
9 minute read
Minimally Invasive Surgery for High-Grade Spondylolisthesis
Current Evidence and Clinical Outcomes
Spondylolisthesis refers to the displacement of a vertebra in relation to the one beneath it. This cranial vertebra may be displaced anteriorly (anterolisthesis), laterally, or posteriorly (retrolisthesis) compared to the caudal vertebra. This condition can occur at any level of the spine and can originate from various causes, including congenital, acquired, or idiopathic factors. Although there is no universally accepted definition, spondylolisthesis is often described in the literature as the forward or backward displacement of a vertebral body relative to the adjacent caudal vertebra by at least 3 mm, 5 mm, or 5%, depending on the diagnostic criteria applied in each study.1 Spondylolisthesis is classified based on the extent of vertebral slippage, with anterolisthesis in the lumbar spine and lumbosacral junction (L5-S1) being the most clinically significant types. The Wiltse classification of spondylolisthesis has 5 types based on underlying etiology: Type I (dysplastic); Type II (isthmic) including fatigue fractures (IIA), elongation from microtrauma (IIB), or acute fractures (IIC); Type III (degenerative); Type IV (traumatic); and Type V (pathologic).2
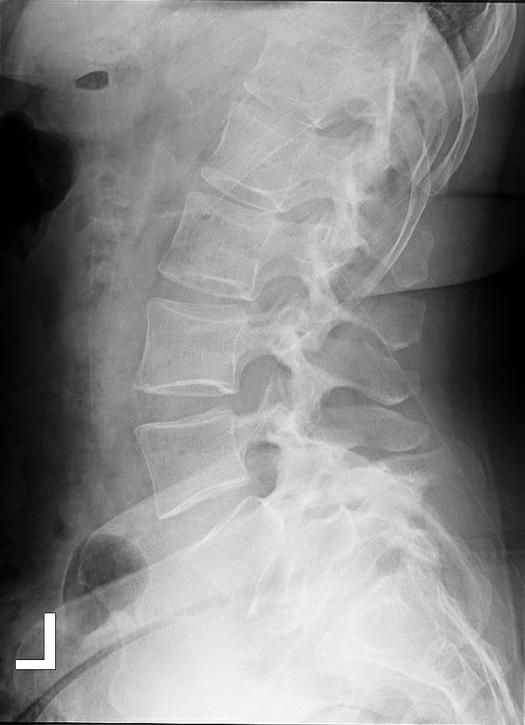
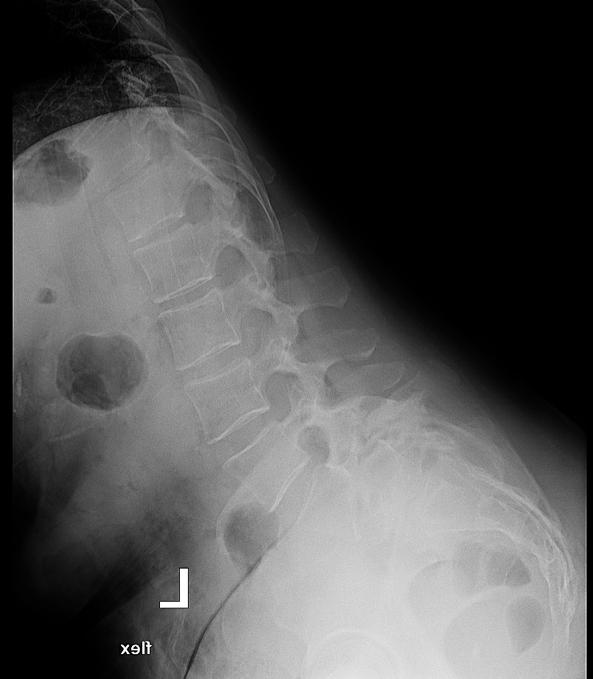
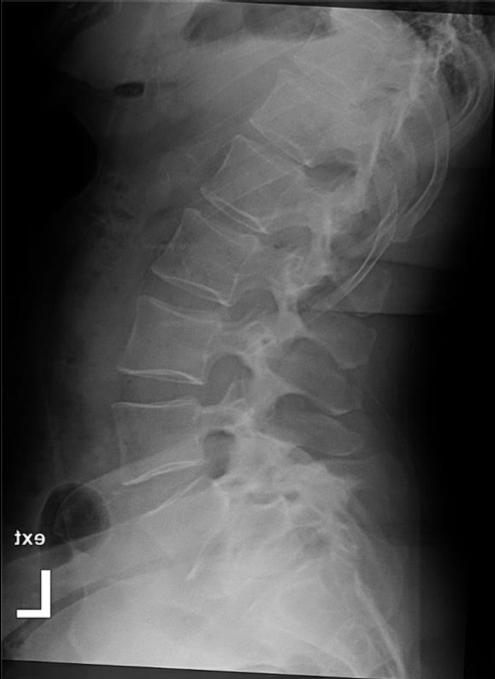
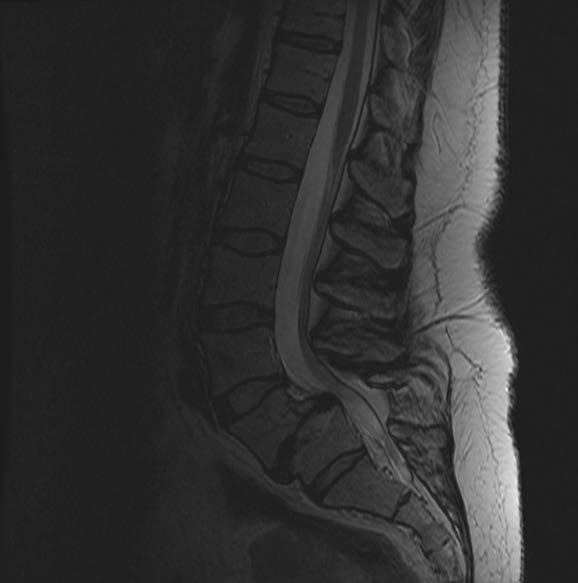
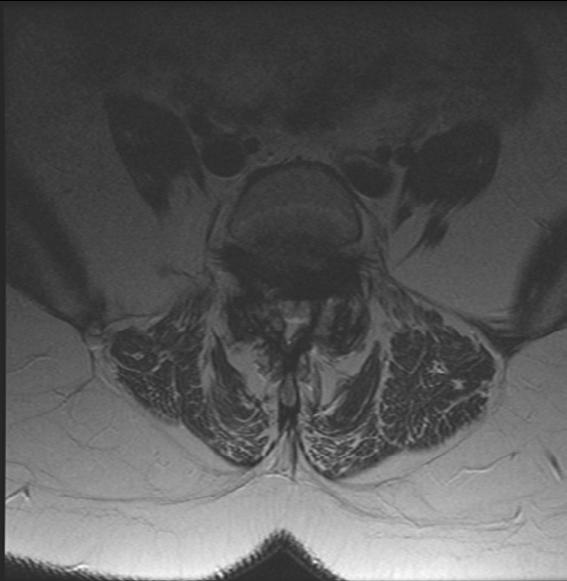
The Meyerding classification categorizes spondylolisthesis according to the extent of anterior vertebral slip seen on standing lateral radiographs. The system classifies slippage into 5 grades: Grade I (0%–25%), Grade II (25%–50%), Grade III (50%–75%), Grade IV (75%–100%), and Grade V (>100%). Grade V, also called spondyloptosis, signifies total vertebral displacement. 3 Grades I and II are categorized as low-grade slips, whereas Grades III to V are identified as high-grade. 4 High-grade spondylolisthesis (HGS) is a more severe form, accounting for approximately 11.3% of cases and typically presenting as nonspecific lower back pain, which may sometimes be associated with radicular symptoms.4,5 Diagnostic evaluation begins with weight-bearing lumbar radiographs, including flexion-extension views to assess instability defined as >4 mm translation or >10° angulation (Figure 1).3 MRI is preferred for evaluating nerve compression, particularly in cases with persistent symptoms (Figure 2).4 The aim of this review is to evaluate the current evidence and clinical outcomes of minimally invasive surgical (MIS) approaches for the treatment of HGS.
Surgical Management of HGS
Surgical options range from decompression alone to fusion with or without instrumentation.4,6 Adults with HGS often develop a stable state (autofusion or ankylosis), which reduces concerns about slip progression. Many are asymptomatic or minimally symptomatic and respond well to physical therapy and selective nerve root injections if radicular symptoms exist. Surgery is advised if conservative treatments fail, especially in HGS with back pain or radicular symptoms.7,8 Unlike low-grade spondylolisthesis (LGS), HGS is associated with secondary changes in pelvic anatomy, potentially leading to global sagittal deformity with intractable back pain or deformity, which may also indicate surgery. Management focuses on functional recovery and restoring spinopelvic sagittal balance, either through full or partial spondylolisthesis reduction.8,9 The best surgical method for HGS is still debated, especially when choosing between reduction and in-situ fusion.4,5 Traditionally, in-situ fusion was preferred because of worries about nerve damage during reduction, with early research showing higher rates of nerve issues, more blood loss, and longer surgeries.4,8 Reduction offers biomechanical benefits by improving sagittal alignment through correcting lumbosacral kyphosis, which turns shear forces into compressive ones, boosts fusion success, and lowers deformity progression risks. It also leads to better cosmetic results and enhances the quality of life.10 Partial reduction has become popular because it can correct the slip angle while reducing nerve injury risk.7 On the other hand, in-situ fusion might still be suitable for certain patients, especially those without major deformity, radiculopathy, or sagittal malalignment. However, it tends to have higher chances of nonunion, ongoing kyphotic deformity, and slip progression, even if a solid fusion is achieved.10
MIS vs Open Surgery
Conventional management of HGS has generally favored open surgical methods to better address anatomical complexity and neurological complications. Patients with LGS typically present with relatively preserved spinal anatomy, making it well suited for MIS approaches.11,12 HGS is associated with significant anatomical distortion, and the severe anterior translation of the vertebral body can make it more difficult to achieve adequate decompression with tubular retractors, potentially impairing visualization through surgical windows.13 This may also lead to difficulties in identifying critical anatomical landmarks, properly positioning interbody devices, and safe navigation around neural structures.
Due to increased complication rates, particularly neurological complications, traditional open surgery remains the preferred option for most HGS cases.13 Lak et al noted higher complication rates with MIS (55%) compared to open (20.6%). However, other studies employing recent MIS techniques have shown minimal or no complications.9,14,15 As such, advancements in MIS techniques, while technically challenging in HGS, have introduced new treatment options.
Technical refinements, optimized patient positioning, and refined reduction strategies are key to successful MIS outcomes. While Lak et al reported better 10-year outcomes with open surgery in 2020, more recent studies have demonstrated the successful application of MIS approaches, yielding good clinical outcomes.13 Chang et al14 and Kulkarni et al15 used MIS-TLIF, while Ramirez Velandia et al 9 adopted a 2-stage ALIF plus posterior approach. Chang et al reported good 1.5-year outcomes, and Kulkarni et al’s 4-year follow-up reported favorable outcomes.9,14,15 Rajakumar et al’s “rocking” technique represents a significant advancement in preserving the benefits of MIS while achieving reduction results that were once believed to require open surgery.16 MIS proponents highlight benefits such as reduced pain, faster recovery, less blood loss, and preserved structural support, which are crucial for spondylolisthesis to avoid destabilizing the surrounding spine support structures.17
These studies have progressively bridged the gap between MIS and open techniques, challenging the notion that complex reductions and sagittal corrections are exclusively achievable through traditional open surgery. However, the predominance of Grade II and III cases in the literature reflects both the higher prevalence of these grades and the technical challenges associated with treating Grade IV and V spondylolisthesis.
Conclusion
MIS approaches, particularly MIS TLIF, represent a promising yet technically challenging strategy for the management of HGS. Recent sophisticated MIS techniques may allow one to achieve faster pain relief, sufficient restoration of sagittal alignment, and successful fusion with minimal complications. However, careful patient selection and surgeon experience are essential, and open approaches may still be preferable in highly complex cases. As MIS technology advances, future research should emphasize multi-center collaboration to gather larger patient cohorts, standardize outcome measures, and extend follow-up periods, helping define optimal treatment strategies for this complex condition.
References
1. Bydon M, Alvi MA, Goyal A. Degenerative lumbar spondylolisthesis. Neurosurg Clin N Am. 2019;30(3):299-304.
2. Wiltse LL, Newman PH, Macnab I. Classification of spondylolisis and spondylolisthesis. Clin Orthop Relat Res. 1976;(117):23-29.
3. Koslosky E, Gendelberg D. Classification in brief: the Meyerding classification system of spondylolisthesis. Clin Orthop Relat Res. 2020;478(5):1125-1130.
4. Kunze KN, Lilly DT, Khan JM, et al. Highgrade spondylolisthesis in adults: current concepts in evaluation and management. Int J Spine Surg. 2020;14(3):327-340.
5. Beck AW, Simpson AK. High-grade lumbar spondylolisthesis. Neurosurg Clin N Am. 2019;30(3):291-298.
6. Morse KW, Steinhaus M, Bovonratwet P, et al. Current treatment and decision-making factors leading to fusion vs decompression for one-level degenerative spondylolisthesis: survey results from members of the Lumbar Spine Research Society and Society of Minimally Invasive Spine Surgery. Spine J. 2022;22(11):1778-1787.
7. DeWald CJ, Vartabedian JE, Rodts MF, Hammerberg KW. Evaluation and management of high-grade spondylolisthesis in adults. Spine. 2005;30(6S):S49-S59.
8. Kasliwal MK, Smith JS, Kanter A, et al. Management of high-grade spondylolisthesis. Neurosurg Clin N Am. 2013;24(2):275-291.
9. Ramirez Velandia F, Gomez Cristancho DC, Urrego Nieto A, et al. Minimally invasive surgery for managing grade IV and V spondylolisthesis. Asian J Neurosurg. 2023;18(03):437-443
10. Elias E, Daoud A, Elias C, Chiu RG, Sanchez JM, Nasser Z. Historical evolution, management, and outcome of surgical treatment for high-grade spondylolisthesis: a systematic review. J Neurosurg Spine. Published online April 1, 2025.
11. Archavlis E, Carvi Y Nievas M. Comparison of minimally invasive fusion and instrumentation versus open surgery for severe stenotic spondylolisthesis with high-grade facet joint osteoarthritis. Eur Spine J. 2013;22(8):1731-1740.
12. Chan AK, Bydon M, Bisson EF, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for grade I lumbar spondylolisthesis: 5-year follow-up from the prospective multicenter quality outcomes database registry. Neurosurg Focus. 2023;54(1):E2.
13. Lak AM, Abunimer AM, Rahimi A, et al. Outcomes of minimally invasive versus open surgery for intermediate to high-grade spondylolisthesis: a 10-year retrospective, multicenter experience. Spine. 2020;45(20):1451-1458.
14. Chang PY, Liao CH, Wu JC, et al. Reduction of high-grade lumbosacral spondylolisthesis by minimally invasive transforaminal lumbar interbody fusion: a technical note. Interdisc Neurosurg. 2015;2(2):79-82.
15. Kulkarni AG, Kumar P, Umarani A, Patil S, Chodavadiya S. Minimally invasive transforaminal interbody fusion for high-grade spondylolisthesis: a retrospective study analysis of a tailor-made solution. Asian Spine J. 2025;19(1):10-20.
16. Rajakumar DV, Hari A, Krishna M, Sharma A, Reddy M. Complete anatomic reduction and monosegmental fusion for lumbar spondylolisthesis of Grade II and higher: use of the minimally invasive “rocking” technique. Neurosurg Focus. 2017;43(2):E12.
17. Lu VM, Kerezoudis P, Gilder HE, McCutcheon BA, Phan K, Bydon M. Minimally invasive surgery versus open surgery spinal fusion for spondylolisthesis: a systematic review and meta-analysis. Spine. 2017;42(3):E177-E185.
18. Rivollier M, Marlier B, Kleiber JC, Eap C, Litre CF. Surgical treatment of highgrade spondylolisthesis: Technique and results. J Orthop. 2020;22:383-389.
Contributors:
Atahan Durbas, MD1
Joshua Zhang, BS1
Raul F. Montes, BS1,2
Tomoyuki Asada, MD, PhD1,3
Sheeraz A. Qureshi, MD1,2
From the 1Department of Orthopaedics at the Hospital for Special Surgery in New York City, New York; 2Weill Cornell Medical College in New York City, New York; and 3Department of Orthopedics at the University of Tsukuba Hospital in Tsukuba, Japan.








