
What is Produced Water, and Why Does it Matter
An Update on the Use of Ozone in Cooling Water Treatment: Part 1
What Materials of Construction Are Needed in the Biopharmaceutical Industry?
When Treating Cooling Towers with ClO2, Is There a Best Way?
A Testing Approach to Validate Legionella-Viable-PCR Technology
Published by Volume 31 Number 1 1300 Piccard Drive, Suite LL 14 • Rockville, MD 20850 Winter 2024 the ANALYST The Voice of the Water Treatment Industry Volume 31 Number 1 Winter 2024




10 What is Produced Water, and Why Does it Matter
Shaun Primeaux, ChampionX
The Association of Water Technologies (AWT) formed the Produced Water Task Force in 2022 to provide membership insight to a major waste stream of the oil and gas production industry. The initial goal of the task force has been to educate the membership with a specific definition and scope of produced water. This article will provide a foundational knowledge and reference point for future discussions.
16 An Update on the Use of Ozone in Cooling Water Treatment: Part 1
Anthony Sacco,
Spartan Environmental Technologies, LLC
The history of ozone in water treatment can be traced back to the early 20th century. Ozone, a powerful oxidizing agent, has since then been used to control microbial growth, remove organic contaminants, and reduce corrosion in cooling water systems. A brief history of the use of ozone in water treatment is covered in this article.
26 What Materials of Construction Are Needed in the Biopharmaceutical Industry?
Alexandra Peters and Marco DeAngelo, Arkema, Inc.
In the past century, the evolution of therapeutics in medicine has greatly extended life expectancy of people across the world. These therapeutics have come in several different forms including those of the pharmaceutical and biopharmaceutical industry. The pharmaceutical industry relies on chemical and synthetics processes, while biopharmaceuticals, also known as biotechnology or biopharma, is rooted in creating drugs from living organisms.
32 When Treating Cooling Towers with ClO2, Is There a Best Way?
Greg D. Simpson, PhD, Pureline Treatment Systems
Chlorine dioxide has been used for microbiological control of cooling water since the early 1970s. Even in these early reports, researchers observed the effectiveness of chlorine dioxide as a bactericide, sporicide, and virucide, and its control of biofilm.
44 A Testing Approach to Validate Legionella-Viable-PCR Technology
Brandon Smith, Environmental Safety Technologies Inc.; and Richard D. Miller, PhD, Environmental Safety Technologies Inc. and Department of Microbiology and Immunology, University of Louisville School of Medicine
The detection of Legionella in building water systems is important in validation of Legionella control as specified in building Water Management Programs for risk management of legionellosis. Culture techniques are the Gold Standard for Legionella analyses because of their detection and quantitation of colony forming units, that are closely equivalent to infectious bacteria, despite the 7-10 day wait for the results. Polymerase chain reaction technology for detecting Legionella in water samples has been around for more than 30 years.

3 the ANALYST Volume 31 Number 1 Cover Bitumen mine's tailing pond. Photo courtesy of ShutterStock. 4 Calendar of Events 7 President’s Message 9 Message From the President-Elect 54 Discovering AWT 57 Making a Splash 58 CWT Spotlight 59 Tales From the Waterside 65 T.U.T.O.R. 69 Beyond Water 72 Capital Eyes 74 What’s (Water) on Your Mind? 78 Advertising Index
2024 Volume 31 Number 1
Winter
1300 Piccard Drive, Suite LL 14
Rockville, MD 20850
(301) 740-1421 • (301) 990-9771 (fax)
www.awt.org
2024 AWT Board of Directors
President
Noah Baskin
President-Elect
John D. Caloritis, CWT
Secretary
Kyle J. Rossi, CWT
Treasurer
Craig Bodenmiller, CWT
Immediate Past President
Steve Hallier, CWT
Directors
Tammy Faber, MBA
Michelle Lunn
Derrick Vandenberg, CWT
Greg Gehrke
Ex-Officio Supplier Representative
Pam Simmons
Past Presidents
Fred Shurtz
Jack Altschuler
John Baum, CWT
R. Trace Blackmore, CWT,
LEED AP
Michael Bourgeois, CWT
D.C. “Chuck” Brandvold, CWT
Thomas Brandvold, CWT
Brent W. Chettle, CWT
Dennis Clayton
Bernadette Combs, CWT,
LEED AP
Matt Copthorne, CWT
James R. Datesh
John E. Davies, CWT
Jay Farmerie, CWT
Gary Glenna
Charles D. Hamrick Jr., CWT
Joseph M. Hannigan Jr., CWT
Staff
Executive Director
Matt Coffindaffer, MBA, CAE
Deputy Executive Director
Sara L. Wood, MBA, CAE
Member Services Director
Angela Pike
Matt Jensen, CWT
Mark R. Juhl
Brian Jutzi, CWT
Bruce T. Ketrick Jr., CWT
Bruce T. Ketrick Sr., CWT
Ron Knestaut
Robert D. Lee, CWT
Mark T. Lewis, CWT
Steven MacCarthy, CWT
Anthony J. McNamara, CWT
James Mulloy
Alfred Nickels
Scott W. Olson, CWT
William E. Pearson II, CWT
William C. Smith
Marc Vermeulen, CWT
David Wagenfuhr
Casey Walton, B.Ch.E, CWT
Larry A. Webb
Member Services Coordinator
Tim Foley
Senior Vice President, Meetings
Tina Schneider, CMP
Meetings Manager
Caroline Bentley
Director of Exhibits and Sponsorship
Jessica Martin
Director of Marketing
Melissa Graham, MBA
Marketing Manager
Taylor Adelsperger
Editorial Services Manager
Heather Rigby
Production Manager
Jansen Vera
Director of Accounting Services
Dawn Rosenfeld
The Analyst Staff
Publisher
Matt Coffindaffer, MBA, CAE
Managing Editor
Heather Rigby, hrigby@msp-amc.com
Production Manager
Jansen Vera
Technical Editor
Michael Henley, mdhenleywater@gmail.com
Advertising Sales Manager
Carol Nettles, carol@awt.org
Calendar of Events
Association Events
2024 Technical Training Seminars (West)
March 6-9, 2024
Embassy Suites Dallas – Frisco Convention Hotel Frisco, Texas
2024 Technical Training Seminar (East)
April 17-20, 2024
Cleveland Marriott Downtown at Key Tower Cleveland, Ohio
2024 Annual Convention and Exposition
September 10–13, 2024
Louisville Convention Center and Omni Louisville Louisville, Kentucky
2025 Technical Training Seminars (West)
February 25–28, 2025
Doubletree Mission Valley San Diego, California
2025 Technical Training (East) TBA
2025 Annual Convention and Exposition
November 12–15, 2025
The Broadmoor Hotel Colorado Spring, Colorado
2026 Annual Convention and Exposition
September 16–19, 2026
Oklahoma Convention Center and Omni Hotel Oklahoma City, Oklahoma
2027 Annual Convention and Exposition
September 8–11, 2027
Cleveland Convention Center Cleveland, Ohio
Also, please note that the following AWT committees meet on a monthly basis. All times shown are Eastern Time. To become active in one of these committees, please contact us at (301) 740-1421.
Second Tuesday of each month, 11:00 am—Legislative/Regulatory Committee
Second Tuesday of each month, 2:30 pm—Convention Committee
Second Wednesday of each month, 11:00 am—Business Resources Committee
Second Friday of each month, Noon—Pretreatment Subcommittee
Second Friday of each month, 10:00 am—Special Projects Subcommittee
Second Friday of each month, 11:00 am—Cooling Subcommittee
Third Monday of each month, 10:00 am—Certification Committee
Third Tuesday of each month, 3:30 pm—Young Professionals Task Force
Third Tuesday of each month, 11am- Women of Water (WOW) Committee
Third Monday of each month, 3:00 pm—Education Committee
Third Friday of each month, Noon—Boiler Subcommittee
Third Friday of every other month, 10:00 am—Technical Committee
Third Friday of each month, 11:00 am—Wastewater Subcommittee
Fourth Friday of each month, 1:00 pm—Education Resources Committee
Other Industry Events
WateReuse Association, March 11–14, 2024, Denver, Colorado
ABMA Boiler Technology Conference & Expo, May 1–3, 2024, Aurora, Colorado Electric Utility Chemistry Workshop, June 7–9, 2024 Champaign, Illinois International Water Conference, November 3–7, 2024, Las Vegas, Nevada
4 the ANALYST Volume 31 Number 1 The Analyst is published quarterly as the official publication of the Association of Water Technologies. Copyright 2023 by the Association of Water Technologies. Materials may not be reproduced without written permission. Contents of the articles are the sole opinions of the author and do not necessarily express the policies and opinions of the publisher, editor or AWT. Authors are responsible for ensuring that the articles are properly released for classification and proprietary information. All advertising will be subject to publisher’s approval, and advertisers will agree to indemnify and relieve publisher of loss or claims resulting from advertising contents. Editorial material in the Analyst may be reproduced in whole or part with prior written permission. Request permission by writing to: Managing Editor, the Analyst, 1300 Piccard Drive, Suite LL 14, Rockville, MD 20850, USA. Annual subscription rate is $100 per year in the U.S. (4 issues). Please add $25 for Canada and Mexico. International subscriptions are $200 in U.S. funds.
Cooling Tower Controllers and Communications
MicroVision EX controllers make life simple. Worry less about installation, programming, and future maintenance. Maintain process control, reduce costs and limit downtime with MicroVision EX, freeing up more time to serve your customers.

Save Money
• Receive notifications of system upsets and make remote changes to parameters without making an unscheduled emergency visit
• Toroidal probe = No annual replacement needed, eliminates expensive service calls
Save Time
• Troubleshoot the system remotely so you can have necessary parts with you to fix the issue the first time
• Toroidal probe = No exposed electrode, no calibration needed, no guessing if the readings are correct

Peristaltic Pumps
•Self-priming
•Inherently degassing
•Reliable metering performance
•Rugged, sealed all metal gear train
Diaphragm Metering Pumps

PULSAtron electronic metering pumps for accurate dosing of liquid chemicals. Highly reliable electronics which keeps your pump operating for year s with minimal maintenance.

• Premium construction with few moving par ts
•Auto-degassing liquid end
• Reliable long-life cycle
•Quick prime models


www.pulsafeeder.com



Talk with one of our Certified Water Technologists Today! 800.296.9102 | www.qualichemwt.com AN ISO 9001:2015 COMPANY SUPPLIER OF THE YEAR The Blender Matters There is a world of difference between a one-size-fits-all toll blender and an experienced, custom water treatment blender like QualiChem. We formulate, manufacture, and ship products to private label water treatment partners internationally. Providing Industry-Recognized Benefits And Services – No Direct Sales – Precision Manufacturing – Formulatory Expertise – Application Support – Custom GHS- Compliant Labels and SDSs A Trusted Business Partner With The Expertise To Help Your Business Grow. To learn more, please visit www.theblendermatters.com.
President’s Message

2023 Recap
Thanks to all who joined us in Grand Rapids for a highly successful AWT Convention & Exposition. I am thrilled to confirm we had a total of 1,154 attendees, including members and exhibitors. Our Business Owners Meeting, held in conjunction with the Convention, was the most well-attended ever with 118 attendees and three corporate sponsors. Our Tech Trainings were a success, as many of you participated in events held in San Diego and Pittsburgh. And by the end of 2023, we had far exceeded our expectations for our new individual membership category (more on that below). My thanks to my predecessor, Steve Hallier, CWT, for leaving AWT in very good shape as I assumed the role of president.
Leadership Meeting
Following the Convention and Exposition, members of the AWT Board, committees, subcommittees, task forces and related trade organizations got together in Annapolis, Maryland. We looked back at our successes in 2023 and reviewed places where there are opportunities for improvement. The group renewed our commitment to our updated critical outcomes and goals: Thriving Members, Influential Representation, Industry Impact, and ongoing and critical charitable endeavors.
The AWT Board of Directors met at the end of January in Clearwater, Florida. More information on that will be coming throughout the year.
AWT Training 2024
AWT Tech Training will be held March 6-9 in Dallas, Texas and April 17-20 in Cleveland, Ohio. It is important to note that programs and sessions are updated each year, based on feedback from attendees. Programs include sessions on Sales, RO Training, Fundamentals and Applications, Wastewater, and Water Treatment Training. Enroll now at www.awt.org.
By Noah Baskin
Individual Member Category
I am very pleased to report that as of December 31, 2023, our new Individual Member Category has grown to 101 members! This area of growth is a huge success story for AWT last year. We continue to field questions and enroll new members daily and we remain very excited about the future of the Individual Member Category. If you want to learn more, visit www.awt.org
I am honored to continue to serve you as president. Please don’t hesitate to reach out to me as I welcome your comments, suggestions, and feedback. I can be reached at: nbaskin@towerwater.com
P.S. There are exciting changes in the works that will improve your experience reading the Analyst! More information will be announced in the Spring issue.
7 the ANALYST Volume 31 Number 1
Why choose Quantrol as your Water Treatment equipment supplier?

-Commitment, to our customers - We got your back.
-Experience, Our sales team averages over 20 years of industry experience.
-Knowledge, of the equipment we offer and how to apply it.
-Accessibility, A real live caring person answers the phone, not a machine.
-Brands, We work with some of the best manufacturers in the industry.
-Stock, 1000’s of items in our Naperville, IL warehouse.









QUALITY EQUIPMENT FOR THE CONTROL OF WATER TREATMENT 9001 Hanslik Ct. Naperv le, IL 60564 | Tel: 630-355-3330 | info@quantrol.net | www.quantrol.com
Message From the President-Elect

Planning for the 2024 Annual Convention & Exposition is already underway. The Convention will be held Tuesday through Friday, September 10–13 in Louisville, Kentucky. Please note that this year—and this year only—our meeting will begin on a Tuesday and end on Friday. We will be headquartered at the Omni Louisville with exhibits at the Louisville Convention Center. We are very excited to visit Louisville this year following a great meeting in Grand Rapids in 2023. We hope you will plan to be there!
Educational Programs
The program for the 2024 convention is shaping up. We are receiving and accepting abstract submissions from members and developing educational programs that reflect the needs identified by our members. The 2024 convention will include new sessions and topics as our industry continues to evolve and the pace of change accelerates. The list of 2024 convention exhibitors, commercial corner underwriters, and sponsors is growing daily.
Golf Tournament
We are working on securing a site for the 2024 golf tournament to be held in conjunction with the Convention. Louisville has no shortage of great golf courses, and we are excited to host the annual golf tournament there. More information is coming soon.
Awards Dinner and Program
This year’s Annual Awards Dinner will be held at historic Churchill Downs Racetrack, the home of the Kentucky Derby. Churchill Downs, which first opened its gates in 1875, will provide a wonderful setting for the Awards Dinner and promises to be a very special event.
Registration for the 2024 Annual Convention & Exposition will open in May. Be on the lookout for the opening announcement and sign-up early to take advantage of discounted rates.
By John Caloritis, CWT
Registration for the 2024 Annual Convention & Exposition will open in May. Be on the lookout for the opening announcement and sign-up early to take advantage of discounted rates.

About Louisville
Louisville is a city that combines heritage with innovation, authenticity with originality and quirkiness with friendliness in a way that is completely unique! Louisville has a booming bourbon scene—it is known as “Bourbon City”—and iconic attractions, world class hotels, a state-of-the-art convention center, and fantastic culinary choices. For more about Louisville, visit www.gotoLouisville.com
As we continue to plan the 2024 Convention & Exposition, I welcome your feedback. I can be reached at jcaloritis@metrogroupinc.com. I look forward to serving as your convention chair this year to work with our convention committee and another successful AWT annual conference for our members.
9 the ANALYST Volume 31 Number 1
What is Produced Water, and Why Does it Matter
Shaun Primeaux, ChampionX

Editor’s note: This is the first article by the AWT’s Produced Water Task Force. Additional articles are planned to discuss different aspects of this water treatment area
Background
AWT formed the Produced Water Task Force in 2022 to provide membership insight to a major waste stream of the oil and gas production industry. The initial goal of the task force has been to educate the membership with a specific definition and scope of produced water. This article, along with the key terms developed by the task force in 2022, is intended to give the membership a foundational knowledge and reference point for future discussions.
Water brought to the surface in the production of oil and/or natural gas is produced water. The produced fluids of oil, gas, and water are split at the surface into hydrocarbon streams of natural gas and oil, and a stream of produced water. The produced water stream may contain reservoir water, water injected into the formation to aid hydrocarbon recovery, and chemicals used in the
drilling or production of a well. The amount of water a well produces varies based on the characteristics of the well and can vary across the life cycle of a well. The amount of water produced is not universally required to be reported of even measured by producers. The following is an overview of the quantity and sources of produced water in the United States and Canada followed by a discussion of how that water is treated.
Produced Water in the U.S.
In 2009, the Argon National Laboratories did a detailed study of produced water quantities in the United States. Though the data may be considered dated, the perspective it provides is valuable. The precision applied to the study is important for a quality understanding of produced water in the United States. This study estimated the volume of water produced in the U.S. to be 20,995,174,000 (nearly 21 billion) barrels per year. Over 80% of this is concentrated in eight states. Overall, when gas production is counted as equivalent barrels of oil, the ratio of oil to water in the U.S. is 1:3.7. Figure 1 identifies the eight states responsible for 84% of United States produced water, along with their respective production rates.

11 the ANALYST Volume 31 Number 1
Figure 1: Eight States Responsible for 84% of the Annual Water Produced in the U.S. Oilfield (Data sourced from 2009 Produced Water Volumes and Management Practices in The United States, Environmental Science Division of Argonne National Laboratory.)
Produced Water in Canada
Canadian oil production is primarily through steamassisted gravity drainage (SAGD) of bitumen in the geographical region of northern Alberta. Bitumen is an extremely viscous, semi-solid, form of petroleum. The process of SAGD entails the drilling of two horizontal wells positioned parallel to each other with one on top of each other. Steam is injected into the reservoir via the upper well. Steam accumulates in the overhead or upper portion of the reservoir forming a steam cap, or chamber, and reducing the viscosity of the bitumen. Oil drains via gravity to the lower horizontal well, which carries it to the surface. Approximately half of Canada’s 3.2 million barrels of daily bitumen production is produced in this way. Figure 2 shows a graphic representation of how a SAGD bottom hole production well works.
Source: M. Ahmadi, Sustainable In-Situ Heavy Oil and Bitumen Recovery

SAGD production allows access to oil reserves previously too deep to mine. The recycling of carrier steam or water decreases the amount of hazardous waste generated. To produce a barrel of oil, SAGD operations use an average of three barrels of water converted to steam that returns to the surface as produced water along with hydrocarbons. More than 90% of this produced water is recycled for steam production. Because 90% of water is recycled, it is not necessarily a waste stream in need of disposal. However, to recycle it, this water still needs treatment and therefore is counted here as total produced water needing treatment.
The remaining half of Canada’s 3.2 million barrels of daily bitumen production, approximately 1.6 million barrels a day, is mined. Bitumen mining operations are similar to SAGD operations in that the heavy bitumen needs to be removed via steam from the solid sands of the reservoir. However, in this operation, the solid material
mixed with bitumen is dug from the earth’s surface, washed from the sand with hot water, then separated from the water. Mining operations also recycle some water for heat transfer. In mining operations, the portion of the water carried off as waste is called tailings. This waste is sent to a “tailings pond” for solids settling and future water treatment. In either the case of SAGD or mining, one can apply a rule of thumb that for every 42-gallon (159 liters) barrel of oil three times as much water is produced. Coincidently, this 1:3 ratio of oil:water is an industry rule of thumb for oil production globally, regardless of production means.
Canadian water production, like in the United States, is not measured directly. This average of 1:3 can be used along with known Canadian oil production to estimate annual water production. Canada produced 5.5 million barrels of oil and other liquid fuels per day in 2021. Based on this oil production, water production is estimated to be 16.5 million barrels per day, or 6,022,250,000 barrels a year. The average water needed to sustain a per person in the United States and Canada is approximately 100 gallons per day. This six billion plus barrels is equivalent to the daily water needs of 6,930,000 people.
In addition to annual produced water used in Canada, there is an accumulation of “tailings”, which is amassed in ponds over the years. Alberta Energy Regulator, the province’s regulatory agency, estimated in 2022 that the volume in these tailings ponds was 1.4 trillion liters. In recent years, there has been a lot of discussion about remediating these ponds, but little has been done to date.
Produced Water in the U.S.A.
In the United States, most water produced offshore is treated for oil removal then discharged overboard while 8% is used for enhanced recovery. This form of “enhanced recovery” entails reinjecting the water into the production reservoir to replenish void space. Doing so helps increases a reservoir’s profitable production life by maintaining pressure. Onshore, 53% of produced water is used for enhanced recovery. In most cases, like offshore, this involves reinjecting to the production reservoir to enhance movement of the oil to the surface. A small percentage of water produced onshore is used to supplement the enhanced recovery method of fracking. Fracking is the use of water and specialty chemicals injected into the reservoir at high pressure to break up underground rock structures and increase their porosity.
More than 40% of water produced onshore in the United States is simply reinjected into a deep well drilled specifically for disposal. This 40% is equal to seven billion
12 the ANALYST Volume 31 Number 1
Figure 2: Graphic Representation of SAGD Production Process.
What is Produced Water, and Why Does it Matter? continued
barrels, 294 billion gallons, of water a year brought to the surface then disposed of by reinjecting it back into the ground. Figure 3 shows the percentage of produced water by disposal method for both land and offshore drilling. Additionally, the specific volumes of produced water by production and disposal method is also provided in Figure 3.
Produced Water Contaminants
The contaminants in produced water are the same broad categories encountered in most any pre- or posttreatment programs. Suspended and dissolved solids, bacteria, and dissolved gases need to be considered. The exact specifications of treated water are dictated by the downstream, or future, use of the water.
Suspended solids are likely from the formation. These can plug a formation if reinjected for void replacement or blind an injection well if reinjected for disposal. Sometimes, when discharged overboard in offshore operations, suspended solids can cause undesirable visual effects on the surface of the water, or they can hold oil and grease and cause an environmental impact by carrying over oil to the surface water. Traditional suspended solids separation equipment such as sock filters or more expensive separation equipment like hydrocylcones and flotation units are used.
Dissolved solids possess an intrinsic ability to crystalize, or precipitate, under certain chemical or physical conditions. As a precipitant, they pose the same risks
as suspended solids already discussed. Therefore, it is best to reduce total dissolved solids (TDS) to a lower risk threshold by precipitating and removing them by filtration at the surface. Sometimes pH adjustment is necessary to drive some contaminants out of solution. Sequestering chemicals can also be used to keep them in solution.
Hydrogen Sulfide (H2S) is the primary gas of concern because of its fatal nature. H2S may originate from the well or develop overtime as a result of microbiological fouling. It is possible to neutralize H2S chemically and remove any unwanted solids with liquid solid separation.
Bacteria can cause microbiologically influenced corrosion (MIC) or fouling of injection wells with sulfate-reducing bacteria (SRBs). In turn, SRBs can sour the reservoir and produced fluids with H2S. Quick use of biocide at the surface to prevent microbiological contamination is best.
Residual oil and grease need to be removed. High oil and grease residual in produced water is the result of poor separation often rooted in bad mechanical or operational techniques upstream in the production process. Residual oil that is disposed of as waste from the produced water is lost revenue for the producer. Separation efficiency and effectiveness can be aided with demulsification chemicals. Remaining residual oil and grease, after good operational techniques, can pose an environmental risk if discharged to surface water or can foul injection wells if pumped into a disposal well. Downstream of separation equipment, traditional waste treatment methods of coagulation, flocculation, and floatation can further remove more oil and grease from the water.
Source: Data sourced from 2009 Produced Water Volumes and Management Practices in the United States, Environmental Science Division of Argon National Laboratory

When reinjecting produced water into a well or reservoir, there is a risk of fluid incompatibility. Produced water that is incompatible with existing fluids or with the reservoir itself can lead to plugging like discussed earlier with regard to TDS and total suspended solids (TSS). Plugging a disposal well can lead to the cost of replacing it by drilling another and/or revenue losses due to decreased (derated) production. Reinjecting into a production reservoir has the same likelihood of plugging risk but a potentially greater consequence of lost revenue, or derated production.
13 the ANALYST Volume 31 Number 1
Figure 3: Annual Barrels of Produced Water in the United States by Disposal Method.
What is Produced Water, and Why Does it Matter? continued
Treating Produced Water as a Waste Stream
The treatment for most of these contaminants, other than H2S scavenging discussed above, is similar to treating most any wastewater stream. The first step is quality jar testing to determine adequate coagulant and flocculant as the proper chemistry to apply. Next, good understanding of the system’s design and holding times help to define proper injection. Specific handling equipment will differ from site to site but is not significantly different from other wastewater treatment facilities.
Corrugated plate interceptors (CPIs), hydrocyclones, flotation units, and in some cases, dewatering equipment are all standard engineered responses one may expect at any production facility. These may be deployed as standalone pieces of equipment or as multi-stage systems, depending on design. It is easy to imagine that the magnitude and complexity can vary greatly for a well pad that contains only a few small producers, to an offshore platform that has to polish to surface water environmental standard, to a SAGD operator recycling 8 million gallons of oil water per day.
In mining and SAGD operations, the produced water has to be conditioned to meet steam generation requirements. Warm lime softening or evaporator technology is used to condition the water for once through steam generators. The water used for transport and the hot water extraction process (HWEP) used in the mining of bitumen also requires specific treatment and separation aids.
Natural evaporation is a common means of reducing the amount of water to dispose of while concentrating the contaminates. This response utilizes a holding pond to store the waste stream in and allow the water to evaporate naturally. Tailings Ponds were referenced earlier as a common method of handling produced water waste in bitumen mining. Tailings Ponds were already characterized as not being effective as they have led to an accumulation of large volumes of hazardous waste over the years. There are companies that provide equipment to speed up and improve evaporation through mechanical misters. By increasing the water surface area exposed to the atmosphere these misters speed up evaporation of the bulk fluid and reduce the cost associated with disposal by reducing the total volume of waste.
The produced water offshore is mostly being discharged overboard and therefore has to meet environmental standards. Because it is not being reused there is no additional pretreatment required. However, the process employed to treat the wastewater can have a big impact
on the production process. The fouling of production equipment, interference with fluid dynamics in separation vessels, and the speed with which water clarifiers work can impact facility revenue and/or profitability.
The pretreatment or feedwater requirements of SAGD, mining, and operational interference in offshore operations are all considered upstream matters of production and operation. Once the water is repurposed beyond the waste stream, it becomes part of the production process where oil quality, flow assurance, and asset integrity are primary customer interests. These are outside of the scope of AWT’s Produced Water Task Force and therefore not examined further in this article.
A More Responsible Use for Produced Water
Until now, we have discussed the treatment of produced water to meet today’s most used disposal methods. The risk of seismic instability due to reinjection, the worsening shortage of fresh water, the simple desire to be better stewards of natural resources are all driving a need for more responsible solutions.
A more desirable reuse would be for human use. If the amount of produced water discussed above were recycled for human needs, it would provide the daily application of more than 26 million people. If recycled to drinking water standards and used for human consumption, then it would hydrate 100 times that many people. This is equal to the drinking water needs of nearly 80% of the United States population. Membrane and thermal technologies exist to treat produced water to these standards for $0.75 to $1.50 USD/barrel. Excluding transportation to the end use, which is 2 to 3 pennies a gallon, neither technology nor purification cost prohibits the use of produced water for human use. The cost of liability and defending produced water for human use in court is the primary obstacle.
Final Thoughts
In Canada and the United States, produced water volumes are high and increasing. As the world demands more hydrocarbon, produced water will increase. As existing production wells age, they will produce higher and higher percentages of water. Water can be cleaned to a quality for both direct and indirect human usage. Furthermore, it can be done at a reasonable financial cost. Sadly, the liability is too great for a producer. Another barrier to more desirable reuse methods is the cost of transporting the water to points of use from remote areas. Until these can be overcome, oil producers in Canda and the United States will continue to treat an increasing volume of 23 billion barrels of water as a waste stream.
14 the ANALYST Volume 31 Number 1
What is Produced Water, and Why Does it Matter? continued
The treatment techniques discussed here are similar to the waste treatment practices AWT members employ across many industries. However, the usual business challenges of geographic location, service load, and profitability are all obstacles for AWTs membership when it comes to treating produced water. Often, producers default to wastewater treatment companies already onsite that understand well their production chemical needs. Whether treating produced water or not, it is incumbent on us as water treating professionals to understand the source, magnitude and value of this resource being produced along with hydrocarbons but being relegated to a waste stream.
Appendix A provides a short list of glossary terms associated with oilfield water treatment concerns.
Bibliography
1. Alberta Energy Regulator (November 2022). “Water Use Performance,” Alberta Energy Regulator, Calgary, Alberta, Canada, accessible at https://www.aer.ca/ protecting-what-matters/holding-industry-accountable/industry-performance/ water-use-performance
2. Ahmadi, M. (2023), Sustainable In-Situ Heavy Oil and Bitumen Recovery: Techniques, Case Studies, and Environmental Considerations, first edition, ISBN: 9780323908481, Elsevier, Amsterdam, the Netherlands.
3. Anderson, D. (June 2022). “Ponds of Toxic Waste in Alberta’s Oilsands Are Bigger than Vancouver and Growing," The Narwhal, accessible at https://thenarwhal.ca/oilsands-tailings-ponds-growth/
4. Bhattacharjee, S. (2015), Water Use In Oil Sands Industry, Advanced Water Research Lab, University of Alberta, Edmonton, Alberta, Canada, accessible at https://awrl.ca/overview/
5. Clark, C.E.; Veil, J.A. (September 2009). “Produced Water Volumes and Management Practices in the United States,” Environmental Science Division of Argon National Laboratory, Lemont, Illinois.
6. McEwen, M. (May 11, 2023). “Produced Water Reuse Could Fend Off Oil Production Cuts,” Midland Reporter Telegram, Midland, Texas, accessible at https://www.mrt.com/business/oil/article/reusing-produced-water-fend-oilproduction-cuts-18094844.php
7. Produced Water Task Force (2022). “Produced Water Terms,” Association of Water Technologies, Rockville, Maryland.
8. U.S. Energy Information Administration (July 2022). Canadian Report. https://www.eia.gov/international/content/analysis/countries_long/Canada/ Canada%20CAXS%202022.pdf

Shaun Primeaux is an industry technical consultant with ChampionX. He has 27 years of global water treating experience, which includes 16 years of work in water treating in upstream oil and gas production. He is a member of the AWT’s Produced Water Task Force. Mr. Primeaux earned a BS in biology and chemistry from Louisiana College. Mr. Primeaux can be reached at shaun.primeaux@championx.com.
Editor’s note: The full list of terms associated with produced water will be available at a later date at the AWT website as the Produced Water Task Force makes deliverables available to AWT membership.
Appendix A: Key Terms From AWT’s Produced Water Task Force Glossary of Terms
Basic Sediments and Water (BS&W): The water and the other extraneous material present in crude oil. Battery (Tank Battery): The production handling equipment on the lease.
Bpd: Barrels per day.
Bopd: Barrels of oil per day.
Bwpd: Barrels of water per day.
Cut oil: Oil that contains water usually in the form of an emulsion. Also called Wet Oil.
Disposal well: A well through which water (usually salt water) is returned to subsurface formations.
Emulsion: A mixture of crude oil and formation water. Generally, requires time and heat, chemicals (called demulsifiers or emulsion breakers), or electricity to separate the water from the oil.
Enhanced oil recovery: Enhanced oil recovery (EOR) improves hydrocarbon recovery by injecting fluids into a hydrocarbon reservoir to increase or maintain reservoir pressure, displace hydrocarbons to production wells, or alter reservoir fluids to improve hydrocarbon flow. There are three major categories of EOR— Thermal Recovery, Gas Injection and Chemical Injection.
Fracturing: Application of hydraulic pressure to the reservoir formation to create fractures through which oil or gas may move to the wellbore.
Gas lift: The raising, or lifting, of liquid from a well by means of injecting gas into the liquid.
Injected gas: High-pressure gas injected into a formation to maintain or restore reservoir pressure or otherwise enhance recovery. Also, gas injected for gas-lift.
Knockout: A kind of tank or vessel used to separate water from oil. A free water knockout (FWKO).
Permeability: The ability of a rock to transmit fluid through the pore spaces; a key influence on the rate of flow, movement, and drainage of the fluid. There is no necessary relationship between porosity and permeability.
Porosity: The percentage that the volume of the pore space bears to the total bulk volume. Pore space determines the amount of space available for storage of fluids.
Saltwater disposal (SWD): The method and system for the disposal of salt water produced with crude oil. A typical system is composed of collection centers and disposal wells in which treated salt water is injected into suitable formation.
Separator: A pressure vessel used for the purpose of separating gas from crude oil and water.
Skim tank: A produced water processing tank designed to skim oil from the surface of the water.
Slickwater: A hydro-fracturing method to increase flow by adding chemicals to the water. The chemicals are used to control viscosity, friction, formation-compatibility, and fluid loss control.
Keywords: CONSERVATION, NATURAL RESOURCES, PETROLEUM, PRODUCED WATER, WASTEWATER, WATER REUSE
Water flooding: One method of enhanced recovery is where the water is injected into an oil reservoir to force additional oil out of the reservoir rock and into the well bores of the producing wells.
15 the ANALYST Volume 31 Number 1
What is Produced Water, and Why Does it Matter? continued
An Update on the Use of Ozone in Cooling Water Treatment: Part 1
Anthony Sacco, Spartan Environmental Technologies, LLC

Editor’s note: This is part 1 of a two-part series. This article provides important information about ozone and its use in water treatment. Part 2 will examine the use of ozone for treating cooling tower water
The history of ozone in water treatment can be traced back to the early 20th century. Ozone, a powerful oxidizing agent, has since then been used to control microbial growth, remove organic contaminants, and reduce corrosion in cooling water systems. A brief history of the use of ozone in water treatment is covered in this article.
The first experiments with ozone were conducted in France and Germany in 1906. Researchers began testing its efficacy in water treatment, including for drinking water and swimming pools. It wasn’t until the 1930s that ozone was used for industrial applications, including cooling water treatment. During the 1940 to 1960 period, ozone generators became more sophisticated, and scientists started to optimize the process of ozone production. The application of ozone in cooling water treatment began to be recognized as an option for industrial facilities, due to its superior disinfection capabilities and low environmental impact.
As concerns about chemical pollution and environmental impact grew in the period of 1970 to 2000, the use of ozone in water treatment increased, including in cooling water. At least one cooling tower manufacturer offered ozone systems as part of their overall cooling tower package. Unfortunately, product quality, operator education and maintenance did not keep up with the increase in cooling water applications, leading to a lack of reliability and a reduced interest in the technology. In other industrial applications and drinking water treatment, ozone technology advanced and the application of ozone grew significantly.
Today, ozone is widely used in a variety of applications safely and effectively. Almost all public aquariums, most bottled water plants, a large number of municipal drinking water plants, and industrial processes use ozone for water treatment. Unfortunately, ozone use in cooling water has declined, probably due to past issues regarding reliability and because of the perception that it is expensive and complicated relative to other treatment alternatives. In addition, some marketers claimed that ozone would offer complete cooling water treatment without the need for any other chemicals or controls. This led to operational issues such as chemical scaling and corrosion.
What Is Ozone?
Ozone is the triatomic form of oxygen, as illustrated in Figure 1. The structure of the molecule is shown below. Its shape has a strong influence on its reactivity (e.g., with respect to the double-carbon bond in various molecules, including aromatic compounds).
Almost all public aquariums, most bottled water plants, a large number of municipal drinking water plants, and industrial processes use ozone for water treatment.
It is a pale blue gas with a strong odor with a solubility in water somewhat higher than oxygen. It is an unstable compound both in air and water. Higher temperatures reduce the half-life. Additional data is shown in Table A.
17 the ANALYST Volume 31 Number 1
Figure 1: Molecular Form of Ozone
Gaseous Dissolved in Water (pH 7) Half-Life Time Temperature (°C) Half-Life Time Temperature (°C) 3 months -50°C 30 min 15°C 18 days -35°C 20 min 20°C 8 days -25°C 15 min 25°C 3 days 20°C 12 min 30°C 1.5 hours 120°C 8 min 35°C 1.5 seconds 250°C
Table A: Half Life of Ozone in Water and Air
The half-life of ozone is also affected by pH with higher pH reducing half-life at any given temperature. This occurs because an equilibrium exists between molecular ozone and the hydroxyl radical. As pH increases, the equilibrium shifts toward the hydroxyl radical that has a very short half-life.
Ozone is a strong, but selective oxidant with an oxidation potential greater than many common oxidants. Table B shows the electrochemical oxidation potential (EOP) of various oxidants. Ozone offers a higher potential than the commonly applied oxidants used in cooling water.
mean that the economies of the ozone are unattractive. On the other hand, if bromide is present in the make-up water, the formation of bromine might make ozone more attractive since less ozone may be needed.
Ozone Water Treatment Technology
To use ozone in water treatment in general, and in cooling water specifically, requires several components integrated into a water treatment system. These include:
Gas preparation
Ozone generation/generator cooling
Ozone water mixing
Ozone off-gas destruct (generally larger systems)
Ozone safety
Instruments and controls
As noted, ozone is a powerful, but selective oxidant. The following reactions are favored: ozonolysis of alkenes and alkynes, oxidation of sulfides, oxidation of amines, oxidation of aromatic rings, and oxidation of alcohols.
On the other hand, carboxylic acids, ketones and aldehyde reactions are generally not favored. Another perspective is that the non-biological oxygen demand (BOD) fraction of chemical oxygen demand (COD) tends to be more reactive to ozone than the BOD fraction. The kinetics of the ammonia reaction with ozone are too slow to be of interest in most applications. Inorganic compounds of Fe, Mn and H2S react quickly with ozone. Ozone can also oxidize bromide ions (Br-) to bromine (Br2). Ozone reactions are discussed in detail by Langlais (1).
Regarding the reaction with bromide, Rice (2, 3) has suggested that the action of ozone in cooling water might be due not just to molecular ozone alone, but also due to oxidation of bromide ion, that can be naturally occurring in some make-up water sources, to bromine, also an effective oxidizing biocide.
The selective nature of ozone reactions is important in considering the use of ozone in cooling water. If the water contains reactive compounds, the demand for ozone might mean that creating the necessary residual for biocidal activity means a large dose of ozone. This might
Gas preparation. Ozone generation requires a dry (-100 degrees dewpoint) oxygen-containing gas. This can be air, purchased oxygen, or oxygen generated on-site from air using a technique such as pressure swing absorption (PSA) oxygen concentration. In practice, the use of oxygen generated on site tends to be the best option for the reasons discussed below. This process can produce 90 to 95% pure oxygen that can meet the dryness required by most ozone generators. The process is illustrated in Figure 2 (4).
Figure 2: PSA Oxygen Concentrator Schematic
Source: Reference 4

Compressed air is fed to a vessel under pressure that is filled with a molecular sieve. The molecular sieve absorbs nitrogen and water leaving oxygen (93%), argon (4%) and some nitrogen (3%). When the first vessel is saturated with water and nitrogen, the process switches
18 the ANALYST Volume 31 Number 1
Oxidizing Agent EOP (V)
radical 2.80 Oxygen (monatomic); 2.42 Ozone 2.08 Hydrogen peroxide 1.78 Hypochlorite 1.49 Chlorine 1.36 Chlorine dioxide 1.27 Oxygen (molecular)
Table B: Oxidation Potential of Various Biocides
Hydroxyl
1.23
An Update on the Use of Ozone in Cooling Water Treatment: Part 1 continued
to a second column by releasing the pressure in the first and pressurizing the second. The first column is then regenerated by using a sweep gas, some of the oxygen produced in the first vessel, to clear out the nitrogen and water. The process continues to cycle back and forth between the two vessels with an oxygen receiver to produce a continuous flow of oxygen. Figure 3 (5) shows the typical equipment layout: compressor, PSA oxygen concentrator, and oxygen receiver.
Ozone generation. While there are various methods of producing ozone, for most commercial applications the ozone is either generated by UV or dielectric barrier plasma discharge (DBD), sometimes referred to as corona discharge. The UV process is not very efficient and does not economically produce the amount of ozone required for cooling water treatment.
The DBD process is illustrated in Figure 4 (6). A high voltage electrode is connected with a dielectric (glass or ceramic). A space is provided for the oxygen containing gas to pass between the dielectric and a grounding electrode. A discharge occurs when the electric field strength is high enough, causing a current to flow through the gas. These discharges cause plasma to form where the oxygen (O2) molecules break up and in some cases form ozone (O3). As the charge builds up, the

current flow stops. Because AC electrical power is used, the process repeats over and over, creating essentially a continuous production of ozone. The electrode geometry is usually either tubular or planar. The photo in Figure 5 (7) shows the blue plasma formed around a tubular glass dielectric in a multi-tubular ozone generator.

19 the ANALYST Volume 31 Number 1
Figure 4: Dielectric Barrier Plasma Discharge (DBD) Ozone Generating Process Figure courtesy of Owens, D., et al., Reference 6
Figure 3: PSA Equipment Layout
An Update on the Use of Ozone in Cooling Water Treatment: Part 1 continued

The process generates considerable heat, so the ozone generator needs to be cooled. Smaller generators can be cooled by air, while larger generators typically use water as a coolant. As the temperature rises for any given ozone generator design, the output decreases (0.5 to 1.0%/°F). So cooling is an important design parameter, including ambient temperature for air-cooled units and available water temperature in water cooled units. Air conditioning units and water chillers are sometime used to control the operating temperature.
Ozone generators using a dry air feed can make ozone with a concentration of 1 to 3 weight percentage (wt%). Using oxygen with >90% concentration can increase the ozone concentration range from 5 to 20 wt%. For practical reasons, operation in the 5 to 12 wt% range is more typical. Higher concentration increases the energy cost but improves the ability to dissolve ozone in water, increasing ozone transfer efficiency, which improves the economics of ozone water treatment.
Ozone water mixing. Ozone is generated on site as a gas. It therefore has to be dissolved in water to be useful. The objective is to maximize the ozone transfer efficiency. This is defined in Equation 1.
Ozone Transfer Efficiency (OTE) = Transferred Dose ÷ Applied Dose Eq. 1
The Applied Dose is the ozone produced at the ozone generator, while Transferred Dose is the ozone that actually dissolves in the water. The ratio is normally expressed as a percentage. The OTE normally is designed to be greater than 90% to use as much ozone as possible. Ozone that does not dissolve leaves the water along with the oxygen and other components of the feed gas. This off gas has to be handled properly especially if it might enter a space where people are present.
Two methods are normally used to dissolve ozone, fine bubble diffusers and venturi injectors. Factors impacting OTE include ozone concentration (Henry’s Law considerations), water temperature, pH, ozone demand of the water, ozone bubble size and the gas volume to liquid volume ratio. For fine bubble diffuser applications, to achieve a high OTE, fine bubble diffusers need up to 16 to 20 feet of water head above the diffuser. The water head provides two benefits: first, the mass transfer of ozone from the bubble to the liquid has sufficient time to occur and the higher pressure at the bottom of the water column reduces the bubble size. Having a 16 to 20-foot water column can be difficult to arrange in many applications, so venturi injectors are often used to draw the ozone gas in contact with the liquid and mix the two phases. If properly designed both methods can achieve OTE >90%.
Ozone safety and environmental considerations. The Occupational Health and Safety Administration (OSHA) regulates worker exposure to ozone. The 40hour exposure limit is 0.10 parts per million volume (ppmv), while the 15-minute (min) limit is 0.30 ppmv. Properly designed systems employ ambient ozone monitors interlocked to shut down production when the workspace exceeds the safe limit of 0.1 ppmv. For smaller systems, the ozone off gas (i.e., undissolved gases) can be vented to the atmosphere without further treatment. In some jurisdictions, depending on the amount of ozone produced, ozone off gases must be treated if released into the environment. This is easily done with catalytic converters using a material called Carulite®, a copper/ manganese oxide catalyst (8).
Instruments and controls are important for efficient and reliable operation of an ozone water treatment system. Some issues with smaller systems in the past have been a result of lack of automation.
Ozone generator output can be paced to the ORP (millivolt [mV]) or dissolved ozone (milligrams per liter [mg/L]) using a proportional-integral-derivative (PID) control loop and a dissolved ozone monitor (DOM) or ORP monitor. This approach provides for a more
20 the ANALYST Volume 31 Number 1
Figure 5: Blue Plasma Inside Operating Ozone Generator Source: Courtesy of DeNora Water Technologies, Reference 7
An Update on the Use of Ozone in Cooling Water Treatment: Part 1 continued
consistent level of ozone in the water versus simple on/off control resulting in better water quality.
Another important instrument is an ambient ozone monitor (AOM), which can indicate ambient ozone levels. As already noted, it is an important part of the ozone safety system. Both electrochemical and UV-based sensors can be used. In either case, a signal can be sent to the process controller to shut down ozone production if ambient ozone levels are too high.
It is important to monitor gas/liquid flow and pressures at various points in the ozone water treatment process. A combination of flow indicators, flow transmitters, pressure indicators and pressure transmitters should be used and linked to the process controller to maintain the process at optimal conditions or shut down the system if the process is out of control.
For larger systems, online continuous monitoring of ozone output using both mass flow meters and high concentration ozone monitors provide definitive ozone output values. Combined with dissolved ozone monitors, trouble shooting of water quality issues can be quickly resolved.
Ozone water treatment system piping & instrumentation diagram (P&ID). Today, system integrators have packaged ozone systems into a single skid for smaller applications and containerized larger applications in a variety of water treatment environments. Many simply require a connection for water in and out with a single power connection simplifying installation. Figure 6 (9) shows a diagram of a P&ID for an ozone water treatment system.
The system includes a booster pump with a venturibased ozone water mixing system. The instrument package includes a gas feed mass flow and a liquid flow transmitter. Various pressure transmitters monitor system performance. All instruments are followed by a PLC, which can shut the system down in case of a failure of any subsystem and alert operators to the issue. Optionally, remote monitoring and reporting can be added for units not regularly attended by an operator. Ambient ozone monitor(s) are included for safety.
The critical features of an ozone water treatment system include the ability to know that the correct amount of oxygen feed gas is flowing to the ozone generator, that
21 the ANALYST Volume 31 Number 1
Oxygen Concentrator Gas Filter 0.01 Ozone Generator Needle Valve Check Valve Liquid Tap Check Valve To Drain (Client Scope) Pressure Gauge Pressure Gauge XXXXX Injector Sample Port Pump MEM PT ACM PI PI PI FT
Figure 6: Example P&ID of an Ozone Water Treatment System
Figure courtesy of Spartan Environmental Technologies, Reference 9
An Update on the Use of Ozone in Cooling Water Treatment: Part 1 continued

the ozone generator can report a fault in operation, the proper motive flow is passing through the venturi, and ambient ozone levels are measured in enclosed spaces where people are present. The system should be able to alert operators to any problems and shut the equipment down in a safe manner if there is a fault.
Significant advances in ozone water treatment systems since the late 1980 to 1995 period, when a significant number of ozone systems were integrated with cooling water circuits, have occurred. The principal changes include:
The use of oxygen feed instead of dry air by means of PSA-type oxygen concentrators. This increased the efficiency of ozone dissolution due to higher ozone concentration (Henry’s Law effect), reduced capital expenses (CAPEX) and improved energy efficiency in ozone production. In addition, experience has shown that the use of air feed, with its higher nitrogen content, can result in more production of nitric acid inside the ozone generator when the dew point of the feed gas rises beyond the target levels. This causes damage to the ozone generator electrodes.
There has also been a shift towards the use of venturi injectors versus bubble diffusers. This has also resulted in an improvement of ozone water dissolution, making the process more efficient (i.e., more ozone dissolves, meaning less needs to be produced).
Modern ozone generators also employ control circuits that better monitor performance and allow for proportional control of ozone production based on input signals from instruments such as ORP probes. This keeps the ozone dosing closer to optimal levels.
Significant advances in ozone water treatment systems since the late 1980s have resulted in much more reliable and lower cost operations and better overall performance.
Operational experience has also improved the selection of materials of construction in terms of electrodes, dielectrics, piping, and seals. In addition, standard operating procedures have been better defined and integrated with PLC control for controlled system shutdowns and operator notification both locally and remotely via telecommunication systems.
The changes have resulted in much more reliable and lower cost operation and better overall performance from the early days when smaller ozone systems were not as developed.
Efficacy of Ozone as a Biocide
Ozone is a highly reactive and powerful oxidizing agent that acts as a biocide, which means it is capable of killing or inactivating various microorganisms such as bacteria, viruses, fungi, and protozoa. Its biocidal properties have made it a popular choice for applications such as water treatment, air purification, and food disinfection.
The action of ozone as a biocide can be described through the following mechanisms:
22 the ANALYST Volume 31 Number 1
Figure 7: Illustration of Ozone Interactions With Bacteria
An Update on the Use of Ozone in Cooling Water Treatment: Part 1 continued
Source: James and Yuan, Reference 10

Oxidation: Ozone is a strong oxidizer, which means it has the ability to gain electrons from other molecules. When it comes into contact with microorganisms, it can cause oxidative damage to their cellular components, such as proteins, lipids, and nucleic acids. This damage leads to the inactivation or death of the microorganisms. The highly reactive nature of ozone allows it to break down the cell walls and membranes of bacteria and other pathogens, making it an effective biocide. The oxidative damage of ozone is shown in the illustration (Figure 7) and photomicrographs in Figure 8 (10).
Ozone can also work via other mechanisms including disruption of cellular processes. This can include interference with essential enzymes and metabolic pathways, as well as damage to genetic material (DNA or RNA). The resulting impact on cellular functions can cause the microorganisms to become inactive or die.
With regard to viruses, ozone has been found to be effective against a variety of viruses. It can inactivate viruses by damaging their protein coat, which in turn prevents the virus from attaching to host cells and replicating. Additionally, ozone can also cause damage to the viral nucleic acids, rendering the virus non-infectious.
The U.S. Environmental Protection Agency (EPA) (11) and various water treatment authorities have published extensive data on the biocidal action of ozone in drinking water and wastewater treatment using the CT concept. Ozone has also been studied widely in the food and beverage industry as a biocide.
The CT concept of disinfection is used in water treatment and disinfection processes to evaluate the efficiency of a disinfectant in inactivating or killing microorganisms. The term “CT” is an abbreviation for “concentration multiplied by time,” representing the product of the concentration of the disinfectant (C) and the contact time (T) it has with the microorganisms. The CT concept is particularly useful for comparing the effectiveness of different disinfectants or determining the optimal conditions for a specific disinfectant to achieve the desired level of inactivation of microorganisms in water. In general, lower CT values for a given log reduction of the microorganism indicate a greater biocide efficiency.
The relationship between CT and disinfection efficiency is influenced by factors such as the type and concentration of the disinfectant, the specific microorganisms being targeted, water temperature, pH, and the presence of other substances in the water that may react with the disinfectant. Different disinfectants have different CT values needed to achieve a particular level of inactivation for a specific microorganism. For instance, chlorine, ozone, bromine, and chlorine dioxide are commonly used disinfectants, and each of these has a unique set of CT values for different microorganisms.
In real-world applications, the concentration of a disinfectant is usually not constant throughout the entire contact time. To account for this variability, the CT value can be calculated using integration. The integration approach provides a more accurate representation of the actual disinfection process by considering the changes in
23 the ANALYST Volume 31 Number 1
Figure 8: Photo Micrographs of Ozone Interactions With Bacteria
The photomicrograph on the left shows bacteria before ozone treatment; the right image shows after ozone treatment
An Update on the Use of Ozone in Cooling Water Treatment: Part 1 continued
concentration over time. Here is a general outline of the mathematical derivation of CT when the concentration is variable over time:
1. Define the concentration of the disinfectant as a function of time, C(t).
2. Determine the time interval during which the disinfectant is in contact with the microorganisms, [t1, t2].
3. Integrate the concentration function, C(t), over the time interval [t1, t2] to calculate the CT value: This is illustrated in Equation 2.
CT = ∫(C(t) dt) from t1 to t2 Eq. 2
The biocidal action of ozone can be illustrated using the CT values for a given log inactivation of various microorganisms. This is shown in Table C.
Regarding Legionella specifically, an important organism for cooling water treatment, several studies have shown that ozone is effective for inactivating the organism and that it action is superior to other biocides (12-14).
Table C: CT Values and their Effect on log Inactivation
Organism
CT Value and Associated Log Inactivation
E. coli 0.02 – 0.06 mg-min/L = CT (2-log)
Streptococeus
faecalia 0.01 – 0.025 mg-min = CT (2-log)
Legionella
pneumophila 0.3 – 1.1 mg-min/L = CT (2-log)
Total Coliform 0.19 mg-min/L = CT (6-log)
HPC 0.19 mg-min/L = CT (3-log)
As noted, and shown in Table C, ozone is effective for inactivating viruses. The data in Table D was developed by the EPA for water treatment applications. Table D shows log reduction of virus for various CT values as a function of temperature. For example, in order to reduce virus by 3 orders of magnitude at 15°C, the required CT value is 0.5 mg-min per liter (mg-min/L).
Comparison of Ozone With Other Oxidizing Biocides
We can compare ozone to some other commonly used oxidizing biocides using CT. The following table compares various biocides for the inactivation of giardia (3-log) and virus (4-log) at 5°C. Lower values are better. Table E (15) shows a comparison of ozone with other common oxidizing biocides.
Environmental Impact
Gomez de Saravia (16) found ozone to be an environmentally friendly biocide for cooling water. Ozone decomposes into oxygen in water without generating harmful by-products such as halogen compounds. Ozone does not contribute to the formation of disinfection by-products (DBPs) such as trihalomethanes (THMs) and haloacetic acids (HAAs), which are linked to various health and environmental risks. This can be an issue in cooling water, depending on where the blowdown is discharged.
Ozone off gas can be readily destroyed in the gas phase using catalytic converters. For many cooling water applications, the off-gas volumes are small enough that they do not require any further treatment other than simple venting out of areas where people would be exposed. Ozone system suppliers should offer off gas ozone destruct as part of their systems if necessary. Where off gas destruction of ozone is required, the limit is normally around 0.1 ppmv. In cooling towers, the tower itself can act as an ozone scrubber to some extent lowering the release of ozone into the environment.
Safety
Ozone is produced on-site, eliminating the need for storage and transportation of hazardous chemicals. It poses minimal risk to operators and has a low potential for accidents if used with the appropriate instruments and controls. Ozone safety and instrumentation were already covered above.
24 the ANALYST Volume 31 Number 1
Temperature (°C) Inactivation <1 5 10 15 20 25 2-log 0.9 0.6 0.5 0.3 0.25 0.15 3-log 1.4 0.9 0.8 0.5 0.4 0.25 4-log 1.8 1.2 1.0 0.6 0.5 0.3
Table D: Virus Inactivation – log Inactivation Versus Temperature
Organism Free Chlorine Chloramine Chlorine Dioxide Ozone (pH 6-7) (pH 8-9) (pH 6-7)_ (pH 6-7) Giardia 122 2,200 26 1.9 Virus 8 1,988 33 1.2 An Update on the Use of Ozone in Cooling Water Treatment: Part 1 continued
Table E: Cooling Tower Values for Different Oxidizing Biocides1
Chlorine, bromine, and chlorine dioxide are strong oxidizing chemicals. Their use can present risks to operators, and accidental spills or leaks can have consequences for human health, the environment, and legal liability. This may be especially important where the biocide is used close to the general public (e.g., a school or other public building). Typically, these chemicals are kept onsite and require careful handling, storage, and transportation.
Part Two
Part 2 of this article series will examine the use of ozone in cooling water treatment. Here are some of the topics that will be addressed in the article:
Limitations on ozone use in cooling water.
Injection of ozone and dosing levels in systems.
Ozone dosing.
Cycles of concentration.
Materials of construction.
System costs.
References
1. Langlais, B.; Reckhow, D.A.; Brink, D.R., editors; AWWA Research Foundation; and Compagnie Generale des Eaux (Paris, France) (1991). Ozone in Water Treatment: Application and Engineering, American Water Works Association Research Foundation and Lewis Publishers, Inc., Boca Raton, Florida.
2. Rice, R.G.; Wilkes, J.F. (1992). “Fundamental Aspects of Ozone Chemistry in Recirculating Cooling Water Systems-Data Evaluation Needs,” Ozone Science & Engineering 14(4), pp. 329-365, accessible at http://doi. org/10.1080/01919519208552276
3. Rice, R.G. (February 3-5, 1992). “Biocidal Aspects of Ozone for Cooling Water Treatment—Probable Impacts of Bromide Ion,” conference presentation, Cooling Tower Institute annual meeting, Houston, Texas.
4. Wikipedia (n.d.). Figure 2, from original image, User: Daniele Pugliesi, conversion from JPEG to SVG, accessible at https://commons.wikimedia. org/w/index.php?curid=19506013
5. AirSep Corp. (2022). Figure 3 image, AirSep Corp., Buffalo, New York, image accessible at https://files.caireinc.com/Lit/ML-IND0031.pdf
6. Owens, D. et al. (September 22-26, 2013). The Physics of Ozone Production in Commercial Ozone Generators and Application Selection,” Proceedings of the World Congress and Exposition, Las Vegas, Nevada, Copyright © 2013, International Ozone Association, International Ultraviolet Association.
7. DeNora Water Technologies (n.d.). Figure 5 courtesy of DeNora Water, a unit of Industrie De Nora S.p.A., Milan, Italy.
8. Carus (October 2008). “CARULITE 200 Granular Catalyst Fact Sheet,” Carus Corp., Peru, Illinois, pdf file available at https://www.carusllc.com/wp-content/ uploads/CARULITE-200-Granular-Catalyst-Data-Sheet.pdf.
9. Spartan Environmental (n.d.). Figure Courtesy of Spartan Environmental Technologies, Mentor, Ohio.
10. James, T.; Yuan, C. (2000). Air Liquide Corp., Chicago Research Center.
11. EPA (April 1999). Alternative Disinfectants and Oxidants Guidance Manual, U.S. Environmental Protection Agency, Office of Water (4607), Washington, D.C.
12. EPA (October 2015). “Draft-Technologies for Legionella Control: Scientific Literature Review,” U.S. Environmental Protection Agency, Office of Water, Washington, D.C., (Office of Water document 4607M) EPA 815-D-15-001.
13. Domingue, E.L.; Tyndall, R.L.; Mayberry, W.R.; Pancorbo, O.C. (1988). “Effects of Three Oxidizing biocides on Legionella pneumophila Sero group 1,” Applied and Environmental Microbiology, 54(3), pp. 741-747.
14. McGrane, W.K.; Ditzler, L. (November 9-11, 1994). “Cooling Tower Legionella pneumophila Study CDC Joint Research Project,” Watertech 1994 Conference, Houston, Texas.
15. Renner, R.C., Hegg, B.A., Schultz, H., Bender, J.H. & Bissonette, E.M. (1991). Handbook: Optimizing water treatment plant performance using the composite correction program. Office of Technology Transfer and Regulatory Support. Office of Research and Development. U.S. Environmental Protection Agency.
16. Gomez de Saravia, S.G.; Guiamet, P.S.; Videla, H.A. (2003). “Prevention and Protection of the Effects of Biocorrosion and Biofouling Minimizing the Environmental Impact,” Revista de Metalurgia, pp. 49-53, accessible at http://revistademetalurgia.revistas.csic.es

Anthony Sacco is the founder and managing director of Spartan Environmental Technologies. He also serves as a board member and vice president of the International Ozone Association. Mr. Sacco has 19 years of experience in the ozone and advanced oxidation industry, and more than 40 years of experience in the process equipment industry overall. He may be contacted at arsacco@spartanwatertreatment.com
Keywords: BACTERIA, BIOCIDES, BIOCONTROL, COOLING TOWERS, LEGIONELLA, OXIDIZING BIOCIDES, OZONE
This paper was presented at the AWT’s Convention and Exposition, which was conducted October 4-6, 2023, in Grand Rapids, Michigan.
25 the ANALYST Volume 31 Number 1
An Update on the Use of Ozone in Cooling Water Treatment: Part 1 continued
What Materials of Construction Are Needed in the Biopharmaceutical Industry?
Alexandra Peters and Marco DeAngelo, Arkema, Inc.

In the past century, the evolution of therapeutics in medicine has greatly extended life expectancy of people across the world. These therapeutics have come in several different forms including those of the pharmaceutical and biopharmaceutical industry. The pharmaceutical industry relies on chemical and synthetics processes, while biopharmaceuticals, also known as biotechnology or biopharma, is rooted in creating drugs from living organisms. Both industries act to research, develop, and manufacture medications to better lives. While they differ in chemical techniques and the means of manufacturing, the design of their manufacturing processes for highpurity systems is often similar.
It is essential for these systems to be made from highpurity materials, and capable of handling both deionized water and other strong chemicals. The water treatment technologies associated with deionized (DI) water include pretreatment, filtration, reverse osmosis (RO), ion exchange (IX), electrodeionization, and ultraviolet (UV) treatment. In all these processes, the main use of DI water is as a washing agent. The filtration processes are used to purify the process water to its high level of purity. Throughout the pharmaceutical manufacturing process, DI water is also prevalent during formulation, manufacturing, and sterilization operations.
DI Water and Chemical Corrosion
DI water is an essential ingredient in pharmaceutical and biopharma manufacturing processes. It is essential that dissolved minerals and other ions, organic compounds, and particles be removed from the high-purity process water and that it meet quality guidelines as set forth by the United States Pharmacopeia (USP) or the pharmacopeia in the region where the plant is located (for plants outside of the U.S.). Facility water treatment systems must be made from materials of construction that will not break down from corrosion or leach contaminants into the water or manufacturing process.
For instance, minerals such as sodium and calcium can cause scaling or corrosion on some materials of construction. Along with the minerals filtered out of DI water, the DI water itself can also be corrosive. Given the low ionic content in the water, DI water will absorb chemicals from both the air or material of a fluid transfer system. When exposed to air, carbon dioxide will dissolve into deionized water, creating carbonic acid, which makes the solution more acidic. DI water will also absorb ions from the material that a piping system or
Editor’s note: In the pharmaceutical/biopharma/ life sciences world, the FDA essentially enforces the standards developed by the USP as they relate to product manufacturing. This relationship came about beginning with the Pure Food and Drugs Act of 1906. In that law, congress essentially gave the standards developed by the USP legal standing. Since that time, congress has maintained that relationship as updated laws have been passed. So, for the pharma world, the USP, while a non-governmental organization, has the role of setting the standards and guidelines that pharma plants, etc. must follow (2). For its part, National Sanitation Foundation (NSF) International certifies products used in different settings, including water treatment and water handling/distribution. For example, if an ion exchange resin or reverse osmosis membrane has NSF certification for Drinking Water (DW) use, then it may be used by a utility to treat drinking water as per the U.S. Environmental Protection Agency’s DW standards
tank is made from; examples include nickel from stainless steel, aluminum from aluminum piping systems, or other chemicals from impure polymeric containers. Thus, it is essential for the material of construction to be of adequate purity for both corrosion and purity concerns.
At the same time, the addition of organic compounds such as bacteria, viruses, and algae can impact the quality and stability of the final products so they must be removed without clogging or creating film buildup in the fluid transfer systems. Thus, non-corroding seamless high-purity systems are the best solution to allow for the utmost purity in final products. For these reasons, the materials of construction for a system are typically required to be low leaching and to be stable during chemical or steam disinfection. They must meet regulations as set forth by the U.S. Food and Drug Administration (FDA), which is the enforcement agency appointed by Congress to enforce the standards as set forth under the USP (1). Likewise, they must also meet the quality guidelines as set forth by NSF International and other certification organizations.
Materials of Construction: Metals Versus Thermopolymers
There is a wide array of both metals and polymers used in the bioprocessing industry. Metals are commonly used in systems requiring high temperature water. However, the use of metals is not always simple since they cannot
27 the ANALYST Volume 31 Number 1
always meet the chemical resistance requirements in this industry. For instance, metals commonly struggle in fluctuating concentrations of acids, leading to corroded systems. On the contrary, polymers do not have the same concerns with corrosion and rusting that can be seen on metals as are inherently non-corroding and do not require passivation. Polymers also have simpler installation as lighter weight materials, making them a great solution in the bioprocessing industry.
According to American Society of Mechanical Engineers Bioprocessing Equipment (ASME BPE), polymeric materials are broken into two categories: thermoplastics and thermosets (3). By definition, thermoplastics are long-chain polymers that are usually not connected by crosslinks. Once they are formed, these materials can be reshaped and reprocessed. While similar in some properties, thermosets are usually connected by crosslinks meaning that materials harden and cannot be reshaped.
Within thermoplastics, there are several different types, including general, polyolefin, elastomeric,
and fluoropolymer thermoplastics. General thermoplastics include polyester (PET), polyamide (nylon), polycarbonate (PC), polysulfone (PSU, PES), and polyether ether ketone (PEEK). Additionally, thermoplastic polyolefins include variations of polypropylene (PP) and polyethylene (PE) while thermoplastic elastomers include blends of ethylene propylene diene monomer (EPDM) rubber with polypropylene, styrene-isobutylene-styrene block polymers, copolymers of ethylene and octane, and ethylene-vinyl acetate copolymer (EVA). For moderate chemical applications at low temperatures, general, polyolefin, elastomeric thermoplastics will likely do the trick. However, for highly corrosive systems requiring strong chemical resistance, fluoropolymers are an upgraded option as they have the highest levels of chemical resistance.
In the family of fluoropolymers, there are several options, including polyvinylidiene fluoride (PVDF), polytetrafluoroethylene (PTFE), ethylene tetrafluoroethylene (ETFE), perfluoroalkoxy (PFA), and
Cost here is product cost and does not include increased maintenance costs for materials with shorter lifespan or higher installation costs.
+++ means no concerns for chemical compatibility. It is important to note that metals often struggle with changes in acid concentrations.
28 the ANALYST Volume 31 Number 1
Polypropylene PVDF / PVDF Copolymer 304 / 316 Stainless Steel Hastelloy / Inconel Comments Upfront Cost $ low $$ medium $$ medium $$$ high
Table A: Material Property Comparison Chart
Chemical Compatibility + +++ ++ +++
Deionized Water +++ +++ +++ +++ Hydrochloric Acid + +++ + + Sulfuric Acid + +++ + ++ Sodium Hypochlorite (Bleach) ++ +++ + +++ Ethylene Oxide + +++ ++ +++ Temperature Range + ++ +++ +++ +++ means no issues at point over 150°C Mechanical Stability with Temperature + ++ +++ +++ +++ means no creeping over time over 150°C Permeation Resistance + ++ +++ +++ Permeation resistance is dependent upon the chemical media and wall thickness of the component. Rusting/Rouging Resistance +++ +++ + + Plastics do not
Range of Components Many Many Many Limited Only polymers provide both rigid and flexible options. Steam Cleaning / Autoclaving No Yes Yes Yes These sterilization techniques assume steam cleaning or autoclaving at 120°C and gamma radiation at 25 kGy. Gamma Radiation No Yes Yes Yes Notes: +++ = Excellent, ++ = Satisfactory, + = Limited
have rusting concerns.
Construction
in the Biopharmaceutical Industry? continued
What
Materials of
Are Needed
fluorinated ethylene propylene (FEP). Each of these fluoropolymers differ in fluorination level, polarity, and crystallinity, leading to different mechanical and chemical resistance properties. The ASME BPE is responsible for outlining approved materials for specific applications within bioprocessing.
Of these ASME BPE listed materials, engineers often choose PVDF as the first fluoropolymer to consider when designing chemical handling systems under pressure. It displays the excellent chemical resistance of fluoropolymers while also having the ability to be used up to 150°C in continuous use applications (4). Not only does PVDF have a wide resistance to many chemicals as seen in Table A, but it is also made without additives, making it a highly pure material with a wide range of flexibility. PVDF can also be used in standalone systems such as piping, tanks, and other components that require high mechanical stability and rigidity. In addition to this, PVDF can withstand sterilization techniques including chemical sterilization, steam cleaning, gamma radiation, and autoclaving (5).
PVDF Homopolymers and Copolymers
PVDF is available in rigid homopolymer form (ASTM D3222) for high strength, injection molded and extruded parts. PVDF homopolymers are commonly used to produce pipes, fittings, valves, pumps, solid tanks, and even membranes. However, for applications seeking additional flexibility, PVDF flexible copolymers (ASTM D5575) offer more ductile and bendable components (6). This includes flexible tubing, tank linings, and even zip ties. This wide range of flexibility offers numerous advantages in the pharmaceutical and biotech industry since a wide variety of parts are offered with excellent chemical resistance, thermal and mechanical stability, and performance characteristics. Figure 1 displays homopolymer PVDF quick disconnect fittings while Figure 2 exhibits copolymer PVDF flexible tubing.
In designing systems, it is important to understand that PVDF can be easily thermoformed, welded or machined. In high-purity systems, beadless welding is a common technique that allows for seamless systems that prevent bacteria and biofilm buildup within the system. For alternative applications, powder or liquid coating versions are available for application to metal.
Figure 1: Homopolymer PVDF Quick Disconnect Fittings Courtesy of Eldon James

Figure 2: Copolymer PVDF Flexible Tubing Courtesy of Eldon James

29 the ANALYST Volume 31 Number 1
What Materials of Construction Are Needed in the Biopharmaceutical Industry? continued
PVDF is commonly used in as a material of construction for fluid handling components, including both pipes and tanks. It’s widespread chemical and abrasion resistance make it an optimal choice for fluid-handling systems ,which also require high purity and minimal leaching (7). The ASME BPE Standard lists piping, vessels, and other components for polymeric systems. Figures 3 and 4 show PVDF piping systems. From high-purity laboratories to vaccine production facilities, PVDF is found in many applications requiring fluid transfer systems with sustained longevity in the pharmaceutical industry.
Properties of PVDF
PVDF homopolymers and copolymers are resistant to a wide range of chemicals at high temperatures. Many acids, halogens, hydrocarbons, alcohols, oxidants, and weak bases can be handled by PVDF-based polymers. PVDF homopolymers have a UL® Relative Thermal Index (RTI) rating up to 150°C and can be used continuously under stress in environments with a pH of <1 up to 12, depending on the chemistry and environment. If there are concerns of stress cracking, or a need for a material with higher ductility and elongation, PVDF copolymers offer a solution as they can be used continuously under stress in environments with a pH of <<1 to 13.5. This characteristic extends the range of uses for basic chemistries. Table B lists common cleaning agents found for water systems and how the chemical resistance of different materials compares.
Compared to other materials in Table B, PVDF has excellent abrasion resistance as seen by testing on a Taber Abrasion wheel. This makes the material an optimal choice for solutions using salts and other abrasive chemistries. PVDF also has excellent permeation resistance to water and oxygen. In addition to this, it has high impact resistance at ambient and colder temperatures. For applications requiring resistance to burning, PVDF is Factory Mutual (FM) 4910 compliant and has an E84 25/25 rating.
PVDF Full Systems
While PVDF has a continuous use rating up to 150°C, it also has a relatively low melting point compared to other fluoropolymers, making it simple to weld and install. PVDF piping systems have several means of joining, including socket fusion, butt fusion, infrared, electrofusion, mechanical, and beadless. The ability for beadless systems allows for seamless joining to prevent bacterial buildup. In designing piping systems, tank systems, or additional components, PVDF has a history of longevity in bioprocessing applications as a high-purity option as both a single-use or multiuse material. The wide


30 the ANALYST Volume 31 Number 1
What Materials of Construction Are Needed in the Biopharmaceutical Industry? continued
Figure 4: PVDF Piping System Courtesy of Georg Fischer Piping Systems
Figure 3: PVDF Piping System Courtesy of Georg Fischer Piping Systems
range of flexibility, excellent chemical resistance, inherent nonrusting, and regulatory approvals make PVDF a good option for material of construction in bioprocessing applications.
Conclusions
When choosing the appropriate material of selection, it is integral to make the decision most suited to the needs of the application. For the biopharmaceutical industry, properties such as chemical compatibility, mechanical stability under temperature or pressure, permeation resistance, rusting concern, steam cleanability, and ability to be autoclaved or gamma radiated are all important considerations. In a highly regulated industry, standards are able to direct on how to make the best selections. PVDF serves as an ASME BPE-listed polymer that makes an excellent material of selection for many applications in this industry, including seamless full piping and tank systems. PVDF provides the excellent chemical resistance of a fluoropolymer with resistance to extremely strong acids to bases with a pH up to 13.5. With a UL RTI rating of 150°C, PVDF can be steam cleaned, gamma irradiated, and autoclaved. It is a phenomenal choice as a material of construction for fluid transfer systems in the pharmaceutical industry.
References
1. AWT (2022). Technical Reference and Training Manual, “Chapter 8: Other Water Treatment Applications,” Association of Water Technologies, Rockville, Maryland.
2. USP (n.d.). “FAQs: USP and its Standards”, “Question 3: What Is the Role of USP Standards in Federal Law?” United States Pharmacopeial Convention Inc., Rockville, Maryland, accessible at https://www.usp.org/frequently-askedquestions/usp-and-its-standards
3. ASME (2022). ASME Bioprocessing Equipment Standard, available at https://www.asme.org/codes-standards/find-codes-standards/bpebioprocessing-equipment-(1)
4. Peters, A.; Palovcak, A.; Seiler, D. (Summer 2021). “Using Plastic Piping to Carry Wastewater Chemicals,” the Analyst, pp. 37-44.
5. Gruen, H.; Burkhart, M.; O’Brien, G.S. (October 2001). “Steam Sterilization of PVDF Piping Systems in PW and WFI for the Pharmaceutical and Biotechnology Applications,” Ultrapure Water Journal 18(8), pp. 31-38.
6. Palovcak, A.; Pomante, J. (September 2018). “PVDF Copolymers for Flexible Fluid Handling Components,” Flow Control Magazine
7. Seiler, D.A.; Barber, L. (May/June 1989). “Pure Materials, Handling HighPurity Fluids with New, Flexible PVDF,” Ultrapure Water Journal 6(4), pp. 37-38.

Alexandra Peters is an applications and standards engineer with Arkema Inc. of King of Prussia, Pennsylvania. Peters received her BS in chemical engineering from Villanova University. In her work, she focuses on PVDF end-use applications and standards. She is a member of ASTM and the ASME B31.3 and BPE committees. Ms. Peters may be reached at alexandra.peters@arkema.com

Marco DeAngelo is an account manager with Arkema in King of Prussia, Pennsylvania. Mr. DeAngelo received his BS degree in chemical engineering from Pennsylvania State University. In his position, he focuses on technical support for users in the Great Lakes, Northwest U.S., and Canada. He is a member of the Material Technology Institute, ASPE, and has presented on PVDF Homopolymer and Copolymer’s for corrosion control. He may be reached at marco.deangelo@arkema.com.
Keywords: CORROSION, MATERIALS OF CONSTRUCTION, PHARMACEUTICALS, PIPING, PVDF, STAINLESS STEEL, THERMOPLASTICS, VALVES
31 the ANALYST Volume 31 Number 1
What Materials of Construction Are Needed in the Biopharmaceutical Industry? continued
Materials Mg Loss PVDF Homopolymer 740 5-10 Polyamide 6-10 (nylon) 4-6 PVC (Rigid) 12-20 Polypropylene 15-20 CPVC 20-25 HDPE 25-30 304 Stainless Steel 50-60 Mild Steel 100-300 PTFE 500-1000
Table B: Abrasion Resistance Using Taber Abrasion Ring CS-10 (mg/1,000 Cycles Using 1 kg Load).
When Treating Cooling Towers with ClO2, Is There a Best Way?
Greg D. Simpson, PhD, Pureline Treatment Systems

Introduction
Chlorine dioxide (ClO2) has been used for microbiological control of cooling water since the early 1970s (1-3). Even in these early reports, researchers observed the effectiveness of ClO2 as a bactericide (4), sporicide (5), and virucide (6), and its control of biofilm (1). Note that later research showed that for biofilm control, chlorine (in whatever form) dosed continuously at low level was found to be best, but for ClO2, short, intermittent doses at higher concentrations (i.e., ~ 1 parts per million [ppm]) was found to be optimal (7).
ClO2 Injection
Injection point, dosage, frequency and treatment duration. Early researchers identified the recirculation pump sump as the best place, generally, to inject the ClO2, with dosages not to exceed approximately 1.0 parts per million (ppm) (8). Sometimes the injection point would vary, depending upon the system (3). Sometimes ClO2 was injected into the makeup (9), into the recirculation pump sump (1), directly before fouled exchangers (10), or into the existing chlorine header to sweep the basin (11). On one or two occasions, ClO2 was injected just prior to the film fill of a tower (12).
Early researchers used different approaches that were dictated by the nature of the system. The method of application varied, including changes in the dosage, changes in the frequency of treatment, and changes in the duration of treatment.
The surprising thing is that despite all the different ways ClO2 has been applied since 1970, it has been highly successful. In the early days, ClO2 was only used when chlorine could not keep the system clean or when the high chlorine levels required to do so would be highly corrosive. Over time, as chlorine gas was being phased out, ClO2 began to compete successfully in an economical way with other, competitive biocides, although from a strictly performance perspective, it has always been a good choice.
The focus of this article is to provide an overview as to how the treatment methodology has evolved over time, primarily to reduce the cost of treatment and to identify the best available technology for treatment.
Injection Points
The location of the injection point can impact the performance of ClO2. In most situations, ClO2 has been injected into the pump suction. But even injecting it into pump suction can be problematic. For example, in the 1980s, in an early, unpublished case history (13), a prototype electrolytic ClO2 generator was evaluated.
Example 1
A natural gas plant in Southern Louisiana had a cooling tower with a microbiological issue. Engineers from the ClO2 generator supplier and plant personnel who had some experience with ClO2 had insisted that it be injected into the pump suction. So, a PVC injection

33 the ANALYST Volume 31 Number 1
Figure 1: Actual Versus Better Injection Point
tube was fabricated through which the solution could be pumped. The ClO2 injection tube was quite long, as the pump sump was unusually deep. The tube was sent to the bottom and then withdrawn about a foot.
The generator was started. After several minutes, testing of the recirculating water showed no ClO2. Generator effluent was tested to ensure that the generator was producing ClO2. It was. After an hour and still no ClO2 residual, it was finally suggested that the ClO2 injection pipe be shortened. The only things that made sense were that the pump sump contained some contaminant that was consuming the ClO2 and the inlet to the recirculating pump was considerably higher than the ClO2 injection point, or that we were injecting into a relatively dead zone, with respect to flow. In either case, the stinger was shortened by about 6 feet, to a point near to pump suction.
Within seconds, a ClO2 residual could be detected in the recirculating pump discharge. This suggested that the injection point was very important; that the injection point should be as near pump suction as possible. This is shown schematically in Figure 1.
Example 2
In another gas plant, the film fill was fouled so badly that treatment with ClO2 resulted in massive sloughing of small biofilm fragments. The block and tackle normally used to remove the screens to clean them was too slow, and so two operators removed the screens rapidly to prevent starvation of the recirculation pumps. Figure 2 shows a picture of one of the duplicate stainless-steel screens that were being fouled so rapidly.
It should be obvious that injecting ClO2 before the screens that are protecting the recirculation pump is wasteful of ClO2. While it will work, it will take more ClO2 to keep these screens clean than to clean them manually.


Example 3
Figure 3 shows the schematic of the cooling system in another natural gas processing plant (gas plant) in the Southwest, before and after an expansion. The water distribution headers were underground. Consequently, the extra pumps that had been added sent the water to the newer part of the plant and in a different direction.
The normal procedure of the ClO2 supplier was to fabricate an injection header so that each pump saw ClO2. But the crew decided to save some PVC piping and effort and fabricated a header to feed only the first three pumps.
When biofilm began to grow on some of the sensors on the expansion part of the plant, the right questions were asked, and corrections were made. This illustrates the importance of making sure each pump sees ClO2. This includes the auxiliary system in power plants.
Example 4
A Western ammonia plant was treated with ClO2 through the existing chlorine header to “sweep” the basin. Virtually every type of biocide or oxidant had been tried in this tower, without success. It was not until ClO2 was used that biological control was achieved.
During each turnaround, some of the exchangers opened with some biofilm, suggesting that the system was being undertreated. But when the cooling basin was drained, the basin was free of sludge or biofilm or any other biological material. The conclusion was that feeding ClO2 to “sweep” the basin is not optimal, and a greater amount of ClO2 is required to control biofilm in plant heat exchangers when ClO2 is fed to sweep the basin. Essentially, sweeping the basin with ClO2 is a very expensive way to keep the basin clean.
34 the ANALYST Volume 31 Number 1
Figure 2: Fouled Screen
When Treating Cooling Towers with ClO2, Is There a Best Way? continued
Figure 3: Original Versus Expansion System
Observations
Determining treatment based on system volume. There are few reports of dosing based on system volumes, although a few exist (3, 7, 14). Treatment was successful, which demonstrated the remarkable performance of ClO2. However, from a scientific point of view, this type of treatment did not account for the relative cleanliness of a system, because the amount of ClO2 required was more a function of surface demand than of bulk water demand (15).
Continuous treatment for several hours per day. Early researchers began to apply ClO2 so that the dosages were high enough to be effective, but sufficiently low so that ClO2 lost over a cooling tower was minimized (1). The injection point was unclear from some of the reports, although the technical manual from this group indicated that pump sump was the appropriate place (6). From the papers cited, Table A provides an overview of the types of cooling water systems that were treated.
Treatment several times per day. As the successes grew, rather than treat once for several hours in a single day, researchers began to treat several times during the day for periods of time (16). Again, the application knowledge increased. Economically, multiple, short durations of treatment were more favorable than a single, treatment of longer duration every day. Table B shows data from facilities treated for multiple times per day, from the papers cited.
Since then, if there were leaks which could be used by bacteria as nutrients, multiple times per day treatment was probably more economical and with better results, although the results of earlier work were acceptable (17).
Intermittent treatment for several hours every few days. About this time, a mobile approach was developed whereby a cooling tower could be treated every few days with a single dose of ClO2 (Figure 4) (18, 19). This type of treatment was done for a duration determined by reaching and maintaining a ClO2 residual for a specific duration. This was done primarily in areas of the country where well water of good bacteriological quality was used as makeup and bleach supplies unreliable. This type of treatment was also very successful (10, 20-23).
This was particularly advantageous for facilities that had multiple cooling towers, where a single generator could be used to treat the entire facility, and where no chemicals were left on site.
The disadvantage of this approach was that the expensive mobile generator and manpower required to operate the generator were tied up for several hours for each treatment. This included time of treatment along with the time it took to travel from one plant to another. In parts of the country where there was a significant drive time between accounts, the best that could be done was three to four towers per day. Despite the advantages, this approach was not found to be economically viable.
35 the ANALYST Volume 31 Number 1
Date First Author Type of Plant Recirc Rate (gpm) Optimal Dose (ppm) Duration (hr/treatment) ClO2 (lb/day) Cl2 (lb/day) 1976 Rauh Aluminum Producer 4,000 Gulf Coast NH3 Plant 80,000 1.2 3 24 1,200-2,400 Veg Oil Refiner 12,000 200 Amine Synthetic Plant 2 1977 Sussman West Coast NH3 Plant 14,500 5 160-300 SW Chemical Plant 20,000 4.6 107 GC Petrochemical 85,000 19 1,000 Mountain States Refinery 10,000 12 250 Midwestern Refinery 30,000 13.5 500 East Coast Refinery 170,000 56 4,200 East Coast Power Plant 110,000 15 200 1978 Ward Midwestern NH3 Plant 40,000 300 Gray Power Plant 1 Continuous
Table A: Summary of Systems Treated—Once/Day for Varying Times
When Treating Cooling Towers with ClO2, Is There a Best Way? continued
Case Histories: Southwestern Gas Plant
This Southwestern natural gas plant used two nonoxidizing biocides for microbiological control, along with chlorine tablets three times/week, as recommended by the supplier. Frequent hydrocarbon leaks resulted in the formation of bacterial slime which required screens to be cleaned once/shift and exchangers to be backwashed daily.
Water used by the plant was of such poor quality that partial zeolite softening was used. The high frequency of exchanger backwashing required a significant amount of makeup water. Water from zeolite regeneration and backwashing of exchangers was sent to two evaporation ponds that were both full and near overflowing. Plans were well in place to build a third evaporation pond at the site.
The chlorine dioxide field trial began with ClO2 being fed to pump suction of the cooling tower through a distribution header to ensure all pumps saw an equal amount of ClO2. In this way, treatment of a cooling system with 1-ppm-based on total recirculation rate did not cause one pump to see more than 1 ppm.
Even with treatment every few days with the mobile generator (Figure 4), required screen cleanings were reduced to once every few days, and then longer between required cleanings as time passed and the system continued to clean up. Backwashing was virtually eliminated, greatly reducing the load on the evaporation ponds. After a few months, because backwashing had been so greatly reduced, one pond was eliminated and the second was reduced to half its original volume and was decreasing in size.
A subsequent bonus was that, because heat transfer had so improved, one fan and one recirculation pump could be turned off. Even with this, plant production increased to a level never before seen in this plant, estimated by plant personnel to be 15%. Plant personnel estimate that this added significantly to their bottom-line profits, not including costs of manpower required and electricity

36 the ANALYST Volume 31 Number 1
Date First Author Type of Plant Optimal Dosage (ppm) Frequency (treatment/days) Duration (hr/treatment) 1984 Freund Grain Alcohol Plant 4 1 1989 Pacheco Ethylene Glycol Cooling Tower 1 6 1 1993 Simpson SW Petroleum Refinery 1 1 3 1 SW Refinery 2 1 3 1 SW Natural Gas Plant 2 1 3 1 SW Natural Gas Plant 3 1 3 1 SW Natural Gas Plant 4 1 4 1 1999 Nalpas UK Nuclear Power Plant 0.2 2 1 2001 Laxton Gulf Coast NH3 Plant 650 mV 8 9 min
Table B: Multiple Treatments per Day
When Treating Cooling Towers with ClO2, Is There a Best Way? continued
Figure 4: Mobile ClO2 System
saved, in addition to the avoidance of the cost to build a new evaporation pond.
Low-level continuous treatment. In this approach, treatment was done at very low levels, sometimes less than 0.1 ppm. This approach has also met with some success, depending upon the system, although there are cautions. Two case histories follow in this section.
Case History: Texas University
One of the larger universities located in Texas wanted to evaluate ClO2 because of its known benefits towards disinfection and biofilm control. The main tower (Tower 1) was used primarily for comfort cooling and had a recirculation rate of 28,000 gpm. Bleach had been used for microbiological control and results were very good.
ClO2 was injected into pump suction at 1 ppm, three times per day for 20 minutes (min) duration. Results continued to be very good.
During this treatment, there was an opportunity to test how low the ClO2 dosage could be and still get good results. So, the dosage was cut in half every week or so for a number of weeks. Bacterial counts as measured by adenosine triphosphate (ATP) was monitored over the next several months, which extended well into the hot summer months.
Figure 5 shows the results of ATP testing (24) (a measurement that is related to the number of bacteria present in a system), which was done over a period of months during this time. The lowest dosage, < 0.1 ppm ClO2, was applied during the hottest months. Makeup water to the towers was city water disinfected with monochloramine.
Note that the ATP numbers remained in the same range, even when the (ClO2) residual was too low to be measured. Figure 5 shows a comparison of ATP data over a similar time for both the makeup water and another tower which was treated with bleach. It is striking that Tower 1 is cleaner, from a biological perspective, than the disinfected makeup water.
Findings. The data show that even with ClO2 being fed at a residual too low to be measured (i.e., << 0.1 ppm) microbiological control was exceptional. Because the residual was too low to be measured accurately, feed was increased to provide a residual of at least 0.3 ppm minimum, just to be safe.

The data also showed that when cooling tower makeup is known to be well disinfected and no vaporous contamination exists, a very low residual treatment of a cooling system can work. However, even with this, some mechanism of monitoring such a low concentration of ClO2 must be used, either Kemio or oxidation reduction potential (ORP).
Case History: Continuous Treatment of a Power Plant
More recently, this approach has been used to treat large power plant cooling towers which have high recirculation rates.
Personnel from a large power plant in the Southeast requested consultation to investigate their issues with low-level ClO2 treatment, as some biofilm had been observed in their main surface condenser, and the tower was green. Their cooling system with a recirculation rate of 330,000 gpm used makeup that came from a nearby tertiary treatment municipal system. The water contained ammonia, and chloramine, as the municipal effluent was chlorinated.
There was no monitoring of the makeup water for chlorine, monochloramine (other chloramines or organic chloramines are poor disinfectants) (25, 26), or bacteriology by the service provider. Testing of water returning to the large tower during ClO2 treatment showed a ClO2 residual of 0.26 ppm, using the DPD (N,N-diethyl-p-phenylenediamine) colorimetric test, but <0.02 ppm by the Kemio test (27). The Kemio unit is a ClO2 monitoring device which uses disposable sensors, much like that of a blood glucose test, to measure ClO2 and chlorite ion up to 50 ppm, and chlorine up to 25 ppm (different sensors). This test has been found to be very reliable in clean systems and has been approved for use by
37 the ANALYST Volume 31 Number 1
When Treating Cooling Towers with ClO2, Is There a Best Way? continued
Figure 5: ATP Measurements of Makeup and Two Cooling Towers
the EPA in potable water. In dirty water, it was found to measure ClO2 reliably, but in some contaminated water, there is interference when testing for the chlorite ion. This confirms other work (20), which showed that DPD is prone to false positive when ClO2 or Cl2 residuals are < about 0.3 ppm, while the Kemio has surprisingly been very good at measuring levels of ClO2 above about 0.03 ppm.
This point is further illustrated in Figure 6. In earlier work, in evaluating a new test for ClO2 using Lissamine Green B dye, water was tested for ClO2 by both the new test and DPD. These results clearly show that at around 0.2 to 0.3 ppm, DPD can become unreliable, especially in contaminated water. Similar results were shown using the Kemio test.
Findings. For systems treated at very low levels of ClO2 (i.e., <0.1 ppm), it is imperative that the quality of the makeup be monitored. It is also imperative that the concentration be monitored by a method that can measure a ClO2 residual of < 0.1 ppm reliably. Use of DPD for such testing is questionable. In addition, any incursion of air contaminated by vapors, crop dust, or coal fines needs to be identified. It is more difficult to account for this than it is to account for questionable makeup water quality and thus treatment at very low levels cannot account for this.
Treatment with Monitoring and Control
Many industrial plants, primarily food, ethanol, refining, petrochemical or others, are susceptible to leaks of one type or another. These leaks provide nutrients to bacteria, algae, and fungi that can cause rapid growth, depending on the severity and nature of the contamination. Losses in heat transfer can therefore occur.
Other towers may have makeup quality of questionable quality from a bacteriological standpoint. Or towers may draw in fines that consume ClO2 or odors that feed bacteria, algae, and especially fungi (28).
One approach which has been very successful has been an intermittent approach, which is at the same time effective, and economical. In this approach, the generator is set to come on at specific times during the day. Rather than set the generator to stay on for a specific duration, the system is monitored for either an ORP setpoint or to reach a residual of ClO2. Once the residual or ORP has reached its user-selectable setpoint, the generator may shut off or it may be allowed to run for a few minutes to ensure the tower “sees” more ClO2. Figures 7 and 8 show how this works.
The advantage of this approach is that the ClO2 generator automatically responds to leaks, changes in makeup water quality, and incursion of air contaminants. These changes
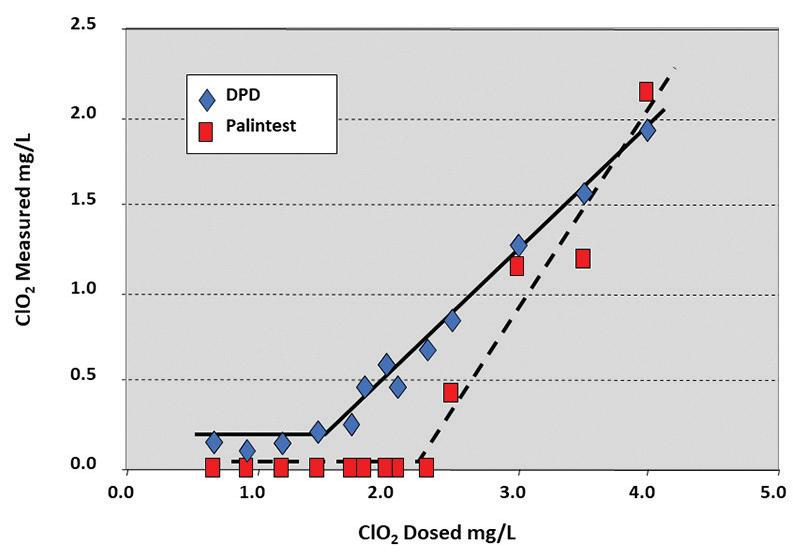
38 the ANALYST Volume 31 Number 1
Figure 6: DPD Versus Lissamine Green B Tests
When Treating Cooling Towers with ClO2, Is There a Best Way? continued


39 the ANALYST Volume 31 Number 1
Figure 8: Response of ORP to Leaks
When Treating Cooling Towers with ClO2, Is There a Best Way? continued
Figure 7: Analysis of a Treatment
may feed microorganisms, which creates a higher demand for ClO2. This means that the time to reach an ORP setpoint takes longer, so that this approach accounts for any change which increases the demand for ClO2.
Case History: Midwest Natural Gas Plant
One midwestern natural gas plant was prone to leaking exchangers. The generator was set to come on every 4 hours and run until the ORP reached a setpoint of ~ 650 millivolts (mV). The first time the generator came on, it ran for approximately 30 minutes before the system was sufficiently free of surface demand (biofilm) to allow the ORP setpoint to be reached.
After that, each time the generator would come on, ClO2 continued to “clean” the system of biofilm, the duration required to reach the setpoint became shorter and shorter. Ultimately, the generator came on for 3 to 5 minutes every 4 hours. This duration remained unchanged until a small light hydrocarbon leak occurred. As a result, bacteria would grow, and the generator would take slightly longer to reach the ClO2 setpoint. In this way, the response of the system to the generation of ClO2 acted as a leak detector. In addition, this “self-tuning” method provided optimum results at lowest possible feedrate.

Final Thoughts
Low-level continuous treatment.
The foregoing case histories should make it apparent that a low-level continuous treatment can work, but conditions must be ideal. For this approach to work, it is essential that the makeup must be well disinfected, no vaporous contamination is possible (28), and no leaks that feed microorganisms is possible. Otherwise, biological material can grow.
An approach that would make this approach more generally applicable would be to feed at a higher rate periodically, which was done at the southeast power plant. But even with this approach, there were issues. Therefore, this approach does not appear to be the best available technology.
Intermittent treatment several times per day with monitoring and control.
This approach is a “self-tuning” approach that ensures biological growth is kept at a minimum by accounting for leaks, changes in makeup water quality and incursion of vaporous contaminants. This approach ensures that economics are optimized.
Providing water treatment chemistry and logistics solutions
Your business has unique needs and challenges. Our in-house technical professionals connect you to our global network and local resources to deliver innovative and effective solutions to support your business.
We offer an extensive portfolio of products and ingredient chemistry, complemented by value-adding services such as toll-blending and private labeling.
brenntag.com
40 the ANALYST Volume 31 Number 1
When Treating Cooling Towers with ClO2, Is There a Best Way? continued
Acknowledgements
This article acknowledges the contributions of Jim Rauh, William Ward, Sidney Sussman, J. W. Lee, Scott Freymark, Harry Gray, and others from Olin Water Services, who pioneered the use of ClO2 for cooling towers.
References
1. Rauh, J. (1976). “Chlorine Dioxide. A Cooling Water Microbiocide,” Proceedings of the Fourth Annual Industrial Pollution Conference, Water Wastewater Equipment Manufacturers Association McLean, Virginia.
2. Sussman, S.; Ward, W. (1977). “Microbiological Control with Chlorine Dioxide Helps Save Energy,” Materials Performance 16(7), p. 24.
3. Gray, H.; Speirs, A. (January 23-25, 1978). “Chlorine Dioxide Use in Cooling Systems Using Sewage Effluent as Make-Up,” Technical Paper 183-A, Cooling Tower Institute.
4. Ridenour, G.; Ingols, R. (June 1947). “Bactericidal Properties of Chlorine Dioxide,” Journal of the American Water Works Association 39(6), p. 561.
5. Ridenour, G.; Ingols, R.; Armbruster, E. (1949). “Sporicidal Properties of Chlorine Dioxide,” Water Works Sewage, 96, p. 276.
6. Smith, J.; McVey, J. (August 26-311973). “Virus Inactivation by Chlorine Dioxide and its Application to Storm Water Overflow,” Division of Environmental Chemistry, American Chemical Society, Chicago, Illinois.
7. Mayack, L.; Soracco, R.; Wilde, E.; Pope, D. (1984). “Comparative Effectiveness of Chlorine and Chlorine Dioxide Regimes for Biofouling Control,” Water Research 18(5), p. 593.
8. Olin Corp. (circa 1978). ClO2 Application Manual, Olin Corp., Clayton, Missouri.
9. Ward, W.; Lee, J.; Freymark, S. (1978). “Advantages of Chlorine Dioxide as Biocide,” Ammonia Plant Safety, 20, p. 64. –
10. McGuire, L.; Dishinger, T. (February 1984). “Chlorine Dioxide Solves Biofouling Problems in a Refinery Cooling Tower Used for Phenol Destruction,” TP 84-06, Cooling Technology Institute, Houston, Texas.
11. Pickrell, W.; Germer, M.; Miller, J.; Simpson, G. (January 27-29, 1999). “Plants Increase Production through Use of Chlorine Dioxide,” First International Conference: Practical Industrial Water Treatment Technology for the New Millennium, Houston, Texas.
12. Simpson, G.D. (September 14-15, 1995). “Biofilm: Removal and Prevention with Chlorine Dioxide,” Third International Symposium on ClO2, New Orleans, Louisiana.
13. Simpson, G. (August 1988). Unpublished results based on personal research.
14. Wadsworth, J. (1991). Personal communication.
15. A more detailed description of “Surface Demand and Bulk Water Demand” can be found in Simpson, G., “What is the Difference Between Bulk Water Demand and System Demand?” Chapter 16 in Practical Chlorine Dioxide: Volume II – Applications, Lightning Source Publishers, La Vergne, Tennessee, 2006.
16. Freund, J.L. Jr.; Booker, J.M. Jr.; Ward, W.J. (October 22-24, 1984). “Biocontrol: Key to Heat Exchanger Efficiency in Grain Alcohol Plant,” Proceedings of the International Water Conference, Pittsburgh, Pennsylvania.
17. Pacheco, A.M.; Durham, H.E.; Dhillon, R.; Edward, C. (1989). “The Use of Chlorine Dioxide to Control Microbiological Growth in an Ethylene Glycol Contaminated Cooling Tower... A Case History,” Paper No. TP89-14, Cooling Technology Institute annual conference.
18. Simpson, G.; Laxton, G.; McCullough, H.; Miller, J. (September 26, 1995). “Biocide Treatment System and Method,” U.S. Patent No. 5,453,207.
19. Simpson, G.; Laxton, G.; McCullough, H.; Miller, J. (June 19, 1995). “Biocide Treatment System and Method,” U.S. Patent No. 5,611,920.
20. Laxton, G.; Simpson, G. (October 18-20, 1993). “Chlorine Dioxide: The Versatile Oxidizer,” presented at the NACE Regional Meeting, Midland, Texas, National Association of Corrosion Engineers (now AMPP).
21. Simpson, G.; Laxton, G.; Miller, R.; Clements, W. (November 10-12, 1993). “A Focus on Chlorine Dioxide: Biocide of Choice for ‘Stressed’ Cooling Water Systems,” WaterTech 93, Houston, Texas.
22. Simpson, G.; Miller, J. (October 31-November 4, 2000). “Control of Biofilm with Chlorine Dioxide,” AWT Annual Convention and Exposition.
23. Simpson, G. (February 15-16, 2001). “Control of Biofilm in Cooling Towers with ClO2,” Fourth International Symposium on Chlorine Dioxide, Las Vegas, Nevada.
24. A more detailed discussion of ATP testing can be found in Simpson, G., Chapter 7. Monitoring, in Practical Chlorine Dioxide: Volume III – Oil & Gas, Lightning Source Publishers, La Vergne, Tennessee, 2021.
25. Jang, H. (May 2009). “Organic Chloramines Formation and Its Disinfection Efficacy,” Doctoral Dissertation, Arizona State University, Tempe, Arizona.
26. Hach Co. (2023). “Disinfection Series 4, Introduction to Chloramination,” Hach Co., Loveland, Colorado.
27. The Kemio unit is a testing device whereby sensors, not unlike blood glucose test strips, are used to perform many tests on a given water over a minute to test ClO2 (up to 50 ppm). This unit can also be used to test for chlorite (up to 50 ppm) and chlorine (up to 10 ppm, although a new sensor is available which can extend this range to 25 ppm). See, for example, https://www.palintest.com/ product-categories/kemio/
28. Simpson, G. (November 12-16, 2023). “Factors that Influence the Successful Treatment of Cooling Towers with ClO2,” presentation at the International Water Conference, San Antonio, Texas.

Greg D. Simpson, PhD, is a scientist, inventor, and author and has consulted globally on a variety of issues to a variety of industries, including petroleum refining, oil field, aerospace, medical waste sterilization, ballast water, pharmaceutical, chemical, pulp and paper, electronics and semiconductor manufacturing, municipal treatment, environmental, food, among others. He began his career as a scientist at a top-secret nuclear weapons facility. Since 1980, Dr. Simpson has been involved in the water treatment industry, serving in a variety of roles. He has authored and / or presented more than 120 technical papers, written three books about chlorine dioxide, coauthored a fourth, and was a major contributor to a fifth. He holds BS and MS degrees from West Texas State University and earned his doctorate from the University of Illinois. Dr. Simpson lives in the Houston area and may be contacted at dr.greg.d.simpson@gmail.com
Keywords: BACTERIA, BIOCIDES, BIOCONTROL, CHLORINE, CHLORINE DIOXIDE, COOLING TOWERS
This paper was presented at the AWT’s Convention and Exposition, which was conducted October 4-6, 2023, in Grand Rapids, Michigan.
41 the ANALYST Volume 31 Number 1
When Treating Cooling Towers with ClO2, Is There a Best Way? continued


A Testing Approach to Validate Legionella
-Viable-PCR Technology
Brandon Smith, Environmental Safety Technologies Inc.; and Richard D. Miller, PhD, Environmental Safety Technologies Inc. and Department of Microbiology and Immunology, University of Louisville School of Medicine

Abstract
The detection of Legionella in building water systems is important in validation of Legionella control as specified in building Water Management Programs for risk management of legionellosis. Culture techniques are the Gold Standard for Legionella analyses because of their detection and quantitation of colony forming units (CFU), that are closely equivalent to infectious bacteria, despite the 7 to 10 day wait for the results. Polymerase chain reaction (PCR) technology for detecting Legionella in water samples has been around for more than 30 years.
Current real-time (quantitative [q]) qPCR has the advantages of quantitation, along with high specificity and speed (same day or next day results), but qPCR has limitations in its broad detection of multiple forms of Legionella DNA in water samples, including live, infectious Legionella, but also non-infectious, dead intact Legionella cells, as well as free Legionella DNA. Viable Legionella PCR techniques (called vPCR) using qPCR in combination with the viable dye, propidium monoazide (PMA) for distinguishing DNA from viable versus nonviable Legionella, have been published in recent years (1, 2). In these and other studies, the vPCR technology results compared favorably to culture and illustrated the over-estimation of Legionella numbers by regular qPCR.
In the current study, we validated the viable Legionella vPCR technology using CDC-ELITE proficiency samples (two rounds of six samples, with each containing defined concentrations of different Legionella species and serogroups. The Legionella pneumophila vPCR test correctly identified the presence/absence of L. pneumophila serogroup 1 and L. pneumophila serogroups 2-15 correctly in every one of the ELITE samples. The quantitative numbers of L. pneumophila detected by vPCR versus culture (ELITE laboratories average and the CDC estimate), were within the correct orders of magnitude. These results confirm our results on actual building water samples in the current study and support the use of vPCR technology for rapid validation of Legionella control measures in building water systems. The continued validation of this technology with CDCELITE and other proficiency testing samples is ongoing.



45 the ANALYST Volume 31 Number 1
Introduction
PCR Milestones. It is widely known that PCR was discovered by Kary Mullis in 1983 at Cetus Corp., along with help from other Cetus scientists. After the patents were secured, the procedure was first published in the journal Science in 1985 (3) and presented the following year at the prestigious Cold Spring Harbor Symposium (4). Once the heat-stable DNA polymerase (Taq polymerase) was introduced into the procedure in 1988 (5), PCR was fully automated very quickly, with the development of the first commercial PCR machine (thermal cycler) by Perkin-Elmer Cetus.
PCR took off from there, and by the end of 1988 it was fully integrated into the Miller Research Laboratory at the University of Louisville as part of a collaborative research project with principal investigator Dr. Ronald Atlas. This research (as funded by Perkin-Elmer Cetus) was intended to develop a PCR procedure for detection of Legionella pneumophila in environmental samples. Four scientific publications (6-9) and one U.S. patent (10) later, the Legionella PCR procedure was fully documented by the Atlas/Miller/Perkin-Elmer Cetus team, with applications for both Legionella clinical diagnostics (11), as well as in Legionella ecology and in the emerging environmental water testing field.
Limitations of Legionella qPCR. Despite its exquisite specificity, sensitivity, quantitation and speed, the one limitation of PCR for Legionella detection has been the stability of DNA as a target. Of course, this is an advantage in the clinical diagnostics field, where Legionella is always viewed as a foreign invader (i.e., an unwanted inhabitant), and the presence of live cells, dead cells or free DNA (with an appropriate clinical presentation), are always viewed as evidence of Legionnaires’ disease.
However, in environmental aquatic locations (e.g., lakes and ponds, among others), Legionella is often a normal inhabitant, living and dying naturally in surface biofilms within free-living amoebae, and often at low numbers. Dead Legionella and free Legionella DNA may persist and accumulate within these environmental biofilms and in the water bathing them.
Consequently, in building water systems, the risk management of Legionnaires’ disease is aided by the ability to discriminate between living versus dead Legionella, where only the living Legionella are potentially infectious, and where the non-infectious
dead Legionella may have accumulated, leading to high numbers of dead (i.e., false-positive) Legionella results. Due to this persistence of DNA (free or within cells) in the environment after cells have lost viability, qPCR and other DNA-based detection methods, cannot differentiate whether positive signals originate from live or dead targets, or from free DNA.
Thus, over the past 30 years of Legionella PCR availability, the culture method has remained as the primary testing method for validating building water management programs for Legionella control, and especially for validating the success of building water disinfection procedures. Legionella qPCR, on the other hand, has been relegated to a limited role of performing Legionella “negative screens”, with a culture follow-up required if the PCR test is positive.
Use of propidium monoazide (PMA) for viable qPCR. As a solution to this dilemma, propidium monoazide (PMA) has been shown (12) to differentiate between live versus dead bacteria by selective penetration of dead cell membranes, and removal of DNA from dead cell PCR reactions. This PMA differentiation has been shown to work with a wide range of species across the bacterial kingdom. In a follow-up article in 2007, Nocker et al. (13) demonstrated the value of the PMA Live/Dead distinction in the field of microbial ecology. Over the past 15 years, there have been hundreds of publications that have documented the successful use of PMAmediated viability PCR (vPCR) for testing a wide variety of bacteria, archaea, yeasts and eukaryotic parasites. (A 200-article bibliography is found at biotium.com/ wp-content/uploads/2017/10/PMA-PMAxxReferences.pdf)
Specifically related to Legionella, at least eight published studies over the past decade have shown the value of the PMA-treatment vPCR technology for detection of viable Legionella in building water samples (1, 2, 14-18), and its use as a new tool for Legionella risk management (19).
Goals of the current study. Our current study was designed to add additional validation data for the Legionella vPCR technology, specifically comparing its qualitative and quantitative results as used for: 1. Testing ELITE Legionella proficiency samples; 2. The testing of real water samples taken from cooling towers and potable water systems for Legionella testing; and finally, 3. Legionella testing to confirm the disinfection of a hospital potable water system performed in order to eliminate the Legionella colonization.
46 the ANALYST Volume 31 Number 1
A Testing Approach to Validate Legionella-Viable-PCR Technology continued
Methods
Since 2020, we have been testing the Legionella viablePCR (vPCR) technology (see Figure 1 for procedural details). A sequence of the mip gene was used for detection of L. pneumophila; and a lpg0774 gene sequence was used to detect L. pneumophila serogroup 1 (all in-house primers and fluorescent probes). We used the Legionella qPCR technology (ISO/TS 12869:2019 with PMAxxTM, Biotium, Inc., Fremont, California) pretreated samples, side-by-side with the standard Legionella culture test (ISO 11731:2017).
Results
The Legionella vPCR technology validation using ELITE proficiency samples. We tested the vPCR on ELITE proficiency samples. Over this two-year period, we have collected 45 sample data points with the results as shown in Table A. The data in the table is based on ELITE proficiency samples during the period of 2020-present. Both procedures had limits of detection of 0.1 CFU per milliliter (CFU/mL).
The results observed with the Legionella culture and vPCR procedures showed that the positive and negative sample results matched up perfectly (100% agreement) when tested on these ELITE samples. Quantitatively, the numbers of L. pneumophila detected by vPCR versus
Of 45 total ELITE samples, the numbers that tested positive for:
L. pneumophila (all serogroups)
L. pneumophila SG1
culture (ELITE laboratories average and the CDC estimate) were within the correct orders of magnitude (data not shown).
Figure 2 shows results from the vPCR technology validation with real building water samples versus data from a cultured analysis. The question addressed is whether the vPCR technology can detect Legionella pneumophila as well as a traditional culture analysis?
The data in Figure 2 showed that vPCR and culture had a 100% agreement on positivity for L. pneumophila in 89 of 399 building water samples. The limits of detection for culture and vPCR were both 10 CFU/mL for cooling tower samples and 0.1 CFU/mL for potable water samples.

47 the ANALYST Volume 31 Number 1
Figure 1: PMA-Mediated PCR Procedure to Detect Legionella pneumophila
Table A: Legionella vPCR Validation With ELITE Samples
Culture 33 15 vPCR 33 15 A Testing Approach to Validate Legionella-Viable-PCR Technology continued


Another question raised in our research was whether or not vPCR can accurately detect the same type of Legionella pneumophila as traditional culture analysis?
Figure 3 shows the data from what we found. These results showed that vPCR and culture had a 100% agreement on positivity for L. pneumophila in 89 of 399 building water samples. Limits of detection for culture
and vPCR were both 10 CFU/mL for cooling tower samples and 0.1 CFU/mL for potable water samples.
Another question we had was whether or not a vPCR result can accurately reflect the approximate quantification of Legionella pneumophila as determined in a traditional culture analysis? This question is examined in Figure 4.
48 the ANALYST Volume 31 Number 1
Figure 2: A Comparison of Analyses by vPCR and a Traditional Culture Analysis
A Testing Approach to Validate Legionella-Viable-PCR Technology continued
Figure 3: Results From an Examination of vPCR and Culture Analyses to Determine if Both Methods Can Identify the Same Type of L. pneumophila
All
is
in CFU/mL. The results both showed linear plots with an R2 coefficient of determination of 0.9483 and 0.8634, respectively. Note: R2 in the text refers to a statistic used in statistical models for prediction of future outcomes, or in the testing of hypotheses based on other related information. R2 provides a measure for researchers on how well observed outcomes are replicated in the model.
Figure 5 provides a statistical measure (R²) of how close the data are to the fitted regression line. This measurement is also known as the coefficient of determination, or the coefficient of multiple determination for multiple regression. A general rule of thumb is the higher the R² value, the more closely the data correctly fits with the statistical analysis. The data shows that the R² values for vPCR compared to culture were similar to the R2 for culture itself (0.89).


49 the ANALYST Volume 31 Number 1
Figure 4 shows the vPCR results for L. pneumophila (mip gene in the top panel) and L. pneumophila serogroup 1(lpg0774 gene in the bottom panel) plotted against culture results.
data
shown
A Testing Approach to Validate Legionella-Viable-PCR Technology continued
Figure 4: vPCR Results (Cfu/Ml) for L. Pneumophila (mip gene in Top Panel) and L. pneumophila Serogroup 1(Lpg 0774 gene; bottom panel) Plotted Against Culture Results (CFU/mL)

Hospital Case Study
In our work, we also compared the vPCR technology validation with real building water samples from a hospital. The vPCR technology was tested against culture and regular qPCR (with no PMA) on samples taken during a hospital disinfection to eliminate L. pneumophila serogroup 1 colonization. The findings are shown in Table B.
The table shows results of pretreatment Legionella pneumophila (culture) analysis and post-disinfection (culture, qPCR and vPCR analysis) of a hospital potable water system. All assays had 0.1 CFU/mL limits of detection.
These hospital potable water case study results clearly showed that the water samples taken immediately after the disinfection procedure ended confirmed that the



50 the ANALYST Volume 31 Number 1
Figure 5: A Statistical Measure Comparing the Accuracy of vPCR Results With a Traditional Culture
A Testing Approach to Validate Legionella-Viable-PCR Technology continued www.aqualyticsreports.com AQUALYTICS Custom service reports and data management for the water treatment industry • Custom trend charts • Images • Control ranges • Inventory tracking • and more! • $39/month • No startup fee • Free trial • Cancel anytime
Legionella Case Study — Disinfection of a Hospital Potable Water System
Pretreatment- culture (1 wk)
IMMEDIATELY
sites positive
Hyperchlorination (25-50 mg/L; 2 hr) Flush → then → Superheat (70°C; 60 min) Flush
Post- treatment- culture -1 wk
L. pneumophila SG 1
Detected
vPCR Compared to a Traditional Culture for Legionella Detection
Validation - Legionella vPCR compared to Culture:
Positivity Statistically identical accuracy in detecting L. pneumophila
Specificity Statistically identical specificity in qualifying the serogroup identity of Legionella pneumophila
culture result (after 7 days of incubation) and the vPCR result (after 6 hours) both showed that the disinfection was successful, and the potable water system had been successfully cleared of the viable L. pneumophila serogroup 1 colonization. This result gave the all-clear for the hospital to resume normal operations within the parameters of its Legionella water management program. In contrast, the qPCR result (with no PMA) still gave three false-positive Legionella results. Only the vPCR results (with PMA) matched the Legionella culture results.
Conclusions
Overall, the results of the testing of ELITE proficiency samples, and a variety of real-world building water samples, have validated the positivity, specificity, and accuracy of the Legionella vPCR technology compared to the standard culture test, and recommend that it can be used accordingly as a valuable option for Legionella risk management. Table C offers an overview summary of our findings.
References
1. Scaturro, M.; Fontana, S.; Dell’eva, I., et.al. (2016). “A Multicenter Study of Viable PCR Using Propidium Monoazide to Detect Legionella in Water Samples,” Diagnostic Microbiology and Infectious Disease Journal, 85, pp. 283-288.
2. Bonetta, S.; Pignata, C.; Bonetta, S.; Meucci, L.; Giacosa, D.; Marino, E.; Gilli, G.; Carraro, E. (April 2017). “Viability of Legionella pneumophila in Water Samples: A Comparison of Propidium Monoazide (PMA) Treatment on Membrane Filters and in Liquid,” International Journal of Environmental Research and Public Health 14(5), p. 467, doi: 10.3390/ ijerph14050467. PMID: 28448459; PMCID: PMC5451918.
3. Saiki, R.K.; Scharf, S.J.; Faloona, F.; Mullis, K.B.; Horn, G.; Erlich, H.A.; Arnheim, N. (1985). “Enzymatic Amplification of β-globin Genomic Sequences and Restriction Site Analysis for Diagnosis of Sickle Cell Anemia,” Science, 230, pp. 13501354.
4. Mullis, K.B.; Faloona, F.; Scharf, S.J.; Saiki, R.K.; Horn, G.; Erlich, H.A. (1986). “Specific Enzymatic Amplification of DNA in vitro: The Polymerase Chain Reaction,” Cold Spring Harbor Symposia on Quantitative Biology, 51, pp. 263-273.
5. Saiki, R.K.; Gelfand, D.H.; Stoffel, S.; Scharf, S.J.; Higuchi, R.; Horn, G.T.; Mullis, K.B.; Erlich, H.A. (1988). “Primer-Directed Enzymatic Amplification of DNA with a Thermostable DNA Polymerase,” Science, 239, pp. 487-491.
6. DiCesare, J.; Haff, L.; Mahbubani, M.H.; Bej, A.K.; Miller, R.D.; Atlas, R.M. (1990). “Detection of Legionella in Environmental Samples Using PCR,” Amplifications, 4, pp. 16-21.
7. Mahbubani, M.H.; Bej, A.K.; Miller, R.D.; Haff, L.; DiCesare, J.; Atlas, R.M. (1990). “Detection of Legionella by Using Polymerase Chain Reaction and Gene Probe Methods,” Molecular and Cellular Probes, 4, pp. 175-187.
8. Bej, A.K.; Mahbubani, M.H.; Miller, R.D.; DiCesare, J.; Haff, L.; Atlas, R.M. (1990). “Multiplex PCR Amplification and Immobilized Capture Probes for Detection of Bacterial Pathogens and Indicators in Water, Molecular and Cellular Probes, 4, pp. 353-365.
9. Mahbubani, M.H.; Bej, A.K.; Miller, R.D.; Atlas, R.M.; Dicesare, J.; Haff, L. (1991). “Detection of Bacterial mRNA Using Polymerase Chain Reaction,” BioTechniques, 10, pp. 48-49.
10. Atlas, R.M.; Bej, A.K.; Mahbubani, M.H.; Miller, R.D.; Steffan, R.J. (March 29, 1994). “Process for Detection of Legionella and other Waterborne Microbial Pathogens and Indicators of Human Fecal Contamination in Water Samples and Kits Thereof,” United States Patent No. 5,298,392.
51 the ANALYST Volume 31 Number 1
20/25
80%
0/25
0% None
qPCR
3/25 positive 12% L. pneumophila SG 1 vPCR (PMA) - 6 hr 0/25 positive 0% None Detected
positive
(no PMA) - 6 hr
Table B: Results of Pretreatment Analysis for Legionella pneumophila and Post-Disinfection Analysis
A Testing Approach to Validate Legionella-Viable-PCR Technology continued
Table C: Summary of
11. Ramirez, J.A.; Ahkee, S.; Tolentino, A.; Miller, R.D.; Summersgill, J.T. (1996). “Diagnosis of Legionella pneumophila, Mycoplasma pneumoniae, or Chlamydia pneumoniae Lower Respiratory Infection Using the Polymerase Chain Reaction on a Single Throat Swab Sample,” Diagnostic Microbiology and Infectious Disease, 24, pp. 7-14.
12. Nocker, A.; Cheung, C.-Y.; Camper, A.K. (2006). “Comparison of Propidium Monoazide with Ethidium Monoazide for Differentiation of Live versus Dead Bacteria by Selective Removal of DNA from Dead Cells,” Journal of Microbiological Methods, 67, pp. 310-320.
13. Nocker, A.; Sossa-Fernandez, P.; Burr, M.D.; Camper, A.K. (2007). “Use of Propidium Monoazide for Live/ Dead Distinction in Microbial Ecology,” Applied and Environmental Microbiology, 73, pp. 5111-5117.
14. Yanez, M.A.; Nocker, A.; Soria-Soria, E.; Murtula, R.; Martinez, L.; Catalan, V. (2011). “Quantification of Viable Legionella pneumophila Cells Using Propidium Monoazide Combined with Quantitative PCR,” Journal of Microbiological Methods, 85, pp. 124-130.
15. Ditommaso, S.; Ricciardi, E.; Giacomuzzi, M.; Arauco Rivera, S.R.; Ceccarelli, A.; Xotti. C.M. (2014). “Overestimation of the Legionella spp. Load in Environmental Samples by Quantitative Real-Time PCR: Pretreatment with Propidium Monoazide as a tool for the Assessment of an Association between Legionella Concentration and Sanitary Risk,” Diagnostic Microbiology and Infectious Disease, 80, pp. 260-266.
16. Li, H.; Xin, H.; Li, S.F.I. (2015). “Multiplex PMA-qPCR Assay with Internal Amplification Control for Simultaneous Detection of Viable Legionella pneumophila, Salmonella typhimurium, and Staphylococcus aureus in Environmental Waters,” Environmental Science & Technology, 49, pp. 14249-14256.
17. Delgado-Viscogliosi, P.; Solignac, L.; Delattre, J.-M. (2015). “Viability PCR, a Culture-Independent Method for Rapid and Selective Quantification of Viable Legionella pneumophila Cells in Environmental Water Samples,” Applied and Environmental Microbiology Journal, 75, pp. 3502-3512.
18. Ditommaso, S.; Giacomuzzi, M.; Ricciardi, E.; Zotti, C.M. (2016). “Cultural and Molecular Evidence of Legionella spp. 8Colonization in Dental Unit Waterlines: Which Is the Best Method for Risk Assessment?”, International Journal of Environmental Research and Public Health, 13, p. 211.
19. Lizana, X.; Lopez, A.; Benito, S.; Agusti, G.; Rios, M.; Pique, N.; Marques, A.M. (2017). “Viability qPCR, a New Tool for Legionella Risk Management,” International Journal of Hygiene and Environmental Health, 220, pp. 1318-1324.

Brandon “Smitty” Smith is the vice president of laboratory services, having spent the last 17 years working with Dr. Miller and EST to create and refine environmental testing. A graduate of the University of Louisville, he has served on AWT and CTI Committees for more than a decade. He has comprehensive knowledge of healthcare pathogens, industry microbial corrosion, and diagnostic microbiology. Mr. Smith may be reached at bsmith@estechlab.com

Richard D. Miller, PhD, is a founder (1993), president, and chief scientist at Environmental Safety Technologies, Inc. (EST), and also recently retired as a tenured teaching/research faculty member of infectious diseases microbiology (teaching medical students since 1977) in the School of Medicine at the University of Louisville. He served on the ASHRAE committees that developed the Legionella Guideline 12:2000, as well as ANSI/ASHRAE Standard 188-2015, Legionellosis: Risk Management for Building Water Systems. Through EST, and with his more than 45 years of experience working with Legionella, he provides environmental testing and risk assessments for Legionella in building water systems nationwide. He also has extensive knowledge of other important healthcare-associated environmental pathogens, their risks for disease, testing methodologies, and interpretation of results. Dr. Miller may be reached at rmiller@estechlab.com
Keywords: BACTERIA, BUILDING WATER, COOLING TOWERS, LEGIONELLA, INSTRUMENTS, MICROORGANISMS, MONITORING
This article is based on a presentation given at the 2022 AWT Convention and Exposition in Vancouver, B.C., which was conducted on September 21-24, 2022.
52 the ANALYST Volume 31 Number 1
A Testing Approach to Validate Legionella-Viable-PCR Technology continued
Discovering AWT
Arthur Freedman Associates, Inc.
1485 Queens Lane
Dyer, IN 46311
(630) 364-3965
www.arthurfreedmanassociates.com
Company History: Arthur J. Freedman, PhD, founded Arthur Freedman Associates, Inc. (AFA) in 1981 after 22 years with Nalco Chemical Co. His career also included work at the Los Alamos Scientific Laboratory, MIT, and at Standard Oil Company of Indiana before joining Nalco. During his career, he contributed to the development of groundbreaking corrosion control and water treatment technologies and received five patents. AFA is now operated by his son, Peter Freedman.
Current Business: AFA continues the work started by Dr. Freedman. Team members each average more than 40 years of experience.
The firm’s scope includes advising clients in industries and sectors such as high-rise and campus facilities, oil and gas, petrochemical, data centers, pharmaceutical, semiconductor, food and other process industries, power generation, and municipal water and wastewater. AFA also provides expert witness services to law firms.
AFA operates independently from chemical or water treatment product companies and uses only third-party labs for testing. AFA consultants work with all parties involved in water treatment system issues in order to find solutions, to keep facilities operating efficiently, and to prevent future problems.


Business Locations: AFA is headquartered in the greater Chicago area (Dyer, Indiana) and has consultant offices across the United States and Canada, serving clients in both North America and internationally.
Recognition and Involvement: Dr. Freedman was involved in the early years when AWT was first formed, and he was one of the authors of the original edition of the Technical Reference & Training Manual, which was published in 2001. Dr. Freedman received the Ray Baum Memorial Award in 2000.
Top Executive: Peter Freedman, President

54 the ANALYST Volume 31 Number 1
AFA founder, Arthur J. Freedman, Ph.D.
Arthur Freedman Associates, Inc. Consultants:
Top row (left to right): Walt Tyler, CWT, Ted Beardwood
Middle row (left to right): Bill Pearson, CWT, Keith Johnson
Bottom row (left to right): Bob Cunningham, PE, Paul Labine
Blue-White Industries
5300 Business Drive
Huntington Beach, CA 92649 (714) 893-8529
www.blue-white.com
Company History: Blue-White Industries was founded by Ozzie King, who had a successful construction business in Los Angeles. The company was incorporated in 1957 and had four permanent employees. Today, the firm has a workforce of 120 individuals, as well as a worldwide network of sales representatives and service centers. The business is still owned and operated by Ozzie King’s heirs, including his grandson—company president and CEO—Rob Gledhill. The firm’s original product was a diaphragm metering pump for chemical injection for swimming pools and spas.
Current Business: Blue-White's product lines now consist of diaphragm and peristaltic type chemical feed pumps, complete skid systems, variable area, digital and ultrasonic flow meters, and water analyzers (turbidity, pH, free chlorine, and temperature). The firm’s chemical feed pumps range from simple mechanical units to ones using the newest technologies. Many of the components used in the manufacture of these pumps are produced in its facility in Huntington Beach. Chemical water treatment applications served by Blue-White include municipal water and wastewater, Industrial wastewater, boiler water, cooling towers, food and beverage, pools and spas, and agriculture and irrigation. The company holds an ISO 9001:2015 certification, which means it follows quality

management system guidelines for its manufacturing processes.
Business Locations: Blue-White is headquartered in Huntington Beach, CA, and maintains a global network.
Involvement and Recognition: Blue-White has been a part of AWT since 2007.
Top Executives: In addition to Mr. Gledhill, other key team members include COO Bill McDowell, CFO Rick Fogarty, VP of Finance Janet King, VP of Advertising Jean Hendrickson, Taylor Gledhill—marketing director, and Danny Sanders—sales director.

55 the ANALYST Volume 31 Number 1
CEO Rob Gledhill on the pump manufacturing line.
Testing of components on a skid system.
Discovering AWT continued




























11335 Lewis Braselton Blvd. Braselton, GA 30517 770-978-1443 (Fax) 770-978-4165 info@biosourceinc.com www.biosourceinc.com 2021 Supplier of the Year • Biocides (oxidizing & non-oxidizing) • Polymers, azoles, phosphonates, sod. molybdate • Repacking (all drum sizes and pails available) • Regulatory and technical assistance Having Product Availability Issues? Let us help you piece it all together. IN BUSINESS SINCE 1991.
Making a Splash
 Tim Elliott, P.Eng. National Sales Manager at Iwaki America Inc.
Tim Elliott, P.Eng. National Sales Manager at Iwaki America Inc.
What prompted you to start volunteering with AWT?
I attended my first AWT convention in 2018, and I was immediately struck by the sense of community and enthusiasm among the members. I’ve looked forward to the event every year since. I was always interested in getting more involved, so when my colleague suggested that I volunteer, I jumped on the opportunity. The Education Committee has turned out to be an especially good fit for me, as I really enjoy sharing ideas about how to solve water treatment problems.
What has been the most rewarding thing about volunteering?
Getting to know the other members that share in the common goal of advancing our industry. This shared enthusiasm has made the volunteering experience not only productive but also enjoyable. It’s really satisfying to be part of a collective effort that goes beyond individual interests, focusing on improving the industry and its professionals.


Why would you encourage others to become a volunteer?
Volunteering with AWT provides a unique platform to actively contribute to the growth and improvement of the water treatment industry. It offers an opportunity to network with professionals who share a passion for the field, and to build lasting connections. Volunteering is also a great way to develop and strengthen leadership and teamwork skills, enhancing your overall professional profile. Beyond personal growth, it’s a chance to give back to the industry and make a positive impact on its future.
Tell us about a current project you or your committee is working on?
As part of the Education Committee, we are currently developing an extensive series of troubleshooting guide videos that can assist water professionals with addressing common water treatment problems. Several of these videos are already available on the “Troubleshooting Guides” section of the AWT members’ website, and more will be added in the coming months. I encourage all members to check out this section of the AWT website so you can take advantage of these great resources!
57 the ANALYST Volume 31 Number 1

Connor Hanrahan, CWT Industrial Water Engineering Albuquerque, NM
What prompted you to obtain your CWT, and when did you begin the process by taking the exam?
The owners of Industrial Water Engineering (IWE) placed a strong emphasis on training and certification since day one. I believe it’s how we stand out in our market, especially against national brands where there is no standard for water treatment excellence. Once I established the relationships with my customers for their recommendation letters, I set out to take the exam. Following the recommendations of the book The Twelve Week Year by Brian Moran (recommended by Trace Blackmore’s Rising Tide Mastermind), I signed up in early January and took the test exactly 12 weeks later.
Why do you feel this credential is important to have?
I worked for a national water treatment company before, and I was disappointed with the lack of training and certification. The CWT is an excellent benchmark for our employees to show that they truly understand the diverse world of water treatment and that they’ve put in the time to prove it.
How did you prepare for the exam?
We followed the example set out in The Twelve Week Year and broke up the Technical Resource & Training Manual (TRTM) into sections based on the timeline. It was a terrific exercise to read through the entirety of the TRTM with our team, and by the time the exam came around, we felt fully prepared. Making a plan and scheduling the date in advance really helped with preparation, it was the first time I didn’t feel like I needed to cram for an exam.
What was the most difficult aspect of the exam?
The most difficult aspect was the length of the exam, there is so much material covered. I highly recommend brining a snack to keep your brain fueled because four hours is a grueling endeavor.
What advice would you give those thinking about taking the exam?
Sign up and form a study group. I worked with my team of account managers and developed a weekly study program to prepare for the test. We gave ourselves 12 weeks from the day we signed up and broke down the TRTM into manageable sections for that timeline. We used the online training and quizzes available through AWT to supplement the reading, the practice exam provided by AWT, and Trace Blackmore’s practice course. Having a group kept us accountable.
58 the ANALYST Volume 31 Number 1 CWT Spotlight

How Neglect of Makeup Water and Condensate Return Chemistry Can Lead to Huge Problems at Industrial Plants
Brad Buecker, Buecker & Associates
From personal experience and discussions over the years with colleagues, I have observed numerous cases where personnel at heavy industries become so focused on process chemistry and engineering that they neglect to put needed resources into steam generator makeup treatment system reliability and protecting boilers from contaminated condensate return. We will examine several case histories that graphically illustrate these issues, and that emphasize the old saying, “An ounce of prevention is worth a pound of cure.”
Makeup Water Issues
High-purity makeup is a necessary requirement for high-pressure utility boilers. Even slight concentrations of impurities can cause serious problems in the harsh environment of these units. However, for lowerpressure industrial drum boilers, higher concentrations of impurities can be tolerated, as the lower heat fluxes reduce the potential for corrosion and deposition. Table A (1) is an extract taken from the recently updated American Society of Mechanical Engineers (ASME) industrial boiler water chemistry guidelines.
Immediately obvious from Table A is that the recommended limits for many impurities decrease with increasing pressure (and boiler temperature). For this discussion, observe the low feedwater hardness limits in all cases. A primary makeup treatment method at many plants is sodium softening to remove calcium and magnesium. From the time humans began heating water for cooking and sanitary purposes, they have undoubtedly observed mineral deposition in heated vessels. These issues became acute following the development and expanding use of steam engines during the Industrial

Revolution of the 18th and 19th centuries. The usual (but not exclusive) culprit is calcium carbonate (CaCO3). Equation 1 shows the reaction of carbonate in boiler water. Ca2+
59 the ANALYST Volume 31 Number 1 Tales From the Waterside
Figure 1. CaCO3 (and Gypsum) Solubility as a Function of Temperature
+ heat → CaCO3↓ + CO2 + H2O Eq. 1
+ 2HCO3-
Note: Special instructions in the table notes.
Superheated
ppb (µg/L) as Na < 20 for all cases.
Note: Consult turbine supplier or qualified consultant for appropriate details for the system.
*Data extracted from Table 1 (Reference 1)— “Suggested Water Chemistry Targets Industrial Water Tube with Superheater.”
Calcium carbonate, which is only slightly soluble in natural waters, becomes even less soluble with increasing temperature.
Figure 1 (2) shows CaCO3 (and gypsum) solubility as a
water temperature.
Yet, this author has directly observed and has seen numerous reports of boilers that have been allowed to operate with frequent and sometimes long-term softener upsets. Figure 2 (2) illustrates deposition from a boiler with multiple hardness excursions.
The effect of scale formation on heat transfer is clearly shown in Figures 3 and 4 (2), with the former illustrating the dramatic increase in tube wall temperature.
As bad as these examples are, the author is acutely aware of several cases in which, as a result of a makeup system mechanical failure, plant management ordered operators to send untreated makeup to the boiler. Tube failures occurred with great rapidity, incurring enormous costs for repairs and lost production.

Even well-operated sodium softeners, by themselves, remove only hardness and related cations from the makeup water. Accordingly, many softening systems are equipped with downstream equipment to remove alkalinity (primarily bicarbonate, HCO3, in natural waters). One common method is forced draft decarbonation (or alternatively ion exchange
60 the ANALYST Volume 31 Number 1 Drum Operating Pressure (psig) 0-300 301-450 451-600 601-750 751-900 Feedwater Dissolved oxygen (ppm [mg/L]) measured before chemical oxygen scavenger addition. <0.007 <0.007 <0.007 <0.007 <0.007 Total iron (ppm [mg/L]) ≤0.10 ≤0.05 ≤0.03 ≤0.025 ≤0.02 Total copper (ppm [mg/L]) ≤0.05 ≤0.025 ≤0.02 ≤0.02 ≤0.015 Total hardness (ppm [mg/L]) as CaCO3 <0.5 ≤0.3 ≤0.2 ≤0.2 ≤0.1 pH at 25°C 8.8-10.5 8.8-10.5 8.8-10.5 8.8-10.0 8.8-10.0 Nonvolatile TOC, including oily matter (ppm [mg/L]) as °C <1 <1 <0.5 <0.5 <0.5 Boiler Water Silica (ppm [mg/L]) SiO2 ≤84 ≤72 ≤40 ≤30 ≤17
alkalinity
Hydroxide
(ppm [mg/L]) as CaCO3
Maximum suggested targeted Specific Conductance—µS/cm @ 25°C without neutralization to comply with steam purity limits ≤1,600 ≤1,400 ≤1,150 ≤920 ≤230 Saturated Steam Purity Target TDS (maximum) (ppm [mg/L]) <0.3 <0.3 <0.3 <0.3 <0.1 Sodium estimated from TDS (ppb [ug/L]) as Na < 100 <100 <100 <100 <30 Maximum mechanical carryover (%) Calculated from TDS 0.029 0.033 0.040 0.050 0.067
Steam Purity Target Sodium,
Table A: Lower-Pressure Boiler Tolerance of Contaminants*
Figure 2: Layered CaCO3 Deposits in a Boiler Tube
function of
Tales from the Waterside continued


dealkalization) that, perhaps with the assistance of a small acid feed upstream, converts most of the alkalinity to CO2 for extraction. This is illustrated in Equation 2.
H+ + HCO3- ⇌ H2CO3 ⇌ CO2↑ + H2O Eq. 2
Even with dealkalization, if softener upsets occur the potential exists for other depositions such as calcium and magnesium silicate. Becoming more popular is reverse osmosis (RO) for industrial makeup water treatment. Just a basic two-stage, single-pass RO unit can remove around 99% of all dissolved ions.
Condensate Return Complexity
Virtually the entire steam volume produced in a dedicated power boiler drives the turbine-generator and then is returned as condensate to the boiler. Apart from a cooling water leak in the steam surface condenser or impurity ingress from a makeup system upset, the boiler feed water remains pristine. The situation is usually much different for cogeneration systems (Figure 5) where condensate may come back from a variety of chemical processes. The blowdown heat exchanger and feedwater heater shown in Figure 5 (2) may not be present in some configurations. Note the multiple condensate return lines.
A large plant often has several steam generators of varying pressure and design scattered around the facility. Some boilers may produce superheated steam for turbines or related high energy applications, but much steam typically goes to process heat exchangers, where chemical in-leakage to condensate can introduce a wide variety of impurities. With that thought in mind, consider the following personal case history.

A number of years ago, the author and a colleague were invited to an organic chemicals plant that had four, 550-pounds per square inch gauge (psig) package boilers with superheaters. The steam provided energy to multiple plant heat exchangers, with recovery of most of the condensate. Each of the boiler superheaters had been failing, on average, every 1.5 to 2 years from internal deposition and subsequent tube overheating. Inspection of an extracted superheater tube bundle revealed deposits of approximately ⅛ to ¼ inch in depth. Additional
61 the ANALYST Volume 31 Number 1
Figure 3: Influence of Deposits on Boiler Tube Wall Temperatures
Figure 4: Blisters on a Boiler Tube From Overheating Due to Internal Deposits
Figure 5: Generic Flow Diagram of a Co-Generation System
Tales from the Waterside continued
inspection revealed foam issuing from the saturated steam sample line of every boiler, whose cause became quickly apparent.
Among the data from water/steam analyses performed by an outside vendor were total organic carbon (TOC) levels of up to 200 milligram per liter (mg/L) in the condensate return. Contrast that with the ≤0.2 mg/L feed water TOC recommendation in Table A. No treatment processes or condensate polishing systems were in place to remove these organics (five phenol derivatives) upstream of the boilers. The feedwater TOC data clearly indicated why foam was issuing from the steam sample lines and why the superheaters rapidly accumulated deposits and then failed from overheating. Furthermore, the operators had rudimentary grab sampling equipment and could only directly analyze one of the organic derivatives. Thus, they could not readily trace what process line or lines were contaminated, and from there narrow down the problem to a specific heat exchanger or exchangers.
Admittedly, any solution would likely have been expensive. Condensate dumping would have required installation of a much larger makeup water treatment system and possibly an upgrade to the plant’s wastewater treatment system. Retrofit of activated carbon filters for polishing may or may not have been effective, per issues related to molecular characteristics of the impurities and reaction kinetics, but lab testing, and perhaps pilot testing, were definitely warranted. There were no magic solutions to this issue.
Extensive condensate return systems, whose typical material of construction is carbon steel, can also suffer from substantial corrosion. A common outcome

is carbonic acid attack caused by CO2 ingress and subsequent reaction with water to form a mildly acidic, but still corrosive to carbon steel, solution (Equation 3).
CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3- Eq. 3
Alert readers will note that this is the reverse of Equation 2. Carbonic acid corrosion often manifests itself as gouging in those areas submerged in the condensate as shown in Figure 6 (2).
Severe pitting is possible in lines subject to oxygen ingress, and particularly if systems are idle for long periods where the attack can become localized. This is shown in Figure 7 (2).
These corrosion mechanisms, besides potentially causing premature failures, generate iron oxide corrosion products that transport to steam generators and form porous deposits on boiler tubes and other internals. The precipitants can become sites for under-deposit corrosion (UDC) initiated by impurities in the boiler water. UDC generally becomes more magnified with increasing boiler pressure and temperature. One UDC phenomenon that has plagued utility boilers is hydrogen damage. Consider the following personal case history. Although it occurred in a power boiler, the operating pressure and temperature were similar to many large industrial steam generators.
An 80-megawatt (MW) unit supplied by a 1,250psig coal-fired boiler had just been returned to service from a scheduled autumn outage. Laboratory personnel discovered that a condenser leak was allowing contaminants to enter the condensate/feedwater, such that total-dissolved-solids (TDS) concentrations at times reached 0.75 parts per million (ppm). Although

62 the ANALYST Volume 31 Number 1
Figure 6: Carbonic Acid Grooving of a Carbon Steel Condensate Return Line
Tales from the Waterside continued
Figure 7: Oxygen Pitting of a Boiler Feedwater Line
the lab staff requested that the boiler be taken off line immediately, the operations managers refused due to load demand issues. The boiler was on congruent phosphate control, so the lab staff increased monitoring frequency and attempted to maintain phosphate and pH levels within recommended guidelines. After approximately three weeks, operators discovered the source of the leak and corrected the problem.
Two months later, boiler waterwall tubes began to fail with alarming frequency. The unit came off numerous times for tube repairs, and in at least one instance had only been back on-line for a few hours when another tube failed. The failures happened so regularly that plant management scheduled an emergency tube replacement during the upcoming spring outage, at huge cost.
The mechanism attributed to these failures was underdeposit hydrogen damage. Interestingly, the leak was not from a failed condenser tube. The condenser hot well was equipped with a drain line that discharges to the cooling water outlet tunnel. During the autumn outage, an operator opened the line to drain the hot well but then forgot to close the isolation valve before startup. Once the unit went on-line, the strong condenser vacuum pulled cooling water into the hot well. Closing this valve was apparently overlooked or not included in the startup procedures list, which resulted in a very expensive lesson learned, although the unit should have been shut down when the leak was first detected.
While cooling water in-leakage can generate several reactions when the contaminated water reaches the boiler, the reaction shown in Equation 4 is quite common and was the documented chemistry in this case.
MgCl2 + 2H2O + heat → Mg(OH)2↓ + 2HCl Eq. 4
Although hydrochloric acid (HCl) can cause general corrosion in and of itself, the compound will concentrate under deposits where the reaction of the acid with iron generates hydrogen, which in turn can lead to hydrogen damage of the tubes. In this mechanism, atomic hydrogen penetrates into the metal wall and then reacts with carbon atoms in the steel to generate methane (CH4) as illustrated by Equation 5.
4H + Fe3C → 3Fe + CH4 Eq.
The formation of gaseous methane and hydrogen molecules induces cracking in the steel, greatly weakening its strength. Hydrogen damage is very troublesome because it cannot be easily detected. After hydrogen damage has occurred, the plant staff may replace tubes only to find that other tubes continue to rupture. Figure 8 (2) shows tube failure from hydrogen damage. Note in the figure the thick-lipped failure. The failure occurred with little metal loss.

Hydrogen damage continues to plague many steam generating systems, and particularly higher-pressure units, around the globe (3).
Conclusion
This article offers several direct examples of serious, sometimes even catastrophic, problems that can occur without proper attention to makeup water and condensate return chemistry for industrial steam generators. Sometimes, plant personnel consider these units to be of secondary importance (cooling towers and cooling systems often fit into this mindset, too), but when failures occur, they can curtail plant production and be enormously expensive to repair.
63 the ANALYST Volume 31 Number 1
5
Figure 8: Hydrogen Damage Tube Failure
Tales from the Waterside continued
References
1. ASME (2021). Consensus on Operating Practices for the Control of Feedwater and Boiler Water Chemistry in Industrial and Institutional Boilers, American Society of Mechanical Engineers, New York, New York. Note: This booklet is inexpensive and can be obtained by contacting the ASME offices.
2. Buecker, B. (2023). Water Essentials Handbook,” ChemTreat, Inc., Glen Allen, Virginia. Note: handbook currently being released in digital format at www.chemtreat.com
3. International Association for the Properties of Water and Steam (2015). Technical Guidance Document: Phosphate and NaOH Treatments for the Steam-Water Circuits of Drum Boilers of Fossil and Combined Cycle/HRSG Power Plants, www.iapws.org.

Brad Buecker is president of Buecker & Associates, LLC, consulting and technical writing/marketing. Most recently he served as senior technical publicist with ChemTreat, Inc. He has many years of experience in or supporting the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering positions with two coal-fired utilities. His work also included 11 years with two engineering firms, Burns & McDonnell and Kiewit, and he spent two years as acting water/wastewater supervisor at a chemical plant. Mr. Buecker has a BS in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He has authored or co-authored more than 250 articles for various technical trade magazines and has written three books on power plant chemistry and air pollution control. He is a member of the ACS, AIChE, AIST, ASME, AWT, NACE (now AMPP), and the Electric Utility Chemistry Workshop planning committee. He may be reached at beakertoo@aol.com
Keywords: BOILERS, COGENERATION, CORROSION, CHEMICAL TREATMENT, POWER, SCALING


64 the ANALYST Volume 31 Number 1
SANIKILL and SaniWare Treatment and control The patented SANIKILL technology now combined with SaniWare water quality monitoring panels! Expand your OPPORTUNITIES! Visit www.sanipur.com - sales@sanipur.com - 484-351-8702 Tales from the Waterside continued
What are the Parameters for Selecting a Filtration Method?
Mike Henley, Technical Editor
Background
Earlier in my career, a wise sage once told me that water treatment basically comes down to two aspects: “chemically or physically treating the water.” He explained that each approach has the same goal— preventing problems inherent to contaminants and characteristics associated with a particular water. After some contemplation, the wisdom of my friend made perfect sense. Now granted, there are water sources that require far more than these two options, but this seemingly simplistic statement is a good beginning point, and a critical element to understanding basic water treatment.
Brief Overview
It is important to understand that whether the treatment technology involves a chemistry or treatment equipment, the ultimate aim is to prevent a problem from developing in the water system, or where the treated water is used. In this article, we will focus on filtration, but before doing so, it seems appropriate to briefly examine the role specialty chemicals play in water treatment.
Please note: This article is taking a “back to basics” look at the subject of water treatment. The discussion will aim to provide a broad overview, so it will not focus in-depth on the particulars associated with the different topics. However, references are included in the Digging Deeper section at the end of the article to provide interested individuals new to water treatment with resources that offer more in-depth knowledge about water treatment—particularly filtration.
Chemical Treatment
To start, before moving to the physical removal of contaminants, we will briefly look at the role of treatment products play to provide clarity to the above observation by my friend.
First it should be noted that treatment chemicals can involve the use of a commodity chemical—in the powder, liquid, or gaseous form. Or it may entail the application of a blended chemical that contains two or more raw materials
"Water treatment basically comes down to two aspects: chemically or physically treating the water.”
blended together as a specialty treatment. Some examples of treatment types include the following:
Corrosion inhibition
Dispersion of foulants
pH control
Foaming control
Biocide/algaecide
Oxygen/gas removal
Flocculation and coagulation
Essentially, the aim when water is chemically treated is to adjust the water chemistry to prevent problems like scaling and corrosion. For instance, an antiscalant works to keep scale-forming minerals in the water suspended so that they are unable to form crystalline structures that cause scaling. Likewise, a corrosion inhibitor works to eliminate or minimize the conditions that lead to the corroding of piping, tubing, heat exchangers, etc., found in water systems.
Examples of end uses associated with chemical treatment include the following:
Cooling towers/cooling water
Boilers
Pools and spas
Wastewater
Drinking water
Mining
Produced water
Hydrofracing (oilfield exploration)
Environmental cleanup
Reverse osmosis (RO) chemicals (e.g., biocontrol, cleaning, and scaling and fouling control)
Ion exchange (IX) resin regenerants
65 the ANALYST Volume 31 Number 1 Technical Updates, Tips, or Reviews T.U.T.O.R.
Physical Separation
This aspect of water treatment involves the use of filtration technologies used to physically remove contaminants from the water. It can be tied with chemical treatments like coagulants and flocculants that cause suspended solids to agglomerate and form floc, which can then be filtered out. The term “physical separation” is associated with macroparticles (and even larger) down to the molecular level. The technologies used are associated with different treatment equipment found at industrial, municipal water and wastewater treatment, lightindustrial and commercial facilities, and even residential applications.
Contaminants
Briefly, here is a basic list of common contaminants found in raw water supplies that are treatment concerns.
Total dissolved solids (TDS). These contaminants are dissolved in the water as ions or chemicals. Some contaminants such as arsenic, lead, nitrates, and others can be considered toxic and dangerous to human health, so there are regulations that set limits for their levels in applications like drinking water. On the other hand, there are cases where ionic forms of elements and minerals considered important for health are added back to say a bottled water where a technology like RO removed them.
Total suspended solids (TSS). These are especially common in surface waters and are small particles of solids found in the water.
Microorganisms. These take on different forms: viruses, bacteria, cysts, amoeba, fungi, algae, and others. Pathogenic microorganisms spread diseases. Cryptosporidium and Giardia are two examples of microbials that can cause diarrhea if present in water and regulations exist that require water prone to contain them to be filtered or treated otherwise to protect human health.
Organics. Surface water, in particular, can be contaminated by organics such as tannins that come from decomposing leaves and other vegetation in the areas where they flow.
Agricultural contaminants from animals and fertilizer.
TSS versus TDS
One key to understanding the aim of filtration technologies is the difference between TSS and TDS. On the one end, certain filtration technologies aim to remove particles that are classified as TSS. It should be noted
that in the list from the previous section, there are some contaminants like organics that can fall under either category, depending on if it is dissolved down to the ionic level, or is a visible particulate material found in a water supply.
Suspended Solids
According to Campbell (2021), TSS refers to waterborne particles 2 micrometers (µm) or larger that either float or remain suspended in the water. TSS impacts water clarity. The higher the amount of TSS in the water, the less clear it will be. Color in rivers, and lakes comes from suspended particles. Examples of TSS can include sand, sediment, algae, bacteria, and plankton. Decaying plants, leaves, and animals release organic particles into water that are often suspended solids. (Note that these organic materials also cause total organic carbon [TOC] in the water source.)
Dissolved Solids
The Water Systems Council (2007) describes TDS as a measure of inorganic and organic substances in a water source. Key sources for TDS in waters include agricultural runoff and residential (urban) runoff, clayrich mountain waters, leaching of soil contamination, and water pollution discharge from industrial or sewage treatment plants. Common chemical forms of TDS include calcium, phosphates, nitrates, sodium, potassium, and chloride. The chemicals may be cations, anions, or molecules.
Metals from piping or plumbing that leach into the water can be another source. In water treatment, conductivity is a common measurement used to determine the level of TDS in the water.
Filtration
Figure 1 shows a chart that reviews filtration technologies appropriate for removing contaminants ranging from the macro particle range down to the micrometer size. Charts like Figure 1 have been available for decades and provide a helpful reference when considering treatment options.
From the chart it can be noted that macro particles are visible to the naked eye, while microparticles (from 1 to less than 100 µm) can be seen in an optical microscope. Scanning electron microscopy (SEM) can be used view contaminants in the molecular and macro molecular size ranges, while a scanning tunneling (ST) microscope is used to detect contaminants in the ionic range.
66 the ANALYST Volume 31 Number 1
T.U.T.O.R. continued

In Figure 1, the top portion shows examples of contaminants, and analysis methods used to detect them. Analytical approaches include ST microscopes (ionic level), SEM (molecular level), optical microscope (micro particle range), and the naked eye (macro particle range). The middle of the chart shows examples of contaminants removed by the filtration technologies from under 0.001 µm in the ionic range up to 1,000 µm for macro particles.
Types of macro particle contaminants removed by filters in the 100 µm to 1,000 µm range would include sand, other sediments, human hair, and granular activated carbon (GAC). Examples of micro particles (1 µm to under 100 µm) that might be removed include human hair, yeast cells, dust, bacteria, Giardia cysts, and Cryptosporidium. In the macro molecular range (1.0 µm to less than 0.1 µm), types of contaminants would consist of bacteria, viruses, colloidal silica, and asbestos. Micro molecular (above 0.01 µm to above 0.001 µm) water impurity examples include viruses, endotoxins, colloidal silica, and carbon black. Molecular level (above 0.001 µm to above 0.01 µm) contaminants include sugar, colloidal silica, endotoxins, synthetic dye, herbicides, and pesticides. Impurities at the ionic range (0.001 µm and under) include salts, herbicides, pesticides, metal ions, and sugar.
As seen in Figure 1, particle filters work in the micro and macro particle range, while microfilters (MFs) are effective between the macro molecular and micro particle size span. Ultrafiltration (UF) is a good option for taking out macro molecule to molecular contaminants, and nanofiltration (NF) will remove well in the molecular and larger ionic ranges. RO is the is made for ionic contaminant removal.
There are also other filtration technologies that work by sorption (adsorption or absorption) (activated carbon and other media) and IX that are not included in this chart but should also be considered.
The value of a chart such as shown in Figure 1 is that it can help guide decisions on the water treatment system based on the types of contaminants that need to be removed, and the final water quality requirements. For example, high-purity water would require several filtration technologies, while a facility without rigorous requirements may be able to either chemically treat the water or could get by with simple depth or cartridge filters for removing macro particles.
67 the ANALYST Volume 31 Number 1
Figure 1: Filtration Spectrum Chart
T.U.T.O.R. continued
Chart courtesy of UltraPure Private Ltd. (Singapore).
A Filtration Definition
For the purposes of this article, we offer a broader definition that considers filtration as the removal of water contaminants from macroparticles that are visible to the human eyes without the aid of a microscope down to the ionic level, which is unseen by the human eye. Under this understanding, treatment technologies such as activated carbon, IX (including electrodeionization), and iron oxide would qualify as types of ionic filters along with RO and NF.
Some might question this definition. However, another way to look at it is that ionic contaminants harm water quality just as much as suspended solids or tannins that color the water. They just happen to be much smaller than say silica or dirt particles. Therefore, to truly filter a water stream, it is needful to use technologies that can remove contaminants from the ionic level all the way up to pollutants seen by the human eye.
Closing Thought
To physically treat water, filtration technologies, as noted in Figure 1, are employed. Filtration for macro particles can range from multimedia gravity filters to molded or cartridge filters or media filters. From there, filtration technologies used at the micrometer range are MF and UF. When one reaches the ionic level, then RO, NF, IX, and activated carbon are choices to consider, based on water quality requirements. In future T.U.T.O.R. articles, we will examine the basics of different filtration technologies referenced in this article.
Digging Deeper Resources
1. Amjad, Z., ed. (1993). Reverse Osmosis: Membrane Technology, Water Chemistry, and Industrial Applications, edited book, Van Nostrand Reinhold, New York, New York.
2. AWT (2022). “External Treatment,” Chapter 2, Technical Reference & Training Manual, 3rd edition, Association of Water Technologies, Rockville, Maryland.
3. Boffardi, B.P. (2003). “Corrosion Inhibitors in the Water Treatment Industry,” chapter in ASM Handbook—Corrosion: Fundamentals, Testing, and Protection, Cramer, S.D.; Covino, B.S., Jr., eds., ASM International, Materials Park, Ohio.
4. Bornak, W.E. (2003). Ion Exchange Deionization for Industrial Users, Tall Oaks Publishing Inc., Littleton, Colorado.
5. Byrne, W. (2002). Reverse Osmosis: A Practical Guide for Industrial Users, 2nd edition, Tall Oaks Publishing Inc., Littleton, Colorado.
6. Campbell, B. (September 9, 2021). “What are Total Suspended Solids (TSS),” Wastewater Digest, accessible at https://www.wwdmag.com/utility-management/ article/10939708/what-is-total-suspended-solids-tss
7. Johnston, P.R. (1990). Fundamentals of Fluid Filtration: A Technical Primer, Tall Oaks Publishing Inc., Littleton, Colorado.
8. Kucera, J. (2010). Reverse Osmosis: Industrial Applications and Processes, John Wiley & Sons, Hoboken, New Jersey.
9. Owens, D.L. (1995). Practical Principles of Ion Exchange Water Treatment, 2nd printing, Tall Oaks Publishing Inc., Littleton, Colorado.
10. Water Systems Council (2007). “Total Dissolved Solids,” Water Systems Council, Washington, D.C., PDF accessible at https://www.watersystemscouncil.org/ download/wellcare_information_sheets/potential_groundwater_contaminant_ information_sheets/2010920TDS_FINAL.pdf

Mike Henley provides consulting services through MD Henley & Associates and serves as technical editor of The Analyst. He formerly was editor of the old Ultrapure Water Journal for 27 years and has been active in several aspects of water treatment and the associated businesses for more than 34 years. Mr. Henley’s background includes helping with the organization of the technical programs at more than 60 UPW conferences, including Water Executive Forums.
Keywords: FILTRATION, ION EXCHANGE, IONIC CONTAMINANTS, MICROFILTERS, NANOFILTERS, PARTICLES, REVERSE OSMOSIS, SPUN FILTERS, ULTRAFILTERS
68 the ANALYST Volume 31 Number 1
T.U.T.O.R. continued
Beyond Water

May We also Be Faithful, Like Those Who Have Gone Before Us
Mike Henley, Technical Editor
Community. That one word conjures a multitude of ideas and concepts. Examples can include the following:
A small city or town
A neighborhood
A church or other group
An industry trade group
One important concept that sets apart a community group from say a large metro area like Houston or Philadelphia is that the group normally shares common interests, values, and goals. Communities obviously are associated with residential areas in a city or a town, as well as within churches or social groups. That said, I believe that the word “community” is also an appropriate way to view the Association of Water Technologies (AWT) as well as the water treatment business in a more general way.
Like in a residential community, one important aspect is that of the different individuals who make up the larger group. While this could be overstating, one important characteristic is that the relationship dynamics can begin to take on those found within a family.
A Water Treatment Family
I come to this conclusion after 34+ years of involvement in the water business in different treatment areas—but always in the role of a technical journalist/editor.
When I first began my first position in 1990, I knew little about the industry dynamics, and did not fully appreciate what “ultrapure” or “high-purity water” really was. But, very soon in my work, I began to meet and be exposed to different individuals, who were truly pioneers and respected subject matter experts (SMEs) in different aspects of water treatment. The first such gentleman I
remember meeting was Dr. Ted Meltzer at the November 1990 Ultrapure Water Expo West in San Jose, California. Ted, as I came to call him, shook my hand and welcomed me to the industry. Needless to say, I was appreciative of that kind greeting, but didn’t fully appreciate Ted’s role in the industry until later. For many years he served as the technical program director for the UPW conferences, but he also was well respected in the water industry and was often invited to lecture—both domestically and overseas—on topics related to high-purity water.
Ted and I developed a wonderful relationship and would exchange phone calls through the years and enjoy visits at conferences. We would speak both about life and our professional work. Ted had a wry sense of humor that he freely shared, both when handling his conference duties, as well as in personal conversations.
During his career, Ted authored several books, two of which Tall Oaks Publishing issued— High-Purity Water Preparation for the Semiconductor, Pharmaceutical, and Power Industries, and Pharmaceutical Water Industries.
Through our friendship, Ted became one of my first mentors in learning about the high-purity water business segment.
Conference Communities
Over time, I slowly began to grasp a special aspect of the water treatment business—the sense of genuine community that is often best illustrated at annual conferences. I well remember attending different conferences and meeting many different individuals who would also become good friends. But this occurred over a period of several years (and continues to the present).
69 the ANALYST Volume 31 Number 1
One interesting characteristic I began noting in the later 1990s was for some attendees that meetings like the AWT, International Water Conference (IWC), Cooling Technology Institute (CTI), UPW, Electric Utility Chemistry Workshop, and others, were more than exhibitions with technical sessions—they also seemed like family reunions. One would see individuals sharing hearty handshakes or even hugs, with expressions like “It’s good to see you. How are things?” And these friendships would deepen over the years.
Fast forward to 2023. One fascinating element about these annual water conference gatherings is that they can take on the aura of importance in the yearly calendar—to the point that one will make it a priority to attend—not so much for the new knowledge from the technical sessions, but for renewing friendships and meeting new colleagues from the water business.
Another interesting aspect is that the older SMEs not only are welcoming to younger entrants in the water industry, but that they are willing to share their wisdom and insights about water treatment, and even life in general. In many cases, unstructured teaching and mentoring occurs simply by the dynamics of the relationships that develop.
Mentoring
Adviser and trainer are important pieces of mentoring, which has become a popular word in recent years, but really goes back to ancient times, when individuals like Jesus had his disciples, and others like Socrates, Plato, and Aristotle were also noted for having followers.
In my case, I did not enter into formal mentoring, but nonetheless gained knowledge and a better grasp of different aspects of water treatment from some of the sages that I have been blessed to meet over the years.
Briefly, here are some areas where I gained a better understanding from these relationships:
Reverse osmosis (RO): The late David Paul helped in understanding better how RO works and what are different concerns to pay attention to for successful operation.
Corrosion: I could give other names, but the late Paul Puckorius taught me some about microbiologically induced corrosion (MIC) and helped me gain a better appreciation for its impact on water systems.








70 the ANALYST Volume 31 Number 1
Beyond Water continued
Observing: The late Arthur Freedman shared some of his water treatment experiences that often related to boiler or cooling water. But the most interesting story he ever shared related to a problem he solved for a pharmaceutical plant where the highly pure Water for Injection was being fouled by organics. He helped solve the problem, not because of his knowledge in the treatment of pharma water, but because of taking time to learn and then observe activities by the pharma system and noting work by a janitor that was causing a solvent to enter a storage tank with pure water.
Ion exchange (IX): Over time, a number of individuals have helped me to better understand the workings of IX systems—Dr. Robert Kunin, Frank McGarvey, and George Crits are some of several examples. Of these three, I particularly remember the zest of Mr. Crits, who in his later years would often have computer disk copies of his “Crits Notes” to share with others. This handbook he developed has been published by Chemical Publishing Co.
Cooling water: Both Dr. Freedman and Mr. Puckorius were helpful in learning more about this important subject. And, Rob Ferguson, co-founder with his wife Janet of French Creek Software, also freely shared his insights.
(Please note that some of this learning also was passed on from technical papers by these individuals that I had the honor of editing and preparing for publication.)
I could go on and share about others, but the above people are simply examples of the value of being in the water treatment community. The point is that such involvement can enrich ones career and offer the opportunity to gain helpful insights from experts who have been active in the industry for many years.
May We Also Be Faithful
In closing, the water community plays an important role in successful water treatment—in part through the knowledge that is shared through personal relationships. All of the individuals named have passed away in the last 15 years, but they left a rich legacy to those who knew them. May those of us who read these words, also be faithful and look for opportunities to share of our wisdom and knowledge, not only in water treatment, but in life itself. Unbeknownst, you may be a help to an individual who goes on to discover a new and better way to control corrosion, treat boiler water, or make some other important contribution to society.
Acknowledgement
I wish to acknowledge the important contributions Dr. Bennett Boffardi, former technical editor of the Analyst, made to the water treatment field, this journal, and the AWT. Because our careers went in different directions, I never had the opportunity to meet Dr. Boffardi in person, but after starting in my work with the AWT, he took the time to call me. He was very gracious, and we had a nice conversation.
Editor’s note: Beyond Water is a column in the Analyst to address life issues that members of the AWT face in addition to their important work in the water treatment business. We welcome contributions from readers. If you have an idea for an article, please feel free to send your suggestion to Mike Henley. We welcome your input.
Mike Henley is a Water Industry Consultant with MD Henley & Associates. As a part of his work, he serves as the technical editor of the Analyst. Mr. Henley has been in the water industry for more than 34 years and was the editor of the old Ultrapure Water Journal for 27 years, and during that time also helped to organize technical programs for more than 60 conferences, including the Water Executive Forum. He may be contacted at mdhenleywater@gmail.com
©2024 MD Henley & Associates.
71 the ANALYST Volume 31 Number 1
Beyond Water continued
Changes to Unified Codes in 2024

The AWT Legislative and Regulatory Committee is dedicated to staying on top of upcoming laws and regulations that may have an impact on the water treatment industry. In this month’s column, the committee has identified an update to the IAPMO UPC
codes. Want to know how you can become more involved in our legislative efforts? Email us at awt@awt.org. We are always looking for more volunteers to be AWT’s eyes and ears regarding issues that may be of interest to the membership.
What is the topic?
International Association of Plumbing and Mechanical Officials (IAPMO) Standards Update
IAMPO writes Unified Codes, such as the Unified Plumbing Code (UPC) and Unified Mechanical Code (UMC), based on the accredited American National Standard (ANS) development process. These codes are adopted into certain states/cities for their own State Codes. In 2024, the UPC and UMC will include aspects of the ANSI/ASHRAE 188 standard to prevent Legionnaires disease from building water systems, with a focus on cooling tower design, maintenance, and testing.
Who could this have an impact on?
APMO UPC codes are typically adopted in these regions: Alaska, Arizona, Phoenix, California, Los Angeles City, San Francisco, Hawaii, Iowa, Minnesota, Missouri, Kansas City, Montana, Nebraska, Nevada, North Dakota, Texas, Washington, and Seattle.
How does this effect you?
States that adopt this standard will require the knowledge of experts to create and implement water management plans.
How can you be involved?
Especially if your state is on the list above, review the Standard and investigate the interest in your state of adoption.
To learn more about these Code changes visit: 2024 UPC, Appendix N
https://epubs.iapmo.org/2024/UPC/
2024 UMC, Appendix H for Professional Qualifications and more https://epubs.iapmo.org/2024/UMC/
72 the ANALYST Volume 31 Number 1 Capital
Eyes
What is the topic?
Toxic Substances Control Act (TSCA) Reporting and Recordkeeping requirements for Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)
On October 11, 2023, the U.S. Environmental Protection Agency (EPA) published its final rule on the Toxic Substances Control Act (TSCA) Reporting and Recordkeeping requirements for Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). This onetime reporting rule requires those that have manufactured (including imported) certain PFAS substances in any year since January 1, 2011, to submit information to EPA regarding PFAS uses, production volumes, byproducts, disposal, exposures, and existing information on environmental or health effects.
How does this effect you?
This may not effect the majority of membership, however it could effect your suppliers and possible your clients.
How can you be involved?
Who could this have an impact on?
Anyone who has manufactured (including imported) a PFAS for a commercial purpose in any year since January 1, 2011, is covered by this rule. As noted in Unit III.B.2, ‘‘manufacture for a commercial purpose’’ includes the coincidental manufacture of PFAS as byproducts or impurities. EPA believes at least portions of the NAICS codes listed in Unit I.A. may be covered by this rule. This rule extends to manufacturers (including importers) only. Importers of PFAS in articles are considered PFAS manufacturers. Persons who have only processed, distributed in commerce, used, and/or disposed of PFAS are not required to report under this rule, unless they also have manufactured PFAS for a commercial purpose. If an entity (such as a wastewater treatment plant) is simply processing PFAS they received domestically, and not also manufacturing PFAS, including as a byproduct, then the entity is not covered by this rule.
Although EPA received several public comments about extending the rule to cover processors (see Unit IV.), TSCA section 8(a)(7) only refers to manufacturers and expanding the rule to processors would be pursuant to EPA’s separate rulemaking authority at TSCA section 8(a)(1), which the Agency is not pursuing at this time.
Review the regulation and verify if it affects you or your client directly. If a client is affected use your newly found knowledge to protect them if they were not already aware.
https://epubs.iapmo.org/2024/UPC/
73 the ANALYST Volume 31 Number 1
learn more about these Code changes visit:
To
Capital Eyes continued
What’s (Water) on Your Mind?

What Could Cause Variations in Drinking Water Quality from Local Utilities?
Compiled by James McDonald, Chem-Aqua
The following discussions come from the Industrial Water Treatment interest group on LinkedIn.
Question of the Week
What could cause variation in the quality of water provided by the local municipality (e.g., city water)?
Luke: Seasonal (or acute) changes in the municipality’s source water (lake, river, etc.). For example, we would see elevated chlorides in the city water in the early spring due to all of the melting and runoff of snow that carried road salt (from road freeze protection) into the local water sources.
Chris: In Carlisle, Pennsylvania, we had higher total dissolved solids (TDS) and hardness in the winter. I attributed this to salting the roads with calcium chloride (CaCl).
Lauren: Heavy rains can stir up sediment in surface water supplied plants, which can lead to incomplete disinfection due to solids in the effluent.
Sachin: Primarily season followed by chemicals added for sediments and disinfection, which result in changes in pH and the Langelier Saturation Index (LSI).
James: Absolutely. Especially in the northeast.
William: A change in raw water quality due to various factors, for example:
Seasonal changes
A treatment plant that is not well maintained
Improper chemical treatment or selection
Poor treatment plant optimization
The following are recommended:
For proper chemical selection, a laboratory test for flocculant selection test must be conducted. This will indicate the needed chemical dosing rate.
For plant optimization, run optimal flow rates based on design to ensure chemical dosing is as recommended by the flocculant selection test.
Peter: A lot of things. Depends on the source, primarily groundwater or surface water. Generally, a process upset. Were the operators playing poker at the time?
Keith: This happens a lot here in the Northeast when municipalities change the location of where they draw source water.
Narayanan: There may be N number of causes for variation in water quality supplying the municipality. Major causes are:
Variation in raw water quality— higher in value of impurities than design value, which is turbidity, pathogenic bacteria, virus, protozoa, color, TDS, heavy metals, pesticides, acids, alkalis, and others.
Variation in raw water flow viz higher or lower raw water flow than water treatment plant (WTP) design value can definitely upset the efficiency of treatment processes.
Poor operation and maintenance of water treatment plant viz untrained plant operators, unguided technicians, ignoring the lab chemist, postponing maintenance team, careless plant managers, human errors etc.
Unexpected breakdowns such as pipeline leaks, or electro mechanical equipment failures.
No strict laws and no severe punishment.
74 the ANALYST Volume 31 Number 1
Using poor quality of electromechanical equipment during erection in WTP and distribution.
Under design of water treatment plant and distribution network, etc.
Ed: Surface waters will vary significantly with amount and type of precipitation. Any municipality using water from rivers or large streams will typically see a reduction of dissolved solids, but with an increase of suspended solids during heavy rainfall. If the precipitation is snowfall, it would then depend on the amount of snow and rate of snow melt. Slow snow melt will significantly reduce dissolved and suspended solids.
If there is a drought, both dissolved and suspended solids can increase. In the Philadelphia area, the further downstream the utility, the greater the increase in solids with extraction and discharge. In severe droughts, we have seen the salt line (where sea water mixes with river water) move up the river, increasing dissolved solids. There are some municipalities that have been forced to switch to primarily well water.
James: Change of seasons if it’s surface water.
Kaushik: I believe apart from all the reasons mentioned above, pipes that transfer and distribute water in the city can be one of the major causes of change in water quality to the user. Pipes aging, rusting, leakages or holes in the pipeline can be a source of water contamination.
Poll of the Week
This poll is a good reminder not to be complacent and assume your incoming water quality is constant. It can change for a plethora of reasons and potentially have negative impacts upon your industrial water systems.
Poll Responses (110
votes)
Have you seen water quality coming from the local municipal water district change over time (e.g., year, month, week, day)?
Question of the Week
When adjusting a dosing pump, what thought should be behind deciding between adjusting the speed versus the stroke or both? In what situations is it important to adjust one over the other?
Ron: It is best to select a pump that supplies a continuous feed of chemicals. Otherwise, the intermittent shots of a chemical can leave the to-be treated components free of any treatment. A good rule of thumb is that the stroke is the size or amount of chemical being injected. The speed is how fast the amounts are being injected. Knowing the desired daily dosage of chemical is the key to sizing a pump properly. It needs to be sized for mid-range, so the user has up and down flexibility with regards to dosage. Just my 2 cents worth.
Stephanus: Like in most cases, no hard rule should be followed without some thought and common sense. Higher speeds (frequency) will lead to more homogeneous chemical mixing but will also lead to more pump wear and tear. Perfect homogeneous mixing at the point of dosing is not always required— for instance, dosing into a stirred tank, recirculated system, or feeding urea into an aerobic digester. I would also avoid short stroke-length dosing for sodium hypochlorite, which can easily, in warmer conditions, form bubbles that could get stuck in the pump.
Mark: Great point about potential issues with pump priming at low stroke length.
Lauren: Think of stroke length and speed like rpms and gears in a manual truck. Both contribute to your truck speed (dosing rate) but get you there in different ways.
Short stroke length/high speed provides a more consistent flow rate with less pulsing. This is very important at very low chemical flow rates (i.e., polymer addition for flocculation).
High stroke length/low speed will get you the same result, but a lot of pulsing and variability. Note that remote control of stroke length and speed are features that cost extra, and sometimes you are only given remote speed and length is field only. For processes that are more predictable this is adequate.
Steve: It depends on what I’m feeding. If there is a large dead band in the system that I’m controlling on a sensor, I prefer much slower speed but higher stroke (output).
James: I don’t find stroke length versus volume pumped to be a linear relationship, so when I want to adjust dosage, I’ll almost always do it with the speed (or adjust the timer up/down).
75 the ANALYST Volume 31 Number 1
YES NO 83% 17% What's (Water) on Your Mind? continued
Paul: I agree with many of the points, especially Steve’s comment on what you are feeding. Changing a biocide feed from slow and continuous (short stroke/higher speed) to slug (long stroke/high speed) would require some calculations and drawdown monitoring. Having pumps with the ability to make these types of changes is critical to success of a program.
Hannes: All relevant points. We found that the viscosity of product dosed will play a role in adjustments.
Question of the Week
What are chemistries that may be added by the local municipality to your makeup water that you need to be aware of?
Ali: It depends on the type of application of makeup water that we are going to use, such as acid to adjust pH, metal on phosphate to precipitate carbonate, or biocide to prevent the growth of bacteria and viruses, etc.
Greg: Chloramines.
Bain: Sodium thiosulfate to reduce free chlorine.
Luke: Compounds and/or formulations containing phosphorus, zinc, and even fluoride can be helpful to know. Then, it could be useful and/or important to know if they are treated with chlorine or chloramines.
Poll of the Week
This poll is a reminder that terminology can mean different things to different people, depending upon the context. If discussing municipal water applications where RO can be the last step in a series of unit operations, RO is not called pretreatment. If discussing boiler or process water applications, RO may very well be called pretreatment.
Poll Responses (671 votes)
Is reverse osmosis considered pretreatment?
Question of the Week
What could cause one’s math to show a cooling tower is running at less than 1 cycle? Is this really possible?
Jakkula: If the makeup water source and quality change, there is possibility of math to show low cycles of concentration (CoC).
Mark:
Massive variation in makeup water quality (did someone accidentally run the RO permeate into the tower sump?).
Heavy rainfall in a low-volume system that usually has high conductivity makeup.
Process variation (example: Styrofoam plant that discharges condensate from the process to use as tower makeup).
Heat exchanger leak (where there is low conductivity water on the other side of the heat exchanger).
Some fool put deionizer (DI) resin in the tower sand filter by accident.
Magic.
Fundamental mathematical ineptitude.
Interesting question...my money goes on “check your math” before making any drastic changes.
Boone: If you are using chemical analysis (and not mass balance) to calculate cycles, then you could theoretically get a value of less than one cycle if there is precipitation and/or deposition of that material.
Mitchell: Use of an alternative water source such as steam or air handler condensate, rain water, or snow melt entering as makeup during low-load conditions.
Loren: Let’s begin with a real-world example. For instance, in Peoria, Illinois, the makeup water conductivity will vary throughout the day. It will vary from 600 to 1,200 micromhos (µmho). In that type of environment, your less than 1 cycle math can occur. I used a cycles controller on the tower instead. It has a conductivity sensor on the makeup water and the tower. Then you dial in a multiplier. It’s not a perfect solution, yet it is an excellent one.
Ramlan: It could be possible when you are adding steam condensate as make up for cooling tower, with condition you are calculating the cycle not by water balance but by water chemistry. Under a situation of having dissolved solids drop out in circulating water due to failure of scale
76 the ANALYST Volume 31 Number 1
YES NO DEPENDS ON CONTEXT 36% 32% 31% What's (Water) on Your Mind? continued
control, you may have calculated cycle of less than 1 if cycles are being calculated based on the water chemistry.
Joseph: My first guess is RO or DI makeup, which could reduce the true cycle count, but not make it less than 1. An unaccounted-for change in the makeup water quality, either seasonally or if the municipal supply changed sources. A tower running at 1 cycle with 50 parts per million (ppm) Ca could read as less than 1 cycle if the tested makeup water recently changed to 60 or 75 ppm Ca.
Question of the Week
What are the uses for running a particle size analysis on a water sample?
Abubaker: Analyzing particle size in water samples is crucial for:
Environmental monitoring: Assessing pollutant impact and compliance.
Water quality assessment: Understanding suspended particle composition.
Treatment optimization: Enhancing water treatment efficiency.
Engineered systems design: Designing effective water management systems.
Research and Studies: Supporting scientific understanding of sediment behavior and impacts.
Sara: Running a particle size analysis on a water sample can provide valuable information about the physical characteristics of the particles in the water, such as their size distribution and total concentration. This information can be useful in a variety of applications.
Keith: I’ve been using PDA/PSA for evaluating and selecting proper water filtration technologies for more than 20 years.
Chaminda: I did this for selecting filter cloths for recess membrane filter presses and selecting belts for belt presses. This helps to find a suitable filter cloth or belt to reject specific particle sizes.

77 the ANALYST Volume 31 Number 1
What's (Water) on Your Mind? continued
Benjamin: In the realm of anaerobic biology, this method helps assess the health of granules in your reactor. How big are these granules, and are they uniformly distributed or is there a lot of flocculated biomass? While various techniques are used to gauge the overall reactor health, this method adds a unique aspect to the analysis.
Lauren: Evaluating filtration technologies (filter belts, specifying throwaway cartridge filters, specifying basket strainers for pretreatment before the main process unit) to provide the necessary removal without quickly blinding the filter.
Farhan: Particle size analysis of a water sample is used to assess water quality, optimize treatment processes, ensure product quality in various industries, study environmental impacts, and more.
Afifa: To find out the sizes of each substances in the water samples. It can be done in different ways such as using particle size analyzer, where there is shouting a laser through a beam, where smaller particles can come through the sample. The particles will be detected, and the results are analyzed using a computer.

Moderator James McDonald, PE, CWT, is Director of Technology & Marketing with Chem-Aqua. He holds an M.S. in chemical engineering and is a Ray Baum Memorial Water Technologist of the Year award winner (2013). Mr. McDonald also chairs the Association of Water Technologies (AWT) Technical Committee.
Keywords: COOLING TOWERS, DRINKING WATER, PRETREATMENT, PUMPS,REVERSE OSMOSIS
78 the ANALYST Volume 31 Number 1 Advertising Index 79 AMSA 50 Aqualytics Reports 56 Bio-source, Inc. 40 Brenntag North America 2 Enviromental Safety Technologies 42 Myron L Company 80 North Metal and Chemical Company 5 Pulsafeeder, Inc. / Idex Corporation 6 Qualichem, Inc 8 Quantrol 64 Sanipur US 71 Scranton Associates 53 Walchem Iwaki America
What's (Water) on Your Mind? continued











AMSA, Inc.® 4714 S. Garfield Rd. • Auburn, Michigan, USA • 48611 Tel: (989) 662-0377 Fax: (989) 662-6461 sales@amsainc.com www.amsainc.com ® Biocides Don’t Do the Job Alone Biocides need help to penetrate and break up biofilm deposits Biocide & BCP® Chemistry Visit www.amsainc.com to learn why pairing AMSA chemistry with a biocide is an effective Biofilm Control Program! ® BEFORE System in Control AFTER Give your customers a great water treatment program using the proven AMSA chemistry for penetration, disaggregation and prevention of organic deposits with a unique mode of action. Satisfied cooling tower owners with great biofilm control will recommend your services to other perceptive potential customers. Biocide Only









































































































 Tim Elliott, P.Eng. National Sales Manager at Iwaki America Inc.
Tim Elliott, P.Eng. National Sales Manager at Iwaki America Inc.







































