
elmi2024 Picture Special: Thanks to all our attendees and sponsors!
Under the Microscope: an account of setting up a new public engagement initiative
Revealing the secrets of the Sands
At the heart of bioimaging: The RMS Life Sciences committee










elmi2024 Picture Special: Thanks to all our attendees and sponsors!
Under the Microscope: an account of setting up a new public engagement initiative
Revealing the secrets of the Sands
At the heart of bioimaging: The RMS Life Sciences committee








The F200 does more to change the accepted views of uncorrected S/TEM than ever before. Its enhanced cold field-emission source, large-area silicon drift detectors, and electron optics stability are the foundation for capabilities that exceed the expected.
• Atomic-scale imaging – even for thick specimens
• High spatial resolution – even at low kV
• Spatially-resolved analytical information with EDS/EELS
• Optimum Bright Field (OBF) STEM – Maximum S/N at low dose
• Light element detection
With the F2, you’ll never have to compromise. Get the performance you need in one powerful TEM that does more than you’d expect.



infocus is the Magazine of the Royal Microscopical Society (RMS) –the only truly international microscopical society. The RMS is dedicated to advancing science, developing careers and supporting wider understanding of science and microscopy.
infocus Magazine
37/38 St Clements
Oxford, OX4 1AJ, UK
Tel. +44 (0)1865 254760
Email: infocus@rms.org.uk Website: www.infocus.org.uk
Scientific Editor
Leandro Lemgruber, University of Glasgow, UK
Editor
Owen Morton
Tel. + (0)1865 254763, Email: editor@infocus.org.uk
Editorial Board
Susan Cox, King’s College, London, UK
Rebecca Higginson, Loughborough University, UK
Laura Fumagalli, University of Manchester, UK
Myfanwy Adams, John Innes Centre, Norwich, UK
Maadhav Kothari, Zeiss Microscopy, UK
Hilary Sandig, Cancer Research, UK
Trevor Almeida, University of Glasgow, UK
Mark Rigby, Nikon UK
Advertising
Email: advertising@infocus.org.uk
ISSN: 1750-4740
© 2024 Royal Microscopical Society
infocus is published four times per year by the RMS. Designed and produced by Creative Design. Reproduction in whole or in part without permission from the RMS is forbidden. Views expressed in the Magazine are those of the individual contributors and do not necessarily reflect those of the RMS.

Dear Readers,
Well, the summer break is over – at least for those of us in the Northern Hemisphere – and for those in the south, I hope you are looking forward to springtime! Either way, September means it’s time for another issue of infocus!
One of the highlights of the last few months was undoubtedly ELMI2024, which took place in Liverpool in early June. The European Light Microscopy Initiative is one of the best meetings in microscopy, bringing together scientists, developers and industry. Organised this year by the RMS, it was a massive success, and we have a colourful ‘picture special’ telling the story in this issue. If you were there, perhaps you will spot yourself!
Elsewhere, Luke Norman gives the lowdown on a public engagement initiative based on microscopy for different audiences in Nottingham. This is well worth a read – especially for anyone interested in setting up outreach projects. One of our regular contributors, Michael Gibson, tells us about the Northamptonshire Natural History Society and their amazing collection of different sand samples (spanning over 8,000!).
One of the greatest strengths in the RMS is our network of Science Committees, representing the various branches of microscopy and flow cytometry across the sciences. In this issue we find out more about the members of the RMS Life Sciences Section - including what they enjoy doing when they’re not looking down a microscope!
As a community it is also important to celebrate the outstanding achievements of individuals. To that end, the RMS recently announced the winners of its Science Section awards for 2025.You can read more about all our winners in this issue.
I hope you enjoy all these articles – as well as our other regular features! Slàinte!
Leandro Lemgruber

COVER IMAGE: A Crystallization Scene of Magnesium Nitrate dissolved in water and ethanol, by Cagri Yalcin, Dutch Society for Microscopy (NGVM).
Winning entry in the 2023 RMS Scientific Imaging Competition, Light Microscopy - Physical Sciences category. Half teaspoon of magnesium nitrate is dissolved in 30 ml of water and ethanol. Two drops are spread on a slide which is heated and then put on an icy surface for cooling down. The image is taken under polarised light using a Euromex Oxion microscope with a 10x objective.

Picture Special: Thanks to all our attendees and sponsors!

RMS reflects on fantastic week in Liverpool
The RMS would like to thank everyone who attended elmi2024 in Liverpool (4 – 7 June), for making it such a special event for the Light Microscopy community.
Against the backdrop of the city’s iconic Albert Dock, more than 640 people arrived at ACC Liverpool for the latest instalment of the popular meeting series. The event featured a fantastic blend of talks, workshops and exhibitors, plus plenty of opportunities for networking.
The European Light Microscopy Initiative (to give elmi its full name) is regarded by hundreds of scientists and developers as an essential fixture in the light microscopy calendar. With tickets having sold out in the first few months of the year, expectations were high, and elmi2024 certainly delivered.
RMS Chief Executive Sali Davis said: “We are so grateful to the Light Microscopy community for supporting this event, and ensuring a truly memorable week for everyone in attendance. The enthusiasm and energy of participants was palpable from start to finish, and I really hope everyone enjoyed themselves in Liverpool.”
She added: “I would like to thank everyone involved in making the event such a success - from our speakers, workshop presenters, exhibitors, and other delegates, to our dedicated volunteers and
the RMS team for helping things run as smoothly as possible during the meeting. I would also like to give special thanks to our team of Scientific Organisers and of course, our generous sponsors, without whom none of this would have been possible.”
With more than 170 abstracts submitted – including poster presentations – a rich scientific programme across six sessions and a dedicated ‘Core Facility Day’ ensured there was something for all interests. Meanwhile, almost 60 companies brought their latest products and technology to the main exhibition hall and Tabletop Exhibition. They also hosted more than 130 well-attended workshops throughout the event – a feature that has become a key attraction of the elmi meeting series.
On the social and networking side, things were just as busy, with evening receptions and buffets within the meeting venue, and a memorable dinner on the last night for more than 400 attendees at the Rum Warehouse. The traditional ‘Industry versus Academy’ football match also took place, with the company representatives emerging comfortable winners on this occasion!
Opposite are some more illuminating facts and figures from elmi2024.
Maybe you can spot yourself in one of our official photos from the event:
elmi2024 in numbers:
175 Abstracts Submitted
130 Posters Presented
645 Attendees registered (all at Early Bird rate, capacity at venue reached)
65% Academic Attendees
32% Company Attendees
35 Countries represented, across five different continents
59 Sponsors 48 in main hall 11 Sponsors attending from15 countries
in tabletop exhibition
50% companies from UK
24 companies presenting 136 workshops on-stand opportunities 6



















































The European Light Microscopy conference 2024 (elmi2024) was held from the 4th to the 7th June 2024 at the ACC in Liverpool, UK, with kind organisation from the Royal Microscopical Society. It managed to bring together scientists from academy and industry with a keen interest in both the development and application of light microscopy.
Attending this conference in the final months of my PhD has been a rather poignant experience, bringing me full circle to where my academic journey began
nine years ago. It has been over four years since I last returned to Liverpool, yet the city retains its charm, evoking a profound sense of nostalgia upon my arrival. This atmosphere was wonderfully complemented by a pre-conference firework show, celebrating the christening of the Queen Anne cruise ship, setting a spectacular tone for the days ahead.
Throughout the week, 645 attendees had the opportunity to hear from researchers at various career stages from both academia and industry. The event featured 130 poster presentations and six sessions of oral talks, highlighting new technologies, refined optimisations, innovative applications, and
the emerging roles of AI in the future of imaging and analysis. Following the three additional sessions dedicated to the invaluable work of the Core Facilities, the meeting was officially kicked off with the keynote speech by Ralf Jungmann. His clear passion for super-resolution and DNA-Paint set a clear standard for the tone of the conference. Each of the following sessions built on this momentum, extending the excitement that it was clear each of the attendees had for microscopy and the many advancements in the field. As well as the seminars and discussions, one incredible opportunity was to be able to take part in several of the 136 company-led workshops giving hands-on learning and personal demonstrations on what they were offering and what was available for researchers to expand their work. The kind engagement that they provided also made it a little bit easier to swallow the loss in the Industry vs Academia football match earlier in the week!
I was lucky to be able to present some of the work conducted as part of my PhD in a flash oral talk and a poster, and the evening’s poster session was a great opportunity to meet with the other delegates, discuss our work and exchange ideas. I also discovered the reputation that elmi has as the “party conference” - something later proven true at the gala dinner at the Rum Warehouse, where speeches and dinner were followed by a night of dancing!
Many thanks to the Royal Microscopical Society and the OLIGOMED consortium (MSCA 956070) for providing the funding to be able to attend elmi2024, I’m already looking forward to the 25th Edition of elmi in Heidelberg, DE!
Joshua McHale International Institute of Molecular Mechanisms and Machines
Polish Academy of Science/University of Udine

We are very pleased to continue offering a range of ‘in-person’ and virtual events this year, in order to maximise accessibility and provide opportunities to those who might not otherwise be able to attend. The following information was correct at the time infocus went to print but could potentially be subject to change in the coming weeks. Please visit our event calendar at www.rms.org.uk for the latest updates.
If you have any questions about a booking you have already made for an event, or need any help or advice, please contact us at info@rms.org.uk
2 – 6 Flow Cytometry Course 2024, York, UK
2 Microscopy: Advances, Innovation, Impact 2024 - incorporating the RMS AGM & Section AGMs, London, UK
15 – 16 Facility Management Training Course 2024, York, UK
November
11 – 12 Frontiers in Bioimaging 2024, Oxford, UK
19 – 20 Frontiers in Physical Imaging 2024, London, UK
December
4 SEMT 2024 Meeting, London, UK (RMS Exhibiting at event)
5 – 6 Virtual European Flow Core Meeting 2024 (online)
6 Super-resolution in the North 2024, Leeds, UK (RMS-hosted event)
8 – 9 Flow Cytometry Facilities Meeting 2025, Edinburgh, UK
6 – 7 EM-UKI 2025, Liverpool, UK
26 – 28 flowcytometryUK 2025, Newcastle, UK
8 – 9 Introduction to Image Analysis 2025, Galway, Ireland (RMS-hosted event)
14 – 16 Advanced imaging techniques in biomineralisation research Faraday Discussion, Edinburgh, UK (RMS sponsored event)
June / July
30 June –
3 July mmc2025: Microscience Microscopy Congress 2025, Manchester, UK
For further information on all these events, please visit our Event Calendar at www.rms.org.uk
Microscopy: Advances, Innovation, Impact 2024 - incorporating the RMS AGM & Section AGMs
2 October, London, UK
(11.00 BST - 19.00 BST, 06.00 - 14.00 EST, 12.00 - 20.00 CEST)
11 – 12 November, Oxford, UK
Scientific organisers: Anjali Kusumbe, University of Oxford; Kurt Anderson, The Francis Crick Institute; Stefania Marcotti, King’s College London
Frontiers in Bioimaging 2024 will focus on the latest developments in optical and electron microscopy as well as image analysis. Sessions
The RMS Annual General Meetings (AGMs) will take place on 2 October 2024.
This meeting will be the sixth one-day meeting in the Microscopy: Advances, Innovation, Impact series, having previously taken place in 2016, 2018, 2020, 2021 and 2022.
will cover novel technical developments and applications of these microscopy-based approaches to key cell and molecular biology questions with an overarching aim to bring insights on how they participate in our understanding of human health and disease. We aim to provide an environment where early-careers and established researchers can meet and engage with a broad range of imaging approaches and to make valuable contacts with leading groups in the field.

19 – 20 November, London, UK
Scientific organisers: Asa Barber, London South Bank University; Thomas Walter, University of Sheffield; Roland Kröger, University of York
The meeting 'Frontiers in Physical Imaging’ aims at providing a forum to discuss state-of-the-art imaging and spectroscopy techniques applied to the characterisation of materials.
Scientific presentations of novel results and educational tutorials to train young scientists
Virtual European Flow Core Meeting 2024
5 – 6 December (Online)
Scientific organisers: Charlotte Petersen Aarhus University; Derek Davies, Derek Davies Cytometry; Désirée Kunel, BIH at Charité; Marjolijn Hameetman, Leiden University Medical Center; Marta Monteiro, Instituto Gulbenkian de Ciência; Peter O’Toole, University of York
This will be the first meeting of what will hopefully become an new tradition - an annual event to meet with fellow flow core colleagues from all over europe. The meeting is aimed at all those that run or work in a Flow Cytometry Core Facility and seeks to address common
in methodological know-how are both highly welcome.
The inaugural meeting will consist of three sessions centred on the broad area of in-situ imaging:
in-situ mechanical testing in SEM & TEM (Asa Barber, City University London),
in-situ studies of biomineralisation (Roland Kröger, University of York),
radiation damage in analytical STEM (Thomas Walther, University of Sheffield)
subject matters, themes, and circumstances within the european community. We will focus on new and emerging technologies as well as operational aspects of running, and working in, a core. We will include talks about national cytometry societies, presentation from current core facilities (Crib talks) and also from our industry colleagues (Technobites). The meeting will run over two days, from lunchtime to lunchtime (5 and 6 December 2024). Although in a virtual format, there will be ample time in the programme for discussion and questions from the community. The meeting will be ‘live’ and will not be recorded, it will be unavailable online afterwards.


ATUMtome Automated Tape
Collecting Ultramicrotome
Collect hundreds to thousands of sections on a continuous tape
Non-destructive to sample
Store sections for years into the future for processing, post-staining, immunogold labeling, correlative imaging - at any work pace desired
Patented Power Drive© for biological samples and extremely hard materials




Luke Norman is the Knowledge Exchange Fellow at the Nanoscale and Microscale Research Centre, University of Nottingham, UK. He translates materials characterisation research and capabilities into public and commercial engagement resources. He is also the curator of Under the Microscope, a public engagement initiative aimed at displaying to the public the wonders of electron microscopy.
When I first came up with the idea for a microscopy based public engagement initiative, I didn’t think I would end up in a wildlife park in Birmingham reaching over a fence trying to obtain a porcupine quill. The things we do for science!
My role as a Knowledge Exchange Fellow at the Nanoscale and Microscale Research Centre (nmRC) is to translate the techniques we use at the centre (including electron microscopy) and the science we carry out into engagement resources for a range of different audiences. This activity, enabled through UKRI’s Higher Education Innovation Funding aims to increase the awareness and visibility of our research, build trust and credibility with the wider community and crucially inspire the next generation of scientists. One of the ways I have done this is through our Under the Microscope public engagement initiative which asks a simple question to its audience “what would you like to see imaged using microscopy?”.

Back in January 2023, I was thinking of new ways I could engage the public with microscopy-based techniques, in particular electron microscopy that is certainly not encountered in day-to-day life for most. At this point, the nmRC was early on in its public engagement journey and we were imaging items related to popular social media marketing days such as Ice Cream for Breakfast Day (first Saturday in February in case you were wondering). However, critical to successful knowledge exchange and public engagement are openness, transparency and interactivity with your audience. We therefore decided to take the plunge and hand over the keys of the nmRC (metaphorically of course) to the public and ask them what they would like to see imaged each month at the centre, and with that, Under the Microscope was born. Electron microscopy is a perfect fit for public engagement activities as it is a very visual technique; you don’t have to understand the detailed optics of the


column to appreciate the beauty of the images it can produce of the world around us.
At the time of writing this article, Under the Microscope has just completed its first year and we’ve imaged 12 items suggested to us by the public. The initiative hasn’t been without its challenges as with the setting up of all new things. However, there are three aspects of running Under the Microscope that I love the most. The first is getting to read the suggestions from the public and more specifically their reasons why they chose that object. We delivered Under the Microscope live as part of a science festival with local children and one of the items that was suggested by them was bread, and their reason… “I eat a lot of it”, this still makes me smile to this day. The second element is obtaining the items themselves – for the second month I came up with a shortlist of the items that had been suggested and put them to a vote with our team. The outright winner being a porcupine quill, which I since found out was selected because the team wanted to see how I would obtain one (Nottingham isn’t particularly known for wild porcupines). Hence why I subsequently ended up on a mission at a wildlife park in Birmingham! The third is that I’ve started to become a bit of an

expert in very specific areas. For example , the 12th suggestion that was chosen was snails (don’t worry, no snails ended up in an electron microscope) and so I contacted the University’s resident snail aficionado Prof. Angus Davison (of 'Jeremy the lefthanded snail' fame) to see what we could image, and he suggested love darts because it was February and would tie in nicely with Valentine’s day. Love darts are what snails use to aid with mating, but a quirky thing about them is that they produce them during the first mating and then use them during the second courtship routine. This fairly random knowledge then ended up being very useful when it was brought to my attention that on an episode of The Tonight Show with Jimmy Fallon, specifically on a segment called pup quiz where a celebrity (in this case Arnold Schwarzenegger) has to answer questions to win puppies to cuddle, there was a question asking which animals produce love darts.
We launched the initiative with a local TV channel called NottsTV who asked if they could suggest the first idea which was a budgie feather belonging to one of their reporters. This didn’t end up being


the last feather we would image as our seventh suggestion was a feather from a peregrine falcon (the fastest animal in the world). This was the first suggestion where the suggestor provided us with the object directly, as it turned out that they had a feather originating from a nest in Belper, Derbyshire. For the SEM imaging, we captured 150 images and stitched them together showcasing how to obtain a larger field of view image.
As mentioned earlier, we don’t always get lucky


having objects sent to us, as was the case of the porcupine quill which still remains the hardest object to have found so far. However, we did find out that if you cut one in half and image the cross section, it has support segments (similar presentation to that of a lemon cut in half). This design from nature is to offer lightweight but strong defensive quills that in the wild will ward against potential predators without encumbering the porcupine!


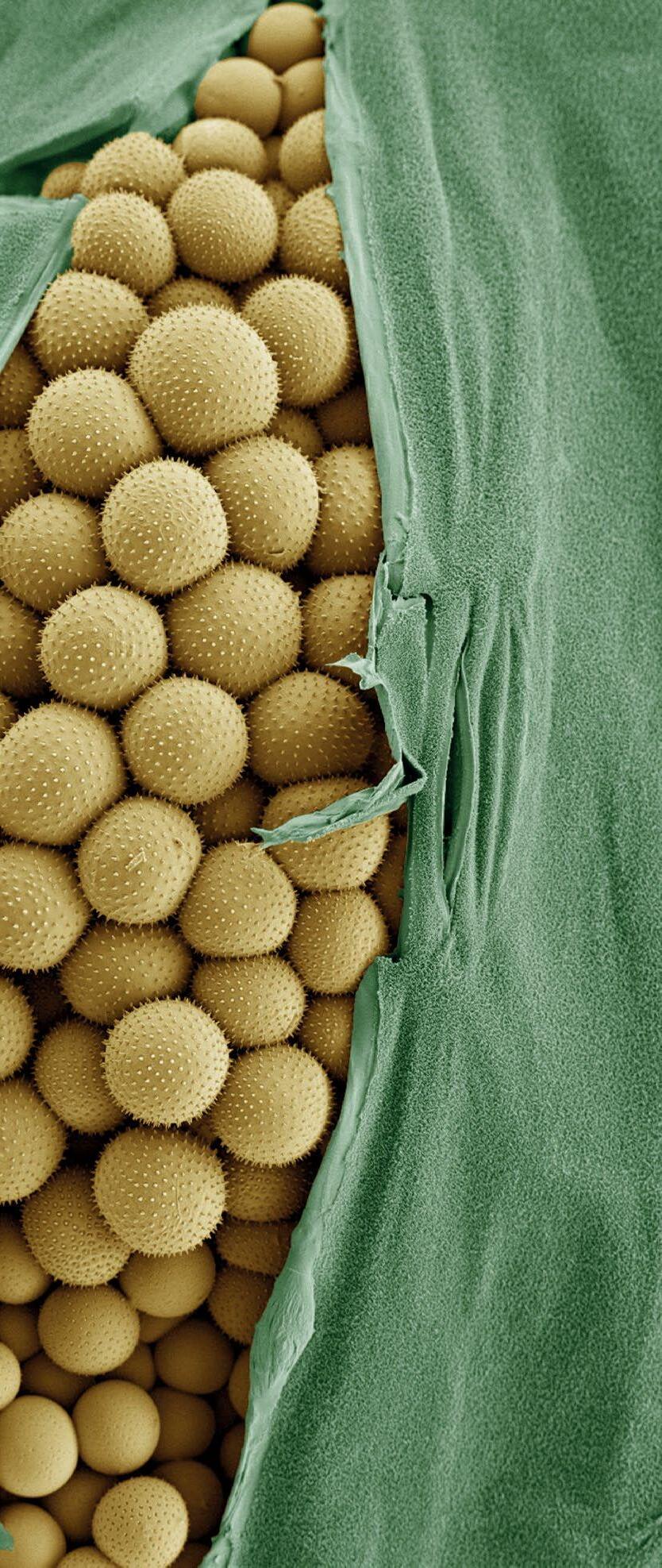
We have ventured into the realms of food science by selecting shortbread biscuits in May 2023. Again this came at a time when our team were picking the winning suggestion from a shortlist and was chosen I think more because it meant biscuits would inevitably be brought into the centre. Pollen has always been a favourite item of mine to image as each variety is completely unique in structure (Daffodil pollen, for example, resembles honeycomb on its outer structure). Pine pollen was suggested to us because the grains bear a


resemblance to Mickey Mouse with two large circular “ears” and a semi-circular face.
A lot of our suggestions from the public are of common everyday items, such as paper with ink or mushrooms, as they want to learn more about what’s around them. Sometimes the reasons are more to do with a fascination of a more specialist topic such as lizard scales which were suggested because “they are shiny and kind of cool”. For this idea, I knew there was only one person who
could help, Dr Tom Hartman of the School of Life Sciences, as there isn’t much of the natural world that he hasn’t imaged. When I approached him to ask if he had ever imaged lizard scales, he replied that not only had he imaged the toes of a crested gecko, but also he had many more frozen specimens in storage if needed.
For the spooky season (October) we chose spider web and collaborated with another UoN Life Sciences academic, Professor Sara Goodacre, who provided us with some samples from her lab. The most striking element was that some of the thinnest threads imaged were just 100 nm in diameter, demonstrating the nanoscale engineering the spiders adopt to create webs.Admittedly, during this imaging session, myself and the operator Nicola Weston got distracted finding objects collected in the web including a wing and an aphid.
As part of my role, I actively try to engage as many people in electron microscopy as possible. Remarkably, this has included King Charles III who was “fascinated” about our use of focused ion beam scanning electron microscopy as part of a Coronation celebration project (the letter from Buckingham Place is pride of place in our display cabinet!). However, a big target on my wish list is the actor Timothy Chalamet, and so for December we imaged popping candy chocolate to coincide with the release of the film Wonka. Cracks had formed on the candy exterior surface upon release of the carbon dioxide which gives the sweet its characteristic crackling sound. Sadly despite tagging Timothy in the post on X, I still await his verdict on those images…
Up to this point, we had been primarily imaging objects using SEM but some suggestions align far better with transmission electron microscopy (TEM), such as the suggestion of car exhaust
particles. Carbon emissions is a hot topic currently and the person who submitted the idea wanted to know more about what was being produced by their vehicle. TEM was perfect for this, as the soot particles could be deposited onto a TEM grid and imaged. This revealed their onion-like layered structure as well as the range of different sizes.
But what does the future hold for Under the Microscope? Well, we aim to keep the initiative going as long as the public remains interested in electron microscopy and we will look to obtain more ambitious objects that have been suggested. We also have plans to take this directly into schools, where the students will be able to suggest ideas and we image them live to them in the classroom. If you would like to submit an idea for us to image, please visit our website www.nottingham.ac.uk/ nmrc to find out more.


Wellington, New Zealand has been dubbed the "coolest little capital in the world," not only for its renowned quality coffee, craft beer, and as the home of Sir Peter Jackson’s movie productions like the "Lord of the Rings" saga.Wellington also boasts a vibrant and well-established scientific community, with universities, crown research institutions (CRI), and independent research institutions.
In a collaborative effort, Alfonso Schmidt (Senior Specialist in Bioimaging at Malaghan Institute of Medical Research) and Maddeleine White (Strategic Partnerships Manager at Gillies McIndoe Research Institute) had the vision to establish the Wellington Bioimaging Group (WBG). This group aims to create a supportive community focused on sample preparation, imaging capabilities, and image analysis. In this spirit, we organised the 1st Wellington Bioimaging Symposium to drive and evaluate interest in the community.


We are confident we delivered a successful 1st Wellington Bioimaging Symposium. Despite being held on the shortest and coldest day in the southern hemisphere, the 60 vibrant and energetic attendees from over 20 different organisations showed genuine interest.
The one-day symposium was divided into two main activities. In the morning, our invited speaker Dr. Ellie Cho from the Biological Optical Microscopy Platform (BOMP) at the University of Melbourne, Australia, delivered a hands-on QuPath Workshop covering the basics and advanced applications of the software. Ellie’s workshop had a significant impact on the local image analysis community by imparting valuable knowledge for robust image analysis.
In the afternoon, the presentations at the symposium covered new bioimaging developments, including quality control in microscopy, macro development and application, and innovative techniques for staining neurons.The second part of the symposium focused on the application of bioimaging in diseases
like cancer and infectious diseases. The entire symposium was free for attendees and provided full catering throughout the day, thanks to our incredible sponsors, including the Royal Microscopical Society, Evident Scientifics, Bio-Strategy/DSKG, InterMed Medical NZ, and Leica Biosystems.
We are convinced that the Wellington Bioimaging Group (WBG) is now a reality. We aim to maintain the momentum of this vibrant community by organising more activities to learn and apply new and exciting bioimaging applications for novel discoveries.
Wellington Bioimaging SymposiumOrganising committee
Alfonso J. Schmidt, Senior Specialist-Bioimaging Hugh Green Technology Centre, Malaghan Institute of Medical Research.
Maddeleine White (PhD), Strategic Partnerships Manager, Gillies McIndoe Research Institute.
The RMS is pleased to announce the winners of its Science
awards for 2025.
The awards, which are given out every two years, celebrate outstanding scientific achievements across all areas of microscopy and flow cytometry, with each RMS Science Section selecting a winner.
RMS President Peter O’Toole said: “It is a real privilege to announce our award-winners, who have all made some incredible contributions across their various scientific fields.
“Each winner has been chosen by one of our Scientific Committees - or ‘Sections’ - which represent all branches of microscopy, imaging and flow cytometr y. As such, these awards are about receiving recognition from others working in the same field - and there’s no higher praise than that.
“The nominations were of exceptional quality and our committees certainly had a difficult job making their final decisions. My warmest congratulations go to all our winners.”
The RMS Section Award-winners for 2025 are as follows:
Sohini has made outstanding contributions to the field of scanning probe microscopy (SPM) through her studies of nanoscale electromechanical properties of novel functional polymer and semiconducting nanostructures. Currently Professor of Device Materials in the Department of Materials Science at the University of Cambridge, she is internationally renowned for her pioneering research on functional materials for energy and biomedical applications. She has extensively used SPM techniques to uncover new functionalities of polymeric materials in particular, at the nanoscale.
Among her many achievements, she led the development of a unique non-destructive piezo-

response force microscopy (PFM) technique applicable to soft nanomaterials, enabling the first direct characterization of nanoscale piezoelectricity using PFM in self-assembled cellulose nanofibers and cross-linked collagen bundles. Her research group also introduced a novel time-resolved, open-circuit conductive atomic force microscopy (cAFM) technique as a new SPM methodology for direct electromechanical characterisation of semiconducting nanomaterials.
Sohini is a world-renowned expert in the use of advanced SPM modes to understand structureproperty and functionality relationships in novel polymer-based piezoelectric nanostructures. She has pioneered the use of piezoresponse force microscopy (PFM), Kelvin probe force microscopy (KPFM) and quantitative nanomechanical mapping (QNM) to reveal the electromechanical properties and lamellar structure of a range of different piezoelectric polymers at the nanoscale, with implications for specific applications in energ y harvesting and sensing.
Sohini’s work has earned her numerous prestigious accolades and awards, including the Royal Society of Chemistry Peter Day Prize (2023) and the 2023 Institute of Physics Lee Lucas Business Award for early-stage medical and healthcare companies. She is a founding Director of ArtioSense Limited (www. artiosense.co.uk), and was recognised as one of the Top 50 Women in Engineering of 2021, by the Women’s Engineering Society.
– Frederick National Laboratory, National Cancer Institute, NIH, USA
Kedar Narayan has been among the leading figures in the development of Volume Electron Microscopy – a high-end EM technique – for several years.
He is a senior scientist and group leader at the

Center for Molecular Microscopy (CMM) at Frederick National Laboratory and National Cancer Institute, NIH, USA. He earned a PhD in immunology, with an emphasis on biophysics and imaging, at the Johns Hopkins School of Medicine in 2008; he also has a background in chemistry, pathology and software engineering. Since 2015 at the CMM - a highly collaborative laboratory with a strong technology development portfolio - his group has developed and applied FIB-SEM and other volume EM technologies to questions in cell biology. Specific areas of focus include correlative imaging, deep learning/AI, and tool development.
Kedar has co-authored more than 50 research papers and has given invited talks around the world about his work. He co-organised the first “Large Data in Cell Biology” symposium and the “volume EM” symposium at Microscopy & Microanalysis in 2019 and he organised a virtual conference in 2020, where worldwide leaders in volume EM worked in a moderated setting to articulate obstacles and wishlists in the field. His community work includes co-organising symposia on volume EM and “large data”, leadership on data working groups, and creating common data and metadata standards for the field.
Kedar also co-edited the first Methods in Cell Biology on vEM. He is on the Scientific Advisory
Board of EuroBioImaging, the EBI Imaging Ecosystem working group, a co-chair of the vEM Data working group, and as a leading member of the vEM community, is committed to the growth and democratisation of the field.
Francisco is a researcher in the fields of analytical electron microscopy, materials science and scientific data analysis.
As the primary creator of HyperSpy (a python package for the analysis of multi-dimensional data, with specific functionality for analysing data collected in electron microscopes) he has made an unparalleled contribution to the analysis of electron microscopy data in physical sciences.
HyperSpy started as a toolbox for the analysis of electron energy loss spectroscopy. Good design meant that a lot of the functionalities could serve as basis for implementing analyses of other types of multi-dimensional data, such as energy dispersive X-ray spectroscopy, scanning electron diffraction, 4D-STEM or atomic resolution images. It has

now become a framework to handle any type of multi-dimensional data, used by a growing number of scientific data analysis software packages. Consequently, it has received more than 1100 citations in peer-reviewed publications.
HyperSpy has a large community of users across the world (~4000 monthly visits to the documentation website) . The success of HyperSpy is in large part due to Francisco’s dedication to building and fostering an inclusive and supportive community around this open-source project, inspiring active participation and contribution. A testament to this is the many users that assist others, submit bug reports, request features and contribute code, therefore transitioning from regular users to developers. Notably, the current lead developer began as a regular user.
Francisco is also a regular speaker at a number of conferences and workshops promoting opensource software development for electron microscopy. Francisco has played a huge role in training this community through his workshops and teaching courses, training thousands of users in not only the use of HyperSpy but also the basics of using python for data analysis. Arguably no other individual has impacted the analysis of electron microscopy data in the physical sciences as much as Francisco over the last decade and a half.
Florian has made a number of outstanding contributions in the field of cytometr y during a relatively short period of time.
His journey from PhD and post-doc, to running a Core facility has been a remarkable one, in which cytometry has been pivotal.
Dr Mair completed his PhD at the University of

Zurich in 2013 and did post-doctoral research there while also working within the University’s Flow Cytometry Facility. In 2017 he moved to the Fred Hutchinson Cancer Research Institute in Seattle in the Lab of Martin Prlic, which looks at T cell responses in inflammation. While in the Prlic Lab, he developed the first 28 colour fluorescence panel to look at human dendritic cells. At the time, this was the maximum number of analytes that could be measured.
He also became a prominent member of ISAC and delivered a well-received tutorial at the CYTO meeting in Vancouver on fluorescence panel development. Additionally, he delivered a talk and tutorial at the Babraham Spectral Cytometry Symposium in 2023. His teaching style is ideal for drawing in the inexperienced without baffling them with too much information.
In May 2022 he moved back to Switzerland to become Scientific Director of the flow cytometry facility at the ETH (Eidgenössische Technische Hochschule) in Zurich. In this position he has utilised the power of spectral cytometry to develop larger panels, particularly in the immunological field, and recently published an OMIP with the first 50-colour spectral panel (still developed at the ‘Fred Hutch’). As experiments become more complex, so do both panel design and data analysis, and Florian has been
developing a new metric to assess spreading error after unmixing, which will have a great impact on the way spectral experiments are devised.
Emilie is a stand-out materials researcher, utilising both optical and electron microscopy for developing new, optically active nanomaterials and establishing new materials for light-assisted catalysis.
Having carried out seminal work in plasmonic nanoparticles in her PhD at Northwestern University, Emilie has pioneered Wulff-construction type principles and user-friendly codes for twinned, alloyed, and kinetically controlled crystal shape prediction.
In her independent career, Emilie has targeted plasmonic metal alternatives to gold and silver for a wide range of applications. She has pioneered the synthesis of well-defined, air-stable plasmonic magnesium nanocrystals and has demonstrated their ability to enhance spectroscopic signals, assist

light-driven catalysis, and efficiently turn light into heat.
Throughout this work, Emilie has developed and applied light and electron microscopy techniques. Her work on plasmonics has made remarkable use of monochromated electron energy loss spectroscopy in the scanning transmission electron microscope (STEM-EELS) for extracting spectra and maps of the plasmonic near-field response characteristics.
Emilie has been an active developer of novel optical microscopy techniques, including hyperspectral and compressive methods for optical imaging and spectroscopy. She also collaborates broadly across multiple disciplines, including several works on magnetic nanoparticles including fossils, airborne particles, and synthetic structures.
Emilie’s broad ranging work has driven forward microscopy to solve materials characterisation challenges, delivering advances in the creation of novel nanomaterials and demonstrating their structure-property relationships directly at the nanoscale for optical, magnetic, and catalysis applications.
– University of New South Wales (UNSW), Australia
Vaishnavi has made a number of remarkable contributions to the field of Cell Biolog y, harnessing wide-ranging microscopy techniques and modalities.
She currently holds a nine-year EMBL Australia Group Leader fellowship and leads a large research group at UNSW.
As a leader in the research fields of motor proteins and their regulation, the roles of the cytoskeleton in organelle remodelling and organisation, and cellular decision making, Vaishnavi has been one of the true pioneers in quantitative single molecule microscopy

to understand the biophysical principles of these processes.
Her research exemplifies the power of microscopy in solving some of the fundamental questions in cell biology. It has consistently featured novel imaging methods such as live-cell single-molecule imaging, and technologies like optogenetics, microfluidics, and micropatterning.
Vaishnavi is a passionate educator and mentor, to-date supervising three Postdoctoral Fellows, seven PhD students, seven MSc students, and 13 undergraduate/Honours students through to completion. She is also a true champion of equity and inclusion, having organised a number of research-specific as well as inclusion-focused events, and is co-founder of BiasWatchIndia, an initiative to document women’s representation in Indian scientific conferences.
Key to her success in building a productive team at UNSW is her approach to inclusive leadership that empowers researchers at all levels in her team and allows them to fulfil their potential and career goals. Her team has published six ground-breaking research papers, four invited review papers and two perspective/comment articles. In recognition of her achievements at UNSW, Vaishnavi was awarded
the Researcher of the Year award in the School of Biomedical Sciences in 2023.
Ilaria is an internationally-recognised multidisciplinary researcher who has made outstanding contributions to the field of superresolution optical imaging. She started her own group – SciLifeLab - in 2015 following the award of a competitive ERC Starter fellowship at the KTH Royal Institute of Technology in Stockholm, Sweden, where she now holds an Associate Professorship. She has led her team in the creation of new and exciting fluorescence nanoscopy technologies aimed at improving the speed and resolution of the light microscope while remaining sufficiently gentle for live cell imaging.
Ilaria’s application of innovative super-resolution microscopy methods in cell biology has been particularly impressive. She has developed new ways to measure rotational diffusivity in cells using photoswitching mechanisms, and has provided new insights into synaptic vesicles endocytosis, as well
as revealing new information on the endoplasmic reticulum, which is notoriously difficult to image in living cells.
Ilaria has an impressive list of publications with an exponential trend in citations. She has also provided open source methods to help lower the barrier to entry to super-resolution light microscopy, including freely available software frameworks for image acquisition, reconstruction and analysis
Ilaria was listed as one of the Top 100 innovators by Photonics100 2024, a global platform providing commentary and analysis on topics of interest for the Photonics industry.

You provide the text and images and we take care of the rest. It’s the ideal way to share your work with the microscopical community.
Full submission information and guidelines are available at www.infocus.org.uk. To submit an idea or if you have any questions about the process please email the Editor (editor@infocus.org.uk)


Celebrating the contributions of women at the forefront of microscopy
The Journal of Microscopy is pleased to announce the publication of Part 2 of our Women in Microscopy special issue series. The issue features papers by women driving the development of microscopy, right across the world and a thought provoking editorial.
The much-anticipated issue has been edited by Michelle Peckham, University of Leeds, Ulla Neumann, Max Planck Institute for Plant Breeding Research, and Sian Culley, King’s College London.
The issue (July 2024) features the following papers:
Editorial (free-to-view)
Introduction to women in microscopy: Volume 2
Michelle Peckham, Ulla Neumann, Siân Culley
Letter to the Editor
Diversity under the microscope: Lessons for building belonging in interdisciplinary spaces from the Women in Imaging + Industry bootcamp
Meagan Esbin, Lena Blackmon
Original Articles
Surface passivation and functionalisation for mass
photometry (Open Access)
Jenny Sülzle, Laila Elfeky, Suliana Manley
Flexible implementation of modulated localisation microscopy based on DMD
Abigail Illand, Pierre Jouchet, Emmanuel Fort, Sandrine Lévêque-Fort
Mesoscale standing wave imaging (Open Access)
Shannan Foylan, Jana Katharina Schniete, Lisa
Sophie Kölln, John Dempster, Carsten Gram Hansen, Michael Shaw, Trevor John Bushell, Gail McConnell
Work smart, not hard: How array tomography can help increase the ultrastructure data output (Open Access)
Irina Kolotuev
Invited Review
Made to measure: An introduction to quantifying microscopy data in the life sciences (Open Access)
Siân Culley, Alicia Cuber Caballero, Jemima J Burden, Virginie Uhlmann
The Journal of Microscopy has been making great strides in 2024, with more ‘open access’ articles than ever before and a number of eye-catching special issues.
In just the first six months of 2024, a total of 45 open access articles (available to view for free) have featured in the peer-reviewed publication. Already, this comfortably exceeds the annual totals for 2023


(32) and 2024 (34). Open Access articles now make up the majority of papers published in the journal (see graph).
The Journal publisher, Wiley, has a number of ‘Transformational Agreements’ in place with institutions and funders to help authors publish open access for free and ensure compliance with open access policies. In the first half of 2024, this has also helped raise the overall number of articles published online to 67 – already approaching the total annual figures for 2022 and 2023.
Journal of Microscopy Editor, Professor Michelle Peckham said: “As we move away from subscriptions towards a fully ‘Open Access’ model, these ‘Transformational Agreements’ are enabling many more authors to publish their articles with us, which is really great.
“We are the world’s oldest journal dedicated to microscopy, and there are many benefits to publishing with us. I would urge prospective authors to check if their institution is affiliated with an open access deal, and get in touch.”
Find out if your institution or funder will cover your open access Article Publication Charges
The Journal of Microscopy is pleased to announce a new special issue featuring papers on “AI in Imaging”.
The advent of artificial intelligence (AI) proposed novel methodologies encompassing image classification and imaging-based predictions, which have the potential to revolutionise the fields of materials science, engineering and biology.
The issue will be guest edited by Peter Soar, Tuan Nguyen and Gianluca Tozzi, all of the University of Greenwich. We plan to publish this special issue in September 2025, and thus would expect articles to be submitted by April 2025.
Please contact Editorial Office Manager Jill Hobbs (journaladmin@rms.org.uk) for more information.
Several more special issues are also in the pipeline, with ‘Calls for Papers’ currently open for the following:
• Microscopy and infectious diseases
• 9th International Conference on Tip-Enhanced Raman Spectroscopy (TERS9)
• AI in Imaging
• Ptychography
Publication types featured in the JoM include full papers, hot topic fast-tracked communications and review articles. Authors considering submitting a review article should contact the editorial office first.
Find out more about the Journal of Microscopy and submit your paper!
























The Journal of Microscopy publishes top quality research articles, review articles and Hot Topic papers covering all aspects of microscopy and analysis. This includes cutting-edge technology and innovative applications in physics, chemistry, material and biological sciences. You can read the latest Early View papers online at www.journalofmicroscopy.org
ORIGINAL ARTICLE (Open Access)
Surpassing light inhomogeneities in structured-illumination microscopy with FlexSIM
Emmanuel Soubies, Alejandro Nogueron, Florence Pelletier, Thomas Mangeat, Christophe Leterrier, Michael Unser, Daniel Sage
Super-resolution structured-illumination microscopy (SIM) is a powerful technique that allows one to surpass the diffraction limit by up to a factor two. Yet, its practical use is hampered by its sensitivity to imaging conditions which makes it prone to reconstruction artefacts. In this work, we present FlexSIM, a flexible SIM reconstruction method capable to handle highly challenging data. Specifically, we demonstrate the ability of FlexSIM to deal with the distortion of patterns, the high level of noise encountered in live imaging, as well as out-of-focus fluorescence. Moreover, we show that FlexSIM achieves state-of-the-art performance over a variety of open SIM datasets.
ORIGINAL ARTICLE
Deep learning for quantifying spatial patterning and formation process of early differentiated human-induced pluripotent stem cells with micropattern images
Slo-Li Chu, Kuniya Abe, Hideo Yokota, Dooseon Cho,Yohei Hayashi, Ming-Dar Tsai
Human-induced pluripotent stem cells (hiPSCs) are an ideal resource for patient specific regenerative medicine. Pluripotency of a hiPSC line has to be analysed before us. Differentiated hiPSCs on a micropattern have been used for the analysis on which hiPSCs form segregated, sorted and radially ordered three germ layers. This study uses DNA-stained fluorescence microscopy images for segmentation of the differentiated hiPSCs, together with fluorescence images staining respective ectoderm, mesoderm



and endoderm to classify a segmented hiPSC as any of the three germ layers. However, cells on a DNA-stained fluorescence micropattern image are low-contrast to background and dense that are not easily segmented by image-processing methods. Meanwhile, regions of fluorescent pixels on the germ-layer fluorescence images overlap, although the hiPSCs differentiating on a micropattern expand in a two-dimensional, nonoverlapped way at the early differentiation stage.
We propose a U-net structure with flexible input micropattern sizes and with designed loss functions to identify the dense differentiated hiPSCs to improve segmentation accuracy. We then use an intensity comparison calculation that compares fluorescence intensities of all the corresponding pixels on the germlayer fluorescence images inside a segmented hiPSC to classify the cell into a nonoverlapped region of any germ layer. Then, distribution (spatial pattering) of a germ layer on the micropattern are quantified using numbers, areas and distances to the micropattern centre of cells inside the germ layer. The U-net is also trained by the labelled cells on DNA-stained fluorescence images with their corresponding brightfield images. This enables the hiPSC segmentation on live-cell time-lapse bright-field micropattern images to quantify spatial patterning changes of the germ layers over time or monitoring of their formation process.
Unveiling the limits of precision in iterative MINFLUX
Carlas Smith, Dylan Kalisvaart, Kirti Prakash
In single-molecule microscopy, a big question is how precisely we can estimate the location of a single molecule. Our research shows that by using iterative localisation microscopy and factoring in the prior information, we can boost precision and reduce the number of photons needed. Leveraging the Van Trees inequality aids in determining the optimal precision achievable. Our approach holds promise for wider application in discerning the optimal precision across diverse imaging scenarios, encompassing various illumination strategies, point spread functions and overarching control methodologies.
Quantifying superimposed protein flow dynamics in live cells using spatial filtering and spatiotemporal image correlation spectroscopy
Rodrigo A. Migueles-Ramírez, Alessandra
Cambi, Arnold Hayer, Paul W. Wiseman, Koen van den Dries
Flow or collective movement is a frequently observed phenomenon for many cellular components including the cytoskeletal proteins actin and myosin. To study protein flow in living cells, we and others have previously used spatiotemporal image correlation spectroscopy (STICS) analysis on fluorescence microscopy image time series. Yet, in cells, multiple protein flows often occur simultaneously on different scales resulting in superimposed fluorescence intensity fluctuations that are challenging to separate using STICS. Here, we exploited the characteristic that distinct protein flows often occur at different spatial scales present in the image series to disentangle superimposed protein flow dynamics. We employed a newly developed and an established spatial filtering algorithm to alternatively accentuate or attenuate local image intensity heterogeneity across different spatial scales. Subsequently, we analysed the spatially filtered time series with STICS, allowing the quantification of two distinct superimposed flows within the image time series. As

a proof of principle of our analysis approach, we used simulated fluorescence intensity fluctuations as well as time series of nonmuscle myosin II in endothelial cells and actin-based podosomes in dendritic cells and revealed simultaneously occurring contiguous and noncontiguous flow dynamics in each of these systems.Altogether, this work extends the application of STICS for the quantification of multiple protein flow dynamics in complex biological systems including the actomyosin cytoskeleton.
Retrograde tracing of breast cancerassociated sensory neurons
Svetllana Kallogjerovic, Inés VelázquezQuesada, Rutva Hadap, Bojana Gligorijevic
Breast cancer is an aggressive disease that affects both women and men throughout the world. While it has been reported that the increasing size of nerves in breast cancer correlates to bad prognosis in patients, the role of nerves, especially sensory nerves, in breast cancer progression, has remained largely understudied. Sensory nerves are responsible for delivering signals such as pain, mechanical forces (pressure, tension, stretch, touch) and temperature to the brain. The human body is densely innervated, and nerves extending into peripheral organs can be as long as a few meters. Nerve classification and function can be very complex, as they contain bundles of extensions (axons) originating in different neuronal bodies (soma). Maintaining neurons and growing axons in cell culture conditions in order to mimic innervation is technically challenging, as it involves multiple organs of the human body. Here, we focus on tracing sensory axons from the breast tumours back to the neuronal soma, located in the dorsal root ganglia, inside the spine. To do so, we are using two different ‘retrograde’ tracers, WGA and CTB, which are proteins with a natural ability to enter axons and travel in a retrograde fashion, arriving at the soma, even if it means to travel distances longer than a meter. Both tracers are fluorescently labelled, making them visible using high-resolution fluorescent microscopy. We show that both WGA and CTB can label sensory neurons in tumours, or in cell culture conditions. The two tracers differ in efficiency of tracing different sensory neurons subpopulations: while WGA is more efficient in tracing small C-fibres (CGRP-positive), CTB is more efficient in tracing
A-fibres (NF200+) of sensory neurons. In summary, we have successfully established retrograde tracing techniques for sensory neurons towards studying and targeting breast cancer innervation.
A comparison of fixation and immunofluorescence protocols for successful reproducibility and improved signal in human left ventricle cardiac tissue
Matthew Taper, Glenn Carrington, Michelle Peckham, Sean Lal, Robert D. Hume
Immunohistochemistry (IHC) and immunofluorescence (IF) are crucial techniques for studying cardiac physiology and disease. The accuracy of these techniques is dependent on various aspects of sample preparation and processing. However, standardised protocols for sample preparation of tissues, particularly for fresh-frozen human left ventricle (LV) tissue, have yet to be established and could potentially lead to differences in staining and interpretation. Thus, this study aimed to optimise the reproducibility and quality of IF staining in freshfrozen human LV tissue by systematically investigating crucial aspects of the sample preparation process. To achieve this, we subjected fresh-frozen human LV tissue to different fixation protocols, primary antibody incubation temperatures, antibody penetration reagents, and fluorescent probes. We found that neutral buffered formalin fixation reduced image artefacts and improved antibody specificity compared to both methanol and acetone fixation. Additionally, incubating primary antibodies at 37°C for 3 h improved fluorescence intensity compared to the commonly practised 4°C overnight incubation. Furthermore, we found that DeepLabel, an antibody penetration reagent, and smaller probes, such as fragmented antibodies and Affimers, improved the visualisation depth of cardiac structures. DeepLabel also improved antibody penetration in CUBIC cleared thick LV tissue fragments. Thus, our data underscores the importance of standardised protocols in IF staining and provides various means of improving staining quality. In addition to contributing to cardiac research by providing methodologies for IF, the findings and processes presented herein also establish a framework by which staining of other tissues may be optimised.

Current opinion on the prospect of mapping electronic orbitals in the transmission electron microscope: State of the art, challenges and perspectives
M. Bugnet, S. Löffler, M. Ederer, D. M. Kepaptsoglou, Q. M. Ramasse
The concept of electronic orbitals has enabled the understanding of a wide range of physical and chemical properties of solids through the definition of, for example, chemical bonding between atoms. In the transmission electron microscope, which is one of the most used and powerful analytical tools for high-spatial-resolution analysis of solids, the accessible quantity is the local distribution of electronic states. However, the interpretation of electronic state maps at atomic resolution in terms of electronic orbitals is far from obvious, not always possible, and often remains a major hurdle preventing a better understanding of the properties of the system of interest. In this review, the current state of the art of the experimental aspects for electronic state mapping and its interpretation as electronic orbitals is presented, considering approaches that rely on elastic and inelastic scattering, in real and reciprocal spaces.This work goes beyond resolving spectral variations between adjacent atomic columns, as it aims at providing deeper information about, for example, the spatial or momentum distributions of the states involved.The advantages and disadvantages of existing experimental approaches are discussed, while the challenges to overcome and future perspectives are explored in an effort to establish the current state of knowledge in this field.The aims


of this review are also to foster the interest of the scientific community and to trigger a global effort to further enhance the current analytical capabilities of transmission electron microscopy for chemical bonding and electronic structure analysis.
1. No submissions fees
2. No page or colour charges
3. No page limit
4. Simple online submission
5. Helpful, friendly editorial team
6. Average time from submission to first decision is less than 50 days
7. High readership figures
8. Online tracking system – authors can easily check the status of an article in production and receive emails at key stages
9. Rapid publication with Early View papers published online in advance of print, significantly shortening time from acceptance to publication
10. Free electronic offprints

Journal of Microscopy App Available for iPhone and Android
Search for Journal of Microscopy on the App Store or Google play and access your personal or institutional subscription wherever you are, whenever you want.
Submit online at https://mc.manuscriptcentral.com/jmi
View the Guidelines for Authors and full submission details online at: www.journalofmicroscopy.org

You‘ll believe it when you see it.

Widefield
LiveCodim
A revolution in super-resolution has arrived in the United Kingdom.
Dr Periklis (Laki) Pantazis is Director of the Imperial College London and Leica Microsystems Imaging Hub, a dedicated biomedical imaging hub focusing on complex dynamics in biological systems.
The hub, which he established in 2021, is a strategic collaboration between Imperial College London and Leica Microsystems in the field of optical imaging and its uses in research and innovation. Both partners collaborate to research, develop and promote scientific applications using Leica’s latest advanced microscopy solutions. Laki is also a member of the RMS Life Sciences Section.
Let’s find out more!
How did you first become interested in science?
I was lucky to have an inspiring science teacher in school who constantly challenged us. He emphasised critical thinking, which was a big part of his teaching style. Since he taught chemistry, which was his main subject, I decided to take it. My interest in life sciences led me to pursue a degree in biochemistry. What was your first experience of using microscopy, and how did you come to focus on microscopy as an academic?
During my biochemistry studies and lab rotations, I found myself growing tired of examining protein bands. However, everything changed when I presented a paper on the improved GFP as a student, which truly captivated me. This newfound interest led me to pursue a PhD in a lab where I had the opportunity to work with optical microscopy, producing some of the most vivid and colourful images.


I previously served as an assistant professor at ETH Zurich in Basel, but it didn’t seem like the right match for me. Consequently, I sought an environment that supported basic research with a robust translational/engineering focus. The Department of Bioengineering at Imperial College London turned out to be the ideal fit. It offered wonderful colleagues, exceptional students and the vibrant setting of a great city.
The establishment of the collaborative imaging centre began quite simply. I was driven by a desire to enhance optical imaging workflows, and initial conversations with Leica’s team in the UK eventually led to its creation. It has been a fantastic journey, and I am grateful that the visions of Leica and Imperial College London align so well.
How does the collaboration work in practice?
The ongoing mission of the Imperial and Leica Imaging Hub is to create a microscopy knowledge centre where experts from academia and industry come together to research, develop and advance scientific applications utilising the microscopy technologies and software provided by Leica. This is typically done through workshops, seminars and events such as the imaging symposium which was held at Imperial earlier this year to showcase and demonstrate the available systems within the Hub. As a result of great feedback on this first event, more events are now being planned for the rest of the year, including opportunities for networking between the RMS and researchers within Imperial.
The primary focus of my laboratory is the development of advanced imaging tools and automated instrumentation, which enables us to utilise imaging for hypothesis-driven research and high-throughput analysis. We are particularly dedicated to creating sophisticated imaging
technologies that facilitate an efficient workflow for both acquisition and interpretation. Our efforts are directed towards mechanistic analysis of biological systems in animal models and organoids, as well as the development of novel diagnostic and therapeutic strategies. Some of our notable contributions include primed conversion (a technique for 3D precise and nontoxic labeling), GenEPi (a mechanical force sensor that reports on Piezo1 function), and bioharmonophores (biocompatible and biodegradable nonlinear imaging probes).
Can you describe the imaging capabilities and technologies available at the hub?
At present, the hub is equipped with three Leica systems: a STELLARIS 8 FALCON microscope, a THUNDER imaging system and a Mica. Typically, two of these systems are always available for use. Additionally, the Aivia AI image analysis software is readily accessible for analysing the images experimenters acquire. There is also a dedicated tissue culture area for sample preparation, with support facilities nearby.
These state-of-the-art microscope systems empower researchers to tackle bold research questions and the optical imaging workflows are enhanced through ongoing communication with Leica application specialists.
In addition to the systems, application support and workshops, the faculty also has the opportunity to collaborate with Leica engineers and software developers. This collaboration enhances Imperial’s research capabilities and helps Leica remain at the cutting edge of innovation.
What, currently, is your favourite microscope / application, and why?
We have collaborated with the lab of Christopher Rowlands on a Raman light sheet microscope that combines the techniques of Raman spectroscopy and light sheet fluorescence microscopy. This
integration allows for highly sensitive chemical imaging of biological samples with minimal damage and deep penetration, providing complementary information that can be crucial for comprehensive biological studies that we are currently exploring.
How many staff are based there, and what are their roles?
I serve as the director, while Miguel Angel Hermida Ayala is the manager of the Imaging Hub.Additionally, an application specialist from Leica is available weekly to address technical issues and questions related to imaging workflows.
Who is generally using the hub, and where are they coming from?
Microscope users come from both academic institutions and some industrial sectors. They are primarily based in institutes throughout the greater London area.
What does the imaging hub offer to users that other facilities do not?
Regular feedback from a Leica application specialist helps you fully utilise the capabilities of the systems. This enables you to rapidly acquire high-quality data, crucial for gathering preliminary results for your next grant application or completing the final revision of a manuscript under review.
How is the facility funded and resourced?
Leica provides the systems on an annual loan basis and includes servicing. New users can be trained completely free, and explore the systems free of charge for a short period of time. Additionally, we offer application development grants to users interested in extended access to the systems.
What do you see as the main benefits of being part of an academic / industry partnership such as this - for both parties?
Combining the theoretical knowledge of academia with the practical, application-oriented focus of

industry can lead to the development of innovative solutions and advancements in optical technology. Imperial College London has a proud history of industry-relevant innovations… it was a wise decision for Leica to team up with us!
What does the medium and long-term future hold for the imaging hub?
In April 2024 we celebrated a significant relaunch of the Imaging Hub with an application symposium. Our objective is to cultivate a robust and engaged user community that can contribute to application notes and whitepapers that can be shared with other researchers to demonstrate the potential of the different systems within the facility. Furthermore, I am eager to see the development of software and hardware materialise through collaborations between Imperial College London and Leica.
do you see yourself in 10 years’ time?
In 10 years’ time, I envision myself still deeply involved in advancing the fields of microscopy and diagnostics. I aim to have successfully contributed to a truly smart microscope, transformative imaging probes and theranostic tools. My goal is to extend the impact of my work beyond the basic science to see tangible benefits in human health, emphasising the translational aspects of my research.
What do you like to do in your spare time?
I love going for runs with my wife in Richmond and Bushy Park. We also enjoy exploring museums and sampling the excellent cuisine in London. Additionally, traveling the world with my family is one of our favourite activities.
The Royal Microscopical Society would like to welcome our new members who have joined us in the last three months. We hope they enjoy a long and rewarding membership with the RMS.
Miss Hannah Walpole
Mr Daniel Trifon
Mr Thomas Brown
Dr Ievgeniia Kovalska
Mr Thomas Selby
Miss Natalia Georgiadou
Mr Luca Verger
Miss Gea van de Kerkhof
Kelly Rogers
Mr Lloyd Groom
Dr Purnima Kumar
Mr Ebinesh Starlin
Mr Robbie Pineda
Dr Arti Tyagi
Dr Renfei Feng
Dr Joanna Tomlinson
Mr Declan Murphy
Miss Tamsin Rowcliffe-Lucas
If you know of anyone who might be interested in becoming a member of the Royal Microscopical Society and if you would like us to contact them, please send their details to our Membership Administrator, Debbie Hunt – debbie@rms.org.uk
Application forms are available to download at www.rms.org.uk/membership
Don't forget you can now log into the RMS website and check your membership status, renew and download receipts. If you have never logged into the RMS website, please enter the email address that is linked to your membership and then click 'forgotten password'.
If you have any queries or questions about your membership please contact Debbie Hunt debbie@rms.org.uk

Name Daniel Trifon
Tell Us About You?
I'm an MRes student at King's College London, specialising in Translational Cancer Medicine. My passion lies in bridging research and practical applications in cancer care. Through rigorous exploration of cancer biology, I aim to revolutionise treatment approaches.
Why did you become a member of the RMS?
Name Naveenkumar M
Tell Us About You?
I joined the RMS because microscopy is pivotal in my research in Translational Cancer Medicine. Being a member grants me access to expertise, resources, and opportunities for professional growth in this field, ensuring I stay updated and contribute effectively to scientific advancements.
How do you feel being an RMS member benefits you?
Being an RMS member grants me access to expertise, resources, and networking opportunities in microscopy. It keeps me updated on advancements, refines my skills, and fosters collaborations, enhancing my research in Translational Cancer Medicine.

I’m a passionate Master's student in plant biology who would like to explore different facets of modern science. Why did you become a member of the RMS?
To widen my knowledge and develop a network of scientific minds in this area.
The RMS is delighted to welcome three new corporate members - Abberior Instruments GmbH, Photonic Solutions Ltd and Hamamatsu Photonics. As corporate members, they join a growing community of companies and organisations

Abberior Instruments GmbH was founded as a spin-off of Nobel laureate Prof. Stefan W. Hell’s research group at the Max Planck Institute in Göttingen, Germany. Besides Stefan Hell, all founding members and decision makers are senior scientists and have shaped the field

Photonic Solutions is an independent supplier of photonics and associated technologies to the UK scientific and industrial market. We are the exclusive distributor for some of the leading manufacturers of scientific and industrial laser systems, cutting edge microscopy and imaging systems, together with research grade spectroscopy solutions.
We offer an extensive range of laser for use in microscopy, from 2p and 3p lasers from Light Conversion for multiphoton imaging, to Oxxius’ range of CW laser engines for confocal and super resolution microscopy. If your application focus is

Hamamatsu Photonics is a world-leading manufacturer of opto-electronic components and systems and employs over 3000 staff worldwide. The corporate headquarters are based in Hamamatsu City, Japan along with various manufacturing plants and central research laboratories. Since its inception in 1953, Hamamatsu Photonics has expanded to now enjoy a global presence throughout Asia, Europe, and North America.
demonstrating support for the Society's activities and objectives.
Corporate membership of the RMS also brings a number of benefits and opportunities associated with events, publications and marketing.
of optical super resolution over decades. abberior is a leading innovator, developer and manufacturer of modern superresolution STED and proprietary MINFLUX microscopes, which allow unique applications in cell and molecular biology. Numerous awards, including the TOP100 Innovation Award 2021, the Innovation Award of German Science and the Focus Growth Champion 2019, underline the success.
Website: www.abberior.rocks
CARS and SRS imaging then we have dedicated ps OPOs from APE GmbH that are optimally configured for these measurement modalities.
For those researchers focusing on time-resolved microscopy, we partner with the technology leader in time-correlated single photon counting, Becker & Hickl to offer their complete laser scanning microscopes for fluorescence lifetime imaging (FLIM).These devices offer single molecule sensitivity with picosecond temporal resolution. Within their suite of instruments you will also find upgrade kits for laser scanning microscopes for all major manufacturers that enable time-resolved applications.
Website: photonicsolutions.co.uk/
Hamamatsu Photonics’ corporate philosophy stresses the advancement of Photonics through extensive research and development. Hundreds of new opto-electronic products are introduced to the market each year and many Hamamatsu products are regarded as state-of-the-art. Hamamatsu sources, detectors and imaging products are designed to cover the entire optical spectrum, from nuclear radiation, x-ray, Ultraviolet (UV), Visible and Infrared radiation. Hamamatsu devices provide solutions for a wide variety of applications including analytical, industrial, and medical instrumentation.
Website: www.hamamatsu.eu
Michael R. Gibson, FRMS
As the celebrated marine biologist and writer Rachel Carson once said, in every grain of sand there is the story of the earth. Here in Northampton, part of that story lies within our society’s unique collection of sands from around the world. The Northamptonshire Natural History Society is one of Northampton’s “hidden gems”, founded in 1876 and located in the centre of the town’s cultural quarter, close to the Guildhall, museum and theatres. In addition to over 8,000 sand samples and prepared slides, the society maintains a number of other unique collections including herbaria, minerals, rocks and fossils, moths, butterflies, shells and weather records.
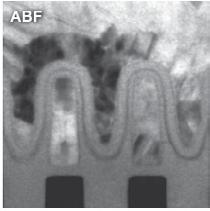
By far the largest collection of sands are those gathered over many years - but mainly during the 1950s - by a former member and society president, Gordon Osborne. A further collection of sands consisting of over a thousand prepared microscope slides by the late Douglas Richardson was donated to the society by him some fifteen years ago now (Figures 1a and 1b). Finally, going back to the late Victorian period, there is a much smaller collection of foraminifera microscope slides (some of which can be seen in Figure 2) collected and prepared by former secretary of the microscopy section, Walter Drawbridge Crick, grandfather of Francis Crick.
Visitors and guests are always welcome to the society’s Humfrey rooms in Northampton by prior arrangement (Figure 3), and can use our

microscopes to study and even photograph many of the more exotic and unusual samples of sand that form part of the collections. Here then, are just a few of the more exotic samples chosen from the Richardson slides together with short descriptive features of note.
The first slide is one of my favourite marine sands, consisting almost entirely of the inorganic remains of microscopic single-celled organisms called foraminifera. Parts of the slide are shown in Figures 4a & 4b, being samples of sand from a remote region of the west coast of Ireland, near Roundstone in Connemara known as “Dog’s Bay”. The actual slide contains a considerable amount of what might loosely be termed “shelly material” made up of bryozoans or “sea mats”, triangular-


shaped sponge spicules, and sea urchin spines as well as many examples of foraminifera - some fragmentary, but still recognisable as such from the shape of their outer shells. When these so-called “tests” are examined with the aid of a stereo

microscope, they can be identified, extracted and arranged into beautiful mounted slide preparations as shown in this lovely example by the late Brian Darnton (Figure 5).
One of the things that make the study of sands


so interesting is that sometimes even samples collected from the same location can have a totally separate origin from one another as illustrated in this next slide also from Dog’s Bay (Figure 6). This type, so-called Lithothamnion calciferum sand , is formed by the production of calcium carbonate inside the cells of red algae of the Corallinacea family. Gradually, blooms of these algae in the sea decompose and leave behind their calcium deposits which form layers of sand on the ocean floor. The exact process involved in the formation of calcium carbonate within the algal cells is not exactly known and somewhat of a mystery.


Our next sample, is also a marine sand, but from Shobata Beach, Sendai, Japan (Figure 7). Like the Dog’s Bay sand, this contains numerous shell debris including foraminiferous tests as well as quartz, and other rock fragments. However, from a purely historical perspective, what makes this sand special is that during World War II the Japanese released several thousand balloons incorporating incendiary devices that were intended to cross the Pacific Ocean at high altitudes carried along on strong air currents with the aim of setting fire to the forests of North America in an effort to hinder the United States in their war effort. However, analysis of the sand ballast found in the balloons narrowed their




origin to five possible areas in Japan, one of them being the island of Sendai. Subsequent bombing by the US Air Force put a stop to the plan and prevented further attacks from taking place.
Another interesting and unusual variety is oolitic sand which forms aquatically from tiny crustacean’s faecal pellets that “seed” through a combined process of crystallisation and accretion, layers of calcium carbonate that result in the production of smooth, rounded egg-shaped individual grains of sand. This particular sample from Key Largo in




Florida (Figures 8 & 9) does indeed look like tiny eggs - hence the name “oolitic” from the Greek meaning “egg stone”.
One last example is a most exotic type of marine sand collected from Platis Gialos Beach, Cephalonia in Greece, and composed almost entirely of coloured, highly polished fragments of marble (shown in Figure 10).


In conclusion, for those readers interested in making their own sand slides, it’s worth mentioning that both glass and cardboard slides can be used to good effect. However, I have found that cardboard slides are a better option for the beginner and ideal especially when working on outreach projects with children or young adults. The Richardson slides are of course exemplary in their detail and preparation, and one should not be put off from trying to make your own! It’s worth noting here that workshops and slide-making sessions for us amateur microscopists are frequently organised by clubs and societies including our own up and down the country from Leeds in the north to the Anglian group in the east, as well as the Quekett Microscopical Club that hosts meetings in and

Dear Readers,
I hope you are well, and that the last few months have been kind to you. If you’re in the Northern Hemisphere like me, perhaps you have managed to enjoy a good summer break; for those in the South, perhaps you are looking forward to later in the year!
For this issue of infocus, I’d like to begin by talking about the important role the RMS has to play in
supporting careers in both the academic and corporate sector – which is very much one of our key priorities as a Society, but one which is perhaps less well known across our membership.

I am acutely aware of the financial problems being faced by many Universities and Institutes in the UK and across the world. From my conversations with colleagues in industry in recent months, I know many are similarly concerned about the challenging economic environment in which they are operating, and of the uncertainty that can bring – including concerns over job security. For those currently feeling the brunt of these effects, I really do hope circumstances improve in the near future.
While national and global economic trends will always have their impact, I’m proud of the work the Society continues to do in support of careers across our membership – covering all aspects of microscopy, imaging and flow cytometry. Initiatives such as our Technical Specialist Job Shadowing programme, mentoring schemes, and of course, the RMS Diploma, do make a real difference for those participating in them. Likewise, many of our training courses, such as the facility management course, and associated events are continuing to help people on their career pathways.
Dr Peter O'Toole.
"The
opportunities provided by our meetings, conferences and courses are also crucial in enabling new connections and potential career development."
The networking opportunities provided by our meetings, conferences and courses are also crucial in enabling new connections and potential career development. With that in mind, it has been an especially busy few months, kicking off with a capacity attendance at elmi2024 in Liverpool. This was a fantastic meeting to be a part of, and it was great to hear the overwhelmingly positive feedback from delegates and exhibitors. Our hugely successful (and similarly sold-out) Electron Microscopy and Light Microscopy Summer Schools shortly followed, and as I write, our Flow Cytometry Course in early September is already at capacity. Our Facility Management Training Course takes place again in October, providing attendees with all the tips and tricks from experienced experts – in short, another great opportunity for career development. All of these events and courses are open to all.
In November we are very much looking forward to the inaugural Frontiers in Physical Imaging Meeting, focusing on in-situ microscopy techniques. This new venture will hopefully replicate the success of the well-established Frontiers in Bioimaging meeting series – the latest instalment of which will also be taking place in November.
Aside from hosting our own events, the Society has been reaching out to the overseas community over the summer, exhibiting at both M&M 2024 in Cleveland, Ohio, and the European Microscopy Congress (emc) 2024 in the Danish capital, Copenhagen. Taking part in these events really brings it home that we are all part of one big community, and it was great to meet so many new people and to strengthen links with other microscopy societies and partner organisations.
On that note, I was proud to learn that our global membership continued to grow in the early part of this year – with more than 100 new members joining from 14 different countries across five different continents. We are keen to see that trend continue – and for the Society to represent a truly diverse membership. Our Science Committees regularly put out an open call for new members, and I would urge any RMS members – whether new or long-standing – to consider applying, and to help shape future RMS activities.
My very best wishes to all, Dr
Peter O’Toole, RMS President.
The RMS was delighted to learn that its Vice President, Professor Grace Burke, has received the 2024 Mishima Award from the American Nuclear Society (ANS)
The award, for “enduring and exceptional contributions to our understanding of irradiationinduced microstructural evolution”, was presented to her during the recent ANS Conference, which took place in Las Vegas from 16 – 19 June.
Grace has made seminal contributions to microscopy and Materials Science throughout her remarkable career, which has included long periods in both industry and academia, in the US and UK.
She served as RMS President from 2019 to 2023, and is the only person to have served as President
for both the RMS and MSA (Microscopy Society of America)
The ANS is committed to advancing, fostering, and promoting the development and application of nuclear sciences and technologies to benefit society.

The RMS enjoyed a booming start to 2024, with 107 new members joining the Society during the first three months.
While the majority of new members are based in the UK, 19 per cent joined from overseas countries – confirming the Society’s status as a truly international organisation.
No fewer than 14 countries across five different continents were represent among our new members. All membership categories – from Students and Early Career members to ‘ordinary’ and ‘Emeritus’ members were also represented among our latest intake.
Around a fifth of new RMS members who joined in the first quarter of 2024, came from overseas countries.
RMS Chief Executive Sali Davis said: “Like many
Societies, the future of the RMS will always depend on its ability to attract new members, and to continue delivering the kind of benefits and opportunities that they want to see.
“It has been really exciting to welcome so many new members so far in 2024, and naturally, we want to ensure that trend continues in the future. The RMS is for the whole of the microscopy, imaging and flow cytometry community, and as a genuinely international society, it is particularly pleasing to welcome new members from right across the globe.
“A thriving Society requires the widest and most diverse possible representation, in order to ensure we can be in tune with the latest trends, challenges and talking points across our international community.”
Find out more about RMS membership and join today!

The RMS was proud to be among the exhibitors at Microscopy and Microanalysis (M&M) 2024, which took place in Cleveland, Ohio, from 28 July to 1 August.
M&M is the premier microscopy event in the United States, organised each year by the Microscopy Society of America (MSA).
This year’s event featured an extensive and dynamic conference programme, spanning advances in instrumentation and techniques development, as well as applications in the analytical, biological, and physical sciences. Meanwhile, many of the world’s leading companies in the field were on hand in the exhibition hall to showcase their latest, cutting-edge instrumentation and accessories.
RMS Chief Executive Sali Davis attended the event along with Honorary Treasurer Rod Shipley and infocus Editor Owen Morton.
Sali said:“This was a great opportunity to strengthen our connections with the microscopy community

on the other side of the Atlantic – and to engage directly with people about everything that we do at the RMS.
“As a truly international society, it’s important to reach out to our overseas audience, and nothing beats the personal interaction of an exhibition stand. It was particularly pleasing to speak to so many students and Early Career attendees about submitting their papers to the Journal of Microscopy – as well as all the benefits of RMS membership.”

She added: “We are very grateful to our partners at the Microscopy Society of America for putting on such a great event and making us so welcome.”
RMS Hon Secretary for Biological Sciences recognised for contributions to biological science
The RMS would like to congratulate Professor Maddy Parsons on her recent election to the Fellowship of the Academy of Medical Sciences.
Maddy, who is currently RMS Honorary Secretary for Biological Science,was among 58 exceptional scientists recognised in 2024 for their remarkable contributions to advancing biomedical and health sciences, delivering benefits for patients and wider society.
Maddy is Professor of Cell Biology and Director of the Nikon Imaging Centre at King's College London. She is internationally recognised for her leadership in multidisciplinary approaches to research, working with physicists, engineers, clinicians, computational scientists and industry to co-develop new ways to image, model, understand and treat complex human disease.
Her discoveries have accelerated understanding of how cells sense their environment and how this leads to diseases such as cancer and inflammatory disorders in both skin and lung. Maddy is also known for her leadership of the BioImagingUK community

and UK EuroBioImaging node, as well as broader strategic roles within UKRI.
She said: “I am deeply honoured by this recognition from the Academy, which I extend to all the wonderful and talented collaborators I have had the privilege of working with during my career. I’m very grateful to the contributions from team members and colleagues who have been instrumental in inspiring and delivering our research.”
The Academy of Medical Sciences is an independent, expert body representing medical science in the UK. Its elected Fellows are among the most influential scientists in the UK and worldwide, drawn from the NHS, academia, industry and public service.
The RMS is delighted to announce the appointment of Noelle Knight as Conference Manager for International Microscopy Congress 21 (IMC21), following the Society’s successful bid to deliver the global microscopy event in 2026.
Noelle, who has a wealth of experience in the events management sector, will be overseeing all aspects of the conference as preparations take shape over the coming two years.

She previously worked as a senior events manager in London, organising large conferences and other events at some of the city’s biggest venues. She also has experience in the fashion industry
– including involvement in both the Caribbean and London Fashion Weeks.
Noelle, who grew up in Jamaica but has now settled in the UK, said: “I’m really happy to be part of the team, and looking forward to all the planning and preparations for IMC21. Hopefully I can bring a new perspective and some fresh ideas for the conference – while also building on its well-established traditions.
“I’m also looking forward to forming some great relationships and working closely with all our partner organisations in the lead up to IMC21 – and also getting to know the wider RMS and microscopy community better.”
The 21st International Microscopy Congress is taking place in Liverpool from 28 August - 05 September 2026

Bookings are now open for the 6th one-day meeting in the Microscopy: Advances, Innovation, Impact series.
All RMS members are invited to attend this FREE event, which incorporates the RMS AGM & Section AGMs.
The meeting will take place in-person on 2nd October at Burlington House, London, and will also be live-streamed via Zoom.
The RMS AGM & Section AGMs will take place at the following times:
11.30 BST - RMS Annual General Meeting
12.40 BST - Early Career Committee, AFM & SPM Section, Flow Cytometry Section
13.00 BST - Data Analysis In Imaging Section, Electron Microscopy Section 14:15 BST – Outreach and Education Committee
The meeting is free to attend for both RMS members and non-members. In-person places are limited, and restricted to RMS members. Virtual attendance is open to all, including non-RMS members.
Alongside the AGMs, a number of scientific talks will be given in the afternoon, including the shortlisted speakers for the 2024 Early Career Award. This will be followed by a drinks reception.
Register for an in-person or virtual ticket and find out more about the meeting programme
Dr Chris Schwalb, COO, Quantum Design Microscopy
Hard-to-reach, sometimes nanosized, sample areas are typically challenging for standard atomic force microscopy (AFM) measurements due to the lack of positioning accuracy using optical microscopes as guidance for the AFM cantilever. Below, we present a novel approach to guide these cantilevers using a truly correlative microscopy platform that seamlessly combines AFM and scanning electron microscopy (SEM). Using the SEM’s high-resolution and wide field of view the AFM cantilever can be positioned with nanometre precision and safely guided to the desired sample area even for challenging threedimensional topologies.We present various examples highlighting the benefits of combined AFM and SEM by analysing diatom structures, measuring side wall roughness of gear wheel structures, as well as studying mechanical properties of individual Vitamin C particles.
Combining different, complementary analytical methods into one instrument is a powerful technique for the contemporaneous acquisition of both structural and functional sample properties. Combined with AFM, SEM can yield new insights for the study of various materials and nanostructures. FusionScope is an easy-to-use and easy-to-learn correlative microscopy platform designed groundup to add the benefits of SEM imaging to a wide range
of AFM and EDS measurement techniques (www. fusionscope.com). Guiding the AFM cantilever with the field of view of SEM is particularly beneficial for the analysis of hard-to-reach sample areas such as Diatoms.
Diatoms are fascinating unicellular organisms that make up a significant amount of the Earth’s biomass. Combining SEM and AFM in a unified

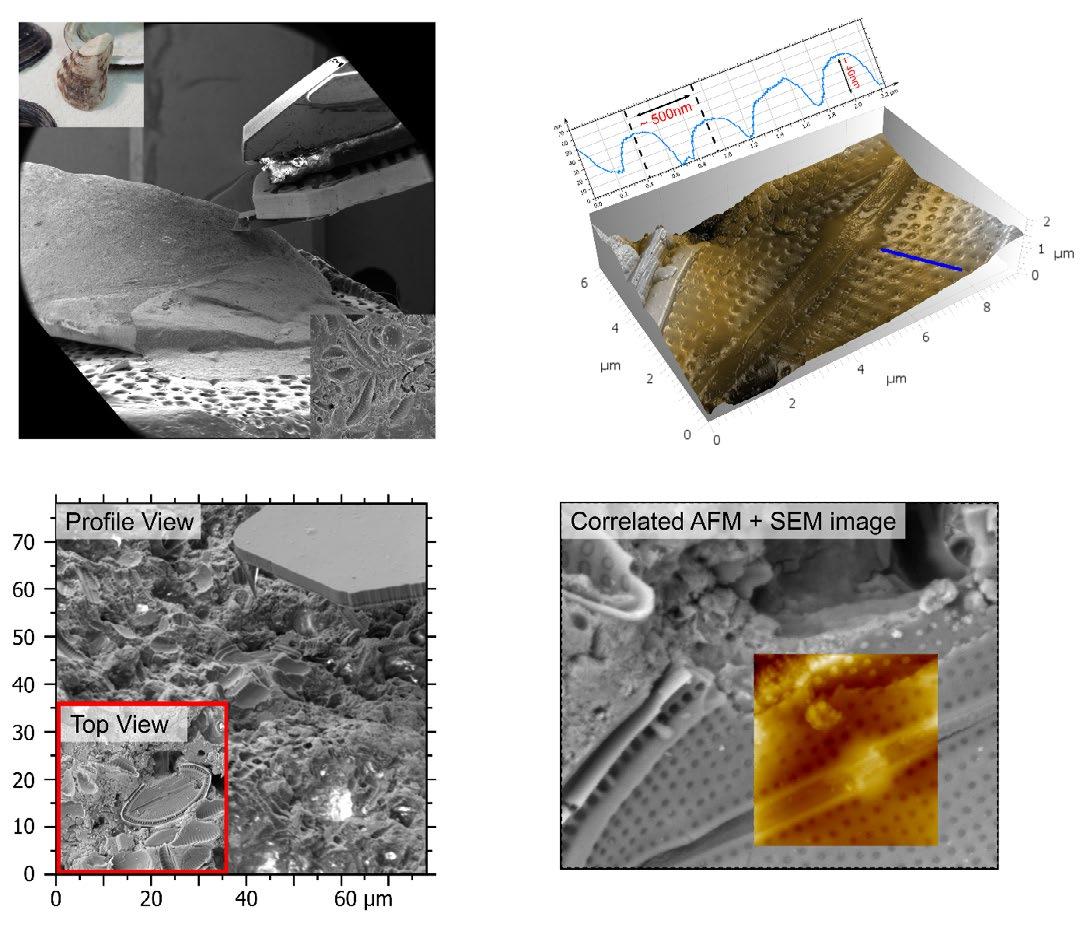
coordinate geometry, allows the diatoms on the surface of a seashell to be easily located, by using the high-resolution SEM to guide the cantilever to an individual diatom and perform a 3D topography analysis of this structure. Above is an example of Dreissena polymorpha, a shell commonly found along the river Rhine. The complete shell can be loaded into the microscope and studied by SEM and AFM.
AFM is ideal for studying 3D metrology with nanometer resolution. However, classical AFM instrumentation can be challenging or even impossible to apply for cases that require cantilever tip navigation to complex sample areas. In Figure 3 (overleaf) we highlight an example for quality control that demonstrates these challenges. Machined gears
produced through techniques such as: casting, sintering, or direct CNC preparation, often undergo stress tests to evaluate performance under variable conditions that align with potential future operating scenarios. It is essential to characterize them before, during, and after these tests without damaging the gears. Traditional AFMs struggle with this task due to difficulties in appropriately positioning the tip at the desired regions. The FusionScope enables SEM identification of relevant areas followed by precise tip approach thanks to its high-resolution and extreme tilt angles. This capability allows for the examination of mechanical properties, such as surface roughness, and features, such as pores and cracks, at different stress levels and locations (e.g., top and flank). It uniquely tracks wear-effects and could aids initial material processing by, for example, detecting unwanted magnetic phases in steel products.

3. AFM approach, analysis, and characterization of intact machined gears. SEM navigation ensures precise placement of the AFM tip on specific locations, enabling detailed investigations of surface characteristics such as roughness, pore and crack analysis on both top and flank parts of the gear. (Data provided by the group of Prof. Harald Plank, FELMI, TU Graz).
Force-distance curves provide insights into the mechanical properties of micro- and nanostructures. However, their interpretation can present considerable challenges especially for small non-flat structures, e.g., particles, fibers, or nanowires, as the cantilever can move or damage the samples during the acquisition. Hence, the ability to observe the cantilever’s force-distance curve generation with high-resolution SEM, allows potential artifacts to be identified easily. As an example, a force-distance measurement on individual Vitamin C particles is shown below. As the cantilever approaches the non-flat particle surface, it first pushes the particle down onto the sample before executing mechanical probing of said particle.This phenomenon culminates in a cantilever bending, as evidenced by the red curve; a distortion that could inadvertently lead to
misinterpretation of the slope as a real mechanical property of the sample. Combining force distance measurements with simultaneous SEM imaging can circumvent these misleading results and provide a comprehensive analysis of mechanical properties of small objects.
Combining AFM and SEM capabilities into a single instrument, in a unified coordinate geometry, provides significant advantages for studying hardto-reach sample areas, prevent misinterpretation of mechanical data, material characterization, or quality control. If you’d like to learn more about the possibilities of combined AFM and SEM for your own research, please contact us at Quantum Design Microscopy or do reach out to your nearest Quantum Design Office and our local team will be happy to help!

Figure 4. SEM images 1 - 4 show the position of the cantilever during the acquisition of a force distance curve on an individual Vitamin C particle. To illustrate the movement of the particle during the force distance curve, horizontal dashed lines were added to the SEM images. The forcedistance curve first exhibits a constant deflection (1)-(2) until the cantilever reaches the particle surface. Then the particle is pushed downward onto the sample (2)-(3) until the real mechanical data can be recorded (3)-(4).

It was with great sadness that we learnt of the passing of Rose Watson on 5/1/2024.

Rose was born in St Catherine, Jamaica in 1944. She came to the UK when she was 15 years old and, on arrival, began attending North Paddington Secondary School in London. On leaving school, Rose’s very first job was in a well-known sausage factory which she disliked immensely and, after seeing how they were made, vowed to never eat sausages again!
Rose’s stint working in a factory was never intended to be a permanent fixture and she began studying histology at night school gaining technician and senior technician qualifications and, after leaving the factory, took employment at The Royal Veterinary College in London. In a passing conversation with me, Rose once mentioned that she hadn’t been sure whether to follow a career in nursing or a laboratory research role. Her decision would become a loss for the nursing world but a huge gain for the EM community.
In the early 1970s Rose joined the Imperial Cancer Research Fund (ICRF) which was later to amalgamate with the Cancer Research Campaign (CRC) in 2002 to form Cancer Research UK (CRUK). On joining ICRF Rose was instrumental in setting up and carrying out electron microscopy with Charles Rowlatt and research pathologist Sammy Franks. Rose later joined the lab of Professor Graham Warren before becoming a member of the EM Facility where she became a senior technician and was eventually promoted to chief technician and co-lead of the EM Facility. In 2006 Lucy Collinson joined CRUK as Head of Electron Microscopy and Rose remained working there with Lucy until her retirement in 2009.
Over the years, Rose helped many PIs, post-docs and PhD students with their research incorporating EM and EM histochemistry and making vital
contributions to many publications over her long career. She worked on a large array of projects including vesicle pathways, cell migration, blood vessels, autophagy, the rab family of GTP-binding proteins, kidney disease, breast, embryonic and ovarian cancer cells and a vast amount of work concentrating on the Golgi apparatus. However, in the later part of her career, Rose probably became best known, admired and respected for her expertise in cryo sectioning and immunolabelling. In the early days, the ultra cryo-microtomes could be found in the department’s main lab alongside the room temperature ultra-microtomes, general prep lab benches, storage and office area. This very full lab was generally a busy hive of activity with no controlled air flow and certainly not the best environment for high precision work but, despite these sometimes challenging conditions, Rose took it all in her stride and consistently produced outstanding results. Sometimes this help would go way beyond her expected role as one colleague, Pat Wilson, remembers:
“When I was rushing to get last minute EM images for a talk she looked after my 2 and 4 year old by


showing them a ribbon of sections cutting on the microtome and preventing them from squirting alcian blue out of a very inviting pipette.”
Rose was taught and trained during an era where skills that have long been forgotten in the lab were part of the teaching. Amongst other things, Rose had been taught how to do glass blowing so, if required, she was able to make her own lab glassware supplies! She had her own unique filing system for grids often consisting of a piece of filter paper in a petri dish divided into sections by nothing but a pencil mark with one carefully selected grid marked simply as “GOOD” or a less impressive “NO.” In anybody else’s hands this could have been disastrous, but it worked for Rose and somehow she always knew where everything was stored.
During the years where I was fortunate enough to work with Rose, I learnt that in this field it isn’t all about the knowledge you learn from formal training or lectures. Just as important are all the tips and tricks that you can pick up along the way, things that aren’t always written down in the text books or captured in publications but instead come from years of experience and practical problem solving in the lab; something that Rose had in abundance. Rose was always generous with her time, always
available to give advice, eternally patient when teaching and willing to pass these skills down to the next generation of electron microscopists.
Rose had a great practicality about her. She was able to look at a problem in the workplace and act quickly to resolve it. One such instance was when Rose was a first aider and, over the tannoy system, a call was made for assistance. A colleague, Ian Summerhayes, had got his tie caught in the automatic photo processor - Rose came to the rescue with a handy pair of scissors! There is some speculation as to whether this quick thinking was to save Ian or the automatic processor!!! Also, she was ever the professional but there could be a fun and mischievous side to her too. There was often much hilarity involved in working with Rose and sometimes, when in meetings or serious conversations, either you would feel a very slight nudge or out of the corner of your eye you would catch Rose, head slightly tilted, almost but not quite smiling giving you a side eye glance with an eyebrow ever so subtly and slightly raised, and a look that could be conveyed as a cynical “really?”
Rose formed long lasting friendships with many colleagues over the years and loved to socialise so it is not surprising that, when talking to past colleagues, many of the anecdotes relate to how sociable Rose was, of the fun that was had outside of the workplace and with her parties being described by many as “legendary.”
Rose was a truly remarkable lady remembered not only for her wealth of knowledge and expertise but also her youthful outlook on life, fun, warmth, openness, generosity, kindness and humour. To quote Terry Pratchett, “No one is finally dead until the ripples they cause in the world die away.” With Rose, for all those who knew her, those ripples will continue for many, many years to come.
Along with the many friends, Rose leaves behind her two children, Andrea and Darren (who are as proud of their mum and all her achievements as she was of them), eight grandchildren and two greatgrandchildren.
By Anne Weston

Steven G Thomas and committee members
Microscopy and biology have always been intimately linked together. One of the driving forces for the development of the very earliest microscopes was our curiosity of the basis of life and our desire to understand it. It was a passion to discover more that drove pioneers such as Robert Hooke and Anton van Leeuwenhoek to use their simple microscopes to observe and describe cells, microscopic organisms, and structures. This curiosity still drives today’s cell biologists to dig deeper into the workings of cells to better understand human health and disease, and the vital role that plants and micro-organisms play in our world.
To support and nurture this link between microscopy and biology, in 1911 the Society established the Biological Section which continued until the 1960s, when the predecessor of the current section, the Histochemical and Cytochemical Section, was formed. Today’s Life Sciences section has evolved from this and it continues to promote microscopical studies of cells including the behaviour of cells and their subcellular components. A key aim is to recognise how the plethora of modern microscopy techniques can be used to expand our understanding of biology.
The current Life Science committee draws together a range of scientists from across bioimaging who work with a broad range of microscopes and techniques. However, they have one thing in common: a passion for biological discovery.We are always looking out for people who share this passion to join the committee - and more details can be found on the RMS website
Below, some of our current committee members introduce themselves to you.

Name: Steve Thomas (Section chair)
Affiliation: University of Birmingham
Area of interest/expertise: My lab focuses on the formation and function of blood platelets and their progenitor cells, the megakaryocyte. I am interested in how the body regulates the production of ~175
billion new platelets every single day and how these cells are produced with such a high level of consistency to ensure that when blood vessels are damaged, your platelets can respond appropriately. More recently we are studying the interactions of these cells when forming blood clots and how different triggers can drive thrombi with different structures. My lab uses a range of microscopy techniques including live cell imaging, super-resolution and light-sheet microscopy to address these challenges.
What I like doing when I’m not doing microscopy: When I’m not in the lab I love being outdoors, camping and hiking in the wild places of the UK. I’m slowly working my way through visiting as many of the Mountains in England,Wales and Scotland as I can, but the list doesn’t seem to be getting any shorter!

Name: Theresa Ward
Affiliation: London School of Hygiene & Tropical Medicine
Area of interest/expertise: I started my PhD at the time of emergence of GFP and capturing organelles in motion is still as potent today. We use different imaging modalities to follow host-pathogen interactions across scales, from molecules to cells to tissues to whole organisms, to better understand disease progression, host response and to investigate therapeutic avenues. Different imaging techniques include confocal microscopy, super-resolution microscopy, correlative microscopy, fluorescence molecular tomography, and whole-organism in vivo imaging. The range of infectious diseases that we
investigate needs to take into account considerations of pathogen containment and how to best enable imaging of the organisms, especially live cell imaging, while ensuring the microscope users are kept safe.
What I like doing when I’m not doing microscopy: good food with good friends is always a winner. And just to sound a bit healthier like my busy colleagues in this feature, a spot of yoga - doing a handstand for the first time in 30 years was a moment of discovery.

Name: Emmanuel Derivery
Affiliation: MRC laboratory of Molecular biology, Cambridge
Area of interest/expertise: My lab focuses on the molecular mechanisms by which cells polarise their cytoskeleton, and this is used to put the right signalling molecules at the right place at the right time. Our approach is highly multidisciplinary, from reconstituted cytoskeleton systems in vitro to highend quantitative imaging of trafficking in vivo during development in flies. To ensure we always have the right tool to address our questions, we also develop novel imaging and bioengineering tools. For instance, we have developed techniques to induce polarity in unpolarised cells using micropatterning and protein design. We also develop novel multispectral imaging modality to dissect the molecular interactome of fast cellular processes.
What I like doing when I’m not doing microscopy: I like to build stuff, in particular small electronic projects I can control with a computer.

Name: Jacquelyn Bond
Affiliation: University of Leeds (UoL) Faculty of Medicine and Health
Area of Interest/ expertise:
Determining how the human brain develops and functions is one of the great scientific questions. I lead the Microcephaly and Neurogenesis Group, which explores the effects of gene mutation on the genes involved in primary autosomal recessive microcephaly (MCPH), a congenital disease of early neuronal development, characterised by reduced brain size and associated intellectual disability. As a cell biologist of >20 years, access to technology platforms that underpin our ability to understand disease processes is paramount. To this end and with the help of colleagues at UoL I have established a high-throughput, high-content library screening process for siRNA, miRNA and small molecule screens. In our investigations into the processes controlling neurogenesis we have relied heavily on confocal, widefield, super resolution, live cell imaging and high content, high-throughput microscopy. Resulting scientific contributions have included: identification of three centrosomal/spindle pole associated disease genes for MCPH including the assembly factor for spindle microtubules (ASPM) gene, mutations in which are the most common cause of MCPH; determining ASPM roles in cytokinesis and spindle orientation processes which are crucial in controlling the ratio of neuronal progenitor cell to neurogenic proliferation in early neurogenesis; identification of ASPM interacting proteins and regulatory proteins and how ASPM is dysregulated in epithelial ovarian cancer and correlates with tumour grade, cancer progression and survival.
What I like doing when I’m not doing microscopy: I spent my pre-university life living in Devon, so I love being by water or surrounded by hills.
I enjoy open water swimming with my friends (but not in winter!), and body boarding/fossil hunting with my teenage sons.

Name: Mark Rigby
Affiliation: Advanced Imaging Manager, Nikon Healthcare UK
Area of interest/ expertise: I have a background in neuroscience research using electrophysiological and microscopy approaches in both London and Kyoto where my research centered around studying changes in voltage in neurons and how these changes impacted neuronal function. A transition to Nikon healthcare meant I provided high end microscopes, as well as application support, to researchers studying a variety of biological questions. This role has greatly expanded my knowledge of different scientific areas. I now relish the opportunity to help researchers make the most of their confocal/multi-photon/TIRF/SMLM experiments and incorporate smart analysis into their acquisition routines.
What I like doing when I’m not doing microscopy: Outside of microscopy I enjoy taking my young children to the trampoline park, and rides out on our bikes. I play squash, football and hockey at a very social level, and like meeting friends in London for runs along the river, and occasionally a beer afterwards.

Name: Alessandro Di Maio
Affiliation: University of Birmingham (Microscopy Facility)
Area of interest/
expertise: My Area of interest is mainly Light Microscopy with applications of LS Confocal, Widefield and Lightsheet microscopy. In my research career I have taken a multidisciplinary approach to science that has allowed me to acquire skills that range from histology to genomic.Those skills have been implemented by a number of microscopy and imaging applications (in vivo and in vitro) resulting in a good level of experience in scientific research. As a facility lead member, my interests widen between experimental design, imaging acquisition and analysis with several components of management and strategy plans.With a Cell Biology background, my main activities currently focus on biological and biomedical applications but with the benefit of working within a multidisciplinary environment, the interests also extend to more specialised microscopy applications within the engineering field.
What I like doing when I’m not doing microscopy: Outside the scientific world I’m an active musician. I’m currently involved in an indiemelodic music project which will soon lead into the publication of new material, and two jazz projects that often lead into live performances.

Name: Periklis (Laki) Pantazis
Affiliation: Imperial College London, Department of Bioengineering
Area of interest/expertise: I enjoy being able to see the processes of life unfold in real time. Much like a painting, sculpture, building or another form of
art, it empowers us, as an audience, to understand and empathise with the subject matter. Developing imaging methods allows us to consume the inner workings of biology consciously and mindfully, from proteins to whole organisms. I am therefore very fortunate to be able to pursue my passion through my work: the introduction of advanced imaging tools and automated instrumentation is the main focus of my laboratory, which enables us to apply imaging for hypothesis-driven research and high-throughput analysis. Specifically, we develop advanced imaging technologies to establish an effective acquisition and interpretation workflow i) for the mechanistic analysis of biological systems in animal models such as mouse and zebrafish and ii) for use in novel diagnostic and therapeutic strategies. Notable contributions include precise conversion of non-toxic labels in 3D, GenEPi (mechanical force sensor reporting Piezo1 function) and bioharmonophores (biocompatible/ biodegradable nonlinear imaging probes).
What I like doing when I’m not doing microscopy: I love to go running with my wife in beautiful Richmond Park and Bushy Park which are close to where we live. Together with our kids we enjoy visiting art exhibitions, events in museums and great food in London as well as around the world.

Name: Morag Rose Hunter
Affiliation: AstraZeneca UK
Area of interest/ expertise: I specialise in designing and running high throughput, high content cell imaging assays. Currently I do this for target identification screens (mostly arrayed CRISPR in 384-well plates) with complex imaging and machine learning / artificial intelligence-based analysis techniques. I was trained as a molecular pharmacologist and used my first
automated microscope during an undergraduate research project. My experiments got larger and more ambitious during my PhD and postdoc years, cementing my love of automation. Nowadays I work on a wide variety of cell biology projects, but my favourites are those related to cellular membrane trafficking and RNA therapeutics. I also support a number of automated confocal microscopes and dozens of active users to ensure that imaging can run 24/7.
What I like doing when I’m not doing microscopy: I enjoy long-distance running, mostly half and full marathons. In the summer I’ll go off-road, but I am not a fan of mud!

Name: Claire Wells
Affiliation: King’s College London (KCL)
Area of interest/ expertise: I lead the Invasion and Metastasis Research Group at KCL which studies how cancer cells are able to dissociate from the primary tumour, invade the surrounding tissue and subsequently metastasise to distal sites. Tissue invasion and migration require cancer cells to reorganise their actin cytoskeleton as well as adhere to and degrade the surrounding extracellular matrix. It is well established that cytoskeletal rearrangement, cell adhesion formation and turnover is regulated by Rho GTPases, Rho, Rac and Cdc42. PAKs are serine/ threonine kinases that operate downstream of Rho GTPases to control cytoskeletal organisation and substratum adhesion. The PAK family can be subdivided into two groups; Group 1 PAKs (1-3) and Group 2 PAKs (4-6) based on sequence homology and members of both groups are activated by growth
factor signalling pathways. We use live cell imaging, biochemical and molecular approaches to investigate the role of PAK family kinases in cancer cell migration, adhesion and invasion. We use both brightfield and fluorescence microscopy to study cancer cells alongside more advanced imaging modalities such as confocal microscopy, super resolution microscopy and light sheet microscopy. My research work is balanced with teaching activities and my role as Faculty Associate Dean for Doctoral studies.
I like doing when I’m not doing microscopy: When I’m not working, I like to attend Zumba dance classes, watch movies and spend time with my friends and family. We enjoy countryside walks and going to music festivals, although I’m not particularly keen on camping.

Name: Stefan Linder
Affiliation: University Medical Center
Hamburg-Eppendorf, Germany
Area of interest/
expertise: I am a cell biologist by training and I am fascinated by observing and
unravelling the inner workings of cells. My group studies molecular mechanisms of cell adhesion, migration, invasion and phagocytosis. Favourite cell type: the primary human macrophage. Favourite organelles: podosomes, the primary adhesion and invasion structures of macrophages, and phagosomes, particularly in the context of Borrelia internalization. Favourite structures: the actin and tubulin cytoskeletons, especially their interaction. Favourite techniques: live cell imaging and super-resolution microscopy, in combination with image analysis (often self-developed). Favourite motto: beautiful images and hard numbers.
What I like doing when I’m not doing microscopy: Running, reading, cooking (mostly not simultaneously).

Name: Ferran Valderrama (Section deputy chair)
Affiliation: St
George’s University of London
Area of interest/ expertise: Our lab is interested in understanding the biology behind the development and progression of cancers of glandular origin, particularly prostate cancer. Over the years we have developed threedimensional (3D) cell culture systems that aim to mimic the glandular structures of the prostate and their modifications at the structural and molecular levels, as they acquire different malignant status.We use a wide range of cell and molecular biology techniques to interrogate our 3D models and visualise the outcomes via different imaging techniques including live bright field, widefield fluorescence, confocal and single plane illumination microscopy. We have identified a set of molecular pathways affecting cell polarity that appear to be key players in determining the progression of prostate cancer ; our final aim is to identify possible targets for therapeutics that may reduce or potentially reverse the progression of the disease.
What I like doing when I’m not doing microscopy: When I’m not working, I like to spend time with my family – I have two boys that enjoy playing football so, my evenings and weekends tend to be invested in travelling across (mainly South) England to bring them to their training or matches. I also like running, so when I’m not taxiing, I like to go for a run!!

Name: Steve Briddon
Affiliation: University of Nottingham
Area of interest/expertise: I am a molecular pharmacologist, with a particular interest in the G protein-coupled receptor (GPCR) family. I ventured into microscopy around 20 years ago as a way of finding out how these cell surface receptors are organised in the membrane, and how their cellular location affects their interaction with signalling molecules, ligands and drugs. Initially this was focused on model systems, but increasingly we have been developing approaches with novel probes and genetics to look at endogenous receptors in cells and primary systems to look at receptor organisation at low levels of expression. To do this, in addition to pharmacological techniques and probe development, we use a range of live cell microscopy and spectroscopy approaches including fluorescence correlation spectroscopy and related techniques and confocal, TIRM and super-resolution microscopy.
What I like doing when I’m not doing microscopy: I’m a keen amateur photographer, so am never happier than when behind a lens (and never less happy than in front of one!). Reading, music, cricket and rugby (sadly now as a spectator) fill any remaining spare time.

Name: Liam Rooney
Affiliation: University of Strathclyde
Area of interest/expertise: My background is in mammalian cell and molecular biology, but since starting my PhD I fell in love with microbeshow they communicate across length scales, how they traverse their environments, and how they colonise their respective niches. However, routine imaging methods weren’t suitable for a lot of the problems I was interested in answering. My main interests lie in the structures formed by dense microbial communities, called biofilms, which pose a significant threat in infection, industrial biofouling, and antimicrobial resistance (AMR). I discovered a system of complex transport channels deep inside these microbial cities during my PhD thanks to the application of the Mesolens to microbiology and, since then, I’ve continued to research these channels and their function alongside my funded research.
My current research aims to develop new manufacturing and characterisation methods for 3D printed lenses, capable of performing similarly to glass lenses, but at a fraction of the price and with open access principles at the forefront. The 3D printed lenses I’ve created have comparable transmissivity, optical performance, and surface profile to their expensive glass counterparts. Moreover, the barrier to entry for 3D printed optics remains low - using lowcost consumer-grade printers and commonly available resources to create high-quality lenses with opensource design specifications.We hope that 3D printed lenses will be combined with other open hardware initiatives to democratise access to high-quality optics
for rapid prototyping and clinical diagnostics in lowresource settings.
What I like doing when I’m not doing microscopy: I find cooking and baking really therapeutic and a great way to tune out. We built a pizza oven in our garden last summer. I’m hoping to refine my skills before the good weather returns, then it’s pizza parties all round! I also love skiing and try to get up to Nevis/Glen Coe/Cairngorm slopes as much as possible. I’m just back from a great trip to the Dolomites in Northern Italy, which was also a great excuse to buy a snazzy 1980s-themed ski suit!

Name: Anjali Kusumbe
Affiliation: University of Oxford
Area of interest/expertise: I investigate vascular-tissue and vascular-immune interactions during aging, regeneration, and metastasis. My research approach is highly multidisciplinary, and we utilise cutting-edge high-resolution tissue imaging techniques, including intravital imaging, and innovate novel methods for imaging. Notably, our pioneering work has advanced clearing and imaging methodologies tailored for large and calcified tissues. Moreover, my laborator y has built extensive tissue maps elucidating vascular and matrix markers across ages, offering an invaluable and freely accessible resource.
What I like doing when I’m not doing microscopy: When I’m not engrossed in microscopy, you’ll find me indulging in the world of movies, cooking, or some much-needed sleep.
Telight is touring universities across the UK, offering researchers and students the chance to experience super-resolution imaging technology firsthand. LiveCodim enhances standard widefield microscopes with super-resolution abilities. This technology allows for detailed and clear imaging of cells with no phototoxicity and photobleaching. Any lab with a standard widefield microscope can be quickly upgraded to a super-resolution imaging platform. The technology is specially tailored to researchers who need to work with samples in their natural environment at high resolution. Their initiative supports scientific research by providing accessible, high-quality microscopy solutions to all the labs, regardless of their size. Researchers and students are encouraged to take advantage of this opportunity organised to showcase the power of gentle imaging in combination with super-resolution technology. Contact Huw Tomas to learn more and see how

this technology can elevate your research. You are welcome to bring your samples for the analysis, just mail us before hand at info@telight.eu
www.telight.eu

You can now find MicroList’s database of resources on FocalPlane. The resources, which have been created by the global imaging community, are presented as cards that are divided into community resources, education resources and tools. The
database is fully searchable using our search bar or can be filtered using a variety of categories. We’ll be adding new resources to the database as we find them and invite the imaging community to continue adding their own resources via FocalPlane. In addition, if there is a resource that you are using for either research or educational purposes, send us an email at focalplane@biologists.com and we’ll add it to the database to help other researchers access the resource or tool. We’d like to thank Jennifer Waters from the Core for Imaging Technology & Education at Harvard Medical School for letting us join the ‘Micro’ team, which now includes Microtutor, the interactive microscopy educational platform hosted by Global BioImaging (https:// microtutor.globalbioimaging.org/).
https://focalplane.biologists.com/ resources/
Here at EM Resolutions we are very proud to be located on Keele University campus. Being part of the Keele University community has created opportunities to grow with, collaborate with, and support other local business and scientists.
We are delighted to have just recruited two graduates from Keele University’s Life Sciences 2024 class. These graduates are settling in and beginning to excel within the EM Resolutions technical team.
This, alongside significant investment in new equipment, better enables us to meet increased demand, ensuring that EM Resolutions continues to scale and maintain our momentum.

This combination of fresh talent and enhanced capabilities enables us to continue to support electron microscopists – lets focus on imaging together.
https://emresolutions.com
Exposing biological samples to the excitation light levels typically used during a fluorescence microscopy experiment can have a detrimental impact on cell health, and consequently data accuracy.
Controlling sample illumination is therefore a crucial factor in minimising photodamage (phototoxicity and photobleaching) caused by fluorescence microscopy – and compared with traditional lamps, CoolLED Illumination Systems include several features to mitigate the risk.

These features include the ability to easily reduce illumination intensity to where the sample is just visible, thanks to
0-100% irradiance control. Moreover, unnecessary sample exposure from shutter latency can be reduced when using high-speed TTL triggering, available on systems such as the 8-channel pE-800 Series.
To help scientists utilise these tools and achieve more accurate and insightful imaging data, CoolLED has a white paper available via www.coolled.com/ photodamage, which explains how:
• Photodamage is not always obvious but can still skew results
• Samples can be easily damaged with mercury or metal halide lamps
• LEDs reduce photodamage via enhanced control and TTL camera synchronisation
• Controlling widefield illumination is also important when setting up confocal experiments
Learn more at www.coolled.com/photodamage
https://www.coolled.com/
The founding father of the Tescan Orsay corporation is leading Telight toward its next success. Jaroslav Klíma has been involved with microscopy technologies practically all his life. He is one of the best-known and most important figures in Brno microscopy. The instruments he has contributed to are used by major institutions around the world, including NASA, Oxford University and car companies such as Porsche.
His passion for microscopy began after graduating from the Faculty of Electrical Engineering at the Technical University in Brno, when he joined Tesla as a service engineer. He later became head of the development department and worked for the company for a total of 19 years.

Jaroslav Klíma has always emphasised organic growth and the quality of his technologies, which is why he received the prestigious Lifetime Achievement Award from the Czechoslovak Microscopy Society in 2019.
We are proud to have such an inspiring founder, whose understanding of the importance of scientific discovery brought so much innovation to the world of microscopy during his tenure.
www.telight.eu
Oxford Instruments has been awarded the King’s Award for Enterprise for Innovation for the Symmetry detector. Symmetry enables a deeper understanding of a material’s structure down to the nanoscale level. Symmetry radically increased the speed with which such in-depth analyses can be performed, resulting in the technique being much more widely adopted.

Symmetry uses a technique called electron backscatter diffraction (EBSD) to analyse surfaces for minuscule weaknesses or flaws in the crystalline structure. This helps understand how they came about and how they can be addressed.
The introduction of Symmetry dramatically increased the speed, sensitivity and resolution of EBSD analysis. Previously, analysing a large surface with EBSD was a painstaking process that could take hours, and a research laboratory had to choose
between high-resolution or rapid results. Now, by adding Symmetry to an electron microscope chamber, analysis is possible in a matter of minutes. This step-change was made possible by a radically new detector design which combined a fibreoptic lens with a CMOS detector. The result was the only instrument to provide high resolution imagery at high speed.
Symmetry has made a niche technology more widely accessible with applications across a variety of sectors and industries – from developing far more robust and long-lasting batteries and semiconductors, to developing stronger aircraft turbine blades. It has even been used to analyse meteorites to help understand how extra-terrestrial rock formed.
“Symmetry was developed to provide our customers with a step change in performance and has enabled them to achieve significant speed and sensitivity gains as they aim to better understand and improve the properties of materials,” said Louise Hughes, EBSD Business Manager. “It was a tremendous team effort, demonstrating innovation at Oxford Instruments at its best, and I am delighted for those efforts to recognised with such a prestigious accolade as the King’s Award for Enterprise.”
https://www.oxinst.com/

HORIBA, renowned industry partner and provider of an extensive array of scientific analysers, is proud to announce their sponsorship of RamanFest 2024. This prestigious event, the 11th International Conference on Advanced Applied Raman Spectroscopy, will take place on 12th- 13th November 2024 in Paris, France.
HORIBA offers a comprehensive portfolio of optical microscopes and analysers for Raman spectroscopy, providing unparalleled chemical imaging information. Moreover, HORIBA also hyphenate their Raman and AFM technology providing not only co-located data but also nanometre resolution Raman imaging with Tip Enhanced Raman Spectroscopy (TERS).
HORIBA’s solutions support a broad spectrum of scientific R&D and QC applications and are at the forefront of research in universities, industries, research institutions, and government agencies across various fields. HORIBA’s instruments can be found within life sciences, pharmaceuticals, materials research, food and agriculture, environmental studies, as well as energy, and water.
RamanFest 2024 promises to be an exceptional event, featuring presentations from world-leading Raman experts and researchers. Attendees will gain insights into the innovative applications of Raman spectroscopy in diverse fields such as life science,
materials science, energy, and environmental analysis. The conference will serve as a global platform for the Raman community to engage and celebrate Raman’s current applications, exploring the pioneering advancements that are set to shape the future.
• Topics at RamanFest 2024 will cover:
• Artificial Intelligence
• Clinics & Health
• Cultural Heritage
• Environment, Recycling & the Circular Economy
• Novel/Renewable Energies
• Raman Methodologies (CARS, SRS, TERS, SERS)
• Regulations
• Semiconductors and Innovative Materials
• Raman and Sports
• Valorisation: From Lab to Business RamanFest offers an unparalleled opportunity to connect, learn, and discuss the latest developments and applications in Raman spectroscopy. For more information about RamanFest 2024, including extended deadlines and early bird registrations, please visit: https://www.ramanfestconf.com/2024/ registration.php#deadlines https://www.horiba.com/
If you would like your Company News to appear on these pages, please contact infocus Magazine at advertising@infocus.org.uk
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
Each year, Super-resolution in the North is a one-day meeting bringing together experts in optical, computational and life sciences across the Midlands and the North of England, Scotland and Wales. On December 8 last year, we welcomed our delegates to York in a crisp, festive atmosphere, at the prestigious premises of the York Medical Society.
[Alex:] It was my privilege to organise and lead the SRITN2023 event, with support from my York Physics of Life colleagues and from the dedicated RMS staff. The best bit was seeing the community cross-section of early career and team research!
SRITN offered free registration and catering for all, at the price of a train ticket, thanks to generous sponsorships from the RMS, IoP, PoLNET, CAIRN and Nikon. In addition to representatives from each of these valued partners, our 45 contributors ranged from academic leaders to core facilities in research institutions from Cardiff to Edinburgh. Following the fine example Sheffield set the previous year, SRITN2023 was an intimate conference with around 50 attendees, which allowed for stimulating conversation in the breaks with a range of microscopists from different walks of life. Thanks to all our contributors!
An impressive range of techniques and applications were on display, presented by a diverse set of speakers at different stages in their career. Delegates were treated to talks about quantitative correlative light-electron microscopy, open-source autofocusing hardware [1], and FRET studies
of nucleic acids and membrane receptors. One contribution - from Kitty Crompton, Newcastleeven sought to explore how astronaut metabolism and health could be influenced by microgravity. Using yeast as a model, her team measured changes in glucose uptake aboard European Space Agency parabolic flights, using a custom-built microscope and automated microfluidics inside a flight case [2].
A particular highlight was the fascinating keynote talk by Séamus Holden. His team presented single molecule tracking in novel microhole cell traps, known as Vertical Cell Imaging by Nanostructure Immobilisation (VerCINI) [3]. They showed how bacterial cells exquisitely remodel their cell walls without rupturing under their own internal pressure!
Sponsor CAIRN presented their adaptable, modular, openFrame microscope [4] and similar interests in autofocus capabilities, and Nikon their NSPARC system [5], which boasts improved signal-to-noise ratio for pixel reassignment-based super-resolution. Another engaging talk came from Ann Wheeler, who outlined the plethora of techniques available at ESRIC, a specialist super-res core facility [6].
[Hannah:] I presented a flash talk entitled “Single Molecule Characterisation of Antimicrobial Peptide Pores in Artificial Bilayers”. Presenting to a room of experts was certainly nerve-racking in the run up, but the talk was well received, and led to interesting discussion during the subsequent pub trip! Most enjoyable for me were the networking and socialisation opportunities, with the last of us staying for a good couple of hours afterwards!
Our next meeting, in December, will again focus
on establishing new super-resolution techniques and correlative approaches, capturing new and early adopters, and broadening new democratised applications of existing super-res techniques to different areas of life and material sciences. We also invite commercial manufacturers and core facilities to establish knowledge exchange, and to explore co-creation of open tools for experiment and analysis.
SRITN2024, organised by Aleks Ponjavic (aleks. ponjavic@leeds.ac.uk) and Michelle Peckham, will take place at the University of Leeds on 6 December 2024 - registration to open soon!
Authors
Alex Payne-Dwyer, PhD. FRMS
Lead Organiser, SRITN2023. Research Fellow at the School of Physics, Engineering and Technology, University of York, alex.payne-dwyer@york.ac.uk
Hannah Baird
Doctoral student, School of Pharmacy and Pharmaceutical Sciences, Cardiff University, BairdHM@cardiff.ac.uk
[1] Rahmani, A. et al. Astigmatism-based active focus stabilisation with universal objective lens compatibility, extended operating range and nanometer precision. Opt. Express 32, 13331-13341 (2024) https://doi.org/10.1364/OE.520845
[2] Wareing, T. et al. Flight-Scope: microscopy with microfluidics in microgravity, BioRxiv (2024) https://doi.org/10.1101/2024.06.12.598447
[3] Whitley, K.D., Middlemiss, S., Jukes, C. et al. Highresolution imaging of bacterial spatial organization with vertical cell imaging by nanostructured immobilization (VerCINI). Nat Protoc 17, 847–869 (2022). https://doi. org/10.1038/s41596-021-00668-1
[4] CAIRN OpenFrame https://cairn-research. co.uk/product/openframe-microscope/
[5] Nikon SPatial ARray Confocal (NSPARC): https:// www.microscope.healthcare.nikon.com/products/ confocal-microscopes/ax/ax-ax-r-with-nsparc
[6] Edinburgh Super-Resolution Imaging Consortium (ESRIC) https://esric.org/

Oxford Instruments Andor has announced the release of Imaris 10.2, the latest version of its market-leading AI-powered image analysis software for microscopic images. In the last decade, Imaris has constantly evolved to improve the visualisation and analysis of large images. Past releases have introduced AI (machine learning) to improve object identification and classification, spatial measurements, correlative data visualisation, and other tools for more precise segmentation and better understanding of microscopy data.
Now, Imaris 10.2 brings significant speed and quality improvements, rendering twice as fast as before, and boosting the productivity of researchers who are interactively visualising the smallest details of their datasets.
To provide a best-in-class experience and support for Mac OS users, this latest release introduces native versions of Imaris, Imaris Viewer (free service), Imaris Stitcher, and Imaris File Converter for ARM-based Mac processors.
Imaris Mac ARM is on average 20% faster compared to its Intel predecessor and is the only commercial microscopy image analysis software equipped with trainable and fast AI segmentation tools natively supporting Apple silicon.
Importantly, Imaris 10.2 improves the widely used Key-Frame Animation tool to ensure more natural rendering flow within 3D datasets of hundreds of gigabytes. The animations in 10.2 enable freeze-time effect for better focus on the structures of interest.
Faster, smarter image analysis software will lead researchers to faster, smarter publications
Anna Paszulewicz, Imaris Product Manager at Oxford Instruments Andor, commented:
“The speed increase in volume rendering is amazing, and it is quite a while since we had such a big jump forward. For me, in mixed scenes I experience nearly double the speed. Faster, smarter image analysis software like Imaris 10.2 will lead researchers across the microscopy world to faster, smarter publications.”
www.oxinst.com
Following our mission to support electron microscopists, we have expanded our range to offer a greater variety of market-leading CVD Graphene coated support films. This welcome addition can be produced in a range of thicknesses from single monolayer sheets to 8 layers, covering the whole TEM grid. These are now available on uncoated 2000 mesh grids, a lacey carbon film, or 2µm Quantifoil grids so there’s a product to suit every need.

Graphene support films are much thinner and more conductive than the average carbon

support film. Although it is a crystalline support film, its contribution to signal formation is relatively low making the single-layer graphene support film especially useful for high resolution imaging, imaging of nanoparticles, and imaging of weak contrast materials and interfaces.
For more information on Graphene support films or to discuss how we can best support you please contact us at info@emresolutions.com.
www.emresolutions.com
CN Tech is delighted to announce its appointment by RMC Boeckeler, the USA-based manufacturer of high-precision ultramicrotomes and other specimen preparation instruments for electron microscopy.
RMC Boeckeler’s PowerTome is a fully modular ultramicrotome, upgradeable from a routine to an advanced instrument. Known for its durability and power-driven cutting stroke, the PowerTome allows you to slice through a wide variety of materials, from softer to extremely hard. This versatility makes it an essential tool for research in many different industries, from life sciences to materials sciences. Its durability and precision make the PowerTome a go-to system for labs across these fields.
unique capability is especially beneficial for 3D reconstruction work, array tomography, and any application requiring high-precision specimen trimming.
The Powertome 3D (PT3D) features a full 1 mm of advance, allowing for uninterrupted, ultrathin sectioning to a depth of one millimetre. This
CN Tech is one of the leading suppliers of electron microscopy (EM) accessories and consumables in the UK and Ireland. Their electron microscopy division offers a growing range of EM preparation instruments and accessories, including SYNTEK diamond knives, plasma cleaners, sputter coaters, and a broad range of EM consumables. Additionally, CN Tech provides passive and active vibration tables/platforms and EMI cancelling systems, ensuring comprehensive support for your electron microscopy needs.
www.cntech.co.uk

CN Tech have been appointed as the official distributor for Syntek diamond knives in the UK and Ireland. CN Tech will have a number of these knives available for trial, making it easier for researchers and industry professionals in these regions to experience their advanced capabilities firsthand.
Syntek diamond knives are indispensable for precise, durable, and versatile cutting in ultramicrotomy, advancing both scientific research and industrial applications.
Advantages of Syntek Diamond Knives for Ultramicrotomy
1. Exceptional Sharpness: Allows for ultra-thin sections (50-100 nanometers) essential for highresolution electron microscopy.
2. High Durability: Made from synthetic diamonds, these knives resist wear and maintain sharpness longer than traditional metal knives, offering cost-effectiveness over time.
3. Consistent Performance: Synthetic diamonds ensure reliable and reproducible cutting, crucial for high-quality, consistent results in research and industry.
4. Versatility: Suitable for various applications including:
• Biological Research: Ideal for TEM and SEM of cells and tissues.
• Materials Science: Effective for cutting metals, polymers, and composites.
• Industrial Use: Enhances quality control and product development in electronics and nanotechnology.
5. Minimal Sample Distortion: Ensures the integrity of specimens, particularly important in biological research.
6. Ease of Maintenance: Proper handling, storage, and cleaning protocols extend their lifespan and effectiveness.
7. High-Quality Edge Geometry: Designed for optimized cutting performance across different sample types.
www.cntech.co.uk
Vision Engineering, the world-leading provider of innovative inspection, metrology, and digital 3D visualisation solutions, is celebrating the 30th anniversary of its best-selling and awardwinning Mantis range of ergonomic optical stereo microscopes.
In 1994, Vision Engineering revolutionised the field of microscopy by introducing the first-ever ‘eyepiece- less’ stereo microscope, Mantis. This innovative instrument was meticulously crafted to bridge the gap between a bench magnifier and a traditional microscope, offering users an unparalleled ergonomic experience.
Its pioneering design not only earned Mantis widespread acclaim but also secured numerous industry-recognised design and innovation awards. As a result, Mantis quickly established itself as the industry standard for high-performance ergonomic stereo magnification, redefining precision and user comfort in industrial microscopy applications within the electronics, medical device, aerospace and automotive industries.
In 2005, Vision Engineering unveiled the second generation of Mantis. Soon after its introduction, Mantis manufacturing moved from Woking to a new manufacturing facility in Connecticut, USA. This
iteration featured notable enhancements, such as a sleek and modern design, improved LED lighting, an expanded field of view, increased magnification capabilities of up to 20x, and stand and accessory options. In 2009, Vision Engineering expanded the Mantis range by introducing the Mantis Elite HD Cam with a built-in camera for image capture.
The third generation Mantis was introduced in June 2023. The newest edition includes improved optics for both hand-to-eye coordination and excellent depth perception, a 3-position turret to house up to 3 objectives ranging from 3x to 15x, an 8x super long working distance lens, and five different illumination options providing flexibility to optimise the lighting to view the perfect image. The Mantis PIXO offers an improved, higher-resolution image and ‘designed-in’ integrated video camera. The system also features an option to switch between white and UV light.
Mantis is designed for precision engineering, electronic engineering, medical device manufacture, and a wide range of other applications that require high-quality images and superior ergonomics and is used in tens of thousands of R&D, manufacturing, and analytical sites worldwide.
https://www.visioneng.com/mantis

European Microscopy Conference 2024, Thermo Fisher Scientific launched the Iliad™ S/TEM and the Apreo™ ChemiSEM™ system
Iliad S/TEM: See beyond the atomic horizon
The new, fully integrated, and advanced Thermo Scientific Iliad (S)TEM features the new Iliad EELS Spectrometer and Energy Filter with the

dedicated Zebra camera as well as the NanoPulser, the new electrostatic beam blanker. The Iliad Spectrometer and Energy Filter stands out with advanced optics and high stability alongside numerous innovations necessary to offer deep integration with the microscope optics and help enable the best experience for EELS data collection for every type of application. Plus, the advanced integration significantly improves the microscope’s serviceability. EELS data collection is extended with easy-to-use Thermo Scientific Velox Software, which makes even the most challenging experiments accessible to every user, including novices. Nearly every component in the Iliad S/TEM can be fully accessed via Python
scripting using Thermo Scientific Autoscript TEM Software, which makes it possible to create new data collection workflows and use AI-based data collection and processing strategies.

Apreo ChemiSEM: High-performance imaging with integrated chemical analysis and structural characterisation
The new Apreo ChemiSEM System is an advanced microscope designed to simplify imaging and provide insights for both expert users and newcomers. Offering integrated workflows and easy access to elemental analyses (EDS) and diffraction (EBSD) results, it supports every aspect of users’ material analysis and delivers consistent and reproducible results, saving time and eliminating daily hassles. thermofisher.com/iliad thermofisher.com/apreo
Linkam Scientific Instruments is showcasing its cryocorrelative portfolio at this year’s M&M conference and will be hosting tutorials and workshops at the event. Linkam will also be presenting two posters during the conference.
Linkam’s CryoGenium plunger system, one of the company’s latest developments, will be shown on the booth.The CryoGenium is a fully automated system which offers a range of features for users of cryoEM, cryo-tomography, single particle tomography (SPT) and cryo-CLEM methods. The novel design replaces the common blotting mechanism to improve process stability and repeatability. Other features of this system include real-time optical monitoring, temperature control over the sample chamber and ethane bath, and control over film thickness and dew point of the sample substrate.
Dr. Michael Schwertner, Senior Research Scientist at Linkam, will be giving poster presentations on the following topics:
CryoGenium – an automated blot-free cryoplunger with optical realtime feedback for singleparticle and cell-based workflows (Poster No. 153; B01.P1)
3:00 PM – 5:00 PM EST, Tuesday, 30th July 2024
Location: Exhibit Hall
Platform for cryo-fluorescence with high stability and improved ease of use (Poster No. PDP-47)
10:00 AM – 12:00 PM EST, Thursday, 1st August 2024
Location: Exhibit Hall
Michael will also lead the workshops on the booth (#1542) at 5:45 PM on Monday 29th – Wednesday 31st July, alongside Linkam’s US distribution partner, McCrone Microscopes and Accessories. Tickets for the workshops will be available on the booth for attendees sign up in advance.
https://www.linkam.co.uk/cryogenium


Oxford Instruments NanoAnalysis has launched Oi View - a cutting-edge digital platform which delivers real-time insights on Oxford Instruments systems to a customer’s phone, tablet, or PC – allowing them to stay informed, wherever they are.
Oi View allows users to stay informed on their system health and utilisation, and on the progress of live analyses. Customers can plan ahead with confidence, making the best use of their time while maximising laboratory productivity. It has been designed and engineered in partnership with digital experts to deliver an easy-to-use, and highly functional website and web app.
“Many facilities invest in Oxford Instruments systems to enhance their analytical capabilities,” explains Iain Anderson, Product Marketing Director for Oxford Instruments NanoAnalysis, “and reliability and efficiency are primary concerns for any facility manager. Through Oi View, they will have access to the health and utilisation of their facility’s Oxford Instruments systems at any time, on any device. Additionally, they can receive periodic reports on this information, to support their own
reports and applications for expansion. This can feed directly into their day-to-day planning, as well as being a key tool as they look to the future.”
Global data insights, delivered to customers wherever they are.
Immediate access means Oi View is great for teams working internationally or remotely, sharing global data insights. And because it works in real-time, with optional notifications, users will know about issues as soon as they happen.
“Their analysis is important to any microscopist,” says Ed Jackson, Oi View Product Manager, “but watching a progress bar to ensure they can intervene if anything goes wrong is not the best use of their time. Oi View gives them confidence to walk away from their machine, knowing they will be informed if anything goes wrong, freeing them up to engage in other activities or to dive into the Oi Library and refresh themself on the best practices or latest applications notes.”
www.nano.oxinst.com/Oiview
Oxford Instruments Andor, a world leader in scientific imaging solutions, announced the launch of two new benchtop microscopes.
Following the successful introduction of the BC43 CF benchtop confocal microscope in 2021, Andor now offers benchtop fluorescence and super resolution models, all have been designed with cost, performance, and accessibility in mind.
The BC43 range will bring instant clarity and depth to research studies, whatever the sample, in ways previously only found in larger, more complex, and more expensive systems. The aim of the portfolio expansion is to make fluorescence, confocal, and super resolution microscopy accessible to a much wider user base across different research areas and experience levels.
More modalities. More performance. More certainty.
The new BC43 WF is a compact, easy-to-use, and affordable widefield microscope, ideal for automated imaging and smaller budgets. This entry-level system enables hassle-free, high-quality fluorescence imaging, and its in-field upgradability allows researchers to extend into confocal and super-resolution microscopy as requirements or budgets increase.
Completing the portfolio is BC43 SR, a microscope that delivers super resolution data quicker and
easier than ever before, resolving samples down to 140 nm.
Crucially, optical performance is backed up by Andor’s new IQ / OQ quality control programme, which ensures that all BC43 microscopes operate within tightly controlled parameters, and provides confidence that data and experiments are reproducible in the future. This has been developed in collaboration with QUAREP-LiMi, an initiative with over 600 members from academia and industry, which is aiming to improve the reproducibility of light microscopy experiments.
The success of BC43 CF, launched in 2021, has inspired the team at Andor to push the boundaries even further by developing two new microscopes that offer maximum performance at minimum cost.
Andor’s Product Manager for Benchtop Microscopes, Dr Geraint Wilde, commented,
“Our benchtop confocal microscope has exceeded our expectations, very quickly being successful in a wide range of research topics such as cancer, neuroscience, development biology, and immunology. This confirms that many researchers are looking for affordable high-quality imaging solutions to add to their regular laboratory.”
https://andor.oxinst.com/
New model delivers up to 33% higher frame rates across all modes and higher full well capacity in Speed Mode
Teledyne Photometrics, a leading manufacturer of scientific CMOS cameras, is pleased to announce a significant update to its popular Kinetix22 CMOS camera. The new and improved Kinetix22 now boasts up to 33% higher frame rates across all modes, along with a higher full well capacity in Speed Mode.
The upgraded Kinetix22 camera is designed to maintain high sensitivity while offering faster speeds, making it an even more powerful tool for imaging. The camera also features a new mode that allows for the collection of more light at high speed, covering the full image output of an optical system.
Major Improvements Include:
• Increased acquisition speeds across all imaging modes at the full field of view:
• Sensitivity: Improved from 88 to 118 fps
• Speed: Enhanced from 498 to 664 fps (requires additional PCIe cable, 554 fps otherwise)

• High Dynamic Range: Increased from 83 to 111 fps
• Sub Electron Mode: Boosted from 5.2 to 6.9 fps
• Higher full well capacity (from 200 eto 1000 e-) in Speed Mode, accessible via a second gain state and suitable when imaging high light levels.
Key Enhancements of the Kinetix22:
• Fast measurement of dynamic samples anywhere in the FOV of your microscope with frame-to-frame intervals of 1.5 ms
• High dynamic range imaging at greater than 110 fps for samples requiring such measurements
With these updates, the Kinetix22 enables imaging of the most dynamic samples, allowing users to discover what they’ve been missing.
All new Kinetix22 orders will include these major improvements. Existing Kinetix22 customers are encouraged to email photometrics.info@teledyne. com or visit the website for more information.
www.teledyne.com
If you would like your new product information to appear on these pages, contact infocus Magazine at advertising@infocus.org.uk
The announcements in this Section are compiled by the manufacturers. They in no way represent a recommendation by the Royal Microscopical Society for any particular instrument or equipment. The Royal Microscopical Society does not endorse, support, recommend or verify the information provided on these pages.
The latest annual gathering of the International Society for the Advancement of Cytometry (ISAC) took place at the Edinburgh International Convention Centre in Scotland. About 2,000 attendees, including students, academics, and exhibitors, participated in the event. True to tradition, the Congress provided a forum to explore the newest developments in flow cytometric hardware, software, and applications. The presentations and posters featured at the event upheld the exceptional quality seen in past years, demonstrating the forefront of research in the field. The meeting is always busy with plenaries, commercial and scientific workshops and parallel sessions which involve tight diary control - and even then, it is not possible to see everything!
First-timers to CYTO 2024 were invited to a morning breakfast where we were introduced to all the emerging leaders, and we were encouraged to engage and network with the Flow Cytometry Community. As a first-timer myself, this was incredibly useful and added extra motivation for me to really make the most of the next five days.
The first day consisted of three scientific tutorial segments, of which you could attend five bespoke sessions ranging from ‘how to think like an engineer’ and mastering cell sorting, to biosafety in Flow Cytometry and data management. The first day of the Congress finished with two social meetings. The first was for SRL (Shared Resource Laboratories)

staff members and was a great opportunity to network with different facilities and talk about shared issues. The open-ticketed President’s Reception was also held on the first day of the Conference and this provided funds for ISACs three Civic Missions –Instruments for Science, Live Education and CYTO Youth. It also gave the opportunity for delegates to meet ISACs senior leadership, Councillors and Committee members and input into the future of the Society.
The Innovation session has been a highlight of recent CYTO meetings. Here, three companies pitched their wares in a Dragon’s Den-style session to a panel of cytometry experts. This year we heard from the finalists who were CytoRecovery, ImageCyte and TerraFlow. It is inspiring to see new approaches to old problems and see technology or data analysis innovations that will benefit us all in the future.
My personal favourite aspect of the conference is the mingling between the companies in the exhibition hall. The two halls were filled with both familiar and new companies, showcasing their new technologies, reagents and software. Attending the exhibition hall is an amazing opportunity to pick the brains of the companies who assist us with our science!
One of the plenary sessions that left a lasting impression on me was delivered by Misty Jenkins. She shared insights into her journey as a woman in STEM and discussed her groundbreaking research on chimeric antigen receptor T cells for treating brain cancer. Jenkins and her team employ state-ofthe-art two-photon microscopy alongside mouse models of brain cancer to delve into the intricacies of the tumour microenvironment and unveil the distinct biology underlying brain tumours. This year’s Hooke lecture was given by Professor Sir Stephen P. Jackson, PhD from the Cancer Research UK Cambridge Institute. He spoke about the Cellular Responses to DNA Damage: Mechanistic Insights and Therapeutic Applications.
The final day also included a session on ‘The Flow you don’t know’, which highlighted the various areas in which you may not expect to encounter flow cytometry. We heard from Hannah Murray from the Scottish Whiskey Research Institute on how they use flow to monitor yeast health; from
James Lambert of the Jet Propulsion Laboratory in California on how flow is used in the search for extraterrestrial life; and from Giuseppe D’Onofrio of the Catholic University of Rome, Italy, who spoke of the way flow cytometry is used to detect blood doping in elite athletes. All were fascinating subjects, showing the power and breadth of flow cytometric applications.
The city of Edinburgh, known for its rich history, literacy and cultural heritage, was also home to the evening CYTO social events. Evening events were dotted all over the city and it was a perfect opportunity to see its stunning architecture with new friends made that day. Looking ahead, excitement builds as the next conference is set to take place in the dynamic city of Denver!
Thanks to CYTO Chairs Rachael Walker (Babraham Institute, Cambridge, UK) and Bill Telford (NIH, USA) and the Organising Committee for delivering a full and impactful programme.
Christopher Chiu, Flow Cytometry Facility Manager, The London School of Hygiene and Tropical Medicine, London, UK
Derek Davies, Cytometry Consultant, Surrey, UK
The authors both acknowledge support from flowcytometryUK to be able to attend the meeting.

infocus is the Royal Microscopical Society’s (RMS) vibrant and striking quarterly magazine for members. It provides a common forum for scientists & technologists who use any form of microscope, including all branches of microscopy. Published four times a year, infocus is free to members of the RMS. infocus features articles on microscopy related topics, techniques and developments, an events calendar, news, event reports, book reviews, new product information, and much more. infocus welcomes submissions of:
Articles - Full articles or reviews of general interest to microscopists, of approximately 30004000 words (excluding references), with images/ figures (as many as appropriate, 4-8 as a guide). Longer articles can also be considered.
Short Articles - Short topical articles, review articles or articles providing hands-on help for microscopy methods.
Primer Articles - Short general articles that are focussed on specific techniques.
Debuts - Student articles publishing emerging results from a project. Results may still be incomplete, but areas of progress/problems should be highlighted, with the aim of provoking feedback.
Book Reviews – if you are a member of the RMS and are interested in writing book reviews for infocus, please contact Owen Morton owen@rms.org.uk.
Please see recent issues of infocus for examples of articles and reviews. To request a sample copy of infocus contact owen@rms.org.uk
If you are interested in submitting to infocus, contact: editor@infocus.org.ukj
• Text should be in a standard font (e.g. Times New Roman or Arial) at a size of 12 pt.
• Articles should begin with a brief summary, which accurately summarises the content and is intelligible without reference to the text.
• Footnotes and appendices should not be used unless absolutely necessary.
• The hierarchy of headings within the text should be clear.
• Spelling should conform with The Concise Oxford Dictionary and SI units must be used.
• Abbreviations should be used sparingly and only if a lengthy name/expression is repeated throughout the article. When used, the abbreviated name or expression should be cited in full at first usage, followed by the abbreviation in parentheses.
• Authors should provide a photograph, brief biography as well as contact information that will be published.
References in the text should be in the form Joy (2000) or Joy & Williams (2000). For three or more authors, use the form Echlin et al. (2000). The reference list should:
• be listed in alphabetical order of first authors’ surnames.
• (where a journal is cited) - include authors’ surnames and initials, date of publication, title of paper, name of journal, volume number, and first and last page numbers.
• (where a book is cited) - include authors’ surnames and initials, title of book, year of publication, edition, followed by publisher and town, county/state (and country if necessary) of publication.
• (where a URL is cited) – include authors’ surnames and initials, year of publication, title of page, URL and date accessed.
• Figures can be one column/half page width, 65.5 mm or two column/full page width, 135 mm.
• Larger images may fill the page/spread. A full page of the magazine is 170 x 250 mm, a double page is 340 x 250 mm.
• Text in figures (labels, axis labels, legends, etc) should be Helvetica or Arial, 8pt size.
• Figure and table captions should be listed numerically at the end of the article text
• Line weights and line strokes should have a maximum value of 1 and a minimum of 0.25.
• For graphs and plots, whenever possible, please submit vectorized images.
• As much as possible, please avoid white spaces.
• All images must be high resolution – 300dpi or more.
• Submission files should be in CMYK format and can be supplied as tiff, jpeg or eps files.
• Images MUST include scale bars or field widths where relevant.
Double page of magazine, 340 x 250mm (Trim size)
• Total number of images/figures/tables should not exceed 15 including tables.
Prior to publication, authors will be sent a PDF of the article by email for approval.
Authors should ensure articles are thoroughly checked before submission – proof amendments should be limited to minor corrections only.
Five hard copies of the issue in which the article is published will be sent to the author, together with an emailed PDF of the article.
Authors are requested to assign copyright to the RMS. However, authors may make copies of their own articles without seeking permission from the RMS, provided that such copies are for free distribution only (they must not be sold) and provided that infocus is properly acknowledged (issue number, month and page number should be given). Permission to reproduce material from infocus in other publications will not be given to third parties except with the consent of the authors concerned.
Authors are responsible for obtaining permission to reproduce copyright material from other sources. Approval for reproduction/modification of any material (including figures and tables) published elsewhere should be obtained by the authors before submission of the manuscript and the source of the material should be properly acknowledged. Authors are responsible for any copyright fee involved.
One column/half page width, 65.5mm
Authors are requested to complete and submit a signed copy of our copyright sign-off form. This is available on the RMS website (www.infocus.org.uk).




