Publications
Fontana, Pierre; Alberio, Lorenzo; Albisetti, Manuela; AngelilloScherrer, Anne; Asmis, Lars M; Casini, Alessandro; Gerber, Bern hard; Graf, Lukas; Hegemann, Inga; Korte, Wolfgang; Martinez, Maria; Studt, Jan-Dirk; Tsakiris, Dimitrios A; Wuillemin, Walter A; Kremer Hovinga, Johanna A (2020). Management of bleeding events and invasive procedures in patients with haemophilia A without inhibitors treated with emicizumab. Swiss Medical Weekly, 150:w20422.
Schwotzer, Rahel; Flammer, Andreas J; Gerull, Sabine; Pabst, Thomas; Arosio, Paolo; Averaimo, Manuela; Bacher, Vera Ulrike; Bode, Peter; Cavalli, Andrea; Concoluci, Adalgisa; Dirnhofer, Ste fan; Djerbi, Nadia; Dobner, Stefan W; Fehr, Thomas; Garofalo, Maura; Gaspert, Ariana; Heimgartner, Raphael; Hbers, Annemarie; Jung, Hans H; Kessler, Chiara; Knpfel, Raphael; Laptseva, Natal lia; Manka, Robert; Mazzucchelli, Luca; Meyer, Martin; Mihaylova, Violeta; Monney, Pierre; Mylonas, Alessio; Nkoulou, Ren; Pazhen kottil, Aju; Seeger, Harald; et al (2020). Expert recommendation from the Swiss Amyloidosis Network (SAN) for systemic AL-amyloi dosis. Swiss Medical Weekly, 150:w20364.
Akhoundova, Dilara; Hiltbrunner, Stefanie; Mader, Cäcilia; Förster, Robert; Kraft, Johannes; Schwanhäusser, Bianca; Bankel, Lorenz; Kollias, Spyros; Treyer, Valerie; Rushing, Elisabeth Jane; Lee, Seok-Yun; Andratschke, Nicolaus; Hüllner, Martin; Curioni-Fonte cedro, Alessandra (2020). 18F-FET PET for Diagnosis of Pseudo progression of Brain Metastases in Patients With Non–Small Cell Lung Cancer. Clinical Nuclear Medicine, 45(2):113-117.
Petrowsky, Henrik; Fritsch, Ralph; Guckenberger, Matthias; De Ol iveira, Michelle L; Dutkowski, Philipp; Clavien, Pierre-Alain (2020). Modern therapeutic approaches for the treatment of malignant liver tumours. Nature Reviews. Gastroenterology & Hepatology, 17(12):755-772.
Popat, S; Curioni-Fontecedro, A; Dafni, Urania; Shah, R; et al; Sta hel, R A (2020). A multicentre randomised phase III trial compar ing pembrolizumab versus single-agent chemotherapy for ad vanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Annals of Oncology, 31(12):1734-1745.
Sellner, Leopold; Schetelig, Johannes; Koster, Linda; Choi, Goda; Blaise, Didier; Beelen, Dietrich; Schianca, Fabrizio Carnevale; Pass weg, Jakob; Schanz, Urs; et al (2020). Idelalisib exposure before allogeneic stem cell transplantation in patients with follicular lym phoma: an EBMT survey. Bone Marrow Transplantation, 55(12):23352338.
Boettcher, Steffen (2020). Wnt to the rescue! A new role in granu lopoiesis. Blood, 136(22):2487-2489.
Petruse, Liliana; Kiesel, Holger; Rampini, Silvana K (2020). CMEAnswers: CME: Chronic Generalized Pruritus without Dermatolog ical Cause. Praxis, 109(15):1177-1178.
Peil, Nils; Varga, Zsuzsanna; Barysch, Marjam J; Brüssow, Cornelia; Dedes, Konstantin J (2020). Ectopic axillary breast cancer in a male patient. Clinical Case Reports, 8(11):2324-2325.
Lukas, Marina; Velten, Britta; Sellner, Leopold; Tomska, Katarzyna; Hüellein, Jennifer; Walther, Tatjana; Wagner, Lena; Muley, Carolin; Wu, Bian; Oleś, Małgorzata; Dietrich, Sascha; Jethwa, Alexander; Bohnenberger, Hanibal; Lu, Junyan; Huber, Wolfgang; Zenz, Thor sten (2020). Survey of ex vivo drug combination effects in chronic lymphocytic leukemia reveals synergistic drug effects and genetic dependencies. Leukemia, 34(11):2934-2950.
Wyvekens, Nicolas; Valtcheva, Nadejda; Mischo, Axel; Helmchen, Birgit; Hermanns, Thomas; Choschzick, Matthias; Hötker, Andreas M; Rauch, Anita; Mühleisen, Beda; Akhoundova, Dilara; Weber, Achim; Moch, Holger; Rupp, Niels J (2020). Novel morphological and genetic features of fumarate hydratase deficient renal cell carcinoma in HLRCC syndrome patients with a tailored therapeutic approach. Genes, Chromosomes and Cancer, 59(11):611-619.
Grogg, Josias Bastian; Schneider, Kym; Bode, Peter-Karl; Kranz bühler, Benedikt; Eberli, Daniel; Sulser, Tullio; Beyer, Joerg; Lorch, Anja; Hermanns, Thomas; Fankhauser, Christian Daniel (2020). Risk factors and treatment outcomes of 239 patients with testic ular granulosa cell tumors: a systematic review of published case series data. Journal of Cancer Research and Clinical Oncology, 146(11):2829-2841.
Marion, William; Boettcher, Steffen; Ruiz-Torres, Sonya; Lummertz da Rocha, Edroaldo; Lundin, Vanessa; Morris, Vivian; Chou, Steph anie; Zhao, Anna M; Kubaczka, Caroline; Aumais, Olivia; Zhang, Yosra; Shimamura, Akiko; Schlaeger, Thorsten M; North, Trista E; Ebert, Benjamin L; Wells, Susanne I; Daley, George Q; Rowe, R Grant (2020). An induced pluripotent stem cell model of Fanconi anemia reveals mechanisms of p53-driven progenitor cell differentiation. Blood advances, 4(19):4679-4692.
Rafiei, Anahita; Wilk, C Matthias; Helbling, Patrick M; Myburgh, Renier; Saito, Yasuyuki; Haralambieva, Eugenia; Soldini, Davide; Chakraborty, Rikhia; Merad, Miriam; Allen, Carl E; Nombela-Arrieta, Cesar; Manz, Markus G (2020). BRAFV 600E or mutant MAP2K1 human CD34+ cells establish Langerhans cell-like histiocytosis in immune-deficient mice. Blood advances, 4(19):4912-4917.
Myburgh, Renier; Kiefer, Jonathan D; Russkamp, Norman F; Mag nani, Chiara F; Nuñez, Nicolás; Simonis, Alexander; Pfister, Surema; Wilk, C Matthias; McHugh, Donal; Friemel, Juliane; Müller, Antonia M; Becher, Burkhard; Münz, Christian; van den Broek, Maries; Neri, Dario; Manz, Markus G (2020). Anti-human CD117 CAR T-cells efficiently eliminate healthy and malignant CD117-expressing hematopoietic cells. Leukemia, 34(10):2688-2703.
Peters, Solange; Danson, Sarah; Hasan, Baktiar; Dafni, Urania; Reinmuth, Niels; et al; Curioni-Fontecedro, Alessandra; Stahel, Rolf A (2020). A Randomized Open-Label Phase III Trial Evaluating the
2020
13
62
Addition of Denosumab to Standard First-Line Treatment in Advanced NSCLC: The European Thoracic Oncology Platform (ETOP) and European Organisation for Research and Treatment of Cancer (EORTC) SPLENDOUR Trial. Journal of Thoracic Oncology, 15(10):1647-1656.
Benz, Rudolf; Zimmermann, Kathrin; Rechsteiner, Markus; Bala banov, Stefan; Manz, Markus G; Widmer, Corinne C (2020). Pe gylated interferon can control myelodysplastic/myeloprolifera tive syndrome with ring sideroblasts and thrombocytosis. Leukemia & Lymphoma, 61(10):2533-2535.
Cerciello, Ferdinando; Choi, Meena; Sinicropi-Yao, Sara L; Lomeo, Katie; Amann, Joseph M; Felley-Bosco, Emanuela; Stahel, Rolf A; Robinson, Bruce W S; Creaney, Jenette; Pass, Harvey I; Vitek, Olga; Carbone, David P (2020). Verification of a Blood-Based Tar geted Proteomics Signature for Malignant Pleural Mesothelioma. Cancer Epidemiology Biomarkers & Prevention, 29(10):1973-1982.
Huls, Gerwin; Chitu, Dana A; Pabst, Thomas; Klein, Saskia K; Stussi, Georg; Griskevicius, Laimonas; Valk, Peter J M; Cloos, Jacqueline; van de Loosdrecht, Arjan A; Breems, Dimitri; van Lammeren-Ven ema, Danielle; van Zeventer, Isabelle; Boersma, Rinske; Jon gen-Lavrencic, Mojca; Fehr, Martin; Hoogendoorn, Mels; Manz, Markus G; Söhne, Maaike; van Marwijk Kooy, Rien; Deeren, Dries; van der Poel, Marjolein W M; Legdeur, Marie Cecile; Tick, Lidwine; Chalandon, Yves; Ammatuna, Emanuele; Blum, Sabine; Löwen berg, Bob; Ossenkoppele, Gert J (2020). Ibrutinib added to 10day decitabine for older patients with AML and higher risk MDS. Blood advances, 4(18):4267-4277.
Giger, Sonja; Kovtonyuk, Larisa V; Utz, Sebastian G; Ramosaj, Mergim; Kovacs, Werner J; Schmid, Emanuel; Ioannidis, Vassilios; Greter, Melanie; Manz, Markus G; Lutolf, Matthias P; Jessberger, Sebastian; Knobloch, Marlen (2020). A single metabolite which modulates lipid metabolism alters hematopoietic stem/progeni tor cell behavior and promotes lymphoid reconstitution. Stem Cell Reports, 15(3):566-576.
Chiesa, Robert; Wang, Junfeng; Blok, Henric-Jan; Hazelaar, Sheree; Neven, Benedicte; Moshous, Despina; Schulz, Ansgar; Hoenig, Manfred; Hauck, Fabian; Al Seraihy, Amal; Gozdzik, Jolanta; Ljungman, Per; Lindemans, Caroline A; Fernandes, Juliana F; Kalwak, Krzysztof; Strahm, Brigitte; Schanz, Urs; Sedlacek, Petr; Sykora, Karl-Walter; Aksoylar, Serap; Locatelli, Franco; Stepensky, Polina; Wynn, Robert; Lum, Su Han; Zecca, Marco; Porta, Fulvio; Taskinen, Mervi; Gibson, Brenda; Matthes, Susanne; Karakukcu, Musa; et al (2020). Hematopoietic cell transplantation in chronic granulomatous disease: a study of 712 children and adults. Blood, 136(10):1201-1211.
Shin, Jiyung J; Schröder, Markus S; Caiado, Francisco; Wyman, Stacia K; Bray, Nicolas L; Bordi, Matteo; Dewitt, Mark A; Vu, Jona than T; Kim, Won-Tae; Hockemeyer, Dirk; Manz, Markus G; Corn, Jacob E (2020). Controlled cycling and quiescence enables effi cient HDR in engraftment-enriched adult hematopoietic stem and progenitor cells. Cell Reports, 32(9):108093.
Oneda, Beatrice; Sirleto, Pietro; Baldinger, Rosa; Taralczak, Malgorzata; Joset, Pascal; Zweier, Markus; Niedrist, Dunja; Azza rello-Burri, Silvia; Britschgi, Christian; Breymann, Christian; Ochsen bein-Kölble, Nicole; Burkhardt, Tilo; Wisser, Josef; Zimmermann, Roland; Steindl, Katharina; Rauch, Anita (2020). Genome-wide non-invasive prenatal testing in single- and multiple-pregnan cies at any risk: Identification of maternal polymorphisms to re duce the number of unnecessary invasive confirmation testing. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 252:19-29.
Gachechiladze, Mariam; Škarda, Josef; Skanderová, Daniela; Überall, Ivo; Kolek, Vítězslav; Smičkova, Petra; Vojta, Petr; Vbrková, Jana; Hajdúch, Marián; Shani, Ilay; Kolář, Zdeněk; Stahel, Rolf; Weder, Walter; Rulle, Undine; Soltermann, Alex; Joerger, Markus (2020). Prognostic value of tumor-infiltrating lymphocytes (TILs) and their association with PD-L1 expression and DNA repair protein RAD51 in patients with resected non-small cell lung carci noma. Lung Cancer, 147:30-38.
Riether, Carsten; Pabst, Thomas; Höpner, Sabine; Bacher, Ulrike; Hinterbrandner, Magdalena; Banz, Yara; Müller, Rouven; Manz, Markus G; Gharib, Walid H; Francisco, David; Bruggmann, Remy; van Rompaey, Luc; Moshir, Mahan; Delahaye, Tim; Gandini, Do menica; Erzeel, Ellen; Hultberg, Anna; Fung, Samson; de Haard, Hans; Leupin, Nicolas; Ochsenbein, Adrian F (2020). Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nature Medicine, 26(9):1459-1467.
Mihic-Probst, Daniela; Reinehr, Michael; Dettwiler, Susanne; Kolm, Isabel; Britschgi, Christian; Kudura, Ken; Maggio, Ewerton Marques; Lenggenhager, Daniela; Rushing, Elisabeth J (2020). The role of macrophages type 2 and T-regs in immune checkpoint inhibitor related adverse events. Immunobiology, 225(5):152009.
Asadollahi, Reza; Britschgi, Christian; Joset, Pascal; Oneda, Be atrice; Schindler, Detlev; Meier, Urs R; Rauch, Anita (2020). Severe reaction to radiotherapy provoked by hypomorphic germline mu tations in ATM (ataxia-telangiectasia mutated gene). Molecular Genetics & Genomic Medicine :e1409.
Wehrle, Julius; Philipp, Ulrike; Jolic, Martina; Follo, Marie; Hussung, Saskia; Waldeck, Silvia; Deuter, Max; Rassner, Michael; Braune, Jan; Rawluk, Justyna; Greil, Christine; Waller, Cornelius F; Becker, Heiko; Duque-Afonso, Jesús; Illert, Anna-L; Fritsch, Ralph M; Meiss, Frank; Duyster, Justus; von Bubnoff, Nikolas; Scherer, Florian (2020). Personalized Treatment Selection and Disease Monitoring Using Circulating Tumor DNA Profiling in Real-World Cancer Patient Management. Diagnostics, 10(8):550.
Rössler, J; Hegemann, I; Schoenrath, F; Seifert, Burkhardt; Kaserer, A; Spahn, G H; Falk, V; Spahn, Donat R (2020). Efficacy of quadru ple treatment on different types of pre-operative anaemia: sec ondary analysis of a randomised controlled trial. Anaesthesia, 75(8):1039-1049.
Hegemann, Inga; Sasselli, Clelia; Valeri, Fabio; Makhro, Asya; Müller, Rouven; Bogdanova, Anna; Manz, Markus G; Gassmann, Max; Goede, Jeroen S (2020). MEMSID: Results from a phase 2 pilot study on memantine treatment for sickle cell disease. HemaSphere, 4(4):e452.
Vu, Diem-Lan; Dayer, Julie-Anne; Masouridi-Levrat, Stavroula; Combescure, Christophe; Boely, Elsa; Khanna, Nina; Mueller, Nico las J; Kleber, Martina; Medinger, Michael; Halter, Joerg; Passweg, Jakob; Müller, Antonia M; Schanz, Urs; Chalandon, Yves; Neofytos, Dionysios; van Delden, Christian; Kaiser, Laurent; Swiss Transplant Cohort Study (2020). Microbiologically documented infections af ter adult allogeneic hematopoietic cell transplantation: a 5-year analysis within the Swiss Transplant Cohort study. Transplant Infectious Disease, 22(4):e13289.
Stahel, Rolf A; Curioni-Fontecedro, A; Rohrmann, S; Dafni, U; Sand ner, U; Opitz, I; Andratschke, N; Franzen, D; Dimopoulou, G; Mat thes, K L; Kohler, Malcolm; Guckenberger, M; Weder, W (2020). Sur vival outcome of non-small cell lung cancer patients: Comparing results between the database of the Comprehensive Cancer Cen ter Zürich and the Epidemiological Cancer Registry Zurich and Zug. Lung Cancer, 146:217-223.
63
Hameister, Erik; Stolz, Sebastian M; Fuhrer, Yvonne; Thienemann, Friedrich; Schaer, Dominik J; Nemeth, Johannes; Schuepbach, Reto A; Goede, Jeroen; Reinhart, Sophie; Schmidt, Adrian; Kahraman, Abdullah; Schmid, Mathias; Moch, Holger; Zoche, Martin; Manz, Markus G; Balabanov, Stefan; Boettcher, Steffen (2020). Clonal hematopoiesis in hospitalized elderly patients with COVID-19. HemaSphere, 4(4):e453.
McHugh, Donal; Myburgh, Renier; Caduff, Nicole; Spohn, Michael; Kok, Yik Lim; Keller, Christian W; Murer, Anita; Chatterjee, Bithi; Rühl, Julia; Engelmann, Christine; Chijioke, Obinna; Quast, Isaak; Shilaih, Mohaned; Strouvelle, Victoria P; Neumann, Kathrin; Menter, Thomas; Dirnhofer, Stephan; Lam, Janice K P; Hui, Kwai F; Bredl, Simon; Schlaepfer, Erika; Sorce, Silvia; Zbinden, Andrea; Capaul, Riccarda; Lünemann, Jan D; Aguzzi, Adriano; Chiang, Alan K S; Kempf, Wer ner; Trkola, Alexandra; Metzner, Karin J; et al (2020). EBV renders B cells susceptible to HIV-1 in humanized mice. Life Science Alliance, 3(8):e202000640.
Gakis, Georgios; Bruins, Harman M; Cathomas, Richard; Compérat, Eva M; Cowan, Nigel C; van der Heijden, Antoine G; Hernández, Virginia; Linares Espinós, Estefania E; Lorch, Anja; Neuzillet, Yann; Ribal, Maria J; Rouanne, Mathieu; Thalmann, George N; Veskimäe, Erik; Witjes, Alfred J (2020). European Association of Urology Guidelines on Primary Urethral Carcinoma-2020 Update. Euro pean Urology Oncology, 3(4):424-432.
Schmid, Sabine; Mauti, Laetitia A; Friedlaender, Alex; Blum, Veron ika; Rothschild, Sacha I; Bouchaab, Hasna; Frösch, Patrizia; Britschgi, Christian; König, David; Wannesson, Luciano; Janthur, Wolf-Dieter; Schär, Sämi; Demmer, Izadora; Addeo, Alfredo; Jochum, Wolfram; Früh, Martin (2020). Outcomes with immune checkpoint inhibitors for relapsed small-cell lung cancer in a Swiss cohort. Cancer Immunology, Immunotherapy, 69(8):1605-1613.
Trinchero, Alice; Sholzberg, Michelle; Matino, Davide (2020). The Evolution of Hemophilia Care: Clinical and Laboratory Advances, Opportunities, and Challenges. Hämostaseologie, 40(3):311-321.
Roos-Weil, Damien; Giacopelli, Brian; Armand, Marine; Della- Valle, Véronique; Ghamlouch, Hussein; Decaudin, Camille; Metzner, Mar len; Lu, Junyan; Le Garff-Tavernier, Magali; Leblond, Véronique; Vyas, Paresh; Zenz, Thorsten; Nguyen-Khac, Florence; Bernard, Olivier A; Oakes, Christopher C (2020). Identification of 2 DNA methylation subtypes of Waldenström macroglobulinemia with plasma and memory B-cell features. Blood, 136(5):585-595.
Sahli, Sebastian D; Rössler, Julian; Tscholl, David W; Studt, JanDirk; Spahn, Donat R; Kaserer, Alexander (2020). Point-of-Care Diagnostics in Coagulation Management. Sensors, 20(15):4254.
Pellegrino, Christian; Favalli, Nicholas; Sandholzer, Michael; Volta, Laura; Bassi, Gabriele; Millul, Jacopo; Cazzamalli, Samuele; Matasci, Mattia; Villa, Alessandra; Myburgh, Renier; Manz, Markus G; Neri, Dario (2020). Impact of ligand size and conjugation chem istry on the performance of universal chimeric antigen receptor T-cells for tumor killing. Bioconjugate Chemistry, 31(7):1775-1783.
Nadeu, Ferran; Mas-de-Les-Valls, Rut; Navarro, Alba; Royo, Ro mina; Martín, Silvia; Villamor, Neus; Suárez-Cisneros, Helena; Mares, Rosó; Lu, Junyan; Enjuanes, Anna; Rivas-Delgado, Alfredo; Aymerich, Marta; Baumann, Tycho; Colomer, Dolors; Delgado, Ju lio; Morin, Ryan D; Zenz, Thorsten; Puente, Xose S; Campbell, Pe ter J; Beà, Sílvia; Maura, Francesco; Campo, Elías (2020). IgCaller for reconstructing immunoglobulin gene rearrangements and oncogenic translocations from whole-genome sequencing in lymphoid neoplasms. Nature Communications, 11:3390.
Takizawa, Hitoshi; Fritsch, Kristin; Kovtonyuk, Larisa V; Saito, Ya suyuki; Yakkala, Chakradhar; Jacobs, Kurt; Ahuja, Akshay K; Lopes, Massimo; Hausmann, Annika; Hardt, Wolf-Dietrich; Gomariz, Álvaro; Nombela-Arrieta, César; Manz, Markus G (2020). Patho gen-induced TLR4-TRIF innate immune signaling in hematopoi etic stem cells promotes proliferation but reduces competitive fitness. Cell Stem Cell, 27(1):177.
Mahkro, Asya; Hegemann, Inga; Seiler, Elena; Simionato, Greta; Claveria, Viviana; Bogdanov, Nikolay; Sasselli, Clelia; Torgeson, Paul; Kaestner, Lars; Manz, Markus G; Goede, Jeroen S; Gassmann, Max; Bogdanova, Anna (2020). A pilot clinical phase II trial Mem SID: Acute and durable changes of red blood cells of sickle cell disease patients on memantine treatment. eJHaem, 1(1):23-34.
Ossenkoppele, G J; Breems, D A; Stuessi, G; van Norden, Y; Bar getzi, M; Biemond, B J; et al; Chalandon, Y; Cloos, J; Deeren, D; Fehr, Mirjam; Pabst, Thomas; Manz, Markus G (2020). Lenalidomide added to standard intensive treatment for older patients with AML and high-risk MDS. Leukemia, 34(7):1751-1759.
Grogg, Josias; Schneider, Kym; Bode, Peter Karl; Kranzbühler, Ben edikt; Eberli, Daniel; Sulser, Tullio; Lorch, Anja; Beyer, Joerg; Her manns, Thomas; Fankhauser, Christian Daniel (2020). Sertoli cell tumors of the testes: systematic literature review and meta-analysis of outcomes in 435 patients. The oncologist, 25(7):585-590.
Lodigiani, Corrado; Iapichino, Giacomo; Carenzo, Luca; Cecconi, Maurizio; Ferrazzi, Paola; Sebastian, Tim; Kucher, Nils; Studt, JanDirk; Sacco, Clara; Alexia, Bertuzzi; Sandri, Maria Teresa; Barco, Stefano; Humanitas COVID-19 Task Force (2020). Venous and ar terial thromboembolic complications in COVID-19 patients admit ted to an academic hospital in Milan, Italy. Thrombosis research, 191:9-14.
Witjes, J Alfred; Babjuk, Marek; Bellmunt, Joaquim; Bruins, H Maxim; De Reijke, Theo M; De Santis, Maria; Gillessen, Silke; James, Nich olas; Maclennan, Steven; Palou, Juan; Powles, Tom; Ribal, Maria J; Shariat, Shahrokh F; Van Der Kwast, Theo; Xylinas, Evanguelos; et al; Lorch, Anja (2020). Corrigendum to ‘EAU-ESMO Consensus Statements on the Management of Advanced and Variant Blad der Cancer-An International Collaborative Multistakeholder Effort Under the Auspices of the EAU-ESMO Guidelines Committees’ [Eu ropean Urology 77 (2020) 223-250]. European Urology, 78(1):e48-e50.
Hussung, Saskia; Follo, Marie; Klar, Rhena F U; Michalczyk, Sandra; Fritsch, Kornelia; Nollmann, Friederike; Hipp, Julian; Duyster, Jus tus; Scherer, Florian; von Bubnoff, Nikolas; Boerries, Melanie; Wit tel, Uwe; Fritsch, Ralph M (2020). Development and Clinical Vali dation of Discriminatory Multitarget Digital Droplet PCR Assays for the Detection of Hot Spot KRAS and NRAS Mutations in CellFree DNA. Journal of Molecular Diagnostics, 22(7):943-956.
Roider, Tobias; Seufert, Julian; Uvarovskii, Alexey; Frauhammer, Felix; Bordas, Marie; Abedpour, Nima; Stolarczyk, Marta; Mallm, Jan-Philipp; Herbst, Sophie A; Bruch, Peter-Martin; Balke-Want, Hyatt; Hundemer, Michael; Rippe, Karsten; Goeppert, Benjamin; Seiffert, Martina; Brors, Benedikt; Mechtersheimer, Gunhild; Zenz, Thorsten; Peifer, Martin; Chapuy, Björn; Schlesner, Matthias; Müller-Tidow, Carsten; Fröhling, Stefan; Huber, Wolfgang; Anders, Simon; Dietrich, Sascha (2020). Dissecting intratumour heteroge neity of nodal B-cell lymphomas at the transcriptional, genetic and drug-response levels. Nature Cell Biology, 22(7):896-906.
Olearo, Flaminia; Kronig, Ilona; Masouridi-Levrat, Stavroula; Cha landon, Yves; Khanna, Nina; Passweg, Jakob; Medinger, Michael; Mueller, Nicolas J; Schanz, Urs; Van Delden, Christian; Neofytos, Dionysios (2020). Optimal treatment duration of Pseudomonas aeruginosa infections in allogeneic hematopoietic cell transplant recipients. Open Forum Infectious Diseases, 7(7):ofaa246.
64
Barco, Stefano; Sollfrank, Stefanie; Trinchero, Alice; Adenaeuer, Anke; Abolghasemi, Hassan; Conti, Laura; Häuser, Friederike; Kremer Hovinga, Johanna A; Lackner, Karl J; Loewecke, Felicia; Miloni, Erwin; Vazifeh Shiran, Nader; Tomao, Luigi; Wuillemin, Wal ter A; Zieger, Barbara; Lämmle, Bernhard; Rossmann, Heidi (2020). Severe plasma prekallikrein deficiency: Clinical characteristics, novel KLKB1 mutations, and estimated prevalence. Journal of Thrombosis and Haemostasis, 18(7):1598-1617.
Dimitriou, Florentia; Schanz, Urs; Nair, Gayathri; Kimeswenger, Susanne; Brüggen, Marie-Charlotte; Hoetzenecker, Wolfram; French, Lars E; Dummer, Reinhard; Cozzio, Antonio; Guenova, Em manuella (2020). Long–term disease control after allogeneic he matopoietic stem cell transplantation in primary cutaneous T–cell lymphoma; results from a single institution analysis. Frontiers in Medicine, 7:290.
Yang, Chia-Lung; Serra-Roma, André; Gualandi, Marco; Bodmer, Nicole; Niggli, Felix; Schulte, Johannes Hubertus; Bode, Peter Karl; Shakhova, Olga (2020). Lineage-restricted sympathoadrenal progenitors confer neuroblastoma origin and its tumorigenicity. OncoTarget, 11(24):2357-2371.
Riesterer, Oliver; Pruschy, Martin; Bender, Sabine; Sharma, Ashish; Bogowicz, Marta; Tanadini-Lang, Stephanie; Stieb, Sonja; Ber togg, Kaja; Weber, Sandra; Ikenberg, Kristian; Huber, Gerhard; Schmid, Stephan; Bredell, Marius; Veit-Haibach, Patrick; Rordorf, Tamara; Held, Ulrike; Glanzmann, Christoph; Studer, Gabriela (2020). Consolidation cetuximab after concurrent triplet radio chemotherapy+cetuximab in patients with advanced head and neck cancer: a randomized phase II study. Radiotherapy and Oncology, 150:62-69.
Lysenko, Veronika; Wildner-Verhey van Wijk, Nicole; Zimmermann, Kathrin; Weller, Marie-Christine; Bühler, Marco; Wildschut, Mat theus H E; Schürch, Patrick; Fritz, Christine; Wagner, Ulrich; Cal abresi, Laura; Psaila, Bethan; Flavell, Richard A; Vannucchi, Ales sandro M; Mead, Adam J; Wild, Peter J; Dirnhofer, Stefan; Manz, Markus G; Theocharides, Alexandre P A (2020). Enhanced engraft ment of human myelofibrosis stem and progenitor cells in MISTRG mice. Blood advances, 4(11):2477-2488.
Buhler, Stéphane; Baldomero, Helen; Ferrari-Lacraz, Sylvie; Nunes, José Manuel; Sanchez-Mazas, Alicia; Massouridi-Levrat, Strav roula; Heim, Dominik; Halter, Jörg; Nair, Gayathri; Chalandon, Yves; Schanz, Urs; Güngör, Tayfun; Nicoloso, Grazia; Tiercy, JeanMarie; Passweg, Jakob; Villard, Jean (2020). Correction: Highresolution HLA phased haplotype frequencies to predict the success of unrelated donor searches and clinical outcome follow ing hematopoietic stem cell transplantation. Bone Marrow Trans plantation, 55(6):1215.
Braun, Lukas M; Lagies, Simon; Klar, Rhena F U; Hussung, Saskia; Fritsch, Ralph M; Wittel, Uwe A (2020). Metabolic Profiling of Early and Late Recurrent Pancreatic Ductal Adenocarcinoma Using Patient-Derived Organoid Cultures. Cancers, 12(6):1440.
Ghazi, Susan; Bourgeois, Soline; Gomariz, Alvaro; Bugarski, Milica; Haenni, Dominik; Martins, Joana R; Nombela-Arrieta, César; Unwin, Robert J; Wagner, Carsten A; Hall, Andrew M; Craigie, Eil idh (2020). Multiparametric imaging reveals that mitochondria-rich intercalated cells in the kidney collecting duct have a very high glycolytic capacity. FASEB Journal, 34(6):8510-8525.
Glatzer, M; Horber, D; Montemurro, M; Winterhalder, R; Inauen, R; Berger, M D; Pestalozzi, B; Pederiva, S; Pless, M; Putora, P M (2020). Choice of first line systemic treatment in pancreatic cancer among national experts. Pancreatology, 20(4):686-690.
Seidel, Christoph; Daugaard, Gedske; Nestler, Tim; Tryakin, Alexey; Fedyanin, Mikhail; Fankhauser, Christian; Hermanns, Thomas; Apa ricio, Jorge; Heinzelbecker, Julia; Paffenholz, Pia; Heidenreich, Axel; De Giorgi, Ugo; Cathomas, Richard; Lorch, Anja; Fingerhut, Anna; Gayer, Fabian; Bremmer, Felix; Giannatempo, Patrizia; Necchi, An drea; Aurilio, Gaetano; Casadei, Chiara; Tran, Ben; Dieckmann, Klaus-Peter; Brito, Margarida; Ruf, Christian; Oing, Christoph; Bo kemeyer, Carsten (2020). Human chorionic gonadotropin-positive seminoma patients: A registry compiled by the global germ cell tumor collaborative group (G3). European Journal of Cancer, 132:127-135.
Serra-Roma, André; Shakhova, Olga (2020). Identification of novel small-molecule kinase modulators for the treatment of neuroblas toma. Oncology and Therapy, 8(1):133-145.
Gualandi, Marco; Iorio, Maria; Engeler, Olivia; Serra-Roma, André; Gasparre, Giuseppe; Schulte, Johannes H; Hohl, Daniel; Shakhova, Olga (2020). Oncogenic ALKF1174L drives tumorigenesis in cutane ous squamous cell carcinoma. Life Science Alliance, 3(6):e201900601.
Pikor, Natalia B; Mörbe, Urs; Lütge, Mechthild; Gil-Cruz, Cristina; Perez-Shibayama, Christian; Novkovic, Mario; Cheng, Hung-Wei; Nombela-Arrieta, César; Nagasawa, Takashi; Linterman, Michelle A; Onder, Lucas; Ludewig, Burkhard (2020). Remodeling of light and dark zone follicular dendritic cells governs germinal center responses. Nature Immunology, 21(6):649-659.
Akhoundova Sanoyan, Dilara. 18F-FET PET for Diagnosis of Pseu doprogression of Brain Metastases in Patients With Non-Small Cell Lung Cancer. 2020, University of Zurich, Medizinische Fakultät.
Halloy, François; Iyer, Pavithra S; Ćwiek, Paulina; Ghidini, Alice; Bar man-Aksözen, Jasmin; Wildner-Verhey van Wijk, Nicole; Theocha rides, Alexandre P A; Minder, Elisabeth I; Schneider-Yin, Xiaoye; Schümperli, Daniel; Hall, Jonathan (2020). Delivery of oligonucle otides to bone marrow to modulate ferrochelatase splicing in a mouse model of erythropoietic protoporphyria. Nucleic Acids Research, 48(9):4658-4671.
Liebers, Nora; Roider, Tobias; Bohn, Jan-Paul; Haberbosch, Isa bella; Pircher, Andreas; Ferstl, Barbara; Ebnöther, Monika; Wendt ner, Clemens-Martin; Dearden, Claire; Follows, George A; Ho, An thony D; Müller-Tidow, Carsten; Dreger, Peter; Troussard, Xavier; Zenz, Thorsten; Dietrich, Sascha (2020). BRAF inhibitor treatment in classic hairy cell leukemia: a long-term follow-up study of pa tients treated outside clinical trials. Leukemia, 34(5):1454-1457.
Thunnissen, Erik; Kerr, Keith M; Dafni, Urania; Bubendorf, Lukas; Finn, Stephen P; Soltermann, Alex; Biernat, Wojciech; Cheney, Rich ard; Verbeken, Erik; Warth, Arne; Marchetti, Antonio; Speel, ErnstJan M; Pokharel, Saraswati; Quinn, Anne Marie; Monkhorst, Kim; Na varro, Atilio; Madsen, Line Bille; Tsourti, Zoi; Geiger, Thomas; Kammler, Roswitha; Peters, Solange; Stahel, Rolf A (2020). Programmed death-ligand 1 expression influenced by tissue sample size. Scor ing based on tissue microarrays’ and cross-validation with resec tions, in patients with, stage I-III, non-small cell lung carcinoma of the European Thoracic Oncology Platform Lungscape cohort. Modern Pathology, 33(5):792-801.
Uka, Rexhep; Britschgi, Christian; Krättli, Anja; Matter, Claudia; Mihic, Daniela; Okoniewski, Michal J; Gualandi, Marco; Stupp, Roger; Cinelli, Paolo; Dummer, Reinhard; Levesque, Mitchell P; Shakhova, Olga (2020). Temporal activation of WNT/β-catenin signaling is sufficient to inhibit SOX10 expression and block mel anoma growth. Oncogene, 39(20):4132-4154.
Ring, Alexander; Zenz, Thorsten (2020). Genetics of «high-risk» chronic lymphocytic leukemia in the times of chemoimmunotherapy. Haematologica, 105(5):1180-1182.
65
Boettcher, Steffen; Wilk, C Matthias; Singer, Jochen; Beier, Fabian; Burcklen, Elodie; Beisel, Christian; Ventura Ferreira, Monica S; Gourri, Elise; Gassner, Christoph; Frey, Beat M; Schanz, Urs; Skoda, Radek C; Ebert, Benjamin L; Brummendorf, Tim H; Beerenwinkel, Niko; Manz, Markus G (2020). Clonal hematopoiesis in donors and long-term survivors of related allogeneic hematopoietic stem cell transplantation. Blood, 135(18):1548-1559.
Fischer, Stefanie; Tandstad, Torgrim; Cohn-Cedermark, Gabriella; Thibault, Constance; Vincenzi, Bruno; Klingbiel, Dirk; Albany, Cos tantine; Necchi, Andrea; Terbuch, Angelika; Lorch, Anja; Aparicio, Jorge; Heidenreich, Axel; Hentrich, Marcus; Wheater, Matthew; Langberg, Carl W; Ståhl, Olof; Fankhauser, Christian Daniel; Ha mid, Anis A; Koutsoukos, Konstantinos; Shamash, Jonathan; White, Jeff; Bokemeyer, Carsten; Beyer, Jörg; Gillessen, Silke (2020). Outcome of Men With Relapses After Adjuvant Bleomycin, Etopo side, and Cisplatin for Clinical Stage I Nonseminoma. Journal of Clinical Oncology, 38(12):1322-1331.
Alberio, Lorenzo; Angelillo-Scherrer, Anne; Asmis, Lars; Casini, Alessandro; Fontana, Pierre; Graf, Lukas; Hegemann, Inga; Kremer Hovinga, Johanna A; Korte, Wolfgang; Lecompte, Thomas; Mar tinez, Martina; Nagler, Michael; Studt, Jan-Dirk; Tsakiris, Dimitrios A; Wuillemin, Walter (2020). Recommendations on the use of an ticoagulants for the treatment of patients with heparin-induced thrombocytopenia in Switzerland. Swiss Medical Weekly, 150:w20210.
Boegeholz, J; Brueggen, C S; Pauli, C; Dimitriou, F; Haralambieva, E; Dummer, R; Manz, M G; Widmer, C C (2020). Challenges in diag nosis and management of neutropenia upon exposure to im mune-checkpoint inhibitors: meta-analysis of a rare immunerelated adverse side effect. BMC Cancer, 20:300.
Casini, Alessandro; Alberio, Lorenzo; Angelillo-Scherrer, Anne; Fon tana, Pierre; Gerber, Bernhard; Graf, Lukas; Hegemann, Inga; Korte, Wolfgang; Kremer Hovinga, Johanna; Lecompte, Thomas; Marti nez, Maria; Nagler, Michael; Studt, Jan-Dirk; Tsakiris, Dimitrios; Wuillemin, Walter; Asmis, Lars (2020). Thromboprophylaxis and laboratory monitoring for in-hospital patients with COVID-19 – a Swiss consensus statement by the Working Party Hemostasis. Swiss Medical Weekly, 150:w20247.
Snowden, John A; Saccardi, Riccardo; Orchard, Kim; et al; Schanz, U (2020). Benchmarking of survival outcomes following haemato poietic stem cell transplantation: A review of existing processes and the introduction of an international system from the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Bone Marrow Transplantation, 55(4):681-694.
Griggio, Valentina; Vitale, Candida; Todaro, Maria; Riganti, Chi ara; Kopecka, Joanna; Salvetti, Chiara; Bomben, Riccardo; Dal Bo, Michele; Magliulo, Daniela; Rossi, Davide; Pozzato, Gabriele; Bonello, Lisa; Marchetti, Monia; Omedè, Paola; Kodipad, Ahad Ahmed; Laurenti, Luca; Del Poeta, Giovanni; Mauro, Francesca Ro mana; Bernardi, Rosa; Zenz, Thorsten; Gattei, Valter; Gaidano, Gi anluca; Foà, Robin; Massaia, Massimo; Boccadoro, Mario; Coscia, Marta (2020). HIF-1 α is overexpressed in leukemic cells from TP53-disrupted patients and is a promising therapeutic target in chronic lymphocytic leukemia. Haematologica, 105(4):1042-1054.
Gomariz, Alvaro; Isringhausen, Stephan; Helbling, Patrick M; Nombela-Arrieta, César (2020). Imaging and spatial analysis of hematopoietic stem cell niches. Annals of the New York Academy of Sciences, 1466(1):5-16.
Bruins, Harman M; Veskimäe, Erik; Hernández, Virginia; Neuzillet, Yann; Cathomas, Richard; Compérat, Eva M; Cowan, Nigel C; Ga kis, Georgios; Espinós, Estefania Linares; Lorch, Anja; Ribal, Maria J; Rouanne, Mathieu; Thalmann, George N; Yuan, Yuhong; van der
Heijden, Antoine G; Witjes, J Alfred (2020). The Importance of Hos pital and Surgeon Volume as Major Determinants of Morbidity and Mortality After Radical Cystectomy for Bladder Cancer: A System atic Review and Recommendations by the European Association of Urology Muscle-invasive and Metastatic Bladder Cancer Guide line Panel. European Urology Oncology, 3(2):131-144.
Deeren, Dries; Balabanov, Stefan; Nickel, Katharina; Giannopoulou, Christina; Gonzalez-McQuire, Sebastian; Kutikova, Lucie; Bouw meester, Walter; Spyridonidis, Alexandros (2020). Management of patients with acute lymphoblastic leukemia in routine clinical prac tice: Minimal residual disease testing, treatment patterns and clinical outcomes in Belgium, Greece and Switzerland. Leukemia Research, 91:106334.
Siebenhüner, Alexander R; Güller, Ulrich; Warschkow, Rene (2020). Population-based SEER analysis of survival in colorectal cancer patients with or without resection of lung and liver metastases. BMC Cancer, 20:246.
Regouc, Manuel; Belge, Gazanfer; Lorch, Anja; Dieckmann, Klaus-Peter; Pichler, Martin (2020). Non-Coding microRNAs as Novel Potential Tumor Markers in Testicular Cancer. Cancers, 12(3):749.
Wierzbinska, Justyna A; Toth, Reka; Ishaque, Naveed; Rippe, Karsten; Mallm, Jan-Philipp; Klett, Lara C; Mertens, Daniel; Zenz, Thorsten; Hielscher, Thomas; Seifert, Marc; Küppers, Ralf; Assenov, Yassen; Lutsik, Pavlo; Stilgenbauer, Stephan; Roessner, Philipp M; Seiffert, Martina; Byrd, John; Oakes, Christopher C; Plass, Chris toph; Lipka, Daniel B (2020). Methylome-based cell-of-origin mod eling (Methyl-COOM) identifies aberrant expression of immune regulatory molecules in CLL. Genome Medicine, 12(1):29.
Satoh, Takashi K; Mellett, Mark; Meier-Schiesser, Barbara; Fenini, Gabriele; Otsuka, Atsushi; Beer, Hans-Dietmar; Rordorf, Tamara; Maul, Julia-Tatjana; Hafner, Jürg; Navarini, Alexander A; Contas sot, Emmanuel; French, Lars E (2020). IL-36 γ drives skin toxicity in duced by EGFR/MEK inhibition and commensal Cutibacterium acnes. Journal of Clinical Investigation, 130(3):1417-1430.
Molina-Vila, Miguel-Angel; Stahel, Rolf A; Dafni, Urania; Jor dana-Ariza, Núria; Balada-Bel, Ariadna; Garzón-Ibáñez, Mónica; García-Peláez, Beatriz; Mayo-de-Las-Casas, Clara; Felip, Enri queta; Curioni Fontecedro, Alessandra; Gautschi, Oliver; Peters, Solange; Massutí, Bartomeu; Palmero, Ramon; Ponce Aix, Santi ago; Carcereny, Enric; Früh, Martin; Pless, Miklos; Popat, Sanjay; Cuffe, Sinead; Bidoli, Paolo; Kammler, Roswitha; Roschitzki-Voser, Heidi; Tsourti, Zoi; Karachaliou, Niki; Rosell, Rafael (2020). Evolu tion and clinical impact of EGFR mutations in circulating free DNA in the BELIEF trial. Journal of Thoracic Oncology, 15(3):416-425.
Bögeholz, Jan; Russkamp, Norman F; Wilk, Christian M; Gourri, Elise; Haralambieva, Eugenia; Schanz, Urs; Mueller, Nicolas J; Manz, Markus G; Müller, Antonia M S (2020). Long-term follow-up of antibody titers against measles, mumps, and rubella in recipients of allogenic hematopoietic cell transplantations. Biology of Blood and Marrow Transplantation, 26(3):581-592.
Stahel, Rolf A; Lacombe, Denis; Cardoso, Fatima; Casali, Paolo G; Negrouk, Anastassia; Marais, Richard; Hiltbrunner, Anita; Vyas, Malvika (2020). Current models, challenges and best practices for work conducted between European academic cooperative groups and industry. ESMO Open, 5(2)::e000628.
Zeller, Carmen. Quantifizierung der BCR-ABL-Transkriptlast von Patienten mit chronisch myeloischer Leukämie mittels digitaler Tröpfchen-Polymerasekttenreaktion. 2020, University of Zurich, Medizinische Fakultät.
66
Okonska, Agata; Bühler, Saskja; Rao, Vasundhara; Ronner, Man uel; Blijlevens, Maxime; van der Meulen-Muileman, Ida H; de Menezes, Renée X; Wipplinger, Martin; Oehl, Kathrin; Smit, Egbert F; Weder, Walter; Stahel, Rolf A; Penengo, Lorenza; van Beusechem, Victor W; Felley-Bosco, Emanuela (2020). Functional genomic screen in mesothelioma reveals that loss of function of BRCA1-as sociated-protein-1 induces chemoresistance to ribonucleotide re ductase inhibition. Molecular Cancer Therapeutics, 19(2):552-563.
Urosevic-Maiwald, Mirjana; Schwotzer, Rahel; Kerl-French, Katrin; Karamachev, Jivko; Hafner, Jürg; French, Lars E; Brüggen, Ma rie-Charlotte (2020). Rapid growth of congenital angioma in an HIV patient with POEMS syndrome. European Journal of Derma tology, 30(1):66-67.
Pavlidis, Nicholas; Peccatori, Fedro; Aapro, Matti; Rolfo, Christian; Cervantes, Andres; Stahel, Rolf; Eniu, Alex; Cavalli, Franco; Costa, Alberto (2020). Changing the education paradigm in oncology: ESO masterclass, 17 years of continuous success. Critical Reviews in Oncology/Hematology, 146:102798.
Witjes, J Alfred; Babjuk, Marek; Bellmunt, Joaquim; Bruins, H Maxim; De Reijke, Theo M; De Santis, Maria; Gillessen, Silke; James, Nich olas; Maclennan, Steven; Palou, Juan; Powles, Tom; Ribal, Maria J; Shariat, Shahrokh F; Van Der Kwast, Theo; Xylinas, Evanguelos; et al; Lorch, Anja (2020). EAU-ESMO consensus statements on the management of advanced and variant bladder cancer-an inter national collaborative Multistakeholder effort: under the auspices of the EAU-ESMO Guidelines Committees. European Urology, 77(2):223-250.
Lausch, C K; Lorch, A; Giertzuch, S; Rieger, A; Knubben-Schweizer, Gabriela; Trefz, F M (2020). Prognostic relevance of pre- and post operative plasma l-lactate measurements in calves with acute abdominal emergencies. Journal of Dairy Science, 103(2):18561865.
Oing, Christoph; Hentrich, Marcus; Lorch, Anja; Gläser, Dietrich; Rumpold, Holger; Ochsenreither, Sebastian; Richter, Stephan; Dieing, Annette; Zschäbitz, Stefanie; Pereira, Ronnie Rodrigues; Bokemeyer, Carsten; Seidel, Christoph (2020). Treatment of refrac tory germ-cell tumours with single-agent cabazitaxel: a German Testicular Cancer Study Group case series. Journal of Cancer Research and Clinical Oncology, 146(2):449-455.
Arlettaz Mieth, Romaine; Gosztonyi, Laura; Hegemann, Inga; Bassler, Dirk; Rueegger, Christoph (2020). Neonatal red blood cell transfusion practices in Switzerland:national survey and review of international recommendations. Swiss Medical Weekly, 150:w20178.
Mylonas, Elena; Yoshida, Kenichi; Frick, Mareike; Hoyer, Kaja; Chris ten, Friederike; Kaeda, Jaspal; Obenaus, Matthias; Noerenberg, Daniel; Hennch, Cornelius; Chan, Willy; Ochi, Yotaro; Shiraishi, Yui chi; Shiozawa, Yusuke; Zenz, Thorsten; Oakes, Christopher C; Saw itzki, Birgit; Schwarz, Michaela; Bullinger, Lars; le Coutre, Philipp; Rose-Zerilli, Matthew J J; Ogawa, Seishi; Damm, Frederik (2020). Single-cell analysis based dissection of clonality in myelofibrosis. Nature Communications, 11:73.
Agarwal, P; Isringhausen, S; Li, H; Paterson, A J; He, J; Gomariz, Á; Nagasawa, T; Nombela-Arrieta, C; Bhatia, R (2020). Erratum: Mes enchymal Niche-Specific Expression of Cxcl12 Controls Quiescence of Treatment-Resistant Leukemia Stem Cells (Cell Stem Cell (2019) 24(5) (769–784.e6), (S1934590919300700), (10.1016/j.stem.2019.02.018)). Cell Stem Cell, 26(1):123.
Hegemann, Inga; Koch, Karin; Clausen, Wan Hui Ong; Ezban, Mi rella; Brand-Staufer, Brigitte (2020). Evaluation of N8-GP activity using a one-stage clotting assay: a single-center experience. Acta Haematologica, 143(5):504-508.
Opitz, Isabelle; Lauk, Olivia; Meerang, Mayura; Jetter, Alexander; Aeschlimann, Beat; Seifert, Burkhardt; Günther, Detlef; Stahel, Rolf A; Weder, Walter (2020). Intracavitary cisplatin-fibrin chemother apy after surgery for malignant pleural mesothelioma: A phase I trial. Journal of Thoracic and Cardiovascular Surgery, 159(1):330340.e4.
Heinhuis, Kimberley M; Carlino, Matteo; Joerger, Markus; Di Nicola, Massimo; Meniawy, Tarek; Rottey, Sylvie; Moreno, Victor; Gazzah, Anas; Delord, Jean-Pierre; Paz-Ares, Luis; Britschgi, Christian; Schil der, Russell J; O’Byrne, Kenneth; Curigliano, Giuseppe; Romano, Emanuela; Patah, Poliana; Wang, Rui; Liu, Yali; Bajaj, Gaurav; Siu, Lillian L (2020). Safety, tolerability, and potential clinical activity of a glucocorticoid-induced TNF receptor-related protein ago nist alone or in combination with nivolumab for patients with ad vanced solid tumors: a phase 1/2a dose-escalation and cohortexpansion clinical trial. JAMA Oncology, 6 (1):100.
Suzuki, Daisuke; Flahou, Charlotte; Yoshikawa, Norihide; Stirblyte, Ieva; Hayashi, Yoshikazu; Sawaguchi, Akira; Akasaka, Marina; Na kamura, Sou; Higashi, Natsumi; Xu, Huaigeng; Matsumoto, Takuya; Fujio, Kosuke; Manz, Markus G; Hotta, Akitsu; Takizawa, Hitoshi; Eto, Koji; Sugimoto, Naoshi (2020). iPSC-Derived Platelets Depleted of HLA Class I Are Inert to Anti-HLA Class I and Natural Killer Cell Immunity. Stem Cell Reports, 14(1):49-59.
Baccin, Chiara; Al-Sabah, Jude; Velten, Lars; Helbling, Patrick M; Grünschläger, Florian; Hernández-Malmierca, Pablo; NombelaArrieta, César; Steinmetz, Lars M; Trumpp, Andreas; Haas, Simon (2020). Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organiza tion. Nature Cell Biology, 22(1):38-48.
Kaserer, Alexander; Kiavialaitis, Greta Emilia; Braun, Julia; Schedler, Andreas; Stein, Philipp; Rössler, Julian; Spahn, Donat R; Studt, Jan-Dirk (2020). Impact of rivaroxaban plasma concentration on perioperative red blood cell loss. Transfusion, 60(1):197-205.
Petersdorf, Effie W; Carrington, Mary; O›hUigin, Colm; Bengtsson, Mats; De Santis, Dianne; Dubois, Valerie; Gooley, Ted; Horowitz, Mary; Hsu, Katharine; Madrigal, J Alejandro; Maiers, Martin J; Malkki, Mari; McKallor, Caroline; Morishima, Yasuo; Oudshoorn, Machteld; Spellman, Stephen R; Villard, Jean; Stevenson, Phil (2020). Role of HLA-B exon 1 in graft-versus-host disease after un related haemopoietic cell transplantation: a retrospective cohort study. The Lancet Haematology, 7(1):e50-e60.
Petruse, Liliana; Kiesel, Holger; Rampini, Silvana K (2020). CME: Chronisch generalisierter Pruritus nicht-dermatologischer Ursa che. Praxis, 109(14):1099-1107.
Friedrich, Marie Sophie; Studt, Jan-Dirk; Braun, Julia; Spahn, Donat R; Kaserer, Alexander (2020). Coronavirus-induced coagulopathy during the course of disease. PLoS ONE, 15(12):e0243409.
Siebenhüner, Alexander; De Dosso, Sara; Meisel, Alexander; Wag ner, Anna Dorothea; Borner, Markus (2020). Metastatic Colorectal Carcinoma after Second Progression and the Role of Trifluridine-Tip iracil (TAS-102) in Switzerland. Oncology Research and Treatment, 43(5):237-244.
Hussung, Saskia; Fritsch, Ralph M (2020). Multi-target ddPCR Assays zur Detektion von KRAS- und NRAS-Mutationen. BIOspektrum, 26(6):629-634.
Britschgi, Christian; Addeo, Alfredo; Rechsteiner, Markus; Delaloye, Raphaël; Früh, Martin; Metro, Giulio; Banini, Marco; Gautschi, Oliver; Rothschild, Sacha I; Wild, Peter J; Banna, Giuseppe L; Curioni-Fontecedro, Alessandra (2020). Real-World Treatment
67
Patterns and Survival Outcome in Advanced Anaplastic Lymphoma Kinase (ALK) Rearranged Non-Small-Cell Lung Cancer Patients. Frontiers in Oncology, 10:1299.
Akhoundova Sanoyan, Dilara; Reiner, Cäcilia S; Papageorgiou, Pa nagiota; Siebenhüner, Alexander R (2020). Sequential Treatment of Metastatic Adenocarcinoma of the Pancreatic Duct with Liver Metastasis Following the NAPOLI-1 Study Protocol with nal-Irino tecan plus 5-FU in the Second Line. Case Reports in Oncology, 13(1):79-84.
Leeksma, Alexander C; Derks, Ingrid A M; Kasem, M Haidar; Kilic, Emine; de Klein, Annelies; Jager, Martine J; van de Loosdrecht, Ar jan A; Jansen, Joop H; Navrkalova, Veronika; Faber, Laura M; Za borsky, Nadja; Egle, Alexander; Zenz, Thorsten; Pospisilova, Sarka; Abdel-Wahab, Omar; Kater, Arnon P; Eldering, Eric (2020). The Effect of SF3B1 Mutation on the DNA Damage Response and Non sense-Mediated mRNA Decay in Cancer. Frontiers in Oncology, 10:609409.
Einwächter, Henrik; Heiseke, Alexander; Schlitzer, Andreas; Gastei ger, Georg; Adler, Heiko; Voehringer, David; Manz, Markus G; Ruzsics, Zsolt; Dölken, Lars; Koszinowski, Ulrich H; Sparwasser, Tim; Reindl, Wolfgang; Jordan, Stefan (2020). The innate immune re sponse to infection induces erythropoietin-dependent replenish ment of the dendritic cell compartment. Frontiers in Immunology, 11:1627.
2021
Hiltbrunner, Stefanie; Spohn, Meta-Lina; Wechsler, Ramona; Ak houndova, Dilara; Bankel, Lorenz; Kasser, Sabrina; Bihr, Svenja; Britschgi, Christian; Maathuis, Marloes H; Curioni-Fontecedro, Alessandra (2021). Comprehensive Statistical Exploration of Prog nostic (Bio-)Markers for Responses to Immune Checkpoint Inhibi tor in Patients with Non-Small Cell Lung Cancer. Cancers, 14:75.
Meier-Abt, Fabienne; Lu, Junyan; Cannizzaro, Ester; Pohly, Marcel F; Kummer, Sandra; Pfammatter, Sibylle; Kunz, Laura; Collins, Ben C; Nadeu, Ferran; Lee, Kwang S; Xue, Peng; Gwerder, Myriam; Roiss, Michael; Hüllein, Jennifer; Scheinost, Sebastian; Dietrich, Sascha; Campo, Elias; Huber, Wolfgang; Aebersold, Ruedi; Zenz, Thorsten (2021). The Protein Landscape of Chronic Lymphocytic Leukemia (CLL). Blood, 138(24):2514-2525.
Gerosa, Rahel; Boettcher, Steffen; Kovtonyuk, Larisa Vladimirovna; Hausmann, Annika; Hardt, Wolf-Dietrich; Hidalgo, Juan; NombelaArrieta, Cesar; Manz, Markus G (2021). CXCL12-abundant Reticu lar Cells are the Major Source of IL-6 Upon LPS-stimulation and Thereby Regulate Hematopoiesis. Blood advances, 5(23):50025015.
Diaz de la Guardia, Rafael; Velasco-Hernandez, Talia; Gutier rez-Agüera, Francisco; Roca-Ho, Heleia; Molina, Oscar; NombelaArrieta, Cesar; Bataller, Alex; Fuster, Jose Luis; Anguita, Eduardo; Vives, Susana; Zamora, Lurdes; Nomdedeu, Josep F; Gomez-Casa res, M Teresa; Ramírez-Orellana, Manuel; Lapillonne, Helene; Ra mos-Mejia, Veronica; Rodríguez-Manzaneque, Juan Carlos; Bueno, Clara; Lopez-Millan, Belen; Menendez, Pablo (2021). Engraftment characterization of risk-stratified AML patients in NSGS mice. Blood advances, 5(23):4842-4854.
Isringhausen, Stephan; Mun, YeVin; Kovtonyuk, Larisa; Kräutler, Nike J; Suessbier, Ute; Gomariz, Alvaro; Spaltro, Gianluca; Helbling, Patrick M; Wong, Hui Chyn; Nagasawa, Takashi; Manz, Markus G; Oxenius, Annette; Nombela-Arrieta, César (2021). Chronic viral infections persistently alter marrow stroma and impair hemat poietic stem cell fitness. Journal of Experimental Medicine, 218(12):e 20192070.
Sellner, Leopold; Schetelig, Johannes; Koster, Linda; Choi, Goda; Blaise, Didier; Beelen, Dietrich; Schianca, Fabrizio Carnevale; Pass weg, Jakob; Schanz, Urs; et al (2021). Correction: Idelalisib expo sure before allogeneic stem cell transplantation in patients with follicular lymphoma: an EBMT survey. Bone Marrow Transplantation, 56(12):3108.
Ceruti, Samuele; Roncador, Marco; Saporito, Andrea; Biggiogero, Maira; Glotta, Andrea; Maida, Pier Andrea; Urso, Patrizia; Bona, Giovanni; Garzoni, Christian; Mauri, Romano; Borgeat, Alain (2021). Low PEEP Mechanical Ventilation and PaO 2/FiO 2 Ratio Evolution in COVID-19 Patients. SN Comprehensive Clinical Medicine, 3(12):2435-2442.
Abela, Irene A; Pasin, Chloé; Schwarzmüller, Magdalena; Epp, Se lina; Sickmann, Michèle E; Schanz, Merle M; Rusert, Peter; Weber, Jacqueline; Schmutz, Stefan; Audigé, Annette; Maliqi, Liridona; Hunziker, Annika; Hesselman, Maria C; Niklaus, Cyrille R; Gottschalk, Jochen; Schindler, Eméry; Wepf, Alexander; Karrer, Urs; Wolfens berger, Aline; Rampini, Silvana K; Meyer Sauteur, Patrick M; Berger, Christoph; Huber, Michael; Böni, Jürg; Braun, Dominique L; Mar conato, Maddalena; Manz, Markus G; Frey, Beat M; Günthard, Hul drych F; Kouyos, Roger D; et al (2021). Multifactorial seroprofiling dissects the contribution of pre-existing human coronaviruses re sponses to SARS-CoV-2 immunity. Nature Communications, 12:6703.
Valtcheva, Nadejda; Nguyen-Sträuli, Bich Doan; Wagner, Ulrich; Freiberger, Sandra N; Varga, Zsuzsanna; Britschgi, Christian; Dedes, Konstantin J; Rechsteiner, Markus P (2021). Setting a diagnostic benchmark for tumor BRCA testing: Detection of BRCA1 and BRCA2 large genomic rearrangements in FFPE tissue - A pilot study. Experimental and Molecular Pathology, 123:104705.
Beattie, Rory; Furrer, Katarzyna; Dolan, Daniel P; Curioni-Fonte cedro, Alessandra; Lee, Daniel N; Frauenfelder, Thomas; Hoeller, Sylvia; Weder, Walter; Bueno, Raphael; Opitz, Isabelle; Swanson, Scott (2021). Two centres experience of lung cancer resection in patients with advanced non-small cell lung cancer upon treat ment with immune checkpoint inhibitors: safety and clinical outcomes. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery, 60(6):1297-1305.
Bassi, Gabriele; Favalli, Nicholas; Pellegrino, Christian; Onda, Yuichi; Scheuermann, Jörg; Cazzamalli, Samuele; Manz, Markus G; Neri, Dario (2021). Specific Inhibitor of Placental Alkaline Phospha tase Isolated from a DNA-Encoded Chemical Library Targets Tumor of the Female Reproductive Tract. Journal of Medicinal Chemistry, 64(21):15799-15809.
El Khawanky, Nadia; Hughes, Amy; Yu, Wenbo; Myburgh, Renier; Matschulla, Tony; Taromi, Sanaz; Aumann, Konrad; Clarson, Jade; Vinnakota, Janaki Manoja; Shoumariyeh, Khalid; Miething, Corne lius; Lopez, Angel F; Brown, Michael P; Duyster, Justus; Hein, Lutz; Manz, Markus G; Hughes, Timothy P; White, Deborah L; Yong, Ag nes S M; Zeiser, Robert (2021). Demethylating therapy increases anti-CD123 CAR T cell cytotoxicity against acute myeloid leuke mia. Nature Communications, 12:6436.
Taromi, Sanaz; Firat, Elke; Simonis, Alexander; Braun, Lukas M; Apostolova, Petya; Elze, Mirjam; Passlick, Bernward; Schumacher, Alicia; Lagies, Simon; Frey, Anna; Schmitt-Graeff, Annette; Burger, Meike; Schmittlutz, Katrin; Follo, Marie; von Elverfeldt, Dominik; Zhu, Xuekai; Kammerer, Bernd; Diederichs, Sven; Duyster, Justus; Manz, Markus G; Niedermann, Gabriele; Zeiser, Robert (2021). Enhanced AC133-specific CAR T cell therapy induces durable remissions in mice with metastatic small cell lung cancer. Cancer Letters, 520:385-399.
68
Birrer, Dominique L; Golcher, Henriette; Casadei, Riccardo; Haile, Sarah R; Fritsch, Ralph; Hussung, Saskia; Brunner, Thomas B; Fiet kau, Rainer; Meyer, Thomas; Grützmann, Robert; Merkel, Susanne; Ricci, Claudio; Ingaldi, Carlo; Di Marco, Mariacristina; Guido, Ales sandra; Serra, Carla; Minni, Francesco; Pestalozzi, Bernhard; Petrowsky, Henrik; DeOliveira, Michelle; Bechstein, Wolf O; Bruns, Christiane J; Oberkofler, Christian E; Puhan, Milo; Lesurtel, Mick aël; Heinrich, Stefan; Clavien, Pierre-Alain (2021). Neoadjuvant Therapy for Resectable Pancreatic Cancer: A New Standard of Care. Pooled Data from Three Randomized Controlled Trials. Annals of Surgery, 274(5):713-720.
Akhoundova, Dilara; Pietge, Heike; Hussung, Saskia; Kiessling, Michael; Britschgi, Christian; Zoche, Martin; Rechsteiner, Markus; Weber, Achim; Fritsch, Ralph M (2021). Targeting Secondary and Tertiary Resistance to BRAF Inhibition in BRAF V600E-Mutated Met astatic Colorectal Cancer. JCO precision oncology, 5:1082-1087.
Condoluci, Adalgisa; Théaudin, Marie; Schwotzer, Rahel; Pazhen kottil, Aju P; Arosio, Paolo; Averaimo, Manuela; Bacher, Ulrike; Bode, Peter; Cavalli, Andrea; Dirnhofer, Stefan; Djerbi, Nadia; Dobner, Stephan; Fehr, Thomas; Garofalo, Maura; Gaspert, Ariana; Gerull, Sabine; Heimgartner, Raphael; Hübers, Annemarie; Jung, Hans H; Kessler, Chiara; Knöpfel, Raphael; Laptseva, Natallia; Magini, Gi ulia; Manka, Robert; Mazzucchelli, Luca; Meyer, Martin; Mihaylova, Violeta; Monney, Pierre; Mylonas, Alessio; Nkoulou, René; Pabst, Thomas; Pfister, Otmar; Rüfer, Axel; Schmidt, Adrian; Seeger, Har ald; Stämpfli, Simon F; Stirnimann, Guido; Suter, Thomas; Treglia, Giorgio; Tzankov, Alexandar; Vetter, Friederike; Zweier, Markus; Flammer, Andreas J; Gerber, Bernhard (2021). Management of transthyretin amyloidosis. Swiss Medical Weekly, 151:w30053.
Vilinovszki, Oliver; Andratschke, Nicolaus; Huellner, Martin; Curi oni-Fontecedro, Alessandra; Kroeze, Stephanie G C (2021). True abscopal effect in a patient with metastatic non-small cell lung cancer. Radiation Oncology, 16:194.
Pavlidis, Nicholas; Peccatori, Fedro A; Aapro, Matti; Cervantes, An dreas; Stahel, Rolf; Eniu, Alex; Cavalli, Franco; Costa, Alberto (2021). Clinical Case Presentation and Discussion During ESO-ESMO Mas terclass: a 10-Year Interactive Educational Experience. Journal of Cancer Education, 36(5):1124-1128.
Spahn, Donat R; Kaserer, Alexander; Studt, Jan-Dirk (2021). Coag ulation Management after Trauma in the Presence of Direct Oral Anticoagulants. Anesthesiology, 135(4):570-572.
Montalban-Arques, Ana; Katkeviciute, Egle; Busenhart, Philipp; Bircher, Anna; Wirbel, Jakob; Zeller, Georg; Morsy, Yasser; Borsig, Lubor; Glaus Garzon, Jesus Francisco; Müller, Anne; Arnold, Isa belle C; Artola-Borán, Mariela; Krauthammer, Michael; Sintsova, Anna; Zamboni, Nicola; Leventhal, Gabriel E; Berchtold, Laura; de Wouters, Tomas; Rogler, Gerhard; Baebler, Katharina; Schwarzfischer, Marlene; Hering, Larissa; Olivares-Rivas, Ivan; Atrott, Kirs tin; Gottier, Claudia; Lang, Silvia; Boyman, Onur; Fritsch, Ralph M; Manz, Markus G; Spalinger, Marianne R; Scharl, Michael (2021). Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host & Microbe, 29(10):1573-1588.e7.
Lewis, Richard; Maurer, H Carlo; Singh, Nikita; et al; Zenz, Thorsten (2021). CXCR4 hyperactivation cooperates with TCL1 in CLL devel opment and aggressiveness. Leukemia, 35(10):2895-2905.
Ingelfinger, Florian; Krishnarajah, Sinduya; Kramer, Michael; Utz, Sebastian G; Galli, Edoardo; Lutz, Mirjam; Zwicky, Pascale; Akarca, Ayse U; Jurado, Nicole Puertas; Ulutekin, Can; Bamert, David; Wid mer, Corinne C; Piccoli, Luca; Sallusto, Federica; Núñez, Nicolás G; Marafioti, Teresa; Schneiter, Didier; Opitz, Isabelle; Lanzavecchia,
Antonio; Jung, Hans H; De Feo, Donatella; Mundt, Sarah; Schreiner, Bettina; Becher, Burkhard (2021). Correction to: Single cell profil ing of myasthenia gravis identifies a pathogenic T cell signature. Acta Neuropathologica, 142(4):789.
Herbst, Friederike; Lang, Tonio J L; Eckert, Elias S P; Wünsche, Peer; Wurm, Alexander A; Kindinger, Tim; Laaber, Karin; Hemmati, Shayda; Hotz-Wagenblatt, Agnes; Zavidij, Oksana; Paruzynski, Anna; Lu, Junyan; von Kalle, Christof; Zenz, Thorsten; Klein, Chris toph; Schmidt, Manfred; Ball, Claudia R; Glimm, Hanno (2021). The balance between the intronic miR-342 and its host gene Evl determines hematopoietic cell fate decision. Leukemia, 35(10):2948-2963.
Buhler, Stéphane; Baldomero, Helen; Ferrari-Lacraz, Sylvie; Ma mez, Anne-Claire; Masouridi-Levrat, Stavroula; Heim, Dominik; Halter, Jörg; Nair, Gayathri; Chalandon, Yves; Schanz, Urs; Güngör, Tayfun; Nicoloso, Grazia; Passweg, Jakob R; Villard, Jean (2021). Analysis of biological models to predict clinical outcomes based on HLA-DPB1 disparities in unrelated transplantation. Blood advances, 5(17):3377-3386.
Sorrentino, Simona; Conesa, Jose Javier; Cuervo, Ana; Melero, Roberto; Martins, Bruno; Fernandez-Gimenez, Estrella; de IsidroGomez, Federico P; de la Morena, Jimenez; Studt, Jan-Dirk; Sor zano, Carlos Oscar S; Eibauer, Matthias; Carazo, Jose Maria; Medalia, Ohad (2021). Structural analysis of receptors and actin polarity in platelet protrusions. Proceedings of the National Acad emy of Sciences of the United States of America, 118(37):e2105004118.
Rothschild, Sacha I; Zippelius, Alfred; Eboulet, Eric I; Savic Prince, Spasenija; Betticher, Daniel; Bettini, Adrienne; Früh, Martin; Jo erger, Markus; Lardinois, Didier; Gelpke, Hans; Mauti, Laetitia A; Britschgi, Christian; Weder, Walter; Peters, Solange; Mark, Michael; Cathomas, Richard; Ochsenbein, Adrian F; Janthur, Wolf-Dieter; Waibel, Christine; Mach, Nicolas; Froesch, Patrizia; Buess, Martin; Bohanes, Pierre; Godar, Gilles; Rusterholz, Corinne; Gonzalez, Michel; Pless, Miklos (2021). SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) NonSmall-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial. Journal of Clinical Oncology, 39(26):2872-2880.
Wang, Weijia; Zhang, Yang; Dettinger, Philip; Reimann, Andreas; Kull, Tobias; Loeffler, Dirk; Manz, Markus G; Lengerke, Claudia; Schroeder, Timm (2021). Cytokine combinations for human blood stem cell expansion induce cell-type- and cytokine-specific sig naling dynamics. Blood, 138(10):847-857.
Grogg, Josias Bastian; Fronzaroli, Jordi Nicola; Oliveira, Pedro; Bode, Peter-Karl; Lorch, Anja; Issa, Allaudin; Beyer, Joerg; Eberli, Daniel; Sangar, Vijay; Hermanns, Thomas; Clarke, Noel William; Fankhauser, Christian Daniel (2021). Clinicopathological charac teristics and outcomes in men with mesothelioma of the tunica vaginalis testis: analysis of published case-series data. Journal of Cancer Research and Clinical Oncology, 147(9):2671-2679.
Ngai, Lok Lam; Ma, Connie Y; Maguire, Orla; Do, An D; Robert, Al berto; Logan, Aaron C; Griffiths, Elizabeth A; Nemeth, Michael J; Green, Cherie; Pourmohamad, Tony; van Kuijk, Bo J; Snel, Alexan der N; Kwidama, Zinia W; Venniker-Punt, Bianca; Cooper, James; Manz, Markus G; Gjertsen, Bjørn T; Smit, Linda; Ossenkoppele, Gert J; Janssen, Jeroen J W M; Cloos, Jacqueline; Sumiyoshi, Teiko (2021). Bimodal expression of potential drug target CLL-1 (CLEC12A) on CD34+ blasts of AML patients. European Journal of Haematology, 107(3):343-353.
Rieger, Max J; Stolz, Sebastian M; Ludwig, Sabine; Benoit, Tobias M; Bissig, Marina; Widmer, Corinne C; Schwotzer, Rahel; Müller, An tonia M; Nair, Gayathri; Hegemann, Inga; Manz, Markus G; Schanz,
69
Urs (2021). Daratumumab in rituximab-refractory autoimmune hae molytic anaemia. British Journal of Haematology, 194(5):931-934.
Gomariz, Alvaro; Portenier, Tiziano; Helbling, Patrick M; Isringhau sen, Stephan; Suessbier, Ute; Nombela-Arrieta, César; Goksel, Or cun (2021). Modality Attention and Sampling Enables Deep Learn ing with Heterogeneous Marker Combinations in Fluorescence Microscopy. Nature Machine Intelligence, 3(9):799-811.
Seidel, Christoph; Daugaard, Gedske; Nestler, Tim; Tryakin, Alexey; Fedyanin, Mikhail; Fankhauser, Christian Daniel; Hermanns, Thomas; Cathomas, Richard; Lorch, Anja; et al (2021). The prog nostic significance of lactate dehydrogenase levels in seminoma patients with advanced disease: an analysis by the Global Germ Cell Tumor Collaborative Group (G3). World Journal of Urology, 39(9):3407-3414.
Hack, Ruben I; Becker, Anton S; Bode-Lesniewska, Beata; Exner, G Ulrich; Müller, Daniel A; Ferraro, Daniela A; Warnock, Geoffrey I; Burger, Irene A; Britschgi, Christian (2021). When SUV Matters: FDG PET/CT at Baseline Correlates with Survival in Soft Tissue and Ewing Sarcoma. Life, 11(9):869.
Beyer, Jörg; Berthold, Dominik; Bode, Peter-Karl; Cathomas, Rich ard; Fankhauser, Christian D; Fischer, Stefanie; Gillessen, Silke; Gross, Tobias; Hermanns, Thomas; Honecker, Friedemann; Lorch, Anja; Omlin, Aurelius; Papachristofilou, Alexandros; Roth, Beat; Ro thermundt, Christian; Seiler, Roland; Spahn, Martin; Stenner, Frank; Bührer, Emanuel (2021). Swiss germ-cell cancer consensus recom mendations. Swiss Medical Weekly, 151:w30023.
Choueiri, Toni K; Tomczak, Piotr; Park, Se Hoon; et al; Lorch, Anja (2021). Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. New England Journal of Medicine, 385(8):683-694.
Lenggenhager, Daniela; Bengs, Susan; Fritsch, Ralph; Hussung, Saskia; Busenhart, Philipp; Endhardt, Katharina; Töpfer, Antonia; The, Frans Olivier; Bütikofer, Simon; Gubler, Christoph; Scharl, Michael; Morell, Bernhard (2021). β 6-Integrin Serves as a Potential Serum Marker for Diagnosis and Prognosis of Pancreatic Adenocarcinoma. Clinical and Translational Gastroenterology, 12(8):e00395.
Puleston, Daniel J; Baixauli, Francesc; Sanin, David E; EdwardsHicks, Joy; Villa, Matteo; Kabat, Agnieszka M; Kamiński, Marcin M; Stanckzak, Michal; Weiss, Hauke J; Grzes, Katarzyna M; iletic, Klara; Field, Cameron S; Corrado, Mauro; Haessler, Fabian; Wang, Chao; Musa, Yaarub; Schimmelpfennig, Lena; Flachsmann, Lea; Mittler, Gerhard; Yosef, Nir; Kuchroo, Vijay K; Buescher, Joerg M; Balabanov, Stefan; Pearce, Edward J; Green, Douglas R; Pearce, Erika L (2021). Polyamine metabolism is a central determinant of helper T cell lin eage fidelity. Cell, 184(16):4186-4202.e20.
Halloy, François; Iyer, Pavithra S; Ghidini, Alice; Lysenko, Veronika; Barman-Aksözen, Jasmin; Grubenmann, Chia-Pei; Jucker, Jes sica; Wildner-Verhey van Wijk, Nicole; Ruepp, Marc- David; Minder, Elisabeth I; Minder, Anna-Elisabeth; Schneider- Yin, Xiaoye; Theo charides, Alexandre P A; Schümperli, Daniel; Hall, Jonathan (2021). Repurposing of glycine transport inhibitors for the treatment of erythropoietic protoporphyria. Cell Chemical Biology, 28(8):12211234.e6.
Todisco, Gabriele; Creignou, Maria; Gallì, Anna; Guglielmelli, Paola; Rumi, Elisa; Roncador, Marco; et al (2021). Co-mutation pattern, clonal hierarchy, and clone size concur to determine disease phe notype of SRSF2 P95-mutated neoplasms. Leukemia, 35(8):23712381.
Lu, Junyan; Cannizzaro, Ester; Meier-Abt, Fabienne; Scheinost, Sebastian; Bruch, Peter-Martin; Giles, Holly A R; Lütge, Almut; Hül lein, Jennifer; Wagner, Lena; Giacopelli, Brian; Nadeu, Ferran; Del gado, Julio; Campo, Elías; Mangolini, Maurizio; Ringshausen, Ingo; Böttcher, Martin; Mougiakakos, Dimitrios; Jacobs, Andrea; Boden miller, Bernd; Dietrich, Sascha; Oakes, Christopher C; Zenz, Thor sten; Huber, Wolfgang (2021). Multi-omics reveals clinically rele vant proliferative drive associated with mTOR-MYC-OXPHOS activity in chronic lymphocytic leukemia. Nature cancer, 2(8):853-864.
Kraljevic, Mateja; Khanna, Nina; Medinger, Michael; Passweg, Jakob; Masouridi-Levrat, Stavroula; Chalandon, Yves; Mueller, Nicolas J; Schanz, Urs; Vernaz, Nathalie; Van Delden, Christian; Neofytos, Dionysios; Swiss Transplant Cohort Study (2021). Clini cal considerations on posaconazole administration and thera peutic drug monitoring in allogeneic hematopoietic cell transplant recipients. Medical Mycology, 59(7):701-711.
Caiado, Francisco; Pietras, Eric M; Manz, Markus G (2021). Inflam mation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. Journal of Experimental Medicine, 218(7):e20201541.
Mayer, Katharina; Hein-Rothweiler, Ralph; Schüpke, Stefanie; Janisch, Marion; Bernlochner, Isabell; et al; Kupka, Danny (2021). Efficacy and Safety of Revacept, a Novel Lesion-Directed Com petitive Antagonist to Platelet Glycoprotein VI, in Patients Under going Elective Percutaneous Coronary Intervention for Stable Ischemic Heart Disease: The Randomized, Double-blind, PlaceboControlled ISAR-PLASTER Phase 2 Trial. JAMA Cardiology, 6(7):753-761.
Sponholz, Stefan; Chalepaki Ntelli, Kyriaki; Karaindros, Georgios; Schirren, Moritz; Lorch, Anja; Hiester, Andreas; Albers, Peter; Schir ren, Joachim (2021). Short-term and long-term outcomes after re section of thoracic growing teratoma syndrome. World Journal of Urology, 39(7):2579-2585.
Grever, Michael; Andritsos, Leslie; Banerji, Versha; et al; Zenz, Thorsten (2021). Hairy cell leukemia and COVID-19 adaptation of treatment guidelines. Leukemia, 35(7):1864-1872.
Favalli, Nicholas; Bassi, Gabriele; Pellegrino, Christian; Millul, Ja copo; De Luca, Roberto; Cazzamalli, Samuele; Yang, Su; Trenner, Anika; Mozaffari, Nour L; Myburgh, Renier; Moroglu, Mustafa; Con way, Stuart J; Sartori, Alessandro A; Manz, Markus G; Lerner, Richard A; Vogt, Peter K; Scheuermann, Jörg; Neri, Dario (2021). Publisher Correction: Stereo- and regiodefined DNA-encoded chemical libraries enable efficient tumour-targeting applications. Nature Chemistry, 13(7):714.
Chanias, Ioannis; Stojkov, Kristina; Stehle, Gregor Th; Daskalakis, Michael; Simeunovic, Helena; Njue, Linet Muthoni; SchneggKaufmann, Annatina S; Porret, Naomi A; Allam, Ramanjaneyulu; Rao, Tata Nageswara; Benz, Rudolf; Ruefer, Axel; Schmidt, Adrian; Adler, Marcel; Rovo, Alicia; Balabanov, Stefan; Stuessi, Georg; Bacher, Ulrike; Bonadies, Nicolas (2021). Myelodysplastic Syn dromes in the Postgenomic Era and Future Perspectives for Pre cision Medicine. Cancers, 13(13):3296.
Schneider, Marcel A; Linecker, Michael; Fritsch, Ralph; Muehlemat ter, Urs J; Stocker, Daniel; Pestalozzi, Bernhard; Samaras, Panag iotis; Jetter, Alexander; Kron, Philipp; Petrowsky, Henrik; Nicolau, Claude; Lehn, Jean-Marie; Humar, Bostjan; Graf, Rolf; Clavien, Pierre-Alain; Limani, Perparim (2021). Phase Ib dose-escalation study of the hypoxia-modifier Myo-inositol trispyrophosphate in patients with hepatopancreatobiliary tumors. Nature Communi cations, 12:3807.
70
Wirsching, Hans-Georg; Weller, Michael; Balabanov, Stefan; Roth, Patrick (2021). Targeted Therapies and Immune Checkpoint Inhib itors in Primary CNS Lymphoma. Cancers, 13(12):3073.
Ecker, Veronika; Stumpf, Martina; Brandmeier, Lisa; Neumayer, Tanja; Pfeuffer, Lisa; Engleitner, Thomas; Ringshausen, Ingo; Nel son, Nina; Jücker, Manfred; Wanninger, Stefan; Zenz, Thorsten; Wendtner, Clemens; Manske, Katrin; Steiger, Katja; Rad, Roland; Müschen, Markus; Ruland, Jürgen; Buchner, Maike (2021). Targeted PI3K/AKT-hyperactivation induces cell death in chronic lympho cytic leukemia. Nature Communications, 12:3526.
Kara, Eleanna; Crimi, Alessandro; Wiedmer, Anne; Emmenegger, Marc; Manzoni, Claudia; Bandres-Ciga, Sara; D’Sa, Karishma; Reynolds, Regina H; Botía, Juan A; Losa, Marco; Lysenko, Veron ika; Carta, Manfredi; Heinzer, Daniel; Avar, Merve; Chincisan, An dra; Blauwendraat, Cornelis; García-Ruiz, Sonia; Pease, Daniel; Mottier, Lorene; Carrella, Alessandra; Beck- Schneider, Dezirae; Magalhães, Andreia D; Aemisegger, Caroline; Theocharides, Alexandre P A; Fan, Zhanyun; Marks, Jordan D; Hopp, Sarah C; Abramov, Andrey Y; Lewis, Patrick A; Ryten, Mina; Hardy, John; Hyman, Bradley T; Aguzzi, A (2021). An integrated genomic ap proach to dissect the genetic landscape regulating the cell-tocell transfer of α-synuclein. Cell Reports, 35(10):109189.
Pavlidis, Nicholas; Peccatori, Fedro; Aapro, Matti; Eniu, Alexandru; Stahel, Rolf; Cervantes, Andres; Cavalli, Franco; Costa, Alberto (2021). ESO-ESMO Masterclass in Clinical Oncology: Analysis and Evaluation of the Learning Self-Assessment Test. Journal of Cancer Education, 36(3):556-560.
Roth, Patrick; Winklhofer, Sebastian; Müller, Antonia M S; Dummer, Reinhard; Mair, Maximilian J; Gramatzki, Dorothee; Le Rhun, Emi lie; Manz, Markus G; Weller, Michael; Preusser, Matthias (2021). Neurological complications of cancer immunotherapy. Cancer Treatment Reviews, 97:102189.
Valerio, Luca; Corsi, Gabriele; Granziera, Serena; Holm, Karin; Hotz, Michel-André; Jankowski, Marius; Konstantinides, Stavros V; Kucher, Nils; Nicoletti, Tommaso; Reinhardt, Christoph; Righini, Christian; Sacco, Clara; Trinchero, Alice; Zane, Federica; Pecci, Alessandro; Barco, Stefano (2021). Sex differences in Lemierre syn drome: Individual patient-level analysis. Thrombosis research, 202:36-39.
Ingelfinger, Florian; Krishnarajah, Sinduya; Kramer, Michael; Utz, Sebastian G; Galli, Edoardo; Lutz, Mirjam; Zwicky, Pascale; Akarca, Ayse U; Jurado, Nicole Puertas; Ulutekin, Can; Bamert, David; Wid mer, Corinne C; Piccoli, Luca; Sallusto, Federica; Núñez, Nicolás G; Marafioti, Teresa; Schneiter, Didier; Opitz, Isabelle; Lanzavecchia, Antonio; Jung, Hans H; De Feo, Donatella; Mundt, Sarah; Schreiner, Bettina; Becher, Burkhard (2021). Single-cell profiling of myasthe nia gravis identifies a pathogenic T cell signature. Acta Neuro pathologica, 141(6):901-915.
Favalli, Nicholas; Bassi, Gabriele; Pellegrino, Christian; Millul, Ja copo; De Luca, Roberto; Cazzamalli, Samuele; Yang, Su; Trenner, Anika; Mozaffari, Nour L; Myburgh, Renier; Moroglu, Mustafa; Con way, Stuart J; Sartori, Alessandro A; Manz, Markus G; Lerner, Rich ard A; Vogt, Peter K; Scheuermann, Jörg; Neri, Dario (2021). Stereo- and regiodefined DNA-encoded chemical libraries enable efficient tumour-targeting applications. Nature Chemistry, 13(6): 540-548.
Willekens, Guido; Studt, Jan-Dirk; Mendez, Adriana; Alberio, Lo renzo; Fontana, Pierre; Wuillemin, Walter A; Schmidt, Adrian; Graf, Lukas; Gerber, Bernhard; Bovet, Cedric; Sauter, Thomas C; Nagler, Michael (2021). A universal anti-Xa assay for rivaroxaban, apix
aban, and edoxaban measurements: method validation, diag nostic accuracy and external validation. British Journal of Hae matology, 193(6):1203-1212.
Nadeu, Ferran; Royo, Romina; Clot, Guillem; Duran-Ferrer, Martí; Navarro, Alba; Martin, Silvia; Lu, Junyan; Zenz, Thorsten; Baumann, Tycho Stephan; Jares, Pedro; Puente, Xose S; Martin-Subero, Iñaki; Delgado, Julio; Campo, Elías (2021). IGLV3-21R110 identifies an aggressive biological subtype of chronic lymphocytic leukemia with intermediate epigenetics. Blood, 137(21):2935-2946.
Kumar, Subodh; Buon, Leutz; Talluri, Srikanth; Roncador, Marco; et al (2021). Integrated genomics and comprehensive validation re veal drivers of genomic evolution in esophageal adenocarcinoma. Communications Biology, 4(1):617.
Gillessen, Silke; Sauvé, Nicolas; Collette, Laurence; Daugaard, Gedske; de Wit, Ronald; et al; Lorch, Anja; Fankhauser, Christian (2021). Predicting Outcomes in Men With Metastatic Nonsemino matous Germ Cell Tumors (NSGCT): Results From the IGCCCG Up date Consortium. Journal of Clinical Oncology, 39(14):1563-1574.
Beyer, Jörg; Collette, Laurence; Sauvé, Nicolas; Daugaard, Gedske; Feldman, Darren R; et al; Lorch, Anja; Fankhauser, Christian; Stark, Daniel; Grimison, Peter; Necchi, Andrea; Cathomas, Richard; Gillessen, Silke (2021). Survival and New Prognosticators in Meta static Seminoma: Results From the IGCCCG-Update Consortium. Journal of Clinical Oncology, 39(14):1553-1562.
Bühler, Marco M; Lu, Junyan; Scheinost, Sebastian; Nadeu, Ferran; Roos-Weil, Damien; Hensel, Manfred; Thavayogarajah, Tharshika; Moch, Holger; Manz, Markus G; Haralambieva, Eugenia; Marques Maggio, Ewerton; Beà, Sílvia; Giné, Eva; Campo, Elías; Bernard, Olivier A; Huber, Wolfgang; Zenz, Thorsten (2021). SAMHD1 muta tions in mantle cell lymphoma are recurrent and confer in vitro re sistance to nucleoside analogues. Leukemia Research, 107:106608. Kirschner, Martin; Vieri, Margherita; Kricheldorf, Kim; Ferreira, Monica S Ventura; Wlodarski, Marcin W; Schwarz, Michaela; Bal abanov, Stefan; Rolles, Benjamin; Isfort, Susanne; Koschmieder, Stef fen; Höchsmann, Britta; Panse, Jens; Brümmendorf, Tim H; Beier, Fabian (2021). Androgen derivatives improve blood counts and elongate telomere length in adult cryptic dyskeratosis congenita. British Journal of Haematology, 193(3):669-673.
Tan, Ge; Meier-Abt, Fabienne (2021). Differential expression of hydroxyurea transporters in normal and polycythemia vera hema topoietic stem and progenitor cell subpopulations. Experimental Hematology, 97:47-56.e5.
De Dosso, Sara; Siebenhüner, Alexander R; Winder, Thomas; Mei sel, Alexander; Fritsch, Ralph; Astaras, Christoforos; Szturz, Petr; Borner, Markus (2021). Treatment landscape of metastatic pan creatic cancer. Cancer Treatment Reviews, 96:102180.
Bigenwald, Camille; Le Berichel, Jessica; Wilk, C Matthias; Chakraborty, Rikhia; Chen, Steven T; Tabachnikova, Alexandra; Mancusi, Rebecca; Abhyankar, Harshal; Casanova-Acebes, Maria; Laface, Ilaria; Akturk, Guray; Jobson, Jenielle; Karoulia, Zoi; Martin, Je rome C; Grout, John; Rafiei, Anahita; Lin, Howard; Manz, Markus G; Baccarini, Alessia; Poulikakos, Poulikos I; Brown, Brian D; Gnjatic, Sacha; Lujambio, Amaia; McClain, Kenneth L; Picarsic, Jennifer; Allen, Carl E; Merad, Miriam (2021). BRAF V600E-induced senes cence drives Langerhans cell histiocytosis pathophysiology. Nature Medicine, 27(5):851-861.
71
Brodard, Justine; Kremer Hovinga, Johanna A; Fontana, Pierre; Studt, Jan-Dirk; Gruel, Yves; Greinacher, Andreas (2021). COVID-19 patients often show high-titer non-platelet-activating anti-PF4/ heparin IgG antibodies. Journal of Thrombosis and Haemostasis, 19(5):1294-1298.
Brune, Magdalena M; Stüssi, Georg; Lundberg, Pontus; Vela, Visar; Heim, Dominik; Manz, Markus G; Haralambieva, Eugenia; et al (2021). Effects of lenalidomide on the bone marrow microenvironment in acute myeloid leukemia: Translational analysis of the HOVON103 AML/SAKK30/10 Swiss trial cohort. Annals of Hematology, 100(5):1169-1179.
Sobottka, Bettina; Lorch, Anja; Silina, Karina; van den Broek, Maries; Moch, Holger (2021). Renal cell carcinoma pathology in 2021: ‘new need for renal cancer immune profiling’. Current Opinion in Urology, 31(3):228-235.
Condoluci, Adalgisa; Alberio, Lorenzo; Gomez, Francisco-Javier; Studt, Jan-Dirk; Orlando, Christelle; Jochmans, Kristin; Gerber, Bernhard (2021). Thrombotic storm under DOAC treatment in a patient with homozygous antithrombin Budapest III mutation. Thrombosis research, 201:161-163.
Freiberger, Sandra N; Brada, Muriel; Fritz, Christine; Höller, Sylvia; Vogetseder, Alexander; Horcic, Milo; Bihl, Michel; Michal, Michal; Lanzer, Martin; Wartenberg, Martin; Borner, Urs; Bode, Peter K; Broglie, Martina A; Rordorf, Tamara; Morand, Grégoire B; Rupp, Niels J (2021). SalvGlandDx - a comprehensive salivary gland neo plasm specific next generation sequencing panel to facilitate di agnosis and identify therapeutic targets. Neoplasia, 23(5):473487.
Manz, Salomon M; Losa, Marco; Fritsch, Ralph; Scharl, Michael (2021). Efficacy and side effects of immune checkpoint inhibitors in the treatment of colorectal cancer. Therapeutic Advances in Gastroenterology, 14:17562848211002018.
Catalano, Antonella; Adlesic, Mojca; Kaltenbacher, Thorsten; Klar, Rhena F U; Albers, Joachim; Seidel, Philipp; Brandt, Laura P; He jhal, Tomas; Busenhart, Philipp; Röhner, Niklas; Zodel, Kyra; Fritsch, Kornelia; Wild, Peter J; Duyster, Justus; Fritsch, Ralph; Brummer, Tilman; Frew, Ian J (2021). Sensitivity and Resistance of Oncogenic RAS-Driven Tumors to Dual MEK and ERK Inhibition. Cancers, 13(8):1852.
Mehra, Tarun; Schönegg, Daphne; Ebner, Julian; Moos, Rudolf M; Schumann, Paul; Gander, Thomas; Essig, Harald; Lanzer, Martin (2021). ASA score and procedure type predict complications and costs in maxillofacial reconstructive surgery: a retrospective study using a hospital administrative database. Swiss Medical Weekly, 151:w20497.
Rittmann, Marie-Claire; Hussung, Saskia; Braun, Lukas M; Klar, Rhena F U; Biesel, Esther A; Fichtner-Feigl, Stefan; Fritsch, Ralph; Wittel, Uwe A; Ruess, Dietrich A (2021). Plasma biomarkers for pre diction of early tumor recurrence after resection of pancreatic ductal adenocarcinoma. Scientific Reports, 11:7499.
Benoit, Tobias Matthieu; Schwotzer, Rahel; Schneiter, Didier; Rü schoff, Jan Hendrik; Franzen, Daniel Peter (2021). Rare cause of emphysema. Thorax, 76(4):421-422.
Cabrini, Matteo; Roncador, Marco; Galbiati, Alessandro; Cipolla, Lina; Maffia, Antonio; Iannelli, Fabio; Sabbioneda, Simone; d’Adda di Fagagna, Fabrizio; Francia, Sofia (2021). DROSHA is recruited to DNA damage sites by the MRN complex to promote non-homol ogous end joining. Journal of Cell Science, 134(6):jcs249706.
Pagella, Pierfrancesco; Nombela-Arrieta, César; Mitsiadis, Thimios A (2021). Distinct Expression Patterns of Cxcl12 in Mesenchymal Stem Cell Niches of Intact and Injured Rodent Teeth. International Journal of Molecular Sciences, 22(6):3024.
Simonis, Alexander; Russkamp, Norman F; Mueller, Jan; Wilk, C Matthias; Wildschut, Mattheus H E; Myburgh, Renier; WildnerVerhey van Wijk, Nicole; Mueller, Rouven; Balabanov, Stefan; Valk, Peter J M; Theocharides, Alexandre P A; Manz, Markus G (2021). Disruption of CSF-1R signaling inhibits growth of AML with inv(16). Blood advances, 5(5):1273-1277.
Hoefflin, Rouven; Lazarou, Adriana; Hess, Maria Elena; Reiser, Meike; Wehrle, Julius; Metzger, Patrick; Frey, Anna Verena; Becker, Heiko; et al; Fritsch, Ralph (2021). Transitioning the Molecular Tumor Board from Proof of Concept to Clinical Routine: A German SingleCenter Analysis. Cancers, 13(5):?-?.
Pietge, Heike; Sánchez-Velázquez, Patricia; Akhoundova, Dilara; Siebenhüner, Alexander; Winder, Thomas; Bachmann, Helga; Nguyen-Kim, Thi Dan Linh; Breitenstein, Stefan; Knuth, Alexander; Petrowsky, Henrik; Pestalozzi, Bernhard; Clavien, Pierre-Alain; Sa maras, Panagiotis (2021). Combination of HAI-FUDR and Systemic Gemcitabine and Cisplatin in Unresectable Cholangiocarcinoma: A Dose Finding Single Center Study. Oncology, 99(5):300-309.
van Cromvoirt, Ankie M; Fenk, Simone; Sadafi, Ario; Melnikova, Eli zaveta V; Lagutkin, Denis A; Dey, Kuntal; Petrushanko, Irina Yu; He gemann, Inga; Goede, Jeroen S; Bogdanova, Anna (2021). Donor age and red cell age contribute to the variance in lorrca indices in healthy donors for next generation ektacytometry: a pilot study. Frontiers in Physiology, 12:639722.
Messerli, Michael; Muehlematter, Urs J; Fassbind, Saskia; Franzen, Daniel; Ferraro, Daniela A; Huellner, Martin W; Treyer, Valerie; Cu rioni-Fontecedro, Alessandra; Burger, Irene A (2021). A pilot study on lung cancer detection based on regional metabolic activity distribution in digital low-dose 18F-FDG PET. British Journal of Radiology, 94(1119):20200244.
Irmisch, Anja; Bonilla, Ximena; Chevrier, Stéphane; Lehmann, Kjong-Van; Singer, Franziska; Toussaint, Nora C; Esposito, Cinzia; Mena, Julien; Milani, Emanuela S; Casanova, Ruben; Stekhoven, Daniel J; Wegmann, Rebekka; Jacob, Francis; Sobottka, Bettina; Goetze, Sandra; Kuipers, Jack; Sarabia del Castillo, Jacobo; Prum mer, Michael; Tuncel, Mustafa A; Menzel, Ulrike; Jacobs, Andrea; Engler, Stefanie; Sivapatham, Sujana; Frei, Anja L; Gut, Gabriele; Ficek, Joanna; Miglino, Nicola; Aebersold, Rudolf; Bacac, Marina; Beerenwinkel, Niko; Moch, Holger; Beisel, Christian; Bodenmiller, Bernd; Dummer, Reinhard; Heinzelmann- Schwarz, Viola; Koelzer, Viktor H; Manz, Markus G; Pelkmans, Lucas; Snijder, Berend; Theo charides, Alexandre P A; Tolnay, Markus; Wicki, Andreas; Wollscheid, Bernd; Rätsch, Gunnar; Levesque, Mitchell P (2021). The Tumor Pro filer Study: integrated, multi-omic, functional tumor profiling for clinical decision support. Cancer Cell, 39(3):288-293.
Danioth, Simona; Schanz, Urs; Greutmann, Matthias (2021). Throm bocytopaenia in cyanotic CHD. Cardiology in the Young, 31(3):429434.
Russkamp, Norman F; Myburgh, Renier; Kiefer, Jonathan D; Neri, Dario; Manz, Markus G (2021). Anti-CD117 immunotherapy to elim inate hematopoietic and leukemia stem cells. Experimental Hematology, 95:31-45.
Rogers, S J; Datta, N R; Puric, E; Timm, O; Marder, D; Khan, S; Ma mot, C; Knuchel, J; Siebenhüner, A; Pestalozzi, B; Guckenberger, M; Bodis, S; Riesterer, O (2021). The addition of deep hyperthermia to
72
gemcitabine-based chemoradiation may achieve enhanced sur vival in unresectable locally advanced adenocarcinoma of the pancreas. Clinical and Translational Radiation Oncology, 27:109113.
Kennedy, Alyssa L; Myers, Kasiani C; Bowman, James; Gibson, Christopher J; Camarda, Nicholas D; Furutani, Elissa; et al; Boettcher, Steffen (2021). Distinct genetic pathways define premalignant versus compensatory clonal hematopoiesis in Shwa chman-Diamond syndrome. Nature Communications, 12(1):1334.
Löwenberg, Bob; Pabst, Thomas; Maertens, Johan; Gradowska, Patrycja; Biemond, Bart J; et al; Manz, Markus G (2021). Addition of lenalidomide to intensive treatment in younger and middleaged adults with newly diagnosed AML: the HOVON-SAKK-132 trial. Blood advances, 5(4):1110-1121.
Birrer, Dominique L; Tschuor, Christoph; Reiner, Caecilia; Fritsch, Ralph; Pfammatter, Thomas; Garcia Schüler, Helena; Pavic, Matea; De Oliveira, Michelle; Petrowsky, Henrik; Dutkowski, Philipp; Oberko fler, Christian; Clavien, Pierre-Alain (2021). Multimodal treatment strategies for colorectal liver metastases. Swiss Medical Weekly, 151:w20390.
Meier-Abt, Fabienne; Wolski, Witold E; Tan, Ge; Kummer, Sandra; Amon, Sabine; Manz, Markus G; Aebersold, Ruedi; Theocharides, Alexandre P A (2021). Reduced CXCL4/PF4 expression as a driver of increased human hematopoietic stem and progenitor cell pro liferation in polycythemia vera. Blood Cancer Journal, 11(2):31.
Rütsche, Cyrill V; Haralambieva, Eugenia; Lysenko, Veronika; Bal abanov, Stefan; Theocharides, Alexandre P A (2021). A patient with a germline GATA2 mutation and primary myelofibrosis. Blood advances, 5(3):791-795.
Alankus, Begüm; Ecker, Veronika; Vahl, Nathalie; Braun, Martina; Weichert, Wilko; Macher-Göppinger, Stephan; Gehring, Torben; Neumayer, Tanja; Zenz, Thorsten; Buchner, Maike; Ruland, Jürgen (2021). Pathological RANK signaling in B cells drives autoimmunity and chronic lymphocytic leukemia. Journal of Experimental Medicine, 218(2):e20200517.
Peters, S; Felip, E; Dafni, U; Tufman, A; Guckenberger, Matthias; Ál varez, R; Nadal, E; Becker, A; Vees, H; Pless, M; Martinez-Marti, A; Lambrecht, M; Andratschke, Nicolaus; Tsourti, Z; Piguet, A-C; Ro schitzki-Voser, H; Gasca-Ruchti, A; Vansteenkiste, J; Stahel, Rolf A; De Ruysscher, Dirk (2021). Progression-free and overall survival for concurrent nivolumab with standard concurrent chemo-radio therapy in locally advanced stage IIIA/B NSCLC: Results from the European Thoracic Oncology Platform NICOLAS phase II trial (ETOP 6-14). Journal of Thoracic Oncology, 16(2):278-288.
Borchmann, Peter; Plütschow, Annette; Kobe, Carsten; Greil, Rich ard; Meissner, Julia; Topp, Max S; et al; Balabanov, Stefan (2021). PET-guided omission of radiotherapy in early-stage unfavourable Hodgkin lymphoma (GHSG HD17): a multicentre, open-label, ran domised, phase 3 trial. Lancet Oncology, 22(2):223-234.
Stremmel, Christopher; Scherer, Clemens; Lüsebrink, Enzo; Kupka, Danny; Schmid, Teresa; Stocker, Thomas; Kellnar, Antonia; Klee berger, Jan; Sinner, Moritz F; Petzold, Tobias; Mehilli, Julinda; Braun, Daniel; Orban, Mathias; Hausleiter, Jörg; Massberg, Steffen; Or ban, Martin (2021). Treatment of acute cardiac tamponade: A ret rospective analysis of classical intermittent versus continuous peri cardial drainage. International journal of cardiology. Heart & vasculature, 32:100722.
Hussung, Saskia; Akhoundova, Dilara; Hipp, Julian; Follo, Marie; Klar, Rhena F U; Philipp, Ulrike; Scherer, Florian; von Bubnoff,
Nikolas; Duyster, Justus; Boerries, Melanie; Wittel, Uwe; Fritsch, Ralph M (2021). Longitudinal analysis of cell-free mutated KRAS and CA 19-9 predicts survival following curative resection of pan creatic cancer. BMC Cancer, 21:49.
Witjes, J Alfred; Bruins, Harman Max; Cathomas, Richard; Com pérat, Eva M; Cowan, Nigel C; Gakis, Georgios; Hernández, Vir ginia; Linares Espinós, Estefania; Lorch, Anja; Neuzillet, Yann; Rouanne, Mathieu; Thalmann, George N; Veskimäe, Erik; Ribal, Ma ria J; van der Heijden, Antoine G (2021). European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. European Urology, 79(1):82-104.
Hiltbrunner, S; Britschgi, C; Schuberth, P; Bankel, L; Nguyen-Kim, T D L; Gulati, P; Weder, W; Opitz, I; Lauk, O; Caviezel, C; Bachmann, H; Tabor, A; Schröder, P; Knuth, A; Münz, C; Stahel, R; Boyman, O; Renner, C; Petrausch, U; Curioni-Fontecedro, A (2021). Local deliv ery of CAR T cells targeting fibroblast activation protein is safe in patients with pleural mesothelioma: first report of FAPME, a phase I clinical trial. Annals of Oncology, 32(1):120-121.
Adenaeuer, Anke; Ezigbo, Eyiuche D; Fawzy Nazir, Hanan; Barco, Stefano; Trinchero, Alice; Laubert-Reh, Dagmar; Strauch, Konstan tin; Wild, Philipp S; Lackner, Karl J; Lämmle, Bernhard; Rossmann, Heidi (2021). c.451dupT in KLKB1 is common in Nigerians, confirm ing a higher prevalence of severe prekallikrein deficiency in Africans compared to Europeans. Journal of Thrombosis and Haemostasis, 19(1):147-152.
Seydoux, Claire; Medinger, Michael; Gerull, Sabine; Halter, Joerg; Heim, Dominik; Chalandon, Yves; Levrat, Stavroula Masouridi; Schanz, Urs; Nair, Gayathri; Ansari, Marc; Simon, Patrick; Passweg, Jakob R; Cantoni, Nathan (2021). Busulfan-cyclophosphamide ver sus cyclophosphamide-busulfan as conditioning regimen before allogeneic hematopoietic cell transplantation: a prospective ran domized trial. Annals of Hematology, 100(1):209-216.
Mun, YeVin; Nombela-Arrieta, César (2021). 3D Microscopy of Mu rine Bone Marrow Hematopoietic Tissues. Methods in Molecular Biology, 2308:127-138.
Meihandoest, Tamana; Studt, Jan-Dirk; Mendez, Adriana; Albe rio, Lorenzo; Fontana, Pierre; Wuillemin, Walter A; Schmidt, Adrian; Graf, Lukas; Gerber, Bernhard; Maeder, Gabriela Monika; Bovet, Cédric; Sauter, Thomas C; Nagler, Michael (2021). Automated Thrombin Generation Assay for Rivaroxaban, Apixaban, and Edox aban Measurements. Frontiers in cardiovascular medicine, 8:717939.
Wilk, C Matthias; Manz, Markus G; Boettcher, Steffen (2021). Clonal hematopoiesis in hematopoietic stem cell transplantation. Current Opinion in Hematology, 28(2):94-100.
Barco, Stefano; Adenaeuer, Anke; Trinchero, Alice; Falter, Tanja; Lackner, Karl J; Lämmle, Bernhard; Rossmann, Heidi (2021). Com ment on «Worldwide Distribution of PK Deficiency: the Defect Seems Mainly Concentrated in West African Countries and the United States». Mediterranean Journal of Hematology and Infectious Diseases, 13(1):e2021027.
Saito, Yasuyuki; Willinger, Tim; Theocharides, Alexandre P A (2021). Editorial: Advances in Human Immune System Mouse Models for Studying Human Hematopoiesis and Cancer Immunotherapy. Frontiers in Immunology, 12:829644.
Zavala, Flora; Nombela-Arrieta, César; Ben Nasr, Moufida; Fiorina, Paolo (2021). Editorial: The Role of Hematopoietic Progenitors in Immune Regulation and Memory. Frontiers in Immunology, 12:789139.
73
Lee, Che-Jui; Wozniak, Agnieszka; Van Cann, Thomas; Timmer mans, Iris; Wellens, Jasmien; Vanleeuw, Ulla; Briaire-de Bruijn, Inge H; Britschgi, Christian; Bovée, Judith V M G; Zlobec, Inti; Sciot, Raf; Schöffski, Patrick (2021). Establishment of an Academic Tissue Microarray Platform as a Tool for Soft Tissue Sarcoma Research. Sarcoma, 2021:6675260.
Kliesch, Sabine; Schmidt, Stefanie; Wilborn, Doris; Aigner, Clem ens; Albrecht, Walter; Hermanns, Thomas; Lorch, Anja; Albers, Pe ter (2021). Management of Germ Cell Tumours of the Testis in Adult Patients. German Clinical Practice Guideline Part I: Epidemiology, Classification, Diagnosis, Prognosis, Fertility Preservation, and Treatment Recommendations for Localized Stages. Urologia internationalis, 105(3-4):169-180.
Koeberle, Dieter; Fritsch, Ralph (2021). Targeting HER2 in Biliary Tract Carcinomas: Challenges and Opportunities. Oncology Research and Treatment, 44(1-2):1-3.
Habib, Marrium; Ludwig, Sabine; Lange, Ute; Prazeres da Costa, Clarissa (2021). The impact of the COVID-19 pandemic on preg nancy, birth and sexual & reproductive health and rights: Perspectives from Germany and Somalia. Journal of Global Health, 11:03085.
Hiltbrunner, Stefanie; Mannarino, Laura; Kirschner, Michaela B; Opitz, Isabelle; Rigutto, Angelica; Laure, Alexander; Lia, Michela; Nozza, Paolo; Maconi, Antonio; Marchini, Sergio; D’Incalci, Maur izio; Curioni-Fontecedro, Alessandra; Grosso, Federica (2021). Tumor Immune Microenvironment and Genetic Alterations in Mesothelioma. Frontiers in Oncology, 11:660039.
Stirm, Kristin; Leary, Peter; Bertram, Katrin; Núñez, Nicolás Gon zalo; Wüst, Daria; Boudesco, Christophe; Verhoeyen, Els; Zenz, Thorsten; Becher, Burkhard; Menter, Thomas; Tzankov, Alexandar; Müller, Anne (2021). Tumor cell-derived IL-10 promotes cell-auton omous growth and immune escape in diffuse large B-cell lym phoma. OncoImmunology, 10(1):2003533.
74
www.usz.ch/fachbereich/medizinische-onkologie-und-haematologie/team
Hier entsteht das neue CCCZ. Wir freuen uns...

Team 14
75
Universitätsspital Zürich Klinik für Medizinische Onkologie und Hämatologie Rämistrasse 100 8091 Zürich
www.usz.ch/moh
Folgen Sie dem USZ unter
Wir wissen weiter.






















































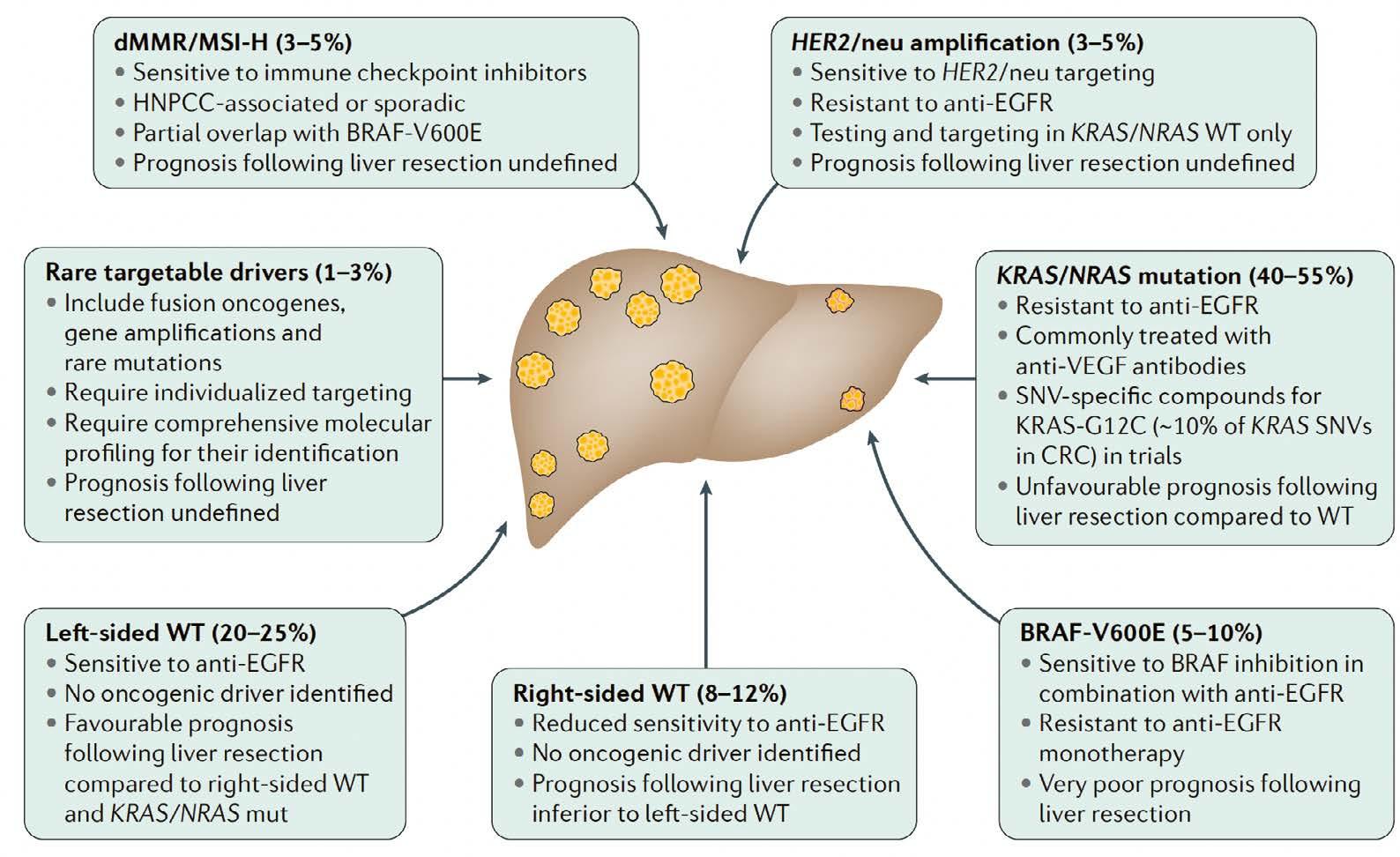








 Andreas Wicki andreas.wicki@usz.ch
Andreas Wicki andreas.wicki@usz.ch




