Respirator Fit Test Training
Learner Guide



In completing this course you will learn how to perform respirator fit testing and know the correct use of equipment.

1.

Learner Guide



In completing this course you will learn how to perform respirator fit testing and know the correct use of equipment.

1.
Respirators are worn by persons who must work in environments where the air is unfit to breathe and either have the function of filtering the air before the user is exposed or they supply a separate source of clean air.
The purpose of a respirator fit test is to protect an individual by ensuring that the respirator creates an effective seal on the face of the user thereby reducing the level of inhaled hazardous particles. The degree of protection can vary depending on the filtration of a particular make and model of respirator. In addition, it is very important that an individual knows how to properly put on (don) and wear a respirator.
A fit test is a validated method of matching a respirator to an individual, and checking the effectiveness of the seal, between the wearer and the outside environment.
The purpose of a respirator fit test is to protect an individual by ensuring that the respirator creates an effective seal on the face of the user thereby reducing the level of inhaled hazardous particles. The degree of protection can vary depending on the filtration of a particular make and model of respirator. In addition, it is very important that an individual knows how to properly put on (don) and wear a respirator.
Respirator fit testing is required for all tight-fitting respirators, including:
• half-face disposable
• half-face reusable
• full-face reusable
• tight-fitting powered air purifying respirators (PAPR)
• self-contained breathing apparatus (SCBA).
Positive-pressure respirators are a special subclass available in both APR (air-purifying respirator) and SAR (supplied-air respirator) versions, which can, in turn, be either tight- or loose-fitting. They provide high levels of protection by pumping air into the mask at all times rather than relying on the wearer’s lung power to draw air in (as do negative-pressure respirators). Tight-fitting positive-pressure respirators are fit tested in “negative-pressure mode” by temporarily converting them with an adapter. This is done because the effectiveness of the seal by itself cannot be evaluated properly while the power is creating positive pressure inside the mask.
There are two types of tests— qualitative and quantitative . A business may choose either method, according to their needs. The use of one or the other type of tests depends on the type of test will depend on the level of respiratory protection required and the resources available. The use of one or the other type of test will depend on the type of respiratory protective equipment (RPE) to be fit tested, the extent of RPE usage, and the resources available.
Qualitative tests are fast, and are easily performed. However, these tests rely on the wearer’s subjective response , and so are not entirely reliable. There are two available tests, QLFT-ATT, which is Qualitative Fit Testing- Aerosol Taste Test, and QLTF- IAA/Isoamyl acetate (banana smell); only for testing respirators with organic vapor cartridges. For the purposes of this course, we will be focusing on QLFT-ATT.
They use a test atmosphere which may be an enclosure into which:
a) The user can enter wearing the equipment
b) A ‘test’ contaminant (of low toxicity) can be placed.
Isoamyl acetate is a low toxicity substance with a banana-like odour. This test is only used on respirators equipped with an organic vapour cartridge. The substance is applied to the cotton wad inside the enclosure. The prospective user should put on the RPE in an area away from the test enclosure so that there is no prior contamination of the filters by ‘pre- exposure’ to the isoamyl acetate. The user enters the chamber and performs each of the following activities for 30 seconds:
a) Normal breathing .
b) Deep breathing , to simulate heavy exertion. This should not be done long enough to cause hyperventilation.
c) Side-to-side and up-and-down head movements . These movements should be exaggerated, but should approximate those that take place on the job.
d) Talking . This is most easily accomplished by reading a prepared text loudly enough to be understood by someone standing nearby.
e) Other exercises may be added depending upon the situation. For example, if the wearer is going to spend a significant time bent over at some task, it may be desirable to include an exercise approximating this bending.
f) Break face-piece seal and expose the wearer to test agent to verify the wearer’s sensitivity.
This test is suitable for respirators incorporating any particulate filter. It relies upon the wearer’s ability to detect a safe aerosol by taste. Individuals vary in their taste threshold; therefore a screening procedure is performed to establish suitability. Saccharin is sweet and Bitrex is bitter.

First, test subjects are tested for sensitivity to the taste of the aerosols. They are tested by wearing the hood, and keeping their mouth open, while the aerosol is sprayed into the hood, in “spray groups” of ten puffs each. If the subject tastes the flavour at any time during the first 10 puffs, they are given a score of 10. If they taste it at any time in the second group of ten puffs, they are given a score of 20. If they taste it in the third group of puffs, (21-30) they are given a score of 30. You stop puffing at any point when they taste the aerosol. If a subject does not taste the aerosol within 30 puffs, they cannot be tested using this method.
A period of at least several minutes should elapse after the sensitivity test before re-testing the subject wearing the RPE. Subjects may rinse their mouths with water at this time.
The test subject, having passed the sensitivity test, is fitted with the appropriate RPE. Since the flavoured mist is an airborne particulate, gas filter RPE should be fitted with a particulate filter for the test. The subject must keep their mouth open and tongue out inside the respirator. The subject places the hood over their head, and the aerosol solution is puffed through the test hole . The test subject will start to do a series of seven exercises (a protocol) similar to the ones outlined in the test above. Each “exercise” takes one full minute. The protocol below is from OSHA:
1) Normal Breathing
2) Heavy Breathing
3) Turn head side to side (stopping for two breaths at each turn)
4) Tilt head up and down, (stopping for two breaths at each apex)
5) Read a written passage (The Rainbow Passage)
6) Bending over
7) Normal breathing
Initially, the tester will deliver the same number of puffs as the subject’s score (10, 20 or 30) Then, the tester will deliver a half set of puffs every 30 seconds, (5, 10 or 15) The timer runs continuously, so, the aerosol gets delivered twice a minute. Since each exercise will be done for one minute, the aerosol should be delivered twice during each exercise in the protocol.
If at any time during the test, the subject can taste the aerosol solution, the test is considered a fail, and should be stopped. At the end of a successful (passed) test, the subject must remove the respirator, and the aerosol is delivered into the hood again, to confirm that the subject can still taste the aerosol.
These tests are relatively easy to perform and the equipment is inexpensive. There are disadvantages to qualitative testing. The test is subjective, and if a person has poor taste sensitivity, or is at risk for an allergic reaction, they cannot be tested this way. It can be time-consuming to perform education and assessment depending on protocols, and the test itself takes no less time than a quantitative test.
Because all qualitative tests rely on recognition of a taste or smell, test subjects should have no food or flavoured drink, no smoking or vaping, for 30 minutes prior to testing.
Quantitative test methods use equipment to measure the efficiency of a respirator in preventing materials in the atmosphere from entering a user’s breathing zone:
• Ambient Aerosol Condensation Nuclei Counting (CNC) using a PortaCount machine.
• Controlled Negative Pressure (CNP) uses a test that creates a vacuum by temporarily cutting off air.
• Generated aerosol uses a non-hazardous aerosol such as corn oil generated in a test chamber.
For the purposes of this course, we will only be discussing Ambient Aerosol Condensation Nuclei Counting (CNC) using a PortaCount machine.

This test measures the particle count found within the respirator and compares it to the particle count outside the mask, (ambient particle count). To do this, you must have an ambient particle count to measure against. You can use naturally occurring or artificially generated atmospheric particles.
The test subject will perform a series of exercises during the test, to challenge the seal of the mask. The machine will continually assess the particle count within the respirator and outside the respirator and compare them.
There will be more discussion of this method further in the course.
Selecting the best fit test method can only be done after considering the pros and cons of each method. For certain respirators, and certain levels of required protection, you will not have the choice, as qualitative testing can only be done up to a required protection level of 10. The table below provides a comparison of qualitative vs. quantitative methods.
Qualitative fit testing (QLFT)
Quantitative fit testing (QNFT)
Suitability for type of RPD
Suitable for disposable and reusable half masks; not suitable for full-face masks.
QNFT-CNC is suitable for disposable and reusable half masks and full-face masks.
QNFT-CNP is suitable for reusable masks only.
Vulnerability/ Objective Outcome
Exposure Limits
Advantages
Based on subjective detection and response by the wearer of the RPD. Fit factor is a “given score” with no numeric measure.
May be used to fit test negative pressure air-purifying respirators if they will only be used in atmospheres less than ten times the WEL, since existing evidence only validates the QLFT protocols to identify respirators that achieve a fit factor of 100.
• Low equipment cost
• Simple pass/fail results
Disadvantages
• Chance of employee deception
• Limited protection-factor verification (maximum fit factor of 100)
• Subjective results may be unreliable
Objective test; provides a numerical measure of how well a face piece seals against a wearer’s face; this is called a fit factor.
Can be used for concentrations higher than ten times the Workplace Exposure Limit.
• High protection factor test, up to 2000 for a full face-piece.
• No chance of employee deception
• Highly reliable
• Documentation of numerical results
• Expensive up-front equipment costs
• QNFT-CNC requires probed face piece or probed adapters, specific to the mask
• QNFT-CNP requires special leak-tight test adapters, specific to the mask
• Annual recalibration of equipment is required.
Trained Staff Fit tester should be trained in the delivery of the test, but interpreting results relies on the ability of the wearer to sense a test agent
Testing Methods
Specialized Equipment
Simple but test results are totally dependent on the user ability to detect the agent, and give an honest response. Unable to achieve a fit factor above 100.
Equipment is relatively inexpensive, but a test agent, either taste or smell, must be obtained. Test hoods are effective, but “homemade” versions will also work.
Requires a highly trained operator who understands how to set up and use the equipment, perform preventive equipment maintenance, and troubleshoot issues.
Both QNFT-CNC and QNFT-CNP are highly sensitive and accurate methods of testing.
QNFT-CNC: Requires a PortaCount machine or similar, and particle generator,
QNFT-CNP: Requires The OHD Quantifit instrument or similar,
Both are expensive up-front investments.
Accuracy of Result
Only Pass/Fail is detected. If a test subject is uncooperative, they may not provide truthful responses.
QNFT-CNC: Extremely accurate, detecting particle leakage of very low amounts
QNFT-CNP: Extremely accurate at measuring the leak rate inside the mask.
Atmospheric Sensitivity
Totally dependent on the subject
A calibrated machine is required, but should reliably detect particles or pressure change, depending on the method used.
The major function of the respiratory system is gas exchanged between the external environment and the circulatory system. This involves taking in oxygen from the air to the blood and releasing carbon dioxide (and other gaseous waste products) from the blood back into the air.
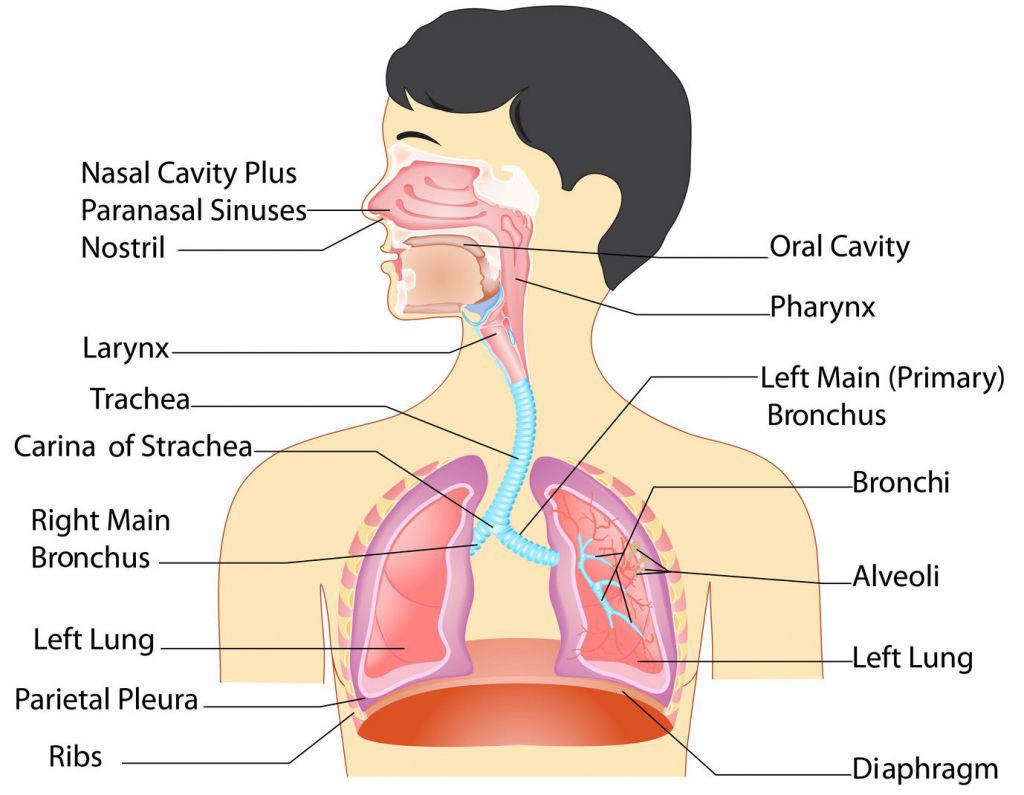
Upon inhalation, gas exchange occurs at the alveoli, the tiny sacs which are the basic functional component of the lungs. The alveolar walls are extremely thin (approx. 0.2 micrometres). These walls are composed of a single layer of epithelial cells in close proximity to the blood capillaries which in turn are composed of a single layer of endothelial cells. The close proximity of these two cell types allows permeability to gases and, hence, gas exchange. Oxygen is taken into the blood whilst excess carbon dioxide is released.
The average person who is moderately active during the daytime breathes about 20,000 litres of air every 24 hours. Inevitably, this air contains potentially harmful particles and gases. Particles, such as dust and soot, mould, fungi, bacteria, and viruses deposit on airway and alveolar surfaces. Fortunately, the respiratory system has defence mechanisms to clean and protect itself. Only extremely small particles, less than 3 to 5 microns in diameter, penetrate to the deep lung.
Cilia, tiny muscular, hair-like projections on the cells that line the airway, are one of the respiratory system’s defence mechanisms. Cilia propel a liquid layer of mucus that covers the airways. This mucus layer traps pathogens (potentially infectious microorganisms) and other particles, preventing them from reaching the lungs. Cilia beat more than 1,000 times a minute, moving the mucus that lines the trachea upwards about 0.5 to 1 centimetre per minute (0.197 to 0.4 inch per minute). Pathogens and particles that are trapped on the mucus layer are coughed out or moved to the mouth and swallowed.
Alveolar macrophages, a type of white blood cell on the surface of alveoli, are another defence mechanism for the lungs. Because of the requirements of gas exchange, alveoli are not protected by mucus and cilia— mucus is too thick and would slow movement of oxygen and carbon dioxide. Instead, alveolar macrophages seek out deposited particles, bind to them, ingest them, kill any that are living, and digest them. When the lungs are exposed to serious threats, additional white blood cells in the circulation, especially neutrophils, can be recruited to help ingest and kill pathogens. For example, when the person inhales a great deal of dust or is fighting a respiratory infection, more macrophages are produced, and neutrophils are recruited.
Rebecca Dezube , MD, MHS, Johns Hopkins University
Coughing is a natural defense mechanism that protects the respiratory tract from inhaling foreign bodies and by clearing excessive bronchial secretions. Sneezing is simply an involuntary release of air that helps the body to get rid of irritants in our nose and throat, like allergens, dirt, and dust.

The four main routes of entry of toxins into the body are via inhalation, ingestion, absorption and injection. With regards to understanding respiratory protection, airborne particulate can only enter the body via inhalation, absorption through skin and eyes, or ingestion (swallowed particulate). Of these, inhalation is the route that poses the greatest threat to health.
1) Ingestion :
Humans have evolved mechanisms in the gut to regulate the uptake of essential elements. Toxic elements may have to compete so that generally only a fraction of any ingested dose is absorbed into the body (often 10% or less). Possible causes of ingestion in industry are mouth pipetting in laboratories, swallowing dust which has been inhaled and cleared by the mucociliary escalator, smoking and eating at the workstation or simply having dirty hands where the hand later comes in contact with the mouth.
2) Inhalation :

In the lung there are no similar mechanisms for selective uptake. Particles less than 10 micron in diameter may reach the alveoli. If soluble, approximately 40% are then absorbed. Insoluble chemicals are relatively safer, for example lead sulphide, whereas lead carbonate is highly soluble and causes poisoning quickly. Larger inhaled particles are less of a risk as absorption higher up the respiratory tract is less efficient. It is important to remember that not only is the lung responsible for the uptake of substances into the body it is also acted on as a target organ. Materials which are not absorbed into the body can remain in the lungs and cause physical and / or chemical damage to them. Inhalation accounts for approximately 90% of industrial poisoning.
3) Absorption :
In the skin there is again no selective uptake. Fat-soluble compounds are absorbed readily as are organic solvents. Percutaneous absorption through healthy intact skin can occur with nitrobenzene, phenol, mercury, and aniline. Absorption of phenol through just a few square inches of intact skin can be lethal. Impervious protective clothing like gloves will increase the rate of absorption if accidental contamination occurs on the inside. Damaged skin also facilitates absorption of toxins.
Once substances have entered the body they can be distributed around the body through the blood supply bound to plasma proteins or to red cells. They may concentrate differentially in the organs. Other toxic materials may be in solution or bound to lipids. Only lipid-soluble substances can pass the blood-brain barrier. (Basic Principles Occ Hyg - 3.3.3)
Airborne contaminants are potentially harmful substances that are either not naturally in the air or are present in an unnaturally high concentration and to which workers may be exposed in their working environment.
Airborne contaminants come in various forms:
• Dust : solid particles generated and dispersed into the air, for example, handling, crushing, grinding of organic or inorganic materials such rock, ore, coal, wood and grain.
• Fibres : Respirable fibres are considered responsible for adverse health effects caused by asbestos. To be respirable, a fibre needs to have a diameter of less than three micrometres and a length of greater than five micrometres; and a length to width ratio of greater than 3:1. These fibres are capable of penetrating to the terminal bronchioles of the gas exchange region of the lung and represent the most significant hazard to human health as they have the potential to cause asbestosis and mesothelioma with only minimal exposure.
• Mists : are suspended liquid droplets generated by condensation of vapour back to the liquid state or by breaking up as a liquid into a dispersed state, such as by splashing or atomising. Mist is the term applied to a finely divided liquid suspended in the atmosphere. Examples are an oil mist produced during cutting and grinding operations, acid mists from electroplating, acid or alkali mists from pickling operations, paint spray mist in painting operations and the condensation of water vapour to form a fog.
• Gases : are formless fluids that expand to occupy the space or enclosure in which they are confined. Examples are nitrogen, oxygen and formaldehyde.
• Fumes : are extremely fine - usually less than 1.0 micrometer in diameter. Fumes are formed when the material from a volatilised solid condenses in cool air. In most cases the hot vapour reacts with the air to form an oxide. Fumes are often associated with molten metals, especially in processes like welding.
• Vapour : is the gaseous form of a substance which is normally in the solid or liquid state at room temperature and pressure.
• Biohazard Aerosols : illnesses spread by tiny pathogens (eg. bacteria, fungi, or viruses) in the air transmitted through airborne contact. In most cases contracted when someone breathes in infected air.
Particles is a generic term used to describe airborne solid or liquid substances in the finely divided state ie, particulate aerosols such as dusts, mists, fumes, fibres and fog as well as microorganisms.
What size dust is a respiratory hazard?
Inhalable dust particles are under 100 microns in diameter.
Thoracic dust particles are under 25 microns and can penetrate the head airways and enter the airways of the lung.
Respirable (breathable) dust fragments are measured in microns. This is the smallest size dust fraction. Respirable dust particles are under 10 microns in diameter.
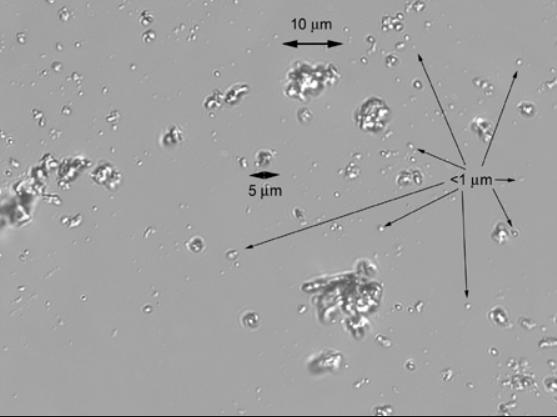
• Determined by having an upper (max) aerodynamic diameter of 10μm (micron)
• Very small respirable 0.5 to 2μm
• Hazardous when deposited in the gas exchange area of the lung (bronchioles and alveoli).
Like the skin and the eye, the lungs are affected by irritants and allergens. They also respond in the forms of fibrotic pneumoconiosis and malignant disease to a variety of industrial agents.
Particles greater than 10 μm in diameter are filtered by the nose. The branching structure of the airways encourages deposition of 2-10 μm particles which can then be cleared by the mucociliary escalator. In the alveoli remaining particles either pass back up the bronchial tree freely or are phagocytosed (a process by which certain living cells called phagocytes ingest or engulf other cells or particles) by macrophages and taken to the mucociliary escalator or to the surrounding lymphatic system. Despite their efficiency, large volumes of particles can overwhelm these defence mechanisms.
Irritation caused by gases and fumes produces inflammation of the respiratory tract and the symptoms tend to be acute (sudden and severe) or delayed, depending on the solubility of the toxic agent. There can also be chronic (ongoing and recurring) effects. Chronic effects from prolonged exposure may be chronic bronchitis or COPD.
Allergic reactions to substances can cause occupational asthma. Symptoms include severe shortness of breath as well as wheezing, coughing and chest tightness. Certain substances such as isocyanates (used in paints), flour dust and various fumes can cause asthma. These substances are called ‘respiratory 20 sensitizers’ or asthmagens. They can cause a change in people’s airways, creating what is known as the ‘hypersensitive state’.
Not everyone who becomes sensitized goes on to get asthma. But once the lungs become hypersensitive, further exposure to the substance, even at quite low levels, may trigger an attack.
Occupational lung diseases are conditions of the respiratory system that have occupational exposure as a risk factor for developing the disease. These diseases may be acute, sub-acute or chronic, and either malignant, non-malignant or infectious in nature.
Pneumoconiosis
The pneumoconioses are a group of interstitial lung diseases caused by the inhalation of certain dusts and the lung tissue’s reaction to the dust. The principal cause of pneumoconiosis is work-place exposure.
Pneumoconiosis diseases may not produce any symptoms for years. However, as the lungs become less flexible and porous their function is greatly reduced. Initially, symptoms include shortness of breath, cough, and general ill feeling. As the diseases progress, shortness of breath, coughing up blood and poor blood oxygenation may occur.
Occupational lung diseases include:
• Aluminosis – pneumoconiosis caused by the presence of dust containing aluminium in the lung tissue.
• Asbestosis, asbestosis induced carcinoma, mesothelioma –diseases caused by inhalation of asbestos.
• Asthma – a condition in which a person’s airways become inflamed, narrow and swell and produce extra mucus, which makes it difficult to breathe.
• Berylliosis (chronic beryllium disease) – pneumoconiosis caused by inhalation of dusts (or vapours) containing beryllium.
• Byssinosis – a pneumoconiosis caused by inhalation of cotton dust.
• Coal workers’ pneumoconiosis (CWP) – pneumoconiosis caused by exposure to respirable coal dust.
• Hard metal pneumoconiosis (hard metal lung disease or HMLD) – fibrotic pneumoconiosis caused by respirable dusts of hard metals such as tungsten, tungsten carbide and cobalt.
• Silicosis – pneumoconiosis caused by exposure to respirable crystalline silica, may lead to lung cancer.
• Talcosis – pneumoconiosis caused by exposure to respirable talc dust.
Safework Australia
Commonly referred to as COPD, this is a group of progressive lung diseases. The most common of these diseases are emphysema and chronic bronchitis. Many people with COPD have both conditions.
Emphysema slowly destroys air sacs in your lungs, which interferes with outward air flow. Bronchitis causes inflammation and narrowing of the bronchial tubes, which allows mucus to build up.
COPD makes it harder to breathe. Symptoms may be mild at first, beginning with intermittent coughing and shortness of breath. As it progresses, symptoms can become more constant to where it can become increasingly difficult to breathe.
Malignant tumours of industrial origin can affect the lungs and surrounding tissues. Lung cancer has been discovered in people working with asbestos (miners, insulators) and this risk is increased by cigarette smoking, arsenic (pesticides), chromium (pigment manufacturers), polycyclic aromatic hydrocarbons (coal gas manufacture, tar workers) and ionising radiation (uranium miners). Wood dust (hardwood furniture makers), leather dust and nickel dust have been directly tied to nasal sinus cancer.
Any person or worker that is required to wear RPE to limit their exposure to workplace airborne contaminants:
• Cuts or drills manufactured stone such as kitchen benchtops
• Blasts, excavates or tunnels into sandstone, clay or granite
• Drills, cuts or chases into concrete and brickwork
• Cuts bricks or tiles dryangle grinds on concrete or masonry
• Jackhammers, scabbles or chisels concrete
• Cleans up the dust and debris created by the above activities, including dried concrete slurry dismantles equipment covered in dust
• Demolishes buildings.
Any person in the health industry that may be exposed to airborne infections includes:
• Medical Staff (doctors, nurses, specialists)
○ Particularly Anaesthetics, Emergency/Trauma, Theatre, Dental, Opthalmology
• Allied Health Staff
• Cleaning and janitorial staff


Two categories of respirators:
1) Air Purifying Respirator (APR) – Removes contaminants before they reach the breathing zone
2) Supplied Air Respirator (SAR) – Provides a source of respirable air which is independent of the working environment.
Air Purifying (APR) Variations:
APR’s work by removing gases, vapors, aerosols (airborne droplets and solid particles) or a combination of contaminants from the air through the use of filters, cartridges, or canisters. These respirators do not supply oxygen from other than the working atmosphere, and therefore cannot be used in an atmosphere that is oxygen-deficient or immediately dangerous to life or health.
• Disposable (filtering facepiece)
• Half face: with filters P1, P2, or P3
• Full face: with filter P3
• Powered Air–Purifying Respirator (PAPR): battery-powered devices that use a blower to pull air through attached filters (for particles) or cartridges (for gases or vapors) to clean it before delivering it to the breathing zone of the wearer. Highefficiency (HE) filters are the only class of particulate filters available for powered airpurifying respirators. The benefits of PAPRs include a low breathing resistance with a high level of protection.
Supplied Air (SAR) Variations:
• Self–Contained Breathing Apparatus (SCBA) For use in situations where the ambient environment is deadly, i.e. under water or highly toxic atmosphere. Provides up to 2 hours of air but can be very heavy to carry.
• Continuous Supplied Air Respirator (airlines or hoses): These are usually long hoses, and air supply is unlimited. The length of the air hose may present issues in certain work environments, where users need to move from one area to another.
• Escape Breathing Apparatus: short term, for emergency evacuations that will not take longer than 15 minutes.
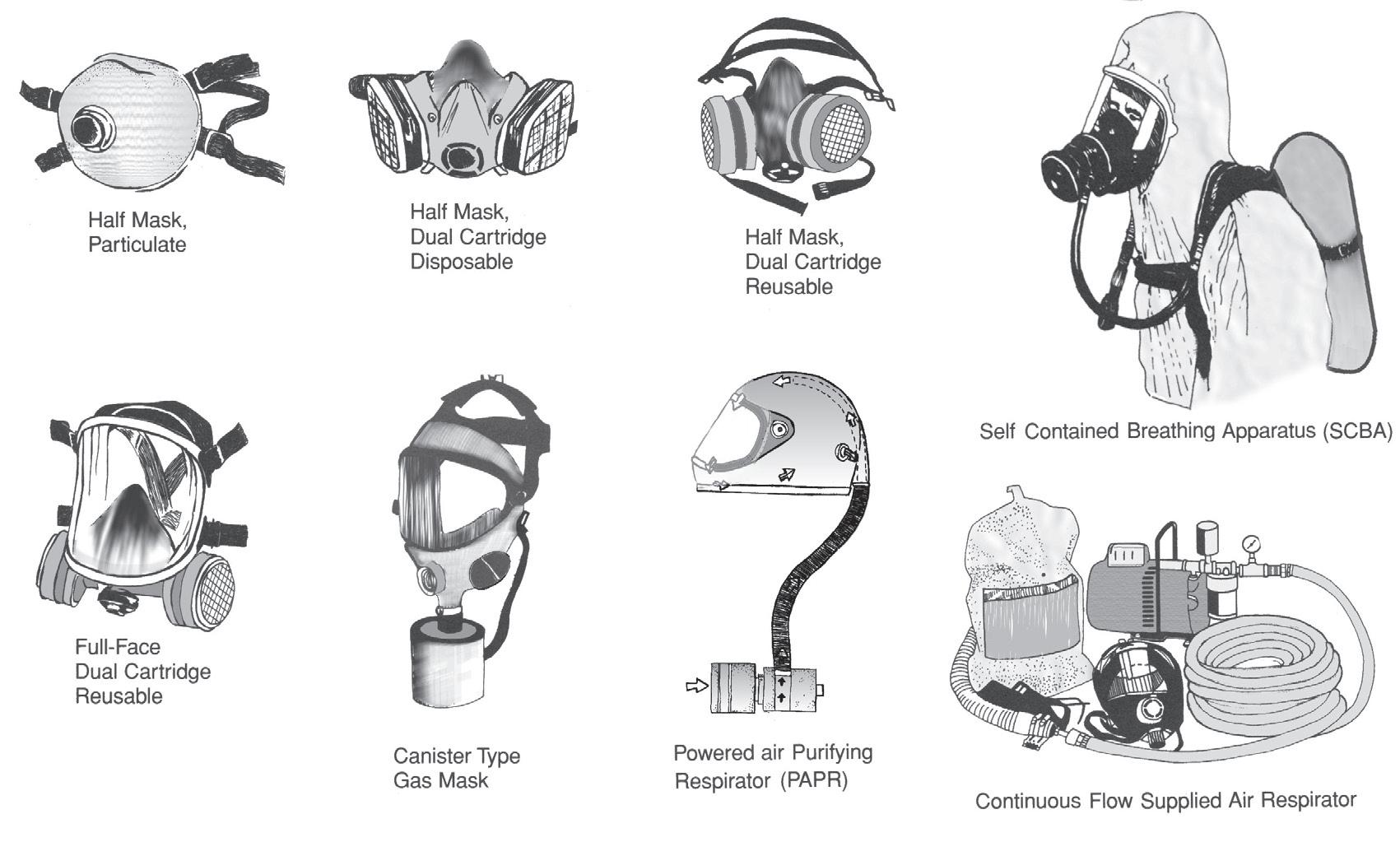
*Air Purifying Respirators (APR)
*Supplied Air Respirators (SAR)
There are two types of filters for air-purifying filters:
1) Particulate Filters that filter out thermally and mechanically generated particulates. The particulate filter is rated according to its efficiency; P1 the lowest efficiency to P3 the highest efficiency. NOTE: Respirator users are advised that an additional gas filter or filters will be required where the process responsible for generating particulates also liberates toxic gases.
a) Class P1 filters : used against mechanically generated particulates, e.g. silica and chrysotile. Three types of Class P1 filter RPE are generally available, i.e. powered type, replaceable filter type and disposable type.
b) Class P2 filters : used for protection against mechanically or thermally generated particulates or both, e.g. metal fumes. Three types of Class P2 RPE are generally available, i.e. powered type, replaceable filter type and disposable type.
c) Class P3 filters : used for protection against highly toxic or highly irritant particulates, such as beryllium. Two types of Class P3 RPE are generally available, e.g. powered type and replaceable filter type. For P3 filter classification, a full facepiece is required for non-powered RPE, and a head covering or full facepiece for a PAPR. When a P3 filter is used in a half facepiece, a protection factor equivalent to a P2 filter is achieved.
2) Gas Filters are used to protect against specific gases and vapours. These filters are coded with letters to indicate the contaminant for which the filter provides protection. See the below table.

Type A – organic vapours
Type B AUS or B1 – acid gases
Type B2 and B3 – acid gases and hydrogen cyanide (HCN)
Type E – sulphur dioxide (SO 2 )
Type G – agriculture chemicals
Type K – Ammonia (NH 3 )
Type AX - Low boiling point organic compounds (below 65°C)
Type NO - Oxides of nitrogen
Type Hg – Mercury
Solvents (with boiling point above 65°C)
Chlorine / sterilization of water, chemical manufacture, hydrogen chloride / chlorinated organic chemical manufacture, steel pickling
Plastics manufacture, gold ore refining, HCN fumigation
SO 2 /casting of metals, bleach manufacture, manufacture of sulphuric acid, fertilizer manufacture, metal cleaning, petroleum refining
Low vapour pressure (below 1.3 Pa at 25°C) organic vapours, pesticide spraying, mixing, manufacture
NH 3 /refrigeration, manufacture of fertilizers, explosives, plastics, low boiling point amines/chemical manufacture
As specified by the manufacturer (e.g. dimethyl ether, vinyl chloride)
Oxides of nitrogen
Metallic mercury/chemical industry, inorganic mercury compounds
Type MB - Methyl bromide Fumigation
Other types
For use against specific chemicals not falling in the above type description as specified by the manufacturer (e.g. hydrogen fluoride)
The mode of air delivery may be either one or a combination of the following:
1) Non-powered : air is drawn through the filter or filters by wearer inhalation. The respirator may consist of a half facepiece with one or more replaceable filters, a filtering (disposable type) facepiece, a full facepiece or head covering with one or more replaceable filters, or a mouthpiece and nose clip, with integral filter.
2) Powered : the contaminated air is drawn through a filter by means of a fan and delivered to the space enclosed by the head covering. This respirator may be a half facepiece, full facepiece or head covering with one or more replaceable filters and an electrically operated blower unit. The air delivery to a wearer may be continuous or on-demand. This is referred to a positive pressure respirator. A low-flow warning device may be fitted to indicate a reduced air supply.
Positive-pressure respirators are special subclass available in both APR and SAR versions, which can in-turn, be either tight- or loose-fitting. They provide high levels of protection by pumping air into the mask at all times rather than relying on the wearer’s lung power to draw air in (as do negative-pressure respirators). Tight-fitting positive-pressure respirators are fit tested in “negative-pressure mode” by temporarily converting them to an APR with appropriate filters. This is done because the effectiveness of the face seal by itself cannot be evaluated properly while the air supply is turned on.
APR and SAR can both be further separated into tight-fitting and loose-fitting styles. Tight-fitting respirators form a tight seal to the wearer’s face and will not provide protection if they do not adequately match the individual’s facial features. Loose-fitting respirators are typically hoods that fit over the entire head and have a neck seal that is not dependent on the wearer’s features.
All tight-fitting respirators must be fit tested .
In order to select appropriate RPE for your workplace, you need to learn how to calculate the level of protection that is required. Let’s look at some of the terminology we will use.
Regulatory agencies set the limits to which workers can be exposed to particular hazards. These are known as “Exposure Standards “. They are numerical values (e.g. milligrams per cubic metre) which represent the exposure levels to which workers may be repeatedly exposed and are regarded as an “acceptable risk”. There are three exposure standards to consider.
1) 8-hour time-weighted average (TWA)
2) Short term exposure limit (STEL)
3) Peak limitation (PEL)
reflects the maximum average exposure a worker can be subjected to without experiencing significant adverse health effects. This may be measured as TWA, STEL, or PEL.
• Time-weighted average (TWA) is a method of calculating a worker’s daily exposure to hazardous substances such as dust, fumes, chemicals, gases, or vapors. It is averaged to an 8-hour workday or 40-hour week, along with the average levels of exposure to the hazardous substance and the time spent in that area.
• Short term exposure limit (STEL) means the time-weighted average maximum airborne concentration of a substance calculated over a 15-minute period. This is the maximum average exposure a worker can be subjected to in a 15-minute period without experiencing significant adverse health effects. Exposures at the STEL must not be longer than 15 minutes and must not be repeated more than four times per day. There must be at least 60 minutes between successive exposures at the STEL.
• Peak Exposure Limit (PEL) means a maximum airborne concentration of a substance determined over the shortest analytically practicable period of time (under 15 minutes)
Note that not all substances are given an STEL or a PEL. Not all chemical substances affect the body in the same way and therefore, some present higher risks to workers than others. The table on the next page gives an example of this. If a substance has a STEL, it must be observed along with the TWA limits.
Where workers have a working day longer than eight hours or work more than 40 hours a week, the person conducting the business or undertaking must determine whether the TWA exposure standard needs to be adjusted to compensate for the greater exposure during the longer work shift, and decreased recovery time between shifts.
Peak limitation or short term exposure limit exposure standards must not be adjusted. TWA exposure standards must not be adjusted (increased) for shorter work shifts.
The following examples are taken from the Safework Australia publication, Workpalce Exposure Standards for Airborne Contaminants
The selection of an appropriate respirator must be done so that the exposure level to the contaminant is reduced to an acceptable level. To understand the contaminant level in the atmosphere an assessment must be undertaken, often by an occupational hygienist. This is called environmental sampling. Once you know the contaminant type and level, you can select RPE.
A protection factor is a measure of how well a mask will filter contaminants. There are several protection factors we must consider when choosing a respirator.
• The Required Minimum Protection Factor (RMPF): is the level of protection required by the respirator to reduce the exposure to an ‘acceptable level’. It is calculated as ambient airborne particle concentration / acceptable exposure level
• Assigned Protection Factor (APF): is the level of protection a respirator will provide that can be expected by 95% of all clean shaven, fit tested and trained wearers on the job. This number is given by the manufacturer of the respirator, and it is used to assist in selecting an appropriate respirator. We multiply the APF by 10 to get the fit factor we wish to achieve. In other words, a respirator with an APF of 10 should have a fit factor of 100 in a fit test. A respirator with an APF of 50 should have a fit factor of 500 in a fit test.
• Fit Factor: is the numerical result produced by a fit test. It is calculated as the number of particles in the ambient atmosphere outside the mask divided by the number of particles inside the mask during a specific set of exercises. It is a score given to determine if a respirator provides a good seal on a user, and is used to match a respirator to an individual. Fit Factor is related to Assigned protection factor in that the required fit factor to pass a mask will be 10x the assigned protection factor.*
• Workplace Protection Factor (WPF): this is the level of protection actually experienced by an individual while working in a hazardous environment. It is expressed as the concentration of REAL ambient hazard outside the respirator divided by the concentration of REAL ambient hazard that leaks into the respirator. WPF’s are usually measured by attaching personal sampling pumps to individuals while they go about their normal work activities. This technique is often used for conducting respirator research.
* This is based on combining filters and masks appropriately, for example, a P3 filter will an APF of 50 will not achieve a fit factor of 500 because it is only a half mask.
Workplace Protection Factor = ambient airborne particle concentration outside the mask divided by particle concentration inside the respirator while working. Requires real-time sampling of respirator in the workplace.
Fit Factor= ambient airborne particle concentration outside the mask divided by the number of particles inside the mask during a specific set of exercises; the result of a Fit Test
The Required Minimum Protection Factor= ambient airborne particle concentration divided by workplace exposure limit.
Remember a passing fit factor is generally 10 times the Assigned Protection Factor of the respirator or filter being tested.
The Workplace Exposure Standard limit for respirable crystalline silica is a Time Weighted Average of 0.05mg/m3
In Workplace X, the current exposure level is 0.45mg/m3.
We calculate the Required Minimum Protection (RMP) by dividing the Current Exposure Level (CEL) by the Workplace Exposure Standard’s limit (WES).
0.45/0.05 = 9
Required minimum protection for this workshop is 9. Therefore, a P2 mask, which has an Assigned Protection Factor of 10, will provide adequate protection. The fit factor we would need to achieve on a fit test is 100.
When looking at protection against micro-organisms such as viruses and bacteria, it is difficult to provide a protection factor as it may not be known what level of exposure is harmful for an individual. Different microorganisms may have a different level of risk, the infectious dose and the individual susceptibility may be varied. In general, N95 masks (P2) are considered to provide adequate protection against microorganisms. This is supported by recent research.
The filtration efficiencies against aerosols containing two different types influenza virus particles (H1N1 and H5N1) were measured for five different models of 3M filtering facepiece respirators. Testing was conducted at the University of Nebraska Medical Center in collaboration with 3M.3638 The results indicate filtering efficiency above 95%, but just below 99%, against an aerosol containing H1N1 virus particles.
The products tested included respirators with NIOSH N95 approval (US) and with EN149 FFP2 and FFP3 approvals (Europe).
36. Lore, M.B., Sambol, A.R., Japuntich, D.A., Franklin, L.M. and S.H. Hinrichs, Inter-laboratory performance between two nanoparticle air filtration systems using scanning mobility particle analyzers. Journal of Nanoparticle Research. 13:15811591; 2011.
37. Lore, M.B., Sebastian, J.M., Brown, T.L., Viner, A.S., McCullough, N.V. and S.H. Hinrichs, Performance of Convential and Antimicrobial-Treated Filtering Facepiece Respirators Challenged with Biological Aerosols. Journal of Occupational and Environmental Hygiene. 9(2):69-80, 2011.
38. Hinrichs, S.H., Lore, M.B. and Brown, T.L. Filtration Peformance of Five Respirator Models when Challenged with H1N1 and H5N1 Influenza Virus Aerosols. 20 October 2010 Report from University of Nebraska Medical Center to 3M Company. 2010.
Several factors need to be considered when selecting RPE as noted within the Australian Standards.
A respiratory protection program includes the following components:
1) Contaminant: level of toxicity, physical form, concentration. Generally, you protect against particulate hazards with a filter and against gas and vapours with a cartridge. If both types of hazards are present, combination cartridges are an option that can filter out both particles and gas or vapours. Also consider flammability of contaminant, whether the contaminant is an eye or skin irritant, or if device failure would be immediately life-threatening.
2) Worker: physical and psychological factors, which will be identified in a medical screen.
3) Work Task: physical aspects, i.e. effort causes faster breathing, or sudden body movements which could affect fit; need for other PPE; vision or communication requirements
4) Work Environment: temperature of environment, or being near flammable substances
5) Equipment Limitations: Does it protect skin, eyes? Is there an air hose or a tank with a limited amount of air? Is it too heavy to wear for any length of time?
6) Special Response to HAZMAT Incidents: These may require further risk assessment and specialized equipment as hazards may include radiation or deadly toxins. It is likely that the respiratory equipment is just one part of the PPE that may be required.
Below is a summary of the information required to make decisions about RPE:
1) Understand the hazard
a) Particulate?
b) Gas or Vapour?
c) Biological?
d) Combination?
2) Understand the exposure
a) Are you certain of the exposure levels?
b) Are they outside the Workplace Exposure Standards?
c) Do you need to assess the environment?
3) Know the level of protection required
a) What level of protection is needed to bring the exposure to below the acceptable level?
4) Select the appropriate respirator
a) Does it meet Australian Standards?
b) Can it be used with the other PPE required?
c) Can one model be used for all individuals?

Whether you work in industry or healthcare, your business is responsible for creating and ensuring a safe workplace, as much as is practicable.
There is extensive legislation in Australia to guide businesses as they manage WHS in the workplace.
The below is a list of the legislation, standards, codes and guidance documents that are in place to manage and outline workplace health risks.
• Work Health and Safety Act 2011
• Work Health and Safety Regulation 2011 (particularly Chapter 3 – General risk and workplace management and 7.1 – Hazardous Chemicals)
• AS/NZS 1715:2009 Selection, use and maintenance of respiratory protective equipment
• Silica and the lung—fact sheet
• Safe Work Australia Managing Risks of Hazardous Chemicals in the Workplace Code of Practice 2012
• Managing risks of hazardous chemicals in the workplace Code of Practice 2013
• Foundry Code of Practice 2004
• Abrasive blasting Code of Practice 2013
• Excavation work Code of Practice 2013
• Silica – Technical guide to managing exposure in the workplace – Work-related Disease Strategy 2012 – 2022
Clinical guidelines provide information on general principles of respiratory protection for healthcare workers and are formally aligned with following legislation and standards.
Australian/New Zealand Standards:
• Standards Australia AS 4381:2015 - Single-use face masks for use in healthcare
• Standards Australia AS/NZS 1715:2009 - Selection, use and maintenance of respiratory protective equipment
• Standards Australia AS/NZS 1716:2012 - Respiratory protective devices
National Safety and Quality Health Service Standards:
• Standard 3 - Preventing and Controlling Healthcare Associated Infections Criterion 3.7.1requires infection prevention and control consultation regarding policies and procedures that address personal protective equipment.
Australian Guidelines for the Prevention & Control of Infection in Healthcare (2019):
• Recommends that where there is a high probability of airborne transmission due to the nature of the infectious agent or procedure then a correctly fitted P2/N95 respirator should be worn
Principles for the Management of Tuberculosis in New South Wales PD 2014:
• Tuberculosis (TB) Services are required to operate in accordance with this policy in conjunction with the current relevant guidelines for the prevention and control of tuberculosis in NSW, which reflect best practice for the clinical and public health management of TB.
Note
Each state and territory will have its specific legislation that must be read in conjunction with the AS and ISO standard.
AS/NZS 1715:2009 & ISO 16975-3
These two Standards are referred to regularly in Australia as guidance for the development of a Respiratory Protection Program in a workplace.
• AS/NZS 1715:2009 Selection, Use and Maintenance of Respiratory Protective Equipment
• International Standard ISO 16975-3, Respiratory protective devices – Selection, use, and maintenance – Part 3: Fit- testing procedures
Risk management refers to the steps that a business and its employees should take to reduce or eliminate safety risks in their workplace.
The hierarchy of control is a system for controlling risks in the workplace. It is a step-by-step approach to eliminating or reducing risks and it ranks risk controls from the highest level of protection and reliability through to the lowest and least reliable protection. The hierarchy consists of hazard control measures broadly grouped into five categories.
Eliminating the hazard and risk is the highest level of control in the hierarchy, followed by reducing the risk through substitution, isolation and engineering controls, then reducing the risk through administrative controls. Reducing the risk through the use of protective personal equipment (PPE) is the lowest level of control and is also considered the least reliable.
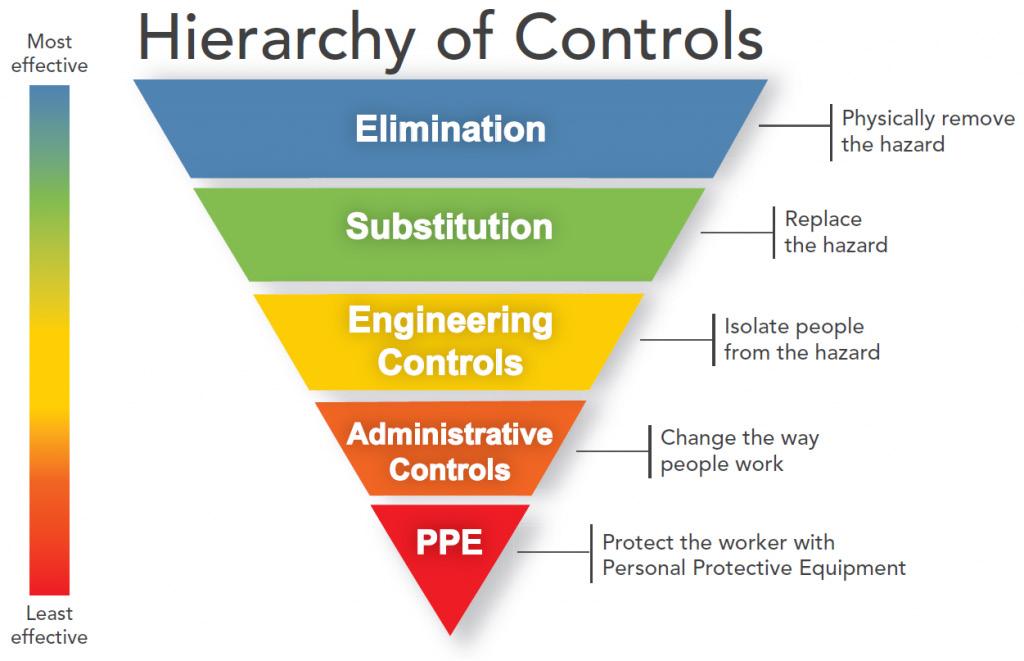
Elimination
• Ensure well-designed work premises and processes. Adopt production processes that generate less dust.
• Treat the dust at its point of generation.
• Have plant equipment that is automated / remote controlled if possible. Substitution
• Substitute with a less hazardous substance, such as the use of pellets rather than powders, or replace sand with garnet as abrasive blasting agent.
• Substitute chemicals and other hazardous substances for less hazardous types, where practicable.
Engineering controls
Administrative controls
• Install effective local exhaust ventilation
• Provide plant with enclosed cabins that filter out air contaminants and are air-conditioned
• Place a physical barrier between the dust-generating task and the worker, such as the use of enclosed cabins to isolate workers.
• Use remotely operated machinery, such as remotely controlled conveyors.
• Install effective local exhaust ventilation
• Provide plant with enclosed cabins that filter out air contaminants and are air-conditioned
• Housekeeping and signage
• Restricting time of exposure, rotation of staff away from dusty areas
• Air quality monitoring
• Safe work procedures and inspections
• Limit eating and drinking
• Vaccination programs and health checks
• Employee training in identifying and managing the risk
Respiratory Protective Equipment
• Select the most appropriate RPE. Available in range from simple P1 or P2 disposable half-face respirator to powered air purifying respirator (PAPR) and air supplied positive pressure demand respirator.
• Minimum required protection factors may need to be determined for correct respirator selection. See AS/NZS 1715 or WHSQ’s silica documentation for further assistance.
• Ensure training, comfort and fit testing are provided before use. Respirators should not be shared. Maintain and store respiratory protective equipment properly.
• Applicable for short term applications. Suitable for all emergency applications, useful when higher order controls cannot fully control the risk.
With regards to the development of a Respiratory Protection Program in a workplace, a significant guide is found in Standards Australia AS/NZS 1715:2009. Selection, use, and maintenance of respiratory protective equipment . This Standard outlines the requirements for fit testing and the creation of a Respiratory Protection Program for a workplace.
One of the best ways to prevent occupational exposure to airborne contaminants is to use a respirator. However, a respirator is only as effective as its seal. Users of respirators must be medically assessed, trained, and fitted to a respirator, as well as taught how to maintain one. In order to ensure that respirators are used correctly in a workplace, a business should have a documented Respiratory Protection Program. A Respiratory Protection Program is a core component of ensuring respiratory safety in a workplace.
Standard AS/NZS 1715:2009 provides a clear outline of the requirements for a proper Respiratory Protection Program. While following this guideline is not required by legislation, Industrial Standards are considered to be “best practice” and a strong indication of a business’s commitment to creating a safe work environment.
According to AS/NZS 1715:2009, a Respiratory Protection Program must include the following components:
1) Appointment of a Program Administrator : This person should have the background and training to enable them to make sound decisions based on an understanding of workplace hazards. Ideally, they should be a WHS professional.
2) Selection of RPE : This process will include considerations of contaminants, operator task requirements, including use of other PPE, communication, vision and comfort.
3) Medical screening of users of RPE : All users of RPE should be screened for conditions that preclude the safe use of RPE.
4) Training : Training must be provided in the safe use and limitations of RPE and should be conducted at least annually.
5) Issue of RPE : Where practicable, RPE shall be for a wearer’s exclusive use. RPE should bear identifying marks.
6) Fitting of equipment : Facial fit tests are required to establish proper fit of RPE.
7) Wearing of RPE (where required) : The RPP shall require that RPE is used properly and worn at all times when required.
8) Maintenance of RPE : Maintenance shall include cleaning and disinfection, inspection, repair, and storage or disposal. All should be done according to manufacturer instructions.
9) Disposal of equipment : Where legislative requirements exist or there are relevant manufacturer guidelines for appropriate disposal, these should be followed.
10) Records for a respiratory protection program : These should include user records, issue of RPE, maintenance and procedural records.
11) Program evaluation : Annual audits are recommended, including a review of the risk assessment, and the results of any biological or atmospheric monitoring.

Using a respirator may place a physiological or psychological burden on employees. The impact of that burden varies with the type of respirator worn, the job and workplace conditions in which the respirator is used, and the medical status of the employee. It is recommended that an initial medical assessment is undertaken to establish if a person is able to wear a respirator. Some factors will preclude the use of RPE entirely.
Physiological considerations
• Chronic lung conditions, i.e. emphysema or asthma
• Circulatory diseases
• Seizure disorders
• Ability to support the weight of the respirator
Psychological considerations
• Claustrophobia
• Isolation
• Anxiety Example Medical Screen https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppC
Factors which can affect the quality of the seal on a respirator:
• Facial hair
• Scars
• Dental issues, i.e. dentures, missing teeth
• Piercings
• Facial characteristics such as high cheekbones, broken nose, hollow cheeks.
• Wearing Glasses
• Other PPE requirements
AS/NZS 1715:2009 Selection, Use and Maintenance of Respiratory Protective Equipment
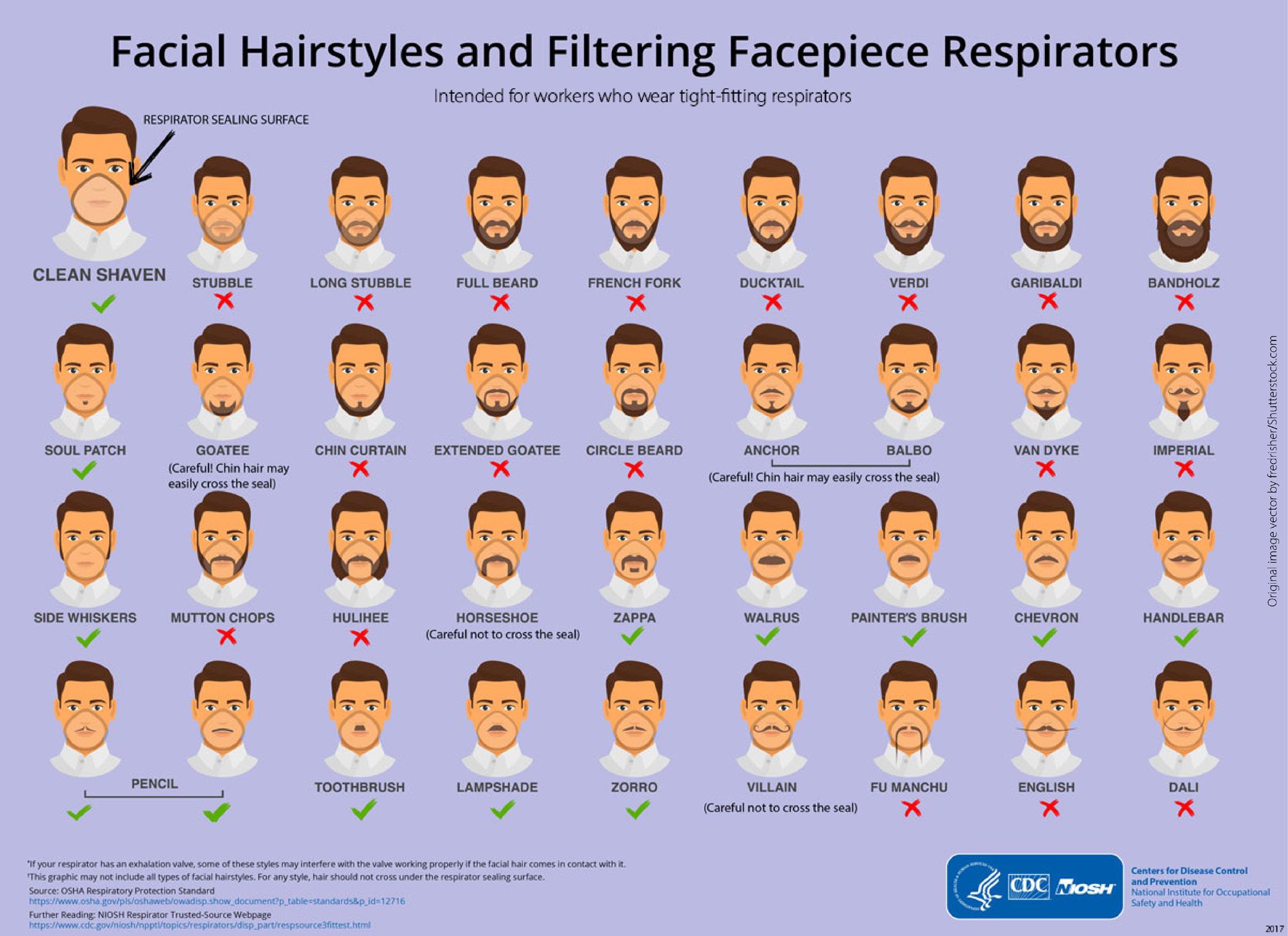
Tight-fitting respirators need to seal securely to the face to create a reliable barrier. Gaps in this face seal let contaminated air inside the respirator. Even a day or two’s growth of stubble can be enough to significantly interfere with respirator fit and create gaps that reduce the protection a respirator can provide. Face seal leakage has been shown to increase from 20 times to 1000 times in the presence of facial hair. Facial hair that does not come between the respirator surface and the skin is not a problem, i.e. a trimmed moustache.
Acceptable Facial Hair Styles
Other PPE such as safety or prescription glasses, hair nets, hard hats and hearing protection are often required to be worn concurrently with a respirator. This can affect the effectiveness of RPE in the following ways:
• Interference with the respirator seal
• Augmentation of physiological factors like body temperature
• Increase in psychological factors such as feelings of claustrophobia
It is important that ALL required PPE be worn during fit testing in order to assess the respirator properly, considering their job role and working environment.
Select RPE for testing that is representative of the RPE used in the workplace. Test subjects may bring their own respirators to be tested, if needed. Ensure proper adapters are available when this is the case.
When using quantitative fit test methods, you must adapt the RPE to be attached to a sample hose. Reusable respirators must be fitted with adapters, before attaching the filter. The filters used for fit testing may be different than the ones used in the workplace. Be aware that the weight of filters and adaptors can affect the fit. Ensure that probes or adapter hoses are placed directly in the breathing zone between nose and mouth.






The RPE user must first be trained in donning and doffing a respirator. After training, they must be given opportunities to practice. They must be able to demonstrate that they can competently don the selected RPE, in accordance with the manufacturer’s instructions.
Prior to the fit test, the last donning must be done unassisted by the wearer. This is very important, as it ensures that the wearer will be able to properly don the respirator unassisted in the work environment.
a filtering face piece




Donning a reusable half-face respirator




The following RPE are suitable for using the negative pressure fit check:
a) Disposable respirators
b) Reusable face-piece
For a disposable face-piece, the wearer completely covers the filter with both hands or a non-permeable substance, e.g. a polythene bag, and inhales sharply. The face pieces should sink onto the face with a very vigorous breath indicating an adequate seal. If an unsatisfactory face seal is indicated by the feel of an airstream channelling through the leak, re-adjust the RPE until a satisfactory seal is indicated.
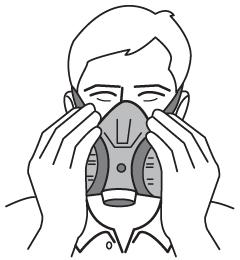
On reusable masks, the user should cover both filters and inhale gently so that the face piece collapses slightly. If the face piece remains slightly collapsed and no inward leakage is detected, the RPE is probably well fitted.
Wear must block the exhalation valve on a half or full face-piece respirator or fully cover the respirator surface on a filtering face-piece, usually by using your hands, and trying to breathe out. If slight pressure builds up, that means air isn’t leaking around the edges of the respirator.
The negative pressure check and the positive pressure fit check should be used each time a respirator is worn but should not be considered reliable fit-tests. They cannot be used in place of a fit test . The wearer should use these fit checks every time, just before entering the hazardous atmosphere. These tests are only suitable for respirators with tight-fitting facepieces.
Although these tests are simple, they have significant drawbacks, the main one being that the wearer may touch the RPE after it has been positioned on the face, possibly modifying the facial seal. Remember, the quality of a user fit check depends on the skill and effort of the user.
According to AS/NZS 1715:2009, fit testing should be conducted annually (recommended minimum) and at additional times when required:
• Before the respirator is issued
• User reports discomfort or suspects poor fit
• When there is a change in the wearer’s facial characteristics:
○ Weight loss or gain;
○ Substantial dental work;
○ Any facial changes (scars, moles, effects of aging, etc.) around the face seal area;
○ Facial piercings;
○ Introduction or change in other head-worn personal protective equipment (PPE);
○ There is a newly identified health issue related to exposure, i.e. a blood test that indicated excess exposure.
There are safety considerations to take into account when conducting fit tests. Awareness of these issues will help you anticipate and prevent any unnecessary risk.
• Test subjects may experience unexpected distress wearing a respirator
• Test subject may experience an allergic reaction to test aerosols.
• A fit-test hood will elevate inhaled carbon dioxide levels and decrease inspired oxygen levels. Ensure test subjects are not experiencing dizziness or headache.
• Test subjects may feel too hot or experience other distress, such as claustrophobia.
Review safety data sheet (SDS), depending on the chemical being used. Understand how to recognise potential reactions to the chemical. If you suspect a subject is having a reaction or is experiencing dizziness or distress, stop the test and remove the hood and RPE.
• Test subjects may experience unexpected distress wearing a respirator
• Risk of tripping over cords
• Alcohol is flammable
• Risk of electric shock
• Placing probes must be done with care to avoid pinches or cuts
• Equipment should be placed with ergonomic considerations in mind, so testers are not leaning over a low desk while conducting tests.
Ensure your initial setup considers ergonomics and limits trip hazards. After refreshing the wick, close and remove sources of alcohol. Conduct regular equipment checks for frayed or damaged electrical cords. Ensure operator is familiar with operations of probe setter tool.
It is imperative that relevant infection control procedures are followed throughout the fit testing process. This includes minimising contact between staff, appropriate donning/doffing procedures, cleaning and disposal protocols for equipment.
The program administrator should ensure that current infection control protocols are observed in maintaining any reusable RPE and testing equipment.
Disinfection may be achieved by using a broad-spectrum disinfectant. The choice of preparation should be made based on recommendations of the RPE manufacturer and medical authorities. Such information should also assist where protection against the transmission of a specific pathogen is required.
With all disinfectants, particular attention should be paid to the manufacturer’s instructions regarding their use, e.g. dilution, temperature, exposure time.
Benchtops and desks:
• All surfaces where the fit testing equipment and RPE will be placed such as benchtops, desks, and tables must be clean and sanitised before commencing a fit test.
• Fitting of a disposable absorbent cloth that covers the table may also be required if the surface is damaged or shows signs of absorbed contamination i.e., stains, etc.
Washing Hands:
• Both fit testers and participants should wash their hands properly prior to engaging in fit testing. Participants should be asked to avoid touching their face during testing. Testers should wash or disinfect hands every time they touch the wearer or their facepiece. Disposable gloves should be worn by the fit tester during the tests and when disinfecting the masks after a test has been completed.
• Disposable gloves must be changed for each test being conducted.
Fit testing hood (QLFT-ATT Only):
• A visual inspection of the hood must be conducted when setting up for fit testing and between fit tests to check for any contaminants and defects.
• Wipe down the hood with a disposable sanitising wipe when preparing for a fit test, between fit tests, and at the end of the day.
• On completion of fit testing, the hood should be adequately cleaned and stored in a sealed bag or container to minimise contamination when stored.
Twin tubing, (QNFT-CNC only):
• A visual inspection of the twin tubing must be conducted when setting up for fit testing and between fit tests to check for any contaminants and defects.
• Wipe down with a disposable sanitising wipe or other suitable cleaning equipment when preparing for a fit test and between fit tests.
• During fit testing, condensation can build up in the twin tubes. If condensation build-up becomes an issue, it may require changing with another clean tube assembly.
• On completion of fit testing, the twin tube should be adequately cleaned and stored in a sealed bag or container to minimise contamination when stored. Do not store twin tubing with moisture inside. TSI, manufacturer of the PortaCount, does not recommend disinfecting of the interior of the tubes between use, however, if you choose to disinfect the tubing, you should rinse the tubing with distilled water.
Adaptors and sample tubes for re-usable masks (QNFT-CNC only):
• A visual inspection of the adaptor must be conducted when setting up for fit testing and between fit tests to check for any contaminants and defects.
• The adaptor and sample tube (the tube from the adaptor to the breathing zone) must be cleaned and sanitised when preparing for a fit test.
• Between fit tests, the adaptor and sample tube must be inspected and sanitised before connecting it to another mask. This includes cleaning and sanitising the suction cap used to hold the sample tube in place during the testing process, if applicable.
Use only clean and disinfected reusable RPE when fit testing. No test subject should don a reusable mask that has not been cleaned and disinfected.
Practice good hand and cough hygiene as per Covid 19 protocols. Fit tests should never be conducted on users who are feeling unwell or appear symptomatic of any respiratory or other illness.
A Fit Test Protocol is the term used to describe the specifics of fit test requirements as per each region’s industrial Standard. These fit test protocols set the fit test frequency, minimum fit factor (pass level) for both half and full-face masks, and they state whether a fit test would be acceptable on a positive pressure mask (converted to negative pressure mode for testing).
Each Standard will have its own test protocol, including what exercises must be completed during the test. These exercises are designed to assure scientifically valid results, when testing the seal on a respirator. The exercise protocols are designed to be used with either fit test method, qualitative or quantitative. All fit tests must be conducted in negative pressure mode, regardless of the type of (tight-fitting) respirator. Businesses should chose the best protocol based on their industry and work task requirements.
In Australia, businesses will commonly refer to these international Standards:
• OSHA 9CFR1910.134 Respiratory Protection Standard
• ISO 16975-3 - Respiratory Protective Devices - Selection, Use And Maintenance - Part 3: Fit Testing Procedures
• ANSI Z88.2-1992 Respiratory Protection with ANSI Z88.10-2001 Respirator Fit Test Methods
• AS/NZS 1715:2009 Selection, use and maintenance of respiratory protective devices
Each protocol’s variations make that protocol more or less appropriate for a business to use when fit testing. For example, if a fit test is performed using the OSHA1910.134 – Appendix A, all exercises are to be performed with the wearer standing on their feet (or jogging). This is a good choice where the worker spends most of their time at work on their feet.
The exercises in each protocol are designed to challenge the seal of the respirator by mimicking the actions that a worker may be required to do while carrying out their normal work tasks. One protocol allows the test to be conducted under higher physical demand (on a treadmill or exercise bike), while another allows a tester to add in elective exercises which may be more job specific. This allows the respirator to be tested dynamically, and to get results that can be assumed to be closely representative of using the respirator in the workplace.
It is important to follow the chosen protocol accurately. If there is a deviation away from instructions contained within the protocol, a tester is unable to consider fit test results to be reliable – irrespective of the method chosen (QLFT – ATT, QNFT – CNC, QNFT – CNP).
See the table below to compare each of the protocols and their test exercises.
Standard/Regulation Test Exercises
OSHA 9CFR1910.134
1) Normal breathing (1min)
2) Deep breathing (1min)
3) Turning head side to side (1min)
4) Moving head up and down (1min)
5) Talking (Rainbow Passage) (1min)
6) Grimace (15s)
7) Bending over (1min)
8) Normal breathing (1min)
OSHA 9CFR1910.134 FAST Half/Full Face:
1) Bending over (50s)
2) Jogging In Place (30s)
3) Turning head side to side (30s)
4) Moving head up and down (39s) FAST Filtering Facepiece:
1) Bending over (50s)
2) Talking (Rainbow Passage) (30s)
3) Turning head side to side (30s)
4) Moving head up and down (39s)
QNFT-CNP QNFT-CNP Redon Exercises:
1) Facing forward (30s)
2) Bending Over (30s)
3) Head shaking (3s several times)
4) Redon 1
5) Redon 2
Bonus Exercises
None
None
Standard/Regulation Test Exercises
ISO 16975-3
QLFT:
1) Normal breathing (1min)
2) Deep breathing (1min)
3) Turning head side to side (Sitting or standing, 1min)
4) Moving head up and down (1min)
5) Talking (Rainbow Passage) (1min)
6) Jogging In Place (1min)
7) Normal breathing (1min)
QNFT:
1) Normal breathing (1min)
2) Deep breathing (1min)
3) Turning head side to side (Sitting or standing, 1min)
4) Moving head up and down (1min)
5) Talking (Rainbow Passage) (1min)
6) Bending over (1min)
7) Normal breathing (1min)
Bonus Exercises
None
Standard/Regulation Test Exercises
ANSI Z88.10-2010
QLFT – ATT
1) Normal breathing (30s)
2) Deep breathing (30s)
3) Turning head side to side (30s)
4) Moving head up and down (30s)
5) Talking (30s)
Elective exercises: Bending over (30s), Jogging in place (30s), Stepping (30s), Vigorous head shake (30s)
QNFT – CNC
1) Normal breathing (30s)
2) Deep breathing (30s)
3) Turning head side to side (30s)
4) Moving head up and down (30s)
5) Bending over (30s)
6) Talking (30s)
Elective exercises: Re-don + normal breathing (30s), Jogging in place (30s), Stepping (30s), Grimace + normal breathing (30s), Vigorous head shake (30s)
QNFT – CNP
1) Normal breathing (30s)
2) Re-don + normal breathing (30s)
3) Bending over (30s)
4) Vigorous head shake (30s)
Elective exercises: Deep breathing (30s), Turning head side to side (30s), Moving head up and down (30s), Stepping (30s), Grimace + normal breathing (30s)
Bonus Exercises
Yes (electives + job specific exercises)
Standard/Regulation Test Exercises
INDG479
1) Normal breathing (1min)
2) Deep breathing (1min)
3) Turning head side to side (Sitting or standing, 1min)
4) Moving head up and down (1min)
5) Talking (Rainbow Passage) (1min)
6) Bending over (1min)
7) Normal breathing (1min)
QLFT: fit test exercises should be performed with the wearer standing.
QNFT: fit test exercises (except for the bending exercise) should be performed while the wearer is:
1) cycling on an exercise bike;
2) walking on a treadmill; or
3) carrying out a stepping exercise.
Bonus Exercises
None
In addition to providing specific test protocols, each Standard also has specified the fit factor that must be achieved for a respirator to pass a fit test. The reason that qualitative fit test methods are limited to a fit factor of 100 is because 100 is the pass level these methods were designed for in the laboratory. This is based on the difference in the concentration of the sensititivity solution to the test solution All legitimate qualitative methods were carefully developed in a laboratory and validated against quantitative method instrumentation.
Full-face masks should not be tested using the Qualitative method, as that method cannot provide a fit factor above 100.
The accepted Standards also state that 100 is the maximum fit factor for all half-masks, (disposable or reusable) when using Quantitative testing. These Standards diverge on the fit factor required to pass a full-face mask(see table above). Some will require a fit factor of 500 while others set the pass level at 2000.
Why doesn’t somebody develop and validate a qualitative fit test method for higher pass levels?
The reason is related to human sensitivity to the chemical stimulants used in this method. For a qualitative fit test method to be validated for 100, the test subjects must be able to detect reliably a challenge agent concentration inside the respirator that is 100 times less than the challenge agent concentration outside the respirator.
Properly designed qualitative protocols include a threshold sensitivity test that each person must pass prior to being fit tested to ensure that he or she can detect the very low in-mask concentration that results when the fit factor is just below 100. If a person cannot pass the threshold test, that qualitative fit test method cannot be used.
Most people have the ability to sense 1/100 concentrations, but the percentage of people who can reliably sense lower concentrations such as 1/500 or less drops off quickly.
Because of this, it is unlikely that a qualitative fit test method will ever be developed for a pass level of 500 or higher.
Remember, disposable filters and filtering face pieces will have an Assigned Protection Factor (APF): which is the level of protection a respirator will provide that can be expected by 95% of all clean shaven, fit tested and trained wearers on the job. If the APF on a filtering face piece is 10, the highest fit factor you can achieve is 100, if the APF is 50, the minimum fit factor you would test for would be 500.
However, no Standard will recognise a fit factor above 100 for anything less than a full-face mask. Even though a half-mask may have a P3 filter with an APF of 50 or 100, the fit factor achieved will never be higher than 100.
Standard/Regulation Issuing organization Fit test frequency
OSHA 9CFR1910.134
Respiratory Protection Standard
ISO 16975-3 - Respiratory Protective Devices - Selection, Use And Maintenance - Part 3: Fit Testing Procedures
ANSI Z88.2-1992
Respiratory Protection with ANSI Z88.10-2001 Respirator Fit Test Methods
NFPA 1500-2007 Fire Department Occupational Safety and Health Program
Safety and Health Administration (USA)
(the International Organization for Standardization)
National Standards Institute (USA)
Fire Protection Association (USA)
NFPA 1404-2006 Standard for a Fire Department SelfContained Breathing Apparatus Program National Fire Protection Association (USA)
CSA Z94.4-02 Selection, Use, and Care of Respirators
INDG479 Guidance on Respiratory Protective Equipment Fit Testing
AS/NZS 1715:2009
Selection, use and maintenance of respiratory protective devices
Standards Association (Canada)
Executive (UK)
EN529:2005 Respiratory protective devicesRecommendations for selection, use, care and maintenance - Guidance document European Committee For Standardization, 36 B-1050 Brussels
fit factor for halfface masks
fit factor for fullface masks QLFT allowed for positivepressure masks
A quantitative fit test is done using very precise measuring equipment. The TSI PortaCount Fit Tester is one such piece of equipment and will be the unit we refer to in this course, although other Condensed Nuclei Counter units are available. This machine can sample the air outside a respirator, (the ambient atmosphere), and the air inside a respirator, which the user is breathing, and compare the number of particles in each. Before we go any further, we need to understand how particles are counted by the PortaCount Fit Tester.
Particle is a generic term used to describe airborne solid or liquid substances in the finely divided state ie, particulate aerosols such as dusts, mists, fumes, fibres and fog as well as microorganisms.
The PortaCount Fit Tester is a condensation particle counter. That means it can count the particulate in the atmosphere both inside and outside a respirator. The working principle of a particle counter is to expose aerosol particles to supersaturated vapor, in this case, reagent-grade isopropyl alcohol, which condenses onto the particles, allowing them to grow to sizes at which they can be optically detected. The alcohol in the PortaCount Fit Tester is delivered by a wick inside an alcohol cartridge. Do not substitute any other alcohol in the PortaCount Fit Tester. Do not use isopropyl alcohol that is less than 99.5% pure.
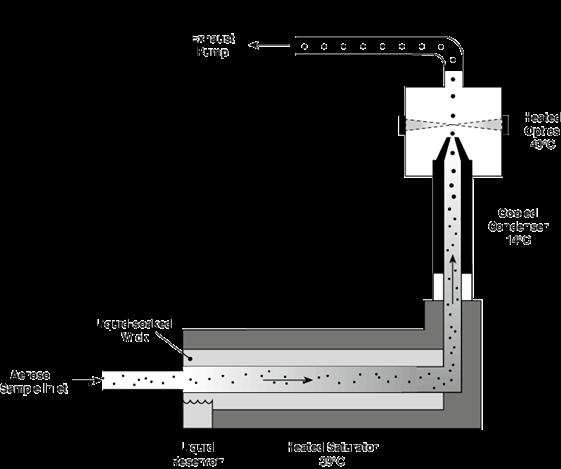
TSI, the manufacturer of the PortaCount Fit Tester, specifies that a minimum ambient concentration of 1000 particles (pt)/cm 3 is needed to properly conduct a fit test. However, when testing with a filter that is <99% efficient, such as P2, or N-95 series masks, the ideal ambient particle concentrations is 100 to 800 pt/cm 3 . The PortaCount Fit Tester has an accessory that can be used to view only a certain size range of particles, called the N95-Companion. This accessory is especially important when working with a particle generator, because the particles have such a wide size range. It is activated in the software when fit testing.
The N95-Companion is an aerosol pre-conditioner that selects particles in a specific size range and passes them on to the PortaCount Fit Tester. Any particles that are not in that size range are discarded, (not counted) because they could incorrectly be counted as seal leakage. Some generated particles are so small, they can penetrate a filter that is <99% efficient, , so we want to screen them out of the actual test. The ambient particle count range you should see when working with the N-95 Companion is therefore much lower.
Ideal ambient particle concentrations is ~100 to 800 pt/cm 3 with N95 Companion running or ~2,000 to 8,000 pt/cm 3 without N95 Companion running. If ambient particle count is too high, it can affect the fit factor results, skewing them towards false positive test results.
In some testing environments, the ambient particle count is very low, because of excellent air filtration, or it is a naturally clean environment. In this case, a particle generator can be used to create an artificial atmosphere. When using a particle generator, testing should be conducted in a room no larger than 7m x 7 m. Placement of the particle generator should be no closer than 2 metres to the PortaCount Fit Tester.

In environments with naturally occurring (no particle generation in use) ambient particle concentrations exceeding 8,000 pt/cc (as per use with full-face, P3 filter fit testing) or 800 pt/cc (as per use with <99% filter efficient masks or half-face masks with a p2 filter, fit testing) you may need to increase the fit test protocol purge times. The purge time allows the PortaCount fit tester to clear the tubing, before counting the particles within the mask. If ambient particle concentration is too high, it may cause failing fit factors. Additionally, concentrations above this may cause the PortaCount Fit Tester to require more frequent cleaning and calibration.
During a fit test, the machine will sample the ambient atmosphere and count the particles that are in the air, (C out). It will also sample the air inside the respirator and count the particles, (C in).
The calculation, simplified, is C out/C in = fit factor
In reality, this calculation is a bit more complex. The machine will sample the ambient air in two moments, which we will call Moment A and Moment B, and average the two to achieve C out. It will sample the air in the mask in between those two moments, to get C in. However, the flow rate is calculated over a specific time period, 1 second, 15 seconds or 40 seconds.* That means the particle count for C in can be below one (1) but above zero, (0). With a 40-second mask sample (factory setting) the minimum measurable concentration is 0.015 particles per cm3.
*for a more detailed explanation of calculating particle concentration, see Appendix B of this guide.
The following example fit test consists of 4 exercises and each (C out) and (C in) sample will be averaged to obtain the fit factor of the respirator.
Formula: C out avg / C in
Exercise 1:
• C out 1 = 135
• C in = 0.92
• C out 2 = 145
Fit factor 1 = 140/0.92 = 152.173
Exercise 2:
• C out 1 = 160
• C in = 0.02
• C out 2 = 140
Fit factor 2 = 150/0.02 = 200 (max fit factor)
Exercise 3:
• C out 1 = 120
• C in = 1.27
• C out 2 = 136
Fit factor 3 = 128/1.27 = 100.787
Exercise 4:
• C out 1 = 135
• C in = 2.5
• C out 2 = 140
Fit factor 4 = 137.5/2.5 = 55
Fit Factor 1 + Fit Factor 2 + Fit Factor 3 + Fit Factor 4 = 507.96
507.96/4 = 126.99 Fit Factor for the complete test.
Once all the exercises in a protocol are completed, the PortaCount will average each fit factor and divide by the number of exercises to get a final Fit Factor and call the test a Pass or Fail. So you can see that even though we did not get a fit factor of 100 on exercise 4, the mask still passed overall.
If the PortaCount does not detect any particles at all for C in during all four samples taken during the test, it will assign a count of 1, to allow a calculation. In that case the calculation would look like this:
• Average C out = 138.875
• Average C in = 1
• Fit Factor = 138.875
The PortaCount will assign a maximum fit factor of 200+ for any exercise. This allows the exercise averages to pass a mask even if some exercise results were slightly below 100, but prevents passing a mask that had a serious leak in some exercises by not bringing the average up falsely.
It should be noted that AIOH, the Australian Institute of Occupational Hygienists, recommends that a fit test not be passed if any exercise is a fail.
No respirator with a filter efficiency of <99% can be expected to provide the highest level of filtration so while a fit factor of 100 would be achieved, you could never achieve a fit factor of 500, 1000 or 2000 with a P2 or N95 mask. Those levels of protection can only be achieved with a minimum of a full-face mask and a P3 filter.
The following tables will help to understand how RPE should be selected, using the calculated Required Minimum Protection Factor.
Up to 10
Up to 50
Up to 100
100+
• P1, P2 or P3 filter half facepiece replaceable filter
• P1 or P2 disposable facepiece
• Powered Air-Purifying Respirator (PAPR)-P1 Filter in PAPR with head covering or face piece
• P2 filter in a full face piece
• PAPR-P2 filter in PAPR with any head covering or full face piece
• PAPR-P3 filter in PAPR with any head covering
• Half face piece with positive pressure demand or continuous flow air-line
• Half face piece – air-hose with electronic blower
• P3 filter in full face piece
• Full face piece air-hose (hose mask) natural breathing type
• PAPR-P3 filter in PAPR with full face piece or head covering and blouse
• Head covering air-hose with electrical blower
• Head covering air-line respirator – continuous flow
• Full face piece air-line respirator – positive pressure demand or continuous flow modes
• Full face piece air-hose with electric blower
• P2 or P3 filter half facepiece replaceable filter
• P2 disposable facepiece
• P2 filter in a full facepiece
• PAPR-P2 filter in PAPR with any head covering or full facepiece
• PAPR-P3 filter in PAPR with any head covering
• Half face piece with positive pressure demand or continuous flow air-line
• Half face piece – air-hose with electronic blower
• P3 filter in full face piece
• Full face piece air-hose (hose mask) natural breathing type
• PAPR-P3 filter in PAPR with full face piece or head covering and blouse
• Head covering air-hose with electrical blower
• Head covering air-line respirator –continuous flow
• Full face piece air-line respirator – positive pressure demand or continuous flow modes
• Full face piece air-hose with electric blower
Suitable Respirator Protective Equipment – Gas and Vapors
Required Minimum Protection Factor Maximum gas/vapour concentration present in air ppm (by volume)
Up to 10 1,000
Up to 50 1,000
Up to 50 5,000
Up to 100 5,000
Up to 100 10,000
100+
Suitable Respirator Protective Equipment
• Class AUS, 1,2 or 3 filter with half facepiece replaceable filter or disposable facepiece
• Class PAPR-AUS, PAPR-1 or PAPR-2 filters in a PAPR with any head covering or facepiece
• Class AUS or Class 1 filter with full facepiece
• Half facepiece air-line respirator with positive pressure demand – or continuous flow
• Half facepiece air-hose with electric blower
• Class 2 filter with full facepiece
• Class PAPR-2 filters, with full facepiece PAPR
• Class 3 filter with full facepiece
• Full facepiece air-line respirator – negative pressure demand
• SCBA negative pressure demand
• Full facepiece air-hose (hose mask) natural breathing type
• Full facepiece, head covering or air-supplied suit with air-line respirator – positive pressure demand or continuous-flow
• SCBA positive pressure demand
• Full facepiece air-hose with electric blower
• Explain the purpose of the fit test, what they will have to do and the meaning of the fit test results. Explain the pass/fail criteria, and that more than one respirator may need to be tested to obtain an acceptable fit.
• When conducting a QLFT-ATT fit test, the test subject should refrain from eating, drinking (except still, unflavoured water), smoking, vaping or chewing gum for at least 30 minutes before the test.
• When you are conducting a QNFT-CNC fit test the wearer should refrain from smoking for at least 30 minutes before the fit test. Smokers will exhale particulate for up 30 minutes after smoking.
• Wearers of RPE must be clean-shaven. Ensure no hair is caught between the seal and the user’s face.
• Ensure that jewellery or other adornments (e.g. piercing) do not interfere with the fit of the facepiece.
• Check that there is no foreign material or substance between the sealing surface of the respiratory interface and the face or neck. Examples include temple bars or straps for eyewear, gels and creams.
• If the wearer needs to use in-facepiece spectacles, they must wear them during the fit test.
• Any other potentially interfering PPE that the wearer uses whilst working must be worn during the fit test, i.e., hardhats, goggles, hair nets
• RPD wearers who have dentures shall be fit tested with dentures, if they wear them while wearing the RPD in the workplace; or without dentures, if they do not wear them while wearing the RPD in the workplace
The 5-minute comfort assessment period is required as part of the overall fit test. Its objective is to allow the respirator to settle on the fit test subject to ensure it is comfortable for the wearer before starting the fit test. If a respirator is not comfortable (even if an adequate fit can be achieved), the wearer is likely to adjust or remove the respirator on the job and protection will be reduced or not achieved at all. Hence, this is a critical step in evaluating a tight-fitting respirators suitability to the wearer. This comfort assessment must be done whether your are doing QLFT or QNFT.
Each of the following Standards specifies this 5-minute comfort assessment period to be conducted as part of the fit testing protocol:
• ISO 16975-3 Section 6.6.2
• OSHA 1910.134 Appendix A Part I.A.5
• ANSI Z88.10-2010 Section 6.6.2
• Allergies to aerosols: Generally, people do not react to aerosols used for Respirator Fit Testing. If a wearer has an allergic reaction to an aerosol during Qualitative Fit Testing (QLFT), the testing must be ceased immediately, and first aid provided. In this case, an alternative aerosol or another fit test method should be chosen.
• Confounders prior to fit testing: Eating, smoking, or chewing gum can all impact the results of (QLFT) for up to 30 minutes because they affect a person’s sense of taste. This could render an Aerosol Taste Test (ATT) ineffective. It is recommended that wearers do not eat, smoke, or chew gum for 30 minutes prior to Qualitative Fit Testing. Subjects may rinse their mouth out with water prior to testing if desired.
• It is recommended that QLFT-ATT testing be conducted in a well ventilated area to prevent a build up of the test solution.
• HVAC Systems: Air-conditioning systems are also air-purifying. That is, they filter the air as it cycles through a closed system. As such, this air can sometimes be too “clean” and may have too few ambient particles. In this situation, a particle generator may be used to increase the ambient particle count for testing.
This can be done with a Particle Generator. It may also be necessary to turn off fans or high powered A/C units while conducting test
• Size of the Room: If fit testing is being performed in a room that is too large, the aerosol mist being created by the Particle Generator may be too diffuse and the ambient particle count will still be too low. If the ambient particle count is not high enough, it can cause a false negative result. Recommended room now larger than 7m x 7m.
• Confounders prior to fit testing: smoking can impact the results of a Quantitative test. Smokers may exhale particulate into the respirator and cause a fit test to fail. Test subjects should not smoke for 30 minutes prior to a fit test.
• Extremely high particle count: In some circumstances, the natural ambient particle count may be very high. If particle count exceeds the recommended maximum, this can result in false positive test results. PortaCount has a recommended maximum for tests done with or without the N95 Companion, and these should not be exceeded, for the most reliable results.
There are some common reasons why a fit test may need to be terminated before completion. Knowing what these situations are and having the confidence to terminate a fit test will save you time and allow you to trouble shoot, or find a better respirator option for the wearer.
1) Poor Seal : When testing using the Qualitative methods, the wearer would have conducted a user check, using both a positive and negative pressure check. When testing using the Quantitative method, you would have confirmed the seal using the “real-time fit check” on the PortaCount Fit Tester. You should not conduct a fit test if the user feels they are unable to get a good seal on a respirator. However, if they have achieved a good seal, but the fit test fails in the first few minutes of the test, stop the test, and check the following:
○ Check for mask size incorrect i.e., too large, or small
○ Ensure no obstruction such as hair, jewelry, or other worn PPE is affecting the seal
○ The mask seal may be twisted or folded over i.e., not sitting flush on the user’s face, etc.
○ Mask is not positioned correctly on the user’s face i.e., too high, low, or off centre, etc.
○ Mask may have damage that was not observed initially
○ If none of these checks solve the problem, try a different respirator.
2) Fit-test subject touched the respirator during a fit test : Any movement of the mask by the user during a test may potentially breach the seal and allow airborne particles within the breathing zone, causing the test to fail. Test should be restarted.
3) The wearer speaks during the fit test in the non-talking exercises : Each protocol must be adhered to and followed in the correct sequence. Talking during exercises that do not require the RPE user to talk may result in the mask moving during the test. This can affect the specificity of the test and potentially invalidate the results. Test should be restarted.
4) There is a low ambient particle count (quantitative tests only) : If the ambient particle count is too low, it can be very difficult to achieve a fit factor above 100. Essentially, the sample is too small to make a valid assessment, using the calculations that the PortaCount Fit Tester will make. That is why the PortaCount Fit Tester will have a minimum particle count to conduct a test, and a recommended range for the greatest accuracy of results. Increase particle count by turning off fans or AC, closing windows, moving to a smaller room, or using a particle generator. Restart test.
5) There is a low alcohol level in the wick (quantitative tests only) : The PortaCount Fit Tester uses the alcohol to enlarge particles to a size which can be counted. When alcohol in the wick gets low, the machine may not “see” the correct particle count, causing an incorrect calculation and resulting test failure. Replenish alcohol in wick. Test should be restarted.
6) Nebuliser was observed to be not releasing any aerosols between replenishments or at the end of the test (qualitative tests only) : If there is no test aerosol being released into the hood, there is nothing for the user to detect, and therefore the resulting “pass” could be false. Replenish aerosols in nebuliser, ensure nebuliser is not clogged, and restart test.
7) F it-test subject unable to taste aerosols at end of the test, (qualitative tests only) : If the user has developed a tolerance for the aerosol, or the sensitivity test was not correct, they may not be able to detect even a significant leak. This speaks to the subjectivity of quantitative testing. In this circumstance, qualitative testing cannot be used with this user. It is important to confirm that the test subject can still taste the aerosol at the end of the test. Have them remove the respirator, and open their mouth. Deliver the test agent into the hood to confirm taste sensitivity.
You may terminate a fit test if any of the issues discussed previously have occurred. Additionally, you must terminate the test if the test subject appears to be distressed or is suffering a reaction.
Remember, if you are testing a different respirator, or have made adjustments to the fit of the respirator, you must conduct another 5-minute comfort assessment prior to the test commencing.

Standard AS/NZS 1715:2009 Section 1.5 provides an index with respiratory protection terminology. Because terminology and commonly used acronyms will vary from region to region, it is helpful if your program uses consistent terminology when referring to contaminants, exposure standards, protective equipment, testing methods and more.
For example, some regions refer to respiratory protection equipment as RPE and others refer to RPD (respiratory protection devices). Some places says Workplace Exposure Limit (WEL), others may say Workplace Exposure Standard (WES)
It is good practice for a Respiratory Protection Program to define the terminology it will use in in an appendix or index. This will ensure consistency in record keeping.
If you are not using a template form or specific software to record your fit test results, you may create your own record. This is the information that would be required:
• Date of the test
• Fit-test method used
• Name of the fit-test operator and fit-test operator’s employer/company name
• Name of the person fit tested
• Details that will uniquely identify the RPE such as make, model, size and material
• Details of other potentially interfering PPE or accessories worn during the fit test such as hard hat, glasses
• Pass/fail criteria; fit factor required
• Results: pass/fail,
• Overall fit factor achieved
• Serial number or other means of identifying test equipment used in the test;
• Any additional information the RPD programme administrator deems pertinent, such as corrective actions taken in the event of a failed fit test.
Standard AS/NZS 1715:2009 Section 2 provides a clear outline of the requirements for a proper Respiratory Protection Program. In this outline, the Standard states that certain records should be kept as part of the documentation of the program. This list is a recommended minimum, and the Program Administrator may feel other records should also be kept.
Records for a respiratory protection program shall include records related to:
Issue of RPE (non-disposable)
• Date
• Identifying marks
User records
• Training
• Fit test date
• Medical screening
Maintenance
• Filter replacement schedule
• RPE maintenance schedule
Program Records
• Procedures used in program
• Audits/evaluations of program
• Atmospheric monitoring records
• Health surveillance records
• Under Work Health and Safety Legislation in Australia, health surveillance records must be retained for 30 years after the date the record is made. Businesses should consider keeping other records for the same period of time. At a minimum, individual fit test records should be kept until the next fit test is completed successfully.
• Wearers of RPE should be given a copy of their personal fit test results. The employer would also keep a copy.
• Any personal employee information gathered as part of the respiratory protection program is subject to the protections provided by The Privacy Act, 1988.
When the message “SERVICE” appears on the display, it can mean one of several things. Usually, it means that the PortaCount Fit Tester is low on alcohol, but there are other reasons that can cause this message to appear. When the service message appears, it does not necessarily mean that the PortaCount Fit Tester will stop working soon. Fit test results are accurate even when this message is on. If none of the suggestions below cause the message to go off, you should schedule a time in the near future to send the PortaCount Fit Tester to TSI for service. It is normal for the service message to stay on most of the time when the PortaCount Fit Tester needs cleaning and recalibration.
The most probable reasons for the service message to be on:
• The PortaCount Plus is low on alcohol. Refill the Alcohol Cartridge.
• Excessive moisture has accumulated in the Alcohol Cartridge. Refer to the instructions from the manufacturer, “Maintenance,” for details on changing the Alcohol Wick.
• The ambient temperature is unusually high. Ignore the service message in this case.
• The battery is low. Ignore the service message and install a fresh battery, (only on battery powered machines. )
• The PortaCount is in need of cleaning and recalibration.
TSI recommends annual recalibration and cleaning of the PortaCount Fit Tester, however, if you frequently conduct testing in areas with extremely high particle counts, this interval should be reduced.
Consumables are supplies that get used up each time you test, i.e. probes, alcohol. You will need to reorder supplies on a regular basis.
Maintaining an adequate supply of alcohol inside the PortaCount Fit Tester is critical to its operation.
• Never transport the PortaCount with the alcohol cartridge inside it. Flooding of the optics may occur.
• Always keep the alcohol cartridge in the alcohol fill capsule during transport and storage. Always keep the alcohol cartridge clean.
• Never leave the cartridge cavity open longer than necessary. Use the storage cap to cover the cartridge cavity when the unit is transported or stored
• Keep the storage cap and alcohol cartridge clean. Always set them down with the end standing up. This precaution prevents dirt or debris from entering the instrument and inhibiting operation. The pin-hole size nozzle inside can easily become clogged.
• TSI recommends that the Zero Check Filter be left attached to the sample line whenever the PORTACOUNT Plus is turned on but not in use. This prevents lint and debris from being drawn into the instrument and blocking the air flow.
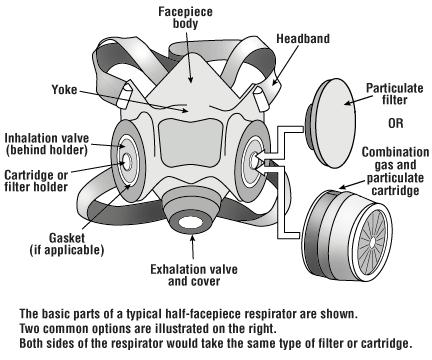
AS/NZS 1715:2009 Section 9 outlines maintenance requirements for RPE. It states that a system of maintenance should include the following elements:
• Cleaning and disinfection
• Inspection
• Repair or replacement of components
• Proper storage
Section 9.2 of the Standard states that non-disposable RPE shall be cleaned after each use, according to the manufacturer’s instructions.
Persons who are required to maintain RPE must be trained in cleaning and disinfection procedures.*
Over time, RPE and its various components may become damaged, due to workplace conditions, poor handling, inadequate storage, and other factors. To extend the working life of RPE, and ensure that it is performing optimally, users should inspect RPE regularly and complete maintenance according to the manufacturer’s instructions.
RPE components must be fit for purpose and kept in good condition. Many components such as head straps, exhalation valves, and seals can wear out, but they may be replaced by contacting the relevant manufacturer. It is good practice for a business to keep replacement parts readily available for their standard issue respirators.
*A centralized cleaning program may be suitable in circumstances where there is extensive use of RPE.
Remove filters from facepiece prior to cleaning. Disassemble facepieces according to the manufacturer’s instructions.
• Wash components in warm (40°C maximum) water with a mild detergent or with a cleaner recommended by the manufacturer. A stiff bristle (not wire) brush may be used to facilitate the removal of dirt.
• Generally, cleaning and disinfecting solutions used should not be hotter than 40°C as higher temperatures can permanently distort facepieces and cause premature deterioration of individual components or the whole assembly.
• Wherever practicable, exhalation valves should be removed from valve seats and cleaned each time the RPE is serviced. Valves and valve seats may be cleaned in cold or warm water; hot water (i.e. greater than 40°C) should be avoided. Valve seats may need to be scrubbed with a suitable brush.
• The cleaned and disinfected RPE should be rinsed thoroughly in clean water to remove all traces of cleaning agent and disinfectant. This is very important to prevent dermatitis or irritation. After rinsing, an anti-fog preparation may be applied to lenses and visors.
• When the cleaner used does not contain a disinfecting agent, and disinfection is required, respirator components should be immersed for about two minutes in one of the following:
○ Hypochlorite solution (50 p.p.m. of chlorine) made by adding approximately 2 mL of laundry bleach to one litre of water at a temperature not greater than 40°C.
○ An aqueous solution of iodine (50 p.p.m. iodine) made by adding approximately 0.8 mL of tincture of iodine (6–8 grams ammonium and/or potassium iodide/100 mL of 40% alcohol (v/v) to one litre of water at a temperature not greater than 40°C.
○ Other commercially available cleansers of equivalent disinfectant quality when used as directed if their use is recommended or approved by the respirator manufacturer. Resuscitators and medical masks should be cleaned by other methods. Check for the most up-to-date information.
After disinfection, rinse components thoroughly in clean water, drain and allow to air dry. Reassemble the facepiece following the manufacturer’s instructions. The RPE should be allowed to air dry away from direct sunlight, on a clean surface, or dried in a low-temperature oven. They may also be hung from a horizontal wire, like drying clothes, but care should be taken not to damage the facepieces.
Clean RPE should be clearly identified as such.
RPE must be inspected before and after use. Regardless of the type (disposable, reusable) all masks, components, attachments, and filter assemblies must be thoroughly inspected to ensure they are fit for purpose.
Regular inspections allow the wearer to identify any issues, damage, or defects that may be present and either replace or repair any defective parts before they enter the hazardous environment.
Never wear RPE that is defective, damaged, missing components, contaminated, or has any issues that impact the sealing surface or its ability to effectively filter and protect the wearer from the hazardous environment.
The following defects may be identified during inspections:
Disposable: Filtering Facepieces:
• Holes or tears in the filter material
• Damage to straps
• Loss of elasticity in straps
• Connection of straps to mask broken
• Defects along the sealing surface of the mask
• Nose piece damaged or defective
• The exhalation valve assembly (if present) damaged, missing, or defective.
Reusable (half face and full face):
• Rubber Face Piece:
○ Cracks, tears, or holes on the sealing surface
○ Manufacturing defects and imperfections along the sealing surface
○ Face seal distorted
○ Excessive contamination such as dirt, paint residue, or other workplace contaminants.
○ Cracks, scratches, or loose-fitting lenses (full-face respirators).
• Head Straps:
○ Breaks or tears
○ Loss of elasticity
○ Frayed edges
○ Broken or malfunctioning buckles
○ Twisted and fatigued straps
○ Connections to masks faulty
• Inhalation and Exhalation valves:
○ Detergent residue on the valve or valve seat
○ Dust particles, dirt, or other contaminants on the valve or valve seat
○ Cracks, tears, or lack of flexibility in the valve material
○ Cracks and flexibility of valve seats.
• Filter element:
○ Incorrect filters for the hazards
○ Filters beyond their service life
○ Damaged or worn threads and connections (filter and facepiece)
○ Cracks or dents in the filter housing
○ Attachments such as piggybacks and pre-filter housings damaged or not secured correctly.
• Batteries and alarms (powered respirators)
○ Batteries low or no charge
○ Poor flow rates
○ Alarms not calibrated or working correctly.
RPE must be always stored following training and the manufacturer’s instructions to ensure the RPE is fit for purpose and ready to use.
Storage or RPE should include the following:
• Store RPE in a clean dry designated place, away from dust, oil, and sunlight to avoid deterioration
• RPE should be stored so that it cannot be crushed or distorted
• Cleaned RPE should be clearly identified and separated from used/contaminated RPE
• Store gas and vapour filters in containers or bags with airtight seals to ensure moisture in the air does not get adsorbed onto the filter material
○ Filters and cartridges must be removed from the respirator and stored in separate bags to prevent cross-contamination;
○ RPE for emergency and rescue work should be maintained in a condition ready for immediate operational use and secured to prevent unauthorized use, tampering or removal. Storage locations must be clearly marked in green and white following the requirements of AS 1319 Safety signs for the occupational environment.
○ Never store a respirator within a fume hood or at a workbench where contaminants are present.
Disposable respirators are for single use and should be disposed after use.
For workplaces that are not high-risk industries, place disposable PPE in a tied-off bag and into a general waste bin. Don’t place PPE in any recycling bins.
Waste from clinical and healthcare services, including RPE, must be disposed of according to existing procedures for disposing clinical waste. Clinical waste is a Prescribed Industrial Waste under EPA Regulations and must be transported by a permitted vehicle and disposed of at a licensed premises.
Reusable RPE will eventually reach the end of its usable life and need to be replaced.
• Filters will need replacing regularly, and proper disposal when they have reached the end of their usable lifespan. For example, 3M filters state that, once opened, maximum use time is 6 months, even if unused, (the carbon will absorb contaminants from the general environment.) They also recommend replacing when contaminant can be detected by smell or taste, or in accordance with your program’s established Filter Change Schedule.
• Some filter’s documentation may state that they are washable and reusable and resistant to sanitizers such as bleach. Refer to the documentation on your own filters before attempting to wash a filter.
• When disposing of used filters, whether particulate or gas, it is important to take steps to ensure that contaminants aren’t released in an area where they could cause harm. Some filters can be recycled. Check recycling requirements in your area before placing filters in a recycling bin.
The specific method of disposal will depend largely on what type of contamination is involved. For extremely dangerous substances, a Hazmat team will have to take custody of the PPE and go through a process tailored to the exact substance. For certain contaminants, i.e., lead, the PPE must be cleaned first to remove this heavy metal. Once properly cleaned, it will be contained and then disposed of properly to avoid the risk of groundwater contamination.
If specialised disposal requirements do not apply, follow manufacturer’s recommendations for proper disposal of worn-out RPE. It is important to ensure the item is properly dismantled or destroyed, however, so that someone doesn’t try to continue to use the equipment. Using outdated or damaged personal protection equipment can put people at major risk for injury.

7. Appendix
The Qualitative fit test methods were discussed in some detail, in section one. To recap, the qualitative test method is the only pass/fail test method that relies on the subject’s sensory response to detect a challenge agent in order. Qualitative fit-tests do not give a numerical indication of fit; no direct measurements of the test and leak concentrations are made. The reliability of the fit test depends upon the wearer’s ability to detect and indicate whether the fit-test agent is sensed. Some wearers may not be sensitive enough to the fit-test agent resulting in face-seal leaks that may not be detected. Therefore, before commencing the fit test, it is necessary to establish whether the wearer is able to detect the fit-test agent at low concentrations. This is called threshold screening. With the use of the threshold-screening step, these qualitative fit-test methods are sensitive enough to ensure fit factors of 100. Where a fit factor greater than 100 is required, qualitative fit-test methods are not suitable and a quantitative fit-test method should be selected.
QLFT methods are suitable for disposable and reusable half masks; they are not suitable for full-face masks.
To achieve a pass in a qualitative fit test the wearer must not taste the test agent at any time during any of the test exercises.
Before carrying out a qualitative fit test using a distinctive taste, establish the taste threshold of the wearer. This screening test is carried out to check that the wearer can detect the taste of the test aerosol. This is often referred to as a sensitivity test. If the wearer cannot detect the taste during the screening test, the fit test method cannot be used and you should choose a different method.
Conduct the fit test as a continuous test, allowing sufficient time between the sensitivity test and the fit test for the wearer to clear their palate. Should a fit tester not wish to continue directly from the sensitivity test to the fit test for practical reasons, they should complete the fit test on the same day. The wearer should refrain from smoking, eating or drinking (except for water) during the intervening period.
The nebulisers used to generate the aerosol for the screening test may clog during use and stop delivering the test substance. Therefore, the fit tester should make periodic checks of the nebulisers during the test to ensure that it is not clogged. If clogging is found at the end of the test session, the test is invalid. Regular cleaning of the nebulisers should help to prevent clogging.
Maintenance and cleaning of the hood and nebulizers should be conducted as per the manufacturer’s recommendations to ensure the equipment is fit for purpose and working correctly.
The following provides guidance on regular maintenance and cleaning protocols for the hood and Nebulizers
HOOD:
• Visually inspect the hood during setup and between tests to ensure there are no defects or damage to the hood face shield or stitching.
• Visually inspect the hood collar during setup and between tests to ensure there are no defects or damage to the collar or hood seal.
• After each session, the hood and collar should be wiped with a damp cloth or paper towel to remove any deposited fit test solution. Cleaning wipes can also be used if required to sanitise the components between tests.
• Visually inspect the nebuliser during set up and between tests to ensure there are no defects or damage to the nebuliser or components such as the O-ring, Insert, reservoir, or bulb.
• Ensure the question-mark-shaped insert is present in the nebuliser reservoir and is pushed down as far as possible on the stem (see nebuliser figure).
• Nebulisers should be rinsed in freshwater after every session or at least every four hours, or if the nebuliser becomes clogged.
• If the nebuliser becomes clogged or obstructed (testing solution may form crystals) the curl of small-gauge wire that came with your fit test kit to remove any crystals that might have formed in the two narrow passageways on the nebuliser tip inside the cup. This should also be done periodically to ensure the passageways are clear of obstructions.

The following form is a sample provided by 3M for QLFT-ATT fit testing , using 3M equipment. This form can be used as a guide for creating a personalized form for a business, or it can be used as it.
Test conducted using 3M FT-10 or 3M FT-30 Fit Test Kit
Name
Company/Department
Make, Model & Size of Respirator
Own face piece, pool or test model used?
Kit Used?
Nil to eat or drink (water ok) 15 min prior to test ?
Test solution - No. of squeezes? Squeezes Initial Squeezes
Fitting of mask and any other PPE (Glasses or Goggles)
Own/Pool/Test
(sweet)/FT30 (bitter)
Yes/No
Test conducted by (Name & Company)
Pass achieved on (Date)
Signed (Tester)
Retests required? Yes/No
If yes, record number and reasons:
The PortaCount Fit Tester is a CNC-counting instrument. CNC-counting instruments are capable of measuring the number concentration of particles in a given aerosol sample by counting single particles. When used for QNFT, the particle concentration of the fit test challenge aerosol around the head and shoulders (Cout) and the particle concentration inside the RPD (Cin) are both measured while the person being fit tested performs a series of exercises designed to stress the face/neck seal in ways that approximate anticipated workplace movements.
The PortaCount, and similar instruments can either use the particles in the ambient air (if testing in a workplace, such as a manufacturing shop, or they can test in very clean environments, like a hospital, with the use of a particle generator.
Upon the completion of the fit test, the instrument provides a pass/fail indication and/or a numeric overall fit factor result for the entire test calculated according to the given formula. The person has passed the fit test if the overall fit factor as calculated using the below formula equals or exceeds the required fit factor as given in the Table (1).
where
• N is the number of exercises
• QNFF 1 is the fit factor for the first exercise
• QNFF 2 is the fit factor for the second exercise
• QNFFn is the fit factor for the nth measured exercise
• Fit the facepiece with a sample probe positioned so that the air sample is drawn from within the respirator, not just inside the valve. Position the open end of the sampling tube in the wearer’s breathing zone, close to the face and approximately mid-way between the nose and mouth. Do not isolate the sample probe from the nose and mouth region by a physical partition; for example, by the inner mask of a full-face mask. For half masks and full-face masks, use a suitable fit test adapter and position the open end of the sampling tube as described above.
• When fitting the sample probes to half and full-face masks, use suitable sampling adapters to avoid puncturing the facepiece. RPE manufacturers and fit test equipment suppliers can provide suitable fit test adapters to fit most facepiece types. These adapters should enable fit testing on wearer-issued facepieces. When fitting the sampling adapter to the facepiece, take care not to block off or restrict the flow of air through the sampling tube.
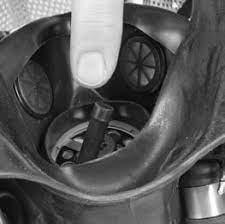
• The positioning and the combined weight of the fit test adapter, sample probe and sample tubes should not interfere with the fit of the facepiece. This is particularly important when fit testing disposable or lightweight half masks. Sample probes should be lightweight and the sample tubes must be supported to avoid any drag on the fit of the facepiece.
• When testing respirators that are expected to achieve 99-100% filtration, the wearer’s exhaled breath can contain particles that can be detected by the particle counting device. These wearergenerated particles can result in a falsely low fit test result. Having an ambient challenge concentration of at least 3000 particles/ cc for fit testing disposable and reusable half masks, and 10,000 particles/cc for fit testing full-face masks, will reduce the likelihood of false fails.
• An ambient particle count that varies significantly over the duration of the test can also give rise to errors in the fit factor. Avoid excessively dusty and smoky environments. Seek further advice from the fit test equipment supplier if necessary.
• Very high fit factors, i.e. numbers over 100,000, could indicate a problem with the application of the fit test; if this happens check the validity of the result. Start with running your daily checks again.
Fit factor is defined as the particle concentration outside the respirator divided by the particle concentration inside the respirator. Because ambient concentration can vary over time, the PortaCount® Pro Respirator Fit Tester calculates the fit factor by taking the average of the ambient concentrations measured before and after the respirator sample and then dividing by the concentration measured in the respirator. This is why the first test cycle (exercise) is longer than additional test cycles in Fit Test Mode. It is necessary to measure the required additional ambient concentration sample before the first fit factor can be calculated. Both the ambient and respirator concentrations are determined by integration. The integrated concentrations are determined by the total number of particles counted during the sample periods.
Fit factor is actually calculated by:
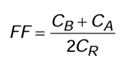
where
• FF = Fit Factor
• C B = particle concentration in the ambient sample before the respirator sample
• C A = particle concentration in the ambient sample after the respirator sample
• C R = particle concentration in the respirator sample
If no particles are counted in the respirator sample, the PortaCount Pro fit tester automatically adds one particle. This prevents dividing the ambient concentration by zero. At the end of a fit test, the overall fit factor is calculated and displayed, based on the individual fit factors for each test cycle.
The following equation is used to calculate the overall fit factor in the PortaCount Pro fit tester:

where
• FF x = Fit Factor for test cycle
• n = number of test cycles (exercises)
Disclaimer: The measurement provided by the PortaCount Pro Respirator Fit Tester is an assessment of respirator fit during a fit test only. Respirator fit at other times will vary. The fit factor value is not intended for use in calculating an individual’s actual exposure to hazardous substances.
Calculating Particle Concentration Particle concentration is calculated by counting the number of particles passing through the sensor in a given period of time. Since the flow rate is known (5.83 cm3/ sec), the particle concentration can be determined.
In 1-second count mode the equation is:
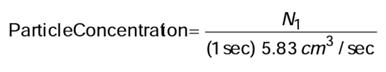
where
• N 1 = the number of particles counted in a 1-second period
Note that the total flow rate of air through the PortaCount® Pro is a nominal 16.7 cm3/sec. The reason that we use 5.83 cm3/sec. in the calculations above is because the flow path inside the PortaCount PRO is divided into two branches, the sensor flow and the bypass flow. The sensor flow is set at precisely 5.83 cm3/sec and the bypass flow is approximately 10.8 cm3/sec. Using the equation above, you can see that the minimum measurable concentration is 0.17 particles per cm3 in 1-Second Count Mode. The equations above can also be used to determine the minimum measurable mask concentration that can be measured in Fit Test Mode given the mask sample time that is used. For example, with a 40 second mask sample (factory setting), the minimum measurable concentration is 0.004 particles per cm3.
Another quantitative fit test device is the Quantifit® made by Occupational Health Dynamics. In order to use the Quantifit, model-specific adapters must be attached to reusable respirator facepieces. Adapters are listed on the OHD website(www.ohdusa.com). A significant disadvantage to this testing method is that filtering facepieces cannot be tested using this method.
No particle filters or cartridges are required, but the facepiece inhalation valves must be either removed or propped open. While the subject remains still and holds their breath, the Quantifit pulls air from the facepiece to create a specified negative pressure, which may be similar to breathing resistance during inhalation. The air flowing out of the respirator is noted as the leak rate. Then the fit factor is determined by dividing a predetermined inhalation flow rate by the leak rate. (Please see the Quantifit User Instructions for more information.)
The person being fit tested shall perform the fit-test exercises described below for the required time. The leakage flow rate measurements are taken after each exercise is completed.
• CNP REDON test exercises. Employers shall ensure that each test subject being fit tested using this protocol follows the fit-test exercise and measurement procedures, including the order of administration, described below.
• Facing Forward. The wearer shall stand and breathe normally, without talking, for 30s. After this breathing normal exercise, the wearer shall face forward, while holding breath for 10s during the fit-test measurement.
• Bending Over. The wearer shall bend at the waist, as if going to touch his or her toes, for 30s. After this exercise, the wearer shall face parallel to the floor, while holding breath for 10s during the fit-test measurement.
• Head Shaking. For about three seconds, the wearer shall shake head back and forth vigorously several times while shouting. After this exercise, the wearer shall face forward, while holding breath for 10 s during the fit-test measurement.
• REDON 1. The wearer shall remove the RPD, loosen all RI straps and then redon the RPD. The wearer shall face forward while holding breath for 10 s during the fit-test measurement.
• REDON 2. The wearer shall remove the RPD, loosen all RI straps and then redon the RPD. The wearer shall face forward while holding breath for 10 s during the fit-test measurement. An optional shortened test method “Redon” involves the following exercises:
• Stand and breathe normally for 30 seconds, then hold breath 10 seconds for measurement.
• Bend at the waist with face parallel to the floor for 30 seconds, then hold breath 10 seconds for measurement.
• Stand upright, shake head back and forth vigorously for about 3 seconds while shouting, then hold breath 10 seconds for measurement.
• Remove the facepiece, loosen all facepiece straps, and then redon the facepiece. Hold breath 10 seconds for measurement.
• Remove the facepiece again, loosen all facepiece straps, and then redon the facepiece again. Hold breath 10 seconds for measurement.
During the CNP measurement the wearer should keep their mouth closed. They should be given time to practise the breath-hold procedure. The CNP method requires the use of the appropriate fit test adapters for the type of filter connection present on the facepiece to be fit tested.
The first time an operator uses the PortaCount Fit Tester, they must download and install the software that TSI has provided. This is called FitPro Ultra, and is available on the TSI website. https://tsi.com/ product-accessories/fitpro-plus-fit-test-software-7001233/
You can set up your software without having the PortaCount attached. You can enter certain details in advance of performing tests.
Go to Global settings
1) Download and install software FitPro Ultra
2) Go To the three line menu and select Global Settings. Set as desired for your area. It is recommended to leave preset minimums and maximums. Select desired test protocols.
3) There is a default database. If you wish to select another database, you will have one prepared on a flash drive. Insert that now.
4) Enter respirator details if available.
With Daily Checks successful, you are now ready to fit test.
1) Connect adaptors to respirators if using re-usable respirators.
2) Attach probes to disposable filtering face piece if using disposable.
3) Fit test subject should have completed a medical screen.
4) Explain the purpose of the fit test, and what results will mean. Describe the exercises and confirm that the subject can perform all exercises. Explain how to mitigate the weight of the hose during the test
5) If fit test subject has not identified a specific respirator that they have been wearing, help them to select a respirator that seems to fit comfortably.
6) Demonstrate how to don and doff a respirator for the user, and how to do a user check on fit and seals. They may do a positive or negative pressure check.
7) Fit test subject should observed donning and doffing a respirator without assistance, to confirm that they are competent.
8) Conduct a real-time fit check on respirator. Select Real Time from the 3 Dot menu. You must select the respirator to commence this check. The Real-Time Fit Check Mode display is helpful for respirator training and troubleshooting. It allows a test subject to experiment with strap tension and other adjustments while watching the direct effect these efforts have in real time. Check the real time gauge for a score that stays in the green. Check the real time graph for a straight line that sits above the red zone and has very little movement
9) Allow user to wear respirator for five minutes to assess comfort of mask. This is the ideal time to enter the person’s details. Select People from the three line menu, select new and add person’s details. After the five minutes, ask user to rate the comfort of the mask on a scale of 1-10. It is advisable to avoid fitting masks that are given a low comfort rating.
10) If you have a good fit and good comfort rating, it is time to conduct the fit test.
Observe the worker donning the respirator for the final time before the fit test. Explain the exercises they will do and ensure they are able to move their head up and down,and side to side without discomfort. Ensure they can bend over without discomfort. Ask if they are comfortable to read aloud, or explain that they may count to 100, or sing a song.
1) Attach respirator to fit test unit.
2) Perform respirator fit check using the testing protocol assigned by the employer or that is required by the work site.
3) Determine whether a worker has passed or failed a respiratory fit test.
Upon successful completion of fit test, send person their fit test report and card.
• Go to 3 Line menu and select fit test history
• Open person’s fit test, it will be at the top
• Select from 3 Dot menu: Print Fit test report
• Select Print to PDF and save file using person’s name
• Email saved file to person.
1) Recalibration Interval
2) Status Messages
3) Low particle message
4) High Concentration message
5) Low alcohol level message
6) Shipping & Storage Precautions
7) Changing the wick
“When the sunlight strikes raindrops in the air, they act like a prism and form a rainbow. The rainbow is a division of white light into many beautiful colors. These take the shape of a long round arch, with its path high above, and its two ends apparently beyond the horizon. There is, according to legend, a boiling pot of gold at one end. People look but no one ever finds it. When a man looks for something beyond his reach, his friends say he is looking for the pot of gold at the end of the rainbow.”
Respirators must be used in workplaces in which employees are exposed to hazardous airborne contaminants. When respiratory protection is required employers must have a respirator protection program as specified in OSHA’s Respiratory Protection standard (29 CFR 1910.134). Before wearing a respirator, workers must first be medically evaluated using the mandatory medical questionnaire or an equivalent method. To facilitate these medical evaluations, this INFOSHEET includes the mandatory medical questionnaire to be used for these evaluations.
The requirements of the medical evaluation and for using the questionnaire are provided below:
• The employer must identify a physician or other licensed health care professional (PLHCP) to perform all medical evaluations using the medical questionnaire in Appendix C of the Respiratory Protection standard or a medical examination that obtains the same information. (See Paragraph (e)(2)(i).)
• The medical evaluation must obtain the information requested in Sections 1 and 2, Part A of Appendix C. The questions in Part B of Appendix C may be added at the discretion of the health care professional. (See Paragraph (e)(2)(ii).)
• The employer must ensure that a followup medical examination is provided for any employee who gives a positive response to any question among questions 1 through 8 in Part A Section 2, of Appendix C, or whose initial medical examination demonstrates the need for a follow-up medical examination. The employer must provide the employee with an opportunity to discuss the questionnaire and examination results with the PLHCP. (See Paragraph (e)(3)(i).)
• The medical questionnaire and examinations must be administered confidentially during the employee’s normal working hours or at a time and place convenient to the employee and in a manner that ensures that he or she understands its content. The employer must not review the employee’s responses, and the questionnaire must be provided directly to the PLHCP. (See Paragraph (e)(4)(i).)
Excerpt from Appendix C of 29 CFR 1910.134: OSHA Respirator Medical Evaluation Questionnaire
To the employer: Answers to questions in Section 1, and to question 9 in Section 2 of Part A, do not require a medical examination.
To the employee: Your employer must allow you to answer this questionnaire during normal working hours, or at a time and place that is convenient to you. To maintain your confidentiality, your employer or supervisor must not look at or review your answers, and your employer must tell you how to deliver or send this questionnaire to the health care professional who will review it.
Once filled out, this form must be given to the PLHCP. This form should not be submitted to OSHA.
look at or review your answers, and your employer must tell you how to deliver or send this questionnaire to the healthcare professional who will review it.
Part A Section 1. (Mandatory) The following information must be provided by every employee who has been selected to use any type of respirator (please print).
1. Today's date:
2. Your name:
3. Your age (to nearest year):
4. Sex (circle one): Male/Female
5. Your height:
6. Your weight:
7. Your job title:
8. A phone number where you can be reached by the health care professional who reviews this questionnaire (include the Area Code):
9. The best time to phone you at this number:
10. Has your employer told you how to contact the health care professional who will review this questionnaire (circle one): Yes/No
11. Check the type of respirator you will use (you can check more than one category):
a. ___ N, R, or P disposable respirator (filter-mask, non-cartridge type only).
b. ___ Other type (for example, half- or full-facepiece type, powered-air purifying, supplied-air, self-contained breathing apparatus).
12. Have you worn a respirator (circle one): Yes/No If “yes,” what type(s):
Part A. Section 2. (Mandatory) Questions 1 through 9 below must be answered by every employee who has been selected to use any type of respirator (please circle “yes” or “no”).
1. Do you currently smoke tobacco, or have you smoked tobacco in the last month?
2. Have you ever had any of the following conditions?
a. Seizures
b. Diabetes (sugar disease)
c. Allergic reactions that interfere with your breathing
d. Claustrophobia (fear of closed-in places)
e. Trouble smelling odors
3. Have you ever had any of the following pulmonary or lung problems?
a. Asbestosis
b. Asthma
c. Chronic bronchitis
d. Emphysema
e. Pneumonia
f. Tuberculosis
g. Silicosis
3. Have you ever had any of the following pulmonary or lung problems?
a. Asbestosis
b. Asthma
c. Chronic bronchitis
1. Do you currently smoke tobacco, or have you smoked tobacco in the last month?
d. Emphysema
2. Have you ever had any of the following conditions?
e. Pneumonia
a. Seizures
f. Tuberculosis
b. Diabetes (sugar disease)
g. Silicosis
c. Allergic reactions that interfere with your breathing
h. Pneumothorax (collapsed lung)
d. Claustrophobia (fear of closed-in places)
i. Lung cancer
e. Trouble smelling odors
j. Broken ribs
3. Have you ever had any of the following pulmonary or lung problems?
k. Any chest injuries or surgeries
a. Asbestosis
l. Any other lung problem that you've been told about
b. Asthma
4. Do you currently have any of the following symptoms of pulmonary or lung illness?
c. Chronic bronchitis
a. Shortness of breath
d. Emphysema
e. Pneumonia
b. Shortness of breath when walking fast on level ground or walking up a slight hill or incline
f. Tuberculosis
c. Shortness of breath when walking with other people at an ordinary pace on level ground
g. Silicosis
d. Have to stop for breath when walking at your own pace on level ground
h. Pneumothorax (collapsed lung)
e. Shortness of breath when washing or dressing yourself
i. Lung cancer
f Shortness of breath that interferes with your job
j. Broken ribs
g Coughing that produces phlegm (thick sputum)
k. Any chest injuries or surgeries
h Coughing that wakes you early in the morning
l. Any other lung problem that you've been told about
i Coughing that occurs mostly when you are lying down
4. Do you currently have any of the following symptoms of pulmonary or lung illness?
j Coughing up blood in the last month
a. Shortness of breath
k. Wheezing
b. Shortness of breath when walking fast on level ground or walking up a slight hill or incline
l Wheezing that interferes with your job
m Chest pain when you breathe deeply
c. Shortness of breath when walking with other people at an ordinary pace on level ground
n Any other symptoms that you think may be related to lung problems
d. Have to stop for breath when walking at your own pace on level ground
5. Have you ever had any of the following cardiovascular or heart problems?
e. Shortness of breath when washing or dressing yourself
a Heart attack
b Stroke
c. Angina
d Heart failure
e Swelling in your legs or feet (not caused by walking)
f Heart arrhythmia (heart beating irregularly)
g High blood pressure
h. Any other heart problem that you've been told about
6 Have you ever had any of the following cardiovascular or heart symptoms?
d Heart failure
e Swelling in your legs or feet (not caused by walking)
1. Do you currently smoke tobacco, or have you smoked tobacco in the last month?
f Heart arrhythmia (heart beating irregularly)
2. Have you ever had any of the following conditions?
g High blood pressure
a. Seizures
h Any other heart problem that you've been told about
b. Diabetes (sugar disease)
6 Have you ever had any of the following cardiovascular or heart symptoms?
c. Allergic reactions that interfere with your breathing
a Frequent pain or tightness in your chest
d. Claustrophobia (fear of closed-in places)
b Pain or tightness in your chest during physical activity
e. Trouble smelling odors
c Pain or tightness in your chest that interferes with your job
3. Have you ever had any of the following pulmonary or lung problems?
d. In the past two years, have you noticed your heart skipping or missing a beat
a. Asbestosis
e Heartburn or indigestion that is not related to eating
b. Asthma
f Any other symptoms that you think may be related to heart or circulation problems
c. Chronic bronchitis
7. Do you currently take medication for any of the following problems?
d. Emphysema
a Breathing or lung problems
e. Pneumonia
b Heart trouble
f. Tuberculosis
c Blood pressure
g. Silicosis
d Seizures
h. Pneumothorax (collapsed lung)
8. If you've used a respirator, have you ever had any of the following problems?
i. Lung cancer
(If you've never used a respirator, check the following space and go to question 9 )
j. Broken ribs
a. Eye irritation
k. Any chest injuries or surgeries
b. Skin allergies or rashes
l. Any other lung problem that you've been told about
c. Anxiety
4. Do you currently have any of the following symptoms of pulmonary or lung illness?
d. General weakness or fatigue
a. Shortness of breath
e. Any other problem that interferes with your use of a respirator
9. Would you like to talk to the health care professional who will review this questionnaire
about your answers to this questionnaire?
b. Shortness of breath when walking fast on level ground or walking up a slight hill or incline
c. Shortness of breath when walking with other people at an ordinary pace on level ground
Questions 10 to 15 below must be answered by every employee who has been selected to use either a full-facepiece respirator or a self-contained breathing apparatus (SCBA). For employees who have been selected to use other t ypes of respirators, answering these questions is voluntary.
d. Have to stop for breath when walking at your own pace on level ground
10. Have you ever lost vision in either eye (temporarily or permanently)?
e. Shortness of breath when washing or dressing yourself
11. Do you currently have any of the following vision problems?
a. Wear contact lenses
b. Wear glasses
c. Color blind
d. Any other eye or vision problem
12. Have you ever had an injury to your ears, including a broken eardrum?
13. Do you currently have any of the following hearing problems?
a. Difficulty hearing
b. Wear a hearing aid
c. Any other hearing or ear problem
b. Wear glasses
c. Color blind
d. Any other eye or vision problem
12. Have you ever had an injury to your ears, including a broken eardrum?
1. Do you currently smoke tobacco, or have you smoked tobacco in the last month?
13. Do you currently have any of the following hearing problems?
2. Have you ever had any of the following conditions?
a. Difficulty hearing
a. Seizures
b. Wear a hearing aid
b. Diabetes (sugar disease)
c. Any other hearing or ear problem
c. Allergic reactions that interfere with your breathing
14. Have you ever had a back injury?
d. Claustrophobia (fear of closed-in places)
15. Do you currently have any of the following musculoskeletal problems?
e. Trouble smelling odors
a. Weakness in any of your arms, hands, legs, or feet
3. Have you ever had any of the following pulmonary or lung problems?
b. Back pain
a. Asbestosis
c. Difficulty fully moving your arms and legs
b. Asthma
d. Pain and stiffness when you lean forward or backward at the waist
c. Chronic bronchitis
e Difficulty fully moving your head up or down
d. Emphysema
f Difficulty fully moving your head side to side
e. Pneumonia
g Difficulty bending at your knees
f. Tuberculosis
h. Difficulty squatting to the ground
g. Silicosis
i Climbing a flight of stairs or a ladder carrying more than 25 lbs
h. Pneumothorax (collapsed lung)
j Any other muscle or skeletal problem that interferes with using a respirator
i. Lung cancer
j. Broken ribs
Part B. Any of the following questions, and other questions not listed, may be added to the questionnaire at the discretion of the healthcare professional who will review the questionnaire.
k. Any chest injuries or surgeries
This infosheet does not include the questions in Part B because they are not mandatory; rather, they may be added to the questionnaire at the discretion of the health care professional who will review the questionnaire.
l. Any other lung problem that you've been told about
Labor, 200 Constitution Avenue, N.W., N-3101, Washington, DC 20210. Telephone (202) 693-1888 or fax to (202) 693-2498.
4. Do you currently have any of the following symptoms of pulmonary or lung illness?
1. In your present job, are you working at high altitudes (over 5,000 feet) or in a place that has lower than normal amounts of oxygen
a. Shortness of breath
OSHA Educational Materials
OSHA has an extensive publications program. For a listing of free items, visit OSHA’s web site at www.osha.gov/publications or contact the OSHA Publications Office, U.S. Department of
If “yes,” do you have feelings of dizziness, shortness of breath, pounding in your chest, or other symptoms when you're working under these conditions
b. Shortness of breath when walking fast on level ground or walking up a slight hill or incline
2. At work or at home, have you ever been exposed to hazardous solvents, hazardous airborne chemicals (e.g., gases, fumes, or dust), or have you come into skin contact with hazardous chemicals
c. Shortness of breath when walking with other people at an ordinary pace on level ground
To report an emergency, file a complaint or seek OSHA advice, assistance or products, call (800) 321-OSHA (6742) or contact your nearest OSHA regional, area, or State Plan office; TTY: 1-877-889-5627.
If “yes,” name the chemicals if you know them:____________, _______________, ______________.
d. Have to stop for breath when walking at your own pace on level ground
3. Have you ever worked with any of the materials, or under any of the conditions, listed below:
e. Shortness of breath when washing or dressing yourself
a Asbestos
This InfoSheet is not a standard or regulation, and it creates no new legal obligations. It contains recommendations as well as descriptions of mandatory safety and health standards. The recommendations are advisory in nature, informational in content, and are intended to assist employers in providing a safe and healthful workplace. The Occupational Safety and Health Act requires employers to comply with safety and health standards and regulations promulgated by OSHA or by a state with an OSHA-approved state plan. In addition, the Act’s General Duty Clause, Section 5(a)(1), requires employers to provide their employees with a workplace free from recognized hazards likely to cause death or serious physical harm.
b Silica (e.g., in sandblasting)
c. Tungsten/cobalt (e.g., grinding or welding this material)
d Beryllium
e Aluminum
f Coal (for example, mining)
g Iron
j


Minimising the risk of infectious respiratory disease transmission in the context of COVID-19: The hierarchy of controls
2 July 2021
This document outlines how to use the hierarchy of controls to manage the risk of COVID-19 transmission. This advice applies to settings where there may be a high risk of COVID-19 transmission such as health care, residential care, and quarantine settings
For more guidance on infection prevention and control during the COVID-19 pandemic, see the Department of Health website
Controlling exposures to occupational hazards is the main way to protect personnel in a workplace. You can use the hierarchy of controls to achieve practical and effective control of workplace hazards
This hierarchy lists different risk avoidance or mitigation strategies in decreasing order of reliability Use multiple control strategies until you eliminate the hazard or it’s effectively minimised. These can be implemented at the same time and/or following on from one another
The hierarchy consists of hazard control measures broadly grouped into five categories. The diagram below 1 shows the most effective measures higher in the list
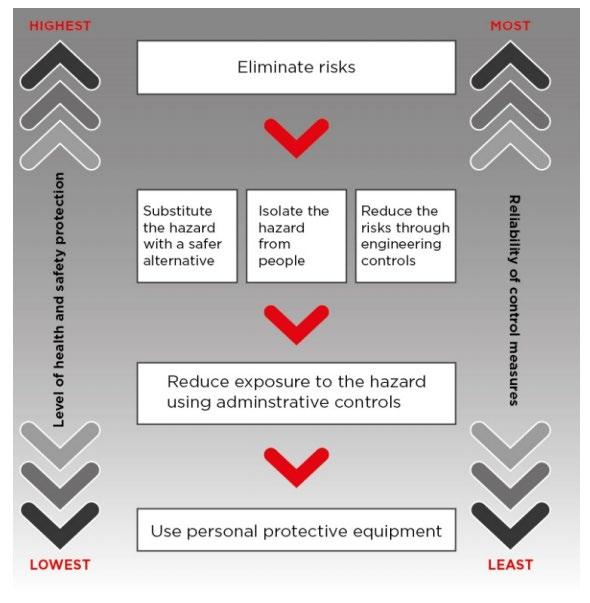
1 Source: Safe Work Australia, How to manage work health and safety risks Code of Practice May 2018 , page 19, Hierarchy of Control Measures

The model code of practice: How to manage work health and safety risks is on the Safe Work Australia website. This provides information on how to do risk assessments, including:
• how to identify hazards; and
• how to apply effective risk controls.
Applying the hierarchy of controls in health care, residential care and quarantine settings
Under the hierarchy, employers have a primary duty of care to do all that is reasonably practicable to eliminate the risk. If this is not possible, minimise risks as far as is reasonably practicable. This can be done by using one or a combination of:
• substitution
• isolation
• engineering controls
Then consider administrative controls and personal protective equipment (PPE)
Specific measures for COVID-19 have been introduced to protect people in health and residential care facilities, quarantine and other relevant high-risk settings. These include:
• advising staff and visitors with relevant symptoms to stay away
• completing health screening questions before entry
• screening body temperature and other measures
Engineering controls to prevent infectious disease transmission are an important part of the hierarchy of controls in high-risk settings. These may include measures such as:
• use of negative pressure rooms and
• optimising ventilation to manage the risk of transmission.
These measures, applied broadly as part of infection prevention and control in health and residential care and in quarantine settings, are very important.
As part of the COVID-19 outbreak response, administrative controls have become more widely adopted in health and residential care facilities.. These include:
• introduction of small staff groups (cohorts),
• using isolation wards or cohorting patients, residents or guests in separate locations to minimise risk of transmission
• making contact tracing more efficient.
Using PPE is an important component of a risk management program to prevent potential COVID-19 exposure. However, administrative controls need to support PPE use to enable timely identification and isolation of potentially infected patients, residents or staff.
PPE must be accessible at the point of care for high-risk individuals or COVID-19 cases. Correct use of PPE in line with situational risk assessment is essential. See the ICEG Guidance on the use of PPE for health care workers in the context of COVID-19 You should consider:
• individual PPE training and competency assessment
• supervising putting on (donning) and removing (doffing) PPE
• auditing PPE use
• anticipating PPE supply needs
Workplaces should adopt a complete PPE program covering all aspects of PPE use.

Standard and transmission-based precautions consist of a range of risk-minimisation strategies to prevent infection. Each strategy used in standard and transmission-based precautions sits in one or more of the five control categories.
You can adopt a range of engineering and administrative controls to reduce or avoid exposure to transmissible respiratory pathogens in health and residential care and quarantine settings Use these and appropriate PPE. Use these measures in:
• patient/resident contact settings
• other shared facilities such as
o lunch/tea rooms
o offices
o foyers
o corridors
o changing areas
o meeting rooms
o toilets
Table 1 has several suggested strategies.
Table 1. Potential risk minimisation strategies for high impact infectious respiratory pathogens, including SARS-Cov-2
Reduce opportunities for the virus to enter the facility
Do not admit SARS-CoV-2-positive patients to hospital unless clinically necessary. Manage care in home or another location if possible.
Limit the number of patients or residents going into hospitals or outpatient settings. For example:
• set up offsite or outdoor fever/testing clinics
• consider telehealth appointments.
Proactively detect and prevent entry to the facility of potentially infectious staff, students, volunteers or visitors. This includes temperature screening, travel risk assessment etc.
Reduce the number of visitors, students and non-essential staff in a facility to a minimum. Promote the use of telelinks for patient/resident visitors, where appropriate.
Reduce the number of entry points into the facility/campus and monitor visitor/staff movements Simplify visitor registration.
Exclude quarantine staff who have been exposed to infection without appropriate PPE.
Use CCTV monitoring in corridors and other high risk areas of quarantine hotels, rather than stationed security personnel.
Find other ways to provide care that will reduce the potential for transmission
Where you can, plan for alternatives to aerosol generating procedures, including high flow oxygen and continuous/bilevel positive airways pressure (CPAP/BiPAP).
Administer aerosolised medicine with spacers instead of nebulisers.

Use physical barriers and other forms of hazard reduction. For example: ventilation controls, patient separation
Review and optimise ventilation and air quality including:
• air exchange rates
• air flow and air filtration systems
• temperature
• ambient humidity.
Use negative pressure rooms with anteroom for SARS-CoV-2-positive patients where available. If a negative pressure room is not available, use a standard isolation room or single room with negative airflow Avoid rooms with positive pressure airflow
In quarantine facilities, ensure:
• sufficient air exchanges in guest rooms
• that room air does not leak significantly into adjacient corridors. Consider grouping SARS-CoV-2-positive patients in dedicated wards or zones separate to:
• uninfected patients/residents
• those with uncertain SARS-CoV-2 status.
If there are multiple COVID-19 patients/clients/residents, consider:
• increasing the distance between patients/residents/guest rooms
• a physical redesign or
• creating a dedicated SARS-CoV-2-positive quarantine area
Place quarantine hotel guests in single rooms with private bathroom facilities rather than shared rooms or bathrooms. Consider immediate transfer of SARS-CoV-2-positive guests to a healthcare facility or “medihotel”.
Consider using safe, temporary barriers to direct wandering residents or quarantine hotel guests into chosen areas.
Redesign work areas to limit number of workers at workstations
Maintain airflow direction away from staff workstations towards patient care areas where possible.
Place physical barriers such as glass or plastic screens in triage and reception areas where physical distancing is difficult to maintain.
Effective and consistent implementation of policies & protocols
Set up clear lines of governance. Assign an organisational lead with overall responsibility for overseeing:
• task analysis
• risk assessments
• ventilation assessments/monitoring indoor air quality where applicable
• infection prevention and control strategies implementation
• promoting and facilitating hand hygiene, respiratory hygeine and cough etiquette

Minimise opportunities for infection transmission
Ensure evidence-based infection prevention and control policies and guidance are in line with national guidance. If needed, adapt the guidance to suit local worker health and safety requirements and other conditions.
Give clear guidance on when to change resident placement For example, residents with signs and symptoms typical of COVID-19 should not have roommates.
In quarantine settings, consider making “safe” and “high-risk” zones to help staff and guest movement.
Ensure staff training and competency assessment in standard and transmission-based precautions is provided.
Give guidance on environmental cleaning and disinfection according to risk. Conduct regular checks with frequency determined by risk.
Give continuing and appropriate education on infection prevention and control to all staff, residents and visitors.
Regularly update residents, family members, staff, other service providers and the broader community on COVID-19 policies
Give policy support to reduce the risk of staff attending when unwell This can include conducive pay and leave arrangements for casual staff
Discourage casual staff from working across different facilities. Ensure that agency staff satisfy infection control training requirements before employment
Use signage (in appropriate languages) at the facility entrance to alert visitors to not attend while unwell.
Consider surveillance testing of asymptomatic staff during periods when community transmission is locally prevalent
Do regular testing of quarantine facility staff to detect infection early and remove staff from duty.
If possible, delay new admissions to residential aged care facilities during periods of community transmission. Give alternative home-care.
Separate care of SARS-CoV-2-positive and unaffected patients or residents. Assign staff to care groups and reduce frequency and number of personnel on ward rounds.
Triage and manage visitors. Ensure they comply with hand hygiene and PPE requirements.
Reduce opportunities for transmission between staff by promoting use of telehealth technology for all staff meetings.
Allocate surgical masks for source control to patients or residents with respiratory symptoms to use when they are outside of their ward or room. Educate patients/residents/guests on safe mask use and disposal.
In quarantine facilities, ensure guests remain in their allocated room, use a surgical mask whenever the door is opened, and maintain physical distancing from staff
Maintain staff wellbeing
Personal Protective Equipment (PPE)
Review PPE policies and guidelines
Set up a respiratory protection program

Manage all workspaces to reduce respiratory transmission risk by adopting measures to improve physical distancing For example, floor markings, spaced seating, maximum room occupancy notices.
Adopt general measures to reduce contact spread, such as education and training. Have enough hand hygiene products and facilities available, and increase cleaning and disinfection of shared areas.
Set up a plan to manage a facility outbreak and ensure all stakeholders are aware of roles and responsibilities.
Use standardised infection control signage for standard and transmission-based precautions
Have enough staff to avoid excessive workloads and ensure staff can take regular breaks.
Know which staff may be vulnerable to severe COVID-19 infection and redeploy if needed.
Develop policy to manage staff and others who become unwell in the workplace.
Ensure all staff providing health care, aged care, and hotel quarantine services are vaccinated against SARS-CoV-2 as soon as practicable.
Provide an employee assistance program that provides psychological support.
Protect the worker
Have risk-assessed PPE recommendations for specific staff roles and activities. Make sure these are consistent with ICEG Guidance on the use of PPE for health care workers in the context of COVID-19
Have enough supply of PPE items and related equipment at the point of use.
Give effective education and communicate on appropriate PPE use for standard, contact, droplet and airborne precautions.
Conduct regular staff PPE donning and doffing competency assessments
Manage the PPE supply chain across all levels of the health service and ensure appropriate PPE ordering by staff.
Fit test staff who may need to wear a particulate filter respirator (P2/N95 or equivalent).
Train staff to perform a fit check (seal check) every time a P2/N95 respirator is used.
Emphasise the importance of eye protection as an essential component of droplet and airborne precautions. Train staff in safe cleaning of reusable eyewear, if used.
Consider the use of reusable powered air purifying respirator (PAPR) or elastomeric respirator where there is high risk from aerosol exposure For example, critical care environments. Train staff in their safe use.

Where PAPR devices or equivalent are available to use, ensure staff:
• are trained and competency assessed for their use
• continue to use the devices to maintain currency of practice
Safe Work Australia. How to manage work health and safety risks Code of Practice MAY 2018. Available at https://www.safeworkaustralia.gov.au/system/files/documents/1901/code_of_practice__how_to_manage_work_health_and_safety_risks_1.pdf
The Australasian Faculty of Occupational and Environmental Medicine (AFOEM) in the Royal Australasian College of Physicians has produced a detailed document on COVID-19 workplace risk mitigation strategies. This is available at: https://www.racp.edu.au/docs/defaultsource/advocacy-library/covid-19-workplace-on-workplace-risk-management.pdf?sfvrsn=88f5f71a_4
Queensland Health. Hierarchy of controls for prevention of COVID-19 transmission in hospitals. Available at: https://www.health.qld.gov.au/__data/assets/pdf_file/0021/1012683/hierarchyof-controls-prevention-covid-19.pdf
SA Health. Strategies for optimising supply of personal protective equipment
Kelaher, et al. How do we Find a “New Normal” for Industry and Business After COVID-19 Shut Downs?, Journal of Occupational and Environmental Medicine: Sept2020, 62 (9),p e531e534, https://journals.lww.com/joem/fulltext/2020/09000/how_do_we_find_a__new_normal__for_industry_and .24.aspx
Australian Guidelines for the Prevention and Control of Infection in Healthcare (2019): https://www.nhmrc.gov.au/about-us/publications/australian-guidelines-prevention-and-control-infectionhealthcare-2019
Australasian Health Facility Guidelines: https://healthfacilityguidelines.com.au/aushfg-parts
Morawska, L. et al. How can transmission of COVID-19 indoors be minimised? Environment International, vol 142, Sept 2020. https://www.sciencedirect.com/science/article/pii/S0160412020317876?via%3Dihub
training@kinnecttraining.com.au | 1300 591 548