Systems Corp.
913 William Leigh Drive
Tullytown, Pennsylvania 19007
(215) 945-0125
www.seko.com
Company History: Seko Dosing Systems was purchased by Lorenzo Folio in 1976. It was a small, local company in Rieti, Italy, with a handful of employees. By 1995, Lorenzo grew it to 35 employees, selling pumps throughout Italy and other countries in the region. Soon after, his two sons joined the company. Stefano and Alessandro Folio, who run the company today, have seen the firm grow to more than 1,300 employees, with 23 global subsidiaries found in 120 countries. Seko is still a privately held, family-run company that manufactures in five countries.


Current Business: Seko is a manufacturer of chemical metering pumps, controllers, sensors, photometric analyzers, side-channel blowers, AODD pumps, and accessories. It has 330 injection molding machines in its global network of manufacturing sites. These products are used in the commercial/industrial water, pool, and institutional (commercial dishwasher and laundry) industries, as well as API oil and gas industries. Seko pump models consist of peristaltic, solenoid diaphragm, motor-driven diaphragm, and piston pumps. These pumps are used consistently along with our controllers and other products in water/wastewater, cooling towers, boilers, and commercial pool industries. The solenoid pumps have PVDF pump heads, injectors, and foot valves. Diaphragm products are made from Teflon®.
Business Locations: Seko’s USA headquarters is located outside of Philadelphia, Pennsylvania, in Tullytown. Seko also has corporate offices in Italy, Spain, Australia, Brazil, China, Bulgaria, Colombia, France, Germany, Hungary, Japan, Mexico, the Netherlands, Romania, Singapore, South Africa, Thailand, Turkey, United Arab Emirates, and the United Kingdom.
Recognition and Involvement: Seko joined AWT in 2015.
Top Executives: Stefano Folio, CEO; Alessandro Folio, president; Claudio Tomassi, general manager—USA; Andrew Pierro, Water Division sales manager—USA.
66 the ANALYST Volume 30 Number 3 Discovering AWT continued
A Seko production cell in Rieti, Italy.
Seko’s headquarters in Rieti, Italy.
Making a Splash

 Stephanie Choury, MBA Enterprise Account Manager at Buckman Digital Water
Stephanie Choury, MBA Enterprise Account Manager at Buckman Digital Water

What inspired you to begin volunteering with AWT?
During my first conference with AWT, I had the pleasure of meeting Pam Simmons, who introduced me to numerous members, including the remarkable Women of Water (WOW) community. From that moment on, I knew I wanted to be more involved with the Association of Water Technologies and work closely with the Women of Water. Over the years, I have developed a supportive network of colleagues who have continuously empowered my professional growth within the industry.
What has been the most fulfilling aspect of your volunteering experience?
Undoubtedly, it is the people! The annual AWT conference is the highlight of my year. Through volunteering, I have the privilege of engaging with individuals I genuinely enjoy, not just during the conference but throughout the year. By actively participating, I stay well-informed about industry advancements and the focus areas for upcoming conferences and training seminars. It brings immense satisfaction to know that together, we can foster continuous growth and expand our influence within the association.
How has volunteering enhanced your professional career?
Being part of AWT and actively volunteering has significantly broadened my professional network within the industry. Not only have I forged meaningful relationships, but I have also had the privilege of tapping into the wealth of knowledge shared generously by experienced professionals. AWT members truly exemplify a network of individuals who believe in collectively uplifting one another. Like rising tides, they elevate the entire industry.
Why would you encourage others to consider volunteering?
I wholeheartedly encourage others to embrace volunteering because this is an industry that will always give back to you more than you put into it. By giving back, you gain valuable insights into the latest industry trends and connect with like-minded professionals. It is an opportunity to contribute, learn, and grow alongside a community that shares your passion and dedication. You will never regret a moment of time you put into your volunteer efforts.
Could you share details about a current project you or your committee are involved in?
Presently, I am collaborating with Paule Genest, the Chair for Women of Water, organizing the upcoming WOW Reception at the 2023 AWT Conference. Women of Water holds immense significance for me and working closely with this group has showcased the incredible achievements we can accomplish together as a team. It is an honor to collaborate with such exceptional women!
How have you leveraged the expanded business connections you’ve established through volunteering?
My expanded network of connections has proven invaluable not only in my professional endeavors but also in my personal life. I have cultivated a circle of trusted individuals who have consistently inspired me to explore uncharted territories. Their knowledge and willingness to assist have been remarkable. Most recently, when contemplating a career change, it was my involvement with AWT that reaffirmed my passion for this industry and led me to where I am today. Volunteering has helped me discover a niche I am deeply passionate about, enabling me to continually learn and contribute to the field of industrial water treatment.
67 the ANALYST Volume 30 Number 3
Kristyn Roseborough Premier Water & Energy Technology, Inc. Jacksonville, FL
What prompted you to obtain your CWT and when did you begin the process of taking the exam?
Having technical competency is important to me. I’ve wanted to obtain this distinction since day one! One of my roles in our company is the facilitator of the Apprentice Program; this responsibility bolstered my familiarity with water treatment concepts, and, subsequently, applying these concepts and really thinking about the various aspects of water treatment while in the field. This was the onset of preparing for the exam.
Why do you feel this credential was important to have?
The CWT grants credentialing as a water treatment professional and enables me to serve at the highest level of competency.

How did you prepare for the exam?
We have a study group at our company that makes learning FUN!!! Talking about the various topics really helps to remember the material. Also sharing real world experiences and encounters related to the material is helpful to the learning process. I also studied on my own. I read through the Technical Manual (very challenging). I found the manual was easier to read if I was looking for information about something in particular, learning to understand a concept. I read Chuck Brandvold’s book, Water Treatment. I read parts
of the Boiler Water Treatment books by Frayne. I think I completed all the Certification Corner questions in the Analyst starting from 2010 (only the older issues have these questions). I took all the quizzes on the AWT website. I reviewed our Apprentice Program Training materials including the AWT Introduction to Water Treatment Training Modules. And last year, I attended the AWT Training Seminars.
What was the most difficult aspect of the exam?
The breadth and depth of knowledge required to pass this exam.
What advice would you give those thinking about taking the exam?
1. Be Prepared. Be Very Prepared.
2. Go through the test once, then go through a second time to check for careless errors (my coworker gave me this advice, and it was helpful).
68 the ANALYST Volume 30 Number 3 CWT Spotlight
Tales From the Waterside

Recovering from an Acid-Feed Excursion
Steve Dunn Process Performance Management
In 2014, I retired from being a full-time power plant chemist (after 34 years) from a two-unit, coal-fired electric generating station located in Northwest Colorado. After my full-time employment ended, I started power plant chemistry consulting with Process Performance Management of Denver, Colorado. On August 23, 2017, I was called to consult on a coal-fired power plant located in far eastern Kansas that had suffered an extremely low pH excursion in the boiler and boiler feed water systems.

The system was a 3,800-pounds per square inch gauge (psig), 3-pass, once-through boiler that was operating on all-volatile treatment (AVT) (ammonia). The boiler feed water was allowed to be slightly oxidized or slightly positive on the oxidation/ reduction scale, this type of feed water chemical program is known as AVT(o). The system was equipped with deep bed condensate polishers and a condensate particulate filter.
The Incident
The plant was first aware of the incident at about 14:30 (2:30 PM) on August 23, when multiple on-line conductivity analyzer alarms started sounding throughout the condensate/feed water system. By 15:00 (3:00 PM), the on-line condensate/
feed water specific conductivity meters were reading 999 microsiemens per centimeter (µS/cm) (pegged out). Wet testing confirmed that the conductivity was extremely high everywhere in the boiler and boiler feed water system. The wet test pH at the condensate pump discharge and the economizer inlet was measured at around 3. The unit load was reduced to 400 megawatts (MW) at 25 MW per minute, there upon the fires were tripped. The unit was offline by about 16:40 (4:40 PM). The condensate storage tank pH was measured at 2.7 in the north tank and 5.4 in the south tank. At 16:45 (4:45 PM), the boiler feed water system pH had risen to about 4, due to ammonia injection. The boiler and boiler feed water systems were then drained as much as possible.
69 the ANALYST
30 Number 3
Volume
Power plant
Cause of the Excursion
A 93% sulfuric acid pump used for polisher regeneration and for polisher regeneration tank neutralization had been turned on earlier in the day for caustic neutralization in the tank. Unknown to the operator that started the pump, a very seldom used acid feed line valve at the neutralizing tank had been closed due to the valve having a leak. The acid pump was of the diaphragm type and was capable of producing exceedingly high pressure in the pump discharge piping.
In this incident, the high pressure in the acid line due to the closed valve at the neutralization tank, was able to flow acid through a 3-inch check valve into the condensate dilution water line used in the polisher regeneration system. The condensate dilution water control valve was open at the time, due to a then unknown fault in the system logic. At the dilution water pumps, there was a recirculation line that was open, that allowed the acid to flow around the pumps into the condensate tank supply line. If the pump recirculation line had been closed, the check ball valves in the dilution water pumps would have stopped the acid at that point. The acid was then pushed back through the condensate supply line to the north condensate storage tank. The south tank was mostly protected by a check valve at the tank.
As one can see, this is one of those perfect-storm scenarios, where things had to be lined up exactly

right to cause the acid to be able to flow back into the condensate storage tank. At several points in the dilution water system, the acid could have been stopped if a particular control point valve had been closed or did not leak through. At the time, the operator doing the neutralization was puzzled by the lack of change in the neutralization tank, as it had always rapidly changed before. An investigation of the neutralization problem was ongoing when the acid contamination incident occurred.
Recovery
After the cause of the condensate storage tank contamination was discovered, the condensate tanks and lines were flushed until the tanks and lines returned to normal conductivity. This operation was not completed until Friday, August 25th. The main boiler and the startup boilers had tube leaks. The condensate polishers were expended.
On Saturday, Sunday, Monday, August 26–28, the plant was flushing the condenser hotwell and other parts of the condensate system while raising the pH with ammonia. Previously dissolved iron, due to the low pH, started to precipitate into the flush water, rapidly plugging the condensate particulate filter and the condensate polishers. The plant was continuously working on restoring the condensate polishers, regeneration required 8–12 hours per polisher. The main boiler tube leaks were repaired, as work continued on the startup boilers tube leaks.
By Tuesday, August 29, the condensate system was considered sufficiently clean to allow the plant to start flushing the boiler feed water system back through the condenser hotwell. Some of the condensate polishers were back in service, however the particulate filter would plug up in eight hours or less. Ammonia addition continued to be used to raise the pH of the flush water. The leaking tubes in the startup boilers were repaired.
70 the ANALYST Volume 30 Number 3 Tales from the Waterside continued
Power plant Control Room
By Monday, September 4, the condensate system and the boiler feed water system were clean enough to allow flushing to start on the boiler. Once again, the flush water pH was driven low, conductivity high, and particulates high. The condensate particulate filter was again plugging rapidly, and the condensate polishers were being expended faster than they could be regenerated.
By Wednesday, September 7, the boiler, boiler feed water, and condensate systems were clean enough to start steaming the boiler. The pH of the water was 9 or above in all the boiler systems, specific conductivity was about 20 µS/cm, cation conductivity was less than 1 µS/cm, and particulates ware less than 100 parts per billion (ppb). As steam from the startup boilers started to heat the main boiler the system, the pH again went low, the conductivity high, and the particulates high. As the main boiler began to steam, the steam was diverted to the hotwell and not allowed to go to the turbine. The struggle to maintain the condensate polishers and the particulate filter in service continued.
By Saturday, September 9, the chemistry of the boiler systems was clean enough to allow some steam to the turbine. This time, as steam pressure steadily increased, system chemistry rapidity improved as condensate polisher and particulate filter run times were able to increase due to lower contaminant load.
By the late afternoon of Monday, September 11, the chemistry conditions were good enough for 300 MW of generation. The economizer inlet pH was 9.5, specific conductivity about 7 µS/cm, cation conductivity less than 0.5 µS/cm, and particulates were less than 100 ppb.
On Friday, September 15, the unit load was able to be raised to 550 MW and chemistry was steadily improving.
The positive trends continued, and by Thursday, September 21, the unit was back to full load. There were no longer any chemistry restrictions on unit operation.
Summary
The incident caused the plant to be away from full load operation for about a month. The incident took a perfect storm of conditions to allow the acid contamination
to occur, but the incident could have been prevented if procedures and equipment had functioned as intended. The conditions that caused the incident had obviously been present for many years without any problems at all.
It makes me wonder how many other plants may be operating in a comparable situation, one closed valve away from disaster. Of course, the plant took many steps to ensure that the incident could not happen again, including procedural and system design changes. However, it was an expensive lesson, many hours of generation lost, plus the cleanup and maintenance expenses.
My main takeaways from this incident were as follows:
Do not be complacent, if something seems wrong (neutralization tank not reacting as expected) shut things down until the issue is resolved.
The importance of communication—if all the operators had known that the acid valve at the neutralization tank was closed, the incident most certainly would have been avoided.
So, if you are operating acid regeneration or other acid dilution systems, take the time to learn from this incident, check piping diagrams for possible paths for contamination and check procedures to ensure that block valves are closed between regenerations.
Author Steve Dunn is a semi-retired (2014) power plant chemist and is still consulting part time on power plant chemistry issues. He has more than 48 years of experience in managing coal-fired power plant boiler, pre-boiler, and cooling water system chemistries. Mr. Dunn has authored a number of presentations for power plant chemistry conferences and several magazine articles over the years. He graduated from Peru State College (Peru, Neraska) in 1980 with a B.S. in physical science.
Note: The power plant pictures used in the layout shown in this article was not the one where in incident discussed in the column occurred.
Keywords: ACID, BOILERS, CAUSTICS, CONDUCTIVITY, ION EXCHANGE, OUTAGES, POWER, REGENERATION
71 the ANALYST Volume 30 Number 3 Tales from the Waterside continued
Lessons Learned from the Power and Industrial Steam Generation Industries
Brad Buecker, Buecker & Associates
A large part of my career (so far) has included nearly two decades at two coal-fired power plants and a chemical plant, another seven years reviewing water treatment and steam generation chemistry specifications and guidelines for combined cycle power plants, and finally investigation of industrial steam generation chemistry issues. That background translates into quite a few lessons learned for this old (I mean, experienced) guy. Many of my fellow Baby Boomers have recently retired or will soon be retiring and are taking much knowledge with them, leaving difficult challenges for new plant personnel. This article offers some of my lessons learned, supplemented by experiences from colleagues. Included are a number of comparisons between high-pressure and low-pressure boiler applications.
Lessons
1. Don’t Neglect Makeup System Operation and Maintenance
For high-pressure (a commonly accepted lower limit is 900 pounds per square inch gauge [psig]) utility/ industrial steam generators, high-purity makeup water (dissolved solids in low parts-per-billion [ppb] concentrations) is essential to minimize corrosion and impurity carryover into steam. The now common core configuration to produce high-purity water is reverse osmosis (RO), followed by mixed-bed ion exchange (MBIX) or electrodeionization (EDI) polishing.
One lesson that is still being learned by some industry personnel is that RO membranes require reliable pretreatment to minimize particulate accumulation, microbiological fouling, and scale formation. The author and a colleague will be addressing many of these details

in a future issue of the Analyst. Accordingly, the focus in this section is on low-pressure makeup treatment and specifically sodium softening.
L ower-pressure boilers (<600 psig) can tolerate moderate levels of impurities, and so basic sodium softening is a common makeup method. However, consider the following example that has been observed many times, including by this author: A water treatment expert is called in because the plant’s boiler(s) is suffering from tube failures. Two items the investigator will quickly examine are the makeup system operational and effluent chemistry logs, if available. The logs often show repeated or sometimes long-term softener upsets, which release hardness ions to the feedwater and boiler. The typical results of such excursions are illustrated in Figures 1 and 2.
72 the ANALYST Volume 30 Number 3 Technical Updates, Tips, or Reviews T.U.T.O.R.
Figure 1: Layered boiler tube deposits from multiple hardness excursions. Photo courtesy of ChemTreat, Inc.
These deposits are very insulating and can induce overheating and metal creep in boiler tubes that lead to blistering and failure (Figure 2).
Much more horrifying are known cases where, during a makeup system malfunction, a manager has directed that the system be bypassed, with direct raw water feed to the boilers. The outcome of such decisions can be catastrophic.
An obvious lesson from this discussion is the importance of conscientious operation and monitoring of makeup water systems. From a more modern perspective, RO is gaining ground as a replacement for sodium softening. A key aspect of RO is that it removes the vast bulk of all ions, not just hardness. This feature typically allows for better control of boiler water chemistry, albeit, as the next section illustrates, condensate return impurity ingress may be a larger concern.
2. Condensate Return Chemistry Can Be a Makeor-Break Issue
For most power units, the boiler/steam/condensate/ feedwater network is nearly a closed loop, with the usually small losses made up with high-purity water. The primary source of potential contamination is the steam surface condenser (unless the unit is equipped with an air-cooled condenser [ACC]). Raw cooling water ingress can, of course, introduce many impurities to condensate, including hardness, chloride, and sulfate, among others. Unfortunately, the author continues to hear of units being allowed to operate with a condenser tube leak. Plant management simply does not fully comprehend the serious drawbacks of such decisions. Consider the following case history from a relatively low-pressure power boiler in the early 1980s.
Case History #1
An 80-megawatt (MW) power generating unit supplied by a 1,250-psig coal-fired drum boiler had just been returned to service from a scheduled autumn outage.
Laboratory personnel discovered that a condenser leak was introducing contaminants to the condensate. Consequently, the condensate/feedwater total dissolved solids (TDS) concentrations at times reached 0.75 partsper-million (ppm). Although the lab staff requested that the boiler be taken offline immediately, operations management refused due to load demand issues.
The boiler was on congruent phosphate treatment at the time, so lab personnel increased monitoring frequency and worked to maintain proper phosphate and pH levels, the latter within a range of 9.2 to 9.6 by essentially feeding tri-sodium phosphate only. After approximately three weeks, an operator discovered the source of the leak (an open valve on the drain line from the condenser hotwell to the cooling water outlet tunnel that allowed the condenser vacuum to pull in cooling water) and corrected the problem. Two months later, boiler waterwall tubes began to fail with alarming frequency. The failures happened so regularly that plant management scheduled an emergency tube replacement during the upcoming spring outage. The repairs, which required a complete boiler re-tubing, cost more than $2,000,000. (Imagine the costs today, and especially for a large unit.)
Subsequent metallurgical analyses confirmed that the failures were due to hydrogen damage. Principally, chloride will concentrate underneath waterwall tube deposits, where the following reaction may occur (Equation 1). MgCl2 +

A product of the reaction is hydrochloric acid. HCl can, of course, cause substantial corrosion by itself, but because the acid concentrates under deposits, it will react with iron to generate hydrogen, which in turn leads to hydrogen damage. In this mechanism, hydrogen atoms penetrate into the metal where they then react with carbon atoms in the steel to generate methane (CH4) as illustrated in Equation 2:
Formation of the gaseous methane causes cracking, greatly weakening the steel’s strength. Hydrogen damage is very troublesome because it cannot be easily detected.
73 the ANALYST Volume 30 Number 3 T.U.T.O.R. continued
Figure 2: Boiler tube blisters caused by internal scale. Photo courtesy of ChemTreat, Inc.
2H2O + heat → Mg(OH)2↓ + 2HCl Eq. 1
4H + Fe3C → 3Fe + CH4 Eq. 2
After corrosion has occurred, the plant staff may replace tubes only to find that other tubes continue to rupture. To re-emphasize, this example was for a low-pressure power boiler. The effects of chemistry upsets usually become more pronounced with increasing pressure and temperature. Under-deposit corrosion/hydrogen damage is still a leading boiler corrosion mechanism globally.

This case history offers another lesson learned: It is apparent that unit startup procedures at that time were not completely comprehensive, as the drain valve from the condenser hotwell to the cooling water discharge should never have been left open upon startup. For any unit, meticulous written shutdown and startup procedures are necessary to prevent such oversights.
Also, highly important for steam generator protection from chemistry upsets is on-line water/steam sample monitoring, with monitoring systems designed to instantly relay data to operators and trained personnel for quick reactions. This topic will appear soon in a future issue of the Analyst
While the steam network for power boilers is essentially a closed loop, the situation is usually much different for co-gen and industrial steam generators, per multiple return condensate lines, as illustrated in Figure 4.
Depending on the products manufactured at the plant, condensate return can contain a wide variety of impurities introduced from leaks in heat exchangers and other equipment. The second case history outlines a classic example of industrial boiler condensate contamination that power industry personnel usually never have to consider.
Case History #2
A number of years ago, the author and a colleague were invited to an organic chemicals plant that had four 550-psig package boilers with superheaters. The steam provided energy to multiple plant heat exchangers, with recovery of most of the condensate. Each of the boiler superheaters failed, on average, every 1.5–2 years from internal deposition within the tubes. Inspection of an extracted superheater tube bundle in a laydown area revealed deposits of approximately ⅛ to ¼ inches in depth.
Furthermore, during our walkaround we observed foam issuing forth from the saturated steam sample line of each boiler. The American Society of Mechanical Engineers (ASME) industrial boiler water guidelines recommend an upper limit of 0.5 ppm total organic carbon (TOC) in the feedwater of boilers at that pressure. (ASME, 1) Previous data from the plant’s water treatment vendor showed TOC levels of up to 200 ppm in the condensate return. Based on this data alone, it was easy to deduce why foam was issuing from the steam sample lines, and why the superheaters rapidly accumulated solids and failed from overheating.
No treatment processes or condensate polishing systems were in place to remove these organics (five phenol derivatives) upstream of the boilers. The plant staff also confirmed that the condensate return was not configured to allow dumping. Admittedly, installation of any treatment or dumping systems would have been costly and caused major operating changes. Significant testing would have been needed to evaluate polishing methods. Activated carbon filtration (ACF) is suitable for some organics, but testing would have been needed to ascertain ACF’s effectiveness on the small phenol molecules.
Alternatively, resins—similar to ion exchange resins but without the active sites—have been developed in which the tiny passageways can adsorb organic molecules. Perhaps such resins would have been effective, but other factors such as capacity, kinetics, and condensate temperature needed evaluation. Condensate dumping would have required installation of a much larger makeup treatment system. Raw water availability and chemistry become critical issues for makeup system modifications. Plant management appeared to be searching for a “magic bullet” that would solve the
74 the ANALYST Volume 30 Number 3 T.U.T.O.R. continued
Figure 3: Tube failure due to hydrogen damage. Note the thick-lipped failure, which is indicative of metal weakening with little metal loss. Photo courtesy of ChemTreat, Inc.
problem, and thus they did not authorize evaluation of these options or others, at least at that time.
A key takeaway from this section and the previous discussion about makeup water treatment is that plant personnel often focus intently on process chemistry and engineering to the neglect of water/steam system treatment and chemistry. Then, when severe upsets or failures occur, management goes into reactionary mode. The same is often also true for cooling systems.

3. Only Use Oxygen Scavengers When Necessary, and Then Be Careful
Ever since humans developed iron and then steel for utensils, tools, and infrastructure purposes, corrosion— visually observable as rust—has been problematic. Of course, many steel alloys have evolved over the years to mitigate oxygen attack. However, in the balance between material strength, economics, and corrosion control, mild steel became the primary choice for much of the piping and other components in power and industrial plants. Uncontrolled oxygen ingress can cause severe corrosion.
Oxygen attack can obviously be very damaging. Accordingly, mechanical deaerators became de rigueur for most steam generating systems. The common guideline for dissolved oxygen (DO) concentration from a properly designed and maintained deaerator is 7 ppb. But this level was still considered excessive and served as the impetus for supplemental use of chemical oxygen scavengers (the proper term is reducing agents) to reduce DO to zero. At one time, the favored compound was hydrazine (N2H4), which reacts with oxygen as shown in

75 the ANALYST Volume 30 Number 3 T.U.T.O.R. continued
Figure 5: Oxygen pitting in a boiler feedwater line. Photo courtesy of ChemTreat, Inc.
Figure 4: General schematic of an industrial steam generation network with steam transport to various processes. Illustration courtesy of ChemTreat.
Equation 3. Reaction kinetics and efficacy increase with increasing temperature.
N2H4 + O2 → 2H2O + N2↑ Eq. 3 Also, hydrazine will passivate oxidized areas of piping and boiler internals as illustrated in Equation 4.
N2H4 + 6Fe2O3 → 4Fe3O4 + N2↑ + 2H2O Eq. 4
Fe3O4 forms the dark magnetite layer that became familiar to steam generation chemists. (Hydrazine will also reduce oxidized copper (cupric oxide, CuO) to its more passive state, cuprous oxide (Cu 2O).
Hydrazine became suspected as a potential carcinogen and is now registered as a hazardous compound. This turn of events led to development of other reducing agents, including carbohydrazide, hydroquinone, and methyl ethyl ketoxime. All of these compounds passivate metals.
Researchers also determined that pH and temperature significantly influence carbon steel corrosion, as illustrated in the following very well-known chart (Figure 6).

We will return to the temperature influence later. In the meantime, for conventional coal-fired power units, ammonia became (but not always exclusively) the primary reagent for feedwater pH control (Equation 5).
NH3 + H2O ⇌ NH4+ + OH - Eq. 5 This is a reversible reaction and ammonia only dissociates to a relatively small extent.
For industrial steam generators, ammonia alternatives are typical for pH control. These are the neutralizing (now often referred to as alkalizing) amines. They are less volatile than ammonia and can often provide better condensate chemistry control. Common compounds are shown in Figure 7.
The combination of ammonia (or alkalizing amine) for pH control and reducing agent feed for oxygen corrosion control is known as all-volatile treatment reducing [AVT(R)]. By the early 1980s, power plant chemists, particularly here in the United States, had settled on this chemistry as the best answer for feedwater corrosion control. This thinking was forever altered in 1986, for “On December 9 of that year, an elbow in the condensate system ruptured at the Surry Nuclear Power Station (near Rushmere, Virginia). The failure caused four fatalities and tens of millions of dollars in repair costs and lost revenues” (4). Researchers learned from that accident, and others since, that the reducing environment produced by oxygen scavengers is the primary ingredient for single-phase flow-accelerated corrosion (FAC) of carbon steel.
76 the ANALYST Volume 30 Number 3 T.U.T.O.R. continued
Figure 6: Influence of temperature and pH on iron dissolution from carbon steel. (Sturla, 2)
Amine Dimethylamine Ethanolamine 5-Aminopentanol 3-Methoxypropylamine Morpholine Cyclohexylamine C2H7N C2H7NO C5H13NO C4H11NO C4H9NO C6H11NH2 45.08 61.08 103.16 89.14 87.1 99.2 HO O NH2 NH2 H2N HN OH Structure Chemical Formula Molecular Weight (g/mol) H3C CH3 NH O N2H H3C
Figure 7: List of common alkalizing amines. A more detailed description of the amine properties, and especially in relation to steam generation chemistry, is available in Reference 3.
The attack occurs at flow disturbances such as elbows in feedwater piping and economizers, feedwater heater drains, locations downstream of valves and reducing fittings, attemperator piping; and, most notably for combined-cycle heat recovery steam generators (HRSG), in economizers and low-pressure evaporators, and particularly the many short-radius elbows. (Note that these locations correspond with the temperature influence shown in Figure 6.) For HRSGs, single-phase FAC (and in some locations a phenomenon known as two-phase FAC) is frequently the leading corrosion mechanism.
This leads to a very important lesson learned, that was adopted at many plants but is still lacking at many others. Researchers in Germany and Russia in the early 1970s discovered that for supercritical steam boilers with high-purity (cation conductivity ≤ 0.15 µS/cm) feedwater and no copper alloys in the condensate/feedwater system, a small amount of deliberately injected dissolved oxygen to the feedwater (and elimination of reducing agent feed) would cause magnetite to become overlayed with a much stronger layer of ferric oxide hydrate (FeOOH) (6). A common term for this program became oxygenated treatment (OT).
The Electric Power Research Institute (EPRI) subsequently developed an offshoot, all-volatile treatment oxidizing (AVT(O)), in which the (typically) small amount of oxygen that enters via condenser air in-leakage is allowed to remain, again with elimination of reducing agent feed. The feedwater purity requirement is slightly relaxed (cation conductivity ≤0.20 µS/cm),
but supplemental oxygen injection, especially to the economizers of multi-pressure HRSGs, may be needed.
The change from reducing to oxidizing chemistry has greatly minimized (but not always eliminated) FAC at many plants. Further details about these programs are beyond the scope of this article, but the critical idea for this discussion is that even though OT and AVT(O) chemistries are well known and documented, many HRSGs around the world are still operated with AVT(R) feedwater chemistry. Very few, if any combined cycle HRSGs have copper alloys in the feedwater network, so there is no need for AVT(R) in these units, and it is not recommended.
Although industrial steam generators operate at lower pressures than utility units, FAC and the potential for failures that can release extremely hot feedwater should not be discounted. Typically, AVT(R) feedwater chemistry is required because makeup water and condensate do not have the purity required for AVT(O). The presence of heat exchangers with copper-alloy tubes also eliminates AVT(O) consideration. But AVT(R) can generate FAC in industrial steam generating networks (7). This reference outlines condensate and feedwater chemistry improvements at a cogeneration plant that supplies approximately half of a university campus and two university hospitals. Recent ultrasonic thickness testing of feedwater elbows and other locations has identified mild FAC. Conditions at other plants may be better or worse.
Technology that has emerged and evolved over the last two decades or so uses film-forming products, amines, or alternative compounds, to protect steam generator metal surfaces. Both success and failure stories have been reported. The film-formers are not a cure-all for corrosion mitigation, and the good chemistry practices mentioned above remain important. (Additional information may be found in Reference 8.)

4. Off-Line Corrosion Protection
Frequently overlooked is off-line corrosion protection, which can be very problematic in many steam generators, including HRSGs that frequently cycle on and off. Units can be protected for short (perhaps overnight) outages if they can be bottled up to retain heat, and if vacuum can be maintained in the condenser. But for other situations,
77 the ANALYST Volume 30 Number 3 T.U.T.O.R. continued
Figure 8: FAC-induced sudden rupture of a feedwater line. Source: Reference 5.
air ingress to steam generators as they cool will initiate serious corrosion of tubes, piping, and additional equipment. Furthermore, and this is a situation I observed many times, water that is allowed to remain in the condenser hotwell after vacuum is broken will generate humid conditions. The humidity moistens salt deposits, typically chlorides and sulfates, on low-pressure turbine blades and rotors. The moistened deposits then can induce pitting, which can lead to stress corrosion cracking and corrosion fatigue. Yet, as EPRI and other organizations have reported, only a minority of power plants have comprehensive shutdown, layup, and startup procedures to protect units while they are off-line.
The discussion below briefly highlights layup measures instituted at a combined-cycle power plant more than a decade ago, but in which the measures are still quite relevant now (9). When a unit comes offline and begins to cool, air may be drawn in at even the smallest openings. The oxygen then attacks metal at stagnant locations, with severe localized corrosion a common result. Upon startup, the corrosion products may then be transported to the boiler and form deposits, which, as we have already seen, may influence under-deposit corrosion.
In many technical guidance documents, the leading recommendation for minimizing air ingress is nitrogen blanketing. The two-unit plant (with dual-pressure HRSGs) in Reference 9 cycles on and off frequently, and often daily. Nitrogen blanketing is implemented during the last stages of shutdown and subsequent short-term layups (<72 hours). Experience has shown that introduction of nitrogen to key points in the system before the pressure has totally decayed will
minimize ingress of air. Key nitrogen injection points are evaporator drum vents, economizers, and feedwater circuits. Nitrogen is sometimes used to “push” water from an HRSG during dry layup draining. A nitrogen pressure of 5 psig is maintained during the dry layup, provided no major tube work is required.
An obvious major concern with nitrogen blanketing is safety. Of course, elemental nitrogen is not poisonous, as it constitutes 78% by volume of earth’s atmosphere. However, an individual who enters a confined space where nitrogen has not been purged may pass out quickly due to lack of oxygen. Death can occur within minutes. To-the-letter adherence of confined space entry procedures is an absolute must for systems protected with nitrogen, as it should be for all other confined spaces.
Another source of oxygen ingress is from atmospherically vented condensate storage tanks. Oxygen-laden water can enter during normal operation and especially during boiler filling. Methods have been developed to limit oxygen ingress to storage tanks, including floating water covers and nitrogen blanketing, but they can be inefficient or present control difficulties.
In this case, plant personnel selected a gas transfer membrane (GTM) technology to treat condensate return and makeup water (Figure 9).
As the condensate flows along the hollow-fiber membranes in the vessel, gases pass through the membrane walls, but water does not. The unit reduces makeup dissolved oxygen concentrations to less than 10 ppb.

78 the ANALYST Volume 30 Number 3 T.U.T.O.R. continued
Figure 9: Cutaway view of GTM unit for dissolved oxygen removal. Source: www.liquicel.com. Note: The site offers an excellent animated view of the process.
The plant staff also addressed protection of the low-pressure turbine from humidity by installing warm, desiccated air circulation to the condenser/LP turbine during all but short-term layups.
References
1. ASME (2021). Consensus on Operating Practices for the Control of Feedwater and Boiler Water Chemistry in Modern Industrial Boilers, The American Society of Mechanical Engineers, New York, New York.
2. Sturla, P. (1973). Proceedings of the Fifth National Feedwater Conference, Prague, Czechoslovakia.
3. Buecker, B.; Shulder, S. (2023). “Remember the 3Ds of Alkalizing Amines: Dissociation, Distribution, and Decomposition,” PPChem Journal, 25/2023 – No. 1.
4. EPRI (2017). “Guidelines for Control of Flow-Accelerated Corrosion in Fossil and Combined Cycle Power Plants,” EPRI Technical Report 3002011569, the Electric Power Research Institute, Palo Alto, California. Note: This document is available to the industry as a free report because FAC is such an important safety issue.
5. Buecker, B.; Shulder, S.; Sieben, A. (June 4-6, 2019). “Fossil Power Plant Cycle Chemistry,” pre-conference seminar for the 39th Annual Electric Utility Chemistry Workshop, Champaign, Illinois.

6. International Association for the Properties of Water and Steam (2015). Technical Guidance Document: Volatile Treatments for the Steam-Water Circuits of Fossil and Combined Cycle/HRSG Power Plants.
7. Buecker, B.; Murphy, F.; Mohammed, N. (July/August 2019). “Steam Chemistry Advancements at UIC,” Industrial WaterWorld (now Water Technology).
8. International Association for the Properties of Water and Steam (n.d.). Technical Guidance Document: Application of Film Forming Amines in Fossil and Combined Cycle Plants, IAPWS TGD8-16.
9. Buecker, B.; Dixon, D. (August 2012). “Combined-Cycle HRSG Shutdown, Layup,
This system can lower the relative humidity from nearly 100% to less than 30% in just a few hours. While protection of the LP turbine from humidity is of primary importance, some guidelines from research organizations outline methods to arrange dry air circulation throughout the entire steam-generating network. However, this involves propping open steam stop valves and other equipment, which may be too complicated or time-consuming for many plants.

This section covered just some of the many details that may be involved in unit shutdowns, layups, and startups. Look for a future article discussing additional layup topics.
Conclusion
This article outlined several critical lessons learned that are often not thoroughly considered by plant designers, owners, and operators. Water and steam are the lifeblood of many plants, but sometimes too much focus is on process issues until a water/steam system failure impacts plant production or threatens employee safety. Injury or death is the ultimate cost.
Brad Buecker is president of Buecker & Associates, LLC, consulting and technical writing/marketing. Most recently he served as senior technical publicist with ChemTreat, Inc. He has more than four decades of experience in or supporting the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering positions at two coal-fired power plants. His work also included 11 years with two engineering firms, Burns & McDonnell and Kiewit, and he also spent two years as acting water/wastewater supervisor at a chemical plant. Mr. Buecker has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He has authored or co-authored more than 250 articles for various technical trade magazines, including the Analyst, Industrial Water Treatment, and Ultrapure Water Journal. Mr. Buecker has also written three books on power plant chemistry and air pollution control. He is a member of the ACS, AIChE, AIST, ASME, NACE (now AMPP), and the Electric Utility Chemistry Workshop planning committee. He may be reached at beakertoo@aol.com .
Keywords: BOILERS, CORROSION, OXYGEN, POWER, SAFETY, SCALING, STEAM
79 the ANALYST Volume 30 Number 3 T.U.T.O.R. continued
Figure 10: Desiccant wheel dehumidifier installed at the plant. Photo by Dan Dixon, formerly of Lincoln Electric System and now EPRI.
and Startup Chemistry Control,” Power Engineering
What’s (Water) on Your Mind?
What is the Impact of Testing Only Calcium Hardness after a Water Softener?
 Compiled by James McDonald, Chem-Aqua
Compiled by James McDonald, Chem-Aqua
Note: The following discussions come from the Industrial Water Treatment interest group on LinkedIn.
Question of the Week
What could be the impact of testing calcium hardness after a water softener INSTEAD of the standard total hardness?
Stephanus: The softening resin’s affinity for calcium is about twice that for magnesium. This means that calcium levels may still seem fine while magnesium levels are rising undetected.
Paul: Total hardness gives you calcium hardness plus magnesium hardness. Calcium hardness is very important in cooling towers; but calcium and magnesium hardness or total hardness is important in boilers, where magnesium hydroxide can form.
Ali: It can be said that almost two-thirds of water hardness is related to calcium hardness.
Arvid: Magnesium hardness, or lack of it, is important in on-site sodium hypochlorite generation.
Question of the Week
How do you calculate the expected pH in a cooling tower when you’re given a makeup water analysis?
Jim: There are several calculations that have been published over the years for converting alkalinity to pH by well-known sources: Caplan; Cavano; Kunz; Vanderpool; AWT; and Calgon.
Todd: Typically, it is associated with the alkalinity of the water, the cycles of concentration, and the amount of acid that is added to neutralize the alkalinity. If one wants to go in depth, one needs to know the concentration of all the di- and polyprotic acids that will buffer the water, the hardness that may scale out the alkalinity, and temperature effects, to name a few.
Mitchell: How can you predict the pH with any accuracy unless you know the contribution to the pH from the treatment chemicals?
Chris: Years ago, I found this relationship: log(MAlk) = 0.55 x pH–2.27. Try it. It worked in most of my cooling tower systems.
Ali: Pourbaix graph.
Question of the Week
What will happen if you load RO membranes backwards, and how can you recover from it?
Stephanus: The lip-seals can only seal in one direction. When installed backwards, a portion of the feed water is bypassed and not forced past the surface of the membrane. Here is what happens: lower crossflow velocity higher polarization more scaling + fouling and lower permeate quality. To correct, remove and swap membranes around. Remember shims on feed side. CIP (clean in place) if required.
Damon: Just to clarify the question, is loading referring to the installation of the physical membrane, with the
80 the ANALYST Volume 30 Number 3
brine seal being placed on the outlet side of the PV (pressure vessel) versus the feed side? Or is it referring if the membrane is being fed through the permeate tube? I would think if it were an orientation issue, you would not damage the membrane but would get feed water bypassing around the membranes, seeing less product water, increased reject, and a not expected pressure drop across the system. To fix the issue you need to remove the membrane(s) and re-install with the brine seal on the feed side of the PV. Damage would be seen if feeding through the permeate tube as the membrane envelope is not designed to flow in that direction.
Todd: The best answer is to start pushing membranes and time to get wet. Hopefully, you catch it quickly and do not damage the membranes.
Joaquim: Well, if the membranes are loaded in the backward direction in the pressure vessel, the membranes will not be protected by the thrust collar since the thrust collar will be on the feed side instead of the end side, in this way the membranes certainly will telescope. To overcome this problem, the membranes position must be changed immediately.
Andy : You will know! The quality coming off the permeate will be poor and at a lower flow rates. If you catch it on startup, simply put them in the right direction. If left in this position too long, they will telescope and only be good for the trash bin. This matter also depends on the type/spec of the membranes.
Sam: I would think if you have membranes being loaded backwards, you have training/QC (quality control) issues that need to be resolved.
Question of the Week
Will carbonate- and bicarbonate-based alkalinity cycle up in cooling tower the same as other cyclable parameters?
Chris: As water cycles up, the pH rises. So, bicarbonate alkalinity will convert to carbonate alkalinity, and all will cycle up as total alkalinity. Acid addition will control alkalinity and pH.
Paul: Cycles of concentration based on sodium, magnesium, nitrate, sulfate, conductivity, and total dissolved solids may give similar numbers: cycles of
concentration based on calcium; M alkalinity; and chloride (heavy chlorination). These are less dependable due to the formation of calcium carbonate or calcium phosphate.
Question of the Week
What are the do’s and don’ts of using oxidizing biocides in closed loops?
Musaab: The use of oxidizing biocides is not a good choice when the inhibitor program contains nitrite, since the oxidant will react quickly with the nitrite. While oxidizing biocides can be used with molybdateonly programs. it is not recommended to use halogens on a regular, frequent basis in a closed system. The repeated use of halogen-based biocides can cause a build-up of chloride or bromide in the system and also can oxidize the copper if the metallurgy is made up of brass. Therefore, non-oxidizing biocides should be used for closed system treatment. Glutaraldehyde and isothiazoline have been found to work very well for microbial control in closed-loop systems. DBNPA is very effective and has been used in some closed systems; however, it is important to understand the half-life of DBNPA is greatly reduced above pH 8.5.
Brett: Depends if this is new closed loop piping or existing. It is common for hospitals or healthcare facilities to perform a chlorinated flush of newly installed piping. However, this water should not be left in the piping and should be flushed and refilled with fresh city water.
Most online closed loops, hot or chilled, should only be fed a non-oxidizing biocide. I would recommend a lower kill rate biocide, such as isothiazoline or glutaraldehyde, in order to reduce the risk of sloughing off too much biofilm at one time, which could plug up heat exchangers and/or filters.
Larger chilled water loops, example: ones connected to a Thermal Energy Storage tank, may be fed Chlorine Dioxide (ClO2). Careful consideration should be made when deciding the location of the ClO2 feed system and whether totes or onsite generators are used.
Javed: Foaming issues are also a concern if we use an oxidizing biocide. That’s the reason I always recommend non-ox biocides.
81 the ANALYST Volume 30 Number 3 What’s (Water) on Your Mind?
Question of the Day
How often should various components of your test kit be calibrated (e.g., conductivity meter, pH meter, colorimeter)?
Frank : The smart alec answer is “RTFM”— Read the Free Manual. The owner’s manual(s) usually states at least daily for pH and less often for conductivity. Most of the folks at the AWT training tell me they calibrate pH daily or weekly and conductivity weekly or monthly. Checking against known standards on all testing meters and kits is a great idea, especially if you notice any inconsistent results. Great topic.
Chris: I think an important addition to this discussion is that 2-point calibrations should always be used and that, best practices, the standards should “bracket” the actual measurements. pH meters measuring cooling waters from, say, 7.5 to 9.2 should use 7 and 10 standards. Conductivity meters measuring pure waters and using a single 3,000 µmhos (micromhos) standard are probably not going to give good results at this low level. My opinion: Calibration of ORP meters are best done at 300 and 600 mV at least monthly. Handheld PTSA and fluoresceine meters daily but continuous analyzers monthly, using DI water and a calibration standard above set point.
Catherine: Depends on usage and specifications of the meter. But generally, colorimeters should have a calibration check yearly. For traditional pH meters and conductivity meters, they should be calibrated as often as is practical but definitely before big changes in measurement ranges. For digital probes, the length of time between calibration can be increased but users should be mindful of the waters they are testing, particularly taking into account residues that could “contaminate the probe” and like traditional meters, jumping between systems with very different ranges can affect calibration.
Steve: More often than I do. If there are no “irregularities” with the many tests done each day, calibration is out of my mind. The ideal person would have calibration done at regular intervals. I cheat by sometimes using client equipment to check against mine.
David: Letter of the law, daily. What happens in reality is another matter.
Craig : Volumetric glassware: Calibrated once, at a specific atm (atmospheric) pressure, temperature and humidity. If temperature changes, then the glassware requires re-calibration. Standardization is irrelevant.
ICP-OES: Standardized every 4 hours, calibrated once a month. pH probe: standardized once a day, OR (and this is important) depending on the pH of the solution. So, for example, if I’m below a pH of 4 for a while, then I’ll re-standardize if I know I’m going above 9. Never calibrated. ORP Probe: Can’t do either, but you can check the drift using a calibrated solution of quinoline at a specific pH. Automatic Pipette: Calibrated once a month, never standardized.
Question of the Week
What is the definition of high-purity water?
Arivintharan: Depends on the set industrial standards, such as in Malaysia where we have either A or B, A being a stricter set of parameters and B being slightly less strict than A, the difference is what industry and location of said industry.
Keith: That depends on who you ask. I’m not being cheeky either.
Stephanus: I do not know if there is a technical definition for “high-purity” water, but ASTM D1139 describes reagent water of Types 1 to 4 with EC of 0.056 to 5 µS/cm (microsiemens per centimeter). (It also has limits for TOC, Si, Na, and others). ISO (International Standards Organization) has a similar standard with that is referred to as pure, ultrapure, among other terms.
Doug : Lacking dissolved and suspended material.
Afifa : The purest form of water that can be produced through filtration, which means that the water does not contain minerals and chemical substances.
Abilash: Depending on application... 0.1 to 5 µS/cm.
Ali: Electrical conductivity is equal to or less than 5 microsiemens.
Lauren: Treatment steps beyond RO such as EDI (electrodeionization).
82 the ANALYST Volume 30 Number 3 What’s (Water) on Your Mind? continued
Nikhil: Water that has undergone extensive purification, with little or no dissolved solids or pollutants, is referred to as high-purity water.
Sachin: Containing no TSS, turbidity, biological contaminants, volatiles, free chemicals, etc., and conductivity (or resistivity) measurements as per water quality requirement. For example, in some applications water is passed through UF (ultrafiltration) even after an RO storage tank to remove any contamination possibility from tanks and pumps.
Laurence: I don’t use that term, but I would assume it is close to distilled water.
Nandini: High-purity water means quality of water nearly distilled water not pure distilled water.
Mike: Ultrapure water is discussed in Wikipedia at this link: https://en.wikipedia.org/wiki/Ultrapure_water. Note: Not all Wikipedia references are peer-reviewed, so they can
have reliability issues or at worst, be untrue. That said, this discussion about UPW was thoroughly peer reviewed by subject matter experts in this segment of water treatment. At this stage, its only need is for some updating because of technological advances—particularly in the microelectronics industry.
Moderator James McDonald, PE, CWT, is Director of Technology & Marketing with Chem-Aqua. He holds an M.S. in chemical engineering and is a Ray Baum Memorial Water Technologist of the Year award winner (2013). Mr. McDonald also chairs the Association of Water Technologies (AWT) Technical Committee.
Keywords: ALKALINITY, CALIBRATION, MAINTENANCE, MEASUREMENTS, MEMBRANES, MONITORING, OPERATIONS, pH, REVERSE OSMOSIS, SOFTENING
Editor’s Note: With the Summer Analyst, we are introducing a new cartoon series that is drawn by Mr. McDonald that will illustrate different aspects of water treatment. We appreciate his contributions to this journal!

83 the ANALYST Volume 30 Number 3 What’s (Water) on Your Mind? continued
Navigating the Waters of Legislative and Regulatory Impact
 Patsy Root, Legislative and Regulatory Committee Member
Patsy Root, Legislative and Regulatory Committee Member
Water treatment professionals are at the forefront of ensuring the effective and safe treatment of building water and wastewater. However, the industry is not immune to the influence of legislative and regulatory activities that fundamentally shape its operations. In response to this challenge, AWT has established the Legislative and Regulatory (Leg/Reg) Committee. This committee serves as a vital platform for professionals to engage in dialogue, access industry resources, and address key issues affecting the water treatment industry and their businesses. In this article, we will take you on a journey through the purpose, goals, and current legislative and regulatory issues tackled by the committee.
The purpose that the Leg/Reg committee fulfills for the AWT membership is outlined in our Charter, which specifically states:
We empower water treatment professionals to:
1. Have an impact on their state and local governmental activities to advocate for commonsense legislation that promotes effective and safe water and wastewater treatment
2. Allow dialog between professional organizations regarding standards, guidelines and recommendations that impact the industrial water treatment industry
3. Maintain current industry resources that are relevant and impactful to member’s profession and business. Periodic updates will be given to the membership.
The primary purpose of the Leg/Reg Committee to have a meaningful impact on state and local governmental activities. By advocating for common-sense regulations and legislation, the committee aims to promote effective and safe water and wastewater treatment practices. Additionally, the committee fosters dialogues between professional organizations, ensuring that industry standards, guidelines, and recommendations align with the needs of the industrial water treatment sector. It also provides members with access to current and relevant industry resources, keeping them informed and equipped to navigate the evolving landscape.
Aligned with the revised AWT strategic plan, the Leg/ Reg Committee primarily focuses on “maintaining an open line of communication and providing industry-related regulatory and legislative information.” Over the next five years, the committee has set forth the following goals:
1. Establishing an Open Forum: Creating a platform where members can openly share industry-related regulatory and legislative information, fostering collaboration and collective knowledge.
84 the ANALYST Volume 30 Number 3 Capital Eyes
2. Dedicated Information Space: Developing a dedicated space where members can easily access industry-related regulatory and legislative updates, ensuring they stay well-informed.
3. Building a Network of Knowledge: Cultivating a robust network within the membership to collect, analyze, and distill legislative and regulatory information, enabling effective dissemination and understanding for the benefit of all members
As with other AWT committees, Leg/Reg exists to support, educate, and promote solid science around water treatment practices. To accomplish this, we meet at least monthly to discuss current topics and members are encouraged to attend our meetings to either learn or share new information.
The Leg/Reg Committee is actively engaged in monitoring and addressing several critical issues impacting the water treatment industry. Here are some of the prominent issues under scrutiny:
1. PFAS (Per- and Polyfluoroalkyl Substances): The presence of PFAS chemicals in water sources and wastewater has raised concerns due to their persistence and potential health risks. The committee is actively working to ensure that legislation and regulations are in place to address the safe handling, treatment, and disposal of PFAS-contaminated water. We additionally monitor activities of the U.S. EPA as they work toward regulations on these compounds and how those rules may affect AWT businesses.
2. Non-Competes: Non-compete agreements can limit professionals’ mobility within the water treatment industry, hindering career growth and knowledge sharing. The committee advocates for fair and reasonable non-compete regulations that strike a balance between protecting intellectual property and fostering professional development.
3. Legionella: Legionella pneumophila bacteria pose a significant health risk when present in
water systems, leading to severe respiratory illnesses, hospitalization, and even death. The committee focuses on encouraging regulators and legislators to leverage existing industry standards, guidelines, and regulations to prevent and control Legionella outbreaks, prioritizing the safety of water treatment processes. We additionally look to local AWT members for support on specific activities and will offer tools to make commenting on these more efficient and effective.
4. The Influence of AI on Water Treatment:
The emergence of artificial intelligence (AI) and machine learning (ML) brings both opportunities and challenges to the water treatment industry. The committee closely monitors AI advancements and their potential impact on water treatment processes, regulations, and workforce requirements
To achieve these goals, the Leg/Reg Committee seeks to increase awareness of AWT, its members, and the water treatment industry among regulatory bodies and lawmakers. By establishing a visible, and highly knowledgeable, presence, the committee aims to influence legislation and regulations that align with AWT member’s needs. Additionally, the committee remains proactive in addressing emerging issues throughout the year, ensuring that members are wellprepared to tackle challenges head-on.
We encourage members to join us this fall at our annual in-person committee meeting to learn more or at our Leg/Reg general session on October 5, 2023.
85 the ANALYST Volume 30 Number 3 Capital Eyes continued
Why choose Quantrol as your Water Treatment equipment supplier?


-Commitment, to our customers - We got your back.
-Experience, Our sales team averages over 20 years of industry experience.




-Knowledge, of the equipment we offer and how to apply it.
-Accessibility, A real live caring person answers the phone, not a machine.



-Brands, We work with some of the best manufacturers in the industry.
-Stock, 1000’s of items in our Naperville, IL warehouse.

QUALITY EQUIPMENT FOR THE CONTROL OF WATER TREATMENT 9001 Hanslik Ct. Naperv le, IL 60564 | Tel: 630-355-3330 | info@quantrol.net | www.quantrol.com









87 the ANALYST Volume 30 Number 3 Advertising Index 14 AMSA 6 AquaPhoenix 44 Brenntag North America 35 CHEMetrics 23 Enviromental Safety Technologies 17 French Creek Software 2 Lutz Jesco America Corporation 42 Myron L Company 51 Pulsafeeder 48 Pyxis 62 Qualichem, Inc 86 Quantrol 58 Sanipur US 87 Scranton Associates 88 Special Pathogens Laboratory 61 Walchem Iwaki America









































 Jeremy Duguay, Harley King, Ph.D., Neil Sharma, Ph.D., and Jordan Schmidt, Ph.D., LuminUltra Technologies Ltd.
Jeremy Duguay, Harley King, Ph.D., Neil Sharma, Ph.D., and Jordan Schmidt, Ph.D., LuminUltra Technologies Ltd.

































 Joshua Utter American Water Chemicals
Joshua Utter American Water Chemicals















































 Compiled by James McDonald, Chem-Aqua
Compiled by James McDonald, Chem-Aqua

 Patsy Root, Legislative and Regulatory Committee Member
Patsy Root, Legislative and Regulatory Committee Member























