Aspergillosis of Sea Fans in a Global Context
Grant No. R-92-2-08
PI: Paul Bayman, PhD
Co-PI: Alberto M. Sabat, PhD
University of Puerto Rico – Río Piedras
FINAL REPORT
Chapter 1: Executive Summary
A one-year no-cost extension of this project was requested and granted. This Report covers work done during the project and during the no-cost extension, as of 28 February 2011. Several aspects of the projects are on-going, so further findings and publications are expected.
This summary is structured around the objectives and hypotheses presented in the original proposal. To distinguish parts cited from the original proposal, they appear here in blue text.
Objective 1. Compare genetic compositions of populations from Aspergillus sydowii and A. flavus in sea fans with populations in sea water, river water, soil and airborne dust using AFLPs.
Hypotheses:
1. Marine and terrestrial strains of A. flavus and A. sydowii will not comprise genetically distinct populations.
2. Strains from sea fans in Puerto Rico will be genetically more similar to strains from soil in Puerto Rico than strains from airborne dust or soil from Africa.
3. Strains isolated from sea fans and seawater will not comprise genetically distinct populations.
We have focused exclusively on Aspergillus flavus, for two reasons: 1) A. sydowii was never isolated from diseased sea fans, and 2) recently published papers on the population biology of A. sydowii suggest there is no population structure. On the other hand, recently published papers on A. flavus have opened new avenues for study because they have demonstrated for the first time the presence of mating types in this
fungus. In particular, we are studying the distribution of mating types in A. flavus isolates from sea fan tissue and seawater, as a test for genetic recombination.
The first and third hypotheses were supported: most marine isolates of A. flavus are in clades that also include terrestrial isolates. There is no evidence of specialization in marine strains. This suggests that all strains may be equally likely to survive in seawater and infect sea fans. However, there is one exception. A group of marine strains from Jobos Bay clusters together. This is probably the result of massive sporulation of a single genetic individual, and the effect is probably short-lived. It is not necessarily due to special traits of this particular genotype or of Jobos Bay. Nonetheless, we would like to take more samples in Jobos Bay and see if this particular genotype is still present. The second hypothesis is not supported: there is no correlation between geographic distance and genetic distance.
A detailed phylogenetic tree has been generated for over 100 isolates of Aspergillus flavus using both DNA sequences and AFLPs. Another 100 isolates have been placed in the DNA sequence trees (based on sequences of three genes, which are not as variable as AFLPs) but not in the AFLP trees. The AFLP markers include nine primerenzyme combinations and >250 phylogenetically informative characters.
About 40 isolates have been grown, extracted and tested for aflatoxin production in vitro. Toxins have been quanitified by TLC with authentic standards. In the next few months we will run these samples with HPLC and test more isolates.
An article based on this Objective was published in the journal Fungal Ecology in 2010; a pdf of the article is attached. Chapter 2 is a more extensive manuscript on the same theme, based on AFLP data from over 100 isolates. This manuscript will be submitted in March 2011.
Objective 2. Find traits that may help A. flavus to colonize sea fans and other marine substrates, using a phylogenetic approach.
Hypotheses:
1. Related strains of A. flavus from sea fans and terrestrial sources will have different alleles for genes involved in salt tolerance and thermotolerance, if those genes help the fungi to colonize marine substrates.
2. Related strains of A. flavus from sea fans and seawater will have different alleles for genes involved in pathogenicity, if those genes help the fungi to infect sea fans.
We have focused on genes involved in aflatoxin formation, because a number of A. flavus isolates from sea fan tissue produce aflatoxins in vitro. Since aflatoxins are immunosuppressive in vertebrates, it is possible that they are involved in the disease if they are also produced in vivo
Several genes in toxin-producing pathways have been sequenced and are phylogenetically informative, but these genes are not present in non-toxin producing strains, so their use for comparative purposes is limited.
An unexpected finding in Chapter 2 is relevant to this Objective as well. Almost all clinical isolates of A. flavus were found to have the same mating type, even though the isolates did not group together on the phylogenetic tree. This suggests that a gene linked to the mating type locus may be involved in pathogenicity (to humans, and perhaps to sea fans as well). Although this relationship is only a correlation with no evidence of causality, it provides a new approach to search for genes involved in pathogenesis.
Objective 3: Determine the effect of water temperature on development of aspergillosis in sea fans.
Hypothesis:
1. When replicate pieces of the same Gorgonia ventalina colony are inoculated with the same fungus at different temperatures, frequency and size of lesions will be greater than in the pieces grown at higher temperatures.
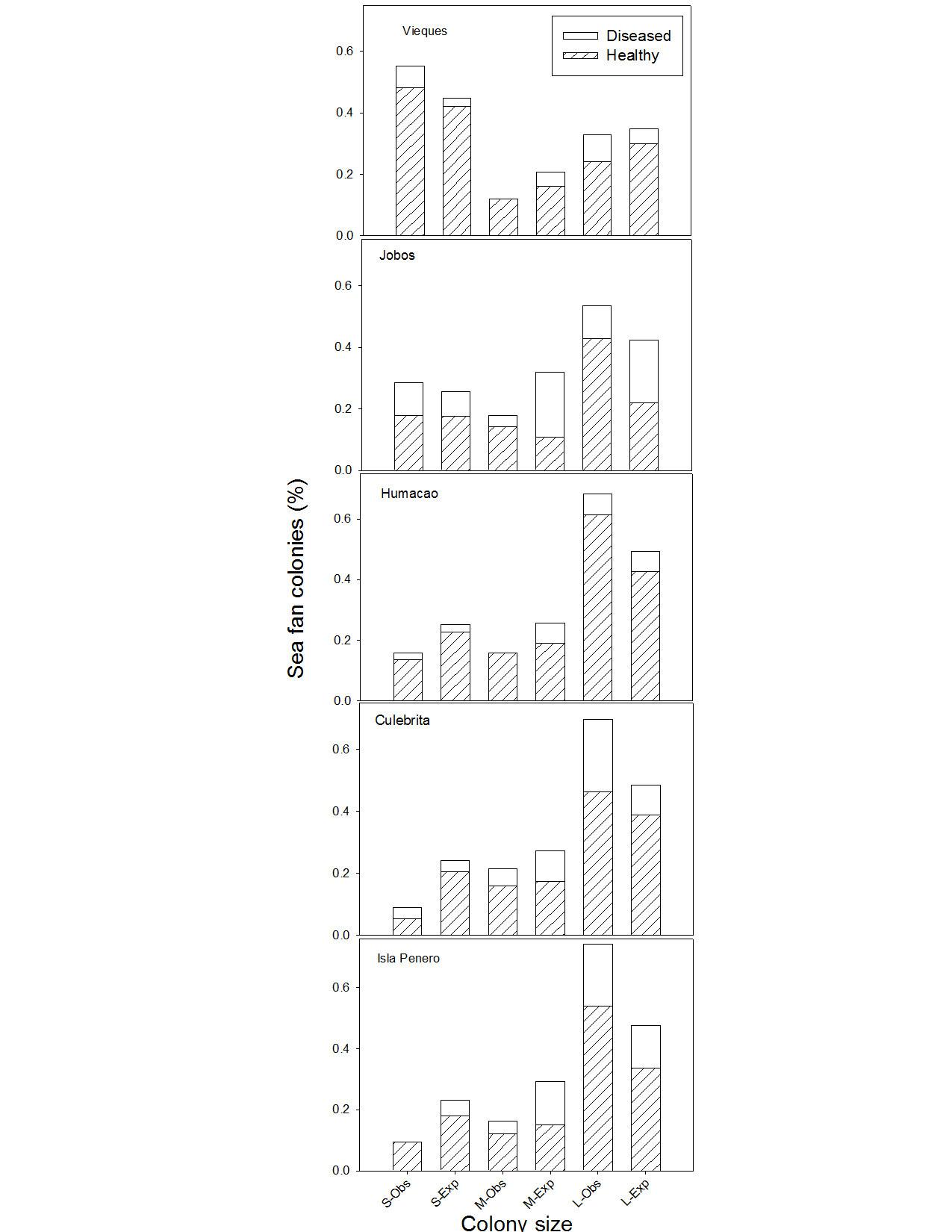
Completion of this objective was not possible because we never successfully induced aspergillosis disease in sea fans. This was despite extensive inoculation experiments, first in vitro (as described in the proposal) and then in the field. Although this is a negative result, it is very interesting because it contradicts the extensive literature on sea fan aspergillosis. Chapter 3 is a manuscript based on these experiments, and ancillary experiments designed to further test these results. In particular, we used ergosterol as a biomarker to measure quantity of fungal tissue in sea fans. This is a new technique for the study of sea fan diseases, and the results support our claim that infection by Aspergillus is not necessary for the disease—in which case the name ‘aspergillosis’ is clearly misleading.
The field component of this study has produced one published paper and a manuscript currently in revision. The published paper is on the demographics of bleaching (attached). The manuscript is a mathematical model to predict the impact of disease on sea fan populations with different levels of a pathogen. The model shows that the survival of the population depends partly on recruitment of new sea fans. Recruitment of sea fans is poorly understood, but we are working to fill this gap. This model is novel for the study of coral diseases and could eventually be used by resource managers to predict which populations are most at risk because of disease.
We believe we have clearly established that the current concept of aspergillosis of sea fans is based on mistaken assumptions. However, we have not identified other pathogens or opportunistic pathogens as alternatives to A. sydowii, the pathogen named in the literature. We are using a metagenomics approach to compare fungi and bacteria in diseased and healthy sea fan tissue. This should give us a list of microorganisms that are potentially pathogens.
Peer-reviewed publications:
Toledo-Hernández C*, Yoshiyoka P, Bayman P, Sabat A,. 2009. Impact of disease and detachment on growth and survival of sea fans Gorgonia ventalina Marine Ecology Progress Series 393: 47-54.
Zuluaga-Montero A*, Toledo-Hernández C*, Rodríguez JA**, Sabat A, Bayman P. 2010. Spatial variation in the fungal community isolated from healthy and diseased sea fans (Gorgonia ventalina) and seawater. Aquatic Biology 8: 151-160.
*Zuluaga-Montero A, *Ramírez-Camejo L, Rauscher J, Bayman P. 2010. Marine isolates of Aspergillus flavus: denizens of the deep or lost at sea? Fungal Ecology 3: 386-391.
*Hernández-Pacheco R, Hernández-Delgado EA, Sabat AM. 2011. Demographics of bleaching in a major Caribbean reef-building coral: Montastraea annularis. Ecosphere 2(1):art9. doi:10.1890/ES10-00065.1
Manuscripts in review and in preparation:
*Ramírez-Camejo LA, Zuluaga-Montero A, **Lázaro-Escudero MA, Bayman P. XXXX. Phylogeography and genetic variation of the cosmopolitan fungus Aspergillus flavus: is everything everywhere? [In prep.]
Sabat AM, Toledo-Hernández C, Zuluaga-Montero A. XXXX. Dynamics and Structure of sea fan populations impacted by disease. [In revision.]
*Toledo-Hernández C, Gulis V, *Ruiz-Díaz CP, Sabat A, Bayman P. XXXX. Critical reinterpretation of aspergillosis disease of sea fans (Gorgonia ventalina). [In revision.]
Poster and oral presentations:
Zuluaga-Montero A*, Toledo-Hernández C*, Rodríguez JA**, Sabat A, Bayman P. 2008. Spatial Variation in the Fungal Community of Healthy and Diseased Sea Fans (Gorgonia ventalina) and Surrounding Seawater. Simposio de Flora y Fauna, UPR Humacao, PR, 4/25. [poster]
Lázaro-Escudero MT**, Hernández-Kendall V**, Zuluaga-Montero A*, Ramírez-Camejo LA*, Bayman-Gupta P. 2008. Fungal composition in Saharan dust: dangerous passengers. X Simposio de Micología, Sociedad Puertorriqueña de Micología, Univ. Turabo, Gurabo PR, 5/3/08. [poster]
* graduate student co-author ** undergraduate student co-author
Poster and oral presentations (continued):
Bayman, P. 2010. Mortandad masiva en abanicos de mar en PR: ¿aspergilosis sin Aspergillus? III Frontiers in Environmental Microbiology: Lessons for Innovation, Universidad del Turabo, Gurabo PR, 19 March. [Invited speaker]
Hernández-Kendall V**, Lázaro-Escudero MT**, Ramirez-Camejo LA*, Paul BaymanGupta P. 2009. A multigene phylogeny of Aspergillus flavus. XI Simposio de Micología, Sociedad Puertorriqueña de Micología, San Juan, PR
Ruíz-Díaz CP*, Toledo-Hernández C*, Bayman P, Sabat A, Marcano M. 2009. Simulation of the interaction between sea fan colony, its immune system and a potential pathogen. 44th ACS Junior Technical Meeting & 29th Puerto Rico Interdisciplinary Scientific Meeting (PRISM). University of Puerto Rico, Río Piedras. [Poster]
Ruíz-Díaz CP*, Toledo-Hernández C*, Bayman P, Sabat A, Marcano M. 2009. Simulation of the interaction between sea fan colony, its immune system and a potential pathogen. 24th SIDIM (Seminario Interdisciplinario de Investigacion en Ciencias y Matematicas). UPR Río Piedras [Poster]
Toledo-Hernández C*, Torres-Vázquez I, Serrano-Vélez J*, Rosa-Molinar E. 2009. New protocol for coral histology using microwave technology. NOAA-Crest Annual Meeting, La Parguera, PR. [Poster]
* graduate student co-author ** undergraduate student co-author
Student participation
Student Degree Date
Current status / future plans
Anabella Zuluaga-Montero PhD 12/08 Instructor, UPR Río Piedras
Carlos Toledo-Hernández PhD 5/09 Postdoc, EPA
Luis A. Ramírez MS 12/10 PhD program, UPR Río Piedras
José A. Rodríguez BA 5/09 PhD program, UMichigan
Adelmarie Bones-González BA 5/09 MS program, Florida
María T. Lázaro-Escudero BA 5/10* PhD program, UCLA
Joan Morales Lappot BA 5/12* Med school*; no longer on project
Verónica Hernández BA 12/11* Graduate school*
*projected
Chapter 2: Phylogeography and genetic variation of the cosmopolitan fungus Aspergillus flavus: is everything everywhere?
Running title: Phylogeography and genetic variation in A. flavus
Address: Department of Biology, University of Puerto Rico — Río Piedras, PO Box 23360, San Juan PR 00931
* Corresponding author: Department of Biology, University of Puerto Rico — Río Piedras, PO Box 23360, San Juan PR 00931; ramirezcamejo@gmail.com
ABSTRACT
Aspergillus flavus is one of the most common eukaryotes on the planet. It is notorious for production of aflatoxins, for causing aspergillosis in humans and animals, and as an opportunistic pathogen of animals and plants. Its role in marine habitats is unclear. Until now, no phylogeographic structure has been detected, except at very local scales, and it appears to fit the classical dictum of microbial biogeography, “Everything is everywhere”. The goal of this study was to use intraspecific genetic relationships among isolates to reveal differences in preferences for: marine vs terrestrial habitats, substrate, clinical vs. environmental sources and phylogeographic structure. In addition,
mating types were determined and frequencies of mating types were compared between populations. Phylogenetic relationships among isolates were estimated Amplified Fragment Length Polymorphisms (AFLPs ) and mating types were determined for a worldwide sample of A. flavus isolates from diverse substrates and geographic locations. All isolates composed a single population, with no significant differentiation of marine vs. terrestrial isolates, clinical vs. enviornmental isolates, or association with substrate or geographic origin. The proportion of mating types was 1:1, supporting the hypothesis of recombination in natural populations. However, a high proportion of MAT1-1 (85%) was found in clinical isolates, suggesting that a gene linked to the MAT1-1 idiomorph could be playing a role in pathogenicity. There was evidence for local clonal reproduction, probably of short duration. These results suggest that a more appropriate description of phylogeography of A. flavus is “everything is everywhere, but not all the time”. The patterns observed in clinical isolates may provide new clues for finding genes involved in pathogenicity.
Key words: Aspergillus flavus, aspergillosis, AFLP, marine fungi, mating type, phylogeography, substrate specificity
INTRODUCTION
Aspergillus flavus is an opportunistic pathogen of humans, animals, and plants. It is notorious for production of aflatoxins (Barros et al. 2007), colonization of a wide array of substrates (Díaz-Guerra et al. 2000, Varga 2006, Hedayati et al. 2007), and tolerance to high temperature and salinity (Barros et al. 2007, Hedayati et al. 2007).
The recent discoveries of mating types and ascocarps in A. flavus (Ramírez-Prado et al. 2008) provide new tools to understand this ubiquitous fungus. The frequency and location of the sexual stage in nature is still unknown. However, A. flavus isolates from a single peanut field in Georgia carried only one idiomorph each, with the MAT1- 1 and MAT1- 2 idiomorphs in equal proportions ((Ramírez-Prado et al. 2008). This suggests that sexual recombination in nature is common. The high level of diversity in natural populations, as measured by either DNA polymorphisms or vegetative compatibility groups (VCGs) is consistent with this conclusion (Bayman and Cotty 1993, Geiser et al. 1998).
However, it is not clear to what extent genetic variation in a local populations of A. flavus can be attributed to recombination as opposed to dispersal. Conidia of A. flavus are small (3-6 µM, Klich 2002) and produced in vast quantities; they can be transported long distances by wind and water. Local populations contain mixtures of numerous VCGs (Bayman and Cotty 1991) that appear to be reproductively isolated from each other (Grubisha and Cotty 2010). This implies that a local population of A. flavus could be composed of genetically distinct, reproductively isolated groups, with adaptations for growth on different substrates.
In nosocomial infections of A. flavus, multiple cases in a single hospital have been shown to be caused by single or a few different strains (Leenders et al.1996, Myoken et al. 2003, Hedayati et al. 2007). However, it has not been possible to discriminate between environmental and clinical strains, suggesting that every strain present in the environment is potentially an opportunistic pathogen if it encounters a susceptible host (Varga 2006). On the other hand, there are significant differences among strains of A. flavus in pathogenicity to plants, and expression of pectinases and other enzymes have
been shown to be related to pathogenicity on corn and cotton (Mellon et al. 2007).
Therefore it is possible that phylogeny of isolates will predict their pathogenicity.
Marine populations of A. flavus have been almost entirely overlooked. We found A. flavus was one of the most commonly isolated fungi from sea water and healthy and diseased sea fan tissue in Puerto Rico (Toledo-Hernández et al. 2008, Zuluaga-Montero et al. 2010a). Salinity is the environmental factor that most determines microbial community structure and diversity, more than temperature, pH or chemical composition (Lozupone and Knight 2007). It is therefore remarkable that A. flavus grows over a wide range of salinity. In some studies agar media with 6% NaCl is used to isolate A. flavus from seeds, more than twice the NaCl concentration of seawater (Doster and Michailides 1994; Bayman et al. 2002). It is not known whether a subset of A. flavus strains have adaptations to salininty that allow them to colonize seawater or whether all isolates are capable of doing so.
Phylogeography of A. flavus. Although A. flavus populations are genetically diverse (Pildain et al. 2004, Chang et al. 2006), genetic variation among populations from different habitats, substrates, and areas is not well understood. Understanding such variation may provide clues as to the source of inoculum and new ideas for the control of A. flavus infection and aflatoxin contamination of host organisms (Karthikeyan et al. 2009). For example, its wide host range implies that A. flavus must have mechanisms to overcome host resistance (Yu et al. 2005) and for resistance to antifungal compounds (Lionakis et al. 2005).
Also, geographical structure in the distribution of S- strains of A. flavus was demonstrated in samples cottonseed in southern Texas (Jaime-García and Cotty 2006).
However, the genetic structure of marine populations of A. flavus is entirely unknown. Being cosmopolitan, with an enormously wide range of hosts and substrates, and being genetically well-characterized, A. flavus is an ideal organism to test the dominant idea of microbial biogeography: “Everything is everywhere; the environment selects” (BaasBecking 1934).
In this study, we used amplified fragment length polymorphisms (AFLPs) to estimate phylogenetic relationships between A. flavus strains from different sites and substrates, in order to answer the following questions: (1) Are there distinct populations of A. flavus in marine vs. terrestrial habitats? We hypothesized that strains of A. flavus from the sea will be more related to each other than to terrestrial strains, because certain adaptations to salinity may give them a competitive advantage over other strains. (2) Do A. flavus clades show substrate specificity? Analyzing several populations of A. flavus from distinct substrates it is possible we will find more genetic variation between than within substrates. The alleles most advantageous in colonizing soil, for example, may be different from those that allow a strain to grow in seawater, plants or insects. 3) Are there similar populations of A. flavus between clinical and environmental strains? We hypothesized that genetic composition of A. flavus populations will be similar in both clinical vs. environmental substrates, because in some disease outbreaks it has been demonstrated that clinical strains are related to single or a few different environmental strains found in the same hospital (Díaz-Guerra et al. 2000, Heinemann et al. 2004) (Hedayati et al. 2007). 4) Are there different frequencies of A. flavus mating types in different substrates and geographic scales? Our hypothesis is that some groups of A. flavus will have equal ratios of mating types, suggesting sexual recombination; other groups will have a biased ratio of mating types, suggesting clonality. 5) Do A.
flavus populations show phylogeographic structure? Because natural selection promotes adaptation to local conditions (Lenormand 2002), we hypothesized that genetic distance among A. flavus strains will be correlated with geographic distance, implying some degree of endemism. We explored the genetic profile using Amplified Fragment Length Polymorphisms (AFLPs) because they are presumably neutral markers and are distributed across the genome.
MATERIALS AND METHODS
Sources of fungi. 117 isolates of A. flavus were isolated and obtained from a variety of substrates and hosts: healthy tissue and diseased tissue of sea fans (Gorgonia ventalina), fresh water, seawater, indoor and outdoor airborne dust, soil, grains and seeds, and clinical strains from culture collections (Table 1). Fungi were isolated from seawater by filtering 250 ml seawater with sterile nitrocellulose membranes of 0.45µm pore size. Seawater filters and sea fan tissue were plated on Glucose Peptone Yeast Agar (GPYA) with 3.3% salt, a standard medium for isolation of marine fungi (ToledoHernández et al. 2008). Indoor and outdoor air air samples were taken with the MicroBio Air sampler (MB-2) from factories of animal and human food in Puerto Rico.
Seeds were plated on water agar (WA) with 1.0% salt (Bayman et al. 2002); soil was diluted and plated on Dichloran Rose Bengal Chloramphenicol Agar (DRBC) (King et al. 1979), and (4) 2% Malt Extract Agar (MEA) (Klich 2002) & 25% Glycerol Nitrate Agar G25N (Pitt 1979), both used to stimulate production of conidia. When more than one A. flavus colony grew on a single plate, only one was isolated. To determine whether the use of different media for different substrates, as indicated by the literature, could have
biased results, we measured the growth rate of five isolates from different substrates on the different isolation media used, in all combinations. No significant differences were found, suggesting that the choice of isolation media does not favor any particular genotypes (data not shown). Plates were incubated at 25° - 30° C for 3 -15 days. For DNA extraction pure cultures of A. flavus were grown in 50 ml Potato Dextrose Broth (PDB) at 30° C for 5 d with agitation.
PCR, sequencing and AFLPs. Mycelia were collected by filtration and DNA was extracted with phenol: chloroform (Lee and Taylor 1990). To confirm that all isolates were A. flavus the nuclear ribosomal ITS region was amplified with primers ITS1F and ITS 4 (Gardes and Bruns 1994?). PCR products were cleaned with Exo-Sap (Fermentas), sequenced in the Sequencing & Genotyping Facility (SGF) at UPR-RP, and corrected with Sequencher (Version 4.8 for Mac). The most similar sequences in GenBank were identified by BLAST searches. All sequences were most similar to A. flavus / A. oryzae with at least 96% identity.
Amplified fragment length polymorphisms (AFLPs) followed instructions of the AFLP Microbial Fingerprinting Kit (Invitrogen Tech) (Zuluaga-Montero et al. 2010).
150-200 ng of genomic DNA of each strain were digested with EcoRI and MseI and fragments were ligated to double-stranded, restriction site-specific adaptors. For selective PCR, over 30 potential primers combinations (EcoRI/MseI) were tested, 8 of which were chosen based on high polymorphism: (1) CA/CA; (2) CC/AC; (3) CC/AG; (4) CC/CA; (5) CC/CG; (6) CG/AG; (7) CG/CA; and (8) CG/CG. Primers were labeled with FAM fluorescent dye (Applied Biosystems) for detection. After amplification, reaction products were diluted, mixed with HI-DI Formamide and 79-560bp ROX size standard,
heated, snap-cooled, and separated by capillary electrophoresis on an ABI 3130xl
Genetic Analyzer (Applied Biosystems). Peaks were extracted with GeneScan Collection
version 3.1.2 software (Applied Biosystems) and the fingerprints were analyzed with Genotyper software (Applied Biosystems). Fragments between 90-500bp were scored as either present or absent using GeneMapper software version 3.5 (Applied Biosystems).
For each primer combination, a panel was generated with assigned allele markers (bins) using a default AFLP analysis. In order to confirm consistency and reproducibility, we compared results to a previous neighbor-joining tree based on AFLP data that included cuantos? of the same isolates and AFLP combinations used in this study (ZuluagaMontero et al. 2010).
Phylogenetic and population analysis. The presence-absence matrix of 460 AFLP characters was analyzed using neighbor-joining (NJ) in PAUP (Version 4.0b 10, Swofford 2002) and the resulting tree was visualized as an unrooted phylogram. In addition, a maximum parsimony tree (MPT) was generated using 100-replicate heuristic search, all characters with equal weight and bootstrapping with 1000 replicates. The MPT was used to test the monophyly hypotheses: e.g., that A. flavus isolates from different substrates formed monophyletic groups (Table 4). For this, a constraint tree was made in PAUP in which all disease-associated isolates or marine-isolated isolates were forced to form a single clade. The significance of differences between the two topologies for each hypothesis was tested with a Templeton test (Wilcoxon signedranks) as implemented in PAUP.
Population structure was tested grouping the samples by substrate and geographic origin. Wright’s F statistics (FST) was calculated with Structure 2.2.3 (Whitlock and
McCauley 1999,Falush et al. 2007). The K values (1-7) were estimated on the log likelihood score and posterior probability of K (Pritchard et al. 2007). Structure was set as follows: length of burning period = 1 million; number of iterations for the Markov Chain Monte Carlo (MCMC) = 300,000; an admixture model was chosen with lambda constant. Correlated allele frequencies among populations were assumed.
Frequencies of mating types. To determine presence/absence of mating type idiomorphs, we amplified segments of MAT 1-1 (396bp) and MAT 1-2 (270 bp) from all isolates (Ramírez-Prado et al. 2008). Amplification was confirmed by gel electrophoresis. In three isolates of each mating type, we sequenced the fragments to confirm it was the desired gene. Differences in proportions of the two mating types between substrates and sites were tested for significance with Fisher’s exact test. The null hypothesis was the proportions of MAT 1-1 and MAT 1-2 were the same in all groups.
Phylogeographic analysis. Latitude and longitude were obtained for the site of origin of each isolate from GoogleEarth and used to calculate distance in km between each pair of isolates with the Geographic Distance Matrix Generator v1.2.3 (http://biodiversityinformatics.amnh.org/open_source/gdmg/index.php). A matrix of genetic distance between pairs of isolates was generated with PAUP (Version 4.0b 10, Swofford 2002). Correlation of geographic distance with genetic distance was analyzed in Statistica7.
RESULTS
Phylogeny of A. flavus inferred from AFLPs. A NJ tree based on AFLP characters for 117 A. flavus from different locations and substrates is shown in Fig. 1. The tree has many well- supported nodes, but lacks bootstrap support at deeper nodes. As a control, trees were compared with a previous AFLP study which included several of the same isolates (Zuluaga-Montero et al. 2010). All terminal clades were similar for these isolates, indicating consistency and reproducibility of results.
Marine vs. terrestrial substrates. There was no differentiation of marine vs. terrestrial isolates among clades; isolates from both substrates were distributed randomly in the AFLP tree (Fig. 1). However, several terminal clades were exclusively or almost exclusively composed of marine isolates (Fig. 1, blue and green branches). The hypotheses of genetic differentiation for marine and terrestrial isolates were both rejected using Templeton tests. Constraint topologies that grouped marine and terrestrial isolates required significantly more steps than the most parsimonious trees (both with 4858 steps vs. 4637 steps for the MP tree) (Table 4).
Substrate specificity. No significant association between substrate and phylogeny was found in AFLP tree (Fig. 1); there was no overall evidence of specificity. However, three clades with bootstrap support were mostly or exclusively composed of isolates from sea fan tissue. In one such clade, all isolates were from gorgonian tissue collected at a single place and time (Fig. 1, see *); in another, from different places in Puerto Rico and including one isolate from seawater (Fig. 1, see **). The hypotheses of monophyly
for A. flavus isolates from each substrate were all rejected using Templeton tests.
Constraint topologies that grouped isolates the same substrate required significantly more steps than the MP tree (seawater 4931 steps, fresh water 4676 steps, sea fan 4919 steps, indoor and outdoor airborne dust 4683 steps, soil 4895 steps, grains and seeds 4849 steps vs. 4637 steps for MP) (Table 4).
Clinical vs. environmental strains. AFLPs did not resolve clinical and environmental isolates into separate clades (Fig. 1). However, the high number of polymorphic loci (460) produced two terminal clades that included mainly clinical isolates. One clade included clinical isolates from three different countries while another included four clinical isolates from México, but neither of these clades had high bootstrap support. The hypotheses of monophyly for clinical isolates were rejected using Templeton tests. Constraint topologies that grouped clinical isolates required significantly more than one of the MP tree (4702 steps vs. 4637 steps) (Table 4). No association was seen between clinical isolates and marine isolates.
Frequencies of mating types. Of the 201 isolates of A. flavus, 185 amplified successfully with primers for mating type MAT 1-1 or MAT 1-2. No isolate produced an amplicon with both sets of primers, indicating only one mating idiomorph per sample. All amplicons sequenced yielded the correct gene. Mating types overall were in roughly equal proportion, with 52 % MAT 1-1 and 48% MAT 1-2 (Table 2). In most cases, proportions of the two mating types were not significantly different between isolates from different substrates, when compared by Fisher’s exact tests (Table 2). However, there were two notable exceptions: clinical isolates were 84.6% MAT 1-1, significantly
different from a pooled sample of all other isolates (Fisher’s exact test, P=0.039).
Isolates from seawater were 80% MAT 1-2, marginally different from a pooled sample of all other isolates (Fisher’s exact test, P=0.058).
When isolates were grouped by country of origin, only Puerto Rico and India showed a roughly 1:1 proportion of mating types (Table 3). Distortions in other areas presumably reflected small sample sizes. Proportions of the two mating types were not significantly different among isolates from different geographic areas, when compared by Fisher’s exact tests (Table 3).
Phylogeography. Clades within A. flavus did not correlate with geographic origin. Most clades included isolates from diverse areas. Only three clades were almost exclusively composed of strains from Puerto Rico, with bootstrap values > 80% (Fig. 2, see asterisks). One of such clade included 12 of 19 isolates from Jobos, Puerto Rico, with a bootstrap support of 83 % (See *, discussed below).
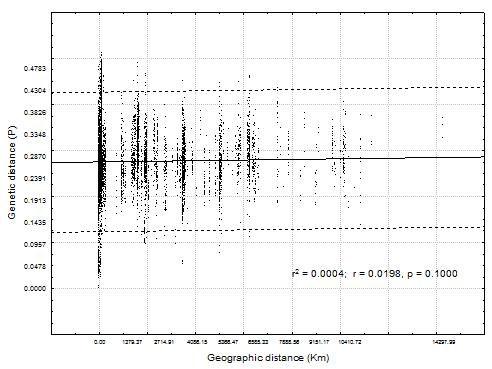
The population genetic analysis, as measured by Fst, revealed low levels of population structure among isolates from the same substrate or geographic region (Fst = 0.157), suggesting that all isolates are part of a single population with high gene flow among isolates. Also, the lack of correlation (r2= 0.0004, P=0.1) between geographical and genetic distances showed a pattern of random mixing and global population structure (Fig. 3).
DISCUSSION
Marine vs. terrestrial substrates. Marine and terrestrial isolates of A. flavus
composed a single population(Fig. 1), suggesting that A. flavus strains found in terrestrial habitats can colonize and survive in marine environments. A preliminary study found the same result, but was not conclusive because only 4 terrestrial samples and no clinical samples were included (Zuluaga-Montero et al. 2010). Similarly, marine and terrestrial strains of A. sydowii were indistinguishable in temperature requirements, susceptibility to crude extracts of sea fans and metabolite profiles (Alker et al. 2001) and in DNA fingerprints (Rypien 2008). However, marine strains of A. sydowii pathogenic on sea fans differed from non-pathogenic terrestrial strains in patterns of carbon source utilization (Alker et al. 2001).
Based on aflatoxin production and size and number of sclerotia produced in vitro, A. flavus isolates can be divided into two groups, S and L (Cotty 1989). In A. flavus populations isolated from soils in cotton fields around USA, S type isolates have ranged in incidence from 3 % to 97 % (Cotty 1997). In Argentina the S strain phenotype were collected mainly from soils, where L strains was associated with the aerial crop parts, suggesting adaptations to different substrates (Barros et al. 2005). It is curious that no S type isolates were among the 57 we isolated from marine substrates. (The S type isolates in Fig. 1, indicated by squares, are reference strains.) This was the only difference in population structure found between marine and terrestrial substrates. It is not clear why only L type isolates were obtained from marine substrates.
On the other hand, four terminal clades were composed entirely of marine isolates; these clades had ≥ 80% bootstrap support and included 4-13 isolates (Fig. 1, see blue and green branches), indicating that some A. flavus genotypes more likely to colonize the marine habitat. It is not clear if this preference is due to adaptation or source of inoculum. In one of these exclusively marine clades, all isolates came from the same
area on the same day. In this case (marked by blue branches in Fig. 1) the grouping may have a historical rather than biological explanation, as discussed below. However, in other clades (marked by green branches in Fig. 1) the isolates came from various sites and dates, suggesting there may be some association between genotype and the marine habitat.
Marine Aspergilli may also be agents of disease. Aspergillosis of sea fans (Gorgonia ventalina) has caused mass mortalities and major changes in community structure of the host (Nagelkerken et al. 1997, Nugues and Nagelkerken 2006). Aspergillus sydowii was reported as the causative agent of sea fan aspergillosis (Smith et al. 1996, Geiser et al. 1998b). In Puerto Rico, however, A. sydowii has not been isolated from diseased tissues of sea fans, despite repeated attempts (Toledo-Hernández et al. 2007, 2008, Zuluaga-Montero et al. 2010a). However, A. flavus was found to be abundant in diseased sea fan tissue, as well as in healthy tissue and seawater, and has been suggested as a potential causal agent of the disease (Toledo-Hernández et al. 2007, Zuluaga et al. 2010a). Given that it is ubiquitous in different substrates and is an opportunistic pathogen of a wide range of organisms, and possibly pathogenic in sea fans, A. flavus can be studied as a model organism for fungi in marine and terrestrial ecosystems.
Substrate specificity. A. flavus strains appear to be generalists in terms of substrate. Host specificity in A. flavus has not been studied previously from a phylogenetic perspective. But in a study of pathogenicity, A. flavus isolates causing disease in humans, insects and plants, produced disease both insects and plants, with no evidence of host specialization (Leger et al. 2000). If there is reduction in sporulation and reproduction by A. flavus on a living host relative to the saprotrophic habit, virulence
and host specificity will selected against (Mehl & Cotty 2010). In sea fans tissues, hyphae of A. flavus have been observed but conidiophores and other reproductive structures have not (Toledo-Hernández et al. in prep.), suggesting that colonization of sea fans may be a dead end for A. flavus in terms of reproduction. It is believed that Aspergilli do not sporulate in the sea (Shinn et al. 2000, Smith et al. 1996); conidiophores have never been observed in the sea and are adapted for aerial dispersal (Raghukumar & Raghukumar 1999).
Clinical vs environmental strains. Several clinical isolates were often most similar in genotype to environmental isolates (Fig. 1). The simplest explanation for this result is that any strain of A. flavus is potentially pathogenic and can cause disease when the environment is favorable and hosts are susceptible or immunocompromised. However, nine of the thirteen clinical isolates grouped in two terminal clades (Fig. 1), although there was no bootstrap support for these clades. In one such clade all isolates came from the culture collection of a single hospital, so there may be a geographic explanation rather than a propensity to clinical infection, but in the other clade the isolates came from three continents. The relatedness of these clinical isolates could allow a first screening to find genes associated with pathogenicity.
Several studies have attempted to establish sources of clinical Aspergillus strains by comparing them to environmental strains from the same hospital or area, using DNA fingerprinting techniques. For example, similar genotypes have been found in clinical and nearby environmental isolates using repetitive DNA (James et al. 2000), randomly amplified polymorphic DNA (RAPDs) (Leenders et al 1996, Díaz-Guerra et al. 2000, Heinemann et al. 2004), and microsatellites (Hadrich et al. 2010). Isolates of A. flavus
recovered from a single local outbreak are often related, implying that in those outbreaks human hosts are infected by the same strains or a few different strains (Hedayati et al. 2007).
Frequencies of mating type. It has been proposed that the anamorphic stages of A. flavus emerge frequently from meiotic lineages which contain both mating-type genes (Geiser et al. 1996), which suggests both mating types should be present in roughly equal frequency. Equal proportions of MAT 1-1 or MAT 1-2 isolates were found in a natural A. flavus population in the soil of a Georgia cotton field (Ramírez-Prado et al. 2008). In this study, the first to determine mating types in isolates from a wide range of substrates and geographic origins, A. flavus likewise demonstrated a 1:1 ratio.
An interesting and novel finding in this study was that clinical isolates were mostly (84.6%) of MAT 1-1 mating type, regardless of phylogenetic position and geographic origin. This skewed ratio suggests that a gene linked to MAT 1-1 is associated with the ability to colonize and perhaps cause illness in a human host. If this is a general phenomenon, the association may be useful to find genes involved in pathogenicity.
The majority of marine isolates from Jobos isolates were MAT 1-1 providing further evidence of a recent clonal origin of this clade, as discussed above.
Phylogeography. “Everything is everywhere; but the environment selects,” has been a paradigm for microbial biogeography ever since it was postulated almost eighty years ago (Bass-Becking 1934). Some microorganisms produce minute propagules in enormous quantities, facilitating dispersal and worldwide distribution. Such species are not expected to show biogeographical structure (Bass-Becking 1934, Pringle et al 2005).
A. flavus provides an ideal system to examine this aphorism: it is ubiquitious, colonizes diverse substrates, and it has been studied extensively.
Evidence in favor of 'Everything is everywhere' in A. flavus. No association between phylogeny and country of origin was observed in worldwide collection of A. flavus (Fig. 2). The low differentiation in Fst and the lack of correlation between genetic distance and geographic distance (Fig. 3) suggest that all strains form a single, global population. These results are supported by previous studies in which phylogenetic analyses was not able to demonstrate phylogeographic structure in A. flavus (Pringle et al 2005, Zuluaga et al. 2010b), A. sydowii (Rypien et al. 2008), and A. fumigatus (Pringle et al. 2005).
Because of this apparent lack of phylogeographic structure, it is not possible to prove or disprove the idea that Aspergillus spores carried from Africa via Saharan dust have a significant impact in the Caribbean, as has been proposed (Jolles et al. 2002, Kellogg and Griffin 2003, Prospero et al. 2005). The fact that two mycology labs in Puerto Rico have not managed to isolate A. flavus or A. sydowii from saharan dust, when it arrived to the Island (Personal communication, B. Bolaños) and in our laboratories from samples collected by the Aerosol and Ocean Science Expeditions III (AEROSE III) in the Atlantic, supports the assertion of Rypien (2008, et al. 2008) that dust clouds from Africa are not significant sources of pathogenic Aspergillus across the Atlantic Ocean.
Evidence against 'Everything is everywhere' in A. flavus. Nonetheless, there are several lines of evidence of phylogeographic structure in A. flavus, especially at the local level. First, as
mentioned above, frequencies of L and S type isolates differ markedly between sites and substrates, and here we found no S type isolates in marine substrates. Second, we found bias of mating type ratios in clincal and marine isolates, significantly so in clinical isolates and marginally so in marine isolates.
Third, a single clade of A. flavus can dominate a local population. Twelve of 19 isolates of A. flavus from Jobos, Puerto Rico are grouped in the same clade, suggesting a local phylogeographic structure (Fig. 2, see *). Each isolate was from a different sea fan colony, but all were isolated from the same area on the same day. However, within this apparently clonal population, there were no two strains with exactly equal genotypes determined by AFLPs. This incongruence could be a technique effect due to DNA degradation in storage, or inconsistency in scoring peaks among sample runs.
A similar case was found in the A. flavus population of Arizona cotton fields, using vegetative compatibility groups (VCGs): one VCG dominated the population of a single cotton field even though it was uncommon in the population at large (Bayman and Cotty 1991). But the following year this VCG was not found, implying local dominance by genotypes that are very successful but are quickly replaced. This may be caused by a sudden appearance of suitable substrates combined with a massive sporulation by a single strain of several closely related strains. These sudden population explosions by single genotypes are presumably localized and temporary, since the following year the genotype may not be found (Bayman and Cotty, 1991). Since models of biogeography were developed to study plants and animals, time is not included as a variable. For organisms with short generation times and enormous reproductive potential like A. flavus, however, the timescale of sampling will influence biogeographic structure. Based in our results we suggest that “everything is everywhere, but not all the time,” for the cosmopolitan fungus A. flavus.
Conclusions. Although our results suggest that all the A. flavus isolates form a single population, we found phylogenetic evidence that certain strains show substrate specificity, as well as some local distribution patterns. Mating type frequencies are of equal proportion on global scale, suggesting frequent recombination. The fact that the clinical strains studied were almost entirely of MAT 1-1 mating type may provide new clues to understanding patogenicity. Improving the resolution of genetic identity of strains and increasing sample size can bring light to dynamics of opportunistic pathogens such as A. flavus.
Acknowledgments. We thank NOAA Sea Grant for support (Grant No. R-92-2-08). We also thank NIH SCORE and NSF CREST-CATEC for support.
Literature Cited
Baas-Becking, L. G. M. 1934. Geobiologie of Inleiding Tot de Milieukunde.W. P. Van Stockum & Zoon N. V., The Hague, The Netherlands.
Barros, G., A. Torres, and S. Chulze. 2007. Aspergillus flavus population isolated from soil of Argentina’s peanut-growing region. Sclerotia production and toxigenic profile. J. Sci. Food Agric. 85:2349-2353.
Bayman, P., and P. J. Cotty. 1991. Vegetative compatibility and genetic diversity in the Aspergillus flavus population of single field. Canadian Journal of Botany-Revue Canadienne De Botanique 69:1707-1711.
Chang, P. K., K. C. Ehrlich, and T. H. Sui-Sheng. 2006. Cladal relatedness among Aspergillus oryzae isolates and Aspergillus flavus S and L morphotype isolates. Int. J. Food Microbiol. 108:172-177.
Criseo, G., R. Cosimo, and R. Orazio. 2008. High genetic variability in non aflatoxigenic A. flavus strains by using Quadruplex PCR-based assay. Int. J. Food Microbiol. 125:341-343.
Cotty, P. J. 1989. Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology 79:808-814.
Cotty, P. J. 1997. Aflatoxin-producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycol. Res. 101:698-704.
Coppin, E., R. Debuchy, S. Arnaise, and M. Picard. 1997. Mating Types and Sexual Development in Filamentous Ascomycetes. Microbiol. Mol. Biol. Rev. 61(4): 411-428.
Di, N. F., M. Gallo, and R. Nigro. 2009. Adsorbents selection for aflatoxins removal in bovine milks. J. Food Process Eng. 95:186-191.
Díaz-Guerra, T. M., E. Mellado, M. Cuenca-Estrella, L. Gaztelurrutia, J. I. Villate Navarro, and J. L. Rodríguez Tudela. 2000. Genetic similarity among one Aspergillus flavus strain isolated from a patient who underwent heart surgery and two environmental strains obtained from the operating room. J. Clin. Microbiol. 38(6):2419-22.
Falush, D., M. Stephens, and J. K. Pritchard. 2007. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol. Ecol. Notes. 7(4):574-578.
Fedorova, N. D., S. Harris, D. Chen, D. W. Denning, J. Yu, P. J. Cotty, and W. C. Nierman. 2009. Using aCGH to study intraspecific genetic variability in two pathogenic molds, Aspergillus fumigatus and Aspergillus flavus. Med. Mycol. 47: S34-S41.
Galagan, J. E., S. E. Calvo, C. Cuomo, L. J. Ma, J. R. Wortman, S. Batzoglou, S. I. Lee, M. Bastürkmen, C. C. Spevak, J. Clutterbuck, V. Kapitonov, J. Jurka, C. Scazzocchio, M. Farman, J. Butler, S. Purcell, S. Harris, G. H. Braus, O. Draht, S. Busch, C. D'Enfert, C. Bouchier, G. H. Goldman, D. Bell-Pedersen, S. Griffiths-Jones, J. H. Doonan, J. Yu, K. Vienken, A. Pain, M. Freitag, E. U. Selker, D. B. Archer, M. Á. Peñalva, B. R. Oakley, M. Momany, T. Tanaka, T. Kumagai, K. Asai, M. Machida, W. C. Nierman, D. W. Denning, M. Caddick, M. Hynes, M. Paoletti, R. Fischer, B. Miller, P. Dyer, M. S. Sachs, S. A. Osmani, and B. W. Birren. 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438: 1105-1115.
Gardes, M., and T. D. Bruns. 1993. ITS primers with enhanced specificity for basidiomycetes- application to the identification of mycorrhizae and rusts. Mol. Ecol. 2:113-118.
Geiser, D. M. 2009. Sexual structures in Aspergillus: morphology, importance and genomics. Med. Mycol. 47:S21-S26.
Geiser, D. M., J. I. Pitt, and J. W. Taylor. 1998a. Cryptic speciation and recombination in the aflatoxin producing fungus Aspergillus flavus. Proc. Natl. Acad. Sci. U.S.A 95:388-393.
Geiser D. M., J. W. Taylor, K. B. Ritchie, and G.W. Smith. 1998b. Cause of sea fan death in the West Indies. Nature 394:137-138.
Geiser, D. M., W. E. Timberlake, and M. L. Arnold. 1996. Loss of meiosis in Aspergillus. Mol. Biol. Evol. 13:809-817.
Grubisha, L.C., and P.J. Cotty. 2010. Genetic isolation among sympatric vegetative compatibility groups of the aflatoxin-producing fungus Aspergillus flavus. Mol. Ecol. 19:269-280.
Hadrich, I., F. Makni, A. Ayadi, and S. Ranque. 2010. Microsatellite typing to trace Aspergillus flavus infections in a haematology Unit. J. Clin. Microbiol. 48(7): 2396-2401.
Hedayati M.T., A. C. Pasqualotto, P. A. Warn, P. Bowyer, and D. W. Denning. 2007. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153:1677-1692.
Heinemann, S., F. Symoens, B. Gordts, H. Jannes, and N. Nolard. 2004. Environmental investigations and molecular typing of Aspergillus flavus during an outbreak of postoperative infections. J. Hosp. Infect. 57:149-155.
Horn, B.W., J. H. Ramírez-Prado, and I. Carbone. 2009. Sexual reproduction and recombination in the aflatoxin-producing fungus Aspergillus parasiticus. Fungal Genet. Biol. 46:169-175.
Jaime-Garcia, R., and P. J. Cotty. 2006. Spatial distribution of Aspergillus flavus and its toxigenic strains on commercial cottonseed from south Texas and its relationship to aflatoxin contamination. Plant Pathol. 55:358-366.
James, M., B. Lasker, M. Mcneil, M. Shelton, D. Warnock, and R. Errol. 2000. Use of a repetitive dna probe to type clinical and environmental isolates of Aspergillus flavus from a cluster of cutaneous infections in a neonatal intensive care unit. J. Clin. Microbiol. 38(10):3612-3618.
Jolles, A.E., P. Sullivan, A. P. Alker, C. Drew Harvell. 2002. Disease transmission of aspergillosis in sea fans: infering procces from spatial pattern. Ecology 83:23732378.
Karthikeyan, M., R. Sandosskumar, S. Mathiyazhagan, M. Mohankumar, V. Valluvaparidasan, S. Kumar, and R. Velazhahan. 2009. Genetic variability and aflatoxigenic potential of Aspergillus flavus isolates from maize. Archives of Phytopathology and Plant Protection 42(1):83-91.
Katz, M. E., A. M. Dougall, K. Weeks, and B. F. Cheetham. 2005. Multiple Genetically Distinct Groups Revealed among Clinical Isolates Identified as Atypical Aspergillus fumigatus. J. Clin. Microbiol. 43(2):551-555.
Kellogg, C., and D. W. Griffin. 2003. African dust carries microbes across the ocean: are they affecting human and ecosystem health? U.S. Geological Survey Report 03028.
King, A. D., A. D. Hocking, and J. I. Pitt. 1979. Dichloran-rose bengal medium for the enumeration and isolation of molds from foods. Appl. Environ. Microbiol. 37:959964.
Klich, M. A. 2000. Identification of common Aspergillus species. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
Leenders, A., A. Van Belkum, S. Janssen, S. De Marie, J. Kluytmans, J. Wielenga, B. Löwenberg, and H. Verbrugh. 1996. Molecular Epidemiology of Apparent Outbreak of Invasive Aspergillosis in a Hematology Ward. J. Clin. Microbiol 34(2):345-351.
Lenormand, T. 2002. Gene flow and the limits to natural selection. Trends Ecol. Evol. (Amst.) 17:183-189.
Lionakis, M. S., R. L. Lewisa, H. A. Torresa, N. D. Alberta, I. I. Raada, and D. P. Kontoyiannisa. 2005. Increased frequency of non-fumigatus Aspergillus species in amphotericin B- or triazole-pre-exposed cancer patients with positive cultures for aspergilli. Diagn. Microbiol. Infect. Dis. 52:15-20.
Lourenço, A., L. Durigon Edison, P. Zanotto, J. E. Gazzinelli Cruz Madeira, A. Palma De Almeida, and B. Correa. 2007. Genetic diversity of environmental Aspergillus flavus strains in the state of São Paulo, Brazil by random amplified polymorphic DNA. Mem. Inst. Oswaldo Cruz, Rio de Janeiro 102(6): 687-692.
Lumbsch, H.T., P. K. Buchanan, T. W. May, and G. M. Mueller. 2008.
Phylogeography and Biogeography of Fungi. Mycol. Res. 112(4):423-424.
Martiny Hughes, J. B., B. J. M. Bohannan, J. M. Brown, R. K. Colwell, J. A. Fuhrman, Green J.L., M. C. Horner-Devine, M. Kane, J. A. Krumins, C. R. Kuske, P. J. Morin, S. Naeem, L. Øvreås, A. L. Reysenbach, V. H. Smith, and J T. Staley. 2006. Microbial biogeography: putting microorganisms on the map. Nature Review Microbiology 4:102-112.
Mehl, H. L, and P. J. Cotty. 2010. Variation in Competitive ability among Isolates of Aspergillus flavus from Different Vegetative Compatibility Groups during Maize Infection. Phytopathology 100:150-159.
Mellon, J. E., P. J. Cotty, and M. K. Dowd. 2007. Aspergillus flavus hydrolases: their roles in pathogenesis and substrate utilization. Appl. Microbiol. Biotechnol. 77:497-504.
Metzenberg, R. L, and N. L Glass. 1990. Mating type and mating strategies in Neurospora. Bioessays 12:53-59.
Moore, G., B. W. Horn, E. Jacalyn, K. Hell, S. Chulze, G. Wright, and M. Naik 2008. Evidence for geographic isolation and distinct patterns of recombination in the aflatoxin gene cluster of Aspergillus flavus. APS Annual Meeting.
Myoken Y., T. Sugata, Y. Fujita, M. Fujihara, T. Kohara, M. Katsu, and Y. Mikami. 2003. Molecular epidemiology of invasive stomatitis due to Aspergillus flavus in patients with acute leukemia J. Oral Pathol. 32(4):215-218.
Nagelkerken, I., K. Buchan, G. W. Smith, K. Bonair, P. Bush, J. Garzón-Ferreira, L. Botero, P. Gayle, C. D. Harvell, C. Heberer, K. Kim, C. Petrovic, L. Pors, and P. Yoshioka. 1997. Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Mar. Ecol. Prog. Ser. 160:255-263.
Nugues, M. M., and I. Nagelkerken. 2006. Status of aspergillosis and sea fan populations in Curaçao ten years after the 1995 Caribbean epizootic. Biol. Trop. 54(3):153-160.
O’Donnell, K., H. C. Kistler, B. K. Tacke, and H. C. Casper. 2000. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc. Natl. Acad. Sci. U.S.A. 97:7905-7910.
Paoletti, M., C. Rydholm, E. U. Schwier, M. J. Anderson, G. Szakacs, F. Lutzoni, J. P. Debeaupuis, J. P. Latgé, D. W. Denning, and P. S. Dyer. 2005. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr. Biol. 15(13):1242-1248.
Pasqualotto Alessandro C. 2009. Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med. Mycol. 47:261-270.
Pildain M.B., G. Vaamonde, and D. Cabral. 2004. Analysis of population structure of Aspergillus flavus from peanut based on vegetative compatibility, geographic origin, mycotoxin and sclerotia production. Int. J. Food Microbiol. 93:31-40.
Pitt, J. I. 1979. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London.
Pritchard. J.K., X. Wen, and D. Falush. 2007. Documentation for Structure Software:Version 2.2.
http://pritch.bsd.uchicago.edu/software/structure22/readme.pdf (accessed 18.02.10).
Pringle, A., D. M. Baker, J. L. Platt, J. P. Wares, J. P. Latge, and J. W. Taylor. 2005. Cryptic speciation in the cosmopolitan and clonal human pathogenic fungus Aspergillus fumigatus. Evolution 59:1886-1899.
Prospero, J. M., E. Blades, G. Mathison, and R. Naidu. 2005. Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia 21(1):1-19.
Raghukumar, S., and C. Raghukumar. 1999. Marine fungi: a critique. Aquatic Microbiology Newsletter 38:26-27.
Ramírez-Prado, J. H., G. G. Moore, B. W. Horn, and I. Carbone. 2008. Characterization and population analysis of the mating-type genes in Aspergillus flavus and Aspergillus parasiticus. Fungal Genet. Biol. 45:1292-1299.
Rypien, K. 2008. African dust is an unlikely source of Aspergillus sydowii, the causative agent of sea fan disease. Mar. Ecol. Prog. Ser. 367:125-131.
Rypien K. L., J. P. Andras, and C. D. Harvell. 2008. Globally panmictic population structure in the opportunistic fungal pathogen Aspergillus sydowii. Mol. Ecol. 17:4068-4078.
Smith, G.W., L. D. Ives, I. A. Nagelkerken, and K. B. Ritchie. 1996. Caribbean sea fan mortalities. Nature 383:487.
Swofford, D. L. 2002. Phylogenetic Analysis Using Parsimony (*and Other Methods). Sinauer, Sunderland, MA.
Toledo-Hernández, C., A. Bones-Gonzáles, O. E. Ortiz-Vázquez, A. M. Sabat, and P. Bayman. 2007. Fungi in the sea fan Gorgonia ventalina: diversity and sampling strategies. Coral Reefs 26:725-730.
Toledo-Hernández, C., A. Zuluaga-Montero, A. Bones-González, J. A. Rodríguez, A. M. Sabat, and P. Bayman. 2008. Fungi in healthy and diseased sea fans (Gorgonia ventalina): is Aspergillus sydowii always the pathogen? Coral Reefs 27:707714.
Varga, J. 2006. Molecular typing of aspergilli: Recent developments and outcomes. Med. Mycol. 44:S149-S161.
White, T.J., T. D. Bruns, S. B. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. p 315-322. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., (Eds) PCR protocols: A guide to methods and applications. Academic Press, New York. Whitlock, M. C., and D. E. McCauley. 1999. Indirect measures of gene flow and migration: FST ≠ 1/(4Nm+1). Heredity 82:117-125.
Yu, J., P. K. Chang , K. C. Ehrlich , J. W. Cary , D. Bhatnagar , T. E. Cleveland , G. A. Payne , J. E. Linz , C. P. Woloshuk , J. W. Bennett . 2004. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 70(3):12531262.
Yu, J., T. E. Cleveland, W. C. Nierman, and J. W. Bennett. 2005. Aspergillus flavus genomics: gateway to human and animal health, food safety, and crop resistance to diseases. Rev. Iberoam. Micol. 22:194-202.
Zuluaga-Montero, A., C. Toledo-Hernández, J. A. Rodríguez, M. Sabat, and P. Bayman. 2010a. Spatial variation in fungal communities isolated from healthy and diseased sea fans Gorgonia ventalina and seawater. Aquat. Biol. 8:151-160.
Zuluaga-Montero, A., L. Ramírez-Camejo, J. Rauscher, and P. Bayman. 2010b. Marine isolates of Aspergillus flavus: Denizens of the deep or lost at sea? Fungal
Ecol. 3:386-391.
Table 1. Aspergillus flavus isolates used in this study.
Culture Substrate
Geographyorigin
1 AB1 Airborne NutriMixFeedCo.,Cataño,PR 2 AB2 Airborne NutriMixFeedCo.,Cataño,PR
3 AB3 Airborne NutriMixFeedCo.,Cataño,PR
4 AB4 Airborne NutriMixFeedCo.,Cataño,PR
5 AB5 Airborne NutriMixFeedCo.,Cataño,PR
6 AB6 Airborne NutriMixFeedCo.,Cataño,PR 7 AB7 Airborne MolinosDePuertoRico,Inc.,Cataño,PR 8 AB8 Airborne NutriMixFeedCo.,Cataño,PR 9 AB9 Airborne NutriMixFeedCo.,Cataño,PR 10 AB10 Airborne NutriMixFeedCo.,Cataño,PR 11 AB11 Airborne NutriMixFeedCo.,Cataño,PR
12 AB12 Airborne NutriMixFeedCo.,Cataño,PR
13
AB13 Airborne NutriMixFeedCo.,Cataño,PR
14 AB14 Airborne NutriMixFeedCo.,Cataño,PR
15 AB16 Airborne NutriMixFeedCo.,Cataño,PR
16 AB17 Airborne NutriMixFeedCo.,Cataño,PR
17 AB18 Airborne NutriMixFeedCo.,Cataño,PR
18 AB19 Airborne NutriMixFeedCo.,Cataño,PR
19 AB20 Airborne NutriMixFeedCo.,Cataño,PR
20 AB21 Airborne NutriMixFeedCo.,Cataño,PR
21 AB22 Airborne NutriMixFeedCo.,Cataño,PR
22 AB23 Airborne NutriMixFeedCo.,Cataño,PR
23 AB24 Airborne NutriMixFeedCo.,Cataño,PR
24 AB25 Airborne NutriMixFeedCo.,Cataño,PR
25 AB27 Airborne NutriMixFeedCo.,Cataño,PR
26 AB28 Airborne NutriMixFeedCo.,Cataño,PR
27 AB29 Airborne NutriMixFeedCo.,Cataño,PR
28 AB30 Airborne NutriMixFeedCo.,Cataño,PR
29 AB31 Airborne NutriMixFeedCo.,Cataño,PR
30 AB32 Airborne MolinosDePuertoRico,Inc.,Cataño,PR
31 AB33 Airborne MolinosDePuertoRico,Inc.,Cataño,PR
32 AB34 Airborne MolinosDePuertoRico,Inc.,Cataño,PR
33 AB35 Airborne MolinosDePuertoRico,Inc.,Cataño,PR
34 AB36 Airborne MolinosDePuertoRico,Inc.,Cataño,PR
35 AB37 Airborne MolinosDePuertoRico,Inc.,Cataño,PR
36 AB39 Airborne MolinosDePuertoRico,Inc.,Cataño,PR
37 AB40 Airborne MolinosDePuertoRico,Inc.,Cataño,PR
38 AB41 Airborne NutriMixFeedCo.,Cataño,PR
39 CN1 Corn ShipdockedatCataño,PR
40 CN2 Corn ShipdockedatCataño,PR
41 CN3 Corn ShipdockedatCataño,PR
42 CN4 Corn ShipdockedatCataño,PR
43 CN5 Corn ShipdockedatCataño,PR
44 CN6 Corn ShipdockedatCataño,PR
45 CN7 Corn ShipdockedatCataño,PR
46 CN8 Corn ShipdockedatCataño,PR
47 CN9 Corn ShipdockedatCataño,PR
48 CN10 Corn ShipdockedatCataño,PR
49 CN11 Corn ShipdockedatCataño,PR
50 CN12 Corn ShipdockedatCataño,PR
51 CN13 Corn ShipdockedatCataño,PR
52 CN14 Corn ShipdockedatCataño,PR
53 CN15 Corn ShipdockedatCataño,PR
54 CN16 Corn ShipdockedatCataño,PR
55 CN17 Corn ShipdockedatCataño,PR
56 CN19 Corn ShipdockedatCataño,PR
57 CN20 Corn ShipdockedatCataño,PR
58 CN21 Corn ShipdockedatCataño,PR
59 JF01 Orchard WolfskillExperimentalFarm,Winters,CA(US)
60 JF31 Orchard WolfskillExperimentalFarm,Winters,CA(US)
61 JF53 Orchard WolfskillExperimentalFarm,Winters,CA(US)
62 JF51 Soil Hilo,Hawaii
63 D12 Airborne UPRRP,PR
64 WC47 Freshwater ElSeñorial.SanJuan,PR
65 WC56 Freshwater ElSeñorial.SanJuan,PR
66 WC44 Freshwater ElSeñorial.SanJuan,PR
67 HP1 Clinical RecintodeCienciasMédicas,PR
68 CB26 Diseased Gorgonia tissue Culebrita,PR
69 CB27 Diseased Gorgonia tissue Culebrita,PR
70 JO28 Diseased Gorgonia tissue Jobos,Salinas,PR
71 JO41 Diseased Gorgonia tissue Jobos,Salinas,PR
72 JO53 Diseased Gorgonia tissue Jobos,Salinas,PR
73 JO26 Diseased Gorgonia tissue Jobos,Salinas,PR
74 JO38 Diseased Gorgonia tissue Jobos,Salinas,PR
75 CB35 Healthy Gorgonia tissue Culebrita,PR
76 IK36 Healthy Gorgonia tissue Icacos,Fajardo,PR
77 IK37 Healthy Gorgonia tissue Icacos,Fajardo,PR
78 JO29 Healthy Gorgonia tissue Jobos,Salinas,PR
79 JO33 Healthy Gorgonia tissue Jobos,Salinas,PR
80 JO34 Healthy Gorgonia tissue Jobos,Salinas,PR
81 JO37 Healthy Gorgonia tissue Jobos,Salinas,PR
82 JO39 Healthy Gorgonia tissue Jobos,Salinas,PR
83 JO40 Healthy Gorgonia tissue Jobos,Salinas,PR
84 JO46 Healthy Gorgonia tissue Jobos,Salinas,PR
85 IK34 Healthy Gorgonia tissue Icacos,Fajardo,PR
86 IK39 Healthy Gorgonia tissue Icacos,Fajardo,PR
87 JO24 Healthy Gorgonia tissue Jobos,Salinas,PR
88 JO25 Healthy Gorgonia tissue Jobos,Salinas,PR
89 JO27 Healthy Gorgonia tissue Jobos,Salinas,PR
90 JO31 Healthy Gorgonia tissue Jobos,Salinas,PR
91 JO49 Healthy Gorgonia tissue Jobos,Salinas,PR
92 DO4 Soil NewDelhi,India
93 WC1 Freshwater ElSeñorial.SanJuan,PR
94 JF78 Soil WolfskillExperimentalFarm,Winters,CA(US)
95 F101 Unknown Unknown
96 WC2 Freshwater ElSeñorial.SanJuan,PR
97 K11 Coffee Driedgreencoffee,PR
98 WC4 Soil ElSeñorial.SanJuan,PR
99 K13 Coffee Driedgreencoffee,PR
100 DO9 Soil NewDehli,India
101 JF75 Orchard WolfskillExperimentalFarm,Winters,CA(US)
102 F161 Unknown Unknown
103 F168 Unknown Unknown
104 VI67 Seawater ViequesIsland,PR
105 655 Diseased Gorgonia tissue Escambrón,SanJuan,PR
106 JO57 Unknown Jobos,Salinas,PR
107 IK12 Seawater Icacos,Fajardo,PR
108 K4 Coffee Driedgreencoffee,PR
109 K20 Coffee Driedgreencoffee,PR
110 DO1 Soil NewDelhi,India
111 DO2 Soil NewDelhi,India
112 DO3 Soil NewDelhi,India
113 DO8 Soil NewDelhi,India
114 MAT1 Microbialmat Salinas,CaboRojoPR
115 CB6 Seawater Culebrita,PR
116 CB9 Seawater Culebrita,PR
117 LP1 Seawater LuisPeña,Culebra,PR
118 MO1 Seawater MonaIsland,PR
119 VI2 Seawater ViequesIsland,PR
120 JO35 Healthy Gorgonia tissue Jobos,Salinas,PR
121 LP7 Healthy Gorgonia tissue LuisPeña,Culebra,PR
122 IK5 Seawater Icacos,Fajardo,PR
123 IK20 Seawater Icacos,Fajardo,PR
124 MO4 Seawater MonaIsland,PR
125 CB14 Seawater Culebrita,PR
126 LP12 Seawater LuisPeña,Culebra,PR
127 DI6 Seawater CayoDiablo,Fajardo,PR
128 IP57 Diseased Gorgonia tissue PiñerosIsland,PR
129 ESC7L Diseased Gorgonia tissue Escambrón,SanJuan,PR
130 JO42 Diseased Gorgonia tissue Jobos,Salinas,PR
131 JO54 Diseased Gorgonia tissue Jobos,Salinas,PR
132 HU7 Diseased Gorgonia tissue Humacao,PR
133 LP3-5L Diseased Gorgonia tissue LuisPeña,Culebra,PR
134 CB32 Healthy Gorgonia tissue Culebrita,PR
135 ESC1 Healthy Gorgonia tissue Escambrón,SanJuan,PR
136 CSJ2 Healthy Gorgonia tissue CabezasdeSanJuan,Fajardo,PR
137 CRO3 Healthy Gorgonia tissue Croabas,PR
138 VI3 Healthy Gorgonia tissue ViequesIsland,PR
139 ESC2 Healthy Gorgonia tissue Escambrón,SanJuan,PR
140 ESC12 Healthy Gorgonia tissue Escambrón,SanJuan,PR
141 LP5 Healthy Gorgonia tissue LuisPeña,Culebra,PR
142 AN6 Soil MonaIsland,PR
143 AN2 Soil MonaIsland,PR
144 K16 Coffee Unknown
145 F29 Airborne Wolfskill,ExperimentalOrchard,Winters,CA(US)
146 F34 Soil Wolfskill,ExperimentalOrchard,Winters,CA(US)
147 GRAC Algae Unknown
148 SAF10 Soil Nigeria,Africa
149 SAF9 Soil Nigeria,Africa
150 SAF25 Soil Nigeria,Africa
151 ESC22 Healthy Gorgonia tissue Escambrón,SanJuan,PR
152 CNPMA Corn Herrera,Laarena-Chitré,Panamá
153 CDPMA Airborne Herrera,Chitré,Panamá
154 FPMA Food Panamá,Panamá
155 ABPMA1 Airborne Herrera,Chitré,Panamá
156 ABPMA2 Airborne Herrera,Chitré,Panamá
157 ABPMA3 Airborne Herrera,Chitré,Panamá
158 SPMA1 Soil Herrera,Chitré,Panamá
159 SPMA2 Soil Herrera,Chitré,Panamá
160 DO12 Tortilla NewDehli,India
161 DO13 Tortilla NewDehli,India
162 H2 Airborne ToaAlta,PR
163 H3 Airborne ToaAlta,PR
164 H4 Airborne ToaAlta,PR
165 H5 Airborne ToaAlta,PR
166 H6 Airborne ToaAlta,PR
167 W1 Wullschlaegelia aphylla ElVerde,RioGrande,PR
168 W2 Wullschlaegelia aphylla ElVerde,RioGrande,PR
169 P1 Soil LaPampa,Argentina
170 P2 Soil LaPampa,Argentina
171 P3 Soil LaPampa,Argentina
172 P4 Soil
173 P8 Soil
174 P9 Soil
175 I1 Soil
176 I3 Soil
177 I8 Soil
LaPampa,Argentina
LaPampa,Argentina
LaPampa,Argentina
Iguazo,Argentina
Iguazo,Argentina
Iguazo,Argentina
178 K12 Coffee PuertoRico
179 MOA Soil
180 MOB Soil
181 MOC Soil
182 MOD Soil
183 MOE Soil
184 MOF Soil
185 MOG Soil
MonaIsland,PR
MonaIsland,PR
MonaIsland,PR
MonaIsland,PR
MonaIsland,PR
MonaIsland,PR
MonaIsland,PR
186 MYA873 Clinical Baracaldo,Spain
187 MYA1758 Clinical Pennsylvania,US
188 MYA3631 Clinical UnitedStates
189 ATCC24133 Clinical NewZealand
190 42wt Cottonseed Yuma,Arizona,US
191 13stockwt Cottonseed Yuma,Arizona,US
192 70stockwt Soil Yuma,Arizona,US
193 11611wt Peanut Nigeria,Africa
194 139M Clinical Hosp.InfantilFedericoGomez,Dist.Federal,Méx
195 30M Clinical Hosp.InfantilFedericoGomez,Dist.Federal,Méx
196 317M Clinical Hosp.InfantilFedericoGomez,Dist.Federal,Méx
197 654M Clinical Hosp.InfantilFedericoGomez,Dist.Federal,Méx
198 250M Clinical Hosp.InfantilFedericoGomez,Dist.Federal,Méx
199 347M Clinical Hosp.InfantilFedericoGomez,Dist.Federal,Méx
200 661M Clinical Hosp.InfantilFedericoGomez,Dist.Federal,Méx
201 631M Clinical Hosp.InfantilFedericoGomez,Dist.Federal,Méx
202 26010wt Unknown YumaArizona,US
Table 2. Distribution of mating type idiomorphs MAT1-1 and MAT1-2 among isolates of A. flavus from various substrates and habitats. The last column (*) shows significance of differences from a pooled sample of all other isolates (Fisher’s exact test).
Table 3. Distribution of mating type idiomorphs MAT1-1 and MAT1-2 among isolates of A. flavus from various geographic locations. The last column shows no significance of differences from a pooled sample of all isolates (Fisher’s exact test).
Table 4. Testing hypotheses of monophyly among isolates of A. flavus from various substrates and type of habitats. Minimum lengths of constraint trees were compared with the most parsimonious tree (MPT) using Templeton tests in PAUP. If the constraint tree was significantly longer than the MPT (4637 steps) and P<0.05, the hypothesis of monophyly was rejected.
Hypotheses (constrainttree)
Z(Typeofdistribution),andP(Probabilityvalue);X=differencebetween#ofStepslongerthanMPTandscoreof besttree(s)found.
Figure 1: Relationships among Aspergillus flavus isolates from different substrates and habitats. The unrooted neighbor-joining tree is based on 460 AFLP characters. Bootstrap values >80 % are shown in red. The S strains are shown in square dot. Marine isolates are exposed in blue and green branches. Tree length = 4637, CI=0.0992, RI=0.5866.
Figure 2: Relationships among Aspergillus flavus isolates from various countries of origin. The unrooted neighbor-joining tree is based on 460 AFLP characters. Bootstrap values >80 % are shown in red. Tree length = 4637, CI=0.0992, RI=0.5866.
Figure 3: Correlation between genetic distance vs geographical distance.
Chapter 3: Critical reinterpretation of aspergillosis disease of sea fans (Gorgonia ventalina)
Carlos Toledo-Hernández, Vladislav Gulis, Claudia Patricia Ruiz-Díaz, Alberto Sabat & Paul Bayman
ABSTRACT
Aspergillosis of sea fans (Gorgonia spp.) is one of the best-characterized coral diseases. The reported pathogen is the soil fungus Aspergillus sydowii. Characteristic signs are tissue necrosis, tissue purpling, and gall and tumor formation. However, recent findings have cast serious doubts about these facts. In this study, we (1) tested the capacity of A. sydowii to induce the characteristics signs of aspergillosis by inoculating tissue fragments of Gorgonia ventalina; (2) tested the transmissibility of aspergillosis by grafting diseased and healthy tissues onto healthy and diseased sea fans; (3) compared fungal biomass in healthy and diseased sea fan tissue; and (4) compared histology of healthy and diseasedtissue. We failed to induce the characteristic signs of aspergillosis when healthy sea fans were inoculated with A. sydowii. Physical contact with diseased tissue induced tissue purpling and necrosis in only one replicate out of 24. These results suggest that the presence of A. sydowii or other pathogens may not be sufficient to produce disease signs. Temporary purpling was frequently observed in the contact area between the grafts and hosts, suggesting that purpling is a generalized immune response and not an exclusive sign of aspergillosis. Histological examination revealed the presence of fungi in both healthy
and diseased tissue. However, ergosterol analyses showed that fungal biomass was higher in healthy than diseased tissue. Within diseased sea fans, fungal biomass was higher in healthy than diseased tissues. Fungi appear to be ubiquitous symbionts of sea fans. The lower concentration of ergosterol in diseased fans suggests that sea fans may suppress growth of all fungi in the affected area, whether beneficial or harmful. These results do not support the notion that sea fan aspergillosis is caused by A. sydowii or any single pathogen. The disease is more likely to be caused by interactions between environmental stress and a variety of opportunistic pathogens that may not necessarily be fungi. Thus, calling this disease aspergillosis is an over simplification at best. Instead, we propose it be called sea fan purpling syndrome.
INTRODUCTION
Coral diseases are on the rise. Since the first reported case of a coral disease three decades ago (Antonius 1973), at least 29 new coral diseases have been described worldwide, three-quarters of which are from the Caribbean (Weil et al 2004).
Three decades of studies have accumulated a wealth of information on the ecology of these diseases (Green & Bruckner 2000; Rosenberg & Ben-Haim 2002; Weil et al 2006). However, remarkably little is known about the etiology and pathology of the majority of them (Work et al 2006, 2008), and even less is known about the immune responses of corals to disease (Mydlarz et al 2008). Nonetheless, with the application of immunological, biochemical, histological and molecular biological techniques in recent years, our understanding of coral diseases has improved (Work et al. 2008).
The causes of some coral diseases may be more complex than originally thought. For instance, the literature on aspergillosis of sea fans (Gorgonia spp.) refers
to Aspergillus sydowii as the sole pathogen (Smith et al 1996; Geiser et al 1998; Rypien 2008). Our recent studies, however, suggest that A. sydowii is not the sole pathogen, and that a primary pathogen model is not congruent with what we now know about this disease (Toledo-Hernández et al 2007, 2008). Furthermore, it has been assumed that necrotized tissue purpled spots, galls and tumors in sea fans with the eventual exposure of the axial skeleton, are exclusive and characteristic signs of aspergillosis (Petes et al 2003; Smith & Weil 2004; Mullen et al 2006), even though interactions with other organisms may induce similar signs as well (Morse et al. 1981). Nevertheless, many authors continue to use the term 'aspergillosis' referring to this disease.
In this study, we repeat the inoculation experiments that were originally done when A. sydowii was proposed as the pathogenof sea fans (Smith et al 1996, Geiser et al 1998). We also repeat grafting experiments used to postulate the transmissibility of aspergillosis from a diseased to a healthy sea fan (Smith et al 1996). In addition, we compare levels of fungal colonization in healthy and diseased tissue by two methods: microscopy and quanitfication of ergosterol (a fungal membrane sterol).
Given the reports of high success of A. sydowii in inducing disease signs in healthy sea fans (Smith et al 1996; Dube et al 2003), we hypothesized that all the inoculation trials in this study would induce disease signs in healthy sea fans. Similarly, given the high transmissibility reported for aspergillosis (Kim and Harvell, Ecology, Smith and Weill 2004), we expected that most of the diseased tissue grafts would induce manifestation of aspergillosis in their host colonies (i.e. tissue purpling followed by tissue necrosis and eventual exposure of the axial skeleton). Due to the ubiquitous nature of fungi in sea fans (Koh et al. 2007, Toledo-Hernández et al. 2008), we
expected to detect fungi in both healthy and diseased sea fans; however, because of increased presence of the pathogen, fungal biomass should be higher in diseased fans.
METHODS
Pure culture inoculation experiment in aquaria
In February 2008, eight healthy G. ventalina colonies (i.e. no lesions, purpling, or any overgrowth by fouling organisms) between 600 and 900 cm2 were collected at Escambrón beach, San Juan, Puerto Rico and brought to the University of Puerto Rico, Río Piedras. In the laboratory, each colony was placed in individual 72.5 L tanks filled with fresh sea water; and checked for the presence of A. sydowii by collecting and culturing one 1cm2 tissue sample from each colony following the methodology of Toledo-Hernandez et al. (2007). Once fungi started to grow from the tissue samples, morphological characteristics were used to identify them (Klich 2002). Only 1 of the 8 colonies was found positive for A. sydowii, and this colony was excluded from the experiment. Six days after establishment in tanks, moments before the inoculation of the sea fans with A. sydowii, one tissue wound was inflicted on each colony by scraping tissue with a scalpel down to the axial skeleton. Wounding was intended to enhance contact between fungal hyphae and internal tissue. Wounds were approximately 2 cm2 and adjacent to the area previously sampled. Experimental fans were inoculated with A. sydowii strain isolated from a sea fan and identified by sequencing the nuclear ribosomal ITS region (GenBank # EU554604, Toledo-Hernandez et al. 2008). Inoculation was conducted by attaching a 2x2 cm block of glucose peptone yeast extract agar (GPYA) overgrown with mycelium to the wounded area. Approximately
100,000 spores of A. sydowii were placed on the agar blocks 5 days before the start of the experiment and incubated at 28o C to allow hyphal growth but not sporulation. As a control, one 2x2 cm GPYA block without A. sydowii was also attached to each fan 2-4 cm from the experimental block. Visual examinations were conducted daily for the next 7 days after which agar blocks were removed. Then, tissue samples (1 cm2 each) were collected from the experimental and control areas of each fan to check for the presence of A. sydowii. Sea fans were monitored for any signs of tissue necrosis and purpling for an additional month.
Horizontal transmission experiment in the field
Previous studies have successfully induced disease in healthy Gorgonia colonies by grafting pieces of diseased tissue (Smith et al. 1996, Smith and Weil 2004). These studies were intended to establish transmissibility of A. sydowii. However, these studies did not consider host immune responses to grafts of nonself tissue, which may resemble characteristic signs of aspergillosis. Also, it is likely that pathogens other than A. sydowii can cause the signs attributed to aspergillosis (Toledo-Hernández et al. 2008), and other pathogens may have been transmitted by the diseased tissue used for grafting.
To test the horizontal transmission of any and all pathogens in diseased sea fan tissue to healthy tissue, two experiments were conducted at El Escambrón beach, Puerto Rico. The first experiment used diseased allograft (nonself) tissue to induce disease in healthy sea fan hosts, while the second experiment used diseased isograft (self) tissue to induce disease in healthy areas of diseased hosts. In both experiments, host colonies ranged in size from 800 to 1200 cm2 and were located within an area of
approx. 50 x 10 m at a depth of 1.5m. Tissue fragments were approximately 4 cm2 and were attached to the host colonies using 10 x 0.2 cm cable ties. In both experiments, visual examination and pictures of each experimental colony were taken every 3 to 7 days for 6 weeks.
For the first experiment, 20 healthy G. ventalina colonies were tagged in May 2008. Two allograft tissue fragments from a nearby diseased colony were attached to each host colony. One of those fragments was diseased (i.e. diseased from a diseased colony) and the other (as a control) was healthy. As an additional control, a third tissue fragment, this time an isograft from the host colony itself, was cut and re-attached adjacent to its original position.
The second experiment used isograft tissue to induce disease in healthy areas of already diseased colonies. In this experiment, 9 diseased G. ventalina colonies were tagged. To compare the reaction of host colonies to diseased and healthy isograft tissue, 2 tissue fragments (one diseased isograft and one healthy isograft) were attached to the same colony. As a control, a third tissue fragment from a nearby healthy colony was attached to a healthy area of each host colony.
Histological examination
Histology was used to compare location of fungal colonization of diseased and healthy sea fan tissue. Twenty tissue fragments of approx. 1 cm2 from 10 G. ventalina colonies were collected at Escambrón beach and Las Cabezas de San Juan, Fajardo, Puerto Rico for histopathological examination. Eight of these fragments were healthy tissues from healthy colonies, 5 were healthy tissues from diseased colonies and 7 were diseased tissues from diseased colonies. Samples were individually stored in sterilized
containers and preserved in Karnowsky’s fixative for an hour. Subsequently, the samples were decalcified in EDTA, dehydrated in alcohols, infiltrated and embedded in paraffin, sliced to 5 µm and stained with Periodic Acid Schiff and Harris`s Hematoxylin and Eosin (Toledo-Hernández in prep). Light microscopic examination was conducted using a Nikon 800 wide field microscope equipped with Differential Interference
Contrast (DIC) and a Q-imaging Ritiga Exi camera with a color filter.
Ergosterol analysis
Ergosterol is a fungus-specific sterol found in plasma membranes, similar to cholesterol found in animals. Concentration of this biomarker can be used to estimate fungal biomass when mycelium and substrate cannot be separated (Gessner & Chauvet 1993). Several studies of sterols from corals did not report ergosterol from these animals, and our laboratory tests with a culture of Symbiodinium sp. showed that these algal symbionts also do not contain ergosterol. Therefore, all ergosterol in sea fans is presumed to be of fungal origin.
In this study, ergosterol analyses were used to compare fungal biomass among healthy tissues from healthy sea fans, healthy tissues from diseased fans and diseased tissues from diseased fans (). To do this, we collected 10 healthy tissue samples from healthy colonies, and 10 healthy and 9 diseased tissue samples from diseased colonies. Fifteen 2 cm2 tissue samples were collected in August 2008 at El Escambrón beach and 29 1cm2 samples were collected at Las Cabezas de San Juan, Fajardo, Puerto Rico in October 2008. The colonies were selected as described above. Tissue fragments were weighed, preserved in 15 ml methanol and shipped overnight on dry ice to Coastal Carolina University. Samples were extracted with alcoholic KOH, lipids were
partitioned into pentane, evaporated to dryness, reconstituted in methanol and filtered (Gulis and Suberkropp 2006). Ergosterol was quantified by HPLC (Shimadzu, Columbia, MD) equipped with a Whatman Partisphere C18 column and an ultraviolet detector set at 282 nm and compared with external ergosterol standards. To estimate dry mass was for each tissue sample, a tissue piece of the same size from the same colony was weighed, decalcified in EDTA and oven-dried to determine the dry weight:wet weight ratio. The differences in fungal biomass among tissues samples were estimated for each site by two-way ANOVA.
RESULTS
Pure culture inoculation experiment in aquaria
No aspergillosis lesions, purpling or tissue necrosis was observed in the experimental corals during the 7 day experimental period nor one month after. In addition, the experimentally inflicted wounds healed completely during the observation period. Aspergillus sydowii was isolated from all fans from underneath the experimental agar blocks (using the isolation technique described in Methods), indicating successful inoculation in all trials. No other fungi were isolated.
Inoculation experiment in the field
Healthy host. Allografts of both healthy and diseased tissue caused purpling by the fourth day (Figure x). However, between the 10th and 12th day, contact areas of all allografts fused to the host colony, and purpling disappeared by the 14th to 16th day. All isograft controls of healthy tissue fused within 4 days after grafting; neither tissue purpling or necrosis was observed in the contact area (Table 1). Aspergillosis lesions,
purpling or tissue necrosis were never observed on the hosts or grafts at any visit. And a visit to the study area almost one year after the conclusion of the experiment revealed that allografts of both diseased and healthy tissue remained fused to their host colonies with no aspergillosis signs. Isografts on diseased hosts
When diseased tissue was used as isografts on healthy areas of the colonies, one of six isografts induced purpling and necrosis by 11 days after grafting. Three of the six isografts induced purpling but then fused to the host colonies 12-16 days after grafting (Table 1). Two diseased tissue isografts never induced any reaction (no purpling, tissue necrosis or fusion) in the host colonies.
When healthy tissue was used as isografts on healthy areas of the colonies (as a control for the effects of colony manipulation), five of the isografts fused in the first 6 days (Table 1). Aspergillosis lesions, purpling and necrosis were not observed in the contact areas at any time. However, one healthy tissue isograft did not fuse, and induced purpling and necrosis 11 days after grafting.
Six allografts of healthy tissue from healthy colonies were placed on healthy areas of diseased colonies as a control for effects of self vs. nonself recognition. Of these, three fused to their host colonies inducing purpling that lasted 3 to 4 weeks. However, this purpling disappeared after 38 days. Two healthy allografts never induced tissue purpling or necrosis, or fused to their host colonies. One healthy tissue allograft control died and induced purpling in its host 6 days after grafting, but after 3 weeks the purpling disappeared (Table 1). Three of the nine colonies detached within the first two weeks, thus no data could be collected from them.
Histological examination
Fungal hyphae were observed in only 8 of the 20 tissue slice preparations. Of these 7 were healthy tissues, whereas one was from a diseased tissue. Fungal hyphae in healthy tissues were detected in cavities within the axial skeleton, whereas in the single diseased tissue, fungal hyphae were detected in cavities within the axial skeleton and the mesoglea (Figure 2A, B ). Identification of fungal hyphae could not be made; however, at least 3 hyphal morphologies were distinguished in a single diseased tissue slice (Figure 2C).
Ergosterol analysis. Ergosterol concentration in G. ventalina tissue ranged from 0.4 to 9.4 mg/g of dry mass, with an overall mean of 2.6 mg/g. Healthy tissue from healthy colonies exhibited the highest ergosterol concentration averaging 3.1 mg/g, followed by healthy tissue from diseased colonies with 2.4 mg/g and diseased tissue with 2.1 mg/g (Figure 3). These differences were statistically significant (ANOVA, F = 65.34, df =2, P < 0.001), contrary to our hypothesis that diseased tissue would be more heavily colonized by fungi.
DISCUSSION
Most of the studies conducted since the first report and description of aspergillosis (Nagelkerken et al. 1997) have assumed that necrotized tissue, purpling, galls and tumors were specific manifestations of fungal infection. Furthermore, most of the studies conducted after A. sydowii was identified as the causative agent of the disease (Smith et al. 1996 and Geiser et al. 1998, Dube et al 2003), assumed that this fungus was always the causative agent of aspergillosis throughout the Caribbean. Consequently, it
was assumed that tissue necrosis, purpling, galls and tumors in sea fans cannot occur without A. sydowii. The same assumption was made for other gorgonians (Smith and Weil 2004), and the presence of A. sydowii in sea fans and other gorgonians was considered sufficient to cause the disease.
The results of this study challenge many, if not all, of the above assumptions. Inoculating healthy colonies of G. ventalina with pure cultures of A. sydowii did not induce the disease, suggesting that this fungus is not always pathogenic to sea fans and that its presence may not be sufficient to cause this disease. Similar conclusions were made in a previous study, after failing to isolate A. sydowii from 81 diseased G. ventalina colonies (Toledo-Hernández et al 2008).
The discrepancies between our results and previous ones may have several explanations. It is possible that G. ventalina populations in Puerto Rico have developed resistance against A. sydowii. Previous studies have reported the ability of some corals to develop resistance against pathogens, e.g. Oculina patagonica may develop resistance against Vibrio shiloi (Rosenberg & Falkovitz 2004; Reshef et al 2006).
However, the mechanism of infection in case of aspergillosis, the immune reactions of sea fans against allogenic organisms, as well as the dynamics and roles of the microbial community of sea fans during the immunological response are poorly understood to conclusively accept this hypothesis.
Another explanation could be that the strain of A. sydowii used in this study was not pathogenic while those in Smith’s and Dube’s studies were. However, a recent phylogenetic study revealed that A. sydowii is an opportunistic pathogen and that genetically diverse isolates are capable of causing disease in corals (Rypien et al 2008).
Previous studies tested horizontal transmission of sea fan aspergillosis by placing allogenic (intraspecific) tissue fragments of diseased sea fans onto healthy sea fan colonies (Smith et al 1996). Horizontal transmission of aspergillosis was considered to occur after the grafted allogenic tissue fragments induced purpling and tissue necrosis to the host colonies. However, these experiments did not consider the capacity for allogenic recognition followed by incompatibility reactions typical of metazoans (Salter-Cid & Bigger 1991). Consequently, it is not clear whether the observed results were truly the immune response of the host colony against pathogenic agent(s) or if it was an immune response against the foreign tissue.
In an attempt to distinguish between these two types of immune responses, we attached healthy and diseased tissue fragments from diseased colonies (allografts) to healthy colonies. Similarly, we cut healthy and diseased tissue fragments from diseased colonies and attached them back to the same colonies (isografts). The results were surprising. Evidence for transmissibility from diseased to healthy colonies was rarely observed. In fact, in the majority of cases diseased tissue did not induce lesions on their host, demonstrating that physical contact is not always sufficient to induce the disease. Alternatively, this may suggest that symptoms are not always caused by an infectious agent, e.g. A. sydowii. However, the fact that on two occasions disease symptoms were induced after grafting healthy or diseased isografts to a diseased host indicates that some lesions are caused by an infectious agent. It also suggests that the infectious agent is spread all over the colony and that the physical contact between a healthy colony and any part of a diseased colony, either healthy or diseased, may be sufficient to transmit the pathogenic agent. On the other hand, tissue purpling was a common
reaction between grafts and hosts regardless of the end results, i.e. tissue necrosis or fusion. Purpling have also been reported to be induced by thermal stress (Mydlarz et al 2008). Since purpling appears to be a generalized immunological or physiological response of sea fans, it cannot be used to diagnose aspergillosis.
In this study, microscopic examinations showed that fungal hyphae in healthy tissues were exclusively detected in skeletal cavities, while in diseased tissue, fungi were observed in the soft tissue as well as in the skeleton. Contrary to our expectations, fungal hyphae were detected in a single sample of diseased tissue only. Since fungi were not always detectable in diseased tissues, it implies that fungi are not always the etiological agents of the disease.
The ergosterol analyses clearly showed that fungi were always present in sea fans. However, fungal biomass was higher in healthy fans, followed by healthy tissues from diseased fans, and it was the lowest in diseased tissues. This also suggests that fungal biomass in the entire sea fan colony may be affected even when a small portion of the colony is diseased. These results are in line with a previous study based on molecular approach for fungal identification that reported lower fungal diversity and species richness in healthy tissues from diseased fans when compared to healthy fans (Toledo-Hernández et al. 2008). Thus, lower fungal biomass, diversity, and species richness in diseased than healthy colonies suggests that sea fans' immune response is generalized, killing or suppressing all fungi in the affected and adjacent area, whether those fungi are beneficial or harmful. Alternatively, these results may suggest that infectious agent(s) (bacteria, protozoa, etc.) are outcompeting other microorganisms, including fungi, that are normally found in sea fans.
Conclusions. We found that (1) A. sydowii was not pathogenic to sea fans or at least was not sufficient to induce disease symptoms in healthy sea fans; (2) physical contact between diseased and healthy fans was also not sufficient to induce the disease in the majority of cases; (3) purpling is a generalized immunological or physiological response in sea fans; and (4) fungi are found in healthy and diseased fans, but fungal abundance is lower in diseased tissues. It is tempting to conclude that A. sydowii is not the etiological agent causing aspergillosis in sea fans. However, we cannot completely rule out this possibility because fungi are indeed present in diseased sea fans. Unfortunately, little is known about the roles of microorganisms in corals, the dynamics of microbial communities, the immune responses of their hosts and interactions among microbes, hosts and the environment. Therefore, molecular-based approaches susch as direct DNA extraction, amplification with fungi-specific primers, DGGE, T-RFLP or cloning and sequencing may help to better understand this syndrome. Given these uncertainties, calling this disease aspergillosis might be inappropriate. Until the pathogen(s) are identified, we propose the name “purpling response syndrome”, which describes the immune reaction of the host as opposed to the hypothetical pathogen that causes the disease.
LITERATURE CITED
Alker AP, Dube D, Harvell CD, Kim K (2004) Localized induction of a generalized response against multiple biotic agents in Caribbean sea fan corals Coral Reefs 23:397405
Ananda K., Sridhar K., Raviraja N., Bärlocher F. (2008) Breakdown of fresh and dried Rhizophora mucronata leaves in a mangrove of Southwest India. Wetlands Ecol Manage 16:1-9
Antonious A. (1973) New observations in coral destruction in reefs. Assoc MarLab Caribb Abstr 10:3-?
Dube D, Kim K, Alker AP, Harvell CD (2002) Size structure and geographicvariation in chemical resistance of sea fan corals Gorgonia ventalina to fungal pathogen. Mar Ecol Prog Ser 231:139-150
Green EP, Bruckner AW (2000) The significance of coral disease epizootiologyfor coral reef conservation. Biol Conserv 96:347-361
Geiser DM, Taylor JW, Richie KB, Smith GW (1998) Cause of sea fan death in the West Indies. Nature 394:137-138
Gessner MO, Chauvet E (1993) Ergosterol to biomass conversion factor for aquatic hyphomycetes. Appl Environ Microbiol 59:502-507
Gessner MO, Gulis V, Kuehn KA, Chauvet E., Suberkropp K. (2007) Fungal decomposers of plant litter in aquatic ecosystems. In: Kubicek C.P. & Druzhinina I.S. (Eds.) The Mycota, Vol. IV, Environmental and Microbial Relationships. 2nd edn., pp. 301-324. Springer, Berlin.
Gulis V, Suberkropp K (2006) Fungi: biomass, production and sporulation ofaquatichyphomycetes. In: Hauer F.R. & Lamberti G.A. (Eds.) Methods in stream ecology, 2nd edn., Academic Press, San Diego, CA p. 311-325
Gunatilaka AAL, Gopichand Y, Schmitz FJ, Djerassi C. 1981. Minor and trace sterols in marine invertebrates. 26. Isolation and structure elucidation of nine new 5α,8α-epidioxy sterols from four marine organisms. J Org Chem 46:3860-3866.
Jolles AE, Sulllivan P, Alker AP, Harvell CD (2002) Disease transmission of aspergillosis in sea fans: Inferring precess from spatial pattern. Ecology 83:2373-2378
Kim K, Harvell CD (2004) The rise and fall of a six-year coral-fungal epizootic. Am Nat 164:S52-63.
Klich MA (2002) Identificationof common Aspergillus species. Centraal bureau voor Schimmelcultures, Utrecht, the Netherlands
Morse DE, Morse A, Duncan H, Trench RK (1981) Algal tumors in the Caribbean Octocorallian gorgonian ventalina. Bull Mar Sci 32:399-409
Mullen KM, Harvell CD, Alker AP, Dube D, Jordán-Dahlgren E, Ward JR, PetesLE 2006. Host range and resistance to aspergillosis in three sea fans species from the Yucatan Mar Biol 149:1355-1365
Mydlarz LD, Holthouse SF, Peters EC, Harvell CD 2008. Cellular responses insea fan corals: Granular amoebocytes react to pathogen and climate stressors. PlosOne 3:1-9
Nagelkerken IK, Smith GW, Bonair K, Bush P, Garzón-Ferriera J, Botero L,Gayle P, Harvell CD,Heberer C, Kim K, Petrovic C, Pors L, Yoshioka P (1997) Widespread disease in Caribbean sea fans: II. Patterns of infection and tissueLoss. Mar Ecol Prog Ser 160:255-263
Petes LE, Harvell CD, Peters EC, Webb MAH, Mullen KM 2003.
PathogensCompromise reproduction and induce melanization in Caribbean sea fans. Mar Ecol Prog Ser 264:167-171
Reshef L, Koren O, Loya Y, Zilber-Rosenberg I, Rosenberg E 2006. The coral probiotic hyphothesis. Environ Microbiol 8:2038-2073
Rosnberg E, Falkowitz L 2004. The Vibrio shiloi/Oculina patagonica model system of coral bleaching. In Annual Review of Microbiology, Vol. 58. Ornston, L.N. Balows, A., and Gottesman S (eds). Palo Alto, CA: Annual Reviews, pp 143-159
Rypien KL, Andras JP, Harvell CD 2008. Globally panmictic population structure in the opportunistic fungal Aspergillus sydowii. Mol Ecol 17:4068-4078
Salter-Cid L & Bigger CH 1991. Alloimmunity in the Gorgonian coral Swiftia exserta. Biol Bull 181:127-134
Shinn EA, Smith GW, Propero JM, Betzer P, Hayes ML, Garrison V, Barber RT2000. African dust and the demises of Caribbean coral reefs. Geophysical Research Latters 27:3029-3032
Smith GW, Ives LD, Nagelkerken IA, Ritchie KB 1996. Caribbean sea fan mortalities.Nature 383:487
Smith GW, Weil E 2004. Aspergillosis in gorgonians. In: Rosenberg E, Loya Y (eds) coral healthy and diseases. Springer-Verlag, Berlin p 279-288
Toledo-Hernández C, Bones-González A, Ortiz-Vázquez O, Sabat AM, Bayman P 2007.Fungi in the sea fan Gorgona ventalina: diversity and sampling strategies. Coral Reefs 26:725-730
Toledo-Hernández C, Zuluaga-Montero A, Bones-González A, Rodríguez J, Sabat AM, Bayman P. 2008. Fungi in healthy and diseased sea fans (Gorgonia ventalina): is Aspergillus sydowii always the pathogen? Coral Reefs 27:707-714
Weil E. 2004. Coral reef diseases in the wider Caribbean. In: Rosenberg E, LoyaY (eds Coral healthy and disease. Springer-Verlag, Berlin, p 35-68Weil E, Smith G, GilAgudelo DL. 2006. Status and progress in coral reef disease sesearch. Dis Aquat Org 69:1-7
Work TM & Aeby GS. 2006. Systematically describing gross lesion in corals. Dis Aquat Org 70:155-160
Work TM, Richardson LL, Reynolds TL, Willis B 2008. Biomedical and veterinary science can increase ourunderstanding of coral disease. J Exp Mar Biol Ecol 262:63-70
Yu SJ, Deng ZW, van Ofwegen L, Proksch P, Lin WH. 2006. 5,8-epidioxysterols and related derivatives from a Chinese soft coral Sinularia flexibilis. Steroids 71:955-959.
Table 1. Immune responses between grafted tissues and healthy or diseased Gorgonia ventalina hosts at El Escambrón, Puerto Rico.

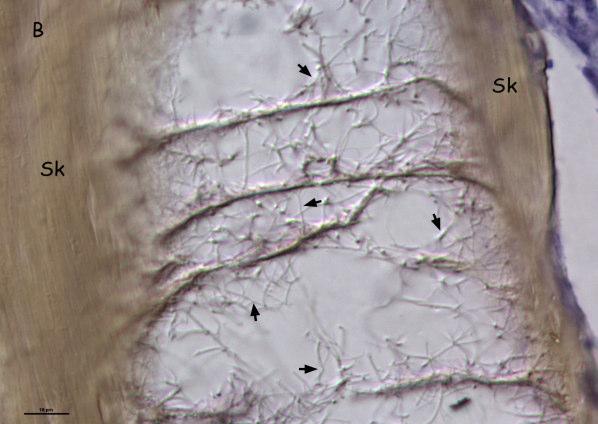
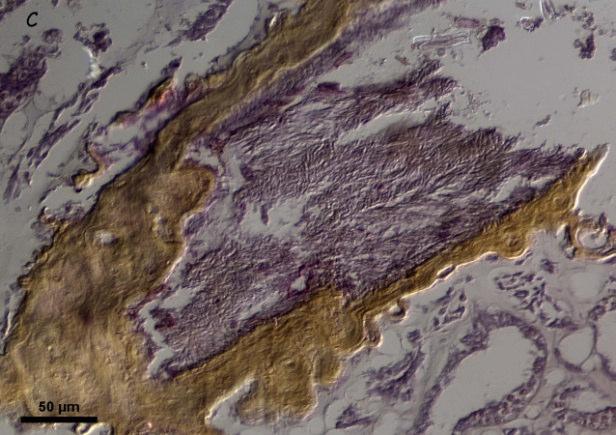
Figure 2. Light micrographs of colony sections showing (A) healthy tissues with hyphae in skeletal cavity, (B) and (C) skeletal cavity of healthy and diseased Gorgonia ventalina colonies, respectively, with fungal hyphae indicated by arrows. Abbreviations are as follows: Amoebocytes (Amb); External epithellium (Ext-Ep); Gastrodermis (Gsds); Gastrodermal channel (Gst Chnl); Mesoglea (Msgl); Sclerited (Scl); Skeleton (Sk); Zooxtanthellae (Zx).
Figure 3. Fungal biomass in healthy (HH) and diseased (HD and DD) Gorgonia ventalina colonies. Means ± 1 SE are shown, n=8-9.
Chapter 4:
Dynamics and Structure of Sea Fan Populations Impacted by Disease
Alberto M. Sabat1, Carlos Toledo-Hernández2 and Anabella Zuluaga-Montero3
Department of Biology, University of Puerto Rico, San Juan, Puerto Rico 00931
1 – E-mail: amsabat@gmail.com
2- E-mail: c_toledo_hernandez@yahoo.com
3- E-mail: abzuluaga@gmail.com
Running head: Modeling the effects of disease on sea fans
Keywords: Sea fan aspergillosis, Gorgonia ventalina, sea fan demography, matrix model, coral diseases, population viability
Abstract
Diseases are emerging as a serious threat to the viability of wild populations. In this study, we analyze the effect of disease incidence and recruitment on the population dynamics and structure of the sea fan Gorgonia ventalina using a matrix model parameterized with data from field studies. Time invariant incidence above 6% will lead to local extinction of sea fans even under high levels recruitment. The viability of a healthy population impacted by an epizootic would not be particularly compromised except at localities with low recruitment or with an initial disease incidence of 90%. Disease prevalence is positively related to incidence; but unexpectedly negatively related to colony recruitment. Prevalence also varied with colony size. Small diseased colonies are rare as result of higher mortality. The model explained 71% of the observed variation in the proportion of healthy and diseased G. ventalina colonies of the three size classes at five localities. We conclude that under current levels of incidence and virulence wide-scale mass mortality events are not expected. However, an epizootic with initial incidence levels of about 90% or higher will cause mass mortalities and lead to widespread local extinctions particularly in localities with low recruitment.
Introduction
Diseases are emerging as a serious threat to the viability of a disquieting number of populations of wild animals. Two particularly dramatic examples of this phenomenon are that of honey bees (Oldroyd 2007; vanEngeldorps et al. 2008) and of amphibians (Lips et al. 2006). There is debate concerning the etiology of these diseases and even if the rise is real, but the consensus is that indeed it is real and that climate change has highly involved with it. (Dobson 2009; Harvell et al. 2009; Lafferty 2009; Ostfeld 2009; Pascual and Bouma 2009;Randolph 2009).
Corals are not immune to this threat (Lafferty et al. 2004; Weil 2004; Sokolow 2009).
Just in the Caribbean, at least 20 coral diseases have been reported to affect no less than 55 scleractinian and octocoral species, some of which have suffered severe population declines attributable to diseases (Richardson and Voss 2005; Croquer and Weil 2009). The increasing contribution of diseases to the worldwide loss of corals has been credited to ocean warming and eutrophication (Bruno et al. 2003; 2007; Selig et al. 2006).
The sea fan Gorgonia ventalina - one of the most abundant octocorals in the Caribbeanis affected by a disease that has been attributed to the soil fungus Aspergillus sydowii (Smith et al. 1996; Geiser et al. 1998; however see Toledo-Hernández et al. 2008 and Zuluaga-Montero et al. 2010). Massive die-offs of sea fans that occurred in the Caribbean in the 1980s and early 1990s (Guzmán & Cortés 1984, Garzón-Ferreira & Zea 1992) have been attributed to this disease (Smith et al. 1996, Nagelkerken et al. 1997), although more recent studies indicate lower levels of prevalence and virulence (Kim and Harvell 2004; Kim et al. 2006; Toledo-Hernández et al. 2009).
In this study, we analyze the effect of disease incidence and recruitment on the population dynamics and structure of the sea fan G. ventalina using a stage-based matrix model (Caswell 2001). We use the model to predict (1) the asymptotic relationship between disease incidence and population growth rate, (2) the number of sea fan recruits that an area would need to receive to keep a local population at equilibrium under increasing disease incidence, and (3) the effect of disease on population structure, including disease prevalence. We also simulate the transient behavior of a healthy sea fan population subjected to epizootic events, and compare the observed and expected proportions of diseased fans in 5 sites that differ in disease incidence and recruitment.
Model
The sea fan population was divided into three size classes based on surface area - small (<500 cm 2), medium (500 ≤ x ≤ 1,000cm2) and large (>1,000cm2) - and two health states (healthy and diseased) for a total of six states. The number of colonies in each of the life cycle stages at t+1 (one year) equals (1)
The contribution of each life cycle stage at time t to all others at t+1 is contained in the 6 x 6 matrix that projects the population vector between t and t+1. P1, P2 and P3 are the probabilities of small, medium and large colonies, respectively, of remaining alive in the same
size class for one year. The subscripts hh, hd, dh and dd signify, respectively, the probability of a healthy colony remaining healthy, of a healthy acquiring disease, of a diseased gaining health, and of a diseased remaining diseased. Thus, P1hh is the probability that a small healthy colony will remain small and healthy (i.e. will survive, but will not grow nor get infected). The Gs represent growth transitions. For example, G2hd is the probability that a medium healthy colony will grow into a large colony and get infected. The Rs are size retrogressions (i.e. become smaller by losing tissue either through disease-caused necrosis or by physical breakage or abrasion), and the Fs are the production of new colonies through sexual reproduction. Notice that (1) diseased colonies do not reproduce (Petes et al. 2003), (2) medium healthy colonies contribute to small healthy ones via sexual reproduction (F2) and via retrogression (R2hh), and (3) small diseased colonies do not grow into medium-sized ones (Toledo-Hernández et al. 2009). We also assume that recruitment of diseased colonies does not occur (i.e. colonies are infected only after settlement).
If σ is annual survivorship, αprobability of infection, βprobability of healing, γ probability of growth, and ρ the probability of retrogression then: Ghh = σγ(1-α) Rhh = σρ(1-α)
= σ(1-γ)(1-α)(1-ρ)
Ghd = σγα Rhd = σρα
= σα(1-γ)(1-ρ) Gdh = σγβ Rdh = σρβ
Gdd = σγ(1-β) Rdd = σρ(1-β)
= σβ(1-γ)(1-ρ)
= σ(1-γ)(1-β)(1-ρ).
For the time invariant asymptotic analysis we calculated the real dominant eigen value of the projection matrix and its corresponding left and right eigen vectors to obtain the asymptotic growth rate of the population (λ), the stable stage distribution (w), and the reproductive value of
each stage (v) (Caswell 2001). For the time varying transient analysis, we iterated equation 1 and calculated λ as Nt+1/Nt, where N is the sum of the population vector.
Parameterization
We estimated the above parameters using data of a field study that measured survivorship, growth and health state transitions in 119 colonies of G. ventalina for one year (ToledoHernández et al. 2009). The study indicates a significant main effect of colony size and a significant interaction between colony size and health state on survivorship. Survivorship increased with size, while disease significantly reduced survivorship but only in small-sized colonies. We used the mean estimates of gain (for γ) or loss (for ρ) of live tissue area for each of the six size-health states to calculate size class transitions by taking the reciprocal of the amount of years it took to either gain or lose 500 cm2. Two out of 72 healthy colonies (0.0278) acquired disease during the study period, and one out of 21 diseased colonies (0.0476) lost all visual signs of disease (Toledo-Hernández et al. 2009). We used these as our initial estimates of αand β , respectively. Values of σ,α,β,γ,and ρ for the three size classes and two health states, and the corresponding life cycle transitions can be found in Table 1.
To estimate recruitment through sexual reproduction (F) it would be necessary to measure not only gamete production as a function of colony size, but also probability of fertilization, survival during the pelagic stage, and probability of successful settlement. These estimates, particularly the latter two, remain elusive (to the best of our knowledge) not only for G. ventalina, but for any marine organism with a pelagic dispersal phase. In lieu, we used the stock-recruitment data for G. ventalina in Yoshioka (1996) as our estimates of F2 and F3.
Yoshioka (1996) measured recruitment of gorgonians as a function of adult abundance for a
period of eight years in two localities in Puerto Rico. His data indicates a peak recruitment event of approximately 1.5 G. ventalina recruits per adult G. ventalina, followed by a sevenfold reduction to baseline levels the following years. We use this estimate of peak recruitment (1.5) and 1/7 this amount (0.2143) as the range of possible values that F2+F3 can attain, assuming that large colonies have twice the contribution of medium ones towards total recruitment.
Results
Asymptotic Analysis
Asymptotic growth rate of sea fan populations (λ) declines exponentially with increasing disease incidence (fig. 1a). The lambdas of the high and low recruitment trajectories approach a value of 0.82 as incidence increases to 100%. For localities with high recruitment, an incidence above 6% will result in population decline (λ<1). Localities with low recruitment will exhibit population decline even with no disease incidence, but the effect of increasing incidence on λis less severe than on localities that receive more recruits (fig. 1a). Within the range of recruitment considered, sea fan populations - even in localities with severe levels of incidence - should not decline at a rate higher than about 18% annually.
How many recruits would a local sea fan population need to receive in order to remain viable (λ>1) for different incidence levels? We answered this question by determining the level of recruitment (F2+F3) necessary for a population to be at equilibrium (λ=1) for levels of incidence between 0 and 50%. The number of recruits required for equilibrium increases exponentially with incidence (fig. 1b). For example, a local population with a disease incidence of 30% would require more than 5 recruits per standing adult to remain viable.
The stable stage vector (w) was calculated for three levels of incidence (0, 0.1, and 0.3) and for high (F2+F3=1. 5) and low (F2+F3=0.2143) recruitment (fig. 2a and b). As expected, the relative abundance of diseased colonies of the three size classes (= prevalence) increases, and that of healthy ones decreases with incidence. Interestingly, disease prevalence is always higher in medium and large colonies than in small ones, particularly at higher incidences. Recruitment also has an interesting effect on disease prevalence. Prevalence is higher in populations with low recruitment – particularly in medium and large colonies– than in populations with higher recruitment. This becomes more evident comparing total prevalence (wSd+wMd+wLd) between high and low recruitment as a function of incidence (fig. 3).
Transient Analysis
The above analyses consider the effects of disease under steady state conditions. In order to simulate the dynamics of a healthy sea fan population experiencing an epizootic, equation 1 was iterated twenty five times (= years) under three scenarios – a growing population (recruitment = 1.5), a declining population (recruitment = 0.2143), and a population at equilibrium (recruitment = 0.9525, see fig. 1b). Initial conditions (t = 0) for each scenario assumed a population of 1,000 colonies with a stable stage distribution corresponding to no incidence (α=0) and the corresponding level of recruitment.
There is no information about incidence values during the epizootic(s) that impacted sea fan populations in the Caribbean 20 to 30 yrs ago. We simulated a weak (α= 0.3), a moderate (α= 0.6) and a strong (α= 0.9) epizootic but only present the results of the moderate case in detail. The dynamics of colony abundance of the weak and strong epizootics are presented in appendix A. The epizootics were assumed to have a duration of three years with peak incidence
occurring only during the first year; declining by half each year during years 2 and 3, reaching and remaining at zero by year 4.
The qualitative effect of such an epizootic on the population growth rate of sea fans was very similar for the three recruitment scenarios. Population growth rate declined precipitously for the three years during which α>0, then rose sharply until attaining the growth rate prior to the epizootic, taking longer the lower the recruitment (figs. 4a, d, g). The effect on colony abundance was unique to each scenario. For the population at equilibrium (fig. 4e) the epizootic decreased the number of healthy colonies to about 35% of its initial size by year 4, then colony abundance rose slightly until reaching equilibrium again at about t=15. For the population with high recruitment (fig. 4b), the epizootic decreased the number of healthy colonies to about 45% of the initial number by year 5, and attained its initial size by year 19. For the population with low recruitment the epizootic was catastrophic, reducing the number of healthy colonies to about 22% of its initial size by year 5 (fig. 4h), and continuing to decline, more slowly, to about 5% of its initial size by t=25.
Disease prevalence for the population at equilibrium, peaked for each size class at between 20% and 17% between years 2 and 4 (first for the small colonies and last for the medium ones); then declined exponentially, more so in the small colonies, until becoming almost imperceptible for the three size classes after year 20 (fig. 4f). Prevalence dynamics for the population with high (fig.4c) and low (fig.4i) recruitment resemble that of the population at equilibrium except that for the high recruitment scenario, peak prevalence is much higher in small colonies than in medium or large ones, and for the low recruitment scenario the reverse is true. Nonetheless, for the three scenarios small diseased colonies are less abundant than either
medium or large ones, particularly in localities with low and intermediate recruitment between years 5 and 15 after an epizootic.
Validation
The observed population size structure and disease prevalence of G. ventalina at 5 sites in Puerto Rico (Zuluaga-Montero 2008) were contrasted with the expected asymptotic structure predicted by the model. Site-specific information on disease incidence and recruitment (as reflected by the relative abundance of small colonies) was used to generate the expected relative abundance of sea fans in the six size-health categories (fig. 5). Overall, the model explained 71% (r=0.8419, observed vs. expected, n=30) of the observed variation in the proportion of healthy and diseased G. ventalina colonies of the three size classes at the five sites.
Discussion
Viability sea fan populations
This study stresses the importance of recruitment together with disease incidence in evaluating the viability of a coral population in a given locality. The critical level of incidence (i.e. that above which a population will start declining), will vary in space and time depending on the level of recruitment. The importance of recruitment has been demonstrated by others (Sokolow et. al. 2009), yet a study that measures incidence and recruitment rates simultaneously for any coral species is still to come. The reason is that measuring these two variables is not trivial; it requires longitudinal studies and long term monitoring.
Only two studies have actually measured incidence of sea fan disease. Kim et al. (2006) report a yearly incidence of close to 30% at a locality in the Florida Keys between 1996 and
1998; and Zuluaga-Montero (2008) report and average incidence of 3.8% at several localities in Puerto Rico between 2006 and 2007. Although the studies differ in their definition of disease (Kim’s definition is broader including sea fans with purple spots but no tissue necrosis; whereas Zuluaga-Montero´s is more conservative including only fans with tissue necrosis.), they suggest that incidence has been declining in the Caribbean. But are current levels viable? Our analysis predicts that sustained background incidence levels above 6% would cause population decline, even for localities that have what may be considered to be high levels of recruitment. Clearly, two studies with estimates of incidence, but lacking recruitment data are not adequate to answer this pressing question. It appears, though, that a decade or more ago incidence was sufficiently high to cause population decline, but that it is currently approaching sustainable levels.
Epizootics may cause mass mortalities and lead to local extinctions even in populations experiencing low baseline incidence levels. The mass mortalities that affected sea fans in the 80s and early 90s (Guzmán and Cortés 1984; Garzón-Ferreira and Zea 1992) and subsequent prevalence dynamics (Kim and Harvell 2004) indicate that epizootics have indeed impacted sea fan populations in the Caribbean. Our transient analysis indicates that only for populations that are already declining due to low levels of recruitment, would a weak or moderate epizootic produce local extinctions. However, an epizootic with initial incidence levels of about 90% or higher would result in mass mortalities and in local extinctions in all localities except those with high recruitment (appendix A).
Recruitment in gorgonians, just as for corals in general, is very variable in space and time (Gotelli 1988; Yoshioka 1996). Thus, the two processes that are essential to understanding the effect of disease on corals – incidence and recruitment – are notoriously variable spatially and
temporaly. This poses a significant but not insurmountable hurdle towards assessing and predicting the viability of coral populations impacted by disease.
Prevalence and size structure
Prevalence, the percent of diseased individuals in a given locality and time period, is commonly measured in studies of infectious diseases. However, interpretation of temporal and spatial patterns of prevalence can be problematic because the underlying processes producing these patterns may be complex and not apparent. For instance, our analysis indicates that – as expected – prevalence in sea fans is related to incidence, but unexpectedly it turned-out to also be influenced by recruitment. The spatial and temporal variability in disease prevalence in Caribbean sea fans has been explained in terms of spatial or temporal variability in incidence (Dube et al. 2002; Jolles et al. 2002; Weil 2004; Kim et al. 2006; Toledo et al. 2007). This study shows that much of this variability is also attributable to spatial and temporal variability in recruitment.
Several studies have documented significantly higher disease prevalence in large sea fan colonies (Nagelkerken et al. 1997; Dube et al. 2002, Kim and Harvell 2002; 2004). The study of Dube et al. (2002) suggests that this is the result of lower incidence in small colonies due to enhanced chemical defenses against fungal pathogens in small sea fans. However, the data of Toledo-Hernández et al. (2009) indicates that mortality due to disease is higher in smaller colonies. Our analysis demonstrates that the higher mortality suffered by small diseased colonies, by itself, can account for their observed relative rareness in the wild.
Validation and caveats
The model did a good job in explaining the observed spatial variability in population structure in the independent study by Zuluaga-Montero (2008) (we used no data from this study to parameterize the model). Especially given that we only had data on disease incidence for one year, and an indirect measure of recruitment. The fact that the model explained more than two thirds of the observed variance in prevalence and size structure, gives credence to the model’s predictions. Nevertheless, the results of our analyses are circumscribed by the demographic and recruitment data we used to parameterize the model. For example, our model only considers the significantly higher mortality due to disease observed in small colonies by Toledo-Hernández et al. (2009). But, the massive number of sea fan skeletons (presumably of all size classes) found by Guzmán & Cortés (1984) and Garzón-Ferreira & Zea (1992) suggest high mortality in all size classes. If true and given our analyses, it is easy to envision the grave effect of such an event should it occur again.
Acknowledgements
This study was financially supported by the NOAA-CRES (NOAA award NA170P2919 ) and Sea Grant (NOAA award NA16RG2278, project R-92-1-04) programs of the UPR.
Literature Cited
Aronson, R. B.,and W. F. Precht. 2001. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38.
Bruno, J. F., L. E. Petes, C. D. Harvell and A. Hettinger. 2003. Nutrient enrichment can increase the severity of coral diseases Ecology Letters 6:1056–1061.
Caswell, H. 2001. Matrix population models: construction, analysis and interpretation. 2nd Ed. Sinauer, Sunderland, MA.
Cróquer, A., and E. Weil. 2009. Changes in Caribbean coral disease prevalence after the 2005 bleaching event. Disease Aquatic Organisms 87:33-43.
Dobson, A. P. 2009. Climate variability, global change, immunity, and the dynamics of infectious diseases. Ecology 90:920–927.
Dube, D., K. Kim, A. P. Alker, C. D. Harvell. 2002. Size structure and geographic variation in chemical resistance of sea fan corals Gorgonia ventalina to a fungal infection. Marine Ecology Progress Series 231:139–150.
Garzón-Ferreira J. and J. S. Zea. 1992. A mass mortality of Gorgonia ventalina (Cnidaria: Gorgonidae) in the Santa Marta area, Caribbean coast of Colombia. Bulletin of Marine Science 50:522–526.
Geiser, D. M., J. W. Taylor, K. B. Ritchie, and G. W. Smith. 1998. Cause of sea fan death in the West Indies. Nature 394: 137–138.
Gotelli, N. J. 1991. Demographic Models for Leptogorgia virgulata, a Shallow-Water Gorgonian. Ecology 72: 457-467.
Gotelli, N. J. 1988. Determinants of Recruitment, Juvenile Growth, and Spatial Distribution of a Shallow-Water Gorgonian. Ecology 69: 157-166.
Guzmán, H. M., and J. Cortés. 1984. Mortandad de Gorgonia flabellum Linnaeus (Octocorallia: Gorgoniidae) en la Costa Caribe de Costa Rica. Revista de Biología Tropical 32:305–308.
Harvell, C. D., S. Altizer, I. M. Cattadori, L. Harrington, and E. Weil. 2009. Climate change and wildlife diseases: When does the host matter the most? Ecology 90:912–920.
Harvell, C. D., E. Jordan-Dahlgren, S. Merkel, L. Raymundo, E. Rosenberg, G. Smith, E. Weil, and B. Willis. 2007. Coral disease, environmental drivers and the balance between coral and microbial associates. Oceanography 20:58–81.
Harvell, C. D. C. E. Mitchell, J. R. Ward, S. Altizer, A. P. Dobson, R. S. Ostfeld, and M. D. Samuel. 2002. Climate Warming and Disease Risks for Terrestrial and Marine Biota. Science 296:2158–2162.
Jolles, A. E., P. Suvillan, A. P. Alker, and C. D. Harvell. 2002. Disease transmission of aspergillosis in sea fans: inferring process from spatial pattern. Ecology 83:2373–2378.
Kim, K., A. P. Alker, K. Shuster, C. Quirolo, and C. D. Harvell. 2006. Longitudinal study of aspergillosis in sea fan corals. Diseases of aquatic organisms 69: 95-99.
Kim, K., and C. D. Harvell. 2004. The rise and fall of a six-year coral fungal epizootic. American Naturalist 164:52–63.
Lafferty, K. D. 2009. The ecology of climate change and infectious diseases. Ecology 90:888–900.
Lafferty, K. D., J. W. Porter, and S. E. Ford. 2004. Are diseases increasing in the ocean? Annual Review of Ecology, Evolution and Systematics 35:31–54.
Lips, K. R., B. Forrest, R. Brenes, J. D. Reeve, R. A. Alford, J. Voyles, C. Carey, L. Livo, A. P. Pessier, and J. P. Collins. 2006. Emerging infectious disease and the loss of
biodiversity in a Neotropical amphibian community. Proceedings of the National Academy of Sciences 103:3165-3170.
Nagelkerken, I. K., G. W. Smith, K. Bonair, and P. Bush. 1997. Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Marine Ecology Progress Series 160:255–263.
Oldroyd P.B. 2007. Unsolved mystery: what’s killing American honey bees? PLoS Biol.,6:1195-1199.
Ostfeld, R. S. 2009. Climate change and the distribution and intensity of infectious diseases. Ecology 90:903–905.
Pascual, M., and M. J. Bouma. 2009. Do rising temperatures matter? Ecology 90:906–912.
Petes, L. E., C. D. Harvell, E. C. Peters, A. M. Webb, K. and M. Mullen. 2003. Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Marine Ecology Progress Series 264:167–171.
Randolph, S. E. 2009. Perspectives on climate change impacts on infectious diseases. Ecology 90:927–931.
Richardson, LL., and J. D. Voss. 2005. Changes in a coral population on reefs of the northern Florida Keys following a coral disease epizootic. Mar Ecol Prog Ser 297:147–156.
Selig, E.R., C.D. Harvell, J.F. Bruno, B.L. Willis, C.A. Page, K.S. Casey, and H. Sweatman. 2006. Analyzing the relationship between ocean temperature anomalies and coral disease outbreaks at broad spatial scales. In: , J.T. Phinney, A. Strong, W. Skrving, J. West, J. Kleypas, and O. Hough-Guldberg, eds. Coral Reefs and Climate Change: Science and Management, Coastal and Estuarine Series, vol. 61, American Geophysical Union Press.
Smith, G. W., L. D. Ives, I. A. Nagelkerken, and K. B. Ritchie. 1996. Caribbean sea-fan mortalities. Nature 383:487.
Sokolow, S. 2009. Effects of a changing climate on the dynamics of coral infectious disease: a review of the evidence. Diseases of Aquatic Organisms 87:5-18
Sokolow, S. H., P. Foley, J. E. Foley, A. Hastings and L. L. Richardson. 2009. Disease dynamics in marine metapopulations: modelling infectious diseases on coral reefs. Journal of Applied Ecology 46:621–631.
Toledo-Hernández, C., P. Yoshioka, P. Bayman, A. M. Sabat. 2009. Impact of disease and detachment on growth and survivorship of sea fans (Gorgonia ventalina). Marine Ecology Progress Series 393:47-54.
Toledo-Hernández, C., A. Zuluaga-Montero, A. Bones-González, A. M. Sabat, and P. Bayman. 2008. Fungi in healthy and diseased sea fans (Gorgonia ventalina): is Aspergillus sydowii always the pathogen? Coral Reef 27:707-714.
Toledo-Hernández, C., A. Bones-González, O. E. Ortiz-Vázquez, A. M. Sabat, and P. Bayman. 2007. Fungi in the sea fan Gorgonia ventalina: diversity and sampling strategies. Coral Reefs 26:725-730.
vanEngelsdorp D., J. Jr. Hayes, R. M. Underwood, and J. Pettis. 2008. A survey of honey bee colony losses in the U.S. Fall 2007 to Spring 2008. PLoS ONE 3(12):1-6.
Yoshioka, P. M. 1996. Variable recruitment and its effects on the population and community structure of shallow-water gorgonians. Bulletin of Marine Science. 59:433-443.
Weil, E. 2004. Coral reef diseases in the wider Caribbean. Pp. 35–68 in E. Rosenberg and Y. Loya, eds. Coral Health and Diseases. Springer Verlag, NY.
Zuluaga-Montero, A., C. Toledo-Hernández, J. A. Rodríguez, A. M. Sabat, and P. Bayman. 2010. Spatial variation in fungal communities isolated from healthy and diseased sea fans Gorgonia ventalina and seawater. Aquatic Biology 8:151-160
Zuluaga-Montero, A. 2008. Aspergillosis in sea fans: fungal community, patterns of prevalence, and genetic variability of A. flavus. Ph.D. dissertation, University of Puerto Rico, San Juan, Puerto Rico.
Table 1. Parameter estimates of life cycle transitions of the demographic model of Gorgonia ventalina presented in equation 1. The model considers three size classes (small, medium and large) and two health states (healthy or diseased). Transition probabilities are a product of annual survivorship (σ ), growth (γ ), retrogression (ρ ), disease incidence (α) and healing (β). See text for further explanations.
Mh Md P2hd
Mh Sd R2hd 0.8889 0 0.2345 0.0278 0 0.0058 Md Mh P2dh 0.8889 0.1707 0.1707 0
Md Md P2dd 0.8889 0.1707 0.1707
Lh Lh P3hh 0.9231 0 0.3446 0.0278 0 0.5882
Lh Ld P3hd 0.9231 0 0.3446 0.0278 0 0.0168
Lh Mh R3hh 0.9231 0 0.3446 0.0278 0 0.3093
Lh Md R3hd 0.9231 0 0.3446 0.0278 0 0.0088
Ld Lh P3dh 0.9231 0 0.3089 0 0.0476 0.0304
Ld Ld P3dd 0.9231 0 0.3089 0 0.0476 0.6076
Ld Mh R3dh 0.9231 0 0.3089 0 0.0476 0.0136
Ld Md R3dd 0.9231 0 0.3089 0 0.0476 0.2716
Figure Legends
Figure 1: (a) Predicted asymptotic population growth rate (λ) in the sea fan Gorgonia ventalina as a function of disease incidence for high and low levels of recruitment. (b) Predicted number of Gorgonia ventalina recruits (per adult) that a locality needs to receive to maintain population at equilibrium (λ=1) for increasing levels of disease incidence.
Figure 2: Predicted asymptotic relative abundance of Gorgonia ventalina colonies of three size classes (small (S), medium (M) and large (L)) and two health states (healthy (h) and diseased (d)) for three levels of disease incidence (0, 0.15 and 0.3) under (a) high and (b) low recruitment.
Figure 3: Predicted asymptotic disease prevalence (i.e. relative abundance of small+medium+large diseased colonies) of Gorgonia ventalina colonies as a function of incidence for high and low recruitment
Figure 4: Simulated transient dynamics of a Gorgonia ventalina population impacted by a moderate epizootic with an initial incidence of 60%. Panels depict population growth rate (a, d, g), abundance of healthy and diseased colonies (b, e, h) and relative abundance of small, medium and large diseased colonies (c, f, l) for high (a, b, c), intermediate (d, e, f) and low (g, h, i) recruitment.
Figure 5: Observed versus expected relative abundance of healthy and diseased Gorgonia ventalina colonies of three size classes – small (S), medium (M) and large (L) – in five localities in Puerto Rico. Observed frequencies were obtained from Zuluaga-Montero (2008). Expected ones were generated by the model. See text for further explanation.
Appendix A: Simulated abundance of healthy and diseased Gorgonia ventalina colonies with high (A1, A4), intermediate (A2, A5) and low (A3, A6) recruitment impacted by a weak (A1, A2, A3) and a strong (A4, A5, A6) epizootic. Initial incidence of weak and strong epizootic is 30% and 90%, respectively.