Neuromyelitis Optica
Celia Oreja-Guevara, Hospital Clinico San Carlos, Spain
Andrew Chan, Ruhr-University Bochum, Germany
Patrick Vermersch, University of Lille, France


Celia Oreja-Guevara, Hospital Clinico San Carlos, Spain
Andrew Chan, Ruhr-University Bochum, Germany
Patrick Vermersch, University of Lille, France

Devic, 1894:


• 45-year-old patient with paraplegia and simultaneous amaurosis

• Demyelination and necrosis: optic nerves, spinal cord
Lennon 2004:
• Characterization of NMO-IgG, antigen: aquaporin IV (AQP4), pathogenetically relevant








 Papadopoulos, M. C. et al. (2014)
Papadopoulos, M. C. et al. (2014)


Down regulation of GLT1



Impaired astrocytic uptake of glutamate, oligodendrocyte injury and demyelination
Reduced Kir 4.1 expression: Impair efflux of K at the astrocytic end feet, impairment of coupling synaptic activity and cerebral blood flow
F:M 10:1 (AQP4-positive NMO)
• Older presentation (34-43 years) than MS
• Very late onset possible
•
30% with other autoimmune diseases
•
3% NMO patients with NMO-relatives
• East Asian/African population
• Severe relapses
• Rare: chronic progressive cases




AQP4-Ab: monophasic or recurrent isolated ON (RION), brainstem encephalitis
Brainstem manifestations: intractable hiccups or vomiting, symptomatic narcolepsy, and neuroendocrine dysfunctions
Posterior reversible encephalopathy syndrome
In children (and sometimes in adults): broader spectrum of encephalitic manifestations (seizures)

1. NMO
2. Limited forms of NMO: - LETM: Longitudinal extensive transverse myelitis
- rON: relapsing bilateral optic neuritis
3. Opticospinal MS (Asia)

4. rON or LETM with systemic autoimmunity
5. „Cerebral“ NMO with lesions at typical topography, with/without rON/LETM
-
Optic chiasm
-
Hypothalamus
-
3./4. Ventricle
-
Brainstem / Area postrema
•
• Area postrema,
• Brainstem lesions




✓ 8/47 (17 %) patients NMO (0/130 MS)
✓ Duration > 48 h (66 %) and/or nausea (80 %)
✓ Before a relapse in 54 %, during a relapse in 29 %
✓ Sometimes isolated and first symptom
✓ Medulla oblongata lesions (47%) +/- extensive myelitis (80 %)



✓ 12 patients AQP4-Ab+ with intractable vomiting (median 4 weeks)

✓ Followed by ON or acute transverse myelitis (NMO diagnosis in 7 cases)
✓ Area postrema lesions in MRI or at autopsy



✓ 12/44 patients (27 %)

✓ Lesions frequently in the superior part of the cervical SC
✓ SC / brainstem

Corticospinal
















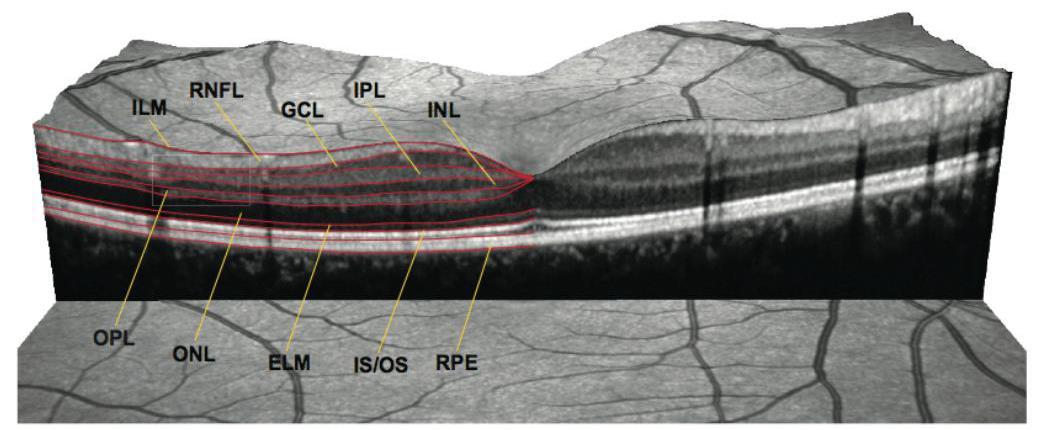
Microcystic macular oedema

MRI lesions


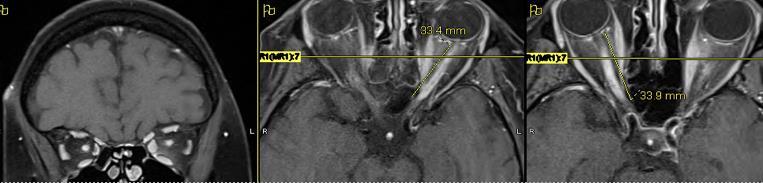
comparing with MS: More extended bilateral Chiasma
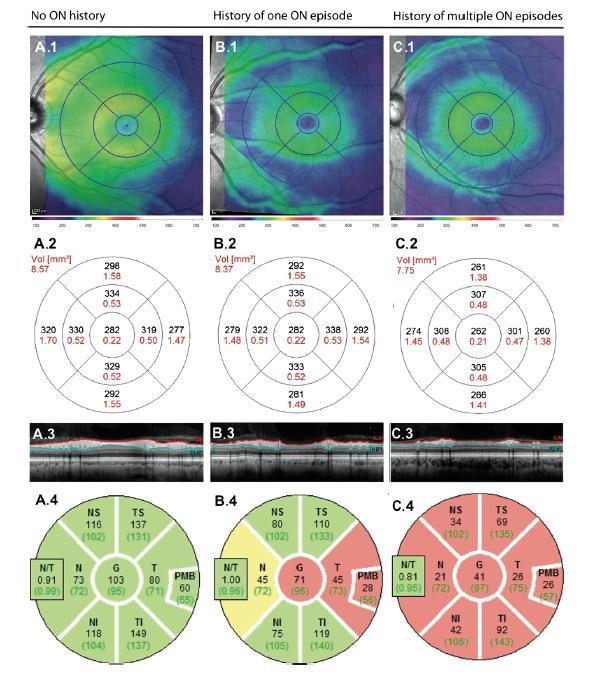
Comparing with MS: bilateral, VA lower, OCT lesions more severe (RNFL, GCL)











58 patients < 18 years old, AQP4-Ab + Brain lesions > 30 %





Pseudo-ADEM

Peri-aqueduccal
Cortico-spinal tracts




NMO associted with other systemic inflammatory disorders

• Bilateral severe optic neuritis, altitudinal defects
− MRI: longer lesions, optic chiasm; OCT: more widespread atrophy
• Brain lesions: up to 60%
−
Cave: Barkhof criteria 10-42%, no cortical lesions
CSF − >50/ml WBC, neutrophils/eosinophils >5/ml, 10-25% OCB (fluctuating), (GFAP, IL6)
• Severe Myelitis, subacute (not peracute/chronic)

−
Cave: 2-3% of MS LETM, 7-14% of NMO initially short lesions




NMO without anti-AQP-4 ab: 20 - 40 % of NMO patients
Among theses 20-40%: Anti-MOG ab is detected in 20%
MOG
unknown
80%: no antibodies detected

Compared with NMO anti-AQP4+, NMO anti-MOG :

▪Are younger
▪First relapse more often multifocal
▪Better outcomes after relapses




Comparing with NMO anti-AQP-4+, NMO anti-MOG :

▪ Fewer relapses
▪ First relapse more frequently monofocal
▪ Better outcomes




High doses IV steroids
➢ Methylprednisolone : 3-10 g/d 3-5 days
➢ Steroid oral tapering : 1 mg/kg/day 10 days-1 month (??)
Plasma exchange (Plex)
➢Severe, worsening or corticosteroid-refractory acute attack
➢5-7 times in alternative days
IVIg
Other options: cetirizine, bevacizumab

TREATMENT: Drugs to avoid
- Standard MS therapies are ineffective for NMO
- NMO worsening was reported with :
natalizumab
fingolimod
alemtuzumab


















Immunosuppressants better than immunomodulators in NMO



















➢ Rituximab is effective

➢ Used in first line : 84.3 % of patients realpse-free, used in second line: 52.3 % relapse-free


➢ Rituximab is effective
➢ Frequent dose: 1 gr iv x 2 , 2 weeks apart
➢ Duration of effect: 6-10 months
➢ Re-treatment timed: ➢ Scheduled: 1 infusion every 6 months

➢ Retreat when CD19> 1% of total lymphocytes
➢ Retreat if CD27>0.05%
➢ Increased infections associated with IgG decrease


Approved: Eculizumab
Not yet approved (clinical trials are finished) :

Satralizumab
Inebilizumab
Other treatments:
Tocilizumab
>Bortezomib









Median time to first adjudicated relapse: not reached (eculizumab) vs. 103 weeks (placebo)
Note: data were censored at end of patient’s time in trial (after a “negatively adjudicated” physician-determined relapse and after discontinuation from trial)
This figure has been adapted for the purposes of this presentation. Copyright is held by The New England Journal of Medicine. CI, confidence interval.




Primary endpoint: significantly reduced risk of adjudicated relapses for eculizumab vs. placebo










• IL-6 is elevated during NMO relapses in serum and CSF
• T cells and monocytes from NMO patients produce IL-6
• IL-6 activates plasmablasts to produce anti-AQP4 antibodies


• Anti-CD20 Abs do not target PBs/cells
• Inhibition of the IL-6R in vitro reduces survival of PBs

➢SA237 is designed to improve pharmacokinetics (PK) by applying “Antibody Recycling technology”


Conventional antibody recycling antibody





Recycling technology


Minimum effective concentration = 1mg/mL
(n=12 each)











Tocilizumab
Eculizumab
Therapeutic trials

Mitoxantrone
Cyclophosphamide
Rituximab + steroids
Mild: Aza, MMF (± low dose prednisone)
Severe: Rituximab
•Rapidly evolving field, clinically/scientifically

•Complex but reliable diagnostic criteria
•Studies difficult (logistics, ethics, methodology): high likelihood to fail
•High unmet need
•Very close to “translation”
•Major implications for understanding of more common disease multiple sclerosis