CPD HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication CPD
Professional Development 2022
Continuing
and 2mg film-coated tablets

This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.Important note: Before prescribing, consult Summary of Product Characteristics (SmPC). Presentation: Tablets: 0.25 mg film-coated tablets corresponding to 0.25 mg siponimod. 2 mg filmcoated tablets corresponding to 2 mg siponimod. ♦Excipient with known effect: Each tablet of 0.25 mg contains 59.1 mg lactose (as monohydrate) and 0.092 mg soya lecithin. Each tablet of 2 mg contains 57.3 mg lactose (as monohydrate) and 0.092 mg soya lecithin. Indications: Treatment of adult patients with secondary progressive multiple sclerosis (SPMS) with active disease evidenced by relapses or imaging features of inflammatory activity. Dosage and administration (D&A): Treatment with Mayzent should be initiated and supervised by a physician experienced in the management of multiple sclerosis. CYP2C9 genotype should be determined before initiation of treatment. Mayzent should not be used in patients with a CYP2C9*3*3 genotype. Treatment initiation with a titration pack that lasts for 5 days. Once daily intake in the morning. On day and 2: 0.25 mg. On day 3: 0.5 mg. On day 4: 0.75 mg. On day 5: 1.25 mg. Maintenance dose starts on day 6. Adults: Maintenance dose: 2 mg once daily. Maintenance dose for CYP2C9 *2*3 or *1*3 genotype: 1 mg once daily. No dose adjustments are needed in patients with renal impairment. Caution should be exercised when initiating treatment in patients with mild or moderate hepatic impairment (see section CI). Mayzent should be used with caution in the elderly patients (65 years or above) due to insufficient data on safety and efficacy. ♦Missed dose and re-initiation: If a dose is missed on one day in the first 6 days of treatment or if 4 or more consecutive daily doses are missed during maintenance therapy, the same initial dose titration and monitoring recommendations should apply. Contraindications (CI): Hypersensitivity to the active substance, or to peanut, soya or any of the excipients listed. ♦Immunodeficiency syndrome. ♦History of progressive multifocal leukoencephalopathy (PML) or cryptococcal meningitis (CM). ♦Active malignancies. ♦Severe liver impairment (Child Pugh class C). ♦Patients who in the previous 6 months had a myocardial infarction (MI), unstable angina pectoris, stroke/transient ischaemic attack (TIA), decompensated heart failure (requiring inpatient treatment), or New York Heart Association (NYHA) class III/IV heart failure. ♦Patients with a history of second degree Mobitz type II atrioventricular (AV) block, third degree AV block, sino atrial heart block or sick sinus syndrome, if they do not wear a pacemaker. ♦Patients homozygous for CYP2C9*3 (CYP2C9*3*3) genotype (poor metaboliser). ♦During pregnancy and in women of childbearing potential not using effective contraception. Warnings and precautions (W&P): ♦Infections: Before initiating treatment with Mayzent, a recent complete blood count (CBC) (i.e. within last 6 months or after discontinuation of prior therapy) should be available. Assessments of CBC are also recommended periodically during treatment. Absolute lymphocyte counts <0.2 x 109/l, if confirmed, should lead to dose reduction to 1 mg. Confirmed absolute lymphocyte counts <0.2 x 109/l in such a patient (already receiving 1 mg) should lead to interruption of Mayzent until the level reaches 0.6 x 109/l when re initiation of Mayzent can be considered. In patients with severe active infection, wait for resolution before initiating treatment. Patients should be instructed to report symptoms of infection to their physician promptly. Effective diagnostic and therapeutic strategies should be used in patients with symptoms of infection while on therapy and up to 3 to 4 weeks after discontinuation. Consider discontinuing therapy if a serious infection develops. Vigilance is advised for clinical symptoms or magnetic resonance imaging (MRI) findings suggestive of PML or for clinical symptoms of CM and, if suspected, Mayzent treatment should be suspended until PML or CM can be excluded. If diagnosed, appropriate treatment should be initiated. Patients without a healthcare professional confirmed history of varicella or without vaccination against varicella zoster virus (VZV) should be tested for antibodies to VZV prior to treatment initiation. ♦Vaccination: VZV vaccination is recommended in antibody-negative patients and initiation of treatment should be postponed for 1 month to allow the full effect of vaccination to occur. Concomitant use is not recommended with live attenuated vaccines and for 4 weeks after stopping Mayzent therapy. Vaccines may be less effective if administered during Mayzent treatment. Treatment discontinuation 1 week prior to planned vaccination until 4 weeks after is recommended. ♦Anti-neoplastic, immune-modulating or immunosuppressive therapies (including corticosteroids): Caution is required when used concomitantly with Mayzent and in the weeks after administration of any of these medicinal products is stopped. ♦Macular edema: Siponimod therapy should not be initiated in patients with macular oedema until resolution. An ophthalmic examination is recommended 3 to 4 months after Mayzent therapy initiation in all patients. In patients with history of diabetes mellitus, uveitis or underlying/co-existing retinal disease Mayzent should be used with caution due to potential increase of risk of macular oedema and an opthalmic examination is recommended prior to and regularly during therapy. Discontinuing therapy is recommended if macular edema develops. After resolution, reinitiation of treatment after discontinuation should be based on the potential benefits and risks for the individual patient. ♦Bradyarrhythmia and Treatment initiation with certain pre existing cardiac conditions: See section CI. ♦Patients with the following cardiac conditions should be observed for a period of 6 hours after the first dose of Mayzent for signs and symptoms of bradycardia: sinus bradycardia (heart rate <55 bpm), history of first- or second- degree (Mobitz type I) AV block, history of myocardial infarction, or history of heart failure (patients with NYHA class I and II). In these patients, it is recommended that an electrocardiogram (ECG) is obtained prior to dosing and at the end of the observation period. If post dose bradyarrhythmia or conduction related symptoms occur or if ECG 6 hours post dose shows new onset second degree or higher AV block or QTc ≥500 msec, appropriate management should be initiated and observation continued until the symptoms/findings have resolved. If pharmacological treatment is required, monitoring should be continued overnight and 6 hour monitoring should be repeated after the second dose. ♦Due to the risk of serious cardiac rhythm disturbances or significant bradycardia Mayzent should not be used in patients with: history of symptomatic bradycardia or recurrent syncope, uncontrolled hypertension, or severe untreated sleep apnoea. In such patients, treatment with siponimod should be considered only if the anticipated benefits outweigh the potential risks, and advice from a cardiologist should be sought prior to initiation of treatment in order to determine the most appropriate monitoring strategy. ♦Mayzent should not be used in patients with significant QT prolongation (QTc >500 msec) or who were treated with QT prolonging medicinal products with known arrhythmogenic properties. ♦Mayzent should not be used in patients with arrhythmias requiring treatment with class Ia (e.g. quinidine, procainamide) or class III (e.g. amiodarone, sotalol) antiarrhythmic medicinal products (risk of torsades de pointes). ♦Mayzent should not be used in patients receiving concurrent therapy with heart-rate-lowering calcium channel blockers (such as verapamil or diltiazem) or other substances that may decrease heart rate (e.g. ivabradine or digoxin) (risk of severe bradycardia and heart block). ♦If concomitant treatment with one of the above substances is being considered during initiation of treatment with Mayzent, advice from a cardiologist should be sought regarding the switch to a nonheart-rate-lowering medicinal product or appropriate monitoring for treatment initiation. ♦At treatment initiation, use with caution in patients receiving
stable dose of beta-blocker if resting heart rate is ≤50 bpm. In this case, beta-blocker should be interrupted until the baseline heart rate is >50 bpm. Mayzent treatment can then be started and treatment with beta blocker can be re-initiated after up-titration to Mayzent maintenance dose. ♦Initiation of Mayzent treatment results in a transient decrease in heart rate and has been associated with transient atrioventricular conduction delays; therefore a titration scheme to reach the maintenance dose on day 6 is applied. After the first dose, the heart rate decrease starts within one hour and the day 1 decline is maximal at approximately 3 to 4 hours. With continued up titration, further heart rate decreases are seen on subsequent days, with maximal decrease reached on day 5 to 6. The highest daily post-dose decrease in absolute hourly mean heart rate is observed on day 1, post-dose declines on the following days are less pronounced. Heart rate returns to placebo levels within 10 days after treatment initiation. ♦Liver function: Recent (i.e. within last 6 months) transaminase and bilirubin levels should be available before initiation of treatment with Mayzent. A liver function test is recommended in patients who develop symptoms suggestive of hepatic dysfunction during treatment and therapy should be discontinued if significant liver injury is confirmed. After resolution, reinitiation of treatment should be based on the potential benefits and risks for the individual patient. Caution should be exercised in patients with a history of significant liver disease. See section CI. ♦Cutaneousneoplasms: Mayzent should not be used in patients receiving concomitant phototherapy with UV-B radiation or PUVA photochemotherapy. Skin examination is recommended for all patients at treatment initiation, and then every 6 to12 months taking into consideration clinical judgement. Patients should be advised to promptly report any suspicious skin lesions to their physician. Caution is required against exposure to sunlight without protection in patients treated with Mayzent. ♦Unexpected neurological signs: Vigilance is warranted for any unexpected neurological or psychiatric symptoms/signs or accelerated neurological deterioration (posterior reversible encephalopathy syndrome). ♦Prior treatment with immunosuppressive or immune modulating therapies: Caution is required when switching patients from other disease modifying therapies (the half-life and mode of action of the other therapy must be considered). A CBC is recommended prior to initiating Mayzent to ensure that immune effects of the previous therapy (i.e. cytopenia) have resolved. Initiating treatment with Mayzent after alemtuzumab is not recommended. ♦Blood pressure: Special care is indicated if patients with uncontrolled hypertension are treated with Mayzent. Blood pressure should be regularly monitored during treatment. ♦Pharmacogenomics: See section CI for patients with CYP2C9*3*3 genotype (approximately 0.3 to 0.4% of population). See section D&A for CYP2C9 *2*3 or *1*3 genotype. ♦Women of childbearing potential: See section CI. Before initiation of treatment, women of childbearing potential must be informed of the risk to the foetus, must have a negative pregnancy test and must use effective contraception during treatment and for at least 10 days after treatment discontinuation.♦Stopping therapy: Patients should be observed for relevant signs of possible severe exacerbation or return of high disease activity upon Mayzent discontinuation and appropriate treatment should be instituted as required. In vast majority of SPMS patients, lymphocyte counts return to the normal range within 10 days of stopping therapy. However, residual pharmacodynamic effects, such as lowering effects on peripheral lymphocyte count, may persist for up to 3 to 4 weeks after the last dose. ♦Interference with haematological testing: Peripheral blood lymphocyte counts cannot be utilised to evaluate the lymphocyte subset status of a patient treated with Mayzent. ♦Excipients: Peanut or soya: see section CI. Lactose: patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose galactose malabsorption should not take Mayzent. Interactions: ♦Anti-neoplastic, immune-modulating or immunosuppressive therapies (including corticosteroids): See section W&P.♦Anti-arrhythmic drugs, QT prolonging drugs, drugs that may decrease heart rate: See section W&P.♦Betablockers: See section W&P. ♦Vaccination: see W&P. ♦CYP2C9 and CYP3A4 inhibitors: Concomitant use with Mayzent is not recommended with moderate CYP2C9 inhibitors and moderate or strong CYP3A4 inhibitors (can consist of a moderate CYP2C9/CYP3A4 dual inhibitor e.g. fluconazole or a moderate CYP2C9 inhibitor in combination with a separate moderate or strong CYP3A4 inhibitor).♦CYP2C9 and CYP3A4 inducers: Caution is required with strong CYP3A4/moderate CYP2C9 inducers (e.g. carbamazepine) in all patients and with moderate inducers of CYP3A4 (e.g. modafinil) in patients with CYP2C9*1*3 and*2*3 genotype (a reduction in siponimod exposure is expected). Fertility, Pregnancy and Lactation: Pregnancy, women of childbearing potential, contraception in females: see section CI. Before initiation of treatment in women of childbearing potential a negative pregnancy test result must be available and counselling should be provided regarding serious risk to the foetus. Women of childbearing potential must use effective contraception during treatment with Mayzent and for at least 10 days after stopping treatment. Mayzent should be stopped at least 10 days before a pregnancy is planned. If a woman becomes pregnant while on treatment, Mayzent must be discontinued. Medical advice should be given regarding the risk of harmful effects to the foetus associated with treatment and ultrasonography examinations should be performed. Embryotoxicity, fetotoxicity and teratogenicity were demonstrated in animal studies. Breast-feeding: Mayzent should not be used during breast feeding (no data in human lactation are available and siponimod is excreted into animal milk). Fertility: The effect of siponimod on human fertility has not been evaluated. Siponimod had no effect on male reproductive organs in rats and monkeys or on fertility parameters in rats. Driving and using machines: Mayzent has no or negligible influence on the ability to drive and use machines. However, dizziness may occasionally occur when initiating therapy. Therefore, patients should not drive or use machines during the first day of treatment initiation with Mayzent. Undesirable effects: Very common (≥10%): Headache, hypertension, liver function test increased. Common (≥1 to <10%): Herpes zoster, melanocytic naevus, Basal
SLOW DOWN SPMS WITH ACTIVE DISEASE.1 IT’S TIME FOR MAYZENT® For adults. Not actual patients. CDP=confirmed disability progression; CI=confidence interval; Gd+=gadolinium-enhancing; HR=hazard ratio; MOA=mechanism of action; SPMS=secondary progressive multiple sclerosis. * EXPAND was a randomized, double-blind, placebo-controlled, Phase III study with a broad range of 1651 patients with SPMS over 24 months, followed by an optional open-label extension.2 † In a subgroup analysis in EXPAND, SPMS with active disease was defined as patients with relapse in the 2 years prior to the study and/or presence of T1 Gd+ lesions at baseline.1 References: 1. MAYZENT [Summary of Product Characteristics]. Novartis Ireland, available from Novartis Ireland Limited, Vista Building, Elm Park Business Campus Merrion Road, Dublin 4. 2. Kappos L, Bar-Or A, Cree BAC, et al; for the EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263-1273. MAYZENT® is the first and only oral treatment specifically indicated for SPMS with active disease1,2*† August 2021 | IE_145831 Mayzent ▼ (Siponimod) 0.25mg
▼
cell carcinoma, lymphopenia, dizziness, seizure, tremor, macular oedema, bradycardia, atrioventricular block (first & second degree), nausea, diarrhoea, pain in extremity, oedema peripheral, asthenia, pulmonary function test decreased. Please see Summary of Product Characteristics for further information on undesirable effects. Frequency not known: In the extension part of the phase 3 study, a case of cryptococcal meningitis has been reported. Marketing Authorisation Holder: Novartis Europharm Ltd, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland. Marketing Authorisation Numbers, Mayzent 0.25 mg film coated tablets: EU/1/19/1414/001 002. Marketing Authorisation Numbers, Mayzent 2 mg film coated tablets: EU/1/19/1414/003. Prescribing information last revised: Feb 2021. ▼ This medicinal product is subject to additional monitoring. Reporting suspected adverse reactions of the medicinal product is important to Novartis and the HPRA. It allows continued monitoring of the benefit/risk profile of the medicinal product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: drugsafety.dublin@novartis.com or by calling 01 2080 612. ▼
60 Second Summary

Epilepsy is “a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures. Older terms such as “simple partial” and “complex partial” seizures have now largely been replaced by terms “focal aware seizures” or “focal seizures with impaired awareness” in the most recent ILAE classification. The correct identification and classification of seizures and epilepsy syndromes is important as it can have major effects on treatment decisions.
The core investigations remain EEG and dedicated epilepsy protocol MRI brain but more extensive investigations such as genomic analysis and autoantibody screening are being increasingly used due to a greater understanding and becoming more cost effective. Despite the availability of investigations, it is important to recognise that epilepsy is primarily diagnosed on careful clinical history and often all investigations can be normal.
The treatment options available to clinicians in seizures has become broader and it is important to recognise the side effects of the more common anti-seizure drugs, as well as their mechanism of action. Many other medications can affect the metabolism of ASMs and it is important to check for drug interaction prior to the commencement of new medications.
The driving regulations regarding seizures is clearly laid out in the RSA guidelines. The general restriction is 1 year for patients with known seizure disorder and 6 months for first unprovoked seizure or seizures in the context of alcohol. The restrictions regarding group 2 vehicles (buses and trucks) are much more restrictive and requires 10 years of seizure freedom with no medications for patients with known seizures and 5 years of medicine free seizure freedom after a single unprovoked seizure.
Written by Dr Gerard Mullane and Dr Albi Chalissery
Dr Gerard Mullane MB MCh BAO MRCPI is a Neurology Registrar in Beaumont Hospital in the RCSI hospital group.

Dr Albi Chalissery MBBS MRCPI MD is a Consultant Neurologist at Beaumont Hospital Dublin and Connolly Hospital, Blanchardstown in RCSI hospital group. Her specialty interest includes Epilepsy, and she recently established a “New seizure” clinic at Beaumont hospital.
 Dr Albi Chalissery
Dr Albi Chalissery
1. REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by HPN.
Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
Novartis has no editorial oversight of the CPD programmes included in these modules
New onset seizure and Epilepsy: Diagnosis and Management
Introduction
In accordance with the International League Against Epilepsy (ILAE) 2005 definition, epilepsy is “a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures and by the neurobiologic, cognitive, psychological, and social consequences of this condition”. It affects approximately 50 million people worldwide1 and 37,000 in Ireland.2 The term epilepsy encompasses a broad range of seizure semiologies, as well as the increasingly recognised epilepsy syndromes. The classification published by the ILAE in 2017 has empowered clinicians to better understand and differentiate between different subtypes of seizures and epilepsy within this heterogenous disease.3
Classification
The updated 2017 classification of seizures acknowledges the limitations of previous classifications, while attempting to simplify the process of diagnostic differentiation. The updated guidelines have advocated a threetier approach: seizure type, epilepsy type and epilepsy syndrome.
Seizure Type
Seizures are due to the altered
electrical activity from different parts of the brain and generally classified as being either focal or generalised in onset.4 Focal seizures originate within the neuronal network in one hemisphere, whereas generalised seizures rapidly engage both cerebral hemispheres. Focal seizures are further subdivided into those with ‘intact awareness’ and those with ‘reduced awareness’. Reduced awareness is generally defined as lack of understanding regarding a patient’s sense of self or their surroundings. Importantly, it does not necessarily refer to lack of awareness that a seizure is occurring. Impaired awareness is believed to be secondary to depressed subcortical arousal systems. The terms “focal aware seizure” and “focal seizure with impaired awareness” have largely replaced the previous terms of “simple partial seizure” and “complex partial seizure” respectively.
Focal seizures can be further classified based on the specific motor or non-motor manifestations they exhibit. These include automatisms (purposeless, repetitive motor activities such as lip smacking or patting), focal atonic seizures (loss of tone of one body part), focal clonic seizures (repeated, regular stereotypical
jerking movements) and focal myoclonic seizures (repeated, irregular, non-rhythmic jerking). Non-motor focal seizures present with a variety of symptoms including alterations in blood pressure and heart rate, sweating, piloerection, rising epigastric sensation, déjà vu, emotional disturbance or changes in sensory phenomena. Finally focal seizures can be classified as to whether or not they progress to a bilateral tonic clonic seizure. In this article the localization of focal seizures based on location of epileptogenic foci are not discussed.
Generalised seizures are similarly classified by motor or non-motor manifestations. Tonic-clonic seizures are the most commonly described type of generalised motor seizures, however it is important to recognise that this term also encompasses tonic, clonic, atonic and myotonic seizures as well as the significant overlap in these subtypes. Nonmotor generalised seizures (also still known as absence seizures) generally present with sudden cessation of activity, occasionally with associated automatisms and followed by an immediate recovery. These seizures generally show evidence of generalised spike- wave activity on Electroencephalograph (EEG).
CPD Continuing Professional Development CPD
31 CPD 83: EPILEPSY
Dr Gerard Mullane
seizures
Focal Onset
Motor Onset
• Automatisms
• Focal atonic seizures
• Focal clonic seizures
• Focal Myoclonic seizures
Nonmotor Onset
• Autonomic dysfunction
• Deja Vu
• Rising epigastric sensation
•
Generalised Onset
Motor
• Tonic Clonic
•
•
• Atonic
• Myotonic
Nonmotor/Abscence
Figure 1. Updated approach to seizure type classification, based on 2017 ILAE guidelines
Epilepsy Type
The second tier of classification concerns epilepsy type. This assumes the patient has a diagnosis of epilepsy. The epilepsy type is largely determined clinically and supported by EEG findings. Epilepsies are classified as generalised or focal. Patients with generalised epilepsy, for example have generalised seizure types with evidence of generalised spike and wave activity on EEG. Patients with one or more focal seizure types are considered to have focal epilepsy. EEG findings in focal epilepsy include focal slowing, or focal epileptiform discharges, however the EEG can often be normal. The newly described “combined generalised and focal epilepsy” is used to describe patients with both generalised and focal seizures, such as in patients with Dravet Syndrome or LennoxGastaut Syndrome. When there is insufficient evidence to classify epilepsy as focal or generalised, the term “unknown ” is used.
Epilepsy Syndrome
The description of several distinct epilepsy syndromes is a new addition to the current classification syndrome and is being increasingly used due to greater availability of investigations, including genetic testing. It is defined as “a cluster of features incorporating seizure
types, EEG and imaging features that tend to occur together.” These include childhood absence epilepsy, juvenile absence epilepsy and juvenile myoclonic epilepsy. Reflex epilepsy syndromes are also described, the most commonly known being photosensitive epilepsy, but reflex reading epilepsy and startle epilepsy have also been described.
Table 1 shows a summary of some of the most important risk factors for developing epilepsy
Table 1: Risk factors
Table 1: Risk factors
Birth/ pregnancy (hypoxic brain injury, asphyxia)
Birth/ pregnancy (hypoxic brain injury, asphyxia)
Febrile convulsion (increase the risk of hippocampal sclerosis)
Febrile convulsion (increase the risk of hippocampal sclerosis)
Traumatic head injury/ Brain tumour/surgery
Traumatic head injury/ Brain tumour/surgery
Family Hx of seizure/epilepsy
Family Hx of seizure/epilepsy
Infection (meningitis/encephalitis)
Infection (meningitis/encephalitis)
Vascular disorder/ Neurodegenerative disorder
Vascular disorder/ Neurodegenerative disorder
A practical approach to the patient with new seizures5
The evaluation of a possible new seizure disorder starts with careful history taking and the diagnosis is mostly clinical, supported by ancillary investigations. The next question that needs to be asked is whether the episode in question is a true epileptic seizure or a seizure mimic. If the event is an epileptic seizure one has to identify whether or not the event was likely an unprovoked event. Consideration must be given to known provoking factors such as sleep deprivation, alcohol or drug use, hypoglycaemia or other metabolic derangements. An extensive assessment of risk factors and provocative factors (Table 1 and 2) can also offer valuable information about relative risk of developing epilepsy. Thorough questioning can reveal a remote history of previously unidentified clinical events, which can be present in approximately 20% of cases. In cases of suspected temporal lobe epilepsy (which is the most common focal epilepsy), these events can be quite subtle, ranging from acute emotional disturbance to transient sensory abnormalities, which the patient themselves may not have remarked on unless questioned directly. Seizures accompanied by acute change in mental status, fever or focal neurological deficit warrant urgent assessment for intracerebral infection or inflammation with laboratory work up, neuroimaging and lumbar puncture.
Investigations
The cornerstone of investigation for patients presenting with a new diagnosis of seizure remains a dedicated high quality MRI brain and EEG. MRI can be
Table 2 shows some of the most encountered provoking factors for seizures
Table 2: Common provocative factors:
Table 2: Common provocative factors:
Sleep deprivation
Sleep deprivation
Alcohol withdrawal Metabolic (hypoNa, HypoCa, hypo/hyper glycaemia)
Alcohol withdrawal
Metabolic (hypoNa, HypoCa, hypo/hyper glycaemia)





Barbiturate or benzodiazepine withdrawal
Barbiturate or benzodiazepine withdrawal
Illicit drug use (cocaine, amphetamines, opioids, phencyclidine)
Illicit drug use (cocaine, amphetamines, opioids, phencyclidine)
Medications (tramadol, imipenem)
Medications (tramadol, imipenem)
Table 1 shows a summary of some of the most important risk factors for developing epilepsy Table 2 shows some of the most encountered provoking factors for seizures Investigations
Table 1 shows a summary of some of the most important risk factors for developing epilepsy
Table 2 shows some of the most encountered provoking factors for seizures
Investigations
The cornerstone of investigation for patients presenting with a new diagnosis of seizure remains a dedicated high quality MRI brain and EEG. MRI can be preceded by CT in certain clinical circumstances, such as acutely altered mental state or focal neurological deficit and is easily available compared to MRI
The cornerstone of investigation for patients presenting with a new diagnosis of seizure remains a dedicated high quality MRI brain and EEG. MRI can be preceded by CT in certain clinical circumstances, such as acutely altered mental state or focal neurological deficit and is easily available compared to MRI
32 CPD 83: EPILEPSY
as
overlap
these subtypes. Non
(also
generally
wave activity
well as the significant
in
motor generalised seizures
still known as absence seizures) generally present with sudden cessation of activity, occasionally with associated automatisms and followed by an immediate recovery. These seizures
show evidence of generalised spike
on Electroencephalograph (EEG)
Sensory disturbance Intact awareness Vs Impaired awarenes +/ progression to bilateral tonic clonic
Pure Tonic
Pure Clonic
Unknown Onset Motor/Nonmotor Manifestations Figure 1. Updated approach to seizure type classification, based on 2017 ILAE guidelines
(A) CT brain axial view (noncontrast) showing bifrontal encephalomalacia secondary to head injury; (B) MRI brain T2 axial showing cavernoma in R frontal lobe; (C) MRI T1 axial, post contrast sequence showing subependymal grey matter heterotopia at the left ventricular atrium; (D) MRI brain FLAIR Coronal sequence showing hippocampal sclerosis (right)
Figure 2: Neuroimaging showing epileptogenic lesions
(A) CT brain axial view (noncontrast) showing bifrontal encephalomalacia secondary to head injury; (B) MRI brain T2 axial showing cavernoma in R frontal lobe; (C) MRI T1 axial, post contrast sequence showing subependymal grey matter heterotopia at the left ventricular atrium; (D) MRI brain FLAIR Coronal sequence showing hippocampal sclerosis (right)

classified as: structural, genetic, metabolic, infectious, immune and unknown.
• Structural epilepsies can be detected on dedicated epilepsy protocol MRI and can therefore be considered for surgical evaluation for potential resection. In patients with persistent focal epilepsy and normal MRI, FDG PET can be useful in looking for hypometabolic regions .
autoantibodies directed at either neuronal cell surface proteins or intracellular antigens. These can often be associated with underlying malignancy. These patients often also present with behavioural or cognitive disturbance, neuropsychiatric symptoms or autonomic dysfunction. Certain specific seizure semiologies have been linked with specific underlying pathogenic antibodies, such as faciobrachial dystonic seizures seen in LGI1 associated encephalitis or orofacial dyskinesia associated with NMDA encephalitis. The evaluation for possible immune mediated seizures includes lumbar puncture for evidence of active inflammatory CSF, testing for autoimmune and paraneoplastic antibodies in serum and CSF, MRI with particular attention paid to temporal lobes and brainstem as well as thorough investigation for possible underlying tumours such as ovarian teratomas in NMDA encephalitis.
Management6
preceded by CT in certain clinical circumstances, such as acutely altered mental state or focal neurological deficit and is easily available compared to MRI in urgent scenarios. The preferred neuroradiological investigation for new onset seizures is 3 Tesla MRI brain with specific epilepsy protocol however many institutions have 1.5 T MRI imaging which would be sufficient in the initial work up. The images should be reviewed by a neuroradiologist provided with detailed clinical information including the suspected clinical epileptic focus. Figure 2 below shows some of the known epileptogenic lesions that can detected on neuroimaging.
EEG remains an essential part of the work up of a patient with new seizures. It can offer a confirmation of suspected diagnosis in some cases, as well as vital information regarding epileptogenic focus and underlying epilepsy type. EEG after a new onset seizure can show epileptiform activity in approximately 29% of patients, however the cumulative rates of detecting epileptiform increases with repeated EEG. The usage of sleep deprived EEG also increases the rates of detecting clinically relevant abnormalities.
EEG has greater utility in the evaluation of generalised seizures
as these patients tend to have a higher frequency of epileptiform discharges when compared to focal seizures. It is important to recognise that routine EEG in the hospital setting can often be normal, even when the clinical suspicion of an underlying seizure disorder is high. EEG provides a relatively short snapshot of an individual patient, and the yield increases with the length of EEG, rising to greater than 90% with prolonged (24-36 hour) EEG monitoring, however this is not generally practicable in the clinical setting. It is also important to note that a number of confounding factors can affect EEG interpretation. Prolonged video EEG, where patients are admitted to a dedicated monitoring unit for usually 3-4 days for continuous EEG recording and video monitoring) remains an invaluable tool, particularly in assessing refractory cases for epilepsy surgery or in cases where non epileptic attack disorder (NEAD) is suspected. Approximately 25% of patients considered to have refractory epilepsy are found to have non epileptic seizures on prolonged video EEG monitoring.
EEG remains an essential part of the work up of a patient with new seizures. It can offer a confirmation of suspected diagnosis in some cases, as well as vital information regarding epileptogenic focus and underlying epilepsy type. EEG after a new onset seizure can show epileptiform activity in approximately
• Genetic epilepsies: The majority of epilepsies are considered to have a genetic basis and the mechanism of inheritance is likely polygenic. Advances in genomic sequencing have become increasingly used in the investigation of epilepsy and specific genetic panels can usually be requested based on the predominant seizure semiology and other clinical features. Whole exome sequencing is also being used increasingly due to improvements in turnaround time and reducing financial costs.
• Metabolic epilepsies usually present at an early age and are associated with cognitive and motor impairment. Careful clinical examination and history can offer clues to the underlying pathology and can guide investigations for specific errors of metabolism.
First ever seizure: When considering the patient presenting with first presentation of a seizure, after it had been established that it was likely a clinical seizure event without provoking factors, the clinical decision regarding drug treatment is raised. After the first seizure, the risk of another seizure is greatest within the first two years and can range from 21-45%. The initiation of an antiseizure medication (ASM) would be quite reasonable if there is a clear epileptiform abnormality in EEG and or epileptogenic focus in neuroimaging. In the case of an isolated seizure with no clear evidence of predisposition to further events, ASM is generally not indicated due to potential side effects associated with these medications, as well as the financial burden on the patient, which can be quite significant.
Underlying aetiology: The aetiology of a suspected epilepsy should be elucidated where possible. These can be broadly
• Infectious epilepsies may result both from acute cerebral insults, such as acute Herpes Simplex Virus encephalitis or bacterial meningitis, and delayed seizure presentations as seen in fungal infections, tuberculosis, toxoplasmosis, malaria, HIV and neurocysticercosis.
• Immune or inflammatory related epilepsy is associated with
“Breakthrough seizures in patients with known epilepsy.” These patients require careful questioning to determine whether or not this represents a true treatment failure. One of the most common causes of “breakthrough seizures” is issues with medication compliance. Medication non-compliance is at approximately 50% worldwide and leads to higher economic costs associated with increased
33
Figure 2: Neuroimaging showing epileptogenic lesions
Type of seizures Commonly used anti seizure medications (Discuss contraception in women and Valproate should not be used in women of childbearing age).
Focal Carbamazepine (CBZ)/CBZ derivatives, Lamotrigine*
healthcare usage. ASM noncompliance can have significant negative effects including an increased risk of “Sudden unexpected death in epilepsy” (SUDEP). Risk factors for nonadherence include polypharmacy in older patients, patients in their late adolescence and early adulthood, as well as concurrent psychiatric comorbidities such as low mood an anxiety. As previously mentioned, known provoking factors such as sleep deprivation, excess alcohol consumption and illicit drug use may increase the risk of seizure and may not represent a true treatment failure.
Anti-seizure medications: The general principle in management of epilepsy is treating with an appropriate ASM based on the suspected underlying seizure type and epilepsy syndrome. There has been a dramatic increase in the number of ASMs available for use in the last 20 years. Whereas previously clinicians would be limited to use of a small number of agents with significant side effects, there are now over 30 agents in use, with a generally more favourable side effect profile. ASMs are broadly divided into narrow and broad-spectrum drugs. Narrow spectrum drugs are generally chosen in clear focal epilepsies but may result in a paradoxical worsening if used in generalised seizures. The number of ASMs continues to increase. Other ASMs approved in more recent years includes perampanel, brivaracetam, cannabidiol, cenobamate and fenfluramine. The ASMs listed below in Table 3 are commonly used in different types of seizures in clinical practice.
Emergency rescue medications such as buccal midazolam (Buccolam 10 mg in 2 ml) is prescribed for patients who had generalized seizure. The aim is
to prevent prolonged seizures or status epilepticus. It is also important to educate family members about the use of buccal midazolam in the event of seizures outside the hospital setting. This drug can be easily administered by a prefilled oral syringe to buccal cavity by family members or carers.
Counselling and Management of the risks of Living with Epilepsy
There is an increased risk for mood disorders and anxiety in people with epilepsy which could negatively impact their quality of life. Mortality is also increased for people suffering from epilepsy and most commonly from sudden unexpected death in epilepsy (SUDEP). The risk is higher with uncontrolled generalised tonic-clonic seizures and hence education about drug compliance from the diagnosis of epilepsy is essential. With increasing duration of ASMs, the risk for fracture increases compared to general population (2-6 times higher in people with epilepsy) and requires monitoring by DEXA scan and management of osteopenia and osteoporosis.
Advice about unsupervised swimming, avoid using bathtub
and use of showers instead and avoiding climbing unguarded heights are also essential to reduce risk of seizure related injury. Epilepsy Ireland, a national organisation established in 1966 providing support to improve quality of life of people with epilepsy in Ireland. They offer a range of services to patients and their families and can be a helpful resource in providing education after a new diagnosis.
Driving Regulations post Seizure
The regulations regarding driving and epilepsy are laid out in the “Sláinte agus Tiomáint Medical Fitness to Drive Guidelines”.8
The guideline makes an important distinction between patients driving group 1 (i.e motorcycles, cars and tractors) and group 2 (Trucks and buses) vehicles.
A number of factors influence the duration of driving restriction; including whether or not the seizure was a first time event or an event in the context of a known seizure disorder, as well as whether alcohol or illicit drug use was related to the event. The restrictions are summarised in Figure 4 but the general rule is that the driving restriction is doubled for those driving group 2 vehicles.
Table 3: Commonly used ASMs in different types of seizures
* Risk of Rash/Hypersensitivity
There are a number of special circumstances that must be considered also. For example: patients who are having ASMs withdrawn entirely are generally advised not to drive during the duration of reduction and for 3 months after. For patients who have very clear provoking factors for a seizure, such as eclampsia or convulsive syncope, sometimes a driving restriction is not required, if the provoking cause is treated but this is decided on a case by case basis at the discretion the the treating clinician.
It is important to recognise that seizures in the context of alcohol excess or illicit drug use do not qualify as clear provoked seizures for licencing purposes. For seizures associated with alcohol or illicit drug use, a 6-month restriction is imposed for group 1 vehicles, and 5 years for group 2.
Restriction for driving could also influence their ability to work, maintain relationship and to live independently. Health professionals managing and promoting health of the patients with epilepsy should be committed to improve quality of life of the person and their family.
References available on request
34 CPD 83: EPILEPSY
Figure 3. Timeline of increasing availability of anti seizure medications over time 8
Myoclonic Levetiracetam, Topiramate,
Commonly used ASMs
different
of
and Management of the risks of Living with Epilepsy: There is an increased risk for mood disorders and anxiety in people with epilepsy which could negatively impact their quality of life. Mortality is also increased for people suffering from epilepsy and most commonly from sudden unexpected death in epilepsy (SUDEP). The risk is higher with uncontrolled generalised tonic clonic seizures and hence education about drug compliance from the diagnosis of epilepsy is essential. With increasing duration of ASMs, the risk for fracture increases compared to Figure 4. Summary of driving restrictions for patients with seizures Restriction for driving could also influence their ability to work, maintain relationship and to live independently Health professionals managing and promoting health of the patients with epilepsy should be committed to improve quality of life of the person and their family. Unprovoked First Seizure Group 1 • 6 months Group 2 • 5 years (with no ASM use) Seizure with a diagnosis (or high probability) of epilpesy Group 1 • 1 year Group 2 • 10 years (with no ASM use) Seizure related to alcohol/ drug use Group 1 • 6 months Group 2 • 5 years (with no ASM use) Figure 4. Summary of driving restrictions for patients with seizures
Generalized Lamotrigine*, Levetiracetam, Sodium Valproate
Sodium Valproate Table 3:
in
types
seizures * Risk of Rash/Hypersensitivity Counselling
other distinct anti-modified protein antibodies (AMPAs) such as antiacetylated protein antibodies have been recently described. Recent



31
copyright, no part of this can be used in any publishers and author. Galapagos UK, has no
Risk factors
Protective factors
at mucosal sites such as the gut, lungs or periodontium and is related to microbiome.2, 3
Window of opportunity to prevent irreversible joint damage by early treatment initiation may be impeded by delays in 1) seeking care by patients when developed symptoms 2) referral to a rheumatologist, and the long waiting-list. It is estimated that a potential savings of ¤237,547 over five-year timeframe can be achieved by the Irish Healthcare system through the reconfiguration of the diagnostic care pathway for suspected RA patients using an early referral pathway.4
The “Pre-RA” Phase
It may take months or years before a subset of susceptible individuals with RA-related antibodies to develop and manifest polyarthritis (polysynovitis). The term “Pre-RA” stage of seropositive individuals (RF and/or ACPA) with other factors (e.g., joint symptoms/ arthralgia without synovitis) was initially suggested by the European Alliance of Associations for Rheumatology (EULAR; formerly known as European League Against Rheumatism) study group in 2012 while predicting the risks and assessing the earliest clinically apparent phases in RA.5
Current data suggest early reactivity to limited number of self-antigens with limited systemic inflammation followed by evolution over time of expanding innate and adaptive responses until certain threshold is crossed where clinically-apparent RA develops.6 The concept has since evolved and many clinical prevention trials are either ongoing or have been completed including a single-dose of Rituximab to delay disease onset.7 Several risk and protective factors have been identified so far to be associated with RA development in individuals with RA-related antibodies (Table 1).
The evidence across many aspects of the concept is however
Obesity or inflammatory diet
currently limited or yet to be robustly validated especially on 1) which subset of high-risk patients would have the greatest benefit from the prevention treatment? 2) which phase of disease should the treatment commence? 3) should treatment strategy focus on once-off therapy or a more frequent regime? 4) is the benefit-to-risk ratio of treatment prevention favourable?
Current Classification Criteria: The 2010 ACR/EULAR Criteria for RA
The 2010 American College of Rheumatology (ACR)/EULAR
classification criteria for RA was not intended to be diagnostic, but has helped to identify early RA and is utilized for conducting clinical trials involving early disease. This relatively new criteria emphasizes on RA characteristics that emerge early in disease and contrasts the older 1987 revised ACR criteria, which relied on chronic disease features such as radiographic erosions and rheumatoid nodules.
The criteria is grouped into four classifications and weighted in favour of more small joints involvement (up to 5 points), high titres of RA-related antibodies (up
to 3 points), and to a lesser extent for larger joints, symptom duration, and acute-phase reactants; classification is satisfied if the total score reaches 6 points (Table 2). As mentioned earlier, the clinical “diagnosis” has to be established by the rheumatologist based upon many other aspects and may be guided by the classification criteria.
Extraarticular manifestations & comorbidities
RA does not exclusively affect the joints. Extraarticular manifestations usually occur in RA patients with more active and severe disease and
32 CPD 84: RHEUMATOID ARTHRITIS
Table
Identified risk and protective factors
antibody
7 Tables Table 1: Identified risk and protective factors associated with RA development in RA related antibody
1:
associated with RA development in RA-related
individuals6
individuals [6]
Female sex Longer duration of breast feeding First
Higher
High titre RA related antibodies or positive in both RF and ACPAs High fatty fish intake and Omega 3 fatty acids
OCP Tobacco exposure (especially long duration, high intensity) Presence of shared epitope (having genes conferring
risk
allele)
Table 2: ACR/EULAR classification criteria for RA Joint distribution (0 5) Points Serology (0 3) Points 1 large joint * 0 Negative RF and ACPA 0 2 10 large joints 1 Low positive RF or ACPA 2 1 3 small joints ** (large joints not counted 2 High positive RF or ACPA 3 4 10 small joints (large joints not counted 3 Symptom Duration (0 1) >10 joints (at least 1 small joint) *** 5 <6 weeks 0 Acute phase reactants (0 1) ≥ 6 weeks 1 Normal CRP and ESR 0 Abnormal CRP or ESR 1 ≥ 6= definite RA. If very short duration of symptoms, other differentials should be considered *Large joints=shoulder, elbow, hip, knee, ankle ** does not include 1st CMC, 1st MTP and DIP joints ***additional joints include TMJ, SC, AC and other joints that are reasonably expected in RA Table 3: Biologic and targeted synthetic DMARD therapy in RA Biologic DMARD therapy bDMARD TNF inhibitors Adalimumab, Infliximab, Etanercept, Certolizumab, Golimumab IL 6 receptor inhibitors Tocilizumab, Sarilumab CD80 and CD86 inhibitor (T cell co Abatacept Table 2: ACR/EULAR classification criteria for RA
degree relatives
parity
higher
such as HLA DR4
Lung disease (airways, parenchyma) Periodontal inflammation
Biologic DMARD therapy
bDMARD
is associated with higher morbidity and mortality. This includes rheumatoid nodules, vasculitis, ocular manifestations (e.g., scleritis, keratitis, keratoconjunctivitis sicca), cardiovascular involvement (such as heart failure, pericarditis, myocarditis, myocardial infarction), pulmonary features (including interstitial lung disease/ILD, pleural disease, brionchiolitis), secondary amyloidosis and Sjogren’s syndrome.
Osteoporosis is one of the common comorbidities of RA, with 60100% increased of fracture risk compared to general population.8 Accumulating evidence points toward RA negatively impacting mental health, not just due to distress and disability but also from immunological alterations.9 Patients with RA also have increased risk of malignancy, mainly of lymphoma and lung cancer, which does not appear to be related to diseasemodifying antirheumatic drugs (DMARD) therapy. The most common cause of premature death among RA patients is CVD, followed by ILD.
Cardiovascular risk
RA is an independent, non-traditional risk factor for cardiovascular disease (CVD). Patients with RA have up to two-times higher risk of developing atherosclerotic CVD compared to general population (similar to diabetes), and almost twice the risk of developing congestive heart failure, with the risk already increased during early disease or disease onset.10 The risk can
be reduced by healthy lifestyle (healthy diet, regular exercise, smoking cessation) and use of DMARDs for optimal disease control; however, caution is required in patients taking NSAIDs or higher doses of Tofacitinib (10mg twice daily), based on recent postmarketing trial results. It is recommended that CVD risk assessment should be performed at least once in every 5 years in low-to moderate risk RA patients but more often in intermediate or higher risk and following major changes in DMARD therapy.11
Management
The treatment strategies in RA have evolved significantly over the past several decades. Early diagnosis, prompt DMARD initiation and regular assessment to achieve remission or low disease state have been shown to improve patients’ outcome. Current therapeutic strategies focus on treat-to-target approach for better disease control, which involves close, frequent monitoring during active disease and prompt DMARD escalation when target is not achieved. The main goal is for early sustained complete remission to prevent irreversible joint damage.
Patients should ideally be seen by a rheumatologist within 6 weeks of symptom(s) onset. Treatment is based on recommended guidelines and upon a shared decision between patient and the rheumatologist. Multidisciplinary team approach is key, and should ideally involve primarily
the rheumatologist, advanced nurse practitioner/clinical nurse specialist, physiotherapist and occupational therapist, but may extend to other specialties depending on patients’ comorbidities such as ILD, CVD or chronic hepatitis.
Methotrexate (MTX) monotherapy is recommended as first line; however, if unresponsive, intolerant, or contraindicated, leflunomide or sulfasalazine should be considered. Short term glucocorticoid as bridging therapy during DMARD initiation or switch should be considered but tapered as soon as clinically reasonable. Additional therapy with either a biological (bDMARD) or targeted synthetic DMARD (tsDMARD) should be considered if disease is not controlled (Table 3).
Majority of these targeted therapies are broadly similar in efficacy but differ in terms of mode and frequency of administration, safety and adverse events (AEs). Most are recommended as adjunct to MTX, and so far, only Tocilizumab and Janus Kinase (JAK) inhibitors have shown efficacy as monotherapy compared to MTX monotherapy.12, 13 Low-dose MTX at 7.5 to 10mg weekly provides added efficacy to anti-TNFs and reduces the incidence of antidrug antibodies (immunogenicity). In case of failure of bDMARD or tsDMARD, another bDMARD (from the same group or a different group), or tsDMARD can be used.14
Table 3: Biologic and targeted synthetic DMARD therapy in RA
Assessment of vaccination status and cardiovascular risk, smoking cessation, dental care, and weight control should be part of patients’ care. Periodic osteoporosis screening should be considered, initially by fracture risk assessment and dual-energy x-ray absorptiometry (DXA), with repeat DXA every 2-3 years in patients 40 years and above and those under 40 years but with high risk for developing osteoporosis.15 The need for orthopaedic intervention has declined with early treat-to-target therapy and the incidence for joint replacements closely resembles those with non-inflammatory disease.
Biosimilars and the small molecules
Following the expiration of patent protections for some native biologic originator (bio-originator; boDMARDs), there have been increasing amount of relatively lowcost biosimilars (bsDMARDs) in the market. These bsDMARDs are deemed highly similar in structure without any clinical meaningful difference to the reference boDMARDs including in its’ safety and efficacy profile. It is costeffective and as part of the solution to reduce national healthcare expenditure, the Irish Government initiated price competition between biosimilar manufacturers (bestvalue biological) and gainshare prescribing incentive initiatives (which can be reinvested back for service development and patients’ care).
Currently there are several bsDMARDs approved for the Irish market including Benepali (Etanercept; others include Erelzi, Lifmior, Nepexto), Idacio, Amgevita, Imraldi, Hulio and Yuflyma (Adalimumab; others include Amsparity, Hefiya, Hyrimoz), Inflectra, Flixabi, Remsima, Zessly (Infliximab; given intravenously however CT-P13 biosimilar which is not yet approved may be given subcutaenously) and for antiCD20 Rituxan (Rituximab; others include Blitzima, Ritemvia, Rixathon, Riximyo, Ruxience, Truxima). The patent for the pegylated monoclonal
33
antibody 8 ** does not include 1st CMC, 1st MTP and DIP joints ***additional joints include TMJ, SC, AC and other joints that are reasonably expected in RA
Table 3: Biologic and targeted synthetic DMARD therapy in RA
tsDMARD
Tofacitinib
TNF inhibitors Adalimumab, Infliximab, Etanercept, Certolizumab, Golimumab IL 6 receptor inhibitors Tocilizumab, Sarilumab CD80 and CD86 inhibitor (T cell co stimulator blockade) Abatacept CD20/B cell inhibitor Rituximab Targeted synthetic DMARD therapy
JAK1/JAK3 inhibitor
JAK1/JAK2 inhibitor Baricitinib Selective JAK1 inhibitors Upadacitinib, Filgotinib TNF: tumour necrosis factor; IL: interleukin; JAK: Janus kinase; DMARD: disease modifying antirheumatic drugs
(certolizumab) which prolongs renal clearance, and stops it from crossing the placenta, making it safer in pregnancy is expected to expire in 2024.
JAK inhibitors (Jakinibs) represents the latest targeted therapies approved for treatment of RA. Currently there are four approved for RA in Ireland; Tofacitinib, Baricitinib, Upadacitinib and Filgotinib. Many phase 3 clinical trials, real-world and long-term data uniformly demonstrated rapid efficacy over time of Jakinibs in RA patients as monotherapy, or in combination to conventional DMARDs, and in anti-TNF partial responders. Recent studies suggested that Jakinibs may also have additional independent effect on non-inflammatory pain.16
Being orally administered, it may offer convenience especially for those frequently air-travel, or have needle-phobia. More compellingly is that its’ shorter half-lives that range in hours means that the AEs are short-lived when it is held or discontinued. There is higher incidence of herpes zoster compared to other mode of actions DMARDs, and emerging postapproval safety data of slight increased risk for 1) major adverse cardiovascular events among patients above 65 years or those who ever smoke and 2) venous thromboembolic events on Tofacitinib.17, 18 Currently there is no definitive data on switching Jakinibs in non or partial responders albeit promising results in several observational studies on switching to another Jakinib.
Refractory
or
“difficult-to-treat” RA
A proportion of RA patients are non-responsive to multiple advanced targeted DMARD therapies. They are known as refractory or “difficult-to-treat” RA patients, and encompasses patients who likely to have cycled through and exhausted all available DMARD therapies due to either 1) persistent joint or systemic inflammation with high disease activity resistant to multiple DMARDs, or requiring moderately high doses of glucocorticoids alongside DMARD therapy to achieve disease control i.e. “Persistent inflammatory refractory RA”, or 2) poor patient adherence to therapies, or 3) persistent measured disease activity mediated predominantly by pain and patient reported outcomes
(PROMs) but without joint and/ or systemic inflammation i.e. “Noninflammatory refractory RA”.19
EULAR task-force recommends a threshold of a failure of ≥2 bDMARDs or tsDMARDs (with different mechanisms of action) as “difficult-to-treat” RA.20 Suboptimal or delayed treatment, having high composite disease activity scores, development of anti-drug antibodies, obesity and unhealthy lifestyle may all favour the development of refractory RA. The disease process of these patients may involve different pathogenetic pathways, which require further research and additional interventions or therapies.
Conclusion
Early diagnosis and treatment advancement have revolutionised the landscape of RA management. Several aspects in disease understanding remain less well studied and currently not supported by robust evidence, mainly in RA prevention for those at risk and those with refractory RA. These unmet needs will continue to serve as a challenge among global scientific community to drive further research and inspire discoveries.
References
1. Lundkvist J, Kastäng F, Kobelt G. The burden of rheumatoid arthritis and access to treatment: health burden and costs. Eur J Health Econ 2008;8 Suppl 2: S49-60
2. Holers VM, Demoruelle MK, Kuhn KA, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol 2018; 14: 542-557
3. Zaiss MM, Joyce Wu HJ, Mauro D, et al. The gut-joint axis in rheumatoid arthritis. Nat Rev Rheumatol 2021; 17: 224-237
4. Kelleher D, Barry L, McGowan B, et al. Budget impact analysis of an early identification and referral model for diagnosing patients with suspected rheumatoid arthritis in Ireland. Rheumatol Adv Pract. 2020; 4: rkaa059
5. Gerlag DM, Raza K, van Baarsen LG, et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis 2012; 71: 638-41
6. Deane KD, Holers VM. Rheumatoid Arthritis Pathogenesis, Prediction, and Prevention: An Emerging Paradigm Shift. Arthritis Rheumatol 2021 Feb; 73: 181-193
7. Greenblatt HK, Kim HA, Bettner LF, et al. Preclinical rheumatoid arthritis and rheumatoid arthritis prevention. Curr Opin Rheumatol 2020; 32: 289-296
8. Jin S, Hsieh E, Peng L, et al. Incidence
of fractures among patients with rheumatoid arthritis: a systematic review and meta-analysis. Osteoporos Int 2018; 29: 1263-1275
9. Nerurkar L, Siebert S, McInnes IB, et al. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry. 2019; 6: 164-173
10. Hansildaar R, Vedder D, Baniaamam M, et al. Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol. 2021; 3: e58-e70
11. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017; 76: 17-28
12. Nam JL, Takase-Minegishi K, Ramiro S, et al. Efficacy of biological diseasemodifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2017; 76: 1113-1136
13. Chatzidionysiou K, Emamikia S, Nam J, et al. Efficacy of glucocorticoids, conventional and targeted synthetic disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2017; 76: 1102-1107
14. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020; 79: 685-699
15. Adami G, Saag KG. Osteoporosis Pathophysiology, Epidemiology, and Screening in Rheumatoid Arthritis. Curr Rheumatol Rep 2019; 21: 34
16. Simon LS, Taylor PC, Choy EH, et al. The Jak/STAT pathway: A focus on pain in rheumatoid arthritis. Semin Arthritis Rheum 2021; 51: 278-284
17. Ytterberg S, Bhatt D, Mikuls T, et al. Safety and Efficacy of Tofacitinib vs TNF Inhibitors in RA Patients Aged 50 Years or Older with One or More Cardiovascular Risks: Results from a Phase 3b/4 Randomized Safety Trial [abstract]. Arthritis Rheumatol 2021; 73 (suppl 10)
18. Charles-Schoeman C, Fleischmann R, Mysler E, et al. The Risk of Venous Thromboembolic Events in Patients with RA Aged ≥ 50 Years with ≥ 1
Cardiovascular Risk Factor: Results from a Phase 3b/4 Randomized Safety Study of Tofacitinib vs TNF Inhibitors [abstract]. Arthritis Rheumatol 2021; 73 (suppl 10)
19. Buch MH, Eyre S, McGonagle D. Persistent inflammatory and noninflammatory mechanisms in refractory rheumatoid arthritis. Nat Rev Rheumatol 2021; 17: 17-33
20. Nagy G, Roodenrijs NMT, Welsing PM, et al. EULAR definition of difficult-totreat rheumatoid arthritis. Ann Rheum Dis. 2021; 80: 31-35
5 Questions
1. Which of the following is false?
Biosimilar DMARDs are more cost effective than biooriginator DMARDs
Osteoporosis and fragility fractures are associated with RA c. RA typically affects the small joint of the hands and wrists
Suspected RA patients should ideally be seen within 6 months of symptom onset
The two commonest causes of premature deaths in RA are CVD and ILD 2. Cardiovascular disease risk assessment in RA patients should be performed:
Has a lower risk of herpes zoster compared to other mode of action DMARDs
Is considered as one of the first-line therapies for newly diagnosed RA patients
34 CPD 84: RHEUMATOID ARTHRITIS
a.
b. Annually
c.
d.
e.
3.
a. Abatacept b. Apremilast c. Guselkumab d. Secukinumab e. Ustekinumab 4.
a. Cigarette
b. High-fatty
c. High-titre
d. Periodontal
e.
5.
a.
b.
c.
d.
e.
a.
b.
d.
e.
After minor changes in antirheumatic therapy
for all patients
Biannually in moderate CVD risk patients
Once every 3 years in all patients
Once every 5 years in low CVD risk patients
Which of the following is a treatment option for rheumatoid arthritis?
Which of the following is not a risk factor for developing RA in individuals with RA-related antibodies?
smoking
fish intake
RA-related antibodies
inflammation
Presence of shared-epitope alleles
Which of the following is true about JAK inhibitors?
Currently licenced treatment for RA includes Baricitinib and Upadacitinib
Has a slower efficacy onset compared to anti-TNFs
Is extremely safe in heavy smoker elderly patients


J O I N O U R M A I L I N G L I S T I N P R I N T A N D O N L I N E , H O S P I T A L P R O F E S S I O N A L N E W S C O V E R S T H E L A T E S T N E W S , I N S I G H T A N D O P I N I O N T H A T S P E A K S T O H E A L T H C A R E P R O F E S S I O N A L S I N T H E I R I S H H O S P I T A L S E C T O R . C O N T A C T E M I L I A @ H O S P I T A L P R O F E S S I O N A L N E W S . I E H O S P I T A L P R O F E S S I O N A L N E W S
Confidence
OTEZLA® (apremilast) 10mg, 20mg and 30mg film coated-tablets Brief Prescribing Information. Refer to the Summary of Product Characteristics (SPC) before prescribing. Further information is available upon request. Presentation: 10mg, 20mg and 30mg film coated-tablets. Indications: Psoriatic arthritis: OTEZLA, alone or in combination with Disease Modifying Antirheumatic Drugs (DMARDs), is indicated for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an inadequate response or who have been intolerant to a prior DMARD therapy. Psoriasis: OTEZLA is indicated for the treatment of moderate to severe chronic plaque psoriasis in adult patients who failed to respond to or who have a contraindication to, or are intolerant to other systemic therapy including ciclosporine, methotrexate or psoralen and ultraviolet-A light (PUVA). Dosage and administration: Treatment with OTEZLA should be initiated by specialists experienced in the diagnosis and treatment of psoriasis or psoriatic arthritis. The recommended dose of OTEZLA is 30mg twice daily taken orally in the AM and PM, approximately 12 hours apart, with no food restrictions. The film-coated tablets should be swallowed whole. An initial dose titration is required per the following schedule: Day 1: 10mg in the AM; Day 2: 10mg in the AM and 10 mg in the PM; Day 3: 10mg in the AM and 20mg in the PM; Day 4: 20mg in the AM and 20mg in the PM; Day 5: 20mg in the AM and 30mg in the PM; Day 6 and thereafter: 30mg twice daily in the AM and PM. No re-titration is required after initial titration. If patients miss a dose, the next dose should be taken as soon as possible. If it is close to the time for their next dose, the missed dose should not be taken and the next dose should be taken at the regular time. Patients with severe renal impairment: The dose of OTEZLA should be reduced to 30mg once daily in patients with severe renal impairment (creatinine clearance of less than 30mL per minute estimated by the Cockcroft-Gault equation). For initial dose titration in this group, it is recommended that OTEZLA is titrated using only the AM doses and the PM doses be skipped. Paediatric population: The safety and efficacy of OTEZLA in children aged 0 to 17 years have not been established. No data is available. Contraindications: Hypersensitivity to the active substance(s) or to any of the excipients. OTEZLA is contraindicated in pregnancy. Pregnancy should be excluded before treatment can be initiated. Special warnings and precautions: Diarrhoea, nausea and vomiting: Severe diarrhoea, nausea, and vomiting associated with the use of OTEZLA have been reported. Most events occurred within the first few weeks of treatment. In some cases, patients were hospitalized. Patients 65 years of age or older may be at a higher risk of complications. Discontinuation of treatment may be necessary. Psychiatric disorders: OTEZLA is associated with an increased risk of psychiatric disorders such as insomnia and depression. Instances of suicidal ideation and behaviour, including suicide, have been observed in patients with or without history of depression. The risks and benefits of starting or continuing treatment with OTEZLA should be carefully assessed if patients report previous or existing psychiatric symptoms or if concomitant treatment with other medicinal products likely to cause psychiatric events is intended. Patients and caregivers should be instructed to notify the prescriber of any changes in behaviour or mood and of any suicidal ideation. If patients suffered from new or worsening psychiatric symptoms, or suicidal ideation or suicidal attempt is identified, it is recommended to discontinue treatment with OTEZLA. Severe renal impairment: See dosage and administration section. Underweight patients: OTEZLA may cause weight loss. Patients who are underweight at the start of treatment should have their body weight monitored regularly. In the event of unexplained and clinically significant weight loss, these patients should be evaluated by a medical practitioner and discontinuation of treatment should be considered. Lactose content: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product. Interactions: Co-administration of strong cytochrome P450 3A4 (CYP3A4) enzyme inducer, rifampicin, resulted in a reduction of systemic exposure of OTEZLA, which may result in a loss of efficacy of OTEZLA. Therefore, the use of strong CYP3A4 enzyme inducers (e.g. rifampicin, phenobarbital, carbamazepine,
phenytoin and St. John’s Wort) with OTEZLA is not recommended. In clinical studies, OTEZLA has been administered concomitantly with topical therapy (including corticosteroids, coal tar shampoo and salicylic acid scalp preparations) and UVB phototherapy. OTEZLA can be co-administered with a potent CYP3A4 inhibitor such as ketoconazole, as well as with methotrexate in psoriatic arthritis patients and with oral contraceptives. Pregnancy, lactation and fertility: Women of childbearing potential should use an effective method of contraception to prevent pregnancy during treatment. OTEZLA should not be used during breast-feeding. No fertility data is available in humans. Undesirable effects: Psychiatric disorders: In clinical studies and post-marketing experience, uncommon cases of suicidal ideation and behaviour, were reported, while completed suicide was reported post-marketing. The most commonly reported adverse reactions with OTEZLA in these indications are gastrointestinal (GI) disorders including diarrhoea (15.7%) and nausea (13.9%). These GI adverse reactions generally occurred within the first 2 weeks of treatment and usually resolved within 4 weeks. Adverse reactions reported in the psoriatic arthritis and/or psoriasis clinical trial programme and post marketing experience include: very common (≥ 1/10) diarrhoea*, nausea*; common (≥1/100 to <1/10) bronchitis, upper respiratory tract infection, nasopharyngitis*, decreased appetite*, insomnia, depression, migraine*, tension headache*, headache*, cough, vomiting*, dyspepsia, frequent bowel movements, upper abdominal pain*, gastroesophageal reflux disease, back pain*, fatigue; uncommon (≥1/1,000 to <1/100) hypersensitivity, suicidal ideation and behaviour, gastrointestinal haemorrhage, rash, urticaria, weight loss; not known (cannot be estimated from the available data) angioedema. *At least one of these adverse reactions was reported as serious. Please consult the SPC for a full description of undesirable events. Pharmaceutical Precautions: Do not store above 30°C. Legal category: POM. Presentation and Marketing Authorisation Numbers: Initiation pack containing 27 film coated tablets (4 x 10mg, 4 x 20mg, 19 x 30mg) - EU/1/14/981/001; 30mg film coated tablets in a pack size of 56 tablets - EU/1/14/981/002.
Marketing Authorisation Holder: Amgen Europe B.V. Minervum 7061, 4817 ZK Breda, The Netherlands. Further information is available from Amgen Ireland Limited, 21 Northwood Court, Santry, Dublin D09 TX31. OTEZLA is a trademark owned or licensed by Amgen Inc., its subsidiaries, or affiliates. Date of preparation: April 2020 (Ref: IE-OTZ-2000019).

Adverse reactions/events should be reported to the Health Products Regulatory Authority (HPRA) using the available methods via www.hpra.ie. Adverse events should also be reported to Amgen Limited on +44 (0)1223 436441.
† Otezla met the primary endpoint of the pivotal trials in psoriasis: PASI-75 response vs placebo at 16 weeks. ESTEEM 1: 33.1% (N=562) vs 5.3% (N=282); ESTEEM 2: 28.8% (N=274) vs 5.8% (N=137), P<0.0001. OTEZLA met the primary endpoint of the pivotal trials in Psoriatic Arthritis: ACR 20 response vs placebo at 16 weeks. PALACE 1: 38% (N=168) vs 19% (N=168), P≤0.001. PALACE 2: 32% (N=162) vs 19% (N=159) P≤0.01; PALACE 3: 41% (N=167) vs 18% (N=169) P≤0.001.
References: 1. Kavanaugh et al. Arthritis Research & Therapy 2019: 21;118. 2. OTEZLA (apremilast). Summary of Product Characteristics.
© 2020 Amgen Inc. All rights reserved. Amgen Ireland Ltd., 21 Northwood Court, Santry, Dublin 9 IE-OTZ-0820-00002 | Date of preparation: September 2020

2
can offer solutions to the challenges in this new environment Make OTEZLA® the positive choice for your psoriasis and psoriatic arthritis patients OTEZLA® has an established efficacy and safety profile for up to 5 years†1,2 No contraindications for concurrent use with live vaccination2 Minimise frequency of clinical appointments No laboratory pre-screening, and no drug specific blood monitoring required2
warnings regarding risk of serious infection2
short half-life of 9 hours
that OTEZLA® is rapidly cleared from the body if administration needs to be stopped2 Immunomodulatory mode of action2
not compromise
anti-inflammatory cytokines
OTEZLA®
No
A
Means
in immune response, down-regulation of proinflammatory cytokines and up-regulation of
AUTHOR: Professor Anne Marie Tobin


 Consultant Dermatologist Tallaght University Hospital Clinical Associate Professor Trinity College Dublin Clinical Lead in Dermatology Health Service Executive
Consultant Dermatologist Tallaght University Hospital Clinical Associate Professor Trinity College Dublin Clinical Lead in Dermatology Health Service Executive
60 Second Summary
Hidradenitis suppurativa (HS), previously known as acne inversa is a chronic inflammatory skin disease that affects approximately 1% of the Irish Population. The condition causes boils, abscesses and scarring in the axillae, groin and inframammary areas of patients.
HS may affect between 1-4% of the population, and a study from Irish dermatology departments indicated a prevalence of 1.4% amongst the patient population attending dermatology clinics.
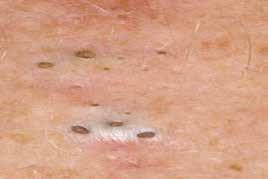

HS begins as small pustules and boils under the arms and in the groin or buttock area, they may be predated by blackheads which are open comedones, the presence of ‘double comedones’, two blackheads beside each other, is pathognomic of the condition. Because the disease begins in puberty it is often not recognized at its beginning. The most commonly areas affected are the axillae, inguinal creases, buttocks, natal cleft and under the breasts or abdominal pannus, any site where skin touches off itself or at sites of friction.

Historically HS was thought to be a disease of apocrine glands it is now recognized as a follicular disorder. It is thought that the initial occlusion is caused by hyperkeratinisation of the pilosebaceous unit leading to occlusion of the follicle with swelling leading to rupture of the follicle and discharge of contents into the dermis.
In a similar paradigm to psoriasis, it is now apparent that HS carries a substantial comorbidity burden, these include obesity, metabolic and cardiovascular disease, inflammatory bowel disease, inflammatory joint dIsease and psychological distress.
Management of Hidradenitis Suppurativa
Hidradenitis suppurativa (HS), previously known as acne inversa is a chronic inflammatory skin disease that affects approximately 1% of the Irish Population. The condition causes boils, abscesses and scarring in the axillae, groin and inframammary areas of patients. HS is chronic, recurrent and debilitating and tends to be progressive. The disease causes significant pain and distress and has a huge impact on patients’ lives affecting their ability to work and intimate relationships.
Epidemiology of Hidradenitis Suppurativa:
HS may affect between 114% of the population, and a study from Irish dermatology departments indicated a prevalence of 1.4% amongst the patient population attending dermatology clinics1. HS is three times more common in women and generally starts around puberty and ameliorates around menopause. One third of patients have a family history of the condition or a family history of pilonidal sinus.


Clinical presentation.
HS begins as small pustules and boils under the arms and in the
groin or buttock area, they may be predated by blackheads which are open comedones, the presence of ‘double comedones’, two blackheads beside each other, is pathognomic of the condition. Because the disease begins in puberty it is often not recognized at its beginning. Pustules enlarge to become abscesses which are
extremely painful and eventually discharge a mixture of pus and blood which is foul-smelling and the pain gradually resolves. As the disease progresses abscesses join up to form sinus tracts and eventually marked scarring. Thus patients suffer both pain and the embarrassment of foul-smelling discharge.
Fig 1: Nodule, abscess, sinus tract and double comedone of HS
Fig 1: Nodule, abscess, sinus tract and double comedone of HS The most commonly areas affected are the axillae, inguinal creases, buttocks, natal cleft and under the breasts or abdominal pannus, any site where skin touches off itself or at sites of friction. The diagnosis of HS is a clinical one and is the presence of: 1. Typical lesions: Primary lesions painful deep seated nodules (blind boils) Secondary lesions abscesses, draining sinuses, bridged scars, “tombstone comedones”
The most commonly areas affected are the axillae, inguinal creases, buttocks, natal cleft and under the breasts or abdominal pannus, any site where skin touches off itself or at sites of friction. The diagnosis of HS is a clinical one and is the presence of:
1. Typical lesions: Primary lesions painful deep seated nodules (blind boils) Secondary lesions abscesses, draining sinuses, bridged scars, “tombstone comedones”
2. Typical localization: Axillae and groin, genitals Under breasts, on buttocks and perineum
2. Typical localization: Axillae and groin, genitals Under breasts, on buttocks and perineum
3. Chronicity and recurrences: Chronic recurrent lesions for more than six months
3. Chronicity and recurrences: Chronic recurrent lesions for more than six months
draining sinuses, bridged scars, “tombstone comedones”
31
CPD 85: HIDRADENITIS SUPPURATIVA
REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice?
IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
PLAN If I have identified a knowledge gap - will this article satisfy those needs - or will more reading be required? 4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs? 5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the CPD Continuing Professional Development CPD 4 previous steps, log and record your findings. Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author. Amgen® has no editorial oversight of the CPD programmes included in these modules Fig 1: Nodule, abscess, sinus tract and double comedone of HS Continuous Professional Development is supported by Amgen® Fig 1: Nodule, abscess, sinus tract and double comedone of HS The most commonly areas affected are the axillae, inguinal creases, buttocks, natal cleft and under the breasts or abdominal pannus, any site where skin touches off itself or at sites of friction. diagnosis of HS is a clinical one and is the presence of: 1. Typical lesions: Primary lesions painful deep seated nodules (blind boils) Secondary lesions Fig 1: Nodule, abscess, sinus tract and double comedone of HS The most commonly areas affected are the axillae, inguinal creases, buttocks, natal cleft and under the breasts or abdominal pannus, any site where skin touches off itself or at sites of friction. diagnosis of HS is a clinical one and is the presence of: 1. Typical lesions: Primary lesions painful deep seated nodules (blind boils) Secondary lesions abscesses,
1.
2.
3.
The most commonly areas affected are the axillae, inguinal creases, buttocks, natal cleft and under the breasts or abdominal pannus, any site where skin touches off itself or at sites of friction. The diagnosis of HS is a clinical one and is the presence of:
1. Typical lesions: Primary lesions - painful deep-seated nodules (blind boils) Secondary lesions - abscesses, draining sinuses, bridged scars, “tombstone comedones”
2. Typical localization: Axillae and groin, genitals Under breasts, on buttocks and perineum

3. Chronicity and recurrences: Chronic recurrent lesions for more than six months
Despite this, diagnosis of HS may be delayed as the chronicity of lesions may not be appreciated and patients may present in different clinical settings, primary care, emergency departments, gynaecology and surgery. It has been estimated that patients may wait eight years for diagnosis, see five different specialities over on average 17 consultations.
This delay in diagnosis leads to irreversible changes of scar, sinus tracts and fistula as the disease progresses, leading to scarring and disability.
Patients with HS may also have other cutaneous disorders such as severe acne and a pilonidal sinus where the spectrum is known as follicular triad, when patients have HS, severe acne, pilonidal sinus and folliculitis de calvens it is known as follicular tetrad.
When two adjacent follicles rupture a sinus tract/tunnel under the skin (Fig 2A and Fig2B)
Severity of HS is most commonly graded using the Hurley Staging
Stage 1 (Mild cases): Single or multiple abscesses
• Painful bumps, pustules and abscesses in the armpits, groin, under the breasts, in between the buttocks or inner thigh;
Stage 3 (Severe cases): Widespread involvement of area, with multiple interconnected tracts and abscesses
When two adjacent follicles rupture a sinus tract/tunnel under the skin (Fig 2B).

a much more heterogeneous immune signature that involves B cells.
• Widespread spots, pustules and abscesses with multiple interconnected skin holes and tunnels (sinus tracts)
• Abscesses can be painful and develop into deep sinus tracts under the skin. Areas of skin involved may split and produce an unpleasant odour
into the dermis (Fig 2A). There may also be abnormalities in the skin’s innate immune system as recent work has shown altered copy numbers of β-defensin, an antimicrobial peptide, was associated with HS. Keratinocytes from the follicles of patients with HS also display differing expression of antimicrobial peptides compared to controls. These follicular contents trigger an immune response leading to redness and inflammation.
Microbial colonisation plays a role and recent work has shown the formation of biofilms in HS lesions and HS skin that is not clinically involved. Bacterial infection may serve a secondary role in the pathogenesis of HS by exacerbating local inflammation via a series of pathogen associated molecular pathways As yet there are no definitive bacterial pathogens identified, but antibiotic treatment is a mainstay of HS treatment.
• These bumps generally start as firm, pea-sized nodules.
Stage 2 (Moderate cases): recurrent abscesses, sinus tract formation

Pathogenesis
Risk Factors for developing HS:
Smoking:
• Recurring spots, pustules and abscesses in multiple areas with scarring and skin tunnelling • Scarring can occur as a result of long-term or repeat occurrences in a single location
Historically HS was thought to be a disease of apocrine glands it is now recognized as a follicular disorder. It is thought that the initial occlusion is caused by hyperkeratinisation of the pilosebaceous unit leading to occlusion of the follicle with swelling leading to rupture of the follicle and discharge of contents
Dysregulation of the innate and adaptive immune system has also been demonstrated based on clinical association with other immune-mediated disorders, its response to biologic therapy in the clinical arena, and from molecular research. Anti-TNF therapy is proving effective in HS and increased lesional expression of IL-17 and IL-23 suggest other pathways and cells may be involved it is clear that HS has
Microbial colonisation plays a role and recent work has shown the formation of biofilms in HS lesions and HS skin that is not clinically involved. Bacterial infection may serve a secondary role in the pathogenesis of HS by exacerbating local inflammation via a series of pathogen associated molecular pathways. As yet there are no definitive bacterial pathogens identified, but antibiotic treatment is a mainstay of HS treatment.
RISK FACTORS FOR DEVELOPING HS: Smoking
Smoking has been associated with a 5 to 12 fold increased risk of HS. In 2011, the specific term “smoker’s boils “for HS lesions in patients who are smokers .
Smoking has been associated with a 5 to 12 fold increased risk of HS In 2011, the specific term “smoker’s boils “for HS lesions in patients who are smokers i This stemmed from a previous randomized, matched pair control group study showing that the percentage of active cigarette smokers among HS patients was 88.9%, with an odds ratio of 9.4 and the percentage of smokers in the matched pair control group was 46%.
32 CPD 85: HIDRADENITIS SUPPURATIVA
Continuous Professional Development is supported by Amgen®
2A 2B
This stemmed from a previous randomized, matched-pair control group study showing that the percentage of active cigarette smokers among HS patients was 88.9%, with an odds ratio of 9.4 and the percentage of smokers in the matched-pair control group was 46%.
Nicotine causes release of tumour necrosis factor alpha causing inflammation, promotion of follicular occlusion and epidermal hyperplasia and contains polyaromatic carbohydrates, that may activate keratinocytes and cells of the immune system.
Obesity
Several studies have confirmed that patients with HS have a higher Body Mass Index (BMI) compared to the general population and this ranges from 12% to 88%, depending on the type of population. In a large retrospective, matched casecontrol study the association between obesity and HS was high with 17.3-fold higher odds of being obese when also afflicted with HS. Overweight individuals tend to have larger and increased

skin folds, leading to subsequent increased mechanical friction and maceration of the overlying skin. In addition, a warm, humid and occlusive environment in these areas favours microbial growth and colonization. In addition obesity is considered to be a state of systemic, low-grade inflammation which contributes to the inflammation in the skin.
Family history
Approximately a third of patients have a family history of the disease and early onset HS is associated with a stronger genetic predisposition ( 55% vs 34 %) and more widespread disease. . This pattern probably represents autosomal dominant inheritance with incomplete penetrance. No definitive genes have been identified apart from rare mutations in gamma secretase gene mutation which leads to a particularly severe clinical presentation.
Hormonal influences
Given that HS is three times more common in women and that some women report premenstrual flare HS can resolve after the menopause, hormonal influences
play a role. This has yet to be fully elucidated and hormonal treatments such as anti-androgens have not proved particularly useful. High rates of Polycystic Ovarian Syndrome among patients with HS also corroborate the likelihood that hormones play a role in pathogenesis.
Co-morbidity in Hidradenitis suppurativa
In a similar paradigm to psoriasis, it is now apparent that HS carries a substantial comorbidity burden, these include obesity, metabolic and cardiovascular disease, inflammatory bowel disease, inflammatory joint dIsease and psychological distress.
Obesity, Metabolic Syndrome and Cardiovascular Disease
Unsurprisingly the increased rates of obesity in HS result in increased rates of metabolic syndrome and also the individual components of metabolic syndrome, including dyslipidemia, hypertension and diabetes, this in turn increases cardiovascular risk. One of the first studies to evaluate CVD risk in HS patients using a validated risk assessment tool, the Framingham
Risk Score (FRS) was an Irish study by Hughes et al. In this study patients were mainly in their thirties yet showed an increased cardiovascular risk than controls.
Inflammatory Bowel Disease
An association between IBD and HS has been reported as early as in 1991. Of 1,076 patients with HS, 3.3% had IBD, with a prevalence of 2.5% for CD and 0.8% for CD. Using the estimated prevalence of IBD in the general it was indicated that the prevalence of IBD is 4-8 times higher in the included HS cohort than in the general population. HS and CD share several similar clinical and pathogenic features. Both conditions are chronic diseases of epithelia which are inhabited by commensal flora and both have demonstrated a clinical response to anti-TNF α therapy. They share a number of predisposing factors including genetic predisposition and smoking association
Fig 3. Algorithm for the treatment of hidradenitis suppurativa, adapted from European S1 Guidelines
33
Continuous Professional Development is supported by Amgen®
Deroofing, LASERs, local excision Wide surgical excision Topical clindamycin Systemic treatment 1. Clindamycin + rifampicine/ Tetracycline 2. Acitretin Systemic treatment Adalimumab/ infliximab Adjuvent therapy Pain management Treatment of superinfections Weight loss and tobacco abstinence
Inflammatory Arthritis
In a French study the estimated prevalence of spondyloarthropathy was 3.7% which far exceeded that of the populationn, 0.3%.
In a retrospective, case-control study of 1,730 HS patients in Massachusetts General Hospital, it was found that 908 patients had spondyloarthritis compared to only 52 in those who did not have HS (52.5% vs. 3.0% p< 0.0001). The pathogenesis underlying the development of SpA in HS patients is unclear. The shared features of dysregulated innate immune response and the role of microbial factors have been hypothesized.
Psychological Distress
HS patients suffer significant pain and foul-smelling suppuration from abscesses or sinuses in affected areas during flares which leads to disruption of activities of daily living as well as work and embarrassment contributing to high levels of psychological distress. Multiple studies have demonstrated high levels of depression and anxiety in patients with HS. These findings support several other studies in the literature proposing a possible link between chronic inflammation and depression.
Treatment of Hidradenitis suppurativa


The publication of the European S1 Guideline for the treatment of hidradenitis suppurativa in 2015 has provided some clarity on how
to manage patients at different Hurley Stage (Fig 3). It clearly suggests a triple-track approach to the management of patients of all stages, i.e. a surgical approach, a medical approach and adjuvant management of pain, suppuration and co-morbidities. The evidence supporting this Guideline was reviewed in an evidence based way and a more complex algorithm generated . This was the first attempt to provide an algorithm for the treatment of patients.
Surgical Treatment
Surgical treatment consists of either lesional treatment: such as de-roofing and carbon dioxide laser ablation, or regional treatment where wide areas of hair-bearing skin are excised and the defect repaired by skin grafting or healing by secondary intention.
Medical treatment
Medical treatments consist of topical clindamycin for mild disease, oral tetracyclines for a prolonged period of up to four months. The combination of rifampicin and clindamycin, both 300mg twice daily for a 10- week period, has proved highly efficacious, though patients must be warned about the risk of developing C. difficile. Adalimumab (Humira®) is recommended as a first-line treatment option in patients with moderate-to-severe HS who were unresponsive or intolerant to oral antibiotics. It should be
administered as 160mg at week zero, 80mg at week two and 40mg each week thereafter, starting from week four and has received a licence for the treatment of HS. Second-line agents for severe disease include infiximab 5mg/ kg, and ustekinumab. The area of biological treatment is being intensely investigated, largely using biologics licensed for other indications.
Adjuvant treatment
The third track of treatment includes treating patients’ symptoms of pain, appropriate wound care and identifying and treating superinfections.
Smoking cessation is important, as research has shown that 92 per cent of patients smoke and smoking is associated with less self-reported remission. The severity of HS was associated with pack years in 846 Dutch patients. Encouraging weight loss is also desired, as weight loss of more than 15 per cent is associated with a significant reduction of disease severity. Clearly treating HS patients requires a multidisciplinary approach involving a dermatologist, a surgeon, nursing support, smoking cessation advice and weight loss programmes.
Although there has been considerable advances in the treatment of HS the disease however remains one for which a definitive treatment have been produced.
Questions
Which of the following statements are true regarding Hidradentis suppurativa: HS is a disease of apocrine glands
HS
severe acne
following Steroids Antibiotics De-roofing Anti TNF NSAIDS HS lesions consist of Comedones Plaques Nodules Abscesses Sinus tracts Risk Factors for the development of
include: Family history Alcohol Smoking Obesity Patients with HS are
likely to suffer from the following conditions: Metabolic syndrome Increased Cardiovascular risk Inflammatory Bowel Disease Depression Rheumatoid arthrtitis
The disease is more common in women HS is an autoimmune disease
affects 5% of the population HS may be associated with
Treatment of HS includes the
HS
more
34 CPD 85: HIDRADENITIS SUPPURATIVA Continuous Professional Development is supported by Amgen®
AUTHORS: Graeme P. Sullivan1, Siobhán Glavey2, Tríona Ní Chonghaile1,3#



1Physiology and Medical Physics, Royal College of Surgeons in Ireland, 123 St. Stephen’s Green, Dublin 2, Ireland.

2Pathology Department, Smurfit Building, Royal College of Surgeons in Ireland, Beaumont Hospital, Dublin 9, Ireland.
3Centre for Systems Medicine, Department of Physiology and Medical Physics, Royal College of Surgeons in Ireland, 123 St Stephen's Green, Dublin 2, Ireland.
#To whom correspondence should be addressed: Tel:+353014028579 Email: tnichonghaile@rcsi.com

60 Second Summary
Multiple myeloma (MM) is a haematological malignancy of terminally differ¬entiated plasma cells, and is the second most common haematological malignancy in the Western World (after non-Hodgkin lym¬phoma). It represents approximately 2% of total cancer cases. Around 361 people are diagnosed with MM each year in Ireland. Men are slightly more at risk (1.2:1.0 sex ratio). The median age at onset is 69 years old, and approximately 63% of patients diagnosed with MM are older than 65 years.
High-dose chemotherapy plus autologous stem-cell transplant (ASCT) remains the standardof-care. Transplant-ineligible patients receive induction regimens according to their frailty status. In both groups, the use of scientifically-informed, evidence-based combinations of novel therapeutic agents including immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs), dexamethasone and more recently monoclonal antibodies (mAbs), has transformed the treatment paradigm. However, genomic and clonal evolution in the tumour-promoting bone marrow milieu underlies relapse in most patients, and new therapies are urgently required.
Novel agents that specifically target and exploit (a) MM tumour cell pathway vulnerabilities and/ or (b) the tumour:bone marrow microenvironment interface are under intense investigation.
Combinatorial approaches targeting MM cells, disrupting MM cell/BM interactions and augmentation of anti-MM immune responses dramatically improve treatment responses and are the focus of ongoing, extensive research efforts.
Prof.
Dr.
Multiple Myeloma: Marrow, Milieus, Microenvironments and Mortality
Introduction
Multiple myeloma (MM) is a haematological malignancy of terminally differ¬entiated plasma cells, and is the second most common haematological malignancy in the Western World (after non-Hodgkin lym¬phoma). It represents approximately 2% of total cancer cases. Around 361 people are diagnosed with MM each year in Ireland. Men are slightly more at risk (1.2:1.0 sex ratio). The median age at onset is 69 years old, and approximately 63% of patients diagnosed with MM are older than 65 years. The lifetime risk of getting MM is approximately 1 in 125 (0.8%). Despite being a rare disease, incidence is increasing – primarily owing to an aging population. There has been a >120% increase in MM cases from the 1990s to now. MM remains an incurable, mortal disease.
Disease development
The clonal plasma cells that causes MM are derived from postgerminal center B cells. In health, following antigen exposure (e.g. viral or bacterial infections), naïve B cells normally proliferate and subsequently undergo somatic hypermutation of the IgH and IgL
VDJ sequences. This process produces long-lived plasma cells that provide durable, sustained antibody production. These cells reside in the bone marrow (BM) and are an important part of humoral immunity. Abnormal clonal plasma cell development mimics these normal biological processes but results in excessive amounts of intact immunoglobulin. These malignant plasma cells chiefly reside in the BM, but can also be found in the peripheral blood and other extramedullary sites, including soft tissue and organs. This is particularly true of the latter stages of the disease. MM is character¬ised by the secretion of a monoclonal immunoglobulin protein (known as M protein or monoclonal protein). This aberrant protein is made by the aforementioned abnormal plasma cells. In 15–20% of patients, the MM cells secrete only monoclonal free light chains, and, in <3% of patients, these cells secrete no monoclonal protein. The clinical features of multiple myeloma are typically driven by monoclonal protein, the malignant cells or cytokines secreted by the malignant cells. Signs of end-organ damage, such as hypercalcaemia (C), renal insufficiency (R), anaemia (A), and/or bone disease (B)
with pathologic lytic lesions or fractures, are commonly referred to as CRAB features and form part of the diagnosis of multiple myeloma.
Multiple myeloma progression
Importantly, MM exists in a spectrum of disorders referred to as monoclonal gammopathies. Within this grouping, monoclonal gammopathy of undetermined significance (MGUS) is characterised by infiltration of clonal plasma cells into the BM and secretion of monoclonal protein, and is the most common form. MGUS is asymptomatic and consistently precedes the development of MM. Typically, MGUS demonstrates low levels of abnormal protein (<3.0 g/dL), abnormal plasma cells (<10%) and patients do not exhibit CRAB features. A proportion of patients experience an intermediate stage, referred to as smouldering multiple myeloma (SMM), with elevated abnormal protein (>3.0 g/dL) and plasma cells (>10 and <60%), but again without CRAB features. Around 15% of MGUS patients subsequently progress to active MM, and approximately 20% progress to myeloma or a related condition (AL amyloidosis, Waldenstrom macroglobulinaemia or a lymphoproliferative
31
CPD 86: MULTIPLE MYELOMA 1. REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice? 2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area. 3. PLAN If I have identified a knowledge gap - will this article satisfy those needs - or will more reading be required? 4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs? 5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the CPD Continuing Professional Development CPD 4 previous steps, log and record your findings. Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
disorder).
Dr. Graeme Sullivan Postdoctoral Research Fellow, Targeted Therapeutics in Cancer Laboratory, RCSI
Dr. Tríona Ní Chonghaile Group Leader, Targeted Therapeutics in Cancer Laboratory, RCSI
Figure 3. Research Team:
Prof. Siobhán Glavey Consultant Haematologist/Clinician Scientist, Beaumont Hospital and RCSI
Dr. Graeme Sullivan, Postdoctoral Research Fellow, Targeted Therapeutics in Cancer Laboratory, RCSI
Dr. Graeme Sullivan Postdoctoral Research Fellow, Targeted Therapeutics in Cancer Laboratory, RCSI
Dr. Tríona Ní Chonghaile Group Leader, Targeted Therapeutics in Cancer Laboratory, RCSI
Pro
Consultant Scientist,
Figure 3. Research Team:
Dr. Graeme Sullivan Postdoctoral Research Fellow, Targeted Therapeutics in Cancer Laboratory, RCSI
Dr. Tríona Ní Chonghaile Group Leader, Targeted Therapeutics in Cancer Laboratory, RCSI
Figure 3. Research Team:
Prof. Siobhán Glavey Consultant Haematologist/Clinician Scientist, Beaumont Hospital and RCSI
Siobhán Glavey, Consultant Haematologist/ Clinician, Scientist, Beaumont Hospital and RCSI
Tríona Ní Chonghaile, Group Leader, Targeted Therapeutics in Cancer Laboratory, RCSI
Specifically, diagnosis of MM requires 10% or more abnormal plasma cells in the BM plus one or more signs of end-organ damage (CRAB). Even without CRAB features, patients who exhibit myeloma-defining events (clonal BM plasma cells of >60%, serum : ratio >100 fold, and/or >1 bone focal lesion on magnetic resonance imaging (MRI) or positron emission tomography (PET)/computed tomography (CT) scan are also treated, as the risk of progression to symptomatic disease is approximately 80% at 2 years. Most recently, nextgeneration sequencing (NGS) analysis of MGUS-SMM-MM patients has proven to be a useful tool to decipher the chronology of disease initiation events. In the near future, the combination of genomic signatures and biomarkers of disease burden might enable identification of those SMM patients who could benefit from early intervention to avoid progression to MM.
Prognostic Factors
The primary genomic events involved in MM are the acquisition of hyperdiploidy or translocations affecting the IGH genes; these events are mutually exclusive. Secondary genomic events include chromosomal translocations, copy-number variations and single-nucleotide variants Genomic events underlying MM affect multiple signalling pathways including MYC, NF κB, and MAPK pathways, plasma-cell differentiation, cell-cycle regulation and DNA-damage sensing and
repair. Clinical and laboratory factors including disease stage, cytogenetic abnormalities, and depth of response to therapy can impact survival of MM patients. Cytogenetic analysis and fluorescence in situ hybridisation (FISH)-based genetic profiling should be routinely performed to evaluate disease biological behavior and prognosis. Among the poor prognostic markers, del(17p) and t(4;14) are the most informative; concomitant secondary cytogenetic abnormalities may impact prognosis. The International Staging System (ISS), based on albumin and β2-microglobulin levels, is most widely used and has been revised (R-ISS) to incorporate lactate dehydrogenase (LDH) and high-risk-FISH abnormalities. Given the genomic complexity of MM, more sophisticated techniques including gene expression profiling, mutational status, and copy number abnormalities have been used, alone or in combination with FISHbased approaches, to more deeply characterise disease biology and prognosis. For example, newly diagnosed MM (NDMM) patients carrying del(17p) may be further stratified using subclonal analysis. Targeted sequencing has been used as an alternative to whole exon sequencing to specifically analyse fractions of the genome and provide more accurate risk stratification. Although not yet widely incorporated into practice, these approaches will help to define future personalised treatment strategies in MM.
Outcomes
Historically, MM was associated with a poor prognosis, with a 5 year age-standardised overall survival rate of 27.5% (according to Irish data from 1994-1998). The subsequent introduction of novel drugs: proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and potent monoclonal antibodies (mAbs) significantly improved the clinical outcomes of patients with MM and drove up the 5-year overall survival to 52.1% in Ireland, which is comparable to other jurisdictions (52.6 % for the U.K., 54.0% for the USA). However, progress has plateaued at this level. There is an urgent need to redouble our research efforts to unstick and build upon the advancements made to date.


Management

High-dose chemotherapy plus autologous stem-cell transplant (ASCT) remains the standardof-care for newly diagnosed patients under 70 years of age with satisfactory cardiac, pulmonary, hepatic and renal function. Transplant-ineligible patients receive induction regimens according to their frailty status. In both groups, the use of scientifically-informed, evidencebased combinations of novel therapeutic agents including IMiDs, PIs, dexamethasone and more recently mAbs, has transformed the treatment paradigm. However, genomic and clonal evolution in the tumour-promoting BM milieu underlies relapse in most patients, and new therapies are required.

LATEST TREATMENTS AND RESEARCH
Exploitation of MM pathway vulnerabilities

Tumour cells rely on survival mechanisms that are imprinted during cellular lineage development: lineagedependency. Bortezomib, a first-in-class proteasome inhibitor (PI), has clearly demonstrated the dependency of MM cells on the protein quality control pathway as a therapeutic target. The ubiquitin-proteasome system (UPS) is the main mechanism for maintaining protein homeostasis. In MM, increased aberrant protein turnover and immunoglobulin production exerts robust pressure on this system, making it exquisitely sensitive to proteasome inhibition. Exploiting this vulnerability with a PI, such as bortezomib, is a logical first-line and (typically) successful treatment approach. However, bortezomib has broad, pleiotropic effects. It triggers intrinsic and extrinsic myeloma cell apoptosis and cell cycle arrest. It also modifies bone turnover and osteoclast activity in the bone marrow. Furthermore, bortezomib inhibits the NF-κB pathway (via degradation of its inhibitor, IκB). NF-κB is a major oncogenic pathway in MM: it mediates MM cell survival and DNA repair, promotes interactions of MM cells with BM stromal cells via the transcription of adhesion molecules, as well as modulating the transcription and release of cytokines in the BM microenvironment (such as IL-6, VEGF, IGF-1), which in turn mediate MM growth and drug resistance, and confer immunosuppression in the BM milieu. Subsequent generations of orally available PI (including carfilizomib and ixazomib) were developed to help overcome bortezomib resistance and offer additional PIs to the armamentarium.
Targeting upstream components of the UPS has also recently emerged as a promising strategy to overcome PI resistance. Therapeutic targeting of deubiquitylating enzymes (DUBs) and the 19S proteasomeassociated ubiquitin receptor Rpn13 overcame PI resistance in preclinical studies. However, the first human trial of USP14/ UCHL5 DUB inhibitor for relapsed/ refractory MM (RRMM) was stopped owing to dose-limiting
32 CPD 86: MULTIPLE MYELOMA
Figure 1. Multiple myeloma tumour cell lines exhibit exquisite sensitivity to proteasome inhibition with bortezomib (Velcade®)
A B C
(A C) A panel of multiple myeloma (MM) cell lines (A) JJN3, (B) MM1S and (C) KMS18 cells were grown overnight and treated with titrations of bortezomib, as indicated, for 24 hrs. After 24 hrs, cells were harvested and analysed by flow cytometry for determination of apoptosis by Annexin V/Propidium Iodide assay.
toxicity. Another novel and attractive approach to overcome PI resistance is the concomitant blockade of the aggresome/ autophagy pathway using an inhibitor of histone deacetylase 6 (HDAC6), which is recruited to maintain proteostasis balance as an adaptive response mechanism. Several groups, including our own, are actively pursuing the development of novel targeted, selective HDAC6 inhibitors with therapeutic potential.
Lineage vulnerabilities in MM also include aberrant transcription factor (TF) regulatory networks controlling the lineage factor IRF4. However, direct targeting of TFs is a challenging strategy and there are no inhibitors for clinical use available at present. Notably, repression of IRF4 transcription is observed posttreatment with lenalidomide, which triggers cereblon (CRBN)mediated degradation of the IRF4 transcriptional activator IKZF3. Building on this knowledge, a new platform technology has been developed to trigger selective protein degradation. Proteolysistargeting chimeras (PROTACS) are designed by conjugating the small-molecule binder of the target protein to an E3 ubiquitin ligase binding scaffold such as the analogs of thalidomide which bind CRBN. This exciting approach will allow for therapeutic degradation of protein substrates that are otherwise difficult to target.
Exploitation of MM cell clone vulnerabilities

MM is characterised by genetic and biological heterogeneity. Despite this, a number of targetable oncogenic dependencies have become apparent, particularly driver mutations and translocations. Alteration of the transcriptional program of MYC, and of its functional collaborators promotes pro-tumour signaling and plasma cell survival. Frequent gene mutations in MM include RAS (KRAS or NRAS) with downstream activation of the MAPK pathway, BRAF, DIS3, and FAM46C. Other signalling pathways affected include the NF-κB pathway (affected by copy number loss, mutation, and translocations) and the PI3K pathway (dysregulated in the absence of genetic change).
Apoptotic pathway dysregulation occurs, with BCL2 dependency in patients with t(11;14) and MCL1 dependency in other patients observed. However, it is worth
(B)
noting that the role of mutations in MM has not been fully elucidated, only TP53 mutation (affecting 6-8% of newly diagnosed patients) demonstrably confers worse outcome. Related to this, tumour-targeted treatments in MM are often compromised by intra-clonal heterogeneity. Deep sequencing has identified a complex subclonal structure in MM with different patterns of clonal evolution impacted by BM, immune response, and therapythus making successful targeting of the above highly difficult. p
Targeting MM epigenetic modifications
Epigenetic alterations affect regulation of gene activity and expression, without altering gene sequence. Such alterations are associated with MM onset and progression, and modulate several important biological processes. Global hypomethylation of the genome characterises the MGUS>MM transition. In contrast, pervasive genome re-methylation occurs in the transition from MM to a more aggressive plasma cell leukaemic stage. Specific epigenetic targets include the type II arginine methyltransferase PRMT5 in MM. Inhibition of PRMT5 results in MM cell killing via NF-κB blockade, thus providing the rationale for clinical trials targeting PRMT5 in MM.
Histone deacetylases (HDAC) are generally hyperactive in MM. HDAC inhibitors are the most studied epigenetic drugs that have led to clinical trials and

approval of the non-selective HDAC inhibitor panobinostat in combination with bortezomib in relapsed/refractory disease. Increased toxicity observed with panobinostat prompted the development of HDAC6selective inhibitors (ricolinostat and citarinostat/ACY-241), which show promising results and lower toxicity in combination with bortezomib and dexamethasone.


Increasing interest surrounds the use of microRNA-targeting strategies in MM. Preclinical data have defined their oncogenic (miR-221/222,-21,-17-92 cluster) or tumour suppressive (miR29b,-34a,-125b,-15,-16) roles in MM associated with repression or overexpression, respectively, of genes involved in essential pro-survival pathways. Several candidates are being tested in other diseases and will soon be evaluated in MM, using miRNA replacement or inhibition strategies. Theoretically, as they are endogenous antisense of mRNAs, their replacement is hypothesised to induce a “natural” effect on the targets, with less off-target effects compared to siRNA-mediated targeting strategies. In addition, the recent availability of suitable in vivo delivery systems now permits their use in clinical trials.

Targeting the tumour-bone marrow microenvironment interface
Disrupting the interactions of MM cells with the BM represents an ideal therapeutic strategy in MM, as demonstrated by
the IMiDs (which remain active against MM even in the protective milieu of the BM. Cellular and non-cellular components of this niche support MM cell proliferation, migration, survival and drug resistance, while also conferring immunosuppression, and therefore represent targets for novel therapeutics under investigation by our group and others. Disease progression is mediated by intrinsic factors of the clonal cells along with factors that mediate a permissive tumour microenvironment. The MM tumour niche comprises the components of the BM microenvironment–cellular (stromal cells, osteoblasts, osteoclasts, endothelial cells and immune cells) and noncellular extracellular matrix (ECM).
Immunomodulatory drugs (IMiDs)
Comprehensive clinical trials have led to the approval of the IMiD thalidomide and its more potent analogs lenalidomide and pomalidomide for treatment of MM and RRMM. These agents exert direct effects on MM cells including growth arrest and caspase-8-mediated apoptosis, associated with CRBN-dependent degradation of IKZF1/3 followed by IRF4 downregulation, as mentioned earlier. In the BM microenvironment, IMiDs abrogate MM cell adhesion to the BM, modulate cytokine and growth factor secretion, inhibit angiogenesis and upregulate T-, NK-, and NK-T-cell-based immunity while downregulating T-reg cells. Mechanistically, binding
33
Figure 2. Ex vivo primary multiple myeloma patient samples respond differentially to a panel of BH3 mimetics
(A) Ex vivo primary patient MM cells were isolated, CD138+ cells sorted, purified and stained by H&E followed by microscopic evaluation.
A B
Primary MM cell cultures, as above, were treated with a panel of BH3 mimetics, as indicated, and analysed by flow cytometry for determination of apoptosis by Annexin V/Propidium Iodide assay.
to CRBN has been implicated in mediating the immune effects of IMiDs, as IKZF1/3 degradation in T cells increases their secretion of cytokines, notably IL-2. This mechanism is also associated with increased NK and NK-T-cell cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) (i.e., antiCD38, daratumumab and -CD20, rituximab) observed after IMiD treatment. Preclinical and clinical studies confirm the strong synergism of IMiDs with PI and with mAb treatment.
Immune-based therapies
Loss of immune surveillance supports MM tumour growth and resistance, and is associated with alterations in nonlymphoid and immune cells in the bone marrow. There is also increasing evidence that evolving immune dysfunction is an important determinant of progression from MGUS/SMM to symptomatic MM. Immune escape is also mediated, at least in part, by increased expression of immune checkpoints, i.e., PD-1/PD-L1, in T cells and MM cells. Alas, results with immune-checkpoint blockade via PD-1/PD-L1 in MM have been disappointing thus far. No single agent activity of the humanised anti-PD-1mAb pembrolizumab has been shown, and importantly, two randomised clinical trials
evaluating pembrolizumab in combination with IMiDs to treat RRMM were terminated owing to excessive mortality.
Thankfully, multiple mAbs that target MM surface antigens and trigger ADCC, antibody-dependent cellular phagocytosis, complement activation, and direct effects on MM cells have proven efficacious. Elotuzumab and daratumumab target SLAMF7 and CD38, respectively. Elotuzumab also activates NK cells and is approved in combination with lenalidomide or pomalidomide in RRMM. Daratumumab, in particular, has shown remarkable efficacy and frequency of response. This has led to its approval as a single agent or in combination with IMiD and PI in both newly diagnosed and RRMM. Notably, a recent (GRIFFIN) trial compared standard lenalidomide-bortezomibdexamethasone with or without daratumumab in transplant eligible patients, and showed deeper responses and minimal residual disease negativity rates in the daratumumab-treated patient cohort. The recent approval of subcutaneous daratumumab will significantly reduce patient treatment times and accessibility. More recently, in March 2021, a new CD38-directed mAb, isatuximab, has been approved in combination with carfilizomib-
dexamethasone to treat RRMM; whether it is effective in daratumumab-refractory disease remains to be determined.

Monoclonal antibody technologies have also provided the framework for the development of Ab-drug conjugates (ADC) and bispecific T-cell engagers (BiTE). B-cell maturation antigen (BCMA)directed ADCs are currently under investigation in both preclinical and clinical settings, and represent a promising approach due to the highly specific expression of BCMA on MM cells and role of the BCMA/ APRIL pathway in supporting MM cell survival in the BM.
Cellular therapies, in particular chimeric antigen receptor (CAR) T-cell approaches are under active investigation and receiving a lot of attention owing to their success in other haematologic conditions (particularly diffuse large B cell lymphoma and mantle lymphoma). Their success is predicated on the identification of unique and highly expressed MM antigenic targets. Among a variety of targets, B-cell maturation antigen (BCMA/TNFRSF17) is the most frequently used due to its selectivity for normal plasma and MM cells. Several CAR-T products have been clinically tested in heavily pre-treated (PI-IMiD-CD38 mAb) RRMM, and have demonstrated remarkable, deep MRD responses. Increased clinical use of and experience with such therapies has also helped to ameliorate the more commonly observed toxicities of CAR-T cells (cytokine release syndrome and neurotoxicity). To date, however, most patients have relapsed, and ongoing research is assessing mechanisms of resistance to CAR-T, utilising combination immune approaches with CAR-T, and using CAR-T earlier in the disease course in order to achieve more durable responses.
Looking to the future
There have been incredible advances in the range of therapies available to clinicians treating MM. Despite this, management of MM patients remains challenging: acquisition of resistance underlies relapse in most patients. The development of second-generation potent drugs of the same class has helped to overcome both PI and IMiD resistance, as have combination therapies
with agents targeting pathways mediating resistance. Identification of biomarkers of patient MM resistance/sensitivity may further inform sequential and combination therapies in the future. The first example of biomarker-driven anti-MM treatment in MM is the Bcl-2 inhibitor venetoclax/ABT199, whose safety and efficacy are predicated upon occurrence of t(11;14) or presence of high levels of BCL2. Several trials in RRMM demonstrate the efficacy of venetoclax, as monotherapy and in combination, restricted to this patient subset.
Agents targeting novel MM vulnerabilities are urgently needed. Resistance is now beginning to appear for immune-based approaches. Loss of targeted antigens (such as BCMA, CD38) is a common event, either due to loss with tumour evolution or to suppression in the face of immune pressure. Multi-antigen targeting may potentially overcome this obstacle, and several trials are evaluating this strategy. Additional resistance may be intrinsic to the technology or modality, for example ongoing efforts are directed at increasing CAR-T cell expansion and persistence in vivo to promote CAR-T cell persistence. Lastly, T-cell exhaustion and the immunosuppressive BM may contribute to both targeted- and immune-therapy resistance. Restoration of host anti-MM immunity represents an important unmet need in MM. With >100 active clinical trials in stage 3 or later development at present, these needs are receiving great attention. Combinatorial approaches targeting MM cells, disrupting MM cell/BM interactions and augmentation of anti-MM immune responses have dramatically improved treatment responses and duration. Key obstacles to overcome MM mortality include (i) constitutive and evolving genomic heterogeneity in MM cells, as well as (ii) the immunosuppressive BM milieu. Going forward, integration of multi-omics sequencing technologies profiling both the MM cell and BM accessory/immune cells will help identify therapeutic targets and translate to novel rationally designed, selective, targeted and immune therapies. Past successes have shown usthe future is bright for MM research!
References on request
34 CPD 86: MULTIPLE MYELOMA
60 Second Summary
Chronic Lymphocytic Leukuemia also known as CLL is a cancer that affects the blood and bone marrow. CLL affects the white blood cells called lymphocytes. It accounts for 2.4% of cancer diagnose in Ireland every year, affects men more than women and is more common in those over the age of 70.
CLL comprises over 40% of all leukaemias, affects older patients and may be asymptomatic for much of its course. It is often detected only through routine blood counts and only picked up if the individual presents to clinical services. Approximately 10% of individuals with CLL report a family history of the condition or a related lymphoproliferative disorder, but the genes which account for increased susceptibility have not been identified.
The course of the disease generally follows a period of “watch and wait”, followed by treatment with periods of relapse and remission. It remains an incurable disease however there are a number of novel treatments that have become available which have changed the course of the disease, improving patient’s response to treatments and overall quality of life.
COVID19 has had a significant impact on CLL patients, many of them being immunodeficient due to the nature of the disease or the multiple lines of treatment received. As such treatments for this high risk group are welcomed.
This CPD article will particularly focus on and examine the evidence on the treatment of CLL with Bruton's tyrosine kinase inhibitors (BTKi’s) particularly in the newly diagnosed CLL setting.
AUTHORS: Mary Kelly, FFNMRCSI, RANP, RNP, Msc, BSc Cancer Nursing, BSc Adult Nursing: Mary is an Advanced Nurse Practitioner in Haematology at the Midlands Regional Hospital, Tullamore. Mary has worked in Haematology Nursing for the past 24 years. She has worked in all Haematology nursing care settings both in the UK and Ireland at St Marys and St Bartholomew’s Hospitals, London, Blackrock Clinic and Midland Regional Hospitals. Mary manages CLL patients as part of her clinical caseload.
Orlaith Cormican RGN, Bsc, MHsc, Post Graduate Certificate in Cancer Care and Haematology: Orlaith is a Clinical Nurse Specialist in Haematology at the Midlands Regional Hospital. Orlaith has worked in cancer care and haematology since 2013. She is currently completing a PhD via the HRB SPHeRE programme. Her areas of interest include chronic haematological malignancies and quality of life in this area. Orlaith works with CLL patients on a daily basis.

Published
Chronic Lymphocytic Leukemia (CLL): An overview of its management and treatment with Bruton's tyrosine kinase Inhibitors


What is it?
CLL is the most common leukaemia in the western world (AlSawaf et al., 2020). CLL is more common in males and is generally associated with those over the age of 70 (NCRI, 2022). All leukaemia’s in Ireland account for 2.4% of all malignant disorders and there are over 500 new diagnoses of CLL made in Ireland every year. The cause of CLL is unknown. Risk factors include family history of haematological malignancy, increasing age and working in farming or hairdressing (Slager et al., 2014).

CLL is characterised by the clonal proliferation and accumulation of neoplastic B-lymphocytes in the blood, bone marrow lymph nodes and spleen (Al-Sawaf et al., 2020). B cell receptor signalling leading to activation of various intracellular signalling pathways plays an important role in the survival of CLL cells (Stevenson & CaligarisCappio, 2004). These pathways are now being successfully exploited as therapeutic targets (Jain & Rai, 2012).
Background and Overview
The majority of CLL cases are diagnosed through blood counts, differential counts, a blood smear and immunophenotyping.
In CLL, CD5 + B cells undergo malignant transformation. They are activated by the acquisition of mutations leading to monoclonal B cell lymphocytosis (MBL). MBL is defined as an asymptomatic condition where the absolute monoclonal B cell count is <5 x 10 9/L. It occurs in 4-5% of healthy adults and is a proposed precursor of CLL. Most cases of CLL are preceded by MBL while only 1-2% with MBL can develop CLL every year (Jain & Rai, 2012). In CLL, further accumulation of genetic abnormalities and subsequent oncogenic transformation of monoclonal B cells occurs (Burger, 2020). The diagnosis of CLL requires the presence of ≥5000 B-lymphocytes/μL in the peripheral blood for duration of at least 3 months. The clonality of the circulating B cells needs is confirmed by flow cytometry. Furthermore, there may be evidence of lymphadenopathy or organomegaly by physical or CT exam (Hallek, 2019).
CLL and small lymphocytic lymphoma (SLL) are different clinical presentations of the same pathological disease and commonly referred to as CLL(Patel & Pagel, 2021). The term CLL is used when the disease manifests primarily in the
blood, whereas SLL is used when involvement is primarily nodal (Rai KR, Stilgenbauer, 2021). Patients with CLL commonly develop complications associated with an intrinsic immune dysfunction resulting in immunodeficiency and autoimmune disorders. Ten per cent of patients will present with autoimmune haemolytic anaemia (Christi, M, 2020). Infection, anaemia and thrombocytopenia are the most common disease related complications. The increased risk of severe infections in CLL patients is primarily related to the disease and treatment related immunodeficiency, mainly bacterial and herpes virus family. Tumour lysis syndrome (TLS) and secondary cancers are less common but also potentially life threatening.
CLL remains an incurable disease but there continues to be progress made in therapeutics, with the emergence of newer therapies improving overall survival (OS) and being well tolerated by patients enabling them to maintain a good quality of life for many years (Sharma & Rai, 2019). For those patients who are relatively asymptomatic, they may follow
31
up
clinical
receiving any therapies
CPD 87: ONCOLOGY CLL
REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice? 2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area. 3. PLAN If I have identified a knowledge gap - will this article satisfy those needs - or will more reading be required? 4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs? 5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the
CPD
the “watch and wait” protocol followed
by
observation without
(Milne et
1.
CPD Continuing Professional Development
your
4 previous steps, log and record
findings.
by HPN. Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
editorial
programmes included in these modules
Abbvie has no
oversight of the CPD
Mary Kelly Orlaith Cormican
Table 1
Stage Characteristics
Stage Characteristics
Low Risk (Stage 0)
Low Risk (Stage 0)
Abnormal increase in the number of lymphocytes in the blood and marrow
Abnormal increase in the number of lymphocytes in the blood and marrow
No enlargement of the lymph nodes, spleen, or liver and with near normal red blood cell and platelet counts.
No enlargement of the lymph nodes, spleen, or liver and with near normal red blood cell and platelet counts.
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Enlarged lymph nodes OR
Enlarged lymph nodes OR
Intermediate Risk (Stages I & II)
Intermediate Risk (Stages I & II)
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Table 1
The IGHV mutation status is also a useful prognostic and treatment indicator; mutated IGHV tends to be less aggressive and especially when combined with additional prognostic factors such as favourable cytogenetics (Puiggros et al., 2014).
High Risk (Stages III & IV)
High Risk (Stages III & IV)
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Enlarged spleen and/or liver
Enlarged spleen and/or liver
The red blood cell or platelets counts are normal or only slightly low.
The red blood cell or platelets counts are normal or only slightly low.
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Anemia (hemoglobin <11g/dL)
Anemia (hemoglobin <11g/dL) OR
Condoluci et al., (2020) developed an International Prognostic score for early stage CLL (IPS-E). The IPS –E aims to predict time to first treatment (TTFT) in patients with early asymptomatic CLL at the time of diagnosis. The CLL-IPI-E combines genetic, biochemical and clinical parameters to categorise patients into risk groups (Table 2). The following three covariates were consistently and independently correlated with TTFT, Unmutated IGHV, absolute lymphocyte count >10x 109/L and presence of palpable lymph nodes. Each covariate is assigned 1 point each and scoring is used to classify risk. Importantly, del (17p) and TP53 mutations were not prognostic for TTFT but are important in predicting treatment response and guiding treatment choice once indicated (Rai KR, Stilgenbauer, 2021).
Treatment and Management
OR
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Thrombocytopenia (platelet counts <100,000/uL)
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Thrombocytopenia (platelet counts <100,000/uL)
Enlarged Lymph nodes, liver or spleen.
Enlarged Lymph nodes, liver or spleen.
al., 2020). Treatment is usually commenced at disease progression or when the patient starts to develop symptoms associated with CLL (Milne et al., 2020). The majority of patients with a diagnosis of CLL will have more than one comorbidity at diagnosis, which may influence treatment selection (Kabel C, 2021). In addition, treatment can be patient- dependent, considering compliance, convenience, financial toxicity and duration of therapy (Murphy, 2021).
Diagnosis, Staging and Prognosis
CLL has a widely variable disease course, influencing prognosis. Some patients die rapidly, within 2-3 years of diagnosis, due to CLL complications. Most patients live 5-10 years, with an initial course that is relatively benign, followed by a terminal, progressive and resistant phase lasting 1-2 years (Christi, M, 2020). Significant morbidity from both the disease and complication of therapy may exist in the later phase (Rai & Barrientos, 2014).
A patient’s prognosis is dependent on disease stage; in addition to the presence or absence of high-risk markers (See Table 1 and 2). The Rai staging system classifies CLL into three separate risk groups. The Binet staging system classifies CLL into three stages (Table 1).
Prognostic indicators
Cytogenetics may detect abnormalities on the chromosomes, which are associated with CLL. Samples are from the blood or bone marrow. FISH analysis may identify abnormalities associated with chromosomes 11, 12 13 and 17: deletion 13q, deletion 11q, trisomy 12, and deletion 17p. Deletion 13q remains the most favourable prognostic group, with deletion 17p being the least favourable (Puiggros et al., 2014).
Furthermore, genomic abnormalities associated with TP53 mutation tend to be characterised as high risk and associated with adverse outcomes
The clinical course of CLL is extremely varied. Clinical trials in early stage CLL have failed to show survival benefit and therefore by international guidelines treatment is not recommended in this cohort. Ongoing randomised trials are evaluating the use of modern therapies in early stage disease and prognostic markers associated with poor clinical outcomes. However, until treatment benefit proves more as beneficial, treating in this setting is reserved for patients enrolled in these clinical trials (Rai KR, Stilgenbauer, 2021).
Indications for treatment outlined by the International working group CLL (IWCLL) criteria include progressive lymphadenopathy, organomeagly, deteriorating blood counts and constitutional symptoms (Hallek et al, 2018). In addition the presence of progressive lymphocytosis, a doubling time of < 6 months, Hb less than 100g/L or a platelet count of < 100 x 109/L or treatment resistant autoimmune cytopenias are indications to commence treatment (Milne et al, 2020).
Lymphocytosis itself, even if extreme, is not a strict indication for treatment if patients have no symptoms and adequate bone marrow function. However, extreme lymphocytosis (>200,000/ micro/L) is associated with an increased risk of hyperviscosity and may serve as an indication for treatment in an otherwise asymptomatic CLL patient (Rai KR, Stilgenbauer, 2021).
Treatment selection

There is no single standard front line therapy regime for all patients with symptomatic or advanced CLL (Rai III-IV, Binet C) or patients with progressive cytopenias. Several initial
Table 2
32 CPD 87: ONCOLOGY CLL
Score Risk Category 5 year cumulative
to
0 Low
1 Intermediate
2-3 High
Table 2
risk
start treatment
8.4%
28.4%
61.2%
Table 1
Chemo Drugs Monclonal Antibodies
Kinase inhibitors
PI3K Inhibitors
B-cell lymphoma-2 (BCL-2) inhibitors
Fludarabine Rituximab Ibrutinib Idelalisib Venetoclax Chlorambucil Obinutuzumab Acalabrutinib
Bedamustine Cyclophosphamide
Common Side Effects
Hair loss
Mouth sores
Loss of appetite
Nausea and vomiting
Low blood counts
Chest pain Heart racing
Swelling of the face and tongue Cough Trouble breathing
Feeling dizzy, lightheaded, or faint
Diarrhoea Nausea
Constipation Fatigue, shortness of breath
Swelling of the feet and hands, body aches, Rash
Low blood counts Atrial Fibrillation
treatments options exist, with varying rates of complete response, time to progression and associated toxicities (See Table 3). Patient’s treatment is individualised based on the patient, tumour characteristics and goals of therapy At least 90% of patients have one or more co-morbidities, therefore an MDT approach involving patients and outlining the potential benefits and risks of a particular treatment is important when considering treatment (Thurmes et al., 2008). Optimising health related quality of life (HRQoL) and managing adverse events are also important considerations in the decision to treat and choice of therapy (Patel & Pagel, 2021). Frey et al., (2016) reports disease severity as a predictor of poor HRQoL. In addition, toxicities associated with long-term targeted treatments have been shown to affect adherence, thus the emergence of limited duration therapies where appropriate is welcome.
Treatment options for CLL include CIT, targeted therapies, radiation therapy and supportive care. Over the past two decades, a dramatic increase in our understanding of the pathogenesis of CLL has led to the development of small molecule inhibitors for CLL targeting the B cell
Diarrhoea Fever Fatigue Nausea Cough Pneumonia Low blood counts Pneumonitis
Tumour Lysis Syndrome
Low blood counts
Diarrhoea Nausea
Respiratory infections
Thrombocytopenia Fatigue
Although FCR is not an effective option for patients with TP53 and del (17q) mutations, for patients with mutated IGHV trials showed that time limited FCR can provide functional cure and therefore despite its diminishing role FCR may still be considered in this patient group.
Bendamustine and rituximab and Obinutuzumab plus chlorambucil are also regimes that are active in CLL. These regimes are also options for older patients with cardiovascular comorbidities where BTK inhibitor therapy could increase the risk of cardiovascular events. CIT may be considered in patients where a time-limited treatment is preferable. Newer treatment options with fixed duration combinations for example Ibrutinib and ventoclax will further challenge the role of chemoimmunotherapy in the near future.
Targeted Treatments
Bruton Tyrosine Kinase (BTK) InhibitorsIbrutinib, Acalabrutinib
The BCR pathway is implicated in the pathogenesis of several B-cell malignancies, including MCL, diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, and CLL. BTK's pivotal role in signalling through the B-cell surface receptors and results in activation of pathways necessary for B-cell trafficking, chemotaxis and adhesion. These BTKi work by forming a covalent bond with a cysteine residue (Cys-481) in the BTK active site, leading to sustained inhibition of BTK enzymatic activity.
Potent, small-molecule inhibitors of BTK developed to treat CLL include, Ibrutinib and Acalabrutinib.
Ibrutinib
receptor pathway and the apoptotic regulator BCL2 (Milne et al, 2020). These targeted therapies, enable patients to be treated with a chemotherapy free regime.
Similar to management in most malignancies, the most suitable treatment for a patient is selected based on genetic features of the disease itself (Milne et al, 2020). Molecular assessment prior to every line of treatment is essential to avoid ineffective treatments.
When planning treatment it is also important to consider the current pandemic. Nevertheless, in those patients with active CLL, treatment should not be withheld, because data suggests that the outcome of patients with COVID-19 is better if their cancer is under control. In patients requiring intervention, BTKi’s may represent the preferred therapeutic option (Roschewski et al., 2020).
Chemo immunotherapy (CIT)
For many years Fludarabine, cyclophosphamide and Rituximab (FCR) were the gold standard treatment for CLL. FCR yielded high overall response rates of up to 95% with some patients remaining in remission for over a decade.
Ibrutinib was the first available oral small molecule inhibitor that binds to BTK preventing its kinase activity. This affects multiple signalling pathways and disrupts the interaction between CLL cells and the microenvironment leading to further apoptosis (Milne et al, 2020). Ibrutinib is indicated for the treatment of CLL and SLL with or without del (17p) (SPC, 2021). Clinical trials demonstrated the clinical benefit of Ibrutinib in a variety of clinical settings (Ghia et al., 2021). The Resonate trial showed improved PFS, OS and ORR for Ibrutinib versus ofatumumab in previously treated patients (Byrd et al., 2014). Resonate 2 showed ibrutinib superiority in PFS, OS and ORR in treatment naïve patients when compared to Chlorambucil. Further Resonate-2 analysis revealed 61% of patients were alive and progression free at 6.5 years and 6.5 year OS was 78%.
Adverse events of clinical interest over time for patients on Ibrutinib included hypertension, atrial fibrillation and less commonly major haemorrhage (Ghia, P, Owen, C et al., 2021).
The iLLUMINATE trial compared Ibrutinib plus obinutuzumab versus chlorambucil and obinutuzumab. The results showed significantly


33
Table:
3
Table
3
improved PFS (Moreno et al., 2019). Ibrutinib is taken orally at the same time each day. Ibrutinib is generally well tolerated; though there are some common side effects (Table 3).
ASCEND trial
Price Variation in Prescription Drugs
patient benefits including limited treatment duration with a shorter period for side effects, outpatient based therapy and reduced financial burden on patients and carers (DePace, 2021).
Acalabrutinib
Acalabrutinib is a highly selective, potent BTK inhibitor (Ghia et al, 2020). In the phase II study that evaluated. Acalabrutinib in relapsed/refractory CLL, Acalabrutinib resulted in an overall response rate (ORR) of 93% in patients with Del (17p) and 90% of those with complex karyotype.
Elevate trial
Prescription drugs are a major source of expenditure for patients in Ireland. High prices can lead to cost-related non-adherence and adverse health outcomes. Researchers at Trinity College Dublin and RCSI University of Medicine and Health Sciences (RCSI) investigated the variation and availability of prescription drug prices in community pharmacies in Ireland. The study is recently published in the journal Research in Social and Administrative Pharmacy.
Pharmaceuticals are one of the main sources of healthcare expenditure for people, with prescription drugs described as a major cause of unaffordable expenditure for the Irish public. The question researchers sought to answer was: how much prices for commonly prescribed drugs varied between pharmacies?
The research team investigated the prices of 12 commonly prescribed drugs by calling 1,500 community pharmacies, emailing 320, and checking the website of 370. A community pharmacy is described
as a retail shop which provides pharmaceutical drugs among other products to the public. Community pharmacies can include both chain pharmacies and independent pharmacies.
Key findings:
The Elevate trial was the first trial of Acalabrutinib versus Ibrutinib in previously treated CLL. Patients were randomised to Acalabrutinib 100mg orally twice daily or Ibrutinib 420mg orally once daily and continued until disease progression or unacceptable toxicity. The primary endpoint was non-inferiority and secondary endpoints included incidence of Atrial fibrillation, grade >/ three infections, Richter’s transformation (RT) and (OS). At a median follow up of 40.9 months, Acalabrutinib was non-inferior to Ibrutinib, with a medium PFS of 38.4 months in both arms. Acalabrutinib demonstrated lower frequencies of adverse events (AE). Cardiovascular events were less common with Acalabrutinib, 9.4% vs 16% for atrial fibrillation (AF) and 9.4% vs 23.2% for hypertension.
ELEVATE-TN Trial
In this randomised, controlled, phase 3 trial the efficacy of Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatmentnaïve CLL patients was compared. At a median follow up of 28.3 months median PFS was longer with Acalabrutinib – obinutuzumab (A-O) and Acalabrutinib monotherapy compared with obinutuzumab-chlorambucil (O-C). Estimated PFS at 24 months was 93% with A-0, 87% for patients on Acalabrutinib monotherapy and 47% in the O-C arm. Patients on A-O and Acalabrutinib tolerated treatment well and had a manageable safety profile, with 79% of patients remaining on therapy after a median follow up of 28.3 months. Sharman et al., (2020) concluded that Acalabrutinib with or without obinutuzumab is efficacious and a well tolerated treatment for patients with treatment – naïve CLL.
Lorraine O’Driscoll FTCD FRSB MRIA, Professor of Pharmacology and Biomedicine at Trinity College Dublin School and Research Lead for Trinity St James's Cancer Institute, is recipient of a ¤4 million award to lead the AllIreland Cancer Liquid Biopsies Consortium (CLuB).
The All-Ireland Cancer Liquid Biopsies Consortium (CLuB)

1,529 pharmacies responded to queries, 1,362 by telephone and 167 by email.
The mean quoted cost for each of the 12 drugs investigated was higher than the HSE reimbursement price for medical card patients. For famciclovir, the average price quoted to us was ¤46.00 which was ¤8.69 higher than the HSE reimbursement price of ¤37.31.
Acalabrutinib proves to be an effective treatment for patients with relapsed/refractory (R/R) disease. The study demonstrated a statistically significant and clinically meaningful PFS with Acalabrutinib compared with Idelalisib plus Rituximab (I-R) or Bendamustine plus Rituximab (B-R). The benefit of Acalabrutinib was seen across all patient subgroups including those with del (17p) or TP53. In addition, a tolerable safety profile was demonstrated. Acalabrutinib is licenced to treat adults with mantle cell lymphoma (MCL) who have received at least one prior treatment for their cancer, or adults with CLL or SLL.
Despite regulatory guidance stating pharmacies should provide medicine prices to patients, no pharmacies had prices displayed on their websites and 12% of pharmacies who answered a call/email did not provide a price.
Based on the above studies and committee appraisal, in April of last year NICE, (2021) recommended the use of Acalabrutinib as a monotherapy for adult patients with:
• 17p deletion or TP53 mutation, or
• No 17p deletion or TP53 mutation, (FCR), or (B-R) is unsuitable.
•
Previously treated CLL patients Administration
The study found that the most expensive pharmacies can charge over double the price of the least expensive pharmacies. For example: Researchers found a typical prescription of prednisolone (a commonly prescribed steroid) costs over ¤5 more (88% higher price) in the most expensive pharmacies, compared to the least expensive pharmacies in Ireland.
Acalabrutinib is a self-administered oral capsule taken twice daily, 12 hourly. Patients are advised to swallow the capsule whole with a glass of water, with or without food.
Prior to commencing BTK treatment a detailed history of past medical history, drug allergies and current medications is obtained. The prescriber assesses the patient’s ability to self-administer and will involve family members /carers in education sessions where appropriate. It is important to check for food and drug interactions when prescribing Ibrutinib and Acalabrutinib. Grapefruit and Seville oranges are contraindicated in patients on Ibrutinib and with Acalabrutinib concomitant use of CYP3A inhibitors or inducers should be avoided. Patients on gastric reducing agents require specific advice due to the potential for drug interaction.
BTK inhibitors and Fixed duration treatment
For each drug, the average price quoted to researchers was higher than the price paid by the state for patients who can access subsidised medicines (medical card holders).
Supportive Care in CLL CLL is generally associated with a gradual decrease in antibody-mediated immunity and the development of hypogammaglobulinaemia. Hypogammaglobulinemia is a condition caused by low serum immunoglobulin or antibody levels. Immunoglobulins have the ability to recognize antigens to trigger a biological response and eradicate infectious sources (Huq M, Bhatnagar NK, 2021).
For nine of the 12 drugs, the price was significantly higher for chain pharmacies compared to independent pharmacies.
James Larkin, PhD Scholar, Department of General Practice, RCSI, and lead author said, "The large price variation for prescription drugs and the lack of transparency from pharmacies is a problem, as it may mean that some people are paying too much for their medication. Or worse, some people are not buying their medication because it costs
Hypogammaglobulinemia is the most common primary immunodeficiency and encompasses a majority of immune-compromised patients. Hypogammaglobulinemia affects up to 85% of all patients with CLL and may affect all immunoglobulin subclasses (IgG, IgA, and IgM). Lower immunoglobulin levels correlate with increased risk for severe and recurrent infections and severity increases with disease progresson. This can be exacerbated by the different treatments a patient receives and the lymphocytopenia associated with same (Hamblin & Hamblin, 2008).
¤4m Award for Professor O’Driscoll
It is important to implement strategies to reduce risk; including vaccination, antibiotic prophylaxis, early pre-emptive antimicrobial therapy and immunoglobulin replacement therapies.
Professor Lorraine O'Driscoll, Taoiseach Micheál Martin and Minister for Further and Higher Education, Research, Innovation and Science, Simon Harris
Furthermore, intravenous immunoglobulins may benefit this population. The dose is generally calculated at 0.4/kg and given intravenously every 4 weeks.
Patient Management/Education
too much. This is particularly concerning given the current cost of living crisis and resulting cost pressures that many are facing. Consideration needs to be given by Government to measures that enforce price transparency or regulating prices.’"
a role in educating the patient and their family about the common side effects seen often in many treatments as well as the risks of developing an oncological emergency such as tumour lysis syndrome or neutropenic sepsis. Some specific targeted therapies have rare side effects such as tumour lysis syndrome associated with venetoclax. Nurses need to empower patients with the correct resources to identify these potential complications rapidly and to escalate if necessary.
CLL and COVID
James O’Mahony, Research Assistant Professor in Cost Effective Analysis, Centre for Health Policy Management, School of Medicine, and senior author, added, “Awareness matters as patients can save money by shopping around. If the state were to regulate prices, or if there was more transparency on prices, it might lead to lower prices and more people taking their medications.
“The pharmaceutical regulator’s current requirements on pharmacies to make prices transparent does not really guarantee any easy access to prices for patients. We’d like to see a greater push for transparency on behalf of the Pharmaceutical Society of Ireland.”
In 2020, the emergence of COVID brought new difficulties for those diagnosed with CLL. CLL generally associated with the older age group is also be linked with immunodeficiency (Montserrat, 2020). This categorises those with a CLL diagnosis as being at risk of severe disease (Montserrat, 2020). With this immunodeficiency it is likely that even with the COVID19 vaccine patients with a diagnosis of CLL will have an impaired or inadequate response with limited antibodies produced (Roeker et al., 2021). However it does appear that response rates and antibody titres do improve with subsequent doses of the vaccine (Herishanu et al., 2022). Due to the limited response in CLL patients, a primary dose followed by a fourth dose 3 months later is recommended (HSE, 2022).
Patients are encouraged to inform their healthcare provide should they test positive for COVID19. The proceeding patient care is assessed based on individualised risk, severity of treatment and current treatments available. The use of treatments e.g. sotrivimab, redensivir and paxlovid will be considered based on each individual patient.
Plan and United Nationals Sustainable Development Goals should help ensure that its patient-centred cancer liquid biopsy research delivers societal and economic impact.
The Future of CLL
emerging hub of excellence is a collaboration between TCD, QUB and NUIG. CLuB will focus on identifying and developing minimally invasive, cost effective, blood tests to complement or -where possible- replace surgical biopsies for cancer diagnosis and optimal treatment selection. It will also use excised tumours as “avatars”, to test the
Drug combinations with fixed duration regimens such as Ibrutinib in combination with venetoclax are producing promising progression free survival data and improved
Patients with CLL require strong psychosocial support due to oftenvarying treatments dependent on their disease. Most medications come with a side effect profile and it is vital that patients are educated about this as well as the symptoms associated with disease. It is well documented that adherence to medications is critical to treatment success (Gast & Mathes, 2019). As we move to more oral based regimes it is imperative we impress on our patients the need for adherence with medications and ensure we ask about adherence at every consultation (Usherwood, 2017). The specialist nurse plays
optimal drug treatment regime for a given patient. CLuB aims to develop a critical mass of scientists, clinicians, nurses, technicians, bioinformaticians/ biostatisticians, and patents advocates for success during this 4-year program and to achieve momentum and expansion for sustainability. CLuB’s alignment with Europe’s Beating Cancer
Professor O’Driscoll is also TCD’s Principal Investigator on a ¤4 million “partnerships of scale” entitled the All-Island cancer Research Initiative (AICRI)Start, led by UCD. AICRIStart brings 10 scientists and clinicians from UCD, TCD, RCSI, DCU, TUD, NUIG, UL, UCC, QUB, UU together to collaborate on cancer research.
Small molecule inhibitors have revolutionised CLL treatment. It is anticipated that they will be used in the majority of patients, early after diagnosis and with curative intent. They are generally well tolerated and enable patients to maintain a good quality of life. Given the success of newer agents such as venetoclax in the clinical setting thus far, it is likely that BCL-2 inhibition will take on an increasingly important role in the treatment of CLL going forward.
The Taoiseach Micheál Martin and Minister for Further and Higher Education, Research, Innovation and Science, Simon Harris, came to Trinity Biomedical Sciences Institute, visiting the School of Pharmacy and Pharmaceutical Sciences laboratories on Level 6, to announce the successful collaborative research projects between academics and institutions in Ireland and in Northern Ireland selected for funding under the North-South Research Programme of the Government’s Shared Island Fund.
References available on request
34 CPD 87: ONCOLOGY CLL
30 JUNE 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
News
60 Second Summary
Chronic Lymphocytic Leukuemia also known as CLL is a cancer that affects the blood and bone marrow. CLL affects the white blood cells called lymphocytes. It accounts for 2.4% of cancer diagnose in Ireland every year, affects men more than women and is more common in those over the age of 70.
CLL comprises over 40% of all leukaemias, affects older patients and may be asymptomatic for much of its course. It is often detected only through routine blood counts and only picked up if the individual presents to clinical services. Approximately 10% of individuals with CLL report a family history of the condition or a related lymphoproliferative disorder, but the genes which account for increased susceptibility have not been identified.
The course of the disease generally follows a period of “watch and wait”, followed by treatment with periods of relapse and remission. It remains an incurable disease however there are a number of novel treatments that have become available which have changed the course of the disease, improving patient’s response to treatments and overall quality of life.
COVID19 has had a significant impact on CLL patients, many of them being immunodeficient due to the nature of the disease or the multiple lines of treatment received. As such treatments for this high risk group are welcomed.
This CPD article will particularly focus on and examine the evidence on the treatment of CLL with Bruton's tyrosine kinase inhibitors (BTKi’s) particularly in the newly diagnosed CLL setting.
AUTHORS: Mary Kelly, FFNMRCSI, RANP, RNP, Msc, BSc Cancer Nursing, BSc Adult Nursing: Mary is an Advanced Nurse Practitioner in Haematology at the Midlands Regional Hospital, Tullamore. Mary has worked in Haematology Nursing for the past 24 years. She has worked in all Haematology nursing care settings both in the UK and Ireland at St Marys and St Bartholomew’s Hospitals, London, Blackrock Clinic and Midland Regional Hospitals. Mary manages CLL patients as part of her clinical caseload.
Orlaith Cormican RGN, Bsc, MHsc, Post Graduate Certificate in Cancer Care and Haematology: Orlaith is a Clinical Nurse Specialist in Haematology at the Midlands Regional Hospital. Orlaith has worked in cancer care and haematology since 2013. She is currently completing a PhD via the HRB SPHeRE programme. Her areas of interest include chronic haematological malignancies and quality of life in this area. Orlaith works with CLL patients on a daily basis.

Published
Chronic Lymphocytic Leukemia (CLL): An overview of its management and treatment with Bruton's tyrosine kinase Inhibitors


What is it?
CLL is the most common leukaemia in the western world (AlSawaf et al., 2020). CLL is more common in males and is generally associated with those over the age of 70 (NCRI, 2022). All leukaemia’s in Ireland account for 2.4% of all malignant disorders and there are over 500 new diagnoses of CLL made in Ireland every year. The cause of CLL is unknown. Risk factors include family history of haematological malignancy, increasing age and working in farming or hairdressing (Slager et al., 2014).
CLL is characterised by the clonal proliferation and accumulation of neoplastic B-lymphocytes in the blood, bone marrow lymph nodes and spleen (Al-Sawaf et al., 2020). B cell receptor signalling leading to activation of various intracellular signalling pathways plays an important role in the survival of CLL cells (Stevenson & CaligarisCappio, 2004). These pathways are now being successfully exploited as therapeutic targets (Jain & Rai, 2012).
Background and Overview
The majority of CLL cases are diagnosed through blood counts, differential counts, a blood smear and immunophenotyping.
In CLL, CD5 + B cells undergo malignant transformation. They are activated by the acquisition of mutations leading to monoclonal B cell lymphocytosis (MBL). MBL is defined as an asymptomatic condition where the absolute monoclonal B cell count is <5 x 10 9/L. It occurs in 4-5% of healthy adults and is a proposed precursor of CLL. Most cases of CLL are preceded by MBL while only 1-2% with MBL can develop CLL every year (Jain & Rai, 2012). In CLL, further accumulation of genetic abnormalities and subsequent oncogenic transformation of monoclonal B cells occurs (Burger, 2020). The diagnosis of CLL requires the presence of ≥5000 B-lymphocytes/μL in the peripheral blood for duration of at least 3 months. The clonality of the circulating B cells needs is confirmed by flow cytometry. Furthermore, there may be evidence of lymphadenopathy or organomegaly by physical or CT exam (Hallek, 2019).
CLL and small lymphocytic lymphoma (SLL) are different clinical presentations of the same pathological disease and commonly referred to as CLL(Patel & Pagel, 2021). The term CLL is used when the disease manifests primarily in the
blood, whereas SLL is used when involvement is primarily nodal (Rai KR, Stilgenbauer, 2021). Patients with CLL commonly develop complications associated with an intrinsic immune dysfunction resulting in immunodeficiency and autoimmune disorders. Ten per cent of patients will present with autoimmune haemolytic anaemia (Christi, M, 2020). Infection, anaemia and thrombocytopenia are the most common disease related complications. The increased risk of severe infections in CLL patients is primarily related to the disease and treatment related immunodeficiency, mainly bacterial and herpes virus family. Tumour lysis syndrome (TLS) and secondary cancers are less common but also potentially life threatening.
CLL remains an incurable disease but there continues to be progress made in therapeutics, with the emergence of newer therapies improving overall survival (OS) and being well tolerated by patients enabling them to maintain a good quality of life for many years (Sharma & Rai, 2019). For those patients who are relatively asymptomatic, they may follow
31
up
clinical
receiving any therapies
CPD 88: ONCOLOGY CLL
REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice? 2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area. 3. PLAN If I have identified a knowledge gap - will this article satisfy those needs - or will more reading be required? 4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs? 5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the
CPD
the “watch and wait” protocol followed
by
observation without
(Milne et
1.
CPD Continuing Professional Development
your
4 previous steps, log and record
findings.
by HPN. Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
programmes included in these modules
Abbvie has no editorial oversight of the CPD
Mary Kelly Orlaith Cormican
Table 1
Stage Characteristics
Stage Characteristics
Low Risk (Stage 0)
Low Risk (Stage 0)
Abnormal increase in the number of lymphocytes in the blood and marrow
Abnormal increase in the number of lymphocytes in the blood and marrow
No enlargement of the lymph nodes, spleen, or liver and with near normal red blood cell and platelet counts.
No enlargement of the lymph nodes, spleen, or liver and with near normal red blood cell and platelet counts.
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Enlarged lymph nodes OR
Enlarged lymph nodes OR
Intermediate Risk (Stages I & II)
Intermediate Risk (Stages I & II)
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Table 1
The IGHV mutation status is also a useful prognostic and treatment indicator; mutated IGHV tends to be less aggressive and especially when combined with additional prognostic factors such as favourable cytogenetics (Puiggros et al., 2014).
High Risk (Stages III & IV)
High Risk (Stages III & IV)
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Enlarged spleen and/or liver
Enlarged spleen and/or liver
The red blood cell or platelets counts are normal or only slightly low.
The red blood cell or platelets counts are normal or only slightly low.
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Anemia (hemoglobin <11g/dL)
Anemia (hemoglobin <11g/dL) OR
Condoluci et al., (2020) developed an International Prognostic score for early stage CLL (IPS-E). The IPS –E aims to predict time to first treatment (TTFT) in patients with early asymptomatic CLL at the time of diagnosis. The CLL-IPI-E combines genetic, biochemical and clinical parameters to categorise patients into risk groups (Table 2). The following three covariates were consistently and independently correlated with TTFT, Unmutated IGHV, absolute lymphocyte count >10x 109/L and presence of palpable lymph nodes. Each covariate is assigned 1 point each and scoring is used to classify risk. Importantly, del (17p) and TP53 mutations were not prognostic for TTFT but are important in predicting treatment response and guiding treatment choice once indicated (Rai KR, Stilgenbauer, 2021).
Treatment and Management
OR
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Thrombocytopenia (platelet counts <100,000/uL)
Abnormal increase in the number of lymphocytes in the circulating blood and the marrow
Thrombocytopenia (platelet counts <100,000/uL)
Enlarged Lymph nodes, liver or spleen.
Enlarged Lymph nodes, liver or spleen.
al., 2020). Treatment is usually commenced at disease progression or when the patient starts to develop symptoms associated with CLL (Milne et al., 2020). The majority of patients with a diagnosis of CLL will have more than one comorbidity at diagnosis, which may influence treatment selection (Kabel C, 2021). In addition, treatment can be patient- dependent, considering compliance, convenience, financial toxicity and duration of therapy (Murphy, 2021).
Diagnosis, Staging and Prognosis
CLL has a widely variable disease course, influencing prognosis. Some patients die rapidly, within 2-3 years of diagnosis, due to CLL complications. Most patients live 5-10 years, with an initial course that is relatively benign, followed by a terminal, progressive and resistant phase lasting 1-2 years (Christi, M, 2020). Significant morbidity from both the disease and complication of therapy may exist in the later phase (Rai & Barrientos, 2014).
A patient’s prognosis is dependent on disease stage; in addition to the presence or absence of high-risk markers (See Table 1 and 2). The Rai staging system classifies CLL into three separate risk groups. The Binet staging system classifies CLL into three stages (Table 1).
Prognostic indicators
Cytogenetics may detect abnormalities on the chromosomes, which are associated with CLL. Samples are from the blood or bone marrow. FISH analysis may identify abnormalities associated with chromosomes 11, 12 13 and 17: deletion 13q, deletion 11q, trisomy 12, and deletion 17p. Deletion 13q remains the most favourable prognostic group, with deletion 17p being the least favourable (Puiggros et al., 2014).
Furthermore, genomic abnormalities associated with TP53 mutation tend to be characterised as high risk and associated with adverse outcomes
The clinical course of CLL is extremely varied. Clinical trials in early stage CLL have failed to show survival benefit and therefore by international guidelines treatment is not recommended in this cohort. Ongoing randomised trials are evaluating the use of modern therapies in early stage disease and prognostic markers associated with poor clinical outcomes. However, until treatment benefit proves more as beneficial, treating in this setting is reserved for patients enrolled in these clinical trials (Rai KR, Stilgenbauer, 2021).
Indications for treatment outlined by the International working group CLL (IWCLL) criteria include progressive lymphadenopathy, organomeagly, deteriorating blood counts and constitutional symptoms (Hallek et al, 2018). In addition the presence of progressive lymphocytosis, a doubling time of < 6 months, Hb less than 100g/L or a platelet count of < 100 x 109/L or treatment resistant autoimmune cytopenias are indications to commence treatment (Milne et al, 2020).
Lymphocytosis itself, even if extreme, is not a strict indication for treatment if patients have no symptoms and adequate bone marrow function. However, extreme lymphocytosis (>200,000/ micro/L) is associated with an increased risk of hyperviscosity and may serve as an indication for treatment in an otherwise asymptomatic CLL patient (Rai KR, Stilgenbauer, 2021).
Treatment selection

There is no single standard front line therapy regime for all patients with symptomatic or advanced CLL (Rai III-IV, Binet C) or patients with progressive cytopenias. Several initial
Table 2
32 CPD 88: ONCOLOGY CLL
Score Risk Category 5 year cumulative
to
0 Low
1 Intermediate
2-3 High
Table 2
risk
start treatment
8.4%
28.4%
61.2%
Table 1
Chemo Drugs Monclonal Antibodies
Kinase inhibitors
PI3K Inhibitors
B-cell lymphoma-2 (BCL-2) inhibitors
Fludarabine Rituximab Ibrutinib Idelalisib Venetoclax Chlorambucil Obinutuzumab Acalabrutinib
Bedamustine Cyclophosphamide
Common Side Effects
Hair loss
Mouth sores
Loss of appetite
Nausea and vomiting
Low blood counts
Chest pain Heart racing
Swelling of the face and tongue Cough Trouble breathing
Feeling dizzy, lightheaded, or faint
Diarrhoea Nausea
Constipation Fatigue, shortness of breath
Swelling of the feet and hands, body aches, Rash
Low blood counts Atrial Fibrillation
treatments options exist, with varying rates of complete response, time to progression and associated toxicities (See Table 3). Patient’s treatment is individualised based on the patient, tumour characteristics and goals of therapy At least 90% of patients have one or more co-morbidities, therefore an MDT approach involving patients and outlining the potential benefits and risks of a particular treatment is important when considering treatment (Thurmes et al., 2008). Optimising health related quality of life (HRQoL) and managing adverse events are also important considerations in the decision to treat and choice of therapy (Patel & Pagel, 2021). Frey et al., (2016) reports disease severity as a predictor of poor HRQoL. In addition, toxicities associated with long-term targeted treatments have been shown to affect adherence, thus the emergence of limited duration therapies where appropriate is welcome.
Treatment options for CLL include CIT, targeted therapies, radiation therapy and supportive care. Over the past two decades, a dramatic increase in our understanding of the pathogenesis of CLL has led to the development of small molecule inhibitors for CLL targeting the B cell
Diarrhoea Fever Fatigue Nausea Cough Pneumonia Low blood counts Pneumonitis
Tumour Lysis Syndrome
Low blood counts
Diarrhoea Nausea
Respiratory infections
Thrombocytopenia Fatigue
Although FCR is not an effective option for patients with TP53 and del (17q) mutations, for patients with mutated IGHV trials showed that time limited FCR can provide functional cure and therefore despite its diminishing role FCR may still be considered in this patient group.
Bendamustine and rituximab and Obinutuzumab plus chlorambucil are also regimes that are active in CLL. These regimes are also options for older patients with cardiovascular comorbidities where BTK inhibitor therapy could increase the risk of cardiovascular events. CIT may be considered in patients where a time-limited treatment is preferable. Newer treatment options with fixed duration combinations for example Ibrutinib and ventoclax will further challenge the role of chemoimmunotherapy in the near future.
Targeted Treatments
Bruton Tyrosine Kinase (BTK) InhibitorsIbrutinib, Acalabrutinib
The BCR pathway is implicated in the pathogenesis of several B-cell malignancies, including MCL, diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, and CLL. BTK's pivotal role in signalling through the B-cell surface receptors and results in activation of pathways necessary for B-cell trafficking, chemotaxis and adhesion. These BTKi work by forming a covalent bond with a cysteine residue (Cys-481) in the BTK active site, leading to sustained inhibition of BTK enzymatic activity.
Potent, small-molecule inhibitors of BTK developed to treat CLL include, Ibrutinib and Acalabrutinib.
Ibrutinib
receptor pathway and the apoptotic regulator BCL2 (Milne et al, 2020). These targeted therapies, enable patients to be treated with a chemotherapy free regime.
Similar to management in most malignancies, the most suitable treatment for a patient is selected based on genetic features of the disease itself (Milne et al, 2020). Molecular assessment prior to every line of treatment is essential to avoid ineffective treatments.
When planning treatment it is also important to consider the current pandemic. Nevertheless, in those patients with active CLL, treatment should not be withheld, because data suggests that the outcome of patients with COVID-19 is better if their cancer is under control. In patients requiring intervention, BTKi’s may represent the preferred therapeutic option (Roschewski et al., 2020).
Chemo immunotherapy (CIT)
For many years Fludarabine, cyclophosphamide and Rituximab (FCR) were the gold standard treatment for CLL. FCR yielded high overall response rates of up to 95% with some patients remaining in remission for over a decade.
Ibrutinib was the first available oral small molecule inhibitor that binds to BTK preventing its kinase activity. This affects multiple signalling pathways and disrupts the interaction between CLL cells and the microenvironment leading to further apoptosis (Milne et al, 2020). Ibrutinib is indicated for the treatment of CLL and SLL with or without del (17p) (SPC, 2021). Clinical trials demonstrated the clinical benefit of Ibrutinib in a variety of clinical settings (Ghia et al., 2021). The Resonate trial showed improved PFS, OS and ORR for Ibrutinib versus ofatumumab in previously treated patients (Byrd et al., 2014). Resonate 2 showed ibrutinib superiority in PFS, OS and ORR in treatment naïve patients when compared to Chlorambucil. Further Resonate-2 analysis revealed 61% of patients were alive and progression free at 6.5 years and 6.5 year OS was 78%.
Adverse events of clinical interest over time for patients on Ibrutinib included hypertension, atrial fibrillation and less commonly major haemorrhage (Ghia, P, Owen, C et al., 2021).
The iLLUMINATE trial compared Ibrutinib plus obinutuzumab versus chlorambucil and obinutuzumab. The results showed significantly


33
Table:
3
Table
3
improved PFS (Moreno et al., 2019). Ibrutinib is taken orally at the same time each day. Ibrutinib is generally well tolerated; though there are some common side effects (Table 3).
Acalabrutinib
Acalabrutinib is a highly selective, potent BTK inhibitor (Ghia et al, 2020). In the phase II study that evaluated. Acalabrutinib in relapsed/refractory CLL, Acalabrutinib resulted in an overall response rate (ORR) of 93% in patients with Del (17p) and 90% of those with complex karyotype.
Elevate trial
The Elevate trial was the first trial of Acalabrutinib versus Ibrutinib in previously treated CLL. Patients were randomised to Acalabrutinib 100mg orally twice daily or Ibrutinib 420mg orally once daily and continued until disease progression or unacceptable toxicity. The primary endpoint was non-inferiority and secondary endpoints included incidence of Atrial fibrillation, grade >/ three infections, Richter’s transformation (RT) and (OS). At a median follow up of 40.9 months, Acalabrutinib was non-inferior to Ibrutinib, with a medium PFS of 38.4 months in both arms. Acalabrutinib demonstrated lower frequencies of adverse events (AE). Cardiovascular events were less common with Acalabrutinib, 9.4% vs 16% for atrial fibrillation (AF) and 9.4% vs 23.2% for hypertension.
ELEVATE-TN Trial
In this randomised, controlled, phase 3 trial the efficacy of Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatmentnaïve CLL patients was compared. At a median follow up of 28.3 months median PFS was longer with Acalabrutinib – obinutuzumab (A-O) and Acalabrutinib monotherapy compared with obinutuzumab-chlorambucil (O-C). Estimated PFS at 24 months was 93% with A-0, 87% for patients on Acalabrutinib monotherapy and 47% in the O-C arm. Patients on A-O and Acalabrutinib tolerated treatment well and had a manageable safety profile, with 79% of patients remaining on therapy after a median follow up of 28.3 months. Sharman et al., (2020) concluded that Acalabrutinib with or without obinutuzumab is efficacious and a well tolerated treatment for patients with treatment – naïve CLL.
ASCEND trial

Acalabrutinib proves to be an effective treatment for patients with relapsed/refractory (R/R) disease. The study demonstrated a statistically significant and clinically meaningful PFS with Acalabrutinib compared with Idelalisib plus Rituximab (I-R) or Bendamustine plus Rituximab (B-R). The benefit of Acalabrutinib was seen across all patient subgroups including those with del (17p) or TP53. In addition, a tolerable safety profile was demonstrated. Acalabrutinib is licenced to treat adults with mantle cell lymphoma (MCL) who have received at least one prior treatment for their cancer, or adults with CLL or SLL.
Based on the above studies and committee appraisal, in April of last year NICE, (2021) recommended the use of Acalabrutinib as a monotherapy for adult patients with:
• 17p deletion or TP53 mutation, or
• No 17p deletion or TP53 mutation, (FCR), or (B-R) is unsuitable.
•
Previously treated CLL patients Administration
Acalabrutinib is a self-administered oral capsule taken twice daily, 12 hourly. Patients are advised to swallow the capsule whole with a glass of water, with or without food.
Prior to commencing BTK treatment a detailed history of past medical history, drug allergies and current medications is obtained. The prescriber assesses the patient’s ability to self-administer and will involve family members /carers in education sessions where appropriate. It is important to check for food and drug interactions when prescribing Ibrutinib and Acalabrutinib. Grapefruit and Seville oranges are contraindicated in patients on Ibrutinib and with Acalabrutinib concomitant use of CYP3A inhibitors or inducers should be avoided. Patients on gastric reducing agents require specific advice due to the potential for drug interaction.
BTK inhibitors and Fixed duration treatment
Drug combinations with fixed duration regimens such as Ibrutinib in combination with venetoclax are producing promising progression free survival data and improved
patient benefits including limited treatment duration with a shorter period for side effects, outpatient based therapy and reduced financial burden on patients and carers (DePace, 2021).
Supportive Care in CLL CLL is generally associated with a gradual decrease in antibody-mediated immunity and the development of hypogammaglobulinaemia. Hypogammaglobulinaemia is a condition caused by low serum immunoglobulin or antibody levels. Immunoglobulins have the ability to recognize antigens to trigger a biological response and eradicate infectious sources (Huq M, Bhatnagar NK, 2021).
Hypogammaglobulinemia is the most common primary immunodeficiency and encompasses a majority of immune-compromised patients. Hypogammaglobulinemia affects up to 85% of all patients with CLL and may affect all immunoglobulin subclasses (IgG, IgA, and IgM). Lower immunoglobulin levels correlate with increased risk for severe and recurrent infections and severity increases with disease progresson. This can be exacerbated by the different treatments a patient receives and the lymphocytopenia associated with same (Hamblin & Hamblin, 2008).
It is important to implement strategies to reduce risk; including vaccination, antibiotic prophylaxis, early pre-emptive antimicrobial therapy and immunoglobulin replacement therapies.
Furthermore, intravenous immunoglobulins may benefit this population. The dose is generally calculated at 0.4/kg and given intravenously every 4 weeks.
Patient Management/Education

Patients with CLL require strong psychosocial support due to oftenvarying treatments dependent on their disease. Most medications come with a side effect profile and it is vital that patients are educated about this as well as the symptoms associated with disease. It is well documented that adherence to medications is critical to treatment success (Gast & Mathes, 2019). As we move to more oral based regimes it is imperative we impress on our patients the need for adherence with medications and ensure we ask about adherence at every consultation (Usherwood, 2017). The specialist nurse plays
a role in educating the patient and their family about the common side effects seen often in many treatments as well as the risks of developing an oncological emergency such as tumour lysis syndrome or neutropenic sepsis. Some specific targeted therapies have rare side effects such as tumour lysis syndrome associated with venetoclax. Nurses need to empower patients with the correct resources to identify these potential complications rapidly and to escalate if necessary.
CLL and COVID
In 2020, the emergence of COVID brought new difficulties for those diagnosed with CLL. CLL generally associated with the older age group is also be linked with immunodeficiency (Montserrat, 2020). This categorises those with a CLL diagnosis as being at risk of severe disease (Montserrat, 2020). With this immunodeficiency it is likely that even with the COVID19 vaccine patients with a diagnosis of CLL will have an impaired or inadequate response with limited antibodies produced (Roeker et al., 2021). However it does appear that response rates and antibody titres do improve with subsequent doses of the vaccine (Herishanu et al., 2022). Due to the limited response in CLL patients, a primary dose followed by a fourth dose 3 months later is recommended (HSE, 2022).
Patients are encouraged to inform their healthcare provide should they test positive for COVID19. The proceeding patient care is assessed based on individualised risk, severity of treatment and current treatments available. The use of treatments e.g. sotrivimab, redensivir and paxlovid will be considered based on each individual patient.
The Future of CLL
Small molecule inhibitors have revolutionised CLL treatment. It is anticipated that they will be used in the majority of patients, early after diagnosis and with curative intent. They are generally well tolerated and enable patients to maintain a good quality of life. Given the success of newer agents such as venetoclax in the clinical setting thus far, it is likely that BCL-2 inhibition will take on an increasingly important role in the treatment of CLL going forward.
References available on request
34 CPD 88: ONCOLOGY CLL
HELP YOUR PATIENTS
THE ITCH & RASH OF MODERATE TO SEVERE ATOPIC DERMATITIS 1


RINVOQ demonstrated in an integrated analysis of the MEASURE UP 1 & 2 monotherapy studies at Week 16:
• EASI 75 skin clearance rates of 76% and 65% for patients treated with RINVOQ 30 mg QD and 15 mg QD, respectively, vs 15% with placebo (p<0.001 for both comparisons)2
• Itch reduction (mean percent change from baseline in Worst Pruritus NRS) of –70% and –58% for RINVOQ 30 mg QD and 15 mg QD, respectively, vs –24% with placebo (p<0.001 for both comparisons)2
2-4

PRESCRIBING

INFORMATION (PI) RINVOQ® (upadacitinib) 15 mg and 30mg prolonged-release tablets. Refer to Summary of Product Characteristics (SmPC) for full prescribing information. PRESENTATION: Each 15 mg prolonged-release tablet contains upadacitinib hemihydrate, equivalent to 15mg of upadacitinib. Each 30mg prolonged-release tablet contains upadacitinib hemihydrate, equivalent to 30 mg of upadacitinib. INDICATION: Treatment of moderate to severe active rheumatoid arthritis (RA) and active psoriatic arthritis (PsA) in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs). RINVOQ may be used as monotherapy or in combination with methotrexate. Treatment of active ankylosing spondylitis (AS) in adult patients who have responded inadequately to conventional therapy. Treatment of moderate to severe atopic dermatitis (AD) in adults and adolescents 12 years and older who are candidates for systemic therapy. DOSAGE AND ADMINISTRATION: Intended for use under guidance and supervision of a physician experienced in diagnosis and treatment of conditions for which upadacitinib is indicated. Dosage: RA, PsA and AS: The recommended oral dose is 15mg once daily. Consideration should be given to discontinuing treatment in patients with AS who have shown no clinical response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve with continued treatment beyond 16 weeks. AD (adults): The recommended oral dose is 15 mg or 30 mg once daily based on individual patient presentation. 30 mg once daily dose may be appropriate for patients with high disease burden and for patients with an inadequate response to 15 mg once daily. The lowest effective dose for maintenance should be considered. For patients ≥ 65 years of age, the recommended dose is 15mg once daily. AD (adolescents from 12 to 17 years): The recommended oral dose is 15mg once daily for adolescents weighing at least 30 kg. Adults and adolescents 12 years and older: Upadacitinib can be used with or without topical corticosteroids. Topical calcineurin inhibitors may be used for sensitive areas such as the face, neck, and intertriginous and genital areas. Consideration should be given to discontinuing upadacitinib treatment in any patient who shows no evidence of therapeutic benefit after 12 weeks of treatment. Special Populations: Elderly: For AD, doses higher than 15mg once daily not recommended in patients aged 65 years and older. Limited data for patients aged 75 and older. Renal: No dose adjustment required in mild-moderate renal impairment. Upadacitinib 15mg once daily should be used with caution in patients with severe renal impairment. Upadacitinib 30 mg once daily is not recommended for patients with severe renal impairment. Upadacitinib has not been studied in subjects with end stage renal disease. Hepatic impairment: No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. Should not be used in patients with severe (Child Pugh C) hepatic impairment. Paediatric Population: No data available in the following paediatric patient populations: children with AD below the age of 12 years; children and adolescents with RA, PsA and AS aged 0 to less than 18 years. CONTRAINDICATIONS: Hypersensitivity to active substance or excipients. Active tuberculosis (TB) or active serious infections. Severe hepatic impairment. Pregnancy. SPECIAL WARNINGS AND PRECAUTIONS: See SmPC for full details. Immunosuppression: Combination use, with immunosuppressants e.g. azathioprine, ciclosporin, tacrolimus, and biologic DMARDs or other JAK inhibitors has not been evaluated in clinical studies and is not recommended as the risk of additive immunosuppression cannot be excluded. Serious infections: Serious and fatal infections have been reported. Caution when treating patients ≥65 years. Frequently reported – pneumonia and cellulitis. Bacterial meningitis has been reported. Opportunistic infections reported –TB, multidermatomal herpes zoster, oral/oesophageal candidiasis, and cryptococcosis. Do not initiate treatment in patients with active, serious infection, including localised infections. Consider the risk/benefit of treatment in patients with: chronic or recurrent infection, history of serious or opportunistic infection, those exposed to TB, those who have resided or travelled in areas of endemic TB or endemic mycoses, those with underlying conditions that may pre-dispose patients to infection. Closely monitor patients and interrupt treatment if there are signs and symptoms of infection pre and post treatment. Tuberculosis: Pre-screen patients for active or latent TB. RINVOQ should not be given to patients with active TB. Consider anti-TB therapy in patients with previously untreated latent TB or in patients with risk factors for TB infection. Consult with a physician with experience in TB therapy to assess whether initiating anti-TB therapy is appropriate for an individual patient. Monitor patients for the development of signs and symptoms of TB, including patients who tested negative for latent TB infection prior to initiating therapy.
Viral reactivation: Cases of herpes virus reactivation have been reported e.g. herpes zoster. If herpes zoster is reactivated, consider interruption of upadacitinib therapy until the episode resolves. Screen for viral hepatitis and monitor regularly for reactivation before starting and during therapy with upadacitinib. Patients, positive for hepatitis C antibody/hepatitis C virus RNA and hepatitis B surface antigen/hepatitis B virus DNA were excluded from clinical studies. If hepatitis B virus DNA is detected while receiving RINVOQ, a liver specialist should be consulted. Vaccination: Use of live, attenuated vaccines during or immediately prior to therapy is not recommended. It is recommended that patients be brought up to date with all immunisations, including
prophylactic zoster vaccinations prior to starting therapy, in agreement with current immunisation guidelines. Malignancy: Immunomodulatory therapies may increase the risk of malignancies including lymphoma and nonmelanoma skin cancer in RA patients. Malignancies were observed in clinical studies. Periodic skin examination recommended for patients who are at increased risk for skin cancer. See SmPC for full details. Haematological abnormalities: Do not start therapy if Absolute Neutrophil Count <1 x 109 cells/L, Absolute Lymphocyte Count <0.5 x 109 cells/L and Hb <8 g/dL. Monitor patients and temporarily stop therapy if abnormal haematological parameters as specified are detected during routine patient management. Diverticulitis: Events of diverticulitis have been reported in clinical trials and post-marketing. Upadacitinib should be used with caution in patients with diverticular disease and especially in patients chronically treated with concomitant medications associated with an increased risk of diverticulitis. Patients presenting with new onset abdominal signs and symptoms should be evaluated promptly for early identification of diverticulitis to prevent gastrointestinal perforation. Cardiovascular Risk: RA patients have an increased risk for cardiovascular disorders. Manage patient’s risk factors for e.g. hypertension, hyperlipidaemia as per usual standard of care. Lipids: Monitor total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) at 12 weeks and thereafter according to international guidelines. Elevated LDL in clinical trials decreased to pre-treatment levels in response to statin therapy, although evidence is limited. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Hepatic transaminase elevations: Monitor liver enzymes at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. In such cases therapy should be interrupted until this diagnosis is excluded.
Venous thromboembolism: Deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving JAKi, including upadacitinib. Use therapy with caution in patients at high risk for DVT/PE. Risk factors should be determined by patients age, obesity and medical history of DVT/PE, patients undergoing major surgery and prolonged immobilisation. If clinical features of DVT/PE occur discontinue therapy, evaluate and treat promptly. INTERACTIONS: Upadacitinib is metabolised mainly by CYP3A4 and plasma exposures may be affected by CYP3A4 inhibitors or inducers. Upadacitinib 15 mg once daily should be used with caution in patients receiving chronic treatment with strong CYP3A4 inhibitors. Upadacitinib 30 mg once daily dose is not recommended for patients receiving chronic treatment with strong CYP3A4 inhibitors. See SmPC for full details. FERTILITY, PREGNANCY AND LACTATION: Upadacitinib is contraindicated during pregnancy. Women of childbearing potential should be advised to use effective contraception during treatment and for 4 weeks following the final dose of upadacitinib. Female paediatric patients and/or their parents/caregivers should be informed about the need to contact the treating physician once the patient experiences menarche while taking upadacitinib. Upadacitinib should not be used during breast-feeding. The effect of upadacitinib on human fertility has not been evaluated. ADVERSE REACTIONS: Refer to SmPC for full details on adverse reactions. Very common adverse reactions (≥1/10): upper respiratory tract infections, acne. Common adverse reactions (≥1/100
herpes zoster, herpes simplex, folliculitis, influenza, anaemia, neutropaenia,
abdominal pain, nausea, urticaria, fatigue, pyrexia,
additional
safety information. Healthcare professionals are asked to report any suspected adverse reactions
LEGAL CLASSIFICATION: POM (S1A). MARKETING AUTHORISATION NUMBER/ PRESENTATION: EU/1/19/1404/001 and EU/1/19/1404/006 – Calendar blister packs containing 28 prolonged-release tablets. MARKETING AUTHORISATION HOLDER: AbbVie Deutschland GmbH & Co. KG, Knollstrasse, 67061 Ludwigshafen, Germany. Further information is available from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. DATE OF REVISION: December 2021. PI-1404-005 Abbreviations: EASI – eczema activity and severity index; NRS – numerical rating scale; QD – once daily; EU European Union. * The recommended dose of RINVOQ is 15mg once daily for adolescents weighing at least 30kg. The safety and efficacy of RINVOQ in children with atopic dermatitis below the age of 12 years have not been established. No data are available. No clinical exposure data are available in adolescents <40kg. The posology in adolescent patients 30kg to <40kg was determined using population pharmacokinetic modelling and
2.
3.
©2022 AbbVie Inc. All rights reserved. IE-RNQ_AD-220017 | February
to <1/10): bronchitis,
hypercholesterolaemia, cough,
increased blood CPK/ALT/AST, increased weight and headache. This medicinal product is subject to
monitoring. This will allow quick identification of new
via HPRA Pharmacovigilance; website: www.hpra.ie.
simulation.2 Reference: 1. Langan S. et al. Atopic dermatitis. The Lancet. 2020;396(10247):345-360. DOI: https://doi.org/10.1016/ S0140-6736(20)31286-1.
RINVOQ Summary of Product Characteristics, available on www.medicines.ie
Baricitinib Summary of Product Characteristics, available on www.medicines.ie 4. Abrocitinib Summary of Product Characteristics, available on www.medicines.ie
2022
RINVOQ, the first and only oral JAK inhibitor indicated for the treatment of both adults and adolescents ≥12 years* with moderate to severe Atopic Dermatitis in the EU
TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR or ALK positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA. KEYTRUDA as monotherapy is indicated for the treatment of adult and paediatric patients aged 3 years and older with relapsed or refractory classical Hodgkin lymphoma (cHL) who have failed autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT is not a treatment option. KEYTRUDA as monotherapy is indicated for the treatment of locally ad vanced or metastatic urothelial carcinoma in adults who have received prior platinum-containing chemotherapy. KEYTRUDA as monotherapy is in dicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who are not eligible for cisplatin-containing chemotherapy and whose tumours express PD L1 with a combined positive score (CPS) ≥ 10. KEYTRUDA as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, is indicated for the first-line treatment of metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC) in adults whose tumours express PD-L1 with a CPS ≥ 1. KEYTRUDA as monotherapy is indicated for the treatment of recurrent or metastatic HNSCC in adults whose tumours express PD-L1 with a ≥ 50% TPS and progressing on or after platinum-containing chemotherapy. KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of advanced renal cell carcinoma (RCC) in adults. KEYTRUDA, in combination with lenvatinib, is indicated for the first line treatment of advanced renal cell carcinoma in adults. KEYTRUDA as monotherapy is indica ted for the adjuvant treatment of adults with renal cell carcinoma at increased risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions. KEYTRUDA as monotherapy is indicated for the first line treatment of metastatic microsatellite instability high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer in adults. KEYTRUDA as monotherapy is indicated for the treatment of the following MSI H or dMMR tumours in adults with (a) unresectable or metastatic colorectal cancer after previous fluoropyrimidine based combination therapy, (b) advan ced or recurrent endometrial carcinoma, who have disease progression on or following prior treatment with a platinum containing therapy in any setting and who are not candidates for curative surgery or radiation, (c) unresectable or metastatic gastric, small intestine, or biliary cancer, who have disease progression on or following at least one prior therapy. KEYTRUDA, in combination with platinum and fluoropyrimidine based chemotherapy, is indicated for the first-line
lethargy, dry eye, cardiac arr hythmia (including atrial fibrillation), hypertension, hyperthyroidism, insomnia, dizziness, dysgeusia, pneumonitis, colitis, dry mouth, severe skin reactions, vitiligo, dry skin, alopecia, eczema, dermatitis acneiform, erythema, dermatitis, myositis, pain in extremity, arthritis, influenza like illness, chills, AST and ALT increases, increase in blood alkaline phosphatase, hypercalcaemia, blood bilirubin increased, blood creatinine increased, infu sion related reaction. In combination with chemotherapy: Very Common: neutropenia, anaemia, thrombocytopenia, leukopenia, hypothyroidism, hypokalaemia, decreased appetite, insomnia, neuropathy peripheral, headache, dizziness, dysgeusia, dyspnoea, cough, nausea, diarrhoea, vomi ting, abdominal pain, constipation, alopecia,


If corticosteroid dosing cannot be reduced to ≤10 mg prednisone or equivalent per day within 12 weeks; (c) If a treatment-related toxicity does not resolve to Grade 0 1 within 12 weeks after last dose of KEYTRUDA; (d) If any event occurs a second time at Grade ≥ 3 severity. Patients must be given the Patient Alert Card and be informed about the risks of KEYTRUDA. Special populations Elderly: No dose adjustment necessary. Renal impairment: No dose adjustment needed for mild or moderate renal impairment. No studies in severe renal impairment. Hepatic impairment: No dose adjustment needed for mild hepatic impairment. No studies in moderate or severe hepatic impairment. Paediatric population: Safety and efficacy in children below 18 years of age not established except in pae diatric patients with cHL. CONTRAINDICATIONS Hypersensitivity to the active substance or to any excipients. PRECAUTIONS AND WARNINGS

KEYTRUDA®
ABRIDGED PRODUCT INFORMATION Refer to Summary of Product Characteristics before prescribing. PRESENTATION KEYTRUDA 25 mg/mL: One vial of 4 mL of concentrate contains 100 mg of pembrolizumab. INDICATIONS KEYTRUDA as monotherapy is indicated for the treatment of advanced (unresectable or metastatic) melanoma in adults. KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with Stage III melanoma and lymph node involvement who have undergone complete resection. KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a ≥50% tumour proportion score (TPS) with no EGFR or ALK positive tumour mutations. KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR or ALK positive mutations. KEYTRUDA, in combi nation with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥1%
every 3 weeks administered as an intravenous infusion over 30 minutes. For use in combination, see the
of Product Characteristics (SmPC)
concomitant therapies. KEYTRUDA must not be administered as an intra venous push or bolus
When
be
Treat
disease
or unacceptable
up
of
if
recurrence, unacceptable toxicity, or for a duration of up
year. Refer to the SmPC for dosing in neoadjuvant and adjuvant treatment of locally advanced, or early stage triple-negative breast
KEYTRUDA, as monotherapy or as combination therapy, should be permanently discontinued (a) For Grade
toxicity except for: endocrinopathies that are controlled with replacement hormones; or haematological toxicity, only in patients with cHL in which KEYTRUDA should be withheld until adverse reactions recover to Grade 0-1; (b)
(pembrolizumab)
treatment of locally advanced unresectable or metastatic carcinoma of the oesophagus or HER-2 negative gastroesopha geal junction adenocarcinoma in adults whose tumours express PD-L1 with a CPS ≥ 10. KEYTRUDA, in combination with chemotherapy as neoad juvant treatment, and then continued as monotherapy as adjuvant treatment after surgery, is indicated for the treatment of adults with locally advan ced, or early stage triple negative breast cancer at high risk of recurrence. KEYTRUDA, in combination with chemotherapy, is indicated for the treatment of locally recurrent unresectable or metastatic triple negative breast cancer in adults whose tumours express PD L1 with a CPS ≥ 10 and who have not received prior chemotherapy for metastatic disease. KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of ad vanced or recurrent endometrial carcinoma in adults who have disease progression on or following prior treatment with a platinum containing thera py in any setting and who are not candidates for curative surgery or radiation. KEYTRUDA, in combination with chemotherapy with or without beva cizumab, is indicated for the treatment of persistent, recurrent, or metastatic cervical cancer in adults whose tumours express PD L1 with a CPS ≥ 1. DOSAGE AND ADMINISTRATION See SmPC for full details. Therapy must be initiated and supervised by specialist physicians experienced in the treatment of cancer. The recommended dose of KEYTRUDA in adults is either 200 mg every 3 weeks or 400 mg every 6 weeks administered as an intravenous infusion over 30 minutes. The recommended dose of KEYTRUDA as monotherapy in paediatric patients aged 3 years and older with cHL is 2 mg/kg bodyweight (up to a maximum of 200 mg),
Summary
for the
injection.
administering KEYTRUDA as part of a combination with intravenous chemotherapy, KEYTRUDA should
administered first.
patients until
progression
toxicity (and
to maximum duration
therapy
specified for an indica tion). For the adjuvant treatment of melanoma or RCC, KEYTRUDA should be administered until disease
to one
cancer at high risk of recurrence.
4
In case of overdose, monitor closely for signs or symptoms of adverse reactions and treat appropriately. INTERACTIONS No formal pharmacokinetic drug interaction studies have been conducted with pembrolizumab. No metabolic drug drug interactions are expected. The use of systemic corticosteroids or immunosuppressants before starting pembrolizumab should be avoided because of their potential interference with the pharmacodynamic activity and efficacy of pembrolizumab. Corticosteroids can be used as premedication, when pembrolizumab is used in combina tion with chemotherapy, as antiemetic
nausea, vomiting, constipation, rash, pruritus, arthralgia, musculoskeletal pain, myositis, pain in extremity, fatigue, asthenia, oedema, pyrexia, lipase increased, alanine aminotransferase increased, aspartate aminotransferase increased, blood creatinine increased. Common: pneumonia, neutropenia, thrombocytopenia, lymphopenia, leukopenia, infusion-related reaction, adrenal insufficiency, hyperthyroidism, thyroiditis, hyponatraemia, hypokalaemia, hypocalcaemia, insomnia, dizziness, neuropathy peripheral, lethar gy, dry eye, cardiac arrhythmia (including atrial fibrillation), pneumonitis, colitis, pancreatitis, gastritis, dry mouth, hepatitis, severe skin reactions, dermatitis, dry skin, erythema, dermatitis acneiform, alopecia, arthritis, nephritis, influenza like illness, chills, amylase increased, blood bilirubin in creased, blood alkaline phosphatase increased, hypercalcaemia. PACKAGE QUANTITIES KEYTRUDA 25 mg/mL: 4 mL of concentrate in a 10 mL Type I clear glass vial. Legal Category: POM. Marketing Authorisation numbers EU/1/15/1024/002 Marketing Authorisation holder Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands. Date of revision: May 2022. © 2022 Merck & Co., Inc., Rahway, NJ, USA and its affiliates.. All rights reserved. Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, Dublin, D18 X5K7 or from www.medicines.ie. II109_II117_II110 Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-2998700) References 1. KEYTRUDA (pembrolizumab) SmPC. Available at: www.medicines.ie. Accessed June 2022 2. Robert C, Carlino MS, McNeil C, et al. 7-Year Follow-Up of KEYNOTE-006: Pembrolizumab Versus Ipilimumab in Advanced Melano ma. Poster 104. Presented at the 18th International Congress of the Society for Melanoma Research; October 28-31, 2021; Virtual 3. C. Robert, A. Ribas, J. Schachter et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019; 20: 1239-1251 Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7, Ireland. IE-KEY-00588 Date of Preparation: June 2022 (pembrolizumab) Infusion 25mg/ml KEYTRUDA as monotherapy is indicated for or metastatic) melanoma in adults.1 the treatment of advanced (unresectable *This is a 7-year follow-up post-hoc analysis and no statistical conclusions can be drawn from these results Survival results from KEYNOTE-006 An open-label, multicentre, randomised, controlled, phase 3 study of KEYTRUDA monotherapy vs Ipilimumab for patients with advanced
Survival
the intention-to-treat population* In participants who completed 2 years of KEYTRUDA and achieved Stable Disease or better*
Assessment of PD-L1 status When assessing the PD-L1 status of the tumour, it is important that a well-validated and robust methodology is chosen to minimise false negative or false positive determinations. Immune-related adverse reactions Immune-related adverse reactions, including severe and fatal cases, have occurred in patients receiving pembrolizumab. Most immune related adverse reactions occurring during treatment with pem brolizumab were reversible and managed with interruptions of pembrolizumab, administration of corticosteroids and/or supportive care. Immune re lated adverse reactions have also occurred after the last dose of pembrolizumab. Immune-related adverse reactions affecting more than one body system can occur simultaneously. Immune-related adverse reactions are immune-related pneumonitis, immune-related colitis, immune-related he patitis, immune-related nephritis, immune-related endocrinopathies (including adrenal insufficiency, hypophysitis, type 1 diabetes mellitus, diabetic ketoacidosis, hypothyroidism, and hyperthyroidism), Immune-related skin adverse reactions (also including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)), Refer to SmPC
for
more information and management of immune-related adverse reactions. Complications of allogeneic Haematopoietic Stem Cell Transplant (HSCT): Cases of graft-versus-host-disease (GVHD) and hepatic veno-occlusive disease (VOD) have been observed in patients with classical Hodgkin lymphoma undergoing allogeneic HSCT after previous exposure to pembrolizumab. Infu sion-related reactions: Grades 1, 2, 3 or 4 infusion reactions including hypersensitivity and anaphylaxis, could be seen with pembrolizumab treat ment. Refer to SmPC for more information and management of infusion-related reactions. Overdose: There is no information on overdose with pembrolizumab.
prophylaxis and/or to alleviate chemotherapy-related adverse reactions. FERTILITY, PREGNANCY AND LACTATION Women of childbearing potential Women of childbearing potential should use effective contraception during treatment with pembrolizu mab and for at least 4 months after the last dose of pembrolizumab. Pregnancy No data on use in pregnant women. Do not use during pregnancy unless the clinical condition of the woman requires treatment with pembrolizumab. Breast-feeding It is unknown whether pembrolizumab is secreted in human milk. A risk to newborns/ infants cannot be excluded. Fertility No clinical data available. SIDE EFFECTS Refer to SmPC for complete infor mation on side effects. Pembrolizumab is most commonly associated with immune-related adverse reactions. Most of these reactions resolved with appropriate medical treatment or withdrawal of pembrolizumab. The most serious adverse reactions were immune-and infusion-related adverse re actions. When pembrolizumab is administered in combination with axitinib or lenvatinib, refer to the SmPC for axitinib or lenvatinib prior to initiation of treatment. For additional lenvatinib safety information related to advanced RCC see the SmPC for Kisplyx and for advanced EC see the SmPC for Lenvima. Monotherapy: Very Common: anaemia, hypothyroidism, decreased appetite, headache, dyspnea, cough, abdominal pain, nausea, vomi ting, constipation, musculoskeletal pain, arthralgia, asthenia, oedema, pyrexia, diarrhoea, pruritus, rash, fatigue. Common: pneumonia, thrombocyto penia, neutropenia, lymphopenia, hyponatraemia, hypokalaemia, hypocalcaemia, insomnia, neuropathy peripheral,
rash, pruritus, arthralgia, musculoskeletal pain, myositis, pyrexia, fatigue, asthenia, oedema, ALT increa se, AST increased. Common: pneumonia, febrile neutropenia, lymphopenia, infusion related reaction, adrenal insufficiency, thyroiditis, hyperthyroi dism, hyponatraemia, hypocalcaemia, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), hypertension, pneumonitis, colitis, gastritis, dry mouth, hepatitis, severe skin reactions, erythema, dermatitis acneiform, dermatitis, dry skin, eczema, pain in extremity, arthritis, acute kidney injury, influenza-like illness, chills, blood creatinine increased, blood alkaline phosphatase increased, hypercalcaemia, blood bilirubin increased. In combination with axitinib or lenvatinib: Very Common: urinary tract infection, anaemia, hypothyroidism, decreased appetite, headache, dysgeu sia, hypertension, dyspnoea, cough, diarrhoea, abdominal pain,
Melanoma.1-3 7-Year Overall
in
KEYTRUDA 37.8% vs Ipilimumab 25.3% HR 0.70 (95 % CI 0.58 to 0.83) 5-year Overall Survival was 92.9% 5-year Progression Free Survival was 70.1% 103/556 (18.5%) of patients obtained stable disease or better In the as-treated population, any grade adverse events of any cause occurred in 442 (80%) of 555 participants in the pembrolizumab group and 190 (74%) of 256 participants in the Ipilimumab group. Grade 3 or worse adverse events of any cause occurred in 96 (17%) in the pembrolizumab group and 50 (20%) in the Ipilimumab group.
60 Second Summary
Malignant melanoma is the fifth most common invasive cancer nationally, with rising incidence. The main risk factor for its development is ultraviolet radiation exposure, especially intense and intermittent exposures and sun burn in fair-skinned individuals. Most cases of melanoma affect middle-aged individuals, though a significant proportion affect young adults. Males over 50 tend to present with thicker tumours, often on the trunk, and have a worse prognosis than females. Most melanomas occur de novo, with approximately 30% arising within a pre-existing mole.
Early detection and prompt excision of melanoma remains the most important element of management, with rapidaccess pigmented lesions set up nationally for this purpose. Early stages of melanoma have excellent prognosis. Historically, the prognosis for advanced and metastatic melanoma was very poor, though dramatic improvements in the landscape of melanoma treatment have been made in the last decade with the advent of a variety of systemic therapies for advanced and metastatic disease. These include targeted therapies such as BRAF and MEK inhibitors for BRAF-mutated melanoma, and immunotherapy agents in the form of immune checkpoint inhibitors and programmed cell-death protein 1 (PD-1) inhibitors.
Primary prevention strategies and public health messaging emphasise the importance of adequately protecting skin from the sun and selfsurveillance for early detection of suspicious lesions.
AUTHOR: Dr Emma Porter. Dermatology Registrar, University Hospital Limerick

1. REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.

3. PLAN If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by HPN.

Copies can be downloaded from www.irishpharmacytraining.ie Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
Malignant Melanoma of the Skin
Introduction
Malignant melanoma is the fifth most common invasive cancer in Ireland, with approximately 20 cases per 100,000 per year - one of the highest incidence rates globally - and rising incidence.1 Ultraviolet (UV) radiation exposure is the main risk factor for development of melanoma, particularly intense and intermittent exposures in fair-skinned individuals.2,3 Sun bed exposure is widely recognised to increased risk of melanoma, particularly when used in younger age groups.4 Further risk factors include multiple melanocytic naevi, dysplastic naevus syndrome, a family history of melanoma, and immunosuppression. Familial genetic mutations with high penetrance are rare, with CDKN2A most frequently reported in these cases. In genome-wide association studies, single nucleotide polymorphisms in multiple genes related to nevogenesis and pigmentation have been associated with melanoma, including melanocortin 1 receptor, the gene which underlies red hair and freckles.5

Median age at diagnosis was 64 years for melanoma between 2011 and 2015 in Ireland. Melanoma patients, particularly females,
tend to have a younger age profile than those with non-melanoma skin cancers, with almost 31% of all female patients and 20% of males diagnosed before age 50. Paediatric melanoma is rare.6 Males over 50 tend to present with thicker tumours, often on the trunk, and have worse prognosis. Epidemiological studies suggest that women with melanoma have a better prognosis, regardless of whether pre or postmenopausal and when adjustments for tumour characteristics are taken into account.7 8
The majority of melanomas occur de novo, with 30% arising within a pre-existing naevus. There is emerging evidence suggesting differing characteristics of these melanomas, with naevusassociated melanoma associated with lower depth of invasion and similarities in body sites.9 Histopathological subtypes of melanoma include superficial spreading, the most common, representing a radial growth phase, and nodular (vertical growth phase). Other types include acral (hands and feet), spitzoid and desmoplastic (spindle cells). In-situ melanoma is considered pre-cancerous, non-invasive and is limited to the epidermis, without evidence of dermal invasion on histology. Lentigo maligna is
a subset of melanoma in situ with histological evidence of photodamage, typically seen on sun-exposed areas such as the face in elderly individuals.
Diagnosis
When diagnosed and treated early, melanoma has an excellent prognosis. Stage 1A carries a 5-year survival rate of 99%. Diagnostic and therapeutic advances contribute to further improvement in survival in all stages, though advanced melanoma still carries a poor prognosis and one fifth of patients are at stage III or IV at the time of diagnosis.1
The National Cancer Control Programme (NCCP) has developed the National Melanoma GP Referral Guidelines (Figure 1) for assessment and referral of suspicious lesions via a standardised pathway to a dermatologist or plastic surgeon, the National Pigmented Lesion GP Referral form.10 11 There are 14 rapid access centres nationally to where referrals can be sent. Standardised referral forms support inclusion of relevant clinical data for pigmented lesions including the ‘ABCDE’ system of lesion abnormalities (see Figure 1) and environmental and genetic risk factors.
31
CPD 89: MELANOMA
CPD Continuing Professional Development CPD
Figure 1. National Melanoma GP Referral Guidelines
identification of occult nodal metastasis with SLNB can allow consideration of systemic adjuvant therapy with an aim to reduce the risk of relapse and improve survival.
Radiological imaging
NICE Guidelines 2015 and European consensus guidelines from 2019 for the management of melanoma outline recommendations for baseline staging imaging for melanoma patients – including stage IIC melanoma and above.12 A summary of these recommendations is illustrated in Table 1 (on page 34). These are general guidelines, and an individualised plan is made for each patient following multidisciplinary review.
Systemic therapy in advanced/ metastatic disease
Figure 1. National Melanoma GP Referral Guidelines

Melanoma is usually clinically suspected before histopathological confirmation. The use of dermoscopy by dermatologists is used as standard in analysis of pigmented lesions and can facilitate differentiation from benign lesions, and prompt excision where atypical features are present.12 In special cases and where available, confocal reflectance microscopy is a further diagnostic tool in improving clinical diagnosis.
The COVID-19 pandemic introduced unprecedented challenges to healthcare systems worldwide, and limited access to non-emergency services resulted in lower levels of referral, and later diagnosis of melanoma and skin cancer in general. 13 14 Many centres report more advanced tumours at diagnosis, though national figures are not currently available.
Management
Primary excision
Early detection of melanoma and prompt, complete excision influences prognosis and survival and is the first line of management. A 2mm surgical margin around the lesions and a cuff of subcutaneous fat is taken on primary excision. Some centres offer sameday excision where resources
allow. After histopathological confirmation, cases are discussed at dedicated multidisciplinary meetings. The diagnosis of melanoma is explained in detail to patients in a clinic setting, and information provided, usually with support from a dedicated skin cancer clinical nurse specialist.
Following primary excision, histopathological findings are used to determine staging – based on the 8th edition of the American Joint Committee on Cancer (AJCC) staging system, shown in Figure 2.15 Breslow thickness, or the depth in millimetres of tumour invasion into the dermis, is the most important pathological feature, followed by the presence of ulceration. Tumours with a Breslow thickness of <0.8mm and without ulceration are pT1a, carrying an excellent prognosis (99% 5-year survival). In contrast, for example, a 50-year-old male with a melanoma with Breslow thickness >4mm with ulceration on an extremity, and no metastatic spread at diagnosis, has a 5-year survival rate of 70%.16
Wide local excision
Wide local excision is performed for all melanomas, with the aim to reduce the risk of local recurrence.
Clinical margins as recommended by NICE Guidelines are 0.5cm for Stage 0 or in situ melanoma, 1cm for Stage I melanoma and 2cm for Stage II.17 In some cases, topical imiquimod can be considered for melanoma in situ, for example if complete surgical excision would cause unacceptable morbidity or disfigurement.
Melanoma is usually clinically suspected before histopathological confirmation. The use of dermoscopy by dermatologists is used as standard in analysis of pigmented lesions and can facilitate differentiation from benign lesions, and prompt excision where atypical features are present. 12 In
Sentinel lymph node biopsy
Sentinel lymph node biopsy (SLNB) involves sampling of the first node(s) in the draining nodal basin of the melanoma, e.g. the axillary nodes for a melanoma on the upper limb. This is a prognostic or staging procedure rather than therapeutic, is typically carried out by plastic surgeons at the time of wide local excision, and requires general anaesthetic. This is offered to patients with tumours ≥0.8mm Breslow thickness, estimated to carry a 5% risk of sentinel lymph node positivity. Preceding 2017, the standard of care was complete lymph node dissection for those with a positive SLNB, however the landmark studies MSLT-I and MSLT-II showed that there is no melanoma-specific survival benefit for those that have SLNB, nor for those with a positive SLNB who undergo immediate lymph node dissection versus ultrasound surveillance.18 19 However,
Metastatic disease has historically been associated with poor survival.20 Ten years ago, median overall survival of those with advanced-stage melanoma was 6-9 months.21 With the advent of targeted therapy and immunotherapy for the treatment of metastatic melanoma in the last decade, outcomes are dramatically improving.22 In 2010, targeted treatment for BRAFmutated melanoma was groundbreaking.23 This has evolved to treatment with a combination of BRAF and MEK inhibition, to reduce resistance seen in BRAF inhibitor monotherapy.24 However, less than 25% of melanomas in Ireland are BRAF-mutated, lower than other countries where this figure is closer to 40%.25
Melanoma is an immunogenic tumour, and a BRAF mutation is not required for immunotherapy. Agents include immune checkpoint inhibitors including anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), such as ipilimumab, and programmed cell-death protein 1 (PD-1) inhibitors such as pembrolizumab and nivolumab.26 Toxicity with treatment, however, is a significant factor considered by oncologists when exploring options with patients with advanced melanoma, and not all patients will be suitable or have a preference for treatment. Several
32 CPD 89: MELANOMA
Guidelines (Figure 1) for assessment and referral of suspicious lesions via a standardised pathway to a dermatologist or plastic surgeon, the National Pigmented Lesion GP Referral form 10, 11 There are 14 rapid access centres nationally to where referrals can be sent. Standardised referral forms support inclusion of relevant clinical data for pigmented lesions including the ‘ABCDE’ system of lesion abnormalities (see Figure 1) and environmental and genetic risk factors.
Figure 2: AJCC 8th edition melanoma staging system. Source: Keung E, Balch C, Gershenwald J, et al. Key changes in the AJCC eighth edition melanoma staging system. Melanoma Lett. 2018;36(1):1–9.
Figure 2: AJCC 8th edition melanoma staging system. Source: Keung E, Balch C, Gershenwald J, et al. Key changes in the AJCC eighth edition melanoma staging system. Melanoma Lett. 2018;36(1):1–9

Wide local excision
Wide local excision is performed for all melanomas, with the aim to reduce the risk of local recurrence.
Clinical margins as recommended by NICE Guidelines are 0.5cm for Stage 0 or in situ melanoma, 1cm for Stage I melanoma and 2cm for Stage II. 17 In some cases, topical imiquimod can be considered for melanoma in situ, for example if complete surgical excision would cause unacceptable morbidity or disfigurement.
33
CPD Questions
True or False: Survival rates for melanoma are better in males than females.
For melanoma with a positive sentinel lymph node biopsy, which of the following is the first management step?
Complete lymph node dissection
Table 1. Summary of recommendations for baseline and surveillance imaging from most recent melanoma management guidelines. Abbreviations: CT TAP – Computed tomography of thorax, abdomen and pelvis. MRI –Magnetic resonance imaging. PET CT – Positron emitted tomography computed tomography. US – Ultrasound.
of the immunotherapy agents are approved for reimbursement by the HSE, including pembrolizumab, ipilimumab, nivolumab, along with multiple agents for BRAF mutation-positive unresectable or metastatic melanoma.27
may only require a small number of clinic reviews over the space of the first year before discharge, as risk of recurrence is much lower.13
Systemic therapy in advanced/metastatic disease
Adjuvant treatment of stage III melanoma
Adjuvant therapy in melanoma aims to reduce the risk of relapse and improve overall survival in patients with moderate to high risk resected melanoma. Funding was made available for nivolumab in February 2021 as monotherapy for the adjuvant treatment of adults with stage III melanoma and lymph node involvement who have undergone complete resection, and pembrolizumab in May 2021. It is anticipated that licensing and funding for further agents will continue to expand in the near future.
Clinical follow up
Patients with melanoma are followed up in a dermatology clinic with full skin examination and assessment of draining lymph node basins on a regular basis, frequency and duration depending on clinical stage. The first five years are most important, as this is the period in which 90% of metastases occur.12 Early stage melanoma, such as melanoma in situ and Stage IA melanoma,
Regular clinical review facilitates identification of recurrent disease, and monitors for development of a second primary melanoma (which can occur in up to 10% of patients) or a non-melanoma skin cancer. It also provides the opportunity for further patient education regarding photoprotection and selfsurveillance, and psychological support. Patients with later stage melanoma may require surveillance imaging (see Table 1).
Table 1. Summary of recommendations for baseline and surveillance imaging from most recent melanoma management guidelines. Abbreviations: CT TAP – Computed tomography of thorax, abdomen and pelvis. MRI – Magnetic resonance imaging. PET-CT – Positron emitted tomography-computed tomography. US – Ultrasound
Commencement of adjuvant immunotherapy
Discussion at multidisciplinary meeting
Metastatic disease has historically been associated with poor survival 20 Ten years ago, median overall survival of those with advanced stage melanoma was 6-9 months 21 With the advent of targeted therapy and immunotherapy for the treatment of metastatic melanoma in the last decade, outcomes are dramatically improving. 22 In 2010, targeted treatment for BRAF mutated melanoma was ground breaking 23 This has evolved to treatment with a combination of BRAF and MEK inhibition, to reduce resistance seen in BRAF inhibitor monotherapy 24 However, less than 25% of melanomas in Ireland are BRAF mutated, lower than other countries where this figure is closer to 40%. 25 Melanoma is an immunogenic tumour, and a BRAF mutation is not required for immunotherapy Agents include
Vitamin D
Following a diagnosis of melanoma, the change in behaviour in reducing sun exposure is likely to result in lower levels of vitamin D synthesis over time, with suboptimal levels common in the general population at a baseline.28 There is some evidence that vitamin D plays a role in melanoma survival; higher serum vitamin D is associated with thinner primary melanoma and better outcome, although a causal mechanism has not been established.29 Serum 25-hydroxyvitamin D3 levels are typically measured for melanoma patients to allow advice on supplementation, with an ideal range considered to be 60-85 nmol/L.
Primary Prevention
Public health campaigns for prevention of melanoma, and skin cancer in general, focus on safe sun practices of avoiding sun burn, seeking shade, covering up, using sunscreens and avoiding the use of sun beds. The SunSmart campaign, with the ‘5 S’s’ of Slip, Slap, Slop, Slide and Seek shade, has been most widely adopted, introduced first in Australia over 30 years ago where it has been shown to have a positive impact on sun-related behaviours.30 This is the primary messaging used in Ireland, promoted by Healthy Ireland, and emphasised each year typically beginning in May with media campaigns. These sun protection practices are recommended especially between 11am and 3pm from April to September even on a cloudy day in Ireland, and when the UV Index is 3 or greater. Children and outdoor workers should have
True or false: Systemic treatment options for a patient with metastatic nonBRAF-mutated melanoma include immunotherapy such as pembrolizumab and nivolumab, and targeted therapies such as BRAF and MEK inhibitors
What is the lowest stage of melanoma for which baseline imaging is recommended by NICE Guidelines 2015?
particular care taken in these measures. Furthermore, healthcare professionals can play an important role in further promoting these primary prevention practices for all patients we encounter.
References available on request
34 CPD 89: MELANOMA
Guidelines 2015
NICE
and European consensus guidelines from 2019 for the management of melanoma outline recommendations for baseline staging imaging for melanoma patients – including stage IIC melanoma and above 15 A summary of these recommendations is illustrated in Table 1. These are general guidelines, and an individualised plan is made for each patient following multidisciplinary review.
CT TAP +/
CT or
CT TAP +/-
CT
CT
Guideline Stage Staging at baseline Surveillance Schedule of surveillance NICE Guidelines 2015 Stage IIC no SLNB and Stage III
Brain
MRI brain if age <24 years
Brain Every 6 months for the first 3 years European consensus guidelines 2019 Stage IIC US
TAP / PET CT MRI brain LN US Every 3 6 months for the first 1 3 years CT TAP / PET CT MRI Brain Every 6 months for the first 1 3 years Stage III & higher US
TAP / PET CT MRI brain LN US 3 6 months for the first 1 3 years CT TAP / PET CT MRI Brain 3 6 months for the first 1 3 years
a.
b.
c.
d.
2.
b.
c.
d.
5.
a. Stage
b.
c. Stage
d.
1. Which of the following is the strongest exogenous risk factor for melanoma development?
Smoking
Chronic low intensity UV exposure
Intense intermittent UV exposure
Multiple melanocytic naevi
3.
a.
Ultrasound surveillance
4.
IB
Stage IIA
IIC
Stage IIIA
involvement of the hands, wrists, but can occasionally predominate. PMR is classically a bilateral and
progresses to involve both sides.
affected, they may not be equally involved, and a substantial minority of patients will report only shoulder inflammatory arthritis conditions.


sitting for prolonged periods – not infrequently on the car journey to their clinic appointment or sitting in the waiting room! Patients with more severe symptoms will often
they will wake with early morning stiffness which will then continue
weight loss, malaise, and anorexia
should prompt a search for one of
49
copyright, no part of this can be used in any publishers and author. Galapagos UK, has no
PMR Mimics
It is perhaps more appropriate to consider PMR as a clinical syndrome rather than a specific disease, at least at initial presentation. This reflects the limitations of our nosological framework of disease categorisation. The entity we consider as PMR is likely composed of a number of different “diseases” which present in similar ways, sometimes this will differentiate into other defined conditions over time. It is likely however that even in those who remain in the PMR diagnostic category that several different disease subsets and pathogeneses are represented.
This being the case, when a patient presents with a polymyalgic syndrome there are a number of important differentials to consider. PMR is frequently considered an easy diagnosis to make, I would argue that it is easy to mis-diagnose PMR given the many different conditions which can present with similar symptom complexes.
Probably the most frequent and important of these to consider is giant cell arteritis (GCA). While it is still debated, PMR and GCA are likely two ends of the spectrum of a single disease process. This is supported by shared pathogenic mechanisms, demographic associations, treatment responses, and particularly by the frequent co-occurrence of the two – 50% of GCA patients have polymyalgic symptoms, while 10-30% of people with PMR will ultimately develop GCA. All patients presenting with PMR should have a clinical assessment for GCA including examination of the temporal arteries and for large vessel arterial bruits. If the history or examination raise concern for GCA, further investigation with imaging and/or temporal artery biopsy should be pursued.
The second classic mimic of PMR is rheumatoid arthritis (RA). RA can present initially with proximal joint symptoms in the same distribution as PMR. This is particularly the case in elderly patients, the very group in whom PMR classically presents. Clues to the presence of RA can include synovitis in the hands and feet, and of course positive rheumatoid factor or anti-cyclic citrullinated peptide
antibodies (these are only present in 70% of rheumatoid arthritis patients however). Marked foot involvement in particular is almost never seen in true PMR.
A number of other conditions can present with initial polymyalgic symptoms. These include ANCAassociated vasculitis (which can subsequently manifest severe organ damage) and idiopathic inflammatory myopathies (which more commonly present with muscle weakness rather than stiffness or pain). Non-rheumatic diseases are important mimics, these include hypothyroidism, Parkinson’s disease, myasthenia gravis, and non-inflammatory myopathies – all of these should particularly be considered in patients with no elevation in inflammatory markers.
The final mimics are those which are drilled into the minds of medical students and trainees the world over – paraneoplastic and infections (particularly infective endocarditis). It is of course vital not to miss these, however, it is unusual that these would present with symptoms of PMR in the absence of other consistent features.
Clinical Examination
Given the predominant symptoms, the clinical examination in PMR will often be dominated by pain and restricted range of motion in the shoulders and hips. It is important to assess for synovitis clinically as this may be a clue as to the presence of rheumatoid arthritis. Palpation of the temporal artery for tenderness, swelling, hardening, or loss of pulsation is important to assess for cranial GCA. Auscultation of the carotid, subclavian, and axillary arteries to assess for vascular bruits may be a clue to large vessel GCA but can also be seen in atherosclerosis. Stigmata of infective endocarditis or signs of an underlying malignancy should not be missed. It is important in patients with known PMR to assess for clinical signs of GCA at each visit due to the high rate (10-30%) of development of GCA in patients with PMR. As diseases may evolve over time, it is also prudent to reassess the peripheral joints for synovitis at each visit, as a diagnosis of rheumatoid arthritis would change management.
Laboratory Investigations
PMR is a systemic inflammatory rheumatic disease. As such acute phase reactants / inflammatory markers are characteristically but not invariably elevated. Erythrocyte sedimentation rate (ESR) is the traditional diagnostic test in PMR but actually, C-reactive protein (CRP) is the more useful test overall. CRP is elevated in 98-99% of patients with PMR. ESR can be normal in up to 20% of patients with untreated PMR. While having both a normal ESR and CRP does occur in PMR, this is distinctly unusual and should prompt reconsideration of the diagnosis. An important pitfall to avoid is being misled by the extent of the inflammatory marker elevation. While it sometimes occurs, PMR does not necessarily need to be associated with very high inflammatory markers, lesser elevations are also significant in the appropriate clinical scenario.
Fibrinogen is another potentially useful acute phase reactant and biomarker in PMR, however its widespread utilisation is limited due to cost implications.2 Patients can also have elevated platelets as an acute phase response, as well as reduced haemoglobin and albumin as negative acute phase reactants.
tenosynovitis in the shoulders, and trochanteric bursitis and hamstring tendinitis in the hips. Capsular inflammation can also be seen.
Treatment
It is a misconception that PMR is an easy disease to treat. It is relatively easy to get a rapid benefit in these patients, but longer-term management and maintenance of initial good responses is much trickier. Negotiation of treatment related adverse events is also a significant challenge.
Our treatment paradigm in PMR continues to be largely glucocorticoid based. In Ireland this will utilise prednisolone as the glucocorticoid of choice. Enteric coated formulations may be associated with less adverse events in individual patients but there is little objective evidence they are beneficial for the wider group of people with PMR and dose tapering is complicated by the absence of lower strength doses of these formulations.
Figure 1: Example of prednisolone
In terms of monitoring, while all of the above acute phase reactants may be elevated, for the individual patient it is likely that one or the other more closely reflects their disease activity. This will most frequently be the CRP, but for some patients ESR, fibrinogen, or even platelets may be more closely related to their symptoms. It is useful to check all available and practical markers at first presentation, and then to choose to monitor the best marker of the patient’s disease longitudinally.
Imaging
Imaging is rarely required to make the diagnosis of PMR. It sometimes reveals the disease when done for other reasons, either incidentally or when investigating unexplained inflammatory marker elevations. Ultrasound, MRI, and PET scan can all demonstrate evidence of inflammation in periarticular structures. This typically consists of subdeltoid/subacromial bursitis and long head of biceps
I recommend a starting dose of prednisolone 15mg daily in PMR; this will be sufficient for complete symptom resolution in most patients. There are a small percentage of PMR patients who will require a higher initial dose of 20mg or even 25mg; glucocorticoids are a weightbased medication, and this is more commonly seen in higher weight individuals. There are two benefits to starting with this relatively low glucocorticoid dose, the lower cumulative glucocorticoid exposure is self-evident. The second benefit is a diagnostic one; 15mg of prednisolone will generally completely resolve “true” PMR but will frequently be less effective in many of the mimics. The glucocorticoid response is typically dramatic in PMR, some individuals will have symptom resolution in hours, and most will in 1-2 days; there are however a smaller percentage of patients where this may take 1-2 weeks, and the absence of a rapid response does not exclude PMR.
Once glucocorticoids have commenced, it is prudent to review the response, either in person or by telehealth, at 2-4 weeks. This again helps to provide diagnostic confirmation. If a response is absent or suboptimal, the diagnostic process should be revisited. If a good response
50 CPD 90: POLYMYALGIA RHEUMATICA
Relapse is common on glucocorticoid particularly if addressed promptly there were no symptoms, while prednisolone dose. Subsequent
has been achieved, this is now the time to formulate a long-term treatment plan. There are many ways in which the glucocorticoid dose can be tapered. My practice is to reduce glucocorticoids at monthly intervals; this obviates the need for the patient to change doses between pharmacy prescriptions – they receive 1 month supply at a time and stay at the same dose until they return for the next dispensing. After 1 month on 15mg, the dose reduces to 12.5mg for 1 month, then 10mg for 1 month, thereafter, reducing by 1mg a month until stopped. If a higher starting dose is needed (20 or 25mg), then this extra reduction should be added initially as extra months, I generally reduce by 5mg a month above 20mg, by 2.5mg a month between 20mg and 10mg, and 1mg a month under 10mg. It is absolutely vital during this process that the patient both knows to, and has the facility, to contact the treating physician if there is a recrudescence of their symptoms.
Relapse is common on glucocorticoid tapering, occurring in up to 50% of patients.3 Many relapses, particularly if addressed promptly will respond to an increase to the last prednisolone dose at which there were no
symptoms, while further delay will often lead to a necessity to return to the initial prednisolone dose. Subsequent tapering as per the original protocol, will generally be successful. If patients continually relapse at a particular glucocorticoid dose, alternative tapering strategies such as alternate day tapering (eg reduce from 5mg to 4mg/5mg on alternate days instead of directly to 4mg) or reducing the dose every two months rather than monthly, may be successful. Even with these strategies many patients will reach a “threshold” glucocorticoid dose below which relapse inevitably and persistently occurs. At this point consideration should be given to the alternative options of long-term maintenance glucocorticoids or addition of a second agent. This decision may be dependent on what the threshold glucocorticoid dose is; glucocorticoids have a multitude of adverse events related to dose and cumulative dose. Generally, anything above 5mg daily of prednisolone is probably inadvisable, but even at lower doses there are increased rates of adverse events.4, 5
Methotrexate has been our traditional second line medication in PMR. There is randomised
1/3 smooth glucocorticoid reduction
Methotrexate has been our traditional second line medication in PMR. There is randomised controlled trial evidence for a steroid sparing effect (6). However, in clinical practice this is often quite modest, and the efficacy of methotrexate in PMR does not approach its efficacy in rheumatoid arthritis. For some patients this modest steroid effect, even if it only results in a reduction of a milligrams in the daily dose, may be clinically meaningful, however. Within the last year we now have evidence from three randomised controlled trials of biologic disease modifying antirheumatic drugs (bDMARDs) to treat PMR. The IL 6 inhibitors tocilizumab and sarilumab, the latter not available in Ireland, appear to have excellent efficacy, mirroring their known effect in GCA (7) surprisingly perhaps, a single dose of rituximab at treatment initiation has been shown to have term benefits in PMR (8) The place of these bDMARDs in the PMR treatment algorithm is still consideration. While it would be intuitively appealing to administer them up front to all patients with the aim to minimise cumulative glucocorticoid exposure as much as possible, this would undoubtedly lead to the over treatment of many patients who would have done just fine with glucocorticoid monotherapy. The disadvantage of waiting until relapses occur, is that by that time
controlled trial evidence for a steroid sparing effect.6 However, in clinical practice this is often quite modest, and the efficacy of methotrexate in PMR does not approach its efficacy in rheumatoid arthritis. For some patients this modest steroid effect, even if it only results in a reduction of a few milligrams in the daily dose, may be clinically meaningful, however. Within the last year we now have evidence from three randomised controlled trials of biologic disease modifying antirheumatic drugs (bDMARDs) to treat PMR. The IL-6 inhibitors tocilizumab and sarilumab, the latter not available in Ireland, appear to have excellent efficacy, mirroring their known effect in GCA.7 More surprisingly perhaps, a single dose of rituximab at treatment initiation has been shown to have long-
term benefits in PMR.8 The place of these bDMARDs in the PMR treatment algorithm is still under consideration. While it would be intuitively appealing to administer them up front to all patients with the aim to minimise cumulative glucocorticoid exposure as much as possible, this would undoubtedly lead to the overtreatment of many patients who would have done just fine with glucocorticoid monotherapy. The disadvantage of waiting until relapses occur, is that by that time a significant accumulation of glucocorticoid adverse events has already occurred. We need better predictive prognostic markers in order to properly risk stratify patients. The role of shared decision making is also crucial, and the input of people with PMR on the risks and benefits of various treatment strategies is crucial given that they will be the ones ultimately experiencing them.
Adjunctive managements are important in all patients with PMR. These are largely directed at minimising glucocorticoid
51
Figure 1: Example of prednisolone tapering regimen in PMR
Figure 2: Prognosis in PMR
tapering regimen in PMR glucocorticoid tapering, occurring in up to 50% of patients (3). Many relapses, promptly will respond to an increase to the last prednisolone dose at which while further delay will often lead to a necessity to return to the initial Subsequent tapering as per the original protocol, will generally be
If 15mg daily x 1 month 12.5mg daily x 1 month 10mg daily x 1 month 9mg daily x 1 month 8mg daily x 1 month 7mg daily x 1 month 6mg daily x 1 month 5mg daily x 1 month 4mg daily x 1 month 3mg daily x 1 month 2mg daily x 1 month 1mg daily x 1 month Then stop
prednisolone
successful.
Figure 2: Prognosis in PMR
adverse events. Long term glucocorticoids, even at relatively modest doses, have a profound negative effect on bone, leading to glucocorticoid induced osteoporosis. All patients should be assessed for the need for calcium and vitamin D supplementation, and have it provided to those who have dietary calcium deficiency and low serum vitamin D respectively. All patients receiving long-term glucocorticoids should receive an anti-resorptive agent, generally a bisphosphonate while on glucocorticoids. People with PMR are generally also in the age range where DXA screening for osteoporosis is recommended, but many will have not had this performed, if this is the case, it should be performed to guide the need for long-term bone protection after glucocorticoids have finished. Those receiving the initial glucocorticoid doses will generally also receive
gastroprotection with a proton pump inhibitor, this can usually be ceased as the glucocorticoid dose reduces. It is important to encourage people with PMR to maintain activity in order to limit glucocorticoid adverse events, particularly myopathy. Patients who have more significant myopathy, either due to disease or glucocorticoid related effects may benefit from physiotherapy. Patient education is important both in terms of the management plan and as there are many pervasive myths around PMR.
Summary
PMR is a common and disabling condition. Dramatic initial treatment responses are tempered by longer term struggles with relapses and treatment related adverse events. Focused optimum management of PMR leads to dramatically improved patient outcomes.
Bone protection DXA PPI
Maintain activity
Physiotherapy
Patient education
1.
References
1. Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, et al. The lifetime risk of adultonset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis and rheumatism. 2011;63(3):633-9.
2. McCarthy EM, MacMullan PA, Al-Mudhaffer S, Madigan A, Donnelly S, McCarthy CJ, et al. Plasma fibrinogen is an accurate marker of disease activity in patients with polymyalgia rheumatica. Rheumatology (Oxford). 2013;52(3):465-71.
3. Salvarani C, Cantini F, Niccoli L, Macchioni P, Consonni D, Bajocchi G, et al. Acute-phase reactants and the risk of relapse/ recurrence in polymyalgia rheumatica: a prospective followup study. Arthritis and rheumatism. 2005;53(1):33-8.
4. Widdifield J, Bernatsky S, Paterson JM, Gunraj N, Thorne JC, Pope J, et al. Serious infections in a population-based cohort of 86,039 seniors with rheumatoid arthritis. Arthritis care & research. 2013;65(3):35361.
5. Kavanaugh A, Wells AF. Benefits and risks of low-dose glucocorticoid treatment in the patient with rheumatoid arthritis. Rheumatology (Oxford). 2014;53(10):1742-51.
6. Caporali R, Cimmino MA, Ferraccioli G, Gerli R, Klersy C, Salvarani C, et al. Prednisone plus methotrexate for polymyalgia rheumatica: a randomized, double-blind, placebo-controlled trial. Annals of internal medicine. 2004;141(7):493-500.
7. Bonelli M, Radner H, Kerschbaumer A, Mrak D, Durechova M, Stieger J, et al. Tocilizumab in patients with new onset polymyalgia rheumatica (PMR-SPARE): a phase 2/3 randomised controlled trial. Annals of the rheumatic diseases. 2022;81(6):838-44.
8. Marsman DE, den Broeder N, van den Hoogen FHJ, den Broeder AA, van der Maas A. Efficacy of rituximab in patients with polymyalgia rheumatica: a double-blind, randomised, placebocontrolled, proof-of-concept trial. The Lancet Rheumatology. 2021;3(11):e758-e66.
52 CPD 90: POLYMYALGIA RHEUMATICA CPD Questions 1. The following are all common clinical features of polymyalgia rheumatica except a. Age ≥ 50 years b. Early morning stiffness c. Worse with activity d. Rapid response to glucocorticoids e. Shoulder and hip stiffness 2. Which of the following may be decreased in PMR? a. C-reactive protein b. Erythrocyte sedimentation rate c. Platelets d. Haemoglobin e. Fibrinogen 3. Standard adjunctive treatments for PMR include all of the following except a. Bisphosphonate b. Calcium/vitamin D c. Statin d. Proton pump inhibitor e. Exercise 4. Which of the following is true regarding glucocorticoid treatment in PMR a. Relapses are rare b. A treatment course of weeks is needed c. High dose steroid (eg 40mg prednisolone) is required initially d. At least a year of treatment is generally needed e. There are minimal adverse events 5. Potential options in refractory/relapsing PMR include all of the following except a. Methotrexate b. Non-steroidal antiinflammatory drugs c. Tocilizumab d. Rituximab e. Long-term low dose glucocorticoid
Figure 3: Adjunctive management in PMR
Figure 3: Adjunctive management in PMR
Summary PMR is a common and disabling condition. Dramatic initial treatment responses are tempered by longer term struggles with relapses and treatment related adverse events. Focused optimum management of PMR leads to dramatically improved patient outcomes. References
Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, et al. The lifetime risk of adult onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis and rheumatism. 2011;63(3):633 9.
2. McCarthy EM, MacMullan PA, Al Mudhaffer S, Madigan A, Donnelly S, McCarthy CJ, et al. Plasma fibrinogen is an accurate marker of disease activity in patients with polymyalgia rheumatica. Rheumatology (Oxford). 2013;52(3):465 71.











Save the Date Saturday, 16th September, 2023 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication
Life-threatening, underrecognized, and underdiagnosed, ATTR-CM is a rare condition found in mostly older patients in which misfolded transthyretin proteins deposit in the heart.1-7 It is vital to recognize the diagnostic clues so you can identify this disease.
heart failure with preserved ejection fraction in patients typically over 60 years old5-7
to standard heart failure therapies (ACEi, ARBs, and beta blockers)8-10
between QRS voltage and left ventricular (LV) wall thickness11-13
of carpal tunnel syndrome or lumbar spinal stenosis3,8,14-20 showing increased LV wall thickness6,13,16,21,22
—autonomic nervous system dysfunction-including

complaints or unexplained weight loss6,16,23,24
J Card Fail. 2016;22(12):996-1003. 10. Castaño A, Drach BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163-178. 11. Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982;49:9-13. 12. Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114(7):1089-1093. 13. Quarta CC, Solomon D, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840-1849. 14. Connors LH, Prokaeva T, Lim A, et al. Cardiac amyloidosis in African Americans: Comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158(4):607-614. 15. Nakagawa M, Sekijima Y, Yazaki M, et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. 2016;23(1):58-63. 16. Rapezzi C,
2018;72(17):
2009;120(13):1203-1212.
Hum Pathol. 2011;42(9):1259-1264.


21. Phelan D, Collier P, Thavendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442-1448. 22. Ternacle J, Bodez D, Guellich A, et al. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. JACC Cardiovasc Imaging 2016;9(2):126-138. 23. Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63-76. 24. Swiecicki PL, Zhen DB, Mauermann ML, et al. Hereditary ATTR amyloidosis: a single-institution experience with 266 patients. Amyloid. 2015;22(2):123-131.

The health information contained in this ad is provided for educational purposes only.
Date of Preparation: February 2021 GCMA code: PP-RDP-IRL-0105
5.
gastrointestinal
References 1. Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23(4):209-213. 2. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357-1377. 3. Connors LH, Sam F, Skinner M, et al. Heart failure due to age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282-290. 4. Pinney JH, Whelan CJ, Petrie A, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098.
Mohammed SF,
Mirzoyev
SA,
Edwards
WD,
et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2(2):113-122. 6. Maurer MS, Hanna M, Grogan M, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016;68(2):161-172.
7. González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585-2594. 8. Narotsky DL, Castano A, Weinsaft JW, Bokhari S, Maurer MS. Wild-type transthyretin cardiac amyloidosis: novel insights from advanced imaging. Can J Cardiol. 2016;32(9):1166.e1-1166.e10. 9. Brunjes DL, Castano A, Clemons A, Rubin J, Maurer MS. Transthyretin cardiac amyloidosis in older Americans.
Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation.
17. Sperry BW, Reyes BA, Ikram A, et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol.
2040-2050. 18. Westermark P, Westermark GT, Suhr OB, Berg S. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci. 2014;119(3):223-228. 19. Yanagisawa A, Ueda M, Sueyoshi T, et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28(2):201-207. 20. Sueyoshi T, Ueda M, Jono H, et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons.
LEARN HOW TO RECOGNIZE THE CLUES OF ATTR-CM AT: S U S P E C T A N D D E T E C T . I E CONSIDER THE FOLLOWING CLINICAL CLUES, ESPECIALLY IN COMBINATION, TO RAISE SUSPICION FOR ATTR-CM AND THE NEED FOR FURTHER TESTING SUSPECT ATTR-CM (TRANSTHYRETIN AMYLOID CARDIOMYOPATHY) A LIFE-THREATENING DISEASE THAT CAN GO UNDETECTED H FpEF I NTOLERANCE DISCORDANCE DIAGNOSIS E CHO N ERVOUS SYSTEM
CPD
CPD
60 Second Summary
Transthyretin amyloidosis (ATTR) is a multi-system fatal disease which occurs as a result of deposition of abnormal transthyretin-derived amyloid fibrils in a variety of body tissues. Both inherited (ATTRv) and acquired i.e. wild-type forms (ATTRwt) can occur. Due to the variety of systems affected there can be a wide variability in symptom manifestation, although cardiac and neurological symptoms generally predominate.

Commonly symptoms are nonspecific and may be mistaken for symptoms of other more frequently occurring causes (e.g. orthostatic hypotension being attributed to medication rather than to an underlying autonomic neuropathy). This, along with a lack of awareness among healthcare professionals, can lead to significant delays in diagnosis. With emergence of disease-modifying treatment, prompt diagnosis is of increasing importance. Licensed treatment options now include transthyretin stabilisers and small interfering RNA therapeutics, while gene editing with CRISPR-Cas9 based therapy is showing promise in clinical trial.
This CPD piece will provide a general overview of ATTR including subtypes, clinical presentation, diagnosis and disease-modifying treatment strategies. Symptomatic management is outside the scope of this piece. The HSE Model of Care for Amyloidosis which aims to improve standards of care for patients with amyloidosis in Ireland is discussed; an important aspect of this is improved awareness and earlier diagnosis. We also discuss methods for earlier or potentially pre-symptomatic identification of patients.
AUTHORS: Dr Zara Togher, Professor Sinéad M Murphy
Dr Zara Togher is a Specialist Registrar in Neurology currently working in University Hospital Limerick. She has an interest in neuropathies and neuromuscular disorders having spent time in dedicated Neuropathy and Neuromuscular clinics in Tallaght University Hospital (TUH).


Prof. Sinead Murphy is a Consultant Neurologist at TUH and Clinical Associate Professor in Trinity College Dublin. She has particular subspecialty interest and expertise in inherited neuropathies and ataxias, runs Neuropathy and Neuromuscular clinics and co-leads the National Ataxia Clinic in TUH.
She is lead of the Irish Consortium for Rare Neurological Disorders, with TUH recently being recognised as a site for the European Reference Network in Rare Neurological Diseases (ERN-RND). She chaired the Neurology sub-group of the HSE Amyloidosis Working Group which developed the Amyloidosis Model of Care and Guideline recently approved by the HSE.

1. REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the
Transthyretin Amyloidosis
Introduction
Transthyretin amyloidosis (ATTR) is a progressive and fatal disease caused by deposition of abnormal transthyretin-derived amyloid fibrils in the extracellular tissues of various organs.1 Transthyretin protein (TTR) is predominantly produced by the liver, although small amounts are also produced in the eye by the retinal and ciliary pigment epithelia, and in the brain by the choroid plexus.2,3 Normally TTR circulates in the form of a tetramer made up of four identical monomeric subunits in both the serum and cerebrospinal fluid (CSF). Its main function is as a transporter of both thyroxin and retinol-binding protein, hence the name.2 In ATTR, dissociation of the tetrameric TTR occurs followed by misfolding, aggregation and formation of insoluble amyloid fibrils which are then deposited systemically. Accumulation of amyloid fibrils in tissues progressively disrupts their structure and function, leading to loss of function in various organs, particularly heart and peripheral nerves. There are two sub-types of ATTR: wild-type ATTR (ATTRwt) and hereditary or variant ATTR (ATTRv).
ATTRv
In ATTRv pathogenic genetic variants destabilise TTR, causing misfolding and aggregation.3 The TTR gene is located on
chromosome 18, and over 120 TTR mutations have been identified to date3,4 Worldwide V30M (p.V50M) is the most frequent, accounting for approximately half of all cases.5 However, with regard to Irish patients, in 1995 Reilly et al. described a cluster of cases concentrated in North West Donegal. This cluster was associated with the T60A (p.T80A) variant. Further population-based studies showed a carrier rate for this mutation of approximately 1.1% in this region.6 This is the most common TTR mutation in the Irish population, although other variants also occur.
It is important to note that there can be significant differences in presentation depending on the particular genetic variant. For example, while the typical presentation of ATTRv is with a combination of cardiomyopathy and neuropathy, patients with ATTRL12P usually present with oculoleptomeningeal disease.7 In addition, penetrance and age of onset varies between populations even with the same mutation. For example, patients with ATTRV30M of Portuguese origin have mean age of onset of 33 years with penetrance of 91% at 70 years, whereas patients of Swedish origin with the same variant have a later age of onset of 56 years and lower penetrance of 36% at 70 years.8
4 previous steps, log and record your findings.
Published by HPN.
Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author. Pfizer has no editorial oversight of the CPD programmes included in these modules
ATTRwt
ATTRwt (previously called senile systemic amyloidosis) is generally considered a disease of older adults in which amyloid fibril deposition occurs due to agerelated structural changes of the TTR protein which cause it to misfold. Studies which examined autopsy specimens revealed increasing amounts of wt TTR deposition in cardiac tissues with age.9,10 It is likely that ATTRwt is significantly underdiagnosed; it is estimated that up to 25% of patients with heart failure with preserved ejection fraction (HFpEF) have ATTRwt. In two autopsy studies specifically looking at supercentenarians (individuals who have reached the age of 110 years or more), the primary cause of death in 70% of patients was related to cardiac deposition of wt TTR.11
Clinical Features
The clinical presentation of ATTR varies between patients depending on the subtype (ATTRwt vs ATTRv) and, in ATTRv, the specific mutation and the origin of the patient. However, the heart and peripheral nerves are the most common clinically involved organs in patients with ATTR. Cardiac symptoms classically consist of heart failure and arrhythmias, whereas neurological symptoms are due to involvement of peripheral
31
CPD 91: ATTR
Continuing Professional Development
Dr Zara Togher Professor Sinéad Murphy
Ocular Pupil abnormalities Dry eyes
Glaucoma
Vitreous opacities
Abnormal conjunctival vessels
Retinal neovascularisation
Orthopaedic/Rheumatological
Spinal stenosis
Neurological
Peripheral neuropathy
Autonomic neuropathy
Carpal tunnel syndrome
Myopathy
misdiagnosis of up to 32-74%.31,32
The Amyloidosis Research Consortium reported that 47% of patients received at least one misdiagnosis before being diagnosed with amyloidosis.33 In this survey, ATTR patients went undiagnosed for more than 4 years (27% ATTRwt and 15% ATTRv). 19% of patients also reported having to travel over 2 hours to get diagnosed. While this is data from the US, this would likely be mirrored in Irish patients, with the majority of patients until recently travelling to the UK for diagnosis.
An amyloid Model of Care has been drafted by the HSE Amyloidosis Working Group to improve the standard of care for patients in Ireland 34. As part of this, a number of symptom ‘gates’ are highlighted where physicians are encouraged to think of a diagnosis of amyloidosis where it may not already be under consideration to aid in timely diagnosis. We will briefly mention here the symptoms of relevance to ATTRv and ATTRwt.
The first of the symptom gates is carpal tunnel syndrome, with a
rationale that patients (in particular with ATTRwt) may present with carpal tunnel syndrome up to 10 years before developing symptomatic cardiomyopathy. Therefore the recommendation is for patients who undergo surgical decompression to have a biopsy of the flexor retinaculum taken at the time of surgery to exclude ATTR. Another symptom gate is that of aortic stenosis with low flow-low gradient. It is advised that these patients be referred for DPD scan to exclude the presence of ATTR. Other presentations that should prompt consideration of ATTR are unexplained spinal stenosis, or an unexplained combination of autonomic and peripheral neuropathy.
Several diagnostic algorithms have been proposed as part of the Model of Care, depending on the presenting symptom, a modified version of the neuropathy algorithm is shown below in Image 2. (page 34)
Disease-modifying treatment
In this section we will focus primarily on disease-modifying
Cardiac Heart failure


Arrhythmia Aortic stenosis
Image 1: Varied systemic manifestations of ATTRv
inflammatory drug which has been repurposed for use in ATTR (off-license) as it stabilises the TTR tetramer in vitro. It has been shown to stabilise neuropathy progression and reduce cardiac dysfunction, however with potential side effects including gastrointestinal symptoms and renal impairment.36
Renal Proteinuria Renal failure

treatment for ATTRv. Symptomatic management is equally important, especially for quality of life, but is outside the scope of this article. Initially, disease-modifying therapy had relied predominantly on liver transplantation, the concept being that this would prevent further variant TTR being produced by the liver. This improved outcomes in some patients (especially those with early-onset ATTRV30M) but was not a viable option for patients with cardiac disease (and therefore was not usually considered in those with ATTRT60A who usually have cardiac disease at presentation).19,35 Thankfully, there have been significant advances in the last number of years in ATTR therapies. Tafamidis is a TTR stabiliser which was approved for use in the treatment of adults with ATTR cardiomyopathy in the European Union in 2016. However, it is not approved for, nor has shown a significant improvement in ATTRv associated neuropathy therefore use is currently limited in Ireland to patients with ATTR (either ATTRwt or ATTRv) with cardiomyopathy.36 Diflusinal is an oral non-steroidal anti-
Newer treatments including gene silencing therapies and gene editing have recently emerged as effective treatment strategies for ATTRv. Gene silencing therapies for ATTRv include antisense oligonucleotides (Inotersen) and small interfering RNAs (siRNAs), namely patisiran. The APOLLO trial37 demonstrated biochemical and clinical efficacy of intravenous patisiran in ATTRv, with significant reductions in circulating TTR and improvements in autonomic neuropathy, quality of life, peripheral neuropathy symptoms, nutritional status and cardiac function. An open label extension reported sustained benefits supporting the long-term effectiveness of this therapy.38 Similar benefits were reported with subcutaneous Inotersen therapy in the NEURO-TTR trial, although it has additional monitoring requirements due to the occurrence of thrombocytopaenia and glomerulonephritis39,40
At present only patisiran is reimbursed in Ireland for patients with ATTRv associated neuropathy and is given by three-weekly intravenous infusion; the initial three doses are given in hospital and subsequent doses can be given at home if tolerated. With regards to gene editing, the first ATTRv patient cohort treated with a single dose of CRISPRCas9 based therapy showed a significant dose-dependent and long-lasting reduction of serum TTR protein levels.41 Clinical outcomes are eagerly awaited.
Given that gene silencing and gene editing therapies only affect TTR production by the liver, as they do not cross the blood brain barrier, there is concern that with prolonged survival will come the development of a new phenotype of ATTRv due to continued unchecked production of mutant
33
Biceps tendon rupture
Rotator cuff tear
Gastrointestinal Diarrhoea Constipation Weight loss Early satiety Nausea/vomiting
TTR in the eye and brain.42 Indeed, symptomatic cerebral amyloid angiopathy has been seen in ATTR patients surviving after liver transplantation.43 In contrast to the systemic circulation where other thyroxin carriers exist, TTR is required for thyroxin transport centrally as well as having some neuroprotective effects. Thus, novel approaches will be needed as prognosis improves with the recent advances in therapy.
Future of ATTR in Ireland
One avenue for improvement is the potential for identification of pre-symptomatic disease in both ATTRwt and ATTRv. As mentioned, carpal tunnel disease may present up to 10 years before development of systemic symptoms. Sperry et al published an observational study of 98 patients where the flexor retinaculum in men ≥50 years and women ≥60 years
undergoing carpal tunnel release surgery was routinely sent for analysis with Congo red staining to determine the prevalence of amyloid.44 This detected amyloid deposits in 10.2% of patients (in this study due to a combination of ATTRv, ATTRwt and light chain amyloidosis). In a similar Japanese study, 34% of patients had amyloid deposition in the tenosynovial tissue sample.45 Similar studies looking at ligamentous tissue in patients undergoing surgery for lumbar spinal stenosis showed amyloid deposition in 88.4%13 and 96%46 of patients respectively. We suggest that routine analysis of surgical tissue would be an easy way to diagnose presymptomatic patients and therefore enable earlier treatment, improving outcomes.
Another consideration is of genetic testing of patients with family
members with ATTRv. Although the dominant genotype in the Irish population (p.T80A) shows incomplete penetrance, screening allows mutation carriers to be followed in order to identify early symptomatic disease and offer early treatment. A recent consensus paper suggests that adult firstdegree relatives of ATTRv patients may be offered genetic counselling and screening and, if positive, should start having neurological and cardiac assessments within 10 years of anticipated onset of symptoms.47 The frequency of such assessments has yet to be clarified.






























As mentioned, a Model of Care has been drafted by the HSE Amyloidosis Working Group34 which aims to improve standard of care for Irish patients with amyloidosis. It aims to reduce burden of disease by improved awareness and earlier diagnosis in order to prevent less end-
organ sequelae. It also aims to avoid patients having to travel abroad (usually to the UK National Amyloid Centre in London) as part of their diagnostic journey by creating a complex care centre or network in Ireland.
With the advent of diseasemodifying treatment, the outlook for patients with this hitherto progressive terminal disease is certainly more hopeful although many challenges remain. An active patient group has been set up (the All Ireland Amyloidosis Support Group), and the first all Ireland ATTR Conference, Emerging from the Shadows, was recently held in Gweedore, Co. Donegal, bringing together patients and their families, amyloidosis experts and other health care professionals interested in learning more about
References available on request
34 CPD 91: ATTR
the disease.
Symptoms, examination, NCS, MR Neurography suggestive of neuropathy due to amyloidosis Screen for presence of monoclonal protein Serum & urine protein electrophoresis Serum free light chains abnormal Refer to Haematology Biopsy of clinically involved organ +/ fat pad Amyloid staining confirmed Amyloid typing Yes No AL, TTR, other Amyloidosis unlikely Tc DPD available Tc DPD abnormal ATTR Genetic testing ATTRv ATTRwt ATTR neuropathy with cardiac phenotype unlikely Consider nerve & muscle biopsy if high suspicion Refer for Tc DPD / nerve & muscle biopsy / carpal tunnel biopsy Amyloid staining confirmed Amyloid typed normal Tc DPD negative or indeterminate yes AL ATTR AGelsolin AApoA no Genetic testing abnormal ATTRv abnormal normal AGelsolin AApoA Image 2 Neuropathy Algorithm
60 Second Summary
• Saint John of God Hospital Pharmacy, the HSE National Medication Safety Programme and the Irish Pharmacy Union have collaborated to launch a national patient information booklet on lithium therapy.
• This initiative is intended to promote and support safer lithium therapy, and empower patients to engage with their Healthcare Professional to discuss all aspects of lithium therapy, monitoring, and side-effects.
• The booklet has been produced by Audrey Purcell, Chief 2 Pharmacist, Saint John of God Hospital, and supported by Professor Dolores Keating, Chief 1 Pharmacist, and the Hospital Drug and Therapeutics Committee.

• It has been reviewed and endorsed by Ciara Kirke, HSE Clinical Lead, National Medication Safety Programme, and the national print supported by the HSE’s National Quality and Patient Safety Directorate.
• It has been edited and reviewed by the National Adult Literacy Agency and has successfully been awarded the plain English mark by NALA.
• It has been reviewed by the Irish Medication Safety Network, Irish Pharmacy Union and the College of Psychiatrists.
• The booklet contains an information section with important safety and clinical information on Lithium therapy, a programme of monitoring, and a record book to record essential information on lithium levels and blood test results.
• Lithium booklets will be distributed from the HSE directly to Hospital Pharmacies, and to Community Pharmacies via the IPU. GPs may sign-post patients to Community Pharmacists to avail of a booklet.
• The electronic template is available on the HSE website www. safermeds.ie and Saint John of God Hospital website https://www. stjohnofgodhospital.ie/images/ National_Lithium_Booklet_final_ version_September_2022.pdf
• Lithium booklets may be reordered from Saint John of God Hospital Pharmacy: hospital. pharmacy@sjog.ie


1. REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN If I have identified a knowledge gap - will this article satisfy those needs - or will more reading be required?
EVALUATE
Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
Lithium Therapy: a national patient safety and quality improvement initiative
31
AUTHORS: Audrey Purcell B(Sc) Pharm. MPSI. MSc. Psych Pharm. Chief 2 Pharmacist, Saint John of God Hospital, Stillorgan, Co Dublin. Honorary Senior Clinical Lecturer, Royal College of Surgeons in Ireland.
CPD 92: LITHIUM MANAGEMENT
5.
4.
Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the 4 previous steps, log and record your findings.
CPD Continuing Professional Development CPD Product: Formulation: Recommended dosing: Lithium level sampling time: Priadel Tablets: 200mg+400mg (200mg tablet may be halved to facilitate 100mg dose ) Lithium Carbonate. 400mg tablet contains 10.8mmol/L Lithium. Once daily: at night time (Prolonged Release) 12 hours post dose (acceptable 10 14 hours) Camcolit tablet EMP 400mg EMP: Exempt Medicinal Product Lithium Carbonate Once daily: at night time (Prolonged Release) 12 hours post dose (acceptable 10 14 hours) Priadel Liquid EMP 520mg/5ml Lithium Citrate 520mg/5ml : equivalent to Lithium Carbonate 204mg/5ml (calculate as 200mg/5ml) Twice daily: morning and night (Immediate Release) Pre morning dose Li Liquid EMP 509mg/5ml Lithium Citrate 509mg/ml: equivalent to Lithium Carbonate 200mg/5ml (calculate as 200mg/5ml) Twice daily: morning and night (Immediate Release) Pre morning dose 1. BACKGROUND AND CLINICAL INFORMATION: Lithium Indications: • Bipolar Disorder: mania, hypomania and prophylaxis of Bipolar Disorder • Recurrent Depressive Disorder: used to augment antidepressants • Reduction of intentional self-harm and suicidality. Lithium products Product: Formulation: Recommended dosing: Lithium level sampling time: Priadel Tablets: 200mg+400mg (200mg tablet may be halved to facilitate 100mg dose ) Lithium Carbonate. 400mg tablet contains 10.8mmol/L Lithium. Once daily: at night time (Prolonged Release) 12 hours post dose (acceptable 10 14 hours) Camcolit tablet EMP 400mg EMP: Exempt Medicinal Product Lithium Carbonate Once daily: at night time (Prolonged Release) 12 hours post dose (acceptable 10 14 hours) Priadel Liquid EMP 520mg/5ml Lithium Citrate 520mg/5ml : equivalent to Lithium Carbonate 204mg/5ml (calculate as 200mg/5ml) Twice daily: morning and night (Immediate Release) Pre morning dose Li-Liquid EMP 509mg/5ml Lithium Citrate 509mg/ml: equivalent to Lithium Carbonate 200mg/5ml (calculate as 200mg/5ml) Twice daily: morning and night (Immediate Release) Pre morning dose
Hypothyroidism: Lithium has varied effects on Thyroid Hormone production and regulation, including inhibition of Iodine uptake in the Thyroid, inhibition of Thyroid Hormone synthesis and release, and hepatic conversion of free Thyroxine. Patient may present with typical hypothyroidism symptoms including lethargy, impaired cognition, weight gain, dry skin, and cold intolerance. Risk factors include females, older adults, family history of hypothyroidism, and presence of anti-thyroid antibodies.

Switching lithium products:
Prescribing:
impairment or those below 50kg in weight, often require lower starting dose (eg 200mg), and maintenance doses.
• Significant change in patient’s sodium or fluid intake
• Last serum lithium level >0.8mmol/L
Plasma levels:
Priadel tablets are recommended for routine use. The tablets have score- lines therefore they can be divided accurately to provide smaller dosage requirements. If a patient is unable to swallow tablets a liquid may be prescribed. It is essential that a switch from tablets to liquid is prescribed by their Doctor, and calculation confirmed by Pharmacist.
Plasma levels:
Starting dose may usually range from 400mg 800mg OD (nocte) in adults, depending on indication. Elderly patients, those with renal impairment or those below 50kg in weight, often require lower starting dose (eg 200mg), and maintenance doses.
Lithium plasma level should be checked 5-7 days after starting, after every dose change, and after addition/discontinuation of medication that can affect level.
Target levels:
• Interacting medicines. Key interacting medicines include: ACEI, ARBs, NSAIDs and Thiazide diuretics. (See summary below and BNF/ Stockley’s for exhaustive list).
Discontinuation:
Lithium plasma level should be checked 5-7 days after starting, after every dose change, and after addition/discontinuation of medication that can affect level.
Target levels:
Example: Switching patient from Priadel tablet (Carbonate) 800mg nocte to Priadel liquid (Citrate) = 400mg (10ml) BD morning and night. Lithium level to be checked 5-7 days post- switch.
Hyperparathyroidism and hypercalcaemia: Hypercalcaemia has been reported with Lithium therapy, which may or may not be related to drug-induced hyperparathyroidism. While lithium has been observed to affect Parathyroid Hormone levels after a single dose, long -term exposure is likely required to observe clinically relevant alterations in Calcium homeostasis.
Baseline work-up:
• ECG : if cardiac history, risk factors for QTc prolongation, concomitant medicines that prolong QTc
The minimum effective plasma level for prophylaxis in adults is 0.4mmol/L; optimal range is 0.60.8 mmol/L. A level of 0.4mmol/L may be effective in unipolar depression; 0.6 -1 mmol/L in Bipolar Disorder, and levels at the higher end of the range in mania (0.8-1mmol/L).
If a decision is made to discontinue lithium, the risk of relapse may be reduced by reducing the dose gradually. It is recommended to reduce the dose slowly over at least 4 weeks or longer, and preferably up to 3 months in Bipolar Disorder; except in medical emergency or overdose.
Polydipsia and polyuria: Common adverse effects associated with lithium. Patients may notice increased urinary frequency (> 3 L in 24 hours) due to poor urine concentration; and increased thirst, which is independent of dry mouth effects of lithium.
The minimum effective plasma level for prophylaxis in adults is 0.4mmol/L; optimal range is 0.6 0.8 mmol/L. A level of 0.4mmol/L may be effective in unipolar depression; 0.6 1 mmol/L in Bipolar Disorder, and levels at the higher end of the range in mania (0.8 1mmol/L).
Monitoring frequency:
• Weight and height
• Urea and Electrolytes
• Serum Creatinine+/-estimated Glomerular Filtration Rate (eGFR)
• Adjusted Calcium
• Thyroid Function Tests (TFTs): include Free T4 and Thyroid Stimulating Hormone (TSH). Patient should be euthyroid before initiation
• Full Blood Count
• Pregnancy test and review of contraception (in women of childbearing age).
Prescribing:
Starting dose may usually range from 400mg-800mg OD (nocte) in adults, depending on indication. Elderly patients, those with renal
TFTs, renal function, Calcium level, and weight check, recommended every 6 months; or every 3 months in at-risk patients.
Once stable, serum lithium levels recommended every 3 months for the first year, then every 6 months; or every 3 months in at-risk patients.
At risk patients include:
• Elderly (> 65 years)
• Have received less than 12 months treatment
• Renal impairment (eGFR<60ml/min)
• Impaired Thyroid function at last test
• Raised Calcium level (adjusted) at last test
• Poor symptom control or suspected poor adherence
ALARM BELL INTERACTIONS: THINK “ANTS” (SEE TABLE PAGE 33)
ADVERSE EFFECTS : ( NOT AN EXHAUSTIVE LIST)
Cardiac: Lithium may cause cardiac arrhythmia, including bradycardia, sinoatrial dysfunction (SA block), abnormal T waves on ECG (T-wave inversion), and STsegment depression.
Dermatological: Lithium may cause acne vulgaris and/or psoriasis (including exacerbation of both) in patients with and without either condition at baseline.
GI: Lithium may cause dyspepsia, diarrhoea, nausea, vomiting, dysgeusia (metallic or salty taste), gastritis and abdominal pain. Some effects (e.g. nausea) may occur early in treatment. Other effects may take longer to develop. Supratherapeutic lithium levels should be suspected with severe nausea, vomiting and diarrhoea.
Renal effects: Up to one-third of patients may develop some degree of decreased kidney function during the course of lithium therapy, with approximately 5% developing significant kidney impairment/failure.
Sexual dysfunction: Studies report rates of the various effects of 5-40 %. Effects can include decreased libido, impaired sexual arousal, and erectile dysfunction. Sexual dysfunction can negatively impact a patient’s quality of life.
Tremor: Lithium can cause tremor in up to 25% of patients, making it one of the most common adverse effects. This is commonly a bilateral, symmetrical hand tremor, which may spontaneously decrease over time as compensatory mechanisms develop within the patient. Course tremor and muscle twitching may be observed in lithium toxicity.
Tremor commonly begins early in treatment, but can develop later in treatment, with or without a dose increase. Risk factors include: higher doses/serum levels, medicines that can increase
32 CPD 92: LITHIUM MANAGEMENT
euthyroid before initiation • Full Blood Count • Pregnancy
test and review of contraception (in women of childbearing age)
•
•
National launch of the Lithium booklet:
• Saint John of God Hospital, the HSE National Medication Safety Programme and the Irish Pharmacy Union have collaborated to implement a national launch of the booklet.
• Lithium booklets will be distributed from the HSE directly to Hospital Pharmacies, and to Community Pharmacies via the IPU Review. GPs may sign-post patients to Community Pharmacists to avail of a booklet.







• Booklets can be ordered from Saint John of God Hospital: hospital.pharmacy@sjog.ie
Acknowledgements:
Many thanks to Aoife Carolan, Senior Pharmacist, Saint John of God Hospital, and Ciara Kirke, HSE Clinical Lead, for article peer-review.
References:
1. Goodwin GM, Haddad PM, Ferrier IN, Aronson JK, Barness TRH, Cipriani A, et al. Evidence-based guidelines for treating Bipolar Disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016: 30(6); 495-553. Available online https://www.bap.org.uk/ pdfs/BAP_Guidelines-Bipolar.pdf (accessed 15/07/2022)
2. The National Institute for Health and Care Excellence (NICE) Guideline Bipolar Disorder: Assessment and Management (NICE 2014). Available online https://www. nice.org.uk/guidance/cg185 (accessed 15/07/2022)
3. Up To Date. Lithium: Drug Information. Available online: www.uptodate.com
4. Taylor D, Barnes T, Young A. The Maudsley Prescribing Guidelines in Psychiatry. 14th ed. West Sussex (England): Wiley Blackwell; 2021.
5. Bazire S. Psychotropic Drug Directory 2020/21. London: Lloyd-Reinhold Publications Limited; 2020.
6. Specialist Pharmacy Service NHS. July 2021. Lithium Monitoring. Available online https://www.sps.nhs.uk/monitorings/ lithium-monitoring/ (accessed 15/07/2022)
7. Specialist Pharmacy Service NHS. October 2021. Considering sodium content of medicines. Available online https://www. sps.nhs.uk/articles/considering-sodiumcontent-of-medicines/#:~:text=This%20 medicine%20contains%20less%20 than,say%20essentially%20 'sodium%2Dfree (accessed 15/07/2022)
8. Choice and Medication. Lithium Patient Information Leaflet. Available online https://www.choiceandmedication. org/stjohnofgodhospital/medication/ lithium-carbonate-and-citrate/ (accessed 15/07/2022)
34 CPD 92: LITHIUM MANAGEMENT
• A record book to record lithium levels and essential blood test results. Healthcare Professionals provide essential support and are recommended to:
Ensure patients have a lithium booklet
Reinforce essential information verbally
• Refer patient to the booklet to be aware of potential side-effects and signs of toxicity
• Ensure the patient understands their own programme of monitoring
There are two parts to this booklet: • an information section, and a record book. The Lithium Record book is on page 19. Page number 1. What is lithium and what is it used for? 3 2. What checks are needed before starting lithium? 4 3. How do I take lithium? 5 4. What blood tests do I have when I take lithium? 8 5. What side effects can lithium cause? 10 6. What happens if lithium level in my blood is too high? 13 7. What can make lithium blood levels too high? 14 8. Pregnancy and lithium: what do I need to know? 16 9. Alcohol and lithium: what do I need to know? 17 About this booklet Information section 2 13 6. What happens if lithium level in my blood is too high? If you have too much lithium in your blood, this is called lithium toxicity (or lithium poisoning). This can make you very ill.
the
list very carefully. If you get one or more of these problems at any time, talk to your doctor straight away. A small number of people may not have any immediate symptoms of toxicity when the lithium in their blood is too high. This is why it is important to have regular checks. Regular checks can prevent long-term problems. Symptoms if there is too much lithium in your system • Severe hand shake (tremor). Vomiting or severe nausea and persistent diarrhoea. • Muscle weakness. • Being unsteady on your feet. • Muscle twitches. • Slurring of words so that it is difficult for others to understand what you are saying. • Blurred vision.
Confusion. Feeling unusually sleepy. Date Daily Dose (mg): Lithium blood level (mmol/L) Kidney Checks (eGFR/ Creatinine) + Ref Range Thyroid checks TSH (mlU/L): + Ref Range Thyroid checks Free T4 (pmol/L): + Ref Range Adjusted Calcium (mmol/L): + Ref Range Weight/ BMI: Date of next blood test: Example 1: 2/3/22 Example 2: 2/3/22 600mg 600mg 0.8 0.8 eGFR 85 ✓ 2.59 (0.27-4.2) ✓ 13 (12-22) ✓ 2.25 (2.1-2.6) ✓ 67kg 67kg 6/6/22 6/6/22 1 2 3 4 5 6 7 8 19 Lithium therapy record book – your healthcare Produced by Audrey Purcell, Chief 2 Pharmacist, Saint John of God Hospital, Stillorgan, Co Dublin. Reviewed and endorsed by: all above Adapted with permission from the National Patient Safety Agency, UK. Version 2: February 2022. Safety Medication Irish Network Produced by Audrey Purcell Senior Pharmacist, and Colleagues, Saint John of God Hospital, Stillorgan, Co Dublin. Adapted with permission from the National Patient Safety Agency, UK. October 2013. To re-order please contact KPW Ballinasloe Tel: 090 9642297 Produced by Audrey Purcell, Chief 2 Pharmacist, Saint John of God Hospital, Stillorgan, Co Dublin. Reviewed and endorsed by: all above Adapted with permission from the National Patient Safety Agency, UK. Version 2: February 2022. Safety Medication Irish Network Produced by Audrey Purcell Senior Pharmacist, and Colleagues, Saint John of God Hospital, Stillorgan, Co Dublin. Adapted with permission from the National Patient Safety Agency, UK. October 2013. To re-order please contact KPW Ballinasloe Tel: 090 9642297 Saint John of God Hospital
• Support patients to engage in appropriate blood test monitoring: keep their record book up to date, and have available at consultations with GP, Consultant, Pharmacist, Nurse.
Read
following
•


J O I N O U R M A I L I N G L I S T I N P R I N T A N D O N L I N E , H O S P I T A L P R O F E S S I O N A L N E W S C O V E R S T H E L A T E S T N E W S , I N S I G H T A N D O P I N I O N T H A T S P E A K S T O H E A L T H C A R E P R O F E S S I O N A L S I N T H E I R I S H H O S P I T A L S E C T O R . C O N T A C T E M I L I A @ H O S P I T A L P R O F E S S I O N A L N E W S . I E H O S P I T A L P R O F E S S I O N A L N E W S
The first and only treatment indicated to reduce: • all-cause mortality • frequency of CV-related hospitalisations in patients with wild-type or hereditary ATTR-CM.1

ATTR-CM=transthyretin amyloid cardiomyopathy; CV=cardiovascular.
References: 1. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016.
2. VYNDAQEL Summary of Product Characteristics. Vyndaqelq 61 mg soft capsules (tafamidis) Prescribing Information: Before prescribing Vyndaqel please refer to the full Summary of Product Characteristics. Presentation: Vyndaqel 61 mg soft capsules. Each soft capsule contains 61 mg tafamidis. Uses: Vyndaqel is indicated for the treatment of wild-type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM). Dosage: Treatment should be initiated under the supervision of a physician knowledgeable in the management of patients with amyloidosis or cardiomyopathy. When there is a suspicion in patients presenting with specific medical history or signs of heart failure or cardiomyopathy, etiologic diagnosis must be done by a physician knowledgeable in the management of amyloidosis or cardiomyopathy to confirm ATTR-CM and exclude AL amyloidosis before starting Vyndaqel, using appropriate assessment tools such as: bone scintigraphy and blood/urine assessment, and/or histological assessment by biopsy, and transthyretin (TTR) genotyping to characterise as wild-type or hereditary. The recommended dose is one capsule of Vyndaqel 61 mg (tafamidis) orally once daily. Vyndaqel 61 mg (tafamidis) corresponds to 80 mg tafamidis meglumine, tafamidis and tafamidis meglumine are not interchangeable on a per mg basis. Vyndaqel should be started as early as possible in the disease course when the clinical benefit on disease progression could be more evident. Conversely, when amyoid-related cardiac damage is more advanced, such as in NYHA Class III, the decision to start or maintain treatment should be taken at the discretion of a physician knowledgeable in the management of patients with amyloidosis or cardiomyopathy. There are limited clinical data in patients with NYHA Class IV. If vomiting occurs after dosing, and the intact Vyndaqel capsule is identified, then an additional dose of Vyndaqel should be administered if possible. If no capsule is identified, then no additional dose is necessary, with resumption of dosing the next day as usual. There are no recommended dosage adjustments for elderly patients or patients with renal or mild and moderate hepatic impairment. Limited data are available in patients with severe renal impairment (creatinine clearance less than or equal to 30 mL/min). Tafamidis has not been studied in patients with severe hepatic impairment and caution is recommended. There is no relevant use of tafamidis in the paediatric population. Method of Administration: The soft capsules should be swallowed whole and not crushed or cut. Vyndaqel may be taken with or without food. Contra-indications: Hypersensitivity to the active substance or to any of the excipients as listed in section 6.1 of SPC. Warnings and Precautions: Contraceptive measures should be used by women of childbearing potential during treatment with tafamidis and for one month after stopping treatment. Tafamidis should be added to the standard of care for the treatment of patients with transthyretin amyloidosis. Physicians should monitor patients and continue to assess the need for other therapy, including the need for organ transplantation, as part of this standard of care. As there are no data available regarding the use of tafamidis in organ transplantation, tafamidis should be discontinued in patients who undergo organ transplantation. Increase in liver function tests and decrease in thyroxine may occur. This medicinal product contains no more than 44 mg sorbitol in each capsule. Sorbitol is a source of fructose. The additive effect of concomitantly administered products containing sorbitol (or fructose) and dietary intake of
Further information available upon request.
PP-VYN-IRL-0159. Date of Preparation: September 2022. © 2022 Pfizer Inc.
sorbitol (or fructose) should be taken into account. The content of sorbitol in medicinal products for oral use may affect the bioavailability of other medicinal products for oral use administered concomitantly. Pregnancy and Lactation: Tafamidis is not recommended during pregnancy and in women of childbearing potential not using contraception.Available data in animals have shown excretion of tafamidis in milk.A risk to the newborns/infants cannot be excluded. Vyndaqel should not be used during breastfeeding. Interactions: In a clinical study in healthy volunteers, 20 mg tafamidis meglumine did not induce or inhibit the cytochrome P450 enzyme CYP3A4. In vitro tafamidis inhibits the efflux transporter BCRP (breast cancer resistant protein) at the 61 mg/day tafamidis dose with IC50=1.16 μM and may cause drug-drug interactions at clinically relevant concentrations with substrates of this transporter (e.g. methotrexate, rosuvastatin, imatinib). In a clinical study in healthy participants, the exposure of the BCRP substrate rosuvastatin increased approximately 2-fold following multiple doses of Page 2 of 2 2020-0065522 61 mg tafamidis daily dosing. Likewise, tafamidis inhibits the uptake transporters OAT1 and OAT3 (organic anion transporters) with IC50=2.9 μM and IC50=2.36 μM,respectively,and may cause drug-drug interactions at clinically relevant concentrations with substrates of these transporters (e.g. non-steroidal anti-inflammatory drugs, bumetanide, furosemide, lamivudine, methotrexate, oseltamivir, tenofovir, ganciclovir, adefovir, cidofovir, zidovudine, zalcitabine). Based on in vitro data, the maximal predicted changes in AUC of OAT1 and OAT3 substrates were determined to be less than 1.25 for the tafamidis 61 mg dose, therefore, inhibition of OAT1 or OAT3 transporters by tafamidis is not expected to result in clinically significant interactions. No interaction studies have been performed evaluating the effect of other medicinal products on tafamidis. Undesirable Effects: The following adverse events were reported more often in 176 ATTR-CM patients treated with tafamidis meglumine 80 mg compared to placebo: flatulence [8 patients (4.5%) versus 3 patients (1.7%)] and liver function test increased [6 patients (3.4%) versus 2 patients (1.1%)]. A causal relationship has not been established. Safety data for tafamidis 61 mg are not available as this formulation was not evaluated in the double-blind, placebo-controlled, randomised phase 3 study. Legal category: S1A. Marketing Authorisation Numbers: EU/1/11/717/003– 61mg (30 capsules). Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at EUMEDINFO@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500. Last revised: 04/2021
q This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Ref: VY 61MG 2_0
Rare Disease
All rights reserved.
ATTR-CMIS LIFE-THREATENING1
for the treatment of
or
in
with
(ATTR-CM). 2 ORAL VYNDAQEL 61MG SOFT CAPSULES
Indicated
wild-type
hereditary transthyretin amyloidosis
adult patients
cardiomyopathy
CPD Continuing
Development CPD
60 Second Summary
Diagnoses of amyloidosis, particularly transthyretin amyloid cardiomyopathy (ATTR-CM), are steadily increasing throughout the world, but the condition remains underdiagnosed.


Patients with amyloidosis may present to a range of medical and surgical specialties, often with multisystemic disease, and a high index of clinical suspicion is required for diagnosis.
Bone scintigraphy and cardiovascular magnetic resonance (CMR) imaging offer highly sensitive and specific imaging modalities for cardiac amyloidosis.
Histological confirmation of amyloid deposition and amyloid type remains the cornerstone of diagnosis for most amyloid types, with transthyretin amyloid cardiomyopathy the exception, which may be diagnosed by validated nonbiopsy diagnostic criteria in the majority. Histological diagnosis of amyloid has been enhanced by laser capture microdissection and tandem mass spectrometry.
Early diagnosis and treatment prior to the development of end-organ damage remains essential to improving morbidity and mortality for patients with amyloidosis.
Diagnoses of amyloidosis, particularly ATTR-CM, are steadily increasing throughout the world, but the condition remains underdiagnosed. Bone scintigraphy and CMR offer highly sensitive and specific imaging modalities for cardiac amyloidosis, and a validated nonbiopsy diagnostic algorithm enables diagnosis in the absence of histology in a majority of patients with ATTR-CM. Histological diagnosis of amyloid has been enhanced by laser capture microdissection and tandem mass spectrometry. Early diagnosis prior to the development of endorgan damage remains crucial to improving morbidity and mortality for patients with amyloidosis.
AUTHORS: Steven Law1, MBBS, BSc and Julian D. Gilmore2, MD, PhD
1National Amyloidosis Centre, Division of Medicine, University College London, UK.
2National Amyloidosis Centre, Division of Medicine, University College London, UK. Electronic address: j.gillmore@ucl.ac.uk.
1. REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by HPN.
Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author. Pfizer has no editorial oversight of the CPD programmes included in these modules
When to Suspect and How to Approach a Diagnosis of Amyloidosis
Introduction
The amyloidoses are a spectrum of diseases with a common final pathological pathway of protein misfolding, insoluble extracellular fibril formation, disruption of tissue structure, and organ dysfunction. Presentation of systemic amyloidosis is heterogeneous with several organ systems potentially affected. Patients present to a range of medical and surgical specialties and a high index of suspicion is required for diagnosis. Following confirmation of amyloid deposition, determining the amyloid fibril type is crucial to guide treatment and inform prognosis. Treatment of many types of amyloid have improved substantially in recent years, although no treatments to accelerate removal of existing amyloid deposits are currently available, and advanced disease at diagnosis continues to carry a poor prognosis.
Reducing diagnostic delay and commencing treatment early is crucial to improving patient outcomes.
Pathology
The key pathological step in amyloid deposition is protein misfolding and aggregation, which leads to extracellular deposition of amyloid fibrils, disruption of local cellular structure, impaired organ function, and symptomatic
amyloidosis. More than 30 “amyloidogenic” proteins have been identified in humans.
The propensity for protein misfolding increases in the presence of abnormal protein structure (eg, abnormal fibrinogen Aα-chain protein in hereditary fibrinogen Aα-chain amyloidosis), excessive concentration of a structurally normal protein (eg, serum amyloid A [SAA] protein in systemic [AA] amyloidosis), or for unknown reasons associated with the aging process (eg, transthyretin [TTR] protein in wild-type transthyretin [ATTR] amyloidosis).
The amyloidogenic protein defines the amyloid type and clinical phenotype and informs diagnosis, treatment, and prognosis.
Light chain amyloidosis (AL), transthyretin amyloidosis (ATTR), and serum amyloid A amyloidosis (AA) are the most common types of amyloidosis.
Amyloid Types

Features of the most common amyloid types are outlined in Table 1, on page 32. Clinical phenotype is dictated by the amyloid type, although there remains variability within the same amyloid type; for example, systemic AL can present with cardiac failure due to deposition of amyloid in the myocardium or nephrotic syndrome due to deposition in the kidney, among other presentations.
Trends in the diagnosis of amyloidosis have changed significantly in recent years.
Systemic AL remains the most commonly diagnosed amyloid type, although there has been a rapid rise in diagnoses of ATTR.
This reflects increased awareness, advances in cardiac magnetic resonance (CMR) imaging and bone scintigraphy, and the widespread adoption of validated nonbiopsy diagnostic criteria.
Conversely, diagnoses of AA have fallen substantially following the widespread use of biologic therapy in the treatment of chronic inflammatory conditions.
AL may be localized or systemic. When AL type amyloid deposition is limited to 1 location in the absence of a systemic B-cell clonal disorder, the condition is termed “localized AL.” Common sites include the bladder, larynx, tonsil, skin, and lung (pulmonary nodules); treatment is with surgical resection if associated with troublesome symptoms, and prognosis is generally excellent.
In systemic AL, an underlying B-cell clonal dyscrasia produces circulating amyloidogenic monoclonal light chains that can deposit as amyloid in almost any tissue. Concomitant multiple myeloma may be confirmed, but often the clonal disorder is subtle and would be otherwise
31
CPD 93: AMYLOIDOSIS
Professional
Julian D. Gilmore
Amyloid type
AL amyloidosis
Fibrillary precursor protein Underlying cause Most common organs involved Treatment
Monoclonal immunoglobulin light chain B cell dyscrasia
Wild type ATTR amyloidosis Wild type transthyretin
Hereditary ATTR amyloidosis Variant transthyretin
Systemic AA amyloidosis
Kidneys, heart, liver, peripheral NS, autonomic NS, soft tissues, gastrointestinal system

Unknown, associated with aging Heart, soft tissues
TTR gene mutation
Serum amyloid A Chronic inflammatory conditions
Heart, peripheral NS, autonomic NS, soft tissues
Kidneys, liver, spleen, heart (<1%)
Chemotherapy and / or autologous stem cell transplantation
Table 1 Summary of the Fibrillary Precursor Protein, Underlying Cause, Clinical Phenotype, and Treatment of the Most Common Amyloid Types
TTR stabilizer
TTR stabilizers, gene silencing therapy
Management of underlying cause of inflammation
Amyloid type
Fibrillary precursor protein
Underlying cause
LECT2 amyloidosis LECT2 Unknown Kidneys, liver
Most common organs involved Treatment
Fibrinogen Aα chain amyloidosis Variant fibrinogen Fibrinogen gene mutation Kidneys, liver
AApoA1 amyloidosis Variant ApoA1
AApoA1 gene mutation Kidneys, liver, heart
Lysozyme Variant lysozyme Lysozyme gene mutation
Gelsolin amyloidosis Variant gelsolin Gelsolin gene mutation
Liver, kidneys, gastrointestinal tract, skin, lacrimal and salivary glands4
Peripheral NS, autonomic NS, cranial nerves, kidneys
Supportive
Supportive
Supportive
Supportive
Supportive
AA = serum amyloid A amyloidosis; AL = light chain amyloidosis; ATTR = transthyretin amyloidosis; LECT2 = leucocyte chemotactic factor 2; NS = nervous system.
considered a monoclonal gammopathy of unknown significance (MGUS). At diagnosis, cardiac and renal involvement are present in up to 70% and 60% of patients, respectively, whereas liver, gastrointestinal, soft tissue, peripheral, and autonomic nerve involvement can also occur.
ATTR occurs when the normal transthyretin tetramer dissociates into amyloidogenic monomers and may be hereditary (hATTR), associated with a TTR gene mutation predisposing to protein misfolding, or wild-type (wtATTR), in which an unmutated TTR gene produces TTR protein that misfolds for unknown reasons.
wtATTR mostly affects elderly males, presenting as a restrictive cardiomyopathy (wtATTR-CM) often with a history of carpal tunnel syndrome or spinal stenosis. hATTR commonly
presents with either neuropathy (ATTR-PN), cardiac failure (ATTR-CM), or both (ATTR-mixed) depending, in part, on the specific TTR mutation. The p.V142I TTR variant is most commonly associated with a dominant cardiac phenotype, whereas p.T80A is typically associated with a mixed phenotype. The p.V50M TTR variant typically causes ATTR-neuropathy when disease onset is younger than 50 years of age, and ATTR-mixed when older than 50 years.
hATTR has an autosomal dominant pattern of inheritance, but disease penetrance is incomplete.
Treatment and the Implication of Diagnostic Delays
The universal aim of treatment in systemic amyloidosis is to reduce ongoing amyloid formation and allow natural amyloid clearance.
At present there are no approved treatments to actively accelerate removal of existing amyloid deposits. Therefore, early diagnosis and commencement of therapy before advanced organ dysfunction occurs is essential. However, diagnostic delay remains common.
Patients have often visited several physicians by the time of diagnosis, and diagnostic delay is associated with more advanced disease at diagnosis.
Reasons for diagnostic delay are multifactorial and include disease rarity and phenotypic heterogeneity, lack of physician awareness, other disease mimicry, and until recently, a lack of sensitive noninvasive investigations.
Presentation and Red Flags
Patients with amyloidosis present to a range of specialties including cardiology, hematology,
nephrology, gastroenterology, neurology, orthopedics, and hand surgery, among others. The likelihood of systemic amyloidosis increases significantly in the presence of multisystemic dysfunction and active inquiry and investigation is required.
Certain populations are at risk of developing amyloidosis and benefit from active monitoring for suggestive signs and symptoms. Patients at risk of systemic AL include those with clonal disorders such as monoclonal gammopathy of uncertain significance, multiple myeloma, Waldenstrom macroglobulinemia, and chronic lymphocytic leukemia. Patients at risk of systemic AA include those with chronic inflammatory conditions such as inflammatory arthropathies, periodic fever syndromes, and inflammatory bowel disease, and either recurrent or chronic infections such as bronchiectasis and tuberculosis. Routine inquiry for symptoms and assessment of renal function, urine dipstick, liver function, and NT-proBNP improve the likelihood of diagnosing amyloidosis early. Patients with a family history of hATTR may be offered predictive genetic testing, and carriers should undergo age-appropriate work up depending on the amyloid type.
Cardiac amyloidosis is most commonly AL or ATTR type and typically presents with symptoms of heart failure or conduction abnormalities. Red flags for cardiac AL include rapid onset heart failure symptoms, systemic symptoms such as weight loss and fatigue, other organ system involvement, and presence of a clonal disorder. ATTR-CM typically has a more indolent heart failure
32 CPD 93: AMYLOIDOSIS
Table 1 Summary of the Fibrillary Precursor Protein, Underlying Cause, Clinical Phenotype, and Treatment of the Most Common Amyloid Types
Table 2 Noninvasive Investigation Options for Systemic Amyloidosis
System Investigation
findings
Table 2 Noninvasive Investigation Options for Systemic Amyloidosis
DPD scintigraphy
CMR
Biventricular hypertrophy, small left ventricular cavity, reduced global longitudinal strain with apical sparing, pericardial effusion, diastolic dysfunction
Cardiac uptake >99% sensitive for ATTR CM also present in ∼30% of cardiac AL amyloidosis21
Elevated T1, increased extracellular volume, late gadolinium enhancement in a subendocardial, diffuse, or transmural pattern22 24
Renal Creatinine and eGFR Frequently abnormal but may be normal
Urinary proteinuria
System Investigation
Peripheral NS Nerve conduction studies
Nephrotic range common in AL and AA amyloidosis. Often minimal in ALECT2 and hereditary amyloidoses. Not a feature of wtATTR amyloidosis.
Suggestive findings
Small fiber neuropathy in early disease, progressing to a large fiber axonal sensorimotor lower limb predominant neuropathy20. Carpal tunnel syndrome, especially in ATTR
Autonomic NS Postural blood pressure Postural blood pressure drop, especially in AL and hATTR
Liver Liver function tests
Raised GGT and alkaline phosphatase. Raised bilirubin in advanced disease Ultrasound abdomen Hepatomegaly and or splenomegaly
Special SAP scintigraphy
Identifies amyloid deposits in the liver, spleen, kidneys, adrenal glands and bones25 Does not provide information on the gastrointestinal tract, NS, or myocardium
Genotyping Consider in all cases of ATTR amyloidosis and in the presence of a suggestive family history
AA = serum amyloid A amyloidosis; AL = light chain amyloidosis; ATTR = transthyretin amyloidosis; ATTR CM = transthyretin amyloid cardiomyopathy; CMR = cardiac magnetic resonance imaging; DPD = 99mTechnetium labeled 3,3 diphosphono 1,2 propanodicarboxylic acid scintigraphy; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; GGT gamma glutamyl transferase; hATTR = hereditary ATTR; NS = nervous system; SAP = 123Iodine labeled serum amyloid P scintigraphy; wtATTR = wild type ATTR.
presentation than cardiac AL but should be considered in elderly Caucasian males or individuals of African ancestry particularly when there is a history of carpal tunnel syndrome, spinal stenosis, or tendon rupture. The most common hATTR-CM is associated with the p.V142I TTR variant, which affects individuals of African ancestry; the population prevalence of this particular variant is nearly 4%, although disease penetrance appears to be low. Other red flags for hATTR include peripheral or autonomic neuropathy, a family history of cardiac disease or
neuropathy, and Irish (p.T80A), Portuguese, Japanese, or Swedish (all p.V50M) ancestry.
Renal amyloidosis presents with proteinuria and or chronic kidney disease depending on the location of amyloid deposits within the kidney. New onset proteinuria in high-risk groups is a red flag for the development of amyloidosis.
Soft tissue amyloid deposition gives rise to nail dystrophy, easy bruising, carpal tunnel syndrome, macroglossia, shoulder infiltration, and periorbital bruising. Both macroglossia and periorbital
purpura are pathognomonic of systemic AL. Carpal tunnel syndrome occurs in both AL and ATTR, and in ATTR especially may predate diagnosis by many years.
Flexor retinaculum histology obtained at carpal tunnel decompression surgery may demonstrate amyloid deposition.
Peripheral neuropathy is present in up to 15% of patients with AL at diagnosis and is often the presenting feature in neuropathic forms of hATTR (eg, associated with p.V50M and p.T80A variants).
Visceral organ amyloidosis presents with nonspecific symptoms such as fatigue, weight loss, anorexia, and abdominal fullness; rarely, advanced liver amyloidosis can cause jaundice.
Approach to Diagnosis
The diagnosis of systemic amyloidosis requires a stepwise approach starting with a high index of suspicion.
A comprehensive history is crucial to identify extent of organ involvement and relevant underlying conditions. This is followed by basic investigations (Table 2) to support the initial clinical suspicion and assess organ function. Subsequent histological demonstration of amyloid deposits and the amyloid fibril protein are usually required to confirm the diagnosis, followed by investigations to identify underlying causes. ATTR-CM is the exception to the requirement for histological confirmation because the diagnosis can often be made using a validated nonbiopsy diagnostic algorithm (discussed below).
Cardiac Investigations


Serial serum NT-proBNP may be used to screen for the development of cardiac amyloidosis in at-risk groups, although they can be normal in early disease. Echocardiography offers a widely available noninvasive tool to screen for features of cardiac amyloidosis such as left ventricular hypertrophy of >12mm
(Figure A) Page 34; ventricular hypertrophy alone is of limited sensitivity and specificity, although suspicion increases
33
ECG Heart
atrial
poor
pseudoinfarction
Suggestive
Cardiac Cardiac biomarkers Persistently elevated serum NT proBNP and troponin
block,
arrhythmia, small QRS complexes,
R wave progression,
pattern Echocardiogram
in the absence of an alternative cause, and with concomitant small or normal QRS voltages on the electrocardiogram. More advanced echocardiographic measures such as global longitudinal strain offer greater specificity for diagnosing cardiac amyloidosis although are not widely acquired and are further limited by significant interoperator variability (Figures B and C). Cardiac uptake by 99mTechnetium labeled 3,3-diphosphono1,2-propanodicarboxylic acid (DPD; Figures E and F) or pyrophosphate (PYP) scintigraphy is >99% sensitive for cardiac ATTR amyloid deposition but only 86% specific due to cardiac uptake in a proportion of patients with cardiac AL.
Gadolinium-enhanced CMR is unique in allowing the characterization of myocardial tissue and is highly sensitive and specific for cardiac amyloidosis. Characteristic findings including raised native T1 signal, increased extracellular volume, and late gadolinium enhancement (Figures G, H, and I, respectively).
Cardiac AL and ATTR-CM cannot be distinguished by CMR or DPD scintigraphy alone, and further workup is essential to avoid incorrect diagnosis and allow early commencement of appropriate treatment.


Investigations of Underlying Disorder
Early assessment for a clonal disorder is essential to identify patients with systemic AL who may benefit from urgent chemotherapy. The combination of serum free light chain assay and serum and urine protein electrophoresis with immunofixation will identify the underlying clonal disease in up to 99% of patients with systemic AL; however, it is important to note that there may be an incidental clonal disease in association with ATTR-CM such that identification of amyloid and a clonal disease alone are insufficient to confirm a diagnosis of AL.
If AA is diagnosed on the basis of histology, focused history and investigations are required to identify the underlying cause of chronic inflammation, a necessary prerequisite for the diagnosis. Serial measurement of the acute phase protein SAA (or C-reactive protein as a surrogate) is required to determine the degree of ongoing inflammation and is used to monitor response to anti-inflammatory treatment. Outcome in AA is directly related to SAA concentration with sustained suppression of SAA after diagnosis to below <3 mg/L conferring the most favorable prognosis.
Nonbiopsy Diagnosis of ATTR-CM
A diagnosis of ATTR-CM can be made without histology if all of the following validated
nonbiopsy diagnostic criteria are met: heart failure, a suggestive or characteristic amyloid echocardiogram or CMR, a Perugini grade 2 or 3 DPD/PYP scan, a normal serum free light chain ratio, absence of a serum paraprotein by electrophoresis and immunofixation, and absence of urinary Bence Jones protein by urine immunofixation.
Diagnosis of ATTR-CM should be followed by sequencing of the TTR gene to distinguish between hATTR-CM and wtATTR-CM. A negative plasma cell dyscrasia workup using all 3 of the tests listed is essential to exclude cardiac AL, which is associated with cardiac uptake on DPD scintigraphy in approximately 30% of patients and a Perugini grade 2 or 3 DPD scan in up to 10% of cases.
Amyloid Confirmation and Typing
Histological identification and typing of amyloid deposits in an affected organ is the gold standard for amyloidosis. When a target organ biopsy is deemed high risk, screening biopsies such as a fat aspirate, bone marrow trephine, or gastrointestinal biopsy may identify amyloid with varying sensitivities.
Amyloid appears as an acellular, eosinophilic material on light microscopy, with randomly orientated nonbranching fibrils of approximately 10 nm in diameter on electron microscopy.
Amyloid deposition is confirmed by observing apple green birefringence following Congo red staining when viewed under cross polarized light. Amyloid type may be determined by immunohistochemical staining using a panel of antibodies, although sensitivity is limited with up to 30% cases showing no immune-specific staining.
Laser capture microdissection and tandem mass spectrometry of amyloidotic tissue is able to identify presence and type of amyloid in >95% of cases including >80% of those in which immunohistochemistry is indeterminate and requires only a small quantity of amyloidotic tissue.
Summary
Diagnoses of amyloidosis, particularly ATTR-CM, are steadily increasing throughout the world, but the condition remains underdiagnosed. Bone scintigraphy and CMR offer highly sensitive and specific imaging modalities for cardiac amyloidosis, and a validated nonbiopsy diagnostic algorithm enables diagnosis in the absence of histology in a majority of patients with ATTR-CM. Histological diagnosis of amyloid has been enhanced by laser capture microdissection and tandem mass spectrometry. Early diagnosis prior to the development of end-organ damage remains crucial to improving morbidity and mortality for patients with amyloidosis.
34 CPD 93: AMYLOIDOSIS
Figure A











Save the Date Saturday, 16th September, 2023 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication
The first and only treatment indicated to reduce: • all-cause mortality • frequency of CV-related hospitalisations in patients with wild-type or hereditary ATTR-CM.1

ATTR-CM=transthyretin amyloid cardiomyopathy; CV=cardiovascular.
References: 1. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016.
2. VYNDAQEL Summary of Product Characteristics. Vyndaqelq 61 mg soft capsules (tafamidis) Prescribing Information: Before prescribing Vyndaqel please refer to the full Summary of Product Characteristics. Presentation: Vyndaqel 61 mg soft capsules. Each soft capsule contains 61 mg tafamidis. Uses: Vyndaqel is indicated for the treatment of wild-type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM). Dosage: Treatment should be initiated under the supervision of a physician knowledgeable in the management of patients with amyloidosis or cardiomyopathy. When there is a suspicion in patients presenting with specific medical history or signs of heart failure or cardiomyopathy, etiologic diagnosis must be done by a physician knowledgeable in the management of amyloidosis or cardiomyopathy to confirm ATTR-CM and exclude AL amyloidosis before starting Vyndaqel, using appropriate assessment tools such as: bone scintigraphy and blood/urine assessment, and/or histological assessment by biopsy, and transthyretin (TTR) genotyping to characterise as wild-type or hereditary. The recommended dose is one capsule of Vyndaqel 61 mg (tafamidis) orally once daily. Vyndaqel 61 mg (tafamidis) corresponds to 80 mg tafamidis meglumine, tafamidis and tafamidis meglumine are not interchangeable on a per mg basis. Vyndaqel should be started as early as possible in the disease course when the clinical benefit on disease progression could be more evident. Conversely, when amyoid-related cardiac damage is more advanced, such as in NYHA Class III, the decision to start or maintain treatment should be taken at the discretion of a physician knowledgeable in the management of patients with amyloidosis or cardiomyopathy. There are limited clinical data in patients with NYHA Class IV. If vomiting occurs after dosing, and the intact Vyndaqel capsule is identified, then an additional dose of Vyndaqel should be administered if possible. If no capsule is identified, then no additional dose is necessary, with resumption of dosing the next day as usual. There are no recommended dosage adjustments for elderly patients or patients with renal or mild and moderate hepatic impairment. Limited data are available in patients with severe renal impairment (creatinine clearance less than or equal to 30 mL/min). Tafamidis has not been studied in patients with severe hepatic impairment and caution is recommended. There is no relevant use of tafamidis in the paediatric population. Method of Administration: The soft capsules should be swallowed whole and not crushed or cut. Vyndaqel may be taken with or without food. Contra-indications: Hypersensitivity to the active substance or to any of the excipients as listed in section 6.1 of SPC. Warnings and Precautions: Contraceptive measures should be used by women of childbearing potential during treatment with tafamidis and for one month after stopping treatment. Tafamidis should be added to the standard of care for the treatment of patients with transthyretin amyloidosis. Physicians should monitor patients and continue to assess the need for other therapy, including the need for organ transplantation, as part of this standard of care. As there are no data available regarding the use of tafamidis in organ transplantation, tafamidis should be discontinued in patients who undergo organ transplantation. Increase in liver function tests and decrease in thyroxine may occur. This medicinal product contains no more than 44 mg sorbitol in each capsule. Sorbitol is a source of fructose. The additive effect of concomitantly administered products containing sorbitol (or fructose) and dietary intake of
Further information available upon request.
PP-VYN-IRL-0159. Date of Preparation: September 2022. © 2022 Pfizer Inc.
sorbitol (or fructose) should be taken into account. The content of sorbitol in medicinal products for oral use may affect the bioavailability of other medicinal products for oral use administered concomitantly. Pregnancy and Lactation: Tafamidis is not recommended during pregnancy and in women of childbearing potential not using contraception.Available data in animals have shown excretion of tafamidis in milk.A risk to the newborns/infants cannot be excluded. Vyndaqel should not be used during breastfeeding. Interactions: In a clinical study in healthy volunteers, 20 mg tafamidis meglumine did not induce or inhibit the cytochrome P450 enzyme CYP3A4. In vitro tafamidis inhibits the efflux transporter BCRP (breast cancer resistant protein) at the 61 mg/day tafamidis dose with IC50=1.16 μM and may cause drug-drug interactions at clinically relevant concentrations with substrates of this transporter (e.g. methotrexate, rosuvastatin, imatinib). In a clinical study in healthy participants, the exposure of the BCRP substrate rosuvastatin increased approximately 2-fold following multiple doses of Page 2 of 2 2020-0065522 61 mg tafamidis daily dosing. Likewise, tafamidis inhibits the uptake transporters OAT1 and OAT3 (organic anion transporters) with IC50=2.9 μM and IC50=2.36 μM,respectively,and may cause drug-drug interactions at clinically relevant concentrations with substrates of these transporters (e.g. non-steroidal anti-inflammatory drugs, bumetanide, furosemide, lamivudine, methotrexate, oseltamivir, tenofovir, ganciclovir, adefovir, cidofovir, zidovudine, zalcitabine). Based on in vitro data, the maximal predicted changes in AUC of OAT1 and OAT3 substrates were determined to be less than 1.25 for the tafamidis 61 mg dose, therefore, inhibition of OAT1 or OAT3 transporters by tafamidis is not expected to result in clinically significant interactions. No interaction studies have been performed evaluating the effect of other medicinal products on tafamidis. Undesirable Effects: The following adverse events were reported more often in 176 ATTR-CM patients treated with tafamidis meglumine 80 mg compared to placebo: flatulence [8 patients (4.5%) versus 3 patients (1.7%)] and liver function test increased [6 patients (3.4%) versus 2 patients (1.1%)]. A causal relationship has not been established. Safety data for tafamidis 61 mg are not available as this formulation was not evaluated in the double-blind, placebo-controlled, randomised phase 3 study. Legal category: S1A. Marketing Authorisation Numbers: EU/1/11/717/003– 61mg (30 capsules). Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at EUMEDINFO@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500. Last revised: 04/2021
q This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Ref: VY 61MG 2_0
Rare Disease
All rights reserved.
ATTR-CMIS LIFE-THREATENING1
for the treatment of
or
in
with
(ATTR-CM). 2 ORAL VYNDAQEL 61MG SOFT CAPSULES
Indicated
wild-type
hereditary transthyretin amyloidosis
adult patients
cardiomyopathy
Continuing Professional Development
CPD
Written by
CPD

60 Second Summary
Atrial Fibrillation (AF) is the commonest cardiac arrhythmia affecting at least 3% of Irish adults over 60 and 6 % over 70. The worldwide prevalence has been rising and is approximately 600 in men and 375 in women per 100,000 population.
While much progress has been made in the last 10 years in particular with collaborative interdisciplinary working and research between cardiologists and stroke physicians, nevertheless there are many unanswered questions and dilemmas in AF.
AUTHORS: Professor Rónán Collins, consultant physician in geriatric and stroke medicine and Associate Clinical Professor in Gerontology Trinity College Dublin.

!! : A trial Fibrillation and Stroke
Professor Collins is Clinical lead National Stroke Programme, Member of the British and Irish Stroke Physicians (BIASP), Fellow of European Stroke Organsiation (FESO), Steering group member for EHRA-PATHS and member of the EHRA writing group for Practical guide to anticoagulation with NOACS in non-vavlulat atrial fribrillation.
1. REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area. 3. PLAN If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required? 4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs? 5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie
in our understanding of the pathophysiology of AF as a truly systemic condition and its association with risk of stroke and other illnesses. AF is associated with a 2 3.5 fold increase in mortality, impaired left ventricular dysfunction in 20 30% of cases, a hazard ratio of 1.6 for the development of dementia independent of previous stroke and associated with an impaired quality of life in almost 60% cases and a hospitalisation rate of 10 40% annually [3]
One in Three!! : Atrial Fibrillation and Stroke
While much progress has been made in the last 10 years in particular with collaborative interdisciplinary working and research between cardiologists and stroke physicians, nevertheless there are many unanswered questions and dilemmas in AF.
Screening for AF has huge potential benefits not least in preventing stroke, development of AF associated symptoms or preventing irreversible remodelling of the atrium and associated atrial or ventricular tachycardia associated cardiomyopathy.
In recent years international guidelines have correctly focused more on stroke prevention as the priority in the management of AF. In their “Atrial Fibrillation Better Care (ABC)” approach to AF the latest ESC guidelines on the management of AF stress the ‘A’ -to Avoid stroke / Anticoagulation as the prime issue of concern before addressing ‘B’ – better symptom control and ‘C’ optimisation of cardiovascular comorbidity and other risk factors.’
Table 1: Original Wilson and Jungner
Atrial Fibrillation (AF) is the commonest cardiac arrhythmia affecting at least 3% of Irish adults over 60 and 6 % over 70.1 The worldwide prevalence has been rising and is approximately 600 in men and 375 in women per 100,000 population.2 The lifetime risk of developing atrial fibrillation is almost 1 in 3 and it is estimated there may be as many as 18 million people with AF in the EU by 2060.


Principles for screening
other illnesses. AF is associated with a 2-3.5 fold increase in mortality, impaired left ventricular dysfunction in 20-30% of cases, a hazard ratio of 1.6 for the development of dementia independent of previous stroke and associated with an impaired quality of life in almost 60% cases and a hospitalisation rate of 10-40% annually.3
unanswered questions and dilemmas in AF.
Much attention has focused on the potential to screen for AF given it fulfils many of the original Wilson and Jungner criteria adopted by the WHO for a disease condition which would be amenable to a screening programme (see table 1.) and more specifically that the condition is common, detectable, associated with serious disease (stroke) which is preventable (with anticoagulation) [4].

Much attention has focused on the potential to screen for AF given it fulfils many of the original Wilson and Jungner criteria adopted by the WHO for a disease condition which would be amenable to a screening programme (see table 1.) and more specifically that the condition is common, detectable, associated with serious disease (stroke) which is preventable (with anticoagulation).4
Table 1: Original Wilson and Jungner Principles for screening
WILSON AND JUNGNER PRINCIPLES FOR SCREENING (1968)
• The condition sought should be an important health problem.
Both biomarkers and echocardiographic variable have been shown to independently predict risk of stroke and may well improve upon the accuracy of the CHA2DS2-VASC score. Secondary prevention for the AF stroke patient is of prime importance and applies to both stroke due to ischaemia and probably surprisingly also in cases of stroke due intracerebral haemorrhage.
ESC guidelines promote an integrated management to the AF patient and in their ‘circle of ideal integration’ list the importance of access to a many specialty model with the patient at the centre of decision making.
• There should be an accepted treatment for patients with recognized disease.
• Facilities for diagnosis and treatment should be available.
• There should be a recognizable latent or early symptomatic stage.
• There should be a suitable test or examination.
• The test should be acceptable to the population.
• The natural history of the condition, including development from latent to declared disease, should be adequately understood.
• There should be an agreed policy on whom to treat as patients.
• The cost of case finding (including diagnosis and treatment of patients diagnosed) should be economically balanced in relation to possible expenditure on medical care as a whole.
• Case finding should be a continuing process and not a ‘once and for all’ project.
43
CPD 94: ATRIAL FIBRILLATION
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author. Pfizer has no editorial oversight of the CPD programmes included in these modules ORAL V ORAL V ATTR CM=transthyretin amyloid cardiomyopathy Reference: 1 VYNDAQEL Summary of Product Characteristics Click here for SmPC and important safety information will allow sionals 4 8 of Indicated yp or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM) 1
One in Three
Atrial Fibrillation (AF) is the commonest cardiac arrhythmia affecting at least 3% of Irish adults over 60 and 6 % over 70 [1]. The worldwide prevalence has been rising and is approximately 600 in men and 375 in women per 100,000 population [2]. The lifetime risk of developing atrial fibrillation is almost 1 in 3 and it is estimated there may be as many as 18 million people with AF in the EU by 2060 Despite this very significant burden of disease we are still in a relative infancy
94 98% 76 95% Smartphone devices /watches 91 99% 84 100%
• Adapted from Hindricks G et al. European Heart Journal 2021;42(5):373 498
Adapted from Hindricks G et al. European Heart Journal 2021;42(5):373–498
Given those facts the national clinical programmes for stroke and chronic disease management in Ireland have introduced case finding as part of the primary care contract to opportunistically pulse check for AF in an older population. The advent of personal digital devices with the potential to detect irregularities of heart rhythm has expanded this potential to detect AF.
Stroke and AF
At present despite a growing body of evidence for use of digital devices, there is no evidence to suggest more strokes would be prevented adopting such strategies over opportunistic screening by pulse checking in high- risk groups. An overview of reported specificities and sensitivities of various detection approaches is outlined below in table 2. All suspected AF must be confirmed by a 12 lead ECG or a 30 second single lead strip read by a physician experienced in AF diagnosis.
Sum Zero game: The Low CHA2DS2-VASC patient
Stroke and AF
tool for risk assessment, there are concerns that it performs modestly and has relatively poor validity (C-statistic of 0.64 at best) and that the emphasis should perhaps be on a positive bias for anticoagulation than not. This author still regularly sees cases of stroke due to atrial fibrillation in people with seemingly low risk as indicated by a CHA2DS2-VASC score of 0 or 1 (for female sex) who are on aspirin or nothing rather than an anticoagulant and this approach is not necessarily at odds with current guidelines.
In recent years international guidelines have correctly focused more on stroke prevention as the priority in the management of AF [7]. In their “Atrial Fibrillation Better Care (ABC)” approach to AF the latest ESC guidelines on the management of AF stress the ‘A’ to Avoid stroke / Anticoagulation as the prime issue of concern before addressing ‘B’ – better symptom control and ‘C’ optimisation of cardiovascular comorbidity and other risk factors.’
A proof of concept study using apples i-phone and watch for example found a positive predictive value 0.84 when a notification was received of an irregular pulse.5 The recently published ‘Smartphonebased screening for atrial fibrillation: a pragmatic randomised clinical trial (eBrave)’ showed a doubling of detection of clinically relevant atrial fibrillation in a cohort of older people who owned a smartphone in both phases of the crossover design.6
disease, obstructive sleep apnoea etc. though these variables are often closely related to the CHA2DS2-VASC risk factors themselves and their inclusion does not seem to improve score predictability. It is unknown as to whether including factors such co-existent malignancy, smoking or obesity would improve reliability. Then as we age, we must recognise that the risk of stroke with AF is a continuum and likely to increase with the duration of AF as the atrium becomes increasingly dilated and dysfunctional with chronic fibrillation.
In addition, many more people now have implantable pacemaker and ICD devices capable of detecting AF if interrogated for same and there is increasing use of implantable recorders in patients with a high index of suspicion for AF. Many such devices pick up brief runs of AF or Atrial High-Rate Episodes (AHREs)that may suggest a risk of developing AF though it is not proven that anticoagulation in such cases would prevent more stroke.
Screening for AF has huge potential benefits not least in preventing stroke, development of AF associated symptoms or preventing irreversible remodelling of the atrium and associated atrial or ventricular tachycardia associated cardiomyopathy. It can also however create unnecessary anxiety, lead to over diagnosis and treatment with anticoagulation or even potentially lead to further invasive investigations that may have associated risks and be unnecessary.
In recent years international guidelines have correctly focused more on stroke prevention as the priority in the management of AF.7 In their “Atrial Fibrillation Better Care (ABC)” approach to AF the latest ESC guidelines on the management of AF stress the ‘A’ -to Avoid stroke / Anticoagulation as the prime issue of concern before addressing ‘B’ – better symptom control and ‘C’ optimisation of cardiovascular comorbidity and other risk factors.’
Sum Zero game: The Low CHA2DS2-VASC patient
While at higher scores the CHA2DS2-VASC performs modestly it does seem to perform more consistently when the a score is low and the incidence of stroke seems < 1% consistently in those with a score of 0 or 1 (for female sex). However, it is increasingly recognised that some populations with low CHA2DS2 VASC scores of 0 or 1 may have appreciably higher risk of stroke than previously thought, (e.g up to 2% risk per annum) and that this risk seems greater in studies of Asian populations and perhaps in other groups too.8,9
For all these reasons it is good practice that people with a low CHA2DS2-VASC score where there may be doubt about initiating anticoagulation, should be referred for a specialist opinion and a thorough assessment of risk.
While the CHA2DS2 VASC score has remained the standard tool for risk assessment, there are concerns that it performs modestly and has relatively poor validity (C statistic of 0.64 at best) and that the emphasis should perhaps be on a positive bias for anticoagulation than not. This author still regularly
This may include assessment of biomarkers such as D-Dimer, Troponin, NT ProBNP, IL-6 and CRP among others, many of which have been shown to independently improve upon the risk stratification of CHA2DS2-VASC alone.
There is no role for aspirin or clopidogrel therapy in the prevention of stroke in AF and indeed it may just increase risk of bleeding without benefit.
While the CHA2DS2-VASC score has remained the standard
A limitation of CHA2DS2-VASC is that it does not include other variables such as chronic kidney
Echocardiographic evaluation of the left atrium to look for dilatation, spontaneous contrast or thrombus in the left atrial appendage, low atrial appendage exit velocity
Suggested best Practice for the CHA2 DS2 VASC = 0 (or 1 for female sex) patient?
• ESC advises no anticoagulation may be reasonable in this population BUT to reassess regularly
• Risk of stroke is < 1% but it is not zero and catastrophic strokes do occur in this population

• Lifestyle advice, weight loss and BP control may reduce burden of Paroxysmal AF
• Seek specialist opinion from an AF service to further assess risk - specific echocardiography evaluation biomarkers e.g Troponin, Pro BNP, D Dimer, CRP IL 6 etc
• Discuss in full with the patient, explain and document indicative risk of stroke and risk of bleeding (HAS BLED score)
• Give your opinion on management when asked – you’re their doctor!
The AF patient with stroke
A third of our strokes in Ireland are associated with AF and AF is a strong predictor of recurrent events Secondary prevention for the AF stroke patient is of prime importance and applies to both stroke due to ischaemia and probably surprisingly also in cases of stroke due intracerebral haemorrhage.
Post stroke AF patient with cerebral infarction
Stroke recurrence is an early risk in AF. A significant decision is when to anticoagulate the AF stroke patient after a stroke due to infarction. This is generally a specialist decision made with the benefit of
44 CPD 94: ATRIAL FIBRILLATION
ORAL VYNDAQEL ORAL VYNDAQEL 61MG SOFT CAPSULES 61MG SOFT CAPSULES ATTR CM=transthyretin amyloid cardiomyopathy Reference: 1 VYNDAQEL Summary of Product Characteristics Click here for SmPC and important safety information This medicinal product is subject to additional monitoring This will allow quick identification of new safety information Healthcare professionals are asked to report any suspected adverse reactions See section 4 8 of the SmPC for how to report adverse reactions Indicated for the treatment of wild type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (
CM) 1 checking in high risk groups. An overview of reported specificities and sensitivities of various detection approaches is outlined below in table 2. All suspected AF must be confirmed by a 12 lead ECG or a 30 second single lead strip read by a physician experienced in AF diagnosis.
Reported
AF
sensitivity specificity Pulse taking 87
70
Automated BP monitors 93
86
Single lead ECG
Table 2 Reported reliabilities of methods of AF detection
ATTR-
Table 2
reliabilities of methods of
detection
97%
81%
100%
92%
and measurement of Total atrial conduction time (PA-TDI) may also be useful in individual risk stratification.10 MR imaging is another growing area of interest in assessing left atrial function.
Both biomarkers and echocardiographic variable have been shown to independently predict risk of stroke and may well improve upon the accuracy of the CHA2DS2-VASC score. A number of more complex risk scores such as Garfield-AF, Anticoagulation and Risk factors in Atrial Fibrillation (ATRIA) and ABC-stroke have been shown to modestly improve upon CHA2DS2-VASC though a pragmatic balance must also be struck between the time involved to use such tools in clinical practice and added expenses of additional measurements versus accruing benefit. At least one biomarker-based risk stratification tools is currently undergoing evaluation in a RCT (ABC-AF Study NCT 03753490).

While acknowledging the ESC guidelines it is important to remember that, as pointed out in the preamble, that a guideline does not robustly cover every situation where there is a paucity of evidence and is not a substitute for physician-based judgement of their individual patient. In discussing the uncertainty of apparent low risk of stroke with patients it is my personal practice to advise anticoagulation in patients with low CHA2DS2-VASC scores (i.e 0 or 1 for female sex alone) with elevated biomarkers or possibly ‘risky’ echocardiographic measurements. All other patients need to understand that the risk of stroke is not Zero, that the risk of stroke in AF is a continuum and unless there is a high risk of bleeding, that anticoagulation may be the best strategy especially where the risk of bleeding is low. Where a decision is made not to anti-coagulate in the CHA2DS2 VASC =0 or 1 (for female sex) patient, this should be reviewed regularly with reassessment of risk factors. Almost 15% of new AF cases will have one new non sex CHA2DS2-VASC risk factor detected at one year the majority of new comorbidities detected within 5 months after diagnosis.11
There is no role for aspirin or clopidogrel therapy in the
prevention of stroke in AF and indeed it may just increase risk of bleeding without benefit.
The AF patient with stroke
A third of our strokes in Ireland are associated with AF and AF is a strong predictor of recurrent events. Secondary prevention for the AF stroke patient is of prime importance and applies to both stroke due to ischaemia and probably surprisingly also in cases of stroke due intracerebral haemorrhage.
Post-stroke AF patient with cerebral infarction
Stroke recurrence is an early risk in AF. A significant decision is when to anticoagulate the AF stroke patient after a stroke due to infarction. This is generally a specialist decision made with the benefit of repeated neuroimaging and assessment of infarct size and the presence or absence of significant haemorrhagic transformation. A traditional role of thumb, largely dictated by concern for haemorrhagic transformation in the era of Warfarin, had been to start anticoagulation in the post-stroke AF patient on the same day -2 days for TIA small infarcts, 4-7 days for moderate sized infarcts and 10-14 days for large volume infarcts. This was always a judgement based
on expert consensus rather than expert science.
More recently the TIMING study published in Circulation,12 showed that it was safe to start anticoagulation with a NOAC in the AF patient with cerebral infarction < 5 days (early) compared to starting > 5 days (delayed). ‘Early’ initiation was not inferior to ‘Delayed’ initiation with a NOAC and though, perhaps not surprisingly, it was associated with less recurrent events numerically, it was not found to be superior. A caveat to the trial was the incidence of intracerebral haemorrhage was very low (only 3 cases and not symptomatic) compared to anticipated 3-4% incidence from previous studies in a warfarin era, and the median NIHSS was only 4 suggesting mainly milder and perhaps lower volume infractions, thought the latter was not detailed. A previous small study initiating rivaroxaban or warfarin within 5 days of infarction showed no difference in rates of recurrence or bleeding on neuroimaging at 4 weeks and the AREST study comparing early initiation with apixaban within 5 days versus delayed initiation with warfarin showed a 2.1 % incidence of haemorrhagic transformation in
the warfarin group versus non with apixaban.13,14
Three further RCTs (OPTIMAS NCT 03759938, ELAN NCT 03148457 and START NCT 03021928) aim to examine the issue of superiority of an early versus delayed strategy to initiation of anticoagulation with a NOAC in the post-stroke AF patient, but the tide is moving to earlier initiation of anticoagulation for AF patients post stroke due to infarction and with a NOAC as the safest option.
The Post stroke AF patient with intracerebral haemorrhage
It seems counter-intuitive and perhaps even dangerous to be considering anticoagulation in the AF patient who has suffered an intracerebral haemorrhage (ICH), but good longitudinal data shows that the AF patient who has suffered a stroke due to ICH does better in terms of recurrent stroke events (both ischemic and haemorrhagic) if started on anticoagulation than not.15,16 A number of caveats need to be stated here, the initiation needs careful risk assessment by a stroke specialist with repeated neuroimaging to ensure the volume of ICH is stable and resolving, that the patient does not have underlying cerebral

45
ORAL VYNDAQEL ORAL VYNDAQEL 61MG SOFT CAPSULES 61MG SOFT CAPSULES ATTR CM=transthyretin amyloid cardiomyopathy Reference: 1 VYNDAQEL Summary of Product Characteristics Click here for SmPC and important safety information This medicinal product is subject to additional monitoring This will allow quick identification of new safety information Healthcare professionals are asked to report any suspected adverse reactions See section 4 8 of the SmPC for how to report adverse reactions Indicated for the treatment of wild type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM) 1
Table 3 Modified EHRA symptom score for AF
Table 3 Modified EHRA symptom score for AF
mEHRA score symptoms description
1
No symtoms 2a Mild
Mild. Daily activity not affected and symptoms not troublesome to the patient 2b Moderate Moderate. Daily activity not affected but symptoms troublesome to the patient 3 Severe Normal daily activity affected 4 Disabling Normal daily activity discontinued
Adapted from Wynn GJ et al. EP Europace, 2014:16(7):965–972, https://doi.org/10.1093/europace/eut395
amyloid angiopathy and that underlying anatomical risks such as aneurysm or AV malformation have been adequately addressed and treated and that blood pressure control is good.
(e.g using the lower dose of dabigatran if on higher dose). In general, a period of 4-8 weeks has been suggested with repeated neuroimaging before restarting anticoagulation in the AF patient who has suffered stroke due to ICH.
The frequency of return assessments at an AF service is largely determined by patients’ symptoms and uncontrolled co morbidities such as hypertension which can increase risk of bleeding, or the presence of troublesome bleeding itself. In all other cases a good rule of thumb is to see the patient a month after initiation of oral anticoagulation and thereafter at monthly intervals suggested by the simple formula of [19]
If the ICH had occurred in the AF patient on an anticoagulant already, then it is important to undertake an assessment of pre-morbid drug adherence; review the time in therapeutic range (TTR) if on warfarin; ensure correct dose of NOAC adjusted for creatinine clearance, age and weight (where indicated); conduct a review of the full prescription for other drugs that increase haemorrhagic risk (e.g concomitant antiplatelet use which may no longer be needed).
Ongoing Management of Atrial Fibrillation


Interval of Routine follow up in months = creatinine clearance (Cockroft Gault) 10
in gerontology and it is intuitive that a properly constructed integrated model of care that includes cardiology, gerontology / strokeneurology , advanced nurse practitioners, clinical pharmacy and clinical nutrition would improve on patient education, medication adherence, better decision making re stroke prevention, AF symptom management, reduced drug errors or unnecessary prescription and reduced burden of AF and symptoms. It is important at each visit that the patients’ CHA2DS2-VASC, HAS-BLED, creatinne clearance as calculated by the Cockroft -Gault equation and the European Heart Rhythm Association (EHRA) Symptom Score (table 3).18
In such cases where the AF patient had been on an oral anticoagulant before the ICH, then consideration should be given to switching from warfarin to a NOAC in all cases (unless patient has true valvular AF* or a mechanical heart valve) as NOACS are associated with a 50% lower risk of intracranial haemorrhage and in vent of iCH outcomes are better on NOACS than Vitamin K antagonists.17 If the patient had been on a NOAC pre-ICH consider switching agent or lowering the dose of anticoagulant where appropriate
ESC guidelines promote an integrated management to the AF patient and in their ‘circle of ideal integration’ list the importance of access to a many speciality model with the patient at the centre of decision making. A criticism personally would be the very obvious glaring omission of geriatric medicine, given that AF is primarily a disease of later life and that many patients with AF have significant comorbidities, polypharmacy, frailty syndromes and may have already had a stroke, be suffering with cognitive impairment and pose some of the more difficult decisions when deciding to initiate anticoagulation and its subsequent management.
While the available research on models of integrated care for AF have had mixed results the components of such care have been heterogenous, none have included a specialist
The frequency of return assessments at an AF service is largely determined by patients’ symptoms and uncontrolled co-morbidities such as hypertension which can increase risk of bleeding, or the presence of troublesome bleeding itself. In all other cases a good rule of thumb is to see the patient a month after initiation of oral anticoagulation and thereafter at monthly intervals suggested by the simple formula of:19 Interval of Routine follow up in months = creatinine clearance (Cockroft Gault)
Many older patients with AF have significant co morbidities such as cancer, hypertension , dementia, polypharmacy or are trauma patients or awaiting surgery . Management of AF within this context can be challenging as oral anticoagulation can pose a risk . The EHRA PATHS project funded by Horizon 2020 aims to address the need for a more holistic approach to the older patient with AF through development of pathways of care that trigger both actions and KPIs for the domains of detected / suspected co morbidity [20]. It is planned to do a RCT on the new proposed pathways of care and you can read more about this important project in the management of AF at https://ehra paths.eu/
Many older patients with AF have significant co-morbidities such as cancer, hypertension , dementia, polypharmacy or are trauma patients or awaiting surgery . Management of AF within this context can be challenging as oral anticoagulation can pose a risk . The EHRA-PATHS project funded by Horizon 2020 aims to address the need for a more holistic approach to the older patient with AF through development of pathways of care that trigger both actions and KPIs for the domains of detected / suspected co-morbidity.20 It is planned to do a RCT on the new proposed pathways of care and you can read more about this important project in the management of AF at https://ehra-paths.eu/
References available on request
46 CPD 94: ATRIAL FIBRILLATION
ORAL VYNDAQEL ORAL VYNDAQEL 61MG SOFT CAPSULES 61MG SOFT CAPSULES ATTR CM=transthyretin amyloid cardiomyopathy Reference: 1 VYNDAQEL Summary of Product Characteristics Click here for SmPC and important safety information This medicinal product is subject to additional monitoring This will allow quick identification of new safety information Healthcare professionals are asked to report any suspected adverse reactions See section 4 8 of the SmPC for how to report adverse reactions Indicated for the treatment of wild type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM) 1
Adapted from Wynn GJ et al. EP Europace, 2014:16(7):965–972, https://doi.org/10.1093/europace/eut395 prevention, AF symptom management, reduced drug errors or unnecessary prescription and reduced burden of AF and symptoms. It is important at each visit that the patients’ CHA2DS2-VASC, HAS-BLED, creatinne clearance as calculated by the Cockroft Gault equation and the European Heart Rhythm Association (EHRA) Symptom Score (table 3) [18]


J O I N O U R M A I L I N G L I S T I N P R I N T A N D O N L I N E , H O S P I T A L P R O F E S S I O N A L N E W S C O V E R S T H E L A T E S T N E W S , I N S I G H T A N D O P I N I O N T H A T S P E A K S T O H E A L T H C A R E P R O F E S S I O N A L S I N T H E I R I S H H O S P I T A L S E C T O R . C O N T A C T E M I L I A @ H O S P I T A L P R O F E S S I O N A L N E W S . I E H O S P I T A L P R O F E S S I O N A L N E W S

HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication Aoife Jackson Events Lead and Custom Editorial Executive Email: aoife@ipn.ie Mobile: 00353 (87) 337 92 53



 Dr Albi Chalissery
Dr Albi Chalissery














 Consultant Dermatologist Tallaght University Hospital Clinical Associate Professor Trinity College Dublin Clinical Lead in Dermatology Health Service Executive
Consultant Dermatologist Tallaght University Hospital Clinical Associate Professor Trinity College Dublin Clinical Lead in Dermatology Health Service Executive












































































