SMOFlipid®
Soya-bean oil, medium-chain triglycerides, olive oil and fish oil1 For the supply of energy and essential fatty acids and omega-3 fatty acids to patients, as part of a parenteral nutrition regimen, when oral or enteral nutrition is impossible, insufficient or contra-indicated.
The links to clinically relevant outcomes
+ additional vitamin E (approx 200 mg α-tocopherol/L) to counteract lipid peroxidation and oxidative stress1
SMOFlipid – a 4-oil mix that provides energy and essential fatty acids, with a profile designed to support recovery.1 No other lipid emulsion brand has the same 4-oil mix and fatty acid profile as SMOFlipid.3
SMOFlipid ® – a mix that works
A meta-analysis in adult patients showed that parenteral nutrition containing omega-3 fatty acids demonstrated clinically relevant effects compared to standard parenteral nutrition.
Shorter length of ICU stay
(Mean stay length reduced by 1.95 days, 95% CI 0.42–3.49; p=0.01)
Shorter length of hospital stay
(Mean stay length reduced by 2.14 days, 95% CI 1.36–2.93; p<0.00001)
40% lower risk of infection
(131 vs 215 events; RR 0.60, 95% CI 0.49–0.72; p<0.00001) - Co-primary endpoint
56% lower sepsis rate
(24 vs 54 events; RR 0.44, 95% CI 0.28–0.70; p=0.0004)
Systematic review and meta-analysis of 49 randomised, controlled trials (3641 ICU or surgical patients. For the outcomes of: infection rate, 24 studies, n=2154, ARR = 8%; 30-day mortality, 20 studies, n=1839, ARR = 2%; sepsis, 9 studies, n=1141, ARR = 6%; LOHS, 26 studies, n-2182; ICU stay, 10 studies, n=822).2 non-ω-3 fatty acid enriched. ARR, absolute risk reduction; CI, confidence interval; ICU, intensive care unit; NS, not significant; PN, parenteral nutrition; RR, relative risk.
PRESCRIBING INFORMATION – SMOFlipid 200mg/ml emulsion for infusion Consult the Summary of Product Characteristics for full information. Additional information is available on request. Active ingredients 1000ml contains: Soya-bean oil (refined) 60g, Medium-chain triglycerides 60g, Olive oil (refined) 50g, Fish oil (rich in omega-3-acids) 30g. 1000ml emulsion contains up to 5 mmol sodium. Indications: Supply of energy and essential fatty acids and omega-3 fatty acids to patients, as part of a parenteral nutrition regimen, when oral or enteral nutrition is impossible, insufficient or contraindicated. Dosage and administration: Intravenous infusion into a peripheral or central vein. The dosage and infusion rate should be governed by the patient’s ability to eliminate fat. Adults – standard dose is 1.0–2.0g fat/kg body weight (bw)/day (5–10 ml/kg bw/day).
Recommended infusion rate is 0.125g fat/kg bw/hour and should not exceed 0.15g fat/kg bw/hour, corresponding to 0.75ml SMOFlipid/kg bw/hour.
Children – infusion rate should not exceed 0.15g fat/kg bw/hour. Increase daily dose gradually over the first week of administration. The maximum recommended daily dose is 3g fat/kg bw/day, corresponding to 15ml SMOFlipid/kg bw/day. Neonates and infants – initial dose should be 0.5–1.0g fat/kg bw/day followed by a successive increase of 0.5–1.0g fat/kg/bw/day up to 3.0g fat/kg bw/day (corresponding to 15ml SMOFlipid/kg bw/day).
The infusion rate should not exceed 0.125g fat/kg bw/hour. In premature and low birthweight neonates, infuse SMOFlipid continuously over about 24 hours. Administer as part of a complete parenteral nutrition treatment including amino acids and glucose. When used in neonates and children below 2 years, the solution (in bags and administration sets) should be protected from light exposure until administration is completed.
Contraindications: Hypersensitivity to fish-, egg-, soya- or peanut protein, or to any of the active substances or excipients, severe hyperlipidaemia, severe liver insufficiency, severe blood coagulation disorders, severe renal insufficiency without access to hemofiltration or dialysis, acute shock, general contraindications to infusion therapy, unstable conditions (see SmPC). Special warnings and precautions for use:
Monitor individual’s capacity to eliminate fat. Dose reduction or cessation of infusion should be considered if serum or plasma triglyceride
IN THIS ISSUE:
NEWS: Irish Heart Attack Audit National Report
Page 5
MEDICINES: Advancing Research on Deprescribing Medicines

Page 7
FEATURE: Update on Acute Coronary Syndromes
Page 16
WOMEN'S HEALTH: Testosterone Therapy in Menopause
Page 25
WOMEN'S HEALTH: Vulvovaginal Candidiasis
Page 34
concentrations during or after infusion exceed 3mmol/L. Use with caution in conditions of impaired lipid metabolism, in patients with marked risk for hyperlipidemia, in neonates and premature neonates with hyperbilirubinemia and/or pulmonary hypertension. Light exposure of solutions for intravenous parenteral nutrition, especially after admixture with trace elements and/or vitamins, may have adverse effects on clinical outcome in neonates, due to generation of peroxides and other degradation products. Contains soya-bean oil, fish oil and egg phospholipids which may rarely cause allergic reactions. Cross allergic reaction has been seen between soya-bean and peanut. Administration of medium-chain fatty acids alone can result in metabolic acidosis; simultaneous infusion of carbohydrate or a carbohydrate-containing amino acid solution is recommended. Laboratory tests generally associated with monitoring of intravenous nutrition should be checked regularly. Monitor blood platelet counts, liver function tests and serum triglycerides in neonates. Any sign or symptom of anaphylactic reaction should lead to immediate interruption of the infusion. High plasma lipid levels may interfere with some laboratory blood tests. Undesirable effects: Common – slight increase in body temperature. Uncommon – lack of appetite, nausea, vomiting, chills. Rare – hypotension, hypertension, dyspnoea, hypersensitivity reactions, heat or cold sensation, paleness, cyanosis, pain in the neck, back, bones, chest and loins. Very rare – priapism. Other adverse reactions can occur (including fat overload syndrome; should signs occur discontinue SMOFlipid), see SmPC for details.
ONCOLOGY: Laryngeal Cancer in Ireland
Page 45
Adverse events should be reported. Reporting forms and information can be found at: yellowcard.mhra.gov.uk www.hpra.ie/homepage/about-us/report-an-issue Adverse events should also be reported to Fresenius Kabi Limited, Cestrian Court, Eastgate Way, Manor Park, Runcorn, Cheshire, WA7 1NT Tel +44 (0)1928 533 533
STUDY: New Insights into Epilepsy
Page 78
HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HPN October 2023 Issue 113 HOSPITALPROFESSIONALNEWS.IE This Publication is for Healthcare Professionals Only
acid requirements Soybean oil covering essential fatty 30% 30% 25% 15% Medium-chain triglycerides (MCT) source of rapidly available energy Olive oil supply of monosaturated fatty acids Fish oil provides ω3-fatty acids especially EPA and DHA
© Fresenius Kabi reland. Job Code IE-SMOF-2300001. Date of preparation: August 2023 Fresenius Kabi Limited. Fresenius Kabi Ireland. Unit 3B Fingal Bay Business Park, Balbriggan, Co. Dublin, Ireland Phone: +353 (0)1 841 3030 Email: enquiries.Ireland@fresenius-kabi com
2
Legal Category POM. Marketing Authorisation Number: UK PL 08828/0166. IE – PA 2059/062/001 (Glass bottle), PA 2059/062/002 (Excel bag) Marketing Authorisation Holder: UK - Fresenius Kabi Limited, Cestrian Court, Eastgate Way, Manor Park, Runcorn, Cheshire WA7 1NT, UK. IE – Fresenius Kabi Deutschland GmbH, Else-Kroener Strasse 1, Bad Homburg v.d.h. 61352, Germany. Package Size and Cost: UK: 100ml £7.44, 250ml £11.90, 500ml £17.43. Further information: Available from Fresenius Kabi Limited, Cestrian Court, Eastgate Way, Manor Park, Runcorn, Cheshire, WA7 1NT. Tel +44 (0)1928 533 533. Date of preparation: October 2020 API/SMOF-01 2 References: SMOFlipid 200mg/ml emulsion for infusion Summary of Product Characteristics. Pradelli L, Mayer K, Klek S, et al. J Parenter Enteral Nutr. 2020 Jan; 44(1):44-57. doi:10/1002/jpen.1672 Anez-Bustillos L, et al. Review: Lipid formulations for the adult and pediatric patient: Understanding the differences. Nutrition in Clinical Practice. 2016;31(5):596–609. 1. 2. 3. * † † 30-day mortality rate (83 vs 101 events; RR 0.84, 95% CI 0.65–1.07; p=NS) - Co-primary endpoint
RINVOQ®▼
FOR ADULTS WITH MODERATE TO SEVERELY ACTIVE ULCERATIVE COLITIS1
RINVOQ® is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1

Full Summary of Product Characteristics is available at www.medicines.ie
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie. Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
AbbVie® is a registered trademark of AbbVie Inc. RINVOQ® and its design are registered trademarks of AbbVie Inc.
REFERENCES: 1. RINVOQ® summary of product characteristics, available at www.medicines.ie. Further information is available from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. Legal Classification: POM(S1A).
IE-RNQG-230034 | August 2023
Contents
¤2.7m Awarded to local Science Researchers P4
Learning from patient safety events P6
Enhanced Community Care Conference P8

European Guidelines on Acute Coronary Syndromes P10
World Hospice and Palliative Care Day P12
Prestigious grant for breast cancer research P14

An overview of Sezary Syndrome P60
REGULARS
Women’s Health: Ovarian Cancer P36
Women’s Health: Diabetes in Pregnancy P37
Women’s Health: Menopause P40
Feature: Heart Failure Care P74
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only. All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd


Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562
GROUP DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood
EDITORIAL
danielle@hospitalprofessionalnews.ie
ACCOUNTS
Fiona Bothwell cs.ipn@btconnect.com
Foreword
Editor
In one of our leading news stories this issue, Dr Frank Moriarty, Senior Lecturer at RCSI School of Pharmacy and Biomolecular Sciences, has received a Career Development Award from Wellcome to advance new methods to research deprescribing–the planned process of reducing or stopping medicines that may no longer be of benefit or may be causing harm.

The research funded in this award will harness the large amounts of information already collected as part of routine healthcare, such as GP and hospital visits. Dr Moriarty said “The support from this award will enable me to build a team to pave the way for high-quality deprescribing research and clinical practice. The evidence we hope to generate through innovative, datadriven approaches will improve the quality of healthcare to benefit population health.”
Turn to page 7 for the full story.
The European Society of Cardiology (ESC) has published new cardiology Guidelines on acute coronary syndromes (ASC). The expert-led taskforce delivering the Guidelines was chaired by Irish heart health expert, Professor Robert Byrne, Director of Cardiology at Mater Private Network and Professor of Cardiovascular Research at RCSI University of Medicine and Health Sciences.
ASC is a key focus area of the new Guidelines, which provide detailed advice on treatment and will be instrumental in paving the way for doctors across Europe as they seek to improve patient care in the field of heart health, and in particular, the management of unstable angina and other related conditions.
SALES EXECUTIVE
Avril Boyd
avril@hospitalprofessionalnews.ie
SALES & TRAINING MANAGER
Swan Mude s.mude@hospitalprofessionalnews.ie
CONTRIBUTORS
Theresa Lowry Lehnen
Dr Julie Ling
JJ Coughlan
Robert A Byrne
Dr Genevieve Ferraris
Laurette L. Bukasa
Mario Cortina-Borja
Helen Peters
Graham P. Taylor
Claire Thorne
Louise Delaney
Sarah Belton
Ciara Coveney
Dr Deirdre Lundy
Dr Kirk Levins DESIGN DIRECTOR

Ian Stoddart Design
This news is featured on page 10, whilst on page 16, we carry an in-depth clinical feature on these updates written by JJ Coughlan and Robert Byrne. “Early identification, diagnosis and risk stratification of ACS allows for appropriate management strategies to be employed. Patients with ACS should receive treatment with antiplatelet therapy and anticoagulation, undergo invasive assessment and revascularisation,” state the authors.
Our Special Focus for October is around women’s health. On page 24, Dr Genevieve Ferraris, GP and Menopause Specialist, The Menopause Hub looks at the use of testosterone therapy in menopausal women, and on page 34, Louise Delany, Antimicrobial Pharmacist with The National Maternity Hospital Dublin authors a clinical overview of vulvovaginal candidiasis –diagnosis and management.
I hope you enjoy the issue.
3 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023 October Issue Issue 113 4
Clinical R&D:
10 8 14 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITALPROFESSIONALNEWS.IE @HospitalProNews HospitalProfessionalNews
P79
Hospital Pharmacists Support Exemption in ATMPs
In July, EAHP co-signed a joint statement on the Hospital Exemption in Advanced Therapy Medicinal Products (ATMPs) development within the Revision of the EU general pharmaceutical legislation with the European Alliance for Vision Research and Ophthalmology (EUEYE) and the European Paediatric Translational Research Infrastructure (EPTRI). The signatory organisations jointly call on the European Commission to maintain a strong focus on the societal aim of health equity and to further support our researchers, clinicians, and hospital pharmacists in their various roles in the ATMP life cycle by:
• defining interests of all stakeholders for a sincere public dialogue and collaborative approach - patients, researchers/developers, healthcare professionals, industry, payers;
• creating a straightforward and affordable authorisation procedure for academic HE- ATMPs drawing from national experiences;
• addressing borderline classification issues to encourage innovation particularly in rare paediatric diseases;
• defining clearly the legal responsibility across the medical practitioners and the hospital management given the multiple factors involved in ATMP evaluation;
• adopting a comprehensive holistic action plan that recognises the key strategic role of multidisciplinary education and training at all levels (development, manufacturing, delivery) including public awareness of availability of treatments;
Critical Care Pharmacy
• fully assessing the impact of the proposed legislation including cost differences between commercial and academic/ non-profit settings; different intellectual property models; and reimbursement sources for HE-ATMPs;
• potentially decoupling cost of development from production according to product characteristics with fine-tuning of relevant regulations related to intellectual property, exclusivity rights, and licensing;
• allowing use of clinical data from observational studies for marketing authorisation;
• support the creation of an industry based on the model of Contract Manufacturing Organizations for selected ATMPs with cost sharing for Good Manufacturing Practice
and Good Laboratory Practice wherever appropriate.
Furthermore, the joint statement underlines that hospital pharmacists play a pivotal role in the handling of ATMPs as licensed medicines since they fall under their responsibility and their knowledge of pharmacoeconomics and clinical evaluations is essential in assessing the added value of an ATMP.
Also, harmonised education and training of healthcare professionals are important for coordinated practices of the hospital exemption including the development of European education and training materials with the integration of ATMP training in pharmacy and medicine schools. The collaboration of scientific societies involved across the entire ATMP spectrum is essential as well as of professional bodies offering continuing education programmes.
The response to the COVID-19 pandemic and the strain it put on critical care services internationally have highlighted the importance of having a competent and trained critical care clinical pharmacy workforce. The University College Hospital Critical Care Pharmacy Team offers a one-year international course in critical care pharmacy, now in its 4th year, called 'The Fundamentals of Critical Care Clinical Pharmacy'.
It comprises 12 monthly sessions on ICU themes, with recorded lectures and live interactive seminars, targeted reading and tasks to complete at one's base unit. The cost is £300 for pharmacists and £150 for pharmacy technicians. A certificate of attendance or competence is offered, the latter based on local sign-off on the completion of tasks/reviews.
The course is intended to:
• Upskill pharmacists who are new to critical care
• Pharmacists covering critical care on weekends/on-call/surge
• Pharmacists in other specialist areas, interested in learning more about critical care
• Pharmacy technicians currently working within critical care. Visit www.eahp.eu for enrolment details.
¤2.7m Awarded to local Science Researchers
University College Cork (UCC) researchers have been awarded ¤2.7m by the European Research Council (ERC) to lead major studies on gut bacteria and the post-war history both of colonialism and of veterancy around the world.
Dr Hilary Browne and Dr Dónal Hassett have been awarded ¤1.5m and ¤1.2m respectively from the ERC for their projects. Their ERC Starter Grants are awarded to early-career researchers, with 2-7 years of experience since completion of PhD, to lead innovative research.

Dr Hilary Browne is a Senior Staff Scientist in Dr. Trevor Lawley’s team at the Wellcome Sanger Institute based outside Cambridge in the UK and will be joining the School of Microbiology and APC Microbiome Ireland in UCC to lead his project
entitled SYNergize – Understanding spore-forming gut bacteria biology to target pathogens.
The project SYNergize will study how beneficial gut bacteria transmit between people. Transmission of bacteria in our gut microbiome is constantly occurring, initially from our mothers when we are born and then later in life with the people that surround us. Despite most gut bacteria dying rapidly when exposed to air, some can make dormant structures called spores that are oxygen tolerant and can re-form a new actively growing bacterial cell when they reach the gut. SYNergize will develop an understanding of these sporeforming gut bacteria as a tool to target harmful pathogens.
Dr Hilary Browne said, “I am excited to conduct my ERC
funded project alongside leading experts in gut microbiome research at UCC and APC Microbiome Ireland. This funding allows me to understand how spore-forming gut bacteria transmit between people and how this impacts their evolution and functions.”
Dr Dónal Hassett, Department of French, School of Languages,
Literatures and Cultures, has received funding for a project entitled COLVET – Ex-soldiers of Empire: Colonial Veterancy of the Interwar world.
4 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Dr Hilary Browne and Dr Dónal Hassett. Photo credit: Provision
Irish Heart Attack Report
Dr Ronan Margey, Clinical Lead of the Irish Heart Attack Audit (IHAA), has launched the Irish Heart Attack Audit National Report 2021 at the National Office of Clinical Audit (NOCA).
A heart attack is a life-threatening medical emergency where the blood supply to the heart is suddenly cut off, usually by a blood clot (thrombosis). In Europe, heart disease is the largest cause of death for both men and women.
Key findings:
• Only 44% of patients called 999/112 for help within 60 minutes of onset of symptoms
• 80% of patients brought directly to the primary percutaneous coronary intervention (PCI) centre by ambulance arrived within the recommended time frame of 90 minutes.
• 79% of patients with a STEMI received treatment with primary PCI.
• Timely primary PCI was higher in patients admitted directly by ambulance to a PCI centre (82%) compared to those transferred to a PCI centre from another hospital (41%)
• The unadjusted in-hospital mortality rate was 5.6%. Timely treatment was associated with reduced mortality (3.5% vs 5.1%).
Pauline O'Shea, Public and Patient Interest Representative

• 39% were smokers at the time of their heart attack, more than double the population rate of smoking (18%). Smokers present with a heart attack nine years earlier than people who have never smoked.
• 66% of eligible patients were referred to cardiac rehabilitation (target is 90%).
Pauline O’Shea, Public and Patient Interest Representative, remarked “As a heart attack patient, and as the Advocacy Campaign Manager at the Irish Heart Foundation representing cardiac patients, I hugely welcome this NOCA audit work, but have some real concerns in relation to some of the findings it reveals. Public education regarding (re)acting promptly to possible heart attack symptoms, the importance of ambulance prehospital ECG diagnosis of STEMI heart attack, the significance of direct ambulance transfer for
Primary PCI and the importance of the timeliness of that treatment, through to outcomes for patients, could literally be costing patients’ lives and affecting survivors' longterm quality of life.”
The report makes 4 key recommendations:
• Develop a public awareness campaign to encourage people with heart attack symptoms to call 112 or 999 immediately for emergency help in order to facilitate pre-hospital
electrocardiogram (ECG) diagnosis of a STEMI.
• There should be a national and regional focus on quality improvement in the STEMI care pathway
• Improve public awareness of the adverse impact of smoking on heart attack risk.
• Support patients with STEMI to reduce the risk of further heart attack by increasing the rate of referral to cardiac rehabilitation phase 3.
Optimising and Progressing Internal Medicine
The Institute of Medicine at the Royal College of Physicians of Ireland has commissioned a review of the Internal Medicine training programme. The review sets out to ensure the training programme is fit-for-purpose and graduates are being provided with the skills and expertise required to meet the needs of patients and society into the future. The review has been titled ‘OPTIMISE’ (Optimisation and Progression of Training in Internal Medicine – In Search of Excellence), and work has been carried out under the leadership of Prof Anthony O’Connor as Clinical Lead.
General internal medicine (GIM) is the core business of Irish hospitals, accounting for approximately
two-thirds of hospital bed days. The preservation of general medical skills in an era of increased specialisation is critical to maintaining standards of care in Ireland, particularly in managing multimorbidity, frailty, and polypharmacy in our growing, ageing population.
Pressures on GIM manifest across the system as longer waiting times in emergency departments, increased numbers of patients placed in beds not suited to their specific needs, decreased efficiency in the discharge process, increased errors, and greater stress within the workplace.
The level of generalist skills in internal medicine is declining,
with some regions prioritising the development of internists or hospitalists. The RCPI is committed to delivering a more structured training programme across BST and HST to better support physicians. A key aim of the RCPI’s Institute of Medicine (IOM), since it was established in 2020, has been to improve the governance and integration of general internal medicine training, and to ensure that training is reviewed, optimised, and benchmarked to international standards, graduating exceptional general physicians of the future.
A formal interim report to detail the findings of our review and set out recommendations for an improved training programme in general
internal medicine is now available. Planning to operationalise and implement the recommendations by 2025 is currently underway.
Five Key Pillars
There are five key pillars to the interim report:
• A New Approach for the Practice of the 2020s and beyond
• A New Approach for the Patients of the 2020s and beyond
• Providing the Resources to Train
• A new paradigm for the TrainerTrainee relationship
• Championing Generalism
5 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
News
Learning from Patient Safety Events
Educational videos of facilitated debriefs following patient safety events, co-designed and developed by RCSI with the HSE National Quality and Patient Safety Directorate, have been published in BMJ Open Quality.
The access article, led by Dr Siobhán McCarthy, Lecturer at the Graduate School of Healthcare Management, RCSI University of Medicine and Health Sciences, provides video resources designed to educate healthcare staff about a methodology known as After Action Review (AAR).

After Action Review is a nonhierarchical facilitated debrief of an event where groups discuss: what did we expect to happen;
what actually happened; why was there a difference; and what have we learnt?
The publication includes access to the online videos of simulated AARs and an associated case scenario of a patient safety event. These have the potential to be used widely to support social and nearto-real-time learning from patient safety and everyday events.
Awareness and skills
Since 2018, the RCSI Graduate School of Healthcare Management has trained up to 500 healthcare staff as AAR facilitators across the Irish health services, using an in-person simulation-based programme
co-designed and funded by the HSE National Quality and Patient Safety Directorate (NQPSD).
Subsequently, RCSI and the HSE formed a research collaboration to assess the implementation and effect of AAR at a hospital site.
Arising from the collaboration, RCSI and the HSE have released a series of videos of simulated AARs to help spread and sustain AAR awareness and facilitation skills. The videos demonstrate the purposes of an AAR, the AAR process, and the skills required to facilitate an AAR effectively.
“By using digital technology and a university-health service partnership approach, we hope that this open-access educational resource will enhance awareness of AAR as a learning tool and of the skills required to facilitate
Consultants Comment on MHC Reports
The Irish Hospital Consultants Association (IHCA) has commented on the publication by the Mental Health Commission of individual reports on the Child and Adolescent Mental Health Services (CAMHS) in the nine Community Healthcare Organisations (CHO)
A spokesperson stated, “The reports published today underline once more the scale and severity of the deficits faced day-today in our child and adolescent mental health services. Details of individual cases and what those involved and their families have to experience are all too familiar to those of us working in this area.
“Regrettably, for consultants working in these services across the country, the capacity deficits in each region have been highlighted by us numerous times. We are trying to provide care in very complex and constrained conditions for young people who
need care for urgent and severe mental health crises. Delivery of timely assessment, care and follow-on monitoring is critical but incredibly challenging in an under resourced service.
“Time and time again, Consultants and others in the service have raised the serious concerns about staffing and capacity shortages and highlighted the impact this has on young people’s mental health and their ongoing care needs. Lessons are not being learned. There is no shortage of reports but very little by way of meaningful action.
“Meanwhile, the growing deficits are stark. Currently CAMHS funding is approximately 0.63% of the overall Health Budget, at just ¤125.18m.
“CAMHS teams are significantly below the recommended staffing levels, some below 50% of
recommended levels. Some of these services are missing a third of the required Psychiatry Consultants, as these permanent posts remain vacant or only filled on a temporary, agency, or locum basis.
There are only 51 CAMHS inpatient beds operational across the country at any one time, falling well short of the 130 beds recommended in the Government’s ‘Vision for Change’ strategy.
The very fact that our benchmarks for staffing and bed levels are rooted in a 17-year-old mental health policy, also points to a lack of priority given to addressing this issue over the past decade and more.
“The current approach is simply not good enough and is failing our youngest and most vulnerable
AARs,” said Dr Siobhán McCarthy. “Effective AAR facilitation skills are important to support staff participating in AARs and to help identify learning and improvement actions.”
Suite of options
Lorraine Schwanberg, Assistant National Director for Incident Management at the HSE NQPSD, emphasised that “AAR forms part of a suite of incident response options as part of the HSE Incident Management Framework and we look forward to using this resource as part of the development of AAR across the health services”. The videos were developed with funding from the Health Research Board Applied Partnership Award (APA-2019024) and includes co-funding from the Health Services Executive.
patients. Our priority must be ensuring all our young people have the best chance of a good quality of life, whether they require lifelong treatment to make that happen or not – this should be the goal of our national health policy.
“There are many strands that have to come together, involving all pillars of the health system –GPs, Consultants, Allied Health Professionals - to ensure these highly vulnerable patients are cared for as required.
“Decision-makers need to use their leadership and resources to drive such a collaborative, wholeof-service approach to ensuring an end-to-end care pathway under the oversight of dedicated clinical leadership for CAMHS. Failure to do so will simply mean similarly stark reports into the future.”
6 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
“By using digital technology and a university-health service partnership approach, we hope that this openaccess educational resource will enhance awareness of AAR as a learning tool and of the skills required to facilitate AARs”
Dr Siobhán McCarthy, Lecturer at the Graduate School of Healthcare Management, RCSI University of Medicine and Health Sciences
¤2.4m to advance research on deprescribing medicines

Dr Frank Moriarty, Senior Lecturer at RCSI School of Pharmacy and Biomolecular Sciences, has received a ¤2.4 million Career Development Award from Wellcome to advance new methods to research deprescribing– the planned process of reducing or stopping medicines that may no longer be of benefit or may be causing harm.
Improvements in healthcare mean people are living longer, and as they get older, people are often prescribed increasing numbers of medicines to prevent and manage disease. This increase in the number of medicines can lead to higher risk of medicine-related adverse effects. It is therefore important to develop robust approaches to identify medicines that might no longer be needed or could be contributing to medicinerelated harm that can be safely stopped or deprescribed.
The research funded in this award will harness the large amounts of information already collected as part of routine healthcare, such as GP and hospital visits. New methods from pharmacoepidemiology will be used to analyse these datasets, to improve our understanding of deprescribing practices. As the
focus of healthcare shifts to more personalised medicine and patientcentered approaches, research in this area will inform the decisions of patients and their healthcare professionals and support optimal treatment. Ultimately, this will help people age better with the right medicines for them.
In addition, as part of the project, which will be known as DIAMOND (Developing Innovative Analytical Methods for research ON Deprescribing), a tool will be developed to identify patients most at risk of side effects from antidepressant medicines. Given people can respond very differently to these medicines, this will help support the monitoring and review of antidepressants to promote the best outcomes for patients with mental health conditions.
Commenting on the announcement, Dr Moriarty said “The support from this award will enable me to build a team to pave the way for high-quality deprescribing research and clinical practice. The evidence we hope to generate through innovative, datadriven approaches will improve the quality of healthcare to benefit population health. We are embedding open science in this project, by sharing our methods
and tools for other researchers to use in future studies and maximise our impact.”
The grant award will run over 8 years, starting next year. As well as supporting research efforts and access to datasets, it will also facilitate the recruitment, training and development of new researchers.
“I would like to congratulate Dr Moriarty on this prestigious funding and acknowledge the support of Wellcome for awarding RCSI our first Career Development Award. This is an important milestone which recognises our dedication to advancing cuttingedge research and fostering talented scientists” said Professor Fergal O’Brien, Deputy Vice Chancellor for Research and Innovation at RCSI.
“I look forward to seeing how this project will apply big data to a space where it has the exciting potential not only to improve medical practices but also to positively impact the lives of patients.”
Wellcome’s Career Development Awards are highly competitive grants supporting mid-career researchers' career progression in biomedical science, health,
and related fields. This award further enriches the RCSI research landscape, signifying the university’s continued commitment to excellence in scientific discovery and complementing previous successes in other Wellcome grant programmes.
On this project, RCSI will collaborate with researchers from University College Cork, University College London, Queen’s University Belfast, the University of British Columbia and Complutense University of Madrid.
Dr Sophie Hawkesworth, Senior Research Manager in Discovery Research at Wellcome commented: “I’m delighted that Wellcome are supporting this exciting award in such an important research area. Our Discovery Research schemes are designed to enable a really broad range of research questions that have the potential to transform our understanding of health and disease.
This award is a great example of the research we are aiming to support that will bring new knowledge and new research tools to a really important and under-studied area. I will be following the progress of the project with interest.”
7 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
News
Transforming healthcare for All
• GPs reviewed 309,778 patients, from January –July 2023, as part of the Chronic Disease Management Programme.
• Community Diagnostics Teams provided 196,988 radiology scans, between Jan –July. It is expected the total scans conducted will surpass the 253,172 conducted in 2022. This is extremely important as timely access to diagnostics meant an an 89% reduction in the number of patients requiring referral to emergency departments or acute medical units.
• In the first seven months of 2023, there were 48,230 patient contacts by the teams supporting older people - and 64% of their patients were discharged home, avoiding a potential hospital admission.
“We will continue to press ahead with the full implementation of this ¤240m programme which underscores our commitment to placing patients at the heart of healthcare; providing care closer to home, while simultaneously alleviating pressure on our hospitals."
The second national HSE Enhanced Community Care Conference (ECC) was held recently, and outlined how hospital attendances and admissions are down as a result of the ECC Programme.

As part of Sláintecare, ECC emphasises general practice, primary care, and communitybased services to provide care closer to home, reducing acute hospital attendance and admission. The work of the ECCs also plays a pivotal role in the Urgent and Emergency Care Plan 2023, as part of key hospitalavoidance measures.
Noel Ridge, a patient at the Galway Integrated Care Hub, where services are provided in an integrated fashion between GPs, practice nurses, community specialist teams and hospital specialist teams, highlighted how the hub had benefitted him: "I've had three heart attacks and been in the hospital nine times in the last 18 months. But I've come through it. The support I receive at the hub provides great comfort because I'm not waiting for hospital appointments that are months or a year apart.
“If I don't feel well, I can call the hub right away and explain what's happening. Overall, visiting the hub
is much more convenient as it is closer to home and appointments are scheduled at specific times, which reduces a lot of stress."
At the conference, held in Dublin Castle, healthcare professionals, policymakers, and stakeholders reflected on the significant progress made. The conference highlighted how service users are moving through the ECC’s integrated pathways of care, resulting in improved outcomes for people, and demonstrating the transformative potential of integrated, community-based healthcare in Ireland.
• 91% of patients with chronic disease are, routinely, fully managed in primary care via the Chronic Disease Management in General Practice programme.
• The investment of clinical frontline staff in the ECC Programme is unprecedented. Over 78% (2,733.2 WTE) of the planned whole time equivalent (full-time) staff have now been secured.
The following roles are onboarded or at an advanced stage of recruitment; 96 CHN Managers and Assistant Directors of Public Health Nursing (ADPHNs); 73 of the 96 General Practitioner (GP) Leads; 30 of the 30 Operational Leads for ICPOP and 27 of the 30 Operational Leads for ICPCD, 61.5 of 80.5 Consultants for ICPOP and ICPCD.
Opening the conference, Minister for Health, Stephen Donnelly TD, said: “The Enhanced Community Care Programme represents our unwavering dedication to providing exceptional healthcare to our communities.
“This crucial part of the Sláintecare Programme is a valuable resource providing both short-term and long-term benefits to the reform of healthcare delivery.
“The ECC programme has played, and will continue to play, an important role in ongoing planning for the Health Service, particularly through the development of alternative pathways, including ECC support for those at risk of hospital admission, expansion of GP out-of-hours service, and the implementation of pathways to support patient discharge.
Speaking at the conference, Bernard Gloster, CEO of the Health Service Executive said: “The ECC Programme continues to demonstrate significant progress in its implementation and daily operations. The core ethos of this ¤240m Programme revolves around delivering timely and effective treatments, while minimising unnecessary referrals and hospital admissions through a 'home first' approach, where safety and appropriateness are paramount. The substantial investment commitment by the Government - as well as the commitment shown by the HSE community to implement the ECC Programme - is now beginning to yield tangible results. The ECC Programme is redefining healthcare delivery, ensuring proximity to the communities we serve."
Keynote speaker at the event, Professor David Colin Thomé OBE, said: “In Ireland and the UK, we stand at a healthcare crossroads, grappling with evolving landscapes. With Ireland’s aging population and increasing chronic conditions, integrated care delivered closer to home becomes pivotal. Sláintecare's ambition for a universal health service aligned with community care is impressive.
“The Enhanced Community Care Programme's goal is bold and well-defined. By shifting focus to general practice, primary care and community-based services, the approach paves the way for comprehensive care pathways, care closer to people’s homes and a reduction in unnecessary hospital admissions. Moreover, as the programme matures, Ireland's distinctive structure empowers community and primary care to drive broader health transformation, potentially reshaping services, and alleviating burdens across the system.”
See www.hse.ie/ECC for more information and to read patient case studies on how ECC has improved their health. Follow the ECC Conference on social @ HSELive #ECCConference23.
8 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Paul Reid, CEO, HSE
“We will continue to press ahead with the full implementation of this ¤240m programme which underscores our commitment to placing patients at the heart of healthcare; providing care closer to home, while simultaneously alleviating pressure on our hospitals.”
Phenylephrine Hydrochloride 10mg/ml Solution for Injection/Infusion
Therapeutic Indication
For the treatment of hypotensive states during spinal anaesthesia or drug-induced hypotension.
Posology and Method of Administration
Subcutaneous and intramuscular injection: Phenylephrine injection may be administered subcutaneously or intramuscularly in a dosage of 2 to 5mg with further doses of 1 to 10mg if necessary according to response.
Intravenous bolus injection: Phenylephrine hydrochloride injection may be administered in a dose of 100 to 500 micrograms by slow intravenous injection as a 0.1% w/v solution, repeated as necessary after at least 15 minutes.
Continuous infusion: 10mg in 500ml of glucose 5% w/v injection or sodium chloride 0.9% w/v injection may be infused intravenously, initially at a rate of up to 180 micrograms per minute, reduced according to response to 30-60 micrograms per minute.
Children: 100 micrograms/kg bodyweight subcutaneously or intramuscularly.
This medicine should only be administered by healthcare professionals with appropriate training and relevant experience.
Available in a pack of 10 x 1ml ampoules.

Each 1ml ampoule contains 10mg phenylephrine hydrochloride equivalent to 8.2mg phenylephrine.
Shelf life : 3 Years unopened. After opening and dilution, chemical and physical in-use stability has been demonstrated for 24 hours at room temperature. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2-8°C.
Prescribing information
Refer to the full Summary of Product Characteristics (SmPC) before prescribing. Name and active ingredients: Phenylephrine Hydrochloride 10mg/ml solution for injection/infusion. Each 1ml ampoule contains 10mg phenylephrine hydrochloride equivalent to 8.2mg phenylephrine. Pharmaceutical form: Solution for injection/infusion. Clear, colourless, sterile solution. Indications: For the treatment of hypotensive states during spinal anaesthesia or drug-induced hypotension. Posology and method of administration: See SmPC section 4.2 for full information concerning dosage, preparation, administration, duration and special populations. Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SmPC. Patients taking non-selective monoamine oxidase inhibitors (MAOIs), or within 14 days of ceasing such treatment due to risk of paroxysmal hypertension and possibly fatal hyperthermia (see section 4.5). Patients with severe hypertension. Patients with severe hyperthyroidism. Patients with peripheral vascular disease due to the risk of ischemic gangrene or vascular thrombosis. Special warnings and precautions for use: Special caution should be exercised when administering phenylephrine to patients with pre-existing cardiovascular disease such as ischaemic heart disease, arrhythmias, arterial hypertension, aneurysms, or non-severe peripheral vascular insufficiency. Anginal pain may be precipitated in patients with angina pectoris. For full details, see SmPC, section 4.4. Adverse reactions: During the use of Phenylephrine, the following adverse reactions have been observed, the frequency of which have not been accurately established: Reflex bradycardia, reflex tachycardia, cardiac arrhythmias, anginal pain, palpitations, cardiac arrest, hypertension, hypotension, flushing, headache, cerebral haemorrhage, vertigo, fainting, head discomfort, dyspnoea, pulmonary oedema, vomiting, salivary hypersecretion, difficulty in urination, urinary retention, sweating, temporary tingling, cold feeling on the skin, alterations in glucose metabolism. Extravasation of phenylephrine can cause tissue necrosis. Presentations: Type 1 amber glass ampoule with ceramic break ring. Pack size: 10 ampoules. Legal Classification: POM. Marketing Authorisation Number: PA0298/027/001 Distributed by Athlone Pharmaceuticals Limited. Date of preparation: April 2022. IE23/001/SmPC Nov 2022.

Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by email medsafety@hpra.ie Adverse events should also be reported to Kent Athlone Pharma Group on (090) 666 1109 or medical@kent-athlone.com. Additional information available on request.
Your partner of choice, when excellence matters.
For further information on this product, please contact Gavin Butler, Key Account Manager, Athlone Pharmaceuticals Ltd., Ballymurray, Co. Roscommon Tel: + 353 (0) 87 3354523 | Email: Gavin.Butler@kent-athlone.com
Date of preparation: May 2023 Ref: IE2023/002/00
European Guidelines on ACS
in the shoulder or arm, and indigestion. We recommend that anyone experiencing these symptoms contact emergency medical services immediately, day or night.
“Studies suggest that women go underdiagnosed for heart problems and thus we hope these Guidelines will serve as call to action to healthcare professionals to ensure female patients receive evidence-based care at all times and remain alert to the symptoms when treating women.”
3. Acute coronary syndrome is a spectrum of conditions - Severity of ACS ranges from unstable angina (no irreversible heart damage) to various levels of myocardial infarction (heart attack), which can involve irreversible heart muscle damage. Despite this, management of conditions follows the same principals in each.
The European Society of Cardiology (ESC) has published new cardiology Guidelines on acute coronary syndromes (ASC). The expert-led taskforce delivering the Guidelines was chaired by Irish heart health expert, Professor Robert Byrne, Director of Cardiology at Mater Private Network and Professor of Cardiovascular Research at RCSI University of Medicine and Health Sciences.
ASC is a key focus area of the new Guidelines, which provide detailed advice on treatment and will be instrumental in paving the way for doctors across Europe as they seek to improve patient care in the field of heart health, and in particular, the management of unstable angina and other related conditions.

Speaking from the European Society of Cardiology Congress in Amsterdam, Professor Robert Bryne of Mater Private Network and RCSI University of Medicine and Health Sciences, commented, “Heart disease is the number one cause of death in women and men globally and as national leaders in cardiovascular care in Ireland, the team at Mater Private and RCSI University are passionate about propelling the field forward to streamline patient care to drive better outcomes for patients.”
“The new Guidelines highlight that time is critical to effective treatment and so, chest pain that lasts for more than 15 minutes and/or recurs within one hour should be taken very seriously. Other symptoms of cardiac distress include sweating, pain
Date for your Diary
The Health Services Research & Pharmacy Practice (HSRPP) conference will take place in University College Cork on 25 – 26 April 2024

This will be the 30th HSRPP conference, with the conference theme as ‘Sustainable Development in Healthcare’, which allows for the submission of research abstracts on a wide variety of interesting research topics. The HSRPP conference has a long legacy of disseminating novel research pertaining to health services, medication use, and pharmacy practice, and places particular emphasis on sharing the work of early career researchers in an encouraging environment.
Abstract submission is now open, with accepted abstracts published in the International Journal of Pharmacy Practice. Please check out our website for more information: https://www.ucc.ie/en/hsrpp2024/ Follow us @HSRPPConference on X/Twitter and use #HSRPP2024
Six key takeaways from the report include:
1. Time is critical for treatmentAnyone experiencing chest pain lasting more than 15 minutes or recurring within one hour, along with other symptoms such as sweating, shoulder/ arm pain, or indigestion, should call emergency services immediately.
2. Women are also at risk - It is a common misconception that acute coronary syndromes primarily affect men. Women are also at risk and should seek immediate medical help if they have symptoms. Knowing this, the report states that healthcare providers must make a concerted effort to ensure that women receive evidencebased care.
4. Long-term management - After an ACS event, continuing care is critical. This is because patients are at increased risk of a repeat event. Medication and the adoption of proactive lifestyle changes such as diet, exercise, smoking cessation, and limiting alcohol will be a factor in preventing future events.
5. Acute coronary syndrome and cancer - Patients with cancer have a higher risk of ACS and the Guidelines include new recommendations for their management. This may involve balancing heart health and cancer treatments, including potentially interrupting cancer therapy if it is contributing to ACS.
6. Patient perspectives are vital - Healthcare professionals are encouraged to respect and incorporate patients' preferences, needs, and values in clinical decisions. Patients should be informed about risks and options and be included in decision-making as much as their condition allows.
10 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Professor Robert Byrne, Director of Cardiology at Mater Private Network 3
INDIC A T E D FOR





































Legal Category: S1A. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com













XELJANZ is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response, or were intolerant to either conventional therapy or a biologic agent.
XELJANZ in combination with methotrexate (MTX) is indicated for the treatment of moderate to severe active rheumatoid arthritis (RA) in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying antirheumatic drugs.
XELJANZ in combination with MTX is indicated for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an





















inadequate response or who have been intolerant to a prior diseasemodifying antirheumatic drug (DMARD) therapy.




XELJANZ is indicated for the treatment of adult patients with active ankylosing spondylitis (AS) who have responded inadequately to conventional therapy.


XELJANZ is indicated for the treatment of active polyarticular juvenile idiopathic arthritis (rheumatoid factor positive [RF+] or negative [RF-] polyarthritis and extended oligoarthritis), and juvenile psoriatic arthritis (PsA) in patients 2 years of age and older, who have responded inadequately to previous therapy with DMARDs.



PP-XEL-IRL-0822 | Date of preparation: October 2022
| UC | PsA | JIA | AS
RA
Celebrating World Hospice and Palliative Care Day
Written by Dr Julie Ling, CEO, European Association for Palliative Care/ Chair, Worldwide Hospice and Palliative Care Alliance

World Hospice and Palliative Care Day is an annual unified day of action to celebrate and support hospice and palliative care around the world. This year's World Day theme is “Compassionate Communities: Together for Palliative Care” and takes place on Saturday 14th October 2023. This aims to highlight the important role that communities have in working compassionately to support the people requiring palliative care, their families, and the bereaved in their communities. In addition to World Day, Ireland is unique, and each year celebrates Palliative Care Week. This year it will take place from 11th – 16th September and the focus of palliative care week is ‘Living for today, planning for tomorrow’ (https://aiihpc.org)

Palliative care is an essential component of national health systems and an essential service within Universal Health Coverage
Safety Notice
reforms. Palliative care includes the physical, psychosocial, and spiritual care of people with lifelimiting conditions and aims to address pain and other symptoms by optimising their quality of life. Palliative care should be integrated early in the course of disease and should be available until the end of their lives and should consider how best to support bereaved families.
In 2014, the World Health Assembly unanimously passed a resolution to strengthen palliative care as a component of comprehensive care throughout the life course and should be available to both children and adults. The World Health Assembly Resolution stated that palliative care is an ethical responsibility of health systems and a key component of universal health coverage. The continuum of universal health coverage under the United Nations Sustainable Development Goal number three includes Promotion, Prevention, Treatment, Rehabilitation, and Palliative essential health services. This also includes the allocation of funding for these services.
In a recent paper that ranked palliative care in 81 countries globally, Ireland ranked second.1 Ireland has a long and proud history of providing palliative care. The national palliative care policy developed in 2001,2 provided a comprehensive blueprint for the
development of palliative care services. In subsequent years, many of the recommendations of the policy have been implemented. As a result, new posts have been developed, hospices have opened, palliative care education and training of health and social care professionals has been established and services have been developed in all regions of Ireland. In 2020, the programme for government, committed to publishing a new palliative care policy for adults. This is expected in the coming months. A policy for children’s palliative care in Ireland was published in 2010 and this has resulted in improved access to palliative care for children and their families.3
Palliative care in Ireland has been enhanced through its recognition as a medical and a nursing specialty and its inclusion in the education and training of healthcare professionals with opportunities for both undergraduate and post-graduate training. In addition, a Palliative Care Competence Framework4 for all health and social care professionals has also been developed. Palliative care services are available throughout Ireland in all settings including hospitals, hospices and in the community as part of primary care. However, these vary within and between geographical regions nationally. Ideally palliative care should be available to all, regardless of
their diagnosis, geographical location and yet in Ireland many people still do not have access to palliative care.
The OECD recently called for better care at end of life,5 they found that despite many people preferring to die at home, half of deaths are in hospitals, and this is often due to a lack of home and community support. Whilst palliative care is available in the community in Ireland, the Lancet commission on the value of death suggests that to refocus death and dying as a part of life, greater community involvement is needed to compliment health and social care services.6 This is at the heart of compassionate communities. At the very simplest level, compassionate communities help to increase awareness of access to palliative care7 and recognise that health and social care services are important, but that care is also the responsibility of others.
Ireland has made huge advances in developing and strengthening palliative care. As we mark World Hospice and Palliative Care Day, this year’s campaign provides the opportunity to celebrate success but also to address the substantial gaps in access to palliative care services, to ensure that there is not only improved access to palliative care in Ireland but also to involve our communities.
References available on request
The marketing authorization holder of Simponi, Janssen Biologics B.V., and the local representative, Merck Sharp & Dohme Ireland (Human Health) Limited, in agreement with the European Medicines Agency and the Health Products Regulatory Authority, would like to inform you of the following: Summary
• Accidental needle stick injuries, bent or hooked needles, and device actuation failure have been reported for the Simponi SmartJect pre-filled pen.
• Instructions for use have therefore been revised as follows:
o Do not put the cap of the pre-filled pen back if removed, to avoid bending the needle.
o Only inject in the thigh or abdomen.
o Use a two-hand approach to administer the injection (one hand to hold the pre-filled pen and the other hand to press the blue button to start the injection).
o Do not pinch the skin, when positioning the pre-filled pen and when administering the injection.
• The device must be pushed against the skin until the green safety sleeve slides completely into the transparent cover BEFORE the blue button is pressed. Only the wider portion of the green safety sleeve remains outside of the transparent cover.
• All patients/caregivers, including those previously trained on the SmartJect pre-filled pen, should be instructed on the proper use of the device in accordance with the revised instructions for use.
12 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Predicting Weight Loss after Bariatric Surgery
First-time patients in need of bariatric surgery will now be able to predict their weight loss ahead of undergoing a gastric bypass or sleeve gastrectomy operation.
A new AI driven web-tool developed by University of Lille and supported by the European Union SOPHIA project led by University College Dublin now allows patients and their doctors to accurately predict weight loss over five years following different types of bariatric surgery.
“Usually, weight loss after bariatric surgery vary widely and predicting weight loss was difficult,” said Professor Carel le Roux, from the UCD School of Medicine, co-ordinator of the SOPHIA consortium.
“Using artificial intelligence, we’ve developed a new pre-surgery prediction tool - created with data from eight counties in Europe, America, and Asia.”
Publishing their findings in The Lancet Digital Health, SOPHIA researchers used data from 9,861 patients as part of the projectwith 385 relevant measures taken to decide on the seven most valuable variables for accurately predicting weight loss post-bariatric surgery.
This include height, weight, type of operation, age, diabetes status, diabetes duration, and smoking status.
The prediction tool is the most accurate approach to predicting weight loss post-surgery created so far, and is likely to only improve over the next two years given the on-going European Union project SOPHIA’s investment.
“The tool will substantially reduce uncertainty for patients as they
can now make a much more informed decision about which surgical option to select. This is why we developed and validated this easy-to-use tool to predict an individual’s 5 year-weight loss after the most common bariatric operations,” said lead author Professor Francois Pattou, Lille University, France.

“Patients and clinicians are now able to make more informed decisions about which surgical option to select.”
Obesity affects 150 million people in Europe and 650 million worldwide, and increases the risk of health complications like heart disease, diabetes and cancer.
The aim of SOPHIA is to improve the ability to predict how patients will respond best to different obesity treatments and to help minimise complications.
The five-year project is funded under the Innovative Medicines Initiative (IMI), Europe’s largest public-private initiative, a joint undertaking between the European Union (represented by the European Commission) and the European pharmaceutical industry (represented by EFPIA, the European Federation of Pharmaceutical Industries and Associations).
IMI facilitates collaboration between the key players involved in healthcare research, including universities, the pharmaceutical and other industries, small and medium-sized enterprises (SMEs), patient organisations, and medicines regulators.
Professor Carel le Roux serves on the advisory boards for Johnson & Johnson, Medtronic, NovoNordisk, Eli Lilly, Boehringer Ingelheim, Keyron, GI Dynamics,
Royal College of Physicians of Ireland Annual Symposium - St Luke’s open for bookings 16 – 20 October 2023 | Hybrid event
The Royal College of Physicians of Ireland Annual Symposium - St Luke’s is open for bookings. The symposium will take place online and in person from 16 to 20 October 2023 touching on the themes of clinical leadership, resilience, and climate and health.

Throughout the symposium, these themes will be explored through a series of exciting events including a public art exhibition, our annual public meeting, the symposium day, and the ever-popular Heritage event. The Annual Stated Meeting will take place on 18 October 2023.
Glia Pharmaceuticals, Rhythm Pharmaceuticals, and Currax Pharma.
The research team stated, “We developed a machine learning model that provides accurate individual weight trajectories expected during 5 years after bariatric surgery, based on seven simple preoperative variables, including age, weight, height, smoking history, type 2 diabetes status and duration, and the type of intervention. These variables are readily available in a variety of clinical settings without interpretation and do not require laboratory tests. The model was validated globally, in eight cohorts and two randomised controlled trials, in Europe, the Americas, and Asia, and incorporated in an easy-to-use and interpretable web-based tool providing individual preoperative prediction of postoperative weight loss trajectory.
“This accessible and interpretable model is the first to provide preoperative predictions of weight trajectories up to 5 years after surgery, simultaneously for three of the most common
types of surgery: RYGB, sleeve gastrectomy, and AGB. Our results highlighted the association of the type of operation and diabetes status with weight trajectories. The present study also showed the impact of diabetes duration and smoking, which were not previously included in weight loss surgery prediction models.
“In summary, we have developed and validated an easy-to-use and interpretable model that provides individual predictions of weight loss trajectory after bariatric surgery. We have shown its generalisability and transportability across multiple cohorts in Europe, the Americas, and Asia, as well as its performance in intervention clinical trials.
“Individual weight loss trajectory prediction appears to be an accurate and simple strategy to inform clinical decisions for both health-care providers and patients before surgery. Our model can also be used postoperatively to identify patients whose actual weight loss trajectories differ from their predicted trajectory, thus allowing the timely implementation of appropriate clinical interventions.”
13 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
News
Professor Carel Le Roux
Prestigious Grant for Research into Breast Cancer
which are small marine organisms, and the closest living relatives to animals. The ERC starting grant is a huge moment for my research and career and will allow me to build a team working on these questions with the resources to do truly excellent science.”
Dr Eoin McEvoy is an Assistant Professor in Biomedical Engineering, whose research work under the ERC Starting Grant will focus on uncovering why tumour growth is sensitive to physical pressure and overcoming related drug resistance.
Two University of Galway academic researchers have secured prestigious grant awards from the European Research Council to pursue scientific research on evolution and breast cancer.
Dr Eoin McEvoy, an Assistant Professor in Biomedical Engineering and Dr James Gahan, who takes up an Associate Professorship in chromosome biology in 2024.

The scientists are amon g eight academics and researchers based in Ireland to be approved for an ERC Starting Grant 2023 and
among 400 across Europe. ERC Starting Grants are awarded over a five year period.
These awards are part of the EU’s Horizon Europe programme and designed to support excellent Principal Investigators who have demonstrated the ground-breaking nature, ambition and feasibility of their research proposal and are at a stage in their career when they are starting their own independent research team or programme.
Dr James Gahan is currently a Research fellow at the University of Oxford and based
at the Michael Sars Centre at the University of Bergen in Norway as a visiting researcher. He takes up an Associate Professor role in the School of Biological and Chemical Sciences and Centre for Chromosome Biology at University of Galway in April 2024 when the ERC Starting Grant commences.
Dr Gahan said, “My research focuses on the very earliest stages of animal evolution, namely how organisms went from being single celled to being composed of many different types of cells with different functions. To study this I work on choanoflagellates,
New Digital Transformation Programme
Blackrock Health Group, which comprises of the Blackrock, Galway, Hermitage and Limerick Clinics, has announced a ¤25 million investment in a new digital transformation programme. The investment includes the introduction of the very latest in electronic health record systems, MEDITECH Expanse, and the introduction of Ireland’s first integrated digital engagement platform that will enable connectivity between patients, consultants and general practitioners (GPs). These innovations will serve to connect patient data for the 325,000 patients it treats annually across its four clinics, enhance clinical care, improve patient safety all while reducing the administration burden and freeing up more time for clinicians to focus on patient care.
The digital transformation project will take three years to implement and will create 60 new jobs across the Blackrock Health group as a direct result of the investment. The partnership builds on the successful rollout of MEDITECH's electronic health record in Galway Clinic in 2017, the first of its kind in Ireland.
The MEDITECH Expanse electronic health record system will help to guide clinical decision-making by offering a comprehensive view of a patient’s health history and will allow patients to attend any of the hospitals within the group with seamless access to their records. The system will also ensure that key information is available to their GP for any follow-up. Using the location agnostic solution, physicians, nurses, and other clinicians at Blackrock Health can use mobile devices to view
patient records, place orders, and document care at any time.
CEO of Blackrock Health, Caroline Whelan said:, “Improving patient care is always at the heart of the decisions that we make at Blackrock Health and that is why we are investing ¤25m to digitally transform the way we work across the Group, which in turn will enhance clinical care and patient safety for the 325,000 patients we treat annually. We are excited to embark on this three-year journey alongside our partners at MEDITECH which will enable staff across our four clinics to work in close collaboration and our patients to access one single health record, at any of our clinics.”
Mike McCann, Chief Information Officer, Blackrock Health noted, “We introduced electronic health
Dr McEvoy added, “Breast cancer remains the second most common cause of cancer-related death in Irish women. This project will develop a new way to diagnose and treat cancer by analysing the physical forces that contribute to tumour growth and drug resistance. By creating computer models and analysing tissue from cancer patients, our team will develop a better understanding of how physical pressure can both prevent tumour progression and reduce drug efficacy. Ultimately, the project aims to create a personalised healthcare tool to predict cancer and treatment outcomes, leading to more effective patient-specific diagnosis and treatment.”
records at the Galway Clinic in 2017 and since then have seen great improvements in the way that we work day-to-day. Based on these results, the natural next step was to implement a group-wide rollout, as we continue to look at ways to integrate technology into healthcare. We look forward to bringing this technology to patients and staff.”
Gina Kerley, Executive Director, MEDITECH added, “For many years, the Galway Clinic has been a leader among Ireland’s healthcare organisations by leveraging technology to improve patient care. We are excited to build on this success and partner with Blackrock Health to provide all four clinics with the innumerable benefits of having a modern electronic health record.”
14 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Dr Eoin McEvoy, credit Aengus McMahon, and Dr James Gahan, credit Melanie Burford
Future of Future of pharmacIE pharmacIE



Friday, 20th October
1pm - 4pm
RCSI, 123 St Stephen
Connect with future pharmacists at APPEL's exclusive careers event. Book your ticket now!


TICKETS

Cardiology
Update on Acute Coronary Syndromes
Written by J.J. Coughlan1,2, Robert A. Byrne1,2
Affiliations:
1. Cardiovascular Research Institute, Mater Private Network, Eccles Street 73, Dublin 7, D07 WKW8, Ireland
2. School of Pharmacy and Biomolecular Sciences, RCSI University of Medicine and Health Sciences, Dublin, Ireland
The 2023 ESC ACS guideline is primarily focused on the management of patients with type 1 MI, which is related to a primary coronary plaque event
(most commonly plaque rupture or erosion). This is sometimes referred to as a ‘spontaneous MI’. UA is not associated with elevated cardiac biomarkers but is defined clinically as myocardial ischaemia at rest or on minimal exertion in the absence of acute
cardiomyocyte injury/necrosis. It is characterized by specific clinical findings of prolonged (>20 min) angina at rest; new onset of severe angina; angina that is increasing in frequency, longer in duration, or lower in threshold; or angina that occurs after a recent episode of MI.
Introduction
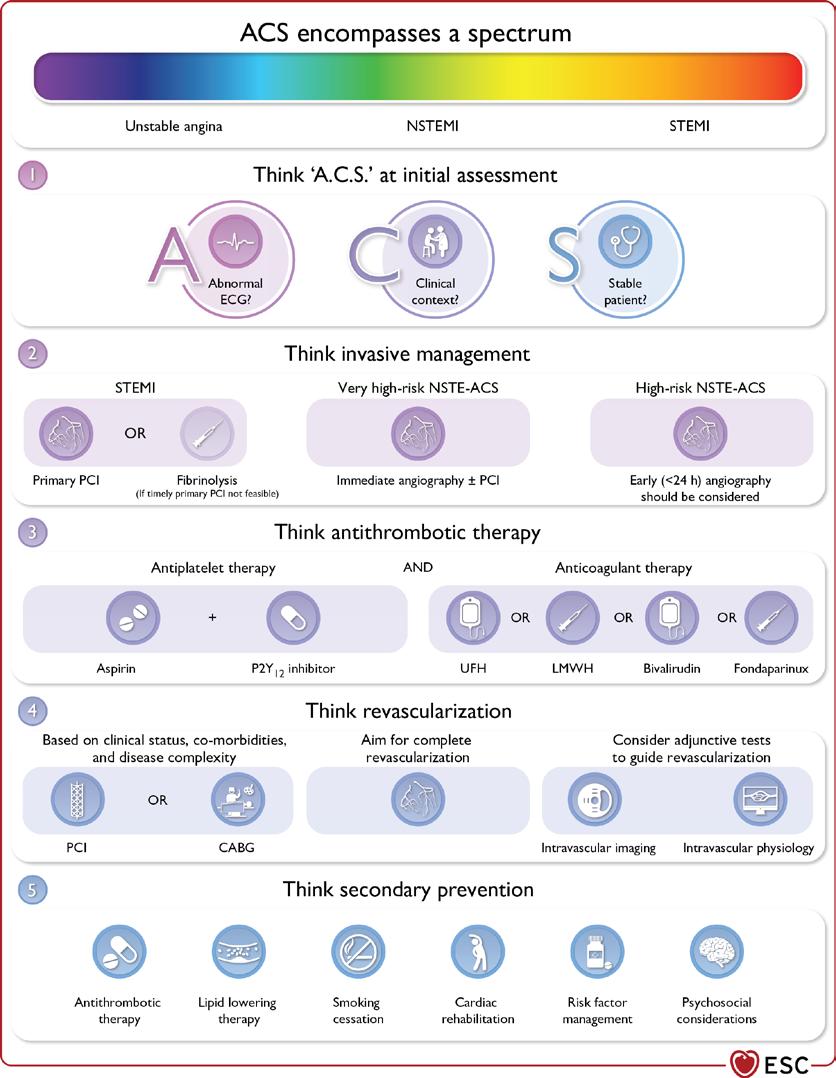
The 2023 European Society of Cardiology (ESC) Guidelines for the management of acute coronary syndromes (ACS) were released at the annual ESC conference in Amsterdam this year. For the first time, all of ACS was covered in one document. This includes ST elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI) and Unstable Angina (UA). This allowed the guideline to highlight the common principles underpinning the identification, diagnosis, and treatment of patients with all three subtypes of ACS. A key aspect of the guideline was the concept that ACS can be best considered as a spectrum. In this article, we will highlight some of the most important messages from the guideline, many of which are summarised in Figure 1

ACS Terminology
The guideline aimed to make the terminology used to describe ACS clear. ACS includes patients presenting with both myocardial infarction (MI) and UA. MI is defined as per the fourth universal definition of MI (UDMI) and is associated with elevations in cardiac biomarkers. In modern practice, high sensitivity cardiac troponin (hs-cTn) is the recommended biomarker to use. The fourth UDMI sub-categorises MI into five categories, as per the aetiology. This is summarised in Table 1
16 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 1. Central Illustra�on
J.J. Coughlan
Figure 1. Central Illustration
Table 1. Fourth universal defini�on of myocardial infarc�on
Fourth universal definition of myocardial infarction
A combination of criteria is required to meet the diagnosis of acute myocardial infarction, namely the detection of an increase and/or decrease of a cardiac biomarker, preferably high-sensitivity cardiac troponin T or I, with at least one value above the 99th percentile of the upper reference limit and at least one of the following:
1. Symptoms of myocardial ischaemia
2. New ischaemic ECG changes
3. Development of pathological Q waves on ECG
4. Imaging evidence of loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischaemic aetiology (i.e., in a vascular bed distribution)
5. Intracoronary thrombus detected on angiography or autopsy.
Type 1 MI
Characterized by atherosclerotic plaque rupture, ulceration, fissure, or erosion with resulting intraluminal thrombus in one or more coronary arteries, leading to decreased myocardial blood flow and/or distal embolization and subsequent myocardial necrosis.
Type 2 MI
Myocardial necrosis in which a condition other than coronary plaque instability causes an imbalance between myocardial oxygen supply and demand. Mechanisms include hypotension, hypertension, tachyarrhythmias, bradyarrhythmias, anaemia, and hypoxaemia, but also by definition coronary artery spasm, spontaneous coronary artery dissection, coronary embolism, and coronary microvascular dysfunction
Type 3 MI MI resulting in cardiac death with symptoms suggestive of myocardial ischaemia when biomarkers are not available, or MI is detected at autopsy
Type 4 MI MI caused by percutaneous coronary intervention.
Type 5 MI MI caused by coronary artery bypass grafting
The common underlying pathophysiology in all three conditions (UA, NSTEMI, STEMI) is thought to be unstable coronary plaque in the majority of cases. This explains why the management of all three conditions shares many common features, particularly after the initial treatment phase.
Initial Classification
The initial classification of ACS is based on the clinical presentation and the ECG. Patients presenting with symptoms suggestive of ACS can be classified as STEMI or NSTE-ACS based on the presence or absence of ST segment elevation (or equivalents) on ECG. It should be noted that the presence of ST elevation on ECG is a surrogate marker for an occluded artery. Like any diagnostic tool, this is imperfect and patients may have an occluded artery without demonstrating ST elevation
on ECG. Other ECG patterns that may prompt triage for immediate angiography include ST depression in V1-V3 (posterior STEMI) and widespread ST depression in 6 or more leads coupled with ST elevation in aVR and/or V1. Recognition of these ECG patterns is important as
Term
Primary PCI
Primary PCI strategy
Rescue PCI
Routine early PCI strategy after fibrinolysis
Pharmacoinvasive strategy
Immediate invasive strategy
Early invasive strategy
Selective invasive strategy
patients with STEMI should be treated with primary percutaneous coronary intervention (PPCI) as soon as possible. If PPCI cannot be performed within 120 minutes, fibrinolysis can be used as an alternative treatment.
Patients with suspected NSTE-ACS and very high-risk
Definition
criteria should also be treated with immediate angiography ± percutaneous coronary intervention (PCI) if indicated. These very high-risk criteria include any of the following: haemodynamic instability or CS, recurrent or refractory chest pain despite medical treatment, life-threatening arrhythmias, mechanical complications of MI, HF clearly related to ACS, and recurrent dynamic ST-segment or T wave changes, particularly with intermittent ST-segment elevation. For patients with NSTE-ACS and high risk criteria (confirmed diagnosis of NSTEMI as per ESC algorithms, GRACE risk score >140, transient ST segment elevation or dynamic ST or T wave changes), an early invasive angiography approach should be considered and inpatient invasive angiography is recommended. For some patients with a low index of suspicion for invasive angina, a selective invasive strategy may be considered. The definitions of the invasive strategies used in the guideline are provided in Table 2.
Emergent PCI with balloon, stent, or other approved device, performed on the IRA without previous fibrinolytic treatment
Emergency coronary angiography and PCI of the IRA if indicated
Emergency PCI performed as soon as possible in cases of failed fibrinolytic treatment
Coronary angiography, with PCI of the IRA if indicated, performed between 2 h and 24 h after successful fibrinolysis
Fibrinolysis combined with rescue PCI (in cases of failed fibrinolysis) or routine early PCI strategy (in cases of successful fibrinolysis)
Immediate coronary angiography (<2 h from diagnosis of ACS) and PCI of the IRA if indicated
Early coronary angiography (<24–72 h from diagnosis of ACS) and PCI of the IRA if indicated
Coronary angiography based on clinical assessment and/or noninvasive testing
17 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Table 1. Fourth universal definition of myocardial infarction
Tables and Figures
Table 2. Invasive strategies in patients with ACS
Table 2. Invasive strategies in pa�ents with ACS
A crucial part of the early management of patients with ACS is the early identification of patients with STEMI and very high risk NSTE-ACS and ensuring that they are treated appropriately. The invasive management of patients with STEMI and NSTE-ACS is summarised in Figure 2 and Figure 3
Diagnostic algorithms for NSTEMI
For patients who do not require immediate invasive management, it is recommended to use an algorithmic approach to rule in/ rule out NSTEMI. The guidelines recommend that the 0/1 hr or 0/2 hr ESC algorithms are used for this. The algorithms are troponin assay specific, but the general principle is the same; using two hs-cTn measurements (taken at the time of arrival to the ED and 1/2 hours later), we can rapidly stratify patients with suspected NSTEMI into one of three groups, rule in, observe or rule out. While
there are several caveats to the use of these algorithms, they facilitate the early identification of patients with NSTEMI and are recommended. All centres should use these algorithms as a key component of their assessment of patients with suspected ACS and this is highlighted in Figure 4

Antithrombotic therapy
All patients with ACS should be treated with antithrombotic therapy, which consists of a combination of antiplatelet therapy and anticoagulant therapy. For patients who do not have an indication for long term anticoagulation (i.e., atrial fibrillation or a mechanical heart valve), anticoagulation will not need to be continued beyond the acute phase of the ACS. However, antiplatelet therapy should be continued beyond the acute phase after ACS in all patients.
Anticoagulation
Anticoagulation is recommended for all patients with ACS at the time of diagnosis. The most well established anticoagulant option for patients with ACS is unfractionated heparin (UFH) and this has a class I recommendation for patients at the time of PCI. For patients with STEMI, alternatives to UFH which should be considered include low molecular weight heparin (LMWH) and bivalirudin, a direct thrombin inhibitor. For patients with NSTEACS, the recommendations for anticoagulation are dependent on the period of time from diagnosis to angiography. For patients with intended angiography within 24 hours, LMWH should be considered as an alternative to UFH. For patients with an anticipated delay to angiography > 24 hours, fondaparinux (a synthetic pentasaccharide factor Xa inhibitor) is recommended. For patients without an indication
for long term anticoagulation, the anticoagulant drug can generally be stopped after the invasive procedure is performed.
Antiplatelet therapy
Dual antiplatelet therapy (DAPT) is indicated for patients with ACS and consists of aspirin and a P2Y12 inhibitor. Available P2Y12 inhibitors include prasugrel, ticagrelor and clopidogrel. It is recommended that patients who are not at high bleeding risk (HBR) receive 12 months of DAPT. Prasugrel and ticagrelor are recommended in preference to clopidogrel and prasugrel should be considered in preference to ticagrelor in patients who are undergoing PCI.
Alternative strategies to 12-month DAPT include DAPT abbreviation and DAPT de-escalation. DAPT abbreviation refers to shortening the duration of DAPT, followed by single antiplatelet therapy (SAPT), which can consist of aspirin or P2Y12 inhibitor monotherapy.
18 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Cardiology
Figure 2. Modes of presenta�on and pathways to invasive management and myocardial revasculariza�on in pa�ents presen�ng with STEMI
Figure 2. Modes of presentation and pathways to invasive management and myocardial revascularization in patients presenting with STEMI
This should be considered in patients who are event free after 3-6 months of DAPT and may be considered after 1 month of DAPT in patients at HBR.
DAPT de-escalation refers to switching from prasugrel or ticagrelor based DAPT to clopidogrel based DAPT and this may also be considered. It should be stressed that these are viewed as alternative strategies in the guideline and should only be employed where there is a clear motivation/rationale for their use, as opposed to being indiscriminately utilised in the wider ACS population. HBR can be defined using the Academic Research Consortium (ARC) HBR criteria, as summarised in Table 3. Patients who meet 1 major criterion or ≥2 minor criteria are considered HBR.
Routine pretreatment with P2Y12 inhibitors (defined as a strategy in which the P2Y12 inhibitor is given before coronary angiography and, therefore, before the
coronary anatomy is known) is not recommended in patients with NSTE-ACS who are scheduled for early (<24 h) invasive angiography but may be considered in patients with STEMI undergoing PPCI and in patients with NSTE-ACS who are not scheduled for early invasive coronary angiography and who are not HBR. Parenteral anticoagulation and loading with aspirin are both recommended at the time of diagnosis for patients with ACS.
Long Term Treatment
Patients who have experienced an ACS event are at a high risk of future cardiovascular events and so require long term treatment to try to reduce this risk. As discussed in the previous section, antiplatelet therapy is an important component of the long-term medication regimen for ACS patients. All patients with ACS should also be treated with lipid lowering agents and the initiation and escalation of the recommended lipid lowering
agents (statins, ezetimibe and proprotein convertase subtilisin/ kexin type 9 [PCSK9] inhibitors) should follow the treatment algorithm outlined in Figure 5

Promotion of healthy lifestyle choices is essential, including smoking cessation, adoption of a healthy diet, regular exercise, achieving a healthy weight and management of psychosocial factors. All patients should undergo a structured cardiac rehabilitation program where they can be educated on these topics. Risk factor treatment targets are also important, including BP (systolic <130mmHg and diastolic <130mmHg) and low-density lipoprotein (<1.4mmol/L).
Patients with ACS and Cancer
This guideline included a dedicated section on the management of patients with ACS and cancer. Cancer and atherosclerotic cardiovascular disease share many common risk factors. More patients with cancer are surviving and so
there are an increasing number of patients presenting with ACS who either have a history of cancer or are undergoing active treatment for cancer. While patients with a history of cancer should be treated similarly to all patients to ACS, patients with active cancer and ACS have some specific considerations that should be taken into account. Some cancer therapies can be associated with ACS. If a cancer therapy is suspected as being a contributing cause of the ACS presentation, this is recommended to be interrupted. It is important that a multi-disciplinary teambased approach is adopted when managing these patients. If patients with cancer and ACS have an expected survival of > 6 months, then an invasive strategy is recommended. However, if the cancer related prognosis is poor (i.e., < 6 months), a conservative approach should be considered.
19 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Figure 3. Selec�on of invasive strategy and reperfusion therapy in pa�ents presen�ng with NSTE-ACS
Figure 3. Selection of invasive strategy and reperfusion therapy in patients presenting with NSTE-ACS
Cardiology
Figure 4. The 0 h/1 h or 0 h/2 h rule-out and rule-in algorithms using high-sensitivity cardiac troponin assays in patients presenting with suspected NSTEMI and without an indication for immediate invasive angiography.
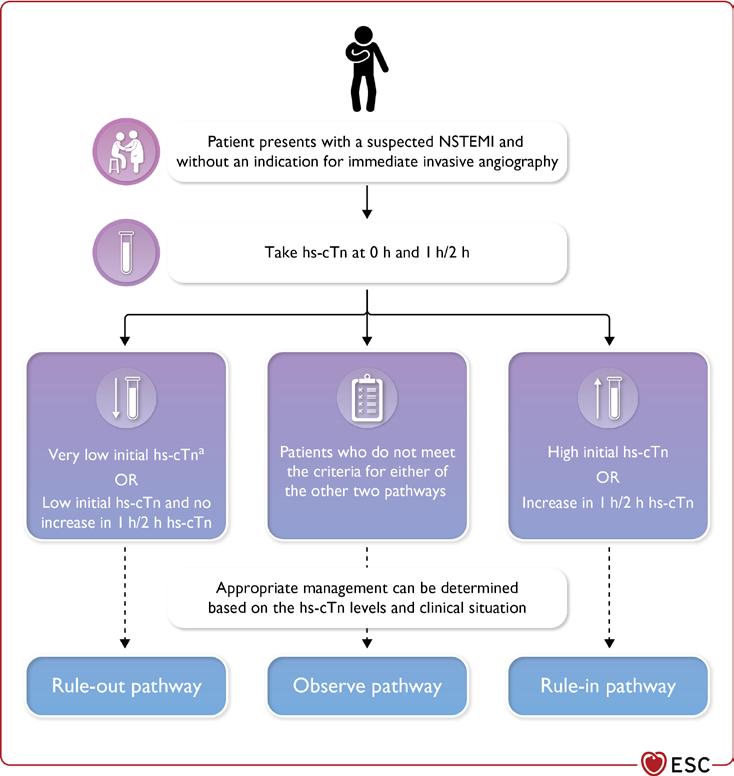
Patient perspectives
For the first time, a section on patient perspectives was included in this ACS guideline. This section highlights that care of patients with ACS should not only strive to employ the most evidence-based practices but should also try to promote care that is respectful of, and responsive to, the individual patients’ preferences, needs and values. This is an important concept for all healthcare professionals involved in the care of patients with ACS to reflect upon. It is always important to consider what is important to the patient and to involve the patient as much as possible in clinical decision making.
Summary
The 2023 ESC Guidelines for the management of ACS covers the whole spectrum of ACS in one guideline for the first time. One of the underlying principles for the guideline was to try to highlight the commonalities in
Major Criteria
Anticipated use of long-term oral anticoagulation
Figure 4. The 0 h/1 h or 0 h/2 h rule-out and rule-in algorithms using high-sensi�vity cardiac troponin assays in pa�ents presen�ng with suspected NSTEMI and without an indica�on for immediate invasive angiography.
Severe or end-stage CKD (eGFR <30 mL/min)
Haemoglobin <11 g/dL
Spontaneous bleeding requiring hospitalization or transfusion in the past 6 months or at any time, if recurrent
Moderate or severe baseline thrombocytopenia (platelet count <100 × 109/L)
Chronic bleeding diathesis
Liver cirrhosis with portal hypertension
Active malignancy (excluding non-melanoma skin cancer) within the past 12 months
Previous spontaneous ICH (at any time)
Previous traumatic ICH within the past 12 months
Presence of a brain arteriovenous malformation
Moderate or severe ischaemic stroke within the past 6 months
Nondeferrable major surgery on DAPT
Recent major surgery or major trauma within 30 d before PCI
Minor Criteria
Age > 75 years
Moderate CKD (eGFR 30–59 mL/min)
Haemoglobin 11–12.9 g/dL for men and 11–11.9 g/dL for women
Spontaneous bleeding requiring hospitalization or transfusion within the past 12 months not meeting the major criterion
Long-term use of oral NSAIDs or steroids
Any ischaemic stroke at any time not meeting the major criterion
20 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Table 3. ARC-HBR criteria
Table 3. ARC-HBR criteria

HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication
Cardiology
the management of patients with UA, NSTEMI and STEMI. Early identification, diagnosis and risk stratification of ACS allows for appropriate management strategies to be employed. Patients with ACS should receive treatment with antiplatelet therapy and anticoagulation,
undergo invasive assessment and revascularisation. After the acute phase, it is important that long term treatment focuses on optimisation of cardiovascular risk, in order to reduce the risk of recurrent events. It is also important to practice patient centred care throughout the ACS
journey, from the time of first medical contact through to long term management. It is hoped that the 2023 ESC Guidelines will assist healthcare providers in delivering optimal care to patients with ACS worldwide. More information can be obtained from the full guideline, available
free of charge on the ESC website. https://www.escardio. org/Guidelines/Clinical-PracticeGuidelines/Acute-CoronarySyndromes-ACS-Guidelines
22 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 5. Lipid-lowering therapy in ACS patients Figure 5. Lipid-lowering therapy in ACS pa�ents
Ground-Breaking Research in Cancer Treatment
Minister for Further and Higher Education, Research, Innovation and Science Simon Harris TD has officially launched Tyndall’s 2022 Annual Report. He commended the ground-breaking research project undertaken by Tyndall in collaboration with Boston Scientific:

“For over 40 years, Tyndall has played a key role in securing Ireland’s international prominence within the ICT industry and the success of this project is further proof of Ireland’s world-leading research capability. Boston Scientific’s continued investment and expansion in Ireland is a strong endorsement of Ireland’s education and research systems.
“This research collaboration illustrates how the application of advanced technology, developed at Tyndall, has a profound effect on the lives of citizens, as well as industry, through smart medical devices, highspeed telecommunications, robotics and automation, and the microelectronic chips that enable all of ICT.
“As a leader in industry-academia collaboration, I am reassured that Tyndall will continue to play its unique role in securing Ireland’s future as a worldwide technology
leader, whilst supporting key technology companies and SMEs, both nationally and internationally.”
Researchers at Tyndall, in partnership with Boston Scientific business partners, clinicians from Cork University Hospital, and designers from Design Partners developed a smart implantable fiducial marker, which can accurately measure radiation at the site of a tumour during radiation therapy.
Boston Scientific Cork said, “Boston Scientific Cork is proud to be part of the ground-breaking research of an implantable and injectable micro sensor, which is capable of supporting radiologists in accurately measuring radiation doses at the target site, while minimising radiation to adjacent healthy tissues. The research is a significant milestone in the development of this type of sensor system, which can be delivered through minimally invasive procedures. The collaborative research programme utilised Tyndall’s world class facilities and expertise in advance technology platforms. This, combined with Tyndall’s access to clinical specialists in radiology and use of design thinking methodology with industry leaders, played a
Minister Simon Harris, TD
significant role in this collaboration for Boston Scientific Cork's innovation strategy.”

This cutting-edge research resulted in a prototype of what is almost certainly the world’s smallest Radiation Field Effect Transistor (RADFET) system, packaged within a fiducial coil. The markers are small metal objects placed on the patient's skin or implanted within the body, in or near a tumour in preparation for radiation therapy, which helps pinpoint the tumour’s location with greater accuracy and allows the treatment team to deliver the maximum radiation dose to the tumour while sparing healthy tissue. This technology could lead to significantly improved treatment outcomes for patients.
Professor William Scanlon, Tyndall CEO, said, "This collaboration between Tyndall and Boston Scientific further strengthens Tyndall’s position as a worldleader in deep-tech research. It is our unique ability to apply our expertise in semiconductor
materials, devices and systems in partnership with industry that underpins successful collaborations like this. We will continue to build on over 40 years of research excellence and industrial impact, while addressing societal challenges through deeptech innovation.”
Board Chair Dr Denis Boyd states in the report that, “Our core aims are to create new knowledge, develop talent, and play a lead role in the creation of a science and technology ecosystem spanning startups, SMEs, large corporates, government, academia, and other stakeholders. By catalysing the activation of that ecosystem, Tyndall and its Board and leadership can play a pivotal role in the reshaped technology world of the next decade and beyond.
“Here at home, Tyndall will continue to promote excellence in research and our planned expansion will give us the scope to develop new areas of research while building on our existing strengths. I am pleased to say that Tyndall is performing well against all of the goals set out in our Tyndall 2025 strategy. We must now double down on that strong performance and play our part in supporting the wider objectives set by government for Ireland’s technology and research ecosystem.”
23 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
News
Tyndall Chair, Dr Denis Doyle and Tyndall CEO, Professor William Scanlon
Emergency Medical Response Charity in Waterford
CRITICAL, the emergency medical response charity, has established a new team in Waterford and now has two doctors volunteering with the service. Pictured are Dr Daragh Mathews and Dr Paul Campbell who have signed up to respond to serious incidents and potentially life-threatening medical emergencies in the county.

now happen at the roadside. This gives patients a greater chance of survival.”
CRITICAL, the emergency medical response charity, has established a new team in Waterford and now has two doctors volunteering with the service in the county. Dr Paul Campbell and Dr Daragh Mathews have signed up to respond to serious incidents and potentially life-threatening medical emergencies in the county.
The doctors work closely with the National Ambulance Service and are tasked to care for the sickest and most seriously injured patients. They regularly respond to road traffic collisions, cardiac arrests, farming accidents and other major traumas within 30km of where they live but can be called further if their expertise and help is required.
Dr Paul Campbell is originally from Tyrone, joined the charity at the start of the summer and has already responded to multiple incidents each week since then. He has been working as a GP in Waterford City since 2012 and has recently completed a Masters in
Pre-Hospital Emergency Medicine from UCD.
Dr Campbell said, “Giving patients early access to specialist care at the scene of emergencies is hugely important. We live in a rural country with many people living considerable distances from hospitals. It’s minutes that make the difference, not just in whether a patient survives but in how quickly they recover afterwards. The equipment provided and funded by public donations to CRITICAL means that I can administer IV medications, take over a person’s breathing or perform other complex procedures at the scene. I’ve already responded to a broad range of calls in Co Waterford including crashes, cardiac arrests, a fall from a height, stroke and sepsis. It’s very rewarding to be able to give back to my local community.”
The owner of the Centra store in Dunmore East has agreed to sponsor the running costs of Dr Campbell’s emergency vehicle in memory of local woman Susan
All Ireland Pharmacy Conference
O’Donoghue who was assisted by the service in August.
Dr Daragh Mathews is a Specialist Registrar in Emergency Medicine with a particular interest in Pre-Hospital Critical Care. He graduated from UCC in 2017 and is currently training to be an Emergency Medicine Consultant. Daragh joined the charity in 2021 and has relocated from Dublin to Waterford in recent weeks.
Dr Mathews added, “It’s been exceptionally busy since I arrived in Waterford City. I’ve been tasked to incidents as far away as Enniscorthy in Co Wexford. The biggest change from Dublin is the distance we’re asked to travel to emergencies and just how far patients can be from the nearest emergency department. This means that we can provide critical care to the patient and stabilise them before arrival at hospital. It puts into perspective how important charities like CRITICAL are in rural parts of the country. Our aim is to get care to the patient early, work in support of the ambulance service but also add a layer of careessentially bringing the emergency department to them. Patients can deteriorate quickly, but being able to provide a critical care doctor to the scene means that interventions which previously would only happen inside of the hospital can
The doctors will soon be joined by a number of off-duty paramedics, EMTs and Advanced Paramedics. Three National Ambulance Service staff from Waterford will be among the first group of Volunteer Emergency Medical Responders across the country to receive vital equipment from CRITICAL. The charity is providing each responder with an Emergency Backpack containing a defibrillator, a resuscitation kit and a medical diagnostic kit at a cost of ¤1,500. Michéal Sheridan, CEO of CRITICAL said, “Our mission is to save lives. We’re focused on establishing a network of Volunteer Emergency Medical Responders in every county in Ireland over the next two years. Waterford is an example of what we would like to replicate in other towns, villages and cities. Here, we now have a specialist in emergency medicine, a GP who has considerable experience in pre-hospital emergency care and within weeks we will also have ‘Off Duty’ National Ambulance Service staff all volunteering to respond to serious incidents in their communities in their free time. Their equipment is entirely funded by donations and fundraisers carried out by members of the public in Waterford and across the country. It costs ¤1,500 to provide the equipment for each off-duty paramedic, ¤25,000 to establish an Advanced Level Doctor and ¤120,000 to put a new Critical Care response vehicle on the road. We are calling on the people of Waterford to support our charity in the knowledge that their donations will save lives in Waterford.”
For more information about CRITICAL or to make a donation visit https://criticalcharity.ie/
The 10th All Ireland Pharmacy Conference (AIPC) will be held at Ballymascanlon House Hotel, Dundalk on 6th and 7th November 2023. The theme for this year’s conference is ‘Creating Momentum & Building Capacity’
This conference is jointly coordinated by the Northern Ireland Centre for Pharmacy Learning and Development and the Irish Institute of Pharmacy. Its focus is to share good practice in pharmaceutical care and practice development across the primary and secondary care sectors. Pharmacists, technicians and qualified assistants are encouraged to exchange ideas for pharmaceutical service development in the Republic of Ireland and Northern Ireland.
On the evening of Monday 6th November, the conference dinner will take place at 8.00pm and provides an opportunity to network with colleagues. The main conference proceedings will start at 9.30am on Tuesday 7th November and will involve parallel oral sessions and poster presentations. Visit www.allirelandpharmacyconference.com for full details.
24 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Picture: Patrick Browne
News
Testosterone Therapy
Use of Testosterone Therapy in Menopausal Women
Menopause awareness is steadily growing, and more women are seeking information from friends, healthcare practitioners and social media to learn about menopause and HRT. An increasing area of interest in menopause is the use of testosterone and the perceived benefits thereof. Women may have heard that testosterone is ‘the missing hormone’ and will come to you asking for more information and or a prescription. It is helpful to understand when testosterone should be used, and the potential benefits and risks.
Testosterone in women comes from three sources:1
1. The ovaries (25%)
2. The adrenal glands (25%)
3. Peripheral conversion of androstendione (from the ovaries and adrenal glands) into testosterone
Levels of testosterone decline gradually with age, with the greatest drop occurring in women between the ages of 20 and 40.2
By menopause, the levels have plateaued and remain mostly stable. Levels only drop abruptly after bilateral oophorectomy.3
Androgens in women are essential for development of female sexual anatomy and physiology,4 as well as modulation of sexual behaviour and desire.3
The decision of when to prescribe testosterone should be based on symptoms and not on serum levels of testosterone. Women may have low testosterone levels and remain asymptomatic –testosterone replacement in this case in not appropriate.4 Currently, the only prescribing indication for testosterone is in postmenopausal women with low libido which
is causing distress (hypoactive sexual desire disorder).3
Hypoactive sexual desire disorder (HSDD) is defined as ‘persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress and interpersonal difficulty’.3 A biopsychosocial approach should be taken in women with low libido as it is very multifactorial. Other causes, including relationship difficulties, psychological concerns, physical symptoms, sociocultural issues and medication side effects should be assessed for and treated appropriately first.3,4
Thus far, studies to date have not demonstrated beneficial effects of testosterone in women for mood, cognition, energy and musculoskeletal health.4 There is a need for good quality studies with these health issues as primary outcomes, as some patients do report an improvement in these areas with the use of testosterone therapy.4 There is also very little data currently available on the use of testosterone in premenopausal women.4
NICE Menopause Guidelines suggest that before a woman is started on testosterone therapy she should be offered a trial of conventional HRT,4 as her symptoms may improve simply with the initiation of an appropriate HRT regime. The incidence of potential adverse side effects may also be higher in women who are on testosterone-only therapy.4

If testosterone is to be used in a patient, there are various preparations available. There are currently no licensed testosterone products available for women in Ireland and the UK. It is therefore
 Written by Dr Genevieve Ferraris, GP and Menopause Specialist, The Menopause Hub
Written by Dr Genevieve Ferraris, GP and Menopause Specialist, The Menopause Hub

reasonable to prescribe licensed male preparations for female patients and down-titrated to an appropriate female dose.3,4 It is important that when counselling patients on the benefits and risks of testosterone that the use of an unlicensed product is also discussed, and recorded in their notes.2 IMC guidance on the prescription of unlicensed medication should be consulted. Of note, there is a 1% testosterone cream for women licensed in Australia (AndroFeme®) but which remains unlicensed outside of Australia.
Transdermal preparations provide the most physiologic form of replacement; intramuscular injections, subcutaneous pellets and oral preparations should be avoided in case of supraphysiologic levels and side effects.3 Transdermal preparations should be dosed to maintain premenopausal physiologic ranges – in most cases this will be one tenth of the prescription male dose.3,4 One of the most commonly prescribed products for women in Ireland is Testogel® 50mg sachets. Women should apply one tenth of a sachet (ie 5mg per day) once daily to the upper outer thigh or buttock. AndroFeme® is also available and is dosed as 0.5ml daily with a dosing applicator. It is applied in the same way as Testogel.
The most common side effects with testosterone are excess hair growth (especially in areas of gel application), acne and weight gain.4 These are reversible with dose reduction or discontinuation of the product. Male pattern baldness, voice deepening and clitoromegaly are very unlikely with physiologic doses.4 Clinical trials have shown no impact on lipid metabolism, cardiometabolic makers, liver function and breast density, however there remains a lack of long term safety data.3
Patients should be monitored for clinical response to treatment, with most patients noting an improvement in sexual function between 6 weeks and 3 months of use.3,4 They should then be followed up every 6 - 12 months, noting both benefits and any side effects.4 Treatment should be discontinued after 6 months of use if there has been no clinically significant improvement in symptoms.3
The British Menopause Society recommends that, prior to initiating treatment, women have blood tests to establish baseline testosterone levels (and to ensure that levels are not actually high before starting therapy).4 Total testosterone levels should then be repeated between 6 weeks and 3 months from starting, and thereafter every 6 – 12 months.4 Levels should be maintained within a physiologic range. Total testosterone levels, rather than free androgen index, provide a more accurate representation of response to treatment.
The menopause transition can be a difficult time in a woman’s life, and she may seek any treatment that could improve her symptoms. While testosterone does have a role to play in managing some of these symptoms, the benefits may have been oversold in the general media and we should remain mindful as practitioners as to when, why and how we prescribe it. However there are good guidelines that exist as above, and in patients where it is appropriate to prescribe testosterone we should not be afraid to do so.
References available on request
25 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Where two worlds meet

VABYSMO® is the first bispecific antibody for the eye that targets two distinct pathways in
VEGF-A, vascular endothelial growth factor A; Ang-2, angiopoietin-2; nAMD, neovascular (wet) age-related macular degeneration; DME, diabetic macular oedema.
Reference: 1. Wykoff CC et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. The Lancet 2022; 399(10326):741-755.
ABRIDGED PRESCRIBING INFORMATION (API)
For full prescribing information refer to the Summary of Product Characteristics [SmPC].
Vabysmo® ▼(faricimab) 120 mg/mL solution for injection (each vial contains 28.8 mg faricimab in 0.24 mL solution. This provides a usable amount to deliver a single dose of 0.05 mL solution containing 6 mg of faricimab).
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See box below for details on how to report. Therapeutic Indications: Vabysmo is indicated for the
treatment of adult patients with: neovascular (wet) age-related macular degeneration (nAMD); visual impairment due to diabetic macular oedema (DME). Posology and Method of Administration: This medicinal product must be administered by a qualified physician experienced in intravitreal injections. Each vial should only be used for the treatment of a single eye. nAMD: the recommended dose is 6 mg (0.05 mL solution) administered by intravitreal injection every 4 weeks (monthly) for the first 4 doses. Thereafter, an assessment of disease activity based on anatomic and/or visual outcomes is recommended 20 and/or 24 weeks after treatment initiation so that treatment can be individualised. In patients without disease activity, administration of faricimab every 16 weeks (4 months) should be considered. In patients with disease activity, treatment every 8 weeks (2 months) or 12 weeks (3 months) should be considered. If anatomic and/or visual outcomes change, the treatment interval should be adjusted accordingly, and interval reduction should be implemented if anatomic and/or visual outcomes deteriorate. There is limited safety data on treatment intervals of 8 weeks or less between injections. Monitoring between the dosing visits should be scheduled based on the patient’s status and at the physician’s discretion, but there is no requirement for monthly monitoring between injections. DME: the recommended dose is 6 mg (0.05 mL solution) administered by intravitreal injection every 4 weeks (monthly) for the first 4 doses. Thereafter, treatment
NOW AVAILABLE*
nAMD and DME1
*Now available in some private health insurer settings. Pharmacovigilance and adverse event reporting information can be found below.
is individualised using a treat-and-extend approach. Based on the physician’s judgement of the patient’s anatomic and/or visual outcomes, the dosing interval may be extended up to every 16 weeks (4 months), in increments of up to 4 weeks. If anatomic and/or visual outcomes change, the treatment interval should be adjusted accordingly, and interval reduction should be implemented if anatomic and/or visual outcomes deteriorate. Treatment intervals shorter than 4 weeks between injections have not been studied. Monitoring between the dosing visits should be scheduled based on the patient’s status and at the physician’s discretion, but there is no requirement for monthly monitoring between injections. Method of administration: for intravitreal use only. Vabysmo should be inspected visually for particulate matter and discoloration prior to administration, and if present, the vial should not be used. Proper aseptic injection techniques must always be used when administering Vabysmo. Refer to Vabysmo SmPC for full details. Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in the SmPC. Active or suspected ocular or periocular infections. Active intraocular inflammation. Special Warnings and Precautions for Use: Refer to Vabysmo SmPC for full details. Traceability: in order to improve the traceability of biological medicinal products, the name and the batch number of the administered medicinal product should be clearly recorded. Intravitreal injection-related reactions: intravitreal injections, including those with faricimab, have been associated with endophthalmitis, intraocular inflammation, rhegmatogenous retinal detachment, retinal tear and iatrogenic traumatic cataract. Proper aseptic injection techniques must always be used when administering Vabysmo. Patients should be instructed to report any symptoms, such as pain, loss of vision, photophobia, blurred vision, floaters, or redness, suggestive of endophthalmitis or any of the abovementioned adverse reactions without delay, to permit prompt and appropriate management. Patients with increased frequency of injections may be at increased risk of procedural complications. Intraocular pressure increases: transient increases in intraocular pressure (IOP) have been seen within 60 minutes of intravitreal injection, including those with faricimab. Special precaution is needed in patients with poorly controlled glaucoma (do not inject Vabysmo while the IOP is ≥ 30 mmHg). In all cases, both the IOP and perfusion of the optic nerve head must be monitored and managed appropriately. Systemic effects: systemic adverse events including arterial thromboembolic events have been reported following intravitreal injection of vascular endothelial growth factor (VEGF) inhibitors and there is a theoretical risk that these may be related to VEGF inhibition. A low incidence rate of arterial thromboembolic events was observed in the faricimab clinical trials in patients with nAMD and DME. There are limited data on the safety of faricimab treatment in DME patients with high blood pressure (≥ 140/90 mmHg) and vascular disease, and in nAMD patients ≥ 85 years of age. Immunogenicity: as this is a therapeutic protein, there is a potential for immunogenicity with faricimab. Patients should be instructed to inform their physician of any signs or symptoms of intraocular inflammation such as vision loss, eye pain, increased sensitivity to light, floaters or worsening eye redness, which might be a clinical sign attributable to hypersensitivity against faricimab. Bilateral treatment: the safety and efficacy of faricimab administered in both eyes concurrently have not been studied. Bilateral treatment could cause bilateral ocular adverse reactions and/or potentially lead to an increase in systemic exposure, which could increase the risk of systemic adverse reactions. Until data for bilateral use become available, this is a theoretical risk for faricimab. Concomitant use of other anti-VEGF: there are no data available on the concomitant use of faricimab with anti-VEGF medicinal products in the same eye. Faricimab should not be administered concurrently with other anti-VEGF medicinal products (systemic or ocular). Withholding treatment: treatment should be withheld in patients with:
• Rhegmatogenous retinal detachment, stage 3 or 4 macular holes, retinal break; treatment should not be resumed until an adequate repair has been performed.
• Treatment related decrease in Best Corrected Visual Acuity (BCVA) of ≥ 30 letters compared with the last assessment of visual acuity; treatment should not be resumed earlier than the next scheduled treatment. • An intraocular pressure of ≥ 30 mmHg. • A subretinal haemorrhage involving the centre of the fovea, or, if the size of the haemorrhage is ≥ 50%, of the total lesion area. • Performed or planned intraocular surgery within the previous or next 28 days; treatment should not be resumed earlier than the next scheduled treatment. Retinal pigment epithelial tear: retinal pigment epithelial (RPE) tear is a complication of pigment epithelial detachment (PED) in patients with nAMD. Risk factors associated with the development of a retinal pigment epithelial tear after antiVEGF therapy for nAMD, include a large and/or high pigment epithelial detachment. When initiating faricimab therapy, caution should be used in patients with these risk factors for retinal pigment epithelial tears. RPE tears are common in nAMD patients with PED, treated with IVT anti-VEGF agents including faricimab. There was a higher rate of RPE tear in the faricimab group (2.9%) compared to aflibercept group (1.5%). The majority of events occurred during the loading phase, and were mild to moderate, without impact on vision. Populations with limited data: there is only limited experience
in the treatment of nAMD patients ≥ 85 years, and DME patients with type I diabetes, patients with HbA1c over 10%, patients with high-risk proliferative diabetic retinopathy (DR), high blood pressure (≥ 140/90 mmHg) and vascular disease, sustained dosing intervals shorter than every 8 weeks (Q8W), or nAMD and DME patients with active systemic infections. There is limited safety information on sustained dosing intervals of 8 weeks or less and these may be associated with a higher risk of ocular and systemic adverse reactions, including serious adverse reactions. There is also no experience of treatment with faricimab in diabetic patients with uncontrolled hypertension. This lack of information should be considered by the physician when treating such patients. Sodium content: This medicinal product contains less than 1 mmol sodium (23 mg) per dose, that is to say essentially “sodium-free”. Interactions: No interaction studies have been performed. Based on the biotransformation and elimination of faricimab, no interactions are expected. However, faricimab should not be administered concurrently with other systemic or ocular anti-VEGF medicinal products. Fertility, Pregnancy and Lactation: Women of childbearing potential: should use effective contraception during treatment and for at least 3 months following the last intravitreal injection of faricimab. Pregnancy: there are no or limited amount of data from the use of faricimab in pregnant women. The systemic exposure to faricimab is low after ocular administration, but due to its mechanism of action (i.e. VEGF inhibition), faricimab must be regarded as potentially teratogenic and embryo-/foetotoxic. Faricimab should not be used during pregnancy unless the potential benefit outweighs the potential risk to the foetus. Breast-feeding: it is unknown whether faricimab is excreted in human milk. A risk to the breast-fed newborn/infant cannot be excluded. Vabysmo should not be used during breast-feeding. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from faricimab therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. Fertility: no effects on reproductive organs or fertility were observed in a 6-month cynomolgus monkey study with faricimab. Effects on ability to drive and use machines: Vabysmo has a minor influence on the ability to drive and use machines. Temporary visual disturbances may occur following the intravitreal injection and the associated eye examination. Patients should not drive or use machines until visual function has recovered sufficiently. Undesirable Effects: The most frequently reported adverse reactions were cataract (13%), conjunctival haemorrhage (8%), vitreous detachment (5%), IOP increased (4%), vitreous floaters (4%), eye pain (3%) and retinal pigment epithelial tear (nAMD only) (3%). The most serious adverse reactions were uveitis (0.6%), endophthalmitis (0.5%), vitritis (0.3%), retinal tear (0.2%), and rhegmatogenous retinal detachment (0.1%) and traumatic cataract (< 0.1%). All grades: Very common (≥ 1/10): cataract. Common (≥1/100 to <1/10): conjunctival haemorrhage, vitreous detachment, vitreous floaters, Retinal pigment epithelial tear (nAMD only), increased intraocular pressure, eye pain, increased lacrimation, corneal abrasion, eye irritation. Uncommon (≥ 1/1,000 to < 1/100): vitreous haemorrhage, ocular discomfort, eye pruritus, ocular hyperaemia, blurred vision, iritis, uveitis, iridocyclitis, vitritis, sensation of foreign body, endophthalmitis, retinal tear, conjunctival hyperaemia, procedural pain, reduced visual acuity, rhegmatogenous retinal detachment. Rare ((≥ 1/10,000 to < 1/1,000): transiently reduced visual acuity, traumatic cataract. Refer to SmPC for full listings of adverse events. Refer to SmPC section 4.8 for instructions on reporting of suspected adverse events. Legal Category: Product subject to prescription which may not be renewed (A). Presentation(s) and Marketing Authorization Number(s): one mL of solution contains 120 mg of faricimab (EU/1/22/1683/001). Marketing Authorisation Holder: Roche Registration GmbH, Emil-Barell-Strasse 1, 79639 Grenzach-Wyhlen, Germany. Vabysmo® is a registered trademark. Further information is available from Roche Products (Ireland) Limited, 3004 Lake Drive, Citywest, Naas Road, Dublin 24. Telephone: (01) 4690700. Date of API Preparation: August 2023.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. In the event of a suspected adverse reaction, please report it to:
The Drug Surveillance Centre
Roche Products (Ireland) Limited
Telephone: (01) 4690700
Email: ireland.drug_surveillance_centre@roche.com
Or alternatively, report to:
HPRA Pharmacovigilance
Website: www.hpra.ie
Vabysmo® Summary of Product Characteristics [SmPC] is available on www. medicines.ie. M-IE-00001609 - based on Vabysmo SmPC dated 22 June 2023
Date of item: August 2023. M-IE-00001420 (v 2.0)
Gestational diabetes in women living with HIV in the UK and Ireland: insights from population-based surveillance data
Written by Laurette L.
Laurette L. Bukasa

risk have been inconsistent.17-20 Using population-based data from the UK and Ireland from 2010 to 2020, our aim was to describe the prevalence of GD in WLWH, assess associated maternal risk factors and examine specific birth outcomes of pregnancies affected by GD.
Methods
Integrated Screening Outcomes Surveillance Service
and received approvals from the West Midlands—Solihull Research Ethics Committee (21/WM/0040) and the Antenatal and Newborn Screening Programme Research Advisory Committee (ANNB_ IDPS_0037).
Analysis dataset
The risk of gestational diabetes (GD) is increasing proportionally to type 2 diabetes in the general population and now affects an estimated 16% of pregnancies globally.1 Pregnancy is a physiological state of reduced insulin sensitivity2 and GD is thought to result from complex mechanisms involving beta-cell dysfunction influenced by genetic, epigenetic and environmental factors.3 Pregnant women in the UK considered to be “at risk” of developing GD are selectively screened at 24–28 weeks’ gestation based on body mass index (BMI) >30 kg/m2, previous macrosomic baby (>4.5 kg), family history of diabetes and belonging to a minority ethnic group with a high prevalence of diabetes.4 In 2015, clinical guidelines recommended a fasting plasma glucose ≥5.6 mmol/l or 2-hour glucose on the oral glucose tolerance test ≥7.8 mmol/l for GD diagnosis, which differs from international criteria (≥5.1 and ≥8.5 mmol/l) and was lowered from ≥7.0 mmol/l for fasting plasma glucose in 2008 based on findings from the HAPO study.5 While the universal risk factors for GD are reasonably well understood forming the basis of screening criteria, questions remain regarding the risk
environment for women living with HIV (WLWH).

In the UK, there are approximately 800 pregnancies in WLWH each year, with 82% resulting in a delivery.6 The vertical transmission rate has decreased by 90%, from 2.1% in 2000–2001 to 0.22% in 2017–2018.7 Guidelines for HIV management, including in pregnancy, have evolved over time alongside the development of new antiretroviral drugs and classes, influencing both the timing of treatment initiation and drugs used in pregnancy.8 Since 2015 and the onset of the universal treatment era, women have increasingly been on treatment prior to conception;9 for example, in the UK, 81% of pregnancies were conceived on antiretroviral therapy (ART) in 2018.10 Furthermore, there have been changes in the characteristics of pregnant WLWH, including increasing maternal age, a growing number with vertically acquired HIV and a decreasing proportion born in sub-Saharan Africa.11, 12 The implications of lifelong treatment for nonpregnant populations may include increased risk of diabetes3, 14 and cardiovascular disease,15, 16 but findings on the effect of treatment in pregnancy, particularly protease inhibitors (PIs), on GD
Integrated Screening Outcomes Surveillance Service (ISOSS) is commissioned by NHS England as part of the NHS Infectious Diseases in Pregnancy Screening Programme to conduct comprehensive populationbased active surveillance of all pregnancies to diagnosed WLWH in the UK and Ireland since 1989 (formerly the National Study of HIV in Pregnancy and Childhood).10 Data from Ireland are included until 2018, and Scotland, Wales and Northern Ireland included until 2019.6 Universal screening for HIV in pregnancy has been a standard of antenatal care since 200121 and maternity reports of all pregnancies in WLWH are submitted by antenatal units at notification (antenatal booking) and at outcome (delivery); they include information on sociodemographics, ART (type and timing), obstetric management and mode of delivery (emergency caesarean section [CS], elective CS and vaginal). Pregnancy complications were defined as any pre-existing (i.e. diabetes and hypertension) or incident (e.g. GD, pre-eclampsia) conditions. Reports are submitted by a named responder in each maternity unit via a secure online portal.10 All data are collected as part of routine antenatal care and selected data are shared with ISOSS without patient consent under Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002.22 This research involved the secondary use of ISOSS data
Analyses were conducted on a pseudonymized dataset of all pregnancies in women with HIV diagnosed before delivery (i.e. before and during pregnancy) with a reported outcome ≥24 weeks’ gestation and estimated date of delivery (EDD) between 1 January 2010 and 30 June 2020 reported by September 2020. Women with a HIV-2 diagnosis, and women with a multiple pregnancy were excluded.
Descriptive analyses used a dataset, including pregnant women with reported GD and pregnancies without a report of GD. Analyses of adverse birth outcomes and risk factors for GD used a dataset, including women with GD and a comparison population of women without GD or any other pregnancy complication reported, Figure 1.
Definitions and classifications
All reports of GD were classified as a case (WLWH-GD). We considered ART use (Yes/No) and any use of the main third agent classes (Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI), Integrase Strand Transfer Inhibitor (INSTI) and PI) in pregnancy (i.e. if a woman had a third agent drug switch in pregnancy, she would be included in all the third agent classes used). HIV-1 RNA viral load (VL) results were recorded at antenatal booking and delivery (from 36 weeks gestation to 7 days postpartum) where available. ISOSS requests a minimum of one CD4 count but up to two VL test results can be reported. We defined the first CD4 count as the earliest reported measurement between 8 weeks before the last menstrual period (LMP) and 1
28 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Gestational Diabetes
Bukasa, Mario Cortina-Borja, Helen Peters, Graham P. Taylor, Claire Thorne
diabetes in women living with HIV in the UK and Ireland: insights from population‐ based surveillance
covariates based on univariable analyses. Multicollinearity within the model was assessed using the variance inflation factor.23
All covariates were included in model selection using penalized regression methods with ART at conception and PI use in pregnancy remaining in all estimation models a priori. Covariates were included based on the most parsimonious model accounting for existing knowledge on possible risk factors.
To account for women with more than one pregnancy in the study, the multivariable independent maternal effects regression model was fitted using generalized estimating equations (GEE). The quasi-likelihood information criterion was used to assess the goodness of fit24 in a completecase analysis of independent effects. Sensitivity analyses were conducted to evaluate time-related effects on risk factors for GD and the effect of women with >1 pregnancy affected by GD. All univariable analyses were conducted in STATA/SE 15.1 (StataCorp LLC, College Station, Texas, USA), with multivariable analyses conducted in R 4.0.4 (R Core Team (2020)).
Results
Journal of the International AIDS Society, Volume: 26, Issue: 4, First published: 03 April 2023, DOI: (10.1002/jia2.26078)
International AIDS Society, Volume: 26, Issue: 4, First published: 03 April 2023, DOI: (10.1002/jia2.26078)
week after delivery, categorized as ≤350 and >350 cells/mm3. LMP was estimated as 280 days prior to EDD. Coinfections were defined as reported hepatitis B, hepatitis C or syphilis, with other infections provided ad hoc. HIV clinical status relates to reported symptoms in pregnancy, including AIDS events. Parity since diagnosis was estimated for each woman based on the number of pregnancies ever recorded in ISOSS as a proxy.
Macrosomia was defined as birthweight ≥4.00 kg and preterm delivery as occurring at <37 gestational weeks. Stillbirth was defined as an intrauterine death occurring at ≥24 gestational weeks. Adverse birth outcomes were described by year group (2010–2014 vs. 2015–2020) to explore the effect of both the universal HIV treatment era (from 2015) and the lowered fasting plasma glucose diagnostic threshold for GD.
Risk factor analyses
The EDD year, maternal sociodemographics (age at delivery, ethnicity, region of origin, parity since diagnosis) and maternal HIV-related characteristics (risk factor for HIV acquisition, timing of diagnosis, coinfections, clinical status, treatment at conception, type of treatment, VL and CD4 count in pregnancy) were included in univariable analyses.
The multivariable independent maternal effects model included
Of the 10,553 pregnancies in 7916 women reported by September 2020, 800 (7.6%) pregnancies were missing GD status and not included in analyses (Figure 1). Pregnancies in WLWH who were missing GD status were more likely to have been pregnant before 2015, to have acquired HIV by injecting drug use (p < 0.001), been diagnosed during pregnancy (p = 0.018) and received PI-based treatment (p < 0.001); maternal age and ethnicity did not differ between pregnancies with and without GD status (Table S1). In the remaining 9753 pregnancies, 460 (4.72%, 95% CI: 4.30%, 5.16%) pregnancies were in women with reported GD and 9293 in women without reported GD, of which 7747 pregnancies were included in a comparison group with no reported pregnancy complications (WLWH-C), Figure 1.
Twenty-eight women had >1 GD pregnancy, contributing 59 (12.8%) of the total 460 cases; of all GD pregnancies in women from Asian ethnic backgrounds, 20.8% (5/24) were in women with >1 GD pregnancy, compared to 14.3% (50/348) of women from Black

29 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Gestational diabetes in women living with HIV in the UK and Ireland: insights from population-based surveillance data
Gestational Diabetes
Gestational diabetes in women living with HIV in the UK and Ireland: insights from population‐ based surveillance data
Journal of the International AIDS Society, Volume: 26, Issue: 4, First published: 03 April 2023, DOI: (10.1002/jia2.26078)
Journal of the International AIDS Society, Volume: 26, Issue: 4, First published: 03 April 2023, DOI: (10.1002/jia2.26078)
ethnic backgrounds (p < 0.001). Overall, GD prevalence increased from 3.39% (95% CI: 2.92–3.91) in 2010–2014 to 6.39% (95% CI: 5.68–7.17) in 2015–2020 with half (49.8%, 229/460) of all GD cases reported since 2016 (Figure 2).
Median maternal age was 33 years (IQR: 8, Q1:29–Q3:37), with 71.8% of pregnancies in Black African women and 64% of pregnancies conceived on treatment. For WLWH with timing of ART initiation available, 96.4% (9901/10,266) had received treatment before the third trimester. PIs were the most common third agent (6188/10,553, 58.6%). A similar number of pregnancies were reported during the study period within the COVID-19 pandemic (March–June 2020, N = 185) compared to the previous four months (November 2019–February 2020, N = 186); GD prevalence across both periods among pregnancies with a GD status was comparable (13.1% [23/175] vs. 8.67% [15/173], p = 0.185).
Overall, 51.3% (235/458) of pregnancies in WLWH-GD were exposed to PIs, with 82.6% exposed from conception (i.e. no drug switches). An increasing proportion of WLWH-GD were on treatment at conception over time (61.4% [62/101] in 2010–2012 vs. 82% [187/228] in 2016–2020). PI-based regimens were used less frequently over time, decreasing from 70.0% (70/100) in 2010–2012 to 42.7% (97/227) in 2016–2020. Over time, an increasing proportion of WLWH-GD were aged ≥35 years (49.5% [50/101] in 2010–2012 vs. 61.8% [141/228] in 2016–2020, p < 0.001) and had an undetectable first VL in pregnancy (48.5% [49/101] in 2010–2012 vs. 66.8% [153/229] in 2016–2020, p = 0.018).
There were no statistically significant differences between pregnancies in WLWH and WLWH-GD regarding exposure to any ART in pregnancy (p = 0.774), concurrent infections (p = 0.349) and HIV clinical status (p = 0.390), but WLWH-GD were more likely to have initiated ART before the third

trimester (p < 0.001), less likely to have received ≥3 drug classes (p = 0.573) and have a lower first VL in pregnancy (p = 0.016) than other WLWH.
Adverse birth outcomes were described for 8207 pregnancies: 460 pregnancies with GD and 7747 pregnancies in WLWH-C. Pregnancies in WLWH-GD were more likely to end in stillbirth (Odds ratio (OR): 5.38, 95% CI: 2.14–13.5) and preterm delivery (OR: 2.54, 95% CI: 1.95–332) than WLWH-C. Emergency CS (OR: 1.54, 95% CI: 1.22–1.94) compared to elective CS, and fetal macrosomia (OR: 1.14, 95% CI: 1.04–1.24) were also more common among WLWH-GD than WLWH-C. When stratified by time period, all statistically significant associations between GD and adverse birth outcomes remained aside from fetal macrosomia.
A GEE model with an exchangeable correlation structure was superior to other correlation structures and was used in the independent effects model based
on a complete case analysis of pregnancies (6880/8207) in WLWH-GD and WLWH-C. After adjusting for covariates, the odds of GD increased by 14% each year over the study period. Pregnancies in women aged ≥35 years (GEEadjusted odds ratio (aOR): 2.87, 95% CI: 1.54–5.34) and from Asian (GEE-aOR: 2.60, 95% CI: 1.46–4.63), Black Caribbean (GEEaOR: 2.63, 95% CI: 1.40–4.93) and Black African (GEE-aOR: 1.55, 95% CI: 1.13–2.12) backgrounds were at greater risk of developing GD compared to women aged <25 years and women from White backgrounds, respectively. Low CD4 cell count (<350) was associated with reduced odds of developing GD (GEE-aOR: 0.73, 95% CI: 0.56–0.96). No relationships were observed for treatment at conception or PI use. The independent maternal effects model with GEE was fitted for 5576 clusters (max size: 5) with some evidence of populationlevel within-cluster correlation for women with multiple pregnancies (α = 0.307, p = 0.06).
30 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Gestational diabetes in women living with HIV in the UK and Ireland: insights from population-based surveillance data
When women with more than one GD pregnancy were excluded (n = 8148), EDD year, age, ethnicity and CD4 count remained significant risk factors; additionally, multiparous WLWH since HIV diagnosis were less likely to have a pregnancy affected by GD (Table S3). Advanced maternal age (≥35 years) (p < 0.001) and Asian ethnicity (p < 0.001) remained significant risk factors for GD in sensitivity analyses across both 2010–2014 (n = 4712) and 2015–2020 (n = 3495) time periods, while the association with CD4 count was only present in 2010–2014 (p = 0.107), Table S3. Pregnancies in WLWH from Black African and Black Caribbean ethnic backgrounds were at greater risk of GD compared to women from White ethnic backgrounds in the 2010–2014 period alone.

Discussion
UK and Ireland national surveillance data were used to characterize and identify risk factors for pregnant WLWH who were affected by GD delivering between 2010 and 2020. There were higher rates of treatment at conception, undetectable VL and CD4 cell counts ≥350 in WLWH-GD pregnancies compared to WLWH without GD or other pregnancy complications. The odds of GD increased by 14% each year even after adjusting for the ageing and increasingly multiparous population.
Pregnancies with GD were more likely to be in women from minority ethnic groups than White women, and in women with CD4 counts ≥350 cells/μl.
The overall prevalence of GD in our study population (4.72%) was comparable with estimates from the general population in the UK.25, 26 The increasing GD prevalence over time (reaching 8.7% by 2019) may be attributed to concurrent changes in HIV-related and universal factors, including HIV/ART effects on insulin resistance,27, 28 the reduction in the fasting plasma glucose threshold for GD diagnosis,4 and increasing obesity observed in both the general population29 and WLWH.30 Our prevalence estimates are comparable with those reported for WLWH in other high-income settings (2.6–11.4%);17, 31-33 however, comparisons must be made with caution given differences in diagnostic criteria. As increases in GD prevalence are
observed in the general obstetric population both in high-income34 and low-income settings,35 challenges remain in determining whether an excess risk exists for WLWH.
While only capturing data on pregnancies in the first 4 months of the COVID-19 pandemic, GD prevalence was comparable with within-study estimates from November 2019 to February 2020 and general population estimates based on hospital admissions data (8.3%),36 despite possible underestimation due to deviation from standard screening guidelines.37
Few studies of WLWH have explored temporal trends in GD prevalence, although findings from earlier periods (1990–2006) reported an upward trend with 2–3% increases over time, with increased coverage with ART considered a contributing factor.38, 39 Here, the temporal increase in GD prevalence persisted despite adjustment for timing and type of ART alongside universal risk factors, such as ethnicity. Use of ART in pregnant WLWH has changed significantly over the study period from treatment by indication (CD4 < 350)40 to lifelong treatment from diagnosis;41 however, sensitivity analyses showed that neither ART use at conception nor use of PIs in pregnancy were associated with GD in these two treatment eras when considered individually. Recent studies have also reported similar findings,42, 43 although PI-based regimens were implicated in earlier studies17, 18 with a meta-analysis suggesting that this association may be limited to studies where firstgeneration PI and the strictest GD diagnostic criteria were used.20
The use of newer drugs in pregnancy, such as some integrase inhibitors, could become increasingly relevant for GD risk in the future although insufficient sample sizes precluded analyses here. Evidence is currently accumulating on the effects of ART on fat mass accrual both in and outside of pregnancy.30
A comparative analysis of randomized clinical trials has shown that weight gain is greater in more recent trials with the use of newer ART regimens,44 which is consistent with post-marketing observational studies of integrase inhibitors.45 Preliminary studies of WLWH on integrase inhibitors
during pregnancy show no cause for concern regarding gestational weight gain;42, 46 however, a risk prediction model based on data from South Africa suggests that treatment emergent obesity with dolutegravir-based regimens (with tenofovir alafenamide or disoproxil and emtricitabine) for obese women (BMI ≥30 kg/m2) versus those with normal BMI could increase the risk of GD four-fold (RR: 4.31, 95% CI: 3.18–5.85).47 Women with CD4 counts <350 were less likely to develop GD than other women in our study. Restoration of the immune system through ART, approximated by higher CD4 counts, could increase the risk of some pregnancy complications.48 The “return to health” effect is associated with increases in CD4 counts and weight gain,44 while lower CD4 counts are associated with lower weight and could potentially reflect poorer ART adherence.49, 50 An alternative pathway could involve reduced albumin levels (associated with lower CD4 counts in the nonpregnant population51), given the link between high albumin levels and insulin resistance.52
Advanced maternal age has been associated with GD both in the general population53, 54 and among WLWH.17, 55, 56 Women ≥35 years were most likely to develop GD in our population, which could be a proxy for age-related weight gain.57 A recent study in Botswana reported similar findings with GD risk increasing linearly with each year of age (aOR: 1.10, 1.04–1.17).42 Maternal age is also related to gravidity, which was accounted for in the modelling strategy; however, evidence from the general population indicates that the incidence of GD is three- to six-fold higher among women over 40 years of age,58 and a significant proportion of women who develop GD in their first pregnancy are at greater risk of developing GD in subsequent pregnancies.59, 60 Approximately 6.5% of women with GD had more than one GD pregnancy, which is substantially lower than recurrent rates of 30–60% reported in the general pregnant population;59, 61, 62 this was likely due to the restricted study period and lack of data on previous pregnancies prior to HIV diagnosis or arrival in the UK that may have been affected by GD. WLWH from Asian backgrounds (representing only 3% of our study population) were at the greatest risk of GD, consistent
with global pooled prevalence estimates.63 Similarly, women from Black African backgrounds were approximately 1.5 times more likely to have GD than White women, consistent with women belonging to this ethnic group being identified as “at risk” of GD in the general population.4
Pregnancies in WLWH-GD were more likely to have adverse birth outcomes compared to women without reported pregnancy complications (WLWH-C). While prevalence of adverse birth outcomes among WLWH-GD from 2010–2014 to 2015–2020 declined, stillbirth, preterm delivery and CS remained more common among WLWH-GD than WLWH-C. These findings are comparable to results from studies in the general population that indicate that women who develop GD in pregnancy are more likely to have a preterm delivery64 and have a CS,65 partly due to fetal macrosomia.66 The CS rate in our study was similar to the general population in other high-income countries, but the preterm delivery and stillbirth rate were greater.67 Stillbirths among WLWH-GD (1.30%) were five times greater than WLWH-C (0.25%) and the general population (0.3%).68 Evidence on stillbirth risk for women affected by GD in the general population is conflicting;69, 70 however, among WLWH, strong relationships between GD and stillbirth have been reported in the UK with a 2.8-fold increased incident rate ratio of stillbirth among women with GD.71 A South African study of pregnant women with diabetes also reported elevated perinatal mortality in WLWH compared with other women (9.4% vs. 1.8%, p < 0.001).72 Collectively, these studies indicate that WLWH may require specific management.
While the use of population-level surveillance data on pregnant WLWH was a study strength, it was also a source of limitations as several important variables were not collected. This includes BMI, a GD risk factor in the general population73, 74 and among WLWH42 that could not be accounted for in our analyses. Approximately 41% of WLWH in the UK are overweight,75 with an increasing trend in general obesity reported nationally,29 highlighting complex interactions with GD that could not be elucidated in our study. Similarly, any GD diagnoses in pregnancies outside of the study
31 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
period, prior to HIV diagnosis and/or arrival in the UK were not available and could, therefore, not be included in adjusted analyses. In the absence of data on historical GD diagnoses, BMI and known risk factors for GD, such as family history of diabetes, we were unable to assess whether these factors influenced GD risk and any interaction with HIV-related factors, such as ART use.
Our analyses of adverse birth outcomes and GD risk factors compared WLWH-GD with a selected sub-group of pregnancies without complications based on
Gestational Diabetes
available data. A more appropriate control group would have been women not meeting the threshold for a GD diagnosis and without any known GD risk factors (for which we lacked data), as women “at risk” of GD based on screening criteria but who are not screened may have a greater risk of adverse birth outcomes than those screened.76 Data were not available on the management of GD which could have impacted the relative risk of adverse birth outcomes.64
Conclusions
WLWH do not appear to be disproportionately affected by GD
Women's Health News
compared to women in the general population. Our findings highlight that there are shared risk factors with the general population and, in the absence of data on other risk factors, maternal age, ethnicity and CD4 count are associated with GD. Timing and type of ART did not affect GD risk, but the lack of data on key risk factors, such as BMI, particularly in the context of newer drugs, warrants further investigation. Optimizing pregnancy and birth outcomes for WLWH is increasingly important in this low-risk era for vertical transmission, as the risk of stillbirth and preterm delivery are
substantially greater in WLWHGD despite updated clinical GD screening guidelines. Effective management of GD can reduce the risk of adverse outcomes76 and thus further research on how GD is managed in this population is required to inform strategies for intervention, alongside future research to understand better the complex relationships between HIV infection and management, GD, pregnancy complications and birth outcomes.
References available on request
Impact of menopausal symptoms on work and careers: a cross-sectional study
Written by M T O’Neill, V Jones, A Reid Occupational Health & Wellbeing Department, Tallaght University Hospital, Dublin
Abstract
Background
Women over 50 years are one of the fastest-growing employment groups. Menopausal symptoms can adversely impact quality of life, work performance and attendance; however, few studies look at the impact of individual menopausal symptoms on work and career development.
Aims
To measure the prevalence of menopausal symptoms in employees in a healthcare setting, to assess the impact of individual symptoms on work, attendance and career development and to explore perceptions about workplace supports.

Methods
In this cross-sectional study of Irish hospital workers, menopausal employees were asked about the frequency of 10 menopausal symptoms and the extent to which each symptom impacted them at work. Impacts on performance, attendance
and career development were assessed, along with the benefits of workplace support.
Results
Responses from 407 women showed that the most common menopausal symptoms affecting employees greater than 50% of the time while at work were fatigue (54%), difficulty sleeping (47%), poor concentration (44%) and poor memory (40%). Work performance was impacted for 65% of respondents and 18% had taken sick leave. There was a significant association between symptom severity at work and reduced work performance, career development decisions and attendance. Manager awareness about menopause (29%) and flexible working times (29%) were selected as the most important workplace supports.
Conclusions
Female employees are negatively impacted by menopausal symptoms while at work, particularly by psychological and neurocognitive symptoms which
were associated with reduced work performance, attendance and career decisions. Manager awareness and flexible schedules were considered the most beneficial workplace supports.
Key learning points
What is already known about this subject:
• Menopausal symptoms can adversely affect quality of life, work performance and attendance.
• Most workers in the health and social care setting are female, a significant proportion of whom are over 50 years of age.
• Studies in menopause at work focus on the frequency of menopausal symptoms in general; however, few studies look at the impact of individual menopausal symptoms on work, attendance and career development.
What this study adds:
• This study demonstrated that fatigue, difficulty sleeping, poor
concentration and poor memory were the menopausal symptoms that impacted employees most at work.
• A significant association was found between symptoms which impacted upon work 50–100% of the time and work performance, career decisions, and attendance.
• Manager awareness about menopause and flexible work schedules were considered the most beneficial workplace supports.
What impact this may have on practice or policy:
• These findings highlight that psychological and neurocognitive symptoms associated with menopause also need to be addressed in the workplace, in addition to vasomotor symptoms.
• Manager training about menopause and the option of flexible work schedules in certain circumstances should be prioritized in employers’ menopause-at-work policies.
32 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE

Candidiasis
Vulvovaginal candidiasis: Diagnosis and Management
Written by Louise Delany –Antimicrobial Pharmacist, The National Maternity Hospital Dublin

• Immunosuppression.
• Genetic factors.
VVC is uncommon in postmenopausal women, unless they are taking oestrogen therapy. It is also uncommon in prepubertal girls.
Clinical Features
VVC typically presents with vulval itch and a non-offensive vaginal discharge.
Introduction
Candidiasis is a fungal infection caused by yeasts that belong to the genus Candida Candida yeasts are present in low numbers on healthy skin in moist areas and are part of the normal flora of the mucous membranes of the respiratory, gastrointestinal and female genital tracts. Overgrowth of Candida species can cause infections. There are over 20 Candida species that can cause such infections in humans, Candida albicans is the most common and causes 80-89% of vulvovaginal candidiasis (VVC) infections in women. Candida glabrata causes the majority of remaining VVC infections. Approximately 75% of women will experience at least one VVC infection in their lifetime and between 40 and 45% will have two or more infections. Infections can occur without any specific precipitating factor, however there are some risk factors which are known to predispose a woman to VVC infections. Known risk factors include;
• Poorly controlled diabetes mellitus.
• Use of broad-spectrum antibiotics. This is due to inhibition of normal bacterial flora, which allows for growth of Candida. VVC infections may occur up to 3 months after a course of antibiotics is taken.
• Increased oestrogen levels, especially during pregnancy (endogenous oestrogen) and with post-menopausal oestrogen therapy (exogenous oestrogen).

Acute infections
Other symptoms may include:
• Soreness or burning of the area
• Superficial dyspareunia
• Cyclical symptoms
Clinical signs may include:
• Erythema
• Fissuring
• Swelling/ oedema
• Vaginal discharge typically non-offensive and curdy but may be thick
• There may also be satellite lesions and excoriation marks
Acute vulvovaginal candidiasis in pregnancy
Clotrimazole 500mg pessary
Clotrimazole 200mg pessary
Diagnosis
VVC diagnosis is based on typical features of the infection being present, supported by laboratory confirmation of Candida species from a vaginal sample. It is good practice to support a diagnosis with routine microscopy for those presenting to secondary care with symptoms. Recurrent VVC is defined as four or more symptomatic episodes over a 12-month period; at least two of these episodes should be confirmed by microscopyor culture. Clinical examination of the external genitalia is recommended in women presenting with symptoms
PV at night (can give for up to 7 consecutive nights if required)*
PV at night (can give for up to 7 consecutive nights if required)*
*Longer courses are recommended in pregnancy to achieve successful cure. Reviews have found that a four-day course will cure just over 50%, whereas a seven-day course cures over 90%.
Acute vulvovaginal candidiasis (not in pregnancy)
Fluconazole 150mg PO STAT (not for use in pregnancy)
Clotrimazole 500mg pessary
Clotrimazole 200mg pessary
Clotrimazole 10% internal vaginal cream
PV STAT at night
PV at night every 24 hours for 3 nights
5g dose (one applicatorful to be inserted into the vagina at night)
Severe acute vulvovaginal candidiasis
Fluconazole 150mg PO on day 1 and day 4 (not for use in pregnancy)
Clotrimazole 500mg pessary
PV at night on day 1 and day 4
34 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Table 1: Acute Infections
Recurrent infections
Recurrent vulvovaginal candidiasis (pessary regime)
Induction
Clotrimazole 200mg pessary PV at night every 24 hours for 10-14 nights
Clotrimazole 500mg pessary PV at night every 24 hours for 7-14 nights
Followed by maintenance if required:
Clotrimazole 500mg pessary PV at night every week for 6 months
Recurrent vulvovaginal candidiasis (oral regime)
Induction
Fluconazole 150mg PO on day 1 and day 4 and day 7 (total 3 doses) (not for use in pregnancy)
Followed by maintenance if required:
Fluconazole 150mg PO weekly for up to 6 months
suggestive of acute VVC and examination should always be carried out for those presenting with recurrent VVC. Clinical examination should be used to exclude alternative or co-existing vulvovaginal pathologies.
A high vaginal swab should be carried out for microscopy and cultures should be carried out
for samples taken from women with recurrent VVC. Resistance to antifungals is rare, however sensitivity testing should be done for those with recurrent VVC.
Management
Women with a diagnosis of VVC, should be advised to avoid using local irritants such as perfumed soaps or wipes, however,
patients can be advised to use an emollient for personal hygiene as a soap substitute, as a moisturiser or as a barrier cream. Patients need to be aware that this is for use in the outer part of the genital area and not for internal use. For those with recurrent infections of VVC, clinicians should include a review of their daily hygiene routine to identify any potential
Table 2: Recurrent Infections
irritants for example washing hair in bath water or excessive cleaning. Medication causes should be considered for patients presenting with symptoms of VVC; women recently started on oestrogen therapy or those that have received a recent course of antibiotics.
Treatment
Treatment of VVC is based on whether it is a first presentation, a recurrent infection and whether the patient is pregnant at the time of infection. The tables below are based on the treatment recommendations as per British Association for Sexual Health and HIV national guideline for the management of vulvovaginal candidiasis (2019). It should be noted that intravaginal and topical treatment can damage latex condoms and diaphragms; extra precautions are advised. During pregnancy the use of an applicator is not advised, and the pessary should be inserted without using an applicator. Oral fluconazole can interact with medications and a medication history should be taken before prescribing this as a treatment option. Topical therapies can cause vulvovaginal irritation, and this should be considered if symptoms worsen or persist. Fluconazole should be avoided if the woman is pregnant, however breastfeeding can be continued if only a single dose of 150mg fluconazole is administered.
Funding for Assisted Human Reproduction Treatment
Minister for Health Stephen Donnelly has announced that fully publicly-funded assisted human reproduction (AHR) treatment commenced last month (September).
Eligible patients will be entitled to one full cycle of IVF (invitro fertilisation) or ICSI (Intracytoplasmic sperm injection) treatment, initially provided in HSE-approved private clinics of their choice ahead of the opening next year of the first public National Advanced AHR Centre.
The Minister has also set out details of eligibility criteria which
sets parameters in respect of the ages of the intending parents, the female patient’s body mass index (BMI), the number of existing children and the number of previous IVF cycles accessed. Patients with known clinical causes of infertility and patients where there is no known clinical cause will be eligible for treatment. Given the complex regulatory and clinical issues still to be addressed in respect of certain categories of AHR treatment, public funding of a number of specific services is being commenced on a structured and phased basis.
Therefore, treatment involving the use of donated gametes (sperm and eggs) will not be available in September for heterosexual or same-sex couples or single female patients. This treatment will become available as soon as possible.
Under the Model of Care for Fertility, people with fertility-related issues should be treated through the public health service at the lowest level of intervention necessary.
In the first instance, people will present to their GP for a consultation and if appropriate they will be referred to their local
Regional Fertility Hub, which provides a range of treatments and interventions for both males and females.
From September, where IVF, ICSI or IUI is clinically indicated, eligible patients will be referred by the Reproductive Medical Consultant in the Regional Fertility Hub for publicly-funded treatment in a HSE-approved private clinic of their choice.
Female patients who meet the criteria will be eligible for treatment if they have been referred by their GP to a regional fertility hub before their 41st birthday.

35 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
News
New and Innovative Service for High Risk Ovarian Cancer Patients
 Written by Sarah Belton, Advanced Nurse Practitioner in Gynaecology Oncology at The National Maternity Hospital
Written by Sarah Belton, Advanced Nurse Practitioner in Gynaecology Oncology at The National Maternity Hospital
consultations bring specialist services to the patient in their own environment, a key aim of Slaintecare. Previously, these patients were seen in the Gynaecology clinic routinely. The new service has increased capacity within the Gynaecology service, at a time when there is significant demand. Patients with a genetic mutation predisposing them to cancer have a high burden of scans and clinic appointments to attend across multiple specialties. In the current cost of living crisis, each of these visits cost the patients’ time and money for fuel, car parking, time off work, potential childcare costs, etc.
devices and using the established platform thisisGO.ie. Future development plans for the service include tailoring information on thisisGO.ie to cover patients’ individual status for hormone replacement therapy, as per the National Women’s Health Action Plan 2022 – 2023, as an example.

The Advanced Nurse Practitioner led Gynaecology Oncology

Family History Clinic is a new, innovative approach to the care of women with BRCA 1 and BRCA 2 germline alterations to manage their ovarian cancer risk reduction strategy. The new service established at The National Maternity Hospital and St Vincent’s University Hospital prioritises prevention of ovarian cancers in these patients, through risk reduction surgery. However, this is a complex issue for pre-menopausal women as it affects fertility and, surgery results in menopause.
Recent Irish research has established that information needs are important to women with BRCA (NCCP, 2023; Warner and Groake, 2022). In its Hereditary Cancer Model of Care document (2023), the National Cancer Control Programme describe the provision of accurate, consistent information as a key theme from research carried out with this patient group. Warner and Groake (2022) found that patients were disempowered by dealing with high levels of uncertainty in their care following BRCA diagnosis, as a result of inconsistent information given by different health care professionals. The NCCP (2023) also states those with a genetic predisposition to ovarian cancer should have access to clear information regarding the symptoms of surgical menopause and longer term health risks associated with this prior to surgery and, that patients should have access to expert, individualised advice regarding their options
for menopause management (NCCP 2023). A recurrent theme from national and international literature is that women do not regret undertaking risk reducing salpingo-opherectomy but repeatedly highlight a lack of information sources particularly around the long term impact of prophylactic surgery in a variety of areas including fertility, menopause (and the use of hormone replacement therapy) bone health, cardiovascular risk and cognitive protection.
The NCCP (2023) Hereditary Cancer Model of Care document describes the role of CNSs and ANPs as key to the provision of cancer predisposition services.
The ANP led Gynae Oncology Family History Clinic, the first of its kind nationally, was established in 2022 and is run on a virtual basis, reducing the need for patients to attend the hospital. From 1st Sept 2022 to 1st June 2023, 95 virtual consultations were offered by the Gynae Oncology ANP. These
As part of the Irish Cancer Society funded Women’s Health Initiative, thisisGO.ie was developed to provide a safe, online, personalised resource that provides current, reputable, relevant and evidence based information for Irish women with gynaecological cancer. Developed with patients, by patients and for patients the site is built on the principle that patient involvement and engagement must be embedded in every stage of the development process from conception to design to implementation. It includes a section dedicated to patients with BRCA mutations. Patients are recommended to consider risk reducing surgery from the age of 40 for BRCA1 mutation, and from the age of 45 for those with a BRCA2 mutation. This demographic is familiar with accessing content and information on mobile internet
The National Cancer Strategy (DoH, 2016) recognised that cancer genetics in Ireland was under-developed and underfunded. However, recent support from the HSE Nursing and Midwifery Practice Development Unit to develop an ANP role, support of stakeholders across The National Maternity Hospital and St Vincent’s University Hospital, establishing a designated nurse led clinic, plus the development of thisisGO. ie patient resource have all contributed to an improved specialist service for patients. Involving service users to develop consistent, evidence based, relevant patient information would further improve the landscape for patients with BRCA mutations nationally, as thisisGO.ie is not restricted to a specific institution or health care setting.
The ANP led Gynae Oncology Family History Clinic has been awarded the Susanna Byrne Perpetual Award by the HSE Nursing and Midwifery Practice Development Unit in Dublin South, Kildare and Wicklow, for demonstrating commitment and excellence in improving the care delivered to patients/clients and their families.
36 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Ovarian cancer
“As part of the Irish Cancer Society funded Women’s Health Initiative, thisisGO. ie was developed to provide a safe, online, personalised resource that provides current, reputable, relevant and evidence based information for Irish women with gynaecological cancer”
Diabetes in Pregnancy
Diabetes in Pregnancy
Pregnancy is a normal physiological process and often an eagerly anticipated life event. Medical conditions can develop in pregnancy, such as Gestational Diabetes (GDM), or pregnancy may exacerbate pre-existing medical conditions such as PreGestational Diabetes (PGDM). Diabetes is the most common significant medical condition to affect pregnant women with up to 12% of pregnancies in Ireland affected by diabetes. This article aims to briefly outline diabetes in pregnancy and look at advances in the management of diabetes in pregnancy.
Pre-Gestational Diabetes
Pre-Gestational Diabetes Mellitus, Type 1 Diabetes Mellitus (T1DM) and Type 2 Diabetes Mellitus (T2DM) occurs in 1-2% of all pregnancies, with rates of new diabetes diagnosis rising in women of child bearing age. PGDM confers significantly greater risk for both the mother and developing fetus but by providing comprehensive Multidisciplinary Team (MDT) care this can help reduce adverse outcomes. Rates of pre-eclampsia, macrosomia, caesarean section, congenital malformation and perinatal mortality are well established to be increased in this group compared to women without glucose abnormalities. We also see greater evidence of vascular disease e.g. nephropathy, retinopathy and hypertension in this cohort of
women which further increases the risks of pregnancy complications.
The number of women presenting with T2DM in pregnancy is increasing in line with a worldwide upward trend in obesity and T2DM now accounts for 30% of women with PGDM in pregnancy. Women with T2DM are more likely to be living with obesity, hypertension and taking pharmacological agents that are contraindicated in pregnancy. Pre-conceptual care is of significant importance in this group to optimise diabetes management and pharmacological management of any pre-existing co-morbidities prior to achieving pregnancy.
Women with PGDM fall within the specialist care pathway within the National Maternity Strategy and require specialist care, booked from early pregnancy, often earlier than the traditional 12 week booking visit. The MDT is comprised of obstetrics, endocrinology, specialist midwifery, dietitians, lactation consultants, ultra-sonographers and ophthalmologists. Women require frequent review in the specialist diabetes clinic, often every 3-4 weeks, in comparison with standard antenatal combined care. The complexity of care required for women with PGDM due to complications and complexity of disease is demanding.
The advent of diabetes healthcare technology has provided
Written by Ciara Coveney, Registered Advanced Midwife Practitioner, Diabetes in Pregnancy at The National Maternity Hospital

both women and healthcare professionals with information to allow for accurate and contemporaneous dosing of insulin on a background of dynamic hormone changes and insulin resistance. Diabetes technology describes devices that are used by women with diabetes to help achieve glycaemic control and improve quality of life. Frequently used devices include Continuous Glucose Monitoring Sensors (CGMs), Continuous Subcutaneous Insulin Pumps (more recently closed loop hybrid systems) and smart insulin pens.

The current NICE guidelines recommends that all pregnant women with PGDM are offered CGMs and upcoming guidance will suggest that all women attempting to achieve pregnancy are offered closed loop hybrid insulin pumps. Insulin pump therapy has demonstrated effectiveness in lowering HbA1c prior to and during pregnancy and can be beneficial in cases of impaired hypoglycaemic awareness. The National Maternity Hospital provides a comprehensive service for women using Continuous Subcutaneous Insulin Pump Therapy (CSII) and continuous glucose monitoring (sensor) technology. The
provision of this service requires expert training and continuous professional development to keep up to date with the multiple technology advances being made in diabetes care internationally. Current figures show that over 50% of women with T1DM attending The National Maternity Hospital are now using insulin pumps and even higher rates of CGMs are observed. Although the numbers of women with PGDM in the service are small compared to the GDM cohort they are under the care of the service from 6 weeks’ gestation and require weekly MDT input.
Gestational Diabetes
Gestational Diabetes (GDM) is diabetes with first onset or recognition during pregnancy. Maternal insulin resistance increases during pregnancy to ensure sufficient nutrition to the developing fetus and may result in impaired insulin action. GDM is a dynamic condition of variable severity and the most current Irish data suggests a prevalence of 12.4% of all pregnancies are affected by GDM. Women are often screened based on identified risk factors and some centres have implemented universal screening for all pregnant women. The increase in GDM prevalence is in line with global upward trends in obesity, advanced maternal age and a decrease in physical activity

37 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
levels. GDM is one of the most common medical conditions to be diagnosed in pregnancy and results in an increased likelihood of pre-eclampsia, birth injury and caesarean section. Offspring of women with GDM are more likely to have a higher birth weight, neonatal hyperinsulinemia, birth trauma and intrauterine death. Lifestyle modification, a combination of medical nutritional therapy and physical activity are the cornerstone of treatment for GDM. These services are often delivered by specialist midwifery, dietitians and endocrinology and are shown to be highly effective in managing GDM. Whilst a yearly increase in gestational diabetes is expected, the numbers of women controlled with conservative measures i.e. lifestyle intervention remains constant in The National Maternity Hospital (~75%) however a proportion of this cohort will require pharmacological treatment.
Diabetes in Pregnancy
The midwifery led team changed the care pathway for women diagnosed with gestational diabetes in The National Maternity Hospital. Our virtual care pathway means that following the initial diagnosis of GDM, women are educated via a live webinar in the comfort of their own surroundings with a specialist midwife and dietitian. Each woman is then followed up with virtual telehealth clinic appointments using the “Attend Anywhere” platform, the frequency of which is tailored to each woman’s gestation and needs.
The diabetes midwifery team sourced a blood glucose monitor, which is bluetooth enabled and syncs to an app that autopopulates to a patient master list. Consequently, for every virtual appointment, each woman’s synched information is readily available. This, in conjunction with the electronic health record, facilitates a comprehensive midwifery led consultation. Our
Women's Health News
colleagues from the Department of Clinical Nutrition and Dietetics also participate in the virtual “Attend Anywhere” clinic. This allows women access to both specialist midwives and specialist dietetics during the same appointment. It addition, this care pathway allows for immediate escalation to the advanced midwife practitioner to review for pharmacological treatment requirement. If insulin therapy is indicated, each woman receives same day education on insulin administration and safety via this virtual platform, enabling immediate commencement of insulin therapy.
From there, women can be referred to Endocrinology and Obstetric care within the diabetes in pregnancy specialist clinic if required. With the twinning of “Attend Anywhere” and the new remote glucose monitoring, the midwifery team aim to deliver evidence-based care on a more personal and easily accessible level to transform the service for women and enhance access to services.
New Irish Life Health Research
More than half of women in Ireland are worried about a female health issue, regardless of the stage of life they are in, according to a new survey. This relates to concerns around contraception, fertility and infertility, menstrual issues as well as perimenopause and menopause. The research was commissioned by Irish Life Health, one of Ireland’s leading health insurance providers. Irish Life Health today announced a new first of its kind female health GP consultation benefit dedicated to meeting the demand for more specialised health services for women.
The survey, which was carried out amongst women aged 18+, revealed insights into perceptions of female healthcare in Ireland. Over half of respondents believe female healthcare is not well serviced in Ireland, while 38% are finding it difficult to get fast access to specialist female health expertise.
The research also revealed the main concerns among different age groups of women in Ireland, which found:
• four in ten 18-24-year-olds in Ireland are worried about menstruation issues
• four in ten 25-34-year-olds are worried about fertility issues
• almost half of 35-54-yearolds are worried about perimenopause or menopause
While the research shows there are significant levels of concern about female health issues, the responses demonstrate confidence in taking action. 89% of women believe they know their body better than anyone, and more than 8 in 10 women are eager to understand more about their health.
The Female Health Consultation benefit by Irish Life Health is the first of its kind in Ireland, offering members access to GPs who specialise in female health, for support and advice in the areas of menstrual health, fertility, contraception, perimenopause and menopause, amongst others.
Dr Maire Finn, one of the specialist GPs delivering this service for Irish
Life Health, comments, “These statistics are not surprising to me as a GP as every day in my practice I hear women’s concerns about their menstrual cycle, their fertility, menopause, and the impact these issues have on their everyday lives. This research tells us that women feel they know their bodies but equally want to know more about health matters that relate specifically to them. It shows that there is work to be done to provide more specialised care in the area of women’s health. This new female health service that Irish Life Health is launching, is a timely and important step in providing the specialised care and information that all women need and want.”
In announcing the new benefit, Liz Rowen, Head of Marketing, Irish Life Health said, “Our research showed that women understand their own bodies, and they know when something isn’t right, but a large proportion of them don’t have the opportunity to talk to someone who really understands their individual concern. Our members will now
At present we are at the write up stage of a research study to evaluate maternal and neonatal outcomes following the implementation of the virtual pathway of care. This aims to examine the use of assistive healthcare technology i.e. Attend Anywhere and bluetooth enabled healthcare technology. We are also evaluating maternal and pregnancy outcomes including interval to treatment, rates of pharmacological treatment, mode of delivery and induction rates. In addition, relevant neonatal outcomes will be evaluated including birth weight, NICU admission rates and neonatal hypoglycaemia rates. This study is the first of its type and size in Ireland that will examine real clinical outcomes for virtual care pathways and we are hopeful it will provide data to support the safe and widespread use of virtual care pathways to improve access to healthcare.
have the opportunity to have their health concerns heard through a dedicated and specialised female health service, which is available on all Irish Life Health hospital plans from the 1st of September.”
Other key findings from the research include:
• Only half of women (51%) feel like they truly understand their menstrual cycle
• 62% of women get their health information from family and friends, while 59% source their information through social media and online platforms
The new specialist female health GP consultation service will offer Irish Life Health members a GP consultation by phone or video, a recommended course of action and, if needed, an in-person appointment at one of Centric Health’s specialist female health clinics across Ireland.
*Research conducted by Red C in July 2023, featuring a panel of 500+ female representatives aged 18+.

38 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Viatris
Empowering people
worldwide to live healthier at every stage of life

With 1,600 people working across five sites in Ireland, Viatris provides access to medicines, develops innovative solutions and improves healthcare for patients.
Newenham Court, Malahide Road, Dublin 17, Dublin, Ireland.
Viatris.ie
Job Code: CC-2023-001
Date of Preparation: July 2023
www.viatris.ie
When Menopause gets Complicated
Written by Dr Deirdre Lundy Specialist in Women’s Health and Menopause at The National Maternity Hospital
Background
As a person begins to experience ovarian hormone disruption (perimenopause) through to and beyond their final menstrual period (post menopause) they may be affected by a variety of symptoms.
The most commonly mentioned symptoms include vasomotor flushing and/or sweating, mood changes (low mood, irritability, feeling overwhelmed), joint aches & pains, fatigue, etc. Urogenital symptoms like vaginal dryness; which might in turn cause discomfort (particularly during exercise or intercourse) can be a problem at this time. Some (peri) menopausal people suffer a worsening in their pre-existing GU problems such as frequency of urination or more frequent UTI’s. These and the more than 50 additional complaints attributed to menopause may have a severe impact on the patients’ quality of life, conversely there are some lucky people who notice very little disruption at this time. It is very hard to predict who will have a rough time in menopause and who won’t but as clinicians we are trained to offer a sympathetic ear and try to help where we can.
For many decades offering menopause support in the form of Hormone Replacement Therapy (HRT) tablets was common practice. But, in 2002, after the publication of an editorial discussing early results of the Women’s Health Initiative (WHI) trial in the USA, concerns were raised about that trial’s use of equine estrogen tablets blended

with the synthetic progestagen ‘medroxy provera acetate’ (MPA) and raised risk of breast cancer as well as thrombotic events including CVA and MI.
During the following 10-15 years many clinicians feared prescribing HRT and a lot of patients weren’t too keen on having to use it. Demand fell, interest in training in menopause waned and patients were left with very few options. Things did slowly improve after the shock waves of the WHI subsided a little. It was known back then but subsequent studies have now affirmed that not all HRT is the same.
Concerns that were raised about the connection with HRT use and breast cancer were somewhat offset by analysis of the absolute risk vs the relative risk. The WHI data showed an increased risk of breast cancer diagnosis with use of oral equine estrogen +MPA HRT use in the order of 1.27. This is about the same risk attached to drinking 2 units of alcohol a day and much lower than the risk attached to being overweight/ obese. So this does not dismiss risk - there is an association - but helps put risk into perspective.
Roehm E. A Reappraisal of Women's Health Initiative Estrogen-Alone Trial: Long-Term Outcomes in Women 50-59 Years of Age. Obstet Gynecol Int. 2015; 2015:713295. doi: 10.1155/2015/713295. Epub 2015

Jan 1. PMID: 25685151; PMCID: PMC4313058
Other information from more recent trials on HRT use and
breast cancer risk data examined the effect of the choice of HRT progestagen on risk. It is thought that more modern, less androgenic progestagens particularly micronised progesterone and dydrogesterone, seem to have less of an impact on breast cancer risk than the powerful MPA used exclusively in the WHI study. Fournier A, Berrino F, ClavelChapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107(1):103-111. doi:10.1007/s10549-007-9523-x
Concerns we had about orally ingested estrogen (e.g. the COCP or oral HRT) do not appear to apply to the low dose, transdermal 17 beta estradiol - the type typically used in modern transdermal HRT. Orally administered estrogen affects the thrombin system and can increase the risk of VTE but modest doses of TD 17 beta estradiol have a neutral effect on clotting.
So in recent years, not only is the fear of HRT use abating, we are seeing the demand for menopause consultations and HRT products outstripping supply. Hamoda H, Panay N, Pedder H, Arya R, Savvas M. The British Menopause Society & Women’s Health Concern
2020 recommendations on hormone replacement therapy in menopausal women. Post Reprod Health. 2020;26(4):181-209.
doi:10.1177/2053369120957514
This has been challenging. Clinicians have not always kept up their confidence surrounding menopause guidelines so may be worried about offering up to date advice. Even for general practitioners with expertise, advising on menopause management options and prescribing HRT (and alternatives) is not really supported in primary care. There are no special considerations for what can be a lengthy and nuanced consultation and perhaps ‘first menopause/ perimenopause visit’ should be added to the chronic disease payment schedule. Added frustration for prescribers is ongoing as the demand for high quality TD estrogens and the more ‘breast friendly’ progestagens has been so high, many patients find that they cannot reliably access their HRT in Irish pharmacies. www.hse.ie/eng/about/who/cspd/ ncps/medicines-management/ guidance-on-shortages/mmpguidance-for-prescribers-on-hrtshortages-september-2022.pdf
Typical Menopause vs ‘Complex’ Menopause
When a person develops symptoms that may be attributable to (peri) menopause there are many well established therapies available. There is no single, best answer when it comes to maintaining wellbeing and improving symptoms in menopause, but the fact remains HRT has been proven most effective for vasomotor symptoms caused by hormonal fluctuations at this transitional time. Maclennan AH. Evidence-based review of therapies at the menopause.
Int J Evid Based Health. 2009 Jun;7(2):112-23. doi: 10.1111/ j.1744-1609.2009.00133. x. PMID: 21631851
But what about prescribing HRT to people with underlying medical conditions? What are the rules?
Well, that’s a little tricky.
Research on (peri) menopause and HRT and their impact on serious medical conditions is staggeringly poor. We are only beginning to see female biological variables being taken into consideration as part of mainstream health research & pharmaceutical studies. Yakerson, A. Women in clinical trials: a review
40 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Menopause
of policy development and health equity in the Canadian context. Int J Equity Health 18, 56 (2019). https://doi.org/10.1186/s12939019-0954-x
Studies on using HRT in groups of people with serious underlying medical conditions are very few and far between. In the absence of actual data, experts look to see what (if any) impact menstruation, pregnancy and use of hormonal contraceptives might have on specific medical conditionsthen try to work out what impact menopausal changes and HRT use might be gleaned from this information. Not ideal and often unhelpful.
So what do we know about use of HRT for females with menopausal symptoms who have background medical issues? There are some guidelines when it comes to chronic manageable conditions as well as the more serious situations where if at all possible referral to a colleague with specific menopause training is advised. The British Menopause Society is generally regarded as the ‘go-to’ resource for up to date guidance and advice in Ireland. They have been enormously supportive of colleagues both in Britain and Ireland. www.bms.org.uk
They advise that expert advice should be sought in a variety of situations including women with complex medical backgrounds (such as women with breast cancer, personal history of venous thrombosis or personal history of stroke, ischemic heart disease, etc.).

Menopause itself is often triggered by medical intervention for serious disease and its treatments. People with female hormonesensitive cancers may undergo oophorectomy on medical advice which can cause sometimes very troublesome menopausal symptoms and if done before 40 yrs. of age could go on the undermine future health outcomes. ESHRE guideline group. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016 May;31(5):926–37
Pelvic radiotherapy and certain forms of chemotherapy can cause temporary or even permanent ovarian failure. Anti-endocrine therapies are often prescribed for females with estrogen sensitive malignancies - most commonly but not exclusively, breast cancer. These medicines improve cancer
outcomes but (depending on the patient) the side effects and the menopausal symptoms they can trigger disimprove quality of life and often limit compliance with the prescribed therapy. Meade E, et.al. Survivorship care for postmenopausal breast cancer women in Ireland: What do women want? Eur J Oncol Nurs. 2017 Jun;28:69–76
Being diagnosed with cancer when already using HRT may become a problem and oncologists might sometimes advise discontinuation of the HRT leading to a rapid return of menopausal symptoms.

Menopausal symptoms can be managed with hormonal, nonhormonal and non-pharmacological therapies. MHT is the most effective method of managing menopausal symptoms but may not be suitable for all after a cancer diagnosis as the role of hormone receptors in many types of cancer and their treatment may complicate the discussion about when MHT should or should not be used.
There is a growing list of therapies and medications that have been shown to be useful in relieving some of the symptoms of menopause for women who are either on anti- estrogen therapies or who have been advised to avoid standard HRT. The British Menopause Society offers guidelines on their “Tools for Clinicians” webpage. Interestingly one of the management options with the best range of meno symptoms relief is Cognitive Behavioural Therapy which has been shown to be effective over placebo in helping with vasomotor flushing and sweating, fatigue, weight gain, musculoskeletal (MSK) pain and sexual function
Franzoi MA, et al Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. 2021 July but access to one on one CBT is limited and so some people may be directed to on online therapy and/ or smart phone apps to seek relief. Other strategies that have been shown to be helpful include:
- SSRI’s & SNRI’s which may help some people with vasomotor symptoms, mood and MSK issues
- Anticonvulsants like gabapentin may help with vasomotor flushing
- Antispasmodics like Oxybutynin can also help with both vasomotor flushing and urinary issues
- Antihistamines with sedating properties may help with sleep disruption
Other options that have shown benefit include yoga and acupuncture, gently physical activity and exercise and hypnosis.
Franzoi MA, et al Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer
New medical therapies designed to reduce vasomotor flushing specifically for people with a breast cancer diagnosis are being launched to market in Europe in 2024. The root cause of VMS appears to be linked to heightened signalling of certain neurotransmitters (particularly neurokinin B & its receptor (neurokinin 3 & 4 receptors)
- these affect the autonomic thermoregulatory pathway. A group of drugs known as the
NK3R antagonists can block NK3R related VMS seemingly as effectively as HRT. The Oasis 4 trial is helping to explore one of these NK antagonist drugs. They are recruiting here in Ireland at time of writing. https://findoasisnow.com/ gb-en/hcp
Applying the research
In our Complex Menopause Clinic at The National Maternity Hospital we aim to offer patients time to discuss the symptoms of menopause that are affecting people with comorbidities or a past diagnosis that might limit typical menopause options. This is a new service funded by the HSE’s National Infant and Women’s Health Program. We are one of six clinics operating around the republic of Ireland.
We try to validate the patients’ individual experience (as some people sadly seem to have been given little forewarning about what the menopausal effects of procedures and treatments for cancers). We explore lifestyle issues and offer support there where we can. We take them through the varied non-HRT strategies that could bring them some relief and allow them improve their QoL. We prescribe where appropriate and review them after 3 months to see if there is any improvement - if they are still struggling we try something else. Some of our patients never really regain their optimal QoL- some we barely help at all I worry - but most patients have expressed a gratitude for having been given a safe space to be listened to, to discuss their symptoms and worries and to be offered treatments wherever we have them.
41 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
PRESCRIBING INFORMATION CIBINQO® ▼ (ABROCITINIB)



















Please refer to full Summary of Product Characteristics (SmPC) before prescribing Cibinqo®. Presentation: Film-coated tablets containing 50 mg, 100 mg, or 200 mg abrocitinib. Each tablet contains 1.37 mg, 2.73 mg, and 5.46 mg of lactose monohydrate, respectively. Indications: For the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy. Dosage: Treatment should be initiated and supervised by a healthcare professional experienced in the diagnosis and treatment of atopic dermatitis. Posology: The recommended starting dose is 100 mg or 200 mg once daily based on individual patient characteristics. A starting dose of 100 mg once daily is recommended for patients at higher risk of venous thromboembolism (VTE), major adverse cardiovascular event (MACE) and malignancy. If the patient does not respond adequately to 100 mg once daily, the dose can be increased to 200 mg once daily. A dose of 200 mg once daily may be appropriate for patients who are not at higher risk of VTE, MACE and malignancy with high disease burden or for patients with an inadequate response to 100 mg once daily. Upon disease control, dose should be decreased to 100 mg once daily. If disease control is not maintained after dose reduction, re-treatment with 200 mg once daily can be considered. The lowest e ective dose for maintenance should be considered. Discontinuation of treatment should be considered in patients who show no evidence of therapeutic benefit after 24 weeks. Cibinqo can be used with or without medicated topical therapies for atopic dermatitis. Treatment initiation: TTreatment should not be initiated in patients with a platelet count < 150 × 103/mm3, an absolute lymphocyte count (ALC) < 0.5 × 103/ mm3, an absolute neutrophil count (ANC) < 1.2 × 103/mm3 or who have a haemoglobin value < 10 g/dL. Dose interruption: If a patient develops a serious infection, sepsis or opportunistic infection, dose interruption should be considered until the infection is controlled. Interruption of dosing may be needed for management of laboratory abnormalities. Missed doses: If a dose is missed, patients should be advised to take the dose as soon as possible unless it is less than 12 hours before the next dose, in which case the patient should not take the missed dose. Thereafter, dosing should be resumed at the regular scheduled time. Interactions: In patients receiving dual strong inhibitors of cytochrome CYP2C19 and moderate inhibitors of CYP2C9, or strong inhibitors of CYP2C19 alone (e.g., fluvoxamine, fluconazole, fluoxetine and ticlopidine), the recommended dose of Cibinqo should be reduced by half to 100 mg or 50 mg once daily. Treatment is not recommended concomitantly with moderate or strong inducers of CYP2C19/CYP2C9 enzymes (e.g., rifampicin, apalutamide, efavirenz, enzalutamide, phenytoin). In patients receiving acid reducing agents (e.g. antacids, proton pump inhibitors and H2 receptor antagonists), 200 mg once daily dose of abrocitinib should be considered (see section 4.5). Renal impairment: No dose adjustment is required in patients with mild renal impairment, i.e., estimated glomerular filtration rate (eGFR) of 60 to < 90 mL/min. In patients with moderate (eGFR 30 to < 60 mL/min) renal impairment, the recommended dose of Cibinqo should be reduced by half to 100 mg or 50 mg once daily. In patients with severe (eGFR < 30 mL/min) renal impairment, 50 mg once daily is the recommended starting dose. The maximum daily dose is 100 mg. Cibinqo has not been studied in patients with end-stage renal disease (ESRD) on renal replacement therapy. Hepatic impairment: No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. Cibinqo must not be used in patients with severe (Child Pugh C) hepatic impairment. Elderly: For patients 65 years of age and older, the recommended dose is 100 mg once daily. Paediatric population: The safety and e cacy of Cibinqo in children under 12 years of age have not yet been established. No data are available. Cibinqo has been studied in adolescents 12 to < 18 years of age. However, because of bone findings in juvenile rats (comparable to a 3 month old human), additional long-term data in growing adolescents is needed to conclude that the benefits outweigh the risks. Method of administration: This medicinal product is to be taken orally once daily with or without food at approximately the same time each day. In patients who experience nausea, taking Cibinqo with food may improve nausea. Tablets should be swallowed whole with water and should not be split, crushed, or chewed because these methods have not been studied in clinical trials. Contra-indications: Hypersensitivity to the active substance or to any of the excipients. Active serious systemic infections, including tuberculosis (TB), severe hepatic impairment, pregnancy, and breast-feeding.
Warnings and Precautions:


Abrocitinib should only be used if no suitable treatment alternatives are available in patients:




• 65 years of age and older;
• patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors (such as current or past long-time smokers);








• patients with malignancy risk factors (e.g. current malignancy or history of malignancy)
Infections/serious infections: Serious infections have been reported in patients receiving Cibinqo. The most frequent serious infections in clinical studies were herpes simplex, herpes zoster and pneumonia. As there is a higher incidence of infections in the elderly and in the diabetic populations in general, caution should be used when treating the elderly and patients with diabetes. In patients 65 years of age and older abrocitinib should only be used if no suitable treatment alternatives are available. Treatment must not be initiated in patients with an active, serious systemic infection. Risks and benefits of treatment prior to initiating Cibinqo should be considered. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with abrocitinib. A patient who develops a new infection during treatment should undergo prompt and complete diagnostic testing and appropriate antimicrobial therapy should be initiated. The patient should be closely monitored, and therapy should be temporarily interrupted if the patient is not responding to standard therapy. Tuberculosis: Tuberculosis was observed in clinical studies with abrocitinib. Patients should be screened for TB before starting treatment and yearly screening for patients in highly endemic areas for TB should be considered. Abrocitinib must not be given to patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, preventive therapy for latent TB should be started prior to initiation of treatment.
Viral reactivation: Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies. The rate of herpes zoster infections was higher in patients who were treated with 200 mg, 65 years of age and older, with a medical history of herpes zoster, with a confirmed ALC < 1 × 103/mm3 prior to the event and patients with severe atopic dermatitis
at baseline. If a patient develops herpes zoster, temporary interruption of treatment should be considered until the episode resolves. Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy and during therapy. Patients with evidence of active hepatitis B or hepatitis C (positive hepatitis C PCR) infection were excluded from clinical studies. Patients who were hepatitis B surface antigen negative, hepatitis B core antibody positive, and hepatitis B surface antibody positive had testing for hepatitis B virus (HBV) DNA. Patients who had HBV DNA above the lower limit of quantification (LLQ) were excluded. Patients who had HBV DNA negative or below LLQ could initiate treatment; such patients had HBV DNA monitored. If HBV DNA is detected, a liver specialist should be consulted. Vaccination: No data are available on the response to vaccination in patients receiving Cibinqo. Use of live, attenuated vaccines should be avoided during or immediately prior to treatment. Prior to initiating treatment with this medicinal product, it is recommended that patients be brought up to date with all immunisations, including prophylactic herpes zoster vaccinations, in agreement with current immunisation guidelines. Venous thromboembolism (VTE): Events of deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving abrocitinib. In a large randomized active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a dose dependent higher rate of VTE including deep venous thrombosis (DVT) and pulmonary embolism (PE) was observed with tofacitinib compared to TNF inhibitors. A higher rate of VTE was observed with abrocitinib 200 mg compared to abrocitinib 100 mg. In patients with cardiovascular or malignancy risk factors abrocitinib should only be used if no suitable treatment alternatives are available. In patients with known VTE risk factors other than cardiovascular or malignancy risk factors, abrocitinib should be used with caution. VTE risk factors other than cardiovascular or malignancy risk factors include previous VTE, patients undergoing major surgery, immobilisation, use of combined hormonal contraceptives or hormone replacement therapy, inherited coagulation disorder. Patients should be re-evaluated periodically during abrocitinib treatment to assess for changes in VTE risk. Promptly evaluate patients with signs and symptoms of VTE and discontinue abrocitinib in patients with suspected VTE, regardless of dose. Major adverse cardiovascular events (MACE): Events of MACE have been observed in patients taking abrocitinib. In a large randomized active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE), defined as cardiovascular death, non-fatal myocardial infarction (MI) and non-fatal stroke, was observed with tofacitinib compared to TNF inhibitors. Therefore, in patients 65 years of age and older, patients who are current or past long-time smokers, and patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors, abrocitinib should only be used if no suitable treatment alternatives are available. Malignancy (excluding non-melanoma skin cancer [NMSC]): Lymphoma and other malignancies have been reported in patients receiving JAK inhibitors, including abrocitinib. In a large randomized active controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a higher rate of malignancies, particularly lung cancer, lymphoma and non-melanoma skin cancer (NMSC) was observed with tofacitinib compared to TNF inhibitors. A higher rate of malignancies (excluding non-melanoma skin cancer, NMSC) was observed with abrocitinib 200 mg compared to abrocitinib 100 mg. In patients 65 years of age and older, patients who are current or past long-time smokers, or with other malignancy risk factors (e.g. current malignancy or history of malignancy), abrocitinib should only be used if no suitable treatment alternatives are available. Non-melanoma skin cancer: NMSCs have been reported in patients receiving abrocitinib. Periodic skin examination is recommended for all patients, particularly those who are at increased risk for skin cancer. Haematologic abnormalities: Confirmed ALC < 0.5 × 103/mm3 and platelet count < 50 × 103/mm3 were observed in less than 0.5% of patients in clinical studies. Treatment with abrocitinib should not be initiated in patients with a platelet count < 150 × 103/mm3, an ALC < 0.5 × 103/mm3, an ANC < 1.2 × 103/mm3 or who have a haemoglobin value < 10 g/dL. Complete blood count should be monitored 4 weeks after initiation of therapy and thereafter according to routine patient management. Lipids: Dose dependent increases in blood lipid parameters were reported in patients treated with abrocitinib compared to placebo. Lipid parameters should be assessed approximately 4 weeks following initiation of therapy and thereafter according to the patient’s risk for cardiovascular disease. The e ect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Patients with abnormal lipid parameters should be further monitored and managed according to clinical guidelines, due to the known cardiovascular risks associated with hyperlipidaemia. Elderly: The safety profile observed in elderly patients was similar to that of the adult population with the following exceptions: a higher proportion of patients 65 years of age and older discontinued from clinical studies and were more likely to have serious adverse reactions compared to younger patients; patients 65 years and older were more likely to develop low platelet and ALC values; the incidence rate of herpes zoster in patients 65 years of age and older was higher than that of younger patients. There are limited data in patients above 75 years of age. Use in patients 65 years of age and older: Considering the increased risk of MACE, malignancies, serious infections, and all-cause mortality in patients 65 years of age and older, as observed in a large randomised study of tofacitinib (another JAK inhibitor), abrocitinib should only be used in these patients if no suitable treatment alternatives are available. Immunosuppressive conditions or medicinal products: Patients with immunodeficiency disorders or a first-degree relative with a hereditary immunodeficiency were excluded from clinical studies and no information on these patients is available. Combination with biologic immunomodulators, potent immunosuppressants such as ciclosporin or other Janus kinase (JAK) inhibitors has not been studied. Their concomitant use with abrocitinib is not recommended as a risk of additive immunosuppression cannot be excluded. Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product. Drug Interactions: Potential for other medicines to a ect pharmacokinetics of abrocitinib: Abrocitinib is metabolised predominantly by CYP2C19 and CYP2C9 enzymes, and to a lesser extent by CYP3A4 and CYP2B6 enzymes, and its active metabolites are renally excreted and are substrates of the organic anion transporter 3 (OAT3). Therefore, exposures of abrocitinib and/or its active metabolites may be a ected by medicinal products that strongly inhibit or induce theses enzymes and transporter. Dose adjustments, as appropriate, may be required. Co-administration with products which increase gastric pH: When abrocitinib 200 mg was administered concomitantly with famotidine 40 mg, an H2-receptor antagonist, abrocitinib active moiety exposures decreased by approximately 35%. The e ect of elevating gastric pH with antacids, or proton pump inhibitors (omeprazole) on the pharmacokinetics of abrocitinib
CIBINQO 200 mg o ers RAPID and SUPERIOR vs dupilumab1 RELIEF
Results through week 2 in a head-to-head study of CIBINQO 200 mg vs dupilumab with background TCS.

Cibinqo is indicated for the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy2




With week 2 PP-NRS4 data: 48% of adults taking CIBINQO 200 mg achieved PP-NRS4 at week 2 compared to 26% of adults taking dupilumab (P<0.0001)
Additional information:
PP-NRS4 response for CIBINQO vs dupilumab at week 2 was a primary endpoint controlled for multiplicity. The onset of pruritus relief was assessed through a step-down approach, day by day, from week 2 to earlier time points once statistical significance was demonstrated at week 2, at the 5% level of significance. P value at day 2 is controlled for multiplicity for the family of PP-NRS4 comparisons. TCS includes low- to medium-potency topical corticosteroids and other medicated topicals.
PP-NRS4 is a 4 point or more improvement in Peak Pruritus Numerical Rating Scale.
















has not been studied and may be similar to that seen with famotidine. The higher 200 mg daily dose should be considered for patients treated concomitantly with products which increase gastric pH, as they may reduce the e cacy of abrocitinib. Potential for Cibinqo to a ect pharmacokinetics of other medicinal products: No clinically significant e ects of Cibinqo were observed in drug interaction studies with oral contraceptives. Caution should be exercised for concomitant use of abrocitinib with dabigatran. Caution should be exercised as the levels of P-gp substrates with a narrow therapeutic index, such as digoxin, may increase. Caution should be exercised when using abrocitinib concomitantly with narrow therapeutic index medicines that are primarily metabolised by CYP2C19 enzyme (e.g. S mephenytoin and clopidogrel). Dose adjustment may be required for other medicines primarily metabolised by CYP2C19 enzyme in accordance with their product information (e.g. citalopram, clobazam, escitalopram and selumetinib). Fertility, pregnancy, and lactation: Women of childbearing potential: Women of reproductive potential should be advised to use e ective contraception during treatment and for 1 month following the final dose of Cibinqo. Pregnancy planning and prevention for females of reproductive potential should be encouraged. Pregnancy: There are no or limited amount of data on the use of abrocitinib in pregnant women. Studies in animals have shown reproductive toxicity. Abrocitinib has been shown to cause embryo-foetal lethality in pregnant rats and rabbits, skeletal variations in the foetuses of pregnant rats and rabbits and to a ect parturition and peri/ postnatal development in rats. Cibinqo is contraindicated during pregnancy. Breast-feeding: There are no data on the presence of abrocitinib in human milk, the e ects on the breast fed infant, or the e ects on milk production. Abrocitinib was secreted in milk of lactating rats. A risk to newborns/infants cannot be excluded and Cibinqo is contraindicated during breast feeding. Fertility: Based on the findings in rats, oral administration of Cibinqo may result in temporary reduced fertility in females of reproductive potential. The e ects on female rat fertility were reversible 1 month after cessation of abrocitinib oral administration. Driving and operating machinery: Cibinqo has no e ect on the ability to drive or use machines. Side E ects: The most commonly reported adverse reactions are nausea (15.1%), headache (7.9%), acne (4.8%), herpes simplex (4.2%), blood creatine phosphokinase increased (3.8%), vomiting (3.5%), dizziness (3.4%) and abdominal pain upper (2.2%). The most frequent serious adverse reactions are infections (0.3%) including herpes simplex, herpes zoster, and pneumonia. Refer to SmPC for further information on side e ects. Legal
Category: S1A. Marketing Authorisation Numbers: EU/1/21/1593/002 - Cibinqo 50 mg (28 film-coated tablets), EU/1/21/1593/007 - Cibinqo 100 mg (28 film-coated tablets); EU/1/21/1593/012 - Cibinqo 200 mg (28 film-coated tablets). Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium.
For further information on this medicine please contact Pfizer Medical Information on 1800 633 363 or at medical.information@ pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 467 6500.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Last revised: 03/2023.



Ref: CQ 5_0 IE.
















References:


1. Reich K, Thyssen JP, Blauvelt A, et al. E cacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, doubleblind, multicentre phase 3 trial. Lancet. 2022;400(10348):273-282.


2. Cibinqo Summary of Product Characteristics.

© 2023 Pfizer Inc. All rights reserved. PP-CIB-IRL-0125 | August 2023.
RAPID ITCH
RELIEF
Pelvic Pain Pelvic Pain in Women
Written by Dr Kirk Levins, Chronic Pain Medicine Consultant
Introduction
Female pelvic pain is a complex and often debilitating condition that affects millions of women worldwide. This condition can have a profound impact on a woman's quality of life, causing physical discomfort, emotional distress, and even disruption of daily activities.
It can originate from various structures within the pelvis, including the reproductive organs, urinary system, musculoskeletal system, or gastrointestinal tract. Two common conditions causing pelvic pain are vulvodynia and endometriosis.
Vulvodynia
Vulvodynia is a chronic pain condition that primarily affects
the vulva, the external genitalia of the female reproductive system. The term "vulvodynia" encompasses various types of vulvar pain disorders that share common features, including pain, burning, stinging, or discomfort in the vulvar area. This pain can be spontaneous or provoked, such as during sexual intercourse or when pressure is applied to the vulvar region. Vulvodynia can be classified into two primary categories, generalized vulvodynia and vestibulodynia (provoked vulvodynia). Generalized vulvodynia involves pain throughout the entire vulvar area. Women with generalized vulvodynia may experience constant or intermittent discomfort. Vestibulodynia is characterized by pain or discomfort primarily at the vaginal entrance or vestibule. It is often provoked by touch or pressure, such as during sexual intercourse or tampon insertion. The exact cause of vulvodynia remains elusive, and it is believed to be a
Women's Health News
multifactorial condition influenced by a combination of factors. Management of vulvodynia can be difficult however combined conservative and interventional approaches have shown promise. Conservative management involves pelvic floor physiotherapy, oral medication such as tricyclic antidepressants, and lifestyle changes. Interventional approaches involve autonomic nerve blocks and ultrasound guided targeted injection of botulinum toxin into the pelvic floor.
Endometriosis Pain
Endometriosis is a chronic and often painful gynecological disorder that affects millions of women worldwide. It is a complex gynecological disorder characterized by the presence of endometrial-like tissue outside the uterus, often leading to chronic pelvic pain and various other symptoms. Pain in endometriosis can manifest in several forms. Two common forms are dyspareunia (painful intercourse) and pelvic girdle pain.
Dyspareunia in endometriosis is a complex and multifaceted issue that requires a comprehensive approach to diagnosis and management. Understanding the interplay between the physical, psychological, and hormonal factors involved in this symptom is essential for healthcare providers to offer effective interventions and support for women with
New Research on Women’s Heart Health
Research carried out by Blackrock Health has found that women are almost half as likely to seek medical attention for heart related symptoms than men. Only 16% of women claimed that they went to a hospital A&E to address heart related symptoms, versus 31% of men.

The findings revealed that heart related symptoms are often dismissed as a sign of ageing amid confusion over what constitutes an urgent cardiac issue. The research, conducted among a nationally representative sample of 1,010 members of the general public aged 18 and over, found that 25%
of people took no action when they experienced heart related symptoms, and 24% thought their symptoms were a general symptom of getting older.
Concerningly, nearly 1 in 5 people (18%) surveyed, ignored potentially life-threatening cardiac symptoms, as they thought they would improve in time. As part of their ongoing Show Your Heart Some Love campaign, Blackrock Health is encouraging people to learn more about potentially concerning cardiac health symptoms.
Dr Carla Canniffe, Consultant Cardiologist at Blackrock Health
said, “Cardiac symptoms are not always recognised by patients when they occur. If left untreated, they can lead to potentially life-threatening situations. This research highlighted several concerning trends; many people simply aren't acting in response to cardiac issues when they experience them, and women in particular, are less likely to seek medical assistance. Symptoms of heart conditions are often confused with other diseases or dismissed by patients as part of ageing or something they have to live with. Accurately recognising and addressing cardiac symptoms
endometriosis. Ultrasound guided injection of Botulinum toxin to the pelvic floor has shown promise in treating dyspareunia due to endometriosis.
Pelvic girdle pain (PGP) refers to discomfort and pain experienced in the area of the pelvis, specifically in the joints and ligaments that support the pelvic region. It is likely caused by a combination of factors including inflammation, high levels of estrogen causing an increase in joint laxity and pain perception, postural changes due to the pain of endometriosis and muscular. Pelvic girdle pain typically presents with pain in the buttock that is exacerbated by transitioning from sitting to standing. The pain can radiate to the level of the knee. Pelvic girdle pain often responds well to injection and lesioning of the nerves supplying the joint.
Dr Kirk Levins is a Chronic Pain Medicine consultant working across SVPH, NMH, NRH and SVUH. He has spent the last 6 years treating women with pelvic pain at both St. Vincents University Hospital and at the NMH. He is also a keen researcher and holds degrees in Chemistry and Neuroscience. He has won awards for his research including the American Academy of Pain Medicine award for research and the Abbvie scholarship. He is involved in several research collaborations with institutions in New Zealand, USA, and the Netherlands.
is essential to improving patient outcomes nationwide."
"During Irish Heart Month, we want to encourage people to learn more about the key symptoms associated with cardiac problems so that people know what to look out for, and to know when something is not right.”
The research found that while over three quarters (77%) of adults claim to have experienced one of the heart related symptoms outlined above, only 53% of those who have experienced cardiac symptoms claim they contacted their doctor.
OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 44
Laryngeal Cancer
Survival in an Era of Organ Preservation: An Update on Laryngeal Cancer in Ireland
Written by Gerard P. Sexton1,2, Paul Walsh3, Frank Moriarty2,4, Paul Lennon5 & James Paul O’Neill1,2
1Department of Otolaryngology, Head and Neck Surgery, Beaumont Hospital, Beaumont Road, Dublin
2Royal College of Surgeons in Ireland, Dublin
3National Cancer Registry Ireland, Cork Airport Business Park, Cork
4School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin
5Department of Otolaryngology, Head and Neck Surgery, St James Hospital, Dublin
The epidemiology of laryngeal cancer is relatively well understood, with much prior work published on the topic in both socially privileged and relatively socially deprived countries.1,2,3 Despite the significant morbidity and quality-of-life impact associated with treatment of both early and advanced disease,4 survival following laryngeal cancer remains favourable relative to other varieties of head and neck cancer (HNC), such as hypopharyngeal and nasopharyngeal cancer. The incidence of laryngeal cancer has been noted in the US and Europe to be falling in recent years.5
Laryngeal cancer of all stages was historically managed with surgery in the first instance, but landmark trials throughout the 1990s and early twentyfirst century proved that organ preservation with non-operative management was a realistic possibility for many patients.6,7,8 In contemporary practice, laryngeal cancer is managed using an adaptive approach taking into account specific anatomy, the extent of disease, and the overall condition and preferences of the patient.9,10,11,12 5-Year survival for laryngeal cancer has been consistently reported at approximately 60% for many years despite advances in treatment;10, 13 concerningly, in some reports following the aforementioned laryngeal preservation trials, survival was noted to drop.14 This begs the question as to whether all larynges are worth preserving.
Aims and objectives
This study aimed to examine the epidemiology of laryngeal cancer patients in Ireland and to evaluate the response of laryngeal squamous cell carcinoma (SCC) to primary treatment
modalities. Specific objectives included description of subsite data, survival trends, trends in choice of treatment modality, and the survival benefit observed in particular groups such as those with T3 disease for whom management may depend on many features.
Materials and methods
Study design
A retrospective cohort study was conducted using STROBE standardised reporting guidelines. The study cohort was derived from a database obtained from the National Cancer Registry of Ireland (NCRI) of HNC patients diagnosed in Ireland between January 1994 and December 2014. The length of diseasespecific follow-up in this instance is until the end of 2015. This database was derived from both
electronic healthcare records and physical charts which the NCRI analyses on a continual basis.
Inclusion/exclusion criteria
The inclusion criteria were adult patients (> 18 years of age) diagnosed with a primary laryngeal cancer (as defined by the American Joint Committee on Cancer TNM Classification of Malignant Tumours (TNM) for Head and Neck Cancer 8th edition15) within the period specified. The primary exclusion criterion was patients with cancers occurring outside this region. Those with histological diagnoses other than SCC were excluded to ensure homogeneity of the data and reduce bias.
Despite being coded under the oropharyngeal site grouping in the International Statistical Classification of Diseases and
Related Health Problems, lesions arising from the anterior epiglottis have here been included as part of the supraglottis. For analysis purposes, disease of unclear subsite has been considered together as ‘other’ and will hereafter be referred to as such.
Statistical methods
Descriptive statistics for included participants’ baseline characteristics were generated. Kaplan–Meier survival analysis was conducted to test the survival benefit of primary radiotherapy compared with primary surgery by T stage at presentation. Primary treatment was determined by the tumour-directed therapeutic modality commenced at the earliest stage following diagnosis. TNM stage at presentation statistics and 5-year survival statistics by disease subsite and
45 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
1
Figure
Laryngeal Cancer

Ethical considerations
Ethical approval was sought from and approved by the RCSI Research Ethics Committee. The database in question already exists, and the NCRI retains legislative authority to analyse data for research, and release data to external parties specifically for this purpose.16 All data derived from the NCRI database are fully anonymised in line with the best practice as outlined by the Data Protection Commission, and their lawful grounds for processing same is pursuant to compliance with a legal obligation.16 Informed consent has not been explicitly sought from any of the patients involved.
Results
overall stage were generated. Hazard ratios adjusted for gender, age, and N- and M-staging as categorical variables were generated using multivariate Cox analysis.
The T4 cohort was also specifically analysed for the effect of primary chemoradiotherapy (defined as no more than 14 days between
commencement of chemotherapy and radiotherapy without the use of primary surgical management) against primary surgery. This was performed due to the prevalent use of these modalities in combination for laryngeal preservation and as a more accurate test of the performance of non-operative management—chemotherapy would usually only be withheld in
this setting due to advanced age or medical comorbidity, and thus, this is expected to mitigate some of the bias in this cohort.
Statistical analyses were conducted using Stata version 16.1 and JoinPoint version 4.9.1.0. Statistical significance was assumed at p < 0.05.
62% (n = 1646) had primary glottic disease, with the supraglottis being the next most common subsite at 25% (n = 665). The 60–64 age group comprised the highest number of cases (n = 447, 16.8%) and also represented the median age group. An overwhelming majority of patients were male (85%, n = 2263). Figure 1 shows the incidence of laryngeal cancer rising over time—the average incidence across all years was 3.06 cases/100000/ year, while the 5-year averages for 1994–1998 and 2010–2014 were 2.78 cases/100000/year and 3.43 cases/100000/year, respectively. This increase was statistically significant (p < 0.05) and represents a significant rise in absolute number of cases—97 cases were recorded in 1994 compared with 170 in 2013.
T1 and T2 diseases were the most commonly encountered overall (31% and 24.4%, respectively) and particularly in the glottis (42.4% and 21.9%, respectively). The glottis also represented the most common source of T3 (n = 199, 47.6%) and T4 (n = 117, 33.9%) disease despite higher prevalence of such disease within other subsites. N0 disease was recorded in 55.2% (n = 1582). This was higher in the glottis (65%, n = 1068) than the supraglottis (44.5%, n = 296) and subglottis (54.2%, n = 32). The prevalence of all N stages of disease was lower in glottic disease than the corresponding level in either supraglottic or subglottic disease. M1 disease was present in 3.3% (n = 87).

46 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 2
Figure 3
Survival
5-year DSS from laryngeal cancer was 60.6% for the period studied, though the glottic region was significantly higher at 71.8% compared with the subglottic region at 40%. 85.9% of those with stage I disease survived to 5 years compared with 5.8% of those with stage IVC. There was no notable difference in 5-year DSS between male and female patients (60.2% vs 62.4%, χ2 = 0.41, p = 0.52).
Survival was noted to increase marginally over time, as shown in Fig. 2, though statistical significance was not achieved (p = 0.48). When broken down by stage, this increase was most notable for stage I disease (p = 0.007) and was negligible for the remainder. A Kaplan–Meier curve of DSS by stage is shown in Fig. 3.
Primary radiotherapy versus surgery
67% (n = 505) of T1 disease was managed with radiotherapy; a similar proportion of T2 (74%, n = 460) and T3 (78%, n = 303) disease was managed as such. T4 disease was managed with primary surgery in 58% (n = 168), with the remainder receiving primary radiotherapy. The proportion of T1 and T4 disease managed with primary radiotherapy by year of incidence is shown in Figs. 4 and 5. Over time, there was a statistically significant increase in the use of primary surgery
rather than radiotherapy for T1 disease (p = 0.003). Use of primary radiotherapy for T4 disease rose significantly in the late 1990s, but later in the study period, this approach became less prevalent. No trends of note were identified for T2 or T3 disease.
T1 disease showed no notable difference in both overall survival (OS) (HR 1.25, p = 0.089) and DSS (HR 1.23, p = 0.297) associated with the use of radiotherapy over surgery. Supraglottic disease showed statistically significantly worse DSS (HR 4.67, p = 0.02) with the use of radiotherapy.
T2 disease showed statistically significantly improved OS with the use of radiotherapy (HR 0.77, p = 0.018). Statistical significance was not achieved for DSS (HR 0.78, p = 0.086). This effect was strongest in the supraglottic group (HR 0.75, p = 0.091). Exclusion of node positive disease yielded a cohort of 288 with T2N0 disease; there was no difference in OS (HR 0.93, p = 0.61) or DSS (HR 0.97, p = 0.86) between the treatment groups for this subgroup.
T3 disease showed no statistically significant difference in OS between radiotherapy and surgery groups (HR 0.98, p = 0.9). There was a statistically significant benefit to DSS associated with the use of radiotherapy in the same group (HR 0.72, p = 0.045). The ‘other’ site group was the only subsite that achieved statistical significance for DSS (HR 0.29, p = 0.004), though this was
narrowly missed in the supraglottis (HR 0.61, p = 0.085).
T4 disease showed no notable difference in either OS (HR 1.18, p = 0.234) or DSS (HR 1.23, p = 0.297) associated with the use of radiotherapy over surgery. No statistically significant difference was noted among any of the subsites with the exception of the subglottis, where radiotherapy was noted to decrease OS (HR 12.37, p = 0.039). Primary chemoradiotherapy did not produce a statistically significant change in OS (HR 0.85, p = 0.51) or DSS (HR 0.74, p = 0.34) when compared with primary surgery.
Discussion
The epidemiology of laryngeal cancer has developed considerably in the recent past, with low- to middle-income countries such as India and Pakistan noting increasing incidence rates and more advanced disease stages at presentation.1 By comparison, in relatively socioeconomically privileged countries, the incidence has either gone largely unchanged or has declined.2, 17 This pattern is not replicated in the presented data, with incidence in Ireland continuing to rise at least during the observed time period. The reasons for this are unclear— multiple high profile public health measures, including a national ban on smoking in the workplace and enclosed public spaces, have been instituted in Ireland during this time frame, though clearly there
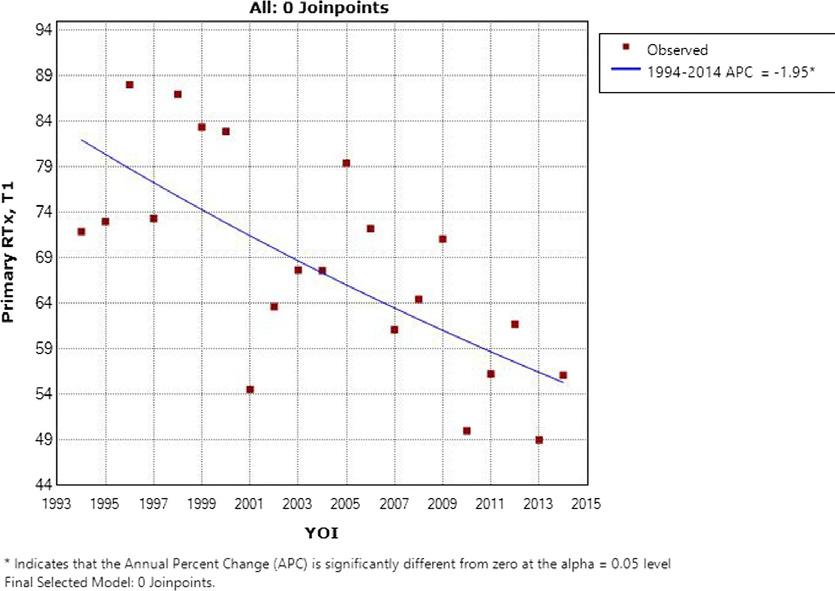
Figure
will be a lag time in the effects of these measures.18 The incidence internationally was recently estimated at 2.76 cases/year per 100,000 inhabitants;5 the Irish incidence was notably higher than this at almost every time point. This phenomenon also occurred during a time of significant increase in the population of Ireland from 3.56 million in 1994 to 4.62 million in 201419—clearly, this represents a significant increase in workload. Notably, the universally observed preponderance of male gender among laryngeal cancer patients is in contrast with some recent reports of poorer survival relative to their female counterparts3—this finding was not observed here, indicating that with adequate and equitable access to healthcare, there is not necessarily a gender bias in laryngeal cancer survivorship. Conservation of the anatomical larynx, as famously described in the landmark publication by Wolf et al.,6 now forms the basis for much of the treatment rationale for advanced laryngeal cancer. It additionally represents one of the more controversial areas in head and neck cancer; despite a strong body of evidence that T4 disease mandates primary ablative surgery,7, 20,21,22 there are multiple recent reports of the successful use of concurrent chemoradiotherapy regimes for organ preservation in such disease.23,24,25 No notable difference in survival was detected in the reported data for patients with T4 disease treated with primary surgery or radiotherapy. It is not clear why this was the case—differentiation of subtyping into T4a and T4b was not available which would have been useful for distinguishing those with probable unresectable primary disease and correction for additional variables as described did not further clarify the issue. In addition, specifically comparing primary chemoradiotherapy against primary surgery did not produce a significant difference. Important changes in the TNM staging system in 2003 likely introduce some bias, as minor erosion of the thyroid cartilage was redesignated as T3 rather than T4 disease, in essence rendering T4 disease more homogeneously advanced.26 This has previously been noted to have ramifications for registrybased data.27 Primary surgery currently represents the standard of care in Ireland for resectable T4a laryngeal cancer without metastatic disease—further inquiry
47 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
4
Laryngeal Cancer
is warranted to explain the lack of a significant difference in survival.
42% of cT4 patients underwent non-surgical management during the period analysed. This follows the international trend towards non-surgical management of such patients during the same time period where survival in the United States was noted to fall.14 It can only be concluded that multiple factors were at play in the survival in Ireland remaining stable overall.
For early stage laryngeal cancer, specifically T2 or earlier, choice of therapy is largely dictated by the specific anatomy of the disease.28 In the presented data, survival for T1 lesions was shown to be similar regardless of choice of therapy, while the same was true of T2N0 lesions. This reflects the role that patient choice, available expertise,
and disease anatomy often play in such decisions. Supraglottic lesions had worse DSS where radiotherapy was employed—this may reflect selection bias, as most supraglottic lesions amenable to laser resection would be smaller and more homogeneous compared to the wider array of T1 glottic lesions. For T2 disease, overall radiotherapy was associated with significantly improved survival, especially in the supraglottic subgroup. A potential lurking variable here is that failure of local control in either modality results in disparate outcomes—where a positive margin following laser excision could be expected to respond to adjuvant radiotherapy, incomplete response to primary radiotherapy will mandate salvage surgery which is itself a poor prognostic marker. It is interesting
to note the increasing prevalence of primary surgery in T1 laryngeal cancer as this modality became more available in Ireland. This occurred without any notable decline in survival for T1 or T2 disease.
For T3 disease, primary radiotherapy with or without chemotherapy represents the standard of care in Ireland and in other countries in Europe.29, 30 It is known that some with T3 disease undergo surgery for reasons including for salvage or in the setting of a non-functional larynx.29,30,31 DSS was significantly improved for T3 disease by the use of primary radiotherapy as opposed to primary surgery; this would be the expected finding as those requiring primary surgery would be expected to have more
NEWS - World Suicide Prevention Day
advanced disease. However OS was the same in both groups, suggesting that patients receiving primary radiotherapy are dying at similar rates for reasons other than disease progression. A possible explanation for this is poorly functioning, post-radiotherapy larynges which are at increased risk of aspiration of secretions and oral intake due to impaired airway protection. This being the case would be highly relevant as it would further emphasise the findings of Kim et al. in 2018 that OS in T3 disease was no different between the total laryngectomy group and the laryngeal preservation group.32 The purpose of this observation is not to suggest that primary radiotherapy should not represent a standard of care in T3 disease, but rather that further investigation is warranted to systematically identify those with T3 disease who are unlikely to benefit from laryngeal preservation to the extent that primary surgery should instead be considered.
Conclusion
Despite innovations in laryngeal surgery and radiotherapy and concerning international survival trends, DSS following laryngeal cancer in Ireland remains largely unchanged at 60.6%. The incidence has increased to approximately 3.43 cases/100000/ year. Primary radiotherapy improves DSS for T3 disease relative to primary surgery but does not improve OS—this may be due to poor organ function postradiotherapy. No survival difference was noted between primary surgery and chemoradiotherapy for T4 disease.

References available on request
Minister of State for Mental Health and Older People, Mary Butler, and the Health Service Executive (HSE) have marked international World Suicide Prevention Day. The global theme for 2023 is ‘Creating Hope through Action’.
Minister Butler and the HSE have three key messages for this World Suicide Prevention Day: Reach In, Reach Out, and Be the Light.
• reach In to someone you know who might be having difficulties – check in with them and tell them you care about them
• reach out if you are feeling low, sad or hopeless – sharing things with someone else will help. Talk about what is going on for you. There are supports online, over the phone, by text or face to face – yourmentalhealth.ie
• finally, you can Be the Light by helping spread supportive messages and get involved in activities that promote positive mental health and wellbeing or suicide prevention in your community
Minister Butler said, "On this World Suicide Prevention Day, we want to spread a message of hope to anyone impacted by suicide or suicidal thoughts. We can make a difference through small but meaningful actions to help those who are struggling.
"Even a single conversation can make a big difference. Every death by suicide is a tragedy, and I am thinking today of those families, friends, and communities bereaved by suicide. Preventing the tragedy of suicide remains a key priority and we will continue to support our colleagues in the HSE National Office for Suicide Prevention and their community partners to ensure that the resources are available to continue their valuable work.”
48 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
5
Figure
DON’T WAIT UNTIL OSTEOPOROSIS STRIKES AGAIN
Rebuild bone before it breaks again—with Movymia®1
THE MEDICINES MANAGEMENT PROGRAMME RECOMMENDS
MOVYMIA® AS A BEST VALUE MEDICINE FOR TERIPARATIDE
RELIABLE: Movymia®’s quality, safety and efficacy is highly similar to its reference product1,3,*

EFFECTIVE: Anabolic MoA effectively rebuilds bone through the stimulation of osteoblasts1,4
AFFORDABLE: Allows more eligible patients to benefit due to its cost advantage5,6
RE-USABLE: One high quality reuseable pen for the entire treatment period1
MOVYMIA 20 MICROGRAMS/80 MICROLITERS SOLUTION FOR INJECTION
Each dose of 80 microliters contains 20 micrograms of teriparatide. One cartridge of 2.4 ml of solution contains 600 micrograms of teriparatide (corresponding to 250 micrograms per ml). Presentation: Glass cartridge. Indications: Movymia is indicated in adults. Treatment of osteoporosis in postmenopausal women and men at increased risk of fracture. In postmenopausal women, a significant reduction in the incidence of vertebral and non-vertebral fractures but not hip fractures has been demonstrated. Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk for fracture. Dosage: The recommended dose is 20 micrograms administered once daily. Patients should receive calcium and vitamin D supplements if dietary intake is inadequate. The maximum total duration of treatment is 24 months. The 24 month course should not be repeated over a patient’s lifetime. Following cessation of teriparatide therapy, patients may be continued on other osteoporosis therapies. Teriparatide must not be used in severe renal impairment. Use with caution in moderate renal impairment and impaired hepatic function. Teriparatide should not be used in paediatric patients (less than 18 years), or young adults with open epiphyses. Method of administration: Movymia should be administered once daily by subcutaneous injection in the thigh or abdomen. It should be administered exclusively with the Movymia Pen reusable, multidose medicine delivery system and the injection needles which are listed as compatible in the instructions provided with the pen. The pen and injection needles are not included with Movymia. However, for treatment initiation a cartridge and pen pack should be used. Movymia must not be used with any other pen. Patients must be trained to use the proper injection techniques. Contraindications: Hypersensitivity to the active substance or excipients. Pregnancy and Breast-feeding Pre-existing hypercalcaemia, severe renal impairment, metabolic bone diseases other than primary osteoporosis or glucocorticoid-induced osteoporosis, unexplained elevations of alkaline phosphatase, prior external beam or implant radiation therapy to the skeleton, patients with skeletal malignancies or bone metastases. Warnings and precautions: In normocalcaemic patients, slight and transient elevations of serum calcium concentrations have been observed following teriparatide injection. Serum calcium concentrations reach a maximum between 4 and 6 hours and return to baseline by 16 to 24 hours after each dose of teriparatide. Therefore, if blood samples for serum calcium measurements are taken, this should be done at least 16 hours after the most recent teriparatide injection. Routine calcium monitoring during therapy is not required. Teriparatide may cause small increases in urinary calcium excretion, but the incidence of hypercalciuria did not differ from that in the placebo-treated patients in clinical trials. Teriparatide should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition. In short-term clinical studies with teriparatide, isolated episodes of transient orthostatic hypotension were observed. Typically, an event began within 4 hours of dosing and spontaneously resolved within a few minutes to a few hours. When transient orthostatic hypotension occurred, it happened within the first several doses, was relieved by placing subjects in a reclining position, and did not preclude continued treatment. Caution should be exercised in patients with moderate renal impairment. Experience in the younger adult population, including premenopausal women, is limited. Treatment should only be initiated if the benefit clearly outweighs risks in this population. Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, teriparatide should be discontinued. The recommended treatment time of 24 months should not be exceeded. Contains sodium. Interactions: Digoxin, digitalis. Fertility, pregnancy and lactation: Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, Movymia should be discontinued. Movymia is contraindicated for use during pregnancy and breast-feeding. The effect of teriparatide on human foetal development has not been studied. The potential risk for humans is unknown. Driving and operation of machinery: Teriparatide has no or negligible influence on the ability to drive and use machines. Transient, orthostatic hypotension or dizziness was observed in some patients. These patients should refrain from driving or the use of machines until symptoms have subsided. Undesirable effects: Nausea, pain in limb, headache, dizziness. Refer to Summary of Product Characteristics for other adverse effects. Pack size: 1. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance. Website: www.hpra.ie Marketing authorisation holder: STADA Arzneimittel AG, Stadastrasse 2-18, 61118 Bad Vilbel, German. Marketing authorisation number: EU/1/16/1161/001-003. Medicinal product subject toedical prescription. Date last revised: January 2022.
1. Movymia® SmPC. 2. Best Value Medicines https://www.hse.ie/eng/about/who/cspd/ncps/medicines-management/best-value-medicines/teriparatide/ Accessed February 2023.
3. Movymia® EPAR – public assessment report, available at: https://www.ema.europa.eu/documents/assessment-report/movymia-epar-public-assessment-report_en.pdf
4. Brixen KT et al. Basic Clin Pharmacol Toxicol. 200494(6):260–70. 5. Lyman GH et al. N Engl J Med. 2018378(21):2036–2044. 6. Janjigian YY et al. Future Oncol. 201814(23):2403–2414.* Forsteo®
Date prepared: March 2023. 2023/ADV/MOV/061H
2
Anaphylaxis
Is Switching of Adrenaline Auto Injector Devices a Concern for Anaphylaxis Management? A CROSS-Sectional Study
Wurttele1,2,*
1Department of Paediatrics and Child Health, University College Cork, T12 DC4A Cork, Ireland
2HRB Clinical Research Facility Cork (CRF-C), Irish Centre for Maternal and Child Health Research (INFANT), Cork University Hospital, T12 DC4A Cork, Ireland
3Gerencia de Atención Primaria Valladolid Este, 54, 47010 Valladolid, Spain
are four brands of AAI available in Ireland—Epipen®, Jext®, and Anapen® are three. Another brand, Emerade®, has been recalled from the market in recent years.9 As the number of different AAI designs has increased, device switches could become a significant clinical issue.6
There has been no previous research in Ireland documenting the prevalence of AAI brands or the frequency at which caregivers switch brands. Similarly, possible factors, which influence the switching of brands of AAI, have not been explored.
The aim of this study is to explore the usage of different brands of adrenaline auto-injectors among caregivers of children with food allergies in Ireland.
Methods
Study Setting and Sample
Introduction
Food allergies have been increasing in prevalence in the last two to three decades, with an overall life-time self-reported prevalence of 6% in Europe.1 Children diagnosed with a food allergy have a negatively impacted health-related quality of life (HRQL)2 and require specialist care from a paediatric allergist, allergy nurse, and dieticians to learn how to best avoid the allergen, as well as to know the correct management of allergic reactions in the case of accidental exposure.

Anaphylaxis is considered to be a severe allergic reaction associated with lower respiratory or cardiovascular features,3 which is rapid in onset and occasionally
fatal.4 The literature reports a global (United Kingdom, Europe, United States, Australia, New Zealand) increase in incidence of hospitalizations for anaphylaxis,5 with the highest incidence of fatal anaphylaxis due to food allergy occurring in the second decade of life.6
Adrenaline is the mainstay of treatment of anaphylaxis.4 In a community setting, an adrenaline auto-injector (AAI) is used.3 Parents and children who have been prescribed an AAI should be given instruction and guidance on when and how to use it by a trained healthcare professional.3 However, in a systematic review, a correct AAI administration technique was shown to be low, with only
32% of parents/caregivers successfully demonstrating correct AAI technique.7 Similar findings were found in a recent review of the literature, regarding the effectiveness of educational intervention on AAI administration ability, where it was found that the AAI administration success among parents ranged significantly, and the success rates varied from 5.6 to 84%.8 This may reflect several issues, including training effectiveness or lack of training, user stress during administration, or inherent differences consequent of the design of the individual auto-injector.3
Adrenaline can be administered by several different brands of AAI, each with a unique design. There
This is a cross-sectional study, which took place in the paediatric allergy clinic in Cork University Hospital, Cork, Ireland. Participants were caregivers of children with food allergies who had attended the paediatric allergy clinic. Inclusion criteria were parents or guardians of children under the age of 18, who had been diagnosed with an IgE-mediated food allergy, who had received previous training in anaphylaxis management and AAI administration, and who were currently prescribed an adrenaline auto injector for their food allergy. Exclusion criteria were patients with drug or venom allergies. Ethical approval was received by the Clinical Research Ethics Committee of the Cork Teaching Hospitals (Reference code: ECM 4 (hh) 12/01/2021 & 3 (w) 09/03/2021).
50 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Written by Caoimhe Cronin1, Ciobha O’Kelly2, Hannah Keohane1, Laura Flores Villarta1, Ciara Tobin2, Roberto Velasco3 and Juan Trujillo
Data Collection Methods
An online questionnaire was designed for data collection in this study. This questionnaire was designed as part of a larger study entitled Telemedicine as an Educational Tool Regarding Adrenaline Auto Injectors and Anaphylaxis Management (TEAAMs), with a section of this questionnaire dedicated to data collection for this study. As there were no such questions found focusing on usage of different brands of AAI in the literature, questions were designed and validated amongst a pilot group of three parents, a consultant paediatric allergist, and an allergy nurse specialist. Small modifications were made based on the feedback from this pilot group before inclusion in the final questionnaire.
Data Collection
Considering the COVID 19 pandemic, participants were identified from a retrospective chart review to minimize patient contact and adhere to social distancing restrictions, and informed consent was obtained remotely.
Potential study participants were contacted initially by phone and asked if they were interested in taking part in the larger TEAAMs study, as well as the present study. Phone call attempts were made to each caregiver at least twice. During this telephone call, they were screened for eligibility, which may not have been apparent in their patient file. Eligible participants were sent an e-mail containing detailed study information. They were given the opportunity to ask further questions by email or telephone.
Once happy to proceed, they remotely completed informed consent and completed the online questions. Emails to remind interested caregivers to complete the questionnaire were sent three times if they had not completed the questionnaire. Google Forms was used to capture study responses. Participants were asked questions relating to: socio-economic demographics (age of child, age of parent, gender of child, relationship to child household income, and level of education reached by caregiver), the child’s allergy history, and questions relating to AAI usage, such as whether participants have changed brands of AAI before and what influenced this change, what training they received from a healthcare professional when they changed the brand of AAI they
used, which brands of AAI they have used, and what preferences they may have and open questions asking why they preferred an AAI brand/found an AAI brand easier to use.
Statistical Analysis
SPSS version 28 (IBM, n.d.) [10] was employed for statistical analysis. Descriptive statistical methods, including observation ranking using mean, was used. These descriptive methods were employed to characterize the population, e.g., age of child, age of parent, type of allergy, and mean household income, and they were used to explore the proportion of caregivers that have different brands of AAI. Chi-square tests were used to explore associations between demographic data and responses, with differences at a level of 95% confidence probability and above regarded as statistically significant. Examples of this include the age of the child and whether they have changed brands of AAI. Free-text responses, regarding why they preferred a particular brand of AAI, were entered into MS Word and were thematically analysed, as outlined by Braun and Clarke 2006.11
Results
Study Participants
A total of 701 parents were contacted between June 2021 and May 2022. Of them, 313 could not be reached by telephone or did not meet eligibility criteria when screened. An invitation email was sent to 388 parents to take part in the study. A total of 164 responses were received to the Google Forms questionnaire. This equates to a response rate of 42.2%. Six responses were excluded from analysis on further inspection, as they responded ‘No’ when asked if their child had been prescribed an AAI. Thus, 158 participants were included in the study. A flow diagram depicting the recruitment of participants to the study is shown in Figure 1.
51 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Figure 1. Flow diagram of participant recruitment. AAI: Adrenaline Auto Injector
Anaphylaxis
1: Remove cap, Step 2: Push to hear a ‘click’, Step 3: Hold for x seconds
expiration date of the new AAI was longer than their previous AAI, seven (9.1%) said the new AAI was recommended by their pharmacy, four (5.2%) stated the new brand was easier to use, two participants stated that their old AAI had been recalled from the market, and one participant stated that the previous AAI was too big and bulky for their child to carry.
Brands of AAI Used by Caregivers
Of the 158 participants whose child was prescribed an AAI, 121 (75.58%) reported their child carried Epipen®, 25 (15.82%) carried Jext® , and 12 (7.59%) carried Anapen®
No child carried Emerade®
Switching Brands of AAI
An amount of 48.73% (n = 77) of caregivers had switched brands of AAI at least once before, with 20.9% (n = 33) switching brands once, 13.9% (n = 22) switching two times, 9.5% (n = 15) switching three times, and 4.43% (n = 7) switching more than three times.
Those who had switched brands in the past were asked the reason they switched brands. An amount of 46 (59.74%) caregivers said this was due to lack of availability of their previous AAI at their pharmacy, 16 (20.7%) said the
An amount of 59.74% (n = 46) parents who had switched brands of AAI in the past did not receive formal training when they were switched to a new brand.
An amount of 153 caregiversresponded when asked what brands they had received training in. An amount of 112 (73.2%) stated they had received training in one brand of AAI in the past (87 Epipen®, 16 Jext®, 7 Anapen®, 2 Emerade®). An amount of 25 (16.3%) had received training in two of the four brands of AAI in the past. An amount of 13 (8.4%) stated to have received training in all three available brands of AAI in Ireland (Epipen®, Anapen®, Jext®). There was an association between caregiver’s age and changing AAI (p = 0.02). In additions, it was found that 32.8% of patients whose parents are less than 40 years old changed AAI brand, while 59.6% of patients whose parents are ≥40 years old did change brands (p < 0.01). Globally,
there was no association between household income and changing brand of AAI (p = 0.32). However, of patients with a house income >100,000 ¤, 14 (70%) changed AAI brand, while 61 (44.9%) of the 136 caregivers with a household income of <100,000 ¤ changed brands (p = 0.04). An association was not found between any history of anaphylaxis and switching AAI brands (p = 0.195). However, a clear trend can be seen between increasing episodes of anaphylaxis and changing AAI brands. However, due to the small number of patients who had experienced multiple episodes of anaphylaxis, no statistical significance was found (p = 0.481). No association was found between changing brands of AAI and the child’s age (p = 0.09) or parent’s educational level (p = 0.44).
Preference of AAI
An amount of 65 caregivers who had switched brands of AAI at least once in the past responded when asked which brand of AAI, if any they preferred. 63.1% (n = 41) caregivers preferred Epipen®, 23.1% (n = 15) preferred Jext® , 6.15% (n = 4) preferred Emerade® , and 7.69 (n = 5) preferred Anapen®
An amount of 53 participants responded to the open question, asking them to explain why they preferred a different brand. When responses were coded, the
most common themes regarding preferred AAI brand referred to the device’s design, layout, training, and instructions.
Design
Many parents referred to the colour coding of the AAI device, which they believed helped them remember the correct steps of AAI administration.
“Different coloured parts, easier to differentiate between steps.”
“…blue to the sky/orange to the thigh.”
Caregivers also preferred AAI brands, which they described as having fewer steps involved in AAI administration and were therefore ‘more straightforward’.
“There were only 2 steps to the process of administering the AAI.”
“It’s a two step process pull out the blue stop and jab and hold.”
Layout
Caregivers preferred AAI brands, which gave clear and simple instructions on the device that could be followed with ease in an emergency.
“Visibly easier to use, as had arrows on pen to point which direction to use first.”
“Clear instructions, label not cluttered.”
52 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 2 Features of the ‘ideal’ AAI device. The device features a clear, colour coded two step process with a link to online instructional resources. The three step process is clearly labelled according to the device’s administration technique, for example Step
Training
Many of the caregivers preferred an AAI brand because this was the brand they had received formal training in from a healthcare professional.
“This is the only brand I was trained in.”
“Because it was the one I was shown how to use in the first place.”
Many parents also appreciated receiving a trainer pen of this brand so that they could simulate its administration.
“I have a trainer pen for this type.”
Some parents also had more confidence in a particular AAI brand, as they have had to administer it in the past.
“I’m just more used to it. We have only ever actually used it once.”
Resources
Caregivers appreciated online resources available on different AAI brand websites. Others appreciated that the company sent a SMS when the device was expiring to remind the parent to obtain a new device.
“Jext have good online information.”
“…the EpiPen website also offers a free SMS expiry reminder system.”
Figure 2 summarises what features the ‘ideal’ AAI device should have based on the responses from caregivers.
Discussion
This study explores the perspective of different AAI devices by caregiversof children with food allergies in a paediatric allergy clinic in the south of Ireland.
Epipen® appears to be the most used AAI among children with food allergies, followed by Jext and then Anapen®. No children carried Emerade®. This is significant, as, since Emerade® was recalled from the Irish market in 2019,9 it was necessary to evaluate whether any parents were not aware of its recall and continued to carry it.
The finding of Epipen® being the most carried pen was in line with our predictions. While the paediatric allergy clinic in Cork University Hospital does not recommend a particular brand when prescribing adrenaline, parents, children, and the general public consider ‘epipen’ to be synonymous with ‘adrenaline auto injector’. The wide availability of Epipen® devices in pharmacies is the likely factor which influences the commonality of this device
among those with food allergy. While no such similar studies have been conducted in Ireland or other European countries to our knowledge, these findings are supported by the previous literature from Canada, which found that Epipen® and Epipen Jnr® were the most commonly dispensed adrenaline devices over a five-year period (68.5% and 17.2%, respectively).12
Switching brands of AAI is a common occurrence among parents of children with allergies, with almost half (48.73%) having switched brands at least once before. Patients of parents older than 40 years of age were more likely to have switched brands in the past. This is most likely associated with the longer duration of their child’s food allergy.
While switching devices occurs commonly in the community, parents’ preference is not the most common reason for switching, rather factors relating to the pharmacy are the most common reasons for switching. The majority of parents stated that their reason for switching devices was because their usual device was not available at their pharmacy or that their usual device had a shorter expiry. This is significant, as, while parents are often required to switch brands due to pharmacy factors, almost 2/3rds of the parents do not receive training in the administration of the new AAI. This is supported by the literature, which explored AAI training practices among pharmacists in the Netherlands, where only half (50%) of pharmacists demonstrated the use of the AAI when asked to fill out a prescription.13 Apart from many not offering training, none of the pharmacists in the study successfully demonstrated the correct use of the AAI.13 There is a need to explore the perspectives, beliefs, and attitudes of pharmacists in Ireland when prescribing adrenaline in order to develop practice guidelines for adequate prescribing of adrenaline in the community.
The high occurrence of switching brands of AAI among caregivers, as well as the suboptimal training in the different brands of AAI, are concerning findings. Research comparing the administration of different AAI devices and the effects of brand switching is sparse, with a recent review finding only three previous such studies.8 One study found that success rates dropped after switching between a one-cap (Epipen® or Jext®) and a two-cap (Anapen®) device without training, (16/45, 36%), compared with participants switched between different
single-cap devices (49/63, 78%; p < 0.0001).14 While the study found that AAI design, as well as adequate AAI training affect successful AAI administration, this study confirms the common occurrence of switching devices without receiving adequate training. There is, therefore, a need to examine the effects of the phenomenon of device switching on AAI administration among caregivers in Ireland.
It was found that most caregivers preferred the Epipen® device (63%). This most likely correlates with Epipen® being the most carried device and the familiarity of Epipen® in the community. When parents who had switched brands in the past were asked why they preferred a particular brand, the main themes that emerged were layout, design, training, and online resources. Based on these findings, a figure representing the ‘ideal’ AAI device was created visually explaining these findings. These factors should also be considered when assessing AAI carriage among parents and young patients, as one recent review found that poor AAI design reduced the likelihood of AAI carriage among adolescents and young adults with food allergies.15 Of note was the high number of parents, which recited the rhyme ‘blue to the sky, orange to the thigh’, highlighting the value of clear and simple colour coding that is easily recalled in an emergency. Although it can be said that Epipen® may be the most preferred AAI due to the high number of parents who carry it, it can be implied from parent responses that this may also be due to having only ever received training in the Epipen® device, or that they have received training in this device most frequently, or even because they have had to use in a real-life scenario. These findings support EACCI recommendation of further study into the benefits of caregiver administration of an adrenaline auto injector during anaphylaxis during a hospital-based oral food challenge [16]. This is supported by a randomised control trial, which found that anaphylaxis at food challenge, followed by self-administration of injected adrenaline, was associated with an increase in health-related quality of life (HRQL) and self-efficacy in young people with peanut allergy.17
Implications for Practitioners
Our study indicates that parents hold training and experience in the use of the AAI under the supervision of a trained healthcare professional in high esteem, regardless of the brand of AAI carried. This study supports the recommendation of EACCI in
the long-term management of anaphylaxis to reinforce the use of the AAI at regular intervals.16 Our study also shows, for the first time, the incidence of switching brands of AAI among caregivers of children with food allergies, and therefore supports the relevance of the EACCI guideline to retrain on the new device when devices are switched.16 Therefore, caregivers should be trained on all available brands of AAI in order to improve self-efficacy and HRQL.
Implications on Future Research
This study highlights the need for the design and validation of educational interventions for caregivers, patients, and healthcare professionals in the use of different brands of adrenaline auto injectors. As device switches have been shown to be a common occurrence, randomized control trials should be conducted to elucidate differences in the successful use of different brands of AAI available in Ireland, as well as the effects of switching brands on administration with or without adequate training. The knowledge and perspectives of pharmacists on training patients and caregivers in different brands of AAI should be explored in more detail. As well as this, knowledge of healthcare professionals in the knowledge of the use of different AAI brands should be assessed in order to determine the readiness of those working in emergency medicine, general practice, allergy, and paediatrics to train caregivers successfully in all AAI brands. Finally, further study should be conducted to assess caregiver-administered adrenaline in a hospital environment on knowledge of anaphylaxis and AAI administration.
Conclusions
This study explored the use of different AAI brands by caregivers, the incidence of switching between AAI brands, and what caregivers preferred about different AAI brands. To our knowledge, this is the first study to document the switching of brands of AAI among caregivers of children with allergies. We have shown that device switching is a common occurrence among caregivers of children with food allergies. Therefore, caregivers should be regularly trained in all available brands of AAI. This study provides the foundation to carry out further study on both the health and economic impact of switching AAI devices, as well as healthcare professionals’ perspectives on the switching of AAI devices.
References available on request
53 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Trinity’s Edward Kennedy Health Policy Award
The Centre for Health Policy and Management is Ireland’s leading academic centre for health systems and policy research and education. Part of its strategy is to provide engagement and dialogue between academics, key health system and policy players, civil society and the public. The Centre hosts events and facilitates national and international dialogue on health system and policy matters as well as ongoing publication of their research findings.
or initiatives that have made meaningful contributions to health system and policy reform, driving positive and sustained changes in healthcare delivery and outcomes. The organisers were very excited to announce that Deputy Róisín Shortall, Social Democrats spokesperson on Health; Finance; & Public Expenditure & Reform is the first recipient of the award.
The Centre for Health Policy and Management hosted the inaugural Edward Kennedy Annual Guest Lecture last night at Trinity College. Deputy Róisín Shortall TD, Social Democrats was presented with the inaugural Edward Kennedy Health Policy Award at the event

to acknowledge her integrity, vision and courage in initiating and advancing the cause of Sláintecare to bring universal health care to Ireland. Deputy Shortall is the party spokesperson on Health, Finance, and Public Expenditure and Reform.

Minister for Health attends Summit
The Centre is part of the Discipline of Public Health and Primary Care in Trinity’s School of Medicine and yesterday welcomed the first cohort of MSc in Health Policy and Management students.
The Edward Kennedy Health Policy Award spotlights the importance of key individuals, organisations
On the award, Professor Steve Thomas, Edward Kennedy Professor of Health Policy and Management at Trinity College said, “Health Policy reform is a highly contested space. It is therefore really important to honour those who have initiated and sustained change in health care for the greater good. Deputy RóisínShortall has shown integrity, vision and courage in initiating and advancing the cause of Sláintecare to bring universal health care to Ireland. We are delighted to present her with the Edward Kennedy Health Policy Award.”
Minister for Health Stephen Donnelly has representing Ireland at a Ukrainian Summit of European Health Ministers.
Ukraine is marking the Summit by announcing an agreement on international medical partnerships to include a proposed pairing, knowledge exchange and cooperation between St James Hospital in Ireland and Kriviy Rig City Hospital, in Ukraine. Health Ministers are also exchanging views at the virtual summit on health workforce challenges as well as country and regional specific needs and how achievements can be scaled up and adapted into best practices in what is the first such meeting organised by Ukraine since Russia’s invasion of the country.
On Ireland’s support to Ukraine, Minister Donnelly said, “Ireland has been unequivocal and steadfast in our support for the people and Government of Ukraine in the face of Russian aggression, and we will continue to stand with Ukraine for as long as it takes.
“We welcome this unique opportunity to share information with international counterparts and to further explore and advance collaboration with Ukraine, as Ireland continues to support the provision of healthcare services in Ukraine and to its displaced citizens.”
Macrosomia on Cardiometabolic Health
Research from Professor Fionnuala McAuliffe and team at the UCD Perinatal Research Centre and SVUH, explores the impact of macrosomia on cardiometabolic health in preteens.
Professor McAuliffe, Professor of Obstetrics and Gynaecology at the UCD School of Medicine and Director of UCD Perinatal Research Centre, says, ‘There is no strong evidence that birthweight over 4kg alone is associated with adverse preteen health.’
Macrosomia (birthweight ≥ 4 kg or ≥ 4.5 kg) is strongly associated with a predisposition to childhood obesity, which in turn is linked with adverse cardiometabolic health. Despite this, there has been a lack of longitudinal investigation
on the impact of high birthweight on cardiometabolic outcomes in youth. The preteen period represents an important window of opportunity to further explore this link, to potentially prevent cardiometabolic profiles worsening during puberty.
With support from Children’s Health Ireland and the National Children’s Research Centre, the team were able to conduct the ROLO longitudinal birth cohort study. The findings show no strong evidence to suggest that macrosomia is associated with adverse preteen cardiometabolic health. Macrosomia alone may not be a long-term cardiometabolic risk factor.
54 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Professor Fionnuala McAuliffe, University College Dublin
Deputy Róisín Shortall TD
ABBREVIATED PRESCRIBING INFORMATION – IRELAND
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Apexxnar®▼ suspension for injection in pre-filled syringe
Pneumococcal polysaccharide conjugate vaccine (20-valent, adsorbed)

Presentation: Each 0.5 mL dose of Apexxnar contains 2.2 micrograms of each of the following polysaccharide serotypes: 1, 3, 4, 5, 6A, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, 33F and 4.4 micrograms of polysaccharide serotype 6B. Each polysaccharide is conjugated to the CRM197 carrier protein and adsorbed on aluminium phosphate. 1 dose (0.5 mL) contains approximately 51 µg CRM197 carrier protein and 0.125 mg aluminium.
Indications: Active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older. Apexxnar should be used in accordance with o cial recommendations taking into consideration the risk of invasive disease and pneumonia in di erent age groups, underlying comorbidities as well as the variability of serotype epidemiology in di erent geographical areas.
Dosage and Administration: For intramuscular injection. Individuals ≥ 18 years of age and older: One single dose. The need for revaccination with a subsequent dose of Apexxnar has not been established. Based on the clinical experience with Prevenar 13, if the use of 23-valent pneumococcal polysaccharide vaccine is considered appropriate, Apexxnar should be given first. Special populations: There are no data with Apexxnar in special populations. Limited experience from clinical studies with Prevenar 13 are available in adults at higher risk of pneumococcal infection either immunocompromised individuals or following bone marrow transplantation. Based on these data the following posology was recommended for Prevenar 13: Individuals at higher risk of pneumococcal infection (e.g., individuals with sickle cell disease or HIV infection), including those previously vaccinated with 1 or more doses of PPSV23, were recommended to receive at least 1 dose of Prevenar 13. In individuals with a hematopoietic stem cell transplant (HSCT), the recommended immunisation series with Prevenar 13 consisted of 4 doses of 0.5 mL each. The primary series consisted of 3 doses, with the first dose given 3 to 6 months after HSCT and with an interval of at least 1 month between doses. A booster dose was recommended 6 months after the third dose.
Contra-indications: Hypersensitivity to the active substances, to any of the excipients, or to diphtheria toxoid.

Warnings and Precautions: Do not administer intravascularly. Appropriate medical treatment and supervision must be available in case of anaphylaxis. Vaccination should be postponed in individuals su ering from acute severe febrile illness. However, the presence of a minor infection, such as a cold, should not result in the deferral of vaccination. It should not be given to individuals with thrombocytopenia or a bleeding disorder that would contraindicate intramuscular injection. The risk of bleeding in patients with coagulation disorders needs to be carefully evaluated before intramuscular administration of any vaccine, and subcutaneous administration should be considered if the potential benefit clearly outweighs the risks. Apexxnar will only protect against Streptococcus pneumoniae serotypes included in the vaccine and will not protect against other microorganisms that cause invasive disease and pneumonia. As with any vaccine, Apexxnar may not protect all individuals receiving the vaccine from pneumococcal disease. Safety and immunogenicity data on Apexxnar are not available for individuals in immunocompromised groups. Vaccination should be considered on an individual basis. Individuals with impaired immune responsiveness, whether due to the use of immuno-suppressive therapy, a genetic
defect, human immunodeficiency virus (HIV) infection, or other causes, may have reduced antibody response to active immunisation. The clinical relevance of this is unknown. In adults across all studied age groups, formal non-inferiority criteria were met although numerically lower geometric mean titres were observed with Apexxnar for most of the serotypes compared to Prevenar 13, however the clinical relevance of this observation for immunocompromised individuals is unknown.
Drug Interactions: Apexxnar may be administered concomitantly with seasonal influenza vaccine (QIV; surface antigen, inactivated, adjuvanted). In subjects with underlying conditions associated with a high risk of developing life-threatening pneumococcal disease, consideration may be given to separating administrations of QIV and Apexxnar (e.g., by approximately 4 weeks). Apexxnar can be administered concomitantly with COVID-19 mRNA vaccine (nucleoside modified).
Fertility, Pregnancy & Lactation: There are no data from the use of Apexxnar in pregnant women. Animal studies do not indicate direct or indirect harmful e ects with respect to reproductive toxicity. Administration of Apexxnar in pregnancy should only be considered when the potential benefits outweigh any potential risks for the mother and foetus. It is unknown whether Apexxnar is excreted in human milk. Side E ects: As Apexxnar contains the same 13 serotype-specific capsular polysaccharide conjugates and the same vaccine excipients as Prevenar 13, the adverse reactions already identified for Prevenar 13 have been adopted for Apexxnar. In clinical trials, the safety profile of Apexxnar was similar to that of Prevenar 13. No new adverse reactions were identified as compared to Prevenar 13. When Apexxnar was administered to adults aged ≥ 65 years together with the third (booster) dose of a COVID-19 mRNA vaccine (nucleoside modified), the tolerability profile generally resembled that of the COVID-19 mRNA vaccine (nucleoside modified) administered alone. Very common (≥ 1/10): Headache, joint pain, muscle pain, vaccination-site pain/tenderness, fatigue. Common (≥ 1/100 to < 1/10): Vaccination-site induration/ swelling, vaccination-site erythema, pyrexia. Uncommon (≥ 1/1,000 to < 1/100): Hypersensitivity reaction including face oedema, dyspnoea, bronchospasm, diarrhoea, nausea, vomiting, rash, angioedema, vaccination-site pruritus, lymphadenopathy, vaccination-site urticaria, chills. The following adverse reactions have been spontaneously reported during the postmarketing use of Prevenar 13, which may also occur with Apexxnar. These events were reported voluntarily from a population of uncertain size, so it is not possible to reliably estimate their frequency or to establish, for all events, a causal relationship to vaccine exposure: Anaphylactic/anaphylactoid reaction, including shock, erythema multiforme, vaccination-site dermatitis. Additional information in special populations in studies with Prevenar 13: Adults with HIV infection have similar frequencies of adverse reactions, except that pyrexia and vomiting were very common and nausea common. Adults with an HSCT have similar frequencies of adverse reactions, except that pyrexia, vomiting, and diarrhoea were very common.
For full prescribing information see the Summary of Product Characteristics.

Legal Category: S1A. Package Quantities: Pack of 1 single-dose pre-filled syringe (with separate needle).

Marketing Authorisation Numbers: EU/1/21/1612/002. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium.
For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500.
Last revised: 12/2022.
Ref: PE 3_0.
chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2017;12:3457-3468.
For more information about APEXXNAR® scan the QR code

PP-PNR-IRL-0027 | Date of Preparation: May 2023
APEXXNAR® is indicated for active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.1
▼
1. APEXXNAR. Summary
2. Froes F, Roche N,
F. Pneumococcal
older.
References
of Product Characteristics.
Blasi
vaccination and
Designed to deliver long-lasting immunity.1,2
Helps protect against the 20 Streptococcus pneumoniae serotypes included in the vaccine.1
Smoking Cessation
Provision of smoking cessation support for patients following a diagnosis of cancer in Ireland
 Written by P. Fitzpatricka b, N. Bhardwajb,
Written by P. Fitzpatricka b, N. Bhardwajb,
M. Masalkhic, A. Lyonsa, K. Frazerd, A. McCannc,e, S. Syeda, V. Niranjanb, C.C. Kellehera f, S. Brennang, P. Kavanaghh, P. Foxd
aDept. of Preventive Medicine and Health Promotion, St Vincent’s University Hospital, D04 T6F4, Elm Park Dublin 4, Ireland
bSchool of Public Health, Physiotherapy and Sports Science, University College Dublin, Belfield Dublin 4, Ireland
cSchool of Medicine, University College Dublin, Belfield Dublin 4, Ireland
dSchool of Nursing, Midwifery and Health Systems, Health Sciences Centre, University College Dublin, Belfield Dublin 4, Ireland
eUCD Conway Institute of Biomolecular and Biomedical Research and UCD School of Medicine, Ireland
fCollege of Health and Agricultural Science (CHAS), University College Dublin, Belfield Dublin 4, Ireland
gSt Luke’s Hospital, Rathgar Dublin 6, Ireland
hHealth Service Executive Tobacco Free Ireland Programme, Strategy and Research, 4th Floor, Jervis House, Jervis Street, Dublin 1, D01 W596, Ireland
Tobacco smoking is not only a cause of many cancers (Gandini et al., 2008); but for those who smoke and develop cancer, it is associated with poorer treatment outcomes (including treatment-related complications and toxicities), increased risk of recurrence (Florou et al., 2014), development of second primaries (Smith et al., 2019), lower survival, and decreased quality of life among continuing smokers (Florou et al., 2014). There is a growing body of evidence that smoking cessation (SC) improves outcomes following diagnosis of a number of cancers (RomaszkoWojtowicz et al., 2018).
Unfortunately, approximately 1 in 5 smokers who have been diagnosed with cancer continue to smoke (Swoboda et al., 2019). Those who continue to smoke are more likely to be younger, less educated, uninsured, of marital status other than married or widowed, and to have survived cervical cancer (Wells et al., 2017). While the benefits of smoking cessation are well-established, and evidence of what constitutes effective stop smoking care is clear, many smokers using health services do not experience quality preventive care in this critical area (Papadakis et al., 2018, Evans et al., 2017). Wells et al. reported that only a small number of patients and families in the United Kingdom (UK) used the available National Health Service (NHS) SC services, with the majority indicating limited discussions related to SC with their oncology
healthcare professionals (HCPs), although they would be agreeable to such discussions (Wells et al., 2017). Barriers to discussions around smoking cessation from the perspective of oncology HCPs include concerns regarding negative impact on the therapeutic relationship and inadequate training (Wells et al., 2017, Warren et al., 2013).
Our previous research in St Vincent’s University Hospital, Ireland, identified a majority of smokers were asked about smoking behaviours; 50% received advice and 51% identified as ‘wanting to quit’. However, 35%
were offered Nicotine Replacement Therapy (NRT) and only 25% provided with an opportunity to speak to a smoking cessation practitioner; in addition, there were few referrals from oncology to smoking cessation services (Fitzpatrick et al., 2014).
Optimal SC services include training of all HCPs, identification of smokers pre or at admission, brief intervention, availability of specialist SC support services and medication, referral to SC support services, provision of one to one advice or group
intervention with follow up to one year (Neubeck, 2006). Ireland has recently published the National Clinical Guidelines for smoking cessation, which recommends that every patient/service user who engages with frontline HCPs, should be asked about their smoking status, the response should be documented, and every smoker should be advised to quit and be offered support at every opportunity (Department of Health, 2022). Hospitals are ideal settings to deliver smoking cessation interventions as admission may trigger a cue to action (Department of Health, 2022). However, patients with a new diagnosis of cancer or undergoing cancer treatment may not fully fit this profile, with high levels of anxiety and worry predominating. Hospital campuses are supportive of quitting because they have been smoke-free since at least 2015 (HSE, n.d.) and
56 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Table 1: Proportion of discharge episodes where current smoking is recorded from hospitals included in the audit (adult patients in specialist cancer hospitals in Ireland 2019).
Hospital Overall proportion of discharge episodes where current smoking is recorded
Patients with cancer only: Proportion of discharge episodes where current smoking is recorded
Discussion
Table 1: Proportion of discharge episodes where current smoking is recorded from hospitals included in the audit (adult patients in specialist cancer hospitals in Ireland 2019).
The results of this audit confirm a suboptimal, non-uniform provision of SC services provided to smokers with cancer. This audit shows that few hospitals ask all patients about smoking and offer SC services, while others asked and offered SC services to only some patients. Some of the SC offered included advice, medication, support and
any hospital visits to a smokefree campus provide important opportunities for HCPs to promote SC (HIQA, 2017).
Although there are many studies looking at ways of managing smoking cessation in patients with cancer there have been no studies to date documenting the SC services provided by specialist cancer hospitals in Ireland for patients with cancer, in addition to very limited published international studies (Frazer et al., 2022). The aim of our study was to document existing SC services for patients with cancer specifically provided by the eight specialist cancer hospitals and one specialist radiotherapy hospital in the context of recently developed National Clinical Guidelines (circulated for consultation 2021, published 2022) (Department of Health, 2022).
Methods
An audit survey was developed using standards outlined in the ‘Stop Smoking National Clinical Guidelines No. 28 Health Services Executive (HSE)’ (Section 3: Recommendations
1-3,) (Department of Health, 2022) and ‘Global Network Self-Audit Questionnaire and Planning Template’, Standard 4 (Global Network for Tobacco Free Healthcare Services, n.d.). The survey sought to document the provision of SC
advice, SC service referral and pharmacotherapy provision. The survey questions were based on the criteria for smoking cessation support provision as set out in the recently published national clinical guidelines for smoking cessation in Ireland (Department of Health, 2022).
In Ireland, all publicly provided adult cancer care is led by eight specialist centres and there is one publicly funded radiation oncology network. All eight specialist cancer centres are part of a tertiary referral university hospital, with the full range of medical and surgical specialties. All patients aged 16 and over diagnosed with cancer in the country are treated at one of these hospitals. No oncology services exist outside these hospitals. The specialist radiotherapy hospital is a standalone centre and is the lead in a network of radiotherapy services nationwide.
The survey was distributed [March-July 2021] to the 9 centres, after obtaining approval from each audit committee [2021]. Qualtrics (Qualtrics, n.d.) was used, and a link was circulated to one key person in each hospital, usually a SC advisor or member of the health promotion staff, to complete the survey, with engagement from other relevant staff as appropriate. The incentive was to contribute to a national study and to receive the study results.
SC information included (i) recording of smoking status, (ii) strategies used to support quit attempts (iii) advice provided on SC supports available within the hospital and in the community; (iv) SC programme available in-hospital (to include any behavioural or pharmacological support; brief intervention, ongoing or intensive SC support, written materials, referral to more intensive specialist supports, and availability of intensive supports onsite). The information gathered was anonymised; MS Excel was used for descriptive analysis.
Results
The response rate was 88.9% with data reported from seven specialist adult cancer hospitals and one specialist radiotherapy centre, all of whom indicated that they had some SC related provision (100%). One hospital could not identify an appropriate person to complete the survey. Another hospital found it difficult to complete the survey in full due to a vacant post, which resulted in the lack of provision of an active in-hospital SC service.
More than half (5) of the hospitals reported that all (3) or some (2) patients overall were asked about their smoking and provided with SC advice. Most hospitals (7) reported some level of SC support or service provision, to all (3) or to most (4) patients. While the data on SC services for overall patients
was relatively complete and many hospitals were doing well against the guidelines, this was not the case for patients with cancer, with limited detailed data available by diagnosis and anecdotal evidence of limited referrals for cancer patients.
In seven hospitals, the service was available for general patients in all (3) or some (4) areas: Inpatient (admission, during hospitalisation, at discharge), outpatients, other visits (attending radiology department, emergency department or other). Some form of SC service or support was provided by all of the following professionals (medical staff, nursing staff, hospital SC staff, community SC staff (for patients referred on discharge), other allied healthcare professionals, other) in four hospitals, while in three it was provided by some of these personnel. When asked if all patients are routinely asked about smoking, three said yes, one said yes except oncology patients, one said yes except outpatients, one said no, one was unsure and two gave no response. There was a lack of information provision across hospitals on the harms of e-cig/vaping use, although use in hospitals is banned.
Stop smoking medications were provided to cancer inpatients in two hospitals, and to cancer patients at outpatients and for patients attending day ward
57 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
collated on a regular basis by the Hospital Inpatient Enquiry (HIPE), part of the Health Service Executive in Ireland.
1 4.7% 7.6% 2 4.7% 2.8% 3 4.9% 1.9% 4 5.7% 3.0% 5 7.3% 8.3% 6 3.5% 3.4% 7 5.5% 5.6% 8 5.4% 5.4% 9 13.4% 13.5%
Smoking Cessation
services in one hospital. Smokers with cancer were referred automatically to the SC service in two hospitals at diagnosis. This corresponded with commencing chemotherapy treatment and in the second hospital commencement of radiotherapy. While stop smoking medications were available 24 hours a day in five hospitals, most hospitals did not stock all three stop smoking medications (NRT, Bupropion, Varenicline). One hospital advised they had data on uptake of SC services for smokers with cancer, but they were unable to identify the percentage uptake. Overall, five hospitals had staff who completed Making Every Contact Count (MECC) training programme provided by the Irish Health Service Executive for all HCPs to complete (Health Service Executive, n.d.).
Table 1 shows the proportion of discharge episodes where current smoking is recorded from hospitals included in the study, both overall patients and among patients with cancer only, as collated on a regular basis by the Hospital Inpatient Enquiry (HIPE), part of the Health Service Executive in Ireland.
Discussion
The results of this audit confirm a suboptimal, non-uniform provision of SC services provided to smokers with cancer. This audit shows that few hospitals ask all patients about smoking and offer SC services, while others asked and offered SC services to only some patients. Some of the SC offered included advice, medication, support and access to an intensive service. One hospital reported recording all relevant smoking data and 55.5% (5/9) of hospitals had data on the uptake of specialist SC services for all patients.
The new clinical guidance recommends that varenicline, alone or in combination with NRT, is offered as a first-line treatment, and if for some reason varenicline is contraindicated, combination NRT should be offered. NRT monotherapy, or bupropion (alone or in combination with NRT) or nortriptyline can also be recommended as a second-line therapy. Our audit did not seek information on nortriptyline as it was not at that time a recommended therapy. Just two hospitals audited offered the provision of all other recommended stop smoking medications while half of hospitals advised that bupropion was not available, and none reported that
stop smoking medications were routinely provided to all patients. It is clear that hospitals who participated in this audit are not fully compliant with the National Clinical Guideline for SC. This may be somewhat expected as the audit was completed just before the national clinical guideline was published, however the value of smoking cessation to patients with cancer is well known.
This study is part of a wider programme of research looking at the feasibility of developing a SC pathway which includes qualitative interviews with patients and oncology HCPs. In the majority of oncology centres included in this report, the oncology service line was embedded within a larger health care system; as part of our audit we looked at the smoking cessation service across the whole hospital, but as there is specific concern about oncology patients the service for these patients was specifically reviewed.
The new national clinical guidance (Department of Health, 2022) has three clear recommendations that directly relate to the general adult population. Firstly, all HCPs should ask about an individual’s smoking behaviour. Secondly, all HCPs should advise all smokers about the harms of smoking for themselves and others and the benefit of quitting, and they should advise that help can be provided or arranged to support a quit attempt. Where a patient is interested in quitting, treatment needs, and preferences should be discussed. HCPs should advise that making an unsupported quit attempt is less effective than using recommended supports, and treatment should be provided or arranged. Thirdly, for people who are currently interested in quitting, all HCPs should recommend that behavioural support, either alone or in combination with pharmacological supports, increases the chances of successful quitting. Smoking status and all discussions, interventions and outcomes should be documented.
There is considerable variation in the smoking rates across hospitals. This reflects three current challenges in accuracy of smoking rates in hospitals in Ireland; the under ascertainment of smoking rates for all patients, which is a feature both nationally (Fitzpatrick et al., 2022) and internationally (Hirvonen et al., 2021); the variation in the prevalence of smoking in the catchment areas of the hospitals,
with smoking rates higher in more deprived areas (Department of Health. Healthy Ireland Survey, 2021); and the fact that the data in Table 2 comes from a hospital discharge data system and each episode is counted, hence those hospitals with radiotherapy may appear to have higher rates due to repeated episodes.
An audit of websites of 62 National Cancer Institute (NCI) designated cancer centres (DCCs) was performed to identify institutions with online evidence of a systemwide tobacco treatment programmes (TTPs) servicing cancer patients (Day et al., 2019). The audit identified 47 NCI-DCCs TTPs. Seventeen TTPs were housed within the cancer centre and 30 TTPs were offered by the primary affiliated institution; among the latter group, only 13 TTPs were identifiable via the NCI-DCC webpage. Just three reported systemwide TTP outcomes. Despite supportive policies from cancer organisations, treatment for tobacco dependence is still not part of standard cancer care (Morgan et al., 2011), and only 7% of cancer clinical trials assess smoking status (Gregorio et al., n.d.). Barriers to the provision of tobacco dependence treatment in the cancer context are common across health care settings (e.g., lack of physician time, concerns around appearing judgmental or eliciting feelings of guilt among patients). This is a particular challenge for people who smoke and develop cancer, for whom a combination of individual and system factors mean that their smoking cessation needs are often not consistently and fully met during cancer care (Papadakis et al., 2018, McPhee and Detmer, 1993).
McPhee et al noted physician forgetfulness, patient refusal, and practice logistical difficulties are among the major reasons that physicians perform cancer prevention activities less frequently than recommended by established guidelines in the United States (US) (McPhee and Detmer, 1993). They found that office systems are effective strategies in promoting cancer prevention activities including in-reach medical record checklists and flow sheets, stickers and alerts, audit with feedback, nurse-initiated reminders, and computer-generated reminders and outreach reminder postcards, letters, telephone calls, and questionnaires. The authors review the literature supporting the efficacy of such office systems.
This audit is the first of its kind published in Ireland whereas the UK has previously audited the provision of hospital-based SC services. (Proctor et al., 2013) Proctor et al, similar to the current audit, found that there was considerable variation in how SC services were staffed and run (Proctor et al., 2013). All of the inpatient wards were able to provide NRT and just over half offered varenicline and a third bupropion. These findings were supported by a British Thoracic Society (BTS) audit of UK hospital SC services in 2019 against National Institute for Health and Care Excellence and BTS standards, noting adherence to these national standards was low (Mangera and Devani, 2020). Neither of these UK studies reported electronic cigarettes information provision, while our current audit showed there was a lack of information provision across hospitals on the harms of e-cig/vaping use.
What are the solutions to the suboptimal smoking cessation services for patients with cancer?
Warren et al in a study of US clinicians working in oncology found that the key barriers to providing cessation support were a lack of clinician education or experience and lack of available resources to refer patients for smoking cessation support (Warren et al., 2015). Ensuring a comprehensive smoking cessation service in each specialist cancer hospital is essential. Embedding of a liaison smoking cessation expert within the oncology service and ensuring all oncology staff take the MECC training which incorporates brief intervention training (Health Service Executive, n.d.) would improve the service without considerable resource implications.
This audit was undertaken in response to the clinical guidelines for smoking cessation published. This work is part of a wider programme of research in the area of smoking cessation for cancer patients. In this further research we have interviewed both patients and health care professionals working in oncology about their own experiences of smoking cessation for patients with cancer.
We have shown considerable variation in the SC information and services provided to cancer patients across the adult cancer specialist centres in Ireland. Audits such as this are essential to demonstrate gaps in service and provide a baseline for improvement.
58 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Palliative Care Study Findings Released
Researchers at Trinity's School of Medicine have investigated how patient and informal caregiver reciprocal support in specialist palliative care impacts on patient and caregiver decision making for care. Their findings indicate that both obligation to each other and feeling constrained by one another can limit patient and caregiver open disclosure in decision making. The study is published in the Journal of Pain and Symptom Management
The team’s previous research informed them that patient and caregiver can reciprocate in supporting one another. This current research now gives fresh insight into the area of decisionmaking in palliative care that had not previously been understood, and ties in with national palliative care week: September 10th to 16th 2023.
Palliative care is an interdisciplinary care approach focused on alleviating symptom distress for people living with life-limiting progressive conditions, and their families. Patients with advanced illness not only depend on healthcare professional support; they also rely heavily on family to take on caregiving roles. Family caregivers are often involved in the decision-making process for treatment and care.
Understanding how supportive behaviours between patients and informal (usually family) caregivers impacts on treatment decisionmaking is necessary to facilitate effective communication between patients and their caregivers, and in turn, optimise care outcomes for both the patient and caregiver.
Researchers now believe the impact of mutual obligation on patient and caregiver decisionmaking needs to be considered when designing interventions for patient care in palliative care settings.
The study involved in-depth qualitative interviews on the topic with patients with advanced illness and their caregivers. All participants were recruited from a large regional specialist palliative care service in Ireland.
Key findings
• Although the patient and caregiver in palliative care can be mutually supportive, how they support one another can have negative connotations for both the patient and caregiver when faced with decisions about care.

• Mutual support between the patient and caregiver was underpinned by mutual obligation.
• For caregivers, obligation to the patient was explained by having little choice but to assume a caregiving role and feeling constrained in a caregiving role.
• For patients, obligation to their caregiver centred on feeling obliged to accept assistance from their caregiver and feeling constrained in a care recipient role.
• Caregivers who felt obliged to care reported difficulty in discussing the patient’s preferences for care with the patient as well as their own wishes for patient care.
Dr Geraldine Foley, Assistant Professor in Occupational Therapy at Trinity
• Some caregivers struggled to engage in conversations with the patient about treatment options.
• Caregivers who felt most obliged to care reported the most distress in their role, feeling burdened and under strain.
• Caregivers who felt supported by the patient, by their own choice and obligation, were more likely to be invited into the decisionmaking process for care.
• Where the caregiver did not feel forced into caregiving and the patient did not feel inhibited by their reliance on the caregiver, open communication about the needs and preferences for care were far more likely.
Dr Geraldine Foley, Assistant Professor in Occupational Therapy at Trinity, primary investigator and corresponding author of the study, said, “The findings from our study pinpoint key dimensions to the patient and caregiver relationship for decision-making in palliative care that we had not fully appreciated prior to conducting the study. We found that caregivers who felt obliged to care had difficulty communicating with the patient about the patient’s preferences, and that patients who felt obliged to accept assistance tended to minimise caregiver involvement in decision making. However, caregivers who assumed
caregiving on their own accord and patients who did not feel constrained in care recipient role, were much more open in their communication with each another and trusting of one another.”
In considering the potential clinical impact of their findings, Dr Foley added, “These findings highlight the importance for healthcare professionals to help the caregiver cope with loss of control and choice in a caregiving role. This includes increasing the level of support available to caregivers but also helping caregivers process and deal with loss of control and choice in a caregiver role.
“Supporting the patient should involve as appropriate open communication between the patient and caregiver because open communication between the patient and caregiver appears to have a positive outcome for the patient as well as the caregiver.”
You can read the full article at the following link: https://www. jpsmjournal.com/article/S08853924(23)00623-1/fulltext
The study was conducted in collaboration with Professor Karen Ryan, Consultant in Palliative Medicine and Clinical Professor at University College Dublin and St. Francis Hospice Dublin, and Dr Regina McQuillan, Consultant in Palliative Medicine at St. Francis Hospice Dublin and Beaumont Hospital Dublin.
The study was supported by a Trinity College Dublin Provost’s Project Award. PhD scholar funded by this award and co-author of the paper is Ms Rachel McCauley, Discipline of Occupational Therapy, School of Medicine, Trinity College Dublin.

59 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
News
An Overview of Sezary Syndrome Sezary Syndrome
presentation with generalised itch and erythroderma may be nonspecific. In up to one-third of skin biopsies later confirmed as SS, the histologic picture may be nonspecific, making diagnosis difficult and consequently delayed.
The exact cause of Sezary syndrome is unknown, and an inheritance pattern has not been determined. The condition occurs in people with no family history of the disorder and is not thought to be inherited in most cases.
Pathogenesis
Pruritus is intense and even high doses of antihistamines cannot provide relief. In more advanced cases there may be alopecia, ectropion, onychodystrophy and palmar/plantar hyperkeratosis.
The cancer can spread to the lungs, liver, spleen, and bone marrow. People with Sezary syndrome are at a higher risk of developing other types of lymphoma or cancers. The disease can also lower the function of the immune system, increasing the risk of infections.
Diagnosis
Sezary syndrome (SS) is a rare and aggressive form of cutaneous T-cell lymphoma (CTCL), characterised by the triad of erythroderma, lymphadenopathy and presence of circulating neoplastic T-cells in the peripheral blood.
We spoke to Theresa Lowry Lehnen, PhD Clinical Nurse Specialist and Associate Lecturer South East Technological University to find out more about this rare disease.

Theresa explains, “Primary cutaneous lymphomas (PCL) are localised to the skin, without extracutaneous involvement at the time of initial diagnosis and are a subset of non-Hodgkin lymphoma (NHL). PCLs can originate from T or B lymphocytes and are called cutaneous T-cell lymphomas or cutaneous B-cell lymphomas.
“Cutaneous T-cell lymphoma is further categorised into two types: an indolent form that includes mycosis fungoides (MF), lymphomatoid papulosis, and anaplastic large T-cell primary cutaneous lymphoma; and an aggressive form that includes Sezary syndrome.
“Mycosis fungoides and Sezary syndrome are the most common forms of cutaneous T-cell lymphoma. Mycosis fungoides originates in the peripheral epidermotropic T-cells, specifically the memory T-cells
(CD45RO+), which express the T-cell receptor (TCR) and CD4+ immunophenotype. Mycosis fungoides has an incidence of around 6 cases per million per year in Europe and the United States, accounting for approximately 4% of all nonHodgkin lymphoma cases.”
Theresa adds that while there is evidence that Sezary syndrome is a distinct disorder from mycosis fungoides beyond merely leukemic presentation, there are patients with MF who develop significant Sezary syndrome at the time of progression. “It is hypothesised that Sezary syndrome can evolve gradually from mycosis fungoides or occur spontaneously,” she notes.
Sezary syndrome is a rare disease, with an annual incidence rate of 1/10,000,000, accounting for approximately 3% of all cases of CTCL. It typically presents in adults over 60 years of age, with a male predominance. Initially the
Theresa adds, “The exact pathogenesis of Sezary syndrome is not fully understood and involves complex interactions between neoplastic T-cells, immune dysregulation, and the microenvironment. Several molecular and cellular mechanisms contribute to the development and progression of the disease.
“The malignant T-cells in SS are typically CD4+ memory T-cells with a Th2 cytokine profile. Aberrant signalling pathways, including STAT3, NF-κB, and JAK/ STAT, play an important role in the survival and proliferation of neoplastic T-cells. The immune dysregulation in SS involves a complex interplay between neoplastic T-cells and the host immune system. The malignant T-cells evade immune surveillance and suppress the antitumor immune response through various mechanisms, including impaired antigen presentation, cytokine secretion, and recruitment of immunosuppressive cells.”
Sezary syndrome incurs significant morbidity and hallmark clinical features include generalized erythroderma, intractable pruritus, lymphadenopathy, and palmoplantar keratoderma.
Theresa told us, “Accurate and timely diagnosis of Sezary syndrome is crucial for appropriate treatment planning and improved patient outcomes. Early identification allows for the initiation of targeted therapies and the avoidance of unnecessary interventions. The condition can be a diagnostic challenge to clinicians as it can mimic benign skin disorders.
“General practitioners rarely see CTCL, and diagnostic delay can occur through misdiagnosis and because CTCL can initially respond to topical corticosteroids like more-common skin conditions. It is usually only on progression of the illness that a dermatology referral occurs, and some general dermatologists and histopathologists may also have little CTCL experience.”
She adds that a multidisciplinary team is needed for complete diagnosis and management, comprising a CTCL-experienced dermatologist, a dermato-and haemato-pathologist experienced in skin and lymphoma, respectively, and an oncologist experienced in delivering CTCLtailored treatments.

60 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
An interview with Theresa Lowry Lehnen, RGN, RNP, PhD, Clinical Nurse Specialist and Associate Lecturer with South East Technological University
Cutaneous features of Sezary Syndrome
“The need for specialist nurses to support patients and liaise with nursing care closer to home is important. The diagnosis of Sezary syndrome requires a comprehensive evaluation involving various diagnostic modalities. SS should be differentiated from mycosis fungoides, psoriasis, pityriasis rubra pilaris, dermatitis, hypereosinophilic syndrome, and adult T-cell leukaemia. Primary skin disorders like scabies, and adverse drug reactions are also considered in the differential.”
A detailed patient medical history and physical examination is carried out. Tests include a full blood count with differential, Sezary blood cell count, HIV test and skin biopsy. More than one skin biopsy may be needed. Other tests that may be carried out include:
• Immunophenotyping using antibodies to identify cancer cells based on the types of antigens or markers on the surface of the cells. Immunophenotyping is used to help diagnose specific types of lymphoma.
• Flow cytometry, a laboratory test that measures the number of cells in a sample, the percentage of live cells in a sample, and certain characteristics of the cells, such as size, shape, and the presence of tumour or other markers on the cell surface. The cells from a sample of a patient’s blood, bone marrow, or other tissue are stained with a fluorescent dye, placed in a fluid, and then passed one at a time through a beam of light. Test results are based on how the cells that were stained with the fluorescent dye react to the beam of light. Flow cytometry is used to help diagnose and manage certain types of cancers, such as leukaemia and lymphoma.
• T-cell receptor (TCR) gene rearrangement test, in which cells in a sample of blood or bone marrow are checked to see if there are certain changes in the genes that make receptors on T cells. Testing for these gene changes can identify whether large numbers of T cells with a certain T-cell receptor are being made.
“After mycosis fungoides or Sezary syndrome has been diagnosed, further tests are necessary to determine if cancer cells have spread from the skin to other parts of the body,” Theresa adds. “Tests include, chest x-ray, CT scan, PET scan, bone marrow aspiration and biopsy, and lymph node biopsy. Excisional lymph node biopsy is preferred, and it can show reactive changes or dermatopathic changes or features suggestive of lymphoma. Secondary skin infections are common because of frequent scratching and compromised skin. Starting antibiotics and taking cultures to rule out methicillin-resistant Staphylococcus aureus may be indicated at diagnosis.”
Staging and Prognosis
The prognosis of patients with mycosis fungoides and Sezary syndrome is based on the TNMB staging system, which considers the extent of skin, lymph node and visceral organ involvement, and blood tumour burden. “The presence of lymphadenopathy and involvement of peripheral blood and viscera increase in likelihood with worsening cutaneous involvement and define poor prognostic groups,” she notes.
Stage I Mycosis Fungoides
Stage I is divided into stages IA and IB:
• Stage IA: Patches, papules and/ or plaques cover less than 10% of the skin surface.
• Stage IB: Patches, papules, and/or plaques cover 10% or more of the skin surface. There may be a low number of Sezary cells in the blood.
Stage II Mycosis Fungoides
Stage II is divided into stages IIA and IIB:
• Stage IIA: Patches, papules and/ or plaques cover any amount of skin surface. Lymph nodes are abnormal, but they are not cancerous.
• Stage IIB: One or more tumours that are 1 centimetre or larger are found on the skin.
Lymph nodes may be abnormal, but they are not cancerous. There may be a low number of Sezary cells in the blood.
Stage III Mycosis Fungoides
In stage III, 80% or more of the skin surface is reddened and may have patches, papules plaques, or tumours. Lymph nodes may be abnormal, but they are not cancerous. There may be a low number of Sezary cells in the blood.
Stage IV Mycosis Fungoides/ Sezary Syndrome
When there is a high number of Sezary cells in the blood, the disease is called Sezary syndrome. Stage IV is divided into stages IVA1, IVA2, and IVB depending on the presence of nodal and visceral involvement.
• Stage IVA1: Patches, papules, plaques, or tumours may cover any amount of the skin surface, and 80% or more of the skin surface may be reddened. The lymph nodes may be abnormal, but they are not cancerous. There is a high number of Sezary cells in the blood.
• Stage IVA2: Patches, papules, plaques, or tumours may cover any amount of the skin surface,
and 80% or more of the skin surface may be reddened. The lymph nodes are very abnormal, or cancer has formed in the lymph nodes. There may be a high number of Sezary cells in the blood.
• Stage IVB: Cancer has spread to other organs in the body, such as the spleen or liver. Patches, papules, plaques, or tumours may cover any amount of the skin surface, and 80% or more of the skin surface may be reddened. The lymph nodes may be abnormal or cancerous. There may be a high number of Sezary cells in the blood.
“For people with early-stage MF, the life span may be normal. Patients with stage IA disease have a median survival of 20 years or more.”
She adds, “Prognosis for Sezary syndrome is generally poor, with a median survival of 2 to 4 years, however, survival has improved with newer treatments. Prognosis can vary depending on age, disease stage, and response to therapy.”
Treatment Strategies
The management of Sezary syndrome requires a multimodal approach, including skin-directed therapies, systemic therapies, and supportive care measures. Skindirected therapies include topical corticosteroids, phototherapy, and electron beam radiation.
Theresa says, “Systemic treatments include interferonalpha, retinoids, histone deacetylase inhibitors, and novel immunotherapies targeting immune checkpoints, such as PD-1 inhibitors. 7 Besides diseasedirected treatment options, pruritis is a major concern in patients with Sezary syndrome.
“Various local and/or systemic options can be used to try and control the pruritis. Patients
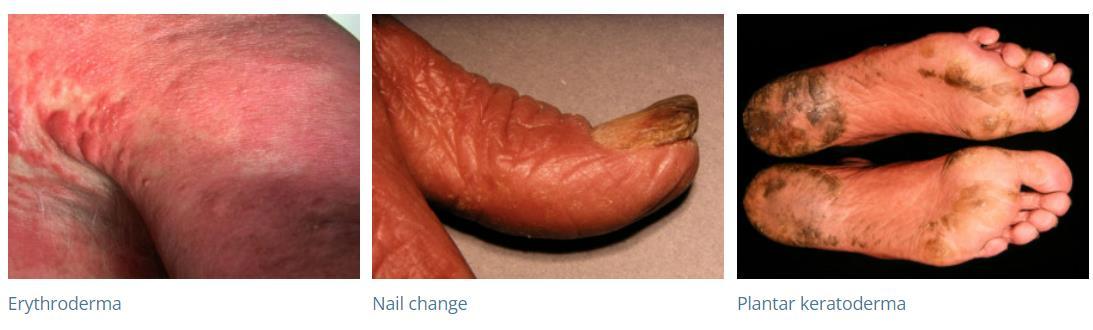
61 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Erythroderma
Nail change
Plantar keratoderma
Sezary Syndrome
with Sezary syndrome are very susceptible to infections from poorly intact skin, colonization, indwelling catheters, and immunosuppression from therapy. Good skin care and avoidance of indwelling catheters are important for minimising these risks.
“Given the leukemic involvement in Sezary syndrome, the treatment is generally systemic. Specific treatment for individual patients is based on a variety of factors, including the patient’s general health and stage of disease.”
Treatments for SS include:
• Phototherapy: PUVA (ultraviolet-A light is directed onto the skin and the patient is given the drug psoralen); UVB (skin directed ultraviolet-B light); NBUVB (skin directed narrow band ultraviolet-B light).
• Biologic or immunotherapy therapy used to stimulate a patient’s own immune system to fight the cancer.
• Retinoids, to slow certain types of cancer cells. Examples are Interferon alpha and Bexarotene.
• Extracorporeal photopheresis (ECP), a procedure used to expose the blood to ultraviolet light.
• Radiation therapy, using high energy X-rays or other types of radiation to kill cancer cells or prevent them from growing.
• Advanced disease treatment can involve chemotherapy, given either orally or through an intravenous infusion to stop the growth of rapidly dividing cancer cells. While the response may be quick, it can be short-lived and require maintenance therapy.
• Haematopoietic stem cell transplant.
Theresa concludes. “Sezary syndrome is generally an incurable condition, and the primary aims of
treatment are symptom control and remission induction. While CTCL is currently deemed incurable, remission has been achieved for some following allogeneic stem cell transplant (ASCT). With new potential therapeutic options on the horizon, it is hoped that these agents will bring improved outcomes and a better quality of life for patients.
“Since the publication of the first WHO-EORTC classification in 2005, much progress has been made, and the 2018 update continues to be a useful guide for clinicians involved in the care of patients with cutaneous lymphomas. Genome-wide genetic studies have contributed to a better understanding of the molecular pathways involved in the pathogenesis of the different types of cutaneous lymphomas and resulted in the recognition of additional diagnostic and prognostic criteria and new potential therapeutic targets
National Shared Care Record
eHealth Standards and Shared Care Records has commenced procurement for a HSE National Shared Care Record. NSCR will aggregate existing digital health information from various sources and present it in a secure and structured way to clinicians, patients, and carers.

It will play a key role in achieving the Sláintecare vision of patient-centred, integrated care by joining up fragmented and siloed digital records.
Initial population of the National Shared Care Record (NSCR) will be from existing digital data available in HSE National Systems and will be aligned to HIQA, EU and International Patient Summary models. Over time the NSCR will be augmented from other data sources, such as GP records once the Health Information Bill is enacted, and expanded with additional categories and details of data beyond the patient summary information, such as lab and screening results and key clinical documents, enabling improved clinical decision-making and selfmanagement of healthcare.
One of the main challenges for the NSCR will be to extract,
transform, and structure data from underlying source systems for display to clinicians and patients. Currently the approach to address data challenges is fragmented as several eHealth work streams are running in parallel involving data quality, record linkage, data standardisation, clinical terminologies, interoperability standards, reference data and data structure modelling.
To reduce risk and deepen understanding of these challenges, as well as to develop further insights into potential tooling and standards, the NSCR Project Team embarked on an 8-week collaborative “Build to Learn” (B2L) project with IIS and A2IHIDs based on open standards using a collection of open-source components within existing controlled secure environments. The exercise was centred on deriving as much insight (to Learn) as possible to advantageously position the NSCR in preparation for a national deployment.
The NSCR B2L project resulted in the successful ingestion of almost 50,000 HealthLink Lab results (no associated demographic information was persisted or surfaced) into a Clinical Data
Repository(CDR) and surfacing them for viewing. The project achieved the following:
• CDR and basic Clinical Viewer deployed and configured.
• HSE data mapped to open standard.
• HealthLink data ingested into CDR.

• Development of technical components to measure content quality and thresholds.
From a clinical knowledge management perspective, the B2L team increased insights into creation of OpenEHR Archetypes (Lab Result) and modelling / development of Templates (specification that defines a tree of one or more Archetypes). Using open-source tooling for both the Clinical Data Repository and Viewer components provided useful information in terms of understanding the installation and configuration process.
Across the end-to-end B2L solution, significant insights have been gained in relation to utilisation of event-based architecture, provision of environments, deployment, and configuration of both a Clinical

“Although genetic markers are becoming increasingly important, integration of histologic, immunophenotypic, genetic, and cutaneouslymphomas clinical data remain essential for an accurate diagnosis. In recent years, a multidisciplinary approach with collaboration among pathologists, dermatologists, haematologists, and radiation oncologists has been crucial for defining new entities and classifications and is the best guarantee for further progress in the diagnosis, treatment, and management of patients with a cutaneous lymphoma.
“More information, support, and the establishment of national and international patient CTCL advice and support groups is necessary for patients with Sezary syndrome and CTCL, who can feel isolated, especially when most people have never even heard of their disease.”
Data Repository and a Clinical Viewer, as well as a deepening understanding of development of Archetypes with supporting mapping activity.
One of the positive aspects of the B2L project was the high level of collaborative working across the eHealth teams involved in the initiative. The intent was not to build a ‘shared record’ but to use the components in an active crossportfolio learning process to surface issues pertinent to the rollout of the NSCR. The insights gained from the B2L project are an important input to inform the Competitive Dialogue of the NCSR procurement currently underway.
If you would like to learn more about the National Shared Care Record or the NCSR B2L project, please contact eHealthStandards. SCRs@hse.ie.
62 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
News



Heart Failure
Challenges in heart failure care in four European countries: a comparative study
Heart failure (HF) is one of the most common cardiovascular diseases worldwide, associated with a high morbidity and mortality.1 In Europe, more than 15 million people live with HF.2 It is expected that the number of HF-patients will increase in the coming years.3 Due to demographic developments, a higher life expectancy, an increasing survival rate of patients with acute cardiac diseases, and advances in HF-diagnostics (resulting in more frequent and earlier detection), the prevalence of HF is increasing, whilst mortality is decreasing due to better therapeutic options.4,5 Diagnosis and treatment of HF are complex and time-consuming, causing high direct and indirect costs, e.g. hospitalizations, medications and loss of labour.6,7 As a chronic disease, HF considerably impairs quality of life. Additionally, HF is often accompanied by comorbidities, such as diabetes.8 Subsequently, HF imposes enormous social, organizational and economic
burden on patients, their informal caregivers and on healthcare systems.9,10 Different countryspecific structures for HF-care have been established in Europe to address these challenges. The report aims to provide an overview of HF-care provision across Germany (DE), Ireland (IRL), the Netherlands (NL) and the UK. The aims of this study are to
(1) map the characteristics of the respective healthcare systems,
(2) compare the approaches and individual challenges of HF-care and
(3) describe the status quo of eHealth and telemedicine implementation.
Methods
A mixed methods approach was implemented that included a literature analysis and interviews with HF-patients, informal caregivers and interdisciplinary experts.
Literature analysis
The literature search took place from January to December 2021 by three researchers (A.N.,
B.S. and Y.P.) with different expertise. To obtain an overview of healthcare systems and map HF-care processes across the countries, information was identified through an open online search of scientific publications, up-to-date electronic newspaper and magazine articles, and (government) reports. Initial searches in PubMed and Google Scholar were supplemented using a snowballing technique. Search terms are composed of the population (patients with HF), the respective country, and the key topics of this report (e.g. diagnosis and management, burden and digital health). Using the information retrieved, search terms were subsequently further refined. In addition to existing reports on individual aspects of HF-care, clinical guidelines were consulted, to identify advised healthcare processes and challenges of HF-care.
Interview study
Data obtained from an qualitative study originally conducted to elicit requirements and needs of
HF-patients and their informal caregivers within the EUfunded project ‘PASSION-HF’ (PAtientSelf-care uSIng eHealth in chrONic Heart Failure; Interreg NWE 702) complemented the analysis.11 Between March and June 2019, a total of 49 HF-patients and 33 informal caregivers were interviewed using a semi-structured design.11 For recruitment, a maximum variation approach was applied with age, gender, social background and disease severity as key determinants. Ten HF-patients were from DE, 9 from IRL, 18 from NL and 12 from Northern Ireland.11 Most were male (76%) aged between 60 and 69 years (43%). Patients and informal caregivers also received questionnaires focusing on healthcare delivery, disease management and attitudes towards technology.11 This information was used to integrate insights into the care process and associated challenges from the perspective of patients and their families.
64 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Written by Bianca Steiner, Anne Neumann, Yannick Pelz, Chantal F Ski, Loreena Hill, David R Thompson, Donna Fitzsimons, Lana J Dixon, Julia Brandts, Marlo Verket, Katharina Schütt, Casper G M J Eurlings, Josiane J J Boyne, Arno J Gingele, Lieven De Maesschalck, Marguerite Murphy, Ermelinda Furtado da Luz, Matthew Barrett, Karen Windle, Thom Hoedemakers, Thomas M Helms, Hans-Peter Brunner-La Rocca, Bettina Zippel-Schultz on behalf of the PASSION-HF Consortium
1 First contact person for patients in case of problems with heart (multiple answers possible)
Eur J Public Health, Volume 33, Issue 3, June 2023, Pages 448–454, https://doi.org/10.1093/eurpub/ckad059
The content of this slide may be subject to copyright: please see the slide notes for details.
possible)
Results
Characteristics of healthcare systems
Expert interviews

Complementary semi-structured interviews were conducted with interdisciplinary experts (DEn = 2, IRL n = 3, NL n = 2, UK n = 2) between December 2021 and January 2022 to clarify and validate country-specific aspects from the literature search. Experts included cardiologists, HF-nurses, health service researchers and medical informatics specialists. Four researchers (A.N., B.S., B.Z. and Y.P.) conducted the interviews. Transcriptions and analyses were presented to the experts in their respective countries for validation.
Healthcare systems across the four countries differ in their insurance systems, e.g. financial structures and organization of service provision. While DE has a social insurance system, IRL and UK have a national health service, and NL has a social insurance system with capitation payments.13
based guidelines aiming to improve cross-sector treatment and quality of care.16 Building on this regulatory framework, insurance companies are allowed to design and offer care programs to patients. However, since the underlying requirements do not reflect medical care reality, the German DMP-HF requires comprehensive revisions and has not yet entered the care process.17
Models of HF-care
The models of HF-care in all countries are principally based on the European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic HF.14 Nevertheless, these models differ in their design and implementation in practice.

Data synthesis
Qualitative and quantitative data were collected and analyzed in a complementary manner. Following a results-based convergent synthesis design, data were first analyzed and presented separately.12 Subsequently, all information collected on the healthcare systems, care models and digital infrastructures was narratively aggregated in individual country reports, which served as the basis for the comparative analysis. Expert interviews were included sequentially.
Since 2009 evidence-based recommendations have been available in DE with the ‘Nationale Versorgungsleitlinie Chronische Herzinsuffizienz’, relating to outpatient care as well as to aspects of inpatient care.15 This focuses on the optimization of therapy to avoid decompensation and hospital admissions and improve coordination of all those involved. In 2018, legal requirements for a Disease Management Program for HF-patients (DMP-HF) were set. They are based on evidence-
In IRL diagnosis and treatment of HF-patients are organized through the national program ‘Heart Failure Model of Care’.18 This model aims to organize most aspects of care from prevention to end-of-life care by defining pathways, clinical guidelines and decision support regarding in- and outpatient care. The model also describes the interaction and responsibilities of specific healthcare professionals within the program. The focus is on minimizing hospitalizations by strengthening collaboration between acute care hospitals, general practitioners (GPs) and community care in the sense of an integrated care approach. However, due to lacking investment, especially in primary care, the model has only been implemented in pilot initiatives and is largely limited to a few large urban centres.19
Health, Volume 33, Issue 3, June 2023, Pages 448–454, https://doi.org/10.1093/eurpub/ckad059 slide may be subject to copyright: please see the slide notes for details.
updated in 2021, and adapted to the Dutch situation to avoid discrepancies between national and regional guidelines mainly used by GPs and international guidelines mainly used by hospital specialist.20,21 To ensure systematic and coordinated treatments, DMPs for HF have been implemented on a national level.22 These cover care in hospitals, outpatient clinics or at home.23 24 More recently, the Connect programme aims at implementing agreements across primary, secondary and tertiary care at regional levels to improve HF-care.25
In the UK, the National Institute for Health and Care Excellence (NICE) has developed clinical guidelines for the diagnosis and management of HF-patients as quality standards to supplement the ESC guidelines.26 NICE guidelines call for interprofessional teamwork—multidisciplinary teams (MDT)—that specialize in HF and work closely with primary care teams. While MDT focus on specialized treatment of HF, the primary care team is responsible for routine patient management, follow-up of HF and ensuring effective communication links between care providers and clinical services involved.
The role of HF-nurses
In the NL, ESC guidelines were integrated into the national guidelines for GPs in 2010,
In IRL, NL and UK, HF-nurses are usually the first point of contact
65 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Figure 1 First contact person for patients in case of problems with the heart (multiple answers
Figure 1 First contact person for patients in case of problems with the heart (multiple answers possible)
Heart Failure
for patients (figure 1). Despite existing initiatives, such structures have not been established in DE yet.27 HF-nurses monitor disease progression and contribute to patient self-management. The role of HF-nurses goes hand-inhand with a delegation of medical tasks. In NL, specialist nurses have prescribed medication in their area of expertise for more than 10 years.13
Although IRL, the NL and the UK have successfully introduced HFnurses, only NL has managed to ensure adequate provision. In IRL as well as in the UK, the personnel capacities are not sufficient to meet the demand,19 resulting in enormous workload for HF-nurses according to the experts. In the UK, for example, HF-nurses report difficulties in patient care due to increases in referrals. Therefore, the British Society for Heart Failure Nurse Forum strongly recommends an increase in the HF-nursing workforce and better integration of community and acute HF-services.28
Regional imbalances
time (figure 2). In DE, differences not only exist between urban and rural areas but also between the formerly separated eastern and western regions.15 This is due, among other things, to an unbalanced distribution of GPs and specialists not meeting the demand.29 Specialists in rural areas have to care for 50–130% more patients than in urban areas.29 Federal states and the Associations of Statutory Health Insurance Physicians have therefore launched programmes to attract colleagues to settle in rural areas.30
This is due to a high degree of decentralization and good infrastructures.33 In NL and UK, specialists, e.g. cardiologists, mostly work in hospital settings.13 As in NL, in UK GPs are accessible at closer distances, specialists tend to be concentrated in metropolitan areas.
Access and supply of healthcare services
valid diagnosis and long-term treatment.37 This is partly due to information and communication gaps between GPs, specialists and hospitals but also due to reimbursement. While, for example, an office- or hospitalbased cardiologist may perform an echocardiogram (ECG),15,38 GPs rarely perform ECGs or specific blood tests as they are usually not reimbursed for them.39
In IRL, NL and UK, primary care is mainly provided on an outpatient basis by GPs who act as gatekeepers.13 Specialist treatment only takes place after referral. DE has not a classic gatekeeping system.34 Patients first consult their GP but may also consult a specialist without receiving a referral.
In DE, IRL and parts of the UK, regional differences exist in availability and quality of care provision. While GPs are accessible at closer distances, specialists tend to be concentrated in metropolitan areas, resulting in increased travel
In IRL, the travel distance of patients to GPs is in a range of up to 10 km, the distance to cardiologists was much greater, even up to 50 km (figure 2). According to the experts, the unequal distribution of resources is increased by insufficient funding to implement the national ‘Heart Failure Model of Care’, especially in primary care.18 Therefore, current efforts are aimed at providing HF-care as part of a new program for the care of chronically ill and shifting treatment of low-risk patients to the outpatient setting.18,31,32
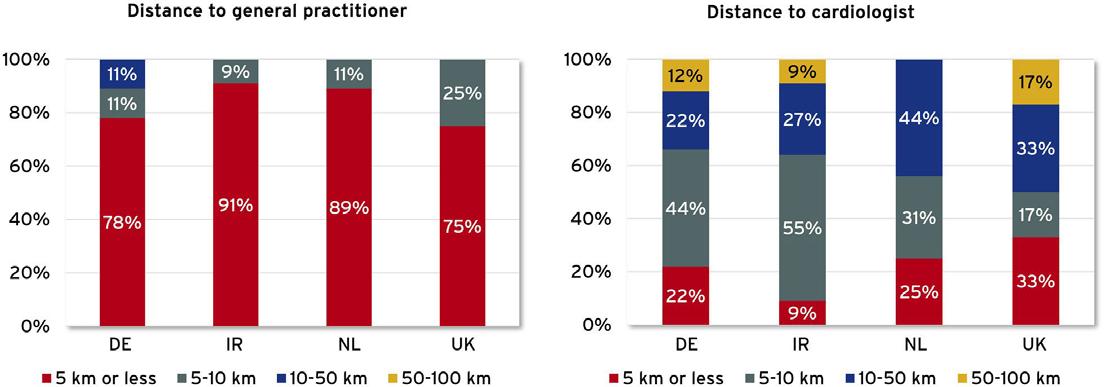
NL shows a slightly different picture. While the results of our study show that GPs are accessible at closer distances and that specialists tend to be concentrated in larger and smaller cities, the country has significantly fewer problems with regional differences in access to care, according to the experts.
Long waiting times for specialist care is evident across all countries. The high number of HF-patients meeting a limited number of specialized physicians and nurses, and unequal geographical distributions are the main reasons.29,35,36 This challenge is particularly visible in IRL. While no more than 4 weeks should pass between referral by GPs and the completion of the diagnosis and therapy plan by a specialist,31 the Irish Health Service Executive cites waiting times for referral to a hospital-based outpatient specialist as averaging 6–9 months.32 According to our interviews, some patients have to wait for up to 2 years.
In IRL, further deficits are prominent in rehabilitation services. Although a multidisciplinary rehabilitation should be offered to every patient,31 less than 1% are transferred to a cardiac rehabilitation facility.40 The Irish Heart Foundation even sees the rehabilitation services in IRL in the middle of an imminent crisis. According to their survey, over 2800 patients are on the national waiting list for cardiac rehabilitation, equalling an increase of 54% since 2013.
2 Distance to general practitioners and cardiologist per
In DE, deficits in care also exist regarding an early and

In NL, although DMPs for HF have been implemented, differences in supply are apparent in delivery of evidence-based treatments between HF outpatient clinics, e.g. regarding device use.41,42 Less than half of the 6666 patients with reduced left ventricular ejection fraction (LVEF; <50%) received an implantable cardioverter defibrillator or cardiac resynchronization therapy, even when LVEF was below or at 30%.42
In the UK, existing deficits in HF-care are mainly due to a high number of patients and limited number of HCPs. Data of the
66 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Eur J Public Health, Volume 33, Issue 3, June 2023, Pages 448–454, https://doi.org/10.1093/eurpub/ckad059 The content of this slide may be subject to copyright: please see the slide notes for details.
Figure 2 Distance to general practitioners and cardiologist per country
Figure 2 Distance to general practitioners and cardiologist per country
National Heart Failure Audit show that after hospitalization only 55% of HF-patients saw a HF-nurse and only 46% had a cardiology follow-up after discharge.35 Experts interviewed, also confirmed that all HF-patients should be seen at least 2–4 times a week for up-titration of evidence-based medication. This is currently not possible.
Information and communication gaps
All countries face the challenge of overcoming information and communication gaps to ensure a coordinated care process that includes all information to provide high quality care and to avoid multiple examinations and wrong decisions. DE has the hurdle of separated budgets, planning logics, and processes of the inpatient and outpatient sectors and a strong subdivision of medical disciplines.43 Those barriers lead to a lack of intersectoral communications and cooperation and subsequently reductions in efficiency and potential misdiagnosis.44,45 According to the experts’ interviews, in IRL, communication problems occur between GPs and HF-nurses, as the nurses in their coordinating role often resort to informal consultations with physicians involved. This complicates adequate documentation due to the lack of a paper trail for the patient’s treatment.
Implementation of telemedicine and digital health applications
All countries have a national eHealth strategy that is implemented to varying degrees. The existing infrastructure and degree of digitalization in healthcare are particularly high in NL and UK. Both countries already support e-prescriptions and electronic patient records.46 However, in NL, since there are more than 3000 different regional electronic patient records connected to the national digital infrastructure AORTA, data exchange between HCPs remains difficult.47 In UK, a summary care record is automatically created for patients once they consult a physician that can be viewed by patients. It contains basic information on allergies, vaccinations and current prescriptions. Data are automatically updated as information is added to the practice-based records via their digital infrastructure NHS Spine.
DE and IR are clearly lagging to establish a digital health
system.47 After several years of developing the national digital infrastructure ‘Telematikinfrastruktur’ in DE, the current infrastructure is considered out of date and is set to be transformed to version 2.0 by 2025.48 Despite the shortcoming of digitalization efforts, there are some providers for telemedical solutions in DE—especially in the field of cardiology.49–51 Those services (e.g. device monitoring or patient management) are often isolated solutions that are regionally and organizationally separated.52 To ensure quality of services, the German Cardiac Society published recommendations that include quality requirements on the staff, organization and evaluation.53,54 Only a few regional telemedicine centres offer their services as part of a certified centre.49,55 The refinancing of digital applications presents a barrier to uptake.55 One step forward is that since January 2022, physician practices are refunded for telemonitoring of HF-patients who are treated with a cardiac implant through statutory health insurance (SHI) funds.56 Another promising approach is the introduction of Digital Health Applications (DiGAs).57,58 Physicians and psychotherapists can prescribe registered and certified eHealth applications to their patients and bill them via SHIs.57 DiGAs are not yet part of routine care.
In IRL, the ‘eHealth Strategy for Ireland’, was published in 2013 focusing on electronic health records, e-prescribing and telemedicine.59 IRL also offers some telemedicine applications for HF-patients. This includes a virtual HF-clinic that consists of interactive web conferences where GPs can consult with hospital cardiologists regarding specific patients.60 However, there are currently no national digital products that target HFpatients specifically.
In the NL, the government places particular emphasis on applications intended to reduce hospital stays and enable and facilitate outpatient treatment.46 The aim is to enable elderly and chronically ill people to be cared for at home for as long as possible. Virtual consultations are in the same way renumerated as face-to-face patient consultations.61 Various telemonitoring systems have been developed for patients with heart diseases. Although there is increasing importance
of telemonitoring from patients’ perspective in NL,62 telemedicine is not yet a widespread reality.47 mHealth apps are only offered locally by hospitals or registered physicians and are not subject to a larger framework.47 Since January 2022, eHealth applications can be reimbursed by health insurers.
In UK, there are insufficient regulations, standards and protocols for telemedicine.63 Currently, some telemonitoring solutions are being tested in pilot projects, but have not yet become an integral part of healthcare provision.64 In terms of eHealth apps, however, the UK is further ahead because policymakers recognized the benefits early on.47 The most popular eHealth app is the official NHS App, that provides access to certain NHS services like patients’ health records, care plans and appointments.65,66 Some GPs and hospitals offer further services to patients, such as direct messaging or consultation via online forms.65
Discussion
Country-specific structures for HF-care have been established in DE, IRL, NL and UK. Models of HF-care in all countries are principally based on the ESC guidelines for the diagnosis and treatment of HF,14 yet they differ in design and implementation. While the gatekeeper model in IRL, NL and UK leads to efficient use of cardiac resources, but carries the risk of delayed specialist care, the German model might result in information gaps and high consumption of resources. However, the challenges are similar and become visible in an unequal distribution of care between urban and rural areas, long waiting times, an inequitable access and supply of healthcare services, information and communication gaps, and insufficient implementation of telemedicine and eHealth. The major challenge is to provide the necessary resources for the increasing number of HF-patients.
A major difference can be seen in the establishment of HF-nurses as focal points in HF-care. If the workforce meets the rising demand, specialist nurses offer a promising approach to facilitate qualitative care. In some cases, they can take over medical tasks such as pharmacological therapy, thus relieving physicians. Successful integration of HF-nurses into care processes can provide much guidance for patients and at the same time relieve the burden on healthcare systems.
The German DiGA concept is unique in Europe and enables reimbursement of specific eHealth applications. However, although the multitude of security and quality standards serve to ensure the development of userfriendly, secure and interoperable applications, they also hamper certification processes. The requirements are difficult to comply with. In addition, only DiGAs classified as risk class I or IIa according to the Medical Device Regulation are eligible. Consequently, the regulation does not apply to many useful applications, such as decision support systems that provide therapy recommendations to empower patients and relieve physicians. Applications for primary prevention are also excluded from reimbursement. Although revision of the legal framework for approval and reimbursement is unavoidable, the DiGA-concept could be a good starting point for further implementation of eHealth solutions in the countries analyzed. A significant contribution could be made to resource-saving structures and self-determined patients by digital solutions.67
Conclusions
HF places a high burden on healthcare systems with significant negative social, organizational and economic impacts. Although promising approaches exist in DE, IRL the NL, and the UK to structure and improve HF-care, implementation is lacking and inconsistent. Insufficient digitalization and a lack of financial resources make it difficult to establish new models of care. Notably, integrating HF-nurses has much potential to improve the care situation. However, to be considered, is the issue of resources available for the increasing number of HF-patients. All countries analyzed have some recognition that digital solutions and the introduction of an electronic health record can partly meet the challenges of HF-care, offering further opportunities to overcome communication and coordination gaps and to strengthen selfmanagement of HF-patients. However, the degree of digital implementation varies greatly. Financing of eHealth applications has shown to be difficult.
References available on request
67 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Colorectal Cancer
Distinct Molecular Profiles of Sporadic Early-Onset Colorectal Cancer: A Population-Based Cohort and Systematic Review
Written by Ashleigh C. Hamilton1, Finian J. Bannon1, Philip D. Dunne2 3, Jacqueline James2 4 5 , Stephen McQuaid2 4, Ronan T. Gray1 6, Manuel Salto-Tellez2 5, Chris R. Cardwell1, Maurice B. Loughrey1
7 and Helen G. Coleman1 2
1Centre for Public Health, Queen’s University Belfast, Northern Ireland, UK 2Patrick G. Johnston Centre for Cancer Research, Queen’s University Belfast, Northern Ireland, UK 3CRUK Beatson Institute, Glasgow, UK 4Northern Ireland Biobank, Belfast, Northern Ireland, UK 5Precision Medicine Centre of Excellence, Queen’s University Belfast, Northern Ireland, UK 6South Eastern Health and Social Care Trust, Northern Ireland, UK 7Department of Cellular Pathology, Belfast Health and Social Care Trust, Northern Ireland, UK
An increase in the incidence of colorectal cancer (CRC) in adults younger than 50 years, known as early-onset CRC (EOCRC), has been observed in high-income countries.1, 2, 3, 4 Studies have suggested that the majority of EOCRC is sporadic in nature, with cases associated with identified germline mutations accounting for up to 35%.5,6 However, not all previous studies have separated sporadic from hereditary cases of EOCRC, and while the molecular pathogenesis of CRC due to inherited conditions such as Lynch syndrome is well defined, the pathways that lead to the development of sporadic EOCRC remain incompletely understood.
CRC is a molecularly heterogeneous disease resulting from stepwise accumulation of mutations in key oncogenes and tumor suppressor genes leading to the development of malignancy via a number of pathways, namely the chromosomal instability pathway (CIN), microsatellite instability (MSI) pathway, and the serrated pathway. Each pathway displays multiple characteristic gene mutations and epigenetic changes. CRCs developing via the CIN pathway are associated with mutations in APC as an early event, with subsequent mutations
in RAS, RAF, PIK3CA, SMAD4, and/or TP53 genes, among others.7 KRAS and NRAS mutation status is used in the clinical setting to inform systemic treatment options.8 CRCs arising through the MSI pathway display deficient mismatch repair (dMMR), which is synonymous with MSI,9 resulting from uncorrected errors during DNA replication.10 Lynch syndrome, a hereditary condition predisposing to the development of several cancers, results in microsatelliteinstability high (MSI-H) CRCs. However, due to the inclusion of both hereditary and sporadic cases of EOCRC in many studies to date, it is unclear how frequently sporadic MSI-H tumors occur in EOCRC cases. CRCs arising via the serrated pathway are MSI-H, associated with an increased prevalence of BRAF mutations (which is a distinguishing feature), and have high levels of CpG island methylation, known as the CpG island methylator phenotype (CIMP).11
A 2019 report reviewed 37 studies with regard to prognosis of EOCRC compared to lateonset CRC (LOCRC) and found conflicting results for a poorer, similar, or better prognosis in younger patients.12 It is possible that survival differences between EOCRC patients and older CRC patients may reflect the different

molecular profiles of tumors occurring in these patients. However, to our knowledge, the evidence for molecular profiles of sporadic EOCRC tumors has not been systematically collated. In the present study, we analyzed a population-based cohort of patients with stage II and III colon cancer to investigate molecular characteristics in sporadic CRCs and survival outcomes according to age categories. We also undertook a systematic review and meta-analysis of the prevalence of MSI status and selected tumor mutations in sporadic EOCRCs.
Methods
Population-Based Cohort Study Patient Population
The study cohort (known as Epi700) was established as previously described.13, 14, 15 In summary, 661 stage II and III colon cancer patients diagnosed in 2 healthcare trusts in Northern Ireland from 2004 to 2008, for whom resection specimens were available to be retrieved from the Northern Ireland Biobank, were identified using the Northern Ireland Cancer Registry. Patients were followed up for recurrence and cause of death to December 31, 2013.
Tumor Pathology Characteristics and Clinical Data Collection
When tumor pathology characteristics were not readily available from routinely extracted cancer registry information, further pathology details, for example, tumor differentiation, were retrieved by manual review of pathology reports.
Clinical variables used in this study including family history of CRC, oncological treatments, Eastern Cooperative Oncology Group performance status, lifestyle information (including smoking and alcohol), and
comorbidities were extracted from the Northern Ireland Clinical Oncology Information System, a prospective electronic record of patient management.
Tumor Molecular Analysis
Following tumor annotation and macrodissection, DNA was extracted according to the manufacturer’s instructions from 5-μm sections of representative whole-tumor blocks using the Maxwell 16 instrument (Promega, Southampton, UK) and Promega DNA extraction kit (Promega, Southampton, UK).
MSI analysis was performed within the Northern Ireland Molecular Pathology Laboratory, using the MSI Analysis System, version 1.2, kit (Promega, Southampton, UK) for 5 mononucleotide repeat markers (BAT-25, BAT-26, NR21, NR-24, and MONO-27).
PCR products were separated by capillary electrophoresis using an ABI 3500 Genetic Analyzer (Fisher Scientific, UK Ltd, Loughborough, UK). The output data were analyzed using GeneMapper v4.1 (Fisher Scientific, UK Ltd, Loughborough, UK) to determine MSI status.9
Tumor samples were analyzed for mutational status of established CRC markers. This included a ColoCarta panel of KRAS, NRAS, BRAF, CMET, and PIK3CA using a validated mass spectrometry–based targeted screening panel of 32 somatic mutations in 6 genes (Agena Bioscience, Hamburg, Germany). Samples were shipped via the Genomics Core Technology Unit (Queen’s University Belfast) and the assays performed by Agena Custom Services Laboratory (Hamburg, Germany).
Statistical Analysis
A statistical analysis was performed using Stata 16 (StataCorp, 2019. Stata Statistical Software: Release 16.
68 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Ashleigh C. Hamilton
2
StataCorp LLC, College Station, TX). Chi-squared tests were used to compare descriptive and molecular characteristics across age categories. A survival analysis was performed using the Cox proportional hazards model to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). The multivariable model for CRCspecific survival included sex, family history of CRC, stage (II/ III), grade/differentiation, adjuvant chemotherapy receipt, Eastern Cooperative Oncology Group performance status, alcohol, smoking, inflammatory bowel disease, and emergency surgery. Results from this study were included in the subsequent systematic review and meta-analysis.
Systematic Review and MetaAnalysis
This study was reported according to the Meta-analysis of Observation Studies in Epidemiology (MOOSE) checklist.16 The review protocol was registered on PROSPERO (CRD42021232567).
Study Population
The population of interest was patients with sporadic EOCRC, defined as adults younger than 50 years at their incident CRC diagnosis who had no identified inherited genetic syndrome that predisposes to CRC. Studies were included if sporadic cases were separated from hereditary cases by the authors or if the article contained information to enable distinction of hereditary from sporadic cases, such as results of genetic testing or family history.
Outcome
The primary outcome was
to estimate the prevalence of MSI-H/dMMR status and KRAS, NRAS, BRAF, PIK3CA, and TP53 mutations in EOCRC. The protocol specified a secondary outcome investigating the influence of molecular profile on survival in EOCRC, but insufficient data were available in potentially eligible articles and so we restricted the reporting of the review to the prevalence of molecular features as outlined.
Search Strategy
The electronic databases Ovid Medline, Embase, and Web of Science were systematically searched from 2000 to April 12, 2021. The full search terms are available in Supplementary Appendix 1. Observational studies, descriptive studies, case series, and interventional studies were eligible for inclusion. All stages of CRC were included, with a focus on colorectal adenocarcinoma as the primary histology, and no language restrictions were imposed. Review articles, editorials, comments, abstract or conference proceedings, individual case studies, and case series with less than 10 patients were excluded.
Articles from the search were imported into Covidence, and duplicates were removed. Titles and abstracts were reviewed by 2 authors independently (A.C.H. and H.G.C.). The full text of all selected articles was read by 2 authors (A.C.H. reviewed all articles; H.G.C. and M.B.L. reviewed independent subsets). Any discrepancies were resolved by discussion among the 2 reviewers of the text, with the third reviewer involved if required.
Data Extraction
Data were extracted by A.C.H. and verified by H.G.C. Data extracted were related to study location, number of sporadic EOCRC cases, definition of sporadic cases, mutation testing, MSI or dMMR testing methods, and the prevalence of each molecular characteristic in the study population, along with sex and anatomical tumor location if available. Where mutation testing had resulted in an unknown or ambiguous result, our approach was to exclude these cases from the analysis. Where a study contained data for both MSI status and MMR proteins, the MSI status results were used. Details of molecular testing undertaken in each study are shown in Table A4. Immunohistochemistry for the p53 protein has been used as a surrogate for TP53 mutation testing,17 where reported in studies. In a change to the study protocol, the Joanna Briggs Institute Checklist for Prevalence Studies was used for quality assessment.
Statistical Analysis
Stata 16 was used to perform meta-analysis to produce pooled estimates of prevalence and 95% CI. The Freeman-Tukey Arcsine Transformation method was used to calculate estimates and standard errors, which were back-transformed to calculate a pooled prevalence.18 The logistic-normal random-effects model was also carried out to ensure the pooled estimates and 95% CI were similar. A sensitivity analysis was performed, and subgroup analysis was carried out by sex and anatomical tumor location. The heterogeneity between studies was determined using the I2 statistic.19 Publication bias was assessed using funnel plots
Results
Population-Based Cohort Study
The number of patients in the cohort for whom a surgical resection specimen was retrieved for molecular analyses was 661. Nine patients had a known hereditary cancer syndrome: Lynch syndrome (n = 6), familial adenomatous polyposis (n = 1), and other familial syndromes (n = 2). These patients were excluded from the analyses, leaving a cohort of 652 patients with presumed sporadic CRCs.
Patients younger than 50 years (n = 35) comprised 5.4% of our cohort of all stage II and III sporadic colon adenocarcinoma patients within the jurisdiction of the Northern Ireland Biobank. The demographic and clinical characteristics of the overall cohort are summarized in Table A1.
The distribution of mutations and molecular features by age category is shown in Figure 1. EOCRC patients did not have any BRAF or NRAS-mutant tumors, and the proportions of these features across age categories were significant (P < .01 and P = .01, respectively). EOCRCs had the highest proportion of MSI-H tumors (25.7%) and PIK3CA mutations (25.7%) of all the age groups, but this did not reach statistical significance.

The results of survival analyses are shown in Table 1. Compared with 60 to 69-year-old patients, EOCRC patients did not have a significantly increased risk of CRC death in stage II/III disease (adjusted HR 1.20, 95% CI 0.61–2.39).
Compared to patients with microsatellite-stable (MSS) tumors, patients with MSI-H tumors had a significantly reduced risk of CRC death (unadjusted HR 0.66, 95% CI 0.45–0.97) in stage II/III disease. In multivariable analysis, patients with MSI-H tumors had a reduced risk of CRC death, but this was not statistically significant (HR 0.71, 95% CI 0.47–1.09). Subgroup analyses of survival by MSI status are shown in Figure 2. EOCRC patients with MSI-H tumors did not have a significantly decreased risk of CRC death (adjusted HR 0.65, 95% CI 0.07–6.32). EOCRC patients with MSS tumors did not have a significantly increased risk of CRC death compared to 60 to 69-year-olds (adjusted HR 1.58, 95% CI 0.71–3.51).
69 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Figure 1 Distribution of molecular characteristics by age category in sporadic stage II and III colon cancer
Colorectal Cancer
Figure 2 Risk of CRC–specific death according to age categories in stage II and III colon cancer cases, by microsatellite instability status.
∗∗Adjusted for sex, adjuvant chemotherapy receipt, stage, tumor differentiation, family history of CRC, ECOG performance status, smoking, alcohol, inflammatory bowel disease and emergency surgery. CI, confidence interval; CRC, colorectal cancer; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; MSI, microsatellite instability
Systematic Review and MetaAnalysis
The search strategy identified 2415 studies, and 1088 duplicates were removed by Covidence, leaving 1327 articles for screening. Following title and abstract screening, 170 studies were eligible for full-text review.
Following the full-text review, 140 studies were excluded for the reasons outlined in Figure 3. Thirty-two articles were included in the review (31 resulting from our search strategy and 1 resulting from our population-based cohort study described above, referred to herein as Hamilton et al, 2022).
RAF Mutations
The results of survival analyses are shown in Table 1. Compared with 60 to 69-year-old patients, EOCRC patients did not have a significantly increased risk of CRC death in stage II/III disease (adjusted HR 1.20, 95% CI 0.61–2.39). Compared to patients with microsatellite-stable (MSS) tumors, patients with MSI-H tumors had a significantly reduced risk of CRC death (unadjusted HR 0.66, 95% CI 0.45–0.97) in stage II/III disease. In multivariable analysis, patients with MSI-H tumors had a reduced risk of CRC death, but this was not statistically significant (HR 0.71, 95% CI 0.47–1.09). Subgroup analyses of survival by MSI status are shown in Figure 2. EOCRC patients with MSI-H tumors did not have a significantly decreased risk of CRC death (adjusted HR 0.65, 95% CI 0.07–6.32). EOCRC patients with MSS tumors did not have a significantly increased risk of CRC death compared to 60 to 69-year-olds (adjusted HR 1.58, 95% CI 0.71–3.51).
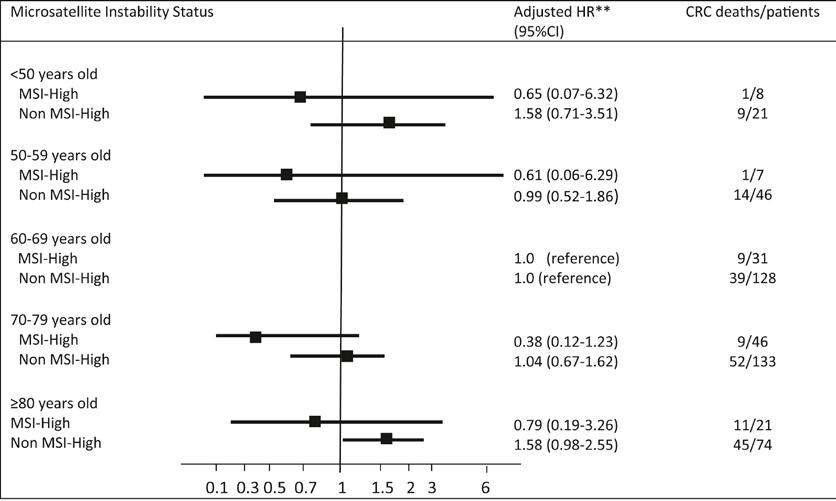
Nine studies reported data on BRAF mutations,22,27,31,33,37,43,44,48 including our population-based cohort study. Pooled analysis revealed a prevalence of 1% (95% CI 0%–3%) for BRAF mutations in sporadic EOCRC. The observed heterogeneity was moderate (I 2 36.78%, P = .12).
KRAS Mutations
Thirteen studies reported data on KRAS mutations,22,24,27,31, 32, 33,37,38,40,43,44,48 including our population-based cohort study. Pooled analysis revealed a prevalence of 32% (95% CI 23%–40%) for KRAS mutations in sporadic EOCRC. The observed heterogeneity was high (I2 84.52%, P < .01).
NRAS Mutations
Four studies reported data on NRAS mutations,43,44,48 including our population-based cohort study. Pooled analysis revealed a prevalence of 3% (95% CI 1%–4%) for NRAS mutations in sporadic EOCRCs. The observed heterogeneity was low (I2 0.00%, P = .42).
PIK3CA Mutations
Five studies reported data on PIK3CA mutations,22,31,44,48 including our population-based cohort study. Pooled analysis revealed a prevalence of 14% (95% CI 5%–25%) for PIK3CA mutations in sporadic EOCRC. The observed heterogeneity was high (I2 83.31%, P < .01).
ECOG, Eastern Cooperative Oncology Group.
a Multivariable model adjusted HR, adjusted for sex, adjuvant chemotherapy receipt, stage, tumor differentiation, family history of CRC, ECOG, performance status, alcohol, smoking, inflammatory bowel disease, and emergency surgery.
b
Multivariable model adjusted for all variables in footnote “a” and Charlson comorbidity score
TP53 Mutations
Seven studies reported data on TP53 mutations.22, 23, 24,31,38,40,44 Pooled analysis revealed a prevalence of 64% (95% CI 56%–71%) for TP53 mutations in sporadic EOCRC. The observed heterogeneity was moderate (I2 57.80%, P = .03).
Figure
caption: Risk of CRC–specific death according to age categories in stage II and III colon cancer cases, by microsatellite instability status. ∗∗Adjusted for sex, adjuvant chemotherapy receipt, stage, tumor differentiation, family history of
70 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Table 1. Survival Analysis by Age Category in Sporadic Stage II and III Colon Cancer
Figure 1 caption: Distribution of molecular characteristics by age category in sporadic stage II and III colon cancer.
Age (y) CRC death Overall survival No of CRC deaths/patients Unadjusted HR (95% CI) Adjusted HRa (95% CI) No of deaths/CRC patients Unadjusted HR (95% CI) Adjusted HRb (95% CI) <50 11/34 1.14 (0.59–2.18) 1.20 (0.61–2.39) 12/35 1.14 (0.79–1.64) 1.19 (0.81–1.75) 50–59 16/58 0.83 (0.47–1.45) 0.83 (0.47–1.46) 19/61 0.75 (0.56–1.01) 0.76 (0.57–1.03) 60–69 52/170 1 (reference) 1 (reference) 61/179 1 (reference) 1 (reference) 70–79 70/193 1.04 (0.73–1.49) 0.88 (0.60–1.28) 115/238 0.98 (0.81–1.19) 0.95 (0.77–1.17) ≥80 61/104 1.96 (1.35–2.84) 1.38 (0.91–2.10) 96/139 1.66 (1.33–2.08) 1.49 (1.16–1.91)
Table 1. Survival Analysis by Age Category in Sporadic Stage II and III Colon Cancer
2
Sensitivity and Subgroup Analyses
Sensitivity analyses were performed by excluding one study at a time for each of the mutations and MSI/MMR status, to assess the robustness of the results. Excluding some studies had a marginal effect on heterogeneity for BRAF, PIK3CA, TP53, and NRAS mutations, but the prevalence of these mutations remained similar.
Subgroup analyses were undertaken by sex for MSI/ MMR status, KRAS, BRAF, and PIK3CA mutations (Table 2). There were insufficient data to undertake subgroup analyses for TP53 and NRAS mutations. The prevalence of MSI-H/dMMR and BRAF and PIK3CA mutations was similar between male and
female patients. The prevalence of KRAS mutations was slightly higher in female patients (35%) than that in male patients (27%).
Subgroup analyses were undertaken by tumor location (Table 2). Analyses for colon were performed for MSI/ MMR status; KRAS, BRAF, and PIK3CA mutations; and for rectum by MSI/MMR status. There were insufficient data for TP53 and NRAS mutations for any subgroup analysis by tumor location or for KRAS, BRAF, and PIK3CA mutations for rectal cancer. Results showed that the prevalence of MSI-H/ dMMR was higher in the colon than in the rectum (16% vs 6%) and higher in the right colon than in the left colon (32% vs 3%). The prevalence of KRAS and BRAF mutations was
similar in the right and left colon, while PIK3CA mutations showed a higher prevalence in the right colon than in the left colon (18% vs 1%).
Discussion
To our knowledge, this is the first systematic review investigating the prevalence of the MSI-H/ dMMR status and somatic mutations in sporadic EOCRC. The importance of distinguishing sporadic from hereditary EOCRC is becoming increasingly recognized, with the molecular pathogenesis, treatment response, and outcomes of sporadic EOCRC less understood than those of hereditary cases.
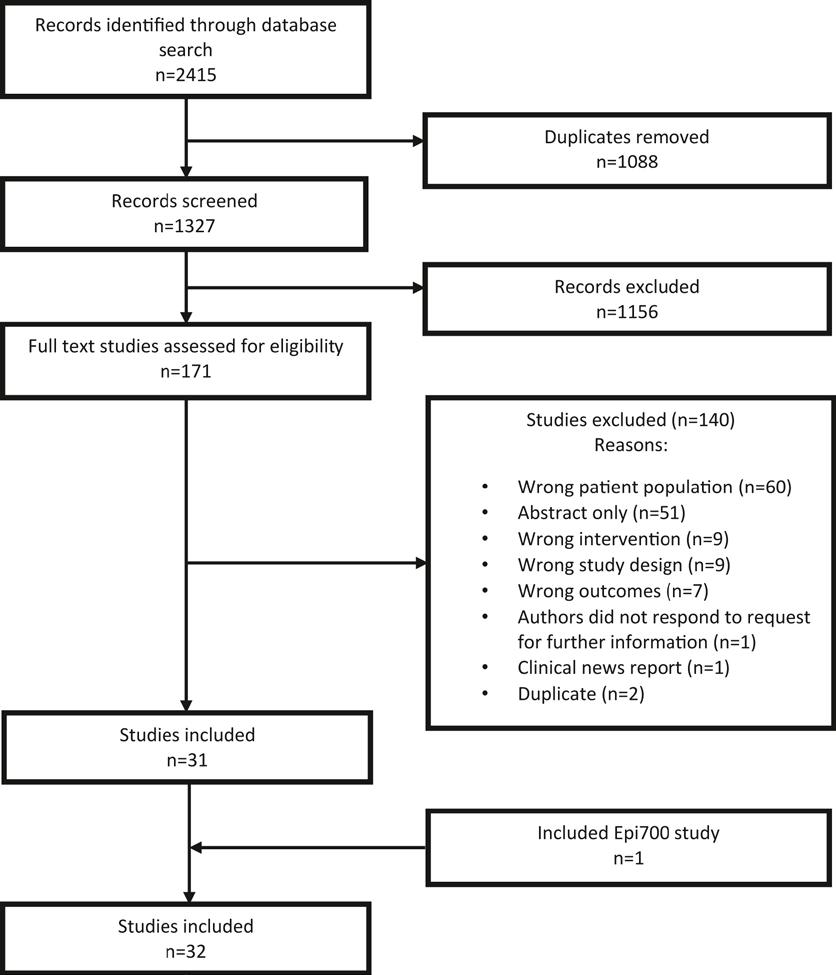
MSI/MMR Status
Our systematic review shows that MSI-H/dMMR has a prevalence of 10% in sporadic EOCRC. These
results were consistent across analyses for MSI-H tumors and dMMR tumors, suggesting that MSI and MMR statuses are highly correlated and that our findings are robust. Other studies have shown near-perfect concordance between immunohistochemistry testing for MMR proteins and MSI testing.9
MSI-H/dMMR CRCs are encountered in 2 clinical settings, representing the phenotypic convergence of 2 clinically distinct pathogeneses. Firstly, such tumors are the hallmark of CRCs arising in the context of Lynch syndrome, resulting from a germline mutation in one of the MMR genes, most commonly MLH1 or MSH2, and young age at cancer diagnosis is regarded as an indicator for a possible hereditary cause of the disease. Our results show that
71 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Figure 3. Flow chart of the selection of articles included in the review
Colorectal Cancer
10% of MSI-H/dMMR tumors in EOCRCs do not arise from Lynch syndrome. However, given the historical case series in reported studies, it is possible that some cases of seemingly sporadic EOCRC in our review may have undiagnosed Lynch syndrome or Lynch-like syndrome.51, 52, 53
Secondly, MSI-H tumors comprise a proportion of sporadic CRCs, and these are considerably more common than Lynch syndrome-related MSI-H CRCs. Sporadic MSI-H CRCs arise via the serrated neoplasia pathway and are strongly associated with BRAF mutations, older age, right-sided tumor location, and high levels of CIMP 54 Given the extremely low prevalence of BRAF mutations in EOCRC, it is unlikely the serrated pathway is the mechanism by which MSI-H tumors develop in younger patients. Similar to Lynch syndrome, results from the subgroup analysis suggest that sporadic MSI-H EOCRCs also have a predilection for the right colon.
CIMP-high CRCs are associated with older-age patients, female sex, proximal tumor location, MSI-H status, and somatic BRAF mutation.55 Evidence regarding CIMP in EOCRCs is sparse, but CIMP-high tumors appear to be less prevalent in younger patients with CRC.56 However, future studies are required to elucidate the role of CIMP in sporadic EOCRC.
BRAF Mutations
BRAF mutations occur in approximately 8% of all CRCs, the vast majority being V600E mutations.57 Our results show that the prevalence of BRAF mutations in sporadic EOCRC is 1%, which is lower than that in LOCRC and indicates this is a rare mutation in younger adults. Given the rarity of BRAF mutations in EOCRC, our results also suggest the association of BRAF mutations with MSI-H tumors seen in CRC in older-age groups58 does not apply to these younger patients.
BRAF mutations are a negative prognostic marker, with worse survival outcomes being reported
in a metastatic disease 59 However, our results suggest a low prevalence of BRAF mutations in EOCRC does not necessarily translate into better survival in this group, and the reasons for this are unclear. In 2021, the National Institute for Health and Care Excellence approved the use of encorafenib, a BRAF inhibitor, in BRAF V600E-mutationpositive metastatic CRC in the United Kingdom.60 However, given the rarity of BRAF mutations in EOCRC, it is likely only a small proportion of young patients will be able to avail this treatment, and optimal treatment strategies for EOCRC remain to be determined.
RAS Mutations
Our results show that the prevalence of KRAS mutations in sporadic EOCRC is 32%. A large Memorial Sloan Kettering Cancer Centre study published after our literature search reported a prevalence of 42.5% for KRAS mutations in sporadic EOCRCs.61 Together with our meta-analysis, these results are comparable to a systematic review investigating KRAS mutations in metastatic CRCs, which reported a pooled prevalence of 35.9%.62 This suggests that the prevalence of KRAS mutations is broadly similar in EOCRC and LOCRC.
Knowledge regarding NRAS mutations in CRC is limited due to its low frequency. A systematic review found a prevalence of 4.1% (95% CI 3.5%–4.8%) of NRAS mutations in metastatic CRC in all ages.62 Our results show that the prevalence of NRAS mutations in tumors in sporadic EOCRCs is 3%, suggesting the prevalence of NRAS mutations is similar in young and older patients.
PIK3CA
Mutations
A 2020 systematic review reported PIK3CA mutations had a prevalence of 12.9% in CRC in patients of all ages.63 Our results show that the prevalence of PIK3CA mutations in tumors in sporadic EOCRC is 14%, suggesting that the proportion of PIK3CA mutations
PMI Annual Charity Lunch

The Pharmaceutical Managers’ Institute will hold their Annual Charity Lunch at the Mansion House, Dawson Street Dublin on Thursday, December 7th, 2023
is similar in EOCRC and LOCRC. However, knowledge regarding PIK3CA mutations in EOCRC remains limited, as shown by the small number of studies in our metaanalysis. PIK3CA mutations currently have no clinical role as predictive or prognostic biomarkers, with a previous systematic review and meta-analysis finding no significant association between PIK3CA mutation status and survival outcomes.64
TP53 Mutations
Mutation of TP53 is a late event in the stepwise development of CRC, most commonly via the CIN pathway.7 TP53 mutations have been shown to be present in up to 60% of CRCs.65 Our results show that the prevalence of TP53 mutations in sporadic EOCRC is 64%, the highest prevalence of any mutation in this study. Similar findings were observed in a wholeexome sequencing study which found TP53 was the most common mutation in EOCRC,66 with subsequent targeted deep sequencing (n = 833) showing a higher frequency of TP53 mutation in EOCRC than in LOCRC (80% vs 72%, Fisher’s exact P = 0.03). TP53 is currently not used as a prognostic or predictive biomarker in clinical practice, and more research is required into its clinical implications.
Survival
Results from our population-based cohort study indicate that stage II and III sporadic EOCRC does not have a significantly worse survival compared with those in patients aged 60–69 years but indicate there may be an aggressive subset within this young age group, driven by MSS tumors. CRCs displaying MSI have a better prognosis in an early-stage disease, with improved 5-year overall survival67 and 5-year recurrence-free survival,68 but less expected benefit from adjuvant chemotherapy 67,69 Results from our population-based cohort study support the conclusion that
non-metastatic MSI-H tumors in sporadic EOCRC patients also carry a better prognosis than MSS tumors, although sample sizes were limited and so results were not statistically significant. We are unable to draw any conclusions about survival in metastatic sporadic EOCRC from this study. Studies have shown that EOCRC patients have a more advanced stage at presentation than older patients, 70 which could be due to more aggressive biology or a delay in diagnosis.
Delayed diagnosis may be caused by a number of factors, including failure of younger patients to seek healthcare, a delay in referral by healthcare professionals, or the exclusion of younger individuals from bowel cancer screening programs.
Further research is urgently needed on outcomes for patients with EOCRC and more specifically on the impact of tumor molecular profile on survival, particularly how this varies by stage of disease.
Conclusion
This systematic review addresses a research gap regarding sporadic EOCRC and provides evidence of differing molecular profiles in younger patients with CRC compared to older patients. Approximately 10% of seemingly sporadic EOCRCs are MSI-H, and BRAF mutations are a rare event in these tumors, having a much lower prevalence than in LOCRC. KRAS, NRAS, PIK3CA, and TP53 mutations have a similar prevalence to LOCRC. The molecular pathogenesis of sporadic EOCRC remains unclear, with the serrated neoplasia pathway unlikely to play a major role. EOCRC patients were not at increased risk of cancerspecific death compared with older patients in our populationbased cohort, but further studies are needed to address whether molecular profiles differentially influence EOCRC patient outcomes in this patient group.
72 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
This year the PMI are proudly supporting the work of LauraLynn as their 2023 charity partner. Visit www.thepmi.com for further details.
Best of ESC Congress 2023
“Joining forces to protect the heart – this was our mission for this year’s ESC Congress,” said Professor Franz Weidinger, ESC President. “Cardiovascular (CV) medicine has been tremendously successful in improving and even prolonging life through paramount achievements over the last decades. Now it’s time to make sure that all of our patients who need treatment do get it –timely, effectively, sustainably. This is why we need to join forces as CV professionals, industry, policymakers, and patients.”
More than 30,000 health professionals from 175 countries attended this year’s meeting, 24,000 onsite and 6,000 online. Novel findings were revealed in more than 3,750 abstracts and clinical cases presented by scientists from 86 countries. Germany led the way with the most abstracts, followed by the United States and Japan. Some 86 studies were simultaneously published in prestigious journals. The shocking cost of CV disease in the EU was revealed in the most comprehensive analysis since 2006. CV disease cost the EU an estimated ¤282 billion in 2021, significantly more than the entire EU budget itself –underscoring the urgent need to act collectively on the European scale to better combat the CV risk of European citizens.
Professor John McMurray, ESC Congress Programme Committee Chair, said: “ESC Congress had a groundbreaking programme featuring the hottest cardiovascular science of the year and covering the entire field of CV medicine.”
Practice-changing research was presented throughout the congress, with major new findings in the Hot Lines. The Hot Line programme kicked off with STEP-HFpEF, the first trial of a pharmacologic agent to specifically target obesity as a treatment strategy for heart failure with preserved ejection fraction, and indicating that obesity is a target for therapeutic intervention in this vulnerable patient group. Next up was NOAH-AFNET 6, which showed that anticoagulants cause bleeding without preventing stroke in patients with atrial high-rate episodes (AHRE), but without electrocardiogram (ECG)diagnosed atrial fibrillation. The results of this major trial suggest
that ECG documentation of atrial fibrillation is needed before starting oral anticoagulation in patients with AHRE. Rounding off Hot Line session 1 was COP-AF, a trial in patients undergoing major noncardiac thoracic surgery, which found that colchicine does not significantly reduce perioperative atrial fibrillation or myocardial injury after non-cardiac surgery.
Definitive conclusions about the role of iron therapy in irondeficient patients with heart failure and reduced ejection fraction were presented in the ferric carboxymaltose metaanalysis, which demonstrated a link with reduced hospitalisation. Another important meta-analysis showed that intravascular imaging-guided percutaneous coronary intervention (PCI) is associated with a lower rate of target lesion failure compared with angiography-guided PCI.
New ESC Guidelines were launched that will influence clinical practice across Europe and beyond. Professor McMurray said: “Four new Guidelines were presented, covering acute coronary syndromes, endocarditis, cardiovascular disease in diabetes and cardiomyopathies – the first guideline of its kind. A focused update on the 2021 heart failure Guideline was also presented at ESC Congress, reflecting the tremendous and rapid progress in this condition even in just the past
two years. Heart failure is both the cause and the consequence of the many conditions that are managed by CV health professionals, and was the spotlight of this year’s congress.”
“Another highlight was the Digital Health Hub, featuring 37 sessions on the application of new technologies to all subspecialties of cardiology,” said Professor McMurray. “Cardiovascular health professionals attending ESC Congress are now equipped with the latest medical knowledge to improve their clinical practice, save lives, improve quality of life and reduce the burden of CV disease around the world.”
ESC Congress 2023 has closed its doors, but ESC staff are already getting ready for ESC Congress 2024, to be held in London, UK from 30 August to 2 September 2024.
Meanwhile, a multi-stakeholder group consisting of 32 European patient organisations, medical associations, research organisations, data collaborations and industry associations met EU officials involved in the legislative negotiations for a Regulation on the European Health Data Space (EHDS) this morning. Attendees included: MEP Tomislav Sokol, Co-Rapporteur, as well as other EU Parliament officials and the representatives of several permanent representations that will
hold upcoming Presidencies of the Council of the European Union. The informal group of organisations have formed a partnership due to a shared concern that the European Commission’s proposal for the EHDS could lose some of its impact and potential value in the co-legislative negotiations. In October 2022, the group put forward recommendations calling for consistent legislation and successful implementation of the EHDS. In June 2023, the group issued a joint statement expressing support for the Commission’s original proposal that strikes a balance between the protection of personal data while allowing the secondary use of electronic health data to enable the creation of tangible benefits for patients in the EU.
In that joint statement, the group raised concerns about the risk that data bias would become systematic within the EHDS from its inception and thus undermine its principal value for secondary use purposes, if an opt-out or optin mechanism were added, and called for an impact assessment of those policy options. Should the legislative negotiations move towards adopting an opt-out mechanism, the statement outlined challenges and recommendations for implementing an effective optout mechanism.
73 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Conference
Age-Related Macular Degeneration
Article contributed by The Irish College of Ophthalmologists

Age related macular degeneration (AMD) is the leading cause of sight loss in the over 50s in Ireland. More than 100,000 people in Ireland aged over 50 are living with AMD. Its prognosis has been greatly improved with modern treatment modalities. Without treatment, severe (wet) AMD can lead to loss of central vision and it is the most common cause of legal blindness in the over 60s.
The severity of the disease varies between individuals. Early detection is critical. Usually, even in its most aggressive form, side or peripheral vision is preserved allowing the patient to move around independently and to continue those tasks that do not require fine central vision.
Early diagnosis and treatment by an ophthalmologist is recommended which results in a better visual outcome for patients.
What is AMD?
Age related macular degeneration is an ageing change in the macula. The macula is the central part of the light sensitive retina. The retina lines the inside of the back of the eye and is responsible for
vision. Light focused on the retina is transformed to an electrical signal that is sent to the brain where ‘seeing’ takes place. The macula is responsible for central and fine vision such as reading, colour vision and recognising facial details.
AMD affects the central vision, where the sharpest vision occurs, and can cause difficulty undertaking daily tasks such as reading, driving and recognising faces.
What causes AMD?
Risk factors for AMD include age, genetic predisposition, dietary and environmental factors such as smoking and cardiovascular disease. Ultraviolet exposure may also have a role. The greatest risk factor is age: a person’s risk typically begins to escalate after age 50. Studies show that after age 75, AMD affects nearly 40 percent of seniors. Smoking poses a significant risk for AMD especially in women.
There are two main types of AMD, ‘Dry’ (atrophic) and ‘Wet’ (neovascular) AMD. Wet AMD represents an advanced stage of the disease. It is almost always preceded by Dry AMD.
Dry AMD
Dry AMD is the most common (80-90%) and the mildest form of the condition. In this type, there are typical age changes such as age spots or ‘Drusen ‘ in the macula. Very often patients with Dry AMD maintain good central vision, needing perhaps more magnification and lighting for reading. A small percentage of patients with Dry AMD will develop more severe patchy dry changes with consequential progressive difficulty with reading and fine vision. At this time there is no known cure for Dry AMD, but progression can be slowed by stopping smoking, eating a healthy diet rich in Lutein (found in the leafy green vegetables), and looking after cardiovascular risk factors such as high blood pressure and elevated cholesterol. Dry AMD usually affects both eyes. Dry AMD can progress to Wet AMD.
Wet AMD
Wet AMD is the less common but more severe form (affects less than 10%). In this form of AMD, an abnormal blood vessel complex grows in under the macula as part of the ageing change. The growth of this complex occurs due to local release of a chemical called Vascular Endothelial Growth Factor(VEGF). This process results in a cycle of leakage, bleeding and scar tissue growth in the macula leading to destruction of central vision and potentially legal blindness without treatment. There is a significant risk of Wet AMD affecting the second eye over time (>50%). The second eye needs to be carefully monitored.
this complex occurs due to local release of This process results in a cycle of leakage, destruction of central vision and potentially of Wet AMD affecting the second eye over
Symptoms:
Symptoms:
In Dry AMD symptoms are often mild, such When progression to Wet AMD occurs, the distortion (due to fluid or blood under the moment to cover or close one eye at a time. seek help promptly from their eye doctor.
Amsler Grid
In Dry AMD symptoms are often mild, such as needing stronger glasses or better lighting for reading. When progression to Wet AMD occurs, the patient may notice the onset of blurring of reading vision with distortion (due to fluid or blood under the macula). Sometimes patients are unaware until they take a moment to cover or close one eye at a time. At this stage, the progression can be rapid and patients should seek help promptly from their eye doctor.
Amsler Grid
An Amsler Grid is a simple test designed to pick up symptoms of Wet AMD. However, it is not a

74 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Eye Health
distortion (due to fluid or blood under the macula). Sometimes patients are unaware until they take a moment to cover or close one eye at a time. At this stage, the progression can be rapid and patients should seek help promptly from their eye doctor.
Amsler
Grid
substitute for an eye examination. The patient checks each eye separately with reading glasses on. If they notice new blurring or distortion of the lines on the grid this could indicate the presence of Wet AMD and they should be examined promptly by their eye doctor.
Looking after general health, being a nonsmoker, eating a healthy diet rich in leafy greens (Lutein), and sensible use of sunglasses can all help to slow the progression of AMD. An ophthalmologist will often recommend a patient take a Lutein based vitamin supplement. Monitoring the vision in each eye separately using an Amsler grid can lead to early detection of AMD and an improved visual outcome.
Knowing the symptoms and signs to be aware of:
Diagnosis of AMD
Diagnosis of AMD
A Fluorescein angiogram is a photographic dye test used to assess AMD. The patient is given an injection of yellow Fluorescein dye into a vein in the arm. The dye is followed with photographs as it goes through the retinal circulation and will show up changes due to AMD. An OCT is a special ultrasound that will instantly detect changes in the macula due to AMD. It is a quick and reliable test and is as easy for the patient as a photograph. FA and OCT are valuable tools in distinguishing Dry from Wet AMD.
slow the progression of early AMD and reduce the risk of conversion to Wet AMD. Supplements containing vitamins C and E, zinc & copper, and lutein and zeaxanthin are recommended.
macula due to a chemical stimulant called vascular endothelial growth factor (VEGF). Intravitreal injections of anti- VEGF drugs, which block vascular endothelial growth factor, can break the cycle of leakage, bleeding and scar tissue growth. Anti-VEGF treatment offers stabilisation and very often improvement in vision. They are delivered through an injection into the eye, administered by an ophthalmologist, usually a retinal specialist. While some pressure will be felt during the injection, the eye is first numbed to minimise any discomfort. The aim of treatment is to preserve existing vision but many patients do have an improvement in vision. Intravitreal anti-VEGF are administered on a monthly basis until stability is achieved. Injections may be needed on a regular basis over several years to maintain vision. There is extensive research in this area and it is likely that new drugs and new drug delivery systems will soon become available.
Laser Photocoagulation
1. Symptoms: look out for straight lines appearing wavy or distorted, e.g. a doorframe. If you see a smudge, gaps or dark spots appearing in your field of vision, or have difficulties in reading small print, even with glasses, or difficulty in recognising faces, it is recommended to make an appointment with an eye doctor, optometrist or GP as soon as possible.
An Amsler Grid is a simple test designed to pick up symptoms of Wet AMD. However, it is not a substitute for an eye examination. The patient checks each eye separately with reading glasses on. If they notice new blurring or distortion of the lines on the grid this could indicate the presence of Wet AMD and they should be examined promptly by their eye doctor.
The AREDS2 study reformulated the AREDS Formulation. Betacarotene is replaced by two anti-oxidants lutein/zeaxanthin, and zinc levels are reduced substantially. The study also showed that although there is evidence that dietary intake of omega-3 fatty acids seem to play important roles in visual health, taking omega-3 as a supplement did not reduce the risk of advanced AMD.
Laser photocoagulation is another treatment modality for Wet AMD. It is much less commonly used now with the advent of intravitreal therapy. Laser light is used to seal the leaking blood vessels. Although laser can be effective, many patients with Wet AMD are not suitable candidates as often the leakage is centrally located and laser is therefore not the treatment of choice. Laser may stabilise the vision but is unlikely to improve it.
2. Risk groups: People over 50 should have a regular eye exam (every 2 years or as directed by the doctor). If you have fair skin, light eyes, high blood pressure, or have been exposed to prolonged periods of sunlight, then you should also have regular check-ups.
A Fluorescein angiogram is a photographic dye test used to assess AMD. The patient is given an injection of yellow Fluorescein dye into a vein in the arm. The dye is followed with photographs as it goes through the retinal circulation and will show up changes due to AMD. An OCT is a special ultrasound that will
Treatment options for AMD
Currently, there are no medical treatments available to reverse Dry AMD, but research is advancing in this area to develop therapies. At this time treatment for Dry AMD is conservative- and includes dietary lutein supplements and assistance with stronger glasses and better lighting where necessary. An iPad or kindle can often make reading more enjoyable.
Supplementation and LifestyleDry AMD
Age-Related Disease Studies
(the National Eye Institute (NEI) of the US National Institutes of Health Age-related Eye Disease Study - AREDS1 and AREDS2) have shown that a combination of vitamins and antioxidants may help
An ophthalmologist will speak to their patient about what supplements may work best for them. They may also recommend a comprehensive dilated eye exam at least once a year. The exam will help determine if the condition is showing signs of progression to the wet form.
In addition, lifestyle changes may help slow the progression of AMD, such as stopping smoking, staying active and eating a healthy diet filled with green leafy vegetables and fish.
Wet AMD Treatment
Intravitreal therapy - Antivascular endothelial growth factor (anti-VEGF) drugs
The current standard treatment, Intravitreal injection therapy has proven to be a major advance for patients with Wet AMD. Without treatment these patients would face irreversible loss of central vision and legal blindness. When Wet AMD develops, abnormal blood vessels grow under the
Photodynamic Therapy (PDT)
PDT is another treatment option for Wet AMD. The patient is given an intravenous infusion of a light sensitive dye called verteporphyrin . When the dye accumulates in the abnormal blood vessel complex under the macula it is activated by a laser light resulting in closure of the blood vessel. PDT is less frequently used now following the advent of intravitreal therapy. PDT can stabilise the vision in certain circumstances but again is unlikely to improve vision.
How to Protect Eye Health & Vision Preservation
Regular eye examination with your ophthalmologist (eye doctor) or optometrists is recommended especially if there a family history of the condition. This should be done every two years for the over 50s and annually if there is any hint of AMD. It is important that patients self-monitor using an Amsler grid between visits.
3. Healthy diet: eat a diet low in saturated fats and rich in omega 3 fatty acids (oily fish and walnuts). Green, leafy vegetables (spinach and kale) contain antioxidants, eggs and yellow/orange-based fruit contain nutrients such as lutein, zeaxanthin and beta-carotene –all work to protect the macula.
4. Vitamin supplements: certain nutritional supplements containing vitamins C and E, zinc, copper, lutein and zeaxanthin can slow down the progression of AMD in people already showing signs of the condition.
5. Get moving and stop smoking: every week we should engage in 150 minutes of medium-level activity such as brisk walking, swimming and light jogging. Smoking poses a significant risk for AMD especially in women.
Further information resources:
• The Irish College of Ophthalmologists www. eyedoctors.ie
• The Macular Society www. macularsociety.org
• Retina International also has an international AMD toolkit on their website
75 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
Psoriasis
The Unspoken Barriers to Treatment for Chronic Plaque Psoriasis
1Department of Dermatology, Tallaght University Hospital, Dublin, Ireland.
Anna Wolinska
I can’t remember exactly when I first noticed the rash on my skin but I know it has been there for years. Within the Irish Traveller Community we have some cures but nothing could ever help me. I was eventually brought to the hospital when I was 15 years old and it was then I was told I had psoriasis. I had light treatment first which was good but it didn’t take long for my rash to come back again. I am now 28 years old. It has had a huge impact on me growing up. I couldn’t wear
the clothes that I liked or that my friends were wearing because my skin was so bad. If I tried to put on fake tan it would stick to my rash and everyone would look at me. It got to a point where I couldn’t go out. I stopped going to weddings because I couldn’t get dressed up. In the Traveller community, because I am not married, I am not allowed to go anywhere without someone from my family coming with me. It can be hard as all of my friends are married now and they can go out whenever they want.
NewsHSE introduces New Pilot
Marking International Overdose
Awareness Day 2023, the HSE in collaboration with St James’s Hospital is introducing NaloxHome: a new pilot initiative designed to save lives and promote harm reduction among people who use drugs.
Nalox-Home provides free naloxone kits to patients who attend the St James’s Hospital Emergency Department (ED) and have experienced or are at risk of an opioid overdose. The pilot is a collaborative effort between the St James’s Hospital Emergency Medicine Department and Inclusion
Health Service, and the HSE National Social Inclusion Office.
Nalox-Home includes a brief overdose awareness intervention delivered to patients at risk of opioid overdose, both upon discharge from the emergency department or after an inpatient admission. Central to the project is the prescription and provision of take-home naloxone, a medication that temporarily reverses the effects of opioids, providing a vital lifeline while awaiting medical assistance.
Speaking at an International Overdose Awareness Day webinar organised by the HSE, Professor
I went back to the hospital a few years ago and I was given an injection for my skin. My rash went away and my skin was perfect. I was so happy but then I couldn’t get anyone to go to the hospital with me and my parents wouldn’t let me go on my own. I missed a few appointments and I couldn’t get my treatment. It didn’t take long for my rash to come back again and it was worse than ever. I enjoyed going to the gym but when my skin got bad again I stopped and I put on weight. I didn’t go anywhere for a long time. Then my brother said he would bring me to my appointments. I felt scared and embarrassed explaining everything to the doctor in the hospital because it sounds silly to people who aren’t Travellers and I didn’t think they would understand. But they have been able to help me a lot - like when my pharmacy wouldn’t give me my injections the doctors were able to sort everything out for me or if I can’t get to an appointment I can call them instead. I hope that when my rash is gone I can start getting dressed up for weddings and things like that again. I hope I will meet someone and have a home of my own. I just want to finally have some independence.
Clinicians Comments
Research in dermatology that includes groups that have been historically marginalised is limited. The Traveller community represents 0.7% of the population in Ireland1. Previous studies have shown that this ethnic group has a significantly lower life expectancy than the general population, the reasons for which are multifactorial. Social inequalities are often stark compared with the rest of the population2,3. This patient’s viewpoint highlights the importance of recognising different cultural expectations and norms that can have an impact on a patient’s access to healthcare. As immigration rates continue to rise with significant population shifts in Europe over recent years4, we as dermatologists should be vigilant to ensure that we are well-informed and respectful of the social and cultural traditions of our patients. In doing so, we can foster a strong patientdoctor relationship with our most vulnerable patients and limit the potential for healthcare disparity across groups that have been historically marginalised.
References available on request
Eamon Keenan, HSE National Lead, Addiction Services, said, “With opioids implicated in 7 out of 10 poisoning deaths identified by the Health Research Board’s (HRB) latest figures in 2020, and 57.5 per cent of those individuals accompanied by someone who may administer naloxone, the availability of naloxone can be crucial while waiting for medical care to arrive.”
Jess Sears, Inclusion Health Clinical Nurse Specialist at St James’s Hospital added, “This pilot has the potential to improve the relationship between hospital staff and the patients we treat, which in

turn may result in them returning for follow up care and improving their overall health outcomes. We wouldn’t hesitate to provide an EpiPen to someone with a severe allergy or a glucagon injection to a diabetic at risk of severe hypoglycaemia. We should follow the same approach for opioid users by training their family and friends on how to identify an emergency and respond with Naloxone, a potentially lifesaving treatment.”
In 2020, Ireland experienced 409 poisoning deaths, a concerning trend that has been on the rise since 2016.
76 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
A.Wolinska1, M. Costa Blasco1, O. Mc Feely1, L. Murphy1, M. Andrawis1, P. Beatty1, C. Doyle1 and AM. Tobin1.
New Bereavement Room at Portiuncula University Hospital
Portiuncula University Hospital has officially opened a new designated room in the Maternity Unit for bereaved families. The Willow Suite has been designed to provide a protected and comfortable space for parents and their families who have experienced pregnancy or infant loss.
The Willow Suite will provide a ‘home from home’ space for parents and families who have experienced a pregnancy or infant loss and for those who require in-patient care. The suite was specifically designed to provide a private environment, within which, the individual needs of each bereaved parent will be respectfully and sensitively cared for.
Director of Midwifery at the hospital, Deirdre Naughton, explains the significance of the new development: “While the birth of a baby is normally a very joyful experience for women and their families, sadly, there are times when a baby dies before or shortly after birth.
“The loss of a baby is an incredibly traumatic event leaving parents with overwhelming emotional needs and is one of the most painful experiences that any parent has to face. It signifies the loss of the future, of hopes and dreams and affects the entire extended family.
“We hope this important new suite will give grieving parents a comfortable space to spend time with their baby and to create
At the official opening of the Willow Suite, a new designated room for bereaved families in the Maternity Unit at Portiuncula University Hospital, from left, Kathy Higgins, Pastoral Care; James Keane, Hospital Manager, Leanne Norton, Bereavement Support Midwife and Deirdre Naughton, Director of Midwifery

memories. It allows a family to grieve with dignity and respect to the personhood of their child,” she added.
The Willow Suite is a single occupancy room with en-suite shower and bathroom facilities, and includes an equipped kitchenette, Bluetooth speakers, oil diffusers and colourful and captivating artwork.
The suite overlooks the hospital gardens and in the coming months a small memory garden space will be completed which will incorporate a seating space around a willow tree to provide families a sanctuary surrounded by nature.
“The name ‘Willow’ was chosen in consultation with staff and women attending our service. Willow is a symbol of the endurance of love and family. It gives hope and a sense of belonging and safety, added Deidre.
Leanne Norton, Bereavement Support Midwife said, “We hope the room will offer some small comfort to parents and their families in times of grief and bereavement. The interior design was undertaken to ensure privacy and dignity and it will allow parents some quiet time to spend with their baby and loved ones, while still getting support and assistance from staff.
“In conjunction with the Willow Suite, we have also upgraded the Rose Room which is located beside the Willow Suite. The Rose Room offers a second space for families at a difficult time in their lives. Its soft furnishings, gentle
National Youth Mental Health Office
Minister for Mental Health and Older People Mary Butler has announced the launch of the new National Youth Mental Health Office in the Health Service Executive (HSE). The new National Office will focus on delivering strengthened and more integrated supports for child and youth mental health care across Ireland. This new HSE office will provide for coordinated input across service, legislative and policy developments for all child and youth mental area areas, from prevention and early intervention to the specialised Child and Adolescent Mental Health Service (CAMHS).
This is a very significant development, and it will improve leadership, operational oversight, and management of all service delivery and improvements. A new HSE National Clinical Lead for Youth Mental Health has already taken up post – Dr Amanda Burke - with a new Assistant National Director for Child and Youth Mental Health starting later this month. They will be supported by additional and dedicated staff for the National Office.
Other priorities for the new National Office will include improved links to other care programmes such as primary care and disability services, as well as external agencies across
the education, childcare and youth justice fields. It will also underpin current and planned initiatives arising from the recent Maskey and Mental Health Commission Reports on CAMHS as well as the forthcoming HSE audits on the current CAMHS Operational Guidelines and Service User experience. The Office will work closely with the Mental Health Commission.
A key focus of the new National Office for Youth Mental Health will be improved and centralised data collection and analysis to help inform new service responses for child and youth mental health.
but alluring artwork and inbuilt custom furniture is designed almost as a small living room and has been used by families as a space for older children and grandparents to wait and rest.”
James Keane, Hospital Manager said, “We are very grateful to the National Infants and Women’s Health programme for the funding to develop the Willow Suite. Person centred bereavement support is integral in the care we provide to parents and families bereaved by pregnancy or infant loss, and we are honoured to provide a dignified and compassionate space within our bereavement care.”
The government remains committed to developing all aspects of mental health, under our national mental health policies and the HSE Annual Service Plans. The overall budget for mental health has increased by nearly 20% during the term of this government to ¤1.2 billion this year. ¤137 million is being provided to CAMHS this year. In addition, more than ¤100 million has been provided to community-based mental health organisations/NGOs this year, with a significant proportion of this dedicated to supporting child and young people.
77 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
News
Epilepsy
New Insights into Epilepsy
Professor Gianpiero Cavalleri, Professor of Human Genetics at RCSI School of Pharmacy and Biomolecular Science and Deputy Director of the SFI FutureNeuro Research Centre

“This identification of epilepsyassociated genetic changes will allow us to improve diagnosis and classification of different epilepsy subtypes. This in turn will guide clinicians in selecting the most beneficial treatment strategies, minimising seizures,” said Professor Colin Doherty, Consultant Neurologist at St James’s Hospital, co-author and clinical investigator at the SFI FutureNeuro Centre.
Unprecedented collaboration
The largest genetic study of its kind, coordinated by the International League Against Epilepsy including scientists from FutureNeuro at RCSI University of Medicine and Health Sciences, has discovered specific changes in our DNA that increase the risk of developing epilepsy.
The research, published in Nature Genetics, greatly advances our knowledge of why epilepsy develops and may inform the development of new treatments for the condition.
Epilepsy, a common brain disorder of which there are many different types, is known to have genetic components and sometimes runs in families. In this new study, researchers compared the DNA from diverse groups of almost 30,000 people with epilepsy
to the DNA of 52,500 people without epilepsy. The differences highlighted areas of our DNA that might be involved in the development of the disorder.
The researchers identified 26 distinct areas in our DNA that appear to be linked to epilepsy. This included 19 that are specific to a particular form of epilepsy called Genetic Generalised Epilepsy (GGE). They were also able to point to 29 genes that are probably contributing to epilepsy within these DNA regions. The scientists found that the genetic picture was quite different when comparing distinct types of epilepsy – in particular, when ‘focal’ and ‘generalised’ epilepsies were compared. The results also suggested that proteins that carry electrical impulse across the gaps
between neurons in our brain make up some of the risk for generalised forms of epilepsy.
Quality of life
“Gaining a better understanding of the genetic underpinnings of epilepsy is key to developing new therapeutic options, and consequently a better quality of life for the over 50 million people globally living with epilepsy,” said Professor Gianpiero Cavalleri, Professor of Human Genetics at RCSI School of Pharmacy and Biomolecular Science and Deputy Director of the Opens in new windowSFI FutureNeuro Research Centre.
“The discoveries we report on here could only be achieved through international collaboration, on a global scale. We are proud of how the global community of scientists working to better understand the genetics of the epilepsies have pooled resources and collaborated effectively, for the benefit of people impacted by the condition.”
The researchers also showed that many of the current medications for epilepsy work by targeting the same epilepsy risk genes that were highlighted in this study. However, based on their data, the researchers were able to propose some potentially effective alternative drugs. These will need to be clinically tested for use in epilepsy as they are normally used for other conditions, but they are known to target some of the other epilepsy risk genes uncovered.
Over 150 researchers based across Europe, Australia, Asia, South America and North America carried out the research. They worked together as part of the International League Against Epilepsy (ILAE) Consortium on Complex Epilepsies. The ILAE Consortium was formed by researchers in 2010, recognising that the complexity of genetic and environmental factors underlying epilepsy would require research across massive datasets, and therefore unprecedented collaboration on an international scale.
“Undertaking such a comprehensive study is a remarkable achievement that RCSI and FutureNeuro are proud to have played a leading role in. The challenge now is to translate the findings of this research to improve the lives of people with epilepsy,” concluded Professor Cavalleri.
“With this study, we have bookmarked parts of our genome that should be the major focus of future epilepsy research. It will form the basis for further work looking at the molecular pathways involved in seizure generation, neuronal dysfunction and altered brain activity,” said Professor Samuel Berkovic, University of Melbourne.
“This is a major milestone for the ILAE Consortium on Complex Epilepsies, demonstrating what can be achieved when scientists openly collaborate and share data from across the world. The outputs are wide-reaching and applicable to epilepsy patients globally,” said Professor Helen Cross, President of the International League Against Epilepsy.
Science Foundation Ireland (SFI) supported the work through its funding of the FutureNeuro Research Centre.
78 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
“This is a major milestone for the ILAE Consortium on Complex Epilepsies, demonstrating what can be achieved when scientists openly collaborate and share data from across the world.
The outputs are wide-reaching and applicable to epilepsy patients globally”
DELL TECHNOLOGIES AND UNIVERSITY OF LIMERICK JOIN FORCES USING AI TO ADVANCE PREDICTIVE AND DIAGNOSTIC CANCER RESEARCH
Dell Technologies (NYSE:DELL) has joined forces with the University of Limerick’s Digital Cancer Research Centre to develop an artificial intelligence (AI) platform and digital twin technology to power predictive and diagnostic research in oncology.
The technology can help clinicians provide more effective cancer care for those with B-cell lymphoma from speeding diagnosis to providing enhanced treatment and improving long-term outcomes for patients in Ireland and worldwide.
Dell created an AI platform for the University that delivers high performance computing power to accelerate oncology and precision medicine research. The platform sits within the Digital Cancer Centre’s multicloud ecosystem. It is powered by Dell’s latest storage arrays and Dell PowerEdge servers optimised for AI to produce cancer patient digital twins for better diagnostics.
With the new AI platform, researchers can:
- Rapidly accelerate biomarker testing for cancer,
- Gain a better understanding of how to treat patients with B-cell lymphoma, and
- Develop personalised therapies based on a person’s tumour characteristics.
By using emerging technologies, researchers at the Digital Pathology Unit at the University of Limerick’s Digital Cancer Research Centre can also better understand the pathogenesis of these malignancies and develop novel therapeutic approaches.
The researchers are particularly interested in the possibility that collagen within the tumour ‘microenvironment’ can cause the cancerous cells to spread around the body and to the central nervous system. Having already identified novel ways to block collagen, the research could lead to new treatments to cure patients before the tumour spreads.
Professor of Molecular Pathology at University of Limerick and Director of the Digital Pathology Unit at the Digital Cancer Research Centre Paul Murray said: “Through our partnership with the Dell Technologies team, we will be able to advance our knowledge of how cells go wrong during cancer development and find new ways to diagnose and treat cancer patients. This is the beginning of a very exciting research project for
the team here at the University of Limerick’s Digital Cancer Research Centre, and we’re looking forward to accelerating this project with the digital support and insights from the team at Dell Technologies.”
EUROPEAN COMMISSION APPROVES TALVEY® (TALQUETAMAB), JANSSEN’S NOVEL BISPECIFIC THERAPY FOR THE TREATMENT OF PATIENTS WITH RELAPSED AND REFRACTORY MULTIPLE MYELOMA
The Janssen Pharmaceutical Companies of Johnson & Johnson, has announced that the European Commission (EC) has granted conditional marketing authorisation (CMA) of TALVEY® (talquetamab) as monotherapy for the treatment of adult patients with relapsed and refractory multiple myeloma (RRMM) who have received at least three prior therapies, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody and have demonstrated disease progression on the last therapy.1
Talquetamab is a bispecific T-cell engaging antibody that binds to CD3, on the surface of T-cells, and G protein-coupled receptor class C group 5 member D (GPRC5D), a novel target expressed on the surface of multiple myeloma cells and hard keratinised tissues, with minimal to no expression detected on B-cells and B-cell precursors.1 Talquetamab is approved as a weekly (QW) or biweekly (Q2W) subcutaneous (SC) injection, after an initial step-up phase.1
Dr Patrick Hayden, Consultant Haematologist and clinical lead for the myeloma service, at St. James’s Hospital said: “Patients with relapsed and refractory multiple myeloma typically experience frequent changes of treatment, short remissions and poor outcomes.3 Every patient’s disease is unique and requires a treatment strategy tailored to their specific needs. The approval of talquetamab represents an important advance for patients living with this difficult-to-treat blood cancer, as this novel compound has a new cellular target called GPRC5D. This approach has been shown to deliver deep responses in patients with advanced myeloma, many of whom were heavily pre-treated.”
The CMA was supported by positive results from the Phase 1/2 MonumenTAL-1 study (Phase 1: NCT03399799; Phase 2: NCT04634552), evaluating the safety and efficacy of talquetamab in patients with RRMM.4,5 The latest data from the study were recently presented at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting
(2-6 June, Chicago) and the 2023 European Haematology Association (EHA) Congress (8-11 June, Frankfurt).
Dr Thorsten Giesecke, General Manager, Commercial Business, Janssen Sciences Ireland UC, said: “Talquetamab’s approval reinforces our focus on strengthening our multiple myeloma offering by investing in cutting-edge research that will help us continue to improve patient outcomes and importantly, their quality of life. Once available in Ireland, Talquetamab will expand the range of therapeutic options available to clinicians when treating patients with advanced multiple myeloma, offering them flexibility for optimising treatment protocols. Janssen’s legacy in innovation and the development of treatments for multiple myeloma spans more than two decades and we are committed to delivering therapeutics which have the potential to transform clinical outcomes for Irish patients.”
Patients in the study (0.8 mg/ kg Q2W: n=145; 0.4 mg/kg QW: n=143) had received a median of five (range, 2-17) prior lines of therapy and showed meaningful overall response rates (ORR) across both doses. With a median follow-up of 12.7 months, 71.7 percent (95 percent Confidence Interval [CI], 63.7-78.9) of response-evaluable patients treated at the 0.8 mg/kg Q2W dose achieved a response, 60.8 percent achieved a very good partial response (VGPR) or better and 38.7 percent achieved a complete response (CR) or better.1 With a median follow-up of 18.8 months, 74.1 percent (95 percent CI, 66.1-81.1) of response-evaluable patients treated with the 0.4 mg/ kg QW dose achieved a response, 59.5 percent achieved a VGPR or better and 33.6 percent achieved a CR or better.1 Responses were durable with a median duration of response not reached (95 percent CI, 13-Not Estimable [NE]) in the 0.8 mg/kg Q2W dose group and 9.5 months (95 percent CI, 6.713.3) in the 0.4 mg/kg QW dose group.1 An estimated 76.3 percent and 51.5 percent of patients maintained a response for at least nine months at the 0.8 mg/kg Q2W and 0.4 mg/kg QW doses, respectively.
1
The MonumenTAL-1 study also included 51 patients with prior T-cell redirection therapy.2 Patients had received a median of five (315) prior lines of therapy, including prior exposure to a bispecific antibody (35.3 percent), CAR-T cell therapy (70.6 percent) or both (six percent).2 With a median duration of follow-up of 14.8 months, 64.7 percent of patients achieved a response, 54.9 percent achieved a
VGPR or better and 35.3 percent achieved a CR or better.2 Median duration of response was 11.9 months (95 percent CI, 4.8-NE) and the 12-month overall survival rate was 62.9 percent.2
The most common adverse events (AEs) observed in the study were cytokine release syndrome (CRS; 77 percent, 1.5 percent Grade 3 or 4), dysgeusia (72 percent, all Grade 1 or 2), hypogammaglobulinaemia (67 percent, all Grade 1 or 2) and nail disorders (56 percent, all Grade 1 or 2).1 In addition, 40 percent of patients experienced weight loss, including 3.2 percent with Grade 3 or 4 weight loss.1 The most common infections were upper respiratory tract infection (29 percent, 2.1 percent Grade 3 or 4) and COVID-19 (19 percent, 2.9 percent Grade 3 or 4).1 Neurologic toxicities were reported in 29 percent of patients, including immune effector cell-associated neurotoxicity syndrome (ICANS; 10 percent, 2.3 percent Grade 3 or 4).1 Adverse reactions leading to treatment discontinuation were mainly due to ICANS (1.1 percent) and weight loss (0.9 percent).1
The EC approval follows the U.S. Food and Drug Administration (FDA) approval of talquetamab for the treatment of adult patients with relapsed or refractory multiple myeloma who received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody, in August 2023.
RCSI MYHEALTH SERIES
RCSI University of Medicine and Health Sciences has launched the 2023/24 programme for RCSI MyHealth, a video and podcast series that brings reliable and trustworthy healthcare information to the public.
The first three episodes in the new series will look at giving children the emotional tools to have a positive return to school, treatment and prevention of stroke, and antibiotic-resistant ‘superbugs’. As the series progresses in 2024, topics will include nutrition, heart health and dementia.
The upcoming episodes of the new 2023/24 RCSI MyHealth series are: · 24 October – Stroke: Prevention and new developments in treatments and rehabilitation· 14 November – Antibiotic resistant ‘superbugs’: Can they be stopped?
RCSI University of Medicine and Health Sciences is ranked first in the world for its contribution to UN Sustainable Development Goal 3, Good Health and Well-being, in the Times Higher Education (THE) University Impact Rankings 2023.
79 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023 Clinical R&D
Clinical R&D
¤25 MILLION INVESTMENT IN A NEW DIGITAL TRANSFORMATION PROGRAMME
Blackrock Health Group, which comprises of the Blackrock, Galway, Hermitage and Limerick Clinics, has announced a ¤25 million investment in a new digital transformation programme. The investment includes the introduction of the very latest in electronic health record systems, MEDITECH Expanse, and the introduction of Ireland’s first integrated digital engagement platform that will enable connectivity between patients, consultants and general practitioners (GPs).
These innovations will serve to connect patient data for the 325,000 patients it treats annually across its four clinics, enhance clinical care, improve patient safety all while reducing the administration burden and freeing up more time for clinicians to focus on patient care.
The digital transformation project will take three years to implement and will create 60 new jobs across the Blackrock Health group as a direct result of the investment. The partnership builds on the successful rollout of MEDITECH's electronic health record in Galway Clinic in 2017, the first of its kind in Ireland.
The MEDITECH Expanse electronic health record system will help to guide clinical decision-making by offering a comprehensive view of a patient’s health history and will allow patients to attend any of the hospitals within the group with seamless access to their records. The system will also ensure that key information is available to their GP for any follow-up. Using the location agnostic solution, physicians, nurses, and other clinicians at Blackrock Health can use mobile devices to view patient records, place orders, and document care at any time.
CEO of Blackrock Health, Caroline Whelan said: “Improving patient care is always at the heart of the decisions that we make at Blackrock Health and that is why we are investing ¤25m to digitally transform the way we work across the Group, which in turn will enhance clinical care and patient safety for the 325,000 patients we treat annually. We are excited to embark on this three-year journey alongside our partners at MEDITECH which will enable staff across our four clinics to work in close collaboration and our patients to access one single health record, at any of our clinics.”
Mike McCann, Chief Information Officer, Blackrock Health said: “We introduced electronic health records at the Galway Clinic in 2017 and since then have seen great improvements in the way that we work day-to-day. Based on these results, the natural next step was to implement a group-wide rollout, as we continue to look at ways to integrate technology into healthcare. We look forward to bringing this technology to patients and staff.”
Gina Kerley, Executive Director, MEDITECH said: “For many years, the Galway Clinic has been a leader among Ireland’s healthcare organisations by leveraging technology to improve patient care. We are excited to build on this success and partner with Blackrock Health to provide all four clinics with the innumerable benefits of having a modern electronic health record.”
ACCORD HEALTHCARE
IRELAND LAUNCH ORGOVYX® (RELUGOLIX) 120 MG THE FIRST AND ONLY ORAL ANDROGEN DEPRIVATION THERAPY FOR PROSTATE CANCER

Accord Healthcare, one of Europe’s fastest growing pharmaceutical companies, is delighted to announce the launch of its latest oncology treatment, ORGOVYX® (relugolix) 120 mg which comes in pack size of 30 film-coated tablets. Relugolix is the first and only oral androgen deprivation therapy (ADT) for advanced hormone-sensitive prostate cancer. 1 in 3 injectable oncology medicines dispensed in Europe are from Accord.1
“Roughly 4,000 men are diagnosed with prostate cancer each year in
Ireland, which means 1 in 7 men will be diagnosed with prostate cancer during their lifetime.2 I am therefore delighted to announce our launch of Orgovyx® onto the Irish market, our 26th High Tech product. We are immensely proud of our ongoing investment in oncology and our drive to shape the future of cancer treatment”, said Padraic O’Brien, MD, Accord Healthcare Ireland.
“Our latest exciting partnership with Myovant, with ORGOVYX®, will enable us to bring a new ADT option to people in Ireland living with prostate cancer. Its oral formulation addresses some of the challenges with current injectables in terms of their in-hospital administration and risk of injection site reactions. We see the potential convenience of relugolix as the first needle-free ADT that can be taken at home, reducing the need for hospital visits which could in turn reduce the burden on both patients and healthcare providers” continued Mr O’Brien.
Relugolix was granted Marketing Authorisation by the European Medicines Agency on 29 April 2022.3 In May 2022, Accord was selected by Myovant Sciences to commercialise this product across Europe (European Economic Area, United Kingdom, Switzerland and Turkey).3
Please refer to the Summary of Product Characteristics (SPC) for further information. The SPC will be available at www.hpra.ie and for Healthcare Professionals at www.accord-healthcare.ie.
Orgovyx® will be available from both full-line wholesalers from launch. For further information contact Accord in Cork on 021-461 9040.
SAFER AND MORE EFFECTIVE BRAIN SURGERIES
After a surgical first, a Swiss company is getting closer to treating severe disorders with its unique electrode grid designed as a safe alternative to existing brain implants.
Under the guidance of neurosurgeon Prof. Nitin Tandon, a clinical study conducted at Memorial Hermann in Houston has marked the inaugural testing of Neurosoft Bioelectronics’ subdural electrodes as they were used for recording the human brain in the context of an epilepsy resective surgery. Two epileptic patients and one brain tumour patient have undergone a successful surgery, while detailed recordings were obtained in parallel from the surface of the brain. These results are very promising as the unique properties of these electrodes could allow for brain insertions in areas where no other implant can safely be inserted today, opening the door to treating indications such as severe tinnitus with no existing cure or validated treatment.
With the density of the electrode sites being 5 times higher and the entire device being 1000 times softer than standard electrodes, the usage of this new technology also holds the potential to address key challenges associated with invasive procedures, notably mitigating side effects such as brain compression, risks of hematomas, as well as scar tissue.
Specifically for the first resective procedure, a sequence of sentences were echoed across the operating room, with the objective of capturing distinct brain activity within the auditory cortex. In addition, specific brain patterns recognized as interictal spikes were recorded within the hippocampus. These atypical neurological discharges also hold tremendous potential as they can help in localising regions of the brain implicated in epilepsy, improving the potential effectiveness of an epilepsy resection as a result.
Furthermore, the application of Neurosoft Bioelectronics’ electrodes presents the possibility of uncovering supplementary biomarkers which could significantly help in providing more effective and safer treatments of brain disorders for future patients. Invasive solutions presently employed for treating brain-related conditions pose substantial risks due to electrodes’ stiffness and thickness. Amidst these challenges, Neurosoft Bioelectronics' soft and elastic electrodes present themselves as
80 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
a promising alternative to existing invasive solutions. The Neurosoft Bioelectronics team has also been studying how these electrodes could address indications like severe tinnitus.
Despite approximately 50 million patients worldwide and an estimated 3.5 million related suicide attempts, there is no effective treatment for severe tinnitus.
"The maturity of our technology has been underscored through its initial human testing. With each subsequent trial, we edge closer to obtaining FDA clearance for some of our initial indications, thereby progressing towards potentially aiding millions of individuals," says
Nicolas Vachicouras, CEO and Co-Founder of Neurosoft Bioelectronics.
Specifically for these first human interventions, “This milestone represents a great de-risking step for the company and is extremely important in bringing the concept of soft brain electrodes from academic laboratories to clinical applications,” says Prof. Lacour, Co-Founder of Neurosoft Bioelectronics and Director of the Neuro-X Institute at EPFL (Swiss Federal Institute of Technology in Lausanne). The study, led by Prof. Nitin Tandon, will continue to move forward in the coming months with additional electrode usage in up to 10 patients, potentially providing additional insights into potential applications of the technology.
"The preliminary findings of our study provide compelling validation for the trajectory of our technology, poised to enhance the safety and efficacy of brain surgery, while validating the ability to record specific information within the auditory cortex. Moving forward, we plan to widen our testing parameters and anchor our findings in a more comprehensive pool of data. Based on the first insights on the usability of our technology, applications for a wide range of treatments can be considered for development, among them tinnitus, deafness, traumatic brain injury and paralysis. In that regard, several other research projects leveraging our electrodes are currently ongoing both within our company, but also with partners at renowned Research Labs across the world," says Nicolas Vachicouras.
VHI MAKES DERMATOLOGY SERVICES AVAILABLE TO CUSTOMERS IN PARTNERSHIP WITH ALLVIEW HEALTHCARE
Vhi has announced the launch of a new rapid access Dermatology service for the diagnosis and treatment of most dermatological conditions on an outpatient care basis.

Delivered in partnership with AllView Healthcare, Vhi’s Dermatology service provides end-to-end care for the diagnosis and treatment of medical dermatological conditions including common skin conditions such as acne, lesions, sun damage and melanoma - the most serious skin cancer.
With lengthy wait times for accessing dermatology services, Vhi’s new Dermatology service offers one of the fastest routes to medical dermatology treatment nationwide, with appointments available within ten days for Vhi members. Currently there are more 45,000 people awaiting a dermatology appointment in Ireland with 43% waiting for 6 months or more.1
By leveraging cutting-edge telehealth and digital technologies, Vhi Dermatology offers a new path to help meet the demand to treat serious skin conditions. Currently every year in Ireland, over 1,000 new cases of melanoma are diagnosed with non-melanoma skin cancer the most common cancer in Ireland. By 2045, it is projected that the number of cases of melanoma diagnosed among males will increase by 177% and among females will increase by 140%.2
New dermatology research commissioned by Vhi shows that 1 in 4 respondents are currently
concerned about the impact of sun damage on their skin. Serious skin conditions also appear to be having a negative impact on people’s health and wellbeing. Close to half (47%) of those surveyed reported that they suffered stress and anxiety as a direct result of having a skin condition.
Dr Rupert Barry, Consultant Dermatologist and Clinical Lead for Vhi Dermatology Services said, “Over recent years, there has been a growing incidence of skin cancer and other skin conditions with an increasing number of patients requiring end-to-end care from diagnosis to surgical excision. This trend is only set to accelerate. That is resulting in considerable wait times for patients looking to access dermatology services in Ireland with 1 in 4 having to wait a year or more for treatment.
“The launch of the Vhi Dermatology service will help give patients access to the care they need, when they need it. By cutting lengthy waiting times, we can rapidly diagnose patients with skin cancer who may be currently waiting for an appointment with a dermatologist and treat patients with skin conditions as quickly as possible.
“Tackling long wait lists and providing timely access to care will be pivotal to ensure the long-term health and wellbeing
of the population. By providing appointments to patients within days our service provides a faster and more effective approach to the management of skin conditions.”
Teledermatology appointments are available for Vhi members nationwide at AllView Healthcare Clinics and use specialist imaging equipment to record a series of high definition microscopic and macroscopic images of the skin complaint.
Following an initial appointment, AllView’s network of dermatology consultants deliver a diagnosis and treatment plan to manage conditions quickly and effectively. If surgery, biopsy or a face-toface appointment is subsequently required, the AllView Healthcare GP will explain this to the patient and give them the fast access option of attending the Vhi Dermatology Clinic at Vhi 360 Health Centre, in Carrickmines, County Dublin.
The Vhi Dermatology Service is one of a number of new services brought by Vhi to its members in recent times as it continues to innovate in the delivery of healthcare – specifically through the introduction of new facilities and services such as Vhi Women’s Health Clinic, Vhi Paediatric Clinic, Vhi Sports, Exercise and Musculoskeletal Medicine Clinic in Vhi 360 Health Centres, digital and
81 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
At the launch of the new dermatology service at the Vhi 360 Health Centre in Carrickmines were (from left): Michele Tait, chief operating officer, Vhi Health and Wellbeing; Dr Rupert Barry, consultant dermatologist and clinical lead for dermatology, Vhi 360 Health Centre Carrickmines; Edina O’Driscoll, head of operations, Vhi 360 Health Centre Carrickmines; and Eoin O’Reilly, chief executive, AllView Healthcare. Photograph: Leon Farrell / Photocall Ireland
Clinical R&D
tele-health services, Vhi Hospital@ Home, Vhi Health Screening, Vhi Midwife Support Service and Vhi’s National Clinical Call Centre.
For more information on Vhi’s Dermatology services, please visit: https://www1.vhi.ie/360health/ dermatology
XWAVE TECHNOLOGIES AND QURE.AI ANNOUNCE STRATEGIC COLLABORATION
Qure.ai, a leading provider of medical artificial intelligence (AI) solutions and xWave Technologies, which has developed a cloudbased Clinical Decision Support (CDS) system for radiology referrals, have today announced a strategic partnership.
The collaboration will focus on enhancing market access, product co-development and integration in order to identify opportunities to deliver advanced healthcare solutions globally that address critical healthcare challenges.
Darren Stephens, Senior VicePresident & Commercial Head UK and Europe, Qure.ai, said, “Together, Qure and xWave can create patient-centric efficiencies in the radiology pathway. By dovetailing clinical decision support with AI in imaging, clinicians will be further supported. Patients will gain quicker results and imaging departments will see reduced demand for unnecessary diagnostic appointments.
This will help to slow the growth of waiting lists, particularly in the NHS. Ordering the right test, first time, along with the efficiency gains of AI, will provide hospitals with powerful support when looking ahead to winter healthcare pressures.”
xWave Technologies, an Irish health technology company, is headquartered at NovaUCD in Dublin.
Mitchell O’Gorman, Chief Executive Officer, xWave Technologies, said, “When we started xWave in 2020, one of the first organisations that we reached out to was Qure.ai. We were struck by the brilliance of its AI technology, the impact it was already delivering across the world and the clear fit between its offering and ours.
The xWave team is now looking forward to working closely with Qure.ai to co-develop innovative diagnostic solutions that aim to solve global health problems at scale.”
Qure.ai, headquartered in India with offices in London and New York, is one of the world’s most deployed medical AI companies, present in over 70 countries having grown in scale and size over seven years. Its certified medical imaging AI solutions for neurocritical trauma or stroke CTs; musculoskeletal x-ray fracture detection; and chest x-ray/CT for detection of lung
cancer, TB or heart failure are used in collaboration with over 25 NHS Trusts and Health Boards in the UK.
xWave’s Clinical Decision Support (CDS) system has been developed in partnership with the European Society of Radiology (iGuide) and the Royal College of Radiologists (iRefer). The CDS system expertly guides clinical referrers to order the best test for patients, ensuring patients get an early diagnosis, tackles radiology waiting lists and reduces clinician burnout.
JANSSEN MARKS FIRST APPROVAL WORLDWIDE FOR AKEEGA® (NIRAPARIB AND ABIRATERONE ACETATE DUAL ACTION TABLET) WITH EC AUTHORISATION FOR THE TREATMENT OF PATIENTS WITH METASTATIC CASTRATION RESISTANT PROSTATE CANCER WITH BRCA1/2 MUTATIONS
The Janssen Pharmaceutical Companies of Johnson & Johnson have announced that the European Commission (EC) has granted marketing authorisation for AKEEGA® (niraparib and abiraterone acetate [AA]), in the form of a dual action tablet (DAT), given with prednisone or prednisolone, for the treatment of adults with metastatic castrationresistant prostate cancer (mCRPC) and BRCA1/2 mutations (germline and/or somatic) in whom chemotherapy is not clinically indicated.2
Prostate cancer is one of the most common cancers in men in Ireland with about 3,940 men diagnosed each year.4 Despite treatment advances, mCRPC remains an incurable, deadly disease.

5,6 BRCA1/2 gene mutations have been identified in approximately 10-15 percent of mCRPC patients7,8 and are more likely to cause aggressive disease, poor outcomes, and a shorter survival time.
9,10,11,12
Professor Ray McDermott, Consultant Medical Oncologist, St Vincent’s University Hospital, Dublin said, “Metastatic castrationresistant prostate cancer remains a lethal disease, with high unmet needs in terms of treatment options, particularly for patients with BRCA1/2 gene mutations. The development of innovative therapies is vital to provide patients with new treatment options. The dual action tablet of niraparib with abiraterone acetate is a promising first line targeted treatment option for men with mCRPC and BRCA1/2 mutations. With BRCA testing now publicly available in Ireland, I hope this medicine will reach the right patients at the right time when available here.”
The EC authorisation, which also marks the first worldwide approval for AKEEGA®, is based on results of the randomised, double-blind, placebo controlled, Phase 3 MAGNITUDE study (NCT03748641).13 The trial assessed whether the addition of niraparib to AAP improved outcomes in those with untreated mCRPC, with or without alterations in homologous recombination repair (HRR) associated genes (which are involved in the repair of damaged DNA), including BRCA1/2.1,13 A total of 423 patients with HRR gene alterations were enrolled, 225 (53.2 percent) of whom had BRCA mutations, making it the largest cohort of BRCA1/2-positive patients with first line mCRPC in any clinical study to date.14
Dr Thorsten Giesecke, General Manager, Commercial Business, Janssen Sciences Ireland UC, said: “Janssen’s legacy in innovation and the development of treatments for prostate cancer spans more than a decade. We remain committed to harnessing the power of science to bring solutions to patients at every stage of their cancer journey. The MAGNITUDE trial was prospectively designed as a precision medicine study to identify the specific population of patients who would most benefit from niraparib with AAP, and potentially increase the likelihood of treatment success. This
82 OCTOBER 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Pictured (l-r) at NovaUCD in Dublin are, Darren Stephens, Senior Vice-President and Commercial Head UK and Europe, Qure.ai and Mitchell O’Gorman, CEO, xWave Technologies. Credit: Paul Sharp, SHARPPIX
approval marks an exciting step in the right direction, changing the outlook for patients with mCRPC for the better.”
The primary endpoint for MAGNITUDE was radiographic progression-free survival (rPFS), as analysed by blinded central review.1,13 Niraparib plus AAP significantly improved rPFS in all HRR-positive patients (Hazard Ratio [HR] 0.73; 95 percent Confidence Interval [CI], 0.56 to 0.96; p=0.022).3 This improvement was most pronounced in patients with BRCA1/2 gene mutations, where a statistically significant 47 percent risk reduction was observed for rPFS.3 With additional median follow-up at 24.8 months in the BRCA subgroup, rPFS by central review demonstrated a consistent and clinically meaningful treatment effect favouring niraparib plus AAP, with a median rPFS of 19.5 months compared with 10.9 months for placebo plus AAP.1 Additionally, there was a trend towards improved overall survival (OS) with niraparib plus AAP, strong improvement in time to symptomatic progression (TSP) and clinically meaningful improvement in time-to-initiation of cytotoxic chemotherapy (TCC).1
The observed safety profile of the combination of niraparib and AAP was consistent with the known safety profile of each agent.3 Of the patients with HRR gene alterations, 67 percent experienced Grade 3/4 adverse events (AEs) in the combination arm versus 46.4 percent in the control arm.15 The most common grade 3 AEs were anaemia (28.3 percent versus 7.6 percent) and hypertension (14.6 percent versus 12.3 percent) with niraparib and AAP versus placebo and AAP, respectively.15The combination of niraparib and AAP also maintained overall quality of life in comparison with placebo and AAP.3,15
Niraparib is a highly selective poly adenosine diphosphateribose polymerase (PARP) inhibitor.13 Together with AA plus prednisone, the combination DAT regimen targets two oncogenic drivers in patients with mCRPC, namely alterations in the androgen receptor axis and in BRCA1/2.13,16,17 This is the first DAT formulation available in the European Union for patients with mCRPC with BRCA mutations.2
Europe is the first region to approve AKEEGA® (niraparib and abiraterone acetate DAT), for the treatment of patients with BRCA-positive mCRPC, globally.
In February 2023, Janssen submitted a New Drug Application
(NDA) to the U.S. Food and Drug Administration (FDA) seeking approval of this niraparib-based combination for the treatment of patients with BRCA-positive mCRPC.18
ABBVIE CELEBRATE EIGHTH COMMUNITY INITIATIVE
AbbVie, a global biopharmaceutical company which employs approximately 80 people at its company offices in southwest Dublin, celebrated its eighth annual community volunteering initiative recently.
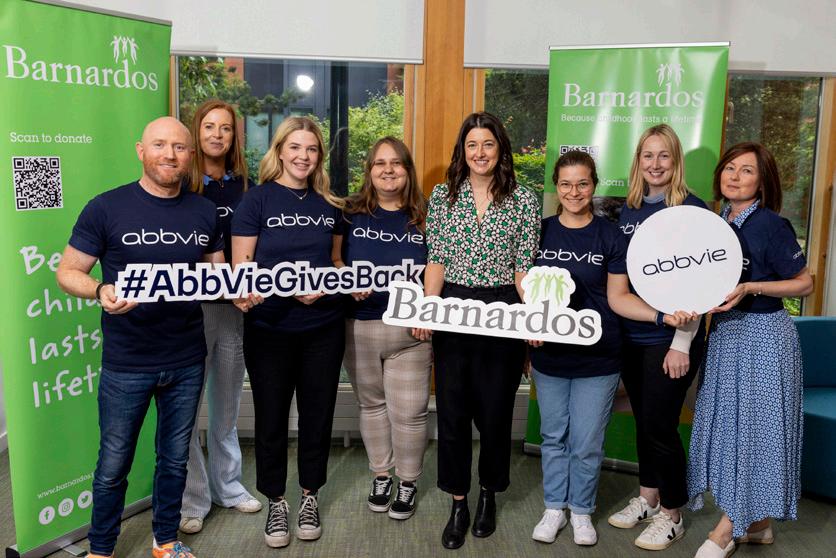
The day-long effort in support of children’s charity Barnardos involved company employees creating 300 activity packs for children going from Early Years Services (aged four-to-five years old) to ‘Big’ School and two different Wellbeing Packs for older children aged eight-to-eleven.
The AbbVie employee activity forms part of the company’s signature Week of Possibilities community volunteering programme. It seeks to empower employees to volunteer whilst at work and personally undertake activities that improve the communities in which AbbVie people work and live.
Barnardos will make 150 Early Years Packs available to children aged four to five who are about to go to school for the first time. Included are pencils, a copybook, eraser, and a whiteboard with markers. Also included are fun items such as stampers, stickers, colouring sheets and additional arts and crafts supplies.
Grow Your Own House
A special feature of the packs is an environmental and sciencefocused activity that allows children to grow their own ‘Sprout House’. AbbVie volunteers cut colourful sponges into size for children to assemble and grow cress from seed. Instructions on how to build their house and grow seeds are also included, alongside helpful pictures.
Two different Wellbeing Packs were assembled for older children accessing services supported by Barnardos. These contain books about how to manage their feelings, educational jigsaws and much, much more. The biopharmaceutical company organised the project in partnership with Volunteer Ireland. The packs will be distributed to Barnardos Services both local to AbbVie’s office in Dublin and across Ireland.
AbbVie is committed to leveraging its science and business expertise to positively influence education in the communities where it is based. All around Ireland, and across the world, it actively engages with schools, colleges, industry, and charity partners to achieve this ambition.
AbbVie believes in making a strong commitment to local communities. Commenting on the project, AbbVie’s General Manager Andres Rodrigo said:
“We are an Irish organisation that works closely with doctors to harness the power of innovation
and science to improve people’s lives. We do that through the provision of medicines that improve outcomes and advance standards of care. Barnardos is a particularly appropriate community partner for AbbVie given the charity was founded by a Dublin-born doctor. The work they undertake on behalf of disadvantaged children in Ireland is outstanding.”
Barnardos CEO Suzanne Connolly said: “Barnardos is delighted to have the support of AbbVie and employee volunteers in creating the Early Years and Wellbeing Packs. These packs will support Barnardos’ programmes which focus on the importance of children’s heart, body and mind, through an holistic approach to education and wellbeing. A huge thank you to the AbbVie team for this donation.”
The event was coordinated by Volunteer Ireland, whose CEO Nina Arwitz joined with AbbVie employees as they created the packs. She said: “The Week of Possibilities demonstrates what’s possible when corporate volunteers come together to work on projects which provide real value in the community. We are delighted to continue our long running partnership with AbbVie supporting their employees to volunteer and make a difference in their communities.”
83 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2023
AbbVie volunteers are pictured with Halina McNabb during the company’s Week of Possibilities initiative

HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication Our annual listing celebrating innovation and excellence in healthcare is coming soon. PROFESSIONAL 100 PROFESSIONAL 100 To request an entry or nomination form please contact: sarah@hospitalprofessionalnews.ie 2 0 2 3 NO M I N A T I O N S NO W O P E N PROFESSIONAL 100 To request an entry or nomination form please contact: kelly-jo@ipn.ie or danielle@hospitalprofessionalnews.ie











































































































 Written by Dr Genevieve Ferraris, GP and Menopause Specialist, The Menopause Hub
Written by Dr Genevieve Ferraris, GP and Menopause Specialist, The Menopause Hub








 Written by Sarah Belton, Advanced Nurse Practitioner in Gynaecology Oncology at The National Maternity Hospital
Written by Sarah Belton, Advanced Nurse Practitioner in Gynaecology Oncology at The National Maternity Hospital




























































































 Written by P. Fitzpatricka b, N. Bhardwajb,
Written by P. Fitzpatricka b, N. Bhardwajb,