Executive Summary
OBJECTIVES (from original proposal)
The overall goal of this project is to understand the causal agents, physiological responses and environmental factors that contribute to aspergillosis disease in the sea fan Gorgonia ventalina. We proposed four steps towards this goal: (1) Monitor incidence of aspergillosis at monthly intervals in sea fan populations at sites with contrasting environmental conditions, and relate disease with water quality parameters such as turbidity and spore load. (2) Determine which species of Aspergillus infect sea fans in Puerto Rico. (3) Determine if Aspergillus isolated from sea fans are pathogenic by fulfilling Koch’s postulates in aquarium experiments. (4) Test the role of stress in susceptibility of sea fans to aspergillosis.
RESULTS
Objective 1: Monitor incidence of aspergillosis at monthly intervals in sea fan populations at sites with contrasting environmental conditions, and relate disease with water quality parameters such as turbidity and spore load.
Carlos Toledo (Ph.D. student) has sampled 203 Gorgonia ventalina colonies, of which 122 were apparently healthy and 81 had lesions suggestive of aspergillosis.
Another Ph.D. student, Anabella Zuluaga, has started to census reefs at monthly intervals to try to correlate incidence of aspergillosis to environmental events such as dust clouds and rainfall (Fig. 1). This may allow us to distinguish between the two principal hypotheses on the source of the inoculum: African dust clouds vs. terrestrial runoff.
Objective 2. Determine which species of Aspergillus infect sea fans in Puerto Rico.
Carlos Toledo (Ph.D. student) has sampled 203 Gorgonia ventalina colonies, of which 122 were apparently healthy and 81 had lesions suggestive of aspergillosis. The healthy colonies are necessary because there is only one article which discusses mycoflora of normal sea fans, and without this baseline information it is hard to evaluate the mycoflora of diseased sea fans. Almost all morphospecies isolated have been identified by sequencing the nuclear ribosomal ITS region; the remaining sequences will be finished in March 2007.
Fungi were as common in pieces of healthy colonies as in diseased colonies (X2=0.233; P0.05,1=0.64). Aspergillus sydowii, described as the sole pathogen in the literature, was more common in healthy tissue than in diseased tissue. In contrast, A. flavus was isolated at much higher frequency than A. sydowii and was more common in diseased tissue than in healthy tissue. We conclude that A. flavus and perhaps other fungi are more likely to be associated with necrosis than the reported pathogen A. sydowii. This
suggests that the fungi may be opportunistic pathogens and that the susceptibility of the sea fans may be more important as determinants of the disease as the presence of a particular fungus.
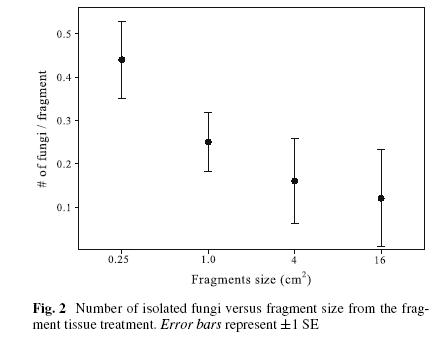
The diversity of fungi in a single Gorgonia colony, and the variability among colonies, means that a defined sampling strategy is needed to study them. How large a piece of sea fan tissue is optimal for revealing the maximum number of fungi. Reducing the size of the pieces significantly increased the number of fungi isolated per piece. (See Fig. 1.) A manuscript on this topic has been submitted to Coral Reefs.
Anabella Zuluaga (Ph.D. student) is comparing fungi found in sea water with those found in sea fans. The hypothesis is that certain fungi infect sea fans at higher frequency than the surrounding water, whereas other common fungi in the water rarely infect sea fans.
Fig. 1. Correlation between size of Gorgonia tissue placed on culture media and the number of fungal colonies isolated.
Fig. 1 Incidence of aspergillosis and other injuries in eight reefs in Puerto Rico. Top: frequency of healthy colonies (blue) vs. colonies with lesions (red). The frequency differed significantly among reefs (P=0.001). Bottom: frequency of aspegillosis lesions (Type 1, brown bars) vs. lesions of unknown origin (Type 2, gold bars),. The relative frequency differed significantly among reefs (P=0.025). Type 2 lesions may include aspergillosis where symptoms are no longer clearly distinguishable. Some previous studies have assumed that all lesions are aspergillosis, whereas the proportion of lesions with clear signs of aspergillosis may be less than 50%.
TYPE 1
TYPE 2
We have isolated more than 50 colonies of Aspergillus from seawater and have identified the following species by sequencing the ITS: A. sydowii, A. ochraceus, A. puniceus, A. versicolor, A. aculeatus, A. japonicus, A. nomius and Penicillium citrinum. If the common pathogens come from African dust clouds (as has been proposed) we expect the fungi that infect sea fans to be uniformly distributed in seawater around Puerto Rico. If, on the other hand, the pathogens come from terrestrial runoff (as has also been proposed), we expect higher prevalence in reefs closer to the coast. Thus far, we have not identified higher prevalence in reefs closer to the coast, supporting the African source hypothesis. However, more data needs to be collected before we can test more rigorously for patterns of distribution of pathogens.
In order to determine relatedness among fungi from different sources (e.g., diseased sea fans, seawater, sediment, dust clouds, soil in Puerto Rico) we have started to use AFLPs (Fig. 2). This experiment will focus on two species: A. flavus and A. sydowii Although not mentioned in the original proposal, AFLPs are consonant with the goals of this objective and will help us determine the source of inoculum.
Fig. 2. AFLPs of Aspergillus. Bands 1-3 (in each set of six) are from a single A. sydowii strain at different DNA concentrations; 4 and is A. flavus; 5 and 6 are positive controls.The four sets of lanes are different primer combinations. Red boxes show some potentially useful bands.
The fungi present in diseased sea fans appear to change over time. In last year's progress report we showed that A. flavus was significantly more common in diseased tissue than in healthy tissue, and that A. sydowii (the reported pathogen) had never been isolated. This year we have seen less A. flavus and more A. niger, and A. sydowii has been isolated several times. A multi-year study would be required to answer this question definitively. However, all the data so far support our original thesis: that there are many species of Aspergillus and other fungi associated with aspergillosis of sea fans, not just A. sydowii as reported in the literature. This finding has major implications for understanding the disease.
ICACOS PALOMINITO
C. LARGO
PINEROS
LOBO MONA PENA
CULEBRITA
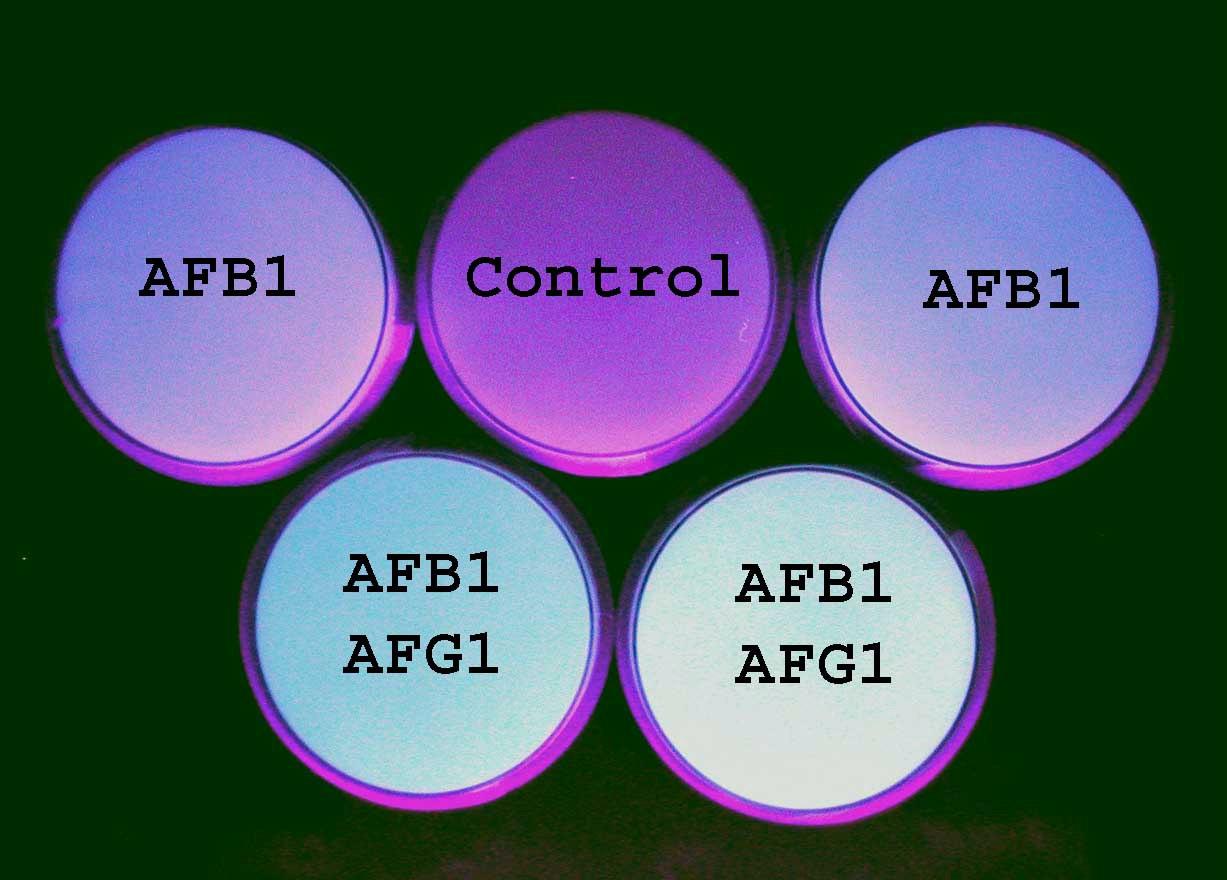
Aspergillus strains have also been characterized for mycotoxin production (Fig. 3). About 50% of A. flavus group isolates appear to produce aflatoxins in liquid
Fig. 3. Aflatoxin production by A. flavus group isolates in vitro. Top: fluorescence of aflatoxins on coconut agar medium under UV light. One plate (control) does not fluoresce; two plates fluoresce blue, indicating the presence of aflatoxin B1; and two plates fluoresce yellow-green, indicating presence of aflatoxins B1 and G1. Bottom: TLC plate of aflatoxins extracted from liquid cultures of fungi. S: authentic standards of aflatoxins; 1, 2, 3: extracts of three fungi, of which only 1 produced aflatoxins.
culture. (Aflatoxin production will be quantified by HPLC.) However, we do not know if aflatoxins and ochratoxins are produced in sea fans infected with Aspergillus. If they are, these mycotoxins may play a role in the disease. These data are novel (this is probably the first evidence of aflatoxin production by fungi from the sea) and raise the question of whether mycotoxins may be involved in pathogenesis. To explore this question we will extract infected tissues (as opposed to cultures of fungi in liquid media) to check for the presence of mycotoxins.
Objective 3. Determine if Aspergillus isolated from sea fans are pathogenic by fulfilling Koch’s postulates.
We have developed a new technique for cultivating sea fans in aquaria (Fig. 4). The technique involves cutting sea fans into 2x2 cm pieces, stringing them on plastic monofilament line, and securing them inside aquarium tanks. The advantage of cutting fans into pieces is that it gives more replicas for inoculation in a single tank. This is significant because culturing sea fans in aquaria is not easy. Another advantage is that it will minimize collection of wild colonies for this experiment. However, we are still testing different methods for inoculating sea fan pieces with fungi, and have not yet generated any data.
Fig. 4. Aquarium tank showing sea fan colonies (red arrows) and monofilament lines with 2x2 cm colony pieces (yellow arrows).
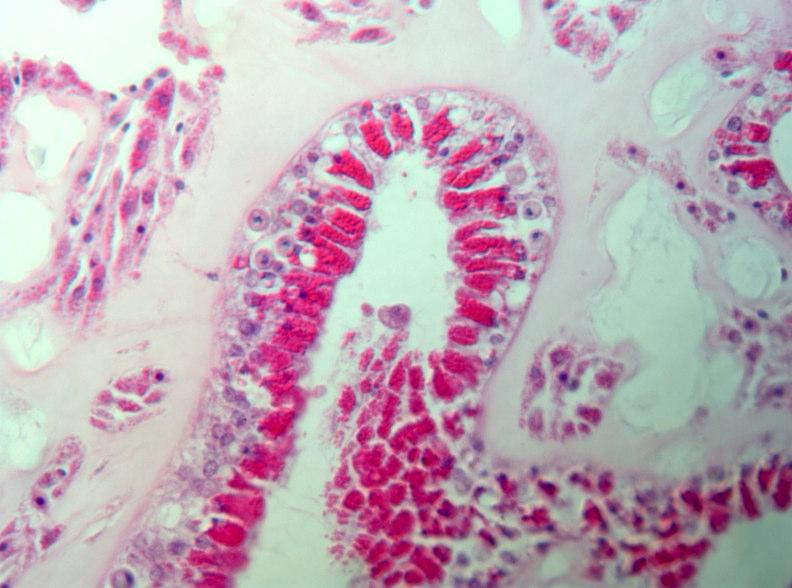
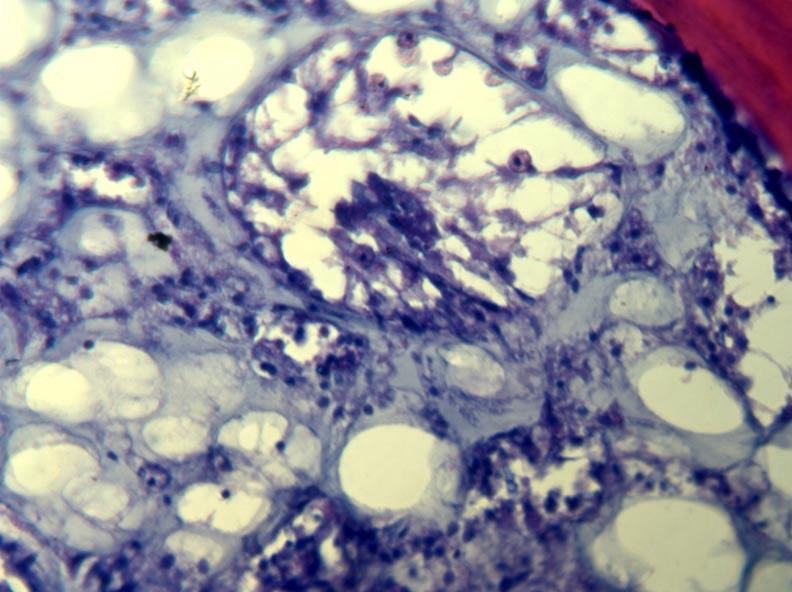
Another way to study pathogenicity and infection processes is by direct observation of infected tissues. In Year 2 we began to do histology on healthy and infected tissues to determine how the disease progresses, and to compare tissues inoculated with different potential pathogens (Fig. 5). A modification of existing methods was developed which uses a microwave oven to process embedding resin. This method is much faster than existing, standard methods, and reduces the use of alcohol and solvents. A brief note describing the method will be submitted to a histology journal this year.
Objective 4. Test the role of stress in susceptibility of sea fans to aspergillosis.
This Specific Aim has not been done, because the development of ELISAs for sea fan heat shock proteins is no longer the best strategy. Since this proposal was written, there have been major changes in the understanding of invertebrate immune systems and many related genes have been identified.
Fig. 5. Sections of healthy (left) and diseased (right) sea fans.
Significance
Comparisons of frequency of fungi in diseased and healthy tissues suggest that, contrary to the literature, several species of fungi are associated with aspergillosis disease of sea fans. There is debate about the source of the inoculum: terrestrial runoff vs. dust clouds from Africa. The use of genetic markers (AFLPs) may help establish patterns of relationships among strains, allowing us to estimate movement and source of the pathogens. Since marine populations have been completely overlooked in studies of movement of Aspergillus species, these studies have implications for epidemiology of aspergillosis and management of mycotoxin contamination. Aflatoxin production by these fungi suggests that secondary metabolites may play a role in the disease, a hypothesis that we will be test.
Manuscripts in press that acknowledge Sea Grant support
Bayman P. 2006. Diversity, scale and variation of endophytic fungi in leaves of tropical plants. In: Microbiol Ecology of Aerial Plant Surfaces [in press]. Bailey MJ, Lilley AK, Timms-Wilson TM, eds. CABI Publishing, UK. [Invited book chapter]
Bayman P, Baker JL. 2006. Ochratoxins: a global perspective. Mycopathologia [in press].
Manuscripts in preparation
Toledo-Hernández C, Sabat A. Environmental correlates of Gorgonia ventalina density. Submitted to Marine Biology.
Toledo-Hernández C, Bayman P, Sabat A. Fungal community of Gorgonia ventalina colonies with and without aspergillosis. To be submitted to Coral Reef.
Other publications
Bayman P, Otero JT. 2006. Endophytes of orchid roots. In: Microbial Root Endophytes. [In press.] Schulz B, Boyle C, Sieber T, eds. Springer Verlag, Berlin. [Invited book chapter]
Santamaría J, Bayman P. 2005. Fungal epiphytes and endophytes of coffee leaves (Coffea arabica). Microbial Ecology 50, 1-8.
Gamboa-Gaitán MA, Wen S, Fetcher N & Bayman P. 2005. Effects of fungicides on endophytic fungi and photosynthesis in seedlings of a tropical tree, Guarea guidonia (Meliaceae). 2005. Acta Biológica Colombiana 10: 45-51.
Otero JT, Bayman P, Ackerman JD. 2005. Variation in mycorrhizal performance in the epiphytic orchid Tolumnia variegata in vitro: the potential for natural selection. Evolutionary Ecology 19: 29-43.
Gamboa-Gaitán MA, Laureano S, Bayman P. 2005. Endophytic Phomopsis strains from leaves of Guarea guidonia (Meliaceae). Caribbean Journal of Science 41: 215-224.
Presentations
Toledo-Hernández C, Bayman P, Sabat A. Multiple Aspergillus species associated with sea fan aspergillosis. Mycological Society of America Annual Meeting, Hilo, Hawaii. Inoculum 2005; 56(4): 59. [Oral]
Bayman P, Toledo-Hernández C, Zuluaga A, Sabat A. Aspergillosis disease of sea fans: pathogens, environment and stress. 1st Annual Symposium for Costal & Marine Applied Research, Recinto Universitario de Mayagüez, Sept. 2, 2005. [Oral]
Zuluaga A, Pérez-Rodríguez I, Bayman P. Microbial communities in sea fans. 1st Annual Symposium for Costal & Marine Applied Research, Recinto Universitario de Mayagüez, Sept. 2, 2005. [Poster]
Fungi in the sea fan Gorgonia ventalina: diversity and sampling strategies
Coral Reefs (2007) 26:725–730
DOI 10.1007/s00338-007-0252-8
NOTE
C. Toledo-Hernández · A. Bones-González ·O. E. Ortiz-Vázquez · A. M. Sabat · P. Bayman
Received:23February2007/Accepted:8May2007/Publishedonline:21June2007
Springer-Verlag2007
Abstract
Fungal communities from gorgonians have been poorly documented and most studies of these communities have lacked defined sampling strategies. The objectives of this study were: (1) to estimate fungal diversity in Gorgonia ventalina; (2) to compare two sampling and tissue processing strategies: tissue fragments of different sizesvs. homogenized tissue. A total of seven genera and fourteen species of fungi were isolated on culture medium and identified by sequencing the nrITS. All but one species were new reports. In both treatments Aspergillus and Penicillium were the most common genera isolated. Most species isolated from fragments were not observed from homogenized tissue and vice versa. Reducing the size fragment increased significantly the number of species isolated per fragment. To better estimate fungal diversity in sea fans a strategy is proposed that combines sampling of small tissue fragments with homogenized tissue, since each technique yielded fungal species not detected by the other.
Keywords Aspergillosis · Coral · Fungal diversity ·Gorgonian · Sampling
Introduction
The diversity of fungi in corals is poorly understood. Most of our limited knowledge of fungi in corals comes from scleractinian corals. For instance, Kendricks et al. (1982) isolated several endolithic Aspergillus spp., Penicillium spp. and Cladosporium spp. (among others) in 15 reefbuilding corals and hydrozoans from the Caribbean and Australia. Betis et al. (2000) reported unknown fungal hyphae associated with internal structures of Pocillopora eydouxi and Acropora cytherea, two reef-building corals from the Pacific Ocean. Priess et al. (2000) reported an Aspergillus-like endolithic fungus associated with the reefbuilding corals Porites lutea and Porites lobata. Kohlmeyer and Volkmann-Kohlmeyer (1987, 1988, 1989, 1990) described several new species of fungi in reef-building corals from the Caribbean and Australia, e.g., Lulworthia calcicola, Halographis runica and five species of Koralionastes.
The mycoflora of gorgonians is even less understoodthan that of scleractinian corals, despite the fact that aspergillosis outbreaks have caused massive mortality in the sea fans Gorgonia ventalina and Gorgonia flabellum (Nagelkerken et al. 1997; Kim and
Harvell 2004). The causal agent of sea fan aspergillosis, Aspergillus sydowii, has been cultured and identified (Smith et al. 1996; Geiser et al. 1998). However, the fungal community of healthy and infected sea fan colonies remains undescribed. The report of 51 types of deuteromycetous fungi from 10 gorgonian species in Singapore (Koh et al. 2000) is suggestive of the rich fungal community to be found in gorgonians.
Previous studies documenting coral mycota have overlooked potential variability within and among colonies. Most reports have not included specific descriptions of the number of coral colonies surveyed, number of tissue fragments sampled per colony, size of the collected fragments, and tissue processing methods, with the exception of Koh et al. (2000). If an investigator wants to approximate the real species diversity of coral fungi should he or she collect a tissue sample from many diVerent coral colonies, or instead collect several samples from the same colony but sample fewer colonies?
Since these issues have not previously been addressed in corals, this study was based on knowledge of mycoflora of plant leaves. All leaves are colonized by diverse communities of fungi, both externally and internally. Some are pathogens, some are mutualists, and many have no apparent effects on their hosts (see Bayman 2006). Effects of sample size, sample distribution, leaf fragment size, and processing techniques have been studied, with the goal of maximizing the diversity while minimizing sampling effort (Carroll 1995; Lodge et al. 1996; Gamboa and Bayman 2001; Gamboa et al. 2002). The same approach may be useful for determining diversity and sampling strategies for fungal communities in corals.
The objectives of this study were to test for variability in fungal diversity within and among coral colonies and to compare sampling and tissue processing strategies. The Caribbean sea fan G. ventalina was chosen to address these questions, for two reasons: (1) its susceptibility to aspergillosis means that understanding its fungal flora is of great importance, and (2) sampling is easier than in hard corals.
Materials and methods
Two treatments were used to estimate fungal diversity associated with the sea fan G. ventalina: tissue fragments and homogenized tissue. Five G. ventalina colonies 400–550 cm2 were collected from Escambrón beach, San Juan, Puerto Rico. In the laboratory, fragments of 16, 4, 1 and 0.25 cm2 were cut from each of 5 positions of the blade of each colony (Fig. 1). Each fragment was surface-sterilized individually in ethanol 70% for 30 s. and then washed in filtered, autoclaved sea water for another 30 s (Sodium hypochlorite was not used for sterilization because it was found to dissolve the mesoglea, and it may kill some fungi (Koh et al. 2000). Since Gorgonia tissues are covered by a mucosal layer, it is not clear if the fungi isolated were in the mucous layer or inside the tissues (see Koh et al. 2000). Fragments were plated on Glucose Peptone Yeast Agar (GPYA, a standard medium for marine fungi) made with Wltered, autoclaved seawater, and observed for fungal growth weekly for 1 month.
For the homogenized tissue treatment, Wve additional tissue fragments of 4 cm2 from the same areas of the same colonies plus an additional colony, were surface sterilized
as described above and homogenized in a sterilized blender with 100 ml filtered, autoclaved sea water. A 500-_l aliquot was plated on GPYA and observed for fungal growth as explained above.
For identification, fungi were isolated from the original petri plate, re-cultured on GPYA, and transferred to liquid Potato dextrose (PD) medium made with filtered, autoclaved seawater. Five days after transfer to liquid PD, fungi were filtered and DNA extracted using a Plant Mini Extraction Kit (Qiagen Sciences). The nuclear ribosomal ITS region was ampliWed and the sequences were identified by BLAST using reference sequences from GenBank. Identifications based on BLAST searches were confirmed by microscopic observations.
To compare the occurrence of fungi among the 16, 4, 1 and 0.25 cm2 tissue fragments, among fragment positions, and between fragments versus homogenized tissue, 2 analyses were conducted. A Kendall Tau rank correlation coefficient was used to measure the degree of correspondence between fragment size and number of fungi observed.
Results and discussion
Diversity of fungi
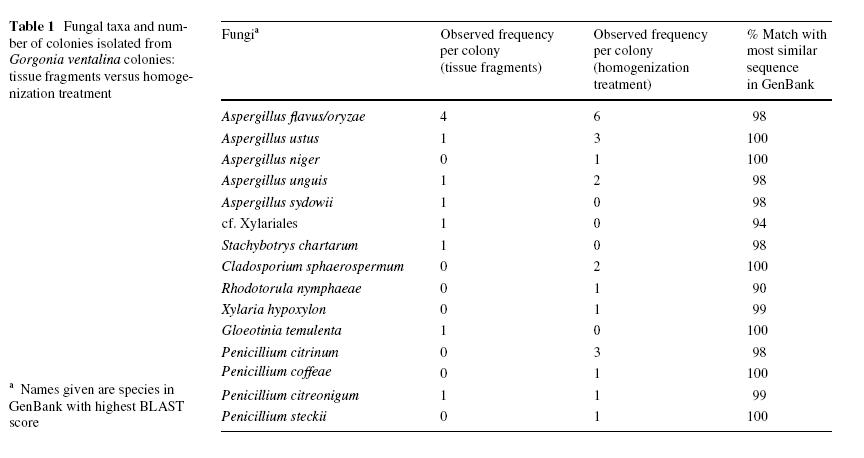
Eight genera of fungi were isolated (data from all treatments combined; Table 1). Aspergillus was the most common genus (20 isolates) and also the most diverse (Five species) (Table 1). Penicillium was less common (7 isolates) but almost as diverse (four species). Other isolated genera (as identified by BLAST searches) were Cladosporium,
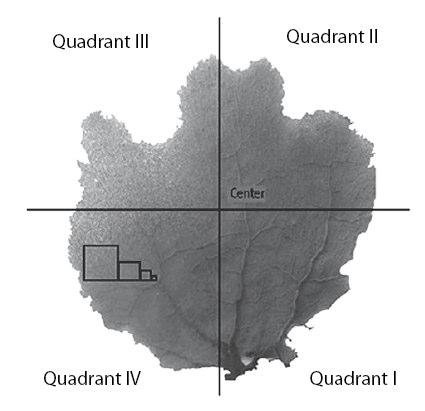
Fig. 1 Schematic representation of Gorgonia ventalina colony sampling strategy. The same number of fragments were collected from each quadrant and from the center. Squares show relative sizes of fragments (16, 4, 1, 0.25 cm2).
Gloeotinia, Rhodotorula, Stachybotrys, Xylaria, and an unknown fungus with affinities to the Xylariales. None of these fungi has been reported previously from G. ventalina, and Rhodotorula, Stachybotrys, Gloeotinia and Xylaria have not been reported from any gorgonian. Of the species of Aspergillus and Penicillium isolated, Aspergillus niger, Aspergillus ustus, Aspergillus unguis, Penicillium coffeae and Penicillium citreonigum have not been reported from gorgonians. However, Aspergillus flavus/oryzae and Penicillium citrinum have been reported in several gorgonians from Singapore (Koh et al. 2000), while A. niger has been isolated from several sponges (Varoglu and Crews 2000).
A. sydowii is the only fungus already reported from G. ventalina, and has been identified as the causative agent of aspergillosis (Smith et al. 1996; Geiser et al. 1998). However, in this study A. sydowii was isolated from colonies without the phenotype associated with aspergillosis (i.e., necrotic tissue surrounded by a purple halo). The presence of these fungi in sea fans is of concern because several are known to produce mycotoxins. Some Stachybotrys species produce toxins such as trichothecenes and stachybotrin. There are no previous reports of Stachybotrys or its toxins from marine substrates, except for a stachybotrin-producing isolate from brackish water in Florida (Pietra 1997). Similarly, although aflatoxin-producing strains of A. flavus are ubiquitous in soils, crops and plant debris, little is known about aflatoxin production in marine organisms (Frisvad and Samson 2004). It is not know if any of these toxins are produced in vivo in infected sea fans. If they are produced in vivo, it is possible they play a role in the aspergillosis disease.
Effects of fragment size
In four of the five sea fan colonies, reduction in fragment size significantly increased the number of fungi isolated per fragment ( 2 = 56.51; P05, 3 < 0.0001; Table 2, Fig. 2) (one G. ventalina colony did not produce fungal colonies from any fragment). Sixty-seven percent of all the fungi were isolated from the smaller (1 x 1 and 0.5 x 0.5 cm) tissue fragments. The number of fungal colonies isolated from fragments was negatively correlated with fragment size (Kendall Tau = ¡1.00; Z = -6.34, P = 0.04). Isolation of multiple species from single fragments was rare. Only two fragments yielded two fungal species growing simultaneously. Nonetheless, at the colony scale, multiple fungal colonization was the rule and not the exception (Table 2). This pattern coincides with that reported for fungal endophytes in leaves (Carroll 1995). In five tropical plant species, reducing the leaf fragment size revealed greater fungal diversity (Gamboa et al. 2002).
Two mechanisms, not mutually exclusive, may explain why decreasing fragment size yields more fungi. First, reducing fragment size may reduce the concentration of antifungal growth compounds produced by sea fans (Kim et al. 2000a, b); these compounds may leach into the agar and inhibit fungal growth. Smaller pieces have higher edge: surface ratio, and therefore a shorter distance for fungus to reach the medium and escape from anti-fungal compounds (Gamboa et al. 2002). Second, fungi with allelopathic properties that grow faster on culture media may inhibit or outcompete the growth of others (Carroll 1995; Gamboa et al. 2002). Competition probably explains why only two fragments produced more than one fungal colony. Reducing fragment size thus reduces the likelihood that viable propagules will not grow because of competition.
Effects of tissue homogenization
A total of 11 species of fungi from 5 genera were isolated and identified in the homogenized tissue treatment, the majority of which were not observed from tissue fragments (Table 1). Aspergillus was the most common and diverse genus from homogenized tissue, just as from tissue fragments (Table 1). However, in contrast to the tissue fragments, the majority of the homogenized tissue produced multiple fungal colonies. Significantly more fungi were isolated from homogenized tissue than from tissue fragments of the same size ( 2 = 26.05; P05, 1 < 0.0001).
Since homogenized tissue can be viewed as a collection of very small fragments, this difference re-enforces the conclusion that smaller pieces yield more fungi and more diversity. With homogenization all fungal propagules are in contact with the culture medium, and any inhibitory compounds in the tissue are diluted. Tissue homogenization may also increase fungi diversity by separating propagules and thus reducing competition. There was no obvious pattern of which fungi were favored by either method.
No association was observed between specific fungi and position in the sea fan blades, either for tissue fragments or homogenized tissue. All parts of the colony are presumably exposed to the same environment, and the modular architecture of gorgonians means that physiological conditions may be relatively uniform throughout the colony, so the lack of association may not be surprising, although Kim et al. (2000a, b) have previously demonstrated a within-colony gradient in antifungal activity.
Implications/conclusions
When sampling the fungal community in sea fans, four points should be considered. First, sample size and fragment size are important. These results show that a single sea fan colony may have multiple colonizations by different fungi throughout its blade. Therefore, increasing the number of fragments sampled from a single sea fan blade is likely to increase diversity. Since no effect of position in the sea fan blade was observed, fragments can be collected at random or as dictated by other considerations. Second, use of smaller tissue fragments increases the number and frequency of fungi isolated and minimizes damage to the sea fan.
The third point to consider is the method of tissue processing. Homogenized tissue yielded almost twice the number of species and one more genus than tissue fragments. (This comparison excludes one colony, which was not used in the tissue fragment treatment). However, the tissue fragments yielded fungal genera and species not observed in the homogenized tissue treatment. Therefore, a combination of both methods might be the best option to estimate fungal diversity.
The fourth and final point refers to tissue collection procedures. The standard sampling methodology of estimating endophytes in plants is by collecting leaves or other organs. In many plants this procedure is harmless because these organs are quickly replaced. However, removing tissue from sea fans might be harmful, since tissue regeneration rates in these corals are extremely slow. For instance, in a tissue regeneration experiment, one tissue fragment of 2 x 2 cm was removed from 20 G. ventalina colonies, and one year later tissue regeneration was still not complete. Only five colonies were sampled in this experiment to avoid damage to populations already under assault from environmental stresses.
So what techniques should be use to estimate diversity of fungi in sea fans? A combination of small fragments and homogenized tissue should be used, since each technique yielded fungal species not revealed by the other. But instead of collecting many fragments from a few colonies, it would be better to collect fewer and smaller fragments per colony and increase the number of colonies surveyed (Lodge et al. 1996).
Molecular techniques such as direct amplification can reveal other microorganisms that do not grow in culture, and should be combined with culturing techniques for optimal eYciency (Bayman 2006; Panthos and Bythell 2006; Ritchie 2006). In Gorgonia spp. the fungus of greatest interest is Aspergillus sydowii , the causal agent of aspergillosis (Smith et al. 1996; Geiser et al. 1998). Since Aspergillus species grow readily in culture, direct amplification was not necessary for this study.
Of previous studies dealing with fungi in gorgonians, only Koh et al. (2000) sampled several tissue fragments from different positions per colony, and they found fungal diversity comparable to or greater than the reported here. However, Koh et al. (2000) did not test effect of fragment size on number of fungi isolated and did not test diversity of fungi within a single Gorgonia colony. Many studies on fungi in corals have either taken a very limited number of samples or have not mentioned sampling strategy at all. Given the diversity and variation of fungi in a single gorgonian colony, a rigorous sampling strategy is clearly desirable.
Acknowledgments This project was supported by UPR Sea Grant (NOAA award NA16RG2278, project R-92-1-04) and an NIH SCoRE award. CTH thanks the RISENIH program for a research fellowship (2 R25 GM061151) and the International Society for Reef Studies (ISRS) for an ISRS/TOC Coral Reef Conservation Award (2006). We thank Miguel A. Gamboa and Anabella Zuluaga for advice on the manuscript. Sequencing was done at the UPR Sequencing & Genotyping Facility, which is funded by a grant from IMBRE.
References
Bayman P (2006) Diversity, scale and variation of endophytic fungi in leaves of tropical plants. In: Bailey MJ, Lilley AK, Timms-Wilson TM (eds) Microbial ecology of aerial plant surfaces. CABI Publishing, Wallingford, pp 37–50
Betis CJ, Kaufman L, Golubic S (2000) Endolithic fungi in reef-building corals (Order: Scleractinian) are common, cosmopolitan, and potentially pathogenic. Biol Bull 198:254–260
Carroll G (1995) Forest endophytes: pattern and process. Can J Bot 73:S1316–S1324
Frisvad JC, Samson RA (2004) Emericella venezuelensis, a new species with stellate ascospores producing sterigmatocystin and aflatoxin B1. Syst Appl Microbiol 27:672–680
Gamboa MA, Bayman P (2001) Communities of endophytic fungi in leaves of a tropical timber tree (Guarea guidonia: Meliaceae). Biotropica 33:352–360
Gamboa MA, Laureano S, Bayman P (2002) Measuring diversity of endophytic fungi in leaf fragments: does size matter? Mycopathologia 156:41–45
Geiser DM, Taylor JW, Ritchie KB, Smith GW (1998) Cause of sea fan death in the West Indies. Nature 394:137–138
Kendricks B, Risk MJ, Michaelides J, Bergman K (1982) Amphibious microborers: Bioeroding fungi isolated from live corals. Bull Mar Sci 32:862–867
Kim K, Harvell CD (2004) The rise and fall of a six-year coral-fungal epizootic. Am Nat 164:S52–S63
Kim K, Harvell CD, Kim PD, Smith GW, Merkel SM (2000a) Fungal disease resistance of Caribbean sea fan corals (Gorgonia spp.). Mar Biol 136:259–267
Kim K, Kim PD, Alker AP, Harvell CD (2000b) Chemical resistance of gorgonian corals against fungal infections. Mar Biol 137:393– 401
Koh LL, Tan TK, Chou LM, Goh NKC (2000) Fungi associated with gorgonians in Singapore. Proc 9th Int Coral Reef Symp 1:521– 526
Kohlmeyer J, Volkmann-Kohlmeyer B (1987) Koralionastetaceae Fam. Nov. (Ascomycetes) from coral rock. Mycologia 79:764– 778
Kohlmeyer J, Volkmann-Kohlmeyer B (1988) Halographis (Opegraphales) a new endolithic lichenoid from corals and snails. Can J Bot 66:1138–1141
Kohlmeyer J, Volkmann-Kohlmeyer B (1989) A new Lulworthia (Ascomycotina) from corals. Mycologia 81:289–292
Kohlmeyer J, Volkmann-Kohlmeyer B (1990) New species of Koralionasters (Ascomycotina) from the Caribbean and Australia. Can J Bot 68:1154–1559
Lodge DJ, Fisher PJ, Sutton BC (1996) Endophytic fungi of Manilkara bidentata leaves in Puerto Rico. Mycologia 88:733–738
Nagelkerken IK, Smith GW, Bonair K, Bush P, Garzón-Ferriera J, Botero L, Gayle P, Harvell CD, Heberer C, Kim K, Petrovic C, Pors L, Yoshioka P (1997) Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Mar Ecol Prog Ser 160:255–263
Pantos O, Bythell JO (2006) Bacterial community structure associated with white band disease in the elkhorn coral Acropora palmata determined using culture-independent 16s rRNA techniques. Dis Aquat Org 69: 79–88
Pietra F (1997) Secondary metabolites from marine microorganisms: bacteria, protozoa, algae and fungi. Achievements and prospects. Nat Prod Rep 14:453–464
Priess K, Le Campion-Alsumard T, Golubic S, Gadel F, Thomassin BA (2000) Fungi in corals:black bands and density-banding of Porites lutea and P.lobata skeleton. Mar Biol 136:19–27
Smith GW, Ives LD, Nagelkerken IA, Ritchie KB (1996) Caribbean sea fan mortalities. Nature 383:487
Ritchie KB (2006) Regulation of microbial population by coral surface mucus and mucus-associated bacteria. Mar Ecol Prog Ser 322:1–14
Varoglu M, Crews P (2000) Biosynthetically diverse compounds from a saltwater culture of sponge-derived Aspergillus niger. J Nat Prod 63:41–43
Fungi in healthy and diseased sea fans (Gorgonia ventalina): is Aspergillus sydowii always the pathogen?
Accepted for publication in Coral Reefs, 5/08
C. Toledo-Hernández *· A. Zuluaga-Montero · A. Bones-González · J.A. Rodríguez · A. M. Sabat · P. Bayman
Keywords: Gorgonia ventalina, Aspergillus sydowii, Aspergillosis, Coral diseases
Abstract
Caribbean corals, including sea fans (Gorgonia spp.), are being affected by severe and apparently new diseases. In the case of sea fans, the pathogen is reported to be the fungus Aspergillus sydowii, and the disease is named aspergillosis. In order to understand coral diseases and pathogens, knowledge of the microbes associated with healthy corals is also necessary. In this study the fungal community of healthy Gorgonia ventalina colonies was contrasted with that of diseased colonies. In addition, the fungal community of healthy and diseased tissue within colonies with aspergillosis was contrasted. Fungi were isolated from healthy and diseased fans from 15 reefs around Puerto Rico, and identified by sequencing the nuclear ribosomal ITS region and by morphology. Thirty fungal species belonging to 15 genera were isolated from 203 G. ventalina colonies. Penicillum and Aspergillus were the most common genera isolated from both healthy and diseased fans. However, the fungal community of healthy fans was distinct and more diverse than that of diseased ones. Within diseased fans, fungal communities from diseased tissues were distinct and more diverse than from healthy tissue. The reduction of fungi in diseased colonies may occur prior to infection due to environmental changes affecting the host, or after infection due to increase in dominance of the pathogen, or because of host responses to infection. Data also indicate that the fungal community of an entire sea fan colony is affected even when only a small portion of the colony suffers from aspergillosis. An unexpected result was that A. sydowii was found in healthy sea fans but never in diseased ones. This result suggests that A. sydowii is not the pathogen causing aspergillosis in the studied colonies, and suggests several fungi common to healthy and diseased colonies as opportunistic pathogens. Given that it is not clear that Aspergillus is the sole pathogen, calling this disease aspergillosis is an oversimplification at best.
Introduction
An alarming increase in diseases of corals has been reported in the last twenty years (Gardner et al. 2003). Since corals are the main structural components of coral reefs, these diseases may threaten this ecosystem in general (Bellwood et al. 2004). Of the coral diseases reported thus far, aspergillosis of sea fans is one of the best understood. This disease was first reported as causing tissue mortality in the Caribbean
sea fans Gorgonia ventalina and Gorgonia flabellum (Nagelkerken et al. 1997).
Aspergillus sydowii was identified as the causative agent of aspergillosis based on sequences of the 18S nuclear ribosomal gene, morphology, and Koch's postulates (Smith et al. 1996; Geiser et al. 1998). Three sources of inoculum have been proposed: 1) spores or hyphae of A. sydowii associated with terrestrial runoff or airborne dust; 2) physical contact between healthy and diseased sea fans, and 3) waterborne infection of healthy colonies by hyphae or spores from an infected colony (Jolles et al. 2002).
Incidence of sea fan aspergillosis has been related, in part, to a reduction in host defense and to an enhancement of fungal growth as a result of increases in water temperature (Alker et al. 2001; Dube et al. 2002; Kim and Harvell 2004). Sea fans aspergillosis is also characterized by high temporal and spatial variability (Kim and Harvell 2004), which complicates its study.
The sea fan aspergillosis literature states or assumes that A. sydowii is the responsible pathogen, as reported by Smith et al. (1996) and Geiser et al. (1998). However, lack of knowledge of the fungal community associated with healthy sea fans undermines an understanding of the roles of fungi when colonies become diseased. Recently, A. sydowii and other potentially pathogenic fungi were isolated from G. ventalina colonies without signs of aspergillosis (Toledo-Hernández et al. 2007). These findings highlight our ignorance of the basic microbial ecology of sea fans.
This study characterizes the fungal community associated with healthy and diseased G. ventalina colonies to address the following questions. Is the fungal community of healthy G. ventalina colonies different in diversity and composition to that of diseased ones? Within colonies with aspergillosis, does the fungal community of healthy tissue differ from that of diseased tissue? Is A. sydowii part of the resident mycoflora of healthy sea fan colonies? Can aspergillosis also be caused by other species of Aspergillus, or other fungi?
Materials and methods
Isolation and identification of fungi
Two hundred and three G. ventalina colonies from 13 reefs distributed around Puerto Rico were surveyed between 2004-06 (Figure 1). The number of colonies surveyed per reef ranged between 12 and 20. Of the 203 colonies surveyed, 122 were healthy colonies (i.e., no lesions, no purpling, nor any tissue overgrowth by fouling organisms), while 81 colonies showed signs of aspergillosis (necrotic area surrounded by a purple halo, as defined by Petes et al. 2003). Colonies were selected within an area of at least 1,500 m2 in each reef taking care not to select close neighbors. Diseased colonies were actively searched for, as they were relatively rare. The size of surveyed sea fans was 900 - 2,000cm2. Since diseased colonies < 900 cm2 were not observed, healthy colonies smaller than this were also excluded. One tissue sample of 2 x 2 cm was collected from each healthy colony while 2 tissue samples of 2 x 2 cm were collected from the diseased colonies, one from a healthy area and the other from an aspergillosis lesion. Tissue samples were immediately placed in individual sterile 50 ml tubes filled with filtered, autoclaved seawater and transported in a cooler with ice for processing within 24 h of collection. To eliminate fungi present on the surface of fan fragments but not colonizing internal tissues, each fragment was surface-sterilized individually in ethanol 70% for 30 sec and then washed in filtered, autoclaved sea water for another 30 sec.
Sodium hypochlorite was not used for sterilization because it dissolves the mesoglea, and may kill some fungi (Koh et al. 2000). Fragments were plated on Glucose Peptone Yeast Agar (GPYA), a standard medium for marine fungi (3 g glucose, 0.3 g yeast extract, 0.3 g peptone and 20 g agar l-1 filtered seawater) and incubated at 28oC in the dark for one month or until fungi were observed (Toledo-Hernández et al. 2007). Fungi were isolated in pure culture and (where possible) identified by morphology. However, some colonies did not sporulate and morphology-based identification was thus not possible; otherwise, identifications based on DNA sequence data agreed with morphology. Representative strains of each morphospecies were chosen for DNA extraction. For DNA extraction, emerging fungi were transferred to liquid Potato Dextrose (PD) medium made with filtered, autoclaved sea water. (PDA is more nutrientrich than GPYA; it is less useful as an isolation medium because it encourages the growth of bacteria, but extracts grown on PDA yield more DNA than GPYA.) After five days DNA was extracted using a Plant Mini Extraction Kit (Qiagen Sciences). The nuclear ribosomal ITS region was amplified using primers ITS1F and ITS4 and annealing temperature of 52-56o C (White et al. 1990) and sequenced. Sequences were corrected using Sequencher and the most similar sequences in GenBank were found using BLAST searches. When the top three matching BLAST hits were from the same species and ≥95% similar to the query sequence, this name was assigned to the culture.
Data analysis
Estimates of the number of fungal species in healthy tissue from healthy colonies (HH), and healthy and diseased tissues from diseased colonies (HD and DD respectively) were made using the Chao2 and Jackknife1 estimators in EstimateS 8.0.0 (Colwell 2006). A richness/species abundance coefficient Bray-Curtis (Sab) was estimated based on presence/absence matrix of fungi isolated from the three tissue types. An analysis of similarity (ANOSIM) and the contribution of each species to the average Bray-Curtis dissimilarity among the three tissue types (SIMPER) were performed using the statistical software PRIMER for Windows version 5.2.9. For this analysis 129 samples were used (no fungi were isolated from the other 74 colonies).
Results
Fungal diversity
Fifteen fungal genera, 30 species, and one unknown isolate with affinities to the Helotiaceae were isolated from the 203 G. ventalina colonies surveyed (Table 1). Aspergillus was the most common and the most diverse genus with 12 species and 64 isolates (Table 1). The second most common genus was Penicillium with 5 species and 33 isolates. Most of the remaining genera occurred as singletons and doubletons. Twenty-two taxa are new reports for G. ventalina and gorgonian corals in general: Aspergillus aculeatus, Aspergillus melleus, Aspergillus ochraceus, Aspergillus tamarii, Aspergillus terreus, Aspergillus versicolor, Candida sp., Chalaropsis sp., Cladosporium cladosporioides, Helotiaceae, Hypocrea lixii, Nectria haematococca, Nectria gliocladioides, Penicillium chrysogenum, Penicillium minioluteum, Pichia guilliermondii, Stachybotrys chlorohalonata, Trichoderma harzianum, and Tritirachium spp. For some fungi, identification inferred from BLAST searches could not distinguish between closely related species. This could reflect limitations of the sequences currently available in
GenBank or taxonomic problems in certain species groups (i.e., Aspergillus flavus vs. Aspergillus oryzae (Geiser et al. 1998, 2000).
Variation in fungal community structure among tissue types
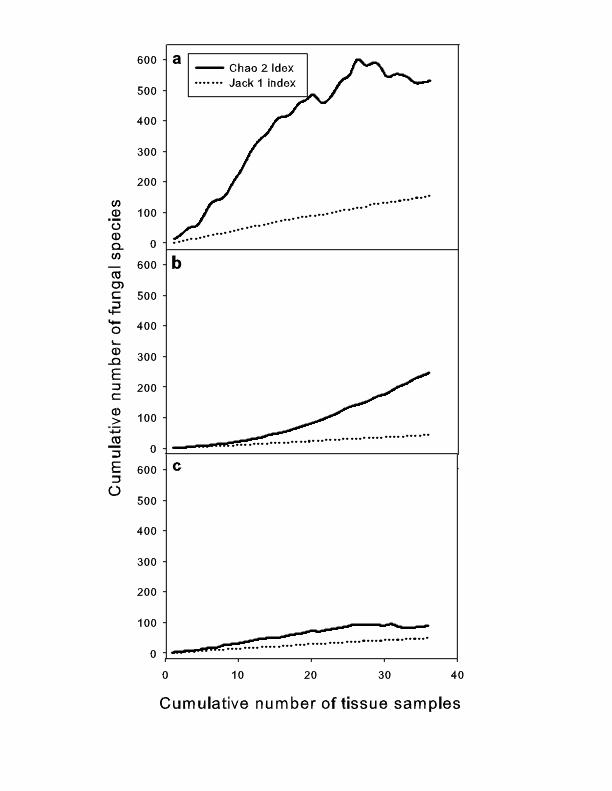
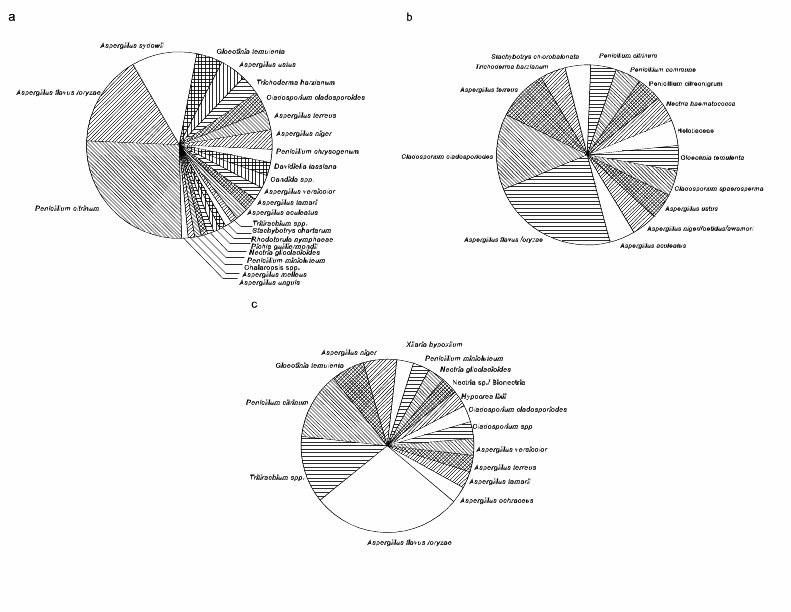
Species richness varied among the three tissue types. HH (healthy tissue from healthy colonies) was highest (25 taxa and 88 isolates, 6 of which could not be identified) followed by DD (diseased tissue from diseased colonies, 15 taxa and 32 isolates, 13 of which could not be identified) and HD (healthy tissue from diseased colonies, 15 taxa and 22 isolates, 6 of which could not be identified) (Table 1). Although no asymptote was reached in any tissue type, the Chao2 and Jackknife 1 indexes predicted the highest species richness for HH (Figure 2). This suggests that the highest fungal species richness in HH was not due to greater sampling effort, but to a natural pattern.
The most common fungi differed among tissue types. A. flavus, A. sydowii and Penicillium citrinum were the most frequently isolated fungi in HH (Table 1; Figure 3). A. sydowii was only isolated from healthy colonies, contrary to expectations. In contrast, A. flavus was the most common fungus in HD followed by C. cladosporoides (Table 1; Figure 3). In DD, A. flavus, P. citrinum and Tritirachium spp. were the most frequently isolated fungi (Table 1; Figure 3). Of all the isolated fungi, 5 were common to all tissue types, 3 were shared exclusively between HH and HD, 6 were shared exclusively between HH and DD, while no fungi were shared exclusively between HD and DD (Table 1). Moreover, 9 fungi were exclusively isolated from HH (including A. sydowii), 7 were exclusively isolated from HD and 4 fungi in DD (Table 1, Figure 3). The ANOSIM test showed significant differences in fungal communities among tissue types (r = 0.034; P = 0.002). The SIMPER analysis showed 93.6% dissimilarity between HH and HD, 90% dissimilarity between HH and DD and 91.3% dissimilarity between HD and DD. HH was distinguished from HD and DD by the greater relative abundance of A. flavus, A. sydowii, and P. citrinum whereas HD was distinguished from DD by the greater relative abundance of A. flavus, P. citrinum, and C. cladosporiodes. Only one fungus was more common in diseased tissue than in other tissue types: Tritirachium sp. (Table 1).
Discussion
Fungal diversity associated with gorgonians
This study, in conjunction with those of Koh et al. (2000) and Toledo-Hernández et al. (2007) shows that sea fans have a diverse and variable fungal community. Overall, 65 fungal taxa have been identified from 11 gorgonian species using culture-dependent techniques. Sixteen fungal genera and 51 species (including two yeasts) were isolated from 10 species of gorgonian corals in Singapore (Koh et al. 2000). All of these fungi were new reports for gorgonians. Eight genera of fungi and 15 species were isolated from the Caribbean sea fan G. ventalina (Toledo-Hernández et al. 2007). Four of these genera, Rhodotorula, Stachybotrys, Gloeotinia and Xylaria, and an unknown fungus with affinities to the Xylariales were new reports for any gorgonian coral. In this study, at least 35 fungal species from 15 genera were identified from 203 G. ventalina colonies. Twenty-three of these species are new reports for G. ventalina or any other gorgonian coral. Here as well as in previous studies (Koh et al. 2000; Toledo-Hernández et al. 2007), Aspergillus and Penicillium were the most frequently isolated and diverse
genera. Aspergillus and Penicillium also appear to be common in other marine invertebrates such as scleractinian corals (Kendrick et al. 1982, Priess et al. 2000) and sponges (Höller et al. 2000). Some of these species (e.g., A. sydowii) are believed to be essentially terrestrial organisms, capable of growing in the sea but incapable of sporulating there (Smith et al. 1996). However, their presence in many marine invertebrates suggests they are ubiquitous in the marine environment.
Differences in fungal diversity between healthy and diseased sea fans
This study shows that (1) the fungal community of healthy sea fans is distinct from, and more diverse than, that of diseased fans, and (2) within afflicted colonies the fungal community of lesions is different and more diverse than that of healthy tissue. Previous studies have reported differences in bacterial communities between healthy and diseased tissue from diseased scleractinian corals (Frías-López et al. 2002; Casas et al. 2004; Breitbart et al. 2005). However, these studies did not include healthy colonies, which were found here to be distinct from healthy tissue in diseased colonies. Only one previous study has contrasted the microbial community between healthy and diseased colonies as well as between healthy and afflicted tissue within diseased colonies (Pantos et al. 2003). Bacterial communities in Montastraea annularis followed the same pattern observed here for fungi in G. ventalina: healthy tissue in healthy colonies was most diverse, healthy tissue from diseased colonies was least diverse, and diseased tissue was intermediate (Pantos et al. 2003). There are three plausible explanations why healthy tissue from diseased colonies showed decreased microbial richness compared to diseased tissue. First, the reduction of microbial diversity in diseased colonies may be directly caused by an increase in dominance of the pathogen (Ward et al. 2007). Second, environmental changes may reduce microbial diversity, making the host more susceptible to pathogens (Pantos et al. 2003). Third, the colony may have a generalized physiological response to a local infection, which could affect commensal microorganisms as well as pathogens. Similarly, localized infections have a general effect on reproduction: even small lesions affect reproductive success of the whole colony (Petes et al. 2003).
Is A. sydowii a pathogen?
The most striking result of this study is that A. sydowii, the reported causal agent of sea fan aspergillosis, was not isolated from diseased G. ventalina colonies, but only from healthy colonies. These observations cast doubt on the role of A. sydowii as the pathogen (either primary or opportunistic) of sea fan aspergillosis, at least in the colonies studied. The most common isolates (e.g., A. flavus and P. citrinum) were found in both healthy and diseased colonies, suggesting they may be opportunistic pathogens capable of causing aspergillosis.
These two species are prime suspects. A. flavus is an opportunistic pathogen of insects, bird, humans and plants (Yu et al. 2005) and produces secondary metabolites called aflatoxins that are toxic, carcinogenic, teratogenic, and immunosuppressive in animals when ingested (Pitt 2000). P. citrinum produces the toxin citrinin, associated with renal toxicity in animals (Malmstrom et al. 2000). It is not known if any of these mycotoxins are produced in vivo in sea fans, but if they are, it is possible they play a role in the disease.
Another possible pathogen is Tritirachium sp., the only species that was more common in diseased tissue than in other tissue types. Tritirachium has not been reported from corals previously, but based on phylogeny, this fungus might be expected to be a pathogen: it belongs to the Clavicipitaceae, all members of which are obligate pathogens of invertebrates or plants.
Alternatively, sea fan aspergillosis may be caused by pathogens other than fungi. However, little or no effort has been devoted to study the bacteria, protists and viruses associated with sea fans, perhaps because A. sydowii is widely assumed to be the primary pathogen. Controlled infection experiments are needed to test pathogenicity of fungi and other microorganisms found in sea fans.
Methodology and sampling issues
In this study, fungi were cultured before being identified, so any fungi that did not grow in culture were overlooked. Estimates of diversity presented here therefore underrepresent total fungal diversity, though it is impossible to say by how much. Studies comparing culturable and nonculturable fungi in other communities suggest that nonculturable species are a much smaller percentage of total diversity for fungi than for bacteria. In fungal endophytes of pine, for example, culturing revealed more major groups of fungi than direct amplification, and was more effective at revealing species richness of certain groups of fungi (Arnold et al. 2007). Since each technique revealed some organisms not revealed by the other, a combination of direct amplification and culturing is the optimal approach for fungal diversity studies (Rohwer et al. 2001; Bayman 2007). Direct amplification of fungi and other microorganisms from sea fan tissue would clearly reveal more species. Nevertheless, this limitation does not affect the most important conclusion of this study, that regarding A. sydowii. Like most species of Aspergillus, A. sydowii grows readily in culture under a wide range of conditions. If anything, culture-based studies may overstate the importance of Aspergillus and Penicillium relative to fungi that do not grow so rapidly and prolifically on agar media.
The present study demonstrates that G. ventalina colonies contain a large, diverse, and mostly unknown fungal community. It also shows that the fungal community of an entire sea fan colony is affected even when only a relatively small portion of the colony suffers from aspergillosis; the fact that this pattern was also observed in bacterial communities in scleractinian corals (Pantos et al. 2003) suggests that it may be a general phenomenon, though the cause is unclear. This study did not find A. sydowii associated with diseased colonies, suggesting that A. sydowii is not the pathogen, at least in Puerto Rico. The presence of several common fungi in both diseased and healthy colonies suggests that aspergillosis may not always be a result of the arrival of new pathogens, but a change in population size or pathogenicity of fungi already present. The importance of environmental stress in the development of aspergillosis has been discussed (Lesser et al. 2007). However, population dynamics of microorganisms in corals and the interaction between microbial communities and stress are not understood.
Initial reports that A. sydowii was the pathogen responsible for aspergillosis in Gorgonia were no doubt accurate (Smith et al. 1996; Geiser et al. 1998); they reflected pathogens of gorgonians in certain areas at certain times. However, subsequent articles generalized these results to mean that A. sydowii was the cause of aspergillosis of
gorgonians throughout the Caribbean, which is clearly not the case. Given that it is not clear that Aspergillus—or any fungus--is the sole pathogen, calling this disease aspergillosis is an oversimplification. Aspergillosis of sea fans has been viewed as one of the best-understood coral diseases; the fact that it turns out to be more complicated than previously thought implies that other coral diseases may be so as well.
Acknowledgments This project was supported by UPR Sea Grant (NOAA award NA16RG2278, project R-92-1-04), NIH SCoRE (2S06GM08102), NSF CREST (HRD 0734826) and NOAA-CRES. CTH thanks the RISE -NIH program for a research fellowship (2 R25 GM061151) and the International Society for Reef Studies (ISRS) for an ISRS/TOC Coral Reef Conservation Award (2006). Sequencing was done at the UPR Sequencing & Genotyping Facility, which is supported in part by NCRR-AABRE Grant #P20 RR16470, NIH-SCORE Grant #S06GM08102, and NSF-CREST. We thank Omara Ortíz-Vázquez for help in the lab, Yogani Govender and Daniel Dávila-Casanova for the map and Paul Yoshioka for advice and inspiration.
References
Alker AP, Smith GW, Kim K (2001) Characterization of Aspergillus sydowii (Thom et Church), a fungal pathogen of Caribbean sea fan corals. Hydrobiologia 460:105111
Arnold AE, Henk DA, Eells RL, Lutzoni F, Vilgalys R. (2007) Diversity and phylogenetic affinities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia 99:185-206
Bayman P (2007) Fungal endophytes. In: Kubicek CP, Druzhinina IS (eds) The Mycota IV. Environmental and microbial relationships. Springer, Berlin, pp 213-227
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827-833
Breitbart M, Bhagooli R, Griffin S, Johnston I, Rohwer F (2005) Microbial communities associated with skeletal tumors on Porites compressa. FEMS Microbiol Lett 243:431-436
Casas V, Kline DI, Wegley L, Yu Y, Breitbart, Rohwer F (2004) Widespread association of a Richettsiales-like bacterium with reef-building corals. Environ Microbiol 6:1137-1148
Colwell RK (2006) EstimateS: statistical estimation of species richness and shared species from sample. Version 7.5. http://viceroy.eeb.uconn.edu/estimates
Dube D, Kim K, Alker AP, Harvell CD (2002) Size structure and geographic variation in chemical resistance of sea fan corals Gorgonia ventalina to fungal pathogen. Mar Ecol Prog Ser 231:139-150
Frías-López J, Aubrey AL, Bonheyo GT, Fouke BW (2002) Partitioning of bacterial communities between seawater and healthy, Black Band Diseased and dead coral surface. Appl Environ Microbiol 68:2214-2228
Höller U, Wright AD,. Matthee GF, Konig GM, Draeger S, Aust HJ, Shulz B (2000) Fungi from marine sponges: diversity, biological activity and secondary metabolites. Microbiol Res 104:1354-1365
Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958-960
Geiser DM, Dorner JW, Horn BW, Taylor JW (2000) The phylogenetics of mycotoxin and sclerotium production in Aspergillus flavus and Aspergillus oryzae. Fungal Genet Biol 31:169-179
Geiser DM, Taylor JW, Ritchie KB, Smith GW (1998) Cause of sea fan death in the West Indies. Nature 394:137-138
Jolles EA, Sullivan P, Alker AP, Harvell CD (2002) Disease transmission of aspergillosis in sea fans: inferring process from spatial pattern. Ecology 83:2373-2378
Kendrick B, Risk MJ, Michaelides J, Bergman K (1982) Amphibious microborers: bioeroding fungi isolated from live corals. Bull Mar Sci 32:862-867
Kim K, Harvell CD (2004) The rise and fall of six-year coral fungal epizootic. Am Nat 164:S52-S63
Koh LL, Tan TK, Chou LM, Goh NKC (2000) Fungi associated with gorgonians in Singapore. Proc 9th Int Coral Reef Symp 1:521-526
Lesser MP, Bythell JC, Gates RD, Johnstone RW, Hoegh-Guldberg O (2007) Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. J Exp Mar Biol Ecol 346:36-44
Malmstrom J, Christophersen C, Frisvad KC (2000) Secondary metabolites characteristic of Penicillium citrinum, Penicillium stechii and related species. Phytochemistry 54:301-309
Nagelkerken IK, Smith GW, Bonair K, Bush P, Garzón-Ferriera J, Botero L, Gayle P, Harvell CD, Heberer C, Kim K, Petrovic C, Pors L, Yoshioka P (1997) Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Mar Ecol Prog Ser 160:255-263
Pantos O, Cooney RP, LeTissier MDA, Barer MR, O’Donnell GO, Bythell JC (2003) The bacterial ecology of a plague-like disease affecting the Caribbean coral Monstastrea annularis. Environ Microbiol 5:370-382
Petes LE, Harvell CD, Peters EC, Webb AM, Mullen KM (2003) Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar Ecol Prog Ser 264:167-171
Pitt JI (2000) Toxigenic fungi: which are important? Med Mycol 38S:17-22
Priess K, Le Campion-Alsumard T, Golubic S, Gadel F, Thomassin BA (2000) Fungi in corals:black bands and density-banding of Porites lutea and P.lobata skeleton. Mar Biol 136:19-27
Rohwer F, Breitbart M, Jara J, Azam F, Knowlton N (2001) Diversity of bacteria associated with the Caribbean coral Montastrea franksi. Coral Reefs 20:85-91
Smith GW, Ives LD, Nagelkerken IA, Ritchie KB (1996) Caribbean sea fan mortalities.Nature 383:487
Toledo-Hernández C, Bones-Gonzáles A, Ortiz-Vázquez OE, Sabat AM, Bayman P (2007) Fungi in the sea fan Gorgonia ventalina: diversity and sampling strategies. Coral Reefs 26:725-730
Ward JR, Kim K, Harvell CD (2007) Temperature affects coral disease resistance anpathogen growth. Mar Ecol Prog Ser 329:115-121
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing offungal ribosomal RNA genes for phylogenetics. Chapter 38. In: Innis AM, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–324
Whitaker RJ, Grogan DW, Taylor JW. 2003. Geographic barriers isolate endemicpopulations of hyperthermophilic Archaea. Science 301:976-978
Yu J, Cleveland TE, Nierman WC, Bennett JW (2005) Aspergillus flavus genomics: gateway to human and animal health, food safety, and crop resistance to diseases. Rev Iberoam Micol 22:194-202
Table 1. Fungi isolated from Gorgonia ventalina as identified by ITS sequences. Three tissue types were used for isolations: healthy tissue from healthy G. ventalina colonies (HH), and healthy tissue (HD) and diseased tissue (DD) from diseased colonies. Range of maximum identity of the closest three matches listed in GenBank (Max ID %); number of base pairs sequences (bp); GenBank accession numbers of isolates from G. ventalina Aspergillus melleusa Aspergillus ochraceusa and Trichoderma harzianuma were identified on the basis of morphology alone. Unknownb fungi were lost before they could be sequenced or identified by morphology
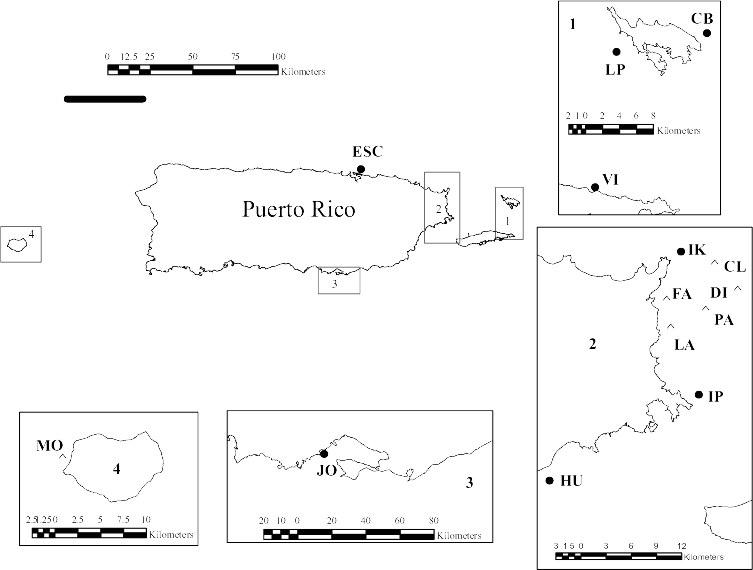
Figure 1. Map of Puerto Rico showing collection sites. LP Luis Peña, CR Carlos
Rosario, CB Culebrita, PT Punta Soldado, VI Vieques Island, ESC Escambrón, PN
Piñones, IK Icacos, IP Isla Piñero, HU Humacao, JO Jobos, MO Mona
Aspergillusmelleus
Aspergillusunguis 1 0 0 99 595 EU554612
Aspergillusustus 4 1 0 99 519 EU554620
Aspergillusversicolor 2 0 1 99-100 450-525 EU554616
Candida sp. 2 0 0 100 747 EU554623 Chalaropsis sp. 1
Cladosporium sp a 0 0 1
Cladosporiumcladosporioides 3 3 1
446 EU554628
538 EU554601
Cladosporiumspaerosperma 0 1 0 99 509 EU554622
Davidiellatassiana 2 0 0 99-100 514-538 EU554613
Gloeotiniatemulenta 4 1 2 99-100 453-574 EU554591
Helotiaceae 0 1 0 98 491 EU554571
Hypocrealixii 0 0 1 99 520 EU554589
Nectria sp./Bionectria 0 0 1 90 520 EU554570
Nectriagliocladioides 1 0 1 98-99 512-516 EU554567
Nectriahaematococca 0 1 0 82 520 EU554568
Penicilliumchrysogenum 2 0 0 100 549 EU554618
Penicilliumcitreonigrum 0 1 0 99 578 EU558538
Penicilliumcommune 0 1 0 100 534 EU554627
Penicilliumminioluteum 1 0 1 99-99 537-529 EU554569
Penicillumcitrinum 23 1 4 99-100 219-543 EU554607
Pichiaguilliermondii 1 0 0 98 536 EU554590
Rhodotorulanymphaeae 1 0 0 99 530 EU558535
Stachybotryschartarum 1 0 0 99 433 EU554595
Stachybotryschlorohalonata 0 1 0 99 491 EU554624
Trichodermaharzianuma 3 1 0
Tritirachium spp. 1 0 4 95-99 509 EU554617
Xylariahypoxylon 0 0 1 99 563 EU558536 Unknownb 6 5 13 Totals 94 27 45
Figu re 1.
Map of Puer to Rico
showing collection sites. LP Luis Peña, CR Carlos Rosario, CB Culebrita, PT Punta
Soldado, VI Vieques Island, ESC Escambrón, PN Piñones, IK Icacos, IP Isla Piñero, HU
Humacao, JO Jobos, MO Mona
Figure 2. Species accumulation curves of Chao2 and Jackknife1 estimators for fungi isolated from healthy tissue of (a) healthy Gorgonia ventalina colonies, (b) healthy tissue, and (c) diseased tissue of diseased
Figure 3. Pie diagram illustrating the observed frequencies in percentages of fungi isolated from healthy tissue from (a) healthy Gorgonia ventalina colonies, (b) healthy tissue, and (c) diseased tissue from diseased colonies.
Spatial Variation in the Fungal Community of Healthy and Diseased Sea Fans (Gorgonia ventalina) and Surrounding Seawater
Anabella Zuluaga-Montero, Carlos Toledo-Hernández, José A. Rodríguez, Alberto Sabat, and Paul Bayman
Submitted to Microbial Ecology, April 22 2008
Abstract
Caribbean sea fans are being attacked by aspergillosis, a disease associated to fungi of Aspergillus group. However, very little is known about the natural microbial community in sea fans, including its fungi. Even less is understood about the temporal and spatial variation in species composition or the source of inoculum. Patterns of spatial and temporal variation may provide important clues to the source of pathogens and etiology of the disease.The objectives of this study were: (1) to measure the spatial variation in the mycoflora associated with diseased and healthy colonies of Gorgonia ventalina and (2) to compare the mycoflora of sea fans with the mycoflora isolated from surrounding seawater in different reefs in Puerto Rico. Samples of seawater were collected from 14 coral reefs. Diseased and healthy sea fan tissue was sampled in eight reefs. Fungi were isolated and identified by morphology and sequencing of the nuclear ribosomal ITS region. Twenty-six species of culturable fungi were identified from seawater and sea fan tissue from all sites. Aspergillus flavus was the most common species isolated from both seawater and sea fan tissue. Higher species richness was found in seawater (23 species) than in sea fans (18 species). The composition of the mycoflora of healthy and diseased sea fans differed significantly among sites; variation in sea fans was much higher in healthy than in diseased colonies. Fungal species richness in seawater was higher inshore than offshore. However, the community composition in seawater did not differ significantly among sites. The fungal community of sea fans was not different from that of the surrounding seawater. The lower diversity in diseased colonies can be explained by one or a few microorganisms becoming dominant and pathogenic. Aspergillus sydowii was not isolated from diseased colonies, suggesting that aspergillosis may be caused by a consortium of opportunistic fungi. The data also suggest that the source of offshore mycoflora is the inshore fungal community, with richness diluted as result of ocean circulation. The higher richness observed in seawater as compared to sea fans suggests that the source of sea fan fungi is the surrounding sea water. The similarity between seawater and sea fans suggests low specificity of sea fans for fungi.
Introduction
Infectious diseases of corals have increased in the last twenty years [11, 18, 33]. Few of these diseases have been characterized in detail, but the pathogens have been identified in some of them. For example, white pox of elkhorn coral (Acropora palmata) has been linked to the bacterium Serratia marcescens [23], bleaching in Oculina patagonica to Vibrio shiloi [29] and aspergillosis in sea fans to Aspergillus sydowii [8]. However, isolation and identification of primary pathogens may not be sufficient to understand the dynamics of coral diseases. Several of the microorganisms that have been identified in diseased colonies and implicated in coral diseases have also been found in healthy colonies [Toledo et al. in press, 7, 14, 16, 20, 34]. Furthermore, some coral diseases are believed to be caused by consortia of microorganisms and not by a single pathogen (e.g. black band disease) [6; 22].
In order to better understand the etiology of coral diseases it is necessary to develop the basic microbial ecology of corals [7]. For example, Aspergillus species are generally considered to be terrestrial fungi that find their way to coral reefs as a result of runoff or Sahara dust deposition [30, 39]. In fact, many fungal species reported in marine organisms are common in terrestrial habitats [13, 17, 20], and relatively little is known about their ubiquity and roles in marine ecosystems [8, 32, 34]. Moreover, spatial variation of these microbial populations is even less understood. To put diseases of corals in an etiological context, it is essential to understand the microflora not only of the hosts, but also of the environment surrounding the hosts. This approach may allow the elucidation of host selectivity and possible reservoirs of inoculum.
In this study, we determined the spatial variation (i.e., differences among reefs) of the mycoflora associated with both diseased and healthy sea fans of Gorgonia ventalina in Puerto Rico and compared it with that of the surrounding seawater. The following questions were addressed: (1) Does the mycoflora of healthy sea fans vary among reefs? (2) Is the same level of variation among reefs found in diseased sea fans or (3) in the surrounding seawater? (4) Are certain fungi more frequent in diseased sea fans than in healthy sea fans? (5) How similar are the fungi communities in sea fans and the surrounding seawater? High spatial variation should be expected in the mycoflora of healthy sea fans and seawater, because local events such as precipitation, runoff and currents will cause variations in arrival of inoculum. Low spatial variation in mycoflora is expected in diseased sea fans due to dominance of A. sydowii across sites. Assuming that seawater is the reservoir and that sea fans are not selective, we expect high similarity between seawater and sea fan mycoflora.
Methods
Sample collection and culturing. We sampled seawater from 14 coral reefs around Puerto Rico from July to September 2006 (Fig. 1). Escambrón on the north coast; Fajardo, Cayo Largo, Palominito Island, Cayo Diablo, Cayo Lobo, Icacos Island and Isla Piñeros on the east coast; and Humacao and Jobos on the southeast coast. In addition to these near-shore sites, we collected at several small islands off Puerto Rico: Luis Peña, Culebrita Island, and Vieques Island at 30, 40 and 35 km off the east coast,
respectively. Samples were also collected at Mona Island located 68 km off the southwest coast of Puerto Rico.
Three samples of water were collected near the sea fan colonies at each site. Samples were collected in 250 ml-sterilized bottles and were stored at 4°C. In the laboratory, fungi were concentrated by filtering water with sterile nitrocellulose membranes of 0.45 µm pore size (Fisher Sci). The filters were cultured in petri plates containing Glucose Peptone Yeast Agar (GPYA) with 3.3% salt (34). All fungi observed were transferred to new plates of GPYA to obtain pure isolates. These isolates were transferred to liquid medium (potato-dextrose broth) for DNA extraction.
Sea fan tissues (Gorgonia ventalina) were collected from eight of the 14 sites where water samples were collected, i.e., Escambrón, Isla Piñeros, Jobos, Humacao, Icacos, Luis Peña, Culebrita, and Vieques (Fig 1). A tissue fragment (4 cm2) from five healthy colonies and five diseased colonies were collected at each site, for a total of 80 tissue samples. Healthy and diseased colonies were distinguished following the criteria described by Toledo et al (in press). Fragments were surface-sterilized in ethanol 70%, then washed in distilled autoclaved water and cultured in GPYA with salt as described by Toledo-Hernández et al. [34]. Fungi were isolated as described above.
DNA extraction, amplification and sequencing. DNA was extracted using a Plant Mini Extraction Kit (Qiagen Sciences). The nuclear ribosomal ITS region was amplified using primers ITS 1F and ITS 4 [39].The PCR amplification was done using Taq PCR master mix kit (Qiagen Sciences). Samples were run using the PCR amplification profile used to amplify Aspergillus from coral tissue [9]. ITS sequences were corrected with Sequencher and used for BLAST searches in GenBank. When the top three matching BLAST hits were from the same species and had ≥95% similarity to the query sequence, this name was assigned to the culture.
Data analyses. Species accumulation curves were used to determine if sampling was sufficient to characterize the culturable fungal community in seawater and sea fan tissue. Multivariate statistical analyses were conducted using Primer 6 software (Plymouth Marine Lab, UK) to test for differences in the fungal communities among reefs and between tissue and seawater. The similarity of each of these fungal communities was analyzed using Bray-Curtis similarity coefficients [3] with presenceabsence data. One-way analysis of similarity (ANOSIM) was done to test for differences in dissimilarities of the fungal communities of healthy and diseased sea fans among reefs [4]. Post hoc analyses of similarities percentage (SIMPER) was used to determine the contribution of each fungal species for those communities that resulted significantly different. The fungal communities in sea fan tissues were compared with a one-way ANOSIM with combination of factors to test for differences between healthy vs. diseased tissue and among reefs [5]. The fungal community in seawater was analyzed with one- way ANOSIM grouping reefs within the inshore and offshore criteria. The distance from the mainland (inshore vs. offshore) was used as the categorical factor for comparison.
A Bray-Curtis analysis was also performed to compare the fungal communities between seawater and sea fan tissue (healthy and diseased). Eight reef sites were included as replicates using presence-absence data. One-way ANOSIM was used to
determine the significance of similarities among sources (seawater vs. tissue). The SIMPER analysis was used to identify the species contributing most to the dissimilarity.
Results
Fungal diversity and sampling A total of 26 species of culturable fungi were identified: 23 from seawater and 15 from sea fan colonies, with ten species shared (GenBank numbers EU 645653-EU 645743). The species accumulation curve for healthy and diseased tissue approached an asymptote (Fig. 2a, 2b), and the jackknife index indicated that sampling effort was sufficient. However, for seawater the curve did not saturate, indicating that the fungal community in seawater is richer and more sampling effort is needed to characterize it (Fig. 2c).
Fungal community in healthy sea fans. Thirteen species of fungi in six genera were isolated. The genus Aspergillus was the most diverse with six species. The most common Aspergillus species in healthy tissue were A. flavus and A. sydowii, found in five and four of the eight sites, respectively (Table 1).
There were significant differences in the fungal community of healthy colonies among sites (ANOSIM, R = 0.174, p = 0.004). According to the SIMPER analysis, Jobos showed significant dissimilarities with Humacao and Luis Peña. Aspergillus flavus, A. niger and Cladosporium sp. were the main species that contributed to these dissimilarities (Table 2a). Other significant dissimilarities were found between Humacao and Luis Peña, and Humacao and Escambrón, where A. niger and Penicillium citrinum contributed most to the dissimilarities, while A. flavus, A. niger and A. sydowii contributed to the dissimilarities between Culebrita and Humacao. Thus, even the most common species contributed to differences among sites.
Fungal community in diseased sea fans. Ten species of fungi in six genera were isolated from diseased sea fans. The most common species was A. flavus, which occurred in six reefs. Surprisingly, A. sydowii was not isolated from diseased tissue from any of the reefs. Also, the diversity of fungi was low and it was common to find singleton species not shared with other diseased colonies in other reefs (Table 1). There were significant differences among sites in the fungal community in diseased tissue (one- way ANOSIM, R = 0.383, p = 0.003). Jobos showed significant dissimilarities with Icacos and Luis Peña (SIMPER analysis). Aspergillus flavus, Gloeotinia temulenta and P. citrinum were the main species that contributed to these dissimilarities (Table 2b).
When the fungal composition was compared between healthy and diseased tissues, significant differences were found between reefs (one-way ANOSIM, Combined factors: type of tissue- site, R = 0.279, p = 0.001). According to the SIMPER analysis, healthy colonies from Jobos showed significant dissimilarities with diseased colonies from Piñeros and Icacos. Likewise, diseased colonies from Jobos showed significant dissimilarities with healthy colonies of Piñeros and Humacao. A. flavus, Cladosporium sp. and G. temulenta were the most important species contributing to these dissimilarities (Table 2c).
Fungal community in seawater. Twenty three species of fungi in six genera were isolated from seawater (Table 3). Water from Escambrón showed the lowest diversity (1 species) and Palominito island the highest (10 species). As in sea fans, Aspergillus was the most diverse genus (9 species). Twenty-two species were found in the seawater of inshore reefs; P. citrinum, A. aculeatus, A. sydowii, A. tamarii and Cladosporium sp. were the most common. Only nine species were found offshore (A. flavus and Penicillium sp. as common species) and almost all offshore species were shared with inshore reefs (Table 3). More species were shared among sites in seawater than were shared among sea fans. No significant difference in fungal communities isolated from inshore and offshore seawater was found (ANOSIM, R = 0.088, p = 0.244).
Comparison of fungal communities among seawater and diseased and healthy sea fans. Ten species were shared among seawater, diseased and healthy tissues, A. flavus being the most common species in both seawater and sea fan tissues Other shared species were A. aculeatus, A. ochraceus, A. niger, A. sydowii, A. tamarii, A. versicolor, Penicillium sp., P. citrinum and Cladosporium sp. No significant differences were found between the fungal communities from healthy sea fans and seawater (ANOSIM, R = -0.011, p = 0.48). Likewise, differences between diseased sea fans and seawater were not significant (ANOSIM, R = 0.141, p = 0.091). There was no evidence that sea fans are selective for a certain subset of the fungi found in seawater.
Discussion
Fungal diversity. Aspergillus and Penicillium appear to be ubiquitous in the sea fan Gorgonia ventalina in Puerto Rico. The most frequent species were A. flavus, A sydowii and P. citrinum. This result is consistent with previous studies in Puerto Rico [34, Toledo et al. in press). Other studies have reported Penicillium as ubiquitous in different gorgonian species [16]. The presence of Aspergillus and Penicillium species in scleractinian corals has also been reported [13]. Penicillium, along with other genera such as Cladosporium, Fusarium, and Acremonium, are frequently found associated with sponges [12]. It appears that these fungal groups are successful at colonizing different hosts and are common in many marine organisms.
Greater diversity probably would have been revealed by using direct amplification of DNA from sea fans and seawater instead of culturing, since microorganisms that do not grow in culture could thus have been identified [2]. However, Aspergillus is the center of attention in this study because A. sydowii is considered the sole causal agent of aspergillosis in sea fans [8,32]. The fact that Aspergillus species grow easily in culture means that direct amplification is not necessary to study their spatial dynamics [34]
Spatial variation in the mycoflora of healthy sea fans. We observed significant differences among sites in the mycoflora associated with healthy sea fans. Three reefs were most responsible for the spatial variation: Jobos, Humacao and Luis Peña. These dissimilarities suggest that local environmental factors may influence the fungal composition of sea fans. Jobos is an estuary, historically impacted by agricultural runoff and a major sugar cane mill, and currently by the chronic discharge of warm
waters from a thermoelectric plant [27]. Sea fans from Jobos had more fungal species and more isolates than other sites, suggesting that the elevated seawater temperatures caused by thermoelectric discharges promote growth and colonization of certain fungi. The average water temperature in Jobos near to our sampling site was 29.7-31.3°C during September- October 2006 (http://cdmo.baruch.sc.edu, station #20), whereas temperatures in coastal waters were 28-30 °C in this period (http://coralreefwatch.noaa.gov). High temperature promotes growth of A. flavus in plants [21] and clinical infections [41]. For example, A. sydowii grows best at 31°C [37], and A. flavus can survive for years at 47°C [15]. Also, Jobos is a large, closed bay with a small entrance, which may limit microbial exchange with adjacent areas and thus promote a distinctive fungal community. On the other hand, Humacao reef is influenced by sewage discharges (Toledo, personal communication), while the Luis Peña reef is a marine reserve located offshore, and is characterized by good water quality [35].
Very little is known about biological, ecological or environmental factors that structure marine microbiological communities, in contrast to terrestrial communities that have been extensively studied [see 18]. However, the spatial variation of the mycoflora in sea fans may be a response of the origin and the dispersal patterns of the inoculum. Dispersal patterns of fungi in the sea have not been studied.
Spatial variation in the mycoflora in diseased sea fans. The mycoflora associated with diseased sea fan colonies also varied significantly among reef sites. Again, Jobos and Luis Peña were sites that contributed to the spatial variation in diseased colonies. The lower number of species found in diseased colonies in all sites was expected because the dominance of certain pathogen(s) may inhibit the growth of other microorganisms which occur in healthy tissues [25]. However, the observed high spatial variation in diseased colonies is contrary to the expectation of dominance by a single (eg. A. sydowii) or few pathogens across sites. Rather, it is consistent with the hypothesis that aspergillosis in sea fans is caused by a consortium of opportunistic fungi varying in space and time (Toledo-Hernández et al. in press).
Studies of bacterial communities of diseased corals have found significant spatial variation, both among geographical areas and at a local scale [6, 36]. Our results show that this pattern also applies to fungal communities associated with diseased corals, which vary among reefs a few kilometers apart. The fact that we also found significant spatial variation among healthy colonies suggests that this variation is a characteristic of marine microbial communities in general, not only those associated with diseased colonies.
Spatial variation in the mycoflora between diseased and healthy sea fans. In this study, the differences in the mycoflora between diseased and healthy colonies were more attributable to differences between reefs than to the health of the colony. In contrast, significant differences were found between fungal communities in healthy and diseased sea fan tissue [Toledo et al in press]. However, the analyses of Toledo-Hernández et al. combined data from several reefs. Our results suggest that the differences they observed may have partly been due to differences among reefs rather than differences between healthy and diseased tissue.
In sclerectinian corals, however bacterial communities differ between healthy and diseased colonies. The bacterial composition in corals may be controlled in part by the coral itself promoting selectivity [14, 22, 25, 26, 28]. However, this pattern was not
observed for fungi. Our study suggests that many fungi found in seawater are successful colonizers of sea fans and provides little evidence for selectivity.
Spatial variation in the mycoflora in seawater. The mycoflora was more diverse in seawater than in sea fans. Moreover, the fact that the species accumulation curve for seawater did not show a tendency towards saturation indicates that the mycoplankton community is highly diverse. P. citrinum, A. aculeatus, A. sydowii A. tamarii and Cladosporium sp. were the most common species isolated from seawater in sites inshore. Of these, A. aculeatus was only found in one offshore site, suggesting that the source of this fungus is terrestrial. Sites offshore were less diverse, with most offshore species shared with inshore sites. These shared species explain why the dissimilarity between the inshore and offshore community was not significant. These data suggest that an important source of offshore mycoflora is the inshore community, with richness diluted as result of ocean circulation. Surveys of inshore and offshore bacterioplankton from Curacao suggest that differences in the proportion of cyanobacteria found between them may reflect the influence of terrestrial runoff, coastal pollution or inorganic sedimentation inshore [7].
Aspergillus species as opportunistic pathogens. Several species of Aspergillus are recognized as opportunistic pathogens. Aspergillus flavus is a pathogen of crops, birds, insects and humans, which has been extensively studied in food safety and clinical contexts [1]. Similarly, Aspergillus sydowii is considered the causal agent of aspergillosis in sea fans, but is an opportunistic pathogen [8, Toledo-Hernández et al. in press]. The fact that A. sydowii was not isolated from diseased colonies from any reef in this study, or in that of Toledo-Hernández et al. (in press) suggests that aspergillosis may be caused by other microorganisms as well. A. sydowii can produce ochratoxins and many strains of A. flavus, the most ubiquitous species found in our study, produce aflatoxins [24]. Several strains of A. flavus that were isolated from healthy and diseased colonies produced aflatoxins in vitro (data not shown). However, it is unknown whether these aflatoxins can be produced in vivo in marine environments.
Given the cosmopolitan nature of genus Aspergillus in terrestrial environments, their salt tolerance, and the tremendous numbers of conidia they produce, their ubiquity in marine environments is not surprising [10]. However, the lack of information about whether they sporulate in the water [30], or how long the fungi can survive pelagically and in corals and other organisms, and produce toxins in vivo, makes it difficult to determine the functional importance of these fungi in marine ecosystems.
Comparison between mycoflora in seawater vs sea fans The similarity between mycoflora of the seawater and sea fans suggests low specificity of sea fans for fungi. Most species found in seawater were also present in sea fans (healthy or diseased). Studies that have related the bacterial community of hard corals and seawater have found more differentiation [7, 28]. Therefore, octocorals may be less selective than scleractinian corals, or fungi may be less specific colonizers than bacteria.
African dust has been suggested as source of microorganisms that cause diseases in coral reefs [39]. However, the results of this study suggest that local environmental factors, rather than broad geographic-scale processes, are influencing the fungal community composition. Out of 97 strains of fungi that we have isolated from African dust storms, only a small fraction (3 species) were common with the fungi
isolated in this study (data not shown); whereas out of ten fungi isolated from local soil, six were shared with species isolated from tissue and seven from seawater. These results suggest that the source of marine fungi is most likely local rather than the African dust. If inoculum comes from local sources and seawater is a reservoir, local land use choices may affect the pathogen load of corals. Thus, local strategies in terrestrial conservation management may be key for the management of coral diseases as well as for the conservation of coral reefs ecosystems in general.
Acknowledgments
We are grateful to A. Herrera for assistance in the field, and J. Acevedo and A. Bones for assistance in the lab, and C. Zambrana for help with the map. Special thanks to A. Dieppa and Don Claudio for help collecting and monitoring of sea fans in Jobos Bay National Estuarine Research Reserve (JOBANER). Comments from A. Cróquer and I. Bejarano greatly improved this manuscript. This study was supported by grants from UPR Sea Grant (NOAA award NA16RG2278, project R-92-1-04), NOAA-CRES program, NIH-SCORE Grant #S06GM08102, and NSF-CREST. AZM thanks the International Society for Reef Studies (ISRS) for an ISRS/TOC Coral Reef Conservation Award (2006) and PBDT fellowship (2007) from Graduate and research Dean Office of UPR Río Piedras. Sequencing was done at the UPR Sequencing & Genotyping Facility, supported in part by NCRR-AABRE Grant #P20 RR16470, and University of Puerto Rico Biology Department.
References
1. Abarca ML,(2000). Taxonomía e identificación de especies implicadas en las aspergilosis nosocomial. Rev. Iberoam. Micol. S79-S84.
2. Bayman P (2006) Diversity, scale and variation of endophytic fungi in leaves of tropical plants. In: Bailey MJ, Lilley AK, Timms-Wilson TM (eds) Microbial ecology of aerial plant surfaces. CABI Publishing, Wallingford, pp 37–50
3. Bray J, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr 34:77-87.
4. Clarke KR, Warwick RM (2001) Change in marine communities: An approach to Statistical Analysis and interpretation, vol 2. Primer –E, Plymouth.
5. Clarke K, Gorley R (2006) Primer V5 (& V6): User Manual/Tutorial, PRIMER-E, Plymouth UK, 192pp
6. Frias-Lopez J, Bonheyo GT, Qusheng J, Fouke BW (2003) Cyanobacteria associated with coral black band disease in Caribbean and Indo -Pacific reefs. Appl Environ Microbiol 69:2409-2413
7. Frias-Lopez J, Zerkle AL, Bonheyo GT, Heikoop JM, Fouke BW (2002) Partitioning of bacterial communities between seawater and healthy, black band disease, and dead coral surfaces. Appl Environ Microbiol 68:2214-2228
8. Geiser D, Taylor JW, Ritchie KB, Smith GW (1998) Cause of sea fan death in the West Indies. Nature 394:137-138.
9. Griffin D, Kellogg CA, Peak KK, Shinn EA. (2002) A rapid and efficient assay for
extracting DNA from fungi. Letters in applied microbiology 34:210-214.
10. Griffin DW, Kellogg CA (2004) Dust storms and their impact on ocean and human health: dust in hearth's atmosphere. Ecohealth 1:284-295
11. Harvell C, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus AD, Overstreet RM, Porter JW, Smith GW, Vasta GR (1999) Emerging marine disease: Climate links and anthropogenic factors. Science 288:15051510.
12. Holler U, Wright AD, Matthée GF, Konig GM, Draeger S, Aust H, Schulz B (2000) Fungi from marine sponges: diversity, biological activity and secondary metabolites. Mycol Res 2:1354-1365.
13. Kendrick B., Risk MJ, Michaelides J, Bergman K (1982) Amphibious microborers: bioeroding fungi isolated from live corals. Bull of Mar Sci 32:862-867
14. Klaus J, Frias-Lopez JF, Bonheyo GT, Heikoop JM, Fouke BW (2005) Bacterial communities inhabiting the healthy tissues of two Caribbean reef corals: interspecific and spatial variation. Coral reefs 24:129-137.
15. Klich M (2002) Biogeography of Aspergillus species in soil and litter. Mycologia 94:2127.
16. Koh L, Tan TK, Chou LM, Goh NK (2000) Fungi associated with gorgonians in Singapore. Proceedings 9th international coral reef symposium 1:521-526.
17. Kohlmeyer J, Volkmann-Kohlmeyer B (2003) Fungi from coral reefs: A commentary. Mycological Research 107:386-387.
18. Kubicek C, Druzinina IS (2007). Environmental and microbial relationships. In: The Mycota. Esser K (Ed). Springer 350 pp.
19. Lafferty K, Porter JW, Ford SE (2004) Are diseases increasing in the Ocean? Annu. Rev. Ecol Evol Syst 35:31-54.
20. Le Campion-Alsumard T, Golubic S, Priess K (1995) Fungi in corals: symbiosis or disease? Interaction between polyps and fungi causes pearl-like skeleton biomineralization. Mar Ecol Prog Ser 117:137-147.
21. Marsh PB, Simpson ME, Craig GO, Donoso J, Ramey HH (1973) Occurrence of Aflatoxins in Cotton Seeds at Harvest in Relation to Location of Growth and Field Temperatures. J Environ Qual 2:276-281
22. Pantos O, Bythell JO (2006) Bacterial community structure associated with white band disease in the elkhorn coral Acropora palmata determined using culture-independent 16s rRNA techniques. Dis Aquat Org 69: 79–88
23. Patterson K, Porter JW, Ritchie KE, Polson SW, Mueller E (2002) The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc Natl Acad Sci 99:8725-8730.
24. Pitt I (2000) Toxigenic fungi: which are important?. Medical Mycology 38 (1):17–22.
25. Ritchie KB. (2006) Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Prog. Ser. 322:1–14
26. Ritchie KB,.Smith GW. (2004) Microbial Communities of Coral Surface Mucopolysaccharide Layers. In: Coral Health and Disease, Springer-Verlag (Berlin) E. Rosenberg and Y. Loya (Eds.) Chapter 13, pp 259-263
27. Robles PO, Gonzalez GM, Laboy E, Capella J (2002) Jobos bay estuarine research reserve. DRNA San Juan, Puerto Rico. Pp?
28. Rohwer F, Seguritan V, Aza F, Knowlton N (2002) Diversity and distribution of coralassociated bacteria. Mar Ecol Prog Ser 243:1-10.
29. Rosenberg E, Falkowitz L (2004). The Vibrio shiloi/Oculina patagonica model system of coral bleaching. Ann. Rev. Microbiol. 58: 143–159
30. Shinn E, Smith GM, Prospero JM, Betzer P, Hayes ML, Garrison V, Barber RT (2000) African dust and the demise of Caribbean coral reefs. Geophys Res lett 27:3129-3032.
31. Smith GW, Ives LD, Nagelkerken IA, Ritchie KB (1996) Caribbean sea-fan mortalities. Nature 383: 487
32. Smith GW, Weil E (2004) Aspergillosis in gorgonians. In: Rosenberg E and Y. Loya (Eds) Coral health and disease, Springer, Heidelberg, pp?
33. Sutherland KP, Porter JW, Torres C (2004) Disease and inmmunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar Ecol Prog Ser 266:273-302.
34. Toledo -Hernández C, Bones-González A, Ortiz-Vázquez OE, Sabat AM, Bayman P (2007) Fungi in the sea fan Gorgonia ventalina: diversity and sampling strategies. Coral reefs 26:725-730.
35. Toledo -Hernández C, Sabat AM, Zuluaga-Montero A (2007) Density, size structure and aspergillosis prevalence in Gorgonia ventalina at six localities in Puerto Rico. Mar Biol
36. Voss J, Mills DK, Myers JL, Remily ER, Richardson L (2007) Black band disease microbial community variation on corals in three regions of the wider Caribbean. Microb Ecol.
37. Ward J, Kim K. Harvell CD (2007) Temperature affects coral disease resistance and pathogen growth. Mar Ecol Prog Ser 329:115-121.
38. Weil E, Smith G, Gil-Agudelo DL (2006) Status and progress in coral reef disease research. Dis Aquat Org 69:1-7.
39. Weir -Brush J, Garrison VH, Smith GW, Shinn EZ (2004) The relationship between gorgonian coral (Cnidaria: Gorgonacea) diseases and African dust storms. Aerobiologia 20:119-126.
40. White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, (Eds) PCR protocols: A guide to methods and applications. Academic Press, New York. pp
41. Yu J, Cleveland TE, Nierman WC, Bennett JW (2005) Aspergillus flavus genomics: gateway to human and animal health, food safety, and crop resistance to diseases. Rev Iberoam Micol 22: 194-202: 194-202.
Table 1. Fungal species isolated from diseased (D) and healthy tissue (H) from G. ventalina at different reef sites.
In-shorereefs | Off-shorereefs
Species Piñeros Island Jobos Humacao Icacos Escambrón Luis Peña Culebrita Island Vieques Island
Aspergillusaculeatus - H,D - - - - - -
A.flavus D H,D D H H,D - H,D H
A.niger - H H - - - - -
A.ochraceus - - - - - D - -
A.sydowii - - H H - H H -
A.tamarii - - - - D H - -
A.terreus - - - - - - D -
A.versicolor - - - - H - D -
Cladosporiumsp. H H - H - - - -
Cl.cladosporioides
Gloeotiniatemulenta
Penicilliumcitrinum - - H - H,D H,D - -
Penicilliumsp. H H - - - - - -
Stachybotryschartarum - - - - - - - H
Tritirachiumsp. - - - - - H,D - -
Table 2a. Significant differences in fungal communities in healthy sea fans among sites. Significance was determined by Analysis of Similarity (ANOSIM) followed by
Analysis of Percent Similarity (SIMPER). Pairwise comparisons that were not significant are not shown.
Table 2b. Results of SIMPER analyses for significant ANOSIM between fungal communities isolated from diseased tissue in G. ventalina by reef sites. Significance was determined by Analysis of Similarity (ANOSIM) followed by Analysis of Percent Similarity (SIMPER). Pairwise comparisons that were not significant are not shown. Group
Table 3. Fungi isolated from seawater from different reef sites. Near- shore reefs: LA
Cayo Largo, PA Palominito Island, DI Cayo Diablo, CL Cayo Lobo, HU Humacao, IK
Icacos Island, IP Isla Piñeros, JO Jobos, FA Fajardo, ESC Escambrón. Offshore reefs: LP Luis Peña, MO Mona Island,VI Vieques Island, CB Culebrita.
Species
Aspergillusaculeatus
A.flavus/oryzae
A.japonicus
A.nigergroup
A.sydowii
Cladosporium cladosporioides
Cladosporiumsp.
Nectriamauritiicola
Penicilliumcitrinum
P.coprophilum
Penicilliumsp
Phomapomorum
Stachybothrysechinata
Table 4. Significant differences in fungal communities between healthy and diseased tissue of sea fans among sites. Significance was determined by Analysis of Similarity (ANOSIM) followed by Analysis of Percent Similarity (SIMPER). Pairwise comparisons that were not significant are not shown. Sites
Figure 1. Map of Puerto Rico showing the study sites. Seawater collection is represented by stars and sea fan tissue (healthy and diseased) collection sites are represented by black circles. Each box shows an amplified view of geographic region of sampling site. ESC Escambrón. (1) CB Culebrita, LP Luis Peña, VI Vieques Island. (2) IK Icacos, IP Piñero Island, CL Cayo Lobo, DI Cayo Diablo, PA Palomino Island, FA Fajardo, LA Cayo Largo, HU Humacao (3) JO Jobos Bay, (4) MO Mona Island.
Figure 2. Species-accumulation curves and Jackknife1 estimators for fungi isolated from (a) healthy tissue, (b) diseased tissue of sea fans and (c) seawater collected from coral reef sites.