





The Award for Best Irish Pharmacy Brand went to Revive Active as part of their hat-trick of wins.
Revive Active is an awardwinning super supplement with 26 active ingredients working together, helping put back what life takes out.
This Irish super supplement is delivered in a powdered format and contains a comprehensive combination of vitamins, minerals and amino acids in one convenient daily powdered sachet. The daily sachet can be added to water, juice or a smoothie and due to the form of ingredients used and the powdered format it offers higher bioavailability as vitamins and minerals dissolved in liquid form are absorbed in the body more easily than in tablet or capsule form.
Each sachet contains a powerful combination of ingredients, delivered in a convenient way to support active people balancing work, home and family life while maintaining a healthy immune system, energy levels and heart and supports a reduction in fatigue.

“A trusted product from a well-established, trusted brand. Well-known brand ambassadors including a GP, increase the chance of sales in pharmacy. Training support to pharmacy is excellent. Nice to have a new flavour”
“The product has very appealing packaging and has a brilliant marketing campaign. I have found that customers come on a repeat basis for this product as they believe it has made a positive impact on their health”




 Revive Active Brand Managers Julieanne Barrett and Joanne Clancy with Head of Brand/Marketing Helen Mannion and Aislinn Connaughton, Senior Digital Marketing Executive
Revive Active Brand Managers Julieanne Barrett and Joanne Clancy with Head of Brand/Marketing Helen Mannion and Aislinn Connaughton, Senior Digital Marketing Executive

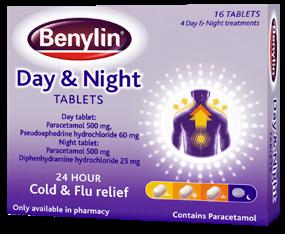




BENYLIN® DAY & NIGHT came out on top within the 2024 Best Cough, Cold and Fly Product category.
BENYLIN® DAY & NIGHT tablets are the only cold/flu remedy to offer 24 hour relief with tablets specifically designed for Day and Night.
Today’s hectic lifestyle means cold sufferers often don’t have the opportunity to take time out from their busy lives. BENYLIN® DAY & NIGHT tablets unique offering helps consumers to keep going throughout the day by relieving their symptoms, while the night tablet relieves cold symptoms and so aids a better night’s sleep.
The product is the only day & night tablets for 24 hour cold and flu relief, and the consumer-friendly packaging clearly demonstrates which tablets are for each occasion, day and night.
Benylin is supported in-store each winter season with brand assets and POS materials as well as TTL media campaign.
“This is a great product for alleviating cold/flu symptoms, enabling people to avoid suffering from symptoms of viral illness and continue with their normal daily lives (relatively) unaffected by the symptoms thanks to the product. Handy that it helps with both day time and night time. Helps aid sleep which is vital for recovery from cold/flu. Good value for money”
“A great product that clearly sets out the time each tablet should be taken. Brilliant to have an option for people who do not want medication that will make them drowsy during the day yet gives them the option to have a medication that will help them to sleep at night, without having to buy a second product”


Best VMS product was awarded to Zest Active - an innovative super supplement from Irish Supplement company Revive Active.

Zest Active is scientifically formulated with 25 active ingredients including vitamins, minerals, amino acids and Beta Glucans, all carefully selected to support energy, the immune system, brain, and muscle function. Each sachet contains a range of B Vitamins (Riboflavin (B2), Niacin (B3), Pantothenic Acid (B5), B6, Folate, B12 which ensures that Zest Active contributes to a normal energy-yielding metabolism.
It contains a natural sweetener, steviol glycosides and is suitable for vegetarian, vegan, diabetic, and gluten free diets and is Halal certified. Zest Active contains no caffeine, binders or fillers. With a refreshing orange flavored blend, Zest Active delivers the daily nutrients you need, to help you unlock your true potential.
“Zest Active has risen to the most popular line in the already popular Revive Active range. This is perfect for all people on the go, and suitable for a wide range of active people looking for the ideal pick me up vitamin drink”
“Excellent product, well known and great tasting. Many repeat customers”
Judges Comments







 Brand Managers with Revive Active Tina Duane and Julieanne Barrett
Brand Managers with Revive Active Tina Duane and Julieanne Barrett





Broncho 5in1 scooped their second award winning the Best Marketing/Training Campaign for 2024..
The cough category has not developed the same way as other categories, there are a lot of single indication SKUs; dry cough, chesty cough cluttering the category. While this may be beneficial for other categories it doesn’t apply to cough as coughs tend to change during the cycle; start with a sore throat which then becomes a dry cough and then a chesty cough.
72% of people don’t know what type of cough they have.
Introducing, NEW Broncho 5in1 Multi-Symptom Action
Syrup for all coughs associated with a cold to relieve: dry cough, chesty cough, sore throat, hoarseness and throat dryness. We lent on the successes of Bronchostop (THR) and Bronchostop Junior (MD) to develop a product that not only targets cough but sore throats; often the first sign
“Excellent marketing / social media campaign to improve brand awareness which has really driven sales”
“A great product that targets all types of cough, strong advertising from brand to support this launch”
Judges Comments


 Sinead Kingston, Brand Manager, Perrigo
Sinead Kingston, Brand Manager, Perrigo





MULTI-SYMPTOM ACTION SYRUP
Marshmallow, Icelandic moss and Hyaluronic acid in 5 1


Throat dr yness associated with a cold to relieve: in 5 1
+ + +
Dr y cough
Ches t y cough
Sore throat
Hoar seness










Broncho 5 in 1 Multi-Symptom Action Syrup is a medical device according to Directive 93/42/EEC, used for the relief of any cough (dry & chesty) associated with a cold, as well as sore throat due to common cold, associated hoarseness, and dryness of the throat. Adults and adolescents from 12 years of age: 15ml up to 3 times daily. Not recommended for children under 12 years. Date of preparation: Feb 2023. IRE BRO 2023 26

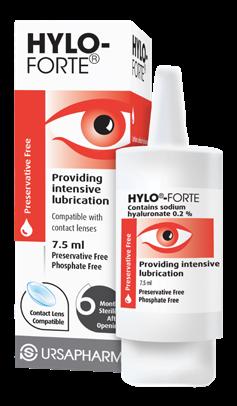



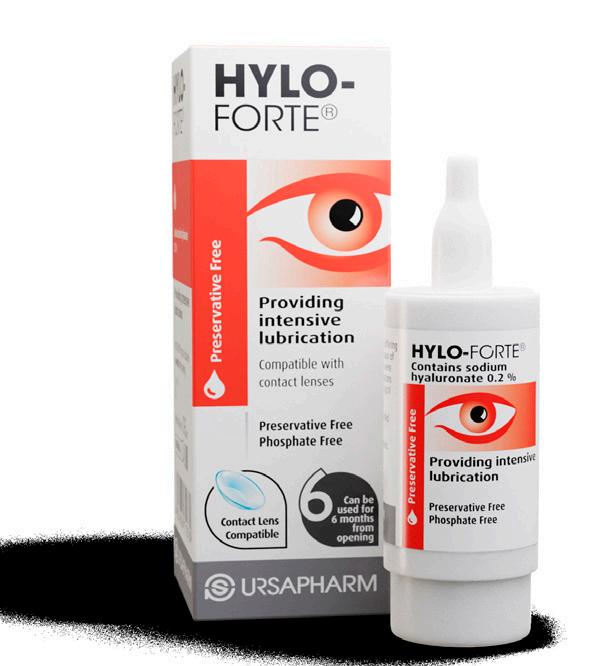
Not to damage their winning streak, Scope Healthcare’s HYLO-FORTE® won the Best Eye Care Product for the third consecutive year.
HYLO-FORTE® is a preservative free, contact lens compatible lubricant containing 0.2% High Molecular Weight Hyaluronic Acid to provide long lasting hydrating relief. The high molecular weight structure of this Hyaluronic Acid helps provide longer lasting benefits to patients with dry eye disease as it is the superior performing hyaluronic acid for ophthalmic use.
HYLO-FORTE® is conveniently presented in the patented COMOD bottle which offers patients precise dosing with a guaranteed 225 drops and 6 months sterility from first opening.
“An unrivalled eye hydration product. The closed valve system enabling use for up to 6 months once open is costeffective for customers and also easier on the eyes, being preservative free. Excellent training available on the Scope website and training support offered to pharmacies is excellent”
“A revolution in eye drops with its packaging which allows use for 6 months. There is great customer feedback from users and many resales. Quite simply the market leading brand in eyecare”




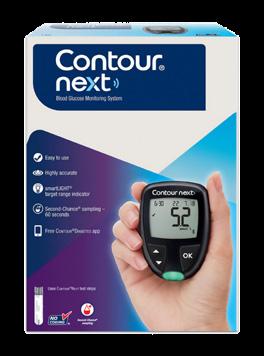


CONTOUR®NEXT Blood Glucose Meter are the winners of the 2024 Best SelfTesting Product.
The CONTOUR®NEXT blood glucose meter is the most used meter in Ireland; with the team behind the brand focused on quality products, improving patient lives and empowering self-management along with providing top class support and education for pharmacists.
The CONTOUR®NEXT is an easy-to-use system:
Easy to read: Big numbers on display.
Easy to use: Large rubber buttons.
No initial set up.
Personalised target ranges with averages on the meter. The unique smartLIGHT® feature makes it quicker and easier to interpret blood glucose readings using coloured lights that clearly identify if the reading is above, within or below your target range.
CONTOUR®NEXT (connected) has a 60 second count down screen that provides patients the opportunity to apply more blood to the same strip if the first sample is insufficient.
“Excellent product. It is an easy to use system and patients like the large print and easy to use system. The use of technology through the app is helping patients keep track of their blood sugars and helps as they can take this information to their healthcare appointments”
“As a Pharmacist, this is my go-to recommendation when a patient presents to the Pharmacy requesting a glucometer. I can see why the brand has a strong customer following”


• Highly accurate1 readings you can trust to guide your patients
• smartLIGHT® feature. Target range indicators have been demonstrated to help improve HbA1c control compared to BGMs without a target range indicator*2
• 60-second Second-Chance® sampling can reduce the need to re-lance and can help save strips.3
• Connects to the CONTOUR®DIABETES app to support self-management







Teva’s Sudocrem Antiseptic Healing Cream won the Award for Best Children and Baby Product.
Trusted by families since 1931, Sudocrem Antiseptic Healing Cream has been parent’s simple, effective skin-care ally for over 90 years. Great for soothing and treating nappy rash symptoms, its gentle formulation is clinically proven to soothe and heal your baby’s delicate skin from birth. A truly versatile product, Sudocrem Antiseptic Healing Cream is a family favourite when it comes to their skincare needs.
Sudocrem Antiseptic Healing Cream is licensed to treat a range of skin conditions including nappy rash, cuts & grazes, eczema, acne, minor burns, sunburn, bedsores & chilblains. Always read the label.
“Sudocrem is a real household name in Ireland. It is used by everyone from birth to old age. It serves as a great barrier and repair cream for damaged skin. I’m pretty sure there’s one in every home”
“Sudocrem has been a staple of many Irish households and has been used for generations. Brand loyalty is exceptionally high and repeat custom is clearly evident. The iconic packaging and variety of pack sizes gives great options for the consumer. It is still one of the leading brands and products in its market category”




A relatively newer product on the market, fabÜ SHROOMS MENO & PERI won the 2024 Best Natural Product Award.
fabÜ SHROOMS MENO & PERI is a unique supplement with seven active ingredients. It is based on the concentrated extract of the functional, edible mushroom Lion's Mane, blended with Vitamin D, Vitamin K2, Vitamin B6, Vitamin B12, Magnesium and Chromium tailored specifically to support a woman's journey through perimenopause and menopause.

This blend was expertly formulated by Laura Dowling, Fabulous Pharmacist, after years of research and development and many more years of listening to women coming into the pharmacy everyday looking for extra support. It is trusted by hundreds of thousands of women across Ireland.
Lion's Mane mushroom, is long revered in ancient practices and eaten around the world. It has been used traditionally in Asia to support women through perimenopause and menopause. This edible mushroom is carefully blended with premium grade vitamins and minerals.
Simple & Safe Usage: Just two mushroom capsules daily with water. Designed with your convenience and safety in mind, our mushroom supplement is the hassle-free solution to support your journey through perimenopause and menopause.
“A great product – we frequently have customers tell us about the improvement and difference this product has made to their lives. This brand provides excellent support and useful online training sessions to pharmacy staff”
“What can I say these products are so well known. People trust the brand now. Repeat sales guaranteed. Great feedback from customers and I hear of excellent results all the time. Laura Dowling is an excellent ambassador for the brand and people really trust the recommendation of their local Irish pharmacist”

Judges Comments




 Laura Dowling, Fabüwellness
Laura Dowling, Fabüwellness





Alflorex® is the winner of the 2024 Best Digestive Product.
Alflorex® is the leading probiotic for IBS, a condition that affects up to 1 in 7 people in Ireland every day. This probiotic is taken daily to manage and prevent recurrence of IBS symptoms. It costs approx. ¤1/day and reduces ALL of the symptoms of IBS including:
• abdominal pain
• bloating & gas
• unpredictable bowel movements like alternating diarrhoea and constipation
The unique 35624® culture in Alflorex is a naturally occurring bacterial strain found in the human gut. This Bifidobacterium longum culture is part of the family of bacteria given by mothers to babies at birth.
The main long-term aim for Alflorex is to strive to have all IBS patients in Ireland take Alflorex for 1 month. The product conversion score is more than 80% (continued usage) and with a high percentage of this group needing direction and treatment we believe Alflorex can change their lives.
“Alflorex is the highest selling brand in our entire Vitamin Portfolio and trusted by many of our customers (and staff members!) Brand support has been exceptional for us. Given the extensive training and sampling support given, our staff are well equipped to be able to recommend this product to our customers” “Excellent product targeted at IBS. Superb training and great PR plan to back it up along with clinical trial evidence support. Gives great clarity to pharmacy team on how to recommend”



















Colief Infant Drops takes the 2024 Award for Best Children and Baby Supplement.
Colief Infant Drops have been trusted by mums and healthcare professionals for over 25 years as a natural remedy for infant colic. Suitable from birth, Colief Infant Drops are completely natural and help break down the lactose in milk into simpler sugars, glucose and galactose, making it easier for baby to digest milk whilst reducing discomfort.
The drops contain the naturally occurring enzyme lactase which helps relieve colic symptoms caused by temporary lactose intolerance – common in many babies as their digestive systems develop. Adding a few drops to infant formula or mum’s expressed breast milk helps to break down the lactose in the milk, making it easier to digest.
“This Irish product is in a league of its own. The natural remedy makes it easy to recommend to distressed parents that present to the Pharmacy suffering from colic. The packaging is very nice and while there is plenty of information on the outer box, it is concise and easy to read”
“I really love this product both as a pharmacist and a parent - I’ve used it for my own children with great success and recommend it often in work”



Adding Colief® Infant Drops to baby milk is a natural way to ease colic symptoms caused by lactose.

Infant colic can have many causes. In some cases it is caused by temporary lactose intolerance. This occurs when babies are unable to fully digest the lactose in milk, resulting in discomfort and extended periods of crying. Suitable from birth, Colief Infant Drops contain lactase enzyme, which breaks down lactose into glucose and galactose, reducing the lactose in milk and making it easier for babies to digest. babies to digest.

















The Colief® range also includes:
Colief® Multibiotic Drops For a happy gut


























































Mums know to call on Colief® facebook.com/ColiefCare www.colief.com
Tel: 01 2968080 | orders@oceanhealthcare.ie | www.oceanhealthcare.ie
Scientifically proven friendly bacteria developed to complement baby’s natural gut flora. Contains Lactobacillus rhamnosus, LGG®, one of the most researched bacteria strains in the world, and Bifidobacterium infantis, a scientifically proven friendly bacteria for babies and children. Suitable for breastfed and formula fed babies, from birth onwards Colief® Multibiotic Drops contains 1.1 billion live cultures per dose.






Best Immunity Product for 2024 goes to Symprove.
Symprove is a water-based digestive health supplement designed to support the digestive system and enhance your overall well-being. Developed by leading experts in the field, Symprove is crafted to help you achieve optimal gut health.
Symprove utilizes a unique formulation of live and active bacteria that work harmoniously with your body's microbiome. Through a patented delivery system, these beneficial bacteria bypass the harsh stomach environment and reach the targeted areas of your gut, where they can flourish and provide their numerous benefits. One shot of Symprove each morning is the ideal way to maintain the balance of bacteria in your gut.
“There are not many products available to rival Symprove. There is a niche audience searching for this product to aid digestion and improve symptoms they are suffering with daily. The marketing campaign is a great success”
“The product is very well packaged and has a convenient once daily dosage formulation which is ideal for most people to remember to take first thing in the morning. The product has an excellent marketing campaign and there are numerous supports available for staff to learn about the product”









The beauty industry is fast-moving and highly saturated. Understanding changing consumer opinions and the behavioural indicators behind global trends is integral to securing the success of any company or brand within the space.
The Award was won by Revive Active’s Beauty Complex.
Beauty Complex is a unique and innovative super supplement which contains a distinctive combination of eight active ingredients including Marine Collagen, Phytoceramides, Hyaluronic Acid and Biotin. It was designed by experts to compensate for declining collagen, ceramides and hyaluronic acid in the body. The type of collagen used in Beauty Complex is Collagen Peptides. These peptides are derived from a 100% natural source, containing more than 97% protein and are the same as the collagen found in our skin and bones. Their hydrolysed form makes them more easily digestible.
The key is the combination of ingredients delivered in optimal quantities that provide synergistic effects. This results in consumers really noticing a difference in their skin, hair and nails.
“Great product – does exactly what it says. Customer feedback on this is always positive regarding how well it works and many are purchasing again making it a high volume selling product within our pharmacy. As a team we always recommend Revive Active products to our customers”










Broncho 5in1 took home the accolade for Best Launch of the Year.
The cough category has not developed the same way as other categories, there are a lot of single indication SKUs; dry cough, chesty cough cluttering the category. While this may be beneficial for other categories it doesn’t apply to cough as coughs tend to change during the cycle; start with a sore throat which then becomes a dry cough and then a chesty cough. 72% of people don’t know what type of cough they have.
Introducing, NEW Broncho 5in1 Multi-Symptom Action Syrup for all coughs associated with a cold to relieve: dry cough, chesty cough, sore throat, hoarseness and throat dryness. We lent on the successes of Bronchostop (THR) and Bronchostop Junior (MD) to develop a product that not only targets cough but sore throats; often the first sign of a cough.
“With it’s clear messaging, Broncho 5in1 puts itself out there as the one-stop shop for your cough relief needs. Bronchostop as a brand is very well respected, and has risen to a top seller every winter. The addition of this multi-relief SKU to the range, can only enhance its appeal”
“Brilliant addition to the category. Excellent support. Clear training for pharmacy team and clarity of use for the patient. Targeted cough and sore throat makes this a unique product”
Judges Comments







Best Women’s Product was voted for by our esteemed judging panel and the winner for 2024 is Optibac For Women.
Optibac For Women is recognised as one of the best probiotics for intimate health. It is Optibac’s best-reviewed supplement, being highly recommended by complementary healthcare practitioners, top UK gynaecologists, and consumers alike.
This specialist supplement is designed specifically for the support of vaginal health. For Women contains highly researched strains of friendly probiotic bacteria, which are scientifically proven to reach the intimate area and complement the natural vaginal flora.
Optibac For Women is recommended for anyone suffering with thrush, Cystitis or BV, and for anyone susceptible to these conditions wishing to maintain vaginal health on an ongoing basis. Ideal for women during all life stages, including pregnancy, menopause and beyond.
“This is an amazing product that I feel very happy recommending to patients. These are a cohort that really struggle as the symptoms around BV, thrush, UTIs can be really debilitating and embarrassing for women. I love the product packaging and I think it is really impressive that they considered recyclable” “Game Changer for women with ongoing BV Issues, particularly liked the combination of ingredients”


 Lauren Byrne, Sales & Training Representative and Madeleine Walsh, Ireland Regional Manager
Lauren Byrne, Sales & Training Representative and Madeleine Walsh, Ireland Regional Manager
“Truly

“Absolutely

“I’m
Helen





Panadol Extra Soluble proved to be the judges choice for the Best Pain Relief Product category 2024.
Made and Trusted in Ireland’. Panadol Extra Soluble is indicated for 5 types of pain, is only available in pharmacies and contains a second active ingredient, caffeine that amplifies the analgesic effect of paracetamol, making it more powerful*. So, when you’ve got a headache you can count on Panadol Extra Soluble to deliver powerful, effective relief. And it’s gentle on your stomach, too. Panadol Extra Soluble is also effective in treating backache, rheumatic pains and muscle pains, period pains and toothache.
Panadol has TV, programmatic digital and Radio advertising running throughout the year. This includes the “Made in Ireland” partnership with RTE.
*than standard paracetamol tablets.
“This product stands the test of time. Often requested by name this soluble is fast acting, working with powerful delivery and yet is gentle on the stomach. It is preferred by many of our customers due to the film coating”
“Having this option to recommend to people presenting in the pharmacy with pain is a gamechanger. The soluble option appeals to so many, and having a non-codeine-containing product which still has the benefit of the added caffeine boost has helped many people move away from codeine-containing products. Great advertising campaigns/window displays also”
Judges Comments




Proceive® Trimester 1 is designed to support mother and baby with optimal nutrition levels during the first trimester. For mother nutrients support the regulation of hormonal activity, energy levels and reduce tiredness and fatigue. While for baby this is a time of rapid growth, nutrients focus on cell division, neural tube development and bone development. The Proceive team goal is to provide the most functional, innovative products to customers, as such they are continually reviewing the latest scientific research. With their latest update, they have further improved the bioavailability of a number of nutrients and certified the Proceive® Pregnancy range as Vegan. Leading innovation in the category, Proceive® were the first pregnancy brand in Ireland to use L-Methylfolate in their products, this is the natural folate form of folic acid which ensures increased absorption of this important nutrient. The brand is proud to be Guaranteed Irish and does not contain fillers, binders, preservatives, artificial colours or flavours offering a pure, comprehensive nutritional supplement for pregnant women.
“Terrific Irish product, we like that they have considered the bioavailability of the vitamins in the product to ensure good absorption and also that the formulation is suitable for vegans. A lot of thought and research has gone into its development”
“I think this product is going to really excel in 2024. The increase in funding for the coming year implies there are great things ahead for this Irish company. I particularly like their packaging”



 Suzanne Acton, Marketing Manager, Affirm Health
Suzanne Acton, Marketing Manager, Affirm Health


Ocean Healthcare scooped another win for Bio-Oil Skincare Oil in the Best Skincare Product category.



Bio-Oil Skincare Oil was the first oil to be submitted to clinical trials and prove that it could improve the appearance of scars and stretch marks. Today, it is the world’s leading scars and stretch mark product, recommended by midwives and dermatologists. In clinical trials, after 8 weeks of use, 97% of participants saw an improvement in the appearance of stretch marks and 92% saw an improvement in the appearance of scars.
The Skincare Oil contains the breakthrough PurCellin Oil for better absorption and includes a combination of plant extract oils like Chamomile, Lavender, Rosemary and Calendula carefully selected for their healing, anti-inflammatory and soothing benefits. It also includes Vitamin A & E to help improve uneven skin tone, ageing and dehydrated skin.

“There are not many products to rival the universal offering of Bio Oil. It is often recommended by doctors and nurses as post-surgical care for scarring. The product has not changed over the years. It is a go to product. The marketing campaign behind this is very good”
“Bio-oil is an excellent product with numerous studies to back up its claims and has a wide range of applications, and customers generally come back on a repeated basis for bio-oil. Bio-oil uses good marketing strategies to promote its product, and is packaged in a straightforward and pleasant way”
Judges Comments


 Skincare by Sacha
Skincare by Sacha
35 years, 4 pioneering skincare products.










Best Baby Skincare Product was won by Child’s Farm Baby OatDerma Moisturiser.
Child’s Farm Baby OatDerma Moisturiser is formulated to hydrate and moisturise even the most sensitive dry and itchy skin. Packed with the goodness of oats, colloidal oatmeal is rich in beta-glucan and avenanthramides which are clinically proven to help soothe and gently nourish the natural balance of delicate skin.
Child’s Farm Baby OatDerma Moisturiser has been created for dry and itchy skin containing colloidal oatmeal, rich in beta-glucan and avenanthramides, it is clinically proven to help soothe and nourish skin. It is also free from parabens, phthalates, artificial colours and dyes, petroleum, formaldehyde.
“This product with its natural ingredients is a firm favourite in our pharmacy. I always quote the packaging when recommending ... “dermatologically tested and paediatrician approved” so as a pharmacist I know I can widely recommend this from babies to all skin conditions with confidence. Bright wellpresented packaging”
“Child’s Farm has quickly become a brand trusted by parents of babies and young children. This is a great fragrance-free product to recommend for babies and young children with dry and itchy skin, a common complaint seen in pharmacies. The pump makes it easy to use. It’s great to see packaging made from recycled materials and even better to know it can be fully recycled again once used”





Best SPF/Tanning Product has been won in 2024 for Bare by Vogue Clear Tan Water.
The Clear Tan Water applies with no colour guide, then gradually develops into a natural looking tan. Whether you’re a seasoned tanner or new to the self-tanning world, Clear Tan Water works for both. The water-based formula and its skin-loving ingredients ensure your skin is drenched in hydration, making the tan effortless to apply and easily glide across the skin. Completely undetectable - wear this during the day and within 4-8 hours you’ll be greeted with a beautiful golden tan that doesn’t need to be rinsed off. It is lightweight, non-sticky and incredibly easy to use - meaning a streak-free application every time.


“Bare is a consistent bestseller in the Tanning Category with a huge Social Media following. This product will easily outsell other tans especially for casual or new customers who would be unfamiliar or new to tanning due to its low commitment and ease of use”
“Excellent promotional material, influencer-led tan. Nice design, easy product to use, convenient onthe-go tan. Packaging stands out on the shelf and this product has a good re-sell opportunity”




Diabetes Ireland have launched the results of the first-ever survey highlighting the lived experience of people with diabetes in Ireland. For further information on “Accessing and Using Diabetes Health Services Survey 2023.”

An anonymous online survey of adults with diabetes or parents/ carers of children with diabetes was conducted between 16 January and 5 February 2023 via social media and standard online communication to ask about experiences of living with diabetes in Ireland. 517 people completed all sections of the survey: 230 adults with type 1 diabetes, 155 with type 2 diabetes and 12 with other types of diabetes, and 120 parents/carers.
Among adults, 37% reported they were already living with diabetesrelated complications and comorbidities. Most commonly these were hypertension (high blood pressure – 23%), diabetes retinopathy (23%), hypothyroidism (17%) and mental health-related issues (14%). During their lifespan living with diabetes, both adults & children living with type 1 diabetes had experienced severe complications: 40% had experienced DKA, mainly at the time of diagnosis, which should be avoidable if diabetes symptoms were recognised early; and 45% of adults experienced at least one episode of severe hypoglycaemia – a critically low glucose level, which usually comes with unconsciousness and requires third party help and possible hospitalisation.
In terms of their ongoing care, the majority of respondents were cared for in the public system but 20% of people with type 1 diabetes and 46% of those with type 2 diabetes pay privately for diabetes care. Only 13% of adult respondents had no review appointment with their healthcare provider in 2022 with a further 29% having only one appointment. However, all children
Dr Kate Gajewskawith type 1 diabetes had at least two or more appointments in the same period, with 67% of them having three appointments or more. Overall, respondents expressed satisfaction with their diabetes care but many highlighted the lack of time given to them by their diabetes healthcare provider and felt the appointments were rushed. The vast majority of respondents (88%) are under the HSE LongTerm Illness Scheme, giving them access to free diabetes-related medications and technologies plus free high blood pressure and high cholesterol medications.
Interestingly, when comparing Dublin with the Rest of the country, there were a number of noticeable differences in people’s daily experience of living with diabetes. More often, people with type 1 diabetes pay privately for their diabetes care if they live outside of Dublin (19% vs. 10%). Among people with type 2 diabetes, those from outside of Dublin were usually receiving their care in general practices (75% vs. 55%). People from Dublin more often were treated in outpatient diabetes hospital clinics (27% vs. 20%) or attended diabetes clinics privately (13% vs. 5%).
Among adults living with type 1 diabetes, those living outside Dublin were more often diagnosed with diabetes-related complications or comorbidities (41% vs. 35%). Among people with type 2 diabetes, those living outside Dublin more often had increased occurrence of diabetes-related complications or comorbidities (48% vs. 20%), mainly hypertension (40% vs. 18%) and mental health-related issues (24% vs. 15%).
Overall, the rates of technology usage (CGM) were very high and there were no differences between Dublin and the rest of the country with insulin pump usage also being similar. However, in openended responses, many people referenced very long waiting lists and difficulties in accessing diabetes-related technology, mainly insulin pump therapy, as well as difficulties in accessing specialist multidisciplinary team members (i.e. dietitians, podiatrists, psychologists etc.).
Generally, people rated their health and well-being as good, but one-third assessed it as fair, poor or very poor. 75% of respondents reported that they do not discuss mental health & wellbeing as part of their diabetes care with half welcoming an opportunity to do so.
Dr Kate Gajewska, Research & Advocacy Manager, Diabetes Ireland who led the research and undertook the survey analysis, said: “We regularly hear from people with diabetes about difficulties in accessing diabetes care services and new technologies across the country. However, we had no real data on this, so we decided to undertake this survey and provide an opportunity for people with diabetes to share their experience of accessing services and living daily with the condition. This survey is the first of its kind in Ireland, and in the absence of a National Diabetes Register and Clinical Audit, it provides very detailed information about experiencing diabetes care by those affected by diabetes in Ireland.”
Alongside the release of the survey findings, Diabetes Ireland is also launching its Pre-Budget Submission 2024 calling on the Minister for Health to set up a task force similar to the Cancer Strategy Taskforce to develop a 10-year National Diabetes Strategy to improve and standardise the delivery of care, access to diabetes services to improve the quality of life of more than 300,000 people living with diabetes in Ireland.
Cormac Devlin TD, Chairperson of the Cross Parliamentary Group on Diabetes said: “The economic burden of diabetes on the Irish healthcare system is now a major challenge for the government and the Health Service Executive.
The high cost of diabetes is mainly caused by the treatment of complications, many of which could be avoided with earlier detection, greater awareness of symptoms, better access to newer treatments and diabetes technologies, multidisciplinary teams, including psychologists, and regular diabetes review appointments. Despite the positive ongoing work of the HSE and Sláintecare, there are still many gaps in current diabetes services, as highlighted by this survey, that need to be tackled strategically in order to provide optimum diabetes care to everyone in need.
Therefore, to improve diabetes care, we need to collectively act now and decide what future care for people with diabetes will look like. “The Cross Parliamentary Group on Diabetes agree that it is time to set up a diabetes task force of relevant stakeholders to develop a 10-year National Diabetes Strategy that provides vision, leadership, direction, goals and priorities, as well as identifying and securing the future funding required to provide optimum care for every person living with diabetes in Ireland. We know this will take some time but we need to be very forwardthinking in our approach to dealing with diabetes long into the future and the immediate setting up of a force will help us achieve it.” – added Mr Devlin.
Diabetes Ireland, alongside the HSE National Clinical Programme, is also calling for additional funding of ¤5m to enable more equitable access to continuous glucose monitoring (CGM) for people with type 1 diabetes. Professor Derek O’Keeffe, National Clinical Programme for Diabetes Lead said: “In line with international best practice, the National Clinical Programme for Diabetes is requesting a broadening of the criteria for the provision of a form of CGM to all individuals living with Type 1 diabetes in Ireland. Bearing in mind that many people already use CGM in Ireland, securing ¤5 million by the Government in the Budget 2024 will facilitate more equitable access and reduce disparities for those living with type 1 diabetes who have not availed of this technology yet, will help them improve their diabetes management, outcomes and quality of life.”








Improves skin elasticity
Easy to take sachet drink
Reduce fine lines












Following on from the March issue Continuing Professional Development on the Pharmacy Role in the Management of Sleep Disorders, this 5-Minute Learning Module is designed to enhance the community pharmacy team understanding and ask further questions as to how you can support and advise patients.

After completing this module, you should recognise the signs and symptoms of differing sleep disorders and how they may present in the pharmacy, the treatments available, indications and suitability for patients and appropriate advice and counselling to be given for each.
Sleep medicine as a clinical speciality and awareness of sleep disorders have grown over the last few decades. The awareness of sleep disorders is not satisfactory, and effort needs to be made for the identification, diagnosis, and treatment of sleep disorders.
The International Classification of Sleep Disorders lists approximately 90 different types of sleep disorders, and sleep breathing disorders like obstructive sleep apnea, and insomnia that result in the loss of sleep duration are the most prevalent ones.
In Ireland, the national prevalence data is not available for clinical sleep disorders including sleep breathing disorders. However, the global trend in the increased prevalence of sleep breathing
Consider:
disorders applies to Ireland and much of the Western countries due to both aging and obesity. International incidence trends also show that men are two and a half to three times more likely to be diagnosed with sleep apnea compared to women.
The reasons for sleep loss are multifactorial. The prominent factors are lifestyle-related factors (irregular sleep schedules, shift-work related), environmental factors, psychosocial factors, and factors associated with sleep disorders (sleep-breathing disorders, insomnia, and other disorders).
Obstructive sleep apnea
The hallmark features of obstructive sleep apnea (OSA) are bouts of total or partial airway collapse accompanied by a drop in oxygen saturation or an increase in arousal from sleep. The frequent and multiple arousals result in nonrestorative sleep with immediate consequences of reduced quality of life, and attention deficits that can result in accidents while engaged in safety-critical tasks.
Am I/my team aware of the licensed indications, maximum recommended duration of treatment, doses and age limitations for OTC products used for the various types of sleep disorders?
Am I/my team aware of the medicines that can cause some sleep disorders, such as insomnia?
Am I/my team confident in the difference between types of sleep disorders?
Are we confident about raising issues of sleep disorders with customers?
The frequent oxygen desaturations and resaturations can result in the development of metabolic disorders and inflammatory responses.
The symptoms of sleep apnea include loud, disruptive snoring, witnessed apneas during sleep, and excessive daytime sleepiness. Screening questionnaires like stop-bang questionnaire is useful for screening for sleep apnea. Excessive daytime sleepiness can be assessed by the Epworth sleepiness scale.
The treatment of OSA usually depends on the etiology and the severity of the disease. Continuous positive Airway Pressure (CPAP therapy) is a common treatment prescribed for individuals with OSA when the symptoms and test reports warrant it.
An insomnia disorder is defined as a persistent difficulty with sleep initiation, duration, or consolidation that occurs despite adequate opportunity and circumstances for sleep and results in concern, dissatisfaction, or perceived daytime impairment, such as fatigue, decreased mood or irritability, general malaise, or cognitive impairment. Among adults with insomnia disorder, sleep complaints typically include difficulties initiating or maintaining sleep. Due to its chronic nature, insomnia is associated with substantial impairment in an individual’s quality of life.
Key Points:
Ensure the pharmacy team knows to be sensitive in dealing with customers who may feel vulnerable as a result of insomnia
Ensure pharmacy staff understand the function and importance of sleep and what effect a lack of sleep has on overall health
Ensure we are all aware of how much sleep is enough for different groups of people
Promote training and education as to the types of sleep disorders and their possible causes
Educate the pharmacy team on how OTC treatments, including herbal remedies, differ
Insomnia is primarily diagnosed by clinical evaluation through a thorough sleep history and detailed medical, substance, and psychiatric history. The sleep history should cover specific insomnia complaints, pre-sleep conditions, sleep-wake patterns, other sleep-related symptoms, and daytime consequences.
The American Academy of Sleep Medicine recommends that clinicians use multicomponent cognitive behavioural therapy for insomnia (CBT-i) for the treatment of chronic insomnia disorder in adults.
For individuals presenting with snoring, witnessed apneas, and excessive daytime sleepiness, explaining the referral pathway and advising them to consult with the GP or a respiratory/sleep physician will enable them to undergo an evaluation, diagnostic tests, and finally establish treatment.
An acute insomnia sufferer stands to benefit from immediate intervention by a pharmacist. Insomnia is perpetuated by the anxiety the individual maintains about sleep. Sleep education will be useful to help them to rationalise their anxieties and reduce hypervigilance which can pave the way to better sleep.
Sleep hygiene measures are also extremely useful in the early stages of poor sleep to improve sleep.
Actions:
Ensure support staff understand the following key points:
The common causes of insomnia, including lifestyle issues, medication and conditions that can cause sleep issues
How to spot sleep disorders and insomnia in both colleagues and customers to get them the help they need
The benefits and limitations of OTC medicines
The importance of good sleep hygiene for everyone
Lifestyle issues that may cause sleep disorders, and tips on improving lifestyle
The importance of regular food intake and sufficient exercise
When to refer customers to the pharmacist.


*Based on IQVIA sales data MAT 11/2023.
Nytol One-A-Night 50 mg Tablets contains diphenhydramine hydrochloride. A symptomatic aid to the relief of temporary sleep disturbance in adults. Adults: One tablet to be taken 20 minutes before going to bed, or as directed by a physician. Do not exceed the maximum dose of one tablet in 24 hours. Elderly patients or patients with liver or kidney problems should consult their doctor before taking this medicine. Children under 18 years: Not recommended. The product should not be taken for more than 7 days without consulting a doctor. Contraindications: hypersensitivity to the active substance or to any of the excipients, stenosing peptic ulcer, pyloroduodenal obstruction, phaeochromocytoma, known acquired or congenital QT interval prolongation, known risk factors for QT interval prolongation. Special warnings and precautions: pregnancy/lactation, renal and hepatic impairment, myasthenia gravis, epilepsy or seizure disorders, narrow-angle glaucoma, prostatic hypertrophy, urinary retention, asthma, bronchitis, COPD. Patients should be advised to promptly report any cardiac symptoms. Tolerance and / or dependence may develop with continuous use. Do not take for more than 7 consecutive nights without consulting a doctor. Should not be used in patients currently receiving MAO inhibitors (MAOI) or patients who have received treatment with MAOIs within the last two weeks. Use in the elderly should be avoided. Avoid concomitant use of alcohol or other antihistamine-containing preparations. Do not drive or operate machines. Cases of abuse and dependence were reported in adolescents or young adults for recreational use and/or in patients with psychiatric dis-orders and/or history of abuse disorders. Contains lactose. May suppress the cutaneous histamine response to allergen extracts and should be stopped several days before skin testing. Interactions: Alcohol, CNS depressants, MAO inhibitors, anticholinergic drugs (e.g. atropine, tricyclic antidepressants), metoprolol and venlafaxine, CYP2D6 inhibitors, Class Ia and Class III anti-arrhythmics. Side effects: dry mouth, fatigue, sedation, drowsiness, disturbance in attention, unsteadiness, dizziness, thrombocytopenia, hypersensitivity reactions, confusion, paradoxical excitation, convulsions, headache, paraesthesia, dyskinesias, blurred vision, tachycardia, palpitations, thickening of bronchial secretions, gastrointestinal disturbance, muscle twitching, urinary difficulty, urinary retention. Product not subject to medical prescription. PA1186/016/001. MAH: Chefaro Ireland DAC. The Sharp Building. Hogan Place. Dublin 2. Ireland. Date of preparation: Nov. 2023. SPC: https://www.medicines.ie/medicines/nytol-one-a-night-50-mg-tablets-34889/spc
MyPro DiaCare Overview - MyPro DiaCare is an oral rehydration and probiotic solution that helps to aid recovery from diarrhoea, dehydration and stomach upset. An orange-flavoured solution, it contains a scientifically balanced mix of glucose, minerals, and probiotics to help rehydrate, support digestion, and restore natural gut flora. The innovative and unique dual-sachet design allows for two independent benefits; it contains a probiotic which helps to swiftly restore the gut to normal while also containing electrolytes for rehydration purposes. This powdered solution easily dissolves in water and can be taken by adults and children from the age of 3 years suffering from any condition that causes loss of fluids.

MyPro DiaCare is a unique food supplement containing a balanced blend of glucose electrolytes, minerals and Lactobacillus Rhamnosus GG.
• Carbohydrate electrolyte solutions enhance the absorption of water during physical exercise.
• MyPro DiaCare contains magnesium which contributes to electrolyte balance.
• Contains chloride which contributes to normal digestion by the production of hydrochloric acid in the stomach.
• Lactobacillus Rhamnosus GG may help to restore and repopulate the equilibrium of intestinal microflora.
MyPro DiaCare is a powder formulation of oral suspension with dual-chambered sachets consisting of the following.
1. Chamber Salts: Dextrose; Sodium citrate; Potassium chloride; Sodium chloride; Flavouring (orange); Magnesium hydroxide; Acidity regulator; citric acid (E 330); Anti-caking agent; silicon dioxide (E 551); Sweetener: sucralose (E 955)
2. Chamber Probiotics: Maltodextrin; Lactobacillus Rhamnosus GG (DSM 21690) Uses for MyPro DiaCare
MyPro DiaCare is an oral rehydration solution that helps to aid recovery from the loss of body fluids in children, adults and the elderly alike. It is useful in the case of effectively treating:
• Diarrhoea
• Persistent vomiting
• Gastroenteritis (tummy bug/ vomiting bug)
• Vomiting and diarrhoea caused by food poisoning
• Fevers accompanied by intense sweating (from colds/flu/ tummy bugs)
• General hydration
• Travel and hot climates
MyPro DiaCare is an orange flavoured, unique food supplement containing a balanced blend of glucose electrolytes, minerals, and Lactobacillus Rhamnosus GG. It contains magnesium which contributes to electrolyte balance, chloride which contributes to normal digestion by the production of hydrochloric acid in the stomach and Carbohydrate electrolyte solutions that enhance the absorption of water during physical exercise.
MyPro DiaCare can help to:
• Reduce the risk of dehydration.
• Restore electrolyte balance in the body.
• Restore and repopulate the equilibrium of intestinal micro flora.
• Provide a source of probioticsLactobacillus Rhamnosus GG.
Who can take MyPro DiaCare?
MyPro DiaCare can be enjoyed by all the family as it gently replenishes electrolytes and restores gut health. Our unique formulation is suitable for ages 3 years plus and has also been effectively used to aid in the recovery of stomach upsets in the elderly.
To prepare one serving, dissolve all content of one dual-chambered sachet in 200ml water. Use the prepared solution within 24 hours and discard any unused solution.
Why choose MyPro DiaCare?
MyPro DiaCare offers several unique selling points (USPs) and benefits that set it apart from other products in the market:
Guaranteed Irish Brand
MyPro DiaCare is proudly Irish, with its headquarters in Mullingar, Co. Westmeath. This localisation reflects a commitment to quality and support for the local economy.
Dual-Chambered Sachet
Its innovative dual-chambered sachet design allows for two independent benefits: probiotic restoration and electrolyte rehydration. This dual-action approach ensures comprehensive relief for stomach upsets and loss of body fluids.
Probiotic Efficiency
MyPro DiaCare contains probiotic yeast, which offers several advantages over traditional bacterial probiotics. It colonizes the gut swiftly within 5-7 days, effectively outnumbering pathogenic species. Additionally,
probiotic yeast is more durable in antibiotic environments and gastric acids, ensuring its effectiveness in various conditions.
Clinical Validation
Supported by clinical trials, MyPro DiaCare's formulation with Lactobacillus Rhamnosus GG has shown significant efficacy. Studies demonstrate that 70%-90% of individuals taking Lactobacillus Rhamnosus GG are protected against bacterial infections, highlighting its preventive benefits.
Oral Rehydration Therapy (ORT)
MyPro DiaCare is recommended as first-line therapy for both mildly and moderately dehydrated children. Its electrolyte content aids in rapid rehydration, making it an essential solution for gastrointestinal discomfort, especially in vulnerable populations.
Adaptability and Health Claims
The product's formula can be adapted to meet specific health claims, particularly in enhancing water absorption during physical exercise. This versatility allows MyPro DiaCare to address a range of needs beyond gastrointestinal discomfort.
WHO/UNICEF Recommendations
MyPro DiaCare's precisely balanced formula adheres to WHO/UNICEF recommendations, ensuring optimal efficacy and safety standards. This endorsement reinforces confidence in its effectiveness and suitability for diverse populations.
MyPro DiaCare offers a holistic solution for stomach upsets and dehydration, combining probiotic restoration and electrolyte rehydration in a uniquely formulated, clinically validated product. Its Irish heritage, innovative design, and adherence to international standards make it a trusted choice for alleviating gastrointestinal discomfort and promoting overall well-being.








Pharmacists are being urged to advise psoriasis patients of a new research study examining the association between the skin condition and psoriatic arthritis.
A research team at University College Dublin, in partnership with the University of Oxford, is aiming to recruit 2,000 people across Ireland, out of a total of 25,000 across Europe, to the study. Psoriasis affects at least 73,000 people here, with one-third of patients going on to develop psoriatic arthritis.
The research study aims to better determine which psoriasis patients may develop psoriatic arthritis in order to prevent it before it occurs. It will also inform wider research efforts to diagnose the condition earlier or predict disease progression, to develop new diagnostic tests, and to pioneer personalised therapies to avert permanent, disabling joint damage. Anyone over 18 years who has been diagnosed with psoriasis, except those already diagnosed with psoriatic arthritis, can take part in the study. Participants will be asked questions about their psoriasis and other medical conditions, any musculoskeletal symptoms arising, treatments they are receiving, and details of their lifestyle.
Taking place completely online, participants can take part from the comfort of their own homes by completing questionnaires every six months over a three-year
period. Some participants may be sent a home blood sampling kit in order to provide a fingerprick blood sample to assist in developing a tool to identify those at higher risk of developing psoriatic arthritis.
The End for Psoriatic Arthritis? Consultant Rheumatologist and study co-lead, Professor Oliver FitzGerald, comments:
“Unfortunately, right now, we don’t know which patients with psoriasis will go on to develop psoriatic arthritis and which will not. There is no diagnostic test or algorithm available to predict PsA. We know that it is important that it be recognised promptly as each day left untreated leads to poorer outcomes.
“By identifying biomarkers associated with psoriatic arthritis, our aim is to develop a blood test to identify it prior to the development of any symptoms. Ultimately, if we can develop ways of better identifying which patients will develop arthritis, we may be able to prevent it through risk factor modification and preventative therapies.”
Patients wishing to take part in the study can find out more information at www.hpos.study

Dr Cathal O’Connor, specialist registrar in dermatology at the South Infirmary Victoria University Hospital, was presented with the Future Leader Award of the European Society for Dermatological Research at the recent academy in Madrid, Spain.
Dr O’Connor presented findings from his PhD study – the ‘assessing sleep in infants with atopic dermatitis’ (SPINDLE) study which was performed in the INFANT research centre in University College Cork.
Dr O’Connor performed detailed research on sleep and neurophysiology in young babies with severe eczema. He said, “our novel findings will be used to increase awareness of the neurological complications of early-onset eczema and the impact on families of babies with eczema.”
The ESDR Future Leaders Academy brings together highly gifted young scientists and renowned senior scientists from dermatological departments all over Europe and beyond for a three day meeting. The goal of this initiative is to encourage excellent young dermatologists to further pursue their academic careers through guidance and mentoring of established scientists in the field.
Dr O’Connor is currently completing his dual specialist training in both dermatology and paediatrics in Cork, before taking up a post as senior clinical fellow in a leading European paediatric dermatology centre. His PhD degree with University College Cork was undertaken as part of the prestigious Irish Clinical and Academic Training programme.
Atopic dermatitis (AD) is the most common chronic inflammatory skin condition in childhood. Most (50-60%) children with AD report sleep disturbance, which is secondary to itch, dry skin, inflammation, and abnormal circadian rhythm. Sleep is essential for brain development, learning, and growth. The aim of this study was to describe in detail the sleep architecture of infants with early-onset atopic dermatitis (AD), compared to controls, by using EEG polysomnography, sleep actigraphy, and parental reporting.

E45 has launched a complete skincare range that offers instant and long-lasting hydration for the face improving the skin’s natural barrier. Suitable for dry, sensitive, and eczema-prone skin. Non-comedogenic and dermatologically tested.










Complete emollient therapy for dry skin conditions
Soothes, calms and protects sensitive dry skin
Suitable for all ages
Complete Emollient Therapy with Hydromol
www.hydromol.co.uk
Living with Eczema
Download now or scan the QR code:


Product information is available on request at this stand
Over my professional career, I amassed 20 years of practical experience in healthcare as a pharmacist and a quality healthcare advocate. As a healthcare professional practicing pharmacy in UAE, I have always been enthusiastic about improving the quality of care. My belief that Patient Safety is a paramount concern and a top priority in healthcare started in 2009 when I completed my MSc in Quality and Safety in Healthcare Management from RCSI Dubai, a branch campus of the Royal College of Surgeons in Ireland (RCSI) in the United Arab Emirates. Since then, as a healthcare professional, I have been seeing and reflecting on the development of patient safety awareness and science in healthcare. In this article, I would like to share a few insights and experiences on this fundamental principle in healthcare.
The global landscape of health care is changing, with health systems working in increasingly complex environments, patient safety is a big and growing global public health challenge and an international healthcare goal.
The United Arab Emirates (UAE) is part of this global community striving for excellence in healthcare and has made significant strides to improve patient safety in its healthcare system.
In UAE healthcare system, the Ministry of Health and Prevention (MoHaP) oversees the national healthcare regulations. The emirates have regional healthcare authorities’ departments, like the Department of Health (DOH) in Abu Dhabi and the Dubai Health Authority (DHA) in Dubai, to regulate each region's medical sectors. The Departments of Health shape the regulatory framework for the health system, inspects against regulations, enforces standards, and encourages the adoption of world–class best practices and performance targets by all healthcare service providers in the Emirate.
In this article, I want to highlight three main facts, that in my opinion, have contributed to the advancement of the quality of care while nurturing patient safety approaches in the healthcare system in the UAE.
First of all, the ‘vision’ to provide world-class health and social care services must exist before any sort of implementation. The UAE does not only have a ‘vision’ but also stringent regulations governing the clinical practices in all healthcare settings to support it. Healthcare providers should adhere to strict guidelines in all process of clinical pathways, including but not limited to best practice clinical
guidelines, correct diagnosis, updated best practice guidelines of medication management, verifying prescriptions, safe medication administration procedures, providing clear instructions to patients, and verifying the authenticity of the medications. The department of health (DoH) implemented standards, policies, and initiatives to promote healthcare quality, implementing a total quality management system to ensure the safe and effective use of medications. JAWDA, which is the Arabic translation of ‘Quality,’ is a program that was launched in 2014. It supplies a framework designed to assess the quality performance of health providers in Abu Dhabi based on a set of quality outcome indicators under main areas like safety, effectiveness of care, timeliness of service delivery, and patient-centric delivery of care.
The Department of Health (DoH) has signed an agreement with the Emirates Classification Society (TASNEEF) to Certify Healthcare Providers in the Emirate of Abu Dhabi for JAWDA Data Certification. JAWDA Data Certification (JDC) is a process to improve the quality and accuracy of health data and clinical coding in the Emirate of Abu Dhabi. It is an initiative of the Department of Health (DoH) to enhance the transparency and trust between healthcare providers, payers, patients, and regulators. JDC involves auditing the claims and coding processes of healthcare facilities, as well as validating the data submission of key performance indicators (KPIs) for quality measurement. The objective of JDC is to ensure that the health data reflects the actual services provided and the outcomes achieved, and to support the improvement of patient
Written by Linda Asaad Ghazal, Pharmacist II, Quality and Patient Safety - BCPS, Msc.QSHM, CPHQ,CPPS - Imperial College London Diabetes Centresafety, clinical effectiveness, and patient experience. JDC is based on a set of standards and guidelines that are aligned with international best practices.
In 2023, The Department of Health - Abu Dhabi announced its accreditation by the prestigious International Society for Quality in Health Care External Evaluation Association (ISQua EEA). DoH received this accreditation for its commitment to quality and patient safety in health and social care. The accreditation recognizes DoH’s adherence to international standards in healthcare services, ensuring continuous improvement and excellence in service delivery.
Another key strategic initiative is the effective integration of technology to improve patient safety. Malaffi is the first health information exchange platform in Abu Dhabi. Malaffi, which means “my file” in Arabic, aims to improve healthcare delivery quality, safety. It has a mobile app that allows patients to access their health information and manage their appointments, prescriptions, and referrals.
By providing instant access to the patient’s medical file, Malaffi empowers healthcare professionals to make better clinical decisions, enhances coordination and avoids unnecessary duplication of tests and procedures. It is one of the most advanced health Information exchange platforms in the world, providing patient’s health information from a routine checkup, consultation or emergency treatment to laboratory

tests or radiology images, all are now collated in their personal file, with their medical history safely and securely stored and instantly accessible when needed.
Malaffi is integrated with the National Unified Medical Record (NUMR) program Riayati and Nabidh, the Dubai Health Authority platforms for the secure exchange of reliable healthcare information among licensed health facilities in Dubai, a step that further supports the delivery of an integrated health system for citizens across the UAE. This initiative not only reduces healthcare costs, but also primarily contributes to patient engagement, reduces the risk of medical error, improves medications safety, supports patient empowerment to aid self-care, informed decisionmaking, enhanced medical compliance, and improved trust between patients and medical professionals.
Adding to this, I believe the different initiatives and collaboration between pharmacovigilance authorities and
healthcare providers, plays an important role in the advancement of providing quality care, an example of this is, healthcare facilities having assigned pharmacovigilance focal points who are tasked with specific responsibilities that include:
• Following up with healthcare professionals within the facility for reporting Medication Errors and send any clarification to DOH when required.
• Identifying and implementing practices to improve the Pharmacovigilance reporting to DOH.
• Educating healthcare professionals on the importance and methods of Medication Errors (ME) to the Pharmacovigilance program.
• Receiving emails of safety alerts and recall circulars and disseminating them to healthcare professionals within the facility.
• Becoming a member of the Abu Dhabi pharmacovigilance networking (ADPVN) and participates proactively in its activities. The ADPVN members meet in comprehensive workshops and advanced training days activities to enhance medication safety and encourage safe patient care practices.
As a pharmacovigilance focal point of my healthcare organization, I have been following and participating in these initiatives and campaigns and observing their positive impact on improving patient safety and quality of care in different settings and contexts. It is also a great learning opportunity from the inspiring stories, best practices of leading experts and peers worldwide committed to making health care safer and better for everyone. Such activities lead to reframing the relationship between healthcare providers and regulators, resulting in effective communications and positive outcomes.
The standard on reporting medication errors, aims to ensuring system thinking and just culture. It ensures that reported errors are studied, reviewed and that procedures are established to prevent them from reoccurring. The standard also requires that healthcare facilities must develop long-term monitoring plans to ensure that changes come into effect after the medication errors occur. The standard adopts the "Culture of Safety", designed to promote the development and implementation of the Continuous Quality Improvement system.
Finally, another important contributing reason to the advancement of quality of care, is the role played by Quality and Patient Safety leaders. Health organisation leaders consider patient safety as a strategic goal as they are aware that patient safety is everyone's responsibility; it is not a one-time event, it is not a theme, it is not about accreditation, but it is a culture, a continuous process of improvement and learning. It is how we do things in our organizations daily.
As a pharmacovigilance focal point of my organization and a quality and patient safety function lead of my pharmacy department, I was supported by my leadership to walk the safety talk at an organizational level. Adding to my responsibilities as the organizations’ pharmacovigilance focal point, my role is to:
1. Encourage and collect adverse events, errors and near misses reporting data.
2. Review and analyse the collected data of medications errors, clinical interventions and near misses.
3. Study reported data, highlight systems gaps, show examples as lessons learned.
4. Prepare monthly slides for discussion at the medication management committee,
5. Discuss and recommend corrective actions that can prevent recurrence.
6. Follow up corrective actions’ implementation and monitoring.
7. Slides then will be circulated to all clinical departments for sharing the good practices and learning from mistakes and near misses.
8. Spread the quality and safety talks, meet with the department colleagues on weekly huddles, and with organizational staff on regular virtual events, discussing Principles of patient safety, quality in healthcare, patient education and engagement, system thinking in analysing medication errors or adverse events, just cultures, medications reconciliations, WHO world patients’ safety campaigns.
9. Mentor other quality champions at different department to disseminate the quality and safety talk.
10. Support improvement projects, continuous educations, and patient safety initiatives.
Patient safety practices have come a long way, with valuable lessons. The way forward is for healthcare providers to focus on investments in advanced technologies like artificial intelligence, machine learning, proactive risks management and preventative mitigation strategies. On the other hand, in recent years, a notable surge in collaborative initiatives between higher education institutions and healthcare providers has appeared, marking a transformative approach to addressing patient safety challenges. As a dedicated safety pharmacist, I keenly see this trend as I explore the evolving healthcare landscape. The constructive collaboration between academia and healthcare fosters innovative solutions that enhance patient safety protocols and bridge the gap between theory and practical application. This dynamic partnership promises to advance pharmaceutical practices and cultivate a culture of continuous improvement in the quest for the best patient care in the UAE.
To conclude, I would like to add that the drive for excellence, be it in any aspect, must come from ‘within’. Personally speaking, my unwavering commitment to pharmacy extends beyond the confines of dispensing medications. As a pharmacist, I am impassioned to pursue quality and patient safety excellence. It is not merely a professional obligation but a personal mission to ensure everyone receives the highest standard of care. Through continuous education, rigorous adherence to protocols, and a relentless dedication to staying informed about advancements in the field, I strive to contribute to a healthcare environment where patient well-being is paramount. In a world where precision and compassion intersect, I find fulfilment knowing that my role extends far beyond the counter — it reaches into the hearts and lives of those entrusting me with their health.





1National Social Inclusion Office, Health Service Executive, D20 KH63 Dublin, Ireland
2HSE Addiction Services, Health Service Executive, V94 PV34 Limerick, Ireland
3Department of Public Health and Primary Care, Trinity College Dublin, D24 DH74 Dublin, Ireland
*Author to whom correspondence should be addressed.
The harm reduction approach underpins drug and alcohol policy in many countries worldwide.1,2,3 In 2022, 105 countries included harm reduction in their national policies.4 Harm reduction is a proven public health response aimed at reducing the health and social harms associated with a range of risk activities. It has been included in most definitions of a public health approach to substance use in the last decade.5 In terms of substance use, this approach focuses on interventions such as needle and syringe exchange programmes, opioid agonist treatment (OAT), overdose antidote provision (naloxone), safer injecting facilities and outreach/ education programmes.6 These aim to reduce the negative consequence of drug use (e.g., overdose and infectious disease transmission from injection drug use), as opposed to stopping drug use itself.7
HIV prevalence among people who inject drugs (PWID) is approximately seven times greater than the adult population.8 In addition, worldwide it is estimated that 53% of PWID are infected with hepatitis C.9 Needle and syringe exchange programmes are a harm reduction measure, which aim to reduce the transmission of HIV
and blood-borne viruses among PWID and prevent the sharing of contaminated equipment. A number of reviews have demonstrated the effectiveness of needle and syringe exchange programmes in terms of preventing HIV and other infectious diseases, increasing access to treatment and increasing the likelihood of users reducing or stopping drug use.10,11 They were first established in Ireland in 1989, when five fixed-site services were established in the Dublin region.12 These services provide access to sterile injecting equipment and a safe way to dispose of used equipment. They are also used to provide onward referral for disease testing, treatment services and other harm reduction interventions.13 In 2022, there were 92 countries providing at least one needle exchange service.4
In Ireland, needle and syringe exchange programmes have been a key component of successive drug and alcohol policies, even before harm reduction was identified as a public health response.14,15 As noted above, these were initially developed in the late 1980s as fixed-site services in areas that experienced high levels of substance use. Some areas developed mobile needle exchange services in response to the spread of injecting drug use beyond inner city Dublin regions. However, there remained large areas of the country with poor access to these services. Government strategy therefore recommended the expansion of the needle exchange programme to community pharmacy settings.14 These provide free access to
sterile injecting equipment from participating pharmacies throughout Ireland during normal trading hours. They also collect used equipment (although returned needles are not required to obtain clean needles) and refer patients for treatment, bloodborne virus testing and hepatitis B vaccinations. Community pharmacies have been established in a number of other countries to help improve the accessibility of needle exchange services.16 By 2012, there were 71 pharmacies actively providing needle exchange services in Ireland,17 with the current strategy recommending further expansion of the programme in areas of need.15 In 2022, there were 90 pharmacies actively providing needle exchange services.












International reviews of the impact of community pharmacy needle exchange services have shown that they have a positive effect in terms of reducing highrisk injecting behaviours such as needle-sharing, in addition to a range of other positive health outcomes.18 However, in Ireland, there is limited up-todate information on the impact of community pharmacy needle exchanges. For example, a review of all needle exchange programmes by Bingham et al.,19 based on 2012 data, highlighted data collection gaps and recommended a standardised reporting mechanism. Bates et al.20 utilised 2013 data to show the positive impact of the Pharmacy Needle Exchange Programme in terms of uptake and referrals to other services. There have been no studies undertaken in Ireland since 2015. In addition, there is a relative absence of studies undertaken elsewhere that monitor patterns of use over time. This is important, as there is a need to determine usage patterns in the context of changing drug markets and user preferences. For example, there has been an increase in cocaine use21 and treatment22 in Ireland in recent years, with crack cocaine use emerging as a problem among marginalised groups.23 Such information is crucial in terms of ensuring that harm reduction initiatives continue to meet the needs of PWID. The study aimed to determine patterns of needle distribution and return in community pharmacies in Ireland.
The Health Service Executive (HSE) in Ireland collects Key Performance Indicators (KPIs) on a quarterly
basis to monitor the performance of each division, set targets for ongoing service delivery, and develop strategic plans for forthcoming years. All community pharmacies that participate in the Community Pharmacy Needle Exchange Programme submit monthly KPI data to the HSE. Each participating pharmacy inputs anonymised data for each free transaction into an audit form or a database. No personal data or unique identifiers are collected from those using the service and registration is not required. Pharmacies receive a payment for participating in the scheme. Following a data validation process, monthly aggregated data are then submitted on a quarterly basis to the HSE Planning and Business Information Unit (PBI). The PBI then construct endof-year totals for each KPI for all pharmacies at regional and national levels. The following data are collected: the number of participating pharmacies, the number of individuals attending the programme each month, the number of needle/syringe packs provided (three different types of packs containing needles, syringes, swabs, vials, citric acid packs, a personal sharps container, and water for one, three and ten injections), the number of needle/syringe packs returned, the percentage of needle/syringe packs returned, the number of clean needles provided each month (collected since 2017) and the average number of clean needles (and accompanying injecting paraphernalia) per individual each month. From these data, the KPIs from 2015 to 2022 for the programme were obtained from the PBI. Yearly
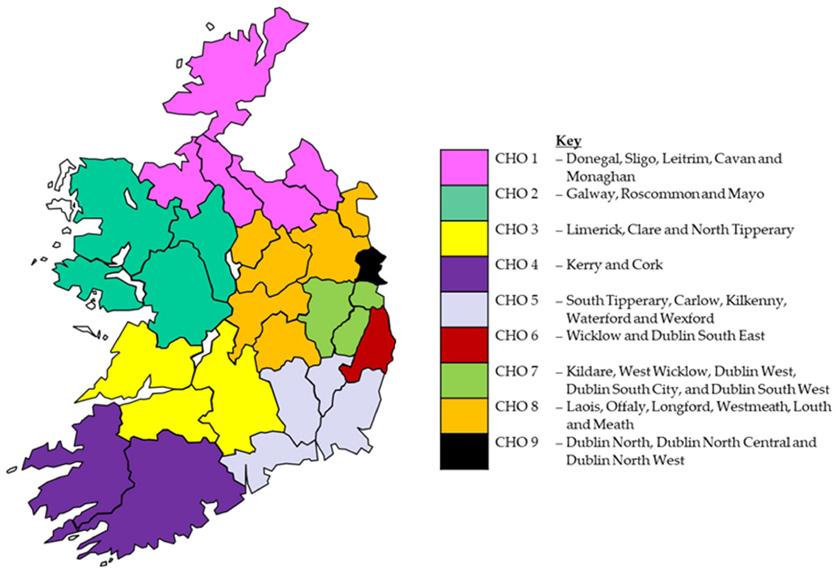
totals of actual usage for each KPI from 2015 to 2022 were calculated for Ireland overall and were disaggregated by Community Health Organisation Area (CHO). These geographical areas are responsible for the delivery of primary and community services in Ireland (see Figure 1). Each CHO contains a mix of urban and rural locations. CHO6, CHO7 and CHO9 do not have any pharmacies included in the programme. These CHOs cover Dublin city and surrounding counties and have other needle exchange services, which were established prior to the introduction of the pharmacy service due to the large number of injecting drug users. The KPIs were compared to yearly targets that had been set for the service by the HSE. The study utilised aggregated KPIs, which did not permit a detailed statistical analysis of patterns. The analysis focused on using counts, percentages, and means to describe patterns from 2015 to 2022. As the analysis was based on aggregate data, it was not deemed necessary to obtain ethical approval for the study. To facilitate the analysis of patterns, the mean of 2015 and 2017 was used to provide an estimate for 2016.
Number of Pharmacies in Programme
In 2022, there were 90 pharmacies in Ireland actively providing the Pharmacy Needle Exchange Programme. This is 5% lower than the KPI target set for 2022 (95 pharmacies). The number of pharmacies providing the programme has reduced by 18% since 2015 (Figure 2), with two sharp declines experienced in 2018 and in 2020. It is below
the KPI target set for every year except 2019.
Number of People Using Programme
In total, 1612 unique individuals per month used the Pharmacy Needle Exchange Programme in 2022 (mean = 17.9 people per pharmacy). This is 7.5% higher than the KPI target set for 2022 (1500 people). The number of unique individuals using the programme increased by 15% from 2015 to 2019, followed by a decline of 16% from 2019 to 2022. Overall, between 2015 and 2022, there has been a 3% decline in the number using the service. In terms of KPI targets, there is a fluctuating pattern, with targets met for five of the years (2015, 2017–2019, 2021), and not met for three of the years (2016, 2020, 2022) included in the analysis.
Packs Provided and Returned
In 2022, 3775 packs per month (containing needles, syringes, swabs, vials, citric acid packs, sharps containers and water) were provided. This represents an overall decline of 19% compared to 2015. The overall proportion of packs returned has also declined, from 23% in 2015 to 16% in 2022. This represents a 28% decline in the proportion of packs returned. Overall KPI targets have not been met in any of the years from 2015 to 2022.
In 2022, the programme provided 21,296 needles per month, with each person attending receiving 9.8 needles on average per month. Compared to 2017, the number of sterile needles provided has declined by 16%. There has been
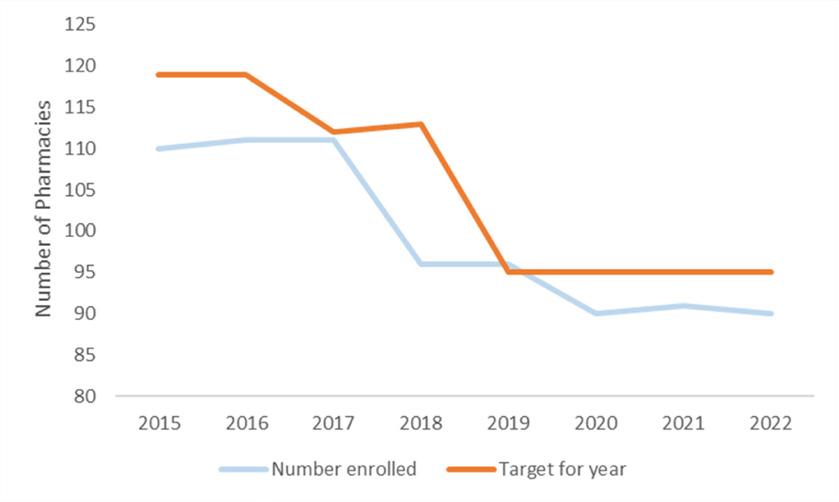



80% of mothers confirm less colic2, thanks to the unique anti-colic base SkinSoft™, flat, symmetrical teat for a latch that is just like mum Self sterilises in just 3 minutes Order now from your sales representative or telephone: 01 405 0900 / 01 450 7771





a 4.2 percentage point reduction in the average number of needles per individual since 2017 (Figure 3). KPI targets have not been met for half of the years included in the analysis (2017, 2018 and 2021).
The greatest proportion of pharmacies providing the programme in 2022 were located in CHO8 (32%, n = 29), CHO4 (18%, n = 16), and CHO5 (16%, n = 14). All of the CHOs involved in the programme have experienced a decline in the number of pharmacies participating since 2015, with the largest decline experienced in CHO1 (25%; from 12 to 9), CHO5 (22%; from 18 to 14) and CHO3 (20%; from 15 to 12). In 2022, the greatest proportion of individuals using the programme were located in CHO4 (48%, n = 774), CHO5 (23%, n = 372) and CHO8 (20%, n = 328). CHO4 (48.4) and CHO5 (26.5) had the highest mean number of people attending per pharmacy in 2022. CHO4 has experienced an increase in people using the programme since 2015 (98%). The number of people using the programme in all other CHOs has declined, with the largest declines experienced in CHO3 (84%), CHO2 (53%) and CHO8 (27%). In terms of returned packs, the CHOs with the largest proportion returned were CHO8 (33%), CHO5 (21%) and CHO1 (15%). With the exception of CHO8 (where the proportion returned increased by 33%), all CHOs have experienced a decline in the proportion of packs returned (23–70%). The mean number of needles per individual was greatest in CHO1 (13.4), CHO5 (11.9) and CHO4
(11.4). Since 2015, the mean number of clean needles per individual has declined in all CHOs, with CHO1 and CHO2 experiencing the largest decline (72% and 70%, respectively).
Community pharmacy needle exchange services are a key component of Ireland’s public health harm reduction approach to reduce the negative impact of injecting drug use. By monitoring usage patterns, the study provides an insight into whether they are meeting the needs of PWID. Overall, the services have experienced a decline in the numbers attending and, in many instances, have not met the targets that were set. The emerging patterns raise a number of issues in terms of policy implications and provide an insight into the provision of harm reduction services in the context of changing drug markets in Ireland.
Between 2015 and 2022, there has been a decline in the number of pharmacies providing (18%), and the number of people (3%) using, the programme. This has coincided with a 19% decline in the number of packs provided and a 16% reduction in the number of sterile needles provided (since 2017).
The declining patterns of use are of concern, since reduced investment in harm reduction initiatives such as needle exchange services has been identified as a factor contributing to HIV outbreaks in Ireland and a number of other countries.24 Indeed, the closure of the largest needle exchange in Glasgow, Scotland, has been
identified as a significant setback in terms of preventing the spread of HIV among PWID.25
Participation by pharmacies in the programme in Ireland is voluntary, which means that they can choose to withdraw, leaving gaps in service provision. Broad coverage has been identified as a key element of effective needle and syringe provision.26 It is important that gaps in service provision are filled, for example by encouraging pharmacies to provide services in areas without a service, or by expanding existing community drug treatment services.
The declining patterns in terms of size and usage also raises the question of whether there has been a change in drug markets in Ireland, with fewer PWID presenting for treatment. Since 2015, there has been a gradual decline in estimates of problematic opioid use in Ireland,27 but this decline has not been significant. During this period there has been an increase in the number of nonfatal overdoses discharged from hospital, with opioids involved in over two thirds of cases.28 In 2020, there were 409 deaths due to poisoning, with 70% involving opioids.29 This represents an 8% increase compared to 2017. Since 2015, there has been an increase in the number of people accessing Opioid Agonist Treatment (OAT) and this was particularly apparent during the COVID-19 pandemic, when individuals were fast-tracked into treatment.30 These patterns suggests that the demand for sterile needles should not have significantly changed during this period.
Declining patterns may be due to some users choosing to obtain injecting equipment elsewhere, such as travelling to the larger needle exchange programmes in Dublin where there would be greater anonymity. The decline in the number of sterile needles provided helps to demonstrate this issue. In England, online purchasing of needles has been suggested as a factor to explain declining utilisation since COVID-19.31 In the current study, the number of individuals using the service did decline in 2020, coinciding with COVID-19, which does suggest that lockdown restrictions may have played a role in declining patterns of usage. During COVID-19, a home delivery needle and syringe service was established,32 along with increased provision of OAT services,33 which may also have meant that fewer people would have needed to attend a needle exchange. Since 2020, patterns have only recovered to 2015 levels, which suggests that COVID-19 may have had an enduring impact on behaviour patterns. In England, the reuse and sharing of equipment has been suggested as a factor to explain the continued decline in people using the service since COVID-19.31
Heroin users can inject multiple times a day and are at high risk of reusing or sharing needles due to cravings if they do not have access to an adequate supply of sterile injecting equipment.34 It has been estimated that a heroin user needs to inject 2.8 times a day,35 which equates to 85 times a month. This is over eight times higher than the number of sterile needles provided per individual using the Pharmacy Needle Exchange Programme in
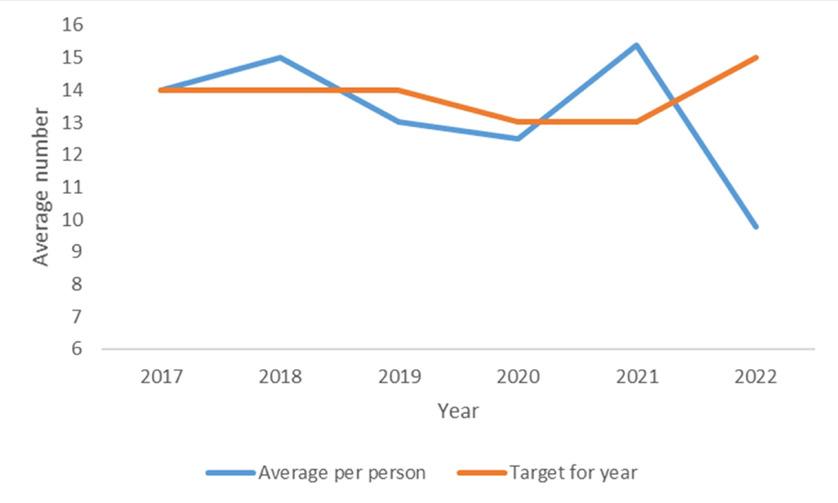






Targeted skin care for dry and fl aky skin.


• Urea 10%
• Emu Oil

• Allantoin

• Urea 30%
• Mandelic Acid
• Allantoin





2022. Although the proportion of active heroin injectors that use the programme is not known, this does nevertheless raise concerns that some individuals are reusing their equipment, and the core messages of the harm reduction advice may need to be reemphasised. Further research could be conducted with this cohort to identify the actual level of injecting and whether there are many situations where people alternate between injecting and smoking.
The reasons behind this declining pattern of usage are unclear and warrant further investigation. In a systematic review of 36 studies, Gionfriddo et al.36 found that perceptions of safety, the potential for improper disposal of syringes, and concerns about other customers are key barriers that discourage pharmacists from selling clean needles to PWID. Recent prevalence studies have highlighted the increase in cocaine use in Ireland and identified the emergence of ‘crack’ cocaine use among a marginalised population that may previously have used heroin.23 Cocaine use can cause intense craving,37 with users also experiencing irritability and anxiety.38 There is a need to investigate whether such behavioural issues are discouraging pharmacies from engaging with the needle exchange service. All pharmacies should have protocols in place to ensure the safety of their employees. PWID may present with challenging behaviour, and staff must possess the necessary skills and have the supporting infrastructure to deal with such challenging behaviour. A review by Lawson et al.39 highlighted stigma and the perception of negative consequences as barriers to accessing sterile injecting equipment.
The withdrawal of pharmacies from the scheme in the current study may have made the service less accessible to PWID, who, as a result, choose more accessible sources of equipment. They may also have additional needs that may not be met by the Pharmacy Needle Exchange Programme. For example, there is evidence that suggests that crack cocaine use is increasing among PWID in Ireland. The EMCDDA have reported this as an emerging pattern in Portuguese drug consumption rooms3 and it has also been reported in Scotland.40 If this is the case, then additional harm reduction initiatives may be required (such as the provision of sterile crack pipes). Such emerging drug use patterns may require additional training for pharmacies, as the needs of IDUs who also
use crack cocaine may present additional challenges.
Packs Returned
Discarded needles and used equipment that was used to inject drugs can increase the risk of injury and the spread of infectious diseases such as hepatitis C and HIV.41 Although these risks are relatively low42,43 (with transmission risk in Ireland also reducing due to a decline in HIV incidence rates44,45), they nevertheless represent a health and safety hazard, causing negative perceptions and anxiety among communities, particularly among parents of young children.42,46 Such discarded injecting paraphernalia can lead to communities opposing the development of services in an area, so it is important that this be addressed in a proactive manner.
The Pharmacy Needle Exchange Programme aims to minimize this impact by encouraging the return of used items from the packs that it provides, particularly needles.19 Although failing to return needles may not mean that they were disposed of unsafely, it is nevertheless of concern that, in 2022, only 16% of packs were returned, which represents a 21% decline compared to 2015. A study of the programme using 2012 data found that 39% of packs were returned,17 which shows that rates for 2015–2022 are considerably lower than those achieved in 2012. Low return rates were also highlighted in a review of the service by Bates et al.20 Data for Northern Ireland needle and syringe exchange services for 2019–2020 show that 31% of CinBins (container for safe disposal of needles which is included in every pack) were returned.47 A review (although somewhat dated) of 26 studies of needle exchange programmes reported an overall return rate of 90%.48 Unpublished data from needle exchange services in Mandura in Western Australia show an overall return rate of 94% from 2021 to date.
These studies suggest that high return rates can be achieved, but barriers in Ireland may need to be overcome. The Centre for Disease Control and Prevention (CDC) in reviewing studies state that PWID may not return needles to exchange programmes due to concerns about being arrested for the possession of syringes, a lack of sharps containers, needles taken by other PWID due to low supply and high demand, homelessness and living conditions.49
In some areas in Ireland, needle disposal bins have been installed
in areas identified as having a problem with drug-related waste that may be more accessible to PWID.50 Although the extent of this initiative is not known, it might be that needles and used items provided by the Pharmacy Needle Exchange Programme are returned to these disposal bins. There are no distinguishing identifiers on products obtained from the Pharmacy Needle Exchange Programme. Users of the programme have to enter the pharmacy and put used equipment (in a sharps container) into secure bins. This may deter returns if users perceive this process to be inconvenient. In addition, if they feel stigmatised for being a drug user, they may try to minimise the number of times they use such facilities. For example, Paquette et al.51 report the significant impact stigma has on syringe access in USA, particularly pharmacies. This issue is also highlighted in a review by Tung et al.46 Tung et al.46 also outline a number of initiatives, such as the use of sharps containers that remove the needle barrel from the needle (potentially preventing crimination), drop boxes and one-way disposable bins in a wider range of locations, the use of vending machines to dispense needles, mail slots in bathrooms and disposal bins designed to look like post boxes (to be less conspicuous). In Mandura in Western Australia, one of the stipulations that contributes to their 94% return rate is the supply of free needles only upon the return of used items.52 Alternative systems of service delivery do need to be considered to improve return rates. In addition, the degree to which the return of equipment is emphasized by pharmacies is not known. It might be that, due to the lowering rates of hepatitis C and HIV among IDUs,44,45 there may be a lack of emphasis of the importance of returning used equipment.
Complacency in relation to bloodborne virus (BBV) transmission among both staff and service users may have contributed to the low return rate. A previous study of the programme emphasised the need for service providers to advise users about the safe disposal of used needles and equipment.20
Although a standardised leaflet is provided to users,53 this does not contain information on the return of packs and there appears to be a lack of standardised procedures in place in terms of facilitating and encouraging users to return packs. Such procedures, if accompanied by training, may help promote the return of used equipment.
In examining the programme by health regions within Ireland (CHO), it can be seen that patterns do differ by area. For example, while CHO8 has the largest proportion of pharmacies (32%), CHO4 has the greatest proportion of individuals using the programme (48%). On average, there are 48 people using each pharmacy in CHO4, which is almost double that of CHO5 (n = 27) and four times that of CHO8 (n = 11). The number of pharmacies in the programme has declined in all CHOs since 2015. Although there may be a rationale to reduce the number of pharmacies providing the service in CHOs with low take-up of the service, for CHOs with large numbers of users (e.g., CHO4), there may be a need to consider expanding the service to more pharmacies. This approach could spread the burden on existing pharmacies that provide the service, and may help to reduce the distance for people to travel (particularly in rural areas). Expansion plans would also need to investigate issues such as the recruitment of pharmacies, to help ensure that sufficient numbers of pharmacies apply to provide the service. A concerning finding from regional comparisons is the variation in the proportion of packs returned by CHO (6–33%) which would warrant further investigation to identify variations in programme implementation at a regional level that may be used to explain and rectify the low proportion of returns.
The declining patterns of usage of the Pharmacy Needle Exchange Programme has raised a number of important issues for consideration. It is hoped that this will help ensure that the needs of those that use it are better met in the future. Despite the declining patterns of usage, it is worth noting that, in 2022, it provided 21,296 sterile needles per month in areas of Ireland that are not served by fixed-site services. As such, the service can be viewed as a significant harm reduction initiative and remains a key component of Ireland’s harm reduction approach to drug use.15 Through regular review and monitoring, it is anticipated that this service can significantly enhance its harm reduction impact in the future. This review was conducted eight years after reviews undertaken by Bingham et al.19 and Bates et al.20 Given the issues that have been identified and the changing drug trends in Ireland, it would be prudent to conduct such reviews on a more regular basis, perhaps every 2–3 years.
References available on request
is a weekly email updating you on stocks, new product launches and other business news, sent directly to your inbox.
Stay up to date, scan the QR code and sign up today.
Or register online at https://cloud.teva-europe.com/TevaSource_Registration.
Did you know?
You can order stock through TevaSource.


Women will soon have the power to tackle devastating menopause symptoms at the touch of a button through a ground-breaking Irish app.

The Menopause Hub App, the first of its kind in this country, includes the latest information and research about the hormonal changes endured by women in their 40s and 50s.
They can also use the mobile app to monitor their own menopausal and perimenopausal symptoms and receive medication reminders.
Launched by award-winning specialists, The Menopause Hub, the app is the brainchild of the clinic’s CEO and founder, Loretta Dignam.
She opened her first clinic in 2019 after finding out for herself the lack of help available to menopausal and perimenopausal women – and said the data gathered by the app can be used by researchers investigating future treatments.
“The aim is that the data collected will provide a valuable insight into the effectiveness of current therapies, such as HRT and/or cognitive behavioural therapies, and this knowledge can also be used by clinicians when prescribing treatments.
Zendra Health CEO, Thomas Coleman, left, and Menopause Hub CEO, Loretta Dignam, with the Menopause Hub App, which will be available to download later this year. Credit: Peter Doyle / Media Consult
“Combining the latest technology with up-to-date data about female health will not only improve outcomes for women today, but will also improve the range and impact of menopause treatments available to future generations,” said Ms Dignam.
“There is a myriad of menopausal symptoms which women in their 40s and 50s experience in varying degrees, including insomnia, hot flushes, anxiety, depression, and brain fog, to name but a few.
“Every women’s menopause will be vastly different. Some will sail through it, while others will struggle to such an extent that they feel they have to give up their careers or their long-term relationships break down.
“It is, therefore, vital that we gather as much clinical data as we can to help medical researchers develop future treatments. For far too long, the impact menopause was having on women’s lives was ignored. But
women are now demanding –and deserve – more help with symptoms.”
The app was developed by medical technology company Zendra Health, which is helping over 80 healthcare services and organisations across the US, UK and Ireland digitalise their care pathways.
CEO, Thomas Coleman, said that the app was co-designed by women going through menopause and clinical experts.
“Hundreds of thousands of lives across Ireland, male and female, are impacted by menopause,” said Mr Coleman, who co-founded the Dún Laoghaire-based tech firm with his twin brother, David. “So we were delighted to develop this streamlined digital care solution for The Menopause Hub. Loretta is one of the thought leaders in this area and we were guided not only by her expertise but by the expertise of her medical team and by the experiences of their clients.”
The Menopause Hub App is available to download from March. See themenopausehub.ie for more details.
The European Microbiome Centres Consortium (EMCC), a network of institutions working to ensure coherence and collaboration among leading experts in the field of human microbiome, launched recently during the final conference of the EU-funded project Human Microbiome Action.

In the past years, several links between changes in the human microbiome and various diseases have been observed, but clear connections are yet unknown. The
European Microbiome Centres Consortium, launched through the EU-funded Human Microbiome Action project, aims to unlock these connections and starts a new phase of European research and innovation in the field. "The EMCC is committed to promoting and harmonizing standards for microbiome research, ensuring robust and reliable methodologies and practices across the research community. We aim to promote regulatory frameworks in Europe, advocating for the acknowledgement of scientific consensus and requirements in the field of microbiome research.
This will foster innovative translational developments, potentially transforming preventive nutrition and healthcare." - Joël Doré, scientific coordinator of the Human Microbiome Action project, and member of INRAE, one of the five founding partners. The EMCC is launched by five founding partners across Europe: EMBL (European Molecular Biology Laboratory), UCC (University College Cork), University of Trento, UCPH (University of Copenhagen) and INRAE (Institut National de Recherche pour l’agriculture, l’alimentation et l’environnement). Their aim after the conference will be to open it to broaden the networks to Europe-wide members who wish to contribute to this topic. In addition to its scientific mission, the EMCC is committed to raising awareness and educating the general public and healthcare professionals about
the importance of the human microbiome in health and disease.
Professor Paul Ross, Director of APC Microbiome Ireland, a world leading SFI Research Centre and co-Director of Food Microbiome and Heath Futures programme at University College Cork highlighted that, “the EMCC will provide an essential partnership involving a collection of Institutions and Centres actively involved in microbiome research across the EU. The mission of the EMCC is to provide guidance and advice on how to advance microbiome science to tackle some of the major challenges that exist across the food-pharma interface. In this respect this initiative should accelerate the science-based translation of microbiome research to its main stakeholders including the public, policy-makers, and food & health-based industries.”









LIVING WITH DONOR KIDNEY TRANSPLANTED 50 YEARS AGO
Staff at Beaumont Hospital had the pleasure of last week welcoming a patient to the Kidney Clinic to celebrate a kidney transplanted 50 years ago.
Christine Kelly and her family were welcomed to Beaumont Hospital last week, 50 years after she received a donor kidney transplant. Christine developed kidney failure in the early 70s and was receiving haemodialysis prior to her transplant in Jervis St Hospital. In 1974 Christine’s sister Mary donated her kidney which was successfully transplanted by Mr Peter McClean FRCSI on 1st March 1974.
The transplant was carried out at Jervis Street whilst Beaumont Hospital has been responsible for Christine’s care since 1987 when Jervis St Hospital moved to Beaumont.
Despite various health challenges over the years Christine has enjoyed good health and quality of life with thanks to the generosity of her sister.
Speaking to mark the occasion
Professor Peter J Conlon, Lead Clinical Director, Beaumont, said, “We were delighted to welcome Christine and her family to the clinic to celebrate 50 years since her successful kidney transplant.

Christine’s story demonstrates the success of organ transplantation and gives hope to all patients with kidney failure.
Christine, with thanks to the generosity of her sister in 1974 has lived successfully with her transplant enjoying good health and quality of life. This is a momentous occasion for Christine, her family and Beaumont and Jervis St Hospitals. Her story underpins the importance of organ donation in saving lives.”
Individuals who wish to support organ donation are encouraged to share their decision with their loved ones and to keep reminders of their decision by carrying the organ donor card, permitting Code 115 to be included on their driver’s licence, or having the ‘digital organ donor card’ App on their smartphone.
DAMIEN O’DOWD APPOINTED
CHIEF EXECUTIVE OF ST JOHN OF GOD HOSPITAL
Saint John of God Hospital announces the appointment of Mr Damien O’Dowd as the new Chief Executive of the Hospital and its associated services Mental Health First Aid Ireland and St Joseph’s Centre, Shankill.
Mr O’Dowd is a senior healthcare executive and director with over twenty years’ experience at leadership and senior level management level within healthcare organisations and services in both the private and voluntary healthcare sectors.
Damien O’Dowd’s appointment sees his return to St John of God Hospital having commenced his career there in the 1990’s. He has most recently held Senior Consultant and Director roles in Vision Consulting and Virtus Health respectively and was Chief Executive of the Bloomfield Health Service between the years 2011 and 2018.
Damien has an in-depth experience in the design and successful implementation of strategies for healthcare organisations focussed on continuous service improvement. He is highly effective in change management and operational management of busy and complex healthcare organisations with particular experience of planning and delivering risk management, quality assurance & accreditation systems. He is equally recognised for his experience in developing systems of governance at both clinical and corporate levels in healthcare and related organisations and also has extensive experience in regulatory preparedness & compliance.
Damien has a strong business growth and service development

acumen with wide experience of financial management & healthcare funding.
Mr O’Dowd holds a Masters Graduate of the School of Law, Queens University Belfast and an MBA Graduate of the Smurfit Graduate School of Business, University College Dublin. He also lectures in management and leadership at undergraduate and post graduate levels.
Speaking about his new appointment, Damien O’Dowd said: “I am delighted to rejoin St. John of God Hospital as CEO, where I first began my career in mental health over 30 years ago and which remains a leading service provider of mental health care in Ireland. I am privileged to be leading an excellent team of highly experienced healthcare professionals and we will continue to work together to elevate the patient experience across all our associated services. The healthcare landscape is everevolving and this is a time of positive change and renewal within our sector. Going forward, we will continue to prioritise service excellence and the well-being of all our patients and the communities we serve.”
NEW PHASE 2B RESULTS FOR AMLITELIMAB SUPPORT POTENTIAL FOR BESTIN-CLASS MAINTENANCE OF RESPONSE IN ATOPIC DERMATITIS
Positive results from Part 2 of the investigational amlitelimab Phase 2b study STREAM-AD showed sustained improvement of signs and symptoms for 28 weeks in adults with moderate to severe AD who previously responded to amlitelimab and continued treatment. High responder rates were also observed in participants who were taken off amlitelimab. The safety profile was consistent
with Part 1 of the study with amlitelimab being well-tolerated and no new safety concerns identified. These results were presented as part of a latebreaking session at the American Academy of Dermatology (AAD) 2024 Conference in San Diego and support the quarterly (every 12-week) dosing of amlitelimab 250 mg with 500 mg loading dose (LD) now being investigated in a larger Phase 3 clinical program (OCEANA).
Professor Stephan Weidinger, M.D, Ph.D, Director, Professor, Chair of Department of Dermatology and Allergy, University Hospital SchleswigHolstein said, “Despite available treatment options, not all patients with moderate-to-severe atopic dermatitis respond sufficiently to these treatments, and many continue to suffer from skin lesions and symptoms such as persistent itch, which can have a high impact on their day-to-day lives. Results from this part of the study indicate amlitelimab’s potential for durable off-drug efficacy which supports the evaluation of a less frequent every 12-week dosing. This could offer an important benefit in the treatment of AD patients.”
In the second part of the doseranging STREAM-AD study, responders to amlitelimab who achieved a 75% improvement in Eczema Area and Severity Index (EASI-75) score and/or Investigator Global Assessment (IGA) score of 0 or 1 during the 24-week treatment period (Part 1) were re-randomized to explore the maintenance of clinical response over an additional 28-week period with continued amlitelimab treatment or amlitelimab withdrawal. Across all dose arms, patients who continued amlitelimab treatment maintained high EASI-75 and/or IGA 0/1, IGA 0/1, and EASI-75 responder rates through 28 weeks. High responder rates were also demonstrated among patients who were taken off treatment.
In 69.2% of patients with continued treatment with amlitelimab 250 mg Q4W with 500 mg loading dose (LD) vs 58.8% of patients withdrawn from treatment IGA 0/1 and/or EASI-75 response was maintained.
An analysis including pooled dose-arms showed that IGA 0/1 response was maintained in 71.9% of patients with continued treatment vs 57% of patients withdrawn from treatment. In this analysis, EASI-75 response was maintained in 69% of patients with continued treatment vs. 61.6% of patients withdrawn from treatment. AD-related biomarkers remained reduced at Week 52 in both
amlitelimab withdrawn and continuing groups, despite amlitelimab reaching negligible levels in serum. Reduction of TARC, eosinophils, and IL22 observed at Week 24 was maintained during withdrawal as well as in patients continuing treatment to Week 52. These biomarker data suggest the modulation of inflammatory T cells via the blockade of OX40L and durable control of AD after amlitelimab withdrawal.
The aggregated safety profile of amlitelimab in Part 2 of this study was consistent with that of Part 1, with amlitelimab being well-tolerated, and no new safety concerns were identified during the 28-week maintenance/withdrawal period. Overall rates of treatmentemergent adverse events (TEAEs) were 69.8% for continued amlitelimab treatment, 71.9% for the amlitelimab withdrawal-arm and 66.7% for placebo. TEAEs more commonly observed included headache (11.6% amlitelimab continuation, 3.9% amlitelimab withdrawal, 6.7% placebo), upper respiratory tract infection (9.3% amlitelimab continuation, 5.5% amlitelimab withdrawal, 20% placebo). No adverse events such as fever or chills, oral ulcers or conjunctivitis were observed across doses.
Amlitelimab is a fully human non-T cell depleting monoclonal antibody that blocks OX40-Ligand, a key immune regulator, and has the potential to be a first- or bestin-class treatment for a range of immune-mediated diseases and inflammatory disorders, including moderate-to-severe atopic dermatitis (Phase 3), asthma (Phase 2), hidradenitis suppurativa (Phase 2), scleroderma, celiac disease, and alopecia (Phase 2 studies to be initiated in 2024). By targeting OX40-Ligand, amlitelimab aims to restore balance between proinflammatory and regulatory T cells. Amlitelimab is currently under clinical investigation, and its safety and efficacy have not been evaluated by any regulatory authority.
AbbVie, the global biopharmaceutical company, has been listed as one of the best places to work in Ireland at the 2024 Great Place to Work Awards. It is the eleventh consecutive year that the company has featured on the list.
AbbVie, which employs approximately 2,600 people across six locations in Ireland, was

Back Row (L-R): Arindam Guha, AbbVie Westport; Clare O’Donnell, AbbVie Westport; Blayne Walsh, AbbVie North Dublin; Andrew Shaw, AbbVie Citywest; Marco Froehlich, AbbVie North Dublin; Elizabeth Grogan, AbbVie Citywest, Dublin; Gary O’Mahoney, AbbVie Ireland; Geraldine McGroary, AbbVie Ballytivnan, Sligo; Gerard Byrne, AbbVie North Dublin; and Denise Bulman, AbbVie Cork.
Front Row (L-R): Andres Rodrigo, AbbVie Citywest, Dublin; and Rakesh Dontireddy, AbbVie Manorhamilton Road, Sligo.
ranked 13th Best Large Company in Ireland at the awards, and was joined by 36 other companies on this prestigious roll call.
Great Place to Work has previously recognised AbbVie as a Best Workplace for Women for the fifth consecutive year in 2023 and was one of 19 organisations included in the most recent Best Workplaces in Pharma & Healthcare list.
Speaking about this new accolade, Andres Rodrigo, General Manager at AbbVie (Ireland), said: “Being named as one of Ireland’s top large workplaces again this year is an incredible accomplishment for the company and an important recognition of the high-performing, inclusive culture that we’ve created over the past eleven years.
“This latest recognition reflects the various AbbVie programmes that support meaningful development, employee well-being, diversity and inclusion, as well as long-standing initiatives which help to support and positively impact our local communities,” he added.
Great Place to Work assesses the policies and practices in place in organisations under key areas including employee development, hiring practices, employee wellbeing and social impact. It then benchmarks these against other organisations in other countries.
Employee development continues to be a key priority area for AbbVie and the company has rolled out a number of mentorship programmes, harnessing the capabilities and experience of leaders, graduates and other colleagues in the organisation.
recipients and the Taoiseach, Leo Varadkar TD. The awards celebrated individuals and partners who have significantly contributed to Vision Ireland's mission, impacting various aspects of everyday life.
Those who were honoured on the evening included:
• Jason Smyth MBE QFA and Karen Byrne, for their remarkable efforts in changing public perceptions through their achievements on "Dancing With The Stars." The duo was lauded for their inspiring performances, highlighting the potential within everyone, regardless of vision impairment.
AbbVie also supports key diversity and inclusion projects, including the launch of the company’s Pride, Asian Leader Network, and Ability, employee groups at its sites across Ireland.
AbbVie’s award-winning Week of Possibilities volunteering initiative and the Employee Giving Campaign also provide employees with opportunities to personally improve and impact the communities in which AbbVie people work and live.
Commenting on the awards, CEO at Great Place to Work Ireland, Cathal Divilly, said, “I'm delighted to extend a huge congratulations to all the exceptional companies recognised as a Best Workplace in Ireland 2024. This achievement is not just a milestone but a reflection of their tireless efforts at crafting workplaces where talent not only flourishes, but also feels valued and empowered. I'd like to congratulate these organisations once again on this outstanding achievement, and may this recognition fuel their continued journey towards excellence and innovation,” he added.
Vision Ireland, the new name for NCBI, is thrilled to announce the resounding success of its 2nd Annual Oireachtas Celebration of Achievement, held on Thursday, 29th February 2024.
Hosted in the Dáil member's dining room by Senator Sen Martin Conway, the evening began with a meet & greet involving the award
• Evie Smurfit Baxter, named Youth Advocate of the Year, for her resilience and advocacy in the face of Stargardt’s disease. Her work, including an animation featured in cinemas, demonstrates the unlimited possibilities for those facing visual challenges.
• Jo Scutt, recognised as Vision Ireland Shop Volunteer of the Year, for her decade-long dedication and fundraising creativity, including marathon running and local charity events, significantly benefiting Vision Ireland.
• Peter Ryan, honoured as Fundraiser of the Year, for his extraordinary effort running from Malin Head to Mizen Head, raising over ¤120,000. His journey from visual impairment to finding a new purpose through sport embodies the spirit of determination.
• Campion Insurance, celebrated for its partnership with Vision Ireland, supporting the innovative Vision Van project, showcasing the power of collaboration in making a tangible difference in the community.
The event also extended heartfelt gratitude to Dr Terry Cross OBEfor his generous auction prize donations, which played a crucial role in raising significant funds during the evening. Also in attendance on the evening was last year's award winner Denise Harris. Both Dr. Cross, Denise Harris’s and all who attended ongoing support exemplifies the community's commitment to Vision Ireland's cause.
The Vision Ireland Foundation extends its sincerest thanks to all guests, honorees, and supporters for their part in making the event memorable. The foundation looks forward to continuing its mission and celebrating more achievements in the years to come.
Accord Healthcare is delighted to announce the launch of Nebivolol 5 mg tablets which come in packs of 28 tablets.
This medicine is indicated for:
• Hypertension
• Treatment of essential hypertension.
• Chronic heart failure (CHF)
Treatment of stable mild and moderate chronic heart failure in addition to standard therapies in elderly patients >70 years
Please refer to the Summary of Product Characteristics (SPC) for further information. The SPC will be available at www.hpra.ie and for Healthcare Professionals atwww.accord-healthcare.ie from launch.
Nebivolol will be available from both full-line wholesalers from launch. For further information please contact Accord Healthcare in Cork on 021-461 9040.
The U.S. Food and Drug Administration (FDA) has accepted for Priority Review the supplemental Biologics License Application (sBLA) for Dupixent® (dupilumab) in a sixth potential indication as an add-on maintenance treatment in certain adult patients with uncontrolled chronic obstructive pulmonary disease (COPD). The target action date for the FDA decision is June 27, 2024. Regulatory submissions are also under review in China and the European Union.
The sBLA, as well as other submissions around the world, is supported by data from the Phase 3 COPD clinical research program evaluating the efficacy and safety of Dupixent in adults who were current or former smokers with uncontrolled COPD with evidence of type 2 inflammation (screening blood eosinophils >300 cells/ microliter). All patients were on background maximal standardof-care inhaled therapy (nearly all on triple therapy). The primary endpoint was met in both trials (BOREAS, NOTUS), showing Dupixent significantly reduced
moderate or severe acute COPD exacerbations by 30% and 34% respectively, compared to placebo. In both trials, Dupixent also rapidly and significantly improved lung function compared to placebo, with improvements sustained at 52 weeks.
Safety results in both trials were generally consistent with the known safety profile of Dupixent in its approved indications. Adverse events more commonly observed with Dupixent (≥5%) compared to placebo in either trial were back pain, COVID-19, diarrhea, headache and nasopharyngitis.
Priority Review is granted to regulatory applications seeking approval for therapies that have the potential to provide significant improvements in the treatment, diagnosis or prevention of serious conditions. The potential use of Dupixent in COPD is currently under clinical development, and its safety and efficacy for this indication have not been fully evaluated by any regulatory authority.
Mater Private Network has completed two new types of robotic-assisted spinal fusion surgeries which mark the inaugural use of this cutting-edge technology in the Republic of Ireland.
Since its introduction in 2019, robotic-assisted spinal surgery has transformed surgical practices at Mater Private Network. This innovative approach empowers surgeons to perform minimally invasive procedures with precision and accuracy, offering patients several important benefits, including reduced incision size and greatly improving post-operative discomfort, as well as minimal blood loss and muscle trauma.
In January 2024, a patient (60s) with adult scoliosis presented to the Mater Private Dublin, having previously undergone surgery on their spine to decompress and relieve pressure on the spinal nerves. Unfortunately, all previous surgeries were unsuccessful, and the patient presented with continued and progressive pain in their lower back and legs. This patient had undergone physiotherapy and had received pain relief injections for chronic pain, but neither provided a satisfactory outcome.
In this context, Mr. Deb Roy, Spinal Surgeon at Mater Private Network, took on the case. He performed an extreme lateral interbody fusion with assistance from the latest in roboticassisted surgical technology.
This minimally invasive surgery involved gaining access to the patient’s spine through a small incision on the side. Once the spine and the nerves around the spine were identified, Mr. Roy removed damaged discs and replaced them using an interbody cage with bone graft.
A second case involved a patient (50s) presenting with progressive pain in the lower back and legs. The pain was so severe that the patient was unable to work or sleep, severely impacting their quality of life. Pain management plans had previously been put in place for the patient, to no avail. To treat this case, Mr. Roy, Spinal Surgeon at Mater Private Network, performed a micro-access interpedicular lumbar fusion. Gaining access to the spine through the patient’s back using a small incision, Mr. Roy thereafter decompressed, stabilised and fused the affected area of the spine.
Mr. Roy commented "It is an honour to have conducted these pioneering surgeries with Mater Private Network. These procedures mark a significant milestone in spinal care in the Republic of Ireland and highlight how we can harness the technologies available to us to drive better outcomes for patients."
Committed to the adoption and utilisation of the latest in medical technology, Mater Private Network continues to show its commitment to innovation and patient-centric care, enhancing patient outcomes across its network in Ireland.
To learn more about the services offered by Mater Private Network, visit www.materprivate.ie
Sligo University Hospital (SUH) has welcomed Minister for Health Stephen Donnelly, TD to formally launch two significant enhancements in gynaecology and maternity services at the hospital.
Minister Donnelly has cut the ribbon on a new Ambulatory Gynaecology clinic in Sligo University Hospital.
The new service in SUH is part of a national plan to improve health outcomes for women through a new model of care. The clinic operates a “see and treat” model which means that women who need gynaecological care can be assessed, treated and discharged on the same day all within this “one stop” setting.
The establishment of the new ambulatory clinic in SUH is reducing the need for women to have multiple gynaecology
appointments for a single episode of care and this in turn is having a positive impact on waiting lists and in-patient bed usage at the hospital, with waiting lists for outpatient appointments reduced by over 50%.
Women are referred to the clinic by their GP and have access to same day diagnostics, investigations, treatments and minor procedures under a team of consultants and specialist nurses. The new unit provides both diagnostic and therapeutic procedures for common gynaecological conditions including hysteroscopies, ultrasound scans, biopsies and management of intrauterine devices.
Speaking at the official opening Minister Donnelly said, “As Minister I’ve made developing better women’s health services a priority during the lifetime of this Government. The unprecedented levels of funding in women’s health services are now delivering for women. I know that this clinic is a positive, on-the-ground improvement, that is already making a real difference to the women of Sligo and the Northwest, reducing waiting times and improving access to services.”
Minister Donnelly also visited the Seomra Suaimhnis / Serenity Room which is a wonderful new labour and delivery suite developed by the maternity service in SUH with the support of the National Women and Infants Programme (NWIP).
The suite is designed around a “home from home” approach, the décor and atmosphere aim to create a relaxed and less clinical environment for women to give birth in. Women also have access to a heated birthing pool in the suite which can help with pain relief during their labour.
Grainne Mc Cann, Hospital Manager at SUH said, “The opening of the Ambulatory Gynaecology clinic in SUH is a major step forward in the delivery of women centred care in our region. Since opening last year we have seen over 320 women in the clinic and have delivered a fully integrated model of care to these patients.
“There is a huge patient benefit involved in the “see and treat” model, the most important being improved access to specialist care for women who need to avail of it.”
Speaking about the Seomra Suaimhnis / Serenity Room
Grainne added, “In SUH we welcome nearly 1,300 babies
into the world every year, I’m delighted we can now offer some of our mums a beautiful, relaxed and homely space to facilitate a positive birth experience.”
Laya healthcare have announced the launch of a new Laya Health & Wellbeing Clinic in Swords Business Campus, Swords, Co Dublin. This will increase the nationwide network of Laya Health and Wellbeing Clinics to four and add an additional clinic to the Dublin area making it the fastest growing urgent care network in Ireland.
The North Dublin clinic will offer a range of health and wellbeing services including walk-in urgent care for the treatment of minor injuries and illnesses where patients as young as 12 months+ will be seen within 60 minutes - available to laya healthcare members and non-members. All Laya Health and Wellbeing Clinics have diagnostic imaging onsite continuing laya healthcare’s promise to improve access and ensure the current and future healthcare needs of members. The Laya Health and Wellbeing Clinic in Swords will provide X-Ray, MRI, Ultrasound and Dexa scan.
The investment of over ¤3million in the opening of this brand-new Laya Health and Wellbeing Clinic in North Co. Dublin will result in a new 25,000 sq ft clinic which will treat approximately 30,000 patients annually. As part of the expansion, 25 new jobs will be created including clinical and administrative teams. As with all Laya Health and Wellbeing clinics, it will be open 365 days per year from 10am – 10pm.
Commenting on the major investment and expansion of the Laya Health and Wellbeing clinic network, John Mc Call, Director of Claims and Provider Relations said, “Contributing to the ambition of Slaintecare, we firmly believe in the importance of delivering the right care at the right time, and that is why our clinics are open 365 days per year with consultant-led care delivered within one hour.”
To find out more about the Laya Health and Wellbeing Clinics visit www.layahealthcare.ie
At an event hosted by Janssen, the Pharmaceutical Companies of Johnson & Johnson, students and lecturers from University

(Stages II-IIIA) and by 73% (HR 0.27; 95% CI 0.21-0.34) in the overall trial population (Stages IB-IIIA). In an updated analysis in 2022, the median DFS was reached at nearly five and a half years (65.8 months) for both the primary and overall populations treated with osimertinib, compared to 21.9 and 28.1 months in the primary and overall populations, respectively, treated with placebo. Consistent DFS results were seen regardless of prior adjuvant chemotherapy use and across all prespecified subgroups. The safety and tolerability of osimertinib in this trial was consistent with previous trials in the metastatic setting.
of Galway’s Health Economics Masters class met with members of Janssen’s Market Access and Government Affairs team, touring the office and participating in presentations and workshops. At the event, in recognition of academic excellence in health economics in 2023, Orla Jenkins of HIQA was the recipient of the Janssen Bursary Medal.
Since 2012, Janssen has collaborated with the University of Galway, which has provided Health Economics students with valuable placement opportunities, contributing to their professional growth. In 2022, Janssen launched the WiSTEM2D mentorship programme with the university, designed to nurture future female leaders in healthcare specifically. WiSTEM2D stands for Women in Science, Technology, Engineering, Math, Manufacturing and Design –the initiative supports women and girls in pursuit of STEM2D studies and careers.
Janssen is proud to support the next generation of talent and to continue their partnership with University of Galway.
APPROVED IN IRELAND
AstraZeneca’s osimertinib is now reimbursed in Ireland for the adjuvant treatment of adult patients with early-stage (IB, II and IIIA) epidermal growth factor receptormutated (EGFRm) non-small cell lung cancer (NSCLC) after complete tumour resection, from
March 1st. Osimertinib is indicated for EGFRm patients whose tumours have exon 19 deletions or exon 21 (L858R) mutations.
While up to 30% of all patients with NSCLC may be diagnosed early enough to have surgery with curative intent, recurrence is still common in early-stage disease. Historically, nearly half of patients diagnosed in Stage IB, and over three quarters of patients diagnosed in Stage IIIA, have experienced disease recurrence within five years. About a fifth of the world’s lung cancer patients are in the EU and among those with NSCLC, approximately 10 - 15% have tumours with an EGFR mutation.
The approval by the European Commission (May 2021) was based on positive results from the ADAURA Phase III trial in which, osimertinib demonstrated a statistically significant and clinically meaningful improvement in disease-free survival (DFS) in the primary analysis population of patients with Stage II and IIIA EGFRm NSCLC. The trial also showed a statistically significant and clinically meaningful improvement in DFS for osimertinib in the overall trial population, a key secondary endpoint.
In the ADAURA trial, adjuvant treatment with osimertinib reduced the risk of disease recurrence or death by 77% (based on a hazard ratio [HR] of 0.23; 95% confidence interval [CI] 0.18-0.30) in the primary analysis population
In June 2023, the final planned overall survival analysis showed osimertinib reduced the risk of death by 51% compared to placebo in both the primary analysis population (Stages II-IIIA) (21% data maturity, OS hazard ratio [HR] of 0.49; 95.03% confidence interval [CI] 0.33-0.73; p=0.0004), and in the overall trial population (Stages IB-IIIA) (18% data maturity, OS HR of 0.49; 95.03% CI 0.34-0.70; p<0.0001).
In the primary analysis population, an estimated 85% of patients treated with osimertinib were alive at five years compared to 73% on placebo. In the overall trial population, an estimated 88% of patients treated with osimertinib were alive at five years compared to 78% on placebo. Median OS was not yet reached in either population or treatment group. Patients on placebo that recurred with metastatic disease had the opportunity to receive osimertinib treatment.
Dr Eynat Dotan, Head of Medical, AstraZeneca, Ireland said: “The ADAURA trial brought the first targeted medicine to patients with early-stage EGFR-mutated non-small cell lung cancer. The exciting overall survival results validate adjuvant osimertinib as the standard of care in this setting and reinforce the importance of early diagnosis and testing for EGFR mutation in lung cancer.”
Osimertinib is now approved to treat early-stage lung cancer in more than fifty countries, including in the US and China, and additional global regulatory reviews are ongoing. Osimertinib is also approved for the 1st-line treatment of patients with locally advanced or metastatic EGFRm NSCLC and for the treatment of locally advanced or metastatic EGFR T790M mutation-positive NSCLC in the EU, the US, Japan, China and many other countries.










