Newsletter February 1, 2023 Issue 1 Encapsulated
Featuring Q&A WITH DR. BRIAN JERMAIN RESUME TIPS & TRICKS INTERNSHIP POSTINGS
ROLES & JARGON
Creators Ange Lu James Sagun Kristina Rodrigues Eli Carbo Ontiveros
INDUSTRY
Table of Contents 3 Mission Statement 4 About Us 8 A Dose of 2022: Industry Updates 10 Definition of Industry Roles and Jargon 12 Introduction to Industry 17 Q&A with Dr. Brian Jermain 26 How to Write a Resume 30 Crossword Puzzle 33 Internship Postings 33 Suggestions
Mission Statement
Encapsulated is an independent, student- run organization committed to providing industryrelated resources, knowledge, and connections for students. We are dedicated to bridging the gap between students and the pharmaceutical industry and creating opportunities for internships and fellowships alike. We hope to encapsulate the field into an easily accessible and digestible format.
Kristina Rodrigues
Co-Founder, Lead Writer
Hi, my name is Kristina Rodrigues, and I’m from Memphis, TN. I am a second-year PharmD student at The University of Texas at Austin College of Pharmacy! I also completed my undergraduate degree in Biology at UT, so I’m excited to be continuing my journey here!

Growing up, I was always involved in science, whether it was dinner table conversations with my parents or doing research in oncology and pharmaceutic labs. After gaining hands-on experience, I learned I have a passion for innovative drug delivery and am eager to grow my knowledge within the Research & Development area of pharmaceutical industry. I’m currently interested in drug development, clinical pharmacology, as well as pharmacometrics.
When I’m not in the classroom or lab, I enjoy anything art-related, such as painting, writing, and playing the violin. These hobbies allow me to be creative, just as I am with my research. I am hoping to further my knowledge and skills during my PharmD career to inspire others who are interested in the industry route.
Ange Lu
Co-Founder, Lead Editor
Hello, my name is Ange Lu, and I’m from Boston, Massachusetts. I completed my pre-pharmacy program at the University of Texas at Austin, and I am now a secondyear student pharmacist at the university’s College of Pharmacy.

Science has always been a tool to help me understand the world and its surroundings. As I grew older, I gained research experience and developed a passion for sharing my knowledge with others through engaging presentations and clear communication. My current area of interest lies in Medical Affairs, specifically in medical science liaisons, medical communications and health economic and outcomes research.
During my free time, I love to create, whether it’s building, drawing, playing an instrument, or taking photographs!
James Sagun
Co-Founder, Lead Writer
Hello! My name is James Sagun, and I was born and raised in Houston, Texas. Currently, I am a second year PharmD student at the University of Texas at Austin College of Pharmacy. Prior to joining the professional program, I completed the prepharmacy program here at UT.

Ever since my undergraduate career, I’ve accumulated a lot of experiences within research and development. From this, my areas of interest within industry include clinical pharmacology, pharmacometrics, and clinical development.
Outside of class, I like to rock climb at Austin Bouldering Project and occasionally venture outdoors in the many bouldering spots in Austin!
Eli Carbo Ontiveros
Co-Founder, Lead Editor
My name is Eli Carbo Ontiveros, and I was born in Mexico City, Mexico. I grew up in Toronto, Canada and now I am a second year PharmD student at The University of Texas at Austin College of Pharmacy! Having lived in different countries has given me a lot of insight into how different healthcare systems work and motivated me to pursue a role with a global impact.

Some of my favorite things to do in my spare time are learning languages and playing with my Siberian husky. Having grown up in different countries, I have had the opportunity to learn 6 languages, and I hope to one day use my linguistic skills in my daily work.
The areas I’m interested in are regulatory affairs and clinical operations. Both of these areas require international communications and have a large-scale impact. I hope that one day, this will allow me to travel to other countries and get to experience different cultures while also using my science skills.
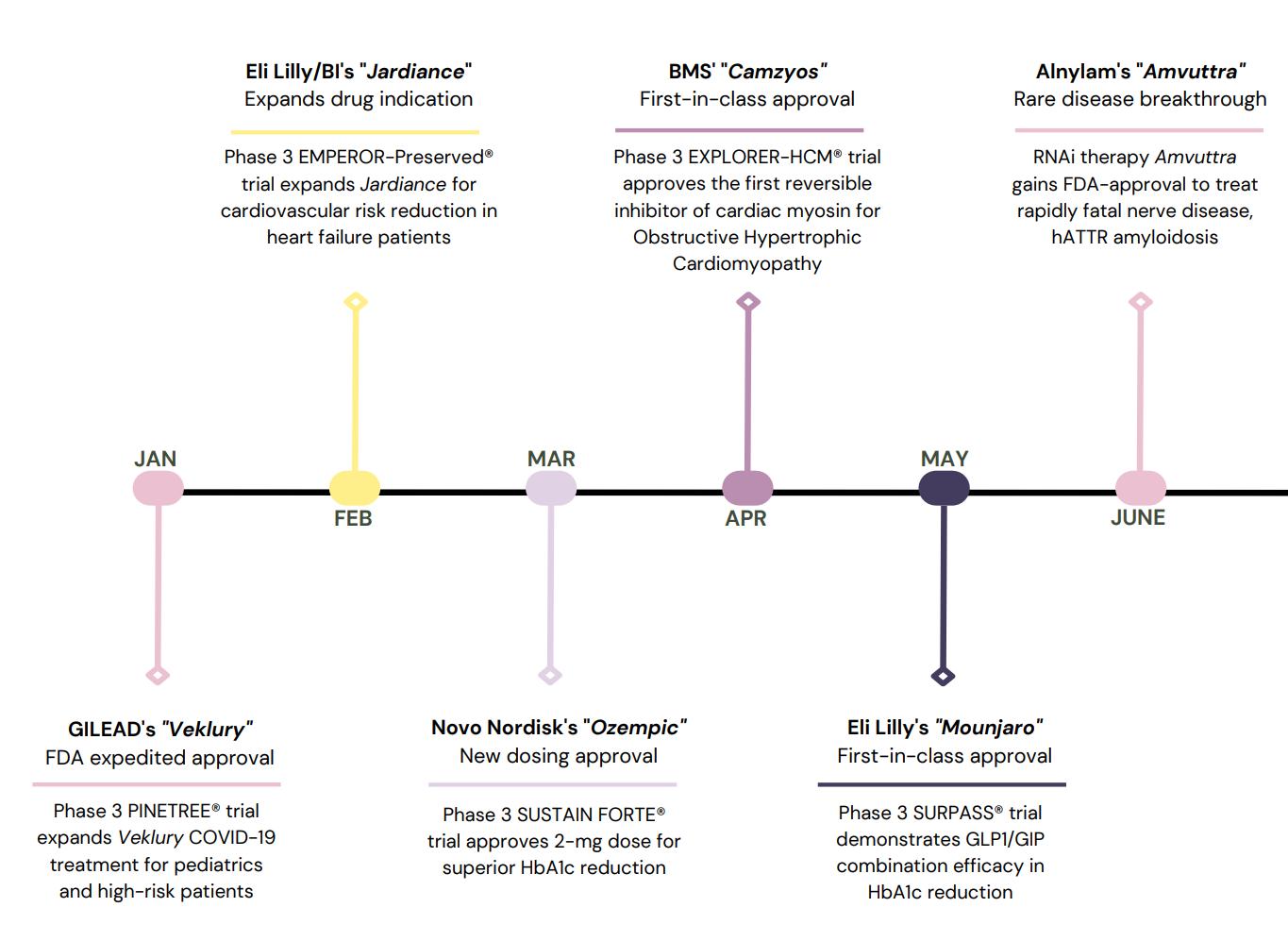
A Dose of 2022


Industry Jargon
Chemistry, Manufacturing, and Controls
(CMC)
Innovator Drug
The chemical composition of the drug, the manufacturing process, and the tests developed to ensure its effectiveness and consistency.
A new brand-name drug that is first made and marketed by a pharmaceutical company. Innovator drugs are usually protected by patents, which give the company that developed the drug exclusive rights to sell it for a certain period of time.
Pipeline Drugs
Drugs in the development stages, but not yet approved the FDA. The term can also be used to refer to drugs that are already approved by the FDA but are still in the process of being marketed and sold.
Key Opinion Leader (KOL)
A well-respected person with experience and expertise in a particular field. In healthcare, these leaders could be physicians, hospital directors, researchers, and people who make clinical guidelines.
Payer A payer is any entity that pays for the use of healthcare services or products delivered. The term usually refers to health insurance companies. Payers determine how patients get access to drugs through their insurance, and how providers get paid for their services.
Claims Data
In HEOR, claims data refers to information collected from insurance claims and other data sources. It includes information on patient demographics, diagnoses, treatments, and costs. It is often used evaluate the effectiveness and cost-effectiveness of healthcare decisions and policies.
Industry Jargon
Endpoint
Event or outcome measured by a clinical trial that is used to find out whether the drug being studied is beneficial
Contract Research
Investigational New Drug (IND)
New Drug Application (NDA)
Biologics License Applications
Code of Federal Regulations (CFR)
Good Clinical Practice (GCP)
An outside company that offers clinical trial, manufacturing, and testing services, usually to larger pharmaceutical companies
An application to the FDA to begin testing a new drug in humans. It includes study data and a clinical trial plan (protocol)
An application to the FDA to get approval to market a new drug in the United States. It includes study data and information on the drug’s safety and effectiveness.
An application to the FDA to market a biological product. It includes information on the product’s safety, effectiveness, and how it is made.
Rules set by the FDA to ensure that drugs are made and controlled in a way that guarantees their strength, quality, and purity.
An international set of regulations that ensures the results and process of a clinical trial are reliable so that patients are protected
When learning about the pharmaceutical industry, it’s easy to get lost in the many acronyms and jargon in presentations, job applications, and resources. As a student, I’ve felt the need to compile a list of some of key pharmaceutical industry definitions to help you better understand an area in the industry.
Introduction to Industry: Functional Areas
In the following pages, the main industry functional areas are briefly introduced with their subgroups and related departments:
The “Branches” of Medical Affairs
The “Umbrella” of Regulatory Affairs
The “Protocols” of Clinical Development
The “Phone” of Commercial and Marketing
Medical Affairs Tree
There are multiple subgroups within medical affairs which includes medical information, medical science liaisons (MSLs), medical communications and publications, and more. This is a diverse branch of industry where many PharmDs gravitate to because they share clinical and scientific advancements to the medical community and guide company direction.
MSLs conduct field visits to share knowledge of the disease and therapeutic knowledge with Key Opinion Leaders (KOLs). Their role fills any gaps in disease state development, and they relay key information to the industry to guide clinical trial direction.
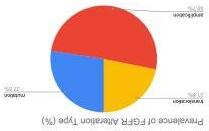
Health Economics and Outcomes Research (HEOR) utilizes pharmacoeconomic data to evaluate the cost-effectiveness of the investigational or approved drug. These researchers work closely with other functional areas like market access and commercial to ensure the treatments are affordable.
Details that HEOR departments consider within their research include real world evidence (RWE), patient-reported outcomes (PROs), claims data, and electronic health record (EHR/EMR) information. With this information, pharmaceutical companies can create contracts with insurance companies and hospitals to include a drug to their formulary.
Medical Science Liaisons 01 02 Medical Information 03 Health Economics and Outcomes Research 04 Medical Communication
The regulatory affairs functional area is one that every company in the pharmaceutical industry must have. Their primary goal is to get the drugs or devices approved by a regulatory agency such as the FDA in the U.S. or EMA in Europe.
This department is responsible for submitting a new drug application (NDA), other necessary documents, and clinical trial data to the regulatory agency.
The pharmaceutical company's regulatory affairs department must work closely with all functional areas to approve outgoing communications and get any necessary data ready for submission. They must also comply and respond to any requests or inquiries the FDA may have regarding their submissions.
The top skills to have in regulatory affairs include knowledge of regulatory guidelines and regulations, excellent project management, strong written and verbal communication, and strong analytical and problem-solving skills
Global Affairs 01 02 Drug Safety 03 Medical Writing 04 Labeling 05 Advertising/ Promotion 06 Strategy
Regulatory Affairs Umbrella
Clinical Operations
Clinical Development
PROTOCOL No. - NCAP-10312022-A




NCT Identifier: “ENCAPSULATED”- Phase 3a

Principal Investigator(s): J.E, K.A
Study Duration: August 2021 - May 2025

Objective: To describe the functional area of Clinical Development

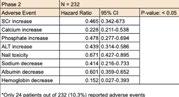




Clinical Development refers to the process of researching, testing, and bringing a new drug to market. This process typically begins with preclinical research, where scientists study the drug's safety and efficacy in pharmacokinetic and pharmacodynamic models.
If the preclinical studies are promising, the drug enters human testing through a series of clinical trials divided into three phases: Phase 1, Phase 2, and Phase 3. After researchers assess patient populations, trial endpoints, and trial eligibility to show safety and efficacy, the drug can be submitted to regulatory agencies such as the FDA for approval to be marketed and sold.


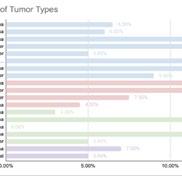

The top skills to have include strong project management, attention to detail, communication, trial rationalization and data analysis, and knowledge of clinical research regulations and guidelines.
Pre-Clinical Development Page 5
Page 4
Page 3
Research & Development
Clinical Pharmacology/Pharmacometrics
Page 2
Page 1
Commercial Departments


Sales Business Development Marketing Market Access







Within the commercial functional area, there are multiple subgroups including marketing, market access, sales, business development, and many more. These areas handle major cost-related decisions and are responsible for the advertisement, promotion, and accessibility of the drug product.



Here, knowledge of payer -based systems, economic strategies, and formulary processes are important in order to have the drug be prescribed and dispensed to patients. This functional area is key for maintaining communication and professional relationships between healthcare providers, KOLs, patients, payers, and the company.
The top skills to have include strong collaborative and entrepreneurial skills, problem solving, and strategic thinking.
4
23
Q&A with Dr. Brian Jermain
Currently, Dr. Jermain is a Pharmacometrician at Pfizer and has been with the company since August 2021. After graduating from the University of Texas at Austin with his PharmD, he completed a two -year postdoctoral fellowship in Pharmacokinetics/Pharmacodynamics (PK/PD) at the University of North Carolina Chapel Hill.
 Dr. Jermain
Dr. Jermain
Tell me what your typical workday is like as a Pharmacometrician?
A typical day as a Pharmacometrician is variable. For the most part, there are around 2-3 hours of meetings to discuss PK analysis or any data generated. The remainder of the day is largely academic, where I’m strictly doing data analysis and creating the PK/PD models using R, NONMEM, and other programs. Other times, I’m reading literature to better understand the modeling landscape and other background research needed.
As a Pharmacometrician, we work closely alongside Clinical Pharmacologists to use the PK/PD data to provide insight and lead strategy in terms of drug dosing, drug interactions, and protocol development.


What soft skills would a PharmD need to be successful in a Pharmacometrics role or industry, in general?




The two soft skills I find important are communication and flexibility. Oftentimes, you need to translate the niche terminology of your project so that your audience can understand why it’s important and what your project can accomplish. Since you also work in a multidisciplinary team, communication is needed to be able to work together effectively. In these teams, people may often not understand the full extent of your abilities, so it’s your responsibility to understand your role in the group and voice where you can provide value.
It’s also good to be flexible, especially with time and your goals. There are times the FDA requires an immediate submission of additional work, which can disrupt your carefully planned schedule. I remember to keep cool and acknowledge that things come up and change. In industry, change is the norm, so it’s good to be adaptable.
What projects did you work on during pharmacy school? How did you prepare for those and make the most out of it?

I was more involved in organizations and leadership than I was in any research projects. There weren’t any opportunities to do PK-related research at the time, so I mainly taught myself and gained new skills that were applicable to my career. For instance, I learned R before applying to pharmacometric-related fellowships.




I did have APPE rotations at Pfizer in Medical Affairs and at PPD (Pharmaceutical Product Development) in clinical trials. My other APPE projects included helping a pharmacy resident at Baylor Scott and White with an infectious disease-related review.

In terms of maximizing your experiences, I took on more challenges and established clear goals of what I wanted to get out of it. The more experiences you get in your rotations, organizations, or life in general, the more you can speak about during your post-graduate interviews. You really just have to put yourself out there, even if it’s awkward at first.

The quicker you can identify something you’re interested in, the better since you can challenge yourself and get out of your comfort zone. You’ll never have all the answers to everything, so don’t be afraid to ask for help along the way. There’s a lot less external pressure than you think!



What was your fellowship application process like? What makes a good candidate for these cycles?
My fellowship interviews were in person at Midyear, though some of them are hybrid now. Midyear would happen the first week of December and you would expect any offers after the new year.

My advice is to do the background research of the fellowships you’re applying to and talk to current fellows to see if you can envision yourself happily in those positions. There’s so many functional areas, so hone in on the program, company, and area you’re interested in.






Once you’re at the interview, be prepared for any situational and behavioral-based questions. Towards the end, ask meaningful questions that you cannot find online to show that you’re invested in the position. Be comfortable presenting since, if you make it far enough, you may do a 10-20 minute presentation over a project or something related.
The best thing to do is to be genuine and be able to speak about your experiences that show you have aspirations and are genuinely invested in what you do. Overall, it’s really nice to see a genuine applicant that finds a way to be themselves, especially during a stressful interview.
After you meet with the team and the company, I would rank your fellowships based on where you’re going to be most supported, since your team and manager can dictate your experiences. At the end of the day, industry experience is industry experience regardless if it’s at a big or small company, so rank the environments you see yourself best working in the highest.
For those who did not receive a fellowship this application cycle, what is your best advice for them if they are still interested in pursuing industry?
Depending on the company, there are certain positions and opportunities available for graduates without a fellowship. You have to use your network and IPhO to find those positions and ensure it matches with your abilities and skills. Getting into industry is the hardest part since, although positions may be available, that doesn’t guarantee you’d make a good match.

Though once you get your foot in the door, it’s easier to move around. You’ll have conversations with your manager about career aspirations, and you should choose a company that values your goals. You’re probably not going to be in the same role for the rest of your life, so choose a company that will support your growth and future plans.
As young leaders, we are often encouraged to say “yes” to opportunities. When was a time you said “yes” to a big risk in your career? Do you have any advice on how to graciously say “no?”
Telling people “no” politely is important because you don’t want to get trapped under unreasonable expectations. My mom advised me that rather than just leaving it at “no,” offer alternate options on what you can do instead. You should be able to compromise and meet in the middle.




My biggest risk was taking on leadership positions. I tend to be more reserved, but you learn different types of leadership when you put yourself out there. The more time you spend in these positions, the more comfortable you’ll be contributing in meetings and leading discussions. Leadership is not always steering conversations but contributing where you can provide value, so you can understand the skill of stepping both in and out of the leadership role. When it comes to gaining a position, you don’t have to shoot for the highest one, like President or VP. Get involved, even if it’s in a subgroup, to gain that experience and get comfortable with leadership.
How did you use your leadership experience to navigate any obstacles and establish yourself as an effective leaders within Pfizer today?



With my positions in Phi Delta Chi, IPhO, and Rho Chi, I learned how to balance many things at once. I had to be flexible in order to get everything done, and the biggest skill that helps me today is planning ahead. Even if your schedule changes due to something spontaneous, you would have developed the skills to handle everything effectively. Aside from this, you must be vigilant of areas that need change. Understand where you can make an impact!

Being reliable, effectively collaborating with others, and building interpersonal relationships are specific strengths that can help you get a lot further than you think. The better you get to know others and be more personable with them, the easier it is to lead. If you don’t spend time building interpersonal relationships, it’s harder to make an impact since people are more likely to collaborate with you if you know them personally.
For instance, I don’t meet a lot of my coworkers in person, but I’ve had more impact with the teams that I’ve gotten to know more about on a personal level despite having virtual meetings.




What advice would you give to yourself as a student and to others, especially if they aren’t able to obtain early experiences like internships, research, and shadowing?
I would’ve loved to get early experiences within Pharmacometrics and possibly circumvent doing a fellowship, but I found out my interests late in P3 year and started learning R in my P4 year in preparation of fellowship applications. If there’s anything you’re interested in, start finding resources and experiences early. These experiences don’t necessarily have to be related to the field you’re interested in, it’s just to gain more knowledge and transferable skills.

I would tell myself to get more involved in organizations and be less worried about classwork. Though it is important to understand the materials and not slack off completely, I would’ve rather gained those experiences and not over prepare for exams.
Overall, just do things that show you’re a go-getter- it doesn’t necessarily have to be learning a coding language. By doing something you’re ambitious about, you’re able to genuinely speak about it, and people appreciate that.




Lastly, what is your biggest accomplishment during your career at Pfizer? What legacy do you want to leave behind?
My biggest accomplishment is that I worked on the analysis to support the pediatric submission for Bosutinib where we had to determine dose selection for pediatrics.



I would like people to think of me as someone that was enjoyable to work with. At the end of the day, work is work, so it’s important to enjoy the people you work with. I’d rather be considered someone fun than the smartest person in the room.
brian.jermain@pfizer.com




Industry Jargon
Crossword Puzzle

3 Team that ensures patients get the proper treatment and benefit from maintained access to drugs at the right price
6 Team that detects, assesses, and prevents drug safety -related problems
9 A trusted, well-respected influencer with proven experience and expertise in a particular field
10 International ethical and scientific quality standard for designing, conducting, recording, and reporting trials involving human subjects’ participation
11 Code of federal regulations
12 Drugs under development by a manufacturer
14 Primary outcome measured by a clinical trial
DOWN
1 Group of people who act as a liaison between the government, the company, and consumers to help ensure that products are safe and effective, and all laws are followed
2 Detailed plan of a scientific experiment, treatment, or procedure. In clinical trials, this states what the study will do, how it will be done, and why it will be done
3 An area-based medical affairs professional whose primary responsibility is interacting with influential healthcare providers
4 An outside supplier or vendor that offers contracted clinical trials, manufacturing, and testing services
5 The department within a pharmaceutical company that communicates accurate information to healthcare providers
7 A field of research that uses economic data to analyze the value of health interventions, including pharmaceuticals
8 A request from a sponsor to obtain authorization from the FDA to administer an investigational drug or biological product to humans
13 A committee that applies research ethics by reviewing the methods for research to ensure that they are ethical
ACROSS
Crossword Puzzle Answer


INTERNSHIP POSTINGS


APPLY NOW! SEND A SUGGESTION




































 Dr. Jermain
Dr. Jermain













