Nutrient, Genetic, and Metabolic Research
Nutrient, Genetic, and Metabolic Research

Nutrient, Genetic, and Metabolic Research
Nutrient, Genetic, and Metabolic Research
NUTRIENT, GENETIC, and METABOLIC RESEARCH

I am pleased to share that this past year was marked by various high-impact and significant scientific discoveries, which culminated in publications from the members of the Sabri Ülker Center, a successful visit from Murat Ülker, the onboarding of new lab members, and the upcoming launch of an academic clinical trial.
It’s exciting to see the renewed energy and enthusiasm in our team after the challenging years of the pandemic. While we still are experiencing delays in our supply chain due to the lingering effects of the pandemic, we are operating at full capacity and are evermore dedicated to advancing and expanding our scientific understanding of chronic metabolic diseases and pushing forward with our translational aspirations.
During the end of 2021 and into 2022, our lab has released five manuscripts onto the publication stage, which you’ll find detailed in this report. Three of these papers focused on the secretion and mechanisms of action of FABP4 hormone and the discovery of Fabkin. These studies revealed previously unanticipated sources and secretory signals driving hormone secretion from adipocytes during the breakdown of lipid stores and endothelial cells as a major new player in FABP4 biology. Another breakthrough was the discovery of a novel hormone complex with FABP4, which we named Fabkin, and how targeting this hormone complex offers remarkable translational opportunities against metabolic diseases, particularly type 1 and type 2 diabetes. Additionally, you’ll find research focused on the mechanisms underlying inflammation and how it emerges and disrupts the function of fat cells. Also, our long-term efforts to explore the significance of subcellular molecular architectural structures in their native tissue environment revealed a whole new concept of metabolic adaptation, and opportunities to restore metabolic function in the liver
by reorganizing the subcellular structure. This has been an extraordinary year for the science emerging from the Center in a way that determines our future areas of priority and brings us one step closer to translating some of our long-term studies for applications in humans. You will see in this report, an exciting development towards the first of such efforts led by one of our physician-scientist colleagues.
Finally, we are excited about the completion of planning of the Fourth Sabri Ülker Symposium on Metabolism and Life, which will be held on the premises of Kadir Has University in Istanbul, May 9-10th, 2023. We have an incredible lineup of international leaders and rising stars in the field of metabolism, with Dr. Randy Schekman of the University of California Berkeley as our Keynote Speaker. As we return to an in-person program, we continue our efforts of promoting global scientific development and providing trainees and the younger generation of scientists with the opportunity to network, collaborate, and learn from groundbreaking scientists. With 2023 being the 100th Anniversary of the Republic of Turkey, we anticipate this Symposium to be the most remarkable yet!
It was truly an honor to have Mr. Murat Ülker visit the Center in June, and to have the opportunity for all of our lab members to introduce themselves and their research projects. We are most grateful for the continued support from the Ülker family and their commitment to advance our pursuits of the most challenging of transitional avenue–targeting metabolic disease, the greatest threat to global human health.
Gökhan S. Hotamışlıgil, MD, PhD Director of the Sabri Ülker Center for Nutrient, Genetic, and Metabolic Research James S. Simmons Professor of Genetics and Metabolism at the Harvard T.H. Chan School of Public Health
“This has been an extraordinary year for the science emerging from the Center in a way that determines our future areas of priority, and brings us one step closer to translating some of our long-term studies for applications in humans.”


Signaling, December 2021 (DOI: 10.1126/scisignal.abf2059)

The role of inflammation in obesity and associated metabolic diseases is well established. This study demonstrates a key missing link between inflammation and metabolic dysregulation in adipocytes that have remained enigmatic for many decades. During chronic overnutrition or obesity, adipocytes, the cells that store lipids, become increasingly enlarged and dysfunctional and cannot effectively buffer nutrients. This, and potential additional stress signals, results in metaflammation, which is the chronic metabolic inflammation originating from target cells such as adipocytes, in response to excess nutrients. This response involves intracellular stress, recruitment of immune cells, and abnormal production and secretion of inflammatory molecules. Over time, this unfavorable situation ultimately results in systemic metabolic failure, including insulin resistance, dyslipidemia, and metabolic disease. Despite playing important roles in obesity, insulin resistance, and diabetes, the initiation and propagation mechanisms of metaflammation are not fully understood and established, thereby limiting the ability to effectively intervene with this critical pathological mechanism. This study clarifies these mechanisms by demonstrating that proper regulation of the calcium (Ca2+) channel inositol triphosphate receptor (IP3R) is a central process for the maintenance of adipocyte metabolic health. Moreover, the study shows that the alteration of this fine balance is a key driver of adipocyte metaflammation and dysfunction in the context of obesity.
Inflammation, either induced by cytokine exposure in cells or by obesity in animals, leads to increased expression and activity of IP3Rs and phosphorylation of a stress-transmitting enzyme called Ca2+/calmodulindependent protein kinase II in adipocytes. This mechanism is dependent on the c-Jun N-terminal kinase (JNK), a molecule we have previously discovered to be a key

mediator of obesity-induced insulin resistance. Studies in cells demonstrated that the JNK-PI3R axis plays a critical role in tumor necrosis factor-alpha’s inflammatory and metabolic activity, a pro-inflammatory cytokine elevated in obese mice and humans. Obese mice, either those fed a high-fat diet or those with a genetic defect causing obesity, have altered Ca2+ homeostasis in adipocytes at least in part due to higher IP3R activity. Moreover, mice lacking IP3R isoforms in adipocytes show protection against adipose tissue inflammation and insulin resistance, despite substantial diet-induced weight gain. This development of increased adiposity uncoupled from metabolic complications, possibly represents a model of metabolically healthy obesity. Interestingly, genetic evidence both in fruit flies and humans, also supports that loss of function of IP3R in body fat also causes increased adiposity. Most intriguingly, human genome-wide association studies have shown that an ITPR genetic polymorphism is associated with increased body-mass index, fat mass, and waist-to-hip ratio, but not metabolic complications. Thus, the regulation of IP3R-mediated Ca2+ homeostasis in adipocytes is a key signal, linking metabolic stress to inflammation and metabolic deterioration in obesity, a mechanism conserved across species.
This study lays the foundation for understanding the impact of intracellular Ca2+ homeostasis on adipocyte metabolism and metaflammation. Also, approaches to prevent aberrant IP3R activity in adipocytes could be a powerful strategy to rescue systemic glucose metabolism and inflammatory stress in obesity. Pharmacological research in this direction is emerging, such as the use of SERCA agonists or Azoramide, although it remains to be elucidated whether targeting this mechanism can be safely and effectively implemented in humans.
Nature, March 2022 (DOI: 10.1038/s41586-022-04488-5)

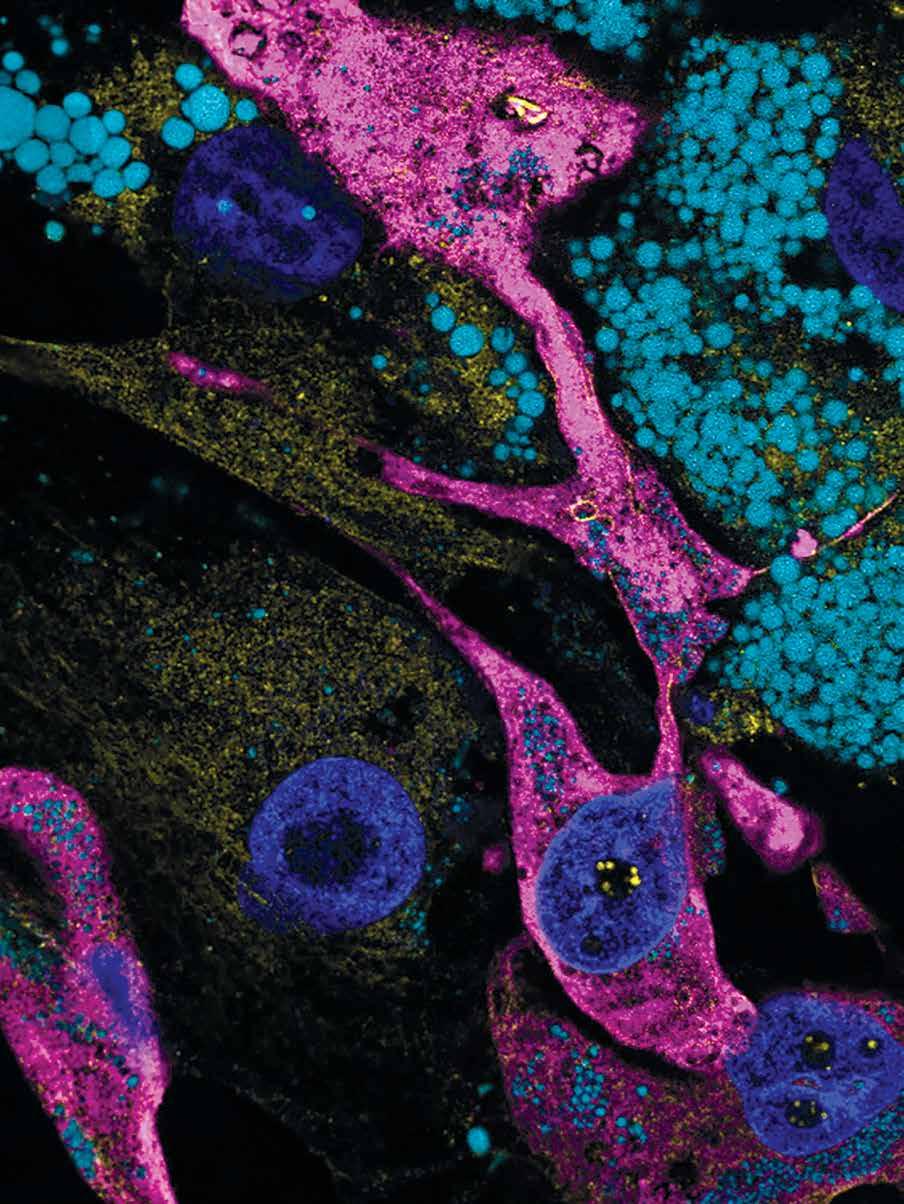
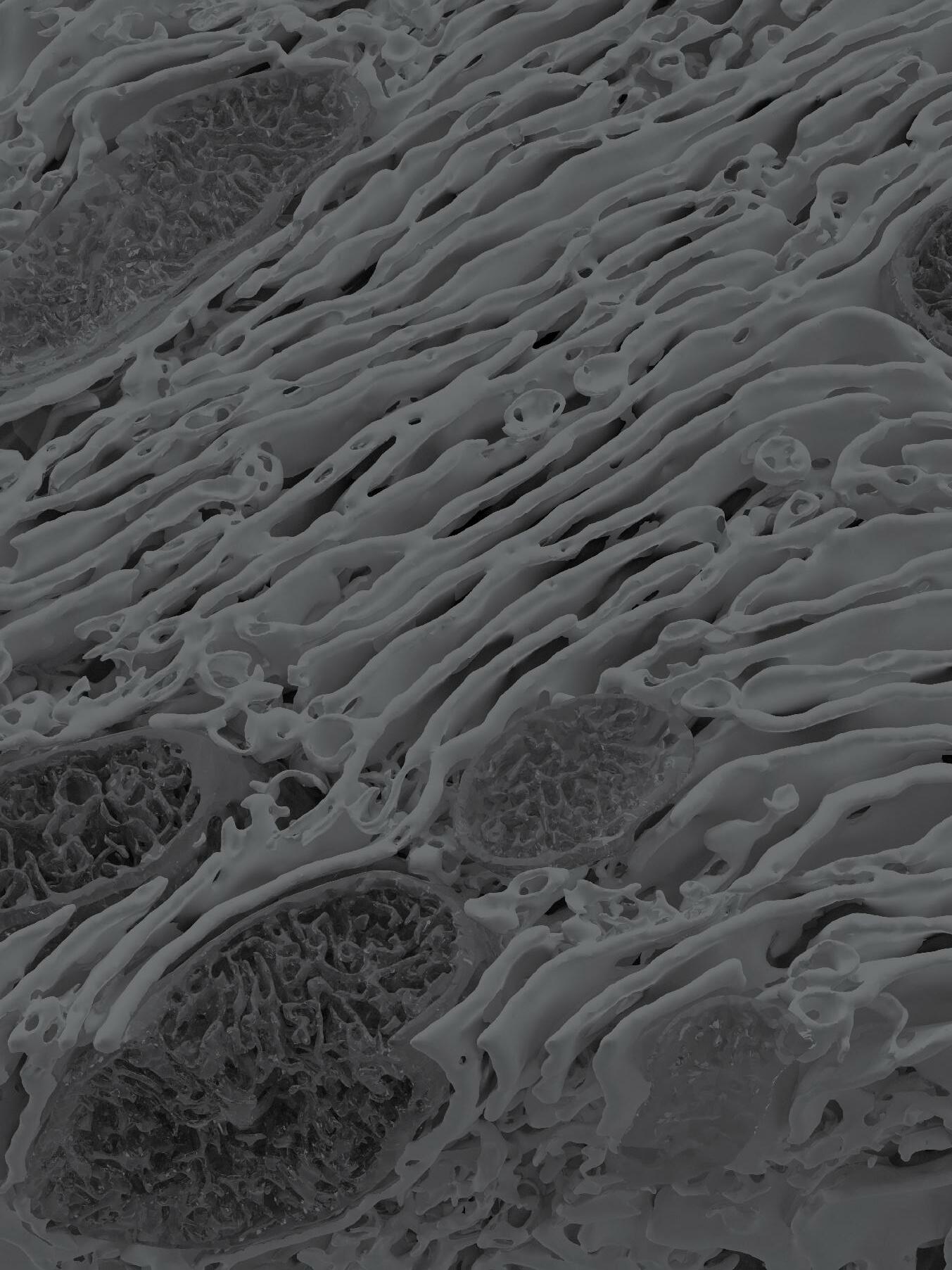
This study reports the most detailed characterization of the subcellular molecular architecture in the liver tissue native environment, in both healthy and obese contexts, shedding critical light on the role of regulation of molecular architectural structure for metabolic programming and homeostasis in health and disease.
Organs and tissues exhibit distinct features of structural organization at various scales to meet functional demands and adapt to challenges for maintaining homeostasis and viability. Within cells, the vast diversity and highly dynamic nature of tasks demand subcellular structural complexity and flexibility. For example, cells that secrete large amounts of protein, such as pancreatic acinar or plasma cells, are filled with rough endoplasmic reticulum (ER) in the form of stacked sheets, whereas Leydig cells, which secrete steroids or lipid hormones, have a vast network of smooth tubular ER. Unfortunately, subcellular structures have been difficult to capture in detail in the native tissue setting, especially for organelles with complex architecture such as the ER, limiting the ability to determine their precise topology and functional profiles of alterations. This study visualized hepatocytes in their native liver tissue environment using enhanced focused ion beam scanning electron microscopy imaging, which made it possible to image large intact volumes of the liver. To identify, segment, and quantify each subcellular structure within the cell, we employed artificial intelligence and machine learning-based approaches, along with convolutional neural networks to automatically segment organelles. Finally, threedimensional reconstruction of the images through multiple
analytical platforms resolved the organization of organelles in unprecedented detail in the liver tissue.

This study also examined structure-function relationships in the context of metabolic homeostasis in health and disease, using obesity as a model. A comparative analysis of subcellular structures in liver tissue of lean and obese mice revealed massive structural reorganization of the ER, characterized by marked disorganization of stacks of ER sheets and predominance of ER tubules. Notably, experimental recovery of the ER’s structural organization through multiple genetic and synthetic interventions resulted in improved ER function, as well as systemic glucose and lipid metabolism. These data demonstrate that the hepatic ER’s molecular architecture is highly dynamic, integrated with the metabolic state, and critical for adaptive homeostasis and tissue health.
Together, our findings indicate that cellular structures can be a prerequisite for metabolic programming, and such regulatory circuits can open new avenues for understanding endocrine and metabolic homeostasis. The fundamental regulatory mechanism identified could be applied to evaluate the susceptibility—or resistance—of individuals to a disease state like obesity, and determine what steps, such as diet, nutrients, or fasting, will reduce, eliminate, or exacerbate these states. Finally, the workflow delineated in this study could be exploited in future studies to further understand the structure-function relationship in native tissue context, and how this may be linked to metabolic function in healthy and diseased tissue.

Nature, December 2021 (DOI: 10.1038/s41586-021-04137-3)

This study reports a new factor—the Fabkin complex—that integrates energy status with the function of metabolic organs, representing a promising target for preventing and treating metabolic disease.
Lipolysis, a process in which adipocytes release energy, is necessary for survival. However, uncontrolled or chronic lipolysis in the context of insulin resistance or insulin insufficiency, can cause disruptions in metabolic homeostasis. We have recently identified a hormone, fatty acid-binding protein 4 (FABP4), as the only hormone known to be released during lipolysis. Our previous work has also shown that mice lacking FABP4 have significantly higher levels of beta cells, which play a central role in the control of lipolysis and the development of diabetes.
FABP4 levels are known to be elevated in type 2 diabetes and correlate with body mass index, yet the role of FABP4 in type 1 diabetes has remained unclear. In this study, we detected significantly higher serum levels of FABP4 in type 1 diabetes patients compared with normoglycemic individuals, indicating that FABP4 may indeed play a role in type 1 diabetes. We added upon these findings by examining non-obese mice, and confirmed that circulating FABP4 serum levels are significantly elevated just before and immediately after impairment of glycemic control, further confirming a potential role of FABP4 in the pathogenesis of both type 1 and type 2 diabetes.
Although circulating FABP4 levels are strongly associated with cardiometabolic diseases in preclinical models and humans, the mechanism of action of FABP4 has not yet been determined. Moreover, although FABP4 influences beta-cell mass and function in vivo, previous studies have not shown a consistent effect of recombinant FABP4 in vitro. These conflicting findings suggest that FABP4 may combine with other proteins to
elicit its biological function. Based on pulldown and mass spectroscopy of serum samples from mice with diabetesinduced obesity, this study revealed that adenosine kinase (ADK) and nucleoside diphosphate kinase (NDPK) bind FABP4 to form a complex (termed Fabkin), that regulates extracellular adenosine triphosphate (ATP) and adenosine diphosphate (ADP) levels. The findings suggest that when FABP4, NDPK, and ADK are combined in a complex, the ADP:ATP ratio decreases, inhibiting purinergic receptors on beta cells and thus, reduces glucose-stimulated insulin secretion. This study also demonstrated that the Fabkin complex alters calcium homeostasis in the endoplasmic reticulum (ER), resulting in ER dysfunction and beta-cell death in vitro, which are critical mechanisms in both.
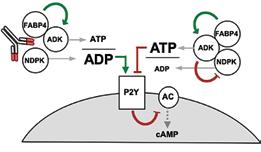
Finally, we demonstrated in this study using a prototype monoclonal antibody, that this complex is critical in both type 1 and type 2 diabetes pathogenesis. After mapping the affinities of each components of the Fabkin complex and antibody interactions, we administered into multiple genetic models of disease to test therapeutic potential. These studies showed that therapautic targeting of the Fabkin complex improves metabolic outcomes, enhances beta-cell function, and preserves beta-cell integrity to prevent both type 1 and type 2 diabetes.
These findings elucidate the mechanism of action of FABP4, demonstrating that FABP4 couples energy fluxes with the metabolic response, and establish an endocrine axis of metabolic regulation. The Fabkin complex likely targets other tissues to influence immunometabolic pathologies such as inflammation, insulin resistance, and cardiac pathophysiologies. Importantly, this discovery also provides a straightforward translational path for applications to human disease.
In this publication, we report the novel discovery that the adipocyte hormone, fatty acid binding protein 4 (FABP4), is also secreted from endothelial cells—the cells which line the blood vessels. Importantly, and unexpectedly, we found that endothelial FABP4 is the major source of basal hormonal FABP4, contributing to ~75% of basal circulating FABP4 levels in lean mice. Moreover, we showed that mice lacking endothelial FABP4 showed altered insulin responses to lipolysis, indicating that endothelial FABP4 is critical for lipolysis-induced insulin secretion.
Our lab previously discovered that FABP4 is a lipid chaperone secreted from adipocytes upon stimulation of lipolysis. Mice lacking FABP4 are protected against obesityassociated metabolic diseases, and circulating FABP4 levels correlate positively with body mass index and indicators of metabolic syndrome in humans, and are increased in mice with dietary or genetic obesity. Taken together, all of these raise the possibility that excessive adipose tissue release of FABP4 may, in fact, be the strong contributor to metabolic dysregulation seen in mice and humans. While adipocytes have always been presumed to be the major source of hormonal FABP4, this question has not been experimentally shown definitively in vivo. To address this question, we generated a new genetic model of mice where FABP4 deletion can be achieved selectively in the desired cells known to express the gene—adipocytes, endothelial cells, myeloid cells, and the whole body—to examine the contribution of these cell types to basal and stimulated plasma FABP4 levels. Unexpectedly, we found that mice with adipocyte deletion of FABP4 only showed ~25% reduction in basal circulating FABP4 levels, whereas mice with deletion of FABP4 from endothelial cells showed ~75% decreases in basal FABP4, compared to wild-type controls. Our data showed, for the first time, that the majority of baseline circulating FABP4 in lean mice is derived from
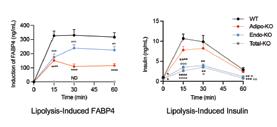
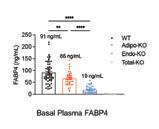
vascular endothelial cells, rather than adipocytes.

FABP4 secretion by cultured adipocytes and in vivo is inducible, stimulated by prolonged fasting and lipolytic signals. Long ago, it was shown that acute β-adrenergic stimulation of lipolysis in vivo is associated with a rapid, strong, and transient induction of insulin secretion in general, this is seen as a pathological response. Interestingly, our previous studies in FABP4-deficient animals demonstrated that the presence of FABP4 is an important requirement for this lipolysis-driven insulin secretion, as insulin responses to lipolysis are markedly blunted in the absence of FABP4 in the whole body. In this study, we found that mice with adipocyte deletion of FABP4 exhibited ~62% reduction in FABP4 responses to lipolysis, while there was only a minimal reduction in mice with endothelial FABP4 deletion, indicating that adipocytes are indeed the main FABP4 source during lipolysis. Surprisingly, though mice with adipocyte deletion had blunted FABP4 responses to lipolysis, insulin secretory responses remained largely intact. In contrast, mice with endothelial FABP4 deletion had nearly intact FABP4 responses but showed blunted lipolysis-induced insulin secretion, identical to whole-body FABP4 deletion mice. These data demonstrated, for the first time, that endothelial FABP4 is a key requirement for lipolysis-induced insulin secretion.
We conclude that the endothelium is the major source of baseline hormonal FABP4, and is required for the insulin response to lipolysis. Endothelial dysfunction is a key aspect of many metabolic diseases, and thus, our discovery of the contribution of the endothelium to circulating FABP4 and its regulatory role in insulin secretion identifies a new role of the endothelium in metabolic regulation, and a potential source for FABP4 in metabolic disease.
10.1101/2022.10.14.512277)
For the vast majority of human history, mankind has faced challenges to survival including famine and disease. As such, various biological pathways have evolved to combat conditions of deprivation, orchestrating incremental responses to promote survival, the central function of the adipose tissue is as a storage depot for excess nutrients, accumulated in periods of nutrient excess to ensure survival in conditions of famine. Liberation of these excess nutrients is not only coupled to nutrient scarcity, but also to conditions of sudden high energy demand, the socalled “fight-or-flight” response mediated by the central nervous system. Whereas ancient man faced a plethora of environmental threats, modern humans overwhelmingly exist in a world of nutrient excess, lack of predation, and low physical demand. Thus, our modern world is at odds with our biology, and metabolic programs that evolved to ensure our survival are now aberrantly promoting metabolic disease.
In recent decades, our understanding of adipose tissue has evolved significantly, with a greater appreciation for the role of adipose tissue as a central signaling organ in the regulation of metabolism. Numerous adipokines have now been identified as playing critical roles in controlling appetite and satiety, glucose and lipid metabolism, as well as inflammation. However, only one recently identified adipokine, Fatty Acid Binding Protein 4 (FABP4), is known to be secreted from the adipose tissue in response to fasting and the breakdown of energy stores in adipocytes. As a lipid-chaperone protein, it was hypothesized that the free fatty acid products of lipolysis may bind FABP4 to potentiate its release. This process of lipolysis involves the breakdown of triglycerides into fatty acids and glycerol, which are subsequently released into circulation to fuel distant tissues, such as the liver and muscle. While essential for survival when starving, this process is paradoxically inappropriately and chronically engaged in obesity, leading to the chronic release of lipolysis products. Concomitantly, circulating FABP4 levels are increased in obesity, and strongly associated with numerous metabolic disorders including diabetes, atherosclerosis, and various cancers in humans. Therefore, it is pertinent to understand the sources and regulation of this protein.
In this study, we investigated the regulation of FABP4 secretion from the adipose tissue in response to lipolysis. To study the contribution of the process of lipolysis to FABP4 secretion in physiology, we generated new genetic models with or without FABP4, and specifically lacking the first enzyme of the lipolysis pathway (ATGL) in their adipose tissue (ATGLAdpKO). Unexpectedly, when a stimulus is given to induce lipolysis in these mice, FABP4 secretion was even greater than the wildtype controls, despite the lack of triglyceride breakdown. However, the adipose tissue, when removed from these mice and stimulated to undergo lipolysis outside the body, was completely deficient in its ability to secrete FABP4. This paradoxical finding raised several possibilities; 1) FABP4 secretion in ATGLAdpKO mice is derived from a non-adipocyte cell type; or 2) a factor present in ATGLAdpKO mice, but not in cell culture conditions, stimulates FABP4 release.
To address the first question, we generated mice specifically lacking both ATGL and FABP4 in the adipose tissue (double knockout, DKO). Upon stimulation of lipolysis, DKO mice exhibited no induction of FABP4 secretion, indicating that the potentiated FABP4 release in the ATGLAdpKO mice was indeed sourced from the adipose tissue. By removing the adipose tissue from the mice, cells are disconnected from the central nervous system. It has been reported previously, that obese mice and humans exhibit hyperactivation of the sympathetic nervous system, which may also be the case in the ATGLdeficient models. Thus, we investigated whether sympathetic nervous system signaling in the mice, which is absent from tissue explants, was associated with enhanced FABP4 secretion. Our experiments using synthetic inhibitors demonstrated that the sympathetic system is, indeed, critical for FABP4 secretion. Therefore, the activity of a key enzymatic step of lipolysis mediated by ATGL, per se, is not required for stimulated in vivo FABP4 secretion from adipocytes, which can be induced through sympathetic signaling. These studies provide us with a novel system wherein we can dissect the biology of FABP4 and the products of lipolysis in generating the function of FABP4 and controlling its secretion.
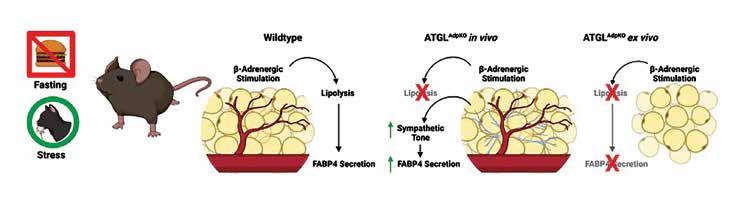

Xian Zhang, Songyuan Luo, Minjie Wang, Qiongqiong Cao, Zhixin Zhang, Qin Huang, Jie Li, Zhiyong Deng, Tianxiao Liu, Cong-Lin Liu, Mathilde Meppen, Amelie Vromman, Richard A. Flavell, Gökhan S. Hotamışlıgil, Jian Liu, Peter Libby, Zhangsuo Liu, Guo-Ping Shi
Nature Communication, December 2022 (DOI: 10.1038/s41467-022-35256-8)
White adipose tissue (WAT) plays a role in storing energy, while brown adipose tissue (BAT) is instrumental in the re-distribution of stored energy when dietary sources are unavailable. Interleukin-18 (IL18) is a cytokine playing a role in T-cell polarization, but also for regulating energy homeostasis via the dimeric IL18 receptor (IL18r) and Na-Cl co-transporter (NCC) on adipocytes. Here we show that IL18 signaling in metabolism is regulated at the level of receptor utilization, with a preferential role for NCC in brown adipose tissue (BAT) and dominantly via IL18r in WAT. In Il18r-/Ncc-/- mice, high-fat diet (HFD) causes more prominent body weight gain and insulin resistance than in wild-type mice. The WAT insulin resistance phenotype of the doubleknockout mice is recapitulated in HFD-fed Il18r-/- mice, whereas decreased thermogenesis in BAT upon HFD is dependent on NCC deletion. BAT-selective depletion of either NCC or IL18 reduces thermogenesis and increases BAT and WAT inflammation. IL18r deletion in WAT reduces insulin signaling and increases WAT inflammation. In summary, our study contributes to the mechanistic understanding of IL18 regulation of energy metabolism and shows clearly discernible roles for its two receptors in brown and white adipose tissues.


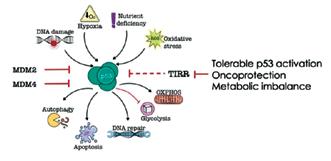
This study identified a novel mechanism linking metabolic health to cancer development.
This study identified a novel mechanism by which reactive oxygen species are dysregulated in obesity and contribute to metabolic disease.

Gökhan S. Hotamışlıgil has been listed as a “Highly Cited Researcher in the field of Cross-Fields” since 2018 by Clarivate Analytics, which is an annual list recognizing significant and influential researchers in the sciences and social sciences around the world. Stanford University’s November 2022 release of the “Updated science-wide author databases of standardized citation indicator” published on Elsevier BV, is a publicly available database that provides standardized information on citations. The data includes career-long and single recent year impact for citations received during calendar year 2021.







This team, consisting of Dr. Gürol Tunçman, Dr. Mehmet Furkan Burak, and Ayşe Aycı, focus on one neglected area related to airway diseases and their metabolic origins. The Centers for Disease Control and Prevention (CDC) cite that “people who are overweight or obese, compared to those with healthy weight, are at increased risk for many serious diseases and health conditions. These include all-causes of death (mortality), high blood pressure (hypertension), high ldl cholesterol, low hdl cholesterol, or high levels of triglycerides (dyslipidemia), type 2 diabetes, coronary heart disease, stroke, gallbladder disease, osteoarthritis (a breakdown of cartilage and bone within a joint), sleep apnea and breathing problems, many types of cancer, low quality of life, mental illness such as clinical depression, anxiety, and other mental disorders, body pain and difficulty with physical functioning.”


One of the goals of our research team is to determine the role of hormonal FABP4 in the development of insulin resistance and diabetes, illuminating the molecular signaling pathways that underlie the well-established connection between obesity and metabolic disease. A better understanding of the mechanism of FABP4 action within the endocrine system will provide unique insights into targetable pathways for the development of new therapies against obesity-related diseases.
Despite the importance of counter-regulatory mechanisms to combat starvation and hypoglycemia, a significant component of this adaptive network, adipose tissue, remains understudied. It is functionally and developmentally conceivable that signals must exist to integrate this major source of energy during fasting or starvation to the rest of the counterregulatory network. Our studies demonstrated that fatty acid binding protein 4 (FABP4) is secreted from adipocytes—its circulating levels rise in fasting and in the context of obesity, and the hormone acts on the liver to promote hepatic glucose production. In this way, the high levels of circulating FABP4 that occur in obese animals appear to have an effect reminiscent of the hyperglucagonemia that characterizes the diabetic state. In our other work, now published (Science TM), we did observe an intriguing pattern in mice after exposure to a short chain fatty acid to promote hepatic glucose production that this response was dependent on both FABP4 and glucagon. The objective of this project has been to understand the contribution of circulating FABP4 in mediating hepatic gluconeogenesis (both synthesis and breakdown) and its contribution to
the diabetic condition. Our overarching hypothesis is that FABP4 is a critical component of “counter-regulatory” machinery and a mediator of the development of diabetes. We conduct several different types of experiments, including the use of multiple genetic mouse models and biochemical, biophysical, and cell-based assays to dissect the function of circulating FABP4 and its interaction with other relevant partner proteins. The potential of this ongoing work lies in pinpointing a novel mechanism of endocrine regulation—the interaction between an adipokine and a glucoregulatory hormone that links the adipose tissue to counterregulatory mechanisms—and carries important implications for metabolic disease pathogenesis.
Most recently, our efforts to identify potential FABP4 binding partner(s) resulted in the discovery of the Fabkin complex as a novel hormone complex and mechanism of action (details of this manuscript are already provided in this report.) While the action of this complex, named Fabkin, is so far studied in beta cells, the biological spectrum and the mechanism of action through purinergic receptors share many relevant features to the counterregulatory system, particularly liver glucose and lipid metabolism in general. We are now investigating these possibilities using new genetic models, as well as human hepatic organoids with multiple cellular populations. We are also pursuing additional translational tools by mapping the interaction sites of the components of the Fabkin complex and gathering 3D structural information.
We are grateful to the National Institutes of Health USA and Juvenile Diabetes Research Foundation for providing funding and logistic support for these projects.
In the face of an epidemic of obesity, the development of novel therapeutic approaches to obesity-related diseases is highly relevant to public health.

Despite the development of effective strategies for lowering cholesterol, atherosclerosis and associated cardiovascular diseases remain a major global health burden. Work in recent years has highlighted the exciting therapeutic potential for interventions that reduce inflammation, as well as the need for identifying novel and selective targets. The Sabri Ülker Center is focused on understanding how lipid metabolism and inflammation interact, and how these interactions can be useful therapeutically. In our earlier work, we discovered Nrf1 protein as a novel cholesterol sensor to defend against cholesterol toxicity and inflammatory damage. The central premise of this project is that Nrf1 in the macrophage is a critical factor for preventing chronic inflammation and maintaining cholesterol homeostasis, and that enhancing these actions of Nrf1 may be beneficial in atherosclerosis with implications for other immunometabolic diseases.
We believe that our research will determine the role of Nrf1 in the regulation of inflammation, specifically investigating the importance of Nrf1 function for controlling inflammatory responses of the macrophages, an immune cell type that plays a very critical role in the development of atherosclerosis.
In ongoing studies, we have obtained substantial new knowledge about the role and action of macrophage Nrf1, in terms of underlying mechanisms of action and impact
on macrophage phenotypes. To follow up on the effect of macrophage Nrf1 deficiency in the context of early and advanced atherosclerosis, we have completed studies in multiple cohorts of genetically modified mouse models of atherosclerosis and deficiency of Nrf1 in specific cells, to demonstrate the critical role of this pathway on disease development. We are also examining the organs of these animals to study cholesterol-induced inflammation and cell death which can be modulated by Nrf1. Our studies, so far, support the intriguing possibility of an involvement of Nrf1 in the crosstalk between cholesterol metabolism and inflammation. In addition to the Nrf1-deficiency models, we have also completed the development of both a genetic mouse model and virus-mediated gene transfer methodology, to test the therapeutic profile of increasing Nrf1 activity in the desired cell types and tissues. This critical milestone is an important development for our translational studies, particularly against liver and heart diseases. We are excited for embarking on these new avenues with the goal of generating new therapeutic possibilities against multiple debilitating and common immunometabolic diseases.
We are grateful to the National Institutes of Health USA and Servier Research Foundation for providing funding and logistic support for these projects.
The Sabri Ülker Center is focused on understanding how lipid metabolism and inflammation interact, and how these interactions can be useful therapeutically.
“The Nrf1 team includes Dr. Isabel Graupera, Dr. Shijun Deng, Jessica Freed, Alanis Carmona, and Dr. Zhe Cao, who focus on new mechanisms of metabolic defense by Nrf1 and how it can be used to treat deadly metabolic diseases. We discovered the role of Nrf1 in two circumstances where the previously known adaptive mechanisms were insufficient, or even dispensable, to account for the adaptation to metabolic stresses specifically in the liver, upon cholesterol overload and in brown adipose tissue upon cold exposure. Nrf1 can sense cholesterol levels in the ER membrane through its direct binding to cholesterol and mount a defensive program to protect the liver tissue. Our studies have shown that Nrf1 has a role in guarding homeostasis through various mechanisms and thus, could be utilized under pathological conditions to restore the health of organs. We are incredibly excited about our ongoing efforts and for the potential of this protein to create novel therapeutic avenues to treat liver diseases and other disorders with underlying organelle maladaptation.”

 Gökhan S. Hotamışlıgil, MD, PhD
Gökhan S. Hotamışlıgil, MD, PhD
This year, we have made important progress towards our translational aspirations as one of our Center members, Mehmet Furkan Burak, MD recently received a $2.5M grant, and launched a double-blind clinical trial with Massachusetts General and Brigham and Women’s Hospital. In this trial, Dr. Burak will test the potential benefits of palmitoleic acid (POA) compared to a placebo in human subjects, focusing on insulin sensitivity, glucose production, and fat formation. One of the most exciting aspects of reaching this stage is the fact that POA was discovered in our Center. POA is a monounsaturated fatty acid naturally found in the body’s adipose tissue, but is also, in specific foods such as certain fish and macadamia nuts. Although it can be consumed through diet, POA contains other complex oils such as palmitic acid, which is generally harmful to the body and counteracts its benefits. Through a purification technique, palmitic acid is separated and thus, creates clinical-grade POA, used in clinical testing.
The initial findings began “at the bench” by exploring a hypothesis that resulted from years of studying fatty acid binding proteins. Our laboratory found that when these proteins are blocked in mice, they start producing higher levels of POA in adipocytes, and when delivered into circulation, the animals do not exhibit any of the diseases associated with obesity. The discovery of POA was reported in the manuscript “Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism” published in Cell and revealed a novel, lipidmediated endocrine network as well as demonstrating that adipose tissue uses lipokines such as C16:1n7-palmitoleate, to communicate with distant organs and regulate systemic metabolic homeostasis. Supplementation of POA in mice improved insulin sensitivity and reversed the lipogenic gene expression. Now, the same methods described in this paper and conducted in our lab are being reformulated for testing in humans, a real-life transition “from bench” to “bedside.”
How will this research be safely translated to clinical trials?


The study will recruit overweight and obese individuals (BMI 25-40) with mild insulin resistance, prediabetes, and/or impaired glucose tolerance. After an initial study screening visit confirms eligibility for the study, an oral glucose tolerance test (OGTT) will be administered to provide for stratified randomization, for better homogeneity between POA and placebo groups. The study aims to have 40 participants, which will consist of 2 main overnight visits consisting of an insulin clamp procedure and a mixed meal tolerance test the night prior; participants will also have a liver MRI and DEXA scan during these two visits. Participants will be asked to consume a palmitoleic acidminimized diet for 10 weeks, which will start 2 weeks before the first overnight visit. This research study will compare insulin sensitivity before, and 8 weeks after taking POA vs placebo in the same individuals. After the first overnight visit, participants will be given either POA or placebo capsules to be taken daily for 8 weeks, until the second overnight visit. There will also be a short blood draw visit 4 weeks after the first overnight. Participants in the study will be appropriately compensated.
Dr. Burak’s road to becoming a principal investigator for clinical trial was not easy, but it was direct. He exhaustively pursued the endpoint to see if bench laboratory discoveries have positive benefits for people. The path to successfully launching an approved clinical trial took 5 years of work toward acquiring the appropriate funding source, as well as developing the protocol and supplying the appropriate data needed to launch this investigator-initiated study. Since this is an academic study, upon completion, the data will be provided to the public so it may be useful in furthering research regarding adipose tissue-related changes in obesity, and how to reverse symptoms, while also potentially translating POA into a viable treatment option.
“It is a privilege to have direct access to human patients and observe, listen, and solve their health problems while also having access to basic science as it allows one to think more mechanistically, instead of focusing on the end results which makes apparent the changes that are not directly visible when working only with patients.”
Mehmet Furkan Burak, MD


Q. How did you decide to pursue a career in research?
Soon after curiosity led me to science, it was clear to me that a career in biological science research was my calling. I love acquiring new knowledge, skills, and technologies related to life science on a daily basis. I enjoy the process of approaching scientific questions through rational thinking and designing, planning, and executing experiments.
Q. Have you encountered particular hurdles, challenges or barriers along the way?
Challenges are an integral part of research AND they are opportunities in disguise. There were countless times when only after days, or even months, of hard work did we realize that our ideas/hypotheses were struck down by unexpected experimental results. However, once we got over the frustration and took another look at the data from a different angle, it often led to new ideas, new directions, and new discoveries.
Q. What attracted you to join the Hotamışlıgil lab at the Sabri Ülker Center?
Gökhan is a brilliant researcher, as well as a devoted mentor. He has dedicated himself to the development of young fledgling trainees into competent, independent investigators. The scientists coming to the lab from diverse backgrounds have created and maintained a stimulating and collaborative atmosphere. The support from the Ülker family and foundation allows us maximal freedom to work on the projects in which we are interested.

Q. What particular scientific questions motivate you?

I’ve been doing research on metabolic syndrome for most of my scientific career. Metabolic syndrome is a cluster of conditions that increase a person’s risk of heart disease, stroke, and diabetes, particularly later in life. These chronic and complex diseases with both genetic and environmental contributing factors make them a daunting, yet intriguing, target. Research in this field has been extremely active, with new breakthroughs coming out almost every day.
The prevalence of metabolic symptoms has increased substantially worldwide over the past decade, posing serious threats to the global healthcare systems. An extensive and in-depth understanding of the complex pathophysiology of disease holds the key to the development of novel therapies to address unmet medical needs and improve human health. Here at the Sabri Ülker Center, our research has led to the identification of a number of molecules and cellular pathways, which play critical roles during the disease initiation and progression. Chemical and biological interventions targeting these factors have demonstrated promising outcomes in preclinical studies, and will hopefully advance to the clinical setting in the foreseeable future.
 Senior Research Scientist
Senior Research Scientist
Q. What attracted you to join the Hotamışlıgil lab at the Sabri Ülker Center?
Passion, drive, and camaraderie are exceptional in the Hotamışlıgil lab at the Sabri Ülker Center. All members have different backgrounds and expertise in science, and the collaborative environment makes a perfect melting pot for scientific discovery. In its field, this lab is one of the top, if not the top, contributor. Gökhan is not only an excellent scientist, but also a mentor with heart. Who wouldn’t be attracted to join?
Q. Have you encountered particular hurdles, challenges or barriers along the way?
Back in the late 1980s in Turkey, a person who goes to Medical School practices as a physician after graduation, which was the norm; if not, then you were seen as a loser. Now, thinking back, our generation probably paved the way for those in medical school to pursue paths other than as practicing physicians, which has positively impacted the following generations. The challenge I encountered was not after I decided to pursue a career in research, but after joining the Hotamışlıgil lab at the Sabri Ülker Center. Probably, except for the Weizmann Institute, I was never surrounded by as many bright, intelligent, and passionate young people pursuing science in my life. Honestly, to adapt and be present in such a high-caliber scientific community was not easy. The search for the next discovery continues and the cycle never stops, which is one of the appeals of working in research.
Q. How did you decide to pursue a career in research?
I was appointed to an Emergency Department after my compulsory service as a General Practitioner in the southeast of Turkey. I always wanted to be a General Surgeon, and the ER was the best place to practice and get experience. But one day, a friend of mine handed me a Human Genetics textbook and told me that there is a PhD program in the Medical School, and that they would open an admittance exam. “You can keep the book if you’re interested in taking the exam.” I had never read any book that fast and continuously in my life before. There were a lot of exciting concepts, tons of unknowns; it just fascinated me. Three days later, I was done with the book and decided to switch to research, instead of practicing medicine, and applied to the Program.
Q. Which particular scientific questions motivate you?
Like many of the scientists in the lab, my daily motivation is to understand what FABP4 protein does in the body after being secreted from the fat deposits, with a particular emphasis on its effect on the airways. When one finds out something and thus makes incremental progress in our understanding of FABP4 biology, we celebrate like kids, but then the next day we come up with more questions. Working on FABP4, those next days occur frequently. That “next day” serves as the biggest motivation—a different, hard-to-explain joy/cycle in science. The search continues for the next discovery and the cycle never stops!

Q. What attracted you to join the Hotamışlıgil lab at the Sabri Ülker Center?

After my undergraduate research experience in computational psychiatry, I decided I wanted to take a translational approach to research and shift my focus toward molecular biology and metabolism. Growing up in Turkey, I was familiar with Dr. Hotamışlıgil’s scientific breakthroughs in metabolic diseases. When I read his papers more in-depth, I was attracted to the unique intersection between metabolism and immunology. More so, I was excited to work in a collaborative and diverse environment with inspiring scientists aiming to solve the most significant public health crises in the world, related to metabolism.

Q. How do you think your time here will affect your career in the long-term?
Tackling scientific challenges every day not only enables me to build on my intellectual development, but to have a better understanding of what it means to be a scientist. Through the guidance of my mentors in the Hotamışlıgil lab at the Sabri Ülker Center, I’m learning how to ask meaningful questions about a problem and how to systematically develop strategies to solve it. As an aspiring physician-scientist, the skills and knowledge I gain here will always be an essential part of my toolbox, especially when I’m dealing with complex disease scenarios.
Q. How did you decide to pursue a career in research?
My curiosity for science started early on in high school through the science classes I took. My very first exposure to research occurred later in college when I joined a lab on mindfulness-based interventions. As soon as I realized that research was all about problem-solving, I was excited to discover other fields of science and find the area I was passionate about. Ever since I started to learn about the multifaceted roles of adipose tissue in disease pathophysiology, science has been an endless puzzle that keeps me fascinated every day.
Q. What do you hope to learn during your time in the lab?
I’m very excited to be learning about the underlying molecular mechanisms of metabolic diseases, and the different experimental approaches and technical platforms on how to further investigate the forces that keep metabolic behavior. Building on this knowledge every day improves my understanding of disease etiology and inspires me to think about the ways we can prevent or treat them. By the end of my work experience here, I hope to have a good base on such concepts.

Many things attracted me to join the Hotamışlıgil lab at the Sabri Ülker Center, including the research, the facility, and the people. Immediately, I was drawn to be a part of the meaningful immunometabolism research being conducted. I felt that the research being done here could truly make a difference to public health. This quality of research was also due to the state-of-the-art equipment and facilities at the Hotamışlıgil lab. I wanted to work somewhere where I wouldn’t feel limited by material resources, and I could tell that the researchers here had all the tools they needed to make groundbreaking discoveries. Finally, the people at the Hotamışlıgil lab were crucial in my decision to join. The Hotamışlıgil lab brings together experts from around the world in a way that is truly unique. Not only is everyone extremely knowledgeable in their field, but they also seemed kind, supportive, and helpful. I had always wanted to positively impact public health, and being a Research Assistant for the Hotamışlıgil lab seemed like the perfect way to do so. I am so happy I joined, and I feel grateful every day for the opportunity to work here.
As I plan for a career in research, I know that my time at the Hotamışlıgil lab will be invaluable. I aim to pursue my PhD after two years as a Research Assistant. Already, my time here has confirmed my interest in molecular metabolism. My confirmed research interest will allow me to apply to programs and labs whose research I know I will enjoy. Additionally, the scientific knowledge and experimental techniques I learn during my time here will prepare me to plan and execute a meaningful research project once I enter graduate school. Instead of spending lots of time learning basic lab techniques and principals, I can focus on my research itself. Most importantly, the people I meet at the Hotamışlıgil lab will be invaluable to my career long-term. Not only am I being connected to a large web of knowledge and resources, but also opportunities for future collaboration. I am very thankful for my time at the Hotamışlıgil lab, as I know it will be extremely impactful to my future career, both in graduate school and beyond.
 Research Assistant
Research Assistant
I chose to pursue a career in research because it is something I am passionate about, enjoy doing, and find meaningful. I have always loved biology and learning. In research, I am continuously learning in order to stay up-todate with the field. Additionally, through my own research, I have the opportunity to learn and discover entirely new things. I also enjoy working hands-on and problemsolving. These are crucial aspects in research. Every day, I am doing different types of experiments, including cell culture, mouse work, or bench work. When things don’t go as planned, I enjoy the opportunity to systematically determine what went wrong and apply this knowledge to future experiments. Finally, I have always wanted to make a positive difference to human health. Research allows me to contribute to a knowledge base that I hope will eventually be translated to disease therapy. This goal gives meaning to my work and motivates me to keep working hard, even on bad days. Because research intersects with so many goals and interests of mine, I have decided to pursue a career in research. I am looking forward to continuing to grow in this career.
There are many things I hope to learn during my time at the Hotamışlıgil lab, most of which are centered around learning what it takes to lead an independent research project. This begins with project planning: establishing goals and proposing an in-depth plan to achieve them. I also hope to learn how to plan and perform meaningful experiments. This includes learning a variety of in vitro and in vivo laboratory techniques, as well as how to determine proper positive and negative controls. I also hope to learn the best ways to analyze a variety of types of datasets, as well as how to effectively present my data and conclusions. Once a project is complete, I also hope to learn what goes into manuscript preparation and publication. Although I have a lot to learn, I am confident I will be able to learn these things from experts in the Hotamışlıgil lab. I have already learned so much, and I am excited to keep learning.


During my biochemistry undergraduate work at Oxford, I had the opportunity to discuss ideas such as Brown and Goldstein hypotheses on insulin resistance and cholesterol homeostasis, and I was therefore initially drawn to the lab’s high-impact publications in the area. I thoroughly enjoyed reading about the concept of metaflammation and felt that the Center, recognized across the wider scientific community as a pioneer in immunometabolism, would serve as a powerful platform where I could explore my interest in the field. The lab’s strong commitment to providing exceptional training and mentorship to younger students interested in finding solutions with far-reaching implications on global health, was also a principal deciding factor for me. I really look forward to being part of an inclusive and trusting culture that nurtures emerging scientists’ potential through a supporting network, which inspires both professional and personal development.
I hope to deepen my understanding of the fundamental cellular and molecular underpinnings of chronic metabolic diseases that pose the most significant challenges to public health. Participating in cutting-edge research at the interface between metabolism and immunology will also enable me to gain insight into the Center’s translational efforts, and develop a comprehensive perspective on novel preventative strategies and therapeutic interventions in the field. Additionally, the opportunity to engage with internationally renowned immunometabolism experts at the front-end of innovation will be invaluable in helping me acquire a dually structured and inventive experimental design framework, and numerous other key competences such as the ability to critically appraise evidence. The Hotamışlıgil lab is a unique environment for young aspiring scientists, like me, to both learn from and contribute to. I am above all, excited at the prospect of my individuality and ideas being bolstered by working in a vigorous intellectual setting with a group of driven, like-minded individuals.

Always having a strong interest in health and science, I pursued medical studies at the Ludwig-Maximilians-University (LMU) and Technical University of Munich (TUM), Germany. For my medical doctoral thesis at the Institute of Clinical Neuroimmunology in Munich, I investigated the function of macrophage phenotypes in Multiple Sclerosis, the most prevalent chronic inflammatory disease of the central nervous system. Coming across the group of fatty acid binding proteins (FABPs), in the context of my experimental work on neuroinflammation, sparked my interest in the field of immunometabolism and drew my attention towards the research conducted by the Hotamışlıgil lab. I was immediately fascinated by their extensive scientific contribution showing the relevance of metabolic health across numerous diseases and disciplines, and became eager to expand my understanding about the link between metabolism and inflammation. Joining the Hotamışlıgil lab offered a great opportunity to pursue this intent. Apart from the cuttingedge research, I was attracted to the diverse working environment and the feeling of equity and inclusion that I experienced during my application process.

Aspiring to pursue a career in research, I’m highly interested in expanding my qualifications and gaining orientation for my scientific career. During my six-month research appointment, I will be working together with Dr. Hatoon Baazim to dissect the role of metabolism in SARS-CoV2 infection. In this context, I am especially interested in learning more about the functions of several proteins and to unravel their complex roles across conditions, ranging from SARSCoV2 infection to neurodegeneration. In addition, I am really interested in scientific exchanges and critical discussions about the different projects performed inside and outside of the lab, and learning from the expertise of my colleagues. As a future clinical scientist, I see exciting potential in applying the knowledge and experience gained during my time in the Hotamışlıgil lab on the research I perform on neuroinflammation, and to put this into the broader context of public health. As a long-term goal, I hope to be able to translate the scientific findings on immunometabolism into the clinical setting and to implement them into the best possible patient care. I am convinced that joining the Hotamışlıgil lab is a great opportunity for me to learn and to grow in a scientific, professional, and personal way and I am looking forward to contributing to this diverse and vibrant environment.
Dr. Nilay Yapıcı, an Assistant Professor at Cornell University and one of the rising stars featured at last year’s symposium, received the 2022 Sabri Ülker International Science Award for her groundbreaking work on Neural Mechanisms Determining Food Choice and Intake.

Dr. Yapıcı earned her BA degree in molecular biology and genetics from Boğaziçi University and went on to earn a PhD in 2008 from the University of Vienna, where she worked with Barry Dickson at the Research Institute of Molecular Pathology. Dr. Yapıcı completed her postdoctoral training with Leslie Vosshall at Rockefeller University, before joining Cornell as an Assistant Professor in 2016. In his opening remarks, Mr. Ali Ülker emphasized the critical importance of fundamental science in generating solutions for the most challenging health problems around the world and the deep commitments of the Sabri Ülker Foundation, Ülker family and the community in supporting science. Dr. Yapıcı began her award acceptance speech by thanking the Sabri Ülker Science Foundation, the Ülker family, Dr. Gökhan Hotamışlıgil, her family, mentors, and colleagues. She also dedicated this award to the young researchers and students in Turkey, and advised them to never give up on their dreams and ideals and to never compromise the quality of science. She then provided a brief description of her research program and its potential applications.
Dr. Yapıcı’s research is focused on discovering novel genes and neural circuit mechanisms in the brain that regulate animal physiology and behavior, with a focus on nutritional choices and eating habits. She discovered novel pathways and neuronal populations that control eating behavior and demonstrated that these pathways could be utilized to control eating behavior and obesity. Dr. Yapıcı works with a variety of model organisms, representing different levels of neuronal complexity, to enable genetic tractability. For example, the fruit fly has 250 thousand, and the mouse has 71 million, neurons, compared to the 86 billion found in humans. The reduced number of neurons in these organisms allows for better observation and understanding of the fundamental principles of how motivational states regulate food intake on the level of molecules, cells, and circuits, and identifies their function and strategies to alter for therapeutic purposes. In investigating these neural processes or genetic pathways, new interventions to combat the effects of metabolic diseases can be identified.
Gökhan S. Hotamışlıgil, MD, PhD
May 2022
Lecturer, 58th International Diabetes Congress. National Diabetes Congress. Virtual
Keynote Lecturer, Sabri Ülker Nutrition for Healthcare Professional Conference. Sabri Ülker Food Research Foundation. Istanbul, Turkey
June 2022
Lecturer, ADA Scientific Sessions. American Diabetes Association. Virtual
Lecturer, Gordon Research Conference on Immunometabolism in Health & Disease. Smithfield, RI
October 2022
Speaker, Academic Year Opening. Izmir Institute of Technology. Virtual
Lecturer, Amgen CMD Scientific Lecture Series.
Virtual
Keynote Speaker, 26th National Pediatric Endocrinology & Diabetes Congress. Virtual
Opening Keynote Speaker, Istanbul Tech Week.
Virtual
November 2022
Speaker, Diabetes Day. Karolinska Institutet.
Virtual
Speaker, November Diabetes Awareness Month. Case Western Reserve University Center for Diabetes, Obesity, and Metabolism. Virtual
Lecturer, Nutrition Seminar. Harvard University. Boston, MA
December 2022
Keynote Speaker, Innovative Approaches in Drug Development. Hacettepe University. Virtual
Mehmet Furkan Burak, MD
February 2022
Plenary speaker, International Endocrinology Congress. Buenos Aires, Argentina
May 2022
Plenary Speaker, 43rd Annual Conference of Turkish Endocrinology and Metabolism Association. Antalya, Turkey
Invited Panel Member and Grant Reviewer. UK Asthma and Lung. London, United Kingdom
June 2022
Grand Round Speaker, Harvard Medical School. Boston, MA
August 2022
NIH p31 NORCH Grant Winner
Brigham & Women’s Hospital Institutional Nominee for the Pathway Award of American Diabetes Association
September 2022
Tersus Life Sciences Clinical Trial Grant ($2.5M) Winner
November 2022
Invited Membership, Massachusetts Health Drug Utilization Board, Commonwealth of Massachusetts. Boston, MA
Plenary Speaker, 3rd International Protein Calorie Restriction Workshop. Brigham & Women’s Hospital, Harvard Medical School. Boston, MA
Eva Tsaousidou, PhD
September 2022
Invited Speaker, Immunometabolism at the Crossroads of Obesity and Cancer. Keystone Symposia Molecular on Molecular and Cellular Biology. Keystone, CO
Kacey Prentice, PhD
June 2022
Speaker, ADA Scientific Sessions. American Diabetes Association. Virtual
December 2022
Global Recipient of One of Two Career Development Awards from the Juvenile Diabetes Research Foundation (JDRF)
Jillian Riveros, B.A.
July 2022
Invited Speaker, Howard Hughes Medical Institute. Excellence Colleges’ Symposium “Elevating Student Voices”. Vassar College. Poughkeepsie, NY
We thoroughly review the results of many repeated experiments and view them through a critical lens. Gathering data then becomes a collaborative process where project teams intensely focus on questions which arise, and then attempt to design pathways that may reveal answers— musicians similarly may pursue an idea or theme in their mind, and attempt to find a way to assemble such sounds and take them from a thought to an auditory experience that others may enjoy. Both science and art, when successful, are ways to amplify the quality of human existence. Both provide enrichment and advantage, and each has the power to heal in its own way. Similarly, our laboratory is driven by the intensity of purpose, dedication to detail, and powered by the creativity and energy of our fellows, students, visiting scientists, research assistants, research associates, and technical and support staff. Opportunities to share and learn from each other keep us all fresh and motivated.

This is why I look forward, with excitement, to May 910, 2023 as the Sabri Ülker Center for Metabolic Research at Harvard University, Harvard T.H. Chan School of Public Health returns to Istanbul for the fourth Sabri Ülker “Metabolism and Life Symposium”. The opportunity to gather in person once again, for a celebration of science

and culture in the 100th Anniversary year of the Republic of Turkey, adds to this significance. The “Metabolism and Life Symposium” brings together a spectacular array of global experts, exploring broad aspects of metabolism in health and disease states and during the aging process.


Thanks to the support of the Ülker family and the Sabri Ülker Foundation, this series of symposia began in 2016, and has become an incubator for the scientific leaders of the future to interact with those international scholars who are currently making discoveries which advance our understanding of the molecular details of metabolic pathways, in search of approaches to translate this exploration into treatments and preventive approaches to fight metabolic disease.
The symposium is a great platform for young and emerging scientists to interact with established experts, and for local and international scholars to build new networks. We are confident that this event will lead to new perspectives, stimulate new collaborations, and inspire future scholarly efforts. We will gather to celebrate science, culture and the arts, in a venue that promises to leverage the beauty and importance of all three, combined.
The pursuit of science, like the pursuit of art, requires an intensity of purpose, a dedication to detail, and most importantly an investment of one’s creativity and energy. Identifying a hypothesis to pursue is an elegant quest that is borne of multiple experiences.

Nutrient, Genetic, and Metabolic Research
Nutrient, Genetic, and Metabolic Research
Harvard T.H. Chan School of Public Health
665 Huntington Avenue Building 1 Room 605 Boston, Massachusetts 02115
Visit us: www.gsh.sph.harvard.edu Follow us: @sabriulkerctr
NUTRIENT, GENETIC, and METABOLIC RESEARCH