

Agenda
Introduction to ICH M9 and the harmonization process
Dissolution in ICH M9
Future perspectives
Assessing bioequivalence risk
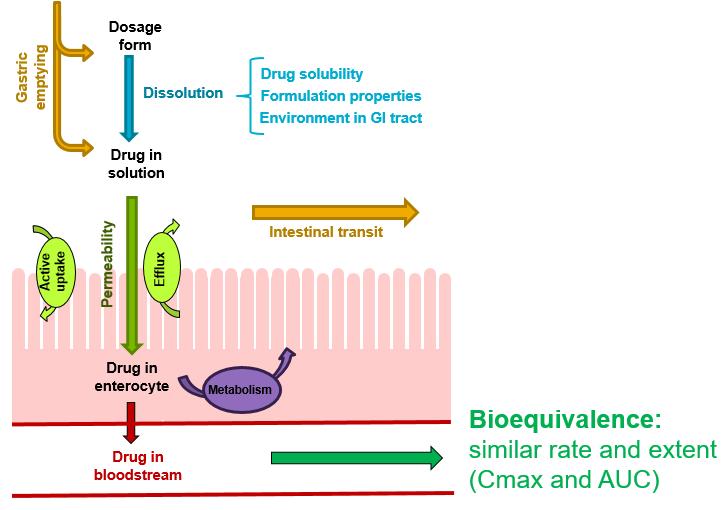
BCS
Biopharmaceutics Classification System
Solubility
High Low
Class 1 (HS, HP)
Biowaiver possible, if dissolution criteria are met
Class 2 (LS, HP)
BCS biowaivers not permitted
Class 3 (HS, LP)
Biowaiver possible, if dissolution criteria and excipient similarity criteria are met
Class 4 (LS, LP)
BCS biowaivers not permitted
ICH M9 – BCS-based biowaivers
Topic endorsed by ICH Management Committee in October 2016
Problem statement from ICH M09 Concept Paper:
“Biopharmaceutics Classification System (BCS)-based biowaivers may be applicable to BCS Class I and III drugs, however BCS-based biowaivers for these two classes are not recognized worldwide. Regulatory guidelines/draft guidance which includes the possibility of BCS-based biowaivers have been issued in, for instance, the EU, US, Canada and within the WHO. Also, Japanese guideline includes the possibility of biowaivers based on the extent of formulation change. However, it appears from these guidelines that BCS based biowaivers may not be recognized globally or that the requested supportive data for such applications differs. In addition, even the classification itself may differ. This means that pharmaceutical companies have to follow different approaches in the different regions.”
Process of harmonisation
Detailed discussions on each topic took place in the Expert Working Group (EWG)
EWG composed of representatives from global Regulatory Agencies (ICH members and observers) and global pharmaceutical industry organisations
reached based on:
Understanding current practice in each region
Scientific data on the topic under discussion
experiences and
positive/productive approach within the EWG












Different comfort levels wrt risk….



Dissolution in ICH M9
Dissolution in ICH M9
Drug products containing BCS Class 1 or 3 drug substances must meet the dissolution criteria described in the guideline to be eligible for a BCS biowaiver.
Test and reference products must be either:
1
• very rapidly dissolving (>85% in 15 minutes),
• or rapidly dissolving (>85% in 30 minutes) and similar, under all test conditions.
3
Test and reference products must be very rapidly dissolving (>85% in 15 minutes) under all test conditions
Dissolution comparisons should be performed in at least three buffers (pH 1.2, 4.5 and 6.8).
Dissolution topics discussed at the EWG
Use of water as a biowaiver media
This was included in the Step 2a document, but removed by Step 4.
Media: can we harmonise the buffer composition?
This falls under the remit of the pharmacopoeias in most territories.
How to address coning
(500ml
Conditions for biowaiver dissolution comparisons
Media
Paddle or basket
Three buffers: pH 1.2, pH 4.5, and pH 6.8.
Pharmacopoeial buffers should be employed.
Additional investigation may be required at the pH of minimum solubility.
Addition of surfactants or organic solvents is not permitted.
Dissolution profile comparisons: demonstration of similarity
Similarity criteria
Very rapidly dissolving: If both test and reference formulation are very rapidly dissolving (i.e. ≥85% dissolution within 15 minutes), no statistical comparison of profiles is necessary, and the two drug products are considered similar. Rapidly dissolving and similar: If both test and reference formulation are rapidly dissolving (i.e. ≥85% dissolution within 30 minutes) AND f2 ≥50, then the two drug products are considered similar.
1 BCS 3
Must meet the similarity criteria in ALL media (i.e. at least pH 1.2, 4.5, 6.8)
Alternative statistical methods for profile comparison are currently NOT allowed in the context of BCS biowaivers (refer to Q&A 3.2.3).
Biowaiver dissolution comparisons: troubleshooting
Coning
The guideline only permits the use of low paddle speeds – 50rpm for paddle and 100rpm for basket apparatus.
If high variability due to coning is seen in the paddle apparatus, recommend trying basket at 100rpm
Alternative approaches may be considered with suitable scientific justification
Crosslinking



“For gelatin capsules or tablets with gelatin coatings where cross-linking has been demonstrated, the use of enzymes may be acceptable, if appropriately justified”

Submitting a BCS biowiver application
it’s not just dissolution!
BCS classification of the drug substance:
• High solubility: highest single therapeutic dose is soluble in ≤ 250 ml of aqueous media across the pH range of 1.2–6.8 at 37±1°C.
• High permeability: demonstrating >85% extent of absorption. Data from human absolute bioavailability or mass balance studies are preferred, and in vitro permeability assessment using Caco-2 cells is also permitted. Data to demonstrate drug substance stability in the GI tract may also be required, depending on the type of data used to demonstrate high permeability.
Excipients:
For BCS 1, qualitative and quantitative differences in excipients are permitted, except for excipients that may affect absorption
For BCS 3, all excipients should be qualitatively the same and quantitatively similar
Examples are given in the M9 guideline.
Future perspectives
Future perspectives
• The way that we perform formulation change ‘biowaiver’ comparisons in drug development looks very different to the regulatory biowaiver landscape.
• There are simple dissolution tools that industry considers to be well-established enough to use as decision-making, that are not currently included in regulatory biowaiver guidance, e.g:
Peak vessels1
Biorelevant media and conditions, e.g. FaSSIF2
Excipient similarity criteria:
• The excipient similarity criteria in ICH M9 are based on SUPAC (1995)
• These limits are somewhat conservative compared to what some territories are already accepting, and represent a stretch for other territories
• However there is a hope that through further analysis and publication of data it may be possible to widen these further in the future.
Mann etal. 2021; 2 Mann etal.2017.
Future perspectives - 2
•
EFPIA White Paper on Biopharmaceutics Modelling as a Fundamental Tool to Support Accelerated Access1:
“There is an opportunity to move beyond traditional BCS thinking to understand invitro/in vivorelationship on a product-specific basis, to enable the establishment of clinically relevant invitrotests and acceptance criteria. This, in combination with insilicomodelling, can be used to facilitate rapid product and process development, with optimisation and scale up based on knowledge of the potential invivoimpact of any changes, and ultimately to define the control strategy to ensure that drug product of suitable clinical quality is always delivered.”
• Two proposals were recently submitted to EMA, highlighting the need for an industry/regulator collaboration on how we can more widely apply biopharmaceutics models (invitrodissolution models and PBBM) in the regulatory setting.
• One of these proposals was selected for presentation to the QWP in May this year, and correspondence is ongoing to discuss next steps.
• EFPIA have proposed a discussion forum/working group to share knowledge between cross-disciplinary experts to establish a framework for application of these models, with the ultimate goal of working towards global harmonisation of their application.
• The IQ Consortium has recently initiated a discussion forum with several international regulatory authorities on applications of PBBM, using 10 case studies previously submitted in files to support bridging or CRDS.
1 EFPIA, 2020.
Future perspectives
3
ICH M13 – bioequivalence studies
Guideline will be developed in 3 parts, with discussion topics divided into 3 ‘tiers’.
• The main focus overall is on the conduct of clinical bioequivalence studies, rather than biowaivers.
• However, Tier 2 will include the topic of biowaivers for additional strengths – this will potentially include some discussion of appropriate dissolution conditions for comparison.
• Discussions on Tier 2 are scheduled to begin in early 2023.
Summary and Conclusions
Summary and conclusions
• The ICH M9 guideline provides a globally harmonized approach for BCS biowaivers.
• The guideline specifies the conditions under which dissolution comparisons must be performed to support biowaiver applications, enabling demonstration of in vitro bioequivalence for drug product changes or introduction of new generic drug products.
• The guideline and associated Question and Answer document were approved in December 2019, and are already implemented in many ICH territories.
• The biowaiver journey is not over – our knowledge and our toolkit for assessing the bioequivalence risk associated with a formulation change continue to evolve.
• Continued dialogue between industry and regulatory agencies globally is needed, to share knowledge and experiences and establish a framework for regulatory application of newer bioharmaceutics tools, with the ultimate goal of working towards a globally harmonized framework.
Acknowledgements
ICH M9 EWG
EFPIA ICH M9 support team
EFPIA MQEG PRIME CMC Team and Biopharmaceutics White Paper co-authors
References
ICH M9 guideline: biopharmaceutics classification system-based biowaivers. https://database.ich.org/sites/default/files/M9_Guideline_Step4_2019_1116.pdf
ICH M9 Q&A document. https://database.ich.org/sites/default/files/M9_QAs_Step4_2021_0106.pdf
Validation of Dissolution Testing with Biorelevant Media: An OrBiTo Study. Mann J. et al. Mol Pharm 2017 14(12):4192-4201.
• Stimuli to the Revision Process: The Case for Apex Vessels. Mann J. et al. Dissolution Technologies 2021 28(4):6.
• EFPIA White Paper: Biopharmaceutics Modelling as a Fundamental Tool to Support Accelerated Access. September 2020, https://www.efpia.eu/media/554809/biopharmaceutics-modelling-as-a-fundamental-tool-to-support-accelerated-access.pdf.
Thank you for your attention.
questions?

