








6 A CATALYST FOR ONESTEP CONVERSION OF METHANE TO METHANOL
A highly selective catalyst can convert methane (a major component of natural gas) into methanol (an easily transportable liquid fuel) — all in a single reaction.
13 COMPRESSED AIR IN THE PHARMACEUTICAL INDUSTRY: PART 2
Learn the key points to observe in the process of renovating an existing or planning a new compressed air system, as well as some tips on optimisation.
18 PARENTS’ DIETS CAN SHAPE THE HEALTH OF THEIR FUTURE OFFSPRING

regulatory oversight from federal agencies.
28 WEARABLE SENSOR CAN DETECT SOLID-STATE SKIN BIOMARKERS
A stretchable, hydrogel-based sensor offers a non-invasive method to monitor health by detecting biomarkers directly on the skin.
31 GENE THERAPY TRIAL RESULTS IN SIGNIFICANT VISION IMPROVEMENTS
The vision of people with a rare inherited condition was anywhere from 100 to 10,000 times better after they received gene therapy.
In addition to finding ways to remain competitive, life science organisations must also consider 13
The diet of expectant mothers can affect the risk of heart disease in their offspring, while fathers’ diets can program certain characteristics even before conception.
20 FDA APPROVES SCHIZOPHRENIA DRUG WITH NEW MECHANISM OF ACTION
Targeting cholinergic receptors as opposed to dopamine receptors, the product is the first in an entirely new class of drugs for schizophrenia patients in more than 70 years.

25 AVOID ADVERSE REGULATORY ACTION WITH COMPREHENSIVE QUALITY
33 ARE IMMUNE CELLS FOCUSED ON THE WRONG PART OF THE FLU VACCINE?
Scientists have discovered why the flu vaccine can perform poorly, having found that a specific type of immune cell indirectly controls the anti-influenza response.

At the time of writing, the highly transmissible XEC strain of COVID-19 has recently been detected in Australia, heralding an all-too-familiar feeling of wariness as we head into summer. Interestingly, the news comes shortly after a breakthrough in determining the animal origins of the virus, based on more than 800 samples collected in and around the Wuhan wet market.
On 1 January 2020, just hours after the market was closed, investigators from the Chinese Center for Disease Control and Prevention (CDC) went to the market to collect samples. They swabbed the floors, walls and other surfaces of the stalls; they came back days later to focus on surfaces in stalls selling wildlife, and also collected samples from the drains and sewers.
The team performed metatranscriptomic sequencing of the samples, a technique aiming to obtain all RNA sequences (and which can pick up DNA as well) from all organisms present in the samples — viruses, bacteria, plants, animals and humans. According to the new analysis of this sequencing data, SARS-CoV-2 was present in some of the same stalls as wildlife sold at the market; in some cases, genetic material from the SARS-CoV-2 virus and these animals was even found on the same swabs.
The international researchers created a shortlist of animal species in the wet market found co-occurring or close to viral samples, which could represent the most likely intermediate hosts for SARS-CoV-2. The common raccoon dog, a species susceptible to SARS-CoV-2 and that carried SARSCoV in 2003, was found to be the most genetically abundant animal in the samples from market wildlife stalls. Genetic material from masked palm civets, which were also associated with the earlier outbreak of SARS-CoV, was also found in a stall with SARS-CoV-2 RNA. Other species such as the Hoary bamboo rat and Malayan porcupines were also found to be present in SARS-CoV-2-positive samples, as well as a multitude of other species — all of which points to the idea that infected animals were introduced into the market in late 2019, which ultimately sparked the pandemic.
In other COVID news, a recent analysis of UK Biobank data has found that COVID-19 infection may increase the risk of serious cardiovascular complications for up to three years after infection. Risk of heart attack, stroke and death was more than two times higher among adults who had COVID-19, and nearly four times greater among adults hospitalised with COVID-19, compared with the group with no history of COVID-19 infection. In some cases, the increased risk was almost as high as having a known cardiovascular risk factor such as type 2 diabetes or peripheral artery disease, with an even higher risk of heart
attack and stroke in people with non-O blood types. It should however be noted that the data was from patients who had the original strain of COVID-19, before vaccines were widely available, which brings hope that vaccination in combination with today’s milder strains may offset this risk.
So what can you do to look after your heart?
As it turns out, our cardiovascular health may in part be determined by our mother’s diet during pregnancy, as revealed in the article on page 18. The good news is, we may in future be able to monitor our heart health using a wearable sensor, which detects certain biomarkers directly on the skin (page 28). Other highlights this issue include a newly approved schizophrenia drug (page 20); a catalyst for one-step methane conversion (page 6); and the second half of our look at compressed air in the pharmaceutical industry (page 13).

Cruciferous vegetables, including broccoli, cabbage, kale and cauliflower, have been found to lower blood pressure in middle-aged and older Australian adults with elevated blood pressure, according to research conducted by Edith Cowan University (ECU) and published in the journal BMC Medicine
As noted by ECU PhD student Emma Connolly, “Hypertension, or high blood pressure, is the leading risk factor for heart disease, with its prevalence increasing with age. Increasing vegetable intake is widely recommended to reduce heart disease risk, and previous observational studies have shown cruciferous vegetables like broccoli, cabbage and Brussels sprouts have stronger relationships with lower heart disease risk than other vegetables.”
Connolly explained that compounds called glucosinolates, which are found almost exclusively in cruciferous vegetables, have been shown to lower blood pressure in animals, but evidence in humans has thus far been limited. Cruciferous vegetables also contain several other components that likely provide additional benefits in lowering blood pressure, such as nitrate and vitamin K.
ECU researchers conducted a randomised, controlled, crossover trial over a six-week period, with participants completing two dietary interventions separated by a ‘washout’ period where they followed their normal diet. During one intervention period participants consumed four serves of cruciferous vegetables per day as soups with lunch and dinner, while during the other intervention period they consumed a root and squash vegetable soup.

The blood pressure of participants was measured continuously for 24 hours before and after both two-week intervention periods, and showed a 2.5 mmHg difference in blood pressure reduction for eating cruciferous vegetables compared to root and squash vegetables, which translates to roughly 5% lower risk of experiencing a heart attack or stroke. Background diet and lifestyle remained consistent throughout the study, indicating the reduction in blood pressure seen was not influenced by these factors.
ECU NHMRC Emerging Leader and Heart Foundation
Postdoctoral Fellow Dr Lauren Blekkenhorst noted that less than one in 15 Australian adults currently meets recommendations for vegetable intake, with cruciferous vegetables the lowest consumed group of vegetables. If people want to lower their blood pressure and reduce their risk of developing heart disease later in life, they should ideally consume these vegetables most days of the week, she said.
Researchers at Ireland’s RCSI University of Medicine and Health Sciences have developed an electroconductive implant that may have the potential to encourage nerve cell (neuron) repair after spinal cord injury. The implant has been described in the journal
Spinal cord injury is a devastating and often paralysing condition. One person suffers a spinal cord injury every week in Ireland, and there are over 2300 individuals and families living with spinal cord injury across Ireland. After injury, the long axonal projections of nerve cells are cut and ‘die back’ from the injury site, and at the same time a lesion or gap forms at the wound site that prevents their regrowth, necessary to restore function.
To address this problem, the research team at RCSI’s Tissue Engineering Research Group (TERG) and the SFI Centre for Advanced Materials and Bioengineering Research (AMBER) at Trinity College Dublin developed an implantable, electroconductive 3D-printed scaffold that can be placed directly into the injury site, bridging the gap.
When electrical stimulation is applied to the implant, it can convey that electrical signal to boost the regrowth of the injured axons. At the same time, the scaffolding and channels of the implant are designed to act as a bridge and direct the axons to grow back in the correct formation.
“Bridging the lesion with an electroconductive biomaterial designed
to mimic the structure of the spinal cord, combined with the application of electrical stimulation, may help injured neurons regrow their axons and reconnect to restore function,” said Professor Fergal O’Brien, who is Head of TERG and Deputy Director of AMBER.
“No such platform exists to date.”
The researchers saw promising results when they put the implant to the test in the lab, according to first author Liam Leahy.
“We could see that when we applied electrical stimulation for a week to neurons growing on this scaffold, they developed long, healthy extensions called neurites,” said Leahy, a PhD candidate at RCSI. “In the body, this kind of growth would be a key step towards repair and recovery after an injury.”
O’Brien concluded, “To date, it has been extremely difficult to promote the regrowth of neurons after spinal cord injury, which is a major obstacle in the development of successful treatments for such debilitating injuries. Our research here represents a promising new approach.”
Scientists at the US Department of Energy’s (DOE) Brookhaven National Laboratory and their collaborators have engineered a highly selective catalyst that can convert methane (a major component of natural gas) into methanol (an easily transportable liquid fuel) — all in a single, onestep reaction.
As described in the Journal of the American Chemical Society, this direct process for methane-to-methanol conversion runs at a temperature lower than required to make tea and exclusively produces methanol without additional by-products. That’s a big advance over more complex traditional conversions that typically require three separate reactions, each under different conditions, including vastly higher temperatures.
“We pretty much throw everything into a pressure cooker, and then the reaction happens spontaneously,” said chemical engineer Juan Jimenez, a postdoctoral fellow in Brookhaven Lab’s Chemistry Division and the lead author on the study.
From basic science to industry-ready The science behind the conversion builds on a decade of collaborative research. The Brookhaven chemists worked with experts at the Lab’s National Synchrotron Light Source II (NSLS-II) and Center for Functional Nanomaterials (CFN) — two DOE Office of Science user facilities that have a wide range of capabilities for tracking the intricacies of chemical reactions and the catalysts that enable them — as well as researchers at DOE’s Ames National Laboratory and international collaborators in Italy and Spain.

Earlier studies worked with simpler ideal versions of the catalyst, consisting of metals on top of oxide supports or inverted oxide on metal materials. The scientists used computational modelling and a range of techniques at NSLS-II and CFN to learn how these catalysts work to break and remake chemical bonds to convert methane to methanol and to elucidate the role of water in the reaction.
“Those earlier studies were done on simplified model catalysts under very pristine conditions,” Jimenez said. They gave the team valuable insights into what the catalysts should look like at the molecular scale and how the reaction would potentially proceed, “but they required translation to what a real-world catalytic material looks like”.
Brookhaven chemist Sanjaya Senanayake, a co-author on the study, explained, “What Juan has done is take those concepts that we learned
about the reaction and optimise them, working with our materials synthesis colleagues at the University of Udine in Italy, theorists at the Institute of Catalysis and Petrochemistry and Valencia Polytechnic University in Spain, and characterisation colleagues here at Brookhaven and Ames Lab. This new work validates the ideas behind the earlier work and translates the lab-scale catalyst synthesis into a much more practical process for making kilogram-scale amounts of catalytic powder that are directly relevant to industrial applications.”
The new recipe for the catalyst contains an additional ingredient: a thin layer of ‘interfacial’ carbon between the metal and oxide.
“Carbon is often overlooked as a catalyst,” Jimenez said. “But in this study, we did a host of experiments and theoretical work that revealed that a fine layer of carbon between palladium and

cerium oxide really drove the chemistry. It was pretty much the secret sauce. It helps the active metal, palladium, convert methane to methanol.”
To explore and ultimately reveal this unique chemistry, the scientists built new research infrastructure both in the Catalysis Reactivity and Structure group’s laboratory in the Chemistry Division and at NSLS-II.
“This is a three-phase reaction with gas, solid and liquid ingredients — namely methane gas, hydrogen peroxide and water as liquids, and the solid powder catalyst — and these three ingredients react under pressure,” Senanayake said. “So, we needed to build new pressurised three-phase reactors so we could monitor those ingredients in real time.”
The team built one reactor in the Chemistry Division and used infrared spectroscopy to measure the reaction rates and to identify the
chemical species that arose on the catalyst surface as the reaction progressed. The chemists also relied on the expertise of NSLS-II scientists who built additional reactors to install at two NSLS-II beamlines — Inner-Shell Spectroscopy (ISS) and in situ and Operando Soft X-ray Spectroscopy (IOS) — so they could also study the reaction using X-ray techniques.
NSLS-II’s Dominik Wierzbicki, a study co-author, worked to design the ISS reactor so the team could study the high-pressure, gas–solid–liquid reaction using X-ray spectroscopy. In this technique, ‘hard’ X-rays, which have relatively high energies, enabled the scientists to follow the active palladium under realistic reaction conditions.
“Typically, this technique requires compromises because measuring the gas–liquid–solid interface is complex, and high pressure adds even more challenges,” Wierzbicki said.
“Adding unique capabilities to address these challenges at NSLS-II is advancing our mechanistic understanding of reactions carried out under high pressure and opening new avenues for synchrotron research.”
Study co-authors Iradwikanari Waluyo and Adrian Hunt, beamline scientists at IOS, also built an in situ setup at their beamline and used it for lower energy ‘soft’ X-ray spectroscopy to study cerium oxide in the gas–solid–liquid interface. These experiments revealed information about the nature of the active catalytic species during simulated reaction conditions.
“Correlating the information from the Chemistry Division to the two beamlines required synergy and is at the heart of the new capabilities,” Senanayake said. “This collaborative effort has yielded unique insights into how the reaction can occur.”
The simplicity of the system could make it particularly useful for tapping natural gas reserves in isolated rural areas, far from the costly infrastructure of pipelines and chemical refineries.

In addition, colleagues Jie Zhang and Long Qi at Ames Lab performed in situ nuclear magnetic resonance studies, which gave the scientists key insights into the early stages of the reaction; and Sooyeon Hwang at CFN produced transmission electron microscopy images to identify the carbon present in the material. The team’s theory colleagues in Spain, led by Verónica GandugliaPirovano and Pablo Lustemberg, provided the theoretical explanation for the catalytic
mechanism by developing a state-of-the-art computational model for the three-phase reaction.
In the end, the team discovered how the active state of their three-component catalyst — made of palladium, cerium oxide and carbon — exploits the complex three-phase, liquid–solid–gas microenvironment to produce the final product. Now, instead of needing three separate reactions in three different reactors operating under three different sets of conditions to produce methanol

from methane with the potential of by-products that require costly separation steps, the team has a three-part catalyst that drives a three-phasereaction, all-in-one reactor with 100% selectivity for methanol production.
“We could scale up this technology and deploy it locally to produce methanol than can be used for fuel, electricity and chemical production,” Senanayake said. The simplicity of the system could make it particularly useful for tapping natural gas reserves in isolated rural areas, far from the costly infrastructure of pipelines and chemical refineries, removing the need to transport high-pressure, flammable liquefied natural gas.
Brookhaven Science Associates and the University of Udine have now filed a patent cooperation treaty application on the use of the catalyst for one-step methane conversion. The team is also exploring ways to work with entrepreneurial partners to bring the technology to market.
“This is a very valuable example of carbonneutral processing,” Senanayake said. “We look forward to seeing this technology deployed at scale to make use of currently untapped sources of methane.”

As tiny nanoplastics continue to build up in the world’s bodies of water, the challenge remains to develop a cost-effective solution to remove them while leaving clean water behind. Now researchers at the University of Missouri have created a liquid-based solution that eliminates more than 98% of these microscopic plastic particles from water, which they have described in the journal ACS Applied Engineering Materials
“Nanoplastics can disrupt aquatic ecosystems and enter the food chain, posing risks to both wildlife and humans,” said Piyuni Ishtaweera, who led the study while earning her doctorate in nano and materials chemistry.
“We’re developing better ways to remove contaminants such as nanoplastics from water.”
The team’s innovative method uses water-repelling solvents made from natural ingredients. Initially, the solvent sits on the water’s surface the way oil floats on water. Once mixed with water and allowed to reseparate, the solvent floats back to the surface, carrying the nanoplastics within its molecular structure.
“These solvents are made from safe, non-toxic components, and their ability to repel water prevents additional contamination of water sources, making them a highly sustainable solution,” Ishtaweera said. The new method is also effective in both fresh and salt water, she added.
The Missouri team tested five different sizes of polystyrene-based nanoplastics, in the process outperforming previous studies that largely focused on just a single size of plastic particles. The researchers simply used a pipette to remove the nanoplastic-laden solvent — leaving behind clean, plastic-free water — but future studies will work to scale up the entire process so that it can be applied to larger bodies of water like lakes and, eventually, oceans.
“Our strategy uses a small amount of designer solvent to absorb plastic particles from a large volume of water,” said Associate Professor Gary Baker, corresponding author on the study. “Currently, the capacity of these solvents is not well understood. In future work, we aim to determine the maximum capacity of the solvent. Additionally, we will explore methods to recycle the solvents, enabling their reuse multiple times if necessary.”
The breakthrough not only offers a practical solution to the pressing issue of nanoplastic pollution but also paves the way for further research and development in advanced water purification technologies. Ishtaweera concluded that “creating effective removal methods fosters innovation in filtration technologies, provides insights into nanomaterial behaviour and supports the development of informed environmental policies”.
MRI scans could replace invasive heart tests, as new research led by the University of East Anglia (UEA) and Queen Mary University of London shows MRIs can reliably estimate pressures inside the heart to predict if a patient will develop heart failure.
A heart MRI is a type of scan that uses powerful magnets and radio waves to create detailed images of the heart, without the use of harmful radiation. Previous research from UEA has shown that heart MRI techniques can estimate pressure in the heart and are linked to symptoms and signs of heart failure; however, to date it remained unknown if MRIderived pressures could predict heart failure risk in a general population.
In this latest work, the researchers analysed heart MRI data from 39,000 UK Biobank participants using artificial intelligence techniques and estimated the pressure inside the heart. They then evaluated each individual’s risk factors and their chance of developing heart failure in the future over a six-year follow-up period. Their results, published in the journal ESC Heart Failure, confirmed that MRI-detected pressure changes can reliably predict if an individual will develop heart failure.
“Participants with higher heart pressure measured by MRI had a fivefold increased risk of developing heart failure over six years,” said co-lead author Dr Pankaj Garg, from UEA’s Norwich Medical School.
“This breakthrough suggests that heart MRI could potentially replace invasive diagnostic tests.”
Additionally, the researchers identified several key risk factors for developing high heart pressure: age over 70, high blood pressure, obesity, alcohol consumption and male gender.
“By combining these factors, we developed a model to predict individual heart failure risk,” said co-lead author Dr Nay Aung, from the William Harvey Research Institute at Queen Mary University of London. “This advancement enables prevention, early detection and treatment of heart failure, which could save many lives.”
A blood test can help predict which preterm babies will go on to develop chronic lung disease, allowing for earlier diagnosis and more targeted treatments. That’s according to new research led by the Murdoch Children’s Research Institute (MCRI), which found that changes in certain blood proteins, alongside gestational age, birth weight and sex, strongly predicated bronchopulmonary dysplasia (BPD) within 72 hours of life.
BPD usually occurs when a baby’s lungs are damaged by respiratory support and the long-term use of oxygen. The disease affects 65% of preterm infants and results in lifelong chronic lung disease and neurodevelopmental disabilities.

early as four hours post-birth,” Pereira-Fantini said. This provided a comprehensive map of what occurred in babies with BPD and gave valuable insight about key biological changes in the first few days of life.
As noted by MCRI’s Dr Prue Pereira-Fantini, “Our ability to predict, prevent and treat BPD is limited”, as the tool currently used for early prediction of BPD severity fails to look at the disease pathology. As a result, “A BPD diagnosis is usually made at 36 weeks post-menstrual age, which limits potential treatments that can minimise lung injury and improve respiratory outcomes,” Pereira-Fantini said.
The MCRI study, which examined 493 blood proteins, involved 23 babies born before 29 weeks’ gestation at The Royal Women’s Hospital. Changes found in 49 of these proteins were detected in babies who later developed BPD, as detailed in the American Journal of Respiratory Cell and Molecular Biology
“Our team was able to identify certain proteins in the blood which, when combined with other key birth measures, may predict BPD as

MCRI Professor David Tingay said the ability to more accurately predict BPD within these first few days may allow for earlier diagnosis, more targeted treatments and better-informed counselling for families.
“Changes in BPD rates can be achieved if appropriate lung protective interventions are provided at the right time,” he said. “We can better tailor the care of these babies when we know how likely they are to experience lung damage and other complications.”
The team are now looking to create a lung injury assessment tool that could be used to assess all preterm babies admitted to a neonatal intensive care unit (NICU) or special care nursery for risk of BPD. According to Pereira-Fantini, “The tool, including a blood test, would provide clinicians with the ability to guide respiratory decisions from birth, giving these babies more chances towards a healthy life.”
Researchers at CSIRO have developed a next-generation uniform prototype that employs nanofibres to safeguard wearers from chemical and biological threats, by filtering out harmful particles while remaining lightweight and breathable.
CSIRO Manufacturing Research Unit Director Dr Marcus Zipper said the textile innovation was the result of collaboration with industry and research partners, including DMTC (formerly known as the Defence Materials Technology Centre).
“Our nanofibre technology, pioneered by CSIRO scientists, has the potential to significantly improve the level of protection soldiers’ uniforms provide and can also be used for non-military applications, including protecting emergency responders and hazmat crews,” Zipper said.
“CSIRO research and development in materials science looks to improve how a particular material functions — we work across a broad range of advanced materials including metals, composites, polymers, adsorbents and nanofibres.”
DMTC’s Head of Program Management, Deepak Ganga, said the new prototype uniform could deliver a significant leap forward in soldier protection, ensuring better comfort and mobility in harsh environments. CSIRO project lead Dr Yen Truong added that the key to the prototype’s success lies in its innovative nanofibre technology.
“In rigorous testing, the prototype surpassed all performance targets for air filtration, air permeability, thermal comfort and chemical protection,” Truong said.
“This means it effectively filters pollutants from the air, allows for breathability, maintains comfortable temperatures even in extreme conditions, and offers superior protection from hazardous chemicals.”
The initial phase of the project was funded by the Department of Defence, while the nanofibre suit prototype was coordinated by DMTC. Other project supporters include Bruck Textiles, Defence Science and Technology Group, and RMIT University.
With DMTC, Truong and the team are seeking funding to progress to the next stage of development, which is expected to involve field testing with Australian Defence Force personnel and further refinement of scaled manufacturing processes with industrial partners.
A research team led by Hamamatsu University School of Medicine has unveiled a link between melatonin secretion and the severity of ADHD (attention deficit hyperactivity disorder) symptoms in children. Their findings, published in the journal Psychiatry Research Communications, suggest that genetic variations affecting melatonin production may play a significant role in the development and exacerbation of ADHD symptoms.
ADHD is a prevalent neurodevelopmental disorder affecting approximately 5% of children worldwide, characterised by persistent patterns of inattention, hyperactivity and impulsivity. While it has long been known that children with ADHD often struggle with sleep disorders, the precise relationship between sleep and ADHD symptoms has remained unclear. Now, researchers have revealed that children with genetic traits that reduce melatonin secretion at night exhibited more severe ADHD symptoms at age 8–9 years using data from the Hamamatsu Birth Cohort for Mothers and Children (HBC Study), which tracks the development of children from birth.
“Our findings indicate that disruptions in melatonin secretion may contribute to the difficulties children with ADHD face in maintaining regular sleep patterns,” said lead author Associate Professor Nagahide Takahashi, from Hamamatsu University School of Medicine. “This could potentially worsen their ADHD symptoms, creating a vicious cycle that can be challenging to break.”
The research thus underscores the importance of good sleep hygiene for children diagnosed with ADHD, such as establishing consistent sleep routines, reducing screen time before bed, and increasing exposure to natural light during the day. Moreover, the study suggests that melatonin supplementation could be a beneficial intervention for managing ADHD symptoms, although further research is needed to confirm its long-term efficacy.
The study also marks a significant step forward in understanding the complex interplay between sleep and neurodevelopmental disorders. As researchers continue to explore these connections, parents and healthcare providers may find new strategies for helping children with ADHD manage their symptoms more effectively.


Italy is home to some of the world’s most iconic spirits and liqueurs, which is no secret to Percento Srl — an Italian R&D lab operating in the food and beverage industry, with particular focus on the barman and mixologist sector. By combining cuttingedge technology with over-the-top manufacturing and design, Percento can provide ongoing R&D operations aimed at improving and optimising the work of professionals in this sector. Liqueurs, extracts, infusions, premixes, distillates and syrups are among the many deliverables for the company’s clients — Italian bars and clubs.
Percento knows that appropriate shaking and mixing is crucial for cocktails preparation, and works in its company laboratory on filtration and clarification of liqueurs, extracts, smoothies and other products by spinning the beverages to separate the solid particles from the liquid. However, dissatisfaction with the equipment was growing. The tools were all underperforming and the whole procedure took too much time without sufficient results. Luckily, OHAUS soon entered to save the day for the Percento mixing business.
Thanks to a local distributor who helped to find the right configuration and the right adapters, the OHAUS Frontier 5000 Multi-Pro 5816 centrifuge with rotor 4 x 250 mL was introduced to the company’s workplace. After a brief training session, it became a real game changer for the liquids clarification procedure.
“Now the clarification takes a few minutes, not an hour like with previously used instruments, and the separation is perfect — solids remain packed on the bottom of the tubes,” said a Percento employee.
OHAUS Frontier 5000 Multi-Pro centrifuges are designed for universal use in multiple applications in research, industrial and clinical laboratories. Available in seven models with capacities from 0.2 to 4 x 750 mL, they include high-speed performance at maximum volume, automatic rotor identification and safety features.
The multipurpose centrifuges offer a high-speed centrifugation platform which can be customised to fit workflow needs using a wide variety of rotors and accessories. The intuitive design of the centrifuges and accessories enables easy access to parameter settings and quick rotation between applications.
OHAUS’s powerful and versatile Frontier centrifuge has thus put a positive spin on Percento Srl’s workflow, in a success story that is worthy of a toast. Salute! Capella Science www.capellascience.com.au
For 85 years, BUCHI has been a leading provider of laboratory technology for R&D, quality control and production worldwide. The company is headquartered in Eastern Switzerland and has R&D, production, sales and service facilities around the world.
BUCHI offers a wide array of solutions for food, beverage and feed solutions on the determination of proximates such as protein, fat and many other parameters in the quality control laboratory, as well as online in production. The company has worked with instruments using rotary evaporation, chromatography, the Kjeldahl method and more, noting intricacies and optimising protocols for customer applications.
BUCHI provides convenient and easy-to-use instruments to fulfil the needs of the food and feed industry. The company strives to provide quality services that are precisely tailored to its customers’ needs.
Bio-Strategy - Part of DKSH Group www.bio-strategy.com

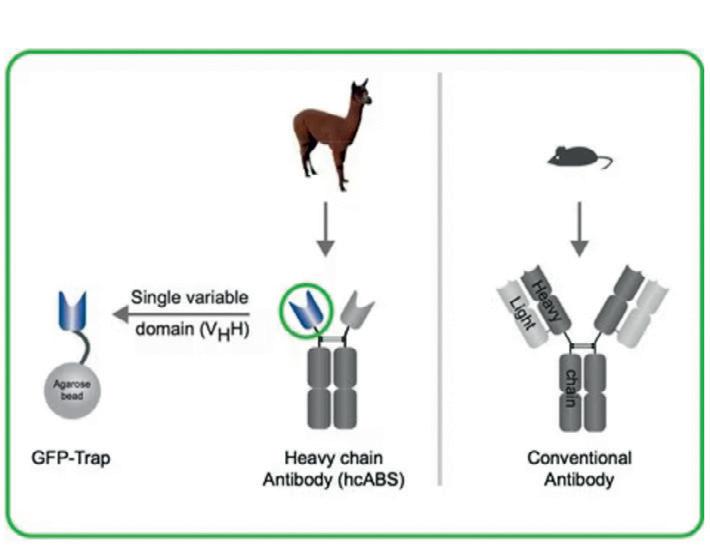
Nanobodies are small, recombinant, versatile, stable and easy to use, with many applications in immunofluorescence, immunoprecipitation, immunohistochemistry, live-cell imaging, western blot, flow cytometry, ELISA and more. Consisting of a single monomeric variable antibody domain fragment, they may be small but they still exhibit full antigen binding capacity.
Compared to conventional antibodies, nanobodies are relatively new and unknown, but that doesn’t mean that they are a niche product. They can do many things that their larger counterparts can do, along with having some advantages.
ChromoTek Nano-Traps are ready-to-use affinity beads for immunoprecipitation (IP) and allow for fast single-band pulldowns of even low-expression proteins. They consist of nanobodies coupled to a variety of resins, including agarose beads, magnetic agarose beads and magnetic particles (M-270).
The ChromoTek GFP-Trap consists of an anti-GFP VHH, derived from alpaca heavy chain antibodies. The anti-GFP VHH (nanobody) coupled to agarose beads is the GFP-Trap and is optimised for the immunoprecipitation of GFP-tagged proteins and their interacting factors.
United Bioresearch Products Pty Ltd www.unitedbioresearch.com.au
Servicebio’s SWE-FP, available from Pacific Laboratory Products, is a low-temperature tissue homogeniser that can reach as low as -50°C in the chamber and is equipped with a freezing table.
A high-speed reciprocating motion creates a vertical shaking system. The frozen sample is mixed with grinding beads in the grinding tube. The resulting grind shear force completely breaks down the tissue sample. Up to 192 samples can be processed simultaneously in 1 min, making it a dedicated device for the rapid processing of multiple samples.
The product has an incorporated slider drive with no wear shaft, providing low-noise operation. Its low-temperature freezing table is suitable for sample storage, electrophoresis, membrane transfer and other low-temperature experiments.

Its imported compressor has a minimum cooling temperature of -50°C, with a low-temperature grinding process that reduces degradation of proteins and RNA. It offers efficient and effective sample grinding with high throughput, with up to 192 samples processed in 1 min (or 24 samples as standard). Multiple adapters are available.
The product measures 650 x 420 x 430 mm and weighs 54 kg. It has a screen size of 5 ″, a minimum temperature of -50°C and precision of ±1°C. With a runtime of 0–9999 s and a pause time of 0–999 s, it can be run 1–99 times in one session. Pacific Laboratory Products www.pacificlab.com.au
compressed air in pharma

When it comes to compressed air, a great deal can often be achieved with just a few measures to increase efficiency and cost savings.
In the second part of this article series, Kaeser Compressors describes the key points to observe in the process of renovating an existing or planning a new compressed air system, and provides some tips on optimisation.
Introduction
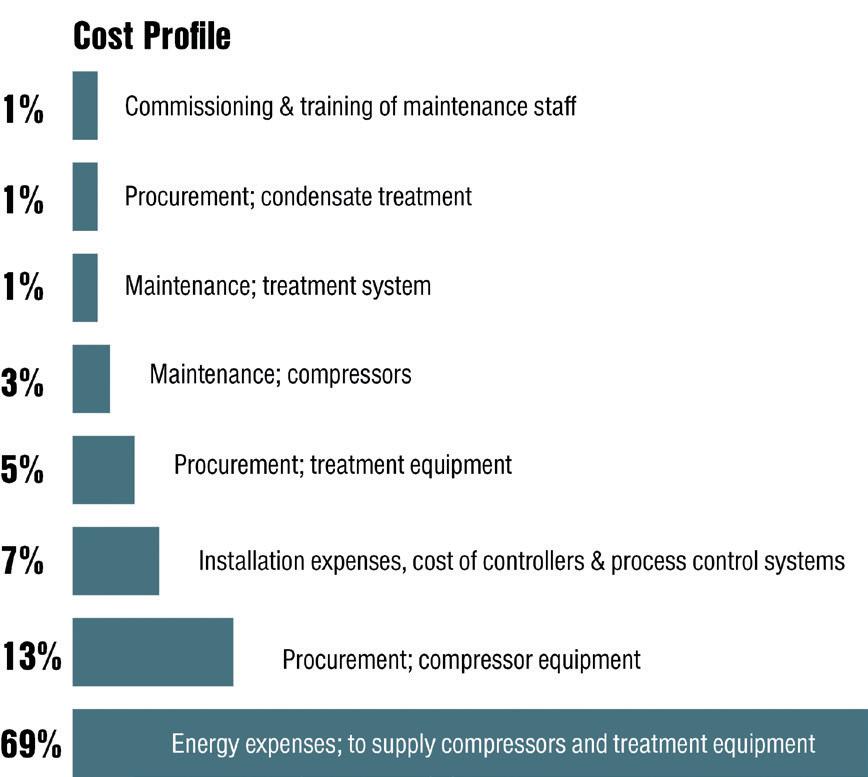
Energy costs account for the lion’s share of total compressed air supply costs. For an optimised compressed air supply produced by a new station with aircooled compressors, the cost profile is divided as follows: commissioning and training of maintenance staff account for around just 1% of total costs;
the same goes for condensate treatment. Installation expenses and the cost of controllers and process control systems come in at 7%, and procurement of treatment equipment at 5%, with that of compressor equipment at around 13%. Maintenance of compressors accounts for 3%, whilst treatment system maintenance comes in at 1%.
The largest cost block by a huge margin derives from energy expenses to supply compressors and treatment equipment, however, at 69% (Figure 1). This striking figure makes clear how energy performance is one of the most important indices for evaluating compressors.

Energy efficiency considerations therefore play a central role in system planning, which always begins with a thorough analysis of the current air demand situation — for both new systems as well as renovations of existing ones. The audit can be carried out either by an external expert or the operator can take on the task internally.
When using an external expert to measure pressure and air consumption, the operation of the compressed air station and of the entire system is analysed for a period of at least 10 days using modern data logger technology (Figure 2). The data logger collects the relevant measured values and transfers them to a computer, which generates a detailed consumption diagram. This includes pressure drops, pressure and consumption fluctuations, idling behaviour, load and compressor standstill times as well as each individual compressor’s contribution in supplying the compressed air consumed.
A specialised program then calculates the optimal configuration and cost saving potential. This analysis goes beyond determining energy consumption at intervals at a certain compressed air demand; rather, it’s possible to obtain a precise picture of the specific power performance of the compressor station during its entire runtime. This means weak points in the partial-load range
are identified and remedied well in advance. The overall result is a clear statement of the achievable cost savings and amortisation details.
Performing analysis independently
Operators who wish to plan their own compressed air supply are advised to begin the analysis at the end of the system, i.e., with the end equipment that is consuming the compressed air, and work backwards step by step to the compressed air production itself. The first aspect to consider is the required compressed air quality and how this will be achieved.
Naturally, the compressed air quality levels required at the end equipment depend first and foremost on the needs of the individual company; the relevant requirements and classifications are defined in ISO 8573. VDMA Guideline 15930-2 provides purity class recommendations by application type and was specially developed for the pharmaceutical industry.
The pipelines themselves are the next system component upstream of the equipment. The important criteria here are: manner of laying the pipe; material; manner of compressed air distribution throughout the company; and the method for joining the pipes to one another and to the components.
The pipeline system should be laid in as many straight lines as possible to save energy. Sharp 90° corners cause major pressure loss and can be easily replaced with generously dimensioned 90° arcs. Instead of the commonly used water shutoff units, ball valves or flap valves with full — not reduced — diameter should be used.
Since the pharmaceutical industry generally has very stringent compressed air quality requirements, pipeline material should be selected with a view to avoiding contamination. The correct joining technique is also very important. Pipeline parts should either be connected to one another by welds, adhesive or using a combination of screws and adhesive.
Leaks in the system are the most active causes of energy waste in a company. Since they’re at work around the clock, 8760 hours per year, leaks unsurprisingly represent the most significant source of loss in the majority of systems. Studies demonstrate that between 25 and 60% of the compressed air produced is lost due to leaks, and leaks occur regularly even in carefully maintained systems. Paying extra attention to building a tight, leak-free system is therefore imperative (Figure 3).
The simplest method of locating leaks is to use an ultrasound measurement device (Figure 4), since the location of leaks can then be pinpointed during production hours. Such a device can be rented from all leading providers in the compressed air sector, if an expert is not specifically engaged to perform this task.

by using an
detector.
Once the focus has shifted upstream to the compressed air station itself, the compressed air treatment becomes the first area to consider. Basically every type of compressor is capable of producing the quality levels required in the pharmaceutical industry. Whether the compression process is oilfree or oil-injected makes no difference since compressed air is nothing other than the ambient air in compressed form — and ambient air always contains contaminants. This means treatment is always necessary to meet the high requirements of the pharmaceutical industry.
In most cases, a precisely coordinated configuration of compressors with different capacities turns out to be the ideal solution. This usually
Identifying and characterising different structural forms of a compound can be crucial. This is especially true for acetaminophen, a common pain-relief medication that exists in multiple polymorphic forms. Process conditions, improper storage and simply time can cause the transition from a stable, efficacious form of a drug substance into an unwanted structural analogue.
Traditional characterisation techniques often struggle to both separate and identify the different species in a mixed system and lack the sensitivity to detect the low levels of polymorphic contaminants that may be present in final products. However, Malvern Panalytical’s Morphologi 4-ID system, which combines automated microscopy and Raman spectroscopy, offers a powerful solution.

5: For truly efficient compressor operation, a combination of base load and peak load machines is essential.
consists of large base load and standby machines, combined with smaller peak load machines (Figure 5). The master controller is responsible for coordinating production of the required compressed air with maximum cost efficiency. Modern master compressed air management systems not only enable real-time monitoring, but also analysis and recommendations to ensure constant optimisation.
If all of these steps are followed, the compressed air supply will not just be efficient, cost-effective and reliable, it will also be compatible with future developments.
To read Kaeser’s entire whitepaper series on compressed air in the pharmaceutical industry, visit https://bit.ly/3WWYyCs.

This advanced approach, known as Morphologically Directed Raman Spectroscopy (MDRS), has been designed to enable the precise identification of individual particles and their composition within a mixture, to assist in determining the long-term stability of a product. By targeting particles with distinct morphologies for Raman analysis, MDRS can enhance measurement sensitivity, allowing for the detection of low-level polymorphic contaminants. Unlike traditional methods, the Morphologi 4 has the ability to combine morphological and chemical analysis in a single platform, providing insights into complex mixtures.
The Morphologi 4 is an effective method for characterising both particle shape and size from less than a micron to over a millimetre, replacing the time-consuming process of manual microscopy. Small differences in particle size and shape can impact both the flowability or cohesion properties and the final product performance, such as drug delivery, dissolution and bioavailability. Therefore, combining measurements should provide a more accurate picture of each individual particle, giving a deeper insight of a sample’s characteristics. This helps to resolve issues during the development of new formulations and products.
ATA Scientific Pty Ltd www.atascientific.com.au
Rotronic, part of Process Sensing Technologies, has launched a new version of the HygroCal humidity validation instrument. Onsite technical and quality control teams can use the lightweight, portable HygroCal100 Advanced (HC100A) to make quick and simple evaluations of up to seven relative humidity (RH) probes simultaneously.

The HC100A is a fully integrated, standalone instrument for validating humidity sensors from 5% to 95%RH. This makes it suitable for a wide range of laboratory and process applications, including those in the pharmaceutical, food and semiconductor sectors.
A re-engineered sealed internal test chamber is designed to ensure both stability and uniformity across the chamber are consistent to within ±0.5%RH. With separate saturation and desiccation reservoirs, changing measurement conditions simply requires ambient air to be passed through either reservoir.
Its internal reference, Rotronic’s advanced HC2A-S probe, is designed to provide accurate humidity and temperature measurements within 0.8% RH and 0.1°C, with low annual drift. The probe’s onboard memory means they can be easily exchanged. Additional reference devices choices are available, with users able to choose from handheld humidity instruments to precision chilled mirrors.
An advanced colour touchscreen interface enables quick and simple configuration and operation of the instrument. Simplifying set-up allows users to define the required calibration procedure. Once set, the calibration procedures will then run automatically. All measurement and reference data can be stored within the internal memory or downloaded to a PC.
Weighing just 3.2 kg, the product is compact, fully automatic, and quick and simple to use.
AMS Instrumentation & Calibration Pty Ltd www.ams-ic.com.au
The Odyssey F imaging system provides highly accurate sensitivity and dynamic range with just a click. The 3- and 10-channel instruments are designed to scan twice as fast as their predecessors to support a broad spectrum of assays beyond qualitative Western blots.
The dual near-infrared (NIR) laser in the instrument is designed to ensure good throughput and performance, making it suitable for quantitative imaging. Additionally, the two visible fluorescence lasers can further enhance users’ insights through powerful laser-based multiplexing for plate-based assays, slide imaging and more.
The imaging system allows users to complete scans in minutes. Users can achieve higher throughput with each scan for reproducible data and experience good sensitivity and increased data confidence thanks to the dynamic range. The enhanced focus offset range of the instrument is designed to ensure compatibility with a wider variety of plates, making it a versatile addition to a lab.
The imaging systems can expand a user’s lab capabilities across multiple applications including EMSA/Gel Shift Assay, Glycoprotein Staining, Nucleic Acid Gel Imaging, Quantitative Western Blots, Gene Knockdown/ Knockout, Protein Arrays, In-Cell Western Assay, Protein Estimation Assays, RNAi Analysis, Tissue Section Imaging and Whole Slide Imaging (WSI). Millennium Science Pty Ltd www.mscience.com.au


Sphere Fluidics is releasing a supercharged version of its Cyto-Mine system, designed to offer the latest innovations in single-cell isolation and analysis. The next-generation Cyto-Mine system will feature enhanced capabilities offering multiplexing and greater assay flexibility to meet users’ requirements; this means users should be able to examine greater numbers of cells and isolate the most valuable ones with high levels of precision. For scientists working in cell line development, protein/antibody discovery, cell and gene therapy or beyond, Sphere Fluidics’ advanced droplet-based microfluidics instruments are designed to help lead the way in cell selection.
Cyto-Mine is a state-of-the-art, automated platform designed to transform biopharmaceutical discovery and cell line development. By incorporating advanced technologies, the system can accelerate and simplify the entire cell line development process, from cell encapsulation and assay integration to highthroughput screening and monoclonality assurance. Capella Science www.capellascience.com.au
Aber Instruments’ OPTURA PALM handheld biomass sensor, designed for non-invasive biomass measurement, allows rapid reading of cell density without sampling. This makes it suitable for laboratory applications such as monitoring seed trains as well as determining optimum cell harvest or virus infection times.
The product harnesses near-infrared (NIR) bio-reflectance for contactless biomass analysis: simply point the sensor at a culture vessel and it generates cell density readings within seconds without having to break the sterile barrier to take samples. The sensor can store up to 1000 readings and data can be easily downloaded via software which comes with the device. This allows scientists to use the product in remote or offsite locations and then transfer data back to the laboratory to create their own custom biomass calibration curves if they require them.

The robust device, consisting of a NIR unit and sensor measurement face, operates by emitting a NIR laser from the sensor into the media. Due to the wavelength used, only cells present in the media reflect light back to the sensor measurement face. Reflectance and biomass are proportionally related so that, for example, a higher reflectance indicates a greater biomass. Since NIR can pass through glass or plastic vessel walls (up to 10 mm) and the laser has a penetration depth of 2.5 cm, it is possible to use the device to measure cells growing inside common labware such as shake flasks, T-flasks, roller bottles and polypropylene tubes in seconds without sampling.
Being able to measure cell density without having to remove cells for offline measurement saves time and effort by eliminating the risk of contaminating the culture and the need to repeat experiments. Additionally, because the product utilises a non-invasive yet quick measurement method, there is no need to use consumables such as single-use pipette tips or cuvettes.
Pryde Measurement Pty Ltd www.pryde.com.au





Babies born to women consuming a high-fat, sugary diet are at risk of cardiovascular disease and diabetes in later life, according to a new study led by the University of South Australia (UniSA).
Researchers identified the link by analysing tissue samples from the fetuses of pregnant baboons fed a high-fat, high-energy diet in a biomedical research institute in the United States. They then compared this to fetuses from baboons on a control diet.
UniSA PhD candidate Melanie Bertossa said the findings, published in The Journal of Physiology, demonstrate a clear link between an unhealthy diet high in saturated fats and sugar and poor cardiovascular health.
“There has been a longstanding debate as to whether high-fat diets induce a hyper- or hypothyroid state in the fetal heart; our evidence points to the latter,” said Bertossa, who served as lead author on the study.
“We found that a maternal high-fat, highenergy diet reduced concentrations of the active

thyroid hormone T3, which acts like a switch around late gestation, telling the fetal heart to start preparing for life after birth. Without this signal, the fetal heart develops differently.”
Bertossa said that diets high in fat and sugar can also alter the molecular pathways involved in insulin signalling and critical proteins involved in glucose uptake in the fetal heart. This increases the risk of cardiac insulin resistance, often leading to diabetes in adulthood. This is despite babies being a normal weight at birth.
“You’re born with all the heart cells you will ever have,” she said. “The heart doesn’t make enough new heart muscle cells after birth to repair any damage, so changes that negatively impact these cells before birth could persist for a lifetime.
“These permanent changes could cause a further decline in heart health once children reach adolescence and adulthood when the heart starts to age.”
UniSA’s Professor Janna Morrison said the study demonstrates the importance of good maternal
nutrition in the lead-up to pregnancy, not only for the mother’s sake but also for the health of the baby.
“Poor cardiac outcomes were seen in babies that had a normal birth weight — a sign that should guide future clinical practice,” said Morrison, senior author on the study.
“Cardiometabolic health screening should be performed on all babies born from these types of pregnancies, not just those born too small or too large, with the goal being to detect heart disease risks earlier.”
The good news is that a separate study, led by Monash University and published in the journal Circulation Research , has found that expectant mothers who eat a high-fibre diet can significantly reduce the risk of cardiovascular disease in their offspring.
“Dietary fibre, which is found in foods such as fruits, vegetables and whole grains, is wellknown for its health benefits,” said lead researcher Professor Francine Marques, from the Monash School of Biological Sciences.

“Our research shows that its impact extends beyond the mother, and can shape the development of her child’s heart.”
The research team found that a high-fibre diet in pregnant mice led to a healthier gut and gut microbiome in both mothers and their offspring; lower levels of inflammation in the heart of the offspring; improved heart function in offspring exposed to high blood pressure; and decreased cardiac fibrosis (scarring) in the heart of the offspring. The work also shed light on how fibre exerts its protective effects across generations, Marques noted.
“Fibre promotes the production of beneficial molecules called short-chain fatty acids (SCFAs) in the gut,” Marques explained.
“These SCFAs travel through the mother’s bloodstream and cross the placenta during development, where they can influence gene activity in the heart, leading to healthier heart development.”
While the study was conducted on mice, Marques said the findings have significant implications for human health, suggesting that a simple dietary change during pregnancy could have lifelong benefits for children. She said the work adds to the growing body of evidence highlighting the importance of a healthy diet during pregnancy, with the researchers planning to continue exploring the intricate relationship between maternal diet, gut health and cardiovascular disease.
And it’s not just expectant mothers who should watch their diet, with a separate study from the international GECKO consortium finding that the macronutrient balance in the diet of male mice affects the level of anxiety-like behaviour of their future sons and the metabolic health of their future daughters. Their results, published in the journal Nature Communications, provide a step towards understanding how the effect of diet can transmit from one generation to the next via a father’s sperm.
Scientists had already discovered that overor under-feeding male mice can affect their offspring’s metabolism and behaviour, as well as their risk of cancer. What is less understood is whether there are diverse types of health impacts on the health of offspring, depending on the type and composition of the diet of male mice before conception. This was the starting point for the GECKO consortium, with lead investigators in Copenhagen, Sydney and Chicago.
At The University of Sydney’s Charles Perkins Centre, researchers fed male mice one of 10 diets differing in the proportions of protein, fats and carbohydrates, then allowed them to mate with females reared on a standard diet. The behaviour and physiology of the resulting pups were then studied.
The scientists found that male mice fed lowprotein and high-carbohydrate diets were more likely to have male offspring with higher levels of anxiety, as measured by time spent in the safety zones of their maze. They also found that male mice that were fed high-fat diets were more likely to have daughters with higher levels of body fat and markers of metabolic disease.
“Our study shows that the type of diet eaten before conception can program specific characteristics of the next generation,” said co-senior author and leader of the GECKO consortium Professor Romain Barrès, from the University of Copenhagen and Université Côte d’Azur.
“It is extraordinary that by titrating mixtures of protein, fat and carbs in the father’s diet we could influence specific features of his sons’ and daughters’ health and behaviour. There is some important biology at play here,” added Professor Stephen Simpson, co-senior author and Academic Director of the Charles Perkins Centre.
The team also observed that males on a lowprotein diet ate more food overall. However, thanks to the study design, they could determine that both the amount of calories, and the macronutrient composition of the males’ diets, influenced the health of their offspring.
The mouse work is part of a broader series of studies within the GECKO consortium, involving humans and other mammals at partner institutions. Barrès described the study as “a step towards establishing dietary guidelines for fathers-to-be, with the ultimate goal of lowering the risk of metabolic disease and mood disorders in the next generation”.
Lauren Davis

In a milestone for schizophrenia treatment, the US Food and Drug Administration (FDA) has approved the drug Cobenfy (xanomeline and trospium chloride) for oral use in adults.
The antipsychotic drug targets cholinergic receptors as opposed to dopamine receptors, which has long been the standard of care. This makes it the first in an entirely new class of drugs for patients with schizophrenia in more than 70 years, with researchers hoping it will benefit those who don’t respond to — or who suffer side effects from — existing drugs.
Schizophrenia is a lifelong, serious and often debilitating mental health disorder that can cause psychotic symptoms including hallucinations (such as hearing voices), difficulty controlling one’s thoughts and being suspicious of others, as well as cognitive problems and difficulty with social interactions and motivation. It is a leading cause of disability, with individuals with schizophrenia at greater risk of dying at a younger age; nearly 5% die by suicide. And while the current standard of care can be effective in managing the disorder, up to 60% of people experience inadequate improvement in symptoms or intolerable side effects during therapy.
Cobenfy’s development was based on a discovery by Australian researchers at The Florey about differences in the brains of people with schizophrenia. Key to this was Professor Brian Dean, whose research in the 1980s and 1990s showed that muscarinic receptors in the brain were involved in the pathology of schizophrenia.
In 1993, Dean discovered that M1 and M4 muscarinic receptors, which are integral to the brain’s cholinergic system, were lower in some people with schizophrenia; such changes would affect their ability to interact with others and their cognition. A drug that activated the muscarinic M1 and M4 receptors could therefore potentially alleviate these symptoms of schizophrenia.
“At that time, no drug treatment was effective in reversing these two symptoms of the disorder,” Dean said. He added that “many people with the disorder are non-responsive and many experience unpleasant side effects such as weight gain, dizziness and sleepiness. I really hoped my discovery could lead to a drug that was effective without side effects to put people off taking it.”
Pharmaceutical company Eli Lilly developed the drug xanomeline that, as suggested by Dean’s research, activated muscarinic M1 and M4 receptors. But while the treatment lessened the positive, negative and cognitive symptoms of schizophrenia in a group of treatment-resistant people, it caused unacceptable side effects. Trials were stopped and Eli Lilly ceased work on xanomeline.
Karuna Therapeutics eventually pursued a treatment combining xanomeline with trospium, a drug to counter xanomeline’s side effects, which they originally dubbed KarXT (this was later changed to Cobenfy).
Cobenfy’s effectiveness for the treatment of schizophrenia in adults was

evaluated in two five-week, randomised, double-blind, placebo-controlled, multi-centre studies in adults with a diagnosis of schizophrenia according to DSM-5 criteria.
The primary efficacy measure was the change from baseline in the Positive and Negative Syndrome Scale (PANSS) total score, the PANSS being a 30-item scale that measures symptoms of schizophrenia. In both studies, the participants who received Cobenfy experienced a meaningful reduction in symptoms from baseline to week five as measured by the PANSS Total Score compared to the placebo group. Patients in a one-year extension trial meanwhile experienced a significant and consistent improvement in symptoms, with the drug favourably impacting their weight and long-term metabolic profiles.
“This is the biggest step forward in the treatment of schizophrenia in decades, because all drugs developed for schizophrenia in the last 70 years have targeted the same limited set of signalling pathways in the brain,” said Professor Ashley Bush, Clinical Lead of Mental Health at The Florey.
“The drug will be used, I expect, alongside existing medications, hopefully creating synergies that will bring even better improvements in outcomes in treating schizophrenia.”
“We will now be entering a very promising new era of studying how Cobenfy is best used, and whether it may be useful for other disorders.”
The FDA’s approval of Cobenfy was granted to Bristol Myers Squibb — which acquired Karuna Therapeutics back in March — over 30 years since Dean’s initial discovery. Other countries are expected to follow suit.




The running costs for a CO2 incubator can easily exceed its purchase price over time; these hidden costs may include regular replacement of fan-associated HEPA-filters or UV lamps, loss of lab space because of low vessel capacity vs footprint ratio, high gas consumption or lack of flexibility for future lab needs. Increased lab downtime risk and potential sample loss due to unreliable con tamination prevention can also add significant costs.
The Eppendorf CellXpert C170i CO2 Incubator can reduce the running costs of a lab. With a fanless design, it can offer up to 25% more usable space for more cell culture vessels in a small footprint, the company says. It also has no expendable parts like fan-associated HEPA filters or UV lamps and, with segmented inner doors, can significantly reduce gas consumption (CO 2 or N2).

Regardless of whether the user requires optimised growth conditions for their cell-based assays or a solid anticontamination concept for long-term cultivation experiments, the GMP-compatible CellXpert family can support their lab’s ambitious goals. With fast temperature and CO2 recovery in less than 5 min after door opening, optimised vibration and air turbulence protection and temperature homogeneity verified at 27 places within the incubator, the CellXpert C170i provides flexibility for different future lab set-ups and applications with its many in-field upgradeable options.
Eppendorf South Pacific Pty Ltd www.eppendorf.com.au

Uni-rAb antibodies are the latest generation of recombinant monoclonal antibodies developed using Proteintech’s Antigen-Specific B-Cell Cloning & Engineering (ABCE) platform. The platform achieves stable and high expression of recombinant monoclonal antibodies by cloning antibody heavy and light chain sequences derived from antigen-specific B cells into high-yield expression vectors followed by their introduction into suitable mammalian expression systems. Antigen-specific single B cell sorting allows the isolation of functional monoclonal antibodies reactive against antigens in their native conformation that predominantly occur in vivo and are difficult to reproduce in vitro. This ultimately leads to the generation of Uni-rAb recombinant antibodies that have native V H -V L pairings, which is critical for maintaining antibody specificity and functionality but is often lost in other antibody development approaches such as phage display. Uni-rAb recombinant monoclonal antibodies offer high lot-to-lot consistency, specificity and affinity, and are available in carrier-free formulations.
United Bioresearch Products Pty Ltd www.unitedbioresearch.com.au
















As the demand for scalable drug delivery solutions grows, research institutes and biotech organisations are stepping up to develop novel disease treatments. From CMOs and CDMOs to lipid manufacturers and pharmaceutical companies, the need for efficient, scalable technology has never been greater. The Micropore Pathfinder and Horizon series encompass these requirements, addressing both research and production needs.
Nanoparticle creation, especially for drug delivery, presents unique challenges. Microfluidic mixers (MF) introduced to the market unfortunately tend to be specific to a formulation construct, making alternative formulations difficult and time-consuming to implement. Even more concerning is their inability to scale efficiently — physics precludes it1
Micropore Pathfinder offers a better way to produce nanoparticles. With customisable, formulation-agnostic technology, the system can pivot to the formulation required rather than changing the formulation to fit a technology. Uninterrupted, it scales on the same mixer from microlitres to thousands of litres — all without sacrificing quality of production.
As noted by keynote speaker Dr Örn Almarsson (Axelyf) at the recent UNSW RNA Institute Symposium, the Advanced Cross Flow (AXF) technology of the Micropore Pathfinder could be a game changer in solving global scaling issues.
The Pathfinder was recently featured at Biomolecular Horizons 20242, where scientists were impressed by its flexibility, simplicity, and scalability. Some noted it’s not just a ‘blackbox’ technology, a similar descriptor Prof Pieter Cullis of University of British Columbia coined. ATA Scientific presented the poster ‘Quality by design considerations in the manufacture of lipid nanoparticles’ — a presentation that was delivered at the Nanomedicines conference in Sydney, plus the Shine Dalgarno Institute at JCSMR, ANU, and at the A-RNA243 conference where the Pathfinder was displayed for further examination.
Evaluation of the AXF technology by the Perrie Group at the University of Strathclyde further validates its potential, showing stunning results — with encapsulation efficiencies above 99%, and optimal biodistribution and efficacy. The manuscript has been accepted and awaits publication4
Built from high-quality stainless steel, the Pathfinder is chemically compatible (with zero leachability), reusable and designed to handle repeated sterilisations — making it a costeffective and environmentally sound solution for scalable nanoparticle production. Operating at just 2 bar pressure (compared to up to 140 bar in other methods), it ensures high-quality nanoparticle production with superior control and precision.
1. Whitepaper: A Better Way to Create Nanoparticles for in Vivo Treatments. ATA Scienti c, 28 Jan 2024, www.atascienti c. com.au/a-better-way-to-create-nanoparticles
2. Biomolecular Horizons 2024 Congress, Melbourne, Australia from 22–26 September 2024, https://www.bmh2024.com
3. Australasian RNA Biology and Biotechnology (A-RNA) Association Meeting. 21–23 Oct 2024 Melbourne Connect. https://www.a-rna24.org.au
4. ‘Production of mRNA lipid nanoparticles using advanced cross ow micromixing’ Hussain et al, Journal of Pharmacy and Pharmacology 2024, https://doi.org/10.1093/jpp/rgae122

With enviable capacity to tune to almost any formulation, the Micropore Pathfinder range offers a package for every stage of your nanoparticle formulation journey. Whether you’re in early-stage research or preparing for clinical trials, the system is flexible enough to meet your needs. The Pathfinder Pro 125 is ideal for discovery, while the Pathfinder Pro 250 supports the transition to clinical trials. The Horizon LNP Mini is GMP-ready for large-scale production.
The global uptake of the AXF technology is growing considerably, with systems in pharmaceutical companies, CMOs and research institutes across the planet reducing the cost of production measurably: democratising medicine. You’re not alone in your search for scalable, high-efficiency nanoparticle technologies. Explore how the Pathfinder can transform your workflow. Contact us for a demo today!
For more detailed information, contact ATA Scientific Pty Ltd Ph: +61 2 9541 3500 enquiries@atascientific.com.au
ATA Scientific Pty Ltd www.atascientific.com.au

The North Dakota State University Veterinary Diagnostic Laboratory (NDSU VDL) has recently implemented the Xybion LIMS (formerly known as the Matrix Gemini LIMS) from Autoscribe Informatics, which has eliminated the vast majority of in-lab paperwork, improved sample tracking and enhanced efficiency. This means the lab can provide results quickly and effectively to its clients, thus providing a more streamlined service for veterinarians, animal producers and the public health sector across North Dakota, South Dakota and Western Minnesota.
The NDSU VDL offers various diagnostic services, including toxicology, bacteriology, virology, molecular diagnostics, pathology, parasitology and serology. As an American Association of Veterinary Laboratory Diagnosticians (AAVLD) accredited laboratory, NDSU VDL provides critical support to veterinarians, animal producers and public health officials to ensure the health and wellbeing of national herds, companion animals and wildlife.

Before the implementation of the Xybion LIMS, NDSU VDL was grappling with several challenges posed by its prior LIMS system, which was causing inefficiencies and bottlenecks due to the significant time required for maintenance and updates. The need for a modern, configurable system to manage the complexities of veterinary diagnostics (including antibiotic susceptibility testing) and support the lab’s high-volume operations was evident, with the Xybion LIMS chosen as the solution.
The introduction of the new LIMS has since transformed laboratory operations, enabling a nearly paperless workflow and interfacing seamlessly with key analytical instruments to enhance data accuracy and reduce manual data entry. Key benefits include a 90% reduction in paper usage, improved sample tracking and seamless integration with vital analytical instruments. This has led to more efficient operations, faster result turnaround times and enhanced reporting accuracy.
The web portal feature has benefited local veterinarians, allowing real-time access to test results and improving patient care. The system’s configurability also allows NDSU VDL to adapt quickly to evolving business needs without waiting for vendor updates.
“Laboratory needs are always dynamic, so it is great we can add fields and make changes ourselves, without waiting for the LIMS vendor to release another version of the software or make the changes for us,” the LIMS administrator said.
According to Autoscribe Informatics, the success of the Xybion LIMS at NDSU VDL is a testament to the effectiveness of the system in addressing the complex needs of veterinary diagnostic laboratories.
Autoscribe Informatics Pty Ltd www.autoscribeinformatics.com.au

The Agilent BioTek LogPhase 600 microbiology reader is designed for measuring microbial growth curves in up to four standard 96-well microplates at a time. It features purpose-built, robust shaking and consistent temperature control, which are critical for optimal bacteria and yeast cell growth and data quality.
The microbiology reader features a shaking mechanism to keep cells in suspension for optimal growth, while its sensor-driven temperature control provides uniform heating, eliminating edge effects and evaporation. A temperature gradient from top to bottom prevents condensation on sealed plates, reducing light scatter and inaccurate readings. This combination delivers reproducible results across replicates and varying test samples.
The LogPhase 600 is an easy-to-implement solution for labs looking to increase throughput in bacterial growth and metabolism studies. Its consistent environmental conditions support accurate bacterial growth curve measurements, which is particularly useful during extended kinetic microbial growth assays.
Controlled via a user-friendly app, the product simplifies data acquisition and microbiology-focused analysis for all plates. The intuitive interface allows new users to start using the instrument with minimal training, and its built-in analysis tools enable simultaneous viewing of multiplate data. The app automatically calculates critical metrics like lag time, maximum rate (OD/min) and time to stationary phase.
The LogPhase 600 is versatile and suitable for a wide range of applications, including yeast and bacterial growth assays, algal research, antimicrobial resistance studies, biofuels research, and food and beverage testing. Its four-plate capacity offers higher throughput compared to some other systems, making it a valuable tool for high-demand laboratory environments.
Millennium Science Pty Ltd www.mscience.com.au

Attention to detail is par for the course within most modern industries and fields, including scientific research. In addition to finding ways to remain competitive in today’s crowded market and maintaining margins in the face of rising R&D costs, life science organisations must also consider regulatory oversight from federal agencies. Regulatory agencies are increasingly holding sponsor companies responsible for quality standards followed at contract organisations.
In 2022, the US FDA issued multiple recalls — more than 130 recalls to temperature abuse, 100 caused by products stored outside appropriate temperature conditions, and 51 associated with manufacturing using a contaminated excipient that had already been recalled by its supplier1. No matter the area, the message is clear: life science quality management is the top priority and is everyone’s responsibility.
The Drug Supply Chain Security Act (DSCSA) was established in 2013 as a crucial regulatory framework providing the FDA with oversight of the pharmaceutical compounding industry, as a result of lapses at a facility that had resulted in fatal consequences. A facility’s reputation can be irrevocably damaged due to poor life science quality management, leading to firms suffering from a tarnished image caused by a loss of public faith.
The Act also protects the integrity of the pharmaceutical supply chain in the United States, with an overarching objective to prevent the diversion and counterfeiting of drug products.
Life science quality management is a continuous process
Given the magnitude of the circumstances leading to the passage of the DSCSA, it would be expected that other compounding facilities would be more vigilant — but this is not the case. The number of Form 483s issued by the FDA, indicating observed conditions or practices in a facility that violate FDA’s requirements, has increased steadily over the years — from 215 in 2021 to 466 in 2022 and 510 in 20232. This shows that inspections at the facilities of organisations remain crucial and continue to reveal multiple violations despite regulatory frameworks in place. While no adverse health effects are necessarily associated with these facilities, the findings (which include microbial contamination and lack of environmental monitoring) lead to negative associations that can easily undermine public confidence.
Increased regulatory oversight puts additional pressure on organisations, but it is proper life science quality management that supports the development and manufacturing of quality products. The reality is that high standards and adherence to protocols can minimise compliance risks. This can be achieved by adopting an automated quality management system with continuous feedback to support timely improvements by flagging any deviations from acceptable limits. This in turn would alert the relevant departments that corrective action is immediately required, acting earlier in the release cycle rather than at the end.
Digital solutions can ease the burden of life science quality management
Maintaining high life science quality management can be a serious investment for many organisations. Quality procedures already make up a signification portion of overhead and can require more resources than firms may anticipate. This could explain why federal agencies are still finding companies lacking on multiple fronts, such as lacking documented procedures to guarantee drug quality or a failure to follow up on out-of-specification results. Inefficient organisational procedures are no excuse and regulatory agencies have demonstrated that companies will be held accountable, even if the offending facility is in another country or a collaborating firm.
To remain compliant while maintaining high quality, organisations stand to benefit greatly from finding ways to streamline their quality management processes. This doesn’t simply mean adopting an automated system to assess quality in each department. The biopharma landscape is increasingly complex, with dynamic regulations, skilled labour shortages, higher demand for personalised medicine and increased competition. Adopting digital solutions like a controlled document management system could greatly help companies by ensuring accurate information throughout the entire product development life cycle.
With controlled document management, organisations can enjoy improved regulatory compliance, version control, document security, electronic signatures and compliance, audit readiness, efficiency and productivity, and product quality. A streamlined process that enhances workflow efficiency goes a long way in ensuring product safety and adherence to quality standards.
At the broader level, a single application available to all key stakeholders involved in quality management can make continuous improvement more manageable. Innovations like the BIOVIA Biopharma Quality Management Analyst Solution can help to bridge this gap by granting easy access to all records and offering analysis capabilities for comprehensive quality management. Implementing digital tools can be a reasonable solution to tackle problems faced, but disjointed adoption can lead to more inefficiency and problems. Rather than disparate processes that can overlap or even be redundant, organisations should strive to implement an integrated solution that supports the maintenance of high-quality standards and meets regulatory compliance in a harmonised manner from start to finish.
From ensuring that materials used onsite are of high quality to strict adherence to quality protocols, the right set of processes can enable efforts to release quality products and avoid adverse regulatory action. Learn how you can streamline all quality-relevant processes in your organisation with a comprehensive life science quality management solution that ensures quality and compliance throughout the entire product life cycle by integration, automation and harmonisation — ultimately helping you maintain quality in the face of rising regulatory oversight.
1. FDA (2022), Fiscal Year 2022 Report on the State of Pharmaceutical Quality
2. FDA (2023), Inspection Observations
The latest Sapphire FL Biomolecular Imager from Azure Biosystems is a multimodal scanner with image resolution now down to just 5 µm.
With customisable and user-changeable laser and filter modules, the product easily supports a broad range of wavelengths from 375 through to 900 nm — allowing UV, phosphor, visible and NIR imaging — and offers an optional chemiluminescent imaging module. A wide depth of field (25 x 25 cm) allows users to image western blots, six 96-well plates, 2D gels, protein and tissue arrays, tissue slides, living small animals, plants, phosphor screens, organ and tumour imaging, and much more.
High sensitivity allows femtogram detection of proteins labelled with common fluorescent dyes. Extended dynamic range EDR, when selected, allows imaging of both bright and weak bands without experiencing saturation. It extends to 24 bits of data, which is useful for samples that feature strong and weak expressing proteins.
The Sapphire FL imager makes imaging of live animals possible. When combined with appropriate tubing and nose cones, living mice can be fluorescently imaged under anaesthesia using one of five built-in anaesthesia ports. The wide imaging platform can accommodate specimens with depth (up to 4 cm), allowing mice, plants, multi-well plates, petri dishes, microscope slides and other large biological samples to be imaged.
The imager offers a 7 mm software-controlled adjustable focal plane, so users can scan their samples at multiple depths to see all their data has to offer. It scans between 5 and 1000 µm resolution for clear visualisation of detailed samples. The chemiluminescence module adds high-resolution, quantitative chemiluminescence and visible imaging. SciTech Pty Ltd www.scitech.com.au


Vaisala introduces the VDL200 Power-over-Ethernet data logger, for environmental monitoring applications where communication security is a priority.
The VDL200 data logger can be used for secure environmental monitoring, especially GxP-regulated applications. With Power-over-Ethernet technology, users can expect uninterrupted data transmission. Whether monitoring temperature, relative humidity or CO2, the VDL200 is designed to deliver accurate and consistent data every time.
The VDL200 is easy to set up, for fast installation and simplified calibration. It is also versatile in its interface options and can be used with Vaisala viewLinc Continuous Monitoring software and Insight PC software.
Key features include: secure Power-over-Ethernet connection; gap-free data with onboard memory and backup battery; easy set-up with USB-C connection and quick configuration with Insight software; flexible user interface (monitor, alarm and report); and simplified calibration, with interchangeable smart probes that are easy to replace or calibrate in place.
Vaisala Pty Ltd www.vaisala.com
Liquid handling automation is essential for enhancing assay reproducibility, maximising throughput and extending walkaway time. In microplate-based assays, the precision of wash steps and reagent dispensing is crucial to minimise background interference from unbound materials and to ensure optimal assay performance.
The Agilent BioTek 406FX Washer Dispenser is a compact instrument offering fast, full plate washing along with up to six reagent dispensers. The product features a Dual-Action manifold that optimises plate washing for a variety of cell types, in addition to bio-magnetic protocols, across a broad range of plate configurations, from 96 to 1536 wells.
Unlike typical microplate dispensers that rely solely on peristaltic or syringe pumps, the 406FX’s innovative Parallel Dispense Technology includes up to four syringe pumps and two peristaltic pump dispensers to enable rapid dispensing of cells and precious reagents. The product offers high levels of flexibility with its independent, hands-free dispensing capabilities, enhancing the performance of multifaceted tasks on the one instrument.
The 406FX’s small footprint allows it to be integrated into existing laminar flow hoods, expanding its adaptability across diverse laboratory environments. The onboard touch screen meanwhile makes protocol creation simple and intuitive.
Maintaining the Agilent BioTek 406 FX Washer Dispenser is easy as a result of features such as the Ultrasonic Advantage, designed to effectively clean manifold tube build-up and prevent operational malfunctions. The product’s versatility and capabilities cater to a wide range of applications, from cell-based assays to ELISA, ELISpot and Meso Scale Discovery (MSD) workflows.
Millennium Science Pty Ltd www.mscience.com.au








Researchers from the National University of Singapore (NUS) and the Agency for Science, Technology and Research (A*STAR) have developed a novel sensor that enables the continuous, real-time detection of solid-state epidermal biomarkers (SEBs), a new category of health indicators.
Led by Assistant Professor Liu Yuxin from NUS and Dr Yang Le from A*STAR, the research team’s wearable, stretchable, hydrogelbased sensor offers a non-invasive method to monitor health by detecting biomarkers such as cholesterol and lactate directly on the skin. As described in the journal Nature Materials, it is a promising alternative for wearable, continuous and real-time health monitoring that could facilitate early detection of conditions such as cardiovascular diseases and stroke.
Monitoring biomarkers traditionally involves analysing biofluids such as blood, urine and sweat; while effective, these methods usually come with challenges. Blood tests are invasive and inconvenient, while urine analyses can be cumbersome and lack real-time capability. Probing biomarkers from sweat, though non-invasive, is limited by the difficulty of inducing sweat in inactive individuals and the discomfort of using sweat-inducing drugs. All of these pose barriers to the early diagnosis and treatment of diseases.
SEBs offer an alternative; these biomarkers are found in the sternum corneum, the outermost layer of the skin, and have shown correlations with diseases such as cardiovascular disease and diabetes. Detecting these biomarkers directly has been difficult. For instance, traditional solid electrodes lack the necessary charge transport pathways to enable electrochemical sensing of SEBs.
The researchers overcame this challenge with their novel sensor design; when the device is worn on the skin, SEBs dissolve into the ionic conductive hydrogel (ICH) layer, diffuse through the hydrogel matrix and undergo electrochemical reactions catalysed by enzymes at the junction between the ICH and electronically conductive hydrogel layer. Relevant physiological data is then transmitted wirelessly to an external user interface via a flexible printed circuit board, providing continuous monitoring capabilities. The sensor is produced using a scalable and cost-effective manufacturing process known as screen printing.
“Our novel hydrogel sensor technology is key to enabling the noninvasive detection of solid-state biomarkers on skin,” said Liu. “The ionic conductive hydrogel layer that solvates the biomarkers and the electronically conductive hydrogel layer facilitates electron transport. This bilayer enables the sequential solvation, diffusion and electrochemical reaction of the biomarkers. Another highlight is the sensor’s sensitivity, with biomarkers being detected precisely even in low amounts.”
“This wearable sensor is the first in the world that can monitor biomarkers on dry or non-sweaty skin,” added Yang. “The sensor’s novel bilayer hydrogel electrode interacts with and detects biomarkers on our skin, allowing them to become a new class of health indicators. The stretchable design enhances comfort and accuracy as well, by adapting to our skin’s natural elasticity. This innovation can change the way we approach health and lifestyle monitoring, particularly for those living with chronic conditions requiring constant health monitoring.”
In clinical studies, the sensor demonstrated strong correlations between the biomarkers detected on the skin and those found in blood samples. This validates the sensor’s accuracy and reliability, suggesting it could be an alternative to blood tests for monitoring chronic diseases such as diabetes, hyperlipoproteinemia and cardiovascular conditions.

The sensor comprises an ionic electronic bilayer hydrogel that can detect solid-state biomarkers from the skin.
The sensor is connected to a flexible printed circuit board which transmits data wirelessly to a user interface.
The sensor can also detect solid-state lactate and cholesterol at low levels; its sensitivity approaches that of mass spectrometry, which ensures the precise monitoring of these biomarkers. The sensor’s design also reduces motion artefacts, which occur when the user’s movements affect the placement of the sensor or its contact pressure to the skin, by three times compared to conventional counterparts.
“One of the possible applications of this technology is to replace the pregnancy diabetic test, commonly known as the glucose tolerance test,” Liu said. “Rather than subject pregnant women to multiple blood draws, our sensor could be used to track real-time sugar levels conveniently in patients’ homes, with a similar level of accuracy as traditional tests. This also can be applied to diabetes in general, replacing the need for regular finger-prick tests.”
“Another potential application is to use the sensor in the daily monitoring of heart health, as cardiovascular disease accounts for almost one-third of deaths in Singapore,” Yang said. “The research team has embarked on a research program to work closely with cardiologists in establishing clinical correlation between biomarkers — lactate, cholesterol and glucose — with heart health.”
The researchers plan to enhance the sensor’s performance by increasing its working time and sensitivity. They also aim to integrate additional solidstate analytes, broadening the sensor’s applicability to other biomarkers. The researchers are also collaborating with hospitals to provide additional clinical validation and bring the technology to patients, particularly for continuous glucose monitoring.
The Micropore Technologies Pathfinder series, using Advanced Cross Flow (AXF) mixing technology, has been designed to create nanoparticles efficiently and with minimal effort. While many turn to readily available lipids to assess size, PDI and encapsulation efficiency (EE), Micropore can encapsulate RNA beyond 99%.
The development of treatments is changing; research institutes and smaller biotech organisations are demanding scale much like traditional CMOs, CDMOs, lipid manufacturers and pharmaceutical companies across the globe. The Micropore Pathfinder and Horizon series encompasses both research and production requirements accommodating this emerging requisite.
The capacity to be customisable to a given formulation means the system can pivot to the formulation rather than changing the formulation to fit a technology. Additionally, the formulation conditions can scale on the same mixer from microlitres to litres of production — even thousands of litres.

The ever-increasing numbers of microfluidic mixers (MF) introduced to the market tend to be specific to a formulation construct; they can require months of research and development to make the required formulation work on the MF device. Another concern is that MF devices cannot scale efficiently; physics precludes it. There are a few that try altering the flow dimensions of the MF devices; however, this is limited in volume and frequently involves manipulation to the base formulation, potentially risking exposure to regulatory authority implications.
The Micropore Technologies Pathfinder range has packages that are suitable for all steps in the formulation journey. For those early in their campaign, the Pathfinder Pro 125 might suit the formulation discovery path. For those who already have a formulation and wish to transition to clinical trials, consider the Pathfinder Pro 250. Furthermore, a small Horizon system is suitable for GMP environments. The technology is flexible enough that there should be a fit for any paradigm.
ATA Scientific Pty Ltd www.atascientific.com.au
Plant & Food Research Rangahau Ahuma-ra
Kai — a New Zealand Government-owned research institute — is on a mission to improve the way food is grown, harvested, prepared and shared, and electron microscopy is a pivotal technology leveraged by the Microscopy and Imaging team in their research.
The team’s electron microscopy capabilities have now been improved with the installation of a TESCAN CLARA scanning electron microscope (SEM) with STEM (scanning transmission electron microscope) and EDS (energy dispersive X-ray spectroscopy) detectors in their microscopy facility in Auckland. This new instrument has consolidated all their electron microscopy requirements into a single instrument as well as opening up new possibilities.

The TESCAN CLARA is a field-emission ultrahigh-resolution SEM that is designed to image conductive, non-conductive and magnetic samples, making it suitable for imaging and analysis of a wide variety of materials. The system specifically came equipped with cryogenic and variable pressure imaging capabilities along with EDS for elemental analysis and mapping.
“When we decided to replace our aging SEM and TEM, the TESCAN CLARA with STEM detector ticked all the boxes,” said Ria Rebstock, Microscopy and Imaging team leader. “The increase in SEM resolution allows us to easily observe structures and answer questions we couldn’t previously.
“Using TESCAN’s BrightBeam column and beam deceleration technology enables us to image biological samples that would otherwise need coating to avoid charging, allowing us to accurately measure microscopic features such as nanocracks in starch. The addition of EDS has added new capability, which we have already leveraged for our own research, commercial clients and industry partners. The EDS software is seamlessly integrated in the TESCAN user interface and caters to beginners and experts alike.”
“The instrument is so easy to use, and I have been able to customise TESCAN’s Essence user interface streamlining our workflow,” added Andrew Chan, research scientist and primary instrument operator. “New users can easily be trained to operate the CLARA in less than an hour, even people with no prior SEM experience.

“We have already had success imaging a diverse range of specimens as part of our research, including plant material such as fruit skins, stems, leaves, microorganisms such as fungi, bacteria and viruses, insects and fish. We’ve also improved the reliability of our workflows for producing TEM-like images using the retractable four quadrant BSE (backscattered electron) detector.
“While the sample preparation process remains largely the same, we now use robust silicon wafers instead of delicate copper or nickel grids to mount sections. We then detect backscattered electrons, which provides a TEM-like image when the contrast is inverted. Using this technique, we no longer contend with grid bars obscuring parts of our sample!”
AXT is TESCAN’s distributor in New Zealand. The company’s Managing Director, Richard Trett commented, “To date, most TESCAN CLARAs are being used to characterise materials. It is exciting to see how truly versatile this SEM is, and how Plant & Food Research have used it so successfully to image biological specimens. It is equally as exciting to see how they have been able to harness the improved imaging capabilities of the system so quickly and we look forward to seeing their applied research being published in the near future.”
AXT Pty Ltd www.axt.com.au

The vision of people with a rare inherited condition that causes them to lose much of their sight early in childhood was anywhere from 100 to 10,000 times better after they received gene therapy to address the genetic mutation causing it, according to a new clinical trial co-led by the University of Pennsylvania and published in The Lancet
Atotal of 15 people participated in the Phase 1/2 trial, including three paediatric patients. Each patient had Leber congenital amaurosis as the result of mutations in the GUCY2D gene, which is essential to producing proteins critical for vision. This condition, which affects less than 100,000 people worldwide and is abbreviated as LCA1, causes significant amount of vision loss as early as infancy.
All subjects had severe vision loss, with their best measure of vision being equal or worse than 20/80 — meaning if a typicallysighted person could see an object clearly at 24 metres, these patients would have to move up to at least six metres to see it. Glasses provide limited benefit to these patients because they correct abnormalities in the optical focusing ability of the eye, and are unable to address
medical causes of vision loss, such as genetic retinal diseases like LCA1.
The trial tested different dosage levels of the gene therapy, ATSN-101, which was adapted from the AAV5 microorganism and was surgically injected under the retina. For the first part of the study, cohorts of three adults each received one of the three different dosages: low, mid, and high. Evaluations were held between each level of dosage to ensure that they were safe before upping the dosage for the next cohort. A second phase of the study involved only administering the high dosage levels to both an adult cohort of three and a paediatric cohort of three, again after safety reviews of the previous cohorts.
Improvements were noticed quickly, often within the first month, after the therapy was applied and lasted for at least 12 months. Three of six high-dosage patients who were tested to navigate a mobility course in varying levels of light achieved the maximum possible score. Other tests used eye charts or measured the dimmest flashes of light patients perceived in a dark environment.
“Even though we previously predicted a large vision improvement potential in LCA1, we did not know how receptive patients’ photoreceptors would be to treatment after decades of blindness,” noted lead author Artur Cideciyan, Co-Director of the Center for Hereditary Retinal Degenerations at the University of Pennsylvania.
Of the nine patients who received the maximum dosage, two had a massive 10,000-fold improvement in vision. According to Cideciyan, “That 10,000-fold improvement is the same as a patient being able to see their surroundings on a moonlit night outdoors as opposed to requiring bright indoor lighting before treatment. One patient reported for the first time being able to navigate at midnight outdoors only with the light of a bonfire.”
Primarily, the study sought to determine the safety of the gene therapy and its varying dosage levels. Researchers did find some patients had side effects, but the overwhelming majority were related to the surgical procedure itself. The most common side effect was conjunctival haemorrhage, the breakage of small blood vessels underneath the clear surface of the eye, which healed. Two patients had eye inflammation that was reversed with a course of steroids. No serious side effects were related to the study drug.
The work comes on the heels of another successful ophthalmological trial at the University of Pennsylvania restoring sight in patients with a different form of LCA. Earlier in 2024, CRISPRCas9 gene editing was used to improve the sight of many patients with a form of LCA tied to mutations in the CEP290 gene. Co-led by one of the new paper’s co-authors, Tomas S Aleman, the study used similar tests and was the first time children were involved in any gene-editing work.
“The treatment success in our most recent clinical trials, together with our earlier experience, brings hope for a viable treatment for about 20% of infantile blindness caused by inherited retinal degenerations,” said Aleman, who is also Co-Director of the Center for Hereditary Retinal Degenerations.
“The focus now is on perfecting the treatments and treating earlier manifestations of these conditions once safety is confirmed. We hope similar approaches will lead to equally positive outcomes in other forms of congenital retinal blindness.”
Moving forward, approval of this experimental medicine for clinical use requires another trial, where participants are randomised to a treatment dose and both patients and those investigating the trial not knowing who gets what. Through that, any possible bias in results could be avoided.

Cayman Chemical’s Microbiome Metabolite Screening Library consists of three plates and contains approximately 140 metabolites of gut microbiota in a 96-well Matrix tube rack format as 10 mM stock solutions in DMSO except for short-chain fatty acid (SCFA) metabolites, which are provided as 1 M stock solutions. The library includes microbiome metabolites categorised as amino acid derivatives, aromatic compounds, hydroxy and branched-chain fatty acids, sphingolipids and nucleotide metabolites, and microbial metabolites of natural products obtained via the diet. Stability data is not available for individual compounds as supplied in the screening library. Panels are routinely re-evaluated to include new catalogue introductions as the research evolves.
Sapphire Bioscience www.sapphirebioscience.com
Cell Biosciences has launched the Biolog PreBioM MicroPlate product line for microbiome analysis and organism selection. The microplates are designed to enable phenotypic characterisation of individual organisms and communities typically found in the gut microbiome, providing insights into microbial behaviour and interactions and allowing researchers to study and produce new probiotics.
The microplates feature a curated selection of prebiotic substrates, ranging from simple sugars to complex dietary fibres. The diverse array of substrates, pre-arrayed on the plates, offers a powerful tool for interrogating the influence of different prebiotics on microbial growth and activity.
When used in conjunction with Biolog’s Odin platform, the microplates enable functional characterisation of living communities of organisms, with data analysis tools that mine the richness of kinetic data, providing insight into how diverse populations change over time or in response to various treatments. Cell Biosciences Pty Ltd www.cellbiosciences.com.au
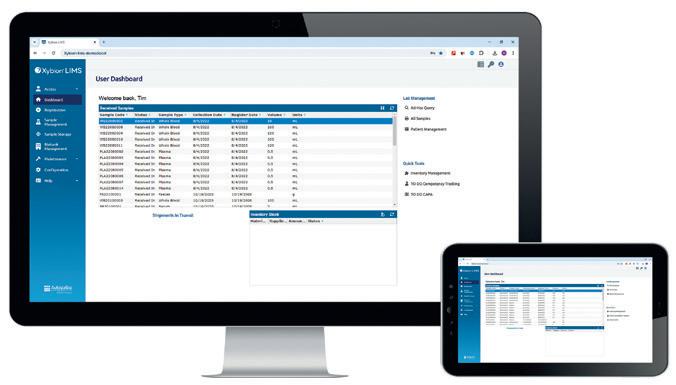
Exum Instruments has released its Massbox Laser Ablation, Laser Ionisation Time of Flight Mass Spectrometry (LALI-TOF-MS) device, featuring patented technology that provides chemical analysis with trace element sensitivity, overcoming the limitations of other elemental analytical techniques to deliver results in minutes via a user-friendly, intuitive interface. Developed by Exum Instruments founder Jeff Williams, LALI-TOF-MS can take any solid material and rapidly identify the full periodic table of its constituents.
Packaged into a compact, desktop instrument, the Massbox delivers quantitative analysis down to trace level for all elements in the periodic table; elemental mapping on areas up to 83 x 83 mm down to 5 µm resolution; depth profiling for analysis of surface modifications, with the system’s laser typically removed tens of nanometres per shot; and rapid screening, allowing users to identify unknown materials or detect trace elements in just seconds. The instrument can be applied to fields such as materials science, battery research, chemistry, and environmental and earth science.
AXT Pty Ltd www.axt.com.au

Autoscribe Informatics’ Xybion LIMS has been specifically designed for biobanks and biorepositories. The product provides a suitable platform to manage and track biospecimens, so that laboratories can collect, store and process samples with ease. The LIMS supports ISBER Best Practice recommendations and complies with stringent regulatory requirements.
Whether collecting organ tissue, blood, urine, skin cells or other samples, the LIMS offers a comprehensive solution for managing the collection, processing and storage of specimens. It enables compliant recording of test data and interpretive information, analysis of results, and distribution of data and findings to stakeholders. It is designed to offer quality, integrity, efficiency, security and regulatory compliance in every aspect of biobanking operations.
Autoscribe Informatics Pty Ltd www.autoscribeinformatics.com.au

Scientists at St. Jude Children’s Research Hospital and Washington University School of Medicine say they have discovered why the flu vaccine can perform poorly, having found that a specific type of immune cell indirectly controls the anti-influenza response. These helper cells often ‘see’ the wrong parts of the virus, likely leading to immunity that is less effective.
Scientists try to adapt the vaccine annually to account for the new surface proteins on the dominant flu strains, while the rest of the formulation contains the virus’s internal proteins and remains constant. But while previous research has hinted at why the flu vaccine has such varied effectiveness, this study — published in the journal Nature Immunology — provides a much clearer mechanism.
Lack of immunity brought to light The ideal immune response against the flu involves antibodies. Antibodies bind to targeted proteins to both prevent their function and attract immune cells. B cells make antibodies, but they do not decide what to target. A different group of immune cells, called T follicular helper cells, activate B cells in lymph nodes. This is the first known study to offer a high-resolution perspective of helper cells taken from the lymph nodes of people who received the flu vaccine, revealing new insights into how these T cells do or do not direct effective immunity to influenza.
“Most studies look at blood, which is easy to sample but not where the main T-cell response happens,” said co-first author Stefan Schattgen, PhD, from the St. Jude Department of HostMicrobe Interactions. “We were able to get a better view by looking at lymph nodes directly over time.”
To look at the immune response to vaccination, the scientists examined lymph node samples from a small group of study participants over the course of two years. The researchers identified the pieces of viral protein to which the T follicular helper cells responded, revealing their preference for internal proteins.
“We found that a large group of T follicular helper cells are responding to the same proteins that they see every year, instead of targeting the surface proteins that we update and want them to target,” said senior co-corresponding author Paul Thomas, PhD, from the St. Jude Department of Host-Microbe Interactions.
The researchers documented how the immune cells in lymph nodes respond to the vaccine, which contrasted with prior research and revealed a new piece of the mechanism underlying poor vaccine performance.
“In blood we only see a small, quick blip of an anti-flu T-cell response, but in lymph nodes, it
can stay for months,” Schattgen said. “This ‘locks in’ the anti-flu immunity. So, if we start with an immune response against the wrong target, it can stay ‘stuck’ on that internal protein instead of the novel surface proteins.”
Together, the findings show that annual influenza vaccine performance can be hindered by an immune response against the wrong proteins; those in the vaccine that are conserved from year to year. Keeping that incorrect immunity longterm prevents the body from creating new, more effective antibodies. This mirrors a well-known immunological phenomenon called antigenic original sin or imprinting, when a person mounts an inappropriate immune response to an influenza infection due to a previous flu exposure.
“We’re finally getting at the underlying mechanism of what antigenic original sin actually is,” Thomas said. “It was advanced by Robert Webster many years ago that the process involved B cells. We’ve now shown T follicular helper cells are likely part of the imprinting process that commits B cells to recall a memory response preferentially.”
With the knowledge of what makes the influenza vaccine less potent in some people, the researchers hope to find ways to use that new understanding to increase vaccine efficacy.
“In the current standard flu vaccine, we have this mix of other proteins that we may not need to be in there,” Thomas said. “New vaccine formulations should focus the T-cell response on just the surface proteins. An easy way to do that is by only giving immune cells those surface proteins to look at, by removing those other offtarget peptides.”
25th Congress of the Australian Institute of Physics (AIP)
December 2–5, Melbourne
The 2024 AIP Congress will mark 50 years since the first Congress, with the biennial event regarded as the largest regular meeting of physicists in Australia. It will this year be co-located with the 17th International Conference on Near-field Optics, Nanophotonics, and Related Techniques (NFO-17), the 2024 Australian and New Zealand Conference on Optics and Photonics (ANZCOP), and the 2024 Conference on Optoelectronic and Microelectronic Materials and Devices (COMMAD), as well as a number of superb satellite conferences. The combined event will bring together those from a range of career stages working across the full breadth of the physical sciences and technology, in research, education, government and industry. This anniversary event will feature a rich program of plenary lectures, parallel specialised sessions, poster sessions, an exhibition, special events, industry events and a gala conference dinner. aipcongress2024.com

ANBUG-AINSE Neutron Scattering Symposium 2024
November 4–6, Lucas Heights www.ansto.gov.au/whats-on/aanss2024-anbugainse-neutron-scattering-symposium-2024
Acoustics 2024 — Acoustics in the Sun November 6–8, Gold Coast www.acoustics2024.org.au
12th International Conference on Environment Pollution and Prevention November 8–10, Brisbane www.icepp.org
AIMS NSW North Coast Div Conference 2024 November 8–10, Port Macquarie www.aims.org.au/Web/Web/Events/UpcomingEvents.aspx
Quantum Thermodynamics
Down Under 2024 (QTDU2024) November 12–15, Brisbane qtdownunder2024.com
Energy Oceania 2024
November 13–15, Melbourne www.energyconferenceaustralia.com
AgCatalyst 2024
November 19–20, Brisbane www.csiro.au/en/work-with-us/industries/ agriculture/AgCatalyst-2024
2024 AAMRI Convention and Dinner November 27–28, Canberra aamri.org.au/news-events/events/2024-aamriconvention/
International Conference on Research Infrastructures
The 8th Asia-Pacific Conference on Unsaturated Soils
December 4–6, Melbourne www.ap-unsat2024.com
Hendra@30 Henipavirus International Conference December 8–11, Geelong www.hendra30.com
EQUS Annual Workshop 2024 December 11–13, Noosa equs.org/events/equs-annual-workshop-2024
Lorne Proteins 2025 February 9–13, Lorne www.lorneproteins.org
Lorne Cancer 2025 February 13–15, Lorne www.lornecancer.org
Lorne Genome 2025 February 16–19, Lorne www.lornegenome.org
Lorne Infection & Immunity 2025 February 19–21, Lorne www.lorneinfectionimmunity.org
Pathology Update 2025 February 21–23, Melbourne www.rcpa.edu.au/Events/Pathology-Update
Positioning Hydrogen 2025 Conference April 2–5, Melbourne hydrogenconferenceaustralia.com
AusMedtech 2025 May 7–8, Sydney www.ausmedtech.com.au
AUS-oMicS 2025 May 18–21, Cairns www.ausomics.com
December 3–5, Brisbane and online icri2024.au Tell the world about your event: email LLS@wfmedia.com.au
Westwick-Farrow Media
A.B.N. 22 152 305 336 www.wfmedia.com.au
Head Office
Unit 5, 6-8 Byfield Street, (Locked Bag 2226) North Ryde BC NSW 1670, AUSTRALIA Ph: +61 2 9168 2500
Editor Lauren Davis LLS@wfmedia.com.au
Publishing Director/MD Janice Williams
Art Director/Production Manager Linda Klobusiak
Art/Production Marija Tutkovska
Circulation
Alex Dalland circulation@wfmedia.com.au
Copy Control Ashna Mehta copy@wfmedia.com.au
Advertising Sales
Sales Manager: Kerrie Robinson Ph:0400 886 311 krobinson@wfmedia.com.au
Ben Calvo Ph: +61 2 9168 2516 bcalvo@wfmedia.com.au
Tim Thompson Ph: 0421 623 958 tthompson@wfmedia.com.au
If you have any queries regarding our privacy policy please email privacy@wfmedia.com.au













Plant basedfoods such as meat allternative

Plant-based foods, such as meat alternatives, are becoming increasingly popular due to growing health and environmental concerns. There are many plant-based alternatives to other animal products, such as cheese, milk , yogur t, butter, and more.
The meat alternative market is experiencing rapid growth, and the role of proteins and fats in food production is crucial. The increasingly popular products are regularly assessed not only for their taste and texture, but also for their nutritional value.


KjelMaster K-375 & KjelSampler K-376 / K-377
Perform potentiometric and colorimetric titrations with the KjelMaster K-375 and a free choice of methods at perfect usability.
Gain high throughput, more automation and sophisticated data management for convenient nitrogen and protein analysis.
Autosampler: either 24 or 48 sample positions
S ample Pa rameters: Total Kjeldahl Nitrogen, Total Volatile Basic Nitrogen, nitrate, nitrite


The FatEx trac tor E-500 is designed for quick and compliant fat ex traction.
R eadily adapt your Fa tEx trac tor E-500 to changing needs with the interchangeable glass assembly and execute ex trac tions according to Soxhlet, Randall or Twisselmann.
Extraction methods:
Soxhlet, Hot extraction, Continuous extraction
Solvents: Petroleum ether, Hexane, Chloroform


ProxiMate, a NIR instrument for the harshest environments, is designed for the food and feed industr y.
T he compac t system can be operated anywhere by anyone thanks to a simple interface and pr e- calibration pack ages that ensur e speed and quality
Ingress protection: IP69
Sample presentation: Up and Down
Wavelength: NIR + VIS