Dissolution Data Integrity



Who did what, when, and where?
Ajay Pydah, Lead Software and Data Management SOTAX
Data Integrity: A Hot Topic
Data Integrity Compliance with Use of Automation
Instrument Automation
Automation












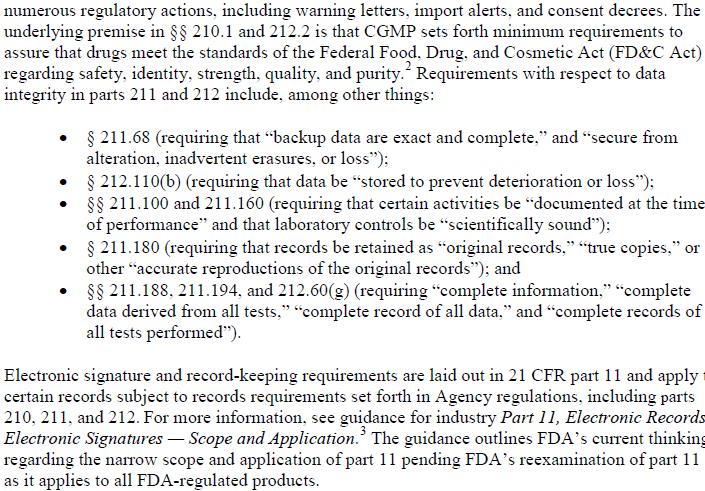
Data Integrity and Compliance with cGMP, Guidance for Industry, April 2016




GMP Data Integrity Definitions and Guidance for Industry, March 2015











Most data integrity concerns observed by the FDA find that the data included in the official records is not the complete data.


Data Integrity Matters: Why is Data Integrity Important?, Heather Longden, Waters, May 2017


Other “versions” of the truth exist in the electronic records, yet never became part of the official record.
These results have been excluded by oversight, without scientifically justified invalidation or deliberately to deceive.




Manufacturers and analytical laboratories should be aware that reverting from automated / computerised to manual / paper based systems will not in itself remove the need for data integrity controls.



21 CFR Part 11 is “only” about electronic data
Code of Federal Regulations Title 21 (21 CFR, Part 11), April 2016
Data Integrity and Compliance with cGMP, Guidance for Industry, April 2016


Today, laboratories need to prove their data integrity and regain the trust of regulators, focusing on the existence of “orphan data” and what it might be hiding.




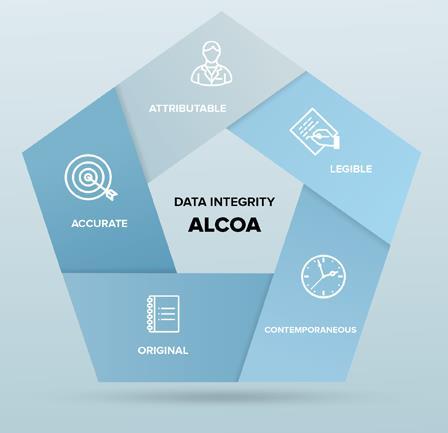
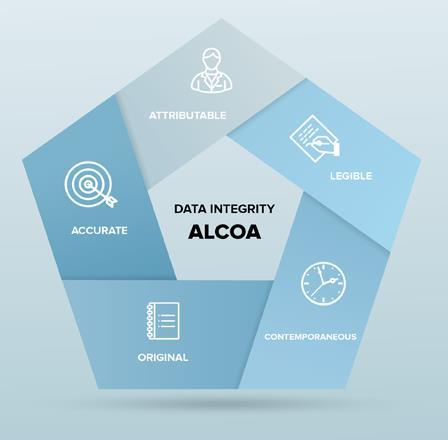
The FDA has been using the ALCOA acronym as a guide to their expectations regarding evidence (both paper-based, electronic, and hybrid) for years and most other health inspectorates have similar expectations. As such, it is immensely useful in developing strategies to prospectively generate strong evidence in both research and manufacturing.

ALCOA
Standard for evidence, T.J. Kuhn, Good Practice for the Pharmaceutical Industry from the Quality Assurance Perspective, July 2008

























































• Data Integrity is the term used to describe the overall accuracy, completeness, and consistency of data generated in the workplace, specifically, the Pharmaceutical industry.
• We uphold this integrity by having a GMP mindset in that we regulate processes, rules and accepted standards.


PHYSICAL INTEGRITY: Protection of the entirety and the accuracy of the data being stored. Protection from fire, flood, theft, format changes, accidental deletion.
LOGICAL INTEGRITY: Data remains unchanged over time regardless of storage or retrievals and protected from human errors and hacker intrusions.









How


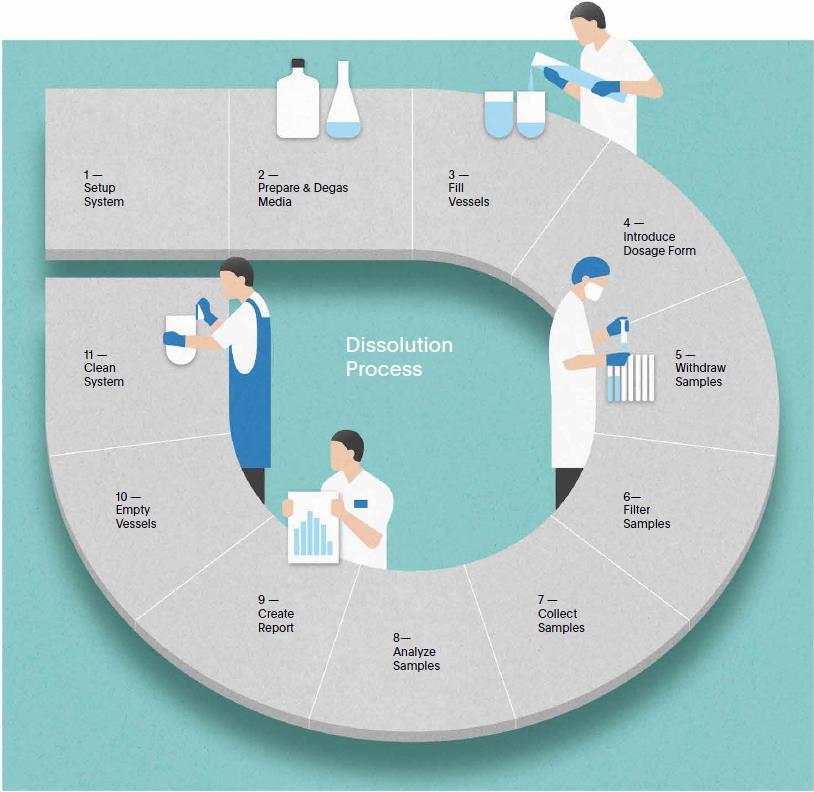

100% manual.
All steps of the dissolution test are performed manually. An automatically generated report documents the test conditions.

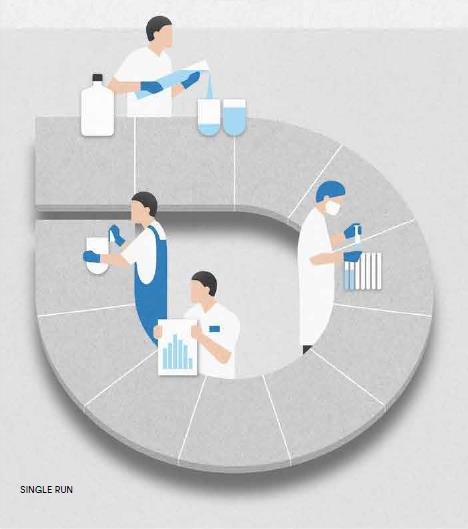


Semi-automated systems automate sampling and filtration for a single test run with integrated analytics or offline sample collection.

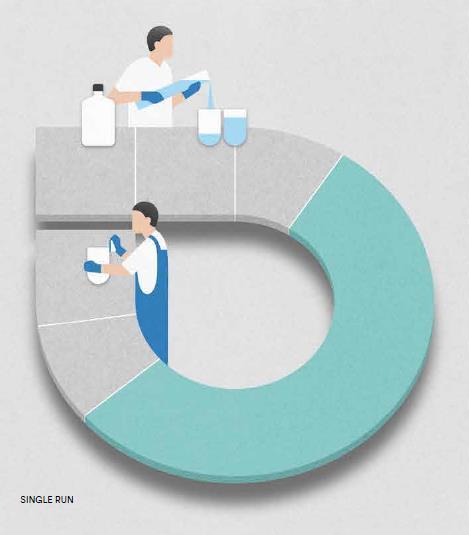

Clean in place the same way every time – and let your system prepare itself for the next run.
Fill vessels automatically and record delivered volumes gravimetrically. Media is dispensed down the vessel wall avoiding reaeration.

Take samples the same way every time from repeatable sampling positioning to automatic filter change and replacement of collected sample volumes, or pH changes.
No need to remove vessels anymore. The self cleaning system automatically empties all vessels on completion of a test run and starts the washing routine.







Methods and your Meta Data are version controlled.
CFR Part 11 Compliant.
Audit trial in a centralized database.


Single Sign-on mechanisms for user authentication.

Data automatically pushed to your
Data Integrity built in!


























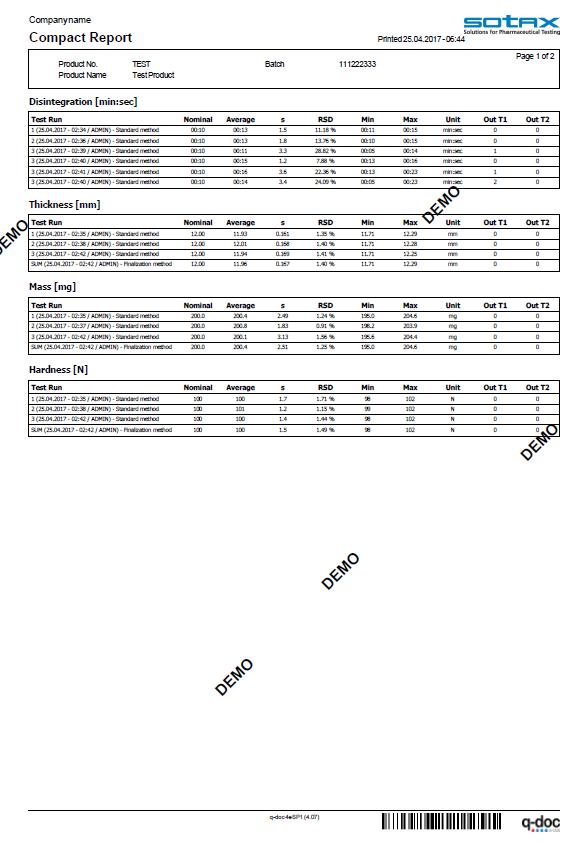
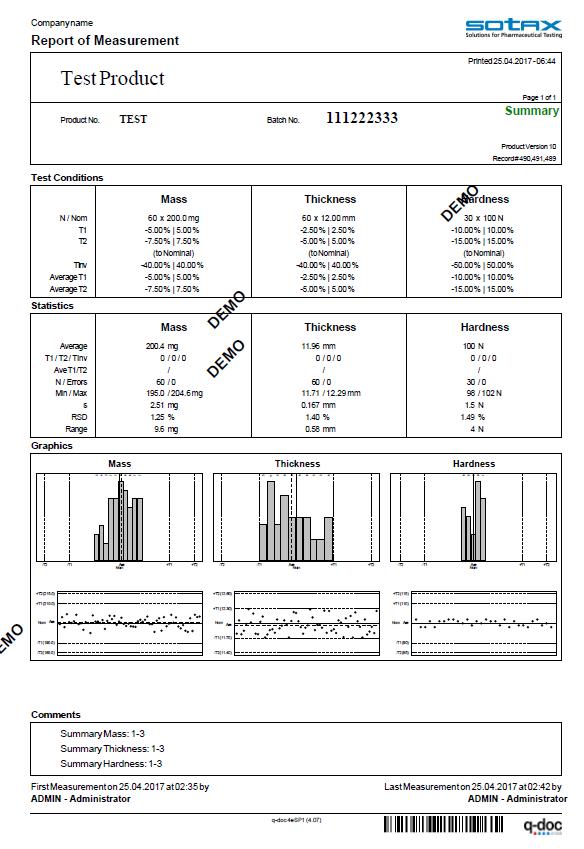
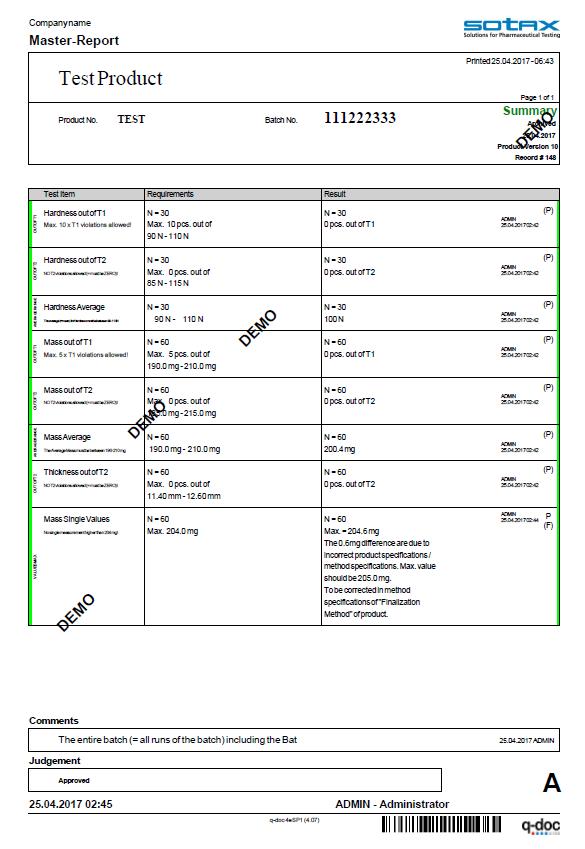




Efficiency included. From audit trail to electronic signatures, product version control, and advanced user management with full LDAP integration
the q-doc® framework is designed for efficient operation with full 21 CFR Part 11 compliance.