DISSO America 2022
Keynote Address




Overview Current Regulatory Paradigm Applications of In Vitro Dissolution studies Dissolution Media: Limitations & ways of Optimization Dissolution Media: Bio-relevant vs. Bio-predictive? Challenges with Dissolution Testing Technology advancements in Dissolution Dissolution in Modeling & Simulations Emerging Trends in Bio-relevant Dissolution Concluding remarks
Current Regulatory Paradigm


USP Monographs • For Immediate release Tablets, USP monograph typically suggests 70% (Q) or 75% (Q) in 45 minutes or 60 minutes in the applicable dissolution method • For an Extended release Tablets, USP monograph typically suggests range of 25% to 40% FDA dissolution methods database • USP II, 50 rpm OR USP I, 100 RPM with 900 ml media (0.1N HCl / pH 4.5 buffer / pH 6.8 buffer with or without solubilizer) • USP I / USP II / USP III using applicable media with extended period of sampling (e.g. 1, 2, 4, 6, 8, 12, 16, 20 and 24 hours) Agency’s current expectation • 80%(Q) or 85%(Q) in 15 or 30 minutes in the applicable dissolution method based on the bio batch dissolution profile • Bio batch dissolution profile with ± 10% range • Wider range requires multiple bio studies with different dissolution profiles with / without supporting PBPK model
Applications of In Vitro Dissolution Studies


1 Compliance with guidelines To support waivers for BE ‘Biowaivers’ To select candidate formulation Surrogate for In Vivo study Quality control procedure To simulate food effects on BA To identify critical manufacturing variables In Vitro In Vivo Correlation (IVIVC) SUPAC and ICH ‘Proof of concept’ to decide suitable candidate To predict the drug release compared to reference product during development Post meal effects and Pharmacokinetic profile An alternative to In Vivo pharmacokinetic Relates between drug release in a dissolution apparatus & amount of drug enters bloodstream Relationship between the dissolution test and biological data Effects of mixing, binders; Excipients role; Production effects associated with NDDS Few applications of In-Vitro dissolution 1 2 3 Formulation and Optimization decisions Equivalence decisions Product compliance In-Vitro Dissolution Studies: Helps in decision making 2 3 4 56 7 8
& ways of Optimization




Dissolution Media: Limitations
Simple aqueous buffers are often not suitable for bioequivalenceBioequivalence cannot be used to simulate the influence of food ingestion on drug releaseEffect of food Aqueous buffers do not represent other key aspects of the composition of the GI contents like osmolality, ionic strength, viscosity, surface tension Media composition When dissolution testing is used to forecast the in vivo performance of a drug, it is critical that the in vitro test mimic the conditions in vivo as closely as possible IVIVC Limitations of dissolution media pH • pH range of 1.2 to 6.8 are recommended Temperature • Normally 37°C temperature is used • Different temperature in cases like suppositories Surfactants • For poorly water soluble drugs, as they increase wetting and solubility Simulated media • More accurate simulation of pharmacokinetic profiles Volume • For USP I and II, 900 mL media is normally used • Using different volume for different strengths may provide good sink condition Geometry • Geometry impacting testing Dissolved Gases • Changes pH, forms bubbles, alteration of the interaction between media and API Optimzation of dissolution media


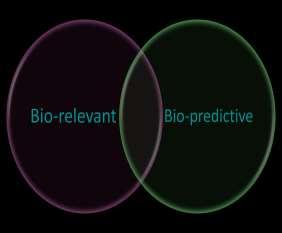
Simulate Gut physiology: Physiologically relevant Non standards experimental conditions: Complexity helps sometimes..! Bio-relevant media Simulate the Clinical PK study outcomes or in vivo release Conventional or nonconventional methods: Simplicity is desirable..! Biopredictive media Dissolution Media: Biorelevant vs. Biopredictive





Challenges with Dissolution Testing 1 Discriminatory power Poorly soluble compounds 2 Variability 3 Types of equipment used 4 Factors affecting drug reactions 5 Bioavailability 7 Postapproval validation 6 Drug stability 8 Future Automation in Mfg. & Need for online testing 9




What it means? • Dissolution method is required to be sensitive to changes in critical quality attributes (CQAs) of a drug product, yet it should be sufficiently rugged and reproducible • Challenging to demonstrate discriminatory power in some cases. For example 1. Soft Gelatin Capsules • Content is drug dissolved or dispersed in oily vehicle along with some inactive like anti oxidants • Hence usually gelatin ribbon thickness is increased intentionally which may affect the capsule opening and hence the dissolution testing outcome 2. Tablet formulation having BCS I API and Direct Compression • High solubility of API and Lack of binder or other inactive, which can help to make intentional meaningful changes to demonstrate discrimination within range of ±10% 20% changes 3. Hard Gelatin Capsule having API and one Lubricant only • Process is simple blending and filling where Only one excipient used, where ±10%–20% changes in concentration may not be adequate to demonstrate discrimination In above cases, dissolution method may be very sensitive due to stringent dissolution condition and hence, it may be over discriminatory to be used as routine QC tool Challenges with Dissolution Testing: Discriminatory power & Poorly soluble compounds (1/3) Discriminatory power1 Poorly soluble compounds2 Choice of dissolution condition should be physiologically relevant Temperature 37℃ Media pH 1.2 to 6.8 Agitation 100rpm Basket 50rpm Paddle For instance Poorly soluble drugs: use of surfactant, co solvent and pH outside physiological range are also used • Glyburide (Non micronized) Tablets: pH 9.5 • Celecoxib capsules: pH 12.0. • Atovaquone Tablets: 40% isopropanol buffered to pH 8.0 with potassium dihydrogen phosphate Use of above media can be explored as QC tool. However these non physiological condition may have poor prediction of in vivo behavior for new developments What can be the implications of additives?
•
Challenges with Dissolution Testing: Variability
Critical parameters
•
•
•
speed
• Vessel or shaft tilt
• Wobble
•
position in
• Coning
•
•
•
Take away
•
Dead-zone
• Computational fluid dynamics studies showed poor hydrodynamics and hence poor mixing
• Dead zone region directly underneath the paddle, and hence therefore position of tablet/capsule has significant effect
Fiber Optics Technology (FOT)
Automation
• For the analysis of Oral solid dosage forms
• Uses 12 dip-type fiber optic probes coupled to 12 separate PDA spectrophotometers to acquire continuous dissolution curves in real time
• System is accurate, quicker and easier to set up when compared with conventional HPLC or UV-sipper systems
•
•
Real-Time Release Testing (RTRT)
• Gives assurance that the product is of intended quality, based on the information collected during the manufacturing process
• Comprise a combination of process controls which may utilise process analytical technology (PAT) tools e.g., near Infrared spectroscopy (NIR) and Raman spectroscopy (usually in combination with multivariate analysis)
RTRT
• Enable real time control and
• Speed & Minimization of analyst to analyst variability
• Eliminating the need for off line sampling
• High sampling rates, and reproducibility
• Hydrodynamics, linear range and light scattering effects
intervention
• Assessment based on larger data
• Reduced overall operational cycle times
Apparatus 2
Shaft centering
Shaft height
Rotational
Vessel variability
Tablet
vessel
Periodical physical calibration of dissolution apparatus
Peak vessel & Tilted vessel
Crescent shaped spindles
Mega paddles & Off centre paddle
Since apparatus 2 is most widely used of all dissolution apparatus, its limitation is of significant importance. Any small changes in tablet / capsule position can produce large differences in dissolution rate and thus in turn may result in significant variability
Efforts to increase discriminatory power (reduced agitation speed, lower volume etc.) would lead to further poor hydrodynamic mixing ability that typically leads to incomplete and variable dissolution
FOT
process
Advantages
(2/3) Source of Variability3
Challenges with Dissolution Testing: Other factors (3/3)



fast
Types of equipment used
formulation

develop “
size
can
affecting
issues in using

analytical
(e.g.
all”
in a
or
•Differences with the equipment used—such as how different types of equipment are maintained, or if there are vibrations within the systems • In
paced
development labs it
be tempting to
one
fits
tests for formulations 4 Factors
drug reactions People tend to have differing reactions to drugs, which can lead to inconsistencies in factors such as: •Timing of the drug’s release, •How long the drug stays in the individual’s gastrointestinal tract •The drug’s acidity level Bioavailability • Dissolution test does not always provide accurate information about the bioavailability of a drug. • For example, Poorly soluble drugs 7 Drug stability • It does not always provide accurate information about the stability of drug • Drug stability can be affected by a variety of factors , including dose, formulation, and exposure to moisture and light 8 5 Post approval validation • Possible
the same facility to perform both dissolution tests and method validation post market approval • Further
method transfers
performing tests
different laboratory,
introducing a new facility for manufacturing) after receiving approval 6 Future Automation & Online testing • Future Automation in Manufacturing • Need for online testing 9
Emerging Trends in Bio-relevant Dissolution


Improved tests to predict low solubility drug behavior in the GI tract which additionally account for factors such as Dynamic media change with GI transit, Supersaturation and precipitation, Motility/ hydrodynamics, Food and digestion, Buffer capacity, Permeation etc. Accounting for permeation in a drug release test Improve inputs for PBPK modelling


Dissolution in Modeling & Simulations Dissolution input in PBPK is the ONLY way to link to drug product quality Bio-predictive dissolution Physicochemical, biopharmaceutic and pharmacokinetic parameters IVIVC/IVIVR & PBPK modeling Bridge the future formulation change Mechanical insights in to critical factors affecting Absorption Biowaiver Bridge the future formulation change CMA Identification End outcome: Accelerated drug product development
advances in Dissolution


Technology
• Automation: PAT tools like RAMAN Pro, Fibre-Optic dissolution testing • RTRT - Real Time Release Testing: As a part of continuous manufacturing, platforms are emerging to replace the traditional dissolution tests • An Artificial Neural Network (ANN) model: To predict the dissolution profile • Multivariate model: Surrogate for conventional dissolution testing • Mechanistic in-vitro dissolution simulation tool (DDD plus™): For in-vitro dissolution experiments


Concluding Remarks • Well established Quality control tool; widely accepted in regulatory processes • Technology advancement in automation • Scope of betterment in terms of Bio-relevant & Bio- predictability • Advancement in dissolution tools help in better predictability of PBPK models • A well qualified model with high confidence can be used to aid regulatory decision-making • The adoption of the harmonized practices and advanced scientific principles will result in better decision-making, ultimately leading to improved patient outcomes with the development of safe and efficacious drug products
Direction for the future



How the Digital Technology and Artificial Intelligence (AI)
can be used to make in-vitro
dissolution more predictive
and
reliable
for
quality and bioavailability


 Zydus Corporate Park, SG Highway, Near Vaishnodevi Circle, Ahmedabad, 382481, India
Zydus Corporate Park, SG Highway, Near Vaishnodevi Circle, Ahmedabad, 382481, India































 Zydus Corporate Park, SG Highway, Near Vaishnodevi Circle, Ahmedabad, 382481, India
Zydus Corporate Park, SG Highway, Near Vaishnodevi Circle, Ahmedabad, 382481, India
