









Consistency is where trust begins. LabDiet® products are proven, stable and most of all reliable. We ensure that you can safely and successfully conduct your studies without concerns over product quality or consistency.
We appreciate your TRUST in US.






Chair: Sarah Hansen
Vice Chair: Karen L. Lencioni
Seminars Chair: Fakhrid-deen S. Muhammad
Vice Chair: Tanya L Herzog
Special Topic Lectures Chair: Steven E. Davison
Vice Chair: Kristin D. Evans
Panel Discussions Chair: Crystal Howard Johnson
Vice Chair: Carissa P. Jones
Workshops Chair: Verda A. Davis
Vice Chair: Derek L. Fong
Facilitators Chair: Courtney Hunter
Vice Chair: Eden Paster
Local Arrangements Chair: Courtney Hunter
BOT Liaison, President, ex oficio: Robert H. Quinn
Abstract Review Subcommittee
Chair: Sharoll L’Italien
Platform Session: Karen R Strait
Poster Session: Karuna Patil
Sarah D. Alaniz, Kristin I. Barba, Kirsten Bell, Kaitlin Cahill, Guiseppe Dell’Anna, Lauren M. Habenicht, Stacey M. Meeker, Kathleen Patterson
Storves, Jaime White-James
EXHIBITOR ADVISORY COUNCIL
Chair: Michael Evans
Vice Chair: Janine Meluso
Past Chair: Perry Spires
Donna Monroe, Merryl D Cramer, Maria Michelina
Cariglia, Priscilla Shirley, Karen M. Froberg-Fejko
Past President: Pam Straeter
BOT Liaison: Kenneth B. Shapiro
EXECUTIVE DIRECTOR
Thomas L. Joseph














On behalf of the National Meeting Program Committee (NMPC), it is my great pleasure to welcome you to the 75th AALAS National Meeting in Music City. From the historic Grand Ole Opry and Ryman Auditorium to the talented new artists performing on Broadway, Nashville is beaming with history, creativity and opportunity. This vibrant setting is a perfect backdrop for our AALAS community, as we gather to celebrate 75 years of laboratory animal medicine. Steeped in the rich knowledge of our history and lessons learned, at this meeting and as always, we will share the most recent scientific advancements as we look forward to our exciting future.
This past spring, the dedicated NMPC convened,rose to the challenge and generated an educational, informative and valuable program for all attendees. Thanks to our tremendous laboratory animal community, submissions in all areas were innovative and compelling, including this year’s focus track on Neurodegenerative Disease. This broad category of diseases is extremely devastating for patients and their families, and carries a massive global financial impact. Bringing emphasis to this area of research serves as a reminder of the essential role that laboratory animal professionals play in the development of effective treatment and cures.
As you prepare for the meeting this year, please remember to vote absentee prior to packing your boots! Then head to Nashville for some great food and even better music - the next Taylor Swift could be playing in a small window front on Broadway right now. With sincere gratitude to our local arrangements committee led by Courtney Hunter, you will be well prepared to have a memorable experience appreciating all Nashville has to offer. Be sure to visit the local establishments, tip your servers and the musicians, enjoy this time reconnecting with colleagues, and hopefully make some new friends along the way. I can’t wait to see you all there!
Sincerely,
Sarah Hansen, DVM, MS, DACLAM Program Committee Chair

I sincerely hope you are as excited about the AALAS National Meeting as I am!
This is the 75th Anniversary of the National Meeting and we are planning a meeting worthy of this milestone. For one thing, it will be in Nashville where there is an endless supply of entertainment and activities. The Music City Center is beautiful, laid out for easy access to everything, and in very close proximity to many hotels. I have no doubt the Local Arrangements Committee, under the leadership of Dr. Courtney Hunter, will be able to provide a wide range of opportunities to take a break from the many educational sessions.
And speaking of education opportunities, the National Meeting has always been the premier source for cutting edge information for our field and this year is no exception. Dr. Sarah Hansen (Chair) and the many, many dedicated and thoughtful members of the National Meeting Program Committee have put together a program packed with so many education and training options than you will hard-pressed to decide where to spend your time.
As if you needed another reason to attend, the opening session will be one to not be missed. In celebration of our 75th anniversary, we are inviting all past presidents to return for what will be a once-in-a-lifetime gathering. On top of that, I have asked Dr. Bob Dysko, one of my first mentors and good friend, to give the key-note address providing a somewhat tongue-in-cheek view of the past 75 years of AALAS. I’m sure those of you who know Dr. Dysko will agree that we will all be entertained and likely learn some things about AALAS that we didn’t know.
Another first for the National Meeting is that we will be presenting the welcome reception within the exhibit hall on Sunday evening. This will give you an early opportunity to interact with the vendors that make this whole meeting possible. The ribbon cutting to open the exhibit hall will occur just after the opening session.
Speaking of the vendors, the Exhibitor Teach and Chat (ETC) was such a big hit last year so it is returning this year. This is an opportunity for vendors to give short presentations about their newest products and innovations. They were very well-attended in Salt Lake City.
I hope I have provided you with more than enough reasons to start working on getting yourself to Nashville. In all my travels this year I’ve been “promising” 5000 attendees at the 75th Anniversary National Meeting……mostly as a taunt to your Past President, Pam Straeter. Please don’t make me a liar. Actually this is all in good fun and no matter how many of you can make it, I’m sure you will not be disappointed. If I don’t see you before, I’ll see you in Nashville!

Robert (Dr. Bob) Quinn, DVM, DACLAM 2024 AALAS President
Looking for quick info on Nashville and the AALAS National Meeting? Here are some tips and links to help you plan for the meeting. Remember, the most current information will be loaded on the AALAS website: www.aalas.org/national-meeting, and in the mobile app once it is launched in early October.
AALAS strongly encourages you to be up-to-date with vaccinations before participating in the National Meeting. During the meeting, everyone will need to comply with CDC and the local Nashville County requirements/recommendations at are in place at that time.
Pre-registration fees are $360 for AALAS national members and $460 for nonmembers. AALAS will again offer up to 100 hours of session recordings. Adding this option at the time you register will give you the lowest rate option for the recordings of $125 for members and $200 for nonmembers. Note: You must be a current member at the time you register to get the membership discount.
Access the online registration portal here: https://www.aalas.org/national-meeting/general-information/registration
Descriptions for the 2024 workshops are available throughout this program or here: https://www.aalas.org/national-meeting/general-information/registration. Workshops may be purchased online during the registration process or on-site in Nashville.
Saturday, November 2, 2 – 6 p.m.
Sunday, November 3, 7:30 a.m. – 7 p.m.
Monday–Wednesday, November 4 – November 6, 7:30 a.m.– 5 p.m.
Thursday, November 7, 7:30 a.m. – noon
Looking for speaker tips, session instructions, and facilitator/moderator guidelines, and more? Visit here: https://www.aalas.org/national-meeting/submission/presenter-information
The National Meeting always needs facilitators. Access the facilitator sign-up form on the above website page. Help us make this the best meeting yet by being a volunteer!
A complete list of updated committee meetings and events will be added to www.aalas.org later this summer and will be included in the Attendee Hub and Mobile App, which are set to launch in late October.
Exhibit Hall set up will take place Saturday, November 2, 8:00 a.m. – 6:00 p.m. through Sunday, November 3, 7:30 a.m. – 4:00 p.m.
The Exhibit Hall Ribbon Cutting will take place on Sunday, November 3 at 6:30 p.m.
Exhbit Hall hours are:
Monday, November 4: 9:00 a.m. – 5:00 p.m.
Tuesday, November 5: 9:00 a.m. – 5:00 p.m.
Wednesday, November 6: 9:00 a.m. – 1:00 p.m.
The Exhbit Hall dismantling will take place Wednesday, November 6, from 1:00 p.m. to 10:00 p.m.


SATURDAY
NOVEMBER 2
Exhibit Hall Exhibitor Set-Up
8:00 AM-6:00 PM, CC, Exhibit Hall BC
First Aid
7:30 AM-6:00 PM, CC, Outside Exhibit Hall B Entrance near Grand Lobby
Mothers Room
2:00 PM-6:00 PM, CC, 2 locations: Level
100 hall before the 100 meeting rooms & Exhibit level - outside Hall D across from Fresh Pick Market
Exhibitor Registration
10:00 AM-6:00 PM, CC, Registration
Registration
2:00 PM-6:00 PM, CC, Registration
COMMITTEE MEETINGS
AALAS Executive Committee Meeting
3:00 PM-5:00 PM, Omni, Gibson Boardroom
AFFILIATE EVENTS
Allied Trade Association (ATA) Board Meeting
3:00 PM-5:00 PM, CC, 210
Allied Trade Association (ATA) New Product Showcase set-up
2:00 PM-6:00 PM, CC, Outside Exhibit Hall Entrance
SUNDAY
NOVEMBER 3
Career Center
Veterinarian Job Fair sponsored by ACLAM, APV and ASLAP (see mobile app for companies participating)
Career Center
8:00 AM-5:00 PM, CC, 208A Veterinarian Job Fair
2:00 PM - 4:00 PM
Exhibit Hall Exhibitor Set-Up
7:30 AM-6:30 PM, CC, Exhibit Hall BC
Exhibit Hall (opening ceremony w/ribbon cutting 6:30 p.m.)
6:30 PM-8:00 PM, CC, Exhibit Hall BC Entrance
First Aid
7:30 AM-8:00 PM, CC, Outside Exhibit Hall B Entrance near Grand Lobby
Mothers Room
7:30 AM-7:00 PM, CC, 2 locations: Level 100 hall before the 100 meeting rooms & Exhibit level - outside Hall D across from Fresh Pick Market
Registration
7:30 AM-7:00 PM, CC, Registration
Speaker Ready Room
12:00 PM-5:00 PM, CC, 203A
SPECIAL EVENTS
Opening General Session / General Membership Meeting
5:00 PM-6:30 PM, CC, Karl F Dean Ballroom A
Welcome Reception
6:30 PM-8:00 PM, CC, Exhibit Hall BC
MEETINGS & EVENTS
AALAS Ask Me Anything (EVERYTHING AALAS QR codes) including AALAS Learning Library, ACE Membership Community, CMAR, ILAM, Educational Products, Registry, Publications
6:30 PM-8:00 PM, CC, Exhibit Hall BC
AALAS Foundation Silent Auction & "In Tune with Research" Contest
9:00 AM-5:00 PM, CC, Across from Exhibit Hall C entrance
District 8 Council
2:00 PM-5:00 PM, CC, 210
Facilitators Meeting
3:00 PM-3:30 PM, CC, 107A
Facility Directors-Yale University
8:30 AM-1:00 PM, Omni, Legends Ballroom B
National Meeting Orientation (first-time attendees, new members, international attendees)
2:00 PM-3:00 PM, CC, Davidson Ballroom A1
Poster Sessions
6:30 PM-8:00 PM, CC, Inside Exhibit Hall
Poster Sessions set-up by presenting author
2:00 PM-5:00 PM, CC, Inside Exhibit Hall
Technical Trade Presentations
Track I: Advancing Research Through Innovation
1:00 PM-4:00 PM, CC, 202A
Technical Trade Presentations
Track II: Facility Enhancements to Maximize Performance
1:00 PM-4:00 PM, CC, 205A
Technician Fun Fair
1:00 PM-5:00 PM, CC, Outside Exhibit Hall Entrance
Tecniplast Welcome Breakfast
8:00 AM-11:00 AM, CC, Davidson Ballroom B
Educational Resources Committee
9:00 AM-11:00 AM, CC, 211 Nominations Committee
10:00 AM-11:30 AM, CC, 210
Online Learning Committee
12:30 PM-2:30 PM, CC, 211 Program Committee Walk Thru 4:00 PM-5:00 PM, CC, meet at Registration
Scientific Advisory Committee
3:00 PM-5:00 PM, CC, 211
AFFILIATE EVENTS
ACLAM Board of Directors
7:30 AM-4:00 PM, Omni, Mockingbird 4
ACLAM Exam Question Writing Committee
8:00 AM-5:00 PM, Omni, Mockingbird 1
ACLAM GRAC
2:00 PM-5:00 PM, Omni, Mockingbird 3
ACLAM Publications Committee
9:00 AM-12:00 PM, Omni, Old Hickory
Allied Trade Association (ATA) New Product Showcase
7:30 AM-7:00 PM, CC, Outside Exhibit Hall Entrance
ASLAP Board of Directors Meeting
7:30 AM-9:30 AM, Omni, Cumberland 4
ASLAP CE Seminar
10:30 AM-4:30 PM, Omni, Cumberland 5
LAMA Board Meeting
11:00 AM-5:00 PM, Omni, Cumberland 4
Vivarium Operational Excellence Network
8:00 AM-3:00 PM, Omni, Cumberland 3
MONDAY
NOVEMBER 4
Career Center
Veterinarian Job Fair sponsored
by ACLAM, APV and ASLAP (see mobile app for companies participating)
Career Center
8:00 AM-5:00 PM, CC, 208A
Veterinarian Job Fair
9:00 AM - 11:00 AM
2:00 PM - 4:00 PM
Exhibit Hall
9:00 AM-5:00 PM, CC, Exhibit Hall BC
First Aid
7:30 AM-6:15 PM, CC, Outside Exhibit Hall B Entrance near Grand Lobby
Mothers Room
7:30 AM-5:00 PM, CC, 2 locations: Level 100 hall before the 100 meeting rooms & Exhibit level - outside Hall D across from Fresh Pick Market"
Poster Sessions
9:00 AM-5:00 PM, CC, Inside Exhibit Hall
Poster Sessions set-up by presenting author
7:30 AM-9:00 AM, CC, Inside Exhibit Hall
Registration
7:30 AM-5:00 PM, CC, Registration
Speaker Ready Room
7:30 AM-4:00 PM, CC, 203A
MEETINGS & EVENTS
AALAS Ask Me Anything
(EVERYTHING AALAS QR codes) including AALAS Learning Library, ACE Membership Community, CMAR, ILAM, Educational Products, Registry, Publications
7:30 AM-5:00 PM, CC, Exhibit Hall BC
AALAS Foundation Silent Auction & "In Tune with Research" Contest
8:00 AM-5:00 PM, CC, Across from Exhibit Hall C entrance
AREA Teachers Program
(Invitation only; RSVP required)
9:30 AM-1:30 PM, CC, Davidson Ballroom A1
Charles River
9:00 AM-4:00 PM, CC, Davidson Ballroom A2
Charles River
7:00 AM-9:00 AM, Omni, Cumberland 2
District 1 Membership Meeting
5:15 PM-6:15 PM, CC, 107A
District 2 Membership Meeting
5:15 PM-6:15 PM, CC, 107B
District 3 Membership Meeting
5:15 PM-6:15 PM, CC, 108
District 4 Membership Meeting
5:15 PM-6:15 PM, CC, 105A
District 5 Membership Meeting
5:15 PM-6:15 PM, CC, 109
District 6 Membership Meeting
5:15 PM-6:15 PM, CC, 110A
District 7 Membership Meeting
5:15 PM-6:15 PM, CC, 106A
District 8 Membership Meeting
5:15 PM-6:15 PM, CC, 110B
Past President's Luncheon
12:30 PM-1:30 PM, Omni, Music Row 3
Technician Fun Fair
8:30 AM-5:00 PM, CC, Outside Exhibit Hall Entrance
Tech Connect Sponsored in part by PMI Lab Diet & Charles River
12:30 PM-2:00 PM, CC, TBD
COMMITTEE MEETINGS
Certification & Registry Board
1:30 PM-3:30 PM, CC, 211
Editorial Staff Meeting
3:00 PM-5:00 PM, CC, 210
AFFILIATE EVENTS
ACLAM Forum Program Committee
10:00 AM-12:00 PM, Omni, Mockingbird 1
ACLAM Outreach Committee
3:00 PM-5:00 PM, Omni, Mockingbird 3
Allied Trade Association (ATA)
New Product Showcase
8:30 AM-5:00 PM, CC, Outside Exhibit Hall Entrance
ASLAP CE Seminar Committee
11:00 AM-11:30 AM, CC, 212
ASLAP LARC Meeting
5:00 PM-6:00 PM, Omni, Mockingbird 2
ASLAP Networking
12:00 PM-2:00 PM, CC, Davidson Ballroom C3
Camp ACLAM Committee
3:00 PM-5:00 PM, Omni, Mockingbird 1
LAWTE General Membership Meeting
3:00 PM-5:00 PM, CC, 214
TUESDAY
NOVEMBER 5
AALAS Ask Me Anything (EVERYTHING AALAS QR codes) including AALAS Learning Library, ACE Membership Community, CMAR, ILAM, Educational Products, Registry, Publications
7:30 AM-5:00 PM, CC, Exhibit Hall BC
AALAS Foundation Silent Auction & "In Tune with Research" Contest
8:00 AM-5:00 PM, CC, Across from Exhibit Hall C entrance
Career Center
Veterinarian Job Fair sponsored by ACLAM, APV and ASLAP (see mobile app for companies participating)
Career Center
8:00 AM-5:00 PM, CC, 208A
Veterinarian Job Fair
9:00 AM - 11:00 AM
2:00 PM - 4:00 PM
Exhibit Hall
9:00 AM-5:00 PM, CC, Exhibit Hall BC
First Aid
7:30 AM-5:00 PM, CC, Outside Exhibit Hall
B Entrance near Grand Lobby
Mothers Room
7:30 AM-5:00 PM, CC, 2 locations: Level 100 hall before the 100 meeting rooms & Exhibit level - outside Hall D across from Fresh Pick Market"
Poster Sessions
9:00 AM-5:00 PM, CC, Inside Exhibit Hall
Poster Sessions Reception w/poster award winners announced at 4:45 PM
4:00 PM-5:00 PM, CC, Inside Exhibit Hall
Registration
7:30 AM-5:00 PM, CC, Registration
Speaker Ready Room
7:30 AM-4:00 PM, CC, 203A
Technician Fun Fair
8:00 AM-5:00 PM, CC, Outside Exhibit Hall Entrance
AALAS/FELASA Executive Group Meeting
9:00 AM-10:00 AM, CC, 210
Branch Leadership Reception
4:30 PM-5:30 PM, CC, 213
Charles River
9:00 AM-5:00 PM, CC, Davidson Ballroom
A2
Lab Animal Breeders Meeting
7:30 AM-9:00 AM, Omni, Old Hickory
Veterinary Tech Student Program
3:30 PM-4:30 PM, CC, 206B
Veterinary Tech Student Program
9:30 AM-10:30 AM, CC, 206B


Veterinary Tech Student Program Luncheon
12:30 PM-1:30 PM, CC, 206B COMMITTEE MEETINGS
Abstract Review Subcommittee (Poster Awards)
12:00 PM-1:30 PM, CC, 210 CMAR Committee
10:00 AM-12:00 PM, CC, 211
Editorial Review Board Meeting
7:30 AM-8:30 AM, CC, 210 ILAM Committee
2:00 PM-5:00 PM, CC, 211
LAS Pro Editorial Advisory Board
2:00 PM-4:00 PM, CC, 210
AFFILIATE EVENTS
AAALAC Ad Hoc/ Conncil/Emeriti Networking (Invitation Only)
7:00 AM-10:30 AM, Omni, Comberland 5 ACLAM Awards Committee
7:30 AM-9:30 AM, Omni, Mockingbird 1
ACLAM General Business Meeting
5:00 PM-7:00 PM, Omni, Legends Ballroom C
ACLAM New Diplomate Orientation
9:00 AM-11:00 AM, Omni, Cumberland 3
Allied Trade Association (ATA) New Product Showcase
9:00 AM-5:00 PM, CC, Outside Exhibit Hall Entrance
VAVMO & VMU
Supervisor's Business Meeting/Luncheon
12:00 PM-2:00 PM, Omni, Cumberland 5
AMP Meet & Greet
3:00 PM-4:30 PM, CC, 212
WEDNESDAY
NOVEMBER 6
Career Center
Veterinarian Job Fair sponsored by ACLAM, APV and ASLAP (see mobile app for companies participating)
Career Center
8:00 AM-5:00 PM, CC, 208A Veterinarian Job Fair
9:00 AM - 11:00 AM
2:00 PM - 4:00 PM
Exhibit Hall
9:00 AM-1:00 PM, CC, Exhibit Hall BC
Exhibit Hall Exhibitor Dismantle
1:00 PM-10:00 PM, CC, Exhibit Hall BC
First Aid
7:30 AM-10:00 PM, CC, Outside Exhibit Hall B Entrance near Grand Lobby
Mothers Room
7:30 AM-5:00 PM, CC, 2 locations: Level 100 hall before the 100 meeting rooms & Exhibit level - outside Hall D across from Fresh Pick Market"
Poster Sessions
9:00 AM-1:00 PM, CC, Inside Exhibit Hall
Poster Sessionsdismantle
1:00 PM-3:00 PM, CC, Inside Exhibit Hall
Registration
7:30 AM-5:00 PM, CC, Registration
Speaker Ready Room
7:30 AM-4:00 PM, CC, 203A
SPECIAL EVENTS
AALAS Foundation Live Auction & Appreciation Reception
6:30 PM-9:00 PM, Omni, Legends D
MEETINGS & EVENTS
AALAS Affiliates Roundtable Conference/Breakfast
(Invitation only; RSVP required)
7:30 AM-9:30 AM, Omni, Mockingbird 3
AALAS Ask Me
Anything (EVERYTHING AALAS QR codes) including AALAS Learning Library, ACE Membership Community, CMAR, ILAM, Educational Products, Registry, Publications
7:30 AM-1:00 PM, CC, Exhibit Hall C
AALAS Foundation & Boot Up for Research
8:00 AM-11:00 AM, CC, Across from Exhibit Hall C entrance
AALAS Foundation
Silent Auction (Auction ends at 1pm)
8:00 AM-1:00 PM, CC, Across from Exhibit Hall C entrance
Biocontainment (ABSL3 and ABSL-4)
4:00 PM-6:00 PM, Omni, Mockingbird 2
Charles River
9:00 AM-12:00 PM, CC, Davidson Ballroom A2
President's Reception (Invitation only; RSPV required)
5:30 PM-6:30 PM, Omni, Legends B
Technician Fun FairWinner Announced
2:00 PM-, CC, Outside Exhibit Hall Entrance COMMITTEE
MEETINGS
CTAD Committee
3:00 PM-4:00 PM, CC, 210 Exhibitor Advisory Council
3:30 PM-5:00 PM, CC, 211
AFFILIATE EVENTS
AAALAC/AALAS/ICLAS International Luncheon (Invitation only; RSPV required)
12:00 PM-2:00 PM, Omni, Legends C
ACLAM TBD
1:30 PM-4:00 PM, Omni, Mockingbird 1
ACLAM/ASLAP Program Committee
8:00 AM-12:00 PM, Omni, Mockingbird 1
Allied Trade Association (ATA) New Product Showcase
9:00 AM-1:00 PM, CC, Outside Exhibit Hall Entrance
THURSDAY
NOVEMBER 7
Career Center
Veterinarian Job Fair sponsored by ACLAM, APV and ASLAP (see mobile app for companies participating)
Career Center
8:00 AM-5:00 PM, CC, 208A Veterinarian Job Fair
9:00 AM - 11:00 AM
First Aid
7:30 AM-5:00 PM, CC, Outside Exhibit Hall B Entrance near Grand Lobby
Mothers Room
"7:30 AM-2:00 PM, CC, 2 locations: Level 100 hall before the 100 meeting rooms & Exhibit level - outside Hall D across from Fresh Pick Market"
Registration
7:30 AM-12:00 PM, CC, Registration
Speaker Ready Room
7:30 AM-1:00 PM, CC, 203A COMMITTEE
MEETINGS
2024/2025 AALAS Program Committee
2:15 PM-4:00 PM, Omni, Cumberland 3
AALAS Executive Committee Meeting
9:00 AM-12:00 PM, Omni, Gibson Boardroom

Having served the research community for more than 50 years, we are well known for creating products that meet the unique needs of research animals.
While we consider all our products to be an important part of animal care, some stand out as absolutely essential. We are proud that our products, both important and essential, are trusted by researchers around the globe!


SUNDAY
NOVEMBER 3
Afternoon
Track I

Research Security 360 - A Complete View
John Michael Nord
1:00 PM - 1:20 PM (TTP)

Vital Signs of Noninvasive Physiological Monitoring
Katherine M Garner
1:20 PM - 1:40 PM (TTP)

Cerebral Open Flow Microperfusion (cOFM) for Perfusion Sampling in Awake, Freely Moving Animals
Shelly Carballo
1:40 PM - 2:00 PM (TTP)

Navigating the Software Maze: How to Choose between Colony, Facility, and Study Management Solutions
Kelly Rodriques
2:00 PM - 2:20 PM (TTP)

Embracing the Future: Digital ID for Laboratory
Animal Identification
Jose R Gadea
2:20 PM - 2:40 PM (TTP)

Use of Artificial Intelligence/ Machine Learning in Animal Health Management Software to Analyze and Predict Health Patterns using Predictive Models
Chandra Devireddy
2:40 PM - 3:00 PM (TTP)

Expanding Diagnostic Capabilities of RFID in Animals with the New TP-500 9mm Micro-Temperature Transponder
Geoffrey Hunt
3:00 PM - 3:20 PM (TTP)

Digital Primate Cadaver for Training and Surgical Planning
Jake Lehman
3:20 PM - 3:40 PM (TTP)

Improvements to Bile Collection Studies in Rats
Brad Gien
3:40 PM - 4:00 PM (TTP)

Revolutionizing Preclinical Studies: A Comprehensive and Scalable Digital Platform for Behavioral Monitoring in Drug Discovery and Development
Michael Ellis
4:00 PM - 4:20 PM (TTP)

Your Data is Only as Clean as Your Bedding
Joel Shepherd
1:00 PM - 1:20 PM (TTP)

Uncovering the Best Decontamination Method for Your Unique Research Program
Kevin Lorcheim
1:20 PM - 1:40 PM (TTP)

Using Data to Support Standards: Evaluating Product Stability to Rework Storage Conditions and Term Recommendations for Purified Diets
Jessie Chouinard
1:40 PM - 2:00 PM (TTP)

Leveraging Silver as a Disinfectant: Enhancing Safety and Material Compatibility in Research Facilities
Nick G Hidell
2:00 PM - 2:20 PM (TTP)

We Are All Animals - But We All Have Different Data Requirements!
Mathew D Sanderson
2:20 PM - 2:40 PM (TTP)

Transforming Vivarium Operations: Cutting-Edge Advances in Automation and AGV Technologies
Fabio Mazzucchelli
2:40 PM - 3:00 PM (TTP)

Learning to Speak Mouse: Universally Compatible, Whole Vivarium Deployable, Home Cage Monitoring
Erik D Dohm
3:00 PM - 3:20 PM (TTP)

Safeguard Research with Rapid Decontamination
Brittany Buchman 3:20 PM - 3:40 PM (TTP)

Dry Heat Sterilization in Vivariums - New Technological Developments
Robert C Davis
3:40 PM - 4:00 PM (TTP)

It’s Not Just a Toy; NHP Enrichment Strategies
Karena Thek
4:00 PM - 4:20 PM (TTP)




MONDAY
NOVEMBER 4
Morning

W-01 Alternative Replacement Training (ART) Methods
April J George 8:00 AM - 12:00 PM (W)

W-02A Microsurgery Skills Training Using Surgical Loupes - A
Robert F Hoyt 8:00 AM - 12:00 PM (W)

W-03 Tips for Perioperative Anesthetic Monitoring
Cholawat Pacharinsak 8:00 AM - 12:00 PM (W)

New Tools for Enhancing Welfare and Research: The Promise of AI in Laboratory Animal Science
Gerry A Hish 8:00 AM - 10:00 AM (S)

Ongoing Changes Within the Animal Research Oversight Environment
B Taylor Bennett 8:00 AM - 10:00 AM (S)

Practical Tips for Importing and Exporting Germ-Free Mice
Steven E Davison 8:00 AM - 10:00 AM (S)

Pathology Quiz Bowl
Marcia L Hart 8:00 AM - 9:15 AM (P)

Keeping it in the FAMily: Facility and Animal Management Quiz Bowl
Erin O’Connor 9:30 AM - 10:45 AM (P)

AALAS Looking Back: Looking Forward
Ann T Turner 10:00 AM - 11:30 AM (P)

A Decade of Openness on Animal Research in the UK: The Good, the Better and the Unexpected. What has changed since the Concordat was published?
Wendy J Jarrett 11:00 AM - 12:00 PM (STL)

Charles C Hunter Lecture: Kangaroo Wrangling - Managing Clinical and Conservation Research at a Zoo
Louden Wright 11:00 AM - 12:00 PM (STL)


Finding the Needle in the Haystack: A Practical Diagnostic Approach to Unexpected Rodent Health Issues
Marcia L Hart
11:00 AM - 12:00 PM (STL)

Responding to FOIA Requests: Balancing the Public’s Right to Know with Staff Safety and Research Integrity
Nancy Halpern
11:00 AM - 12:00 PM (STL)
MONDAY
Afternoon

AALAS and FELASA: Working Together for the Laboratory Animal Science Community
Scott A Mischler
12:30 PM - 2:00 PM (P)

From Expert to Leader: Navigating the Leap from Individual Contributor to Manager
Mary Spencer
12:30 PM - 2:00 PM (P)

Integrating Automated Genotyping and Colony Management Software into the “Standard of Care” for Mouse Colonies
Jeanne M Wallace
12:30 PM - 2:00 PM (P)

Pushing the Boundaries of Environmental Health Monitoring: What’s Next?
Kerith R Luchins
12:30 PM - 2:00 PM (P)

The NCI Comparative Oncology Trials Consortium: An Infrastructure for Implementation of Comparative Oncology Clinical Trials Consortium: An Infrastructure for Implementation of Comparative Oncology Clinical Trials in Pet Dogs to Advance Studies in Cancer Drug Development and Biology
Amy K LeBlanc
12:30 PM - 2:00 PM (P)

W-04 Beginning Training Methods & Techniques for NHPs - Part 1
Lisa A Houser
1:00 PM - 5:00 PM (W)

W-05 Communication & Media Training for Animal Research Institutions
Eva C Maciejewski
1:00 PM - 5:00 PM (W)

W-02B Microsurgery Skills Training Using Surgical Loupes - B
Robert F Hoyt
1:00 PM - 5:00 PM (W)

W-06 Zebrafish Husbandry and Facility Management 101: The Basics
Logan Fehrenbach
1:00 PM - 5:00 PM (W)

W-07 AALAS Foundation 101: Come Learn About the Foundation and How It Can Support Your Public Outreach Efforts - Find Out What’s New!
Vicki Campbell
2:30 PM - 5:00 PM (W)

A Global Look at Current & Emerging Animal Rights Trends: Identifying the Threats & Advice for Combatting Them
Paula A Clifford
3:00 PM - 5:00 PM (S)

Implementing Inclusion, Diversity, Equity, and Accessibility into Your Organization
Emily I Weston
3:00 PM - 5:00 PM (S)

It’s Okay to Get a Little Dirty: The Laboratory Animal Professional’s Guide to Working with and Caring for ‘Dirty’ Mice
Victoria K Baxter
3:00 PM - 5:00 PM (S)

Organization, Operations, and Financial Sustainability of Academic Animal Care and Use Programs
Jann Hau 3:00 PM - 5:00 PM (S)
TUESDAY
NOVEMBER 5
Morning

W-08 3D Printing for Animal Welfare: Creating Custom Equipment Without the Factory
Constance J. Woodman
8:00 AM - 12:00 PM (W)

W-04 cont. Beginning Training Methods & Techniques for NHPs - Part 2
Lisa A Houser
8:00 AM - 12:00 PM (W)

W-09 Occupational Health and Safety Considerations in Animal Research: Learning Through Interactive Case Studies - Part 1
Lesley A Colby
8:00 AM - 12:00 PM (W)

Embracing Variability to Ensure Reproducibility, Validity, and Translation
F Claire Hankenson
8:00 AM - 10:00 AM (S)

Establishing and Growing a Successful Gnotobiotics Program
Allison R Rogala
8:00 AM - 10:00 AM (S)

Top-Down: From Regulations to Researcher Compliance in Zebrafish Facilities
Michelle L Altemara
8:00 AM - 10:00 AM (S)

Transforming Vivarium Operations: Advancement in Technology for Enhanced Human Capital and Workflow Optimization
Sylvia I Gografe 8:00 AM - 10:00 AM (S)

Advancing Neuroscience Research through Vertical Vivaria Design: Lessons from Washington University’s Neuroscience Research Building
Trevor Calarco 11:00 AM - 12:00 PM (STL)

Charles River Ethics and Animal Welfare Lecture: The 3Hs Initiative: Housing, Handling and Habituation Methods for Laboratory Rodents
Emma Robinson 11:00 AM - 12:00 PM (STL)

Murine Models of Experimental Autoimmune Encephalomyelitis (EAE)
Shawn P O’Neil 11:00 AM - 12:00 PM (STL)

Wallace P Rowe Lecture TBN 11:00 AM - 12:00 PM (STL)
TUESDAY
Afternoon

Animal Research & Internal Communications: How Effectively Communicating within your Organization about Animal Studies Can Improve Morale, Operations, Reputation and Security
Valerie Hill 12:30 PM - 2:00 PM (P)

Best Practices in IACUC Membership Composition, Recruitment, and Retention
Scott D Bury 12:30 PM - 2:00 PM (P)

Enhancing Neurodegenerative Disease Modeling Through Continuous Home Cage Monitoring: Insights into Behavioral and Physiological Biomarkers
Sean Maguire 12:30 PM - 2:00 PM (P)

Navigating USDA, APHIS, AC Appeals
Nancy E Halpern 12:30 PM - 2:00 PM (P)

Step Up YOUR Laboratory Animal Anesthetic/Analgesic Game! Practical Techniques to Improve YOUR Investigations and Data!
Rebecca A Johnson 12:30 PM - 2:00 PM (P)



W-10 Positive Reinforcement Training and Temperament Testing: Preparing Monkeys for Restraint Procedures - Part 1
Jaine E Perlman
1:00 PM - 5:00 PM (W)

W-11 Tools to Achieve Sustainable Diversity, Equity, Inclusion, and Belonging in the Workplace; A Multi-Organization Collaboration
Crystal H Johnson
1:00 PM - 5:00 PM (W)

W-12 Working with Difficult Personalities: How to Survive and Thrive
Laura A Conour
1:00 PM - 5:00 PM (W)

W-13 Zebrafish Husbandry and Facility Management 201: Advanced Care Techniques
Tannia S Clark
1:00 PM - 5:00 PM (W)

Chlamydia Muridarum: It’s Likely in Your Colonies and You Should Care
Neil S Lipman
3:00 PM - 5:00 PM (SEM)

From the FDA Trenches: Designing, Conducting, and Reporting the Results of Animal Studies to Support Medical Devices - A Regulatory Perspective
Susanne Bush
3:00 PM - 5:00 PM (SEM)

Smart Animal Facilities: Navigating the Future with Hi-Tech Solutions in Animal Research
Harshan R Pisharath
3:00 PM - 5:00 PM (SEM)

Techniques in Miniature Swine for a New Era
Derek Brocksmith
3:00 PM - 5:00 PM (SEM)
WEDNESDAY NOVEMBER 6
Morning

W-14 AALAS Foundation 201: Take the Next Step - Put Foundation Outreach Materials to Action!
Vicki C Campbell
8:00 AM - 10:30 AM (W)

W-15 Animal Facility, Design, Processes, Decisions, and Technology
Chad Zuberbuhler
8:00 AM - 12:00 PM (W)

W-16 Not Just a Document: The Art of Crafting SOPS That Work
Amy L Dryman
8:00 AM - 12:00 PM (W)

W-09 cont. Occupational Health and Safety Considerations in Animal Research: Learning Through Interactive Case Studies - Part 2
Lesley A Colby
8:00 AM - 12:00 PM (W)

W-10 cont. Positive Reinforcement Training and Temperament Testing: Preparing Monkeys for Restraint Procedures - Part 2
Jaine E Perlman
8:00 AM - 12:00 PM (W)

Heroes Behind the Scenes: The Crucial Contribution of Laboratory Animal Professionals in Therapeutic Development for Neurologic and Neurodegenerative Disorders
Sarah Hansen
8:00 AM - 10:00 AM (S)

Outbreak! Navigating through the Storm of an Unexpected Infectious Agent Detection
Kenneth S Henderson
8:00 AM - 10:00 AM (S)

The Digital In Vivo Alliance - Leveraging Machine-Learning (ML)-Defined Digital Biomarkers to Improve the Reproducibility and Translation of Animal Studies
Brian R. Berridge
8:00 AM - 10:00 AM (S)

The Evolution of Lab Animal Medicine and Unique Career Pathways
Valerie K Bergdall
8:00 AM -10:00 AM (S)

Alternative Replacement Training (ART) Methods: Implementing the 3R’s Into Our Training Program
April J George 11:00 AM - 12:00 PM (SLC)

Nathan E Brewer Lecture: TBN 11:00 AM - 12:00 PM (STL)

Should Ethics be Included in the Next Guide? The Pros, the Cons, and Why Members of the Research
Jerrold Tannenbaum 11:00 AM - 12:00 PM (STL)

Should it Stay or Should it Go? The Clashing Trends of Tolerating Some Infectious Agents and Excluding Others
Kenneth S Henderson 11:00 AM - 12:00 PM (STL)


WEDNESDAY
Afternoon

Adapting to Unconventional Scenarios: Navigating Regulatory Challenges in Laboratory Animal Research
Summer Boyd 12:30 PM - 2:00 PM (P)

Cultivating a Culture of Belonging Through Engagement: Strategies for Today’s Workplace
Donna M Jarrell 12:30 PM - 2:00 PM (P)

Danio Zoom Live 2: The Return
Christine Archer 12:30 PM - 2:00 PM (P)

Effective Communication with the General Public About Scientific Research that Requires the Care and Use of Animals - Takehome Messages from a Workshop
Nia Johnson 12:30 PM - 2:00 PM (P)

W-17 A Practical Guide to Prevent and Combat Compassion Fatigue in Biomedical Facilities
Danielle Adney 1:00 PM - 5:00 PM (W)

W-18 Participation in a Discussion-Based Disaster Preparedness Exercise
Evan T Shukan 1:00 PM - 5:00 PM (W)

W-19 The Art of Communication: Transform Your Leadership and Level Up Your Vivarium
Andrea Abrams 1:00 PM - 5:00 PM (W)

W-20 Would You Like to Improve Your Suturing and Rodent Surgery Aseptic Technique?
Marcel I Perret-Gentil 1:00 PM - 5:00 PM (W)

Assessing Success in Animal Enrichment, Behavior, and Social Housing: Strategies and Challenges
Kristina Bartley 3:00 PM - 5:00 PM (S)

Navigating Diverse Aquatic Husbandry: Insights from Multi-Species Facilities
Michelle L Altemara 3:00 PM - 5:00 PM (S)

NHP Housing Trends and Determinations
John J Hasenau 3:00 PM - 5:00 PM (S)




Refining Human-Animal Interactions and Housing of Nonhuman Primates in Research: A Practical Approach
Elizabeth A Nunamaker
3:00 PM - 5:00 PM (S)
THURSDAY
NOVEMBER 7
Morning

All Things Outreach: Starting, Maintaining, and Promoting Outreach for any Size Program
Julie E Roller
8:00 AM - 10:00 AM (S)

Current Trends in Gnotobiotics: Adapting to Challenges
Joshua M Frost
8:00 AM - 10:00 AM (S)

Focusing the Light on Lighting in Animal Facilities, A Major Extrinsic Factor
John J Hasenau
8:00 AM - 10:00 AM (S)

Maintaining the Highest Level of Genetic Integrity in Laboratory Animal Colonies in the 21st Century
Bart MG Smits
8:00 AM - 10:00 AM (S)

An in Depth Look at the AVMA Working Group on the Psychological Impacts of Humane Endings
Nathaniel Socrates Kollias
11:00 AM - 12:00 PM (STL)

Navigating the Storm: Strategies for Research Institutions Facing Animal Rights Extremism
John Sancenito
11:00 AM - 12:00 PM (STL)

Which is the Best Animal Model to Study Osteoarthritis (OA)?
Bertrand Lussier
11:00 AM - 12:00 PM (STL)
- Post resumes for employers to review (use push pins located in the room).
- Bring plenty of resumes for employers to take and to replenish the board.
- Don’t want your resume/CV removed? Prominently note this on the top of your resume/CV.
- Include a cell phone/contact number to assist with scheduling a brief on-site interview.
EMPLOYERS—POSITIONS OPEN BULLETIN BOARDS
- Post job openings, not to exceed 11 x 17 inches (use push pins located in the room).
- Check out the Positions Wanted bulletin boards to find the perfect candidate.
- Do not remove the posted Open Positions ads.
- Include a cell phone/contact number to assist with scheduling a brief on-site interview.
This is a self-service operation which offers employers a place to publicize job openings, interview schedules, and allows job seekers to post résumé and other descriptions.
Sunday-Wednesday, 8:00 AM-5:00 PM Thursday, 8:00 AM-2:00 PM
VETERINARIAN JOB FAIR IS SPONSORED BY

THURSDAY
Afternoon

Building Compassion Fatigue Programs Within Large Organizations
Michelle Creamer-Hente 12:30 PM - 2:00 PM (P)

Through the Looking Glass: Perspectives on Openness in Animal Research
Kirsten Bell 12:30 PM - 2:00 PM (P)

Welcome to CUSP: An Online Repository of Animal Research Methodologies and Procedures
Michelle Brot 12:30 PM - 2:00 PM (P)

When Your Emergency Response Plan Gets Washed Away: Lessons Learned from an Unprecedented Vivarium-Level Flooding Event
Timothy J Scott 12:30 PM - 2:00 PM (P)

Music City Center | Sunday, November 3, 2024
5:00-6:30 PM | Music City Center, Karl F Dean, Ballroom A
• Welcome from AALAS President, Bob Quinn
• AALAS Awards presentations
• Recognition of guests and Board of Trustees members
• Gavel ceremony introducing the new president

• Introduction of new trustees
• Incoming president, Jim Macy’s address
• Keynote Speaker Bob Dysko, DVM, DACLAM
• Welcome Reception from 6:30-8:00 PM, Music City Center, Exhibit Hall
Welcome Reception, Exhibit Hall, Sunday, November 3, 2024
District membership meetings will be held on Monday, November 4th at 5:15 PM. See the mobile app for room numbers.
We would like to announce that Bob Dysko will be presenting this year’s keynote speech, “75 Years of AALAS” at our 75th annual National Meeting.
Robert (Bob) Dysko is a Past-President of AALAS, a board-certified laboratory animal veterinarian, and an Active Emeritus Professor at the University of Michigan (U-M), having retired from full-time service in January 2024. He received his veterinary degree from Iowa State University in 1983, completed a residency in laboratory animal and comparative medicine at U-M in 1986, and became board-certified by the American College of Laboratory Animal Medicine (ACLAM) in 1987. He was a faculty veterinarian at Wake Forest University from 1986-1990, and then returned to U-M as an Assistant Professor in 1990. During his tenure there he has had many major responsibilities within the Unit for Laboratory Animal Medicine (ULAM) including oversight of all campus animal facility design and construction projects, direction of the rodent health surveillance program, membership on the university’s and the Ann Arbor Veterans Affair’s animal care and use committees, and director of the program for training graduate veterinarians in laboratory animal medicine and comparative medical research. From July 2012 - June 2017 he was the fourth Director in ULAM’s 50-year+ history. Bob has been active in both ACLAM and AALAS during his career, having served on multiple committees for both organizations. He was on the ACLAM Board of Directors from 2000-2003 and on the AALAS Executive Board from 2008-2012, serving as President of AALAS from 2010-2011. He has also been a member of the Editorial Review Board for the AALAS journals since 1994. He served on the Board of Directors from the Amer-

ican Association of Veterinary Medical Colleges from 2013-2017 as the at-large representative for Departments of Comparative Medicine. He has been involved with the Institute for Laboratory Animal Research (ILAR) of the National Academies (of Science, Engineering, and Medicine) since 2014, when he joined the Roundtable on Science and Welfare in Laboratory Animal Use. He then joined the ILAR Council in 2019 and was named Council Chair in 2021. ILAR was recently converted to a Board within the National Academies, with a name change to the Board on Animal Health Sciences, Con servation, and Research (BAHSCR), and Bob now serves as co-chair of this Board.













It seems like 2024 just started, and here we are looking ahead toward another AALAS National Meeting. But this isn’t just another annual meeting. This year we arrive at a milestone – our 75th anniversary, an exciting and dynamic event coming this November in Nashville!
The kick-off starts Sunday with a busy day including Technical Trade Presentations (TTPs). These informative presentations are given by representatives of our exhibiting companies. The TTPs provide a variety of beneficial tips, best practices, and product-related information relevant to a variety of industry applications. Thank you to all the vendors who submitted topics this year! Don’t miss them!
New this year, and in celebration of the 75th anniversary, the Sunday evening Welcome Reception is being held in the exhibition hall. This will offer attendees the opportunity to socialize, mingle, and enjoy drinks and hors d’oeuvres. Plus, you’ll get a sneak peek at the exhibit hall!
The week itself will be filled with a variety of educational programs and sessions, including Exhibitor Teach & Chats (ETC). Be sure to visit the exhibit hall each day for these 20-minute presentations covering a wide range of applicable topics. They’re a great opportunity to keep up with trends in the industry.
If you have a little spare time and are up for a short walk, you’ll have the chance to see and hear why Nashville is nicknamed ‘The Music City’. There are numerous restaurants, bars, and venues that offer great food and live music. For those that enjoy history, the Country Music Hall of Fame and Museum is right next door to the convention center, and the Johnny Cash Museum is a gem for any music fan.
The AALAS National Meeting is always a productive time to learn and connect. I certainly encourage you to attend this year. Not just because it is our 75th anniversary, but because the opportunity to discover, explore, and network within one of our liveliest cities is not to be missed.
I’m truly excited and hope to see you in Nashville!

Michael Evans Chair, Exhibitor Advisory Committee
Approximately 200 companies (almost 400 booths) exhibiting at the AALAS National Meeting. Exhibitors interact with people from the academic community, research institutions, government organizations, and commercial companies. Visit the Exhibitors section of nationalmeeting.aalas.org for a prospectus, a list of previous exhibitors, sponsorship opportunities, and other information.
RIBBON CUTTING CEREMONY
COMMERCIAL COMPANY BOOTHS
AFFILIATE BOOTHS
REFRESHMENT BREAKS
POSTER SESSIONS
Sunday, November 3, 6:30 p.m.
Learn about the latest products and services offered by vendors in the field.
Visit with our affiliate members and check out their public outreach and educational materials.
Monday: 9:00 a.m. – 11:00 a.m. (sponsored by NEPCO) 2:00 p.m. – 4:00 p.m. (sponsorsed by Charles River)
Tuesday: 9:00 a.m. – 11:00 a.m. (sponsored by The Jackson Laboratory) 1:00 p.m. – 3:00 p.m. (sponsored by TBD)
Wednesday: 9:00 a.m. – 11:00 a.m. (sponsored by TBD)
Come view this year’s poster sessions, and don’t miss the Poster Reception on Tuesday from 4:00 p.m. – 5:00 p.m. Meet the authors, enjoy some refreshments, and see who won this year’s Poster Awards (winners will be announced at 4:45 p.m.)
EXHIBIT HALL HOURS
Sunday, November 3: 6:30 p.m. - 8:00 p.m. (Welcome Reception inside Exhibit Hall)
Monday, November 4: 9:00 a.m. – 5:00 p.m.
Tuesday, November 5: 9:00 a.m. – 5:00 p.m.
Wednesday, November 6: 9:00 a.m. – 1:00 p.m.


Advanced Wall Solutions
Alfa Wassermann Diagnostic Technologies
Allentown
Bronze Level Sponsor
Allometrics
Alternative Design Manufacturing & Supply, Inc.
ALZET® Osmotic Pumps/DURECT Corp.
American Protective Products LLC
Bronze Level Sponsor
Anatomage, Inc.
ANCARE CORP.
Anesthesia Equipment Supply, Inc.
Animal Identification & Marking Systems, Inc.
Animal Welfare Institute
AnimalCare Software
Aquaneering Inc.
Arcoplast
ARES Distribution
Art’s Way Scientific
a-tune Software Inc
Avid Identification Systems, Inc
AVIDITY SCIENCE
BASi Research Products
Benchling
Beta Star Life Science Equipment
Bio Serv
Wi-Fi Sponsor
Gold Level Sponsor
bioBUBBLE, Inc.
BioInfoRx, Inc.
BioSAFE Engineering
BMT USA
BodyCAP
Brain & Software International-BSI
Braintree Scientific, Inc.
Britz & Company
CannonDesign
Carter2 Systems, Inc.
CATCH GLOVE by ROTANO
Cayuse
Charles River
Tech Connect Reception Sponsor
Refreshment Break Sponsor
Tote Bag Sponsor
President’s Level Sponsor
CITI PROGRAM, a division of BRANY
Class Biologically Clean Ltd
ClearH2O
Gold Level Sponsor
Colonial Medical Supply Co., Inc.
Contec, Inc
CURIS System
Datesand Ltd
Dechra Veterinary Products
Drexel University Master of Laboratory Animal Science (MLAS) Program
DVM Consulting and Recruiting
Eastern Virginia Medical School
Ecolab
Elm Hill Labs
ETC Sterilization Systems
EZ-Systems/ Euthanex
Fidelis Animal Health, Inc
Fine Science Tools
Foot Master Casters & Wheels
GemPharmatech
GenoTyping Center of America
Bronze Level Sponsor
GenVault
GerVetUSA Inc
Getinge
Girton
Gnotobiotic Containment Solutions
Gruenberg - TPS
Hilltop Lab Animals, Inc
HistoWiz
HotDog Patient Warming
Hundred Inc.
Hybex Innovations Inc
IDEXX BioAnalytics
InfoEd Global
InfoPathways
Innovive
Inotiv
Instech Laboratories, Inc.
Kent Scientific Corporation
Key Solutions, Inc.
Lab Products, LLC
Lab Supply
LabDiet
Tech Connect Reception Sponsor
Neck Cord Sponsor
President’s Level Sponsor
LABEX of MA
LabVoice
LBS (serving Biotechnology) Ltd
Lenderking Caging Products
LGL Animal Care Products, Inc.
Life Science Products, Inc
Lighthouse Environmental Infection Prevention
Lithgow Laboratory Services
Lomir Biomedical Inc
MacCauley Suffolks LLC
MadgeTech
MAI Animal Health/Vetcorder
Marshall BioResources
Medline Industries, LP
Memmert USA LLC
Micro Photonics
Microchip ID LLC
Mispro
MOLECUBES
NEPCO
Refreshment Break Sponsor
Silver Level Sponsor
New England Ovis


Newco Distributors
NKP Isotec
NuAire, Inc.
Oak Hill Genetics, LLC
Optimize Courier
Otto Environmental
P. J. Murphy Forest Products
Patterson Scientific
Perimed Inc
Pharmacal Research Laboratories, Inc.
PLAS-LABS
PreLabs
Premier BioSource
Priority One Services, Inc
PureLine

Quip Laboratories
Exhibit Hall Aisle Sign Sponsor
National Meeting Pen Sponsor
Gold Level Sponsor
RapID Lab Red Plank Software
Research Diets, Inc.
Res-Tek, Inc.
RICA Surgical Products, Inc.
Ridglan Farms
Roboz Surgical Instrument Co.
Rochester Midland Corporation
RockStep Solutions
RSM-Equipment
RWD Life Science
SAFE Complete Care Competence, formerly
BioFresh
Safe Haven Lab Cages
SAI Infusion Technologies
Sanitation Strategies
Exhibit Hall Aisle Sign Sponsor
Silver Level Sponsor
SARSTEDT
SCANBUR
SensoScientific, Inc.
Shepherd Specialty Papers
Shinva Medical Instrument Co., Ltd
Shoe Cover Magic
Sika Corporation
Sinclair Bio Resources, LLC
SoftMouse.NET
SOMARK Innovations
Sound
SPIRE INTEGRATED SOLUTIONS
Vet Tech Student Program Sponsor
Bronze Level Sponsor
SteraMist Disinfection
STERIS
Stoelting Co
Studylog Animal Study Workflow Software
Suburban Surgical Co Inc
Systems Engineering
TBJ, Inc.
Tecniplast
The Andersons
The Jackson Laboratory
Refreshment Break Sponsor
Silver Level Sponsor
Thoren Caging, Inc..
TOPAZ Technologies
Translite, LLC
Transnetyx
Tricorder Array Technologies LLC
Turner Scientific
Unified Information Devices
ValuMax Protective Apparel Inc
Verona Safety
Vet and Tech /Search A Vet
VetEquip, Inc.
Tote Bag Sponsor
Silver Level Sponsor
Vivus Technologies LLC
Voda IQ
VRL Laboratories
AI Chat Bot Sponsor
Silver Level Sponsor
Wedgewood
WF Fisher & Son
Worldwide Primates, Inc.
XpressBio
Xybion Digital
Zeigler Bros Inc
Non-Exhibiting Sponsors
Animal Specialties and Provisions LLC
Tote Bag Sponsor
Silver Level Sponsor
Pfizer
Area Program Sponsor
Mosaic Vivarium (Virtual Chemistry, Inc)
Tote Bag Sponsor
Silver Level Sponsor
Affiliate Exhibitors
AAALAC International
American College of Lab Animal Medicine (ACLAM)
American Society of Laboratory Animal Practitioners (ASLAP)
Americans for Medical Progress (AMP)
Association of Primate Veterinarians (APV)
Board on Animal Health Sciences, Conservation, and Research (BAHSCR)
FESAHANCCCAL
Foundation for Biomedical Research (FBR)
International Council for Laboratory Animal Science (ICLAS)
Laboratory Animal Management Association (LAMA)
Laboratory Animal Welfare Training Exchange (LAWTE)
National Association for Biomedical Research (NABR)
Scientists Center for Animal Welfare (SCAW)
Thai Association for Laboratory Animal Science (TALAS)
Vivarium Operational Excellence Network/OpExAHC (VOE)
Zebrafish Husbandry Association (ZHA)






Low-Flow Anesthesia

Physiological Monitoring



























Noninvasive Blood Pressure
Ventilators
Animal Warming



















Surgical Platforms



























































































1:00 PM - 1:20 PM/Room: 202A
Speaker: John M Nord
Moderator: Michael Evans
This session will discuss research security, including a deep dive into the newest developments regarding NSPM-33 and the CHIPS and Science Act of 2022. A few of the questions we will address include: What are the key things we need to be confident we comply with? What types of research grants does this apply to? What departments need to be involved in setting up our research security program? The presenter will also give a 360-degree view of a holistic research security program and the elements you should look for at your institutions. We will also explain a partnership solution offered by Cayuse and IP Talons, ThreatShield, that addresses many topics discussed during this time. This Technical Trade Presentation is sponsored in part by Cayuse.

1:20 PM - 1:40 PM/Room: 202A
Speaker: Katherine M Garner
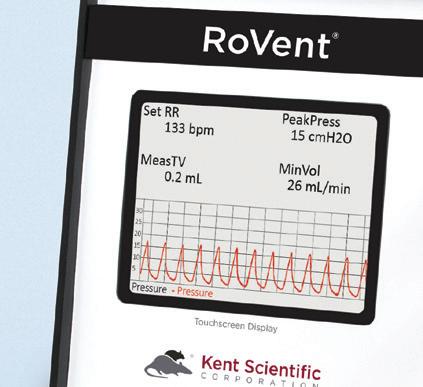
Moderator: David Poldiak
Appropriate perioperative animal monitoring is crucial to ensure well-being and control research variables. Determining what signs to monitor, how to monitor them, and how to interpret the results can be intimidating, especially to newer surgeons. Research animals, in particular, often have unique challenges that are not found in other settings. When compounded with the variety of systems on the market that specialize in different parameters, users may be overwhelmed with options. They would benefit from additional understanding of monitoring purposes and options. Here, we briefly discuss the benefits of optimized non-invasive physiological monitoring, focusing on how the Kent Scientific PhysioSuite can accomplish monitoring goals for small laboratory rodents.
This Technical Trade Presentation is sponsored in part by Kent Scientific Corporation.

Open Flow Microperfusion (cOFM) for Perfusion Sampling in Awake, Freely Moving Animals
1:40 PM - 2:00 PM/Room: 202A
Speaker: Shelly Carballo
Moderator: Candace A Rohde-Johnson
Pharmaceutical development traditionally focuses on blood collection, serving as a conduit throughout the body. However, drugs often target specific tissues rather than blood itself. Current methods for studying target tissue are typically limited to terminal collection or imaging, providing only a snapshot or limited perspective over time. Open flow microperfusion (OFM) technology allows real-time
monitoring of drugs or endogenous molecules within tissues. OFM offers a refinement, enabling observation of ongoing processes in a single animal, potentially reducing the number of animals required for studies. This presentation will provide an overview of OFM study data and capabilities, mainly focusing on cerebral OFM (cOFM) use to study neurodegenerative diseases in brain tissue.
This Technical Trade Presentation is sponsored in part by BASi Research Products.

Navigating the Software Maze: How to Choose between Colony, Facility, and Study Management Solutions
2:00 PM - 2:20 PM/Room: 202A
Speaker: Kelly Rodriques
Moderator: Bernard Chambon
In the evolving landscape of animal management software, navigating the plethora of options can be daunting. Join us to explore the distinct features and functionalities of colony management, facility management, and in vivo study software. We’ll dive into the core features of each software category, shedding light on how they cater to distinct aspects of research operations. We’ll explore areas of overlap between these software types, highlighting potential synergies and considerations for integration. By understanding how these software solutions complement each other, attendees will gain insights into creating seamless workflows and maximizing efficiency in their research endeavors while upholding the highest animal welfare standards. By examining real-world use cases and scenarios, we’ll help attendees identify the best-suited solution for their specific needs, whether it involves managing breeding colonies, optimizing facility resources, orchestrating in vivo experiments, or all of the above!
This Technical Trade Presentation is sponsored in part by SoftMouse.NET.

2:20 PM - 2:40 PM/Room: 202A
Speaker: Jose Gadea
Moderator: Matt Ruiter
In the realm of laboratory research, the identification and tracking of animals are pivotal for scientific progress. Traditional methods, while effective, often come with limitations such as potential inaccuracies, labor-intensive processes, and ethical concerns. However, emerging technologies offer a promising solution: digital identification (ID) systems. This presentation will delve into the transformative potential of digital ID for laboratory animal identification, highlighting its role as the future standard in research practices. We will explore the myriad benefits of digital ID, including enhanced accuracy, seamless integration with technology, comprehensive data storage, and improved animal welfare. Moreover, we will discuss how digital ID systems facilitate remote monitoring, enable advanced data analytics, and promote traceability, ultimately fostering more efficient and ethical research methodologies. Through case studies and real-world examples, attendees will gain insights into the practical implementation of digital ID and its profound implications for the future of laboratory animal research. Join us as we navigate the frontier of scientific innovation and embrace the transformative power of digital ID in advancing scientific discovery and animal welfare alike.
This Technical Trade Presentation is sponsored in part by Unified Information Devices.






Use of Artificial Intelligence/Machine Learning in Animal Health Management Software to Analyze and Predict Health Patterns using Predictive Models
2:40 PM - 3:00 PM/Room: 202A
Speaker: Chandra Devireddy
Moderator: Chittaranjan Mallipeddi
The use of Artificial Intelligence (AI) and Machine Learning (ML) in identifying behavioral and health patterns from the data collected in animal health management software applications can help in advancing preclinical research, improving animal welfare, and enhancing the relevance of experimental findings to animal health. AI and ML techniques can be used to develop predictive models that forecast future health outcomes based on current behavioral and physiological data. ML algorithms can predict the likelihood of certain health conditions or the efficacy of treatment interventions in research animals, helping researchers make informed decisions about experimental design and animal care. By integrating diverse datasets from preclinical studies, including pharmacokinetic data, pharmacodynamic data, and clinical observations, and using AI/ML technology, researchers can uncover complex relationships between drug treatments and animal responses, leading to the identification of treatment strategies. Predictive analytics can predict disease outbreaks and potential health issues by analyzing historical data and identifying patterns, helping researchers take proactive measures to prevent or mitigate these issues. Animal health management software powered by AI can integrate and analyze data from within software to provide a comprehensive view of research animals’ health and behavior.
This Technical Trade Presentation is sponsored in part by Key Solutions, Inc.

Diagnostic Capabilities of RFID in Animals with the New TP-500 9mm MicroTemperature Transponder
3:00 PM - 3:20 PM/Room: 202A
Speaker/Moderator: Geoffrey Hunt
Temperature Evaluation has always been crucial in assessing animal health and their reactions to external factors. Historically, measuring temperature has been limited to adult mice due to the size of the transponder and needle cannula. With new improvements in micro-circuitry, there is now an option for a smaller 9mm transponder option with temperature capabilities. Measurement of temperature in animal models smaller than five grams or even neonatal rodent pups can be achieved using the TP-500 Temperature Programmable Transponder developed by Avidity Science.
This Technical Trade Presentation is sponsored in part by Avidity Science.

Primate Cadaver for Training and Surgical Planning
3:20 PM - 3:40 PM/Room: 202A
Speaker: Jake Lehman
Moderator: TBN
There is a need for better technology and resources for training primate anatomy. With over a decade of experience with 3D visualization of real human cadavers, Anatomage has created a digital primate cadaver developed from an actual primate cadaver specimen. With over 1,100 segmented structures, the digital primate will enable
the visualization of highly detailed and accurate primate anatomy for training and surgical planning. During the session, attendees can dissect and explore skeletal, muscular, cardiovascular, nervous, and major organ systems and learn about the origins of the digital primate cadaver.
This Technical Trade Presentation is sponsored in part by Anatomage, Inc.

3:40 PM - 4:00 PM/Room: 202A
Speaker: Brad Gien
Moderator: Steve C Denault
Our session will begin with a brief overview of the purpose behind rodent bile collection studies and their importance in scientific research. We’ll then explore how the innovative technology developed by SAI is revolutionizing the process, making the notoriously challenging task of bile collection significantly easier. Attendees will be introduced to SAI’s products that streamline bile studies, improve animal welfare, and ensure a less stressful experience for researchers and the animals involved.
This Technical Trade Presentation is sponsored in part by SAI Infusion Technologies.

4:00 PM - 4:20 PM/Room: 202A
Speaker/Moderator: Michael Ellis
Computer vision-based behavioral monitoring is a highly underutilized technology in drug development despite its potential to enhance preclinical studies through dynamic rodent phenotyping. This presentation introduces a new digitally enabled platform for home cage monitoring, integrating rodent cages with advanced cameras, cloud-based infrastructure, and machine learning algorithms for digital biomarker (DB) development. This system features continuous monitoring of metrics, including a multidimensional activity profile, respiratory rate, loss of righting reflex detection, food and water level alerting, and water in/out and cage in/out detection, with additional metrics in development. These digital measures can be used to enhance the translational relevance, generalizability, reproducibility, and 3Rs impact of animal studies. Ultra-high-definition cameras live stream animal activity to the cloud, providing veterinary professionals and in vivo scientists with real-time access through an intuitive interface. This interface enables live monitoring and visualization of DBs, identifying significant events such as behavioral changes and physiologic responses. Collaboration between investigators and between investigators and veterinary personnel is facilitated through simplified annotation and study-sharing features. The scalable design supports multi-cage stacks, making it suitable for complex investigations, DB development, and industrial-scale deployment. This technology significantly advances rodent preclinical research and husbandry, offering improved efficiency, real-time analytics, and a holistic approach to phenotyping. This presentation will provide an overview of this novel technology, including design, application, and outcomes of proof-of-concept vignettes demonstrating the unique value of digital home cage measures.
This Technical Trade Presentation is sponsored in part by The Jackson Laboratory.



Your Data is Only as Clean as Your Bedding
1:00 PM - 1:20 PM/Room: 205A
Speaker: Joel M Shepherd
Moderator: Cindy A Buckmaster
In this presentation, we will discuss the potential impact of bedding quality on the integrity of research outcomes. Participants will learn how beddings commonly used in biomedical research are manufactured, tested, and certified- focusing on what distinguishes one bedding from another and how that may impact meaningful biomedical progress. This will be an open and honest discussion about how we can work together to minimize study variability and increase the translatability of our findings for future medical advancements.
This Technical Trade Presentation is sponsored in part by Shepherd Specialty Papers.

1:20 PM - 1:40 PM/Room: 205A
Speaker: Kevin Lorcheim
Moderator: Scott Hoy
Are you wondering which decontamination method is right for your facility? For starters, it’s not a one-size-fits-all situation. Your research program has unique needs, goals, and operations that influence which type of decontamination best fits your research facility. Attend this session to learn the crucial factors to consider before choosing your facility’s decontamination method: the target pathogen, application area size, shape, item quantity, materials, and timeline. Explore your options with decontamination expert Kevin Lorchiem, Senior Manager at ClorDiSys, an Allentown company. Discover manual spray techniques, ultraviolet light technology, fog/vapor, and gaseous methods – and which solutions are most compatible with your vivarium and program. So, whether you’re a decision-maker in laboratory animal science overseeing decontamination action or a laboratory animal professional eager to expand your knowledge on decontamination, this talk is a must-attend.
This Technical Trade Presentation is sponsored in part by Allentown LLC.

1:40 PM - 2:00 PM/Room: 205A
Speaker/Moderator: Jessie Chouinard
Purified diets are an important part of many areas of biomedical research. Created from purified ingredients, such as casein, corn starch, cellulose, vitamins, and minerals, and with each individual nutrient provided by one particular ingredient, purified diets allow for greater control over the nutrient content of the diet than grain-based diets. These formulas are “open” (published), remaining constant and unchanged, thus allowing researchers to report the ingredient composition and increase study reproducibility. Nutrient stability of grain-based laboratory animal diets in various settings has been well documented over the years, but there is limited data on purified diets. When purified diets were first developed around the 1970s, recommendations focused on using the diets within six months from
the date of manufacture. Unlike grain-based laboratory animal diets, where abundant data supports our current recommendations, those set for purified diets were somewhat arbitrary. While we still use those standards today, we would like to review the current standard and use data to provide recommendations based on a better understanding of the nutrient stability of various purified diets. Therefore, we evaluated the nutrient stability of four different representative purified diets for 12 months at three different storage conditions (ambient, 4°C, and -18°C). This presentation will cover the results from this trial and provide recommendations on properly storing various purified diets. The target audience will include researchers, facility managers, and veterinarians, who may be involved in decisions about the storage of these custom diets.
This Technical Trade Presentation is sponsored in part by PMI Nutrition International (LabDiet®).

Leveraging Silver as a Disinfectant: Enhancing Safety and Material Compatibility in Research Facilities
2:00 PM - 2:20 PM/Room: 205A
Speaker: Nick G Hidell
Moderator: Perry L Spires
This technical trade presentation will present research on the efficacy of silver-stabilized hydrogen peroxide, with a focus on the role of silver in Halosil NXT’s HaloMist disinfectant. Attendees will gain insights into the unique properties of silver that enhance biocidal efficacy and its compatibility with lower concentrations of hydrogen peroxide. The presentation will explore how this integration enhances safety, improves material compatibility, and elevates the overall effectiveness of the Halo Disinfection System. During our presentation, we will share research regarding the antibacterial properties and mechanism of activity of silver-stabilized hydrogen peroxide, as well as practical knowledge about and insights into the Halo Disinfection System. The target audience for this Technical Trade Presentation includes facility directors and managers overseeing hygiene protocols, laboratory technicians and hygiene specialists involved in daily disinfection tasks, infection control officers developing prevention programs, and safety officers maintaining safe working environments in life sciences and research facilities.
This Technical Trade Presentation is sponsored in part by Quip Labs.

We are all Animals - But we all have Different Data Requirements!
2:20 PM - 2:40 PM/Room: 205A
Speaker: Mat Sanderson
Moderator: Leo Herlin
How we work with different species and collect data is a considerable challenge when implementing facility management software. What works for rodent facilities does not necessarily work for a large animal facility. What about a facility with both small and large animals where the data requirements vary greatly? A lot of software is heavily focused on rodent breeding and rodent procedures, but there are still a lot of non-rodent species being worked on that need efficient and accurate data collection methods. During this fun presentation, we will share our experiences of working with non-rodent facilities with some examples of how the workflows for large animal species can be achieved in a real-time situation where multiple people are involved in the process to record some of the more ‘uncommon’ activities which may be specific to larger animals. We will also discuss how these can be implemented in a multi-species facility. Topics covered will include ordering animals from internal or external sources, easily and accu-





rately collecting data in real-time, retrospectively, or using paper records, and generating documentation or final reports to be used elsewhere. The target audience is technicians, veterinary care staff, vivarium managers, and researchers.
This Technical Trade Presentation is sponsored in part by Brain & Software International - enos.

Transforming Vivarium Operations: Cutting-Edge Advances in Automation and AGV Technologies
2:40 PM - 3:00 PM/Room: 205A
Speaker: Fabio Mazzucchelli
Moderator: Mike Dvorak
This presentation explores the profound influence of cage automation and Automated Guided Vehicle (AGV) systems within the laboratory animal industry. AGVs epitomize leading-edge technology integrated into cage wash areas, effectively streamlining cage and component cleaning processes. This advancement enhances staff ergonomics and safety and markedly diminishes labor-intensive tasks. Furthermore, the integration of AGV systems optimizes facility design by improving space utilization and enhancing HVAC system dimensioning. Consequently, this presentation delves into the tangible benefits of automation and AGV systems, encompassing reduced capital and operational expenditures alongside heightened operational efficiency.
This Technical Trade Presentation is sponsored in part by Tecniplast.

3:00 PM - 3:20 PM/Room: 205A
Speaker: Erik D Dohm
Moderator: Robert J (Robby) Tindal
Abstract: Integrating advanced technologies has become imperative for ensuring the well-being of mice used in research and efficient operations. It is now possible to design monitoring systems in a price range compatible with vivarium-wide home cage deployment to bring advanced technology to the cage side. Artificial Intelligence (AI) and machine learning can effectively interrogate 24/7 data streams from individual cages to interpret mouse ultrasonic vocalizations (mUSV) and cage microenvironmental conditions of temperature, humidity, and light to paint a clear picture of animal welfare and detect possible biomarkers. Advanced individual home cage monitoring helps to ensure experimental reproducibility and the integrity of data collection points. In addition, advanced technologies such as these create a treasure trove of new data previously unavailable, impacting all levels of research, from vivarium management up through the research itself. This talk targets principal investigators, laboratory/facility/operations managers, veterinarians, and dedicated laboratory animal professionals. Join us in exploring the future of mouse animal facility home cage monitoring.
This Technical Trade Presentation is sponsored in part by Tricorder Array Technologies LLC PenPal™.

3:20 PM - 3:40 PM/Room: 205A
Speaker: Brittany Buchman
Moderator: Michael Matthews
Current processes of cleaning gnotobiotic chambers and their surroundings require considerable time and effort, which disrupts core disinfection schedules. Enhancing the efficiency of the decontami-
nation process could significantly improve research productivity by minimizing the elapsed time and potential errors associated with physical labor. This presentation unveils the innovation and greaterthan-six-log reductions (Geobacillus stearothermophilus) of ionized Hydrogen Peroxide (iHP) decontamination and SteraMist systems, backed by scientific research findings. In collaboration with the Department of Homeland Security (DHS) Science and Technology Directorate’s (S&T’s) Plum Island Animal Disease Center (PIADC), iHP has demonstrated efficacy against Foot-and-Mouth Disease Virus, African Swine Fever and other problematic pathogens that showcase a promising potential to revolutionize and centralize decontamination routines research settings. The necessity of turnkey decontamination solutions that align with the changing demands of vivarium facilities (from standard to modular configurations) highlights a potential space for regulated, high-log contamination control that doesn’t sacrifice innovation and results. With the recent EPA movement to eliminate hazardous substances like ethylene oxide sterilization, it’s crucial to ensure facility adherence to current and emerging standards (GxP, Annex 1) in viral vector and fill-finish manufacturing to vivarium cleaning and equipment sterilization while considering future standards. Discover how iHP technology can safeguard research with a quick, efficacious, turnkey decontamination solution.
This Technical Trade Presentation is sponsored in part by SteraMist Disinfection & Decontamination.

3:40 PM - 4:00 PM/Room: 205A
Speaker: Bob Davis
Moderator: Perry L Spires
The presentation focuses on the newest developments in using dry heat instead of steam for sterilizing laboratory animal cages, IVC racks, water, BL2 and BL3 waste, enrichment, and other items used in the vivarium. The outline of the talk is as follows: What is dry heat sterilization? How does it work with laboratory animal cages, IVC racks, and related items? How does dry heat sterilization compare to steam sterilization? What are the benefits of dry heat sterilization? Developments in Gruenberg’s dry heat sterilizers; Sterilization of water; BL2 configurations and application notes; BL3configurations and application; notes; VHP/Gaseous decontamination configurations and application notes; How are these systems validated? Review of a recent study on preventing Murine Norovirus in Mice using dry-heat sterilization.
This Technical Trade Presentation is sponsored in part by Gruenberg - TPS.

4:00 PM - 4:20 PM/Room: 205A
Speaker: Karena Thek
Moderator: Karen M Froberg-Fejko Environmental Enrichment (EE) is a crucial aspect of good husbandry and care of all laboratory animals in our care. The challenge when considering EE is understanding the purpose of enrichment and knowing what kind of enrichment works in your environment. Primate enrichment can be challenging due to their complex behaviors and limited attention span. This discussion will include why we provide enrichment, how to broaden your enrichment program, the methodology of thinking of toys as more than just toys, and some ideas for thinking outside the box about enrichment.
This Technical Trade Presentation is sponsored in part by Bio-Serv.



8:00 AM - 12:00 PM/Room: 107A
Leader: April J George
Faculty: Michael L Fallon, Mandy N Sexton
Facilitator: TBN
Workshop Fee: $150 Workshop Limit: 50
Alternative Replacement Training (ART) Methods are one impactful way to implement the 3R’s principles within our training curriculums. ART methods can reduce the number of animals needed to achieve competency, improve a facility’s culture of care, and refine training approaches by improving efficiencies and skill development. During this workshop, we will allow attendees to experience training models and emerging technologies firsthand. Facilitators will provide data regarding the benefits of these training modalities while stimulating conversations between attendees regarding impressions and experience. Participants will get the opportunity to experience and discuss the creation process and discuss the benefits of training models, the Anatomage Virtual Dissection Table, 3D scanning, 3D printing, and virtual reality for learning. Attendees will rotate between stations to permit hands-on experience with all the above-mentioned ART methods and learn more about incorporating these tools into training programs. The target audience is anyone who performs training as part of their job role or wants to expand their animal welfare and 3R’s initiatives into training curriculums and improve training efficiencies.

(offered twice, also Monday 1:00 PM)
8:00 AM - 12:00 PM/Room: 110B
Leader: Robert F Hoyt Jr
Faculty: Tannia S Clark, Tanya L Herzog, Kenneth R Jeffries, Karen J Keeran, Audrey Noguchi, Marvin L Thomas, Gayle Z Nugent, Randall R Clevenger, Shawn M Kozlov, Victoria L Frasier
Facilitator: Bertrand Lussier
Workshop Fee: $250 Workshop Limit: 20
Performing surgical procedures with magnification has gained widespread use in human medicine over the past 30+ years. Using surgical loupes, surgeons can now routinely perform procedures on tiny structures considered impossible a few decades ago. Within the past 20 years, using magnification to perform microsurgical techniques to increase precision has spread to other health disciplines, including dental specialties and dermatology, such that using magnification for performing routine procedures is now considered the standard of care. Unfortunately, the use of microsurgery and its value to biomedical research has only begun to be realized. Because of their small structures, laboratory animals, such as rats and mice, have generally not been considered animal models for many surgical procedures routinely performed in biomedical research. Investigators have routinely elected to use larger species such as dogs, pigs, sheep, rabbits, and nonhuman primates for such modeling because surgical equipment is more readily available, and the surgical techniques are more familiar to the support personnel. The recent shift to using genetically engineered rodents, especially mice, has increased researchers’ desire to use these smaller animals in more sophisticated modeling procedures. Rather than being limited to simple procedures, such as IM, IP, or IV injections, microsurgery researchers can now perform complex surgical procedures on many rodent organ systems, such as the heart, lungs, and gastrointestinal tract.

This workshop will introduce the basic techniques, equipment, and general microsurgery applications using surgical loupes. Hands-on training will be conducted in two phases: 1) teaching students to develop technical skills by performing exercises using surgical loupes, and 2) applying these skills to perform simple surgical procedures using rodents and organ surrogates. To enhance student success, we have significantly increased the teacher-student ratio.
This Workshop is sponsored in part by Q-Optics, Supramid Suture, and RICA Surgical Products.

W-03 Tips for Perioperative Anesthetic Monitoring
8:00 AM - 12:00 PM/Room: 109
Leader: Cholawat Pacharinsak
Faculty: Patrick E Sharp
Facilitator: TBN
Workshop Fee: $150 Workshop Limit: 30
General anesthesia alters multiple physiologic responses of the body. Therefore, anesthetists should monitor patients from premedication to recovery to gain optimal anesthesia outcomes. Monitoring requires anesthetists to thoroughly understand the various monitoring equipment used and how to interpret the information generated. Anesthetic monitoring alerts anesthetists to arising complications so anesthetists can take the necessary actions to correct the complications before they become irreversible. This workshop will focus on basic monitoring methods, including ECG, direct and indirect blood pressure, ETCO2, %SpO2, and body temperature. Troubleshooting anesthetic complications will also be discussed. This dry workshop is suited for veterinarians, veterinary technicians, IACUC members, and scientists.
8:00 AM - 10:45 AM
Platform Session abstracts will be available on www.aalas.org in July. They will also be included in both the mobile app and the National Meeting Final Program.

New Tools for Enhancing Welfare and Research: The Promise of AI in Laboratory Animal Science
8:00 AM - 10:00 AM/Room: 101A
Leaders: Gerry A Hish, Oliver He
Moderator: William W King
Facilitator: TBN
Artificial intelligence (AI) and machine learning (ML) have emerged as transformative forces in research and healthcare, offering unprecedented opportunities for advancements in various applications. Despite the recent popularity of generative AI tools like ChatGPT, many other uses of AI - both potential and already realized - are only sometimes appreciated by those who might benefit most from their use. As these technologies become more accessible, professionals working in laboratory animal science (LAS) will be in a unique position to identify areas that could benefit from AI and ensure its optimal use to enhance animal welfare, streamline research methodologies, and improve data management and interpretation. This seminar series will focus on understanding the potential applications of AI and ML through a review of its current uses in related fields and by identifying opportunities for its use in LAS. Speakers will share their experiences applying these technologies to varied areas




of LAS based on their roles and areas of expertise. These include a bioinformaticist who will discuss the importance of using standardized biomedical ontologies to enhance natural language processing and improve the ability of AI to mine complex datasets, a veterinary pathologist who will demonstrate how the pathology service core at their institution is utilizing AI-based chatbots to enhance the service it provides to its customers, and a clinical veterinarian that is using a publicly available AI tool and computer vision to improve the assessment of animal behaviors and enhance welfare. This session and the discourse it will facilitate is intended for anyone interested in learning more about the challenges, successes, and future directions that AI might have in revolutionizing laboratory animal science and how it will contribute to the broader field of biomedical research.
Speakers/Topics:
8:00 William W King Welcome and Introductions
8:10 Marc S Hulin
8:30 Oliver He
8:50 Yao Lee
9:10 Gerry A Hish
The Use of AI in Laboratory Animal Science: Current and Potential Applications
Making data FAIR and AI-ready in Lab Animal Medicine
Pathology Empowered: Optimizing Core Services with AI Tools
Enhancing the Analysis of Laboratory Animal Behaviors (And Preserving My Sanity) Through AI-based Video Assessment
9:30 All Questions & Answers

Ongoing Changes within the Animal
8:00 AM - 10:00 AM/Room: 104A
Leader/Moderator: B Taylor Bennett
Facilitator: Brandon Morton
This past year has again presented challenges in keeping up with issues that impact the animal research oversight environment. There continues to be an unprecedented number of bills introduced at the state and federal level with the potential to affect animal research. There has been the need to adapt to the recent revisions to the Animal Welfare Regulations. At the same time, the process for addressing the proposed actions in the joint Report on Reducing Administrative Burden for Researchers: Animal Care and Use In Research (released 8/28/2019) continues, and AAALAC has had additional changes to its leadership. The discussion surrounding the process for updating the Guide for the Care and Use of Laboratory Animals continues. Efforts by those opposed to animal research targeting large animal and nonhuman primates appear to continue to be an issue. This has impacted how animal care and use programs are managed going forward. This seminar will provide the attendees with an opportunity to hear from representatives of the USDA, OLAW, AAALAC International, the DOD, FDA, and NABR regarding these ongoing issues and possible changes within their organizations and to discuss with those representatives how their organizations’ activities impact the environment in which we work and what changes to expect in the future. Questions for the panelists can be submitted to btbdvm@yahoo.com. The target audience will be those who need to keep current with the regulations and requirements for conducting animal-based biomedical research.
Speakers/Topics:
8:00 B Taylor Bennett Welcome and Introductions
8:10 Elizabeth C Theodorson USDA Update
8:25 Neera V Gopee OLAW Update
8:40 Gary L Borkowski AAALAC Update
8:55 Shannon T Marko DOD Update
9:10 Dorothy Bailey FDA Update
9:25 Matthew R Bailey NABR Update
9:40 B Taylor Bennett Discussion
This Seminar is sponsored in part by National Association for Biomedical Research (NABR), The U.S. Department of Agriculture (USDA), Animal and Plant Health Inspection Service Animal Care, National Institutes of Health Office of Laboratory Animal Welfare, AAALAC International, and Department of Defense, Food and Drug Administration.

8:00 AM - 10:00 AM/Room: 207A
Leader/Moderator: Steven E Davison
Facilitator: Sarah Hansen
The use of germ-free and gnotobiotic mice in research is ever-increasing due to the rapidly evolving data implicating the microbiome’s influence on various diseases and health conditions. Institutions and researchers may need to share germ-free animals for several reasons, including the need for unique genetic strains, establishment of breeding colonies, and use in pilot experiments. However, facilities to maintain or establish germ-free colonies are often costly and may be cost-prohibitive for some institutions. Additionally, there is limited availability and very few strains of germ-free mice available from commercial vendors. For all these reasons, the import and export of germ-free mice between institutions is occurring at higher rates. Sharing these animals presents specific challenges that institutions may be unprepared for if personnel are unfamiliar with the process. This seminar will provide researchers, veterinarians, and husbandry staff with practical tips and considerations for sharing and housing these specialized animals. Topics will include health and disease status information that both the exporter and importer should consider and regulatory and experimental forethought required before sharing germ-free mice. Additional information will include basic information regarding the purchase of germ-free mice from vendors and technical tips for shipping germ-free mice in various specialized shipping devices. Housing considerations will be discussed, including housing designed for germ-free or gnotobiotic mice and temporary housing options for facilities that do not have germ-free facilities where needed. We will also cover some basic information regarding purchasing germ-free mice from vendors.
8:00 Steven E Davison Welcome and Introductions
8:05 Steven E Davison Overview of Why Investigators and Institutions Share Germ-Free Animals
8:35 Erin N Yu Receiving Germ-Free Mice: Colony Health and Housing Considerations
8:55 LaTisha V Moody Shipping Options for Germ-Free Mice
9:35 Lucy H Kennedy Considerations and Procedures for the Exporting Institution


Exhibit Hall Refreshment Break!
9:00 a.m.– 11:00 a.m.
Sponsored by NEPCO

Pathology Quiz Bowl
8:00 AM - 9:15 AM/Room: 209A
Leader: Marcia L Hart
Moderator: Marcus J Crim
Facilitator: TBN
Panelist: Marcia L Hart, Bettina A Gentry
This panel discussion will consist of an informal review of the pathology of laboratory animals in the form of an image-based interactive Mentimeter quiz. Topics will include lesions of well-described infectious and non-infectious diseases, pathological manifestations of emerging diseases, and selected phenotypic characteristics of important genetically engineered animal models. Images will be educational and challenging to laboratory animal specialists at all levels of pathology expertise. The targeted audience is comparative medicine trainees, laboratory animal veterinarians, pathologists, and scientists. Participants from comparative medicine training programs can receive a fabulous cash prize for the highest score. Participants will learn the gross and histologic pathology of laboratory animals.

Keeping it in the FAMily: Facility and Animal Management Quiz Bowl
9:30 AM - 10:45 AM/Room: 209A
Leader: Erin K O’Connor
Moderator: Samantha A Gerb
Facilitator: Craig L Franklin
Panelist: Samantha A Gerb, Scott W Korte, Erin K O’Connor, Sarah N
Schlink, Dana E Weir-Guffey
This panel discussion is a partner to the long-standing Pathology Quiz Bowl and will consist of an informal review of elements of facility operations and experimental animal models in the form of an image-based quiz using Mentimeter. Topics include facility equipment, environmental conditions, husbandry considerations, animal


models, and experimental techniques. Images will be educational and challenging to laboratory animal specialists at all levels of laboratory animal expertise. The targeted audience is comparative medicine trainees, laboratory animal veterinarians, laboratory animal care professionals, and scientists. Participants can receive a fabulous cash prize for the highest score.
This Panel Discussion is sponsored in part by IDEXXX BioAnalytics.

10:00 AM - 11:30 AM/Room: 106C
Leader/Moderator: Ann T Turner
Facilitator: TBN
Panelist: B Taylor Bennett, Dennis M Stark, Tom E Darby, Cindy A Pekow, Chip H Price, Mark A Suckow, Bob C Dysko, Tracy A Parker
Have you ever wondered how GLAS came about? Are you curious about why the AALAS Foundation was established – and when? What is the Leadership Summit, and how does AALAS plan strategically? Why is AALAS involved in international lab animal science, and how has that role evolved? Why does AALAS have vendor members when most associations do not allow suppliers to be active members? What about all the specialty lab animal associations, and how does AALAS fit in? How does AALAS balance board composition? A panel of Past AALAS Presidents will discuss these topics plus others to provide a first-hand glimpse of significant milestones on the AALAS journey, from a small group of veterinarians meeting in one room to discuss research rodent nutrition to the multifaceted organization of today. While a detailed history of AALAS is presented in the iconic book 50 Years of Laboratory Animal Science: 1950-2000, this discussion will focus on the more recent history. According to William Shakespeare in The Tempest, “What’s past is prologue,” meaning history sets the stage for the present and future. The presenters will share stories from the past and perspectives for the future of AALAS. This session will interest anyone attending the national meeting, no matter how many years they have been involved with AALAS.

ClearH2O
10:10 a.m. – 10:30 a.m.
(Presentation title, product, and description to come)

Allentown, LLC
10:35 a.m. – 10:55 a.m.
(Presentation title, product, and description to come)
DVM CHARLES C. HUNTER LECTURE
Dr. Louden Wright received his DVM from the University of Tennessee College of Veterinary Medicine in 2015. Following this, he completed internships at the University of Wisconsin and Kansas State University veterinary teaching hospitals and a residency in zoo and wildlife medicine through the University of California-Davis and the San Diego Zoo Wildlife Alliance. He has since achieved diplomat status in the American College of Zoological Medicine. Post-residency, he worked as the Veterinary Director for the Great Plains Zoo in Sioux Falls, South Dakota and as a clinical veterinarian for Sanford Research. He is currently the associate veterinarian for the Nashville Zoo, where in addition to his clinical duties, he coordinates veterinary involvement in ongoing wildlife research. His own research interests are in pharmacokinetic and pharmacodynamic assessments of analgesics in wildlife species and anesthetic protocol development in amphibians.





A Decade of Openness on Animal Research in the UK: The Good, the Better and the Unexpected. What Has Changed Since the Concordat Was Published?
11:00 AM - 12:00 PM/Room: 101A
Speaker: Wendy J Jarrett
Moderator: Paula A Clifford
Facilitator: TBN
The last ten years have seen a concerted effort in the UK to improve public communication about the use of animals in scientific research, driven mainly by the Concordat on Openness, published in May 2014. This was the world’s first openness agreement, which has since been adapted and adopted in nine countries. Work on a US Animal Research Openness Initiative is also well underway. More than 125 UK organizations have now committed to being more open about using animals in research. How has this drive for openness changed the way our sector communicates? Does the UK public now have access to better information about animal research? How has the Concordat affected staff morale? How is openness helping to dispel misinformation and misrepresentation? Have there been any unintended consequences or negatives? This presentation will aim to answer these questions and more, providing attendees with an update on how the Concordat on Openness has affected UK bioscience in its first decade. It will interest animal care staff, veterinarians, facility managers, communications professionals, and anyone interested in helping the public understand the realities of animal research rather than the myths.
This Special Topic Lecture is sponsored in part by Understanding Animal Research.

Charles C Hunter Lecture: Kangaroo Wrangling - Managing Clinical and Conservation Research at a Zoo
11:00 AM - 12:00 PM/Room: 209A
Speaker: Louden Wright
Moderator: Amanda E Sparks
Facilitator: TBN
All zoological facilities accredited by the Association of Zoos and Aquariums must maintain a commitment to scientific study in proportion to their size and scope. With small population sizes and the limitations of coordinating research on heavily regulated endangered species, scientific study in zoos can look slightly different than in most research facilities. From assisted reproductive techniques in leopards to radiotracking hellbenders in Tennessee rivers to pharmacokinetic studies on kangaroos, Nashville Zoo has developed a wide-ranging program of scientific advancement. Come and hear how this AZA-accredited facility in the heart of Nashville manages and encourages its research program and the impact that program is having on animals at our zoo and beyond. This presentation should appeal to anyone attending the 2024 AALAS National Meeting. It should also be interesting for anyone who designs and performs research, as well as those who participate in it and provide oversight for projects.
This Special Topic Lecture is sponsored in part by the Committee for Technician Awareness and Development (CTAD).

11:00 AM - 12:00 PM/Room: 207A
Speaker: Marcia L Hart
Moderator: Robert “Bob” S Livingston
Facilitator: Crysti Reed
Unexpected morbidity and mortality within contemporary rodent research colonies can significantly slow or completely halt research projects for affected investigators. Animal loss or confounding research data generated from diseased animals can render experimental data difficult to interpret or unusable. In many cases, these outcomes also impact investigators sharing housing or workspace. Unexpected morbidity or mortality can be caused by or related to multiple factors, including husbandry, the environment, experimental manipulation, genetics, or infectious disease, with most infectious agents being rare or opportunistic agents not typically found by routine animal health monitoring. Developing an appropriate and timely diagnostic approach for a sick animal or widespread illness/ death within a rodent colony can help mitigate the situation and rapidly resume research. This presentation will utilize various diagnostic case scenarios to outline best practice approaches for sample collection methods and techniques, sample handling guidelines, and expectations regarding the next steps. This presentation will contain helpful information for veterinarians, veterinary technicians, facility managers, and scientists.

11:00 AM - 12:00 PM/Room: 104A
Speaker: Nancy E Halpern
Moderator: Jim Newman
Facilitator: Thomas A Leach
This lecture will discuss the current status of FOIA requests to regulators, preparing and submitting responses, legal remedies for protecting confidential information, and staff privacy while providing responsive information as requested. Regulated research facilities are increasingly the target of Freedom of Information Act (FOIA) requests, often related to the same situation and requiring significant resources to provide timely responses while protecting staff safety and the integrity of research projects. Participants will learn the various legal approaches to responding to such requests and how to build protections into existing administrative procedures in anticipation of continued requests in the future. Target audience: Attending Veterinarians, Veterinary Staff, Animal Health and Welfare Staff, Compliance Staff, IACUC members, Administrative Staff, General Counsel and Staff.



AALAS and FELASA: Working Together for the Laboratory Animal Science Community
12:30 PM - 2:00 PM/Room: 106C
Leader: Scott A Mischler
Moderator: Elizabeth A Nunamaker
Facilitator: TBN
Panelist: Scott A Mischler, Elizabeth A Nunamaker, Klas Abelson, TBN
The Laboratory Animal Science (LAS) community, represented by the Federation of European Laboratory Animal Science Associations (FELASA) in Europe and the American Association for Laboratory Animal Science (AALAS) in the United States, have been working together to harmonize animal care and research recommendations. This session will provide an overview of the structure and relationship between FELASA and AALAS, as well as the value of this harmonization effort to the laboratory animal community. Brief updates on the ongoing joint activities of the associations will be presented. Finally, ample time for panel and audience participation will be provided to explore future collaborative efforts between the associations. Topics will include the History of the relationship between AALAS and FELASA, FELASA and Working Group establishment, updates on the Pig Conditioning Working Group, the value of harmonization and how you can get involved, and an open microphone discussion on potential future collaborations and topics. The target audience will include AALAS and European researchers and laboratory animal scientists interested in learning about the joint efforts and accomplishments of AALAS and FELASA (Federation of European Laboratory Animal Science Associations) in harmonizing guidelines between the world regions. Additionally, audience participation will be critical in defining future directions and potential participants for joint efforts.

From Expert to Leader: Navigating the Leap from Individual Contributor to Manager
12:30 PM - 2:00 PM/Room: 102A
Leaders: Mary T Spencer, Jarrod Nichol
Moderator: Steven J LaMacchia
Facilitator: TBN
Panelist: Jarrod Nichol, Mary T Spencer, Lindsay G Andrews, Renee L Thompson
In the world of work, standout performers are often catapulted into management roles, riding the wave of their success with little preparation for the challenges ahead. This leap of faith by employers rests on the assumption that excellence in one’s field naturally translates to effective leadership. Yet, the reality is more complex. Without a structured development plan, coaching, or mentorship, this transition can become a daunting obstacle course for the newly minted manager and a potential pitfall for the organization. This engaging panel discussion will delve into the critical support structures necessary for smoothing the journey from individual contributor to manager. We’ll explore the indispensable role of targeted training and skill development, ensuring that new managers survive and thrive in their roles. How do we cultivate an ecosystem within our organizations that not only supports this pivotal transition but also fosters an atmosphere of growth, mentorship, and lifelong learning? Join us as we unravel the strategies for empowering your top talent to embrace the mantle of leadership, transforming expert performers into visionary managers who can inspire their teams and drive organizational success. The target audience for this discussion is Frontline Staff and New Managers.
This Panel Discussion is sponsored in part by Vivarium Operational Excellence Network (VOEN).


12:30 PM - 2:00 PM/Room: 103A
Leader/Moderator: Jeanne M Wallace
Facilitator: Kristin Uyl
Panelist: Ronald Emeson, Jessica Janeczek, Karen Jackson, Todd Ellis
This panel will discuss “MyColony,” an initiative at Vanderbilt University Medical Center in partnership with Transnetyx. NIH directives inspired the initiative to improve the rigor and reproducibility of animal studies and recognize that genotyping and breeding colony management have become a standard part of maintaining mouse colonies. Gel-based PCR and poor colony management introduce unnecessary experimental variability, reducing productivity and increasing costs while hindering housing space utilization. Automated Genotyping and Colony + Artificial Mouse Intelligence (AMI) software address these issues. After a piloting phase and multiple stakeholder consultations, a new “standard of care” for breeding mouse cages was established, encompassing routine services and unlimited access to Automated Genotyping and Colony + AMI software.
A MyColony per diem rate, instituted over two years, was selected as the cost recovery model. The program launched in December 2022, and the first breeding cage per diem increase occurred in July 2023. To date, 158 out of 180 labs have utilized the MyColony services. The target audience includes IACUC/research administrators, animal resource directors/managers, breeding core managers, and researchers. Participants will learn about Automated Genotyping and Colony + AMI›s advantages over traditional methods and how these services enhance research rigor/reproducibility, reduce wasteful overproduction, and ensure efficient use of vivarium space. The presentation also covers the initiative’s change management plan and cost recovery model and examines the pros, cons, and potential improvements. Supported by the ACLAM/ASLAP Joint Program Committee.
This Panel Discussion is sponsored in part by The American College of Laboratory Animal Medicine (ACLAM) and the American Society of Laboratory Animal Practitioners (ASLAP).

12:30 PM - 2:00 PM/Room: 105A
Leader: Kerith R Luchins
Moderator: Brianne M Hibl
Facilitator: Julita A Ramirez
Panelist: Joseph P Garner, Chris A Manuel, Wai Hanson, Patricia L Foley
As environmental health monitoring (EHM) for rodent colony health becomes increasingly common, there continue to be new applications, questions related to, and case studies for this technology. In this session, participants will hear from subject matter experts from the 3Rs Collaboratives Environmental Health Monitoring Initiative on what’s next for EHM. We will begin with an overview of the ever-expanding number of methods and definitions of EHM. Topics include case studies on how to use EHM to detect and eliminate critical pathogens, a brief review of newly updated systematic review data, the application of EHM for rats, and experiences with false positives. Finally, IACUC considerations for EHM will be discussed. The target audience for this presentation is broad. It includes veterinarians, veterinarians in training, vivarium managers and supervisors, animal care technicians, and IACUC members at any institution with a rodent health monitoring program.
This Panel Discussion is sponsored in part by The 3Rs Collaborative.





The NCI Comparative Oncology Trials Consortium: An Infrastructure for Implementation of Comparative Oncology Clinical Trials Consortium: An Infrastructure for Implementation of Comparative Oncology Clinical Trials in Pet Dogs to Advance Studies in Cancer Drug Development and Biology
12:30 PM - 2:00 PM/Room: 106A
Leader: Amy K LeBlanc
Moderator: Amy K LeBlanc
Facilitator: TBN
Panelist: Amy K LeBlanc, Jessica Beck, Christina Mazcko
Pet dogs spontaneously develop cancer within the context of a natural lifespan, shared home environment with humans, and intact, educated immune system. In recent years, canine cancer patients have emerged as an attractive bridge species between mouse models and human patients. Clinical studies conducted in these canine patients are known as comparative oncology clinical trials. These trials are designed to generate complementary datasets that inform the human cancer drug development process regarding in vivo target modulation, pharmacokinetic-pharmacodynamic relationships, tolerability, and efficacy. In this session, speakers from the NCI Comparative Oncology Program will discuss the entire process of comparative oncology trial conception, design, and implementation in detail. Emphasis is placed on the scientific and research questions that form the basis of the trial, in addition to logistical considerations, including ACUC approval and biosafety issues, determination of trial endpoints, and biologic sample collection and processing to ensure the trial culminates with valid, interpretable data. Specific examples of NCI Comparative Oncology Trials Consortium studies will be presented, covering a range of trial concepts (small molecules, immunotherapies, and medical devices). This session is appropriate for investigators, veterinarians, veterinary technicians/technologists, IACUC members, and anyone interested in translational medicine. This Seminar is sponsored by the ACLAM/ASLAP Joint Program Committee
This Panel Discussion is sponsored in part by The American College of Laboratory Animal Medicine (ACLAM) and the American Society of Laboratory Animal Practitioners (ASLAP).

W-04 Beginning Training Methods & Techniques for NHPs - Part 1
(8-hour workshop continued Tuesday 8:00 AM)
1:00 PM - 5:00 PM/Room: 108
Leader: Lisa A Houser
Faculty: Jaine E Perlman, Stacey Smith, Bess Carlson
Facilitator: TBN
Workshop Fee: $250 Workshop Limit: 50
Positive reinforcement training (PRT) improves the lives and welfare of non-human primates (NHPs) by allowing them to cooperate with clinical, husbandry, and research procedures and providing a form of cognitive enrichment. However, PRT can be daunting in a research environment, especially for staff who need prior training experience. In this 8-hour workshop, participants will gain knowledge and expertise to form a foundation to implement basic PRT for clinical, husbandry, and research-related procedures. Lectures will be supplemented with hands-on experience through interactive activities and demonstrations to implement basic training concepts and techniques. This workshop will provide an understanding of fundamental concepts associated with training: how to set yourself
and your NHP up for success in training, how to utilize clickers to enhance your training sessions, how to shape behavior, how to use targets to train a new behavior; how to implement desensitization techniques to overcome fear in animals; and how to troubleshoot training issues. Participants will gain a strong foundation of training terminology and techniques to use with NHPs in the laboratory. This workshop is designed for beginners with little to no primate training experience and anyone who has an interest in learning how to use positive reinforcement training to train NHPs for clinical, husbandry, and research-related procedures, including animal care technicians, animal care managers, behavior specialists, veterinary technicians, veterinarians, and research technicians.
This Workshop is sponsored in part by Bio-Serv, LabDiet, Lomir, and Allentown.

1:00 PM - 5:00 PM/Room: 107A
Leaders/Faculty: Kirk Leech, Eva C Maciejewski
Facilitator: Leah K Yonkovich
Workshop Fee: $150 Workshop Limit: 50
This communication and media training workshop is designed for all communicators who work at animal research institutions. The topic leaders will share best practices and insights from experience to communicate effectively with the public and the media about animal research. The topic leaders will also discuss messaging and strategic media outreach in a crisis communication situation at a research institution. Following the presentation, the topic leaders will divide the audience into small groups to practice talking about the animal research program at their institution and using the messages they developed in conversation with the members of their group. A second exercise will follow, in which the topic leaders will present a real-life crisis communication scenario at a research institution. They will ask the participants to discuss the scenario and develop messaging and a communications strategy to address it. Topic leaders will invite individual participants to share messaging and their proposed crisis communication strategy with all the participants. Participants will receive the FBR communication manual and the EARA-FBR Crisis Communication Pocket Guide® ahead of the workshop. Hence, they have the tools to refer to as they engage in the workshop’s group exercises. Communicators at animal research institutions will come out of this workshop with a better understanding of how to develop impactful messages to talk about their institution’s animal research and testing program.
This Workshop is sponsored in part by European Animal Research Association (EARA) and the Foundation for Biomedical Research (FBR).

W-02B Microsurgery Skills Training Using Surgical Loupes
(offered twice, also Monday 8:00 AM)
1:00 PM - 5:00 PM/Room: 110B
Leader: Robert F Hoyt Jr
Faculty: Tannia S Clark, Tanya L Herzog, Kenneth R Jeffries, Karen J Keeran, Audrey Noguchi, Marvin L Thomas, Gayle Z Nugent, Randall R
Clevenger, Shawn M Kozlov, Victoria L Frasier
Facilitator: Bertrand Lussier
Workshop Fee: $250 Workshop Limit: 20
See Monday AM for description.
This Workshop is sponsored in part by Q-Optics, Supramid Suture, and RICA Surgical Products.



W-06 Zebrafish Husbandry and Facility Management 101: The Basics
1:00 PM - 5:00 PM/Room: 110A
Leaders: Logan Fehrenbach, Raphael A Malbrue
Faculty: Lindsey T Ferguson, Toi A Collins, Alexander G Kramer, Jonathon R Byington
Facilitator: TBN
Workshop Fee: $150 Workshop Limit: 50
This Workshop is an introductory hands-on experience working with zebrafish in a research setting. The target audience is laboratory animal professionals (i.e., technicians, veterinarians, researchers) new to working with zebrafish or searching for a refresher course to review current introductory practices. Attendees will gain exposure to daily husbandry, clinical, and operational duties from laboratory animal veterinarians and aquatic technical staff to accomplish the following learning objectives: History of Zebrafish Research and Role in Biomedical Research, Introduction to Zebrafish Biology, Introduction to Zebrafish Husbandry, Introduction to Zebrafish Life Support Systems, Zebrafish Facility Management, Zebrafish Nutrition and Feeding Programs, Introduction to Biosecurity and Health Surveillance Programs and Regulations, Policies, and Guidelines About Zebrafish in Research. The workshop will incorporate a series of interactive lectures focused on the listed objectives (i.e., case studies and virtual video simulations). Attendees will also receive hands-on experience with basic husbandry techniques such as setting up rotifer polycultures and basic water quality testing. Participants are encouraged to register for the Zebrafish Husbandry and Facility Management 201: “Advanced Care Techniques” workshop which is on Tuesday afternoon. Attendees confident with zebrafish husbandry and facility management basics are encouraged to enroll directly in the 201 workshop.
This Workshop is sponsored in part by VRL Diagnostics, Iwaki Aquatic Systems, and Aquaneering.

W-07 AALAS Foundation 101: Come Learn About the Foundation and How It Can Support Your Public Outreach Efforts - Find Out What’s New!
2:30 PM - 5:00 PM/Room: 107B
Leader: Vicki C Campbell
Faculty: Debra L Hickman, Larry J Shelton
Facilitator: Joanne C Drew
Workshop Fee: Free Workshop Limit: 50
Learn about the AALAS Foundation - what it does, and how you can get involved in its public outreach mission. Anyone interested in learning more about all of the free and low-cost public outreach materials the AALAS Foundation has available should plan to attend this workshop. Attendees of this workshop will also be able to preview our new public outreach program, “Careers in LAS,” which offers new videos and fully scripted PowerPoint templates that are aimed at helping inform students about the variety of career opportunities in LAS. Samples of the AALAS Foundation’s free outreach materials will be available for workshop attendees. Samples of low-cost “giveaway” items, such as “Careers in LAS” mouse stress balls, cell phone wallets, and more, will be available for review.
This Workshop is sponsored in part by AALAS Foundation.
Exhibit Hall Refreshment Break! 2:00 p.m.– 4:00 p.m.
Sponsored by Charles River

Innovive
2:10 p.m. – 2:30 p.m.
(Presentation title, product, and description to come)

Cayuse
2:35 p.m. – 2:55 p.m.
(Presentation title, product, and description to come)
2:45 PM - 5:00 PM
Platform Session abstracts will be available on www.aalas.org in July. They will also be included in both the mobile app and the National Meeting Final Program.

A Global Look at Current & Emerging Animal Rights Trends: Identifying the Threats & Advice for Combatting Them
3:00 PM - 5:00 PM/Room: 101A
Leader/Moderator: Paula A Clifford
Facilitator: TBN
From years-long protests to brazen animal thefts, targeting animal suppliers, and using the courts as a PR mouthpiece, the animal rights movement is going through a transformational period. As a result, research universities and companies are navigating new waters while responding to and protecting critical animal studies. This session will investigate current and emerging animal rights tactics to provide an up-to-date status report on the primary threats to biomedical research involving animals. We will also explore how activism overseas - primarily taking place in the UK - influences events in the United States. Finally, presenters will offer proactive advice on how organizations and individuals can monitor and protect against these emerging threats. Q&A during the session’s last part will allow attendees to gain advice and insights on specific concerns or issues affecting them or their institutions. Those who attend the session will walk away with a comprehensive understanding of the current threats to biomedical research and how to respond. Leaders who attend will also be armed with information on how to better protect their organizations and staff against these new and emerging tactics.









Target Audience: Veterinarians, Administrators, Animal Care and IACUC Leadership, Institutional Officials, Technicians (Husbandry, Clinical, Research, etc.)
Speakers/Topics:
3:00 Paula A Clifford Welcome and Introductions
3:05 Paula A Clifford
Understanding the Threats to Biomedical Research in Order to Effectively Protect Your Organization and Yourself
3:35 Wendy J Jarrett New and Emerging Animal Rights Tactics in the UK and Monitoring Them
4:05 Jim Newman Current and Emerging Animal Rights Tactics in the United States and How to Respond
4:35 All Questions & Answers
This Seminar is sponsored in part by Americans for Medical Progress (AMP), and Understanding Animal Research.

Implementing Inclusion, Diversity, Equity, and Accessibility into Your Organization
3:00 PM - 5:00 PM/Room: 104A
Leaders: Emily I Weston, Chandra D Williams
Moderator: Tracy A Parker
Facilitator: Jarmareo D Chisley, Sherrie Jean, Mark A Suckow Previously, the IDEA Advisory Council surveyed and collected ideas from members and panel participants on DE&I topics within the AALAS organization and held a workshop to develop ideas and strategies to increase inclusion, diversity, equity, and accessibility (IDEA) within our professional community. This seminar will provide opportunities to hear from leaders within the field on how they have implemented strategies and partnered with internal/ external stakeholders to create a dynamic and diverse culture within their organizations. IDEA topics in this seminar include developing and using employee resource groups (ERGs), leveraging partnerships with local nonprofit and educational organizations to enhance and increase diverse talent in the workplace, and strategies for stakeholder acceptance of diversity programming. The target audience includes veterinarians, technicians, animal care staff, researchers, IACUC staff, and vendors.
Speakers/Topics:
3:00 Chandra D Williams Welcome and Introductions
3:05 Donna M Jarrell Championing Diversity in a Large Academic Setting
3:35 Morika D Williams
4:05 Tasha M Thomas
Empowering Tomorrow’s Leaders: Enhancing Diversity in Biomedical Careers Through Intentional Partnerships
Cultivating a Culture of Belonging and Diversity Through Employee Resource Groups (ERGs)
4:35 All Questions & Answers

It’s Okay to Get a Little Dirty: The Laboratory Animal Professional’s Guide to
3:00 PM - 5:00 PM/Room: 207A
Leader/Moderator: Victoria K Baxter
Facilitator: Marlena Holter
Over the last few decades, the laboratory animal science community has made a concerted effort to ‘clean up’ laboratory rodent colonies and rid them of most endogenous pathogens known to affect research studies. However, the ability of standard specific pathogen-free (SPF) laboratory mice to reflect human responses to disease, vaccines, and therapeutics is often criticized, with their minimally diverse microbiomes and immature immune systems given as potential reasons for this lack of translatability. In response, a ‘dirty’ mouse movement has developed, where laboratory mice are exposed to a more natural and diverse array of pathogens, microbiomes, and environments. However, because these ‘dirty’ mice harbor many endogenous pathogens excluded in standard SPF mouse colonies and require more complex housing environments, institutions are often hesitant to establish and manage such colonies for fear of cross-contamination with SPF mice and due to the additional effort and personnel costs associated with properly caring for these ‘dirty’ mice. This seminar will demonstrate how ‘dirty’ mouse models have been used in studies to better reflect human responses, with worldwide leaders in the ‘dirty’ mouse field introducing three of the most commonly used models: 1) the pet store co-housing model, 2) the re-wilded mouse model, and 3) the wildling mouse model. The speakers will provide practical, first-hand accounts of what it is like to establish and administer ‘dirty’ mouse programs, both investigator-managed satellite colonies and colonies centrally managed by the institution’s animal care program. This seminar should interest a wide range of laboratory animal personnel involved in the care and management of rodent colonies, from IACUC administrators to facility managers to laboratory animal veterinarians. The goal is to reduce the mystique surrounding ‘dirty’ mouse models, educate laboratory animal professionals on how to successfully manage ‘dirty’ mouse colonies, and support investigators using these mice in their research.
3:00 Victoria K Baxter Welcome and Introductions
3:05 Victoria K Baxter An Introduction to the World of ‘Dirty’ Mice
3:15 Sara Hamilton Hart Lessons Learned from Mingling with ‘Dirty’ Mice
3:35 Andrea Graham
The Re-Wilded Mouse Model: Immunological Impacts of Farm Microbes and Outdoor Life
3:55 Stephan Rosshart Born to be Wild: How to Establish, Maintain, and Manage a Wildling Facility
4:15 Peter C Smith
Mitigating the Microbiological Risk
Associated with Housing and Utilizing Pet Store Mice in a Centrally Managed Laboratory Animal Facility
4:35 All Questions & Answers






3:00 PM - 5:00 PM/Room: 209A
Leader: Jann Hau
Moderator: Patricia Preisig
Facilitator: Steven J Schapiro
The seminar will focus on the importance of the practical organization of academic animal resource centers (ARCs) for operational efficiency and financial sustainability. Survey data from US and EU university ARCs show that per diem rates are generally set too low to allow recovery of costs. EU programs have a small positive net cash flow, while US programs average a large deficit. Cost accounting is the most influential factor in impacting per diem rates in US and EU programs. US and EU programs are reluctant to raise per diem rates to recover costs, resulting in the animal program being ‘caught in the middle’ between the competing financial challenges of investigator ‘affordability’ and the animal program’s fiduciary responsibility to the institution. This challenges ARC’s senior management to ensure the program’s stability, continuous improvement, and sustainability. The participants will be presented with results from recent US and EU academic ARCs analyses focusing on organization, operation, services offered, and finances. Strengths and weaknesses of the organization of ARCs focusing on institutional commitment to longterm robustness and sustainability will be presented and discussed. The importance of quality R&D as an integral component of the ARC will be given. An MBA will be developed at Manchester University for managers of ARCs and AAALAC’s views on the importance of effective
organization to ensure animal welfare and research quality. This seminar is relevant for senior managerial staff in ARCs, veterinarians, and scientists contemplating a managerial career in comparative medicine and laboratory animal science.
Speakers/Topics:
3:00 Patricia Preisig Welcome and Introductions
3:05 Jann Hau
3:10 Patricia Preisig, James D Macy
3:30 James G Fox
3:45 Maria Kamper
4:00 Javier Guillén
Introduction to Academic Animal Care and Use Programs
Experiences Drawn from The Yale Animal Resource Cost and Benchmarking Surveys and Experiences Drawn from a Comparative Benchmarking Analysis of US and EU Institutions
Importance of R&D as an Integrated Component of Programs
Development of an MBA at Manchester University for Managers of Programs
Importance of Effective Organization to Ensure Compliance and Timely Implementation of 3Rs Initiatives
4:10 All Questions & Answers







W-08 3D Printing for Animal Welfare: Creating Custom Equipment Without the Factory
8:00 AM - 12:00 PM/Room: 110B
Leaders: Constance J Woodman, Kristina C Bartley
Faculty: Adam Bower, Olivia Tian
Facilitator: TBN
Workshop Fee: $150 Workshop Limit: 50
Lab animal professionals play an indispensable role in acquiring welfare equipment for animals. However, there are challenges in acquiring enrichment toys, husbandry tools, and training aids. Animal caretakers and researchers can produce their on-demand custom equipment through new technology. In this workshop, beginner attendees will produce custom objects related to animal welfare, either a small equipment piece or a small enrichment device. Attendees’ training will be guided through two phases of learning. First, they will follow the object creation process using free online model libraries and free browser-based software to edit models, and then we will use 3D printers to produce items. While the items are printed, the second phase will be presentation and Q&Abased, addressing the technical aspects of safety, application, and hygiene needs. Participants will go home with a small custom object, an outline of the tools and steps used to create and produce the custom equipment, and copies of a research report about 3D printing and safety for animal welfare. The purpose of this workshop is to find solutions for challenges, including the high cost of specialty equipment, back ordered or discontinued products, and allows for affordable custom items. Learn to stop animal welfare burnout by making your items on demand! We suggest participants bring their laptop to work on. A PC, Mac, or Chromebook will all work.
This Workshop is sponsored in part by Raise 3D.

W-04 cont. Beginning Training Methods & Techniques for NHPs - Part 2
(8-hour workshop continued from Monday 1:00 PM)
8:00 AM - 12:00 PM/Room: 108
Leader: Lisa A Houser
Faculty: Jaine E Perlman, Stacey Smith, Bess Carlson
Facilitator: TBN
See Monday 1:00 PM for pricing and description.
This Workshop is sponsored in part by Bio-Serv, LabDiet, Lomir, and Allentown.

W-09 Occupational Health and Safety Considerations in Animal Research: Learning Through Interactive Case Studies - Part 1
(8-hour workshop continued Wednesday 8:00 AM)
8:00 AM - 12:00 PM/Room: 110A
Leaders/Faculty: Lesley A Colby, Susan B Harper
Facilitator: TBN
Workshop Fee: $200 Workshop Limit: 50
The animal research environment poses many potential risks to personnel health and safety. In partnership with environmental health and safety professionals, animal care and support personnel are instrumental in identifying and controlling these risks to ensure a safe environment for themselves, their staff, and others who enter or work in animal facilities. This 8-hour workshop will focus on identifying, assessing, and controlling biological, chemical, radiologi-

cal, and physical hazards commonly encountered in animal research programs. Topics discussed include conducting a risk assessment, essential components of an occupational health program, managing animals administered infectious agents and chemicals, humanized animals, equipment-related hazards, and safe housing and handling of select animal species. Facility design and disaster planning, as they pertain to occupational health and safety considerations, will be briefly discussed, while high containment (ABSL3 and ABSL4) will not be addressed. The workshop will be highly interactive, allowing participants to work in small groups and extensive class discussions. Through a mixture of case studies, group discussions, and interactive exercises, participants will evaluate “real world” examples and be guided through strategies for identifying potential hazards, assessing the magnitude and extent of induced risks, and developing effective control measures that protect the safety of workers, animals, and the environment. Before the course, participants will be encouraged to submit relevant scenarios and questions for discussion during the course. The target audience includes vivarium managers, supervisors, lead technicians, trainers, animal care and veterinary technicians, and biosafety professionals.
8:00 AM - 10:45 AM
Platform Session abstracts will be available on www.aalas.org in July. They will also be included in both the mobile app and the National Meeting Final Program.

Embracing Variability to Ensure Reproducibility, Validity, and Translation
8:00 AM - 10:00 AM/Room: 101A
Leaders: F Claire Hankenson, Joseph P Garner
Moderator: Natalie A Bratcher-Petersen
Facilitator: Cindy A Buckmaster
Individual variation is the fundamental essence of life. Without it, there would be no evolution or biology. Human clinical research has long focused on individual variation as the source of knowledge, and modern medicine is pivoting from one-size-never-fits-all interventions to highly personalized biomarker-based interventions. Despite this progress in the clinical space, preclinical researchers frequently aim to minimize variability rather than embrace it. Yet, it’s crucial to acknowledge that individual variability cannot be controlled, and it holds immense value as a subject of study. A growing body of evidence shows that the poor reproducibility of preclinical research and the poorer forward translation of animal results to humans (i.e., external validity) stems from a need for more tractable biological variation in experimental paradigms. This seminar addresses some of the multifactorial intricacies that limit reproducibility, stimulate dialogue, and propose innovative strategies for enhancing the relevance and translatability of preclinical research findings to clinical applications. This seminar will present considerations and discussion around various solution-based, practical approaches such as advocating for variability and embracing systematic heterogeneity, using home cage digital measures to assess variability in animal behavior within and between cages as well as across sites, inclusion of both sexes and addressing misconceptions and barriers to sex inclusive research; utilizing naturally an existing genetic variation in experimental design; and promoting practices to improve generalizability (i.e., effective comparisons between animals and humans), including suggestions for improving rigor and transparency in animal research,




encompassing experimental design, statistical assessment, and reporting factors to ensure broader applicability of comparative outcomes. This seminar is for researchers, veterinarians, data scientists, support and care staff, vivarium managers, welfare scientists, ethics committee members, and those interested in improving research reproducibility and the translational relevance of animal studies.
Speakers/Topics:
8:00 Natalie A Bratcher-Petersen Welcome and Introductions
8:05 Joseph P Garner
Leaving Stepford Behind: Why We Should, and How We Can, Combine the Best of Human and Animal Experimental Design
8:30 F Claire Hankenson Advocating for Generalizability: Recognizing What Can Confound Expected Research Outcomes
8:55 Michael Saul
9:20 Brianna N Gaskill
Measuring the Variability We Know Exists: Using Innovative Home Cage Digital Measures to Assess Sources of Experimental Variability Including Individual, Cage, and Genetic Effects, and Implications for Study Design and Statistical Analysis
Turning Knowing, Into Doing: Evaluation of a Workshop to Train Scientists How to Appropriately Include Sex as a Biological Variable
9:35 All Questions & Answers
This Seminar is sponsored in part by The Jackson Laboratory.

Establishing and Growing a Successful Gnotobiotics Program
8:00 AM - 10:00 AM/Room: 104A
Leader/Moderator: Allison R Rogala
Facilitator: Stephanie W Fowler
Demand for the use of gnotobiotic animal models is as strong as ever and continues to expand at a rapid pace. Subsequently, those in the laboratory animal community are frequently given the opportunity for involvement in this rapidly growing and exciting field when asked to establish or support a gnotobiotic program at their institution. Yet, operating and maintaining a Gnotobiotic facility is vastly complex and expensive to establish and maintain successfully. Therefore, adequately understanding the complexity and critical factors contributing to success is essential. This seminar brings speakers who are leading experts from the gnotobiotic trenches who have not only built, grown, and maintained successful gnotobiotic programs but who currently train and consult others in doing the same. The session will set the foundation by introducing the importance of gnotobiotic models and the necessary components for conducting germ-free and gnotobiotic work. Attendees will learn how to design and develop a germ-free program tailored to individual or institutional-specific needs. Specifically, they will learn about the essential equipment necessary and some alternatives to using traditional gnotobiotic isolators, how to select and maintain staff to fit the rigorous demands of germ-free work, and how to develop a cost structure for your program as well as how to garner support for the at various stages of the process. The focus will be on mouse gnotobiotic animal models and facilities; however, basic concepts may be extrapolated as a foundation for other species. The individual speakers will be available for a question-and-answer session to give the audience pearls of wisdom from the panelists and attendees’
varied institutions and backgrounds. Target audience: This seminar targets anyone in the laboratory animal field interested in learning more about germ-free and gnotobiotic facility design, management, and operation, including husbandry technicians, managers, directors, veterinary technicians, veterinarians, research scientists, vendors, and more. This seminar integrates well with the proposed “Current Topics in Gnotobiotics.”
8:00 Allison R Rogala Welcome and Introductions
8:05 Allison R Rogala Importance and Contributions of Gnotobiotic Animal Models
8:20 Joshua M Frost The Essential Components of the Gnotobiotic Program
8:50 Jessica K Lang Recruiting, Training, and Retaining Gnotobiotic Staff
9:20 Betty R Theriault Gaining Institutional Support and Determination of Cost Structure
9:50 All Questions & Answers
This Seminar is sponsored in part by the Association for Gnotobiotics.

8:00 AM - 10:00 AM/Room: 209A
Leader: Michelle L Altemara
Moderator: Joshua R Barber
Facilitator: Emma Liechty
Achieving reproducible research and ethical utilization of live animals necessitates collaboration among regulators, researchers, husbandry staff, and veterinarians. These presentations offer an insightful overview of the regulatory landscape governing aquatic animal care and research and the publications that influence these regulations. Topics include federal laws, the pivotal role of Institutional Animal Care and Use Committees (IACUC) in ensuring ethical research practices, and the significance of evidence-based publications in harmonizing husbandry practices across facilities. Emphasizing communication and collaboration among stakeholders, the final presentation focuses on the collective responsibility for animal welfare and the promotion of reproducible research practices. Anyone involved in the operation of an aquatics facility, whether lab animal technicians, managers, veterinarians, or IACUC members, will gain a deeper understanding of the distinct challenges aquatic life encounters and the essential publications required to inform decision-making processes.
Speakers/Topics:
8:00 Joshua R Barber Welcome and Introductions
8:05 Amber P Chiodini
8:30 Michelle L Altemara
8:55 Arrow S Megginson
Charting Waters: Navigating Regulations in Aquatic Animal Care and Research
Harmonizing Zebrafish Husbandry Parameters: Bridging Gaps in Published Literature
Kraken the Deep Mysteries of Ethics, Husbandry, and Record-keeping of Aquatic Animals

TUESDAY MORNING TUESDAY MORNING

9:20 Dante M D’India
Swimming Together: Enhancing Communication and Collaboration in Zebrafish Research
9:45 All Questions & Answers

Transforming Vivarium Operations: Advancement in Technology for Enhanced Human Capital and Workflow Optimization
8:00 AM - 10:00 AM/Room: 207A
Leader/Moderator: Sylvia I Gografe
Facilitator: Kenneth J Salleng
Join us in discovering the future of vivarium management in this engaging seminar tailored for directors, managers, and anyone interested in new technologies. The talks will discuss implementation strategies to guarantee excellent investment returns, overcoming struggles with employee retention, and how to accommodate flexibility in a fast-paced research environment. With a keen focus on improving personnel work experiences and streamlining vivarium processes, our expert speakers will delve into the transformative impact of advanced technology on daily operations. We’d like to explore with our first speaker how the sensible implementation of digital home cage solutions can streamline operations, minimize repetitive tasks, and optimize the organization of animal rooms. Empowering staff with these innovative tools cultivates job satisfaction, boosts productivity, and enables a shift towards more meaningful and high-value activities within a vivarium. Our second speaker was involved in a case study, which discovered that automated systems in the cage-washing area could revolutionize cage movement. Witness how Mt Sinai School of Medicine reduced labor dependency, mitigated ergonomic risks, and created a safer and more appealing work environment, ultimately increasing retention and interest in vital cagewash positions. The talk of our third speaker explores the hurdles faced in transitioning traditional animal facilities into modern and automated environments with a focus on personnel perspectives. They will discuss addressing challenges related to outdated infrastructure, comfort zone mentality, change management, and the need for innovative solutions to enhance animal welfare, research efficiency, and overall scientific outcomes. Entering the era of cutting-edge AGV (Automated Guided Vehicle) technology, our fourth speaker highlights how AGV technology minimizes human interaction by automating rack and material movements. This state-of-the-art technology effectively enhances operational workflows and personnel working conditions, including better ergonomics and reduced allergen exposure. Explore the positive impact on staff resources as personnel can shift their focus to tasks that add significant value to vivarium operations.
Speakers/Topics:
8:00 Sylvia I Gografe Welcome and Introductions
8:10 Matthew L Keller Digital Home Cage Solutions: Streamlining Workflow for Enhanced Efficiency
8:35 Zachary L Jones Automated Cage Handling: Redefining Dirty Washing Processes
8:55 Odell D Jones
Revolutionizing Animal Facilities: Overcoming Challenges in Transforming Traditional Environments into Modern and Automated Spaces

9:15 Melanie Hierweger AGV Technology in Animal Facilities: Optimizing Rack Movements for Efficiency and Flexibility
9:40 All Questions & Answers
Exhibit Hall Refreshment Break!
9:00 a.m.– 11:00 a.m.
Sponsored by The Jackson Laboratory

Micro Photonics
10:10 a.m. – 10:30 a.m.
(Presentation title, product, and description to come)

The Andersons Lab Bedding Products
10:35 a.m. – 10:55 a.m.
(Presentation title, product, and description to come)

Advancing Neuroscience Research through Vertical Vivaria Design: Lessons from Washington University’s Neuroscience Research Building 11:00 AM - 12:00 PM/Room: 104A
Speaker: Trevor Calarco
Moderator: Kenneth R Boschert
Facilitator: TBN
As the demand for neuroscience research continually escalates, there is a continual demand for novel approaches to facilitate breakthroughs. Traditional vivarium setups often present logistical challenges that hinder collaboration and efficiency. In response, in partnership with CannonDesign, Washington University embraced design innovation by integrating a vertical vivarium within its neuroscience research building (NRB). The NRB’s vertical vivarium and central breeding and core facility revolutionize research practices by offering cubicle housing for study animals and multiple behavioral/testing suites on each floor. This configuration fosters direct researcher access, eliminating the need for dispersed satellite vivaria across campus. Housing a diverse array of species alongside cutting-edge technologies like MRI and in-vivo imaging, the facility accommodates nearly 40,000 cages. The meticulous attention to detail in design and implementation is critical to its success. Particular emphasis was placed on researcher accessibility while upholding stringent security and environmental protocols. Extensive vibration and noise analyses mitigated potential disruptions from adjacent infrastructure, ensuring optimal research conditions. By breaking away from tradition, embracing novel design principles, and addressing unique research needs, this innovative approach is helping propel Wash U and the NRB into an era of unprecedented collaboration and discovery. The targeted audience is Administrators, Managers, Researchers, Planners, and Designers.
This Special Topic Lecture is sponsored in part by CannonDesign.







TUESDAY MORNING TUESDAY MORNING


Charles River Ethics and Animal Welfare Lecture: The 3Hs Initiative: Housing, Handling and Habituation Methods for Laboratory Rodents
11:00 AM - 12:00 PM/Room: 101A
Speaker: Emma Robinson
Moderator: Patricia V Turner
Facilitator: TBD
Refinement encompasses the lifetime experience of laboratory animals, and improving welfare needs to extend beyond consideration of the most refined methods for specific procedures. To address this, we have developed a framework that considers the lifetime experience of laboratory animals and improves their welfare by targeting the 3Hs: Housing, Handling, and Habituation.
To promote natural behaviors and enhance positive welfare, both rats and mice benefit from being housed in highly enriched caging with sexually mature male mice housed individually to reduce social stress. Recognizing the space restrictions of standard rodent caging, we also incorporate access to playpens for added enrichment. We have modified our handling and restraint techniques to eliminate the need for physical restraint in rats or tail handling in mice, including substance administration. We have developed an oral dosing protocol that eliminates the need for restraint and gavage as the test substance is combined with a palatable substance that the animal drinks directly from the syringe. We have implemented habituation protocols for mice and rats, including basic handling, modified restraint methods, and exposure to experimental apparatus combined with positive reinforcement. To objectively assess the welfare benefits of these approaches, we use our affective bias test and reward learning assay, which provide a sensitive measure of both short-term and chronic changes in affective state, respectively. We have demonstrated that refinements can be achieved when we take a more holistic approach. By enhancing a positive relationship between the animals’ environment and research staff, we can reduce the negative impacts experienced across the animal’s entire lifetime. Implementing the 3Hs has been shown to have scientific and welfare benefits and helps researchers meet their commitments to the 3Rs.
Participants will learn about the 3Hs initiative and how to use the new continuous professional development resource to support the integration of refined methods into their research.
Who is the intended audience: All AALAS attendees
This Special Topic Lecture is sponsored in part by Charles River.

11:00 AM - 12:00 PM/Room: 207A
Speaker: Shawn P O’Neil
Moderator: Carissa P Jones
Facilitator: TBN
For nearly a century, experimental autoimmune encephalomyelitis (EAE) has been used in various species as a model of neuroinflammatory demyelination. Murine models of EAE continue to play a central role in the evolution of our understanding of the pathogenesis of multiple sclerosis (MS) and in developing methods for treating this complex disease. In this presentation, we will review and compare the fundamental pathological features of MS versus EAE and discuss how different mouse strains and experimental designs are selected to focus on the specific clinical phases of MS that are observed in patients (relapsing/remitting, secondary progressive, primary progressive) or to target specific pathological features of the disease, such as the contributions of cell-mediated versus antibody-mediated inflammation, demyelination, axonal injury, and repair. We will also review the design of prophylactic, semi-therapeutic, and therapeutic experiments for evaluating the efficacy of prospective new drugs in development, the use of current therapeutics as positive control tool compounds in EAE experiments, and the endpoints that are typically used to evaluate the outcome of these studies. The target audience would be lab animal veterinarians, a broad spectrum of scientists involved in neuroscience, immunology, vaccine development, pharmacology, and drug development, and investigative and toxicologic pathologists and histology personnel. Attendees will learn fundamental concepts of MS pathogenesis, including histopathological features of MS versus EAE; strains of mice used in EAE experiments (C57BL/6, SJL, NOD, Biozzi); the different experimental approaches used in EAE studies (neuroinflammatory (MOG, PLP-induced), adoptive transfer) versus toxicological demyelination (cuprizone induced); prophylactic versus therapeutic studies and what types of scientific endpoints are used to evaluate the outcome of EAE experiments.
This Special Topic Lecture is sponsored in part by The American College of Laboratory Animal Medicine (ACLAM) and the American Society of Laboratory Animal Practitioners (ASLAP).

Wallace P Rowe Lecture
11:00 AM - 12:00 PM/Room: 209A
Speaker and description will be available after the Award Selection Committee selects the Bhatt Award Recipient. This session information will be available in the Mobile App and the Final Program.
This Special Topic Lecture is sponsored in part by the AALAS Awards Selection Committee (ASC).



Emma is Professor of Psychopharmacology at the University of Bristol where she also completed her BSc and PhD. Following a 5-year fellowship which included working in Experimental Psychology in Cambridge, Emma established her independent research in the School of Physiology, Pharmacology & Neuroscience. Here Emma’s research focuses on investigating the neural and neurochemical mediators of normal cognitive and emotional behaviour and how these may be disrupted in psychiatric disorders. Complementing the psychopharmacology research, the group runs a programme using objective measures of affective state to better understand animal welfare. The 3Hs initiative emerged from both these research programmes and aims to provide researchers with validated methods which benefit both scientific objectives and animal welfare.




Braintree Scientic, Inc. has been providing research equipment to the Life Sciences Industry for over 40 years.




Prepared and Neutral Microtubes





Our unique, hand-picked selection of products for mice and rats is extensive and has evolved with researchers’ needs and changing technology. If there are additional products you require, please let us know and we will source them.


TUESDAY AFTERNOON TUESDAY AFTERNOON


Animal Research & Internal Communications: How
Effectively Communicating within your Organization about Animal Studies Can Improve Morale, Operations, Reputation and Security
12:30 PM - 2:00 PM/Room: 102A
Leader: Valerie Hill
Moderator: Thierry Decelle
Facilitator: Paula Clifford
Panelist: Jim Newman, Amy Puffenberger, Shawn DeLong, Timothy John Jameson
When it comes to communications about animal research, much of the focus tends to be discussions with the public, the press, legislators, and other key external parties. However, communications within the walls of your facility are just as important, or perhaps even more critical. The session will feature a facilitated discussion with panelists from varying types of organizations, from a large university with a decentralized campus to a large industry perspective where there is a need to communicate with several sites globally to a small company with limited communications resources based primarily in one location. Topics will include two-way interactions within the animal care unit and between leadership and staff. These discussions might pertain to new or updated policies, preparing for an AAALAC visit, reporting activities of the IACUC, updates on items of improvements and concern (news stories, anti-research claims, etc.); communications with non-animal staff to ensure that all employees, including those who work outside of the vivarium and labs, understand how and why your organization studies animals; communications with administrators including the executive leadership and communications teams to assist these key offices in navigating improved standards, questions and concerns about animal studies and welfare and initiatives to increase animal research openness; ensuring an effective internal communication program through an education and re-education component in order to continually build understanding, trust, and partnerships with key leaders as individuals join or leave your organization. A portion of the presentation will also focus on practical actions, resources, and structural decisions, such as having a specialized internal communications role dedicated to animal studies. In addition, survey results gathering information on the current structures and resources allocated to animal research communications will be shared. The target audience is Directors, Clinical Care and Husbandry Supervisors/Management, Lead Technicians, Administrators, and Communicators.
This Panel Discussion is sponsored in part by Americans for Medical Progress (AMP).

12:30 PM - 2:00 PM/Room: 103A
Leader: Scott D Bury
Moderator: Wai Hanson
Facilitator: Erica Armstrong
Panelist: Matthew Rassette, Scott D Bury, Rebeccah Wood
IACUC membership criteria are well defined in the regulations, but beyond meeting these requirements, member composition varies widely among institutions. In this session, participants will learn about the current demographics of IACUC memberships regarding inclusion and representation. Commonly, one of the most challenging members to recruit is the community member, and this panel

will address best practices in community member recruitment and development. However, ethical considerations for retaining this member for an extended period will be discussed once recruited and developed. The target audience for this presentation is broad and includes IACUC members, veterinarians, veterinarians in training, researchers, and institutional leaders in animal welfare.
This Panel Discussion is sponsored in part by The American College of Laboratory Animal Medicine (ACLAM) and the American Society of Laboratory Animal Practitioners (ASLAP).

Enhancing Neurodegenerative Disease Modeling Through Continuous Home Cage Monitoring: Insights into Behavioral and Physiological Biomarkers
12:30 PM - 2:00 PM/Room: 105A
Leader/Moderator: Sean Maguire
Facilitator: Giorgio Rosati
Panelist: Jason S Villano, Frank J Jenkins, Jeetendra R Eswaraka
The accurate characterization of neurodegenerative disease models is necessary for advancing neuroscience research, particularly in understanding the pathophysiology and progression of these disorders. Recent advancements in continuous home cage monitoring technologies have opened new avenues for in-depth, continuous observation of animal models, providing a wealth of data previously unattainable without digital biomarker technology. This seminar will explore the novel insights and methodological advancements brought forth by continuous home cage monitoring in the study of neurodegenerative diseases. The panel will include 1) a brief exploration into the technological underpinnings of home cage monitoring systems and how these systems enable the collection of continuous behavioral and physiological data and the outputs of digital biomarkers; 2) how continuous monitoring facilitates the early detection of subtle behavioral changes, such as alterations in circadian rhythms, activity levels, and social interactions, which are critical in understanding the onset and progression of neurodegenerative conditions; 3) case studies showing the utility of home cage monitoring in drug efficacy testing and genetic model validation and how the sensitivity and reliability of preclinical trials can be enhanced; 4) open discussion. The target audience is researchers, veterinary, in vivo, and operations staff. Oversight body personnel and this panel will provide an understanding of the technologies and current use cases, followed by an open discussion with the audience and panelists to explore further the transformative impact of continuous home cage monitoring and digital biomarkers on the characterization of neurodegenerative disease models. This panel will highlight the opportunity these advanced monitoring techniques provide to improve our understanding of neurodegenerative diseases and therapies while advancing animal welfare.

Navigating USDA, APHIS, AC Appeals
12:30 PM - 2:00 PM/Room: 106A
Leader: Nancy E Halpern
Moderator: Thomas Leach
Facilitator: Karen M Froberg-Fejko
Panelist: Nancy E Halpern, James H Lister, B Taylor Bennett This panel discussion will be about the USDA inspection appeals process from a legal perspective, the pros and cons of engaging in the process, the likelihood of success, and animal care and welfare improvements. Participants will learn the Animal Welfare Act compliance is essential and fully transparent via access to the United States Department of Agriculture Animal and Plant Health Inspection Service Animal Care Public Search Tool, available at https://aphis. my.site.com/PublicSearchTool/s/. Participants will learn different legal perspectives from attorneys working with registrants and licensees and the various legal approaches to the current Appeals process, potential avenues for improvement, and the recommended




path to help minimize the realignment of resources from life-saving medical research and animal care and welfare to regulatory defense. The target audience is Attending Veterinarians, Veterinary Staff, Animal Health and Welfare Staff, Compliance Staff, IACUC members, Administrative Staff, General Counsel, and Staff.

Step Up YOUR Laboratory Animal Anesthetic/ Analgesic Game! Practical Techniques to Improve YOUR Investigations and Data!
12:30 PM - 2:00 PM/Room: 209A
Leader/Moderator: Rebecca A Johnson
Facilitator: TBN
Panelist: Rebecca A Johnson, Cholawat Pacharinsak
This popular panel discussion focuses on answering audience questions about the newest yet practical anesthetic/analgesic techniques for laboratory animals, including models of neurodegenerative disease. Attendee participation is highly encouraged as dialogue will begin with panelist-led case presentations incorporating questions submitted to panelists over the past year, then will progress to a real-time Question and Answer session. We will focus on common yet challenging anesthetic cases that veterinary personnel frequently encounter in the research setting that help the research team “step up their game” regarding anesthesia/analgesia. Topics discussed will depend on specific audience questions but may include novel and validated pain scoring systems, advances in clinical anesthetic/analgesic techniques (including innovative use of pharmacologic agents), and the most helpful anesthetic monitoring systems since prompt and accurate diagnosis and treatment of physiological disturbances during anesthesia is paramount to improve animal morbidity, mortality, and experimental outcomes, especially regarding neurodegenerative diseases. Participants should obtain a deeper understanding of appropriate and current lab animal anesthetic techniques and how to properly recognize and treat acute and chronic pain states in various laboratory animals using the most up-to-date clinically available analgesic techniques. The information gained will be valuable for all veterinary personnel caring for laboratory animal species throughout a laboratory procedure, including those responsible for providing anesthetic techniques pre- and intra-procedure, as well as personnel relied upon for post-procedural care and analgesic monitoring.

W-10 Positive Reinforcement Training and Temperament Testing: Preparing Monkeys for Restraint Procedures - Part 1
(8-hour workshop continued Wednesday 8:00 AM)
1:00 PM - 5:00 PM/Room: 108
Leader: Jaine E Perlman
Faculty: Mollie A Bloomsmith, Kristine Coleman, Lisa A Houser, Jennifer L McMillan
Facilitator: TBN
Workshop Fee: $250 Workshop Limit: 50
The workshop includes 8 hours of instruction on using positive reinforcement training (PRT) to teach monkeys to cooperate with restraint procedures and provide information about utilizing temperament testing to assist in selecting subjects and planning for their training. Participants will learn approaches to training laboratory primates to cooperate with restraint for sample collection (e.g., blood), administration (e.g., injections), and chair restraint. PRT is a significant refinement in the care of nonhuman primates and an
effective means of improving their welfare. However, animals respond differently to restraint, and measuring temperament provides insight into how individuals might respond to these procedures, allowing for individualized and more effective training plans. Workshop goals:1) review animal training terminology and techniques; 2)teach PRT techniques as they apply to restraint procedures, such as the use of the cage squeeze back mechanism and chair restraint; 3)teach methods to assess and quantify temperament in monkeys and to use this information to develop individualized training plans. Participants will learn how to establish a foundation for successful restraint training using PRT and incorporate alternative techniques, such as negative reinforcement, to meet research timelines. They will learn to identify monkeys engaged in the training process and how to increase the involvement of monkeys who seem uninterested in training. Participants will learn how to shape restraint behaviors, apply desensitization techniques, maintain trained behaviors, and transfer trained behaviors among multiple staff members. Participants will learn how temperament can impact training approaches and the anticipated timelines for training to cooperate with restraint. Understanding the intersection of individual differences in temperament and animal training will aid in designing more efficient animal training programs. This workshop is designed for those with basic animal training terminology and techniques and with animal training experience, including behavior specialists, animal caregivers, research technicians, animal managers, veterinarians, and investigators.
This Workshop is sponsored in part by Carter 2 Systems, Britz and Company, NC3Rs, and Lomir.

W-11 Tools to Achieve Sustainable Diversity, Equity, Inclusion, and Belonging in the Workplace; A MultiOrganization Collaboration
1:00 PM - 5:00 PM/Room: 107A
Leader: Crystal H Johnson
Faculty: Sally Thompson-Iritani, Chandra D Williams, Janet L Steele, Donna M Jarrell, Lee-Ronn Paluch, Gregory W Salyards, Erin NZ Yu, Tanise L Jackson, Sharron M Kirchain, Temeri D Wilder-Kofie
Facilitator: TBN
Workshop Fee: Free Workshop Limit: 50 Diversity, Equity, Inclusion, and Belonging (DEIB) continues to be a societal issue that impacts our workforce and requires actionable steps to ensure meaningful change. Many laboratory animal research affiliations have published DEIB statements and formed committees within their respective organizations. While each organization (AALAS, APV, ACLAM, ASLAP, VOEN) has developed individual plans to move their respective agendas forward, a collaborative approach leads to sustainability and increased impact to achieve like-minded initiatives. This workshop is a joint effort between AALAS, ACLAM, APV, ASLAP, and VOEN to provide an intimate setting for a collegial discussion about diversity, equity, inclusion, and belonging. The attendees will participate in breakout sessions that foster small group discussions, including real-life case-based scenarios on the DEIB responsibilities of management and leadership in the workplace, unconscious bias and the associated barriers, and allyship parameters. Participants are expected to gain practical knowledge and understanding of diversity, equity, and inclusion. This interactive workshop will showcase tools that can be applied daily to encourage a more inclusive culture and belonging within the workplace. The target audience includes veterinarians, technicians, animal care staff, researchers, IACUC staff, and vendors.
This Workshop is sponsored in part by the AALAS Inclusion, Diversity, Equity & Accessibility (IDEA) Advisory Council.



W-12 Working with Difficult Personalities: How to Survive and Thrive
1:00 PM - 5:00 PM/Room: 107B
Leader/Faculty: Laura A Conour, Pamela A Straeter
Facilitator: TBN
Workshop Fee: $150 Workshop Limit: 50
Do you have a PI, colleague, or employee you dread working with? Is your day ruined when you see this person looming in your office door and stomping into your personal space? Have you tried deep breathing exercises but still find yourself grinding your teeth after a non-productive encounter? Are you starting to ask yourself, “Is it me?” This workshop should appeal to you if you answer “yes” to even one of these questions. Take charge of the situation and learn how to manage these interactions in a manner that keeps you from screaming and doesn’t ruin your work day. This workshop is structured for managers and directors of laboratory animal care and compliance departments. Using a combination of didactic learning, case studies, and role-playing scenarios, we will present you with the tools needed to recognize the personality type of individuals that are challenging to interact with and strategize how to manage these interactions such that they are constructive, productive and structured in a manner that preserves your sanity. Workshop attendees should come prepared with examples of challenging interactions and be ready to participate in this highly interactive workshop.

Husbandry and Facility Management 201: Advanced Care
1:00 PM - 5:00 PM/Room: 110A
Leaders: Tannia S Clark, Lauren M Pandolfo
Faculty: Lauren M Pandolfo, Logan Fehrenbach, Jeff D Wyatt, Stephen C Frederickson, Raphael A Malbrue, Toi A Collins
Facilitator: TBN
Workshop Fee: $150 Workshop Limit: 50
This Workshop is a more in-depth and advanced hands-on experience working with zebrafish in a research setting. This course builds on the foundation taught in Zebrafish Husbandry and Facility Management 101: “The Basics,” (scheduled on Monday afternoon), focusing on in-depth topic discussion and a hands-on approach that builds upon the foundational knowledge. The target audience is laboratory animal professionals (i.e., technicians, veterinarians, researchers) new to working with zebrafish or experienced users searching for a refresher course to review current practices. Attendees will learn advanced facility operations such as how to identify and create a clinical health monitoring program, navigate regulatory oversite language such as establishment of humane endpoints, and determine the needs for facility design and research execution from laboratory animal veterinarians, researchers, and aquatic technical staff to accomplish the following learning objectives: Facility Construction/Design Requirements, Disaster Planning, Clinical Health Monitoring and Diseases of Concern, Advanced Anesthesia and Restraint, Research Techniques, and Diagnostic/Post Approval Monitoring (PAM). The workshop will incorporate interactive lectures focused on the listed objectives:(i.e., case studies and virtual video simulations). Attendees will also receive hands-on experience with zebrafish handling techniques for research and diagnostics, such as handling fish for lab or veterinary procedures like fin clipping. This will be achieved using simulated model fish (3D printed models and silicon components).
This Workshop is sponsored in part by Aquaneering, VRL Diagnostics, and Iwaki.

Exhibit Hall Refreshment Break! 2:00 p.m.– 4:00 p.m.
Sponsored by TBD

Inotiv
2:10 p.m. – 2:30 p.m.
(Presentation title, product, and description to come)

Transnetyx
2:35 p.m. – 2:55 p.m.
(Presentation title, product, and description to come)
2:45 PM - 5:00 PM
Platform Session abstracts will be available on www.aalas.org in July. They will also be included in both the mobile app and the National Meeting Final Program.

Chlamydia Muridarum: It’s Likely in Your Colonies and You Should Care
3:00 PM - 5:00 PM/Room: 104A
Leader/Moderator: Neil S Lipman
Facilitator: TBN
Clinical disease associated with the bacterium Chlamydia muridarum, identified as mouse pneunomitis virus at its discovery, was initially described four score years ago. The causative agent was subsequently determined to be a bacterium, not a virus. Until recently, references to C. muridarum (Cm) in the literature were limited to its use in a murine model to study the sexually transmitted human reproductive tract disease associated with a similar bacterium, C. trachomatis, by which Cm was also previously known. Its lapse into obscurity was presumably a result of the establishment of closed mouse breeding colonies in which the Cm-associated disease was no longer observed, and testing for the bacterium, which does not grow on artificial media, was extremely challenging. Therefore, it was surprising to recently find that Cm is prevalent in and within academic mouse colonies across the globe. In this seminar, the attendee will learn about the biology of the bacterium, its prevalence and how to assess for its presence, its’ epidemiologic characteristics, the risks it poses to the studies in which infected mice are used, and methods available to prevent its introduction or, if present, how to eliminate it. The target audience for this seminar is Veterinarians, Facility Managers, and Veterinary Technicians interested in biosecurity.
Speakers/Topics:
3:00 Neil S Lipman Welcome and Introductions
3:05 Neil S Lipman


1940’s to Present: Where has it Been?


3:25 Noah G Mishkin
Pathophysiology: Experimental vs Natural Infections
3:45 Rodolfo J Ricart Arbona Diagnosis and Epidemiology
4:05 Glory Leung Impact on Experimental Models
4:35 Rodolfo J Ricart Arbona Control and Eradication
4:55 All Questions & Answers

From the FDA Trenches: Designing, Conducting, and Reporting the Results of Animal Studies to Support Medical Devices - A Regulatory Perspective
3:00 PM - 5:00 PM/Room: 101A
Leader/Moderator: Susanne Bush
Facilitator: Sara Thompson
In March 2023, FDA’s Center for Devices and Radiological Health (CDRH) issued a guidance document entitled “General Considerations for Animal Studies Intended to Evaluate Medical Devices,” which is intended to assist sponsors and testing facilities in designing, conducting, and reporting the results of animal studies used to evaluate medical devices and support premarket submissions. In this seminar, we will share our unique perspective as FDA animal study reviewers on the regulatory framework for animal studies. This will include an overview of the new animal studies guidance, insights on standard Good Laboratory Practice (GLP) study concerns, the preparation of a final study report, and pathologic assessment of medical devices. The presentations will draw on the FDA professional’s experience to help sponsors and testing facilities avoid common pitfalls encountered in our animal study reviews. Finally, we will discuss how to work with our stakeholders to conduct efficient and effective GLP animal studies supporting medical device premarket submissions to the FDA. What participants will learn: Participants will learn about CDRH’s new animal studies guidance and gain insight from FDA animal study reviewers regarding study design, GLP compliance issues, and the use of animal performance studies to address other endpoints. Target Audience: veterinarians, study directors, medical device professionals, and other laboratory professionals involved in animal studies intended for regulatory submissions.
Speakers/Topics:
3:00 Susanne Bush Welcome and Introductions
3:05 Susanne Bush Introduction to CDRH and Device Regulation
3:15 Natalie Miller Overview of New CDRH Animal Study Guidance
3:35 Diane Cordray The Importance of GLP
3:55 Maureen O’Brien Pathology Best Practices
4:15 Kate Gardner The Complete Final Study Report
4:35 All Questions & Answers

3:00 PM - 5:00 PM/Room: 207A
Leader/Moderator: Harshan R Pisharath
Facilitator: TBN
Embark on a journey of innovation and efficiency at our seminar, where we explore the dynamic intersection of cutting-edge technology and animal research management. Digital home cage monitoring technologies have evolved from standalone equipment used by researchers to mainstream individually ventilated racks (IVC) with
integrated Machine Learning (ML)/Artificial Intelligence (AI) capabilities. These advancements offer unprecedented tools to animal facility managers, driving operational efficiency, improving animal welfare, and enhancing research rigor and reproducibility. While these technologies hold transformative potential for animal care, their adoption can be daunting due to concerns about cost, return on investment (ROI), and staff expertise. Join us for an enriching experience where participants will acquire a comprehensive understanding of how technology can drive financial returns, improve breeding outcomes, and facilitate NIH grant support in the dynamic landscape of animal vivarium operations. Take advantage of this opportunity to revolutionize your approach to research facility management. This session is tailored for facility directors, managers, veterinarians, and scientists seeking to upgrade vivarium operations and technology.
Speakers/Topics:
3:00 Harshan R Pisharath Welcome and Introductions
3:05 Jeetendra R Eswaraka Maximizing ROI: The Financial Impact of Hi-Tech Integration
3:35 Jason S Villano Breeding Beyond Boundaries: HiTech Benefits in Production Areas
4:05 Frank J Jenkins Navigating NIH Grants: A Roadmap to Funding for Hi-Tech Vivarium Initiatives
4:35 All Questions & Answers

Techniques in Miniature Swine for a New Era
3:00 PM - 5:00 PM/Room: 209A
Leader: Derek Brocksmith
Moderator: Vikki Wehmeier
Facilitator: Stacy Qualls
The Sinclair Nano Pig is a miniature swine comparable to the size of a Beagle. It is a game changer for safety and efficacy studies but also improves the husbandry and staff ergonomics. This seminar will address various areas of the use of miniature swine in biomedical research. Problem-solving will be highlighted, including best practices for surgical modeling, behavior training, selecting the proper vascular access, and husbandry management. Miniature swine applications in medical device and neurological model development will be other areas of emphasis. Participants can expect to learn the technical aspects and practices of anesthesia, peri/post-op complications, handling, husbandry, and sample collection. This will include proper selection of anesthetics and analgesics, behavior training for ease of handling, husbandry management, and a new era of gene-edited models. Answers to frequently asked questions about husbandry and housing management will be given. Lastly, primary surgical considerations for medical device and other modeling will be covered. We expect a broad audience to benefit from these presentations, including laboratory animal veterinarians, attending veterinarians, veterinary and animal technicians, facility managers, graduate students, and scientists interested in miniature swine model research and use.
Speakers/Topics:
3:00 Vikki Wehmeier Welcome and Introductions
3:05 Derek Brocksmith Housing and Husbandry Management of Miniature Swine
3:25 Alicia Braxton Behavioral Training in Swine
3:45 Guy Bouchard
Stress-Free Blood Collection and Common Vascular Access


4:05 Bhanu Telugu Gene Edited Miniature Swine Models of Alzheimer’s
4:25 M Michael Swindle Medical Device Surgery, Anesthesia, and Common Peri/Postoperative Complications
4:45 All Questions & Answers
This Seminar is sponsored in part by Sinclair Bio Resources, LLC.

W-14 AALAS Foundation 201: Take the Next StepPut Foundation Outreach Materials to Action!
8:00 AM - 10:30 AM/Room: 107A
Leader: Vicki C Campbell
Faculty: Debra L Hickman, Larry J Shelton
Facilitator: Kelly S Patterson
Workshop Fee: Free Workshop Limit: 50
Ready to take that first step and Speak Up & Reach Out to the public? Or, you’re simply looking for some great tips on improving and taking your public outreach efforts to the next level. If so, then this is a suitable workshop for you! Join us and learn how to plan your public outreach activities and effectively use the AALAS Foundation’s free outreach materials. With customizable PowerPoint templates, you’ll learn how to edit and use our “What’s Happening in Research?” and “Careers in LAS” fully scripted slides. You’ll also be presented with great tips on overcoming your fear of public speaking. Attendees of this workshop will be armed with helpful guidance on responding to the “tough questions” often asked by the public about the work done in LAS. This workshop aims to ensure you feel inspired, motivated, and confident in speaking to the public and using the AALAS Foundation’s free resources.
This Workshop is sponsored in part by AALAS Foundation.

W-15 Animal Facility, Design, Processes, Decisions, and Technology
8:00 AM - 12:00 PM/Room: 110B
Leader: Chad Zuberbuhler
Faculty: Lauri Tyrrell, Danielle Yeaton, Mark A Corey, Clifford R Roberts, Laura Halverson, Katie L McGimpsey, Mitch Hickmann
Facilitator: TBN
Workshop Fee: $150 Workshop Limit: 50
This session will benefit those involved with animal facility design and operations by describing the process, decisions, and technologies involved in the design and construction of animal facilities. The workshop will begin with a discussion of the facility design process—who should be involved, the objectives and level of effort by stakeholders, milestone decisions to be reached, and the anticipated duration of the various process phases. Current trends in the industry will be explored through discussions about planning, interior construction, acoustics, and finishes. Throughout the session, we will frame the conversation from the occupant’s perspective and their experiences in the real world. We will include critical mechanical, electrical, and piping design and operations. This section will focus on the mechanical, electrical, and plumbing risks associated with compromised animal welfare, research loss, facility resiliency, and how engineering
decisions affect each parameter. The lessons learned will help participants make more informed decisions as they develop and operate their facilities. Conversations will focus on vivarium operations and facility sanitation and safety. We will also discuss energy and water conservation strategies that are being effectively implemented in animal facilities and the derived long-term benefits.

W-16 Not Just a Document: The Art of Crafting SOPS That Work
8:00 AM - 12:00 PM/Room: 107B
Leader/Faculty: Amy L Dryman
Facilitator: TBN
Workshop Fee: $150 Workshop Limit: 50
This workshop will cover how to build SOPs to fit and function for your team and program. We will explore SOP anatomy and function, solve the riddle of when an SOP is not an SOP, and dive into how to develop and then write an SOP that’s a valuable tool and reference. While we won’t get from the first draft to a finalized tool, we will examine key points to remember when developing, writing, editing, and finalizing SOPs. Participants will get the chance to put those points to the test during the workshop. The audience for this session is anyone tasked with writing SOPs for their program or interested in learning to create an SOP from scratch. This workshop is not intended for an audience looking to learn how to cover GLP specifics.

W-09 Occupational Health and Safety Considerations in Animal Research: Learning Through Interactive Case Studies - Part 2
(8-hour workshop continued from Tuesday 8:00 AM)
8:00 AM - 12:00 PM/Room: 110A
Leader/Faculty: Lesley A Colby, Susan B Harper
Facilitator: TBN
See Tuesday 8:00 AM for pricing and description.

W-10 Positive Reinforcement Training and Temperament Testing: Preparing Monkeys for Restraint Procedures - Part 2
(8-hour workshop continued from Tuesday 1:00 PM)
8:00 AM - 12:00 PM/Room: 108
Leader: Jaine E Perlman
Faculty: Mollie A Bloomsmith, Kristine Coleman, Lisa A Houser, Jennifer L McMillan
Facilitator: TBN
See Tuesday 1:00 PM for pricing and description.
This Workshop is sponsored in part by Carter 2 Systems, Britz and Company, NC3Rs, and Lomir.
8:00 AM - 10:00 AM
Platform Session abstracts will be available on www.aalas.org in July. They will also be included in both the mobile app and the National Meeting Final Program.






Heroes Behind the Scenes: The Crucial Contribution of Laboratory Animal Professionals in Therapeutic Development for Neurologic and Neurodegenerative Disorders
8:00 AM - 10:00 AM/Room: 101A
Leader/Moderator: Sarah Hansen
Facilitator: Marcia L Hart
It’s estimated that physical and cognitive disability due to neurologic disorders affects up to 15% of the population, a number expected to rise. Therapeutics and effective interventions for these devastating diseases remain insufficient. From basic science through bedside treatments, collaborative teams approaching research questions face significant challenges. At a basic level, the development of animal models requires careful evaluation to ensure that disease states are readily recapitulated. Further translation to humans requires large animal models that likewise pose challenges regarding administration, care, and endpoints. Once a therapeutic has reached the late preclinical stage, preliminary animal data is revisited and expanded to develop critical targeted dosing regimes and evaluate potential adverse effects. At every stage, data must be adequately characterized and repeatable to facilitate necessary studies for advancement to human clinical trials. Told through the lens of veterinary staff directly involved in animal models ranging from mice to non-human primates, this seminar shares the critical role of laboratory animal professionals in neurologic and neurodegenerative research. Each speaker will discuss the background and devastating effects of a human neurologic disorder and their involvement in research directed toward clinical interventions. In this seminar, participants will gain insights into the pivotal contributions of laboratory animal professionals in developing essential interventions for neurodegenerative diseases, as well as the profound personal impact these studies can have on individuals involved. The target audience includes veterinarians, technicians, IACUC members, and anyone working in laboratory animal medicine who hopes to hear more about how their work makes a difference in the lives of humans.
Speakers/Topics:
8:00 Sarah Hansen Welcome and Introductions
8:15 Verda A Davis Behavioral Assessment of Rodent Models of Alzheimer’s Disease
8:35 Steven E Davison Challenges Associated with Post-Surgical Care in a Swine Model of Spinal Cord Injury
8:55 Allison M Ostdiek
9:15 Benjamin J Olthoff
Translation of Intracortical Visual Prosthesis from Animal Research to Human Medicine to Enhance Quality of Life for the Visually Impaired
Safety Assessment of Neurodegenerative Disease Therapeutics: Potential Pitfalls of Delivery and Their Clinical Management
9:35 All Questions & Answers

Outbreak! Navigating through the Storm of an Unexpected Infectious Agent Detection
8:00 AM - 10:00 AM/Room: 104A
Leader/Moderator: Kenneth S Henderson
Facilitator: Cheryl L Woods
A routine rodent health monitoring program alone does not prevent the introduction of adventitious agents. Still, it is a vital tool to provide insight into the success of your biosecurity program and allows you to detect and eliminate unwelcome visitors when they appear. It is not “if” but “when” you will obtain an unexpected positive for an agent on your exclusion list. The impact of a surprising finding on the research conducted at your vivarium or the ability of the agent to spread beyond the initial ground zero location is dependent on the agent as much as it is on the rodent models used, housing system, facility design, and biosecurity program employed at the facility. This seminar will address the initial steps when confronted with an unexpected detection of an infectious agent, agent-targeted strategies to methodically determine the extent of infection within your vivarium, feasibility and process of eradication through treatment, depopulation, or relocation, and post-investigation through rootcause analysis to mitigate future introductions of excluded agents. The target audience is veterinarians, rodent vivarium managers, and those overseeing rodent health monitoring programs.
8:00 Kenneth S Henderson Welcome and Introductions
8:05 Marnie Silverstein Metzler
Fecal Forensics: Catching the Culprit Through Confirmation Testing and Biosecurity Assessments
8:30 Kenneth S Henderson
Know Thy Enemy: Strategies for Hunting Down and Determining the Extent of an Outbreak
8:55 Jason S Villano Decision Tree for Treatment, Eradication, or Toleration
9:20 Guy B Mulder
Root Cause and CAPA: Identifying Cause and Preventing Similar Events in the Future
9:45 All Questions & Answers

The Digital In Vivo Alliance - Leveraging MachineLearning (ML)-Defined Digital Biomarkers to Improve the Reproducibility and Translation of Animal Studies
8:00 AM - 10:00 AM/Room: 207A
Leader: Brian R Berridge
Moderator: Natalie A Bratcher-Petersen
Facilitator: Paige A Ebert
Optimal drug development is a seamless progression from basic research to preclinical modeling to human studies. The proliferation of digital ‘wearables’ in clinical medicine will redefine our understanding of human health and disease. Home cage application of analogous preclinical digital biomarkers could enhance animal studies’ translational relevance, generalizability, reproducibility, and 3Rs impact. The Digital In Vivo Alliance (DIVA) is a multi-disciplinary collaboration to advance digital biomarkers through their


development, validation, adoption, and regulatory acceptance. Animal studies modeling drug-induced neuroactivity, Amyotrophic Lateral Sclerosis, Friedreich’s Ataxia, and the epileptogenic Dravet Syndrome support the development of digital biomarkers of activity, locomotion, and seizure. The outcomes of these studies are used to iteratively train ML-based algorithms and provide a substrate for their analytical and clinical validation. Cross-site studies demonstrate the enhanced reproducibility of these measures. This session will share the design, application, and outcomes of these studies as proof-ofconcept vignettes demonstrating the unique value of digital home cage measures as well as the paradigm-defining approach of DIVA. This seminar is for researchers, veterinarians, data scientists, support and care staff, vivarium managers, welfare scientists, ethics committee members, and those interested in how emerging technologies and digital biomarkers can be used to enhance the translational relevance, generalizability, reproducibility, and 3Rs impact of animal studies.
8:00 Natalie A Bratcher-Petersen Welcome and Introductions
8:05 Brian R Berridge
8:30 Vivek Kumar
Enhancing In Vivo Toxicology Studies: Leveraging Machine Learning-Informed Digital Biomarkers for Improved Sensitivity and Translational Relevance
Advancing Animal Research Through Innovation: Leveraging the V3 Framework to Build Confidence in Computer-Vision Derived Digital Biomarkers to Assess Animal Behavior
8:55 Jennifer Leedy
9:20 Michael Saul
Addressing Challenges in Preclinical Models: Enhancing Relevance in Neuroscience Disease Research through Home Cage Digital Measures
Understanding Cross-Site Reproducibility Challenges: Utilizing Home Cage Digital Measures to Discover and Reassess Sources of Cross-Site Variance
9:35 All Questions & Answers
This Seminar is sponsored in part by The Jackson Laboratory.

8:00 AM - 10:00 AM/Room: 209A
Leader: Valerie K Bergdall
Moderator: Judy M Hickman-Davis
Facilitator: Nicholas Alexander Lordi
The 75th National AALAS Meeting celebrates a commitment to animal welfare and science. Veterinarians play a critical role in creating and accepting animal care standards. Increased regulations and the fast pace of new technology have forced a rapid evolution of lab animal science, resulting in innovative career opportunities. These inno-
vative career paths range from improved research animal welfare in the lab to wildlife conservation in Madagascar. Novel venues require veterinary input on ethics, risk mitigation, regional permits, and indigenous community communication. A local Public Health Department›s unique role in providing oversight of research institutions using animals allows veterinary input on zoonotic diseases and environmental health. Veterinarians are an essential component of the military medical research team, contributing to developing life-saving medical products and procedures. They support public health through research, surgical care, food safety, and defense. Veterinarians in academia and industry perform independent research or serve as collaborators for basic research, as well as drug design and development. Veterinarians play a vital role in animal welfare as AAALAC International site visitors for the accreditation of programs using animals in science and education. The rise of “One-Health-One Medicine” over the last 20 years highlights the interconnectedness of humans, animals, and the environment to promote ecosystem health. Promoting human and animal health through the lens of climate change and advancing a community-designed strategy can reverse poverty and transform the health of people and animals. This session will discuss how laboratory animal medicine has changed, focusing on innovative career pathways. We will highlight how the veterinary profession and research animal oversight evolve together. We will review opportunities in public health, the military, industry, accreditation, and One-Health One Medicine. The targeted audience is Veterinarians, Veterinary Students, Veterinary Training Program Directors, and Organizers. Anyone interested in the past and future employment opportunities within lab animal medicine and care.
8:00 Judy M Hickman-Davis Welcome and Introductions
8:05 Judy M Hickman-Davis
8:20 Erin M Bryant Hall
8:40 Christian C Hofer
9:00 Gary L Borkowski
9:20 Jeff D Wyatt
An Introduction to Unique Careers: a Brief History of Lab Animal Medicine as a Profession
What Does the Public Health Department Have to do with Lab Animal Care?
How Do Deserts, Donkeys and Working Dogs Lead You to Lab Animal?
Academia, Pharmaceuticals and Accreditation, Excellence in Animal Welfare and Science
One Health One Medicine, Building a Career from the Human-Animal Bond
9:40 All Questions & Answers
Exhibit Hall Refreshment Break!
9:00 a.m.– 11:00 a.m.
Sponsored by TBD






The Jackson Laboratory
10:10 a.m. – 10:30 a.m.
(Presentation title, product, and description to come)

Contec Inc.
10:35 a.m. – 10:55 a.m.
(Presentation title, product, and description to come)

Alternative Replacement Training (ART) Methods: Implementing the 3R’s Into Our Training Program
11:00 AM - 12:00 PM/Room: 104A
Speaker: April J George
Moderator: Michael L Fallon
Facilitator: Mandy N Sexton
Training research technicians to work with animals poses various challenges. However, these challenges create an excellent opportunity to assess practices, enhance efficiencies, and integrate the 3R’s principles (refinement, replacement, and reduction) into our training programs. Incorporating Alternative Replacement Training (ART) Methods is one impactful way to implement the 3R’s principles within our training curriculums. The use of ART methods can reduce the number of animals needed to achieve competency, improve a facility’s culture of care, and refine training approaches by enhancing efficiencies and skill development. During this conversation, we will give examples of ART Methods, discuss the benefits, and provide the results of an evaluation project that will open your mind to these training modes. The targeted audience would be anyone who performs training as part of their job role or wants to expand their animal welfare and 3R’s initiatives into training curriculums and improve training efficiencies. Attendees will learn about Alternative Replacement Training (ART) Methods that create the opportunity to train without live animals. They will learn that this includes not just simulators/training models but everything from training documents and videos that describe a process to the most modern technologies (like virtual reality, 3D scanning, 3D printing, and virtual cadavers). They will also learn how to incorporate ART Methods into their training program and be provided evidence of their effectiveness as training tools.

Nathan E Brewer Lecture
11:00 AM - 12:00 PM/Room: 209A
Speaker and description will be available after the Award Selection Committee selects the Nathan Brewer Award Recipient. This session information will be available in the mobile app and the Final Program. This Special Topic Lecture is sponsored in part by the AALAS Awards Selection Committee (ASC).

Should Ethics be Included in the Next Guide? The Pros, the Cons, and Why Members of the Research
11:00 AM - 12:00 PM/Room: 101A
Speaker: Jerrold Tannenbaum
Moderator: B Taylor Bennett
Facilitator: TBN
The process of writing the next edition of the Guide for the Care and Use of Laboratory Animals is advancing slowly but surely. A critically important and fraught question the authors of the next edition will face is whether or to what extent ethical principles or rules for investigators, IACUCs, veterinarians, and animal care staff should be included. This presentation explains why the future success of animal research may depend on how this question is answered. The presentation also argues that a satisfactory answer will only be achieved if research community members make their voices heard.
The discussion will review the treatment of ethics in the current Guide and identify strengths and flaws. Arguments for and against including certain ethical principles in the next edition will be considered. Among the challenges to the inclusion of the ethical tenets is the fact that ethical principles and positions involve value judgments about which there can be disagreement and controversy that the statute pursuant to which the Guide is applied to IACUCs was not intended to allow IACUCs to engage in the ethical assessment of research; that IACUCs do not have particular expertise about ethical issues; that different IACUCs could well disagree about whether certain kinds of animal research or particular experiments or experimental techniques are ethically acceptable; and that attempts to resolve such disagreements in the Guide – and enforcement of such solutions by OLAW or other government or private bodies that require adherence to the Guide – could hinder or threaten necessary research. So-called “harm-benefit analysis” and research involving nonhuman primates will be among the examples used to illustrate these challenges. The presentation will suggest how the next edition of the Guide could address these potential problems while affording appropriate attention to ethics. The audience would be anyone involved in biomedical research. The presenter is a member of the NASEM Standing Committee for the Care and Use of Animals in Research, which has begun producing a new Guide. The views he will express are his own and do not necessarily reflect those of other members of the Standing Committee or the Committee as a whole.
This Special Topic Lecture is sponsored in part by National Association for Biomedical Research (NABR).

11:00 AM - 12:00 PM/Room: 207A
Speaker: Kenneth S Henderson, Chris A Manuel
Moderator: Karuna Patil
Facilitator: TBN
Since the mid-1990s, many new infectious agents have been discovered, covering all classes of organisms that infect rodents. However, compared to agents discovered before the mid-90s, only a few newer agents have been added to vivarium exclusion lists or in health monitoring programs. Thus, research institutions tolerate most of


these new agents. Some of these agents are benign, while others are opportunistic and even primary pathogens of rodents. If not excluded outright, agents designated as tolerated are at most isolated within dedicated “low health status” or “dirty” areas within institutions. Prevalence data, recently reported for commonly screened agents gathered over the past 20 years, show a dramatic drop in the prevalence for widely excluded agents compared to tolerated ones. Are we helping ourselves by tolerating so many agents? How should we systematically determine “should it stay or should it go?” This lecture will introduce you to and review currently tolerated or ignored agents and their known or unknown impacts on animal health and research outcomes. Then, we will introduce the participants to 12 key factors, which either actively or passively funnel current and future infectious agents of rodents into the pigeonholes of tolerated and excluded. The target audience includes animal care and veterinary technicians, veterinarians, program directors, and diagnosticians who develop the assays used for agent detection, fund health monitoring programs, make decisions on agents to tolerate and exclude, and those who physically make rodents’ health monitoring programs work daily.

Adapting to Unconventional Scenarios: Navigating Regulatory Challenges in Laboratory Animal Research
12:30 PM - 2:00 PM/Room: 102A
Leader: Summer Boyd
Moderator: Deborah Calantropio-Covington
Facilitator: Victoria Elam
Panelist: Summer Boyd, Diana Medina, Deborah Calantropio-Covington, Jodi Ternes
This panel is tailored to cater to Veterinary Technicians and those with any clinical oversight role. This session provides valuable insights and resources for attendees responsible for ensuring the welfare and compliance of animals in research settings, including research staff, animal care personnel, and departmental supervisors directly engaged in laboratory animal facility inspections and site visits. Moreover, individuals tasked with leading tours or escorting guests through laboratory animal facilities will find the discussion both relevant and insightful, offering practical strategies to enhance their roles in facilitating transparent and compliant operations through various situations. This interactive panel discussion will delve into the complexities of working through unique species, including unique housing environments for salamanders, anoles, spiny mice, and poison dart frogs; the single housing and re-pairing of rabbits, mice, and rats, and non-human primate colony management changes and the challenges that pose. Other potential circumstances encountered in laboratory animal research with special attention to navigating the intricacies of laboratory animal facility inspections and site visits. Our panel of expert Veterinary Technician Specialists (VTS) will offer insights and resources to help attendees understand the laws, guidelines, and nuances of various species and special research or husbandry needs. From regulatory agencies to accreditation bodies, we’ll explore the various entities conducting inspections, empowering attendees to navigate these encounters with confidence and PAW-sitive compliance. This session promises to equip attendees with a holistic understanding of the inspection process, foster team collaboration, and enhance preparedness within the lab animal community.
This Panel Discussion is sponsored in part by Academy of Laboratory Animal Veterinary Technician and Nurses (ALAVTN).


Cultivating a Culture of Belonging Through Engagement: Strategies for Today’s Workplace 12:30 PM - 2:00 PM/Room: 103A
Leader: Donna M Jarrell, Tanise L Jackson, Mary T Spencer
Moderator: Donna M Jarrell
Facilitator: Jarrod Nichol
Panelist: Donna M Jarrell, Tanise L Jackson, Mary T Spencer
Creating a culture of belonging is essential in an era where employee engagement and retention are critical to organizational success. This panel discussion will explore effective strategies to foster such a culture of operational excellence, focusing on accommodating diverse workstyles, the impact of servant leadership, and the importance of self-care as a retention tool. We’ll discuss how recognizing and supporting diverse workstyles can boost creativity and satisfaction, making every team member feel valued. The conversation will then shift to how servant leadership can motivate employees by prioritizing their needs and development, enhancing trust and purpose within the team. Lastly, we’ll examine the role of self-care in preventing burnout and promoting resilience, highlighting its significance in today’s high-stress work environments. Organizations can reduce turnover and foster a supportive workplace by encouraging a healthy worklife balance. The discussion aims to provide an integrated approach, showcasing how these strategies work together to build a cohesive culture of belonging that benefits employees and the organization. Through this holistic approach, we can create more engaging, supportive, and sustainable workplaces. The target audience comprises managers and leaders overseeing Animal Care and Use Programs.

Danio Zoom Live 2: The Return
12:30 PM - 2:00 PM/Room: 105A
Leader/Moderator: Christine Archer
Facilitator: Michelle Altemara
Panelist: Christine Archer, Emma Liechty, Joshua Barber, Hugh Hammer, Logan Fehrenbach
The Zebrafish Husbandry Association returns for 2024 AALAS with a new set of panelists from various roles and facility types to answer all your zebrafish and aquatic facility queries. This panel is a live extension of the ZHA’s popular bi-weekly online meetups where questions are answered, collaborations are formed, and difficult topics are discussed. As aquatic animals grow in importance in the laboratory landscape, it is more important than ever to come together to share our experience and expertise. Panelists will prompt the audience with common topics of discussion such as enrichment, diet, larviculture, and life support systems, as well as provide an opportunity for open questions from the audience for a more organic discussion tailored to the attendee’s needs. During this panel, attendees are welcome to bring any questions about aquatic animals. Panelists will provide different points of view, from that of a large multi-species facility manager, a smaller zebrafish-only facility manager, to a facility veterinarian. Participants will learn about broad and specific aspects of aquatic animal husbandry, health, and welfare. This session will be of interest to animal care technicians, facility managers, veterinary technicians, veterinarians, IACUC members, and anyone working in a lab animal setting who encounters aquatic animals, with a special focus on attendees who might only be working with zebrafish part-time or helping to cover the species as part of the weekend or holiday work.
This Panel Discussion is sponsored in part by Zebrafish Husbandry Association (ZHA).






Opioid Analgesic For subcutaneous use only in mice, rats, and ferrets, and non-human primates.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
LEGAL STATUS—In order to be legally marketed, a new animal drug intended for a minor species must be Approved, Conditionally Approved, or Indexed by the Food and Drug Administration. THIS PRODUCT IS INDEXED—MIF 900-014. Extra-label use is prohibited. This product is not to be used in animals intended for use as food for humans or food-producing animals.
HUMAN SAFETY WARNING
Abuse Potential
ETHIQA XR contains buprenorphine, an opioid that exposes humans to risks of misuse, abuse, and addiction, which can lead to overdose and death. Use of buprenorphine may lead to physical dependence. The risk of abuse by humans should be considered when storing, administering, and disposing of ETHIQA XR. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drugs or alcohol) or mental illness (e.g., depression).
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression may occur with accidental exposure to or with misuse or abuse of ETHIQA XR. Monitor for respiratory depression if human exposure to buprenorphine occurs. Misuse or abuse of buprenorphine by swallowing, snorting, or injecting poses a significant risk of overdose and death.
Accidental Exposure
Because of the potential for adverse reactions associated with accidental exposure, ETHIQA XR should only be administered by veterinarians, veterinary technicians, or laboratory staff who are trained in the handling of potent opioids. Accidental exposure to ETHIQA XR, especially in children, can result in a fatal overdose of buprenorphine.
Risks From Concurrent Misuse or Abuse with Benzodiazepines or Other CNS Depressants
Concurrent misuse or abuse of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death.
See HUMAN SAFETY WARNINGS for detailed information.
DESCRIPTION
Ethiqa XR is an injectable suspension of extended-release buprenorphine. Buprenorphine hydrochloride, an opioid analgesic, is the active ingredient in Ethiqa XR. Lipid-bound buprenorphine hydrochloride is suspended in medium chain fatty acid triglyceride (MCT) oil. Lipids encapsulate the buprenorphine limiting diffusion which provides for larger doses and prolonged action.1,2 Ethiqa XR has a slightly yellow to white opaque appearance. Each mL contains approximately 1.3 mg buprenorphine hydrochloride. The sterile product contains cholesterol, benzyl alcohol, glyceryl tristearate, and buprenorphine hydrochloride suspended in MCT oil. Buprenorphine belongs to the opioid class of drugs and is a narcotic under the Controlled Substances Act due to its chemical derivation from thebaine.
Buprenorphine Formula C29H41NO4
INDICATIONS

Ethiqa XR is indicated for the control of post-procedural pain in mice, rats, ferrets, and non-human primates.
DOSAGE AND ADMINISTRATION
Wear protective clothing when administering Ethiqa XR.
Do not dispense Ethiqa XR for administration at home by the pet owner (see HUMAN SAFETY WARNINGS).
Dosing
At the doses stated in the table below, therapeutic blood levels are maintained for 72 hours after the initial dose. If needed, a single repeat dose may be administered 72 hours after the initial dose.
Administer Ethiqa XR subcutaneously according to the dosage for the appropriate species listed in the Dosing Chart below.
DOSING TABLE FOR SUBCUTANEOUS INJECTION OF ETHIQA XR
Species Mice
Rats Ferrets Non-human primates
Administration
Animals may exhibit an obtunded response to stimuli up to 4 hours after receiving Ethiqa XR.
When using Ethiqa XR, an opiate antagonist such as naloxone, should be available in case reversal is required.
Ethiqa XR may cause sedation, decreased blood pressure, decreased heart rate, decreased gastrointestinal mobility, and respiratory depression. Use caution with concomitant administration of Ethiqa XR with drugs that cause respiratory depression.
Animals should be monitored for signs of decreased cardiovascular and respiratory function when receiving Ethiqa XR.
The safety of Ethiqa XR has not been evaluated in pregnant, lactating, neonatal, or immune-compromised animals.
ADVERSE REACTIONS
Two Laboratory Studies in Mice
No adverse reactions were observed in sixteen of 20-to-25-gram young adult mice (8 males, 8 females) after a single subcutaneous injection of 16.25 mg/kg Ethiqa XR (5X). Laboratory parameters evaluated in the study included hematology and clinical chemistry; histopathology was also performed.11 In a second study, 16 adult mice (8 males, 8 females) received 16.25 mg/Kg (5X) Ethiqa XR subcutaneous for three doses at four-day intervals. A surgical procedure was performed on the mice prior to receiving each of the three doses of Ethiqa XR. Mortality was seen in two male mice after the third surgical procedure and third dose of Ethiqa XR (total dose of 49 mg buprenorphine/Kg body weight in 8 days). Weight loss was observed postprocedurally in mice administered Ethiqa XR.11
Two Laboratory Studies in Rats
Adverse reactions were evaluated in twenty-four 180-to-200-gram young adult rats (12 male, 12 female) after a single subcutaneous injection of Ethiqa XR. A surgical procedure was performed on the rats prior to receiving a single dose of Ethiqa XR of 0.65 (1X), 1.3 (2X), 3.9 (6X), or 6.5 mg/ Kg (10X); Six in each group (3 male and 3 females).12
Adverse reactions also were evaluated in 24 young adult rats (8-weeks at start). There were 12 male and 12 females. The female rats weighed between 128-164 grams and males weighed between 169-219 grams. Each rat received a subcutaneous injection of 1.3 (2X), 3.9 (6X), or 6.5 mg/Kg (10X) Ethiqa XR for three doses at four-day intervals (8 rats per group; 4 males, 4 females). A surgical procedure was performed on the rats prior to receiving each of the three doses of Ethiqa XR. Laboratory parameters evaluated in the study included hematology, clinical chemistry, urinalysis, histopathology, and bodyweight.3,12 Signs of nausea were observed at all dose levels (1 rat at 1.3 mg/Kg, 3 rats at 3.9 mg/Kg, 2 rats at 6.5 mg/Kg) within 24 hours of the dose. Signs included self-licking, self-gnawing and efforts to eat wood-chip bedding. Mortality was seen in 1 of 36 rats exposed to wood chip bedding. Necropsy revealed the stomach and esophagus were compacted with bedding, the bladder was abnormally distended, and the urine contained blood.3,12 3 out of 222 rats (the 222 rats are from five (5) pharmacokinetic and safety studies) were observed to bleed profusely from the jugular vein, which was used for obtaining blood samples, and subsequently died.
Two Laboratory Studies in Ferrets
No studies have been published administering Ethiqa XR to ferrets. One unpublished study reports that no adverse reactions were observed after 4 adult female ferrets received a single subcutaneous injection of 0.6 mg/Kg of Ethiqa XR.13
In a pharmacokinetic single-dose study, no adverse reactions were observed in 6 male, approximately 1-year old, ferrets after receiving 0.04 mg/Kg buprenorphine immediate-release.9
Two Laboratory Studies in Non-Human Primates
In a pharmacokinetic study, 25 adult common marmosets received a single SQ dose of Buprenorphine SR (0.15 mg/Kg, N=8) or Ethiqa XR (0.1 mg/kg, N=6, 0.15 mg/Kg, N=3, and 0.2 mg/kg, N=8). Injection site reactions were scored based on gross examination of erythema and swelling. Mild sedation was noted at 8- and 24-hours post-dose in all groups. Body weights decreased relative to baseline in all groups except Ethiqa XR 0.15 mg/Kg; however, these decreases were not clinically significant (<10% of body weight). Buprenorphine injections of either formulation resulted in increased cage movement that was dose dependent. Both Buprenorphine SR and Ethiqa XR acute injection sites exhibited acute necrosis and inflammation. The degree of inflammation was overall similar for chronic both drugs; however, qualitatively different. The Burprenorphine SR injection sites were associated with mainly macrophages and neutophils, while the Ethiqa XR sites were associated with macrophages and multinucleated giant cells and cholesterol clefts in response to the vehicular medium.6 In a pharmacokinetic study, four adult male cynomolgus monkeys were administered a single dose of Ethiqa XR (0.2 mg/Kg) SQ. No abnormal behaviors or clinical signs were observed up to 120-hours post injection.7
CONTACT INFORMATION
Contact Fidelis Animal Health at 1-833-384-4729 or www.ethiqaxr.com. To report suspected adverse drug experiences, contact Fidelis Animal Health at 1-833-384-4729.
For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
CLINICAL PHARMACOLOGY3
Mechanism of Action: Buprenorphine exerts its analgesic effect via high affinity binding to various subclasses of opiate receptors particularly mu, in the central nervous system. Buprenorphine analgesic and adverse reactions are mediated by mu opioid receptor agonism. Due to its partial agonist activity, buprenorphine exhibits a ceiling affect to its actions and thus has a greater therapeutic index compared to full mu opioid receptor agonists such as morphine. Buprenorphine binds tightly to and dissociates slowly from the opioid receptor. Therefore, the pharmacological effects of buprenorphine are not directly related to plasma concentrations.
3.25 mg/Kg
0.65 mg/Kg
Dose mg/Kg Body Weight Time to Reach Therapeutic Blood Levels after Administration Comments 30 minutes10 6 hours4 30 minutes13 15 minutes6
Ethiqa XR can be administered 30 minutes prior to painful stimulus10
0.6 mg/Kg 0.2 mg/Kg
Ethiqa XR can be administered 15 minutes prior to painful stimulus6
Shake the vial well before each use to ensure uniform suspension. If stored refrigerated, bring to room temperature before use.
Use aseptic technique to subcutaneously administer Ethiqa XR by utilizing minimally stressful restraint techniques or sedation.
An oily sheen may be observed in the fur after injection due to leakage of Ethiqa XR, which is an oil-based drug suspension, from the injection site. The oily sheen may last for 4 to 5 days post-injection. Leakage from the injection site can be minimized by slowly injecting Ethiqa XR into the subcutaneous space.
Do not return any unused drug suspension from the syringe back into the vial. The animal can be returned to its cage immediately after receiving Ethiqa XR. (See CONTRAINDICATIONS, PRECAUTIONS, and ADVERSE REACTIONS for additional information on bedding.)
CONTRAINDICATIONS
Only administer Ethiqa XR by subcutaneous injection. Ethiqa XR is not intended for intravenous, intra-arterial, intrathecal, intramuscular, or intraperitoneal injection.
Do not use in animals with pre-existing respiratory compromise.
Do not house rats on wood chip-type bedding after administration of Ethiqa XR. Signs of nausea, including pica, have been observed in rats for up to 3 days post-treatment with Ethiqa XR. Pica involving wood chip type bedding can be lethal (see ADVERSE REACTIONS).
HUMAN SAFETY WARNINGS
Not for use in humans. Keep this and all medications out of reach of children and pets.
Human User Safety While Handling Ethiqa XR in the Hospital: Ethiqa XR should only be handled and administered by a veterinarian, veterinary technician, or laboratory staff trained in the handling of potent opioids.
To prevent human adverse reactions or abuse, at least 2 trained administrators should be present during injection of Ethiqa XR. Wear protective clothing when administering Ethiqa XR.
Mucous Membrane or Eye Contact During Application:
Direct contact of Ethiqa XR with the eyes, oral, or other mucous membranes could result in absorption of buprenorphine and the potential for adverse reactions. If accidental eye, oral, or other mucous membrane contact is made during application, flush the area with water and contact a physician immediately. If wearing contact lenses, flush the eye first and then remove the contact lens.
Skin Contact During Application:
If human skin is accidentally exposed to ETHIQA XR, wash the exposed area immediately with soap and water and contact a physician. Accidental exposure could result in absorption of buprenorphine and the potential for adverse reactions.
Drug Abuse, Addiction, and Diversion of Opioids:
Controlled Substance:
Ethiqa XR contains buprenorphine, a Schedule III controlled substance with an abuse potential similar to other Schedule III opioids. Abuse:
Ethiqa XR contains buprenorphine, an opioid substance, that can be abused and is subject to misuse, abuse, and addiction, which may lead to overdose and death. This risk is increased with concurrent use of alcohol and other central nervous system depressants, including other opioids and benzodiazepines.
Ethiqa XR should be handled appropriately to minimize the risk of diversion, including restriction of access, the use of accounting procedures, and proper disposal methods, as appropriate to the clinical setting and as required by law.
Prescription drug abuse is the intentional, non-therapeutic use of a prescription drug, even once, for its rewarding psychological or physiological effects. Buprenorphine has been diverted for non-medical use into illicit channels of distribution. All people handling opioids require careful monitoring for signs of abuse.
Storage and Disposal:
Ethiqa XR is a Schedule III opioid. Store in a locked cabinet according to federal and state controlled substance requirements/guidelines. Discard any broached vials after 90 days. Any unused or expired vials must be destroyed by a reverse distributor; for further information, contact your local DEA field office or call Fidelis Animal Health at 1-833-384-4729.
Information for Physician:
Ethiqa XR contains a mu opioid partial agonist (1.3 mg buprenorphine/mL). In the case of an emergency, provide the physician with this package insert. Naloxone may not be effective in reversing respiratory depression produced by buprenorphine. The onset of naloxone effect may be delayed by 30 minutes or more. Doxapram hydrochloride has also been used as a respiratory stimulant.
PRECAUTIONS
Death has been reported when non-steroidal anti-inflammatory drugs (NSAIDs such as meloxicam and carprofen) and Ethiqa XR have been administered concomitantly in mice.5
The use of paper or soft bedding for up to 3 days following administration of Ethiqa XR should be considered (see CONTRAINDICATIONS and ADVERSE REACTIONS).
Buprenorphine is excreted in the feces (see CLINICAL PHARMACOLOGY). Coprophagy may lead to ingestion of buprenorphine or its metabolites by animals treated with Ethiqa XR and untreated cage mates. Ethiqa XR forms a depot near the injection site. Granulomatous inflammatory nodules have been observed in naked-skinned mice and rats administered Ethiqa XR.4,5 Injection site reactions including inflammation and necrosis have been observed in common marmosets.6
Buprenorphine can act as an agonist and antagonist at different classes of opioid receptors. Agonism at the mu opioid receptor and, in some cases, antagonism at the kappa or delta opioid receptors are possible underlying mechanisms for the ceiling effect and bell-shaped dose-response curve of buprenorphine. Studies with knockout mice have shown that the antinociceptive effect of buprenorphine, which is mediated primarily by the mu opioid receptor, is attenuated by the ability of the drug to activate the opioid receptor like (ORL-1) receptor. The drug can be described as a ‘full’ and a ‘partial’ agonist at the same receptor depending on the specific assay. There appears to be no ceiling effect for analgesia, but there is a ceiling effect for respiratory depression. Pharmacokinetic studies with bolus injections of buprenorphine in mice and rats provide similar models. After bolus intravenous administration, plasma levels decline tri-exponentially. The drug is n-dealkylated in the liver to norbuprenorphine (NBN), an active metabolite. Studies have shown that glucuronide metabolites of buprenorphine and NBN are also metabolically active, and can approximate or exceed the concentration of the parent drug. Un-metabolized drug excreted in the urine and feces one week after injection was 1.9 and 22.4% of the dose, respectively, and 92% of the dose was accounted for in one week.3
Mice
Pharmacokinetic parameters of Ethiqa XR were studied in 6-8 week old male and female Balb/c mice following a single subcutaneous injection of 3.25 mg/kg bodyweight. Therapeutic blood levels were observed up to 72 hours after subcutaneous injection.
Rats
Pharmacokinetic parameters of Ethiqa XR were studied in 8 week old male and female Fischer rats following a single subcutaneous injection of 0.65 mg/kg bodyweight. Therapeutic blood levels were observed up to 72 hours after subcutaneous injection.
Ferrets
Pharmacokinetic parameters of Ethiqa XR were studied in 4 adult female ferrets following a single subcutaneous injection of 0.6 mg/Kg body weight. Therapeutic significant blood levels were observed within 30 minutes up to 72 hours after administration.13
Non-Human Primates
In a pharmacokinetic study, 25 adult common marmosets were evaluated after receiving a single SQ dose of Buprenorphine SR (0.15 mg/Kg, N=8) or Ethiqa XR (0.1 mg/kg, N=6, 0.15 mg/Kg, N=3, and 0.2 mg/kg, N=8). Therapeutic blood levels were observed within 30 minutes to 72 hours after subcutaneous injection.6
In a pharmacokinetic study, four adult male cynomolgus monkeys (6.41-9.58 Kg) were administered a single dose of Ethiqa XR (0.2 mg/Kg) SQ. Therapeutic blood levels peaked above 0.5 ng/mL for at least 96-hours and remained in the significant range.7
In adult baboons (5 male and 5 females), onset of concentrations of buprenorphine hypothesized to produce analgesia (0.1 ng/mL) occurred within 30 minutes of SQ administration of Buprenorphine SR (0.2 mg/Kg) and remained there for at least 120-hours.8
HOW SUPPLIED
Ethiqa XR is supplied in a 5 mL glass vial containing 3 mL of injectable drug suspension.
STORAGE INFORMATION
Store between 15° and 25°C +/- 2°C (59° and 77°F) or refrigerated. DO NOT FREEZE. If stored refrigerated, bring to room temperature before use. Once broached, the multi-dose vial should be discarded after 90 days.
Product could change its physical properties if not stored within the specified storage conditions and original vial container.
REFERENCES
1. Mishra et al. Engineering solid lipid nanaparticles for improved drug delivery: promises and challenges of translational research. Drug Deliv. and Transl. Res, 2: 238-253; 2012.
2. Bethune et al., The role of drug-lipid interactions on the disposition of liposome-formulated opioid analgesics in vitro and in vivo. Anesth Analg. 93(4):928-33; 2001.
3. Guarnieri et al.,Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Animal, 41(11): 337-343; 2012.
4. Levinson BL, Leary SL, Bassett BJ, Cook CJ, GormanGS, Coward LU. Pharmacokinetic and Histopathologic Study of an Extended-Release, Injectable formulation of Buprenorphine in Sprague-Dawley Rats. J AM Assoc Lab Anim Sci. Jan 1, 61(1): 81-8; 2022.
5. Fidelis’ postmarketing surveillance database.
6. Fabian NJ et al. Evaluation and comparison of pharmacokinetic profiles and safety of two extended-release buprenorphine formulations in common marmosets (Callithrix jacchus). Scientific Reports, 13, 11864; 2023.
7. Klein H. et al. A pharmacokinetic study of extended-release buprenorphine in Cynomolgus monkeys (M. fasicularis). Journal of Medical Primatology. 52(6):369-373; 2023.
8. Williams W et al. Pharmacokinetics of sustained-release buprenorphine in adult baboons (Papio Anubis). 2021 National Meeting of the Am Assoc for Lab Anim Sci (virtual).
9. Katzenbach JE, Wittenburg LA, Allweiler SI, Gustafson DL, Johnson MS. Pharmacokinetics of single-dose buprenorphine, butorphanol, and hyromorphone in the domestic ferret (Mustela putorius furo). J Exotic Pet Med 27:95-102; 2018.
10. Chan G et al. Assessment of the Safety and Efficacy of Pre-emptive Use of Extended-release Buprenorphine for Mouse Laparotomy. J Am Assoc Lab Anim Sci 99(99): 1-7;2022.
11. Traul KA et al. Safety studies of post-surgical buprenorphine therapy for mice. Lab Anim. 49(2):100-110;2015.
12. Cowan A et al. Lack of adverse effects during a target animal safety trial of extended-release buprenorphine in Fisher 344 rats. Nature America, Inc. Jan Vol 45(1):28-34; 2016.
13. Plunkard J, Jimenez I, Craney M, Villano J. Pharmacokinetics and efficacy of extended-release buprenorphine for post-operative pain management in the domestic ferret (Mustela putorius furo). Submitted for publication; 2024.
MANUFACTURED FOR Fidelis Animal Health 685 US Highway One, Suite 265 North Brunswick, NJ 08902 833-384-4729 www.EthiqaXR.com Fidelis, Fidelis Animal Health, and Ethiqa XR are trademarks of Fidelis Animal Health, Inc., a Delaware Corporation. NDC 86084-100-30. U.S. Patent Nos. 10,555,899; 11,058,629 FID-ETH-PIMA016 April 2024

You always strive for the highest quality research that delivers the most consistent, predictable outcomes possible. So, when it comes to providing up to 3 days of pain relief for mice, rats, ferrets, and non-human primates, choose Ethiqa XR (buprenorphine extended-release injectable suspension) 1.3 mg/mL CIII.
Unlike compounded forms of buprenorphine, only Ethiqa XR meets the highest FDA standards for quality in efficacy, safety, purity, and manufacturing.
Ethiqa XR. Pain relief that won’t compromise…just like you.
FDA-Indexed
Up to 3 days pain relief Pharmaceutical Grade cGMP Not compounded

Order Now

Ethiqa XR is a subcutaneously-administered opioid analgesic indicated for the control of post-procedural pain in mice, rats, ferrets, and non-human primates.
HUMAN SAFETY WARNING
Abuse Potential
ETHIQA XR contains buprenorphine, an opioid that exposes humans to risks of misuse, abuse, and addiction, which can lead to overdose and death. Use of buprenorphine may lead to physical dependence. The risk of abuse by humans should be considered when storing, administering, and disposing of ETHIQA XR. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drugs or alcohol) or mental illness (e.g., depression).
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression may occur with accidental exposure to or with misuse or abuse of ETHIQA XR. Monitor for respiratory depression if human exposure to buprenorphine occurs. Misuse or abuse of buprenorphine by swallowing, snorting, or injecting poses a significant risk of overdose and death.
Accidental Exposure
Because of the potential for adverse reactions associated with accidental exposure, ETHIQA XR should only be administered by veterinarians, veterinary technicians, or laboratory staff who are trained in the handling of potent opioids. Accidental exposure to ETHIQA XR, especially in children, can result in a fatal overdose of buprenorphine.
Risks From Concurrent Misuse or Abuse with Benzodiazepines or Other CNS Depressants
Concurrent misuse or abuse of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. See HUMAN SAFETY WARNINGS for detailed information.
Important Safety Information For Rats, Mice, Ferrets, and Non-human Primates:
Only administer Ethiqa XR® by subcutaneous injection. Ethiqa XR is not intended for intravenous, intra-arterial, intrathecal, intramuscular, or intra-peritoneal injection. Do not use in animals with pre-existing respiratory compromise.
Death has been reported when non-steroidal anti-inflammatory drugs (NSAIDs such as meloxicam and carprofen) and Ethiqa XR have been administered concomitantly in mice.
Do not house rats on wood chip-type bedding after administration of Ethiqa XR. Pica involving wood chip type bedding can be lethal.
Ethiqa XR may cause sedation, decreased blood pressure, decreased heart rate, decreased gastrointestinal mobility, and respiratory depression. Use caution with concomitant administration of Ethiqa XR with drugs that cause respiratory depression. Animals should be monitored for signs of decreased cardiovascular and respiratory function when receiving Ethiqa XR.
The safety of Ethiqa XR has not been evaluated in pregnant, lactating, neonatal, or immune-compromised animals.
For Humans:
Not for use in humans. Keep out of reach of children and pets.
Ethiqa XR contains buprenorphine, a Schedule III controlled substance with an abuse potential similar to other Schedule III opioids, which may lead to overdose and death.
Ethiqa XR should be handled appropriately to minimize the risk of misuse, abuse, addiction, and criminal diversion, including restriction of access, the use of accounting procedures, and proper disposal methods as appropriate to the laboratory setting and as required by law.
Ethiqa XR should only be handled and administered by a veterinarian, veterinarian technician, or laboratory staff trained in the handling of potent opioids. Wear protective clothing when administering Ethiqa XR to avoid direct contact with human skin, eyes, oral, or other mucus membranes which could result in absorption of buprenorphine and adverse reactions.
For more information, consult the Prescribing Information including the Boxed Warning.



Effective Communication with the General Public About Scientific Research that Requires the Care and Use of Animals - Take-home Messages from a Workshop
12:30 PM - 2:00 PM/Room: 106A
Leader/Moderator: Nia Johnson
Facilitator: Mariah Waul
Panelist: Alice Huang, Paula Clifford, Eva Maciejewski, Nicole Navratil
This panel discussion will review the take-home messages developed from a 2-day workshop on “Effective Communication with the General Public about Scientific Research that Requires the Care and Use of Animals” presented by the National Academies Roundtable on Science and Welfare in Laboratory Animal Use on December 19-20, 2023. The workshop aimed to facilitate the synthesis of information, tools, and strategies for improving communication about scientific research involving animals. This panel discussion will focus on sharing the best practices and key takeaways for addressing public concerns about (1) proper care and use of animals in research, (2) the ethics of this work, (3) the implications of the scientific method in research with animals, and (4) the enhancement of the well-being of both animals and humans that continues to result from this work. The panel discussion will focus on the key takeaways useful to individual participants in their efforts to enhance public understanding of what research with animals involves, the ethical, veterinary, and scientific principles that guide how it is done, and what it accomplishes in terms of the well-being of both animals and humans. The target audience is the animal research community.

W-17 A Practical Guide to Prevent and Combat Compassion Fatigue in Biomedical Facilities
1:00 PM - 5:00 PM/Room: 108
Leader: Danielle Adney
Faculty: Makenzie Peterson, Alexandria Hernandez, Linda Waterman
Facilitator: Michael Campagna
Workshop Fee: $150 Workshop Limit: 50
As a deeper understanding of compassion fatigue, vicarious trauma, moral injury, and burnout grows in workplaces, biomedical research facilities have often been left out of these conversations. These facilities navigate unique stressors uncommon in the general American workplace, which can contribute to the retention, safety, and overall well-being of facility staff. Despite robust evidence and data about trauma-informed practices broadly, there needs to be more data describing best practices to prevent and mitigate its impact in laboratory animal research settings. Join us in gaining better understanding of factors contributing to compassion fatigue in research, how to recognize it, and concrete steps to mitigate it in your facility. Speakers will include a cross-disciplinary pairing of organizational well-being specialists and veterinary representatives from The Jackson Laboratory to demonstrate real-life organizational application. Participants will walk away with a concrete understanding of the value of implementing a tailored employee well-being program for biomedical research facilities. The learning objectives include recognizing the signs of individual and organizational workplace stress and
trauma, describing evidence-based practices to assess and improve overall well-being, and understanding the importance of strategic organizational change initiatives. This workshop would be appropriate for anyone in the LAS field.

W-18 Participation in a Discussion-Based Disaster Preparedness Exercise
1:00 PM - 5:00 PM/Room: 107A
Leaders: Evan T Shukan, Hana Bao
Faculty: Hana M Petersen, Shanon V Wilson
Facilitator: TBN
Workshop Fee: $150 Workshop Limit: 50
We will guide participants through a discussion-based disaster preparedness exercise, using an evolving scenario and timed smallgroup and larger-group discussions to help participants understand the strengths and weaknesses of their programs. The target audience is anyone with a leadership, response, or oversight role in their animal program, including supervisors, veterinarians, business managers, and IACUC members. We will also focus on helping participants learn how to assemble and conduct their discussion-based disaster preparedness exercises to ensure compliance with the USDA Contingency Planning Guidance. This will be a new scenario for those who attended this workshop at AALAS NM 2023, so repeat attendance is encouraged.

W-19 The Art of Communication: Transform Your Leadership and Level Up Your Vivarium
1:00 PM - 5:00 PM/Room: 107B
Leader/Faculty: Andrea Abrams
Facilitator: Gregory W Lawson
Workshop Fee: $150 Workshop Limit: 50
You and your team are experts on animal welfare, veterinary science, regulatory compliance, and customer service to your stakeholders. And yet, the world of laboratory animal science also requires another vital skill: the art of communication. You will leave this dynamic workshop with straightforward tactics you can immediately implement at your vivarium to improve your team’s morale, ensure regulatory compliance, ace your following interview, and protect your and your team’s boundaries from the email energy drain. Communications SME Andrea Abrams, MA, MBCP, CCMP (25 years in the communications industry, including 25 years as a Comms expert for the Federal Reserve Bank and clients including Stanford University and University of California, Berkeley) is a communications coach to executives and front-line staff. In this workshop, Andrea will provide actionable tactics for mastering interviews, conflict resolution, unlocking your team’s potential, and leveling up your team’s culture for improved performance and morale. Attendees will learn the four elements of communication guaranteed to work every time, the physical and choreographic aspects of communication that will level up your confidence and charisma, the art of nailing every interview, whether you’re already an executive or new to the leadership ranks, the formula to end the cycle of email essays, and how to integrate communications training at your vivarium. Andrea will also comment on the University of California, Berkeley Office of Laboratory Animal Care case study in January 2024, in which communications training for all vivarium team members yielded immediate positive results. The target audience is leaders (Executive Director, Director, Managing Veterinarian, Research Directors, Animal Technician Supervisors, Managers, Assistant Managers).






W-20 Would You Like to Improve Your Suturing and Rodent Surgery Aseptic Technique?
1:00 PM - 5:00 PM/Room: 110A
Leader: Marcel I Perret-Gentil
Faculty: Laurie A Long, Raphael A Malbrue, Kelvin Moore, Vittoria M Capria, Szczepan W Baran, Miguel A Torres, Haley Wrightnour, Lindsey T Ferguson
Facilitator: TBN
Workshop Fee: $150 Workshop Limit: 30
You may feel proficient, even confident, in performing rodent surgery; however, you may be surprised how minor improvements can significantly impact your animal’s recovery and data. During this workshop, participants will learn and refine commonly used suture, knot-tying, and rodent surgical draping techniques. The workshop will focus on appropriate hand-eye coordination to improve suturing skills and provide updates from recent scientific studies on the benefits of using Press’n Seal and Reynold Wrap aluminum foil in routine rodent surgical aseptic procedures. A state-of-the-art inanimate model will be introduced and utilized during the suture practice. Easy-to-apply hands-on exercises will be put into practice, and they have been shown to improve aseptic technique significantly with Press’n Seal and Reynolds Wrap. This workshop is designed for individuals with minimal or no suturing skills. It is also an excellent opportunity for those with considerable experience wanting to upgrade their skills and teach others enhanced techniques. It is also for those who wish to improve and teach rodent surgery aseptic techniques with simple-to-implement methods and trainers who want to pass these skills to their students.
This Workshop is sponsored in part by Kent Scientific, Atramax, SurgiReal.
2:45 PM - 5:00 PM
Platform Session abstracts will be available on www.aalas.org in July. They will also be included in both the mobile app and the National Meeting Final Program.

Assessing Success in Animal Enrichment, Behavior, and Social Housing: Strategies and Challenges
3:00 PM - 5:00 PM/Room: 101A
Leaders: Kristina C Bartley, Jennifer N Camacho
Moderator: Kristina C Bartley
Facilitator: Chineta Pullin
Advancements in Laboratory Animal Care have led to intricate designs for enrichment, sophisticated social housing practices, and diverse methods for assessing animal behavior to gauge the intended benefits. Despite the wealth of available information, coupled with ambiguous guidelines from regulatory bodies, obligations to animal welfare, and regulatory pressures, assessing the effectiveness of chosen measures remains a daunting task. This seminar will feature speakers addressing various facets of measuring success in enrichment, social housing, and behavior management across diverse species, including nonhuman primates, rabbits, rodents, and farm animals. They will explore quantitative and qualitative
approaches for evaluating animal behavior, aiding in identifying and mitigating abnormal behavior, assessing social housing conditions, and enhancing animal welfare. Furthermore, these methodologies will enable the evaluation of the efficacy of environmental enrichment strategies. Speakers will share successful tools that others can adopt to validate, refine, or establish criteria for measuring success in their own programs. This collaborative discussion promotes informed decision-making, and fosters improved animal care practices within laboratory settings. The target audience will be Laboratory Animal Behaviors, Enrichment Coordinators, Supervisors, Managers, Directors, and Veterinary Technicians.
Speakers/Topics:
3:00 Kristina C Bartley Welcome and Introductions
3:05 Brianna N Gaskill
3:25 Corrine K Lutz
3:45 Jennifer A Jones
4:05 Jennifer N Camacho
4:25 Kimberly Bagley, Rebecca L Harveson
Mouse Ethogram; How Do You Measure Behavior, Success of Enrichment, Social Housing and Behavior in Laboratory Rodents
Measuring the Effectiveness of Behavioral Management Practices for Nonhuman Primates
Rabbit Social Housing; What Are The Measures? And Additional Tips for a Successful Enrichment and Behavior Management Program
Practical Tools to Quantify Behavior, Social Housing and Enrichment in Multiple Species
Digital Tools That Can Improve Efficiency in Animal Behavior Observation and Assessments
4:45 All Questions & Answers

Navigating Diverse Aquatic Husbandry: Insights from Multi-Species Facilities
3:00 PM - 5:00 PM/Room: 207A
Leader/Moderator: Michelle L Altemara
Facilitator: Christine Archer
As research expands into new realms, zebrafish facilities are evolving to accommodate diverse aquatic species. This expansion presents unique challenges for facility staff, who must adapt to varying husbandry practices tailored to each species. Our presentations delve into the intricacies of husbandry practices for a spectrum of aquatic inhabitants, including bettafish, cuttlefish, sea lampreys, medaka, and killifish. We also address crucial considerations for maintaining biosecurity and preventing infectious diseases within multi-species facilities. Bettafish, with their centuries-long history of selective breeding, serves as valuable subjects for behavioral experiments to understand aggression and parental care dynamics. Their intricate social behaviors offer insights into broader animal behavior and social dynamics questions. Cuttlefish, renowned for their remarkable ability to camouflage and mimic their surroundings, intrigue researchers seeking to unravel the mysteries of their visual perception and skin patterning. Scientists gain valuable insights into the mechanisms behind this captivating phenomenon by studying how cuttlefish interpret their environment and alter their appearance accordingly. These presentations provide invaluable guidance for facility staff and veterinarians managing diverse aquatic populations effectively. By understanding each species’ specific needs and


behaviors, professionals can optimize husbandry practices, enhance animal welfare, and support the success of research endeavors in aquatic biology and behavior.
Speakers/Topics:
3:00 Michelle L Altemara Welcome and Introductions
3:05 Joshua R Barber
Beyond the Ordinary: Delving into the Realms of Laboratory Betta Fish and Cuttlefish
3:30 Hugh S Hammer And Now for Something Completely Different: Sea Lamprey Husbandry
3:55 Kara Maloney Three’s Company: Zebrafish, Medaka, and Killifish in One Facility
4:20 Marcus J Crim Infectious Disease and Biosecurity of Multi-species Aquatic Facilities
4:45 All Questions & Answers

3:00 PM - 5:00 PM/Room: 104A
Leader/Moderator: John J Hasenau
Facilitator: TBD
Nonhuman Primate (NHP) space allotment continues to be an essential area of focus for all concerned with welfare, care, and management. Yet, there is limited data evaluating these animals’ optimal space allocations and diverse housing arrangements. Overall, space is one concern, as is the complexity and functionality of the enclosures for promoting species-typical behavior and thus enhancing welfare. This presentation will outline different infrastructural arrangements for Macaque species (M. Mulatta, M. fascicularis, M. nemestrina) and introduce some evaluation tools that can contribute to better empirical determinations. The evaluations allow a greater understanding of the utilization of existing housing options and encourage the refinement of enclosure design for optimal animal welfare while balancing animal care and research staff needs. The evaluations have been designed to allow for multi-center use, focusing on consistency and repeatability to enable comparative data interpretation through interobserver reliability across facilities and species. Practical applications for use on existing housing systems will be emphasized. Data output, management, and strides in making this an open-source access will also be presented. Discussion on the development of the techniques to capture clinical components (biomarkers) needed for further evaluation of housing will be encouraged. Future infrastructure design concepts will also be discussed, highlighting work done at Neuralink regarding housing design, use, and research data collection. This seminar will encourage individuals to understand better and evaluate their current NHP housing and maintenance and use programs. It will give attendees a better understanding of assessing their programs and encouraging optimal use of existing housing systems while hopefully planning for future housing systems to allow optimal NHP care, welfare, and management. This session is appropriate for all individuals engaged in research involving NHPs, especially those involved in housing and study designs (DVMs, behaviorists, animal care technicians, animal study technicians, facility managers, and facility directors).
Speakers/Topics:
3:00 John J Hasenau Welcome and Introductions
3:05 John J Hasenau NHP Housing Consortium, History and Goals

3:15 Lydia M Hopper, Alexis Roach
3:40 Mollie A Bloomsmith
4:00 Dawn M Abney
4:15 Sam Baker
Development, Implementation, and Evaluation of a New Behavioral Tool to Assess Primate Space Use, Activity, and Welfare Across Different Enclosure Designs
The Costs and Benefits of Cross-institutional Studies: Comparing Enclosure Use by Rhesus Between Two Facilities
Implementation of the Primate Welfare Assessment Tool (PWAT) to Set Standards and Create Goals for Improved NHP Housing
Evaluating Productivity and Health, in a Work from Home Environment
4:40 All Questions & Answers

Refining Human-Animal Interactions and Housing of Nonhuman Primates in Research: A Practical Approach
3:00 PM - 5:00 PM/Room: 209A
Leader/Moderator: Elizabeth A Nunamaker
Facilitator: TBN
In this session, we present a comprehensive examination of the imperative to refine human-animal interactions and housing conditions for nonhuman primates (NHPs) in research, focusing on practical implementation. Starting with refining human-animal interactions, we delve into the multidimensional aspects of improving interactions between researchers and NHPs, exploring better welfare, better science, and better operations. This underscores the ethical, scientific, and operational motivations and benefits for continuous evolution in housing and husbandry practices change. Then, moving on to the case for refining housing, we dissect the critical impact of housing conditions on NHPs. This underscores the need for advancements in housing practices to enhance the physiological and psychological well-being of NHPs, aligning with ethical, scientific, and operational imperatives. We focus on practical recommendations and provide actionable strategies for refinements that foster positive interactions with NHPs and improve physiological and psychological well-being. Recommendations to be covered include positive reinforcement training programs, animal restraint techniques, and housing refinements, including social housing, cage sizes, novelty, and creativity. Additionally, we cover veterinary care, compassion fatigue, and empathy, offering a comprehensive guide to implementing positive changes in NHP research practices. The session concludes by exploring the diverse roles of stakeholders in NHP research and provides practical recommendations for key stakeholders. This includes critical tips for (1) IACUCs focusing on institutional oversight and (2) Technicians, offering practical insights for those directly involved in animal care. This collaborative approach aims to create a unified front among stakeholders, promoting the collective responsibility of refining human-animal interactions and housing conditions for NHPs. This interdisciplinary exploration with practical guidance intends to inspire positive change in NHP research, bridging the gap between theory and application for the betterment of scientific endeavors and non-human primates’ welfare.
Speakers/Topics:
3:00 Elizabeth A Nunamaker Welcome and Introductions
3:05 Christina L Cruzen


Refining Human-Animal Interactions


National Meeting 2024


This program is an extension of our extremely popular Demo Day, but in-person!

Monday, November 4:
Clear H2O 10:10am - 10:30am
Allentown, LLC. 10:35am - 10:55am
Innovive 2:10pm - 2:30pm
Cayuse 2:35pm - 2:55pm
Tuesday, November 5:
Micro Photonics 10:10am - 10:30am

The Andersons Lab Bedding Products 10:35am - 10:55am
Inotiv 2:10pm - 2:30pm
Transnetyx 2:35pm - 2:55pm
Wednesday, November 5:
The Jackson Laboratory 10:10am - 10:30am
Contec Inc. 10:35am - 10:55am


3:35 Noel O Dybdal Refining Nonhuman Primate Housing
4:05 Elizabeth A Nunamaker Practical Recommendations for Stakeholders in Nonhuman Primate Research
4:35 All Questions & Answers
This Seminar is sponsored in part by The 3Rs Collaborative.
8:00 AM - 10:45 AM
Platform Session abstracts will be available on www.aalas.org in July. They will also be included in both the mobile app and the National Meeting Final Program.

All Things Outreach: Starting, Maintaining, and Promoting Outreach for any Size Program
8:00 AM - 10:00 AM/Room: 207A
Leader: Julie E Roller
Moderator: Larry J Shelton
Facilitator: Sarah J Gilliam
This seminar is going to cover all things outreach! As outreach is a critical part of educating the public and also identifying future career professionals for LAS, we feel that it is critical to educate attendees about all of the current AALAS and AALAS Foundation initiatives surrounding public outreach. Each of the panelists has been actively working with the AALAS Foundation and/ or doing outreach in their own institution and out in the community. There will be four main content areas covered, and we hope to have some good group discussions to be able to help a target audience of those that are wanting to get started in outreach or are already performing outreach activities but need some support. For those in the audience just starting out, we hope to advise on how to identify opportunities and educate them on the resources that already exist so that they don’t have to develop materials from scratch. For those already doing outreach at their institutions, we hope to give some examples of successful programs and what those programs include, to help them identify target areas to improve in their own programs. Main topic areas that will be covered include: How to start/build an outreach program, offering outreach opportunities to staff, finding community resources to plug into for outreach activities, current examples of the types of outreach being performed, and working towards the AALAS Foundation POE award, AALAS resources that prepare you for outreach, and AALAS Foundation Resources. We hope that through our presentations and discussion with the audience, participants will walk away with some tips and resources that they were not aware of to help them build or further develop their outreach programs.
Speakers/Topics:
8:00 Larry J Shelton Welcome and Introductions
8:05 Julie E Roller Maintaining a Successful Outreach Program and Identifying Outreach Opportunities
8:30 Matthew M Taylor Building Community Among Employees
9:00 Lindsay S Holmes
Preparation is Key: Using AALAS Resources in Preparation to Perform Outreach
9:30 Debra L Hickman What Do I Say? AALAS Foundation Resources to Support Public Outreach

8:00 AM - 10:00 AM/Room: 101A
Leader/Moderator: Joshua M Frost
Facilitator: Jessica K Lang
Gnotobiotic facilities continually encounter numerous technical challenges due to the need for continued microbial exclusion and the use of specialized equipment. Simultaneously, the use of gnotobiotic animal models in research continues to expand rapidly, not only in numbers but also in the scope of the technical demands necessary to carry out advanced studies. This seminar is designed to present the audience with the most current trends and challenges in the field and provide proven solutions from the collective experience of the speakers, who have extensive experience establishing and leading some of the largest gnotobiotic programs in the country. In addition, the panelists are actively involved in consulting and training other facilities and will address the specific problems faced in these facilities and how they were ultimately addressed. This will include challenges in implementing gnotobiotic isolator caging systems, alternative sterilization methodologies, the use of rederivation to generate germ-free mouse lines, and a discussion of how standard facility processes and procedures were adapted to meet study-specific requirements. Attendees will learn from the technical approaches in these real-world scenarios, which could be cross-applied in other situations. The discussion session will encourage audience sharing and provide additional perspectives for attendees. Target Audience: This seminar targets managers, veterinarians, research scientists, and program administrators. While foundational concepts in gnotobiotics will not be directly covered, discussing real-world problems will be highly informative to seasoned veterans and those who might become involved in gnotobiotics. Committee note: Although this seminar was planned to allow it to stand alone, the topics are meant to complement the other seminar proposed by this group, “Establishing and Growing a Successful Gnotobiotics Program.” Ideally, the current submission, due to its more advanced content, would follow the other submission if both were to be accepted.
8:00 Joshua M Frost Welcome and Introductions
8:05 Allison R Rogala Best Practices in Quality Assurance
8:30 Stephanie W Fowler Rederivation and Procurement of Gnotobiotic Mice
8:55 Alton G Swennes Best Sterilization Methods and Validation
9:20 Rickesha S Bell Beyond Isolators: Successful Use of Gnotobiotic Isolator Caging System
9:45 All Questions & Answers
This Seminar is sponsored in part by Association for Gnotobiotics.






Focusing the Light on Lighting in Animal Facilities, A Major Extrinsic Factor
8:00 AM - 10:00 AM/Room: 104A
Leader/Moderator: John J Hasenau
Facilitator: TBD
Appropriate lighting in animal facilities spans many concerns: technology, safety, animal welfare, consistency, durability, intensity, duration, quality (wavelength), and effects on the actual research outcomes. Currently, lighting technology in research animal facilities is rapidly transitioning from conventional white fluorescent to solid-state LED lighting to address primarily financial and improved energy efficiency concerns. This seminar provides an ethological perspective on rodent and zebrafish lighting, the nature of light and circadian rhythms, current industry practices and standards, and our understanding of the neurophysiology of the visual and non-visual systems. The implications of this extrinsic factor will also be considered for vivarium measurement, production, and technological application of light. Known LED lighting research results will be mentioned. Additional consideration will be provided for the emerging Zebrafish model and the practicality of light deprivation and husbandry for mice with an influence on neurological development and gene expression. We will suggest simple recommendations for converting to LED artificial lighting for lighting manufacturers, designers, engineers, DVMs, researchers, facility managers, directors, and research care staff that ensure best practices for optimizing research animal health and wellbeing and, ultimately, improved scientific outcomes. This session is appropriate for all individuals engaged in research, primarily rodents and zebrafish, especially those involved in housing designs and study outcomes.
Speakers/Topics:
8:00 John J Hasenau Welcome and Introductions
8:05 John J Hasenau Animal Facility Trends for Lighting
8:15 Robert T Dauchy Introduction to Light and the Influence of LED Lighting Technology on Research Animals
8:45 Brianna N Gaskill An Ethological Perspective to Rodent Lighting Conditions
9:05 Lauren M Pandolfo What About Our Aquatic Species, Does it Matter?
9:30 Amanda M Coldwell Does Light Deprivation Affect Neuronal Development in Rodents?
9:50 All Questions & Answers

Maintaining the Highest Level of Genetic Integrity in Laboratory Animal Colonies in the 21st Century
8:00 AM - 10:00 AM/Room: 209A
Leader/Moderator: Bart MG Smits
Facilitator: TBN
Ensuring the genetic integrity of rodent breeding colonies is critical to maintaining the quality of preclinical research. The seminar aims to provide a complete overview of designing and managing a modern genetic integrity program. We will discuss key risk points for genetic integrity and strategies to mitigate the risk as applied by a large commercial rodent breeder. This seminar will review foundational concepts in rodent colony management, including background strain, substrain, strain containment, and genetic drift. Next, we will introduce breeding best practices and operational perspectives on genetic risk mitigation. An essential part of maintaining colonies at the highest level of genetic integrity is genetic monitoring and a colony refreshment program. We will present the latest technologies
for genetic monitoring. The seminar will conclude with an overview of the AWARE Program, designed to proactively optimize genetic integrity and animal welfare to achieve production goals. The key learning objectives are the importance of genetic quality to research outcomes; key risk points for genetic integrity breaches, as well as strategies to mitigate the risk; how to incorporate husbandry best practices to support genetic quality; how genetic monitoring and refresh practices vary by strain/stock type; how new technologies can be applied to genetic monitoring; management of genetic integrity and animal welfare to achieve production goals. The target audience is facility directors, transgenic core directors and staff, animal care technicians, quality control analysts, principal investigators, scientists, veterinarians, and veterinary nurses.
Speakers/Topics:
8:00 Bart M Smits Welcome and Introductions
8:05 Bart M Smits Introduction and Foundational Concepts in Genetic Quality
8:20 Sue-Ellen Bethmann Operational Perspectives on Genetic Quality
8:40 Bart M Smits The Importance of Genetic Monitoring and Colony Refreshes
9:00 Adam Navis Advances in Genetic Monitoring Technologies
9:30 Cara A Clouse AWARE Program: Proactive Management of Genetic Integrity and Animal Welfare to Achieve Production Goals
9:55 All Questions & Answers

An in Depth Look at the AVMA Working Group on the Psychological Impacts of Humane Endings
11:00 AM - 12:00 PM/Room: 101A
Speaker: Nathaniel Socrates Kollias
Moderator: TBN
Facilitator: TBN
The psychological impacts of humane endings on veterinarians, animal health and production professionals, and support staff are a growing concern within the veterinary community. The COVID-19 pandemic further highlighted the need for resources and compassion for our colleagues involved in the care and wellbeing of animals. Whether humane ending activities are related to the euthanasia of individual animals, slaughter for human consumption, or rare emergencies warranting depopulation, it is often the veterinarian’s responsibility to make and oversee these determinations. The AVMA has historically and continues to lead the charge on humane endings and the various issues and difficulties surrounding this delicate topic. This continued work led to creating a working group addressing the concerns of its members, veterinary medicine, and animal health professionals. This working group uses end-of-life decision information to lay the foundation and rationale to support and prepare veterinary professionals impacted by depopulation events. While there are inevitable mental health challenges shared by all people involved in end-of-life decisions, it is crucial to recognize that there are stressors unique to veterinarians working in settings outside private practice. This working group continues to take the necessary steps to acknowledge and address the associated psychological impacts on the veterinary, research, and production animal community during


emergencies. The Psychological Impacts of Humane Endings working group’s goal is to develop practical materials to support veterinarians in successfully handling the psychological impacts of animal death, with emphasis on depopulation events. This AVMA working group has developed tools that can be utilized for depopulation events as well as resources for managing the stress of individuals involved in these emergencies. The audience will learn how the AVMA formed the Psychological Impacts of Humane Endings working group and how their work is helping to guide the profession in developing compassion and resources for veterinary professionals during depopulation events. The targeted audience will be Veterinarians and animal care staff.
This Special Topic Lecture is sponsored in part by American Veterinary Medical Association (AVMA).

11:00 AM - 12:00 PM/Room: 104A
Speaker: John J Sancenito
Moderator: Sarah Hansen
Facilitator: TBN
Research institutions utilizing animals face a pervasive threat from animal rights extremists. While many animal rights activists engage in peaceful protests, a segment of extremists resort to more violent actions, creating a complex and nuanced challenge for research programs with live animals. The presentation offers an in-depth analysis of the infrastructure,
strategies, and tactics these radical groups leverage. We will explore the mechanisms behind their campaign organization, providing a detailed look into their operational blueprints. The presentation will feature comprehensive statistics to chart the progression and current landscape of protest actions, unlawful activities, and instances where these groups have successfully infiltrated research institutions. The core of the presentation is dedicated to outlining effective strategies that research institutions can employ to counter these threats. This approach will ensure a holistic defense mechanism against these extremist factions’ physical and reputational damages. The targeted audience is all Animal Lab Facility Employees, Facility Managers, Laboratory Managers, Laboratory Supervisors, and Security Specialists.

Which is the Best Animal Model to Study Osteoarthritis (OA)?
11:00 AM - 12:00 PM/Room: 207A
Speaker: Bertrand Lussier
Moderator: Karen L Lencioni
Facilitator: TBN
Osteoarthritis (OA) is a crippling disease. It affects more than 50% of the population over 50 years old. It has been studied for years using numerous animal models. The murine model, more specifically the mouse model, has been used extensively to understand the disease’s pathophysiology, especially in genetically modified models. The rat has also been studied because of its ease of handling, availability, and size. The rabbit is considered a large animal model; it was used several years ago but has been neglected for several reasons. The canine






Need something to isten to?


an AALAS podcast
AALAS is proud to present a rand-new podcast that eatures some o our communit 's rightest minds discussing their research.

Listen here:




model is ethically problematic; the IACUC tend to reject protocols using dogs if an alternative is possible. The ovine model has become interesting but tedious to use. Lately, the porcine model, which uses minipigs, has emerged. OA model selection should be based on the purpose of the study, concentrating on a specific question (primary outcome) and derived questions (secondary outcomes). Once these questions are answered, it becomes easier to select an appropriate model. This lecture will review the most frequently used models in mice, rats, pigs, sheep, and dogs. We will also present a novel model, the MI-RAT. Finally, the translational value of models will be discussed. The target audience is researchers, veterinarians, and technicians working on and developing models of osteoarthritis.

Building Compassion Fatigue Programs Within Large Organizations
12:30 PM - 2:00 PM/Room: 102A
Leader: Michelle Creamer-Hente
Moderator: Harutyun Avsaroglu
Facilitator: Brittany Steele
Panelist: Elizabeth Houston, DezShonna Kinloch, Michelle Creamer-Hente
Compassion fatigue, a prevalent issue in laboratory animal science, poses significant challenges to the well-being of laboratory science professionals. Recognizing the impact on staff, three sites within a large preclinical toxicology contract research organization collaborated to develop and implement Compassion Fatigue/Compassion Resiliency Programs. This presentation explores our journey to establish these programs, highlighting the collaborative efforts across sites and how they spread to a larger corporate initiative. The session will delve into the unique challenges faced in large organizations, such as gaining local and global buy-in, embracing site-specific needs and culture, and the strategies employed to overcome such obstacles. Our approach involved fostering a sense of shared responsibility within each site, pooling resources, and leveraging cross-site expertise to develop comprehensive compassion fatigue initiatives. The success of our efforts culminated in more robust compassion fatigue programs at individual sites and the adoption of a corporate-level initiative, extending compassion fatigue and compassion resiliency programs to all sites within the organization. Key topics covered include program development and evolution, the importance of education and resource availability, implementation strategies, and the role of cross-site collaboration. We will share insights gained from our experiences, addressing the importance of tailoring programs to the specific needs of laboratory animal professionals and the unique challenges at each site. Attendees will gain practical knowledge on initiating and sustaining compassion fatigue programs compatible with large or small institutions. We aim to inspire other laboratory animal science community members to consider implementing compassion fatigue programs tailored to their institutional needs. Join us in exploring the journey from site-specific initiatives to a corporate-wide commitment, emphasizing the significance of prioritizing laboratory animal professionals’ mental health and resilience. The target audience is Veterinarians, Operations Managers, Trainers, Quality Assurance, and Program Managers.

12:30 PM - 2:00 PM/Room: 103A
Leaders: Kirsten Bell, Megan Gerhardt, J. Preston Van Hooser
Moderator: Megan Gerhardt
Facilitator: TBN
Panelist: Madeline Van Hooser, Allison Charron, Lisa Garrett, Kirsten Bell
Knowing your audience is critical to developing an effective animal research openness program. Understanding who they are, what they may (or may not) know about animal research, and any preconceived ideas or biases can be helpful during the design process. This information can also influence the message(s) we want to deliver about our work and how we deliver them. But how well do we really know our audience? Engaging in conversations with the public can be challenging because we don’t know how we will be received. Without those conversations, we try to put ourselves in the public’s shoes, remember what we thought before working with research animals, and use that as our starting point. As we move forward from that point, however, we have the opportunity to learn and improve. By inviting participants to share their thoughts and experiences with us, we can begin to understand what’s important to them and refine our programs to meet the needs of our audience. We can only hope to secure public understanding and trust in our work by giving them the information they need to make informed decisions. In this session, an openness champion, high school and higher education students, and teachers/faculty will share their perspectives on openness and what’s most important to them in an experience. Session attendees will learn about openness program design, delivery methods, participant expectations, and how programs have been received. In addition, they will appreciate the diversity of perspectives and the fact that openness programs are not “one size fits all.” The target audience includes institutional and animal care and use leadership, outreach specialists, and others interested in pursuing openness in animal research.

Welcome to CUSP: An Online Repository of Animal Research Methodologies and Procedures
12:30 PM - 2:00 PM/Room: 105A
Leaders: Michelle Brot, Scott D Bury, Aubrey Schoenleben
Moderator: Scott D Bury
Facilitator: Erica Armstrong
Panelist: Thomas E Todd, Monika K Huss, Kristyn A Hoffman 2024 marks an exciting year as the Compliance Use Standard Procedure (CUSP) Sharing site is being launched after six years of development. CUSP is supported by the National Institutes of Health (NIH) and the Federal Demonstration Partnership (FDP) as a burden-reducing initiative of the 21st Century Cures Act. CUSP is a valuable resource to researchers, IACUCs, administrators, and veterinarians by providing free, easy-to-access information covering a wide range of field and laboratory research procedures, husbandry, and veterinary care for common and uncommon species, including cephalopods. This resource will not only be valuable to users in traditional research environments but also for those in or interested in entering smaller niche fields, such as spaceflight preclinical research. Users can incorporate CUSP descriptions into individual animal protocols or adopt them into institutional procedure libraries, thereby saving time and disseminating information within and between institutions. CUSP represents a unique and exciting opportunity to share information, knowledge, and best practices with hundreds of other researchers and veterinarians nationwide while positively impacting animal welfare and reducing administrative burden. The audience





will learn about the CUSP Sharing Site and how this resource can benefit them and the greater research community. We will also be sharing pilot and early user feedback during the session. They will dive into opportunities for researchers using atypical species such as bats, marsupials, and cephalopods and anyone working in atypical environments to share procedures that promote good animal welfare in areas of husbandry, veterinary care, enrichment, handling techniques, and more, gain a deeper understanding of the roles and responsibilities of both individuals and institutions that join the site, and participate in a hands-on, live guided demonstration of the CUSP Sharing Site, gaining direct interactive experience with the site. The target audience is Lab Animal and Veterinary Staff; IACUC Administrators, Veterinarians, Managers, and Staff; IACUC Members, Chairs, and Vice Chairs; and Researchers and Research Staff.

AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY
12:30 PM - 2:00 PM/Room: 106A
AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COM -
Leader/Moderator: Timothy J Scott
EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY
Facilitator: TBN
Panelist: Renee L Thompson, Lisa A Quinn
AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COM -
EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY
AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COM -
In February 2023, our institution was confronted with an unprecedented flooding event. This incident, marking the most severe flooding event in the program’s history, prompted an immediate, multifaceted response to protect animals, staff, and the future of crit-
EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS
EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE
COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY
ical research endeavors. This presentation will offer a comprehensive overview of the critical moments that defined this crisis, starting with examining the circumstances that led to the flooding event. It will then pivot to an in-depth analysis of the emergency response efforts mobilized by the team, highlighting the swift and strategic actions taken to manage the crisis in real time. The innovative processes and strategies implemented to mitigate the impact of the flood on the institution’s research activities will be key to our discussion. We will explore the operational, veterinary, and administrative measures that were swiftly enacted, showcasing how these interventions served to minimize disruptions, protect valuable research outcomes, and promote animal welfare. Furthermore, this talk will provide insights into the significant transformations made to the Emergency Preparedness Planning for the Center for Comparative Medicine in the aftermath of the flood. By reflecting on the lessons learned and the proactive steps taken to enhance resilience, we aim to shed light on the importance of preparedness, adaptability, and innovative thinking in navigating the complexities of emergency management within a research-intensive healthcare environment. This panel serves as an opportunity for us to share our experiences and help other laboratory animal professionals who may find themselves in a similar situation, as well as a chance to explore the steps we’ve taken to create a more robust emergency response plan with the hopes of imparting knowledge that others can incorporate into their programs. The target audience is anyone working in a vivarium, and it is most useful to those responsible for designing, maintaining, and updating their institutional disaster plan.
AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COM -
EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS
AALAS
COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMEXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE
EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY EXCHANGE AALAS COMMUNITY




REGISTER EARLY AND SAVE
Attendees who register for the 2024 National Meeting before August 1 will receive a $125 discount off the advance registration fee. Attendees who register between August 2 and October 7 save $125 off the on-site registration fee. Besides the cost savings, another advantage to registering early is the time you will save once you get to Nashville! You will receive your badge and registration materials by simply showing your photo identification at the registration counter.
Name badges are issued to all registered attendees to access education sessions and the exhibit hall; it also records your contact information at vendor booths. If you registered for a workshop, it will be printed on the back of your badge. Badges must be scanned to enter the workshop.
LOST BADGE FEE
There is a $50.00 fee to replace a lost, misplaced, or forgotten badge.
FIRST NATIONAL MEETING? START HERE!
Attending your first National Meeting can be an overwhelming experience. Join us for the National Meeting orientation session on Sunday, November 3, from 2 – 3 p.m. This session is for first-timers, new members, international members, and anyone else who wants to get the most from the National Meeting. You will receive tips on how to plan your week as well as information on AALAS programs and opportunities.
Before you reserve your hotel room, be sure to finalize your travel plans! Fees may apply if you cancel your reservation or check out early.
Air Canada: www.aircanada.com
Alaska Airlines: www.alaskaair.com
Allegiant Air: www.allegiantair.com
American: www.aa.com
Delta: www.delta.com
Frontier: www.flyfrontier.com
Southwest: www.southwest.com
Spirit: www.spirit.com
United: www.united.com
Select the session recordings at the time you register for the National Meeting, and you will have access to up to 100 hours of recorded educational sessions (as released for inclusion). Bonus: When you use the recorded sessions to teach a seminar, both the presenter and attendees receive CEUs for use in maintaining your technician certification registry status or CMAR certification.
VISA APPLICATIONS AND INVITATION LETTERS
International registrants who plan to attend the AALAS National Meeting are urged to research visa application requirements for their country and to apply early. AALAS cannot assist with visa applications or with the embassies but will gladly provide invitation letters to attend the meeting.
To request an invitation letter, send an email to sheldon.williams@aalas.org; include in the request the applicant’s full name and complete mailing address. Submit your request by August 14 to allow for timely visa application, facilitate advance registration for the meeting at a lower rate, and increase the chances of booking a hotel in the AALAS block. Invitation letters will be mailed via regular U.S. post; there is an additional cost for expedited delivery.
DON’T MISS THE NATIONAL MEETING ORIENTATION
International attendees are invited to join us for the National Meeting orientation on Sunday, November 3, from 2–3 p.m. See the Final Program for location when it becomes available.
BEGIN YOUR TECH WEEK PLANNING
The 25th annual International Laboratory Animal Technician Week will be held from January 26 – February 1, 2025 and will be called “Technicians Uplift Research”. All Tech Week items are available at the AALAS Bookstore. For those facilities wanting to do a little something extra for their technicians, additional gifts will be available for purchase in the AALAS Bookstore. Let your technicians know you appreciate them.
The Charles C Hunter Lecture will take place on Monday, November 4 from 11:00 a.m. to noon. The topic “Kangaroo Wrangling –Managing Clinical and Conservation Research at a Zoo” will be presented by: Dr. Louden Wright, DVM, DACZM: Associate Veterinarian at Nashville Zoo at Grassmere.
2024 TECHNICIAN FUN FAIR
WHO: All technicians!
WHEN: Starting Monday, November 3 through Wednesday, November 6, 2024.
WHERE: Fun Fair registration will be onsite at the Technician Fun Fair Booth. You will visit the on-site exhibitor booths to find the answers to the questions. The exam will be accessed through the link provided when you register or scan the QR code to open the exam.
WHY: To learn more about AALAS and your field, meet other technicians and make new friends, check out exhibitors’ new products and services, win cool prizes, become active in your organization, and earn CEUs! For more details, visit www.aalas.org/national-meeting or email Nancy Dorcy at nancy.dorcy@aalas.org
Here’s a great opportunity for the technicians attending the National Meeting! Hosted by CTAD and sponsored by Charles River and PMI LabDiet, the Tech Connect Reception is on Monday, November 4 from 12:30 – 2:00 p.m. Join us for an exclusive networking reception where you can connect with other technicians, enjoy interactive games like Bingo and Kahoot, and stand a chance to win incredible prizes! We are thrilled to announce that AALAS President Dr. Robert ‘Bob’ Quinn will be joining us to gain valuable insights into the indispensable role of technicians in the Laboratory Animal Research field. Don’t miss this opportunity that offers both enjoyment and profes-
sional development. Seats are limited, so pre-register soon. Please note: Reservations can only be made after you have registered for the National Meeting. Reserve your spot today by registering online or by contacting Nancy Dorcy at nancy.dorcy@aalas.org.

Dr. Louden Wright received his DVM from the University of Tennessee College of Veterinary Medicine in 2015. Following this, he completed internships at the University of Wisconsin and Kansas State University veterinary teaching hospitals and a residency in zoo and wildlife medicine through the University of California-Davis and the San Diego Zoo Wildlife Alliance. He has since achieved diplomat status in the American College of Zoological Medicine. Post-residency, he worked as the Veterinary Director for the Great Plains Zoo in Sioux Falls, South Dakota and as a clinical veterinarian for Sanford Research. He is currently the associate veterinarian for the Nashville Zoo, where in addition to his clinical duties, he coordinates veterinary involvement in ongoing wildlife research. His own research interests are in pharmacokinetic and pharmacodynamic assessments of analgesics in wildlife species and anesthetic protocol development in amphibians.
All winners will be announced and prizes will be awarded at the AALAS Foundation’s Appreciation Reception, held on November 6, 2024, at the AALAS National Meeting in Nashville, Tennessee.
Visit www.aalasfoundation.org for more information and to register for the contest. The deadline to enter the contest is September 3, 2024.
We’re very thankful to Charles River Laboratories for sponsoring this year’s “In Tune With Research” contest!”
The AALAS Foundation is excited to announce its hybrid Silent Auction at the National Meeting in Nashville, Tennessee. All auction items will be on display at the Foundation’s Booth at the National Meeting; however, all bidding will be conducted via mobile device. This means that even if you are unable to attend the AALAS National Meeting, you will still be able to participate and bid on the fabulous auction items that will be up for bid!





AALAS FOUNDATION “IN TUNE WITH RESEARCH” CONTEST
Gather your paintbrushes, put on your creative thinking cap, and register to enter the “In Tune With Research” contest! The “In Tune with Research” contest challenges contestants to creatively paint/ decorate a wooden 14” guitar! The contest includes four categories: Individual, Corporate, Branch, and Institution/Organization. A winner will be selected from each category. Additionally, a Best of Show winner will be chosen by AALAS leadership. A Fan Favorite will be awarded to the entry whose photo receives the most “likes” in the “In Tune With Research” contest photo gallery on the AALAS Foundation’s Facebook page.
If you are attending the National Meeting, you will pick up your winning auction items in person. If you are not attending the AALAS National Meeting, a shipping charge will be included at time of payment, and your items will be shipped to you at the conclusion of the National Meeting. Auction donors making pledges by August 15, 2024, will be recognized in the AALAS Final National Meeting Program.
We need your help to make this year’s auction a success, so please consider donating an auction item!
Not sure what to donate? Items that always prove popular are animal-related gifts, event tickets, jewelry, gift baskets, gift certificates, hotel getaways, travel packages, sporting goods & memorabilia, unique items from your area, and the latest electronics.
Go to our online auction donation page at https://tinyurl.com/AF2024-Auction to pledge your auction item donation and for more information..
“AALAS FOUNDATION APPRECIATION RECEPTION & LIVE AUCTION”
The AALAS Foundation will be conducting its Appreciation Reception & Live Auction on Wednesday, November 6, from 6:30-9:00 pm at the AALAS National Meeting in Nashville, Tennessee.
The Appreciation Reception & Live Auction is always a fun and lively event. This year’s Appreciation Reception theme is “The Grand Ole Opry,” so plan on dressing as your favorite Grand Ole Opry star and join us for a casual and fun evening!
Appreciation Reception Program Agenda
6:30 PM - Opening Remarks/Recognition of Sponsors & Platinum+ Donors /Recognition of Memorial Wall of Honor Recipients/Awards/ Contest Winners Announced (PLEASE REMAIN QUIET DURING THIS PORTION OF THE PROGRAM)
7:00 PM - Live Auction Check In/Cash Bars Open/Appetizers Served
7:30 PM - Live Auction Begins


We are thankful for the following sponsors and supporters of this year’s Appreciation Reception & Live Auction:
$10,000 LEVEL SPONSORS

$5,000 LEVEL SUPPORTERS
The AALAS Foundation is looking forward to conducting its Animal Research and Education Awareness (AREA) Program this year in Nashville, Tennessee. This important educational program is being sponsored by Pfizer and will take place on Monday, November 4, from 9:30 a.m. to 1:30 p.m. The Foundation needs volunteer tour guides to lead small groups on a tour of the National Meeting Exhibit Hall from 8:30 a.m. until 1:30 p.m. Tour guides also participate in small group discussions with the students about career opportunities and the care and use of animals in research. Additionally, tour guides will assist students in playing Pfizer’s educational game, “The Long Road to Drug Discovery.”
To register as a Tour Guide, please visit: https://tinyurl.com/2024-AF-AREA-PROGRAM
$1,000 LEVEL SUPPORTERS


The money raised through the AALAS Foundation’s annual contests and auctions helps support the AALAS Foundation and its mission to inform the public about the vital role of animals in research, the vast career opportunities available in biomedical research, and to educate the public about all the compassionate professionals working in the field of laboratory animal science.
Learn about recent actions taken by the AALAS Board of Trustees and provide feedback to AALAS national leadership by attending your district membership meeting, conducted by your district trustees. They will lead discussions on recent board decisions, issues, policies, and procedures. Check the list to find your district and who your trustees are; for example, individuals living in Maryland belong to District 3. District membership meetings will be held Monday, November 4, from 5:15–6:15 p.m.; room assignments will be listed in the Final Program. International members of AALAS will have a designated meeting room as well and will meet at the same time as the districts.
Partnering with one of the leading conference platform providers, Cvent, our mobile app provides users with the following features, plus many more.
• General meeting information
• Schedule by day, tracks, session type, and speaker
• Posters and platforms
• Exhibitor listings by name and category
• Maps
• Social media access
• QR scanner
• Speaker listings
The AALAS Foundation is excited to announce it is celebrating the zebrafish and its role in infertility research this year! A “Celebrate the Zebrafish” lapel pin has been designed, and anyone making a $5 or more in-person donation at the AALAS Foundation booth during the National Meeting in Nashville will receive a lapel pin. Anyone making a $10 or more online donation to the AALAS Foundation between November 8, 2024, and December 31, 2024, will also receive a lapel pin while supplies last.

We’re very thankful to the Zebrafish Husbandry Association for sponsoring our 2023 “Celebrate the Zebrafish” program.

Learn about recent actions taken by the AALAS Board of Trustees and provide feedback to AALAS national leadership by attending your district membership meeting, conducted by your district trustees. They will lead discussion on recent board decisions, issues, policies, and procedures.
Check the list to see which district you’re in and who your trustees are; for example, individuals living in Maryland belong to District 3. District membership meetings will be held Monday, November 4, from 5:15–6:15 p.m.; room assignments will be listed in the Final Program. International members of AALAS will have a designated meeting room as well and will meet at the same time as the districts.
District 1
District 2
District 3
District 4
District 5
District 6
District 7
District 8
Branches
Metro New York, New England, Northern Mountain, Southern New England, Upstate New York
Branches Delaware Valley, New Jersey, Three Rivers
Branches Greater Virginia, National Capital Area
Branches
Appalachian, Florida, Mid-South, Research Triangle, Southeastern
Branches Ohio, Indiana, Kentucky, Michigan
Branches Central Illinois, Chicago, Iowa, Minnesota, Nebraska
Branches Kansas City, Louisiana, Mid-Missouri, Mile High, Oklahoma, Texas
Branches Arizona, Mountain West, Northern California, Northernn Rocky Mountain, Oregon, Sacramento Valley, San Diego, Southern California, Washington
At Large Trustees
States Connecticut, Maine, Massachusetts, New Hampshire, New York, Rhode Island, Vermont
States Delaware, New Jersey, Pennsylvania, West Virginia
States Maryland, Virginia, Washington, DC
States Alabama, Florida, Georgia, Mississippi, North Carolina, South Carolina, Tennessee, US Commonwealth of Puerto Rico
States Indiana, Kentucky, Michigan, Ohio
States Illinois, Iowa, Minnesota, Nebraska, North Dakota South Dakota, Wisconsin, Wyoming
States Arkansas, Colorado, Kansas, Louisiana, Missouri, New Mexico, Oklahoma, Texas
States
Alaska, Arizona, California, Hawaii, Idaho, Montana, Nevada, Oregon, Utah, Washington
Deb Hickman, Kenneth Shapiro, Gordon Yee, Jason Villano
Trustee
Jennifer Asher
Trustee
Erin Vogelsong
Trustee
Donna Tignor
Trustee
Janet Steele
Trustee
Stacy Cantrell
Trustee
Stephen Levin
Trustee
Adrienne Duran
Trustee
Katherine Marshall

Essential online training for investigators, technicians, veterinarians, managers, and IACUC members in the laboratory animal science field.
260+ courses on animal research and compliance – courses customizable
Translatable instantly to 100+ languages – website and courses
Specialty certificate programs on animal biosafety, transgenics, and research support
Training documentation and management

WE’ M T YA THERE!
Join us for the 75th AALAS National Meeting in Nashville, Tennessee. Each fall since 1950, the American Association for Laboratory Animal Science has held its annual National Meeting. During the five days of the meeting, members and nonmembers come together to enjoy the workshops, lectures, poster sessions, and exhibits. The program is designed to have topics relevant to the entire membership. Exhibitors have an opportunity to interact with AALAS members from the academic community, research institutions, government organizations, and commerical companies.
The AALAS National Meeting is the largest gathering in the world of professionals concerned with the production, care, and use of laboratory animals.
Join us in achieving our 25th Anniversary goal by contributing a $25 donation on the 25th of each month. Don't worry - if you miss the 25th, your donation on any other date in the month still counts! Consider the convenience of signing up for automatic monthly donations to effortlessly support our cause. Together, let's make a meaningful impact!
With your help, we look forward to another successful twenty-five years of advocacy and providing free resources to assist AALAS members with their public outreach activities.