1 minute read
Medical Affairs Tree
There are multiple subgroups within medical affairs which includes medical information, medical science liaisons (MSLs), medical communications and publications, and more. This is a diverse branch of industry where many PharmDs gravitate to because they share clinical and scientific advancements to the medical community and guide company direction.
MSLs conduct field visits to share knowledge of the disease and therapeutic knowledge with Key Opinion Leaders (KOLs). Their role fills any gaps in disease state development, and they relay key information to the industry to guide clinical trial direction.
Advertisement
Health Economics and Outcomes Research (HEOR) utilizes pharmacoeconomic data to evaluate the cost-effectiveness of the investigational or approved drug. These researchers work closely with other functional areas like market access and commercial to ensure the treatments are affordable.
Details that HEOR departments consider within their research include real world evidence (RWE), patient-reported outcomes (PROs), claims data, and electronic health record (EHR/EMR) information. With this information, pharmaceutical companies can create contracts with insurance companies and hospitals to include a drug to their formulary.
The regulatory affairs functional area is one that every company in the pharmaceutical industry must have. Their primary goal is to get the drugs or devices approved by a regulatory agency such as the FDA in the U.S. or EMA in Europe.
This department is responsible for submitting a new drug application (NDA), other necessary documents, and clinical trial data to the regulatory agency.
The pharmaceutical company's regulatory affairs department must work closely with all functional areas to approve outgoing communications and get any necessary data ready for submission. They must also comply and respond to any requests or inquiries the FDA may have regarding their submissions.
The top skills to have in regulatory affairs include knowledge of regulatory guidelines and regulations, excellent project management, strong written and verbal communication, and strong analytical and problem-solving skills
Clinical Operations
Clinical Development
PROTOCOL No. - NCAP-10312022-A
NCT Identifier: “ENCAPSULATED”- Phase 3a
Principal Investigator(s): J.E, K.A
Study Duration: August 2021 - May 2025
Objective: To describe the functional area of Clinical Development
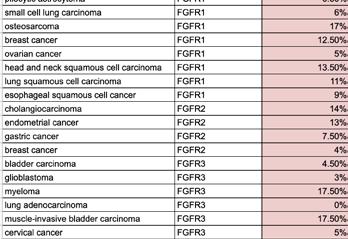
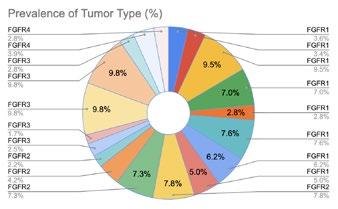
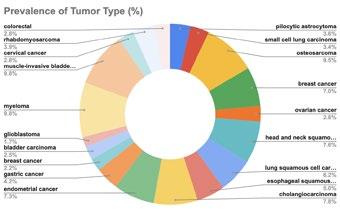
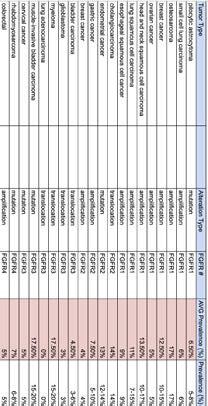
Clinical Development refers to the process of researching, testing, and bringing a new drug to market. This process typically begins with preclinical research, where scientists study the drug's safety and efficacy in pharmacokinetic and pharmacodynamic models.
If the preclinical studies are promising, the drug enters human testing through a series of clinical trials divided into three phases: Phase 1, Phase 2, and Phase 3. After researchers assess patient populations, trial endpoints, and trial eligibility to show safety and efficacy, the drug can be submitted to regulatory agencies such as the FDA for approval to be marketed and sold.
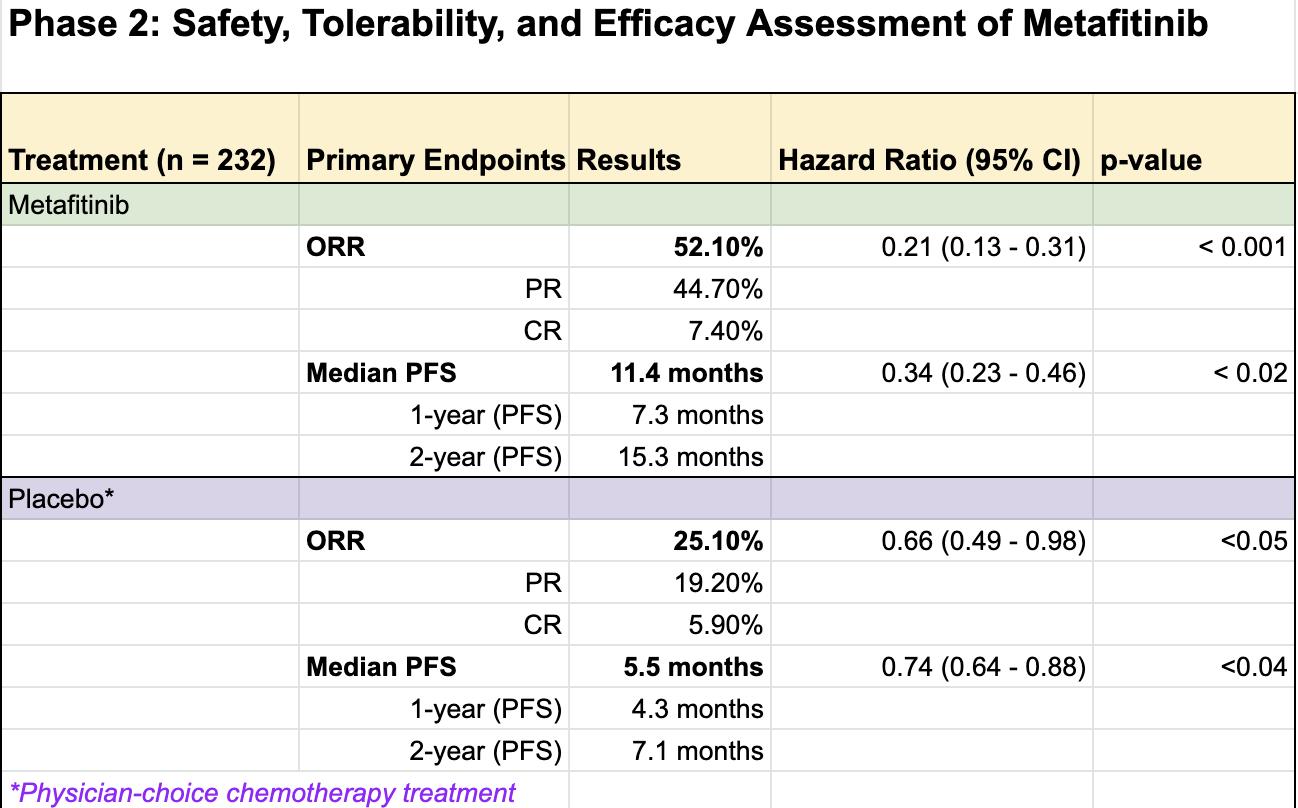
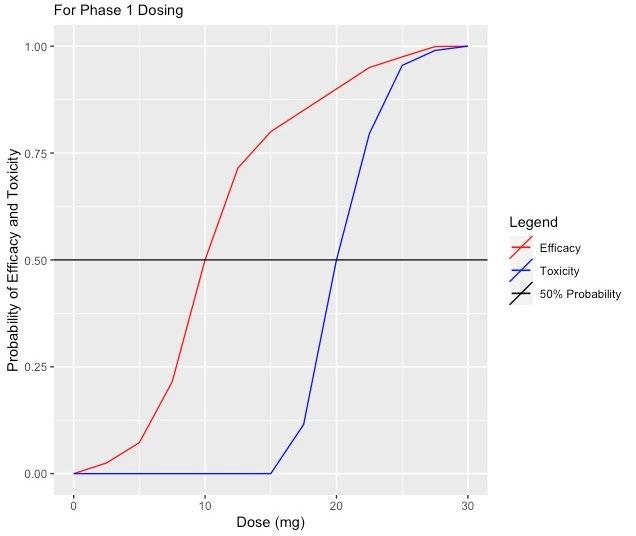
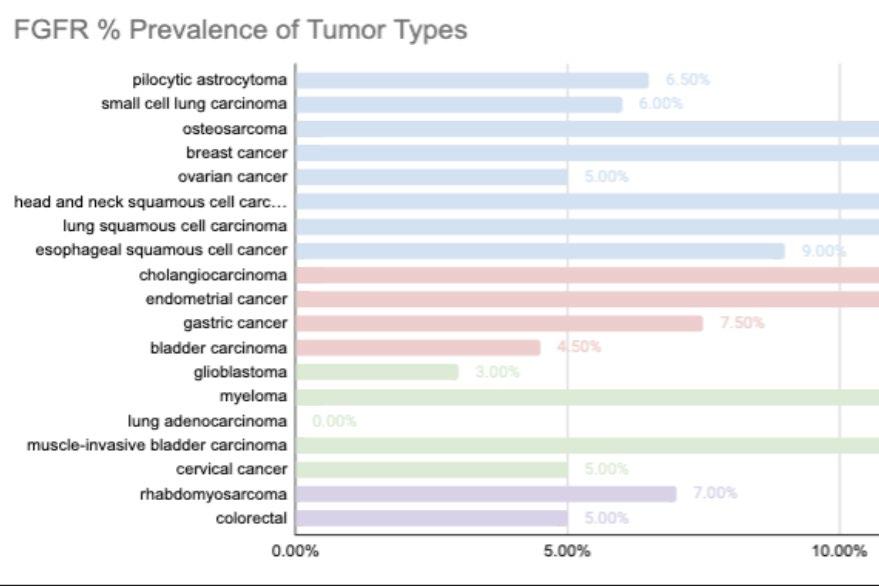
The top skills to have include strong project management, attention to detail, communication, trial rationalization and data analysis, and knowledge of clinical research regulations and guidelines.








