Final Report for PR SeaGrant
Sea Grant College Program
University of Puerto Rico Mayaguez PO Box 9011 Mayaguez, PR 00681-9011.
Project title: Rapid Detection and Quantification of Fecal Pollution in the Caribbean Region.Date: date summary was prepared Project Number: R-92-1-08
Investigators and affiliation
Dave Bachoon, PI Associate Professor
Department of Biology & Environmental Sciences Milledgeville, Georgia
Georgia College & State University
Ernesto Otero, Co-PI Department of Marine Science University of Puerto Rico, Mayaguez Mayaguez, Puerto Rico
Adash Ramsubhag Co-PI Lecturer in Microbiology Department of Life Sciences University of the West Indies St. Augustine, Trinidad and Tobago
Dates Covered: 1 Mar 2008- 15 Mar 2011
Executive Summary
A. Summary of Impacts and Contributions including:
Objectives:
Adapt and develop QPCR protocols to quantify the abundance of B. adolescentis, a known indicator of human fecal contamination, in marine environments of Puerto Rico, Florida, Georgia and Trinidad;
Nucleic acid primers specific for B. adolescentis, were developed and used in samples from Puerto Rico, Georgia and Trinidad. The primers were used in qPCR with cybraGreen to rapidly detect B. adolescentis in water samples in less than two hours.
Provide evidence of the applicability of the developed method to a diverse environmental settings, thus the inclusion of PR, Florida, Georgia and Trinidad.
Samples from different settings were analyzed with the developed methods. The samples shipped from Trinidad and Puerto Rico were used to detect B. adolescentiss in surface waters. Although the PCR method worker in all samples. The detection in samples from Trinidad was not very efficient as the samples from Puerto Rico and the Georgia coast.
Compare the new method with standard protocols of enterococci and E. coli. For this targeted sampling will be conducted at previously identified sites.
Samples from Trinidad, PR and GA were successfully analyzed for enterococci and E. coli using standard culture techniques and used with PCR based detection of Bifidobacter and B. adolescentis to evaluate human derived fecal contamination in coastal waters. A paper related to this work is under review and one was recently published. The RT PCR approach was recently tested and was able to detect enterococci and B. adolescentis, in all samples.
Demonstrate the usefulness of this approach by means of a short workshop aimed to students, agencies and resource managers.
The workshop have not been conducted up to date; however, we are contacting local managers to make them aware of the applicability of the nested approach used here to identify potential sources of fecal contamination in different coastal and estuarine environments. We recently, contacted Puerto Rico DNER, USEPA anfd EQB officials
in order to make them aware of our published results and the locally developed capability. An unofficial collaboration with USEPA Ecosystem Research Division personnel have resulted in the proposal of a special session for the next ASLO Meeting to be held in San Juan, PR in which part of our results will be presented.
Additional NEW Goals
The fluorescence time series of samples amended with commercially available fluorescence substrates targeting enterococci were examined closely during the funding period. We have determined preliminarily that these fluorometric assays may be adapted as an early warning (3-6 hours) for the detection potential of fecal contamination. The results are being analyzed and will be sent for publication in the near future. Officials from the local EQB have shown interest in the subject and will be kept informed of the results.
Advancement of the Field: A 200 to 500 word summary of the interpretation of what the project findings mean to the discipline. New discoveries or methods should be included.
The usefulness of Bifidobacterium sp and B. adolescentis as a warm blooded animal and human derived fecal contamination was confirmed. Recently, other groups of microbial indicators have been used by other researchers which considered Bifidobacteria prone to false positive identification of human fecal contamination. Basically, our research suggest that Bifidobacteria are as prone to false positive identification of human derived contamination as other indicators and thus should not be ruled out as a sole or complementary test.
Furthermore, our research indicates underscores the importance of using a nested approach in the assessment of fecal contamination and source assessment as a cost effective approach by combining traditional plate counts to develop preliminary evidence of fecal contamination with more expensive molecular approaches to confirm sources.
Results for Puerto Rico indicate widespread fecal contamination in coastal areas. This contamination was confirmed to be frequently of human origins, but in many occasions wildlife (birds) are suspected. Nonetheless, this findings indicate widespread sources of biological contamination which combined with the putative accompanying chemical contaminants (i.e. nutrients) may relate to the observed shifts in coastal communities, This connection, although discussed in recent published works have not been tested rigorously.
Problems encountered (if any): Describe any major problems and how they were addressed during the study. Problems with experimental protocols and how they were overcome should also be included.
No major problems were encountered.
Research Impacts: A detailed summary or list of research impacts. Research impacts refer to cases where the information discovered is applied to an important problem or produces a change in established practices. Impacts are also seen in cases where new information has a beneficial effect on resource quality, or provides a way to optimize industrial procedures. Another way of looking at impacts is as a high order societal/scientific benefit.
Provided the means to increase awareness of sources of fecal contamination in sites around Puerto Rico. Communication of these research will be made available to managers of resources impacted (DNER, EQB, USEPA). This evidencecan be used in support of agencies goals to sustain a healthy environment and human population.
Furthermore, this research provided the opportunity to evaluate and streamline an early warning (3-6 hours) approach for the detection potential of fecal contamination which could be adopted by local agencies to speed up assessment of contamination of public beaches while results of regular testing are made available.
Other important impacts or products:
List the students supported, their level (Pre bachelor or Graduate), their role in the research project, amounts paid by Sea Grant and Match, time period, number of weeks, and number of hours.
A. The following students were involved in DNA extraction, PCR preparation in Georgia, as well as organizing PCR results.
Cortney Miller (undergraduate; GCSU); 1 month pay, 20 hrs/ wk.
Daniel Burt (Graduate student ;GCSU); 1 month pay,20 hrs/ wk.
Shanu Markand (Graduate Student; GCSU) ;6 months pay, 20 hrs/ wk.
Lixian Chen (Graduate Student; GCSU) ;1 month pay, 20 hrs/ wk.
B. The following students were involved in sampling and general microbiology lab work in Puerto Rico.
Carolina Hincapie (Graduate Student; UPRM). SWorked for 15 hours a week from January-May 2008. Dissertation not related to the proposed work.
Brenda Soler (Graduate Student; UPRM.; In additrion to the above she was in charge of early phases of the fluorometric assay for fast detection of enterococci. She worked
for 15 hours a week During January-May 2008 and Summer 2009. Her dissertation is not related to the project.
The following student worked in Trinidad conducting general laboratory work, involving sampling and determining fecal contamination levels.
C. Garvin Perry (Graduate Student; UWI-Trinidad); worked 40 hrs/wk since the inception of the grant.
Include a list of theses or dissertations, and send a copy of the abstract when complete. Sea Grant needs to be acknowledged and include project number.
Shanu Markand (MS Thesis; GCSU 2010) Evaluation of fecal contamination in Puerto Rico, Trinidad and Georgia, USA.
Garvin Perry is scheduled to complete laboratory work for his master's degree by the end of September 2010 and should submit his thesis for examination by December, 2010. His research title is "Strain selectivity of common methods of enumerating E. coli and enterococci in marine waters of Trinidad".
List presentations
• Shanu Markand , Samender Sherchen , Lisa Gentit , Dave Bachoon. Comparison of Enterococcus measurements in GA , Trinidad and Puerto Rico by culture based methods and quantitative polymerase chain reaction. Georgia College & State University Research Conference. April. 2010.
• Miller, C. M., Riggins, L. Phillips, T. and Bachoon, D. 2008. Comparison of Three Molecular Methods used for the Detection of Bifidobacterium adolescentis as an Indicator of Human Fecal Pollution. American Society of Microbiology Meeting. November 6-8, 2008. Jacksonville, FL
• Kristen Green, Shanu Markand, Ernesto Otero, Adash Ramsubagh, Gavin Perry and Dave S. Bachoon (2009) Comparison of Traditional and Molecular Methods for Assessing Fecal Pollution in Georgia and the Caribbean Region . 109th General Meeting, American Society of Microbiology Meeting. May, 2009. Philadelphia, Pa.
• Shanu Markand, Ernesto Otero, Adash Ramsubagh, Gavin Perry and Dave S. Bachoon Enumeration and Molecular Source Tracking of Fecal Pollution in Popular Public Beaches and Estuaries in Georgia, Puerto Rico and Trinidad. Georgia College & State University Research Conference. April. 2009. Winner of an American Society for Microbiology Travel Grant Award
• Dave Bachoon, D. S.; Ernesto Otero, E.; Adesh Ramsubaugh, A.; Trisha Philips, T.; Samendra Prasad Sherchan, S. 2011. RAPID DETECTION AND QUANTIFICATION OF FECAL INDICATOR BACTERIA ESCHERICHIA COLI O157:H7 AND ATRAZINE DEGRADERS IN THE CARIBBEAN. ASLO Aquatic Sciences Meeting. San Juan, PR 13-18 Feb. 2011.
• Zepp, R. G.; Cyterski, M.; Molina, M.; White, E.; Otero, E.; Wolfe, K.; Parmar, R. 2011. PREDICTIVE MODELING OF CULTURABLE AND QPCR ENTEROCOCCI AT BOQUERNN BEACH, PUERTO RICO. ASLO Aquatic Sciences Meeting. San Juan, PR 13-18 Feb. 2011.
Publications (journal articles, articles from proceedings, book or book chapters).
Bachoon, D.S. S. Markand, E. Otero, G. Perry and A. Ramsubaugh. 2010. Assessment of non-point sources of fecal pollution in coastal waters of Puerto Rico and Trinidad. Mar. Poll. Bull. 60: 1117-1121.
Bachoon, D. C. Miller, C. Green and E. Otero. 2010. Comparison of Four Polymerase Chain Reaction Methods for the Rapid Detection of Human Fecal Pollution in Marine and Inland Waters. I. J. Microbiol. doi:10.1155/2010/595692
Any patents, copyrights, disclosures of invention, technology licensing should also be listed with appropriate description. None
List honors awarded to project personnel as a result of this work. None. Indicate sources of matching funds: List names and amounts. Indicate if any sponsors identified in the original proposal have changed. No changes in matching funds were made.
New extramural funds in addition to match: List all the titles, funds duration and sponsors of all grants and contracts secured as a follow-up or adjunct to this Sea Grant project. Indicate if they are in effect or pending. Only indicate contributions directly linked to the project. This information is used to determine activity in leveraging Sea Grant support to obtain additional funding.
None. We are in the process of identifying other sources of funds to practice knoledge and skills acquired during our work.
Benefits: Additional impacts and contributions that go beyond those described above. Impacts that provide physical, environmental, economic or societal gains have important outreach potential and should be described. Any benefit consonant with the Sea Grant mission should be included. New products and
processes that increase revenues, jobs, health or tools, technologies and information that improve environmental management or models that improve forecasting or management of ecosystem stressors are benefits consonant with the Sea Grant mission and need to be mentioned.
1. In many Caribbean islands such as Puerto Rico and Trinidad the local economy is heavily based on tourism and the island tourism is linked to marine activities. Therefore a healthy functioning marine environment is critical for tourism in the Caribbean islands. Our research has indicated for the first time that fecal pollution in Trinidad is present in popular beaches of the island. Furthermore our research suggests that the risk of fecal contamination is widespread in both islands. Results obtained from the combined approach of molecular PCR technologies with traditional methods of fecal bacteria assessment have provided us with new insights into the usefulness of methodologies for evaluating the level of fecal pollution in marine environments. Our results support the idea of chronic impairment of coastal water quality by biological contamination and/or by accompanying chemical contaminants. Our work strengthen the limited collectionof peer reviewed data evidencing the necessity to take remediative action regarding the health of coastal ecosystems by directing our focus towards lowering biological pollution inputs into Caribbean coastal areas. Lowering such inputs will prove beneficial to managed resources such as critical marine habitats as well as those used for recreation.
2. During the first year we were contacted informally by the Rincon´s Surf Rider Chapter (Steve Tamar) and conducted an intercalibration activity in support of their monitoring activities. Recently we have been briefly consulted by Wes Marten (Chairperson) in matters related to water quality and fecal contamination in relation to a new series of monitoring efforts.


Intercalibration excercise with members of Surf Riders, Rincón. Left: Steve and Judith Tamar and Ernesto Otero (center) at Barrero Beach. Right: John Folden at the sampling site in Tres Palmas.
3. Groups of students from Georgia College and State University and University of West Indies have visited Trinidad and Tobago during summers as part of a course on Microbial Diversity. Activities included hands on experience on water quality analysis (measurement of physical, chemical and biological parameters at several beaches. As part of the activity, samples were analyzed at the University of the West Indies where Dr. Ramsubhag and his graduate student assisted in the enumeration of fecal bacteria using the IDEXX system, which were used in part for our research.


4. Other Benefits
4.1. Dr. Bachoon has discussed our findings with researches and managers from the University of Georgia MAREX and shared our findings with Thomas J.
Goreau, PhD President, Global Coral Reef Alliance. I was contacted by Dr Phil Smith, Director from Aquatonics Ltd Glenthorne, they are working on benthic pollution in Trinidad and Tobago and were interested in our findings. In addition I have been discussing our research with members of the Trinidad government agencies with the hope of brining the issue of water quality and fecal pollution on their agenda.
4.2. During the past year, Dr. Otero has shared his views with personnel from various governmental and non governmental groups related to fecal contamination through coastal sites in PR. As mentioned earlier, the Surfrider organization contacted us asking our opinion about certain ideas related to water quality monitoring in sites of their interest including some associated with Tres Palmas Reserve. We understand that our conversations were important guidance in supports of this organizations initiative, unique in PR. Furthermore, Otero have talked with EQB personnel such as Annette Feliberty Ruiz, (Chief, Point Sources Permits Division), indicating interest in the subject and sharing our published work with her colleagues at the agency. Similary, Evelyne Huertas, from the local USEPA office had transmitted our published work to the EPA ORD Office in Cincinnati, USA to researches conducting research in bacterial source tracking. This communication has the potential to increase awareness at the US level of PR fecal contamination issues. In addition, during our work an opportunity for a brief informal collaboration arose during May-August 2009 with the USEPA Ecosystem Research Division in Athens, GA. During this collaboration, EPA officials conducted monitoring at Boquerón Beach and we complemented their efforts using fast enetrococci fluorometric assays in blind test in comparison with their results. Qualitatively the results indicated this fast method promises effectiveness as a technically simple evaluation tool for detection of fecal contamination potential within a few hours of sampling. Finally, Otero is cochair of a Special Session during ASLO spring Aquatic Sciences Meeting to be held in San Juan, PR. (S90: Biological Contamination of Puerto Rican Streams and Coastal Waters: Sources, Fate, Monitoring, and Predictive Modeling), an opportunity to increase the global impacts of our research.
4.3. In Trinidad, the project is valuable to local regulatory agencies in developing new water quality standard using fecal coliforms. Currently, there are no formal regulatory standards for recreational water in Trinidad and Tobago. We would have discussions with our Environmental Management Agency and other stakeholders with regard to suitability and other issues related to the methods used for detecting fecal pollution.
4.4 During the final stages of the rpoposed work, including the no-cost extension, Otero, coordinated interests of various agencies and one NGO in order to produce a collaborative effort through Seagrant. Organizations that demonstrated interests
included the San Juan Bay Estuary Program, PR EQB, PR DNER. Personel of US-EPA in Athens agreed to collaborate by conducting analysis of submitted samples, thus adding value to the proposed work.
B. Final Report Narrative Statement of Problem
The marine environment of many of the islands of the Caribbean, including Puerto Rico and Trinidad and Tobago, is under threat from pollution due to the discharge of untreated or poorly treated domestic and industrial wastewaters into the ocean. Some of the major pollutant sources are the microbial contaminants and pathogens found in the feces of humans and animals. Quantifying the levels of human fecal contamination is a high priority for local officials responsible for both recreational and shellfish growing waters. The adoption of new biological sensors or fecal indicator bacteria, such as fecal enterococci (Stewart, et al. 2003), which replaced total coliforms and E. coli as the microbiological standard for coastal bathing or swimming waters, has potentially increased the number of beach advisories in many many places, including Puerto Rico. Recent research has demonstrated the potential for a large proportion of false positive results for enterococci in coastal waters and sediments in Puerto Rico (Hartel et al, 2005). This is due to the fact that standard methods used to test for these microbial contaminants are based on culturing techniques that are unable to inhibit the growth of nontarget bacteria. As a result, nonenterococci bacteria may grow and mimic enterococci, especially under conditions found in the environment. Furthermore, other species of enterococci might be present, which may also grow in enterococci media and trigger false alarms of human fecal contamination. For example, Rivera-Ortiz (personal communication) had confirmed the presence high numbers of Enterococci avium in a mangrove key used as rookery by cattle egrets in La Parguera, PR. These errors are more critical when results are close to threshold values established by regulation, thus increasing the incidence of contamination alerts and beach closures. Analysis of enterococci are frequently positive in beaches monitored by the local Environmental Quality Board, (i.e. > 60%
03-Ene-0604-Mar-0603-May-0602-Jul-0631-Ago-0630-Oct-0629-Dic-0627-Feb-0728-Abr-0727-Jun-07
Date
1. MPN Enterococci from January 2006 to Early June 2007 at the Patillas Public Beach (EQB, 2006; 2007)
of the samples collected in Patillas Beach were over the accepted levels from Jan-Jul 2006; EQB, 2006 and Figure 1). This situation poses a potential economic hardship for the Caribbean region, particularly to the booming coastal tourism industry. Resource managers need a reliable sensor that can provide a quantitative measure of fecal pollution as well as its source. Bifidobacteria has recently emerged as a good candidate for monitoring fecal pollution in marine environments (King et al. 2007).
Bifidobacteria are anaerobic bacteria found in the intestinal microflora of humans and some animals, making up a significant portion of all the microorganisms present.
Certain species, such as B. adolescentis, have been shown to grow exclusively in the intestines of humans (Bonjoch, et al. 2004). This makes Bifidobacteria a good sensor for distinguishing between human and non human fecal contamination. However, the anaerobic and fastidious nature of these microbes (Requena et al, 2002) has limited the use of Bifidobacteria as a biological indicator of fecal pollution by standard plate count techniques. With the use of currently available molecular techniques it is possible to circumvent the limitations imposed by plate count techniques and assay Bifidobacteria in environmental samples without culturing.
We have successfully used the detection of a 297 bp PCR amplicon of B. adolescentis as a molecular sensor of human fecal pollution in Georgia estuaries (Fig.2.) and in New Orleans (Sinigalliano et al. 2007).
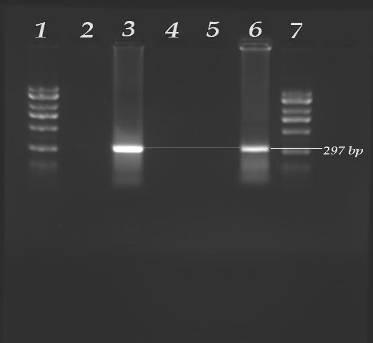
Figure 2 –Detection of Bifidobacteria adolescentis by PCR in agarose gels after electrophoresis – Lane 1 –2kb Ladder, Lane 2 = Cow Feces, Lane 3 = Positive control (sewage), Lane 4 = Altamaha River Sound, Lane 5 = West Point – Fedrica River, Lane 6 = Dunbar Creek, Lane 7 = 2kb Ladder.
Although it is relatively simple to detect human fecal pollution by non-quantitative PCR assays for B. adolescentis, it is more challenging to determine the number of these bacteria per volume of water. The recent development of quantitative PCR (qPCR) or Real-Time PCR assays to reliably quantify bacteria populations in samples, has provided scientists the means to circumvent the lengthy and tedious process, related to culturing and subculturing, to quantify and verify the identity or source of fecal bacteria found in natural environments. Quantitative PCR or real-time PCR is carried out using different reaction components and instruments compared to standard non quantitative PCR. When performing QPCR, DNA amplification can be observed during the PCR process, this eliminates the need to determine successful DNA amplification by agarose gel-electrophoresis. Thus QPCR can be used to detect bacteria in environmental samples faster than nonquantitative PCR or heterotrophic plate counts. QPCR protocols have been
developed and used to quantified bacteria such as enterococci, E. coli, Vibrio and Bifidobacteria in environmental samples (Marinen et al. 2003; Gueimonde et al. 2004; Sinigalliano et al. 2007). Haugland et al. 2004., have used QPCR to determine the levels of Enterococcus in the waters of Lake Michigan and Lake Erie in as little as three hours. In addition to being rapid, studies have shown that there is a good correlation between QPCR estimates of bacterial abundance and Enterococcus colony-forming –units (CFU) by plate counts (Haugland et al. 2004). We have previously used real-time qPCR assays to enumerate a variety of indicators and pathogens in environmental samples, including assessing the abundance of Enterococcus fecalis and Escherichia coli in the waters and soil of New Orleans after Hurricane Katrina (Sinigalliano et al. 2007).
To date, QPCR have been used successfully for the quantification of Bifidobacteria species in human fecal samples with Bifidobacterium genus- and species-specific primers (Marinen et al. 2003; Gueimonde et al. 2004; Matsuki et al. 2004).
Although QPCR analysis of bifidobacteria abundance in fecal samples have been demonstrated to be rapid and very sensitive (Marinen et al. 2003), this techniques have not been used to quantify Bifidobacteria in marine environments. Herein, we propose to evaluate the level of fecal pollution in marine environments by estimating the abundance of Bifidobacteria in water samples using qPCR. The establishment of a quantitative assay for an alternative fecal indicator bacterium such as B. adolescentis can potentially provide another tool to water quality managers for increased confidence in management decisions. This approach may be particularly beneficial in subtropical and tropical climates where traditional indicators such as E. coli and enterococci have proven especially problematical and do not correspond well to point-source sewage contamination (Stewart et al. 2003). Contrary to the definition of a good indicator bacterium, these two organisms have been shown to persist and even re-grow in these environments in the absence of human fecal contamination (Solo-Gabriele et al, 2000 and Desmarais et al, 2002). Current preliminary work had been successful in utilizing PCR based techniques to identify and corroborate human derived fecal contamination along the coast of Georgia (Table 1). These preliminary results show no detection of bifidobacteria under conditions where lower but still reportable levels of enterococci were found suggesting a false positive identification of fecal contamination from human sources. At present, work conducted at Bachoon´s laboratory is expanding the PCR detection towards QPCR, in which abundance of bifidobacteria assessments will be possible from coastal waters.
Table 1: Determination of human microbial contamination in various locations along the Georgia coast. The combination of enterococci counts and detection of B. adolescentis PCR amplification serves provide better estimates of human sources of microbial contamination (see also Fig.2.).
Sample Site – Georgia Estuaries
Little Satilla Tributary – Buck Swamp Road
Enterococci Count (CFUs/100ml)
Human Fecal Contamination?
516 Yes
Black Bank Creek 160 Yes
Little Satilla River 86 No
Turtle River Tributary – Highway 82 58 No
Turtle River Tributary – Head Drive 55 No
Dunbar Creek 22 Yes
West Point – Fedrica River 16 No
Altamaha River Sound Too Few to Detect No
Methods used
The approach of this study relies on the parallel determination of the abundance of fecal contamination microbes by standard and a QPCR approach, the subject of this study. Replicate 100 mL aliquots for the determination of enterococci, E. coli and B. adolescentis will be collected using sterile containers (e.g. Whirl-pak bags) at each sampling site in Georgia, Puerto Rico and Trinidad-Tobago. Figure 3 shows some of the potential sampling sites, however, a final decision of sampling sites will depend on preliminary findings. Other sites in Puerto Rico such as San Juan Bay to the north and Río Fajardo to the east will be considered. The final selection of sites include healthy and contaminated rivers, estuaries, beaches and reef systems. Samples will be collected and kept cold at (5ºC) until reaching the laboratory. Samples will be processes within 6 hours of collection (see flowgram).
Standard Methodology
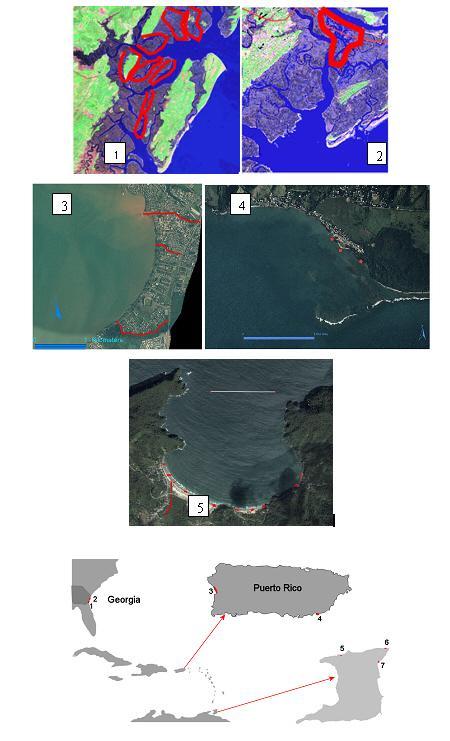
Figure 3. The area of study encompasses a wide range of environments from estuarios and marshes in Georgia (southeastern US) and coastal systems of Puerto Rico and Trinidad-Tobago in the Caribbean. The numbers indicate potential areas of sampling in all three countries. 1. Mckintosh county near Sapelo Is.; 2; Chatham county near Skidaway Is.; 3. Mayagüez Bay Bay tributaries such as the Yagüez River and Savat and Sabalos Creeks. 4. Patillas Public Beach; 5. Maracas Bay; 6. Toco; 7. Salybia. Samples will be collected in areas shown in red.
Enterococci numbers will be estimated using the Enterolert system and fecal coliforms and E. coli will be estimated using the Colisure system (IDEXX Laboratories). Both methods are EPA approved. Three dilutions to a total volume of 100mL will be tested (10-1, 10-2 and 10-3) using manufacturer supplied sterile bottles. The dry incubation media is added to each bottle. The capped bottle is mixed well and poured into Quantitray 97 well trays and sealed using a quantitray sealer. During the sealing process the samples are distributed uniformly in all wells. Incubation will proceed for 24h @ 41ºC for enterococci and 24h @37ºC for fecal coliforms followed by an additional 24h of incubation at the same temperature to estimate E. coli abundance. After the incubation time, positive wells (those in which the targeted bacteria grew) will be determined by fluorescence under a UV light lamp. Most probable number tables will be used to estimate abundance of these bacterial groups.
DNA Extraction
One hundred milliliters of each water sample will be filtered in triplicate through a 0.22-μm Type GS nitrocellulose filter (Millipore, Billerica, MA). Genomic DNA Hill be extracted from the filtered water samples using the MoBio Ultraclean™Soil DNA Kit (Carlsbad, CA) with modifications as described previously (King et al., 2006). Extracted DNA Hill be quantified by a Nanodrop ND-1000 Spectrophotometer (Wilmington, DE) and checked for integrity on a 2% agarose gel stained and with ethidium bromide for visualization under UV light. Afterwhich, DNA extracts will be stored at -20°C until further use.
24h@41ºC Enterococci (fluoresce)
10-1, 10-2 10-3 dilutions
24h@37ºC coliforms (red)
E.coli (fluoresce)
Collection of water samples
Filtration and DNA extraction
Re-count additional 24h@37ºC
Counts of positive wells and MPN determination
Addition of exogenous internal control
Bifidobacteria adolescentis qPCR if previous step positive
Figure 4. Logistics of laboratory work.
In addition, the efficiency of the DNA extraction procedure will be determined for water simples from each region using Lactococcus lactis as an exogenous purification control. Five milliliters of a known density of L. lactis will be added to 95 ml of sample water in a sterile 1L polypropylene container and mixed be inverting the container 10 times. DNA will be extracted from the 100 ml solution as previouslydescribed. This procedure wll be performed in triplicate to ensure that accurate purification efficiency will be obtained. Quantitative PCR will be performed and the extraction efficiency will be calculated.
Quantitative PCR
Initial non-quantitative PCR assays already developed for specific detection of B. adolescentis (King et. al. 2006) will be used as a preliminary starting point for the adaptation to qPCR protocols. Primers and probes specific to B. adolescentis are already been adapted to QPCR with laboratory cultures of B. adolescentis in our lab at Georgia College & State University. We will test the best candidate oligonucleotide primers with artificial culture assemblages to optimize the physical assay conditions. Currently we have begun evaluating the QPCR protocol with filed samples from the Georgia coast.
Quantitative PCR reactions will be prepared in 100 µl optical tubes by addition of the following components to a final volume of 25 µl: 12.5 µl Stratagene® FullVelocity® SYBR® Green QPCR Master Mix, a 2X concentrated mixture of archaeal DNA polymerase, dNTPs (GAUC), stabilizers, neutralizing hot start monoclonal antibodies,
a thermostable accessory protein, 5mM MgCl2; 0.1 µM of each primer, and template DNA. The reactions will be monitored in a MJ/MiniOpticon Real Time PCR Detection System (BioRad®, Hercules, CA), under the following conditions: 95°C for 8 min; 40 cycles of 95°C for 20 s, Annealing Temp for 45 s (See Table 2 for specific
Table 2. Sequences of the primers used in this study and their specific annealing temperatures.
Primer
Lactis Forward
Lactis Reverse
SketaF2
SketaR3
8F
3’)
GCTGAAGGTTGGTACTTGTA
TCAGGTCGGCTATGTATCAT
AGAAATTCCAAACGAACTTG
CAGTGCTCTACCTCCATCATT
338R AGA GTT TGA TCC TGG CTC AG
TCA CCT ACG GGA GGC AGC
Bifido5’
Bifido3’ GAT TCT GGC TCA GGA TGA AAC GC
ADO1
ADO2
CTG ATA GGA CGC GAC CCC AT
CTC CAG TTG GAT GCA TGT
CGA AGG TTG CTC CCA GT
Sinigalliano et al. 2007
°C Haugland et al., 2004
°C Amman et al., 1990
°C Gueimonde et al., 2004
°C Bonjoch et al., 2004
annealing temperatures), followed by melting curve analysis at 45-95°C every 1°C for 10 s. Cycle threshold (CT) will be determined automatically by the machine following manual adjustment of the threshold fluorescence value to 10 units. Standards and samples, including controls, will be run in triplicate.
Exogenous Internal Control: The DNA extracted from water samples will be tested for the presence of PCR inhibitors by using salmon testes DNA from Oncerhynchus keta (Sigma®, St. Louis, MO) as an internal control. Six milligrams of lypholized, salmon testes DNA will be resuspended in 1 ml of sterile distilled and de-ionized H20 to a final concentration of 6 mg/ml and stored at -20°C until further use. Quantitative PCR reactions will be performed with the addition of 1 µl of DNA from each water sample, and 0.6 ng salmon testes DNA as template in a single 25 µl qPCR reaction. The internal control forward primer SketaF2 and the reverse primer SketaR3 are specific to the rRNA transcriber region 2 of O. keta and their sequences can be found in Table 2. (Haugland et al., 2004). Quantitative PCR results for each water sample will be obtained and the mean and standard deviation Hill be calculated to determine the amount of inhibition in each sample.
Eubacteria QPCR: The total community of eubacteria Will be enumerated in each water sample using QPCR to normalize the quantification data of enterococci, bifidobacteria, and Bifidobacterium adolescentis using the same method. DNA sodium salt from Escherichia coli strain B (ATCC® strain #11303) (Sigma®, St.
Louis, MO) will be resuspended in 1 ml of sterile ddH20 to a final concentration of 50ng/µl and stored at -20°C until further use. Fresh, 10-fold serial dilutions will be performed in sterile ddH20 to create a standard curve. Quantitative PCR will be performed by adding 1 µl of DNA from each water sample into a single 25 µl qPCR reaction. Forward primer 8F and reverse primer 338R are specific to a conserved 16S rRNA region in all eubacteria, and their sequences can be found in Table 2.
Bifidobacteria qPCR
Bifidobacteria will be quantified via qPCR in all water samples to determine their relative abundance in each location and to determine the amount of fecal pollution per site. B. adolescentis genomic DNA ATCC® (Manassas, VA) number 15703D will be used to prepare the standard curve. Quantitative PCR will be performed by adding 1 µl of DNA from each water sample into a single 25 µl qPCR reaction. Sequence data for the forward primer Bifido5’ and reverse primer Bifido3’ can be found in Table 2., and are specific to a 16S rRNA region of Bifidobacterium spp. (Gueimonde et al., 2004).
Bifidobacterium adolescentis
qPCR
Only water samples testing positive in the bifidobacteria specific qPCR will be evaluated in the B. adolescentis specific QPCR. Quantitative PCR Hill be performed by adding 1 µl of DNA from each water sample into a single 25 µl qPCR reaction. Forward primer ADO1 and reverse primer ADO2 are specific to the 16S rRNA region of B. adolescentis as reported in Table 2. (Bonjoch et al., 2004).
Targeted Sampling
We will apply the qPCR technique, under field conditions, in parallel with the standard methodologies for enterococci, fecal coliforms and E. coli described above. We will collect samples from areas with persistent problems of fecal contamination. In addition we will analyze sites with no apparent human fecal contamination but which might contain contamination from other natural sources such as bird rookeries in salt and freshwater wetlands ( i.e. bird rookeries in mangroves). Once we find areas of significant fecal contamination, we will revisit the sites to conduct more detailed sampling in order to corroborate and define potential point sources of fecal contamination.
Relationship Among Different Methods
We will tabulate enterococci, fecal coliforms and E. coli results together with abundance of Bifidobacter adolescentis by the qPCR approach. We will conduct parametric and non-parametric regression analysis in order to assess the covariation of these results throughout the study sites. In addition, we will use multiple comparison
analyses to establish differences between different sampling locations and establish if each technique indicates similar patterns of contamination.
Results and Findings
A. Comparison of Four Molecular Methods for the Detection of Human Fecal Pollution in Marine and Inland Waters.
Quantity and quality of the DNA extracts. There were no large differences between
Table 3. DNA recovered and qPCR estimation of Bifidobacterium adolescentis in sewage sample
DNA extracts from 0.22 µm and 0.45 µm of filters.
Sample (n; number of samples)
DNA Extracted (avg. ± 1SD; ng/μL)
the total DNA recovered from 100ml of sewage samples that have been diluted from 10-1 to 10-4 and filtered through 0.22 and 0.45 μm-pore membrane filters. (Table 3).
Analysis of PCR inhibition in the DNA extracts amended with salmon testes DNA indicated that all samples had similar Ct (Ct =22-24; data not shown). Quantitative PCR estimates of the amount of B. adolescentis DNA in the sewage-DNA extract from the 0.22-μm-pore membrane filters were significantly greater than that extracted from 0.45 -μm-pore membrane filters (Table 3). No significant differences were observed between filter pore sizes in each of the exogenous B. adolescentis DNA additions. Differences in Ct among DNA addition were proportional to the treatments (Table 4).
PCR detection of human fecal bacteria in sewage The molecular methods for detecting human fecal bacteria in environmental samples were initially evaluated and compared using municipal sewage DNA and animal fecal DNA samples. Detection of B. adolescentis as a marker of human fecal bacteria in sewage samples with a single PCR (Matsuki et al. 1999) showed positive bands in only 2 out of 3 raw sewage samples (Table 5). Nested PCR detection methods were more successful at detecting B. adolescentis in sewage samples. The results in Table 4, indicate that the nested PCR approach of Bonjock et al. 2004 detected B. adolescentis in over half of the
Table 4. Testing for potential qPCR inhibitors in natural samples. Filters of different pore sizes were extracted and aliquots were spiked with different amounts of B. adolescentis target DNA. Ct is the Ct difference between the amended and un-amended samples.
sewage samples and the incidence of false negative results increased with higher dilution of sewage samples. False positive detection of B. adolescentis was observed in 3 out of 8 pig fecal samples. Using the nested PCR method developed by King et al. 2007., B. adolescentis was detected in 100% of the raw sewage samples and B. adolescentis was not detected in non-human fecal samples. It was observed with the nested PCR approaches that in the first PCR the putative marker of Bifidobacteria was not always visible in samples that were positive for B. adolescentis after the second round of PCR. HuBac was detected in 8 out of 27 sewage samples analyzed and in most of the pig fecal DNA samples. None of the sewage sample DNA recovered from the 0.45 -μm-pore membrane filters showed positive bands for HuBac and the detection of HuBac decreased in sewage samples with increasing dilution (Table 5).
Detection of Human fecal pollution in aquatic environments. Several environmental sites that are currently being monitored for fecal pollution were chosen for this study. Molecular detection of B. adolescentis and HuBac in DNA samples recovered from marine and freshwater environments using 0.22-μm-pore membrane filters with known concentration of E.coli and enterococci indicated that B. adolescentis was detected in five of the marine sites using the method of King et. al 2007 and in a freshwater sample from Lacey Mill Road (Table 6). All of these five sites had elevated levels of fecal indicator bacteria. Using the method of Matsuki et al.1999, B. adolescentis was not detected in any of the environmental samples (Table 6). The method of Bonjock et al. 2004 detected B. adolescentis only in one freshwater site at Lacey Mill Road. The HuBac marker was detected in three samples from the marine environment that had elevated levels of fecal indicator bacteria and in one of the freshwater samples from Lacey Mill Road site 2.
Table 5. Detection of Human Fecal Bacteria (B. adolescentis and HuBAC) PCR Products in Environmental Samples by Nested (Bonjoch et al. 2004 and King et al. 2007) and single PCR assays.
Sample (n)
et al. (1999)
Table 6. Detection of human fecal bacteria B. adolescentis and HuBac in environmental samples. Nimbers represent average of colonies per 100ml filtered samples.
et al. (2004)
et al. (2007) Layton et al. (2006)
Parguera este pueblo, costa
Lacey Mill Road site
Lacey Mill Road
Discussion
It is well establish that the concentration and quality of DNA recovered from water samples influences any subsequent PCR analysis of that DNA for the detection of specific bacteria (King et al. 2007; Sinigalliano et al. 2008). Currently in microbial source tracking of fecal pollution, numerous methods are used for the recovery of fecal bacteria DNA from water samples. Often the water samples are filtered through membrane filters of pore sizes ranging from 0.2 to 0.45 µm for concentrating the bacteria prior to DNA extraction (Blanch et al. 2006; King et al 2007, Bachoon et al. 2009). Municipal sewage samples were used to evaluate the influence of membrane filter type on the concentration of DNA recovered and on PCR-detection of fecal bacteria. The concentration of DNA extracted from the 0.22-μm-pore nitrocellulose membrane filter (Type GS) was not significantly different from the concentration of DNA extracted from the 0.45 -μm-pore membrane filter (GN-6) for most of the sewage samples (Table 3). The similarity among different sample dilutions points toward saturation of the adsorbing matrix used in the kits used. That is, the DNA retention capacity of cartridges used from the kits was surpassed by most of the dilutions used. Even though there was no significant difference in the concentration of DNA recovered from the filters, it was possible that the presence of PCR inhibitors in the DNA extracts could have differentially inhibited PCR or qPCR assays. As mentioned above, no evidence was found of such inhibition during our determinations based on the expected Ct values for the different targets used (salmon testes and B. adolescentis DNA) covering a wide of target concentrations. The lack of qPCR inhibition in DNA extracted from environmental samples using 0.22 um or 0.45 um filters and the MoBio kit have been reported in previous studies (Morrison et al. 2008; Sinigalliano et al. 2007). However collecting bacteria on a 0.22-μm-pore nitrocellulose membrane filter increased the amount of B. adolescentis DNA detected in diluted sewage samples compared to the detection of these bacteria
in sewage samples filtered through a 0.45 -μm-pore membrane filter. This seems contradictory considering how similar the concentrations of DNA recovered from both types of filters were. However, the quality or source of DNA is not necessarily represented by the amount DNA recovered when using filters during concentration steps as some cells may pass different pore sizes. In our case, it seems that B. adolescentis could pass through 0.45um pores as our analysis shows an order of magnitud higher detection using 0.22 um pore size filters than 0.45 um ones (Table 3). These results agree with previous observation that suggested using 0.22-μm-pore nitrocellulose membrane filter (Type GS) instead of 0.45 -μm-pore membrane filter (GN-6) for DNA extraction from environmental samples (King et al. 2007). In general, our results indicates the advantage of using smaller volumes of water and 0.22 pore size filters over larger volumes and 0.45um pore filters.
PCR detection of host-specific fecal bacteria has become very useful in tracking the source of fecal pollution in environmental samples. Currently there is no standard protocol for PCR detection of fecal indicator bacteria. Comparison of three PCR methods for the detection of the fecal bacteria B. adolescentis indicated that direct detection of B. adolescentis by a single step PCR (Matsuki et al. 1999) was only effective for detecting the bacteria in raw or sewage samples concentrated on a 0.22 μm filters. When PCR was performed on DNA recovered from diluted sewage samples filtered thorugh 0.45 μm filters a single step PCR method did not detect the marker for B. adolescentis. These results were not surprising because the original method of Matsuki et al. (1999) was developed for use with cell cultures and human fecal samples. These results are supported by reports of Lamandella et al. 2008, which showed that B. adolescentis was not detected in some human fecal samples using the PCR method of Matsuki et al. 1999. Other reports have indicated that the detection of B. adolescentis in environmental samples required multiplex PCR (Bonjock et al., 2004; King et al., 2007; Amador et al., 2008). In contrast, the nested PCR detection methods for B. adolescentis of Bonjock et al. 2004 and King et al.2007 readily detected B. adolescentis in raw and diluted sewage samples (Table 3). The method of King et al. (2007) was the most reliable of the three methods for detecting B. adolescentis in sewage samples. The higher sensivity of the King et al. (2007) compared to the Bonjock et al. 2004 method for detecting B. adolescentis in parallel DNA extracts was attributed to the amplicon in the first PCR being smaller (777 bp) than the amplicon generated by the Bonjock (1350 bp) PCR assay (Fig. 1). Although not evaluated in this study, previous research have demonstrated that qPCR detection methods are capable of detecting B. adolescentis in environmental samples without multiplexing (Morrison et al. 2008; Bonjock et al. 2009). The HuBac PCR assay performed well with undiluted sewage samples but was prone to false positive amplicons with pig and cow fecal (faint bands) samples. Cross amplification of the HuBac primers with swine feces have been reported previously (Layton et al. 2006).
Molecular source tracking methodologies are often used to identify the source of fecal pollution in marine and freshwater environments. However before using any
molecular source tracking method with environmental samples it is critical to evaluate the performance of these PCR bacteria detection methods in environmental samples. Environmental sites from freshwater and marine locations with historically low to high levels of fecal indicator bacteria concentrations were chosen for this study. Analysis of marine and freshwater samples from Puerto Rico and Georgia with known concentrations of the fecal indicator bacteria, E. coil and Enterococcus sp. indicated that the PCR method of King et al. 2007, and the Layton method for HuBac were in agreement in detecting human fecal pollution in most sites with elevated levels of E. coli and Enterococcus sp. In West Luquillo beach there was high levels of fecal bacteria but only the method of King et al. 2007 indicated the presence of human fecal pollution. In contrast the Bonjock et al. (2004) assay detected B. adolescentis in only one site at Lacey Mill Road. The location and close proximity of some of these samples such as Patillas, Costa Azul Creek and Tubo -DRNA to human activity supports the finding of human fecal bacteria in those samples. In addition B. adolescentis was detected previously in the Little River site and the Lacey Mill Road sites (unpuplished data).
B. Assessment of Non-point Sources of Fecal Pollution in Coastal Waters of Puerto Rico and Trinidad
Enumeration of fecal indicator bacteria
The concentration of fecal indicator bacteria E.coli and enterococci in coastal waters of Trinidad and Puerto Rico is shown in Table 7 and 8. In Trinidad water samples were obtained from some of the most popular rural beaches on the northeast that were not impacted by housing or agriculture and from marine waters in the west and south coast of the island. At Las Cuevas Beach and Toco Bay (Figure 5) the concentrations of E.coli and enterococci (<20 cfu/100 ml) were below the EPA regulatory standard for recreational waters and indicated that these beaches were at the time of sampling safe for recreational use (Table 7). The low levels of fecal bacteria detected in these beaches of Trinidad was likely due to the lack of urban development in Las Cuevas Beach and Toco Bay. This agrees with previous reports that have indicated that urban development or animal farming are often the source of fecal bacteria in the environment (King et al., 2007; Bonkosky et al., 2008;). In spite of large variability,
probably due the inclusion of variable amounts of suspended solids in replicates, the average concentration of enterococci at Maracas Bay, Salybia Beach, Mosquito Creek, Balandra and Quinam Bay exceeded the EPA recommended levels of 104 CFU per 100 ml for full body contact bathing in recreational waters (USEPA 2004). In addition although enterococci counts were low (<10 cfu/100 ml) for the Carioni Swamp sites, the level of E.coli in the Caroni swamp was relatively high (> 700 cfu/100 ml). Similar disagreement between quantification of E.coli and enterococci have been reported in previous studies of fecal pollution in sub-tropical and tropical regions (Shebata et al. 2004; Yamahara et al. 2009). The fact that no nearby urban development nor animal farms are found nearby this site, the elevated level of E.coli at the Caroni Swamp is most probably derived from the extensive bird rookeries typical of this sanctuary.
In Puerto Rico fecal pollution ranged from low <10 cfu/100 ml to extremely high > 100,000 CFU/100ml. (Table 8). Based on single sample thresholds for infrequently used recreational waters, enterococci and E. coli levels indicated that the water quality of 30-50% of the sites visited was impaired. Using the one time threshold for designated swimming areas increased the proportion of impairment to 40-60%. Very
high numbers of enterococci (>1000) were found in rural, suburban and urban areas. Creeks associated with beaches contained high numbers of enterococci and E. coli (P5, 10, 14) probably due to contamination from septic systems or sewers as many of these creeks flow and/or drain many populated areas. Increased pluviosity prior to sampling might have increased coastal contamination in some sites (P9 and P11) as no apparent source of contamination was evident when sampling. However, enterococci levels over the threshold for designated swimming areas (EQB, 2009) at P11 have been frequently observed. Another reason for high contamination was
Table 7. Average bacterial densities (CFU 100m1-1) in waters associated to coastal sites of Trinidad. Numbers in parenthesis are standard deviation and number of replicates (SD)(n).
T1 Balandra Bay 113 (147)(6)
T2 Salybia Bay 147 (341)(7) 1160 (na)(1)
T3 Salybia River Mouth 50 (50)(6) nd
T4 Toco Bay 13 (12)(6)
T5 Las Cuevas Bay 15 (18)(7)
Maracas Bay 121 (204)(7)
Maracas River Mouth 166 (167) (7) 198 (na)(1)
T8 Chaguaramas Bay 9 (5)(6) nd
T9 Mosquito Creek 215 (-)(1) 2200 (na)(1)
T10 Carli Bay 129 (108)(7) 1680(na)(1)
T11 Caroni Swamp 8 (3)(2) 713(760)(2)
T12 Caroni swamp-Boat Dock 3 (-)(1) 3100 (na)(1)
T13 Quinam Bay 108 (124)(6) nd
T14 Morne Diablo Bay 36 (75)(6) nd
• Refer to Figure 1 for location; ** SU= suburban; U= Urban, R= Rural; -= not detected; += detected; nd= not determined; na= not applicable.
observed in association to bird rookeries (P3, 27) since these sites are isolated from human sources of contamination and inland and marine species of birds, use the area as resting areas during the nights. Fecal contamination in the outflow of Joyuda Lagoon might be a combination of human and bird sources since bird rookeries are common in the area as well as increased housing development along the tidal creek connecting to the sea.
Microbial Source Tracking. A major concern with fecal pollution studies in coastal environments is the host-specificity of the marker used to identify the source of the fecal pollution. Microbial markers for B. adolescentis and HuBac were used to increase the reliability of detecting areas impacted by human fecal contamination (Layton et al., 2006; King et al., 2007; Bonkosky et al., 2008; Bomjock et al. 2009;). The specificity of the B. adolescentis and HuBac PCR assays was optimized and established using pig and cattle fecal DNA. Under the PCR conditions used, the marker for B. adolescentis was not detected in pig and cattle fecal samples in agreement with previous work (King et al., 2007) However, other researchers have
detected B. adolescentis in pig fecal DNA extracts using PCR conditions unlike those reported here (Lamendella et al. 2007; Dorai-Raj et al. 2009). In contrast, the HuBac assay resulted in an infrequent (1 out of 3) faint product band while testing pig fecal DNA extracts. The specificity of the HuBac PCR assay used in this study could have been improved by using fluorescent probe detection (TaqMan-based Real-Time PCR assay) but it was outside the scope of the present work. Even so, previous reports have indicated that the detection of HuBac by conventional PCR and qPCR is prone to false positive results with pig fecal samples (Layton et al., 2006; Dorai-Raj et al. 2009). Consequently, in sites were HuBac was detected the presence of B. adolescentis was used to confirm the presence of human fecal pollution.
Bifidobacteria were detected in 11 out of 14 sites in Trinidad. This is not surprising because Bifidobacteria are more abundant in animal feces than E.coli and enterococci (Matsuki et al. 1999). In addition PCR assay were demonstrated to be very effective for detecting these bacteria in marine environments (King et al., 2007; Morrison et al., 2008; Bonjock et al., 2009). However, B. adolescentis was not detected in any of the Trinidad samples (Table 1) and suggest that the fecal pollution in Trinidad was probably from a non-human source. This agrees with the low levels of fecal enterococci and E.coli recorded at these sites. In contrast, HuBac was detected in a sample from Carli Bay that had very high levels of E.coli (16850 CFU/100 ml) but low concentration of enterococci (70 CFU/100 ml). The lack of housing developments in Carli Bay and the inability to detect B. adolescentis in parallel water samples suggest that the detection of HuBac in Cali Bay is likely a false positive indication of human fecal pollution.
It is not uncommon for a few heads of cattle to be found grazing in close proximity to Cali Bay, Salybia or the Caroni swamp. None of the Tirnidad sites contained detectable levels of cattle fecal contamination (Table 7). Consequently, the high level of fecal bacteria in the Caroni swamp was attributed to the large flocks of birds that inhibit the swamp. However, additional MST studies with markers for other potential fecal sources are needed to track the fecal pollution in the Caroni Swamp and Cali Bay.
Out of the 27 sites from Puerto Rico, 11 were positive for the presence of B. adolescentis (Table 8). In general, sites where B. adolescentis was not detected had lower numbers of both enterococci and E. coli with the exception of P12 where only E. coli numbers were high. Similarly to mEI counts, an independent concurrent MPN based assay was unable to detect enterococci in P12. Testing of B. adolescentis detection using the present approach on water from areas affected by known sewage spills (> 105 CFU 100 ml-1 enterococci and E. coli) and on fecal material from known animal sources (pig, chicken, cow, rabbit, and horse) indicated high specificity of the test (data not shown).
In contrast to the detection of B. adolescentis, HuBac was only detected in Puerto Rico in four occasions including mostly sites consistently affected by fecal contamination including P11 (EQB, 2009), P8 ( Webb and Gomez-Gomez, 1998) and P12 (Hartel et al., 2005). Although the last site where HuBac was detected (P17) may receive human fecal contamination from upriver sources, the overall low colony counts found in this river system (P16, P18, P19) and the lack of B. adolescentis
Table 8. Average bacterial densities (CFU 100m1-1) in waters associated to coastal sites of Puerto Rico. Numbers in parenthesis are standard deviation and number of replicates (SD)(n). Station
East La Parguera 1 (2)(4) 0 (0)(3)
West La Parguera 16 (29)(4) 25 (7)(2)
West of Bird Rookery 45 (29)( 5)
P6 Tres Palmas Reserve Beach 12 (5)(5) 47 (14)(3)
P7 Public Beach Rincón 3 (4)(2) 16 (23)(2)
P8 Central Park; San Juan 6,027 (1,532)(6)
P12 Mouth Yaguez River 1 0 (0)(2) 1,800
P15 Joyuda Lagoon Creek 1
P16 Mouth Manatí River
P17 West Manati River Mouth
P18 Bridge NW Manatí River Mouth
P19 Channel Manatí Town
P20 Boca Vieja; Aguadilla
P23 Mouth Yaguez River 2
P26 Joyuda Lagoon Creek 2
P27 West of Bird Rookery
(17)(4)
(46) (2)
(25) (2)
(71) (2)
(742) (2)
(71) (2)
(883) (2)
detection do not confirm humans as dominant source of fecal contamination at this site. Similarly, BoBac was also detected at P17 but not at other upriver sites, closer to
potential sources of bovine fecal contamination. Other reports have indicated a positive correlation between the detection of B. adolescentis and HuBac and elevated levels of fecal bacteria in marine environments (King et al., 2007; Morison et al., 2008; Sinigaliano et al., 2008).
Table 3. Matrix of chi square results of 2x2 contingency tables (df=1) examining differences between the detection of fecal pollution by the different methods in different sampling areas. Non significant differences (P<0.05) are interpreted as no differences between the capacity of each method to indicate the presence of contamination. Significant differences are shown in bold. Only the upper half of the matrix is presented
Location
Puerto Rico
* Marginally significant; F= confirmed using Fisher Exact Test due to low frequency of observation; ** same frequency distribution for the combination of factors.
Comparison of plate counts with PCR methods. Our data show general agreement between MeI and MI counts suggesting that both types of counts are useful to detect the presence of fecal pollution in marine waters. Since data is missing for MI counts for Trinidad, we compared the detection of Bifidobacteria, B. adolescentis , HuBac and BoBac with the detection of significant fecal contamination based only on mEI counts, using as threshold for detection the one-time sampling average of 104 CFU/100ml. A chi square analysis using presence/absence, location, and detection protocol for classification suggests a dependence between some of these variables and detection of fecal contamination (X2= 69.47, df=13, P<<0.01), that is, dissimilar detection patterns or disagreement among tests. Chi square analyses comparing each test combination (Table 3) discern the discrepancies among the detection frequencies of enterococci/Bifidobacter/B.adolescentis vs. HuBac/ BoBac indicators of fecal contamination in Trinidad and Puerto Rico. This is due to the lower incidence of detection of Hubac and BoBac, which would suggest limited human or bovine sources of pollution even in samples where fecal counts were in the thousands CFU 100ml-1.
Similarly, the detection of B. adolescentis in Trinidad was unrelated to counts of mEI in contrast to results from Puerto Rico. Overall, the pattern of detection of fecal contamination using MeI, Bifidobacteria and B. adolescentis is similar in Puerto Rico while B. adolescentis described a different pattern in Trinidad. These results support a lower level of human derived pollution in the samples analyzed in Trinidad than in Puerto Rico, where the mEI counts and incidence of B. adolescentis detection were higher. Comparisons of fecal detection between different methods in Trinidad and Puerto Rico show agreement with the exception of B. adolescentis results. The difference in patterns of B. adolescentis detection found during this study in the two study sites suggests real differences of the sources of fecal pollution intrinsic to the sample sets of each island. This is supported by the absence of urban sites in the data set from Trinidad while a 1/3 of the samples analyzed from Puerto Rico were derived from urban sites. Also, the overall higher counts in Puerto Rico, in contrast to significant but lower numbers from Trinidad suggest a higher potential for human fecal contamination of the waters studied in Puerto Rico.
C. Early Warning of Fecal Contamination Using Fluorometric Detection of enterococci
Samples collected were filtered and exposed to commercially available fluorogenic substrates preparations. Time series analysis reveals the potential of this approach for the detection of enterococci at levels similar to those used in present USEPA guidelines in less than 6 hours (Fig. 6). Further analysis is been conducted to evaluate species dependent variability and usefulness under different environmental scenarios.
Figure 6 Fluorescence production by enterococci from natural waters. The number in the leyend represent estimates of enterococci using accepted standard methodologies based on MPN.
Objectives accomplished or not and why (See Executive Summary example)
Discussion of project impacts and products
1. This projectprovides the means to increase awareness of sources of fecal contamination in sites around Puerto Rico. At presen,t the government agencies of Puerto Rico conduct regular monitoring for fecal pollution in mostly in public beaches and other localities which primary designated use is for recreation involving swimming primarily (REF)). A limited number of reports are available evaluating fecal contamination in other site (See Description and Results above). Recent reports (refs) have evaluated the potential reach of fecal contamination in a limited geographic scale, however, we are not aware of recent work that systematically examine the level and potential human derived fecal contamination at a broad scale, both spatially and environmentally, as presented here. The dissemination of the present work through the different management agencies will provide the opportunity to increase the awareness to expand monitoring programs at a wider scale in order to support the goals of the different agencies towards a healthier environmental and human population. The results of this research will be communicated to managers of resources impacted (DNER, EQB, USEPA).
2. Informal conversations held by with USEPA and EQB personnel demosntrated the usefulness and the potential interest for the application of the work described here, both the use of Bifidobacter adolescentis as a marker of human derived fecal contamination and the fast fluorometric examination of enterococci. The fast enterococci determination is of interest since in a matter of hourse it can indicate the presence of a potential problem in public beaches by means of a technically simple and quite inexpensive approach. The results shown here have a high potential of impact in helping management of coastal resources.
Furthermore, this research provided the opportunity to evaluate and streamline an early warning (3-6 hours) approach for the detection potential of fecal contamination which could be adopted by local agencies to speed up assessment of contamination of public beaches while results of regular testing are made available.
Recommendations
Bidifidobacter adolescentis is usefull in the evaluation of humans derived contamination in coastal waters. A battery of other markers can be used in addition to evaluate independently possible sources.
The evidence of frequent contamination of coastal waters indicates a large incidence of fecal sources to coastal sources. These contamination can be considered and impairment for effective maintenance of natural ecosystem health (i.e. coral reef and associated habitats) as well as for human recreation.
Our results indicate the feasibility of detection of fecal contamination using a fluorometric approach that offers a technically simple and inexpensive way to monitor coastal waters. This approach, is complementary to standard culture and culture independent apporaches and can be used as an early warning system to identify moments where other resources should be used to narrow down possible sources and establish specific levels of contamination
C. Bibliography
Amador J. A., Sotomayor-Ramı´rez D., Martı´nez G., Chen L. & Bachoon D. (2008) Tracking human faecal contamination in tropical reservoirs in Puerto Rico. Lakes Reserv. Res. Manage. 2008 (13), 301–17.
Amann R. I., Ludwig W. & Schleifer K.-H. (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59,143–69.
Bachoon D.S.,Nichols T.W., Manoylov K.M. & Oetter D. (2009). Assessment of fecal pollution and relative algal abundances in Lakes Oconee and Sinclair, Georgia, USA. Lakes & Reservoirs: Research and Management 2009 14: 139–149.
Blanch, A. R., Belanche-Muñoz, L., Bonjoch, X., Ebdon, J., Gantzer, C., Lucena, F., Ottoson, J., Kourtis, C., Iversen, A., Kühn, I., Mocé, L., Muniesa, M., Schwartzbrod, J., Skraber, S., Papageorgiou, G.T., Taylor, H., Wallis, J., Jofre, J. (2006). Integrated analysis of established and novel microbial and chemical methods for microbial source tracking. Appl. Environ. Microbiol. 72, 59155926.
Bonjoch, X., Ballesté, E., Blanch, A.R. (2004). Multiplex PCR with 16S rRNA genetargeted primers of Bifidobacterium spp. to identify sources of fecal pollution. Appl. Environ. Microbiol. 70, 3171-3175.
Bonjoch, X., E. Ballesté, and A.R. Blanch. 2004. Multiplex PCR with 16S rRNA gene-targeted primers of Bifidobacterium spp. to identify sources of fecal pollution. Appl. Environ. Microbiol. 70:3171-3175.
Bonjoch, X., Lucena, F., & Blanch, A.R. 2009. The persistence of bifidobacteria populations in a river measured by molecular and culture techniques. Journ of Appl. Microbiol. 107, 1178-1185
Bonkosky, M., E.A. Hernandez-Delgado, B. Sandoz, I.e. Robledo, J. Norat-Ramirez and H. Mattei. 2008.Detection of spatial fluctuations of non-point source fecal pollution in coral reef surrounding waters in southwestern Puerto Rico using PCR-based assays. Mar. Poll. Bull. 58: 45–54.
Bullock, C.A. & Moonesar, I., 2005. Potential sources of bacteriological pollution for two bays with marinas in Trinidad Rev. Biol. Trop v.53 supl.1 San José mayo 2005
Desmarais, T.R., H.M. Solo-Gabriele and C.J. Palmer. 2002. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Appl. Environ. Microbiol. 68: 1165-1172.
Dick, L. & Field, K. 2004. Rapid estimation of numbers of fecal Bacteroides by use of a quantitative PCR assay for 16s rRNA genes. Appl. Environ. Microbiol. 70, 5695-5697.
EQB, 2007. Resultados Monitoria de playa 8 junio 2007. < http://www.gobierno.pr/ NR/rdonlyres/9BE372A9-5785-42EC-8F90-07501B4EAC84/0 /BeachMonitoring8dejuniode2007.pdf> Accessed 2007 June 1.
EQB, 2009. Resultados de las Monitorias. Año 2009. Programa de Monitorías de Playas y Notificacion Pública. http://www.gobierno.pr/NR/rdonlyres/20C9388F-7B8F-4059-87CE92A120E85D32/0/ResultadosMonitoriaActualizadoal3dediciembrede2009.pdf (downloaded on 3 December 2009).
EQB. Beach monitoring 2006 Dic 22. <http://www.gobierno.pr/NR/rdonlyres/ 2DC6D09F-B5F8-49F7-96E8-0D53738662F2/0/ BEACHMONITORINGDIC 222006.pdf> Accessed 2007 Mar 17.
Gavini, F., V. Delcenserie, K. Kopeinig, S. Pollinger, H. Beerens, C. Nonaparte and M. Upmann. Bifidobacterium species isolated from animal feces and from beef and pork meat. J. Food Protect. 69: 871-877.
Gueimonde, M., Tölkkö S., Korpimäki, T., Salminen S., 2004. New real-time quantitative PCR procedure for quantification of Bifidobacteria in human fecal samples. Appl. Environ. Microbiol. 70, 4165-4169.
Hartel, P.G., K. Rodgers, J. A. Fisher, J. L. McDonald, L.C. Gentit, C. N. Belcher, E. Otero, Y. Rivera-Torres, T. L. Bryant, and S. H. Jones. 2005. Survival and regrowth of fecal enterococci in desiccated and rewetted sediments.
Proceedings of the 2005 Georgia Water Resources Conference, held April 2527, 2005, at the University of Georgia. Kathryn J. Hatcher, editor, Institute Ecology, The University of Georgia, Athens, Georgia.
Hartel, P.G., J.L. McDonald, L.C. Gentit, S. N. J. Hemmings, K. Rodgers, K. Smith, C.N. Belcher, R.L. Kunts, Y. Rivera-Torres, E. Otero and E.C. Schroder. 2007. Improving Fluorometry as a Source Tracking Method to Detect Human Fecal Contamination . Estuaries and Coasts. 30:551-561.
Haugland, R., Siefring, S., Wymer, L., Brenner, K., Dufour, A., 2004. Comparison of Enterococcus measurements in freshwater at two recreational beaches by quantitative polymerase chain reaction and membrane filter culture analysis. Water Res. 39, 559-568.
Hong, P.H., Wu, J.H, & Lui, W.T. 2008. Relative abundance of Bacteroides ssp. in stools and wastewaters as deterimined by hierarchical oligonucleotide primer extention. Appl. Environ. Microbiol, 74, 2882-2893
Kaufmann, P., A. Pfefferkorn, M. Teuber, and L. Meile. 1997. Identification and quantification of Bifidobacterium species isolated from food with genus specific 16S rRNA-targeted probes by colony hybridization and PCR. Appl. Environ. Microbiol. 63:1268–1273.
King, E.L., Bachoon, D.S., Gates, K.W., 2007. Rapid detection of human fecal contamination in estuarine environments by PCR targeting of Bifidobacterium adolescentis. J. Microbiol. Methods. 68, 76-81.
Klijn, N., Weerkamp, A.H., de Vos, W.M., 1995. Detection and characterization of lactose-utilizing Lactococcus spp. in natural ecosystems. Appl. Environ. Microbiol. 61, 788-92.
Lamendella, R., Santo Domingo, J.W., Kelty, C., Oerther, D.B., 2008. Bifidobacteria in feces and environmental waters. Appl. Environ. Microbiol. 74, 575-584.
Langendijk, P.S., Schut, F., Jansen, G.J., Raangs, G.C., Kamphuis, G.R., Wilkinson, M.H., Welling, G.W., 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 8, 3069-3075.
Layton A., McKay L., Williams D., Garrett V., Gentry R. & Sayler G. (2006) Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl. Environ. Microbiol. 72, 4214–24.
Lipp, E.K., J.L. Jarrell, D.W. Griffin, J.Lukasik, J. Jacukeiwics and J. B. Rose. (2002). Preliminary evidence for human fecal contamination in corals of the Florida Keys, USA. Mar. Poll. Bull. 44: 666-670.
Lynch, P.A., B.J. Gilpin, L.W. Sinton and M.G. Saville. 2002. The detection of Bifidomacterium adolescentis by colony hybridization as an indicator of human faecal pollution. J. Appl. Microbiol. 92: 526-533.
Matsuki T., Watanabe K., Tanaka R., Fukuda M. & Oyaizu H. (1999) Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNAgene- targeted species-specific primers. Appl. Environ. Microbiol. 65, 4506–12
Meays, C.L., K. Broersma, R. Nordin and A. Mazumber. Source tracking fecal bacteria in water: a critical review of currnt methods. J. Environ. Man
Morrison C., Bachoon, D., Gates, K., 2008. Quantification of enterococci and bifidobacteria in Georgia estuaries using conventional and molecular methods. Water Research. 42, 4001-4009
Nebra, Y., Bonjoch, X., Blanch, A.R., 2003. Use of Bifidobacterium dentium as an indicator of the origin of fecal water pollution. Appl. Environ.Microbiol. 69, Patterson K.L., Porter, J.W., Ritchie, KB, Polson, S.W., Mueller, E., Peters, E.C., Santavy, D.L., Smith, G.W. 2002. The etiology of white pox a lethal disease of the Caribbean elkhorn coral Acropora palmata. Proc Natl Acad Sci USA 99:8725–8730
Requena, T., J. Burton, T. Matsuki, K. Munro, M.A. Simon, R. Tanaka, K. Watanabe, and G.W. Tannock. 2002. Identification, detection amd enum,eration of
human Bifidobacterium species by PCR targeting the transaldolase gene. Appl. Environm. Microbiol. 68: 2420-2427.
Scott, T.M., J.B.Rose, T.M.Jenkins, S.R.Farrah and J. Lukasik. 2002.Microbial Source Tracking: Current Methodologies and Future Directions. Appl. Environ. Microbiol. 5796-5803
Shanks, O.C., Kelty, C.A., Sivaganesan, M., Varma, M., & Haugland, R. 2009. Quantitative PCR for genetic markers of human fecal pollution. Appl. Environ. Microbiol. 75, 5507-5513
Shibata, T., H. M. Solo-Gabriele, L. E. Fleming, and S. Elmir. 2004. Monitoring marine water recreational water quality using multiple microbial indicators in an urban tropical watershed. Water Res. 38:3119–3131.
Sinigalliano, C.D., Gidley, M.L., Shibata, T., Whitman, D., Dixon, T.H., Laws, E., Hou, A., Bachoon, D., Brand, L., Amaral-Zettler, L., Gast, R.J., Steward, G.F., Nigro, O.D., Fujioka, R., Betancourt, W.Q., Vithanage, G., Mathews, J., Fleming, L.E.,Solo-Gabriele, H.M., 2007. Impacts of Hurricanes Katrina and Rita on the microbial landscape of the New Orleans area. Proc. Natl. Acad. Sci. U S A. 104, 9029-9034.
Solo-Gabriele, H., M.A. Wolfert, T.R. Desmarais and C.J. Palmer. 2000. Sources of Escherichia coli in a coastal subtropical environment. Appl. Environ. Microbiol. 66:230-237.
Stewart, J.R., R.D. Ellender, J.A. Gooch, S. Jiang, S.P. Myoda, and S.B Weisberg. 2003. Recommendations for microbial source tracking: Lessons from a methods comparison study. J. Wat. Health. 1(4):225-231.
USEPA, 2004. United States Environmental Protection Agency (EPA), 2004. Water quality standards for coastal and great lakes recreation waters; Final Rule (40 CFR Part 131). http://www.epa.gov/EPA-WATER/2004/November/Day16/w25303.htm. (Accessed on Nov 3, 2009)
Webb, M.T.R. and F. Gomez-Gomez. 1998. Synoptic survey of water quality and bottom sediments, San Juan Bay Estuary System, Puerto Rico, December 1994-July 1995. Water Resources Investigation Report 97-4144. 69pp.
Yamahara, K. M. Walters S. P., and A. B. Boehm. 2009. Growth of Enterococci in Unaltered, Unseede Beach Sands Subjected to Tidal Wetting. 75:1517-1524
