





LARVIVA is a complete range of hatchery feeds. It is specially developed to maximize the success of the hatchery operations by giving your larvae a strong start ensuring high quality, robust and performing fry ready for the grow out stages. www.larviva.com

By Ruby Gonzalez

By Nancy Erickson
















BY JEAN KO DIN
Ihave been soaking up a series of presentations, webinars, and even in-person conversations about what is exciting in the world of genetics and broodstock at the moment. And, suffice to say, there is a lot!
For those in the industry who know me well enough, they know that my science background is limited to say the least. But, I am passionate about my role in bringing the most relevant information that will service the largest group of our readership community. It’s a difficult challenge for me on most days but because of my role as editor at Hatchery International, I have access to some of the most authoritative experts in the field.
This is what has inspired a new project for our publication. I want to call it HI Advantage. These webinars will be about creating a resource library of advanced education for hatchery professionals.
Our first project will be a three-part series on Advanced Genetics. We will invite researchers and geneticists from all over to provide teachings on new advancements in the world of genetics.
The webinar series will launch at the end of our online Genetics theme week (April 21-25) with an exploration on the latest tools in genotyping. We are bringing in experts to talk about the latest genomic resources, advanced genome editing techniques, sterility breeding innovations and other hot topics in the world of genetics. I am still in the early stages of putting this together in consultation with very knowledgable geneticists in the industry. My hope is that these webinars will bring you new tools for understanding long-term success in aquaculture breeding programs.
This will not be the same, baseline subject matter that we would cover with our free Hatchery 101: Genetics & broodstock webinar (available now). That is for more practical application for our hatchery workers. This HI Advantage series is about higher professional learning.
You will gain access to live presentations

from genetics experts, Q&A opportunities catered to your interests, plus a package of rich worksheets, questionnaires, and other downloadable resources.
Much of the content will be inspired by the industry conferences I’ve attended where the latest posters and research results are being presented to the regional aquaculture community in attendance. But when travel is not an option, we wanted to bring these expertise to you.
We believe in that the content that we are putting together will be worth the investment. There is so much expertise out there and through HI Advantage, we feel we can help save you time pouring over the latest journal articles, attending the latest poster presentations, and even reading new study summaries in our pages. Although we admit that it won’t fully replace the benefits of these things that I’ve mentioned, we believe that we can curate all that knowledge and share it in the most accessible format we can provide to our international audience.
I’m personally excited to try this new project idea for this year. I believe I can truly bring good value to our audience and I believe there is potential for it to continue to grow as a resource library for advanced professional learning.
Genetics, genomics, and broodstock management are the bread and butter of hatchery management. There are so many areas within this subject that we want to explore more deeply and I think this webinar series is our best opportunity to do so.
And if this new project gets a good response from this audience, I think it has endless potential to dive deeper in other areas of work, as well. You can let us know what you would like to see. We truly value reader feedback and you’ll be surprised what one idea can snowball into.If our team ever misses the mark, please don’t hesitate to reach out and give your two cents.
As I always like to say, my email inbox is always open at jkodin@annexbusinessmedia. com. | HI
VOLUME 26, ISSUE 3 | MAY/JUNE 2025
Reader Service
Print and digital subscription inquiries or changes, please contact customer service
Angelita Potal
Tel: (416) 510-5113
Fax: (416) 510-6875
Email: apotal@annexbusinessmedia.com
Mail: 111 Gordon Baker Rd., Suite 400, Toronto, ON M2H 3R1
Editor Jean Ko Din jkodin@annexbusinessmedia.com
Associate Editor Seyitan Moritiwon smoritiwon@annexbusinessmedia.com
Contributors Nancy Erickson, Ruby Gonzalez, Nicole Kirchhoff, Christine Lepine, Zimri Oseland, Rakesh Ranjan, Kata Sharrer, Magida Tabbara
Associate Publisher / Advertising Manager Jeremy Thain jthain@annexbusinessmedia.com +1-250-474-3982
Account Manager Morgen Balch mbalch@annexbusinessmedia.com +1-416-606-6964
Account Coordinator Catherine Giles cgiles@annexbusinessmedia.com
Media Designer Brooke Shaw bshaw@annexbusinessmedia.com
Audience Development Manager Urszula Grzyb ugrzyb@annexbusinessmedia.com 416-510-5180
Group Publisher Anne Beswick abeswick@annexbusinessmedia.com 416-410-5248
CEO Scott Jamieson sjamieson@annexbusinessmedia.com
PUBLISHED BY ANNEX BUSINESS MEDIA 105 Donly Drive South, Simcoe, ON N3Y 4N5
Subscription rates (six issues) Canada: $37.74+Tax
Printed in Canada ISSN
Publications Mail Agreement #PM40065710
UNDELIVERABLE CANADIAN ADDRESSES TO 111 Gordon Baker Rd., Suite 400, Toronto, ON M2H 3R1
Annex Privacy Officer
Privacy@annexbusinessmedia.com Tel: 800-668-2374
The contents of Hatchery International are copyright ©2025 by Annex Business Media and may not be reproduced in whole or part without written consent. Annex Business Media disclaims any warranty as to the accuracy, completeness or currency of the contents of this publication and disclaims all liability in respect of the results of any action taken or not taken in reliance upon information in this publication.
Next Ad Deadline
The advertising deadline for the May/June issue is March 11. Don’t miss the opportunity to be part of this exciting aquaculture publication. For more information, or to reserve space in the next issue, call our advertising department at +1.250.474.3982 jthain@annexbusinessmedia.com
Next Editorial Deadline
The editorial deadline for the May/June issue is March 14. Contact Jean Ko Din at jkodin@annexbusinessmedia.com for details. Material should be submitted electronically with prior arrangement with the editor.

CFEED, a Norwegian producer of copepods for marine larvae, is expanding its production facility in Vanvikan, Norway.
The company specializes in hatchery-stage live feed, producing Acartia tonsa copepods for over 15 species, including sea bream cod, grouper, seriola, seabass, tuna, and more. It made a decision two years ago to expand its production capabilities.
The new facility is set to open on Aug. 20, after construction is completed earlier in the month.
According to CFEED, this expansion will significantly increase capacity and create new job opportunities for the local community. It will also strengthen its presence as a producer of hatchery-stage feed for marine fish larvae.
“This project strengthens our

presence in the aquaculture market and ensures a sustainable, cutting-edge production facility that can scale efficiently. By improved technology and boosting of capacity, we can provide a safe, modern workplace for our employees while continuing to supply premium products to our customers. As part of its long-term vision, CFEED is reinforcing its position as a global leader in sustainable aquafeed solutions,” said Tore Remman, CEO of CFEED.
The company was founded in 2014. It develops and produces live feed solutions for fish hatcheries to enhance global aquaculture.
“At CFEED, we are privileged to have the trust of our customers, who rely on our product and expertise. They recognize our continuous efforts in research and develop-
ment to enhance our product quality and services. This expansion allows us to scale up production while exploring new markets to better serve our growing customer base,” said Linn Baardsgaard, sales and marketing director.
The company will have an official grand opening event on Aug. 20 and is welcoming industry partners and stakeholders, who will be able to tour the new facility on that day.
The Iowa Department of Natural Resources (DNR) is investigating a fish kill at Dry Run Creek near Decorah in Winneshiek County.
On March 11, the DNR Field Office in Manchester learnt of a possible manure release and fish kill in an unnamed tributary of Dry Run Creek. Together with Decorah Fish Hatchery, they responded to the incident and observed dead fish
and very murky conditions in the tributary.
Officials identified overland runoff from an animal feeding operation near the headwaters of the tributary as the source of the release. According to a March 13 press release from DNR, they’re currently unsure about the amount of manure released.
Dead fish have been observed for several miles in the creek, and efforts to clean up have begun. The responsible party has been notified of the investigation and has started working to control the manure runoff.
Downstream water users are advised to avoid using water from Dry Run Creek.
The public is urged to call the DNR’s 24-hour spill line at 515-7258694 if they see dead or stressed fish in a lake or river, as quick reporting can help DNR staff identify the cause of a fish kill and potentially stop a fish kill in progress.



BRF Ingredients and Symrise have won the F3 Krill Replacement Challenge top prize for developing promising alternatives to krill.
Symrise won for its protein hydrolysate ingredients and BRF Ingredients for its chicken hydrolysate. Their products were the top-performing krill replacements in a 12-week feed trial on Atlantic salmon, demonstrating superior growth, feed consumption, and survival rates.
Each company won US$50,000 (US$100,000 total) at the World Aquaculture Society’s Aquaculture 2025 meeting in New Orleans, La.
“The F3 Krill Replacement Challenge has highlighted the incredible innovation and potential within the aquaculture
industry, demonstrating that there are multiple solutions to replace krill. These alternatives will help protect our oceans while ensuring the continued growth of aquaculture,” said Kevin Fitzsimmons, chair of the F3 – Future of Fish Feed Initiative.
The two winners were chosen from a pool of 10 finalists, selected from 40 global companies that entered the F3 Challenge to test their krill meal alternatives to offer innovative solutions that could transform feed production and reduce the environmental impact of traditional krill sourcing.
Overfishing, climate change, and industrial harvesting have been attributed to the decline in krill stocks, threatening their role as a key food source for many marine species. More sustainable alternatives are essential to re-
duce reliance on krill and ensure the future of aquaculture without further depleting ocean resources. It was confirmed that all products being used in the trial were free of marine animal ingredients.
“For us at BRF Ingredients, this recognition reinforces our commitment to feed the world with sustainable, high-quality and global-standard Ingredients. We congratulate all participants while celebrating our hydrolyzed products team’s technical skills and talent,” said Marcel Sacco, vice president of marketing and new channels at BRF Ingredients.
“As a firm believer in the immense potential of byproduct valourization, I see this award as a testament to our ability to develop natural ingredients from circularly sourced raw materials,” said Vincent Percier, marketing director at Symrise Aqua Feed.
“This innovation enables us to replace wild and endangered species like krill in feed formulations, contributing to a more sustainable global food production footprint.”
AquaTactics, the Washington-based aquaculture division of Bimeda, has entered the autogenous fish vaccine sector after receiving USDA approval.
Autogenous fish vaccine production and wet-lab safety testing will happen at Bimeda’s vaccine


facility, Bimeda Biologicals, in San Angelo, Texas, which already has experience in terrestrial animal vaccine production.
According to a press release from AquaTactics, its site in Kirkland, Washington, will remain key for fish vaccine development and customer support.
“With USDA approval, we can now expand our quality offering and provide custom vaccine solutions that meet the specific needs of our clients. This is an important milestone in our mission to enhance fish health and productivity for aquaculture operations across the USA,” said Sam Dash, general manager of AquaTactics.
AquaTactics provides a range of fish health services, including:
• Veterinary services
• Vaccination protocol design
• Fish health products
• Pathology services
“I am excited to see AquaTac-
tics further our ability to support the U.S. aquaculture industry and the aquatic veterinary profession. We are now able to provide high-quality fish vaccines to veterinarians, fish health professionals, and the facilities they service in all 50 states,” said Katharine “Ono” Onofryton, AquaTactics’ veterinarian.
The Maryland Department of Natural Resources’ annual Fall Oyster Survey has shown that oyster populations in the Chesapeake Bay are doing well.
For the fifth consecutive year, the survey’s 2024 spatfall intensity index–a measure of reproductive success and potential population growth for oysters–was above the 39-year median. This was at-
tributed to a “remarkable year for oyster reproduction” in 2023.
The 2023 year class oysters were abundant in many areas, a good sign for both the fishery and the Bay’s oyster sanctuaries. Just a little mortality in their class was observed, although lower salinities stunted their growth.
Also, disease levels in Maryland were reported to have decreased due to a wet start to 2024.
“The 2024 Fall Survey confirms Maryland’s oyster population is doing well, with the key indicators showing encouraging results,” said Christopher Judy, director of the Department of Natural Resources Shellfish Division.
“Certain low salinity regions still need to improve, but overall the findings are promising for the near-term future of this vital species.“
This survey started 85 years ago and is one of the longest-running


continuous oyster monitoring programs in the world.
The 2024 fall survey was done from Oct. 8 to Nov. 25 throughout the oyster-growing waters of the Maryland portion of Chesapeake Bay and its tributaries, including the Potomac River.
Biologists collected 364 samples from about 300 oyster bars, monitored locations that included natural oyster bars, oyster seed production areas, seed and shell planting sites, harvest areas, and
sanctuaries. Of these, 95 oyster bars within 38 sanctuaries were sampled during the survey.
It uses five indicators to assess the status of Maryland’s oyster populations: spatfall intensity (reproductive success), oyster disease, total observed mortality, biomass (number and weight of oysters), and cultch (a measure of habitat). The spatfall intensity and cultch indexes were derived from 53 long-term monitoring sites.
The preliminary results of the survey as reported to the Oyster Advisory Committee will be available on the Maryland Department of Natural Resources’ website.
Find the latest news, features and more web content from across the world.
HatcheryInternational.com


By Ruby Gonzalez
Broodstock improvement is an ongoing goal, propped by different objectives. There is genetic diversification and much more specific reasons, as shown in researches from Chile, China and India.
”Raw materials of marine origin”
Salmo studies conducted in Chile, a global powerhouse in salmon production, provided insights on factors affecting quality of broodstock and early evaluation of eggs.
In spawning Atlantic salmon, for instance, there is a need to adapt existing commercial broodstock diets to offer high-quality nutrition during gonadal maturation, according to “Effect of diet composition on maturation rate of female Atlantic salmon (Salmo salar) during gonadal maturation” by Maritza Pérez-Atehortúa et al.
Results showed that while commercial diets during gonadal maturation may produce spawned Atlantic salmon females, high-quality nutrition improves reproductive performance, producing a high number of spawned females.
“We think that experimental diets with raw materials of marine origin allow greater availability of key nutrients for the production of hormones that activate the sexual maturity of fish, such as cholesterol, which is the basis of steroid hormones to synthesize
hormones that synchronize the final sexual maturity of animals,” corresponding author, Prof. Iván Valdebenito, told Hatchery International.
Four different formulations were tested. Diet 1 contained exclusively marine-origin protein and lipid ingredients. Diet 2 substituted 65 per cent of the protein and 51 per cent of the lipid by terrestrial animal and vegetable sources. Diets 3 and 4 were commercial diets.
Diets 1 and 2 produced 73 per cent and 67 per cent spawned females, respectively. It was lower in Diets 3 and 4 with 53 per cent and 50 per cent, respectively.
While the commercial diets produce enhanced size, the study notes these “do not adequately fulfill the essential needs of the broodstock in terms of promoting successful gonadal maturation.” As such, these are categorized as growth diets.
Marine-origin raw materials such as fishmeal and fish oil are traditionally the key protein and lipid sources for salmon broodstock diets. Substitutes for these major materials are being made in commercials diets because of dwindling resources.
The quality of embryo may be predicted by presence of inclusions, according to the study,
also done in Chile, “The presence of inclusions in blastodiscs of coho salmon embryos (Oncorhynchus kisutch) is associated with low rates of fertility and embryo survival”.
“In the current study, it was established that there is a relationship between blastomere inclusions, symmetry, fertility and survival rate,” said authors Leydy Sandoval-Vargas et al. in the short communication.
Comparing results in cell samples with and without inclusions, all parameters were “significantly lower” in the former. It was also observed that “increases in the percentage of cells with inclusions were associated with low fertility rates.”
The “inclusion” cited in the study refer to structures of one or more holes of variable size and depth, which can pass right through the cell. This can be seen as a free space within the cell membrane such as, say, a cleavage or doughnut.
Study findings may provide the salmon aquaculture industry another set of safeguard protocols impacting the bottom line.
“In species with long incubation periods, such as salmonids, it is necessary to perform early evaluation of the egg quality both before fertilization and shortly after fertilization to optimize space and investment. In Chile, some salmonids-breeding companies habitually carry out those assessments,” the authors explained.
There are hypotheses on how the hole in
salmonid cell may be formed. It might be traced replacement of marine ingredients in aquafeeds with terrestrial source. “It could be speculated that some oil drops could give rise to the formation of fat vesicles, which in turn could create the holes.”
It might also be formed by mechanisms related to oocyte aging.
Both of the Chile researches were published on Aquaculture.
A research team in India initiated to standardize breeding and rearing of Indian catfish Clarias magur (Hamilton, 1822) to contribute to the sustainability and growing supply that is badly needed in the country.
In “Standardization of breeding and rearing of the endangered Indian catfish Clarias magur (Hamilton, 1822) for juvenile production,” authors, Kamal Sarma et al., sought to standardise and develop induced breeding technique and subsequently, rearing of fry for the production of juveniles to sort out the seed production related issues for the production of table size fish and broodstock.
Sub-experiments were done on the standardisation of the induced breeding technique; and rearing and feeding management of fry for production of fingerlings; and production of magur juveniles using different protein level diets.
The extent of standardisation considered wide-ranging facets, starting with selection of broodstock and pond design down to water exchange and estimation of quality parameters.
Decrease in supply of this popular species in India has been so noticeable that is has been categorized as endangered. Contributing factors are decline in wild population and unregulated fishing, among others.
Production is said to be held back at ground zero. Commercial volume of quality seeds is hard to come by. Induced spawning has been found to be “more inefficient” compared other species. Even in occurrences of successful induced breeding, low survival rates in fry, fingerling and juvenile.
Standardisation of the breeding performance was initiated using the WOVA-FH. Highest spawning fecundity was observed with 1.0 ml of WOVA-FH hormone dose.
“Hence, it can be suggested that if maturity of fish and environmental conditions are favourable, 1.0-1.5 ml kg−1 of WOVA FH will be the best dose for induced breeding, spawning fecundity, fertilization and hatching of C.
magur,” wrote Kamal Sarma et al. WOVA-FH contains a synthetic version of the gonadotropin hormone, which plays a crucial role in regulating reproductive processes in fish.
Larval rearing is most crucial phase for seed production of this species. Rearing trial revealed that C. magur require higher protein – 35 per cent – feed for better growth.
The study was published on Aquaculture Reports
Improving feed adaptability, as well as growth rate, in largemouth bass Micropterus salmoides was achieved in China through selective breeding.
By the fifth generation, the experiment produced animal with improvement in growth rate of almost 40 per cent and feed adaptability of over 20 per cent.
“During the breeding process, each generation undergoes standardized artificial feed adaptation training, and growth rate was used as the target trait. After five generations of
selective breeding, the breeding population exhibited higher food intake and growth rates compared to the non-breeding population,” cited authors Tao Zhu et al. in the article, “Whole genome resequencing reveals the correlation between selection signatures and adaptability of Micropterus salmoides to artificial fed”. This was published on Scientific Reports
Animal sourced from the wild was selected as the parent for the selective breeding.
The breeding group showed “greater willingness to eat’ during the feed adaptation. The assessment of feed adaptability indicated that the domestication success rate was 96 per cent for the breeding group and 74 per cent for the non-breeding group.”
External factors were likewise considered. During the entirety of the breeding process, the experiment ensured the presence of human activity.
“We speculate that largemouth bass individuals unable to adapt to human activities may experience excessive fear, leading to reduced food intake, which renders them unsuitable as candidate parents,” they explained. | HI













BY ZIMRI OSELAND, WYOMING GAME & FISH DEPARTMENT
Robertson drip incubators were first designed in 1938 by Fred J. Foster, a regional director for the U.S. Fish and Wildlife Service (Simon & Roberts, 1941). Foster developed the incubator to be used at Lake Station, which was operated by the Bureau of Fisheries in Yellowstone National Park (Simon & Roberts, 1941). These drip incubators are designed to incubate a large number of green eggs to the eyed stage with minimal water usage.
The incubators have a simplistic design of columns and rows that hold shallow trays full of eggs. Capacities can vary, but they generally accommodate several columns and up to seven rows per column.
Each column has its own water supply, and each row can hold a maximum of six egg trays. Beginning at the top of the column, a perforated spreader pan distributes the water evenly to the first tray of eggs. Perforations in the trays allow water to flow through the eggs and drip down over the eggs below. Each subsequent row has another perforated spreader pan on the top of that stack of egg trays, which serves to evenly distribute the water for the eggs below.
The Wyoming Game & Fish Department constructed and installed their first Robertson drip incubator at the Dubois Fish Hatchery in 1939 and then installed the next units in 1941 (Simon & Roberts, 1941) at the Story Fish Hatchery.
Today, the Wyoming Game & Fish Department uses two types of Robertson drip incubators: the original flowthrough style and


recirculating. The following will be a comparison of the two.
Flowthrough drip incubators can be constructed based on the available space and water flows. If a hatchery has an ample water supply, the flowthrough drip incubators are an excellent option that require little to no equipment. Egg development can be monitored with ease by gently sliding trays out for observation as needed.
Flowthrough incubators are typically set up to operate on five gallons per minute (gpm) per
column. Formalin treatments are easily administered by injecting chemical directly into the inflow at the recommended concentrations.
As with any incubator setup, there are limitations to consider with this type of incubator. Incubation can only occur from the green egg stage to the eyed stage (no hatching). Obtaining a specific water temperature by mixing water sources can be difficult for the first tray of eggs. Sediment and organics can clog the spreader pans and choke off flowthrough egg trays, so your water source must be clean.
Recirculating drip incubators
operate very similarly to their flowthrough counterpart. With the addition of a catch basin and a recirculation pump, this incubator only requires 0.25-0.5 gpm for operation, or about 5-10 per cent of the flow required to operate the flowthrough style. This makes them a great option for hatcheries with limited flow. Set inside the catch basin, the recirculation pump sends water back to the spreader pan at the top of the column, and builds flow to the desired five gpm. With the 0.25-0.5 gpm of fresh flow coming into the system at all times, the catch basin will overflow this amount when flows are stable in the system.
Limitations mentioned for the flowthrough incubator still apply for the recirculation incubator. Additionally, an electrical source will need to be close by for the recirculation pumps and alarm system. Administering and calculating formalin treatments can be more challenging, and mixing water sources to achieve a specific temperature will take more time.
Personnel at the Daniel Fish Hatchery fabricated two Robertson Drip Incubators out of fiberglass sheets, aluminum angle, PVC, and small nuts and bolts. Other facilities in Wyoming have used PVC plastic sheets or aluminum sheets to build these incubators.
Each incubator measures 51 inches tall by 15 inches deep by 45 inches wide and has three vertical columns with seven rows per column. Egg trays measure 1/2” inch deep by 16 inches wide by 14 inches long and can hold 1.5
quarts of eggs. To promote longevity and effective disinfection, the trays were constructed with PVC. The solid and perforated PVC materials were fabricated with a PVC porta welder.
All green and eyed eggs incubated in Robertson drip incubators are treated with formalin daily to minimize the growth of Saprolegnia
Medical tubing from the manifold is plumbed directly into the water source which helps get the formalin into solution before it flows over the eggs. Capillary tubes are used to determine how much formalin flows to each column to reach the targeted concentration of 1,667 ppm for 15 minutes.
A backflow preventer eliminates siphoning from the manifold back into the source barrel. Quarter inch Murdock valves are installed on the manifold to give personnel the option to only treat columns that have eggs.
The Daniel Hatchery began using a Masterflex pump in 2021, replacing both chicken waterers and IV bottles for their formalin treatments. The pumps are plumbed with medical and capillary tubing, a backflow preventer, and a manifold system to distribute formalin to eggs. The pump pulls formalin from a source barrel, sends it through a manifold, and then distributes it to each column of the drip incubator. This was implemented to limit formalin exposure for employees. The variable speed pump allows the user to increase or decrease flow rates depending on how many columns you need to treat.

The Daniel Hatchery uses on-demand water heaters to manipulate water temperature and egg development during incubation. By using this tool, employees have more flexibility to bring groups of eggs together developmentally, which increases the overall efficiency of the incubator and hatchery production.
When considering an on-demand water heater, there are a few things to keep in mind such as: available space, type of water heater (electric or gas/propane), cost versus benefit.
Robertson drip incubators have been utilized for a long time and have proven to be an effective way to incubate eggs. The Wyoming Game & Fish Department has used the flowthrough incubators since 1939 and the recirculation incubators since 2012, each with great success. These incubators can be customized to fit your available floor space and water flows. Additional columns and rows within the incubator can be easily added or removed to fit your needs. Keeping track of egg development is as easy as gently pulling a tray of eggs out from the stack and carefully checking them. The egg trays are easily cleaned and disinfected after use. Water heaters, chiller units, and formalin treatment systems are easily incorporated into the design. Consider a Robertson drip incubator for your next incubator room upgrade for a reasonable cost. | HI
Simon, J. R., & Roberts, F. (1941). A Drip Incubator and Heater Combination . The Progressive Fish-Culturist, Volume 8, Issue 56, Pages 10-13.

Smarter, stronger, more economical drum filters
The Hydrotech Drum Filter Value series focuses on reduced maintenance, increased component quality and simplified operation – all to give your plant maximum filtration performance at a minimum operational cost.
Let us help you!
Call +46 (0)40 42 95 30, or visit www.hydrotech.se
By Nancy Erickson
Much like the life cycle of Alaska’s salmon, the state’s oldest operating salmon hatchery that began life as a two-year aquaculture and fisheries science program in 1972 at Sitka’s Sheldon Jackson College, has evolved into what is now the Sitka Sound Science Center (SSSC). It is the only aquaculture educational facility of its scope in Alaska.
Sheldon Jackson was founded by Presbyterian missionaries in the 1880s to train Alaskan Natives in vocational skills. Over the years it served as a vocational school, grade school and high school, a two- and ultimately, a fouryear college.
Now occupied by the SSSC, the Sage building, one of many structures on campus, was constructed in 1929 and served many fields of study from art, astronomy, carpentry, shoe making, and of course, science classrooms, according to Lisa Teas Conaway, SSSC communications coordinator. The basement was renovated in 1974 to create an educational salmon hatchery space that obtained the first state-issued salmon hatchery permit in Alaska. A small public aquarium was added in 2005.
Sheldon Jackson College closed its doors in 2007, after which SSSC was formed as a non-profit focusing on scientist research and education and increasing awareness of our natural world, added Teas Conaway. “We have continued to be an educational facility aimed at training students to become skilled employees in a growing workforce.”
“The Sheldon Jackson Hatchery (SJH) naturally fits well within our mission with its ability to help scientists expand their knowledge of the salmon we rear and the ability to bring in students of all grade levels to learn about the salmon life cycle and the hatcheries place within it,” she said.
“Salmon are a vital part of our community with deeply engrained ties to our local indigenous population,” said Teas Conaway. “We strive to ‘do salmon right’ by putting the

needs of the fish first and at all costs, raise them to be as healthy and viable as possible.”
William Coltharp has been SSSC’s aquaculture director since 2018, but his life path didn’t start out that way.
He arrived in Sitka in 1980 to study forestry at Sheldon Jackson College, but soon learned forestry jobs in Southeast Alaska were more about logging than planting trees - and the industry was slowing down.
“In 1987, I went back to school for a Bachelor of Science degree in aquaculture with a minor in mariculture and doing work study at the Sheldon Jackson Hatchery,” explained Coltharp.
After working remotely at area lakes, he fell in love raising fish. When the managerial
position opened at SJH in 1990, Coltharp jumped on it. During his 10-year tenure, he ran the hatchery with only college workstudy students as help, while also teaching fish husbandry and hatchery practicum.
Coltharp left the hatchery in 2000 and worked for the Northern Southeast Regional Aquaculture Association (NSRAA), conducting salmon enhancement projects throughout northern Southeast Alaska for 19 years before returning to SSSC.
Sheldon Jackson Hatchery is the oldest and smallest hatchery operating in Alaska and one of only two training facilities on the west coast, according to Coltharp. But the hatchery is also unique in its “fish first perspective.”
“We approach each day with a fish first
perspective,” said the director. “I start every day based on tides, weather, seasons and the needs of the fish.”
SJH Fish Culturist Haley Jenkins concurs.
“Sheldon Jackson Hatchery was built and operated by students and for students. It has remained that way ever since the 70s’,” said Jenkins. ”This hatchery, unlike others in the state, is an educational and training facility. We have two permanent staff members, a one-year full-time apprentice and several interns (high school and college age). This essentially means that the majority of our staff are learning and training every day.”
typical work days
The hatchery is permitted to rear and release on site three million pink salmon, three million chum salmon and 250,000 coho salmon, according to their web site. In addition to fish reared and released from the hatchery, nine million chum salmon are reared and released at Deep Inlet, a remote site south of Sitka, under the care of NSRAA.
A typical workday changes with the seasons.

“In late summer/early fall, we are spawning pink and chum (taking eggs and fertilizing),” explained Jenkins. “We take three million eggs from each of those species. By October, we are picking through those eggs to get any of the unfertilized out. November is when we spawn the adult coho and take 250,000 eggs. By January, the pink and chum have hatched and are almost ready to be transported to their saltwater nets. February to May we are out on a boat five times a day, every single day,

feeding those pink and chum fry. Summertime is for special projects and taking care of baby coho.”
“It’s so difficult to describe a typical workday at a hatchery because we operate on the salmon life cycle,” she added. “It is one of my favorite things about working in aquaculture.”
“To work in salmon aquaculture in Alaska, you must be able to adapt and act quickly when things happen,” said the Virginia native. “We are supporting the lives of millions of




fish, so expecting the unexpected every day is normal, but it can be a real challenge at times.”
Jenkins started in an entry-level job at SJH after graduating from Virginia Tech and was promoted to the fish culturist position after one year, where she is in charge of the day-today aspects of raising fish.
“Our goal is to not just incubate fish but also the future of the mariculture industry,” so states the SSSC web site.
That future includes students of all ages.
Pre-kindergarten programs involve experiencing salmon anatomy – showing eggs during spawning season and includes art fish pressings, said Teas Conaway. Sitka’s first graders visit the hatchery multiple times throughout the year, the first visit coinciding with spawning and observing “their” baby salmon grow to the point of release in spring.
Fifth grade students get a salmon ecology lesson, learning about the role hatcheries play in the fishing industry, Teas Conaway continued. Seventh graders learn about the bio-genetics of salmon and get hands-on data collection opportunities through measuring weight and length.
“Sitka High’s field science aquaculture classes visit our hatchery weekly and help with the day-to-day operations of the hatchery crew from spawning adults to feeding babies,” said the communications coordinator. Internship opportunities are open to all three high schools in Sitka.
Don’t forget the public.
A “Salmon Release Party” is celebrated in May where the whole community can help carry little containers with smolt (young salmon) to pour into the ocean along Sika’s beach for their first big swim. Wild Alaska Salmon Day is celebrated in August with art activities and a hatchery tour for the community.
“We start all of our hatchery tours with

discussing food webs and helping visitors relate this concept with what goes on within their own homes,” said Teas Conaway. “From cows in the mid-west to fruit orchards in the south, we all have food webs around us. Here in Southeast Alaska, salmon play a vital role in our food web and have been immensely important to our communities since time immemorial.”
Wild salmon stocks began feeling the strain when commercial fishing started growing in the 1940s.
“We got into this business producing hatchery fish to relieve fishing pressure on the wild stocks and in that respect we have had some success, mainly with pink, chum and some stocks of coho and sockeye,” said Coltharp.
“It’s so difficult to describe a typical workday at a hatchery because we operate on the salmon life cycle. It is one of my favorite things about working in aquaculture.”
“Overall, hatchery salmon are known to have less genetic diversity and we in the scientific community do not want hatchery and wild salmon to interact,” said Jenkins. “This is obviously very difficult to control in some ways, but luckily salmon like to return to the same waters that they were born in.”
“I do think wild salmon and hatchery salmon can and do co-exist, but I also think there is a limit to production and we may be close to that point,” advised Coltharp. “Good research and sound science should be in the forefront to determine that.”
“We strive for good health, low mortality




and high ocean survival,” said Jenkins. “While we are permitted to raise a relatively low number of fish, we aim to contribute as much as we can to the fisheries and to the community.”
The newly constructed Spawning Platform and Incubation Facility has upgraded
hatchery operations to the top of modern standards, taking in its first broodstock in fall 2023.
“The facility has greatly increased efficiency, increasing our ability to care for our salmon,” said Teas Conaway. “Our students will now be learning on modern equipment and will be better prepared with the skills and knowledge needed to enter the workforce.” | HI


BY KATA SHARRER AND RAKESH RANJAN, FRESHWATER INSTITUTE
Consumers often associate the vibrant pinkred colour of salmon and trout fillets with freshness and premium quality. Therefore, having reliable, repeatable, and objective methods for assessing fillet colour is critical.
Determining the fish fillet colour has traditionally been a subjective process: a person visually inspects the fillet with a standardized colour reference card and assigns a score based on the closest matching shade. Anyone who has ever undertaken this task knows that colour reference cards and fillet colours often do not match perfectly. Additionally, scoring can be impacted by limited colour memory, eye fatigue, and differences in colour perception between observers.
These factors can lead to biases and inconsistencies in the colour grading process. Another commercially available optical instrument used for precise and objective colour measurement is a colorimeter. However, this tool doesn’t offer accurate colour assessment for fish, as the non-uniform colour and texture of fillets make invasive and single-spot measurements from a colorimeter unreliable.
In either case, whether using a colour reference card or a colorimeter, these traditional colour scoring methods can be inaccurate, labor-intensive, and unsuitable for large-scale fish processing.
A more comprehensive approach involves computer vision and image processing techniques for colour assessment. These technologies have been adopted in industries requiring

precise colour measurement and quality control — like printing, textiles, and cosmetics. Recently, research studies have demonstrated that these technologies can also be adopted to assess the quality of meat and fish products. However, conventional image processing techniques often fall short when it comes to accurately capturing complex colour details, incorporating texture and shape information, and
maintaining consistency under varying lighting conditions.
With recent advancements in Artificial Intelligence (AI), Convolutional Neural Networks (CNNs) can offer a solution to the limitations posed by traditional image processing techniques.
Leveraging this technology, researchers at The Conservation Fund Freshwater Institute have developed FilletCam AI, an
AI-powered handheld device designed for objective, accurate, and high-throughput fish fillet colour assessment.
FilletCam was designed and developed at Freshwater Institute using an off-the-shelf RGB camera, edge computing device, touchscreen monitor, power bank, and other low-cost hardware, all housed in a custom 3D-printed enclosure.
Our team developed this AI tool over a multi-step process: 1) collecting images and training a custom CNN object detection model to recognize fillets and reference colour palettes; 2) identifying various colour comparison metrics and their performance evaluation; and, 3) comparing the performance of the FilletCam AI with tradition approach and implementing further modifications.
A graphical user interface (GUI) was developed to streamline data collection, processing, and visualization. With just one click, authorized users can instantly share the processed results via email.
First, images of rainbow trout and Atlantic salmon fillet portions were captured with a
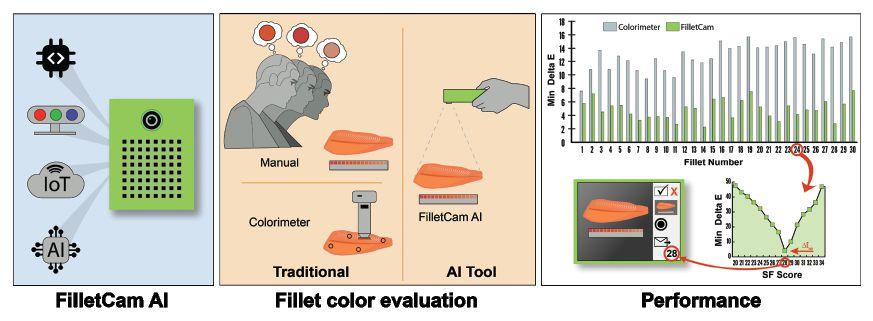
SalmoFan lineal card and ColorChecker colour calibration card in the frame to create the custom model training dataset.
A total of 104 images were annotated by manually drawing the bounding boxes around region of interests (ROIs): one for the fillet and one for each of the 15 colour palettes on the SalmoFan card. The annotated images were then divided into a 70:20:10 ratio for model training, validation, and testing. The training set was further augmented with rotation, cropping, saturation adjustments, blurring, and brightness changes, resulting in a total of 582 training images. Finally, a YOLOv8 object detection model by Ultralytics was trained, and its performance was analyzed.
The trained CNN model achieved a high detection accuracy with mAP0.5 score of 99.5 per cent and an F1 score of 0.99. The model detected fillet portions and colour palettes were masked and converted to Lab* colour space for colour comparison. Lab* is a sensor-independent colour space that can accurately compare the colours of objects captured by various computer vision devices.
The Delta E metric was adopted to assess fillet colour scores; a lower Δ E error indicates a better colour match between two objects. Among the different Δ E algorithms evaluated (Δ E1976, Δ E1994, Δ E2000), Δ E2000 produced the lowest error and was deemed the most suitable for this application. Δ E errors were calculated between the fish fillet and 15 ROIs representing different shades on the SF card. Finally, the colour score for the fillet was assigned based on the ROI with the lowest Δ E value.
Three experts from the Freshwater Institute evaluated the colour of 30 fish fillet portions sourced from local grocery stores, and their scores were
compared with FilletCam’s assessment.
Colour values from a colourimeter were also collected for further comparison. For 21 out of 30 fillets, the colour score from FilletCam exactly matched the expert score range. The predicted score deviated by one point from the expert range for six out of 30 fillets, whereas for only two out of 30 fillets, the deviation in the scores was two points.
The deviation in predicted score of this AI tool may be attributed to the subjective nature of expert colour scoring, with scores varying by one to three points for this study. This highlights the subjective aspect of manual scoring and emphasizes the need for objective measurement.
The colourimeter accuracy was notably lower, with only nine of 30 fillets accurately predicting the colour score, while 13 of 30 deviated by more than two points.
Overall, this proof-of-concept device could minimize subjectivity and bias in colour-based quality inspections of fish fillets across processing lines, seafood retail outlets, and grocery stores. With further commercial refinements, it holds significant promise as a valuable AI tool for the processing industry.
Our ongoing effort focuses on incorporating additional features into the model, such as evaluating fillet gaping severity, melanosis, and blood spots, to expand the viability of this AI tool for comprehensive fillet quality analysis. This technology could further be refined to create species-specific models that would broaden its utility in the seafood industry. | HI
For more details about this work, see the full, open-access journal article published in December 2024:

Pioneer Fish Farm P/L for sale WIWO. PFF has over 30 years of continuous production of silver perch. Three generations of family have worked on the farm.
Hatchery and grow-out licenses. NSW DPI approved.
• Total Land Area: 38.8 hectares
• Production Ponds: 23 covering 8 hectares and 3 water storage ponds
• Water Licence: Substantial, from Gloucester River (which flows from Barrington Tops National Park)
• Facilities: Complete hatchery, purging shed with lab, cool room, ice room, etc.
• Accommodation: Shed also contains an open-plan house with 3 bedrooms, 2 bathrooms, 2 lounge rooms, large sunroom, and lots of area.
• Infrastructure: All operational ponds, electricity, etc.
• Equipment: Plant, machinery, and equipment to cover all aspects of silver perch production, including transport truck and ute, tractors, excavator, loader, quad, etc.
• Harvesting Equipment: All included.
• Stock: Farm fully stocked and operational. Produces both live and chilled product.
Sydney is only a 3.5-hour drive from PFF, providing a huge market. The farm is 5 minutes from Gloucester Township, offering easy access to schools, shops, medical facilities, hospitals, etc.
Present owners will provide ongoing support and training to ensure continuing production. Selling due to owner’s health and desire to retire.
Scan to watch a video of the property
Barton Cha Founder, Director, Business Broker barton.cha@sinosmart.com.au
+61 0478 824 168

BY MAGIDA TABBARA
Fish larvae are the basic building blocks of a successful hatchery operation. Their survival and development into healthy adults is a big asset for hatcheries and, eventually, seafood consumers. Proper development and growth of fish larvae depends on a variety of factors, that can be physiological, or environmental, or a combination of the two.
Think of the larvae as babies: they need time to grow and develop but also require a lot of attention and appropriate environmental factors and feed to grow in good health. Feed is a broad umbrella under which falls a lot of criteria that we need to be mindful of in order to ensure optimal growth of our larvae.
Such criteria include but are not limited to the nutritional composition of the feed, the quality of the ingredients, palatability, particle size, position in the water column (i.e., floating or sinking feed), quantity offered, and feeding frequency. These criteria need to be adjusted to meet the needs of the fish without compounding production expenses.
Larvae in their early stages of development are extremely sensitive to feed deprivation.
As soon as they start losing their yolk sac, the larvae become in desperate need of a rich source of nutrients that will meet their need for energy. After all, they’re in a state of rapid growth, and they’re in need of all necessary building blocks to develop properly.
Therefore, it’s always recommended to start weaning the fish early, so that they can get used to exogenous sources of nutrients. Those sources, however, need to match the yolk reserves in a variety of aspects: energy density, nutrient availability, quantity, and accessibility at any time needed.
Accessibility is often confused with overfeeding. Overfeeding larval fish can be detrimental for a variety of reasons. Other than the usual decrease in water quality, offering larvae excess feed results in a confusion effect, which can often be overlooked.
Larvae by instinct are programmed to forage. However, if they constantly find feed dropping within their field of vision, they end

up overlooking it while searching for it. That eventually results in them not consuming anything.
All of that increases production expenses, as more labour is needed to feed, and all that feed offered is just wasted. Accordingly, research efforts have been put into finding the perfect balance between feed quantity and feeding rates.
Larval digestive physiology has been studied in order to optimize the environmental factors surrounding the fish for better digestion and growth.
Studies have shown that feeding time can differ greatly among species. Some larvae feed the most at sunrise and sunset, while others have the ability to eat at any point during the light cycle.
In general, it seems that larval fish prefer to eat during light time, as a lot of the enzymes are produced and work on digesting feed during the night. Accordingly, hatcheries work on extending the photoperiod in rooms containing larval fish to stimulate fish feeding and indirectly, larval growth. But that doesn’t stop at feeding periods.
Studies showed that offering larval fish feed
multiple times a day significantly improves their survival and their specific growth rate.
In a study evaluating feeding frequency of yellow croaker larvae, the authors concluded that the best specific growth rate can be obtained when the larvae are offered feed eight to 12 times a day. Such big number of feedings ensures enough feed is offered to the fish to sustain their growth. Additionally, the increased number of feedings gives the opportunity to almost all the larvae to obtain feed to satiation, which allows for better growth.
If we solve the issue of multiple feedings and increased labour by relying on automatic feeders, we still need to account for feed ration issues. Larvae have the tendency to “gobble up” when feed is offered, especially when offered in big quantities, trying to use all they can as fast as they can. The problem with that is decreased digestion, which eventually compromises their growth.
Feed needs to spend a sufficient time in their digestive tract, allowing for proper mechanical and chemical digestion and later on absorption at the gut level. Depending on the fish species in question, there is no alternative to the traditional hand feeding method. Some fish are continuous eaters, while others prefer to eat at specific times.
“Larvae tend to ‘gobble up’ when feed is offered, trying to use all they can as fast as they can. The problem with that is decreased digestion and compromised growth.”
Additionally, their requirements for feed will definitely differ with their growth. Therefore, a skilled technician needs to keep an eye out on the larvae, offering them feed to apparent satiation while recording how much feed is being dispensed.
Proper feed quantity records, in tandem with growth over time data, will allow for the formation of feeding tables that help the hatchery operate at maximum efficiency. After a while, the needed feed quantities at the optimal rates will become a part of the hatchery routine.

There’s no substitute for proper data keeping and feed management. After all, we all would like our fish to grow well, hence maximize our benefits, while minimizing waste and expense.
For that, we need to master the “art” of aquaculture.
When working with fish larvae, we need to keep in mind that they’re no different than babies and their feeding is very sensitive. This sensitivity requires an artistic touch of the technician in charge of feeding that will



promote the larvae to feed.
Some species need to be trained on how to feed as their instincts become different when they’re reared in captivity. But with proper feed training for both the fish and the technicians in charge, the hatchery will observe an increased efficiency and a decrease in wastage.
Don’t let your larvae gobble up! Slow and patient feeding in adequate amounts will get them all to grow well and healthily. | HI
Cho, S. H., Lim, Y. S., Lee, J. H., Lee, J. K., Park, S., & Lee, S. M. (2003). Effects of feeding rate and feeding frequency on survival, growth, and body composition of Ayu post‐larvae Plecoglossus altivelis. Journal of the World Aquaculture Society, 34(1), 85-91.
Papandroulakis, N., Dimitris, P., & Pascal, D. (2002). An automated feeding system for intensive hatcheries. Aquacultural Engineering, 26(1), 13-26.
Rønnestad, I., Yúfera, M., Ueberschär, B., Ribeiro, L., Sæle, Ø., & Boglione, C. (2013). Feeding behaviour and digestive physiology in larval fish: current knowledge, and gaps and bottlenecks in research. Reviews in Aquaculture, 5, S59-S98.
Xie, F., Ai, Q., Mai, K., Xu, W., & Ma, H. (2011). The optimal feeding frequency of large yellow croaker (Pseudosciaena crocea, Richardson) larvae. Aquaculture, 311(1-4), 162-167.


The Institute for Feed Education and Research (IFEEDER) has released a report that states that in 2023, U.S. livestock, poultry and farmed aquaculture consumed approximately 283.6 million tons of feed.
The feed consumption data, done in collaboration with the American Feed Industry Association (AFIA) and Fats and Proteins Research Foundation (FPRF), demonstrates the role that feed mills, ingredient suppliers, and liquid feed manufacturers play in keeping animals fed.
“On behalf of our project partners AFIA and FPRF, IFEEDER is pleased to make this data readily available as part of its ongoing mission to advance understanding and trust in a sustainable animal feed supply chain,” said Lara Moody, IFEEDER’s executive director.
The project, conducted by researchers at Decision Innovation Solutions (DIS), used a ration cost optimization model to quantify the consumption of feed ingredients for the major animal species.
Excluding harvested forages and roughages, the study found that in 2023, beef cattle consumed the most feed at 76.7 million tons, and aquaculture consumed 615,800 tons.
After examininig about 70 unique feed ingredients used among the studied species, the researchers found that by weight, corn tops the list at 159.4 million tons, followed by
soybean meal at 35.4 million tons, corn distillers’ dried grains at 32.6 million tons, wheat middlings at 5.6 million tons, and canola meal at 5.2 million tons.
The full report, along with infographics and a multimedia map, are available at ifeeder.org/ consumption-report.

Garware Technical Fibres is celebrating 10 years as a supplier to the Chilean salmon farming industry.
Globally, Garware has been operating and manufacturing technical textiles since the late 1990s and in Chile since 2014. It says aquaculture is its most significant business area.
“The early years were challenging, primarily in gaining the support of salmon companies to conduct initial product trials. During that introduction phase, we tested nets with Mowi and Camanchaca to showcase our solutions,” said MarcosJofré, business associate of Garware Technical Fibres.
Garware said it had to adapt its products to the Chilean salmon farming market, with a special focus on predators, higher fouling levels, the ectoparasite Caligus rogercresseyi high-energy and exposed farming sites, square metal cages, longer tides, and a distinct and evolving regulatory framework.
As it continues in the industry, it says the main challenges are regulatory changes, a greater focus on sustainability, continuous fish protection and offering quality products.
“We are well-positioned to meet the evolution of the industry. Our corporate social responsibility efforts in Chile also stand out, supported by initiatives linked to SalmonChile,” said Francisco Serra, commercial manager of Garware Technical Fibres Chile, adding that the company has a wide range of products in development, which will be launched soon.

Unibio’s microbial single-cell protein, Uniprotein, has been officially approved for aquaculture applications in the Kingdom of Saudi Arabia (KSA) for fish and crustaceans by the Saudi Food and Drug Authority (SFDA).
The SFDA confirmed that Uniprotein can be used for fish and shrimp feed after a comprehensive review process that started in April 2023. As a result, Uniprotein is cleared for sale to the Saudi feed industry and can be tested in commercial conditions, reinforcing the significant benefits of microbial single-cell protein.
“This is a significant step into KSA, a key region that is working towards feed and food self-sufficiency under the Vision 2030 program while increasing local fish production,”





said Olivier Hartz, chief commercial officer at Unibio.
Uniprotein is a sustainable protein alternative to traditional protein sources such as fishmeal and soy. Its amino acid profile closely mirrors that of fishmeal, and it has already been approved for use in feed for both terrestrial and aquatic animals in the European Union. Global approvals are ongoing.
Uniprotein is said to be free of pesticides and antibiotics, full traceability, and non-GMO status. Unibio says the product has generated strong interest from customers around the world and is currently under development for potential direct human consumption.
expands AI biomass tool with seabream algorithm

Innovasea has added a new species to BiomassPro, an AI-powered solution that estimates the size and weight of fish stocks.
According to a press release from the company, it is offering the ‘first commercially available seabream algorithm for biomass estimation.’
In recent trials with French aquaculture farmer, Aquafrais Cannes, the algorithm delivered over 95 per cent accuracy for gilthead seabream weight estimations.
Innovasea’s BiomassPro now supports six species—including Atlantic salmon (Salmo salar), Cobia (Rachycentron canadum), Gilthead seabream (Sparus aurata), King salmon
> Premium quality, disease-free eyed eggs available all year round > Rainbow trout all female (diploid or triploid) and other salmonids



(Oncorhynchus tshawytscha), Red snapper (Lutjanus peru) and Yellowtail (Seriola rivoliana)—with plans to add European seabass later in the year.
MiAlgae appoints Jerome Quiohilag to boost production efficiency

MiAlgae has appointed Jerome Quiohilag as associate director to scale its production capacity and enhance manufacturing efficiency.
The company repurposes whisky industry by-products to grow microalgae as a sustainable, cost-effective alternative to fish-derived Omega-3 sources.
Quiohilag joins MiAlgae from Veranova, a manufacturer of active pharmaceutical ingredients for pharmaceutical and biotech customers. There, he oversaw the production of medicinal products and ensured compliance with strict quality and safety standards.
He has over a decade of experience in pharmaceutical manufacturing. He has led high-performing teams across diverse man-

ufacturing environments, directing operations to meet strategic business objectives whilst driving efficiency and process optimisation.
“We’re thrilled to welcome Jerome at this exciting time for MiAlgae. His expertise will be crucial as we scale up to meet growing demand and continue driving biotech innovation,” said Douglas Martin, founder and managing director of MiAlgae.
The company is preparing for its next major milestone: the development of an industrial-scale production facility in Scotland. The first phase is set to be completed in 2025.
“This is a unique opportunity to join a fast-growing company with a clear mission and global ambition. I look forward to applying my expertise to scale operations and contribute to a greener, more sustainable future,” Quiohilag said.


BY NICOLE KIRCHHOFF
Whether during business planning, valuing your business for sale, or calculating insurance value, at some point every hatchery will be asked to put a dollar value to their broodstock.
This is often where the debate begins, not only because each of these valuations is entirely different, but also because there is no agreed-upon market price for broodstock in our industry.
This past month, I called on three other industry experts to discuss this critical topic:
1. Nick King, general manager for SalmoGen;
2. Carlos Lopez, director of commercial sales for Spring Genetics;
3. Brad Gentner, a natural resource economist and president of the Gentner Group.
It is clear, that valuing our broodstock is not easy. As Lopez exclaimed, “It is something we struggle with every year.”
First, let’s talk about a business valuation of broodstock, for financial planning and setting a required market price for products. For business reasons, you may calculate the production costs of raising a broodstock to their current age, condition, and fertility. As King explains you need to “differentiate those first couple of years when they are raised basically as a production fish, from the final year or time leading up to their maturation.”
For example, a five-pound mature tilapia broodstock may have cost a business US$9 in production costs. Or you may instead calculate the production-based value of these broodstock in terms of their offspring production potential, i.e. fecundity, survival rates, and market price of the fish.
“We know the fair market value of [salmon] eggs,” King elaborates “and we can put a value to that. A 10-kg salmon can produce 1200 eggs per kilogram, so presumably 12,000 eggs at whatever the market price is. So that is the value potential of that broodstock.”
This is much more complicated with
species such as marine fish that don’t die after they produce eggs, who don’t have an established market price for eggs or brood, and who may have a second market production price for fingerlings.
Instead, an operator must use the best available data, including your business records, to calculate and justify these valuations. Calculating these values helps businesses decide how much broodstock they can afford to maintain and their earning potential for a business over time.
These values, however, may be entirely different, yet somewhat related, to the sales price of this broodstock. Broodstock can be sold either as an ongoing product offering or as an asset of a company up for sale. As a sales item themselves, broodstock may have an established market value.
For example, Spring Genetics sells their tilapia breeders on the world market at a current market price of US$2.50-$3.50 apiece. Broodstock may also be valued as part of the assets of a company at sale, i.e. their future sale potential over the next five to 10 years.
Let’s talk about a real world example. According to public disclosure documents of the recent Benchmark sale of their Salmon broodstock asset, they valued their broodstock assets at $33.4 million for 1,517 MT biomass or $22.02/kg (compare this to a farmgate value of $8.69 per kg as reported for Chilean farmed Salmon in 2024). In this example, no company sold/sells broodstock salmon on the market, and therefore this value required an internal assessment of value.
Finally, insurance companies require valuation as part of a damage assessment. This value may be much lower than the actual value of a brood, but will financially allow a company to recover at the time of catastrophic loss. For companies with more common wild or F1-sourced broodstock, insurance may be a viable and affordable solution.
Gentner explains damage assessment incorporates “all of the losses, the cost of producing that product and their future earning
potential, for tax purposes.” As you can imagine, for companies with decades-long breeding programs in the millions, it may be both financially and biologically impossible to put an insurance recovery value on that asset.
As Lopez explains, “You have to self-insure the 12 generation deep genetic material with a back up somewhere else in case of total wipe out, as it is not feasible for insurance companies to value or help recover that asset accurately.”
Therefore, companies must decide if they insure their broodstock in an insurance market or if they invest in a back-up location for their broodstock.
Where it gets complicated, is putting a value to attributes that set your broodstock apart. These are attributes such as genetic value through selection programs which can impact fecundity rates, fertilization rates, spawning consistency, disease resistance, physical condition or appearance, market demand, and growth rate.
For some species, such as salmon, seabream and tilapia, breeding programs have made such large strides, that baselines of many of these production traits have set hard entry barriers into the industry for those who don’t have selected offspring.
King explained, “Genetic potential is accounted for in the market price for the eggs. An egg isn’t worth $0.35 without the genetics that comes with that egg.”
For newer industries, breeding programs will need to show their increased value with hard data. However, if you can demonstrate increased production value for your eggs or offspring, then you can calculate potential cost recovery through your production (i.e. if you are a vertically integrated farm) or through an increased market price of eggs and offspring.
As our discussion concluded, we all agreed that the validation of broodstock is not an easy or static endeavour for any aquaculture business. But hopefully, this short guide will make this complex topic a little easier to understand. | HI





