
Enabling a Healthier World Public In Vivo Predictive Dissolution Media Selection Using Drug Formulation and Physiological Properties Deanna Mudie | September 2022
GI fluid and drug substance properties

impacting dissolution
Dissolution medium selection methodology based upon interplay between drug, formulation, and GI fluid properties
Summary - Knowledge of drug
formulation - GI fluid property interplay can streamline media selection and enable right-first-time development
Agenda
1 2 3
Solid Oral Dosage Forms Must


Tract
Transport to blood
Public
Dissolve and Absorb In The GI
Deanna Mudie | September 2022 Disintegration & dissolution
Dissolution & precipitation Intestinal transit GI membrane absorption Gastric emptying 3
Physiological,
Public
Drug Substance and Excipient Properties Affect in vivo Solubility & Dissolution Rate Deanna Mudie | September 2022 Intrinsic solubility Acidity, Basicity pKa Lipophilicity Melt temperature Crystal form Polymorph Salt form Swellability drug substance & excipient properties physiological properties pH Buffer species & concentration Mixed lipidic colloidal species Osmolality Surface tension Viscosity 4
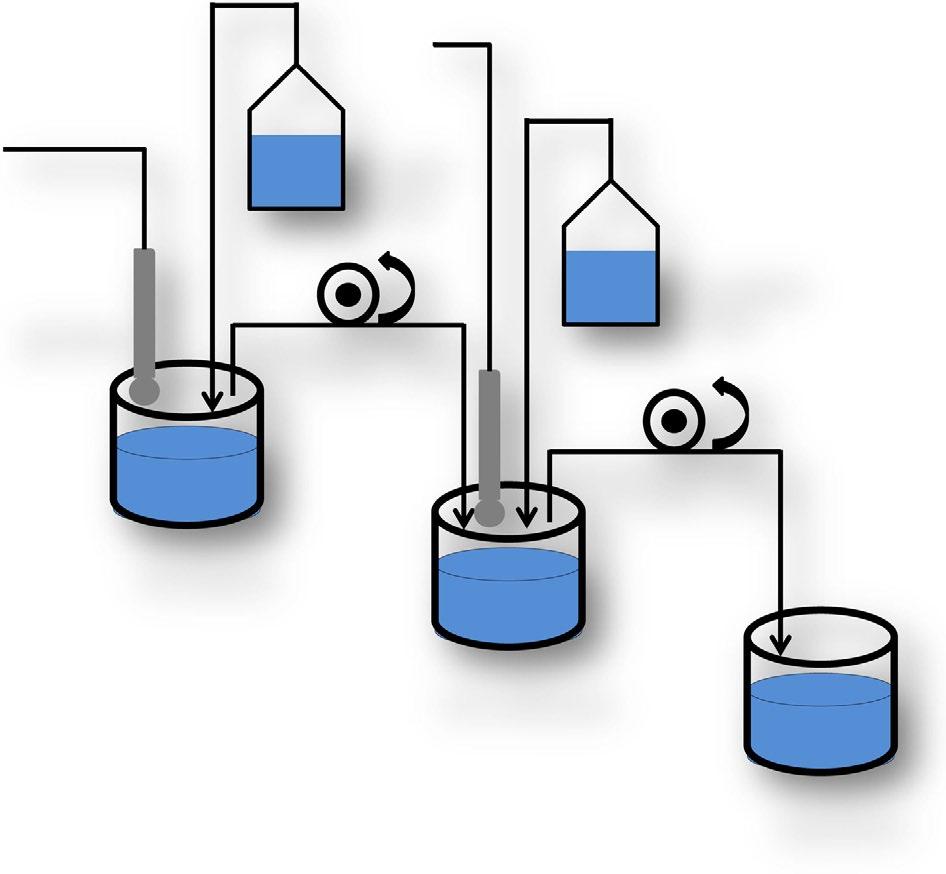
PublicDeanna Mudie | September 2022 Single fluid USP Multi-fluid transfer Dissolution-permeation Artificial Stomach-Duodenum or GIS D/P System In Vitro Dissolution Testing is the Gold Standard for Assessing Oral Bioperformance Tsume, Y. et al., Eur J Pharm Sci, 76, 203 212 (2015) Carino, S.R., et al., J Pharm Sci, 95, 116-125 (2006) Kataoka, M. et al., Pharm Res, 20, 1674 1680 (2003) 5
PublicDeanna Mudie | September 2022 Example: simulated human fasted state intestinal fluid Mudie et al. AAPS J. 2020 Jan 27;22(2):34, Mudie et al. Mol Pharm. 2010 Sep 7;7(5), Fuchs et al. J Pharm Sci. 2014 Nov;103(11) Increasing physiological relevance, increasing complexity *Jejunal segment **Median, range of 2 8 USP SIF, TS FaSSIF FaSSIF-V2 FaSSIF-V3 Year 1996 1998 2008 2015 pH 6.8 6.5 6.5 6.7 Buffer species Phosphate Phosphate Maleate Maleate or phosphate Buffer concentration (mM) 50 28.7 19.1 10.3 or 13.5 Buffer capacity (mM/ΔpH) 18.4 12 10 5.6 Bile salt(s) (mM) 3 (TC) 3 (TC) 1.4 (TC), 1.4 (GC) Phospholipid(s) (mM) 0.75 (PC) 0.2 (PC) 0.035 (PC), 0.315 (LPC) Fatty acids (mM) 0.315 Lipids (mM) 0.2 Human intestinal fluid* 5.5 7** Bicarbonate 6 - 20 0.3 – 6.3 1.4 5.5 0.2 0.1 0.6 0.1 – 0.3 Biorelevant Dissolution Media Have Evolved and Differ in Properties And Composition 6
Public Method for Selecting Practical, Yet Biorelevant Dissolution Media for Poorly Soluble (i.e. BCS 2/4) Drugs Deanna Mudie | September 2022 Selection methodology based upon GI fluid –drug/excipient property interplay: Acidity/ Basicity pKa Acidity/ Basicity pKa & intrinsic solubility log D pH Buffer species & concentration Bile salts and lipids GI fluid property drug substance or excipient property 7
Selection of Medium pH

Deanna Mudie | September 2022
PublicDeanna Mudie | September 2022 pH 1 –3 pH 5.5 –7 1 10 100 1,000 1 2 3 4 5 6 7 8 Relative solubility pH Weak acid HA A + H+ 0 10 1 2 3 4 5 6 7 8 Relative solubility pH Neutral (8 < pKa < 1) C C + H2O 1 10 100 1,000 10,000 100,000 1,000,000 1 2 3 4 5 6 7 8 Relative solubility pH Weak base Tsume, Y. et al. Eur J Pharm Sci. 2014., Koenigsknecht et al. Mol Pharm. 2017., Bergstrom CAS et al. Eur J Pharm Sci. 2014 �������� �������� = ���������������� ����(����) ��������� (����) � ��������(����) −�������� ���� Interplay Between Drug/Excipient and Fluid pH – Impact on Solubility and Dissolution Rate 9
Recommendations for pH Selection
Weak acids Weak bases Neutral
Low dissolution at gastric pH, high at intestinal pH
• Simplify media by only testing at intestinal pH
• Consider intestinal pH range of 5.5 – 7 when pKa ≤ 7
High dissolution at gastric pH, possible precipitation at intestinal pH
• Test starting at gastric pH, then transfer to intestinal pH
• Consider intestinal pH range of 5.5 – 7 when pKa ≥ 5.5
pH independent dissolution
• Simplify media by testing at any single pH
Public
Deanna Mudie | September 2022
Mudie et al. AAPS J. 2020 Jan 27;22(2):34 10
Selection of Buffer Species & Concentration

Deanna Mudie | September 2022
of Haloperidol
bicarbonate
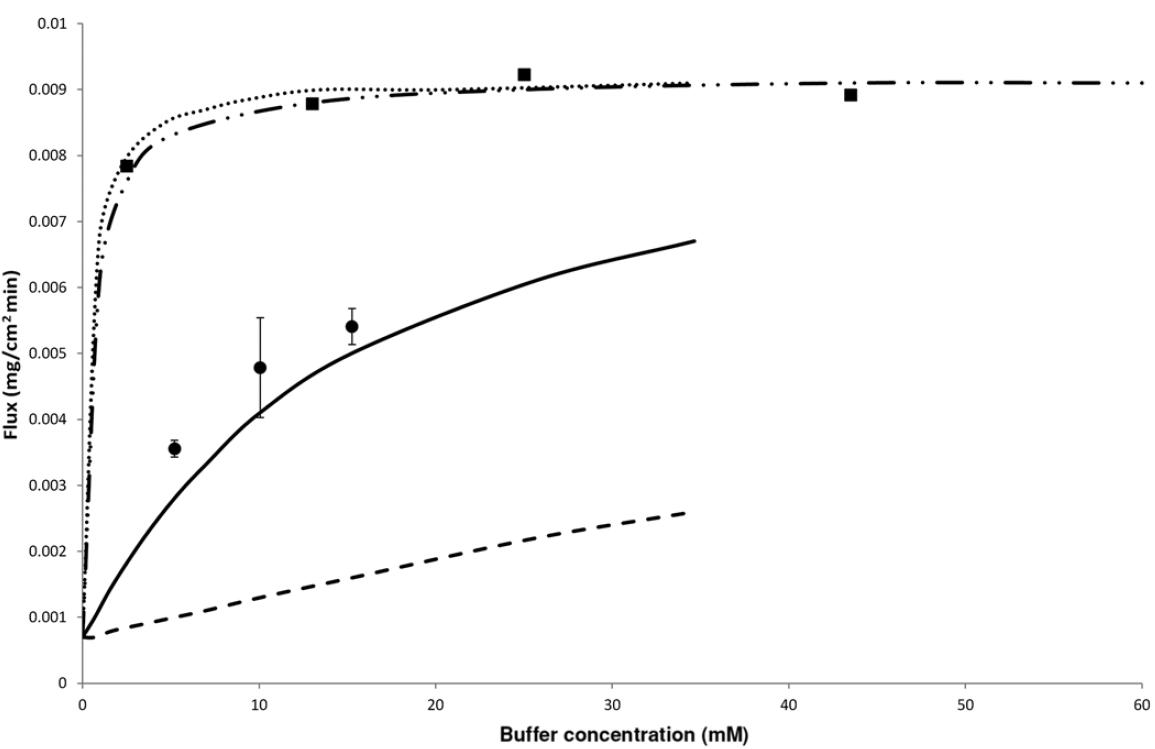
Public Interplay Between Drug/Excipient and Fluid Buffer Species & Concentration Deanna Mudie | September 2022 0.5 – 20 mM HCl 6 – 20 mM bicarbonate Flux
(base) in
buffer and phosphate buffer at bulk pH 6.5 Figure from Krieg et al., J. Pharm. Sci. (2015), 1 11 Mudie et al. AAPS J. 2020 Jan 27;22(2):34 12
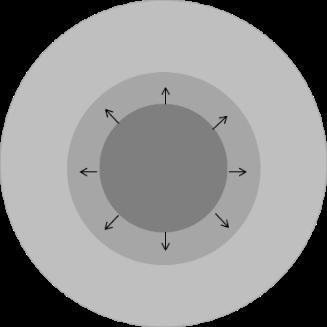
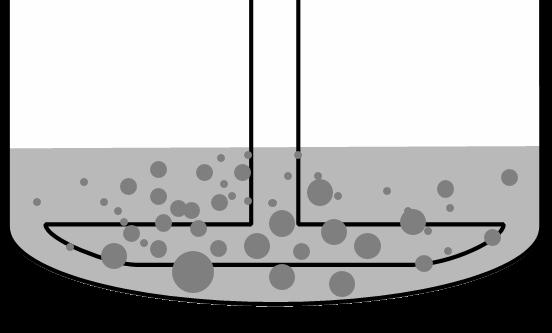
Public �������� �������� = ���������������� ���� � �������� (����) � �������� − �������� ���� Mechanism for Buffer Effects – Solid Surface pH Deanna Mudie | September 2022 Cs = ʄ(pHs) Cb = ʄ(pHb) • pHs ≠ pHb for some acids & bases • Lowers dissolution driving force pKa S0 Dissolution Rate Weak Acid pHs < pHb Weak Base pHs > pHb Mudie et al. AAPS J. 2020 Jan 27;22(2):34 pKa S0 Dissolution Rate 13
Public Buffer Effects Example with Weak Acids Deanna Mudie | September 2022 ������������. �������������������������������������������� ���������������� = �������� @������������ �������� �������� �������� @������������ ���� �������� pKa S0 Dissolution Rate Weak Acid pHs < pHb 0 2 4 6 8 10 12 14 16 18 20 4 5 6 7 8 9 10 11 12 13 Relative dissolution rate alpha (=pKa logSo) pKa = 4 pKa = 6 Increasing So pKa = 3 pKa = 5 Buffer concentration matters when pKa < 6 and alpha (=pKa - log So) < 10 Mudie et al. AAPS J. 2020 Jan 27;22(2):34 14
Recommendations for Buffer Selection
Weak
Weak
Neutral
Public
Deanna Mudie | September 2022
acids • Buffer species & concentration matter when pKa < 6 and alpha (= pKa- log So ) < 10 • When outside this range • Use low buffer concentration buffer, designed to capture dissolution in physiological bicarbonate* • Simplify to any buffer species & concentration
bases • Buffer species & concentration matter when pKa > 7 and beta (= pKw - pKa- log So ) < 11 • When outside this range • Use low buffer concentration buffer, designed to capture dissolution in physiological bicarbonate* • Simplify to any buffer species & concentration
• Buffer species & concentration do not matter • Simplify to any buffer species & concentration *Krieg et al., J. Pharm. Sci. (2015), 1-11, Mudie et al. AAPS J. 2020 Jan 27;22(2):34 15
Selection of Bile Salts and Lipids

Deanna Mudie | September 2022
Public Interplay Between Drug/Excipient and Fluid Bile Salts and Lipids Deanna Mudie | September 2022 Solubility increase due to solubilization in mixed lipidic aggregates From Fagerberg and Bergström, Ther. Deliv. (2015) 6(8), 935-959 1 10 100 -1 0 1 2 3 4 5 6 C s FaSSIF/ C s blank buffer log D pH 6.5 0 – 0.8 mM bile salts 0.03 mM phospholipids 1.4 5.9 mM bile salts 0.2 mM phospholipids Free drug 17
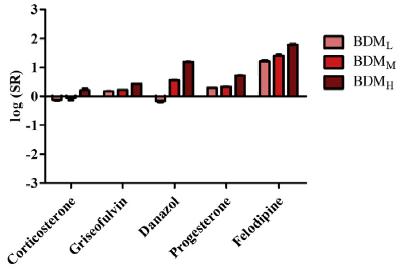
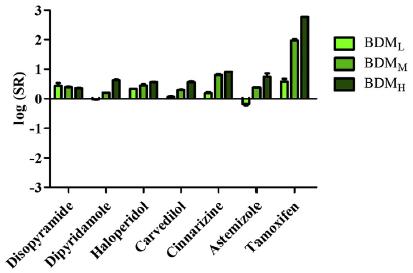
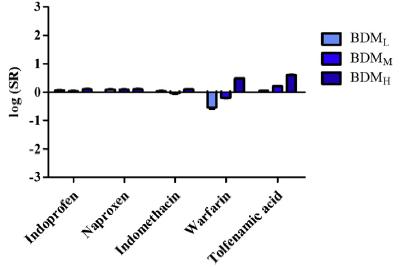
Public Interplay Between Drug/Excipient and Fluid Bile Salts and Lipids Deanna Mudie | September 2022 Study by Fagerberg et al., 2021 Figures from Fagerberg et al., J Pharm Sci (2021) 110, 186 197 Drugs with log D6.5 < 3 show < ~4-fold increase in solubility in presence of BS/PL BS (mM) BDML 0.08 BDMM 1.5 BDMH 7.5 Neutral Weak bases Weak acids log D6.5 > 3 log D6.5 > 3 log D6.5 > 3 18

Public Interplay Between Drug/Excipient and Fluid Bile Salts and Lipids Deanna Mudie | September 2022 Study by Fagerberg et al., 2021 Figure from Fagerberg et al., J Pharm Sci (202) 110, 186 197 Weak acids showed a more complex interplay Weak bases show similar influences of log D6.5 and Tm log D6.5 is most influential drug property on drug solubilization in BS/PL for neutral drugs Variable Influence of Projection (VIP) 19
Public Impact of Bile Salts and Lipids on Dissolution Rate Versus Apparent Solubility Deanna Mudie | September 2022 ������������. �������������������� ���������������� = ��������,������������������������ ��������,�������������������� ∗ ���������������� �������� 0 10 20 30 40 1 10 100 1,000 Relative dissolution rate Cs FaSSIF/Cs blank Increasing log D Increasing free drug diffusivity (Du)Competing impact of Deff and Cs on dissolution rate Du ~ 5 X 10 6 cm2/s Free drug Deff ↑ Cs ↓ Dm ~ 1 X 10 7 cm2/s Drug bound in micelles Deff ↓ Cs ↑ �������� �������� = ���������������� ����(����) � �������� (����) � ��������(����) − �������� ���� ���������������� = �������� � �������� + �������� � �������� 20
Recommendations for Including Bile Salts and Lipids
Weak acids, bases and neutral compounds
Bile salts and lipids have minimal effect on dissolution rate when
Simplify medium by leaving out bile salts and lipids when measuring dissolution rate
When determining extent of dissolution is important, add bile salts and lipids to dissolution test or apparent solubility measurement

Public
Deanna Mudie | September 2022
log D6.5 < 3
Public Impact of Buffer Concentration and BS/PL on Model Drug Dissolution Rate (pH 6.5 phosphate) Deanna Mudie | September 2022 Neutral drug danazolWeak base dipyridamoleWeak acid Ibuprofen alpha < 10, log D6.5 <3 beta > 11, log D6.5 > 3 log D6.5 > 3 0 20 40 60 80 100 0 2 4 6 % Dissolved Time (min) 50 mM + BS/PL 50 mM blank 3.5 mM blank 3.5 mM + BS/PL 0 10 20 30 40 50 0 10 20 30 % Dissolved Time (h) 50 mM blank 50 mM + BS/PL 0.23 mM + BS/PL 0.23 mM blank 0 10 20 30 0 5 10 15 20 % Dissolved Time (min) 10 mM blank 50 mM blank 50 mM + BS/PL 10 mM + BS/PL Mudie et al. AAPS J. 2020 Jan 27;22(2):34 22
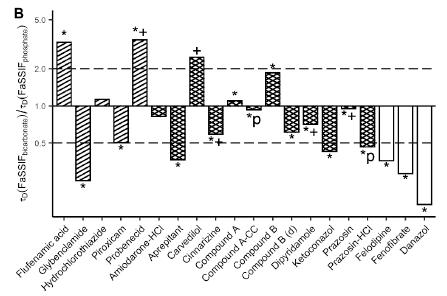
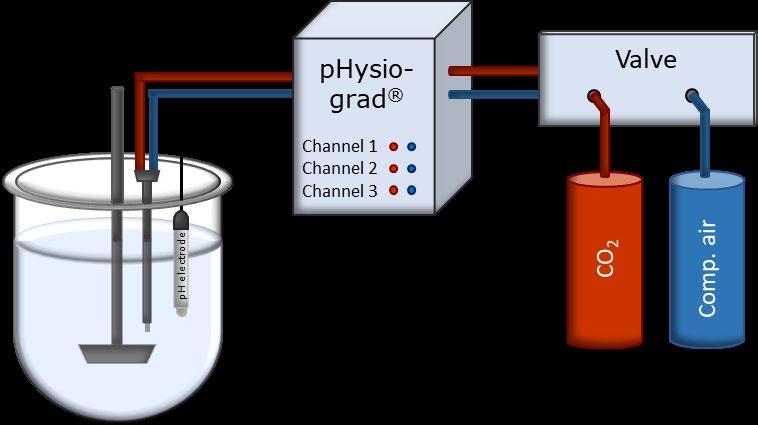
Public Impact of Bicarbonate Versus Phosphate Buffer on Drug Dissolution Deanna Mudie | September 2022 Study by Krollik et al. (2022) Figures from Krollik et al. Eur J Pharm Biopharm ,171 (2022) 90 101 Non-sink tests conducted in bicarbonate vs. phosphate τD (time to 63% dissolved) determined for each test τD bicarbonate /τD phosphate Results in line with recommendations for buffer selection for 11 drugs 7 drugs showed >2 fold faster dissolution in bicarbonate FaSSIFbicarb or FaSSIFphosphate pH 6.5 10 – 12 mM/ΔpH 3 mM BS 23
Benefits and Drawbacks of Streamlined Versus Most Complex Media?
Moderate-to-high
media
Designed to mimic GI fluids
medium
Bicarbonate
Higher time and cost
Potential to simplify media based upon recommendations
drug and excipient
Designed to mimic most important aspect
Standardized media & workflows
fluids
Better chance for biorelevance?
tailored to drug formulation of interest
Lower time and cost
Uncertainty in drug properties in early development?
Buffer species impacts (e.g. complexation) on performance?
Public
Deanna Mudie | September 2022
complexity ‘biorelevant’ dissolution
(i.e.
properties) – still “biorelevant”
– one
fits all
of GI
-
pH 1 3 HCl bile salts pH 5.5 7
bile salts e.g. pH 6.5 phosphate 25
Summary
Selecting dissolution media that can rank or test formulation robustness is important for efficient development of oral drug products
Relative advantage of ‘one medium fits all’ versus tailored medium approach may depend upon organizational priorities and stage of development
Knowledge of key physiological and drug formulation properties can be combined to streamline media selection and drug formulation understanding, enabling right first-time development, while minimizing fluid complexity
Selection approach can ultimately reduce cost and increase development speed of medicines
Public
Deanna Mudie | September 2022
26
Acknowledgments

Deanna Mudie | September 2022 Mudie DM, Samiei N, Marshall DJ, Amidon GE, Bergström CAS. Selection of In Vivo Predictive Dissolution Media Using Drug Substance and Physiological Properties. AAPS J. 2020 Jan 27;22(2):34. Christel Bergström (Uppsala University) Nasim Samiei (Uppsala University) Aaron Stewart (Lonza) David Vodak (Lonza) Gregory Amidon (U. Michigan) Derrick Marshall (formerly Lonza) Brian Krieg (formerly U. Michigan) Mike Morgen (Lonza) Gordon Amidon (U. Michigan) Michael Grass (Lonza)
Disclaimer and Forward-looking Statements
Webinar: Streamlined Selection of In Vivo Predictive Dissolution Media

This presentation (“Presentation”) is the property of Lonza AG and its affiliates (“Lonza”) and any unauthorized use or interception of this Presentation is illegal.
The information contained herein are believed to be correct. However, no warranty is made, either expressed or implied, regarding its accuracy or the results to be obtained from the use of such information. Lonza disclaims any liability for the use of this presentation and the use of the information contained herein is at your own risk.
All trademarks belong to Lonza or its affiliates or to their respective third party owners and are only being used for informational purposes. All copyrighted material has been reproduced with permission from their respective owners, all other materials ©2021 Lonza. All rights reserved.
Certain matters discussed in this Presentation may constitute forward looking statements. These statements are based on current expectations and estimates of Lonza Group Ltd., although Lonza Group Ltd. can give no assurance that these expectations and estimates will be achieved. Investors are cautioned that all forward-looking statements involve risks and uncertainty and are qualified in their entirety. The actual results may differ materially in the future from the forward-looking statements included in this Presentation due to various factors. Furthermore, except as otherwise required by law, Lonza Group Ltd. disclaims any intention or obligation to update the statements contained in this Presentation.
Public
Deanna Mudie | September 2022 28


Deanna Mudie | September 2022 Thanks! Any questions? Contact us: small.molecules@lonza.com lonza.com





















