


Farewell to

Fair Lady

Curious Science Writers

Let There Be Light
All About Tech







































As I sat down to begin writing this final Publisher’s Note, I couldn’t help but be overwhelmed with joy and gratitude for my tenure here at AALAS. Since starting in 2001, I have seen consistent growth in the face of various challenges with this organization, and I feel an immense sense of pride in the work that I and many others have done to advance the laboratory animal science field. As the final months of my time here are coming to a close, I have taken the time to look back and reflect.
When I started here, I felt ready. My education and experience had prepared me well. However, six weeks into my position, our great nation was attacked on September 11. For a moment, I panicked, wondering how this would permanently alter the world we knew and loved. How would security look? What about travel? We had the National Meeting in six weeks, and almost everyone planned to fly. Nevertheless, we all persevered and held our National Meeting in the scheduled city. I was confident that if I could make it through this, I could make it through anything. In the following years, we had continued success with our meetings, but when Covid reared its ugly head at the start of 2020, I remembered that we had done it before, and we could do it again.
And that we did, each and every year I’ve been here. Our association has achieved such great heights throughout the years by working together and keeping our common goal in mind. I truly believe in our mission and that our work helps make the world a better place for both humans and animals, and I will always be grateful to you all for you playing your part. Working for this association is one of the biggest honors of my life.
The National Meeting was phenomenal for me. I love speaking with people from all over and getting to know them, and I really enjoyed renewing my friendships with past presidents and branch leaders. Many people came to the Front Porch. I got to see many people I have worked with in leadership; however, the most heartwarming were the people who started off with, “You probably don’t remember me, but I met you one year at our branch meeting, and you changed my life.” Most people don’t get the chance to meet people whom they have influenced in that way, and I was fortunate enough to meet so many!
AALAS, it has been an honor, and I loved every minute of it. I am truly blessed.
Staff
Publisher Ann Turner

Associate Publisher Chris Lyons
Managing Editor John Farrar
Associate Editor Morgan McCloud
Ad Sales John Farrar
Design/Production Zara Garza
Andrew Burich Benaroya Research Institute
Bob Dauchy Tulane Univ School of Medicine
David DeOrnellis Champions Oncology
Penny Devlin Pennsylvania State Univ College of Med
Sonia Doss Duke Univ Medical Center
Kelly Ethun Emory University
Glenn Jackson Cornell University
Richard Marble NAMSA
Elizabeth Nunamaker Charles River Laboratories
Sara Oglesby AbbVie Inc.
Jane Olin Edwards Lifesciences
Karuna Patil Seattle Children's Research Institute
Amy Pierce Tulane Univ School of Medicine
Stacy Pritt UT Southwestern Medical Center
Robin Tucker Georgetown Univ
Laboratory Animal Science Professional (LAS Pro) is the official magazine for American Association for Laboratory Animal Science members. LAS Pro provides a wide range of useful resources and knowledge to the association’s 14,000 laboratory animal science professionals who are involved in advancing responsible laboratory animal care and use to benefit people and animals. All signed articles, including, committee reports, news, and commentary, reflect the individual views of the authors and are not official views of AALAS.
Authorization to photocopy portions for personal or internal use is granted by the American Association for Laboratory Animal Science. Photocopying for purposes of resale or outside distribution is prohibited unless written approval is obtained from the AALAS Director of Communications.
Copyright 2022 by the American Association for Laboratory Animal Science.

Laboratory Animal Science Professional (USPS 010-730) is published bimonthly by the American Association for Laboratory Animal Science, 9190 Crestwyn Hills Drive, Memphis, TN 38125. Periodicals Postage paid at Memphis, TN 38101 and additional mailing offices.

POSTMASTER: Send address changes to AALAS, 9190 Crestwyn Hills Drive, Memphis, TN 38125-8538.
Publisher
Executive Director American Association for Laboratory Animal Science

American Association for Laboratory Animal Science 9190 Crestwyn Hills Drive Memphis, TN 38125-8538
Phone: 901-754-8620 Fax: 901-753-0046 E-mail: info@aalas.org Web: www.aalas.org

I’m Kieya Dionne Griffith, the new Executive Administrative Assistant for AALAS. I recently joined the team in August 2022. My role here is to manage and support the administrative needs of the Board of Trustees and sub-committees. I also function as the Human Resources Manager for the AALAS Staff. I’m originally from Compton, California. I attended the University of California at Irvine where I was a Social Science Major. At the time, my aspiration was to go into politics/city government. I would later become engaged in non-profit work that would make an impression on me to focus on the human interest and assets of a positive human condition. That would shape the career pursuits and volunteer engagements that followed. Some of my volunteer affiliations are the Women’s Foundation for Greater Memphis, the National Civil Rights Museum, Power Ministries, TN Disciples Women Ministry, and TN Disciples of Christ Lay Pastor.
My name is Sarah Maxwell, and I joined AALAS as the Meeting Planner for the Meetings & Financial Services department. I completed my B.S.Ed in Health Promotion & Lifestyle Management at the University of Memphis and worked as an Event Manager at a country club for several years following graduation. I joined the AALAS team back in 2018, but moved to Birmingham, AL shortly after for my husband’s new job. I continued to work as an Event Manager at a museum there until we made the decision to move back to the Memphis area earlier this year. I was thrilled when I saw a position was becoming available at AALAS! My role here is to plan and coordinate details of AALAS meetings and events, such as committee meetings, ILAM, and pieces of the National Meeting. Outside of work, I enjoy spending time with my husband, our three dogs, and our friends and family.


My name is Lisa Lissner, and I joined AALAS as a Membership Assistant in the Membership Department. I previously attended the University of Memphis as a biology major. While a student, I worked at the University of Tennessee Department of Physiology and Biophysics as a Lab Assistant, in which I assisted in testing and care of our valued laboratory research animals. Later, I attended Methodist Hospital’s Laboratory Liaison Technician Program and received certification as a phlebotomist. After several years of working in hospitals and doctor's offices, I decided to switch gears. I started a career at FedEx Express in export clearance and attended Southwest Community College and received a technical certificate in Customs Brokerage. After 23 years of service at FedEx, I decided to retire. After my retirement, I attended Tech 901 and completed an IT Foundations course, as well as completed and received a CompTIA Project+ certification. Outside of work, I enjoy kayaking, hiking, antiquing, and gardening. I also volunteer and serve as a board member of a non-profit, A Betor Way, and I volunteer at Lisieux Community. Any free time I have left, I enjoy spending time with friends and family, especially my three grandchildren.
My name is Sheldon Williams, and I joined AALAS as a Membership Assistant working in the Professional Services Department. I am currently completing my Bachelor of Arts in Mass Communication with a concentration in Public Relations at Louisiana State University Online. Once graduated with my BA I want to continue my education by attending law school. Other than working at AALAS and school, I enjoy spending time with family, reading about politics, and exploring new places in Memphis.

ClearH2O has recently been named as one of the 2022 Best Places to Work in Maine. Known for its leadership and innovation in manufacturing essential animal nutrition supplements and diets, ClearH2O’s products are used by veterinarians and animal care professionals around the world to advance animal health and welfare while improving medical research and livestock production.
Commenting on making the 2022 Best Places to Work in Maine list, the company’s president, Kathie Dioli, states, ‘I believe this award acknowledges the great work environment and team we have built at ClearH2O. The work we do is meaningful, having a direct impact on improving the lives of animals and mankind. We place a high standard on personal character, integrity, and performance expectations while offering a fast-paced, collaborative, and collegial work environment. We love what we do, knowing that we’re making a difference in the world…And, we have fun doing it, too’, says Kathie.

The awards program was created in 2006 and is a project of the Society for Human Resource Management - Maine State Council (MESHRM) and Best Companies Group. Partners endorsing the program include: Mainebiz, the
Maine State Chamber of Commerce and Maine HR Convention.
This statewide survey and awards program was designed to identify, recognize and honor the best places of employment in Maine, benefiting the state's economy, its workforce and businesses. The 2022 Best Places to Work in Maine list is made up of 100 companies in three size categories: 34 small winners (15-49 U.S. employees), 45 medium winners (50-249 U.S. employees) and 21 large winners (250+ U.S. employees).
Companies from across the state entered the two-part process to determine the Best Places to Work in Maine. The first part consisted of evaluating each nominated company's workplace policies, practices, and demographics. This part of the process was worth approximately 25% of the total evaluation. The second part consisted of an employee survey
to measure the employee experience. This part of the process was worth approximately 75% of the total evaluation. The combined scores determined the top companies and the final rankings. Best Companies Group managed the overall registration and survey process in Maine and also analyzed the data and used their expertise to determine the final rankings.
ClearH2O will be recognized in the October 17th edition of Mainebiz where the rankings will be revealed for the first time.

For more information on ClearH2O, visit www.clearh2o.com or contact William T. Thomas at 207-245-6316.
For more information on the Best Places to Work in Maine program, visit www.BestPlacestoWorkME.com or contact Jackie Miller at 717-323-5237.

As your global solutions provider, we know how important ergonomics are in the workplace. Ergonomic equipment can make or break the success of any operation. And this is especially true in lab animal science. Ergonomic equipment in lab animal research helps improve employee comfort, decrease stress, and increase productivity. ¹ Couldn’t we all benefit from a more ergonomic work environment?
Adding a successful ergonomics process to your facility can help improve these common issues. Furthermore, a successful ergonomics process supports research goals, reduces employee stress, minimizes human errors, and improves human performance by removing barriers between people and their tasks. ¹
That’s where FlexFlow comes in. FlexFlow is a bedding dispenser that can help make your research program more ergonomic in the following ways.
Operator errors happen, and people make mistakes. It’s only natural. But repeated errors leave operators feeling unsatisfied and stressed with their work. All of these can negatively impact their productivity, efficiency, and overall well-being.
The good news is there are solutions. You can create more ways to help operators make fewer mistakes in the workplace by providing them with more ergonomic work materials. And adding FlexFlow to your facility is one way to do just that.
For example, FlexFlow helps minimize errors through its intuitive human-machine interface (HMI) software. FlexFlow features a large, colorful touchscreen that is as easy to use as any smartphone. This easy-to-use HMI software makes selecting settings and troubleshooting FlexFlow simple. And more simplicity means fewer errors.
An efficient and ergonomic lab animal work environment should include direct routes, with minimal distance to maneuver carts and racks, from sanitation stations to related operational areas, including bedding dispensers. ²

Utilizing FlexFlow in your facility could be an effective solution for improving organization and direction in your work environment. How? Well, FlexFlow is the most compact bedding dispenser in the industry. In half the footprint of other units, it maintains the same throughput capabilities.
FlexFlow can immediately increase your floor space — creating more opportunities for improving workflow. Additionally, one or multiple units can be strategically placed within operational areas to enhance accessibility.
It’s our privilege to provide the lab animal science community with monthly educational webinars and blog stories. Nearly 700 professionals across the globe attend our webinar series to connect and learn from laboratory animal experts. Some of our recent guest speakers include Hilton Klein, Karen Froberg-Fejko, David W. Brammer, and Jori K. Leszczynski. To get updates about our webinars and blogs, follow us on LinkedIn @Allentownllc.

What’s more, FlexFlow’s side handles are removable. So, you can connect multiple units to increase throughput, depending on your facility’s needs. Simply remove the side panels and attach as many FlexFlow bedding dispensers as you need. Or you can position single units strategically in various locations for a more dynamic workflow, depending on the nature of your research.
Stress and strain in lab animal research may increase “injuries, errors, and worker fatigue.” ¹ Are you looking for new ways to reduce workplace injuries, errors, and fatigue in your facility? Introducing FlexFlow to your laboratory could help. By design, FlexFlow alleviates the strain on employees caused by manually distributing bedding to individual cages. Instead, employees can simply place cages under FlexFlow’s chute. Then, FlexFlow automatically dispenses the bedding for them, which helps minimize physical stress.
1. Kerst, J. “An Ergonomics Process for the Care and Use of Research Animals.” ILAR Journal, vol. 44, no. 1, 2003, pp. 3–12., https://doi.org/10.1093/ilar.44.1.3.
2. Festing, Michael F. W., and Douglas G. Altman. “Guidelines for the Design and Statistical Analysis of Experiments Using Laboratory Animals.” OUP Academic, Oxford University Press, 1 Oct. 2002, https://academic.oup.com/ilarjournal/article/43/4/244/981872.
What companion animals do you have?
As a child, I had dogs/cats, and as an adult, we had 2 cats.
Best binge-watching TV series? Outlander.
What are your favorite hobbies?
Gardening has been my most favorite hobby, I have been planting and growing new vegetables and herbs.
Where is your dream vacation spot?
Anywhere tropical. I love the beach, ocean, and sun.
What is your favorite dessert?
I love sweets and am an equal opportunity eater of all, but if I had to pick, it would be a tie between cheesecake and cake.

How did you get in this field? I wanted to find a career that allowed me to work with animals and people and grow professionally. I shared my wants with my cousin who happened to already be working at Upenn, and she referred me for a temp animal caretaker position 16 years ago.

Who were your mentors? I have been fortunate enough to have a few great managers who invested in my career and believed in my potential. They allowed me to explore responsibilities outside of my role which gave me opportunities to meet new people, learn new processes, and build my resume.
What are your current interests in animal science? I had been working on ways to reduce grinding caused by stress in our colonies. During construction projects, we have seen abnormal behaviors which have been affecting some studies. I want to support the researchers by helping our mice feel at ease.
Where do you see yourself in 5 years? I am working toward a position that allows me to better support my department, my colleagues, and staff by offering in person support. I want to assist with additional coverage to cover leaves of leadership, project managing, and training. I see myself continuing to work in ULAR at UPenn.
What is your favorite part of your job? Being on our Wellness committee has been a great opportunity. We are promoting worklife balance and helping staff learn about opportunities that serve them as a person with a life outside of their role. We have
done several events that were well received, and I’m excited about the future of this committee.
What advice do you have for others just beginning their animal science career? Be and stay driven, always do your best when no one is looking. People may not recognize your greatest at first, but your time will come if you are patient and stay focused.
What is the most rewarding aspect of your career? Seeing my staff grow and develop into their best version of themselves has been rewarding. Helping my staff meet their goals personally and professionally has been an amazing experience and allows me to pay it forward
What is something unexpectedly interesting about your career? Something unexpected, and interesting is seeing the effects of open communication with the research community. I have been reminded over and over how initiating contact can create an amazing partnership and platform to collaborate on SOPs, training, building relationships, and creating processes.many momentous experiences in my life.

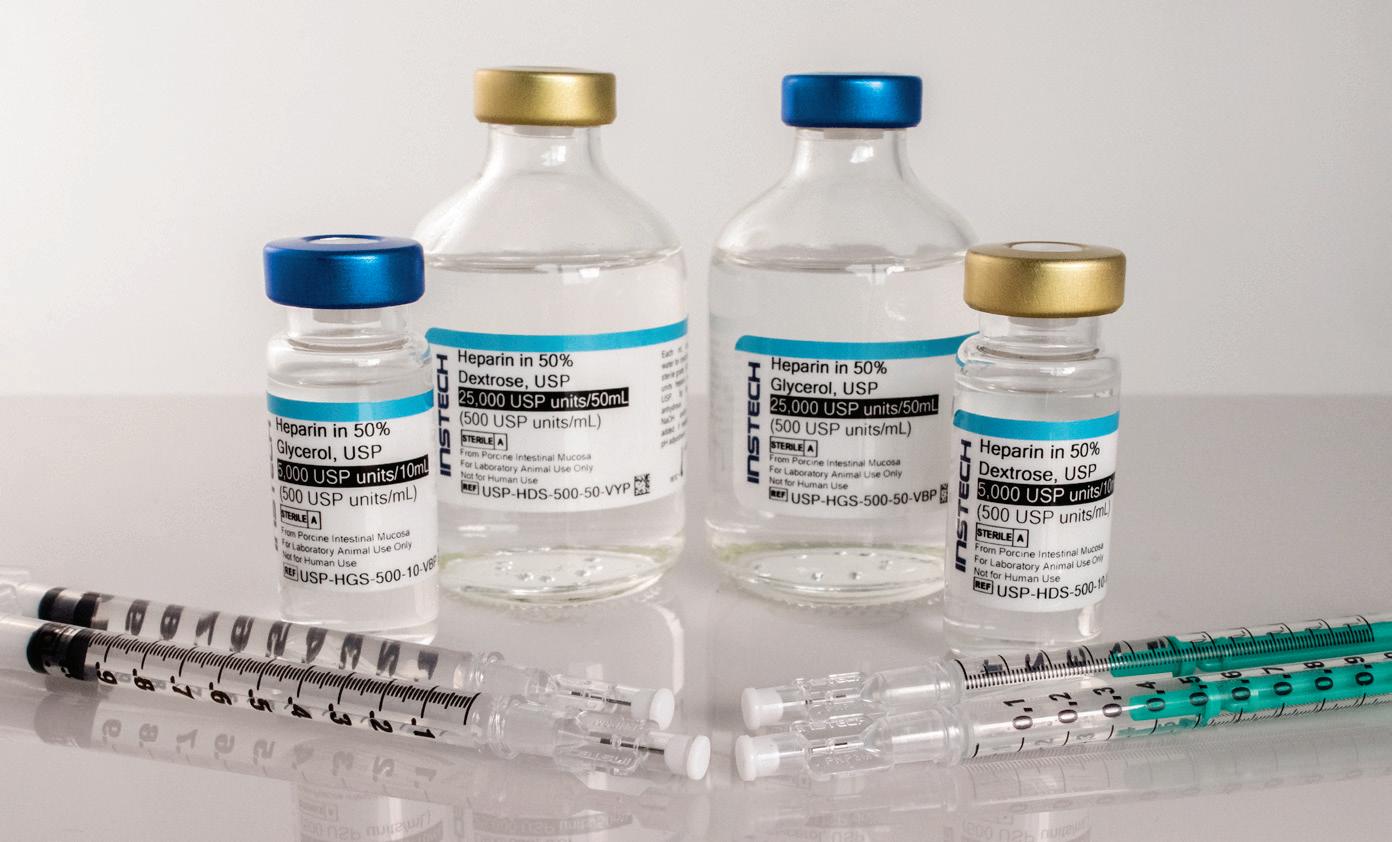
Beginning with sterile ingredients does not guarantee sterility of the lock and flush solutions you are infusing into your animals. Look for “USP” on the label to ensure they have been prepared to pharmaceutical-grade standards. It’s good for your science, good for animal welfare, and recommended by AAALAC.


After 21 wonderful years in her role, Dr. Ann Turner has decided it’s time to retire. Before she leaves, we thought it would be great to ask her a few questions about what has happened and what she has learned during her tenure as AALAS Executive Director. Let’s take a peek into the world of Ann Turner.

Q: Do you remember your first day (if not day, then month or year) as the Executive Director at AALAS? What stands out to you when you reflect on that time?
A: My first year was very eventful. Just a few weeks after I started, September 11th happened, and that was only six weeks before our National Meeting. I remember walking into Betty’s office, and she and I both had “deer in headlights” looks on our faces. She said, “You know, we may have to cancel the National Meeting,” and I looked at her and said, “Yes, I know but lets keep it to ourselves right now.” After consulation with President Richard Simmonds, we decided this was a time when the AALAS community needed to be together and persevered. What a start to my tenure!
Q: How do you feel your previous work experience prepared you for this role?
A: I feel that my previous work experience prepared me for this role by putting me in situations where I was doing something controversial. I started in the 70s as a sex ed-
ucation teacher, which was certainly not a popular career choice then. I also worked with nursing homes, which people have very loaded and strong feelings towards, so I was not very fun at cocktail parties. I also worked for the National Rehabilitation Association the year that the ADA was passed, and there was a lot of resistance to making changes for accommodation. I was working for the people that stood up for others with disabilities, and that gave me a strong sense of pride. All of that, on top of my education, I’ve truly been preparing for this all of my life.
Q: What’s some of the best advice you’ve been given during your time here and why?
A: The best advice I got was about retiring. I had just hit a milestone age, and I was feeling pressure to retire based on my age to make way for younger people. John Graham was the ED of ASAE about ten years ago. I asked him about his thoughts, and he said, “Ann, there is no reason to retire until you find something that you want to do more than what you are doing right now.” Retirement is not leaving something; it’s about what you want to move towards.
Q: What was your first National Meeting like? How did you feel when you first walked out onto the stage?
A: In the wake of our national tragedy, we were excited to be around each other, but it was certainly tense getting
onto the plane to get there. But when I got there and walked onto the stage, I felt welcomed, like I had found my home. I was really blown away by the exhibit hall. I had no idea that the equipment that’s used in this field was as vast as it was.
Q: What is your favorite thing about the National Meeting and why?
A: I love seeing a plan come together. We plan for the National Meeting up to seven years before it happens. There are stages of planning for each individual meeting that happens every year, so when you get to the final year of the planning, it gets expedited. It’s nice seeing the people of course, but I love seeing a plan come together like that.
Q: Now, what is your favorite thing about AALAS and why?
A: The people. I really like AALAS folks. What I love about AALAS is that while we are a large organization, our community has a small town feel to it.

Q: What is something that many people may not expect to hear that comes with your job?
A: Very few people have knowledge about what any association executive does. Working for an association is not something that happens often
outside of state capitals, especially Washington, DC. It is a vast and highly involved job, and it’s not something most people relate to. An association is not the same as owning a business, working for a university, or working in entertainment, but you do pull skills from those and many other jobs to ensure that everything gets done and is done well.
Q: How did you manage a solid work-life balance in this role?
A: I got better at it with age. It’s easier when your children leave home, certainly. One piece of advice that I stand by is to schedule vacations away from where you work. Do not schedule stay-cations because you will open up your email and get sucked in. Schedule your vacations where you have to do something, and preferably somewhere with bad internet so you don’t feel inclined to check your email. Another thing I’ve done to maintain that balance is by volunteering at my local church and in my leadership association. You have to work hard at not being overcommitted. Find something you like to do and do it. Q: What advice do you have for the incoming Executive Director?
A: Listen. Listen to the board, past presidents, staff, members, almost
everyone. My rule of thumb is that you don’t do anything drastic or major for the first six months until you find out what the culture is. Sometimes things may appear to need changing and do not, and sometimes things may emerge that do need changing. So, again, listen.
Q: What advice do you have for individuals who are new to the LAS field?

A: Get involved! You can be involved on the local level and move up to the branches and eventually to the national level. Be involved where you want to be involved.
Q: Now for some fun questions! What do you most look forward to in retirement?

A: That is easy. Sleeping in late. I hate getting up early in the morning; I like to stay up late at night and sleep in late. My new mantra is that I want to get up at the crack of nine. I am also looking forward to developing new skills through volunteer work. I am also going to learn to play pickleball.
Q: How have you been preparing for retirement?
A: I have been thinking a lot about what I want to do and when I want to do it. I have remained open to the possibility of working part-time or consulting at some point, if the opportunity presents itself. My husband and I are planning to move to Virginia to be nearer family, especially grandchildren. Other than that, I am keeping an open mind abou the next part of my life journey.
Q: What’s the first trip you plan to take that isn’t work-related?
A: I will be going to visit my grandchildren in January, and that has already been planned. I think next we’d like to go on a cruise somewhere.
Q: What will be the next book you read?
A: Right now, I am rereading Where the Crawdads Sing. The first time, I listened to the audiobook, so now I am reading the physical copy of the book. I feel like I am learning so much more and picking up on things I missed while listening to the book, so that has been very fun! Next will probably be a light romance novel by Danielle Steele.


 Written by Morgan McCloud and Paula Clifford
Written by Morgan McCloud and Paula Clifford
Former English teacher Jayne Mackta developed Curious Science Writers (cSw) while leading a local advocacy group, the New Jersey Association for Biomedical Research (NJABR). She has always had a passion for increasing science literacy in the world, and she imagined training high schoolers to write science for the public. Thus, this program was founded in 2010. Each year, a group of high school students is selected through a competitive application process to engage in this innovative training program. While the program distinguishes writers in the title, editors, graphic designers, and social media coordinators are also included in the program. Early articles focused on more exotic animals in research, and over time, the topics continued expanding. This program has soared.
Eventually, in 2019, Jayne retired, and the program needed a new home. Americans for Medical Progress (AMP) saw significant value in the program and adopted cSw in 2019. This robust in-person program moved from New Jersey to Washington, DC, and just as promptly, became an interna-


tional virtual program in the wake of the Covid-19 pandemic. As a result, cSw has hosted students from across the United States and India, China, South Korea, and Canada. A hybrid program was piloted in 2021 and will be implemented in 2022. This will offer the opportunity for students, in some areas, to attend an in-person experience as part of the summer boot camp.
Students begin applying at the beginning of the calendar year, and enrollment continues until May or when all slots are filled. Then the students are assigned a biomedical research topic to cover and begin gathering information from reliable sources. The official boot camp takes place over the summer, so as not to interfere with the school year. Mornings consist of content, guest speakers, and interactive activities. Afternoons are dedicated to independent and group work. From July and into August, students are working with professional mentors to create a biomedical science story for a more general audience. Finally, students are working towards publication alongside their mentor from August to May of the following year. To strengthen the cSw community, a monthly Curious Science Writers Gathering has been implemented. The event features program updates, a guest speaker, a scicomm activity, and networking.
You can learn more about the program at www.curioussciencewriters.org" OR To share this program with a student you know, or to volunteer as a mentor or guest speaker, email info@curioussciencewriters.org






Morgan McCloud, BA, is the Communications Coordinator for AALAS in Memphis, TN.
Paula Clifford, RLATG, is the Executive Director for Americans for Medical Progress (AMP) in Washington, DC.
 By Robert T. Dauchy, MS, CMAR, RLATG
By Robert T. Dauchy, MS, CMAR, RLATG
Light is an essential extrinsic factor, an external environmental condition much like noise, vibration, temperature and humidity, to be considered in the design and operation of animal room facilities. Light, in an intensity-, wavelength-, and duration-dependent manner, exerts profound influence on circadian regulation of neurohormonal and neurobehavioral rhythms in all life.8 And yet, artificial light emitted by any of a variety of lighting technologies such as incandescent, cool white fluorescent, or the emerging light-emitting diode (LED) technology, is often times not adequately monitored, measured, or reported in laboratory animal science (LAS) and biomedical research investigations.
Interestingly, The Guide for Care and Use of Laboratory Animals (The Guide)11 provides only limited guidance on how to manage light and lighting protocol concerns and focuses primarily on rodents (Sprague-Dawley rats) based on information available prior to 1985 pertaining to visual system knowledge of the primary optic tract (POT) and related phototoxic retinopathy studies.2,4 Little-to-no information relating to the involvement of the more recently discovered non-visual system of the Retinohypothalamic Tract (RHT)/intrinsically-photosensitive Retinal Ganglion Cell (ipRPG) system is addressed.3 Our team at Tulane University has been investigating the impact of light at night (LAN) on human and animal metabolism and physiology in normal and neoplastic tissues for over 30 years. More recently, in our AALAS Grants for Laboratory
Animal Science (GLAS)-supported investigations, we’ve turned our attention to the influence of exposure to blue-enriched (465-480 nm) light-emitting diode (LED) light at daytime (bLAD) on laboratory rodents.1,6-8,10,13 Blue-enriched LED lighting technology is currently the most commonly transitioned to from cool white fluorescent (CWF) lighting in the community, workplace, and home settings, to include vivaria around the world. Our studies revealed that exposure of rodents to bLAD compared with CWF has a marked positive effect on the circadian regulation of neuroendocrine, metabolic, and physiologic parameters associated with the promotion of animal health and wellbeing that may influence scientific outcomes. This work has led to the first clinical trials, funded by the Department of Energy and now underway, to examine

the influence of bLAD exposure, compared to daytime CWF lighting, on human metabolism and physiology.
Recently, we reviewed the nature of light and circadian rhythms, lighting technologies, current industry practices and standards, and our present understanding of the neurophysiology of the visual and non-visual systems.9 We considered the implications of this extrinsic factor for vivarium measurement, production, and technological application of light, and provided simple recommendations for artificial lighting for use by regulatory authorities, lighting manufacturers, designers, engineers, researchers, and research care staff that ensure the best practices for optimizing laboratory animal health and wellbeing and, ultimately, improved scientific outcomes.
In February 2023, our team will participate in an expert working group on the effects of light on rodent physiology and behavior to be held in Manchester, England. Currently, international guidelines for lighting that are incorporated into building design, as established by the Commission Internationale de l’Eclairage (CIE)5 and the Illuminating Engineering Society of North America (IESNA),12 are actually based more upon staff requirements rather than laboratory animal physiology. Even the units most commonly used to measure light in the vivarium setting (lux) are inappropriate as they are based on human visual sensitivity and do not affect what animals, particularly rodents, see or how light affects their biology.9,10 The aim of this international meeting will be to develop simple lighting guidelines for the housing and testing of laboratory rodents based on the scientific consensus of the published data that may be incorporated into building design.
Science relies on accurate measurements utilizing the best technologies available, which evolve over time. Our goal is to provide as comprehensive a description of light and lighting protocols as possible as experienced by the circadian, neuroendocrine, and neurobehavioral systems of our laboratory animals using the currently available technology. Clearly, at some point in the near future, The Guide11 parameters will require revisiting in more detail. We are honored and pleased that the AALAS GLAS Program has supported our team over the years in the study of the extrinsic factor of light. For us in the laboratory animal science and biomedical research communities, improved understanding and reporting of vivarium light and lighting protocols that positively influence the health and wellbeing of our laboratory animals remains our noble goal and continued challenge.
Sprague-Dawley rats. J Am Assoc Lab Anim Sci 61(4):333-343. PMID: 35738839.
2. Bellhorn RW. 1980. Lighting in the animal environment. Lab Anim Sci 30:385-450. PMID: 7052389
3. Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295: 1070-1073. Doi: 10.1126/science.1067262. PMID: 11834835.
4. Clough G. 1982. Environmental effects on animals used in biomedical research. Biol Rev Camb Philos Soc 57(Pt 3): 487-523. Doi: 10.1111/j.1469-185x.1982.tb00705.x. PMID: 6982731.
5. Commission Internationale de l’Eclairage. 2018. CIE System for metrology of optical radiation for ipRGC-influenced responses to light. CIE S 026/E:2018. Vienna (Austria): CIE. Doi: 10.25039/S026.2018
6. Dauchy RT, Hoffman AE, Wren MA, Hanifin J, Warfield B, Brainard GC, Xiang S, Yuan L, Hill SM, Belancio VP, Dauchy EM, Smith K, Blask DE. 2015. Daytime blue light enhances nighttime circadian melatonin inhibition of human prostate cancer growth progression. Comp Med 65(6): 473-485. PMID: 26678364.
7. Dauchy RT, Wren-Dail M, Hoffman AE, Hanifin JP, Warfield B, Brainard, GC, Hill SM, Belancio VP, Dauchy EM, Blask DE. 2016. Daytime blue-enriched LED light exposure enhances the nighttime melatonin amplitude: consequences for circadian regulation of rodent metabolism and physiology. Comp Med 66(5): 373-383. PMID: 27780004
8. Dauchy RT, Blask DE, Hoffman AE, Xiang S, Hanifin JP, Warfield B, Brainard GC, Anbalagan M, Dupepe LM, Dobek GL, Belancio VP, Dauchy EM, Hill SM. 2019. Influence of daytime light exposure on circadian regulatory dynamics of metabolism and physiology in mice. Comp Med 69(5): 350-379. PMID31540584
9. Dauchy RT, Blask DE. 2022. Vivarium lighting as an important extrinsic factor: recommendations for a measurement plan in the age of melanopsin. Comp Med (in press).
10. Hanifin JP, Dauchy RT, Blask DE, Hill SM, Brainard, GC. 2020. Relevance of Electrical Light on Circadian, Neuroendocrine, and Neurobehavioral Regulation in Laboratory Animal Facilities. ILAR Journal 60(2): 150-158. PMID: 33094817
11. Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
12. Illuminating Engineering Society of North America. 2008. Light and human health: An overview of the impact of optical radiation on visual, circadian, neuroendocrine, and neurobehavioral responses. IED TM-18-08. New York (NY): Illuminating Engineering Society of North America.
1. Allen AA, Pierce AP, Dauchy RT, Voros GB, and Dobek GD. 2022. Influence of daytime LED light exposure on circadian regulation of neuroendocrine hormones in adolescent
13. Voros GB, Dauchy RT, Myers L, Hill SM, Blask DE, Dobek GD. 2021. Impact of daytime blue-enriched light on physiological parameters of three common mouse strains maintained on an IVC system. J Am Assoc Lab Ani Science 60(3): 259-271. PMID: 33673880
Innovive provides the only simple, full-cycle clean environment for accelerated science without the stress and complexity of a washroom.
Less Washing, More Science
innovive.com | (866) 432-2437
 By Amanda Darbyshire, Andrea Osborne, Glenn Jackson, Derek Fong, Misty Williams-Fritze, Erica Feldman, and Chris Manuel
By Amanda Darbyshire, Andrea Osborne, Glenn Jackson, Derek Fong, Misty Williams-Fritze, Erica Feldman, and Chris Manuel
For those who work in research, obtaining a standard CD1 or even the highly immune compromised NSG mouse can be as simple as ordering food online – you enter the number needed, a form of payment, and click “order now.” If you are ordering from a reputable vendor the expectation is that you will receive the requested mice in the amount ordered within a reasonable amount of time. This mouse order is also assumed to be free of pathogens of concern and transported in a manner that meets local, state, and federal regulations. Mice are the “bread and butter” of research…so what about those species that are less commonly used? Enter: the research chinchilla (Chinchilla lanigera, C. chinchilla), a rodent that fits a niche, yet critical, area of research for which very little species specific regulatory guidance is available.
The chinchilla is classified into its own family Chinchillidae. Chinchillas originate from the mountains of South America and are considered endangered in the wild.1 One of the complicating factors to successful chinchilla procurement is a very long gestational length and small litter size. The sexual maturity of chinchillas varies depending on the season in which they are born, gestation is one of the longest for rodents, and produces only 1 to 3 precocious kits.4 Comparatively, chinchillas require larger caging, prefer different feed pellet length, and require a large number of breeding pairs to meet the research demand and replenish breeder stocks. Overall, the process is much longer with fewer offspring as compared to mouse or rat production in a research setting.
To anyone living in the United States, the fact that chinchillas are considered endangered would be difficult to comprehend. The pet industry, fancier shows, and fur trade of chinchillas are abundant – even a quick online search uncovers options per state to

adopt a chinchilla from a “reputable breeder” (https://beyondthetreat. com/chinchilla-breeders/). The relative abundance of “reputable breeders” does not translate to a stable supply of animals for use in biomedical research. Some breeders prefer not to sell to research institutions or do not have the required USDA licensure to do so. Animal health, vendor reliability, and legal transportation are “well-oiled” for standard research animals but become limitations for non-standard research species like chinchillas. Out of this interesting conundrum, The Chinchilla Consortium (hereafter referred to as Consortium) was established.
The Consortium first met in January 2021 as a group of like-minded veterinarians, purchasing staff, and research support staff all with a common goal – to help the researchers at our various institutions reliably and legally obtain healthy chinchillas. At the initial meeting 11 research institutions, both academic and Contract Research Organizations, were represented. Since the initial meeting, the Consortium has met regularly, growing to include representation from 19 research institutions. Discussions include chinchilla housing, husbandry, welfare, clinical veterinary care and available sources from which to purchase chinchillas.
Researchers breed their own mice and rats all the time! Why not just start a breeding colony? Chinchilla breeding colonies in research settings are rare largely due to the practical challenges of chinchillas’ long gestation (105 to 120 days) and smaller litter size (average 2-3 kits). Females and males reach sexual maturity at 4-5 months and 7-9 months of age, respectively. It is recommended to begin breeding chinchillas around 8-9 months of age. Chinchillas are seasonally polyestrous from November to May only (Northern hemisphere) with the estrus cycle lasting an average of 35 days. They have a post-partum estrus from 12 hours to 2 days after parturition.2
One Consortium institution recently maintained a colony of 11 breeding pairs of chinchillas for one breeding season. All animals were on an IACUC approved protocol. While it’s not a large sample size, the scarcity of chinchilla breeding in a research setting and corresponding knowledge about breeding program logistics and efficiency makes it worth describing. Of the 11 breeding pairs, 8 (77%) successfully produced a litter. Breeders were of unknown age and originally sourced from a commercial breeder. Males and females were gradually introduced to each other to ensure compatibility, and most pairs were compatible. However, it’s important to closely monitor introductions as some aggression did lead to separations and attempts at re-pairing with a different partner. Generally, animals were placed into a common playpen and observed for compatibility for a few hours. If all went well, they were returned to a normal housing unit, and checked 2-3 times/day for compatibility. If there were no concerns after 48 hours of pairing, then routine daily monitoring was resumed.

Males were checked for penile fur rings every 1-2 weeks and gently cleaned with water-based lube and a cotton applicator, as needed. Larger breeding schemes may utilize harem breeding with specialized breeding cage set-ups which prevent females from leaving their home cage but allow males to pass through the cage freely.
To monitor for pregnancy, weights were monitored and abdominal palpation was performed. Pregnancy was also confirmed via abdominal radiographs based on rates of weight gain. Radiographs were successful to confirm pregnancy in the last month of gestation. In general, 100 g or more of weight gain within a month appeared to correlate with late gestation. It has been reported that fetal mineralization occurs by gestational day 66.3 To avoid post-partum estrus pregnancies which were not desired, males were separated once pregnancy was confirmed. Dust baths were not provided in the week before estimated parturition and until 1 week after parturition to minimize the potential risk of pyometra. Females were also provided with solid flooring to prevent foot entrapment of precocial chinchilla kits. To better support the high metabolic demands of late pregnancy, females were provided with daily timothy hay (vs. typical 3 days per week) once confirmed pregnant. Animals were paired in February and March, and the average time from pairing to parturition was 154 days with a range of 107 to 210 days.
Kits were generally found in the morning and no parturition complications were observed in this cohort. Dystocia is reportedly uncommon in chinchillas. The average litter size was 2 kits with a range of 1-3 kits. Birth weights ranged from 38-71 g. It has been reported that kits <25 g are unlikely to survive. Unlike many other species that exhibit an initial drop in body weight after parturition, most chinchilla kits gained weight from day 0. To provide more nutritional support to the dams and kits, daily hay was continued and Clear H2O® DietGel® Criticare with Timothy Hay was provided. Not surprisingly, there was an inverse correlation between litter size and birth weight. Kits from litters of 3 may need additional supportive care and monitoring. However, even those animals that needed supportive care tended to do well with
treatment. Supportive care for kits that failed to thrive generally involved heat support and supplemental feedings with Kitten Milk Replacer (KMR, PetAg, Hampshire, IL) twice per day. Once kits started to flourish, the supplemental feedings were tapered over time based on clinical discretion. For housing units, there should not be more than a 0.5-inch opening to ensure smaller kits cannot escape their primary enclosure. Animals were weaned at approximately 8 weeks and there were not any complications or weight loss noted post-weaning. Chinchillas were successfully socially housed with same sex partners that included other recently weaned kits or mother-daughter pairings.
A survey of Consortium members indicated a combined total need of roughly 1,200 chinchillas per year for research. The survey also showed institutions have a common interest in sourcing specific pathogen free (SPF) chinchillas. An SPF chinchilla colony would lead to less variability in research data and would reduce zoonotic risks. Potential agents to be screened for would include S. zooepidemicus and ringworm (Trichophyton or Microsporum spp). The Consortium has approached a variety of laboratory animal vendors to gauge their interest in developing an SPF chinchilla commercial production colony. At this time, the largest obstacle for a potential SPF chinchilla vendor is the economics of developing and maintaining an SPF breeding colony, affected by the unique reproductive physiology, housing requirements, and current demand for the use of chinchillas. It is difficult to turn a profit when breeding an animal with a long gestation period and small litter size.
Despite the expected low profitability, some members of the Consortium have moved forward in developing a small and/or temporary chinchilla breeding colony in order to provide animals for investigator projects that could not be accommodated by vendors/breeders.
Here are factors to consider when establishing a breeding colony:
• Upfront cost of purchasing breeder pairs
• Establishing a facility or designated area to house the colony
• Ensuring appropriate and safe caging is available (for adults and kits)
• Substrate for cage bottoms (if needed based on available caging)
• Feed costs (chinchilla specific or rodent pelleted diets and timothy hay)
• Dust baths
• Additional enrichment
• Testing requirements for maintaining an SPF colony
• Labor costs
Many of these costs are comparable across the country while other costs such as facility-related expenses and labor can differ significantly between institutions. This discrepancy in cost can
impact whether or not chinchilla breeding is a profitable endeavor. For example, one institution calculated the total cost was approximately $800 per chinchilla weaned. For comparison, chinchillas from small, local breeders may cost $100-200 per animal. Although small breeders may be less expensive, they are not without challenges including inconsistent availability of animals and unknown or questionable health status.
NIH has been approached about the availability of funding to develop a resource center for research chinchillas, but such grants are no longer available. The Consortium has found success in sharing information on small breeders that will supply chinchillas for research.

The Chinchilla Consortium meets on a quarterly basis to discuss the challenges and successes to acquiring, breeding, and housing chinchillas in the laboratory environment. Although there is a paucity of caging standards and commercial chinchilla vendors, chinchillas continue to remain an important species for research institutions. In our discussions, we have identified areas to address when developing breeding colonies and introducing chinchillas to an institution. In partnership with IDEXX BioAnalytics, the Consortium has also established a standard recommended chinchilla pathogen testing panel. The Consortium intends to continue to share the knowledge and experiences of its members with the at-large laboratory animal science community to assist in developing standards of practice and recommendations for the chinchilla. Please check the next issue of LASPro for part 2 of The Chinchilla Consortium’s story that will provide information on standardization of animal and veterinary care for the research chinchilla.
The listed authors would like to acknowledge the support of the entire Chinchilla Consortium when compiling the information in this
article and for their continued support of the laboratory chinchilla (in alphabetical order): Dr. Rudy Beiler, Dr. Charlette Cain, Lucie Cote, Dr. Gina Dobek, Dr. Aurore Dodelet-Devillers, Dr. Emily Drake, Dr. Misha Dunbar, Dr. Mike Fink, Dave Goldberg, Dr. Laurie Goodchild, Dr. Lauren Habenicht, Dr. Karen Krueger, Dianne Larson, Dr. Raphael Malbrue, Matt Mellini, Dr. Erin Mitchell, Dr. Andie Moffitt, Dr. Arthur Nedder, Imelda Norton, Marie Ortega, Jim Pomonis, Dr. Colleen Thurman, Dr. Jason Villano, Dr. Wendy Williams, and Jiajie Xu.


Amanda Darbyshire, DVM, DACLAM is the Assistant Director of Clinical Medicine of the Laboratory Animal Program at Purdue University in West Lafayette, IN.
Andrea Osborne, DVM, MS, DACLAM works as a Senior Clinical Laboratory Animal Veterinarian at the University of Alabama at Birmingham in Birmingham, AL.
Glenn Jackson, DVM, PhD, DACLAM is an Assistant Director at the Center for Animal Resources and Education at Cornell University in Ithaca, NY.
Derek Fong, DVM, DACLAM is the Associate Director of Veterinary Services for the Office of Laboratory Animal Resources at the University of Colorado Anschutz Medical Campus in Aurora, CO.
Misty Williams-Fritze, DVM, MS, DACLAM is the Director of Veterinary Services & Technical Operations at CBSET, Inc. in Lexington, MA.
Erica R Feldman, DVM, DACLAM works as a Clinical Veterinarian at the Center for Animal Resources and Education at Cornell University in Ithaca, NY.
Chris Manuel, DVM, PhD, DACLAM is the Senior Associate Director for the Office of Laboratory Animal Resources at the University of Colorado Anschutz Medical Campus, in Aurora, CO.
1. CITES Appendices. [Internet]. 2021. CITES. [Cited 10 June 2022]. Available at: https://cites.org/eng/app/appendices. php
2. Greco A, Ragucci M, Liuzzi R, Prota M, Cocchia N, Fatone G, Mancini M, Brunetti A, Meomartino L. 2019. Noninvasive Ultrasound Monitoring of Embryonic and Fetal Development in Chinchilla lanigera to Predict Gestational Age: Preliminary Evaluation of This Species as a Novel Animal Model of Human Pregnancy. Contrast Media and Molecular Imaging. Volume 2019, article 6319476.
3. Hsu C, Chan M, Wheler C. 2015. Biology and Diseases of Chinchillas. p 387-405. In: Fox J, Anderson L, Otto G, Pritchett-Corning K, & Whary M, eds. Laboratory Animal Medicine, 3rd edition. San Diego (CA): Elsevier, Inc.
4. Suckow M, Stevens K, & Wilson R, eds. 2012. Part V: Chinchillas. p 949-1028. In: The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents, 1st edition. San Diego (CA): Elsevier, Inc.

The Iditarod is an approximately 1000-mile dogsled race that travels from Anchorage, AK, to Nome AK, along one of two routes every year. It is a commemoration of the 1925 Serum Run that delivered anti-serum to Nome from Anchorage to help treat a diphtheria outbreak. 674 miles of the roughly 1000-mile delivery was made by teams of sled dogs working in a relay fashion. The almost exclusively indigenous Athabascan mushers and their dogs covered those 674 miles of extremely challenging terrain and weather conditions in only 127 hours to deliver this life-saving medication – and not a single vial was broken or lost along the way.
Iditarod’s inaugural race happened in 1973 and has been an annual event ever since. The 2022 race was the 50th consecutive running! It was originally organized by Joe Redington Sr, who moved to Alaska in 1948 and lived there continuously until his death in 1999. In addition to organizing, Joe Sr ran the race 19 times – his last race was in 1997 at age 80!
Sled dogs and their human companions (mushers) compete in races ranging from just a few miles all the way to long-distance races like Iditarod. The other major long-distance races include the Yukon Quest between eastern Alaska and the Yukon in Canada and the Finnmarkslopet in Norway.

My first contact with Iditarod was listening to a LAM colleague, Bob Baker, DVM, DACLAM, tell me about his experiences volunteering as a veterinarian for Iditarod in about 2013. I had always wanted to see Alaska, and I love unique and active travel experiences, so this seemed like a fit. After some encouragement from Bob,
I applied and was accepted as a trail veterinarian for the March 2015 Iditarod. Including that race, I have now worked in 6 of the last eight races, including the 2020 race, which just happened to coincide with the escalation of the COVID pandemic in the USA. Iditarod’s motto is “The Last Great Race.” That year it seemed like the last sporting event, professional sports including the NBA, were busy canceling their seasons while Iditarod carried on and finished the race. Strange times indeed! Okay, enough history, let’s talk about the interesting part: the dogs! These dogs are the ultimate super-athlete. The average Iditarod dog is an Alaskan Husky, which is more a phenotype than an actual breed. They are about 50 pounds, lean, long-legged, and have a thick dense coat. Beyond that, anything goes! All coat colors and both sexes compete. I have seen dogs from 2-12 years old on the trail. As a group, they are lean, tremendously strong, and very well socialized with both other dogs and humans. They have voracious appetites and an incredible
drive to do what they love – go down the trail with their human and canine pack.
While racing, these dogs will consume about 10,000 calories per day and generally operate on a roughly 4-hour run and 4-hour rest schedule. Their metabolism is incredible. Both humans and these canine athletes see the same metabolic changes with strenuous exercise; stress hormone increases, glycogen depletion, blood markers of cellular injury, and metabolic stress. The difference between these dogs and humans is simple but amazing; these changes will persist in humans until we rest to recover. Sled dog athletes’ metabolic profiles return to the pre-race status in about 4 days while they are still racing! They also can utilize about 4 times the amount of oxygen of a competitive human cyclist and twice that of equine endurance athletes. What they can physically accomplish and the absolute joy they take in doing it is the main reason I will continue to be involved in Iditarod.
I called the sled dog team and musher a “pack,” and it is true in every sense of the word. From the outside, it may look like the musher is in charge and is making the dogs perform. Nothing could be further from the truth. The primary jobs of the musher are to take care of every need the dog has and to be a cheerleader for the team. And when I say “every need” I mean every need. When the dogs need to have a hot meal prepared for them every 4 hours, the musher cooks, serves and cleans up after. When the dogs have a sore joint or a paw pad issue, the
musher is a physical therapist/masseuse and nurse. When the dogs need to lie down and nap, the musher’s job is to make sure everyone has a comfortable bed of straw to snooze on.

The dogs may have s a 4-hour rest schedule; the mushers do not. Mushers only rest when chores are done and all the dogs have what they need and are already asleep. Mushers rarely sleep more than 4 hours at a stretch during the race, with 1-2 hours being much more common. Whether you are a musher, a trail veterinarian, or another trail volunteer, the #1 rule is the same: the dogs come first! Being witness to the incredible bonding and relationship of mutual caretaking between musher and their dogs has been a great privilege and blessing.
I clearly remember during my first race watching a musher come into a checkpoint that was -40° and extremely windy at 3 AM. He and the dogs were all covered in frost. He was undoubtedly freezing, exhausted, and hungry, but the dogs came first. As I watched him start getting his dogs settled down to rest while doing all the usual chores, I saw something I will never forget. As he was moving through his team taking off booties and settling them in to nap, each dog got about 30 seconds of absolutely undivided direct attention. Each dog was told how proud the musher was of them and how great a job they were doing. Human eyes were locked with canine eyes, and that dog clearly knew that it was the most important thing in the world to that musher at that moment. What I saw that night, and have seen many

times since then, will always be my definition of the human-animal bond.
Okay, on to what veterinarians do on the trail. Much the same as we do in the animal facility or veterinary clinic – be part of a team of people with different skills who work together to care for animals and ensure their well-being. We just happen to be doing it in rotating 6-hour shifts around the clock in sometimes quite adverse to us wimpy humans weather conditions. A checkpoint team consists of veterinarians, communications staff (comms), and trail crew (trails). Trails get teams parked, check them in and out of the checkpoint, make sure needed supplies are available, sometimes delivered directly to the team, and clean up the dog yard after teams leave the checkpoint. Comms relay information about teams in and out of the checkpoint and numbers of dogs and arrange for transportation of dogs and humans as needed. The veterinarian’s job is to ensure that each dog coming in or going out is healthy and well. The goal is to perform a physical exam of each dog at each checkpoint, but mushers are not required to stay at checkpoints – they can check in and immediately leave. At a minimum, there is a visual assessment of every dog, heart and lungs are listened to, and the veterinarian and musher have a brief conversation to ensure that the dogs are doing well and there are no concerns.
If dogs in a checkpoint are found to have minor medical problems (sore feet and diarrhea are far and away the most common), we can prescribe certain medications to help.. If a dog requires any sort of pain control, it cannot continue racing; it will be turned over to the veterinary staff at the checkpoint, and we will be responsible for the care of the dog. Responsibility for the dog means giving any medications/treatments needed, feeding 3 hot meals a day, full physical exam at least once daily, and a visual assessment of each “returned” dog a least hourly. All are performed by the veterinarians working the checkpoint.
The list of medications that cannot be used in a dog that is still racing is extensive, and there is an active drug testing program (that covers both dogs and mushers) to prevent “doping.” There are folks from comms, trails, and vets working 24 hours a day – for the vets, it is usually 6 hours on, 6 hours off rotation. But if the checkpoint is really busy, everyone pitches in. I worked 22 hours straight at a checkpoint my second year, right alongside my colleagues who were all doing the same.
To summarize and wrap up, most years, I spend about 2 weeks in central Alaska sleeping on floors, wooden benches, or sometimes in a tent. We work 6 hours on, 6 hours off, are often quite cold, eat what we can when we can, as the wonderful food is frequently on offer from the fantastic people of central Alaska, but sometimes you throw together a baloney sandwich at 3 AM and are happy to have it. We crawl around on our knees doing physical exams on sleepy dogs, make sure the mushers are doing alright and have what they need, and pitch in to do whatever work needs doing. You may not be able to bathe for 10 days at a stretch or more. You may have to use an outhouse at -40 degrees. It’s exhausting and physically difficult sometimes. It is also one of the most meaningful and satisfying experiences of my life. The mushers, other volunteers, and especially the residents of Alaska, the dogs, and the environment all recharge my soul and make me feel lucky to be a small part of this incredible event.

Special thank you to Mack Boyd for the photographs and permissions.
Steven Shipley, DVM, DACLAM, is the Associate Director of Vet Services, Division of Comparative Medicine, and the Associate Professor of Pathology and Lab Medicine at UNC at Chapel Hill, NC.

































 By Gennifer Caesar, BS, ALAT
By Gennifer Caesar, BS, ALAT



Having been a part of the Laboratory Animal Research (LAR) community for over 10 years, one observation is that LAR organizations are slow in adopting new technologies. For instance, some institutions still use pen and paper to track animal census, animal health records, and facility tasks. One might wonder why, in the age of digital technology being applied in almost all areas of our lives, why some institutions are slow to adopt digital transformations.

Before exploring the “why,” having a definition of “what” will lay the foundation to answer that question. So, what is digital transformation? One definition of digital transformation (DX) is the process of integrating digital solutions that significantly change how a business operates and achieves its goals. The definition in and of itself identifies the main component of this growing concern: change. Fear of the unknown can often lead to misconceptions rather than being open to possibilities. Let’s explore some common misconceptions centered around changing how organizations operate.
• “We’ve always done it this way” – A common consideration. It brings to focus the uncomfortable nature of changing processes that have seemingly worked and that have been mastered by employees. A digital transformation strategy would identify the improvement areas of current processes and design a plan to improve those areas with a workable digital solution. Additionally, this may also provide a way to measure how effective the changes are in order to make adjustments in a timely manner.

• “That sounds expensive. Is it really necessary?” – Budgetary restraints can be a real nemesis when acquiring a digital solution. Often, even when an institution recognizes the need for a new solution, it must make profitability “sense” to upper management. The justification and buy-in needed from the financial gatekeepers can delay adoption. Addi-

tionally, there can be a disconnect between the value added from adoption and the upfront costs. With clearly defined goals and key performance metrics, the value of investing in a digital solution will most undoubtedly pay for itself down the road by reducing errors, streamlining processes, and easily managing workloads.


• “This is going to cause more work” – As with the adoption of any new solution, it will require more initial effort on the front end. However, the goal of digital transformation is to reduce the overall amount of effort on the part of the end user in completing their regular tasks. Proper strategy and training of staff and stakeholders will change the way they complete their tasks and result in less future effort and mistakes. For example, completing an animal census by manually counting, cage by cage, may require from 30 minutes to 2 hours or more, depending on the number of cages in the room, the number of interruptions, and the process for reconciling missing cages. Applying a digital solution that not only changes the counting technique but also allows for immediate reconciliation of missing cages would significantly reduce this time requirement and minimize counting mistakes.
• “We aren’t sure what we’re looking for just yet” - Often the decision to acquire software or a digital solution is admin-









istrative and requires further study. The caveat is that the facility or laboratory may not know what they are looking for in order to decipher between one solution and another. Additionally, there may be no one within the organization that has the understanding or time to undertake a project of this magnitude in addition to their existing responsibilities. Finding a great resource to focus on the solutions that would best fit your organization’s needs would mitigate the burden of time and expertise when seeking to employ a digital transformation.
These misconceptions often stem from fear of the unknown. Although understandable, believing these misconceptions can be crippling to an organization’s advancement and reduce the opportunity for innovation and operational efficiency. Improving how we do our work may the way for more medical discoveries and advancements that benefit our society. Digital transformations will lead us into a new age and, with proper strategy and implementation, provide everyone the knowledge, skills, and desire to achieve enhanced operational efficiency.
FFor subcutaneous use in mice and rats only.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
LEGAL STATUS--In order to be legally marketed, a new animal drug intended for a minor species must be Approved, Conditionally Approved, or Indexed by the Food and Drug Administration. THIS PRODUCT IS INDEXED--MIF # 900-014. Extra-label use is prohibited. This product is not to be used in animals intended for use as food for humans or food-producing animals.
Ethiqa XR contains buprenorphine, a high concentration (1.3 mg/mL) opioid agonist and Schedule III controlled substance with an abuse potential similar to other Schedule III opioids. The high concentration of Ethiqa XR may be a particular target for human abuse. Buprenorphine has opioid properties that in humans may lead to dependence of the morphine type. Abuse of buprenorphine may lead to low or moderate physical dependence or high psychological dependence. The risk of abuse by humans should be considered when storing, administering, and disposing of Ethiqa XR. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (suicidal depression).
Because of human safety risks, this drug should be used only with veterinary supervision. Do not dispense Ethiqa XR
The concentration of buprenorphine in Ethiqa XR is 1.3 mg/mL. Respiratory depression, including fatal cases, may occur with abuse of Ethiqa XR Ethiqa XR has additive CNS depressant effects when used with alcohol, other opioids, or illicit drugs that cause central nervous system depression.
Because of the potential for adverse reactions associated with accidental injection, Ethiqa XR should only be administered by a veterinarian or laboratory staff trained in the handling of potent opioids.
Ethiqa XR is an injectable suspension of extended-release buprenorphine. Buprenorphine hydrochloride, an opioid analgesic, is the active ingredient in Ethiqa XR. Lipid-bound buprenorphine hydrochloride is suspended in medium chain fatty acid triglyceride (MCT) oil. Lipids encapsulate the buprenorphine limiting diffusion which provides for larger doses and prolonged action.1,2 Ethiqa XR has a slightly yellow to white opaque appearance. Each mL contains approximately 1.3 mg buprenorphine hydrochloride. The sterile product contains cholesterol, gl yceryl tristearate, and buprenorphine hydrochloride suspended in MCT oil.
Buprenorphine Formula C29 H41NO 4
Ethiqa XR is indicated for the control of post-procedural pain in mice and rats.
Wear protective clothing when administering Ethiqa XR (see Human Safety Warnings)
Shake the vial briefly before each use to ensure uniform suspension. If stored refrigerated, bring to room temperature before use.
Use aseptic techniques to withdraw the dose into a disposable 0.5 or 1 mL syringe. A 20 to 23 gauge needle should be used for injections due to the viscosity of the drug suspension.
The dosage of Ethiqa XR is a single subcutaneous injection of 0.05 mL per 20 gram mouse (3.25 mg/kg body weight). Therapeutic drug concentrations are maintained for 72 hours after the initial dose. If needed, a single repeat dose may be administered 72 hours after the initial dose.
Secure the mouse in a scruff-of-the-neck hold. Insert the needle into the dorsal subcutaneous space created by the scruff hold.
Inject the entire dose into the dorsal subcutaneous space. An oily sheen may be observed in the dorsal fur of the mouse after injection due to leakage of the oil-based drug suspension from the injection site. The oily sheen may last for 4 to 5 days post-injection. Leakage from the injection site can be minimized by slowly injecting Ethiqa XR into the subcutaneous space.
The mouse can be returned to its cage immediately after receiving Ethiqa XR
Do not return any unused drug suspension from the syringe back into the vial.
Once the vial is broached, Ethiqa XR can be stored at 15° to 25°C (59° – 77°F) or refrigerated for 28 days. DO NOT FREEZE.
Product could change its physical properties if not stored within the specified storage conditions and original vial container.
Wear protective clothing when administering Ethiqa XR (see Human Safety Warnings) Shake the vial briefly before each use to ensure uniform suspension. If stored refrigerated, bring to room temperature before use.
Use aseptic techniques to withdraw the dose into a disposable 0.5 or 1 mL syringe. A 20 to 23 gauge needle should be used for injections due to the viscosity of the drug suspension.
The dosage of Ethiqa XR is a single subcutaneous injection of 0.1 mL per 200 gram rat (0.65 mg/kg body weight). Therapeutic drug concentrations are maintained for 72 hours after the initial dose. If needed, a single repeat dose may be administered 72 hours after the initial dose.
Secure the rat in a passive restraint tube or by holding with a heavy glove with one person to secure the rat and a second person to administer the drug. Insert the needle in the dorsal subcutaneous space. Inject the entire dose into the dorsal subcutaneous space. An oily sheen may be observed in the dorsal fur after injection due to leakage of the oil-based drug suspension from the injection site. The oily sheen may last for 4 to 5 days post-injection. Leakage from the injection site can be minimized by slowly injecting Ethiqa XR into the subcutaneous space. The rat can be returned to its cage immediately after receiving Ethiqa XR See CONTRAINDICATIONS and Rat PRECAUTIONS for additional information on bedding.
Do not return any unused drug suspension from the syringe back into the vial.
Once the vial is broached, Ethiqa XR can be stored at 15° to 25°C (59° – 77°F) or refrigerated for 28 days. DO NOT FREEZE. Product could change its physical properties if not stored within the specified storage conditions and original vial container.
Only administer Ethiqa XR by subcutaneous injection. Ethiqa XR is not intended for intravenous, intra-arterial, intrathecal, intramuscular, or intra-peritoneal injection.
Do not use on mice or rats with pre-existing respiratory deficiencies.
Do not keep rats on wood chip-type bedding after administration of Ethiqa XR
Not for use in humans. Keep out of the reach of children.
Human User Safety while handling Ethiqa XR:
Two trained staff for administration: Ethiqa XR should only be handled and administered by a veterinarian, veterinary technician, or laboratory staff trained in the handling of potent opioids. To prevent human adverse reactions or abuse, at least 2 trained administrators should be present during injection of Ethiqa XR
Protective covering: To prevent direct contact of Ethiqa XR with human skin or mucous membranes when handling the suspension, protective clothing is recommended.
Mucous membrane or eye contact during administration: Direct contact of Ethiqa XR with the eyes, oral or other mucous membranes of humans could result in absorption of buprenorphine and the potential for adverse reactions. If accidental eye, oral or other mucous membrane contact is made during administration, flush the area with water and contact a physician.
Skin contact during administration: If human skin is accidentally exposed to Ethiqa XR, wash the exposed area with soap and water and contact a physician. Accidental exposure could result in absorption of buprenorphine and the potential for adverse reactions.
Controlled Substance: Ethiqa XR contains buprenorphine, a mu opioid partial agonist and Schedule III controlled substance with an abuse potential similar to other Schedule III opioids. Ethiqa XR can be abused and is subject to misuse, abuse, addiction, and criminal diversion. Ethiqa XR should be handled appropriately to minimize the risk of diversion, including restriction of access, the use of accounting procedures, and proper disposal methods, as appropriate to the laboratory setting and as required by law.
Abuse: Abuse of Ethiqa XR poses a hazard of overdose and death. This risk is increased with concurrent abuse of alcohol and other substances including other opioids and benzodiazepines. Buprenorphine has been diverted for non-medical use into illicit channels of distribution. All people handling opioids require careful monitoring for signs of abuse. Drug abuse is the intentional non-therapeutic use of a prescription drug for its rewarding psychological or physiological effects. Abuse of opioids can occur in the absence of true addiction.
Storage and Discard: Ethiqa XR is a Class III opioid. Store in a locked, substantially constructed cabinet according to DEA and local controlled substance guidelines. Discard broached vials after 28 days. Any unused or expired vials must be destroyed by a DEA registered reverse distributor; for further information, call 1-833-384-4729.
Physician information: Ethiqa XR injectable suspension is a mu-opioid partial agonist (1.3 mg buprenorphine/mL). In the case of an emergency, provide the physician with the package insert. Naloxone may not be effective in reversing respiratory depression produced by buprenorphine. The onset of naloxone effect may be delayed by 30 minutes or more. Doxapram hydrochloride has also been used as a respiratory stimulant.
The safety of Ethiqa XR has not been evaluated in pregnant, lactating, neonatal, or immune-compromised mice. As with other opioids, buprenorphine may cause sedation, decreased blood pressure, decreased heart rate, decreased gastrointestinal mobility, and respiratory depression. Use caution with concomitant administration of Ethiqa XR with drugs that cause respiratory depression. The use of paper or soft bedding for up to 3 days following administration of Ethiqa XR should be considered.
Normal mice may exhibit an obtunded response to stimuli up to 4 hours after receiving Ethiqa XR
Buprenorphine is excreted in the feces (see Clinical Pharmacology section below). Coprophagy may lead to ingestion of buprenorphine or its metabolites by mice treated with Ethiqa XR and untreated cage mates.
The safety of Ethiqa XR has not been evaluated in pregnant, lactating, neonatal, or immune-compromised rats. As with other opioids, buprenorphine may cause sedation, decreased blood pressure, decreased heart rate, decreased gastrointestinal mobility, and respiratory depression. Use caution with concomitant administration of Ethiqa XR with drugs that cause respiratory depression.
Rats may exhibit signs of nausea including pica up to 3 days post-treatment. Rats should be maintained on paper or soft bedding to avoid ingestion of wood chip-type bedding after administration of Ethiqa XR Pica involving wood chip-type bedding can be lethal in rats.
Buprenorphine is excreted in the feces (see Clinical Pharmacology section below). Coprophagy may lead to ingestion of buprenorphine or its metabolites by rats treated with Ethiqa XR and untreated cage mates.
No adverse reactions were observed in 20 to 25 gram young adult male and female mice after a single subcutaneous injection of Ethiqa XR at a dose 5 times the indicated dose. Laboratory parameters evaluated in the study included hematology and clinical chemistry; histopathology was also performed. In a second study, adult male and female mice received Ethiqa XR subcutaneously at 5 times the indicated dose for three doses at four day intervals. A surgical procedure was performed on the study mice prior to receiving each of the three doses of Ethiqa XR
Mortality was seen in two male mice after the third surgical procedure and dose of Ethiqa XR (total dose of 49 mg buprenorphine/ kg body weight in 8 days).
Weight loss has been observed in mice treated post-procedurally with Ethiqa XR
Rats
Adverse reactions were evaluated in 180 to 200 gram young adult male and female rats after a single injection of Ethiqa XR. A surgical procedure was performed on the rats prior to administration of a single dose at the intended dose of 0.65 mg/kg or a single dose of 2, 6 or 10-fold excess dose. Adverse reactions also were evaluated in male and female rats administered 2, 6 and 10 times the intended dose for three doses at four day intervals. A surgical procedure was performed on the rats prior to administration of the first of three doses. Laboratory parameters evaluated in the study included hematology, clinical chemistry, urinalysis, histopathology, and bodyweight.
Signs of nausea were observed at all dose levels within 24 hours of the dose. Signs included self-licking, self-gnawing and efforts to eat wood-chip bedding.
Mortality was seen in 1 of 36 rats exposed to wood chip bedding. Necropsy revealed the stomach and esophagus were compacted with bedding, the bladder was abnormally distended and the urine contained blood.
Mortality was seen in 3 of 222 rats treated with Ethiqa XR due to technical complications with serial bleeding of the jugular vein. For technical assistance, or to report an adverse drug reaction, please call Fidelis Pharmaceuticals LLC at 1-833-384-4729.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http:// www.fda.gov/AnimalVeterinary/SafetyHealth.
Buprenorphine can act as an agonist and antagonist at different classes of opioid receptors. Agonism at the mu opioid receptor and, in some cases, antagonism at the kappa or delta opioid receptors are possible underlying mechanisms for the ceiling effect and bell-shaped dose-response curve of buprenorphine. Studies with knockout mice have shown that the antinociceptive effect of buprenorphine, which is mediated primarily by the mu opioid receptor, is attenuated by the ability of the drug to activate the opioid receptor like (ORL-1) receptor. The drug can be described as a ‘full’ and a ‘partial’ agonist at the same receptor depending on the specific assay. There appears to be no ceiling effect for analgesia, but there is a ceiling effect for respiratory depression. Pharmacokinetic studies with bolus injections of buprenorphine in mice and rats provide similar models. After bolus intravenous administration, plasma levels decline tri-exponentially. The drug is n-deakylated in the liver to norbuprenorphine (NBN), an active metabolite. Studies have shown that glucuronide metabolites of buprenorphine and NBN are also metabolically active, and can approximate or exceed the concentration of the parent drug. Un-metabolized drug excreted in the urine and feces one week after injection was 1.9 and 22.4% of the dose, respectively, and 92% of the dose was accounted for in one week.3
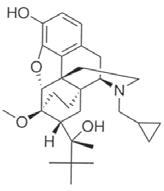
Pharmacokinetic parameters of Ethiqa XR were studied in 6-8 week old male and female Balb/c mice following a single subcutaneous injection of 3.25 mg/kg bodyweight. Clinically significant blood levels were observed up to 72 hours after subcutaneous injection.
Pharmacokinetic parameters of Ethiqa XR were studied in 8 week old male and female Fischer rats following a single subcutaneous injection of 0.65 mg/kg bodyweight. Clinically significant blood levels were observed up to 72 hours after subcutaneous injection.
Ethiqa XR is supplied in a 5 mL glass vial containing 3.0 mL of injectable drug suspension (NDC 86084-100-30).
U.S. Patent No. 8,461,173; 10,555,899
STORAGE INFORMATION
Store between 15° and 25°C (59° – 77°F) or refrigerated. DO NOT FREEZE. If stored refrigerated, bring to room temperature before use. Once broached, the multi-dose vial should be discarded after 28 days.
1. Mishra et al., Drug Delivery and Transl. Res, 2:238-253; 2012.
2. Bethune et al., The role of drug-lipid interactions on the disposition of liposome-formulated opioid analgesics in vitro and in vivo. Anesth Analg. 93(4):928-33; 2001.
3. Guarnieri et al., Lab Animal, 41(11): 337-343; 2012.
Manufactured for:
Fidelis Pharmaceuticals
675 Highway One Suite B113
North Brunkswick, NJ 08902 833-384-4729
www.EthiqaXR.com
Fidelis Pharmaceuticals® and EthiqaXR® are registered trademarks of Fidelis Pharmaceuticals LLC, a Delaware Corporation. August 2021 FID-ETH-PI007
Opioid Analgesic

.
Ethiqa XR® is the first and only pharmaceutical grade extended-release buprenorphine that’s FDA-indexed to control post-procedural pain for mice and rats up to 72 hours with just one injection.
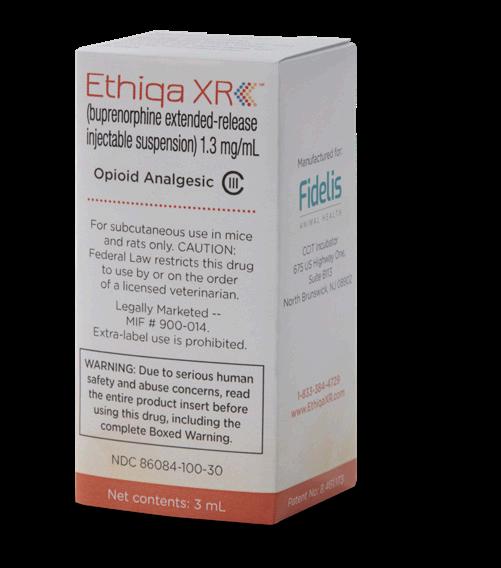

This formulation contains buprenorphine, a high-concentration (1.3 mg/mL) opioid agonist and Schedule III controlled substance with an abuse potential similar to other Schedule III opioids. The high concentration may be a particular target for human abuse. Buprenorphine has opioid properties that in humans may lead to dependence of the morphine type. Abuse of buprenorphine may lead to low or moderate physical dependence or high psychological dependence. The risk of abuse by humans should be considered when storing, administering, and disposing of Ethiqa XR. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (suicidal depression). Because of human safety risks, this drug should be used only with veterinary supervision. Do not dispense Ethiqa XR.
The concentration of buprenorphine in Ethiqa XR is 1.3 mg/mL. Respiratory depression, including fatal cases, may occur with abuse of Ethiqa XR. Ethiqa XR has additive CNS depressant effects when used with alcohol, other opioids, or illicit drugs that cause central nervous system depression. Because of the potential for adverse reactions associated with accidental injection, Ethiqa XR should only be administered by a veterinarian or laboratory staff trained in the handling of potent opioids.
For Rats and Mice: Only administer Ethiqa XR by subcutaneous injection. Ethiqa XR is not intended for intravenous, intra-arterial, intrathecal, intramuscular, or intra-peritoneal injection. Do not use on mice or rats with pre-existing respiratory deficiencies. Do not keep rats on wood chip-type bedding after administration of Ethiqa XR. Use caution with concomitant administration of Ethiqa XR with drugs that cause respiratory depression. For Humans: Ethiqa XR should only be administered by a veterinarian or laboratory staff trained in the handling of potent opioids. Protective clothing is recommended to avoid direct contact with human skin or mucus membranes which could result in absorption of buprenorphine and adverse reactions. Not for use in humans.
For more information, consult the Prescribing Information including the boxed warning located on the next page.
An upgrade in quality that upholds your high standards.
Without a doubt, the laboratory animal research community recognizes the tremendous value of training for IACUC Committee members and administrators. Training frequency, methodology, topics, and other details are often discussed during AAALAC site visits, regulatory visits, and animal welfare audits of animal care and use programs. What, then, can an IACUC do to ensure that training for these individuals is tracked and documented?
In this issue of Laboratory Animal Science Professional, two members of the IACUC Office at the University of Texas Southwestern Medical Center discuss their rigorous process for identifying external training opportunities, creating internal training sessions for IACUC, and documenting training for IACUC members and administrators. Their processes provide an auditable overview of all training provided, including training topics and venues, as well as attendance. This approach to training oversight provides numerous benefits. For example, the accessibility and availability of training documentation that can be provided to internal administrators, external auditors and AAALAC site visitors is made more efficient. As well, the ability to provide the information for administrators renewing their PRIM&R’s Certified Professional in IACUC Administration (CPIA) designation, as well as for veterinary staff requiring continuing education units to various licensing boards, is another professional advantage. Overall, we think that this is a win-win situation, and you will too! Enjoy!
Stacy Pritt, DVM, MS, MBA, CPIA, CHRC, DACAW, is the Associate Vice President of Research Support & Regulatory Management at UT Southwestern Medical Center in Dallas, TX.

F. Claire Hankenson, DVM, MS, DACLAM, is the Associate Vice Provost for Research and Attending Veterinarian and Executive Director, University Laboratory Animal Resources, at the University of Pennsylvania in Philadelphia, PA.

Institutional Animal Use and Care Committees (IACUC) are charged with oversight of an organization’s animal care and use program by the Public Health Service (PHS) Policy on the Humane Care and Use of Laboratory Animals3 and the United States Department of Agriculture (USDA) Animal Welfare Act and Animal Welfare Regulations1. These regulations also highlight the need to train IACUC members on their expected duties and engage them through continuing education efforts. Specifically, according to the Guide for the Care and Use of Laboratory Animals (Guide)2, the IACUC is responsible for training its members so they are fully prepared to execute their tasks of assessing their organization’s animal care and use program. It is expected that “ongoing opportunities to enhance their understanding of animal care and use in science should also be provided” 2 (p17). Similarly, the Animal Welfare Act and Animal Welfare Regulations1 published by USDA’s Animal and Plant Health Inspection Service (APHIS), also known as the “Blue Book”, section 2143 (b)(1), states that “… members shall possess sufficient ability to assess animal care, treatment, and practices in experimental research as determined by the needs of the research facility and shall represent society’s concerns regarding the welfare of animal subjects used at such facility”1. Further credence to the importance of ongoing training of IACUC members is demonstrated by institutional accreditation from AAALAC International, which uses the Guide to set a specific standard for operation and function of animal care and use programs.
With the constant evolution of laboratory
animal science and medicine, including its associated guidelines and regulations, animal care and use programs should be pro-active in offering opportunities to assist committee members with program oversight. In this column, we will describe how the IACUC Office at the University of Texas Southwestern Medical Center (UTSW) identifies and documents the participation of IACUC members and staff in continuing education opportunities. The prioritization of continuing education (CE) in our animal care and use program fosters professional development, encourages active group discussion on a variety of animal-related topics, and promotes group cohesiveness for support and delivery of best practices in animal welfare.
One of the IACUC Office’s goals at UTSW is to pro-actively identify CE opportunities for members and professional staff. We use announcements and articles published by regulatory agencies, biomedical research support groups, professional societies and IACUC administrator groups (e.g. Yammer), to inventory training opportunities. If webinars, conferences, or meetings offered by organizations outside of UTSW are identified, these may be communicated directly to IACUC members and staff, with follow-up communication as described below.
Additionally, the IACUC Office prepares CE presentations for monthly IACUC meeting participants. These presentations are a standing item in our IACUC meeting agenda and may cover relevant case studies, examples of protocol review scenarios, or changes in federal policies or guidance documents that may impact our institution. Rather than present this information didactically, we have
Date
Webinar Host: Title (IACUC Staff-only Example)
Date Discussion Topic held at Convened IACUC meeting
Scientist
Non-Institution Member
5th IACUC member X
Alternate Member #1 X
Guests of the IACUC IACUC Staff #1 X X
IACUC Staff #2 X X
Figure 1. Documentation of IACUC Participation for Continuing Education Events. The constitution of IACUC members follows OLAW requirements.
found that an active-learning format with open-ended questions facilitates group interaction and discussion.
For the past several years, our IACUC Office has utilized a focused structure for CE documentation by designating a staff member to actively document participation in CE for IACUC members and IACUC Office staff. Assigning this role to a specific Office staff member removes the burden of self-documentation so that research support resources may be more efficiently utilized. For IACUC administrators, documentation of CE takes on an additional purpose, as it is a crucial part of obtaining and maintaining CPIA (Certified Professional in IACUC Administration) certification, a quality standard offered by the nonprofit Public Responsibility in Medicine and Research (PRIM&R). In lieu of retaking the exam, recertification may be achieved through the completion of continuing education requirements. Therefore, accurate documentation of administrator participation in eligible CE events is of distinct importance.
Our designated staff member captures participation in CE for IACUC members and professional staff using an intradepartmental spreadsheet that lists IACUC members, alternate members, and guests, as well as the date and title of each CE presentation (Figure 1). By creating specific tabs labeled by fiscal year within the spreadsheet, multiple years of CE training may be easily catalogued for review, which helps to prevent the presentation of duplicative topics at convened IACUC meetings and provides a comprehensive review document for AAALAC and other auditors that visit the institution. In addition, the CE designee also maintains a digital document that exclusively catalogues CE events external to IACUC meetings, which allows professional staff to quickly and easily identify events that may be applied towards CPIA recertification. Indeed, each staff member of our IACUC Office that has been eligible for CPIA recertification has successfully moved through the process without needing to retake the certification exam.
Of final note, our office also implements a more personal touch for CE events held outside of convened IACUC
meetings. Our CE designee utilizes a digital calendar to track these external CE events and after the event has concluded , reaches out to staff and IACUC members via email to query attendance. CE participation may be captured by a certificate of attendance from the CE event or with a digital document containing event details and listing all IACUC member and staff attendees (Figure 2). As many of these external CE events are held online and the majority of our Office staff work remotely, this unique outreach promotes group engagement and a sense of community.
Title: Animal Care Date: September 30, 2022 Time: 12:00pm – 1:00pm CDT Presenter(s): Dr. Iman X. Pert
Name Signature Kathryn Cavanaugh
Julia Wilkerson
Figure 2. Documentation of IACUC Participation for externally held Continuing Education Events, e.g., webinars.
As shown in Figure 3, our structured CE identification and documentation process benefits IACUC members and professional staff alike. Our Office identifies opportunities for continuing education for IACUC members and staff by utilizing multiple resources. CE participation is documented and confirmed using attendance logs for convened IACUC meetings or staff-attended CE events. By designating a point person in our Office to diligently and proactively document CE training events, we have eliminated or lightened the workload for the majority of IACUC members and staff, while ensuring that documentation is easily
accessible when needed. In our experience, this has led to increased office efficiency and productivity, benefitting not only the workforce, but the institution as well.
Julia Wilkerson, PhD, is a Regulatory Analyst at UT Southwestern Medical Center in Dallas, TX

Kathryn Cavanaugh, BS, CPIA, is the Assistant Director of the IACUC and SCRO at UT Southwestern Medical Center in Dallas, TX

1. Animal and Plant Health Inspection Service. United States Department of Agriculture. Animal Welfare Act and Animal Welfare Regulations. https://www.aphis.usda.gov/animal_welfare/ downloads/AC_BlueBook_AWA_508_comp_version.pdf
2. Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals. 8th Ed. Washington DC; National Academy Press.
3. Office of Laboratory Animal Welfare. National Institutes of Health. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Bethesda, MD. U.S. Department of Health and Human Services. https://olaw.nih.gov/sites/default/ files/PHSPolicyLabAnimals.pdf
Nutritional quality of animal feed degrades over time, even under ideal storage conditions. Scientists and medical researchers are rightly concerned about animal food quality— valid experimental results depend on product consistency. However, reliance on a customary 180-day shelf life for LabDiet® products is overly cautious, costly, and wasteful.


Vitamins are the most sensitive nutrients. Extensive research of LabDiet® products under real-world conditions demonstrates vitamin quality is sustained over longer periods, even beyond 12 months.
Caution of lab animal professionals derives from research conducted decades ago. The most recent edition of Guide for the Care and Use of Laboratory Animals summarizes this, stating: “Most natural-ingredient, dry laboratory animal diets stored properly can be used up to 6 months after manufacture.”
Importantly, the Guide continues with, “food storage time should be reduced to the lowest practical period and the manufacturers’ recommendations considered.”
Previous research showed LabDiet® products are nutritionally complete up to 18 months from the date of manufacture (DOM) when stored under recommended temperature (<72⁰F/21⁰C) and relative humidity (RH; ≤ 50%).
What about long-term exposure to real-world conditions?
Global distribution involves ocean freight with bags packed in uninsulated steel shipping containers exposed to elements for weeks, sometimes months. Once in-country additional delays, unpredictable transportation, and variable storage conditions are realistic. Comprehensive studies of vitamin stability in LabDiet® products were performed on shipments to subtropical Taiwan and the temperate UK.
Pacific transit: During the 50-day journey from US to Taiwan, temperature and humidity levels exceeded recommendations by up to 20⁰F and 20% RH (pictured to the right). After arrival, the Certified LabDiet® Dealer stored diets under recommended conditions.
Atlantic transit: During the ~25 day journey to the UK, products were also exposed to great temperature and humidity variation. Unlike in Taiwan, continued storage conditions were variable.
Degradation of labile vitamins A and E were greater in the Taiwan study. Thiamin loss was higher in the UK study. Regardless of loss observed, all diets continued to meet or exceed minimum nutrient requirements of the animals at the end of the 18 or 24 month testing periods.
LabDiet® formulates to meet or safely exceed animal requirements, despite suboptimal transportation and storage conditions. Results of these, and other studies illustrate LabDiet® products are nutritionally sound even when exposed to conditions that deviate beyond normal.
Using this proven safety margin, LabDiet® assures an expiration date for standard products at 9 months from DOM and 12 months from DOM for all irradiated diets. LabDiet® supports facilities adopting a similar “internal shelf life” protocol to dramatically reduce facility costs and product waste while assuring the integrity of lab animal nutrition to support research.
For more information about the studies summarized above, including full citations, as well as nutrient stability after exposure to extreme temperature and humidity in the short-term, visit https://www.labdiet.com/ ProductStability/index.html or contact your LabDiet® representative.
 By Nicole Madera, Donnie Adams, Shonn Offord, and Amy Funk
By Nicole Madera, Donnie Adams, Shonn Offord, and Amy Funk
Ferrets (Mustela putorius furo) are recognized as the gold standard animal model for studying respiratory diseases. While the hallmark signs of these types of diseases are similar between ferrets and humans, their natural behavior is not. The behavioral characteristics of ferrets can be very distinct from other species as well and challenging to recreate in a research facility. The best types of enrichment for lab animals are ones that encourage the expression of positive species-specific behavior. A recent paper showed that ferrets in enriched cages produce more exploratory behavior similar to polecats in the wild, with 17.5% of their time spent with exploratory behavior.2 When compared to unenriched cages, time budgets are spent mostly with inactivity, with eating, drinking, and exploratory behavior accounting for only 0.4% of their total time budget in a day.2 Ferrets were domesticated for their hunting prowess to control vermin and ability to tunnel narrow holes to ferret out prey. They demonstrate similar unique behaviors to their weasel counterparts. While most of them are undesirable, such as hissing, screaming, biting, and attacking, there is one, the Weasel War Dance, also known as the Dance of Joy, that, when seen, is positively correlated to a ferret that is thoroughly enjoying themselves. Members of the weasel subfamily hunt in packs, and this behavior is thought to be seen when they are celebrating triumph and victory after
a hunt. To satisfy their natural behavior as tunnel dwellers, we sought to build a custom complex enrichment device (Figure 1) that would provide them with opportunities to display their natural behavior and enjoyment of tubes and tunnels (Figures 2 and 3). Ferrets have binocular vision with horizontally slit pupils common in species that chase prey with gaits characterized by a hopping motion. This biology could explain their fascination with balls,3 and we thought this would aid in increasing exploratory behavior. Ball pits are a common enrichment for young humans, and it was shown to increase a positive emotional state in rats.1
All ferrets that experienced time in this novel enrichment device were on a St. Jude Children’s Research Hospital IACUC-approved study where enrichment would not interfere with study parameters. No ferrets experienced pain or distress. Ferrets on campus are socially housed in 3 tier racks (Allentown, Allentown, New Jersey) with 6 banks on a 12:12h light-dark cycle with ad libitum food (Purina Lab Diet SL14 High Density Ferret Diet, St. Louis, MO) and water in an AAALACi- accredited facility.

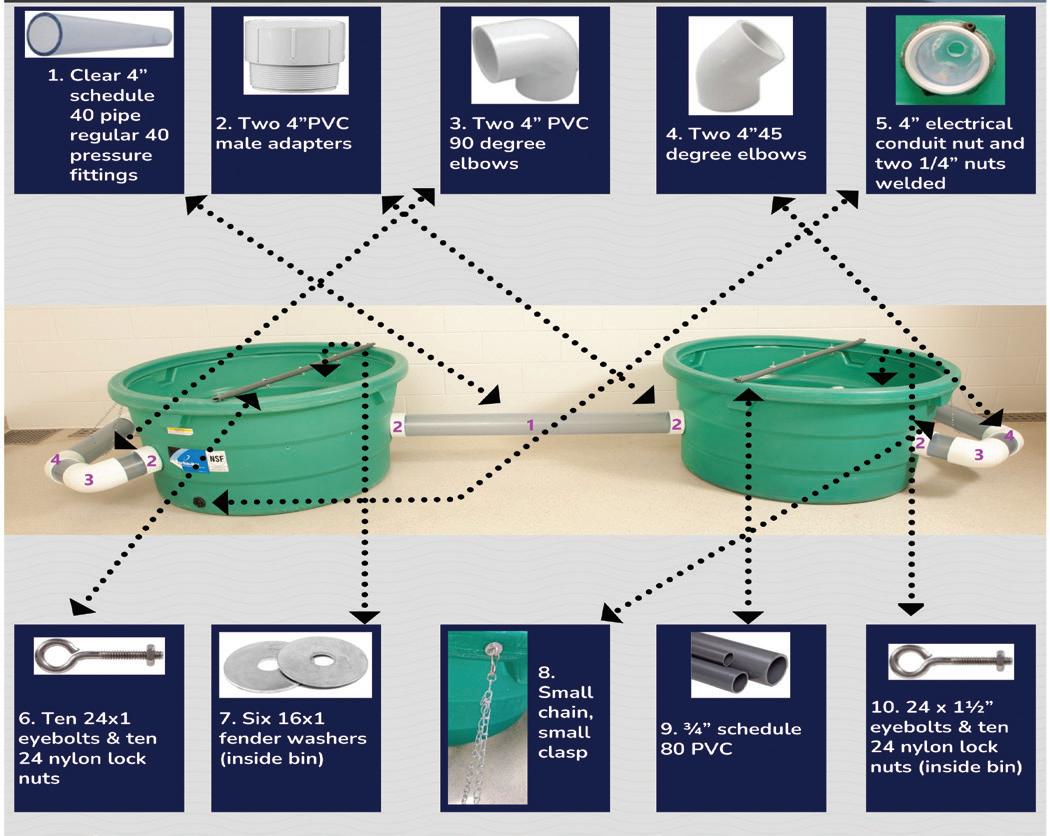
The ball pit was built using two 120-gallon stock tanks (Plastic-Mart, Hoppers, IA) joined together by clear 4” schedule 40 PVC pipes (Ferguson Plumbing Supply, Memphis, TN). Each of the stock tanks (Figure 3) had, two of the following: 4” PVC male adapters, 4” PVC ninety-degree elbows, and 4” forty-five-degree elbows (Spears PVC Pipe Supplies, Olive Branch MS) securing them together as one unit. A 4” electrical conduit nut (Industrial Sales Co., Bartlett, TN) was welded with two ¼” nuts to screw in two ¼” bolts for easy installation and removal when sanitizing the ball pit. To attach the tunnels to the main stock tanks (Figure 4), a total of ten each was used: 24x1 eyebolts, 24 nylon lock nuts, and 3/16x1 fender washers. A small chain and clasp were used to add support for the middle of the tunnel.

Stainless steel Kong toys on a 5 1/2” quick link chain was (Bio-Serv,3 Foster Lane, NJ) suspended over each stock tank with a PVC pipe. The bar going across the top was made with a ¾” schedule 80 PVC with ten each of the following: 24x1 1/2” eyebolts, 24 nylon lock nuts, and 3/16”x1” fender washers. This bar was secured to the stock tanks by drilling two holes with a diameter of 4 ½” and connecting them with male adapters and electrical nuts. The stock tanks were filled with non-crushable plastic balls (Little Tikes Balls for Kids® , Memphis, TN) to add environmental complexity. Sanitation of this equipment was accomplished by soaking the balls in a 10% bleach solution for a minimum 30-minute contact time. This hand-sanitation process was validated with PocketSwab® Plus ATP swabs (Charm Sciences, Lawrence, MA). The rest of the equipment was disassembled and sent through cage wash

but could be hand sanitized in a similar manner as the plastic balls.
Four male adult ferrets were tested to see if they preferred the stock tank with the balls or one without balls and more positive behaviors were seen in the ball pit, such as sniffing (Figure 5), exploratory behavior (Figure 6) prey capture, and the Weasel War Dance (Figure 7) as compared to a stock tank without balls. This innovative ball pit was constructed with low-cost plumbing supplies and served to elicit positive behaviors not seen in the home cage of ferrets. More work is needed to better understand what other types of enrichment would encourage more of these affiliative behaviors.




Special thanks to Biomedical Engineer, Bradley Lindley in Facility Operations, who developed the design with us to ensure safety for our animals. Much appreciation to Biomedical Communications photographer, Ann M. Hedges for the excellent photos of ferrets in action. Thank you, Felecia Moore, Animal Services Supervisor, for improving our diagram of the ball pit. We are grateful to you for your skills and endless energy.
Nicole Madera is a Veterinary Technologist II in the Animal Resources Center at St. Jude Children’s Research Hospital in Memphis, Tennessee.
Donnie Adams is an Animal Health Technician I in the Animal Resources Center at St. Jude Children’s Research Hospital in Memphis, Tennessee.
Shonn Offord is a Husbandry Floor Supervisor in the Animal Resources Center at St. Jude Children’s Research Hospital in Memphis, Tennessee.
Amy Funk, DVM, DACLAM is a Staff Veterinarian in the Animal Resources Center at St. Jude Children’s Research Hospital in Memphis, Tennessee.
1. Hinchcliffe, JK, MG Jackson, and ES Robinson. 2022. The use of ball pits and playpens in laboratory Lister Hooded male rats induces ultrasonic vocalisations indicating a more positive affective state and can reduce the welfare impacts of aversive procedures. Lab Anim, 2022: p. 236772211065920.
2. Larrat, S and N Summa. 2021. Ferret Behavior Medicine. Vet Clin North Am Exot Anim Pract, 2021. 24(1): p. 37-51.
3. Talbot, S, R Freire, and S Wassens. 2021. Effect of captivity and management on behaviour of the domestic ferret (Mustela putorius furo). Applied Animal Behaviour Science, 2014. 151: p. 94-101.

Sheep are commonly used in research for bioimplants, wound healing, orthopedic, and cardiology research. The Abigail Wexner Research Institute at Nationwide Children's Hospital (NCH) Animal Resources Core (ARC) sheep are used primarily for cardiac models and hosts for vascular grafts. The Institutional Animal Care and Use Committee (IACUC) reviews all procedures and is in full accordance with the Animal Welfare Act and the Health Research Extension Act to ensure the humane treatment of animal subjects in research.
• Ketamine hydrochloride injection; Hospira, Inc. Lake Forest, Il
• Diazepam; Hospira, Inc. Lake Forest, Il
• Butorphanol tartrate injection, Torbugesic; Zoetis Parsippany, NJ
• Laryngoscope; Miller Blade #4, 180 mm; Welch Allyn, Milwaukee, WI
• BAAM, Beck Airway Airflow Monitor; Great Plains Ballistics Inc.
• Gentle-Lock™ Oral Endotracheal Tube Holder, 81802; Teleflex In. Morrisville, NC
• iStat 1 Point of care blood gas analyzer; Abbott Laboratories: Abbott Park, Illinois, U.S.
When working with large species, a system of stretchers and transport beds are utilized to get sedated or recovering animals from housing to procedures or operating rooms. This aligns with ARC goals for staff safety and ergonomics.
Due to the unique hemodynamics of the sheep cardiac models, and their numerous post-surgical sedations for imaging, NCH uses primarily an induction cocktail of Ketamine (4mg/kg), Diazepam (0.5mg/kg), and Butorphanol (0.1mg/kg) as the intravenous cocktail of choice for rapid sedation and intubation.1
Endotracheal intubation is performed in the housing room aisle or anteroom space immediately post injection. Animals are manually restrained until relaxation, then rolled onto a fabric stretcher and moved to a raised bed for intubation and transport. Supplies used for endotracheal intubation include a laryngoscope with #4 blade, cetacaine spray, an appropriately sized intubation tube, Beck Airway Airflow Monitor (BAAM), and intubation tube holder/bite guard device [Fig 1.0]. These items help
to quickly determine correct placement of the endotracheal tube and secure the endotracheal tube for protection from mild chewing until transported to a procedure room with additional inhalant anesthetics available [Fig2.0]. These items all fit into a small caddy which is very easily transported for intubation in a variety of
spaces and situations. Most patients require suction after intubating and before connecting an anesthesia circuit to ensure the tubing is clear of secretions. Surgical preparation rooms are outfitted with a wall mounted vacuum system and a suction cannister with French suction tubing is available for ruminants due to the abundant secretions produced.
Small ruminants undergoing anesthesia will be fasted for a minimum of 12 hours prior to procedure start time. Once intubated, the animals will have an esophageal gastric tube placed to help with gas expulsion and gastric drainage during all sedated procedures. Typical set-up of gastric tubes involves utilizing a large, clear PVC gavage tubing with rounded tip to prevent trauma during placement and a urine drainage bag with the tip cut off to allow attachment to the gastric tube’s open end [Fig3.0]. The urine drainage bag has multiple benefits including cleanliness, ability to save ruminal fluid, release valve for fluid or gas, and monitoring of total output. Gastric tube placement allows the technicians to keep animals from bloating and provides access for manual expression of gas build up that could impact surgeons. For complicated procedures or anticipated post-operative complications, the rumen fluid will be preserved and administered back to the animal at the conclusion of the procedure. Ruminal fluids can also be used for transfaunation of sheep in intensive care when sedated animals do not require their fluid back. In such cases, the fluid bag is clamped before removal from donor animal to reduce oxygen exposure2 and subsequently saved for up to three days in refrigeration.3 The urine drainage bag makes it possible to maintain fluids with minimal oxidation and dead space for preservation, making it easy to take the bag off and drain it back into the gastric tube using gravity. The
tube is occluded and removed from the patient prior to extubation of endotracheal tube.

Complex surgeries require additional monitoring such as catheterization of auricular artery for invasive blood pressure readings and non-invasive blood pressure cuff placed over the radial artery. Electrocardiogram adhesives dots are placed on the back of each leg between the heel and dew claws (area shaved for contact) and then secured with tape for continuous ECG monitoring during surgery. Other parameters being monitored include SPO2, EtCO2, ventilation parameters, temperature, heart rate, tympany, and in some cases blood gases. Blood gases are used to confirm oxygen saturations and acid-base disturbances. Protocols that require clamping of a major vessel will also require blood gas and injection of countermeasure when unclamping due to the sudden release of built-up blood waste from the clamped vessel.
Emergency drug lists are made available in surgery areas and on emergency drug carts. The list includes drugs for cardiac interventions, reversals for induction drugs, emergency dose ranges, and drug concentrations for the dosages listed. Some drugs also contain a quick reference such as “1mL/10lbs”- and all have columns designating what each drug can be used to treat. Surgical monitoring records include a set list of parameters filled in prior to induction for all animals including tidal volume, oxygen flow rate, rebreathing bag size, ranges for anesthetics used, and fluid rates (all calculated using most recent weight).
Following a procedure, animals are monitored in the operating room until spontaneous respirations occur before attempting transport to recovery. Intubation tubes are left in until animals begin to chew actively, at which time the partially inflated intubation tube is removed-

the choice keep the cuff partially inflated is due to the secretions common in this species and to help clear the airway on the way out. In recovery runs, a portable ventilator and anesthesia machine with monitoring is available for recovering after invasive procedures. Recovery runs are fitted with rubber floor mats for better footing, and a V-shaped foam support to keep animals sternal and reduce the strain on technicians supporting the animals. The foam support also allows technicians to keep the head angled slightly downward to drain any secretions in recovery should the need arise. Animals are recovered until ambulatory- often with the encouragement of grain upon standing.
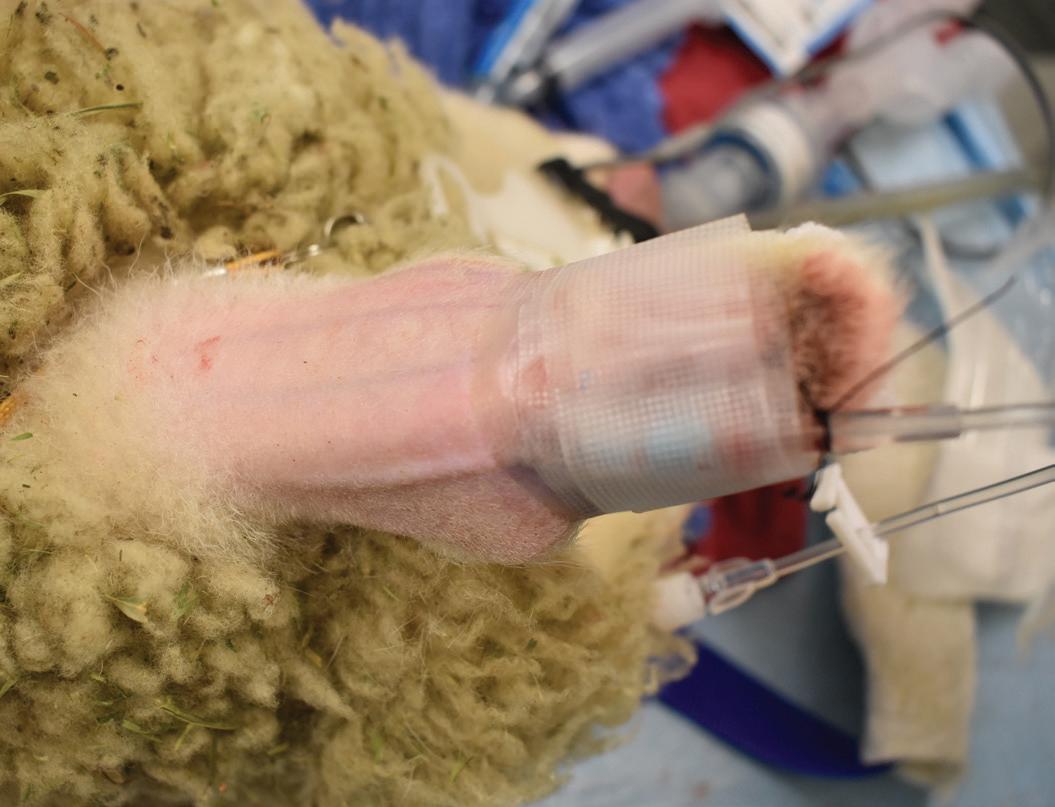

ARC’s main objectives are high quality research, safety, and welfare. These objectives are reached through a collaborative effort with research staff to understand the demands of their procedures; planning in the form of emergency drugs, route of sedated animal to surgery, etc; and ergonomic supports such as stretchers, inflation lift beds, hospital beds, and other forms of wheeled and hydraulic tables to allow animals to be lifted minimally during sedation, prep, and transfer to operation and recovery. In this way, ARC has been able to reduce the risk to staff and animals alike while performing small ruminant procedures regularly and meeting the needs of cardiovascular research.
Ashley Klinger, B.S., RLATG, Veterinary Technical Specialist in the Animal Resources Core at Nationwide Children’s Hospital- Abigail Wexner Research Institute, Columbus, Ohio.
Carmen Arsuaga- Zorrilla, DVM, M.S., DACLAM, Senior Clinical Veterinarian in the Animal Resources Core at Nationwide Children’s Hospital- Abigail Wexner Research Institute, Columbus, Ohio.
1. Allen Richard C, Becker Charleen, Morgan Paula. 2000. Formulary Colorado State University Teaching Hospital. Fort Collins (CO): Colorado State University
2. DePeters EJ, George LW. 2014. Rumen transfaunation, Immunology Letters, 162:2. Cited 30 August 2022.
3. Fabro Carla, Sarnata Chiara, Spanghero Mauro. 2020. Impacts of rumen fluid, refrigerated or reconstituted from a refrigerated pellet, on gas production measured at 24h of fermentation. Animal Feed Science and Technology, Volume 268. Cited 30 August 2022.
The North American 3Rs Collaborative’s (NA3RsC) is a non-profit whose mission is to improve the lives of research animals, maximize good data in experiments with animals, and replace animals with alternative technologies when possible. One of our replacement focused initiatives is on microphysiological systems (MPS). Our MPS Initiative seeks to advance MPS technologies' wide adoption and regulatory acceptance. It is unique in the field as it combines most developers with commercially available MPS. It is also clearly situated in an organization committed to advancing all 3Rs (refinement, reduction, replacement) and recognizes animal research's current value and necessity. Our initiative has four main aims:
• Interfacing with end-users by coordinating webinars, workshops, and discussions.
• Promoting regulatory acceptance through discussion, consensus building, and communications.
• Creating, maintaining, and improving a user-friendly technology expo where end-users can see all commercial developers in one place and sort via filters (na3rsc.org/mps-tech-hub).
• Develop regular, widespread educational resources for all stakeholders.
MPS is a rapidly evolving technology that facilitates crucial scientific research by mimicking organ function, cellular metabolism, and/or cellular dynamics. These 3D tissue cultures can be created from animal or human cells. They often incorporate extracellular matrix (ECM) and stromal components. In some cases, MPS can live for up to three months in culture. Their composition allows cell-cell and cell-ECM communication and interactions, simulating the in vivo behavior of many different organ types.6,8,9 They can also model both healthy and disease states. Approximately 18 organs/organ systems are commercially available, including liver, CNS and neuro, kidney, gut, tumor, lung, etc.
The complex nature of MPS can lead to some advantages over traditional in vitro or in vivo models, including improved physiological relevance and complexity, control, and reproducibility.3
Compared to traditional 2D cell cultures, MPS can more closely mimic organ morphology and function over an extended period (up to several months for some models); this allows researchers to examine changes in tissue structure and function in response to compounds.4,5 They can even simulate the drug administration routes patients use in the clinic – for example, oral versus intravenous versus topical – which facilitates better studies of drug stability and activation.2 Additionally, some MPS can incorporate aspects of the human immune system better than certain animal models, which are particularly important for investigating immune-oncology agents and cellular therapies,1 inflammation, and immune-related diseases (Type 1 diabetes, etc.). Additionally, since MPS use cells isolated directly from human tissue, their gene expression is generally more similar to human disease states
than some animal models that incorporate human cells; this can result in experimental results that may more closely mimic human patient results.10 MPS can also be linked in multi-organ systems to replicate organ-organ interactions and more systemic in vitro models. Due to these factors, in some cases, MPS may be better predictors of human effects that may not be apparent in some animal models.
MPS can be extremely beneficial in the early stages of drug discovery and basic research before starting expensive and time-consuming animal studies and clinical trials. Using MPS early can allow a company to determine which drugs may behave differently in animal models versus human patients. In turn, this can affect the number of animal studies needed before filing regulatory materials and proceeding to clinical trials. In particular, if early MPS trials show that a drug is likely to fail in later clinical trials for human safety or efficacy reasons, then the decision can be made to not proceed to animal trials therefore reducing the number of animals needed to develop an effective therapeutic. Scalable MPS can also support large scale batch testing and can provide answers more quickly.7,11
As with all model systems, MPS also has some disadvantages. First, they typically only recapitulate some features of an organ or a limited number of organs. Therefore, they may not always be predictive of whole-body drug reactions. Second, MPS are housed in incubators and have no variable external conditions. Thus, these systems are not shaped by external stimuli in the same way that whole animal models or humans are. That is, animals and humans are affected by external conditions such as their housing, interactions with other individuals, nutrition, and many other factors. Although these factors in animal models can cause variability, they are more representative of a human patient population. Third, while they are often less expensive than animal studies, they are more costly than traditional cell cultures and require specialized equipment and/or expertise. Finally, there are certain conditions that MPS cannot mimic, such as whole body behavioral responses, large bone fractures, or mental health conditions. For example, although an MPS could be used in safety testing for an antidepressant, it cannot be used to determine whether a drug can help resolve depression.
Due to the advantages of MPS systems and the extensive work performed by developers to characterize them, they are becoming more widely accepted by regulatory agencies for developing treatments for patients. At the time of this writing, data from MPS have been used to support 4 applications to the FDA for Investigational New Drugs (IND), applications necessary before initiating clinical trials. Furthermore, MPS data was used exclusively by Vertex to expand the label of a previously FDA-approved drug for cystic fibrosis.
In conclusion, microphysiological systems can mimic either human or animal systems in terms of physiology, toxicity, disease states, and efficacy. They can be practical to use, saving companies
time and money in the long run. Ultimately, MPS can be used to refine, reduce, and replace some aspects of animal research while still ensuring patient health and safety.
Monica Badea is a student at Kutztown University in Kutztown, PA.
Sally Thompson-Iritani (she/her), DVM/PhD, CPIA, CCFP, is the Asst. Vice Provost, Animal Care, Outreach & 3Rs, Clinical Associate Professor, DEOHS, SPH, at the University of Washington.
Tessa DesRochers is the Chief Scientific Officer at KIYATEC, Inc in Greenville, SC.
Megan LaFollette, MS, PhD, is the Program Director of The North American 3Rs Collaborative in Denver, CO.
1. Appleton KM, Elrod AK, Lassahn KA, Shuford S, Holmes LM, and DesRochers TM. (2021) PD-1/PD-L1 checkpoint inhibitors in combination with olaparib display antitumor activity in ovarian cancer patient-derived three-dimensional spheroid cultures. Cancer Immunol Immunother 70:843-856.
2. Azizgolshani H, Coppeta JR, Vedula EM, Marr EE, Cain BP, Luu RJ, Lech MP, Kann SH, Mulhern TJ, Tandon V, Tan K, Haroutunian NJ, Keegan P, Rogers M, Gard AL, Baldwin KB, de Souza JC, Hoefler BC, Bale SS, Kratchman LB, Zorn A, Patterson A, Kim ES, Petrie TA, Wiellette EL, Williams C, Isenberg BC, and Charest JL. (2021) High-throughput organ-on-chip platform with integrated programmable fluid flow and real-time sensing for complex tissue models in drug development workflows. Lab Chip 21:1454-1474.
3. Hoarau-Véchot J, Rafii A, Touboul C, Pasquier J. (2018). Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions?. International journal of molecular sciences, 19(1), 181.
4. Horowitz LF, Rodriguez AD, Ray T, Folch A. (2020). Microfluidics for interrogating live intact tissues. Microsyst Nanoeng 6, 69.
5. Kwak T, Wang F, Deng H, Condamine T, Kumar V, Perego M, Kossenkov A, Montaner LJ, Xu X, Xu W, Sheng C, Schuchter LM, Amaravadi RK, Mitchell TC, Karakousis GC, Mulligan C, Nam B, Masters G, Nockstein N, Bennet J, Jefedova Y, Gabrilovich DI. (2020). Distinct populations of immune-suppressive macrophages differentiate from monocytic myeloid-derived suppressor cells in cancer. Cell reports, 33(13), 108571.
6. Li AP, Bode C, and Sakai Y (2004) A novel in vitro system, the integrated discrete multiple organ cell culture (IdMOC) system, for the evaluation of human drug toxicity: comparative cytotoxicity of tamoxifen towards normal human cells from five major organs and MCF-7 adenocarcinoma breast cancer cells. Chem Biol Interact 150:129-136.
7. Marx U, Akabane T, Andersson TB, Baker E, Beilmann M, Beken S, Brendler-Schwaab S, Cirit M, David R, Dehne E-M, et al. 2020. Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. ALTEX 37:365–394.
8. Miller PG and Shuler ML (2016) Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol Bioeng 113:2213-2227.
9. Prantil-Baun R, Novak R, Das D, Somayaji MR, Przekwas A, and Ingber DE (2018) Physiologically Based Pharmacokinetic and Pharmacodynamic Analysis Enabled by Microfluidically Linked Organs-on-Chips. Annu Rev Pharmacol Toxicol 58:3764.
10. Ridky TW, Chow JM, Wong DJ, Khavari PA. (2010). Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nature medicine, 16(12), 1450–1455.
11. Roth A & MPS-WS Berlin 2019 (2021). Human microphysiological systems for drug development. Science (New York, N.Y.), 373(6561), 1304–1306. https://doi.org/10.1126/science.abc3734
In our gnotobiotic facility, we routinely perform size match procedures, which require multiple cages to be open in the biosafety cabinet simultaneously. In this situation, it is a challenge to get an adequate grip on wire bars with one hand while keeping cages in place. These procedures are completed in a sterile setting, requiring surgical gloves constantly covered in chemical sterilant, which can add to the challenges of manipulating animals and caging.

Size matching is the reorganization of mice based on average tumor size and standard deviation. It is vital for oncology studies by reducing the experimental variability that comes with tumor growth. Generally, size matching requires multiple cages opened in quick succession. With Allentown Sealed Positive Pressure (SPP) caging, it is challenging to lift the wire bar due to the unique sealing of the cage to maintain the microenvironment.
We utilize spay hooks to facilitate a more efficient way of opening the wire bar while working in a biosafety cabinet. A spay hook (Figure 1) is stainless steel and may already be used in laboratory animal facilities, making it an easily obtainable option. We use Spay Snook Hooks for their softer, thicker curves, but other options could be considered.
Within the sterile biosafety cabinet, cages are set perpendicular to the work surface, and each cage is opened (Figure 2). Lids can either be slid back approximately two inches or set aside if proper airflow is maintained in the BSC. Water bottles are left empty in the clean cages until the size match event is complete. This improves ergonomics and efficiency by minimizing the total weight of the wire bar and preventing water from dripping into the cages. An eight-inch spay hook is inserted through each wire bar and rests on the bottom of the cage. One hand lifts the spay hook, hooking the wire bar and lifting

Have you ever found yourself fumbling with chemical sterilant-covered hands in a biosafety cabinet (BSC) while attempting to maneuver a wire bar lid to perform a multi-cage procedure? Wish you had another hand or two?Figure 1: Eight inch Spay Snook Hook used in Sealed Positive Pressure during a size match event. Figure 2: Sealed Positive Pressure cages staged on a biosafety cabinet with Spay Snook Hooks through the wirebars.
just enough to get the mouse inside the cage (Figure 3). The wire bar is then lowered closed for the next mouse to be sorted, leaving the spay hook in place in the wire bar. After all, mice have reorganized, the spay hook is removed from the wire bar, and the cage lid is placed and secured.
Using this method, up to six SPP cages will fit inside a 6-foot BSC simultaneously, each with its own spay hook. One person can efficiently handle restraining mice, tumor measurements, and placing the mice into their new caging groups. The spay hook is autoclavable, fits the hand, reduces touch points, and is reasonably sized for the task. After use within a cage, the spay hook can be wiped and cleaned with many facility approved disinfectants and can be placed inside a clean cage or in an autoclave pouch for autoclave sterilization for next use.

It has been demonstrated during our procedures that the spay hook tool is a viable option to manipulate wire bars quickly and efficiently to support tumor size matching, as well as other manipulations such as dosing or weaning. Animal studies were approved by AbbVie's Institutional Animal Care and Use Committee or Ethics Committee. Animal studies were conducted in an AAALAC-accredited program where veterinary care and oversight were provided to ensure appropriate animal care.
Andrea Kachellek, BS, RLATG, is a Senior Supervisor at Abbvie in Chicago, IL.
Nicole Martinec, BS, RLATG, is a Lead Animal Care Technician at Abbvie in Chicago, IL. She is currently serving as the Chicago AALAS branch President-Elect.

The last several years have brought about a lot of changes because of the pandemic on staffing, remote work, and changes in priorities.
Will your institution be asking these questions in 2023?
• Have salaries at your institution gone up, stayed the same, or decreased in the last 2 years?
• What is the trend in the laboratory animal community?
One way to find out is to get data to compare your salaries and benefits to other institutions using the AALAS Laboratory Animal Facility Compensation Survey.

Since 1997, AALAS has conducted 11 Surveys. This survey is an important service provided to allow your organization to easily compare your compensation levels and benefits with your peers.
Question: How can AALAS provide the best data for your organization?
Answer: With your institution’s company’s participation!
As with any research, data is the key. We need your organization’s participation to ensure accurate information for all positions.
The study highlights compensation levels and organizational practices for 18 laboratory animal facility-related positions throughout the United States. These positions include director, assistant/associate director, manager, administrative/business manager, supervisor, clinical veterinarian, facility compliance manager, IACUC coordinator, training coordinator, research technician, animal health care technician, senior-level laboratory animal technician, mid-level laboratory animal technician, entry-level laboratory animal technician, shipping coordinator, purchasing coordinator, administrative support assistant, and cage washer.
What information is included in the final report?
The information is displayed in two sections. First, the institution, laboratory animal facility, benefits information, salary administration, and technician certification data are summarized.
Then the personnel compensation information is reported for all respondents, as well as segregated by geographic region, type of institution, number of employees at the institution, and number of employees in the department.

The survey will once again be conducted by an independent company, Industry Insights, Inc., to ensure the confidentiality of your data.
Survey invitations will be sent to AALAS member institutions in January. If you would like more information about your institution participating, please email info@aalas.org.
Please encourage your facility management or human resources department to participate in the 2023 AALAS Compensation Survey!
The GLAS (Grants for Laboratory Animal Science) mission is to enhance scientific knowledge in laboratory animal health and welfare through research and to promote collaborative efforts by the AALAS membership within the broader scientific community. The GLAS program aims to advance responsible laboratory animal care and use to benefit people and animals in such areas as environmental conditions, housing and enrichment, pain and distress, health and welfare, euthanasia, and animal care and use. Since the first grants were awarded in 2007, the GLAS program has awarded 92 grants totaling $1,743,310. As a result of the GLAS program, a total of 384 cited presentations, publications, and posters stemming from GLAS-sponsored projects have been produced and are listed on the AALAS website. Through these outcomes, AALAS has had a visible role in advancing research and knowledge in laboratory animal science.
The GLAS program provides two types of research grants: the standard grant for up to $50,000 for studies with a sound hypothesis and preliminary data; and the small grant for up to $7,500 for innovative or pilot studies. The GLAS program requires that the principal investigator be an AALAS member at any level, but it is not restricted geographically.
Application forms and instructions will be available online starting December 1! Applications are due on February 1.
To optimize your chances for a GLAS award, watch the recordings of the AALAS Scientific Advisory Committee’s perspectives and suggestions to prospective GLAS candidates on how to craft a successful GLAS application. The recordings are the panel discussion and Q&A session from the 2020 National Meeting. You may also want to view the online GLAS Tutorial for details on preparing your application.
To access the application form and these informational resources, visit www.aalas.org/glas.
If you have a proposal to improve laboratory animal health and welfare in research, we hope you will submit your idea in a GLAS application!
The Texas Branch of AALAS was excited to hold its 59th Annual Conference on August 10-12, 2022. The event was held at the Marriot Hotel in Sugarland, TX, and the theme of the conference was “Texas Strong – Experts in Emergency Response.” This was an exciting event for the branch, as it was the first in-person event that has been held since the pandemic. TBAALAS hosted over 200 attendees and provided some great talks, posters and other fun activities. There were a wide variety of topics covered in the talks and posters, including topics in the areas of husbandry, training, veterinary care, staffing, employee and animal enrichment, and of course some talks surrounding our theme of emergency and disaster response. We were very happy to have Tecniplast sponsor, Dr. Doug Brining, as our Keynote Speaker, gave a great talk on disaster response at UTMB during the pandemic. Marc Hulin, National AALAS President was our guest for the conference, and we appreciated him making the trip to Texas, as well as participating in the program and being our MC for our awards banquet. A huge thank you, Marc!

Activities in addition to the poster/presentation program included a wet lab on rodent palpation, a leadership workshop
on emotional intelligence, and some off-site activities including a tour at Brazos Bend State Park and a behind-the-scenes tour of the primate area at the Houston Zoo that our attendees could participate in. A new and fun addition this year was a human Hungry Hungry Hippos tournament – which provided some extra fun and excitement for both the participants and the spectators!
The Helen Jordan Memorial Vendor Hall welcomed over 50 vendors, and attendees had lots of time in the hall to spend getting to know the vendors and their products. The vendor hall was a hopping place, also hosting a happy hour with the vendors and a social event fiesta to welcome the attendees. A Past-Presidents Reception welcomed back many of the previous TBAALAS Presidents and they were recognized during the social event. A silent auction was held in the vendor hall that raised money to support our Technician Scholarship Award for next year to be able to send a deserving individual to National AALAS for the first time. This year’s Technician Scholarship winner is Delali Miwodor (UT Southwestern Medical Center), and she will be attending the national meeting in Louisville this year!


At the awards banquet and presentation, we recognized many winners of technician recognition awards and presentation awards. Award winners Included:

Presentation award winners (sponsored by Animal Care Systems, Alternative Design, Envigo, LGL Animal Care Products, and Quip Laboratories):
• Morgan Fields (UT Southwestern Medical Center)
• Julie Roller (UT Southwestern Medical Center)
• Katherine Head (UT Southwestern Medical Center)
• Manuel Aguilar (Texas Biomedical Research Institute)
• Lindsey Edwards (UT Health Science Center at Houston)
• Level I Rookie Award - Georgia “Ann Dee” McVicker (Texas Tech University)
• Level II Non-Supervisor Award - Cindy Wang (MD Anderson Cancer Center)
• Level III Supervisor Award - Christa Harper (UT Southwestern Medical Center)
• Allentown Manager of the Year Award - Morgan Fields (UT Southwestern Medical Center)
• Charles River Outstanding Clinical Veterinarian Award - Dr. Christopher Jannsen (UT Health Science Center at Houston)
• A-Tune (a Transnetyx Company) Advancing Technology to Support Research Award - Chetna Patel (MD Anderson Cancer Center)
• Hungry Hungry Hippos Tournament Winners – Fred Rock (Avidity Science), Mitch Moore (Allentown), and Bill McDonald (Ancare).
The group also congratulated some new lifetime members for TBAALAS.

• Dr. Ken Gray
• Dale Weiss
• Lane Watkins
• Valeri Lansford
• Pat Sikes
These folks were also recognized for their retirement since the start of the pandemic, and this list also included John Donaho, who was specially recognized by Cordelia Rasa for his help as TBAALAS Webmaster for many years. The President’s Recognition Award was given to Sheri Brodie by Julie Roller for her great support during Julie’s presidency. The gavel was passed on from the outgoing President, Julie Roller to the incoming President, Mona Jaffari, and we celebrated the work done by both the Board and the Program Committee over the past 2 years.
This was a great program, and it was wonderful to get to spend time with old friends, as well as get to meet some new faces! Thank you to the Program Committee for all their hard work in planning and executing this great event and thank all the attendees and vendors for making our meeting a great success! We have already started planning our next conference, to be held in February 2024. For more information about TBAALAS, please visit our website at www.TBAALAS.net.
3 Where are the testes located in the male bird? 6 Floor housing is appropriate for what type of birds used in research? 7 What is a male turkey called? 8 What are the largest muscles in the birds body? 10 What is a common method of identifying individual birds? 11 Removal of the distal tip of a bird’s wing is called 14 What item is a necessary component of all bird diets? 15 A distinctive fea ture of female birds is that only the left is functional 17 What gland produces an oily substance that is used when a bird preens its feathers to make them waterproof? 18 What order do parrots belong to? 19 The body temperature in birds is than most mammals
What is the involuntary bristling of fur or feathers called? 23 Due to their robust immune system, the yolk of which bird’s eggs are used for antibody development?
DOWN 1 What are special heated cages used for young chicks called? 2 A nail that is not associated with a toe and is used for fighting in male Galliformes is called a(n) 4 A featherless is a breeding season sex indicator in birds 5 Social hierarchy or dominance of birds is known as a “ ” 6 What helps birds cool themselves when they get too hot? 9 What bird’s song is studied to understand learning, memory, and language development? 12 What condition can result if a sparrow is held too tightly around the thorax? 13 The process of shedding old, worn feathers for new plumage is called what?
Birds are in the taxonomic class of 20 Kidney and toxicity is a potential side effect of NSAIDs in birds
control cannibalism and fighting in chickens, the of th e upper beak is sometimes removed
Glad you asked!
These are mandatory forms we have been requested to include in the magazine in compliance with the United States Postal Service. Thanks!



 American Association for Laboratory Animal Science 9190 Crestwyn Hills Dr, Memphis, TN 38125
American Association for Laboratory Animal Science 9190 Crestwyn Hills Dr, Memphis, TN 38125

The AALAS Foundation is excited to announce the launch of its Ann Turner CARE Academy – a free public outreach training program designed to train and prepare AALAS members to be more successful and confident in their public outreach efforts.
Registration is now open to attend this ground-breaking public outreach initiative- in honor of Dr. Ann Turner who has long been the face of the AALAS and laboratory animal science community.
Attendance is limited to the first twenty-five (25) AALAS members who register to attend this biennial program to be held March 9th – March 10th, 2023, at the AALAS National Headquarters in Memphis, Tennessee.
Attendees must pay their own travel expenses to and from Memphis. The AALAS Foundation will cover the following expenses:
• One (1) night hotel room for each attendee (breakfast provided by hotel)
• Shuttle expenses to/from hotel/airport and to AALAS Offices
• Dinner provided on the evening of Training Academy Day 1
• Lunch provided on Training Academy Day 2
Attendees of this split day training program will learn to feel more confident and comfortable utilizing AALAS Foundation public outreach resource materials, and responding to tough questions, typically asked when speaking to the public about the important role of animals in biomedical research.
Training will be conducted by a professional trained in public speaking. Topics will include overcoming the fear of speaking in public about laboratory animal science, how to successfully present the AALAS Foundation’s “What’s Hap-
pening in Research?” fully scripted, Power Point presentations, how to answer “tough” questions regarding the use of animals in research, and much more!
Why should you or your employees attend this important training? The future of Laboratory Animal Science is greatly dependent on the public’s view and opinion of animals in biomedical research – and a strong future work force is dependent on the effective education of young students about career opportunities available in laboratory animal science.
The Ann Turner CARE Academy hopes to offer a solution to help change public opinion and educate students about the important role of animals in research and about the vast array of amazing career opportunities in the field of lab animal science.
We look forward to working with our 2023 academy attendees and celebrating the successful completion of the first graduates of the Ann Turner CARE Academy!
Register to enroll in the Ann Turner CARE Academy today at the link below or email vicki.campbell@aalas.org for more information: https://tinyurl.com/CARE-ACADEMY
The AALAS Foundation is also seeking corporate sponsors to ensure the continued success of this program. Email vicki. campbell@aalas.org for more information about sponsorship opportunities.
Allentown LLC www.allentowninc.com
Ancare Corp www.ancare.com
Avidity Science LLC www.avidityscience.com 31
Bio-Serv www.bio-serv.com 29
Braintree Scientific Inc. www.braintreesci.com 43
Clear H2O Inc www.clearh2o.com 3
Fidelis Pharmaceuticals www.ethiqaxr.com 34/35
Innovive www.innovive.com 21
Instech Laboratories Inc www.instechlabs.com 11
Kent Scientific Corporation www.kentscientific.com 32/33
Lomir Biomedical Inc www.lomir.com inside back cover
Onset Computer www.onsetcomp.com 47
PlasLabs Inc. www.plas-labs.com 38
PMI LabDiet www.labdiet.com
Priority One Services www.priorityoneservices.com 8
Virox Technologies www.peroxigard.com 1
VRL Laboratories-USA www.vrlsat.com 25
Contact



The moment that I saw Wally, I knew he was mine. My mother-in-law frequently fosters, and he was the newest addition to the group. After we left, I kept telling my husband stories about things Wally and I would do together once we adopted him, to which I got some pushback. Eventually, he listened to reason, and he surprised me by bringing Wally home in mid-December. He quickly became my best companion on neighborhood walks and couch cuddles, and he often frequents the AALAS office when he isn’t at daycare.
Wally loves everyone, which is fitting since everyone loves him back! He is goofy, kind, and very affectionate. He likes to use his paws as hands, encouraging you to give him more pets or scratches and pets you back. He is the silliest, goofiest, kindest dog I’ve ever met, and I feel insanely fortunate to call him my perfect son.


