Characterization and Quality Comparison of Liposome Formulations







Schwendeman, PhD

Anna
Disso America 2022 @AnnaSchwendy
on Complex






Center for Research
Generics http://www.complexgenerics.org https://www.youtube.com/channel/UCbF2EkiP5lbttBzsSu4eQRg @complexgenerics.org
Access to newer equipment, methodologies, software, models

Support from FDA and AAM
3
Housed at UMICH and UMB Neutral ground for communication
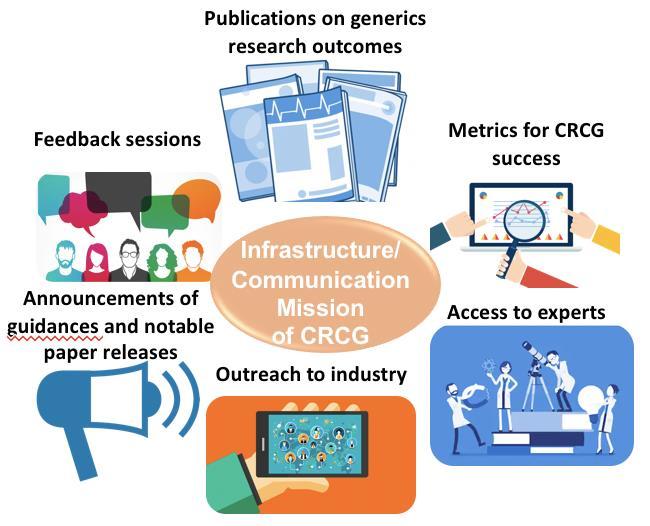
Our Mission
“To increase access to safe and effective generic drugs through enhanced infrastructure/communication, education, and research collaboration across industry, academia and the FDA”.
CRCG Aims:
Communication: Establish core program infrastructure and gain the support of stakeholders to ensure CRCG success.
Education: Promote generic industry training through workshops, webinars and hands-on demonstration and engage fellows, students and public in complex generics research.

Research: Conduct collaborative research and technique development that facilitate complex generics.

4
Research Efforts in Complex Generics

Complex injectables, formulations and nanomaterials
Quantitative clinical
Complex mixtures and peptides
Inhalation and nasal products
Long-acting injectables and implants
Drug-device combination products
Ophthalmic products
Topical dermatological drug products
Patients substitution of generic drugs
Locally-acting physiologically-based pharmacokinetics modeling
Data analytics

Oral absorption Models and bioequivalence
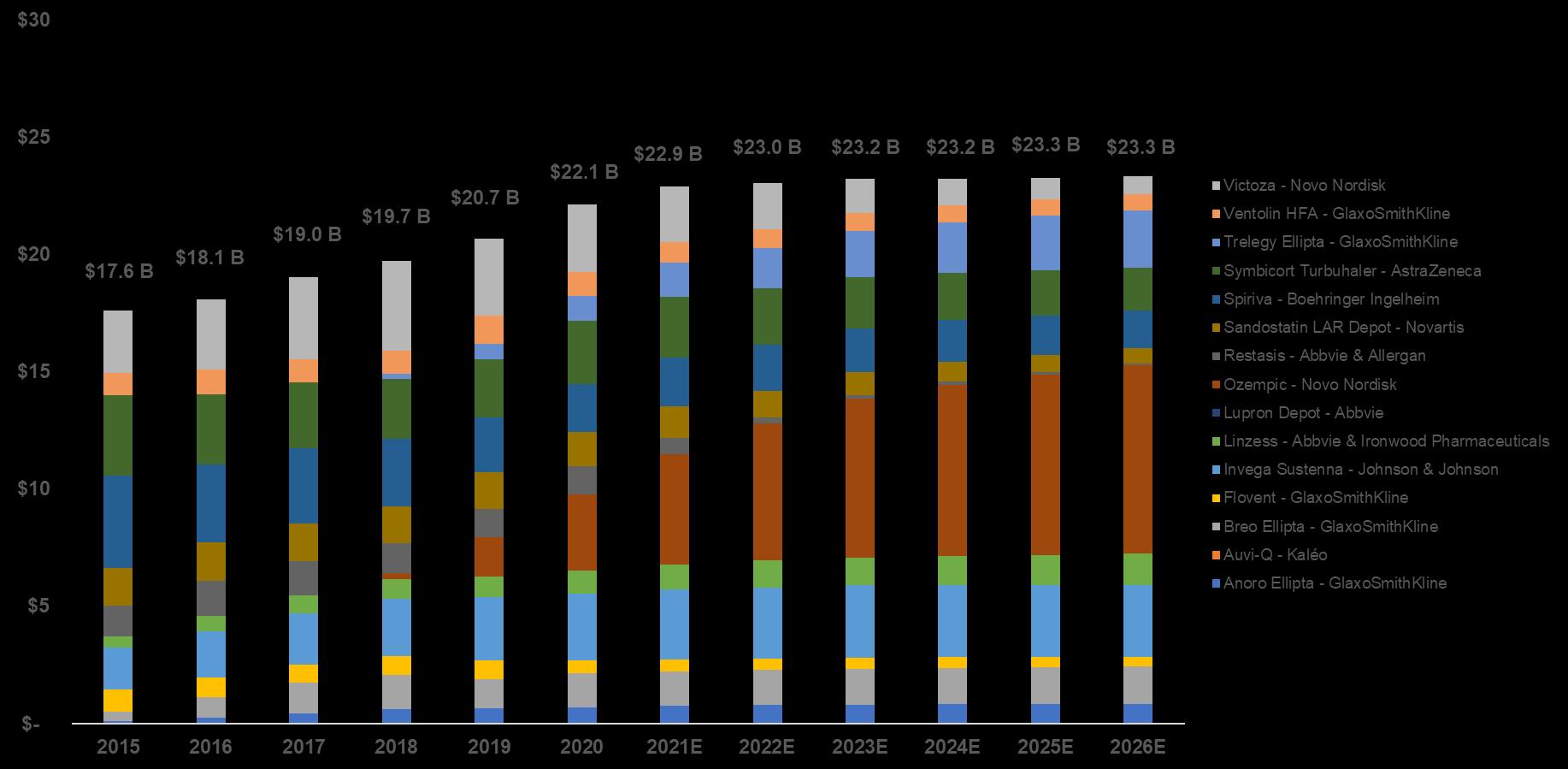
Top15 complex product worldwide annual net sales

Communication Mission

Injectables

Company Name Large Specialty Trade Org Type of products mentioned Perrigo X Oral, topical, inhalations, injectables Apotex X Oral, injectables, inhalations, drug-device combo Teva X All types of products Viatris X All types of products Sandoz X All types of products Fresenius Kabi X Injectables (lipid and polymer based) Cosette X Topical, locally acting orals, suppositories Solaris Pharma X Topical Nexus X Injectables Xellia X
AAM X Drug-device combo, orals, injectables PBOA X Drug-device combo, inhalations, orals, injectables Lassman Law X PSG for complex products SAAM X Oral, inhalations, ophthalmic
Training Mission


Research Mission
















Development of Doxil® USP-4 IVR Assay
Case Study

Doxil® Shortage 2011-2012 Date Event Nov2011 BenVenuevoluntarysuspendsall manufacturing Dec2011 Congressionalcommitteehearing Nov2011–May 2012 FDAallowstemporaryimportationof drugs Nov2011–May 2012 FDAworkswithmanufacturersto expeditelotreleases Feb2012 “DrugShortagePreventionAct”is introduced May2012 FDAconditionallyapprovesLipodox
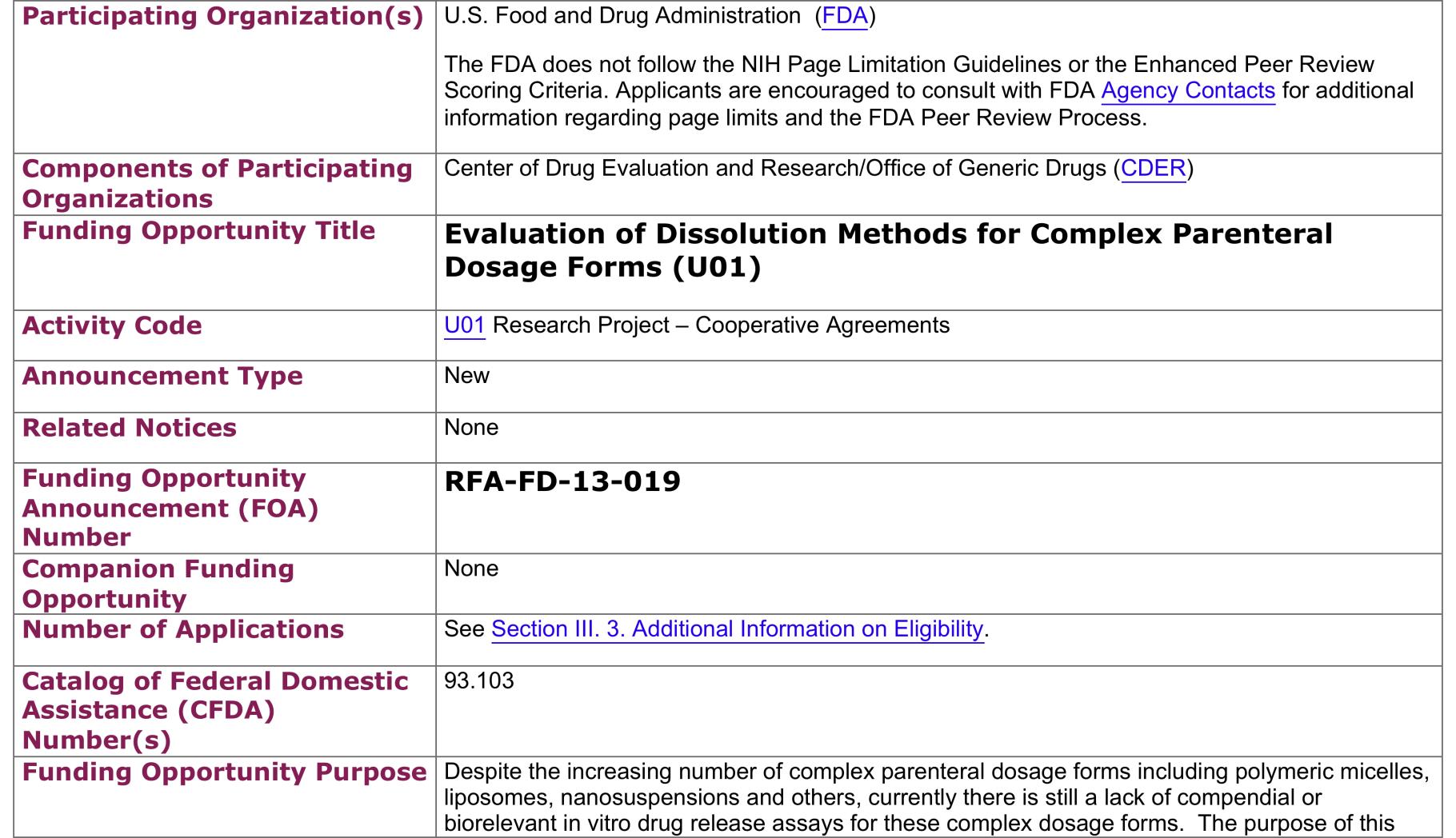
FDA RFA on Doxil® Dissolution Assay
 Dr. Frank Szoka
Dr. Frank Szoka

Project Objective
The objective of this project is to develop an in vitro release (IVR) assay for evaluating the biosimilarity of generic liposomal doxorubicin formulations to the innovator Doxil.
1
Liposome Formulation - performed by ZoneOne Pharma (Z1P) 0-4 months
2
Develop Release Conditions to performed by Z1P and UM 0-6 months
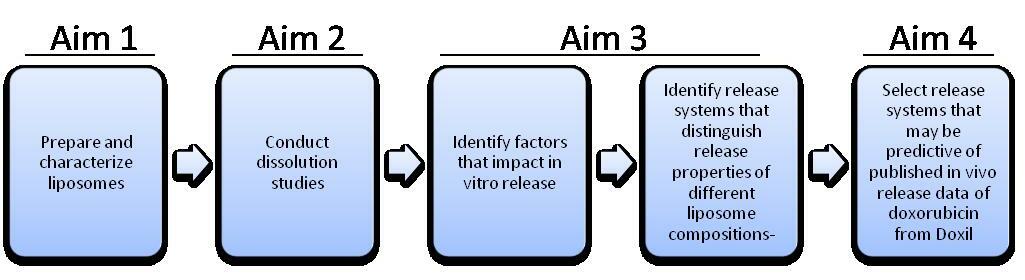
Aim
.
Aim
.
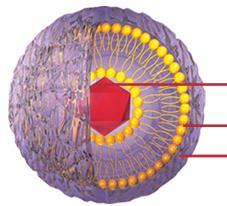
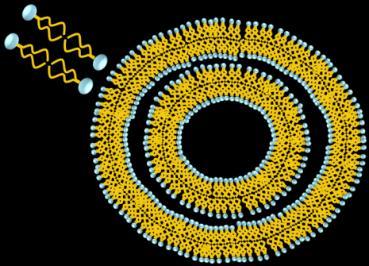
Composition of Doxil®
Doxorubicin
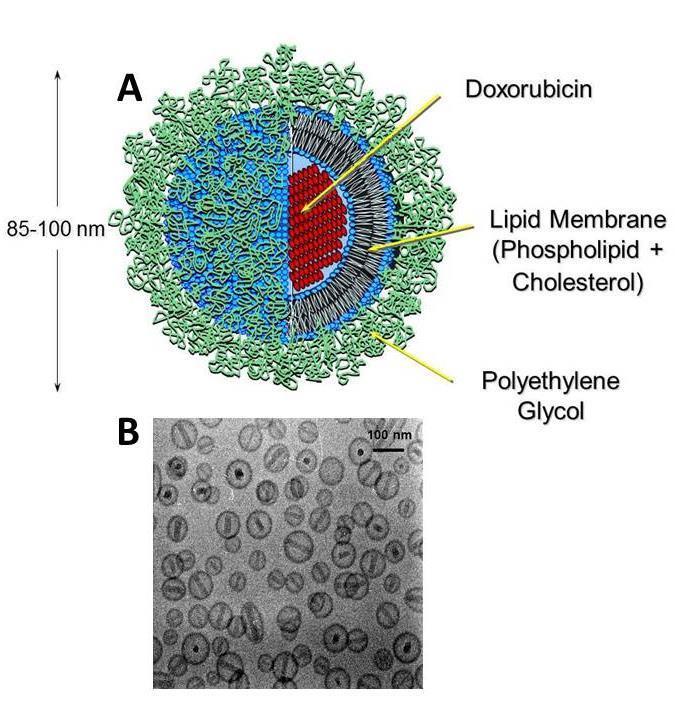
Crystals
Lipid
Membrane
Polyethylene
Glycol
Example of DOXIL® Manufacturing
Varied liposome formation processes (red box) but not the composition www.doxil.com
Lipids
Dissolved in ethanol Mixed with aqueous solution






MLV formation
High Pressure Extrusion

Doxil® DOX loading
doxorubicin liposome PEG
Purification/ Sterilization
SUV formation
18
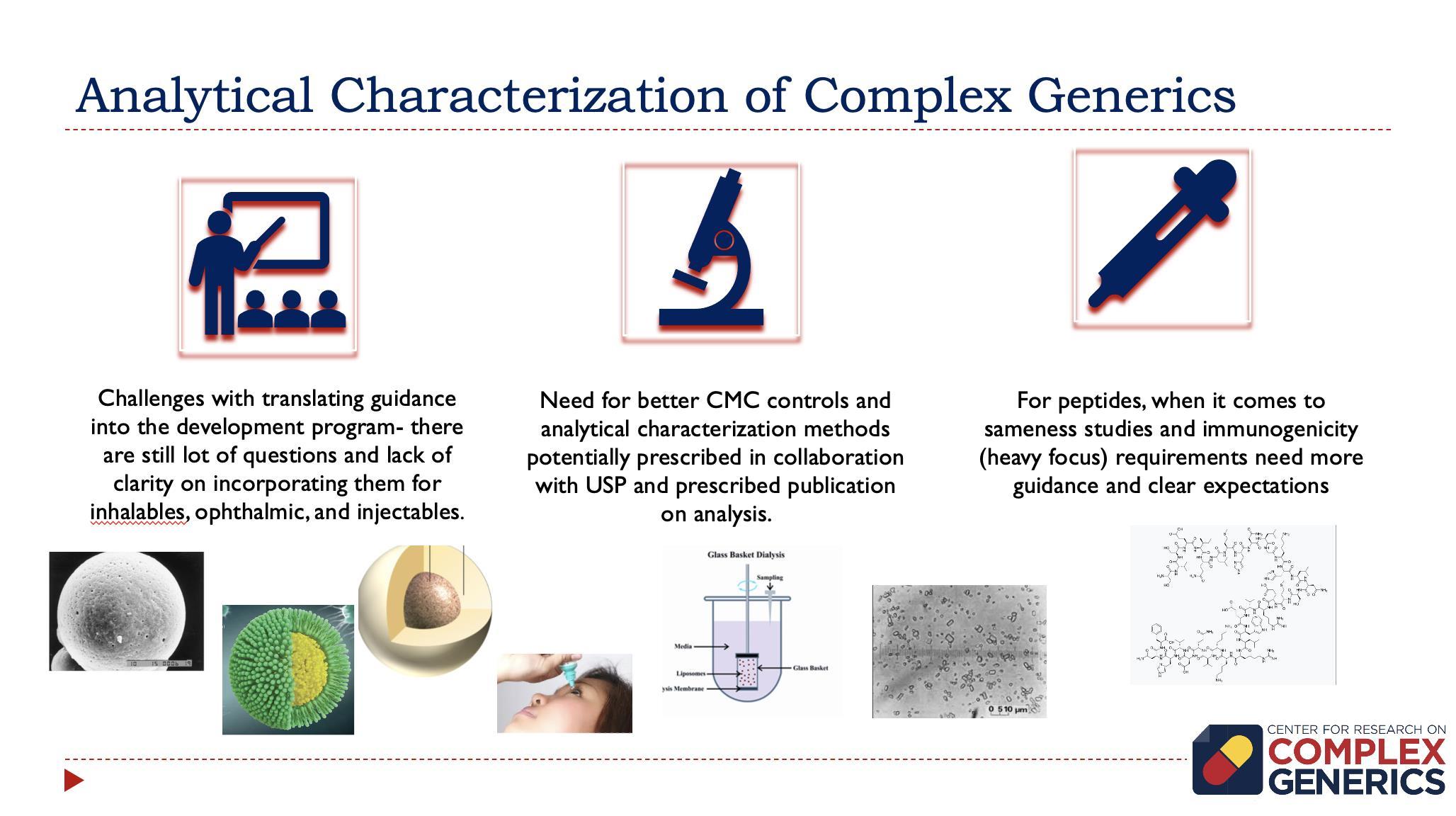
Importance and Challenges of Development In Vitro Release (IVR) Assays
• IVR performance is used to guide product and process development • IVR assay should be able to discriminate between formulations of the same compositions but different in vivo performances • Development of in in vitro-in vivo correlation (IVIVC) • Most approved nanomedicine products are incredibly stable and release drug after macrophage uptake
Most
set-ups for
assays
common
IVR
• “Sample and Separate” – Nanoformulation is mixed with release media – Samples removed, release media and nanoformulation separated and analyzed – Could be performed in centrifuge tubes (1 mL) or USP I/II apparatus (~1 L) • “Dialysis Bag” – Nanoformulation is separated from the media by dialysis membrane – Normal dialysis, reverse dialysis, side-by-side dialysis cell • “Flow Through Systems” – USP 4 apparatus with various adaptors to hold nanoformulation and flow media continuously Shen, Burgess, Drug Deliv Trans Res, 3(5): 409-415 (2013)
Consideration for Dialysis Bag IVR Assays


• Selection of dialysis bag type, molecular weight cut-off and membrane composition • Bag introduces diffusion barrier for the drug • Hydrophobic drug adsorbs to the bag • Maintain sink conditions – Media composition to avoid drug precipitation – Volume of the release media – Drug concentration in release media vs. limit of detection • The rate and type of agitation
impact of dialysis bag material



• CE Float-A-Lyzer adsorbs hydrophobic drugs No bag RC –bag (10 kDa) CE- Float-A-Lyzer (10 kDa)0.00% 20.00% 40.00% 60.00% 80.00% 100.00% 0 4 8 12 16 20 24 Time (h) University of 14 0 h 24 h Conventional Dialysis Method For Liposomes The
The Impact of Dialysis Membrane Pore Size
DOX(control)
0 20 40 60 80 100 0 4 8 12 16 20 24 Cumulative release (%) Time (h) 300kD CE 100kD CE 50kD CE 8-10kD CE Free
Flow Through Methods

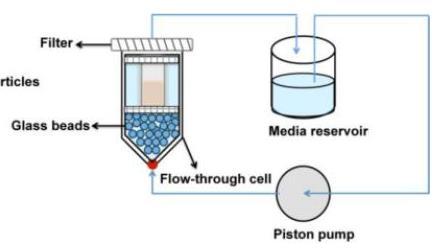
Shen, Burgess, Drug Deliv Trans Res, 3(5): 409-415 (2013)
Consideration for Flow Through Methods
design of USP-4 cell
• The
• Open or closed system • Online or of-line detection • Flow rate • Media composition • Drug concentration in release media vs. limit of detection
No drug release from Doxil® PBS at 37oC

100% free DOX DOX solution in 100KD Float-ALyzer Doxil liposomePOPC Doxil® similar

0 0.2 0.4 0.6 0.8 0 4 8 12 16 20 24 Absorbance (AU) Time (h)
Ammonium Bicarbonate Induces Doxil® Release
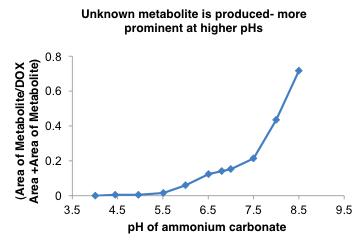
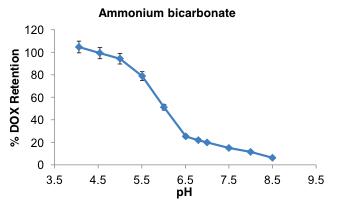
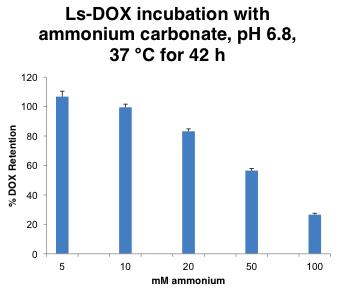
Doxil® release in NH

at 37oC but precipitate in USP-4 System (Sotax)
liposome--
HCO
0 0.2 0.4 0.6 0.8 0 4 8 12 16 20 24 Absorbance (AU) Time (h) DOX
POPC Doxil® similar 100% free DOX control
4
3
Optimized


Release Media 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0 12 24 36 48 OD Time (h) Free DOX (control 1) Free DOX with stir bar(control 2) L-Doxil® in 300KD Float-A-Lyzer pH 5.998 pH 5.998 pH 5.998 pH 6.271 pH 6.306 pH 6.283 • Add acceptor HP-CD to avoid precipitation • Use NH4HCO3 to facilitate doxorubicin release from Doxil • Control of pH and OD by MES buffer (pH 6) • Adapt new release media to USP-4 IVR Yuan, AAPS J, 19(1), 2017
40.00%
30.00%
20.00%
Back to USP-4

Temperature Effect
–
0.00% 10.00%
50.00% 60.00% 70.00% 80.00% 90.00% 100.00% 110.00% 0 4 8 12 16 20 24 Release Time (h) No precipitation 55°C 45°C 37°C Yuan, AAPS J, 19(1), 2017
The effects of
release and assay reproducibility
conditions on
0 20 40 60 80 100 0 4 8 12 16 20 24 Cumulative release % Time (h) L-DOX 10µg/ml in total media L-DOX 20µg/ml in total media L-DOX 40µg/ml in total media
A0 20 40 60 80 100 120 0 4 8 12 16 20 24 Cumulative release % Time (h) 5% γ CD 5% HP-CD C 0 20 40 60 80 100 0 4 8 12 16 20 24 Cumulative r elease % Time (h) 1.5% γ-CD 1.5% β-CD 1.5% HP-CD B D
IVR
DOX
0 20 40 60 80 100 0 4 8 12 16 20 24 Cumulative release % Time (h) Day 1 Day 2 Day 3 Yuan, AAPS J, 19(1), 2017
0 20 40 60 80 100 0 4 8 12 16 20 24 Cumulative release % Time (h) POPC L-DOX Doxil® 23: 51-2(Homogenizer made) L-DOX (23:68-1) Optimized IVR assay distinguishes between different liposomal Dox formulations Media composition: 100 mM NH4HCO3, 75 mM MES, 5% w/v HP-CD 5% w/v sucrose, 0.02% w/v NaN3 (pH 6) Temperature: 45°C; Flow rate: 16 ml/min; Cell: Float-A-Lyzer, CE, 300 kDa Media volume: 80 mL; Dox concentration: 10 mg/mL f2 = 50 log 100 1+σ��=1 �� (����−����)2 �� n is number of time points, Rt is cumulative release for ref Tt is cumulative release for test Yuan, AAPS J, 19(1), 2017
Summary


• The developed USP-4 assay is capable of distinguishing between some liposomal doxorubicin formulations and predicts similarity for others.
• Due to high stability of Doxil ® and solubility limitations USP-4 assay is performed at 45oC in presence of HP-CD and ammonia bicarbonate.
• The USP-4 assay has difficulty in method validation due to large lotto-lot differences in Float-A-Lyzer and extensive maintenance required for equipment operating at 45oC.
• The developed assay is not physiologically relevant. It only a quality control and comparability assessment tool.
• Similar approaches had been utilized by us for development of IVR for AmBisome® , Exparel®, liposomal cyclosporin and other complex products.


@AnnaSchwendy
Univ of Michigan




Wenmin Yuan
Jie Tang Yue Yuan
Rui Kuai Zone One Pharma







Frank Szoka
Mark Hayes Charles Noble
Zhipeng Dai
Acknowledgements





Jiang
Nan

FDA Wenlei
Zheng


































































 Dr. Frank Szoka
Dr. Frank Szoka



















































