




Arthropen Vet® 250


Alljoints®
Arthropen Vet® (Horse and Dog)
Zoldronic Acid™ * Equinate™ IA & Equinate™ IV Matrix 6000® IV *Available under ACVM import permit in NZ *Not available in Aus/NZ


First and foremost, I would like to thank you, our valued partners in business, for your continued support over the past year. It was another busy year for us all, highlighted for us by the opportunities we had to meet with you face-to-face after the challenging period we lived through linked to the Global Pandemic.
One major challenge for most of us that has emerged recently is inflation, and we have worked hard to maintain prices wherever possible, in many cases absorbing significant increases in raw material, manufacturing packaging and transport costs. Most of
our products (around two-thirds) had no change to their price in the last 12 months.
We continue to work hard to deliver new products to support equine veterinary practice. As usual the past year has been no exception, and we have again introduced several new products to the market including Tri-Sulfox Injection (sulfadoxine + trimethoprim), Lethaton Euthanasia Solution, and Tri-Sil Powder (sulfadimidine + trimethoprim). Our latest product Oxytetracycline Injection 100mL is hitting the market place as I write this, with the 250mL presentation due soon.
Randlab remains committed to veterinary education and has continued its role as the major sponsor of equine veterinary education in Australia, New Zealand and a major sponsor globally.
We are excited at what lays ahead for Randlab and our customers for the remainder of 2023 and into 2024, with an exciting range of new initiatives and products to come.
Finally, I would like to again thank you for your continued support and wish you, your families, and businesses all the success in the world as we navigate these uncertain times together. Stay safe and well, Angelo
The largest supporter of equine veterinary eduction in Australasia and a major sponsor globally.










Subscribe to our Newsletter by sending an email to randlab@randlab.com.au

















The information contained in this catalogue is believed to be correct at the time of publication. However, veterinarians should rely on their own research prior to administering or prescribing any of the products.
Check out Randlab’s extensive gastric ulcer and gastroscopy resources online:
All files are available for free downloading.
Cardiac Opening (entrance to the stomach)
Oesophagus
Proximal Duodenum

Plicatus
Greater curvature of the stomach
Body of the stomach

Equine Squamous Gastric Disease (ESGD):
The horse is a continuous secretor of gastric acid, with the parietal cells of the glandular gastric mucosa producing an astonishing 40L of gastric acid per day. The glandular part of the stomach is protected from acid damage by an integrated array of defences including a thick protective mucus layer and local secretion of sodium bicarbonate which forms a buffer zone immediately adjacent to the epithelium. The upper squamous portion of the stomach has no such innate defences.
In nature horses are known to graze upwards of 18 hours a day. This constant intake of fibre forms a fibre slurry or fibre ball in the stomach that not only absorbs any free gastric acid, but also forms a physical barrier to prevent gastric acid splashing.
With intermittent feeding associated with domestication and routine horse husbandry practices, the stomach may remain relatively empty for much of the day. This provides the potential for free gastric acid to “splash” on the unprotected squamous mucosa. This is exacerbated by exercise and transport with some acid splash occurring even at the walk.
Over time, this increased acid exposure leads to accumulated acid damage resulting in true gastric ulceration of the squamous mucosa (ESGD).




Pylorus (exit from the stomach)
Pyloric Antrum
Lesser curvature of the stomach
The lower region of the stomach, know as the glandular region, is permanently exposed to gastric acid and has innate protective mechanisms which protect against acid damage. Although the exact pathogenesis of gastric glandular/pyloric disease is not known, it is likely multifactorial with an underlying inflammatory component. Stress is also believed to play an important role. Lesions are often proliferative and true ulceration rarely occurs. Acid suppression remains an important part of management of EGGD. The current consensus recommendation for treatment of EGGD is high dose omeprazole plus sucralfate (4-20mg/kg bid-qid) for a minimum of 8 weeks.





















Never outperformed in 4 university trials on 3 continents (Australia, USA & Europe)
APVMA Approval No. 81799 (Australia) | ACVM No. A010916 (New Zealand)
Available in:
The “workhorse” of ulcer treatment.
Increased absorption compared to competitors in 4 x University trials means better bioavailability.
Active Constituent
Omeprazole 370 mg/g in an acid protective paste
Pack Size
Pack of 6 x 33g multidose syringes*
Pail of 50 x 33g multidose syringes
* One box of Ulcershield is enough to treat one 600kg horse for 30 days.
For accurate diagnosis of gastric ulcers and to differentiate ESGD from EGGD, direct endoscopic examination of the gastric mucosa (gastroscopy) is recommended.
Indications
For the treatment and prevention of gastric ulcers in the horse.
Clinical signs of gastric ulceration include:

Always administer Ulcershield on a relatively empty stomach. Administer a minimum of 20 min and preferably > 60 min prior to feeding.



• poor body condition
• poor performance
• depressed appetite
• reluctance to train
• poor hair coat
• girth pain
• skin sensitivity
• chronic diarrhoea
• behavioural changes
• recurrent low grade colic
• intermittent loose faeces
• crib biting / windsucking
Equine Glandular (Pyloric) Gastric Disease [EGGD] is a specific type of gastric disease that affects approximately 40% of racing and sports horses. The disease is characterised by inflammatory lesions, which are most commonly found in the pyloric region of the stomach.
The treatment of EGGD is problematic. Current recommendations include the use of enhanced gastric acid suppression due to the more acidic nature of the gastric fluid found in the pyloric region of the stomach. This is commonly used in combination with a gastroprotectant such as sucralfate. Longer term treatment (> 8 weeks) with the combination is generally required for EGGD.
Pharmacology
Omeprazole is a proton-pump inhibitor. Omeprazole suppresses gastric acid by specific inhibition of the H+/K+/ ATPase enzyme system at the secretory surface of the gastric parietal cell. As this is the last step in the secretory pathway, omeprazole will block gastric acid secretion irrespective of the stimulus. Maximum acid suppression occurs after 3-5 days of treatment.
As omeprazole is subject to degradation by gastric acid, the omeprazole in Ulcershield is protected using a unique, patented formulation. Omeprazole needs to pass through the stomach to the small intestine, where it is absorbed. The presence of significant amounts of food in the stomach, will reduce the amount of omeprazole absorbed.
Ulcershield should always be administered on a relatively empty stomach. Do not feed for 20, and preferably 60, minutes post Ulcershield administration.
Ulcershield has a wide safety margin in horses and foals and can be used in foals from one month old.
Ulcershield can be safely administered to stallions used for breeding. Safety in pregnant or lactating mares has not been established. The safety of Ulcershield for longer than 91 days treatment has not been determined in clinical studies. Complications of long-term administration of omeprazole have not been reported in the horse.
Dosage
Treatment Dose: 4 mg/kg. Adult horses (up to 600 kg) give 6 mL daily for 28 days.
Prevention/Maintenance Dose: 2 mg/kg*.
Adult horses (up to 600 kg) give 3.0 mL daily for 28 days.
*The maintenance dose may be insufficient to prevent the recurrence of ulceration in horses subjected to increased stress such as intense training, heavy competition schedule, transport, etc. In such cases, ongoing treatment with the full treatment dose may be necessary.
Withdrawal: It is recommended that horses be weaned off omeprazole over a minimum period of two weeks, by halving the dose weekly.
Check out Randlab’s extensive gastric ulcer and gastroscopy resources online: All files are available for free downloading.

EVER WONDERED...
APVMA Approval No. 82722 (Australia) | ACVM No. A010775 (New Zealand)
Available in:
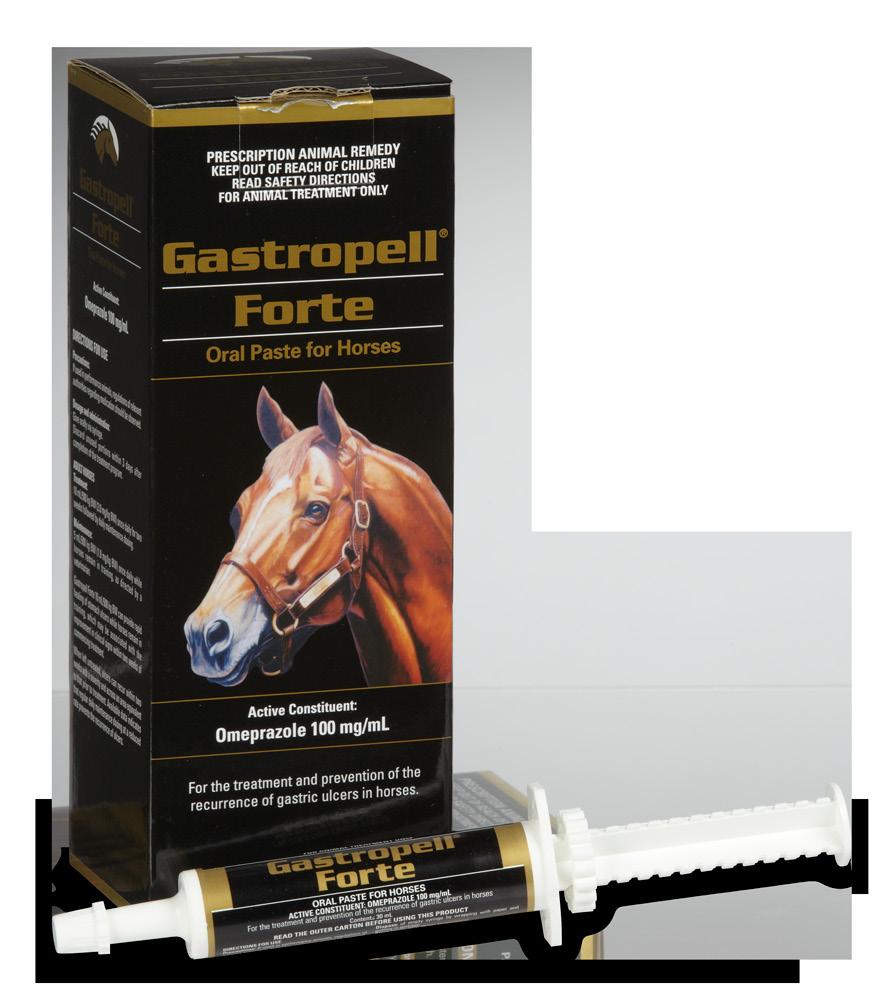
Fortified, premium product for high performance horses, chronic recalcitrant ulcers or wherever a rapid response is required.
Active Constituent
Enteric-coated Omeprazole 100 mg/mL
Pack Size
5 x 30mL multidose syringes*
* A box of Gastropell Forte is enough medication to treat a 500kg horse for 15 days.
For accurate diagnosis of gastric ulcers and to differentiate ESGD from EGGD, direct endoscopic examination of the gastric mucosa (gastroscopy) is recommended.
Gastropell Forte has been shown to be effective in high performance horses (specific APVMA registration). It is also the product of choice in chronic, recurrent or severe gastric ulcers or whenever rapid resolution of gastric ulceration is required.
Clinical signs of gastric ulceration include:
• poor body condition
• poor performance
• depressed appetite
• reluctant to train
• poor hair coat
• girth pain
• skin sensitivity
• chronic diarrhoea
• behavioural changes
• recurrent low grade colic
• intermittent loose faeces
• crib biting / windsucking
Equine Glandular (Pyloric) Gastric Disease [EGGD] is a specific type of gastric disease that affects approximately 40% of racing and sports horses. The disease is characterised by inflammatory lesions, which are most commonly found in the pyloric region of the stomach.
The treatment of EGGD is problematic. Current recommendations involve the use of enhanced gastric acid suppression (such as Gastropell Forte) due to the more acidic nature of the gastric fluid found in the pyloric region of the stomach. This is commonly used in combination with a gastroprotectant such as sucralfate. Longer term treatment (8-12 weeks) with the combination is generally required for EGGD.
Gastropell Forte is the only registered double-strength omeprazole product on the market. Omeprazole is a dose-dependent inhibitor of gastric acid secretion, so double the strength means double the action. Clinical trial data indicates that omeprazole will inhibit gastric acid secretion to maintain gastric acidity at a nonaggressive level, allowing existing ulcers to heal within 2-4 weeks.
For the performance horse given two or three feeds a day, the stomach is left for long periods without ingestion of food and without the mat of ingesta that helps to contain and absorb the acidic fluid. This exposure to acid is then increased during intense exercise when the stomach volume is reduced to less than half that of the resting horse and the gastric acid is forced up into the squamous region where it damages the squamous stomach lining.
The stomach lining is in a continual cycle of erosion and repair depending on the acidity of the stomach fluid and the horse’s feeding and exercise regimen. Even though it is dosed orally, Gastropell Forte does not act directly on the gastric fluid. The drug passes through the stomach to the small intestine, where it is
absorbed and travels via the bloodstream to the acid secreting parietal cells of the stomach.
The powerful antisecretory effect of Gastropell Forte causes a dramatic reduction in the acidity of stomach fluid within 2-3 days of commencing treatment, and because the gastric fluid is no longer corrosive to the gastric lining, ulcers then heal spontaneously within 2-4 weeks. Continuation with daily dosing at a reduced dose rate then prevents ulcers from recurring.
Gastropell Forte can provide rapid healing of stomach ulcers. This is likely to be associated with improvement/resolution of clinical signs within two weeks of commencing treatment.
When left untreated, ulcers can recur within two weeks with a severity and across an area equivalent to that prior to treatment. Available data indicates that regular daily maintenance dosing at a reduced rate prevents the recurrence of ulcers.
Always administer Gastropell Forte on a relatively empty stomach. Administer a minimum of 20 min and preferably > 60 min prior to feeding.
The enteric coated omeprazole beads in Gastropell Forte may be damaged by chewing. Always administer at the back of the mouth over the base of the tongue. Avoid chewing.
Treatment Dose (ESGD): 2.0 mg/kg BW (10ml/500 Kg BW) once daily for 2 to 4 weeks followed by daily maintenance dosing.
Treatment Dose (EGGD): 2.0 mg/kg BW (10ml/500 Kg BW) once daily for 6 to 8+ weeks.
Prevention/Maintenance Dose: 5 mL/500 kg BW (1.0 mg/kg BW) once daily whilst horse remains in training.
Withdrawal: It is recommended that horses be weaned off omeprazole over a minimum period of two weeks, by halving the dose weekly.
Horse Hack: The treatment of Equine Glandular (Pyloric) Gastric Disease remains problematic. Current recommendations include the use of high dose omeprazole combined with sucralfate (20mg/kg bid-qid) for a minimum of 8 weeks treatment. Other medications such as misoprostol (5μg/kg PO bid) have also been recommended. Husbandry changes are also an essential part of EGGD management (see middle spread of this catalogue). Horses should undergo a follow up gastroscopy at the end of the prescribed treatment period.
APVMA Approval No. 62558 (Australia) | ACVM No. A010165 (New Zealand)
Available in:
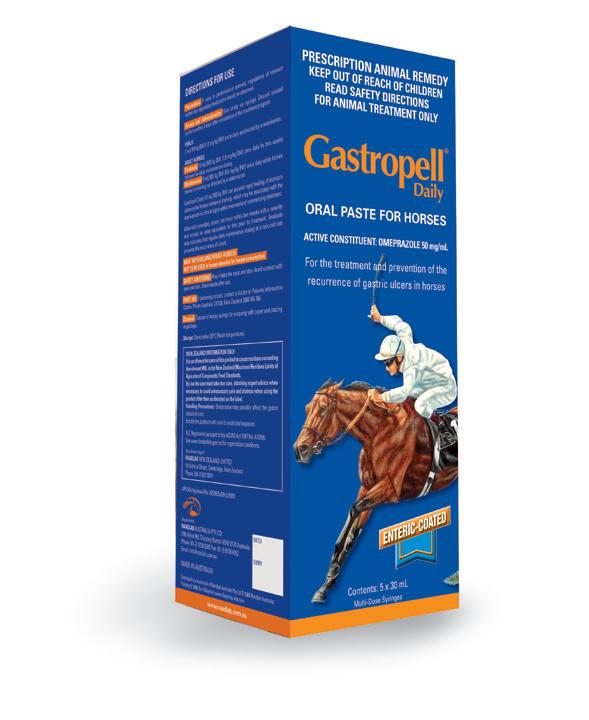
Entry level treatment for EGUS.
Ideal for horses in light work, horses under low stress scenarios or low body weight dosing (foals and ponies).
Active Constituent
Enteric-coated Omeprazole 50 mg/mL
Pack Size
Pack of 5 x 30mL multidose syringes*
Pail of 50 x 30mL multidose syringes

* A box of Gastropell Daily is enough medication to treat a 500kg horse for 15 days.
For accurate diagnosis of gastric ulcers and to differentiate ESGD from EGGD, direct endoscopic examination of the gastric mucosa (gastroscopy) is recommended.
Indications
Gastropell Daily is the product of choice for the treatment and prevention of low grade gastric ulceration. Ideal for use in horses in light work or under low stress scenarios and for dosing low body weight horses such as foals and ponies.
Clinical signs of gastric disease include:
• poor body condition
• poor performance
• depressed appetite
• reluctant to train
Signs in foals include:
• depressed appetite
• dribbling saliva
• sternal recumbency
Pharmacology
• poor hair coat
• girth pain
• skin sensitivity
• chronic diarrhoea
• inappetence
• colic
• dog sitting
• behavioural changes
• recurrent low grade colic
• intermittent loose faeces
• crib biting/ windsucking
• teeth grinding
• diarrhoea
• weakness
Gastropell Daily contains omeprazole, a dose-dependant inhibitor of gastric acid secretion. Clinical trial data indicates that Gastropell Daily will inhibit gastric acid secretion to maintain gastric acidity at a non-aggressive level, which allows existing ulcers to heal within two to four weeks. A reduced daily dose may then prevent recurrence of ulcers.
Even though it is dosed orally, Gastropell Daily does not act directly on the gastric mucosa. The drug must pass through the stomach to the small intestine, where it is absorbed and travels via the bloodstream to the acid secreting parietal cells of the stomach. The presence of food in the stomach will significantly reduce the absorption of Gastropell Daily and the drug should always be administered on a relatively empty stomach.
The antisecretory effect of Gastropell Daily causes a dramatic reduction in the acidity of gastric fluid within three days of commencing treatment, and because the gastric fluid is no longer damaging to the stomach lining, squamous ulcers then heal spontaneously within 2-4 weeks.
Gastropell Daily suppresses acid suppression for 18-24 hours and daily dosing is essential.
When left untreated, ulcers can recur within two weeks with a severity and across an area equivalent to that prior to treatment. Available data indicates that regular

daily maintenance dosing at a reduced rate prevents the recurrence of ulcers.
Administration
Always administer Gastropell Daily on a relatively empty stomach. Administer a minimum of 20 min and preferably > 60 min prior to feeding.
The enteric coated omeprazole beads in Gastropell Daily may be damaged by chewing. Always administer at the back of the mouth over the base of the tongue. Avoid chewing.
Withdrawal: It is recommended that horses be weaned off omeprazole over a minimum period of two weeks, by halving the dose weekly.
Adult Horses: Treatment Dose, 1.0 mg/kg BW (10ml/500 Kg BW) once daily for 2 to 4 weeks followed by daily maintenance dosing.
Maintenance Dose, 0.5 mg/kg BW (5ml/500 Kg BW) once daily while horse remains in training, or as directed by a `veterinarian.
Foals: 1.0 mg/kg BW (2mL/100Kg Kg BW) once daily or as directed by a veterinarian.


HAVE YOU EVER WONDERED... Who the horse on the Gastropell Daily box is?
(FRA)
Goldikova was a champion French miler and the only horse to win three Breeders' Cup Mile races (2008-2010).
In total she had 27 starts for 17 wins, nine placings and $7.2M in prizemoney. Included in her wins were 14 Group 1 victories, which even today remains a record for a European trained horse.
Product image is from an original painting by renowned US horse artist Tom Chapman.
Available:
Adjunct treatment for gastric ulcers (esp glandular ulcers) and other ulcerative disorders of the intestinal tract.
Active Constituent
Sucralfate (aluminium sucrose sulfate)
Pack Size
500g multidose jar
Sucralfate has been used in the treatment of oral, oesophageal, gastric and duodenal ulcers. It is particularly indicated as an adjunct therapy in the treatment of Equine Glandular Gastric Disease (EGGD) including glandular and pyloric disease and ulcers.
Sucralfate has also been used for the treatment and prevention of ulcerative colitis/typhlitis, especially due to prolonged and/or high doses of NSAID’s and especially in foals.
Pharmacology
Sucralfate is a basic aluminium complex of sucrose sulfate. It is practically insoluble in water and alcohol. It dissociates in the acid environment of the stomach to form sucrose octasulfate and aluminium hydroxide.
Sucrose octasulfate polymerises to a viscous sticky substance that creates a protective barrier by binding to ulcerated mucosa. It has an affinity for negatively charged injured tissue. It protects the mucosa by preventing back-diffusion of hydrogen ions and inactivates pepsin and adsorbs bile acid. The duration of action (ie binding to the ulcer site) may persist up to 6 hours after oral dosing.
There is some evidence that sucralfate may also have a direct cytoprotectant effect via promoting increased prostaglandin synthesis.
Sucralfate acts locally rather than systemically. After oral administration, sucralfate reacts with hydrochloric acid in the stomach to form a paste-like complex that will bind to the proteinaceous exudates that generally are found at ulcer sites. This insoluble complex forms a barrier at the site and protects the ulcer from further damage caused by pepsin, gastric acid or bile.
Sucralfate is also known to inactivate pepsin and bind bile acids.
Sucralfate may have some cytoprotective effects, possibly by stimulation of prostaglandin E2 and I2 production. The increase in PGE synthesis stimulates mucus secretion from the glandular mucosa and increases mucosal blood flow.
Only 3-5% of the oral dose of Sucralfate is absorbed. This is largely excreted unchanged in urine within 48 hours. By reacting with hydrochloric acid in the gut, the remainder of the drug is converted to sucrose-sulfate which is excreted in the faeces within 48 hours.
Sucralfate has no known adverse effects in the horse.
Sucralfate alone is not beneficial in the treatment of equine Equine Gastric Ulcer Syndrome (EGUS), but can be used in conjunction with a proton pump inhibitor (PPI) such as omeprazole (Ulcershield/Gastropell Forte/Gastropell Daily).
The administration of sucralfate close to the administration of omeprazole may reduce the absorption of omeprazole from the small intestine.
Sucralfate may also decrease the absorption of other orally administered drugs such as fluoroquinolones (eg enrofloxacin) and tetracyclines (eg doxycycline). These drugs should be administered at least two hours prior to sucralfate administration.

The administration of omeprazole prior to sucralfate is likely to decrease the gastric pH which in turn will decrease the gel formation of the sucralfate.
Ideally sucralfate should be suspended in water and given over the back of the horse’s tongue prior to feeding. This protects the ulcerated areas from abrasion and acid damage associated with eating. However, sucralfate may also be given at the time of eating in the feed.
Sucralfate has a wide dose range varying from 4-40mg/kg bid to qid. The most frequently used dose rates are 12mg/kg tid or 20mg/kg bid.
Adjunct treatment of gastric ulcer with omeprazole (esp EGGD): 4-40mg/kg PO bid-qid.
Right dorsal colitis: 22mg/kg PO q6-8h
Foals: In association with omeprazole for preventing gastric ulcers 10-20mg/kg PO q6-8h
Horse Hack: Sucralfate has also been successfully used for the treatment of chronic equine diarrhoea of unknown aetiology. Some cases of chronic diarrhea will respond to a short course (two weeks) of Sucralfate along with appropriate dietary management. A dose rate of 20-40mg/kg bid-qid is recommended.




DID YOU KNOW THAT... Camel racing is more popular than horse racing in the UAE and the camels are ridden by mechanical jockeys built out of battery operated drills?
CAMEL RACING (UAE)

Robotic jockeys were introduced into camel racing in the Gulf in 2001. The original models were based on industrial drills with an L-shaped arm connected to a whip. The drills were strapped to the camel’s saddles and operated by remote control by the camel trainers, who drive on an inner track alongside the race in their SUVs. The trainers also blast their car horns to encourage the camels. The robotic jockeys have built in walkie-talkies that allow the owners to deliver commands to the camels.
The camels were initially apprehensive about the robotic jockeys. The designs were then modified to include more human-like features such as a mannequinlike face, sunglasses, hats and perfumes. As with horse racing, the robotic camel jockeys are dressed in the racing silks of their respective owners. Most of the colours would be well known to horse racing fans.
Some of the more sophisticated robotics transmit the speed and heart rate of the camel. The robots originally weighed 16-18kg but now only weigh 2-3kg.
Randlab’s TechTalks are independent reviews written by world authorities in their field.
Check out Randlab’s TechTalks on Polysulfated Glycosaminoglycan (Zycan), Pentosan Polysulfate (Arthropen) and HA (Equinate & Matrix) in the Resources section on the Randlab website: See also AllJoints in Supplements section.


APVMA Approval No. 91413 (Australia)
Available:
For the management of arthritis, allergies and dermatologic disorders in horses, dogs and cats
Active Constituent
Triamcinolone Acetonide 6 mg/mL
Pack Size
12 x 3mL (=18mg) vials
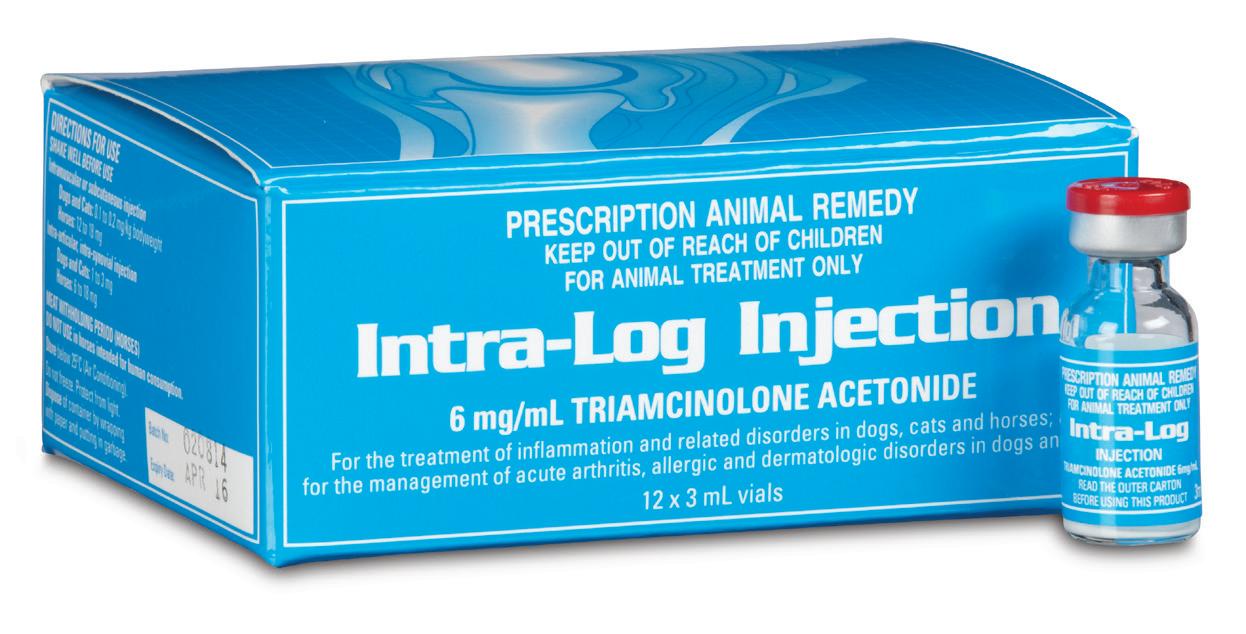
Intra-Log is a potent synthetic corticosteroid that is registered for intra-articular, intra-synovial, intramuscular and subcutaneous use. It is commonly used to treat arthritis, including acute synovitis, osteoarthritis, etc in horses by the intra-articular route.
Intra-Log is also an anti-inflammatory agent, an immunosuppressive agent and a replacement for glucorcorticoid activity in patients with adrenal insufficiency.
Intra-Log is indicated in the treatment of the following disease categories:
• Inflammatory conditions
• Dermatologic disorders
• Allergic disorders
• Arthritis
Intra-Log Injection is a highly potent synthetic glucocorticoid with an intermediate onset and long duration of action. It has 5-10 times the glucocorticoid potency of cortisone and no mineralocorticoid activity.
Triamcinolone has effects on virtually every cell type and cell system in mammals. It has been used in the treatment of:
Endocrine conditions (e.g. adrenal insufficiency), rheumatoid arthritis, collagen diseases (e.g. systemic lupus), allergic conditions, respiratory diseases (e.g. asthma), dermatologic diseases (e.g. pemphigus, allergic dermatoses), hematologic disorders (e.g. thrombocytopaenia, autoimmune haemolytic anaemia), neoplasia, CNS disorders, GIT disorders (e.g. ulcerative colitis) and renal diseases (e.g. nephrotic syndrome), etc.
Triamcinolone injections have been rarely implicated as a cause of equine laminitis. Frequency is rare and a direct causal association has not been established. However, appropriate caution should be taken and the owner informed about the risk whenever injecting triamcinolone. This is particularly true in horses/ponies with a previous history or increased risk factors (eg EMS, PPID) for the development of laminitis.
The total dose of triamcinolone should not exceed 20mg in any single administration. If multiple joint injections requiring a total dose >20mg is require, it is recommended that the injections be separated by a week or more.
Administration of triamcinolone during the last trimester of pregnancy may induce the first stage of parturition and may precipitate premature parturition followed by dystocia, foetal death, retained placenta and metritis.
WARNING: The injection of triamcinolone into a ligament (either intentionally or inadvertently) may result in a prolonged excretion time and lead to a positive doping test in performance horses.
Total dose should not exceed 20mg in any single administration. If using triamcinolone systemically, the aim is to use the minimum amount required for as short a time as possible. Delay any further administration for > 7 days.
Intramuscular or subcutaneous injection:
Horses: 12 to 18 mg (2-3 mL).
Dogs and Cats: 0.1 to 0.2 mg/kg bodyweight.
Intra-articular/ intra-synovial injection:
Horses: 6 to 18 mg per joint depending on joint size.
Dogs and Cats: 1 to 3 mg.
Horse Hack: Horses administered triamcinolone may go off their feed for 2-5 days following administration.
The administration of Intra-Log Injection to horses may affect the peripheral blood count for a period of up to two weeks. The changes are typical of a stress leucogram
(leucocytosis or leucopaenia associated with a relative neutrophilia and or lymphopaenia, increase in ALP, etc). These changes may be observed even when the triamcinolone is administered intra-articularly.

DID YOU KNOW THAT...

An Australian horse that cost $1250 and was trained by a taxi driver from Queanbeyan went on to win over $6 million in prizemoney and meet The Queen?
TAKEOVER TARGET AKA 'ARCHIE' (JOE JANIAK, AUS)
See page 68 for the full story


APVMA Approval No. 87359 (Australia)
Available:
Worldwide exclusive.
The first registered generic to Adequan, the most trusted joint medication in the USA.
Active Constituent
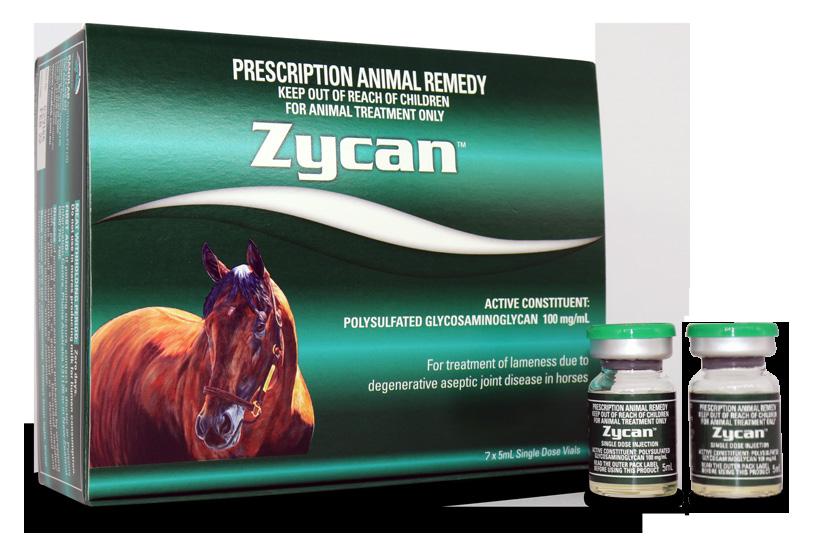
Polysulfated Glycosaminoglycan 100 mg/mL
Pack Size
7 x 5mL single dose vials
Zycan is the first registered generic to Adequan. Adequan has been registered in the USA for over 35 years and is the favoured joint medication of US veterinarians. There are over 50 publications supporting the use of Adequan in horses, humans and canines.
Zycan is a Disease Modifying Osteoarthritis Drug (DMOAD) for the treatment and prevention of clinical signs attributable to degenerative and/or traumatic aseptic joint disease in horses. DMOADs are intended to prevent, retard or reverse the morphologic cartilaginous lesions associated with degenerative joint disease (DJD).
PSGAGs, such as Zycan have been advocated for the (i) prevention and treatment of joint disease (ii) joint maintenance programs and (iii) post-operative care of horses returning to training following joint surgery.
Zycan is a semi-synthetic polysulfated glycosaminoglycan (PSGAG) and is physiologically similar to the natural mucopolysaccharides found in joint cartilage. The low molecular weight of Zycan facilitates the distribution of the PSGAG from the bloodstream to the synovial fluid. Distribution from the synovial fluid to the cartilage then takes place by diffusion. PSGAG is deposited in all layers of articular cartilage and is preferentially taken up by osteoarthritic cartilage. When administered IM, synovial levels exceed serum levels, peak in 48 hours and persist for up to 96 hours. The latter is the rationale behind every fourth day dosing.
PSGAGs exert their chondroprotective effects by inhibiting the detrimental effects of cytokines and PG’s on cartilage and connective tissue, reducing proteoglycan breakdown, stimulating HA synthesis and enhancing the production of glycosaminoglycan, proteoglycan and collagen.
In multiple studies, PSGAGs have been shown to:
• Inhibit various degradative proteolytic enzymes, including glycanohydrolases, glycosidases and MMPs.
• Inhibit MMP-3 more effectively than phenylbutazone, flunixin, betamethasone and HA.
• Increase collagen and GAG synthesis in articular cartilage explants and cell culture.
• Stimulate synthesis of HA by synoviocytes, restoring synovial fluid viscosity.
• Inhibit PGE synthesis and the influx of leucocytes into inflammatory sites.
• Inhibit the production of superoxide radicals and pro-inflammatory IL-1.
• Increases the synthesis of proteoglycans by acting as a precursor.
• Decrease articular fibrillation and erosion.
• Decrease chondrocyte death.
• No change in partial or full thickness articular cartilage lesions has been reported.
PSGAGs (250mg) have also been used intra-articularly for the treatment of acute synovitis, including post-arthroscopy (McIllwraith 2016).
ONLY REGISTERED GENERIC TO ADEQUAN® WORLDWIDE
IM PSGAG administration may occasionally result in transient localised swelling and discomfort at the injection site. As PSGAGs have heparinoid-like activity, do not administer when a pre-existent tendency to haemorrhage or increased coagulation time is expected. Do not administer within 24 hours of surgery.
IA PSGAG administration may cause post-injection joint flares (joint pain, effusion, swelling and lameness) secondary to sensitivity reactions. A slightly increased risk of joint infections following IA injection of 250mg PSGAG has been reported compared with corticosteroids + HA.
This product is NOT registered for intra-articular use.
Safety in pregnant or lactating animals or in foals has not been established. Use with caution in these groups.
Intra-muscular injection: 500mg (5mL) per 500kg horse, repeated every 4th day for 7 injections (i.e. 28 days). Weekly injections have also been shown to be effective. The series may be repeated as needed upon recurrence of the clinical signs of DJD and associated lameness. Otherwise, twice yearly courses are recommended.
Weekly maintenance injections after the initial priming course have also been advocated.
Horse Hack: Zycan can be used as a substitute for pentosan (eg Arthropen) and as such, can be used as you would pentosan.
Zycan appears to cause fewer injection site reactions than pentosan. Zycan is not known to cause discolouration of the hair coat at the injection site.
Zycan is also a good alternative to pentosan in horses that have had previous neck reactions to pentosan.

American Pharoah is the most celebrated US racehorse of the past few decades. And not just because he features as the product model on Randlab’s Zycan box. The horse is one of only 13 horses to win America’s fabled Triple Crown (Kentucky Derby, Preakness Stakes, Belmont Stakes) and only the second in the last 40 years. He is the only horse in history to also win the Grand Slam by subsequently taking out the Breeders’ Cup Classic.
The image on the product box is from an original painting by acclaimed US horse artist Tom Chapman.
APVMA Approval No. 63265 (Australia) | ACVM No. A010081 (New Zealand)
Available in:
Most highly sulfated pentosan on the market. Higher sulfation means increased efficacy.
Active Constituent
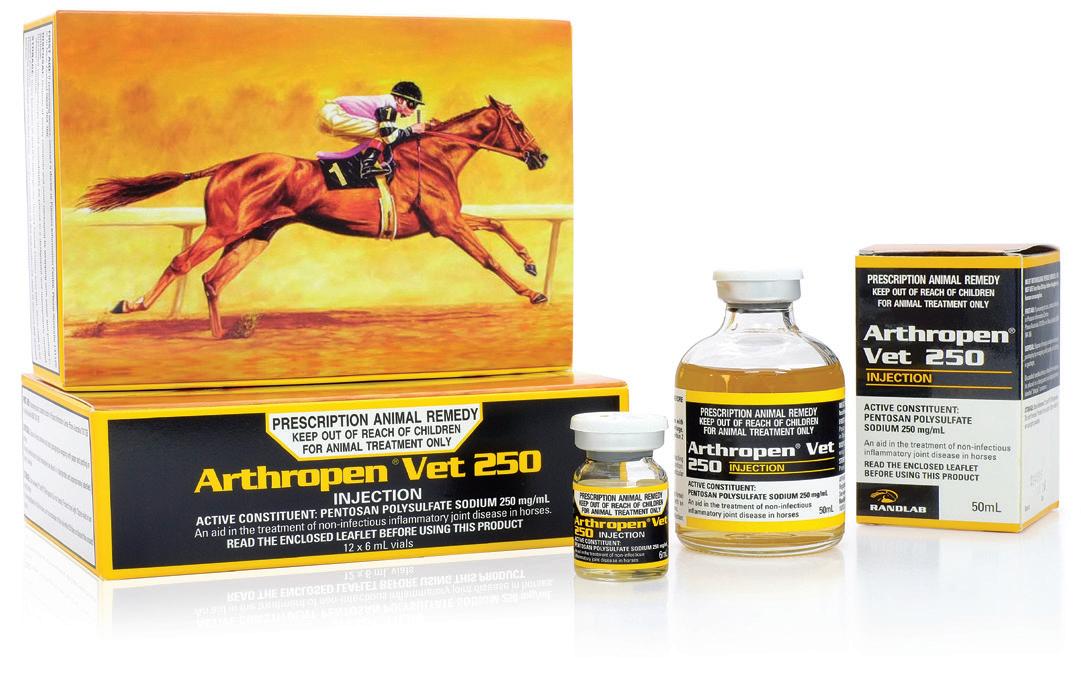
Pentosan Polysulfate Sodium 250 mg/mL
Pack Size
12 x 6mL single dose vials
50mL multidose vial (8 doses/500kg horse)
Early treatment is an important step in preventing the products of inflammation from damaging articular cartilage and causing irreversible joint disease.
Arthropen Vet 250 Injection is indicated as an aid in the treatment of noninfectious, inflammatory joint disease in the horse.
Clinical applications include the following conditions:
• osteoarthritis (OA)
• traumatic joint disease
• multiple or non specific joint disease
• osteochondritis dissecans (OCD)
• synovitis
• degenerative joint disease
Arthropen Vet 250 Injection is a Disease Modifying Osteoarthritis Drug (DMOAD).
The high concentration of Pentosan Polysulphate in Arthropen Vet 250 has been specifically designed for low volume administration in the horse.
Arthropen Vet is especially useful in treating conditions affecting multiple joints or where joint pain is suspected but cannot be localised.
Pentosan Polysulfate Sodium (PPS) possesses anti-inflammatory, anti-arthritic and chondroprotective activities. It is a semi-synthetic polysaccharide with a linear xylan (pentosan) backbone. Xylan is a hemicellulose derived from plant origin by extraction from the cell walls of hardwoods. The xylan backbone then undergoes sulfation. The degree of sulfation is believed to modulate the potency of the pentosan molecule. Arthropen Vet 250 Injection is the only fully sulfated PPS on the market.
The beneficial effects of pentosan polysulfate sodium in joint disease are due to the following actions:
• Anti-inflammatory activity.
• Inhibition of neutrophil migration into the joint.
• Inhibition of enzymes implicated in cartilage destruction.
• Stimulation of hyaluronic acid synthesis by synovial fibroblasts, resulting in increased volume and viscosity of synovial fluid.
• Stimulation of proteoglycan synthesis by chondrocytes.
• Fibrinolytic activity, resulting in improved circulation to subchondral bone and peri-articular structures.
• Reduction in Matrix Metalloproteinases (MMPs).
• Reduction in bone oedema.
WARNING: The intravenous use of pentosan polysulfate may rarely result in anaphylaxis and death. Do not use intravenously!
Administration and Precautions
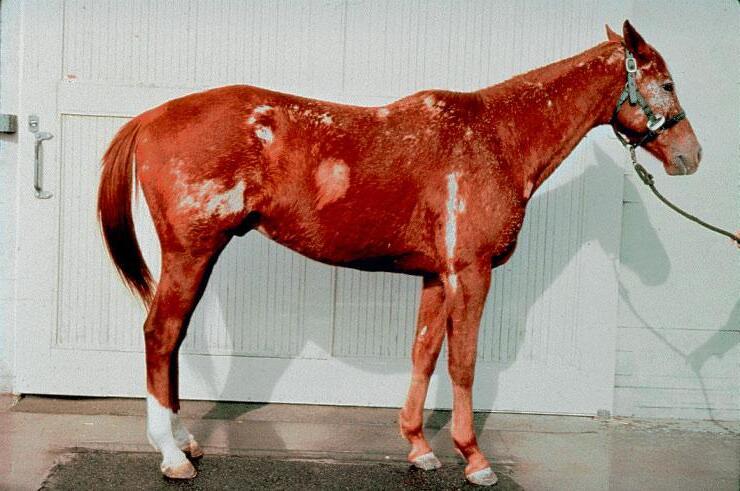
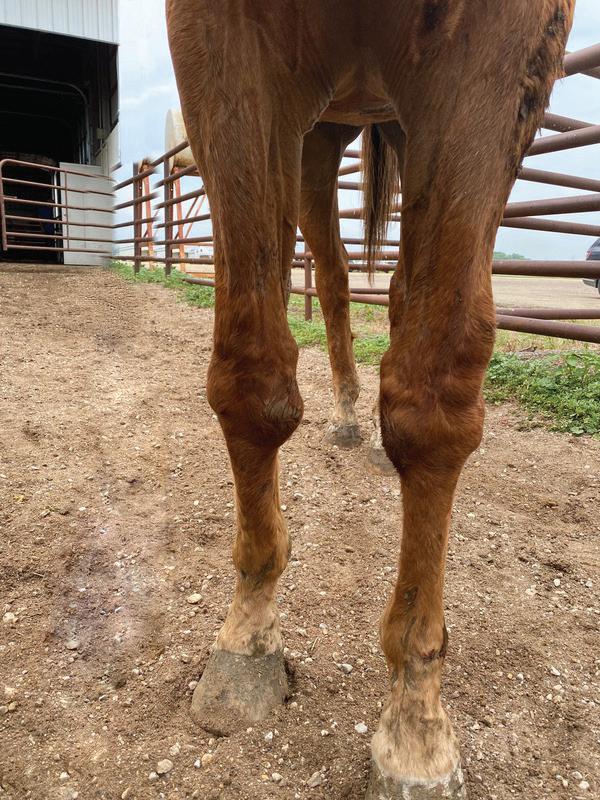
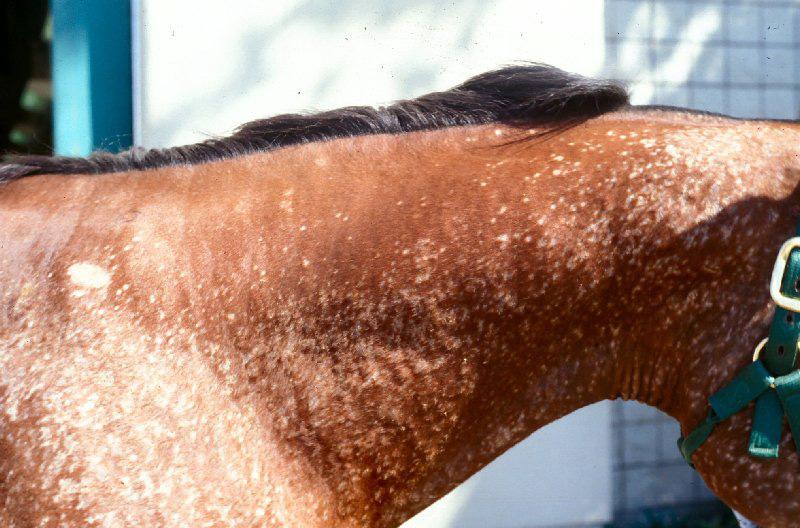
Reversible, discolouration of the hair coat and hair loss may be seen at the injection site following long-term use of PPS. To minimise the risk of such reactions, the injection site should be alternated and PPS given by deep IM injection.
AIRCON
Because of its heparinoid activity, Arthropen is best not used for a period of 40 hours prior to strenuous exercise in horses with a history of Exercise Induced Pulmonary Haemorrhage (EIPH). Contraindicated for use in horses with clotting defects, traumatic haemorrhage, infection, liver/kidney failure, or within two days of surgery.
Intra-articular use of Arthropen may result in temporary joint flares. If used in performance animals, the regulations of the relevant authorities regarding medication control should be observed.
An initial course of four injections a week apart is recommended. This is generally followed by a program of either weekly injections or injections spaced at 2-4 week intervals depending on the intensity of the horse’s exercise/competition program and the response to treatment. Horses undergoing heavy training or competition schedules or horses with a chronic lameness problem are likely to benefit from ongoing weekly Arthropen injections.
Dose - 3mg/kg bodyweight (6mL/500kg horse) by intramuscular injection on four occasions with an interval of 5-7 days between injections. Preferable to alternate injection sites from week to week. Best given by deep intramuscular injection. To avoid haemorrhage associated with injection, a small needle (e.g. 21 gauge) is recommended.
Intra-articular: 1.0mL by intra-articular injection. May be repeated at weekly intervals for 3 to 4 treatments. More than one joint may be treated at the one time. Joint flares are common after intra-articular PPS.
Horse Hack: The storage conditions for Arthropen have changed. It no longer needs to be refrigerated and can be kept in air conditioning (store below 25oC, do not freeze). In house testing has also shown the product to be stable at even higher temperatures.

HAVE YOU EVER WONDERED... Who the horse on the Arthropen 250 Injection box is?
AFFIRMED (USA) US Triple Crown winner, winning the 1973 Kentucky Derby.
Affirmed was only the 11th horse to win the fabled US Triple Crown (1978). He was equally well known for his racetrack rivalry with another champion horse Alydar. The pair met on ten occasions, with Affirmed winning seven of the clashes, including each of the three Triple Crown races. After Affirmed won the Triple Crown, there was a 37-year hiatus until American Pharoah swept the series in 2015.
The image on the Arthropen box is from an original painting by acclaimed US horse artist Tom Chapman.
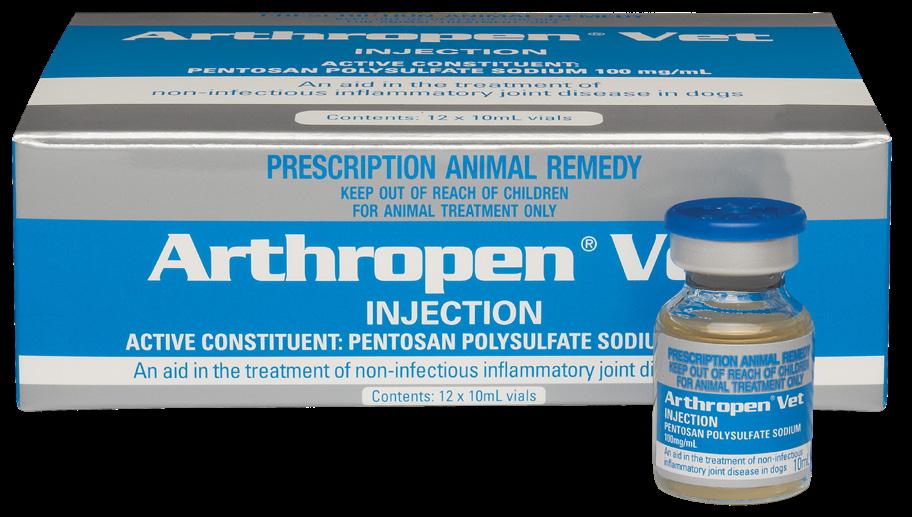
Available in:
An aid in the treatment and prevention of non-infectious inflammatory joint disease in
Low concentration pentosan means less tissue reactive. Highest level of sulfation for increased activity.
Active Constituent
Pentosan Polysulfate Sodium 100 mg/mL
Pack Size
12 x 10mL multidose vials
Early treatment is an important step in preventing the products of inflammation from damaging articular cartilage and causing irreversible joint disease.
Arthropen Vet Injection is a low concentration formulation of pentosan polysulfate (PPS) suitable for use in dogs and horses. It aids in the treatment and prevention of non-infectious inflammatory joint disease in conditions such as:
• osteoarthritis (OA)
• traumatic joint disease
• multiple or non specific joint disease
• osteochondritis dissecans (OCD)
• synovitis
• degenerative joint disease
Arthropen Vet Injection is a Disease Modifying Osteoarthritis Drug (DMOAD). The low concentration of pentosan polysulfate in Arthropen Vet Injection may cause less tissue irritation at the injection site.
Arthropen Vet Injection is especially useful in treating conditions affecting multiple joints or where joint pain is suspected but cannot be localised.
Pentosan Polysulfate Sodium (PPS) possesses anti-inflammatory, anti-arthritic and chondroprotective activities. It is a semi-synthetic polysaccharide with a linear xylan (pentosan) backbone. Xylan is a hemicellulose derived from plant origin by extraction from the cell walls of hardwoods. The xylan backbone then undergoes sulfation. The degree of sulfation is believed to modulate the potency of the pentosan molecule. Arthropen Vet Injection is the only fully sulfated PPS on the market.
The beneficial effects of pentosan polysulfate sodium in joint disease are due to the following actions:
• Anti-inflammatory activity.
• Inhibition of neutrophil migration into the joint.
• Inhibition of enzymes implicated in cartilage destruction.
• Stimulation of hyaluronic acid synthesis by synovial fibroblasts, resulting in increased volume and viscosity of synovial fluid.
• Stimulation of proteoglycan synthesis by chondrocytes.
• Fibrinolytic activity, resulting in improved circulation to subchondral bone and peri-articular structures.
• Reduction in Matrix Metalloproteinases (MMPs).
• Reduction in bone oedema.
Reversible, localised discolouration of the hair coat and hair loss may be seen at the injection site following long-term use of PPS. To minimise the risk of such reactions, the injection site should be alternated and PPS given by deep IM injection.
Because of its heparinoid activity, Arthropen is best not used for a period of 40 hours prior to strenuous exercise in horses with a history of Exercise-Induced Pulmonary Haemorrhage (EIPH). Contraindicated for use in horses with clotting
defects, traumatic haemorrhage, infection, liver/kidney failure, or within two days of surgery.
Intra-articular use of Arthropen may result in temporary joint flares. If used in performance animals, the regulations of the relevant authorities regarding medication control should be observed.
An initial course of four injections a week apart is recommended. This is generally followed by a program of either weekly injections or injections spaced at 3-4 week intervals depending on the intensity of the horse’s exercise/competition program and the response to treatment. Horses undergoing heavy training or competition schedules or horses with a chronic lameness problem are likely to benefit from ongoing weekly Arthropen injections.
Dose - 2-3mg/kg bodyweight by intramuscular injection on four occasions with an interval of 5-7 days between injections. Preferable to alternate injection sites from week to week. Best given by deep intramuscular injection. To avoid haemorrhage associated with injection, a small needle (e.g. 21 gauge) is recommended.
Intra-articular: 2.5mL by intra-articular injection. May be repeated at weekly intervals for 3 to 4 treatments. More than one joint may be treated at the one time. Joint flares are common after intra-articular PPS.
WARNING: The intravenous use of pentosan polysulfate may rarely result in anaphylaxis and death. Do not use intravenously!
Arthropen Vet works even better in dogs than horses.


DID YOU KNOW THAT...
In 1959, when the 14th Dalai Lama fled Tibet after a crackdown by the Chinese army, he did so on horseback?
DALAI LAMA (TIBET)
APVMA Approval No. 70144 (Australia) | ACVM No. A011191 (New Zealand)
Available in:
High molecular weight and high viscosity HA means high performance.
Active Constituent
Sodium Hyaluronate 10 mg/mL
Pack Size
6 x 6mL single dose vials
12 x 6mL single dose vials

Early treatment is an important step in preventing the products of inflammation from damaging articular cartilage and causing irreversible joint disease.
Indications
Higher molecular weight formulations of HA such as Matrix 6000, are known to have increased clinical effects.
Matrix 6000 IV Injection is a high molecular weight, high viscosity formulation of sodium hyaluronate. It is ideal for intravenous use in the treatment and prevention of lameness in horses due to non-infectious synovitis including those associated with early equine degenerative joint disease.
Ideal for use pre-competition or pre-race to help restore joint function.
Hyaluronic Acid (HA) is a natural and essential component of articular cartilage and synovial fluid. It is produced by both synoviocytes within the synovial membrane and by chondrocytes within the articular cartilage matrix.
The hyaluronic acid produced by bacteria is of the same structure and configuration as that found in mammals. The active ingredient in Matrix 6000 IV injection is extracted from the capsule of a selected micro-organism and purified to produce an ultrapure form of sodium hyaluronate that is essentially free of protein and nucleic acids. Since this product originates from a microbial source, there is no potential for contamination with dermatan or chondroitin sulphate or any other glycosaminoglycan.
Sodium hyaluronate restores lubrication of the joint fluid and regulates the normal cellular constituents. This effect decreases the impact of exudation, enzyme release and subsequent degradation of joint integrity.
HA has the ability to confer extraordinary compressive strength to the articular cartilage when functioning as the core molecule for proteoglycan aggregates. Articular surfaces are covered with a fine layer of HA, which exerts resistance to cartilage compression while still retaining its elasticity. HA also confers viscoelastic and lubricating properties to synovial fluid and is responsible for the boundary lubrication of the synovial membrane and the lubrication of articular cartilage. These latter properties are believed to be further increased by higher molecular weight formulations of HA, such as in Matrix 6000.
Hyaluronate molecules are long chains which form a filter matrix interspersed with normal cellular fluids. This supplements the viscoelastic properties of normal joint fluid. Sodium hyaluronate exerts a slight anti-inflammatory action by providing a steric barrier that limits the movement of granulocytes and macrophages into the joint.
Administration and Precautions
Not for Intra-articular use.
Dosage
Adult horse (450-700 kg): Administer 6 mL (60 mg) intravenously. Treatment may be repeated at weekly intervals.
In performance horses, Matrix 6000 may be administered prior to competition as an aid in reducing joint inflammation and restoring joint function. The regulations of the relevant regulatory authority regarding medication control should always be observed.
This product does not contain any antimicrobial preservative. Any solution remaining in the vial after administration of the required dose should be discarded.


DID YOU KNOW THAT... The rise of the Italian dictator Mussolini changed the entire thoroughbred breeding industry for the next 85 years?
NEARCO (FREDERICO TESIO, ITA)
See page 68 for the full story

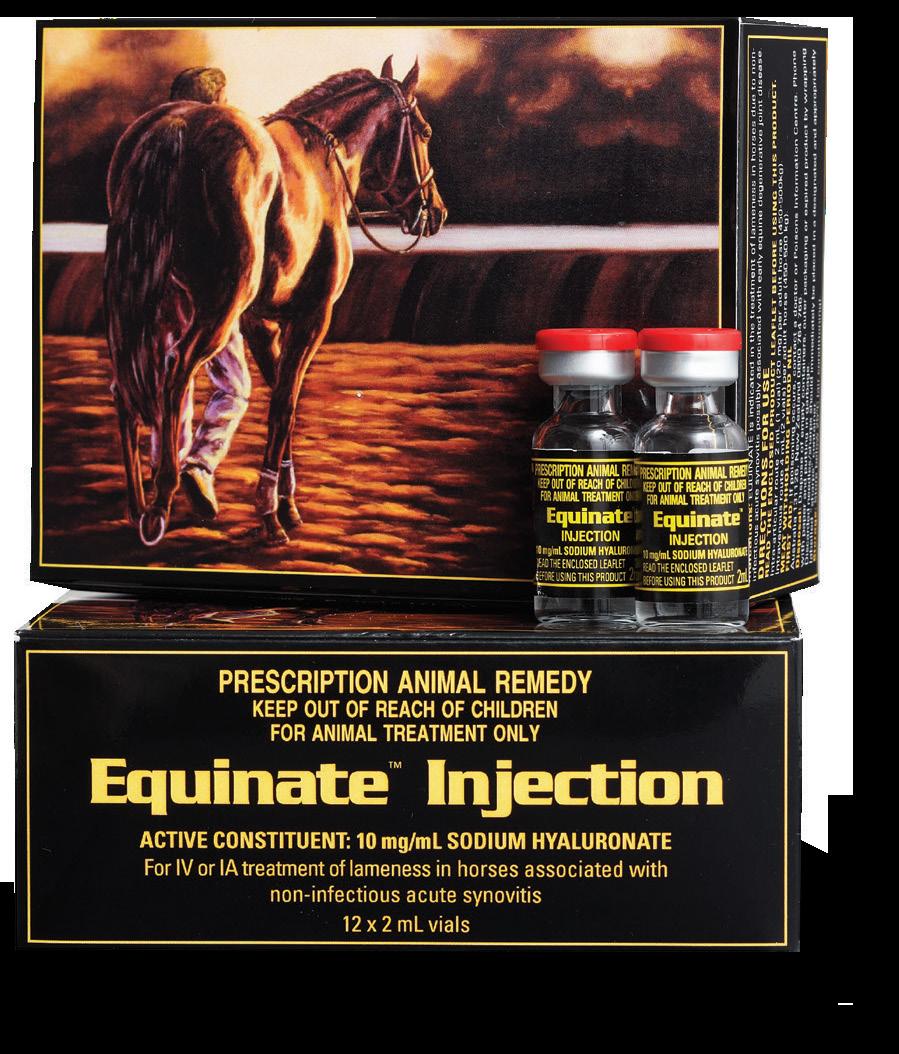
Available in:
Intra-articular HA of choice.
Protein free to reduce joint flares.
High molecular weight & viscosity.
Active Constituent
Sodium Hyaluronate 10 mg/mL
Pack Size
12 x 2mL single dose vials
Early treatment is an important step in preventing the products of inflammation from damaging articular cartilage and causing irreversible joint disease.
Indications
Equinate Injection is the Hyaluronic Acid (HA) product to meet all your HA requirements. Equinate Injection is indicated in the treatment and prevention of lameness in horses due to non-infectious synovitis including those associated with early equine degenerative joint disease. It is designed for intra-articular administration but may also be used intravenously.
Hyaluronic Acid is a natural and essential component of articular cartilage and synovial fluid. It is produced by both synoviocytes within the synovial membrane and by chondrocytes within the articular cartilage matrix.
The hyaluronic acid produced by bacteria is of the same structure and configuration as that found in mammals. The active ingredient in Equinate Injection is extracted from the capsule of a selected micro-organism and purified to produce an ultrapure form of sodium hyaluronate that is essentially free of protein and nucleic acids. Since this product originates from a microbial source, there is no potential for contamination with dermatan or chondroitin sulphate or any other glycosaminoglycan. This reduces the flare reactions sometimes associated with IA injections of sodium hyaluronate. It contains no preservative.
Sodium hyaluronate restores lubrication of the joint fluid and regulates the normal cellular constituents. This effect decreases the impact of exudation, enzyme release and subsequent degradation of joint integrity.
HA has the ability to confer extraordinary compressive strength to the articular cartilage when functioning as the core molecule for proteoglycan aggregates. Articular surfaces are covered with a fine layer of HA, which exerts resistance to cartilage compression while still retaining its elasticity. HA also confers viscoelastic and lubricating properties to synovial fluid and is responsible for the boundary lubrication of synovial membranes and the lubrication of articular cartilage. These latter properties are believed to be further increased by higher molecular weight formulations of HA, such as is found in Equinate Injection.
Hyaluronate molecules are long chains which form a filter matrix interspersed with normal cellular fluids. This supplements the viscoelastic properties of normal joint fluid. Sodium hyaluronate also exerts a slight anti-inflammatory action by providing a steric barrier that limits the movement of granulocytes and macrophages into the joint. APVMA Approval No. 65128 (Australia) | ACVM No. A010491 (New Zealand)
Strict aseptic technique should be observed when injecting Equinate Injection intra-articularly.
To achieve best results in cases of intra-articular or intravenous administration, horses should be rested following treatment.
Intra-articular Injection - The recommended dosage for intra-articular injection is 2mL (1 vial/20mg) per joint. A greater volume e.g. 4mL (2 vials/40mg) may be required in larger joints such as the stifle or shoulder. Treatment may be repeated at weekly intervals for a total of three treatments. As with any intra-articular procedure, proper injection site disinfection and animal restraint are important. Excess joint fluid should be aseptically removed prior to intra-articular injection. Care should be taken not to scratch the cartilage surface with the injection needle. Use the smallest gauge needle possible (e.g. 21 or 20 gauge).
Intravenous Injection - 4mL (2 vials/40mg) per adult horse (450-500kg). Treatment may be repeated at weekly intervals for a total of three treatments or be used pre-competition or race to alleviate joint inflammation.


DID YOU KNOW THAT... The second largest empire the world has ever known was built on a diet of equine milk and blood?
APVMA Approval No. 62557 (Australia) | ACVM No. A010089 (New Zealand)
Available in:
Suitable for weekly/regular IV maintenance or precompetition dosing.
Low molecular weight IV formulation.
Active Constituent
Sodium Hyaluronate 10 mg/mL
Pack Size
12 x 4mL single dose vials
Early treatment is an important step in preventing the products of inflammation from damaging articular cartilage and causing irreversible joint disease.
Equinate I.V. Injection is indicated in the intravenous treatment or prevention of lameness in horses due to non-infectious synovitis including those associated with early equine degenerative joint disease.
Hyaluronic Acid (HA) is a natural and essential component of articular cartilage and synovial fluid. It is produced by both synoviocytes within the synovial membrane and by chondrocytes within the articular cartilage matrix.
The hyaluronic acid produced by bacteria is of the same structure and configuration as that found in mammals. The active ingredient in Equinate IV Injection is extracted from the capsule of a selected micro-organism and purified to produce an ultrapure form of sodium hyaluronate that is essentially free of protein and nucleic acids. Since this product originates from a microbial source, there is no potential for contamination with dermatan or chondroitin sulphate or any other glycosaminoglycan. The solution is pyrogen free and sterile.
Sodium hyaluronate restores lubrication of the joint fluid and regulates the normal cellular constituents. This effect decreases the impact of exudation, enzyme release and subsequent degradation of joint integrity.
HA has the ability to confer extraordinary compressive strength to the articular cartilage when functioning as the core molecule for proteoglycan aggregates. Articular surfaces are covered with a fine layer of HA, which exerts resistance to cartilage compression while still retaining its elasticity. HA also confers viscoelastic and lubricating properties to synovial fluid and is responsible for the boundary lubrication of synovial membranes and the lubrication of articular cartilage. Hyaluronate molecules are long chains which form a filter matrix interspersed with normal cellular fluids. This supplements the viscoelastic properties of normal joint fluid. Sodium hyaluronate exerts a slight anti-inflammatory action by providing a steric barrier that limits the movement of granulocytes and macrophages into the joint.
Studies have shown that weekly treatments with intravenous hyaluronic acid for a total of three injections to be effective.
This product does not contain any antimicrobial preservative. Any solution remaining in the vial after administration of the required dose should be discarded.

Intravenous Route - 4 mL (40 mg) per adult horse (450-500 kg). Treatment may be repeated at weekly intervals or as required.
In performance horses, Equinate IV Injection may be administered prior to competition or racing as an aid in reducing joint inflammation and restoring joint function.
The regulations of the relevant competition Authority regarding medication control should always be observed.
To achieve best results in cases of intravenous administration, horses should be rested during treatment.

DID YOU KNOW THAT...
The 1976 Great American Horse Race Was Won By A Mule Named Lord Fauntleroy?
LORD FAUNTLEROY AKA
USA)


In 1976, the USA was in the grips of its bicentennial celebrations and ready to party. To commemorate its pioneering spirit and acknowledge the role equids had played in the development of the fledging nation, The Great American Horse Race was launched. The race would start in New York, cover 3,500 miles (5,633km) through 13 states and take 14 weeks. Billed as “The race of a lifetime” it also offered the winner $25K (today’s equivalent ~ Au$190K). Each rider was allowed two horses and a support crew. Ninety-one teams from around the world lined up to share in the adventure and compete for the lucrative purse. Teams of horses from Iceland, France, Australia, Denmark and Japan would participate, all convinced that their nation’s home breed would win.
Amongst all the ceremony was 54yo Virl Norton, a steeplejack and part-time donkey/mule wrangler from San Jose and his mule with the fancy name Lord Fauntleroy (aka ‘Leroy’). Unlike most other teams, Virl’s support team only consisted of one - his 16yo son riding the backup mule, Lady Eloise. Much to the consternation of the breed associations, it was Leroy who was awarded first place honours with a total ride time of 315.47 hours. The next five placings were held by Arabians.
Some of the breed associations accused Norton of cheating to which the wily cowboy retorted by offering, “to wire his winnings to a bank in New York and that they could race back to it”.
Available in:
product no longer available in aus & nZ
Highly potent, long-acting bisphosphonate injection.
Active Constituent
Zoledronic Acid (as monohydrate) 0.45 mg/mL
Pack Size
100mL vial
Zoldronic Acid is a long-acting bisphosphonate injection used for the treatment of skeletal diseases associated with high osteoclast activity and accelerated bone turnover, prevention of skeletal fractures and alleviation of non-specific bone pain. Zoldronic Acid has been indicated in the treatment of conditions such as equine Palmar/Plantar Osteochondral Disease (POD) and as an aid in the prevention of consequent metacarpal/metatarsal condylar fractures.
Zoledronic acid is reported as the strongest bisphosphonate currently available with a duration of action exceeding 12 months and a potency exceeding 100x other commonly used bisphosphonates.
Zoledronate is a third-generation nitrogen-containing bisphosphonate that has high tissue specificity for bone hydroxyapatite. Once absorbed into the bone, the distribution of the drug is not homogenous, with the highest concentrations being found at sites of high remodeling and cancellous bone, rather than in bone cortex. Here the zoledronic acid causes osteoclast apoptosis as well as inhibition of osteoclastic bone resorption. Bone resorption is the essential first step in the process of bone remodeling.
Zoledronic acid is largely excreted unchanged by the kidneys. Up to 50% of the administrated dose may be eliminated in the urine within the first 48 hours with the remainder being incorporated into the bone matrix for a prolonged period. The effects of zoledronic acid are long-lasting and may exceed 12 months.
As bisphosphonates are bound to the bone matrix, their release into the circulation may be highly random and intermittent. Under some circumstances this may result in both a prolonged (up to three years) and intermittent excretion.
In some thoroughbred racing jurisdictions, the administration of bisphosphonates to horses under four years of age is not permitted.
WARNING: Zoledronic acid is currently a banned substance for horses competing under FEI rules and for horses competing in some thoroughbred racing jurisdictions (eg Racing Australia). Check the rules of the local Regulatory Authority prior to administration.

The use of bisphosphonates in thoroughbred weanling and yearling horses being sold at public auction is not permitted.
Catastrophic fractures have been reported in speed racing horses (thoroughbred and endurance horses) that have been administered bisphosphonates. Although a direct linkage between occurrence of fractures and bisphosphonates has not been established, careful consideration should be exercised prior to administration in these horses.
Zoledronic acid is rapidly processed by the kidneys, consequently, its administration is not recommended in animals with reduced renal function or kidney disease.
The administration of Non Steroidal Anti-inflammatory Drugs (NSAIDs) in association with bisphosphonates should be avoided due to their possible effect on the kidneys.
Horses with administered bisphosphonates may occasionally exhibit polyuria/ polydypsia, that may persist for up to two weeks.
The safety of zoledronic acid in pregnant and lactating mares or in growing horses has not been established.
Rarely horses that have been administered zoledronic acid may exhibit hypocalcaemia. It is recommended that horses treated with zoledronic acid have their serum calcium measured approximately 14 days post administration.
Use with caution in horses with hypocalcaemia or cardiac dysfunction.
Excretion times for zoledronic acid have not been published but due to its strong bone binding affinity, may be intermittent and prolonged (up to three years).
DO NOT USE in horses with reduced renal function.
Dose: 0.057-0.075mg/kg in 1L 0.9% saline by slow intravenous infusion over 30-45minutes. The lower dose rate (0.057mg/kg) is recommended.
Dose should not be repeated more frequently than annually.

HAVE YOU EVER WONDERED... Who the horse on the Zoldronic Acid box is?
WE DON'T KNOW EITHER.
Check out Dr Ellie Crispe’s TechTalk on the latest research on Exercise Induced Pulmonary Haemorrhage (EIPH/ “bleeders”) in performance horses in the Resources section of Randlab’s website:

AT THE BLAST OF THE TRUMPET IT SNORTS, ‘AHA!’ IT CATCHES THE SCENT OF BATTLE FROM AFAR, THE SHOUT OF COMMANDERS AND THE BATTLE CRY.
See also Optimise X on page 64.
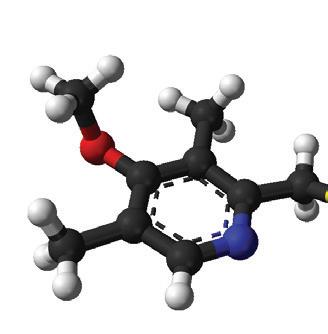
(Clenbuterol Hydrochloride 21.44 µg/g, Sulfadiazine 335 mg/g, Trimethoprim 67 mg/g)
Oral bronchodilator and antibacterial for the treatment of respiratory infections.
(Clenbuterol Hydrochloride 41 µg/mL)
Oral bronchodilating agent


(Sulfadimidine 430 mg/g, Trimethoprim 86 mg/g, Bromhexine Hydrochloride 8.6 mg/g)
Oral mucolytic and antibacterial for the treatment of respiratory infections
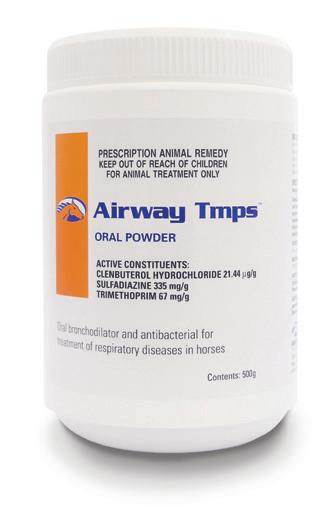
APVMA Approval No. 61474 (Australia) | ACVM No. A010158 (New Zealand)
Available in:
Whenever antibiotic cover and bronchodilation is required. For the treatment of respiratory infections caused by TMPS sensitive organisms.
Active Constituents
Clenbuterol Hydrochloride 21.44 µg/g
Sulfadiazine 335 mg/g
Trimethoprim 67 mg/g
Pack Size
500g multidose jar
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Randlab’s Airway Tmps. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
For the treatment of respiratory disease in horses caused by organisms susceptible to a sufadiazine/trimethoprim antibiotic combination. Treatment of respiratory conditions characterised by restriction of the airways, including bronchospasm and obstruction resulting from bacterial and viral infection, bronchitis and bronchopneumonia.
Airway Tmps Oral Powder is formulated for the treatment of respiratory disease in horses caused by organisms susceptible to the sufadiazine/trimethoprim antibiotic combination. These include; Staphylococci, Streptococci, Fusobacterium, Enterobacter, Corynebacterium (excluding Rhodococcus equi), Salmonella, Shigella, Klebsiella, Pasteurella, Haemophilus, Proteus spp and most E.coli, and some Brucella and Nocardia spp. MostPseudomonasspparenotsensitive.
Clenbuterol is a bronchodilator with minimal effect on the cardiovascular system. Clenbuterol also has a stabilising effect on mast cells, preventing the release of histamine, serotonin and bradykinin and also improves mucociliary clearance of airway discharge by increasing ciliary beat frequency.
Clenbuterol will potentiate the action of corticosteroids and antagonise the effects of prostaglandin F2α and oxytocin. Beta adrenergic agents adversely affect the action of clenbuterol. Concurrent treatment of horses with these medications is not recommended. Airway Tmps Oral Powder should not be used in horses during the last 14 days of gestation, as uterine contractions may be prevented.
Dosage
Dose: The label dose rate is one level scoop (9g) orally per 250kg bodyweight in feed twice daily for 6 to 10 days.
One level scoop contains 9g of powder.
Twice daily dosing is recommended to maintain therapeutic levels.
Administer on damp food with honey or molasses to avoid sifting of powder. Alternatively, Airway Tmps may be made up into a paste with molasses, honey or Karo (corn) Syrup and administered over the back of the tongue.
Randlab supports responsible Antimicrobial Stewardship.
Horse Hack: Airway Tmps can be easily administered as a “powder sandwich” over the back of the tongue. Cut the top off a 60mL syringe at the 2mL mark with a bread knife or hacksaw. Withdraw the plunger to 60mL. Put two teaspoons of molasses, honey or apple sauce in the syringe, followed by the prescribed dose of Airway Tmps and a further two teaspoons of molasses or honey. Administer to the horse over the back of the tongue. As well as adding some flavouring, the molasses or honey stops the horse spitting out the dose.



DID YOU KNOW THAT... American racehorse Man o’War was so popular that Coca Cola used him in an advertising campaign?
MAN
o'WAR
(SAMUEL D. RIDDLE, USA)

Man o’War was an American racehorse who won 20 of his 21 starts. His only defeat coming when unluckily beaten by a horse who was prophetically named ‘Upset’.
His match race against Sir Barton (the first ever US Triple Crown winner) in 1920 was the first time that a horse race was filmed in its entirety.
In 1920 he shared The New York Times outstanding athlete of the year award with Babe Ruth.
In 1921, Man O’War’s owner turned down an offer of US$1M for the stallion (about AU$24M in current money). The offer was subsequently raised to a ‘blank cheque’, but his owner still declined to sell.
Man O’War would go on to be a success at stud, with the champion War Admiral amongst his progeny. He died in 1947 at the age of 30 with his funeral being broadcast live on NBC Radio. The Coca Cola company, feeling the mood of the nation, honoured his death with a memorial poster, featuring “Big Red”.
Man o' War was embalmed and buried in a casket lined with his racing silks. He was originally interred at Faraway Farm in Lexington, but in the early 1970s, he was re-interred at a new burial site at the Kentucky Horse Park, where his grave is marked with a statue.
APVMA Approval No. 62490 (Australia) | ACVM No. A010112 (New Zealand)
Available in:
Combination oral antibiotic and mucolytic.
For treatment of respiratory infections caused by TMPS sensitive organisms.
Active Constituents
Sulfadimidine 430 mg/g
Trimethoprim 86 mg/g
Bromhexine Hydrochloride 8.6 mg/g
Pack Size
500g multidose jar
2kg multidose pail
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Randlab’s Bromo Tmps. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
For the treatment of respiratory infections in horses due to organisms susceptible to the combination of sulfadimidine and trimethoprim and where mucolytic activity is also desirable.
MICs of the sulfadimidine-trimethoprim combination vary widely for the range of respiratory pathogens which are of significance in equine respiratory disease. In order to ensure efficacy against the major equine respiratory pathogens, the dose of Bromo Tmps Oral Powder has been calculated to produce drug levels which would be therapeutic against those pathogens with the highest MICs when given twice daily.
Pharmacokinetic trials in horses have established that both sulfadimidine and trimethoprim are readily and rapidly absorbed after oral administration and reach therapeutic plasma levels within approximately 30 minutes of administration.
Trial results demonstrate that the formulation is efficiently absorbed even when administered with food.
Twice daily dosing of Bromo Tmps Oral Powder is recommended in preference to once daily dosing as the minimum (trough) concentrations of sulfadimidine and trimethoprim are potentially subtherapeutic for some sensitive pathogens under a once daily dose regimen.
Bromhexine decreases the viscosity and tenacity of respiratory mucus, facilitating expectoration and suppressing tissue irritation. It also increases the permeability of the alveolor capillary membrane increasing the concentration of antibacterial agents in respiratory secretions.
Administration and Precautions
Administer on damp food with honey or molasses to avoid sifting of powder. Alternatively, Bromo Tmps may be made up into a paste with molasses, honey or Karo (corn) Syrup and administered over the back of the tongue.
Dosage
Dose: 30mg/kg twice daily [e.g. (30mg/kg x 500kg) / (430+86)] = 29g.
Add one level scoop (12g) per 200kg twice daily in feed. Twice daily dosing is recommended to maintain therapeutic levels of active drugs in all situations.
Randlab supports responsible Antimicrobial Stewardship.


Horse Hack: Bromo Tmps can be easily administered as a “powder sandwich” over the back of the tongue. Cut the top off a 60mL syringe at the 2mL mark with a bread knife or hacksaw. Withdraw the plunger to 60mL. Put two teaspoons of molasses, honey or apple sauce in the syringe, followed by the prescribed dose of Bromo Tmps and a further two teaspoons of molasses or honey. Administer to the horse over the back of the tongue. As well as adding some flavouring, the molasses or honey stops the horse spitting out the dose.

DID YOU KNOW THAT... The long-held doctrine that the thoroughbred is based on three foundation Arabian stallions has recently been shown to be incorrect?
TURKOMAN HORSE (EXTINCT)

Thoroughbred studbooks trace the origins of the modern thoroughbred racehorse to three foundation Arabian stallions. These stallions were brought to England and bred to native mares, resulting in an animal with athleticism, speed and strength.
However, new genetic research disputes this traditional belief. The research shows that modern horse Y chromosome lineages split about 700 years ago and can be traced back to a genetic mutation in one stallion or a family of closely related Turkoman stallions. The English Thoroughbred was derived from the Turkoman lineage, which is responsible for the predominance of this haplotype in most modern horse breeds.
The Turkoman horse originated on the steppes of the Turkoman desert and was noted for its endurance, toughness and refined appearance. Ironically, the breed that gave rise to most modern-day horses became extinct in the early 18th century.
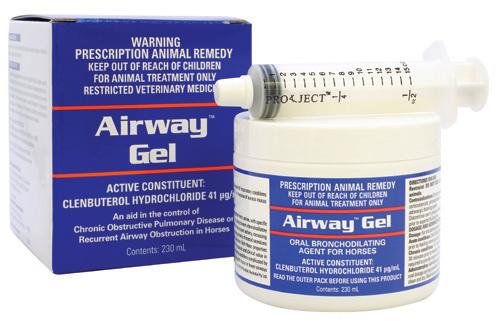
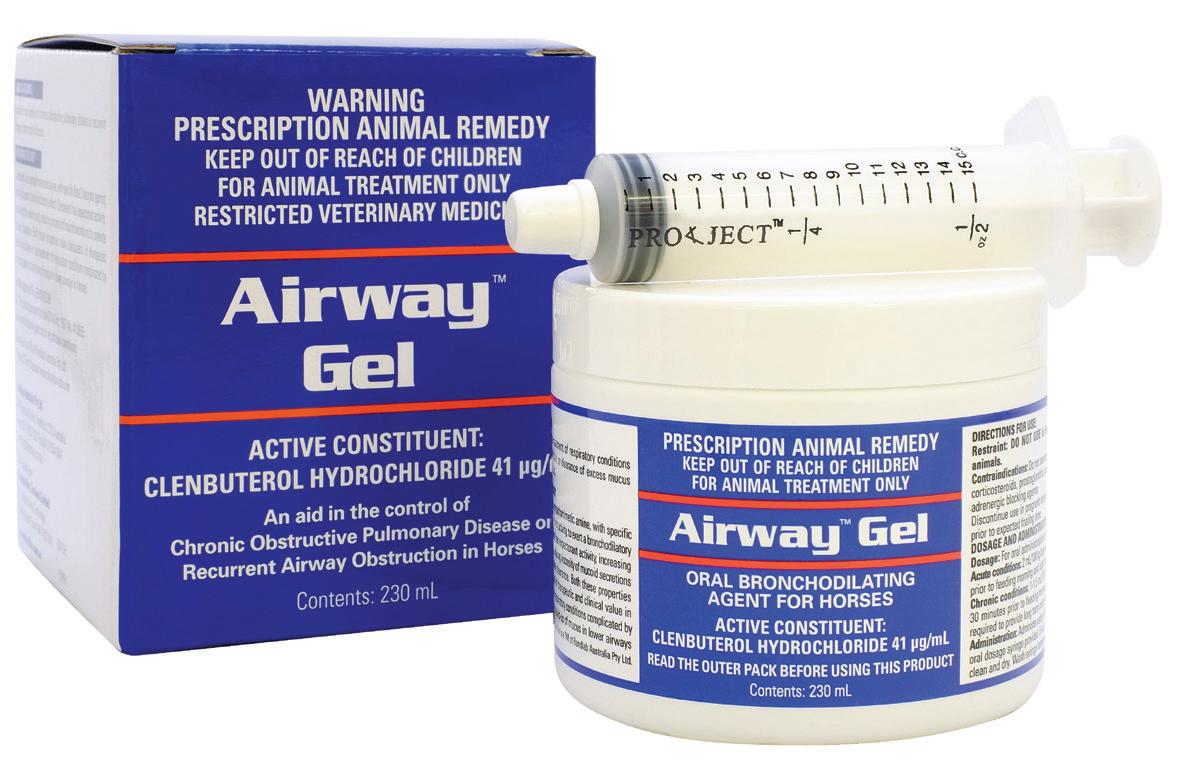
APVMA Approval No. 65910 (Australia) | ACVM No. A010655 (New Zealand)
Available in:
Easy to use gel formulation for bronchodilation and the clearance of airway secretions.
Active Constituent
Clenbuterol Hydrochloride 41 µg/mL
Pack Size
230mL multidose jar
Indications
As an aid in the treatment of respiratory conditions where bronchodilation or clearance of excess mucus would be beneficial. Useful in the treatment of conditions such as Equine Asthma, Inflammatory Airway Disease, Recurrent Airway Obstruction, exercise induced bronchospasm, pneumonitis and bronchopneumonia. Promotes clearance of blood from the respiratory tract following an episode of EIPH.
Pharmacology
Horse Hack: Horses may develop some tolerance to the pharmacological effects of clenbuterol and pulsed administration (e.g. 10 days on, 5-10 days off) is recommended over continuous administration in chronic conditions.
Clenbuterol is a sympathomimetic amine, with specific 2-receptor agonist activity. Clenbuterol has both bronchodilatory and expectorant activity and aids in the clearance of mucus and secretions from the airways.
The prime targets for clenbuterol are the lungs and uterus. This selectively allows intense bronchodilating properties with minimum effect on the cardiovascular system. Clenbuterol promotes airway clearance by increasing the volume and reducing the viscosity of mucoid secretions and increasing the bronchial ciliary beat frequency. It also stabilises mast cells, preventing release of histamine, bradykinin and serotonin.
These properties make clenbuterol of value in the treatment of respiratory conditions complicated by bronchospasm or build-up of mucus, pus or blood in the lower airways of horses.
As clenbuterol dilates airways and increases ciliary beat frequency, Airway Gel is useful for the clearance of blood from the respiratory tract in the days immediately following an episode of EIPH, reducing the risk of secondary bacterial pneumonia.
Discontinue use in pregnant mares at least seven days prior to expected foaling times as uterine contractions may be prevented. Do not use concomitantly with corticosteroids, prostaglandins and other betaadrenergic blocking agents. Clenbuterol may inhibit the effect of PGF2α and oxytocin.
WARNING: Some horses may be initially sensitive to the sympathomimetic effects of clenbuterol. This may manifest as agitation, including “colicky” signs, sweating, increased heart rate and tremors. These symptoms are self-limiting and usually resolve within an hour. The horse may be continued on the Airway Gel at a reduced rate (start with a half dose) for a few days. The dose should then be slowly increased until the horse tolerates the full recommended dose.
Acute conditions: PO 0.8 μg/kg (10mL @ 500Kg BW) bid initially. May be increased up to 3.2 μg/kg in recalcitrant cases. Administer prior to feeding. Chronic Conditions: 1-1.5 mL/100 kg bodyweight 30 minutes prior to feeding morning and night as required to provide long term control of symptoms.


HAVE YOU EVER WONDERED... Who the horse on the Airway Gel box is?



DID YOU KNOW THAT... The World’s biggest “get well” card was signed by 22,000 people in one afternoon in honour of a horse?




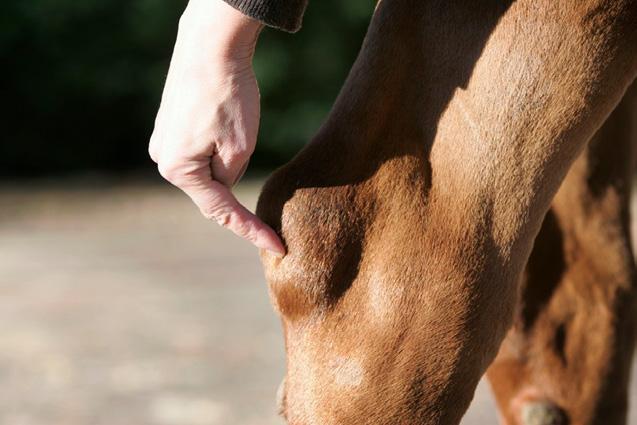



One stop shop for all your anti-inflammatory requirements.
See also Intra-log Injection (triamcinolone acetate) on page 12 of this catalogue.
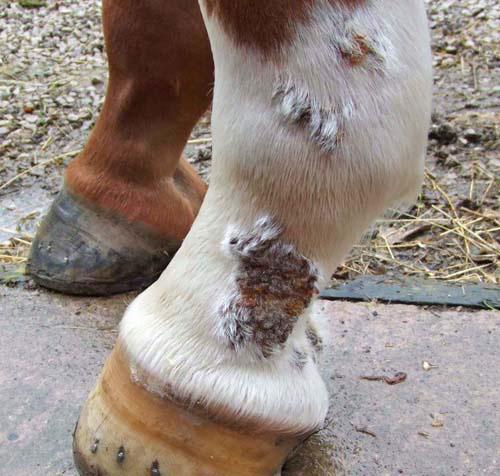









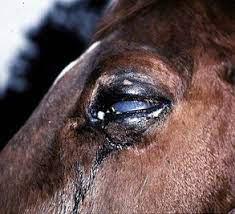

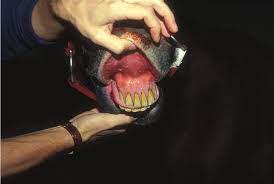











Somedaysyoureallywill need it




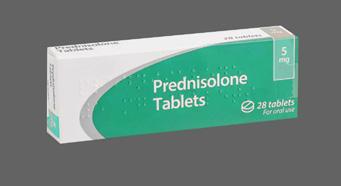
When administered orally dexamethasone provides better absorption than prednisolone granules/tablets.
APVMA Approval No. 89900 (Australia) | ACVM No. A011809 (New Zealand)
Available in:
Potent, short-acting glucocorticosteroid.
Active Constituent
Dexamethasone Sodium Phosphate 5mg/mL
Pack Size
50mL multidose vial

Glucocorticoids have an effect on virtually every cell type and system in the body. The primary pharmacological actions of dexamethasone in the horse are as an anti-inflammatory and immunosuppressive agent. As such, it has been used in the treatment of a wide variety of conditions including:
Anti-inflammatory:
• Orthopaedic conditions. May be used alone or to augment the effect of non-steroidal anti-inflammatory drugs (such as phenylbutazone or meloxicam). Useful in the treatment of conditions such as osteoarthritis, bursitis, tenosynovitis, tendinitis, desmitis, rhabdomyolyis, etc.
• Intra-articularly for inflammatory joint disease or osteoarthritis either in combination with hyaluronic acid or another corticosteroid (eg triamcinolone) or a biologic (eg IRAP or PRP), especially when a post-injection flare is anticipated.
Immunosuppression:
• Equine Asthma including IAD and RAO.
• Dermatitis including allergic dermatitis, pruritis, pemphigus and insect bite dermatitis.
• Auto-immune and immune-mediated diseases such as systemic lupus, rheumatoid arthritis, immune-mediated haemolytic anaemia (IMHA), thrombocytopaenia, polyneuritis equi, purpura haemorrhagica, lupus and other immune-mediated vasculitides.
• Adrenal insufficiency
• CNS disorders and trauma characterised by increased CSF pressure.
• Neoplasia for amelioration of symptoms and secondary inflammation and regression of some lymphoid neoplasias.
• Shock incl endotoxic, anaphylactic, etc.
• Overnight Dexamethasone Suppression Test for diagnosis of PPID.
Dex 5 Injection is an aqueous solution of dexamethasone sodium phosphate (DSP) for intravenous or intramuscular administration. It has powerful anti-inflammatory, antishock and gluconeogenic activity. Its anti-inflammatory and immunosuppressive effects are approximately 25x more potent than cortisol. It has no mineralocorticoid activity.
Glucocorticoids, such as dexamethasone have a broad range of pharmacological and physiological effects. They are the most efficacious anti-inflammatory drugs.
Dexamethasone injection may be given orally with a bioavailability of 42-61% in the fasted horse. Feeding decreases the bioavailability.
DSP may also be administered by nebulisation for treatment of diseases of the respiratory tract, such as Equine Asthma (EA), Inflammatory Airways Disease (IAD) or Recurrent Obstructive Airways Disease (ROA)/heaves. Nebulised DSP has the benefits of minimal systemic bioavailability and not inducing lower airway inflammation or inducing HPA axis suppression whilst still achieving the desired decrease in neutrophil and immune cell counts in BAL fluid.
DSP has also been administered intra-articularly, usually in combination with another corticosteroid (eg triamcinolone), hyaluronic acid or a biologic (IRAP, PRP). It is particularly useful when a rapid, albeit transient, resolution of joint inflammation is required or when a joint flare is anticipated following the IA injection of biologics.
Dexamethasone activity lasts up to 48 hours, although most is excreted in the first 24 hours with a plasma half-life of 3-6 hours.
EXCLUSIVE NEW EXTENDED BROACHING PERIOD (28 DAYS) AND SHELF-LIFE (36 MONTHS)
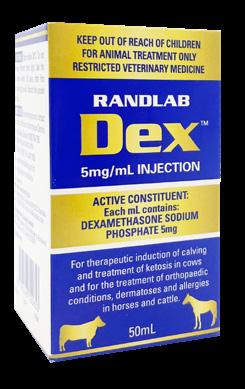
Now approved by APVMA for 28 days usage following the first broaching of the vial. Other dexamethasone 5mg/mL injections are only approved for 24 hours.
Although a direct causal relationship between dexamethasone and laminitis has not been demonstrated, it is recommended that DSP be avoided, especially at higher doses or over a prolonged period, in any horse or pony that may have risk factors for laminitis.
DSP is contraindicated for use in animals with diabetes mellitus, cardiac disease, renal disease and animals with latent or suspected infections unless accompanied by effective antibiotic cover.
Use with caution during the last trimester of pregnancy as may induce parturition. To avoid adrenal suppression, doses should be tapered after long-term administration.
Dosing schedules are based on desired effect and vary according to the condition being treated. The response to dexamethasone is known to vary considerably from horse to horse. Due to DSP’s high potency and multiple untargeted systemic effects, use the lowest dose possible for the shortest period of time to achieve the desired results.
Anti-inflammatory effects: 0.02-0.2mg/kg sid IV or IM.
0.05-0.1mg/kg (5-10mL/500kg BW would be a typical dose).
Immunosuppressive effects: 0.2-0.5mg/kg sid IV or IM.
Shock (endotoxic/anaphylactic): 4-6mg/kg IV.
Vasculitis: 0.05-0.2mg/kg IV or IM q12-24h for 2 weeks, then taper over 4-6 weeks.
Purpura haemorrhagica: 0.04-0.2mg/kg sid or bid.
Respiratory disease such as EA/RAO/IAD: 0.05-0.1mg/kg sid IV or IM for 3-4 days and then taper over 3-4 weeks.
Interstitial pneumonia incl Equine multinodular pulmonary fibrosis: 0.02–0.04 mg/kg IV or IM.
Inflammatory Bowel Disease (IBD): 005-0.2mg/kg IM for 2-4 weeks.
Dermatitis/pruritis/pemphigus: 0.05-0.1mg/kg sid.
Head/CNS trauma: 0.1-0.3mg/kg IV q6-24h.
Uveitis: 2mg q24-36h subconjunctivally.
Immune-mediated Haemolytic Anaemia: 0.05-0.1mg/kg sid IV or IM for 3-5 days then taper over 7-14 days.
Lymphoma: 0.2mg/kg IV for 5 days initially followed by ongoing immunosuppression.
Intra-articularly: 2-4mg/joint usually in conjunction with another corticosteroid, biological (eg. IRAP, PRP) or hyaluronic acid. Use a new bottle.
Overnight Dexamethasone Suppression Test (DMT) for PPID: Collect baseline serum sample in late afternoon. Administer dexamethasone 0.04mg/kg IM. Collect second serum sample ~20 hours post DSP administration. Failure to suppress cortisol levels by < 30mmol/L supports a diagnosis of PPID.
Horse Hack: The oral absorption of dexamethasone is superior to prednisolone. Injectable DSP may be administered orally in the fasted horse at a dose rate of approx. 150% the systemic dose.
Feeding reduces absorption.
The membrane stabilising effects of dexamethasone are valuable in the treatment of acute rhabdomyolysis ("tie up") and results in a rapid decrease in plasma muscle enzymes.
APVMA Approval No. 68303 (Australia)
Available in: P
Rapid onset.
Prolonged effects.
Active Constituents
Phenylbutazone Sodium 200 mg/mL
(equivalent to Phenylbutazone Base 186 mg/mL)
Sodium Salicylate 50 mg/mL
Pack Size
100mL multidose vial
Non-steroidal, anti-inflammatory, antipyretic and analgesic agent for the relief of inflammation and painful musculoskeletal conditions in horses, including:
• arthritis • bursitis
• tendonitis • osteitis

Store between 2° and 8°C (Refrigerate. Do not freeze).
• arthrosis
• tenosynovitis
• laminitis • rhabdomyolysis • skin inflammation
• post-operative pain/inflammation • soft tissue inflammation
Phenylbutazone is a nonsteroidal anti-inflammatory drug (NSAID) with antipyretic and analgesic activity. Sodium salicylate is an antipyretic analgesic agent. The combination of these two drugs provides both rapid action (sodium salicylate) and prolonged effect (phenylbutazone).
Phenylbutazone binds irreversibly to the enzyme cyclooxygenase, inhibiting its activity in the biosynthesis of prostaglandins. Prostaglandins are the primary mediators of the inflammatory response causing tissue swelling and inflammation, pain and fever. Further production of prostaglandin cannot occur until new enzyme is synthesised; resulting in a reduced concentration of prostaglandins at sites of inflammation and reduced inflammatory responses.
The safety of Platinum Bute IV has been evaluated at doses of up to 20 mL/225 kg (Day 1) and 10 mL/225 kg (Days 2-5), without any adverse effect. Store between 2 and 8°C. (Refrigerate do not freeze).
Administration
When used for the relief of febrile conditions, treatment of the underlying cause should be provided concurrently with Platinum Bute IV Injection.
This product is contraindicated for use in horses with serious cardiac, hepatic or renal dysfunction, history of blood disorders (especially haemoglobinuria) or gastro-intestinal disturbances.
Toxic reactions to Platinum Bute IV Injection are uncommon, but may be expressed as oedema, jaundice, blood dyscrasia, gastric irritation or ulceration of the large intestine.
Dosage
Administer by slow intravenous injection of solution at room temperature. Horses and Foals: Initially give 1-2mL/45kg (10-20mL/450kg) per day.
Subsequent doses, depending on response, may be reduced to 0.5-1mL/45kg (5-10mL/450kg) per day. Treatment may be continued for 5-6 days as required.




DID YOU KNOW THAT... International fashion powerhouses Hermés and Gucci both started as saddle makers?
HERMÉS & GUCCI
The French luxury design house Hermés was established in 1837 as a harness workshop in Paris, dedicated to creating high-quality wrought harnesses and bridles for carriage horses used by European noblemen.
In 1880, Hermés moved their premises to Saint-Honoré, Paris where it remains today. With the move, Hermés started selling saddlery and began to retail. By 1914 up to 80 saddle craftsmen were employed.
Soon after, Hermés was granted the exclusive rights to use zippers for leather goods and clothing in Europe. It was not until 1922 that Hermés introduced the first of its iconic leather handbags.
The Hermés logo features a horse and Duc carriage (a roofless carriage with a low open body, once favoured for taking in the city air).

High-end luxury Italian fashion house Gucci was founded in Florence, Italy in 1921. The brand started as a manufacturer of luxury leather luggage as well as equestrian equipment such as saddles.
The Gucci logo is a double G in honour of the company’s founder Guccio Gucci. The logo also symbolises a horse’s bit as homage to the company’s saddlery beginnings.
Both companies continue to make high-end equestrian leather goods.
Saddles generally retail from $12K-$50K.
A case of ‘orse couture.
Available in:
Four convenient pack sizes.
Apple flavoured for increased palatability.
Consistency allows for easy administration and minimal dessication.
Active Constituent
Phenylbutazone 200 mg/mL
Pack Size
250mL jar 1L multidose pail
500mL jar 30mL multidose syringe
Anti-inflammatory, analgesic and antipyretic oral paste for the relief of pain and fever.
The analgesic and anti-inflammatory effects are indicated in the treatment of a wide variety of conditions in the horse including traumatic injuries and postsurgery.
Suitable for the treatment of musculoskeletal conditions including bone and joint disorders, soft tissue injuries and inflammation including tendinitis, acute tenosynovitis, desmitis, myositi (incl rhabdomyolysis), capsulitis, bursitis, acute and chronic laminitis and osteoarthritis.
Pharmacology
Phenylbutazone is a nonsteroidal anti-inflammatory drug (NSAID) with antipyretic and analgesic activity. It binds irreversibly to the enzyme cyclooxygenase, inhibiting its activity in the biosynthesis of prostaglandins. Prostaglandins are the primary mediators of the inflammatory response causing tissue swelling and inflammation, pain, and fever. Further production of prostaglandin cannot occur until new enzyme is synthesised; resulting in a reduced concentration of prostaglandins at sites on inflammation and a reduced inflammatory response.
Phenylbutazone is well absorbed following oral administration. The drug is distributed throughout the body, with highest levels being attained in the liver, lungs, heart, kidneys and plasma. Phenylbutazone is up to 95% plasma protein bound. The increased vascular permeability to plasma proteins during an inflammatory process results in concentration of phenylbutazone in the inflammatory exudate.
The serum half-life of phenylbutazone is dose dependent, ranging in the horse from 3.5 hours at a dose of 2g/450kg to 6 hours at a dose of 8g/450kg. Clinical response to the drug can be seen within a few hours of oral dosage, while optimum effects occur approximately 12 hours post administration. Therapeutic efficacy may last for more than 24 hours, due probably to the irreversible binding of the drug to cyclooxygenase in the tissues.
Phenylbutazone is almost completely metabolised in the horse. The principal metabolites are oxyphenbutazone, with a pharmacological effect similar to phenylbutazone, and the pharmacologically inactive -hydroxyphenylbutazone. APVMA
Horse Hack: Misoprostol (5μg/kg bid) and/or Sucralfate (20mg/kg bid-qid) are recommended for the prevention or treatment of NSAID-induced gastrointestinal ulceration during long-term administration of phenylbutazone.



Ponies and foals exhibit an increased sensitivity to the toxic effects of phenylbutazone.
This product is contraindicated in animals with cardiac, renal or hepatic disorders, blood dyscrasias or pre-existing gastrointestinal ulceration.
Phenylbutazone should not be combined with other NSAIDs or other highly protein bound drugs, such as digoxin, warfarin, and sulfonamides.
Potential adverse effects of phenylbutazone include anorexia, depression, colic, lethargy, weight loss, ventral oedema, oral and gastrointestinal lesions including ulcers and renal papillary necrosis.
If any of these signs occur discontinue treatment immediately.
Horses - Administer orally prior to feeding. 4.4 mg/kg (10 mL/450 kg) bodyweight twice daily for the first day; 2.2 mg/kg (5 mL/450 kg) bodyweight twice daily for the next four days; 2.2 mg/kg (5 mL/450 kg) once daily on alternate days or as directed by a veterinarian. Maximum daily dose should not exceed 4 grams (20 mL).
Ponies - Administer orally prior to feeding. 2.2 mg/kg (2.5 mL/225 kg) bodyweight twice daily for four days; 2.2 mg/kg (2.5 mL/225 kg) once daily on alternate days or as directed by a veterinarian.
Do not exceed the recommended dose, as ponies exhibit an increased sensitivity to the toxic effects of phenylbutazone.

DID YOU KNOW THAT... A slipped saddle cloth robbed an Australian Stockhorse of an Olympic Silver Medal?

APVMA Approval No. 68070 (Australia) | ACVM No. A010915 (New Zealand)
Available in:
A preferential
non-steroidal anti-inflammatory, analgesic and antipyretic,
Increased safety profile.
Decreased withholding time pre-competition.
Active Constituent
Meloxicam 20 mg/mL
Pack Size
100mL multidose vial
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) for rapid initiation of therapy for any condition requiring anti-inflammatory activity, whilst preserving COX-1 function.
Suitable for the treatment of musculoskeletal disorders, including joint, bone and soft tissue injuries and relief of pain associated with colic and ophthalmic conditions.
Meloxicam is effective for treating inflammation and pain associated with surgery and during post-surgery recovery.
Meloxicam Injection may be used as a short course or as an initial loading dose prior to changing to oral meloxicam.
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) of the oxicam class which acts by inhibition of prostaglandin synthesis, thereby exerting antiinflammatory, anti-endotoxic, anti-exudative, analgesic and antipyretic properties. It has preferential COX-2 inhibitory properties, thus preserving the important biological functions of the COX-1 enzyme in the synthesis of regulatory prostaglandins responsible for maintaining a healthy gastrointestinal tract, renal function, platelet function, etc.
Meloxicam provides effective pain relief for colic without impeding recovery of ischaemic bowel.
Avoid use in dehydrated, hypovolaemic or hypotensive horses as there is a potential risk of renal toxicity.
Use with caution in pregnant or lactating mares and in foals under 6 weeks of age as the safety of this product in these classes of horse has not been established.
Use in animals suffering from haemorrhagic gastrointestinal disorders, impaired hepatic, cardiac or renal function and haemorrhagic disorders, or where there is evidence of individual hypersensitivity to the product is contraindicated.
Concurrent administration with glucocorticosteroids, other non-steroidal antiinflammatory drugs or with anti-coagulant agents is contraindicated. Use with caution in conjunction with other highly protein bound drugs.
Horse Hack: The withholding period for Meloxicam Injection prior to competition is considered less than other NSAID’s such as phenylbutazone. If using in performance horses, the regulations of the relevant authorities should be consulted.
Dosage
Horses: 0.6mg/kg BW (3mL/100kg) meloxicam by intravenous injection
Cattle: Single use only by subcutaneous or intravenous injection.
0.5 mg meloxicam/kg bodyweight (i.e. 2.5 mL/100kg BW) in combination with antibiotic therapy, as appropriate.
Pigs: Single use only by intramuscular injection in the anterior half of the neck. (Can be repeated once after 24 hours if necessary).
0.4 mg meloxicam/kg BW (i.e. 2.0 mL/100kg) in combination with antibiotic therapy, as appropriate.
Sheep: Single use only by subcutaneous injection high on the neck behind the ear. For sheep and lambs from 14 days of age. 1.0 mg/kg BW (1.0 mL/20kg) meloxicam.


DID YOU KNOW THAT...
The most famous horse in the world today is technically a pony?
METALLIC CAT AKA “DENVER” (ROCKING P RANCH, USA)

Champion US Cutting horse sire Metallic Cat is probably the most well-known horse in the world today. The red roan stallion never lost a cow in the cutting pen. He has been even more successful as a stallion, where his progeny have amassed over US$60M in prizemoney. He is valued in excess of US$32M. His current breeding fee is US$11K and is limited to 200 mares per season. The American Quarter Horse stallion is now 18 years old and only stands 14.1hh, so is technically a pony. The horse’s celebrity was honoured in 2019 when he appeared as himself in the world-wide TV phenomenon, Yellowstone.
APVMA Approval No. 82561 (Australia) | ACVM No. A011118 (New Zealand)
Available in:
Paste formulation for ease of administration.
High bioavailability in both fed and fasted horses.
Active Constituent
Meloxicam 20 mg/mL
Pack Size
230mL multidose jar
Non-steroidal anti-inflammatory drug (NSAID) for any condition requiring antiinflammatory activity, whilst preserving COX-1 function.
Suitable for the treatment of musculoskeletal disorders, including joint, bone and soft tissue injuries and relief of pain associated with colic and ophthalmic conditions.

Horse Hack: The withholding period for Meloxicam Paste prior to competition is considered less than other NSAID’s such as phenylbutazone. If using in performance horses, the regulations of the relevant authorities should be consulted.
Meloxicam is effective for treating inflammation and pain associated with surgery and during post-surgery recovery.
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) of the oxicam class which acts by inhibition of prostaglandin synthesis, thereby exerting antiinflammatory, anti-endotoxic, anti-exudative, analgesic and antipyretic properties. It has preferential COX-2 inhibitory properties, thus preserving the important biological functions of the COX-1 enzyme in the synthesis of regulatory prostaglandins responsible for maintaining a healthy gastrointestinal tract, renal function, platelet function, etc.
Meloxicam provides effective pain relief for colic without impeding recovery of ischaemic bowel.
Avoid use in dehydrated, hypovolaemic or hypotensive horses as there is a potential risk of renal toxicity.
Use with caution in pregnant or lactating mares and in foals under 6 weeks of age as the safety of this product in these classes of horse has not been established. Use in animals suffering from haemorrhagic gastrointestinal disorders, impaired hepatic, cardiac or renal function and haemorrhagic disorders, or where there is evidence of individual hypersensitivity to the product is contraindicated.
Concurrent administration with glucocorticosteroids, other non-steroidal antiinflammatory drugs or with anti-coagulant agents is contraindicated. Use with caution in conjunction with other highly protein bound drugs.
Dosage
Horses: Daily dose of 0.6 mg/kg bodyweight (i.e. 3.0 mL/100 kg bodyweight) for up to 14 days.
Foals: 0.6mg/kg twice daily dosing is required in foals < 7 weeks old due to more rapid clearance.


Who the horse on the Meloxicam paste and Meloxicam Forte box is?
SECRETARIAT (USA)
The horse nominated by Google as the Greatest Of All Time.
It is also the image on the cover of the annual Randlab catalogue.
Secretariat won the 1973 US Triple Crown, including the Belmont Stakes by an extraordinary 31 lengths.
Overall, the stallion had 21 start for 16 wins and 4 placings. No horse in the history of horse racing has captured the enduring attention of the general public and racing aficionado’s as much as “Big Red”.
The image on the Meloxicam box is an original painting by well-known US horse artist, Tom Chapman. It is from a photo taken of Secretariat at stud.
DID YOU KNOW THAT...

Secretariat was not the first choice of name for the horse rated the Greatest Of All Time?
SECRETARIAT (PENNY CHENERY, USA)

‘Secretariat’ was not the first choice of name for the chestnut yearling son of Bold Ruler and Somethingroyal. If fact it was the sixth choice behind, ‘Scepter’, ‘Royal Line’, ‘Something Special’, ‘Games of Chance’ and ‘Deo Volente’. The name Secretariat was suggested by owner Penny Chenery’s secretary, who once worked for the League of Nations Secretariat in Geneva. SecretARIAT would ultimately give the back part of his name to the world’s largest-selling equestrian brand ARIAT. The company started in 1993 as a boot company applying athletic shoe technology to English and Western Riding Boots. Ariat’s turnover is now in excess of US$500M per year. The company was recently sold to Gap Inc who also own other well known clothing brands such as Old Navy, Banana Republic and of course The GAP.
Available in:
Oral suspension for ease of administration.
Suitable for top dressing in feed.
High bioavailability in both fed and fasted horses.
Active Constituent
Meloxicam 30 mg/mL
Pack Size
250mL multidose bottle
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) for any condition requiring anti-inflammatory activity, whilst preserving COX-1 function.
Suitable for the treatment of musculoskeletal disorders, including joint, bone and soft tissue injuries and relief of pain associated with colic and ophthalmic conditions.
Meloxicam is effective for treating inflammation and pain associated with surgery and during post-surgery recovery.
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) of the oxicam class which acts by inhibition of prostaglandin synthesis, thereby exerting antiinflammatory, anti-endotoxic, anti-exudative, analgesic and antipyretic properties. It has preferential COX-2 inhibitory properties, thus preserving the important biological functions of the COX-1 enzyme in the synthesis of regulatory prostaglandins responsible for maintaining a healthy gastrointestinal tract, renal function, platelet function, etc.
Meloxicam provides effective pain relief for colic without impeding recovery of ischaemic bowel.
Avoid use in dehydrated, hypovolaemic or hypotensive horses as there is a potential risk of renal toxicity.
Use with caution in pregnant or lactating mares and in foals under 6 weeks of age as the safety of this product in these classes of horse has not been established.
Use in animals suffering from haemorrhagic gastrointestinal disorders, impaired hepatic, cardiac or renal function and haemorrhagic disorders, or where there is evidence of individual hypersensitivity to the product is contraindicated.
Concurrent administration with glucocorticosteroids, other non-steroidal antiinflammatory drugs or with anti-coagulant agents is contraindicated. Use with caution in conjunction with other highly protein bound drugs.
Dosage
Shake well before use.
Horses: Daily dose of 0.6 mg/kg bodyweight (i.e. 10mL suspension/500kg BW) for up to 14 days.
Foals: 0.6mg/kg (i.e. 2ml suspension/100kg BW) twice daily for up to 7 days. Twice daily dosing is required in foals < 7 weeks old due to more rapid clearance.

Horse Hack: The withholding period for Meloxicam Oral Suspension prior to competition is considered less than other NSAID’s such as phenylbutazone. If using in performance horses, the regulations of the relevant authorities should be consulted.

DID
YOU KNOW THAT...
A Shetland pony is part of Sydney’s famed café latte set?
HARLEY THE HORSE (ERIC FINDLAY, AUS)

The sleepy suburban Sydney village of Summer Hill is known for its restaurants and cafes and lack of parking. Situated in Sydney’s inner west, it lies a mere 7km from the heart of Sydney’s CBD and exemplifies urban living. It is the not the type of place you expect to hear hoof beats whilst ordering your green matcha latte with oat milk. A winter Sunday afternoon stroll in a local park adjacent to the train line was interrupted by what sounded remarkably like the clopping of tiny feet on the concrete pathway. Somewhat confused, I turned around to see an older man leading a piebald Shetland pony off his pushbike.
I hail down the bike rider and his pony to find out more. But the man (subsequently found to be Eric Findlay) is in no mood for a chat with a stranger about his pony (apparently named “Harley”) and cycles off on his journey to who knows where.
“That’s the Summer Hill pony”, my walking companion, who is a local, declares. “Surely you have heard of him? Even Albo (local Federal member and now Australian Prime Minister, Anthony Albanese) mentioned him in one of his pre-election speeches last month”.
Despite protestations by neighbours and the local council about flies, the smell and “noisy pee” the pony has been living in Summer Hill for about 20 years. The last 17 under a Local Environmental Court Order. Whilst the Albanese Government remains in power, Harley remains a protected urban species.
Available in:
Potent anti-inflammatory for the control of severe intestinal pain and musculoskeletal and ocular disorders.
Suitable for use in horses, cattle, pigs and dogs.
Active Constituent
Flunixin meglumine equivalent to Flunixin 50 mg/mL
Pack Size
100mL multidose vial
For the alleviation of visceral pain and inflammation associated with colic and the normalisation of peristalsis.
For the alleviation of inflammation and pain associated with musculoskeletal disorders.
For the treatment of inflammatory ocular conditions such as uveitis and pre- and post-eye surgery.
At lower doses than used for anti-inflammatory effects, flunixin reduces the haemodynamic changes associated with endotoxaemia/endotoxic shock.
Flunixin is considered to be the most potent cyclooxygenase inhibitor of the NSAID’s. It is also reported to be a more potent analgesic than phenylbutazone or pethidine and to provide comparable analgesic effects to morphine.
Flunixin does not significantly alter gastrointestinal motility, BP or cardiac rhythm. Flunixin has been used successfully to reduce the adverse haemodynamic changes associated with toxic shock by decreasing endotoxin induced lactic acidosis, reducing the severity of arterial hypertension and endothelial cell injury and improving venous return.
Flunixin provides effective anti-inflammatory and analgesia in a wide range of musculoskeletal disorders.
It also results in effective visceral analgesia in cases of equine colic due to flatulence or inflammatory conditions. Flunixin is considered to be a more potent analgesic for the treatment of colic than many of the narcotic or other NSAID’s and opioids.
Intravenous administration of flunixin has been advocated in therapy of ocular inflammatory conditions and may be used pre- and post-operatively to reduce inflammation resulting from ocular surgery and the accumulation of prostacyclin in the aqueous humour. Flunixin alone or in combination with corticosteroids, is a useful treatment for inflammatory eye conditions such as uveitis. Flunixin may also be administered subconjunctivally prior to intraocular surgery or in cases of uveitis or other inflammatory eye conditions.
Flunixin has a short plasma half-life (1.6 hrs) with a peak response reached at 1216 hours after both oral and IV administration and a long pharmacological duration of action (up to 36 hours). Therapeutic effects are maintained even at low plasma concentrations due to irreversible cyclooxygenase binding in the tissues.
Avoid intramuscular administration in the horse.
In horses with colic, flunixin may mask the behavioural and cardiopulmonary signs associated with endotoxaemia or intestinal devitalisation. This must be considered in any pre colic surgery assessment.
In the absence of any specific studies in pregnant animals, such use is not recommended. Concurrent use with other anti-inflammatory drugs should be avoided. Use with caution in animals with renal, hepatic or haematologic disease. Excessive doses or prolonged administration may result in gastrointestinal (right dorsal colon, caecum or stomach) ulceration.
Injection reactions or clostridial myositis may develop following intramuscular injections of flunixin in horses and should be avoided.
Musculoskeletal disorders: 1.1 mg per kg (1mL/45kg) bodyweight one to three times daily by intravenous injection for up to 5 days.
Colic: Alleviation of pain associated with colic: 1.1 mg per kg bodyweight I.V administration is recommended for prompt relief. May be repeated if signs of colic recur. Cause of colic should be determined and treated with appropriate therapy. Endotoxaemia (incl at risk of endotoxaemia): 0.25mg/kg q8h administered IV to interrupt eicosanoid production and inhibit the associated endotoxin-induced haemodynamic effects.
Horse Hack: Colix Injection may be administered orally at a dose rate of 1.1-1.3mg/kg. The injection can be administered mixed with molasses or squirted directly over the back of the horse’s tongue. The onset of action after oral dosing is generally within 2 hours, peak response 12-16 hours and duration of action up to 30 hours.
WARNING: Prolonged detection times leading to a positive drug test following the administration of flunixin have been reported in the horse. This is believed to be due to drug “recycling” with the horse ingesting previously excreted flunixin. It is recommended that boxed competition horses be moved to a different stall after the normal excretion period (~96 hours) to prevent potential reingestion of excreted flunixin.
Sedatives, Anaesthesia and Analgesia 33
An expanding range of sedative and analgesic options to cover every situation from low grade sedation to general anaesthesia.


Available in:
Strong analgesia. Enhanced sedation.
Active Constituent
Butorphanol base as tartrate 10mg/mL
Pack Size
10mL multidose vial
50mL multidose vial
*when used in conjunction with an alpha-2 agonist.
Butorphanol tartrate is a synthetic, mixed opioid agonist/antagonist with analgesic and sedative properties. It is generally used in combination with a sedative (such as an alpha-2 agonist) to avoid initial excitatory activity. Butorphanol has wide application in equine veterinary practice including:
• Restraint during standing surgery
• Restraint during minor surgical procedures
• Restraint during diagnostic procedures (eg yearling repository x-rays)
• Relief of pain associated with colic for up to four hours
• Anaesthetic pre-medication in combination with an α2-agonist
• Component in balanced general anaesthesia (esp for painful procedures)
• Post-operative and general pain management
• Potent anti-tussive
Butorphanol Injection (butorphanol tartrate) is a totally synthetic, centrally acting, narcotic agonist/antagonist with analgesic, sedative and antitussive activity. At higher doses it is analgesic and at a lower dose it has a predictable sedative effect. Following IV doses, the onset of action is approximately 3 minutes with a peak analgesic effect at 15-30 minutes. The duration of action in horses is typically 1-2 hours but may last up to 4 hours after a single dose (esp in combination with an alpha-2). The dose may be repeated as required every 3-4 hours, but it is not recommended that treatment be continued beyond 48 hours.
Butorphanol at the recommended dose rate causes minimal cardiopulmonary depression. Furthermore, the cardiopulmonary effects of butorphanol are not linearly dose related and reach a plateau beyond which further dosage increases result in relatively lesser effects.
Analgesia; Butorphanol is useful for the relief of pain associated with colic, postpartum treatment of mares, surgical sedation (both standing and as a pre-anaesthetic agent), castration and many other veterinary procedures.
When used for the relief of pain, butorphanol may be used alone or in combination with tranquilisers, such as xylazine, detomidine, etc. It begins to alleviate pain within 15 minutes of administration and can be effective for up to four hours. It is five times more effective in controlling pain than morphine.
In horses with colic; Butorphanol provides prompt analgesia within 15 minutes of injection, thus facilitating a thorough examination and treatment and limiting self-inflicted injury. The level of pain relief is not so strong or long-lasting to mask the need for surgery. Butorphanol also produces fewer changes in gastrointestinal function and motility than other opioids. Compared with an IV bolus, a constant rate infusion of butorphanol induces fewer GI side effects while providing good analgesia. Sedation; Butorphanol is also used for enhancement of sedation in combination with another sedative, such as xylazine or detomidine for standing chemical restraint and as part of premedication protocols for general anaesthesia.
Cough suppressant; Butorphanol may also be used as a cough suppressant where it acts centrally to reduce cough and may therefore also be useful in procedures such as tracheal washes and bronchoalveolar lavage (BAL). APVMA Approval No. 87965 (Australia)

Intravenous dosing of butorphanol in the unsedated horse has been associated with excitement, increased ambulation, ataxia, increased response to auditory stimuli and decreased gastrointestinal motility.
WARNING: Butorphanol should not be used in patients with liver disease as the drug cannot be efficiently eliminated and may accumulate to toxic levels.
Animals with diseases of the lungs characterised by excessive mucus production should not receive butorphanol as it may prevent removal of mucus by cough.
Butorphanol depresses brain function and should not be used in patients with injuries to the head, those in a coma or with an increase in CNS pressure.
Butorphanol should be used with caution in animals that have kidney disease, preexisting cardiac dysrhythmia or bradycardia.
Safety in breeding stock and foals has not been proven.
Do not mix with other products in same syringe.
Butorphanol may be administered intramuscularly at a higher dose and/or more frequently as systemic absorption via this route is only about 37%. However, dose and dosing interval should be based on the response to treatment.
Analgesia: 0.1-0.4mg/kg q3-4h IV or 0.04-0.2mg/kg IM.
CRI: Initial loading dose 17.8µg/kg followed by 13-24µg/kg/hr.
COLIC PAIN: 0.01-0.02mg/kg IV alone or in combination with an alpha-2 agonist (eg xylazine 0.02-0.1mg/kg IV).
Sedation: ALONE; 0.1-0.13mg/kg (=1-1.3mL/100kg BW) by intravenous injection q34h. IN COMBINATION; 0.02-0.05mg/kg IV in combination with xylazine (0.5-1.0mg/kg), detomidine (10-40 µg/kg), romifidine (80-100 µg/kg) or acepromazine (0.02- 0.05mg/ kg).
PRE-ANAESTHETIC; 0.01-0.04mg/kg IV following administration of an alpha-2 agonist (eg xylazine 0.1-0.5mg/kg IV).
FIELD ANAESTHESIA: Sedate with xylazine (1mg/kg IV or 2mg/kg IM) followed by butorphanol (0.02-0.04mg/kg IV). Wait until horse heavily sedated (~10 minutes) before administering ketamine (2.2mg/kg IV). Combination improves induction, increases analgesia and increases recumbency time by 5-10 minutes
Antitussive: 0.02 mg/kg IM bid-tid.
Foals: Alone; 0.1-0.2mg/kg IV or IM. In Combination; 0.5 mg/kg xylazine followed by 0.04 mg/kg butorphanol.
Horse Hack: Premedication with butorphanol reduces cough associated with invasive airway procedures such as bronchoalveolar lavage (BAL) and tracheal washes.
APVMA Approval No. 87454 (Australia)
Available in:
A dissociative anaesthetic and analgesic for horses.
Active Constituent
Ketamine (as Hydrochloride) 100mg/mL
Pack Size
50mL multidose vial
Ketamine-based anaesthesia is the most common anaesthetic technique for short procedures in the field. The duration of anaesthesia is generally less than 30 min.
Ketamine-based anaesthesia can also be used as an induction agent prior to intubation and inhalation anaesthesia. Intravenous anaesthesia may also be maintained following α2-agonist + ketamine induction with “Triple Drip” Continuous Rate Infusion (CRI). See Dosage section.
Ketamine also inhibits NMDA-receptors so may be adjunctively useful to control pain.
Ketamine anaesthesia is characterised by analgesia, normal pharyngeallaryngeal reflexes, mild cardiac stimulation, respiratory depression and increased sympathetic tone. Protective reflexes (such as coughing, swallowing and corneal) are maintained and the eyes normally remain open with a dilated pupil. Nystagmus is common.
Ketamine will increase heart rate and blood pressure due to increased sympathetic tone. This results in an increase in cardiac output compared with other anaesthetic agents. Ketamine has a wide therapeutic index (e.g. 5x pentobarbital).
Ketamine has been shown to cross the placenta, and depression of neonates may occur if ketamine is used for induction of anaesthesia for caesarean section.
Recovery from ketamine-based anaesthesia is considered to be good and superior to thiobarbiturate or inhalation anaesthesia.
Administration and Precautions
Ketamine should always be used following sedation with an α2-agonist. Do not use alone for anaesthesia as may cause hyperexcitability and involuntary muscle reactions.
Ketamine may cause pain at the injection site if administered intramuscularly. Ketamine-based anaesthesia should not be used as the sole anaesthetic mode for major surgery and should always be supplemented by gaseous or barbiturate anaesthetics or muscle relaxants.
This product is contraindicated for use in horses with prior hypersensitivity to ketamine, renal or hepatic insufficiency, history of epilepsy, cardiovascular insufficiency, myocardial damage or serious cardiac arrhythmia or horses with glaucoma, hypovolaemic animals, patients prone to seizures or with cerebral trauma or intracranial lesions.
Horses should not be fed until they have fully recovered from general anaesthesia due to the risk of oesophageal choke.
Dosage
Sedatives other than xylazine may be used prior to ketamine anaesthesia, e.g. detomidine (0.02 mg/kg) or romifidine (0.1 mg/kg).
Induction of anaesthesia: Premedicate with an α2-agonist (e.g. xylazine 1.1 mg/kg IV or 2.0 mg/kg IM) given 5-10 minutes before induction of anaesthesia with ketamine. Horse must be adequately sedated before administering the ketamine (2.2 mg/kg IV). Total anaesthetic time is short but can be prolonged by administering one-third to one-half of the induction dose or by instigating “Triple Drip” CRI (see below).
Butorphanol (0.02-0.04 mg/kg IV) may also be added to the original xylazine in highly strung horses (such as young horses, Thoroughbreds or Arab) 5-10 minutes prior to Ketamine administration.
Diazepam (0.03 – 0.1 mg/kg IV) may also be administered along with xylazine prior to ketamine to improve induction, improve muscle relaxation during anaesthesia and prolong anaesthesia by about 5-10 minutes.
Field anaesthesia/“Triple Drip”: Add 500mg xylazine and 2000mg ketamine to 1L of 5% guaifenesin in dextrose or 0.9% saline.
INDUCTION: 1.1 mL/kg IV rapidly.
MAINTENANCE: 2.0-4.5 mL/kg/hr to effect. “Triple drip” should not be used for anaesthetics greater than 1 hour in duration unless oxygen supplementation and respiratory support is provided.
For foals & ponies: Add 250mg xylazine and 500mg ketamine to 500mL 5% guaifenesin solution. For induction give 1.1 mL/kg IV rapidly. For maintenance 2-3mL/kg/hr to effect.
CRI for analgesia: Loading dose of 0.6 mg/kg IV followed by CRI of 0.4-0.8 mg/ kg/hr. Can be increased to 1.2mg/kg/hr if required. To prepare CRI solution, add 30mL ketamine to 1L bag of saline (= 3mg/mL) and administer IV over 8 hours at a rate of 125 mL per hour for a 500kg horse.
Foals treatment of seizures: 0.02 mg/kg/min CRI.



APVMA Approval No. 82623 (Australia) | ACVM No. A011244 (New Zealand)
Available in:
Rapid prolonged and potent sedation.
For horses and cattle*.
(*NZ Only)
Active Constituent
Detomidine Hydrochloride 10mg/mL
Pack Size
20mL multidose vial

Sedator is a dose controlled sedative and analgesic. It may be used to facilitate examinations, x-rays, minor surgical procedures, transport etc. It may also be used to control pain including that of the uncomplicated equine colic case. Sedator has a role in providing prolonged analgesia (up to 12 hours) in horses.
Sedator is a sedative and analgesic that may be used to facilitate:
• Examinations e.g. endoscopy, rectal palpation, gynaecological examinations, lameness examinations and all diagnostic imaging procedures.
• Minor surgical procedures e.g. debridement and suturing of wounds, removal of skin tumours, hoof care and castration.
• Pre-anaesthetic sedative when used in combination with anaesthetic agents
• Treatment and procedures in young or fractious horses e.g. nasogastric tubing, clipping and shoeing.
• Effective analgesia in treatment of colic cases.
• Sedation and analgesia in the management of serious injuries.
• Dental examinations and procedures.
• Safe handling of fractious animals.
0
WARNING: Intravenous potentiated sulphonamides are contraindicated in sedated or anaesthetised horses as potentially fatal dysrhythmias may occur.
Sedator is not recommended for use in pregnant mares.
Sedator may cause penile relaxation. Use with caution in male animals, particularly stallions during the breeding season.
Clinicians should anticipate the tendency of the horse’s head to drop under the influence of detomidine and the horse should be accommodated appropriately. Immediately following administration there may be a tendency to stagger, particularly if high dose rates are used.
The safe handling of horses can be improved with Sedator. Although the animal is easy to handle under detomidine sedation, normal precautions are recommended. Local anaesthetics or additional analgesia should be used when required for painful procedures, particularly surgery, as the duration of analgesia from Sedator may not equate with the duration of sedation.
Horse Hack: Horses should not be fed until they have fully recovered from sedation due to the risk of oesophageal choke.
A risk/benefit assessment should be undertaken by a veterinarian before using Sedator in animals suffering from cardiac disease (with pre-existing bradycardia and risk of atrioventricular block), respiratory, liver or renal insufficiencies or shock.
Overdosage and Side Effects: Sympathomimetic properties, particularly at higher doses, may include piloerection, sweating, diuresis & occasional slight tremors. The symptoms may be relieved by atropine or a specific alpha-2 antagonist (e.g. tolazoline).
Dosage should be determined depending on the desired depth and duration of sedation required, temperament of the horse and concurrent environmental stimuli.
LOWER DOSES MAY BE ADEQUATE.
Dosage: 0.1 mL to 0.8 mL per 100 kg bodyweight. Administration is by slow intravenous or the intramuscular route.
For analgesia in COLIC until diagnosis is confirmed, recommended dose is 20-40 μg/kg BW (0.2-0.4 mL/100 kg BW).
The full analgesic effect is established by 5-15 minutes following administration. If the desired level of sedation is not achieved following administration of a low dose, a further additive dose may be given.
The dose response can be estimated as follows:
Teetering, sweating, piloerection, muscle spasms
Constant Rate Infusion (CRI): Constant Rate Infusion (CRI) for analgesia: Loading dose 0.01mg/kg followed by 15mg detomidine (1.5mL) in 1L of Ringers Solution administered at a constant rate infusion of 0.6μg/kg/min. This equates to approximate 6 drops/second IV. Adjust to effect.
APVMA Approval No. 87320 (Australia) | ACVM No. A011802 (New Zealand)
Available in:
Quick acting, short duration sedation and analgesia.
Active Constituent
Xylazine (as hydrochloride) 100 mg/mL
Pack Size
50mL multidose vial

Randlab Xylazine 100 is an injection containing 100 mg/mL (10% w/v) of Xylazine for use as a sedative, analgesic and skeletal muscle relaxant in horses.
Randlab Xylazine 100 is an extremely effective sedative in the horse, allowing a number of procedures to be more easily conducted e.g. ophthalmic, dental or endoscopic examinations, wound suturing, rectal examination, stomach tubing, examination of fractious patients (incl lameness exam), diagnostic imaging, bandage application and removal etc. Xylazine in combination with appropriate local anaesthesia may be suitable for minor standing surgeries such as castration, dental procedures, draining abscesses, etc.
Xylazine may provide short acting analgesia in cases of colic facilitating examination and transport. The visceral analgesia effect of xylazine has been demonstrated to be superior to that produced by butorphanol. Xylazine decreases digital blood flow for up to 8 hours after administration.
Randlab Xylazine 100 is also a very effective premedicant for both ketamine and barbiturate anaesthesia.
Randlab Xylazine 100 produces dose dependent sedation and analgesia through an alphamimetic and direct opioid effect. It also produces muscle relaxation by inhibition of the intraneuronal transmission of impulses centrally in the CNS.
Onset of action is rapid with effective sedation present within 3-5 minutes after intravenous injection and within 10-15 minutes following intramuscular or subcutaneous administration.
The sedative effect usually lasts 1-2 hours, while the analgesic effect only lasts 30-40 minutes. The usual response to Xylazine 100 administration in the horse is drooping of the head, ataxia without recumbency, penile prolapse which does not progress to paraphimosis, and pendulous lower lip. Complete recovery varies with the dose administered, however, it is usually uneventful and requires minimal supervision.
Xylazine causes cardiopulmonary depression manifested by a decrease in heart rate, stroke volume, cardiac output and respiratory rate together with an increase in total peripheral resistance. These effects are transitory and of no clinical significance in healthy horses. However, it is not recommended to use xylazine where there is cardiovascular disease including conditions of shock, or respiratory depression.
A number of cardiac dysrhythmias such as second degree AV block have been encountered following xylazine administration due to increased vagal tone. These are largely transient and benign.
Horses should not be fed until they have fully recovered from sedation due to the risk of oesophageal choke.
Horse Hack: Inadvertent intra-arterial (e.g. carotid artery) administration of xylazine will result in extreme excitement and convulsions. The horse will normally recover within 10 minutes but is at high risk of sustaining an injury whilst convulsing.
WARNING: Some horses may exhibit “xylazine rage” following administration of the drug. This usually manifests as aggression, such as kicking or biting, especially after any sudden or unexpected moves. “Xylaine rage” represents a risk to handlers. It can be minimised by using the lowest appropriate dose of xylazine or by combining xylazine with either acepromazine or butorphanol.
Horses: Dose dependent effect: 2.0-5.0mL per 450kg BW by slow IV injection. Draft breeds are more sensitive to the effects of xylazine and the dose should be lowered accordingly.
Sedation: 1.1 mg/kg IV or 2.2 mg/kg IM. Allow animal to rest quietly until full effect is reached.
Colic: 0.2-0.5mg/kg IV will provide analgesia for 20-30 minutes. For longer duration, 0.6-1mg/kg IM will provide analgesia for 1-2 hours.
CRI for analgesia: 0.5-1.1 mg/kg IV followed by 0.72 mg/kg/hour.
Caudal epidural: 0.03-0.35 mg/kg in first coccygeal space. 3-5 hour duration of effect.
Field induction: 1.1mg/kg IV. Wait until full sedation is achieved (5-10 min). If adequate sedation does not occur, re-dose with half the original dose of xylazine. Then follow with 2.2mg/kg IV of ketamine. Butorphanol (0.02-0.04mg/kg IV) or diazepam (0.03 - 0.1mg/kg IV) may be added before the ketamine for additional sedation, analgesia or muscle relaxation respectively.
“Triple drip” anaesthesia: Add 500mg xylazine and 2000mg ketamine to 1L of 5% guaifenesin in dextrose or 0.9% saline.
Induction: 1.1 mL/kg IV rapidly.
Maintenance: 2.0 - 4.5 mL/kg/hr to effect. “Triple drip” should not be used for anaesthetics greater than 1 hour in duration unless oxygen supplementation and respiratory support is provided.
Foals & Ponies: Add 250mg xylazine and 500mg ketamine to 500mL 5% guaifenesin solution. For induction give 1.1 mL/kg IV rapidly. For maintenance 2-3mL/kg/hr to effect.
Reversal: Tolazoline, atipamezole or yohimbine may be used alone or in combination to reverse the effects of xylazine or speed recovery.

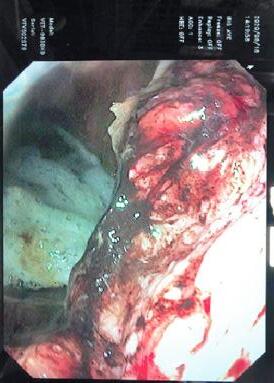
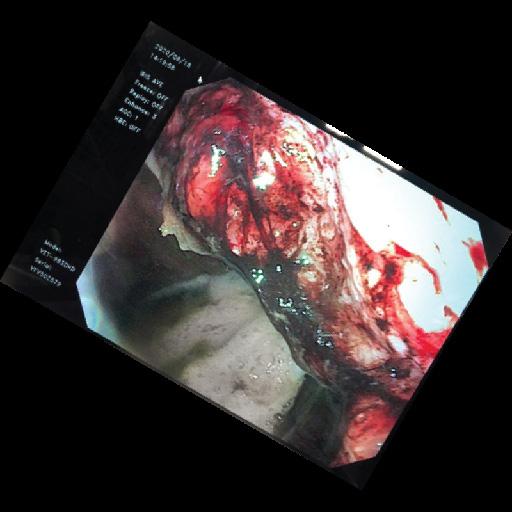
Always administer ulcer pastes on a relatively empty stomach. This will usually be first thing in the morning
Don’t allow the horse to chew on Gastropell paste (not an issue with Ulcershield)
Wait 30-60 minutes before feeding
Feed horse (at least 2kg/3L roughage or hay) prior to exercise or travel
There is no need to fast the horse overnight















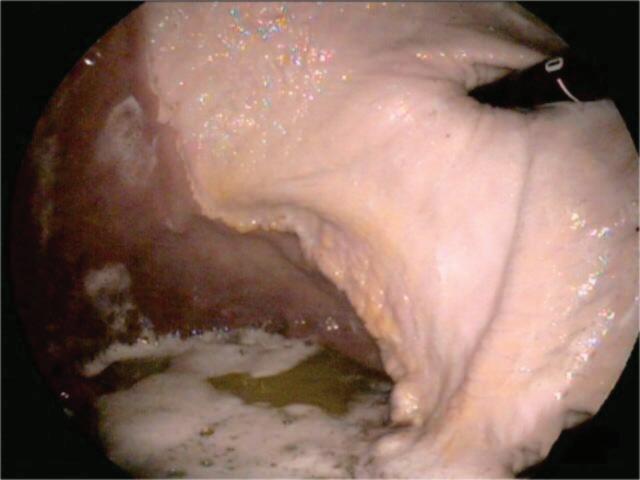

Gastroscopy is the only way to accurately diagnose gastric ulcers, make the important differentiation between squamous and glandular/pyloric lesions, grade any lesions, determine the appropriate treatment course and monitor any treatment.
Gastric ulcers adversely affect horse's performance as well as appetite, wellbeing and behaviour.
Horses naturally decrease their feed intake between about 10:00PM and dawn.
Food in the stomach (esp. hay) will inhibit the absorption of ulcer pastes.

Horses should always be fed some roughage (~ 2kg/3L) before working or traveling. This food forms a slurry or ball in the stomach which prevents acid splash.
Horses should be fed 1-2% dry weight of their bodyweight in roughage/hay per day (5-10kg for a 500kg horse). This helps prevent gastric ulcers, maximises hindgut health and minimises boredom.
Lucerne hay is the hay of choice as it has added acid buffering capacity. Ideally hay should be fed using a slow hay feeder.
APVMA Approval No. 80410 (Australia) | ACVM No. A011152 (New Zealand)
Available in:
Sedation in a convenient oral gel.
Active Constituent
Acepromazine Maleate 12 mg/mL
Pack Size
30mL multidose syringe
• An aid in training fractious horses.
• To calm nervous horses and reduce stress.
The required dosage of SED-Ace Oral Tranquiliser Gel may vary depending on the individual temperament of the animal, the level of sedation required and concurrent environmental stimulus.
• In minor surgical procedures in which a general anaesthetic is not required.
• To sedate horses that will not allow IV or IM sedation.
• In non surgical procedures such as shoeing, clipping and dentistry.
• Handling mares during mating or other gynaecological procedures.
• Calming of horses to assist in lameness examinations and ensure consistency of gait.
• Horses that have wounds which cannot heal due to constant irritation (biting, licking etc.)
• After major surgery to reduce straining.
• Useful aid in the treatment of colic and tetanus.
• To calm horses during transport.
• To calm horses during turn out.
• As a hypotensive agent and to improve peripheral blood flow.
SED-Ace Oral Gel (acepromazine maleate) provides dose-dependent tranquillisation, relaxation and sedative effects. It provides a safe, convenient and effective method of tranquillising and sedating horses.
Low doses of oral acepromazine maleate (ACP) promote a mild tranquillising effect, useful as an aid in quietening excitable, nervous and hard-to-handle horses. It can provide a beneficial “calming” effect during handling, training, transport and confinement. It reduces excitability, whilst retaining co-ordination and awareness of environment.
Acepromazine maleate also mediates hypotensive, hypothermic and antispasmodic actions. It has little, if any, analgesic effect, so that painful procedures must be avoided unless accompanied by an analgesic, particularly where animals are known to have unpredictable temperaments.
On an empty stomach, the tranquillisation effect will be apparent in horses in 20 - 40 minutes, and with a duration of up to 3 hours. On a full stomach, the full tranquillisation effect will be apparent within 40 - 60 minutes or longer.
The side effects of oral ACP are less than with intravenous administration. During sedation, horses will normally retain visual and auditory function, so that loud sounds or rapid movements should be avoided. Exceeding the recommended dosage will not necessarily intensify the sedative effects of Sed-Ace Oral Gel.
Paralysis of the retractor penis muscle has been associated with the use of intravenously administered acepromazine maleate in horses. When this occurs steps should be taken immediately to reduce any penile swelling and return the penis to the prepuce.
Due to the risk of potential irreversible penile prolapse, avoid using in male horses (stallions and geldings) or use the lowest dose possible to produce the required effect.
In some horses, lower doses of SED-Ace may be adequate.
Dose will also vary depending on desired level of sedation.
Horses: To be given orally. 2-10 mL per 450 kg bodyweight (0.05 to 0.26 mg per kg body weight).
Horse Hack: For best results, avoid excitement or stimulation of the animal prior to administration and preferably administer on an empty stomach.


DID YOU KNOW THAT... Japan’s burgeoning thoroughbred breeding industry had its roots in a failed crop harvest?
INTAGLIO (KOIWAI FARM, JAPAN)

Japan is undoubtedly the most prosperous racing country in the World. All the more remarkable given that the first thoroughbreds were only introduced into Japan in 1907.
A stallion named Intaglio and 20 mares were imported by sea into Japan from the UK by Koiwai Farm in Shizukuishi, Iwate Prefecture. The Koiwai Farm is better known these days as a gourmet dairy brand.
The farm was established in 1892 as a cropping farm. But the soil quality proved to be too poor and eight years later the operation’s focus shifted to livestock.
In 1905 the Japanese Government gave “informal permission” for betting on horse racing. Horses were still the main means of transportation in Japan at that time and any horse was eligible to race.
One of the partners in Koiwai Farm was Yanosuke Iwasaki, second president of Mitsubishi Corp. It was he who selected the British thoroughbreds for importation, with Anglo-Arabs also being put up as an alternative. The entire course of Japanese horse racing may have been very different had Iwasaki elected to import the Anglo-Arabs instead. When betting was again rescinded in 1908, the Koiwai Farm survived by selling thoroughbred x hackney crossbreds.
Koiwai Farm went on to produce six Japanese Derby winners, including St Lite who was the first horse to win the Japanese Triple Crown in 1941.
APVMA Approval No. 88500 (Australia)
Available in:
Low tissue irritation.
Convenient 20mL vial size for less wastage.
Active Constituent
Prilocaine Hydrochloride 20 mg/mL
Pack Size
6 x 20mL multidose vials
Prilocaine causes less tissue irritation, has a faster onset of action and longer duration of action compared to other local anaesthetics such as lignocaine and mepivicaine.
Indications
Medicaine is indicated whenever local anaesthesia is required, including:
• Infiltration anaesthesia
• Nerve blocks
• Intravenous regional limb perfusion
• Standing surgeries including arthroscopy, laparoscopy, standing laparotomy, fracture repair, genital/perineal surgery, hoof resections, castrations, respiratory surgery (incl laryngoplasty and sinus surgery), "kissing spines" surgery
• Topical anaesthesia including minor dermatological procedures and wound debridement, repair and suturing
• Biopsy/excision/debulking of skin tumours including sarcoids, melanomas, SCC, etc.
• Dentistry including fixation of broken jaws, tooth extraction
• As an adjunct to general anaesthesia to reduce the dose of parenteral/inhaled anaesthetic agents
• Post-operative pain management
• Bronchoalveolar lavage (BAL) to reduce cough
• Skin grafts
• Biopsies (skin, liver, muscle, etc)
• Rectal relaxant prior to rectal examination
• Foal transphyseal screw placement
• Relief of back muscle pain/spasm
Pharmacology
Prilocaine, the active ingredient in Randlab Medicaine Injection, is a synthetic, sterile, isotonic local anaesthetic agent of the amide class. It has low tissue irritability compared to many other local anaesthetics. It inhibits nerve conduction via sodium channel blockade.
Prilocaine shows fast onset (within minutes) and intermediate duration of action (100-130 minutes). Prilocaine metabolism is similar to lidocaine, being rapidly metabolised by the liver and excreted in the urine.
Prilocaine shows the least systemic toxicity of all the amide local anaesthetics. It has low cardiac and neurological toxicity compared with other amide local anaesthetics and therefore is commonly used for intravenous regional limb perfusion.
Administration and Precautions
Discard unused portion within 24 hour of first broaching. A new bottle should always be used for any aseptic technique or whenever a sterile surgical field is required.
injection.
Horse Hack: Intra-rectal administration of prilocaine is an effective rectal relaxant prior to performing a rectal examination or rectal surgery. A dose of 50-60mL is usually administered via syringe after inserting a gloved hand through the anus.
Many local anaesthetic agents, including prilocaine, possess antimicrobial activity and synergistic activity when used in conjunction with some antibiotics (check compatibilities). Consider using a combination of prilocaine and an appropriate antibiotic to reduce MIC’s of known equine pathogens in procedures such as joint injections for septic arthritis or regional perfusions for sepsis.
The dose of Medicaine should be adjusted according to the route of administration, the site of administration and the response of the patient. The smallest dose producing the required effect should be given.
WARNING: There are currently no local anaesthetic agents registered with the Australian Pesticides & Veterinary Medicine Authority (APVMA) for intra-articular use. Although these agents are commonly used by intra-articular injection (eg joint block, joint surgery, etc), veterinarians should be aware that this is an offlabel usage. This applies to prilocaine, mepivacaine and lidocaine/ lignocaine.

DID YOU KNOW THAT...

In the late 80’s Harvey Wallbanger went on to become a sprinting sensation winning 79 of 93 starts?... Oh, and did I mention he was an American Bison?
HARVEY WALLBANGER (COLLIN THORSTENSON, USA) See page 71 for the full story

Scan the QR code for more information on equine euthanasia preparedness. Miscellaneous Suite

Scan the QR code for more information on equine euthanasia preparedness.
APVMA Approval No. 88008 (Australia)
Available in:
Used to induce euthanasia smoothly and quickly with minimal discomfort to the animal.
Active Constituents
Pentobarbitone Sodium 300 mg/mL (equivalent to 273 mg/mL pentobarbitone)
Pack Size
480mL Vial
WARNING: Lethaton should not be used for general anaesthesia.
Scan the QR code for more information on equine euthanasia preparedness.
Pentobarbital or a pentobarbital combinations are the principal choice for equine euthanasia by chemical means.
Lethaton Euthanasia Solution (Pentobarbitone sodium) is for the humane euthanasia of all animals. From the class of drugs known as barbiturates, it is used to induce euthanasia smoothly and quickly, with minimal discomfort to the animal.
“Euthanasia” is a Greek term meaning “good death”. This objective is met when death is induced which causes no pain or distress to the animal. To achieve this goal the techniques used should result in immediate loss of consciousness followed by cardiac and respiratory arrest ultimately resulting in loss of brain function and death. The drugs must act quickly and effectively. Veterinarians who perform this task must be technically proficient and well prepared.
Intravenous injection of a barbituric acid derivative (eg, pentobarbitone) is the preferred method for euthanasia for most domestic animal species. Barbiturates administered IV may be given alone as the sole agent of euthanasia or as the second step after sedation or general anaesthesia.
Most commonly, euthanasia is achieved by injecting a barbiturate anaesthetic in a dose sufficient to shut down the horse's central nervous system. The drug renders the horse unconscious, the horse's heart stops beating, and the horse stops breathing.
Following intravenous injection of Lethaton, there is a rapid onset of action and progressive loss of consciousness, proceeding through anaesthesia, deep anaesthesia, apnea due to depression of the respiratory centre, and cardiac arrest. A typical timeline would be:
1) Injection time < 17 seconds
2) Collapse < 26 seconds
3) Absence of auscultable heartbeat < 84 seconds
4) Lack of corneal reflex < 160 seconds
Horses show variability in how they react during the euthanasia procedure. Most horses simply drop and lay still, maybe taking one or two deep agonal breaths before expiring. Some horses continue to take occasional breaths for a minute or so, and there may be some excitatory movement of the limbs such as paddling or rigors. Even though the horse is deeply unconscious and may no longer have a heartbeat, seeing these apparent signs of life may be upsetting for some owners. However, they do not indicate that the horse is conscious or has any sense of feeling; they are simply involuntary reflexes by the body in its final moments.
Must not be used in animals intended for food purposes for either human or animal consumption including inadvertent wildlife consumption.
Onset of action may be delayed if the horse is (i) excited, agitated or in pain at the time of administration (ii) has been premedicated with a tranquiliser that decreases cardiac output or (iii) has heart failure.
Aesthetically disturbing terminal gasping, vocalisation and excitatory muscular activity (such as paddling and rigor) may occur in the unconscious animal following barbiturate euthanasia. These may be distressing to observers. Barbiturates persist in the animal’s remains and may cause sedation or even death of animals that consume the body. Carcasses from barbiturate euthanasias

remain a hazard for wildlife and pets that may consume them, aquatic animals (if carcass is disposed of near a waterway) and the environment.
For equids euthanised with pentobarbital, disposal of remains must be carried out promptly through on-farm burial, incineration or cremation, direct haul to a solid waste landfill, or biodigestion. This will help prevent exposure of wildlife and domestic animals to potentially toxic barbiturate residues. Disposal of remains must be conducted in accord with all federal, state, and local regulations.
Tissue artefacts (such as splenomegaly) are likely to be present at post-mortem in horses euthanised with barbiturates.
Pentobarbital or a pentobarbital combination is the principal choice for equine euthanasia by chemical means. Because a large volume of solution must be injected, use of an IV catheter placed in the jugular vein will facilitate the procedure. To facilitate catheterisation of an excitable or fractious equid, a tranquiliser, such as acepromazine, or an α2-adrenergic receptor agonist can be administered, but these drugs may prolong time to loss of consciousness because of their effect on circulation and may result in varying degrees of muscular activity and agonal gasping. Opioid agonists or agonist-antagonists in conjunction with α2-adrenergic receptor agonists may further facilitate restraint.
Wherever possible the horses should be euthanised in a quiet, secluded and secure place. If this is not possible, or if the horse is already distressed (eg following severe injury or trauma) then it is recommended that the horse be administered an effective dose of a tranquilliser (eg Sedator or Xylazine 100) prior to Lethaton.
A neuromuscular blocking agent such as suxamethonium chloride (succinylcholine) may be added at 0.1 mg/kg to facilitate paralysis and rapid collapse to the ground.
There should be minimal number of personnel involved in the procedure. An experienced horse handler on the horse’s head is preferred.
The horse’s halter should be fitted tightly so that it will not slip over the horse’s head if the horse falls backwards.
To minimise the chances of perivascular administration and to facilitate rapid administration, it is preferable to use a long, large bore catheter (eg 10-14G, 3½5¼" catheter).
Pull up the required dose of Lethaton in 50mL syringes. It may be useful to have at least one spare syringe prepared.
Administer the full dose of the Lethaton rapidly via the catheter and then move a safe distance away from the horse.
The horse handler should maintain constant pressure on the lead rope so as to try and direct the horse’s fall and prevent the horse flipping over backwards.
Once the horse is recumbent, check for the absence of a pulse and heart beat, pupillary dilatation and loss of a palpebral and corneal reflexes.
Horses: 1-2mL/5kg (100-200mL @ 500kg BW) by rapid intravenous injection.
Horse Hack: When horses are euthanised, consideration should always be given to the unpredictability of how the horse is likely to fall and the possibility of some thrashing
activity once recumbent. Some degree of exaggerated muscular activity is expected after the horse falls, even if the horse is not experiencing pain or distress. Appropriate precautions should always be taken to minimise any unnecessary risk to personnel and facilities.
See also: Airways TMPS Oral Powder (page 22) Bromo TMPS Oral Powder (page 23)
Randlab supports responsible Antimicrobial Stewardship.
For further information about antibiotic selection, dosing and antimicrobial stewardship, follow this QR code.

Using my new AI Technology I was able to generate an image of Randlab's ox tetra-cycling. Is there anything else I can help you with?


No Siri... I said draw Randlab’s

Available:
APVMA Approval No. 90134 (Australia)
Active Constituent
Oxytetracycline (as the hydrochloride) 100 mg/mL
Pack Size
100mL multidose vial
250mL multidose vial
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Randlab’s Oxytetracycline Injection. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
Oxytetracycline is a broad-spectrum antibiotic with activity against a wide range of both Gram-negative and Gram-positive bacteria.
At lower dose rates it is bacteriostatic but is bactericidal at higher dose rates. It has good anaerobic coverage but a variable effect against Bacteroides and Clostridium spp.
Oxytetracycline is the drug of choice for Lawsonia intracellularis (Equine Proliferative Enteropathy) and Actinobacillus equuli (a common cause of peritonitis in adult horses) infection.
Oxytetracycline distributes well throughout the body with therapeutic levels achieved in most body fluids.
High dose oxytetracycline causes tendon relaxation in foals with congenital (but not acquired) contracted tendons and is most efficacious when given in the first 3 days of life (20 mg/kg IV).
Oxytetracycline has mild anti-inflammatory properties. Oxytetracycline is concentrated in bone and teeth.
Oxytetracycline has antibacterial activity against a wide range of bacteria. Wherever possible, antibiotic selection should be based on culture and sensitivity testing. Expected sensitivity to oxytetracycline is outlined in the table to the right. Following intravenous administration, oxytetracycline is distributed widely throughout the body including bone, heart, kidneys, lungs, muscle, pleural fluid, bronchial secretions, sputum, bile, saliva, urine, synovial fluid, ascitic fluid and aqueous and vitreous humour. It crosses the placenta and is concentrated in the milk. Oxytetracycline only penetrates the CNS if the meninges are inflamed.
Tetracylines have been shown to have anti-inflammatory properties in humans where they may be used as adjunct therapy for arthritis and autoimmune diseases. In horses oxytetracyline appears to be a potent inhibitor of matrix metalloproteinase-9 and a modest inhibitor of matrix melalopreteinase-2. Some of the response observed in treating chronic Lyme Disease in horses has been attributed to the anti-inflammatory effects rather than its antimicrobial activity. The drug is excreted primarily unchanged in urine with some also excreted through the bile and other non-biliary gastrointestinal pathways.
Horse Hack: Oxytetracycline has been administered IV at high doses (20-70mg/kg) every 48 hr to newborn foals for the treatment of contracted flexor tendons. The method of action is believed to involve calcium chelation. Treatment is more effective when initiated in the first three days of life.
Randlab supports responsible Antimicrobial Stewardship.
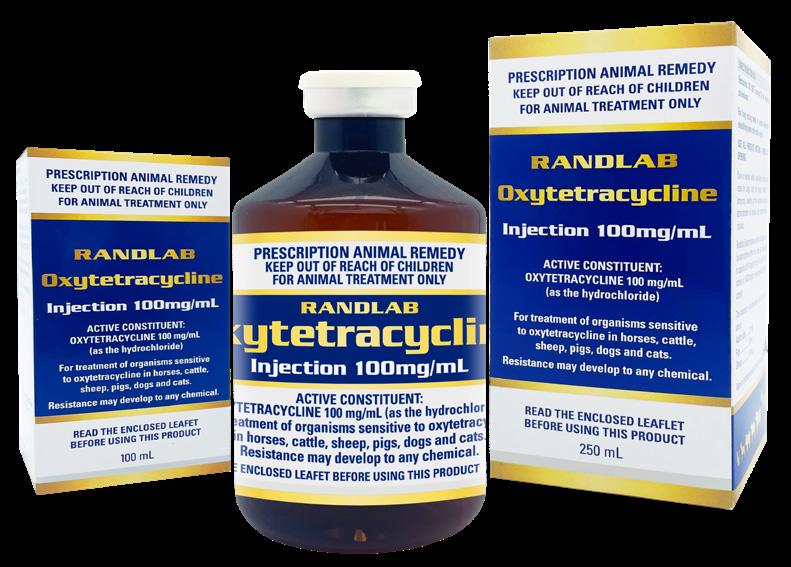
(Equine Proliferative Enteropathy)
Actinobacillus equuli
Pasteurella spp
Neorickettsia risticii (Potomac Horse Fever)
Ehrlichia spp
Streptococcus Enterococci
Staphylococcus (inc S. aureus) E. coli
Gram negatives incl Bacteroides spp, Brucella, Bartonella, Haemophilus spp, Pasturella multocida, Shigella & Yersinia pestis
Chlamydia
Klebsiella
Psuedomonas aeruginosa
Mycoplasma Bacteroides spp
Proteus
Spirochetes (inc Lyme Disease)
Rickettsia spp
Clostridium spp
Protozoa spp (inc Babesia equi)
Bacillus anthracis
Clostridium perfringens
Clostridium tetani
Listeria monocytogenes
Nocardia spp
Actinomyces spp
Intramuscular administration is not recommended in the horse because effective serum concentrations are unlikely to be maintained and intramuscular injection is associated with severe tissue irritation.
Hypotension and collapse may be seen following rapid IV administration of oxytetracycline. Renal tubular necrosis may occur with high doses (eg neonatal foals with contracted tendons). Care should be taken in dehydrated foals due to potential renal effects.
Bone and deciduous tooth discolouration is common in developing animals with oxytetracycline and should only be used during the last half of pregnancy.
When long term administration of a tetracycline is required (eg Lawsonia intracellularis EPE infection), it is common to follow a 5–10-day course of IV oxytetracycline by a course of oral doxycycline (10-20mg/kg q12-24hr).
Adult: 5.0-6.6mg/kg q12hr or 10-20mg/kg q24hr. In cases of severe infection, the initial high blood serum concentrations after a long-acting dose may result in a better response to the treatment.
Foals: 5-10mg/kg IV q12hr diluted in 1L Hartmann’s and then given slowly or 1020mg/kg IV q24H diluted and then given slowly.






















































APVMA Approval No. 91644 (Australia)
Available:
For the treatment and control of bacterial infections susceptible to the combination of sulfadoxine and trimethoprim in horses, cattle, sheep, goats, pigs and dogs.
Excellent broad-spectrum activity against a wide range of grampositive and gram-negative bacteria
Active Constituents
Sulfadoxine 200mg/mL
Trimethoprim 40mg/mL
Pack Size
100mL multidose vial
Randlab supports responsible Antimicrobial Stewardship.
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Randlab’s Tri-Sulfox. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.

Tri-Sulfox Injection is irritant when administered subcutaneously or by the intrauterine or intramuscular routes and these routes should be avoided. Inadvertent perivascular injection is also irritant and likely to result in significant thrombophlebitis.
Indications



Tri-Sulfox Injection has broad-spectrum activity against a wide range of both grampositive and gram-negative bacteria incl:
• Actinobacillus equuli
• Actinomyces spp
• Bacillus anthracis
• Bordetella spp
• Brucella spp
• Campylobacter spp
• Clostridium spp
• Corynebacterium spp
• Enterobacter spp
• Escherichia coli
• Fusiformis spp
• Haemophilus spp
• Klebsiella spp
• Pasteurella spp
• Pneumococcus spp
• Proteus spp
• Salmonella spp
• Shigella spp
• Staphylococcus spp (incl some S. aureus)
• Streptococcus spp
Concurrent administration of TMPS and detomidine may result in dysrhythmias (esp under general anaesthesia), hypotension and death.
Concurrent use of penicillin is antagonistic to sulphonamides.
Label dose: 3mL/50kg BW bid by IV Injection.
TMPS is a time-dependent antibiotic, therefore twice daily dosing is required.
Current NCAS recommended dose rate: 30mg/kg BW bid by slow IV Injection.


Pseudomonas aeruginosa, Enterococcus faecalis and Enterococcus faecium are intrinsically resistant to Tri-Sulfox. Mycobacterium tuberculosis, Mycoplasma spp, Leptospira are also resistant. In vivo activity against anaerobes is generally poor.
Pharmacology
The combination of sufladoxine and trimethoprim act synergistically and Tri-Sulfox Injection is bactericidal against most gram-positive and gram-negative bacteria incl E. coli, Haemophilus, Pasteurella, Salmonella, Staphylococcus and Streptococcus spp. Bacterial resistance to TMPS combinations is still, surprisingly, relatively low.
Both compounds affect bacterial purine synthesis at different stages of the bacterial metabolic pathway. This results in a double blockade of bacterial metabolism and a broader spectrum of activity than either component acting alone.
Tri-Sulfox Injection is distributed widely throughout the body and may be used in the treatment of bacterial infections of the respiratory tract (incl pneumonia, bronchitis and secondary bacterial infections following viral pneumonia), urogenital tract infections (incl placentitis, cystitis, vaginitis, urethritis, nephritis and metritis), gastrointestinal tract infections (incl E. coli and salmonellosis). Tri-Sulfox may be used for treatment and prophylaxis peri-operatively and for most peri-parturient problems requiring broad-spectrum antibiotic coverage as well as for wound infections and septicaemias.
Tri-Sulfox is excreted in the urine and therefore useful for treatment of urinary tract infections.
Tri-Sulfox is inactivated in the presence of purulent material and abscesses, etc should be surgically drained and lavaged prior to using Tri-Sulfox.
The concentration of TMPS in the bottle of Tri- Sulfox Injection is 200+40=240mg/mL.
Therefore a 500kg horse will require (30x 500)/240 =62mL bid.
For more information about antibiotic selection, dosing, etc and antimicrobial stewardship, scan the NCAS QR Code.
Horse Hack: Solution is irritant if given perivascularly. The use of catheters/cannulas will reduce the risk of inadvertent perivascular administration and is strongly recommended.
WARNING: TMPS antibiotics should not be contemporaneously administered to anaesthetised or sedated horses.
Rarely these horses may develop dysrhythmias, leading to hypotension and even death.
APVMA Approval No. 92858 (Australia) | ACVM Approval No. A011661 (New Zealand)
Available in:
Oral antibiotic powder for the treatment of infections known or suspected to be sensitive to the sulphadimidine and trimethoprim combination in horses, calves, pigs & poultry
Combination of sulphadimidine and trimethoprim working together to give greater bactericidal activity.
Active Constituents
Sulphadimidine 430mg/g
Trimethoprim 86mg/g
Pack Size
500g jar
Randlab supports responsible Antimicrobial Stewardship.
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Tri-Sil Powder. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
For the treatment of respiratory, urogenital, gastrointestinal, synovial and other infections in horses due to sulphadimidine and trimethoprim susceptible organisms.
Suitable for use in horses, calves, pigs and poultry.
The combination of sulphadimidine and trimephoprim in the ratio of 5:1 has been shown to be effective in treating many of the major bacterial pathogens considered of clinical significance in the horse. These include: Staphylococcus spp (incl S. aureus and S. intermedius), Streptococcus equi (incl subspecies S.equi equi and zooepidemicus), Actinobacillus spp, most Escherichia coli, Pasteurella spp, Proteus spp, Fusobacterium spp, Enterobacter spp, Corynebacterium spp (excluding Corynebacterium (Rhodococcus) equi), Salmonella spp, Shigella spp, Klebsiella spp and Haemophilus spp. Some Brucella and Nocardia spp are also susceptible. Most Pseudomonas spp are not sensitive.
The minimum inhibitory concentrations (MICs) of the combination for susceptible bacteria are substantially lower than those of either of the individual agents. The combination of sulfonamide and trimethoprim is bactericidal while either drug alone is bacteriostatic. Folate synthesising bacteria which are resistant or moderately resistant to either drug alone are frequently susceptible to the combination.
Bacterial resistance to the TMPS combination is surprisingly rare given the extensive use of the combination in veterinary and human medicine over the past 50 years.
Sulphadimidine and trimethoprim have similar antibacterial spectra, trimethoprim being approximately 20 times more potent than sulphadimidine. The combination blocks two sequential obligate enzymatic reactions in the microbial folate synthesis pathway. Mammalian cells require preformed folic acid and thus are unaffected by sulfonamides.
A synergistic action is demonstrated by the enhanced antimicrobial activity (potentiation) of the combination compared with the antimicrobial activity of either agent alone. Antimicrobial activity of the combination of sulphadimidine and trimethoprim was found to be up to 1024 times that of sulphadimidine alone, and up to 128 times that of trimethoprim alone. Potentiated sulfonamides present a lower risk of toxicosis in long-term therapy than do sulfonamides alone, as a lower dose of sulfonamide is required to achieve the same clinical effect.


Pharmacokinetic trials in horses have established that sulphadimidine and trimethoprim are readily and rapidly absorbed after oral administration and reach therapeutic plasma levels within approximately 30 minutes of administration. Trial results demonstrate that the formulation is efficiently absorbed even when administered with food.
TMPS is widely distributed to all body tissues and fluids. Concentrations are above plasma levels in kidney; similar to plasma levels in pleural, peritoneal, synovial and ocular fluids; slightly lower in CSF, muscle and milk.
The parent compound and its metabolites are excreted predominantly in the urine by glomerular filtration. Plasma half-life in the horse has been variously estimated at between 6.4 and 9.8 hours.
For oral use only. Give on dampened food mixed with honey or molasses or reconstitute as a paste and administer over the back of the horse’s tongue.
The antibacterial actions of sulfonamides are reduced in the presence of blood, pus and tissue breakdown products, which contain purines and thymidine, as the bacterial requirement for folic acid is decreased in such media.
Trimethoprim/sulfonamide (TMPS) are contraindicated in horses suffering from severe hepatic and renal insufficiency, urolithiasis or sulfonamide sensitivity.
Use the enclosed scoop.
One level large scoop contains 5 g sulfadimidine and 1 g trimethoprim. One level small scoop contains 1.25 g sulfadimidine and 0.25 g trimethoprim.
HORSES: One level large scoop per 200 kg bodyweight twice daily.
The 500g jar contains enough TMPS for an 8-day course for a 500kg horse. Before filling scoop, rotate container horizontally to ensure consistent filling weight.
WARNING: Oral administration of sulfonamides and trimethoprim may rarely be associated with the development of severe, acute diarrhoea in the horse. This may even occur several weeks after finishing the course.


































APVMA Approval No. 83452 (Australia)
Available in:
Easy to use paste ensures accurate and reliable dosing. Three convenient pack sizes.
Active Constituents
Sulfadiazine 400 mg/g
Trimethoprim 86 mg/g
Pack Size
250g multidose jar
500g multidose jar
1kg multidose tub
Randlab supports responsible Antimicrobial Stewardship.
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with TMPS Oral Paste. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
Indications
Randlab’s TMPS Oral Paste has a broad spectrum of activity against the common Gram-positive and Gram-negative bacteria including:
• Streptococci
• Escherichia coli
• Bordetella
• Corynebacterium
• Hemophilus
• Listeria monocytogenes
• Pasteurella
• Shigella
• Proteus spp.
• Dermatophilus congolensis
• Bacillus
• Brucella
• Fusiformis
• Klebsiella spp
• Nocardia
• Salmonella
• Staphylococci
The combination also has some effect against infections caused by protozoa (e.g. coccidia and Toxoplasma infection)
InfectionscausedbyLeptospira,Psuedomonas,MycobacteriumandErysipelothrix aregenerallynotsusceptible.
Randlab’s TMPS Oral Paste is useful in the treatment of respiratory infections, with high lung tissue levels achieved (although Randlab’s Airway Tmps and Bromo Tmps are designed specifically for respiratory infections), soft tissue infections, abdominal infections, joint infections, infections of the CNS and infections of the uterus/placenta and urinary tract.
The combination of sulfadiazine + trimethoprim (TMPS) act synergistically to kill a wide range of susceptible bacteria and reduces the possibility of bacterial resistance developing.
The combination blocks the synthesis of bacterial folates at two critical points, which are essential for bacterial cell multiplication and survival. The combination of sulfadiazine + trimethoprim is bactericidal, while either drug alone is bacteriostatic. Folate synthesising bacteria which are resistant or moderately resistant to either drug alone are frequently susceptible to the combination.
WARNING: Oral administration of trimethoprim+ sulfonamides may rarely be associated with the development of severe, acute febrile diarrhoea in the horse. This may even occur several weeks after finishing the course.

TMPS is readily absorbed following oral administration with peak levels occurring about 1-4 hours after dosing. TMPS is widely distributed to all body tissues and fluids with levels similar to plasma found in pleural, peritoneal, synovial and ocular fluids with lower levels in CSF and muscle.
Both drugs cross the placenta and are present in the milk.
Sulfadiazine is excreted largely unchanged in the urine with higher levels than plasma present in the kidney and urine.
The antibacterial actions of sulfonamides are reduced in the presence of blood, pus and tissue breakdown products as the bacterial requirements for folic acid is decreased in such media.
This product is not recommended for use in treatment of infections or abcessess caused by anaerobic bacteria.
TMPS should not be used in horses with marked liver parenchymal damage, blood dyscrasias or with a history of sulfonamide sensitivity.
Rarely TMPS may cause idiosyncratic neurological reactions consisting of behaviour changes, gait abnormalities and hyperaesthesia. These effects are generally reversed soon after discontinuing the medication.
Label dose rate: 5mL/100kg twice daily.
1mL of TMPS Paste weighs approx 1.2g. Therefore a 250g pot contains approx 208mL of paste and so forth.
Administer directly into the mouth from a dosing syringe.
Adult: 24-30mg/kg twice daily combined sulfonamide+ trimethoprim dose.
[e.g. 500kg horse = 30mg x 500kg/(400+86 mg/g) =31g.
31g of paste = 26mL twice daily.]
Foals: 15-30mg/kg twice daily.
Dose rate equivalent to 5-6mL/100 kg twice daily (12 hourly).
For more information about antibiotic selection, dosing, etc and antimicrobial stewardship, scan the NCAS QR Code.
APVMA Approval No. 90730 (Australia)
Available:
For the control of gentamicinsusceptible bacterial infections in dogs, cats and horses.
The drug of choice in the treatment of gram negative infections.
Active Constituent
Gentamicin (as sulphate) 100mg/mL
Pack Size
100mL multidose vial
Randlab supports responsible Antimicrobial Stewardship.
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Randlab’s Gentamicin Injection. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
Indications
Gentamicin is active against a wide range of bacteria. It is the drug of choice for treatment of gram-negative infections (incl. Pseudomonas spp, Proteus, Klebsiella). It is also effective against Staphylococci.
Gentamicin has no activity against anaerobes, streptococci and enterococci and limited activity against Salmonella spp.
Gentamicin is most frequently used in combination with a beta-lactam antibiotic (eg penicillin, cephalosporin, ampicillin) to achieve a broader spectrum of activity.
Gentamicin is commonly used by intra-articular injection. It may also be used for regional limb perfusion (either IV or intraosseous) or impregnated in PMMA beads.
Gentamicin may be administered by nebuliser for treatment of respiratory tract infections including pneumonia.
Gentamicin is commonly used as part of an intrauterine flush or for the intrauterine treatment of uterine infections.
Pharmacology
Gentamicin sulphate is an aminoglycoside antibiotic that has good activity against a variety of gram negative aerobic bacilli (incl E. coli, Klebsiella spp, Proteus spp, Pseudomonas aeruginosa, some Salmonella spp, Enterobacter, Serratia, Shigella), many Staphylococci strains, Mycoplasma and spirochetes.
It is the drug of choice for treatment of E. coli, Klebsiella spp, Pseudomonas spp (incl Pseudomonas aeruginosa), Actinobacillus equuli and Pasteurella spp.
Gentamicin must penetrate the cell wall and enter the bacterial cell to assert its effect. The concomitant administration of antibiotics that interfere with cell wall synthesis (eg penicillin) will enhance the efficacy of gentamicin.
Gentamicin is primarily distributed in the extracellular fluid and is found in peritoneal, pleural, pericardial, ascitic and synovial fluids, sputum and bronchial secretions.
Therapeutic levels are also found in bone, heart and lung.
Gentamicin does not readily cross the blood-brain barrier or penetrate ocular tissue. CSF levels are variable and may be from 0-50% of those found in the serum.
Gentamicin tends to accumulate in the inner ear and kidneys, which makes these organs particularly sensitive to gentamicin toxicity.
Gentamicin crosses the placenta but no appreciable levels are present in neonatal foals of mares treated with gentamicin. Small amounts of gentamicin may be excreted into the milk with minimal risk to the nursing foal.

Gentamicin is inactivated by purulent material and other low oxygen environments. Gentamicin is eliminated almost entirely by glomerular filtration with a half life in horses of 1.82-3.25 hours.
Intravenous is preferred over intramuscular administration as horses may develop some localised muscle soreness following repeated IM injection.
Known potential adverse effects of gentamicin include nephrotoxicity and ototoxicity. The latter is of unknown significance in the horse.
Do not administer to animals with compromised renal function.
The risk of nephrotoxicity is increased if the horse has pre-existing renal disease, in neonates and geriatrics or if the horse has fever, sepsis or dehydration.
To avoid potential renal toxicity, ensure that treated horses are well hydrated and have adequate access to water and electrolytes during the treatment period. High levels of calcium and protein in the diet may decrease the risk of nephrotoxicity by increasing glomerular filtration rate.
Horses with decreased renal function may have a significantly prolonged gentamicin half-life and dosing frequency should be reduced.
Although many Salmonella spp may exhibit in vitro sensitivity, they are rarely sensitive in vivo.
Adult; 6.6-9.7mg/kg sid by IV or IM injection.
Foal (<14 weeks); 8.8-12mg/kg q24-36h
Intrauterine; 1-2g buffered with equal volume of 7.5% sodium bicarbonate and diluted in 200mL isotonic saline daily for 3-7 treatments.
Intra-articular; 100-500mg per joint depending on joint size. Diluted 1:2 with sterile saline.
Regional Limb Perfusion: Add 10mL gentamicin to 50mL saline or prilocaine. Nebuliser: 100-200mg diluted 1:1 with isotonic saline sid-qid.
Horse Hack: The label dose for Gentamicin Injection is 2.0mg/kg BW by IV or IM injection tid. Like many antibiotics, the label doses are no longer considered valid.
Gentamicin is a concentration-dependent antibiotic and as such higher doses at less frequent intervals is recommended. This increases bacterial killing, increases bacterial susceptibility and decreases potential toxic effects and the risk of developing resistant bacterial strains..
Available in: P P
Product available in Australia under APVMA Permit No. 84510
For the treatment of anaerobic bacterial and protozoal infections caused by metronidazole-sensitive organisms
The only equine metronidazole product on the market.
Active Constituent
Metronidazole 500 mg/g*
*1g Metronidazole paste is equivalent to 1mL
Pack Size
1kg multidose pail
Randlab supports responsible Antimicrobial Stewardship.
Don't forget to return your completed Metronidazole APVMA permit form to Randlab. Spare copies of the form are available at randlab.com on the Metronidazole page.
For the treatment of infections in horses caused by anaerobic bacteria such as Clostridium spp, Bacteroides spp (incl penicillin resistant B. fragilis), Fusobacterium and protozoa such as Giardia and Trichomonas spp.
For the treatment of infections where anaerobic bacteria are implicated or suspected such as pleuropneumonia, sinusitis, tooth root infections, enteritis, colitis and some cases of deep foot infection and metritis.
Metronidazole is the antimicrobial of choice for the treatment of Acute Febrile Diarrhoea or chronic diarrhoea due to anaerobic infections such as Clostridial infections.
Metronidazole in combination with either oxytetracycline or chloramphenicol has been suggested for the treatment of proliferative enteropathy caused by Lawsonia intracellularis.
Metronidazole reaches therapeutic concentrations in abscesses, bone, peritoneal fluid, milk and the CNS. It crosses the placental barrier.
Oral absorption in horses is 80-100%. Rectal absorption is 30-50%. The rectal dose should therefore be adjusted accordingly.
The half-life of metronidazole is 2-4 hours in adult horses and 9-12 hours in foals.
Therapeutic concentrations of metronidazole are attained after administration per rectum. This may be the route of choice in horses with impaired gastrointestinal status/ileus or to reduce the risk of anorexia.
Do not use in animals with renal or hepatic disorders or dysfunction.
Over-dosage (high doses or prolonged treatment beyond the specified five days) may result in CNS symptoms such as depression, ataxia, tremors, seizures and weakness. These are generally reversible after ceasing the metronidazole.
WARNING: Anorexia is common following oral Metronidazole use. It is less common with rectal administration but is still likely to occur.

Other side effects reported include peripheral neuropathy and impaired hepatic function. Signs are generally reversible after a few days once the treatment has been stopped. Rarely this may not be reversible.
Insufficient data is available on use in pregnant mares to recommend the use of the product during pregnancy.
Administer orally. Give 20mL Equine Metronidazole Paste (equivalent to 10g metronidazole) per 500kg bodyweight every 12 hours for 5 days or as directed by a veterinarian.
Adult: 15mg/kg qid or 20mg/kg bid orally
Foal: 10-20mg/kg bid orally
Horse Hack: Metronidazole Paste may also be administered per rectum at approximately double the oral dose rate.

DID YOU KNOW... There's a connection between this iconic scene from the Man From Snowy River movie and a well known equine vet?
DENNY (CHARLIE LOVICK, AUS) See page 71 for the full

APVMA Approval No. 62637 (Australia)
Available in:
Broad spectrum topical anti-microbial ointment.
Active Constituents
Povidone-Iodine 100 mg/g (equivalent to available Iodine 10 mg/g)
Pack Size
250g jar
Vetedine Ointment is a povidone iodine (known as an iodophor) with potent prolonged antibacterial, antifungal and virucidal activity on contact, suitable for topical disinfection and antisepsis in horses.
Use Vetedine Ointment as an aid in the treatment or prevention of local infections in wounds, abscesses, burns and fungal infections such as ringworm, disinfection of the navel of newborns, dehorning, castration, disinfection prior to surgery, girth galls, abrasions and puncture wounds.
• Has rapid action, even in presence of blood, pus, oils, soap, etc.
• Effective anti- bacterial, fungal and viral activity
• No resistance by any micro-organisms
• Does not stain. Washes off with water
• Does not delay wound healing or granulation
• Does not sting or irritate wounds
• Does not sensitise tissues
• Can be bandaged without danger of burns or irritation
• Has prolonged germicidal action
• Has no odour
• Has prolonged activity, even in the presence of pus or blood
• Forms an adherent film over wounds
• The characteristic colour delineates the treated area
Vetedine Ointment acts by releasing iodine slowly. Povidone iodine is as effective as iodine against a broad spectrum of disease causing micro-organisms, but it is less irritating to skin, and any stains wash out in water. There has been no reported resistance to povidone iodine in human or animals. It is non-sensitising and does not cause pain on application to wounds or mucus membranes. It forms a film to protect open wounds, and is soluble in water.
Povidone iodine is particularly effective in treating mixed skin infections, wound cleansing, treatment of burns and ulcers, and in skin antisepsis. It does not delay healing or granulation, and does not lose antimicrobial activity in the presence of organic matter such as blood, pus, oils, soaps, etc.
Administration and Precautions
Apply to the affected area twice daily or by the veterinary surgeon. Care should be exercised using iodine on extensive wounds or deep burns especially over extended periods. Does not permanently stain skin or natural fibres. Wash with soap and water to remove.


DID YOU KNOW THAT...


The only Kentucky Derby winner to be disqualified has been linked to the death of Martin Luther King?
DANCER’S IMAGE (PETER D. FULLER, USA)
The Kentucky Derby has been described as the most exciting two minutes in sport. There have been 147 running’s of America’s most fabled race, but only one winner has ever been disqualified.
Dancer’s Image was first past the post in the 1968 Derby but was disqualified a few days later when traces of the relatively new drug phenylbutazone were found in his blood sample.
Although the horse was known to have been administered the ‘Bute’ by his veterinarian six days earlier (by use of a pill gun), Dancer’s Image’s connections blamed the positive swab on skullduggery fueled by revenge. In the weeks prior to the race, the horse’s prominent Massachusetts owner Peter D. Fuller had given the horse’s winnings (Au$90K) from his previous start to Coretta Scott King, the widow of civil rights activist Martin Luther King Jr., who had been assassinated on the same day as the colt had won.
The previous year, King had held a sit-in in Kentucky against housing discrimination which had disrupted Derby week.
Dancer’s Image’s connections, including his renowned US veterinarian Dr Alex Harthill, believed the Derby disqualification following the positive swab was in retribution for the donation which was widely seen as interference of a wealthy Northerner businessman in Southern politics.
Ironically the use of Bute was legal on Kentucky racetracks the year before the 1968 Derby and was made legal again the year after.
Dancer’s Image was also disqualified at his next race when running third in the Preakness Stakes after causing interference.
He was retired to stud after that race where the billboard at the entrance to the stud defiantly declared, “Dancer’s Image: Winner, 1968 Kentucky Derby”.

From control of oestrous behaviour, synchronisation of oestrus/ovulation and for the maintenance of pregnancies in “at risk” mares, Randlab’s reproduction suite has got your mare covered.
Injectable GnRH analogue for the reliable induction of ovulation Deslorelin as acetate 1.25mg/mL (~10 doses) APVMA
Australian Owned Australasian Made

Weekly altrenogest injection Altrenogest 50 mg/mL (~10 doses) APVMA Approval No. 83216 | ACVM No. A11475 (NZ)



Ovu-Mate now also available!

Sizes: 125mL, 1L & 2L
Oral progestagen for the regulation of oestrus and maintenance of pregnancy
APVMA Approval No. 89636 (Australia)
Available in:
Convenient, long-acting deslorelin injection for the accurate timing of ovulation.
Active Constituent
1.25 mg/mL deslorelin (as deslorelin acetate)
Pack Size
10mL multidose vial (=10 x 1mL doses)
The induction and timing of ovulation in mares supporting a follicle greater than 30mm in diameter and signs of oestrous behaviour and/or uterine and cervical oestral changes on ultrasound.
Upwards of 94% of suitable mares would be expected to ovulate within 48 hours of Biorelin administration, with an additional percentage (< 6%) up to 54 hours. Few mares will ovulate in the first 24 hours and most will ovulate around 40 hours post-injection.
Biorelin Injection (slow release deslorelin acetate) is a synthetic Gonadatrophin Releasing Hormone (GnRH) for induction and timing of ovulation in oestrual mares.
The long duration of oestrus in mares, and the varying time from the onset of oestrus to ovulation, typically results in the need for multiple breedings or inseminations in order to achieve conception. Biorelin Injection induces ovulation within 48 hours after treatment in oestrous mares with a developing follicle greater than 30mm in diameter by increasing the levels of endogenous luteinising hormone (LH).
Biorelin Injection is a sustained release, non-aqueous formulation and is indicated for the induction of ovulation in oestrous mares. It is intended to optimise breeding management by shortening and synchronising the oestrous period, thereby allowing for stallions and assisted breeding programs to be more efficiently managed.
Following the use of Biorelin, the length of the inter-ovulatory interval between cycles is not altered.
The pregnancy rate of mares treated with Biorelin is similar to that of control mares.
Effectiveness is contingent upon accurate diagnosis of oestrus and detection of a developing follicle between 30 and 40mm in diameter. Ideally, mares should be regularly (every second day) checked prior to Biorelin administration and should demonstrate an increase in follicular size of 3-5mm per day.
The follicle should also be producing oestrogen and the mare displaying oestrous behaviour and/or uterine (oedema 3/3) and cervical (softening) changes on ultrasound indicative of oestrus.
Injection site reactions have been observed in ~15% of treated mares. The reactions are mild to moderate swellings at the injection site which may be painful on palpation. These reactions typically resolve within 36 hours.
Pregnant women and others of childbearing age should exercise caution when handling this product. Accidental administration may lead to a disruption of the menstrual cycle. Direct contact with the skin should therefore be avoided and any
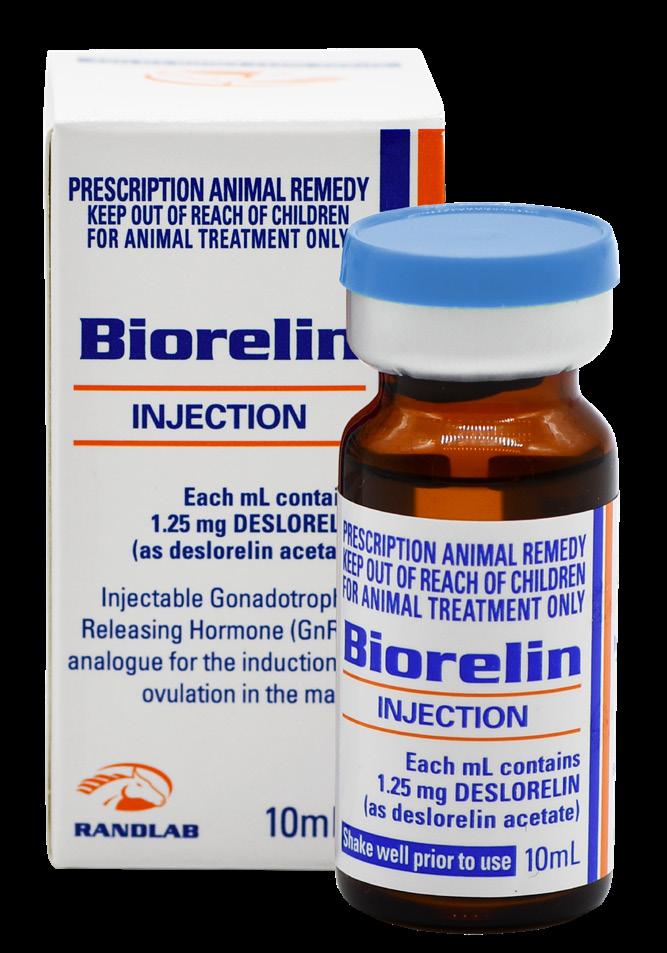
contact areas should be washed immediately with soap and water if exposure does occur. Routine precautions should be employed by practitioners when handling and using this product to prevent accidental injection.
Dosage
Shake vial vigorously before administration. Inadequate re-suspension may affect efficacy.
For administration by intramuscular injection only.
Discard unused portion within 28 days of broaching.
1.0 ml by intramuscular (IM) injection (1.25 mg deslorelin per dose). Only 1 mL should be administered per mare during any one oestrus cycle.
Horse Hack: Mares should be bred ~ 24 hours post Biorelin injection for chilled or fresh semen.
Mares should be bred ~ 40 hours post Biorelin injection for frozen semen.
During the transitional oestrus period, a higher dose of Biorelin (1.9mg = 1.5mL) may improve ovulation rate.

Before the 1956 Olympics, no member of the Aus eventing team had ever competed in a 3-day event?

1956 AUSTRALIAN EVENTING TEAM
Australia first mounted an Olympic Equestrian Team for the 1956 Games. The Games that year were held in Melbourne, Australia, but Australia’s strict quarantine laws meant that the Equestrian sports were held in Stockholm. So whilst the rest of the world was heading down under, Australia’s equestrian team embarked on a six week sea voyage to England. As the photo above shows, the team horses were later flown on to Stockholm.
The team consisted of six horses that had been purchased by or donated to the Equestrian Federation Australia. Austrian Franz Mairinger had been appointed team coach 15 months previously. Mairinger arrived in Australia to discover that none of the riders earmarked for the Olympics had ever competed at dressage nor had they competed in a three-day-event.
Their first and only three-day-event before the Olympic Games was at Badminton, which they also used as a qualifier for the Games.
In a remarkable achievement the Aussies defied all odds and finished a proud fourth at the Games and paved the way for an Olympic Gold in eventing at the following Games in Rome.
Available in:
Convenient, long-acting, weekly progestagen injection.
Active Constituent
Altrenogest 50 mg/mL
Pack Size
30mL multidose vial
Altrenogest acts similarly to the natural hormone, progesterone, by suppressing the normal oestrous cycle and preventing signs of oestrus and ovulation. Mares return to heat and release natural hormones again once treatment stops. Treatment with altrenogest makes it possible to regulate the breeding of mares.
Ovu-Mate injection may be used in fillies and mares to:
• Delay the onset of oestrus
• Suppress oestrous behaviour
• Synchronisation of oestrus for the efficient use of stallions or assisted- breeding techniques.
• As an adjunct to the treatment of placentitis.
Following intramuscular administration of Ovu-Mate Injection high blood levels are rapidly reached. Levels achieved initially exceed the levels achieved by daily oral dosing sevenfold and decline steadily over the next 5-7 days.
Pharmacokinetic data from mares treated with Ovu-Mate Injection have shown blood levels of altrenogest are maintained for 5 days above the oral therapeutic level following administration of the injection. Repeated weekly injections may result in progressive accumulation of plasma levels of altrenogest.
Studies have been conducted to support suppression of behavioural signs of oestrus in cycling mares for 7 days following administration of altrenogest injection.
Use rubber gloves when handling this product.
Women who are pregnant or suspected of being pregnant should not handle the product. Women of childbearing age should handle the product with extreme care. Not to be handled by persons with known or suspected progesterone dependent tumours or thromboembolic disorders.
Avoid skin contact. Do not inhale or swallow. Rubber gloves should be worn when handling this product. APVMA Approval No. 83216 (Australia) | ACVM No. A011475 (New Zealand)
WARNING: If altrenogest is used in performance animals, the regulations of the relevant authorities regarding medication should be observed. In some jurisdictions and under some circumstances this product may be regarded as a prohibited substance due to the presence of low levels of the male anabolic steroids trenbolone and trendione in all formulations of altrenogest. Contact Randlab's Veterinary & Technical Director, Dr Michael Robinson on mrobinson@randlab.com.au or +61 451481050 for further information.
Dosage
Suppression of oestrus: 3mL per 500kg bodyweight (0.3mg/kg) by intramuscular injection once every 5-7 days or as required to suppress signs of oestrus. The dose rate should be extrapolated according to bodyweight if treating lighter or heavier horses.


Horse Hack: As with any long-acting depot injections, some minor reactions may occasionally occur at the injection site, especially in mares on weekly dosing.
Alternating injection sites from week to week will assist in minimising these reactions. The gluteal muscles may also be used as an alternative to the neck.
A system such as injections on the left on even calendar days and on the right on odd calendar days will help to average out the number of injections at each site. This is a particularly useful system if a majority of injections are going to be administered by stud staff or different personnel.


DID YOU KNOW THAT... Thoroughbred foundation stallion Godolphin Arabian was once used as a carthorse by a French king?
ARABIAN (EARL OF GODOLPHIN, UK)
Few horses can claim to have had a greater impact on the modern thoroughbred than Godolphin Arabian. The stallion is considered one of the three foundation stallions of the thoroughbred breed.
The small golden-bay Arabian stallion was born on the Barbary Coast (AF) around 1724 and, along with four other horses, was a diplomatic gift to King Louis XV of France in 1730. The stallion was named “Scham” by his new owners. However, the King was unimpressed with his gift and is said to have used him as a carthorse.
The stallion was eventually imported into England by the Duke of Devonshire, where he served his first mare. The resulting foal, “Lath”, was a splitting image of his father and said to have “run like the wind”.
Following the death of the Duke of Devonshire, the Earl of Godolphin inherited most of the Duke’s equine interests, including Scham. He bred two full brothers to Lath, all of whom resembled their father and had superior speed. Scham would go on to sire 100 foals and eventually become known as the Godolphin Arabian. He died on Christmas Day in 1753 aged 29yo. His grave can still be visited at the entrance to the stable block of what was once the Earl of Godolphin’s stud in the Gog Magog Hills in Cambridgeshire, England.
This legendary stallion has had a lasting impact on Thoroughbred bloodlines through champions such as Eclipse, Silky Sullivan, Man o’ War, War Admiral and Seabiscuit. The legacy of Godolphin Arabian is also honoured through the vast racing and breeding empire of Sheikh Mohammed bin Rashid Al Maktoum (Vice President & Prime Minister of the UAE and Ruler of Dubai) which is branded Godolphin.


APVMA Approval No. 65653 (Australia) | ACVM No. A010159 (New Zealand)
Available in:
Three convenient pack sizes.
Active Constituent
Altrenogest 2.2 mg/mL
Pack Size
125mL
1 Litre
2 Litre



For regulation and control of the breeding cycle of mares and the maintenance of pregnancy in habitually aborting mares or mares at risk of early abortion.
• To induce ovulatory oestrus early in the breeding season in mares where some follicular activity exists.
• For the suppression of oestrus either during prolonged oestrus or in normally cycling mares.
• For the control of the ovarian cycle in breeding mares to allow the most efficient use of the stallion or assisted-breeding techniques.
• For suppression of oestrous behaviour in fillies and mares engaged in competition.
• For the maintenance of pregnancy in habitually aborting mares or mares at risk of early embryonic death or abortion.
• For behavioural modification in non-competition stallions and geldings.
Pharmacology
For regulation and control of the breeding cycle of mares
• For the induction of ovulatory oestrus or to induce cyclical ovarian activity in mares with some follicle activity early in the breeding season: administer OvuMate daily for 10 consecutive days.
• For the suppression of oestrus in normally cycling mares: administer Ovu-Mate daily for 15 consecutive days.
• For the suppression of prolonged oestrus: administer Ovu-Mate daily for 10 consecutive days.
• To control the breeding cycle of mares to allow programmed use of the stallion or efficient use of assisted-breeding programs: administer Ovu-Mate daily for 15 consecutive days.
For the treatment of habitually aborting mares, or mares at risk of early embryonic death or abortion
• Administer Ovu-Mate daily, from the 2nd or 3rd day after ovulation in the mated or inseminated mare, continuing until day 120 of gestation.
• Ovu-Mate may be used as part of the treatment regimen in mares with placentitis.
Ultrasound scanning to confirm pregnancy is recommended on day 14 or 15 after ovulation. If the mare is non-pregnant, it is advised to cease Ovu-Mate administration and to administer an injection of prostaglandin F2 to destroy any luteal tissue that may still be present, allowing the mare to return to oestrus and be re-mated.


Porcine Ovu-Mate now also available!
Pack Size
1 Litre (4mg/mL)
WARNING: If altrenogest is used in performance animals, the regulations of the relevant authorities regarding medication should be observed. In some jurisdictions and under some circumstances this product may be regarded as a prohibited substance due to the presence of low levels of the male anabolic steroids trenbolone and trendione in all formulations of altrenogest. Levels are lower in oral formulations of altrenogest compared to the longacting injectable formulations.
Contact Randlab's Veterinary & Technical Director, Dr Michael Robinson on mrobinson@randlab.com.au or +61 451481050 for further information.
This product is contraindicated for use in mares suffering from uterine infections. If administered in feed, Ovu-Mate should be added to the feed immediately prior to consumption and not stored.
Unused feed must be destroyed and not given to any other animals.
Care should be taken to avoid contact between the solution and women of child bearing age. When using the product wear rubber gloves. Product is harmful if absorbed by skin contact, inhaled or swallowed. Avoid contact with eyes and skin. Do not inhale. After use and before eating, drinking or smoking, wash hands, arms and face thoroughly with soap and water. After each day’s use wash gloves.
Dosage for oral administration. Administer 1 mL Ovu-Mate per 50 kg bodyweight (equivalent to 0.044 mg Altrenogest per kg) daily (12.5 mL per 625 kg mare). Ovu-Mate may be administered in the feed or orally over the back of the tongue by syringe.
Horse Hack: Mares may continue to cycle and ovulate whilst on altrenogest but will not exhibit oestrous behaviour.
Mares will generally begin to show oestrous behaviour 3-5 days after cessation of treatment with Ovu-Mate and ovulate 10-12 days after cessation of treatment.
Whenever possible the decision to deworm should be based on the results of an individual and/or herd Faecal Egg Count (FEC).

Randlab supports the responsible use of anthelmintics




APVMA Approval No. 89307 (Australia)
Available in:
Active Constituents
Moxidectin 20 mg/mL
Praziquantel 125 mg/mL
Pack Size
11.8g syringe
Potent, long-acting dewormer effective against encysted larvae
Premium product for use when macrocyclic lactone resistance is identified or strongly suspected.
Randlab supports the responsible use of anthelmintics

Whenever possible the decision to deworm should be based on the results of a Faecal Egg Count (FEC). Consult a veterinarian when developing a worm control program to suit your specific needs.
Pradectin with Tape Gel Wormer is effective for the treatment and control of small strongyles (adults and larvae including encysted stages), large strongyles, tapeworm, pinworms, ascarids, Habronema spp, stomach bots, hairworm, intestinal threadworm and cutaneous onchocerciasis.
Pradectin with Tape Gel Wormer has a prolonged Egg Reappearance Period (ERP) of at least 14 weeks, which means that recontamination of the pasture by strongyle eggs is significantly reduced during this period.
Moxidectin is also an effective arachnicide against feeding ticks.
Pradectin with Tape Gel Wormer Long Acting Horse Wormer and Boticide Gel contains moxidectin, a second-generation macrocyclic lactone, and praziquantel, a synthetic isoquinoline-pyrazine derivative in a palatable gel formulation.
Pradectin with Tape Gel Wormer is effective as a single dose against encysted stages of small strongyles including developing stages (DL), late encysted stages (LL3/EL4) and aids in control of early encysted stages (EL3) including inhibited larvae (90% effective).
At the recommended dose rate Pradectin with Tape Gel Wormer is effective in the treatment and control of the following parasites:
• Small strongyles (adults and larvae): Cyathostomum spp incl benzimidazole resistant strains and as a single dose against encysted stages of small strongyles incl developing stages (DL), late encysted stages (LL3/EL4) and aids in control of early encysted stages (EL3) including inhibited larvae (90% effective).
• Large Strongyles: Strongylus vulgaris (adults, L3 and L4 arterial larval stages), Strongylus edentatus (adults and L4 visceral larval stages), Strongylus equinus (adults), Triodontophorus spp. (adults) including T. brevicauda, T. serratus and T. tenuicollis, Craterostomum acuticaudatum (adults).
• Tapeworms: Anoplocephala perfoliata and Paranoplocephala mamillana
• Stomach bots: Gasterophilus intestinalis (2 & 3 stage larvae),Gasterophilus nasalis (2 & 3 stage larvae)
• Ascarids: Parascaris equorum (adults and intestinal stages (L4))
• Habronema spp: gastric and cutaneous (summer sore)
• Lungworms: Dictyocaulus arnfieldi (adult and immature)
• Onchocerca spp. microfilariae (cutaneous onchocerciasis)
• Trichostrongylus axei (adults)
• Intestinal Threadworms: Strongyloides westeri
• Pinworms: Oxyuris equi (adults and L3/L4 larval stages)
• External parasites: Feeding ticks, lice and mites
Administration and Precautions
When used as directed, Pradectin with Tape Gel Wormer is safe to use in foals over four weeks of age and in all breeding stock, including stallions and pregnant mares.
Do not administer the full syringe to horses under 400Kg.
Use with caution in foals and smaller breeds by carefully calibrating the dose to the horse’s body weight to avoid overdosing.
Dosage
Pradectin with Tape Gel Wormer is administered orally at a dose rate of 0.4 mg/kg moxidectin and 2.5 mg/kg praziquantel.
It is supplied in a ready to use syringe, calibrated according to the body weight of the horse to be treated in 25 kg body weight increments. Use of this calibration will deliver the correct recommended dose.
One syringe is sufficient to treat a horse weighing 575 kg. For a heavy horse (weight exceeding 575 kg) it will be necessary to apply more than one syringe. For example, a 725 kg horse would require 1¼ syringes for treatment.
To avoid over- or underdosing, use the correct dose based on the horse’s measured/estimated body weight.
If gel remains in the syringe once the full dose has been delivered, replace the barrel cap; store the remaining gel below 30°C (room temperature) for later use, but do not use after the expiry date shown on the label.
Pradectin with Tape Gel Wormer is the anthelminthic of choice for horses (especially young horses) where heavy strongyle burdens (especially encysted cyathostomin larvae) are suspected.
The mass simultaneous emergence from the large intestinal wall of encysted larvae (eg following worming with an anthelminthic that is not effective against encysted larvae) may result in a syndrome known as larval cyathostominosis.
This syndrome is characterised by an acute generalised typhlocolitis and a profound inflammatory reaction resulting in profuse, watery diarrhoea which may occasionally progress to death.
WARNING: To minimise emerging anthelminthic resistance to moxidectin, it is strongly recommended that moxidectin-containing wormers (such as Pradectin with Tape) not be used for routine deworming of horses. Moxidectin should be reserved for cases where known or suspected resistance to other macrocyclic lactones (such as ivermectin, abamectin) has been established or where there is a strong likelihood of encysted and dormant larvae. Moxidectin’s use as routine dewormer should be discouraged.
APVMA Approval No. 93028 (Australia)
Available:
For the treatment and control of susceptible strains of all common worms of horses, including tapeworms
Strategic wormer for rotational worming. Alternative to mectin based wormers.
Active Constituents
Oxfendazole 7.0 g
Pyrantel Embonate 9.1 g
Pack Size
35mL syringe (up to 700kg BW)

Whenever possible the decisions to worm should be based on individual and herd Faecal Egg Counts (FEC). Consult a veterinarian when developing a worm control program to suit your specific needs.
Indications
For the treatment and control of susceptible strains of all common worms of horses including tapeworms and adult stages of ivermectin, moxidectin and abamectin resistant strains of Parascaris equorum.
Pharmacology
Oxfendazole belongs to the benzimidazole class of antiparasitic drugs. It has a broad spectrum of activity against a variety of pathogenic internal parasites, although it is not effective against tapeworm or bots.
Pyrantel acts as a depolarising neuromuscular blocking agent in susceptible parasites, which paralyses the organism. At double the normal dose rate (as present in Oxfendoate Broad Spectrum Worm Paste), it is effective against tapeworm.
At the recommended dose rate, Oxfendoate is effective against the following parasites:
• Small Strongyles including benzimidalzole resistant (adults and immature): Cyathostomum spp., Cylicocyclus spp., Cylicostephanus spp., Cylicodontophorus spp., Gyalocephalus spp. etc
• Large Strongyles: Strongylus vulgaris (adults and arterial larval stages), Strongylus edentatus (adults and tissue stages), Strongylus equinus (adults) and Tridontophorus spp. (adults).
• Bots: Gasterophilus spp. (oral and gastric stages)
• Ascarids: Parascaris equorum (adult and immature)
• Habronema muscae (adult)
• Onchocerca spp. (microfilariae)
• Hairworms: Trichostrongylus axei (adult)
• Intestinal Threadworms: Strongyloides westeri (adult)
• Lungworms: Dictyocaulus arnfieldi (adult and immature)
• Pinworms: Oxyuris equi (adult and immature)
• Tapeworms: Anoplocephala perfoliata, Anoplocephala magna, Paranoplocephala mamillana (adult, immature, heads, segments).
This product is most effective against adult stages of ivermectin, moxidectin and abamectin resistant strains of Parascaris equorum. Egg shedding may recommence 2-4 weeks after treatment. A re-treatment interval of 2-4 weeks
Randlab supports the responsible use of anthelmintics
will provide better control of the immature (juvenile) stages of macrocyclic lactone (mectins) resistant Parascaris equorum.
Safe to use in foals, pregnant mares and breeding stallions.
Oxfendoate has shown a wide safety margin at the recommended dose level. It may be used in horses of any age. Safe to use in pregnant mares and stallions.
Withdrawal of feed is not required prior to administration.
All horses should be included in a regular parasite control program with particular attention being paid to mares, foals and yearlings.
Mare and Foal Worming Schedule
Pregnant mares should be wormed two weeks prior to expected foaling date. It is recommended to regularly treat foals in conjunction with their dams from 4 weeks of age.
Oxfendoate Oral Broad Spectrum Worm Paste for Horses is given at the recommended dose level of 1 mL per 20 kg bodyweight.
The dose of 1 mL per 20 kg bodyweight delivers 10 mg/kg of Oxfendazole and 13 mg/kg of Pyrantel Embonate.
The contents of this syringe will treat one horse at 700 kg bodyweight.
Each weight marking on the syringe plunger will deliver 5 mL of paste, which is sufficient to treat 100 kg bodyweight.
Depress the plunger to the chosen dose, depositing the dose on the base of the tongue.

Ostensibly they would seem
DID YOU KNOW THAT... The iconic Aussie bush wear manufacturer and retailer, R. M Williams was once owned by the French luxury fashion house Louis Vuitton? R. M WILLIAMS (AUS)

APVMA Approval No. 87987 (Australia)
Available in:
For the treatment and control of tapeworms, roundworms, bots and skin lesions caused by Habronema, Draschia,
Traditional broad-spectrum wormer.
Active Constituents
Abamectin 3.7 mg/g
Praziquantel 46.2 mg/g
Pack Size
32.4g syringe
Randlab supports the responsible use of anthelmintics

Whenever possible the decision to deworm should be based on the results of a Faecal Egg Count (FEC). Consult a veterinarian when developing a worm control program to suit your specific needs.
Indications
At the recommended dose rate Promax All Wormer is effective in the treatment and control of the following parasites: tapeworms, roundworms (including arterial larval stages of Strongylus vulgaris and benzimidazole resistant small strongyles) and bots.
ProMax All Wormer for Horses also effectively controls skin lesions caused by Habronema and Draschia spp. cutaneous larvae (summer sores), and Onchocerca spp. microfilariae (cutaneous onchocerciasis).
Pharmacology
At the recommended dose rate, ProMax All Wormer is effective aginst the following parasites:
• Small Strongyles including benzimidalzole resistant (adults and immature): Cyathostomum spp., Cylicocyclus spp., Cylicostephanus spp., Cylicodontophorus spp., Gyalocephalus spp. etc
• Large Strongyles: Strongylus vulgaris (adults and arterial larval stages), Strongylus edentatus (adults and tissue stages), Strongylus equinus (adults) and Tridontophorus spp. (adults).
• Bots: Gasterophilus spp. (oral and gastric stages)
• Ascarids: Parascaris equorum (adult and immature)
• Habronema muscae (adult)
• Onchocerca spp. (microfilariae)
• Hairworms: Trichostrongylus axei (adult)
• Intestinal Threadworms: Strongyloides westeri (adult)
• Lungworms: Dictyocaulus arnfieldi (adult and immature)
• Pinworms: Oxyuris equi (adult and immature)
• Tapeworms: Anoplocephala perfoliata, Anoplocephala magna, Paranoplocephala mamillana (adult, immature, heads, segments).
ProMax All Wormer is safe to use in foals, pregnant mares and breeding stallions.
Dosage And Administration
Dose orally at the recommended rate of 0.2 mg/kg abamectin and 2.5 mg/kg praziquantel.
Each weight marking on the syringe plunger will deliver sufficient paste to treat 50 kg bodyweight.
The contents of the syringe will treat a total of 600 kg bodyweight.


YOU KNOW THAT... A filly who cost only $10K as a yearling has spawned an empire worth $16.3 Million?

As she entered the auction ring, the most remarkable thing about the brown yearling thoroughbred filly was that she was unremarkable.
She stood a mere 14.2hh and her pedigree page struggled to show any of the black type so favoured by racing connoisseurs. The filly was soon knocked down for the price of an average pony at $10K.
Named Yankee Rose, she would win her first two race starts before being unluckily beaten into second place in Capitalist’s Golden Slipper. She would win her first Group One race (the ATC Sires Produce Stakes) at her next start.
The following Spring the diminutive filly won her second Group One race (The Champion Stakes) as well as placing third in Australia’s premier weight-for-age contest, the Cox Plate, behind champions Winx and Hartnell.
Yankee Rose was subsequently purchased by Japan’s Northern Farm for an estimated $2.3M. She retired to stud having raced on only 10 occasions for 4 wins and over $2M in prizemoney.
But it is at stud that Yankee Rose has really excelled, with her first foal selling for AU$2.4M (Romneya a filly by Deep Impact/$315K in prizemoney) and her third foal for AU$4.48M (a colt by Lord Kanaloa).
However, it is Yankee Rose’s second foal that is flying the flag for the Aussie mare. The filly named Liberty Island (by Duramente) was Japan’s Champion two-year-old last season and is set to become Japan’s champion three-year-old. She has thus far had 7 starts for 5 wins (incl 3 x Group 1’s), two seconds and over AU$5.5M in prizemoney.
Yankee Rose’s $10K purchase price has now compounded into an extraordinary AU$17M, with more to come.
Cheers, to all the Yankee Rose’s of the world. Winners of the genetic lottery that fuel the dreams that keep horsemen saddling up each waking morning.
APVMA Approval No. 88042 (Australia)
Available in:
For the treatment and control of roundworms, tapeworms, bots, nematode skin lesions and ticks in horses
Low volume wormer for all your worming needs. Suitable for horses weighing < 700kg or 2 x 350kg horses.
Active Constituents
Ivermectin 18.7 mg/g
Praziquantel 140 mg/g
Pack Size
7.49g syringe
Whenever possible the decision to deworm should be based on the results of a Faecal Egg Count (FEC). Consult a veterinarian when developing a worm control program to suit your specific needs.
Low volume, broad-spectrum dewormer
Randlab supports the responsible use of anthelmintics

All horses should be subject to regular worming to maintain good health and peak condition. Decisions to worm should be based on individual and herd Faecal Egg Counts (FEC).
Goldmectin LV Ivermectin Oral Paste is highly effective in the treatment and control of most types of equine helminths including tapeworms, gastrointestinal, cutaneous and pulmonary nematodes and bots in horses of any age.
Susceptible parasites include: tapeworms, roundworms (including arterial larval stages of Strongylus vulgaris and benzimidazole resistant small strongyles), bots, and skin lesions caused by Habronema and Draschia spp. (summer sores) and Onchocerca spp. microfilariae (cutaneous onchocerciasis).
Ivermectin may also be used as an arachnicide to remove feeding ticks.
At the dosage rate of 1 mL per 100 kg bodyweight Goldmectin LV delivers 0.2 mg/kg of ivermectin and 1.5 mg/kg of praziquantel.
For the treatment and control of the following parasites in horses:
• Small Strongyles including benzimidazole resistant (adults and immature): Cyathostomum spp., Cylicocyclus spp., Cylicostephanus spp., Cylicodontophorus spp., Gyalocephalus spp. etc
• Large Strongyles: Strongylus vulgaris (adults and arterial larval stages), Strongylus edentatus (adults and tissue stages), Strongylus equinus (adults) and Tridontophorus spp. (adults).
• Bots: Gasterophilus spp. (oral and gastric stages)
• Ascarids: Parascaris equorum (adult and immature)
• Habronema muscae (adult)
• Onchocerca spp. (microfilariae)
• Hairworms: Trichostrongylus axei (adult)
• Intestinal Threadworms: Strongyloides westeri (adult)
• Lungworms: Dictyocaulus arnfieldi (adult and immature)
• Pinworms: Oxyuris equi (adult and immature)
• Tapeworms: Anoplocephala perfoliata, Anoplocephala magna, Paranoplocephala mamillana (adult, immature, heads, segments).
• External parasites: Ticks, mites and lice.
Goldmectin LV for Horses also controls skin lesions caused by Habronema and Draschia spp. cutaneous larvae (summer sores) and Onchocerca spp. microfilariae (cutaneous onchocerciasis).
Particular attention should be given to young horses and mares. Foals should be treated initially at 6 to 8 weeks of age and this treatment repeated regularly as required.
Goldmectin LV has shown a wide safety margin at the recommended dose level. It may be used in horses of all ages. Mares may be treated at any stage of pregnancy. Stallions may be treated without adversely affecting their fertility. Suitable for foals older than 6 weeks old.
Goldmectin LV is given orally at the recommended dose level of 1ml/100 kg bodyweight. The dose of 1ml/100 kg bodyweight delivers 0.2 mg/kg of ivermectin and 1.5 mg/kg of praziquantel. The contents of this syringe will treat one horse at 700 kg bodyweight. Each weight marking on the syringe plunger will deliver 1.07 g of paste which is sufficient to treat 100 kg bodyweight.
Safety Directions
Protection of Wildlife, Fish, Crustaceans and the Environment: Ivermectin is extremely toxic to aquatic species. Do not contaminate dams, rivers, streams or other waterways with the chemical or used syringe.


DID YOU KNOW THAT...
The European avantgarde art movement “Dadaism” is French slang for “hobby horse”?
DADAISM (FRANCE)
The name came about when German artist Richard Huelsenbeck randomly slid a letter-opener into a dictionary and it landed on the word “dada” , meaning 'hobby horse'.
Dadaism evolved in the aftermath of World War I. The movement rejected the logic, reason, and aestheticism of modern capitalist society and chose to express itself through nonsense, irrationality and anti-bourgeois sentiments. Media spanned visual, literary, sound media, collage, sound poetry, cut-up writing and sculpture.


Whether it be recovery post training or competition or joint health, Randlab has a range of supplements to get you where you need to be.
Available in:
Unique proprietary blend of natural botanical products and adaptogens which may assist in optimising general health and recovery from strenuous exercise and competition.
May be beneficial in reducing oxidative stress and muscle fatigue caused by free radicals generated during exercise.
Active Constituents
Pinus densiflora, Actinidia chinensis, Aloe vera, Agave inulin, Papain
Pack Size
1L bottle
WARNING: This product is believed to contain no prohibited substances, but veterinarians should check with any governing authority before prescribing in competition horses. It is WADA and HASTA approved.
As Optimise X is a nutritional supplement, we make no specific claims about its use. However, Optimise X has been shown in humans to:
• Optimise general health
• Aid recovery from strenuous exercise/training/competition
• Reduce oxidative stress
• Reduce muscle fatigue
• Improve muscle contractility
• Improve endurance
• Improve joint mobility and decrease joint pain
• Regulate insulin
• Cardiovascular protective
• Value in the prevention and treatment of pathologies associated with oxidative stress, cancer and aging.
In horses, Optimise-X may be beneficial in reducing oxidative stress and muscle fatigue caused by free radicals generated during exercise, promoting general health and allowing horses to perform at their optimum. Its cardiovascular affects may be of benefit in conditions of the respiratory system such as Exercise Induced Pulmonary Haemorrhage (EIPH / “bleeders”).
Optimise X contains a unique blend of natural ingredients which are believed to function as adaptogens. An adaptogen is a natural substance, often derived from plants, that is considered to help the body adapt to stress, fatigue, and aging by acting upon vital tissues and organs and promoting or restoring normal physiological functioning. Research in vitro and in the human literature would indicate the key ingredients in Optimise X have the following properties:
Pinus densiflora; antioxidant, antimicrobial, anti-inflammatory, insulin regulation, neuroprotective, antimutagenic.
Actinidia chinensis; antioxidant, anti-inflammatory, immunoregulatory, antimutagenic, antihyperlipidemic, insulin regulation and cardiovascular protective. Good source of triterpenoids, polyphenols, Vit C, amino acids and minerals.
Aloe vera; antioxidant, antibacterial, anti-inflammatory, insulin regulation, antimutagenic, antihyperlipidemic. Contains polyphenols which are powerful antioxidants.
Agave inulin; fibre source, pre-biotic, improves digestive health Papain; anti-inflammatory
In a series of double-blinded, placebo-controlled human exercise physiology studies, Optimise X has been shown to:
• Increase time to fatigue of trained and untrained cyclists on a cycle ergometer.
• Reduce oxidative stress induced suppression of aerobic respiration thereby increasing the efficacy of ATP production.

CONTAINS NO PROHIBITED SUBSTANCES
• Blunt the decrease in force generating capacity that occurs during an acute bout of resistance free weight training, which improved the quality of work during the training session.
• Reduce blood lactate concentration during exercise at 70% of peak power output.
• Reduce perceived exertion at 70% and 95% of peak power output.
Shake bottle well before each use.
In some jurisdictions it is a requirement for supplements to be administered in the horse’s feed so that it may be voluntarily consumed. Administration by syringe over the back of the tongue withdraws the Regulator’s requirement for voluntary consumption and would require registration of this product as a medicine.
Most horses find Optimise X palatable and will eat it readily in their feed.
The recommended daily dose is 25mL twice daily mixed in the horse’s feed.
At times of acute stress (eg heavy competition, following an episode of EIPH/”bleeding”, long-distance travel, “tie up”/rhabdomyolysis, etc), this dose may be increased two to fourfold.
We are interested to hear your feedback on this product. Please either email Dr Michael Robinson on mrobinson@randlab.com.au or ring on +61 (0)451 481 050.


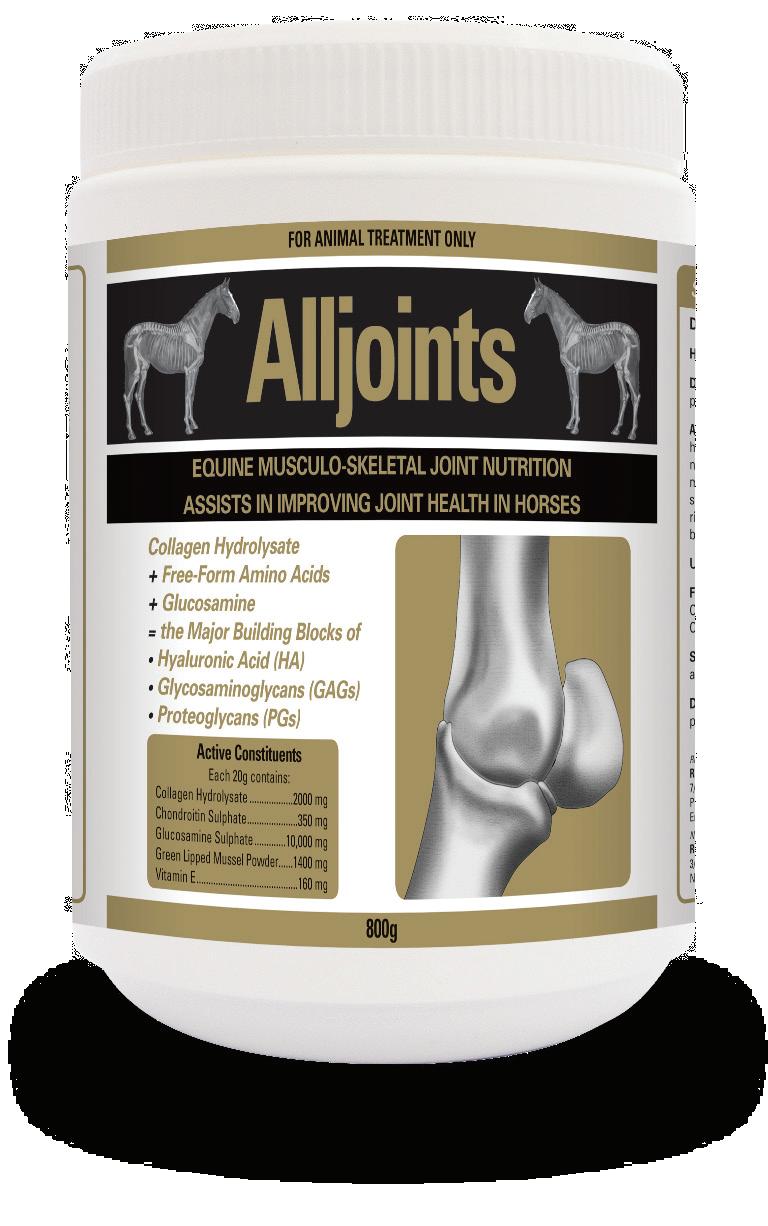
Unique formulation combining key active ingredients. Supports joint cartilage repair and maintenance in horses.
Active Constituents
Each 20g contains: Collagen
Hydrolysate 2000 mg, Chondroitin
Sulphate 350 mg, Glucosamine
Sulphate 10,000 mg, Green Lipped
Mussel Powder 1400 mg, Vitamin E 160 mg
Pack Size
800g jar
One level scoop provides the daily dose of 20g
Randlab products to aid recovery post competition and training
Available in:
Great value electrolyte, B-group vitamins and antioxidant supplement with a high concentration of ingredients. Suitable for use as a feed supplement or as a recovery drench.
Active Constituents
Each 30g dose provides;
Sodium 3.60g, Potassium 0.40g, Calcium 2.50g, Magnesium 0.40g, Chloride 3.40g, Phosphate 3.80g, Sulphate 1.30g, Bicarbonate 10.6g, Thiamine (B1) 0.11g, Riboflavin (B2) 0.11g, Niacin (B3) 0.32g, Pyridoxine (B6) 6.00mg, Vitamin B12 0.43mg, Choline 0.16g, Folic Acid 24.48mg, Inositol 0.27g, Vitamin E 0.22g
Pack Size
15kg pail
One level scoop provides the daily dose for in feed supplementation of 30g. This should be given twice daily in times of heavy sweating.
Recovery drench administer 150-200g (5-7 scoops) diluted in 1-2L of water by stomach tube.

Oral Electrolyte paste with B-Group
Vitamins, Vitamin E and Folic Acid. To aid recovery in hard working or dehydrated horses.
Active Constituents
Each g contains: Sodium 63.0mg, Chloride 208.8mg, Potassium 81.3mg, Magnesium 2.96mg, Calcium 21.2mg, Zinc 1.34mg, DL-tocopheryl acetate (Vitamin E) 18.22mg, Folic acid 0.35mg, Thiamine mononitrate (Vitamin B1) 1.70mg, Riboflavin (Vitamin B2) 1.44mg, Nicotinamide (Vitamin B3) 5.55mg, Calcium pantothenate (Vitamin B5) 3.00mg, Pyridoxine hydrochloride (Vitamin B6) 0.17mg, Cyanocobalamin (Vitamin B12) 0.68mg
Pack Size
60g syringe or box of 12 x 60g syringes


Active Constituents
Electrolytes, B vitamins and energy saline drench for horses.
Each 300g sachet provides Vitamin B Complex, balanced electrolytes; sodium, potassium, magnesium, calcium, chloride, sulphates, volatile fatty acids (propionate, acetate), buffering agent (citrate) and an anti-oxidant (Vitamin E), all in a rapid and completely water soluble form.
Pack Size
300g powder in a sealed foil sachet
Highest concentration of branched chain amino acids for optimum muscle recovery and treatment of “tie up”.
Active Constituents
L-Leucine 22.00g, L-Isoleucine 5.50g, L-Valine 5.50g, L-Glutamine 0.68g, L-Carnitine 0.43g, Aniseed oil 25g/L
Pack Size
60mL adjustable dose syringe
Available in:
Omeprazole paste for treatment and prevention of gastric ulcers.
Convenient single dose syringe.
25% better absorption than competitors in 3 x University trials means better bioavailability.
Active Constituents
Omeprazole 370 mg/g pH-buffered Paste
Pack Size

*In some countries, Goldcort Injection may be marketed as Ferra-Cort Injection.
Injectable corticosteroid (flumethasone) for intramuscular or intra-articular use.
For the treatment of acute musculoskeletal conditions in horses, dogs and cats.
Active Constituent
Flumethasone 0.5 mg/mL

10 x 6mL single dose syringe

Pack Size 12 x 3mL single dose vials


Lipotrope injection for horses and camels with liver disease.
Amino acid blend to help restore liver function. Hoof Gold Premium product for maintenance of healthy hooves, hair and skin.
Active Constituents
Each 30g scoop contains:
Biotin 30mg, Zinc 250mg, Methionine 500mg, Arginine 100mg, Lysine 100mg, MSM 2000mg, Lecithin 200mg, Folic Acid 50mg, Magnesium 150mg, Copper 75mg, Vit E 500iu, Vit C 1000mg, Calcium 4200mg, Phosphorous 3860mg
Pack Size
1.5kg tub or 6kg tub
Active Constituents
L-Methionine 40mg/mL, Inositol 50mg/mL, Carnitine 50mg/mL, Arginine 50mg/mL
Pack Size
100mL multidose vial

Anti-inflammatory, analgesic and antipyretic oral paste for pain relief from musculoskeletal conditions.
Aids in the treatment of musculo-skeletal conditions. Convenient multi dose syringe.
Active Constituent
Phenylbutazone 200 mg/mL
30mL multidose syringe
Horses In

There was not much to recommend the unraced three-year-old gelding. He was on the small side, gangly, had bad knees and on paper, his pedigree was uninspiring.
Originally retained by his breeder, the gelding was paradoxically named Takeover Target. He had had five barrier trials and two lots of knee surgery before he found his way into a tried horse sale at the Inglis Newmarket Complex.
Joe Janiak, a part time horse trainer and full-time taxi driver, living out of a caravan in the NSW rural town of Queanbeyan, admits that he bought the gelding on spec, paying a mere $1250. “Plus GST”, Joe is quick to add. Their relationship did not necessarily get off to a great start with the gelding striking Janiak in the head and delivering a wound that required 30 stitches.
It would be a year before the horse made it onto a racetrack, winning his first seven starts by large margins and culminating in an elite Group 1 victory. It was then that the $1M plus offers began to flow to buy the gelding.
But Joe decided to retain the horse he called “Archie” and he would go on to take his taxi driver trainer on an unprecedented journey around the world winning group one races in every State of Australia as well as the UK, Singapore and Japan. His win in the 2006 King’s Stand Stakes at Royal Ascot scored Janiak an invitation into the Royal Box to lunch with The Queen.
Takeover Target would eventually be retired as a ten-year-old after fracturing a hind cannon bone in a race at Newmarket (UK). The injury required six screws to be placed in the condylar fracture by veterinarians at the Newmarket Equine Hospital (UK). Once fit to travel, Takeover Target returned to Australia and retirement.
In a career that spanned six seasons, Takeover Target won 21 of his 41 races (including 8 Group 1’s) and was placed on a further 10 occasions. He accumulated over $6M in prizemoney, was Australia’s champion sprinter on three occasions as well as being the World’s Highest Rated Turf Sprinter and Global Sprint Challenge champion.
Takeover Target the $1250 (+GST) Aussie battler horse who took on the world. And won.

page 17
Frederico Tesio was a fabled Italian breeder, geneticist, and racehorse trainer of the early 1900’s. No doubt he also dressed well. His somewhat unconventional views on breeding racehorses resulted in several champion racehorses and stallions such as Botticelli and Ribot. But none more important than the breed-shaping stallion Nearco (f. 1935). In two seasons of racing, the Tesio bred and trained Nearco was undefeated in 13 starts in Italy from 1000m to 3000m, including Italy’s most prestigious race, The Italian Derby. Tesio then decided to take on the world and set the stunning brown colt for the Grand Prix de Paris.
With the rise of Mussolini and the recent association of the Italian National Fascist Party to the German Nazi Party, Tesio had to receive permission to race the colt in France from none lesser than Mussolini himself.
The Grand Prix de Paris (1938) became somewhat of an international incident with the French people believing that the Italian stallion somehow represented the spreading black heart of fascism. The parochial French racegoers booed when Nearco strode onto the course and cheered wildly when his French rivals took to the track. Nearco would win the race convincingly from 18 challengers, including the English Derby winner Bois Roussel. The situation was further fuelled when Nearco’s Italian jockey gave the Fascist salute on returning to scale.
Fearing reprisal and wanting the best for his colt, Tesio sold Nearco four days later to the British owner of Beech House Stud on the outskirts of Newmarket for a then record of £60,000 (approx AU$7.7M in today’s money).
As a stallion, Nearco proved difficult to handle and was widely regarded as dangerous. In despair, his owner called on the service on veteran stud groom Ernie Lee who tamed the stallion with kindness and within weeks the pair became inseparable.
At the outbreak of World War II, Nearco was considered so valuable that a paddock was constructed at Beech House Stud with a cement underground bomb shelter. Lee trained Nearco to walk underground at the sound of any air raid siren.
At stud Nearco would sire Neartic who in turn would sire Northern Dancer, arguably the most influential sire of the 20th Century. Another of his sons Nasrullah would sire the US champion sire Bold Ruler who in turn sired the ‘Greatest of All Time’ and Randlab poster boy, Secretariat.
Other notable racehorses and stallions descended from Nearco’s male lines included Nijinsky, Lyphard, Nureyev, Storm Bird, Danzig, Danehill, Sadler’s Wells, Seattle Slew, Spectacular Bid, Cigar, Nashua, Sunday Silence, Deep Impact, Ruffian, Riva Ridge, Slew o’Gold, Royal Charger, Grey Sovereign, Red God, Foolish Pleasure, Halo, Roberto, Mill Reef, Sir Percy, Dalakhani, Nyquist, California Chrome, Blushing Groom, Justify, Galileo, Ouija Board, Sea The Stars, Black Caviar, Treve, Frankel and Winx.
It is estimated that less than 1% of horses in GB and Ireland do not have some Nearco blood in their pedigree. None of which is likely to have happened had Nearco remained in his homeland of Italy as his breeder/owner/trainer Tesio had first intended.
Horses In History Part I available in the Randlab 2020-21 catalogue & Horses In History Part II available in the Randlab 2021-22 catalogue.

The Mongol Empire covered 24 million square kilometers (17.81% of the world’s surface area) and was the largest contiguous land empire in history. The Empire encompassed most of Asia, the Middle East and Russia. The Empire was established by Genghis Khan in 1206 and grew rapidly under his descendants. Behind only the British Empire, it remains the second largest empire the world has known. However, the Empire only survived about 190 years.
Horses made the Empire possible, allowing conquest of areas previously not accessible by other means. The Mongol horsemen were responsible for the invention of the steel stirrup which provided warriors with a stable platform allowing them to ride up to 130km per day and to shoot arrows when retreating, a revolutionary battle tactic at the time. The stirrup is seen as the single technological breakthrough that helped establish the Empire.
Every soldier travelled on horseback making the army the most mobile military force in the world. The Mongol horses could travel long distances each day on little to graze.
The Mongol cavalrymen preferred mares as their mounts as their milk provided nourishment during extended military campaigns. The milk was often fermented to produce a potent alcoholic drink.
The Mongols would also cut a vein in their horse’s necks at the end of every day, taking a few swallows of blood for sustenance (haematophagia).
But horses were not only integral to the expansion of the Empire. They also facilitated an era of unprecedented travel, trade and cultural exchange (“The Ancient Silk Road”). In the era of Kublai Khan (Genghis Khan’s grandson) a visitor could travel the lengths of the empire on a Mongol pass. This consisted of over 10 000 outposts (yams) and over 300,000 horses. Travelers could swap their horses for fresh horses at each of the yams. The yams also provided a rapid postal and messenger service. A messenger would ride a horse as hard as possible to reach the next yam as fast as possible. They rode so intensely that they often had to tie themselves to the horse with tight bands around their head, chest, and stomachs to prevent falling off.
At least forty horses were sacrificed at Genghis Khan’s entombment, as the Mongols believed that the horses would carry him in the afterlife, as they had in his lifetime.

page 24
Seen here with Richardson's then surgical resident Dr Steve Zedler, now at the University of Queensland. In May 2006, Barbaro became the 132nd winner of the Kentucky Derby by an emphatic 6.5 lengths and only the sixth horse in history to win the race undefeated.
Two weeks later the colt would line up as a 2-1 on favourite in the second leg of the US Triple Crown, the Preakness Stakes. An air of optimism surrounded Barbaro’s attempt to become the first winner of the fabled Triple Crown in 28 years.
The colt seemed unusually on edge come Preakness day and broke through the barrier stalls as the last horse was being loaded. He was pulled up quickly by jockey Edgar Prado and, after being briefly examined by the track veterinarian, was cleared to race before being replaced in the starting gates.
The field had barely travelled a furlong when, directly in front of the public grandstand, jockey Prado was again pulling the colt to a standstill. The jockey had felt the colt faulter and looked around to see his off hindleg flaying around in the air like a limp flag. Barbaro had suffered a comminuted fracture of his off hind pastern (which had exploded into more than 20 pieces), cannon and sesamoid.
There would be no better man for the repair of Barbaro’s shattered leg than Professor Dean Richardson from the New Bolton Center of Veterinary Medicine, University of Pennsylvania. As fate would have it, the Center, was only 90 min away by horse ambulance. Amongst Richardson’s support team on that day was his surgical resident Dr Steve Zedler, who is now based at the University of Queensland. Richardson performed the seven-hours of intricate surgery by methodically recreating a bony scaffold using a 12” LCP and 23 screws. The limb was then cast.
Zedler recalled, “It was a piece-by-piece, step-by-step thing. I'll take that and screw it to that. Richardson had to fuse both the fetlock and pastern joints as well.”
The world anxiously watched every step of Barbero’s recovery. The colt seemed to galvanise horsemen and the general public alike in a wave of unprecedented emotion and hope. By the time the last leg of the Triple Crown was to be run at Belmont Park (NY) three weeks on, a giant card had been installed at the racetrack for well-wishers to express their concerns. The card measured 19m wide and 2m high and featured four life-size images of Barbaro.
New Yorkers are a hardened breed but on one afternoon in early June 2006, 22,000 of them turned up in force to sign a giant card that simply stated what the rest of the world was hoping, “Get well Barbaro! We love you”.

28
Australia’s Olympic Eventing Team is renowned for punching above its weight. Australia first competed at the Olympic Games in Equestrian in 1956. Although the Games were held in Melbourne that year, the equestrian events were held in Stockholm due to Australia’s rigid animal quarantine laws. The next Games (Rome 1960) saw Australia win its first Gold in Equestrian with Laurie Morgan (Gold Individual & Team), Neale Lavis (Silver individual/Gold Team) and Bill Roycroft (Gold Team) all bringing home Eventing medals.
But only a hop, skip and jump across the Olympic arena, the Australian showjumping team has not been as successful. The high-priced sales of many high-class Aussie showjumpers into the European and American markets has meant that the Australian showjumping team is often left threadbare. Bonvale was a wiry little black Australian Stockhorse gelding. Station-bred from generations of cattle horses. Highly strung for a stockhorse. But 15yo John Fahey, son of a dairy farmer from the mid North Coast of NSW, had jumped the horse for his previous owner and convinced his father to buy the gelding for £550 when he came up for sale as part of a deceased estate. “That was a lot of money for a stockhorse back in those days. He was only just under 15hh, but we just knew he wanted to jump”.
Soon after purchasing Bonvale, 15yo Fahey took Bonvale to the local Taree Show and put him in the high jump. The pair won the class clearing 2.133m(!), confirming the little stockhorse was made of the right stuff.
Fahey had an unorthodox ritual he used to employ before jumping Bonvale. The horse had a habit of dropping his off hind leg, which sometimes caused him to pull a rail. To counter this problem Fahey would shift his weight to the left when going over a jump. But this caused his right leg to kick up. To counteract this, Fahey used to tie his right leg down to stop his body from moving too far to the left.
In 1964, twenty-year-old Fahey riding Bonvale won the final Olympic selection event and suddenly the country boy and his stockhorse were on their way to the Olympic Games. The journey to Tokyo was by ship and would take ten days. “We used to exercise the horses by walking them below deck”. Fahey was extremely unlucky not to win an individual Olympic silver medal after knocking a couple of rails that John says he “never should have had down.”
On the day of the event each member of the showjumping team was presented with a sheepskin saddlecloth by the Equestrian Federation of Australia and told they were only to be used during their competition round.
Fahey and Bonvale went clear to the 12th jump, when the sheepskin saddlecloth moved back and as Bonvale went to jump the water the saddlecloth came over his tail and just took his attention.
The horse uncharacteristically got the next two fences down. This meant that Fahey and Bonvale ended up with eight faults after two rounds and equal third place with one of the world’s outstanding combinations, Peter Robeson and Firecrest (GBR).
The Ground Jury could have awarded both combinations a bronze medal, but instead determined there would be an additional jump off round. Unfortunately, Robeson then jumped clear to take the Bronze Medal and Fahey ultimately finished in 4th place.
Sixty years later, their performance remains the best performance by an Australian showjumper at the Olympic Games. It was also the best performance of the entire Australian equestrian team at the Tokyo Games.
“Bonvale was such a versatile horse, he could do anything, jump 1.82m in a six bar, win a speed class and the Grand Prix; he could do it all!”, Fahey commented. Bonvale and Fahey would again represent Australia at the next Olympics in Mexico. They also won at Hickstead International Showjumping on three consecutive days - a feat that had never been done before.
A few years later Bonvale was retired from competitive jumping after bowing a tendon in a speed round at Madison Square Garden. The little black stockhorse was inducted into Equestrian Australia’s Hall of Fame in 2014.

Prince Charles started secretly dating Lady Diana Spencer in the Summer of 19801. Around the same time, Charles bought an old racehorse called Allibar with a view to riding him in some amateur point-to-point races. At the time, the Prince declared his motivation as being, “I wanted to understand what it was like from a jockey’s perspective. You have no idea until you do it what incredibly hard work it is.” Others believe, it was an effort to impress the much younger (13 years difference) Diana. As if being the next King of England was not impressive enough.
"All those years ago I remember the struggle to try and get fit, fit enough to ride in these races. I ran, I also bicycled endlessly, on a bicycle, where you take the seat off so you can’t sit down." A Palace spokesperson declined to comment.
The Prince’s first ride was at Plumpton in March and he finished a good second. Four days later he finished fourth in a steeplechase at Sandown.
The Prince would not have another ride until October, where once again he finished a creditable second on his own horse Allibar. The day became of greater historical import as it was the first time Charles and Lady Di were seen in public together, whilst his ex-girlfriend, Camilla Parker Bowles, watched from the sidelines. Nonetheless, Charles’ jockey career was off to a good start with three top four placings from as many rides. But, as is often the case, racing can be a fickle master. Whilst preparing for his next start at the popular Cheltenham Festival, Charles rode Allibar in a morning gallop. Towards the end of the workout he felt Allibar slow before crashing to the ground. “I held his head while the life drained out of it. I couldn’t bear it because you see the life going out of his eyes. It nearly finished me. I’ve never had that happen”.
The Prince found a replacement horse for Allibar in the optimistically-named Good Prospect. Charles’ next two rides did not go as well with the Prince falling off twice in the space of five days, the first time at Cheltenham in front of a large jeering crowd.
The then Prince of Wales said that after he fell to the ground, other horses “galloped over him and disappeared so fast, that you’re left in silent despair!” But only after Charles was seen whipping the ground and shouting "bloody hell".
Charles sixth and final race ride was on his grandmother’s horse and he finished ninth.
Charles and Diana married the following July and that was the end of his jockey career. But that's a whole different story.
Horses In History Part I available in the Randlab 2020-21 catalogue & Horses In History Part II available in the Randlab 2021-22 catalogue.

page 41
The bison calf’s life did not get off to a great start when his mother was killed by poachers, leaving him an orphan. His peril was noted by an American cowboy/railroad worker by the name of Collin ‘TC’ Thorstenson, who decided to adopt the calf. TC had grown up on a Sioux Indian reservation in North Dakota and had a natural affinity with animals. TC elected to bottle feed the calf every hour around the clock and raised him like a horse. It wasn’t long before the bison had imprinted on the cowboy and followed him everywhere. He named him Harvey Wallbanger because he would bang his body against the stable wall when left alone.
By 1985, the bison had become a local celebrity and was invited to participate in a match race over 100m against a quarter horse. A full-grown male bison may weigh up to 900kg and stand 1.4m tall. Despite their massive size and unathletic build, bison are known to be able to run up to 72km/h and jump as high as 1.8m. They are also strong swimmers.
Harvey Wallbanger won that first match race by 2.5 lengths and so the legend began.
Harvey campaigned in America, Canada and Mexico winning 79 of his 93 starts against quarter horses, thoroughbreds and standardbreds. In one year alone he won over $108K (~ AU$342K in today’s money) in prizemoney. He was well known for his speed out of the gates and his fighting spirit in a close finish. His races were won as much by intimidating the opposition as by speed.
Harvey died after eating some hay contaminated with oleander at the age of 13. His value was confirmed when the Courts granted TC $475K (approx AU$1.5M in today’s money) in compensation.
Harvey Wallbanger, the bison who thought he was a racehorse. And that’s no BS.

page 52
Pic shows stunt double Gerald Egan on Denny.
The movie The Man from Snowy River (1982) was both a box office and critically acclaimed success. Based on the iconic poem by Banjo Patterson of the same name, the movie came to globally symbolise what was quintessentially Australian.
Contrary to popular misconception, neither the poem nor the movie is set in the Snowy Mountains of NSW. That was just where ‘The Man’ originated from. Patterson never revealed who ‘The Man’ was based on. Unlike the other characters in the poem such as Harrison and Clancy of the Overflow, ‘The Man’ is singularly referred to as such. He is likely a synthesis of several of the mountain horsemen Patterson encountered on his many journeys. Men such as Charlie Lovick and Gerald Egan. Bushmen from some place that was always ‘near somewhere’. In this case, near-Mansfield in Victoria’s High Country.
Denny, the buckskin Australian Stockhorse, had been signed up to play the equine lead in the movie early doors. Five other horses were designated to be his understudy for the less challenging shots.
Like so many stockhorses, Denny was not prone to hogging the limelight. Little is known about Denny, except that he was bred by the Lovick’s, was about 10yo when the film was shot and was easily trainable.
When Tom Burlinson signed up to play the lead role of the fictitious Jim Craig (aka ‘The Man’), he had barely ridden a horse. It was left to Lovick to teach the star to ride like a cattleman and make it believable. Lovick tagged Denny for the job and over the course of six weeks, Lovick and Denny were able to break Burlinson to saddle. Gerald Egan was another near-Mansfield local recruited for the occasion. Gerald is a man for all seasons. A bushman, breaker, racehorse trainer and masterful apprentice jockey master. He is the type of guy that Banjo would have liked to write about.
Egan is also the father of well-known and uber popular Western Australian equine veterinarian, Dr Jack Egan. Egan senior was charged with riding Denny in the iconic scene in the movie known as the “jump into the terrible descent”. Although Burlinson later claimed he had ridden the horse in the scene, he had, in fact, only done the less demanding profile shots, which used a tilted camera to make the descent appear steeper.
In preparation for the dangerous stunt, Egan spent the day walking up and down the mountainside with Denny, letting the horse know and sense every step. Multiple retakes from different camera angles of the treacherous jump were required to assemble adequate footage for the final edit of what has become one of the most iconic scenes in Australia's cinematic history.

page 64
So who IS the most famous horse in history?
Somewhat ironically, the most famous horse (or more accurately equid) in history did not have a name and is generally referred to as Jesus’ donkey.
It was no historical accident that Jesus of Nazareth chose a donkey to make His final return to Jerusalem. In ancient times, entering a city on a donkey signified entry in peace, rather than as a conquering king upon a horse.
Jesus’ donkey is believed to have been a Nubian wild ass (Equus africanus africanus), a now extinct species and an ancestor of the domestic donkey (Equus africanus asinus). In keeping with the custom at the time, domesticated animals were not given names. According to the New Testament as Jesus approached Jerusalem, he beseeched two of his disciples, “Go to the village ahead of you, and at once you will find a donkey tied there, with her colt by her. Untie them and bring them to me.”
The arrival of the King of Zion by donkey had also previously been referenced by the Prophet Zechariah (9.9.-17) some five hundred years earlier.
The donkey is the only animal with a single cross on its dorsum. All donkeys have this cross, although they may be hard to see in donkeys with darker hair coats. The cross is part of the classification of a donkey and it is not present in any other equid, including mules. Why donkeys bear this ancient marking is not certain, but biologists speculate that it is a form of camouflage similar to zebra stripes.
In Christianity the donkey cross marking has come to symbolise the shadow of the cross at Cavalry.
Our commitment to quality starts at sourcing only the finest raw materials and pharmaceutical grade ingredients for our products. We manufacture all our products in facilities that are GMP approved through the Australian regulator, the APVMA. We carefully follow each batch of our manufactured product with strict Quality Assurance (QA) testing in government-approved independent laboratories.
















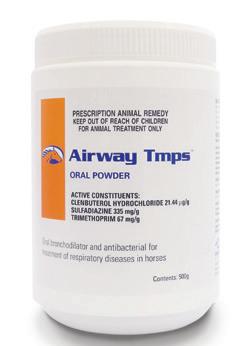










Anti-Inflammatory Suite page 25
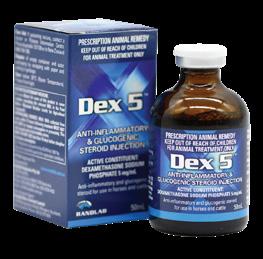


Misc Suite page 42







Repro Suite page 54








Sedatives & Anaesthesia Suite page 33
Antimicrobial Suite page 44


DISCLAIMER:









: Available in Aus : Available in NZ : International export product
: Available in Aus under permit only
The information contained in this catalogue is believed to be correct at the time of publication. However, veterinarians should rely on their own research prior to administering or prescribing any of the products. © Copyright Randlab 2023 Content & Editor: Dr Michael Robinson Design: Design Studio Ninety8
:Available in NZ under ACVM import permit



Alex Macpherson (VIC/TAS/SA)
Phone: 0421 829 101
Email: alex@randlab.com.au
Andrew Grant (QLD/NT)
Phone: 0426 407 117
Email: agrant@randlab.com.au
John Dalton (NSW/ACT)
Phone: 0404 221 776
Email: john@randlab.com.au



Dr Michael Robinson
Global Technical Director/Veterinarian
Phone: + 61 (0) 451 481 050
Email: mrobinson@randlab.com.au

Rebecca Puvanendran (WA)
Phone: 0420 349 301
Email: rebecca@randlab.com.au
Bruce Bell
Assistant General Manager
Phone: + 61 (0) 404 137 514
Email: bruce.bell@randlab.com.au
Randlab has a global distribution network of partners committed to serving veterinarians and their clients wherever they may be.



Rest of the World (Randlab Headquarters)
NEW ADDRESS
71 Milperra Road Revesby, NSW, 2212
Australia
Phone: (+61-2) 9728 3505
Fax: (+61-2) 9728 4352
Email: info@randlab.com
Randlab Europe (Eu Randlab Sprl)
24 Rue Tayant
Ransart 6043 Belgium
Phone: (+32) 475 933 457
Email: bernard.stoffel@gmail.com
Randlab Europe (Joint Products only)
Nupsala Veterinary Services
Mobile: + 44 (0) 1865 922 227 Email: info@nupsala.com www.nupsala.com



Randlab Central Asia
Horsepower Sydney Pty Ltd, Room 404 Chandmani Centre, Jamiyan Gun St, 1st khoroo Sukhbaatar District, Ulaanbaatar, Mongolia
Phone: (+976) 998 812 05 or (+976) 981 112 05
Email: Horsepowersyd@gmail.com
Randlab in Singapore
Mano Equestrian Services pte ltd 135 Woodlands Industrial Park E5, Woodlands E-Terrace Singapore 757504
Phone: (+65) 6363 4236
E: contact@manoequestrian.com.sg
Randlab China 兰德实验室中国 Mobile: (+86) 1801 975 3936
Email: sophia@randlab.com.cn




Chris Barris (NZ)
Phone: (+64-9) 275 5657
Mobile: +64 (0)21 157 2249
Email: chris@randlab.co.nz
Rest of the World (Randlab Headquarters)
NEW ADDRESS
71 Milperra Road
Revesby, NSW, 2212
Australia
Phone: (+61-2) 9728 3505
Fax: (+61-2) 9728 4352
Email: info@randlab.com
Randlab in Japan
EBM Trading, Yokohama-City Higashiasahina, Kanazawa-Ward 1-52-16 Japan, 236-0033
Phone: +81 (0) 80 4364 4002
Email: h.echigo@ebmtrading.com
Randlab Middle East / Gulf Warehouse 5, Al Qusais
Industrial Estate, Area 3, Dubai, United Arab Emirates
Phone: (+971) 426 666 48
Email: omneia@randlab.com
Randlab New Zealand 3/180 Montgomerie Road, Mangere, Auckland, New Zealand
Phone: (64-9) 275 5657
Mobile: +64 (0)21 157 2249
Email: chris@randlab.co.nz
We hope you like this catalogue �� and find it a useful resource to refer to throughout the year ��
But if you decide to discard it ��, please recycle it.

When Secretariat was sold, he was worth more than his weight in gold?
So let’s finish where it all began…
In 2021, I somewhat randomly googled “The most famous horse in history”. It was a Covid thing. And so I disappeared down a wombat hole of famous horses.
According to Google the answer to my original question was Secretariat, the great American racehorse who won the US Triple Crown in 1973.
Rather fortuitously, Secretariat also happened to be the cover boy (original painting by US artist Tom Chapman) for the Randlab annual catalogue, Randlab’s Meloxicam Paste & Suspension and Gastropell Forte. So I kept reading and stumbled across the bizarre twist of fate (a losing coin toss that became racing's biggest win) that determined Secretariat’s ultimate ownership even before he was conceived.
Since then we have featured the unique stories of nearly 100 horses in the Horses In History series. Thankfully, you seem to have enjoyed reading the stories as
much as I have writing them.
This June (2023) marks the 50th anniversary of Secretariat’s historic 31-length victory in the Belmont Stakes, generally regarded as the most emphatic win EVER recorded by a racehorse. It was that day more than any, that the big red horse transcended stardom to become a legend.
Like Elvis, he had the looks, the moves and the swagger of greatness.
WHATEVER HAPPENED TO THE GREATEST OF ALL TIME?
The big red horse was at the peak of his powers in the spring of 1973. Something that could not be said for the USA, which was buckling under the psychological and financial weight of the Vietnam War and The Watergate Scandal. For the first time in its history, America was beginning to doubt itself.
Like Phar Lap (who had also carried the "Big Red" epithet) had done during the Great Depression in Australia, Secretariat became a living metaphor for the might and power of the ailing Nation. The horse was featured on the covers of TIME, SPORTS ILLUSTRATED and Newsweek all in the same week.
It was a fledging 23yo Seth Hancock, newly anointed president of Claiborne Farm, who announced at the end of Secretariat’s two-year-old season that the colt had been syndicated for a record US$6.08M into 32 shares. At 524Kg, he was literally worth more than his weight in gold. After racing on 21 occasions for 16 wins and 4 placings, a career that only lasted a fleeting 15 months closed and Secretariat was retired to the stallion barn at the famous stud in Paris, Kentucky. The same farm where his sire, Bold Ruler, had also stood.
By all standards, Secretariat’s stud career was successful but could never approach his prodigious performances on the track. He sired 582 offspring for 41 stakes winners. He is best known in Australia as the sire of the 1990 Melbourne Cup winner Kingston Rule, who still holds the race record for Australia’s most famous race.
But it was as a broodmare sire that Secretariat really excelled. This is perhaps not surprising as the horse was found at postmortem to have an enormous heart (10kg vs average thoroughbred of 3.9kg), a trait believed to be carried on the X-chromosome.
Secretariat was the maternal sire of such great horses/stallions as Storm Cat, A.P. Indy, Gone West, Dehere, Volksraad, etc and also appears on the maternal line of recent US Triple Crown winners American Pharoah and Justify.
On Wednesday 4th October 1989 at 11:45AM, Secretariat was humanely euthanised, approximately a month after suffering a bout of acute laminitis. His death was one of those. “Where were you when moments…”.
On hearing of his death, people who had never seen the horse were known to weep. More so than any other, he was the horse that had transcended racing and became a cultural phenomenon.
And perhaps for the first time the reality hit. Even legends are ultimately not immortal.











