Assessment of Dissolution Data in Drug Product’s Development and Life-Cycle: FDA’s Regulatory Perspective
Mei Ou, Ph.D.
Senior Biopharmaceutics Reviewer
Division of Biopharmaceutics

Office of New Drug Products Office of Pharmaceutical Quality CDER-FDA
SPDS US Conference
September 20, 2022 www.fda.gov



Disclaimer
This presentation reflects the views of the presenter and should not be construed to repr esent FDA’s views or policies.

3
Outline
Division of Biopharmaceutics in FDA

for new drug products
for
assessment of
products
data
assessment and structured application (KASA) review
4
• The
• Dissolution
• Dissolution
generic drug
• Regulatory
dissolution
• Knowledge-aided
• General recommendations
Division of Biopharmaceutics in CDER
Center for Drug Evaluation and Research (CDER)
Office of Policy for Pharmaceutical Quality (OPPQ)
Office of Program and Regulatory Operations (OPRO)
Office of Administrative Operations (OAO)
Office Quality Surveillance (OQS)
Office of Infectious Diseases (OID)
Office of Oncologic Diseases (OOD)
Office of Neuroscience (ON)
Office of Immunology and Inflammation (OII)
Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine (ORPURM)

Office of Cardiology, Hematology, Endocrinology and Nephrology (OCHEM)
Office of Specialty Medicine (OSM)
Office of Nonprescription Drugs (ONPD)
Office Quality Surveillance (OQS)

ice
Office of Pharmaceutical Quality (OPQ)
Office of New Drug Products (ONDP)
Office of New Drug Product (ONDP)
Office of Lifecycle Drug Products (OLDP)
Office of Pharmaceutical Manufacturing Assessment (OPMA)

Office of Testing and Research (OTR)
Office of Biotechnology Products (OBP)
Office of Generic Drugs
CDRH Consults
5
Division of Lifecycle API Division of New Drug API Division of New Drug Products I Division of New Drug Products II Division of New Drug Products III
Division of Biopharmaceutics
Branch 1
Branch
2 Branch 3
Office of New Drugs
•
Division of Biopharmaceutics Overview
Biopharmaceutics: Examines the interrelationship of the physical/chemical properties of the drug, the dosage form (drug product) in which the dr ug is given, and the route of administration on the rate and extent of systemic drug absorption. The importance of the drug substance and the drug formulation on absorption, and in vivo distribution of the drug to the site of action, is described as a sequence of events that precede elicitation of a drug's therapeutic effect 1 .
•
Clinically relevant specifications:
–
Ensure the delivery of the intended dose of drug to the site of action to assure consistent safety and efficacy for the marketed product relative to those achieved by the clinical trial formulation, and/or,
–
Identify and reject drug product batches that are likely to perform inadequately in the indicated patient population, and/or,
– Impact/enhance drug product lifecycle management (e.g., post-approval changes).

• Biopharmaceutics review responsibilities: Assessment of biowaiver requests, scientific bridging throughout drug product’s development, 505(b)(1)/505(b)(2) NDA submissions and 505(j) ANDA submissions (pre-and post-approval), BCS class 1 designation, BCS class 1 and 3 based biowaiver requests for new drug products, in vitro dissolution/drug release, in vitro alcohol dosedumping for oral MR products, evaluation of IVIVC, IVIVR, PBPK, other model proposals, ER-claims, disintegration in lieu of dissolution, counsels (CDRH, OND, OGD, OCP, etc.), citizen petitions, drug shortages, regulation and guidance, etc.
1Applied
6
Biopharmaceutics &
Pharmacokinetics 6th Edition, Shargel, Wu-Pong, Yu.
Role of Dissolution in Regulatory Review
• Quality Control (QC) test:
To ensure batch to batch consistency from pivotal clinical batches to commercial batches

To ensure drug product’s quality at release, on stability and during shelf-life
• Bio-relevant or Clinical-relevant:
Identify potential factors affecting bioavailability
Signal drug product that may not be bioequivalent to reference product
Assess the need for potential BA/BE studies throughout product’s life cycle (pre-and post-approval)
• Surrogate for clinical performance:
BA/BE waivers or scientific bridge
7
–
–
–
–
–
–
Dissolution for New Drug Products
Discovery IND NDA Postapproval

For pre-approval changes: Scientific Bridge: Based on the principles of FDA’s SUPAC and other guidances, reviewers assess the proposed change(s) (minor or major) and the supporting information (in vitro and in vivo) to establish the scientific bridge between drug products used during product’s development [505(b)(1) and 505(b)(2)] or between the proposed drug product and the listed drug product [505(b)(2)].
Biowaiver: A biowaiver request means that the CFR’s requirement to provide data from in vivo BA or BE studies can be waived. Specifically, per 21 CFR 320.21, any person submitting a full NDA to the FDA shall include in the application either evidence measuring the in vivo BA or BE of the drug product that is the subject of the applications; or information to permit FDA to waive the submission of evidence measuring in vivo BA or BE.
For post-approval changes: Per FDA SUPAC guidances,
BA/BE studies are needed or • Biowaiver: Applicants need to provide adequate supporting information to permit FDA to waive the submission of evidence measuring in vivo BA or BE.

8
•
Dissolution for Generic Drug Products
OGD
Office of Bioequivalence
ONDP
Division of Biopharmaceutics

Original ANDA
Dissolution similarity between the proposed generic product vs. RLD
Dissolution as a QC test for the proposed generic drug product



Dissolution as a QC test and to support biowaiver requests
Post-approval
Per FDA’s SUPAC guidances, • BA/BE studies are needed or
• Biowaiver requests included, data supporting bridging, including comparative dissolution, etc.
9
Dissolution/Drug Release Method and Acceptance Criterion/Criteria
• New Drug Products:
–
Dissolution/drug release method development and validation report(s) should be submitted in IND and/or NDA
–
Setting of acceptance criterion/criteria is based on dissolution/drug release data in NDA
• Generic Drug Products:
–
For immediate release (IR) solid oral dosage forms: USP/FDA dissolution methods can be used as a starting point, but the suitability of the selected method should be demonstrated for the proposed products. Otherwise, Applicants are requested to optimize or develop the dissolution/drug release method for the proposed generic drug products.
– For modified release (MR) solid oral dosage forms and complex dosage forms: usually Applicants are recommended to develop an appropriate product-specific dissolution/drug release method, but on a case-by-case basis.
–
Setting of the acceptance criterion/criteria is based on the data provided in the ANDA submission.
• Other Complex Dosage Forms: in vitro release testing and/or in vitro permeation test are encouraged, but case-be-case basis.

–
Nanoparticle, suspension, semi-solid dosage forms (i.e., cream, ointment, lotion, shampoo, gel), implants, topical patches, transdermal, vaginal rings, otic, ophthalmic implants, etc.
10
Product Specific Dissolution/Drug Release Method
Key Components:
• Evaluation of the proposed dissolution/drug release methodology

• Assessment of the discriminating ability of the selected method
• Setting of the acceptance criterion/criteria
11
Evaluation of the Proposed Dissolution/Drug Release Method

• Drug substance solubility profile in the appropriate physiological pH range
• Sink conditions (recommended but NOT required)
• Selection of dissolution/drug release method parameters (i.e., equipment/apparatus, agitation/rotation speed, medium, surfactant, enzyme, concentration, temperature, etc.)
• Selection of a physiologically relevant dissolution medium
– pH 1.2, 4.5, 6.8 buffers, simulated gastric fluid (SGF), simulated intestinal fluid (SIF), vaginal fluid, tear fluid, saliva, 32oC, 37oC, etc.
12
Assessment of Discriminating Ability
• Discriminating ability of the method with regards to relevant critical material attributes (CMAs), critical formulation variables (CFVs), and critical process parameters (CPPs) that could affect dissolution, and/or critical factors/attributes that could affect bioavailability (CBAs) .
• In general, the testing conducted to demonstrate the discriminating ability of the selected dissolution method should compare the dissolution profiles of the reference (target) drug product and the test products that are intentionally manufactured with meaningful variations (e.g., ±10-20% change outside the specified acceptance ranges for the tested variables).
• Complete information/data supporting the discriminating ability of the selected method should be included in the dissolution method development report.

• If available, data showing that the selected method can identify and reject product that is not bioequivalent to the reference/target drug product should be included in the report.
• It is noted that the discriminating ability of a dissolution method is not only based on the selected dissolution testin g methodology, but also on the selected sampling time point(s) and acceptance limits for the proposed acceptance criterion/criteria.
13
Setting of Acceptance Criterion/Criteria
• Multi-point dissolution data from the pivotal clinical batches and primary registration/stability batches should be used for setting of the acceptance criterion/criteria.
• The setting of the dissolution acceptance cr iterion/criteria should be based on the average in vitro dissolution data of each batch/lot under study, equivalent to USP Stage 2 testing (n = 12), occasionally USP Stage 3 testing (n = 24) would be needed.
• For immediate-release (IR) products, the selection of the sampling time point should be where Q = 80% dissolution occurs.
• For extended-release (ER) products, the establishment of at least three specification time-points covering the initial, middle, and terminal phases of the complete dissolution profile data should be set.
• For delayed-release (DR) products, in general, no more than 10% dissolved at 2 hours in acid stage; where Q = 80% dissolution occurs (for IR) or three time points of criteria (for ER) in buffer stage.

14
Case Study –Discriminating Ability


Immediate Release Tablets

15
• Dissolution Method: USP Apparatus II (Paddle), 50 rpm, 900 mL of pH 4.5 acetate buffer, 37oC • Proposed Dissolution Acceptance Criterion: Q=75% in 30 minutes • Recommended Dissolution Acceptance Criterion: Q=80% in 20 minutes 0 20 40 60 80 100 120 0102030405060708090100 % Dissolved Time (min) Batch - Within DS PSD specification Batch - Out of specification DS PSD 0 20 40 60 80 100 120 0102030405060708090100 % Dissolved Time (min) Batch - Target water amount in wet granulation Batch - High water amount in wet granulation
Common Deficiencies
vitro
release method development report is not included in the
ability is not
method issues
release methodology validation issues

release is
Data fully support the setting of a tighter acceptance criterion/criteria
16
• In
dissolution/drug
submission • Discriminating
demonstrated • Analytical
• Dissolution/drug
• Dissolution/drug
incomplete •
Current Dissolution Review for Original ANDAs
Knowledge-Aided Assessment and Structured Application (KASA)
• Implemented in February 2021 for the Biopharmaceutics assessment of solid oral dosage forms under ANDA submissions.
– KASA provides transparency and consistency on the selection of the dissolution method and setting of the acceptance criterion/criteria across ANDA submissions based on understanding of the biopharmaceutics risk of the drug product from development throughout lifecycle management.
• Perform computer-aided analyses of applications to ensure consistency and effectiveness of regulatory review.

• Knowledge management from drug product development throughout life cycle.
• Facilitate structured review.
• Guide biopharmaceutics risk assessment and mitigation strategies.
17
KASA Webpage Overview
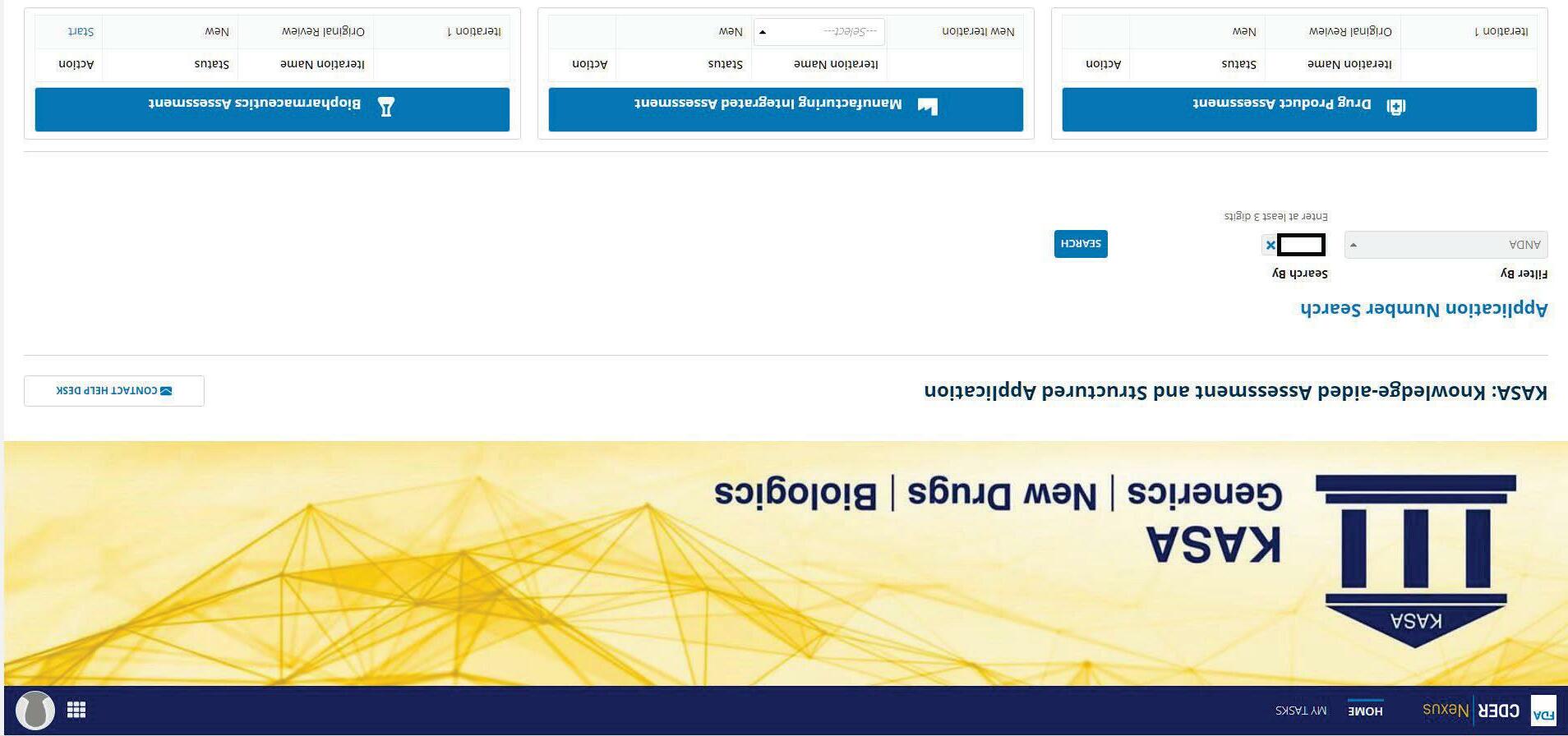

18
Biopharmaceutics initial risk assessment decision tree for immediate release solid oral dosage forms (non-NTI or non-rapid onset)
Is drug substance’s solubility high per M9 guidance?
Is the in vitro dissolution rapid in 500 mL of 0.1N HCl in aqueous medium (without surfactant)?
Very low risk
No Low risk
Is drug substance’s permeability high?
Does the in vitro dissolution of the highest strength show rapid dissolution in a medium in the pH range 4.5-6.8 (without surfactant)?
Could critical bioavailability attribute(s) be clearly identified, detected and controlled?
Low risk
PBBM: Physiologically-Based Biopharmaceutics model
Critical bioavailability attributes (CBAs): critical material attributes (CMAs), critical formulation variables (CFVs),
critical process parameters (CFF)s, etc.: formulation or process variables that may impact the bioavailability (absorption rate and extent) of a drug product.
risk
Could IVIVC/R be established with PBBM and/or any available in vitro and in vivo data?
High risk Very High risk

19
Yes
Yes
No
Yes No Yes
No Yes
Yes Medium
No
No
Biopharmaceutics initial risk assessment decision tree for extended-release solid oral dosage forms (non-NTI)
Yes
Is in vitro dissolution independent of test condition (e.g., medium pH, rotation speed)? No
Medium risk Yes
Could critical bioavailability attribute(s) be clearly identified, detected and controlled? No
Medium risk
Could IVIVC/R be established with PBBM and/or any available in vitro and in vivo data?
High risk

Very high risk
20
NoYes

21
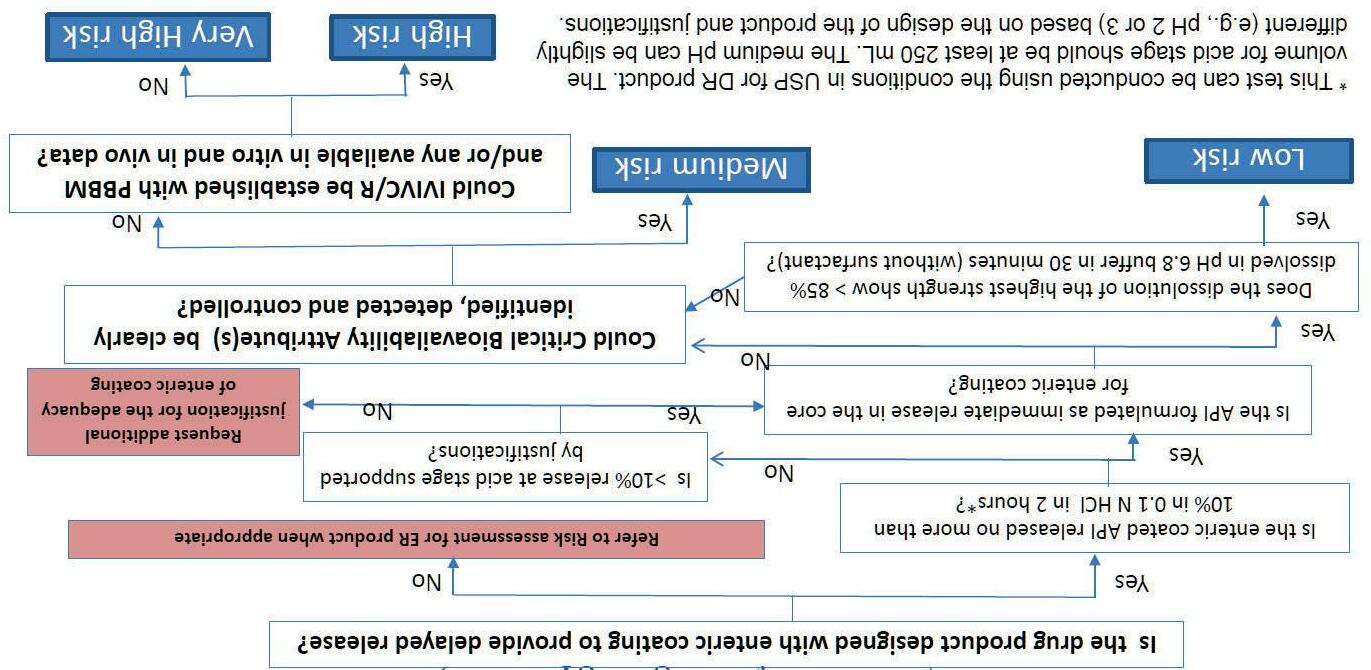
Biopharmaceutics initial risk assessment decision tree for delayed-release solid oral dosage forms (non-locally acting)
Biopharmaceutics Risk Mitigation Strategies
Risk levelMitigation strategies
Very LowStandardized dissolution method and acceptance criterion per FDA Dissolution Guidance August 2018.
LowAdequate method development to justify dissolution method and acceptance criterion/criteria.
MediumOptimized dissolution/drug release method to detect meaningful changes in identified critical bioavailability attributes (CBAs) to reject batches that are not bioequivalent and predict the in vivo performance accurately.
HighIVIVR is used to support patient-centric dissolution test (based on available in vitro/in vivo data and/or PBBM).
Very HighIn vivo studies are used to develop IVIVC or IVIVR to support patient-centric dissolution test.

22
Summary and Challenges
• Dissolution is often independent of in vivo performance/ assessment
• Dissolution methodology sometimes oversimplified for high-risk products
• Sometimes the selected methodology can be under-or overdiscriminating. Either scenario should be avoided.
• Acceptable IVIVC, safe space, BE, or in silico-PBBM information could provide regulatory flexibility and lead to a wider acceptance limit(s).

23
General Recommendations
• Understand the factors/attributes influencing in vitro dissolution/drug release and in vivo bioavailability.
• The selected dissolution/drug release method should be reliable, robust, and reproducible to be used as a QC test for drug products.
• The selected method should be able to measure product’s QC performance throughout its shelf-life and predict pre-and post-approval changes.
• It is advised to start the development of the dissolution/drug release method as early as possible in the drug product development.
• Submit the method development report and seek FDA’s feedback (Division of Biopharmaceutics) in early stage (pre-NDA or pre-ANDA).
• Limit changes (e.g., formulation, manufacturing process, and site changes, etc.) between the pivotal clinical batches and the commercial drug product.
• Set appropriate acceptance criterion/criteria.
• Complete dissolution data/profiles with multi-time points of registration batches should be collected to support the stability program.
• Biowaiver is possible if there is a validated IVIVC and/or a “safe space” built via bracketing approach.
• IVIVC study should be planned a priori, and the model should be adequately validated.

24
FDA Guidances
• Dissolution Testing of Immediate Release Solid Oral Dosage Forms, August 1997
• Dissolution Testing and Acceptance Criteria for Immedi ate-Release Solid Oral Dosage Form Drug Products Containing High Solubility Drug Substances, August 2018
• M9 Biopharmaceutics Classification System-Based Biowaivers, May 2021

• Bioavailability Studies Submitted in NDAs or INDs-General Considerations, April 2022
• Liposome Drug Products, Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation, April 2018
• Drug Products, Including Biological Products , that Contain Nanomaterials, April 2022
• Benefit-Risk Considerations for Product Quality Asse ssments Guidance for Industry, Draft Guidance, May 2022
• The Use of Physiologically Based Pharmacokinetic Analyses-Biopharmaceutics Applications for Oral Drug Product Development, Manufacturing Changes, and Controls, Draft Guidance, October 2020
• Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations, September 1997
• SUPAC-IR -Immediate Release Solid Oral Dosage Form s: Scale-Up and Post-Approval Changes: Chemistry, Manufacturing, and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation, November 1995
• SUPAC-MR: Modified Release Solid Oral Dosage Forms: Scale-Up and Post-Approval Changes: Chemistry, Manufacturing, and Controls, In Vitro Dissolution Te sting, and In Vivo Bioequivalence Documentation Bioavailability and Bioequivalence Studies for Orally Administered Drug Products, General Considerations, September 1997
• SUPAC-SS: Nonsterile Semisolid Dosage Forms: Scale-Up and PostapprovalChanges: Chemistry, Manufacturing, and Controls; In Vitro Release Testing and In Vivo Bioequivalence Documentation, May 1997
25
Dissolution Sources
• FDA Dissolution Method Database: The dissolution methods contained in the database are recommended methods that are not binding on FDA or others. We will consider alternate methods when supported by appropriate data.
• USP:United States Pharmacopeia, a legally recognized compendium of standards for drugs, published by the United States Pharmacopeial Convention, Inc., and revised periodically; it also includes assays and tests for determination of strength, quality, and purity.
• Drug Bank: The DrugBankdatabase is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information.
• Code of Federal Regulations Title 21: Title 21 is the portion of the CFR that governs food and drugs within the US for the FDA
• FDA Guidance: Guidance documents represent the Agency's current thinking on a particular subject. They do not create or confer any rights for or on any person and do not operate to bind FDA or the public.

• The Orange Book: The publication identifies drug products approved on the basis of safety and effectiveness by the FDA under the Federal Food, Drug, and Cosmetic Act (the Act) and related patent and exclusivity information.
• Drugs@FDA: Drugs@FDAincludes most of the drug products approved since 1939. The majority of patient information, labels, approval letters, reviews, and other information are available for drug products approved since 1998.
26
Acknowledgement
-Dr. Bhagwant Rege, Director, Division of Biopharmaceutics
-Dr. Angelica Dorantes, Branch Chief, Division of Biopharmaceutics Branch 1
-Dr. Elsbeth Chikhale, Senior Pharmaceutical Quality Assessor, Branch 1
-Dr. Anitha Govada, Senior Pharmaceutical Quality Assessor, Branch 1
-Dr. Banu Zolnik, Senior Pharmacologist, Clinical Pharmacology
-All Biopharmaceutics Colleagues

27
Thank you for your attention!


28














