
17 minute read
Tech Tips
Considerations for Preparation and Monitoring of a Sheep Surgical Model
By Ashley Klinger, BS, RLATG, and Carmen Arsuaga-Zorrilla, DVM, MS, DACLAM
Sheep are commonly used in research for bioimplants, wound healing, orthopedic, and cardiology research. The Abigail Wexner Research Institute at Nationwide Children's Hospital (NCH) Animal Resources Core (ARC) sheep are used primarily for cardiac models and hosts for vascular grafts. The Institutional Animal Care and Use Committee (IACUC) reviews all procedures and is in full accordance with the Animal Welfare Act and the Health Research Extension Act to ensure the humane treatment of animal subjects in research.
Materials
• Ketamine hydrochloride injection;
Hospira, Inc. Lake Forest, Il • Diazepam; Hospira, Inc. Lake Forest, Il • Butorphanol tartrate injection, Torbugesic;
Zoetis Parsippany, NJ • Laryngoscope; Miller Blade #4, 180 mm;
Welch Allyn, Milwaukee, WI • BAAM, Beck Airway Airflow Monitor;
Great Plains Ballistics Inc. • Gentle-Lock™ Oral Endotracheal Tube Holder, 81802; Teleflex In. Morrisville, NC • iStat 1 Point of care blood gas analyzer; Abbott
Laboratories: Abbott Park, Illinois, U.S.
Methods
When working with large species, a system of stretchers and transport beds are utilized to get sedated or recovering animals from housing to procedures or operating rooms. This aligns with ARC goals for staff safety and ergonomics.
Due to the unique hemodynamics of the sheep cardiac models, and their numerous post-surgical sedations for imaging, NCH uses primarily an induction cocktail of Ketamine (4mg/kg), Diazepam (0.5mg/kg), and Butorphanol (0.1mg/kg) as the intravenous cocktail of choice for rapid sedation and intubation.1
Endotracheal intubation is performed in the housing room aisle or anteroom space immediately post injection. Animals are manually restrained until relaxation, then rolled onto a fabric stretcher and moved to a raised bed for intubation and transport. Supplies used for endotracheal intubation include a laryngoscope with #4 blade, cetacaine spray, an appropriately sized intubation tube, Beck Airway Airflow Monitor (BAAM), and intubation tube holder/bite guard device [Fig 1.0]. These items help to quickly determine correct placement of the endotracheal tube and secure the endotracheal tube for protection from mild chewing until transported to a procedure room with additional inhalant anesthetics available [Fig2.0]. These items all fit into a small caddy which is very easily transported for intubation in a variety of
Figure 1: Blanket, clipboard with monitoring form and normal ranges.
5 2 3
Figure 2: Ventilator circuit with HME, Bite guard with original MRI safe Velcro holder; SPO2.

spaces and situations. Most patients require suction after intubating and before connecting an anesthesia circuit to ensure the tubing is clear of secretions. Surgical preparation rooms are outfitted with a wall mounted vacuum system and a suction cannister with French suction tubing is available for ruminants due to the abundant secretions produced.
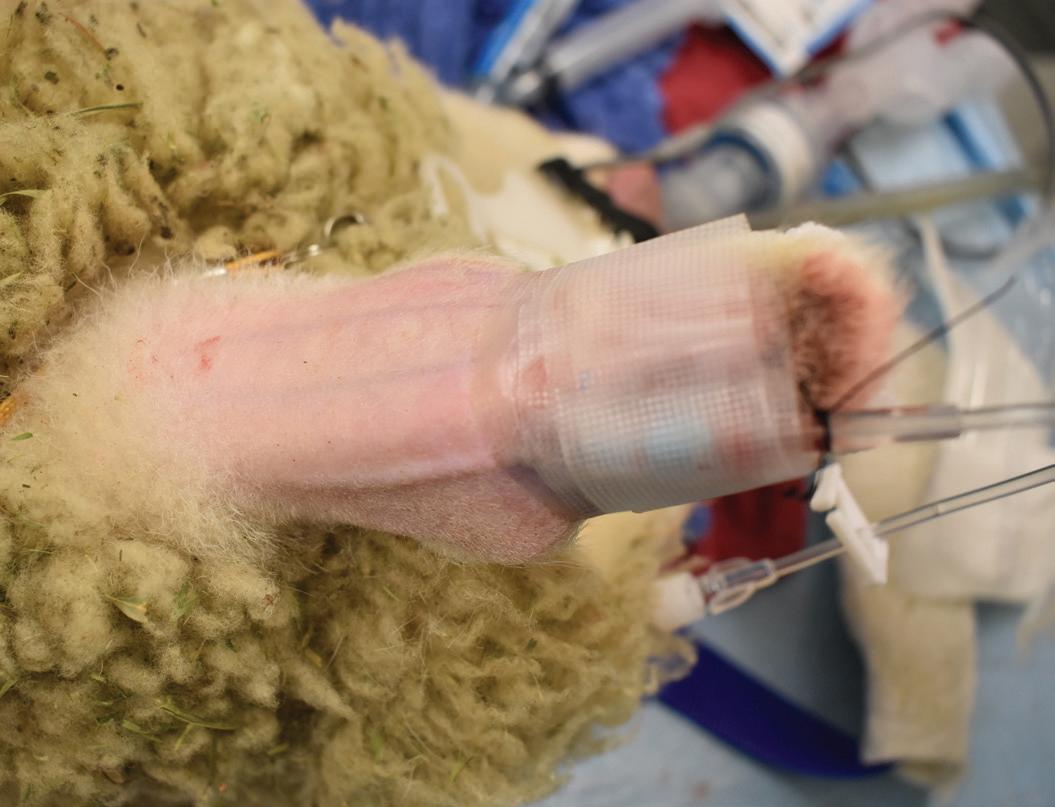
Small ruminants undergoing anesthesia will be fasted for a minimum of 12 hours prior to procedure start time. Once intubated, the animals will have an esophageal gastric tube placed to help with gas expulsion and gastric drainage during all sedated procedures. Typical set-up of gastric tubes involves utilizing a large, clear PVC gavage tubing with rounded tip to prevent trauma during placement and a urine drainage bag with the tip cut off to allow attachment to the gastric tube’s open end [Fig3.0]. The urine drainage bag has multiple benefits including cleanliness, ability to save ruminal fluid, release valve for fluid or gas, and monitoring of total output. Gastric tube placement allows the technicians to keep animals from bloating and provides access for manual expression of gas build up that could impact surgeons. For complicated procedures or anticipated post-operative complications, the rumen fluid will be preserved and administered back to the animal at the conclusion of the procedure. Ruminal fluids can also be used for transfaunation of sheep in intensive care when sedated animals do not require their fluid back. In such cases, the fluid bag is clamped before removal from donor animal to reduce oxygen exposure2 and subsequently saved for up to three days in refrigeration.3 The urine drainage bag makes it possible to maintain fluids with minimal oxidation and dead space for preservation, making it easy to take the bag off and drain it back into the gastric tube using gravity. The tube is occluded and removed from the patient prior to extubation of endotracheal tube.
Complex surgeries require additional monitoring such as catheterization of auricular artery for invasive blood pressure readings and non-invasive blood pressure cuff placed over the radial artery. Electrocardiogram adhesives dots are placed on the back of each leg between the heel and dew claws (area shaved for contact) and then secured with tape for continuous ECG monitoring during surgery. Other parameters being monitored include SPO2, EtCO2, ventilation parameters, temperature, heart rate, tympany, and in some cases blood gases. Blood gases are used to confirm oxygen saturations and acid-base disturbances. Protocols that require clamping of a major vessel will also require blood gas and injection of countermeasure when unclamping due to the sudden release of built-up blood waste from the clamped vessel.
Emergency drug lists are made available in surgery areas and on emergency drug carts. The list includes drugs for cardiac interventions, reversals for induction drugs, emergency dose ranges, and drug concentrations for the dosages listed. Some drugs also contain a quick reference such as “1mL/10lbs”- and all have columns designating what each drug can be used to treat. Surgical monitoring records include a set list of parameters filled in prior to induction for all animals including tidal volume, oxygen flow rate, rebreathing bag size, ranges for anesthetics used, and fluid rates (all calculated using most recent weight).
Following a procedure, animals are monitored in the operating room until spontaneous respirations occur before attempting transport to recovery. Intubation tubes are left in until animals begin to chew actively, at which time the partially inflated intubation tube is removed-
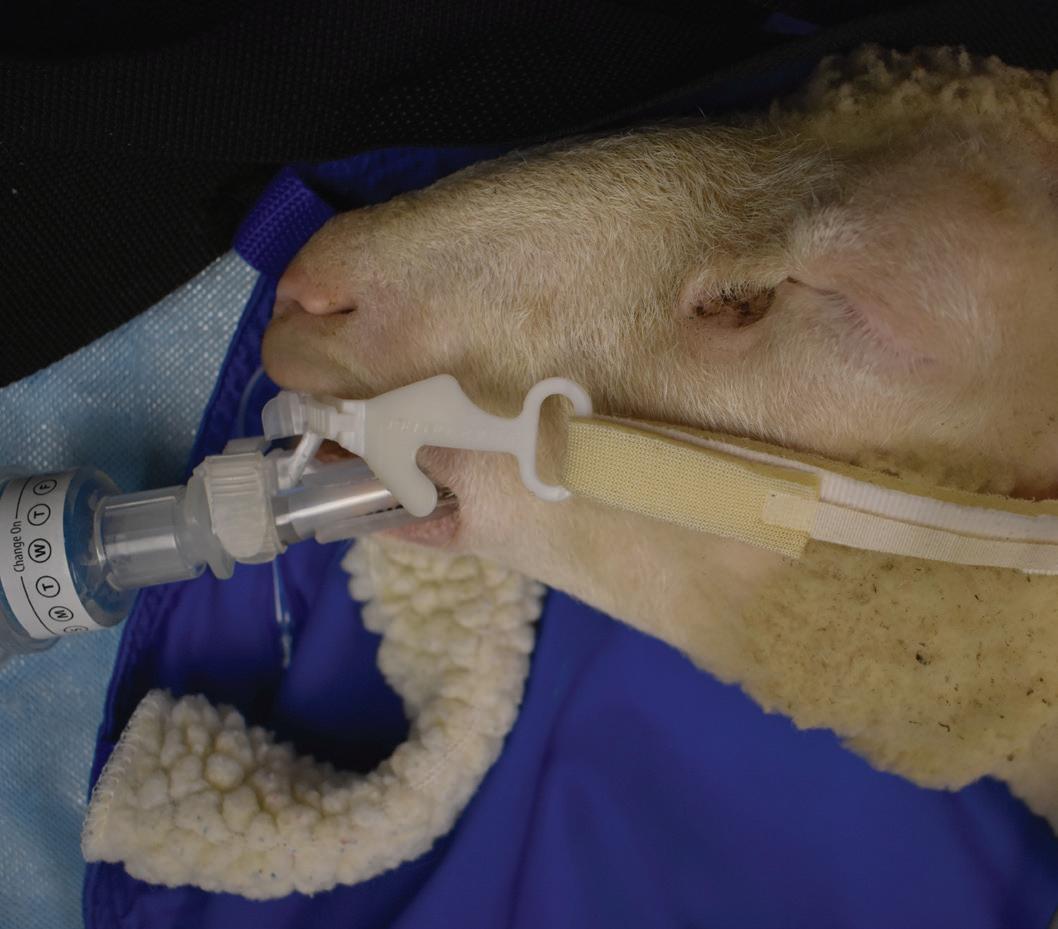
Figure 3: Gastric tube attached to cut urine drainage bag for rumen tubing.
the choice keep the cuff partially inflated is due to the secretions common in this species and to help clear the airway on the way out. In recovery runs, a portable ventilator and anesthesia machine with monitoring is available for recovering after invasive procedures. Recovery runs are fitted with rubber floor mats for better footing, and a V-shaped foam support to keep animals sternal and reduce the strain on technicians supporting the animals. The foam support also allows technicians to keep the head angled slightly downward to drain any secretions in recovery should the need arise. Animals are recovered until ambulatory- often with the encouragement of grain upon standing.
Conclusion
ARC’s main objectives are high quality research, safety, and welfare. These objectives are reached through a collaborative effort with research staff to understand the demands of their procedures; planning in the form of emergency drugs, route of sedated animal to surgery, etc; and ergonomic supports such as stretchers, inflation lift beds, hospital beds, and other forms of wheeled and hydraulic tables to allow animals to be lifted minimally during sedation, prep, and transfer to operation and recovery. In this way, ARC has been able to reduce the risk to staff and animals alike while performing small ruminant procedures regularly and meeting the needs of cardiovascular research.

Ashley Klinger, B.S., RLATG, Veterinary Technical Specialist in the Animal Resources Core at Nationwide Children’s Hospital- Abigail Wexner Research Institute, Columbus, Ohio. Carmen Arsuaga- Zorrilla, DVM, M.S., DACLAM, Senior Clinical Veterinarian in the Animal Resources Core at Nationwide Children’s Hospital- Abigail Wexner Research Institute, Columbus, Ohio.
REFERENCES
1. Allen Richard C, Becker Charleen, Morgan Paula.
2000. Formulary Colorado State University Teaching Hospital. Fort Collins (CO): Colorado State University 2. DePeters EJ, George LW. 2014. Rumen transfaunation, Immunology Letters, 162:2. Cited 30 August 2022. 3. Fabro Carla, Sarnata Chiara, Spanghero Mauro. 2020. Impacts of rumen fluid, refrigerated or reconstituted from a refrigerated pellet, on gas production measured at 24h of fermentation. Animal Feed Science and Technology, Volume 268. Cited 30 August 2022.
4
Figure 4: Auricular artery invasive blood pressure monitoring line (22g catheter and t-port)
5
Figure 5: Sheep recovering in foam V-board after graft surgery (stretchers and warming blanket visible, bandages to cover catheter site and chest drainage tube post-op.
ONSET COMP AD HERE
Microphysiological Systems and Animal Replacement
By Monica Badea, Sally Thompson-Iritani, Tessa DesRochers, and Megan LaFollette
The North American 3Rs Collaborative’s (NA3RsC) is a non-profit whose mission is to improve the lives of research animals, maximize good data in experiments with animals, and replace animals with alternative technologies when possible. One of our replacement focused initiatives is on microphysiological systems (MPS). Our MPS Initiative seeks to advance MPS technologies' wide adoption and regulatory acceptance. It is unique in the field as it combines most developers with commercially available MPS. It is also clearly situated in an organization committed to advancing all 3Rs (refinement, reduction, replacement) and recognizes animal research's current value and necessity. Our initiative has four main aims:
• Interfacing with end-users by coordinating webinars, workshops, and discussions. • Promoting regulatory acceptance through discussion, consensus building, and communications. • Creating, maintaining, and improving a user-friendly technology expo where end-users can see all commercial developers in one place and sort via filters (na3rsc.org/mps-tech-hub). • Develop regular, widespread educational resources for all stakeholders.
MPS is a rapidly evolving technology that facilitates crucial scientific research by mimicking organ function, cellular metabolism, and/or cellular dynamics. These 3D tissue cultures can be created from animal or human cells. They often incorporate extracellular matrix (ECM) and stromal components. In some cases, MPS can live for up to three months in culture. Their composition allows cell-cell and cell-ECM communication and interactions, simulating the in vivo behavior of many different organ types.6,8,9 They can also model both healthy and disease states. Approximately 18 organs/organ systems are commercially available, including liver, CNS and neuro, kidney, gut, tumor, lung, etc.
The complex nature of MPS can lead to some advantages over traditional in vitro or in vivo models, including improved physiological relevance and complexity, control, and reproducibility.3 Compared to traditional 2D cell cultures, MPS can more closely mimic organ morphology and function over an extended period (up to several months for some models); this allows researchers to examine changes in tissue structure and function in response to compounds.4,5 They can even simulate the drug administration routes patients use in the clinic – for example, oral versus intravenous versus topical – which facilitates better studies of drug stability and activation.2 Additionally, some MPS can incorporate aspects of the human immune system better than certain animal models, which are particularly important for investigating immune-oncology agents and cellular therapies,1 inflammation, and immune-related diseases (Type 1 diabetes, etc.). Additionally, since MPS use cells isolated directly from human tissue, their gene expression is generally more similar to human disease states than some animal models that incorporate human cells; this can result in experimental results that may more closely mimic human patient results.10 MPS can also be linked in multi-organ systems to replicate organ-organ interactions and more systemic in vitro models. Due to these factors, in some cases, MPS may be better predictors of human effects that may not be apparent in some animal models. MPS can be extremely beneficial in the early stages of drug discovery and basic research before starting expensive and time-consuming animal studies and clinical trials. Using MPS early can allow a company to determine which drugs may behave differently in animal models versus human patients. In turn, this can affect the number of animal studies needed before filing regulatory materials and proceeding to clinical trials. In particular, if early MPS trials show that a drug is likely to fail in later clinical trials for human safety or efficacy reasons, then the decision can be made to not proceed to animal trials therefore reducing the number of animals needed to develop an effective therapeutic. Scalable MPS can also support large scale batch testing and can provide answers more quickly.7,11
As with all model systems, MPS also has some disadvantages. First, they typically only recapitulate some features of an organ or a limited number of organs. Therefore, they may not always be predictive of whole-body drug reactions. Second, MPS are housed in incubators and have no variable external conditions. Thus, these systems are not shaped by external stimuli in the same way that whole animal models or humans are. That is, animals and humans are affected by external conditions such as their housing, interactions with other individuals, nutrition, and many other factors. Although these factors in animal models can cause variability, they are more representative of a human patient population. Third, while they are often less expensive than animal studies, they are more costly than traditional cell cultures and require specialized equipment and/or expertise. Finally, there are certain conditions that MPS cannot mimic, such as whole body behavioral responses, large bone fractures, or mental health conditions. For example, although an MPS could be used in safety testing for an antidepressant, it cannot be used to determine whether a drug can help resolve depression.
Due to the advantages of MPS systems and the extensive work performed by developers to characterize them, they are becoming more widely accepted by regulatory agencies for developing treatments for patients. At the time of this writing, data from MPS have been used to support 4 applications to the FDA for Investigational New Drugs (IND), applications necessary before initiating clinical trials. Furthermore, MPS data was used exclusively by Vertex to expand the label of a previously FDA-approved drug for cystic fibrosis.
In conclusion, microphysiological systems can mimic either human or animal systems in terms of physiology, toxicity, disease states, and efficacy. They can be practical to use, saving companies
time and money in the long run. Ultimately, MPS can be used to refine, reduce, and replace some aspects of animal research while still ensuring patient health and safety.
Monica Badea is a student at Kutztown University in Kutztown, PA. Sally Thompson-Iritani (she/her), DVM/PhD, CPIA, CCFP, is the Asst. Vice Provost, Animal Care, Outreach & 3Rs, Clinical Associate Professor, DEOHS, SPH, at the University of Washington. Tessa DesRochers is the Chief Scientific Officer at KIYATEC, Inc in Greenville, SC. Megan LaFollette, MS, PhD, is the Program Director of The North American 3Rs Collaborative in Denver, CO.
REFERENCES
1. Appleton KM, Elrod AK, Lassahn KA, Shuford S, Holmes
LM, and DesRochers TM. (2021) PD-1/PD-L1 checkpoint inhibitors in combination with olaparib display antitumor activity in ovarian cancer patient-derived three-dimensional spheroid cultures. Cancer Immunol Immunother 70:843-856.
2. Azizgolshani H, Coppeta JR, Vedula EM, Marr EE, Cain BP, Luu RJ, Lech MP, Kann SH, Mulhern TJ, Tandon V, Tan K, Haroutunian NJ, Keegan P, Rogers M, Gard AL, Baldwin KB, de Souza JC, Hoefler BC, Bale SS, Kratchman LB, Zorn A, Patterson A, Kim ES, Petrie TA, Wiellette EL, Williams C, Isenberg
BC, and Charest JL. (2021) High-throughput organ-on-chip platform with integrated programmable fluid flow and real-time sensing for complex tissue models in drug development workflows. Lab Chip 21:1454-1474. 3. Hoarau-Véchot J, Rafii A, Touboul C, Pasquier J. (2018). Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions?. International journal of molecular sciences, 19(1), 181. 4. Horowitz LF, Rodriguez AD, Ray T, Folch A. (2020). Microfluidics for interrogating live intact tissues. Microsyst Nanoeng 6, 69.
5. Kwak T, Wang F, Deng H, Condamine T, Kumar V, Perego M, Kossenkov A, Montaner LJ, Xu X, Xu W, Sheng C, Schuchter LM, Amaravadi RK, Mitchell TC, Karakousis GC, Mulligan C, Nam B, Masters G, Nockstein N, Bennet J, Jefedova Y, Gabri-
lovich DI. (2020). Distinct populations of immune-suppressive macrophages differentiate from monocytic myeloid-derived suppressor cells in cancer. Cell reports, 33(13), 108571. 6. Li AP, Bode C, and Sakai Y (2004) A novel in vitro system, the integrated discrete multiple organ cell culture (IdMOC) system, for the evaluation of human drug toxicity: comparative cytotoxicity of tamoxifen towards normal human cells from five major organs and MCF-7 adenocarcinoma breast cancer cells. Chem Biol Interact 150:129-136.
7. Marx U, Akabane T, Andersson TB, Baker E, Beilmann M, Beken S, Brendler-Schwaab S, Cirit M, David R, Dehne E-M,
et al. 2020. Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. ALTEX 37:365–394. 8. Miller PG and Shuler ML (2016) Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol Bioeng 113:2213-2227.
9. Prantil-Baun R, Novak R, Das D, Somayaji MR, Przekwas A,
and Ingber DE (2018) Physiologically Based Pharmacokinetic and Pharmacodynamic Analysis Enabled by Microfluidically Linked Organs-on-Chips. Annu Rev Pharmacol Toxicol 58:3764. 10.Ridky TW, Chow JM, Wong DJ, Khavari PA. (2010). Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nature medicine, 16(12), 1450–1455. 11.Roth A & MPS-WS Berlin 2019 (2021). Human microphysiological systems for drug development. Science (New York, N.Y.), 373(6561), 1304–1306. https://doi.org/10.1126/science.abc3734
Utilizing A Spay Hook Surgical Tool to Manipulate Sealed Positive Pressure Cages During a Size Match
By Nicole Martinec, BS, LATG, and Andrea Kachellek, BS RLATG
Have you ever found yourself fumbling with chemical sterilant-covered hands in a biosafety cabinet (BSC) while attempting to maneuver a wire bar lid to perform a multi-cage procedure? Wish you had another hand or two?
In our gnotobiotic facility, we routinely perform size match procedures, which require multiple cages to be open in the biosafety cabinet simultaneously. In this situation, it is a challenge to get an adequate grip on wire bars with one hand while keeping cages in place. These procedures are completed in a sterile setting, requiring surgical gloves constantly covered in chemical sterilant, which can add to the challenges of manipulating animals and caging.
Size matching is the reorganization of mice based on average tumor size and standard deviation. It is vital for oncology studies by reducing the experimental variability that comes with tumor growth. Generally, size matching requires multiple cages opened in quick succession. With Allentown Sealed Positive Pressure (SPP) caging, it is challenging to lift the wire bar due to the unique sealing of the cage to maintain the microenvironment.
We utilize spay hooks to facilitate a more efficient way of opening the wire bar while working in a biosafety cabinet. A spay hook (Figure 1) is stainless steel and may already be used in laboratory animal facilities, making it an easily obtainable option. We use Spay Snook Hooks for their softer, thicker curves, but other options could be considered.
Within the sterile biosafety cabinet, cages are set perpendicular to the work surface, and each cage is opened (Figure 2). Lids can either be slid back approximately two inches or set aside if proper airflow is maintained in the BSC. Water bottles are left empty in the clean cages until the size match event is complete. This improves ergonomics and efficiency by minimizing the total weight of the wire bar and preventing water from dripping into the cages. An eight-inch spay hook is inserted through each wire bar and rests on the bottom of the cage. One hand lifts the spay hook, hooking the wire bar and lifting

Figure 1: Eight inch Spay Snook Hook used in Sealed Positive Pressure during a size match event.
Figure 2: Sealed Positive Pressure cages staged on a biosafety cabinet with Spay Snook Hooks through the wirebars.


Figure 3: Use of spay hook to life the wirebar with one hand, allows the other hand to safely place mouse inside the cage post manipulation.
just enough to get the mouse inside the cage (Figure 3). The wire bar is then lowered closed for the next mouse to be sorted, leaving the spay hook in place in the wire bar. After all, mice have reorganized, the spay hook is removed from the wire bar, and the cage lid is placed and secured.
Using this method, up to six SPP cages will fit inside a 6-foot BSC simultaneously, each with its own spay hook. One person can efficiently handle restraining mice, tumor measurements, and placing the mice into their new caging groups. The spay hook is autoclavable, fits the hand, reduces touch points, and is reasonably sized for the task. After use within a cage, the spay hook can be wiped and cleaned with many facility approved disinfectants and can be placed inside a clean cage or in an autoclave pouch for autoclave sterilization for next use.
It has been demonstrated during our procedures that the spay hook tool is a viable option to manipulate wire bars quickly and efficiently to support tumor size matching, as well as other manipulations such as dosing or weaning. Animal studies were approved by AbbVie's Institutional Animal Care and Use Committee or Ethics Committee. Animal studies were conducted in an AAALAC-accredited program where veterinary care and oversight were provided to ensure appropriate animal care.
Andrea Kachellek, BS, RLATG, is a Senior Supervisor at Abbvie in Chicago, IL. Nicole Martinec, BS, RLATG, is a Lead Animal Care Technician at Abbvie in Chicago, IL. She is currently serving as the Chicago AALAS branch President-Elect.








