Encapsulated REGULATORY AFFAIRS
Q&A WITH DR. ALEX CHEUNG
INTERVIEW TIPS
SUMMER 2023 UPDATES


Q&A WITH DR. ALEX CHEUNG
INTERVIEW TIPS
SUMMER 2023 UPDATES
Encapsulated is an independent, student-run organization committed to providing industry-related resources, knowledge, and connections for students . We are dedicated to bridging the gap between students and the pharmaceutical industry and creating opportunities for internships and fellowships alike We hope to encapsulate the field into an easily accessible and digestible format.

Issue 3
Published September 2023
Contributors
Lead Writer
James Sagun
Kristina Rodrigues
Lead Editor

Eli Carbo Ontiveros
Ange Lu

June 2, 2023
July 13, 2023

August 2, 2023
September 12, 2023

This can demonstrate your understanding of the industry and your ability to apply your knowledge professionally.
Critical thinking, communication skills, teamwork, and time management skills are examples of transferable skills that companies value. Provide examples of using these skills in academic or professional settings.
As a student, securing an internship in the pharmaceutical industry can be a great way to gain experience and build your career . The interview process can be nerve -wracking, but you can impress potential employers with the proper preparation and approach . Here are some tips and topics to discuss to help you stand out during the interview process.


Discuss any relevant coursework, research experience, or interns- hips you have completed that relate to the position you are interviewing for .
This can demonstrate your ability to evaluate scientific literature and communicate complex concepts effectively and critically
You are unique from undergraduate students in that you can talk more specifically about research practicees, pharmacokinetics, and more advanced topics relevant to pharmacy.
Highlight courses and topics that interest you. Many clinical courses have many transferable skills in industry, depending on your area of interest

Companies value candidates with research experience, so talk about it if you have experience in a lab or other area!

Demonstrate your ability to manage projects, work collaboratively with others, and effectively communicate with diverse stakeholders This is very important as it can help you stand out from other candidates and show you are a leader .
Mention your current job, such as being a pharmacy technician, and how it has helped you develop skills for working in a team . Highlight transferable skills, such as teamwork, communication, and problem solving.

FDA The Food and Drug Administration, a regulatory agency in the United States responsible for ensuring the safety, efficacy, and quality of food, drugs, medical devices, and cosmetics.
NDA New Drug Application, a submission made to the FDA for approval to market a new drug in the United States.

IND Investigational New Drug, a submission made to the FDA to request permission to conduct clinical trials of a new drug.
CMC Chemistry, Manufacturing, and Controls, the section of a regulatory submission that provides information on the chemical composition of a drug, the manufacturing process, and quality control procedures.
EMA European Medicines Agency, a regulatory agency in the European Union responsible for evaluating and regulating medicines for human and animal use.
PMA Premarket Approval, a submission made to the FDA for approval to market a new medical device in the United States.
Promotional Labeling
Any printed, audiovisual, or electronic material that is distributed or intended to be distributed to healthcare professionals (HCPs) to promote a drug.
Fair Balance
Drug advertising and promotion must present a balanced portrayal of a drug's benefits and risks.
Off-Label Promotion Promotion of a drug for uses that the FDA has not approved.
Comparative Claims Claims made in drug advertising that compare the drug to other drugs or treatments.
Substantiation

The requirement that drug advertising and promotion be supported by reliable scientific evidence.
OPDP Office of Prescription Drug Promotion, a division within the FDA that regulates prescription drug advertising and promotion.

Regulatory Affairs (RA) is a broad function in the pharmaceutical industry and encompasses a wide range of responsibilities related to regulatory compliance Depending on the size and scope of a pharmaceutical company, the regulatory affairs function may be organized into sub-types or departments Many of these functions can also be combined.
They are responsible for managing regulatory applications and submissions for drugs and devices, including formatting, publishing, and submitting documents to health authorities like the FDA.
They develop and implement strategies for new drug development programs that speed up the approval process through the FDA or other agencies, such as expedited approvals and post-approval activities, such as label changes.
This branch manages the Chemistry, Manufacturing, and Controls (CMC) regulatory submissions and any compliance activities related to drug product manufacturing, production site compliance, and chemistry of the products.
This area manages regulatory submissions and compliance activities related to clinical trials, including clinical trial applications, protocol amendments, and clinical study reports
They ensure all promotional materials like TV, print, and social media ads comply with regulatory requirements, including FDA regulations and guidelines, and that all claims are truthful and not misleading.
They ensure the product labeling and packaging comply with regulatory requirements, including FDA regulations and guidelines, and accurately reflect the drug's approved indications, dosing, and safety information.

This branch is responsible for monitoring and reporting adverse drug reactions (ADRs) associated with a company's products and ensuring appropriate risk management plans are in place.
They ensure the company complies with all relevant regulations and guidelines, including FDA regulationns, ICH guidelines, and countryspecific requirements.

The Regulatory Affairs (RA) department is an important pharmaceutical department responsible for obtaining drug approvals and maintaining legislative compliance. Asso-
ciates here act as the liaison between the pharmaceutical industry and the regulatory authorities across the world, such as the EMA in Europe, FDA in America, and PMDA in Japan.
The Regulatory Affairs team plays a key role in evaluating clinical trial information and global scientific and
legal requirements to ensure public safety in both pre- and post-market approval. This functional area works closely with Clinical Development and Commercial to establish a welldetailed drug product plan.
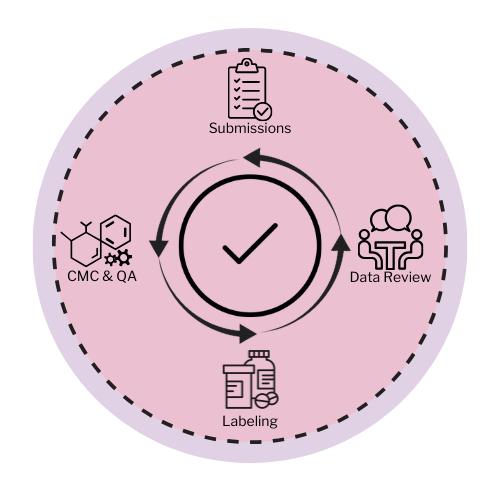
Organizing a new drug application requires detailed preparation and effective communication between many groups within the Regulatory Affairs department. Professionals in these areas have strong communication and negotiation skills and are equipped with strong regulatory knowledge on a global and domestic perspective.
These groups are incorporated along the entire drug lifecycle and include areas such as Pharmacovigilance, Regulatory Strategy, Advertising and Promotion (Ad/Promo), Labeling, Submissions, Global Affairs, Regulatory Policy, and many more These groups interact both internally and externally within the company to ensure that the drug product is approved with favorable labeling that is aligned with the company’s marketing goals and business objectives.
process, the Regulatory Strategy team provides direction for various tasks such as product development and portfolio expansion, optimal approval pathway designation, and field investigation of other products For instance, if your drug product has the potential to be a best-inclass or first-in -class therapy, the Strategy team determines what expedited review program is chosen, such as Fast Track or Accelerated Approval, and how to best explain safety and efficacy of trial data to regulatory authorities.
During the pre-approval phase, many regulatory documents must be compiled, reviewed, and revised prior to submissions for events such as Investigational New Drug Applications (INDs) and New Drug Applications (NDAs) This is accomplished by the Submissions, Labeling , and many other regulatory affairs teams that work closely with Medical Writers to gather clinical trial information for application approvals.
These pre-approval teams verify that false claims are not made in the drug product labeling. Many data review meetings are conducted to ensure the NDA is filed correctly and the manufacturing facility is compliant
with standard operating procedures (SOP) and good manufacturing practices (GMP).
To date, some companies are developing in- house management systems that have the potential to streamline the data handling of trial information for the automated publication of regulatory documents This will allow regulatory professionals to leverage their time for insights generation and data analysis of the drug product’s riskbenefit profile
Prior to submission, the company must structure the documents in a well -organized format. One format that the regulatory team can utilize is the “Common Technical Document” (CTD), which is broken down into 5 modules to manage the entire metadata of the drug product. The five modules encompass topics including administrative/prescribing information, clinical and non-clinical overviews, quality (pharmaceutical documentation), pre-clinical pharmacology and toxicology reports, and clinical safety and efficacy reports. This CTD format is agreed upon internationally by several regulatory authorities when applying for a new drug product.

During the post-approval phase, drug advertisements on TV commercials and magazines must be accurate or else the company faces massive penalties The Ad/Promo team ensures that each direct-toconsumer (DTC) advertisement is carefully written with a “major statement” of the product’s primary risks and reviewed by regulatory authorities prior to dissemination. Every detail is cons-idered, from the person, demographic, and story
advertised to ensure that product claims are consistent with the approved indication.
The company is also expected to conduct a detailed periodic report of any unexpected adverse events to update the drug’s safety profile. This is done by the Pharmacovigilance team. This group monitors and analyzes adverse events on a single case and aggregate level and determines whether risk mitigation is needed By doing so, they play a key role in initiating product recalls, trial halts, and boxed warnings.
Additional regulatory duties after commercialization include filings for new product indications, filings to switch a product classification to over -the- counter, and updating the manufacturing and supply chain for continuous improvements
Overall, the general strategy within Regulatory Affairs is multifaceted and multifunctional where various groups work together to shorten the timeline to approval for potentially life changing products.
Although the global pharmaceutical market is largely concentrated in North America, both Europe and the Asian Pacific are growing markets
that companies can expand their products in. A 2023 projection study estimated that 45% of the global pharmaceutical market is located in North America, 24% in the Asian Pacific, and 20 % in Europe Because of this, every regulatory strategy team should have a plan that is applicable to international markets and adaptable to submission challenges and setbacks
With the emergence of new technology and focus on patient-centric drug development, there are many evolving trends in the regulatory and pharmaceuticals space. These include the implementation of artificial intelligence (AI) and a greater harmonization between global regulatory agencies.
The use of machine learning and advanced technology in the creation of “digital twins” for modeling disease progression has allowed companies to predict drug effects even before administering to human patients. This can enhance precision medicine by determining the most optimal dose, route of administration, and timing of a drug product while reducing the amount of adverse events and ethical or operational challenges However, with the implementation of these in silico clinical
data, regulatory professionals must routinely work with the clinical development team to ensure that the integrity of data collected is up to regulatory standards.
Since many late-stage clinical trials are conducted globally, there can be variability in clinical trial regulation due to a country’s economy, company legal jurisdictions, and population samples . Because of this, many countries, like those in the EU, USA, and Japan, have collaborated to harmonize the regulatory process by integrating national and international standards through the ICH
(International Conference on Harmonization)
Recently, a collaborative review program, called Project Orbis, was developed by the US with several countries to improve the timeline of approval for oncology therapeutics.
The program provided the framework for the global submission and review and has given earlier access to oncology therapy to countries where significant regulatory delays can occur . So far, over 60 oncology drug products have received global approval by Project Orbis in countries such as the UK, Switzerland, Australia, Singapore, Canada, and many more
Although the use of new technology and global harmonization is seen to improve the regulatory approval process, there are some challenges that can arise with these trends.
The rise of advanced technology introduces two new challenges : (1) ethical concerns about patient privacy and data transparency, and (2)

digital literacy and competency within the company staff. These issues can be addressed by incorporating strong governance over large data and implementing continuous professional development within the company For example, companies can ensure governance by establishing quality assurance and auditing procedures for these technologies and supporting their staff in the training of managing digital systems . By doing so, regulatory teams can be confident in staying up to date with the evolving therapeutic and regulatory landscape while promoting a supportive work environment.
Global harmonization relies on the cooperation between countries and their respective regulatory authorities . Because of this, harmonization is heavily dependent on the economic stability and infrastructure of these countries. For example, political disagreements, global pandemics, and other destabilization factors can impact the ability of countries to work together . Thus, it is imperative that developed countries support and include developing countries in harmonization to facilitate greater access to healthcare and information exchange.

Alex Cheung earned his PharmD from St John’s University in Queens, New York. Upon graduating from St. John's, Alex completed a post-doctoral fellowship in U.S Commercial Regulatory Affairs - Advertising and Promotion at Bristol Myers Squibb (BMS), and has since stayed with the company as a Senior Manager in the same group In this role, Alex provides strategic regulatory guidance to Commercial and Medical stakeholders to ensure that the company's prescription drug promotion is accurate, truthful, and non-misleading. At BMS, Alex also serves as a preceptor for the same post-doctoral Fellowship program he completed in 2021 In addition, he is the co-lead for the Headquarters' Pan Asian Network - an employee resource group focused on promoting a workplace environment that fully values the contributions of Asian employees and helps the organization deepen the understanding of Asian patients, customers and other stakeholders
 Dr. Alex Cheung
Dr. Alex Cheung






How did you navigate your way into the pharmaceutical industry? What was your journey to narrowing it down to Advertising and Promotion?
I was exposed to pharmacy at an early age since my dad was a research chemist at a pharma company for 18 years. After he saw that PharmDs were moving up in the orgs, he encouraged my sister and I to pursue pharmacy. I attended St. John’s for my PharmD and actually thought I wanted to be a clinical pharmacist until I did my APPEs.
When I was completing my industry APPEs, I fell in love with the projects and initiatives that were involved in being an ad-promo reviewer. I felt "alive" from an intellectual and creative perspective in ways that I hadn't experienced during my clinical experiences. I eventually realized that the skill sets that I could utilize in pharma as an ad-promo reviewer would be more inline with my skills than if I were a clinical pharmacist.
My advice is to start by finding an area you feel alive in. Reflect why you feel that way and use it to pursue a path you’re passionate about.
What experiences and skills – whether from pharmacy school or elsewhere –helped you the most when preparing for your role within industry?
This is something I talked about in my fellowship interviews, but for me, it was theater. I have both acting and directing experience, and these roles required me to naturally translate written text to a performance on stage and give it meaning. For example, if a character pauses, how does the actor illustrate their vision while preserving their and the playwright’s perspective.


In my job as a regulatory advisor, I am responsible for interpreting and analyzing how promotional claims may or may not be truthful or misleading given the words that are used, the way they are packaged, the graphics that accompany them, etc. I analyze promotional material in much the same way that I would analyze word choice or punctuation in a play, just with a scientific lens.
After completing your PharmD, what preparations did you make before starting your fellowship with BMS?




Recruitment is where the bulk of the work comes in. In order to be considered a qualified candidate for a fellowship, you have to convey a strong understanding of what the role you're applying to entails and demonstrate your capabilities to do that job well.

Once you actually begin the fellowship, you will learn everything you need to know to be able to excel in your field.

Having experience as a pharmacy student who went on rotations to now precepting fellows, how has your perspective changed?






I realized there is a lot of planning and accountability that comes with the role of precepting Fellows. We are in charge of the fellow's growth and development, and we have to be strategic to ensure we allow them to reach their ultimate goal by the end of the fellowship. When I was a fellow and even a pharmacy student, I think I took for granted just how much work went into ensuring the student/fellow got a truly well-rounded experience that would be valuable to them.
As a preceptor, I aim to prepare the fellow for full time employment. I do everything I can to give the Fellow all of the experiences necessary to demonstrate their value as a full time employee.
As a preceptor evaluating potential fellowship candidates, what qualities or experiences have made the biggest impression on you?


Aside from objective skills, humility is a great quality to have. A fellow is a little fish in a big pond, and there's so much to learn.
A good regulatory advisor always questions their way of thinking or their assumptions to ensure that we are promoting our products compliantly and ethically. Of course, we don't want fellows to be insecure and completely unsure of themselves. We want to instill confidence in the fellows by teaching them how to do the job, but it would be detrimental if a fellow (or anyone for that matter) no longer felt the need to question their thinking.
How did you handle the learning curve transitioning into industry?





The learning curve is massive, and it's not identical for everyone. I definitely experienced imposter syndrome. For basically the entire first half of my fellowship, I questioned how I would ever be as competent as my colleagues at doing the job. I completed my fellowship virtually; in isolation, it was easy to doubt my skills and experiences.
To combat this, I always prepared questions for meetings and communicated areas that I needed more experience in. This would give my preceptor the opportunity to assess my progress and develop any action plans to close the gaps in my development. Uncertainty is ultimately part of the process, and it’s hard showing vulnerability, but it’s good to open up about what you don't know so that you can address those knowledge gaps.
How has social media impacted the pharmaceutical industry, especially regulatory advertising?
I entered industry in 2020, so social media was already well established. However, in regulatory advertising, the reg ad and promo rules were written in a time before social media, so CRA needs to understand how to apply these regulations to an evolving platform. If we want to spread a message on TikTok, we have to run through the risks and see if this platform includes features that would allow us to promote in a compliant way. This is where being creative comes in handy. Regulatory rules don't change; however, the arena in which we apply the regulatory principles always fluctuates.


How did you measure success, and what is the biggest successful risk you’ve taken to get to where you are now?
I measure success based on feeling. If I'm doing something well and stretching my limits to learn more, I feel successful. I feel like I've achieved something I didn't know I could do. For example, at work, I co-lead the Pan Asian Network, and lead a number of initiatives focused on difficult conversations around identity and belonging for Asian Americans. This role made me feel challenged, it made me question my abilities. There is no greater reward than overcoming those challenges.
Actually, choosing to apply to reg and promo was the biggest successful risk I took. Within the spectrum of felllowships, there were only seven ad-promo fellowships, versus the hundreds of medical affairs fellowships in the same year. I was unsure if I should take the risk to apply to ad promo, so I wrote out my letter of intent for each program. My reg ad promo LOI was far more nuanced and convincing, which showed me that my passion for ad-promo was well worth taking a risk on.
If you could go back in time and give your younger self any advice, what would it be?
As someone who is half Chinese and half Italian, I never felt like I truly belonged with either cohort. As such, I tried to cram myself into the cohorts, rather than accepting that I am my own unique mixed-race individual. I would have advised myself to create the mixed-race archetype, rather than trying to fit into the mold of Chinese or Italian.






This translates very much into the workplace. There is a lot of pressure to conform to what other people expect you to be, but there is so much value in having team members with diverse perspectives, backgrounds, and skills! Bringing your authenticity and personality into work can make you a great and dynamic leader, so don't be afraid to be yourself!
What is your biggest accomplishment that you’re most proud of? What legacy do you want to leave behind?
I went to a 6 -year pharmacy school where you start your PharmD curriculum in your “junior” year of college. This was a pivotal year since I transitioned into a professional student. During my P1 year, I landed a lead role in a play called “Speech and Debate.” Given the time requirements, I wondered if I would be able to balance everything, but it went well, and it was a good reminder that my personal life was just as important as my professional career. Find ways you can still interweave what’s important to you because that’s who you are.
As for legacy, I want to be remembered as authentic. I aim to bring my personality and humor into my work while also being professional. When I retire, I want someone to say, “Alex always brought who he was into his career.”






That’s a wonderful legacy to leave behind! Thank you for sharing your experiences and taking the time to have this interview with us!
 Dr. Alex Cheung in
Dr. Alex Cheung in

