







First and foremost, I would like to thank you, our valued partners in business, for your continued support over the past year. It seems we might be through the most challenging period linked to the Global Pandemic, and while we now face some other emerging challenges, our industry has continued to be resilient.
Coming out of COVID, we had expected the global supply chain to bounce back quickly, but we have had
to be even more nimble and decisive to ensure ongoing supply of our products. Our long-held strategy to manufacture our products in Australia has certainly helped us avoid significant supply disruption over the recent past.
We continue to work hard to deliver new products to support equine veterinary practice. As usual the past year has been no exception, and we have introduced a number of new products to the market including Butorphanol Injection, Medicaine (prilocaine injection), Pradectin (moxidectin + praziquantel), a new pail presentation for Ulcershield and our latest releases Gentamicin, Dex-5 and Intra-Log (triamcinolone acetate) Injections. Our trimethoprim/sulfadoxine and oxytetracycline injections should be available early in 2023. In a significant expansion for the Company, Randlab Middle East started trading directly in the key equine markets in the region earlier this year. A warehouse was renovated, stocked, and opened in Dubai, and three highly experienced staff hired to service the local needs of the region.
Randlab remains committed to veterinary education and has continued its role as the major sponsor of equine veterinary education in Australia, New Zealand and a major sponsor globally.
Randlab will also be operating from new premises when we reopen in the first week of January 2023. With our ever-increasing product range, the move will hopefully allow us to serve our customers better.
We are excited at what lays ahead for Randlab and our customers in 2023, with a number of new initiatives and products.
Finally, I would like to again thank you for your continued support and wish you, your families, and businesses all the success in the world as we navigate these uncertain times together. Stay safe and well, Angelo
January 2023 will see a new era for Randlab as we move to our new expanded premises in Sydney. This should deliver increased efficiencies and help us to provide an even better service to our customers.
The new address is 71 Milperra Rd, Revesby, NSW 2212. The phone number remains the same, (+61-2) 9728 3505. Drop in and say hello! There is always a fresh pot of Greek coffee on the burner.
Our local team of experienced Territory Managers are here to assist you with all your enquiries and are a great place to start. They are backed up by a team of internal experts and international consultants. From high calibre specialist veterinarians to experts in the equine market we have your needs covered. Refer to the contact list on pages 5 and 71.
The centrepiece and one-stop site for all things Randlab. Here you will find details about all of Randlab’s products, MSDS safety forms, access to scientific papers, newsletters and contact details. Our popular TechTalks, Trainer's Tips and Stable Talk technical brochures are also archived on the website under Resources. This section also contains our comprehensive gastroscopy resources. For more information go to www.randlab.com In addition, our contact form is available on our website (www.randlab.com/contact-us) and is manned 24/7.
Here we showcase the results from our Gastroscopy Clinic Days, highlights from events that we have sponsored and feature upcoming events. Search on Facebook for “Randlab” to connect with us. Like us to keep up to date with the latest Randlab events and offers.
The information contained in this catalogue is believed to be correct at the time of publication. However, veterinarians should rely on their own research prior to administering or prescribing any of the products.



Phone:
Phone:

Phone: (+61-2) 9728 3505 Fax: (+61-2) 9728 4352 Email: info@randlab.com

NEW ADDRESS 71 Milperra Rd Revesby, NSW, 2212

Phone:















Randlab is committed to assisting the equine veterinary profession grow.

Randlab is proud to be the largest supporter of equine veterinary education in Australasia. Your education is our passion.



Sponsorships include every EVA State Meeting, EVA Bain Fallon Silver Sponsorship, AVA Equine events, AVA Hunter Branch Equine Meetings, ANZCVS Equine Stream, NZEVA, Equine Veterinary & Dental Services (EVDS)Conferences, Toothfest 2023, Vet Prac and VetPD.

Randlab is excited to announce its new partnership with the International Society of Equine Reproduction as it prepares to launch its new global education webinar platform.
In addition, Randlab sponsors Local Practitioner’s Meetings, Journal Clubs, University Student Equine Special Interest Groups and Client Information Evenings/Events by request. Such is our commitment that most weeks you will find Randlab support at least two events around Australasia.
Randlab also sponsors equine veterinarians as they continue their volunteer work both in Australia and abroad in developing countries.
But it is not all hard work and education, Randlab likes to see veterinarians getting some well deserved R&R and sponsors the AVA Sailing Day, Sydney City To Surf teams, Chairman’s Lounge luncheon at the Adelaide 3DE and the many dinners for which Randlab is famous.
Randlab will continue its sponsorship of the Gala Dinner and the Prof Hutchins Newshour at the Bain Fallon Lectures and the equine dinners at the NZVA Conference and the Karaka Sales.
Randlab
UQ Practioners Conference and Sports Medicine & Rehab Journal Club
















The horse is a continuous secretor of gastric acid, with the parietal cells of the glandular gastric mucosa producing an astonishing 40L of gastric acid per day. The glandular part of the stomach is protected from acid damage by an integrated array of defences including a thick protective mucus layer and local secretion of sodium bicarbonate which forms a buffer zone immediately adjacent to the epithelium. The upper squamous portion of the stomach has no such innate defences.

In nature horses are known to graze upwards of 18 hours a day. This constant intake of fibre forms a fibre slurry or fibre ball in the stomach that not only absorbs any free gastric acid, but also forms a physical barrier to prevent gastric acid splashing.
With intermittent feeding associated with domestication and routine horse husbandry practices, the stomach may remain relatively empty for much of the day. This provides the potential for free gastric acid to “splash” on the unprotected squamous mucosa. This is exacerbated by exercise and transport with some acid splash occurring even at the walk.
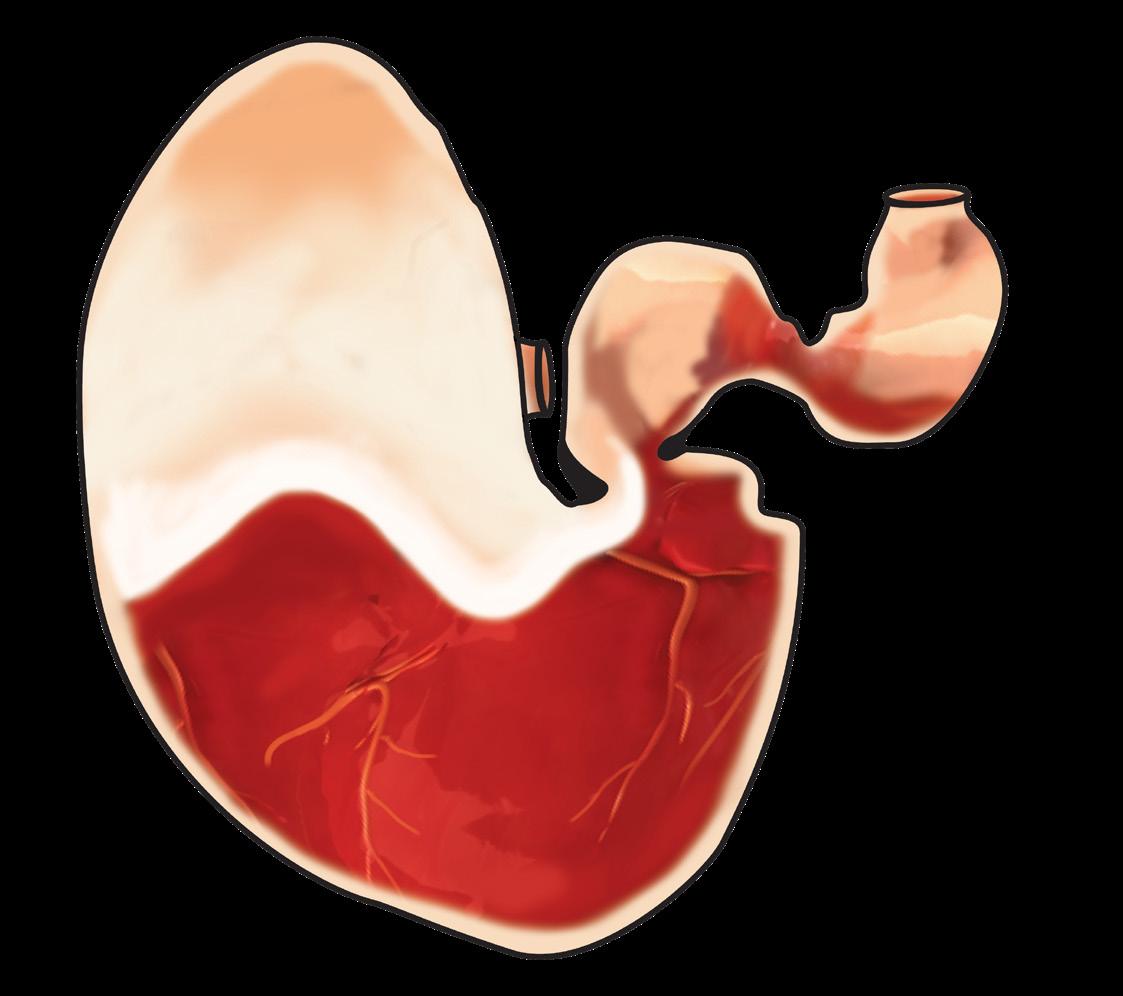

Over time, this increased acid exposure leads to accumulated acid damage resulting in true gastric ulceration of the squamous mucosa (ESGD).
The lower region of the stomach, know as the glandular region, is permanently exposed to gastric acid and has innate protective mechanisms which protect against acid damage. Although the exact pathogenesis of gastric glandular/pyloric disease is not known, it is likely multifactorial with an underlying inflammatory component. Stress is also believed to play an important role. Lesions are often proliferative and true ulceration rarely occurs. Acid suppression remains an important part of management of EGGD. The current consensus recommendation for treatment of EGGD is high dose omeprazole plus sucralfate (4-20mg/kg bid-qid) for a minimum of 8 weeks.

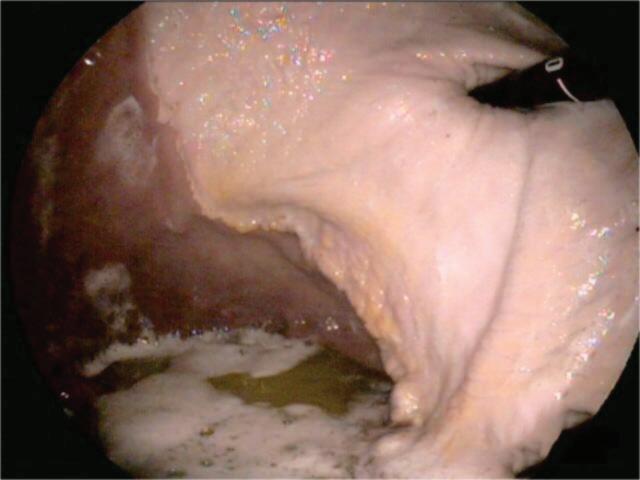
For accurate diagnosis of gastric ulcers and to differentiate ESGD from EGGD, direct endoscopic examination of the gastric mucosa (gastroscopy) is recommended.
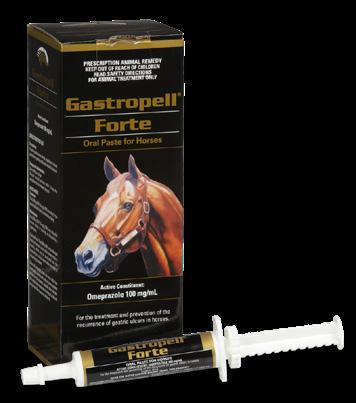
For the treatment and prevention of gastric ulcers in the horse.
Clinical signs of gastric ulceration include:
• poor body condition • poor hair coat
• behavioural changes
Always administer Ulcershield on a relatively empty stomach. Administer a minimum of 20 min and preferably > 60 min prior to feeding.
Ulcershield can be safely administered to stallions used for breeding. Safety in pregnant or lactating mares has not been established. The safety of Ulcershield for longer than 91 days treatment has not been determined in clinical studies.

Complications of long-term administration of omeprazole have not been reported in the horse.
• poor performance • girth pain
• recurrent low grade colic • depressed appetite • skin sensitivity • intermittent loose faeces • reluctance to train • chronic diarrhoea • crib biting / windsucking
Equine Glandular (Pyloric) Gastric Disease [EGGD] is a specific type of gastric disease that affects approximately 40% of racing and sports horses. The disease is characterised by inflammatory lesions, which are most commonly found in the pyloric region of the stomach.
The treatment of EGGD is problematic. Current recommendations include the use of enhanced gastric acid suppression due to the more acidic nature of the gastric fluid found in the pyloric region of the stomach. This is commonly used in combination with a gastroprotectant such as sucralfate. Longer term treatment (> 8 weeks) with the combination is generally required for EGGD.
Omeprazole is a proton-pump inhibitor. Omeprazole suppresses gastric acid by specific inhibition of the H+/K+/ ATPase enzyme system at the secretory surface of the gastric parietal cell. As this is the last step in the secretory pathway, omeprazole will block gastric acid secretion irrespective of the stimulus. Maximum acid suppression occurs after 3-5 days of treatment.
As omeprazole is subject to degradation by gastric acid, the omeprazole in Ulcershield is protected using a unique, patented formulation.
Omeprazole needs to pass through the stomach to the small intestine, where it is absorbed. The presence of significant amounts of food in the stomach, will reduce the amount of omeprazole absorbed.
Ulcershield should always be administered on a relatively empty stomach. Do not feed for 30, and preferably 60, minutes post Ulcershield administration.
Ulcershield has a wide safety margin in horses and foals and can be used in foals from one month old.
Treatment Dose: 4 mg/kg. Adult horses (up to 600 kg) give 6 mL daily for 28 days. Prevention/Maintenance Dose: 2 mg/kg*.
Adult horses (up to 600 kg) give 3.0 mL daily for 28 days.

*The maintenance dose may be insufficient to prevent the recurrence of ulceration in horses subjected to increased stress such as intense training, heavy competition schedule, transport, etc. In such cases, ongoing treatment with the full treatment dose may be necessary.
Withdrawal: It is recommended that horses be weaned off omeprazole over a minimum period of two weeks, by halving the dose weekly.

APVMA Approval No. 82722 (Australia) | ACVM No. A10775 (New Zealand)
For accurate diagnosis of gastric ulcers and to differentiate ESGD from EGGD, direct endoscopic examination of the gastric mucosa (gastroscopy) is recommended.
Gastropell Forte has been shown to be effective in high performance horses (specific APVMA registration). It is also the product of choice in chronic, recurrent or severe gastric ulcers or whenever rapid resolution of gastric ulceration is required.
Clinical signs of gastric ulceration include:
• poor body condition • poor hair coat • behavioural changes • poor performance • girth pain • recurrent low grade colic • depressed appetite • skin sensitivity • intermittent loose faeces • reluctant to train • chronic diarrhoea • crib biting / windsucking
Equine Glandular (Pyloric) Gastric Disease [EGGD] is a specific type of gastric disease that affects approximately 40% of racing and sports horses. The disease is characterised by inflammatory lesions, which are most commonly found in the pyloric region of the stomach.
The treatment of EGGD is problematic. Current recommendations involve the use of enhanced gastric acid suppression (such as Gastropell Forte) due to the more acidic nature of the gastric fluid found in the pyloric region of the stomach. This is commonly used in combination with a gastroprotectant such as sucralfate. Longer term treatment (8-12 weeks) with the combination is generally required for EGGD.
Gastropell Forte is the only registered double-strength omeprazole product on the market. Omeprazole is a dose-dependent inhibitor of gastric acid secretion, so double the strength means double the action. Clinical trial data indicates that omeprazole will inhibit gastric acid secretion to maintain gastric acidity at a nonaggressive level, allowing existing ulcers to heal within 2-4 weeks.
For the performance horse given two or three feeds a day, the stomach is left for long periods without ingestion of food and without the mat of ingesta that helps to contain and absorb the acidic fluid. This exposure to acid is then increased during intense exercise when the stomach volume is reducedto less than half that of the resting horse and the gastric acid is forced up into the squamous region where it damages the squamous stomach lining.
The stomach lining is in a continual cycle of erosion and repair depending on the acidity of the stomach fluid and the horse’s feeding and exercise regimen.
Even though it is dosed orally, Gastropell Forte does not act directly on the gastric fluid. The drug passes through the stomach to the small intestine, where it is
absorbed and travels via the bloodstream to the acid secreting parietal cells of the stomach.
The powerful antisecretory effect of Gastropell Forte causes a dramatic reduction in the acidity of stomach fluid within 2-3 days of commencing treatment, and because the gastric fluid is no longer corrosive to the gastric lining, ulcers then heal spontaneously within 2-4 weeks. Continuation with daily dosing at a reduced dose rate then prevents ulcers from recurring.
Gastropell Forte can provide rapid healing of stomach ulcers. This is likely to be associated with improvement/resolution of clinical signs within two weeks of commencing treatment.
When left untreated, ulcers can recur within two weeks with a severity and across an area equivalent to that prior to treatment. Available data indicates that regular daily maintenance dosing at a reduced rate prevents the recurrence of ulcers.
Always administer Gastropell Forte on a relatively empty stomach. Administer a minimum of 20 min and preferably > 60 min prior to feeding.
The enteric coated omeprazole beads in Gastropell Forte may be damaged by chewing. Always administer at the back of the mouth over the base of the tongue. Avoid chewing.
Treatment Dose (ESGD): 2.0 mg/kg BW (10ml/500 Kg BW) once daily for 2 to 4 weeks followed by daily maintenance dosing.
Treatment Dose (EGGD): 2.0 mg/kg BW (10ml/500 Kg BW) once daily for 6 to 8+ weeks.
Prevention/Maintenance Dose: 5 mL/500 kg BW (1.0 mg/kg BW) once daily whilst horse remains in training.
Withdrawal: It is recommended that horses be weaned off omeprazole over a minimum period of two weeks, by halving
Horse Hack: The treatment of Equine Glandular (Pyloric) Gastric Disease remains problematic. Current recommendations include the use of high dose omeprazole combined with sucralfate (20mg/kg bid-qid) for a minimum of 8 weeks treatment. Other medications such as misoprostol (5μg/kg PO bid) have also been recommended. Husbandry changes are also an essential part of EGGD management. Horses should undergo a follow up gastroscopy at the end of the prescribed treatment period.
APVMA Approval No. 62558 (Australia) | ACVM No. A10165 (New Zealand) Ulcer Treatments | Gastropell® Daily Oral Paste Available in:
Entry
For accurate diagnosis of gastric ulcers and to differentiate ESGD from EGGD, direct endoscopic examination of the gastric mucosa (gastroscopy) is recommended.
Gastropell Daily is the product of choice for the treatment and prevention of low grade gastric ulceration. Ideal for use in horses in light work or under low stress scenarios and for dosing low body weight horses such as foals and ponies.

Clinical signs of gastric disease include: • poor body condition • poor hair coat • behavioural changes • poor performance • girth pain • recurrent low grade colic • depressed appetite • skin sensitivity • intermittent loose faeces • reluctant to train • chronic diarrhoea • crib biting/ windsucking
Signs in foals include:
• depressed appetite • inappetence • teeth grinding • dribbling saliva • colic • diarrhoea • sternal recumbency • dog sitting • weakness
Gastropell Daily contains omeprazole, a dose-dependant inhibitor of gastric acid secretion. Clinical trial data indicates that Gastropell Daily will inhibit gastric acid secretion to maintain gastric acidity at a non-aggressive level, which allows existing ulcers to heal within two to four weeks. A reduced daily dose may then prevent recurrence of ulcers.
Even though it is dosed orally, Gastropell Daily does not act directly on the gastric mucosa. The drug must pass through the stomach to the small intestine, where it is absorbed and travels via the bloodstream to the acid secreting parietal cells of the stomach. The presence of food in the stomach will significantly reduce the absorption of Gastropell Daily and the drug should always be administered on a relatively empty stomach. The antisecretory effect of Gastropell Daily causes a dramatic reduction in the acidity of gastric fluid within three days of commencing treatment, and because the gastric fluid is no longer damaging to the stomach lining, squamous ulcers then heal spontaneously within 2-4 weeks.

Gastropell Daily suppresses acid suppression for 18-24 hours and daily dosing is essential.
daily maintenance dosing at a reduced rate prevents the recurrence of ulcers.
Always administer Gastropell Daily on a relatively empty stomach. Administer a minimum of 20 min and preferably > 60 min prior to feeding.
The enteric coated omeprazole beads in Gastropell Daily may be damaged by chewing. Always administer at the back of the mouth over the base of the tongue. Avoid chewing.
Withdrawal: It is recommended that horses be weaned off omeprazole over a minimum period of two weeks, by halving the dose weekly.
Adult Horses: Treatment Dose, 1.0 mg/kg BW (10ml/500 Kg BW) once daily for 2 to 4 weeks followed by daily maintenance dosing.
Maintenance Dose, 0.5 mg/kg BW (5ml/500 Kg BW) once daily while horse remains in training, or as directed by a `veterinarian.
Foals: 1.0 mg/kg BW (2mL/100Kg Kg BW) once daily or as directed by a veterinarian.


HAVE YOU EVER WONDERED... Who the horse on the Gastropell Daily box is?
Goldikova was a champion French miler and the only horse to win three Breeders' Cup Mile races (2008-2010).
Product image is from an original painting by renowned US horse artist Tom Chapman.
Gastric ulcers adversely affect horse's performance as well as appetite, wellbeing and behaviour.




Horses naturally decrease their feed intake between about 10:00PM and dawn.


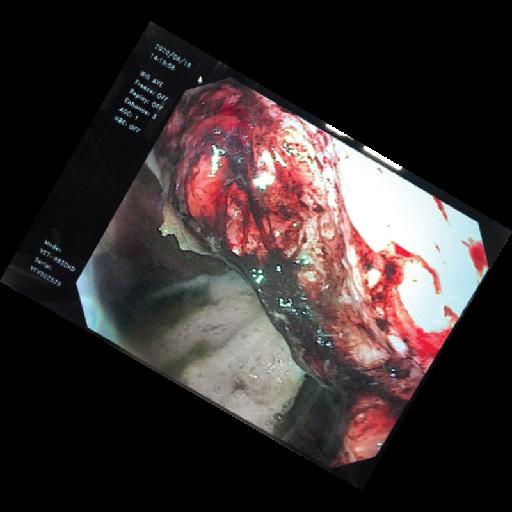


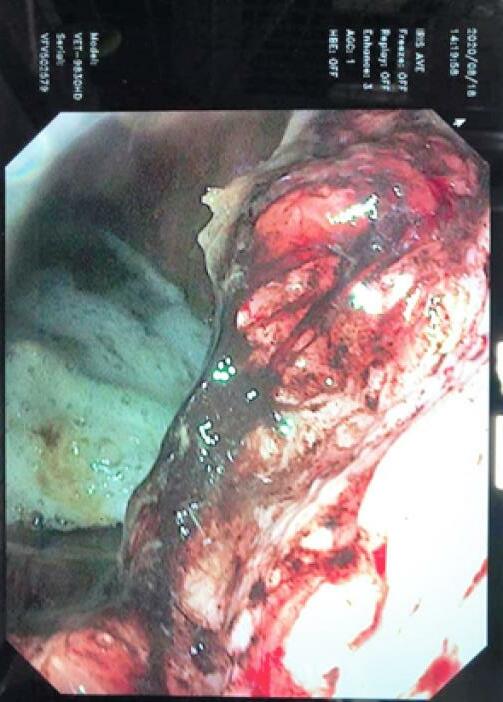

Food in the stomach (esp. hay) will inhibit the absorption of ulcer pastes.
Horses should always be fed some roughage (~ 2kg/3L) before working or traveling. This food forms a slurry or ball in the stomach which prevents acid splash.
Gastroscopy is the only way to accurately diagnose gastric ulcers, make the important differentiation between squamous and glandular/pyloric lesions, grade any lesions, determine the appropriate treatment course and monitor any treatment. ~
Horses should be fed 1-2% dry weight of their bodyweight in roughage/hay per day (5-10kg for a 500kg horse). This helps prevent gastric ulcers, maximises hindgut health and minimises boredom.
Lucerne hay is the hay of choice as it has added acid buffering capacity. Ideally hay should be fed using a slow hay feeder.
Current management recommendations for glandular/pyloric disease (EGGD) include:
Always administer ulcer pastes on a relatively empty stomach. This will usually be first thing in the morning
Administer Gastropell pastes over the base of the tongue as far back in the horse's mouth as possible. Then raise the horse's head. Do not allow the horse to chew on Gastropell pastes (not an issue with Ulcershield)








Wait 30-60 minutes before feeding
Feed horse (at least 2kg/3L roughage or hay) prior to exercise or travel
There is no need to fast the horse overnight
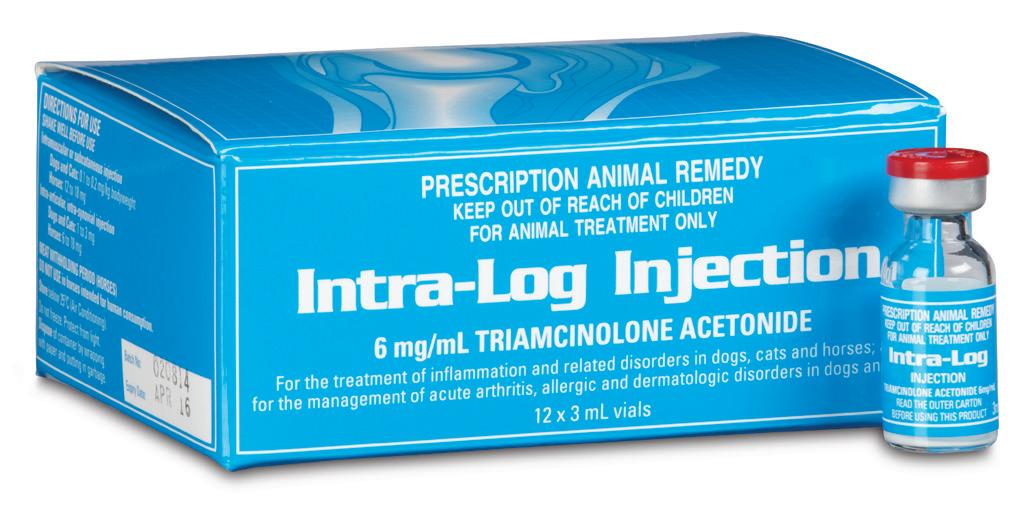
Intra-Log is a potent synthetic corticosteroid that is registered for intra-articular, intra-synovial, intramuscular and subcutaneous use. It is commonly used to treat arthritis, including acute synovitis, osteoarthritis, etc) in horses by the intraarticular route.
Intra-log is also an anti-inflammatory agent, an immunosuppressive agent and a replacement for glucorcorticoid activity in patients with adrenal insufficiency.
Intra-Log is indicated in the treatment of the following disease categories:
• Inflammatory conditions
• Dermatologic disorders
• Allergic disorders
• Arthritis
Intra-log Injection is a highly potent synthetic glucocorticoid with an intermediate onset and long duration of action. It has 5-10 times the glucocorticoid potency of cortisone and no mineralocorticoid activity.

Triamcinolone has effects on virtually every cell type and cell system in mammals. It has been used in the treatment of:
Endocrine conditions (e.g. adrenal insufficiency), rheumatoid arthritis, collagen diseases (e.g. systemic lupus), allergic conditions, respiratory diseases (e.g. asthma), dermatologic diseases (e.g. pemphigus, allergic dermatoses), hematologic disorders (e.g. thrombocytopaenia, autoimmune haemolytic anaemia), neoplasia, CNS disorders, GIT disorders (e.g. ulcerative colitis) and renal diseases (e.g. nephrotic syndrome), etc.
Triamcinolone injections have been rarely implicated as a cause of equine laminitis. Frequency is rare and a direct causal association has not been established. However, appropriate caution should be taken and the owner informed about the risk whenever injecting triamcinolone. This is particularly true in horses/ponies with a previous history or increased risk factors (eg EMS, PPID) for the development of laminitis.
The total dose of triamcinolone should not exceed 20mg in any single administration. If multiple joint injections requiring a total dose >20mg is require, it is recommended that the injections be separated by a week or more. Administration of triamcinolone during the last trimester of pregnancy may induce the first stage of parturition and may precipitate premature parturition followed by dystocia, foetal death, retained placenta and metritis.
WARNING:
Total dose should not exceed 20mg in any single administration. If using triamcinolone systemically, the aim is to use the minimum amount required for as short a time as possible. Delay any further administration for > 7 days.
Intramuscular or subcutaneous injection: Horses: 12 to 18 mg (2-3 mL).
Dogs and Cats: 0.1 to 0.2 mg/kg bodyweight.
Intra-articular/ intra-synovial injection: Horses: 6 to 18 mg per joint depending on joint size.
Dogs and Cats: 1 to 3 mg.

Horse Hack: Horses administered triamcinolone may go off their feed for 2-5 days following administration.
The administration of Intra-Log Injection to horses may affect the peripheral blood count for a period of up to two weeks. The changes are typical of a stress leucogram
(leucocytosis or leucopaenia associated with a relative neutrophilia and or lymphopaenia, increase in ALP, etc). These changes may be observed even when the triamcinolone is administered intra-articularly.

DID YOU KNOW THAT... Australia’s most famous horse most likely died after being administered the wrong medicine by his unqualified 'vet'?

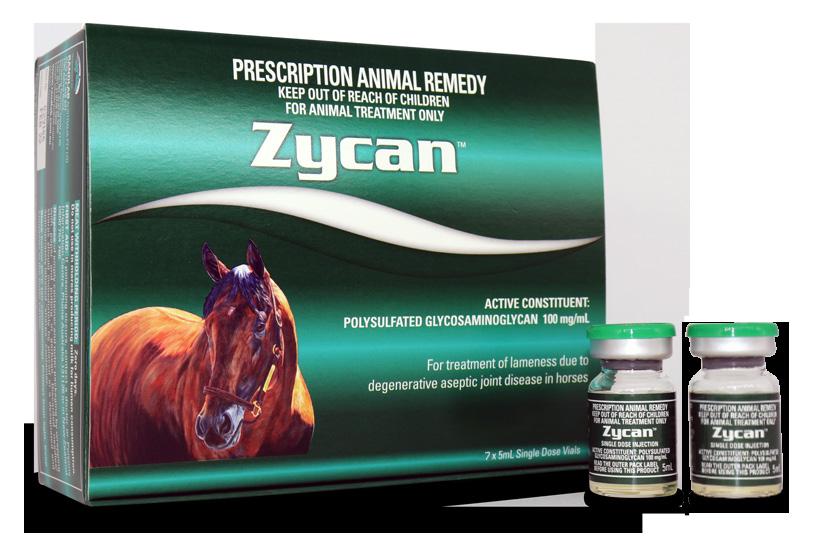
Zycan is the first registered generic to Adequan. Adequan has been registered in the USA for over 35 years and is the favoured joint medication of US veterinarians. There are over 50 publications supporting the use of Adequan in horses, humans and canines.
Zycan is a Disease Modifying Osteoarthritis Drug (DMOAD) for the treatment and prevention of clinical signs attributable to degenerative and/or traumatic aseptic joint disease in horses. DMOADs are intended to prevent, retard or reverse the morphologic cartilaginous lesions associated with degenerative joint disease (DJD).
PSGAGs, such as Zycan have been advocated for the (i) prevention and treatment of joint disease (ii) joint maintenance programs and (iii) post-operative care of horses returning to training following joint surgery.
Zycan is a semi-synthetic polysulfated glycosaminoglycan (PSGAG) and is physiologically similar to the natural mucopolysaccharides found in joint cartilage. The low molecular weight of Zycan facilitates the distribution of the PSGAG from the bloodstream to the synovial fluid. Distribution from the synovial fluid to the cartilage then takes place by diffusion. PSGAG is deposited in all layers of articular cartilage and is preferentially taken up by osteoarthritic cartilage. When administered IM, synovial levels exceed serum levels, peak in 48 hours and persist for up to 96 hours. The latter is the rationale behind every fourth day dosing. PSGAGs exert their chondroprotective effects by inhibiting the detrimental effects of cytokines and PG’s on cartilage and connective tissue, reducing proteoglycan breakdown, stimulating HA synthesis and enhancing the production of glycosaminoglycan, proteoglycan and collagen.
In multiple studies, PSGAGs have been shown to:
• Inhibit various degradative proteolytic enzymes, including glycanohydrolases, glycosidases and MMPs.
• Inhibit MMP-3 more effectively than phenylbutazone, flunixin, betamethasone and HA.
• Increase collagen and GAG synthesis in articular cartilage explants and cell culture.
• Stimulate synthesis of HA by synoviocytes, restoring synovial fluid viscosity.
• Inhibit PGE synthesis and the influx of leucocytes into inflammatory sites.
• Inhibit the production of superoxide radicals and pro-inflammatory IL-1.
• Increases the synthesis of proteoglycans by acting as a precursor.
• Decrease articular fibrillation and erosion.
• Decrease chondrocyte death.
• No change in partial or full thickness articular cartilage lesions has been reported.
PSGAGs (250mg) have also been used intra-articularly for the treatment of acute synovitis, including post-arthroscopy (McIllwraith 2016).
IM PSGAG administration may occasionally result in transient localised swelling and discomfort at the injection site. As PSGAGs have heparinoid-like activity, do not administer when a pre-existent tendency to haemorrhage or increased coagulation time is expected. Do not administer within 24 hours of surgery.
IA PSGAG administration may cause post-injection joint flares (joint pain, effusion, swelling and lameness) secondary to sensitivity reactions. A slightly increased risk of joint infections following IA injection of 250mg PSGAG has been reported compared with corticosteroids + HA.
This product is NOT registered for intra-articular use. Safety in pregnant or lactating animals or in foals has not been established. Use with caution in these groups.
Intra-muscular injection: 500mg (5mL) per 500kg horse, repeated every 4th day for 7 injections (i.e. 28 days). Weekly injections have also been shown to be effective. The series may be repeated as needed upon recurrence of the clinical signs of DJD and associated lameness. Otherwise, twice yearly courses are recommended.
Weekly maintenance injections after the initial priming course have also been advocated.
Horse Hack: Zycan can be used as a substitute for pentosan (eg Arthropen) and as such, can be used as you would pentosan.
Zycan appears to cause fewer injection reactions than pentosan. Zycan is not known to cause discolouration of the hair coat at the injection site.
Zycan is also a good alternative to pentosan in horses that have had previous neck reactions to pentosan.
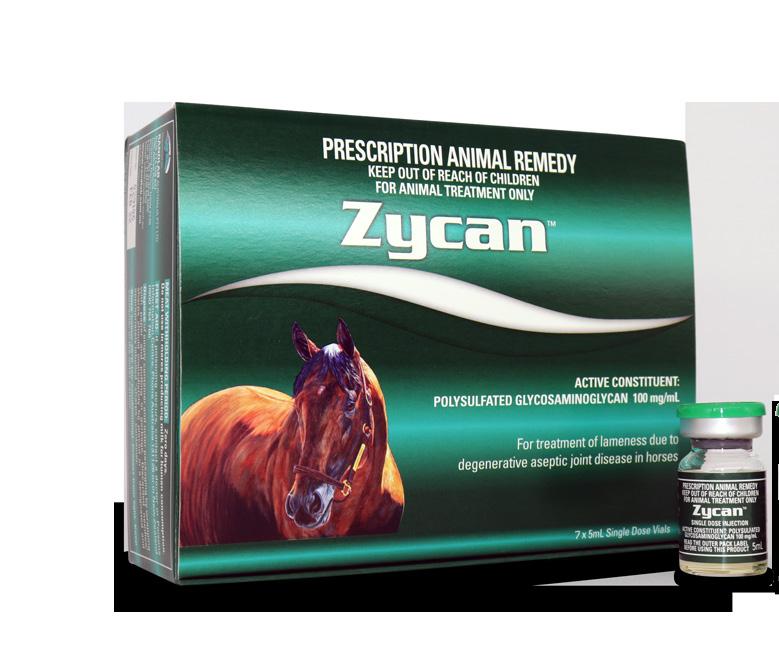

American Pharoah is the most celebrated US racehorse of the past few decades. And not just because he features as the product model on Randlab’s Zycan box. The horse is one of only 13 horses to win America’s fabled Triple Crown (Kentucky Derby, Preakness Stakes, Belmont Stakes) and only the second in the last 40 years. He is the only horse in history to also win the Grand Slam by subsequently taking out the Breeders’ Cup Classic.
The image on the product box is from an original painting by acclaimed US horse artist Tom Chapman.
HAVE YOU EVER WONDERED... Who the horse on the Zycan box is?
Most highly sulfated pentosan on the market. Higher sulfation means increased efficacy.
Early treatment is an important step in preventing the products of inflammation from damaging articular cartilage and causing irreversible joint disease.
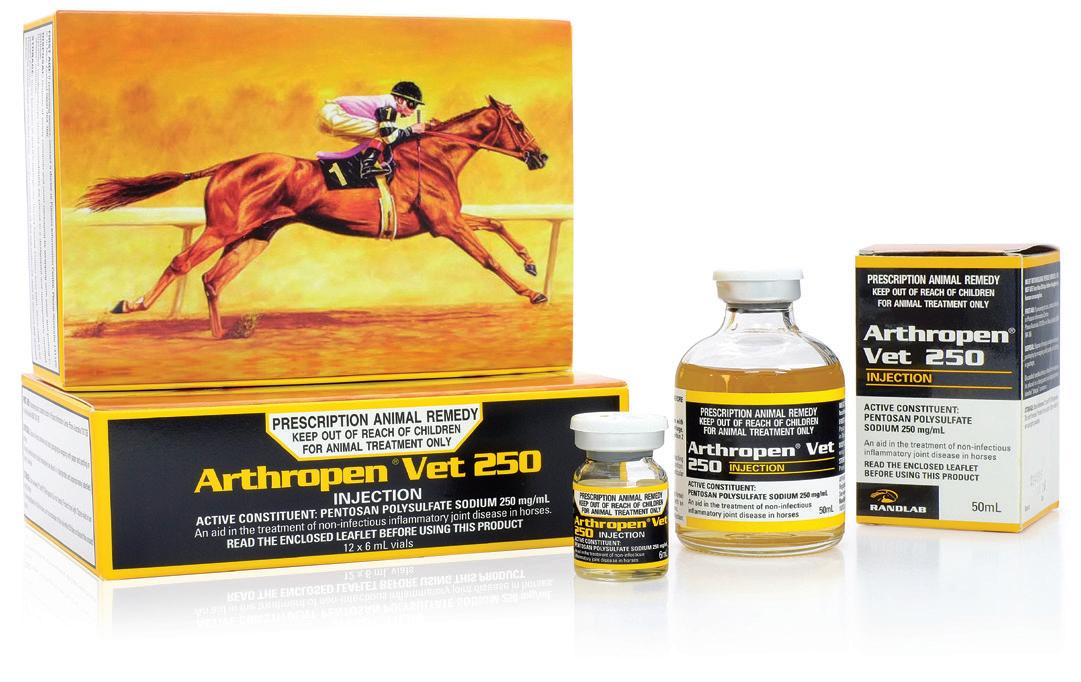
Arthropen Vet 250 Injection is indicated as an aid in the treatment of noninfectious, inflammatory joint disease in the horse.
Clinical applications include the following conditions:
• osteoarthritis (OA)
• traumatic joint disease
• multiple or non specific joint disease
• osteochondritis dissecans (OCD)
• synovitis
• degenerative joint disease
Arthropen Vet 250 Injection is a Disease Modifying Osteoarthritis Drug (DMOAD).
The high concentration of Pentosan Polysulphate in Arthropen Vet 250 has been specifically designed for low volume administration in the horse.
Arthropen Vet is especially useful in treating conditions affecting multiple joints or where joint pain is suspected but cannot be localised.
Pentosan Polysulfate Sodium (PPS) possesses anti-inflammatory, anti-arthritic and chondroprotective activities. It is a semi-synthetic polysaccharide with a linear xylan (pentosan) backbone. Xylan is a hemicellulose derived from plant origin by extraction from the cell walls of hardwoods. The xylan backbone then undergoes sulfation. The degree of sulfation is believed to modulate the potency of the pentosan molecule. Arthropen Vet 250 Injection is the only fully sulfated PPS on the market.
The beneficial effects of pentosan polysulfate sodium in joint disease are due to the following actions:
• Anti-inflammatory activity.
• Inhibition of neutrophil migration into the joint.
• Inhibition of enzymes implicated in cartilage destruction.
• Stimulation of hyaluronic acid synthesis by synovial fibroblasts, resulting in increased volume and viscosity of synovial fluid.
• Stimulation of proteoglycan synthesis by chondrocytes.
• Fibrinolytic activity, resulting in improved circulation to subchondral bone and peri-articular structures.
• Reduction in Matrix Metalloproteinases (MMPs).
• Reduction in bone oedema.
Reversible, discolouration of the hair coat and hair loss may be seen at the injection site following long-term use of PPS. To minimise the risk of such reactions, the injection site should be alternated and PPS given by deep IM injection.
Because of its heparinoid activity, Arthropen is best not used for a period of 40 hours prior to strenuous exercise in horses with a history of Exercise Induced Pulmonary Haemorrhage (EIPH). Contraindicated for use in horses with clotting defects, traumatic haemorrhage, infection, liver/kidney failure, or within two days of surgery.
Intra-articular use of Arthropen may result in temporary joint flares. If used in performance animals, the regulations of the relevant authorities regarding medication control should be observed.
An initial course of four injections a week apart is recommended. This is generally followed by a program of either weekly injections or injections spaced at 2-4 week intervals depending on the intensity of the horse’s exercise/competition program and the response to treatment. Horses undergoing heavy training or competition schedules or horses with a chronic lameness problem are likely to benefit from ongoing weekly Arthropen injections.
Dose - 3mg/kg bodyweight (6mL/500kg horse) by intramuscular injection on four occasions with an interval of 5-7 days between injections. Preferable to alternate injection sites from week to week. Best given by deep intramuscular injection. To avoid haemorrhage associated with injection, a small needle (e.g. 21 gauge) is recommended.
Intra-articular: 1.0mL by intra-articular injection. May be repeated at weekly intervals for 3 to 4 treatments. More than one joint may be treated at the one time. Joint flares are common after intra-articular PPS.
Horse Hack: The storage conditions for Arthropen have changed. It no longer needs to be refrigerated and can be kept in air conditioning (store below 25oC, do not freeze). In house testing has also shown the product to be stable at even higher temperatures.

Affirmed was only the 11th horse to win the fabled US Triple Crown (1978). He was equally well known for his racetrack rivalry with another champion horse Alydar. The pair met on ten occasions, with Affirmed winning seven of the clashes, including each of the three Triple Crown races. After Affirmed won the Triple Crown, there was a 37-year hiatus until American Pharoah swept the series in 2015.
The image on the Arthropen box is from an original painting by acclaimed US horse artist Tom Chapman.
WARNING: The intravenous use of pentosan
may rarely result in anaphylaxis and death. Do not use intravenously!
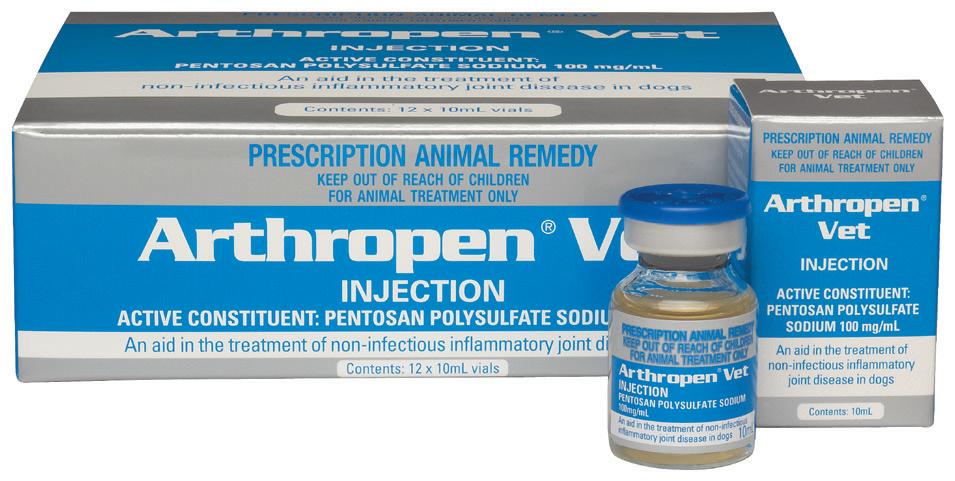
Early treatment is an important step in preventing the products of inflammation from damaging articular cartilage and causing irreversible joint disease.
Arthropen Vet Injection is a low concentration formulation of pentosan polysulfate (PPS) suitable for use in dogs and horses. It aids in the treatment and prevention of non-infectious inflammatory joint disease in conditions such as:
• osteoarthritis (OA)
• traumatic joint disease
• multiple or non specific joint disease
• osteochondritis dissecans (OCD)
• synovitis
• degenerative joint disease
Arthropen Vet Injection is a Disease Modifying Osteoarthritis Drug (DMOAD). The low concentration of pentosan polysulfate in Arthropen Vet Injection may cause less tissue irritation at the injection site.
Arthropen Vet Injection is especially useful in treating conditions affecting multiple joints or where joint pain is suspected but cannot be localised.
Pentosan Polysulfate Sodium (PPS) possesses anti-inflammatory, anti-arthritic and chondroprotective activities. It is a semi-synthetic polysaccharide with a linear xylan (pentosan) backbone. Xylan is a hemicellulose derived from plant origin by extraction from the cell walls of hardwoods. The xylan backbone then undergoes sulfation. The degree of sulfation is believed to modulate the potency of the pentosan molecule. Arthropen Vet Injection is the only fully sulfated PPS on the market.
The beneficial effects of pentosan polysulfate sodium in joint disease are due to the following actions:
• Anti-inflammatory activity.
• Inhibition of neutrophil migration into the joint.
• Inhibition of enzymes implicated in cartilage destruction.
• Stimulation of hyaluronic acid synthesis by synovial fibroblasts, resulting in increased volume and viscosity of synovial fluid.
• Stimulation of proteoglycan synthesis by chondrocytes.
• Fibrinolytic activity, resulting in improved circulation to subchondral bone and peri-articular structures.
• Reduction in Matrix Metalloproteinases (MMPs).
• Reduction in bone oedema.
Reversible, localised discolouration of the hair coat and hair loss may be seen at the injection site following long-term use of PPS. To minimise the risk of such reactions, the injection site should be alternated and PPS given by deep IM injection.
Because of its heparinoid activity, Arthropen is best not used for a period of 40 hours prior to strenuous exercise in horses with a history of Exercise-Induced Pulmonary Haemorrhage (EIPH). Contraindicated for use in horses with clotting
defects, traumatic haemorrhage, infection, liver/kidney failure, or within two days of surgery.
Intra-articular use of Arthropen may result in temporary joint flares. If used in performance animals, the regulations of the relevant authorities regarding medication control should be observed.
An initial course of four injections a week apart is recommended. This is generally followed by a program of either weekly injections or injections spaced at 3-4 week intervals depending on the intensity of the horse’s exercise/competition program and the response to treatment. Horses undergoing heavy training or competition schedules or horses with a chronic lameness problem are likely to benefit from ongoing weekly Arthropen injections.
Dose - 2-3mg/kg bodyweight by intramuscular injection on four occasions with an interval of 5-7 days between injections. Preferable to alternate injection sites from week to week. Best given by deep intramuscular injection. To avoid haemorrhage associated with injection, a small needle (e.g. 21 gauge) is recommended.
Intra-articular: 2.5mL by intra-articular injection. May be repeated at weekly intervals for 3 to 4 treatments. More than one joint may be treated at the one time. Joint flares are common after intra-articular PPS.
Arthropen

The oldest known pants come from Central Asia between 3,000 and 3,300 years ago and were attributed to nomadic herdsman in Western China.
The invention of bifurcated lower body garments was related to the new epoch of mounted warfare and allowed the warriors additional protection of their lower abdomen and genitals.
Pants also became popular among the nomadic riders for long-distance riding soon thereafter.
APVMA Approval No. 70144 (Australia) | ACVM No. A011191 (New Zealand)

Early treatment is an important step in preventing the products of inflammation from damaging articular cartilage and causing irreversible joint disease.
Higher molecular weight formulations of HA such as Matrix 6000, are known to have increased clinical effects.
Matrix 6000 IV Injection is a high molecular weight, high viscosity formulation of sodium hyaluronate. It is ideal for intravenous use in the treatment and prevention of lameness in horses due to non-infectious synovitis including those associated with early equine degenerative joint disease.

Ideal for use pre-competition or pre-race to help restore joint function.
Hyaluronic Acid (HA) is a natural and essential component of articular cartilage and synovial fluid. It is produced by both synoviocytes within the synovial membrane and by chondrocytes within the articular cartilage matrix.
The hyaluronic acid produced by bacteria is of the same structure and configuration as that found in mammals. The active ingredient in Matrix 6000 IV injection is extracted from the capsule of a selected micro-organism and purified to produce an ultrapure form of sodium hyaluronate that is essentially free of protein and nucleic acids. Since this product originates from a microbial source, there is no potential for contamination with dermatan or chondroitin sulphate or any other glycosaminoglycan.
Sodium hyaluronate restores lubrication of the joint fluid and regulates the normal cellular constituents. This effect decreases the impact of exudation, enzyme release and subsequent degradation of joint integrity.
HA has the ability to confer extraordinary compressive strength to the articular cartilage when functioning as the core molecule for proteoglycan aggregates. Articular surfaces are covered with a fine layer of HA, which exerts resistance to cartilage compression while still retaining its elasticity. HA also confers viscoelastic and lubricating properties to synovial fluid and is responsible for the boundary lubrication of the synovial membrane and the lubrication of articular cartilage. These latter properties are believed to be further increased by higher molecular weight formulations of HA, such as in Matrix 6000.
Hyaluronate molecules are long chains which form a filter matrix interspersed with normal cellular fluids. This supplements the viscoelastic properties of normal joint fluid. Sodium hyaluronate exerts a slight anti-inflammatory action by providing a steric barrier that limits the movement of granulocytes and macrophages into the joint.
Adult horse (450-700 kg): Administer 6 mL (60 mg) intravenously. Treatment may be repeated at weekly intervals.
In performance horses, Matrix 6000 may be administered prior to competition as an aid in reducing joint inflammation and restoring joint function. The regulations of the relevant regulatory authority regarding medication control should always be observed.
This product does not contain any antimicrobial preservative. Any solution remaining in the vial after administration of the required dose should be discarded.

Approval No. 65128 (Australia) | ACVM No. A10491 (New Zealand)

Early treatment is an important step in preventing the products of inflammation from damaging articular cartilage and causing irreversible joint disease.
Equinate Injection is the Hyaluronic Acid (HA) product to meet all your HA requirements. Equinate Injection is indicated in the treatment and prevention of lameness in horses due to non-infectious synovitis including those associated with early equine degenerative joint disease. It is designed for intra-articular administration but may also be used intravenously.
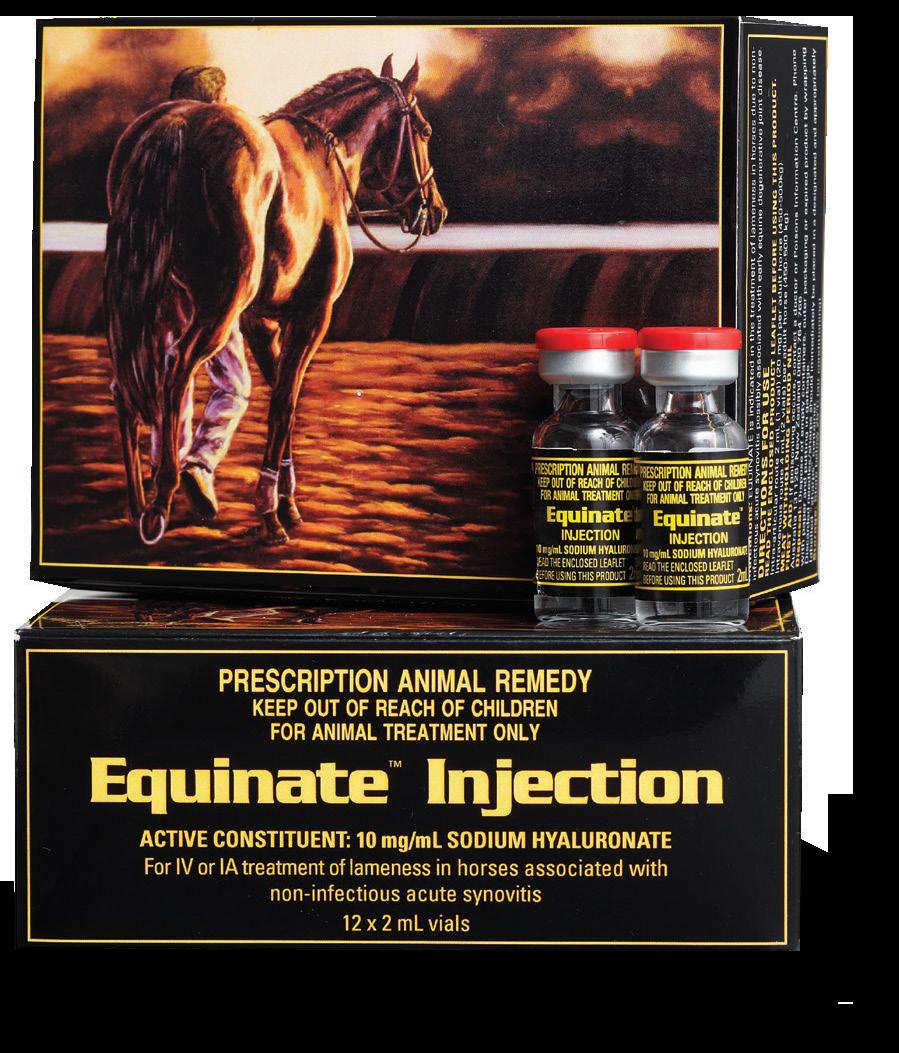
Hyaluronic Acid is a natural and essential component of articular cartilage and synovial fluid. It is produced by both synoviocytes within the synovial membrane and by chondrocytes within the articular cartilage matrix.
The hyaluronic acid produced by bacteria is of the same structure and configuration as that found in mammals. The active ingredient in Equinate Injection is extracted from the capsule of a selected micro-organism and purified to produce an ultrapure form of sodium hyaluronate that is essentially free of protein and nucleic acids. Since this product originates from a microbial source, there is no potential for contamination with dermatan or chondroitin sulphate or any other glycosaminoglycan. This reduces the flare reactions sometimes associated with IA injections of sodium hyaluronate. It contains no preservative.
Sodium hyaluronate restores lubrication of the joint fluid and regulates the normal cellular constituents. This effect decreases the impact of exudation, enzyme release and subsequent degradation of joint integrity.
HA has the ability to confer extraordinary compressive strength to the articular cartilage when functioning as the core molecule for proteoglycan aggregates. Articular surfaces are covered with a fine layer of HA, which exerts resistance to cartilage compression while still retaining its elasticity. HA also confers viscoelastic and lubricating properties to synovial fluid and is responsible for the boundary lubrication of synovial membranes and the lubrication of articular cartilage. These latter properties are believed to be further increased by higher molecular weight formulations of HA, such as is found in Equinate Injection.
Hyaluronate molecules are long chains which form a filter matrix interspersed with normal cellular fluids. This supplements the viscoelastic properties of normal joint fluid. Sodium hyaluronate also exerts a slight anti-inflammatory action by providing a steric barrier that limits the movement of granulocytes and macrophages into the joint.
Strict aseptic technique should be observed when injecting Equinate Injection intra-articularly.
To achieve best results in cases of intra-articular or intravenous administration, horses should be rested following treatment.
Intra-articular Injection - The recommended dosage for intra-articular injection is 2mL (1 vial/20mg) per joint. A greater volume e.g. 4mL (2 vials/40mg) may be required in larger joints such as the stifle or shoulder. Treatment may be repeated at weekly intervals for a total of three treatments. As with any intra-articular procedure, proper injection site disinfection and animal restraint are important. Excess joint fluid should be aseptically removed prior to intra-articular injection. Care should be taken not to scratch the cartilage surface with the injection needle. Use the smallest gauge needle possible (e.g. 21 or 20 gauge).
Intravenous Injection - 4mL (2 vials/40mg) per adult horse (450-500kg). Treatment may be repeated at weekly intervals for a total of three treatments or be used pre-competition or race to alleviate joint inflammation.

Suitable for weekly/regular IV maintenance or pre-competition dosing. Low molecular weight IV formulation.
Active Constituent
Sodium Hyaluronate 10 mg/mL
Pack Size 12 x 4mL single dose vials
Early treatment is an important step in preventing the products of inflammation from damaging articular cartilage and causing irreversible joint disease.
Equinate I.V. Injection is indicated in the intravenous treatment or prevention of lameness in horses due to non-infectious synovitis including those associated with early equine degenerative joint disease.

Hyaluronic Acid (HA) is a natural and essential component of articular cartilage and synovial fluid. It is produced by both synoviocytes within the synovial membrane and by chondrocytes within the articular cartilage matrix. The hyaluronic acid produced by bacteria is of the same structure and configuration as that found in mammals. The active ingredient in Equinate IV Injection is extracted from the capsule of a selected micro-organism and purified to produce an ultrapure form of sodium hyaluronate that is essentially free of protein and nucleic acids. Since this product originates from a microbial source, there is no potential for contamination with dermatan or chondroitin sulphate or any other glycosaminoglycan. The solution is pyrogen free and sterile.
Sodium hyaluronate restores lubrication of the joint fluid and regulates the normal cellular constituents. This effect decreases the impact of exudation, enzyme release and subsequent degradation of joint integrity.
HA has the ability to confer extraordinary compressive strength to the articular cartilage when functioning as the core molecule for proteoglycan aggregates. Articular surfaces are covered with a fine layer of HA, which exerts resistance to cartilage compression while still retaining its elasticity. HA also confers viscoelastic and lubricating properties to synovial fluid and is responsible for the boundary lubrication of synovial membranes and the lubrication of articular cartilage. Hyaluronate molecules are long chains which form a filter matrix interspersed with normal cellular fluids. This supplements the viscoelastic properties of normal joint fluid. Sodium hyaluronate exerts a slight anti-inflammatory action by providing a steric barrier that limits the movement of granulocytes and macrophages into the joint.
Studies have shown that weekly treatments with intravenous hyaluronic acid for a total of three injections to be effective.
This product does not contain any antimicrobial preservative. Discard after opening.
Intravenous Route - 4 mL (40 mg) per adult horse (450-500 kg). Treatment may be repeated at weekly intervals or as required.
In performance horses, Equinate IV Injection may be administered prior to competition or racing as an aid in reducing joint inflammation and restoring joint function.
The regulations of the relevant competition Authority regarding medication control should always be observed.


A few months after competing at the 1986 World Equestrian Championships, Di Schaeffer purchased an off-the-track thoroughbred for her 11yo daughter Wendy. The horse stood only 15.3hh and had the right temperament to become a successful pony club horse.
But Sunburst and Wendy had other ideas! Under Di’s guidance, the pair soon progressed through the junior and young riders ranks to be competing at advanced senior by the time Wendy was seventeen. Spurred on by Australia’s 1992 Eventing Gold at the Barcelona Games, 18yo Schaeffer set an audacious plan for herself and Sunburst towards the Atlanta Olympics.
However, the plan nearly came unstuck when, with only nine weeks to go before the Olympics, Schaeffer made the unorthodox decision to return home to compete at the Naracoorte Horse Trials, leaving Sunburst behind in the UK. During the XC, one of Schaeffer's young horses crushed her leg breaking both her tibia and fibula. To all intents and purposes, it was Olympic game over!
Wendy had a plate and nine screws inserted in her fractured tibia and a customised hinge-brace fitted to protect the leg. Somewhat defiantly, she was back on a horse 2.5 weeks after the surgery and back training with Sunburst a further two weeks later.
Despite their unusual preparation, Wendy somehow managed to convince the selectors to include her on the Olympic Team alongside Andrew Hoy, Matt Ryan and Phillip Dutton.
Throughout the Opening Ceremony, Wendy struggled walking with the Australian Team. Some of the team officials expressed doubt about her fitness to ride and there was a push to have Wendy and Sunburst replaced by the reserve, Gillian Rolton and her horse. But Wendy trusted Sunburst and had every confidence he could get the job done. With her leg heavily strapped, Wendy completed her dressage test with the highest score of the Aussies but in 18th place overall. The pair then finished with the fourth fastest clear round on cross country and went clear in show-jumping.
Wendy and Sunburst managed to achieve the best result of the whole team, bringing home the team gold for Australia. It was only a change of the rules from the previous Olympics that cost Wendy the individual gold. Wendy was only 21yo and the youngest rider to ever win an equestrian gold medal.
In a tragic and ironic twist, Sunburst himself had to be euthanised less than a year later when he broke his leg in a paddock accident. Sunburst was inducted into the Equestrian Australia Hall of Fame in 2013.
For treatment of lameness associated with noninfectious synovitis and degenerative joint disease
DID YOU KNOW THAT... That a South Australian pony club horse and his rider went all the way together to Olympic Gold glory?

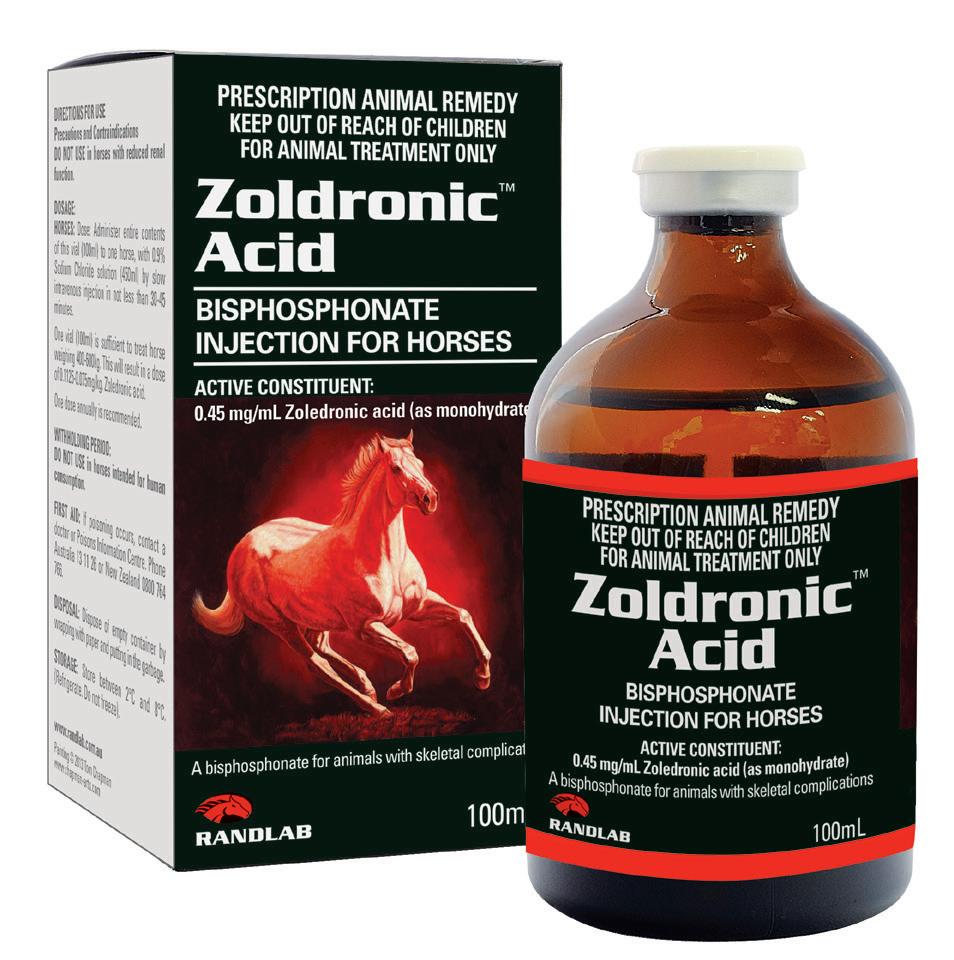
Zoldronic Acid is a long-acting bisphosphonate injection used for the treatment of skeletal diseases associated with high osteoclast activity and accelerated bone turnover, prevention of skeletal fractures and alleviation of non-specific bone pain.
Zoldronic Acid has been indicated in the treatment of conditions such as equine Palmar/Plantar Osteochondral Disease (POD) and as an aid in the prevention of consequent metacarpal/metatarsal condylar fractures.
Zoledronic acid is reported as the strongest bisphosphonate currently available with a duration of action exceeding 12 months and a potency exceeding 100x other commonly used bisphosphonates.
Zoledronate is a third-generation nitrogen-containing bisphosphonate that has high tissue specificity for bone hydroxyapatite. Once absorbed into the bone, the distribution of the drug is not homogenous, with the highest concentrations being found at sites of high remodeling and cancellous bone, rather than in bone cortex. Here the zoledronic acid causes osteoclast apoptosis as well as inhibition of osteoclastic bone resorption. Bone resorption is the essential first step in the process of bone remodeling.
Zoledronic acid is largely excreted unchanged by the kidneys. Up to 50% of the administrated dose may be eliminated in the urine within the first 48 hours with the remainder being incorporated into the bone matrix for a prolonged period. The effects of zoledronic acid are long-lasting and may exceed 12 months.
As bisphosphonates are bound to the bone matrix, their release into the circulation may be highly random and intermittent. Under some circumstances this may result in both a prolonged (up to three years) and intermittent excretion. In some thoroughbred racing jurisdictions, the administration of bisphosphonates to horses under four years of age is not permitted.
WARNING: Zoledronic acid is currently a banned substance for horses competing under FEI rules and for horses competing in some thoroughbred racing jurisdictions (eg Racing Australia). Check the rules of the local Regulatory Authority prior to administration.
The use of bisphosphonates in thoroughbred weanling and yearling horses being sold at public auction is not permitted.
Catastrophic fractures have been reported in speed racing horses (thoroughbred and endurance horses) that have been administered bisphosphonates. Although a direct linkage between occurrence of fractures and bisphosphonates has not been established, careful consideration should be exercised prior to administration in these horses.
Zoledronic acid is rapidly processed by the kidneys, consequently, its administration is not recommended in animals with reduced renal function or kidney disease.
The administration of Non Steroidal Anti-inflammatory Drugs (NSAIDs) in association with bisphosphonates should be avoided due to their possible effect on the kidneys.
Horses with administered bisphosphonates may occasionally exhibit polyuria/ polydypsia, that may persist for up to two weeks.
The safety of zoledronic acid in pregnant and lactating mares or in growing horses has not been established.
Rarely horses that have been administered zoledronic acid may exhibit hypocalcaemia. It is recommended that horses treated with zoledronic acid have their serum calcium measured approximately 14 days post administration. Use with caution in horses with hypocalcaemia or cardiac dysfunction.
Excretion times for zoledronic acid have not been published but due to its strong bone binding affinity, may be intermittent and prolonged (up to three years).
DO
Dose: 0.057-0.075mg/kg in 1L 0.9% saline by slow intravenous infusion over 30-45minutes. The lower dose rate (0.057mg/kg) is recommended.
Dose should not be repeated more frequently than annually.
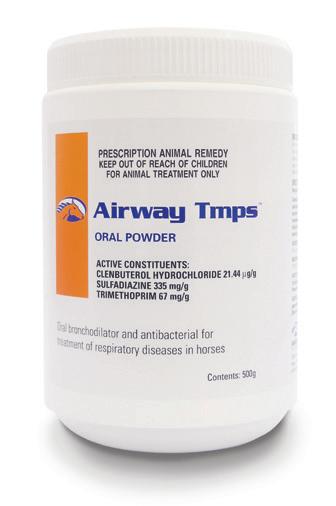
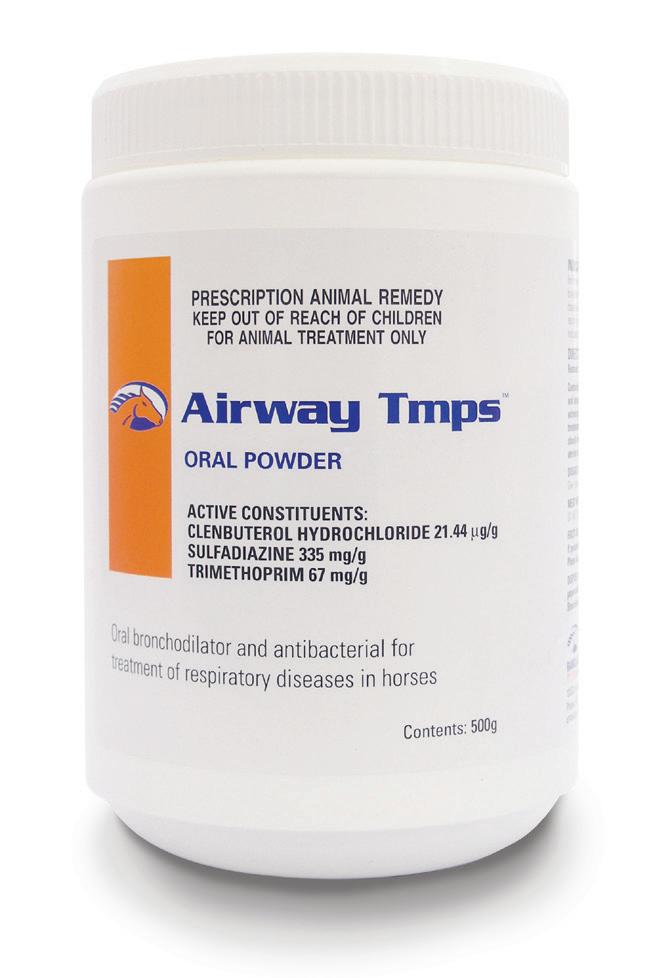
(Clenbuterol Hydrochloride 21.44 µg/g, Sulfadiazine 335 mg/g, Trimethoprim 67 mg/g)
Oral bronchodilator and antibacterial for the treatment of respiratory infections.
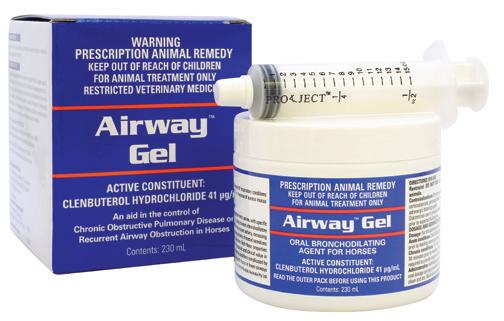



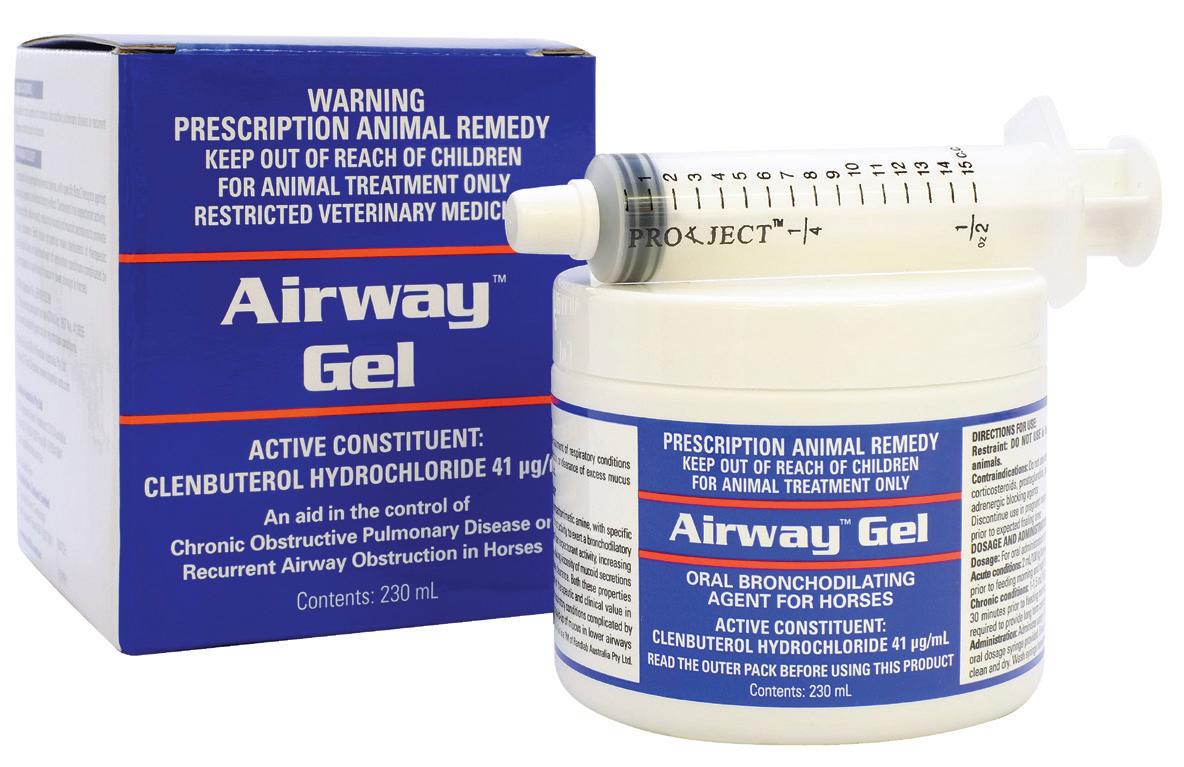
(Clenbuterol Hydrochloride 41 µg/mL)
Oral bronchodilating agent
Randlab supports responsible Antimicrobial Stewardship.
(Sulfadimidine 430 mg/g, Trimethoprim 86 mg/g, Bromhexine Hydrochloride 8.6 mg/g)
Oral mucolytic and antibacterial for the treatment of respiratory infections
APVMA Approval No. 61474 (Australia) | ACVM No. A10158 (New Zealand)
Available in:
Randlab supports responsible Antimicrobial Stewardship.
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Randlab’s Airway Tmps. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
For the treatment of respiratory disease in horses caused by organisms susceptible to a sufadiazine/trimethoprim antibiotic combination. Treatment of respiratory conditions characterised by restriction of the airways, including bronchospasm and obstruction resulting from bacterial and viral infection, bronchitis and bronchopneumonia.
Airway Tmps Oral Powder is formulated for the treatment of respiratory disease in horses caused by organisms susceptible to the sufadiazine/trimethoprim antibiotic combination. These include; Staphylococci, Streptococci, Fusobacterium, Enterobacter, Corynebacterium (excluding Rhodococcus equi), Salmonella, Shigella, Klebsiella, Pasteurella, Haemophilus, Proteus spp and most E.coli, and some Brucella and Nocardia spp. MostPseudomonasspparenotsensitive. Clenbuterol is a bronchodilator with minimal effect on the cardiovascular system. Clenbuterol also has a stabilising effect on mast cells, preventing the release of histamine, serotonin and bradykinin and also improves mucociliary clearance of airway discharge by increasing ciliary beat frequency.
Clenbuterol will potentiate the action of corticosteroids and antagonise the effects of prostaglandin F2α and oxytocin. Beta adrenergic agents adversely affect the action of clenbuterol. Concurrent treatment of horses with these medications is not recommended. Airway Tmps Oral Powder should not be used in horses during the last 14 days of gestation, as uterine contractions may be prevented.
Dose: The label dose rate is one level scoop (9g) orally per 250kg bodyweight in feed twice daily for 6 to 10 days.
One level scoop contains 9g of powder.

Twice daily dosing is recommended to maintain therapeutic levels. Administer on damp food with honey or molasses to avoid sifting of powder. Alternatively, Airway Tmps may be made up into a paste with molasses, honey or Karo (corn) Syrup and administered over the back of the tongue.
Horse Hack: Airway Tmps can be easily administered as a “powder sandwich” over the back of the tongue. Cut the top off a 60mL syringe at the 2mL mark with a bread knife or hacksaw. Withdraw the plunger to 60mL. Put two teaspoons of molasses, honey or apple sauce in the syringe, followed by the prescribed dose of Airway Tmps and a further two teaspoons of molasses or honey. Administer to the horse over the back of the tongue. As well as adding some flavouring, the molasses or honey stops the horse spitting out the dose.
In 1894 William “Doc” Carver, a carnival cowboy entrepeneur debuted an act featuring diving horses in Kansas City. The act perversely proved very popular and routinely drew large crowds
The show eventually found a permanent home at the fabled Steel Pier in Atlantic City, New Jersey where it was performed four times a day, seven days a week from 1928 to 1978. That's 50 years!

In the act, horses ascended a ramp to a platform 40 or 60 feet in the air before plunging into a pool in the Atlantic Ocean. Horses would either free dive or be ridden by a diving girl who mounted the horse at full canter dressed only in a bathing suit and swim cap before plunging into the cold Atlantic water 40 feet below.
The most famous of these riders was Sonora Webster. After being the leading rider-diver for seven years, Sonora was blinded when her horse Red Lips hit the water askew, which resulted in Sonora suffering bilateral retinal detatchement and total blindness. She continued to perform in the act for a further 11 years, keeping her blindness a secret from the public until after her retirement.
Horse high diving eventually ceased at Steel Peer in 1978 due to declining interest and pressure from animal rights activitsts.
Sonora’s life was made into a Disney biopic called Wild Hearts Can't Be Broken (1991).
DID YOU KNOW THAT...
Horse high diving was a popular tourist attraction in the US up until 1978?
RED LIPS (SONORA WEBSTER-CARVER, USA)


Available in:
Combination oral antibiotic and mucolytic. For treatment of respiratory infections caused by TMPS sensitive organisms.
Sulfadimidine 430 mg/g
Trimethoprim 86 mg/g
Bromhexine Hydrochloride 8.6 mg/g
Pack Size
500g multidose jar
2kg multidose pail
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Randlab’s Bromo Tmps. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
For the treatment of respiratory infections in horses due to organisms susceptible to the combination of sulfadimidine and trimethoprim and where mucolytic activity is also desirable.
MICs of the sulfadimidine-trimethoprim combination vary widely for the range of respiratory pathogens which are of significance in equine respiratory disease. In order to ensure efficacy against the major equine respiratory pathogens, the dose of Bromo Tmps Oral Powder has been calculated to produce drug levels which would be therapeutic against those pathogens with the highest MICs when given twice daily.
Pharmacokinetic trials in horses have established that both sulfadimidine and trimethoprim are readily and rapidly absorbed after oral administration and reach therapeutic plasma levels within approximately 30 minutes of administration.
Trial results demonstrate that the formulation is efficiently absorbed even when administered with food.
Twice daily dosing of Bromo Tmps Oral Powder is recommended in preference to once daily dosing as the minimum (trough) concentrations of sulfadimidine and trimethoprim are potentially subtherapeutic for some sensitive pathogens under a once daily dose regimen.
Bromhexine decreases the viscosity and tenacity of respiratory mucus, facilitating expectoration and suppressing tissue irritation. It also increases the permeability of the alveolor capillary membrane increasing the concentration of antibacterial agents in respiratory secretions.
Administer on damp food with honey or molasses to avoid sifting of powder. Alternatively, Bromo Tmps may be made up into a paste with molasses, honey or Karo (corn) Syrup and administered over the back of the tongue.
Dose: 30mg/kg twice daily [e.g. (30mg/kg x 500kg) / (430+86)] = 29ml.
Add one level scoop (12g) per 200kg twice daily in feed. Twice daily dosing is recommended to maintain therapeutic levels of active drugs in all situations.
Randlab supports responsible Antimicrobial Stewardship.
Horse Hack: Bromo Tmps can be easily administered as a “powder sandwich” over the back of the tongue. Cut the top off a 60mL syringe at the 2mL mark with a bread knife or hacksaw. Withdraw the plunger to 60mL. Put two teaspoons of molasses, honey or apple sauce in the syringe, followed by the prescribed dose of Bromo Tmps and a further two teaspoons of molasses or honey. Administer to the horse over the back of the tongue. As well as adding some flavouring, the molasses or honey stops the horse spitting out the dose.


After the Olympic Games, the Asian Games is the largest sporting event on the world stage. Held every four years in the alternative biennial to The Olympics, 45 countries from around Asia participate in 40 sports. The 2006 Asian Games (XV Asiad) held in Doha, was the debut on the world stage for the fledging Gulf country, Qatar. It was also the litmus test for Qatar's capability of hosting the 2022 FIFA World Cup.
One of the highlights of any Games is the traditional lighting of the cauldron during the Opening Ceremony. There has probably been no more enthralling Opening than the 2006 Asian Games. Emerging from a subterranean pod, resembling a spaceship, an Australian Arabian gelding by the name of Ace trained by Aussie Steve Jefferys and ridden by a secret horseman in traditional Arabian dress, bows before the Amir and the one billion TV audience. The rider removes his mask to reveal his identity as the Amir’s son, HE Sheikh Mohammed Bin Hamad Al-Thani. The pair then ascend a 60m, 26o ramp with the torch flame to light the cauldron and declare the Games officially open.
Rain is a rare event in Qatar during December, but on this night, it rained making the horse’s ascent of the steep wooden ramp treacherous. The courageous Ace struggled to maintain his purchase, stumbled to his knees on several occasions and almost collapsed before reaching the summit. You can watch the spectacular sequence of events by googling 'Doha Asian Games - lighting the torch'.
See if you can spot Randlab's Dr Michael Robinson!
DID YOU KNOW THAT... An Australian Arabian horse tripped and stumbled with his Royal rider to open the 2006 Asian Games?
ACE (HE SHEIKH MOHAMMED BIN HAMAD AL-THANI, QAT)
Available in:
Easy to use gel formulation for bronchodilation and the clearance of airway secretions.
Active Constituent
Clenbuterol Hydrochloride 41 µg/mL
Pack Size 230mL multidose jar
As an aid in the treatment of respiratory conditions where bronchodilation or clearance of excess mucus would be beneficial. Useful in the treatment of conditions such as Equine Asthma, Inflammatory Airway Disease, Recurrent Airway Obstruction, exercise induced bronchospasm, pneumonitis and bronchopneumonia. Promotes clearance of blood from the respiratory tract following an episode of EIPH.
Clenbuterol is a sympathomimetic amine, with specific 2-receptor agonist activity. Clenbuterol has both bronchodilatory and expectorant activity and aids in the clearance of mucus and secretions from the airways.
The prime targets for clenbuterol are the lungs and uterus. This selectively allows intense bronchodilating properties with minimum effect on the cardiovascular system. Clenbuterol promotes airway clearance by increasing the volume and reducing the viscosity of mucoid secretions and increasing the bronchial ciliary beat frequency. It also stabilises mast cells, preventing release of histamine, bradykinin and serotonin.
These properties make clenbuterol of value in the treatment of respiratory conditions complicated by bronchospasm or build-up of mucus, pus or blood in the lower airways of horses.
As clenbuterol dilates airways and increases ciliary beat frequency, Airway Gel is useful for the clearance of blood from the respiratory tract in the days immediately following an episode of EIPH, reducing the risk of secondary bacterial pneumonia.
Discontinue use in pregnant mares at least seven days prior to expected foaling times as uterine contractions may be prevented. Do not use concomitantly with corticosteroids, prostaglandins and other betaadrenergic blocking agents. Clenbuterol may inhibit the effect of PGF2α and oxytocin.
WARNING: Some horses may be initially sensitive to the sympathomimetic effects of clenbuterol. This may manifest as agitation, including “colicky” signs, sweating, increased heart rate and tremors. These symptoms are self-limiting and usually resolve within an hour. The horse may be continued on the Airway Gel at a reduced rate (start with a half dose) for a few days. The dose should then be slowly increased until the horse tolerates the full recommended dose.
Acute conditions: PO 0.8 ug/kg (10mL @ 500Kg BW) bid initially. May be increased up to 3.2 μg/kg in recalcitrant cases. Administer prior to feeding. Chronic Conditions: 1-1.5 mL/100 kg bodyweight 30 minutes prior to feeding morning and night as required to provide long term control of symptoms.
Horse Hack: Horses may develop some tolerance to the pharmacological effects of clenbuterol and pulsed administration (e.g. 10 days on, 5-10 days off) is recommended over continuous administration in chronic conditions.



Emily Davison was a well-known suffragette who vigorously campaigned for women’s rights. Her life motto was “deeds not words” and perhaps it was this phrase that motivated her protest to charge on to the track during the running of the 1913 English Derby.
As the field thundered around the home turn, Davison attempted to attach a scarf in the suffragette colours to the bridle of the Royal runner, Anmer. Davison was struck to the ground by the horse and died four days later from head injuries.
The King later recorded in his diary that it was "a most regrettable and scandalous proceeding" and in her journal the Queen (Mary) described Davison as a "horrid woman", which did little for the suffragette cause. A subsequent inquest found Davison’s death to be by misadventure rather than a reckless act of martyrdom.
It was another five years before women over the age of 30 and who had the prerequisite amount of property, were granted the vote.
The mud-stained silk scarf that Davison was wearing at the time she fell to the turf is on display in the public gallery of the British House of Commons.
DID YOU KNOW THAT...
A renowned British suffragette was killed during the running of the English Derby by a horse owned by King George V?
ANMER (KING GEORGE V, UK)








Available in: P
Active Constituent
Dexamethasone Sodium Phosphate 5mg/mL
Pack Size
50mL multidose
Glucocorticoids have an effect on virtually every cell type and system in the body. The primary pharmacological actions of dexamethasone in the horse are as an anti-inflammatory and immunosuppressive agent. As such, it has been used in the treatment of a wide variety of conditions including:
Anti-inflammatory:
• Orthopaedic conditions. May be used alone or to augment the effect of non-steroidal anti-inflammatory drugs (such as phenylbutazone or meloxicam). Useful in the treatment of conditions such as osteoarthritis, bursitis, tenosynovitis, tendinitis, desmitis, rhabdomyolyis, etc.
• Intra-articularly for inflammatory joint disease or osteoarthritis either in combination with hyaluronic acid or another corticosteroid (eg triamcinolone) or a biologic (eg IRAP or PRP), especially when a post-injection flare is anticipated.
Immunosuppression:
• Equine Asthma including IAD and RAO.
• Dermatitis including allergic dermatitis, pruritis, pemphigus and insect bite dermatitis.
• Auto-immune and immune-mediated diseases such as systemic lupus, rheumatoid arthritis, immune-mediated haemolytic anaemia (IMHA), thrombocytopaenia, polyneuritis equi, purpura haemorrhagica, lupus and other immune-mediated vasculitides.
• Adrenal insufficiency
• CNS disorders and trauma characterised by increased CSF pressure.
• Neoplasia for amelioration of symptoms and secondary inflammation and regression of some lymphoid neoplasias.
• Shock incl endotoxic, anaphylactic, etc.
• Overnight Dexamethasone Suppression Test for diagnosis of PPID.
Dex 5 Injection is an aqueous solution of dexamethasone sodium phosphate (DSP) for intravenous or intramuscular administration. It has powerful anti-inflammatory, antishock and gluconeogenic activity. Its anti-inflammatory and immunosuppressive effects are approximately 25x more potent than cortisol. It has no mineralocorticoid activity.

Glucocorticoids, such as dexamethasone have a broad range of pharmacological and physiological effects. They are the most efficacious anti-inflammatory drugs.
Dexamethasone injection may be given orally with a bioavailability of 42-61% in the fasted horse. Feeding decreases the bioavailability.
DSP may also be administered by nebulisation for treatment of diseases of the respiratory tract, such as Equine Asthma (EA), Inflammatory Airways Disease (IAD) or Recurrent Obstructive Airways Disease (ROA)/heaves. Nebulised DSP has the benefits of minimal systemic bioavailability and not inducing lower airway inflammation or inducing HPA axis suppression whilst still achieving the desired decrease in neutrophil and immune cell counts in BAL fluid.
DSP has also been administered intra-articularly, usually in combination with another corticosteroid (eg triamcinolone), hyaluronic acid or a biologic (IRAP, PRP). It is particularly useful when a rapid, albeit transient, resolution of joint inflammation is required or when a joint flare is anticipated following the IA injection of biologics.
Dexamethasone activity lasts up to 48 hours, although most is excreted in the first 24 hours with a plasma half-life of 3-6 hours.
Although a direct causal relationship between dexamethasone and laminitis has not been demonstrated, it is recommended that DSP be avoided, especially at higher doses or over a prolonged period, in any horse or pony that may have risk factors for laminitis.
DSP is contraindicated for use in animals with diabetes mellitus, cardiac disease, renal disease and animals with latent or suspected infections unless accompanied by effective antibiotic cover.
Use with caution during the last trimester of pregnancy as may induce parturition. To avoid adrenal suppression, doses should be tapered after long-term administration.
Dosing schedules are based on desired effect and vary according to the condition being treated. The response to dexamethasone is known to vary considerably from horse to horse. Due to DSP’s high potency and multiple untargeted systemic effects, use the lowest dose possible for the shortest period of time to achieve the desired results.
Anti-inflammatory effects: 0.02-0.2mg/kg sid IV or IM. 5mL @ 500Kg BW would be a typical dose.
Immunosuppressive effects: 0.2-0.5mg/kg sid IV or IM.
Shock (endotoxic/anaphylactic): 4-6mg/kg IV.
Vasculitis: 0.05-0.2mg/kg IV or IM q12-24h for 2 weeks, then taper over 4-6 weeks.
Purpura haemorrhagica: 0.04-0.2mg/kg sid or bid
Respiratory disease such as EA/RAO/IAD: 0.05-0.1mg/kg sid IV or IM for 3-4 days and then taper over 3-4 weeks. Interstitial pneumonia incl Equine multinodular pulmonary fibrosis: 0.02–0.04 mg/kg IV or IM.
Inflammatory Bowel Disease (IBD): 005-0.2mg/kg IM for 2-4 weeks.
Dermatitis/pruritis/pemphigus: 0.05-0.1mg/kg sid
Head/CNS trauma: 0.1-0.3mg/kg IV q6-24h
Uveitis; 2mg q24-36h subconjunctivally
Immune-mediated Haemolytic Anaemia: 0.05-0.1mg/kg sid IV or IM for 3-5 days then taper over 7-14 days.
Lymphoma: 0.2mg/kg IV for 5 days initially followed by ongoing immunosuppression. Intra-articularly: 2-4mg/joint usually in conjunction with another corticosteroid, biological or hyaluronic acid.
Overnight Dexamethasone Suppression Test (DMT) for PPID: Collect baseline serum sample in late afternoon. Administer dexamethasone 0.04mg/kg IM. Collect second serum sample ~20 hours post DSP administration. Failure to suppress cortisol levels by < 30mmol/L supports a diagnosis of PPID.
Horse Hack: The oral absorption of dexamethasone is superior to prednisolone. Injectable DSP may be administered orally in the fasted horse at a dose rate of approx. 150% the systemic dose. Feeding reduces absorption. The membrane stabilising effects of dexamethasone are valuable in the treatment of acute rhabdomyolysis ("tie up") and results in a rapid decrease in plasma muscle enzymes.

APVMA Approval No. 68303 (Australia)
Available in:
Rapid onset.
Prolonged effects.
Phenylbutazone Sodium 200 mg/mL
(equivalent to Phenylbutazone Base 186 mg/mL)
Sodium Salicylate 50 mg/mL
Pack Size
100mL multidose vial
Non-steroidal, anti-inflammatory, antipyretic and analgesic agent for the relief of inflammation and painful musculoskeletal conditions in horses, including:
• arthritis • bursitis
• arthrosis
• tendonitis • osteitis • tenosynovitis
• laminitis • rhabdomyolysis • skin inflammation
• post-operative pain/inflammation • soft tissue inflammation
Phenylbutazone is a nonsteroidal anti-inflammatory drug (NSAID) with antipyretic and analgesic activity. Sodium salicylate is an antipyretic analgesic agent. The combination of these two drugs provides both rapid action (sodium salicylate) and prolonged effect (phenylbutazone).
Phenylbutazone binds irreversibly to the enzyme cyclooxygenase, inhibiting its activity in the biosynthesis of prostaglandins. Prostaglandins are the primary mediators of the inflammatory response causing tissue swelling and inflammation, pain and fever. Further production of prostaglandin cannot occur until new enzyme is synthesised; resulting in a reduced concentration of prostaglandins at sites of inflammation and reduced inflammatory responses.
The safety of Platinum Bute IV has been evaluated at doses of up to 20 mL/225 kg (Day 1) and 10 mL/225 kg (Days 2-5), without any adverse effect.
Store between 2 and 8°C. (Refrigerate do not freeze).
When used for the relief of febrile conditions, treatment of the underlying cause should be provided concurrently with Platinum Bute IV Injection.
This product is contraindicated for use in horses with serious cardiac, hepatic or renal dysfunction, history of blood disorders (especially haemoglobinuria) or gastro-intestinal disturbances.
Toxic reactions to Platinum Bute IV Injection are uncommon, but may be expressed as oedema, jaundice, blood dyscrasia, gastric irritation or ulceration of the large intestine.
Administer by slow intravenous injection of solution at room temperature. Horses and Foals: Initially give 1-2mL/45kg (10-20mL/450kg) per day.
Subsequent doses, depending on response, may be reduced to 0.5-1mL/45kg (5-10mL/450kg) per day. Treatment may be continued for 5-6 days as required.
Store between 2° and 8°C (Refrigerate. Do not freeze).
Drongo was an Australian racehorse that raced during the early 1920’s.
He in turn was named after a type of bird.


Drongo had 37 starts but never won a race.
The term “drongo” has become part of the Aussie vernacular and means "stupid", a “loser” or “no-hoper”.
But the expression is a misnomer given that Drongo was actually placed in seven races (including a second in the Victorian Derby, which today would have been worth $380K). He was also placed in two other stakes races and earnt over £2200 (equivalent today to $163K).
You would have to be a drongo not to be happy with that.
For the rapid relief of inflammation and painful musculoskeletal
DID YOU KNOW THAT... A maiden racehorse became part of the Aussie vernacular?
DRONGO (DOROTHY WOOD, AUS)
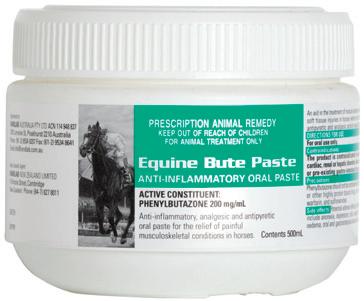


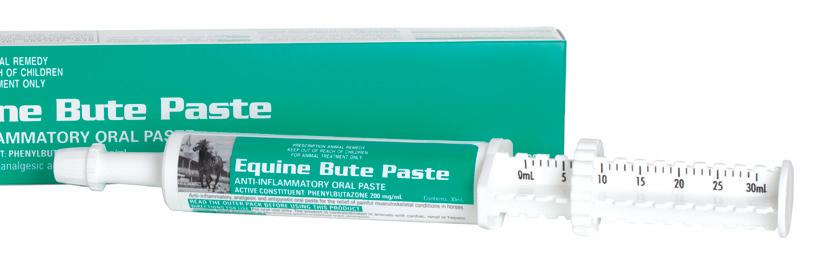

Anti-inflammatory, analgesic and antipyretic oral paste for the relief of pain and fever.
The analgesic and anti-inflammatory effects are indicated in the treatment of a wide variety of conditions in the horse including traumatic injuries and postsurgery.
Suitable for the treatment of musculoskeletal conditions including bone and joint disorders, soft tissue injuries and inflammation including tendinitis, acute tenosynovitis, desmitis, myositi (incl rhabdomyolysis), capsulitis, bursitis, acute and chronic laminitis and osteoarthritis.
Phenylbutazone is a nonsteroidal anti-inflammatory drug (NSAID) with antipyretic and analgesic activity. It binds irreversibly to the enzyme cyclooxygenase, inhibiting its activity in the biosynthesis of prostaglandins. Prostaglandins are the primary mediators of the inflammatory response causing tissue swelling and inflammation, pain, and fever. Further production of prostaglandin cannot occur until new enzyme is synthesised; resulting in a reduced concentration of prostaglandins at sites on inflammation and a reduced inflammatory response.
Phenylbutazone is well absorbed following oral administration. The drug is distributed throughout the body, with highest levels being attained in the liver, lungs, heart, kidneys and plasma. Phenylbutazone is up to 95% plasma protein bound. The increased vascular permeability to plasma proteins during an inflammatory process results in concentration of phenylbutazone in the inflammatory exudate.
The serum half-life of phenylbutazone is dose dependent, ranging in the horse from 3.5 hours at a dose of 2g/450kg to 6 hours at a dose of 8g/450kg. Clinical response to the drug can be seen within a few hours of oral dosage, while optimum effects occur approximately 12 hours post administration. Therapeutic efficacy may last for more than 24 hours, due probably to the irreversible binding of the drug to cyclooxygenase in the tissues.
Phenylbutazone is almost completely metabolised in the horse. The principal metabolites are oxyphenbutazone, with a pharmacological effect similar to phenylbutazone, and the pharmacologically inactive -hydroxyphenylbutazone.
Ponies and foals exhibit an increased sensitivity to the toxic effects of phenylbutazone.
This product is contraindicated in animals with cardiac, renal or hepatic disorders, blood dyscrasias or pre-existing gastrointestinal ulceration.
Phenylbutazone should not be combined with other NSAIDs or other highly protein bound drugs, such as digoxin, warfarin, and sulfonamides.
Potential adverse effects of phenylbutazone include anorexia, depression, colic, lethargy, weight loss, ventral oedema, oral and gastrointestinal lesions including ulcers and renal papillary necrosis.
If any of these signs occur discontinue treatment immediately.


Horses - Administer orally prior to feeding. 4.4 mg/kg (10 mL/450 kg) bodyweight twice daily for the first day; 2.2 mg/kg (5 mL/450 kg) bodyweight twice daily for the next four days; 2.2 mg/kg (5 mL/450 kg) once daily on alternate days or as directed by a veterinarian. Maximum daily dose should not exceed 4 grams (20 mL).
Ponies - Administer orally prior to feeding. 2.2 mg/kg (2.5 mL/225 kg) bodyweight twice daily for four days; 2.2 mg/kg (2.5 mL/225 kg) once daily on alternate days or as directed by a veterinarian.
Do not exceed the recommended dose, as ponies exhibit an increased sensitivity to the toxic effects of phenylbutazone.
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) for rapid initiation of therapy for any condition requiring anti-inflammatory activity, whilst preserving COX-1 function.
Suitable for the treatment of musculoskeletal disorders, including joint, bone and soft tissue injuries and relief of pain associated with colic and ophthalmic conditions.
Meloxicam is effective for treating inflammation and pain associated with surgery and during post-surgery recovery.
Meloxicam Injection may be used as a short course or as an initial loading dose prior to changing to oral meloxicam.
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) of the oxicam class which acts by inhibition of prostaglandin synthesis, thereby exerting antiinflammatory, anti-endotoxic, anti-exudative, analgesic and antipyretic properties. It has preferential COX-2 inhibitory properties, thus preserving the important biological functions of the COX-1 enzyme in the synthesis of regulatory prostaglandins responsible for maintaining a healthy gastrointestinal tract, renal function, platelet function, etc.
Meloxicam provides effective pain relief for colic without impeding recovery of ischaemic bowel.
Avoid use in dehydrated, hypovolaemic or hypotensive horses as there is a potential risk of renal toxicity.
Use with caution in pregnant or lactating mares and in foals under 6 weeks of age as the safety of this product in these classes of horse has not been established.
Use in animals suffering from haemorrhagic gastrointestinal disorders, impaired hepatic, cardiac or renal function and haemorrhagic disorders, or where there is evidence of individual hypersensitivity to the product is contraindicated.
Concurrent administration with glucocorticosteroids, other non-steroidal antiinflammatory drugs or with anti-coagulant agents is contraindicated. Use with caution in conjunction with other highly protein bound drugs.
Horse Hack: The withholding period for Meloxicam Injection prior to competition is considered less than other NSAID’s such as phenylbutazone. If using in performance horses, the regulations of the relevant authorities should be consulted.
Horses: 0.6mg/kg BW (3mL/100kg) meloxicam by intravenous injection
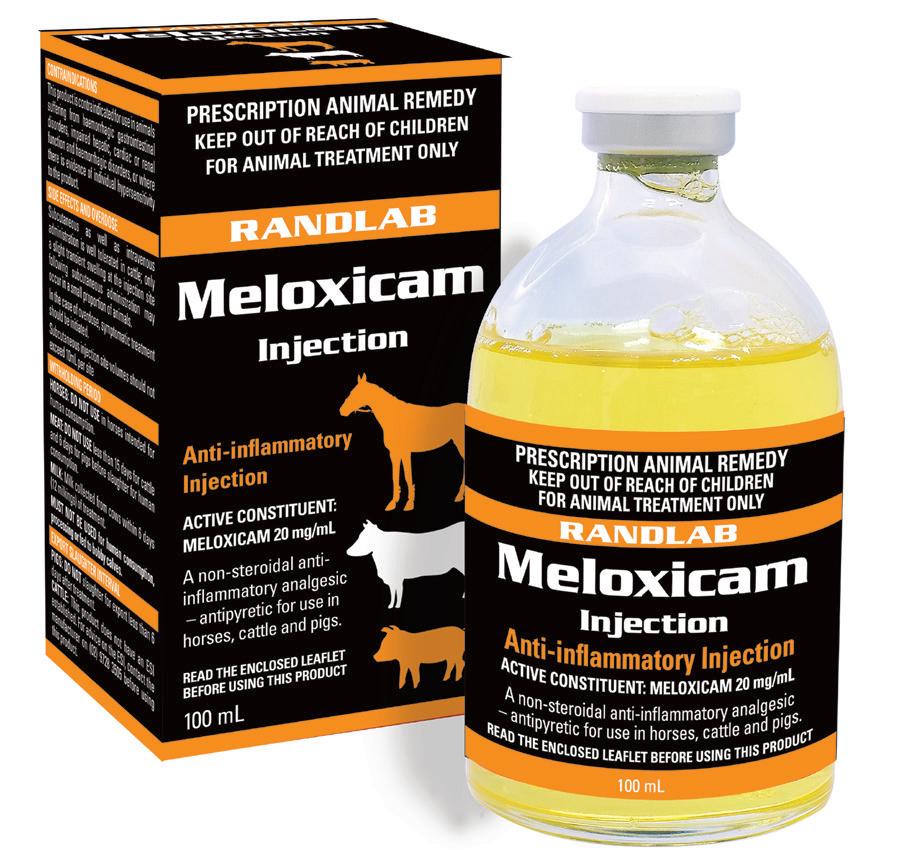
Cattle: Single use only by subcutaneous or intravenous injection. 0.5 mg meloxicam/kg bodyweight (i.e. 2.5 mL/100kg BW) in combination with antibiotic therapy, as appropriate.
Pigs: Single use only by intramuscular injection in the anterior half of the neck. (Can be repeated once after 24 hours if necessary). 0.4 mg meloxicam/kg BW (i.e. 2.0 mL/100kg) in combination with antibiotic therapy, as appropriate.
Sheep: Single use only by subcutaneous injection high on the neck behind the ear. For sheep and lambs from 14 days of age. 1.0 mg/kg BW (1.0 mL/20kg) meloxicam.

The phrase, “A horse, a horse, my Kingdom for a horse” has become one of the most quoted in the Shakespearean cannon. Based on historical records, the line is fictitiously uttered by the dying King Richard III as he lies bludgeoned on the battleground at the Battle of Bosworth Field. The last King to die in battle.
Richard’s horse a grey courser, referenced as “White Surrey”, had become stuck in the mire and subsequently killed, leaving the King disadvantaged as he continued to fight on foot. Coursers were swift and strong horses, frequently used during the Middle Ages as a warhorse and much favoured by Knights and Kings. The nursery rhyme Humpty Dumpty is often credited to being about the demise of the deformed King Richard , with the wall his metaphorical horse.

A
DID YOU KNOW THAT... The loss of his horse really did cost Richard III his Kingdom?
KING RICHARD III (WHITE SURREY, UK)


Non-steroidal anti-inflammatory drug (NSAID) for any condition requiring antiinflammatory activity, whilst preserving COX-1 function.
Suitable for the treatment of musculoskeletal disorders, including joint, bone and soft tissue injuries and relief of pain associated with colic and ophthalmic conditions.
Meloxicam is effective for treating inflammation and pain associated with surgery and during post-surgery recovery.
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) of the oxicam class which acts by inhibition of prostaglandin synthesis, thereby exerting antiinflammatory, anti-endotoxic, anti-exudative, analgesic and antipyretic properties. It has preferential COX-2 inhibitory properties, thus preserving the important biological functions of the COX-1 enzyme in the synthesis of regulatory prostaglandins responsible for maintaining a healthy gastrointestinal tract, renal function, platelet function, etc.
Meloxicam provides effective pain relief for colic without impeding recovery of ischaemic bowel.
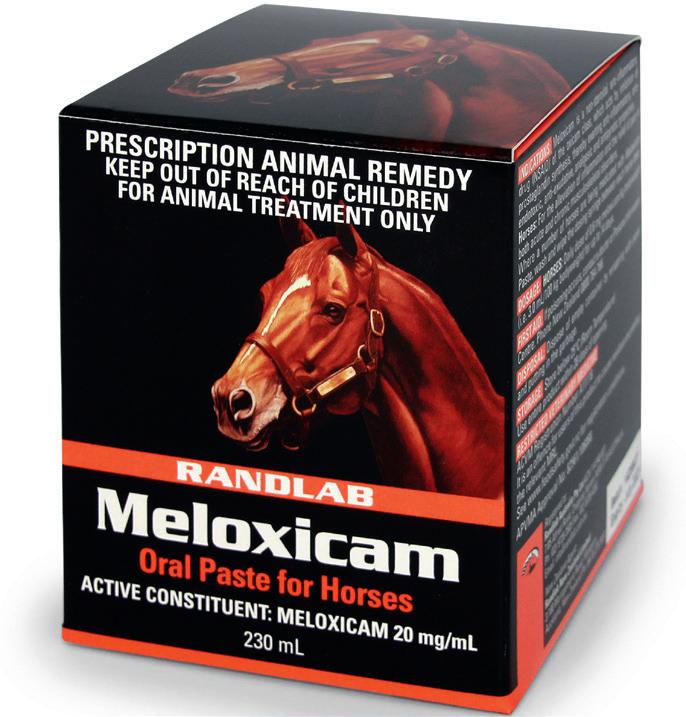
Avoid use in dehydrated, hypovolaemic or hypotensive horses as there is a potential risk of renal toxicity.
Use with caution in pregnant or lactating mares and in foals under 6 weeks of age as the safety of this product in these classes of horse has not been established.
Use in animals suffering from haemorrhagic gastrointestinal disorders, impaired hepatic, cardiac or renal function and haemorrhagic disorders, or where there is evidence of individual hypersensitivity to the product is contraindicated.
Concurrent administration with glucocorticosteroids, other non-steroidal antiinflammatory drugs or with anti-coagulant agents is contraindicated. Use with caution in conjunction with other highly protein bound drugs.

Horses: Daily dose of 0.6 mg/kg bodyweight (i.e. 3.0 mL/100 kg bodyweight) for up to 14 days.


Foals: 0.6mg/kg twice daily dosing is required in foals < 7 weeks old due to more rapid clearance.
It is also the image on the cover of the annual Randlab catalogue. Secretariat won the 1973 US Triple Crown, including the Belmont Stakes by an extraordinary 31 lengths. Overall, the stallion had 21 start for 16 wins and 4 placings. No horse in the history of horse racing has captured the enduring attention of the general public and racing aficionado’s as much as “Big Red”. The image on the Meloxicam box is an original painting by well-known US horse artist, Tom Chapman. It is from a photo taken of Secretariat at stud.
Oral suspension for ease of administration. Suitable for top dressing in feed. High bioavailability in both fed and fasted horses.
Active Constituent
Meloxicam 30 mg/mL
Pack Size
250mL bottle
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) for any condition requiring anti-inflammatory activity, whilst preserving COX-1 function.
Suitable for the treatment of musculoskeletal disorders, including joint, bone and soft tissue injuries and relief of pain associated with colic and ophthalmic conditions.
Meloxicam is effective for treating inflammation and pain associated with surgery and during post-surgery recovery.
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) of the oxicam class which acts by inhibition of prostaglandin synthesis, thereby exerting antiinflammatory, anti-endotoxic, anti-exudative, analgesic and antipyretic properties. It has preferential COX-2 inhibitory properties, thus preserving the important biological functions of the COX-1 enzyme in the synthesis of regulatory prostaglandins responsible for maintaining a healthy gastrointestinal tract, renal function, platelet function, etc.
Meloxicam provides effective pain relief for colic without impeding recovery of ischaemic bowel.
Avoid use in dehydrated, hypovolaemic or hypotensive horses as there is a potential risk of renal toxicity.
Use with caution in pregnant or lactating mares and in foals under 6 weeks of age as the safety of this product in these classes of horse has not been established. Use in animals suffering from haemorrhagic gastrointestinal disorders, impaired hepatic, cardiac or renal function and haemorrhagic disorders, or where there is evidence of individual hypersensitivity to the product is contraindicated.
Concurrent administration with glucocorticosteroids, other non-steroidal antiinflammatory drugs or with anti-coagulant agents is contraindicated. Use with caution in conjunction with other highly protein bound drugs.
Shake well before use.
Horses: Daily dose of 0.6 mg/kg bodyweight (i.e. 10mL suspension/500kg BW) for up to 14 days.
Foals: 0.6mg/kg (i.e. 2ml suspension/100kg BW) twice daily for up to 7 days. Twice daily dosing is required in foals < 7 weeks old due to more rapid clearance.
Horse Hack: The withholding period for Meloxicam Oral Suspension prior to competition is considered less than other NSAID’s such as phenylbutazone. If using in performance horses, the regulations of the relevant authorities should be consulted.


The descendants of the Icelandic horse came to Iceland with the country’s first settlers from Norway 1100 years ago. They were descended from an ancient breed of horses that is now extinct.

The preservation of the Icelandic breed is guaranteed with the country’s Parliament passing a law in 982AD prohibiting the importation of horses into the island nation. The Icelandic horse is the only breed of horse in Iceland.
The horses are becoming more popular worldwide with about 2500 horses exported globally last year. These horses cannot return to Iceland.

The average Icelandic horse stands 14hh and weighs between 330-450kg. Although only small, they are classified as a horse and not a pony. There are over 100 recognised coat colours and patterns.
The Icelandic horse is 5-gaited with two additional gaits; the tölt and the skeið. The latter is a flying pace where horses may reach speeds of upto 50km/h.
There are currently about 90,000 Icelandic horses in Iceland and 350,000 people (that’s one horse every 4 head of population) . There are 215 vets.
A non-steroidal anti-inflammatory, analgesic and antipyretic with preferential COX-2 inhibition
DID YOU KNOW THAT... The last horse to be imported into Iceland was 1040 years ago? ICELANDIC HORSE (ICELAND)
Available in:
For the alleviation of visceral pain and inflammation associated with colic and the normalisation of peristalsis.
For the alleviation of inflammation and pain associated with musculoskeletal disorders.
For the treatment of inflammatory ocular conditions such as uveitis and pre- and post-eye surgery.
At lower doses than used for anti-inflammatory effects, flunixin reduces the haemodynamic changes associated with endotoxaemia/endotoxic shock.
Flunixin is considered to be the most potent cyclooxygenase inhibitor of the NSAID’s. It is also reported to be a more potent analgesic than phenylbutazone or pethidine and to provide comparable analgesic effects to morphine.
Flunixin does not significantly alter gastrointestinal motility, BP or cardiac rhythm. Flunixin has been used successfully to reduce the adverse haemodynamic changes associated with toxic shock by decreasing endotoxin induced lactic acidosis, reducing the severity of arterial hypertension and endothelial cell injury and improving venous return.
Flunixin provides effective anti-inflammatory and analgesia in a wide range of musculoskeletal disorders.
It also results in effective visceral analgesia in cases of equine colic due to flatulence or inflammatory conditions. Flunixin is considered to be a more potent analgesic for the treatment of colic than many of the narcotic or other NSAID’s and opioids.
Intravenous administration of flunixin has been advocated in therapy of ocular inflammatory conditions and may be used pre- and post-operatively to reduce inflammation resulting from ocular surgery and the accumulation of prostacyclin in the aqueous humour. Flunixin alone or in combination with corticosteroids, is a useful treatment for inflammatory eye conditions such as uveitis. Flunixin may also be administered subconjunctivally prior to intraocular surgery or in cases of uveitis or other inflammatory eye conditions.
Flunixin has a short plasma half-life (1.6 hrs) with a peak response reached at 1216 hours after both oral and IV administration and a long pharmacological duration of action (up to 36 hours). Therapeutic effects are maintained even at low plasma concentrations due to irreversible cyclooxygenase binding in the tissues.
Avoid intramuscular administration in the horse.
In horses with colic, flunixin may mask the behavioural and cardiopulmonary signs associated with endotoxaemia or intestinal devitalisation. This must be considered in any pre colic surgery assessment.
In the absence of any specific studies in pregnant animals, such use is not recommended. Concurrent use with other anti-inflammatory drugs should be avoided. Use with caution in animals with renal, hepatic or haematologic disease. Excessive doses or prolonged administration may result in gastrointestinal (right dorsal colon, caecum or stomach) ulceration.
Injection reactions or clostridial myositis may develop following intramuscular injections of flunixin in horses and should be avoided.
Musculoskeletal disorders: 1.1 mg per kg (1mL/45kg) bodyweight one to three times daily by intravenous injection for up to 5 days.
Colic: Alleviation of pain associated with colic: 1.1 mg per kg bodyweight I.V administration is recommended for prompt relief. May be repeated if signs of colic recur. Cause of colic should be determined and treated with appropriate therapy. Endotoxaemia (incl at risk of endotoxaemia): 0.25mg/kg q8h administered IV to interrupt eicosanoid production and inhibit the associated endotoxin-induced haemodynamic effects.
Horse Hack: Colix Injection may be administered orally at a dose rate of 1.1-1.3mg/kg. The injection can be administered mixed with molasses or squirted directly over the back of the horse’s tongue. The onset of action after oral dosing is generally within 2 hours, peak response 12-16 hours and duration of action up to 30 hours.
WARNING: Prolonged detection times leading to a positive drug test have been reported in the horse. This is believed to be due to drug “recycling” with the horse ingesting previously excreted flunixin. It is recommended that boxed competition horses be moved to a different stall after the normal excretion period (~96 hours) to prevent potential re-ingestion of excreted flunixin.
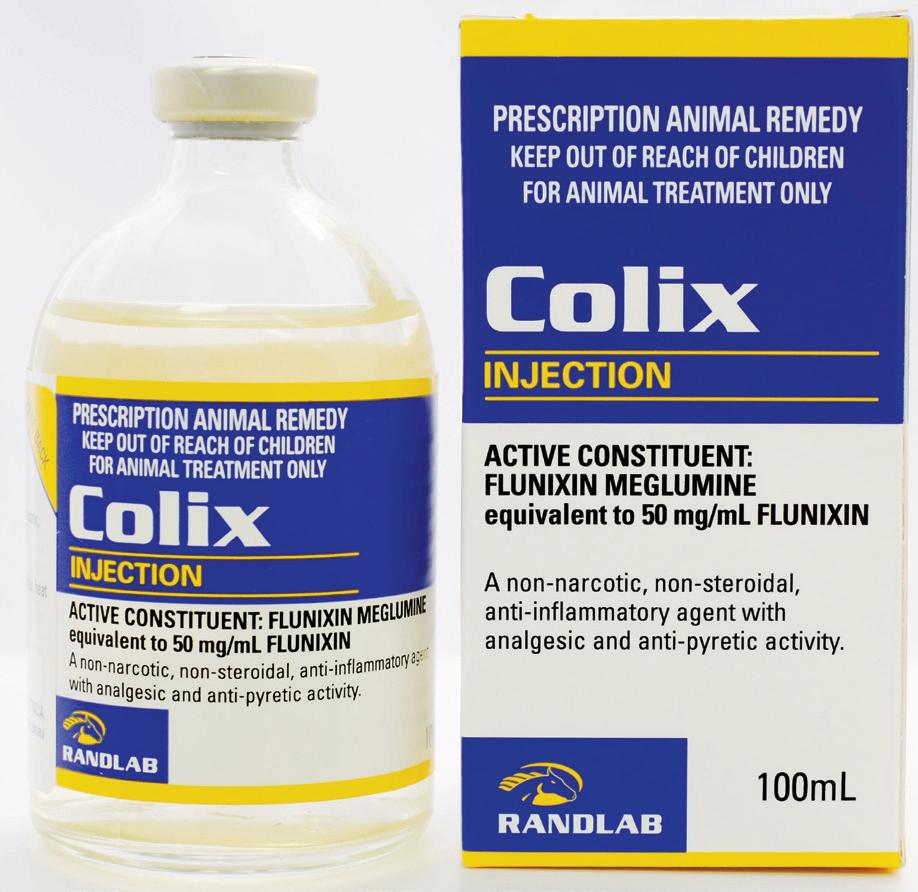

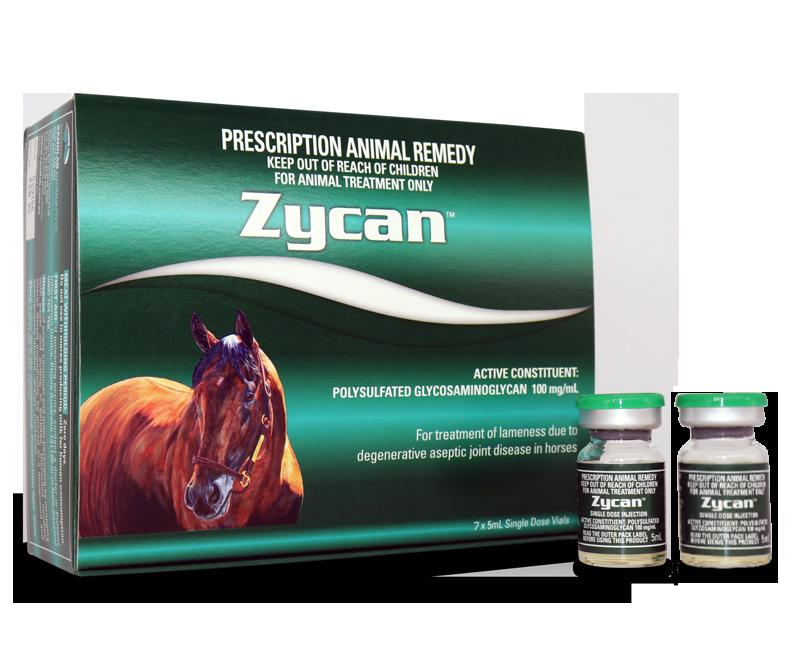
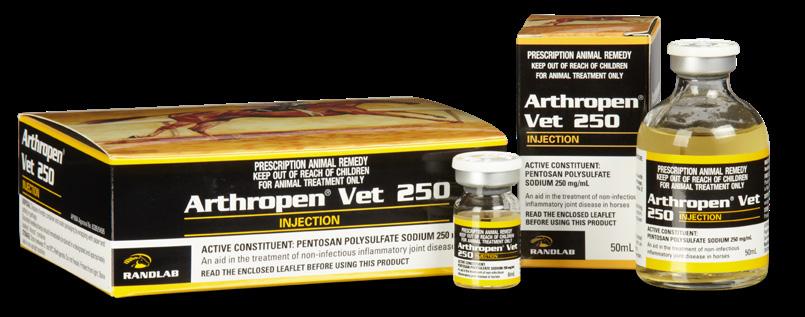
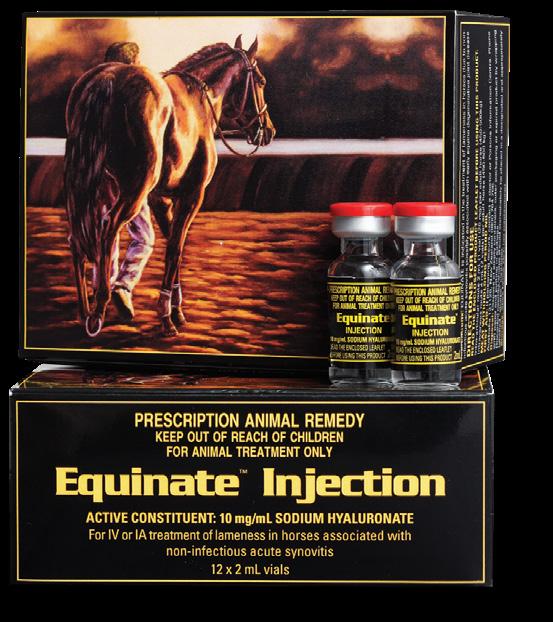



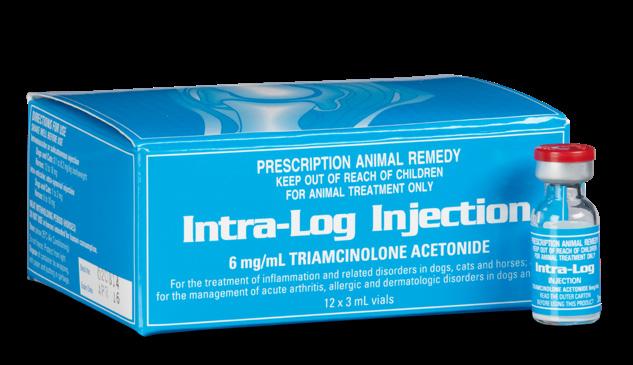
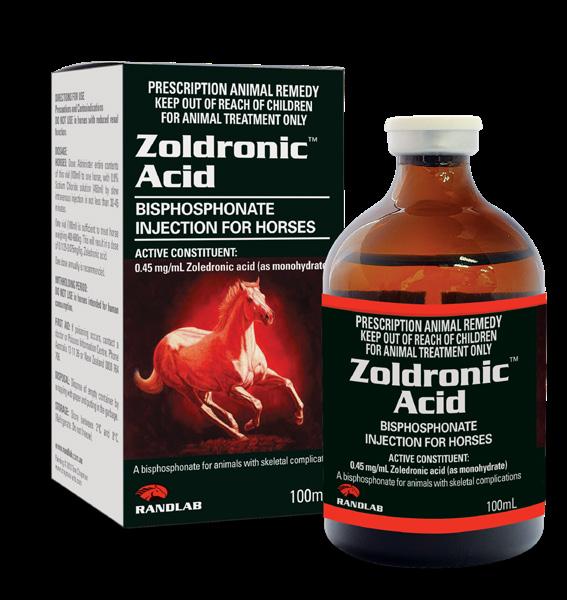
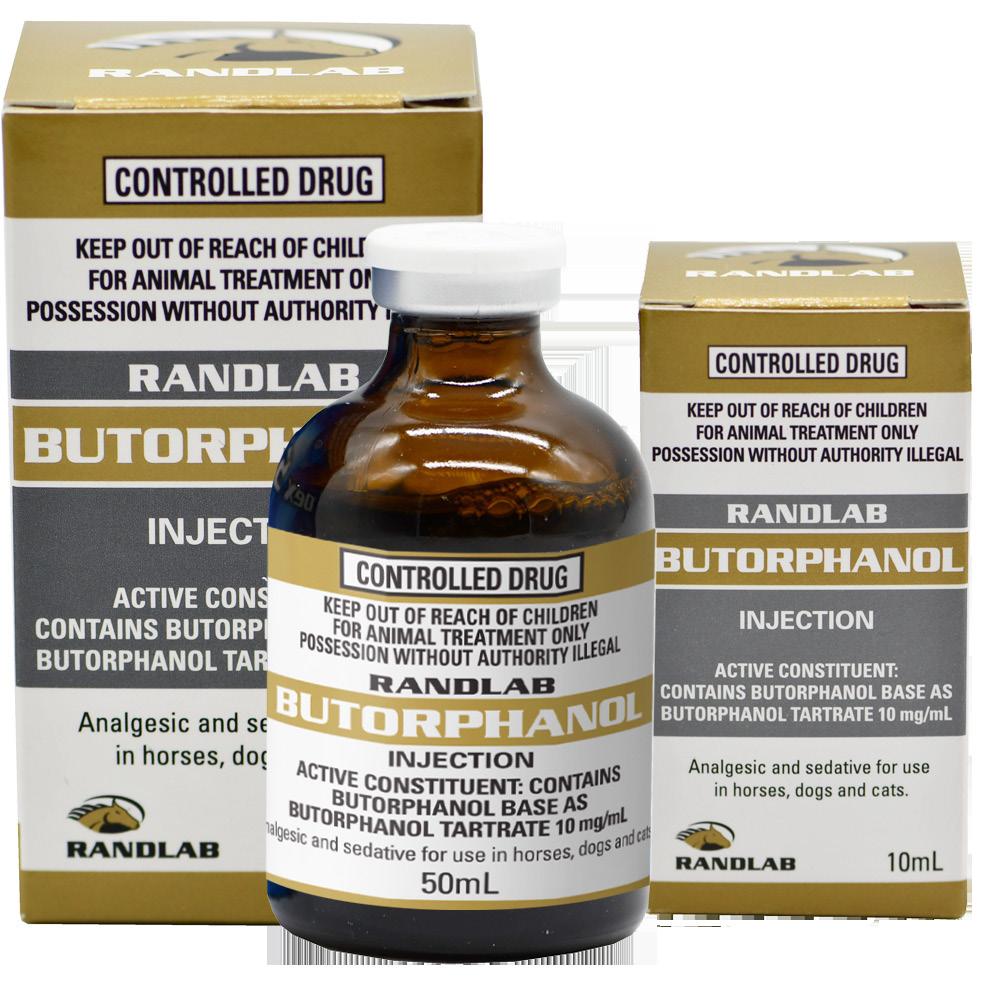
Butorphanol tartrate is a synthetic, mixed opioid agonist/antagonist with analgesic and sedative properties. It is generally used in combination with a sedative (such as an alpha-2 agonist) to avoid initial excitatory activity. Butorphanol has wide application in equine veterinary practice including:
• Restraint during standing surgery
• Restraint during minor surgical procedures
• Restraint during diagnostic procedures (eg yearling repository x-rays)
• Relief of pain associated with colic for up to four hours
• Anaesthetic pre-medication in combination with an α2-agonist
• Component in balanced general anaesthesia (esp for painful procedures)
• Post-operative and general pain management
• Potent anti-tussive
Butorphanol Injection (butorphanol tartrate) is a totally synthetic, centrally acting, narcotic agonist/antagonist with analgesic, sedative and antitussive activity. At higher doses it is analgesic and at a lower dose it has a predictable sedative effect. Following IV doses, the onset of action is approximately 3 minutes with a peak analgesic effect at 15-30 minutes. The duration of action in horses is typically 1-2 hours but may last up to 4 hours after a single dose (esp in combination with an alpha-2). The dose may be repeated as required every 3-4 hours, but it is not recommended that treatment be continued beyond 48 hours.
Butorphanol at the recommended dose rate causes minimal cardiopulmonary depression. Furthermore, the cardiopulmonary effects of butorphanol are not linearly dose related and reach a plateau beyond which further dosage increases result in relatively lesser effects.
Analgesia; Butorphanol is useful for the relief of pain associated with colic, postpartum treatment of mares, surgical sedation (both standing and as a pre-anaesthetic agent), castration and many other veterinary procedures.
When used for the relief of pain, butorphanol may be used alone or in combination with tranquilisers, such as xylazine, detomidine, etc. It begins to alleviate pain within 15 minutes of administration and can be effective for up to four hours. It is five times more effective in controlling pain than morphine.
In horses with colic; Butorphanol provides prompt analgesia within 15 minutes of injection, thus facilitating a thorough examination and treatment and limiting self-inflicted injury. The level of pain relief is not so strong or long-lasting to mask the need for surgery. Butorphanol also produces fewer changes in gastrointestinal function and motility than other opioids. Compared with an IV bolus, a constant rate infusion of butorphanol induces fewer GI side effects while providing good analgesia. Sedation; Butorphanol is also used for enhancement of sedation in combination with another sedative, such as xylazine or detomidine for standing chemical restraint and as part of premedication protocols for general anaesthesia.

Cough suppressant; Butorphanol may also be used as a cough suppressant where it acts centrally to reduce cough and may therefore also be useful in procedures such as tracheal washes and bronchoalveolar lavage (BAL).
Intravenous dosing of butorphanol in the unsedated horse has been associated with excitement, increased ambulation, ataxia, increased response to auditory stimuli and decreased gastrointestinal motility.
WARNING:
Animals with diseases of the lungs characterised by excessive mucus production should not receive butorphanol as it may prevent removal of mucus by cough. Butorphanol depresses brain function and should not be used in patients with injuries to the head, those in a coma or with an increase in CNS pressure.
Butorphanol should be used with caution in animals that have kidney disease, preexisting cardiac dysrhythmia or bradycardia.
Safety in breeding stock and foals has not been proven.
Do not mix with other products in same syringe.
Butorphanol may be administered intramuscularly at a higher dose and/or more frequently as systemic absorption via this route is only about 37%. However, dose and dosing interval should be based on the response to treatment.
Analgesia: 0.1-0.4mg/kg q3-4h IV or 0.04-0.2mg/kg IM.
CRI: Initial loading dose 17.8µg/kg followed by 13-24µg/kg/hr.
COLIC PAIN: 0.01-0.02mg/kg IV alone or in combination with an alpha-2 agonist (eg xylazine 0.02-0.1mg/kg IV).
Sedation: ALONE; 0.1-0.13mg/kg (=1-1.3mL/100kg BW) by intravenous injection q34h. IN COMBINATION; 0.02-0.05mg/kg IV in combination with xylazine (0.5-1.0mg/kg), detomidine (10-40 µg/kg), romifidine (80-100 µg/kg) or acepromazine (0.02- 0.05mg/ kg).
PRE-ANAESTHETIC; 0.01-0.04mg/kg IV following administration of an alpha-2 agonist (eg xylazine 0.1-0.5mg/kg IV).
FIELD ANAESTHESIA: Sedate with xylazine (1mg/kg IV or 2mg/kg IM) followed by butorphanol (0.02-0.04mg/kg IV). Wait until horse heavily sedated (~10 minutes) before administering ketamine (2.2mg/kg IV). Combination improves induction, increases analgesia and increases recumbency time by 5-10 minutes
Antitussive: 0.02 mg/kg IM bid-tid.
Foals: Alone; 0.1-0.2mg/kg IV or IM. In Combination; 0.5 mg/kg xylazine followed by 0.04 mg/kg butorphanol.
Horse Hack: Premedication with butorphanol reduces cough associated with invasive airway procedures such as bronchoalveolar lavage (BAL) and tracheal washes.
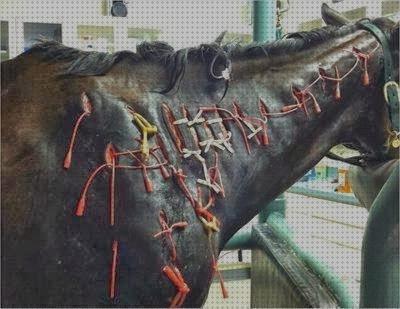
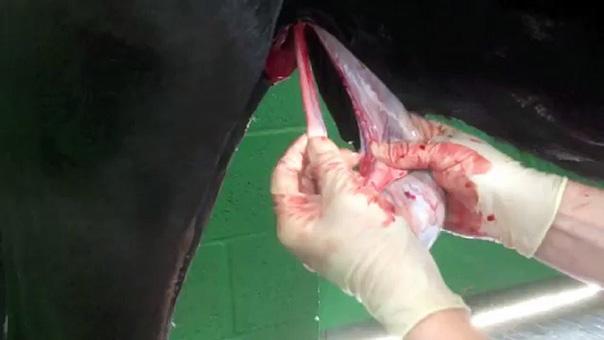
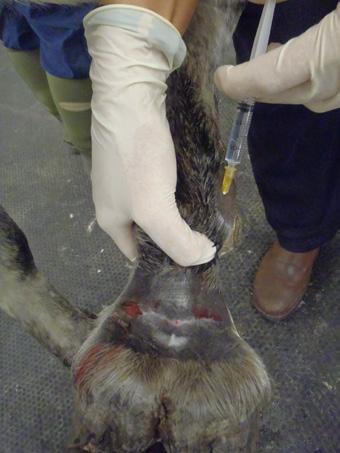
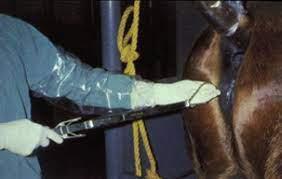



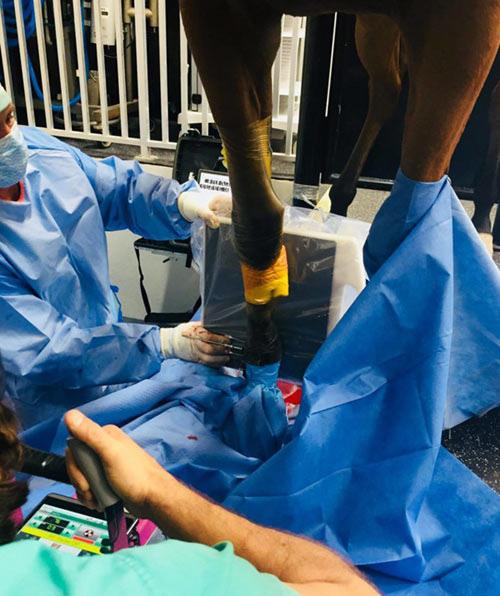
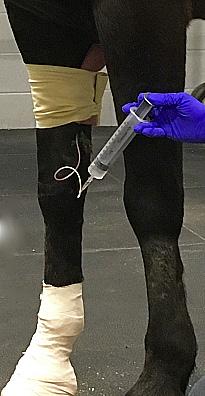

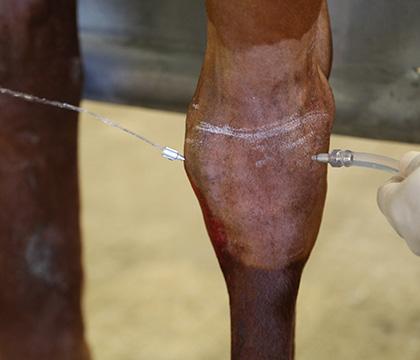
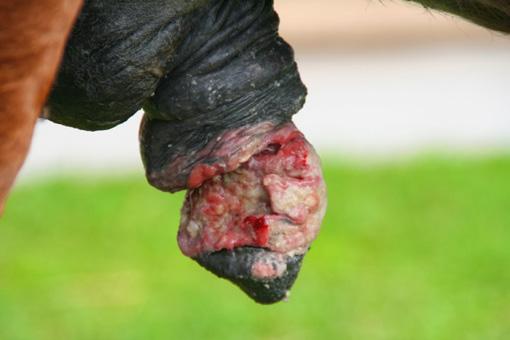

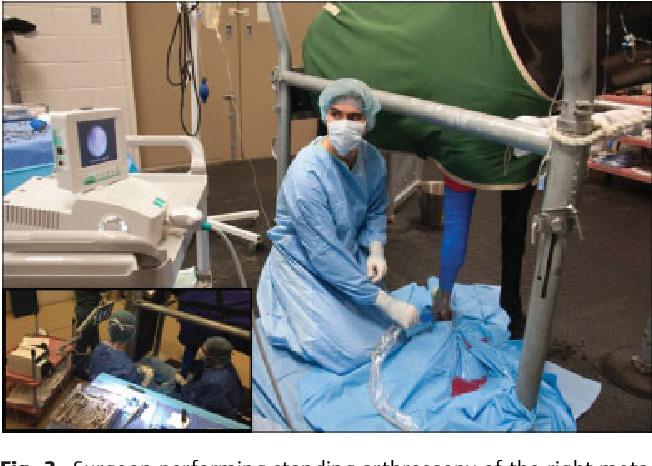
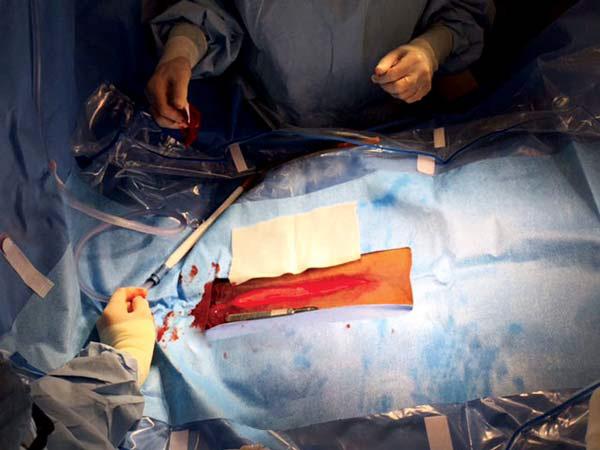
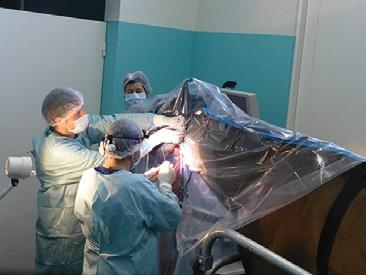
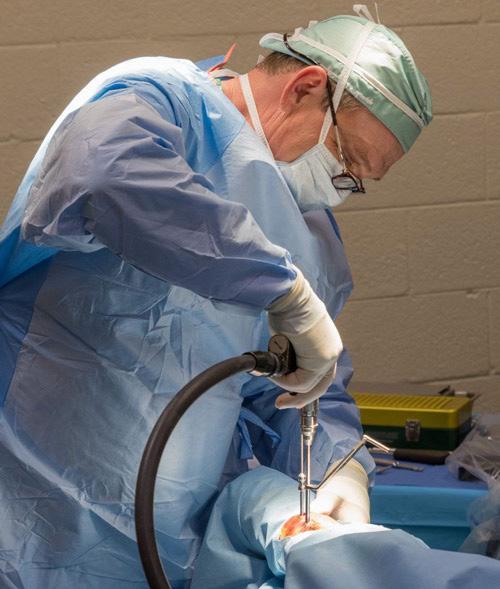


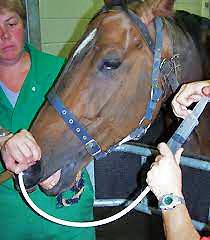
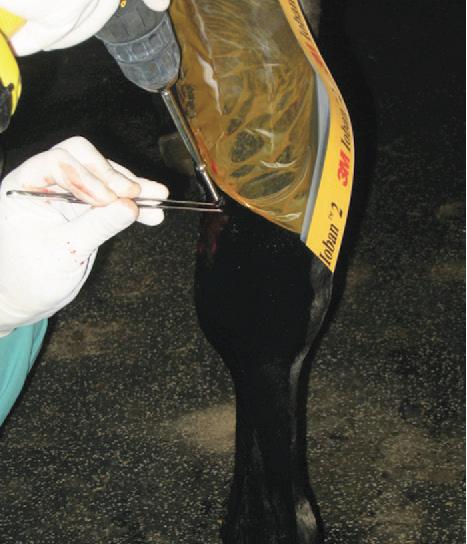
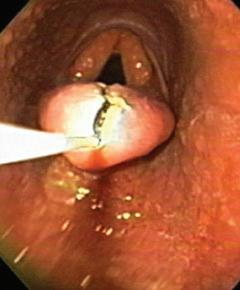
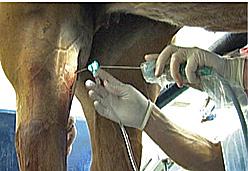
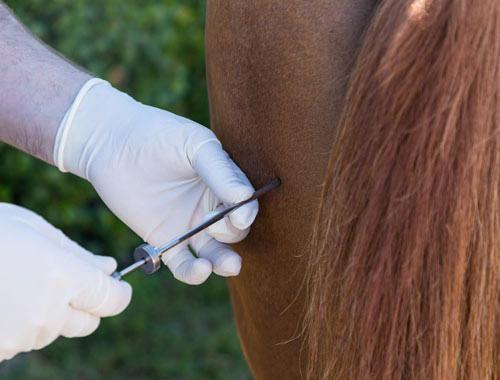
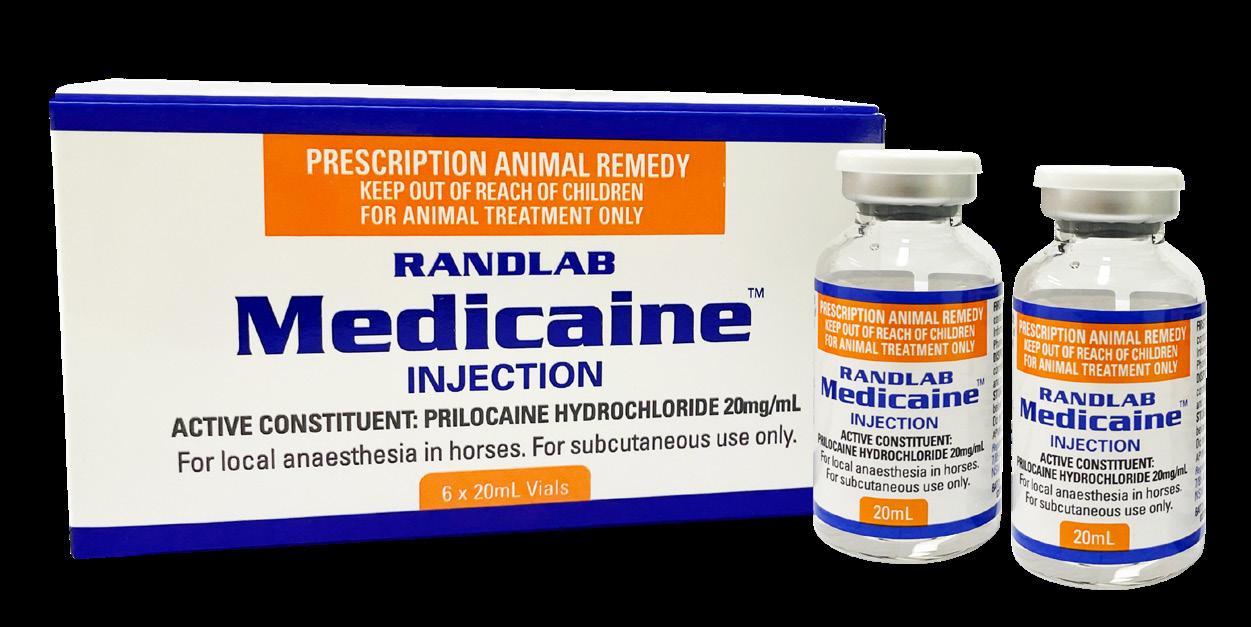

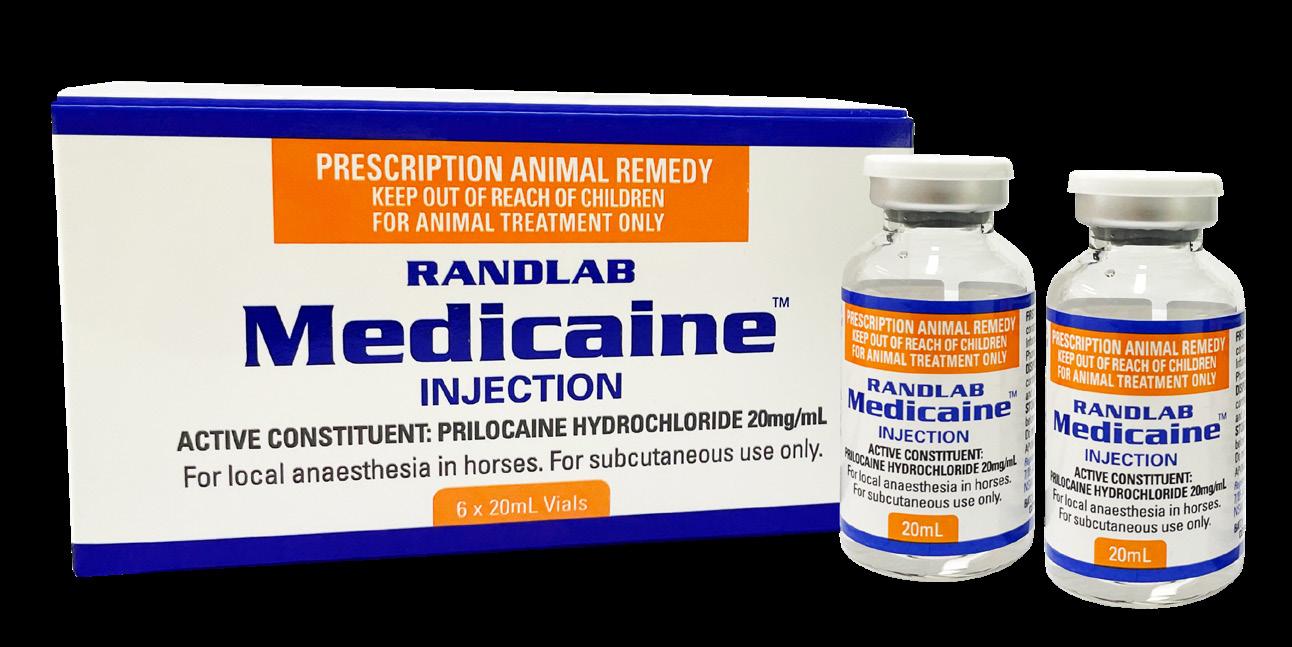
APVMA Approval No. 88500 (Australia)

Prilocaine Hydrochloride 20 mg/mL
Pack Size
6 x 20mL multidose
Prilocaine causes less tissue irritation, has a faster onset of action and longer duration of action compared to other local anaesthetics such as lignocaine and mepivicaine.
Medicaine is indicated whenever local anaesthesia is required, including:
• Infiltration anaesthesia
• Nerve blocks
• Intravenous regional limb perfusion
• Standing surgeries including arthroscopy, laparoscopy, standing laparotomy, fracture repair, genital/perineal surgery, hoof resections, castrations, respiratory surgery (incl laryngoplasty and sinus surgery), "kissing spines" surgery
• Topical anaesthesia including minor dermatological procedures and wound debridement, repair and suturing
• Biopsy/excision/debulking of skin tumours including sarcoids, melanomas, SCC, etc.
• Dentistry including fixation of broken jaws, tooth extraction
• As an adjunct to general anaesthesia to reduce the dose of parenteral/inhaled anaesthetic agents
• Post-operative pain management
• Bronchoalveolar lavage (BAL) to reduce cough
• Skin grafts
• Biopsies (skin, liver, muscle, etc)
• Rectal relaxant prior to rectal examination
• Foal transphyseal screw placement
• Relief of back muscle pain/spasm
Prilocaine, the active ingredient in Randlab Medicaine Injection, is a synthetic, sterile, isotonic local anaesthetic agent of the amide class. It has low tissue irritability compared to many other local anaesthetics. It inhibits nerve conduction via sodium channel blockade.
Prilocaine shows fast onset (within minutes) and intermediate duration of action (100-130 minutes). Prilocaine metabolism is similar to lidocaine, being rapidly metabolised by the liver and excreted in the urine.
Prilocaine shows the least systemic toxicity of all the amide local anaesthetics. It has low cardiac and neurological toxicity compared with other amide local anaesthetics and therefore is commonly used for intravenous regional limb perfusion.
Discard unused portion within 24 hour of first broaching. A new bottle should always be used for any aseptic technique or whenever a sterile surgical field is required.
Local anaesthetics should be avoided when there is inflammation at the site of the proposed injection.
Horse Hack: Intra-rectal administration of prilocaine is an effective rectal relaxant prior to performing a rectal examination or rectal surgery. A dose of 50-60mL is usually administered via syringe after inserting a gloved hand through the anus.
The

DID YOU KNOW THAT... Australia sent 136,000 horses to WWI. Only one came home?
Ketamine-based anaesthesia is the most common anaesthetic technique for short procedures in the field. The duration of anaesthesia is generally less than 30 min.
Ketamine-based anaesthesia can also be used as an induction agent prior to intubation and inhalation anaesthesia. Intravenous anaesthesia may also be maintained following α2-agonist + ketamine induction with “Triple Drip” Continuous Rate Infusion (CRI). See Dosage section.
Ketamine also inhibits NMDA-receptors so may be adjunctively useful to control pain.
Ketamine anaesthesia is characterised by analgesia, normal pharyngeallaryngeal reflexes, mild cardiac stimulation, respiratory depression and increased sympathetic tone. Protective reflexes (such as coughing, swallowing and corneal) are maintained and the eyes normally remain open with a dilated pupil. Nystagmus is common.
Ketamine will increase heart rate and blood pressure due to increased sympathetic tone. This results in an increase in cardiac output compared with other anaesthetic agents. Ketamine has a wide therapeutic index (e.g. 5x pentobarbital).
Ketamine has been shown to cross the placenta, and depression of neonates may occur if ketamine is used for induction of anaesthesia for caesarean section. Recovery from ketamine-based anaesthesia is considered to be good and superior to thiobarbiturate or inhalation anaesthesia.
Ketamine should always be used following sedation with an α2-agonist. Do not use alone for anaesthesia as may cause hyperexcitability and involuntary muscle reactions.
Ketamine may cause pain at the injection site if administered intramuscularly. Ketamine-based anaesthesia should not be used as the sole anaesthetic mode for major surgery and should always be supplemented by gaseous or barbiturate anaesthetics or muscle relaxants.
This product is contraindicated for use in horses with prior hypersensitivity to ketamine, renal or hepatic insufficiency, history of epilepsy, cardiovascular insufficiency, myocardial damage or serious cardiac arrhythmia or horses with glaucoma, hypovolaemic animals, patients prone to seizures or with cerebral trauma or intracranial lesions.
Horses should not be fed until they have fully recovered from general anaesthesia due to the risk of oesophageal choke.
Sedatives other than xylazine may be used prior to ketamine anaesthesia, e.g. detomidine (0.02 mg/kg) or romifidine (0.1 mg/kg).

Induction of anaesthesia: Premedicate with an α2-agonist (e.g. xylazine 1.1 mg/kg IV or 2.0 mg/kg IM) given 5-10 minutes before induction of anaesthesia with ketamine. Horse must be adequately sedated before administering the ketamine (2.2 mg/kg IV). Total anaesthetic time is short but can be prolonged by administering one-third to one-half of the induction dose or by instigating “Triple Drip” CRI (see below).

Butorphanol (0.02-0.04 mg/kg IV) may also be added to the original xylazine in highly strung horses (such as young horses, Thoroughbreds or Arab) 5-10 minutes prior to Ketamine administration.
Diazepam (0.03 – 0.1 mg/kg IV) may also be administered along with xylazine prior to ketamine to improve induction, improve muscle relaxation during anaesthesia and prolong anaesthesia by about 5-10 minutes.
Field anaesthesia/“Triple Drip”: Add 500mg xylazine and 2000mg ketamine to 1L of 5% guaifenesin in dextrose or 0.9% saline.

INDUCTION: 1.1 mL/kg IV rapidly.
MAINTENANCE: 2.0-4.5 mL/kg/hr to effect. “Triple drip” should not be used for anaesthetics greater than 1 hour in duration unless oxygen supplementation and respiratory support is provided.
For foals & ponies: Add 250mg xylazine and 500mg ketamine to 500mL 5% guaifenesin solution. For induction give 1.1 mL/kg IV rapidly. For maintenance 2-3mL/kg/hr to effect.
CRI for analgesia: Loading dose of 0.6 mg/kg IV followed by CRI of 0.4-0.8 mg/ kg/hr. Can be increased to 1.2mg/kg/hr if required. To prepare CRI solution, add 30mL ketamine to 1L bag of saline (= 3mg/mL) and administer IV over 8 hours at a rate of 125 mL per hour for a 500kg horse.
Foals treatment of seizures: 0.02 mg/kg/min CRI.
Old Billy (presumably that was not his name originally) was born in Lancashire England in 1760. The shire horse spent his entire working life as a barge horse dragging barges along the canals from the shore. But all the hard work did Old Billy no harm as he allegedly lived until the ripe old age of 62 years, 2-3 times the life expectancy of the average horse.
His cut away skull showing his dentition is on display in the Manchester Museum.
DID YOU KNOW THAT... The oldest known horse allegedly lived to an age of 62 years?
OLD BILLY (MERSEY & IRWELL NAVIGATION, UK)
APVMA Approval No. 82623 (Australia) | ACVM No. A011244 (New Zealand)
Sedator is a dose controlled sedative and analgesic. It may be used to facilitate examinations, x-rays, minor surgical procedures, transport etc. It may also be used to control pain including that of the uncomplicated equine colic case. Sedator has a role in providing prolonged analgesia (up to 12 hours) in horses.
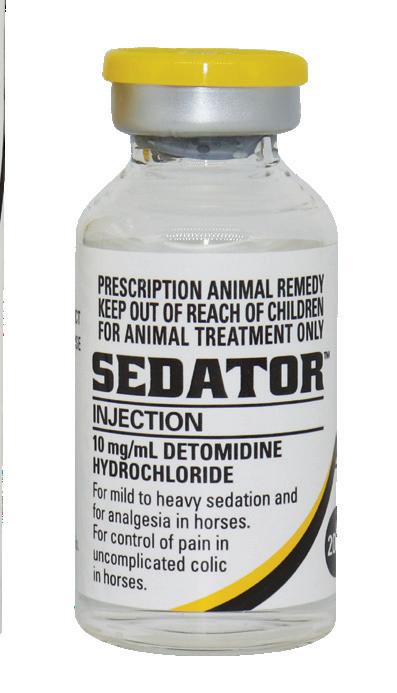

Sedator is a sedative and analgesic that may be used to facilitate:
• Examinations e.g. endoscopy, rectal palpation, gynaecological examinations, lameness examinations and all diagnostic imaging procedures.
• Minor surgical procedures e.g. debridement and suturing of wounds, removal of skin tumours, hoof care and castration.
• Pre-anaesthetic sedative when used in combination with anaesthetic agents
• Treatment and procedures in young or fractious horses e.g. nasogastric tubing, clipping and shoeing.
• Effective analgesia in treatment of colic cases.
• Sedation and analgesia in the management of serious injuries.
• Dental examinations and procedures.
• Safe handling of fractious animals.
0
Sedator is not recommended for use in pregnant mares.
Sedator may cause penile relaxation. Use with caution in male animals, particularly stallions during the breeding season.
Clinicians should anticipate the tendency of the horse’s head to drop under the influence of detomidine and the horse should be accommodated appropriately.
Immediately following administration there may be a tendency to stagger, particularly if high dose rates are used.
The safe handling of horses can be improved with Sedator. Although the animal is easy to handle under detomidine sedation, normal precautions are recommended.
Local anaesthetics or additional analgesia should be used when required for painful procedures, particularly surgery, as the duration of analgesia from Sedator may not equate with the duration of sedation.
A risk/benefit assessment should be undertaken by a veterinarian before using Sedator in animals suffering from cardiac disease (with pre-existing bradycardia and risk of atrioventricular block), respiratory, liver or renal insufficiencies or shock.
Overdosage and Side Effects: Sympathomimetic properties, particularly at higher doses, may include piloerection, sweating, diuresis & occasional slight tremors. The symptoms may be relieved by atropine or a specific alpha-2 antagonist (e.g. tolazoline).
Dosage should be determined depending on the desired depth and duration of sedation required, temperament of the horse and concurrent environmental stimuli.
LOWER DOSES MAY BE ADEQUATE.
Dosage: 0.1 mL to 0.8 mL per 100 kg bodyweight. Administration is by slow intravenous or the intramuscular route.
For analgesia in COLIC until diagnosis is confirmed, recommended dose is 20-40 μg/kg BW (0.2-0.4 mL/100 kg BW).
The full analgesic effect is established by 5-15 minutes following administration. If the desired level of sedation is not achieved following administration of a low dose, a further additive dose may be given.
The dose response can be estimated as follows:
Degree of sedation Effects begin (mins) Duration of action (hrs) Other effects μg/kg mL/ 100 kg
10-20 0.10.2 Mild: easy to handle 3-5 0.3-1.0 Slight teetering 20-40 0.20.4 Moderate: easy to handle 3-5 0.5-1.0 Slight teetering 40-80 0.40.8 Heavy: easy to handle 2-5 0.5-2.0
Teetering, sweating, piloerection, muscle spasms
Constant Rate Infusion (CRI): Constant Rate Infusion (CRI) for analgesia: Loading dose 0.01mg/kg followed by 15mg detomidine (1.5mL) in 1L of Ringers Solution administered at a constant rate infusion of 0.6μg/kg/min. This equates to approximate 6 drops/second IV. Adjust to effect.
Approval No. 87320 (Australia)
Quick acting, short duration sedation and analgesia.
Active Constituent
Xylazine (as hydrochloride) 100 mg/mL
Pack Size
50mL multidose vial
Randlab Xylazine 100 is an injection containing 100 mg/mL (10% w/v) of Xylazine for use as a sedative, analgesic and skeletal muscle relaxant in horses.
Randlab Xylazine 100 is an extremely effective sedative in the horse, allowing a number of procedures to be more easily conducted e.g. ophthalmic, dental or endoscopic examinations, wound suturing, rectal examination, stomach tubing, examination of fractious patients (incl lameness exam), diagnostic imaging, bandage application and removal etc. Xylazine in combination with appropriate local anaesthesia may be suitable for minor standing surgeries such as castration, dental procedures, draining abscesses, etc.
Xylazine may provide short acting analgesia in cases of colic facilitating examination and transport. The visceral analgesia effect of xylazine has been demonstrated to be superior to that produced by butorphanol. Xylazine decreases digital blood flow for up to 8 hours after administration.
Randlab Xylazine 100 is also a very effective premedicant for both ketamine and barbiturate anaesthesia.
Randlab Xylazine 100 produces dose dependent sedation and analgesia through an alphamimetic and direct opioid effect. It also produces muscle relaxation by inhibition of the intraneuronal transmission of impulses centrally in the CNS. Onset of action is rapid with effective sedation present within 3-5 minutes after intravenous injection and within 10-15 minutes following intramuscular or subcutaneous administration.

The sedative effect usually lasts 1-2 hours, while the analgesic effect only lasts 30-40 minutes. The usual response to Xylazine 100 administration in the horse is drooping of the head, ataxia without recumbency, penile prolapse which does not progress to paraphimosis, and pendulous lower lip. Complete recovery varies with the dose administered, however, it is usually uneventful and requires minimal supervision.
Xylazine causes cardiopulmonary depression manifested by a decrease in heart rate, stroke volume, cardiac output and respiratory rate together with an increase
Horse Hack: Inadvertent intra-arterial (e.g. carotid artery) administration of xylazine will result in extreme excitement and convulsions. The horse will normally recover within 10 minutes but is at high risk of sustaining an injury whilst convulsing.
in total peripheral resistance. These effects are transitory and of no clinical significance in healthy horses. However, it is not recommended to use xylazine where there is cardiovascular disease including conditions of shock, or respiratory depression.
A number of cardiac dysrhythmias such as second degree AV block have been encountered following xylazine administration due to increased vagal tone. These are largely transient and benign.
Horses should not be fed until they have fully recovered from sedation due to the risk of oesophageal choke.
Horses: Dose dependent effect: 2.0-5.0mL per 450kg BW by slow IV injection. Draft breeds are more sensitive to the effects of xylazine and the dose should be lowered accordingly.
Sedation: 1.1 mg/kg IV or 2.2 mg/kg IM. Allow animal to rest quietly until full effect is reached.
Colic: 0.2-0.5mg/kg IV will provide analgesia for 20-30 minutes. For longer duration, 0.6-1mg/kg IM will provide analgesia for 1-2 hours.
CRI for analgesia: 0.5-1.1 mg/kg IV followed by 0.72 mg/kg/hour. Caudal epidural: 0.03-0.35 mg/kg in first coccygeal space. 3-5 hour duration of effect.
Field induction: 1.1mg/kg IV. Wait until full sedation is achieved (5-10 min). If adequate sedation does not occur, re-dose with half the original dose of xylazine. Then follow with 2.2mg/kg IV of ketamine. Butorphanol (0.02-0.04mg/kg IV) or diazepam (0.03 - 0.1mg/kg IV) may be added before the ketamine for additional sedation, analgesia or muscle relaxation respectively.
“Triple drip” anaesthesia: Add 500mg xylazine and 2000mg ketamine to 1L of 5% guaifenesin in dextrose or 0.9% saline.
Induction: 1.1 mL/kg IV rapidly.
Maintenance: 2.0 - 4.5 mL/kg/hr to effect. “Triple drip” should not be used for anaesthetics greater than 1 hour in duration unless oxygen supplementation and respiratory support is provided.
Foals & Ponies: Add 250mg xylazine and 500mg ketamine to 500mL 5% guaifenesin solution. For induction give 1.1 mL/kg IV rapidly. For maintenance 2-3mL/kg/hr to effect.
Reversal: Tolazoline, atipamezole or yohimbine may be used alone or in combination to reverse the effects of xylazine or speed recovery.
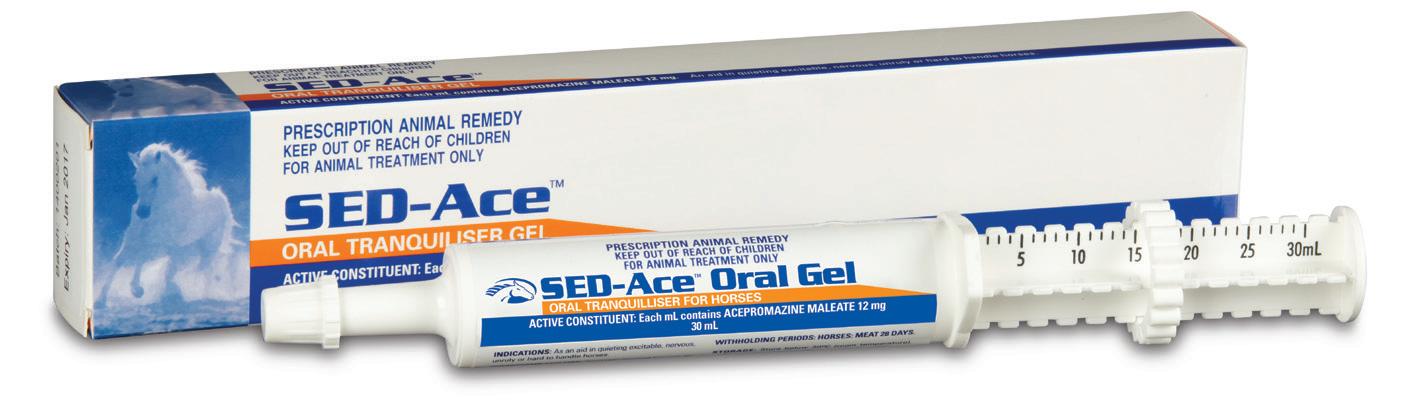
Approval No. 80410 (Australia) | ACVM No. A011152 (New Zealand)
• An aid in training fractious horses.
• To calm nervous horses and reduce stress.
• In minor surgical procedures in which a general anaesthetic is not required.
• To sedate horses that will not allow IV or IM sedation.
• In non surgical procedures such as shoeing, clipping and dentistry.
• Handling mares during mating or other gynaecological procedures.
• Calming of horses to assist in lameness examinations and ensure consistency of gait.
• Horses that have wounds which cannot heal due to constant irritation (biting, licking etc.)
• After major surgery to reduce straining.
• Useful aid in the treatment of colic and tetanus.
• To calm horses during transport.
• To calm horses during turn out.
• As a hypotensive agent and to improve peripheral blood flow.
SED-Ace Oral Gel (acepromazine maleate) provides dose-dependent tranquillisation, relaxation and sedative effects. It provides a safe, convenient and effective method of tranquillising and sedating horses.
Low doses of oral acepromazine maleate (ACP) promote a mild tranquillising effect, useful as an aid in quietening excitable, nervous and hard-to-handle horses. It can provide a beneficial “calming” effect during handling, training, transport and confinement. It reduces excitability, whilst retaining co-ordination and awareness of environment.
Acepromazine maleate also mediates hypotensive, hypothermic and antispasmodic actions. It has little, if any, analgesic effect, so that painful procedures must be avoided unless accompanied by an analgesic, particularly where animals are known to have unpredictable temperaments.
On an empty stomach, the tranquillisation effect will be apparent in horses in 20 - 40 minutes, and with a duration of up to 3 hours. On a full stomach, the full tranquillisation effect will be apparent within 40 - 60 minutes or longer.
The side effects of oral ACP are less than with intravenous administration.
During sedation, horses will normally retain visual and auditory function, so that loud sounds or rapid movements should be avoided. Exceeding the recommended dosage will not necessarily intensify the sedative effects of Sed-Ace Oral Gel.
Paralysis of the retractor penis muscle has been associated with the use of intravenously administered acepromazine maleate in horses. When this occurs steps should be taken immediately to reduce any penile swelling and return the penis to the prepuce.
Due to the risk of potential irreversible penile prolapse, avoid using in male horses (stallions and geldings) or use the lowest dose possible to produce the required effect.
The required dosage of SED-Ace Oral Tranquiliser Gel may vary depending on the individual temperament of the animal, the level of sedation required and concurrent environmental stimulus.
In some horses, lower doses of SED-Ace may be adequate.
Dose will also vary depending on desired level of sedation.
Horses: To be given orally. 2-10 mL per 450 kg bodyweight (0.05 to 0.26 mg per kg body weight).
Horse Hack: For best results, avoid excitement or stimulation of the animal prior to administration and preferably administer on an empty stomach.

At the time of her passing, Queen Elizabeth II owned approximately 140 horses including racing and breeding thoroughbreds, Highland, Shetland and Fell ponies, etc. This was in addition to the horses owned by the Royal Mews.
As The Queen became frailer, she chose to ride British native ponies who were known to be more docile and closer to the ground. Important when your only head protection is a scarf.
The Queen had owned Carltonlima Emma for 18 years and she had become her regular mount around the grounds of Windsor Castle. The Queen rode Emma for the last time in July 2022, just two months prior to her passing.

As The Queen’s funeral cortege arrived at Windsor Castle, Emma and The Queen’s groom, Terry Pendry, stood alone and silently between the beds of flowers. The mare remained motionless until The Queen’s casket drew level and Pendry bowed his head. At which time, Emma became unsettled and appeared to salute her former Master with her right foreleg.
The moment remains one of the most poignant at the Monarch’s funeral.
DID YOU KNOW THAT... Queen Elizabeth’s favourite pony saluted her casket as it passed her by?
UK)


APVMA Approval No. 90730 (Australia)
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Randlab’s Gentamicin Injection. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
Gentamicin is active against a wide range of bacteria. It is the drug of choice for treatment of gram-negative infections (incl. Pseudomonas spp, Proteus, Klebsiella. It is also effective against Staphylococci.
Gentamicin has no activity against anaerobes, streptococci and enterococci and limited activity against Salmonella spp.
Gentamicin is most frequently used in combination with a beta-lactam antibiotic (eg penicillin, cephalosporin, ampicillin) to achieve a broader spectrum of activity.
Gentamicin is commonly used by intra-articular injection. It may also be used for regional limb perfusion (either IV or intraosseous) or impregnated in PMMA beads.
Gentamicin may be administered by nebuliser for treatment of respiratory tract infections including pneumonia.
Gentamicin is commonly used as part of an intrauterine flush or for the intrauterine treatment of uterine infections.
Gentamicin sulphate is an aminoglycoside antibiotic that has good activity against a variety of gram negative aerobic bacilli (incl E. coli, Klebsiella spp, Proteus spp, Pseudomonas aeruginosa, some Salmonella spp, Enterobacter, Serratia, Shigella), many Staphylococci strains, Mycoplasma and spirochetes.
It is the drug of choice for treatment of E. coli, Klebsiella spp, Pseudomonas spp (incl Pseudomonas aeruginosa), Actinobacillus equuli and Pasteurella spp.
Gentamicin must penetrate the cell wall and enter the bacterial cell to assert its effect. The concomitant administration of antibiotics that interfere with cell wall synthesis (eg penicillin) will enhance the efficacy of gentamicin.
Gentamicin is primarily distributed in the extracellular fluid and is found in peritoneal, pleural, pericardial, ascitic and synovial fluids, sputum and bronchial secretions. Therapeutic levels are also found in bone, heart and lung.

Gentamicin does not readily cross the blood-brain barrier or penetrate ocular tissue. CSF levels are variable and may be from 0-50% of those found in the serum.
Gentamicin tends to accumulate in the inner ear and kidneys, which makes these organs particularly sensitive to gentamicin toxicity.
Gentamicin crosses the placenta but no appreciable levels are present in neonatal foals of mares treated with gentamicin. Small amounts of gentamicin may be excreted into the milk with minimal risk to the nursing foal.
Gentamicin is inactivated by purulent material and other low oxygen environments. Gentamicin is eliminated almost entirely by glomerular filtration with a half life in horses of 1.82-3.25 hours.
Intravenous is preferred over intramuscular administration as horses may develop some localised muscle soreness following repeated IM injection.
Known potential adverse effects of gentamicin include nephrotoxicity and ototoxicity. The latter is of unknown significance in the horse.
Do not administer to animals with compromised renal function.
The risk of nephrotoxicity is increased if the horse has pre-existing renal disease, in neonates and geriatrics or if horse has fever, sepsis or dehydration.
To avoid potential renal toxicity, ensure that treated horses are well hydrated and have adequate access to water and electrolytes during the treatment period. High levels of calcium and protein in the diet may decrease the risk of nephrotoxicity by increasing glomerular filtration rate.
Horses with decreased renal function may have a significantly prolonged gentamicin half-life and dosing frequency should be reduced.
Although many Salmonella spp may exhibit in vitro sensitivity, they are rarely sensitive in vivo.
Adult; 6.6-9.7mg/kg sid by IV or IM injection.
Foal (<14 weeks); 8.8-12mg/kg q24-36h
Intrauterine; 1-2g buffered with equal volume of 7.5% sodium bicarbonate and diluted in 200mL isotonic saline daily for 3-7 treatments.
Intra-articular; 100-500mg per joint depending on joint size. Diluted 1:2 with sterile saline.
Regional Limb Perfusion: Add 10mL gentamicin to 50mL saline or prilocaine.
Nebuliser: 100-200mg diluted 1:1 with isotonic saline sid-qid.
Horse Hack: The label dose for Gentamicin Injection is 2.0mg/kg BW by IV or IM injection tid. Like many antibiotics, the label doses are no longer considered valid.
Gentamicin is a concentration-dependent antibiotic and as such higher doses at less frequent intervals is recommended. This increases bacterial killing, increases bacterial susceptibility and decreases potential toxic effects and the risk of developing resistant bacterial strains..
(Australia)
Available:

Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Randlab’s Tri-Sulfox. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
Tri-Sulfox Injection has broad-spectrum activity against a wide range of both grampositive and gram-negative bacteria incl:
• Actinobacillus equuli
• Actinomyces spp
• Bacillus anthracis
• Bordetella spp
• Brucella spp
• Campylobacter spp
• Clostridium spp
• Corynebacterium spp
• Enterobacter spp
• Escherichia coli
• Fusiformis spp
• Haemophilus spp
• Klebsiella spp
• Pasteurella spp
• Pneumococcus spp
• Proteus spp
• Salmonella spp
• Shigella spp
• Staphylococcus spp (incl some S. aureus)
• Streptococcus spp
Pseudomonas aeruginosa, Enterococcus faecalis and Enterococcus faecium are intrinsically resistant to Tri-Sulfox. Mycobacterium tuberculosis, Mycoplasma spp, Leptospira are also resistant. In vivo activity against anaerobes is generally poor.
The combination of sufladoxine and trimethoprim act synergistically and Tri-Sulfox Injection is bactericidal against most gram-positive and gram-negative bacteria incl E. coli, Haemophilus, Pasteurella, Salmonella, Staphylococcus and Streptococcus spp. Bacterial resistance to TMPS combinations is still, surprisingly, relatively low.
Both compounds affect bacterial purine synthesis at different stages of the bacterial metabolic pathway. This results in a double blockade of bacterial metabolism and a broader spectrum of activity than either component acting alone.
Tri-Sulfox Injection is distributed widely throughout the body and may be used in the treatment of bacterial infections of the respiratory tract (incl pneumonia, bronchitis and secondary bacterial infections following viral pneumonia), urogenital tract infections (incl placentitis, cystitis, vaginitis, urethritis, nephritis and metritis), gastrointestinal tract infections (incl E. coli and salmonellosis). Tri-Sulfox may be used for treatment and prophylaxis peri-operatively and for most peri-parturient problems requiring broad-spectrum antibiotic coverage as well as for wound infections and septicaemias.

Tri-Sulfox is excreted in the urine and therefore useful for treatment of urinary tract infections.
Tri-Sulfox is inactivated in the presence of purulent material and abscesses, etc should be surgically drained and lavaged prior to using Tri-Sulfox.
Tri-Sulfox Injection is irritant when administered subcutaneously or by the intrauterine or intramuscular routes and these routes should be avoided.
Inadvertent perivascular injection is also irritant and likely to result in significant thrombophlebitis.
Concurrent administration of TMPS and detomidine may result in dysrhythmias (esp under general anaesthesia), hypotension and death.
Concurrent use of penicillin is antagonistic to sulphonamides.
TMPS is a time-dependent antibiotic, therefore twice daily dosing is required. 30mg/kg bid by slow IV Injection.
The concentration of TMPS in the bottle of Tri- Sulfox Injection is 200+40=240mg/mL.

Therefore, a 500Kg horse will require (30x 500)/240 =36mL bid.
Horse Hack: Solution is irritant if given perivascularly. The use of catheters/cannulas will reduce the risk of inadvertent perivascular administration and is strongly recommended.
The rarest horse breed in the world is the Sorraia? SORRAIA

Available in:
Combination of sulphadimidine and trimethoprim working together to give greater bactericidal activity.
Active Constituent
Sulphadimidine 430mg/g Trimethoprim 86mg/g Pack Size 500g jar
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Tri-Sil Powder. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
For the treatment of respiratory, urogenital, gastrointestinal, synovial and other infections in horses due to sulphadimidine and trimethoprim susceptible organisms.
Suitable for use in horses, calves, pigs and poultry.
Randlab supports responsible Antimicrobial Stewardship.
Pharmacokinetic trials in horses have established that sulphadimidine and trimethoprim are readily and rapidly absorbed after oral administration and reach therapeutic plasma levels within approximately 30 minutes of administration. Trial results demonstrate that the formulation is efficiently absorbed even when administered with food.
TMPS is widely distributed to all body tissues and fluids. Concentrations are above plasma levels in kidney; similar to plasma levels in pleural, peritoneal, synovial and ocular fluids; slightly lower in CSF, muscle and milk.
The parent compound and its metabolites are excreted predominantly in the urine by glomerular filtration. Plasma half-life in the horse has been variously estimated at between 6.4 and 9.8 hours.
The combination of sulphadimidine and trimephoprim in the ratio of 5:1 has been shown to be effective in treating many of the major bacterial pathogens considered of clinical significance in the horse. These include: Staphylococcus spp (incl S. aureus and S. intermedius), Streptococcus equi (incl subspecies S.equi equi and zooepidemicus), Actinobacillus spp, most Escherichia coli, Pasteurella spp, Proteus spp, Fusobacterium spp, Enterobacter spp, Corynebacterium spp (excluding Corynebacterium (Rhodococcus) equi), Salmonella spp, Shigella spp, Klebsiella spp and Haemophilus spp. Some Brucella and Nocardia spp are also susceptible. Most Pseudomonas spp are not sensitive.
The minimum inhibitory concentrations (MICs) of the combination for susceptible bacteria are substantially lower than those of either of the individual agents. The combination of sulfonamide and trimethoprim is bactericidal while either drug alone is bacteriostatic. Folate synthesising bacteria which are resistant or moderately resistant to either drug alone are frequently susceptible to the combination.
Bacterial resistance to the TMPS combination is surprisingly rare given the extensive use of the combination in veterinary and human medicine over the past 50 years.
Sulphadimidine and trimethoprim have similar antibacterial spectra, trimethoprim being approximately 20 times more potent than sulphadimidine. The combination blocks two sequential obligate enzymatic reactions in the microbial folate synthesis pathway. Mammalian cells require preformed folic acid and thus are unaffected by sulfonamides.
A synergistic action is demonstrated by the enhanced antimicrobial activity (potentiation) of the combination compared with the antimicrobial activity of either agent alone. Antimicrobial activity of the combination of sulphadimidine and trimethoprim was found to be up to 1024 times that of sulphadimidine alone, and up to 128 times that of trimethoprim alone. Potentiated sulfonamides present a lower risk of toxicosis in long-term therapy than do sulfonamides alone, as a lower dose of sulfonamide is required to achieve the same clinical effect.
For oral use only. Give on dampened food mixed with honey or molasses or reconstitute as a paste and administer over the back of the horse’s tongue.
The antibacterial actions of sulfonamides are reduced in the presence of blood, pus and tissue breakdown products, which contain purines and thymidine, as the bacterial requirement for folic acid is decreased in such media.
Trimethoprim/sulfonamide (TMPS) are contraindicated in horses suffering from severe hepatic and renal insufficiency, urolithiasis or sulfonamide sensitivity.
Use the enclosed scoop.
One level scoop provides 5g sulphadimidine plus 1g trimethoprim.
HORSE: 30mg/kg twice daily combined sulphadimidine + trimethoprim dose.
For example, 500kg horse: 30mg x 500kg = 15g
Each scoop contains 6g of combined active. 15g =2.5 scoops
The 500g jar contains enough TMPS for an 8-day course for a 500kg horse.
Before filling scoop, rotate container horizontally to ensure consistent filling weight.

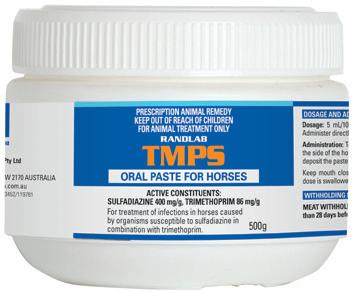
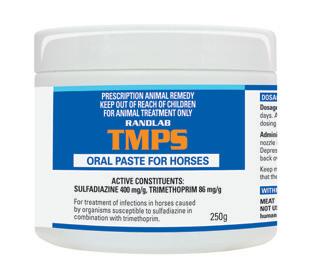
APVMA Approval No. 83452 (Australia)
Easy to use paste ensures accurate and reliable dosing. Three convenient pack sizes.
Active Constituent
Sulfadiazine 400 mg/g
Trimethoprim 86 mg/g
Pack Size 250g jar 500g jar 1kg tub
Randlab supports responsible Antimicrobial Stewardship.
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with TMPS Oral Paste. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
Randlab’s TMPS Oral Paste has a broad spectrum of activity against the common Gram-positive and Gram-negative bacteria including:
• Streptococci
• Dermatophilus congolensis
• Escherichia coli • Bacillus
• Bordetella
• Corynebacterium
• Hemophilus
• Listeria monocytogenes
• Pasteurella
• Shigella
• Proteus spp.
• Brucella
• Fusiformis
• Klebsiella spp
• Nocardia
• Salmonella
• Staphylococci
The combination also has some effect against infections caused by protozoa (e.g. coccidia and Toxoplasma infection)
InfectionscausedbyLeptospira,Psuedomonas,MycobacteriumandErysipelothrix aregenerallynotsusceptible.
Randlab’s TMPS Oral Paste is useful in the treatment of respiratory infections, with high lung tissue levels achieved (although Randlab’s Airway Tmps and Bromo Tmps are designed specifically for respiratory infections), soft tissue infections, abdominal infections, joint infections, infections of the CNS and infections of the uterus/placenta and urinary tract.
The combination of sulfadiazine + trimethoprim (TMPS) act synergistically to kill a wide range of susceptible bacteria and reduces the possibility of bacterial resistance developing.
The combination blocks the synthesis of bacterial folates at two critical points,
WARNING: Oral administration of trimethoprim+ sulfonamides may rarely be associated with the development of severe, acute febrile diarrhoea in the horse. This may even occur several weeks after finishing the course.
which are essential for bacterial cell multiplication and survival. The combination of sulfadiazine + trimethoprim is bactericidal, while either drug alone is bacteriostatic. Folate synthesising bacteria which are resistant or moderately resistant to either drug alone are frequently susceptible to the combination.
TMPS is readily absorbed following oral administration with peak levels occurring about 1-4 hours after dosing. TMPS is widely distributed to all body tissues and fluids with levels similar to plasma found in pleural, peritoneal, synovial and ocular fluids with lower levels in CSF and muscle.
Both drugs cross the placenta and are present in the milk.
Sulfadiazine is excreted largely unchanged in the urine with higher levels than plasma present in the kidney and urine.
The antibacterial actions of sulfonamides are reduced in the presence of blood, pus and tissue breakdown products as the bacterial requirements for folic acid is decreased in such media.
This product is not recommended for use in treatment of infections or abcessess caused by anaerobic bacteria.
TMPS should not be used in horses with marked liver parenchymal damage, blood dyscrasias or with a history of sulfonamide sensitivity.
Rarely TMPS may cause idiosyncratic neurological reactions consisting of behaviour changes, gait abnormalities and hyperaesthesia. These effects are generally reversed soon after discontinuing the medication.
1mL of TMPS Paste weighs approx 1.2g. Therefore a 250g pot contains approx 208mL of paste and so forth.
Administer directly into the mouth from a dosing syringe.
Adult: 24-30mg/kg twice daily combined sulfonamide+ trimethoprim dose. [e.g. 500kg horse = 30mg x 500kg/(400+86 mg/mL) =31mL].
Foals: 15-30mg/kg twice daily. Dose rate equivalent to 5-6mL/100 kg twice daily (12 hourly).
Available:
APVMA Approval No. 83452 (Australia)
Whenever possible, bacterial culture and sensitivity testing should be carried out prior to initiating treatment with Randlab’s Oxytetracycline Injection. Antibiotic sensitivity testing should also be undertaken in cases of non-responsive or chronic infections.
Oxytetracycline is a broad-spectrum antibiotic with activity against a wide range of both Gram-negative and Gram-positive bacteria.
At lower dose rates it is bacteriostatic but is bactericidal at higher dose rates. It has good anaerobic coverage but a variable effect against Bacteroides and Clostridium spp.
Oxytetracycline is the drug of choice for Lawsonia intracellularis (Equine Proliferative Enteropathy) and Actinobacillus equuli (a common cause of peritonitis in adult horses) infection.
Oxytetracycline distributes well throughout the body with therapeutic levels achieved in most body fluids.
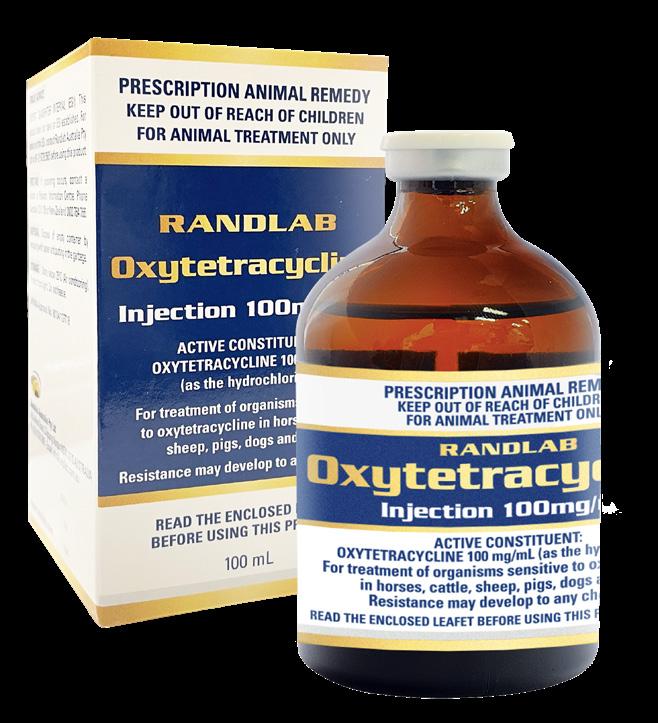
High dose oxytetracycline causes tendon relaxation in foals with congenital (but not acquired) contracted tendons and is most efficacious when given in the first 3 days of life (20 mg/kg IV).
Oxytetracycline has mild anti-inflammatory properties. Oxytetracycline is concentrated in bone and teeth.
Oxytetracycline has antibacterial activity against a wide range of bacteria. Wherever possible, antibiotic selection should be based on culture and sensitivity testing. Expected sensitivity to oxytetracycline is outlined in the table to the right. Following intravenous administration, oxytetracycline is distributed widely throughout the body including bone, heart, kidneys, lungs, muscle, pleural fluid, bronchial secretions, sputum, bile, saliva, urine, synovial fluid, ascitic fluid and aqueous and vitreous humour. It crosses the placenta and is concentrated in the milk. Oxytetracycline only penetrates the CNS if the meninges are inflamed. Tetracylines have been shown to have anti-inflammatory properties in humans where they may be used as adjunct therapy for arthritis and autoimmune diseases. In horses oxytetracyline appears to be a potent inhibitor of matrix metalloproteinase-9 and a modest inhibitor of matrix melalopreteinase-2. Some of the response observed in treating chronic Lyme Disease in horses has been attributed to the anti-inflammatory effects rather than its antimicrobial activity. The drug is excreted primarily unchanged in urine with some also excreted through the bile and other non-biliary gastrointestinal pathways.
Horse Hack: Oxytetracycline has been administered IV at high doses (20-70mg/kg) every 48 hr to newborn foals for the treatment of contracted flexor tendons. The method of action is believed to involve calcium chelation. Treatment is more effective when initiated in the first three days of life.
Antibiotic of choice Some activity expected (consult C&S)
Lawsonia intercellularis (Equine Proliferative Enteropathy) Streptococcus Enterococci
Actinobacillus equuli Staphylococcus (inc S. aureus) E. coli
Pasteurella spp
Gram negatives incl Bacteroides spp, Brucella, Bartonella, Haemophilus spp, Pasturella multocida, Shigella & Yersina pestis Klebsiella
Neorickettsia risticii (Potomac Horse Fever) Chlamydia
Psuedomonas aeruginosa Ehrlichia spp Mycoplasma Bacteroides spp Proteus
Spirochetes (inc Lyme Disease)
Rickettsia spp Clostridium spp
Protozoa spp (inc Babesia equi)
Bacillus anthracis
Clostridium perfringens
Clostridium tetani
Listeria monocytogenes Nocardia spp Actinomyces spp
Intramuscular administration is not recommended in the horse because effective serum concentrations are unlikely to be maintained and intramuscular injection is associated with severe tissue irritation.
Hypotension and collapse may be seen following rapid IV administration of oxytetracycline. Renal tubular necrosis may occur with high doses (eg neonatal foals with contracted tendons). Care should be taken in dehydrated foals due to potential renal effects.
Bone and deciduous tooth discolouration is common in developing animals with oxytetracycline and should only be used during the last half of pregnancy.
When long term administration of a tetracycline is required (eg Lawsonia intracellularis EPE infection), it is common to follow a 5–10-day course of IV oxytetracycline by a course of oral doxycycline (10-20mg/kg q12-24hr).
Adult: 5.0-6.6mg/kg q12hr or 10-20mg/kg q24hr. In cases of severe infection, the initial high blood serum concentrations after a long-acting dose may result in a better response to the treatment.
Foals: 5-10mg/kg IV q12hr diluted in 1L Hartmann’s and then given slowly or 1020mg/kg IV q24H diluted and then given slowly.

This product is not registered, but is supplied under APVMA Permit No. 84510. Read the permit before using.
The only equine metronidazole product on the market. Randlab supports responsible Antimicrobial Stewardship.
Don't forget to return your completed Metronidazole APVMA permit form to Randlab. Spare copies of the form are available at randlab.com on the Metronidazole page.
For the treatment of infections in horses caused by anaerobic bacteria such as Clostridium spp, Bacteroides spp (incl penicillin resistant B. fragilis), Fusobacterium and protozoa such as Giardia and Trichomonas spp.
For the treatment of infections where anaerobic bacteria are implicated or suspected such as pleuropneumonia, sinusitis, tooth root infections, enteritis, colitis and some cases of deep foot infection and metritis.
Metronidazole is the antimicrobial of choice for the treatment of Acute Febrile Diarrhoea or chronic diarrhoea due to anaerobic infections such as Clostridial infections.
Metronidazole in combination with either oxytetracycline or chloramphenicol has been suggested for the treatment of proliferative enteropathy caused by Lawsonia intracellularis.
Metronidazole reaches therapeutic concentrations in abscesses, bone, peritoneal fluid, milk and the CNS. It crosses the placental barrier.
Oral absorption in horses is 80-100%. Rectal absorption is 30-50%. The rectal dose should therefore be adjusted accordingly.


The half-life of metronidazole is 2-4 hours in adult horses and 9-12 hours in foals.
Therapeutic concentrations of metronidazole are attained after administration per rectum. This may be the route of choice in horses with impaired gastrointestinal status/ileus or to reduce the risk of anorexia.
Do not use in animals with renal or hepatic disorders or dysfunction.
Over-dosage (high doses or prolonged treatment beyond the specified five days) may result in CNS symptoms such as depression, ataxia, tremors, seizures and weakness. These are generally reversible after ceasing the metronidazole.
WARNING: Anorexia is common following oral Metronidazole use. It is less common with rectal administration but is still likely to occur.
Other side effects reported include peripheral neuropathy and impaired hepatic function. Signs are generally reversible after a few days once the treatment has been stopped. Rarely this may not be reversible.
Insufficient data is available on use in pregnant mares to recommend the use of the product during pregnancy.

Administer orally. Give 20mL Equine Metronidazole Paste (equivalent to 10g metronidazole) per 500kg bodyweight every 12 hours for 5 days or as directed by a veterinarian.
Adult: 15mg/kg qid or 20mg/kg bid orally Foal: 10-20mg/kg bid orally
Horse Hack: Metronidazole Paste may also be administered per rectum at approximately double the oral dose rate.
DID YOU KNOW THAT... New Zealand’s most successful sire almost perished in a fire en route to NZ?
APVMA Approval No. 62637 (Australia)
Available in:
Broad spectrum topical anti-microbial ointment.
Active Constituent
Povidone-Iodine 100 mg/g
(equivalent to available Iodine 10 mg/g)
Pack Size 250g jar
Vetedine Ointment is a povidone iodine (known as an iodophor) with potent prolonged antibacterial, antifungal and virucidal activity on contact, suitable for topical disinfection and antisepsis in horses.
Use Vetedine Ointment as an aid in the treatment or prevention of local infections in wounds, abscesses, burns and fungal infections such as ringworm, disinfection of the navel of newborns, dehorning, castration, disinfection prior to surgery, girth galls, abrasions and puncture wounds.
• Has rapid action, even in presence of blood, pus, oils, soap, etc.
• Effective anti- bacterial, fungal and viral activity
• No resistance by any micro-organisms
• Does not stain. Washes off with water
• Does not delay wound healing or granulation
• Does not sting or irritate wounds
• Does not sensitise tissues
• Can be bandaged without danger of burns or irritation
• Has prolonged germicidal action
• Has no odour
• Has prolonged activity, even in the presence of pus or blood
• Forms an adherent film over wounds
• The characteristic colour delineates the treated area
Vetedine Ointment acts by releasing iodine slowly. Povidone iodine is as effective as iodine against a broad spectrum of disease causing micro-organisms, but it is less irritating to skin, and any stains wash out in water. There has been no reported resistance to povidone iodine in human or animals. It is non-sensitising and does not cause pain on application to wounds or mucus membranes. It forms a film to protect open wounds, and is soluble in water.

Povidone iodine is particularly effective in treating mixed skin infections, wound cleansing, treatment of burns and ulcers, and in skin antisepsis. It does not delay healing or granulation, and does not lose antimicrobial activity in the presence of organic matter such as blood, pus, oils, soaps, etc.

Apply to the affected area twice daily or by the veterinary surgeon. Care should be exercised using iodine on extensive wounds or deep burns especially over extended periods. Does not permanently stain skin or natural fibres. Wash with soap and water to remove.

The chestnut Quarter Horse colt foaled in 1956 by the renowned Lightning Bar carried a heavy influence of thoroughbred blood in his pedigree and was bred to run. Fast.
The colt was named Doc Bar paying homage to his paternal line heritage, but in four race starts the horse only placed once earning a meagre $95.
His disillusioned owners gave the colt to a friend to show in halter classes, where, despite his small stature (14.3hh) and “little fox ears”, he proved to be somewhat of a success, winning nine of his fifteen shows.
In 1963, the stallion was purchased for US$30K to stand at stud. As with many pre-potent stallions, Doc Bar hit the ground running and was an immediate and surprising success. His influence is said to have “revolutionised the cutting industry in a way never seen before or since”.
Doc Bar’s blood flows through nearly every horse in the cutting horse industry, with horses often carrying multiple crosses to the great stallion through his many successful sons and daughters.
His impact has also been felt in the world of cow horses and reining horses, but it is in cutting where he reigned supreme.

Doc Bar died in 1992 at 36yo. He was inducted into the American Quarter Horse Hall of Fame the following year.
Doc Bar. A cut well above the ordinary.
DID YOU KNOW THAT...
A Quarter Horse that failed dismally at his chosen career as a racehorse went on to become a breed-changing cutting horse stallion.
DOC BAR (TOM FINLEY, USA)





APVMA Approval No. 89636 (Australia)
The induction and timing of ovulation in mares supporting a follicle greater than 30mm in diameter and signs of oestrous behaviour and/or uterine and cervical oestral changes on ultrasound.
Upwards of 94% of suitable mares would be expected to ovulate within 48 hours of Biorelin administration, with an additional percentage (< 6%) up to 54 hours. Few mares will ovulate in the first 24 hours and most will ovulate around 40 hours post-injection.

Biorelin Injection (slow release deslorelin acetate) is a synthetic Gonadatrophin Releasing Hormone (GnRH) for induction and timing of ovulation in oestrual mares.
The long duration of oestrus in mares, and the varying time from the onset of oestrus to ovulation, typically results in the need for multiple breedings or inseminations in order to achieve conception. Biorelin Injection induces ovulation within 48 hours after treatment in oestrous mares with a developing follicle greater than 30mm in diameter by increasing the levels of endogenous luteinising hormone (LH).
Biorelin Injection is a sustained release, non-aqueous formulation and is indicated for the induction of ovulation in oestrous mares. It is intended to optimise breeding management by shortening and synchronising the osetrous period, thereby allowing for stallions and assisted breeding programs to be more efficiently managed.
Following the use of Biorelin, the length of the inter-ovulatory interval between cycles is not altered.
The pregnancy rate of mares treated with Biorelin is similar to that of control mares.
Effectiveness is contingent upon accurate diagnosis of oestrus and detection of a developing follicle between 30 and 40mm in diameter.
Ideally, mares should be regularly (every second day) checked prior to Biorelin administration and should demonstrate an increase in follicular size of 3-5mm per day.
The follicle should also be producing oestrogen and the mare displaying oestrous behaviour and/or uterine (oedema 3/3) and cervical (softening) changes on ultrasound indicative of oestrus.
Injection site reactions have been observed in ~15% of treated mares. The reactions are mild to moderate swellings at the injection site which may be painful on palpation. These reactions typically resolve within 36 hours.
Pregnant women and others of childbearing age should exercise caution when handling this product. Accidental administration may lead to a disruption of the menstrual cycle. Direct contact with the skin should therefore be avoided and any
contact areas should be washed immediately with soap and water if exposure does occur. Routine precautions should be employed by practitioners when handling and using this product to prevent accidental injection.
Shake vial vigorously before administration. Inadequate re-suspension may affect efficacy.
For administration by intramuscular injection only. Discard unused portion within 28 days of broaching.
1.0 ml by intramuscular (IM) injection (1.25 mg deslorelin per dose).
Only 1 mL should be administered per mare during any one oestrus cycle.
Horse Hack: Mares should be bred ~ 24 hours post Biorelin injection for chilled or fresh semen.
Mares should be bred ~ 40 hours post Biorelin injection for frozen semen.
During the transitional oestrus period, a higher dose of Biorelin (1.9mg = 1.5mL) may improve ovulation rate.

Charles Darwin coined the phrase "natural selection" whilst reading a book on horse breeding?
(CHARLES DARWIN, UK)
“This preservation of favourable variations and the destruction of injurious variations, I call Natural Selection, or the Survival of the Fittest. Variations neither useful nor injurious would not be affected by natural selection and would be left a fluctuating element.”Charles Darwin, Origin Of Species, 1869.
Altrenogest acts similarly to the natural hormone, progesterone, by suppressing the normal oestrous cycle and preventing signs of oestrus and ovulation. Mares return to heat and release natural hormones again once treatment stops.
Treatment with altrenogest makes it possible to regulate the breeding of mares. Ovu-Mate injection may be used in fillies and mares to:




• Delay the onset of oestrus
• Suppress oestrous behaviour
• Synchronisation of oestrus for the efficient use of stallions or assisted- breeding techniques.
• As an adjunct to the treatment of placentitis.
Following intramuscular administration of Ovu-Mate Injection high blood levels are rapidly reached. Levels achieved initially exceed the levels achieved by daily oral dosing sevenfold and decline steadily over the next 5-7 days.
Pharmacokinetic data from mares treated with Ovu-Mate Injection have shown blood levels of altrenogest are maintained for 5 days above the oral therapeutic level following administration of the injection. Repeated weekly injections may result in progressive accumulation of plasma levels of altrenogest.
Studies have been conducted to support suppression of behavioural signs of oestrus in cycling mares for 7 days following administration of altrenogest injection.
Use rubber gloves when handling this product.
Women who are pregnant or suspected of being pregnant should not handle the product. Women of childbearing age should handle the product with extreme care. Not to be handled by persons with known or suspected progesterone dependent tumours or thromboembolic disorders.
Avoid skin contact. Do not inhale or swallow. Rubber gloves should be worn when handling this product.
WARNING: If used in performance animals, the regulations of the relevant authorities regarding medication should be observed. In some jurisdictions and under some circumstances this product may be regarded as a prohibited substance due to the presence of low levels of the male anabolic steroids trenbolone and trendione in all formulations of altrenogest.
Contact Randlab's Veterinary & Technical Director, Dr Michael Robinson on mrobinson@randlab.com.au or +61 451481050 for further information.
Suppression of oestrus: 3mL per 500kg bodyweight (0.3mg/kg) by intramuscular injection once every 5-7 days or as required to suppress signs of oestrus.
Horse Hack: As with any long-acting depot injections, some minor reactions may occasionally occur at the injection site, especially in mares on weekly dosing.
Alternating injection sites from week to week will assist in minimising these reactions. The gluteal muscles may also be used as an alternative to the neck.
A system such as injections on the left on even calendar days and on the right on odd calendar days will help to average out the number of injections at each site. This is a particularly useful system if a majority of injections are going to be administered by stud staff or different personnel.
you realise there’s some pretty good sorts up there. And if you like them you wink… and if she likes you she winks back”.
Maybe that is why the wonder mare occasionally liked to kick up her heels.
DID YOU KNOW THAT... All-time great, Winx was named after a Vegas burlesque dancer?
WINX (RICHARD TREWEEKE, AUS)
APVMA Approval No. 65653 (Australia) | ACVM No. A10159 (New Zealand)
For regulation and control of the breeding cycle of mares and the maintenance of pregnancy in habitually aborting mares or mares at risk of early abortion.
• To induce ovulatory oestrus early in the breeding season in mares where some follicular activity exists.
• For the suppression of oestrus either during prolonged oestrus or in normally cycling mares.
• For the control of the ovarian cycle in breeding mares to allow the most efficient use of the stallion or assisted-breeding techniques.
• For suppression of oestrous behaviour in fillies and mares engaged in competition.
• For the maintenance of pregnancy in habitually aborting mares or mares at risk of early embryonic death or abortion.
• For behavioural modification in non-competition stallions and geldings.
For regulation and control of the breeding cycle of mares
• For the induction of ovulatory oestrus or to induce cyclical ovarian activity in mares with some follicle activity early in the breeding season: administer OvuMate daily for 10 consecutive days.
• For the suppression of oestrus in normally cycling mares: administer Ovu- Mate daily for 15 consecutive days.




• For the suppression of prolonged oestrus: administer Ovu-Mate daily for 10 consecutive days.
• To control the breeding cycle of mares to allow programmed use of the stallion or efficient use of assisted-breeding programs: administer Ovu-Mate daily for 15 consecutive days.
For the treatment of habitually aborting mares, or mares at risk of early embryonic death or abortion
• Administer Ovu-Mate daily, from the 2nd or 3rd day after ovulation in the mated or inseminated mare, continuing until day 120 of gestation.
• Ovu-Mate may be used as part of the treatment regimen in mares with placentitis.
Ultrasound scanning to confirm pregnancy is recommended on day 14 or 15 after ovulation. If the mare is non-pregnant, it is advised to cease Ovu-Mate administration and to administer an injection of prostaglandin F2 to destroy any luteal tissue that may still be present, allowing the mare to return to oestrus and be re-mated.
This product is contraindicated for use in mares suffering from uterine infections. If administered in feed, Ovu-Mate should be added to the feed immediately prior to consumption and not stored.

Unused feed must be destroyed and not given to any other animals.
Care should be taken to avoid contact between the solution and women of child bearing age. When using the product wear rubber gloves. Product is harmful if absorbed by skin contact, inhaled or swallowed. Avoid contact with eyes and skin. Do not inhale. After use and before eating, drinking or smoking, wash hands, arms and face thoroughly with soap and water. After each day’s use wash gloves.
Dosage for oral administration. Administer 1 mL Ovu-Mate per 50 kg bodyweight (equivalent to 0.044 mg Altrenogest per kg) daily (12.5


(Abamectin

APVMA
Available in:
Moxidectin 20 mg/mL
Praziquantel 125 mg/mL Pack Size 11.8g syringe
Whenever possible the decision to deworm should be based on the results of a Faecal Egg Count (FEC). Consult a veterinarian when developing a worm control program to suit your specific needs.
Pradectin with Tape Gel Wormer is effective for the treatment and control of small strongyles (adults and larvae including encysted stages), large strongyles, tapeworm, pinworms, ascarids, Habronema spp, stomach bots, hairworm, intestinal threadworm and cutaneous onchocerciasis.
Pradectin with Tape Gel Wormer has a prolonged Egg Reappearance Period (ERP) of at least 14 weeks, which means that recontamination of the pasture by strongyle eggs is significantly reduced during this period.
Moxidectin is also an effective arachnicide against feeding ticks.
Pradectin with Tape Gel Wormer Long Acting Horse Wormer and Boticide Gel contains moxidectin, a second-generation macrocyclic lactone, and praziquantel, a synthetic isoquinoline-pyrazine derivative in a palatable gel formulation.
Pradectin with Tape Gel Wormer is effective as a single dose against encysted stages of small strongyles including developing stages (DL), late encysted stages (LL3/EL4) and aids in control of early encysted stages (EL3) including inhibited larvae (90% effective).
At the recommended dose rate Pradectin with Tape Gel Wormer is effective in the treatment and control of the following parasites:
• Small strongyles (adults and larvae): Cyathostomum spp incl benzimidazole resistant strains and as a single dose against encysted stages of small strongyles incl developing stages (DL), late encysted stages (LL3/EL4) and aids in control of early encysted stages (EL3) including inhibited larvae (90% effective).
• Large Strongyles: Strongylus vulgaris (adults, L3 and L4 arterial larval stages), Strongylus edentatus (adults and L4 visceral larval stages), Strongylus equinus (adults), Triodontophorus spp. (adults) including T. brevicauda, T. serratus and T. tenuicollis, Craterostomum acuticaudatum (adults).
• Tapeworms: Anoplocephala perfoliata and Paranoplocephala mamillana
• Stomach bots: Gasterophilus intestinalis (2 & 3 stage larvae),Gasterophilus nasalis (2 & 3 stage larvae)
• Ascarids: Parascaris equorum (adults and intestinal stages (L4))
• Habronema spp: gastric and cutaneous (summer sore)
• Lungworms: Dictyocaulus arnfieldi (adult and immature)
• Onchocerca spp. microfilariae (cutaneous onchocerciasis)
• Trichostrongylus axei (adults)
• Intestinal Threadworms: Strongyloides westeri
• Pinworms: Oxyuris equi (adults and L3/L4 larval stages)
• External parasites: Feeding ticks, lice and mites
When used as directed, Pradectin with Tape Gel Wormer is safe to use in foals over four weeks of age and in all breeding stock, including stallions and pregnant mares.
Do not administer the full syringe to horses under 400Kg. Use with caution in foals and smaller breeds by carefully calibrating the dose to the horse’s body weight to avoid overdosing.
Pradectin with Tape Gel Wormer is administered orally at a dose rate of 0.4 mg/kg moxidectin and 2.5 mg/kg praziquantel.

It is supplied in a ready to use syringe, calibrated according to the body weight of the horse to be treated in 25 kg body weight increments. Use of this calibration will deliver the correct recommended dose.
One syringe is sufficient to treat a horse weighing 575 kg. For a heavy horse (weight exceeding 575 kg) it will be necessary to apply more than one syringe. For example, a 725 kg horse would require 1¼ syringes for treatment.
To avoid over- or underdosing, use the correct dose based on the horse’s measured/estimated body weight.
If gel remains in the syringe once the full dose has been delivered, replace the barrel cap; store the remaining gel below 30°C (room temperature) for later use, but do not use after the expiry date shown on the label.
Pradectin with Tape Gel Wormer is the anthelminthic of choice for horses (especially young horses) where heavy strongyle burdens (especially encysted cyathostomin larvae) are suspected.
The mass simultaneous emergence from the large intestinal wall of encysted larvae (eg following worming with an anthelminthic that is not effective against encysted larvae) may result in a syndrome known as larval cyathostominosis.
This syndrome is characterised by an acute generalised typhlocolitis and a profound inflammatory reaction resulting in profuse, watery diarrhoea which may occasionally progress to death.
WARNING: To minimise emerging anthelminthic resistance to moxidectin, it is strongly recommended that moxidectin-containing wormers (such as Pradectin with Tape) not be used for routine deworming of horses.
Moxidectin should be reserved for cases where known or suspected resistance to other macrocyclic lactones (such as ivermectin, abamectin) has been established or where there is a strong likelihood of encysted and dormant larvae. Moxidectin’s use as routine dewormer should be discouraged.
supports the responsible use of anthelmintics.
APVMA Approval No. 87987 (Australia)
For
Whenever possible the decision to deworm should be based on the results of a Faecal Egg Count (FEC). Consult a veterinarian when developing a worm control program to suit your specific needs.
At the recommended dose rate Promax All Wormer is effective in the treatment and control of the following parasites: tapeworms, roundworms (including arterial larval stages of Strongylus vulgaris and benzimidazole resistant small strongyles) and bots.
ProMax All Wormer for Horses also effectively controls skin lesions caused by Habronema and Draschia spp. cutaneous larvae (summer sores), and Onchocerca spp. microfilariae (cutaneous onchocerciasis).
At the recommended dose rate, ProMax All Wormer is effective aginst the following parasites:
• Small Strongyles including benzimidalzole resistant (adults and immature): Cyathostomum spp., Cylicocyclus spp., Cylicostephanus spp., Cylicodontophorus spp., Gyalocephalus spp. etc
• Large Strongyles: Strongylus vulgaris (adults and arterial larval stages), Strongylus edentatus (adults and tissue stages), Strongylus equinus (adults) and Tridontophorus spp. (adults).
• Bots: Gasterophilus spp. (oral and gastric stages)
• Ascarids: Parascaris equorum (adult and immature)
• Habronema muscae (adult)
• Onchocerca spp. (microfilariae)
• Hairworms: Trichostrongylus axei (adult)
• Intestinal Threadworms: Strongyloides westeri (adult)
• Lungworms: Dictyocaulus arnfieldi (adult and immature)
• Pinworms: Oxyuris equi (adult and immature)
• Tapeworms: Anoplocephala perfoliata, Anoplocephala magna, Paranoplocephala mamillana (adult, immature, heads, segments).
ProMax All Wormer is safe to use in foals, pregnant mares and breeding stallions.

Dose orally at the recommended rate of 0.2 mg/kg abamectin and 2.5 mg/kg praziquantel.
Each weight marking on the syringe plunger will deliver sufficient paste to treat 50 kg bodyweight.
The contents of the syringe will treat a total of 600 kg bodyweight.
Bundy is a twelve-year-old, barely 14.2hh pinto pony that flies over 1.25m jumps and spins on a dime. He is fast, furious and fearless and the “pony of a lifetime” for his devoted owner, fifteen-year-old Sydney girl Jasmine.

Bundy was captured as a foal at foot along with his sire and dam in the Guy Fawkes River National Park near Coffs Harbour, Australia. The brumby foal could easily have ended up at a knackery had he not been rescued by his previous owners.
The pony had several owners before Jasmine’s mother responded to an advertisement on Gumtree about three years ago.

Bundy was Jasmine’s first horse and in just three years has taken her from an inexperienced novice to a consistent winner on the junior, Interschools and open showjumping circuits.
Most weekends you will find Jasmine and Bundy out competing and more often than not, they will bring home a blue ribbon against their bigger thoroughbred and warmblood oppostion.
There are 40 million Bundy’s in the world, each with their own unique story.
Here’s to all the Bundy’s! The best horses in the history of the world.
DID YOU KNOW THAT... There is only one Bundy in the world but there are 40 million Bundy’s?
AVENLY BUNDY AKA ‘BUNDY THE BRUMBY’ (JASMINE SPIRO, AUS)
Randlab supports the responsible use of anthelmintics.
APVMA Approval No. 88042 (Australia)
Ivermectin
Low volume, broad-spectrum dewormer
Whenever possible the decision to deworm should be based on the results of a Faecal Egg Count (FEC). Consult a veterinarian when developing a worm control program to suit your specific needs.
Goldmectin LV Ivermectin Oral Paste is highly effective in the treatment and control of most types of equine helminths including tapeworms, gastrointestinal, cutaneous and pulmonary nematodes and bots in horses of any age.

Susceptible parasites include: tapeworms, roundworms (including arterial larval stages of Strongylus vulgaris and benzimidazole resistant small strongyles), bots, and skin lesions caused by Habronema and Draschia spp. (summer sores) and Onchocerca spp. microfilariae (cutaneous onchocerciasis). Ivermectin may also be used as an arachnicide to remove feeding ticks.
At the dosage rate of 1 mL per 100 kg bodyweight Goldmectin LV delivers 0.2 mg/kg of ivermectin and 1.5 mg/kg of praziquantel.
For the treatment and control of the following parasites in horses:
• Small Strongyles including benzimidazole resistant (adults and immature): Cyathostomum spp., Cylicocyclus spp., Cylicostephanus spp., Cylicodontophorus spp., Gyalocephalus spp. etc
• Large Strongyles: Strongylus vulgaris (adults and arterial larval stages), Strongylus edentatus (adults and tissue stages), Strongylus equinus (adults) and Tridontophorus spp. (adults).
• Bots: Gasterophilus spp. (oral and gastric stages)
• Ascarids: Parascaris equorum (adult and immature)
• Habronema muscae (adult)
• Onchocerca spp. (microfilariae)
• Hairworms: Trichostrongylus axei (adult)
• Intestinal Threadworms: Strongyloides westeri (adult)
• Lungworms: Dictyocaulus arnfieldi (adult and immature)
• Pinworms: Oxyuris equi (adult and immature)
• Tapeworms: Anoplocephala perfoliata, Anoplocephala magna, Paranoplocephala mamillana (adult, immature, heads, segments).
• External parasites: Ticks, mites and lice.
Goldmectin LV for Horses also controls skin lesions caused by Habronema and Draschia spp. cutaneous larvae (summer sores) and Onchocerca spp. microfilariae (cutaneous onchocerciasis).
All horses should be subject to regular worming to maintain good health and peak condition. Decisions to worm should be based on individual and herd Faecal Egg Counts (FEC).
Particular attention should be given to young horses and mares. Foals should be treated initially at 6 to 8 weeks of age and this treatment repeated regularly as required.
Goldmectin LV has shown a wide safety margin at the recommended dose level. It may be used in horses of all ages. Mares may be treated at any stage of pregnancy. Stallions may be treated without adversely affecting their fertility. Suitable for foals older than 6 weeks old.
Goldmectin LV is given orally at the recommended dose level of 1ml/100 kg bodyweight. The dose of 1ml/100 kg bodyweight delivers 0.2 mg/kg of ivermectin and 1.5 mg/kg of praziquantel. The contents of this syringe will treat one horse at 700 kg bodyweight. Each weight marking on the syringe plunger will deliver 1.07 g of paste which is sufficient to treat 100 kg bodyweight.
Protection of Wildlife, Fish, Crustaceans and the Environment: Ivermectin is extremely toxic to aquatic species. Do not contaminate dams, rivers, streams or other waterways with the chemical or used syringe.



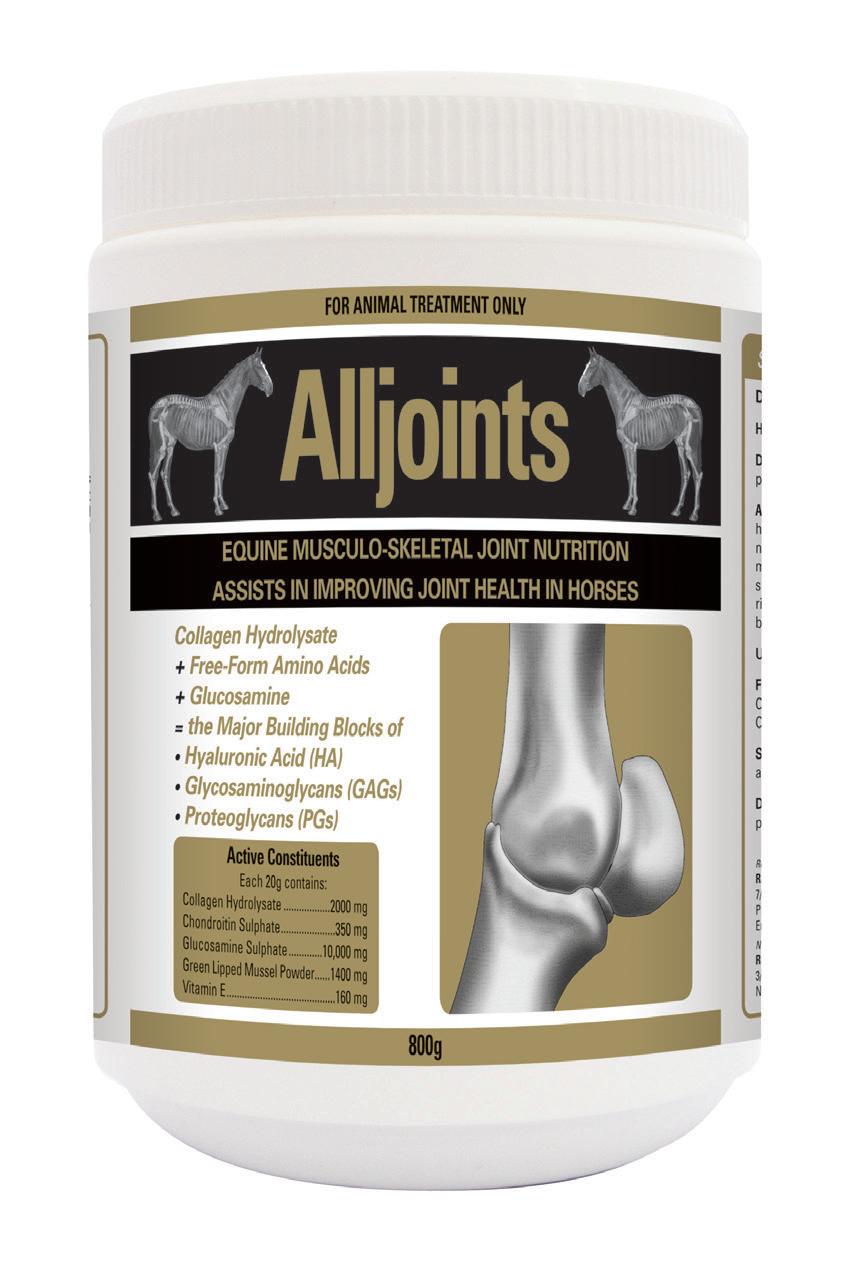
Randlab products to aid recovery post competition and training
Great value electrolyte, B-group vitamins and antioxidant supplement with a high concentration of ingredients. Suitable for use as a feed supplement or as a recovery drench.
Active Constituent
Each 30g dose provides;
Sodium 3.60g, Potassium 0.40g, Calcium 2.50g, Magnesium 0.40g, Chloride 3.40g, Phosphate 3.80g, Sulphate 1.30g, Bicarbonate 10.6g, Thiamine (B1) 0.11g, Riboflavin (B2) 0.11g, Niacin (B3) 0.32g, Pyridoxine (B6) 6.00mg, Vitamin B12 0.43mg, Choline 0.16g, Folic Acid 24.48mg, Inositol 0.27g, Vitamin E 0.22g
Pack Size
15kg pail
One level scoop provides the daily dose for in feed supplementation of 30g. This should be given twice daily in times of heavy sweating.
Recovery drench administer 150-200g (5-7 scoops) diluted in 1-2L of water by stomach tube.
Oral Electrolyte paste with B-Group Vitamins, Vitamin E and Folic Acid. To aid recovery in hard working or dehydrated horses.

Active Constituent
Each g contains: Sodium 63.0mg, Chloride 208.8mg, Potassium 81.3mg, Magnesium 2.96mg, Calcium 21.2mg, Zinc 1.34mg, DL-tocopheryl acetate (Vitamin E) 18.22mg, Folic acid 0.35mg, Thiamine mononitrate (Vitamin B1) 1.70mg, Riboflavin (Vitamin B2) 1.44mg, Nicotinamide (Vitamin B3) 5.55mg, Calcium pantothenate (Vitamin B5) 3.00mg, Pyridoxine hydrochloride (Vitamin B6) 0.17mg, Cyanocobalamin (Vitamin B12) 0.68mg
Pack Size
90g syringe or box of 12 x 90g syringes
Electrolytes, B vitamins and energy saline drench for horses. Aids in recovery from strenuous exercise.


Each 300g sachet provides Vitamin B Complex, balanced electrolytes sodium, potassium, magnesium, calcium, chloride, sulphates, volatile fatty acids (propionate, acetate), buffering agent (citrate) and an anti-oxidant (Vitamin E), all in a rapid and completely water soluble form.
Pack Size
300g powder in a sealed foil sachet
page 15
As Australia’s greatest racehorse much has been written about the champion’s mysterious death. Sixteen days earlier Phar Lap had won the richest horse race in the world at his first start in North America, after which he returned. He returned to a private training facility in Southern California to prepare for a lucrative campaign on the US Eastern Seaboard.

In the early hours of the morning of the 5th April 1932, Phar Lap’s trainer and constant companion, Tommy Woodcock, awakened to find the horse with a minor temperature and mild colic. He immediately roused Australian ‘horse doctor’ William Nielson, a member of the Phar Lap inner sanctum, who had accompanied horse and trainer to the US on their sea voyage. Only 90 minutes earlier, Woodcock had assisted a drunk Nielson to his bed after the 'doc' had yet another night out drinking and gambling.
Nielson had not studied veterinary science at university but was field trained and a renowned 'horse doctor'. Since arriving in the US and with only one horse to look after, Nielson had taken advantage of the local hospitality on most nights. All night binges at the illegal speakeasies which also usually involved gambling and prostitution, were the norm.
Upon being woken by Woodcock, the still drunk Nielson attended the great horse administering a horse “ball” which he filled based on the horse’s symptoms. “Balling” was the most common way of administering drugs to horses back then. It involved filling a 5cm gelatine capsule with the prescribed drug(s), grabbing the horse’s tongue and throwing the ball into the back of the horse’s throat. The horse 'doc' then returned to his bed.
After being re-summoned to examine Phar Lap later in the day, Nielson headed into town, a 40 min drive, seeking the assistance of the local horse vet. Why Nielson, did not just ring the vet on the house phone remains a mystery. Nielson returned to the farm some 3 hours later, having stopped into the local speakeasy for a drink en route. Meanwhile, Phar Lap had died an agonising death in the cradled arms of Woodcock.
Post mortem results revealed the horse had died from colic caused by inflammation of the stomach and intestine. Arsenic poisoning was suspected by many with much conjecture as to whether it had been administered accidentally or deliberately.
The cause of the intestine haemorrhage and inflammation has never been definitely determined. Many blamed Woodcock for administering an arsenic-based “tonic” which was common at the time. However, Woodcock denies the horse ever received such tonics. Others have claimed the arsenic had blown on to the grass where Phar Lap had grazed from a neighbouring orchid where it had been sprayed earlier as a pesticide.
Many claim the administration of the arsenic was more sinister and involved local gangsters/mafia that had already lost a lot of money when the big red horse had won at Agua Caliente and stood to lose more at his subsequent starts.
The 'horse doc' Bill Nielson blamed Woodcock for allowing the world’s best horse of his day to graze wet grass causing a gastric impaction. Many veterinarians from around the world have examined the reports of the post-mortem that was done on the farm immediately after the horse's death and the results of the examination of his organs some days later at the University of California and subsequent toxicology reports from the FDA. The majority of these veterinarians believe the horse had died from proximal duodenitis-jejunitis, a condition that was only recognised by the veterinary community some 50 years after the horse’s death. High levels of arsenic were confirmed in the horse's hair follicles by high speed x-rays as recently as 2006 and again in 2008.
In an interview in the weeks prior to his death in 1985, the normally reserved Tommy Woodcock voiced his long held secret concerns that the horse had died as a result of the drugs (including arsenic) that had been incorporated into the ball and administered by the drunk 'horse doc' Bill Nielson earlier that day.

Make up your own mind: Listen to the full exposé on Phar Lap’s death, including the Woodcock interview, by downloading the podcast “Killing Phar Lap: A Forensic Investigation” by scanning this QR code:
page 20
On his second birthday, Lennie Gwyther was given a chestnut pony by his grandfather. The pony and Lennie shared the same birthday. Lennie named the pony Ginger Mick after a favourite book character.
The pair grew up in the small town of Leongatha in rural Victoria during the Great Depression. Following an injury to his war hero father, Lennie was expected to help maintain the family farm.
By the time Lennie was nine, Ginger Mick and he had formed a formidable team. Like many Australians, young Lennie became increasingly interested in the construction of the Sydney Harbour Bridge as its official opening drew closer. The Bridge had taken nine years to construct.
As a reward for his hard work on the farm, Lennie’s parents agreed to his wish to travel to Sydney to watch the opening of The Bridge. However, the family could not afford the cost of the train ticket, so 9yo Lennie pleaded with his parents to allow him to make the 1000km journey on Ginger Mick.
The pair left Leongatha on 3 February 1932 and headed to Sydney. Lennie carried a haversack that included his toothbrush, silk pyjamas, spare clothes and a water bottle.
The expedition took seven weeks to complete and soon attracted wide-spread attention. Lennie and Ginger Mick captured the heart of the nation and become a symbol of the "aussie resilience". A courageous good news story amidst the constant shroud of the Great Depression.
Upon his arrival at Martin Place, Lennie was surprised to see 10000 cheering citizens had gathered to greet him, and even more surprised to learn that he and Ginger Mick had been invited to take part in the official opening of The Bridge.
By the time Lennie left Sydney for home a month later, he had become one of the most famous figures in a country craving uplifting news. The pair made the long journey back to Leongatha once more on foot.
In 2017 a bronze statue commemorating the pair’s epic journey was erected in Leongatha.
We can’t help feeling that Lennie would have been secretively happy when he saw renegade Captain Francis de Groot charge through the crowd on his horse, also called “Mick”, to break the ribbon, depriving the Premier Jack Lang of the honour of opening The Bridge (see full story Horses In History Part I, 2021 Randlab catalogue).
Horses In History Part I available in the Randlab 2020-21 catalogue.
DASHING SOLITAIRE page 21
If Phar Lap is Australia’s most famous horse, then Fine Cotton is the most infamous. Fine Cotton, a non-descript racehorse, was the centrepiece in Australia’s most notorious racing scandal when in 1984 he won a race in Brisbane and in so doing landed a massive Australia-wide betting plunge. His official trainer was Hayden Haitana. Soon after the horse crossed the line (some say even before the race) an elaborate plot involving racing royalty, politicians, police, life bans, jail sentences and the brutal murder of popular racehorse trainer George Brown began to emerge. The original ‘ring in’ was many months in the planning and involved substitution of the poorly performed Fine Cotton with the better performed Dashing Solitaire, who bore a strong resemblance to Fine Cotton. However only weeks before the scheduled race, Dashing Solitaire was injured when a kangaroo jumped into his paddock at Coffs Harbour, spooking the horse who then ran through a fence and was unable to continue in training. At this point the well contrived conspiracy turned to debacle. The perpetrators panicked and selected Bold Personality as the ringer. But he bore little physical resemblance to Fine Cotton. He was younger, bay (not brown like Fine Cotton) and had no white markings. Their clumsy attempts to masquerade Bold Personality with hair dye and white paint on raceday were quickly uncovered after the race and led to the early unravelling of the full conspiracy.
page 31
Following Earnest Shackleton’s previous unsuccessful attempt to reach the South Pole in 1903, the Anglo-Irish explorer decided to use ponies to do the bulk of the provision hauling for his 1907 return mission.
Four Manchurian ponies were selected, with each of the four men on the expedition having their own pony and supply sledge. The ponies were fed on a diet that largely consisted of Maujee, a British military ration consisting of dried beef, carrots, milk, currants and sugar. Occasionally the ponies would also share the scraps from the human plate of seal meat, blubber and fish.

The ponies themselves were on a one-way ticket with a contingency plan for each pony to provide up to 55kg of pony meat, a crucial aspect of the men’s provisions supply on the return journey.
One by one the ponies became too frail to continue and were shot until only one, by the name of Socks, remained. When Socks was struggling to keep his footing on the treacherous ice of the Beardmore Glacier, Shackleton and his men began contemplating putting him out of its misery. But before they could perform the deed, Socks disappeared into a crevasse, dragging one of the explorers (Frank Wild) and his provisions sledge with him.
Fortunately, the pony’s harness snapped and the sledge jammed across the opening and was able to be retrieved. Luckily, Wild was also able to be pulled to safety. However, Socks disappeared into the void along with the all-important meat his carcass would have provided.
The four men continued to forge southwards, but were gradually running out of food. On January 9, 1909, Shackleton made the decision to turn back and abandon the attempt to be the first to reach the end of the Earth. At the time, they were only 156km from the South Pole. All four men were physically capable of continuing but, Shackleton determined they would have all died of starvation on the return march.
Shackleton’s party had come agonisingly close, and had they been able to cache Socks’ meat, it is likely that they would have been the first to stand at the Pole and Antarctic history would read quite differently. The tragic and triumphant stories of Scott and Amundsen’s journeys would not have been written, as neither man would have had reason to set foot on the ice continent.
Socks came closer to reaching the South Pole than any other equine before or since.
page 33
Here at Randlab we are unabashed enthusiasts of the athletic horse. Secretariat, considered by Google, as the Greatest Of All Time, is the cover boy of the annual Randlab catalogue as well as the product model for Gastropell Forte and Meloxicam.

Following the death of Secretariat’s original owner (Cristopher Chenerey) towards the end of the colt's two year old career the horse had to be sold by his Estate to pay death duties. So at the beginning of his three-year-old career, Secretariat was syndicated for a record-breaking US$6.1M (equivalent today to Au$55.5M), on the condition he be retired from racing at the end of his 3yo career.
Secretariat’s last racetrack appearance was in the Woodbine International Stakes on 28 October 1973. He won pulling up by 6.5 lengths. He retired having won 16 of his 21 starts and being placed on 4 other occasions.

When Secretariat first retired to Claiborne Farm, his sperm showed some signs of immaturity on multiple occasions. He was test bred to three non-thoroughbred mares in December 1973 to check his fertility. One of these, an appaloosa named Leola, produced Secretariat's first foal in November 1974. The foal was a chestnut like his sire, but had a spotted blanket like his dam and was named First Secretary.
Before First Secretary’s birth, there was a bidding war between appaloosa enthusiasts who badly wanted Leola and the special foal she carried. Jack Nankivil of Minnesota prevailed after taking out a mortgage on his home to pay for the unborn foal. In order to recoup some of his investment, Nankivil immediately sold 15 lifetime breeding rights to the foal before anyone even knew whether it was a colt or a filly.

Understandably, Nankivil was thrilled when Leola delivered a colt who looked like his sire, but with an appaloosa blanket. However, the American Appaloosa registry refused to identify Secretariat as the sire because no breeding certificate had been issued for the mating and Nankivil could only get the colt registered with the Canadian Appaloosa registry.
Secretariat went on to produce 663 named foals over 15 years. That’s only an average of 44 foals per year. Secretariat sired a number of major stakes winners, but nothing that even approached his own prodigious talent.
First Secretary also proved to be a success at stud siring many winners both on the (quarter horse) racetrack and in the show ring.
Horses In History Part I available in the Randlab 2020-21 catalogue.
page 41
During WWI, eight million horses were killed either in battle or in the aftermath.
Australia sent 136,000 horses overseas to fight.
Only one came home. Sandy.
Sandy was the remount of Australian, General Sir William Throsby Bridges, until the General was killed at Gallipoli. The horse was then shipped to France where he was attached to the Australian Veterinary Corps Hospital at Calais and ridden by the vets trying to reach wounded animals.


It was the wish of the much-lauded war hero Bridges that his horse be returned to Australia.
page 52
Cambridge Stud on New Zealand's North island is one of the great studs in the world, largely through the deeds of its two flagship stallions Sir Tristram and his son Zabeel.
The stud was founded in 1975 by (Sir) Patrick Hogan who went to England, Ireland, France and the US in search of a foundation stallion. He returned empty handed having not been able to find a stallion within his budget of NZ$200K. Hard to believe considering the $20M plus price tag paid for stallion prospects these days.
Some time after, Hogan would receive the pedigree of a stallion by mail. The horse in question was Sir Tristram (Ire) who was by Sir Ivor out of a Round Table mare. His racetrack performances were ordinary having won only two non-descript races in France from 19 race starts (no Group One’s!). Ironically, Hogan had stood within 20m of Sir Tristram in France whilst on his stallion search tour, but had not been shown the stallion as he was not deemed good enough.
But Hogan believed Sir Tristram had a strong stallion’s pedigree. He was duly purchased for NZ$160K.
Sir Tristram was lucky to ever make it back to NZ. A fire broke out in the stable where he was serving his quarantine in England. Several horses perished in the fire. Sir Tristram was found badly burnt sometime later on a property four farms from his incinerated stable in a paddock full of mares.
In the aftermath of the fire and the subsequent prolonged recovery, Sir Tristram eventually arrived in NZ in poor condition. Many of the original shareholders were unimpressed with what they saw and opted out. The stallion was described as being, “weak, with bent hocks, and on the walk from behind one hock rolls out badly. He is a most unattractive horse".
Sir Tristram went on to sire an extraordinary 45 Group One winners, including three Melbourne Cup winners, and 140 individual stakes winners, and was awarded 17 Leading Australasian Sire premierships. Most importantly, he sired Zabeel who went on to be his successor as Australasia’s premier sire of stayers.
The stallion they called "Paddy" was humanely euthanised in 1997 after breaking his shoulder in a paddock accident. A priest conducted the 40-minute burial service, after which he was buried standing in the vertical position with his tail facing the rising sun, the ultimate sign of honour.
When the last yearling by Zabeel was sold at auction in 2015 it was Sir Patrick Hogan who symbolically lead the colt into the Karaka sale ring. On the fall of the hammer, the purchase price was NZ$160K, the same price Hogan had paid for Sir Tristram. Thus, ending the dynasty as it had started 40 years earlier.

page 61
The phrase “A heart as big as Phar Lap” has become a popular part of the Aussie vernacular for over 50 years since information relating to the extraordinary size of his heart (6.35kg – nearly double the size of a normal thoroughbred) was first published.
The famously large heart is on permanent display at the National Museum of Australia (NMA) in Canberra, where it is the Museum’s most visited exhibit and has acquired almost a cult relic following.
The heart, along with Phar Lap’s other organs were removed from his body following his suspicious and sudden death in 1932 by the horse’s “veterinarian” William Nielson. Nielson was not a university-trained veterinarian but was known as a 'horse doc', having learnt his craft in the field.
Nielson accompanied Phar Lap on his fatal trip to race in the USA. After the horse’s death, Phar Lap’s organs underwent several post mortem examinations before the heart was preserved in formalin and shipped backed to The University of Sydney.
But more recent information may have revealed that the heart on display was actually a “ring in” and belonged to a Clydesdale.
William Nielson’s daughter quotes her deceased father as saying that the Phar Lap’s heart was cut into too many pieces at the time of post-mortem and was substituted by a Clydesdale’s heart. Conspiracy theorists and some reputable journalists say that the substitution may be part of a bigger cover up to try and conceal the true nature of the horse’s demise.
Recent attempts to get a DNA analysis of Phar Lap’s heart by an investigative journalist who was also friends with the horse’s former trainer Tommy Woodcock have been seemingly blocked by the NMA on technological grounds.







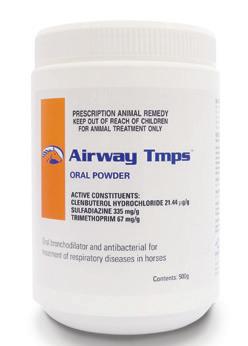











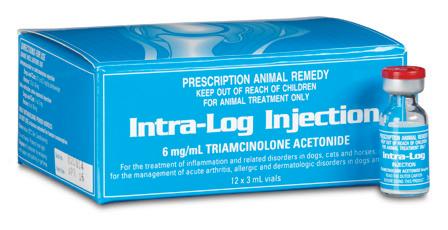
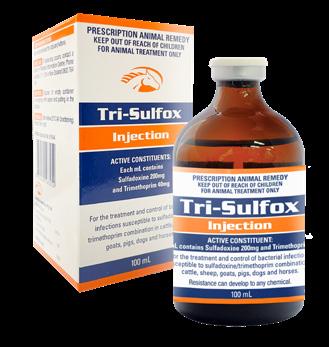





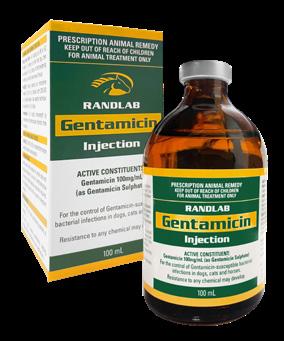




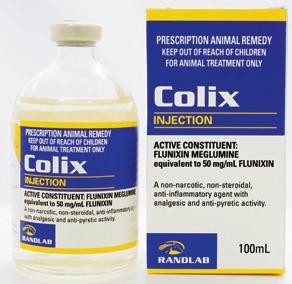














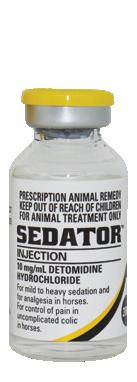

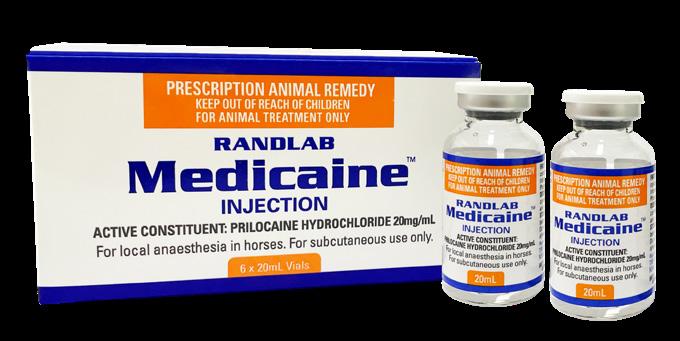



Randlab has a global distribution network of partners committed to serving veterinarians and their clients wherever they may be.
Randlab Europe (Eu Randlab Sprl) 24 Rue Tayant Ransart 6043 Belgium Phone: (+32) 475 933 457 Email: bernard.stoffel@gmail.com

Randlab Middle East / Gulf Warehouse 5, Al Qusais Industrial Estate, Area 3, Dubai, United Arab Emirates Phone: (+971) 426 666 48 Email: regulatory@randlab.com


Randlab South Asia
39 Woodlands Close, #08-68 (Suite A) MEGA@Woodlands Singapore 737856 Phone: (+65) 8797 8900 Email: info@randlab.asia
Rest of the World (Randlab Headquarters)
NEW ADDRESS
71 Milperra Road Revesby, NSW, 2212 Australia

Phone: (+61-2) 9728 3505 Fax: (+61-2) 9728 4352 Email: info@randlab.com
Randlab Central Asia


Horsepower Sydney Pty Ltd, Room 404 Chandmani Centre, Jamiyan Gun St, 1st khoroo Sukhbaatar District, Ulaanbaatar, Mongolia Phone: (+976) 998 812 05 or (+976) 981 112 05 Email: Horsepowersyd@gmail.com
Randlab China 兰德实验室中国 Mobile: (+86) 1801 975 3936 Email: sophia@randlab.com.cn



Randlab in Japan
EBM Trading, Yokohama-City Higashiasahina, Kanazawa-Ward 1-52-16 Japan, 236-0033 Phone: +81 (0) 80 4364 4002 Email: h.echigo@ebmtrading.com
Randlab New Zealand 3/180 Montgomerie Road, Mangere, Auckland, New Zealand Phone: (+64-9) 275 5657 Email: chris@randlab.co.nz
Randlab Europe (Joint Products only) Nupsala Veterinary Services Mobile: + 44 (0) 1865 922 227 Email: info@nupsala.com www.nupsala.com
Our local team of experienced Territory Managers
Alex Macpherson (VIC/TAS/SA)
Phone: 0421 829 101
Email: alex@randlab.com.au
Andrew Grant (QLD/NT)
Phone: 0426 407 117
Email: agrant@randlab.com.au
John Dalton (NSW/ACT)
Phone: 0404 221 776 Email: john@randlab.com.au
Dr Michael Robinson



Global Technical Director/Veterinarian



Phone: + 61 (0) 451 481 050
Email: mrobinson@randlab.com.au
Rebecca Puvanendran (WA)
Phone: 0420 349 301 Email: rebecca@randlab.com.au
Bruce Bell
Assistant General Manager
Phone: + 61 (0) 404 137 514
Email: bruce.bell@randlab.com.au
Chris Barris (NZ)
Phone: (+64-9) 275 5657 Email: chris@randlab.co.nz
Rest of the World (Randlab Headquarters)
NEW ADDRESS
71 Milperra Road Revesby, NSW, 2212
Australia Phone: (+61-2) 9728 3505 Fax: (+61-2) 9728 4352 Email: info@randlab.com
We hope you like this catalogue �� and find it a useful resource to refer to throughout the year �� But if you decide to discard it ��, please recycle it.
