

TAFINLAR + MEKINIST
ABBREVIATED PRESCRIBING INFORMATION
Non-fixed dose combination of MEKINIST® and TAFINLAR®
Before prescribing Mekinist and Tafinlar in combination, please refer to the Summary of Product Characteristics (SmPC) of both products.
Presentation of each product: MEKINIST (trametinib) 0.5 mg and 2.0 mg film-coated tablets. Each film-coated tablet contains trametinib dimethyl sulfoxide equivalent to 0.5 mg and 2.0 mg of trametinib respectively. TAFINLAR (dabrafenib) 50 mg and 75 mg hard capsules. Each capsule contains dabrafenib mesilate, equivalent to 50 mg and 75 mg of dabrafenib respectively.
Indications: Combination of Mekinist and Tafinlar:

• for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.
• for the adjuvant treatment of adult patients with stage III melanoma with a BRAF V600 mutation following complete resection.
• for the treatment of adult patients with advanced non-small cell lung cancer (NSCLC) with a BRAF V600 mutation.
Dosage and Administration: Adults: recommended dose of Mekinist is 2mg once daily in combination with Tafinlar 150mg twice daily. Dose modification: Management of ADRs may require treatment interruption, dose reduction, or discontinuation. Elderly: No initial dose adjustment required in patients >65 years. Paediatrics: Safety and efficacy not established in patients <18 years. Renal impairment: No dose adjustment required in mild or moderate impairment. Caution advised in severe renal impairment. Hepatic impairment: No dose adjustment required in mild hepatic impairment. Caution advised in moderate and severe hepatic impairment. In the adjuvant melanoma setting, patients should be treated for a period of 12 months unless there is disease recurrence or unacceptable toxicity. Contraindications: Hypersensitivity to active substance or excipients. Warnings/Precautions for Mekinist (used as a monotherapy or in combination with Tafinlar): Left ventricular ejection fraction (LVEF) reduction/Left ventricular dysfunction: Cases of LVEF decrease have been reported. Should be used with caution when conditions could impair left ventricular fraction. All patients should be evaluated for LVEF prior to initiation of treatment with continued evaluation during treatment. Consider dose modification guidelines. In patients receiving Mekinist in combination with Tafinlar, there have been occasional reports of acute, severe LVEF due to myocarditis. Full recovery was observed when stopping treatment. Physicians should be alert to the possibility of myocarditis in patients who develop new or worsening cardiac signs or symptoms. Haemorrhage: Haemorrhagic events including major and fatal haemorrhagic events occurred in patients taking Mekinist as monotherapy and in combination with Tafinlar. Hypertension: Blood pressure should be measured at baseline and monitored during treatment with Mekinist, with control of hypertension by standard therapy as appropriate. Interstitial lung disease (ILD)/Pneumonitis: Mekinist should be withheld in patients with suspected ILD or pneumonitis, including patients presenting with new or progressive pulmonary symptoms and findings including cough, dyspnoea, hypoxia, pleural effusion, or infiltrates, pending clinical investigations. Mekinist should be permanently discontinued for patients diagnosed with treatment-related ILD or pneumonitis. If Mekinist is being used in combination with Tafinlar then therapy with Tafinlar may be continued at the same dose. Rhabdomyolysis: Signs or symptoms of rhabdomyolysis should warrant an appropriate clinical evaluation and treatment as indicated. Visual impairment: visual disturbances, including chorioretinopathy or retinal pigment epithelial detachment (RPED) and retinal vein occlusion (RVO) have been observed. Not recommended for patients with history of RVO. Ophthalmological evaluation should be performed at baseline and during treatment if clinically warranted. If retinal abnormality is observed, treatment should be interrupted immediately and referral to specialist should be considered. Permanently discontinue treatment if RVO is noticed. Rash: observed in 60% of patients in monotherapy and 24% of patients in combination with Tafinlar. Severe cutaneous adverse reactions (SCARs): SCARs, including Stevens-Johnson syndrome, and drug reaction with eosinophilia and systemic symptoms (DRESS), which can be life-threatening or fatal, have been reported with Mekinist in combination with Tafinlar. Before initiating treatment, patients should be advised of the signs and symptoms and monitored closely for skin reactions. If signs and symptoms
suggestive of SCARs appear, Mekinist and Tafinlar should be withdrawn. Deep vein thrombosis (DVT)/pulmonary embolism (PE): can occur when used as a monotherapy or in combination with Tafinlar. Seek immediate medical care if patients develop symptoms of DVT or PE. Pyrexia: Pyrexia including severe rigors, dehydration and hypotension (including acute renal insufficiency) reported. Incidence and severity increased when Mekinist used in combination with Tafinlar. Monitoring serum creatinine and other evidence of renal function impairment during and following severe pyrexia events. Serious non-infectious febrile events observed. For management of pyrexia, therapy should be interrupted if the patient’s temperature is ≥38°C (100.4°F). In case of recurrence, therapy can also be interrupted at the first symptom of pyrexia. Colitis and gastrointestinal perforation: Colitis and gastrointestinal perforation, including fatal outcome, have been reported. Treatment with Mekinist monotherapy or in combination with Tafinlar should be used with caution in patients with risk factors for gastrointestinal perforation, including a history of diverticulitis, metastases to the gastrointestinal tract and concomitant use of medications with a recognized risk of gastrointestinal perforation. If patients develop symptoms of colitis and gastrointestinal perforation they should immediately seek medical care. Sarcoidosis: Cases of sarcoidosis have been reported in patients treated with trametinib in combination with dabrafenib, mostly involving the skin, lung, eye and lymph nodes. In the majority of the cases, treatment with trametinib and dabrafenib was maintained. In case of a diagnosis of sarcoidosis, relevant treatment should be considered. It is important not to misinterpret sarcoidosis as disease progression Sodium: This medicine contains less than 1mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium free’. Warnings/Precautions for Tafinlar (used as a monotherapy or in combination with Mekinist): Pyrexia including severe rigors, dehydration and hypotension (including acute renal insufficiency) reported. Incidence and severity increased when used in combination with Mekinist. During and following severe pyrexia events, serum creatinine and other evidence of renal function should be monitored. Serious non-infections febrile events have been observed. For management of pyrexia therapy should be interrupted if the patient’s temperature is ≥38°C (100.4°F). In case of recurrence, therapy can also be interrupted at the first symptom of pyrexia. Cutaneous squamous cell carcinoma (cuSCC) and new primary melanoma: skin examination prior, during and for 6 months after discontinuation of treatment or until initiation of another anti-neoplastic therapy. Non-cutaneous secondary/recurrent malignancy: monitoring as clinically appropriate for up to 6 months after discontinuation of Tafinlar or until initiation of another anti-neoplastic therapy. Pancreatitis: unexplained abdominal pain should be promptly investigated to include measurement of serum amylase & lipase. Close monitoring when re-starting Tafinlar. Uveitis: monitoring patients for visual signs and symptoms during therapy. Colitis and gastrointestinal perforation: Colitis and gastrointestinal perforation, including fatal outcome, have been reported in patients taking dabrafenib in combination with trametinib. Sarcoidosis: Cases of sarcoidosis have been reported in patients treated with dabrafenib in combination with trametinib, mostly involving the skin, lung, eye and lymph nodes. In the majority of the cases, treatment with dabrafenib and trametinib was maintained. In case of a diagnosis of sarcoidosis, relevant treatment should be considered. It is important not to misinterpret sarcoidosis as disease progression. Interactions: Mekinist: Effect of trametinib on other medicinal products: Based on clinical data, no loss of efficacy of hormonal contraceptives is expected when co-administered with trametinib monotherapy Tafinlar: Effect of other medicinal products on dabrafenib: Caution with co-administration of strong inhibitors (e.g. ketoconazole, gemfibrozil, nefazodone, clarithromycin, ritonavir, saquinavir, telithromycin, itraconazole, voriconazole, posaconazole, atazanavir) of CYP2C8 or CYP3A4. Avoid co-administration with strong inducers (e.g. rifampicin, phenytoin, carbamazepine, phenobarbital, or St John’s wort (Hypericum perforatum) of CYP2C8 and CYP3A4. Avoid agents that increase gastric pH, when possible. Effect of dabrafenib on other medicinal products: Avoid concomitant use with medicinal products that are sensitive substrates of certain metabolising enzymes or transporters, if monitoring for efficacy and dose adjustment is not possible. Please refer to Section 4.5 of SPC for groups of medicinal products that can be affected. A drug utilisation review (DUR) is essential on initiating dabrafenib treatment. Exercise caution when co-administering with warfarin and consider additional INR (lnternational Normalised Ratio) monitoring. Exercise caution
when co-administering with digoxin and additional monitoring is recommended. Effects of dabrafenib on substance transport systems: Tafinlar inhibits OATP1B1 and OATP1B3. Monitoring recommended of drugs that are sensitive substrates of OATP1B1 and OATP1B3 and known to have a narrow therapeutic index with regards to high peak concentrations (Cmax). Fertility, Pregnancy & Lactation: Women of child-bearing potential: When Mekinist and Tafinlar are use in combination, use effective methods of contraception during therapy and for at least 16 weeks after stopping treatment. Tafinlar may decrease the efficacy of oral or any systemic hormonal contraceptives; use an effective alternative method of contraception. Pregnancy: Caution should be exercised by considering the expected benefit to the mother against possible risk to the foetus. Breastfeeding: Caution should be exercised by considering the benefit of breastfeeding for the child and the benefit of therapy for the woman. Fertility: Mekinist may impair human fertility. Tafinlar represents a potential risk for impaired spermatogenesis, which may be irreversible. Ability to Drive and Use Machines: Trametinib and Dabrafenib have minor influence. Patients should be made aware of the potential for fatigue, dizziness and eye problems. Adverse reactions with Mekinist monotherapy in metastatic melanoma: Very common (≥ 1/10): hypertension, haemorrhage, cough; dyspnoea, diarrhoea, nausea, vomiting, constipation, abdominal pain, dry mouth, rash, dermatitis acneiform, dry skin, pruritus, alopecia, fatigue, oedema peripheral, pyrexia, asparate aminotransferase increased. common (≥ 1/100, < 1/10): folliculitis; paronychia; cellulitis; rash pustular; anaemia; hypersensitivity; dehydration; vision blurred; periorbital oedema; visual impairment; left ventricular dysfunction; ejection fraction decreased; bradycardia; lymphoedema; pneumonitis; stomatitis; erythema; palmar-plantar erythrodysaesthesia syndrome; skin fissures; skin chapped; face oedema; mucosal inflammation; asthenia; alanine aminotransferase increased; blood alkaline phosphatase increased; blood creatine phosphokinase increased. Adverse reactions with Tafinlar monotherapy: Very common (≥ 1/10): papilloma; decreased appetite; headache; cough; nausea, vomiting, diarrhoea; hyperkeratosis, alopecia, rash, PPE, arthralgia, myalgia, pain in extremity, pyrexia, fatigue, chills, asthenia. common (≥ 1/100, < 1/10): cuSCC; seborrhoeic keratosis; skin tags; basal cell carcinoma; hypophosphataemia; hyperglycaemia; constipation; dry skin; pruritus; actinic keratosis; skin lesion; erythema; photosensitivity reaction; influenza-like illness. Adverse reactions observed when Mekinist and Tafinlar are used in combination: Very common (≥ 1/10): nasopharyngitis, decreased appetite, headache, dizziness, hypertension, haemorrhage, cough; abdominal pain, constipation, diarrhoea, nausea, vomiting, alanine aminotransferase increased, aspartate aminotransferase increased, dry skin, pruritus, rash, erythema, arthralgia, myalgia, pain in extremity, muscle spasms, fatigue, oedema peripheral, pyrexia, chills, asthenia, influenza-like illness. common (≥ 1/100, < 1/10): urinary tract infection, cellulitis; folliculitis; paronychia; rash pustular; cuSCC; papilloma; seborrhoeic keratosis; neutropenia,; anaemia; thrombocytopenia; leukopenia; dehydration; hyponatraemia; hypophosphataemia; hyperglycaemia; vision blurred; visual impairment; uveitis; ejection fraction decreased; hypotension; lymphoedema; dyspnoea; dry mouth; stomatitis; dermatitis acneiform; actinic keratosis; night sweats; hyperkeratosis; alopecia; palmar-plantar erythrodysaesthesia syndrome; skin lesion; hyperhidrosis; panniculitis; skin fissures; photosensitivity; mucosal inflammation; face oedema; blood alkaline; phosphatase increased; gamma-glutamyltransferase increased; blood creatine phosphokinase increased. For more details on adverse reactions, please see SmPC. Pack Size: Mekinist is supplied in bottles of 30 tablets. Tafinlar is supplied in packs of 120 hard capsules. Legal category: POM. Marketing Authorisation Numbers: Mekinist: EU/1/14/931/002 (0.5mg), EU/1/14/931/006 (2.0mg). Tafinlar: EU/1/13/865/002 (50 mg), EU/1/13/865/004 (75 mg). Marketing Authorisation
Holder: Novartis Europharm Ltd, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland.
Date of revision of text: Aug 2021.
Full prescribing information is available upon request from: Novartis Ireland Limited, Vista Building, Elm Park Business Park, Elm Park, Dublin 4. Tel: 01-2601255 or at www. medicines.ie. Detailed information on this product is also available on the website of the European Medicines Agency http://www.ema.europa.eu.
Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med
TAFINLAR
Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Merrion Road, Dublin 4, D04 A9N6

Kirkwood JM, Manola J, Ibrahim J, et al; for Eastern Cooperative Oncology Group. A pooled analysis of Eastern
and Intergroup
of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10(5):1670-1677.

of Product Characteristics. Novartis
Braftovi Summary of Product Characteristics.
relapse-free survival
Medicament
Clin Oncol. 2018;36(35):3441-3449.
available at www.medicines.ie
Summary of Product Characteristics.
at www.medicines.ie
Fabre Medicament
at www.medicines.ie
Zelboraf Summary of Product
available at www.medicines.ie
Mektovi Summary of Product
Hauschild A, Dummer R, Schadendorf D, et al. Longer follow-up
with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600–mutant stage III melanoma.
Contents Foreword
Winter Plan becoming a ‘Media Exercise’ P5
Electronic Health Record (EHR) platform launched in Ireland P8


Budget 2023 falls short for new medicines P10
National Cancer Registry publishes National Trends report P12
Professor Stephen Lane gives an Overview of Asthma P14
Cases of HIV continue to increase P22
Financial burden of chronic health conditions detailed P25
REGULARS
CPD: Amyloidosis P31
Men’s Health Focus: Erectile Dysfunction P40
Men’s Health Focus: Mental Health P44
Men’s Health Focus: Cancer Survivorship P48
Feature: Inflammatory Bowel
P64
Clinical R&D:
Editor
The HSE Winter Plan was published recently, and with it came a wave of criticism from the hospital sector that not enough is being done. President of the Irish Medical Organisation, Dr Clive Kilgallen has said the annual unveiling of the Plan for the health services has become increasingly ‘unmoored from the daily reality of the services.’ He adds, “It’s now more about branding than a meaningful intervention to deal with the capacity issues, workforce crisis and patient waiting lists.”
Meanwhile Professor Robert Landers, President of the Irish Hospital Consultants Association stated that the Plan fails to address the threat of a ‘twindemic.’


“There are two key things that our patients need: hospital beds and hospital Consultants. Unfortunately, this Winter Plan is unlikely to make in-roads in delivering the significant numbers required of both of these resources,” he says.
Turn to page 5 for the full story.
Meanwhile, the Budget 2023 has also come under fire. On page 10 of this issue, the Irish Pharmaceutical Healthcare Association determines that the Government’s Budget allocation of ¤18 million for new medicines falls short of the amount needed to optimise patients’ access to new medicines next year and to help Ireland catch up with peer European countries.
Michael O’Connell, IPHA President says, “We recognise that the Government faced extraordinary pressures in framing Budget 2023, especially in dealing with a cost-of-living crisis and the consequences of the appalling invasion of Ukraine. But the ¤18 million allocation is about half of what will be needed next year to give patients access to the broadest range of treatment options.”
In clinical news, the National Cancer Registry has published a new cancer trends report. Breast, cervical and colorectal cancer 19942019: National trends for cancers with population-based screening programmes in Ireland, focuses on cancer incidence, mortality, stage and survival for patients diagnosed with female breast cancer, cervical cancer and colorectal cancer.
“There is clear evidence that programmatic cancer screening improves cancer outcomes and saves lives. This report demonstrates that the trends in Ireland are consistent with these international findings which is reassuring for service users, providers and policy makers,” says NCRI Director Professor Deirdre Murray. You can read more about this on page 12.
Our Special Focus for November is on Men’s Health and we have some excellent clinical content contributed by local specialists including Professor Brendan Kelly on men’s mental health issues, Dr Brendan Noonan on developing a cancer survivorship pathway for men, Dr Kate McCann on obesity in the male population and Theresa Lowry-Lehnen on Testicular Cancer.
I hope you enjoy the issue.
Hospital Pharmacy Position Paper on Trials
Clinical trials are essential for continuously improving patient outcomes and their quality of life. Hospital pharmacists, as members of the multidisciplinary team, are needed to safely manage them. With the entry into application of the Clinical Trial Regulation, EAHP decided to update its Position Paper on Clinical Trials in 2022.
One of the key contributions of the pharmacist is the promotion of patient safety by collaborating in the development of a research protocol, reviewing as a member of an advisory committee, establishing mechanisms that contribute to safety, and assuring compliance with local
and national regulations and standards. Hospital pharmacists are at the forefront of patient care and consequently also have a significant impact on patient management and thus should be further integrated into the work of ethics committees.
The position paper of EAHP touches on the role of hospital pharmacists in clinical trials, the involvement of different patient groups, the improvements in Europe's clinical trial landscape and the role of ethics committees in clinical trials. It contains several calls to action, namely
• EAHP calls on national governments to recognise the
important roles that hospital pharmacists play in clinical trials by requiring their involvement to increase patient safety
• EAHP encourages regulators to further improve training on clinical trials by anchoring it into both undergraduate and continuing education of pharmacists.
• EAHP recognises that not all patient groups are suitable candidates or fully represented in clinical trials. Where appropriate, efforts should be made – taking into account also all relevant constraints – to create clinical trials that also study the effects of new
treatment options in diverse patient populations, so that also these groups could be provided with access to new medicines once approved.
• EAHP underlines the importance of utilising the full potential of the EU Clinical Trial Regulation by swiftly putting all necessary measures in place at the national level to successfully transition to this new regime.
• EAHP urges the Member States to ensure that the role of ethics committees under the new Clinical Trial Regulation remains strong in the interest of clinical trial participants.
Presenting at FIP World Congress
Professor Martin Henman, Associate Professor, Practice of Pharmacy at Trinity College Dublin and Dr Silvia Ravera, European Directorate for the Quality of Medicines and Healthcare (EDQM), pictured right, recently presented at the International Pharmaceutical Federation (FIP) World Congress.

The 80th World Congress of Pharmacy and Pharmaceutical Sciences took place in Seville. Professor Henman and Dr Ravera presented on the work of EDQM.
Posters of both the Resolution CM/Res(2020)3 on the implementation of pharmaceutical care for the benefit of patients and health services (09000016809cdf26 (coe.int) ) and the ongoing project to develop Guidelines concerning Medication Review were displayed in the Social and Administrative Pharmacy Section area.
Budget 2023: Disappointing and Lacks Essential Basics
The Irish Hospital Consultants Association (IHCA) has acknowledged the provisions in Budget 2023, but it warns that it does not address the decade-long public hospital capacity deficits that are the root causes of the unacceptable public hospital waiting lists, which now stand at almost 1 million people awaiting assessment and treatment.
Professor Robert Landers, President of the IHCA, said, “Budget 2023 fails to address the overwhelming capacity deficits including the severe shortage of Hospital Consultants, public hospital beds, theatre and other
frontline facilities which are needed to provide timely, safe care to patients.
“The Government’s commitment to deliver just 250 additional acute and community beds in 2023 falls well short of what is needed to provide care to admitted patients who currently receive care on a hospital trolley as no bed is available and the large number of patients on public hospital waiting lists.
“The allocation of ¤443 million specifically to address waiting lists in 2023 shows the Government has run out of ideas when it comes to tackling the
record public hospital waiting lists. The ¤350 million Waiting List Action Plan for 2022 has spectacularly failed to reach any of its targets of reducing waiting lists for outpatient appointments, inpatient and day case treatment, and GI (gastrointestinal) scopes despite the significant outlay.
“Budget 2023 will do nothing to address the 900 permanent hospital Consultant posts (22% of the total) that are vacant or filled on a temporary basis. Filling these posts with permanent appointees is essential to effectively provide care to the nearly 1 million people currently on waiting lists, the
growing delays in treatment and the resulting poorer patient outcomes.
“Budget 2023 fails to end the Consultant pay inequity imposed unilaterally by the Minister for Health in 2012, despite the unambiguous commitment of the Minster Stephen Donnelly two years ago and the clear evidence that this is driving the highly trained specialists our public hospitals need to pursue their careers abroad.
“Continuing the same mistakes year after year – with the same resulting impact on delayed treatment and increased risk to patient safety – is not the solution.”
Winter Plan – Time for a reality check
The Irish Medical Organisation (IMO) has warned that the Winter Plan has increasingly become a media exercise rather than a meaningful intervention to alleviate the year round pressures in our health services.
Dr Clive Kilgallen, President of the IMO said, “The annual unveiling of the Winter Plan for the health services has become increasingly unmoored from the daily reality of the services. It’s now more about branding than a meaningful intervention to deal with the capacity issues, workforce crisis and patient waiting lists.”
Dr Kilgallen said the plan was a series of rehashed announcements; “the monies promised today were previously announced in the budget and are simply insufficient to tackle the enormity of the year round challenge facing our health service, let alone cope with the additional pressures expected over the coming months. We already have almost 900 vacant consultant posts in our services so the promise of a further 50 ED Consultants in a short space of time seems ambitious to say the least, or simply unachievable in real terms. Unless and until we have sufficient capacity in
the system in terms of beds, adequate numbers of consultants, fully resourced GP services and other community services
these announcements become meaningless to those working in our health services and the patients who need care.”
Winter Plan: Fails to address ‘Twindemic’ Threat
Commenting on the publication of the HSE Winter Plan 202223 Professor Robert Landers, President of the Irish Hospital Consultants Association (IHCA) said, “The provision of ¤169 million announced in the Winter Plan 2022-2023 is welcome, but much of this ‘additional funding’ has been previously included in existing allocations. We have grave concerns that it will not be nearly sufficient enough to address the magnitude of deficits in our public hospitals and the enormity of the challenges they face this autumn and winter.

“There are two key things that our patients need: hospital beds and hospital Consultants. Unfortunately, this Winter Plan is unlikely to make in-roads in delivering the significant numbers required of both of these resources.
“This summer record numbers of admitted patients have had to be
treated on trolleys while waiting for admission to a hospital bed because our wards were full. This was the worst June and August for ED overcrowding in the past 16 years. It is now expected the situation could get significantly worse as we face into a potential ‘twindemic’ of flu combined with further expected Covid cases.
“Talk of ‘prioritising’ the opening of outstanding hospital beds already promised but not delivered will not address the problem or the impact of bed shortages on patients in our wards this winter.
“There are still 250 inpatient beds to be opened as part of the additional 1,146 hospital beds which were funded and committed to two years ago in Budget 2021.1 Minister Donnelly’s suggestion that an additional 160 public hospital beds per year is sufficient is now
far too little too late given what we now know post-Covid. We need an immediate and front-loaded expansion of hospital bed capacity.
“We fear plans to recruit an additional 51 Consultants in Emergency Medicine, will also have little to no impact - given the current average timeline of over 500 days to recruit a Consultant. This means if any candidates are found, they may not even be in post to deliver on next year’s Winter Plan.
“If the Minister for Health does not, as unequivocally committed to two years ago, end the discrimination against consultants appointed since 2012, the critical breach of trust that is driving our highly trained specialists away from our public hospitals will not be repaired. All the while, other countries are moving
ahead and attracting away those specialists our public hospitals desperately need.
“Based on the current mistaken approaches, it will be extremely challenging for the HSE to attract the number and calibre of specialists required to fill not only the proposed 51 posts, but also the 900 existing permanent consultant posts that cannot be filled. The reality is that far too many of our highly trained doctors are opting to emigrate and work with more collaborative terms and conditions elsewhere, rather than apply for posts in Irish public hospitals.
“Filling these posts with permanent appointees is essential to provide timely care and to continue to deliver essential services to patients year-round, including in the most challenging winter period.”
“It’s now more about branding than a meaningful intervention to deal with the capacity issues, workforce crisis and patient waiting lists.”Dr Clive Kilgallen, President of the IMO
EU Legislation Reform Critical to Biosimilars Competition
The EU plans to update its 20-yearold pharmaceutical legislation early next year to improve access to medicines and to make innovation more affordable.
Medicines for Europe have now said that the introduction of biosimilar medicines was one of the pioneering and most successful reforms of the previous legislation in 2004. “Now we need to update the legislation to make it fit for the future,” the organisation has stated.
The smart use of biosimilar medicines increases the number of treated patients and can encourage huge investments in R&D in Europe. This contributes to lower healthcare budgets.
The statement adds, “The first biosimilar medicine was introduced over 15 years ago, starting a transformation in therapy by offering broader and earlier access to lifechanging biological therapies. Grassroots cooperation among stakeholders was also a key success factor for the uptake of biosimilar medicines in Europe. The success of biosimilar medicines access will be further supported by the recent European Medicines Agency (EMA-HMA) publication that healthcare professionals and patients can confidently choose from any authorised version of a biological medicine.
“Looking forward, the cancer and other non-communicable disease
communities are calling for more integrated care frameworks for patients. This can be enabled by the use of biosimilar medicines. The ¤18 billion (at list price meaning that real savings are higher) savings biosimilar medicines have generated for European health systems can be reinvested in better care pathways to improve patient outcomes. The reform of EU pharmaceutical legislation can guarantee future biosimilar medicines competition by updating EU regulations with the latest scientific regulatory developments in the field.”
Commenting ahead of the Biosimilar medicines conference BIOS22, the Chair of the Biosimilar
Outstanding Impact in Public Health Award
Dr Karen DeSalvo, Google Chief Health Officermedicines group, Isabell Remus said, “We are on the verge of an unprecedented opportunity to drive further patient access to life-changing biologic medicines. About one in two medicines that will face competition in Europe in the next decade is a biologic. We owe it to the patients and healthcare systems we serve to grasp this opportunity with both hands. The time for action is now: the revision of the EU pharmaceutical legislation is a unique chance to create a framework that supports the development and authorisation of biosimilars – ensuring patients can benefit from their full potential over the next 20 years.”
Dr Karen DeSalvo, Google Chief Health Officer, has received the Emily Winifred Dickson Award from RCSI University of Medicine and Health Sciences, in recognition of her outstanding impact in public health.

Emily Winifred Dickson broke boundaries when she became a
Fellow of RCSI in 1893, making her the first female Fellow of any of the surgical royal colleges in Britain and Ireland. RCSI established the Emily Winifred Dickson Award in honour of the achievements of this pioneering woman. The award recognises women who have made an outstanding contribution to their field.
Dr Karen DeSalvo is a doctor of internal medicine and health leader working at the intersection of medicine, public health, and information technology. She has dedicated her career to improving health outcomes for all, with a focus on solutions that address all the determinants of health.
During her time at the US Department of Health and Human Services, Dr DeSalvo focused on creating a more consumer-oriented,
Pharmacy Expressions of Interest
Over the coming months the Pharmaceutical Society of Ireland will be gathering input for their project examining Emerging Risks to the Future Pharmacy Workforce.
This is a complex issue with many contributing factors and multiple stakeholders. Ensuring that the PSI hear from pharmacists and others will be an important part of the work. The PSI will be holding focus groups for pharmacists and final year pharmacy students and invite those interested to consider taking part.

They are also looking for one pharmacist member for a Working Group that will play a strategic role in the project. If you would like to know more visit www.thepsi.ie
transparent and value-based health system. She served as the New Orleans Health Commissioner following Hurricane Katrina.
Dr DeSalvo continues to be a powerful voice and advocate for eliminating inequities and improving the public’s health. At Google, she leads a team of health professionals who provide guidance for the development of inclusive research, products and services, and has been part of the Google COVID response team.
Before joining Google, Dr DeSalvo was National Coordinator for Health Information Technology and Assistant Secretary for Health (Acting) in the Obama Administration. She was previously Vice Dean for Community Affairs and Health Policy at the Tulane School of Medicine where she was a practicing internal medicine physician, educator, researcher and leader.
BRING PROTECTION TO LIFE
BY REDUCING THE RISK OF THE COMPOSITE OF DECLINING KIDNEY FUNCTION (BY ≥ 50%), ESKD, AND RENAL OR CV DEATH IN CONJUNCTION WITH OTHER CKD THERAPIES WHEN COMPARED TO PLACEBO*1
FORXIGA IS INDICATED IN ADULT PATIENTS FOR THE TREATMENT OF CKD.2
treatment
GFR mL/min/1.73m2
FORXIGA 10 mg
Once daily
No titration required except in patients with severe hepatic impairment
Applicable for CKD, HFrEF, or T2D.2
*The DAPA-CKD study was an international, multicentre, randomised, double-blind, placebo-controlled study. In DAPA-CKD, the primary endpoint showed that FORXIGA in conjunction with other CKD therapies reduced the relative risk of the composite endpoint of ≥50% sustained decline in eGFR, reaching ESKD, and renal or CV death by 39% (5.3% ARR) vs placebo with other CKD therapies in 4304 adult patients with CKD with an eGFR of 75 to 25 mL/ min/1.73m2 and albuminuria (UACR ≥ 200 and ≤ 5000 mg/g) (median follow-up of 2.4 years; 9.2% vs 14.5%; HR 0.61; 95% CI (0.51 - 0.72; p<0.001).1 ESKD defined as sustained eGFR < 15 mL/min/1.73m2, chronic dialysis treatment or receiving a renal transplant. DAPA-CKD was stopped early due to efficacy benefit, because of the unplanned early stop, the secondary endpoints are considered nominally significant; Secondary endpoints showed that FORXIGA in conjunction with other CKD therapies reduced the relative risk of the composite of CV death or hHF by 29% (1.8% ARR: HR 0.71; 95% CI, 0.55 to 0.92; nominal p=0.009) and also reduced the relative risk of all-cause mortality by 31% (2.1% ARR; HR 0.69; 95% CI, 0.53 to 0.88; nominal p=0.004) vs placebo with other CKD therapies. There were comparable rates of the individual component of CV death, FORXIGA vs placebo (3.0% vs 3.7%; HR 0.81; 95% CI, 0.58, 1.12)1
The overall safety profile of dapagliflozin in patients with chronic kidney disease was consistent with the known safety profile of dapagliflozin.2 ©AstraZeneca 2022. All Rights Reserved.
ABRIDGED PRESCRIBING INFORMATION
FORXIGA® (dapagliflozin) 5MG & 10MG FILMCOATED TABLETS.
Consult Summary of Product Characteristics (SmPC) before prescribing.
Indications: Adults and children aged 10 years and above: Type 2 diabetes mellitus: For the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise, as monotherapy when metformin is considered inappropriate due to intolerance, or in addition to other medicinal products for the treatment of type 2 diabetes. Adults: Heart Failure: Indicated in adults for the treatment of symptomatic chronic heart failure with reduced ejection fraction. Chronic kidney disease: Indicated in adults for the treatment of chronic kidney disease.
Presentation: Film-coated tablets. 5mg or 10mg of dapagliflozin (as propanediol monohydrate). Each 5mg tablet contains 25mg of lactose. Each 10mg tablet contains 50mg of lactose.
Dosage and Administration: Adults: Type 2 diabetes mellitus: The recommended dose is 10mg once daily. Consider a lower dose of insulin or insulin secretagogue such as a sulphonylurea when used in combination with dapagliflozin to reduce the risk of hypoglycaemia. Children and adolescents: No dose adjustment in children aged 10 years and above. No safety and efficacy data available for children below 10 years of age. Heart Failure: The recommended dose is 10mg once daily. Chronic kidney disease: The recommended dose is 10 mg once daily. Children and adolescents: <18 years: Safety and efficacy not yet established. Elderly: ≥65 years: No dose adjustment is recommended based on age. Renal impairment: No dose adjustment is required based on renal function. Due to limited experience, it is not recommended to initiate treatment with dapagliflozin in patients with GFR < 25 mL/min. If GFR falls below 45 mL/min, additional glucose lowering treatment should be considered in patients with diabetes mellitus if further glycaemic control is needed. Mild or moderate hepatic impairment: No dose adjustment. Severe hepatic impairment: Starting dose of 5mg is recommended, if well tolerated, dose may be increased to 10mg.
Method of administration: Forxiga can be taken orally at any time of day with or without food. Tablets should be swallowed whole.
Contraindications: Hypersensitivity to dapagliflozin, or excipients. Warnings and Precautions: Renal impairment: Due to limited experience, it is not recommended to initiate treatment with dapagliflozin in patients with GFR < 25 mL/min. The glucose lowering efficacy of dapagliflozin is dependent on renal function, and is reduced in patients with GFR < 45 mL/min and is likely absent in patients with severe renal impairment. Hepatic impairment: Exposure is increased in patients with severe hepatic impairment. Use in patients at risk of volume depletion and/or hypotension: Dapagliflozin increases diuresis which may lead to a modest decrease in blood pressure, it may be more pronounced in patients with very high blood glucose concentrations. Exercise caution in patients for whom a dapagliflozin induced drop in blood pressure could pose a risk, such as patients on anti hypertensive therapy with a history of hypotension or elderly patients. Careful monitoring of volume status and electrolytes is recommended in conditions leading to volume depletion, such as acute gastrointestinal illness. In volume depleted patients temporary interruption of dapagliflozin is recommended until volume depletion is corrected. Diabetic ketoacidosis (DKA): Rare cases of DKA, including life-threatening and fatal cases, have been reported in patients treated with SGLT2 inhibitors, including dapagliflozin. In a
number of cases, the presentation of the condition was atypical with only moderately increased blood glucose values, below 14mmol/L (250mg/dL). The risk of DKA must be considered in the event of nonspecific symptoms such as nausea, vomiting, anorexia, abdominal pain, excessive thirst, difficulty breathing, confusion, unusual fatigue or sleepiness. Patients should be assessed for ketoacidosis immediately if these symptoms occur, regardless of blood glucose level. In patients where DKA is suspected or diagnosed, dapagliflozin treatment should be stopped immediately. Treatment should be interrupted in patients who are hospitalised for major surgical procedures or acute serious medical illnesses. Monitoring of ketones is recommended in these patients. Measurement of blood ketone levels is preferred to urine. Treatment with dapagliflozin may be restarted when the ketone values are normal and the patient’s condition has stabilised. Before initiating dapagliflozin, factors in patient history that may predispose to ketoacidosis should be considered. Patients who may be at higher risk of DKA include patients with a low beta cell function reserve (e.g. patients with low C peptide or latent autoimmune diabetes in adults (LADA) or patients with a history of pancreatitis), patients with conditions that lead to restricted food intake or severe dehydration, patients for whom insulin doses are reduced and patients with increased insulin requirements due to acute medical illness, surgery or alcohol abuse. SGLT2 inhibitors should be used with caution in these patients. Restarting SGLT2 inhibitor treatment in patients experiencing a DKA while on SGLT2 inhibitor treatment is not recommended, unless another clear precipitating factor is identified and resolved. Dapagliflozin should not be used for treatment of patients with type 1 diabetes. Necrotising fasciitis of the perineum (Fournier’s gangrene): Postmarketing cases have been reported in female and male patients taking SGLT2 inhibitors. Urgent surgical intervention and antibiotic treatment is required. Advise patients to seek medical attention if they experience a combination of pain, tenderness, erythema, or swelling in the genital or perineal area, with fever or malaise. Either uro-genital infection or perineal abscess may precede necrotising fasciitis. If suspected, discontinue Forxiga and institute prompt treatment (including antibiotics and surgical debridement). Urinary tract infections: Temporary interruption of dapagliflozin should be considered when treating pyelonephritis or urosepsis. Elderly (≥65 years): Elderly patients are more likely to have impaired renal function, be treated with medicines such as anti-hypertensives or diuretics, and be at a greater risk of volume depletion. Cardiac failure: Experience with dapagliflozin in NYHA class IV is limited. Chronic kidney disease: There is no experience with dapagliflozin for the treatment of chronic kidney disease in patients without diabetes who do not have albuminuria. Patients with albuminuria may benefit more from treatment with dapagliflozin. Lower limb amputations: Counsel patients with diabetes on routine preventative foot care as an increase in cases of lower limb amputation (primarily of the toe) has been observed in long term, clinical studies in type 2 diabetes mellitus with SGLT2 inhibitors. Urine laboratory assessments: Patients will test positive for glucose in the urine due to mechanism of action. Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take Forxiga.
Drug Interactions: Diuretics: Dapagliflozin may add to the diuretic effect of thiazide and loop diuretics and may increase the risk of dehydration and hypotension. Insulin and insulin secretagogues: Consider a lower dose of insulin or insulin secretagogue when used in combination with dapagliflozin to reduce the risk of hypoglycaemia. Interference with 1,5 AG assay: Monitoring glycaemic control with 1,5-AG assay is not recommended as measurements of 1,5 AG are
unreliable in assessing glycaemic control in patients taking SGLT2 inhibitors. Alternative methods should be used. Interference with lithium: Dapagliflozin may increase renal lithium excretion and the blood lithium levels may be decreased. Serum concentration of lithium should be monitored more frequently after dapagliflozin initiation and dose changes. Please refer the patient to the lithium prescribing doctor in order to monitor serum concentration of lithium. Pregnancy and Lactation: Not recommended during the second and third trimesters of pregnancy. Treatment should be discontinued when pregnancy is detected. Do not use whilst breast-feeding. Ability to Drive and Use Machines: Alert patients on the risk of hypoglycaemia when dapagliflozin is used in combination with a sulphonylurea or insulin.
Undesirable Events: Consult SmPC for full list of side effects. Very common (≥1/10): Hypoglycaemia (when used with SU or insulin). Common (≥1/100 to <1/10): Vulvovaginitis, balanitis and related genital infections, urinary tract infection, dizziness, rash, back pain, dysuria, polyuria, haematocrit increased, creatinine renal clearance decreased during initial treatment, dyslipidaemia. Uncommon (≥1/1,000 to < 1/100): Fungal infection, volume depletion, thirst, constipation, dry mouth, nocturia, vulvovaginal pruritus, pruritus genital, blood creatinine increased during initial treatment, blood urea increased, weight decreased. Rare (≥ 1/10,000 to < 1/1,000): Diabetic ketoacidosis (when used in type 2 diabetes mellitus). Very Rare (< 1/10,000): Angioedema, necrotising fasciitis of the perineum (Fournier’s gangrene), Tubulointerstitial nephritis.
Legal Category: Product subject to prescription which may be renewed (B).
Marketing Authorisation Number: EU/1/12/795/002; EU/1/12/795/007.
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden.
Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 71 00.
FORXIGA is a trademark of the AstraZeneca group of companies. Date of API preparation: 07/2022 Veeva ID: IE-4049
Adverse events should be reported directly to: HPRA Pharmacovigilance, Website: www.hpra.ie

Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
References: 1. Heerspink HJL et al. N Engl J Med. 2020;383(15):1436–1446.
2. FORXIGA 10 mg film-coated tablets. Summary of product characteristics.
ARR = absolute risk reduction; CKD = chronic kidney disease; CV = cardiovascular; DAPA-CKD = Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease; eGFR = estimated glomerular filtration rate; ESKD = end-stage kidney disease; hHF = hospitalisation for heart failure; HR = hazard ratio. HFrEF = heart failure with reduced ejection fraction; T2D = type 2 diabetes
Ireland’s most advanced Electronic Health Records System
Dr Róisín Ní Muirceartaigh, Consultant Anaesthesiologist and Chief Medical Information OfficerMater Private Network (MPN) has embarked on delivering Ireland’s most ambitious and comprehensive Electronic Health Record (EHR) platform, MEDITECH Expanse. Backed by an investment of ¤26m, Mater Private Network will integrate MEDITECH Expanse across its entire Irish network, including its flagship Dublin and Cork hospitals, the Mid-Western Radiation Oncology Centre, Limerick, both Day Hospitals and clinics in counties Limerick, Offaly, Meath and Louth.
PMI Christmas Charity Lunch
The Pharmaceutical Managers’ Institute is holding its Annual Charity Lunch on Thursday, December 8th in the Mansion House Dublin.
The Charity Lunch makes a welcome return after two years on hold due to Covid-19. The perfect start to the Christmas festivities.

The charity partner for 2022 is Our Lady’s Hospice & Care Services – Harold’s Cross, Blackrock and Wicklow. Visit www.thepmi.com for further details.
John Hurley, CEO, Mater Private Network, said “This investment of ¤26m demonstrates a significant level of commitment from our shareholder, Infravia, to the integration of transformational technology across the full scope of our network. The ultimate beneficiaries of our investment in digital transformation are our patients. The EHR will facilitate clinical integration across our network, enhance access and patient participation in their own care. Seamless personalised care across our network will further improve our excellent clinical outcomes and patient experience.”
Lead clinician on the project, Dr. Róisín Ní Muirceartaigh, Consultant Anaesthesiologist and Chief Medical Information

Officer, said “Every step in the transfer or communication of medical and healthcare information is a significant contributor to clinical efficiency. EHR is about introducing a single, unified patient record which will encompass the entirety of the patient’s journey with us. It also ensures any information brought in from other institutions has a single, safe, easy to find, digital location in the patient’s new e-Chart, quickly and easily accessed by every professional involved in their care.”
Working with their chosen provider MEDITECH, a large multidisciplinary team drawn from across the Mater Private Network will collaborate to deliver Meditech Expanse across its entire hospital network by 2024.
Three in Five Expect another Wave
Almost three-fifths of people believe that there will be another ‘wave’ of Covid-19 this year, according to new research carried out by Ipsos for the Irish Pharmaceutical Healthcare Association (IPHA).
The research, measuring public attitudes to Covid-19 vaccination and to vaccination in general, shows that 58% of people believe another significant rise in Covid-19 cases this year is either fairly likely or very likely. Just 28% of people believe a significant rise in Covid-19 cases is unlikely. The rest said it was neither likely nor unlikely or they did not know.
Most people, or 70%, believe that medical science can respond to future Covid-19 variants while 68%
say that they would feel protected against the disease if there is another ‘wave’.
Over four-fifths of people, or 84%, believe that Ireland would be in a better position to deal with another pandemic because of our experience with Covid-19.
Trust in vaccination remains high, with 79% of people saying that vaccines in general are effective. That figure is largely unchanged since Ipsos asked the same question in June. Three in four people, or 74%, trust the medical evidence about vaccines.
Doctors or nurses, at 85%, remain the most trusted people among those listed for information about vaccines, down six points since Ipsos asked the same question
in February. They are followed by pharmacists, at 80%, scientists or vaccine specialists, at 79%, and official health websites, at 71%. Online user-generated content and politicians are among the least trusted when it comes to information about vaccines, registering 7% and 14%, respectively.
The survey finds that 91% of people have been vaccinated for Covid-19, with 44% saying they have had two or more booster doses. About one in three, or 34%, have received a single booster dose for Covid-19.
Michael O’Connell, IPHA’s President, said, “Public trust in vaccination remains high and our experience of living through
Covid-19 gives us confidence in dealing with another pandemic. But Covid-19 is still with us so it is encouraging to find that most people believe in the ability of medical science to respond to new variants.
“We know that the formula for scientific invention is intellectual property. That formula helped the industry, with others, to find answers fast for Covid-19, and it is helping us to develop new medicines for a range of other serious medical conditions all the time. It is vital that we continue to guard the conditions for innovation, especially in the context of the EU Pharmaceutical Strategy on which policymakers in Brussels are working.”


Budget’s ¤18m for new Medicines falls Short
especially in dealing with a cost-ofliving crisis and the consequences of the appalling invasion of Ukraine. But the ¤18 million allocation is about half of what will be needed next year to give patients access to the broadest range of treatment options.
from authorisation by the European Medicines Agency to reimbursement for patients. Medicines subject to a full health technology assessment – for example, cell and gene therapies – are taking 900 days. That is much longer than in peer European countries.
The Government’s Budget allocation of ¤18 million for new medicines falls short of the amount needed to optimise patients’ access to new medicines next year and to help Ireland catch up with peer European countries.
The industry acknowledged the extraordinary pressures faced by the Government when framing the Budget, especially in dealing with a cost-of-living crisis. It acknowledged, too, that though ¤18 million falls well short of the funding needed, it is still an investment in innovation, building on the ¤80 million allocated in the past two Budgets.
IPHA member companies expect to launch 30 new medicines next year, potentially treating more than 7,000 patients. These medicines are for a range of serious medical conditions, including ulcerative colitis, heart disease, lung disease and many forms of cancer. The industry has estimated the cost of
these new medicines at ¤35 million – twice as much as what has been allocated by the Government in Budget 2023. IPHA members have implemented a range of price cuts and rebates this year under the new Framework Agreement for the Supply and Pricing of Medicines to contribute to creating headroom to fund new medicines.
The concern now is that the process for reimbursement of new medicines remains too slow because funding is inadequate. The industry wants Ireland to be as fast as peer European countries in adopting new medicines in the health services.
Michael O’Connell, IPHA’s President, said the Budget allocation falls short of the amount needed to optimise patients’ access to new medicines.
“We recognise that the Government faced extraordinary pressures in framing Budget 2023,
“Last December, we concluded a new medicines supply Agreement. That Agreement, allied with adequate and sustained funding, can improve Ireland’s capacity to deliver the latest treatments to patients. IPHA members have implemented a lot of price cuts and new rebates under the Agreement already this year.
“In parallel, it is vital that the medicines reimbursement system be improved. It is not designed to bring innovation to patients as fast as possible. We must narrow the gap between the completion of health technology assessments and the availability of new medicines. As an industry, we are not bystanders in solving the problem. We have an obligation to bring forward proposals for a fitter and faster reimbursement system. As we prepare to do that, we want to collaborate with health policymakers on solutions that work for everyone,” said Mr O’Connell.
IPHA’s analysis shows that some medicines are still taking 650 days, on average,
Record Funding for Mental Health
Minister for Mental Health and Older People, Mary Butler, has announced another record year with further increases in the Mental Health budget for 2023.
Minister Mary Butler commented, “Reflecting the government’s strong commitment to Mental Health, Budget 2023 sees an additional ¤72.8 million of funding for Mental Health services into 2023. This brings our total investment for Mental Health to over ¤1.2 billion and including ¤14 million of new developments and ¤43.8 million for existing level of services.
“This historic funding will provide for continued Mental Health supports in both community and acute settings, with a continued focus on Clinical Programmes for eating disorders, early intervention in psychosis, Attention Deficit Hyperactivity Disorder (ADHD) and self-harm.”
Minister Butler concluded, “Importantly, this funding will also ensure a dedicated approach to waiting lists, especially for Child and Adolescent Mental Health Services (CAMHS) and primary care psychology for young people.
“Funding will also be made available to community-based
voluntary organisations that support health and social care, including Mental Health. This support is being made available in recognition of the challenges being faced by the sector in delivering and maintaining key health and social care services against a backdrop of increased inflationary pressures affecting energy, heating and related costs.”
Announced additional supports include:
• ¤10 million in Mental Health
Capital funding and ¤4 million for improving regulatory compliance in mental health in-patient
Ireland is almost four months slower to reimburse and make new cancer medicines available to patients compared to the average across the European Union’s 27 members, according to the most recent EFPIA Patients WAIT Indicator Survey. The survey, which analysed data for the four years between 2017 and 2020 using centrally authorised medicines, found that, among western European countries, only Portugal was slower than Ireland to make new medicines available to patients. Denmark and Germany were three and four times faster, respectively, than Ireland to make new medicines available to patients.
Oliver O’Connor, IPHA’s CEO, said the latest Budget funding was still investment but the amount is not near enough.
“Ireland has a poor record on speed of access to new medicines. Adequate funding and reimbursement system reforms can improve the environment. While we have been making progress on funding, today’s announcement falls well short of expectations. The new medicines supply Agreement was a step on the road to an access environment that can be competitive with peer countries in Europe.”
services in alignment with Mental Health Commission regulations
• ¤750,000 for Counselling and Psychology Training Places in a new HSE initiative
• ¤9 million for New Mental Health Developments
• ¤2 million has been provided for new accommodation, ¤5 million has been provided for emergency placements over 2023 by the HSE, and ¤3 million funding under New Development funding for emergency placement additional capacity.






National Trends for Cancer Report
BreastCheck
BreastCheck began screening for breast cancer in the eastern half of the country in 2000 and then extended nationally from 2007. Screening was offered initially every two years to women aged 50 to 64 years, with an extension of the age eligibility to 69 years starting in 2015.
• The trends in incidence show what would be expected following the introduction/ expansion of a screening programme
The National Cancer Registry (NCRI) has just published a new cancer trends report entitled Breast, cervical and colorectal cancer 1994-2019: National trends for cancers with population-based screening programmes in Ireland
The report focuses on cancer incidence, mortality, stage and survival for patients diagnosed with female breast cancer, cervical cancer and colorectal cancer. All three cancers have populationbased screening programmes in Ireland.
National screening programmes organised by the Health Service Executive’s National Screening Service began inviting participants in 2000 (BreastCheck), 2008 (CervicalCheck) and 2012 (BowelScreen). Screening programmes aim to prevent or detect cancers early, therefore ensuring better outcomes for patients.
Key Findings
• All three cancers show favourable trends in incidence, stage, survival and/or mortality consistent with improvements in early detection and outcomes, with clear evidence for additional or more recent benefits of screening.
• Almost a third of cervical cancer cases and a quarter of female breast cancer cases diagnosed during 2017-2019 were detected as a result of screening. A smaller proportion of colorectal cancers were screen detected.

• The introduction of a populationbased screening programme
would be expected to decrease incidence in cervical and colorectal cancers, but not breast cancer. The incidence trends seen in our report are consistent with this.
• Screen-detected cancer cases were, on average, detected at a substantially earlier stage than other cases diagnosed at the same ages. Survival has improved for all three cancers, with the biggest improvements in seen in the age-groups targeted by the national screening programmes.
• Death rates of all three cancers have fallen significantly. Decreases in the age-groups targeted for screening have been more substantial than the overall decreases for all three cancers.
Cervical Check
CervicalCheck was introduced in 2008 and now uses HPV testing as the primary screening test to screen women aged between 25-65 years for cervical cancer.
• Overall, the rates of cervical cancer have shown a significant decreasing trend of 2.8% per year since 2009 following the introduction of screening, reversing the previous trend of a significant increase from 1999 to 2009.
• The proportion of cases diagnosed with early-stage cancers (Stage 1 and Stage 2) is much higher in women in the screening age group (88%) than in the non-screening group (52%)
• There has been a significant decreasing trend in mortality by on average 1.1% per year over the 1994-2019 period.
BowelScreen
BowelScreen was introduced in 2012 and offers free at-home screening (FIT test) for bowel cancer men and women aged from 60-69.
• Overall, the rates of colorectal cancer in men have shown a significant downward trend of 2.5% per year following the introduction of screening, with a smaller but still decreasing trend (0.3% per year) in women since 1994.
• The proportion of cases diagnosed with early-stage cancers (Stage 1 and Stage 2) is much higher in screened men (64%) and women (62%) compared to non-screened age groups of men (37%) and women (39%)
• There has been a significant decreasing trend in mortality over the 1994-2019 period and particularly noticeable in the 70+ year male age group (3.7% per year since 2011).
• The proportion of women in diagnosed with early stage cancers (stage 1 and stage 2) is higher (93%) in women in the screened age group compared to the non-screened age groups of <50 (78%) and > 70 years (75%).
• Mortality rates show a significant decreasing trend (by on average 1.8% per annum).
NCRI Director Professor Deirdre Murray said, “This is the first time that the NCRI has undertaken an in depth analysis on those cancers that have an associated screening programme. Internationally, there is clear evidence that programmatic cancer screening improves cancer outcomes and saves lives. This report demonstrates that the trends in Ireland are consistent with these international findings which is reassuring for service users, providers and policy makers.”
NSS Chief Executive Fiona Murphy welcomed the report and added, “The NCRI report is the first of its kind to analyse the impact of population screening programmes on cancer trends in Ireland. It is agreed that since the 1990s there has been a collective improvement in better awareness of cancer symptoms, new treatments, improvements in care and changes in underlying risks for cancer which have all had a positive impact on improved survival. However, with the publication of this report and the detailed analysis of the various screened populations we can now confidently say that a portion of that improvement is directly attributable to CervicalCheck, BowelScreen and BreastCheck. The public can be reassured that our screening programmes are effective.”
“This is the first time that the NCRI has undertaken an in depth analysis on those cancers that have an associated screening programme. Internationally, there is clear evidence that programmatic cancer screening improves cancer outcomes and saves lives. This report demonstrates that the trends in Ireland are consistent with these international findings which is reassuring for service users, providers and policy makers.”
trifluridine/tipiracil
Lonsurf® (Trifluridine/ tipiracil): Abbreviated Prescribing Information: Please refer to the Summary of Product Characteristics before prescribing COMPOSITION*: Lonsurf 15 mg/6.14 mg: film-coated tablet containing 15 mg trifluridine and 6.14 mg tipiracil (as hydrochloride). Lonsurf 20 mg/8.19 mg: film-coated tablet containing 20 mg trifluridine and 8.19 mg tipiracil (as hydrochloride). INDICATION*: As monotherapy for the treatment of adult patients with metastatic colorectal cancer who have been previously treated with, or are not considered candidates for, available therapies including fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapies, anti-VEGF agents, and anti-EGFR agents. As monotherapy for the treatment of adult patients with metastatic gastric cancer including adenocarcinoma of the gastroesophageal junction, who have been previously treated with at least two prior systemic treatment regimens for advanced disease. DOSAGE AND ADMINISTRATION*:
Recommended starting dose: 35 mg/m2/dose taken orally twice daily on Days 1 to 5 and Days 8 to 12 of each 28-day cycle, within 1 hour after completion of the morning and evening meals (20mg/m2/dose for patients with severe renal impairment). Dosage calculated according to body surface area, not exceeding 80 mg/dose. Possible dosing adjustments based on individual safety and tolerability: permitted dose reductions to a minimum dose of 20 mg/m2 twice daily (15mg/m2/dose for patients with severe renal impairment), dose escalation not permitted after a dose reduction.
CONTRAINDICATIONS*: Hypersensitivity to the active substances or to any of the excipients. WARNINGS *: Bone marrow suppres sion: Complete blood cell counts must be obtained prior to initiation of therapy, prior to each cycle and as needed. Treatment must not be start ed if absolute neutrophil count < 1.5 x 109/L, if platelet counts < 75 x 109/L, or if unresolved Grade 3 or 4 non-haematological clinically relevant toxicity. Patient should be monitored closely for infections, appropriate measures should be administered as clinically indicated. Gastrointestinal toxicity: anti-emetic, anti-diarrhoeal and other measures should be administered as clinically indicated, dose modifications should be applied as necessary. Renal impairment: not recommended if end-stage renal disease. Patients with renal impairment should be monitored closely; patients with moderate or severe renal impairment should be more frequently monitored for haematological toxicities. Hepatic impairment: not recommended if baseline moderate or severe hepatic impairment. Proteinuria: monitoring by dipstick urinalysis recommended prior to starting and during therapy. Excipients: contain lactose. INTERACTIONS*: Precautions: medicinal products that interact with nucleoside transporters CNT1, ENT1 and ENT2, inhibitors of OCT2 or MATE1, human thymidine kinase substrates (e.g. zidovudine), hormonal contraceptives. FERTILITY*. PREGNANCY AND BREASTFEEDING*: Not recommended. CONTRACEPTION*: For women and men, highly effective contraceptive measures must be used during treatment and for 6 months after stopping treatment. DRIVE & USE MACHINES*: Fatigue, dizziness or malaise may occur.
UNDESIRABLE EFFECTS*: Very common: Neutropenia, leukopenia, anaemia, thrombocytopenia, decreased appetite, diarrhoea, nausea, vomiting, fatigue. Common: Lower respiratory tract infection, febrile neutropenia, lymphopenia, hypoalbuminaemia, dysgeusia, neurop athy peripheral, dyspnoea, abdominal pain, constipation, stomatitis, oral disorder, hyperbilirubinaemia, Palmar-plantarerythrodysaesthesia syn drome, rash, alopecia, pruritus, dry skin, proteinuria, pyrexia, oedema, mucosal inflammation, malaise, hepatic enzyme increased, blood alkaline phosphatase increased, weight decreased. Uncommon: Septic shock, enteritis infectious, lung infection, biliary tract infection, influenza, urinary tract infection, gingivitis, herpes zoster, tinea pedis, candida infection, bacterial infection, infection, neutropenic sepsis, upper respiratory tract infection, conjunctivitis, cancer pain, pancytopenia, granulocytopenia, monocytopenia, erythropenia, leukocytosis, monocytosis, dehydration, hyperglycaemia, hyperkalaemia, hypokalaemia, hypophosphataemia, hypernatraemia, hyponatraemia, hypocalcaemia, gout, anxiety, insomnia, neurotoxicity, dysaesthesia, hyperaesthesia, hypoaesthesia, syncope, paraesthesia, burning sensation, lethargy, dizziness, headache, visual acuity reduced, vision blurred, diplopia, cataract, dry eye, vertigo, ear discomfort, angina pectoris, arrhythmia, palpitations, embolism, hyperten sion, hypotension, flushing, pulmonary embolism, pleural effusion, rhinorrhoea, dysphonia, oropharyngeal pain, epistaxis, cough, enterocolitis haemorrhagic, gastrointestinal haemorrhage, pancreatitis acute, ascites, ileus, subileus, colitis, gastritis, reflux gastritis, oesophagitis, impaired gastric emptying, abdominal distension, anal inflammation, mouth ulceration, dyspepsia, gastrooesophageal reflux disease, proctalgia, buccal polyp, gingival bleeding, glossitis, periodontal disease, tooth disorder, retching, flatulence, breath odour, hepatotoxicity, biliary dilatation, skin exfoliation, urticaria, photosensitivity reaction, erythema, acne, hyperhidrosis, blister, nail disorder, joint swelling, arthralgia, bone pain, myalgia, musculoskeletal pain, muscular weakness, muscle spasms, pain in extremity, renal failure, cystitis noninfective, micturition disorder, haema turia, leukocyturia, menstrual disorder, general physical health deterioration, pain, feeling of body temperature change, xerosis, discomfort, blood creatinine increased, electrocardiogram QT prolonged, international normalised ratio increased, activated partial thromboplastin time prolonged, blood urea increased, blood lactate dehydrogenase increased, protein total decreased, C-reactive protein increased, haematocrit decreased. Post-marketing experience: interstitial lung disease. OVERDOSE* PROPERTIES*: Trifluridine is an antineoplastic thymidine-based nucleoside analogue and tipiracil hydrochloride is a thymidine phosphorylase (TPase) inhibitor. Following uptake into cancer cells, trifluridine, is phosphorylated by thymidine

hence the
of the

Authorisation Holder LES LABORATOIRES
tion:
An Overview of Asthma Care with Professor Stephen Lane
where compliance and adherence to medication is one of the biggest problems we face. This is because asthmatics do not use their inhalers once they are feeling well; this is the most common reason for asthma to be uncontrolled.

maximal inhaled treatment and whose asthma is associated with high levels of eosinophils on their blood count. Most asthmatics will not need this treatment but for those who do, it is very effective.
Ireland has one of the highest rates of asthma prevalence in the world. It is estimated that approximately 500,000 people in Ireland have a degree of asthma, though usually mild. It is the most common chronic respiratory disease in adults.
Asthma may affect a person all their lives, but it can be managed so effectively that a person has a high quality of life and health. However, many people with asthma live and work with avoidable asthma illness, due to having uncontrolled disease.
This is despite the availability of excellent national and international evidenced-based guidelines and effective, accessible and safe medicines.
We recently spoke to Professor Stephen Lane to understand more about the future of this condition within Ireland.
Professor Lane’s main interest is asthma, related allergies, and COPD.
Professor Lane is Consultant Respiratory Physician at Tallaght University Hospital and Peamount Healthcare and Professor of Respiratory Medicine at Trinity College Dublin. He is Chair of the Clinical Advisory Group of the National Clinical Program for Respiratory Medicine.

Digitalisation of Respiratory Care
Over the past few decades, technology and improvements in artificial intelligence have
dramatically changed major sectors of our day-to-day lives, including the field of healthcare. E-health includes a wide range of subdomains, such as wearables, smart-inhalers, portable electronic spirometers, digital stethoscopes, and clinical decision support systems. E-health has been consistently shown to enhance the quality of care, improve adherence to therapy, and allow early detection of worsening in chronic pulmonary diseases.
Professor Lane states, “This is a relatively new field. There are currently a number of devices that can be prescribed to improve adherence and allow doctors to remotely control patients’ compliance particularly in asthma
“Within the field of digitalisation, there is certainly a way forward whereby you might have an inhaler that records each dose that might be connected to the patient’s phone via Bluetooth technology and the patients can then interrogate that or send it to their doctor. Whilst I do see a role for it, it hasn’t taken off as a standard of care yet but anything that improves adherence to treatment when well is to be welcomed.
“This is, however, a clear opportunity for the future albeit with some challenges. Patients will need to be able to use Bluetooth, use the peak flow meter and the dose counter. Clearly digitalisation is a way forward both for recording lung function like peakflow and oxygen saturations.
New Developments in Asthma Therapy
Anti-interleukin 5 therapy
“This is a new class of treatment that is indicated in patients with severe asthma who are getting frequent exacerbations despite
“These are revolutionary treatments that are licensed and funded. Currently there are 3: Benralizumab, Mepolizumab and Reslizumab. One key thing to note about these drugs is that Benralizumab, and Mepolizumab are available through the PCRS once they have been prescribed by a physician working within the Severe Asthma Network, a part of the National Clinical Programme for Respiratory Medicine.”
Move away from mono reliever therapy with short acting bronchodilators (SABINA Project) to MART
Professor Lane explains the SABa IN Asthma (SABINA) programme aims to describe the magnitude of SABA (blue inhalers) overuse globally and its impact on clinical asthma outcomes and healthcare utilisation. The SABINA project aims to highlight the dangers of SABA therapy alone. Overuse of SABAs is associated with increased mortality from asthma. Maintenance and Reliever Therapy (MART) with the combination of formoterol and inhaled

















corticosteroids (ICS) has an established scientific rationale and demonstrated to reduce asthma exacerbations. Reliever treatment is moving toward combining the long acting bronchodilator (LABA) formoteral with an inhaled corticosteroid (ICS) for reliever use in asthma. Formoterol is available in the following combination inhalers e.g Symbicort, Flutiform, Bufomix, DuoResp etc. These treatment treat underlying asthma as well as relieving symptoms. Formoterol is a very safe LABA when used with ICS. LABAs are not to be use alone in asthma, although they have a role in COPD.
He adds, “Pharmacists should always be on the look-out for patients who are using LABAs as a treatment on their own. These type of drugs on their own in an asthmatic context are dangerous.”
“Grass pollen SLIT with Grazax or Oralair, grass pollen molecules, is indicated for the treatment of
refractory, seasonal rhinitis which is known to everybody as summer hay fever in May, June and July. We usually start treatment in the autumn, out of season ad continue it for 3 years. It can treat hay fever, can get rid of it for good, and possibly prevent the development of asthma. It is a very effective treatment which is very under prescribed in Ireland.
“House dust mite SLIT is effective for all year round rhinitis in patients who are allergic to HDM It is also indicated for difficult asthma. Acarizax, a HDM product, is now available in Ireland but not yet on the GMS so prices can vary between pharmacies.
“But I would hope that the pharmacists would see an increase in prescriptions for grass pollen and HDM products going forward. Everybody is looking for the instant fix, but it needs to be explained to patients that these treatments are designed for long-term use.
Triple Therapy for patients with COPD, NOT asthma or asthma COPD overla)
“The two main treatments for COPD are LABAs alone or LAMAs (long acting anti muscarinic agents) alone or usually as a LABA-LAMA combination. But despite the LABA-LAMA treatment, patients with COPD continue to have day-to-day symptoms and frequent exacerbations even though they are doing all the right things such as stopping smoking. The next level is to add in an ICS. In selected patients adding an ICS to LAMA and LABA i.e. triple therapy further improves systems, further reduce exacerbations, but the most important thing it can do is reduce mortality. When triple therapy comes in the one device this can improve patient adherence (Trelegy), however, many patients prefer twice daily dosing (Trixeo).
“In my view, I would put everybody eligible patient with COPD on
triple treatment, as there are studies showing monotherapy with a LAMA is not as good as dual therapy with LABA-LAMA which in turn is not as good asas good as triple therapy.”
What do pharmacists need to know about these?
Be aware of move from SABAs to MART therapy
Monitor how many SABA cannisters a patient with asthma is collecting. More than 3 year indicates poorly controlled asthma
Be aware of new triple therapies in COPD
Be aware of increasing use of SLIT for rhinitis and asthma
Be aware of new monoclonal anti=IL5 treatments for asthma which are now given in the community via the High Tech Scheme
Tallaght University Hospital presents Hero Awards
Tallaght University Hospital (TUH) has just announced the winners of its fifth annual Hero Awards, which recognise staff whose work has made an incredible difference to patients and their families, as well as their colleagues.
Announcing the awards, Chief Executive of TUH, Lucy Nugent said: “Tallaght University Hospital is nothing without the people who work here, which is why these awards are so important. I am very proud of our staff and their commitment and true heartfelt desire to make things better for our patients. These awards give patients, families and colleagues the chance to say thank you and to recognise the excellence demonstrated by the staff here at TUH, something I know will remain the case, as the hospital continues to expand its services.”
Winner of the Patient Experience Award - Nurse Vangie Heroso, Post Anaesthesia Care Unit
A family member spoke about how the winner of this year’s Patient Experience Award, Staff Nurse Heroso cared for her brother during his final days in the Post Anaesthesia Care Unit at TUH.
Praising her invaluable support at a very difficult time this family member described Vangie as “A real earth angel who should be recognised for her hard work and kind heart for the love & dignity she showed me and especially my brother during his last days. There was no other family there just me and my brother, so she was a great comfort, and she will always be a part of the memories I have of my last moments with my beautiful big brother.”
Winners of the People Caring for People Award – Alison Baker Kerrigan, Arts Officer and Clara Monahan, Music Therapist in the Arts & Health Department
Winner of the Unsung Hero Award – Alison Loughnane Health Care Assistant, William Stokes Unit
The winner of the Service Excellence Award – Professor Tara Coughlan, Consultant Geriatrician
In a tribute, one colleague described the winner of the Service Excellence Award in the following way: “Professor Coughlan has worked tirelessly for the older person in TUH for most of her career. She is exemplary
as a clinician and stands very strongly amongst her peers. She is an inspirational person and clinician and it is a great privilege to work alongside her. She never asks anyone to do a job that she cannot complete herself and inspires those around her to be the best that they can be. She respects colleagues from all disciplines and works in a truly multidisciplinary way.”
Winner of the Mentoring Award – Dr Ann Leonard, Quality Innovation Manager
After nominating Dr Leonard as the winner of this year’s Mentoring Award one colleague remarked “Ann is innovative, and inclusive and gets individuals involved with interesting projects. She enables these individuals to develop skills outside of their department by getting them engaged in these side projects and is both empowering and motivating.”
Winner of the Teamwork Award – Ann Dwyer, Clinical Nurse Manager, Franks Ward
In a tribute to Ann Dwyer who won the teamwork award, one colleague said “Ann is a great team player, she is selfless, sees the big picture, has initiative and finds solutions. She always
has a positive attitude to her job and always has the patient's best interest at the centre of her management of Franks Ward. Her positivity in the face of challenges is inspiring and uplifting. She has a powerful can-do approach to every difficulty and never complains.”
Winner of Team of the Year –Patient Food Services Team
CEO Awards
There were two further awards nominated by Chief Executive Lucy Nugent and the management team to recognise individuals who go above and beyond, often behind the scenes, which may not be evident to all staff.
The first recipient of this award was Dr Maria Donnelly, Consultant Intensivist. In her tribute, Ms Nugent said, “Dr Donnelly is receiving this award in recognition of her many years of dedicated service and more recently the leadership she has demonstrated in being the clinical lead for the ICU expansion. I have no doubt that her attention to detail and tenacity has ensured the development of this new world-class unit for our patients and staff.”
The second recipient was Mr Broc Delaney, Porter.
Fresenius Kabi Oncology

Trust, connection and commitment

The best plans can be created when both partners collaborate to combine insights, expertise and perspectives. We are dedicated to working in partnership with you to choreograph effective and comprehensive support to meet the needs of your oncology patients.
Fresenius Kabi Limited
Fresenius Kabi Ireland, Unit 3B Fingal Bay, Balbriggan, Co. Dublin, Ireland
T: +353 (0)1 8413030
F: +353 (0)1 8496949
www.fresenius-kabi.com/ie
Management of Hidradenitis Suppurativa
Written by Professor Anne Marie Tobin, Consultant Dermatologist Tallaght University Hospital

Clinical Associate Professor Trinity College Dublin
Clinical Lead in Dermatology Health Service Executive
1. Typical lesions: Primary lesions - painful deep-seated nodules (blind boils) Secondary lesions - abscesses, draining sinuses, bridged scars, “tombstone comedones”
2. Typical localization: Axillae and groin, genitals Under breasts, on buttocks and perineum
Pathogenesis
Management of Hidradenitis Suppurativa
HS is three times more common in women and generally starts around puberty and ameliorates around menopause. One third of patients have a family history of the condition or a family history of pilonidal sinus.
Clinical presentation
3. Chronicity and recurrences: Chronic recurrent lesions for more than six months
Hidradenitis suppurativa (HS), previously known as acne inversa is a chronic inflammatory skin disease that affects approximately 1% of the Irish Population. The condition causes boils, abscesses and scarring in the axillae, groin and inframammary areas of patients. HS is chronic, recurrent and debilitating and tends to be progressive. The disease causes significant pain and distress and has a huge impact on patients’ lives affecting their ability to work and intimate relationships.
Hidradenitis suppurativa (HS), previously known as acne inversa is a chronic inflammatory skin disease that affects approximately 1% of the Irish Population. The condition causes boils, abscesses and scarring in the axillae, groin and inframammary areas of patients. HS is chronic, recurrent and debilitating and tends to be progressive. The disease causes significant pain and distress and has a huge impact on patients’ lives affecting their ability to work and intimate relationships.
Epidemiology of Hidradenitis Suppurativa:
Epidemiology of Hidradenitis Suppurativa:
Hidradenitis suppurativa (HS), previously known as acne inversa is a chronic inflammatory skin disease that affects approximately 1% of the Irish Population. The condition causes boils, abscesses and scarring in the axillae, groin and inframammary areas of patients. HS is chronic, recurrent and debilitating and tends to be progressive. The disease causes significant pain and distress and has a huge impact on patients’ lives affecting their ability to work and intimate relationships.
Despite this, diagnosis of HS may be delayed as the chronicity of lesions may not be appreciated and patients may present in different clinical settings, primary care, emergency departments, gynaecology and surgery. It has been estimated that patients may wait eight years for diagnosis, see five different specialities over on average 17 consultations. This delay in diagnosis leads to irreversible changes of scar, sinus tracts and fistula as the disease progresses, leading to scarring and disability.
HS may affect between 1−4% of the population, and a study from Irish dermatology departments indicated a prevalence of 1.4% amongst the patient population attending dermatology clinics1. HS is three times more common in women and generally starts around puberty and ameliorates around menopause. One third of patients have a family history of the condition or a family history of pilonidal sinus.
HS may affect between 1−4% of the population, and a study from Irish dermatology departments indicated a prevalence of 1.4% amongst the patient population attending dermatology clinics1. HS is three times more common in women and generally starts around puberty and ameliorates around menopause. One third of patients have a family history of the condition or a family history of pilonidal sinus.
Epidemiology of Hidradenitis Suppurativa:
Clinical presentation.
Clinical presentation.
HS may affect between 1-4% of the population, and a study from Irish dermatology departments indicated a prevalence of 1.4% amongst the patient population attending dermatology clinics1.
HS begins as small pustules and boils under the arms and in the groin or buttock area, they may be predated by blackheads which are ope comedones, the presence of ‘double comedones’, two blackheads beside each other, is pathognomic of the condition. Because the disease begins in puberty it is often not recognized at its beginning. Pustules enlarge to become abscesses which are extremely painful and eventually discharge a mixture of pus and blood which is foul-smelling and the pain gradually resolves. As the disease progresses abscesses join up to form sinus tracts and eventually marked scarring. Thus patients suffer both pain and the embarrassment of foul-smelling discharge
Patients with HS may also have other cutaneous disorders such as severe acne and a pilonidal sinus where the spectrum is known as follicular triad, when patients have HS, severe acne, pilonidal sinus and folliculitis de calvens it is known as follicular tetrad.

Historically HS was thought to be a disease of apocrine glands it is now recognized as a follicular disorder. It is thought that the initial occlusion is caused by hyperkeratinisation of the pilosebaceous unit leading to occlusion of the follicle with swelling leading to rupture of the follicle and discharge of contents into the dermis (Fig 2A). There may also be abnormalities in the skin’s innate immune system as recent work has shown altered copy numbers of β-defensin, an antimicrobial peptide, was associated with HS. Keratinocytes from the follicles of patients with HS also display differing expression of antimicrobial peptides compared to controls. These follicular contents trigger an immune response leading to redness and inflammation.
unit leading to occlusion of the follicle with swelling leading to rupture of the follicle and discharge contents into the dermis (Fig 2A) There may also be abnormalities in the skin’s innate immune as recent work has shown altered copy numbers of β defensin, an antimicrobial peptide, associated with HS. Keratinocytes from the follicles of patients with HS also display expression of antimicrobial peptides compared to controls. These follicular contents immune response leading to redness and inflammation
HS begins as small pustules and boils under the arms and in the groin or buttock area, they may be predated by blackheads which are ope comedones, the presence of ‘double comedones’, two blackheads beside each other, is pathognomic of the condition. Because the disease begins in puberty it is often not recognized at its beginning. Pustules enlarge to become abscesses which are extremely painful and eventually discharge a mixture of pus and blood which is foul smelling and the pain gradually resolves. As the disease progresses abscesses join up to form sinus tracts and eventually marked scarring. Thus patients suffer both pain and the embarrassment of foul smelling discharge
Fig 1: Nodule, abscess, sinus tract and double comedone of HS
The most commonly areas affected are the axillae, inguinal creases, buttocks, natal cleft and under the breasts or abdominal pannus, any site where skin touches off itself or at sites of friction. The diagnosis of HS is a clinical one and is the presence of:
HS begins as small pustules and boils under the arms and in the groin or buttock area, they may be predated by blackheads which are ope comedones, the presence of ‘double comedones’, two blackheads beside each other, is pathognomic of the condition. Because the disease begins in puberty it is often not recognized at its beginning. Pustules enlarge to become abscesses which are extremely painful and eventually discharge a mixture of pus and blood which is foul smelling and the pain gradually resolves. As the disease progresses abscesses join up to form sinus tracts and eventually marked scarring. Thus patients suffer both pain and the embarrassment of foul smelling discharge


Severity of HS is most commonly graded using the Hurley Staging Stage 1 (Mild cases): Single or multiple abscesses • Painful bumps, pustules and abscesses in the armpits, groin, under the breasts, in between the buttocks or inner thigh; • These bumps generally start as firm, pea-sized nodules.

Dysregulation of the innate and adaptive immune system has also been demonstrated based on clinical association with other immune-mediated disorders, its response to biologic therapy in the clinical arena, and from molecular research. Anti-TNF therapy is proving effective in HS and increased lesional expression of IL-17 and IL-23 suggest other pathways and cells may be involved it is clear that HS has a much more heterogeneous immune signature that involves B cells.
Dysregulation of the innate and adaptive immune system has also been demonstrated clinical association with other immune mediated disorders, its response to biologic therapy clinical arena, and from molecular research. Anti TNF therapy is proving effective in HS and lesional expression of IL 17 and IL 23 suggest other pathways and cells may be involved it is HS has a much more heterogeneous immune signature that involves B cells
When two adjacent follicles rupture a sinus tract/tunnel under the skin (Fig 2B).
Stage 2 (Moderate cases): Recurrent abscesses, sinus tract formation • Recurring spots, pustules and abscesses in multiple areas with scarring and skin tunnelling • Scarring can occur as a result of long-term or repeat occurrences in a single location
Stage 3 (Severe cases): Widespread involvement of area, with multiple interconnected tracts and abscesses • Widespread spots, pustules and abscesses with multiple interconnected skin holes and tunnels (sinus tracts) • Abscesses can be painful and develop into deep sinus tracts under the skin. Areas of skin involved may split and produce an unpleasant odour.
Fig 1: Nodule, abscess, sinus tract and double comedone of HS
Fig 1: Nodule, abscess, sinus tract and double comedone of HS
The most commonly areas affected are the axillae, inguinal creases, buttocks, natal cleft and under the breasts or abdominal pannus, any site where skin touches off itself or at sites of friction. The diagnosis of HS is a clinical one and is the presence of:
The most commonly areas affected are the axillae, inguinal creases, buttocks, natal cleft and under the breasts or abdominal pannus, any site where skin touches off itself or at sites of friction. The diagnosis of HS is a clinical one and is the presence of:
When two adjacent follicles rupture a sinus tract/tunnel under the skin (Fig 2B). Microbial colonisation plays a role and recent work has shown the formation of biofilms in and HS skin that is not clinically involved. Bacterial infection may serve a secondary role pathogenesis of HS by exacerbating local inflammation via a series of pathogen associated molecular pathways As yet there are no definitive bacterial pathogens identified, but treatment is a mainstay of HS treatment.
Microbial colonisation plays a role and recent work has shown the formation of biofilms in HS lesions and HS skin that is not clinically involved. Bacterial infection may serve a secondary


TIME TO PRESS PLAY
Each pre-filled syringe contains 150 mg of tralokinumab in 1 mL
(150 mg/mL). Dosage and administration:
The recommended dose of tralokinumab is an initial dose of 600 mg (four 150 mg injections) followed by 300 mg (two 150 mg injections) administered every other week as subcutaneous injection. Every fourth week dosing may be considered for patients who achieve clear or almost clear skin after 16 weeks of treatment. Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve further with continued treatment every other week beyond 16 weeks. Tralokinumab can be used with or without topical corticosteroids. The use of topical corticosteroids, when appropriate, may provide an additional effect to the overall efficacy of tralokinumab. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas. If a dose is missed, the dose should be administered as soon as possible and then dosing should be resumed at the regular scheduled time. No dose adjustment is recommended for elderly patients, patients with renal impairment or patients with hepatic impairment. For patients with high body weight (>100 kg), who achieve clear or almost clear skin after 16 weeks of treatment, reducing the dosage to every fourth week might not be appropriate. The safety and efficacy of tralokinumab in children below the age of 18 years have not yet been established. Method of administration: Subcutaneous use. The pre-filled syringe should be not shaken. After removing the pre-filled syringes from the refrigerator, they should be allowed to reach room temperature by waiting for 30 minutes before injecting. Tralokinumab is administered by subcutaneous injection into the thigh or abdomen, except the 5 cm around the navel. If somebody else administers the injection, the upper arm can also be used. For the initial 600 mg dose, four 150 mg tralokinumab injections should be administered consecutively in different injection sites. It is recommended to rotate the



injection site with each dose. Tralokinumab should not be injected into skin that is tender, damaged or has bruises or scars. A patient may self-inject tralokinumab or the patient’s caregiver may administer tralokinumab if their healthcare professional determines that this is appropriate. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: If a systemic hypersensitivity reaction (immediate or delayed) occurs, administration of tralokinumab should be discontinued and appropriate therapy initiated. Patients treated with tralokinumab who develop conjunctivitis that does not resolve following standard treatment should undergo ophthalmological examination. Patients with pre-existing helminth infections should be treated before initiating treatment with tralokinumab. If patients become infected while receiving tralokinumab and do not respond to antihelminth treatment, treatment with tralokinumab should be discontinued until infection resolves. Live and live attenuated vaccines should not be given concurrently with tralokinumab. Fertility, pregnancy and lactation: There is limited data from the use of tralokinumab in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. As a precautionary measure, it is preferable to avoid the use of tralokinumab during pregnancy. It is unknown whether tralokinumab is excreted in human milk or absorbed systemically after ingestion. Animal studies did not show any effects on male and female reproductive organs and on sperm count, motility and morphology. Side effects: Very common (≥1/10): Upper respiratory tract infections. Common (≥1/100 to <1/10): conjunctivitis, conjunctivitis allergic, eosinophilia, injection site reaction. Uncommon (≥1/1,000 to <1/100): keratitis. Precautions for storage: Store in a refrigerator (2°C-8°C). Do not freeze. Store in the original package in order to protect from light. Legal category: POM. Marketing authorisation number and holder: EU/1/21/1554/002. LEO Pharma A/S, Ballerup, Denmark. Last revised: June 2021 Reference number: REF-19238(1)
Reporting of Suspected Adverse Reactions
Adverse events should be reported.
Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie
Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com

role in the pathogenesis of HS by exacerbating local inflammation via a series of pathogen associated molecular pathways. As yet there are no definitive bacterial pathogens identified, but antibiotic treatment is a mainstay of HS treatment.
Risk Factors for developing HS Smoking
Smoking has been associated with a 5 to 12 fold increased risk of HS. In 2011, the specific term “smoker’s boils “for HS lesions in patients who are smokers . This stemmed from a previous randomized, matched-pair control group study showing that the percentage of active cigarette smokers among HS patients was 88.9%, with an odds ratio of 9.4 and the percentage of smokers in the matched-pair control group was 46%.
Nicotine causes release of tumour necrosis factor alpha causing inflammation, promotion of follicular occlusion and epidermal hyperplasia and contains polyaromatic carbohydrates, that may activate keratinocytes and cells of the immune system.
Obesity
Several studies have confirmed that patients with HS have a higher Body Mass Index (BMI) compared to the general population and this ranges from 12% to 88%, depending on the type of population. In a large retrospective, matched case-control study the association between obesity and HS was high with 17.3-fold higher odds of being obese when also afflicted with HS. Overweight individuals tend to have larger and increased skin folds, leading to subsequent increased mechanical friction and maceration of the overlying skin. In addition, a warm, humid and occlusive environment in these areas favours microbial growth and colonization. In addition obesity is considered to be a state of systemic, low-grade inflammation which contributes to the inflammation in the skin.
Family history
Approximately a third of patients have a family history of the disease and early onset HS is associated with a stronger genetic predisposition ( 55% vs 34 %) and more widespread disease. . This pattern probably represents autosomal dominant inheritance with incomplete penetrance. No definitive genes have been identified apart from rare mutations in gamma secretase gene mutation which leads to a particularly severe clinical presentation.
Hormonal influences
Given that HS is three times more common in women and that
some women report premenstrual flare HS can resolve after the menopause, hormonal influences play a role. This has yet to be fully elucidated and hormonal treatments such as anti-androgens have not proved particularly useful. High rates of Polycystic Ovarian Syndrome among patients with HS also corroborate the likelihood that hormones play a role in pathogenesis.
Co-morbidity in Hidradenitis suppurativa
In a similar paradigm to psoriasis, it is now apparent that HS carries a substantial comorbidity burden, these include obesity, metabolic and cardiovascular disease, inflammatory bowel disease, inflammatory joint dIsease and psychological distress.
Obesity, Metabolic Syndrome and Cardiovascular Disease
Unsurprisingly the increased rates of obesity in HS result in increased rates of metabolic syndrome and also the individual components of metabolic syndrome, including dyslipidemia, hypertension and diabetes, this in turn increases cardiovascular risk. One of the first studies to evaluate CVD risk in HS patients using a validated risk assessment tool, the Framingham Risk Score (FRS) was an Irish study by Hughes et al. In this study patients were mainly in their thirties yet showed an increased cardiovascular risk than controls.
Inflammatory Bowel Disease
An association between IBD and HS has been reported as early as in 1991. Of 1,076 patients with HS, 3.3% had IBD, with a prevalence of 2.5% for CD and 0.8% for CD. Using the estimated prevalence of IBD in the general it was indicated that the prevalence of IBD is 4-8 times higher in the included HS cohort than in the general population. HS and CD share several similar clinical
and pathogenic features. Both conditions are chronic diseases of epithelia which are inhabited by commensal flora and both have demonstrated a clinical response to anti-TNF α therapy. They share a number of predisposing factors including genetic predisposition and smoking association
Inflammatory Arthritis
In a French study the estimated prevalence of spondyloarthropathy was 3.7% which far exceeded that of the populationn, 0.3%.
In a retrospective, case-control study of 1,730 HS patients in Massachusetts General Hospital, it was found that 908 patients had spondyloarthritis compared to only 52 in those who did not have HS (52.5% vs. 3.0% p< 0.0001). The pathogenesis underlying the development of SpA in HS patients is unclear. The shared features of dysregulated innate immune response and the role of microbial factors have been hypothesized.
Psychological Distress
HS patients suffer significant pain and foul-smelling suppuration from abscesses or sinuses in affected areas during flares which leads to disruption of activities of daily living as well as work and embarrassment contributing to high levels of psychological distress. Multiple studies have demonstrated high levels of depression and anxiety in patients with HS. These findings support several other studies in the literature proposing a possible link between chronic inflammation and depression.
Treatment of Hidradenitis suppurativa
The publication of the European S1 Guideline for the treatment of hidradenitis suppurativa in 2015 has provided some clarity on how to manage patients at different Hurley Stage (Fig 3). It clearly suggests a triple-track approach to the management of patients of all stages, i.e. a surgical approach, a medical approach and adjuvant management of pain, suppuration
and co-morbidities. The evidence supporting this Guideline was reviewed in an evidence based way and a more complex algorithm generated . This was the first attempt to provide an algorithm for the treatment of patients.
Surgical Treatment
Surgical treatment consists of either lesional treatment: such as de-roofing and carbon dioxide laser ablation, or regional treatment where wide areas of hair-bearing skin are excised and the defect repaired by skin grafting or healing by secondary intention.
Medical treatment
Medical treatments consist of topical clindamycin for mild disease, oral tetracyclines for a prolonged period of up to four months. The combination of rifampicin and clindamycin, both 300mg twice daily for a 10- week period, has proved highly efficacious, though patients must be warned about the risk of developing C. difficile. Adalimumab (Humira®) is recommended as a first-line treatment option in patients with moderate-to-severe HS who were unresponsive or intolerant to oral antibiotics. It should be administered as 160mg at week zero, 80mg at week two and 40mg each week thereafter, starting from week four and has received a licence for the treatment of HS. Second-line agents for severe disease include infiximab 5mg/ kg, and ustekinumab. The area of biological treatment is being intensely investigated, largely using biologics licensed for other indications.
Adjuvant treatment
The third track of treatment includes treating patients’ symptoms of pain, appropriate wound care and identifying and treating superinfections.
Smoking cessation is important, as research has shown that 92 per cent of patients smoke and smoking is associated with less self-reported remission. The severity of HS was associated with pack years in 846 Dutch patients. Encouraging weight loss is also desired, as weight loss of more than 15 per cent is associated with a significant reduction of disease severity. Clearly treating HS patients requires a multidisciplinary approach involving a dermatologist, a surgeon, nursing support, smoking cessation advice and weight loss programmes.
Although there has been considerable advances in the treatment of HS the disease however remains one for which a definitive treatment have been produced.
PRESCRIBING INFORMATION (PI) RINVOQ® (upadacitinib) 15 mg and 30mg prolonged-release tablets. Refer to Summary of Product Characteristics (SmPC) for full prescribing information. PRESENTATION: Each 15 mg prolonged-release tablet contains upadacitinib hemihydrate, equivalent to 15mg of upadacitinib. Each 30mg prolonged-release tablet contains upadacitinib hemihydrate, equivalent to 30 mg of upadacitinib. INDICATION:
Treatment of moderate to severe active rheumatoid arthritis (RA) and active psoriatic arthritis (PsA) in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs). RINVOQ may be used as monotherapy or in combination with methotrexate. Treatment of active ankylosing spondylitis (AS) in adult patients who have responded inadequately to conventional therapy. Treatment of moderate to severe atopic dermatitis (AD) in adults and adolescents 12 years and older who are candidates for systemic therapy. DOSAGE AND ADMINISTRATION: Intended for use under guidance and supervision of a physician experienced in diagnosis and treatment of conditions for which upadacitinib is indicated. Dosage: RA, PsA and AS: The recommended oral dose is 15mg once daily. Consideration should be given to discontinuing treatment in patients with AS who have shown no clinical response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve with continued treatment beyond 16 weeks. AD (adults): The recommended oral dose is 15 mg or 30 mg once daily based on individual patient presentation. 30 mg once daily dose may be appropriate for patients with high disease burden and for patients with an inadequate response to 15 mg once daily. The lowest effective dose for maintenance should be considered. For patients ≥ 65 years of age, the recommended dose is 15mg once daily. AD (adolescents from 12 to 17 years): The recommended oral dose is 15mg once daily for adolescents weighing at least 30 kg. Adults and adolescents 12 years and older: Upadacitinib can be used with or without topical corticosteroids. Topical calcineurin inhibitors may be used for sensitive areas such as the face, neck, and intertriginous and genital areas. Consideration should be given to discontinuing upadacitinib treatment in any patient who shows no evidence of therapeutic benefit after 12 weeks of treatment. Special Populations: Elderly: For AD, doses higher than 15mg once daily not recommended in patients aged 65 years and older. Limited data for patients aged 75 and older. Renal: No dose adjustment required in mild-moderate renal impairment. Upadacitinib 15mg once daily should be used with caution in patients with severe renal impairment. Upadacitinib 30 mg once daily is not recommended for patients with severe renal impairment. Upadacitinib has not been studied in subjects with end stage renal disease. Hepatic impairment: No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. Should not be used in patients with severe (Child Pugh C) hepatic impairment. Paediatric Population: No data available in the following paediatric patient populations: children with AD below the age of 12 years; children and adolescents with RA, PsA and AS aged 0 to less than 18 years. CONTRAINDICATIONS: Hypersensitivity to active substance or excipients. Active tuberculosis (TB) or active serious infections. Severe hepatic impairment. Pregnancy. SPECIAL WARNINGS AND PRECAUTIONS: See SmPC for full details. Immunosuppression: Combination use, with immunosuppressants e.g. azathioprine, ciclosporin, tacrolimus, and biologic DMARDs or other JAK inhibitors has not been evaluated in clinical studies and is not recommended as the risk of additive immunosuppression cannot be excluded. Serious infections: Serious and fatal infections have been reported. Caution when treating patients ≥65 years. Frequently reported – pneumonia and cellulitis. Bacterial meningitis has been reported. Opportunistic infections reported –TB, multidermatomal herpes zoster, oral/oesophageal candidiasis, and cryptococcosis. Do not initiate treatment in patients with active, serious infection, including localised infections. Consider the risk/benefit of treatment in patients with: chronic or recurrent infection, history of serious or opportunistic infection, those exposed to TB, those who have resided or travelled in areas of endemic TB or endemic mycoses, those with underlying conditions that may pre-dispose patients to infection. Closely monitor patients and interrupt treatment if there are signs and symptoms of infection pre and post treatment. Tuberculosis: Pre-screen patients for active or latent TB. RINVOQ should not be given to patients with active TB. Consider anti-TB therapy in patients with previously untreated latent TB or in patients with risk factors for TB infection. Consult with a physician with experience in TB therapy to assess whether initiating anti-TB therapy is appropriate for an individual patient. Monitor patients for the development of signs and symptoms of TB, including patients who tested negative for latent TB infection prior to initiating therapy. Viral reactivation: Cases of herpes virus reactivation have been reported e.g. herpes zoster. If herpes zoster is reactivated, consider interruption of upadacitinib therapy until the episode resolves. Screen for viral hepatitis and monitor regularly for reactivation before starting and during therapy with upadacitinib. Patients, positive for hepatitis C antibody/hepatitis C virus RNA and hepatitis B surface antigen/hepatitis B virus DNA were excluded from clinical studies. If hepatitis B virus DNA is detected while receiving RINVOQ, a liver specialist should be consulted. Vaccination: Use of live, attenuated vaccines during or immediately prior to therapy is not recommended. It is recommended that patients be brought up to date with all immunisations, including

prophylactic



melanoma skin cancer in RA patients. Malignancies were observed in clinical studies. Periodic skin examination recommended for patients who are at increased risk for skin cancer. See SmPC for full details. Haematological abnormalities: Do not start therapy if Absolute Neutrophil Count <1 x 109 cells/L, Absolute Lymphocyte Count <0.5 x 109 cells/L and Hb <8 g/dL. Monitor patients and temporarily stop therapy if abnormal haematological parameters as specified are detected during routine patient management. Diverticulitis: Events of diverticulitis have been reported in clinical trials and post-marketing. Upadacitinib should be used with caution in patients with diverticular disease and especially in patients chronically treated with concomitant medications associated with an increased risk of diverticulitis. Patients presenting with new onset abdominal signs and symptoms should be evaluated promptly for early identification of diverticulitis to prevent gastrointestinal perforation. Cardiovascular Risk: RA patients have an increased risk for cardiovascular disorders. Manage patient’s risk factors for e.g. hypertension, hyperlipidaemia as per usual standard of care. Lipids: Monitor total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) at 12 weeks and thereafter according to international guidelines. Elevated LDL in clinical trials decreased to pre-treatment levels in response to statin therapy, although evidence is limited. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined.
Hepatic transaminase elevations: Monitor liver enzymes at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. In such cases therapy should be interrupted until this diagnosis is excluded.
Venous thromboembolism: Deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving JAKi, including upadacitinib. Use therapy with caution in patients at high risk for DVT/PE. Risk factors should be determined by patients age, obesity and medical history of DVT/PE, patients undergoing major surgery and prolonged immobilisation. If clinical features of DVT/PE occur discontinue therapy, evaluate and treat promptly. INTERACTIONS: Upadacitinib is metabolised mainly by CYP3A4 and plasma exposures may be affected by CYP3A4 inhibitors or inducers. Upadacitinib 15 mg once daily should be used with caution in patients receiving chronic treatment with strong CYP3A4 inhibitors. Upadacitinib 30 mg once daily dose is not recommended for patients receiving chronic treatment with strong CYP3A4 inhibitors. See SmPC for full details. FERTILITY, PREGNANCY AND LACTATION: Upadacitinib is contraindicated during pregnancy. Women of childbearing potential should be advised to use effective contraception during treatment and for 4 weeks following the final dose of upadacitinib. Female paediatric patients and/or their parents/caregivers should be informed about the need to contact the treating physician once the patient experiences menarche while taking upadacitinib. Upadacitinib should not be used during breast-feeding. The effect of upadacitinib on human fertility has not been evaluated. ADVERSE REACTIONS: Refer to SmPC for full details on adverse reactions. Very common adverse reactions (≥1/10): upper respiratory tract infections, acne. Common adverse reactions (≥1/100
Financial Burden of Chronic Health Conditions
Researchers at Trinity, along with colleagues in RCSI University of Medicine and Health Sciences and the Economic and Social Research Institute (ESRI) have found that patients with more than one chronic health condition are under a significantly higher financial burden with healthcare expenses than those without a chronic health condition. Their findings, published in the journal BMJ Open, are particularly pertinent in the current cost-of-living crisis.
Healthcare is a major source of expenditure for patients in Ireland. The majority of people (59%) in Ireland aged 50 years or over have more than one chronic health condition. Chronic health conditions are health issues that can last a long duration, generally progress slowly, and are not passed from person to person. Examples include diabetes, arthritis, or depression. Individuals with more than one health condition are likely to have higher healthcare needs and higher out of pocket payments for healthcare. These high costs can lead to people choosing not to buy their medicines or not attending their healthcare appointments.
The question researchers sought to answer was: How much more do people with multiple health conditions spend on healthcare, compared to those with no conditions?
The research team used data from The Irish Longitudinal Study of Ageing (TILDA) from 2016 to investigate the healthcare expenditure of a nationally representative sample of 5,899 adults aged 50 years or over.
Key findings:
• People with two chronic conditions had an average annual expenditure of ¤806.80, and people with three or more conditions spent an average of ¤885.80. This compared to ¤580.30 for people with no chronic conditions. Therefore, people with two chronic conditions on average spent 39% more on healthcare than people with no chronic conditions, and people with three or more conditions spent on average spent 53% more than people with no chronic conditions. These figures are likely an underestimate as household adaptations and travel costs to access healthcare were not included in the analysis.
• The biggest contributor to increased expenditure was medicines, accounting for approximately half of

expenditure for people with two or more conditions.
• People with more than one condition had lower incomes, which means that some people with multiple conditions are spending a very large proportion of their income on healthcare. For example, we found that almost 1 in 10 people with three or more conditions spent more than 20% of their income on healthcare.
• Having a medical card reduced healthcare expenditure by approximately half.
• Though data was collected in 2016, there have been few changes to the medical card entitlements system in Ireland since then.
James Larkin, PhD Scholar, Department of General Practice, RCSI, and lead author said, “What is particularly concerning is that people with more than one chronic condition, not only have to spend more on healthcare, they also have lower incomes on average, meaning they do not have the ability to pay for some healthcare costs. Increasing the income threshold for the medical card, would likely be beneficial to people with more chronic conditions, especially considering the threshold has changed very little in the last 8 years. Furthermore, we know from previous Irish research that 31% of those entitled to a medical card are not availing of it. So to reduce financial burden, barriers to medical card uptake should be addressed. These barriers include lack of awareness of entitlement, potential stigma, and large administrative burdens.”
New Appointment for Bon Secours

Sharon Morrow, CEO, Bon Secours Dublin
before taking up her most recent appointment as Director of the All-Island CHD Network, a unique cross jurisdictional collaboration between Northern Ireland and the Republic of Ireland.
hospital, which recently announced plans to open a new ¤14 million surgical and oncology day ward that will create 80 new jobs when it opens in June next year.
The appointment of Sharon Morrow as the new CEO of Bon Secours Dublin, one of the leading private hospitals in Ireland, has been announced.
She takes the role in a period of significant expansion for the
Sharon trained as a nurse in the Adelaide Hospital, Dublin and after several clinical positions became Project Manager at Tallaght University Hospital. Subsequently she worked as a Directorate Business Manager at St James’s Hospital and then as National Clinical Care Programme Manager with the HSE, before returning to Tallaght University Hospital as Deputy Chief Executive / Chief Operations Officer. Sharon was Chief Executive Officer at Laura Lynn Children’s Hospice,
Speaking about her appointment, Sharon Morrow said, “I am delighted to be joining Bon Secours Health System Group at a time of great expansion and am excited to work with, and contribute to, the experienced team in Glasnevin to help them continue the exceptional care for which they are known.”
Welcoming the appointment, Bon Secours Health System Group CEO Bill Maher, said: “We are delighted that Sharon has come on board to bring her wealth of experience to our hospital in Glasnevin. She will be a valuable member of the team and I look forward to working with her.”
The new ¤14 million surgical and oncology day ward planned at the hospital is part of a ¤300 million national investment by Bon Secours Health System in its services. Driven by its new strategy, “Resilience, Reliability and Readiness – The 2025 Plan”, this includes new capital projects, state-of-the-art equipment, IT infrastructure, and the creation of 450 new jobs across the country.
Bon Secours opened also new ¤10 million operating theatres in Cork in January, with the hospital group also in the process of developing a new 150-bed medical facility in Limerick City which will greatly expand services currently offered to patients in the mid-west. Bon Secours Tralee also plans to open a new ¤10 million Medical Assessment Unit and surgical day ward in 2024.
treatment of HIV-1
Make Doravirine a Part of Every Day


DELSTRIGO® is indicated for the treatment of adults infected with HIV-1 without past or present evidence of resistance to the NNRTI class, lamivudine, or tenofovir. Delstrigo is also indicated for the treatment of adolescents aged 12 years and older weighing at least 35kg
with
of resistance to the NNRTI

toxicities which preclude the use of

lamivudine, or tenofovir and who have
12


NNRTI class, lamivudine, or tenofovir and who have experienced toxicities which preclude the use of other regimens that do not contain tenofovir disoproxil. DOSAGE AND ADMINISTRATION Therapy should be initiated by a physician experienced in HIV infection management. Pifeltro: One 100 mg tablet once daily. Delstrigo: One 100/300/245 mg tablet once daily. Pifeltro and Delstrigo: If co-administered with rifabutin or other moderate CYP3A inducers, increase doravirine dose to 100 mg twice daily (12 hours apart). Pifeltro: Elderly: No dose adjustment necessary. Renal impairment: No dose adjustment necessary. Hepatic impairment: mild to moderate: no dosage adjustment required; severe: use with caution. Delstrigo: Elderly: Special care advised. Renal impairment: estimated creatinine clearance (CrCl) ≥ 50 mL/min: no dose adjustment necessary; estimated CrCl <50 mL/min: not recommended. Hepatic impairment: mild to moderate: no adjustment required; severe hepatic: use with caution. CONTRAINDICATIONS Hyper sensitivity to the active substance or excipients. Co-administration with strong CYP3A inducers. PRECAUTIONS AND WARNINGS A residual risk of sexual transmission of HIV-1 cannot be excluded and precautions should be taken in accordance with national guidelines. Use with CYP3A inducers may reduce the exposure of doravirine. Autoimmune disorders (such as autoimmune hepatitis) and immune reactivation syndrome have been reported in patients treated with combination antiretroviral therapy which may require investigation and treatment. Contains lactose monohydrate. Delstrigo: Post-treatment exacerbation of HBV (including hepatic decompensation and liver failure) have been reported in patients co-infected with HIV-1 and HBV following discontinuation of lamivudine or tenofovir disproxil. Monitor patients co-infected with HIV-1 and HBV after discontinuation of Delstrigo and if appropriate initiate anti-HBV therapy. Renal impairment, including acute renal failure and Fanconi syndrome have been reported with tenofovir disoproxil. Assess estimated CrCl prior to initiation and during therapy. In patients at risk of renal dysfunction, serum phosphorus, urine glu cose, and urine protein should also be assessed. Discontinue therapy if estimated CrCl declines below 50 mL/min. Avoid with concurrent or recent use of nephrotoxic medicinal products (e.g. high-dose or multiple NSAIDs). Evaluation of renal function is recommended for persistent or worsening bone pain, pain in extremities, fractures, and/or muscular pain or weakness. Assessment of bone mineral density should be considered for patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss. Hypophosphatemia and osteomalacia secondary to proximal renal tubulopathy should be considered in patients at risk of renal dysfunction who present with persistent or worsening bone or muscle symptoms. Doravirine/lamivudine/tenofovir disoproxil must not be co-administered with other medicinal products containing lamivudine, tenofovir disoproxil, or tenofovir alafenamide or with adefovir dipivoxil. Doravirine/lamivudine/tenofovir disoproxil should not be administered with doravirine unless needed for dose adjustment (e.g. co-administered with rifabutin). Drug interactions: Refer to SmPC for full information on drug interactions.
Pifeltro and Delstrigo: Doravirine is metabolized primarily by CYP3A. Do not co-administer with strong CYP3A enzyme inducers. If co-administra tion with rifabutin or other moderate CYP3A inducers cannot be avoided, increase doravirine dose to 100 mg twice daily (taken 12 hours apart). Use with caution when co-administering doravirine with medicinal products that are sensitive CYP3A substrates that have a narrow therapeutic window (e.g., midazolam, tacrolimus and sirolimus). Delstrigo: Do not administer with other antiretroviral medicinal products. Co-administration of doravir ine/lamivudine/tenofovir disoproxil with medicinal products that reduce renal function or compete for active tubular secretion may increase serum concentrations of lamivudine. Co-administration of doravirine/lamivudine/tenofovir disoproxil with medicinal products that reduce renal function or compete for active tubular secretion via OAT1, OAT3 or MRP4 may increase serum concentrations of tenofovir. Avoid with concurrent or recent use of nephrotoxic medicinal products. Pregnancy and Lactation: Pifeltro and Delstrigo: Avoid use during pregnancy. An Antiretroviral Pregnancy Registry has been established. Breastfeeding is not recommended. SIDE EFFECTS Refer to SmPC for complete information on side-effects. Pifeltro and Delstrigo: Common: abnormal dreams, insomnia, headache, dizziness, somnolence, nausea, diarrhoea, abdominal pain, flatulence, vomiting, rash, fatigue alanine aminotransferase increased. Uncommon: hypophosphataemia, nightmare, depression, suicidal ideation, paraes thesia, asthenia. Rare: hypomagnesaemia, blood creatine phosphokinase increased. Delstrigo: Common: cough, nasal symptoms, alopecia, muscle disorders, fever. Uncommon: neutropenia, anaemia, thrombocytopenia, pancreatitis, rhabdomyolysis, proximal renal tubulopathy (including Fanconi syndrome), aspartate aminotransferase increased. Rare: lactic acidosis, hepatitis, angioedema, myopathy, acute renal failure, renal failure, acute tubular necrosis, nephritis (including acute interstitial) and nephrogenic diabetes insipidus. Very Rare: pure red cell aplasia, peripheral neu ropathy (or paraesthesia). Lactic acidosis Cases of lactic acidosis have been reported with tenofovir disoproxil alone or in combination with other antiretrovirals. Patients with predisposing factors such as patients with decompensated liver disease, or patients receiving concomitant medications known to induce lactic acidosis are at increased risk of experiencing severe lactic acidosis during tenofovir disoproxil treatment, including fatal outcomes. PACKAGE QUANTITIES Bottle of 30 tablets Legal Category: POM Marketing Authorisation numbers: Pifeltro: EU/1/18/1332/001 Delstrigo: EU/1/18/1333/001 Marketing Authorisation Holder: Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands Date of

from: MSD,
2022
2022
NJ, USA and

information is available on
D18 X5K7 or from www.medicines.ie.



Ireland’s Increased Support to Global Fund
Minister for Foreign Affairs Simon Coveney T.D. announced in New York recently that Ireland will increase its contribution to the Global Fund to fight AIDS, TB and Malaria by 30% for the period 2023-2025, reaffirming Ireland's commitment to ending the three diseases by 2030.
One of the founding members of the Global Fund since its inception in 2002, Ireland has contributed over ¤273 million in funding to-date. This new funding commitment of ¤65 million amounts to a 30% increase in funding from Ireland to help combat AIDS, TB and Malaria.
Speaking at the Global Fund's
Seventh Replenishment Conference in New York today, Minister Coveney said, "As a founding member of the Global Fund, I am proud of Ireland's long-term partnership. We have seen first-hand the life-changing work of the organisation. 50 million lives saved since 2002 - that is ten times the population of Ireland."
"Ireland will play its part in the global efforts to do what is needed to end HIV and AIDs, to end TB, and to end malaria. I am delighted to confirm that the Government of Ireland will increase its contribution to the Global Fund by 30%. We will play our part in the effort to save 20 million more lives."
Speaking on the announcement of the increase, Minister of State for Overseas Development Aid and Diaspora, Colm Brophy T.D., said, "We must continue to work together to regain ground lost against HIV, TB and malaria during the COVID-19 pandemic. It is critical to get back on track in achieving the UN's Sustainable Development Goal targets by 2030. The Global Fund is Ireland's largest global health partner. The results it has achieved over the last 20 years have been remarkable. By increasing our funding by 30%, we are signalling Ireland's commitment to ending AIDS, TB and malaria."
Cases of HIV Continue to Increase
Stephen O' Hare, Executive Director of HIV IrelandThe Global Fund to fight AIDS, Tuberculosis and Malaria (Global Fund), founded in 2002, is a leading international funding body for these three diseases.
As Ireland's largest partner in global health, the Global Fund has achieved remarkable results over the last 20 years in the fight against HIV, TB and malaria and has consistently represented a strong investment for Ireland. Health programmes supported by the Global Fund partnership have saved at least 50 million lives to date.
Ireland has contributed ¤50 million to the Global Fund for the period 2020-2022.
Notified cases of HIV continue to increase in Ireland, the most recent figures from the Health Protection Surveillance Centre show.
The summary of HIV infections reported for week 39 of 2022 show 652 cases of HIV, up from
268 cases in the same period in 2021. Charities across Ireland have recently described these increases as worrying.
Stephen O' Hare, Executive Director of HIV Ireland, said, “Our concern is that what we are seeing this year is a very rapid development in terms of the number of cases being presented.

“And that can be explained somewhat by the opening up of social venues and somewhat by the re-emerging of testing facilities, but it is a worry still we're seeing so many cases at this time of the year.”
Mr O'Hare is also calling for the medication PrEP (pre-exposure prophylaxis) to be made more widely available saying research published in 2022 by HIV Ireland points to ongoing difficulties in accessing it.
“PrEP is highly effective at preventing a person who is HIV negative from acquiring HIV through sexual intercourse and is a vital component in our strategy to reduce HIV transmissions,” Mr O'Hare said.
“Delays in access to services is detrimental to communities and will further reduce Ireland's ability to meets its ambitious target of ending new HIV transmissions by 2030.”
It is now 40 years since the first case of AIDS was diagnosed in Ireland.




Footnotes:
*BIKTARVY® was assessed in four Phase 3, randomised clinical trials: two double-blind trials in treatment-naive adults through to 144 weeks (Study 1489 [BIKTARVY® vs ABC/3TC/DTG, n=629] and Study 1490 [BIKTARVY® vs DTG + FTC/ TAF, n=645]) and two in virologically suppressed adults through to 48 weeks (Study 1844 [double-blind trial, switching from DTG + ABC/3TC or ABC/3TC/DTG to BIKTARVY®, n=563] and Study 1878 [openlabel trial, switching from ABC/3TC or FTC/TDF plus boosted ATV or DRV to BIKTARVY®, n=577]).1 The primary endpoint of Studies 1489 and 1490 was HIV-1 RNA <50 copies/mL at Week 48, as defined by the US FDA snapshot algorithm, with a prespecified non-inferiority margin of –12%.3,4 The primary endpoint of Studies 1844 and 1878 was HIV-1 RNA ≥50 copies/mL at Week 48, as defined by the US FDA snapshot algorithm, with a prespecified noninferiority margin of 4%.5,6 Efficacy defined as viral load <50 copies/mL.1
**Defined as maintained efficacy, which is dependent on patient adherence. Adherence is impacted by tolerability and simplicity of treatment.8–11
†At Week 144, in Study 1489 (BIKTARVY® [n=314] vs ABC/3TC/DTG [n=315]) efficacy was 82% vs 84% (95% CI: –2.6 [–8.5– 3.4]) and in Study 1490 (BIKTARVY® [n=320] vs DTG + FTC/TAF [n=325]) efficacy was 81% vs 84% (95% CI: –1.9 [–7.8–3.9]), with BIKTARVY® demonstrating noninferior efficacy vs comparator in both trials. 2 At Week 48, in Study 1844 (BIKTARVY® [n=282] vs ABC/3TC/DTG [n=281]) efficacy was 94% vs 95% (95% CI: –1.4 [–5.5–2.6]) and in Study 1878 (BIKTARVY® [n=290] vs ABC/3TC or FTC/TDF plus boosted ATV or DRV [n=287]) efficacy was 92% vs 89% (95% CI: 3.2 [–1.6–8.2]).1
‡At Week 144, in pooled treatment-naïve patient data from Study 1489 (n=629) and Study 1490 (n=645), and Week 48 in Study 1844 (n=563), there were 0 cases of treatment emergent resistance to study regimens [BIKTARVY® (n=0/834), ABC/3TC/DTG (n=0/315) and DTG + FTC/ TAF (n=0/325)].2,5 At Week 24, in Study 1878 (n=577) one participant in the ABC/3TC plus boosted DRV group developed treatment-emergent resistance; no participants in the BIKTARVY® group developed treatmentemergent resistance through Week 48.6
§ At Week 144, in patients receiving BIKTARVY®, the most frequently reported study-drug-related adverse reactions (≥5%) in Study 1489 were diarrhoea 6% (n=19/314), nausea 6% (n=18/314) and headache 5% (n=16/314); in Study 1490 they were headache 4% (n=14/320), diarrhoea 3% (n=10/320) and nausea 3% (n=10/320).2
ll 44-week data on AEs leading to study drug discontinuation % (n). Study 1489 (vs ABC/3TC/DTG): 0% (n=314) vs 2% (n=5 /315); Study 1490 (vs FTC/TAF + DTG): 2% (n=6/320) vs 2% (n=6/325).2 48-week data on AEs leading to study drug discontinuation % (n). No discontinuations due to weight gain in Study 1489 or Study 1490. Study 1844 (vs ABC/3TC/DTG): 2% (n=6/282) vs 1% (n=2/281); Study 1878 (vs boosted DRV or ATV + 2 NRTIs): 1% (n=2/290) vs <1% (n=1/287).5,6
¶Small STR with flexible daily dosing. Each BIKTARVY® tablet is approximately 15 mm x 8 mm.1
References:
1. BIKTARVY® (BIC/FTC/TAF) Summary of Product Characteristics.
2. Orkin C, et al. Lancet HIV. 2020; 7: e389 e400.
3. Sax PE, et al. Lancet. 2017; 390: 2073–2082.
4. Gallant J, et al. Lancet. 2017; 390: 2063–2072.
5. Molina JM, et al. Lancet HIV. 2018; 5: e357–e365.
6. Daar ES, et al. Lancet HIV. 2018; 5: e347–e356.
7. Wohl D. et al. Patient. 2018; 11: 561–573.
8. US Department of Health and Human Sciences (DHHS). Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. June 2021. Available from: https://clinicalinfo.hiv. gov/sites/default/ files/guidelines/documents/ AdultandAdolescentGL.pdf (Accessed June 2021)
9. Cihlar T and Fordyce M. Curr Opin Virol. 2016; 18: 50–56.
10. Trottier B, et al. J Int AIDS Soc. 2014; 17(4 Suppl 3): 19765.
11. Orkin C, et al. HIV Med. 2018; 19: 18–32.
Abbreviations:
3TC, lamivudine
ABC, abacavir
AE, adverse event
ATV, atazanavir
BIC, bictegravir
CI, confidence interval
DRV, darunavir
DTG, dolutegravir
FDA, Food and Drug Administration
FTC, emtricitabine
PLWH, people living with HIV RNA, ribonucleic acid
STR, single-tablet regimen
TAF, tenofovir-alafenamide fumarate
TFV, tenofovir
TDF, tenofovir disoproxil fumarate
US, United States
Prescribing Information:
Consult the Summary of Product Characteristics (SmPC) before prescribing. BIKTARVY® bictegravir 50mg/emtricitabine 200mg/ tenofovir alafenamide 25mg film-coated tablets.
INDICATION: Treatment of adults with HIV-1 infection without present or past evidence of viral resistance to the integrase inhibitor class, emtricitabine or tenofovir DOSAGE: Adults: One tablet, once daily, taken orally and whole with/without food. Elderly: No dose adjustment is required in patients ≥ 65 years. Renal impairment: Patients with estimated creatinine clearance (CrCl) ≥ 30 mL/min: No dose adjustment required. Patients with CrCl ≥ 15 mL/min and < 30 mL/min or Patients with end stage renal disease (ESRD, CrCl < 15 mL/min) who are not receiving chronic haemodialysis: Should be avoided. Patients with ESRD on chronic haemodialysis: No dose adjustment, but only use if potential benefit outweighs the potential risks. Hepatic impairment: Mild/moderate hepatic impairment: no dose adjustment required. Severe hepatic impairment: not recommended. Paediatric population (<18 years): Safety and efficacy has not been established. Refer to SmPC for full information CONTRAINDICATIONS: Hypersensitivity to active substances / any excipients. Co-administration with rifampicin and St John’s wort. Refer to SmPC for full information. WARNINGS/ PRECAUTIONS: Should not be co-administered with other antiretroviral products. Limited safety and efficacy data in HCV co-infection. Tenofovir alafenamide is active against HBV. Co-infected HIV/HBV patients should be closely monitored for at least several months following discontinuation for symptoms of severe acute exacerbations of hepatitis. Should not be administered simultaneously with magnesium/aluminium-containing antacids or iron supplements under fasted conditions. See SmPC for more information on liver disease, weight and metabolic parameters, risk of mitochondrial dysfunction following exposure in utero, immune reactivation syndrome, opportunistic infections, osteonecrosis with CART therapy or nephrotoxicity and patients with ESRD on chronic haemodialysis. Co- administration: Biktarvy is not recommended for coadministration with atazanavir, carbamazepine, ciclosporin (IV or oral use), oxcarbazepine, phenobarbital, phenytoin, rifabutin, rifapentine, or sucralfate. Excipients: Contains less than 1 mmol sodium (23 mg) per tablet, i.e. essentially ‘sodium free’. Refer to SmPC for full information on warnings and precautions. INTERACTIONS: See SmPC for full list. PREGNANCY/LACTATION: Use only if potential benefit justifies the potential risk. Breast-feeding: not recommended. Refer to SmPC for full information. DRIVING/USING MACHINERY: dizziness has been reported. SIDE EFFECTS: Refer to SmPC for full information Common (≥1/100 to <1/10): depression, abnormal dreams, headache, dizziness, diarrhoea, nausea, fatigue. Serious adverse events: suicidal ideation, suicide attempt behaviour (particularly in patients with a pre-existing history of depression or psychiatric illness), anxiety, sleep disorders, angioedema and Stevens-Johnson syndrome. LEGAL CATEGORY: POM. PACK: Bottle of 30 film-coated tablets. PRICE: UK NHS List Price - £879.51; Éire/ Ireland – POA. MARKETING AUTHORISATION NUMBER: Great Britain: PLGB 11972/0008 Ireland and United Kingdom (Northern Ireland: EU/1/18/1289/001 & EU/1/18/1289/002 FURTHER INFORMATION: Gilead Sciences Ltd, 280 High Holborn, London, WC1V 7EE, UK; Great Britain & Northern Ireland: +44 (0) 8000 113700, For Ireland: +353 214 825 999. E-mail: ukmedinfo@gilead.com Biktarvy is a trademark. DATE OF PREPARATION: March 2021; UK-HIV-2021-03-0032. Additional monitoring required.
Adverse events should be reported. For Great Britain and Northern Ireland, reporting forms and information can be found at www.mhra. gov.uk/yellowcard/ or via the Yellow Card app (download from the Apple App Store or Google Play Store). Adverse events should be reported to Gilead (safety_FC@gilead.com) or +44 (0) 1223 897500.
Adverse events should be reported. For Ireland, reporting forms and information can be found at www.hpra.ie and can be reported to HPRA on +353 1 6764971. Adverse events should be reported to Gilead (safety_FC@gilead.com) or +44 (0) 1223 897500.
A Burning Issue
What is GORD?
GORD is caused by acid leaking up from the stomach and coming into contact with the oesophagus. While the stomach is able to withstand litres of acid without pain, the gullet is not. GORD has a significant impact on quality of life and productivity, with sufferers reporting impaired sleep and interference with social activities and work.
Incidence of GORD is certainly rising as obesity rates accelerate. People are also binge drinking and eating more fatty or calorific food, which increases acidity levels and contributes to poorer digestive health.
Common Symptoms
GORD is typically a very treatable disease, but many people don’t know they have it because its symptoms are associated with numerous other conditions.
symptoms of GORD include:
discomfort
sore throat, and/
nighttime
in the back of the
It’s normal to experience reflux symptoms every now and then, especially after a large meal. Acid reflux is considered GORD if symptoms occur at least twice per week or moderate to severe symptoms occur once a week.
Other symptoms include vomiting, halitosis, anorexia, dysphagia, cough and respiratory or oropharyngeal symptoms. Theresa says, “it is estimated that between

Theresa Lowry Lehnen talks all things GORD
20% and 40% of patients with heartburn will have a diagnosis of GERD (Patrick, 2011). it is estimated that between 20% and 40% of patients with heartburn will have a diagnosis of GERD (Patrick, 2011). GORD may be just an occasional symptom for some people, but for others it can be a severe, lifelong condition.
Left untreated, GORD can cause considerable discomfort and a poor quality-of-life. Medical attention should be sought and symptoms investigated when GORD is severe, occurs several times a week, overthe-counter medications are not helping, dysphagia or symptoms such as vomiting, haematemesis, anaemia or unexplained weight loss occur, that could suggest a more serious problem.1, 10
“Several factors may increase the risk of developing GORD. First degree relatives of patients with GORD are four times more likely to develop symptoms, raising the possibility of a strong genetic contribution to the aetiology.11
“Medicines such as calciumchannel blockers, nitrates and non-steroidal antiinflammatory drugs (NSAIDs) can cause GORD or make the symptoms worse.”10
Diagnosing GORD
She adds, “A presumptive diagnosis of GORD can be made based on the typical symptoms of heartburn and acid regurgitation.
Tests for GORD include, endoscopy, barium swallow or meal, manometry, 24 hour pH monitoring and blood tests. A Full Blood Count should be taken to assess for anaemia, which could be a sign of internal bleeding.8
“GORD can be classified according to the presence or absence of erosions on endoscopic examination. Absence of erosions are classified as non-erosive (NERD), whereas GORD symptoms with erosions is classified as erosive oesophagitis.2 The primary role of endoscopy is to look for complications and to exclude other diagnoses.
“Manometry is used to assess how well muscle at the distal end of the oesophagus is functioning. A tube containing pressure sensors can measure the pressures in the oesophagus and help determine whether surgery may be necessary.8
“A barium swallow, or barium meal, may be required to assess
swallowing ability and look for any blockages or abnormalities in the oesophagus.8
“24 hour pH monitoring may be necessary to measure the acidity level in the oesophagus and confirm a diagnosis of GORD. It is the gold standard and most objective test to diagnose the reflux disease and allows monitoring of GORD patients in their response to medical or surgical treatment.8
“A urea breath test is the examination of choice for patients under the age of 50 years presenting with dyspepsia. It is recommended as a non-invasive test for active H.pylori infection, but does not confirm or establish a diagnosis of GORD.”2
Treatment and Management
The management of GORD includes pharmacotherapy, dietary and lifestyle changes and in some cases surgery,” Theresa explains. “Initial treatment is guided by the severity of symptoms and treatment is adjusted according to response. The extent of healing depends on disease severity, treatments chosen and the duration of therapy.
“Patients should be advised about lifestyle changes, avoidance of excess alcohol and consumption of aggravating foods such as fats. Other measures include smoking cessation and weight reduction if applicable, raising the head of the bed when sleeping, and the avoidance of wearing tight fitting clothing and bending down after a meal.5, 12
“Initial management for mild symptoms, may include the use of antacids and alginates which reduce reflux and protect the oesophageal mucosa. Histamine H2 –receptor antagonists such as ranitidine may be used to relieve
symptoms and permit reduction in antacid consumption.
“For more severe symptoms and patients with oesophagitis, oesophageal ulceration, oesophagopharyngeal reflux and Barrett’s oesophagus, treatment involves the use of Proton pump inhibitors (PPI)
“If GORD is unresponsive to diet and lifestyle changes in pregnant women, antacids or alginates can be used. If this is ineffective ranitidine a H2 receptor antagonists may be used. Omeprazole is reserved for pregnant women with severe or complicated reflux disease.”
GORD in Infants
In most babies, reflux is nothing to worry about (as long as they are healthy and gaining weight as expected). However, in some cases (though very few) reflux can cause a lot of pain when strong acid travels up into the food pipe. When reflux becomes painful and it happens frequently, this is known as ‘gastro-oesophageal reflux disease’ (GORD).
Baby reflux symptoms include:
constant or sudden crying when feeding
bringing up milk during or after feeds (regularly)
frequent ear infections
lots of hiccups or coughing
refusing, gagging or choking during feeds
poor weight gain
frequent waking at night
Theresa adds, “If necessary, suitable alginate preparations can be used instead of thickened feeds. For older children lifestyle changes may be helpful, followed if necessary by an alginate containing preparation. Infants or children who do not respond to these measures or who have complications such as oesophagitis or a respiratory disorder, need to be referred to hospital as a H2 receptor antagonists may be required to reduce acid secretion.”
She continues, “Antacids containing aluminium or magnesium compounds can often relieve symptoms in ulcer dyspepsia and non-erosive GORD. Antacids are best given when symptoms occur or are
expected, usually between meals and at bedtime. Conventional doses of liquid magnesium aluminium antacids 3-4 times daily promote ulcer healing but are not as effective as antisecretory medication. Magnesium containing antacids tend to be laxative in nature, while aluminium containing antacids can be constipating. Antacid products containing both magnesium and aluminium can reduce these colonic side effects. Sodium bicarbonate should no longer be prescribed alone for the relief of dyspepsia, but is present as an ingredient in many indigestion remedies.
“Caution must always be maintained with the use of H2 receptor antagonists and proton pump inhibitors as they
5th International Pharmacy Conference

The 5th International Pharmacy and Pharmaceutical Conference takes place from December 12th-13th, 2022 in Dublin.
This is an advanced Pharmaceutics and clinical research Congress composed of well-organized scientific sessions, plenary sessions, Oral presentations, Poster presentations, one to one meetings, networking sessions, e-poster presentation, Young Researcher Forums (YRFs), B2B meetings, International workshops, Symposiums and Industrial sessions.
On 23 May 2022 it
can mask the symptoms of gastric cancer. Particular care is required in patients presenting with alarm features.
“In such cases, malignancy should be out ruled before treatment commences. Side effects of H2 receptor antagonists include diarrhoea, headache and dizziness and the H2 receptor antagonist cimetidine should be avoided in patients stabilised on warfarin, phenytoin and theophylline. Side effects of PPIs include GI disturbances, dizzyness, headache and sleep disturbances. Patients at risk of osteoporosis on PPIs should maintain an adequate intake of calcium and vitamin D and if necessary receive other preventative therapy. Long-term use of PPIs has been linked to
complications, such as vitamin and mineral malabsorption, pernicious anaemia, gastrointestinal infections, gastric cancer and dementia.”2, 5 Theresa concludes, “Gastroesophageal reflux disease is a common disorder, and is one of the most frequent conditions encountered in primary care. Treatment and management of GORD symptoms is important and early intervention has the potential to reduce serious complications.
“The goal of treatment is to effectively control symptoms, prevent complications and improve the patient’s quality of life. The goal of antireflux treatment is to effectively control GERD symptoms, prevent complications of GERD, and improve quality of life Special attention should focus on reducing the rate of refractory GORD and complications such as Barrett’s oesophagus and adenocarcinoma. Knowledge and understanding of the safe and effective use of medications in the treatment of GORD especially PPIs, prevents inappropriate use, and addresses their adverse reactions and interactions with other medications. The clinical benefits and risk of using PPIs should be evaluated for each individual. Assessment, monitoring, audit and evaluation for disease activity, progression, and effects of the therapeutic regime on a patient with GORD is important and a continuous process. For patients requiring long term PPI therapy the clinical effects should be reviewed regularly and treatment adjusted as required. The lowest dose of a PPI that controls symptoms should be used. Implementing personcentred care, monitoring and evaluating symptoms, outcomes and responses to therapy plays a pivotal role in managing the illness and improving the patient’s quality of life.”
References available on request


suspicion in patients presenting with specific medical history or signs of heart failure or cardiomyopathy, etiologic diagnosis must be done by a physician knowledgeable in the management of amyloidosis or cardiomyopathy to confirm ATTR-CM and exclude AL amyloidosis before starting Vyndaqel, using appropriate assessment tools such as: bone scintigraphy and blood/urine assessment, and/or histological assessment by biopsy, and transthyretin (TTR) genotyping to characterise as wild-type or hereditary. The recommended dose is one capsule of Vyndaqel 61 mg (tafamidis) orally once daily. Vyndaqel 61 mg (tafamidis) corresponds to 80 mg tafamidis meglumine, tafamidis and tafamidis meglumine are not interchangeable on a per mg basis. Vyndaqel should be started as early as possible in the disease course when the clinical benefit on disease progression could be more evident. Conversely, when amyoid-related cardiac damage is more advanced, such as in NYHA Class III, the decision to start or maintain treatment should be taken at the discretion of a physician knowledgeable in the management of patients with amyloidosis or cardiomyopathy. There are limited clinical data in patients with NYHA Class IV. If vomiting occurs after dosing, and the intact Vyndaqel capsule is identified, then an additional dose of Vyndaqel should be administered if possible. If no capsule is identified, then no additional dose is necessary, with resumption of dosing the next day as usual. There are no recommended dosage adjustments for elderly patients or patients with renal or mild and moderate hepatic impairment. Limited data are available in patients with severe renal impairment (creatinine clearance less than or equal to 30 mL/min). Tafamidis has not been studied in patients with severe hepatic impairment and caution is recommended. There is no relevant use of tafamidis in the paediatric population. Method of Administration: The soft capsules should be swallowed whole and not crushed or cut. Vyndaqel may be taken with or without food. Contra-indications: Hypersensitivity to the active substance or to any of the excipients as listed in section 6.1 of SPC. Warnings and Precautions: Contraceptive measures should be used by women of childbearing potential during treatment with tafamidis and for one month after stopping treatment. Tafamidis should be added to the standard of care for the treatment of patients with transthyretin amyloidosis. Physicians should monitor patients and continue to assess the need for other therapy, including the need for organ transplantation, as part of this standard of care. As there are no data available regarding the use of tafamidis in organ transplantation, tafamidis should be discontinued in patients who undergo organ transplantation. Increase in liver function tests and decrease in thyroxine may occur. This medicinal product contains no more than 44 mg sorbitol in each capsule. Sorbitol is a source of fructose. The additive effect of concomitantly administered products containing sorbitol (or fructose) and dietary intake of

sorbitol (or fructose) should be taken into account. The content of sorbitol in medicinal products for oral use may affect the bioavailability of other medicinal products for oral use administered concomitantly. Pregnancy and Lactation: Tafamidis is not recommended during pregnancy and in women of childbearing potential not using contraception.Available data in animals have shown excretion of tafamidis in milk.A risk to the newborns/infants cannot be excluded. Vyndaqel should not be used during breastfeeding. Interactions: In a clinical study in healthy volunteers, 20 mg tafamidis meglumine did not induce or inhibit the cytochrome P450 enzyme CYP3A4. In vitro tafamidis inhibits the efflux transporter BCRP (breast cancer resistant protein) at the 61 mg/day tafamidis dose with IC50=1.16 μM and may cause drug-drug interactions at clinically relevant concentrations with substrates of this transporter (e.g. methotrexate, rosuvastatin, imatinib). In a clinical study in healthy participants, the exposure of the BCRP substrate rosuvastatin increased approximately 2-fold following multiple doses of Page 2 of 2 2020-0065522 61 mg tafamidis daily dosing. Likewise, tafamidis inhibits the uptake transporters OAT1 and OAT3 (organic anion transporters) with IC50=2.9 μM and IC50=2.36 μM,respectively,and may cause drug-drug interactions at clinically relevant concentrations with substrates of these transporters (e.g. non-steroidal anti-inflammatory drugs, bumetanide, furosemide, lamivudine, methotrexate, oseltamivir, tenofovir, ganciclovir, adefovir, cidofovir, zidovudine, zalcitabine). Based on in vitro data, the maximal predicted changes in AUC of OAT1 and OAT3 substrates were determined to be less than 1.25 for the tafamidis 61 mg dose, therefore, inhibition of OAT1 or OAT3 transporters by tafamidis is not expected to result in clinically significant interactions. No interaction studies have been performed evaluating the effect of other medicinal products on tafamidis. Undesirable Effects: The following adverse events were reported more often in 176 ATTR-CM patients treated with tafamidis meglumine 80 mg compared to placebo: flatulence [8 patients (4.5%) versus 3 patients (1.7%)] and liver function test increased [6 patients (3.4%) versus 2 patients (1.1%)]. A causal relationship has not been established. Safety data for tafamidis 61 mg are not available as this formulation was not evaluated in the double-blind, placebo-controlled, randomised phase 3 study. Legal category: S1A. Marketing Authorisation Numbers: EU/1/11/717/003– 61mg (30 capsules). Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at EUMEDINFO@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500. Last revised:
q
allow
identification of new safety
See section 4.8 of the
60 Second Summary
Diagnoses of amyloidosis, particularly transthyretin amyloid cardiomyopathy (ATTR-CM), are steadily increasing throughout the world, but the condition remains underdiagnosed.


Patients with amyloidosis may present to a range of medical and surgical specialties, often with multisystemic disease, and a high index of clinical suspicion is required for diagnosis.
Bone scintigraphy and cardiovascular magnetic resonance (CMR) imaging offer highly sensitive and specific imaging modalities for cardiac amyloidosis.
Histological confirmation of amyloid deposition and amyloid type remains the cornerstone of diagnosis for most amyloid types, with transthyretin amyloid cardiomyopathy the exception, which may be diagnosed by validated nonbiopsy diagnostic criteria in the majority. Histological diagnosis of amyloid has been enhanced by laser capture microdissection and tandem mass spectrometry.
Early diagnosis and treatment prior to the development of end-organ damage remains essential to improving morbidity and mortality for patients with amyloidosis.
Diagnoses of amyloidosis, particularly ATTR-CM, are steadily increasing throughout the world, but the condition remains underdiagnosed. Bone scintigraphy and CMR offer highly sensitive and specific imaging modalities for cardiac amyloidosis, and a validated nonbiopsy diagnostic algorithm enables diagnosis in the absence of histology in a majority of patients with ATTR-CM. Histological diagnosis of amyloid has been enhanced by laser capture microdissection and tandem mass spectrometry. Early diagnosis prior to the development of endorgan damage remains crucial to improving morbidity and mortality for patients with amyloidosis.
1National Amyloidosis Centre, Division of Medicine, University College London, UK.
2National Amyloidosis Centre, Division of Medicine, University College London, UK. Electronic address: j.gillmore@ucl.ac.uk.
1. REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by HPN.
Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author. Pfizer has no editorial oversight of the CPD programmes included in these modules
When to Suspect and How to Approach a Diagnosis of Amyloidosis

Introduction
The amyloidoses are a spectrum of diseases with a common final pathological pathway of protein misfolding, insoluble extracellular fibril formation, disruption of tissue structure, and organ dysfunction.
Presentation of systemic amyloidosis is heterogeneous with several organ systems potentially affected. Patients present to a range of medical and surgical specialties and a high index of suspicion is required for diagnosis. Following confirmation of amyloid deposition, determining the amyloid fibril type is crucial to guide treatment and inform prognosis. Treatment of many types of amyloid have improved substantially in recent years, although no treatments to accelerate removal of existing amyloid deposits are currently available, and advanced disease at diagnosis continues to carry a poor prognosis.
Reducing diagnostic delay and commencing treatment early is crucial to improving patient outcomes.
Pathology
The key pathological step in amyloid deposition is protein misfolding and aggregation, which leads to extracellular deposition of amyloid fibrils, disruption of local cellular structure, impaired organ function, and symptomatic
amyloidosis. More than 30 “amyloidogenic” proteins have been identified in humans.
The propensity for protein misfolding increases in the presence of abnormal protein structure (eg, abnormal fibrinogen Aα-chain protein in hereditary fibrinogen Aα-chain amyloidosis), excessive concentration of a structurally normal protein (eg, serum amyloid A [SAA] protein in systemic [AA] amyloidosis), or for unknown reasons associated with the aging process (eg, transthyretin [TTR] protein in wild-type transthyretin [ATTR] amyloidosis). The amyloidogenic protein defines the amyloid type and clinical phenotype and informs diagnosis, treatment, and prognosis. Light chain amyloidosis (AL), transthyretin amyloidosis (ATTR), and serum amyloid A amyloidosis (AA) are the most common types of amyloidosis.
Amyloid Types
Features of the most common amyloid types are outlined in Table 1, on page 32. Clinical phenotype is dictated by the amyloid type, although there remains variability within the same amyloid type; for example, systemic AL can present with cardiac failure due to deposition of amyloid in the myocardium or nephrotic syndrome due to deposition in the kidney, among other presentations.
Trends in the diagnosis of amyloidosis have changed significantly in recent years. Systemic AL remains the most commonly diagnosed amyloid type, although there has been a rapid rise in diagnoses of ATTR.
This reflects increased awareness, advances in cardiac magnetic resonance (CMR) imaging and bone scintigraphy, and the widespread adoption of validated nonbiopsy diagnostic criteria.
Conversely, diagnoses of AA have fallen substantially following the widespread use of biologic therapy in the treatment of chronic inflammatory conditions.
AL may be localized or systemic. When AL type amyloid deposition is limited to 1 location in the absence of a systemic B-cell clonal disorder, the condition is termed “localized AL.” Common sites include the bladder, larynx, tonsil, skin, and lung (pulmonary nodules); treatment is with surgical resection if associated with troublesome symptoms, and prognosis is generally excellent.
In systemic AL, an underlying B-cell clonal dyscrasia produces circulating amyloidogenic monoclonal light chains that can deposit as amyloid in almost any tissue. Concomitant multiple myeloma may be confirmed, but often the clonal disorder is subtle and would be otherwise
Hereditary
amyloidosis
Systemic
amyloidosis
LECT2 amyloidosis
Fibrinogen Aα chain amyloidosis
AApoA1 amyloidosis
Serum
transthyretin
Kidneys,
tissues
Heart, peripheral NS, autonomic NS, soft tissues

Kidneys, liver, spleen, heart (<1%)
organs
Chemotherapy
Table 1 Summary of the Fibrillary Precursor Protein, Underlying Cause, Clinical Phenotype, and Treatment of the Most Common Amyloid Types
TTR stabilizer
TTR stabilizers, gene silencing therapy
Management of underlying cause of inflammation
Variant fibrinogen
ApoA1
gene mutation
gene mutation
Kidneys, liver
Kidneys, liver
Kidneys, liver, heart
Lysozyme
Gelsolin amyloidosis
lysozyme
gene mutation
Variant gelsolin Gelsolin gene mutation
Liver, kidneys, gastrointestinal tract, skin, lacrimal and salivary glands4
Peripheral NS, autonomic NS, cranial nerves, kidneys
Supportive
Treatment
Supportive
Supportive
Supportive
Supportive
AA = serum amyloid A amyloidosis; AL = light chain amyloidosis; ATTR = transthyretin amyloidosis; LECT2 = leucocyte chemotactic factor 2; NS = nervous system.
considered a monoclonal gammopathy of unknown significance (MGUS). At diagnosis, cardiac and renal involvement are present in up to 70% and 60% of patients, respectively, whereas liver, gastrointestinal, soft tissue, peripheral, and autonomic nerve involvement can also occur.
ATTR occurs when the normal transthyretin tetramer dissociates into amyloidogenic monomers and may be hereditary (hATTR), associated with a TTR gene mutation predisposing to protein misfolding, or wild-type (wtATTR), in which an unmutated TTR gene produces TTR protein that misfolds for unknown reasons.
wtATTR mostly affects elderly males, presenting as a restrictive cardiomyopathy (wtATTR-CM) often with a history of carpal tunnel syndrome or spinal stenosis. hATTR commonly
presents with either neuropathy (ATTR-PN), cardiac failure (ATTR-CM), or both (ATTR-mixed) depending, in part, on the specific TTR mutation. The p.V142I TTR variant is most commonly associated with a dominant cardiac phenotype, whereas p.T80A is typically associated with a mixed phenotype. The p.V50M TTR variant typically causes ATTR-neuropathy when disease onset is younger than 50 years of age, and ATTR-mixed when older than 50 years.
hATTR has an autosomal dominant pattern of inheritance, but disease penetrance is incomplete.
Treatment and the Implication of Diagnostic Delays
The universal aim of treatment in systemic amyloidosis is to reduce ongoing amyloid formation and allow natural amyloid clearance.
At present there are no approved treatments to actively accelerate removal of existing amyloid deposits. Therefore, early diagnosis and commencement of therapy before advanced organ dysfunction occurs is essential. However, diagnostic delay remains common.
Patients have often visited several physicians by the time of diagnosis, and diagnostic delay is associated with more advanced disease at diagnosis.
Reasons for diagnostic delay are multifactorial and include disease rarity and phenotypic heterogeneity, lack of physician awareness, other disease mimicry, and until recently, a lack of sensitive noninvasive investigations.
Presentation and Red Flags
Patients with amyloidosis present to a range of specialties including cardiology, hematology,
nephrology, gastroenterology, neurology, orthopedics, and hand surgery, among others.
The likelihood of systemic amyloidosis increases significantly in the presence of multisystemic dysfunction and active inquiry and investigation is required.
Certain populations are at risk of developing amyloidosis and benefit from active monitoring for suggestive signs and symptoms. Patients at risk of systemic AL include those with clonal disorders such as monoclonal gammopathy of uncertain significance, multiple myeloma, Waldenstrom macroglobulinemia, and chronic lymphocytic leukemia. Patients at risk of systemic AA include those with chronic inflammatory conditions such as inflammatory arthropathies, periodic fever syndromes, and inflammatory bowel disease, and either recurrent or chronic infections such as bronchiectasis and tuberculosis. Routine inquiry for symptoms and assessment of renal function, urine dipstick, liver function, and NT-proBNP improve the likelihood of diagnosing amyloidosis early. Patients with a family history of hATTR may be offered predictive genetic testing, and carriers should undergo age-appropriate work up depending on the amyloid type.
Cardiac amyloidosis is most commonly AL or ATTR type and typically presents with symptoms of heart failure or conduction abnormalities. Red flags for cardiac AL include rapid onset heart failure symptoms, systemic symptoms such as weight loss and fatigue, other organ system involvement, and presence of a clonal disorder. ATTR-CM typically has a more indolent heart failure
Table 2 Noninvasive Investigation Options for Systemic Amyloidosis
Peripheral
Autonomic
Liver
Ultrasound abdomen
Special SAP scintigraphy
Small
Postural
alkaline
Hepatomegaly and or splenomegaly
wtATTR
in ALECT2 and
to a large fiber axonal
neuropathy20. Carpal tunnel syndrome,
hATTR
Raised bilirubin in advanced disease
Visceral organ amyloidosis presents with nonspecific symptoms such as fatigue, weight loss, anorexia, and abdominal fullness; rarely, advanced liver amyloidosis can cause jaundice.
Approach to Diagnosis
The diagnosis of systemic amyloidosis requires a stepwise approach starting with a high index of suspicion.
Genotyping
Identifies amyloid deposits in the liver, spleen, kidneys, adrenal glands and bones25 Does not provide information on the gastrointestinal tract, NS, or myocardium
Consider in all cases of ATTR amyloidosis and in the presence of a suggestive family history
AA = serum amyloid A amyloidosis; AL = light chain amyloidosis; ATTR = transthyretin amyloidosis; ATTR CM = transthyretin amyloid cardiomyopathy; CMR = cardiac magnetic resonance imaging; DPD = 99mTechnetium labeled 3,3 diphosphono 1,2 propanodicarboxylic acid scintigraphy; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; GGT gamma glutamyl transferase; hATTR = hereditary ATTR; NS = nervous system; SAP = 123Iodine labeled serum amyloid P scintigraphy; wtATTR = wild type ATTR.
presentation than cardiac AL but should be considered in elderly Caucasian males or individuals of African ancestry particularly when there is a history of carpal tunnel syndrome, spinal stenosis, or tendon rupture. The most common hATTR-CM is associated with the p.V142I TTR variant, which affects individuals of African ancestry; the population prevalence of this particular variant is nearly 4%, although disease penetrance appears to be low. Other red flags for hATTR include peripheral or autonomic neuropathy, a family history of cardiac disease or
neuropathy, and Irish (p.T80A), Portuguese, Japanese, or Swedish (all p.V50M) ancestry.
Renal amyloidosis presents with proteinuria and or chronic kidney disease depending on the location of amyloid deposits within the kidney. New onset proteinuria in high-risk groups is a red flag for the development of amyloidosis.
Soft tissue amyloid deposition gives rise to nail dystrophy, easy bruising, carpal tunnel syndrome, macroglossia, shoulder infiltration, and periorbital bruising. Both macroglossia and periorbital
purpura are pathognomonic of systemic AL. Carpal tunnel syndrome occurs in both AL and ATTR, and in ATTR especially may predate diagnosis by many years.
Flexor retinaculum histology obtained at carpal tunnel decompression surgery may demonstrate amyloid deposition.
Peripheral neuropathy is present in up to 15% of patients with AL at diagnosis and is often the presenting feature in neuropathic forms of hATTR (eg, associated with p.V50M and p.T80A variants).
A comprehensive history is crucial to identify extent of organ involvement and relevant underlying conditions. This is followed by basic investigations (Table 2) to support the initial clinical suspicion and assess organ function. Subsequent histological demonstration of amyloid deposits and the amyloid fibril protein are usually required to confirm the diagnosis, followed by investigations to identify underlying causes. ATTR-CM is the exception to the requirement for histological confirmation because the diagnosis can often be made using a validated nonbiopsy diagnostic algorithm (discussed below).
Cardiac Investigations

Serial serum NT-proBNP may be used to screen for the development of cardiac amyloidosis in at-risk groups, although they can be normal in early disease. Echocardiography offers a widely available noninvasive tool to screen for features of cardiac amyloidosis such as left ventricular hypertrophy of >12mm
(Figure A) Page 34; ventricular hypertrophy alone is of limited sensitivity and specificity, although suspicion increases
in the absence of an alternative cause, and with concomitant small or normal QRS voltages on the electrocardiogram. More advanced echocardiographic measures such as global longitudinal strain offer greater specificity for diagnosing cardiac amyloidosis although are not widely acquired and are further limited by significant interoperator variability (Figures B and C). Cardiac uptake by 99mTechnetium labeled 3,3-diphosphono1,2-propanodicarboxylic acid (DPD; Figures E and F) or pyrophosphate (PYP) scintigraphy is >99% sensitive for cardiac ATTR amyloid deposition but only 86% specific due to cardiac uptake in a proportion of patients with cardiac AL.
Gadolinium-enhanced CMR is unique in allowing the characterization of myocardial tissue and is highly sensitive and specific for cardiac amyloidosis. Characteristic findings including raised native T1 signal, increased extracellular volume, and late gadolinium enhancement (Figures G, H, and I, respectively).
Cardiac AL and ATTR-CM cannot be distinguished by CMR or DPD scintigraphy alone, and further workup is essential to avoid incorrect diagnosis and allow early commencement of appropriate treatment.


Investigations of Underlying Disorder
Early assessment for a clonal disorder is essential to identify patients with systemic AL who may benefit from urgent chemotherapy. The combination of serum free light chain assay and serum and urine protein electrophoresis with immunofixation will identify the underlying clonal disease in up to 99% of patients with systemic AL; however, it is important to note that there may be an incidental clonal disease in association with ATTR-CM such that identification of amyloid and a clonal disease alone are insufficient to confirm a diagnosis of AL.
If AA is diagnosed on the basis of histology, focused history and investigations are required to identify the underlying cause of chronic inflammation, a necessary prerequisite for the diagnosis. Serial measurement of the acute phase protein SAA (or C-reactive protein as a surrogate) is required to determine the degree of ongoing inflammation and is used to monitor response to anti-inflammatory treatment. Outcome in AA is directly related to SAA concentration with sustained suppression of SAA after diagnosis to below <3 mg/L conferring the most favorable prognosis.
Nonbiopsy Diagnosis of ATTR-CM
A diagnosis of ATTR-CM can be made without histology if all of the following validated
nonbiopsy diagnostic criteria are met: heart failure, a suggestive or characteristic amyloid echocardiogram or CMR, a Perugini grade 2 or 3 DPD/PYP scan, a normal serum free light chain ratio, absence of a serum paraprotein by electrophoresis and immunofixation, and absence of urinary Bence Jones protein by urine immunofixation.
Diagnosis of ATTR-CM should be followed by sequencing of the TTR gene to distinguish between hATTR-CM and wtATTR-CM. A negative plasma cell dyscrasia workup using all 3 of the tests listed is essential to exclude cardiac AL, which is associated with cardiac uptake on DPD scintigraphy in approximately 30% of patients and a Perugini grade 2 or 3 DPD scan in up to 10% of cases.
Amyloid Confirmation and Typing
Histological identification and typing of amyloid deposits in an affected organ is the gold standard for amyloidosis. When a target organ biopsy is deemed high risk, screening biopsies such as a fat aspirate, bone marrow trephine, or gastrointestinal biopsy may identify amyloid with varying sensitivities.
Amyloid appears as an acellular, eosinophilic material on light microscopy, with randomly orientated nonbranching fibrils of approximately 10 nm in diameter on electron microscopy.
Amyloid deposition is confirmed by observing apple green birefringence following Congo red staining when viewed under cross polarized light. Amyloid type may be determined by immunohistochemical staining using a panel of antibodies, although sensitivity is limited with up to 30% cases showing no immune-specific staining.
Laser capture microdissection and tandem mass spectrometry of amyloidotic tissue is able to identify presence and type of amyloid in >95% of cases including >80% of those in which immunohistochemistry is indeterminate and requires only a small quantity of amyloidotic tissue.
Summary
Diagnoses of amyloidosis, particularly ATTR-CM, are steadily increasing throughout the world, but the condition remains underdiagnosed. Bone scintigraphy and CMR offer highly sensitive and specific imaging modalities for cardiac amyloidosis, and a validated nonbiopsy diagnostic algorithm enables diagnosis in the absence of histology in a majority of patients with ATTR-CM. Histological diagnosis of amyloid has been enhanced by laser capture microdissection and tandem mass spectrometry. Early diagnosis prior to the development of end-organ damage remains crucial to improving morbidity and mortality for patients with amyloidosis.
Rare Diseases: Shining a light on Hereditary ATTR Amyloidosis (HATTR)

In this short article I want to shine a light on one rare disease namely Hereditary ATTR Amyloidosis and raise awareness on the importance to patients and their families of prompt diagnosis access to treatment and genetic testing and counselling for family members.
In 1993 my late mother was diagnosed with that was then known as Familial Amyloid Polyneuropathy (FAP). Today Familial Amyloid Polyneuropathy now known as Hereditary ATTR Amyloidosis (HATTR) has a significant presence within my immediate family circle. I am also a member of the All-Ireland ATTR Amyloidosis support group and I link with Rare Diseases Ireland for this group. In May 2020 a small group all of whom were patients or family members of individuals with hereditary amyloidosis led by Rosaline Callaghan came together to raise awareness and support patients with the condition and their families and carers. The group lobbied successfully with others to have the drug Patisiran licensed for patients in Ireland. There was a glaring inequality in that patients in the North of Ireland had access to this medication whilst their colleagues in the south did not. Thankfully, this is no longer the case, Patisiran is now available within a managed accessed programme to all patients within the HSE considered suitable for this treatment by their consultant. In May 2022 in conjunction with the Irish Heart Foundation the group hosted a one-day conference in Gweeedore Co Donegal The conference theme Emerging from the Shadows presented a hopeful and positive future for patients and their families and the conference speakers endorsed this positive message. The location of the conference gave recognition to the original families and their antecedents. (See below) The group continues to support patients and their families with Hereditary Amyloidosis.
What is Hereditary ATTR Amyloidosis
The term ‘amyloidosis’ is a general term for a group of conditions where an abnormal protein, called amyloid, accumulates in the tissues. Different types of amyloidosis are named according to the type of amyloid protein which is produced. All begin with the letter ‘A’ which stands for
Amyloidosis. The ‘A’ is followed by one or more other letters, which name the amyloid protein. for example: AL amyloidosis, AA amyloidosis and ATTR(Hereditary) amyloidosis https://www.ukmf
Hereditary ATTR Amyloidosis is a rare disease that if left untreated is progressive and fatal. It is an autosomal dominant condition whereby offspring of an affected parent have a 50/50 chance of inheriting the mutation in the TTR (Transthyretin) gene that causes the condition. Hereditary Amyloidosis accounts for 20% of Amyloidosis Case worldwide.
There are over 100 mutations of the TTR(Transthyretin) gene linked to Hereditary ATTR Amyloidosis. The term ATTR Amyloidosis covers all the mutations. Time of onset of symptoms varies depending on the specific mutation. Patients with one of the most common variants the V50M which is found in families from Portugal, France, and Spain also Sweden and Japan can experience symptoms in their 30/40s The V141 variant is most common in African Americans is a later onset disease as are the two variants that are common to the island of Ireland and to people of Irish descent worldwide.
The most common and best known of these, the Thr Ala 60 variant has its origins in Northwest Donegal in an area around modern-day Gweedore. The presence of a cluster of cases of what was then known as Familial Amyloid Polyneuropathy in Northwest Ireland is recognised in literature by Staunton et al (1987 ,1991). Reilly, Staunton and Harding (1995) identified ThrAla 60 the variant as the causative mutation of the TTR gene in this study cohort. ThrAla 60 also known as The Donegal Mutation or colloquially as ‘Donegal Amy’ is still the predominant TTR mutation for Hereditary ATTR Amyloidosis on the Island of Ireland and beyond. It has followed the Irish Diaspora to many parts of the UK, North America Canada and Australia where it has emerged within families of Irish Descent in these parts.
A second lesser-known mutation H110D (H90D) has now appeared within the population in county Cork and its environs. Professor Aisling Ryan and her colleagues in Cork University Hospital have found this variant in a group
Written by Mary Halpin Byrne
ATTR
of families, and it has been recognised in the literature by Jimenez-Zepeda et al (2014).
Of course, given the more multicultural population demographic in Ireland today it is possible that a patient may present with any of given variants depending on their ethnicity.
There is also an acquired condition known as Wild Type Amyloidosis which is not inherited this is often to linked to ageing and can be underdiagnosed.
Hereditary ATTR Amyloidosis previously known as Familial Amyloid Polyneuropathy is a multisystem condition Cardiac Symptoms can be more predominant in the ALA60 variant for example while Neurological Symptoms can be the first symptoms in the H90D variant, but this is not definitive. Patients can present with multiple symptoms and in varying degrees of severity depending on the progression of their condition. For example, Bi-Lateral Carpal tunnel syndrome in an individual might raise a red flag for Hereditary Amyloidosis.
Today the future is much brighter for patients with ATTR Amyloidosis. Thanks to the dedication of professionals and researchers and to the patients who took part in clinical trials the future is so much more hopeful for patients and their families. Whilst there is no cure the condition can be stabilised
and its progression halted thanks to the advent of new medication and the innovative advances in Gene Silencing treatments all of which offer such hope to patients and their families with improved quality of life and the reduction of symptoms and distress.
Factors in Raising Awareness Education of Health Professionals
For patients with Hereditary ATTR Amyloidosis in common with other rare conditions of a complex nature the path to diagnosis is not always straightforward. The education of health professionals to recognise the possibility of a condition such as ATTR Amyloidosis and make the proper referral to have the diagnosis verified is crucial. Dedicated services are now available to patients with Amyloidosis within the HSE structure, provided by a team of specialist professionals led by Professor Emer Joyce at the Mater Hospital (Cardiology), Professor Sinead Murphy Tallaght Hospital (Neurology) and Professor Aishling Murphy at Cork University Hospital. Up to very recently patients had to travel to the National Amyloidosis Centre (NAC) at the Royal Free Hospital in London under the auspices of the treatment abroad scheme (TAS) administered by the HSE.
The National Amyloidosis Centre is a world-renowned international centre of excellence and Irish Patients have received excellent
Organising Committee for the Emerging from the Shadows Conference (May 2022) with Chris Macey Director of Advocacy and Patient Support, Irish Heart Foundation.
Back Row: Paddy Doherty, James Green, Chris Macey and Eibhlin NI Chuinneagain, Front Row: Mary Halpin Byrne, Bernie Cannon Cunningham, Rosaline Callaghan (Group Founder) and Ann Deegan

genetic counselling for patients and their families is a priority to confirm the hereditary dimension of the condition.
Access to specialised consultants and diagnostics and the availability of new treatments has heralded a more positive outlook for patients with ATTR Amyloidosis and their families.
To ensure continuity to patients these services should be publicity funded with dedicated ring-fenced funding this will allow continuous development of diagnostic pathways and protocols for managing Hereditary Amyloidosis including guidelines for genetic testing of family members. It would also support staff training and development, recruitment and retention of specialist staff research and development and patient and family support
treatment in this centre. There is still a considerable degree of co-operations between the professionals within the NAC and the HSE in a collaborative effort to continue to find new and better treatments and guidelines to manage HATTR Amyloidosis. However, a well-funded dedicated home-based service which negates the need to travel must be the best option for patients and their families.
Family History
Another key factor is the importance of family history: Hereditary Amyloidosis is an autosomal dominant condition which means there is a 50/50
chance of inheritance for offspring of affected persons inheriting the mutation of the TTR gene.
Health professionals need to be aware that family history can be an oral tradition often shared in anecdotes and stories.
Taking time to listen and probe appropriately can yield dividends for both patient and health professionals and hasten the progress to a correct diagnosis.
Genetic Testing /Counselling
Because of the hereditary nature of the condition, clinical diagnosis should be backed up by genetic testing and by genetic counselling for patients and family members. Accessing genetic counselling is
News Hospital Pharmacy Survey
Project MADAM (Model-Adjusted Doses for All Mothers) has launched a survey that aims to explore whether healthcare practitioners are willing to follow dose recommendations that are primarily based on model predictions and to ensure that the envisioned pregnancy formulary meets their needs. Healthcare professionals are invited to participate in this survey.
Most women use medication at least once during their pregnancy. Because of physiological changes in women's bodies as part of pregnancy, the pharmacokinetics (the absorption, distribution, metabolism and elimination) of medications can be altered during that time. In some cases,
this may justify changes in the medication dose that is received during pregnancy. However, because pregnant women are often excluded from clinical studies, evidence-based dose recommendations in pregnancy are missing.
Project MADAM is a collaboration between Radboud and Maastricht University Medical Centres and the Dutch Teratology Information Service (Lareb) with funding from the Gates Foundation. Our goal is to investigate how a new kind of evidence can be used to make dose recommendations for pregnant women. Pharmacokinetic models, that is, computer models that integrate the physiological changes in women's bodies and
not always straightforward and there can be long waiting list for this service.
Development of clear guidelines and protocols as to when genetic testing of family members should be a priority.
In summary Hereditary Amyloidosis can be difficult to recognise and diagnose, not all health professionals are familiar with the symptomology or history of the condition and the presenting symptoms can be similar to other medical conditions.
Family history is a key indicator that a person may have the condition genetic testing and
A patient centred dedicated multidisciplinary co-located and accessible service should not be denied to patients and their families, it should be a taken as given for all patients with rare disease who have to deal with the challenges inherent in their condition not only for themselves but very often for their immediate and extended family.
Thanks to all those who are working tirelessly to research new treatments and develop guidelines for the on-going management of Hereditary Amyloidosis which is the focus of this article but is no less relevant to making similar efforts for all rare diseases.
References available on request
information on fetal development, are used to determine optimal dose regimens in pregnancy. Model predictions are examined by an editorial board of experts alongside other kinds of evidence on the safety and effectiveness of the medication. Pregnancyadjusted dose recommendations can then be issued. We intend to build an international pregnancy formulary using this approach.
The survey is anonymous and last approximately ten minutes. You will be presented with different statements for which you can indicate if you agree or disagree. Important themes include your current practices and information needs with regards to medication in pregnancy. The use of

computer models as evidence for medication dosing in pregnancy will be explained.
Access the survey by visiting www.eahp.eu
Pregnancy-adjusted doses: what should a pregnancy formulary look like?
Decade of Despair
Patients have been subjected to a ‘decade of despair’ by the growing exodus of Ireland’s highly skilled medical and surgical talent abroad to more competitive healthcare services.
As the latest figures from the National Treatment Purchase Fund (NTPF) released today (Friday 14 October) show a near record 907,700 people on some form of hospital waiting list,1 the IHCA said patients will unfortunately continue to languish on ever growing waiting lists, and suffer poorer outcome as a result, until the Government ends the pay discrimination, as the Minister for Health unequivocally committed to do two years ago.
The ongoing pay discrimination imposed by Government on Consultants in October 2012 is one of the root causes of the current recruitment and retention crisis of hospital specialists, says the IHCA, as it reveals new analysis showing that the number of people awaiting an outpatient

appointment has increased by 240,000 over the past decade since the pay inequity was imposed – an increase of 62%.2
The inpatient/day case waiting list has increased by 60% over the same period, with an additional 29,700 patients now waiting for essential hospital treatment compared with 10 years ago.
The number of adults waiting over a year for treatment has multiplied more than 100-fold over the past decade, increasing from just 118 waiting 12 months or longer to 12,800 at the end of September. This is clearly leading to poorer outcomes for patients, the Association warned.
Minister Stephen Donnelly recently suggested the Government was 65,000 behind its targeted reduction in outpatient numbers outlined in the Waiting List Action Plan for 2022.3 In fact, today’s NTPF figures confirm the Government is a massive 138,000 off reaching its projected end
of year position for outpatient waiting lists.4 This means almost 46,000 people on the outpatient waiting list would need to be assessed and treated each month until the end of the year for the Action Plan’s reduction goals to be met.
Commenting on today’s waiting lists, IHCA President Professor Robert Landers said:
“Irish patients have suffered a decade of despair because they are continually denied the timely hospital treatment that they need due to the increasing shortage of hospital Consultants.
“Since the salary inequity was imposed on newly appointed Consultants, the number of permanent Consultant posts that are not filled as needed has increased exponentially to over 900, 22% of the total number of approved posts.
“Three successive Governments are culpable of creating and
perpetuating the Consultant recruitment and retention crisis which is undermining the quality and safety of care that public hospitals provide.
“Government policy is driving Consultants abroad, where they are welcomed with adequate staffing levels, funded services and better conditions. Meanwhile back home, we have an increasing number of individuals employed as Consultants without having the approved qualifications or registration status, 111 in total.
“It is astonishing that the Government keeps trying to impose decade-long discriminatory terms on new Consultants and expects a different outcome. Such action is in stark contrast to the terms and collaborative approach applied in other countries who are successfully competing in attracting a greater number of our highly trained specialists to pursue their careers abroad instead of in our public hospitals.”
Reducing treatment-resistant High Blood Pressure
A clinical trial to evaluate a new treatment for patients with high blood pressure that cannot be controlled with medication (treatment-resistant or refractory high blood pressure) has started at the Mater Private Network in Dublin and four patients have already been enrolled and received a study treatment.
The new treatment which involves a procedure carried out under mild sedation is aimed at patients who have high blood pressure (more than 150-180 over 90 mmHg) that is uncontrolled despite being on up to five medications.
Professor Robert Byrne, Director of Cardiology at Mater Private and Professor of Cardiovascular Research at RCSI , is the principal investigator leading the study at Mater Private Network.
Professor Byrne explained, “This new investigational technique for treating high blood pressure is a minimally invasive procedure and is carried out under mild sedation. We use a special catheter which is inserted through a small incision in the groin and guided to the renal arteries using X-ray guidance. The
Professor Robert Byrne, Director of Cardiology at Mater Private and Professor of Cardiovascular Research
catheter then delivers a small dose of medical-grade alcohol to the area just outside the renal artery where the nerves that contribute to the increase in blood pressure are located. The alcohol has the effect of deactivating the over-signaling of the nerves and this, in turn, reduces the blood pressure.”
According to Professor Byrne, it is estimated that 10% of people with high blood pressure would have refractory high blood pressure.
He explained that if successful, a patient would only have to undergo the procedure once as it appears to have a long-lasting effect. Patients would still have to continue taking medication for their blood pressure but the hope is that with this procedure it would then be controlled.
If this new treatment proves safe and effective it could also potentially be a step towards a time at some stage in the future
when patients with high blood pressure may no longer need to take medication for their condition.
“If the treatment were to prove successful in the group of patients that are resistant to medications, down the line, it might be an option for people who say listen, I just want to control my blood pressure with a single procedure rather than have to take a couple
of pills every day. So that is a direction of investigation as well,” Prof Byrne said.
“It could be a step on the road… to eliminate requirements for blood pressure tablets, but we’re still a long way off,” he added.
The clinical trial for this new treatment for high blood pressure is underway in 100 hospitals in Europe and the United States.
HIQA’s monitoring approach against the National Standards for Safer Better Healthcare
Written by John Tuffy, HIQA’s Health of Healthcare
Almost 300 staff from public acute and community healthcare settings attended a webinar outlining the new programme.
As part of the initial phase of monitoring, HIQA’s core assessment includes assessment of 11 standards relating to:
• Governance, leadership and management,
• Support of workforce,
• Person-centred care,
Earlier this year, the Health Information and Quality Authority (HIQA) commenced a new monitoring and inspection programme in all public acute hospitals and rehabilitation and community inpatient healthcare services in Ireland. Read on to find out everything you need to know.
Background
Since the National Standards for Safer Better Healthcare were published in 2012, HIQA has strived to drive improvements in the quality and safety of healthcare services in Ireland through its role in monitoring public hospitals. In the last number of years, HIQA has focussed its monitoring role on thematic programmes of inspection, such as the assessment of medication safety practices, infection prevention and control, nutrition and hydration, and maternity services
Over the last number of years, the focus on compliance with national standards, combined with increased investment and dedicated resourcing by the HSE in enacting better, more effective systems of oversight of performance in services — such as ongoing surveillance and audit — has contributed to improved quality and safety of healthcare services. HIQA had seen notable improvements in services in the areas of infection prevention and control and medication safety. For example, in infection prevention and control there have been improvements in governance and leadership structures at hospital and national level, strengthened coordination of infection prevention and control at national HSE level, aided by
the Antimicrobial Resistance and Infection Control (AMRIC) team and increases in numbers of specialised staff to implement antimicrobial stewardship, infection prevention and control and medication safety measures.
Rationale for change
Despite improvements in certain areas, healthcare services have faced numerous challenges in the past few years, most notably COVID-19, the cyberattack and capacity issues. These have all had a lasting impact on the health service, and major efforts are required to enable services to fully recover. HIQA recognised the need to develop and commence a new monitoring programme as services adapt to the changing healthcare landscape, while also establishing a proactive and responsive regulatory role.
The experience and learning gathered from the thematic inspections conducted over the years has informed the revised consolidated approach which aims to evaluate if care is person centred, safe and effective in public acute and community healthcare services.
The programme focuses on key known areas of harm, governance of services and the voices of patients, while also being adaptable to any healthcare setting and can be applied to all potential services HIQA will monitor in future as our role expands.
Revised monitoring approach
A new programme of inspection against the National Standards for Safer Better Healthcare was launched in April 2022. HIQA published a Guide to the Assessment Judgment Framework for monitoring healthcare services against the National Standards for Safer Better Healthcare and a short monitoring guidance which explained the new approach.
• Effective care
• Safe care (protecting patients from harm), with a particular focus on four key risk areas
o Infection prevention and control
o Medication safety
o Transitions of care (into the hospital, within the hospital and discharges out of the service)
o Assessment of the deteriorating patient, including use of early warning scores and assessment of sepsis.
The inspection approach will also ensure that the voices of patients and staff are considered in evaluating the safety and culture of our healthcare services. This will incorporate a ‘floor-to-board approach’, which means that inspectors will focus on everyday practice and the experiences of people using the healthcare service, and less time speaking with managers of the services.
In order to make judgments about compliance, inspectors will:
• communicate with people who use the service, whenever it is appropriate, to find out about their views and experience of the service
• observe practice to see if it reflects what is outlined in relevant documents such as national guidelines and local policies
• review documents to see if appropriate records are being kept which reflect practice and what people have stated
• speak with staff and management to find out how they plan, deliver and evaluate care and services. These conversations may concentrate on:
o the structures and processes in place
o how care is delivered and how outcomes are measured and monitored
o other aspects of their role, such as their experience and training.
Looking forward
As we continue to navigate the very difficult times facing the healthcare sector, adapting our monitoring approach will help ensure that healthcare professionals are supported by hospital management to deliver safe effective healthcare for everyone who uses the service.
Ongoing challenges such as increased waiting lists, emergency department overcrowding and the continuing consequences associated with the COVID-19 pandemic have undoubtedly created a difficult environment for clinicians, nurses and health and social care professionals to further progress clinical and alternate pathways to address the pressures on the health system. As regulator in this area, HIQA is keen to utilise the learning from our previous monitoring programmes to build on systems already implemented and drive quality improvement. The vast learning and coordinated systemic application of best practice that has been gleaned throughout the COVID-19 pandemic will become all the more important in preparation for the winter season and beyond.
As we hopefully move out of the acute phase of the COVID-19 pandemic, designated resourcing and applied learning will hopefully maintain focus on the full implementation of effective infection prevention and control practice and other supports in relation to the key known areas of harm.
This new monitoring programme places emphasis on a forwardfacing approach to monitoring which aims to drive quality improvement while also continuing to respond to concerns as they arise. It aims to enable a proactive, consistent and effective approach to monitoring of compliance.
The first inspection reports under the new monitoring programme have been published and are available on www.hiqa.ie.






















MEN’S HEALTH: ERECTILE DYSFUNCTION
Management and Treatment of Erectile Dysfunction

An interview with Theresa LowryLehnen (PhD), CNS, GPN, RNP, South East Technological University

had low (<21) Sexual Health Inventory for Men (SHIM) scores.
“In the past, erectile dysfunction was almost always considered a psychogenic disorder. However, evidence now suggests that more than 80% of cases have an organic aetiology. While most patients with ED have organic disease, some do have a primary psychological cause, particularly younger men. Even when ED is organic in nature, there are almost always psychological consequences regarding relationship issues, cultural norms and expectations, loss of self-esteem, shame, and anxiety and depression related to sexual performance.”
hypertension, and endothelial dysfunction,” she explains.
“Erectile dysfunction and vascular disease are thought to be linked at the level of the endothelium. Endothelial dysfunction, results in the inability of smooth muscle cells lining the arterioles to relax and prevents vasodilatation. The endothelial cell is known to affect vascular tone and impact the process of atherosclerosis and impacting ED, CVD and peripheral vascular disease. Cardiovascular disease and hypertension are very significant risk factors for erectile dysfunction.”
Erectile dysfunction (ED) is a common condition occurring in males over 40 years of age, although it can occur earlier. It is estimated that at least 150 million men globally have ED. It is difficult to obtain accurate values for the true prevalence of erectile dysfunction however, as many patients fail to seek medical attention, and many clinicians are reluctant to ask patients about their sexual health.
We recently spoke to Theresa Lowry Lehnen, RGN, GPN, RNP, BSc, MSc, M. Ed, PhD Clinical Nurse Specialist and Associate Lecturer South East Technological University to understand more about this condition and its impact on males in Ireland.
ED can have a substantial negative impact on a man’s quality of life, Theresa reflects. She says, “Erectile dysfunction is the inability to achieve or maintain an erection for satisfactory sexual performance, and affects a considerable proportion of men at least occasionally. It is often treatable, however, if left untreated, ED can be a source of severe emotional stress for both the man and their partner.”
Theresa notes that erectile dysfunction is often an under recognised, yet important, cardiovascular risk factor. She says, “Owing to its strong association with metabolic syndrome and cardiovascular disease, cardiac assessment is warranted in men with symptoms of ED.”
Aetiology of Erectile Dysfunction

Although most men will experience periodic episodes of erectile dysfunction, it tends to become more frequent with advancing age.
“Many factors can contribute to sexual dysfunction in older men, including physical and psychological conditions, comorbidities and polypharmacy,” she adds. “Aspects of an ageing man’s lifestyle behaviour and androgen deficiency, most often decreasing testosterone levels, can affect sexual function.
“Studies have shown that the percentage of men who engage in some form of sexual activity, decreases from 73% in men aged 57–64 years to 26% for men aged 75–85 years. The aetiology for this decline in male sexual activity is multifactorial, and is in part related to female partners menopause at approximately 52 years of age, leading to a significant decline in female libido and desire to engage in sexual activity.”
While ED is associated with ageing, many studies and largescale surveys have concluded that ED is a major health concern among young men.
Theresa adds, “One study in 2013 reported that 1: 4 men seeking medical help for erectile dysfunction in the real-life setting, is < 40 years of age. Another study in 2016 concluded that 22.1% of men < 40 years of age
Erectile dysfunction is multidimensional in nature, and Theresa says it can be broadly divided into endocrine and nonendocrine causes.
“The condition can be caused by any disease process which affects penile arteries, nerves, hormone levels, smooth muscle tissue, corporal endothelium, or tunica albuginea. It is closely related to cardiovascular disease, diabetes mellitus, hyperlipidaemia,
Besides cardiovascular disease, there are strong correlations between ED and hyperlipidaemia, diabetes, hypogonadism, obesity, smoking, alcoholism, benign prostatic hyperplasia (BPH) with lower urinary symptoms (LUTS), depression, and premature ejaculation. Diabetes is a common aetiology of sexual dysfunction, because it can affect both the blood vessels and the nerves that supply the penis. Men with diabetes are four times more likely to experience erectile
dysfunction, and on average, experience it 15 years earlier than men without diabetes.
“Obesity is also correlated to the development of several types of dysfunction, including a decrease in sex drive and an increase in episodes of ED,” she continues. “Neurogenic erectile dysfunction is caused by a deficit in nerve signalling to the corpora cavernosa. Such deficits can be secondary to spinal cord injury, multiple sclerosis, Parkinson disease, lumbar disc disease, traumatic brain injury, radical pelvic surgery and diabetes. Men being treated for prostate cancer with treatments such as radical prostatectomy, radiation therapy or the use of lutenising hormonereleasing hormone (LHRH) agonists and antagonists often experience ED.”
Numerous medications are listed with erectile dysfunction and/ or a decreased libido as a side effect. Drugs that can cause ED include hydrochlorothiazide’s and beta-blocking agents. Medications used to treat depression, particularly the SSRIs such as citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine and sertraline, can also contribute to ED. The severity of erectile dysfunction is often described as mild, moderate or severe according to the five-item International Index of Erectile Function (IIEF-5) questionnaire, with a score of 1–7 indicating severe, 8–11 moderate, 12–16 mild–moderate, 17–21 mild and 22–25 no erectile dysfunction.
The International Index of Erectile Function (IIEF-5) Questionnaire
The IIEF is a multidimensional validated questionnaire with 15 questions in the five domains of sexual function (erectile and orgasmic functions, sexual desire, satisfaction with intercourse and overall sexual satisfaction), and there is also an abbreviated format of five questions in the Sexual Health Inventory for Men (SHIM).
Investigations and Diagnosis
Theresa continues, “A thorough medical history, detailed sexual history, and physical examination are required before commencing treatment or further investigations. It is important to distinguish between psychological and
organic causes of ED, as well as to ensure that the patient has erectile dysfunction and not another disorder. History that points towards a psychological aetiology include, sudden onset of erectile dysfunction especially if it is related to a new partner or a major life-changing event, situational ED, normal erections with masturbation or a different partner, presence of morning erections and high daily variability in erectile rigidity.
“The main differential diagnosis for erectile dysfunction is hypogonadism, loss of libido, depression with low mood, and other psychological conditions. It may also be the first manifestation of diabetes or cardiovascular disease as well as depression.

It is important to differentiate between true erectile dysfunction and other sexual disorders such as premature ejaculation, and this is usually assessed by obtaining a good sexual history.
other antihypertensives. ACE inhibitors and calcium channel blockers are the least likely to cause ED. Beta-blockers are only a minor contributor, while alpha-blockers can improve erectile function.”
Vascular risk factors such as hypertension and diabetes and lifestyle factors such as smoking, activity level, alcohol intake, and the use of any recreational drugs should be assessed, Theresa adds. A full general and cardiovascular examination should be undertaken, as erectile dysfunction can be the first symptom of underlying vascular disease. Peripheral pulses should be checked and blood pressure measured. The genitalia should be carefully inspected for hypogonadism, signs of infection, the presence of penile fibrosis or plaques, and phimosis.
“Testosterone supplementation is more effective as a treatment for low libido than for ED. For most men with both ED and hypogonadism, oral PDE5 inhibitors alone are recommended as the initial therapy. Testosterone supplementation is reasonable in men with proven hypogonadism and ED who have already failed PDE5 inhibitor therapy or who also have low libido. Hypogonadal patients with borderline erectile rigidity are most likely to benefit from testosterone supplementation.
The International Index of Erectile Function (IIEF -5) Questionnaire
“A complete medication list including supplements should be checked with the patient. ED can be a result of prescription or other medications. Prescription drugs that can cause ED include, antidepressants especially SSRIs, cimetidine, ketoconazole, spironolactone, sympathetic blockers, thiazide diuretics, and
Theresa continues, “The role of testosterone replacement therapy (TRT) as a potential to improve erectile function in ED remains an issue for clinicians who are comfortable treating androgen deficiency. Androgens are known to have a significant impact on the function of the smooth musculature within the corpus spongiosum.
“Testosterone replacement therapy may cause increased levels of haemoglobin or haematocrit which is associated with an increased risk of heart attack, stroke and blood clots. Testosterone treatment can also cause an enlarged prostate or other prostate disorders. During TRT treatment, the prostate specific antigen (PSA) will be measured to monitor for any changes and this is particularly important in men over 45 years of age.
“As a result of using testosterone replacement, natural production of testosterone may be
MEN’S HEALTH: ERECTILE DYSFUNCTION

reduced. This may lead to a reduction in sperm production and fertility. Other side effects of TRT include: weight gain, increased appetite, hot flushes, acne, depression, restlessness, irritability, aggression, tiredness, general weakness and excessive sweating. Lifestyle modifications are considered first-line therapy for ED, and men should be encouraged to make the necessary changes to benefit both their sexual function and their overall health.
“PDE5 inhibitors are highly effective and have an overall success rate of up to 76%. PDE5 inhibitors are contraindicated in patients taking nitrates, but otherwise are safe and effective.
When PDE5 inhibitors are co-administered with nitrates, pronounced systemic vasodilation and severe hypotension can occur.
“PDE5 inhibitors and α-adrenergic receptor blockers, often used for treatment of BPH, need to be taken at least 4 hours apart. Among second-line therapies, external vacuum devices (VCDs) are a good, non-surgical option for patients with ED. VCDs are clear plastic chambers placed over the penis, tightened against the lower abdomen with a
mechanism to create a vacuum inside the chamber. This directs blood into the penis. If an adequate erection occurs inside the chamber, the patient slips a small constriction band off the end of the VCD and onto the base of the penis. An erection beyond 30 minutes is not recommended. While cumbersome, these devices are considered safe.
“Other second-line therapy includes the use of either intracavernosal injection (ICI) or intraurethral suppositories (IUS). A small needle is used to inject the ICI medication into the lateral aspect of the penis through a small-gauge needle. These vasoactive agents include prostaglandin E1, papaverine and phentolamine and sometimes atropine, which work alone or in combination to elicit an erection. Response is dose related, usually occurs within 10– 15 minutes, and does not require stimulation. A concern with ICI use is priapism, and if this occurs the patient will need to seek urgent medical attention. Bruising can also occur, due to it being an injected medication. The intraurethral suppository consists of a tiny pellet of prostaglandin E1 inserted into the urethral meatus. Response is dose related, and onset usually occurs within 10–15
minutes. Patients need to be trained on the technique of the IUS before use, and should be advised that pain or burning may occur with this medication.
“In men who fail to respond to first or second-line therapy, or who are not interested in conservative therapies, penile prosthesis implantation is available. Penile implants include malleable and inflatable devices, although most implants used are of the inflatable variety. Adverse effects including malfunction and infection are rare, and patient satisfaction is high.”
Outlook
Theresa told us that future Therapies for ED Clinical studies in gene therapy are looking towards replacing proteins that may not be functioning properly in the penile tissue of men with erectile dysfunction.
She says, “Replacement of these proteins may result in improvement in ED. Experimental animal models have demonstrated improvement in erectile function with gene therapy. Human studies may demonstrate success with this therapy in the future, however, gene therapy in humans is controversial, and can take a long time for regulatory approval and public acceptance.
“Stem cell studies may also provide advancements in the
treatment of ED in the future. The mechanism of action of stem cells is to generate angiogenesis with subsequent increase in cavernosal smooth muscle cells within the corporal bodies.
“The clinical studies published to date provide encouraging results, with improvement of sexual function reported with no side effects. Although pioneering, stem cell studies to date are small scale, with a short follow up period, various aetiologies of ED and without a control group. Melanocortin activators are drugs that act through the central nervous system, and have been shown in animal studies to produce an erection. Initial studies in humans suggest that the drug (PT-141) can be effective if given intranasally in men with psychological rather than physical causes, and mild to moderate ED.
“Larger studies are necessary, however, to demonstrate the safety and overall effectiveness of these drugs. Another potential new treatment for ED, is penile low intensity shock wave lithotripsy. This consists of 1500 shocks twice a week for 3–6 weeks. The purpose is to stimulate neovascularisation to the corporal bodies with improvement in penile blood flow and endothelial function. The use of low-intensity shock wave lithotripsy may convert PDE5 inhibitor non-responders to responders.”
The
Roche Products Ireland Limited (RPIL) is pleased to announce that The European Commission has authorised VABYSMO® (faricimab) injection for the treatment of adult patients with neovascular (wet) age-related macular degeneration (nAMD) and visual impairment due to diabetic macular edema (DME). AMD and diabetic retinopathy (of which DME is a complication) are two of the leading causes of vision loss in Ireland.
Faricimab is the first bispecific antibody for the treatment of wet AMD and visual impairment due to DME. It targets and inhibits two disease pathways linked to a number of vision-
threatening retinal conditions by neutralising Ang-2 and VEGF-A. By independently blocking both pathways involving Ang-2 and VEGF-A, faricimab is designed to stabilise blood vessels and thereby reduce inflammation, leakage and abnormal vessel growth (neovascularisation) more than inhibition of VEGF-A alone.
The European Commission decision is based on results across four phase III studies: TENAYA and LUCERNE in nAMD, and YOSEMITE and RHINE in DME. The studies showed that faricimab was non-inferior to aflibercept in respect of the primary endpoint, the average
authorises
change in best-corrected visual acuity (BCVA) from baseline. Faricimab given at intervals of up to every 16 weeks was shown to offer visual acuity gains that were non-inferior to aflibercept given every 8 weeks.
In TENAYA and LUCERNE, approximately 45% of faricimabtreated patients were able to extend treatment to every 16 weeks, and approximately 80% up to every 12 weeks or 16 weeks. In YOSEMITE and RHINE, more than 50% of patients received faricimab every 16 weeks and more than 70% every 12 weeks or 16 weeks. Of note, current standards of care for
AMD typically require eye injections every four to eight weeks.
Faricimab was generally well tolerated across the four studies, with a comparable safety profile to aflibercept. The most frequently reported adverse reactions were cataract (11%), conjunctival haemorrhage (7%), IOP increased (4%), vitreous floaters (4%), eye pain (3%) and retinal pigment epithelial tear (nAMD only) (3%).
The most serious adverse reactions were uveitis (0.5%), vitritis (0.3%), endophthalmitis (0.3%), retinal tear (0.2%), and rhegmatogenous retinal detachment (< 0.1%). Safety results were consistent across study arms. See full SmPC for details.












Men’s Mental Health
Written by Professor Brendan KellyMEN’S HEALTH: MENTAL HEALTH

Recent years have seen a welcome increase in public awareness about psychological wellbeing, mental health, and treatment of mental illness. There is growing recognition that a healthy lifestyle, good diet, and reasonable exercise pattern all help to maintain mental health and deal with life’s difficulties when they present themselves. The Covid-19 pandemic generated additional challenges for many people, but also served as a reminder of the value of social connection and mutual support in sustaining wellness.
Notwithstanding these positive developments, there is still room for improvement, especially in recognising the continued impact of common mental illnesses and issues relating to misuse of alcohol and other substances. This article provides an overview of these topics from the point of view of men’s mental health, which has become the subject of enhanced focus in recent years (Castle and Coghill, 2021).
Common mental illnesses in men
The World Health Organization (WHO) points out that one in every eight people in the world live with a mental disorder (WHO, 2022).
Anxiety disorders and depression are the most common conditions. In 2019, 301 million people were living with an anxiety disorder around the world (including 58 million children and adolescents) and 280 million people were living with depression (including 23
million children and adolescents). These are enormous numbers, especially when many people do not have access to effective care in some parts of the world.
From the point of view of men’s mental health, it is noteworthy that while depression and nonfatal deliberate self-harm are more common in women than men, completed suicide is more common among men. In Ireland, the HSE National Office for Suicide Prevention (NOSP) Annual Report 2021 presents provisional data for 2021 (excluding late registered deaths) and shows that 302 males and 97 females died by suicide in 2021. Final data are available for 2019 and reveal a similar picture: 408 males and 116 females died by suicide in 2019. This gender ratio is broadly consistent over time.
Better treatment of depression must form part of broader efforts to address mental health problems, deliberate selfharm, and suicide, in both men and women. Anti-depressant medication and psychological therapies such as cognitive behaviour therapy (CBT) have both been proven effective for depression, once they are accessed in an appropriate, timely manner. Despite this, there is still a reluctance among many men to acknowledge symptoms and to come forward for treatment. Public education has a vital role to play in addressing these issues and increasing uptake of mental health services and psychological supports among men.
For everyone with depression, a careful consideration of the social environment and social reengagement is another useful step on the road to recovery. Self-help
groups and organisations such as Aware (www.aware.ie) are extremely valuable. For men, Men’s Sheds also offer psychological support and social outlets, and can be accessed through the Irish Men’s Sheds Association (https://menssheds.ie).
Alcohol and other substance misuse in men
Substance misuse, including alcohol, present problems for both women and men but are generally more common among men. The Healthy Ireland Survey 2021 found that “almost two out of every three (61%) men who drank alcohol in the last six months did so at least once a week, with 53% of women drinking this frequently”. The report also points out that “men remain more likely than women to binge drink on a typical drinking occasion (35% and 10%, respectively), and younger people remain more likely to do so than older people (31% of those aged under 25, compared with 13% of those aged 65 and older)”.
This problem is not confined to alcohol: men are also more likely than women to enter specialised treatment for cannabis, cocaine, heroin, and amphetamine misuse. Therefore, while alcohol and substance misuse present
problems for both men and women, it is probable that they make a particular contribution to the higher rate of suicide among men.

Treatment for alcohol misuse or dependence is provided through local addiction services which can be accessed following referral by a GP, psychiatrist, or other health professional, as well as through self-referral in certain circumstances. Abstinence is the usual and most sensible goal of treatment. The precise supports offered depend on the stage of the person’s addiction, their general life circumstances, any previous experiences of treatment, and their readiness for change on this occasion.
Overall, while men face specific challenges in relation to mental health, especially substance misuse and suicide, treatments and solutions are available. Services need to improve in many respects, but wider public education would be a good first step towards utilising the services we have and improving mental health outcomes for men.
Brendan Kelly is Professor of Psychiatry at Trinity College Dublin and author of “In Search of Madness: A Psychiatrist’s Travels Through the History of Mental Illness” (Gill Books, 2022).

MEN’S HEALTH: KIDNEY DISEASE

Mental Health in Men with Chronic Kidney Disease

Written by Aoife Smith, Psychotherapist MIACP Coordinator of Counselling Services, Irish Kidney Association

Chronic kidney disease has been recognised as a leading public health problem. More than 850 million people worldwide have some form of kidney disease. This is roughly double the number of people who live with diabetes (422 million) and 20 times the prevalence of cancer worldwide (42 million) . Chronic kidney disease (CKD) is characterised by a progressive and irreversible loss of kidney function. It is often referred to as a ‘silent illness’, with few signs or symptoms in the early stages, resulting in many patients only being diagnosed when their kidneys have almost failed.
Our mental health plays a central role in how we experience and how we cope with a chronic illness. Research has widely shown that loss of health is a major contributing factor in developing mental health problems.
Neuropsychiatric conditions including depression, anxiety disorders and cognitive impairment have all been shown to be prevalent in patients with chronic kidney disease . These conditions are independently associated with poor clinical outcomes, including decrease in health-related quality of life, longer hospitalisation and higher risk of mortality . The
prevalence of depression in CKD patients is three to four times higher when compared with the general public and two to three times higher when compared to other chronic diseases including diabetes, coronary artery disease and chronic obstructive pulmonary disease . Women, when suffering with a chronic illness, are more likely to ask for help than men with the same illness and are also more likely to ask for that help sooner.
When it comes to kidney disease specifically, studies show that both male and female can suffer from depression and anxiety. Some more recent smaller studies show that women with CKD are more likely to suffer from anxiety, while men with CKD are more likely to suffer from depression .
Depression changes how we think, feel and function. Of course it is normal, when living with a chronic illness, for anyone to feel down.
Dips in mood are an ordinary reaction to losses, setbacks, and disappointments. Depression interferes with how we care for ourselves, impacts relationships, sleep, diet and overall enjoyment of life. Depression can manifest in both physical and mental symptoms. There are various types of depression with respect
to symptoms but there are some typical signs of depression: Feelings of hopelessness and helplessness; Low energy; Loss of interest in friends, activities, and things you used to enjoy; Feeling more irritable, short-tempered or aggressive than usual; Consuming more alcohol, engaging in reckless behaviour, or self-medicating; Feelings of restlessness and/or agitation; Sleep patterns, weight or appetite changes; Difficulty concentrating or finding it hard to make decisions; Difficulty controlling negative thoughts.
Unfortunately, depression in men can often get overlooked, as it can be masked by increased anger, violence or alcohol/ substance use. Many men find it difficult to talk about their feelings, they don’t want to be seen as weak or tearful as this is perceived to be incompatible with the typical societal male role. Men tend to focus more on the physical symptoms that often accompany male depression, such as back pain, headaches, difficulty sleeping, or sexual problems. These can all result in the underlying depression going untreated. In kidney patients, depression can be even more difficult to identify because key symptoms of depression like low energy, loss of weight or appetite and sleeping difficulties can be attributed to kidney disease itself.
CKD patients with depression have reported some of the following typical feelings and experiences:
• Regular pain and difficulty sleeping
• Feeling that their life is in the hands of those who care for them and their disease
• Unwanted feelings of dependence on health care providers and the dialysis machine
• Feelings of powerlessness over the disease
• Feelings of lack of control over their schedule and lives
• Inability to stand up to social pressures at the expense of their own health care needs, such as not following a renal food and fluid plan when out with friends
• Feelings of hopelessness that there are only treatment options rather than a cure for kidney disease
For men living with chronic kidney disease the risk of depression significantly increases and it can be intense and unrelenting. Despite its high prevalence and the significant clinical burden there is research to show that depression in CKD patients is underrecognised and an undertreated problem.
Screening patients who show signs of depression or screening all CKD patients periodically, using screening tools like the patient health questionnaire (PHQ-9)
or Beck’s Depression Inventory assessment (BDI), might help to identify patients who need support.
The Irish Kidney Association provides a free counselling service to all those affected by and living with end stage chronic kidney disease, their families and carers. Please email counselling@ika.ie for further information.
MEN’S HEALTH: MALE BEHAVIOUR
How do behavior and emotional state affect the lifestyle, quality and length of men's lives?
Written by Tatiana Iarmak – http:// orcid.org/0000-0001-5371-2958.

Lecturer of the Department of Advanced Training of Junior Medical Specialists at the Municipal Health Care Institution 'Kharkiv Regional Medical Vocational College', Kharkiv, Ukraine. An independent trainer, consultant to medical facilities and non-medical facilities to instruct medical staff on how to safely provide services to patients/clients.
Corresponding Author details: iarmak.tat@gmail.com
and Finland. Behavioral cultural factors play an important role in both improving and worsening of the men’s health.
be infection with HIV infection, hepatitis B, C and other infections, transmitted through blood, infected instrument, staff hands.

With the development of the society, people have become more and more interested in the topic of health. The philosophers of antiquity were the first ones to study health. In ancient Greece, a huge contribution was made by Hippocrates, Plato, Socrates, Pythagoras and others. Hippocrates was a founder of a healthy lifestyle, whose postulates were set out in his book ‘Health program: A Proven Guide to Healthful Living’. Pythagoras, the father of valeology - the science of the health of a healthy person and of a 'virtuous life', which in modern society is called a healthy lifestyle, was the first one to study the health of a healthy, not of a sick person. In ancient Rome, Asclepiades, Celsus, Galen, and others, as well as scientists from ancient India, Tibet, and China, made their contribution to the study of health. Philosophers and scientists of antiquity, studying health, came to a conclusion that health depends not only on a healthy lifestyle, but also on the behavior and activities a person is engaged in. Confucius (China BC) created the doctrine of human behavior in various life situations. The Buddha's teaching has been based on human behavior and experience.
The World Health Organization has defined health as ‘A state of complete physical, mental and social well-being and not merely the absence of disease or infirmity’. The formation of health occurs under the influence of various factors: living and working conditions, nutrition, behavior, the presence or absence of bad habits, and much more. Men, just like women, create their own health. Behavior plays a big role in men's lives. It has an impact on all areas of life: health, family, work, finances, environment, recreation, etc. The quality and longevity of men’s lives depends on an individual behavior of a man, and results in their level of success, moral and material well-being, social status. In September 2021, as a part of the 71st session of the WHO Regional Committee for Europe, WHO/Europe hold an event 'Health literacy in the assessment of the analysis of behavioral and cultural factors'. In 2022, WHO/Europe launched the Behavioral and Cultural Factors Research Unit (behavioral cultural factors are a set of psychological, cultural, social and other factors) - a new flagship initiative that identifies behaviors which affect health. Such departments have already been established in the Netherlands, Slovakia, Wales
In recent decades, a new term sanogenic-healing or health-saving behavior, which promotes physical and psychological health, has appeared. Pathogenic behavior is a behavior, which leads to health disorders: weakening of the immune system, the appearance of diseases and a possible death. Destructive behavior is aimed at destroying one's own personality (alcoholism, drug addiction, etc.), personal relationships, ties with society, which can lead to degradation. The risky behavior of men can harm not only their own health, but also the health of other people. For example, men change sexual partners more often than women, which can lead to sexually transmitted infections (STIs) and the spread of those infections; malfunction of the reproductive function and reproductive system in men as a result of skin and venereal diseases; violate traffic rules; get behind the wheel in a state of intoxication or when are unwell. More often men are involved in extreme sports such as base jumping, one of the most dangerous sports - jumping with a special parachute from low objects: bridges, roofs of multi-storey buildings, rocks, etc.; heliskiing - jumping from a helicopter and riding on the untouched snowy slopes of the mountains, etc. All of the abovementioned can lead to serious consequences up to injuries incompatible with life. Also, men are engaged in activities, such as working at night, having little sleep, which results in the psychological overload and stress and can cause a heart attack, stroke, blood pressure instability, chronic diseases of the cardiovascular system. Various forms of behavior may depend on age, for instance, adolescents, young people are more likely to expose their body to the negative effects associated with pain - piercing, tattoos, etc., the consequence of which may
Men and women react differently to the environment, the people who surround them. Life is not without emotions. The lifestyle defines what emotions will dominate. Emotions affect all organs and systems of the body, physical and psychological health. There is no universal classification of emotions. Scientists from the University of California, Berkley identified 27 categories, both positive - joy, admiration, surprise, interest, etc., and negativeanger, fear, horror, disgust, etc. Emotions can be explicit, hidden, controlled and uncontrolled. They are directly related to behavior, and behavior - to thinking, which can also be sanogenic (positive), pathogenic (negative) and destructive. The higher the level of culture, intellect, moral and ethical qualities, the state of the psyche of men, the better their health and quality of life is.
Lisa Feldman Barrett, PhD, professor of psychology at Northeastern University in Boston, winner of the National Institutes of Health Director's Pioneer Award for research on emotions, Fellow of the Royal Society of Canada, showed in her book 'How Emotions Are Born' that people themselves create emotions and themselves manage them based on their own knowledge, experience, culture of the country, family and upbringing. British scientist neurophysiologist, Nobel laureate Charles Sherington proved the connection of emotions with physical changes in the body. Negative manifestations in men: anger, aggressiveness, irritability, jealousy, fear, etc. can lead to an increase in blood pressure, body temperature; stroke, heart attack, disorder of the immune, endocrine systems; liver, kidneys, bladder and more. Positive emotions, such as laughter, joy, delight give a surge of strength, energy,
‘Each man lived his own life and paid his own price for living it.’ Oscar Wilde
cheer up, as a consequence, the health indicators (pulse, pressure, blood sugar) are normalized; the condition of the skin, hair improved, the hormonal background is restored, etc. With the manifestation of positive emotions, the hormones of happiness (endorphin, dopamine) are released, which prolongs life. Dopamine is a hormone of confidence, which is necessary for making decisions. From a positive (optimist) or negative (pessimist) attitude to life, emotional state, the decision made may prevent or create health problems, improve or worsen the quality of life, so psychologists do not recommend making decisions based on emotions, even positive ones. Hidden or unmanifested emotions are more inherent in men. According to the research by American scientists, men have a lower level of social adaptation than women. Men do not like to 'show emotions', they try to 'keep everything in themselves.' It is difficult for them to ask for support or help, to share their problems or experiences, life events, even positive ones. Emotions can be 'hidden', but they won’t go away, so unexpressed suppressed emotions, especially negative ones - resentment, guilt, jealousy, etc., can contribute to depression, mental illness, oncology, premature death (heart attack), suicide. Emotions cannot be kept in oneself, they must be manifested - released. It is also necessary to learn how to control emotions. Uncontrolled emotions lead to aggression, a heat of passion, negative consequences up to physical violence. Emotions and mental health are closely related. Even the ancients said that the soul and body are dependent on each other.
In recent years, obesity has become a very serious problem in the world. It claims millions of human lives, which leads to social and economic losses. Overweight and obesity are a social problem in all countries of the world, associated with lifestyle and recognized as a disease (WHO data). In recent years, obesity has acquired the character of a pandemic - Globesity, regardless of the economic level of countries. Mexico, USA, South Korea, Canada, Brazil, India are currently in the lead. In European countries, the largest number of
men is registered in the UK, Ireland, Germany, Greece, Spain, the Czech Republic, and Ukraine. There is a trend towards an increase in the number of overweight and obese children and adolescents (especially boys), which is a risk factor and guarantees weight problems in adulthood.

In June 2022, the 75th World Health Assembly (WHA) hosted the 'Tipping the Scales: Global Response to Obesity' event, which aims at preventing the spread of obesity. A new WHO/ Europe Policy Brief 'Obesity in the WHO European Region' was presented, providing the latest data on obesity and approaches to preventing and controlling obesity. One of the main causes of overweight and obesity are physical inactivity or lack of physical activity. Many men lead a sedentary lifestyle, resulting in overweight, leading to obesity. Obesity in men can contribute to an increase in blood cholesterol levels, which leads to the occurrence of cardiovascular disease; problems with body systems: respiratoryPickwick's syndrome - shortness of breath, daytime sleepiness; musculoskeletal - osteoarthritis of the hip and knee joints; varicose veins in the legs; endocrinehormonal imbalance, the risk of hormone-dependent tumors; the work of the thyroid, pancreas (type 2 diabetes mellitus - in men it is noted twice as often as in women (statistical data); metabolic disorders provoke oncology - cancer of the prostate, testicles, stomach; psychological stress; sexual dysfunction; a decrease in cognitive abilities - a deterioration in memory, the ability to concentrate; premature death - life expectancy is shortened by 5 - 15 years, depending on the degree of obesity. In addition, men who are overweight or obese face discrimination when applying for a job, feel discomfort when traveling in transport, difficulties in purchasing clothes, which lowers their self-esteem and can lead to severe depression and other psychological disorders, suicide.
The medieval physician, philosopher Avicenna (Abu Ali ibn Sina) considered exercise, nutrition and sleep to be essential for maintaining health. Asklepiad - singled out walking and running as beneficial for health, Plato -
advised to do physical exercises for beauty and health all your life. Thanks to physical exercises, blood circulation improves, the brain retains mobility and inquisitiveness of the mind for a longer time, which means prolonged youth. The second important cause of overweight and obesity, especially in men, is malnutrition: drinking alcohol, high-calorie foods - confectionery, fatty foods, sugary carbonated drinks (Coca-Cola, etc.), fast food; salty food - cheeses, sausages and other. A late dinner, large amounts of food and a lack of focus on food, negatively affect men’s health: men like to watch TV while eating, work at a computer, 'surf on the internet, speak on the phone,' etc. The human brain is designed in such a way that it reacts to a stronger stimulus, so being distracted from food and all eaten is converted into fat. Types of nutrition for healthy people of different ages, given the seasons, were laid down by Hippocrates. Hippocrates said: 'I am what I eat' and believed that the life expectancy of obese people is short. Nutrition and health are intertwined. Everything that a person eats is reflected in the psychological and physical health, appearance. American scientist, philosopher Benjamin Franklin said: 'We must eat to live, not live to eat. The more food, the more disease.' Indian 'Ayurveda'; Tibetan, Chinese folk medicine attribute healing significance to nutrition. By 2030, 1 billion people in the world are predicted to be obese, of which one in seven is a man. Obesity can become the main risk factor for cancer (WHO data), and by 2050, 45% of the world's population will be overweight, and 16% will suffer from obesity (data from the World Obesity Federation - WFO).
Also, a passive or in other words, a sedentary lifestyle can lead to obesity. Sitting causes a chronic increase in the stress hormone cortisol, which leads to obesity and depression. Even a short sitting impairs brain activity, disrupts metabolism, the level of the lipoprotein lipase enzyme in the blood decreases and calories are stored in fat, the level of 'healthy' cholesterol falls. Long sitting increases the risk of
type 2 diabetes, cardiovascular disease, blood clots in the legs, osteoporosis, and premature death (Australian studies). Men often sit in front of the TV, in the office, driving a car, etc. As a result, the circulation of venous blood in the pelvic organs is disturbed, stagnation and oxygen starvation occur in the prostate gland, which leads to prostatitis. With prolonged sitting, the overheating of the inguinal region and testicles increases (their temperature is lower than body temperature), which affects erection, sperm reproduction, thus, the production of the male sex hormone, testosterone, decreases. Consequently, it is harmful for men to use a heated seat. It is undesirable to sit cross-legged as the vessels are compressed, the blood circulation of the inguinal region worsens. When sitting, the load on the spine rises- back pain appears, pressure on the intervertebral discs increases and posture is disturbed. One in ten people die from a sedentary lifestyle (studies from Queen's University of Belfast, published in the Journal of Epidemiology & Community Health.). Scientists from Cornell University (USA) formulated the principle of prevention: 'healthy activity during the day.'
Conclusions:
Urbanization, computerization of all spheres of life, the influence of society, environment; behavioral and cultural factors, emotional state; hypodynamia or decreased physical activity, improper or unhealthy diet has a tremendous impact on the health and quality of life of men. On the contrary, healthy lifestyle: constant physical activity throughout life, rational nutrition, culture of behavior, control of emotions; following the programs of the WHO and other healthcare structures involved in prevention of diseases of the population; staying up to date with the researches of the world scientists who study the health of a healthy person will allow men strengthen and maintain their men's health, increase life expectancy, and improve the level and quality of life.
MEN’S HEALTH: CANCER SURVIVAL
Developing a Cancer Survivorship Pathway for Men

Prostate cancer is the most common male cancer in Ireland. One in eight men in Ireland will be affected by the disease and over 3,500 men are diagnosed with prostate cancer here every year. However, prostate cancer also has one of the best survival rates of all cancers. Thanks to early detection and advances in treatment, there are over 26,000 prostate cancer survivors in Ireland today with 90% of men diagnosed with the disease surviving.
The consequences of cancer and its treatment can result in significant, often lifelong, effects on health and quality of life. Male cancer survivors may experience effects of cancer treatment that can have a considerable impact on masculinity such as erectile dysfunction, weight gain and muscle loss, sleep disturbance and continence issues, and the psychological impact of change of body image, which is further compounded by the lack of supports to express this distress. The health care system will be able to respond to these needs in a more coordinated way if a cancer survivorship pathway is formalised with particular emphasis being placed upon dealing with troublesome symptoms, supporting individuals to transition through the various stages of the cancer journey, encouraging the active participation of patients in care and helping individuals to live well with, through and beyond a cancer diagnosis.
At a recent stakeholder workshop held to examine the unmet needs of men affected by cancer in Ireland, contributors highlighted the glaring issues and disparities facing male cancer survivors, and particularly emphasised the challenge of supporting men to engage with cancer survivorship services to derive an optimal outcome for their illness. In line with this, Cork University Hospital and University College Cork Cancer Trials Group in association with the Irish Cancer Society (ICS) are working together to help improve the experiences, quality of life and outcomes of men impacted by advanced genitourinary cancer. The Irish Cancer Society funded Liam Mc Trial (short for Linking In with Advice and supports for Men with Metastatic Cancer) will pilot a dedicated integrated survivorship research programme aimed at better engaging with and supporting men in Ireland postcancer treatment. As part of this new initiative, the trial team seek to address the key gaps and unmet survivorship needs of men affected by cancer. The initial phase of the Liam Mc Trial commencing in November 2023 will focus on men with genitourinary cancer malignancies (cancers of the urinary and reproductive system such as prostate, bladder and testicular cancer) with selected eligible patients referred by their hospital teams.
An important aspect of the programme is to demonstrate how to improve the survivorship supports and services for underserved communities of men who have not traditionally been the focus of such
initiatives and are recognised as experiencing disparities in terms of cancer incidence, prognosis, outcome and/or quality of life. Unfortunately, there are many such communities, but these might include, for example, members of the Travelling community, the LGBT+ community, ethnic minority and migrant communities, communities with social disadvantage and/or socioeconomic challenges, or specific mental health issues likely to impact their ability to have a positive outcome from a cancer diagnosis. The focus of this programme will be centred on provision of evidence to drive improvement in the survivorship supports and services for those cancers that yield a significant burden on quality of life due to morbidity related to tumour burden, local treatment effects, and/or systemic treatment effects such as androgen deprivation, and for which there are still considerable challenges and resources issues.
The LIAM Mc trial will aim to address some of the most common unmet needs of male cancer survivors (physical, social, or psychosocial) and address their cancer-related symptoms within a holistic and personcentred approach. Recruitment of participants will include a gateway introduction which will allow the
men to express any concerns or discuss areas where they require more support. In the initial phase, the project team will enroll a cohort of advanced or metastatic prostate cancer survivors as they often experience a significant burden from the side effects of treatment and their advanced cancer status. The larger expansion phase will enrol men with any advanced genitourinary malignancy. The programme, which will be underpinned by a robust research infrastructure, will coordinate resources, be readily accessible and seek to develop and improve evidence-based post-treatment services for these men.
The Cork University Hospital-based research trial will see men receive specialist nurse and dietician support weekly, as well as twiceweekly physiotherapy sessions to empower them to maintain physical activity so important for quality of life. This will be complemented by access to social work and psychological supports to ensure their practical and emotional as well as physical needs are met. Based out of the state-of-the-art cardio rehab gym at Cork University Hospital (CUH), it will be supported by the UCC Cancer Trials Group and overseen by a team of researchers from UCC and CUH under the direction of Consultant Medical Oncologist Dr Richard Bambury, and Lecturer Practitioner in Nursing Dr Brendan Noonan.


Written by Dr Brendan Noonan, Lecturer Practitioner, School of Nursing and Midwifery, University College CorkIrish Cancer Society Liam Mc Trial team
Men urged to look after their hearts

Over 4,000 men died from cardiovascular disease last year according to the latest data from the CSO.
Not only are more men than women dying from heart disease and stroke they are also dying earlier than their female counterparts. We know that men are almost three times more likely than women to die young (under the age of 65) from heart disease and stroke.
Despite the devastating impact heart disease and stroke can have on men’s lives, a survey carried out by the Irish Heart Foundation revealed that one in four men do not prioritise their heart health.
To help combat this the Irish Heart Foundation runs a ‘Reboot Your Life’ campaign which aims to encourage men to review their lifestyles and make vital changes to improve their heart health. The website has plenty of tips for how men can make small changes for a healthier heart. We’ve also created a booklet full of tips to help workplaces and community groups spread the word.
The campaign is supported by the HSE and Healthy Ireland as well as Rugby Players Ireland.
While one in four men in Ireland die from heart disease and stroke the good news is that 80 per cent of these premature deaths are preventable by adopting healthy lifestyles.
The older you get, the higher your risk – and so we encourage men, particularly men in their 40s and
50s, to take stock and Reboot their lifestyles by identifying what small changes they can make now to benefit their heart health into the future.
Make that change
There are a number of things men can do to reduce their risk of heart disease and stroke. These include, stopping smoking, reducing alcohol intake, getting their blood pressure and cholesterol checked, eating a healthy diet, being a healthy weight and being physically active.
Quit smoking
Smoking is a major risk factor for heart disease and stroke. The benefits from quitting happen almost immediately, even 20 minutes after you quit that last cigarette. Your blood pressure and pulse return to normal while the risk of heart attack beings to fall just one day later.
Rethink Your Drink
Alcohol is high in sugar and calories, and drinking more than the recommended amount can be harmful to your heart. A bottle of wine has 550 calories which is about the same as a burger and fries.
Get Active
Being physically active can help reduce blood pressure, cholesterol levels and weight which can in turn help to reduce your risk of heart disease and stroke. It is recommended that adults get at least 30 minutes
of moderate intensity physical activity five days a week.
Get your blood pressure and cholesterol checked
High blood pressure and cholesterol are major risk factors for heart disease and stroke. Its important to get these checked regularly to reduce your risk of heart attack and stroke. High blood pressure is also a risk factor for a number of other conditions including kidney damage and dementia.
Eat for your heart
Its best to aim for a diet rich in fruit, vegetables and wholegrains. Limit processed salty meats such as sausages, bacon and ham and take very small amounts of unsaturated reduced fat spreads and oils. Try also to eat less foods and drinks high in fat sugar and salt.
Engaging with Men
It is often assumed that men don’t engage in health programmes. However, the Irish Heart Foundation has a long track record of initiatives to improve men’s heart health delivered in partnership with stakeholders including the Irish Men’s Sheds Association, Pavee Point, the HSE and the Men’s Development Network. Through working with men to understand their needs,
we design and deliver relevant, innovative approaches that reduce men’s cardiovascular risk.
Farmers Have Hearts
One example of a tailored approach to men’s health is the Farmer Have Hearts programme, a health check programme for farmers developed and led by the Irish Heart Foundation since 2013. It takes a targeted approach, engaging with farmers in marts. The programme aims to identify farmers’ cardiovascular risk, encourage them to make positive lifestyle changes and refer those at high risk to their GP. In recent years, we have built on this work with partners including Teagasc and South East Technological University to design and roll out a successful health behaviour change intervention, which will be scaled up in the future.
For more information, please see www.irishheart.ie
Scrapping VAT on Defibrillators Welcomed
The Budget decision to scrap VAT on defibrillators has been hailed as a “victory for common sense” by the Irish Heart Foundation which led the campaign against the tax.
The Irish Heart Foundation’s Director of Advocacy, Chris Macey, insisted lives will be saved as a result of the decision.

Automated external defibrillators (AEDs) are easy-to-use portable
devices that, in some cardiac arrests, can deliver a shock to the heart to allow it to resume its normal rhythm. However, AEDs on average cost ¤1,500, including ¤345 VAT – making them unaffordable for many community organisations.
“Every minute counts when someone has a cardiac arrest –and without CPR or defibrillation, the chances of survival decrease
by 10 per cent. The earlier an AED is used, the better a person’s chances of surviving a cardiac arrest,” Mr Macey stated.
“The decision to remove the 23% rate of VAT on AEDs (from January 1) is a victory for common sense. More lives will now be saved as a result as they will become more accessible to clubs and community organisations,” he added.
However, while also welcoming the VAT removal on nicotine replacement products, Mr Macey said the 50c rise in the price of a packet of cigarettes was a sign Ireland had become “complacent” in the battle against nicotine addiction.
Budget 2023, he said, was as a “missed opportunity” to get the ball rolling on bringing the cost of a packet to ¤20 by 2025.
MEN’S HEALTH: UROLOGY
Talking about Urology
Written by Serena Wyman, Fundraising and Events Manager, The Urology Foundationpatients and their families - and all those with an interest in urological diseases. This year
TUF commissioned an independent survey of 2,000 people into the public’s awareness of urology conditions. Some of the results reinforce the need for more education:
• 1 in 3 men would not seek advice or know that Erectile Dysfunction could be a sign of a serious medical condition
The Urology Foundation (TUF) is a charity dedicated to improving urological health across all urological conditions through investment in cuttingedge research, the training and education of urology professionals, awareness and campaigning.
Diseases and cancers of the kidneys, bladder, prostate and male reproductive organs are becoming more prevalent and devastating the lives of thousands of men, women and children in the UK and Ireland. TUF is committed to finding better treatments and cures, nurturing urology professionals to deliver better care and raising awareness of the signs and symptoms of these diseases and encouraging an early diagnosis.
Our mission is to increase survival and improve quality of life for people with urology disease.
In order to achieve this
• We invest in ground-breaking, innovative research to find new way to diagnose, treatment and manage these diseases, including setting up the TUF Trials Unit in 2021.
• We support and equip urology profession (i.e. registrars, nurses, consultants, trainees, or surgeons) to develop the skills, knowledge and expertise to be able to offer the highest quality care to patients and their loved ones. In September we launched a series of videos on good communication specifically for healthcare professionals.
You can find the videos on our website theurologyfoundation.org
• Engage with influencers and decisions makers including participation in All Party Parliamentary Groups (APPGs) and events. For example, this September we had an Early Day Motion to draw the attention of Parliamentarians to urological disease.
• Collaborations with those working in or with an interest in urological conditions
• We work with patients and their families to drive change
• Run and support public awareness campaigns
• Work to breakdown the taboo around urology disease
The delivery of our public awareness campaigns takes a number of forms including our website, social media content, providing expert speakers and commentary on topical issues, supporting urology associated health campaigns such as World Continence Week in addition to our own two regular health campaigns – Urology Awareness Month and TUF Nuts Tuesday.
TUF organised the first Urology Awareness Month in 2014 which now runs annually every September. It is an opportunity to bring together the urology community – researchers, urologists, nurses and allied healthcare professionals, influencers and decision makers,
• Despite the fact that urinary track infections are the second most common kind of infection 25% of those surveyed did not know what could put them at risk of developing a UTI
• 1 in 3 in three people surveyed did not know what could contribute to the development of kidney stones or what lifestyle choices they could make to lower their likelihood of them doing so
The second campaign that we run is on testicular cancer.
Past research we commissioned suggests that a quarter of people in the UK would not seek medical advice for a urological condition because of embarrassment and that 20% of people who are suffering from, or know someone suffering from, a urological condition, feel ashamed.
We know there can be many barriers to men accessing health information and support. These
include poor understanding of specific diseases, a fear of being diagnosed with a serious illness, cultural differences, stigma and a reluctance to engage in health or well-being programmes. Add to this the fact that men are often loathe to discuss their health, especially anything to do with their sexual organs, and it is likely that that this percentage is increased in men, with men delaying seeing their GP due to embarrassment. With early detection of testicular cancer being key to survival rates TUF launched the #TUFNutsTuesday campaign.
The idea behind the campaign is to promote the fact that testicular cancer has a 98% survival rate if it is caught early and therefore men should regularly check themselves, to know what is normal for them, and if they spot any changes to see their GP as a matter of urgency. We ask men to get into the habit of checking themselves on the first Tuesday of every month. We use social media posts as a reminder for them to do so and direct them to our website for advice on how best to check themselves.


Healthcare professionals can access a range of downloadable education resources and posters on our website, theurologyfoundation.org, or order a free resource pack which includes posters, leaflets, flyers and newsletters by emailing TUF at info@theurologyfoundation.org

Prescribing
once daily
30 to 59 mL/min/1.73 m2): 50 mg. Moderate renal impairment and concomitant strong CYP3A inhibitors: Reduce dose to 25 mg. Mild renal impairment (GFR 60 to 89 mL/min/1.73 m2): 50 mg. Mild renal impairment and concomitant strong CYP3A inhibitors: Reduce dose to 25 mg. Hepatic impairment: Severe hepatic impairment (Child-Pugh Class C): Not recommended. Moderate hepatic impairment (Child-Pugh B): Reduce dose to 25 mg. Moderate hepatic impairment and concomitant strong CYP3A inhibitors: Not recommended. Mild hepatic impairment (Child-Pugh A): 50 mg. Mild hepatic impairment and concomitant strong CYP3A inhibitors: Reduce dose to 25 mg. The tablet is to be taken once daily, with liquids, swallowed whole and is not to be chewed, divided, or crushed. It may be taken with or without food Contraindications: Hypersensitivity to the active substance or to any of the excipients (see the SPC for a list of excipients). Severe uncontrolled hypertension defined as systolic blood pressure ≥180 mm Hg and/or diastolic blood pressure ≥110 mm Hg. Special warnings and precautions for use: Renal impairment: Betmiga has not been studied in patients with end stage renal disease (GFR < 15 mL/min/1.73 m2 or patients requiring haemodialysis) and, therefore, it is not recommended for use in this patient population. Data are limited in patients with severe renal impairment (GFR 15 to 29 mL/min/1.73 m2); based on a pharmacokinetic study a dose reduction to 25 mg is recommended in this population. This medicinal product is not recommended for use in patients with severe renal impairment (GFR 15 to 29 mL/min/1.73 m2) concomitantly receiving strong CYP3A inhibitors. Hepatic impairment: Betmiga has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) and, therefore, it is not recommended for use in this patient population. This medicinal product is not recommended for use in patients with moderate hepatic impairment (Child-Pugh B) concomitantly receiving
strong CYP3A inhibitors. Hypertension: Mirabegron can increase blood pressure. Blood pressure should be measured at baseline and periodically during treatment with mirabegron, especially in hypertensive patients. Data are limited in patients with stage 2 hypertension (systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 100 mm Hg). Patients with congenital or acquired QT prolongation: Betmiga, at therapeutic doses, has not demonstrated clinically relevant QT prolongation in clinical studies. However, since patients with a known history of QT prolongation or patients who are taking medicinal products known to prolong the QT interval were not included in these studies, the effects of mirabegron in these patients is unknown. Caution should be exercised when administering mirabegron in these patients. Patients with bladder outlet obstruction and patients taking antimuscarinic medicinal products for OAB: Urinary retention in patients with bladder outlet obstruction (BOO) and in patients taking antimuscarinic medicinal products for the treatment of OAB has been reported in postmarketing experience in patients taking mirabegron. A controlled clinical safety study in patients with BOO did not demonstrate increased urinary retention in patients treated with Betmiga; however, Betmiga should be administered with caution to patients with clinically significant BOO. Betmiga should also be administered with caution to patients taking antimuscarinic medicinal products for the treatment of OAB. Interactions: Pharmacokinetic interactions: Mirabegron is a substrate for CYP3A4, CYP2D6, butyrylcholinesterase, uridine diphosphoglucuronosyltransferases (UGT), the efflux transporter P-glycoprotein (P-gp) and the influx organic cation transporters (OCT) OCT1, OCT2, and OCT3. Pharmacokinetic interactions involving the potential for other medicinal products to affect mirabegron exposures: Increases in mirabegron exposure due to drug-drug interactions may be associated with increases in pulse rate. Strong CYP3A inhibitors See Posology and administration above for dose adjustments recommended during concomitant use of strong CYP3A inhibitors in patients with renal or hepatic impairment. Mirabegron exposure (AUC) was increased 1.8-fold in the presence of the strong inhibitor of CYP3A/P-gp ketoconazole. CYP2D6 inhibitors: No dose adjustment is needed for mirabegron when administered with CYP2D6 inhibitors (or in patients who are CYP2D6 poor metabolisers). Inducers: Inducers of CYP3A (such as rifampicin) or P-gp may decrease the plasma concentrations of mirabegron. No dose adjustment of mirabegron is required as this effect is not expected to be clinically relevant.
Pharmacokinetic interactions involving the potential for mirabegron to affect exposures to other medicinal products: Inhibition of CYP2D6: Moderate and time dependent inhibition of CYP2D6 by mirabegron may result in clinically relevant drug interactions. CYP2D6 activity recovers within 15 days after discontinuation of mirabegron. Caution is advised if mirabegron is co-administered with medicinal
products metabolized by CYP2D6 with a narrow therapeutic index such as thioridazine, Type 1C antiarrhythmics (e.g.flecainide, propafenone) and tricyclic antidepressants (e.g., imipramine, desipramine). Caution is also advised if mirabegron is co-administered with CYP2D6 substrates that are individually dose titrated. Inhibition of P-gp: Mirabegron is a weak inhibitor of P-gp. For patients who are initiating a combination of Betmiga and digoxin, the lowest dose for digoxin should be prescribed initially. Serum digoxin concentrations should be monitored and used for titration of the digoxin dose to obtain the desired clinical effect. The potential for inhibition of P-gp by mirabegron should be considered when Betmiga is combined with sensitive P-gp substrates e.g. dabigatran. Fertility, pregnancy and lactation: The effect of mirabegron on human fertility has not been established. Betmiga is not recommended during pregnancy and in women of child-bearing potential not using contraception. Mirabegron should not be administered during breast feeding. Refer to SPC for full guidance. Driving and use of machines: Betmiga has no or negligible influence on the ability to drive and use machines. Undesirable effects: Summary of the Safety Profile: the safety of Betmiga was evaluated in 8433 patients with OAB, of which 5648 received at least one dose of mirabegron in the phase 2/3 clinical program, and 622 patients received Betmiga for at least 1 year (365 days). In the three 12-week phase 3 double blind, placebo controlled studies, 88% of the patients completed treatment with this medicinal product, and 4% of the patients discontinued due to adverse events. Most adverse reactions were mild to moderate in severity. The most common adverse reactions reported for patients treated with Betmiga 50 mg during the three 12-week phase 3 double blind, placebo controlled studies are tachycardia and urinary tract infections. The frequency of tachycardia was 1.2% in patients receiving Betmiga 50 mg. Tachycardia led to discontinuation in 0.1% patients receiving Betmiga 50 mg. The frequency of urinary tract infections was 2.9% in patients receiving Betmiga 50 mg. Urinary tract infections led to discontinuation in none of the patients receiving Betmiga 50 mg. Serious adverse reactions included atrial fibrillation (0.2%). Adverse reactions observed during the 1-year (long term) active controlled (muscarinic antagonist) study were similar in type and severity to those observed in the three 12-week phase 3 double blind, placebo controlled studies. The following adverse reactions were observed with mirabegron in the three 12-week phase 3 double blind, placebo controlled studies. The frequency of adverse reactions is defined as follows: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be established from the available data) Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. The adverse events
are grouped by MedDRA system organ class. Infections and infestations: Common: urinary tract infection Uncommon: vaginal infection, cystitis Psychiatric disorders: Not known: Insomnia*, confusional state* Nervous system disorders: Common: headache* dizziness* Eye disorders: Rare: eyelid oedema Cardiac disorders: Common: tachycardia Uncommon: palpitation, atrial fibrillation Vascular disorders: Very rare: Hypertensive crisis* Gastrointestinal disorders: Common: nausea*, constipation*, diarrhoea* Uncommon: dyspepsia, gastritis Rare: lip oedema Skin and subcutaneous tissue disorders: Uncommon: urticaria, rash, rash macular, rash papular, pruritus Rare: leukocytoclastic vasculitis, purpura, angioedema* Musculoskeletal and connective tissue disorders: Uncommon: joint swelling Renal and urinary disorders: Rare: urinary retention* Reproductive system and breast disorders: Uncommon: vulvovaginal pruritus Investigations Uncommon: blood pressure increased, GGT increased, AST increased, ALT increased (*observed during post-marketing experience) Reporting of suspected adverse reactions: see below. Legal category: POM (S1B) Marketing Authorisation number: EU/1/12/809/003 - 25mg EU/1/12/809/010 - 50mg. Marketing Authorisation holder: Astellas Pharma Europe B.V. Sylviusweg 62, 2333 BE Leiden, The Netherlands. Further information is available from: Astellas Pharma Co., Ltd, 5 Waterside, Citywest Business Campus, Dublin 24. Phone: +3531 467 1555. Summary of Product Characteristics with full prescribing information available upon request. Job number: BET_2019_0002_IE Date of preparation of API: 27 May 2019.
Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via:
HPRA Pharmacovigilance Astellas Pharma Co. Ltd
Earlsfort Terrace, IRL - Dublin 2 Tel: + 353 1 467 1555 Tel: +353 1 6764971 E-mail: Irishdrugsafety@astellas.com Fax: +353 1 6762517

Website: www.hpra.ie E-mail: medsafety@hpra.ie.

MEN’S HEALTH: OBESITY IN MEN

Obesity – Where are all the Men?
Written by Dr Kate McCann, Lifestyle Medicine Physician, Emdoc Health
Before I started writing about obesity and men, I had a quick look at my patient list just to confirm when I already knew: my weight management patients are overwhelmingly women. So, is this unique to my practice or Ireland? No, studies from the NHS found that only 10% of referrals to weight management clinics are men. So, where are all the men?
Is it that we don’t know that there is a difference in health outcomes between males and females? No. Actually, Ireland has been a pioneer; obesity was included when we were the first country in the world back in 2009 to publish a National Men’s Health Policy.
Is it that men in Ireland are just less likely to be above a healthy weight? No: Men are more likely to be above a healthy weight or obese. 43% of men versus 31% of women are above a healthy weight. 25% of men versus 22% of women are obese. By age: 65% of adolescent and young men (age 15-24) are a healthy weight. This dramatically decreases to 35% by age 24; fewer than 20% of men are a healthy weight by age 64. Obesity in 51- 64 year old Irish men has risen from 11% to 42% .
Is it that men aren’t referred because they don’t need supports to lose weight? No: A recent NIHR study found that the likelihood of men returning to healthy weight was only 1: 210 compared to 1:124 in women.
Is it just that our use of BMI overestimating the obesity rates in men? In short, probably not. In a recent article, one US obesity expert notes that while BMI is inherently biased, the disease of obesity is only 9 years old! Our current obesity research and treatment guidelines are based on BMI. While our use of BMI is indisputably flawed, BMI does remain, for the moment, an important metric when used with other measures of health, including waist circumference, etc.
Is weight stigma the problem? It is probably a contributing factor. However, both men and women with obesity may hesitate to access healthcare due to weight stigma.
Do men need referral for weight management if it is just a matter of “Eat Less, Move More”?
Obesity is a complex, chronic and recurring disease. While lifestyle modification is an essential part in both obesity prevention and management, it consists of far more than that out-dated phrase. Evidence-based lifestyle interventions should consider the patient’s goals, and target sustainable, long-term changes. It may require input from dietitians, physiotherapists, and psychologists. Patients who have metabolic factors such as insulin resistance will need that addressed first. Use of GLP-1 analogues such as semaglutide can -and should - be considered when indicated. Referral to tertiary level, multi-disciplinary bariatric services can also be discussed. Patients deserve to know their evidence-based and safe options out there.
We know there is a difference in obesity between men and women. In short, though, it is still an underresearched area. We still have an incomplete understanding as to why obesity rates between men and women are different. The causes are multifactorial, including socioeconomic, environment – including access to healthy food, adequate cooking facilities, exercise facilities and green space - education, cultural,and genetics. Other areas for further study include lifestyle factors in men versus women. For example, studies suggest that men include a higher alcohol consumption and a higher meat-based protein consumption. Further research is also needed to look at more diverse cohorts of men.
When it comes to obesity treatment, studies have found that the motivation for men to seek treatment is to reduce the risk of heart disease and stroke. Men were more likely to engage in programmes promoting healthy

eating and physical activity rather than weight loss groups or any programme using the term “dieting”. Groups that are exclusively for men, programme associated with sports clubs, and programmes that were community based - not health centre basedwere more successful at engaging male patients.
Addressing obesity in men is an imperative not only for patient care, but health economics. Globally, healthcare systems will be under pressure as increasing rates of obesity puts further demands on resources. The current estimated cost of obesity to the Irish economy is in excess of ¤1 billion each year, including loss of productivity, increased health service use, and premature death.
As healthcare professionals, what do we do to address this health disparity? We need to provide compassionate, patient-centred, and evidence-based care.
MEN’S HEALTH: PELVIC TRAINING
Pelvic Floor Muscle Training: A Men’s Health Solution!
Stress Incontinence is leakage of urine with coughing, sneezing or exercise. One in eight men can have this embarrassing and very bothersome problem. Prostate Cancer Surgery, Enlargement of the Prostate, Prostatitis and Aging are the main causes of this type of leakage. Men with incontinence spend much on costly incontinence pads. They hide the problem by wearing dark coloured clothing. Stress incontinence is treatable and preventable with pelvic floor muscle training.

Home Pelvic Routine for Men is an expert designed on-line series of exercise videos and e-booklets, a step-by-step series of exercises and routines, delivered from the Home Pelvic Routine Platform, for Men to train their weak pelvic floor muscles, in comfort and privacy at home, on any device, phone, tablet or laptop. The exercises are given in the right order, at the right pace, treating the root cause and ensuring excellent results.
Aoife’s Home Pelvic Routine for Men, to treat stress urinary incontinence and other pelvic conditions has been created from learnings of 25 years of successfully treating men with pelvic floor muscle dysfunction. Aoife sees over 200 hundred men every year through her hands. Aoife’s uses an evidence-based approach and knowledge from her vast experience to ensure optimal results. The Home Pelvic Routine for Men is for sale online via the Home Pelvic Routine
Written by Aoife Ni Eochaidh, Chartered Physiotherapiste learning platform, courses. homepelvicroutines.com and online from pharmacies and physiotherapy clinics here in Ireland and in the UK.
People that have purchased it have described it as awesome, life changing, simple and easy to use! People have encouraged their friends to buy it. People like that they can use the Home Pelvic Routine on their phone, their laptop or PC. The videos can be watched repeatedly, and the E-booklets can be downloaded and printed.
Aoife’s Home Pelvic Routine product is affordable, it retails at ¤70 (inc VAT). There is a shortages of specialist pelvic physiotherapists globally. Home Pelvic Routine allows people to access high quality pelvic floor muscle training from anywhere in the world, in comfort and in privacy at an affordable price. It is more convenient than attending
the physiotherapist for every single appointment, or even attending at all.
The product is targeted at men, including those going through or after prostate cancer surgery, and we have created a female version too. We are excited to have developed a range of products, including our Home Pelvic Routine Advanced Courses and our soon to launch Home Pelvic Routines Maintenance Products. Men of any age can use the product from, New Dads, New Grandads, Athletes, Sporty teenagers, and or Men recovering from illness. It is aimed particular at men with stress urinary incontinence both to prevent and solve pelvic floor muscle weakness and thus improving men’s health, fitness, and quality of life. All men need
is an email address to access their course, to create a password enabled account. A friendly IPPM Physiotherapist is available at the end of the phone for any queries. One to one in person and online pelvic physiotherapy appointments are also available with Aoife, from her private clinic based at Suite 16, Bon Secour Consultant’s Clinic Renmore, Galway.
For further information please email aoife@ippm.ie. To purchase a Home Pelvic Routine Course for Men please log on to courses. homepelvicroutines.com or ring 087 3469491 to make an appointment. Please see the IPPM website for more information www. ippm.ie. With Incontinence the problems are awful but with Home Pelvic Routine Pelvic Floor Muscle Training, the results are great!

Aoife Ni Eochaidh is a multiaward-winning chartered physiotherapist and clinical specialist women’s and men’s health or pelvic physiotherapist. Aoife founded a company, International Pelvic Physiotherapy Management Ltd (IPPM) which provides a portfolio of services in the pelvic physiotherapy space.
Aoife also has created a range of online pelvic floor muscle training courses, called Home Pelvic Routines, they are a series of videos and e-books which are a solution for urinary incontinence and other pelvic conditions including bowel incontinence and erectile dysfunction. A range is also available for women.

“Men
drugs. Can be given as monotherapy in case of intolerance to MTX or when treatment with MTX is inappropriate. In combination with MTX for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an inadequate response or who have been intolerant to a prior disease modifying antirheumatic drug (DMARD) therapy. For the treatment of adult patients with active ankylosing spondylitis (AS) who have responded inadequately to conventional therapy. For the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response, or were intolerant to either conventional therapy or a biologic agent. For the treatment of active polyarticular juvenile idiopathic arthritis (JIA) (rheumatoid factor positive [RF+] or negative [RF-] polyarthritis, and extended oligoarthritis), and juvenile psoriatic arthritis in patients 2 years of age and older, who have responded inadequately to previous therapy with DMARDs. Can be given in combination with MTX or as monotherapy in case of intolerance to MTX or where continued treatment with MTX is inappropriate. Dosage: Treatment should be initiated and supervised by specialist physicians experienced in the diagnosis and treatment of the condition for which tofacitinib is indicated. Tofacitinib is given orally, with or without food. RA, PsA and AS: The recommended dose is 5 mg twice daily or 11 mg once daily which should not be exceeded. Treatment with tofacitinib 5 mg film coated tablets twice daily and tofacitinib 11 mg prolonged release tablet once daily may be switched between each other on the day following the last dose of either tablet. Available data suggest that clinical improvement in AS is observed within 16 weeks of initiation of treatment. Continued therapy should be carefully reconsidered in AS patients exhibiting no clinical improvement within this timeframe. UC: The recommended dose is 10 mg twice daily for induction for 8 weeks. For patients who do not achieve adequate therapeutic benefit by week 8, the induction dose of 10 mg twice daily can be extended for an additional 8 weeks (16 weeks total), followed by 5 mg twice daily for maintenance. Tofacitinib induction therapy should be discontinued in any patient who shows no evidence of therapeutic benefit by week 16. The recommended dose for maintenance treatment is tofacitinib 5 mg twice daily. Tofacitinib 10 mg twice daily for maintenance treatment is not recommended in patients with UC who have known venous thromboembolism (VTE) risk factors, unless there is no suitable alternative treatment available. For patients with UC who are not at increased risk for VTE, tofacitinib 10 mg twice daily may be considered if the patient experiences a decrease in response on tofacitinib 5 mg twice daily and failed to respond to alternative treatment options for UC such as tumour necrosis factor (TNF) inhibitor treatment. Tofacitinib 10 mg twice daily for maintenance treatment should be used for the shortest duration possible. The lowest effective dose needed to maintain response should be used. Polyarticular JIA and juvenile PsA: The recommended dose in patients 2 years of age and older: 10 kg - < 20 kg: 3.2 mg (3.2 mL oral solution) twice daily, 20 kg - < 40 kg: 4 mg (4 mL oral solution) twice daily, and ≥ 40 kg 5 mg (5 mL oral solution or 5 mg tablet) twice daily. Patients ≥ 40 kg treated with tofacitinib 5 mL oral solution twice daily may be switched to tofacitinib 5 mg tablets twice daily. Available data suggest that clinical improvement is observed in paediatric patients within 18 weeks of initiation. Continued therapy should be carefully reconsidered in a paediatric patient exhibiting no clinical improvement within this timeframe. Dose interruption and adjustment:
Tofacitinib treatment should be interrupted if a patient develops a serious infection until the infection is controlled. Interruption of dosing may be needed for management of dose-related laboratory abnormalities including lymphopenia, neutropenia, and anaemia. It is recommended not to initiate dosing in patients with an absolute lymphocyte count (ALC) less than 0.75×10 /L, an absolute neutrophil count (ANC) less than 1×10 /L or with haemoglobin less than 9 g/dL. It is recommended not to initiate dosing in paediatric patients with an absolute neutrophil count (ANC) less than 1.2×10 9/L or with haemoglobin less than 10 g/dL. Renal impairment: No dose adjustment is required in patients with mild or moderate renal impairment. Patients with severe renal impairment the dose should be reduced to 5 mg once daily when the indicated dose in the presence of normal renal function is 5 mg twice daily or 11 mg prolonged-release tablet once daily. Dose should be reduced to 5 mg twice daily when the indicated dose in the presence of normal renal function is 10 mg twice daily. Patients with severe renal impairment should remain on a reduced dose even after haemodialysis. Hepatic impairment: No dose adjustment is required in patients with mild hepatic impairment. Patients with moderate hepatic impairment dose should be reduced to 5 mg once daily when the indicated dose in the presence of normal hepatic function is 5 mg twice daily or 11 mg prolonged-release tablet once daily. Dose should be reduced to 5 mg twice daily when the indicated dose in the presence of normal hepatic function is 10 mg twice daily. Tofacitinib should not be used in patients with severe hepatic impairment. Elderly: No dose adjustment is required in patients aged 65 years and older. Use with caution as increased risk and severity of adverse events. See also Warnings & Precautions for use in patients over 65 years of age. Paediatric population: The safety and efficacy of tofacitinib in children less than 2 years of age with polyarticular JIA and juvenile PsA has not been established. The safety and efficacy of tofacitinib in children less than 18 years of age with other indications (e.g. ulcerative colitis) has not been established. The safety and efficacy of tofacitinib prolonged-release formulation in children aged less than 18 years have not been established. Interactions: Tofacitinib total daily dose should be reduced by half in patients receiving potent inhibitors of cytochrome P450 (CYP) 3A4 (e.g. ketoconazole) and in patients receiving 1 or more products that result in both moderate inhibition of CYP3A4 and potent inhibition of CYP2C19 (e.g. fluconazole). Coadministration with potent CYP inducers (e.g. rifampicin) may result in a loss of or reduced clinical response. Coadministration with potent inducers of CYP3A4 is not recommended. Contraindications: Hypersensitivity to any of the ingredients, active tuberculosis (TB), serious infections such as sepsis, or opportunistic infections, severe hepatic impairment, pregnancy and lactation. Warnings and Precautions: Patients treated with tofacitinib should be given a patient alert card. Use in patients over 65 years of age: Considering the increased risk of serious infections, myocardial infarction, and malignancies with tofacitinib in patients over 65 years of age, tofacitinib should only be used in patients over 65 years of age if no suitable treatment alternatives are available. Combination with other therapies: There was a higher incidence of adverse events for the combination of tofacitinib with MTX versus tofacitinib as monotherapy in RA clinical studies. Tofacitinib should be avoided in combination with biologics and potent immunosuppressants such as azathioprine, 6-mercaptopurine, ciclosporin and tacrolimus. Venous thromboembolism (VTE): Serious VTE events including pulmonary embolism (PE), some of which were fatal, and deep vein thrombosis (DVT), have been observed in patients taking tofacitinib. In a randomised post authorisation safety study in patients with rheumatoid arthritis who were 50 years of age or older with at least one additional
3


(19% [88/476] vs 8% [10/122]; P=0.007) and OCTAVE Induction 2 (17% [71/429] vs 4% [4/112]; P<0.001) XELJANZ
XELJANZ
(60% [285/476]

33% [40/122]; P<0.001) and OCTAVE Induction 2 (55% [236/429] vs 29% [32/112]; P<0.001) 1
week 52 (primary endpoint) vs placebo (34% [68/198] vs 11% [22/198];P<0.001).1


In OCTAVE

to a decision on treatment continuation with tofacitinib. Tofacitinib should be used with caution in patients with known risk factors for VTE, regardless of indication and dose. Tofacitinib 10 mg twice daily for maintenance treatment is not recommended in patients with UC who have known VTE risk factors, unless there is no suitable alternative treatment available. Promptly evaluate patients with signs and symptoms of VTE and discontinue tofacitinib in patients with suspected VTE, regardless of dose or indication. Retinal venous thrombosis (RVT): Patients experiencing symptoms suggestive of RVT should be advised to promptly seek medical care. Infections: Serious and sometimes fatal infections have been reported in patients administered tofacitinib. Rheumatoid arthritis patients taking corticosteroids may be predisposed to infection. Patients should be closely monitored for infections, with prompt diagnosis and treatment. Treatment should be interrupted if a serious infection develops. As there is a higher incidence of infections in the elderly and in the diabetic populations in general, caution should be used when treating the elderly and patients with diabetes. Tuberculosis: Patients should be evaluated for both active and latent TB prior to being treated with tofacitinib. Patients who test positive for latent TB should be treated with standard antimycobacterial therapy before administering tofacitinib. Viral Reactivation: Viral reactivation and cases of herpes zoster have been observed. Screening for viral hepatitis should be performed in accordance with clinical guidelines prior to starting therapy with tofacitinib. The impact on chronic viral hepatitis is not known. Major adverse cardiovascular events (MACE): MACE have been observed in patients taking tofacitinib. In a randomised post authorisation safety study in patients with RA who were 50 years of age or older with at least one additional cardiovascular risk factor, an increased incidence of myocardial infarctions was observed with tofacitinib compared to TNF inhibitors. In patients over 65 years of age, patients who are current or past smokers, and patients with other cardiovascular risk factors, tofacitinib should only be used if no suitable treatment alternatives are available. Diabetes: Dose adjustment of anti-diabetic medication may be necessary in the event that hypoglycaemia occurs following initiation of tofacitinib. Vaccinations: Prior to initiating tofacitinib it is recommended that all patients, particularly pJIA and jPsA patients, be brought up to date with all immunisations in agreement with current immunisation guidelines. Live vaccines should not be given concurrently with tofacitinib. Malignancy and lymphoproliferative disorder: Tofacitinib may affect host defences against malignancies. In a randomised post authorisation safety study in patients with RA who were 50 years of age or older with at least one additional cardiovascular risk factor, an increased incidence of malignancies excluding non-melanoma skin cancer (NMSC), particularly lung cancer and lymphoma, was observed with tofacitinib compared to TNF inhibitors. Lung cancers and lymphoma in patients treated with tofacitinib have also been observed in other clinical studies and in the post marketing setting. Other malignancies in patients treated with tofacitinib were observed in clinical studies and the post marketing setting, including, but not limited
to, breast cancer, melanoma, prostate cancer, and pancreatic cancer. In patients over 65 years of age, patients who are current or past smokers, and patients with other malignancy risk factors tofacitinib should only be used if no suitable treatment alternatives are available. NMSCs have been reported in patients treated with tofacitinib; the risk of NMSC may be higher in patients treated with tofacitinib 10 mg twice daily than in patients treated with 5 mg twice daily. Periodic skin examination is recommended in patients at increased risk for skin cancer. Interstitial lung disease: Caution is recommended in patients with a history of chronic lung disease as they may be more prone to infection. Asian patients are known to be at higher risk of ILD, caution should be exercised with these patients. Gastrointestinal perforations: Tofacitinib should be used with caution in patients who may be at increased risk, e.g. history of diverticulitis or concomitant use of corticosteroids or NSAIDs. Hypersensitivity: Cases of hypersensitivity associated with tofacitinib administration have been reported. Allergic reactions included angioedema and urticaria; serious reactions have occurred. If any serious allergic or anaphylactic reaction occurs, tofacitinib should be discontinued immediately. Laboratory Parameters: Increased incidence of lymphopenia and neutropenia have been reported, and decreases in haemoglobin, which should be monitored in accordance with the SmPC. Monitor ANC and haemoglobin at baseline, 4-8 weeks and 3 monthly, ALC at baseline and 3 monthly. Tofacitinib has been associated with increases in lipid parameters, maximal effects were observed within 6 weeks. Monitoring should be performed 8 weeks after initiation and managed according to hyperlipidaemia guidelines. Tofacitinib has been associated with liver enzyme elevations; use caution if initiating in patients with elevated liver enzymes particularly in combination with potentially hepatotoxic products. Routine monitoring of liver tests and prompt investigation of any observed liver enzyme elevations are recommended to identify potential cases of drug-induced liver injury. Gastrointestinal obstruction with a non-deformable prolonged-release formulation: Caution should be used when administering tofacitinib 11 mg prolonged release tablets to patients with pre-existing severe gastrointestinal narrowing (pathologic or iatrogenic). Pregnancy & Lactation: Use of tofacitinib during pregnancy and breast-feeding is contraindicated. Undesirable Effects: RA, PsA and A: The most common serious adverse reactions were serious infections; pneumonia, herpes zoster, UTIs, cellulitis, diverticulitis, appendicitis and opportunistic infections.
most
reported adverse reactions during the first 3 months in controlled clinical studies were headache, upper respiratory tract infections, diarrhoea, nausea and

The most
sinusitis, bronchitis, nasopharyngitis, pharyngitis,


reactions
to <1/10)

all indications were pneumonia, influenza, herpes zoster, urinary tract
reported adverse reactions in patients receiving tofacitinib 10 mg twice daily were headache, nasopharyngitis, nausea, and arthralgia.
MEN’S HEALTH: TESTICULAR CANCER
Management and Treatment of Testicular Cancer
An interview with Theresa Lowry
RGN,
(QTS),
BSc, MSc,
“Testicular Cancer includes several types of cancer, such as germ cell tumours (GCT), sex cord-gonadal stromal tumours and secondary testicular tumours. 90-95% of testicular tumours arise from germ cells to generate the “GCT”, followed by 5-10% gonadal stromal tumour, mixed GCT and secondary tumours,” she says.
Anatomy and Physiology
Testicular cancer is rare, but it’s the most common cancer in young men aged between 15 and 34. Almost 170 men are diagnosed with testicular cancer every year in Ireland.
We recently spoke with Theresa Lowry Lehnen PhD, Clinical Nurse Practitioner and Associate Lecturer South East Technological University to find out more about this condition and its current management and treatment in Ireland.

Testicular cancer (TC) is a rare disease but the most common cancer found in young men aged between 15 and 34 years. Theresa explains, “Mortality associated with TC is relatively low at
approximately 0.1% of all annual cancers, and cure rates are greater than 90% for all stages with over 95% five-year survival rate.”
The incidence of testicular cancer has increased in Western and Industrialised countries in recent years, possibly due to an increased exposure to etiologic factors. The highest incidence of testicular cancer is observed in Western and Northern Europe at 8.7 and 7.2 per 100,000 men, respectively.
Theresa adds however that survival rates are excellent due to testicular cancers chemo-sensitivity, staging at diagnosis, adequate early treatment and rigorous follow up and recovery therapies.
The testes are located in a skin-covered, highly pigmented, muscular sack called the scrotum that extends from the body behind the penis. Paired ovals, the testes are approximately 4 to 5 cm in length and are surrounded by two distinct layers of protective connective tissue. The outer tunica vaginalis is a serous membrane that has both a parietal and a thin visceral layer.
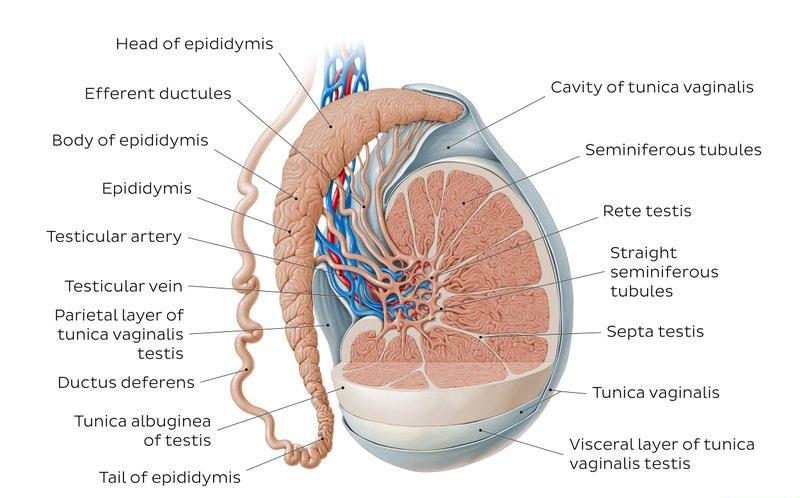

Theresa continues, “Beneath the tunica vaginalis is the tunica albuginea, a tough, white, dense connective tissue layer covering the testis which also inverts to form septa that divide the testis into 300 to 400 structures called lobules. Within the lobules, sperm develop in structures called seminiferous tubules. During the seventh month of the developmental period of a male foetus, each testis moves through the abdominal musculature to descend into the scrotal cavity.
“Tightly coiled seminiferous tubules form the bulk of each
testis. They are composed of developing sperm cells surrounding a lumen, the hollow centre of the tubule, where formed sperm are released into the duct system of the testis. From the lumens of the seminiferous tubules, sperm move into the tubuli recti, and from there into a fine meshwork of tubules called the rete testes. Sperm leave the rete testes, and the testis itself, through the 15 to 20 efferent ductules that cross the tunica albuginea. Inside the seminiferous tubules are six different cell types. These include supporting cells called sustentacular cells, as well as five types of germ cells. Germ cell development progresses from the basement membrane at the perimeter of the tubule toward the lumen.”
Risk Factors
The main non-modifiable risk factors for testicular cancer are cryptorchidism, family history, previous history of testicular cancer, genetic predisposition, ethnicity, congenital abnormalities and infertility.

Cryptorchidism is the major risk factor associated with GCT. “The undescended testicle remains in the abdomen or groin, and the risk of developing the disease does not change even after surgery to move the testicle into the scrotum,” Theresa adds.
In patients with cryptorchidism, the relative risk of developing
testicular cancer ranges from 2.9 to 6.3. The risk is increased in both testes, although the risk is much higher in the ipsilateral testis (6.3 vs. 1.7).
“A person diagnosed with cancer in one testicle, is also more likely to get cancer in the other testicle. Patients with a personal history of testicular cancer have a 12-times greater risk of developing a contralateral testicular cancer than the general population. However, the greatest risk is in the first five years after diagnosis, and the 15-year cumulative risk is 1.9%. Genetic and environmental factors play an important role in the genesis and development of testicular cancer. Several genes are implicated in its pathogenesis and different environmental factors have been investigated. Klinefelter’s syndrome, caused by a chromosomal abnormality has been associated with TC and other cancer types, and congenital abnormalities of the testicles, penis or kidneys may also contribute to an increased risk of testicular cancer. Age represents one of the most frequent factors of TC occurrence and the highest incidence of GCT has been found in men between 15 and 35 years old. Infertility is strongly associated with TC. Caucasian men have a higher chance of getting the disease than Afro-Caribbean or Asian men.”
Symptoms of Testicular Cancer
• Small lumps or hardness on the front or side of a testis.
• Swelling or enlargement of the testis.
• An increase in firmness of the testicle.
• A sensation of dragging or heaviness in the scrotum.
• A dull ache in the lower abdomen or groin.
Theresa adds, “Testicular cancer may present as a painless scrotal mass, an incidental radiologic finding, posttraumatic symptom, or scrotal pain. An enlarged testicle or a small lump or area of hardness are usually the first signs of testicular cancer. Any lump, enlargement, hardness, pain, or tenderness in the testicle should be evaluated as soon as possible. Other symptoms of testicular cancer usually do not appear until after the cancer has spread to other parts of the body.
“When cancer spreads to other sites such as the lungs, brain, abdomen or neck symptoms including nausea, vomiting, gastric upset, cough, shortness of breath, weakness, sensory disturbances, abdominal pain, lumps in the neck/groin areas,
and back pain, can occur. Prompt evaluation is important to ensure early diagnosis and treatment and decrease the burden of treatment in advanced disease.”
Testicular cancers are defined based on their cell type. The most common histology of TC is germinal- seminoma and non-seminoma. About 95% of testicular cancers begin in germ cells, specialised cells in the testicles that make sperm. While these tumours typically start in the testicles they also occasionally arise in the abdomen, chest, or other areas of the body, even if there’s no evidence of cancer in or near the testicles. Seminomas make up about half of all germ cell tumours. They usually grow slowly and are less likely to metastasise to other parts of the body. Non-seminomas are often more aggressive than seminomas, and more likely to spread beyond the testicle.
Diagnosis of Testicular Cancer
Evaluation by clinicians is guided by a complete history of the presenting symptoms, physical examination and assessment for risk factors. Theresa explains, “Testicular examination should include the affected and unaffected testis for comparison. The normal testis is 3.5 - 5 cm in length, smooth, homogenous, movable and detached from the epididymis. Hard, firm, or fixed areas within or adjacent to the testes are abnormal and warrant further investigation. Physical examination should also include evaluation of the inguinal and supra-clavicular lymph nodes, the abdomen, and the chest for gynecomastia. It is important to ask specifically about the history of cryptorchidism, orchiopexy, or inguinal hernia repair as an infant. A family history of testicular cancer in father or brother should be elicited. Physical examination findings of any solid intratesticular mass should be considered testicular cancer until proven otherwise.
“Many signs and symptoms of testicular cancer are similar to those caused by noncancerous conditions such as a spermatocele, variocele, hydrocele, inguinal hernia, lymphoma, epididymo-orchitis or epididymitis and differential diagnosis is important.
“Blood tests can detect ‘tumour markers’-chemicals released by some testicular cancers. Tumour markers include Alpha-fetoprotein (AFP), Beta human chorionic gonadotrophin (bHCG) and Lactate dehydrogenase (LDH).
“Scrotal ultrasonography can confirm the presence of a mass, determine intra V’s extra testicular location, and explore the contralateral testis. Ultrasonography has a sensitivity of 92% to 98% and specificity of 95% to 99.8%. Once a solid intratesticular tumour is identified, radical inguinal orchiectomy is performed both for diagnostic and therapeutic purposes. A biopsy of the suspect mass will be carried out and when suspicion for metastatic disease is present, additional imaging with computed tomography (CT) of the chest and abdomen may be done for staging. Staging is determined by the size of the tumour, lymph node involvement, whether the cancer has spread and if tumour markers are present.”
Staging
Stages are based on four categories:
T (Tumour): This describes whether the tumour has spread to tissues near the testicle.
N (Node): Indicates whether the testicular cancer cells have spread to regional lymph nodes.
M (Metastasis): This refers to whether the cancer has metastasised.
S (Serum): This indicates the level of tumour marker proteins in the serum, or blood.
Once the individual T, N, M and S components are scored, they are combined to determine the overall testicular cancer stage group. The stages of testicular cancer are:
Stage 0: The cancer cells have not spread beyond the testicle. At this stage, tumours are also referred to as carcinomas in situ.
Stage 1 testicular cancer: The cancer has invaded tissues next to the testicle, but has not spread to lymph nodes, or more distant sites in the body. Levels of tumour marker proteins may be normal or elevated. The three subcategories of stage 1 testicular cancer are:
• Stage 1A: The tumour may have grown through the inner layer of tissue surrounding the testicle, but not the outer layer, and it has not spread to blood or lymph vessels. Serum levels of tumour markers are normal.
• Stage 1B: Tumours at this stage may have spread to blood or lymph vessels or may have invaded the outer layer
surrounding the testicle, the spermatic cord or the scrotum. Serum levels of tumour markers are normal.
• Stage 1C: These cancers can demonstrate any degree of invasion of nearby tissues, and levels of tumour markers measured after the tumour has been removed by surgery are elevated.
Stage 2 testicular cancer:
Testicular cancers at this stage have invaded tissues next to the testicle and can now be found in at least one nearby lymph node. Tumour marker levels may be normal or slightly elevated.
Stage 2 testicular cancer has three subcategories:
• Stage 2A: Tumours at this stage have spread to one or more lymph nodes, but no node is larger than 2 cm.
• Stage 2B: Tumours at this stage have spread to at least one lymph node, which is between 2 cm and 5 cm in size.
• Stage 2C: These tumours have spread to at least one lymph node larger than 5 cm.
Stage 3 testicular cancer:
Testicular cancers at this stage have spread to distant lymph nodes or organs. Stage 3 testicular cancer has three subcategories:
• Stage 3A: These cancers have spread to a distant lymph node or the lungs. Tumour marker protein levels are normal or slightly elevated.
• Stage 3B: At this stage of testicular cancer, patients have moderately elevated levels of tumour marker proteins, and the disease has either spread to nearby or distant lymph nodes, or the lungs.
• Stage 3C: These cancers have high levels of tumour marker proteins and may have spread to nearby or distant lymph nodes, or the lungs. Alternatively, they may have spread to other distant organs, such as the liver or the brain, but in this case serum tumour markers can be at any level.
Treatment and Prognosis
“Treatment options for testicular cancer include surgery, radiation therapy, chemotherapy and stem cell transplant. Sometimes more

MEN’S HEALTH: TESTICULAR CANCER

than one type of treatment might be used including chemotherapy and/ or radiotherapy,” Theresa says.
“The management of seminomas depends on the extent of spread of the cancer. Surgery, radiotherapy and chemotherapy are used to treat seminoma and the stage of cancer will decide treatment options. Nonseminomas are usually treated with surgery and chemotherapy.
“When a diagnosis of testicular cancer is suspected based on physical examination and ultrasound findings, radical inguinal orchiectomy is performed, which removes the testicle, epididymis, and spermatic cord up to the level of the internal inguinal ring. In this procedure, these structures are delivered through an inguinal incision made along Langer's lines in the groin. If the mass is too large to pass through a standard 3 to 5 cm inguinal incision, the incision can be carried inferiorly to the anterior scrotum to allow for removal of the testis in its tunics along with the spermatic cord. Trans-scrotal orchiectomy
or biopsy is contraindicated, as doing so alters the lymphatic drainage patterns and impacts further management. Further surgery or radiotherapy or chemotherapy will be based on the disease's stage and response to the initial management.
“Removing one testicle does not affect libido or the ability to have an erection providing the remaining testicle is normal. The removal of a testicle may be traumatic especially for a young man and a testicular prosthesis can be placed in the scrotum at the time of surgery. Fertility can be compromised by testicular cancer treatment. However, the potential to father children should not be greatly affected provided the other testicle is normal. Chemotherapy, however, does affect sperm production in this testicle and it is recommended that patients with testicular cancer arrange to freeze sperm in case there are problems with fertility later on.
“Prognosis is determined by the histology, extent of distant tumour spread, and extent of tumour marker elevations. For men with disseminated seminomas, the main adverse prognostic variable
is the presence of metastases to visceral organs other than the lungs. A tumour that originated in the mediastinum has a worse prognosis when compared to a tumour that originated within the testicle.”
Patient Education and Testicular Self-Examination (TSE)
There is no national screening programme for detecting testicular cancer in Ireland. Testicular self-examination (TSE) is one of the simplest and most effective ways to identify TC early, although there is controversy about its efficacy.
“It is important to educate men of all ages about testicular cancer and TSE and that HCP’s especially nurses are well informed and able to discuss it with their patients,” she notes. “HCP’s play a key role in providing information about testicular cancer risk factors and symptoms and in explaining the importance of TSE.”
Testicular Self-Examination

• Often the best place to check is in the bath or shower where the scrotum is relaxed and the testicles can be felt easily.
• Hold the scrotum in both hands.
• Use your fingers and a thumb to examine the testicles.
• It is common for one testicle to be slightly larger than the other.
• Gently feel each testicle, one at a time.
• You should be able to feel a soft tube at the top and back of both testicles. This tube called the epididymis carries the sperm. It may be slightly tender but do not confuse this with an abnormal lump in the testicles.
• If you notice a lump or anything unusual contact your GP immediately. The GP will be able to assess and if necessary, refer to a consultant for further investigations.
• Do not be embarrassed or nervous.
• Remember early detection of the disease is the best chance of a cure.
• TSE should be performed every month
Men tend to seek help late for testicular problems for many reasons. This includes anxiety, concern and fear of receiving an undesired diagnosis. Others feel ashamed, deprived of their masculinity and are too embarrassed to talk about it with anyone even a partner. Researchers have found that when men are properly educated about testicular self-examination they are more likely to carry out TSE and recognise symptoms of testicular cancer.
Outlook
Theresa concludes, “Testicular cancer is the most common malignancy in men aged 15 to 45 years and represents one of the most common curable malignancies when identified promptly and treated with a multimodal approach.
“It is one of the most curable cancers with excellent survival rates and having awareness about the disease and seeking prompt healthcare attention is of utmost importance. Since treatment is successful for most people with testicular cancer, one of the major future goals is to reduce the side effects of treatment for people with early-stage cancer. In addition, treatments for poor-risk and recurrent cancers are being studied in clinical trials, along with research on the causes and genetics of testicular cancer.
“Stem cell transplant is most often used to treat testicular cancers that have re-occurred after treatment with chemotherapy. Current studies are looking at whether a stem cell transplant may be valuable as part of the first treatment for some patients with advanced germ cell cancers. Clinical trials are also underway to find better ways of reducing symptoms and side effects of current testicular cancer treatments that can improve patients comfort and quality of life.”
References available on request
ABBREVIATED PRESCRIBING INFORMATION

Please refer to the Summary of Product Characteristics (SmPC) before prescribing Pelgraz▼(peg lgrastim) 6 mg solution for injection in pre- lled syringe or prelled injector. Presentation: Each pre- lled syringe or pre- lled injector contains 6 mg of peg lgrastim* in 0.6 mL solution for injection. The concentration is 10 mg/mL based on protein only**. *Produced in Escherichia coli cells by recombinant DNA technology followed by conjugation with polyethylene glycol (PEG). ** The concentration is 20 mg/mL if the PEG moiety is included. Indications: Reduction in the duration of neutropenia and the incidence of febrile neutropenia in adult patients treated with cytotoxic chemotherapy for malignancy (with the exception of chronic myeloid leukaemia and myelodysplastic syndromes). Dosage and Administration: Pelgraz therapy should be initiated and supervised by physicians experienced in oncology and/or haematology.

Posology: One 6 mg dose (a single pre- lled syringe or pre- lled injector) of Pelgraz is recommended for each chemotherapy cycle, given at least 24 hours after cytotoxic chemotherapy. Safety and e cacy of Pelgraz in children and adolescents has not yet been established and no recommendation on a posology can be made. No dose change is recommended in patients with renal impairment, including those with end-stage renal disease. Method of administration: Pelgraz is for subcutaneous use. The injections should be given subcutaneously into the thigh, abdomen or upper arm. See SmPC for instructions on handling of the medicinal product before administration.
Contraindications: Hypersensitivity to peg lgrastim or any of the excipients in Pelgraz.

Warnings and precautions: To improve the traceability of biological medicinal products, the trade name of the administered product should be clearly recorded. The long-term e ects of peg lgrastim have not been established in acute myeloid leukaemia (AML); therefore, it should be used with caution in this patient population. Granulocytecolony stimulating factor (G-CSF) can promote growth of myeloid cells in vitro and similar e ects may be seen on some non-myeloid cells in vitro. The safety and e cacy of peg lgrastim have not been investigated in patients with myelodysplastic syndrome, chronic myelogenous leukaemia, and in patients with secondary AML; therefore, it should not be used in such patients. Particular care should be taken to distinguish the diagnosis of blast transformation of chronic myeloid leukaemia from AML. The safety and e cacy of peg lgrastim administration in de novo AML patients aged < 55 years with cytogenetics t(15;17) have not been established. The safety and e cacy of peg lgrastim have not been investigated in patients receiving high dose chemotherapy. This medicinal product should not be used to increase the dose of cytotoxic chemotherapy beyond established dose regimens. Pulmonary adverse reactions, in particular interstitial pneumonia, have been reported after G-CSF administration. Patients with a recent history of pulmonary in ltrates or pneumonia may be at higher risk. The onset of pulmonary signs such as cough, fever, and dyspnoea in association with radiological signs of pulmonary in ltrates, and deterioration in pulmonary function along with increased neutrophil count may be preliminary signs of adult respiratory distress syndrome (ARDS). In such circumstances peg lgrastim should be discontinued at the discretion of the physician and the appropriate treatment given. Glomerulonephritis has been reported in patients receiving lgrastim and peg lgrastim. Generally, glomerulonephritis resolved after dose reduction
or withdrawal of lgrastim and peg lgrastim. Urinalysis monitoring is recommended. Capillary leak syndrome has been reported after G-CSF administration and is characterised by hypotension, hypoalbuminaemia, oedema and haemoconcentration. Patients who develop symptoms of capillary leak syndrome should be closely monitored and receive standard symptomatic treatment, which may include a need for intensive care. Generally asymptomatic cases of splenomegaly and cases of splenic rupture, including some fatal cases, have been reported following administration of peg lgrastim. Spleen size should be carefully monitored (e.g. clinical examination, ultrasound). A diagnosis of splenic rupture should be considered in patients reporting left upper abdominal pain or shoulder tip pain. Treatment with peg lgrastim alone does not preclude thrombocytopenia and anaemia because full dose myelosuppressive chemotherapy is maintained on the prescribed schedule. Regular monitoring of platelet count and haematocrit is recommended. Special care should be taken when administering single or combination chemotherapeutic medicinal products which are known to cause severe thrombocytopenia. Peg lgrastim in conjunction with chemotherapy and/or radiotherapy has been associated with development of myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML) in breast and lung cancer patients. Patients treated in these settings should be monitored for signs and symptoms of MDS/AML. Sickle cell crises have been associated with the use of peg lgrastim in patients with sickle cell trait or sickle cell disease. Therefore, use caution when prescribing peg lgrastim in patients with sickle cell trait or sickle cell disease, monitor appropriate clinical parameters and laboratory status and be attentive to the possible association of this medicinal product with splenic enlargement and vasoocclusive crisis. White blood cell (WBC) counts of 100 × 109/L or greater have been observed in less than 1% of patients receiving peg lgrastim. No adverse reactions directly attributable to this degree of leukocytosis have been reported. Such elevation in WBCs is transient, typically seen 24 to 48 hours after administration and is consistent with the pharmacodynamic e ects of this medicinal product. Consistent with the clinical e ects and the potential for leukocytosis, a WBC count should be performed at regular intervals during therapy. If leukocyte counts exceed 50 × 109/L after the expected nadir, this medicinal product should be discontinued immediately. Hypersensitivity, including anaphylactic reactions, have been reported with peg lgrastim. Permanently discontinue peg lgrastim in patients with clinically signi cant hypersensitivity. Do not administer peg lgrastim to patients with a history of hypersensitivity to peg lgrastim or lgrastim. If a serious allergic reaction occurs, appropriate therapy should be administered, with close patient follow-up over several days. Stevens-Johnson syndrome (SJS), which can be life-threatening or fatal, has been reported rarely in association with peg lgrastim treatment. If the patient has developed SJS with the use of peg lgrastim, treatment must not be restarted at any time. As with all therapeutic proteins, there is a potential for immunogenicity. Rates of generation of antibodies against peg lgrastim is generally low. Binding antibodies do occur as expected with all biologics; however, they have not been associated with neutralising activity at present. Aortitis has been reported after lgrastim or peg lgrastim administration in healthy subjects and in cancer patients. The symptoms experienced included fever, abdominal pain, malaise, back pain and increased in ammatory markers (e.g. C-reactive protein and WBC count). In most cases aortitis was
diagnosed by CT scan and generally resolved after withdrawal of lgrastim or peg lgrastim. The safety and e cacy of Pelgraz for the mobilisation of blood progenitor cells in patients or healthy donors has not been adequately evaluated. Increased haematopoietic activity of the bone marrow in response to growth factor therapy has been associated with transient positive bone-imaging ndings. This should be considered when interpreting bone-imaging results. The additive e ect of concomitantly administered products containing sorbitol (or fructose) and dietary intake of sorbitol (or fructose) should be taken into account. Pelgraz contains less than 1 mmol sodium (23 mg) per 6 mg dose, that is to say essentially ‘sodium-free’. The needle cover contains dry natural rubber (a derivative of latex), which may cause allergic reactions. Pregnancy and Lactation: Peg lgrastim is not recommended during pregnancy and in women of childbearing potential not using contraception. A decision must be made whether to discontinue breastfeeding or to discontinue/abstain from peg lgrastim therapy taking into account the bene t of breastfeeding for the child and the bene t of therapy for the woman. Adverse Events include: Adverse events which could be considered serious include: Common: Thrombocytopenia. Uncommon: Myelodysplastic syndrome, acute myeloid leukaemia, sickle cell anaemia with crisis, capillary leak syndrome, glomerulonephritis, hypersensitivity reactions (including angioedema, dyspnoea, anaphylaxis), splenic rupture (including some fatal cases), Sweet’s syndrome (acute febrile neutrophilic dermatosis), pulmonary adverse reactions including interstitial pneumonia, pulmonary oedema and pulmonary brosis have been reported. Uncommonly cases have resulted in respiratory failure or ARDS which may be fatal. Rare: Aortitis, pulmonary haemorrhage, Stevens-Johnson syndrome. Other Very Common adverse events: Headache, nausea, bone pain. Other Common adverse events: Leukocytosis, musculoskeletal pain (myalgia, arthralgia, pain in extremity, back pain, musculoskeletal pain, neck pain), injection site pain, non-cardiac chest pain. See SmPC for details of other adverse events. Shelf Life: 3 years. Store in a refrigerator (2∞C – 8∞C). Pelgraz may be exposed to room temperature (not above 25°C ± 2°C) for a maximum single period of up to 72 hours. Pelgraz left at room temperature for more than 72 hours should be discarded. Do not freeze. Accidental exposure to freezing temperatures for a single period of less than 24 hours does not adversely a ect the stability of Pelgraz. Keep the container in the outer carton in order to protect from light. Pack Size: One pre lled syringe or pre lled syringe injector with one alcohol swab, in a blistered packaging.
Marketing Authorisation Numbers: Pre- lled syringe: EU/1/18/1313/001, Prelled injector: EU/1/18/1313/002. Marketing Authorisation Holder (MAH): Accord Healthcare S.L.U, World Trade Center, Moll de Barcelona, s/n, Edi ci Est, 6a planta, Barcelona, 08039 Spain. Legal Category: POM. Full prescribing information including the SmPC is available on request from Accord Healthcare Ireland Ltd, Euro House, Little Island, Co. Cork, Tel: 021-4619040 or www.accord-healthcare.ie/products Adverse reactions can be reported to Medical Information at Accord Healthcare Ltd. via E-mail: medinfo@accord-healthcare.com or Tel: +44(0)1271385257.





Date of Generation of API: May 2021. IE-01426





Adverse events should be reported. Reporting forms and





can be found on the HPRA website (www.hpra.ie),

by e-mailing medsafety@hpra.ie. Adverse events should also be




to
email;

or tel:0044 (0) 1271 385257









MEN’S HEALTH: PROSTATE CANCER

Stand Up for Their Prostate and Stand up for Yourself
In 2020 3,890 people were diagnosed with prostate cancer, making it the most common cancer in Ireland excluding nonmelanoma skin cancer.
Worldwide, more than 1 million men are diagnosed with prostate cancer each year and more than 300,000 die of the disease. Startling statistics show that 1 in 6 men will be diagnosed with prostate cancer during their lifetime. However, when detected early, prostate cancer has very promising five-year survival rates of 92%. But like many other forms of cancer, these survival rates are all dependent on when the cancer is detected, and we know that early detection saves lives and improves survival outcomes.
The Marie Keating, is encouraging men to be more open about their health and to speak to their GP about a PSA test when they turn 50, or 45 with a family history of prostate cancer.
Urology Nurse Specialist and Director of Nursing Services at the Marie Keating Foundation, Helen Forristal explains:
“Men typically get a bad rap for not speaking about their health, but when resources and supports are put in place, we see that this is not the case. Our Stand Up for Your Prostate campaign is designed to give men an opening to speak about their health with

others, and this can be exactly what some men need to go to their GP. Our message this year is a simple one, if you are 50, ask your GP to consider checking your PSA levels. If you are 45 with a family history of prostate or breast cancer in the family, have that conversation. It could save your life.”
A PSA or Prostate-Specific Antigen test is a simple blood test that monitors possible changes in your prostate which may need further investigations and follow up. By having regular PSA levels checked as instructed by your GP or Urologist, it can help to detect prostate problems or cancer in its earliest stages, even if you are experiencing no symptoms.
This year The Marie Keating Foundations Stand Up for your Prostate campaign was fronted by courageous campaign ambassador John Wall, a man living with Stage 4 Prostate cancer. He bravely shares his experiences in the hopes that it will drive others to stand up for their prostate, listen to the advice, go get checked and have conversations with others about prostate health. He tirelessly campaigns to support prostate cancer awareness and early detection. His powerful campaign message was to “Stand up for your prostate and stand up for yourself”.
Speaking on why he felt this campaign was so important John said “The Marie Keating Foundation has given me the opportunity to have an honest discussion about the impact of an advanced cancer diagnosis & how I now live a life that will inevitably be shortened as a result. By doing so I hope that others will not only benefit from my lived experience, but also potentially avoid such a devastating impact by simply recognising the warning signs & acting upon them.”
Prostate Cancer Facts:
• Prostate cancer is the most common male cancer in Ireland.
• Many men with early prostate cancer have no symptoms at all.
• Because you may not have symptoms, if you are a man over the age of 45 it is important to talk to your doctor about PSA testing.
• There has been a significant increase in the number of men diagnosed in recent years due to the increasing use of the PSA blood test.
• Prostate cancer responds well to treatment and, if detected early, it can be treated successfully. When prostate cancer is detected early, over 92% of men survive.
* If your father or brother has had prostate cancer, you are twice as likely to get prostate cancer at some point in your life.
This year’s Stand Up for Your Prostate campaign aimed to encourage men to take an active role in their health, and to speak to their GP if they have any concerns or worries about a change in their bodies.
Prostate Cancer’s early warning signs (note that there may not be any) can include:
• frequency passing urine
• getting up a night-time to go to the toilet
• blood in your urine or semen at any time that is otherwise unexplained.
For more information on prostate cancer, go to mariekeating.i.e
John Wall’s Story – Man living with Stage 4 Prostate Cancer
Helen Forristal, MSc (ANP) BSc (Professional Nursing) ENB 237 (Oncology Nursing), Director of Nursing Services at The Marie Keating Foundation

• pain on passing urine,
• difficulty passing urine,
• your flow has become weak or intermittent
At 50, John Wall is a father and husband living life with an advanced prostate cancer diagnosis. When John heard about the Marie Keating Foundation’s “Stand Up For Your Prostate” campaign and learned about how the campaign was encouraging the men of Ireland to be more open about their health and take the stigma out of prostate cancer, John wanted to share his story.
For 46 years of my life, I never thought that my health would ever be an issue. I was happily married with a loving family, a good job and a great future to look forward to.
Don’t get me wrong, I wasn’t exactly the fittest person in the world, but I had given up drinking and smoking many years ago and also had an annual medical at work each year, so I thought I was fine. That was me living blissfully unaware of what lay ahead.
I noticed during the early months of 2017 that I was having to pay frequent visits to the loo, especially at night. I never paid much attention to it. As awkward as it became, I thought it was just part of my middle-aged status. I did go to my GP twice but because of my age, nothing was considered other than an infection, so off I trotted with some antibiotics. I did
A combined
against

Abbreviated Prescribing Information - Vesomni 6 mg/0.4 mg modified release tablets. Please read the Summary of Product Characteristics (SPC) before prescribing. Presentation: Each tablet contains a layer of 6 mg solifenacin succinate, corresponding to 4.5 mg solifenacin free base and a layer of 0.4 mg tamsulosin hydrochloride, corresponding to 0.37 mg of tamsulosin free base. Indication: Treatment of moderate to severe storage symptoms (urgency, increased micturition frequency) and voiding symptoms associated with benign prostatic hyperplasia (BPH) in men who are not adequately responding to treatment with monotherapy. Posology and method of administration: Adultmales,includingolderpeople: One Vesomni tablet (6 mg/0.4 mg) once daily taken orally with or without food. The maximum daily dose is one Vesomni tablet. The tablet must be swallowed whole, intact without biting or chewing. Do not crush the tablet. Special populations (see also contraindications below): Renal impairment: Severe renal impairment (creatinine clearance ≤ 30 mL/min): Treat with caution, maximum daily dose in these patients is one Vesomni tablet.
Hepatic impairment: Moderate hepatic impairment (Child-Pugh score of 7-9): Treat with caution, maximum daily dose in these patients is one Vesomni tablet. In patients with severe hepatic impairment (Child-Pugh score > 9), the use of Vesomni is contraindicated. Concomitant treatment withmoderateandstronginhibitorsofCYP4503A4: e.g. verapamil, ketoconazole, ritonavir, nelfinavir, itraconazole: Treat with caution, maximum daily dose should be limited to one Vesomni tablet.
Paediatricpopulation:There is no relevant indication for use of Vesomni in children and adolescents.
Contraindications: Patients with hypersensitivy to the active substance(s) or to any of the excipients (see SPC). Patients undergoing haemodialysis. Patients with severe hepatic impairment. Patients with severe renal impairment who are also treated with a strong cytochrome P450 (CYP)3A4 inhibitor e.g. ketoconazole. Patients with moderate hepatic impairment who are also treated with a strong CYP3A4 inhibitor e.g. ketoconazole. Patients with severe gastrointestinal conditions (including toxic megacolon), myasthenia gravis or narrow-angle glaucoma and patients at risk for these conditions. Patients with a history of orthostatic hypotension. Special Warnings and Precautions for Use: Vesomni should be used with caution in patients with: severe renal impairment; risk of urinary retention; gastrointestinal obstructive disorders; risk of decreased gastrointestinal motility; hiatus hernia/ gastroesophageal reflux and/or who are concurrently taking medicinal products (such as bisphosphonates) that can cause or exacerbate oesophagitis; autonomic neuropathy. The patient should be examined in order to exclude the presence of other conditions, which can cause similar symptoms to benign prostatic hyperplasia. Other causes of frequent urination (heart failure or renal disease) should be assessed before treatment with Vesomni is initiated. If a urinary tract infection is present, appropriate antibacterial therapy should be started. QT prolongation and Torsade de Pointes have been observed in patients with risk factors, such as pre-existing long QT syndrome and hypokalaemia, who are treated with solifenacin succinate. Angioedema with airway obstruction has been reported in some patients on solifenacin succinate and tamsulosin. If angioedema occurs, Vesomni should be discontinued and not restarted. Appropriate therapy and/or measures should be taken. Anaphylactic reaction has been reported in some patients treated with solifenacin succinate. In patients who develop anaphylactic reactions, Vesomni should be discontinued and appropriate therapy and/or measures should be taken. As with other alpha1-adrenoceptor antagonists, a reduction in blood pressure can occur in individual cases during treatment with tamsulosin, as a result of which, rarely, syncope can occur. Patients starting treatment with Vesomni should be cautioned to sit or lie down at the first signs of orthostatic hypotension (dizziness, weakness) until the symptoms have disappeared. The ‘Intraoperative Floppy Iris Syndrome’ (IFIS, a variant of small pupil syndrome) has been observed during cataract and glaucoma surgery in some patients on or previously treated with tamsulosin hydrochloride. IFIS may increase the risk of eye complications during and after the operation. Therefore, the initiation of therapy with Vesomni in patients for whom cataract or glaucoma surgery is scheduled is not recommended. Discontinuing treatment with Vesomni 1-2 weeks prior to cataract or glaucoma surgery is anecdotally considered helpful, but the benefit of treatment discontinuation has not been established. During pre-operative assessment, surgeons and ophthalmic teams should consider whether patients scheduled for cataract or glaucoma surgery are being or have been treated with Vesomni in order to ensure that appropriate measures will be in place to manage IFIS during surgery. Vesomni should be used with caution in combination with moderate and strong inhibitors of CYP3A4 and it should not be used in combination with strong inhibitors of CYP3A4, e.g., ketoconazole, in patients who are of the CYP2D6 poor metaboliser phenotype or who are using strong inhibitors of CYP2D6, e.g., paroxetine.
Interactions: Pharmacological interactions: Concomitant medication with other anticholinergic medicinal products may result in more pronounced therapeutic and undesirable effects. Allow approximately one week after stopping treatment with Vesomni before commencing any anticholinergic therapy. The therapeutic effect of solifenacin may be reduced by concomitant administration of cholinergic receptor agonists. Pharmacokinetic interactions: Pharmacokinetic interactions involving the potential for other medicinal products to affect Vesomni exposures: InteractionswithCYP3A4andCYP2D6inhibitors:See Contraindications, Posology and administration and Special warnings and precautions above. Concomitant administration may lead to increased exposure to both solifenacin (ketoconazole 400 mg/day resulted in a 1.5-fold increase in Cmax and a 2.8-fold increase in AUC). and tamsulosin (ketoconazole 400 mg/day resulted in a 2.2-fold increase in Cmax and a 2.8-fold increase in AUC). Vesomni should be used with caution in combination with strong CYP3A4 inhibitors. Vesomni should not be given together with strong CYP3A4 inhibitors in patients who are also CYP2D6 poor metabolizer phenotype or who are using strong CYP2D6 inhibitors. See SPC for details of the effects of other CYP3A4 and CYP2D6 inhibitors. Inducers: Inducers of CYP3A4 (e.g. rifampicin) may decrease the plasma concentrations of solifenacin and tamsulosin. Information available for the individual active substances Solifenacin can reduce the effect of medicinal products that stimulate the motility of the gastrointestinal tract, such as metoclopramide and cisapride. Solifenacin did not affect the pharmacokinetics of digoxin, or the pharmacokinetics or effect on prothrombin time of R- or S-warfarin. Co-administration of tamsulosin and other alpha1-adrenoreceptor antagonists could lead to hypotensive effects. Diclofenac and warfarin may increase the elimination rate of tamsulosin. No interactions have been seen when tamsulosin was given concurrently with atenolol, enalapril or theophylline. Fertility, pregnancy and lactation: The effect of Vesomni on fertility has not been established. Ejaculation disorders have been observed in short and long term clinical studies with tamsulosin. Events of ejaculation disorder, retrograde ejaculation and ejaculation failure have been reported in the post authorization phase. Vesomni is not indicated for use in women. Driving and use of machines: No studies have been performed, however patients should be informed about the possible occurrence of dizziness, blurred vision, fatigue and uncommonly somnolence, which may negatively affect the ability to drive or use machines. Undesirable Effects: Summary of the safety profile: Vesomni may cause anticholinergic undesirable effects of, in general, mild to moderate severity. The most frequently reported adverse reactions during the clinical studies performed for the development of Vesomni were dry mouth (9.5%), followed by constipation (3.2%) and dyspepsia (including abdominal pain; 2.4%). Other common undesirable effects are dizziness (including vertigo; 1.4%), vision blurred (1.2%), fatigue (1.2%), and ejaculation disorder (including retrograde ejaculation; 1.5%). Acute urinary retention (0.3%, uncommon) is the most serious adverse drug reaction that has been observed during treatment with Vesomni in clinical studies. List of adverse reactions: the ‘Vesomni frequency’ below reflects adverse drug reactions that have been observed during the double-blind clinical studies performed for the development of Vesomni (based on reports of treatment-related adverse events, which have been reported by at least two patients and occurred with a frequency higher than for placebo in the double-blind studies). The ‘solifenacin frequency’ and ‘tamsulosin frequency’ below reflect adverse drug reactions (ADRs) previously reported with one of the individual components (as presented in the Summary of Product Characteristics (SmPCs) of solifenacin 5 and 10 mg and tamsulosin 0.4 mg respectively that may also occur when receiving Vesomni (some of these have not been observed during the clinical development program of Vesomni). The frequency of adverse reactions is defined as follows: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data). The adverse events are grouped by MedDRA system organ class preferred term (PT). Vesomni frequency: Nervous system disorders: Common: dizziness Eye disorders: Common: vision blurred Gastrointestinal disorders: Common: dry mouth, dyspepsia, constipation Skin and subcutaneous tissue disorders: Uncommon: pruritus Renal and urinary disorders: Uncommon: Urinary retention*** Reproductive system and breast disorders: Common: ejaculation disorders including retrograde ejaculation and ejaculation failure General disorders and administration site conditions: Common: fatigue Solifenacin5mg&10mgfrequency#: Infections and infestations: Uncommon: urinary tract infection, cystitis Immune system disorders: Not known: anaphylactic reaction* Metabolism and nutrition disorders: Not known: decreased appetite*, hyperkalemia* Psychiatric disorders: Very rare: hallucination*, confusional state* Not known:
dermatitis* Musculoskeletal and connective tissue disorders: Not known: muscular weakness* Renal and urinary disorders: Uncommon: difficulty in micturition Rare: urinary retention***Not known: renal impairment* General disorders and administration site conditions: Uncommon: fatigue, perhiperal oedema Tamsulosin 0.4mg frequency#: Nervous system disorders: Common: dizziness Uncommon: headache Rare: syncope Eye disorders: Not known: vision blurred*, Intraoperative Floppy Iris Syndrome (IFIS)**, visual impairment* Cardiac disorders: Uncommon: palpitations Not known: atrial fibrillation*, arrhythmia*, tachycardia* Vascular disorders: Uncommon: orthostatic hypotension Respiratory, thoracic and mediastinal disorders: Uncommon: rhinitis Not known: dyspnoea*, epistaxis* Gastrointestinal disorders: Uncommon: constipation, nausea, diarrhea, vomiting Skin and subcutaneous tissue disorders: Uncommon: pruritus, rash, urticaria Rare: angioedema Very Rare: Stevens-Johnson syndrome Not known: erythema multiforme*, exfoliative dermatitis* Reproductive system and breast disorders: Common: ejaculation disorders including retrograde ejaculation and ejaculation failure Veryrare: priapism General disorders and administration site conditions: Uncommon: asthenia. #: The ADRs from solifenacin and tamsulosin included are the ADRs listed in the summary of product characteristics of both products. *: from post-marketing reporting. Because these spontaneously reported events are from the worldwide post-marketing experience, the frequency of events and the role of solifenacin or tamsulosin and their causation cannot be reliably determined. **: from post-marketing reporting, observed during cataract and glaucoma surgery. ***: see Special warnings and precautions for use. Long-term safety of Vesomni: The profile of undesirable effects seen with treatment up to 1 year was similar to that observed in the 12-week studies. The product is well-tolerated and no specific adverse reactions have been associated with long-term use. Description of selected adverse reactions: For urinary retention see Special warnings and precautions for use. Older people: The therapeutic indication of Vesomni, moderate to severe storage symptoms (urgency, increased micturition frequency) and voiding symptoms associated with BPH, is a disease affecting elderly men. The clinical development of Vesomni has been performed in patients 45 to 91 years of age, with an average age of 65 years. Adverse reactions in the elderly population were similar to the younger population. Reporting of suspected adverse reactions: see below. Overdose: Overdosage with the combination of solifenacin and tamsulosin can potentially result in severe anticholinergic effects plus acute hypotension. Refer to SPC for details of treatment of overdose. Legal Category: Prescription Only Medicine (SIB). Nature and contents of container: Aluminium blister packs containing 30 tablets. Product Authorisation Number: PA1241/016/001. Marketing Authorisation holder: Astellas Pharma Co. Ltd. Further information is available from: Astellas Pharma Co. Ltd, 5 Waterside, Citywest Business Campus, Naas Road, Dublin 24. Phone: +3531 467 1555. Summary of Product Characteristics with full prescribing information available upon request. Job number: VESOM_2019_0001_IE Date of preparation of API: 24 May 2019
Reporting
reactions after authorisation of the medicinal product is important.
monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals
allows
Pharmacovigilance Astellas
www.hpra.ie
medsafety@hpra.ie.
via:
Co. Ltd
Irishdrugsafety@astellas.com
have my PSA (Prostate-Specific Antigen) checked as part of a blood test and the results were off the charts but I was not a candidate for Prostate Cancer. I didn’t fit the age profile. I was too young. Oh, the benefit of hindsight!
In July 2017 I began to experience slight pains in my right leg. Initially didn’t think much of it, but it got to the point where even whilst sitting, I had difficulty lifting the leg. I’m was never one to head straight to my GP on a whim, but my instinct told me this time was an exception. That evening, I was referred for a scan that would change our lives more than we could ever have imagined. A few hours after talking with my GP, my urologist Syed Jaffry, (my new best friend) was asking me the dreaded question, “Do you have anyone else with you?” Without Syed’s advice, I may not be writing this right now.
My scan showed an enlarged prostate, along with a significant enlargement of my lymph nodes. Trouble with a capital “T” lay ahead. After several days of poking, prodding and discussions using words that we couldn’t even spell, our worst fears were confirmed.
The diagnosis was Stage 4 Prostate Cancer with advanced secondaries in my lymph nodes. There was a lot of gasps not only from my wife and I but also my
primary care team. I was only 46 years old. Surely not?
What next? We wanted second opinions, so we got several!
Travelled to Dublin, Cork, Limerick and Galway. All agreed that chemotherapy was the first line of defence but only one agreed on what should happen next. My new best friend, Mr Jaffry advised that I would have no option but to travel abroad for radical surgery which would be dependent on the success of the chemotherapy. All other advice basically centred around prolonging my life so that I could get my affairs in order. Needless to say we chose the former!
Fortunately, after several rounds of Chemotherapy, we made arrangements to travel to University Hospital Leuven, a European Centre of Urology Excellence to meet Dr Steven Joniau, my surgeon. That was in February 2018. After a thorough consultation, he told us to come back the following month for surgery. On March 15th I had a radical open prostatectomy where along with my beloved prostate, 61 of my lymph nodes were also removed. My wife Deirdre stayed with me throughout and looking back, I wouldn’t have pulled through without her support. It was major surgery that undoubtedly has significantly prolonged my life. After 2 weeks we travelled back
MEN’S HEALTH: PROSTATE CANCER


and since that trip, we’ve never looked back.
We’re not done yet! After a trip to Lourdes in September 2018, I had 39 cycles of ARC Radiation in The Galway Clinic. It must be said that the advice elsewhere was not to proceed with this, as it would involve radiating a very large area but upon the advice of my surgeon we proceeded. Sure, what could go wrong? Well, let me tell you, absolutely nothing. I feel better today than I’ve ever felt in my life.
Today the only treatment I’m on is called ADT (Androgen Deprivation Therapy). It suppresses my male hormones, induces the menopause and makes me sweat whether I’m in a freezer or a furnace. But hey, I’m alive and living a good life. That’ll do me.
During all of this, I met the amazing Laura Brennan whom I was incredibly fortunate to become good mates with, and without her advice and friendship, I really would doubt that I’d be living with such positivity. She has practically single handily changed the attitudes of so many regarding the HPV Vaccine and inspired so many others to make the most of what you have while you have it. As Laura helped others and raised awareness, I am trying in my own way to do what I can, while I can.
Recently I’ve reached out to other men on Twitter to offer advice based on my experiences. Let me tell you, I was amazed at the
response. We the men of Ireland need to talk, talk to each other, support each other. I’ve come across some amazing people that all going well, will be meeting after the summer. A group of us have supported each other online and are planning a little get together later in the year which I am looking forward to immensely.
We have no idea what the future holds except that my illness is not curable, but at least for the time being, is responding well to treatment. I raise awareness surrounding vaccines and prostate cancer through social media, primarily Twitter and also through radio and newspaper interviews. I find it’s incredibly therapeutic to talk. Helping others helps me.
This episode of my life has taught me the real meaning of life. Live every day, enjoy every day. Cherish those close to you and let them cherish you.
My family and I have been incredibly fortunate that I have an employer as understanding as The IAA, without whose assistance my quality of life would be remarkably different. Not for a moment do we take for granted all that has and is being done for us. We simply cannot thank you all enough for your support.
Finally, especially to my family, my friends and work colleagues, Thank You. A hundred thousand thank you’s.”

































Performance measures for colonoscopy in inflammatory bowel disease patients: European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative

Dekker, Evelien; Nass, Karlijn J.; Iacucci, Marietta; Murino, Alberto; Sabino, João; Bugajski, Marek; Carretero, Cristina; Cortas, George; Despott, Edward J.; East, James E.; Kaminski, Michal F.; Karstensen, John Gásdal; Keuchel, Martin; Löwenberg, Mark; Monged, Ashraf; Nardone, Olga M.; Neumann, Helmut; Omar, Mahmoud M.; Pellisé, Maria; Peyrin-Biroulet, Laurent; Rutter, Matthew D.; Bisschops, Raf
for a different indication, which then raises the suspicion of IBD. Surveillance colonoscopy is recommended in longstanding IBD patients (8 years after disease onset).10 In each clinical category, performance measures were defined for the following three quality domains: preprocedure, completeness of the procedure, and identification of pathology. One or two performance measures were defined per domain.
Performance measures for colonoscopy in IBD patients
The European Society of Gastrointestinal Endoscopy (ESGE) and United European Gastroenterology (UEG) have identified monitoring and evaluation of the quality of endoscopy as a major priority.1 To this aim, the ESGE and UEG have developed several performance measures for different types and aspects of gastrointestinal (GI) endoscopy over the past few years.2, 3, 4, 5, 6 Current performance measures for colonoscopy have mainly focused on optimal detection of (pre)malignant lesions.4 However, the detection of (pre)malignant lesions is not the primary aim in colonoscopies performed in patients with a clinical suspicion of inflammatory bowel disease (IBD), nor when assessing endoscopic disease activity in known IBD patients. To date, no endoscopy performance measures have been identified for IBD patients. Furthermore, the current performance measures for colonoscopy do not include surveillance of longstanding IBD patients. Although several
recommendations have been published for surveillance colonoscopy in IBD patients,7,8,9 these recommendations are numerous and not consistently measurable in community endoscopy practices.
The aim of the IBD taskforce within the colonoscopy working group of the ESGE Quality Improvement Committee was to identify performance measures for colonoscopy in IBD patients that are widely applicable to endoscopy services throughout Europe and other interested countries. These performance measures would ideally meet the following criteria: have a proven impact on clinical outcomes; be well-defined, reliable, simple, and user-friendly; provide an opportunity for improvement; and be widely applicable to all levels of endoscopy services.
This paper reports the consensus-based list of key performance measures for colonoscopy in IBD patients and describes the methodological
process applied in the development of these measures. Performance measures are divided into key performance measures and minor performance measures.
Methodology
The multistep procedure to develop performance measures has been previously described.1 In short, a modified Delphi consensus process was used to develop performance measures for colonoscopy in IBD patients. These performance measures were categorized into performance measures for three clinical settings: clinical suspicion of IBD, endoscopic assessment of disease activity in known IBD patients, and surveillance. Clinical suspicion of IBD can be defined as: either a clinical suspicion of IBD prior to colonoscopy (i. e. symptoms of diarrhea, iron deficiency anemia, or raised biomarkers), which may be confirmed by endoscopic signs of inflammation; or the finding of signs suggestive of IBD during a colonoscopy initially performed
The input from the working group members and the evidence derived from the literature search resulted in a total of 16 statements and 11 potential performance measures that were considered relevant for IBD colonoscopies (see Supporting information). The working group members considered several other performance measures, such as measures on patient tolerance, sedation, standard terminology, and complications; however, the working group members agreed that these performance measures were not essential to assure high quality colonoscopy explicitly for IBD patients. Therefore, general colonoscopy recommendations and standards for these measures should be considered for IBD colonoscopy.4
The statements and performance measures were categorized into three clinical categories and six domains. To minimize overlap between the different categories, some statements and potential performance measures were combined into a “general IBD colonoscopy” category after the first voting round. After three voting rounds, a total of 15 statements, eight key performance measures, and one minor performance measure were accepted ([Fig. 1]). The process of the development of these statements and performance measures can be reviewed in the Supporting information. The performance measures are presented below using the descriptive framework proposed
by the Quality Improvement Committee and a short summary of the available literature.1 The performance measures are listed according to the clinical categories and domains to which they were attributed.
General IBD colonoscopy: preprocedure
The acceptance of this performance measure is based on agreement with the following statement:
• For colonoscopies performed in IBD patients, the endoscopy report should explicitly include the indication for the procedure: i. e. clinical suspicion of IBD, endoscopic assessment of disease activity, or surveillance.
Agreement: 100 %
Inappropriate referral for colonoscopy might lead to the misuse of limited endoscopic resources, an increase in potential harm to patients from unnecessary invasive procedures, and an increase in healthcare costs.
In general, colonoscopies with an appropriate indication are
associated with significantly higher diagnostic yields for relevant lesions than colonoscopies without an appropriate indication.4 There is also literature that supports these findings specifically for IBD colonoscopies. The diagnostic yield for IBDrelated lesions is significantly higher in colonoscopies with an appropriate indication compared with colonoscopies without an appropriate indication.12, 13 The proposed minimum standard rate for reporting of the indication for colonoscopy (≥ 95 %) was set because this is a prerequisite for the monitoring and evaluation of explicit performance measures in each clinical category for IBD patients.
The acceptance of this performance measure is based on agreement with the following statements:
• For colonoscopies performed in IBD patients, the endoscopy report should include the adequacy of bowel preparation using a validated score.
Agreement: 100 %
• Adequate bowel preparation should be obtained in 90 % of the colonoscopies performed in IBD patients. Agreement: 95 % Inadequate bowel preparation has a detrimental effect on all quality aspects of colonoscopy.14 Adequate bowel preparation in IBD patients is essential for disease assessment and for the detection of dysplasia during colonoscopy.14 A successful surveillance colonoscopy requires adequate bowel preparation to detect any nonpolypoid flat lesions hidden by debris and stool.15 A recent study has shown that inadequate bowel preparation and active colonic inflammation were the most frequent factors resulting in unsuccessful chromoendoscopy in surveillance colonoscopies in IBD patients.16
The quality of bowel preparation should be assessed with a validated scale, as has also been recommended by the ESGE for general colonoscopy.4 Three scales have been comprehensively validated: the Boston Bowel Preparation Scale (BBPS), the
Ottawa Scale, and the Aronchick Scale. Adequate bowel preparation may be defined as: BBPS ≥ 6; Ottawa Scale ≤ 7; or Aronchick Scale excellent, good, or fair.4
The proposed minimum standard of adequate bowel preparation for colonoscopy in IBD patients (≥ 90 %) was adopted from the ESGE guideline on performance measures for lower GI endoscopy,4 as no evidence was found to support adjusted standards for the subpopulation of IBD patients. Few data explored an association between IBD disease activity and the quality of bowel preparation. Hence, there is no definitive proof that patients with IBD have an increased likelihood of inadequate bowel preparation. In a retrospective analysis of 348 colonoscopies from 169 consecutively enrolled IBD patients, no differences were found in the quality of bowel preparation between patients with active disease and those with mucosal healing, suggesting that the efficacy of bowel preparation is not influenced by disease inflammation.17
General IBD colonoscopy: completeness of procedure and identification of pathology
The acceptance of this performance measure is based on agreement with the following statements:
• When colonoscopies are performed because of endoscopic suspicion of IBD or for endoscopic assessment of disease activity in IBD patients, at least one image should be recorded per segment.
Agreement: 89 %
• For surveillance colonoscopies in longstanding IBD patients, at least one annotated image should be recorded for every lesion biopsied or resected.
Agreement: 95 %
Photodocumentation of endoscopic landmarks or lesions during colonoscopy is embedded in several quality recommendations for GI endoscopy.4, 5 It allows continuous monitoring for quality purposes and it should be considered to be as important as text descriptions for endoscopic findings.18 Despite the lack of supporting evidence, the working group members agreed that photodocumentation supports quality control in colonoscopy in IBD patients.
Photodocumentation of each inspected segment (i. e. ileum, cecum, ascending, transverse, descending, and sigmoid colon, and rectum) could support optimal diagnosis, assessment of disease activity, and the assessment of future changes in IBD patients, as low interobserver agreement exists regarding endoscopic assessment of disease activity.19, 20
Annotated photodocumentation of every lesion (biopsied or resected) facilitates accurate interpretation, assists with onward referral, and enables direct comparison if subsequent follow-up procedures are required. The working group members agreed on the definition of annotation, meaning anything that indicates where the picture is taken. Annotation should be interpreted in its most simple form, for example it could be written on the pictures or simply described in the endoscopy report. A minimum standard of 90 % is recommended for adequate photodocumentation in colonoscopy in IBD patients.
When endoscopic software and endoscopy reporting systems support videodocumentation during colonoscopy, this might be superior to photodocumentation in certain situations.21 However, videodocumentation is not yet widely available and not
always easy to incorporate in the endoscopy report. Where videodocumentation is used, annotation by marking the colon segments is recommended to support the interpretation of the videos afterward.
Clinical suspicion of IBD: completeness of procedure
The acceptance of this performance measure is based on agreement with the following statement:
• The terminal ileum should be reached in colonoscopies in patients with suspected IBD.
Agreement: 95 %
Ileal intubation is essential for identifying ileal Crohn’s disease.22 Most studies support that ileoscopy increases the diagnostic yield when evaluating suspected IBD.23, 24, 25, 26 Reported rates for ileal intubation in colonoscopies in patients with diarrhea have varied widely from 46 % to 96 %.24, 25, 26 There is a scarcity of data regarding the preferred depth of ileal intubation and patient discomfort with ileal intubation in correlation with the sedation used. Furthermore, the existing guidelines do not comment on this subject.27, 28 Despite the absence of concrete supporting evidence, the members of this working group recommend that endoscopists should aim to achieve terminal ileal intubation in suspected IBD patients (minimum standard: ≥ 80 %; target standard ≥ 90 %).
Clinical suspicion of IBD: identification of pathology
The acceptance of this performance measure is based on agreement with the following statements:
• Adequate biopsies should be taken in patients with a clinical suspicion of IBD, as these are essential for correct diagnosis.
Agreement: 89 %
• Adequate biopsies in patients with endoscopic suspicion of IBD should include two biopsies from each of the ileum, cecum, ascending colon, transverse colon, descending colon, sigmoid, and rectum, including affected and macroscopically normal (if present) mucosa.
Agreement: 95 %
• Adequate biopsies in patients with clinically suspected IBD and endoscopically normal mucosa should include at least two biopsies from the terminal ileum in a separate vial.
Agreement: 84 %
• Adequate biopsies in patients with suspected Crohn’s disease
should include biopsies taken from the largest ulcers. Agreement: 95 %
For the clinical category “Endoscopic assessment of disease activity in known IBD,” the working group members reached consensus on the following statement:
• Adequate biopsies to assess disease activity in ulcerative colitis (UC) patients with endoscopic inflammation, should include at least two biopsies from the most affected area. Agreement: 100 %
According to clinical practice, evidence from the literature, and statements in relevant guidelines, ileocolonoscopy with histology is the fundamental basis for diagnosing IBD.27, 28,29 Histology plays a pivotal role in the differentiation between Crohn’s disease and UC. Within this context, the distribution and extent of histological pathology can further aid in the differential diagnosis of IBD. This requires a sufficient number of biopsies that are collected separately from the ileum, all colonic segments, and the rectum, as well from endoscopically affected areas and macroscopically normal areas.30 Providing the pathologist with endoscopic and clinical information further aids in establishing a diagnosis.30 Biopsies are also crucial for differentiating IBD from other diseases, such as intestinal tuberculosis, amebiasis, amyloidosis, and strongyloidiasis.31, 32, 33, 34, 35
The added value of terminal ileal biopsies in patients with clinically suspected IBD and endoscopically normal mucosa was supported by the literature.36 Baker et al. reported, in a retrospective analysis, that histological inflammation in biopsies of endoscopically normal terminal ileum was significantly associated with the development of Crohn’s disease during a mean follow-up of 6 years compared with the finding of normal histology. Furthermore, no real disadvantages for biopsies in the terminal ileum exist when there is a clinical suspicion of IBD. Therefore, terminal ileal biopsies were recommended to histologically confirm a normal ileum and prevent a patient undergoing a second colonoscopy to exclude IBD in the future.
In active Crohn’s disease, histological disease activity scores, proinflammatory gene expression levels, and numbers of myeloperoxidase-positive cells were significantly higher in biopsies from the ulcer edge in the
colon and ileum, with decreasing gradients observed with distance from the ulcer edge.37
In an endoscopically completely normal colon, biopsies are also important to rule out microscopic colitis. Here, ESGE recommends two biopsies from the left colon and two biopsies from the right colon, placed in separate containers and labelled as such.30 This is supported by the finding of lymphocytic and collagenous colitis presenting histologically as pancolitis, excluding the rectum.38
The recently published ESGE guideline on tissue sampling in the lower GI tract recommends biopsies in UC patients to evaluate disease activity.30 A minimum of two biopsies from the worst affected area or the most representative area of mucosal healing, preferably at the edge of any ulcers was recommended. The worst affected area might include an ulcerated anastomosis, where biopsies might differentiate between an IBD-associated ulcer or an ischemic lesion. Histological assessment of biopsies can be used to assess disease activity, the presence of cytomegalovirus, or histological healing, and to optimize therapy by either escalation or exit strategies, predict long-term adverse outcome, and manage patients to achieve treatment targets.30
Although data on actual adequate biopsies rates are lacking, based on available evidence and expert opinion, a minimum standard of ≥ 80 % was considered appropriate by the working group members.
Endoscopic assessment of disease activity in known IBD: identification of pathology
The acceptance of this performance measure is based on agreement with the following statement:
• An endoscopic activity score should be used for the assessment of disease activity in ulcerative colitis, the endoscopy report should explicitly include the score used. Agreement: 100 %
Accurate assessment of disease activity and disease extent in patients with IBD is of paramount importance for planning and tailoring treatment strategies.39
The use of endoscopic disease activity indices to evaluate the prognosis and efficacy of medical treatment in UC patients has been recommended by international guidelines.39 There are insufficient data to set the minimum and target standards reliably, but the proposed values for the use of an endoscopic activity score for the
assessment of disease activity in UC patients of ≥ 90 % and ≥ 95 %, respectively, seem achievable.
Nineteen different endoscopic scoring indices have been partially validated.40 Among these, the most commonly used are the Mayo Endoscopic score (MES) and the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Both have been validated for reliability, construct validity, and responsiveness.19, 41, 42, 43, 44 The operating properties of both scores are comparable. However, because the MES is easier to use, it remains the outcome of choice for clinical trials and daily practice.43 Electronic chromoendoscopybased scores, such as the Paddington International Virtual Chromoendoscopy Score (PICaSSO), require more real-life, treatment-related studies for their full establishment in both daily practice and clinical trials.45
Endoscopic activity scores for Crohn’s disease are more complex to use; hence their broad implementation into routine clinical practice might be difficult.39 Therefore, the working group members agreed not to include activity scores for Crohn’s disease in the performance measure and statements. Nevertheless, whenever feasible, the working group members recommend using the Simple Endoscopic Score for Crohn’s Disease (SES-CD) to assess disease activity in Crohn’s disease.46
Surveillance: identification of pathology
The acceptance of this performance measure is based on agreement with the following statement:
• High definition endoscopy should be used for surveillance in longstanding colitis.
Agreement: 100 %
Patients with longstanding IBD are at increased risk of developing colorectal cancer, with an estimated risk of approximately 18 % after 30 years with the diagnosis.47,48 Consequently, patients are recommended to undergo screening colonoscopy with the aim of detecting premalignant dysplastic lesions.8, 28 The use of high definition endoscopy is strongly recommended in current guidelines for surveillance in longstanding IBD patients.8, 27, 28, 29 High definition endoscopy significantly improves the detection of dysplastic lesions in surveillance colonoscopy in IBD patients compared with standard definition endoscopy.49 The improved visualization of the mucosa enables detection of most dysplastic lesions.50, 51 This
improved visualization, combined with a lack of adverse effects when using high definition endoscopy, resulted in a proposed minimum standard of ≥ 90 % and target standard of ≥ 95 % for the use of high definition endoscopy in longstanding IBD patients.
The acceptance of this performance measure is based on agreement with the following statement:
• Dye-based or virtual chromoendoscopy in combination with targeted biopsies should be used in surveillance colonoscopy in longstanding IBD patients.
Agreement: 95 %
The routine use of dye-based pancolonic chromoendoscopy or virtual chromoendoscopy with targeted biopsies for neoplasia surveillance in patients with longstanding colitis, in the situation of quiescent disease activity and adequate bowel preparation, has already been recommended by the ESGE Guideline on advanced imaging for detection and differentiation of colorectal neoplasia.52 Virtual chromoendoscopy has emerged as an attractive alternative to overcome the laboriousness of dye-based chromoendoscopy. The current evidence showed no significant difference between the two techniques for dysplasia detection.53, 54, 55
Numerous academic studies, predominantly at tertiary centers, have demonstrated the low yield of nontargeted biopsies for dysplasia detection.56, 57, 58, 59 In addition, nontargeted random biopsies cause a significant workload for both endoscopists and pathologists. The value of continuing four-quadrant biopsies, both in terms of effort and cost, has been questioned as their yield is so low compared with targeted approaches, on the basis of both dysplasia detected per patient and dysplasia detected per sample. However, the literature supports that, for certain high risk subsets of IBD patients (i. e. primary sclerosing cholangitis), four-quadrant or random biopsies may still have a role.60, 61 Therefore, when using chromoendoscopy for IBD surveillance, the use of targeted biopsies only is recommended as an easily measurable quality indicator.
A minimum standard of ≥ 70 % may seem relatively low. However, it allows a different strategy to be followed in a selected number of colonoscopies. For example, in high risk patients with a family history of colonic neoplasia, a tubular-appearing colon, or
primary sclerosing cholangitis, where endoscopists may opt to take random biopsies in addition to targeted biopsies, as suggested in the ESGE tissue sampling guideline for the lower GI tract.30
Although no significant learning curve was observed for the use of chromoendoscopy,62 the working group members agreed that endoscopists should be adequately trained according to the recently published ESGE curriculum.63
The acceptance of this performance measure is based on agreement with the following statement:
• The detection rate of neoplastic lesions in surveillance colonoscopies in longstanding IBD patients should be more than 8 %. Agreement: 89 %
Current surveillance strategies in IBD patients aim to identify dysplasia and prevent progression to CRC. Interval cancers are significantly more frequent in IBD patients compared with non-IBD patients and are most likely due to undetected or incompletely resected dysplastic lesions.8, 64,65 While the correlation between the adenoma detection rate and the risk of developing interval cancers is solid in a screening population,66, 67 it is still debatable in IBD. Nevertheless, applying a neoplasia detection rate as a performance measure for surveillance colonoscopy in IBD patients seems reasonable.
The neoplasia detection rate has already been incorporated into the ESGE curriculum for optical diagnosis.63 In the literature, neoplasia detection rates vary between 10 % and 26 % in surveillance colonoscopies in longstanding IBD patients.53, 62,68
Current literature on neoplasia detection rates in longstanding IBD patients comes mainly from academic services and it can be assumed that there will likely be differences in the prevalence of dysplasia and treatment preferences between countries.69, 70 Furthermore, owing to improved treatment of IBD, the prevalence of neoplasia might also fall and, with frequent surveillance, it seems unlikely that many dysplastic lesions will be found in longstanding IBD patients. Therefore, the working group members considered a minimum standard of ≥ 8 % achievable for the neoplasia detection rate in surveillance colonoscopies in longstanding IBD patients. In addition, because of the uncertainty of the prevalence and incidence in a nontertiary setting, this quality
indicator was qualified as a minor performance measure.
Conclusions
This paper describes the key performance measures for colonoscopy in IBD patients. These measures were supported by the available evidence where possible or based on an expert consensus between the working group members and were regarded as feasible to measure in endoscopy services throughout Europe and other interested countries. As there is limited evidence to support performance measures for all clinical categories for colonoscopy in IBD patients, most evidence was graded as moderate or low quality. This generated future research priorities, primarily to audit the proposed performance measures and to evaluate if these proposed measures do actually improve the care of IBD patients.
Similarly to the previously published ESGE quality improvement initiatives, the first step should be to implement these key performance measures for colonoscopy in IBD patients in endoscopy services throughout Europe and other interested countries. The ESGE recently published recommendations to overcome barriers in dissemination and implementation of quality measures for GI endoscopy.71 The dissemination and implementation of performance measures are important to identify services and endoscopists with substandard levels of performance. Furthermore, the ESGE recommendations on endoscopy reporting systems will support endoscopy services to facilitate quality monitoring in daily practice.72 Adequate quality monitoring will enable the principle of audit and feedback; this principle has been proven to improve the quality of care.73
Financial or logistical issues may cause barriers for optimal implementation of quality control systems. However, in an era where hospital accreditation is becoming increasingly important, hospital administrations are expected to be more inclined to support the need for such developments. Furthermore, investments in hardware will support endoscopy services in broad quality assessment for all types of endoscopy. Moreover, we should overcome financial, individual, or logistical barriers to aim for the highest possible quality in our endoscopy services to ensure the best possible outcomes for our patients.
References available on request
Obesity as a Disease
Therese Coleman
Obesity is recognised as a disease that requires treatment by World Obesity, the American Medical Association, Obesity Canada and the European Association for the Study of Obesity.
Overweight and obesity are defined as abnormal or excessive fat accumulation that presents a risk to health. A body mass index (BMI) over 25 kg/m2 is considered overweight, and over 30 kg/ m2 is diagnostic of obesity. The prevalence of overweight and obesity is increasing globally with an estimated 1.9 billion adults considered overweight and 650 million people living with obesity. Ireland has one of the highest levels of obesity in Europe, with 60% of adults, and more than 20% of children and young people living with overweight and obesity.
The causes of obesity are complex and multifactorial, but include biological pre-disposition (including genetic susceptibility) and environmental factors. The biological pre-disposition to obesity is highly inheritable; up to 70-80% of our BMI is determined by our genes. For people with a genetic predisposition to obesity, excess energy from food leads to an accumulation of fat in fat cells. The distribution of fat as visceral fat, cardiac fat and fat in muscles may occur as fat cells reach their maximal storage capacity. These enlarging fat
cells modify core metabolic and inflammatory processes which can in turn lead to a variety of other metabolic disturbances and diseases including dyslipidaemia, hypertension and diabetes.
For those that have obesity, the physiological basis for their disease is well described. Most individuals with obesity have impaired metabolic pathways that result in disordered signaling for hunger, satiety (the feeling of fullness), and fullness (the state of fullness). For many, efforts to lose weight using diets are met with unyielding resistance or disappointing weight regain as a result of a maladapted physiology that increases hunger and reduces the metabolic rate in response to reduced calorie intake. This makes it very difficult to lose weight once it is gained, which is why the environmental changes in the last few decades have uncovered this biological pre-disposition to obesity in our population.
Our modern environment is obesogenic as a result of the wide availability of calorie dense foods, inhibition of physical activity, higher levels of stress and fewer hours of sleep, all of which can contribute to the development of obesity. These factors promote greater calorie intake and reduced physical activity, which facilitates weight gain. For those that gain
weight, and are biologically pre-disposed to the disease of obesity, weight loss becomes extremely difficult. This is why preventing obesity is better than treating obesity.
If obesity develops then treatment should be considered if there is a present health impact or a risk of a decline on health. Obesity is associated with adverse health outcomes including effects on physical health, social health, mental health and interpersonal relationships. In addition to these health effects, people living with obesity experience a stigma not associated with other chronic diseases.
People with obesity are blamed for their obesity, resulting in shame and loss of self-esteem. This blame can negatively impact relationships, limit opportunities in the world of work or education, and discourage social participation. This decline in psychosocial function often seen in people with obesity can be associated with a decline in mental health.
By recognising obesity as a disease we can facilitate people to receive a medical diagnosis, access treatment and overcome stigma. The recognition of obesity as a disease may also help to reduce weight bias and stigma in the media, among health professionals, among policy makers and Governments, and in wider society. While the disease of obesity can be difficult to treat, treatments are available. The treatments for obesity can be divided into three main categories
1.
Specialist dietary interventions can be used to treat obesity, either individually, or as an adjunct to pharmacotherapy or surgery. Dietary interventions have been shown to support up to 15% weight loss. The LOOK AHEAD (Action for Health on Diabetes) trial was a randomised control trial of intensive


diet and lifestyle intervention that aimed to evaluate the reduced incidence of adverse cardiovascular events among overweight or obese people with diabetes.
This study recruited over 5000 overweight individuals with diabetes and followed them up for over 9 years. The trial was stopped early as the intervention did not show a reduction in the primary outcome of adverse cardiovascular events in the intervention arm. It is worth noting that the cardiovascular event rate during the study was lower than expected which may have affected the study power. However, the study did demonstrate significant weight loss and reduced prevalence of cardiovascular risk factors and remains a gold standard in terms of dietary treatment for obesity. The intervention group achieved the most weight loss in the first year (8.6% vs 0.7% in the control group). Average weight loss after 8 years was 5% in the intervention group
However weight loss maintenance is a major challenge. Most weight was regained in the first five years post intervention at which point it stabilised at about 4-5% weight loss. A smaller proportion of people maintained greater than 10% weight loss after 8 years. An analysis of the Look AHEAD data suggested that treatment response in the first two months of treatment is strongly correlated with results after 1 year. Individuals who lose < 2% or < 3% of their bodyweight in month 2 and 3 respectively are less likely to achieve 10% weight loss after 1 year. Reviewing patients after three months on a diet and lifestyle programme would therefore be appropriate for assessing their response to the treatment.
Diets with 800 kcal per day or less are consistent with the term very low-calorie diet (VLCD). Diets with reduced calorie intakes, but with absolute intakes of over 800 kcal per day, can be termed low-calorie diets. VLCDs and low-calorie diets are usually delivered as specially formulated food products, usually in the form of milkshakes, soups or nutritional bars. The use of VLCDs

and low-calorie diets can result in significant weight loss in the short to medium-term. In most studies, there is a significant drop-out rate as people can find the associated side effects of these interventions, such as constipation, to be intolerable.

However, in those who do tolerate the interventions, obesity can be successfully treated with a weight reduction of between 10 to 15% over 12 to 20 weeks of treatment. Weight maintenance following cessation of treatment can be challenging but is achievable.
Weight regain following dietary intervention appears to be mediated by physiological changes and metabolic adaptations that result in increased hunger and reduced basal metabolic rates following a period of reduced energy intake. However, despite metabolic adaptations that promote weight regain, studies have shown that weight loss maintenance is possible with ongoing dietary modification, the addition of pharmacotherapy, and concurrent exercise programmes. It should be noted that while exercise therapy may not be proven to significantly enhance weight loss during obesity treatment programmes, exercise therapy has been shown to assist with weight maintenance in the medium to long-term, which is why it is an integral element of obesity treatment programmes.
The Diabetes Remission Clinical Trial (DIRECT) was a randomised controlled study of a low calorie diet intervention completed in a primary care setting in the UK. In DIRECT, the aim was to induce remission or improve control of type II diabetes using a low-calorie diet. In this study, the intervention was a low-calorie diet of between 800 and 900 kcal per day which was prescribed for at least 12 weeks and continued for up to 5
months. Following the intervention, the participants progressed to a staged reintroduction of an isocaloric diet. A structured weight maintenance programme was then introduced and continued for up to 12 months. This intervention was compared with standard diabetes and obesity treatment that was based on lifestyle advice and diabetic pharmacotherapy.
This landmark study demonstrated that a low-calorie dietary intervention, delivered in a primary care setting with specialist support from dieticians and GPs, could deliver weight loss of 15 kg or more in 24% of participants. Almost 50% of people in DIRECT achieved remission of their Type II diabetes. This cohort were first diagnosed with Type II diabetes within six years of recruitment and diabetes remission was defined as a HbA1c of less than 6.5% (48 mmol/mol) while off all diabetes pharmacotherapy for at least two months.
While DIRECT demonstrated that such an intervention could be safely delivered in the primary care setting, it is resource intensive. Therefore, at present, the multidisciplinary framework needed for implementation of such a therapeutic intervention remains in development in Ireland, and therefore outside of specialist hospital-based clinics, these interventions are not widely available.
2. Pharmacotherapy
There are four medications licenced for the treatment of obesity in Ireland: orlistat (Xenical®). liraglutide 3 mg (Saxenda®), semaglutide 2.4mg (Wegovy®), and naltrexone/ bupropion (Mysimba®). All four medications have been shown to be effective in producing weight loss greater than placebo for a duration of at least one year.
Orlistat is an inhibitor of pancreatic lipase, and inhibits the digestion of fat. Orlistat does not target appetite or satiety mechanisms but can result in almost 3% weight loss in addition to weight lost via a diet and exercise programme. More than 15% of people using orlistat will lose more than 10% of their body weight.
Liraglutide is a daily, subcutaneously administered, human glucagon-like peptide 1 (GLP-1) analogue that acts centrally to reduce appetite and increase satiety. It results in an average weight loss of more than 6% in addition to weight lost via diet and exercise based regimen. More than 30% of people using Liraglutide lose more than 10% of their body weight.
Semaglutide is a once weekly, subcutaneously administered, GLP-1 analogue that, like Liraglutide, acts centrally to increase satiety and reduce hunger. Using the obesity treatment dose of 2.4mg weekly, average weight loss at one year is in excess of 12% and more than 60% reduce their body weight by 10% (including 50% who lose more than 15% of their body weight).
Naltrexone hydrochloride/ bupropion hydrochloride enhances satiety centrally and modulates the hedonic responses to eating, therefore reducing cravings. This medication can result in over 5% weight loss in addition to weight lost via a diet and exercise programme. More than 15% of people using Naltrexone hydrochloride/bupropion hydrochloride will lose more than 10% of their body weight.
While these obesity treatments are licensed for the treatment of obesity in people with or without diabetes, at the time of writing, they are not covered by the GMS or the drug payment schemes, and so people must fund their treatment out of pocket.
3. Bariatric and Metabolic Surgery
Bariatric and metabolic surgery is the most effective intervention in treating obesity and obesity-associated diabetes. All procedures are performed laparoscopically with a very low rate of complications. In diabetes, surgery is a more effective treatment in treating type 2 diabetes than medical care alone. Surgery improves glycaemic control in the medium-term, but also reduces mortality and maintains control of diabetes in the long-term. Therefore, surgery is increasingly used to primarily treat diabetes rather than the associated obesity.
Weight loss after surgery usually reaches a maximum twelve months post-operatively, with a mean ten-year weight reduction of 25%. The weight loss associated with surgery is durable in general, although weight regain can occur in a minority of surgical recipients. The weight loss occurs in tandem with significant improvements in multiple health outcomes including a reduction in all-cause mortality.
Weight loss after surgery is not a result of physical restriction or calorie malabsorption, as is commonly believed. The major mechanism of weight loss after surgery is increased satiety and decreased hunger, which is associated with changes in gastrointestinal hormones such as GLP-1. There is also evidence that surgery increases energy expenditure, despite reduced food intake. Therefore, surgery directly addresses the two major components of the disease of obesity.
Surgery is the only treatment with evidence for long-term weight loss, and reduction in all cause mortality, and therefore should be considered in all people with obesity. While the peri-operative risks are as low as general elective surgery, these procedures are not ‘the easy option’.
Before proceeding a thorough multidisciplinary assessment is needed to ensure candidates are fully prepared to have an optimal response to surgery.
Conclusion
Obesity is a disease that affects all aspects of human health that can result in secondary disease and disability. However, obesity can be successfully treated. The treatment of obesity needs to be individualised, and can include diet, exercise and behavioural interventions, pharmacotherapy, and surgical interventions.
Given that there are treatment options available, people with obesity need to be recognised and offered referral for treatment. As a healthcare system, we need to provide greater access to treatment to every person living with this disease. This year the HSE published a new Model of Care for Obesity. It is hoped that this is the start of a new phase of public sector investment in obesity care that will increase availability of therapy and offer people living with obesity treatment options that will reduce the morbidity and mortality associated with this insidious chronic disease.
References available on request
The Mayo Clinic Model: Delivering High Performance Cardiac Rehabilitation in Ireland
 Written by Deirdre O’Reilly, President, Irish Association of Cardiac Rehabilitation
Written by Deirdre O’Reilly, President, Irish Association of Cardiac Rehabilitation
of Irish CR providers in 2020, and numerous meetings over the following two years between Mayo Clinic Faculty (Cardiovascular Medicine) and Irish CR experts enabled the final programme format and content to be devised.
Implementation
Cardiac Rehabilitation (CR) is recognised as an essential component of secondary prevention for patients with cardiovascular disease (CVD). CR enables each patient to recover fully after their cardiac event (e.g. myocardial infarction) and to actively return to everyday life as quickly as possible. This is achieved by providing a wideranging programme of integrated care encompassing individually tailored exercise prescription, education, risk factor modification and psychosocial support.
Modern healthcare increasingly emphasises the need for high performance and value-based care, and in keeping with this the Irish Association of Cardiac Rehabilitation (IACR) continues to promote the highest international standard of care to ensure that patient outcomes are optimised during CR.

Feedback from frontline CR staff has also consistently identified the need for a short, intensive evidence-based training programme to comprehensively educate emerging cardiology nursing staff to deliver this standard of care.
Responding to this need, the IACR in collaboration with Royal College of Surgeons (RCSI) coordinated and delivered an advanced CR training workshop to support the majority of CR centres in Ireland. We successfully partnered with the recognised international leaders of CR delivery - the Mayo Clinic (Rochester, Minnesota) – who travelled to Ireland to deliver a cutting-edge, comprehensive and in-person CR training programme over 3 full days.
Objectives
The overarching goal for the ‘Cardiac Rehabilitation Workshop: Mayo Clinic Model’ was to make a national impact on the quality of CR delivered in Ireland by sharing international best practices with frontline CR nursing staff from every region of the country. This extended to CR programme

development and administration, CR assessment tools and CR outcome measurements.
Key learning objectives were:
1. To identify key components of patient engagement and patient assessment in CR.
2. To develop appropriate and effective individualized treatment plans (ITP) for CR patients.
3. To summarize the rationale and benefits of tracking patient outcomes in CR .
4. To prescribe an appropriate and effective exercise prescription for each patient in CR.
IACR facilitates regular communication with CR colleagues nationally via fortnightly online meetings. Via this forum and a national assessment of CR quality conducted in collaboration with the Irish Heart Foundation (Charles et al., 2021), a training deficit was identified. Additionally, feedback from frontline CR staff directly informed the eventual format of the advanced CR training workshop provided by the Mayo Clinic.
IACR explored the possibility of partnering with the Mayo Clinic to meet the educational requirements
Support from the RCSI and local cardiologists enabled the training programme to be hosted in the RCSI Education & Research Centre (Beaumont Hospital), and partnership with industry colleagues covered logistical expenses (e.g. travel, accommodation, equipment and subsidised registration fees). Every effort was made to minimise barriers to attendance for participants (e.g. parking fees). As participant feedback had expressed a strong preference for hands-on in person ‘practical’ training’ (in addition to didactic educational sessions), a ‘hybrid’ training programme was developed in partnership with the Mayo Clinic faculty to provide attendees with a combination of engaging presentations outlining cutting-edge science in CR and practical hands-on experience of delivering both inpatient and outpatient CR. Furthermore, this internationally recognised high-performance CR model was adapted specifically for the Irish healthcare setting.
Knowledge and skills acquisition was conducted face to face in both a classroom and CR gym
setting with expert supervision from 8 members of the Mayo Clinic Cardiovascular faculty (Rochester, Minnesota, USA).
Outcomes
The training workshop was held on September 7th-9th with 36 attendees. In addition to full capacity attendance, participants represented 22 of the 34 CR centres (65%) providing outpatient CR programmes nationally. Open-ended participant feedback indicated that this face-to-face ‘practical’ training workshop was highly engaging and well-received by attendees. Formal evaluations completed via the Mayo Clinic IT platform further indicated that 100% of attendees rated the course as ‘excellent’, 100% would recommend the course to their CR colleagues and 100% felt that the educational content matched their current or potential scope of practice.
This exceptional standard of intensive supervised training has bolstered the existing knowledge and skills of Irish CR staff and will ensure that the majority of CR centres nationwide can deliver comprehensive and ‘high performance’ CR in their local settings.
Mayo Clinic Faculty with Conference organising committee and participants
OSTEOPOROSIS STRIKES
20 micrograms administered once daily. Patients should receive calcium and vitamin D supplements if dietary intake is inadequate. The maximum total duration of treatment is 24 months. The 24 month course should not be repeated over a patient’s lifetime. Following cessation of teriparatide therapy, patients may be continued on other osteoporosis therapies. Teriparatide must not be used in severe renal impairment. Use with caution in moderate renal impairment and impaired hepatic function. Teriparatide should not be used in paediatric patients (less than 18 years), or young adults with open epiphyses. Method of administration: Movymia should be administered once daily by subcutaneous injection in the thigh or abdomen. It should be administered exclusively with the Movymia Pen reusable, multidose medicine delivery system and the injection needles which are listed as compatible in the instructions provided with the pen. The pen and injection needles are not included with Movymia. However, for treatment initiation a cartridge and pen pack should be used. Movymia must not be used with any other pen. Patients must be trained to use the proper injection techniques. Contraindications: Hypersensitivity to the active substance or excipients. Pregnancy and Breast-feeding Pre-existing hypercalcaemia, severe renal impairment, metabolic bone diseases other than primary osteoporosis or glucocorticoid-induced osteoporosis, unexplained elevations of alkaline phosphatase, prior external beam or implant radiation therapy to the skeleton, patients with skeletal malignancies or bone metastases. Warnings and precautions: In normocalcaemic patients, slight and transient elevations of serum calcium concentrations have been observed following teriparatide injection. Serum calcium concentrations reach a maximum between 4 and 6 hours and return to baseline by 16 to 24 hours after each dose of teriparatide. Therefore, if blood samples for serum calcium measurements are taken, this should be done at least 16 hours after the most recent teriparatide injection. Routine calcium monitoring during therapy is not required. Teripa ratide may cause small increases in urinary calcium excretion, but the incidence of hypercalciuria did not differ from that in the placebo-treated patients in clinical trials. Teriparatide should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition. In short-term clinical studies with teriparatide, isolated episodes of transient orthostatic hypotension were observed. Typically, an event began within 4 hours of dosing and spontaneously resolved within a few minutes to a few hours. When transient orthostatic hypotension occurred, it happened within the first several doses, was relieved by placing subjects in a reclining position, and did not preclude continued treatment. Caution should be exercised in patients with moderate renal impairment. Experience in the younger adult population, including premenopausal women, is limited. Treatment should only be initiated if the benefit clearly outweighs risks in this population. Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, teriparatide should be discontinued. The recommended treatment time of 24 months should not be exceeded. Contains sodium. Interactions: Digoxin, digitalis. Fertility, pregnancy and lactation: Women of child bearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, Movymia should be discontinued. Movymia is contraindicated for use during pregnancy and breast-feeding. The effect of teriparatide on human foetal development has not been studied. The potential risk for humans is unknown. Driving and operation of machinery: Teriparatide has no or negligible influence on the ability to drive and use machines. Transient, orthostatic hypotension or dizziness was observed in some patients. These patients should refrain from driving or the use of machines until symptoms have subsided. Undesirable effects: Nausea, pain in limb, headache, dizziness. Refer to Summary of Product Characteristics for other adverse effects. Pack size: 1. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie. Marketing authorisation holder: STADA Arzneimittel AG, Stadastrasse 2-18, 61118 Bad Vilbel, German. Marketing authorisation number: EU/1/16/1161/001-003. Medicinal product subject to medical prescription. Date last revised: July 2019.


Insulin prescribing, administration, and glucose monitoring trends in a hospital setting
AUTHORS: D. Hogan-Murphy1, L. Reddington2, M.C. Dennedy2,3, L. Egan3,4, J. Okiro2, J. Given1, S. Donnellan1, M.R. Salehmohamed2
1. Department of Pharmacy, Galway University Hospitals, Galway, Ireland
2. Department of Endocrinology, Diabetes and Metabolism, Galway University Hospitals, Galway, Ireland
3. Department of Pharmacology and Therapeutics, School of Medicine, National University of Ireland, Galway, Ireland
4. Department of Gastroenterology, Galway University Hospitals, Galway, Ireland
ABSTRACT
Aims: Insulin is a high-alert critical medication which can cause significant patient harm when used inappropriately. The aim of this study was to conduct a prospective audit on insulin prescribing, administration, and glucose monitoring trends in Galway University Hospitals.
Methods: This audit was conducted over one day in March 2022. The audit was approved by the local Clinical Audit Committee, piloted on two inpatients, and communicated to all data collectors prior to commencement. Generated data were anonymous and securely stored. Independent analysis was conducted by three researchers to confirm reliability of results.
Results: Four hundred and fifty-four inpatients were reviewed of which 17% [75] had diabetes and 9% [41] were prescribed insulin. The overall insulin error rate with one or more errors comprising prescribing and/or administration per inpatient drug record was 90% [37]. In total, 95% [235] insulin brand names and 89% [220] dose units were clearly prescribed, 84% [208] administration times were clearly specified by a prescriber, 87% [214] orders were signed, 58% [25] prescribers clearly documented their registration number/bleep/name at least once for contact purposes, 35% [30] meal time supplements were documented clearly by a nurse, 70% [202] administrations were double checked by a second person, 53% [142] administration times were documented by a nurse, and 26% [10] of inpatients were administered insulin by a nurse when not prescribed.
Conclusion: Results will assist in developing quality improvement initiatives to optimise patient care.
DM/Insulin Prevalence
than half a billion adults live with DM worldwide, a rise of 16% since previous estimates in 20196. This is predicted to escalate to almost 800 million by 20456. In Ireland, in the absence of a national DM registry, the current approximate projection is 5.6%7
DM is a leading cause of death globally8 and described as the most challenging health problem in the 21st century9,10 driven primarily by rising levels of obesity and an ageing population9,11. A systematic review and meta-analysis on the epidemiology of DM and its complications amongst adults in Ireland found variables from 7–25% for retinopathy; 3–32% for neuropathy; and 3-5% for nephropathy12. The economic burden also plays heavily with Ireland ranked 7 th in the world for
DM related health expenditure per person7, and as high as ¤1.4 billion annually with costs mostly associated with hospitalisations and treatment of complications6
Insulin is a high-alert medicine used in the treatment of DM which bears a heightened risk of causing significant patient harm. With limited Irish data availability and few local incidents reported, anecdotal evidence suggests insulin accounts for a substantial number of medication errors13,14. A review by the States Claims Agency of over 20,000 medication incidents reported by Irish acute hospitals in 2017 and 2018 found insulin was the fourth most commonly implicated therapeutic subgroup15, many comprising omissions leading to hyperglycaemia and inaccurate
dosing leading to hyperglycaemia and hypoglycaemia16
Insulin has been identified as a significant medication safety concern in Galway University Hospitals (GUH). The aim of this study was to conduct a prospective audit on insulin prescribing, administration, and glucose monitoring trends in GUH in order to identify and develop agreed quality improvement initiatives to enhance patient care.

Methods
A prospective audit on insulin prescribing, administration, and glucose monitoring was conducted over one day in March 2022 on 24 wards in GUH. GUH comprises University Hospital Galway (UHG), a Model 4 public hospital, and Merlin Park University Hospital
Introduction
Diabetes mellitus (DM) is a heterogeneous complex metabolic condition characterised by hyperglycaemia with a degenerative potential resulting from changes in the production, secretion and/or inability of insulin to adequately exercise its effects1 The most common classifications include Type 1 DM and Type 2 DM, the latter accounting for more than 90% of all cases2. Type 2 DM is characterised by insulin resistance and a relative deficiency of insulin secretion which progressively worsens over time3,4 Type 1 DM results in an absolute deficiency in beta-cell function with autoimmune destruction of beta-cells a common origin5. Current estimates suggest more
Twenty-five inpatients [61%; n=41] had changes to their insulin regimen made during their inpatient stay. This included changes to a brand name of insulin [4; n=37; 11%], the initiation of insulin and/or a meal time supplement [14; n=37; 38%], and/or a dose change [9; n=32; 28%; median 3; range 1-6]. Three inpatients had their insulin omitted after an episode of hypoglycaemia (blood glucose <4 mmol/L).
Discussion
Figure 1: DM/Insulin prevalence
(MPUH), a Model 2 public hospital, and provides a comprehensive range of services to emergency and elective patients within the Saolta University Healthcare Group in the West of Ireland.
as presented in Table 1.
Table 1: Overall insulin error rate per inpatient drug record
brand
Insulin name not clearly prescribed
dose units not clearly prescribed
commencement. All audit forms were anonymous and securely stored in a locked cabinet and all generated data were securely stored on an encrypted password protected work computer. Any audit records will be destroyed after full dissemination of audit findings. Independent analysis was conducted by the two primary researchers and a specialist registrar in endocrinology to confirm reliability of results. This process involved independently inputting content of paper audit forms into excel, analysing data, and comparing results. No significant discrepancies were identified.
as pre-admission, four inpatients [n=37; 11%] were not prescribed the same insulin as pre-admission, and 14 inpatients [n=37; 38%] were not on insulin pre-admission and were either prescribed a meal time supplement [10 inpatients; n=37; 27%] or were newly prescribed insulin on admission [4 inpatients; n=37; 11%].
This study identified 17% of inpatients had DM on audit day in GUH of which 9% were insulin dependent and 55% of all inpatients with DM were treated with insulin. This is similar to the most recently published National Health Service (NHS) National Diabetes Inpatient Audit (NaDIA) England 2019 report which found 18% of all inpatients in 188 NHS hospitals had documented DM17 The NaDIA England and Wales 2015 report identified a lower prevalence of 6% of all inpatients in 206 NHS hospitals were insulin dependent and 36% of inpatients with DM were treated with insulin18
Insulin administration times not clearly specified by the prescriber
Results
orders not signed by the prescriber
General participation and prevalence
for contact
A total of 247 insulin doses were prescribed of which 235 orders [95%] had the insulin name clearly documented. Two hundred and twenty [89%] insulin dose units were clearly prescribed, 208 [84%] administration times were clearly specified by a prescriber, and 214 [87%] orders were signed by a prescriber (Figure 2).
The majority of inpatients in this audit were medical and prescribed the same insulin as pre-admission. All inpatients not prescribed the same insulin as pre-admission were altered by the DM team during admission and were therefore appropriately changed. The remaining inpatients were not on insulin pre-admission and were either prescribed a meal time supplement or were newly prescribed insulin on admission.
time supplement
Inclusion criteria comprised inpatients prescribed/administered insulin in UHG and MPUH for the previous 72 hours until 9am on the morning of audit. Exclusion criteria comprised non-admitted patients, Day Wards, Emergency Department, Acute Medical Unit, Short Stay Unit, Emergency Surgical Unit, Critical Care including Post Anaesthetic Care Unit, Maternity Department, and Psychiatry Department. Content of the audit protocol and tool was informed by the research objective, local practices, and existing evidence-based international and national literature. The audit tool was piloted on a medical ward in UHG with two random inpatients prescribed and administered insulin. Minor amendments were made to its content and the pilot was excluded from data analysis.
incorrectly
administration not double checked by a 2nd person
administration times not documented
Insulin administered when not prescribed
Insulin prescribing patterns
In total, 454 inpatients were reviewed of which 41 [9%] were prescribed insulin and included in the audit. The number of inpatients using an Insulin and Glucose Monitoring Record was 117 [26%] of which 75 inpatients [17%] had a documented history of DM (Figure 1). This equates to 55% of all inpatients with DM were treated with insulin. Patient specialty comprised medical [27, 66%], surgical [12, 29%], and paediatric [2, 5%].
Twenty-five prescribers [n=43; 58%] clearly documented their medical council registration number (MCRN)/bleep/name on the insulin drug record at least once for contact purposes and 25 inpatients [61%] had the meal time supplement signed by a prescriber. The meal time supplement was documented clearly 30 times out of a total of 86 entries [35%].
The overall insulin error rate comprising both prescribing [80%] and administration [89%] per inpatient drug record was 90%. This is significantly higher than the NaDIA 2019 report which found 18% of inpatient drug records had one or more insulin errors17
The audit was led by two lead researchers and conducted by 29 data collectors comprising endocrine consultant and nonconsultant hospital doctors, diabetes nurse specialists, and pharmacists. The audit tool was guided by the protocol which was communicated to all data collectors prior to the audit via video conferencing and faceto-face meetings and emails. This audit was conducted in accordance with the HSE Code of Governance (2021) and HSE Healthcare Audit Quality Assurance and Verification Standards (2019) and was approved by the GUH Clinical Audit Committee prior to
Insulin administration patterns
Overall insulin error rate per inpatient drug record
The overall insulin error rate with one or more errors comprising prescribing and/or administration per inpatient drug record was 90% [n=41] as presented in Table 1.
Two hundred and eighty-seven doses were administered of which 202 [70%] were double checked by an independent second person. Administration times were documented for 142 doses [n=270; 53%] as illustrated in Figure 3. Ten inpatients [n=39; 26%] were administered insulin by a nurse when not prescribed.
This figure is also higher than findings from an analysis of the National Reporting and Learning System database of patient safety incidents concerning insulin reported from NHS providers in England and Wales over a six year period which found 61% of incidents occurred at the administration stage and 17% at the
stage19
most common medication
types were wrong dose,
Nineteen inpatients [n=37; 51%] were prescribed the same insulin as pre admission, four inpatients [n=37; 11%] were not prescribed the same insulin as pre-admission, and 14 inpatients [n=37; 38%] were not on insulin pre admission and were either prescribed a meal time supplement [10 inpatients; n=37; 27%] or were newly prescribed insulin on admission [4 inpatients; n=37; 11%].
Glucose management
followed by omitted or delayed insulin19
Most prescriptions in this audit had the insulin name, dose,
Insulin prescribing patterns
Nineteen inpatients [n=37; 51%] were prescribed the same insulin
The GUH DM team reviewed/was contacted to review 21 inpatients insulin drug record [55%, n=38].
clearly documented and signed by the prescriber which are positive
A total of 247 insulin doses were prescribed of which 235 orders [95%] had the insulin name clearly documented. Two hundred and twenty [89%] insulin dose units were clearly prescribed, 208 [84%] administration times were clearly specified by a prescriber, and 214 [87%] orders were signed by a prescriber (Figure 2).
Insulin Prescribing Patterns
What number of insulin orders have been signed by the prescriber?
number of insulin administration times are clearly specified by the prescriber?
number of insulin dose units are clearly prescribed?
Insulin Prescribing Patterns
number of insulin orders have been signed by the
number of insulin orders have the name clearly prescribed?
number of insulin doses have been prescribed?
Figure 2: Insulin prescribing patterns
number of insulin administration times are clearly specified by the prescriber?
number of insulin dose units are clearly prescribed?
number of insulin orders have the name clearly prescribed?
findings. However, less than three out of five prescribers clearly documented their MCRN/ bleep/name on the insulin drug record at least once for contact purposes leading to possible time delays and patient care issues if communication is required.
Insulin administration patterns
Whilst the majority of inpatients were prescribed a meal time supplement, it was only documented clearly by nurses for approximately one in every three inpatients potentiating administration errors and significant patient harm. Almost one in three insulin doses were also not double checked by an independent second person and almost half administration times were not documented. Insulin is a highalert medication which requires a two-person check of both dose preparation and administration at the bedside as well as precise administration timing to minimise hypoglycaemia, hyperglycaemia, wide glycaemic excursions, and diabetic ketoacidosis.
episode of hypoglycaemia. This is lower than a study evaluating insulin information on discharge summaries in a United Kingdom (UK) hospital which found 33 out of 42 patients [79%] had changes made including the initiation and/or discontinuation of insulin therapy, insulin dose changes, and insulin preparation/brand20
number of insulin doses have been prescribed?
Figure 2: Insulin prescribing patterns
Twenty five prescribers [n=43; 58%] clearly documented their medical council registration number (MCRN)/bleep/name on the insulin drug record at least once for contact purposes and 25 inpatients [61%] had the meal time supplement signed by a prescriber. The meal time supplement was documented clearly 30 times out of a total of 86 entries [35%].
Future quality improvement interventions for consideration to optimise patient care include implementing a dedicated insulin safety team that comprises a broad membership to facilitate sustainability and spread of interventions to improve insulin prescribing and administration practice; electronic prescribing as a part of the ongoing implementation of a new pharmacy system; an insulin inpatient and discharge checklist; and promotion of an annual national audit on insulin prescribing, administration, and glucose monitoring trends similar to the UK. Once interventions are in situ it is anticipated that results of a reaudit in GUH will be favourable.
Declaration of Conflicts of Interest
Two hundred and eighty-seven doses were administered of which 202 [70%] were double checked by an independent second person. Administration times were documented for 142 doses [n=270; 53%] as illustrated in Figure 3 Ten inpatients [n=39; 26%] were administered insulin by a nurse when not prescribed.
Insulin Administration Patterns
documented?
The authors report no conflict of interest.
Twenty five prescribers [n=43; 58%] clearly documented their medical council (MCRN)/bleep/name on the insulin drug record at least once for contact purposes and had the meal time supplement signed by a prescriber. The meal time supplement was 30 times out of a total of 86 entries [35%]
Insulin
number of doses with administration times
Based on audit results as well as evidence from local insulin error reporting, local practices, and best practice, some of the current interventions implemented specific to insulin in GUH with support from hospital management and medication safety committees which are transferrable to other hospitals include an updated inpatient Insulin and Glucose Monitoring Drug Record;
number of doses are double checked by an independent second person?
recruitment of a clinical pharmacist with a special interest in diabetes; implementation of mandatory e-learning for nurses and doctors on insulin prescribing and administration; updated/newly approved policies, procedures, protocols, and guidelines; piloting of a self-administration policy; continuous education and training to patients as well as medical, nursing, and pharmacy undergraduate students and employees in GUH; expansion of a medication safety portal inclusive of educational videos; use of hospital screens and social media to disseminate prudent information; and further promotion of error reporting. No challenges were encountered as all key stakeholders were involved from the onset.
Corresponding Author: Dr Diana Hogan-Murphy Department of Pharmacy University Hospital Galway Galway E-Mail: diana.hogan-murphy@hse.ie References available on request
hundred and eighty seven doses were administered of which 202 [70%] were double independent second person. Administration times were documented for 142 doses
in Figure 3. Ten inpatients [n=39; 26%] were administered insulin by a nurse
What number of doses have been administered?
Figure 3: Insulin administration patterns
Glucose management
More than one in four inpatients were administered insulin by a nurse when not prescribed. A nurse may only administer a non-prescribed medication in a situation that requires immediate intervention in lifethreatening situations and there is no immediate access to an appropriate prescriber. All insulin doses should be prescribed prior to nurse administration.
number of doses with
number of doses are double checked by an independent second person?
The GUH DM team reviewed/was contacted to review 21 inpatients insulin drug record [55%, n=38]. Twentyfive inpatients [61%; n=41] had changes to their insulin regimen made during their inpatient stay. This included changes to a brand name of insulin [4; n=37; 11%], the initiation of insulin and/or a meal time supplement [14; n=37; 38%], and/or a dose change [9; n=32; 28%; median 3; range 1 6] Three inpatients had their insulin omitted after an episode of hypoglycaemia (blood glucose <4 mmol/L).
The GUH DM team reviewed or was contacted to review more than half of the inpatients prescribed insulin. More than three out of five inpatients had changes to their insulin regimen made during their inpatient stay including brand of insulin, the initiation of insulin, and dose changes. Three inpatients had their insulin omitted after an
number of doses have been
50 mg/mL concentrate for solution for infusion
In a post-hoc subgroup analysis of the PACIFIC study, WITH IMFINZI, the median overall survival (OS) at 5 years, was 63.1 months versus 29.6 months for the placebo arm (HR: 0. 61; 95% CI, 0.44-0.85)1*

*Patients were retrospectively tested for PD-L1 expression on tumour cell using the Ventana PD-L1 (SP263) IHC assay, where available. 63% of patients provided a tissue sample of sufficient quality and quantity to determine PD-L1 expression and 37% were unknown. This exploratory post-hoc 5-year OS analysis was conducted at ~5 years after last patient was randomised and was not powered to show statistical significance.
Primary analysis in the ITT population
Median OS†: Not reached vs 28.7 months with
(HR=0.68; 99.73% CI, 0.47-0.997); P=0.00252
Median PFS ‡: 16.8 vs 5.6 months with placebo (HR=0.52; 95% CI, 0.42-0.65; P<0.0001)1
†The primary 2-year OS analysis was conducted after 299 deaths for 42% maturity (61% of targeted events) with a median follow-up of 25.2 months. Reduction in the risk of death vs placebo was 32% (95% CI, 0.53-0.87). Median OS was NR with IMFINZI (95% CI, 34.7-NR) vs 28.7 months with placebo (95% CI, 22.9-NR).1,2 ‡Measured based on RECIST v1.1 criteria by BICR. The primary PFS analysis was conducted after 371 events (81% of targeted 458 events) with a median follow-up of 14.5 months. Reduction in the risk of progression or death vs placebo was 48% (HR=0.52; 95% CI, 0.42-0.65). Median PFS was 16.8 months with IMFINZI (95% CI, 13.0-18.1) vs 5.6 months with placebo (95% CI, 4.6-7.8).3
SAFETY INFORMATION
The most frequent any grade (>10%) adverse reactions were cough/productive cough (21.5%), diarrhoea (16.3%), rash (16.0%), pyrexia (13.8%), upper respiratory tract infections (13.5%), abdominal pain (12.7%), pruritus (10.8%), and hypothyroidism (10.1%)1 See SmPC for recommended treatment modifications for IMFINZI and management considerations.

IMFINZI® (durvalumab) 50mg/ml Concentrate for Solution for Infusion Consult Summary of Product Characteristics (SmPC) before prescribing.
Indication: IMFINZI as monotherapy is indicated for the treatment of locally advanced, unresectable non-small cell lung cancer (NSCLC) in adults whose tumours express PD-L1 on ≥ 1% of tumour cells and whose disease has not progressed following platinum-based chemoradiation therapy. IMFINZI in combination with etoposide and either carboplatin or cisplatin is indicated for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).
Presentation: Each ml of concentrate for solution for infusion contains 50mg durvalumab.
Dosage and administration: Treatment must be initiated and supervised by a physician experienced in the treatment of cancer. Patients with locally advanced NSCLC should be evaluated for treatment based on the tumour expression of PD-L1 confirmed by a validated test. For locally advanced NSCLC, the recommended dose of IMFINZI is 10 mg/kg administered as an intravenous infusion over 1 hour every 2 weeks or 1500 mg every 4 weeks, until disease progression, unacceptable toxicity, or a maximum of 12 months. For ES-SCLC, the recommended dose for IMFINZI in combination with chemotherapy (etoposide and either carboplatin or cisplatin) is 1500 mg, administered as an intravenous infusion over 1 hour, every 3 weeks (21 days) for 4 cycles, followed by 1500 mg every 4 weeks as monotherapy, until disease progression or unacceptable toxicity. Patients with a body weight of 30kg or less must receive weight-based dosing, please consult Section 4.2 in the SmPC for more information. Dose escalation or reduction is not recommended. Dose withholding or discontinuation may be required based on individual safety and tolerability. Suspected immune-mediated adverse reactions: Adequate evaluation should be performed to confirm aetiology or exclude alternate aetiologies. Based on the severity of the adverse reaction, IMFINZI should be withheld and corticosteroids administered. Guidelines for the management of immune mediated adverse reactions are described in Table 2, Section 4.2 in the SmPC. Consider increasing dose of corticosteroids and/or using additional systemic immunosuppressants if there is worsening or no improvement. Upon improvement to ≤ Grade 1, corticosteroid taper should be initiated and continued over at least 1 month. After withhold, IMFINZI can be resumed within 12 weeks if the adverse reactions improved to ≤ Grade 1 and the corticosteroid dose has been reduced to ≤ 10 mg prednisone or equivalent per day. IMFINZI should be permanently discontinued for recurrent Grade 3 (severe) immune-mediated adverse reactions and for any Grade 4 (life-threatening) immune-mediated adverse reactions, except for endocrinopathies that are controlled with replacement hormones. For myasthenia gravis, if there are signs of respiratory or autonomic insufficiency, IMFINZI should be permanently discontinued. Non-immune-mediated adverse reactions: Withhold IMFINZI for Grade 2 and 3 adverse reactions until ≤ Grade 1 or baseline. IMFINZI should be discontinued for Grade 4 adverse reactions. Special populations: Elderly: No dose adjustment is required for elderly patients (≥ 65 years of age). Renal impairment: No dose adjustment is recommended in patients with mild or moderate renal impairment. Data from patients with severe renal impairment are too limited to draw conclusions on this population. Hepatic impairment: Data from patients with moderate and severe hepatic impairment are limited. Due to minor involvement of hepatic processes in the clearance of durvalumab no dose adjustment of IMFINZI is recommended for patients with hepatic impairment as no difference in exposure is expected.
Contraindications: Hypersensitivity to the active substance or to any of the excipients.

Warnings and precautions: Traceability: In order to improve the traceability of biological medicinal products, the tradename and the batch number of the administered product should be clearly recorded. Immune-mediated pneumonitis: Monitor for signs and symptoms of pneumonitis or radiation pneumonitis. Suspected pneumonitis should be confirmed with radiographic imaging and other infectious and disease-related aetiologies excluded. Immune-mediated hepatitis: Monitor for abnormal liver tests prior to and periodically during treatment with IMFINZI, and as indicated based on clinical evaluation. Immune-mediated colitis: Monitor for signs and symptoms of colitis or diarrhoea. Immune-mediated hypothyroidism, hyperthyroidism and thyroiditis: Monitor for abnormal thyroid function tests prior to and periodically during treatment and as indicated based on clinical evaluation. Immune-mediated adrenal insufficiency: Monitor for clinical signs and symptoms of adrenal insufficiency. Immune-mediated type 1 diabetes mellitus: Monitor for clinical signs and symptoms of type 1 diabetes mellitus including diabetic ketoacidosis. Immune-mediated hypophysitis/hypopituitarism: Monitor for clinical signs and symptoms of hypophysitis or hypopituitarism. Immune-mediated nephritis: Monitor for abnormal renal function tests prior to and periodically during treatment with IMFINZI. Immune-mediated rash: Events of Stevens-Johnson Syndrome or toxic epidermal necrolysis have been reported in patients treated with PD-1 inhibitors. Patients should be monitored for signs and symptoms of rash or dermatitis (including pemphigoid). Immune-mediated myocarditis: Patients should be monitored for signs and symptoms of immunemediated myocarditis. Other immune-mediated adverse reactions: Myasthenia gravis, myositis, polymyositis, meningitis, encephalitis Guillain-Barre syndrome, immune thrombocytopenia, cystitis noninfective and pancreatitis have been observed. Monitor for signs and symptoms. Infusion related reactions: Monitor for signs and symptoms of infusion related reactions.
All immune-mediated adverse events should be managed as recommended in Table 2 Section 4.2 of the SmPC. Patients excluded from clinical trials: Patients with: a baseline ECOG performance score ≥ 2; active or prior documented autoimmune disease within 2 years of initiation of the study; a history of immunodeficiency; a history of severe immune-mediated adverse reactions; medical conditions that required systemic immunosuppression, except physiological dose of systemic corticosteroids (≤ 10 mg/day prednisone or equivalent); uncontrolled intercurrent illnesses; active tuberculosis or hepatitis B or C or HIV infection or patients receiving live attenuated vaccine within 30 days before or after the start of IMFINZI. Durvalumab should be used with caution in these populations after careful consideration of the potential benefit/risk on an individual basis. The safety of concurrent prophylactic cranial irradiation (PCI) with IMFINZI in patients with ES-SCLC is unknown.
Drug Interactions: The use of systemic corticosteroids or immunosuppressants before starting durvalumab, except physiological dose of systemic corticosteroids (≤ 10 mg/day prednisone or equivalent), is not recommended because of their potential interference with the pharmacodynamic activity and efficacy of durvalumab. However, systemic corticosteroids or other immunosuppressants can be used after starting durvalumab to treat immune-related adverse reactions.
PK drug-drug interaction between durvalumab and chemotherapy was assessed and showed concomitant treatment did not impact the PK of any of the drugs involved. Additionally, based on population PK analysis, concomitant chemotherapy treatment did not meaningfully impact the PK of durvalumab.
Pregnancy and Lactation: Durvalumab may cause foetal harm when administered to a pregnant woman and is not recommended during pregnancy and in women of childbearing potential not using effective contraception during treatment and for at least 3 months after the last dose. A decision must be made whether to discontinue breast feeding or to discontinue or abstain from durvalumab therapy taking into account the benefit of breast feeding for the child and the benefit of therapy for the woman.
Undesirable events: Consult SmPC for full list of adverse events. IMFINZI Monotherapy: Very common (≥ 1/10; any grades): Upper respiratory tract infections, hypothyroidism, cough/productive cough, diarrhoea, abdominal pain, rash, pruritus, pyrexia, arthralgia. Common (≥ 1/100 to < 1/10; any grades ): Pneumonia, dental and oral soft tissue infections, oral candidiasis, influenza, hyperthyroidism, pneumonitis, dysphonia, aspartate aminotransferase increased or alanine aminotransferase increased, night sweats, myalgia, blood creatinine increased, dysuria, peripheral oedema, infusion related reaction. Uncommon (≥ 1/1,000 to < 1/100; any grades): Thyroiditis, adrenal insufficiency, interstitial lung disease, colitis, pancreatitis, hepatitis, dermatitis, myositis, nephritis. Rare (≥ 1/10,000 to < 1/1000; any grades): Type 1 diabetes mellitius, hypophysitis/hypopituitarism, diabetes insipidus, myocarditis, polymyositis, myasthenia gravis, meningitis, pemphigoid, immune thrombocytopenia, cystitis noninfective. Not known: Noninfective encephalitis, Guillain-Barre syndrome. IMFINZI Combined with Chemotherapy: Very common (≥ 1/10; any grades): Neutropenia, anaemia, thrombocytopenia, leukopenia, decreased appetite, cough/productive cough, nausea, constipation, vomiting, alopecia, fatigue. Common (≥ 1/100 to < 1/10; any grades): Upper respiratory tract infections, pneumonia, dental and oral soft tissue infections, febrile neutropenia, pancytopenia, hypothyroidism, hyperthyroidism, thyroiditis, adrenal insufficiency, pneumonitis, diarrhoea, abdominal pain, stomatitis, aspartate aminotransferase increased or alanine aminotransferase increased, hepatitis, rash, pruritus, dermatitis,
reaction.
interstitial

Lung Cancer
An Update on Small Cell Lung Cancer


NCT01450761, which showed no benefit in overall survival for the addition of Ipilimumab to standard chemotherapy.
 Dr Laurann Rabbitt
Dr Laurann Rabbitt
There are approximately 2700 new cases of lung cancer in Ireland each year with small cell lung cancer accounting for 13-15%. Most patients present with extensive stage disease, which is defined as spread of disease beyond what can be reasonably covered in a single radiation field. For the last three decades treatment options for these patients have been limited to platinum based chemotherapy. 5 year survival rates remained stubbornly low at 5%, with 1 year survival rates of 25.7% in Ireland over the period 20042007. This has changed in the last five years with the addition of immunotherapy now becoming the standard of care in the extensive stage setting.
There were several challenges associated with improving outcomes for people with small cell lung cancer. It often presents with rapid onset of symptoms and at a late stage, and although usually initially sensitive to platinum based chemotherapy it relapses quickly. It also occurs almost exclusively in those with a heavy smoking history and if a patient’s baseline health is poor, they may be unable to tolerate second or third line treatment.
On a molecular level it is very different to non-small cell lung cancer. It is characterised by a high mutation load, high mitotic index and rapid proliferation rate. While the identification of
targetable driver mutations has revolutionised care for many with metastatic non-small cell lung cancer (NSCLC), small cell is largely driven by loss of function mutations such as p53 and Rb deletions which are less amenable as drug targets.
Immunotherapy is particularly effective in malignancies with a high tumour mutational burden (TMB) such as melanoma, and in treatment for NSCLC a higher TMB score is associated with a longer duration of response compared to those with a lower score. Immunotherapy should have been a natural option for small cell lung cancer given its high TMB, but the benefit was slow to be proven. One reason may be that with the high burden of disease and rapidity of progression, the time needed to mount an effective anti-tumour response (often 6 months with immunotherapy) may exceed the overall survival of these patients.
Alternatively, there may be other immunologic mechanisms by which small cell lung cancer evades immune surveillance that are not as easily targeted. Initial trials in the first line setting were not encouraging. One phase II trial, NCT02359019, found no benefit in overall survival for maintenance Pembrolizumab after initial platinum chemotherapy in patients with extensive stage disease. There was also a negative phase III trial,
However in 2018 a trial emerged which showed benefit of upfront immunotherapy in combination with chemotherapy in the setting of treatment-naive extensive stage small cell lung cancer. In IMPower 133, 403 patients with extensive stage small cell lung cancer were randomised to receive an induction phase of either four cycles of carboplatin and etoposide alone, or with the addition of Atezolizumab, an immune checkpoint inhibitor. Following this they entered a maintenance phase to receive either placebo or Atezolizumab four weekly until progression of disease or unacceptable toxicity. The median overall survival was 12.3 months in the Atezolizumab group compared to 10.3 months in the placebo group At 18 months, 34.0% of the Atezolizumab-chemotherapy compared with 21.0% of the standard treatment group were alive. Toxicities were similar in both groups, although with increased immune-related adverse events in the Atezolizumab group.
The CASPIAN trial was a similar phase III trial published in November 2019. It randomised 805 patients to receive either platinum based standard of care treatment or with the addition of Durvalumab, followed by a phase of either maintenance Durvalumab or placebo. Durvalumab is an
immune checkpoint inhibitor monoclonal antibody which blocks the interaction of programmed death ligand 1. Immune mediated toxicities included rash, thyroid dysfunction, type 1 diabetes and pneumonitis. The overall survival in the Durvalumab group was 13 months compared to 10.3 months in the chemotherapy alone group. While three months may seem a small benefit, the durability of response is seen in the 3 year survival data. The 36-month overall survival rate was 17.6% in the Durvalumab group versus 5.8% in the control group – in other words, triple the amount of patients who received Durvalumab were alive at three years.
At present the only immunotherapy reimbursed in small cell lung cancer in Ireland is Atezolizumab. Globally, further trials are ongoing to investigate the role of immunotherapy monotherapy, dual agent immunotherapy regimes, and immunotherapy in the limited stage setting. There are also novel agents under development such as PARP inhibitors and antibody-drug conjugates such as sacituzumab-govotecan. It may be several years before these are translated in to standard clinical practice in Ireland, particularly because of the high costs associated with these drugs, however they represent exciting developments after many years of stagnation in the treatment for small cell lung cancer.
with (a) unresectable or metastatic colorectal cancer after previous fluoropyrimidine based combination therapy, (b) advan ced or recurrent endometrial carcinoma, who have disease progression on or following prior treatment with a platinum containing therapy in any setting and who are not candidates for curative surgery or radiation, (c) unresectable or metastatic gastric, small intestine, or biliary cancer, who have disease progression on or following at least one prior therapy.
KEYTRUDA, in combination with platinum and fluoropyrimidine based chemotherapy, is indicated for the first-line treatment of locally advanced unresectable or metastatic carcinoma of the oesophagus or HER-2 negative gastroesopha geal junction adenocarcinoma in adults whose tumours express PD-L1 with a CPS ≥ 10.

in combination with chemotherapy as neoad juvant treatment, and then continued as monotherapy as adjuvant treatment after surgery, is indicated for the treatment of adults with locally advan ced, or early stage triple negative breast cancer at high risk of recurrence.
in combination with chemotherapy, is indicated for the treatment of locally recurrent unresectable or metastatic triple negative breast cancer in adults whose tumours express PD L1 with a CPS ≥ 10 and who have not received prior chemotherapy for metastatic disease. KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of ad vanced or recurrent endometrial carcinoma in adults who have disease progression on or following prior treatment with a platinum containing thera py in any setting and who are not candidates for curative surgery or radiation.
in combination with chemotherapy with or without beva cizumab, is indicated for the treatment of persistent, recurrent, or metastatic cervical cancer in adults whose tumours express PD L1 with a CPS ≥ 1. DOSAGE AND ADMINISTRATION See SmPC for full details. Therapy must be initiated and supervised by specialist physicians experienced in the treatment of cancer. The recommended dose of KEYTRUDA in adults is either 200 mg every 3 weeks or 400 mg every 6 weeks administered as an intravenous infusion over 30 minutes. The recommended dose of KEYTRUDA as monotherapy in paediatric patients aged 3 years and older with cHL is 2 mg/kg bodyweight (up to a maximum of 200 mg), every 3 weeks administered as an intravenous infusion over 30 minutes. For use in combination, see the Summary of Product Characteristics (SmPC) for the concomitant therapies. KEYTRUDA must not be administered as an intra venous push or bolus injection. When administering KEYTRUDA as part of a combination with intravenous chemotherapy, KEYTRUDA should be administered first. Treat patients until disease progression or unacceptable toxicity (and up to maximum duration of therapy if specified for an indica tion). For the adjuvant treatment of melanoma or RCC, KEYTRUDA should be administered until disease recurrence, unacceptable toxicity, or for a duration of up to one year. Refer to the SmPC for dosing in neoadjuvant and adjuvant treatment of locally advanced, or early stage triple-negative breast cancer at high risk of recurrence. KEYTRUDA, as monotherapy or as combination therapy, should be permanently discontinued (a) For Grade 4 toxicity except for: endocrinopathies that are controlled with replacement hormones; or haematological toxicity, only in patients with cHL in which KEYTRUDA should be withheld until adverse reactions recover to Grade 0-1; (b) If corticosteroid dosing cannot be reduced to ≤10 mg prednisone or equivalent per day within 12 weeks; (c) If a treatment-related toxicity does not resolve to Grade 0 1 within 12 weeks after last dose of KEYTRUDA; (d) If any event occurs a second time at Grade ≥ 3 severity. Patients must be given the Patient Alert Card and be informed about the risks of KEYTRUDA. Special populations Elderly: No dose adjustment necessary. Renal impairment: No dose adjustment needed for mild or moderate renal impairment. No studies in severe renal impairment. Hepatic impairment: No dose adjustment needed for mild hepatic impairment. No studies in moderate or severe hepatic impairment. Paediatric population: Safety and efficacy in children below 18 years of age not established except in pae diatric patients with cHL. CONTRAINDICATIONS Hypersensitivity to the active substance or to any excipients. PRECAUTIONS AND WARNINGS Assessment of PD-L1 status When assessing the PD-L1 status of the tumour, it is important that a well-validated and robust methodology is chosen to minimise false negative or false positive determinations. Immune-related adverse reactions Immune-related adverse reactions, including severe and fatal cases, have occurred in patients receiving pembrolizumab. Most immune related adverse reactions occurring during treatment with pem brolizumab were reversible and managed with interruptions of pembrolizumab, administration of corticosteroids and/or supportive care. Immune re lated adverse reactions have also occurred after the last dose of pembrolizumab. Immune-related adverse reactions affecting more than one body system can occur simultaneously. Immune-related adverse reactions are immune-related pneumonitis, immune-related colitis, immune-related
infants cannot be excluded. Fertility No clinical data available. SIDE EFFECTS Refer to SmPC for complete infor mation on side effects. Pembrolizumab is most commonly associated with immune-related adverse reactions. Most of these reactions resolved with appropriate medical treatment or withdrawal of pembrolizumab. The most serious adverse reactions were immune-and infusion-related adverse re actions. When pembrolizumab is administered in combination with axitinib or lenvatinib, refer to the SmPC for axitinib or lenvatinib prior to initiation of
lymphopenia, hyponatraemia, hypokalaemia, hypocalcaemia, insomnia, neuropathy peripheral, lethargy, dry eye, cardiac arr hythmia (including atrial fibrillation), hypertension, hyperthyroidism, insomnia, dizziness, dysgeusia, pneumonitis, colitis, dry mouth, severe skin reactions, vitiligo, dry skin, alopecia, eczema, dermatitis acneiform, erythema, dermatitis, myositis, pain in extremity, arthritis, influenza like illness, chills, AST and ALT increases, increase in blood alkaline phosphatase, hypercalcaemia, blood bilirubin increased, blood creatinine increased, infu sion related reaction. In combination with chemotherapy: Very Common: neutropenia, anaemia, thrombocytopenia, leukopenia, hypothyroidism, hypokalaemia, decreased appetite, insomnia, neuropathy peripheral, headache, dizziness, dysgeusia, dyspnoea, cough, nausea, diarrhoea, vomi ting, abdominal pain, constipation, alopecia, rash, pruritus, arthralgia, musculoskeletal pain, myositis, pyrexia, fatigue, asthenia, oedema, ALT increa se, AST increased. Common: pneumonia, febrile neutropenia, lymphopenia, infusion related reaction, adrenal insufficiency, thyroiditis, hyperthyroi dism, hyponatraemia, hypocalcaemia, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), hypertension, pneumonitis, colitis, gastritis, dry mouth, hepatitis, severe skin reactions, erythema, dermatitis acneiform, dermatitis, dry skin, eczema, pain in extremity, arthritis, acute kidney injury, influenza-like illness, chills, blood creatinine increased, blood alkaline phosphatase increased, hypercalcaemia, blood bilirubin increased. In combination with axitinib or lenvatinib: Very Common: urinary tract infection, anaemia, hypothyroidism, decreased appetite, headache, dysgeu sia, hypertension, dyspnoea, cough, diarrhoea, abdominal pain,

BEACON LIMERICK OFFICIALLY OPENS
Limerick’s most advanced private Cardiology, diagnostic imaging and health screening centre, Beacon Limerick, was officially opened last week by Limerick's Senior Hurling Manager John Kiely.

Beacon Hospital is renowned for its dedicated teams of highly skilled medical professionals, and its world leading technologies and equipment.
The new satellite clinic on Barrington Street offers stateof-the-art healthcare services including a comprehensive Cardiology Service, Diagnostic Radiology, Vascular Services, HealthCheck, Endocrinology and a minor surgical day unit.
The Team of Consultant Cardiologists at Beacon Limerick provide comprehensive cardiac consultation and diagnostics services including, ECG, Echocardiograms, Stress ECG, Pacemaker Checks and Holter Monitoring.
Beacon Limerick offers an extensive diagnostic imaging service using the most technologically advanced radiology equipment in Ireland. It is run by a team of experienced Limerick-based Consultant Radiologists and Radiographers offering cardiac CT (only centre in the Mid-West), general CT, Ultrasounds, DEXA Scans,
vascular scanning and X-Rays.
Additionally, Beacon Limerick’s HealthCheck offers a premier health screening service enabling patients to take a proactive approach to their health in order to help identify and make any changes necessary to stay well.
Michael Cullen, CEO of Beacon Hospital said, “We’re delighted to officially open Beacon Limerick today. The clinic is equipped with state-of-the-art modern medical and surgical equipment.
Our consultants, nurses, radiographers, physiologists and administrative staff are top of their field and will bring leading patient care to the people of Limerick and the Mid-West.
This marks a significant milestone for Beacon Hospital as we continue to invest and expand in both our Dublin campus and our regional satellite clinics, with Beacon Limerick a key part of our future.”
CROÍ WELCOMES MEASURES
IN BUDGET 2023 TO IMPROVE DIAGNOSTIC SERVICES FOR HEART VALVE PATIENTS
Following the announcement of Budget 2023, Croí, the cardiac and stroke foundation, has welcomed measures that will improve access to diagnostic services, such as echocardiography, for patients with structural heart conditions, including heart valve disease.
The announcement comes following a Croí report which highlighted inequalities in the availability of echocardiography services and making clear and achievable recommendations to improve access.
The report published by Croí earlier this month also outlined the
need to meet additional demand through recruitment, training, and upskilling of the workforce. Specifically, a structured postgraduate programme in echocardiography aimed at the 35% of cardiac physiologists not yet trained in this important test.
In a positive move, the Government has allocated ¤47 million to facilitate GPs in accessing diagnostic tests, with major implications for access to radiology tests as well as up to 266,500 tests across areas such as echocardiography, spirometry and natriuretic peptide blood tests. The current budget estimates do not include provision for workforce planning however, which will make it more challenging to achieve the best outcome from the funding allocated by the Department of Health.
The charity launched the report, based on data from the National Survey on Echocardiography Services in Leinster House to an audience of TDs, Senators and political staff. The report highlighted enormous disparities in terms of access to echocardiography services for public and private patients, and recommended GP direct access, via resourced community diagnostics, as a means to address them, reduce waiting times and lower the burden on hospital services.
Neil Johnson, Croí Chief Executive responded: “Croí believes that all patients requiring echocardiography services should have equitable and timely access. Our report provided an evidence base to uphold our values and advocate on them, for the benefit of heart patients. We wish to thank Minister Donnelly for taking on board our recommendations, and also his Oireachtas colleagues who listened so intently to our presentation the week before last. We will continue to work constructively with both the Department of Health, the Department of Further and Higher Education, and their respective Oireachtas committees to deliver the best outcomes for heart and stroke patients.”
BAYER NOTICE: SUPPLY OF MICROLITE 100/20 MICROGRAM TABLETS - SUPPLY OF YASMIN 0.03MG/3MG FILM-COATED TABLETS
Bayer Limited regrets to advise that there will be a temporary interruption in the supply of Microlite 100/20 Microgram Tablets and Yasmin 0.03mg/3mg FilmCoated Tablets.
The anticipated out-of-stock date is currently mid-October 2022, but this may change if demand decreases or increases. At this point the earliest stock is expected to return to the market is the midNovember 2022.
The return to supply will be notified through the IPU medicines shortages list and the HPRA Medicinal Product Shortages webpage.
We suggest that patients and users affected by the temporary shortage consult their treating physicians.
Bayer apologises for any inconvenience caused by this supply shortage.
For queries regarding availability of stock please contact customer service at Tel: +353 1 206 1520 or ordersireland@bayer.com For other queries regarding these products please contact Bayer Ltd., 1st Floor The Grange Offices, The Grange, Brewery Road, Stillorgan, Co. Dublin, A94 H2K7; Tel: +353 1 216 3300;
Fax: +353 1 206 1456; E-mail: info.ireland@bayer.com
EUROPEAN COMMISSION APPROVES UPADACITINIB
FOR THE TREATMENT OF ADULTS WITH MODERATE TO SEVERELY ACTIVE ULCERATIVE COLITIS
This medicinal product is subject to additional monitoring. This will allow for quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA pharmacovigilance website www.hpra.ie . Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
AbbVie (NYSE: ABBV) has announced the European Commission (EC) approval of Upadacitinib (45 mg [induction dose] and 15 mg and 30 mg [maintenance doses]) for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.*
"Our years of experience and long-term investment in IBD research have given us invaluable insights into the challenges that ulcerative colitis patients face, and a deep understanding of the ongoing need for additional treatment options to help those
still suffering,” said Andres Rodrigo, General Manager of AbbVie in Ireland. "We celebrate the approval of Upadacitinib by the EC as it meaningfully expands our ability to help indicated patients in need of relief from ulcerative colitis."
The EC approval is supported by data from two induction studies, U-ACHIEVE induction and U-ACCOMPLISH, and one maintenance study, U-ACHIEVE maintenance.2 Statistical significance was achieved for the primary endpoint and all secondary endpoints with Upadacitinib 45 mg in the two induction studies and both Upadacitinib 15 mg and 30 mg in the maintenance study.
Clinical Remission†
• During the U-ACHIEVE and U-ACCOMPLISH induction trials, 26 percent and 33 percent of patients treated with Upadacitinib 45 mg achieved clinical remission at week 8, the primary endpoint, compared to 5 percent and 4 percent of patients who received placebo.2,11,12
• During the U-ACHIEVE maintenance trial, 42 percent and 52 percent of patients treated with Upadacitinib 15 mg or 30 mg, respectively, achieved clinical remission at week 52, the primary endpoint, compared to 12 percent of patients who received placebo.2,13
• Additionally, 57 percent and 68 percent of patients receiving Upadacitinib 15 mg or 30 mg, respectively, achieved corticosteroid-free remission, defined as clinical remission (per Adapted Mayo Score) and corticosteroid free for ≥90 days immediately preceding week 52 among patients who achieved clinical remission at the end of the induction treatment, compared to 22 percent of patients on placebo.2,13
Clinical Response & Mucosal Healing‡§
• Seventy-three and 74 percent of patients treated with Upadacitinib 45 mg achieved clinical response (per Adapted Mayo Score) at week 8 compared to 27 and 25 percent of patients receiving placebo during the U-ACHIEVE and U-ACCOMPLISH induction trials, respectively.1,2,11,12,14
• In both trials, a significantly greater proportion of patients experienced clinical response per partial Adapted Mayo Score
(symptomatic response) as early as week 2 (U-ACHIEVE: 60 percent vs 27 percent and U-ACCOMPLISH: 63 percent vs 26 percent).1,2,11,12,14
• Mucosal healing was observed in 36 percent and 44 percent of patients treated with Upadacitinib 45 mg in U-ACHIEVE and UACCOMPLISH, respectively, at week 8, compared to 7 percent and 8 percent of patients, respectively, who received placebo.1,2
• In the maintenance study at week 52, mucosal healing was observed in 49 percent and 62 percent of patients treated with Upadacitinib 15 mg and 30 mg, respectively, compared to 14 percent who received placebo.1,2,13
HALEON LAUNCHES CENTRE FOR HUMAN SCIENCES
Haleon (LSE/NYSE: HLN) has launched the Haleon Centre for Human Sciences (“HCHS”), bringing together the expertise of practising health professionals and scientists, to address the behavioural challenges impacting everyday health.
Unveiled at the International Pharmaceutical Federation (FIP) congress in Seville, the Centre will operate as a community of health professionals and specialists in behavioural science, health psychology and the social sciences. This collaboration will aim to solve some of the most pressing everyday health challenges.
The HCHS is the first major initiative for health professionals since Haleon’s launch as an independent, global leader in consumer health. Its mission is to support practicing health professionals - pharmacists, pharmacy assistants and dental professionals - in serving their patients and communities.
Leveraging Haleon’s competitive advantage of combining trusted science with deep human understanding, the Centre will bring expertise in physiological science together with human sciences to deliver real world-solutions and tangible interventions, resulting in measurable improvements in health outcomes through sustained behavioural change.
The launch of the HCHS comes at a crucial time. A recent survey undertaken by Haleon revealed that 90% of pharmacists across Europe and the US believe that
the role of the pharmacist has changed, requiring them to have greater expertise in influencing human behaviour and choices in patients’ health.
How It Works
The HCHS community will design, pilot, implement and evaluate solutions in real world settings. It will tackle issues one at a time, starting with pain management for the inaugural programme. Pain is the most common reason people seek healthcare and is the leading cause of disability in the world [Lancet 2021].[i] Haleon has long championed the role of pharmacists and pharmacy services in pain management and strongly believes that with the right support, an enhanced role would be of great benefit to patients and healthcare systems more broadly. Facilitated by Robert Horne, Professor of Behavioural Medicine at UCL School of Pharmacy, pharmacists can expect to see the first solution from the HCHS delivered in 2023.
Tess Player, VP Global Head of Expert/Health Professionals at Haleon, said, “As a global leader in consumer health, Haleon’s purpose is to deliver better everyday health with humanity. We know that some of the barriers to that are behavioural. The potential for the application of human sciences to help improve people’s health by influencing behavioural change, is astounding. The key to unlocking this lies with health professionals. While primarily students of medicine, their changing roles requires them to become experts in influencing patient behaviour. This is where we can help. We have long heard from practising health professionals that they would benefit from greater opportunity and resources to upskill themselves in behavioural and social sciences in an easily accessible way. 91% of pharmacists would like more support to develop strategies for helping patients adopt new and sustainable health behaviours. With the launch of our Centre for Human Sciences, we are committing to removing this barrier so they can focus on improving everyday health outcomes in the communities they serve.”
“Through a deep understanding of the human factors behind health decisions and behaviours, and the development of practical solutions, the Centre’s community can tackle some of the very real challenges pharmacy professionals experience. This is just one example of how Haleon
is delivering on its purpose and raising the bar in the level of support for health professionals, patients, and everyday health.”
Late-breaking positive results from a Phase 3 trial evaluating the investigational use of Dupixent® (dupilumab) in children aged 1 to 11 years with active eosinophilic esophagitis (EoE) will be presented at United European Gastroenterology (UEG) Week 2022. The data will be submitted to regulatory authorities around the world, starting with the U.S. Food and Drug Administration (FDA) in 2023. In May 2022, Dupixent 300 mg weekly was approved by the FDA to treat EoE in people aged 12 years and older, weighing at least 40 kg.
Dupixent led to significant improvements in the primary efficacy measure for higher (n=37) and lower (n=31) dose groups at 16 weeks in the randomized, placebo-controlled Phase 3 trial. Among children treated with Dupixent, 68% of children on higher dose and 58% of patients on lower dose achieved the primary endpoint of significant histological disease remission, compared to 3% for placebo (both p<0.0001). Children on the higher dose regimen also experienced significant improvements in abnormal endoscopic findings of their esophagus, with a reduction of 3.5 points compared to an increase of 0.3 points for placebo (p<0.0001). Symptomatically, higher dose Dupixent led to a numerical improvement in the proportion of days children experienced disease symptoms from baseline as reported by their caregivers compared to placebo, though not statistically significant. Additionally, a prespecified exploratory analysis was presented which found higher dose Dupixent led to a 3.09 percentile increase in body weight for age percentile from baseline, compared to 0.29 for placebo.
Safety results were generally consistent with the known safety profile of Dupixent in its approved EoE indication for children and adults aged 12 years and older who weigh at least 40 kg. For the 16-week treatment period, the overall rates of adverse events (AEs) were 79% (higher dose
DUPIXENT® (DUPILUMAB) LATE-BREAKING PHASE 3 DATA PRESENTED AT UEG WEEK 2022 SHOWED SIGNIFICANT HISTOLOGICAL REMISSION OF EOSINOPHILIC ESOPHAGITIS (EOE) IN CHILDREN 1 TO 11 YEARS OLD
n=27/37, lower dose n=26/30) for Dupixent and 91% (n=31/34) for placebo. AEs most commonly observed with Dupixent (≥5%) compared to placebo included COVID-19 (higher dose n=5/37, lower dose n=9/30, placebo n=0/34; all cases were mild or moderate and did not lead to study discontinuation), rash (higher dose n=3/37, lower dose n=3/30, placebo n=2/34), headache (higher dose n=1/37, lower dose n=4/30, placebo n=1/34), viral gastroenteritis (higher dose n=4/37, lower dose n=0/30, placebo n=1/34), diarrhea (higher dose n=2/37, lower dose n=2/30, placebo n=1/34) and nausea (higher dose n=1/37, lower dose n=3/30, placebo n=0/34). Rates of treatment discontinuation due to AEs prior to week 16 were 0% (higher dose n=0/37, lower dose n=0/30) for Dupixent and 6% (n=2/34) for placebo.
The potential use of Dupixent in children with EoE aged 1 to 11 years is currently under clinical development, and the safety and efficacy have not been evaluated by any regulatory authority.
TILRAY MEDICAL RELAUNCHES CANNABIS ORAL SOLUTION ACROSS IRELAND
Tilray Brands, Inc. ("Tilray" or the "Company") (Nasdaq: TLRY; TSX: TLRY), a leading global cannabis company inspiring and empowering the worldwide community to live their very best life, have announced that it has successfully relaunched its EU GMP-produced medical cannabis oral solution in Ireland under the country’s Medical Cannabis Access Program (MCAP). Tilray’s approved product has also recently received reimbursement approval, which gives patients cost reimbursement and ensures more eligible patients can access quality medical cannabis under Ireland’s MCAP.
Denise Faltischek, Tilray Brands’ Chief Strategy Officer and Head of International Business, said, “We are extremely proud to relaunch in Ireland with an expanded footprint. The MCAP reimbursement approval is a tremendous step in providing patients with greater access to Tilray’s high-quality medical cannabis products that address their needs. We remain dedicated to do our part to increase access for patients in need around the world.”
Patients in Ireland may now access Tilray’s full-spectrum medical cannabis oil solution through the MCAP. Tilray intends to supply Irish patients with its full suite of
medical cannabis products when local regulations allow.
Tilray Medical is a leading provider of EU-GMP certified medical cannabis products in 22 countries with a comprehensive portfolio of THC and CBD products. Each medical cannabis product that Tilray offers has been selected to ensure patients can receive both the highest product quality as well as consistency when it comes to supply of their medicinal cannabis products.
THE LAUNCH OF SOLFEROL CAPSULES, A PRESCRIPTION RANGE OF VITAMIN D3 MEDICINES

Windzor Pharma Ireland Ltd is please to announce the launch of SOLFEROL Softgel Capsules, a range of Vitamin D3 medicines, for the treatment of Vitamin D deficiency.
This brand offers the widest range of presentations on the market 5 different strengths, including two unique strengths, 400iu for the treatment of pregnant and breast feeding women, and 20,000iu for patients who are severely deficient in Vitamin D3.
The demand for Vitamin D3 has grown strongly in recent years due to the Covid pandemic and the need for bone health in elderly patients.
Solferol Softgel Capsules were developed in Ireland and are sold all across Europe.
The product range is now available in all pharma wholesalers and listed on all software prescribing systems nationally. All five presentations are available on the reimbursement schemes, with a better price for all pack sizes.
The full range is as follows:
SOLFEROL Softgel Capsules 400iu x 90
SOLFEROL Softgel Capsules 800iu x 30
SOLFEROL Softgel Capsules 800iu x 90
SOLFEROL Softgel Capsules 1000iu x 28
SOLFEROL Softgel Capsules 20000iu x 4.
Full prescribing information available on www.windzorpharma.com
WALK IN MY SHOES
As Walk in My Shoes, the flagship awareness-raising campaign of St Patrick’s Mental Health Services, celebrated World Mental Health Day on 10 October with the return of its WIMS Live initiative, the campaign’s newest ambassador has been announced. Grace Mongey Gernon of Instagram’s FacesbyGrace, and mental health advocate, will be working with Walk in My Shoes over the coming year to promote youth mental health and to tackle mental health stigma.
Using her skills and experience as a social media content creator, Grace will help Walk in My Shoes to amplify their message and support them in promoting positivity online through campaigns
such as #MindYourSelfie and WIMS Live.
Speaking about Walk in My Shoes’ newest ambassador, Paul Gilligan, CEO, St Patrick’s Mental Health Services, said: “We are delighted to welcome Grace on board as the new Walk in My Shoes ambassador. We look forward to working with Grace over the coming year to empower young people to live mentally healthy lives, to tackle mental health stigma and to raise awareness of mental health difficulties.
For the past 10 years, Walk in My Shoes has been at the forefront of promoting youth mental health through our education and awareness initiatives, and each year, the Walk in My Shoes ambassador plays a significant role in helping us to educate and advocate for young people in their mental health journeys. We are very lucky to have so many supporters and advocates willing to lend their support to the Walk in My Shoes campaign, and we are very grateful for the important work that they do.”
The announcement of Grace Mongey as the latest Walk in My Shoes ambassador comes as WIMS Live, an exciting online programme of wellbeing events, activities and curated content, returned this year to mark World Mental Health Day on 10 October.
Running from 10 October to 16 October, this year’s WIMS Live theme focused on shining a light on the lesser understood or commonly misrepresented mental health difficulties such as psychosis, schizophrenia and bipolar disorder.

ACCORD LAUNCH SONDELBAY®
(TERIPARATIDE) IN IRELAND
Sondelbay® – is a self-administered, once-a-day subcutaneous injection for patients with osteoporosis using a pen device.

Accord Healthcare Ireland have announced the launch of Sondelbay® to the Irish market. Sondelbay® adds to the company’s existing portfolio of High-Tech medicines and is the companies fourth biosimilar product, it is also the companies first branded product in the new Bone Health franchise representing another great milestone in Accord Healthcare Irelands growing portfolio.
In January Accord Healthcare received a positive opinion recommending the marketing authorisation for Sondelbay® as a biosimilar of the reference product Forsteo®. It is indicated for treatment of osteoporosis in postmenopausal women and in men at increased risk of fracture. In postmenopausal women, a significant reduction in the incidence of vertebral and nonvertebral fractures but not hip fractures has been demonstrated. Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk for fracture.1
Tracy Kivlehan, Accord Healthcare Ireland, Head of Hospitals and Specialty Brands, said “We are proud to launch Sondelbay® in Ireland, as an addition to our emerging Bone Health franchise. We know the problems patients with osteoporosis face such as weak hands, poor grip and diminished dexterity, and that’s why we are delighted to bring our product to the market. We have taken patient and HCP concerns into consideration when creating our device and this has influenced the design, our pen comes with a dose counter and side injection button.2 We truly believe in putting the patient at the heart of everything we do and we are committed to delivering for patients and Healthcare Professionals across the country”.
Accord, part of the Intas group, will manufacture the medicine in its own state of the art facility, where they now have the second highest number of biosimilars in phase III to approval in the world.
XBIOTECH ANNOUNCES FIRST PATIENT ENROLLED INTO THE FRENCH NATIONAL CANCER INSTITUTE (INCA) SPONSORED PHASE I/II/III CLINICAL STUDY FOR NATRUNIX™ THERAPY FOR COLORECTAL CANCER
INCA funded Phase I/II/III Study for Natrunix in Combination with Trifluridine/Tipiracil, the TASKIN Study, Launches at 20 Leading Medical Centers in France
XBiotech (NASDAQ: XBIT) has announced the enrollment of the first patient in a multicenter, randomized clinical study for Natrunix in combination with trifluridine/tipiracil for the treatment of colorectal cancer. The much anticipated clinical study for XBiotech’s candidate cancer treatment is being funded by the French National Cancer Institute (INCA). The study is headed by renown oncologists Dr. François Ghiringhelli and Dr. Come Lepage, Professor in Medical Oncology and Director of the INSERM research team at the GeorgesFrancois Leclerc Cancer Centre, and Professor at the Department of Gastroenterology and Digestive Oncology, University Hospital Dijon, Dijon, France, respectively.
Investigators are combining XBiotech’s Natrunix with trifluridine/tipiracil as a new candidate therapy for metastatic colorectal cancer. Subjects receiving the experimental therapy have failed earlier treatment with oxaliplatin, irinotecan, and fluoropyrimidine. Subjects are randomized to receive Natrunix plus trifluridine/tipiracil chemotherapy or placebo plus the chemotherapy. The study is designed to seamlessly proceed through Phase III development based on achievement of certain efficacy milestones in the Phase I/ II portions.
Natrunix is a therapeutic monoclonal antibody discovered, manufactured, and undergoing clinical development by XBiotech. The antibody blocks the activity of substance produced by tumors and inflammatory cells that stimulates new blood vessel formation and breaks down connective tissue at the site of the tumor, allowing tumors to grow and spread. The same substance also activates blood vessels, making them sticky to enhance migration of circulating tumor cells to new sites of metastasis.
Natrunix potently blocks the action of the substance, known as IL-1, which is also produced in response to chemotherapy.
Colorectal cancer is one of the most common forms of cancer in Europe and the United States, with the American Cancer Society’s estimating over 151,000 new cases and over 52,000 deaths annually in the United States alone.
ACCORD HEALTHCARE LAUNCH ZOLSKETIL® PEGYLATED LIPOSOMAL DOXORUBICIN 2 MG/ML

Accord Healthcare is delighted to announce the launch of Zolsketil® pegylated liposomal 2 mg/ml concentrate for dispersion for infusion in a 20 mg/10 mL and 50 mg/ 25 mL vial.
Zolsketil® is the first ever generic version of Caelyx® in Europe1,2 and is crafted to the equally exacting standards as the originator product1,3
This medicine is indicated for the treatment of several cancers:1
• As monotherapy for patients with metastatic breast cancer with increased cardiac risk
• For treatment of advanced ovarian cancer in women who have failed a first-line platinumbased chemotherapy regimen
• In combination with bortezomib for the treatment of progressive multiple myeloma in patients who have received at least one prior therapy and who have already undergone or are unsuitable for bone marrow transplant
• For treatment of AIDS-related Kaposi’s sarcoma in patients with low CD4 counts (<200 CD4 lymphocytes/mm3) and extensive mucocutaneous or visceral disease
Please refer to the Summary of Product Characteristics (SPC) for further information. The SPC will be available from the launch date at www.hpra.ie and for Healthcare Professionals on the Accord Healthcare website www.accord-healthcare.ie
For further information please contact Accord in Cork on 021-461 9040 or visit their website www.accord-healthcare.ie
TIZIANA LIFE SCIENCES PLANS TO SUBMIT IND FOR PHASE 1 TRIAL OF INTRANASAL FORALUMAB IN ALZHEIMER'S DISEASE PATIENTS
Tiziana Life Sciences Ltd. (Nasdaq: TLSA) (“Tiziana” or the “Company”), a biotechnology company developing breakthrough immunomodulation therapies via novel routes of drug delivery, has announced that it plans to submit an Investigational New Drug Application (IND) for a Phase 1 Trial of intranasal foralumab in Alzheimer's disease patients after receiving an affirmative written response from the FDA on a Pre-Investigational New Drug Application (PIND). Tiziana plans on filing the IND for Alzheimer’s disease by the third quarter of 2023 upon the completion of requested toxicology studies, then starting its Phase 1 program by the end of 2023.
“I am thrilled to see the company advancing foralumab into another promising central nervous system (CNS) -related therapeutic area with high unmet need,” commented Gabriele Cerrone, Executive Chairman and interim Chief Executive Officer of Tiziana Life Sciences. “Intranasal foralumab’s unique action on regulatory T-cells should allow us to study multiple CNS inflammatory pathology indications for this unique drug, including in Multiple Sclerosis (MS) and Amyotrophic Lateral Sclerosis (ALS).”
“We have spent years studying intranasal anti-CD3, or foralumab, in inflammatory CNS-related disease models in animals,” said Howard L. Weiner, M.D., Co-Director of the Ann Romney Center for Neurologic Diseases at Brigham and Women’s Hospital. “We look forward to evaluating intranasal foralumab in patients with early symptomatic Alzheimer’s disease, where we believe its locally acting antiinflammatory mechanism of action will be relevant.”
“Tiziana strongly believes that many inflammatory CNS disease pathologies could improve with intranasally administered foralumab and as such, we hope to study additional neurological disease states over time,” stated Matthew Davis, M.D., RPh, Chief Scientific Officer and Chief Medical Officer of Tiziana Life Sciences.
the FLAURA Study,
showed
months median

1st-generation EGFR TKIs* in the FLAURA study
0.799; 95.05% CI, 0.641 to 0.997; P=0.0462)1
Adapted from: TAGRISSO. Summary of Product Characteristics.
and erlotinib
THE FLAURA
Progression-free survival was the primary endpoint. Overall survival was a prespecified secondary endpoint.2
AS MONOTHERAPY IS INDICATED FOR:
• the adjuvant treatment after complete tumour resection in adult patients with stage IB-IIIA non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations.†
• the first-line treatment of adult patients with locally advanced or metastatic NSCLC with activating EGFR mutations.
the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC.
substitution mutations; the first-line treatment of adult patients with locally advanced or metastatic non-small-cell lung cancer (NSCLC) with activating EGFR mutations and the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC. Presentation: 40mg and 80mg osimertinib (as mesylate) film-coated tablets. Dosage and Administration: Treatment should be initiated by a physician experienced in the use of anticancer therapies. When considering the use of TAGRISSO, EGFR mutation status (in tumour specimens for adjuvant treatment and tumour or plasma specimens for locally advanced or metastatic setting) should be determined using a validated test method. The recommended dose is 80mg once a day, taken with or without food at the same time each day. Patients in the adjuvant setting should receive treatment until disease recurrence or unacceptable toxicity. Treatment duration for more than 3 years was not studied. Patients with locally advanced or metastatic lung cancer should receive treatment until disease progression or unacceptable toxicity. If a dose is missed, the dose should be made up unless the next dose is due within 12 hours. Should be swallowed whole with water and it should not be crushed, split or chewed. If patients are unable to swallow, the tablet may be dispersed in water (non-carbonated). No other liquids should be used. The dispersion can also be administered through a nasogastric tube. Refer to SmPC for full instructions.
Dose adjustments: If required to manage safety and tolerability, then dose should be reduced to 40mg taken once daily. Special populations: Hepatic impairment: No dose adjustments are necessary in patients with mild hepatic impairment (Child Pugh A) (total bilirubin ≤upper limit of normal (ULN) and aspartate aminotransferase (AST) >ULN or total bilirubin >1.0 to 1.5x ULN and any AST) or moderate hepatic impairment (Child Pugh B) (total bilirubin between 1.5 to 3 times ULN and any AST). Use in patients with severe hepatic impairment is not recommended. Renal impairment: No dose adjustments are necessary in patients with mild, moderate, or severe renal impairment. Caution should be exercised when treating patients with severe and end stage renal impairment. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Should not be used with St. John’s Wort. Warnings and Precautions: Interstitial Lung Disease (ILD): Patients with acute onset and/or unexplained worsening of pulmonary symptoms (dyspnoea, cough, fever) should have their treatment interrupted while investigations are performed to exclude ILD. If ILD is diagnosed, TAGRISSO should be discontinued and appropriate treatment initiated as necessary. Reintroduction should be considered only after careful consideration of the individual patient’s benefits and risk. Stevens-Johnson syndrome (SJS): Before initiating treatment, patients should be advised of signs and symptoms of SJS. If suggestive, TAGRISSO should be interrupted or discontinued immediately. QTc interval prolongation: May lead to an increased risk for ventricular tachyarrhythmias (e.g. torsade de pointes) or sudden death. When possible, TAGRISSO should be avoided in patients with congenital long QT syndrome. Periodic monitoring with electrocardiograms and electrolytes should be considered in patients with congestive heart failure, electrolyte abnormalities, or those who are taking medicinal products that are known to prolong the QTc interval. Treatment should be withheld in patients who develop a QTc interval greater than 500 msec on at least 2 separate ECGs until the QTc interval is less than 481 msec or recovery to baseline if the QTc interval is greater than or equal to
481 msec, then resume TAGRISSO at a reduced dose. TAGRISSO should be permanently discontinued in patients who develop QTc interval prolongation in combination with any of the following: torsade de pointes, polymorphic ventricular tachycardia, signs/symptoms of serious arrhythmia. Changes in cardiac contractility: In patients with cardiac risk factors and those with conditions that can affect LVEF, cardiac monitoring, including an assessment of LVEF at baseline and during treatment, should be considered. In patients who develop relevant cardiac signs/symptoms during treatment, cardiac monitoring including LVEF assessment should be considered. Keratitis: Patients with signs and symptoms suggestive of keratitis such as acute or worsening: eye inflammation, lacrimation, light sensitivity, blurred vision, eye pain and/or red eye should be referred promptly to an ophthalmology specialist. Age and body weight: Elderly patients (>65 years) or patients with low body weight (<50 kg) may be at increased risk of developing adverse events of Grade 3 or higher. Close monitoring is recommended. Drug Interactions: Concomitant use of strong CYP3A inducers (e.g. phenytoin, rifampicin and carbamazepine) should be avoided. Moderate CYP3A4 inducers (e.g. bosentan, efavirenz, etravirine, modafinil) should be used with caution, or avoided when possible. Closely monitor patients taking concomitant medicinal products with disposition dependent upon breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) and with narrow therapeutic index. Concomitant use of St. John’s Wort is contraindicated. Pregnancy and Lactation: TAGRISSO should not be used during pregnancy unless the clinical condition of the woman requires treatment with TAGRISSO. Discontinue breast-feeding during treatment with TAGRISSO. Women of childbearing potential should be advised to avoid becoming pregnant if they or their partner are being treated with TAGRISSO. Undesirable Events: Consult SmPC for full list of adverse events. Very common (all grades): Decreased appetite, diarrhoea, stomatitis, rash, dry skin, paronychia, pruritus, platelet count decreased, leucocytes decreased, lymphocytes decreased, neutrophils decreased. Common (all grades): Epistaxis, interstitial lung disease, alopecia, urticaria, palmar-plantar erythrodysaesthesia syndrome, blood creatine phosphokinase increased, blood creatinine increased. Uncommon (all grades): Keratitis, QTc interval prolongation, erythema multiforme, cutaneous vasculitis. Rare (all grades): Stevens-Johnson syndrome, myositis. Legal Category: Product subject to prescription which may not be renewed (A). Marketing Authorisation Number: EU/1/16/1086/001 and EU/1/16/1086/002. Marketing Authorisation Holder: AstraZeneca AB, SE-151 85, Södertälje, Sweden. Further information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 7100. TAGRISSO is a trade mark of the AstraZeneca group of companies. Date of API Preparation: 03/2022 Veeva ID: IE-3782
Adverse events
reported directly to: HPRA Pharmacovigilance. Website: www.hpra.ie. Adverse
to AstraZeneca Patient Safety on Freephone 1800 800 899.
