CHALLENGES DISSOLUTION SCIENCE IS FACING IN THE BRAND NAME PHARMACEUTICAL INDUSTRY Xujin Lu, Ph.D. Drug Product Development Bristol Myers Squibb Company SPDS Disso America 2022
Challenges to Brand-Name Drug Developers
2 Discover and develop original innovative pharmaceutical products Establish the original clinical safety and efficacy of the drugs Determine the characteristics of the dosage form of the drugs • manufacturing process • drug stability • purity • strength • drug release and dissolution Accept the most restrictive review and approval from regulatory agencies Distribute drug products the first time to market that could be global with many country-specific requirements
Challenges in Dissolution 3 (1) Regulatory and compendial requirements related • International harmonization • Dissolution acceptance criteria • Compendial devices (2) New manufacturing technology demands • Continuous manufactures • Real time release testing (3) Predictive dissolution for drug product development • Biorelevant dissolution • Integrated biopharm risk assessment: in-vitro, in-silico, and in-animal • In-vitro justification for relative BA and biowaiver (4) Performance testing of novel formulations • Drug release of topical, nasal, LAI, etc. • Different drug delivery routes
Outline Challenges in dissolution in brand name pharmaceutical industry (1) International harmonization of dissolution acceptance criteria (2) Apex vessel implementation (3) Dissolution in continuous manufacturing (4) Predictive dissolution for drug product development (5) Drug release and performance of injectables Summary 4
(1) USP <711> vs ChP <0931>: Dissolution Acceptance Criteria 5 USP <711> ChP <0931> Stage Test units Criteria for Immediate-Release Dosage Forms Stage Test units Criteria for Conventional-Release Dosage Forms S1 6 (S1) Each unit ≥ Q + 5% S1 6 (S1) Each unit ≥ Q S2 12 = 6 (S1) + 6 (S2) Average of 12 units (S1 + S2) ≥ Q, and no unit < Q – 15%. If 1-2 units < Q, but ≥ Q - 10% and average of the 6 units ≥ Q S3 24 = 6 (S1) + 6 (S2) + 12 (S3) Average of 24 units (S1 + S2 + S3) ≥ Q, not more than 2 units < Q – 15%, and no unit < Q – 25%. S2 6 (S2) If 1-2 units < Q and only 1 < Q - 10% but ≥ Q - 20%, and average of the 6 units (S1) ≥ Q, test 6 additional units (S2); If 1-3 units of the total of 12 units (S1 + S2) < Q and only 1 unit < Q – 10% but not < Q – 20%, and the average of the 12 units ≥ Q
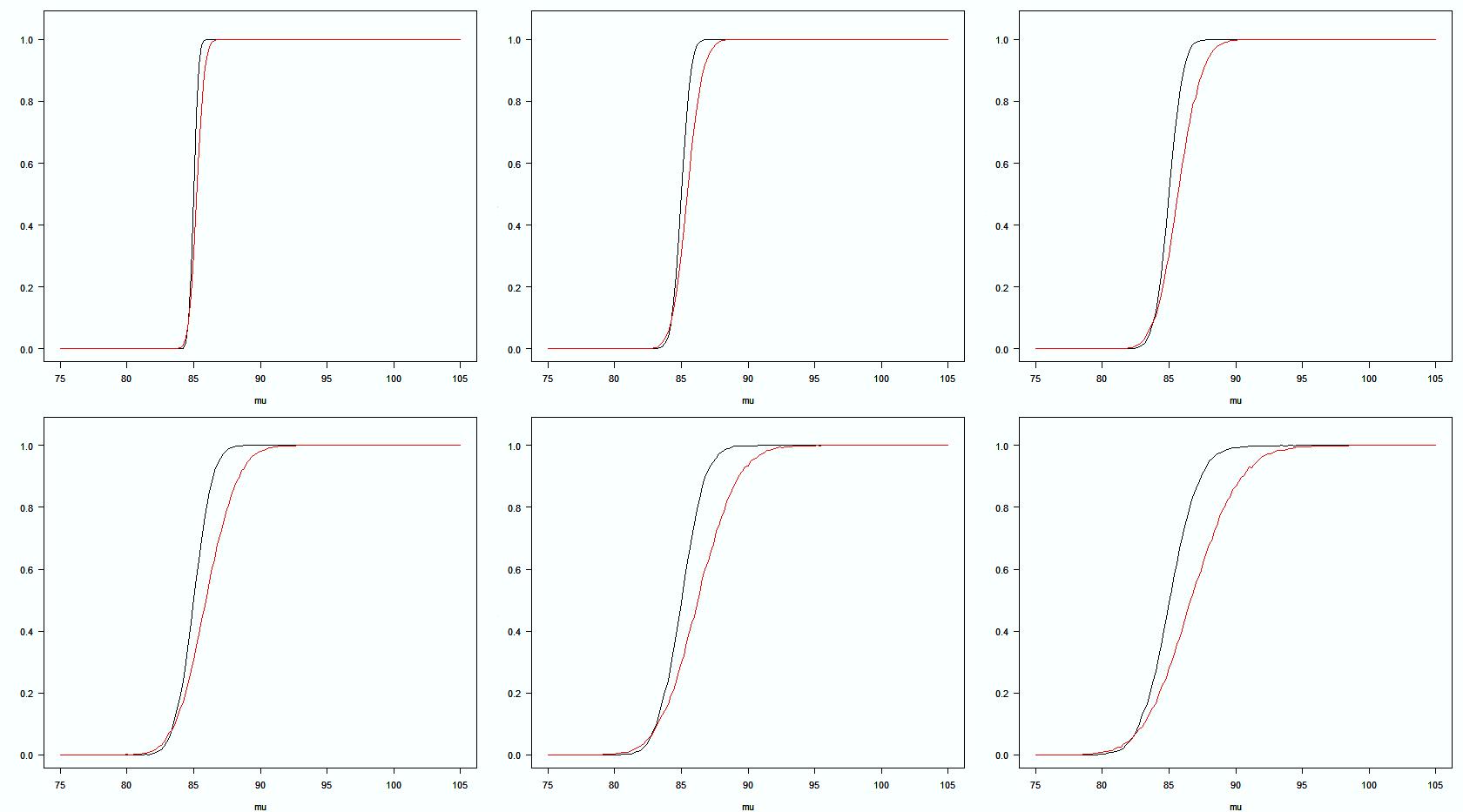
USP Stage1 + 2 CHP Stage1 ● At this stage, ChP acceptance probability is lower than USP. ● The difference of probability is related to the varibility. ● Evaluated by using Monte Carlo simulation generated Operating Characteristic Curves. ● Simulation condition: Q=85.0%, Drug release 75.0%-105.0% Std Dev (σ):1,2,3,4,5,6 Acceptance Probability for Dissolution Testing: USP Stage 1 + Stage 2 vs ChP Stage 1 σ=1 σ=2 σ=3 σ=4 σ=5 σ=6 % Drug release Acceptance probability Ref. B.M. Ning, 2021 AAPS PharmSci360 6
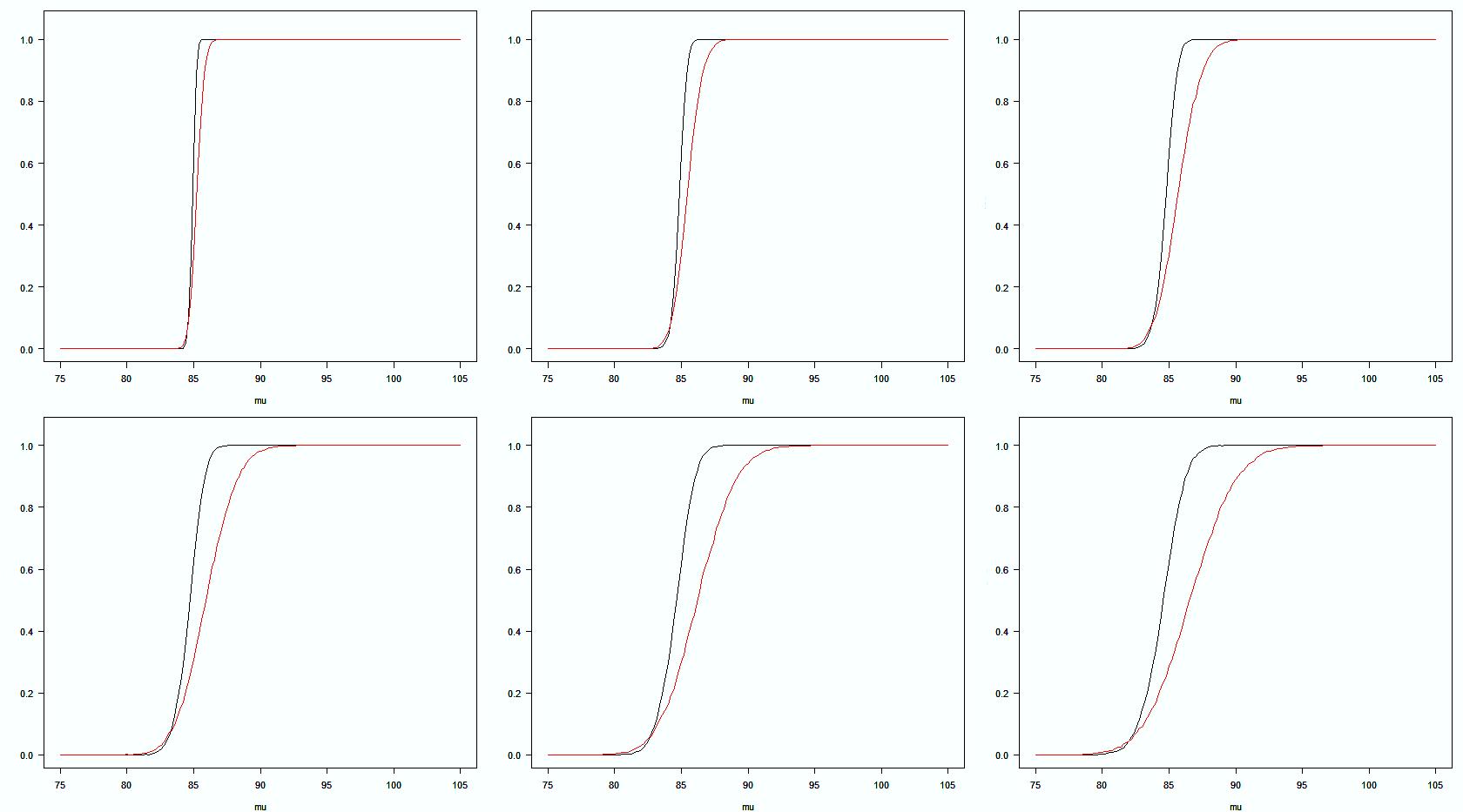
USP Overall Criteria CHP Overall Criteria ● Overall, the total acceptance probability of ChP (2 stages) is lower than USP (3 stages). Total Acceptance Probability for Dissolution Testing: USP Stage 1 + 2 + 3 vs ChP Stage 1 +2 σ=1 σ=2 σ=3 σ=4 σ=5 σ=6 % Drug release Acceptance probability Ref. B.M. Ning, 2021 AAPS PharmSci360 7
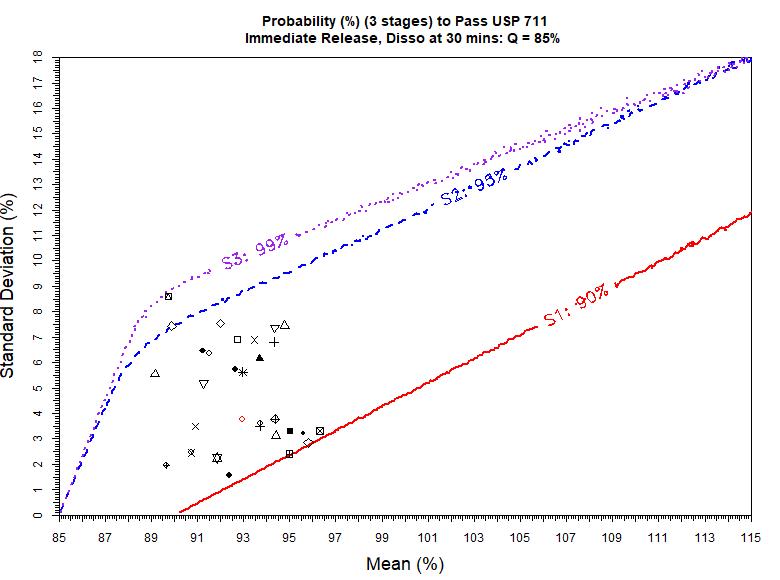
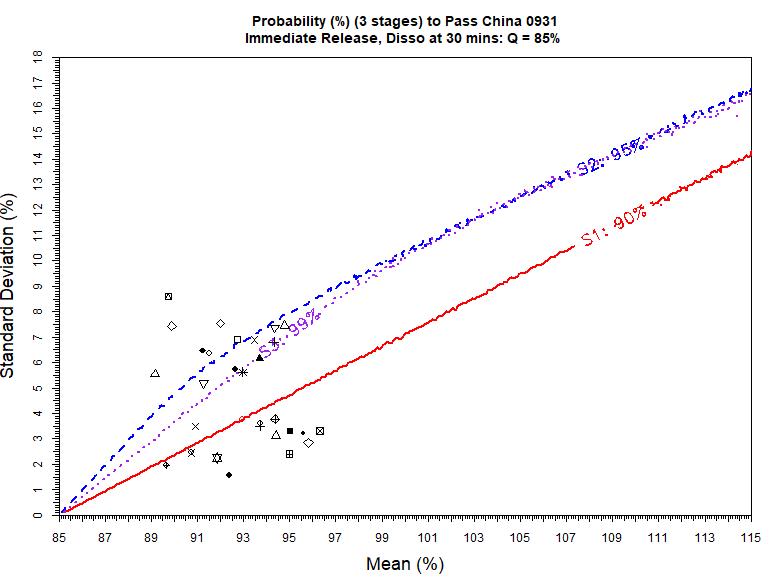
USP <711> vs. ChP <0931> Example 30 batch data Ref. F.S. Li, 2021 AAPS PharmSci360 Stage 1 0 (0%) batch pass USP with > 90% chance Almost 15 (50%) batches pass ChP with >90% chance Stage 2 29 (97%) batch pass USP with > 95% chance 23 (77%) batches pass ChP with > 95% chance Stage 3 30 (100%) batches pass USP with > 99% chance 18 (60%) batches pass ChP with > 99% chance In General Stage 1, USP <711> is more difficult to pass than ChP <0931> Stage 2 and Stage 3, USP <711> may be easier to pass than ChP <0931> Be cautious, for the same batch of the same drug product, if tested against USP <711> and ChP <0931> with the same Q value, the results could be very different – very likely pass USP but fail ChP (or vice versa!) 8
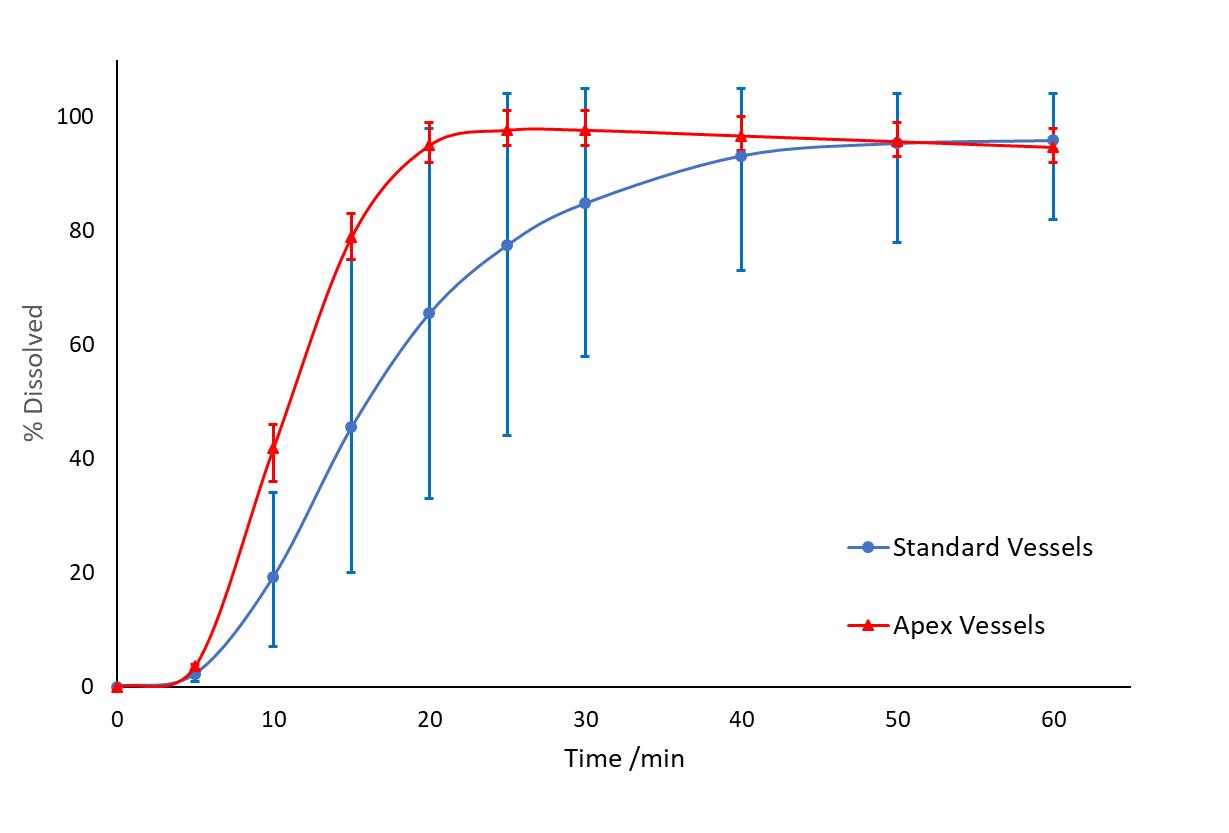
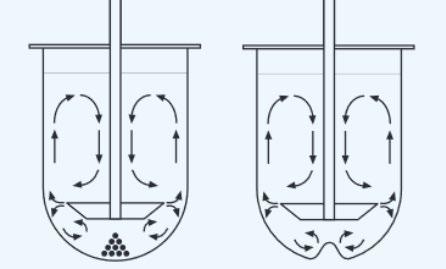
(2) Use of Apex Vessels in Dissolution Testing 9 Formulation A in Standard Vessels vs. Apex Vessels at 100 rpm (n=6, error bars = max/min) in 1 L of pH 6.8 phosphate buffer Apex vessel - an important element of the dissolution scientist’s toolbox Significant benefit in overcoming coning for formulations that contain dense insoluble excipients Useful in development but not widely used in approved QC methods Ref. J. Mann, M. Cohen, A. Abend, et al, 2021 Pharmacopeia Forum 47 (6)
Apex

in Dissolution Testing

Vessels
10 IQ Consortium and AAPS IVRDT Community collaboration study and stimuli discussion in 2020 – 2021 Stimuli article on Pharmacopeial Forum in Nov 2021 with expectation leading to greater acceptance of the apex vessel Consideration for inclusion in future USP 1092 and further in USP 711 as an alternative vessel to the standard 1L vessel? Industrial case studies – Demonstrated benefits such as improved clinical relevance, more robust and discriminatory methods, and streamlined in vitro bridging strategies. Inter-laboratory study – Minimal differences in dissolution performance between 11 partners when a controlled protocol was executed. CFD modeling and simulation – No significant differences between apex vessels made by different manufacturers. Apex vessel specification – Manufactures proposed alongside a qualification procedure for the use of the vessels. Ref. J. Mann, M. Cohen, A. Abend, et al, 2021 Pharmacopeia Forum 47 (6)
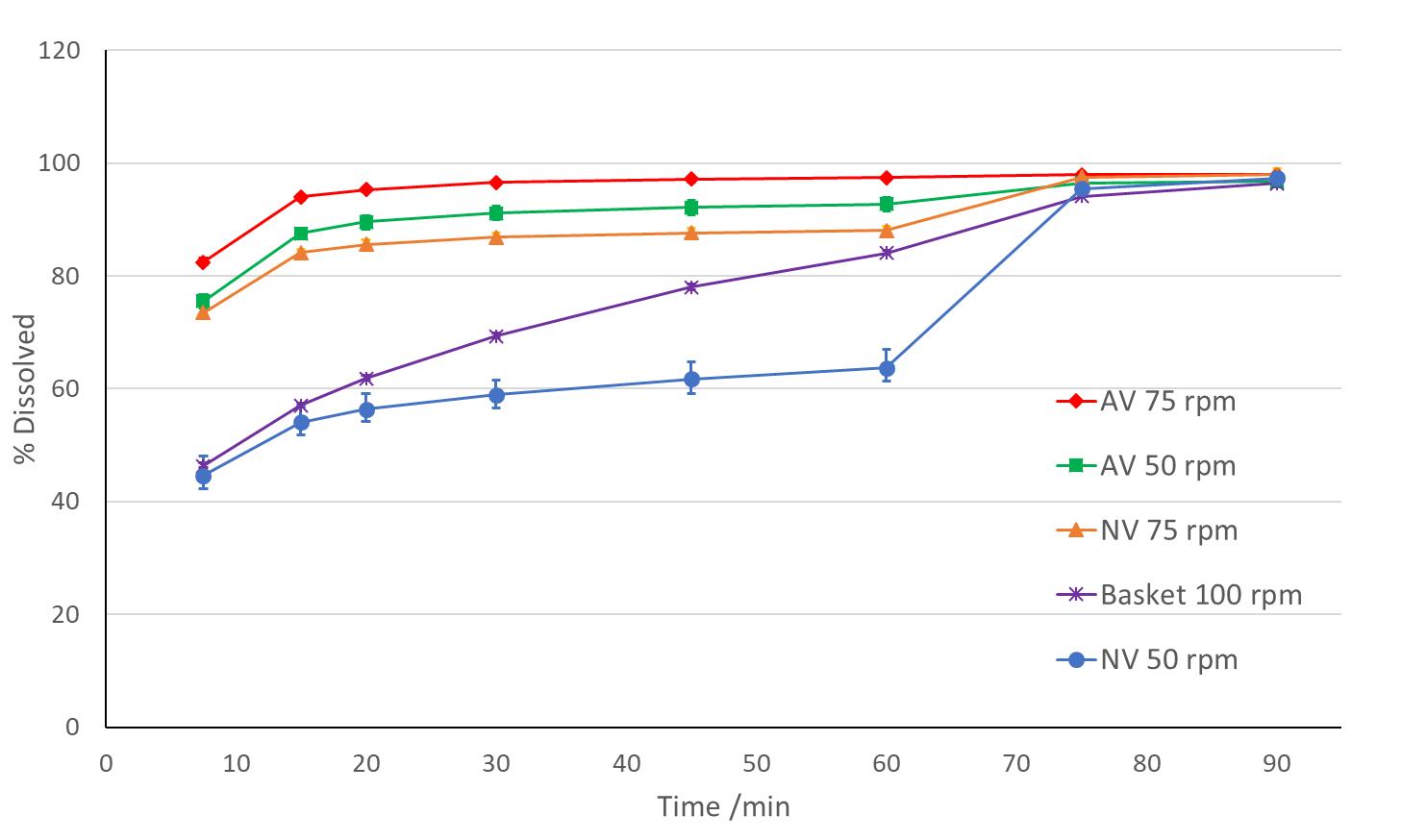

Use of Apex Vessels for biowaiver of a BCS 1 drug 11 Comparison of hydrodynamic conditions at pH 6.8 (n=3, AV = apex vessel, NV = normal vessel) Formulation: 100 mg tablet Drug solubility: A minimum of 2 mg/mL at pH 6.8 Required dissolution by ICH M9: - 900 mL or less of medium - 50 rpm paddle or 100 rpm basket, t - 3 media – pH 1.2, 4.5 and 6.8 Required drug release by ICH M9: - For BCS 1 drug: ≥ 85% dissolved in ≤ 30 min - Dissolution in pH 1.2 and 4.5 passed - Failed in pH 6.8 due to coning Ref. J. Mann, M. Cohen, A. Abend, et al, 2021 Pharmacopeia Forum 47 (6)
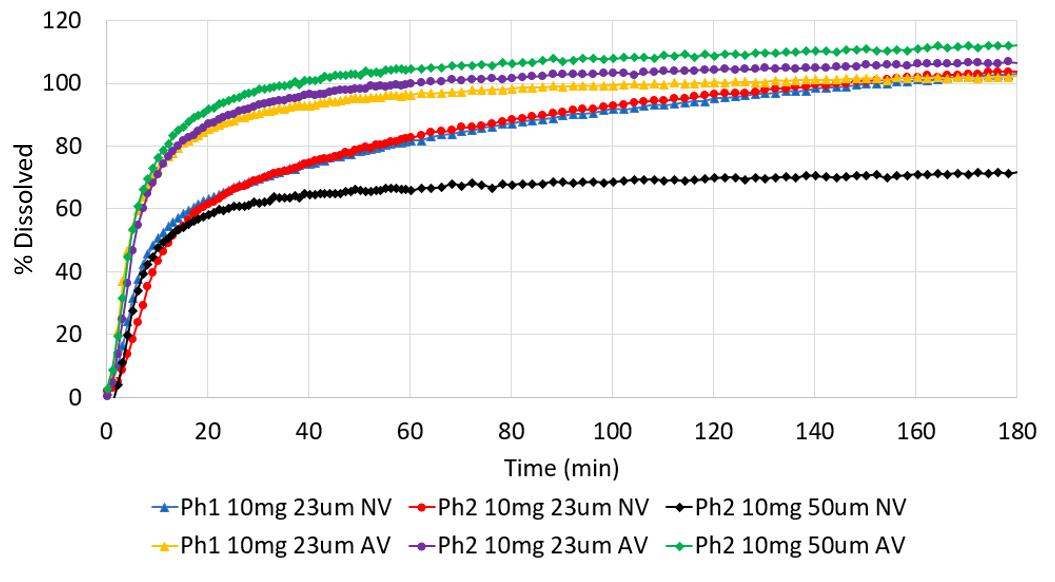
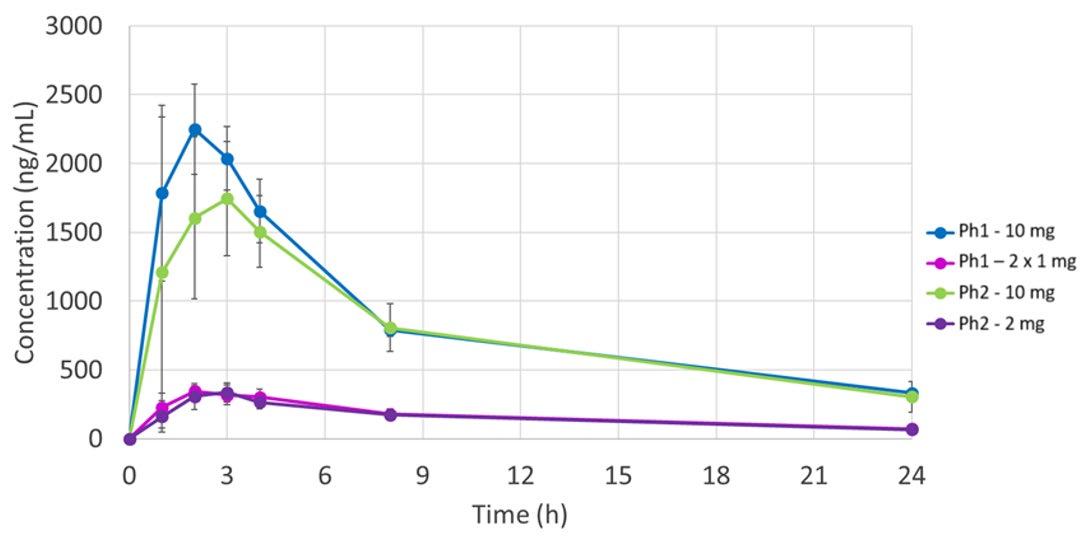

Use of Apex Vessels for biopharmaceutics risk assessment of a formulation change in early development 12 Mean dissolution profiles (n=3), Ph2 tablets (2 mg and 10 mg) vs Ph1 tablets (2 x 1 mg and 10 mg) in FaSSIF-V1, 500 mL, 50 rpm, normal vessel (NV) vs apex vessel (AV) Dog PK (n=4) exposure of Ph1 (2 x 1 mg and 10 mg) and Ph2 (2 mg and 10 mg) tablets. 2 mg tablets: Ph1 vs Ph2 10 mg tablets: Ph1 vs Ph2 Ref. J. Mann, M. Cohen, A. Abend, et al, 2021 Pharmacopeia Forum 47 (6)
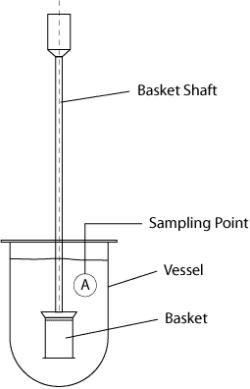
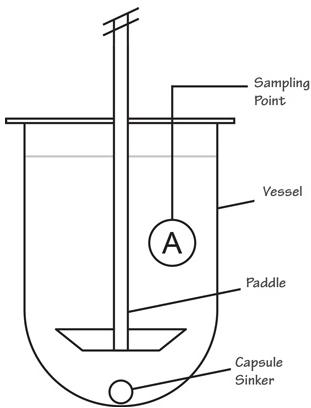

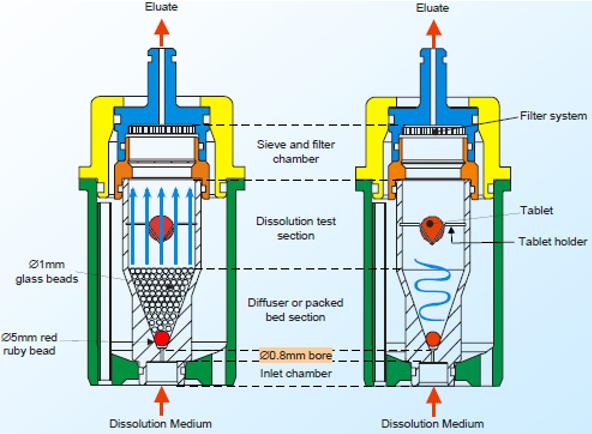
(3) Dissolution in Continuous Manufacturing 13 Dissolution – an important testing for finished drug product, yet a time-consuming measurement ◦ Required for drug product release testing ◦ Requires use of compendial device and setting ◦ Not suitable for on-line or in-line measurement Ref. Quality Lab Accessories LLC USP I Ref. Quality Lab Accessories LLC USP II Ref. B.R. Pezzini et al, Braz. J. Pharm. Sci. 51(2) 2015 USP III Ref. M. Kakhi, Eur. J. Pham. Sci. 37(5) 2009 USP IV
RTRt: Model Based Dissolution
release testing (RTRt):
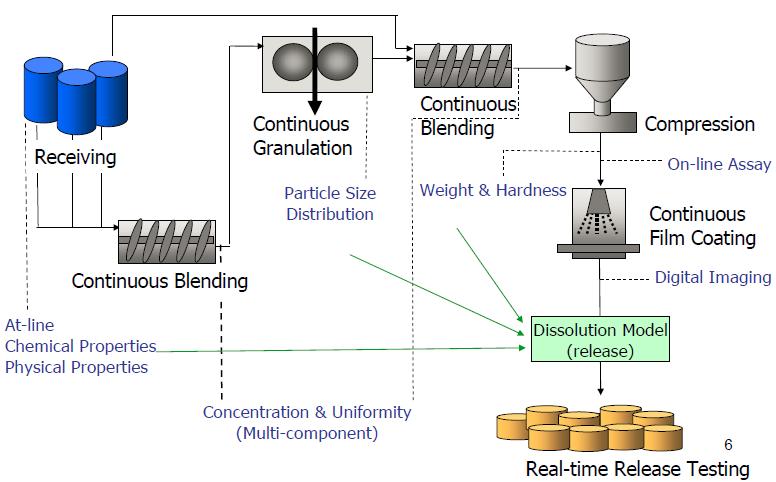
14 Real time
“The ability to evaluate and ensure the quality of inprocess and/or final product based on process data –Typically include a valid combination of measured material attributes and process controls”. --- ICH Q8(R2) Ref. Sau Lee, MIT CMAC 2nd International Symposium on Continuous Manufacturing of Pharmaceuticals, Sept. 2016. Ref. ICH Q13 “Continuous Manufacturing Of Drug Substances And Drug Products” Draft, June 2021.
Dissolution Modeling for RTRt
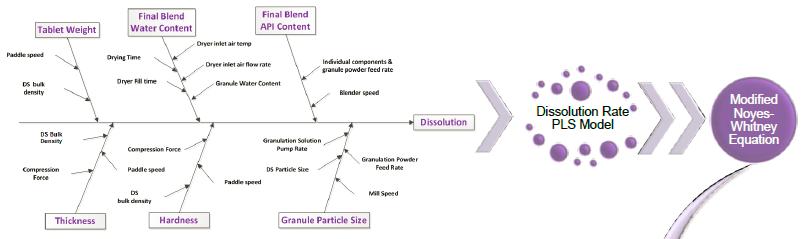


15 Ref. H Li, J Prichard, KA Swinney “Dissolution Modeling for Real Time Release Testing (RTRT)”, Vertex, 2016. Cases from industry

Determine if a method to dissolution can be implemented 16 Ref. H. Li, I. Nir, A. Hermans, et al, “In Vitro Performance Tests for Continuous Manufacturing: The Impact on the Current Compendial Framework”, PF 2022, 48 (3) • Viewpoint of the USP Expert Panel of New Advancements in Product Performance Testing

(4) Predictive Dissolution for Drug Product Development • Individual mean results from 16 partners • Dissolution of ibuprofen from Brufen Forte 600 mg tablets in the two-stage dissolution test • Experiment: USP 2, paddle 75 rpm, 37 °C, 250 mL FaSSGF for S1, add 250 mL FaSSIF concentrate at 30 min for S2. • A: For data with 95% confidence interval • B: With an absolute deviation of 10% from the overall mean. Predictive dissolution variations in different labs OrBiTo Study (2017): “Validation of Dissolution Testing with Biorelevant Media” Ref. J. Mann, et al, 2017 Mol. Pharmaceutics, 14, 4192−4201. 17
Predictive Dissolution for Drug Product Development

18 Problem statement: The same oral drug dosage forms may have different predictive dissolution profiles due to testing methods difference. Compare the difference in dissolution profiles generated through detailed dissolution methodologies and conditions in different labs. PBPK modeling using the dissolution profiles to determine if those dissolution profiles will be all BE or not. Determine the need for loosely specifying or regulating the predictive dissolution methodologies. Lead to harmonized dissolution methodologies, improve oral product development, and reduce animal studies. PQRI Working Group (2020 -2022) “Standardization of In Vivo Predictive Dissolution Methodologies and In Silico Bioequivalent Study”
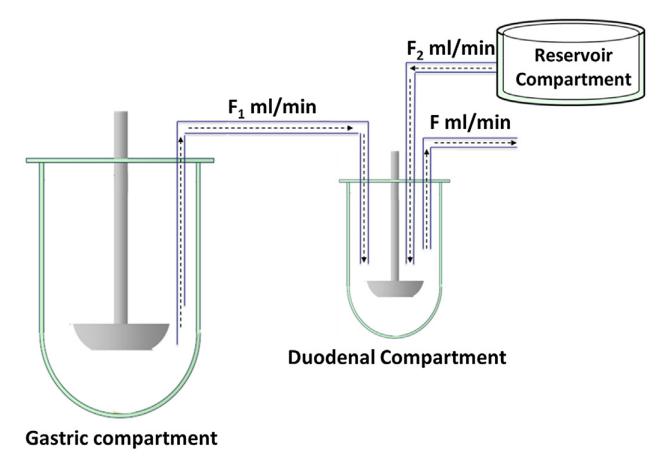

Predictive Dissolution Standardization? 19 PQRI Working Group (2020 -2022) Study Plan: Approach 1. In vivo predictive dissolution profiles from participant’s dissolution methodologies Model drug (IR tablet) from one source (the same drug, same manufacture and same lot). Well-studied and human clinical data including plasma profiles available . Two-stage method or transfer method used by individual lab. Approach 2. Simulations of plasma profiles and virtual bioequivalence (BE) test based on dissolution profiles Study is in progress. Ref. T. Fiolka, et al, 20.20 J Pharm Sci, 109, 2512-2526. Ref. A. Kourentas, et al, 2016 JEPS, 80, 106-114.

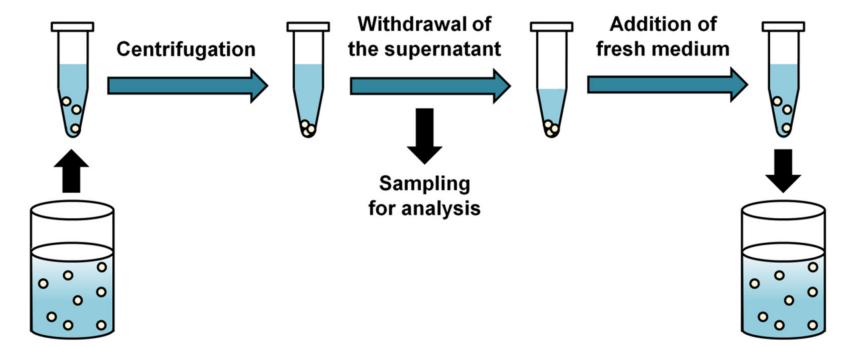

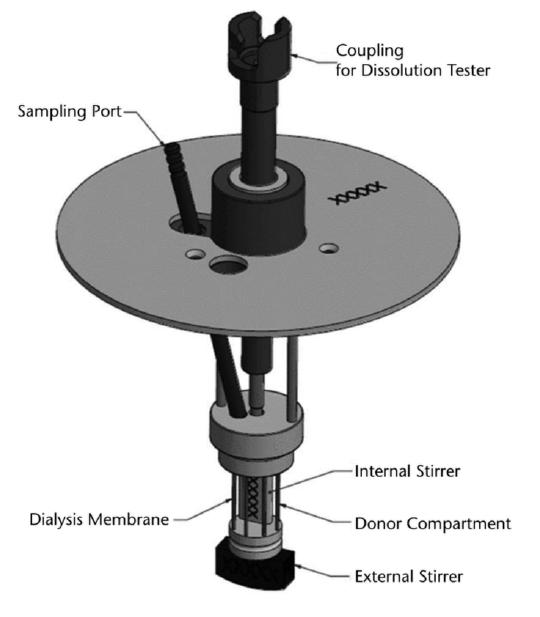
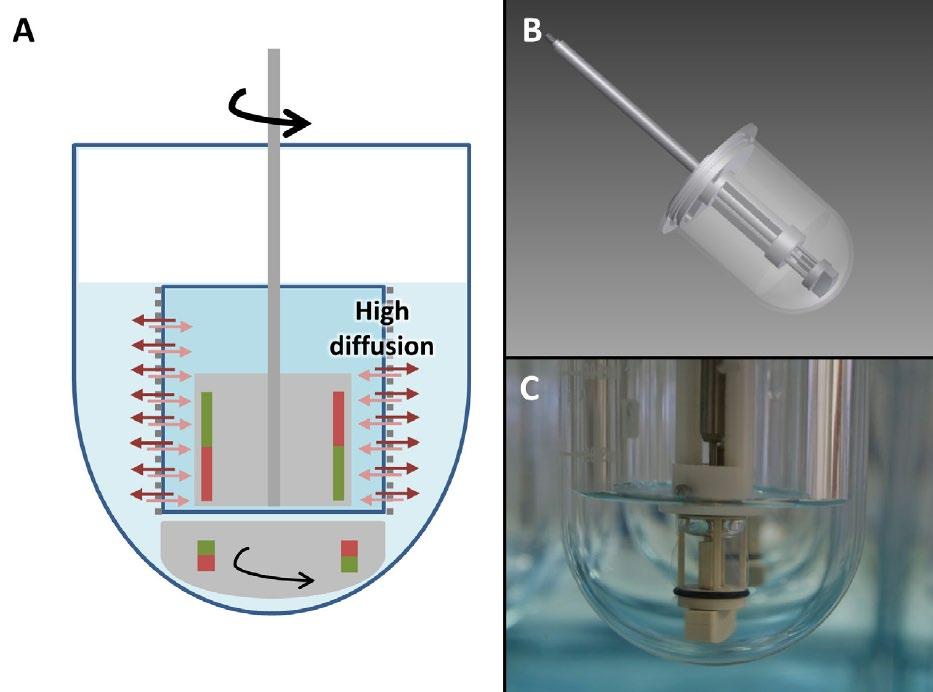
(5) Drug Release and Performance of Injectables Current Methodologies Ref. Y. Kim, E.J. Park, T.W. Kim et al, Pharmaceutics 2021, 13, 1313. Ref. M.G. Wacker, X. Lu, M. Burke, et al, PF, Nov. 2021 . 20
Drug


of
Release and Performance
Injectables New Development – Dialysis based Ref. C, Janas, M-P. Mast, L. Kirsamer, et al, 2017 EJPS, 115, 73–83. Ref. M.G. Wacker, X. Lu, M. Burke, et al, PF, Nov. 2021. 21 Cumulative drug release of flurbiprofen from drug solution and polymeric nanoparticles. Dispersion Releaser (Pharma Test)


Drug Release and Performance of Injectables New Development – Hybrid method Ref. X. Lu, L. Lo, et al, AAPS PharmSci 360, 2021. 22 0 20 40 60 80 100 0 5 10 15 20 25 30 Drug Release (%) Time (hour) 3g 4g 6g 9g Time (hour) Drug Release (%) Drug in PLGA microsphere, n = 6
Drug Release and Performance of Injectables

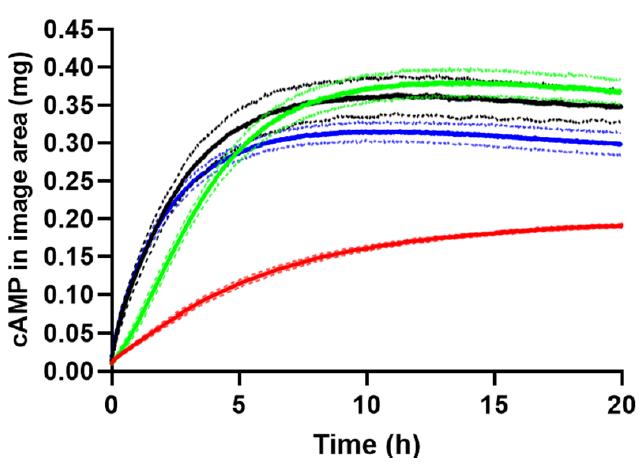


New Development – UV imaging 23 Ref. F. Bock, J.P. Botker , S.W. Larsen, X. Lu, J. Ostergaard, Int. J. Mol. Sci. 2022, 23, 3599.
Summary
Dissolution and drug release testing face many challenges in the brand name pharmaceutical industry.
Some of the challenges are similar with what are in the generic pharmaceutical industry. But many of them have different circumstances.
Harmonization and standardization are helpful for dealing with the challenges.
Development of new drug release methodologies are demanded to meet the industry need.
Various technologies including PAT, QbD, and modeling are changing the way of thinking, doing, and applying dissolution testing.
Predictive dissolution plays more and more important role for biopharmaceutics risk assessment, IVIVC development, and justification of relative BA and biowaiver.
24
Acknowledgement AAPS IVRDT Community IQ Consortium Dissolution WG USP Expert Panel of New Advancements in Product Performance Testing PORI WG for Standardization of In Vivo Predictive Dissolution Methodologies and In Silico Bioequivalent Study William Fish Suliman Chawdry Lili Lo Jigna Patel Vincent Cicale Fredrik Bock Johan Peter Bøtker Susan Weng Larsen Jesper Østergaard 25
