

WELCOME & INTRODUCTION
S. Vincent Rajkumar, MD
Mayo Clinic, Rochester


imwg.org
imwg.net
imwg.info
imwg.online
imwg.ai





imwg.org
imwg.net
imwg.info
imwg.online
imwg.ai



• Bone Disease Committee (Terpos/Hillengass)
• Immunotherapy Committee (Martin/Lin)
• Mass Spectrometry Committee (Murray)
• SMM Committee (Mateos/Kumar)
• MRD Committee (Paiva/Munshi)
• QOL/PRO Committee (Zweegman/Sidana)










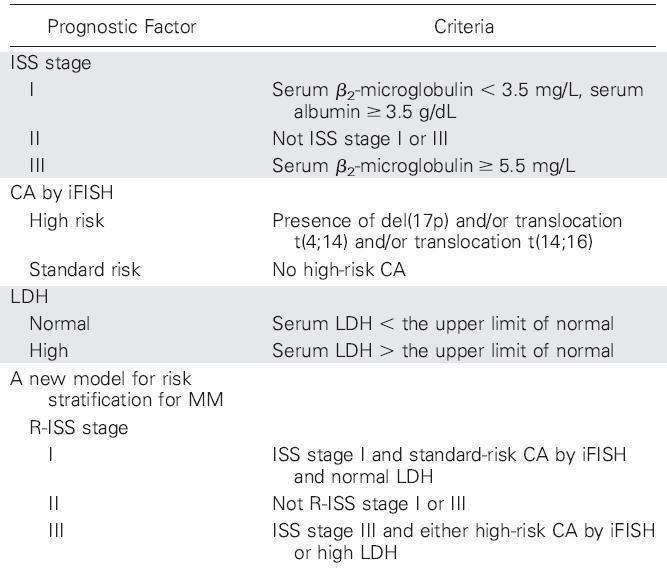
IMWG Guidelines for Newly Diagnosed Myeloma
IMWG Consensus on Solitary Plasmacytoma
IMWG Guidelines for Smoldering Myeloma
IMWG Recommendations for Dose and Schedule of Myeloma
Drugs and Regimens

Immunotherapy




Session Chair: S. Vincent Rajkumar, MD Mayo Clinic, Rochester

Francesco Maura, MD
Sloan Kettering Cancer Center
Francesco Maura, M.D. Memorial Sloan Kettering Cancer Center, NY Twitter: @FrancescoMaura4 email: mauraf@mskcc.org

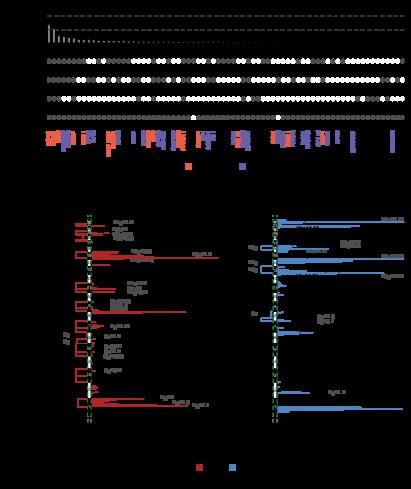
~2000 patients with WES/targeted sequencing data

>150 genomic drivers



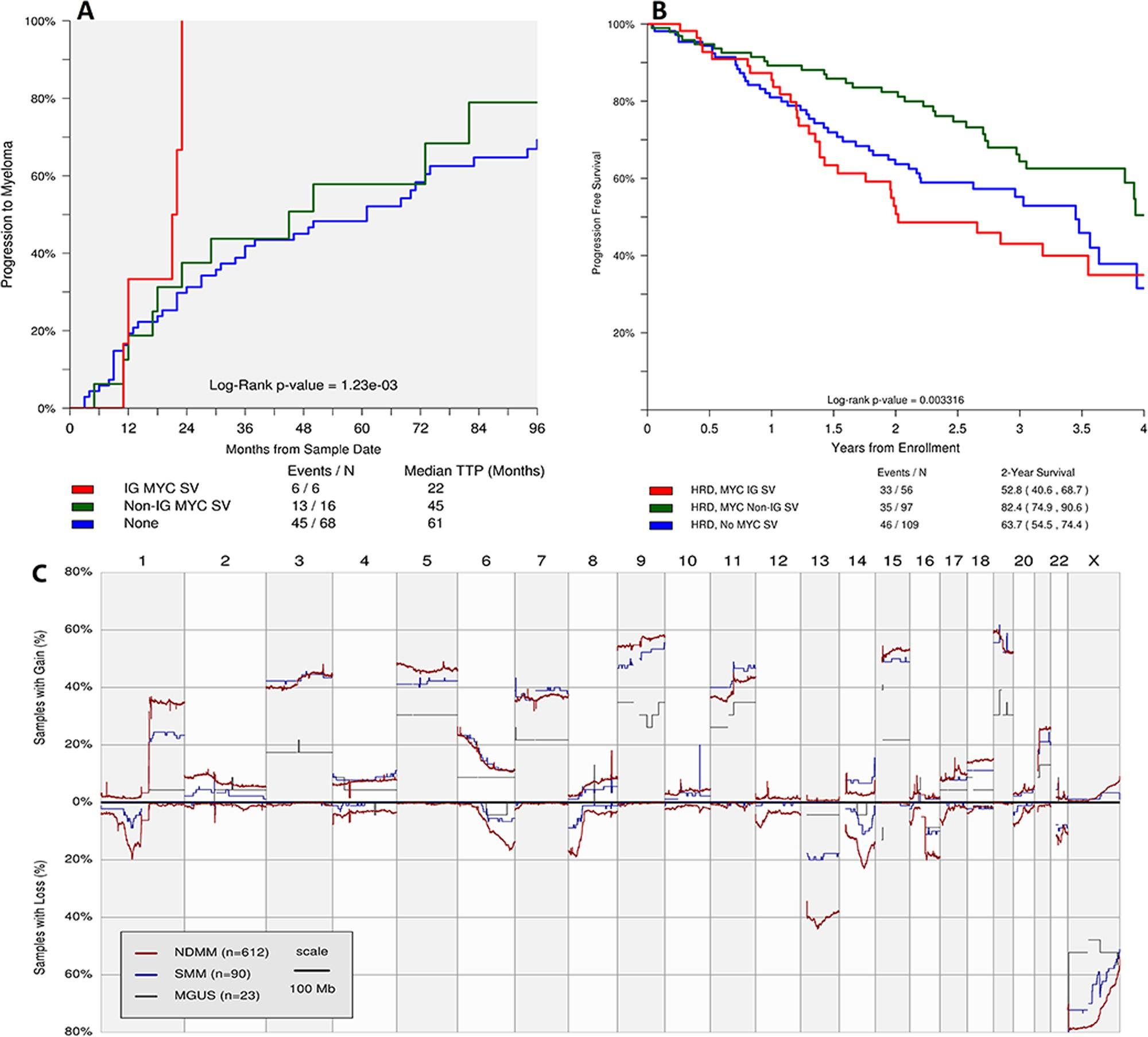

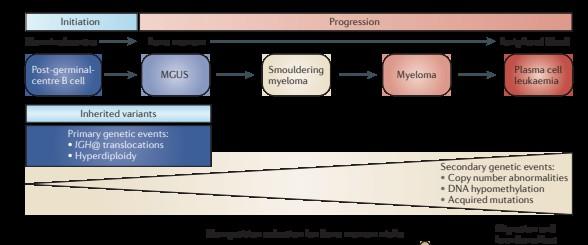
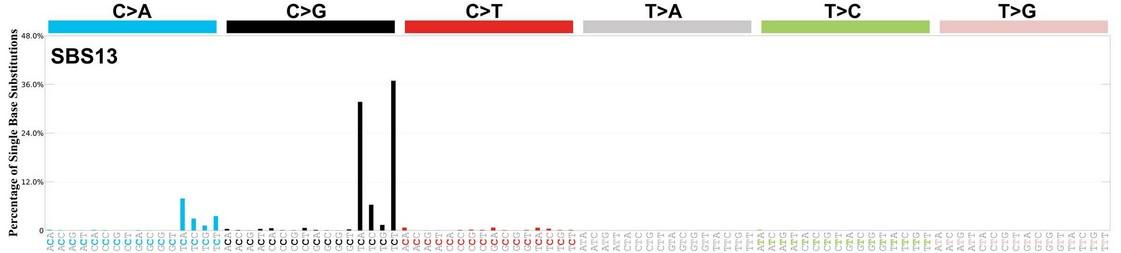
SBS signatures in normal B- and T-cells

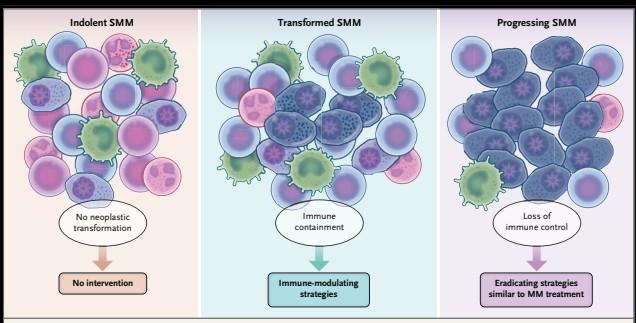
MPC: myeloma precursor conditions
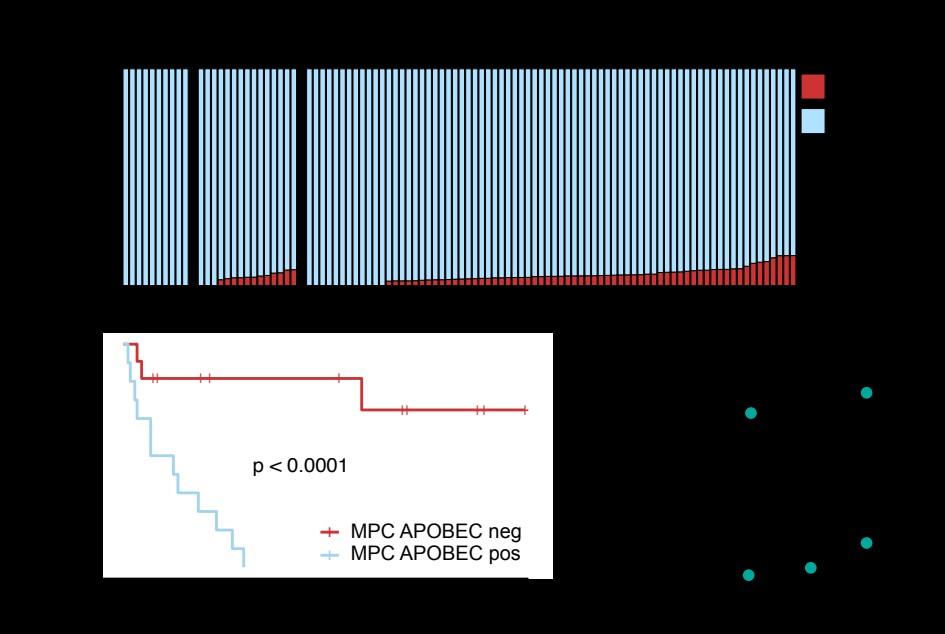
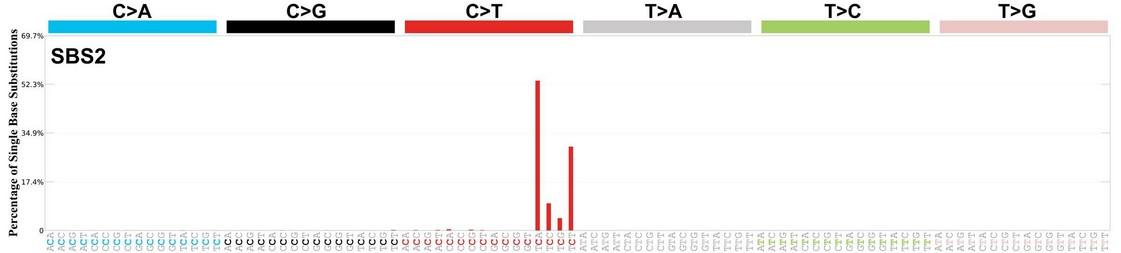
APOBEC is not detectable in stable myeloma precursor conditions and normal B-cells
Oben B. et al. Nature Comm 2021 Machado H. et al.
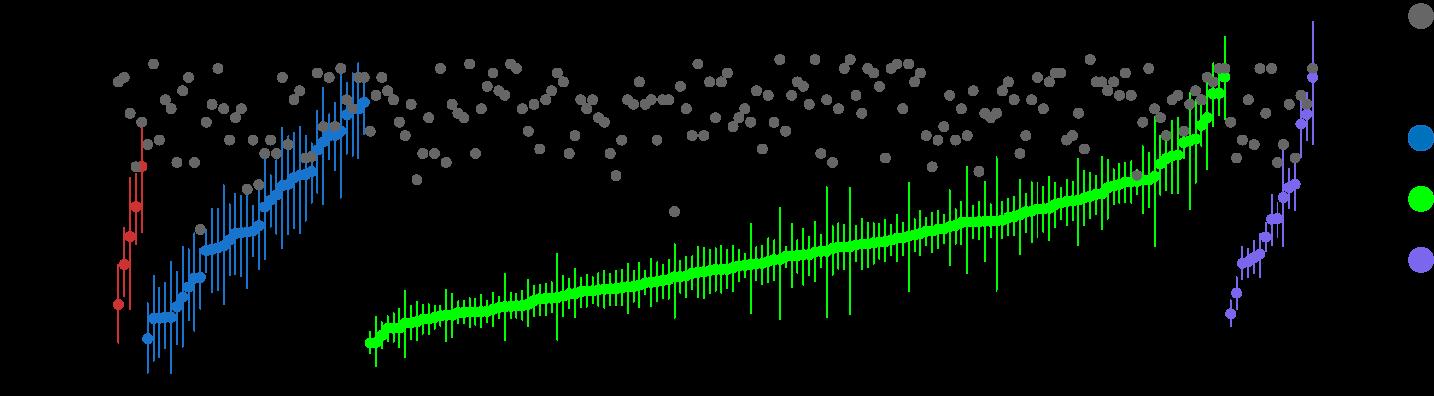

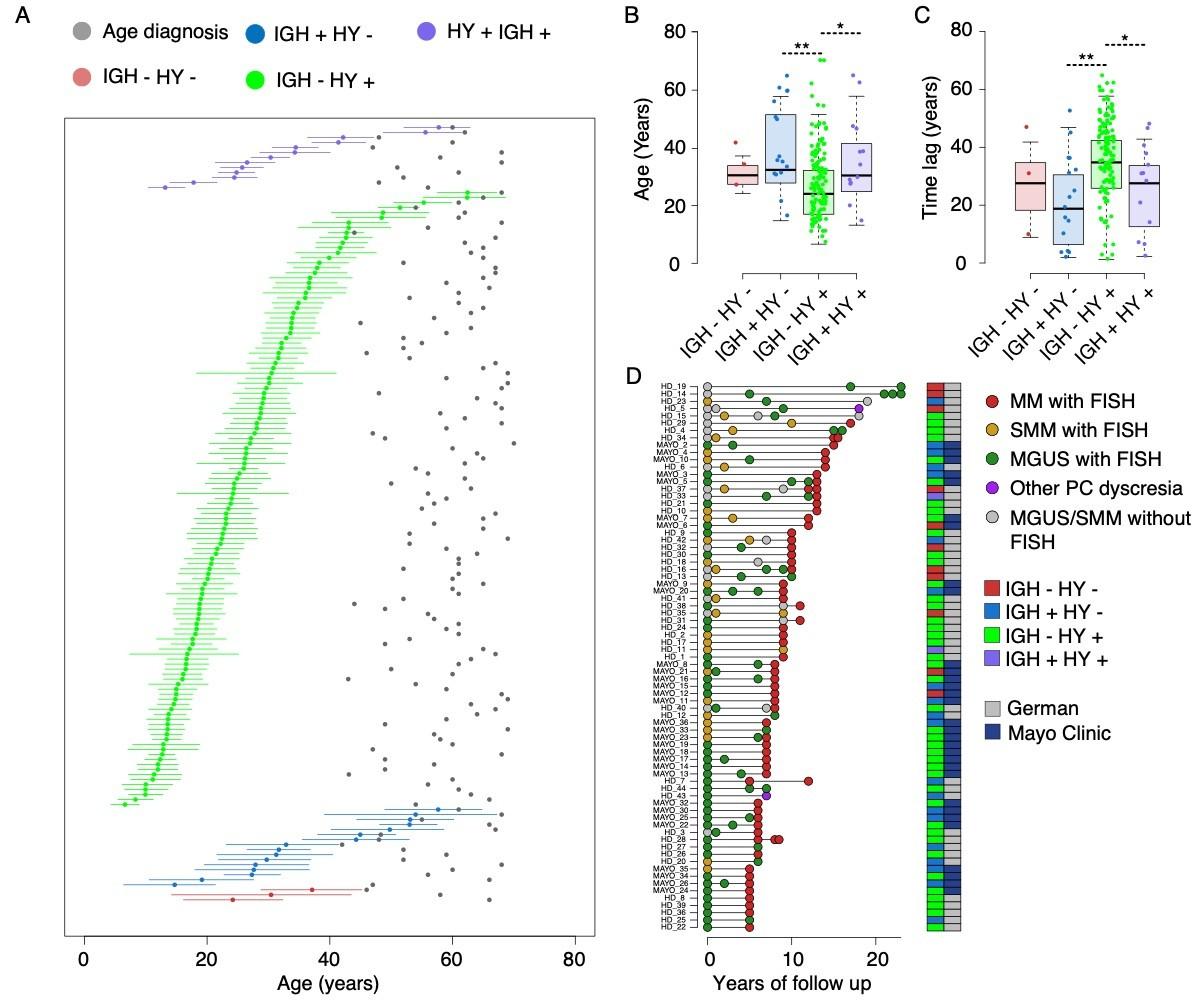



Age of first chromosomal gain to age of sample collection was median 30 years.



Myeloma

• 374 patients
• 301 with no early intervention

MSKCC
Saad Usmani
Ross Firestone
Juan Jose Garces
Kylee H Maclachlan
Andrew Mcpherson
Andriy Derkach
Alexander Lesokhin
Malin L. Hultcrantz
Urvi Shah
Heather Landau
Ahmet Dogan
Yanmin Zhang
Misha Roshal
Sham Malinakody
Sohrab Shah
Moffit Cancer Center
Ken Shain
Fred Locke
Ciara Freeman
Ariosto Silva
Marilena Tauro
Mark B Meads
Mike Jain
Danny De Avila
Praneeth Reddy Sudalagunta
Rafael Renatino Canevarolo
Erin M. Siegel
Phaedra Agius
Jamie Teer
Rachid Baz
Doris Hansen
Leif Begsagel
Marta Chesi
Esteban Braggio
Erin Wiedmeier-Nutor
Linda Baughn
Yusuke Yamashita
Yan Asmann
Shaji Kumar
Rafael Fonseca
Vincent Rajkumar
MDACC
Robert Orlwoski
Krina Patel
Sanger Institute
Peter Campell
Daniel Leongamornlert
UM – SCCC
Ola Landgren
Bachisio Ziccheddu
Benjamin Diamond
Marios Papadimitrioui
Michael Durante
University of Calgary
Nizar Bahlis
Paola Neri
Holly Lee
Mansour Poorebrahim
And many many more….
Heidelberg & GMMG
Niels Weinhold
Alexandra Poos
Elias K. Mai
Hartmut Goldschmidt
Katja C. Weisel
Roland Fenk
Marc-Andrea Bärtsch
Marc S. Raab

NYU
Gareth Morgan
Patrick Blaney
University of Milan
Niccolò Bolli
Matteo Da Via
Giancarlo Castellano
Alessio Marella
Akihiro Maeda
Marta Lionetti
Antonio Matera
Stefania Pioggia
Matteo Claudio Da Vià
Claudio de Magistris
Hasselt University
Benedith Oben
Guy Froyen
University of Würzburg
Leo Rasche
Hermann Einsele









Twitter/X: @FrancescoMaura4
Email: mauraf@mskcc.org


Meletios(Thanos)Dimopoulos, MD
Professor and Chairman
Department
Plasma
National

• median follow-up: ~6.2 years
• 29% cumulative progression rate at 2 years
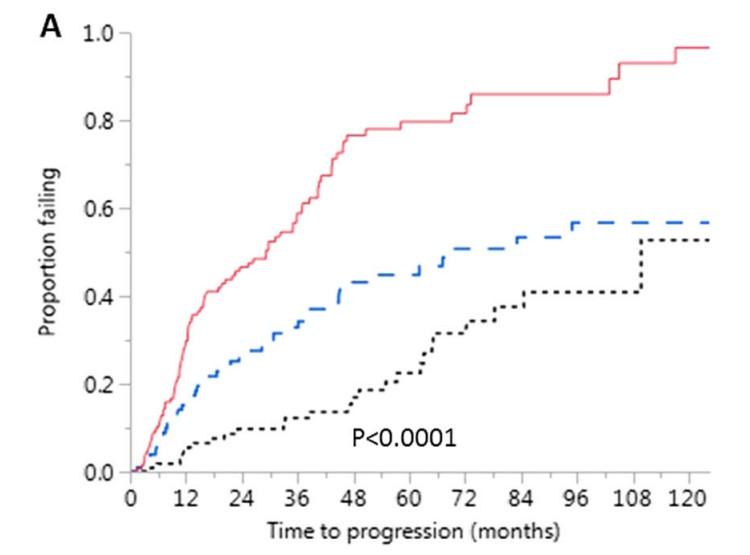
• Single center
• n = 421 patients, with SMM diagnosis in 2003-2015
• Excluded those with SLiM (retrospectively)
• Advanced imaging in 124 (29.4%)
• median follow-up: 3 years
• 22% cumulative progression rate at 3 years
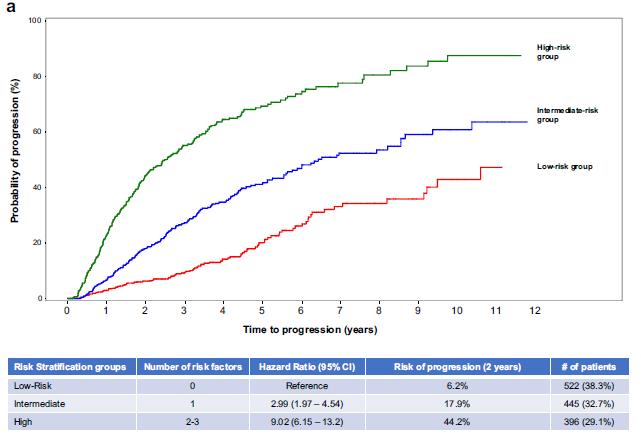
• Multicenter
• n = 1363 patients, with SMM diagnosis in 20042018
• Excluded those with SLiM
• Advanced imaging in unknown number
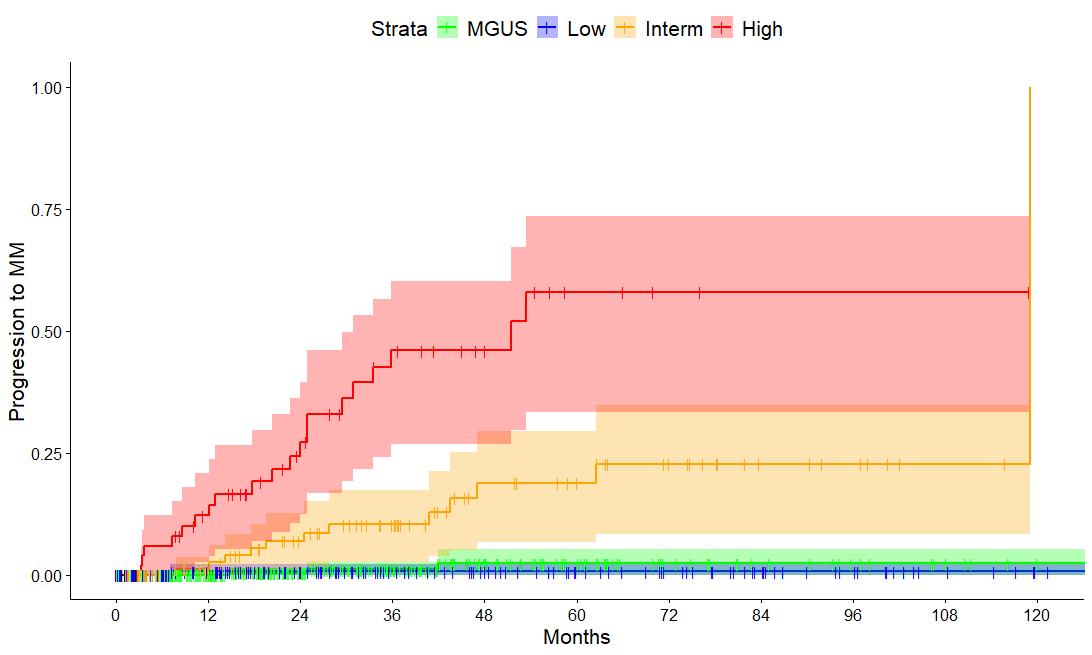
median follow-up : 3 years
12% cumulative progression rate at 3 years
• n = 427 SMM patients diagnosed after 2014
• Advanced imaging in 100%
• PD based on SLiM-CRAB criteria
• Majority low risk
• 12% cumulative PD rate at 3 years
• 43% of progressions by SLiM only
• TTP of Low risk SMM ~ MGUS indicating that the current cutoff of 10% BMPC is arbitrary
2-year progression rate among high risk per 20-2-20 according to % of those with advanced imaging in the respective cohort (more advanced imaging = lower progression rate)
• PANGEA and PANGEA 2.0 models: Trajectories of age, serum creatinine concentration, hemoglobin, paraprotein levels and FLC ratios, with or without BMPC% (Cowan A et al Lancet Haematol 2023 Chabrun F et al ASH 2024)
• Circulating plasma cells (risk stratification and non invasive assessment of evolution): Cutoffs of >0.015% (Terminι R et al) or 0.0014% (Kastritis E et al) (Termini R et al Clin Cancer Res 2022;Kastritis E et al ASH 2024/Submitted)
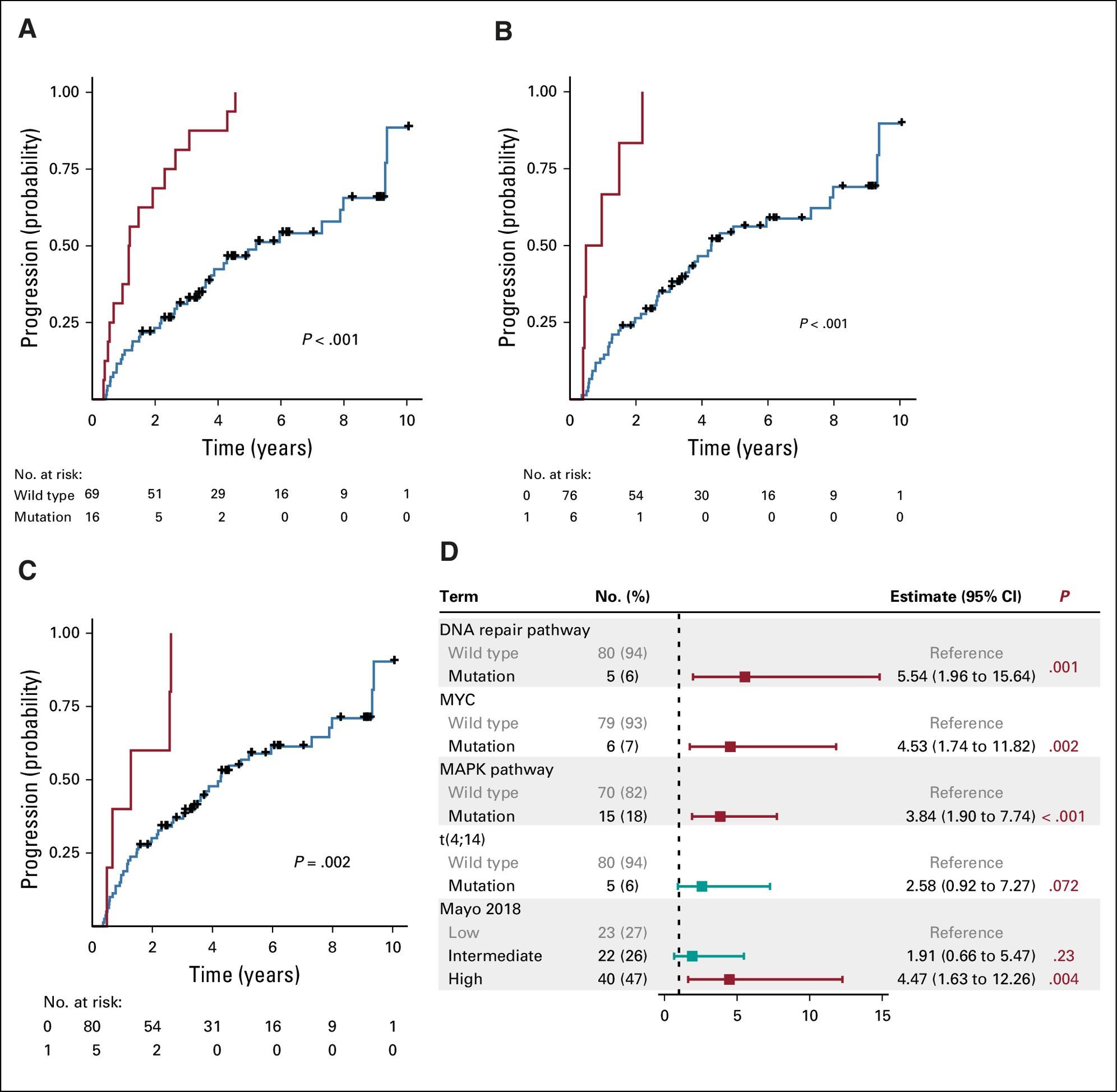
• Genomic stratification models: Alterations of the MAPK pathway , DNA repair pathway and MYC and APOBEC associated mutations ; distinct genetic subtypes based on mRNA signatures; single cell transcriptional signatures (Bustoros M et al J Clin Oncol. 2020; Bustoros M et al Nat Commun. 2022; Sklavenitis-Pistofidis R et al ASH 2024)
• Immune biomarkers (Bidikian N et al ASH 2024)
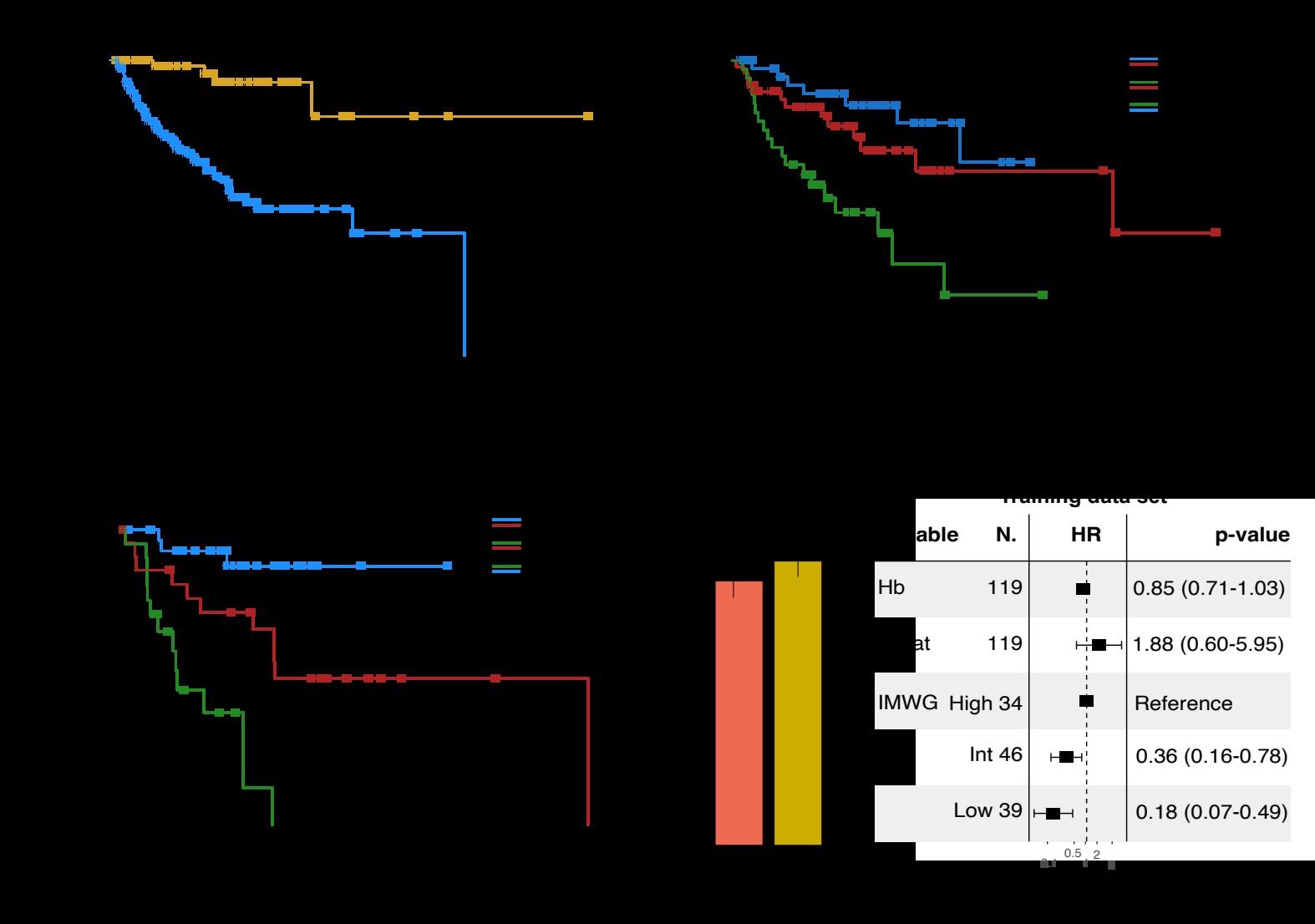
High risk SMM : Len-dex vs observation (n = 119)
Median follow-up: 12.5 years
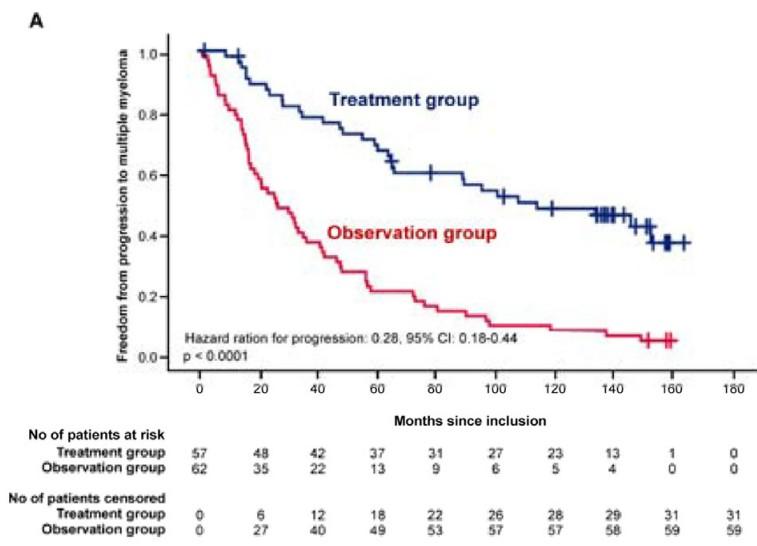
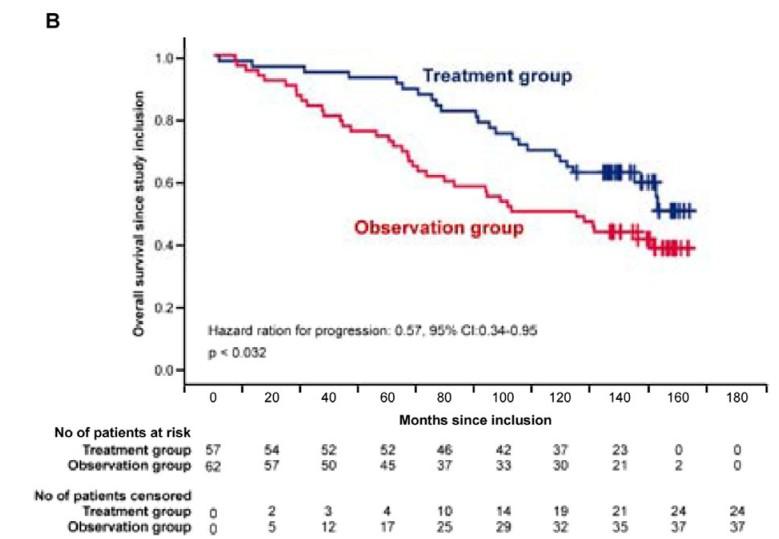
Randomized Trial of Lenalidomide vs observation in SMM
Mateos MV, et al. Eur J Cancer 2022
Mateos MV, et al. Lancet Oncology 2016
Mateos MV, et al. NEJM 2013
HR: 0.28 (95%CI 0.120.62)
Median follow-up: ~3 years (A) high risk (B) intermediate risk (C) low risk.
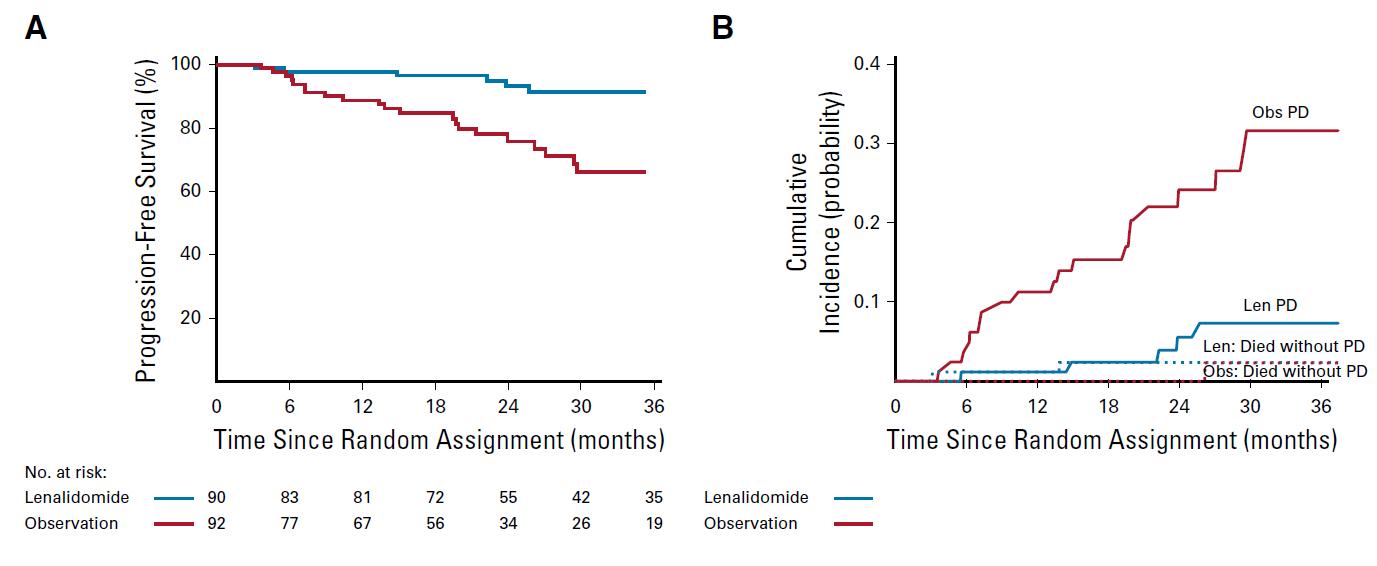
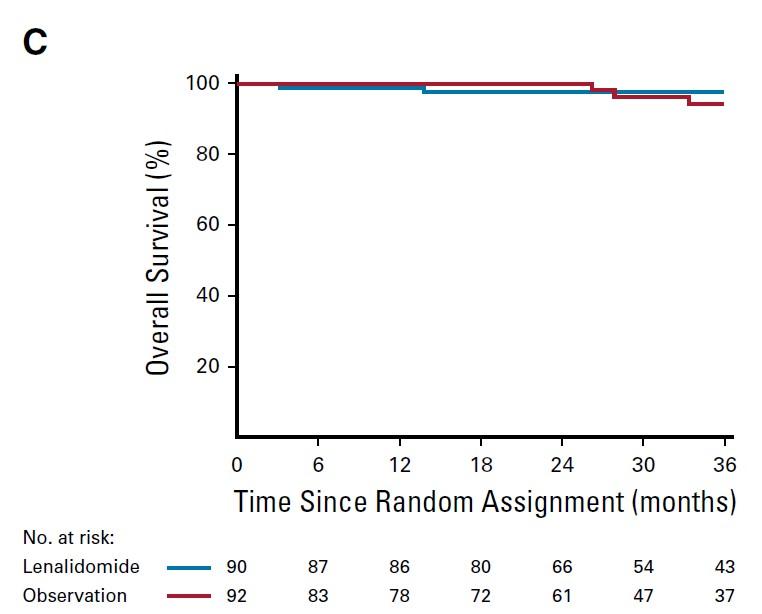
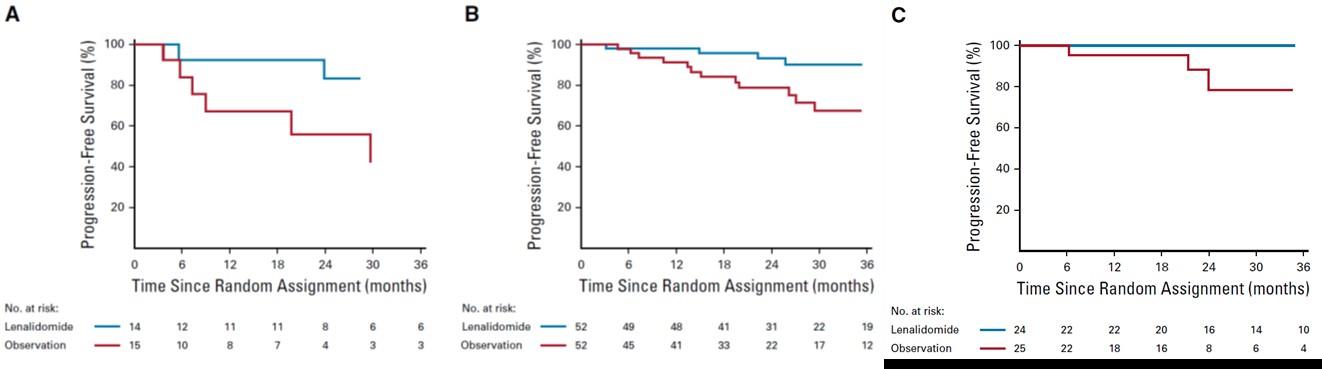
PD: required biochemical PD + by IMWG criteria for MM and evidence of endorgan damage
Lonial S et al J Clin Oncol 2019
• Disease definition: patients with active MM per IMWG 2014 were included (i.e. BMPCs>60%, FLCr>100)
• Risk stratification was based on older tools
• Non-advanced imaging was used for initial assessments and follow up
• Follow up was less stringent according to current standards
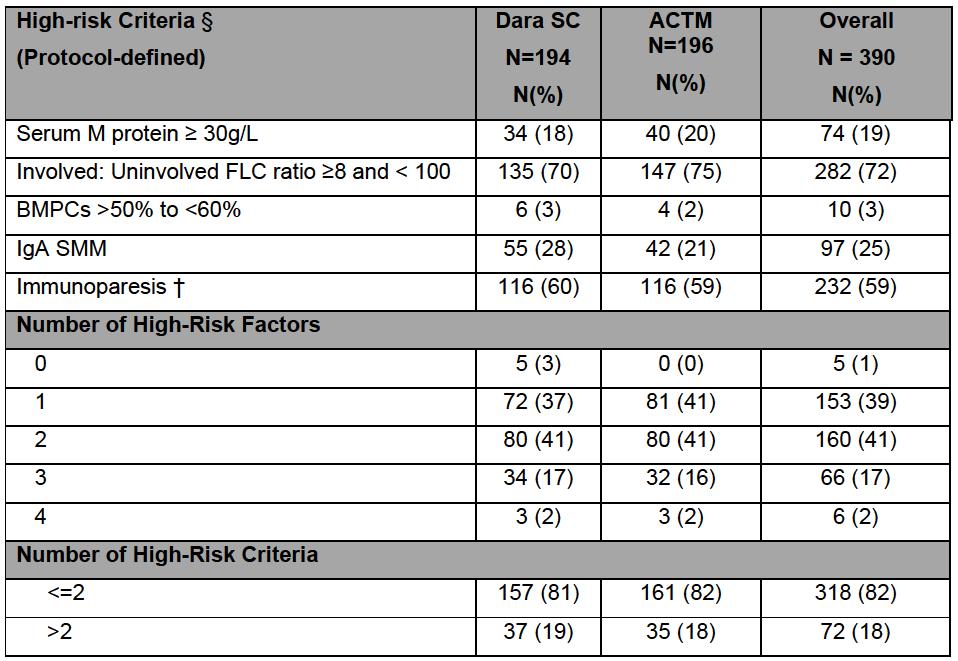
• SMM defined by current criteria (IMWG 2014)
• High risk criteria were protocol defined (before 2018)
• Central labs every three months
• Advanced imaging used at baseline and every year
• Bone marrow biopsies performed at least every two years
• IMWG criteria were used to define symptomatic MM
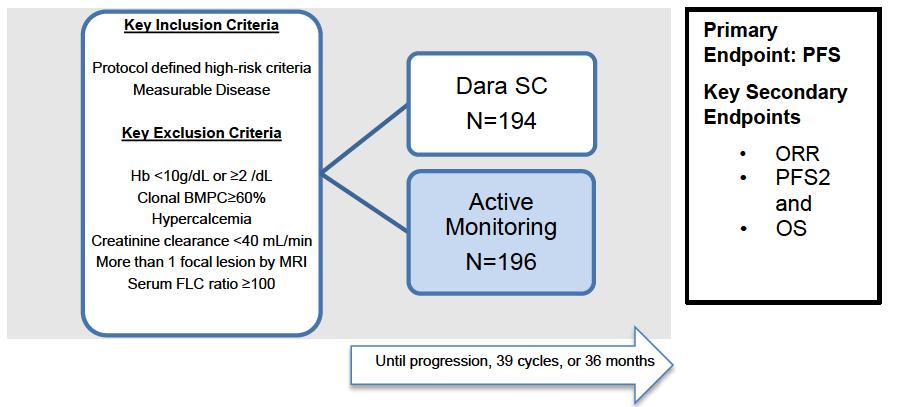
Are AQUILA results applicable to current SMM population?
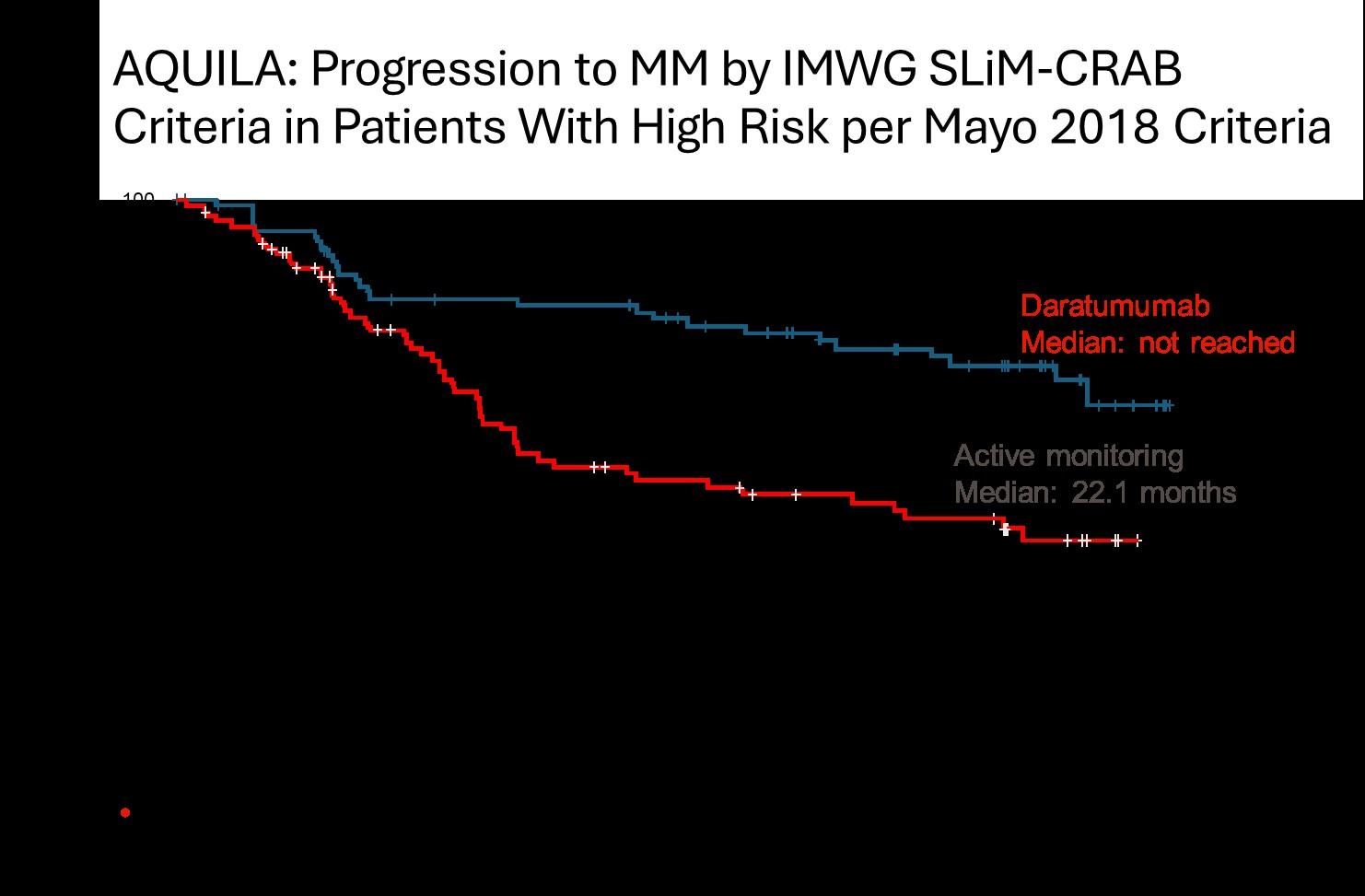
• DARA significantly reduced the risk of progression to MM or death by 51% versus active monitoring for all subsets and risk categories, more pronounced in high risk SMM
• The benefit continued beyond 36 months
• May 20, 2025: FDA ODAC positive vote
N=427 patients diagnosed between 2014-2023, median follow up: 3 years M-protein <3 vs >3 gr/dl FLC ratio <8 vs >8-<100
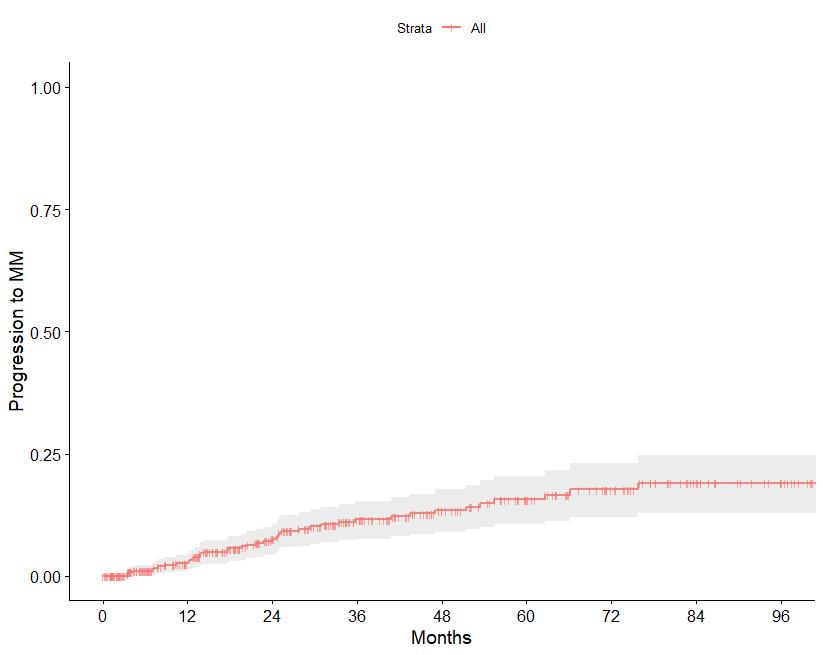
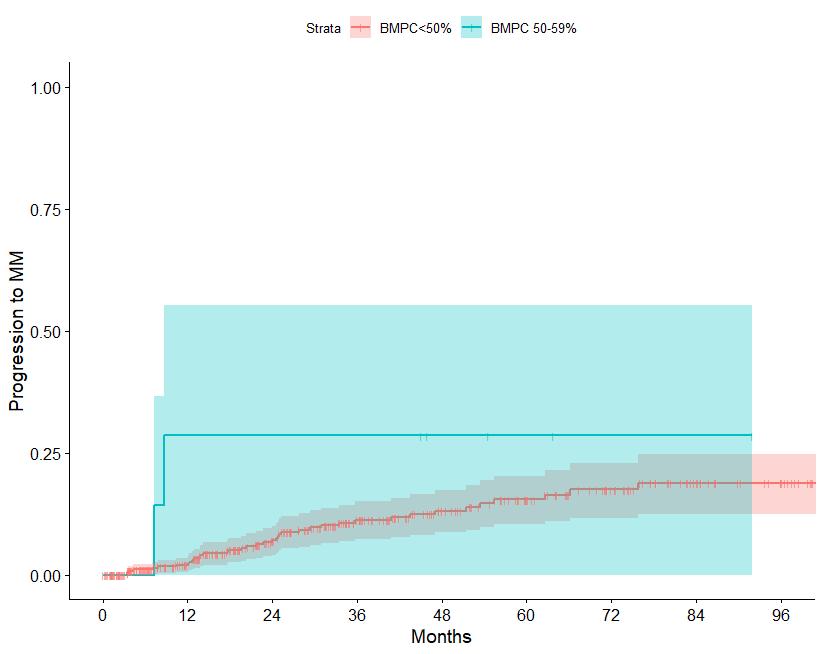
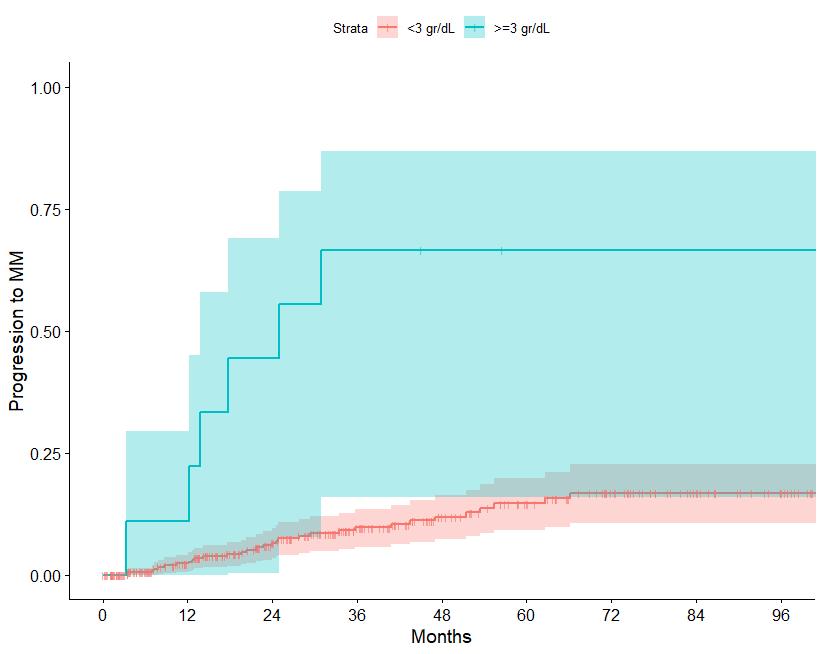
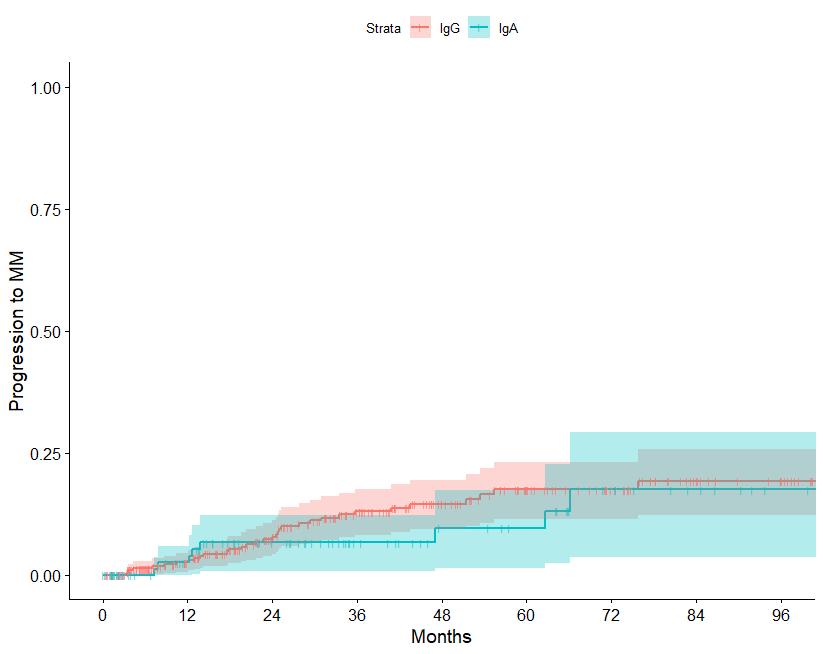
Source: Department of Clinical Therapeutics ; Kastritis E et al Blood Adv 2025
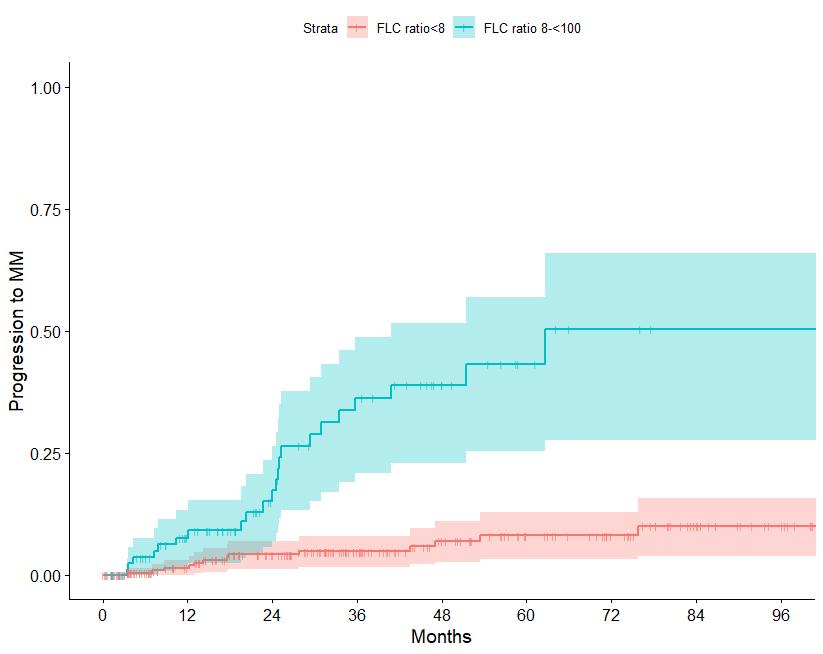
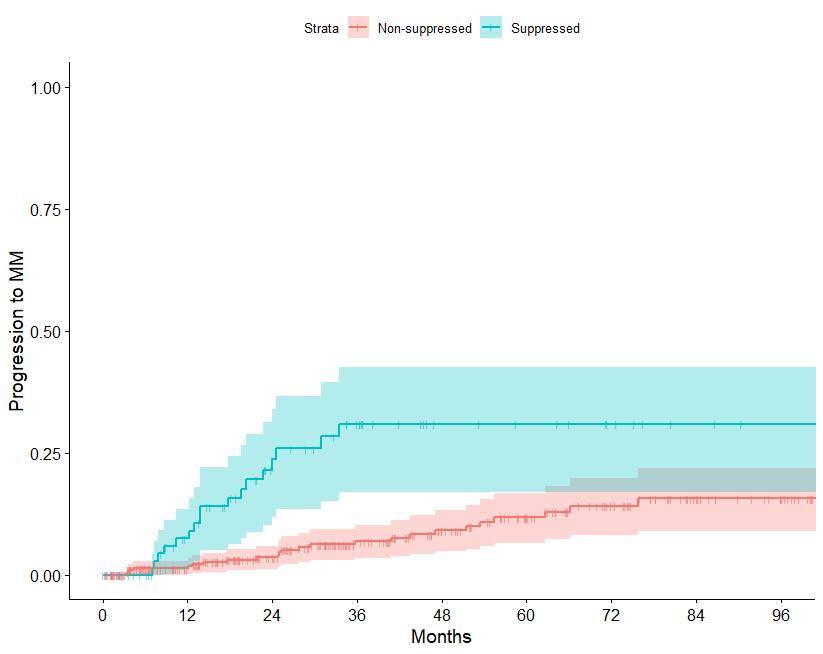
Patterns of progression among 427 Smoldering Myeloma patients diagnosed after 2014: importance of monitoring
With modern criteria, use of advanced imaging at baseline and follow-up, the majority of patients are detected by SLiM criteria or asymptomatic bone lesions (no bone fractures)
• Data from small studies
• SMM populations not meeting current definitions
• High risk SMM was minority
• PO administration
• Single agent activity ~55% ORR
• High discontinuation rate (~30->40%)
• Slightly increased risk of second primary malignancies
• Low cost
• Data from large study
• SMM populations per current definitions
• High risk SMM was minority
• SC administration
• Single agent activity ~64% ORR
• Low discontinuation rate (<5%)
• No increased risk of second primary malignancies
• High cost
Delaying disease progression
• Randomized trials available
• No cure
• Fixed duration treatment
• Low toxicity
• Many and increasing salvage options
• Low/high costs
• Applicable to elderly/frail
Curative strategy
• Only phase 2 studies so far
• Some patients may be cured?
• Fixed duration treatment
• Higher toxicity depending on regimen
• Salvage options may be less, but increasing
• High costs
• Applicable to younger/fitter
• Teclistamab monotherapy in high risk SMM (Immuno-PRISM trial, NCT05469893).
• N=12, ORR: 100% ; 42% CR, 25% VGPR, 33% PR.
• Elranatamab in patients with previously untreated high-risk SMM / EMN34 study (NCT06183489)
• Linvoseltamab in SMM (NCT05955508)
• Teclistamab or Talquetamab in Combination With Daratumumab for High-Risk Smoldering Myeloma (REVIVE Study)(NCT06100237)
• Cilta-Cel in SMM
• CAR- PRISM trial, NCT05767359)
• CAR-HiRiSMM tria, NCT06574126
• A Study of Autologous Expanded Natural Killer Cell Therapy for Asymptomatic Multiple Myeloma (UARK 2013-05, NCT01884688
• We now have four options for HR SMM
• Early intervention with daratumumab (when approved)
• Close follow up with labs (Q 3-4 months) and modern imaging techniques (Q year)
• Enrollment in trials with combinations of current agents active in MM (PI,IMiDs,antiCD38)
• Enrollment in trials with curable intent (HDT, CARs, Bispecifics)
• Decisions may be based on patient’s wish, fitness/frailty, comorbidities, life expectancy
• No need for trials in low risk SMM
• For intermediate risk SMM: we need trials with innovative approaches to intercept evolution to myeloma
• Research to identify risk factors at baseline that may move patients to an early myeloma category
• Research to develop dynamic models which are more informative for the daily management of patients (evolving patterns)



National and Kapodistrian University of Athens

Department of Clinical Therapeutics
(HORIZON-MISS-2021-CANCER-02-03)
Elucidation of risk factors and health determinants associated with progression of monoclonal gammopathies to multiple myeloma

(HORIZON-MISS-2021-CANCER-0201)
Early detection and screening of hematological malignancies

Dana-Farber Cancer Institute


Jill Corre, PharmD, PhD

Jill CORRE
Unit for Genomics in Myeloma, Toulouse, FRANCE




Honoraria: Janssen, Takeda, Amgen, Sanofi, Bristol Myers Squibb, Pfizer, Adaptive, Abbvie
Consulting or advisory board : None
Research funding: Sanofi, Bristol Myers Squibb
Travel Support: Janssen, Sanofi, Bristol Myers Squibb, Pfizer
3340

Barcelona July 7-8, 2023

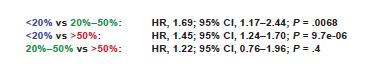
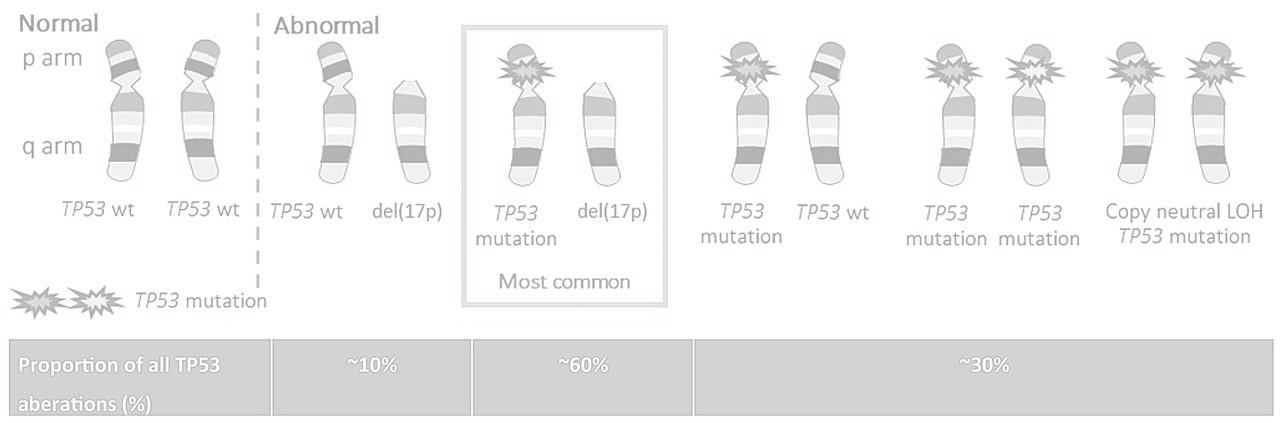
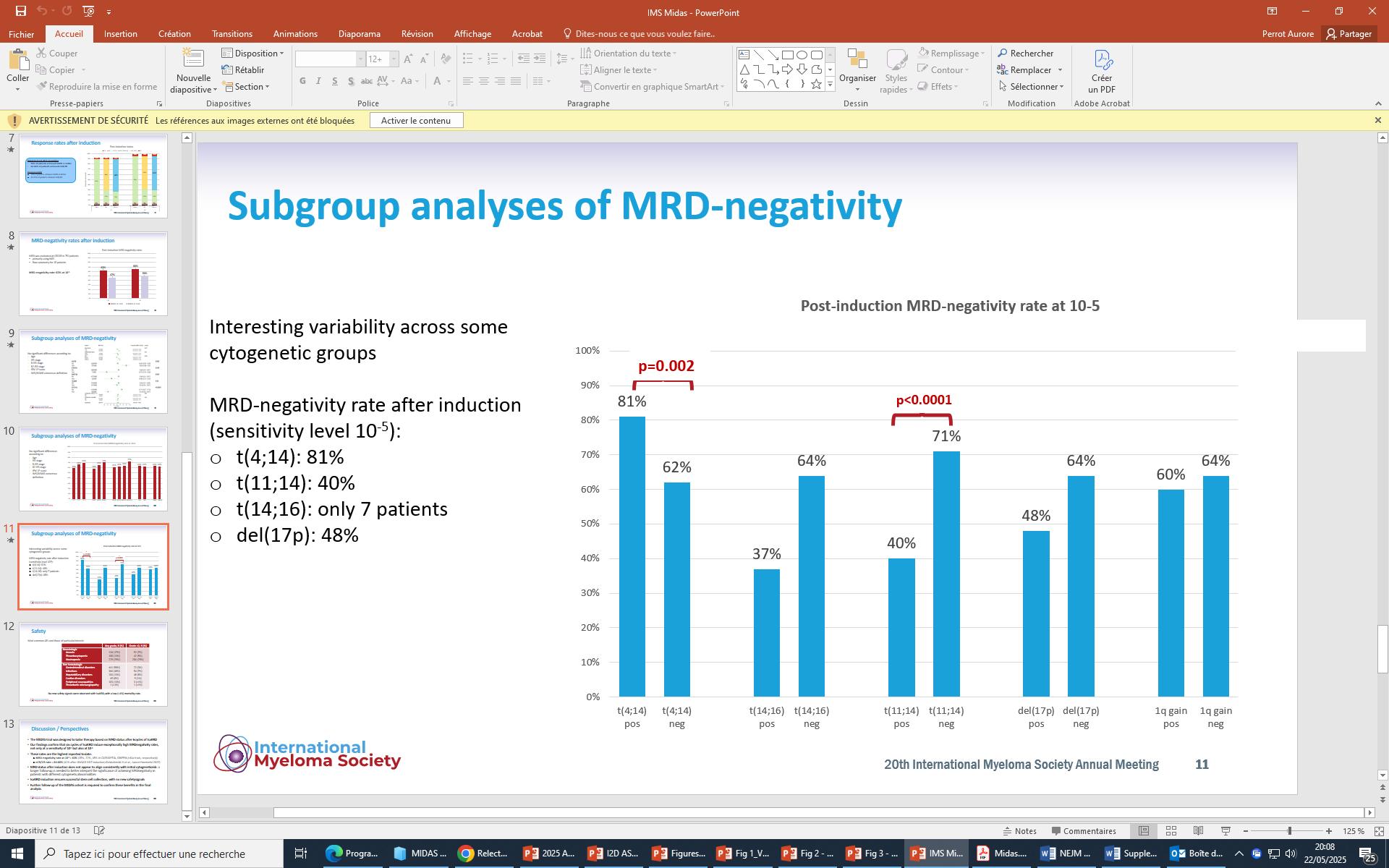
in more than 20% of sorted plasma cells
Association of 2 among t(4;14) or t(14;16) or t(14;20)
Del17p in more than 20% of sorted plasma cells
TP53 mut (no threshold VAF)
Biallelic Del(1p32)
Association of 2 among t(4;14) or t(14;16) or t(14;20)
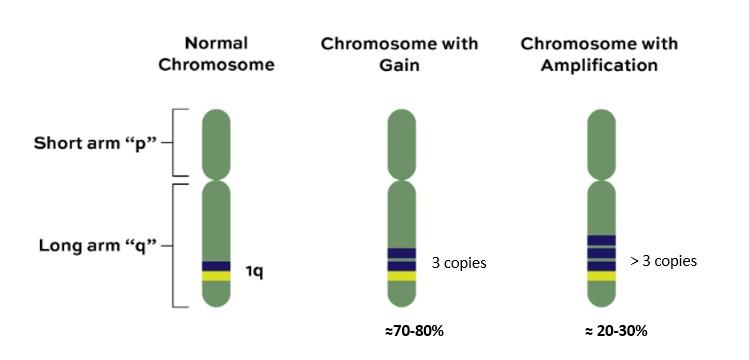
Gain/Amp 1q Monoallelic del(1p32)
Avet-Loiseau et al. J Clin Oncol, in

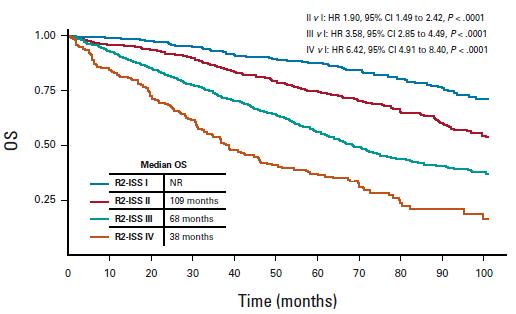
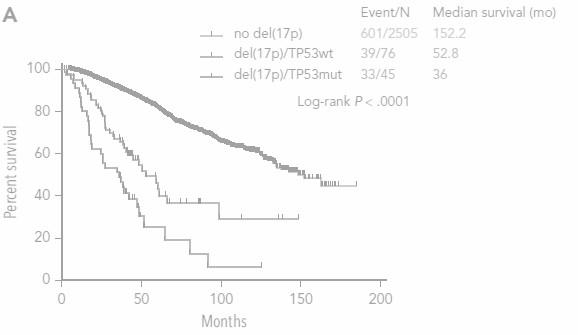
Del17p ISS stage I/II are NOT defined high risk
ISS in 877 NDMM patients with del(17p)
Unpublished data
Heterogeneity of intermediate risk subroup (R-ISS II)
ISS in 877 NDMM patients with del(17p)
Unpublished data

Heterogeneity of intermediate risk subroup (R-ISS II) No del17p Del17p
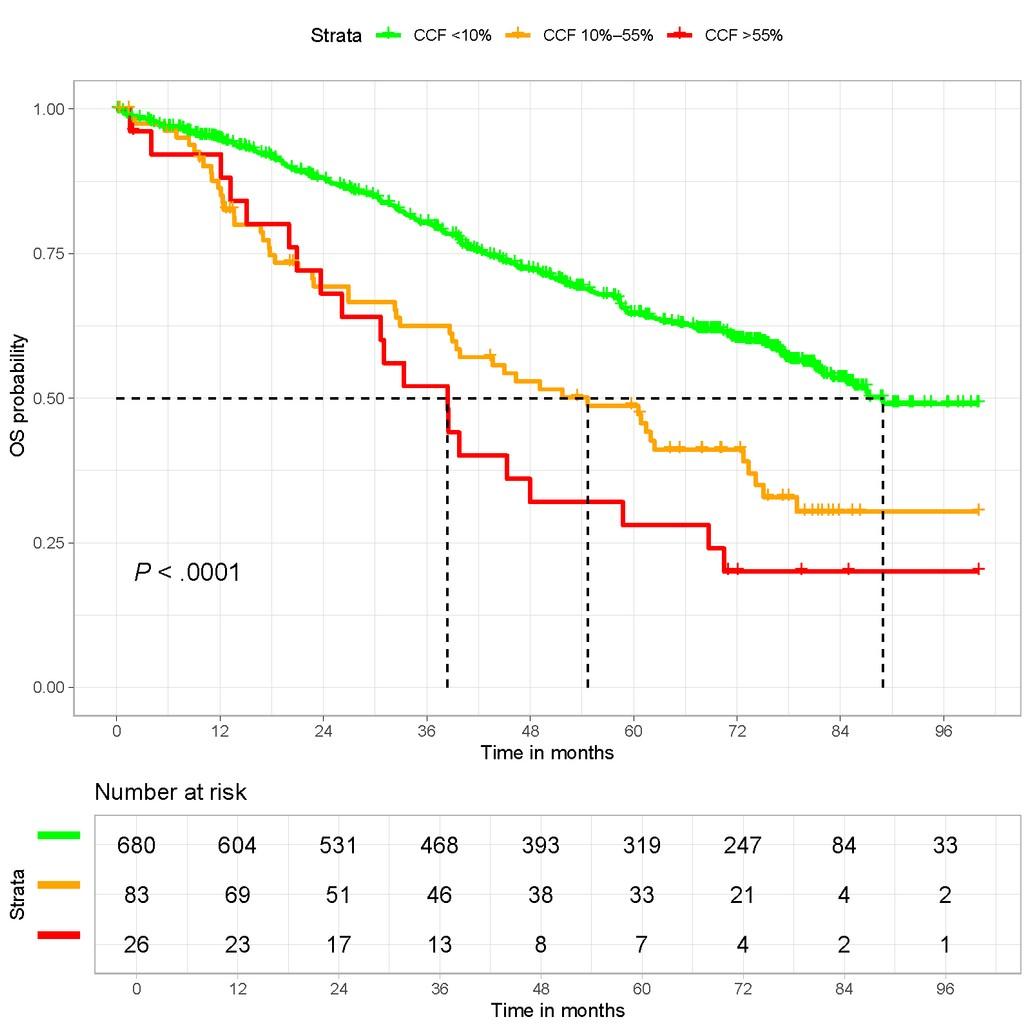
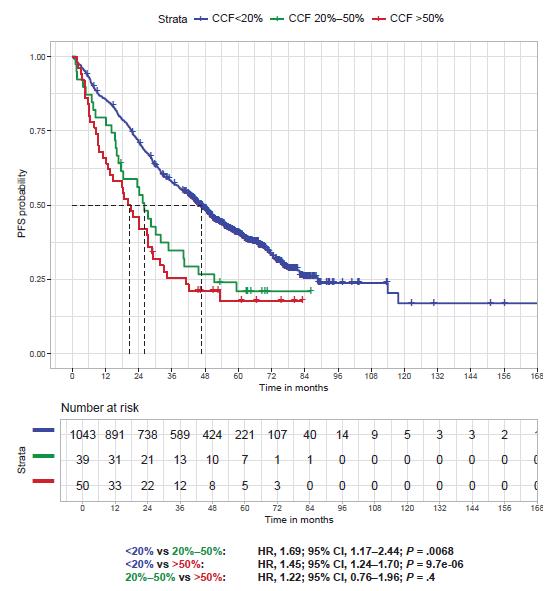
• R-ISS : « higher that the cutoff threshold defined by each lab »
• R2-ISS : 10 to 20%
• IFM : 50-55%
• Until 1% in some clinical studies !
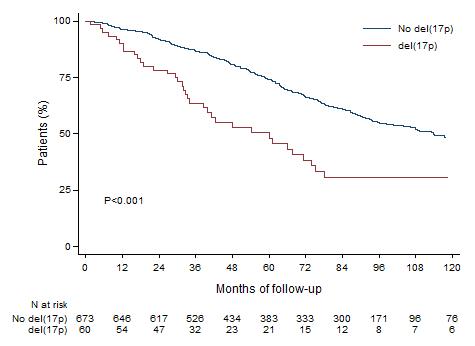
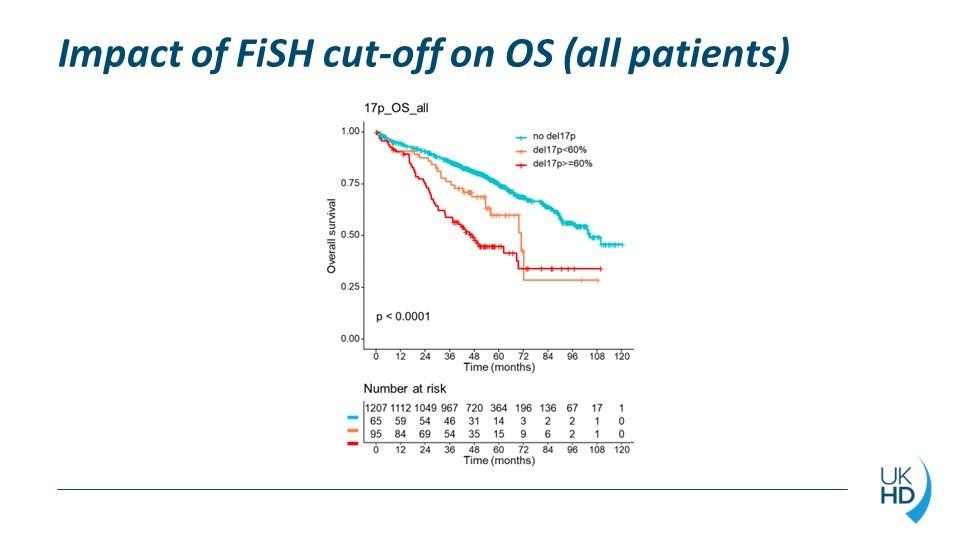
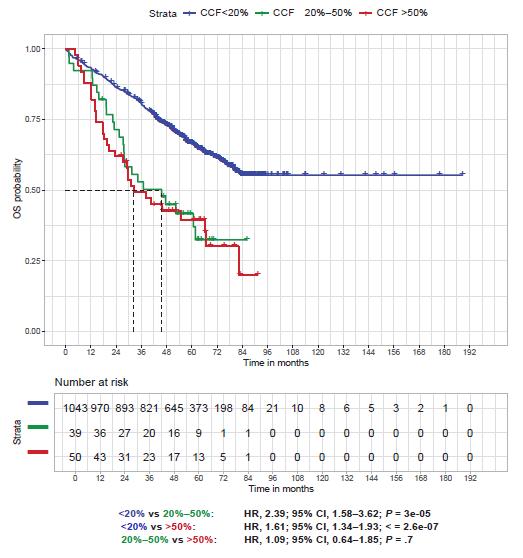
OS according to del17p


>1000 del(17p) patients
Del(17p) 0-29%
Del(17p) 30-49%
Del(17p) 50-100%
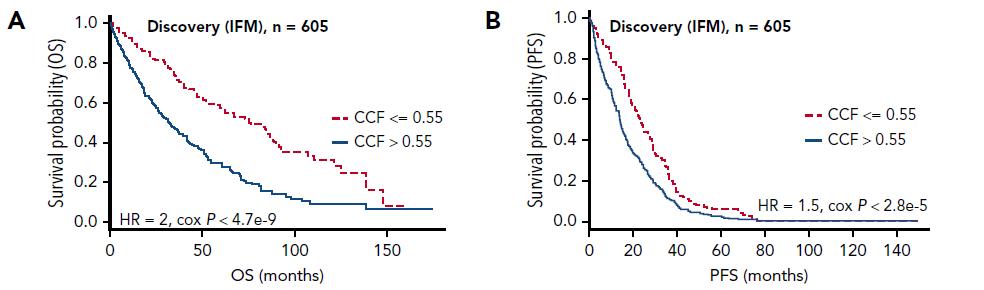
Thakurta et al. Blood 2019

Del17p in more than 20% of sorted plasma cells
Association of 2 among t(4;14) or t(14;16) or t(14;20)
Gain/Amp 1q Monoallelic del(1p32)
Avet-Loiseau et al. J Clin Oncol, in

n=34 n=25 n=43 n=541 Do we also need TP53 mutational status ?


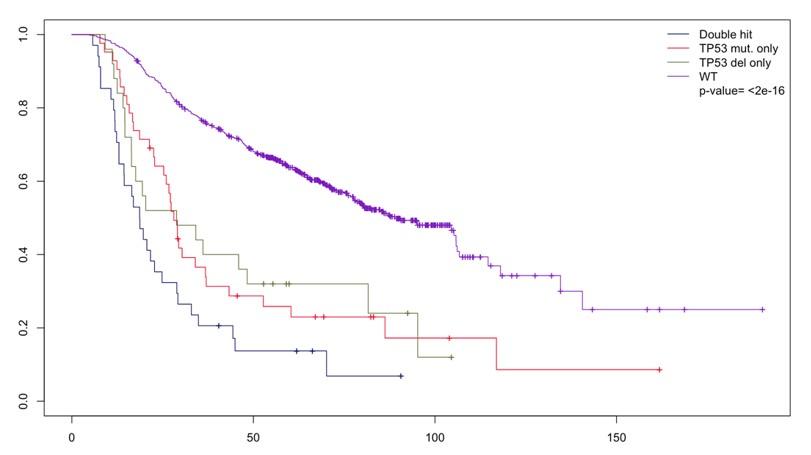


Do we also need TP53 mutational status ?






n=34 n=25 n=43 n=541

Del17p in more than 20% of sorted plasma cells
TP53 mut (no threshold VAF)
Association of 2 among t(4;14) or t(14;16) or t(14;20)
Gain/Amp 1q Monoallelic del(1p32)
Avet-Loiseau et al. J Clin Oncol, in
Prognostic impact of del(1p32) : 10% of NDMM, including 1/5 biallelic
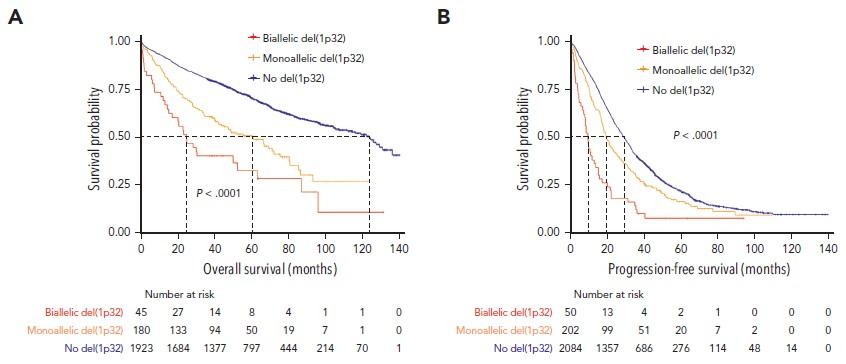

Prognostic impact of del(1p32) : 10% of NDMM, including 1/5 biallelic


Del17p in more than 20% of sorted plasma cells
TP53 mut (no threshold VAF)
Biallelic Del(1p32)
Association of 2 among t(4;14) or t(14;16) or t(14;20)
Gain/Amp 1q
Monoallelic del(1p32)
Avet-Loiseau et al. J Clin Oncol, in

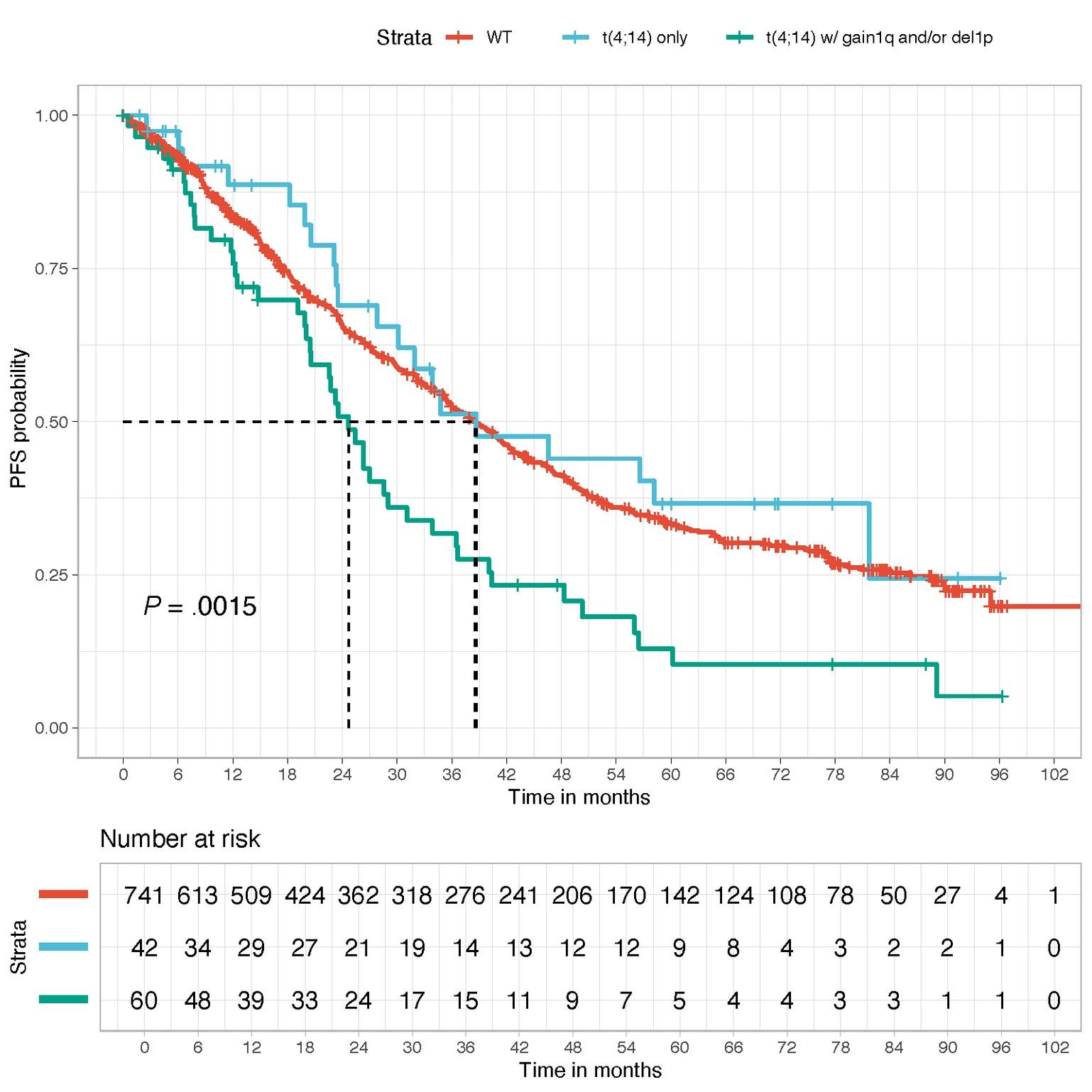
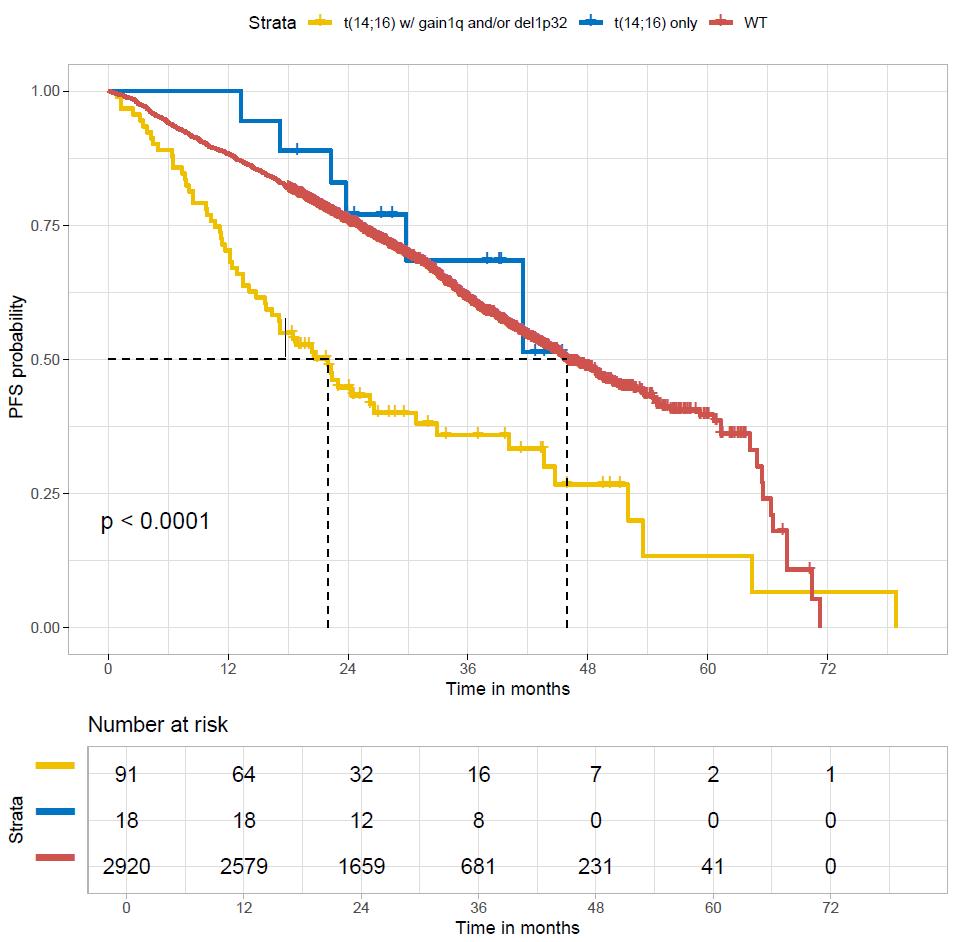
WT
t(4;14) only
t(4;14) with gain 1q or del1p32
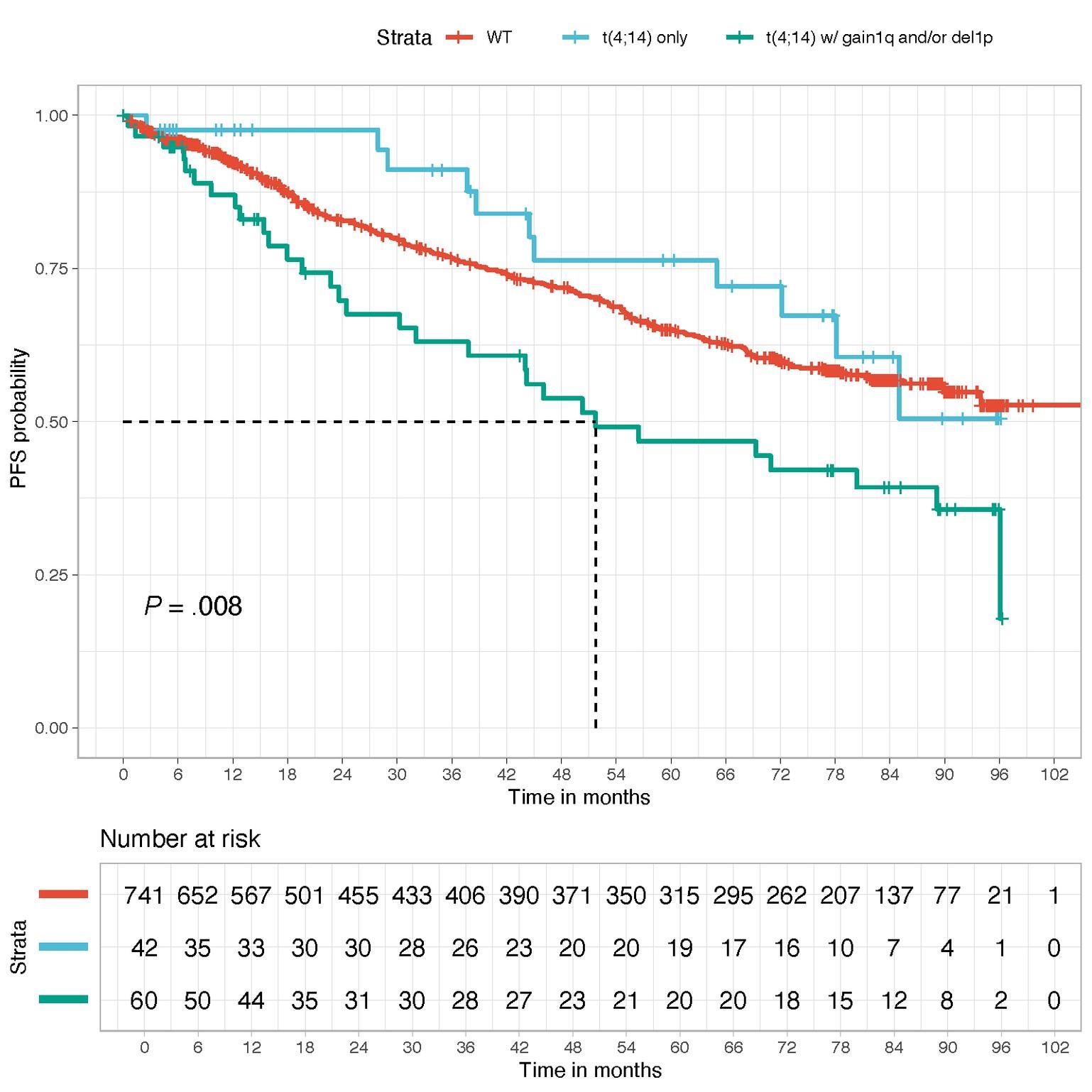


WT
t(4;14) only
t(4;14) with gain 1q or del1p32
t(4;14)=highriskifassociatedtoanotherintermediatelesion (1qgain,del1p32)

in more than 20% of sorted plasma cells
Association of 2 among t(4;14) or t(14;16) or t(14;20)
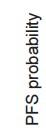
without Renal Failure (Creatinine <1.2 mg/dL)
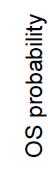
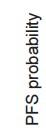
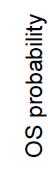
FORTE study
in more than 20% of sorted plasma cells
2 among t(4;14) or t(14;16) or t(14;20)
• The IMS Panel recommends the use of this HRMM definition in all clinical trials going forward and in routine practice.
• The HR subset should represent around 20% of NDMM patients.
• Represents an important step toward risk-stratified therapeutic approaches in routine.
• We hope that this definition will promote the design and conduct of clinical trials FOCUSED on patients with HRMM.
• NGS-based definition, but available data with FISH (report the cut-offs positivity used).
• Risk features should be also evaluated at relapse* and prior to participation in a clinical trial.
(*the risk status at relapse supplants risk status at diagnosis, unless the patient was deemed high-risk at diagnosis).
• Do we need an ultra high risk definition ?

definition




t(8;14)

Chromothripsis




t(4;14) breakpoint


t(x;22) (light chains) Immune profile





Number and size of PET or MRI lesions at diagnosis Extra medullary disease



Functional Risk Dynamic response to therapy - MRD



Team Genomic and immunology of myeloma
Ludovic Martinet
Aurore Perrot
Anaïs Schavgoulidze
Laure Derrier
Sabrina Maheo
Céline Mazzotti
Luka Pavageau
Chloé Cerutti
Antoine Graffeuil
Coralie Franck
Lesluyes Tom

Nadège Carrié-Constantin
Marie-Véronique Joubert
Liliana Lucca
Pierre-Paul Arixa
Marine Cuisinier
Cazes Amélie
Daunes Hélène
Charlotte Théral
Sandy Bernou
Laurine Martin
Marie Cortez
Marie-Anne Marsili
Jennifer Amassi
Marie-Sarah Mascaraque
Lou-anne Larichaudy
Stéphanie Laffaure
Tambi Ralamboarivony
Emmanuelle Le Trionnaire
Charlotte Avet-Loiseau
Aurélie Berberian
Sophie Renaudin
Jean-Michel Herrera






Nikhil Munshi
Mehmet Kemal Samur
Anil Aktas-Samur
Feature/Method Consideration(s)
NGS-based methods
iFISH
Sample source/analyte
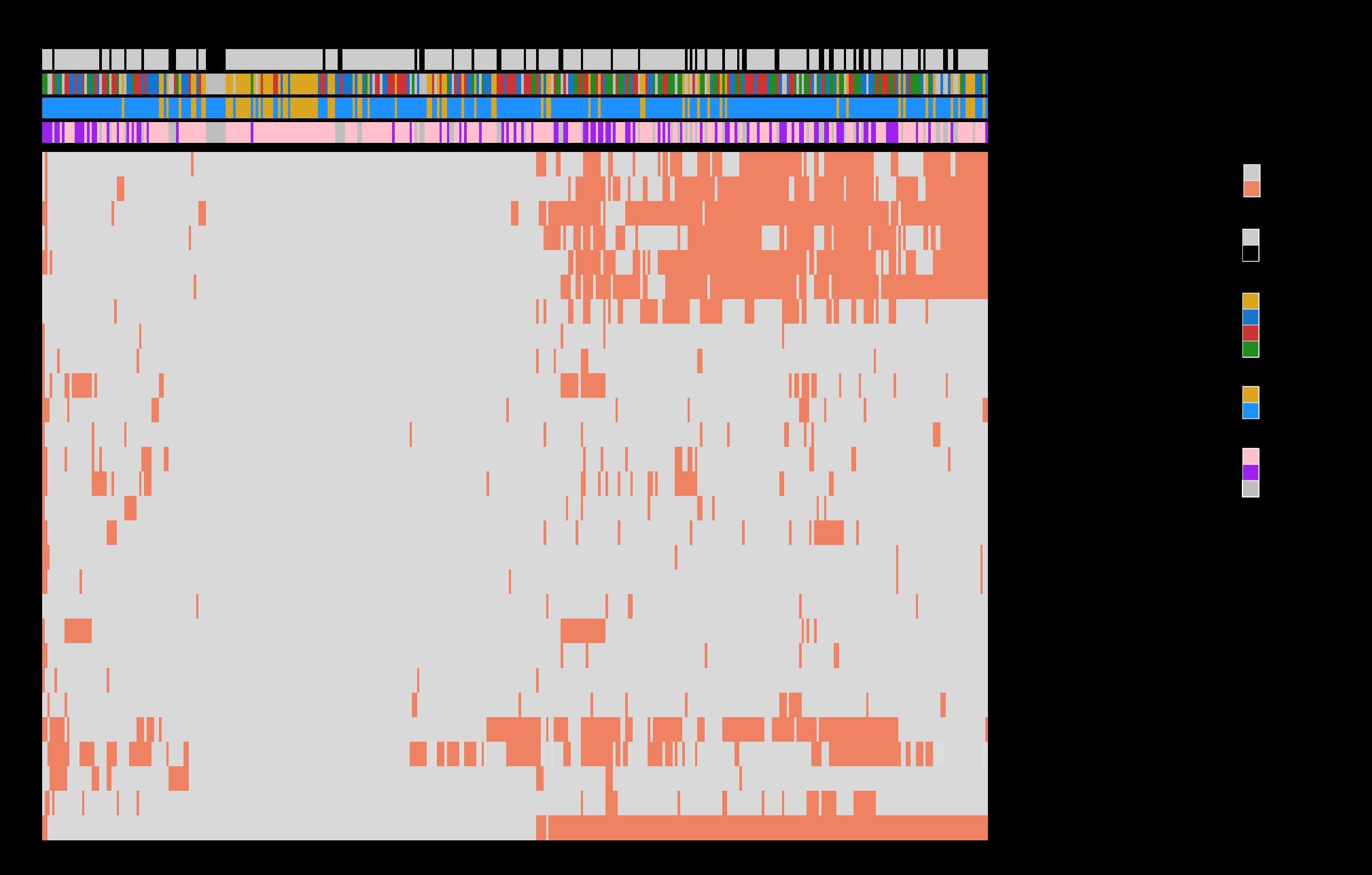
WGS/targeted NGS should now be used for broad molecular profiling, similar to other hematological malignancies.
iFISH is not sufficient to fully risk-stratify MM patients.
If performed, selected/enriched plasma cells, with ≥70% purity, should be used.
Percentage of cells with abnormalities reported should be corrected based on purity of the sample.
Mutations in p53 and a small deletion in 1p requires sequencing and iFISH alone is no longer sufficient to detect these changes.
Although data suggest that the molecular profile (CAs, mutations, other genomic alterations) is spatially heterogeneous (i.e., different tumor sites can have different molecular profiles or clonal distributions), currently, bone marrow should be used for assessing the molecular profile.
Timing of risk assessment and risk status definition
It is important to perform risk assessment at diagnosis and at relapse.
Risk features should be evaluated at relapse and prior to participation in a clinical trial.
The risk status at relapse supplants risk status at diagnosis, unless the patient was deemed high-risk at diagnosis.
CA, chromosomal abnormality; GEP, gene expression profiling; iFISH, interphase fluorescence in situ hybridization; MM, multiple myeloma
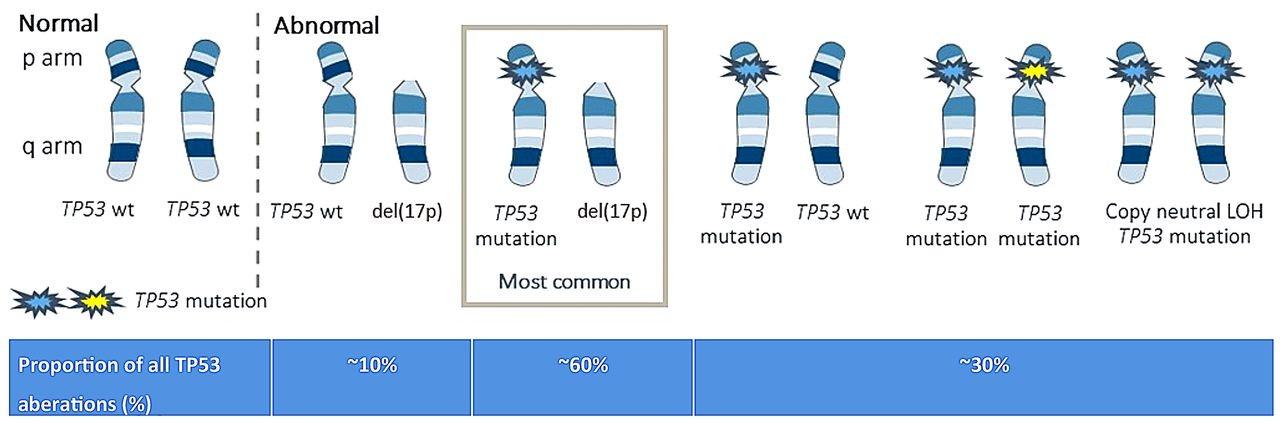
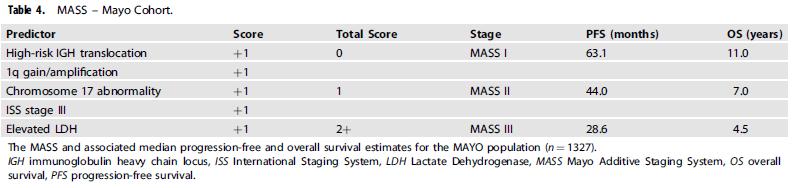
(Nuclear Receptor SET Domain Protein 2 = histone methyl transferase)
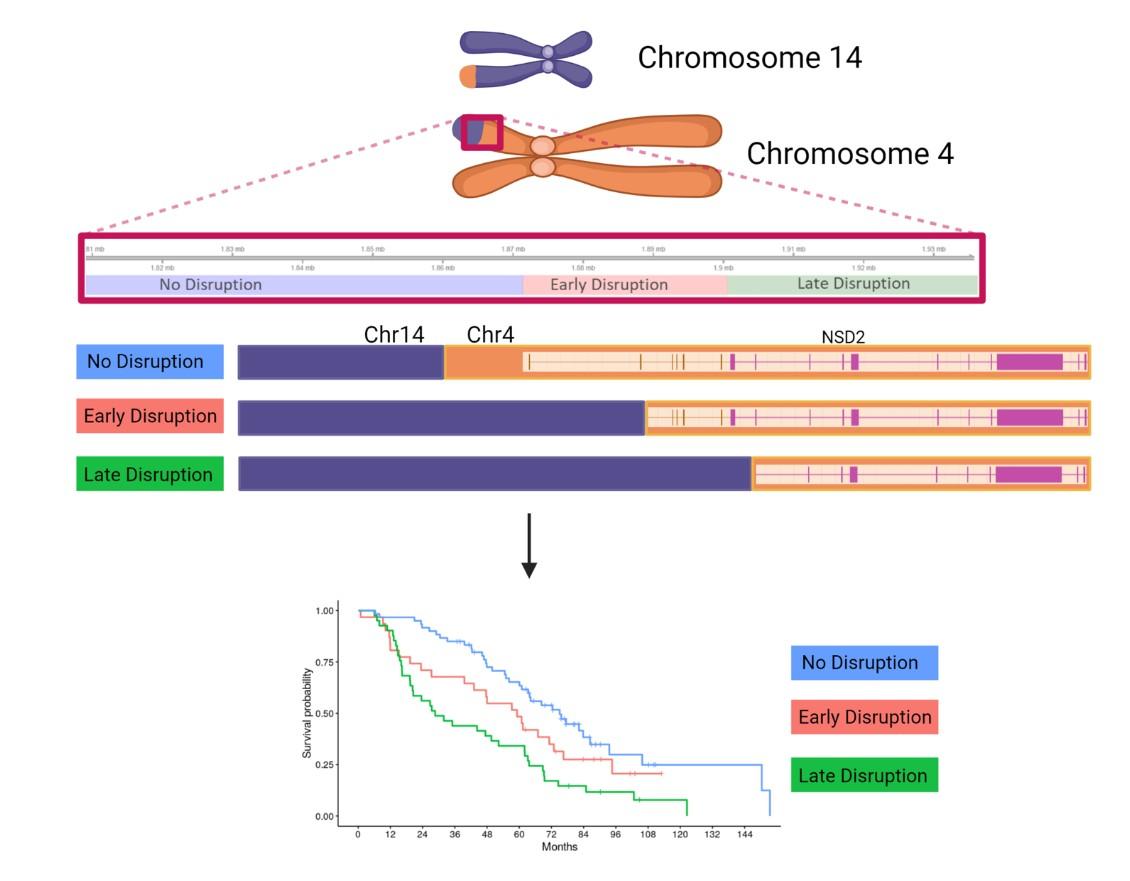
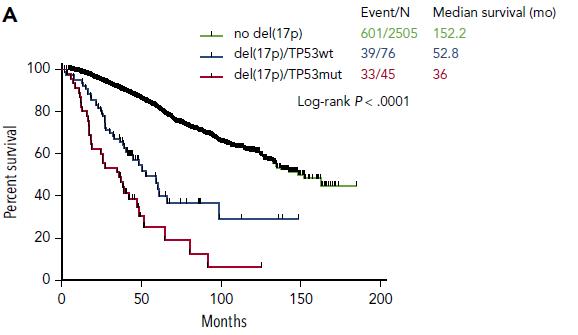
75,1 months (≈ 45%)
59,4 months (≈ 25%)
28,6 months (≈ 30%)
p=0.00019
FISH (gold standard) Next Generation Sequencing
20 000 plasma cells / probe [cryostor]
≥ 45 000 plasma cells [dry pellet]
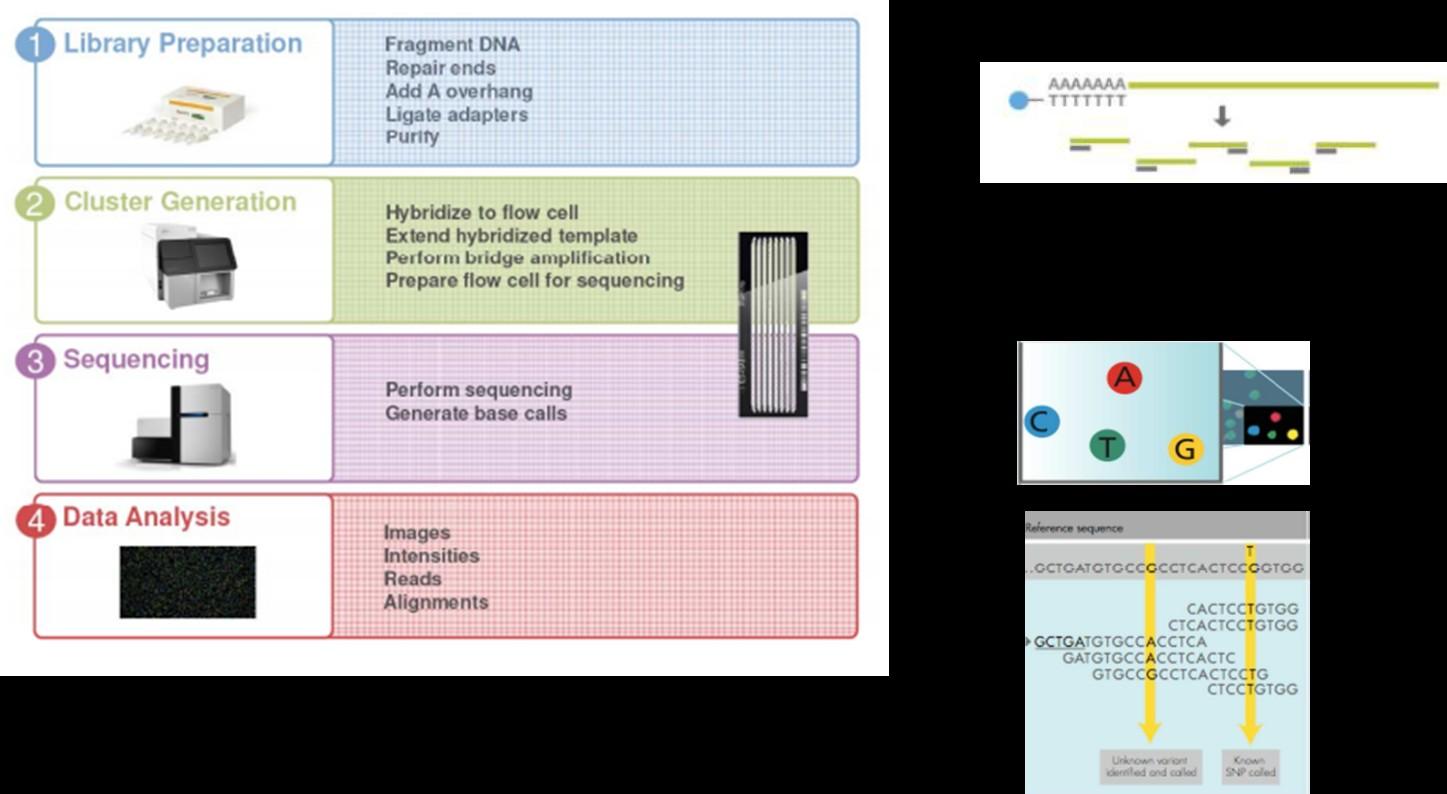
Targeted sequencing panel (3Mb) including :
≈200x depth
- ≈ 2400 SNPs : ALL COPY NUMBER VARIATIONS = del(17p), gain 1q, del(1p32)...
- all IGH sequence: 14q32 TRANSLOCATIONS = t(11;14), t(4;14), t(14;16), t(14;20), t(6;14), t(8;14)
- MUTATIONAL PROFIL
- TP53, NRAS, KRAS, BRAF, FAM46C, DIS3, ATM, ATR, MYC, TRAF3, BIRC2, BIRC3, CYLD, IRF4, CDKN2C…
- CRBN, BCMA, GPRC5D, FCRH5
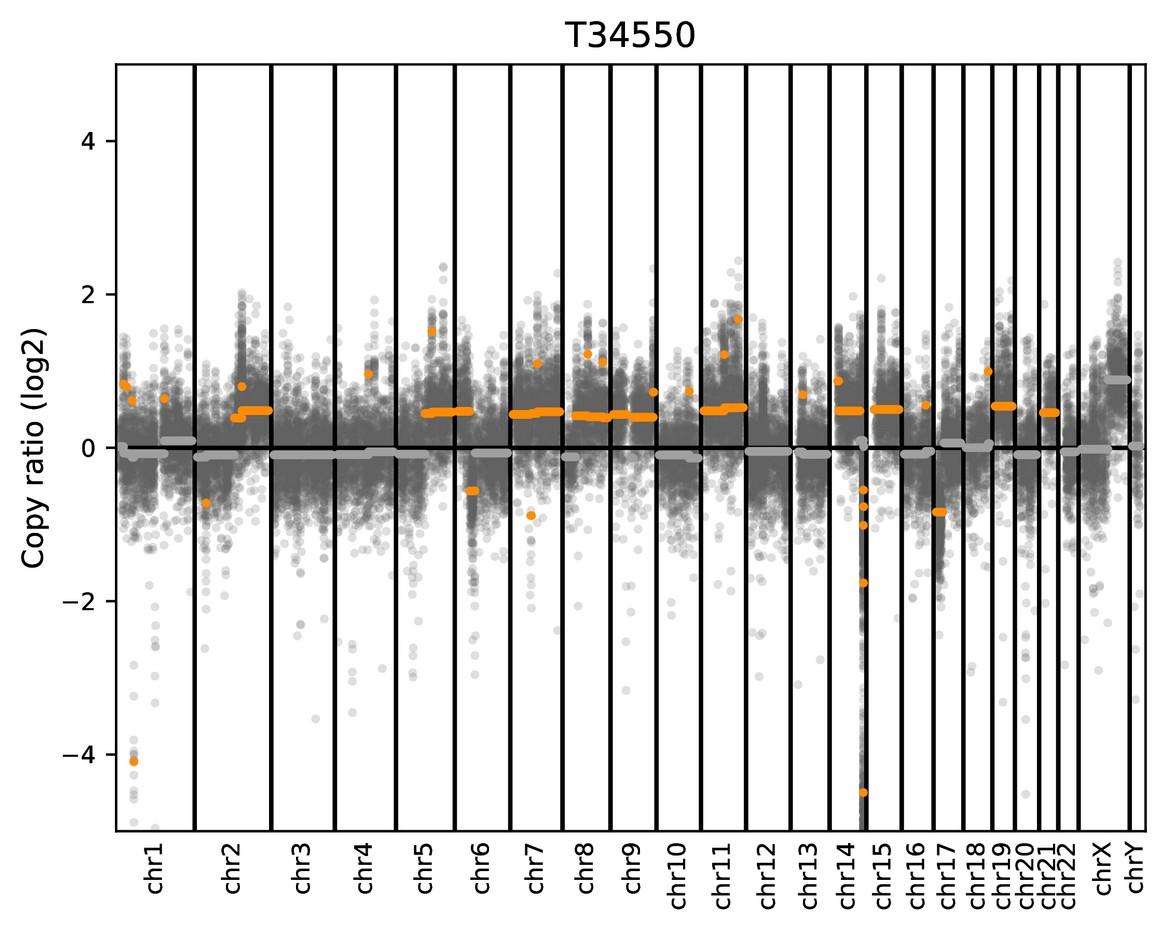
Double focal deletion of CDKN2C (1p32)
Not detected by FISH
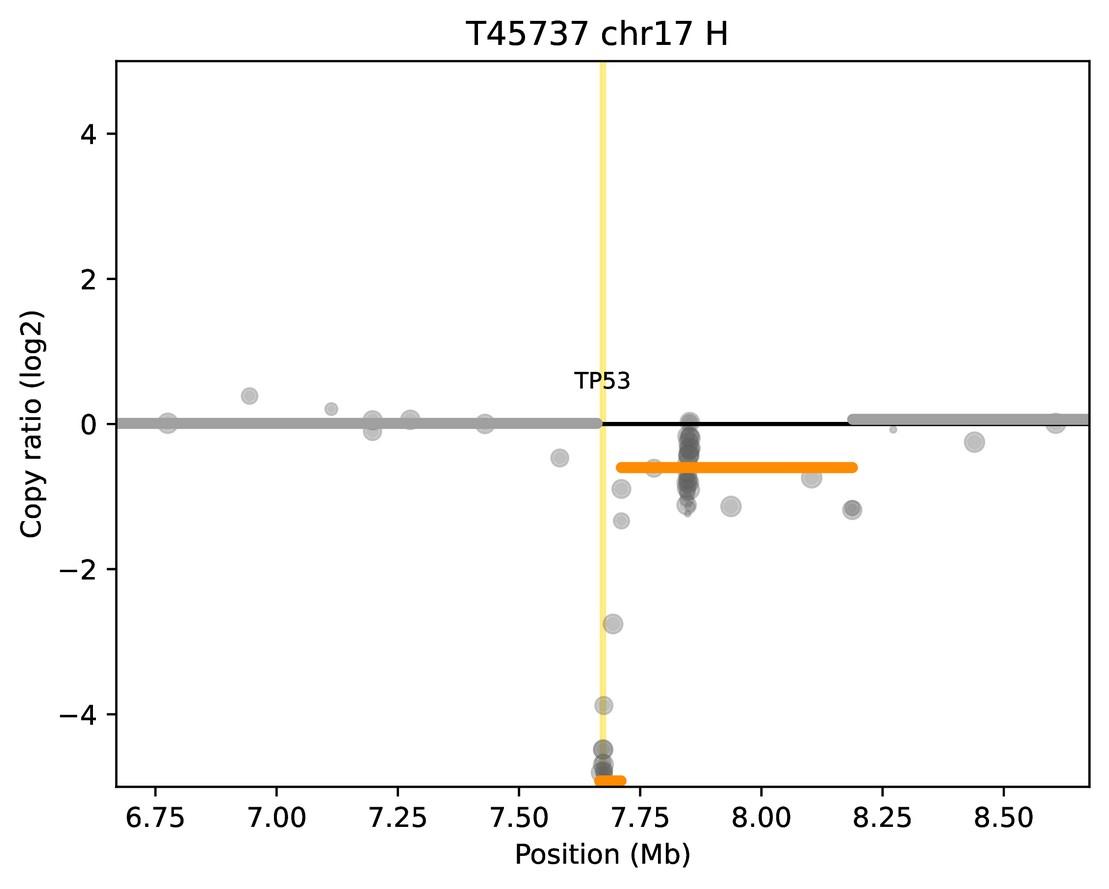
Double focal TP53 deletion Not detected by FISH
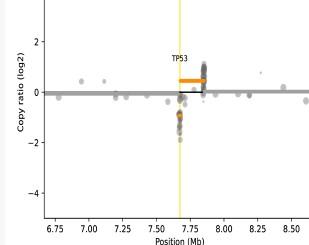
Focal TP53 deletion
Not detected by FISH

Risk features should be evaluated at relapse and prior to participation in a clinical trial.
The risk status at relapse supplants risk status at diagnosis, unless the patient was deemed high-risk at diagnosis.
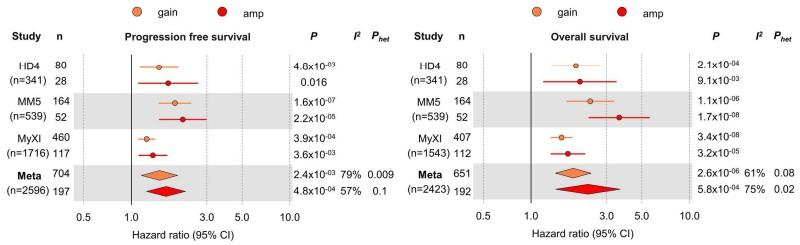
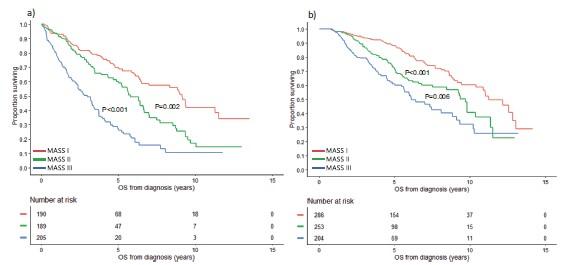
Niels Weinhold et al., Haematologica 2021
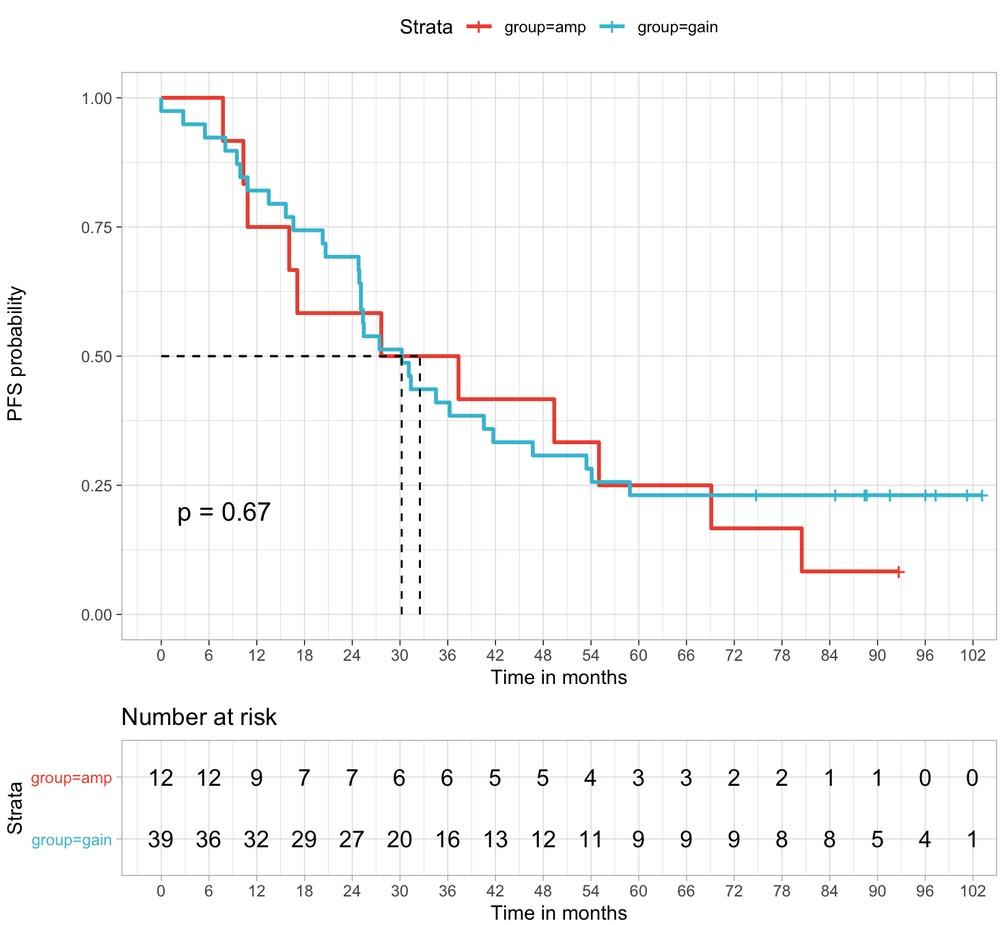
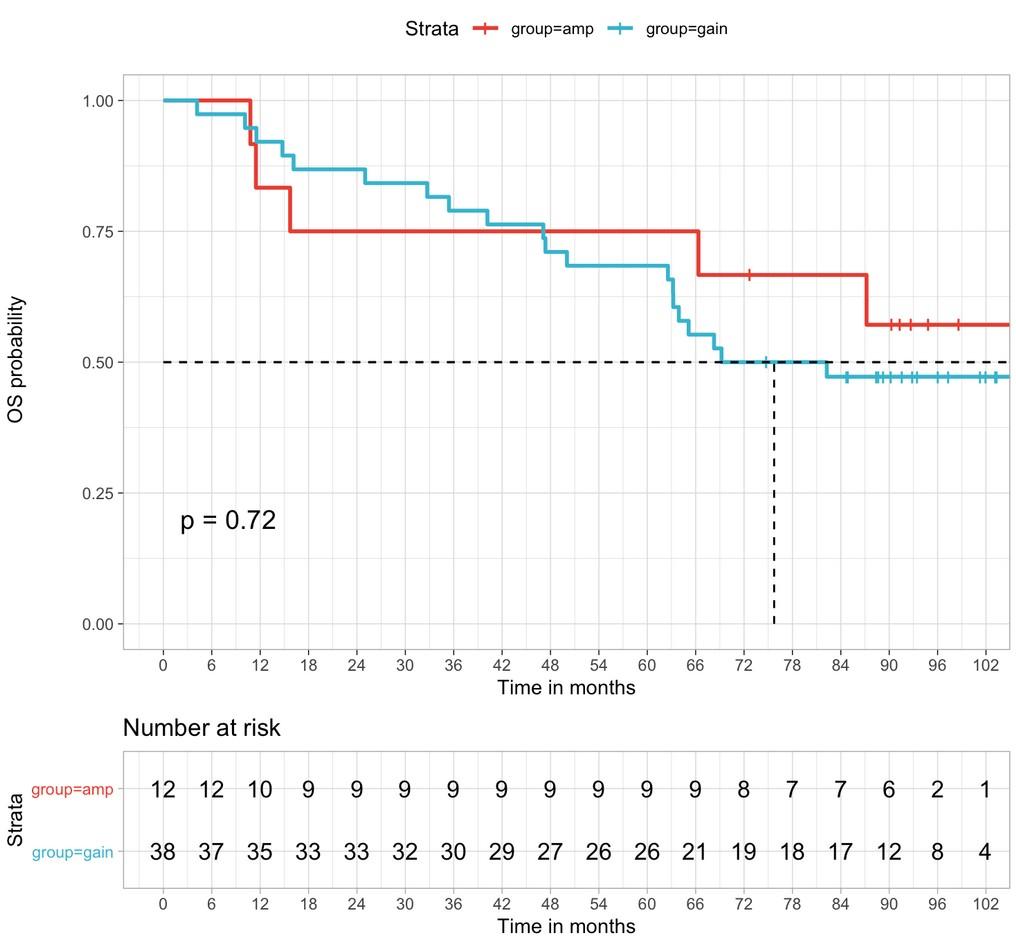
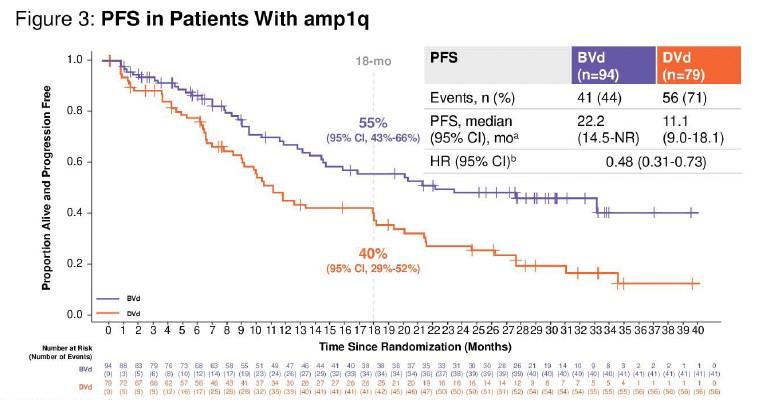
amp(1q) vs. gain(1q) vs. normal 1q: PFS
amp(1q) vs.
amp(1q) vs. normal 1q: HR 4.40, 95% CI 2.59–7.49, p<0.0001 amp(1q) vs. gain(1q): HR 3.22, 95% CI 1.89–5.49, p<0.0001 gain(1q) vs. normal 1q: HR 1.37, 95% CI 0.81–2.32, p=0.25
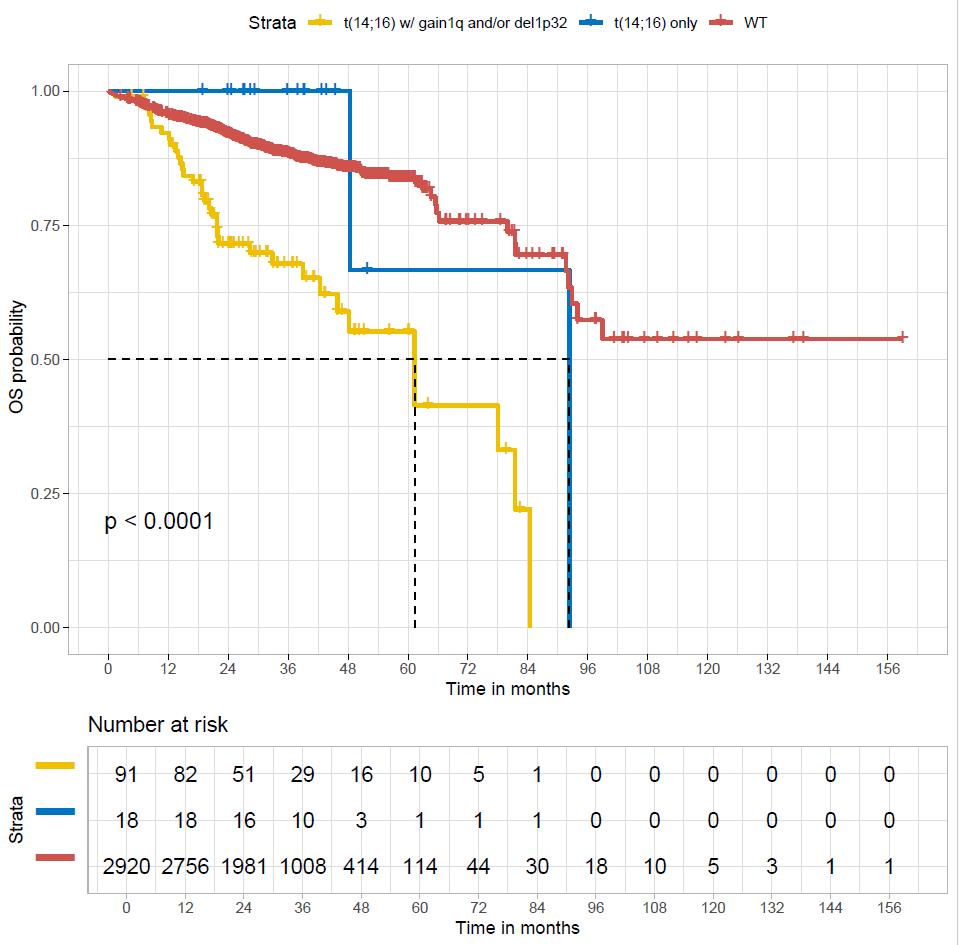
Schavgoulidze et al. Blood Cancer
Multivariate analysis of all recurrent abnormalities → Construction and validation of a prognostic model
6 independent variables weighted by a specific coefficient:
• Trisomy 5 - 0.3
• Trisomy 21 0.3
• t(4;14) 0.4
• gain 1q 0.5
• del(1p32) 0.8
• del(17p) 1.2
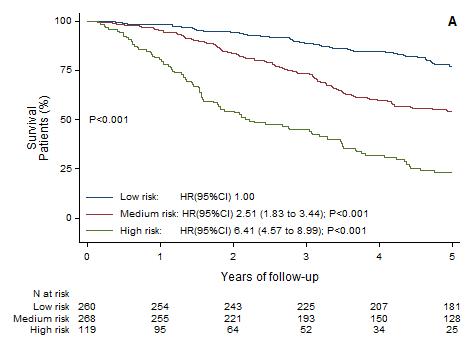
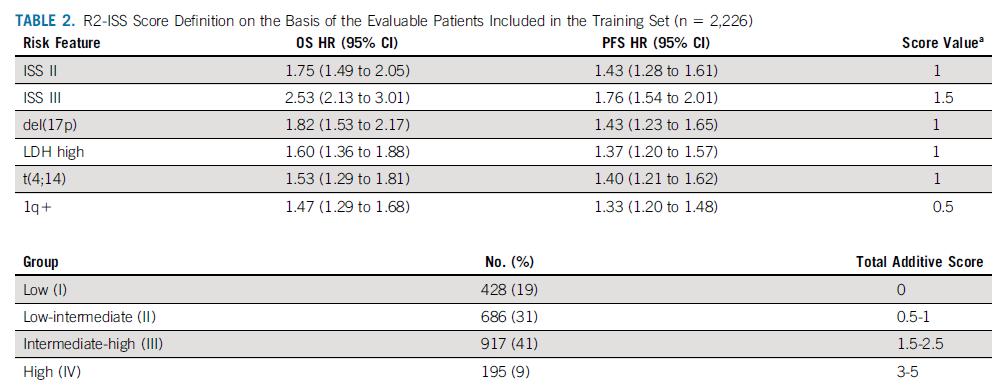
Score ≤ 0: LOW RISK (≈55%)
Score < 0 & ≤ 1: INTERMEDIATE RISK (≈35%)
Score > 1: HIGH RISK (≈15%)
trisomy 21 trisomy 5 t(4;14) t(14;16)
t(4;14), t(14;16), t(14;20)
et al. Blood Cancer J 2022

Shaji Kumar, M.D.
Mark and Judy. Mullins Professor of Hematological
Chair, Myeloma and Amyloidosis Group Mayo Clinic

Scottsdale, Arizona

Rochester, Minnesota

Jacksonville, Florida

• Research funding for clinical trials to the institution: Celgene, Takeda, Janssen, BMS, KITE, Merck, Abbvie, Medimmune, Novartis, RocheGenentech, Amgen, Tenebio, Carsgen
• Consulting/Advisory Board participation: (with no personal payments) Celgene, Takeda, Janssen, Abbvie, Genentech, Amgen, Molecular Partners and (with personal payment) Oncopeptides, Genecentrix, Cellectar.

• Response assessment is critical to assess treatment efficacy
• Uniform assessment allows for comparison of treatment approaches
• Response depth has prognostic value
• Increasingly response depth allows us to adapt treatment approaches

Serum, Urine
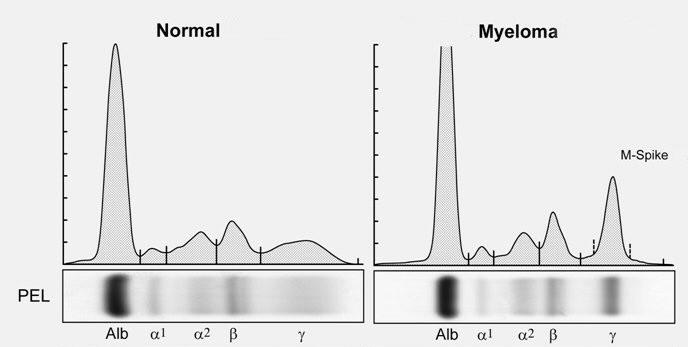
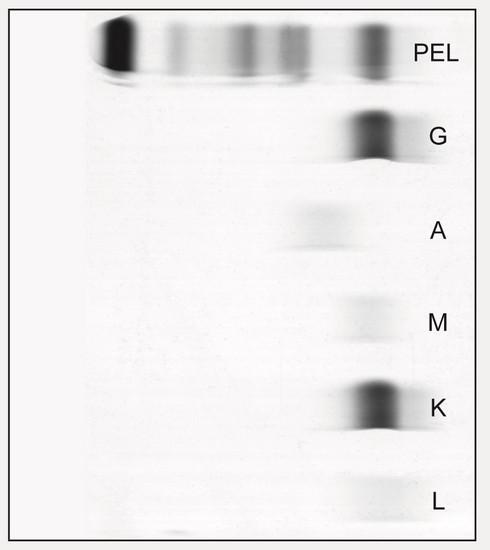
Protein
Marrow, Blood, Extramedullary
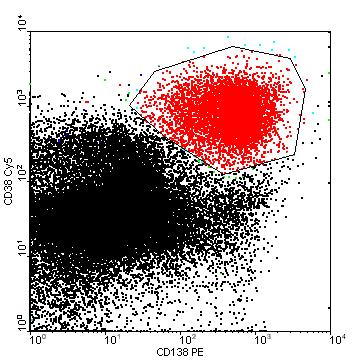
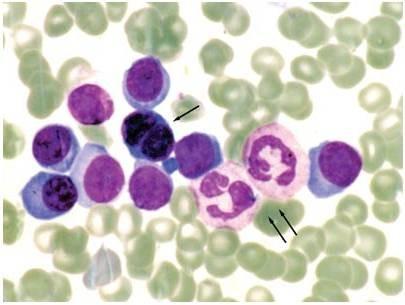
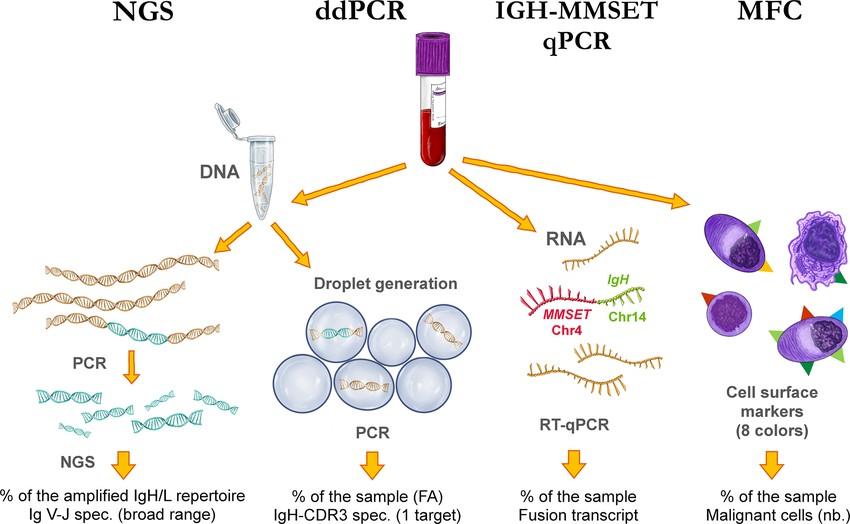
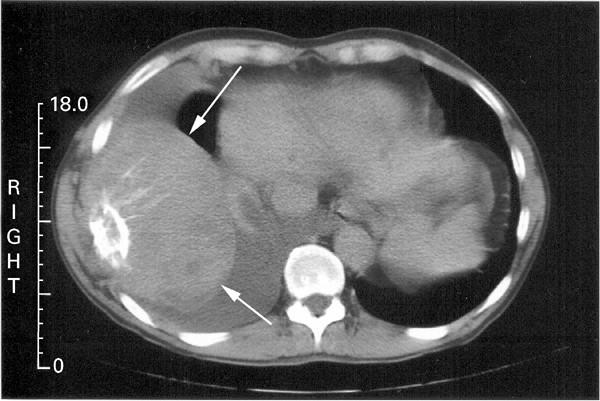
• SPEP ≥ 1g/dl (≥0.5 allowed in relapsed disease)
• Serum iFLC ≥10 mg/dL with an abnormal ratio
• 24-hour urine UPEP ≥200 mg/24 hours can be used in place of FLC assay if not available
• If selected should be followed for response
If none of the above, then -
• BMPC≥30%
• Soft tissue mass (para-skeletal or EMD) with one dimension
≥2 cm
• Stable disease - Not meeting criteria for complete response, very good partial response, partial response, or progressive disease
• Nor recommended for use as an indicator of response; stability of disease is best described by providing the time-toprogression estimates.
• Minor Response - ≥25% but ≤49% reduction of serum M-protein and reduction in 24-h urine M-protein by 50–89%.
• Partial Response
• If serum is measurable by SPEP - ≥50% reduction of serum M-protein
• If baseline Serum M spike was ≥0.5 and <1, then a reduction to <0.3 gm/dL
• If serum FLC is measurable - ≥50% decrease in the difference between involved and uninvolved FLC levels
• If 24 h urinary M-protein is used as measurable disease, a reduction by ≥90% or to <200 mg per 24 h
• If serum and urine M-protein and serum-free light assay are unmeasurable, ≥50% reduction in plasma cells (provided baseline PC% is > 30%)
• Very Good Partial Response (VGPR)
• If serum is measurable by SPEP - ≥90% reduction of serum Mprotein or immunofixation positive only
• If baseline Serum M spike was ≥0.5 and <1, then a reduction to 0 gm/dL
• If serum FLC is measurable - ≥90% decrease in the difference between involved and uninvolved FLC levels or immunofixation positive only
• If 24 h urinary M-protein is used as measurable disease, a reduction to <100 mg per 24 h or immunofixation positive only
• Complete Response
• Negative immunofixation on the serum
• normal FLC ratio or iFLC < ULN
• Negative immunofixation on the 24-hour urine
• and <5% plasma cells in bone marrow aspirates
• sCR (stringent Complete response)
• Complete response as above plus normal FLC ratio iFLC < ULN and absence of clonal cells in bone marrow
* Bone marrow results from within 3 months of the M protein/FLC assessment can be included for CR assessment
• Recommendation for functional imaging (PET/CT or DWI-WBMRI) and move away from SPD
• For PSD/EMD functional imaging mandatory to allow response evaluation. MRI preferred for CNS.
• Same technique recommended pre and post therapy.
• Measures of the lesion(s) needed only if no other disease parameters to be quantified available
• 3 months window (+ or -) allowed to match with other tests (BM/PB)
PET/CT/DWI-
MRI response Definition
CR (MRD)
PR
SD
PD
Uptake ≤ liver pool (Deauville scale < 4) by PET or RAC-1 by DWI-MRI in all locations (Marrow and focal lesions)
Decrease of either number of focal lesions + stable SUV (DS 4-5),
OR Decrease in activity, with stable number of focal lesions, OR both or RAC-2 on MRI, compared to baseline
No significant change of BM/FL FDG uptake or at MRI RAC-3 compared with baseline
New lesion (FL/EMD/PSD) compared with baseline imaging, both in the functional imaging (PET DS > 4 or MRI RAC 4 or 5) or on CT
PET/CT/DWI-
MRI response Definition
CR (MRD)
PR
SD
PD
Uptake ≤ liver pool (Deauville scale < 4) by PET or RAC-1 by DWI-MRI in all locations (BM/FL/PSD/EMD) irrespective of the reduction of soft-tissue plasmacytomas size
Decrease of either number of FLs/PSD/EMD + stable SUV (DS 4-5),
OR Decrease in activity, with stable number of FLs/PMD/EMD, OR both or RAC-2 on MRI, compared to baseline irrespective of the reduction of soft-tissue plasmacytomas size.
No significant change in BM/FL/EMD/PSD uptake or at MRI (RAC-3) compared with baseline, irrespective of soft-tissue plasmacytoma size
New lesion (FL/EMD/PSD) compared with baseline imaging, both in the functional part (PET DS > 4 or MRI RAC 4 or 5) or on CT
First evaluation after 3 months from the start of therapy (to avoid as much as possible background influence/tumor flare after CART/ bone regeneration). If PR/SD: repeat imaging every 3-6 months until CMR/RAC-1. Once CR established, no other evaluation requested until suspect of progression
• MRD negative CR - Absence of aberrant clonal plasma cells by NGF or NGS on bone marrow aspirates with a minimum sensitivity of at least 1 in 10⁵ nucleated cells
• MRD rate at 10-6 rates should be reported when feasible designated as MRD negative CR 10-6
• If MRD negative as defined above but no negative immunofixation, should be defined as NGF/NGS negative (without CR) at either threshold
• Imaging negative MRD neg – MRD as defined above at either threshold with a functional imaging (PET-CT or WB-DWI MRI) negative
• Sustained MRD negative CR– MRD negative CR as defined above at either threshold with two negative MRD tests at least 24 months apart, and without any positive test in between
• MRD at 10-6 by NGS
• Negative by functional imaging (PET-CT or WB-DWI MRI)
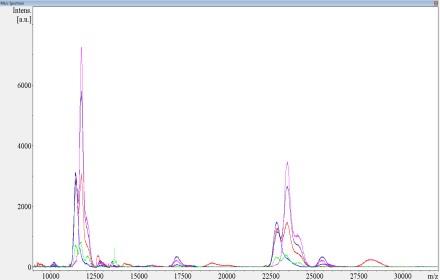
• No monoclonal protein by mass spec
• No circulating plasma cells
• This will form the building block for a cure definition in future, when sustained for 5 years without therapy
• ≥25% increase in the level of the serum monoclonal paraprotein, which must also be an absolute increase of at least 5 g/l
• ≥25% increase in the difference between involved and uninvolved FLC levels. The absolute increase must be ≥10 mg/dl
• ≥25% increase in the 24 h urinary light chain excretion, which must also be an absolute increase of at least 200 mg/24 h
•Definite increase in the size of existing bone lesions or soft tissue plasmacytomas (Deauville score> 4 (increase of SUV > liver) in (or MRI DWI RAC >= 3) in one or more existing lesion)
•≥25% increase in plasma cells in a bone marrow, which must also be an absolute increase of at least 10% only in patients without measurable disease by serum and urine
•Development of new bone lesions or soft tissue or para-skeletal plasmacytomas
•≥50% increase in circulating plasma cells (minimum of 200 cells per μL) if this is the only measure of disease
• Confirmation of monoclonal protein measurements are required for every category, but sequential confirmation can be replaced by simultaneous confirmation – two measurements
• 24 hour urine measurements are required at baseline, and if M protein present, will need retesting only to confirm CR


More sensitive mass spectrometry (using expected protein sequence)


Circulating tumor/free DNA
Novel imaging techniques


Tisch Cancer Institute, Mount Sinai

Session Chair: Philippe Moreau, MD
University Hospital Nantes


University Hospital of Toulouse


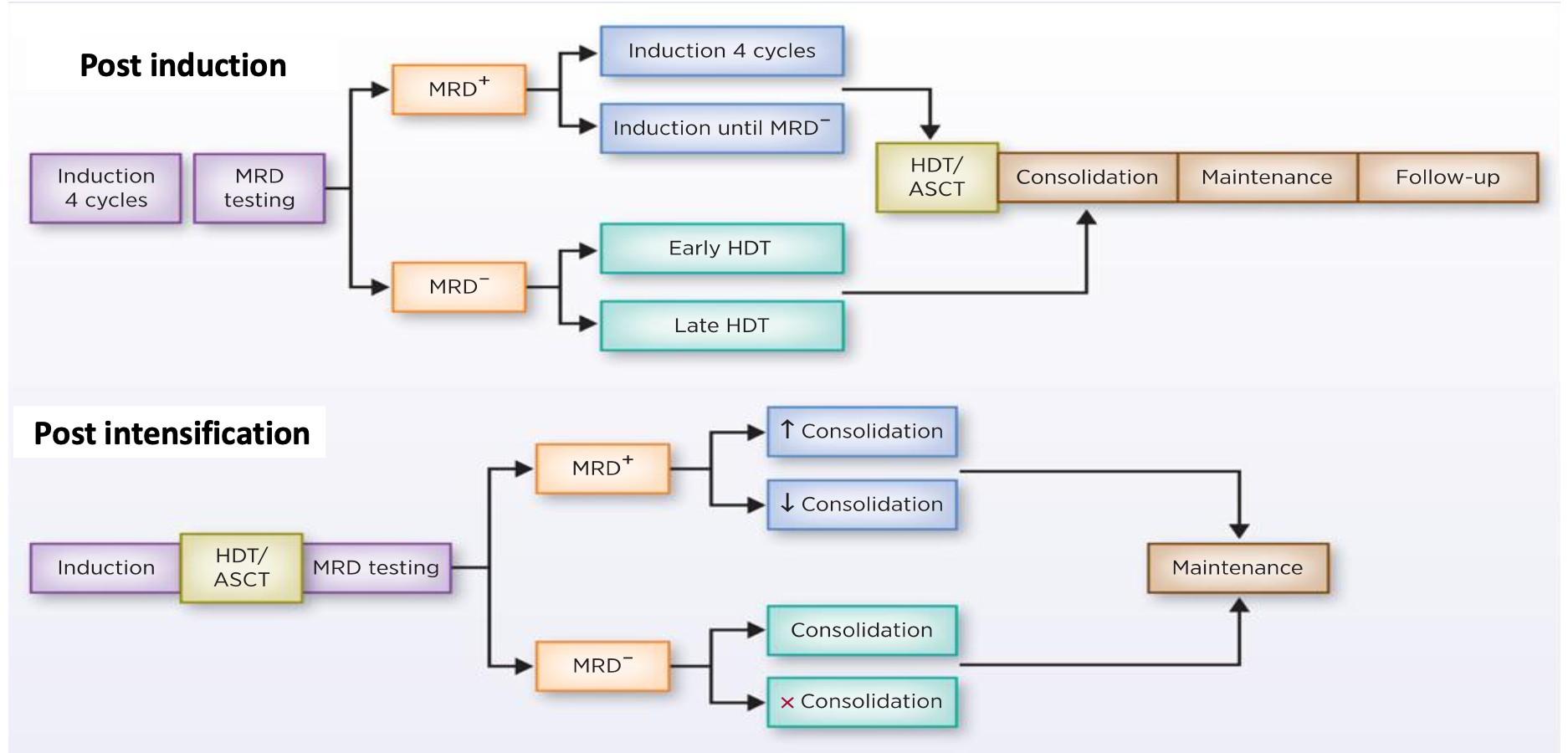
After induction
After transplant
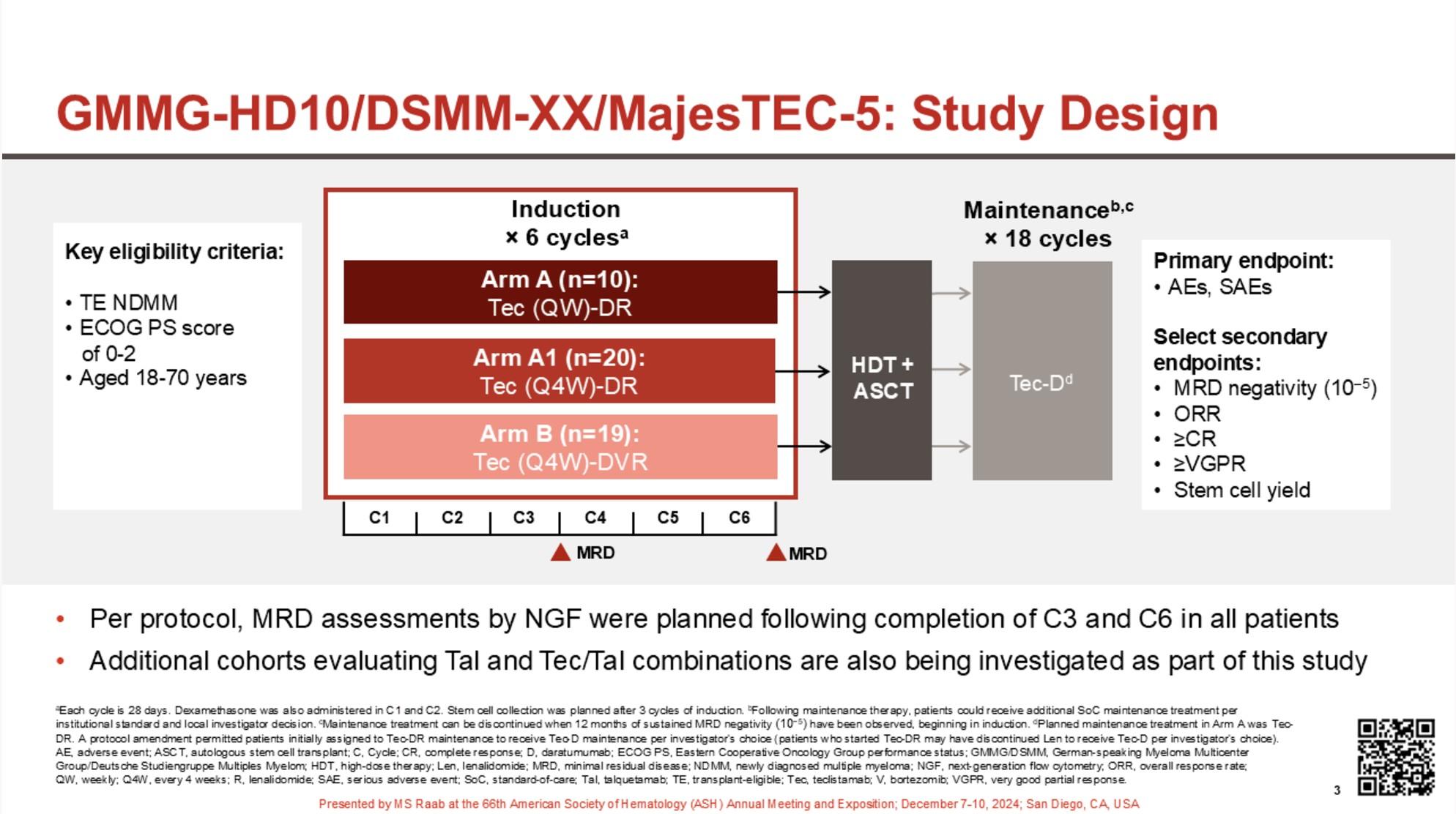
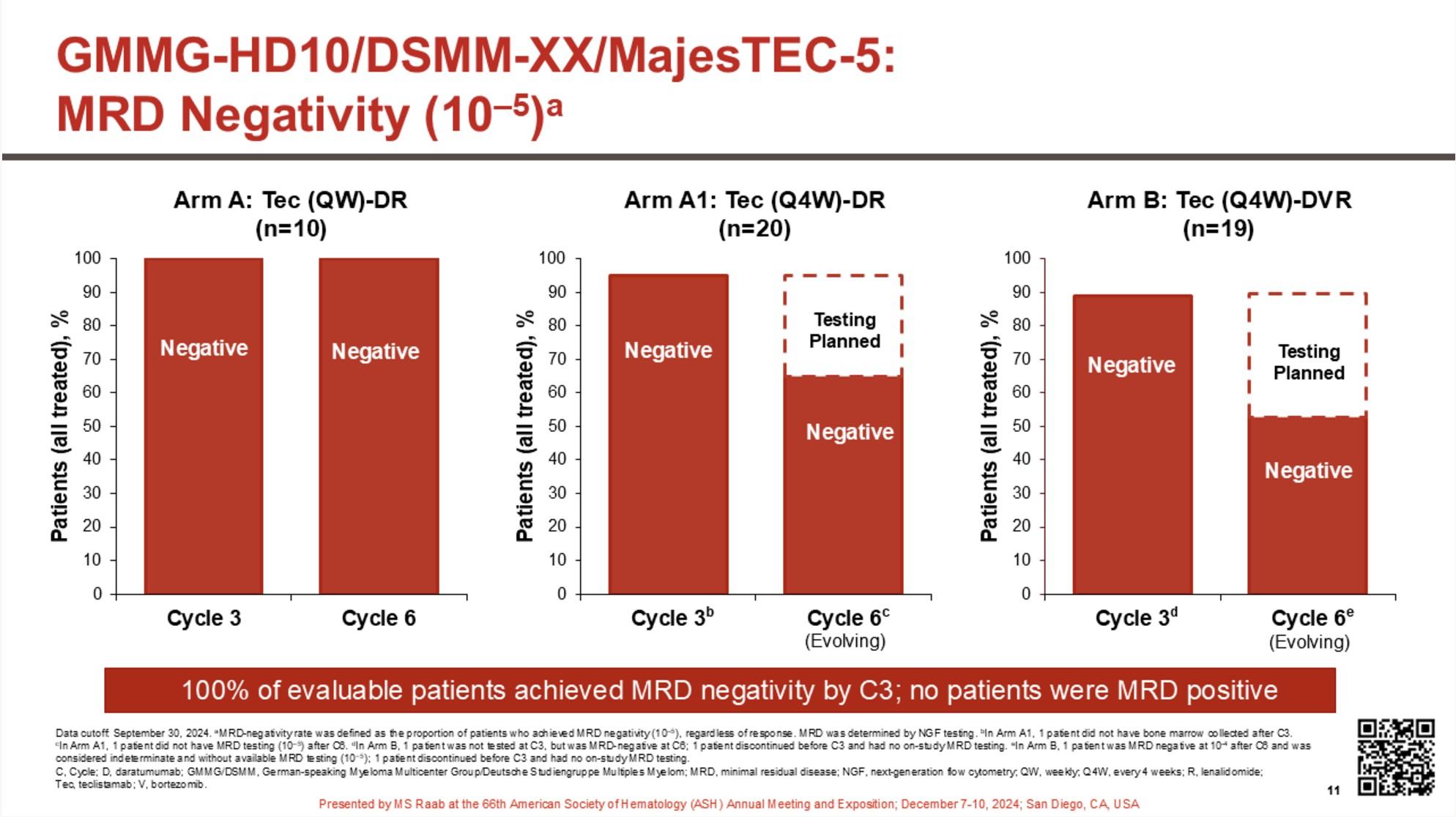
eligibility criteria:
Transplant-
Primary endpoint: PFSc
Key secondary endpoints: Overall CR rate,c overall MRD-negativity rate,d OS
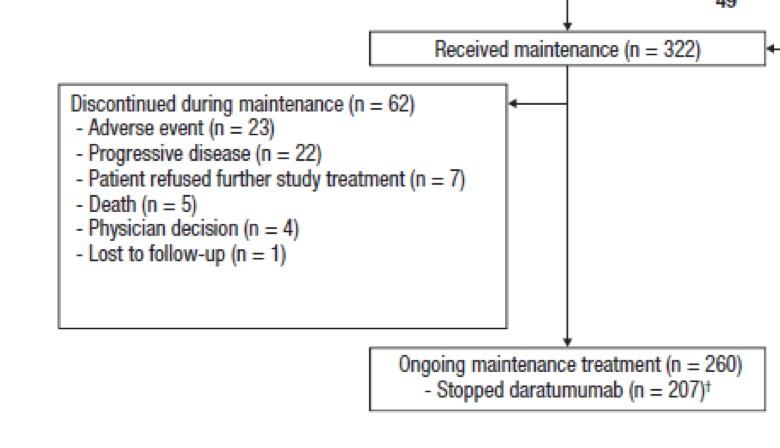
Discontinue DARA therapy only after 24 months of D-R maintenance for patients with CR and 12 months of sustained MRD negativity
Restart DARA therapy upon confirmed loss of CR without PD or recurrence of MRD
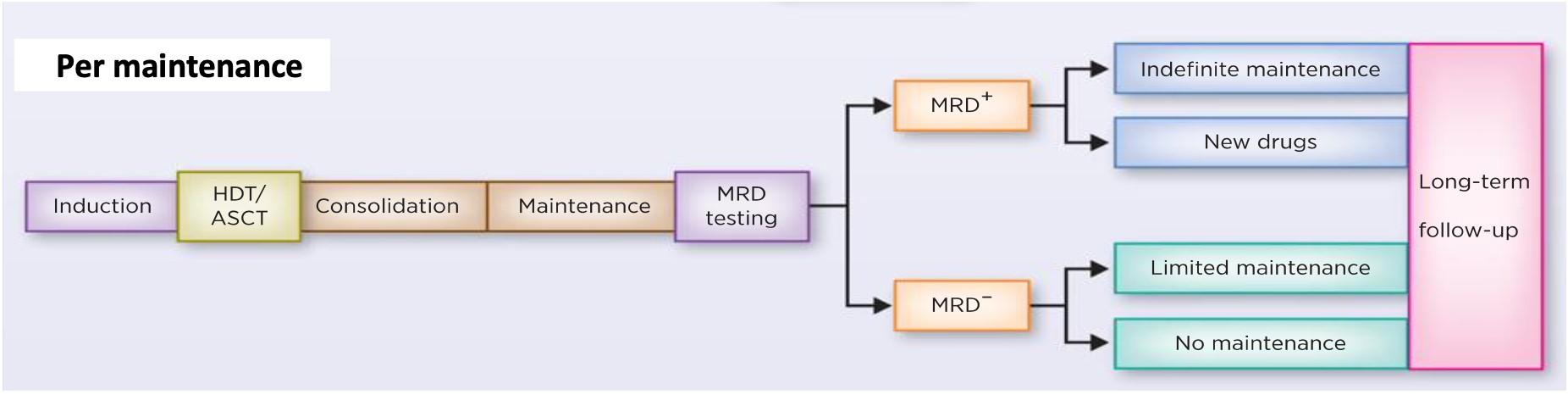
Maintenance
DARA: 1,800 mg SCb Q4W
R: 10 mg PO Days 1-28
MRD positive
Continue D-R until PD
MRD negative
Discontinue DARA therapy only
28-day cycles
Discontinue DARA therapy only
after 24 months of D-R maintenance for patients with CR and 12 months of sustained MRD negativity
Restart DARA therapy upon confirmed loss of CR without PD or recurrence of MRD
Treatment interruption if « sustained » MRD negative
Early interruption:
Not suitable for patients with high-risk cytogenetics
Phase 3 MRD-driven trial
Induction
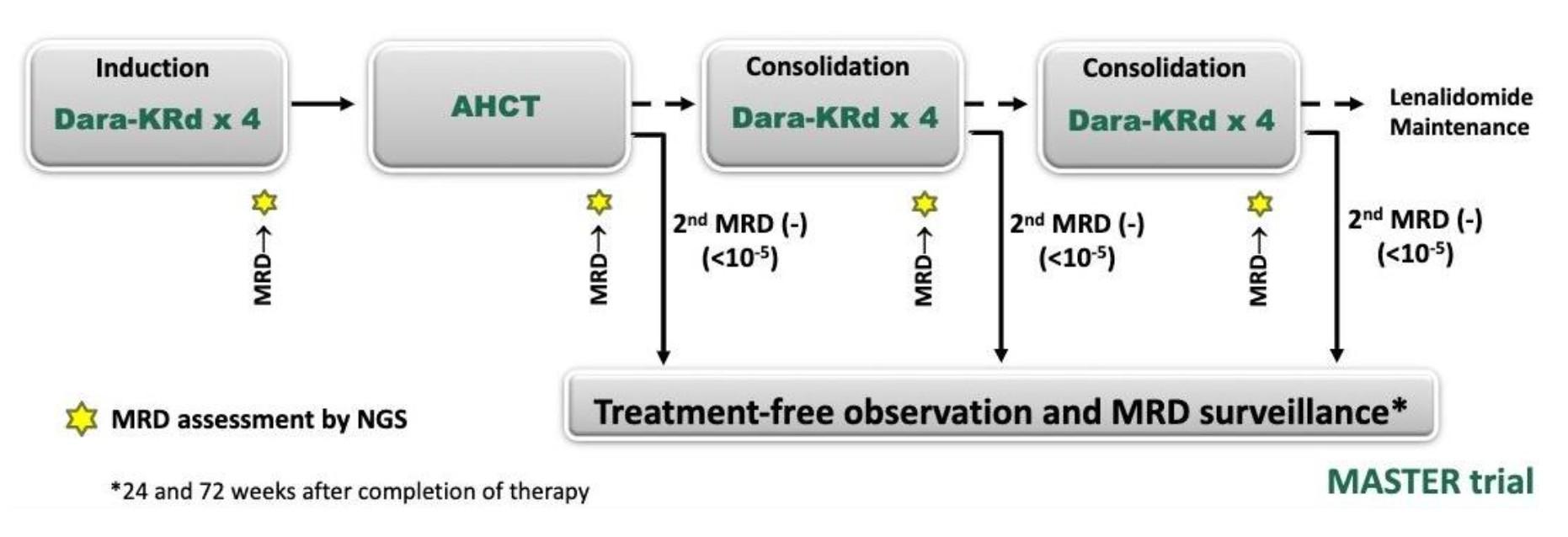
MIDAS = MInimal residual Disease Adapted Strategy
MRD evaluation
x 6 1:1
IsaKRD x 6 (28d cycles) MRD
Stem cell collection after cycle 3 (G-CSF+/- plerixafor)

Risk-adapted consolidation and maintenance Standard risk (MRD <10-5) High risk (MRD >10-5)
ASCT + IsaKRD x 2
ASCT + IsaKRD x 2
(3 years)
Key eligibility criteria
NDMM < 66y
Transplant-eligible
ECOG 0-2
No active cardiac disease
. 791 patients included in 72 centers (Dec 2021 – Jul 2023) . 757 completed induction . 761 patients did at least one stem cell harvest

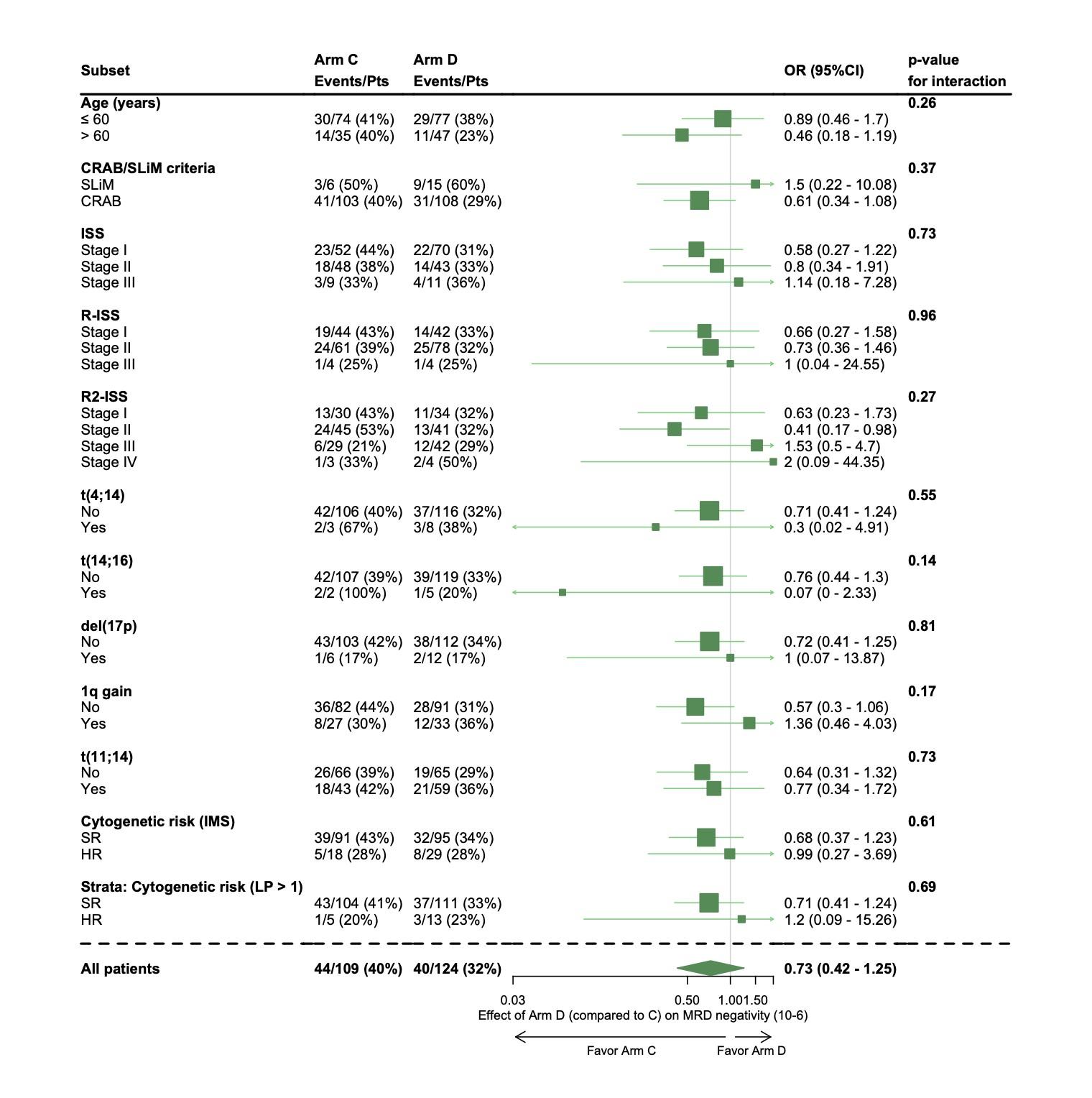
High-risk
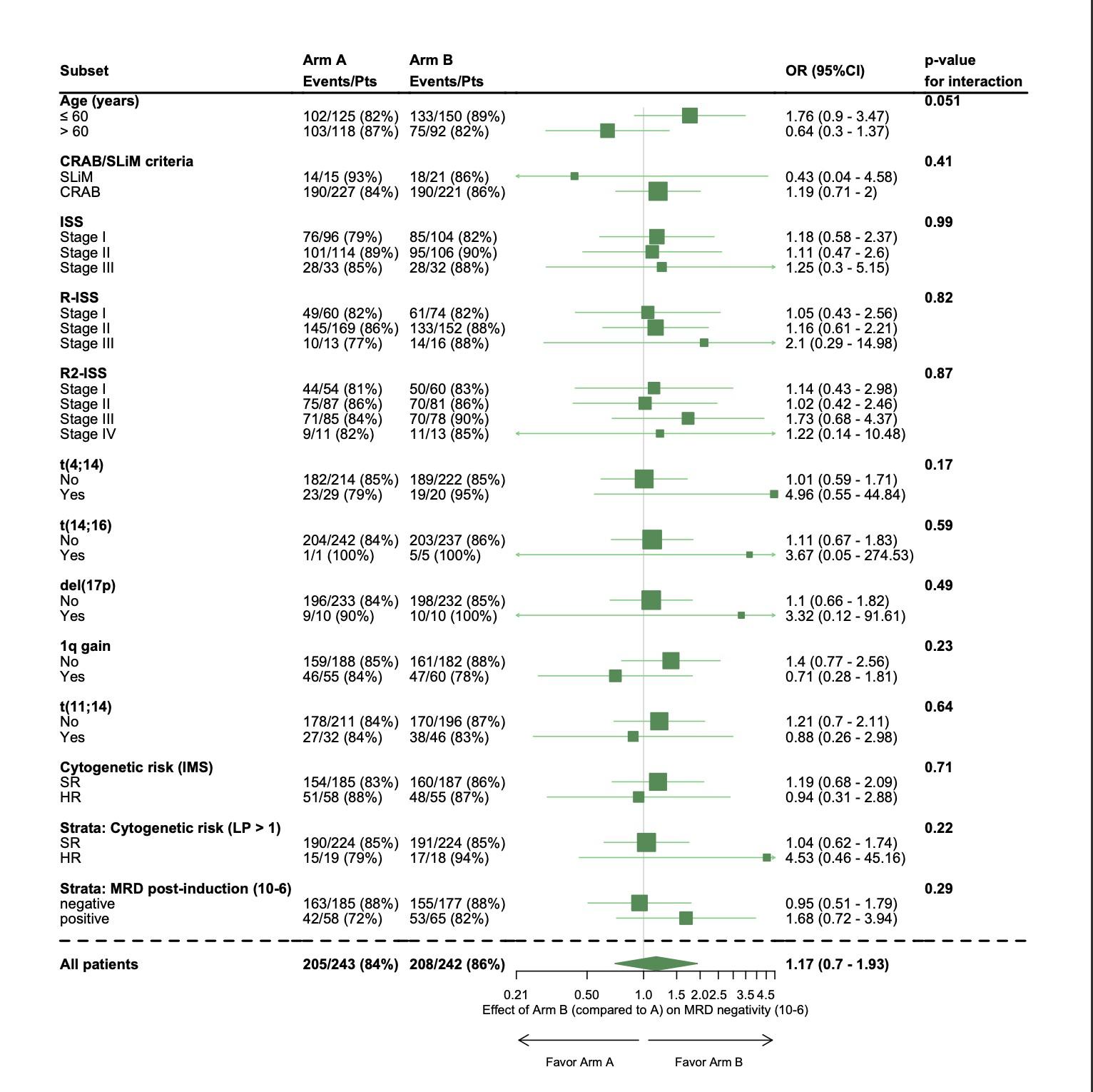
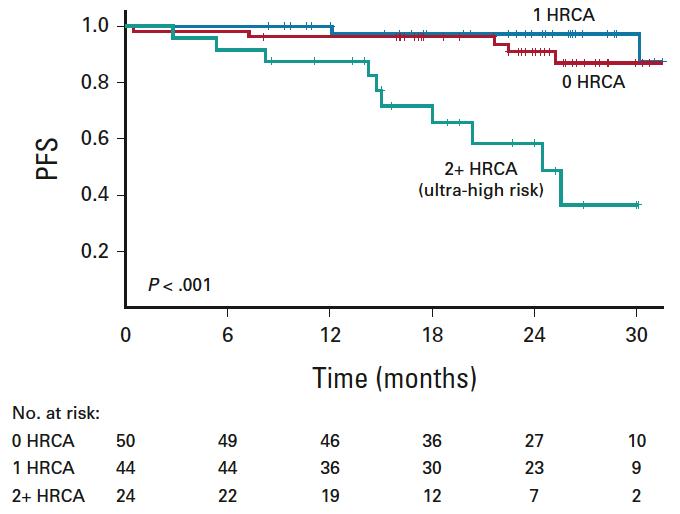
Per protocol analysis
Per protocol analysis
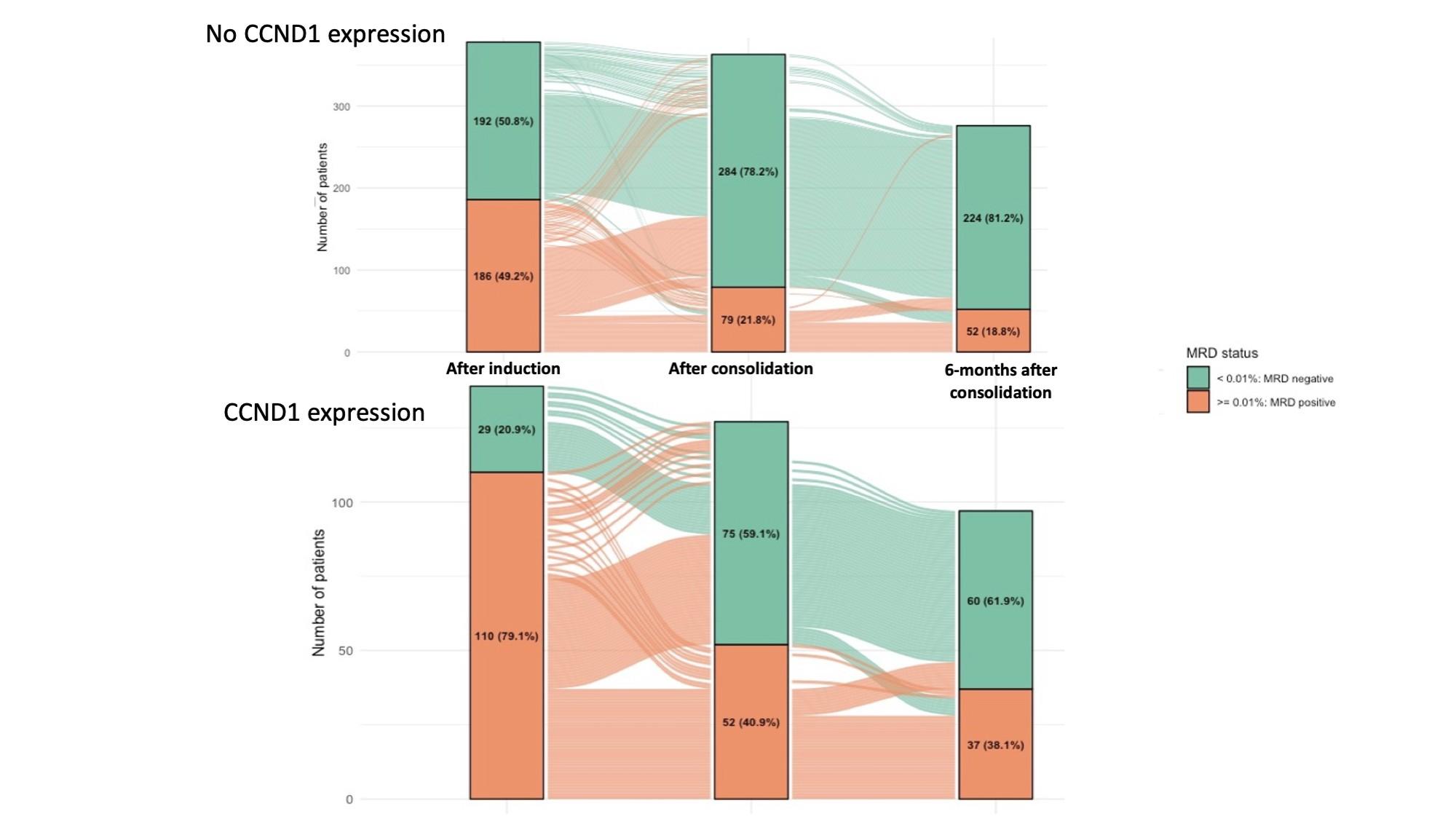
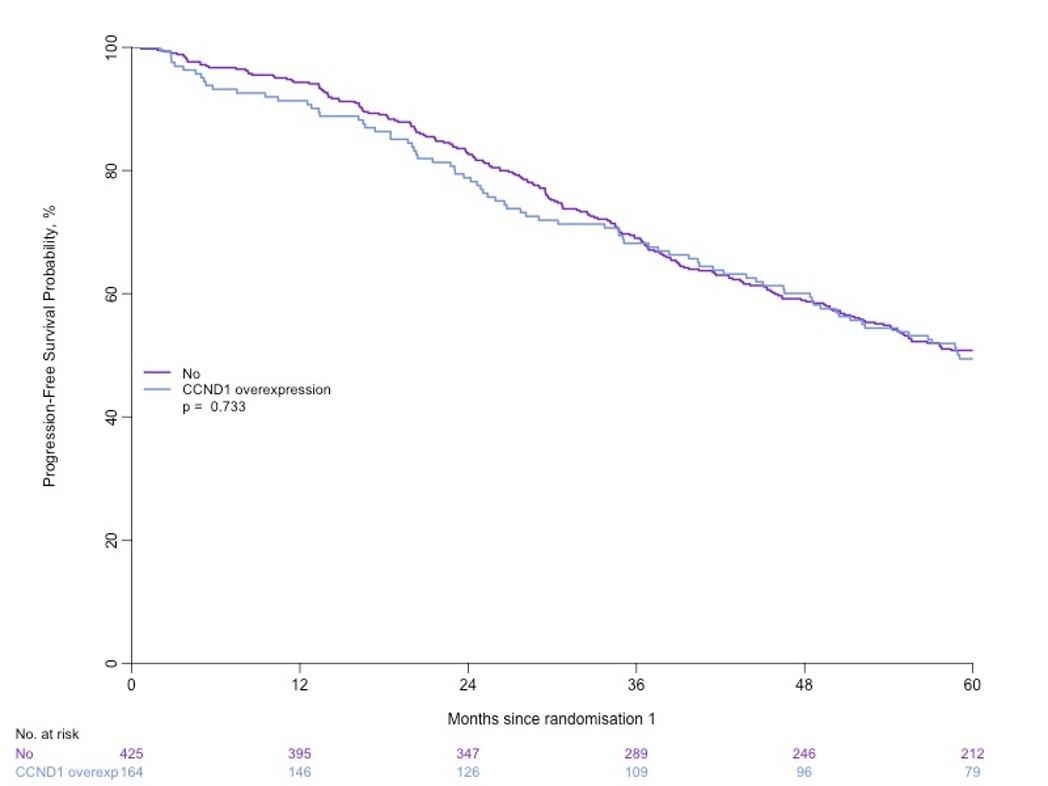
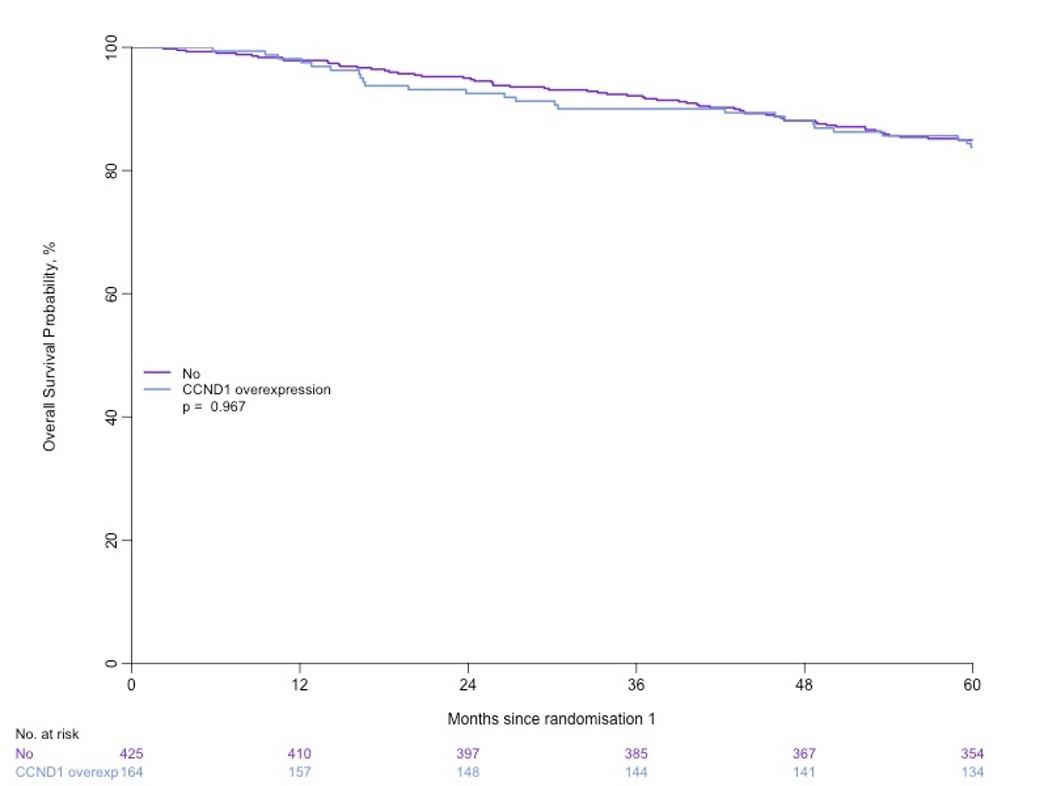
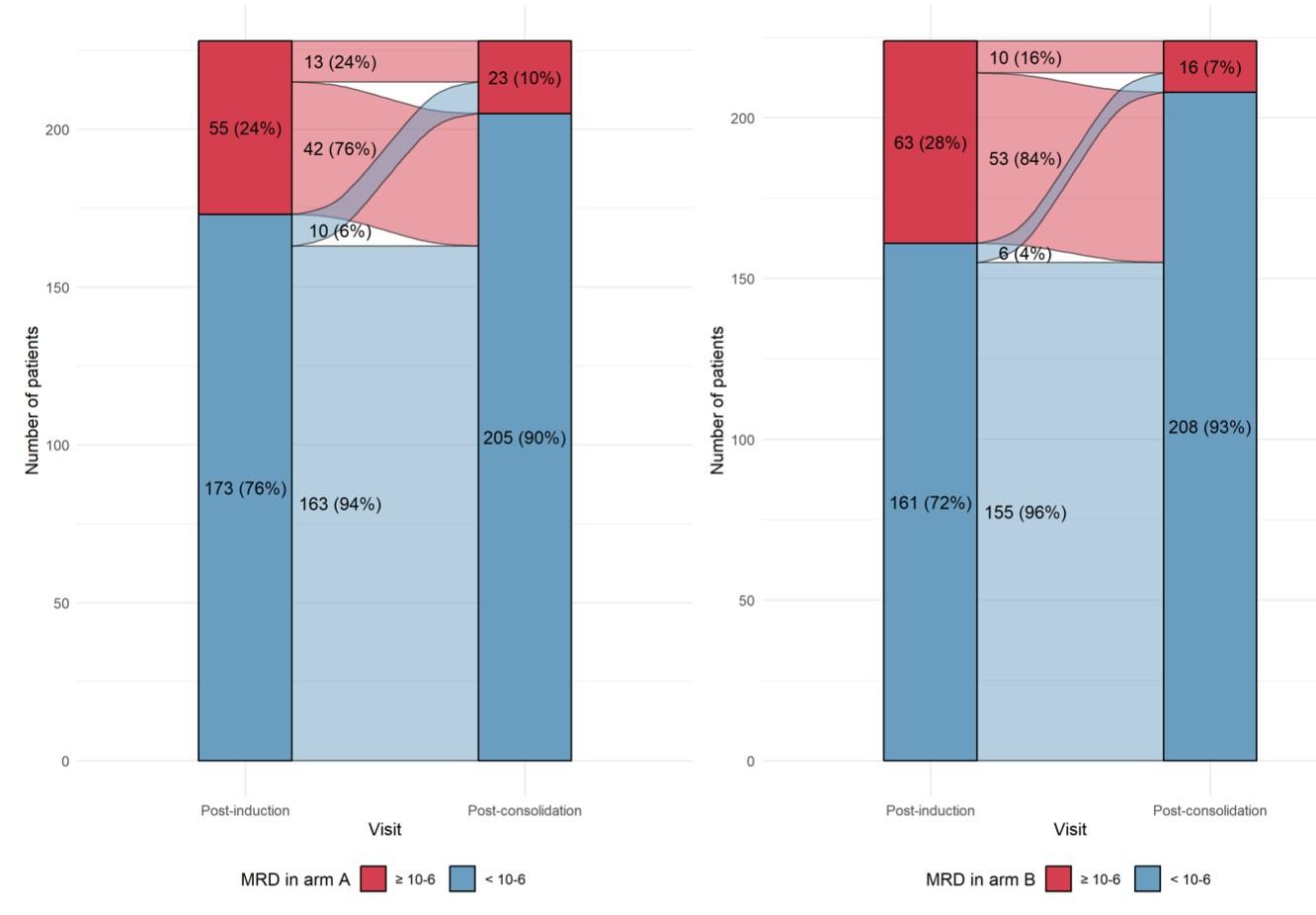
MASTER trial: No early treatment discontinuation despite MRD negativity in patients with high-risk cytogenetics
– Can transplant be differed in MRD-negative patients after induction without PFS data?
– Tandem transplant can probably be abandonned
PERSEUS trial
Early relapses observed after discontinuation of daratumumab during maintenance?
Open Questions
Should we consider different strategies for high-risk cytogenetics?
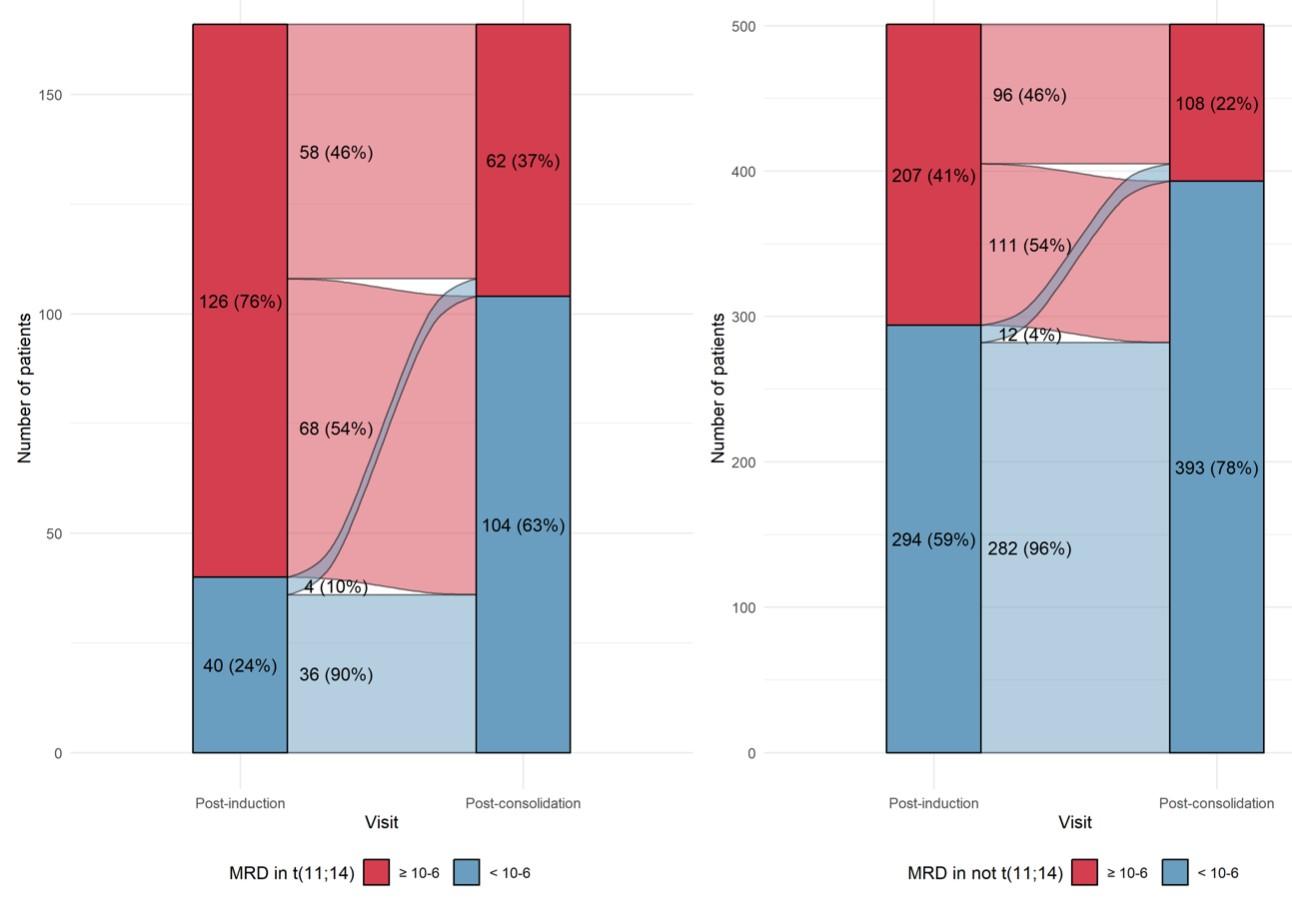
Could MRD after induction serve as an early stratification factor in slow responders such as patients with t(11;14)?



Professor and Chair
Department of Hematology and Medical Oncology
Anne and Bernard Gray Professor in Cancer Chief Medical Officer, Winship Cancer Institute
Emory University School of Medicine


• What is the right regimen
• Is there a difference between transplant ineligible and frail?
• How long to treat
• Optimal endpoint and timing (early vs late)
• Are we ready to de escalate without clear cure?



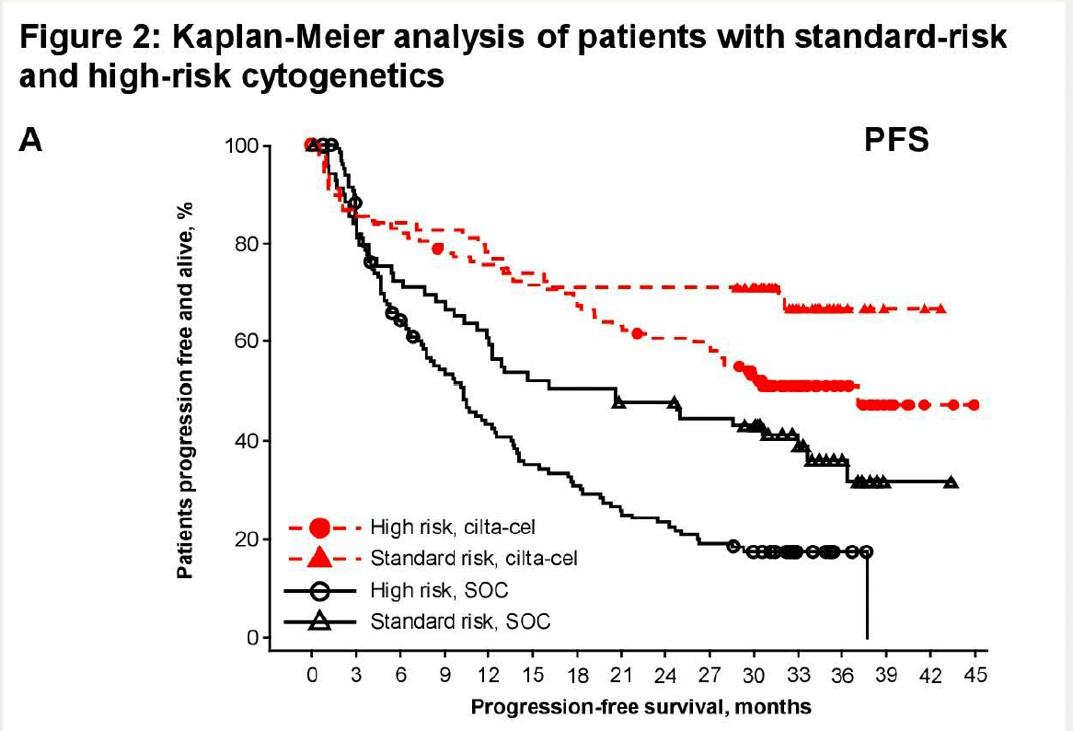
• High risk
– Deletion 17p >20% and/or p53 mutation
– Deletion 1p and +1q (1 extra copy of 1q not high risk alone)
– High risk 14q32 trans and (+1q or deletion 1p)
• Standard risk
Hyperdiploidy
t(11;14) Is there an intermediate category?

1. Richardson PG, et al. New Engl J Med. 2022;387:132-147. 2. Voorhees PM, et al. Lancet Haematol. 2023;10(10):e825-837. 3. Sonneveld P, et al. N Engl J Med. 2024;3990(4):301-313.
DVRd-Exponential
VRd-Exponential
PERSEUS ITT
PERSEUS ITT
DVRd - PERSEUS ITT
VRd - PERSEUS ITT
DVRd-Exponential
VRd-Exponential
CEPHEUS TIE
CEPHEUS TIE
DVRd - CEPHEUS TIE
VRd - CEPHEUS TIE
Estimated PFS, DVRd vs VRd PERSEUS: 205 months (17.1 yrs) vs 87 months (7.3 yrs)
CEPHEUS: 100 months (8.3 yrs) vs 53 months (4.4 yrs)
MRD-negative
Median: 61.9 vs 34.4 months
60-month PFS rate: 52% vs 30% (P<0.001) 5-year rate: 63% vs 45% (P<0.001) 24-month rate: 85% vs 80% 54-month rate: 68% v. 50% (p<0.001)

• Continuous therapy has carried the load
• Limited duration is the future
• Do we have the tools to define who has had enough therapy?
• Is the benefit of combinations for standard risk limited duration?
• follow up after discontinuation needs to be robust to detect failures


• If so how long and what interval and depth?
• Can all trials be reported in a similar way to enhance comparisons – Importance of fixed timepoints and consistent reporting
• Does MRD win over risk and genetics when considering duration?


• Are Early and Late MRD the same?
• What is the role of PFS ?
• What is the role of OS?
• Are we ready to de escalate without knowing how many patients we are curing.
• As we seek to create more “patient friendly” regimens, how do we continue to improve outcomes (rather than staying the same?

• What is the right regimen CD38 + IMID+PI
• Is there a difference between transplant ineligible and frail?
– Maybe but not as clear. More than age cut off
• How long to treat
– MRD directed may be of use, but follow up limited
• Optimal endpoint and timing (early vs late)
– Does escalation or de-escalation change natural history?
• Are we ready to de escalate without clear cure?
– Caution about losing the gains without long term follow up


Jonathan
Ajay
Nisha
Richa
Nishi
Craig
Vikas
Charise
Danielle
Donald










Tom Martin, MD
University of California San Francisco
Thomas G. Martin, MD
Clinical Professor of Medicine
Associate Chief, Division of Hematology
Co-Lead, Cancer Immunology and Immunotherapy
Helen Diller Family Comprehensive Cancer Center
University of California, San Francisco
San Francisco, California


Thomas G. Martin, MD
Clinical Professor of Medicine
Associate Chief, Hematology
Co-Lead, Cancer Immunology and Immunotherapy
Helen Diller Family Comprehensive Cancer Center
University of California, San Francisco Medical Center San Francisco, California
Thomas G. Martin, MD, has disclosed that he has received consulting fees from Pfizer, Lilly, AstraZeneca and GSK and funds for research support from Amgen, Johnson & Johnson – Janssen, Sanofi, and Seattle Genetics.
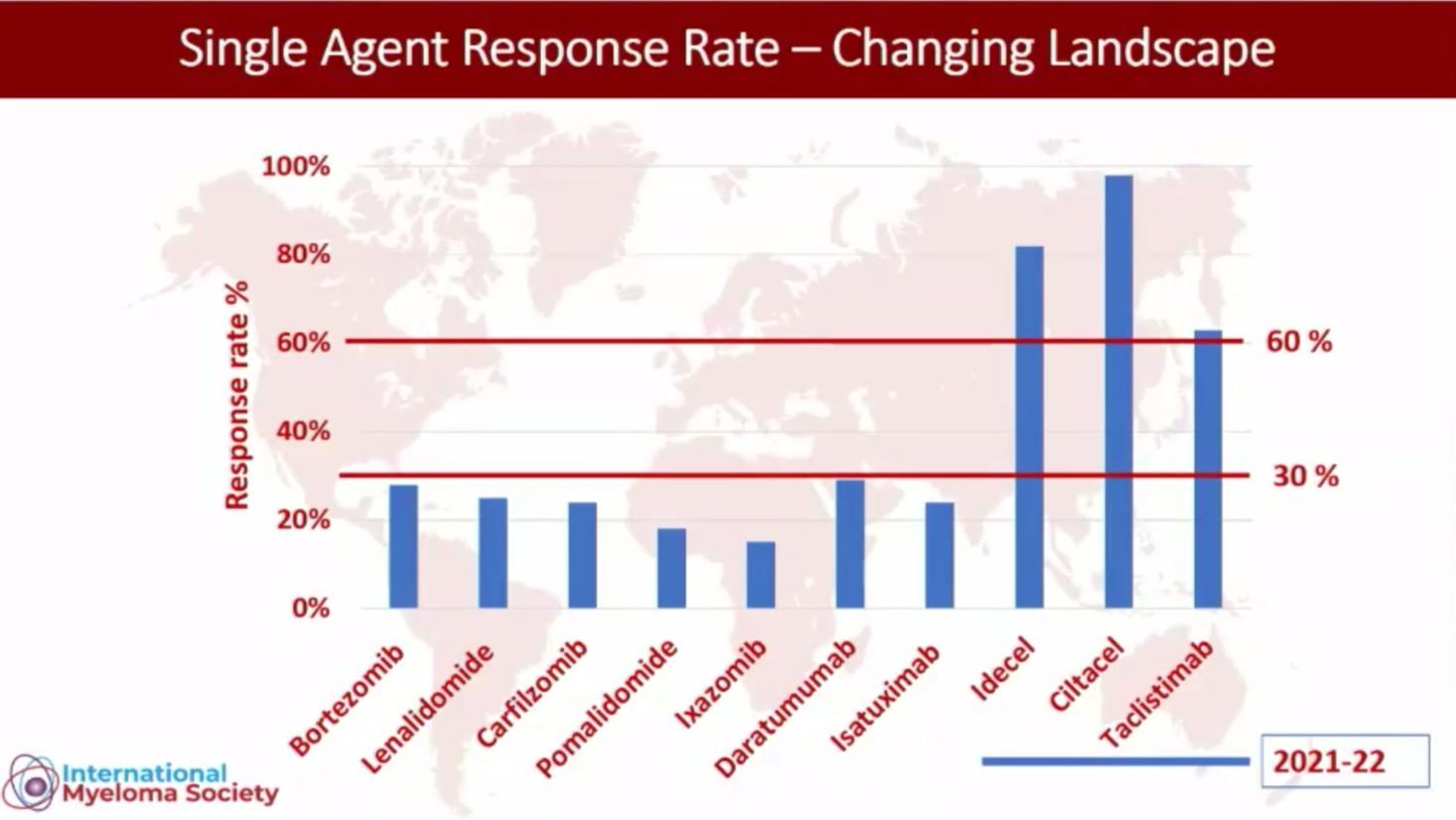
Where will each agent have its BEST/Biggest Impact?
Tom Martin
• Real-world assessment of lines of therapy and outcomes based on 3 US insurance claims databases
Will these drugs have curative potential in NDMM/Early RRMM!!!


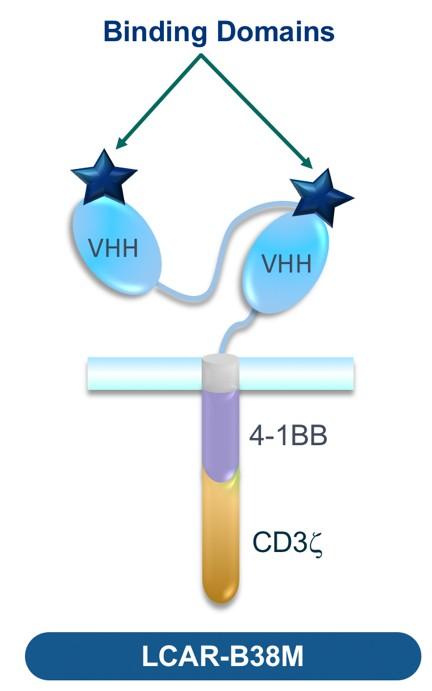


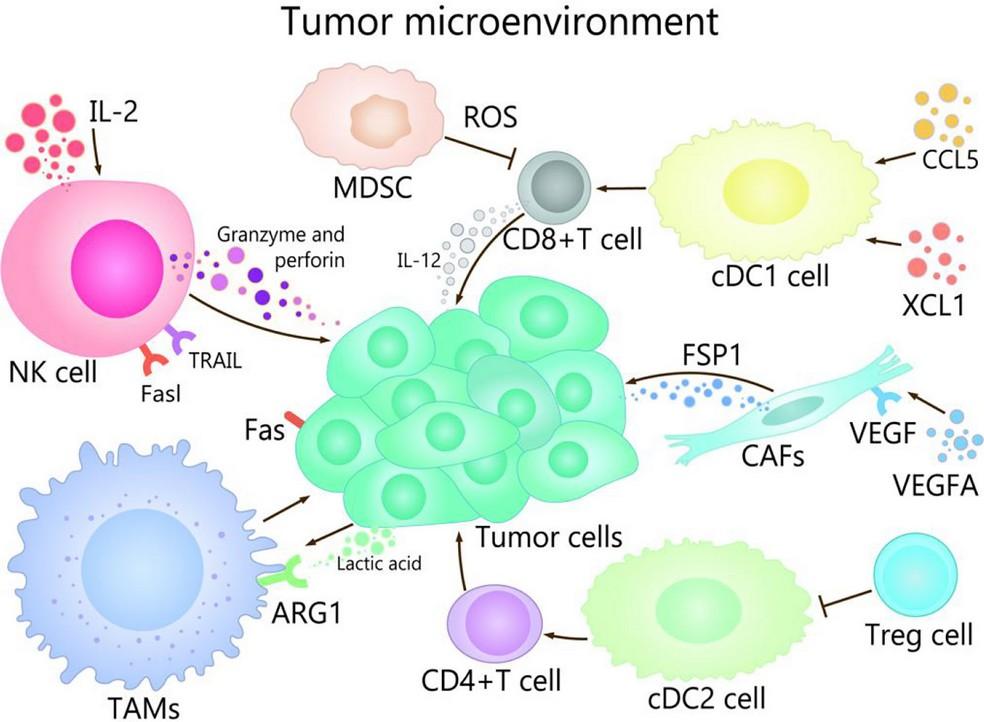
We need to interrogate the BM-ME
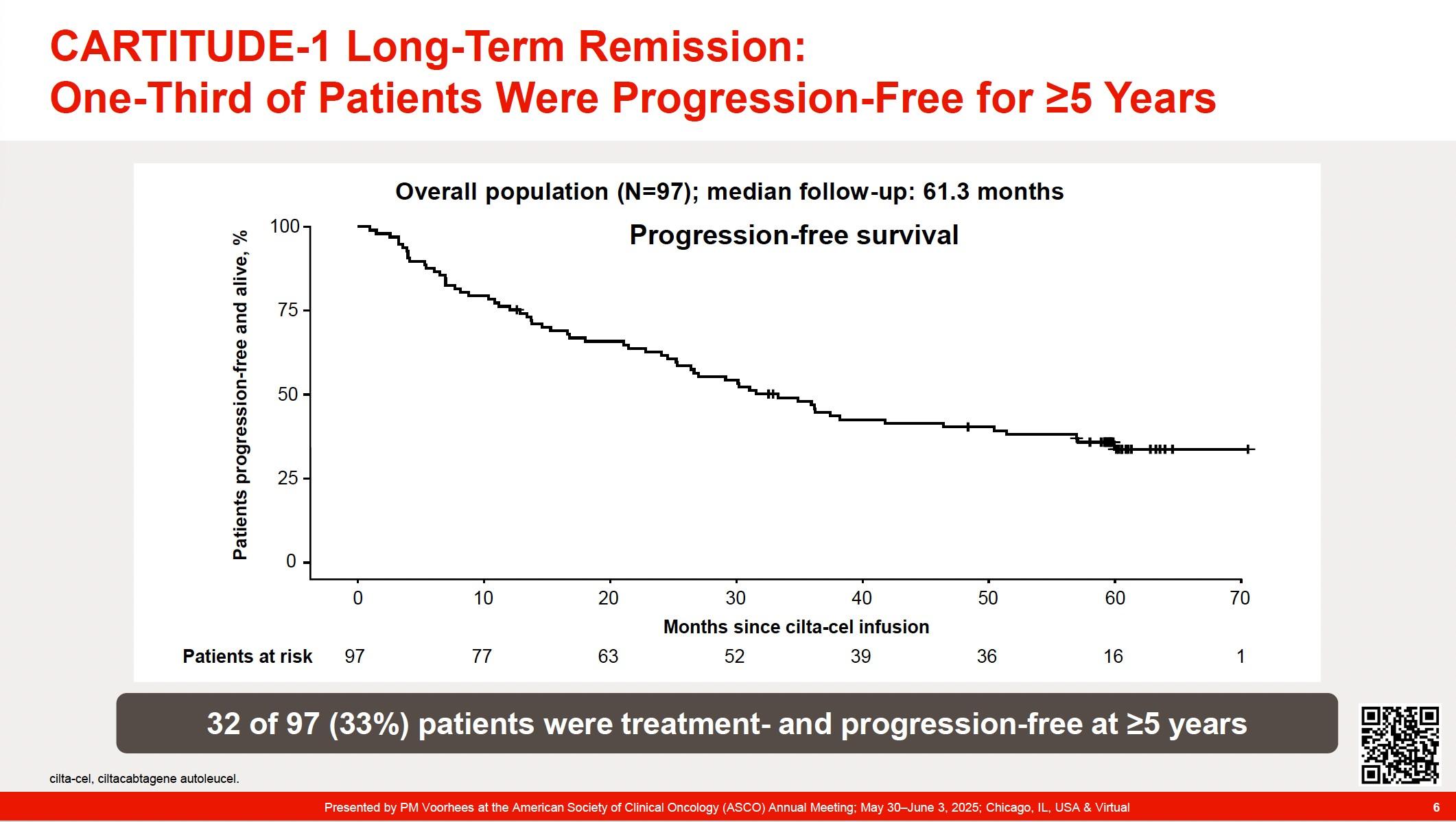
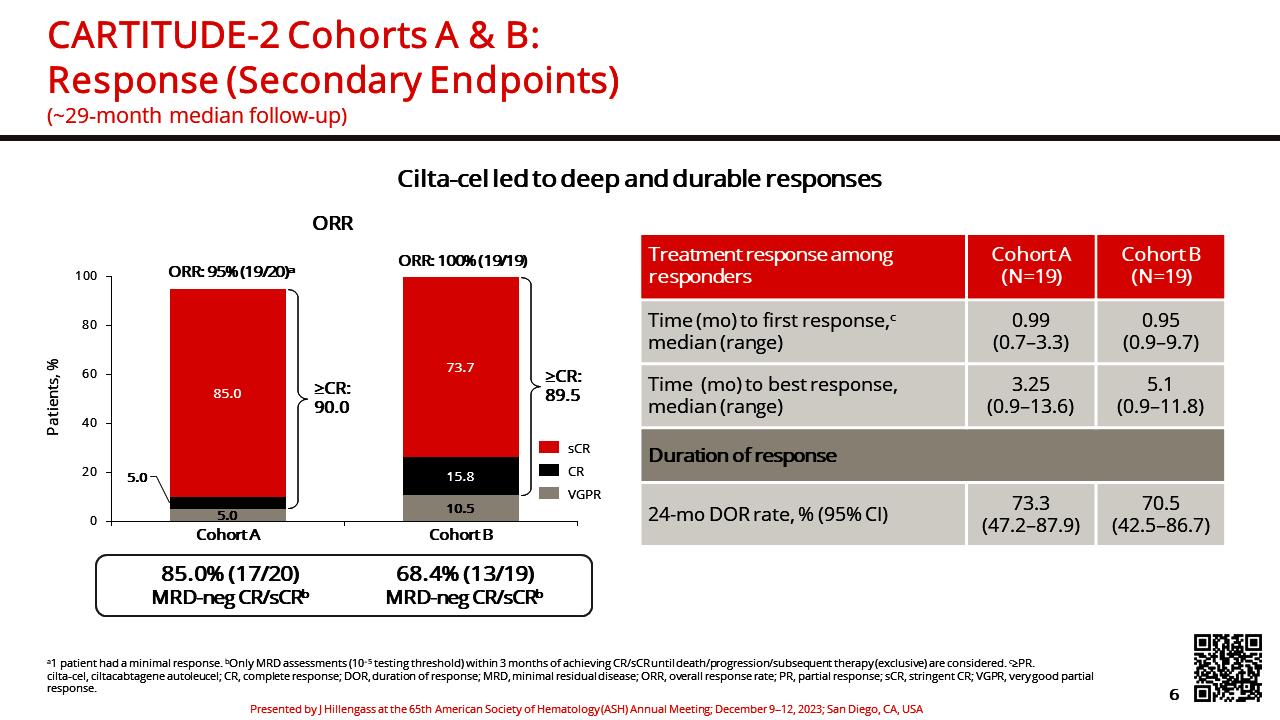
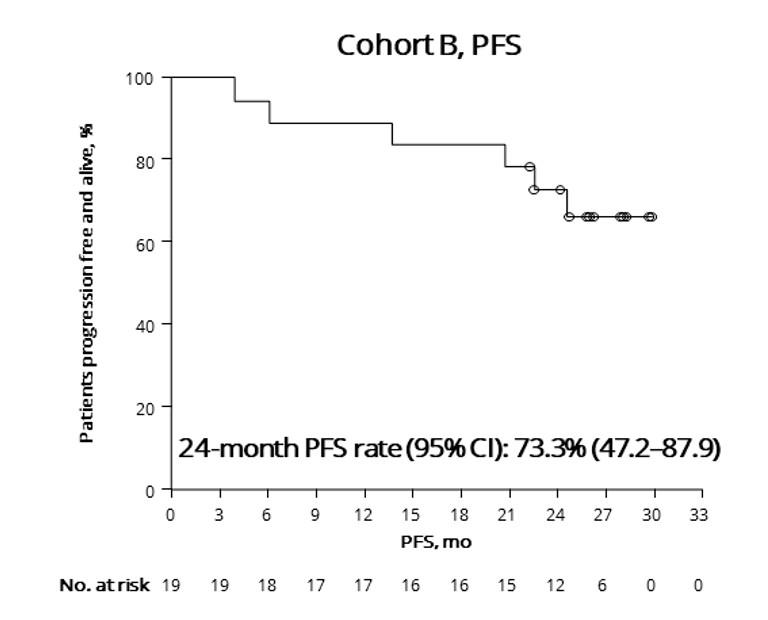
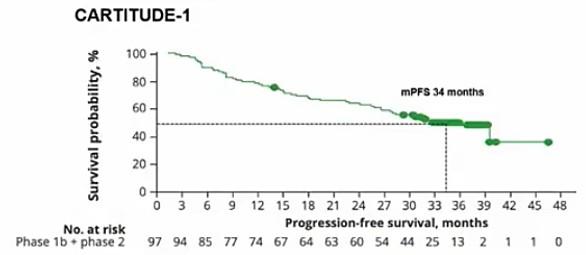
• CARTITUDE-1 (n=97) Accelerated FDA Approval: 2/22/2022. ?? Cure in RRMM
• CARTITUDE-2 (multi-cohort)
• A - Cilta-cel in patients with RRMM having received 1-3 PLT, Len refractory
• B – Cilta-cel in patients with RRMM s/p 1 PLT AND early relapse (<12m)
• C – Cilta-cel in TCE patients and exposure to BCMA therapy (non-CAR)
• D- Cilta-cel in NDMM patients without CR after Ind/SCT/Consol (4-8 cycles)
• E – HR-NDMM => D-RVd + Ciltacel + DR maintenance as frontline therapy
• CARTITUDE-4 (n=419) CART vs. SOC triplets Full FDA Approval
• CARTITUDE-5 (n=650) Phase 3: TI - VRD+Rd vs. VRD+Cilta-cel (Accrued)
• CARTITUDE-6 (n=750) Phase 3: TE – D-VRd/ASCT vs. D-VRD+ Cilta (Accrued)
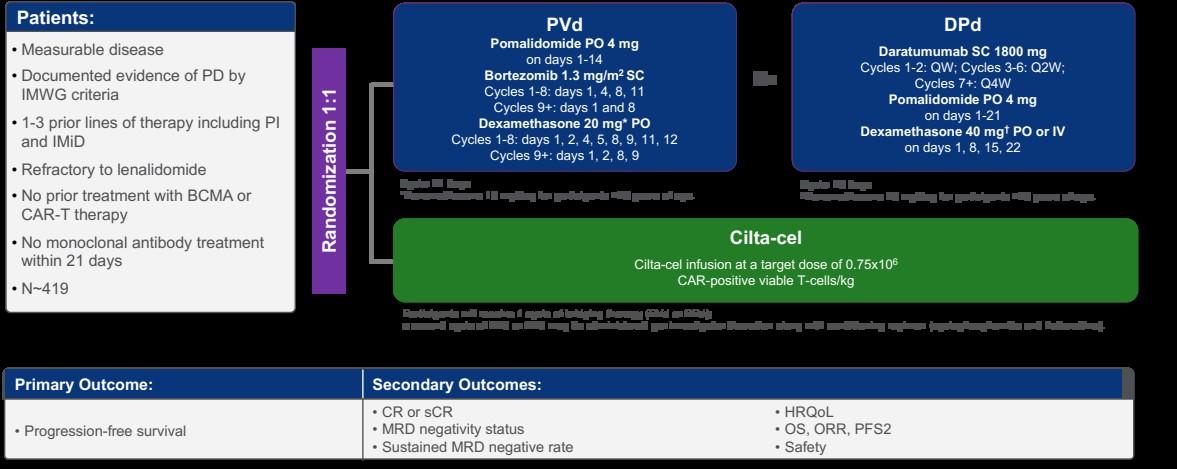
Primary endpoint of PFS was met and study now reported 1. https://clinicaltrials.gov/ct2/show/NCT04181827 2.
https://www.prnewswire.com/news-releases/janssen-announces-unblinding-of-phase-3-cartitude-4-study-of-carvykti-cilta-cel-as-primary-endpoint-met-in-treatment-of-patie nts-with-relapsed-and-refractory-multiple-myeloma-301732398.html . San Miguel j. et al. NEJM 2023
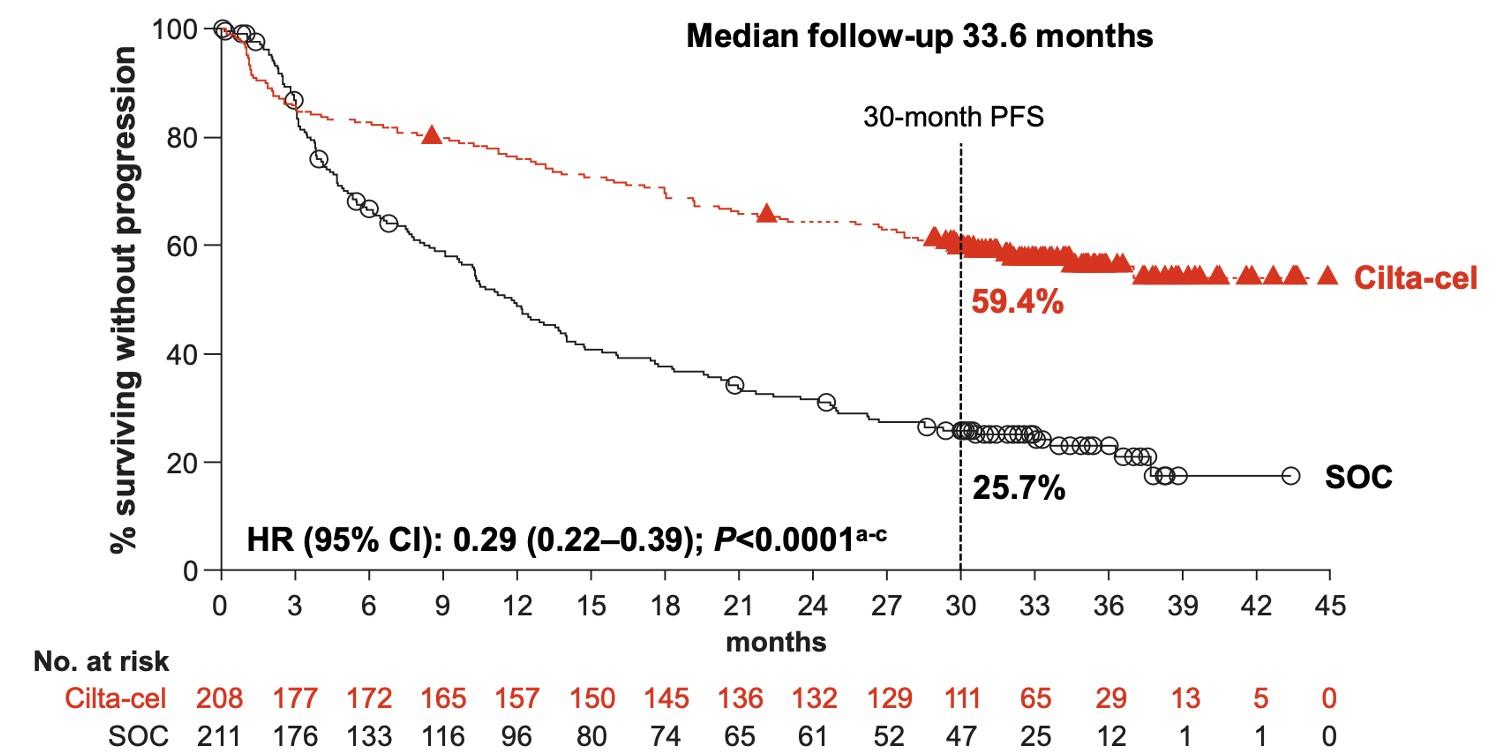
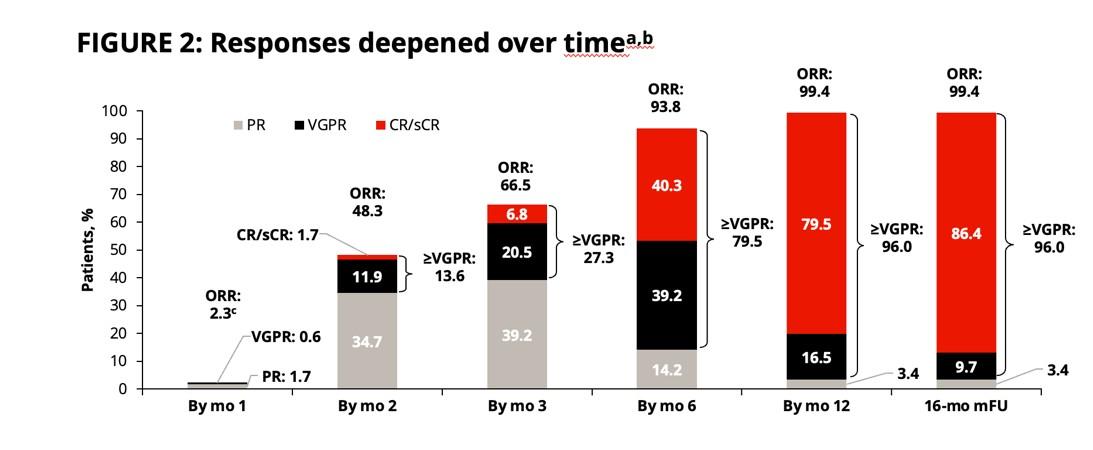
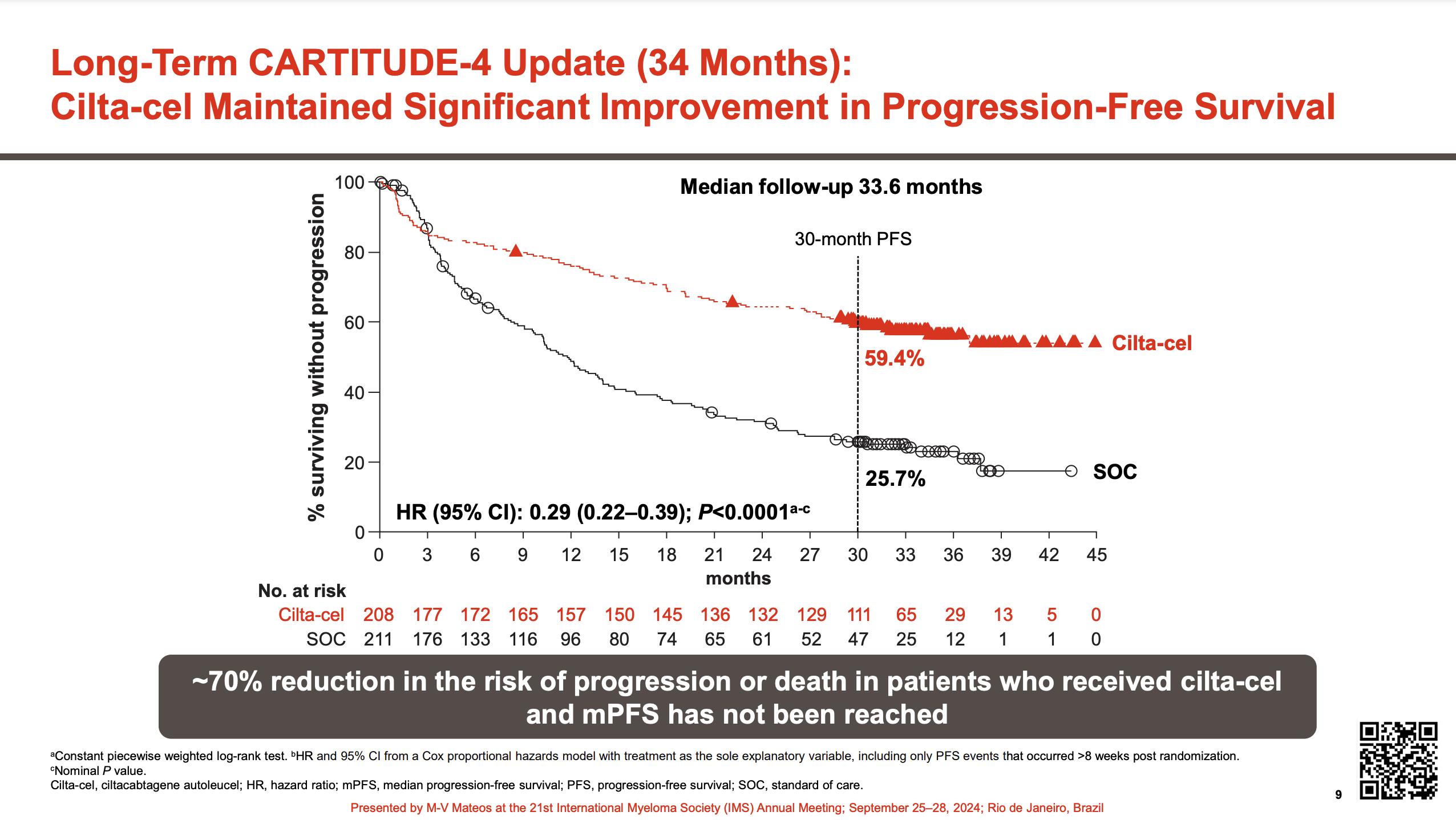
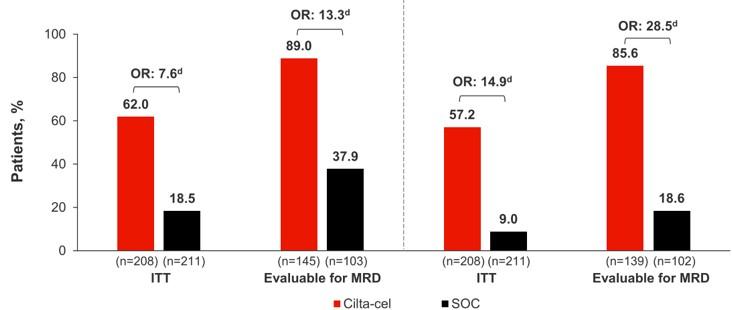
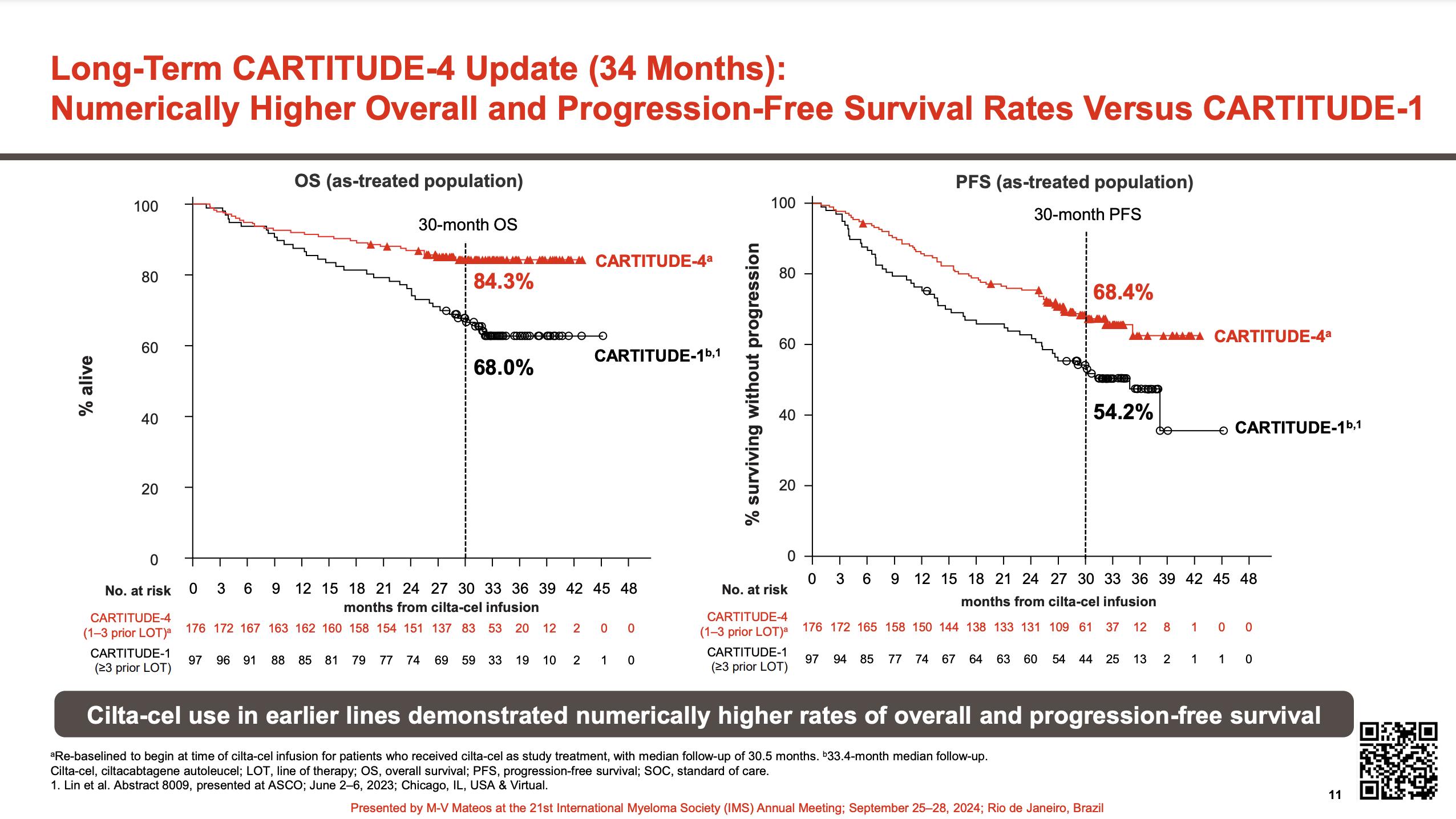
•Median follow-up 33.6 months
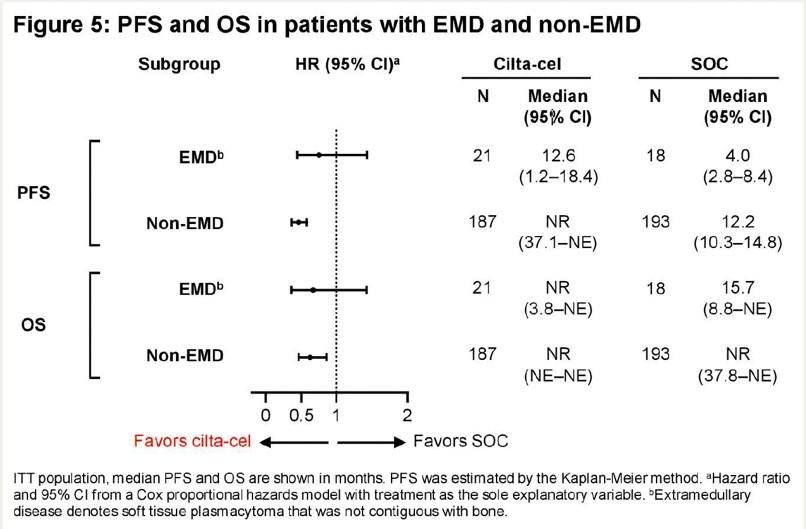
•Improved PFS in sub-groups
• LOT
• EMD
• HR cytogenetics
Study population
• 208 patients cilta-cel arm (ITT) and 176 received ciltacel (as-treated population)
• 211 patients SOC
Safety in the cilta-cel as-treated population
• CRS occurred in 76.1% of patients and were mostly grade 1/2; all cases resolved3,4 (Table 3)
• CAR-T cell neurotoxicity occurred in 20.5% of patients; none were fatal3,4
• ICANS occurred in 4.5% of patients; all were grade 1/2 and resolved3,4
• Cranial nerve palsy (9.1%), peripheral neuropathy (2.8%), and movement and neurocognitive treatment-emergent AEs (MNTs) (0.6%) were mostly grade 1/23,4
• By the CCO, all but 2 of the cranial nerve palsy and 2 of the peripheral neuropathy cases had resolved; the MNT case (grade 1) had not yet resolved by the CCO3,4
• Secondary Malignancies
Cilta-cel vs. SOC
Overall: 9(4.3) vs. 14 (6.7)
Cutaneous: 5 vs. 10
1st yr./ 2nd yr

Phase1/2: single arm, Qwk => Q2/Q4wk 30-month mFu Toxicity
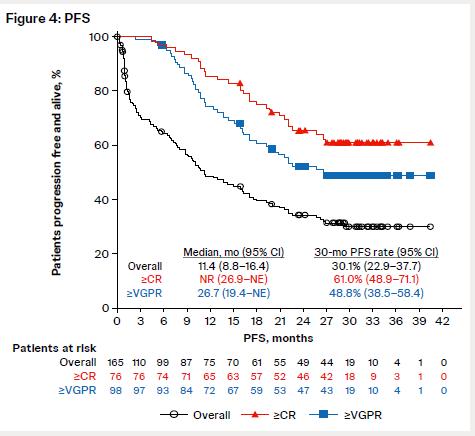
Nooka Et al.

• Pooled analysis of 4 MagnetisMM trials; median time to response 1.7 mo (range: 0.3-9.3) • Response
Bispecific Antibody
Anemia
Thrombocytopenia
ICANS #
Hypogamma/IVIg Other
(0.7%)
(0%)
(2.0%)
(28%)
(53%)
(25%)
(29%)
(1.8%)
0 due to AEs NR/10%
Dysgeusia 77% (N/A)
(0%)
63% (0%)
(0%)
(26%)
(26%)
(20%)
(0%) 1 due to AE
(4%)
(2%)
(3.2%)
(53%)
(61%)
(34%)
(29%)
(? 1)
(N/A)
(0%)
(0%)
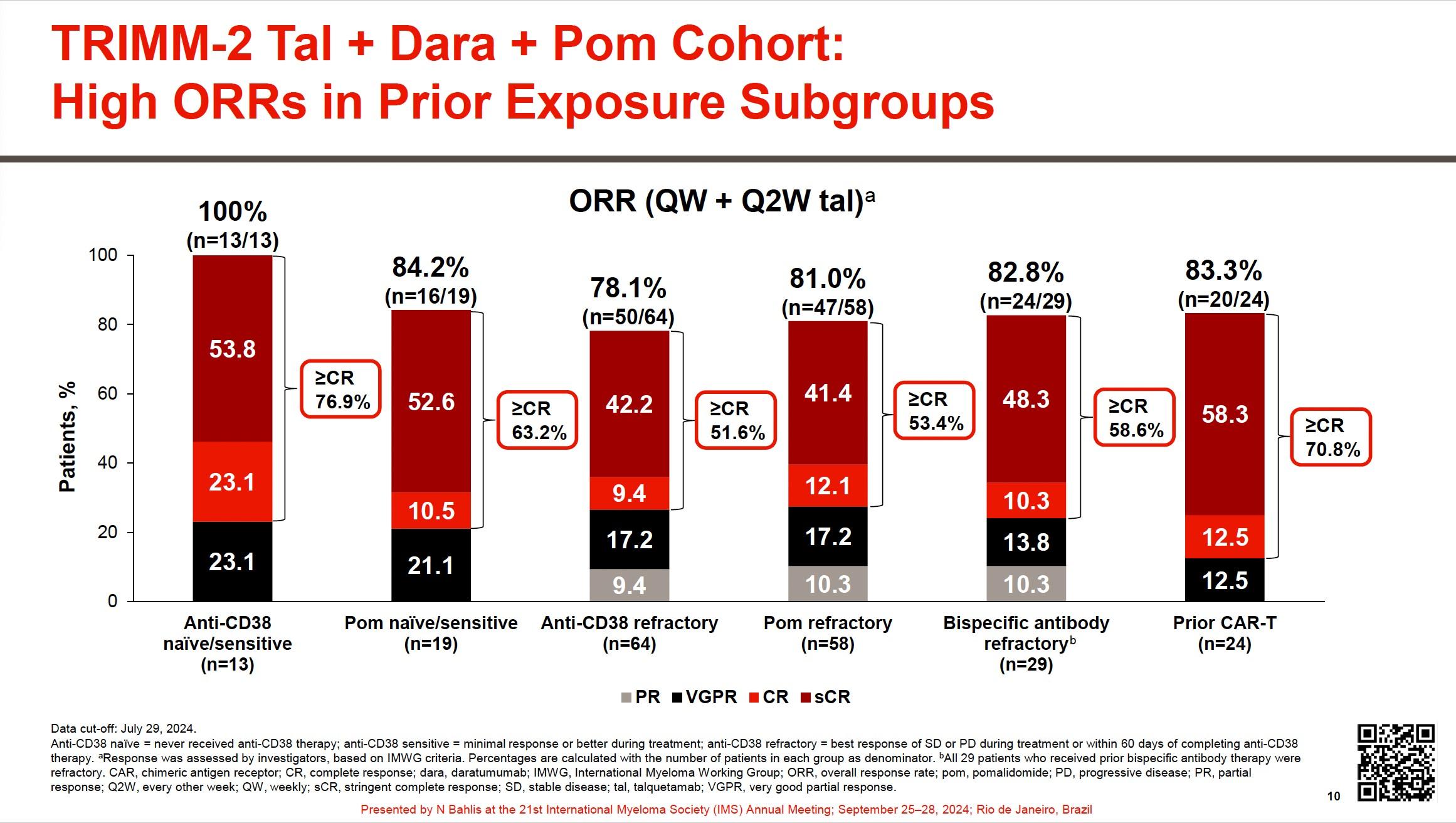
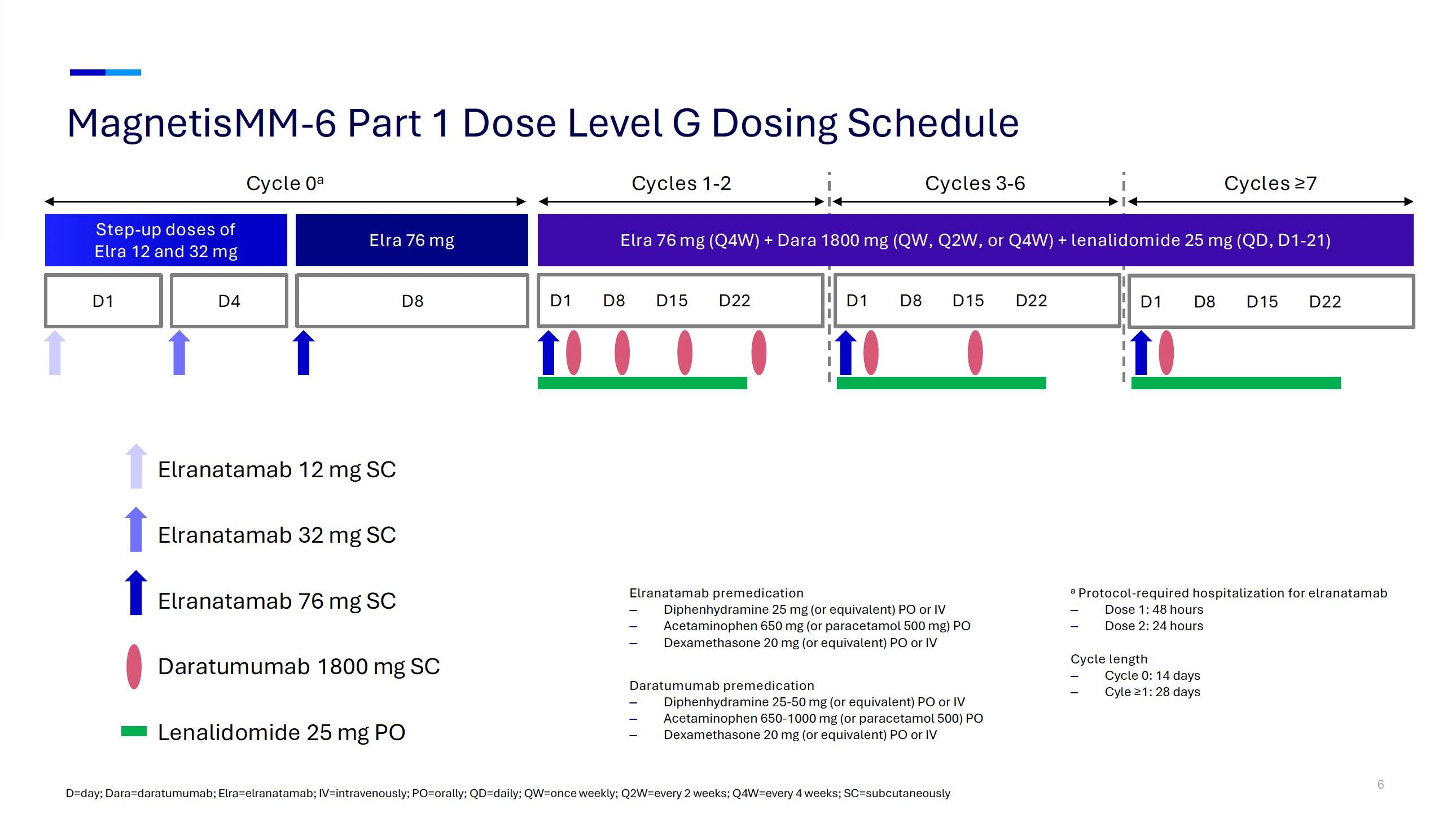
• Phase 1b trial conducted across 39 sites in USA, France, Greece, and Spain
Inclusion criteria:
• Age ≥18 years
• RRMM
• ≥3 LoT, or ≥2 LoT if either TCE or DCR (IMiD + PI)
• Prior CFZ treatment permitted if previously tolerated and ≥6 months had elapsed since last exposure
• CFZ-refractory patients were allowed during dose finding



• DLTs • Incidence/severity of TEAEs
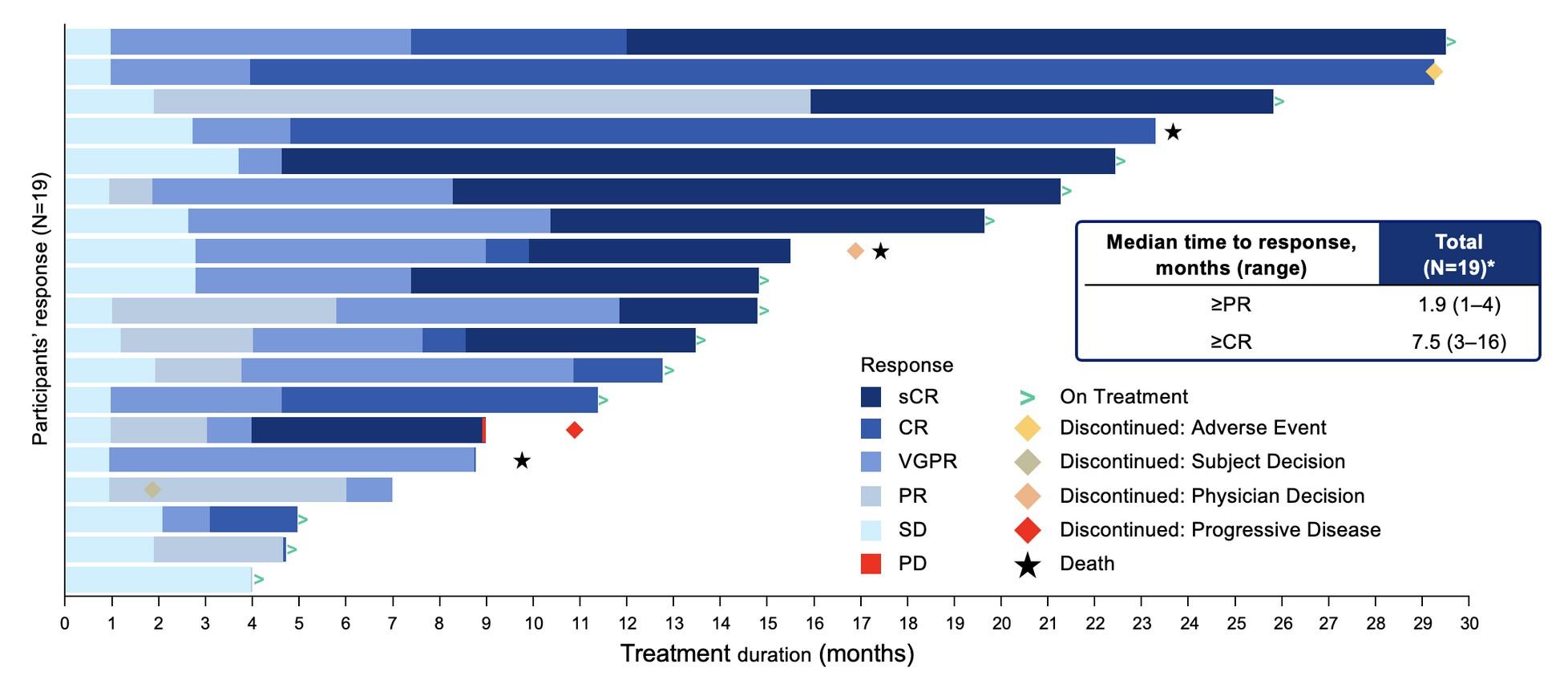
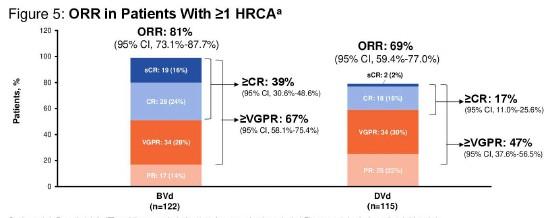
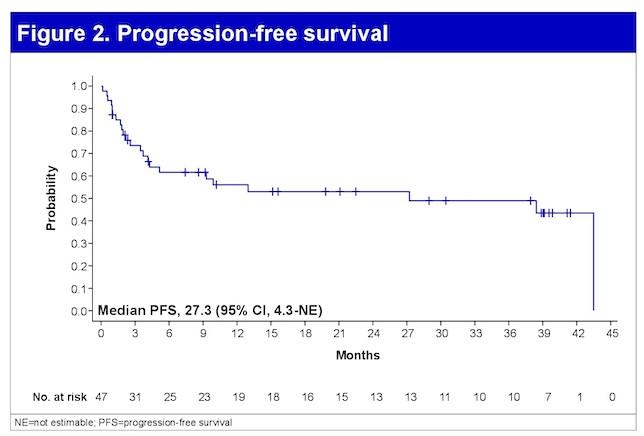
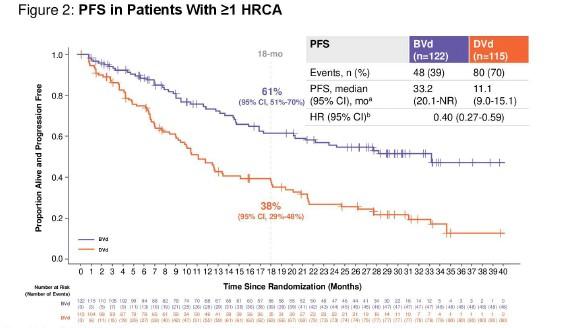
HR Cyto = Median PFS 33.2 mo (95% CI. 20.1-NR)
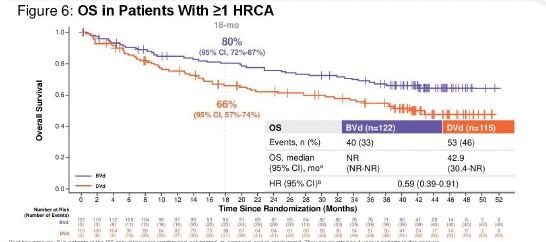
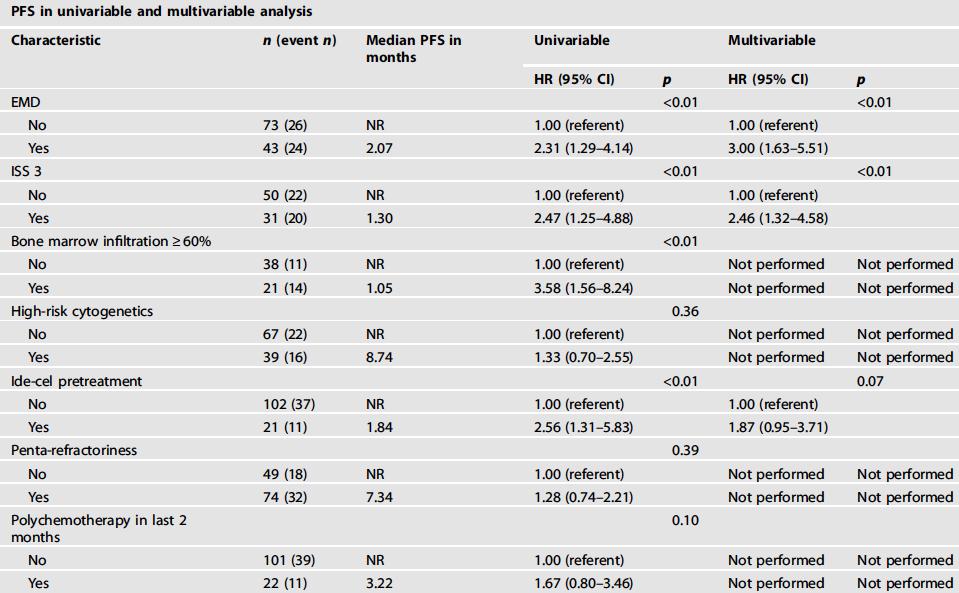
Median OS NR (18 mo CI. 80%)
Current and planned (not inclusive of all trials)
MajesTEC-7: Tec-D vs. DRd
•
M-Tec-3: Tec-D vs. DPd/DVd M-Tal-1: Tal SQ
Mag-6: Elran-DR vs. DRd M-Tec-4: R vs. Tec vs. Tec-R
Linvo: Phase I/II
Frontline – maintenance. Early RR RRMM (TCE)
Mag-7: Elran vs. Len
Linvo: Linker MM-4
Linvo: Combinations
Mag-3: Elran (single)
Mag-5: Elran, Elran +D, Dara+Pd
Camma-1: Cevo, CevoPd, CevoDd
Biomarkers and resistance important for all timepoints
Camma-3: Cevo SQ
Frontline: time-limited, no CART!
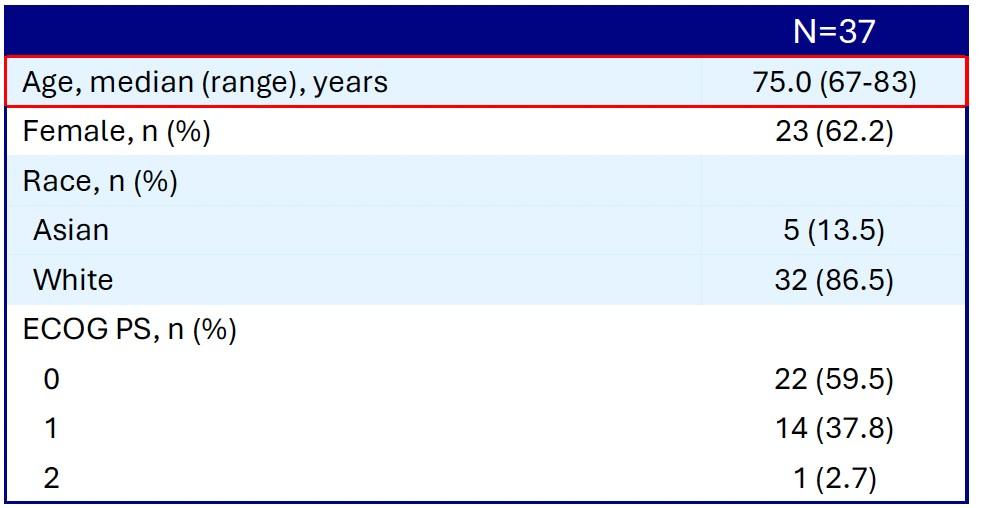
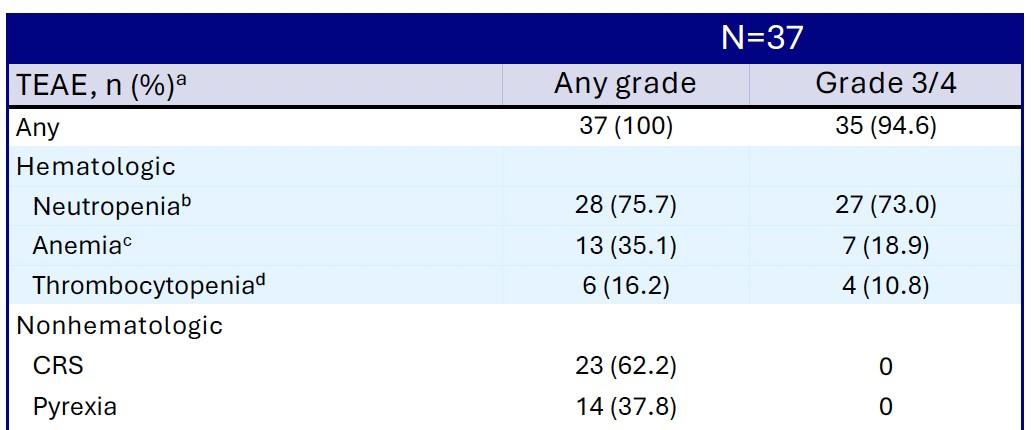

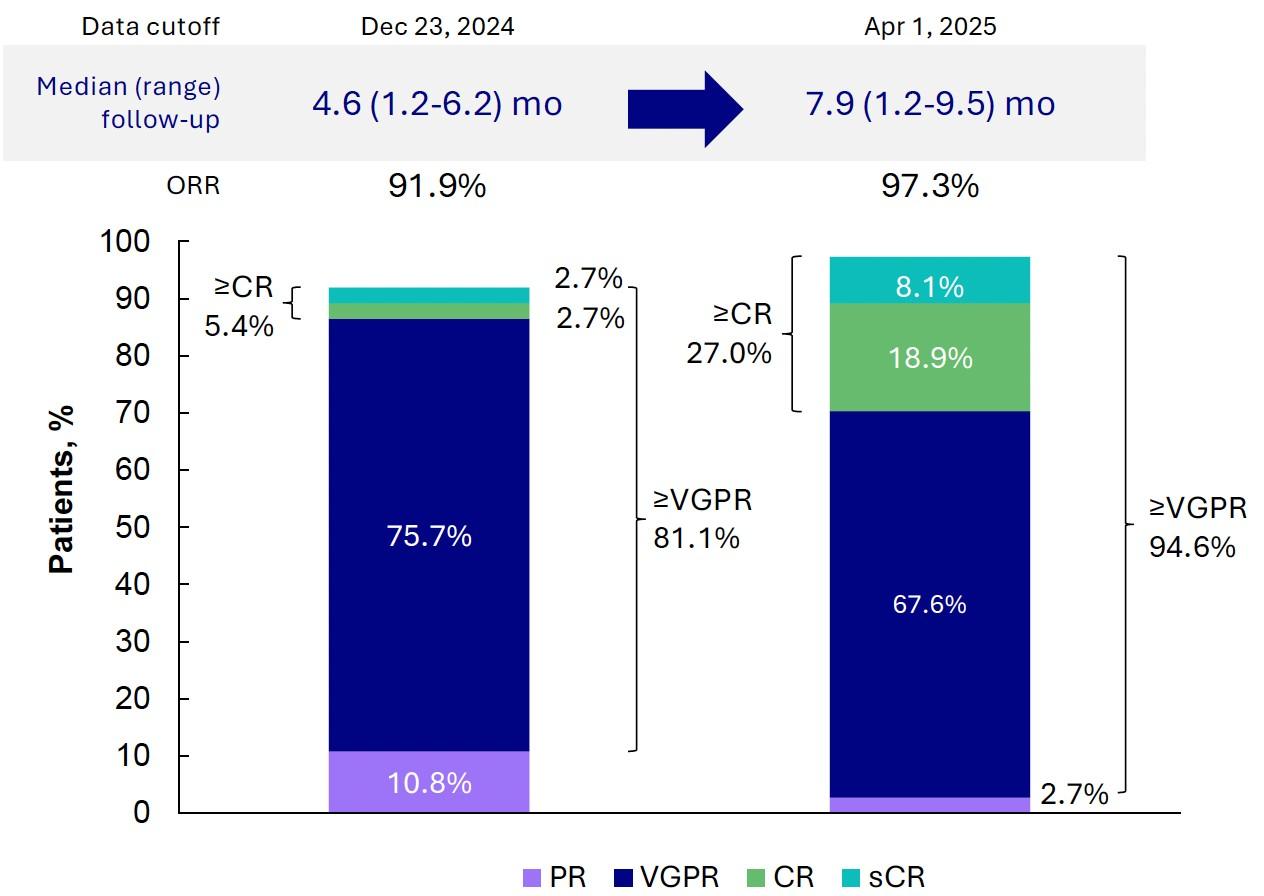
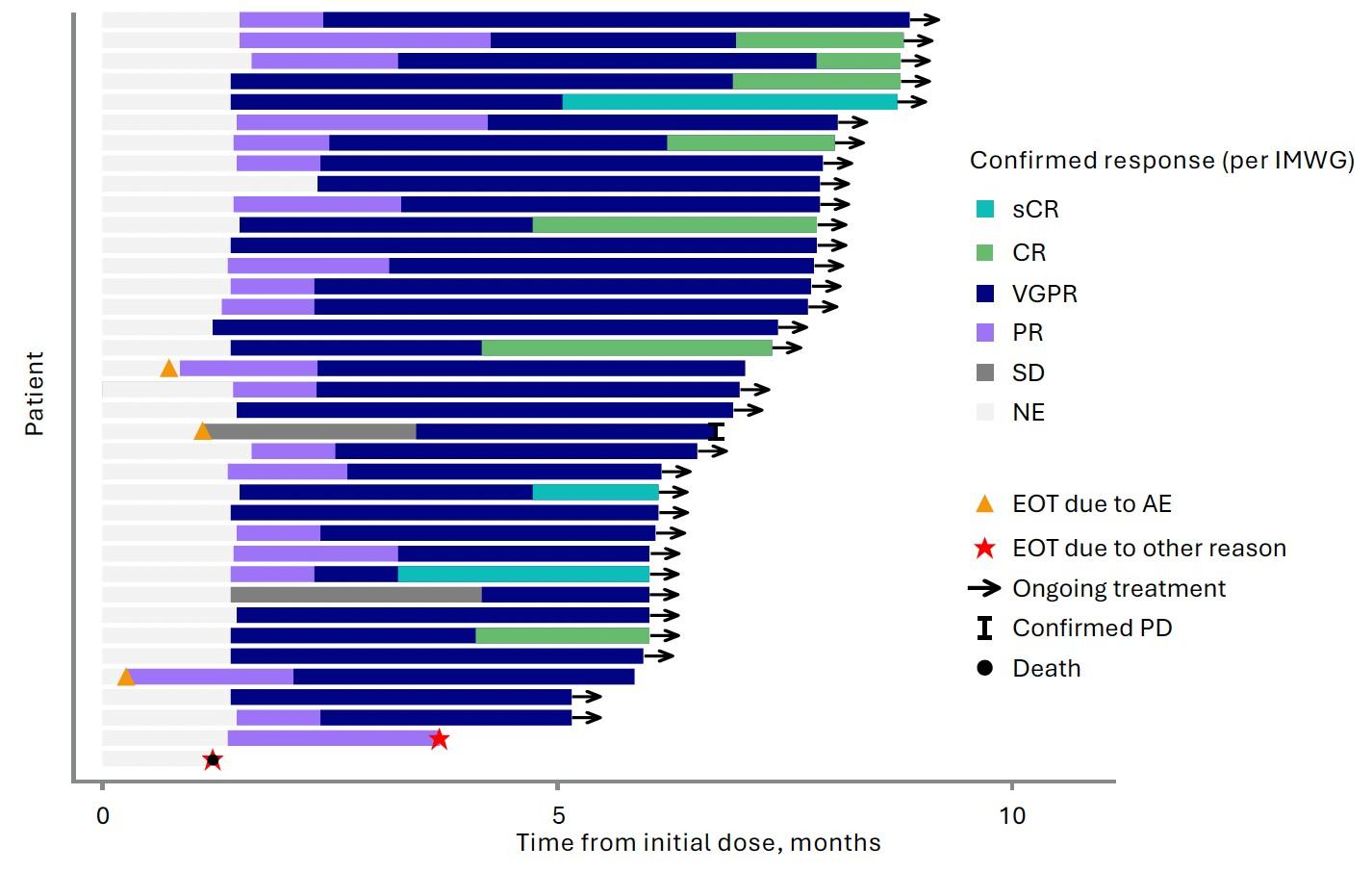




Induction
Len +/- CD38
Maintenance
BiSp +/-CD38
Maintenance
BiSp +/-CD38
Maintenance
Len +/- CD38
Maintenance QUAD Induction
QUAD -BiSp

Triplet/Quad Induction


BsAb +/- partner
BiSp +/-CD38
Maintenance

BsAb (different target) Bela maintenance
QUAD -Bela Bela +/- partnermaintenance

• CAR is currently the front-runner for BCMA Therapy for RRMM in BCMA Naïve patients
• Is there room for more CARs YES
• Wait for Hermann’s talk!!!
• Convenience and lowest TRM/morbidity and best QOL
Frontline is the space with the greatest potential impact
• “Cures” win – whatever the platform/Sequence
• We shouldn’t change our practices/advances based on the changes at FDA...
• We should continue our partnership and collaboration
• We need to solidify endpoints OS, PFS, MRD
• Can we agree on a definition of “CURE” ?
• We shouldn’t change our practices/advances based on the changes at FDA...
• We should continue our partnership and collaboration
• We need to solidify endpoints OS, PFS, MRD
• Can we agree on a definition of “CURE” ?
• Example from last Thursday 6/5/2025
• IND 31367
• Submitted 5/5
• Approved 6/5

And optimize the CAR design
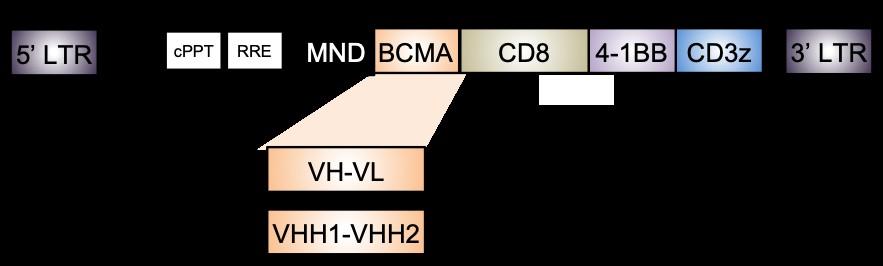
CARVYKTI


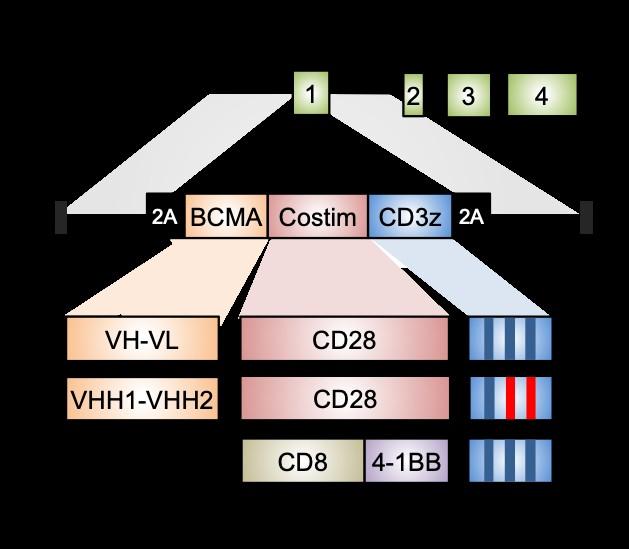
Clinical scale manufacturing UCCAR-1
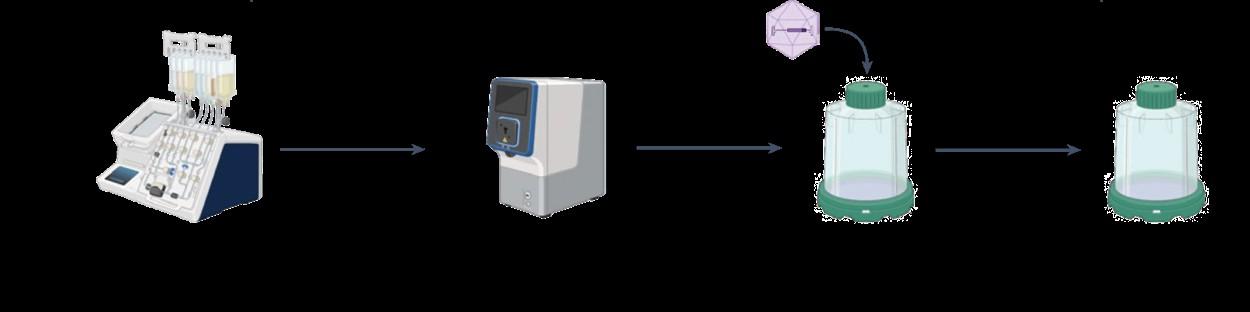
1XX based on Feucht et al., Nat. Med 2019
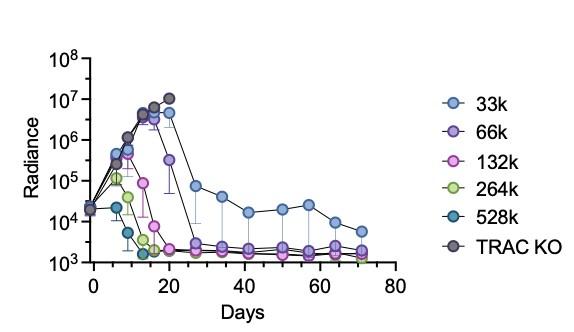
Talbot et al., unpublished. Shy et al. unpublished

Nizar Jacques Bahlis, MD
University of Calgary

IMWG 2025 Summit, Milan
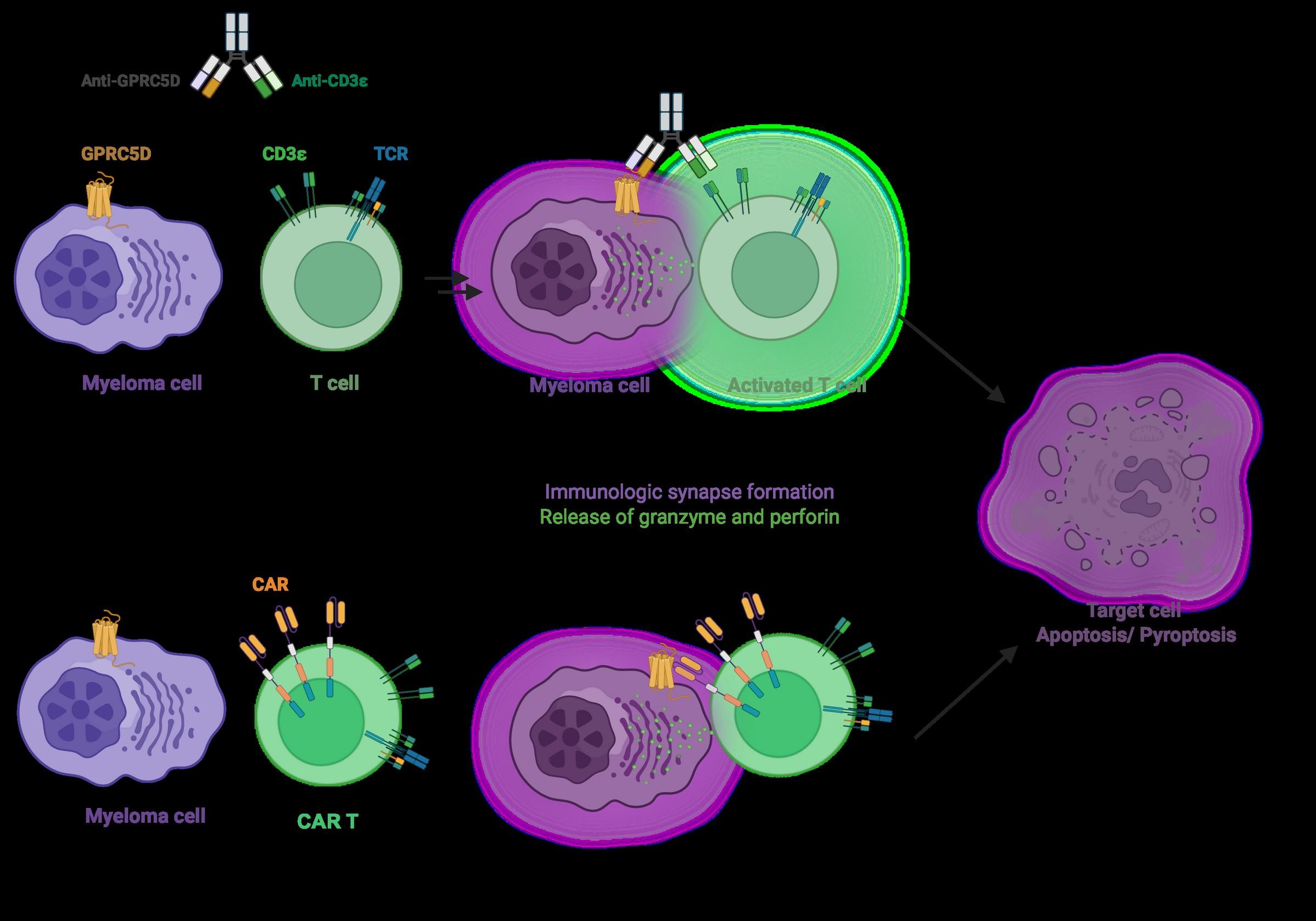
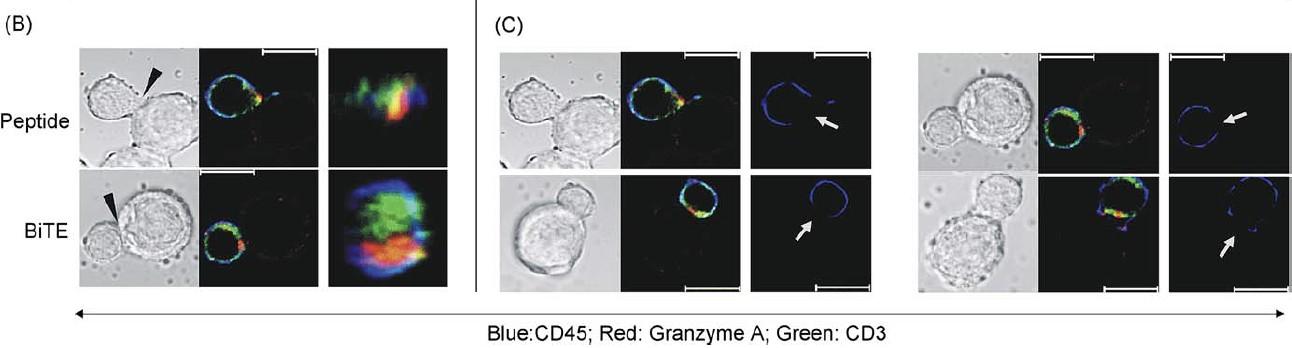
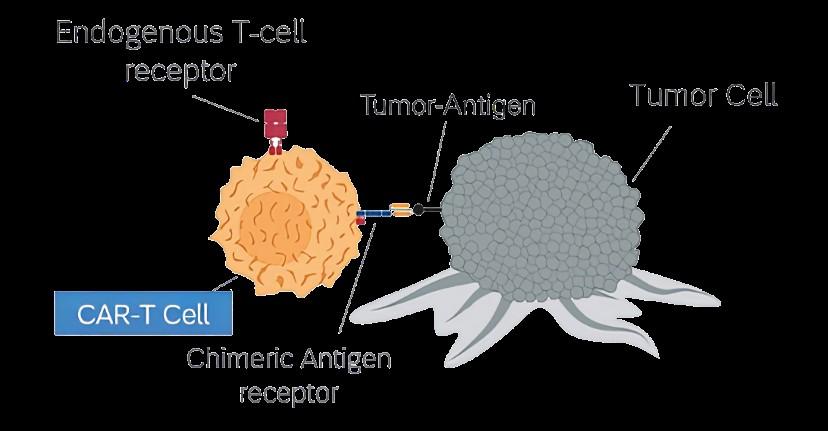
Cytolytic synapses induced by BsAbs or CART have the same composition, subdomain arrangement and size from synapses induced by regular components of cytotoxic T cell recognition.
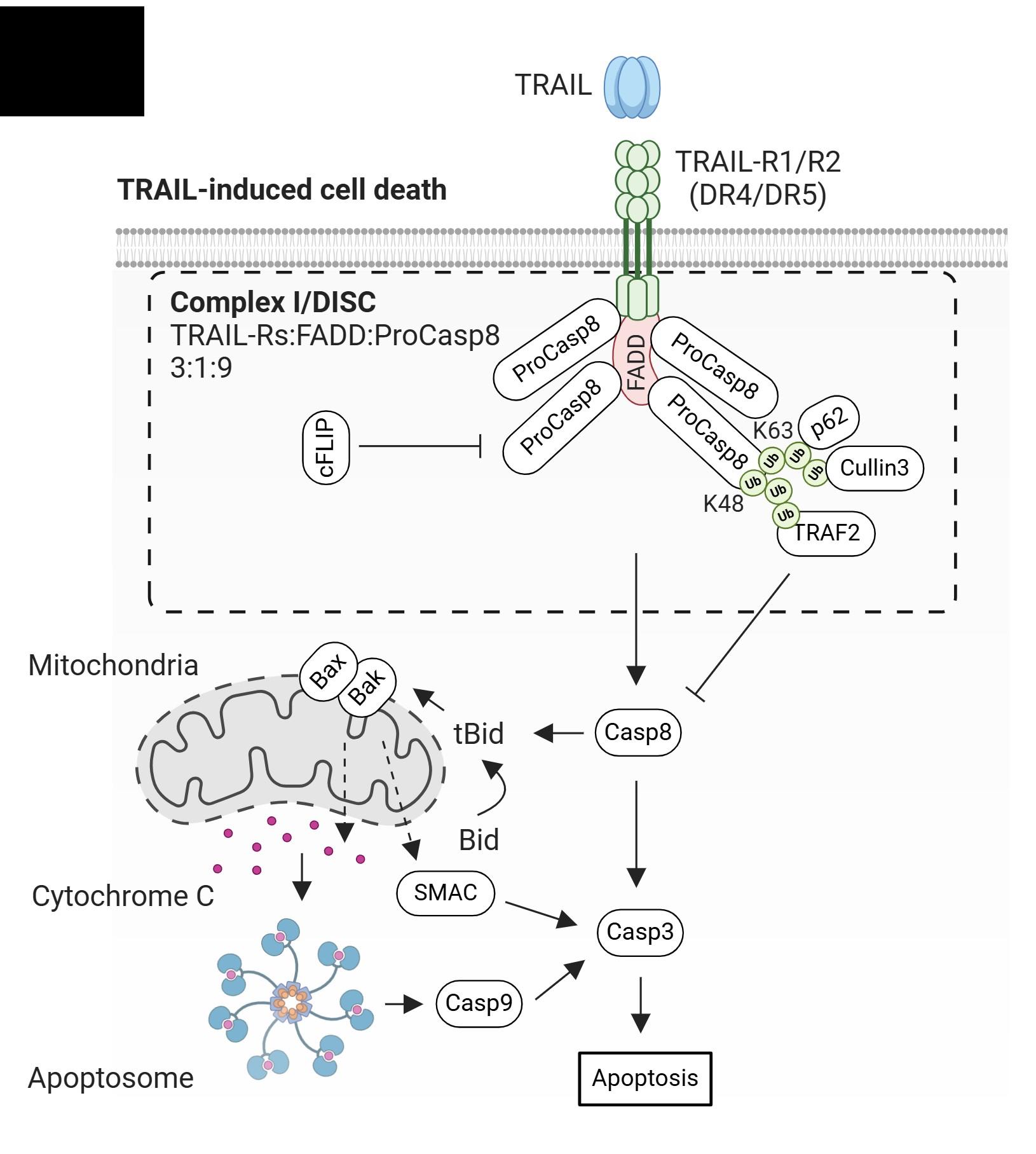


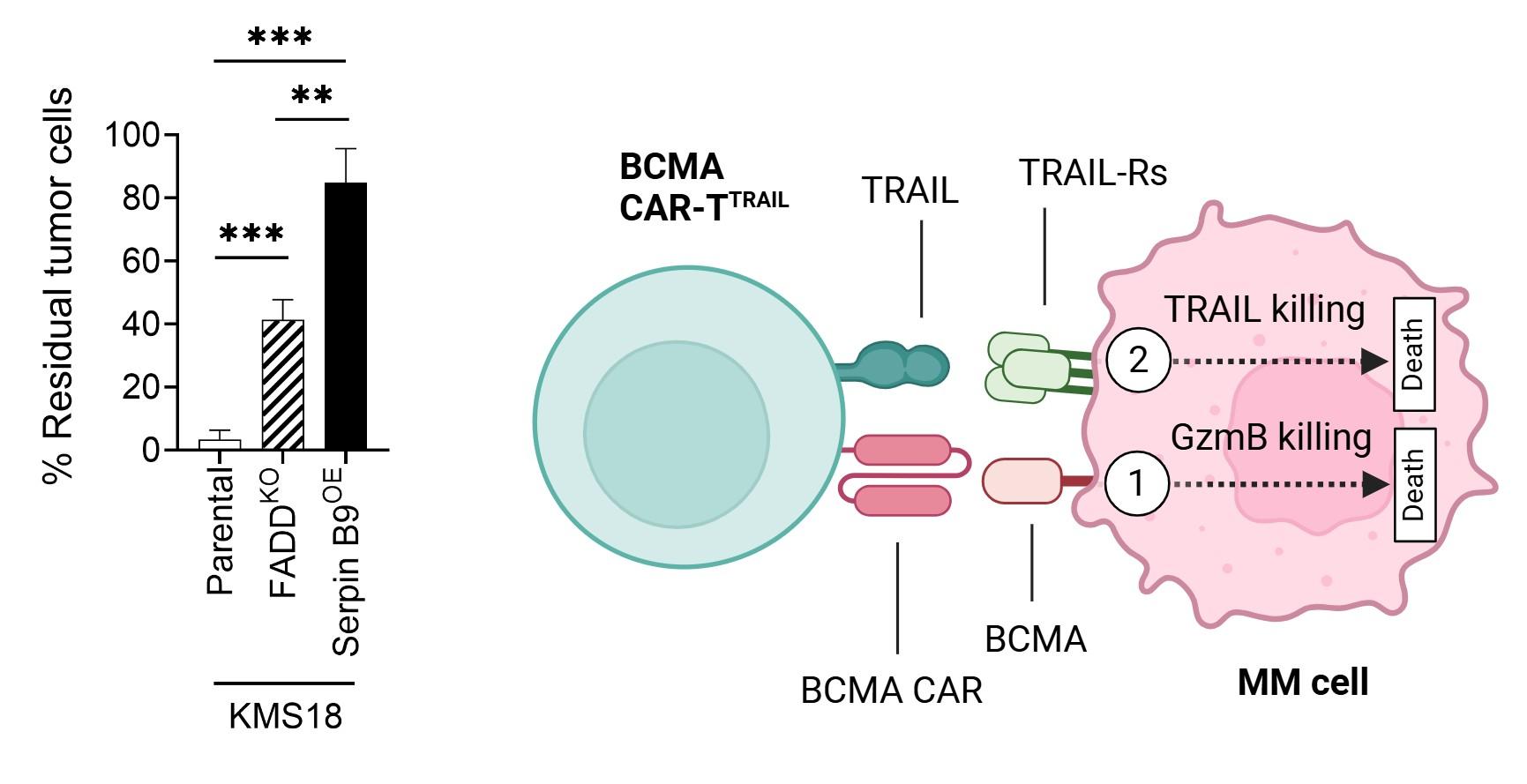
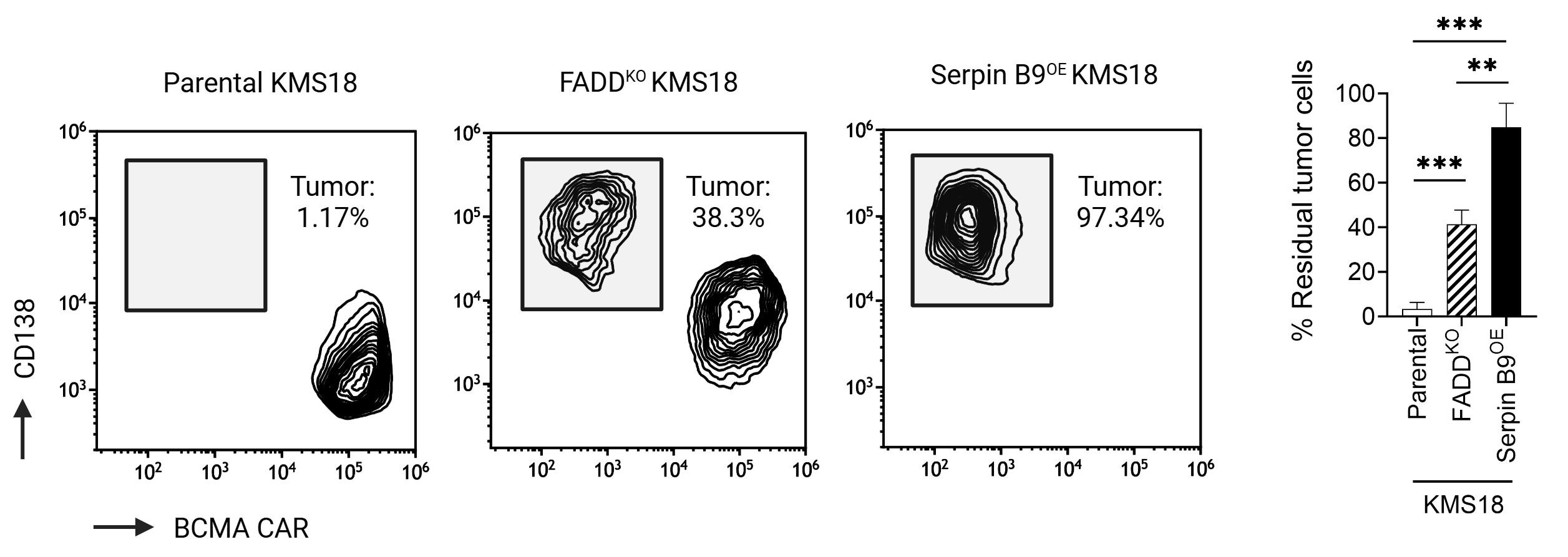
Pourbrahim M. unpublished data Boise L provided Serpin B9 and FADD-KO cells
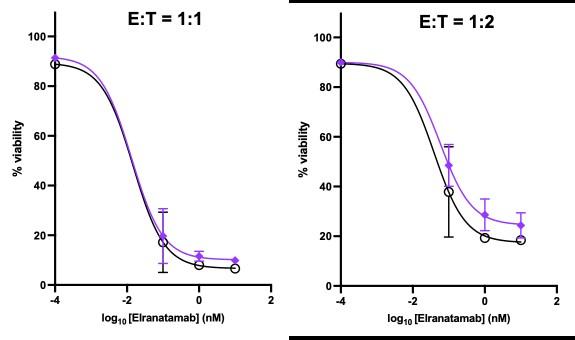
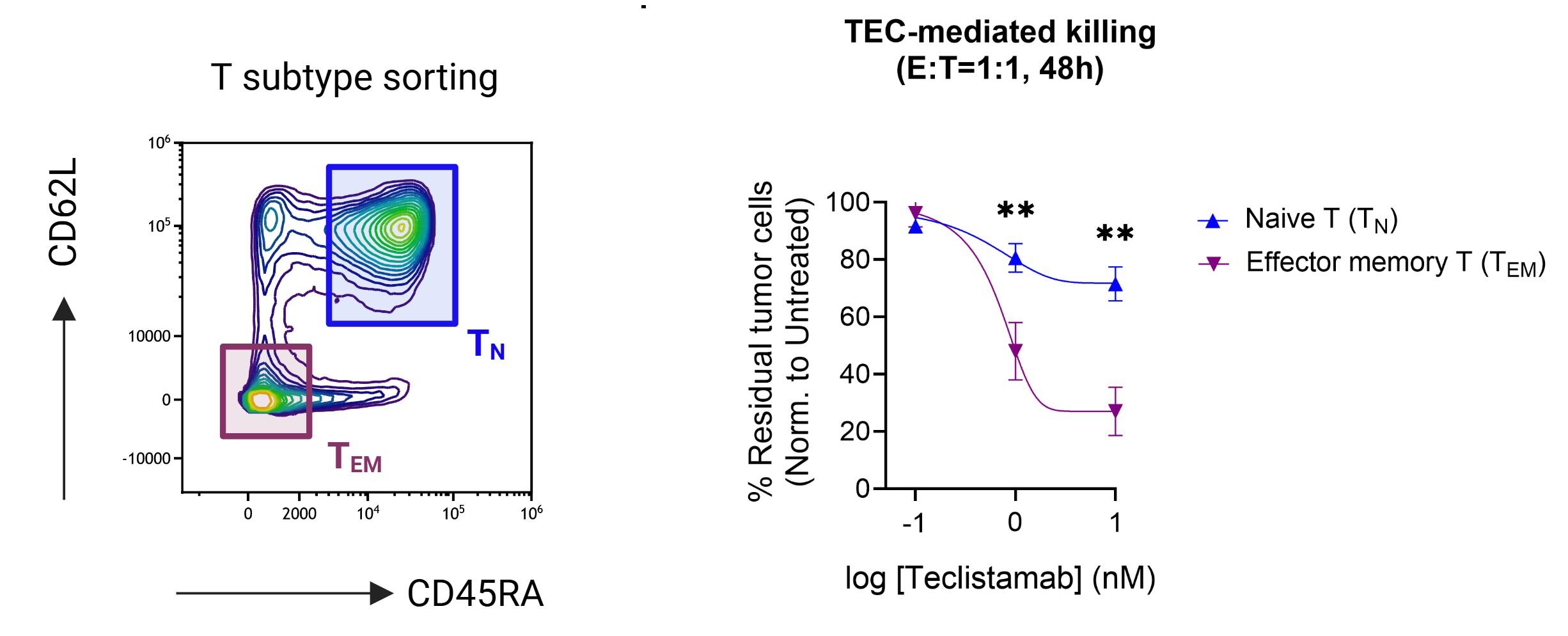
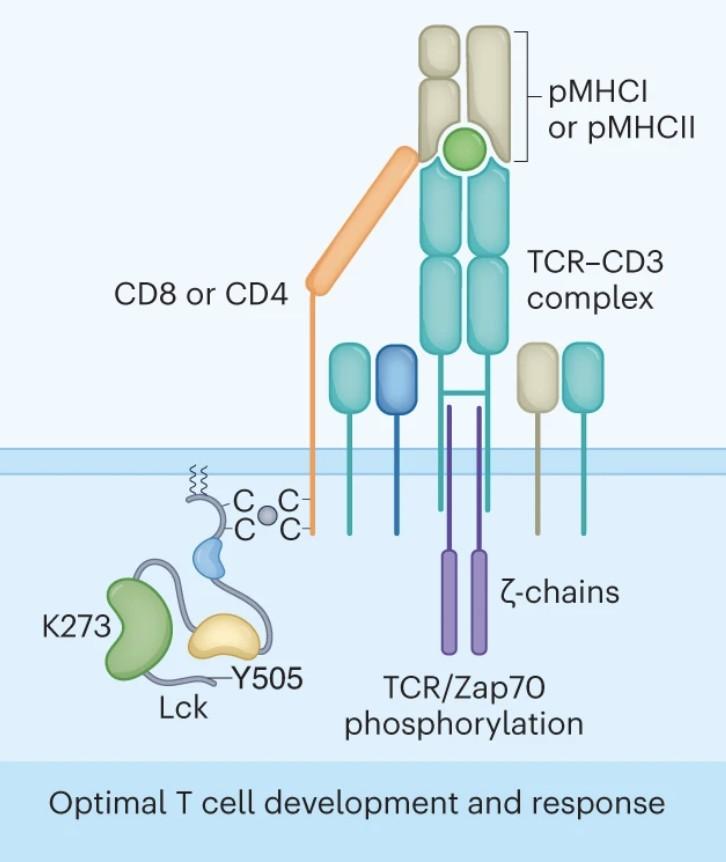
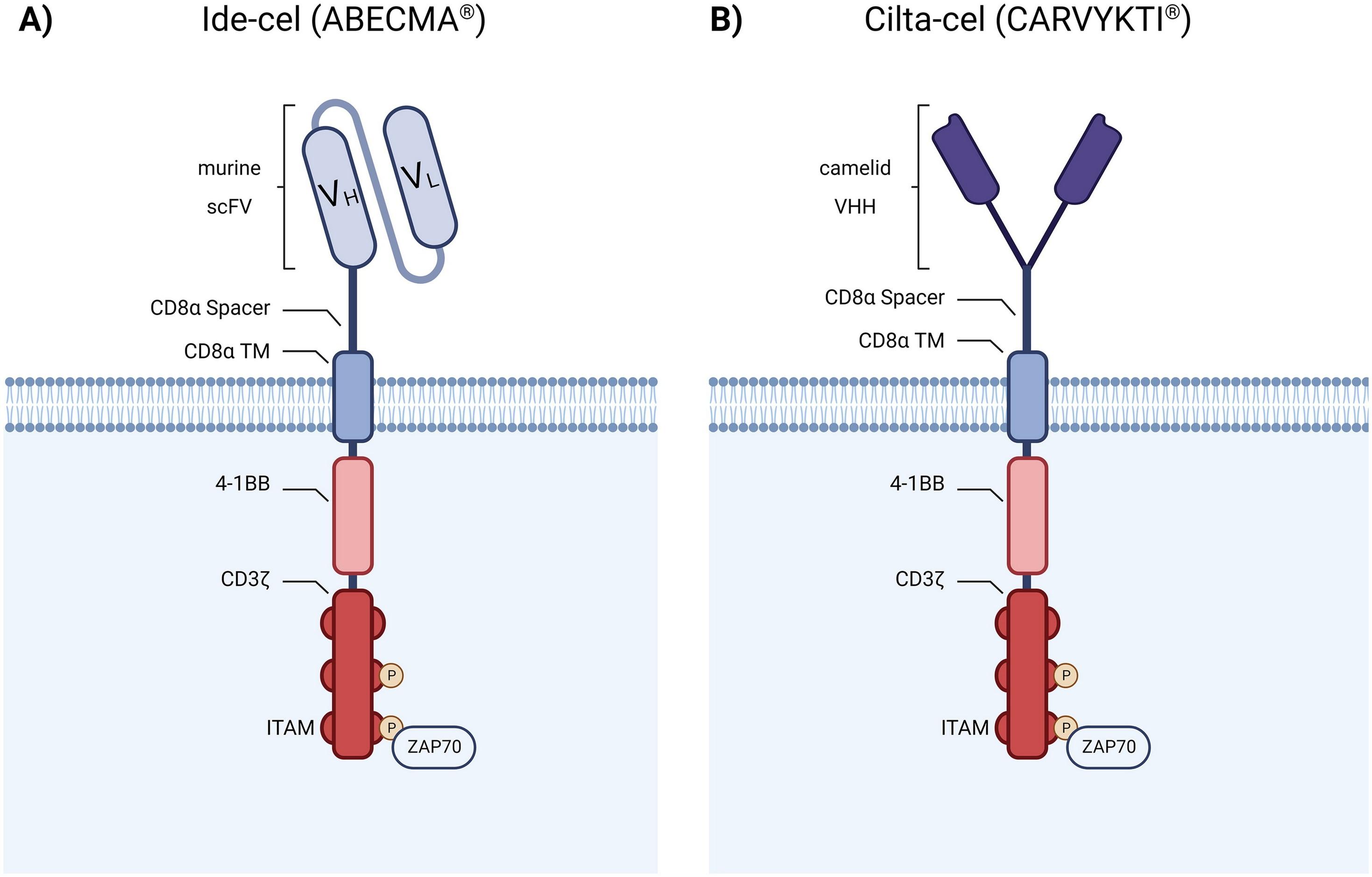
Scheller L et al, Leuk Lymphoma. 2024 Feb
Efficacy
Bispecific CAR T
Activate Tem >>> T naïve
+++ CD8 >> CD4 dependent
↑ absolute CD3 count
↓ Texhaused (TCF1 - TOX +)
Higher E:T ratio +++
Higher CD3/sBCMA ratio +++
Efficacy reduced with ↑ Tregs
Equally activate Tnaive & Tem
↑ Tnaive/Tcm in leukopheresis ++
↑ Tem in product +++
CD4/CD8 ratio 50/50 leukopheresis and product
Higher E:T ratio +++
Higher Cmax/sBCMA ratio +++
Efficacy reduced with ↑ Tregs
Effect on T cells
CD8 Tem /TEMRA ↑
CD4 / CD8 ratio ↓
Lymphodepletion (BM pooling)
diversity ↓ CD4 / CD8 ratio ↓ (LD chemo) Lymphodepletion (LD chemo)
diversity ↓
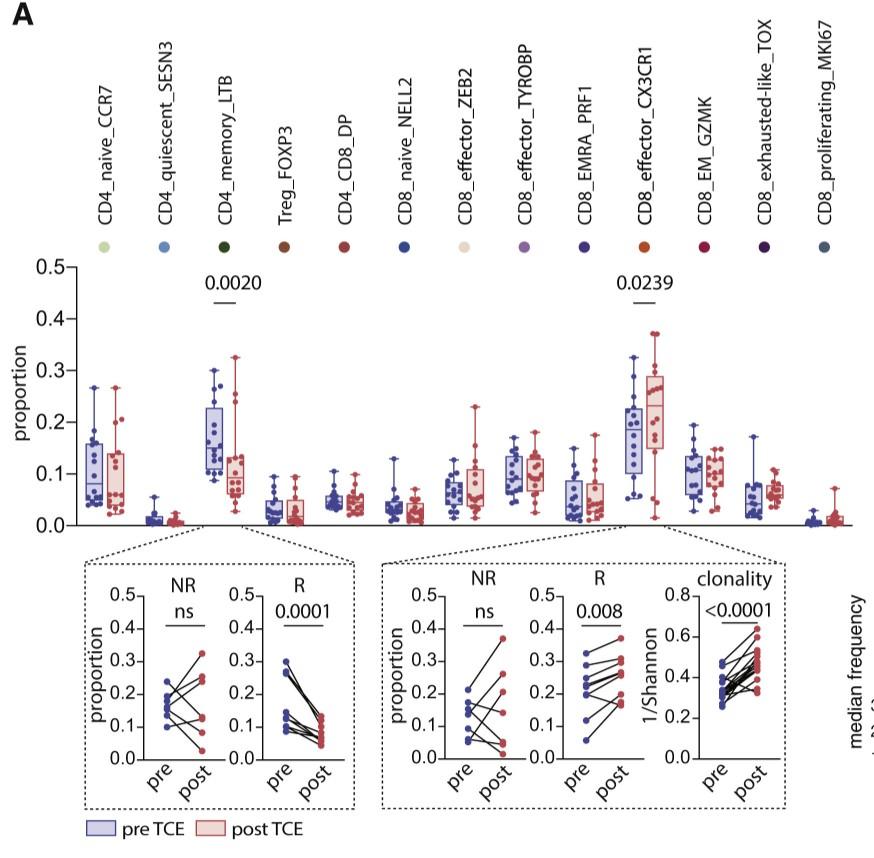
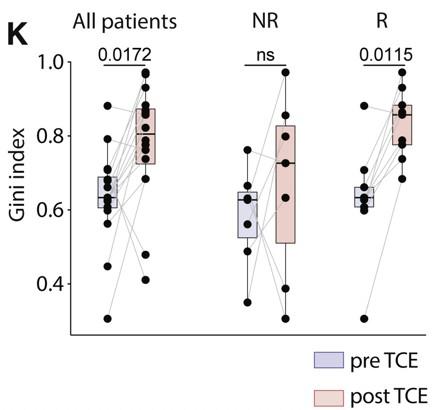

need to demonstrate functional exhaustion
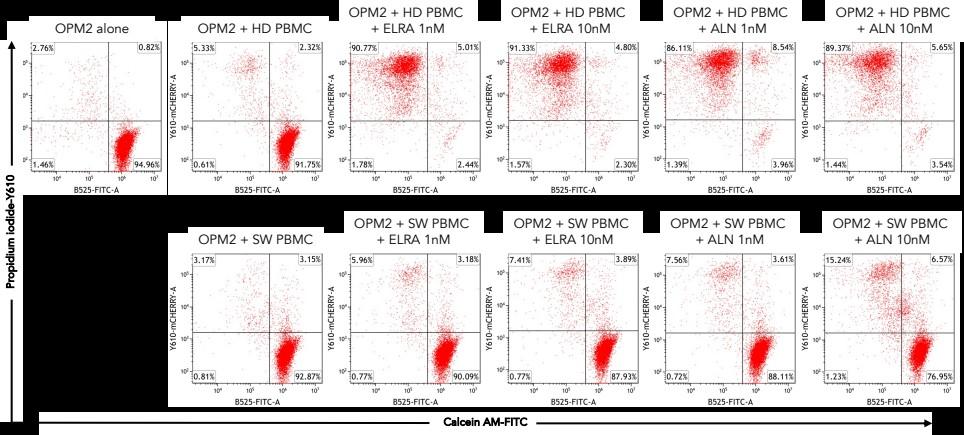
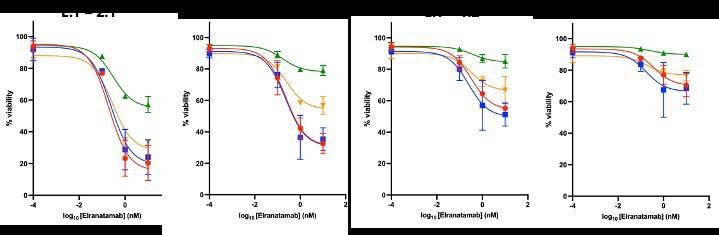
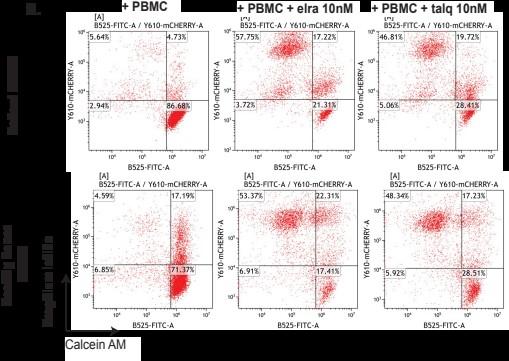
Healthy donor (HD) or patient (SW) peripheral blood mononuclear cells PBMC) co-cultured with OPM2 cells (CTV stained) with or without elranatamab (ELRA) or alnuctamab (ALN). Effector to target ratio 10:1. OPM2 viability was assesses at 48 hours.
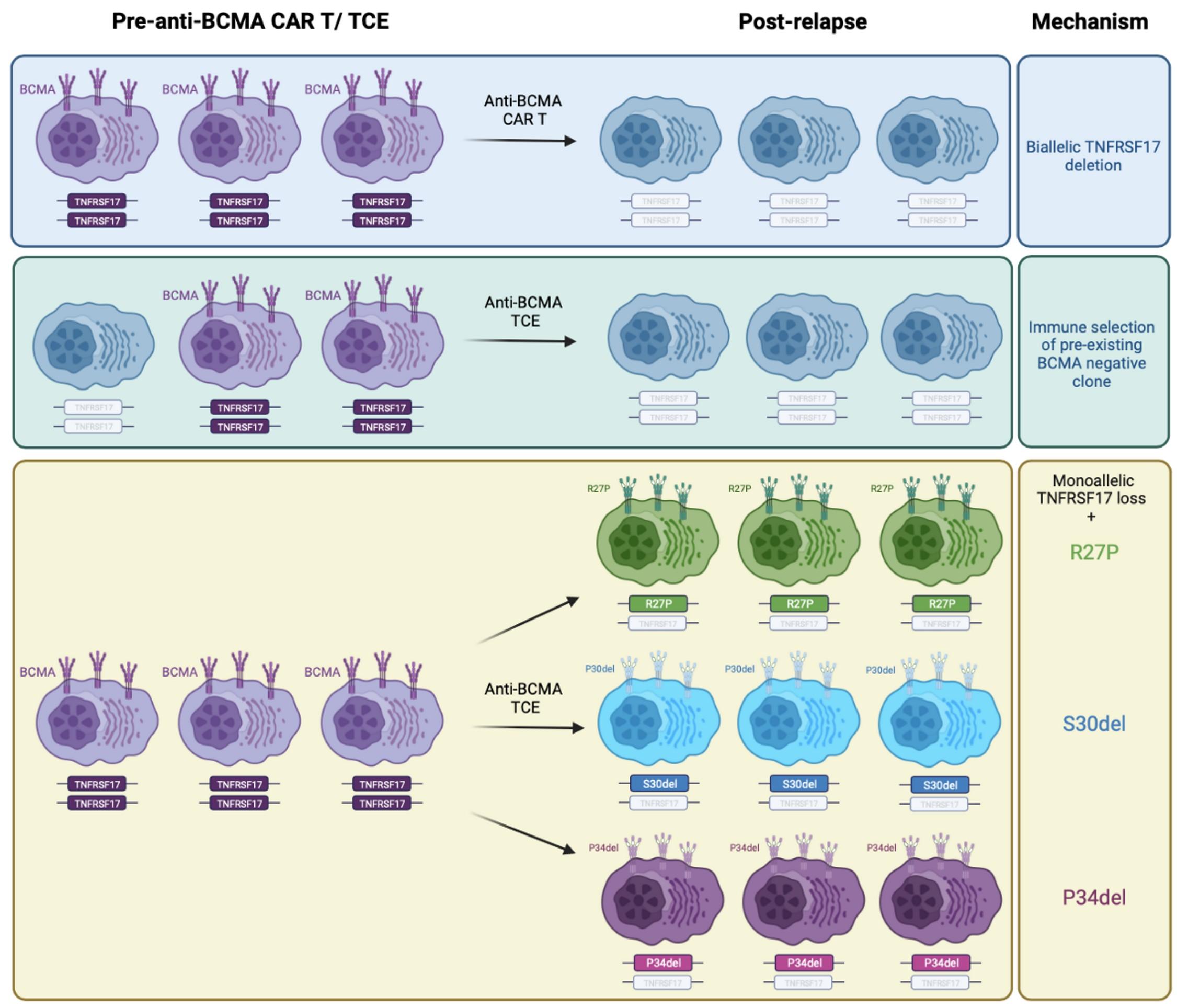
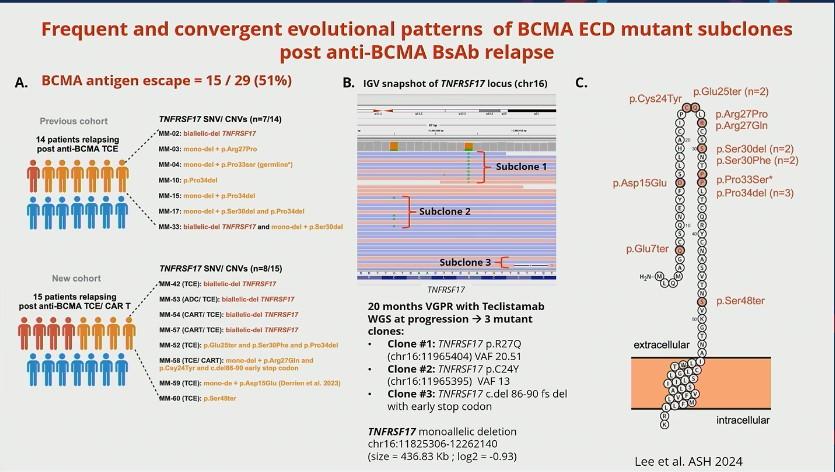
FACT #4 Target antigen loss is the major mechanism of acquired resistance to anti-BCMA/ –GPRC5D TCEs and anti-GPRC5D CAR T
(NOT the case post anti-BCMA CAR T)
A.BCMA antigen escape = 24 / 40 (60 %)


GPRC5D antigen escape is identified in 61.9% of cases relapsing after talquetamab (13/21)
No target Ag switch:
1. Anti-BCMA TCE / ADC anti-BCMA CAR
2. Anti-BCMA CAR T / ADC anti-BCMA TCE
Target Ag switch:
3. Anti-BCMA TCE anti-GPRC5D TCE or CAR T
4. Anti-BCMA CAR T / ADC anti-GPRC5D TCE

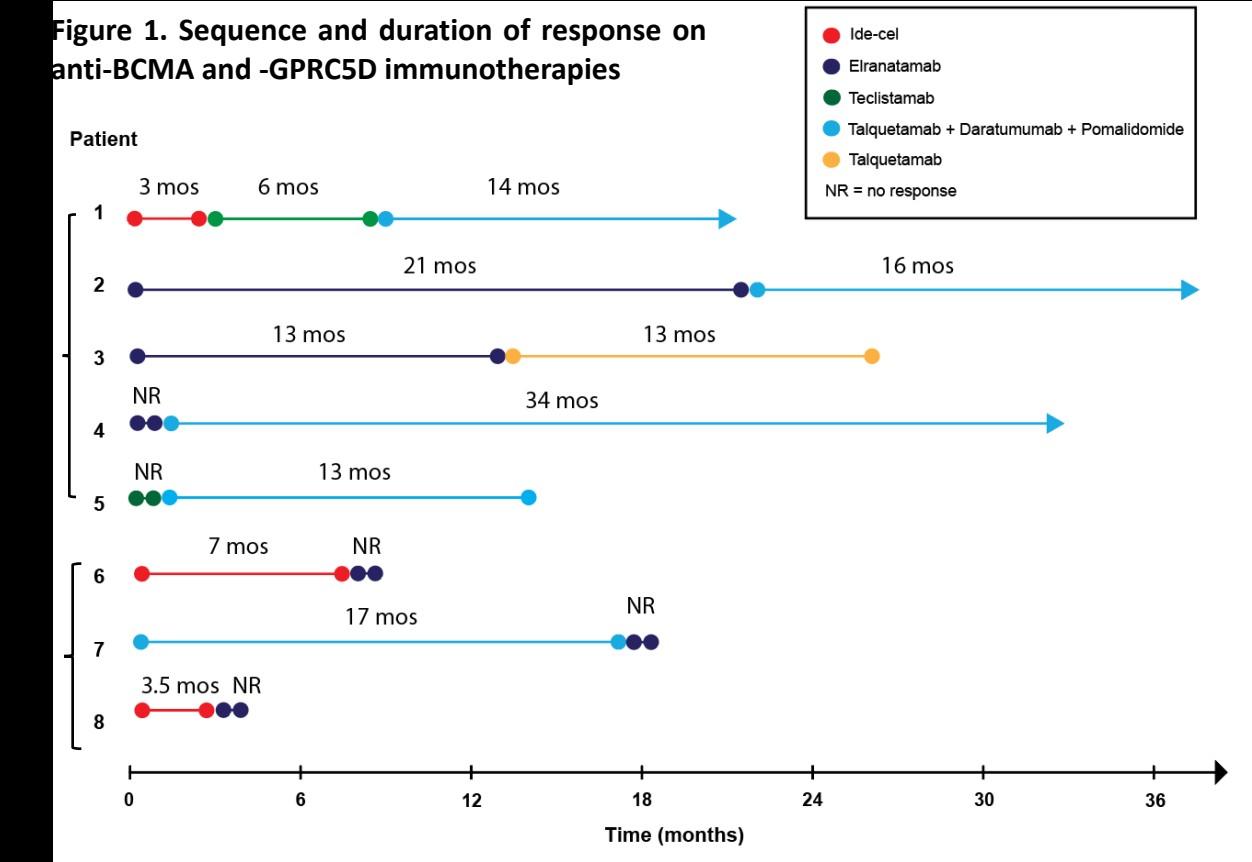
Patient 1:
65 yo female Multiple Myeloma IgG kappa R-ISS III
Treatments:
1.Dara-VTD Mel 200 VGPR; DOR ~ 14 months
2. Carfilzomib_Len-Dex VGPR; DOR ~ 23 months
3. Dara_Pom_Dex VGPR; DOR ~ 21 months.
4. Teclistamab + Cetrelimab ~ 20 months remission
71 yo with PENTA REFRACTORY MM including anti-BCMA TCE
5. Ide-Cel ~ 3 months post anti-BCMA TCE, March 26, 2024
ongoing remission ~ 15 months
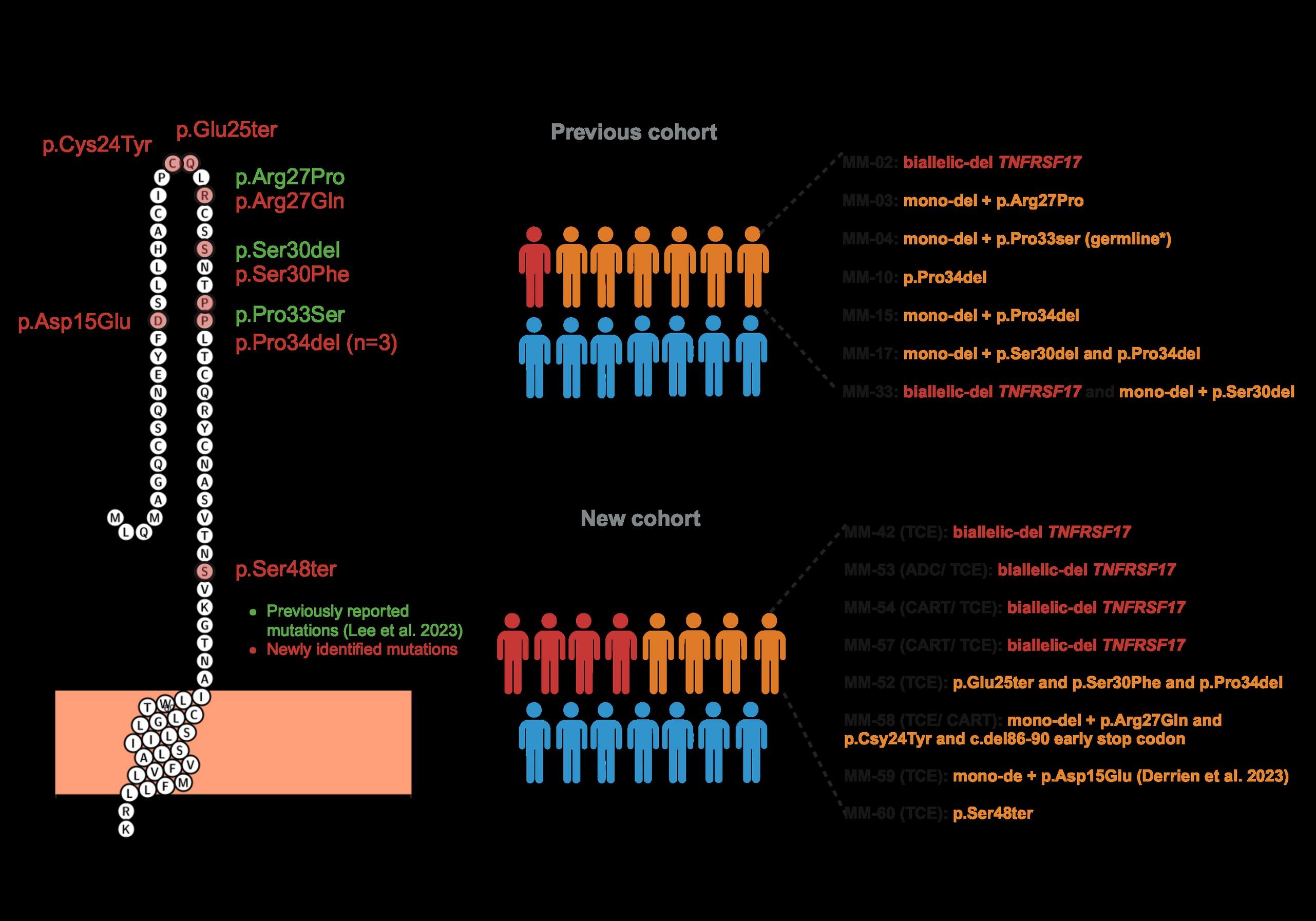
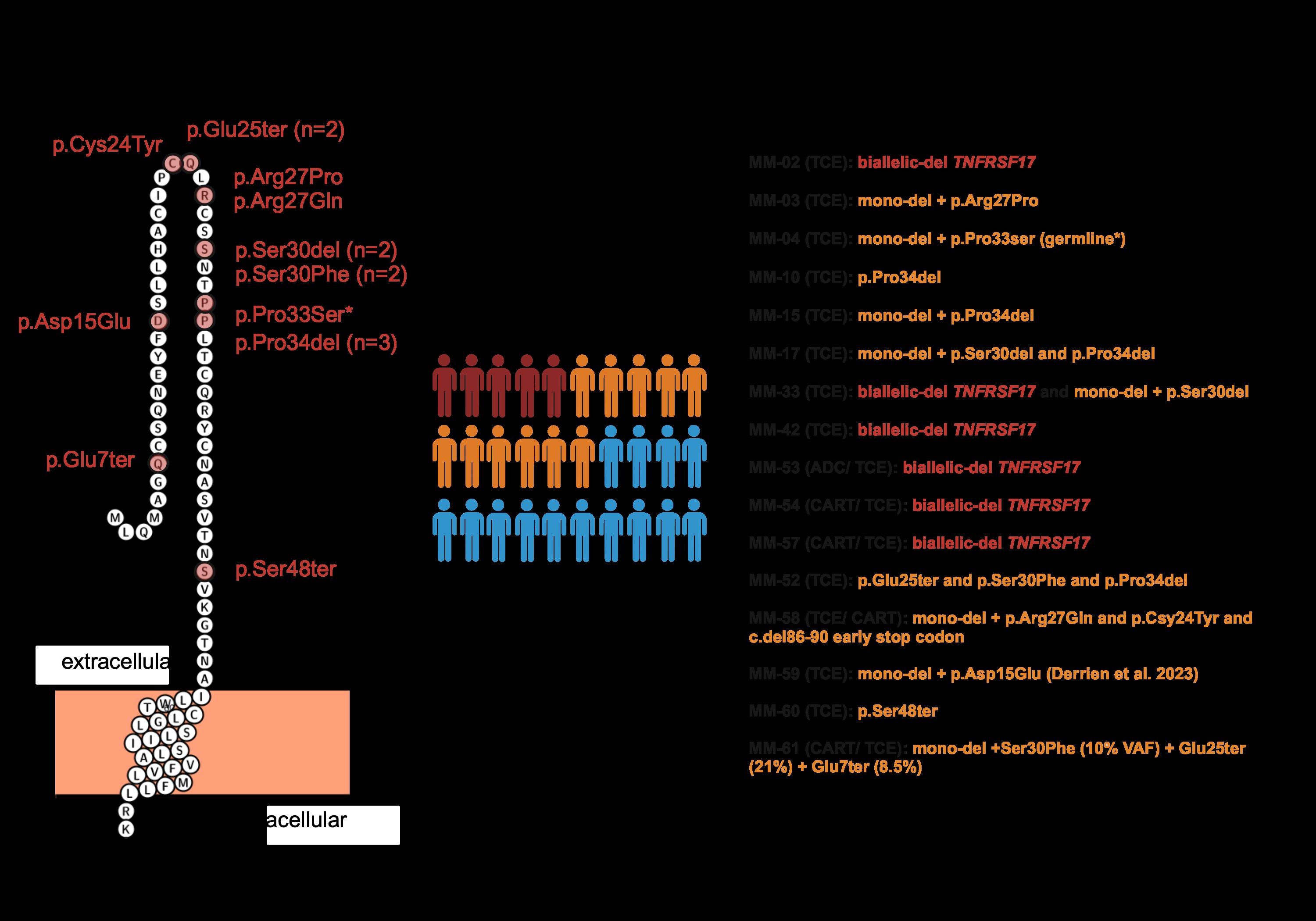
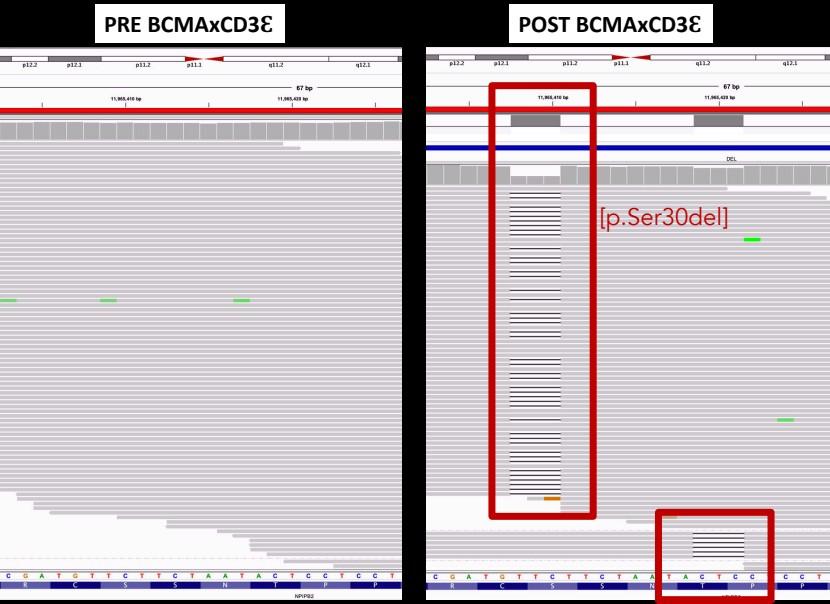
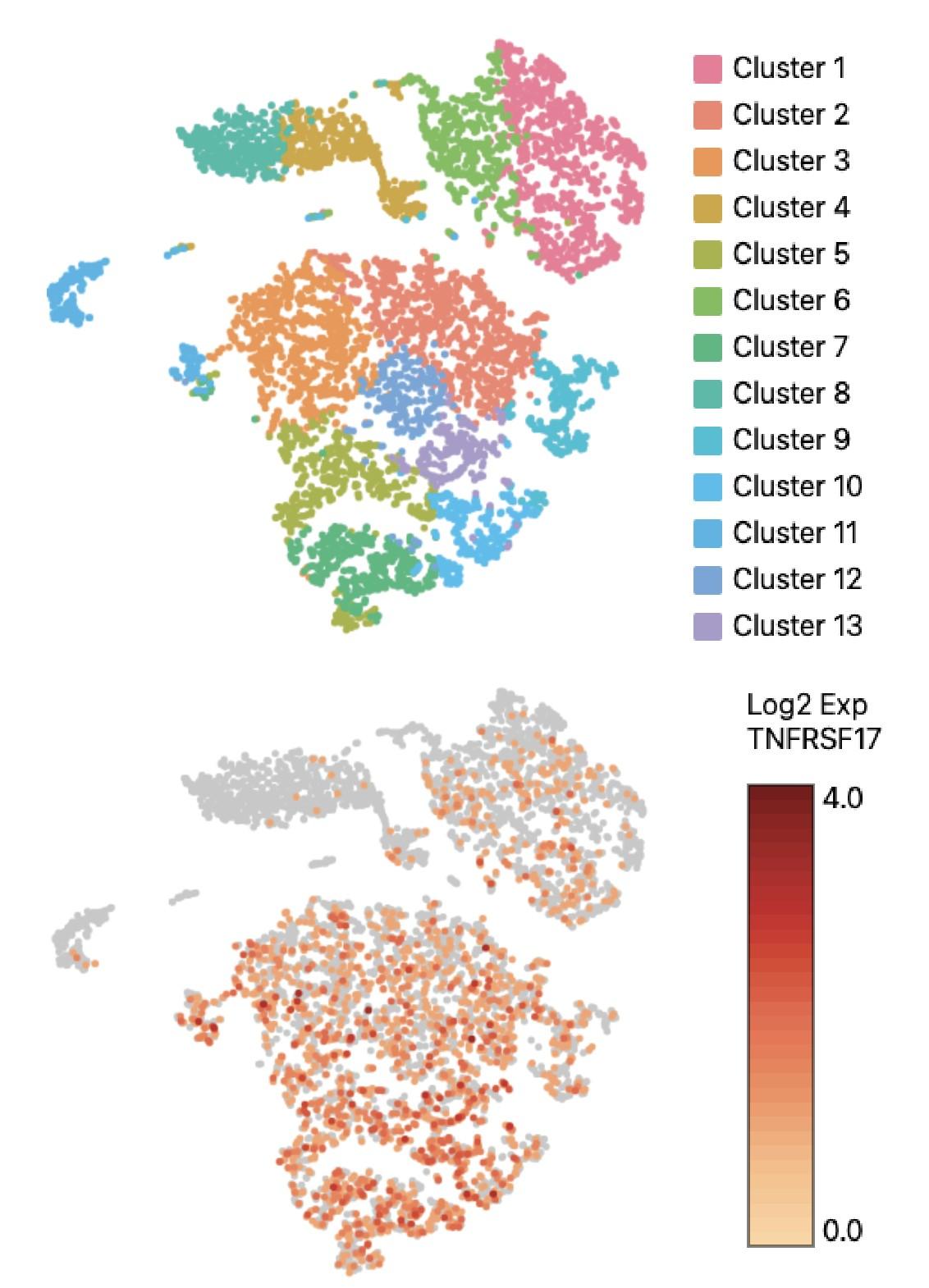
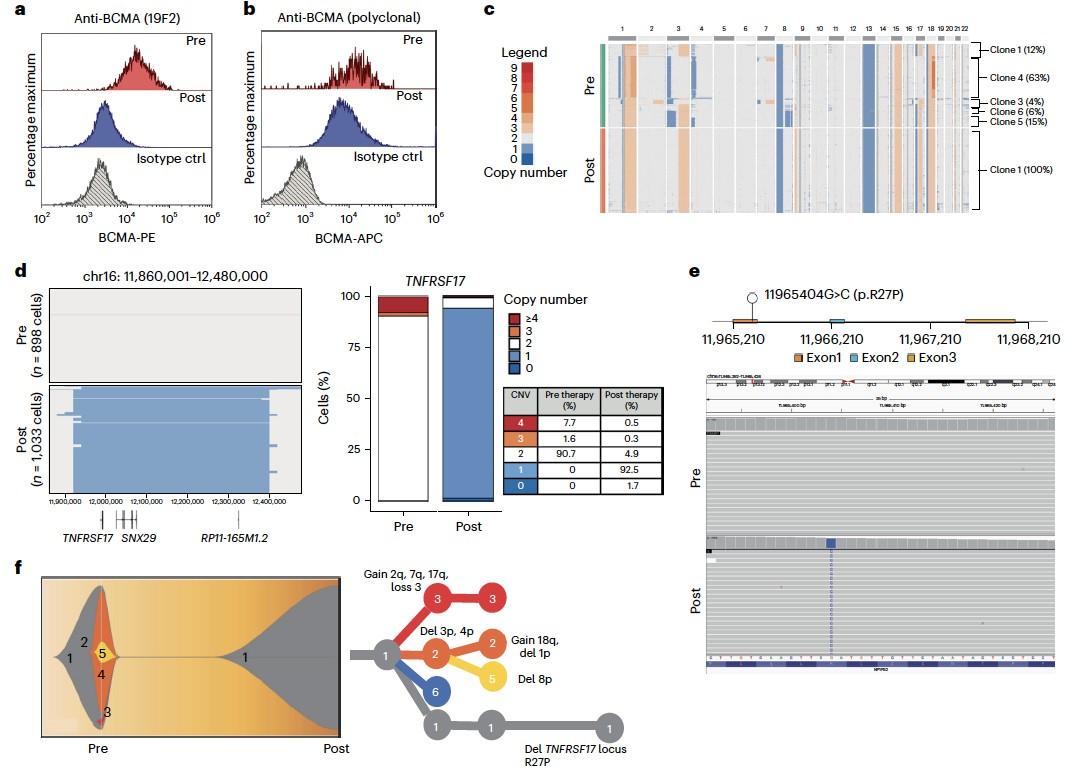
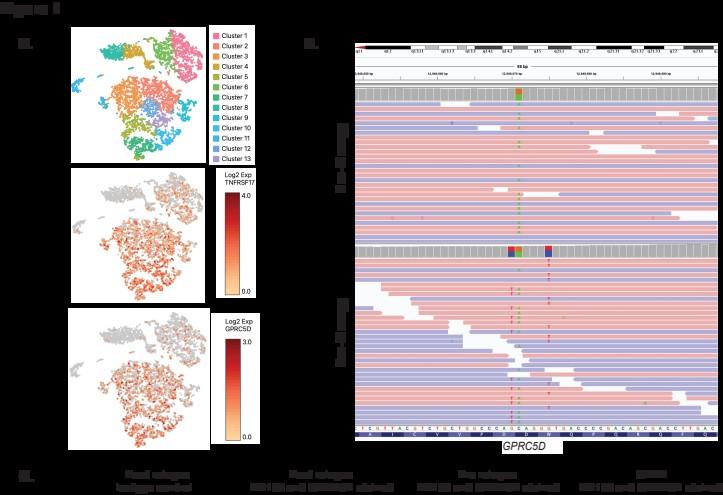
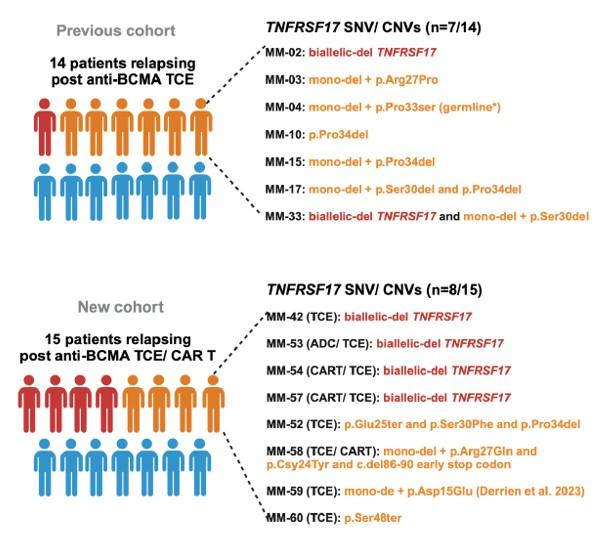
WGS at progression post Teclistamab : 3 TNFRSF17 mutant clones:
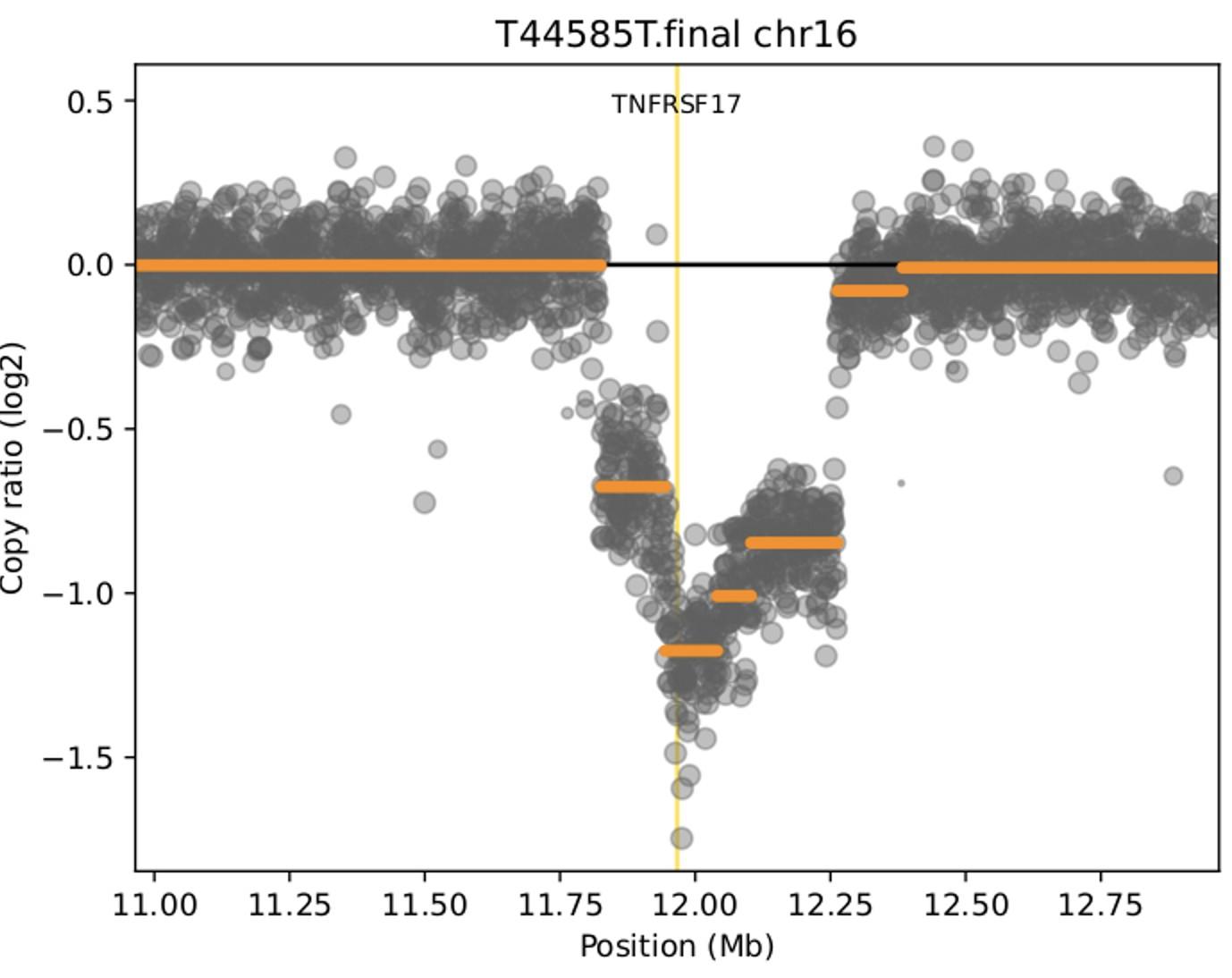
• Clone #1: TNFRSF17 p.R27Q (chr16:11965404) VAF 20.51%
• Clone #2: TNFRSF17 p.C24Y (chr16:11965395) VAF 13 %
• Clone #3: TNFRSF17 c.del 86-90 fs del with early stop codon VAF 10%
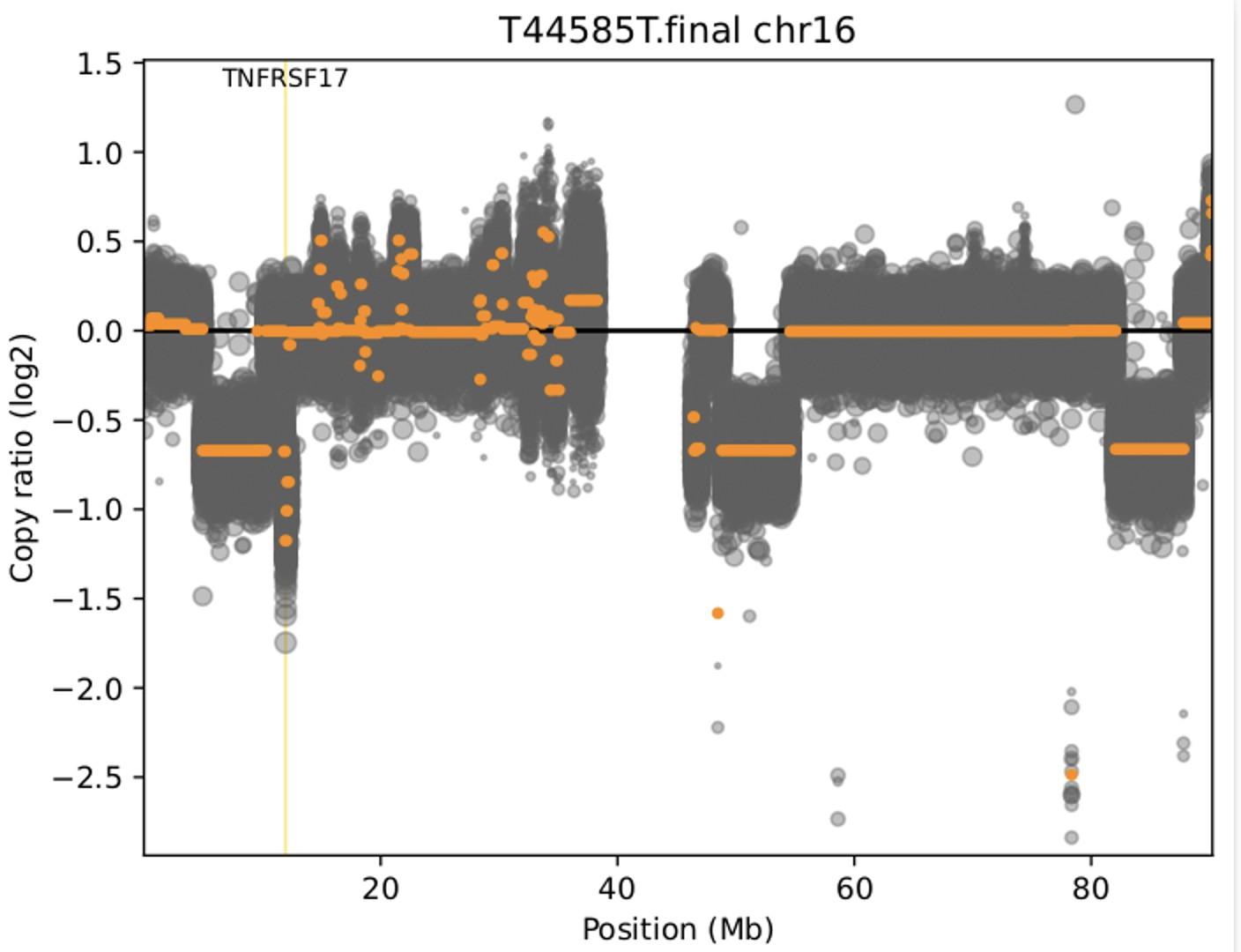
TNFRSF17 monoallelic deletion chr16:11825306-12262140
(size = 436.83 Kb ; log2 = -0.93)

Treatments:
1.Dara-VTD Mel 200 VGPR; DOR ~ 14 months
2. Carfilzomib_Len-Dex VGPR; DOR ~ 23 months
3. Dara_Pom_Dex VGPR; DOR ~ 21 months.
4. Teclistamab + Cetrelimab (PD1 inhibitor) ~ 20 months remission 5.
Allow Sufficient washout time for lymphocytes count recovery and adequate CD4/CD8 ratio prior to apheresis; may vary between patients (role for IMiDs , CELMoDs, stem cell boost?) optimal CAR-T product
Confirm retained target expression (FCM and/or NGS)
Bridging with TCE prior to CAR T infusion does not negatively impact outcomes (Fandrei D, Blood Cancer Discovery 2024, Dhakal B et al Blood 2025))
No target Ag switch:
1. Anti-BCMA TCE / ADC anti-BCMA CAR
2. Anti-BCMA CAR T / ADC anti-BCMA TCE
Target Ag switch:
3. Anti-BCMA TCE anti-GPRC5D TCE or CAR T
4. Anti-BCMA CAR T / ADC anti-GPRC5D TCE
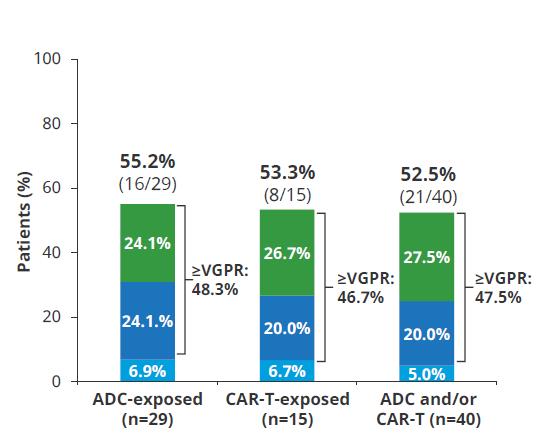
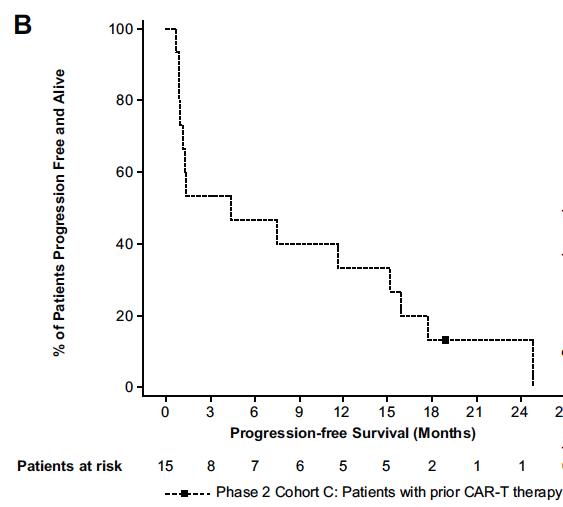
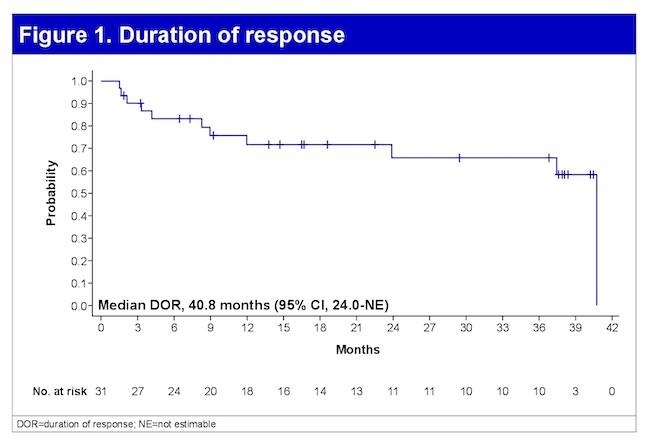
mDOR 14.8 mos (95% CI , 6.222.6)
mPFS 7.3 mos (95% CI, 1.316) Touzeau C et al Blood Aug 2024
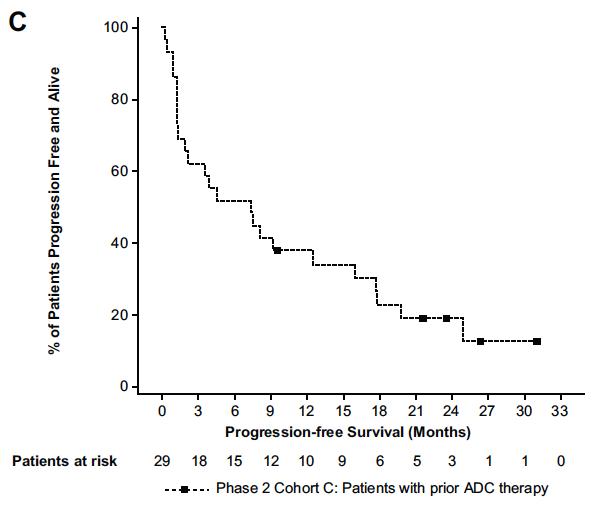
PD-1 & TIM-3 were NOT upregulated on CD4+/CD8+ T cells from patients receiving a prior anti-BCMA CAR-T or ADC
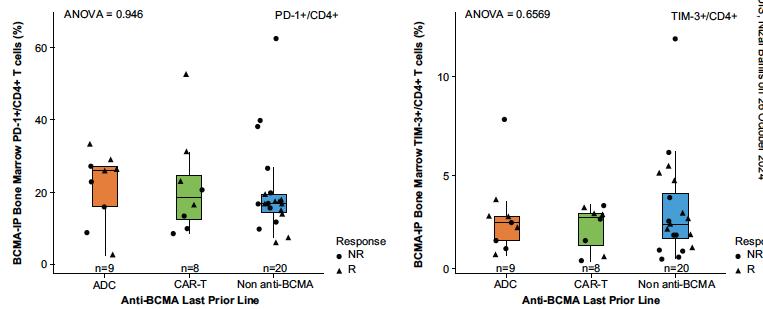
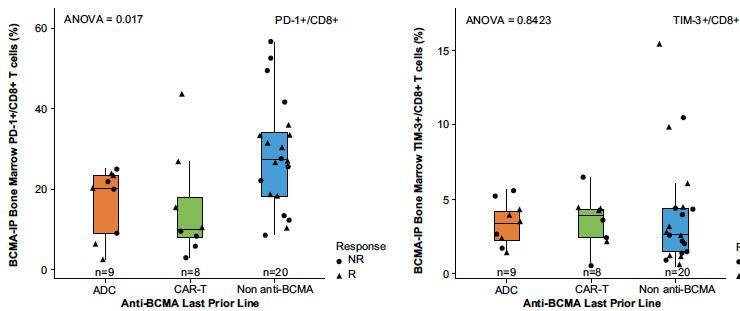
Touzeau C et al Blood Aug 2024

Patient 2:
65 yo male Multiple Myeloma kappa light chain R-ISS II, t(11;14)
positive Treatments:
1.CyborD Vel- Mel 200 Feb 2015 Len maint 3 yrs VGPR; DOR ~ 4 years
2. Dara_Len_Dex , Feb 2019 VGPR, PFS ~ 1 year
3. BB2121-MM03 trial, Ide-Cel, Aug 2020, cell dose 563 x 10^6. SD PD Dec 2020
4.TECLI-MMY1001 trial with Teclistamab Jan 20, 2021. Best response as sCR ~ 1 year. PD June, 2022
5.TRIMM-2: Daratumumab +Talquetamab + dexamethasone, pomalidomide. Sep 70 yo with double refractory MM progressed 3 months post antiBCMA CAR
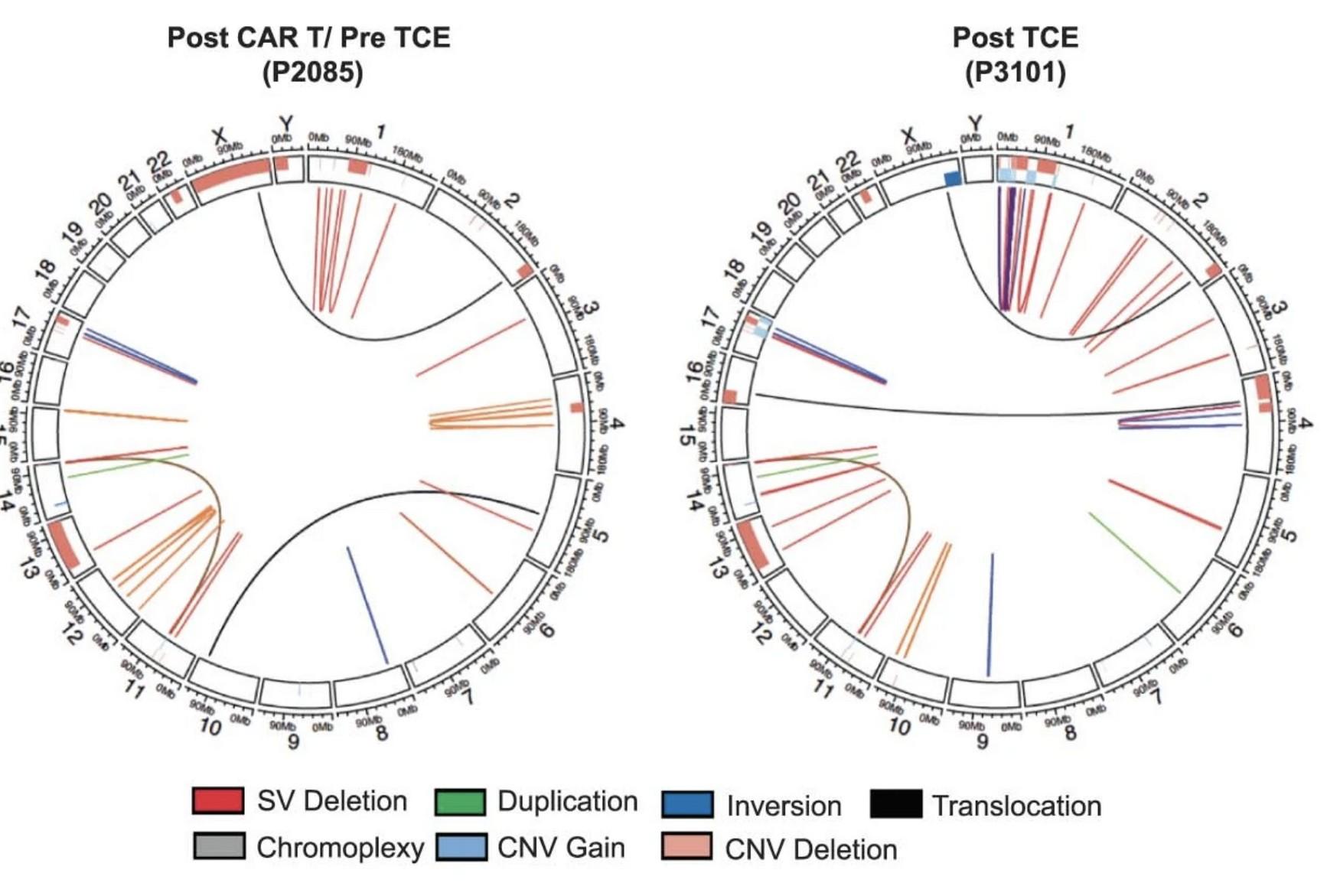
Lee H et al. Nat Med. 2023;29:2295-2306

No target Ag switch:
1. Anti-BCMA TCE / ADC anti-BCMA CAR
2. Anti-BCMA CAR T / ADC anti-BCMA TCE
Target Ag switch:
3. Anti-BCMA TCE anti-GPRC5D TCE or CAR T
4. Anti-BCMA CAR T / ADC anti-GPRC5D TCE





Patient 2: 65 yo male Multiple Myeloma kappa light chain R-ISS II, t(11;14) positive Treatments:
1.CyborD Vel- Mel 200 Feb 2015 Len maint 3 yrs VGPR; DOR ~ 4 years
2. Dara_Len_Dex , Feb 2019 VGPR, PFS ~ 1 year
3. BB2121-MM03 trial, Ide-Cel, Aug 2020, cell dose 563 x 10^6. SD PD Dec 2020 4.TECLI-MMY1001 trial with Teclistamab Jan 20, 2021. Best response as sCR ~ 1 year. PD June, 2022
2022. sCR ongoing ~ 4 years 70 yo with double refractory MM progressed 3 months post anti-

Treatments:
1.CyBorD
2.Dara_Len-Dex

Double hit, penta refractory MM
Therapy: anti BCMAxCD3
IMWG response: CR; DOR 13 months
Elranatamab
Lee H et al. Nature Medicine. 2023;29:2295-2306





Lee H et al. Nature Medicine. 2023;29:2295-2306



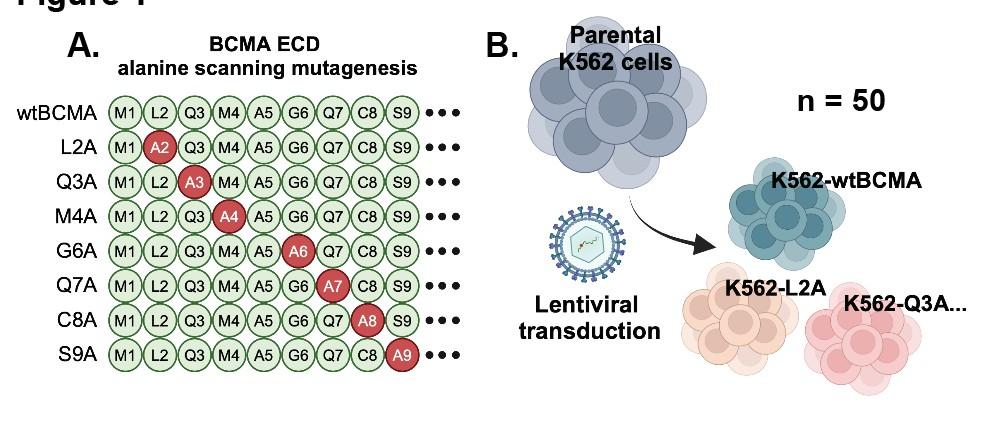

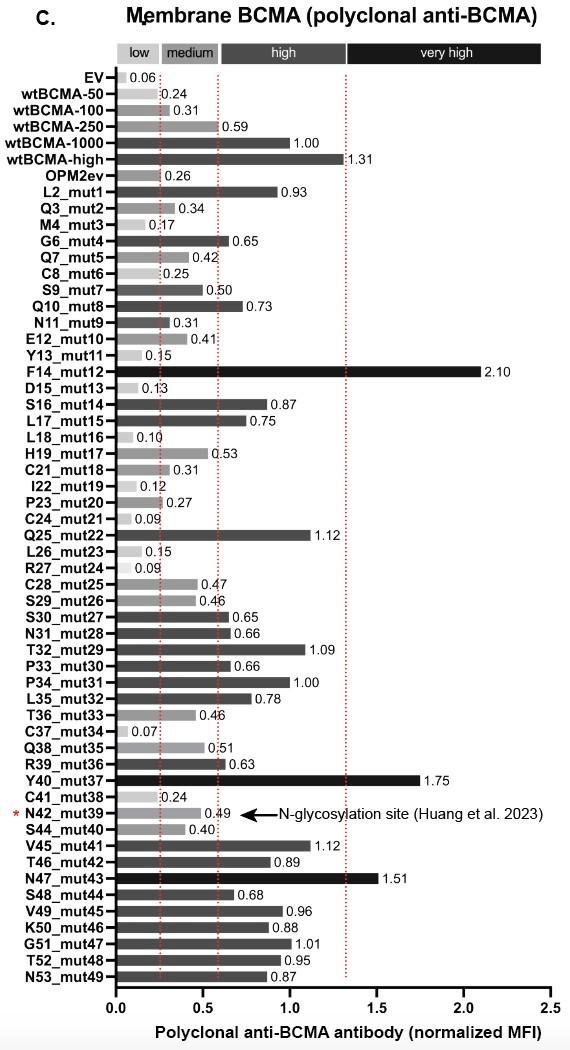
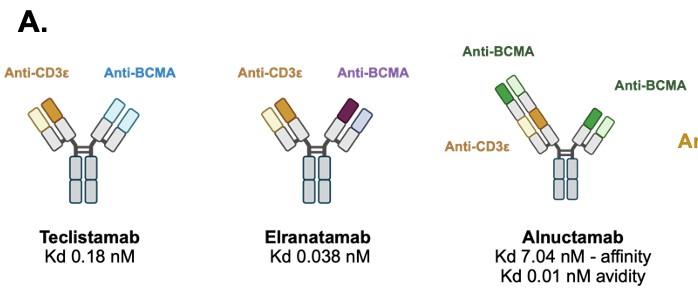

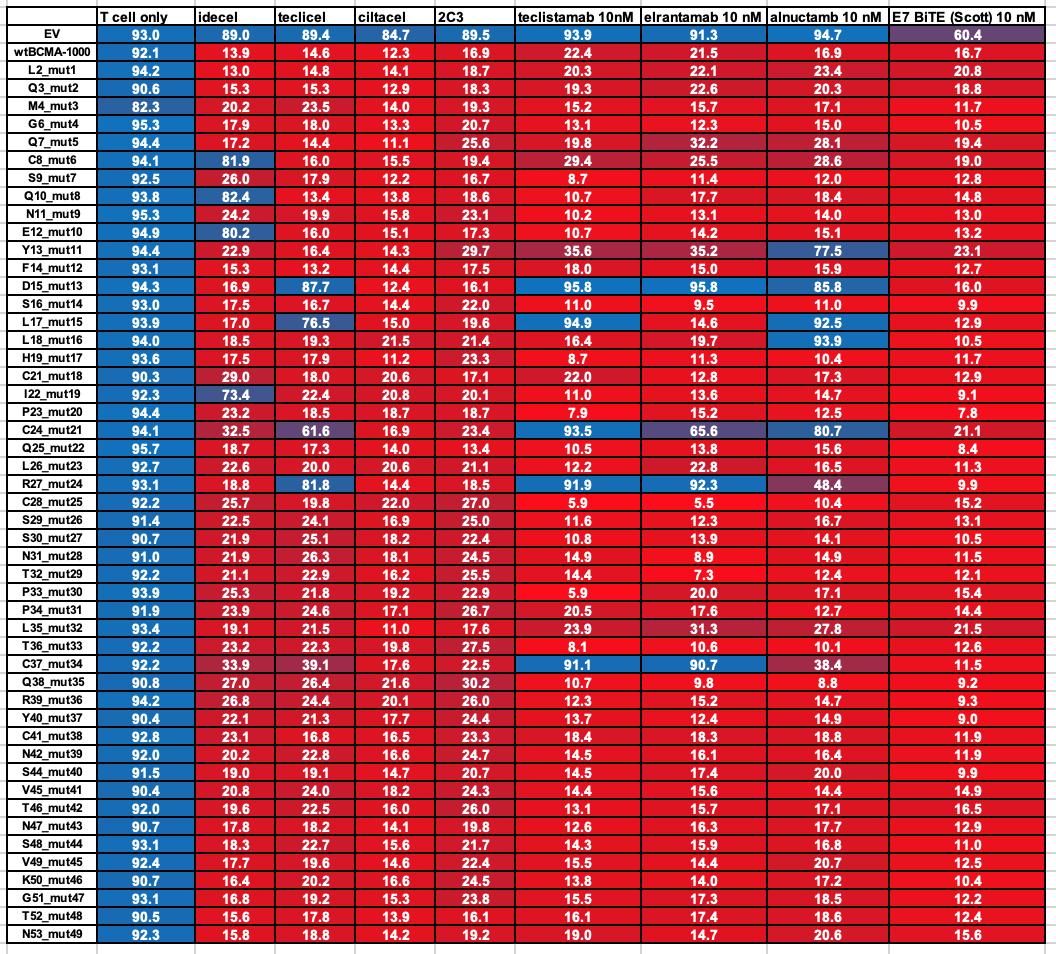

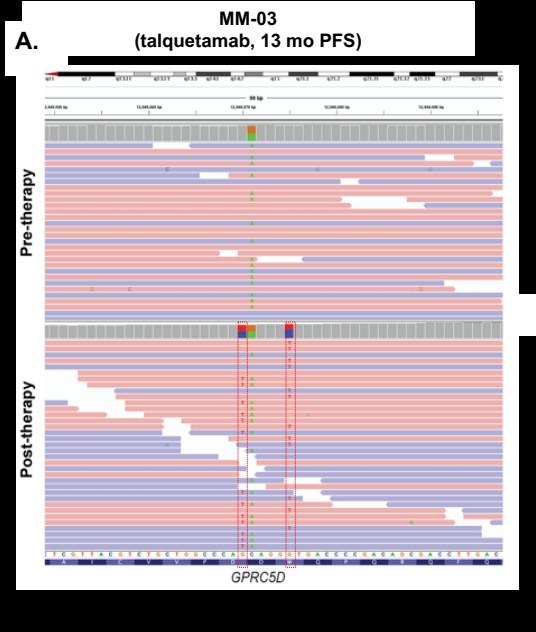
75 yo female Multiple Myeloma IgG Lambda R-ISS III
t(14;16) pos
Treatments:
1.CyBorD VGPR; DOR ~ 18 months
2.Dara_Len-Dex VGPR; DOR ~ 16 months
3.Pom_Dex MR; DOR ~ 4 months.
4.Carfilzomib_Dexamethasone PR; DOR ~ 14 months + del17p 50.8%
80 yo with Double hit PENTA REFRACTORY MM
5. Elranatamab 76 mg sCR; DOR ~ 13 months
6. Talquetamab q2w, Daratumumab & pomalidomide (2 mg added C2D1)
sCR; , DOR ~ 13 months

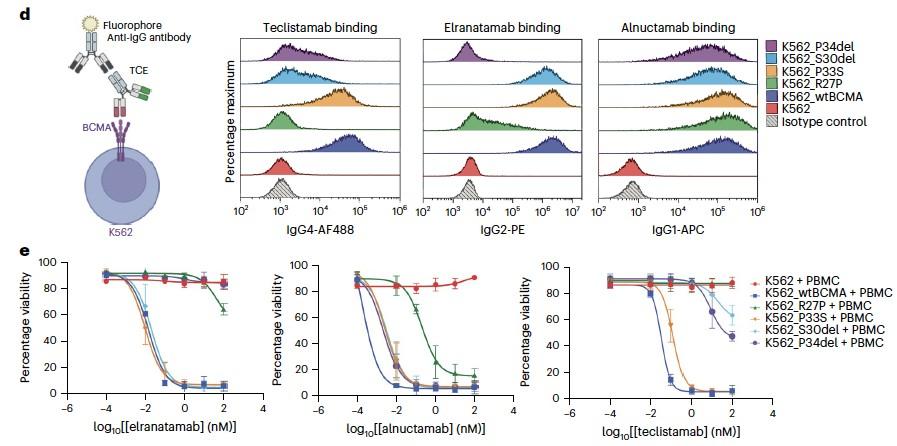
Anti-HA Anti-GPRC5D

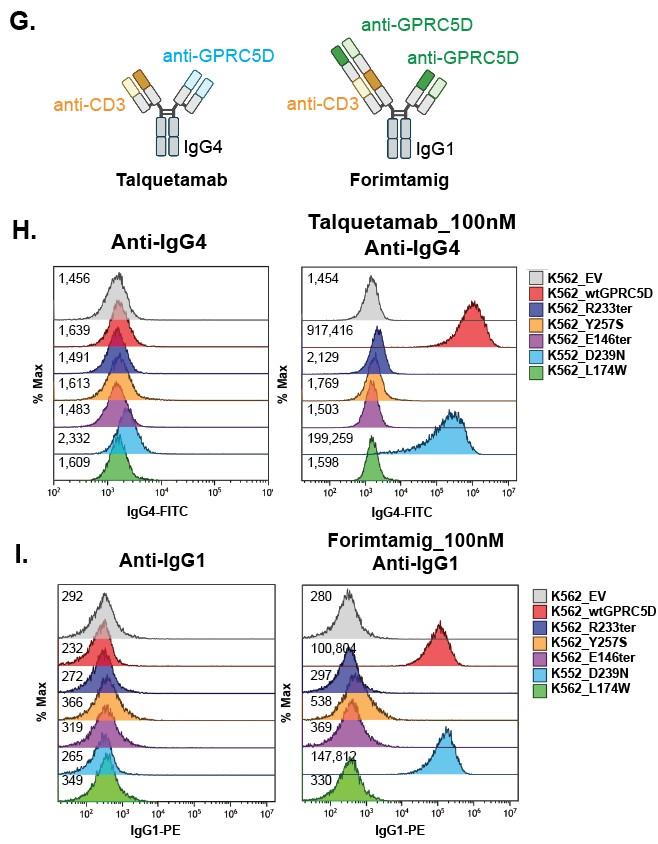
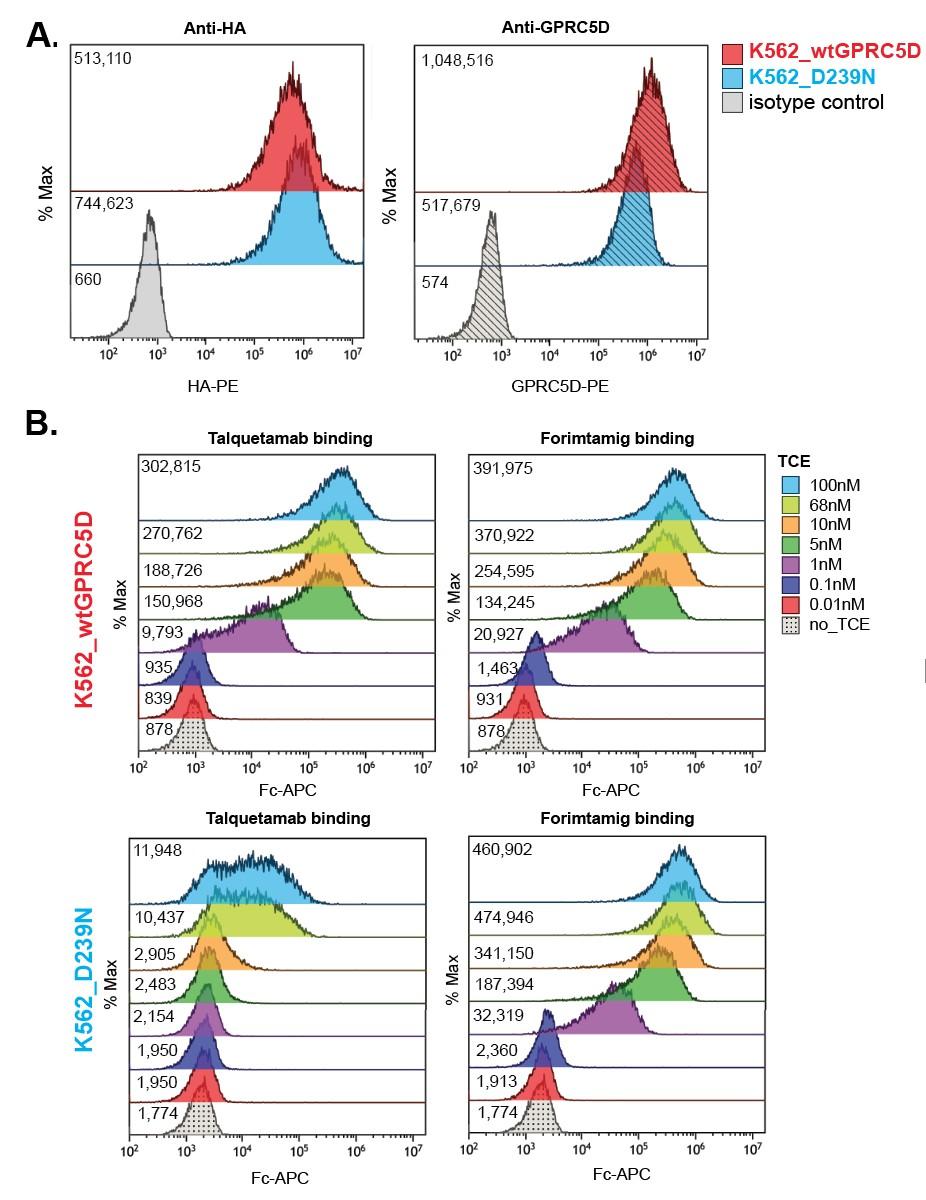
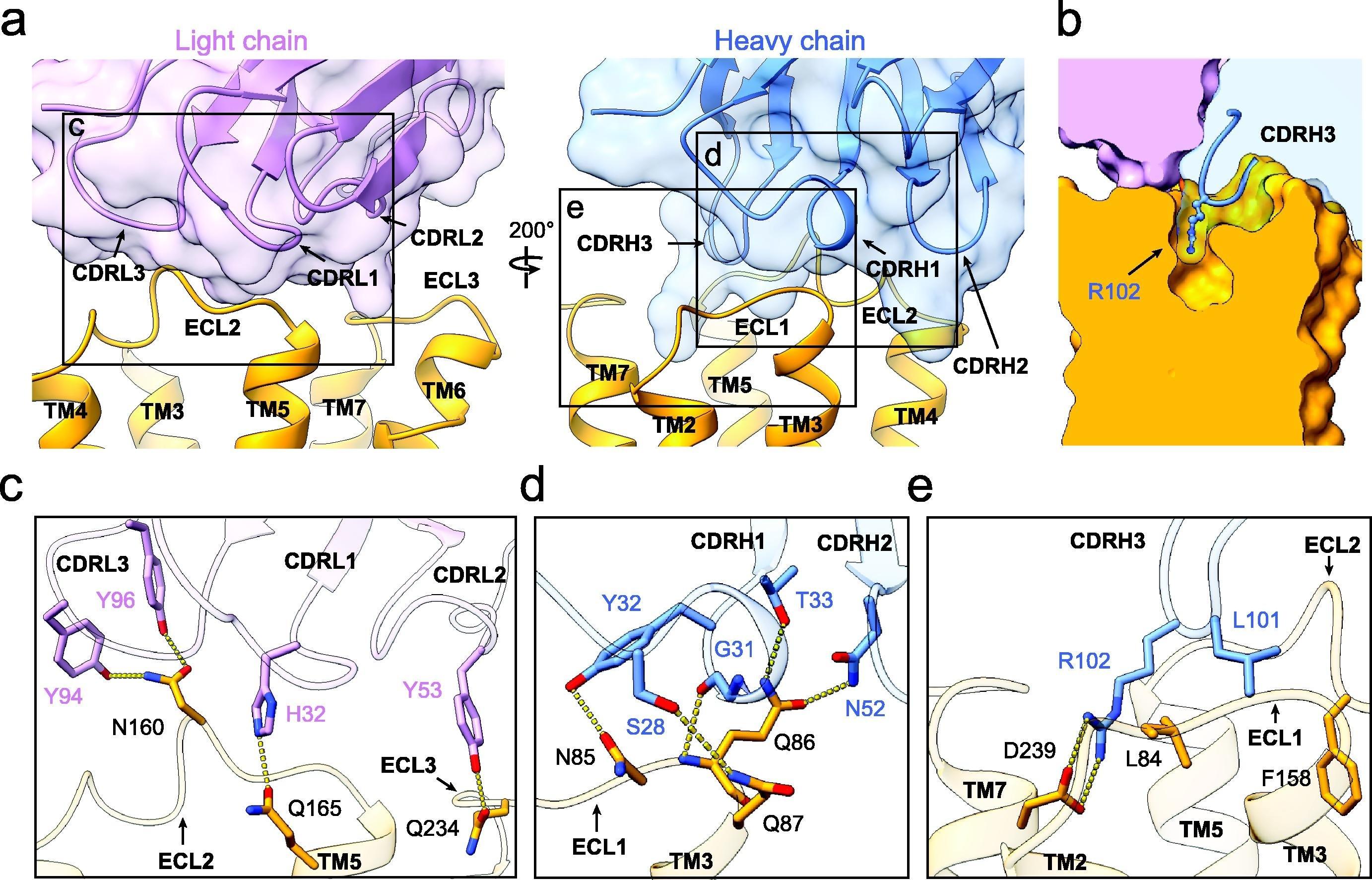
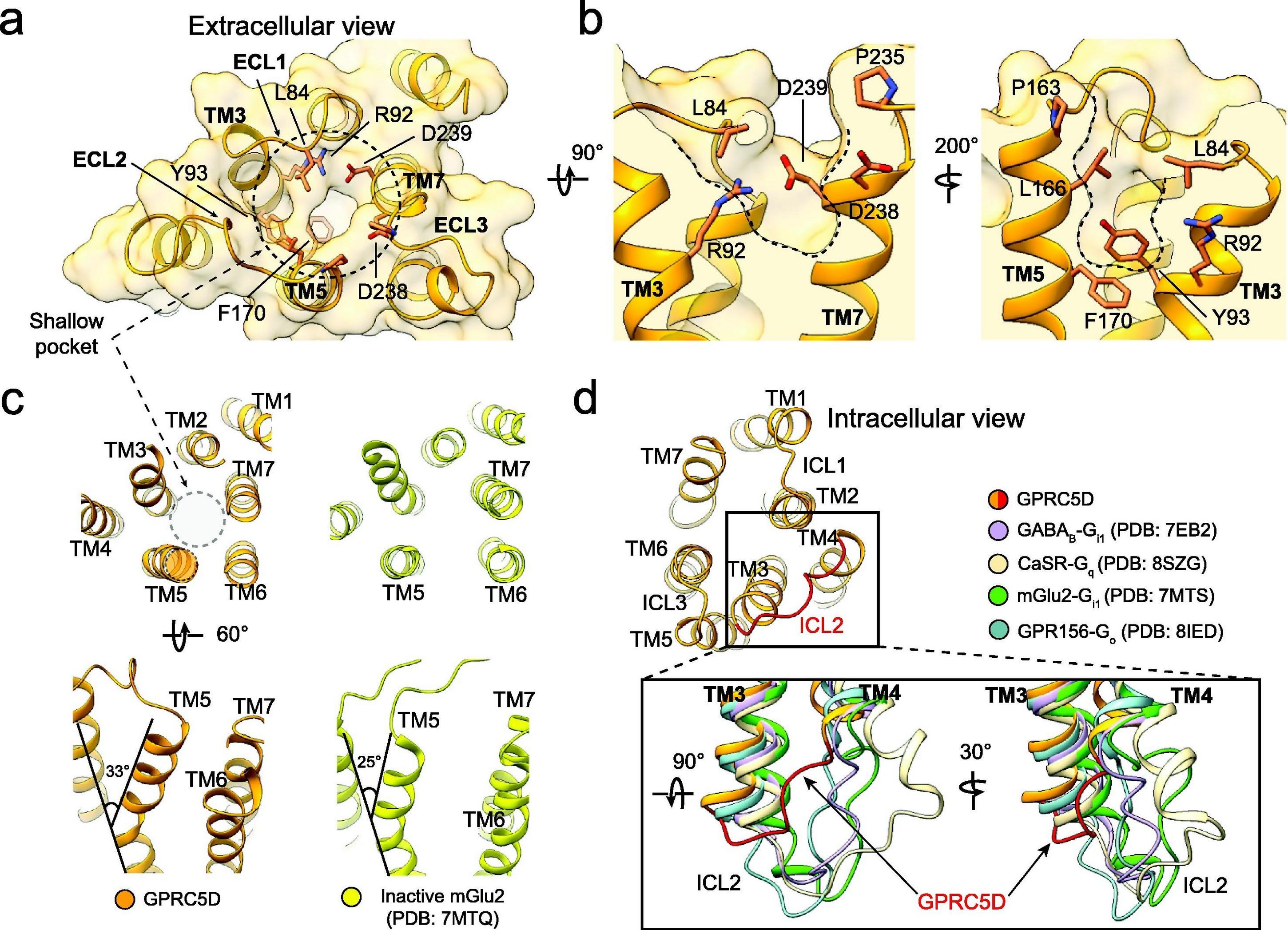
Shallow pocket in GPRC5D extracellular domain comprised of: - ECL 1, ECL2, TM3, TM5, and TM7 - Walls of the pocket formed by:-
R102 of talquetamab intrudes into pocket forming an ion pair with D239 of GPRC5D

Confirm retained target expression (FCM and/or NGS)
Ensure adequate absolute CD3 T cell count (CD8 Tem)
Sequential treatment with anti-BCMA CAR –T TCE or TCE
TCE can yield durable responses if adequate T cell count (Tem)
Switching targeted antigen with Talquetamab if TNFRSF17 mutational status is unknown.
Optimal duration of therapy with bispecific antibodies
Better define patients who respond to sequential TCE based therapies?
Immune profiling
Should we combine TCEs targeting different MM Ag? Combine TCE +/- anti-CD38 mAb to target antigenic escape? Or with CelMODs to expand Tem?
Surveillance for emerging BCMA or GPRC5D mutants
switching treatment









Hermann Einsele, MD,
Würzburg University Hospital
June 10th 2025, Milan



Prof. Dr. Hermann Einsele Department of Internal Medicine II University Hospital Würzburg

To improve efficacy
• T-cell exhaustion
• Target antigen loss
• Tumor microenvironment
To reduce Toxicities
CRS
ICANS
CNP/MNT
To improve availability
Long manufacturing time (CAR-T Cells)
Cost

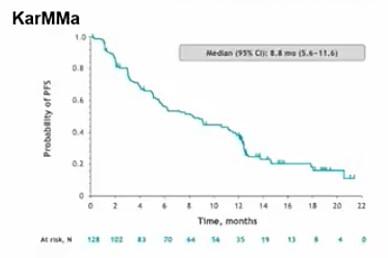
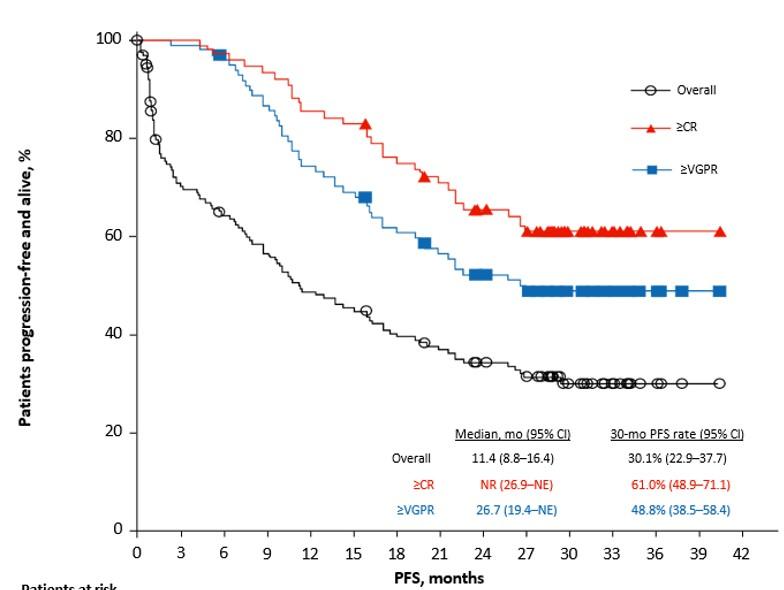
Median (95% CI): 8.8 mo. (5.6 – 11.6)
CRS grade >2 3-5%
ICANS grade >2 3-5%, CNP/MNTs 1-10%
Bispecifics:
Require hospitalization for step-up dosing
Infection Rate Grade >2 40-70% Safety Majestic-1
To improve efficacy
• T Cell exhaustion
• Target antigen loss
• Tumor microenvironment
To reduce Toxicities
CRS
ICANS
CNP/MNT
Combinations
Novel constructs
(binders, modulation of Transcription factors, Inhibitory molecules)

To improve availability
Long manufacturing time (CAR-T Cells) • Cost


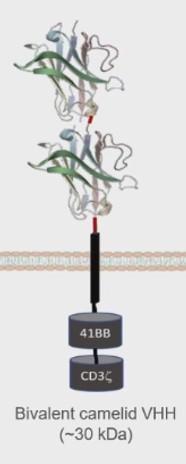
(BTKi,


Small D-Domain construct facilitates high transduction efficiency and CAR positivity, with low total cell dose
D-Domain CARs stable and lack tonic signaling
D-Domain binder fast off-rate, promoting tumor cell killing without prolonged inflammation
→ reduced neurotoxicity?!
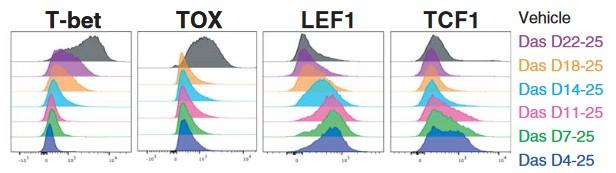
At median follow-up of 34 months:
ORR 100%, CR 79%
Median PFS for all pts. 30.2 mo. / for CR/sCR pts. 34.3 mo.
No delayed or non-ICANS neurotoxicities
(Parkinsonism, cranial nerve palsies, GBS)
CRS 95 % (≥ Grade 2 47%), ICANS 18% (Grade ≥ 2 6%)



To install genetic program for sustained anti-myeloma efficacy and longevity in CAR-Ts,

• restoration of IL2-production
• exhaustion resistance ↑
• terminal differentiation ↓
• potency in Aglow settings ↑


Knock-in screening
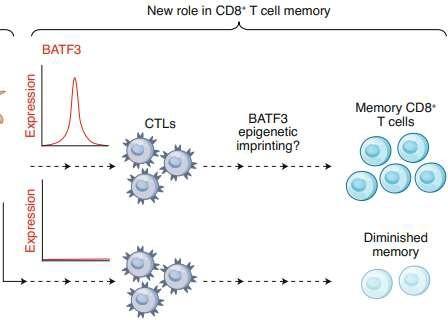
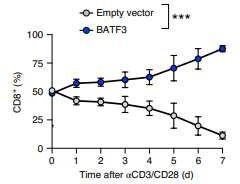
Batf3-deficiency
Memory formation (transition-to and quality-of) ↓
contraction after response ↑ pro-apoptotic factor BIM ↑

100 TFs (and TFxTF) in TCR and CAR + different stimulations TFAP4 (+BATF/BATF3) promotes proliferative, stem celllike memory state
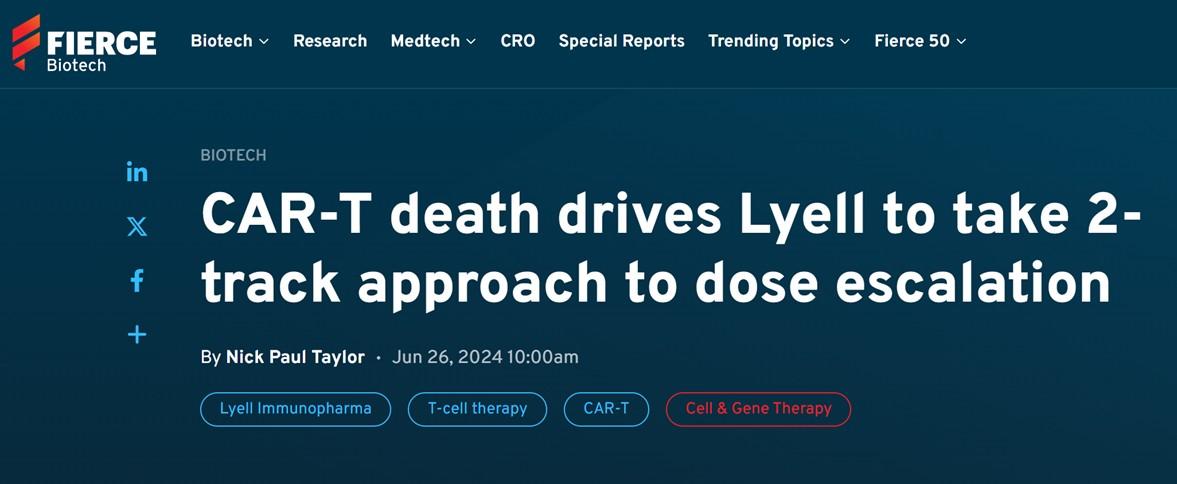

Transcription factor Batf3 regulates T-cell apoptosis and longevity via the proapoptotic factor BIM Batf3-transduced CAR-T Cells targeting ROR2 in r/rMM
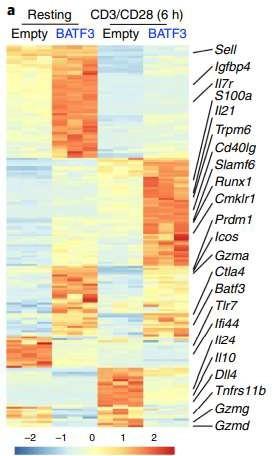
BCMA-CART Cells

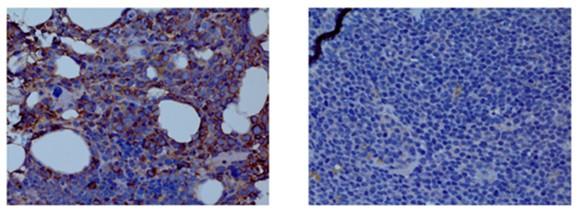



BCMA-targeting TCE
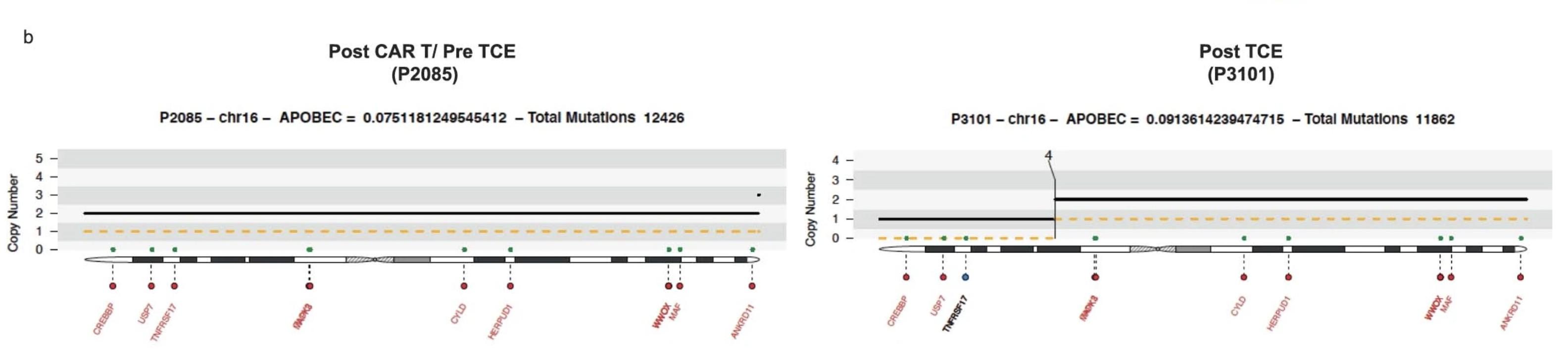
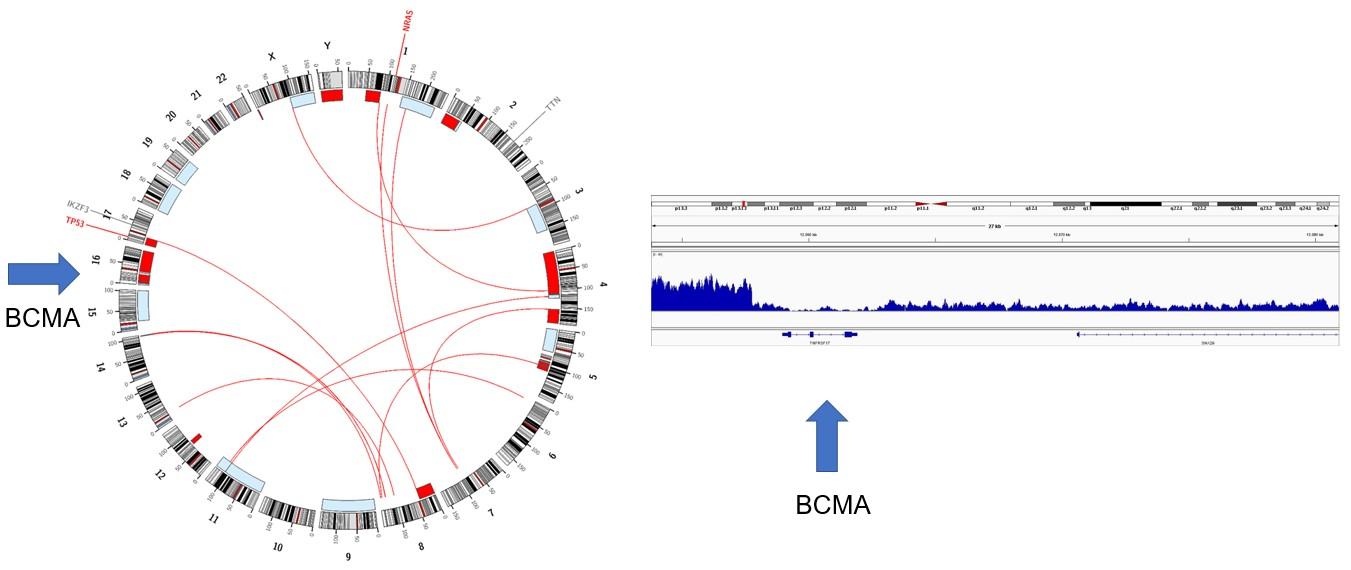
Whole genome sequencing
4-6 % irreversible BCMA loss Biallelic deletion TFRSF17
Arlo-Cel for r/rMM (No/prior BCMA)
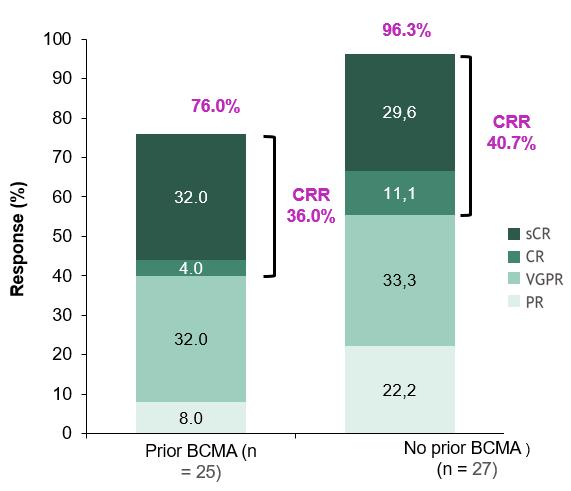
Response in TCR-naïve and -exposed cohorts1
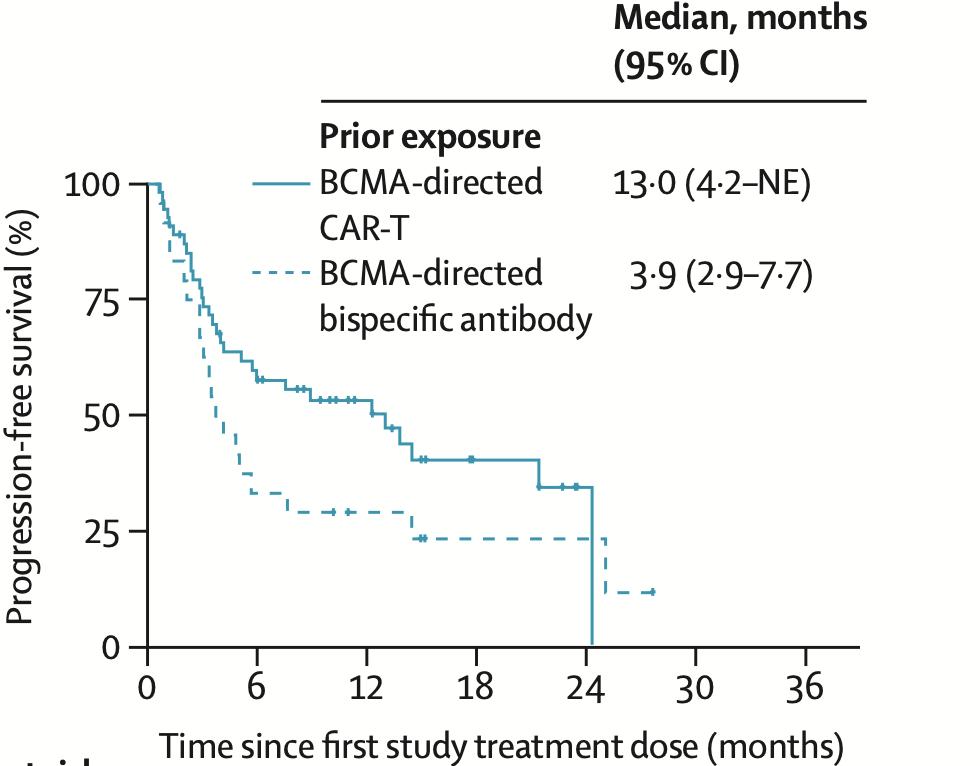
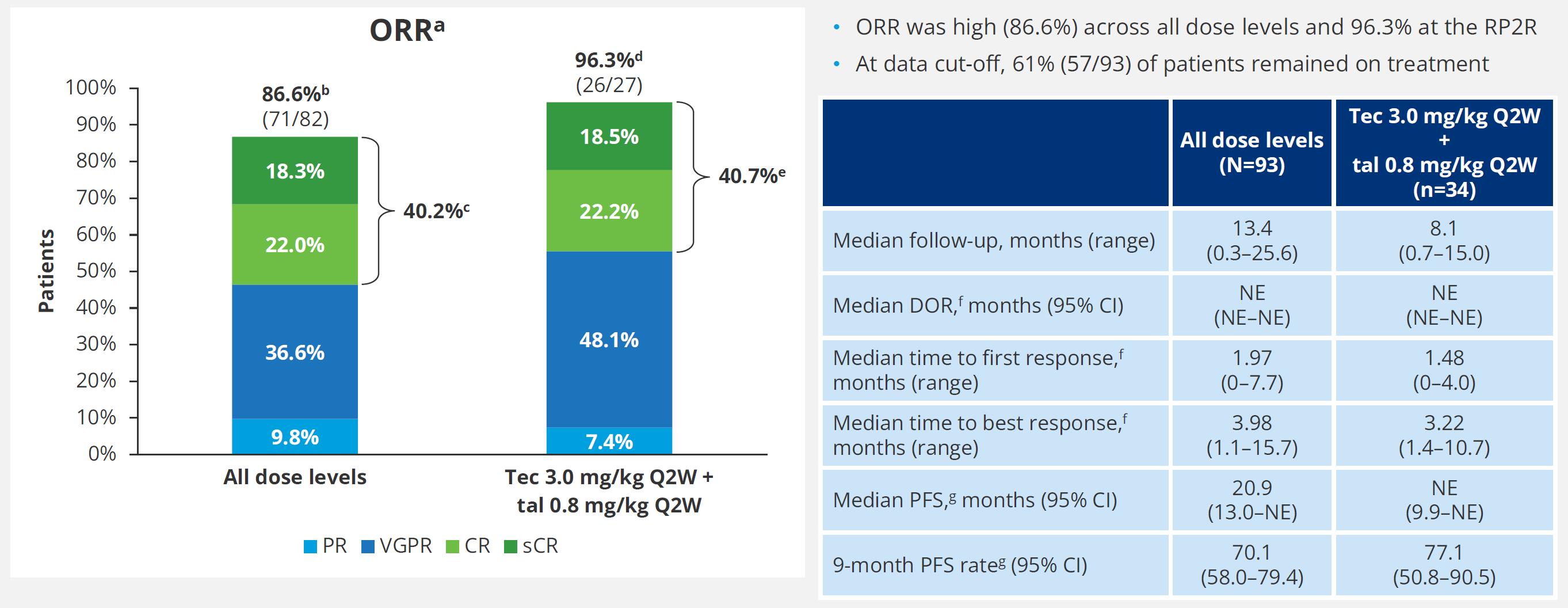
0.8 mg/kg Q2W cohort, 23.4-month mFU; Prior TCR cohort, 20.5-month mFU ORR: 66.7% (n=52/78)

Bal S. et al., ASH 2024
Chari A. et al., Lancet Haematol 2025
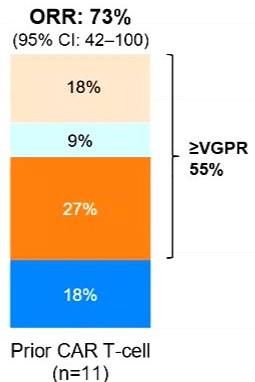
Talquetamab for r/rMM (no/prior BCMA) Lee et al.,
Cevostamab for r/rMM - FCRH5 targeting (no/prior BCMA)

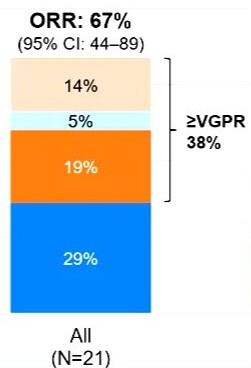
Library. Kumar S. 06/13/2024
But: GPRC5D-targeting T cell engaging therapy is also associated with target antigen loss. 13/17 cases relapsing during Talquetamab therapy and 8/10 patients relapsing after Arlo-cel showed GPRC5D Ag Escape
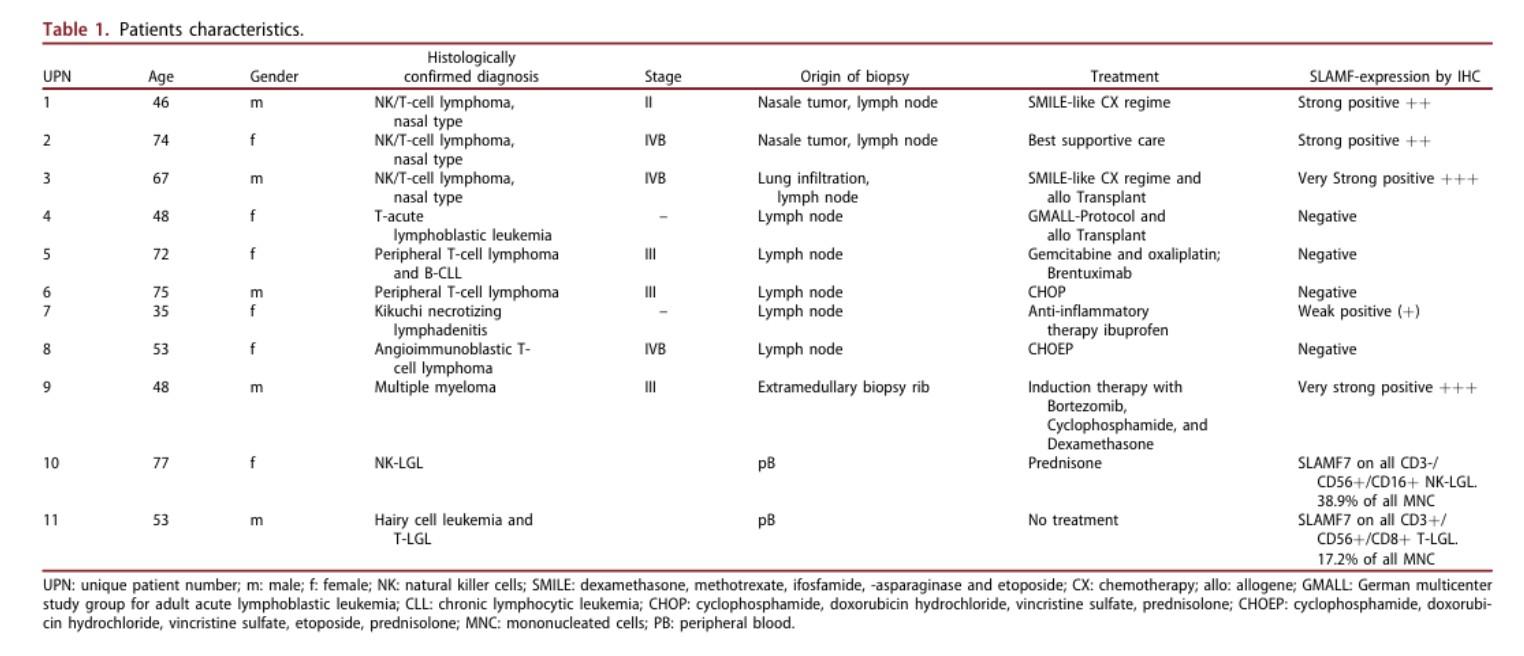
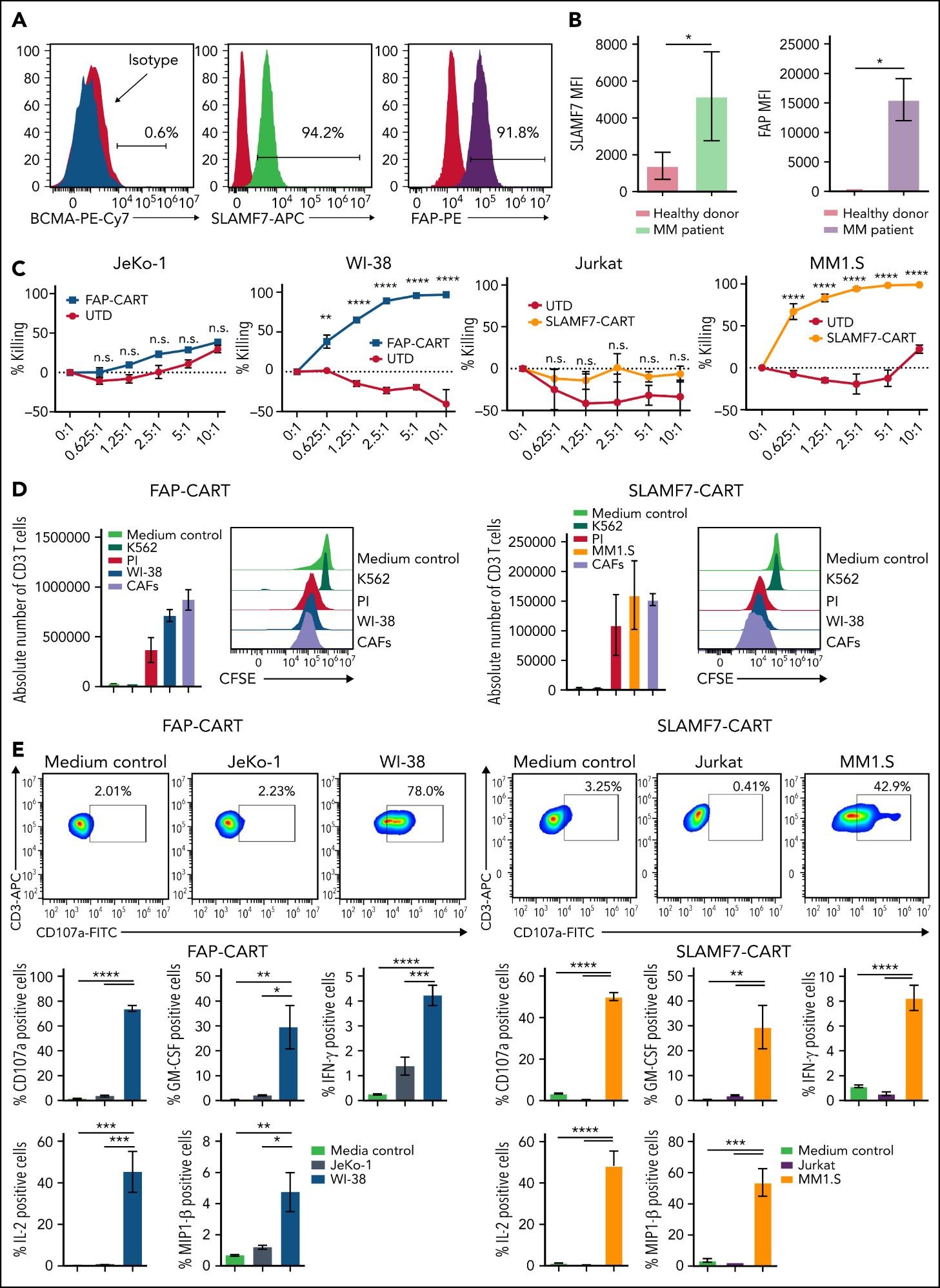
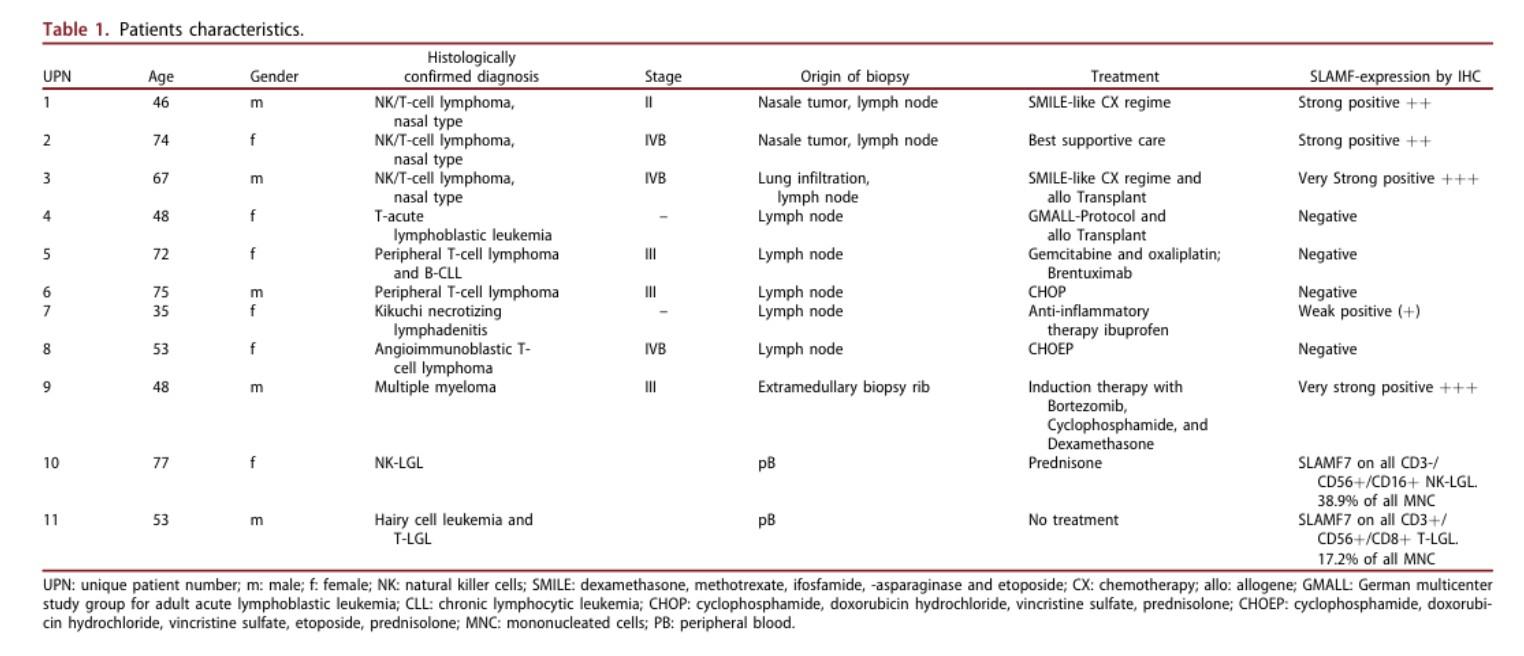
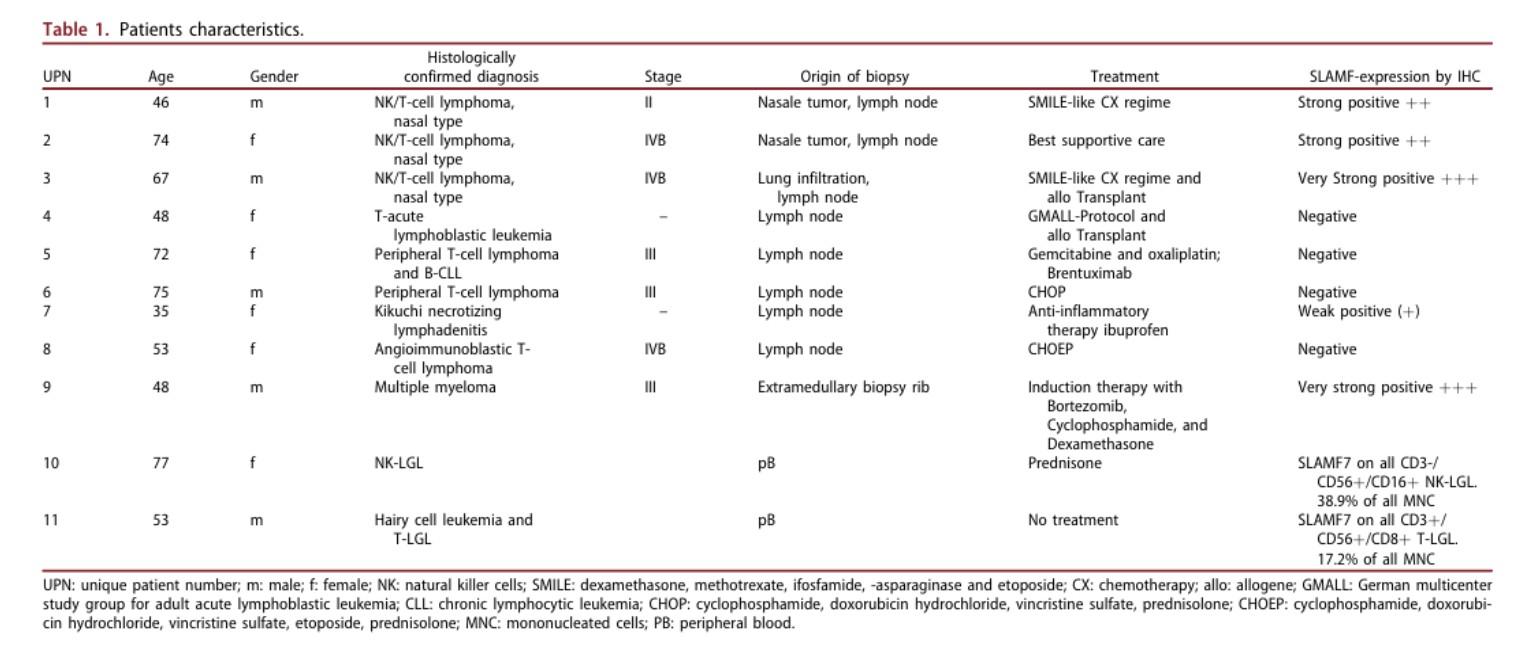
CARAMBA-1: SLAMF7-directed CAR T Cells
SLAMF7 is a strong CAR-T target in MM
• Sustained high level expression on MM/EMD,
• no interference from soluble SLAMF7
CARAMBA-1 is a First-in-Human Phase I/IIa trial of SLAMF7 CAR-T therapy Dose escalation is ongoing
• Safety: favorable safety signal, no DLTs

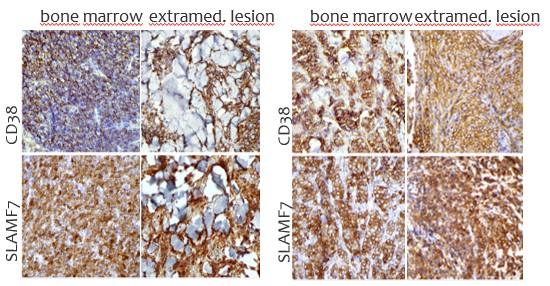



• Efficacy: SLAMF7 CAR-T engraftment, responses in heavily pretreated MM
• But: SLAMF7 Expression on activated T Cells / CAR-T Cell fratricide

SLAMF7 on … bone marrow fibroblasts & macrophages (1)
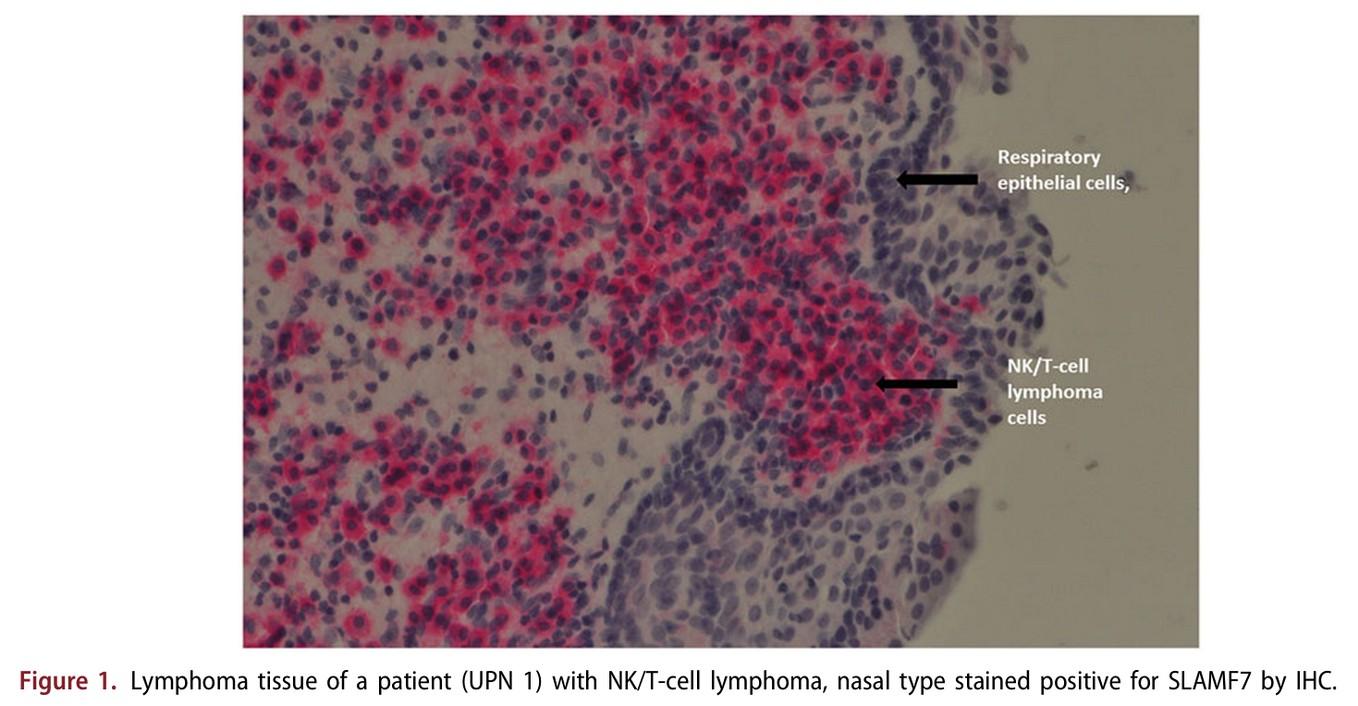
SLAMF7 on … NKT cell lymphoma & T cell lymphoma after CAR T Therapy(2)
Modified from Sakemura, Blood 2022

Elmagaacli et al. Leuk Lymphoma 2019
ROR2 expression early stages of embryogenesis
Absent on most adult-human tissues
Oncofetal antigen – associated with invasiveness
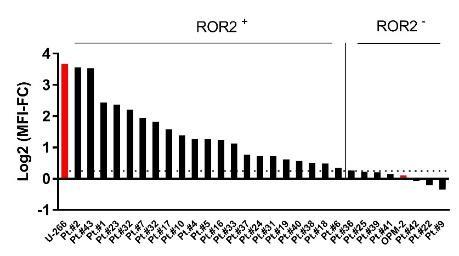
90 % of primary MM samples show ROR2 expression above threshold

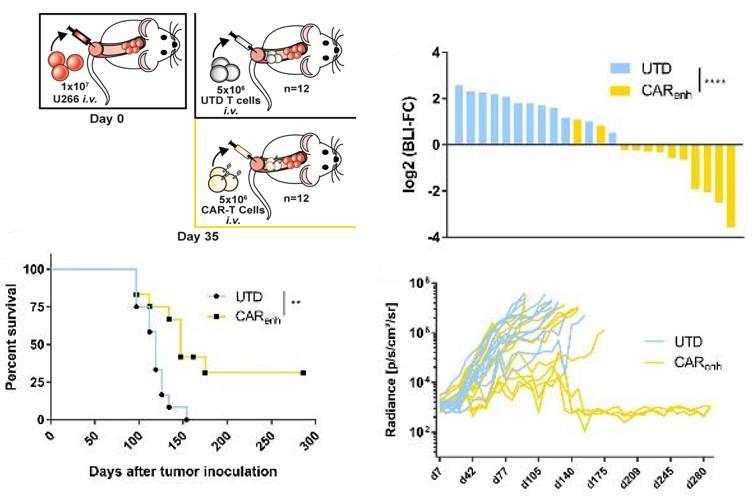
ROR2-specific CAR-T cells elicit long-term tumor control and a curative effect on mice treated in a model of advanced MM (U-266 xenograft)
J. Weber, M. Hudecek; unpublished data; do NOT post

Dual-targeting BCMA-CD19 (Gracell): FasTCAR-T GC012F
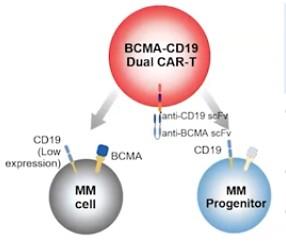
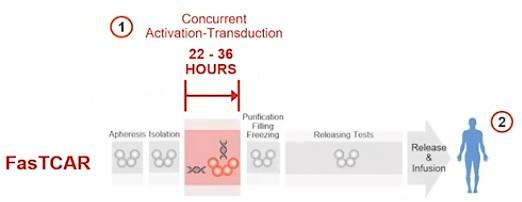
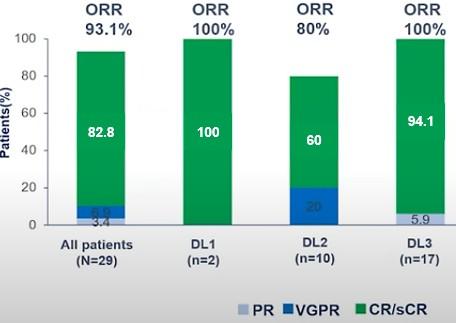
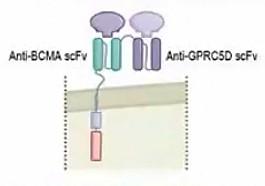
BCMA/GPRC5D bispecific CAR-T Cells


Deep and Lasting Responses Observed at ≥ 50 μg/kg /

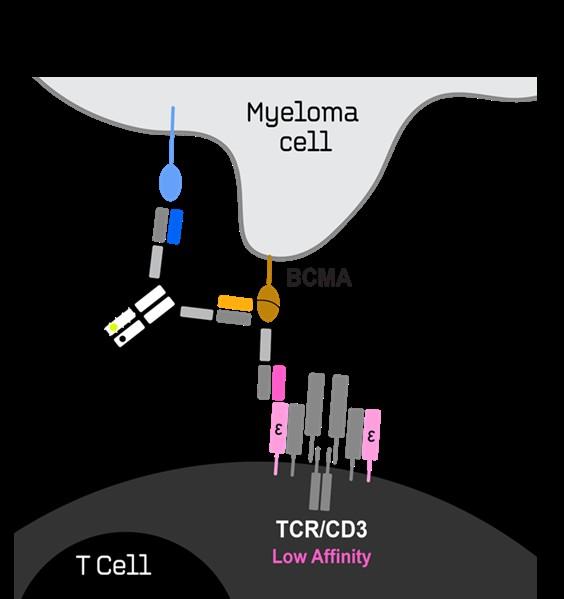
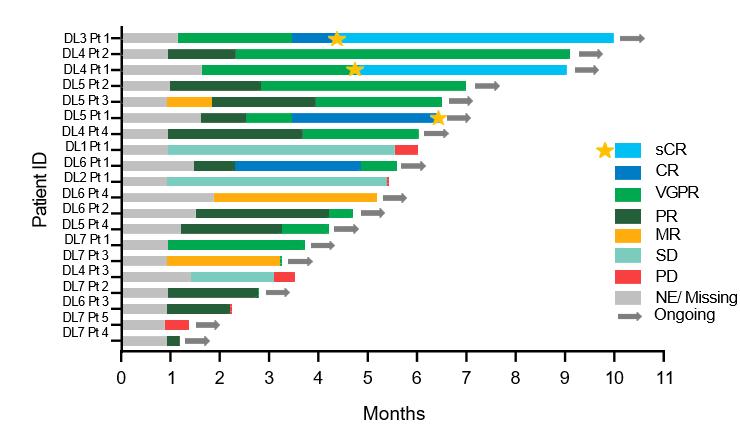
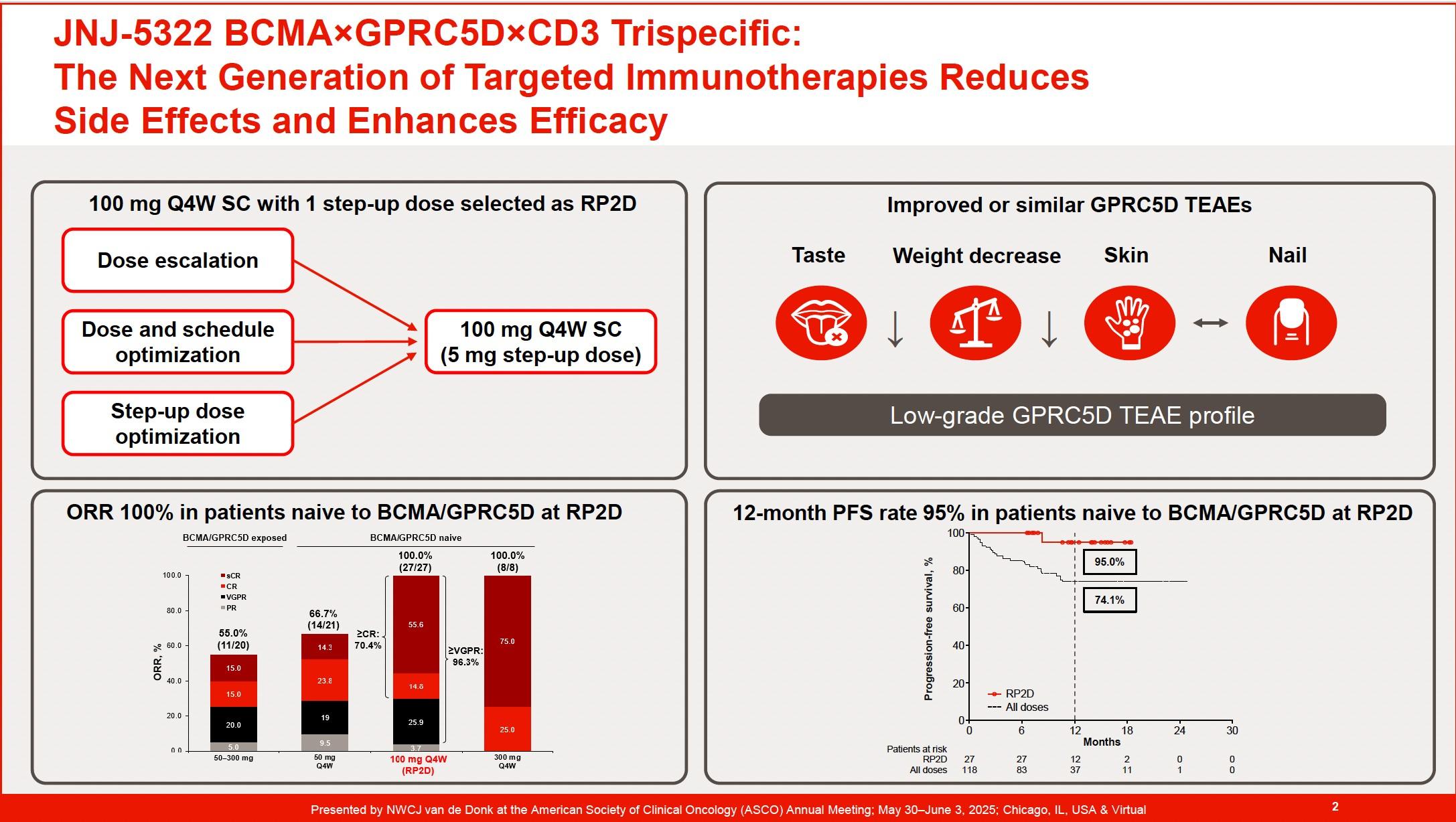
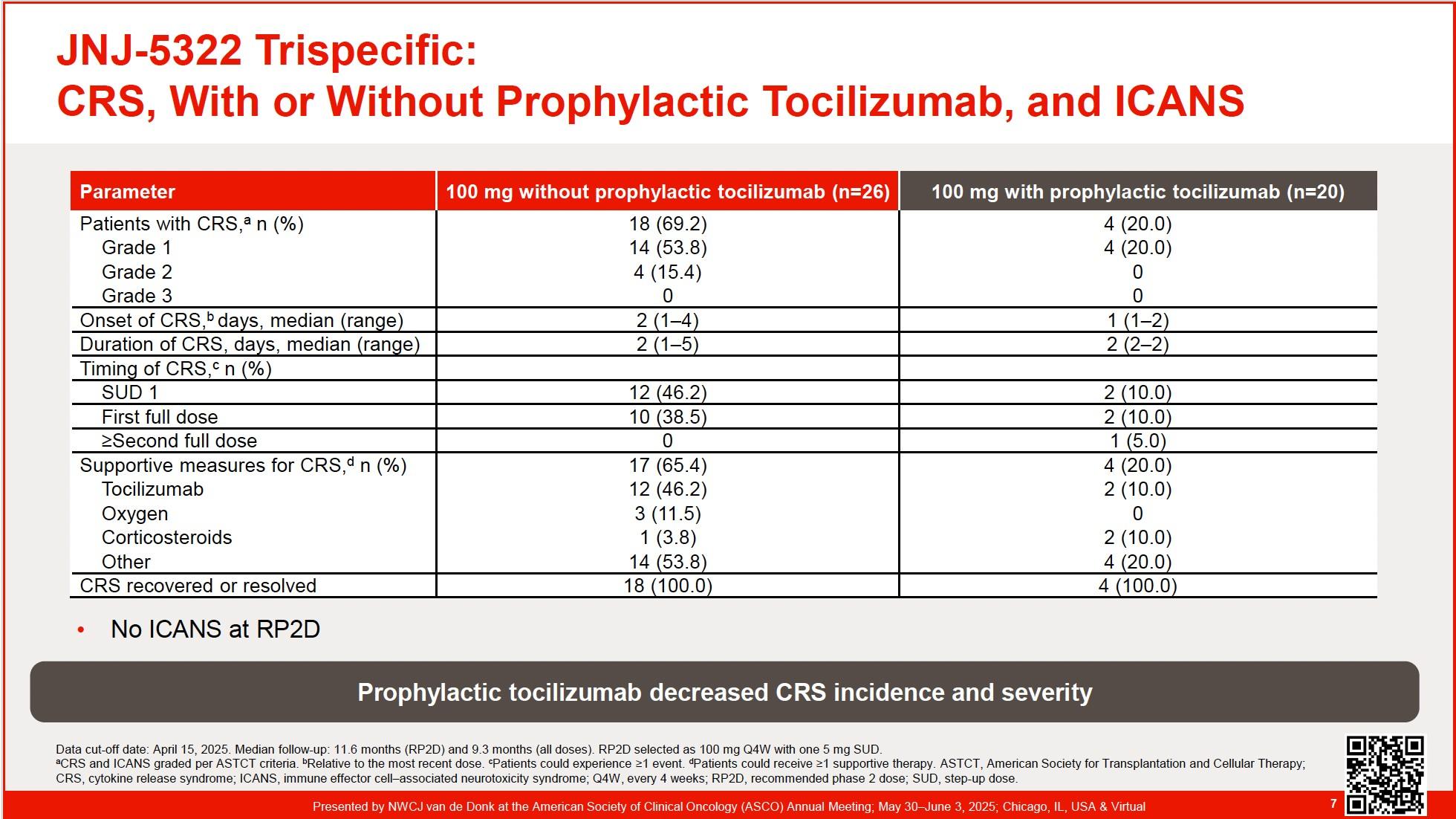
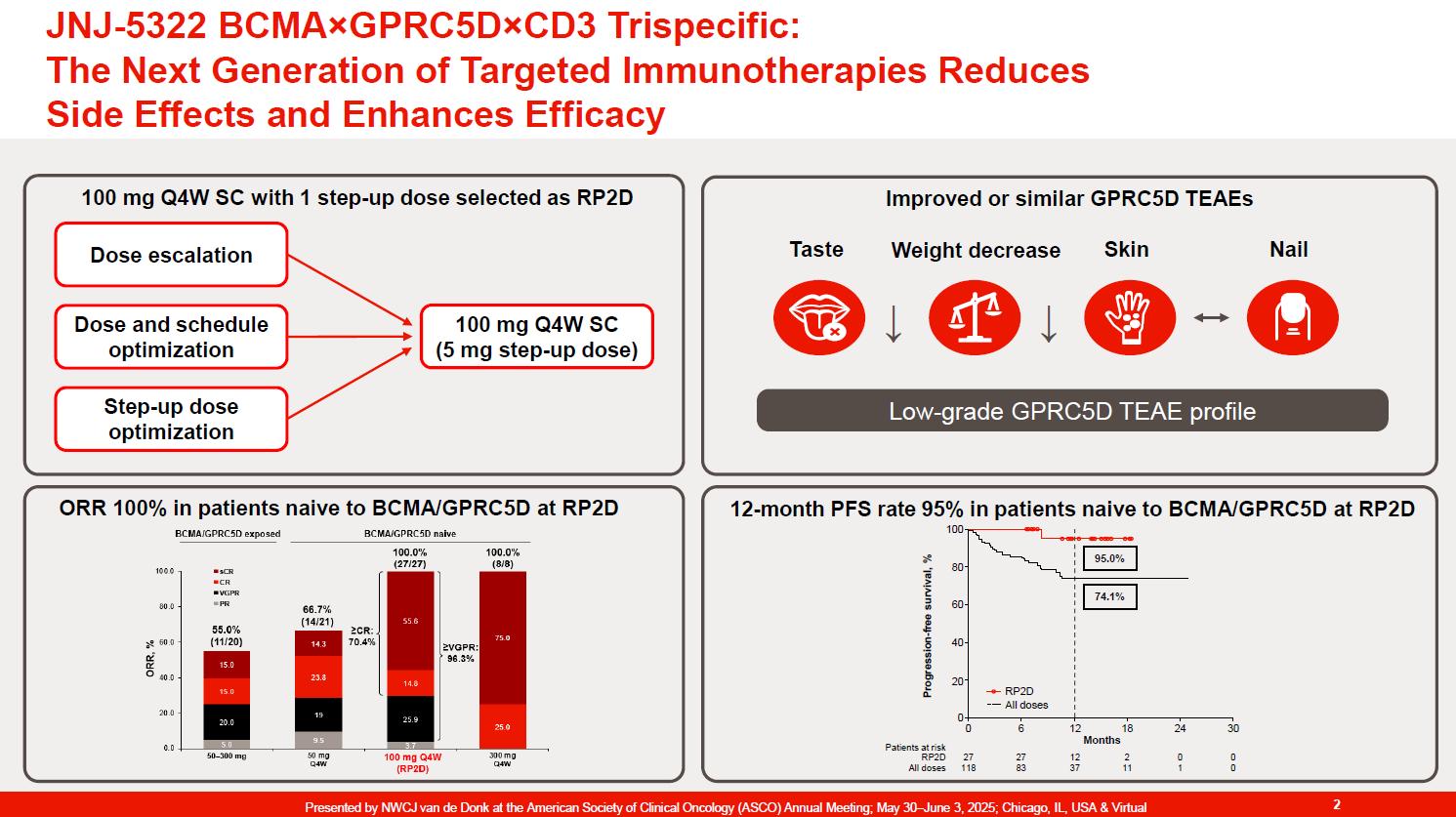
The Next Generation of Targeted Immunotherapies reduces Side Effects and Enhances Efficacy in r/rMM (4

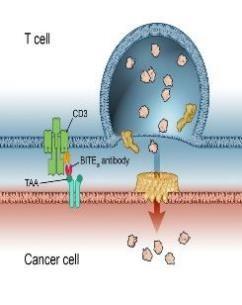
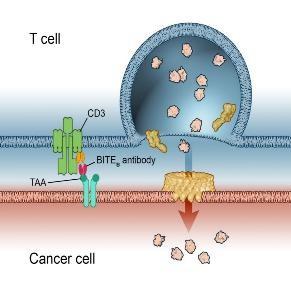
Antibody mediated therapy today
Hemibody Solution
• IgG
• BiTEs, DARTS, Tribodies etc.
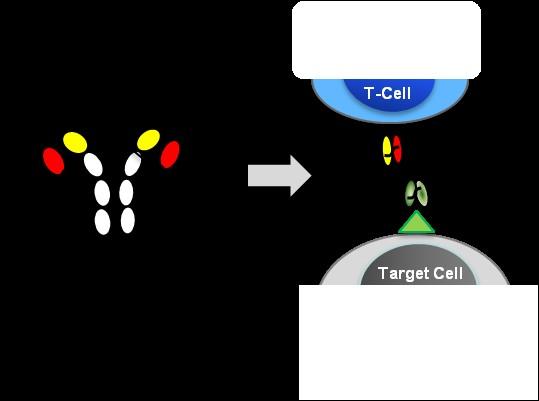

Problem
• No singular tumor antigens
• Poor specificity
• Active CD3 binding side
• Unspecific T Cell activation
• CRS /ICANS

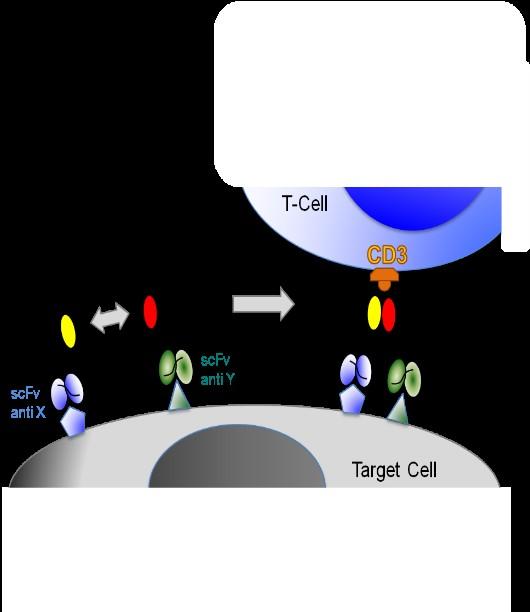
• Higher specificity
• Targeting a broad range of Ags
• No unspecific T Cell activation
• Prevent CRS /ICANS)
• Reduce Infections


T Cell engagement induced by Hemibodies and BiTEs
Banaszek, A. et al., Nat Comm 2019 Geis, M., et al., Commun Biol 4, 44, 2021
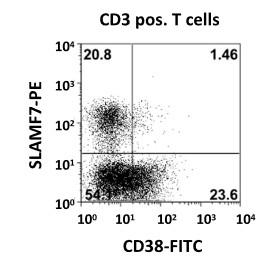
CD38 and SLAMF7 are stably expressed on MM

• Dual CD38/SLAMF7 expr. exclusively on MM cells
• All CD138 pos. MM Cells strongly express CD38 and SLAMF7
• No co-expression on T, NK or hematopoieticprogenitor cells
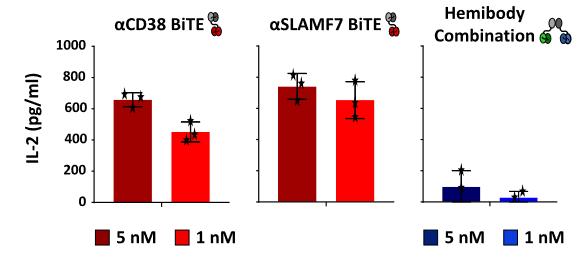
Patients >18y suffering refractory/relapsed (RR) MM, n=30
• No Ag shedding, very low risk of target antigen loss
Inclusion criteria:
• Measurable disease after 3rd line therapy
• Allowed: proteasome Inhib., Imids, ASC, including CD38 and SLAMF7 antibodies
• Prior CAR-T or bispecifics allowed except for anti CD38 or SLAMF7 directed T Cell engaging therapies
Primary endpoint:
• Determination of safety and tolerability (AA/SAA according to CTCAE V5.0)
• MTD and DLT
Secondary endpoints:
• Efficacy: ORR/ CR-rate/MRD negativity
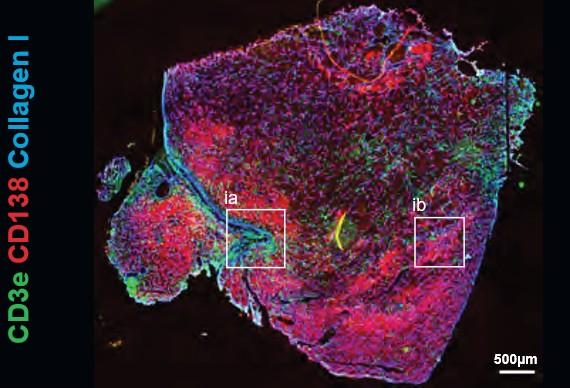
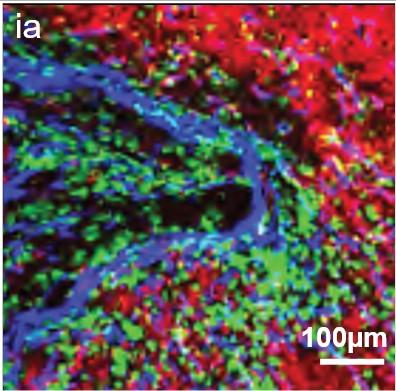
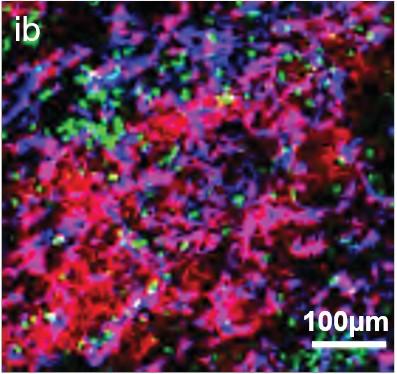

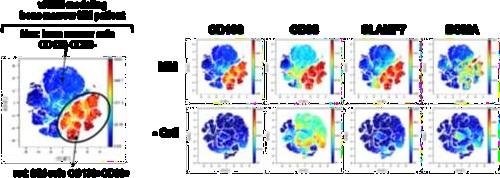
• EMD lesions are infiltrated by immune cells that are anatomically confined to distinct niches.
• T Cells found in the vicinity of PCs showed signs of T Cell dysfunction.

Transgenic proteins released by chimeric antigen receptor (CAR) T Cells upon activation impact tumor environment
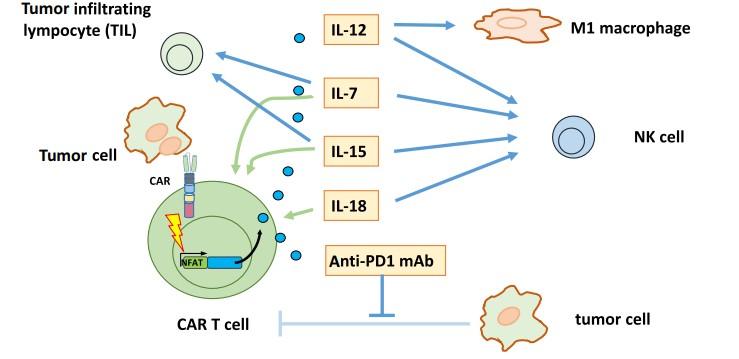
H. et al., Adv Cell Gene Ther. 2020
Three-Month Response (all patients and by lymphoma subtype)
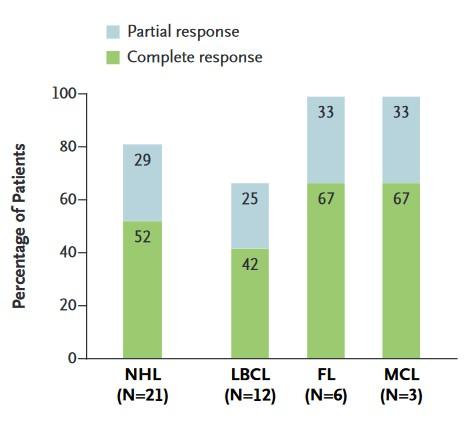
Responses in patients with NHL resistant to CD19-CART cell therapy
huCART19-IL18 Expansion and Persistence According to Dose Level

Shorter manufacturing time Humanized CD19 CAR Local IL-18 production
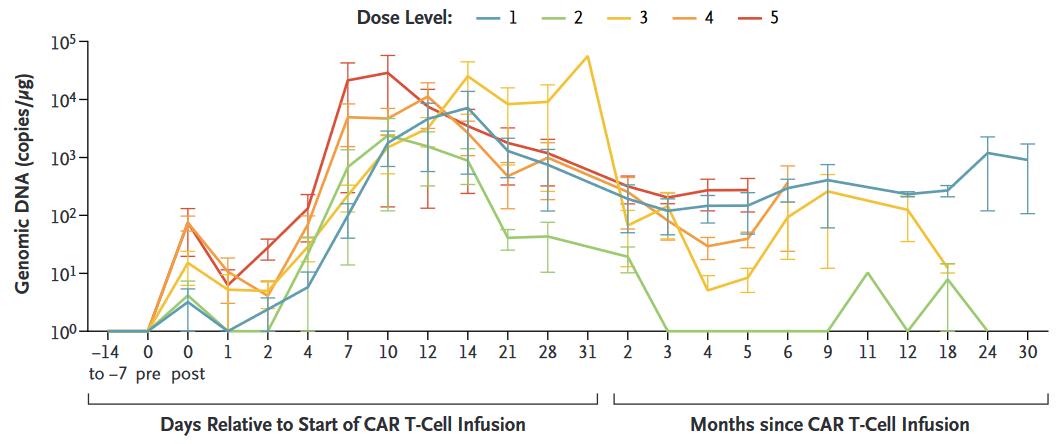
3/4 patients evaluable showed CAR-T Cell persistence > 2 yrs
Median DOR: 9.6 mo.
• Manufacturing time for CAR-T Cells: 6-8 weeks
10-15 % do not receive CAR-T (progression/death while awaiting manufacturing)
• High Costs restrictive use of CAR-T Cells
Strategies:
• More rapid Generation of autologous CAR-T Cells
• Academic CAR-T Cell production (ARI002h, SLAMF7)
• Allogeneic CAR-T Cells
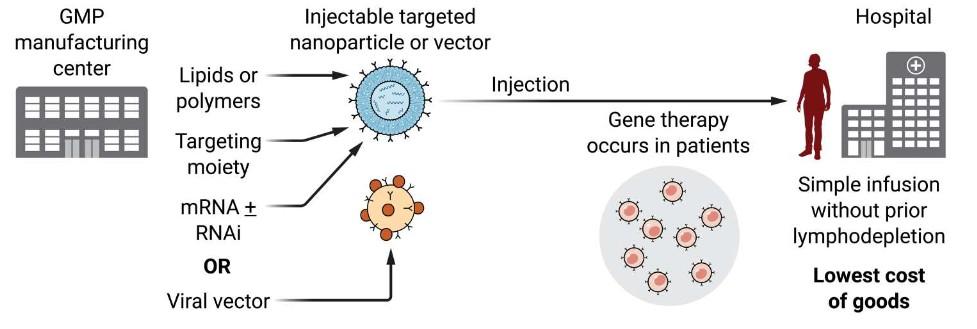
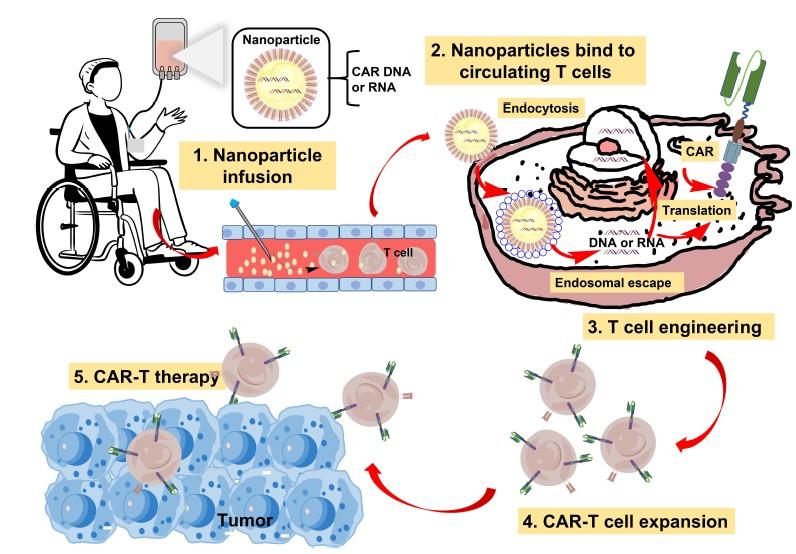
• In vivo CAR-T Cell production


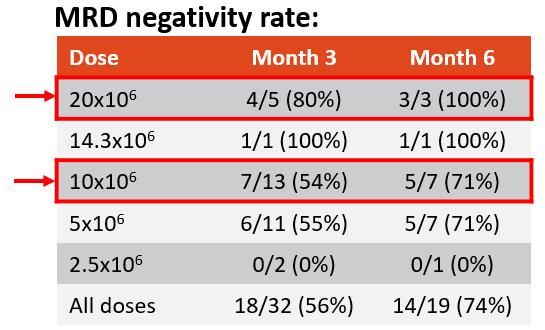
MRD Negativity Rate
T-charge
• Innovative platform for CAR-T
• Reduces manufacturing time < 2d
• Fully human BCMA CAR
• Preserves T Cell stemness
• Long presistence (71% at 12 mo.)
• Deep responses 100% ORR (up to 100% MRD neg,)
• Ongoing for > 2 years in several patients
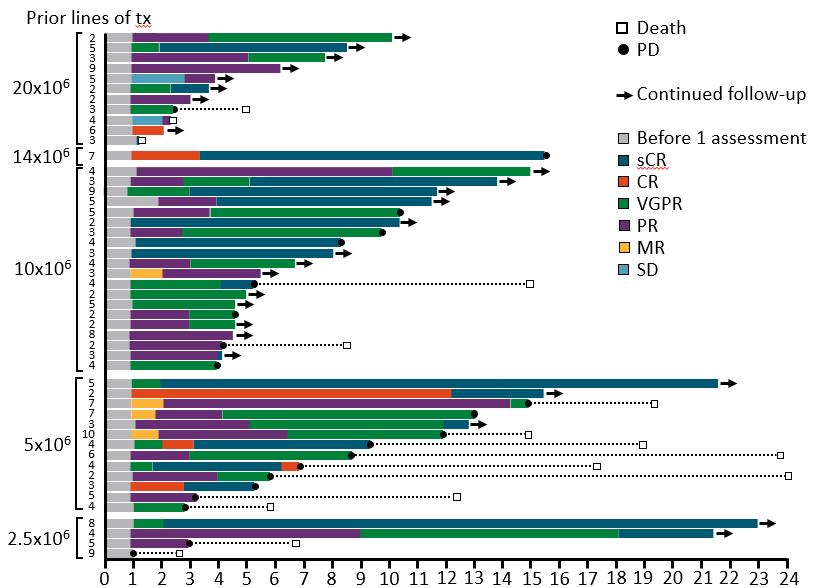
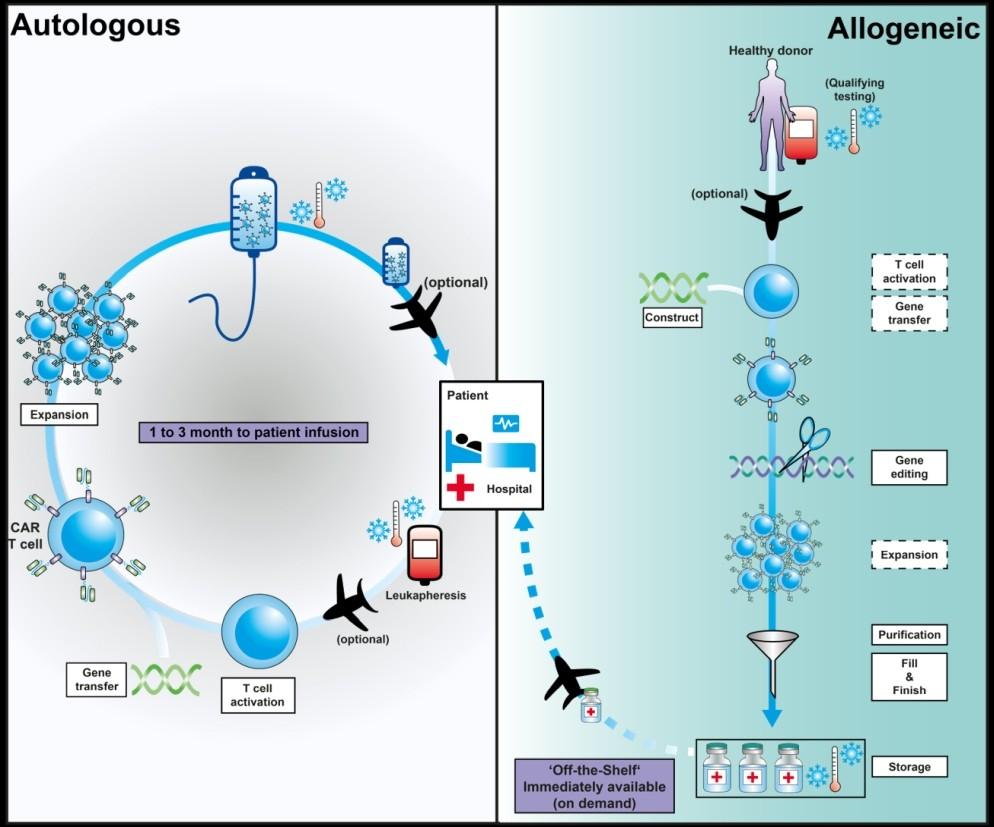
Allogeneic CAR-T Cells
= off the shelf CAR-T Cells
Extensive genetic engineering
• Function?
• Persistence?
Allogeneic cells
• Immune-mediated rejection
• Persistence?



In the FCA 320M CAR+ Cell dose group, 17 patients (71%) ORR
11 (46%) VGPR+
6 (25%) CR/sCR Median Duration of Response: 8.3 mo.

Mai, D. et al., Sci. Transl. Med. 14, eabo3603 (2022)

Viral-based
non-viral
mRNAbased
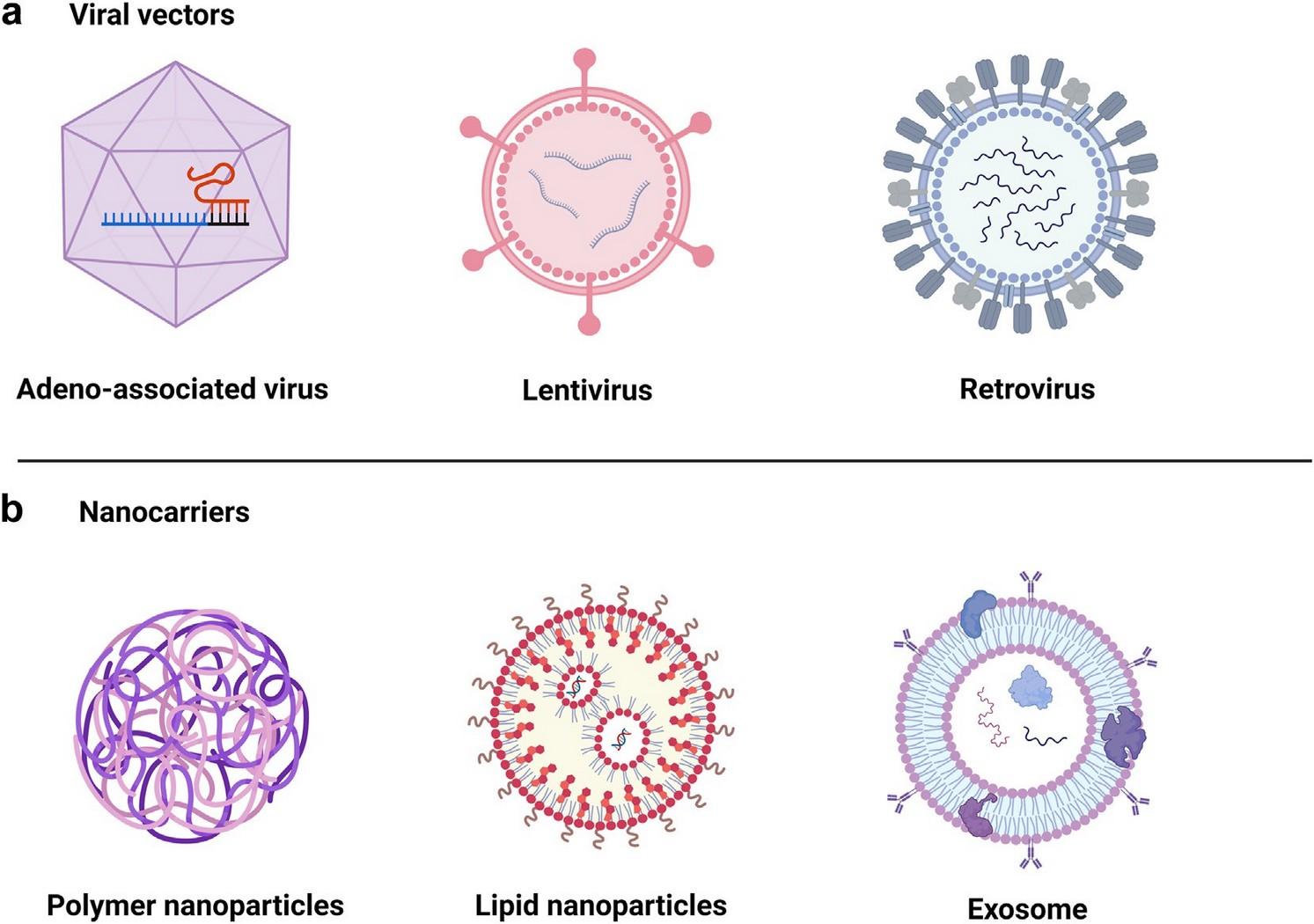
From: Bui et al. (2024) eBioMedicine 106, 105266
novel transposon-LNPs
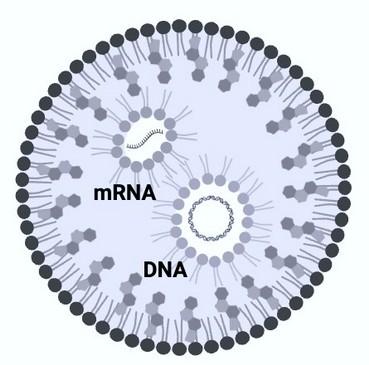
mRNA & mcDNA co-encapsulated

General challenges of in vivo CAR-T approach Challenges with viral vectors Challenges with mRNA-LNPs
Precise targeting of T cells
General Safety due to off-target gene delivery
Distribution to / loss of vector in highly vascularized organs
Need for GLP-tox studies in relevant tox species (NHPs ?)
Efficiency of CAR-T generation, are sufficient numbers possible ?
Immunogenicity
LV tropism for other cells (esp. hepatocytes)
Anti-viral immunity (incl. pre-existing for certain AAV serotypes)
LV & RV: generally secondary leukemias
Repeat dosing required due to transient expression of mRNA
toxicity of LNP components (PEG, cationic lipids)
Repeat dosing causes increased immunogenicity
Possibility of generating fatal CAR-leukemia cells efficiency of gene transfer into resting T cells
LV/RVs need proliferating cells for efficient vector integration
Viral vectors are still complex to manufacture and expensive
Transposon-LNP technology combines the «best of both worlds»: (i) stable CAR expression ( LVs), (ii) scalable CAR-Ts ( mRNA-LNPs), but safe

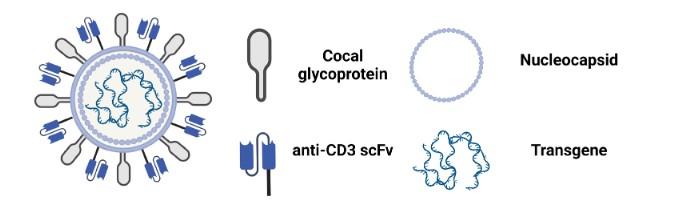
+ Transposase (Sleeping Beauty)
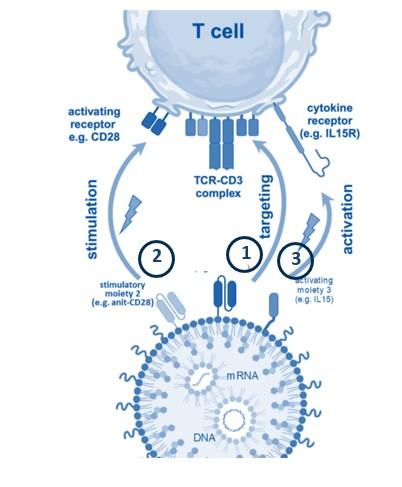
Gene transfer into resting T cells + local Cytokine production

• Combination therapies (TKIs, IMiDs, CELMoDs, ICPi etc.) will further improve persistence and the efficacy of CAR-T Cells/ T Cell engaging Antibodies
• Additional MM targets and multitargeting to reduce antigen loss (Novel targets, Dual CAR-T, trispecific Antibodies)
• More rapid and less expensive production of CAR-T Cells (Academic CAR-T production, allo CAR-T)
• In Vivo generation of CAR-T Cells to increase global availability (off-the-shelf, reduced cost)
Medizinische Klink/Poliklinik II, Würzburg
M. Topp M. Hudecek
T. Bumm S. Danhof
L. Rasche M. Kortüm
G. Stuhler A. Beilhack
J. Waldschmidt T. Steinbrunn
Immunologie, Würzburg
W. Kastenmüller G. Gasteiger
Medizinische Klinik III, Regensburg
W. Herr M. Edinger
Med. Mikrobiologie, TUM
D. Busch
Universitätsklinikum Heidelberg
M. Raab H. Goldschmidt
E. Mai
UKE Hamburg
K. Weisel L. Leypoldt
Wilhelminen Cancer Research Institute, Wien
H. Ludwig
Zelltherapie u. Immunologie, Leipzig
U. Köhl
Medizinische Universität Wien
M. Krauth
Princess Margaret Cancer Centre, Toronto
K. Stewart S. Trudel
University of Calgary
N. Bahlis P. Neri
H. Lee
Università di Torino
F. Gay M. Boccadoro
R. Mina
Ospedale San Raffaele, Mailand
M. Casucci C. Bonini
Memorial Sloan-Kettering Cancer Center, NYC
S. Usmani F. Maura
M. Merz
Dana-Farber Cancer Institute, Boston
N. Munshi K. Anderson
S. Treon C. Mitsiades
Fred Hutchinson Cancer Research Center, Seattle
S.R. Riddell M. Jenssen

UCSF Helen Diller Family Comprehensive Cancer Center
Tom Martin A. Chari
Myeloma Research Rotterdam
P. Sonneveld T. Cupedo
A. Broijl
Amsterdam UMC
N. van de Donk S. Zweegman
Patients and family members
Funding Agencies









Many thanks for your kind attention!






Ajai Chari, MD

Professor of Clinical Medicine
Director of Multiple Myeloma Program
Co-Director of Clinical Research Hematology/Oncology
Helen Diller Comprehensive Cancer Center
University of California, San Francisco San Francisco, California
Research Support/P.I. Janssen Employee N/A
Consultant/Scientific Advisory Board
Abbvie, Adaptive, Amgen, Antengene, Bristol Myers Squibb, Forus, Genetech/Roche, Glaxo Smith Klein, Janssen, Karyopharm, Millenium/Takeda,
Sanofi/Genzyme
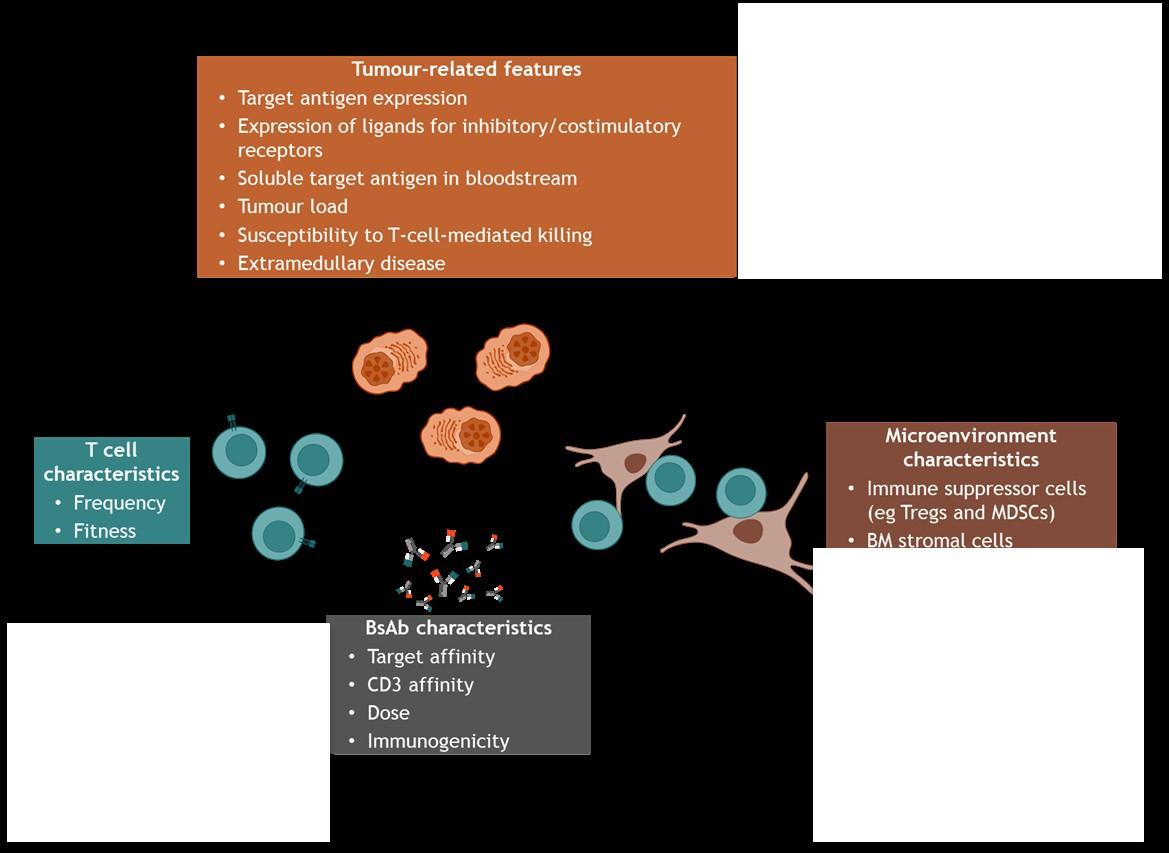
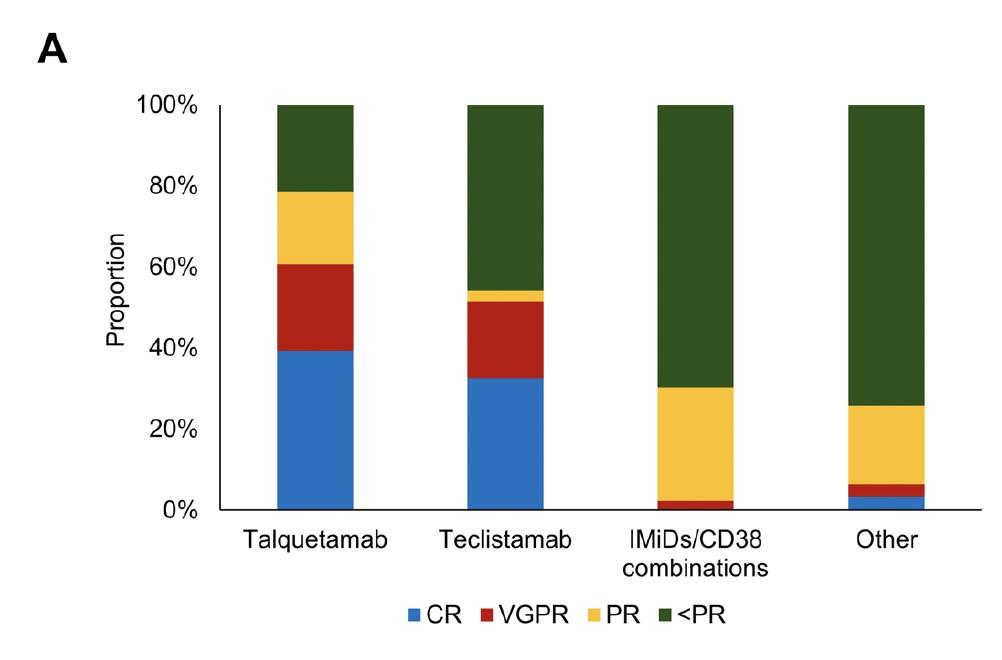
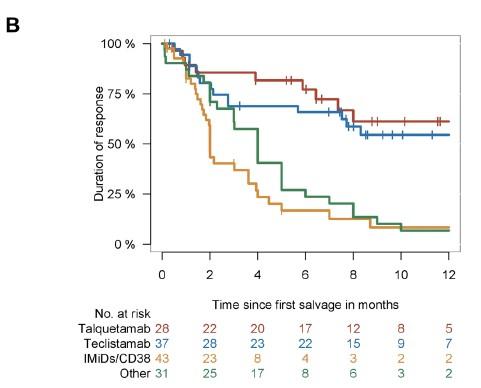
T cell redirection therapies
For > 4 LOT and IMID/PI/anti CD38 exposed


Siegel DS et al. Blood 2012;120(14): 2817–2825
Lonial S et al. Lancet 2016;387:1551-1560
Chari A et al. N Eng J Med 2019;381:727-738
Rasche et al EHA 2024
Van De Donk et al IMS 2023
Lesohkin et al Nat Med 2023
Anderson L et al. ASCO 2021;abstract 8016 (poster presentation)
Usmani S et al ASCO 2022;abstract 8028 (poster presentation)
For > 4 LOT and IMID/PI/anti CD38 exposed


Lonial S et al. Lancet 2016;387:1551-1560
Chari A et al. N Eng J
Rasche et al EHA 2024
Van De Donk et al IMS 2023
Lesohkin et al Nat Med 2023
Anderson L et al. ASCO 2021;abstract 8016 (poster presentation)
Usmani S et al ASCO 2022;abstract 8028 (poster presentation)
1. Anderson L et al. 2021 ASCO. Abstract 8016. 2. Ferreri CJ et al. 2021 ASH. Abstract 766. 3. Berdeja J et al. Lancet. 2021;398;314-324. 4. Lin Y et al. 2022 EHA. Abstract P961. 5. Cohen AD, et al. Blood Adv. 2019;3:2487-2490;
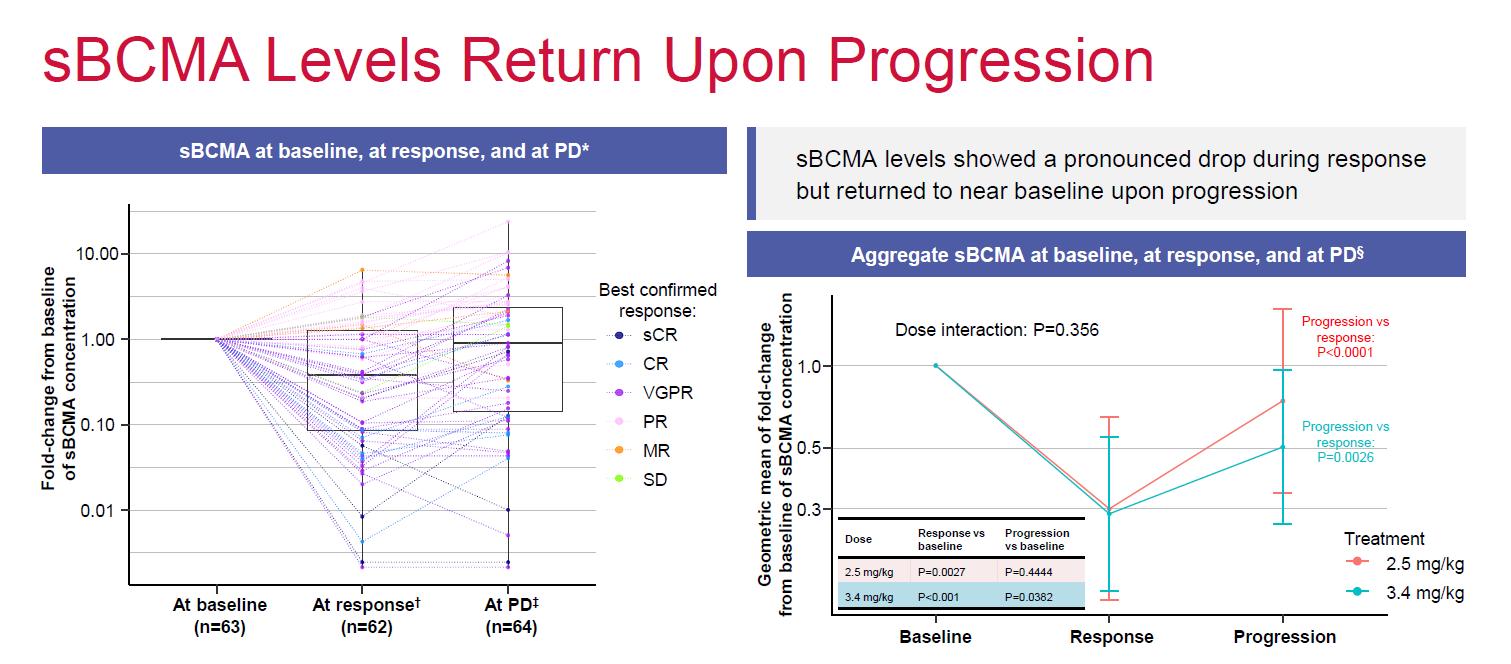

• longitudinal monitoring of immune cell populations showed no change in immune cell ratios or major cell populations, regardless of response status
• sBCMA levels showed pronounced drop during response but returned to baseline upon progression
• Impact on functional loss of BCMA unclear
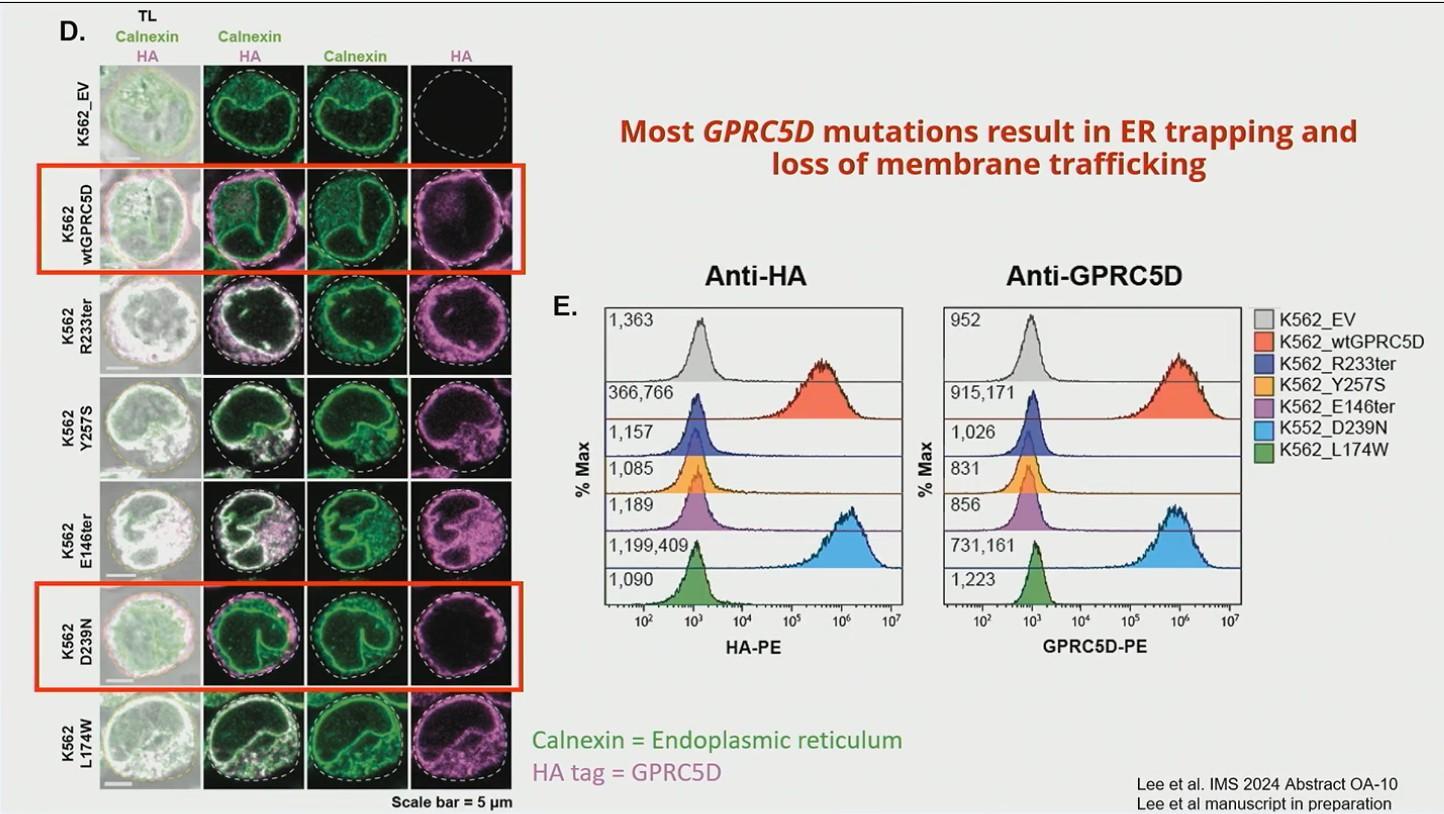
• In NDMM (COMMPASS) Monoallelic loss of GPRC5d on chromosome 12p: 13.17%
• 8/9 patients had GPRC loss after treatment with talquetamab
• 2 had biallelic loss
• 6 had SNV +/- monoallelic loss
• Many GPRC5d mutations result in ER trapping and loss of membrane trafficking
• 1 had alteration in ionic binding with GRPC5d and talquetamab
• Epigenetic silencing also possible


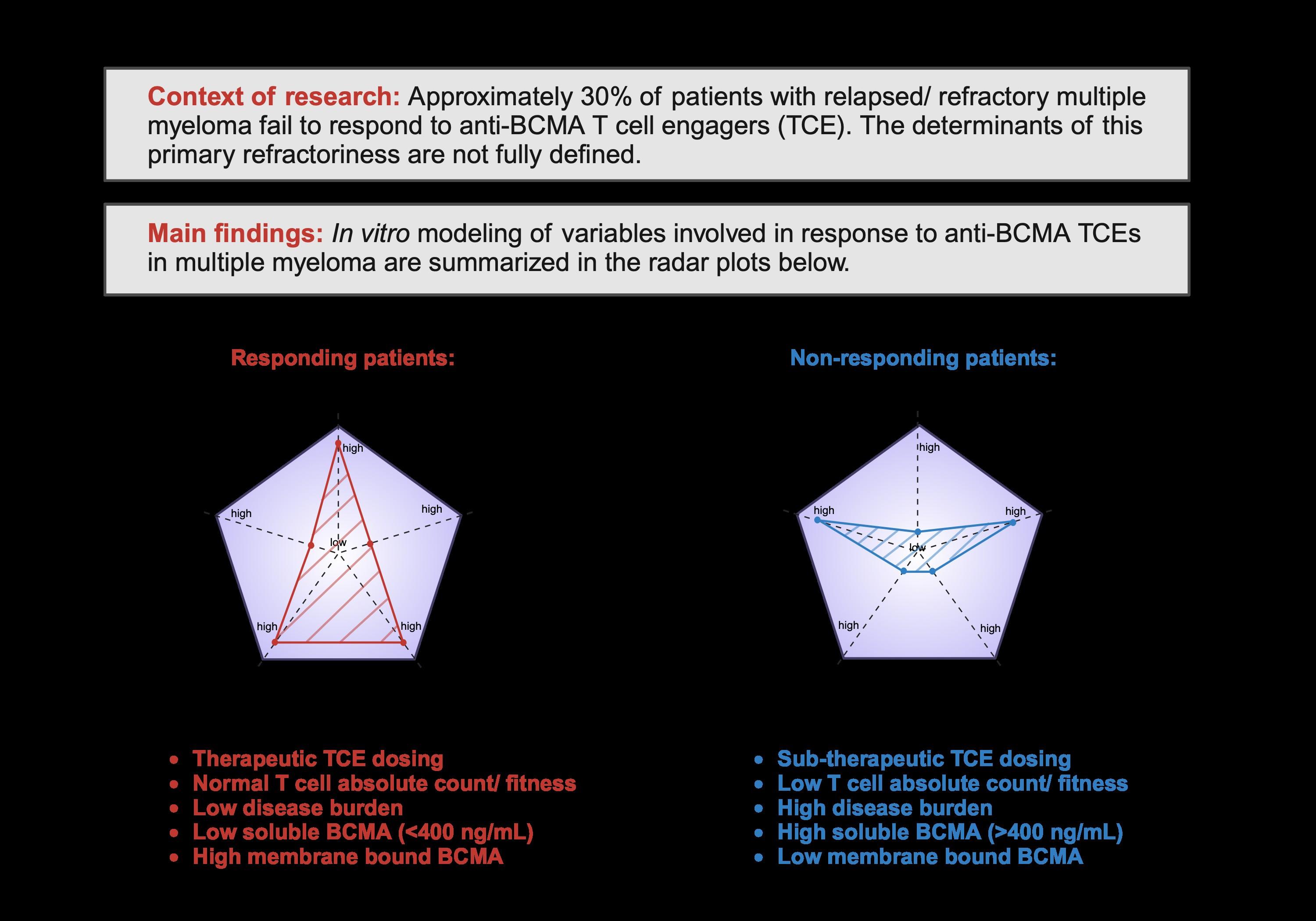
• Efficacy/Primary refractoriness appears to improve in earlier lines of therapy
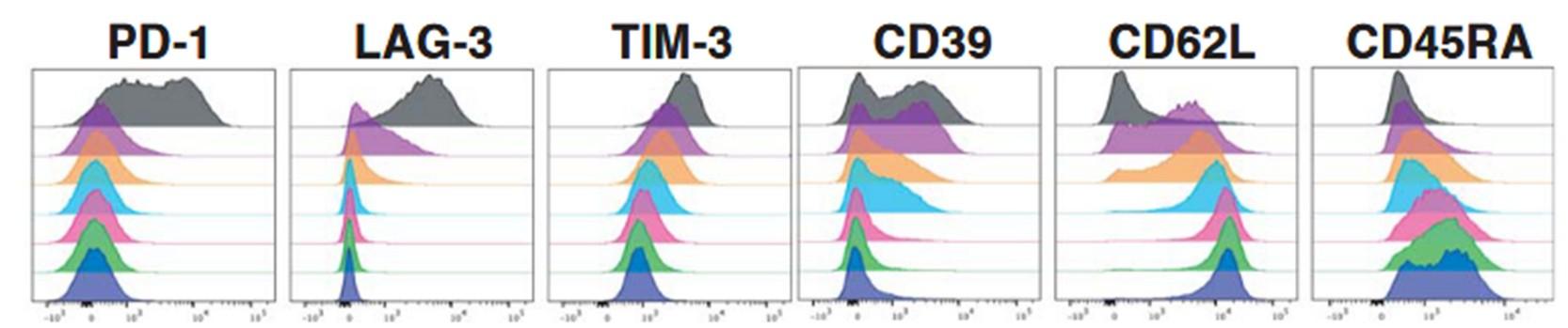
• As compared to NDMM, RRMM have lower CD4/CD8 ratio and CD8 + clonality (Friedrich et al Cancer Cell 2023)
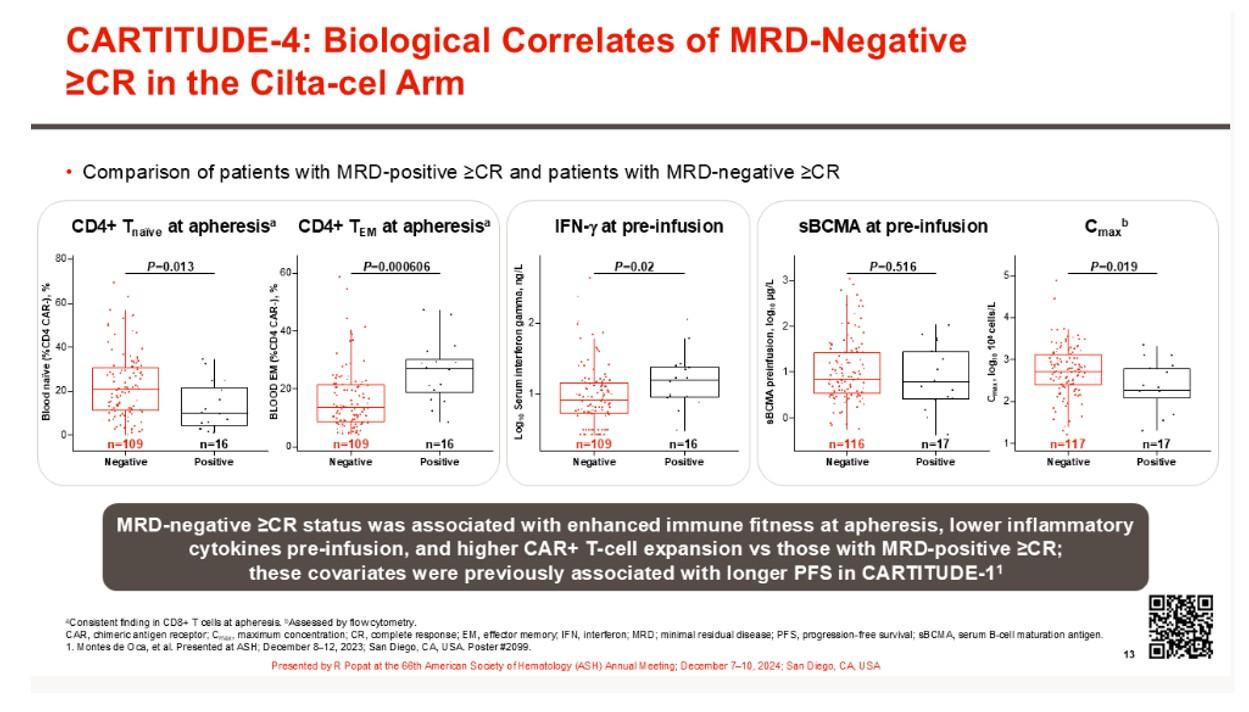

MRD Negative vs. MRD positive post CART
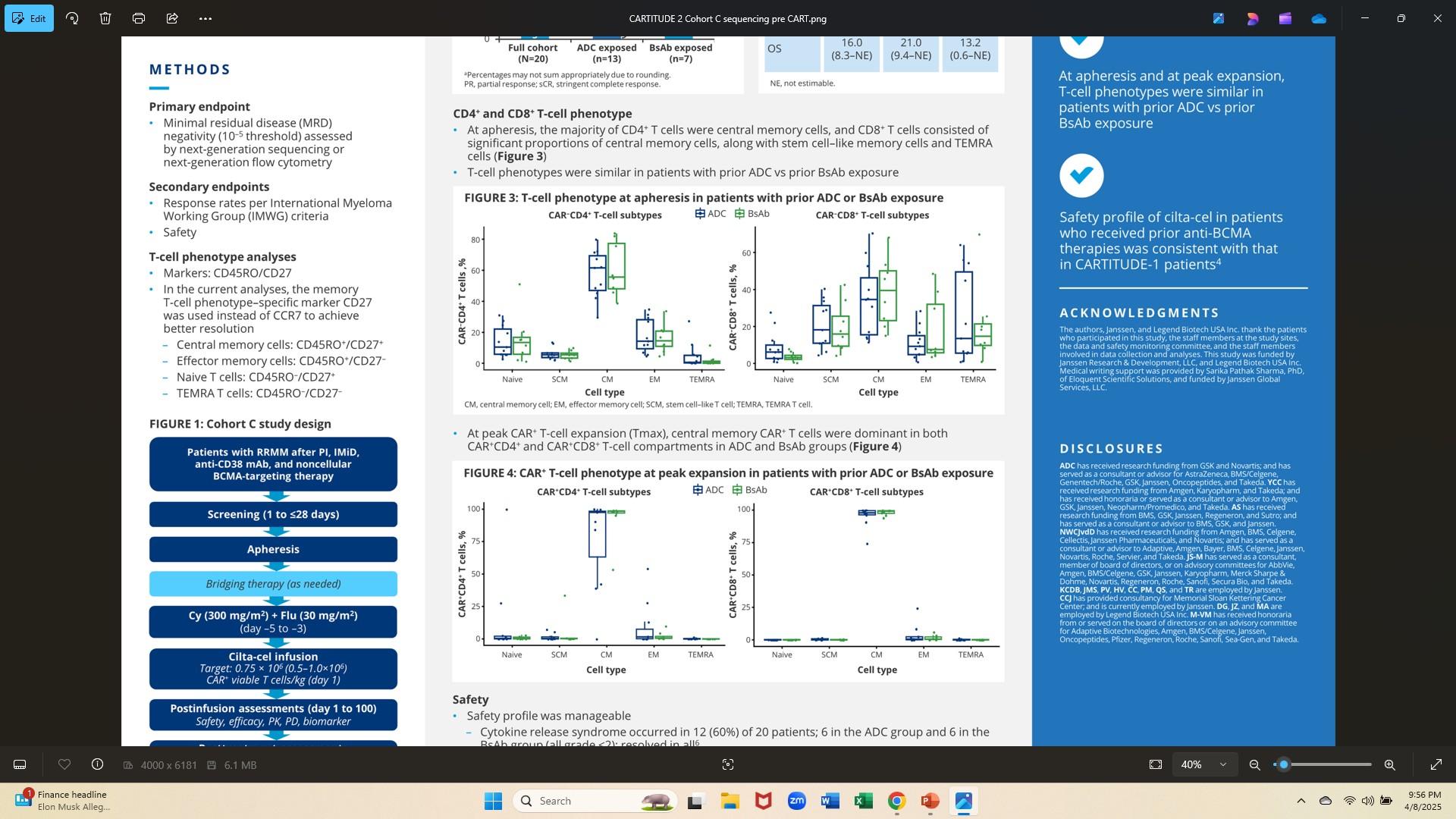
CARTITUDE 2: at apheresis, majority CD4+ T cells central memory; CD8+ T cells CM & stem-cell like memory and TEMRA cells - T cell phenotypes similar in pts with prior ADCs vs prior BsAB
Talquetamab Phase ½
MonumenTAL-1 Study
(phase 1 study)
(Phase ½
)
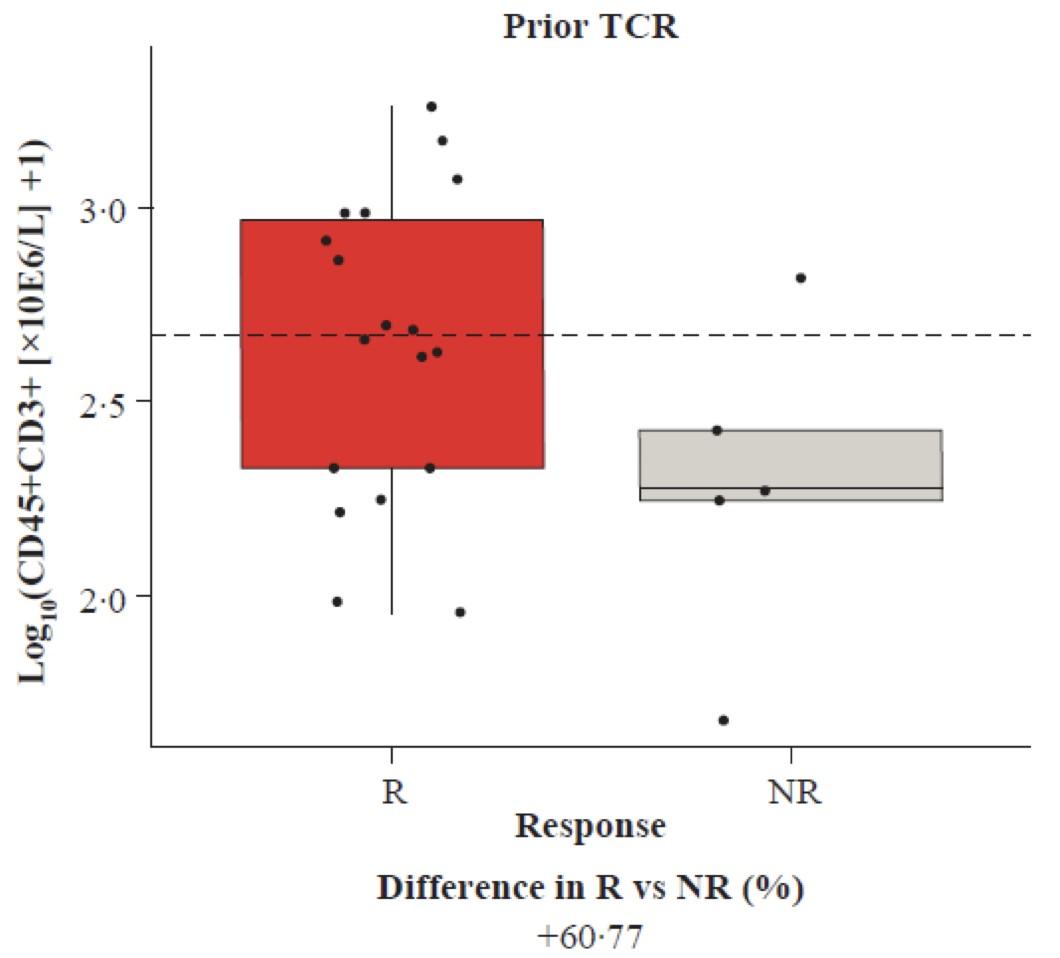
• At baseline, prior TCR pts have less favorable immune phenotype vs TCR-naïve
• prior CAR-T pts have greater T-cell activation in 1st cycle vs BsAb group
• Preliminary data shows marked improvement with Dara + talquetamab PFS 19 mos (including prior bsAB resistant pts)
In the prior BsAb group, a higher PD1+ CD8 % cells across all T-cell subsets, and a higher frequency of TIGIT+ CD8 T cells for TSCM and Tnaive, suggesting a systemic T-cell dysfunction
12-week washout required between discontinuation of last prior BsAb therapy and 1st cevostamab dose in CAMMA 2 may not be sufficient to overcome this dysfunction
% PD1+ CD8+ T-Cell Subsets
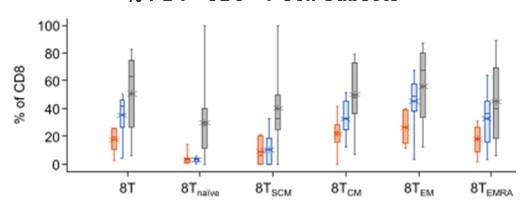
% TIGIT+ CD8+ T-Cell Subsets
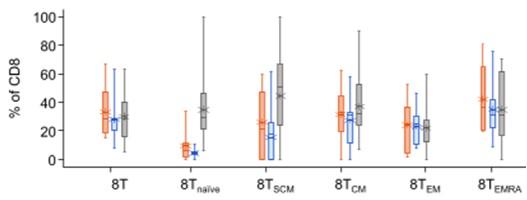
* *
Prior ADC (n = 7) Prior CAR-T (n = 9) Prior BsAb (n = 17)
CART-hematotox score: >2 high risk (Hthigh) vs 0–1 low risk (HTlow)
One point : ANC ≤ 1.2, Hg ≤ 9, plt 76–175k, CRP ≥ 3.0 mg/dl, ferritin 650 to 2000 ng/ml.
Two points: plt ≤ 75 ferritin ≥ 2000 ng/ml.
•CAR-Hematotox higher scores correlate with worse efficacy:
•inferior ORR 44% vs. 70% p = 0.01)
•inferior PFS (median 5 vs. 15 mos, p < 0.001)
•inferior OS (median 10.5 mos vs. NR, p < 0.001)
Rajeski et al. J Hematol Oncol . 2023 Jul 31;16:88
Analysis of patients receiving BCMA directed CAR-T therapy
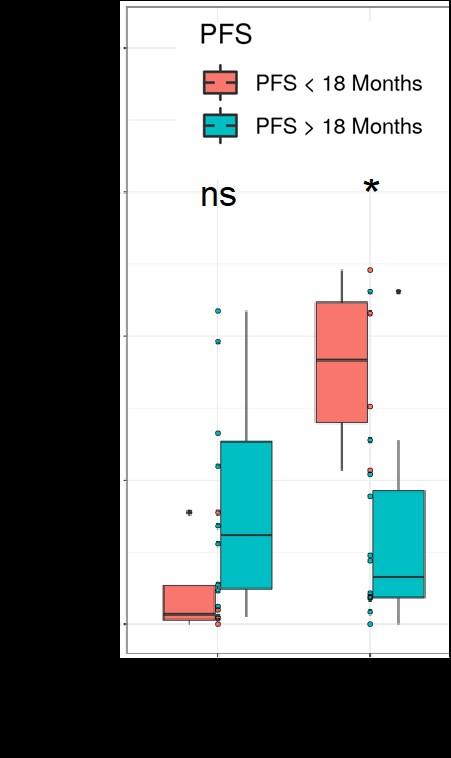
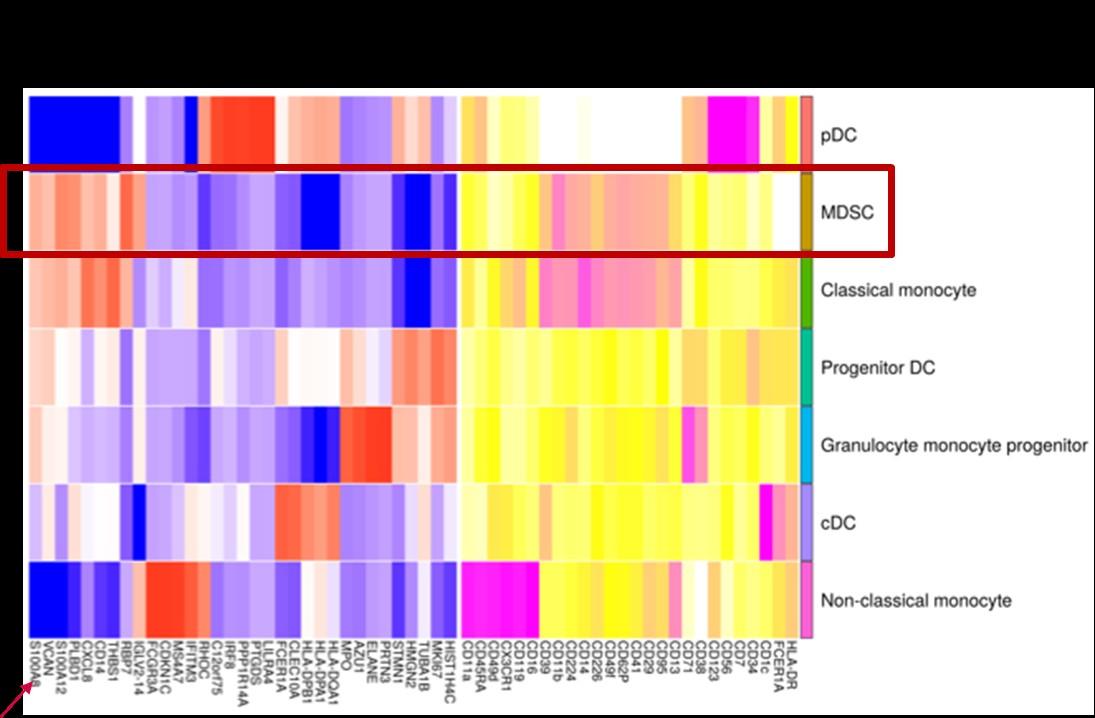
• S100A8 is one of most elevated transcripts in MDSCs in MM pts
• S100A8 is an alarmin, part of the DAMP (Damage
Associated Molecular Pattern) family released in response to tissue injury
• Secreted by myeloid cells, mesenchymal cells, PMNS
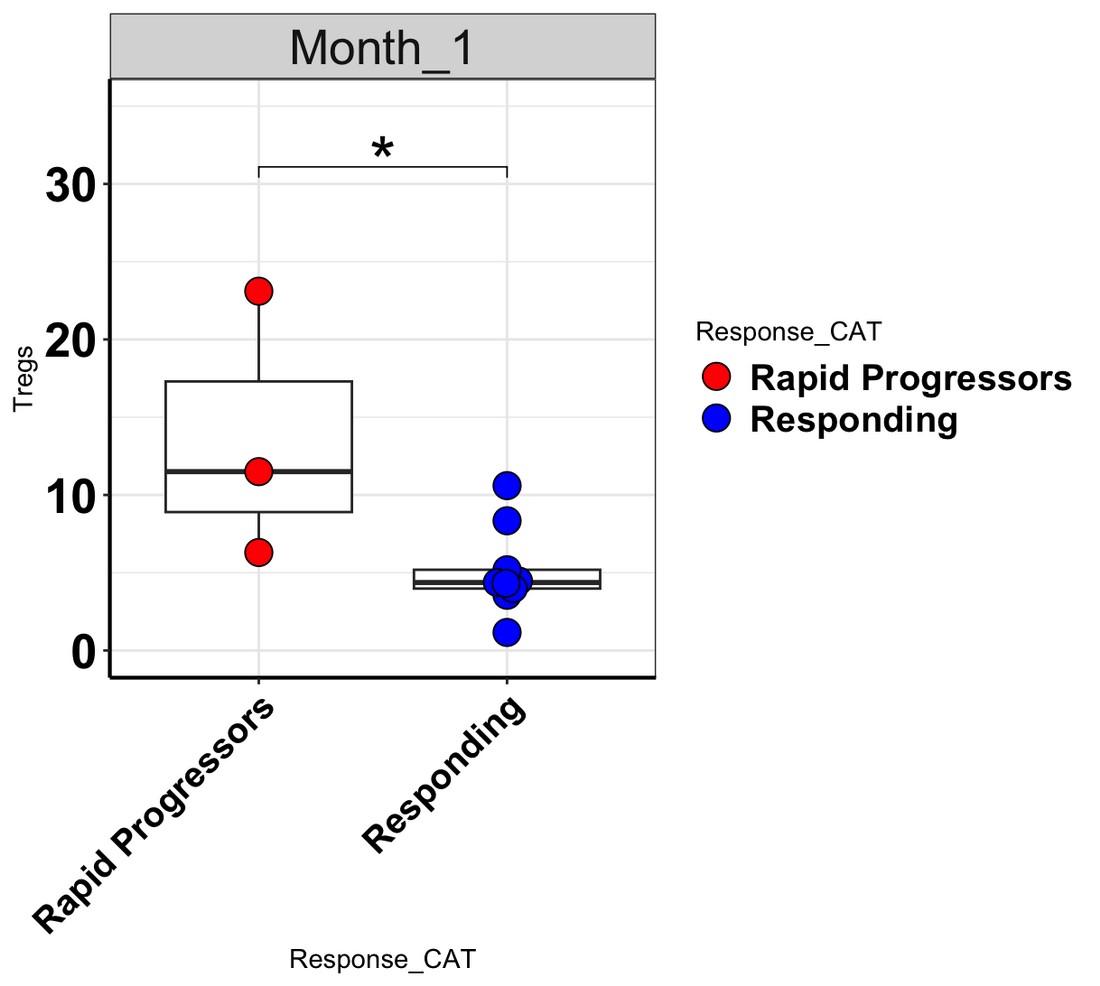
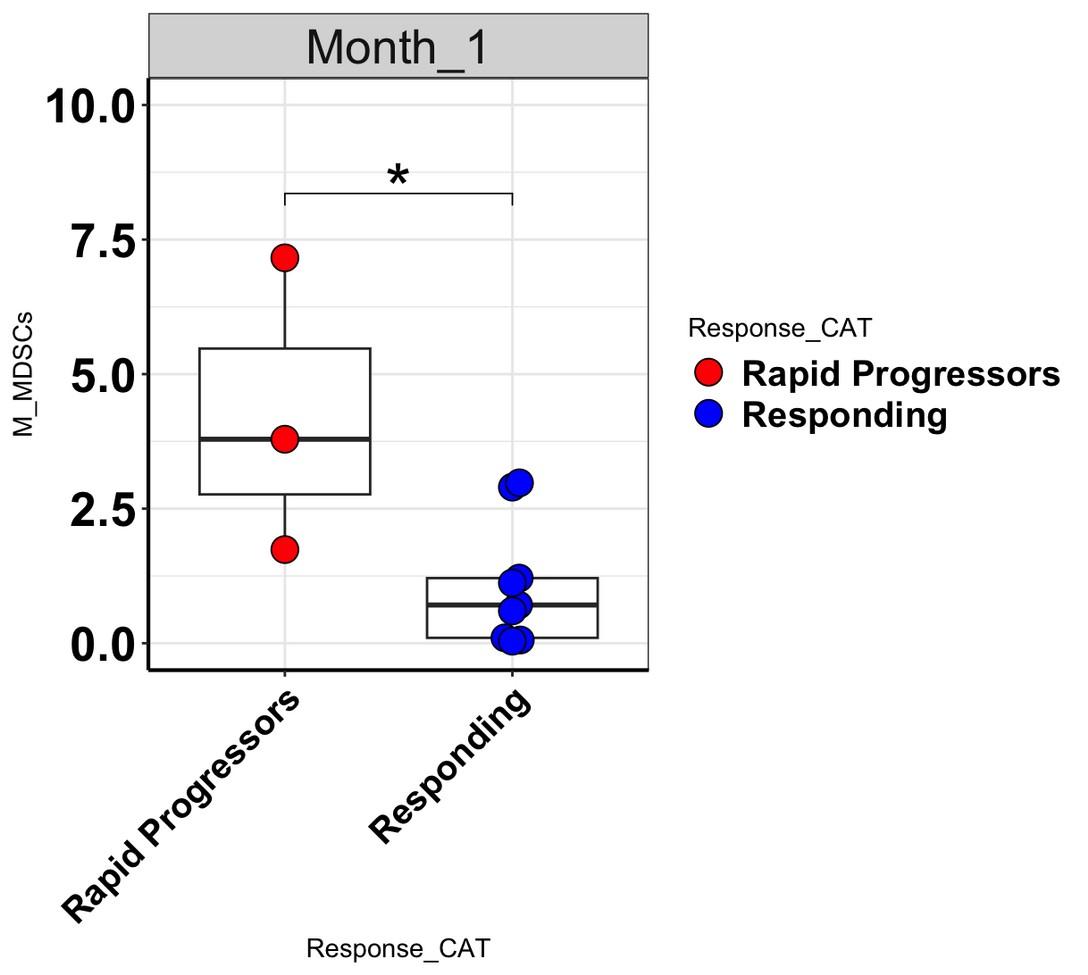
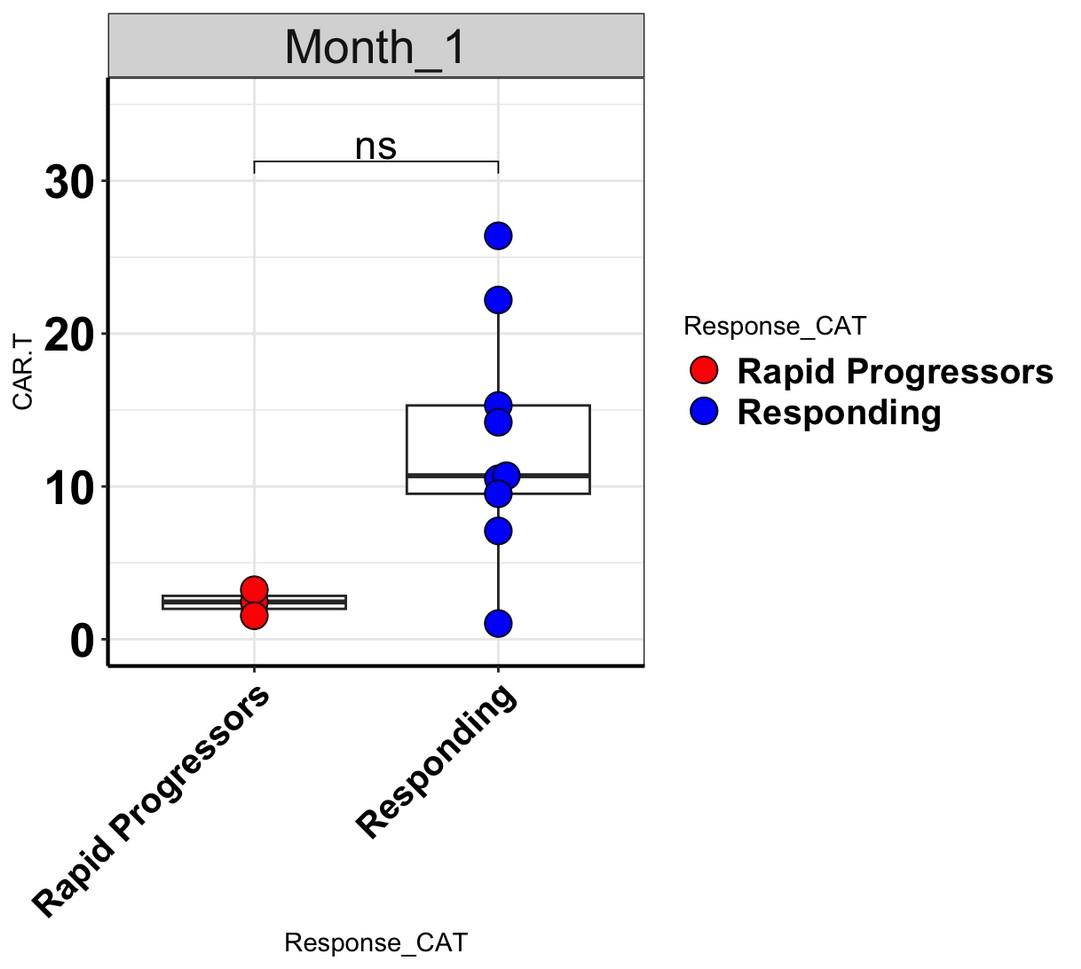
levels increase at Month 1 in rapid progressors
• the decrease of CAR-T cytotoxicity with addition of S100A8/A9 is increased in a dose dependent manner
• suboptimal responders to bispecifics also have higher S100 at baseline and during therapy
• S100 promotes T reg differentiation
• addition of anti-S100A8/A9 mAb erases S100
inhibitory impact in both CART & bispecifics and also reduces T cell exhaustion
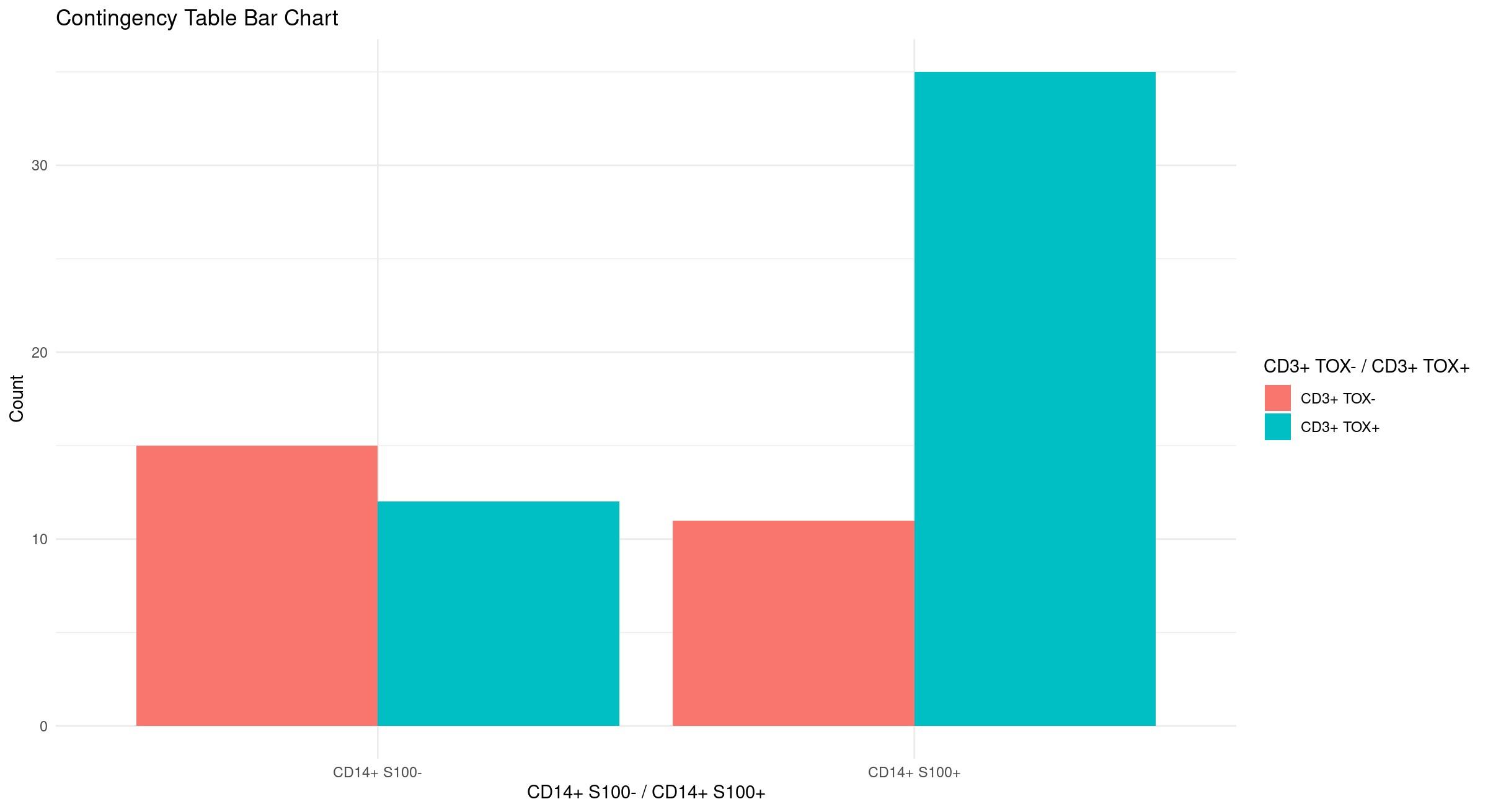
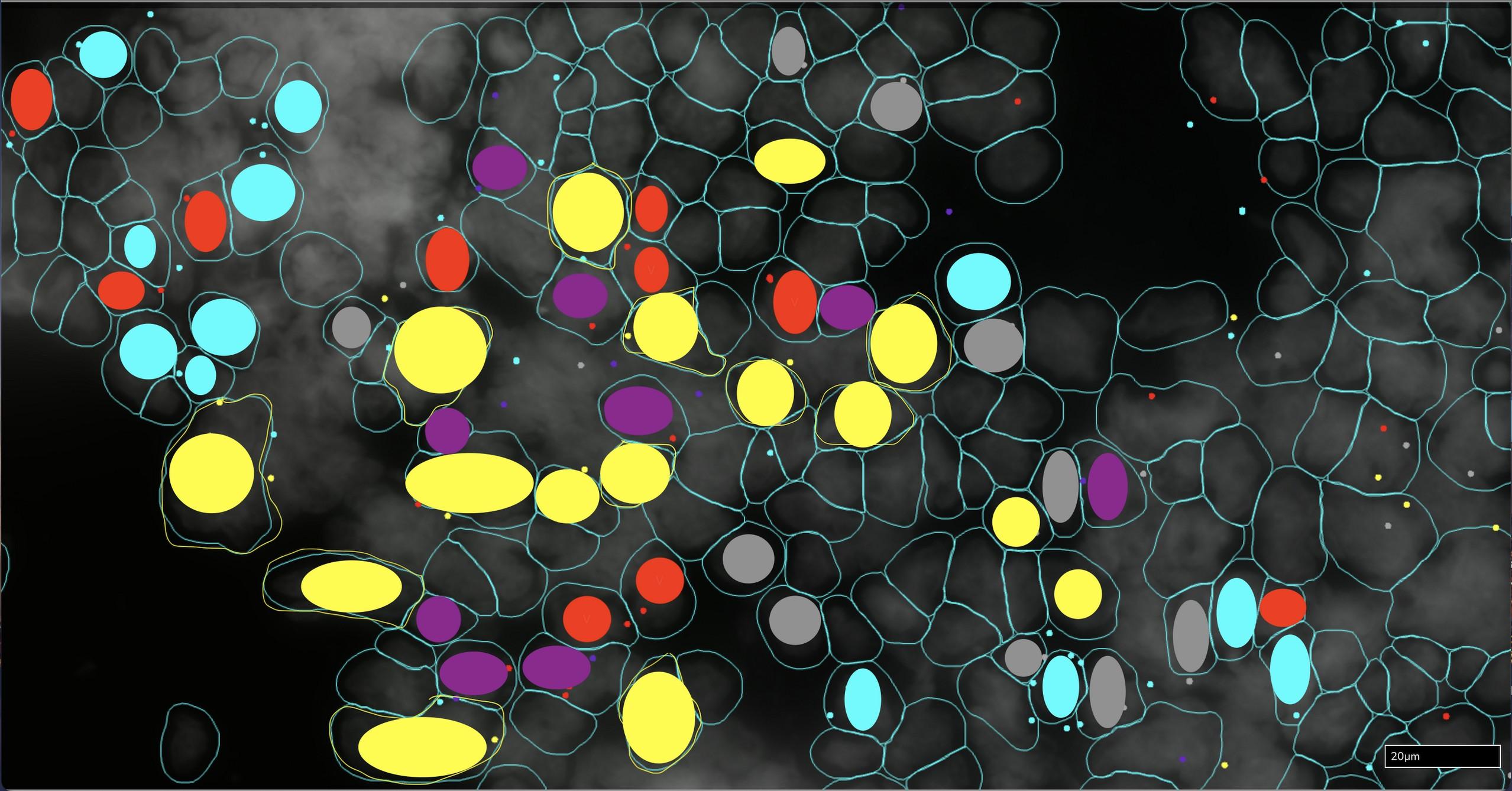
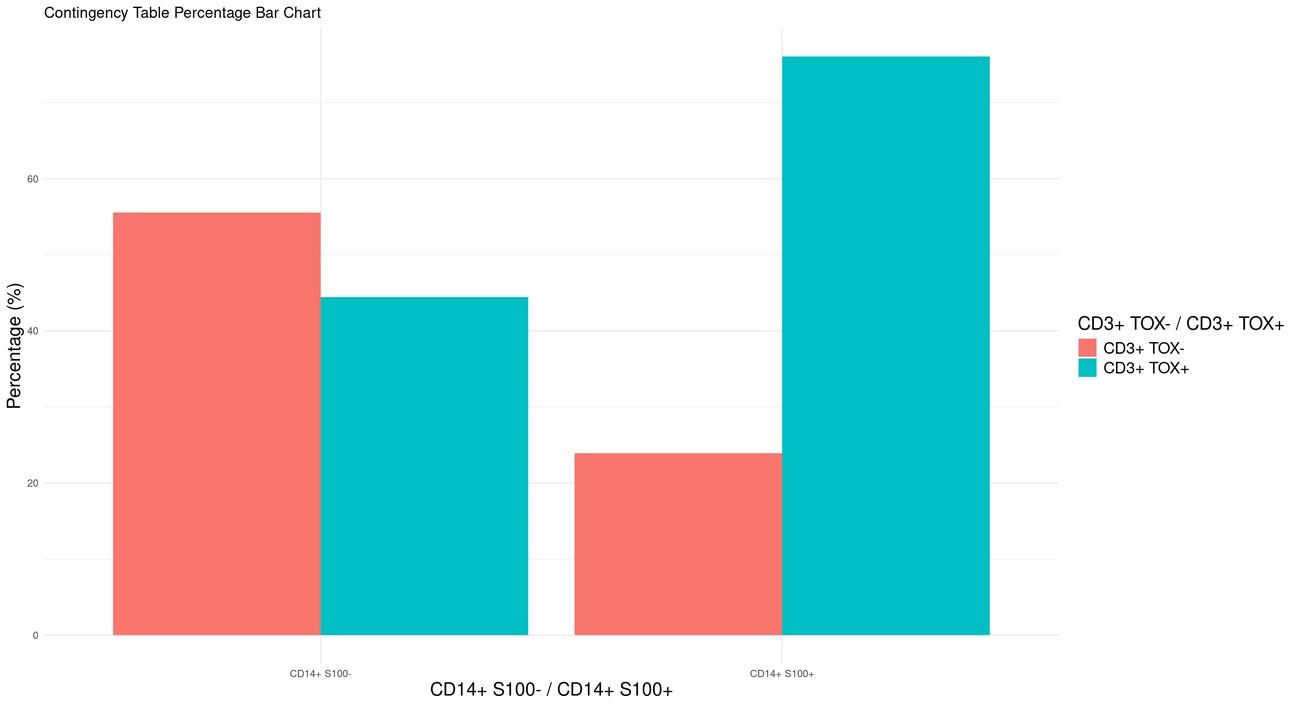
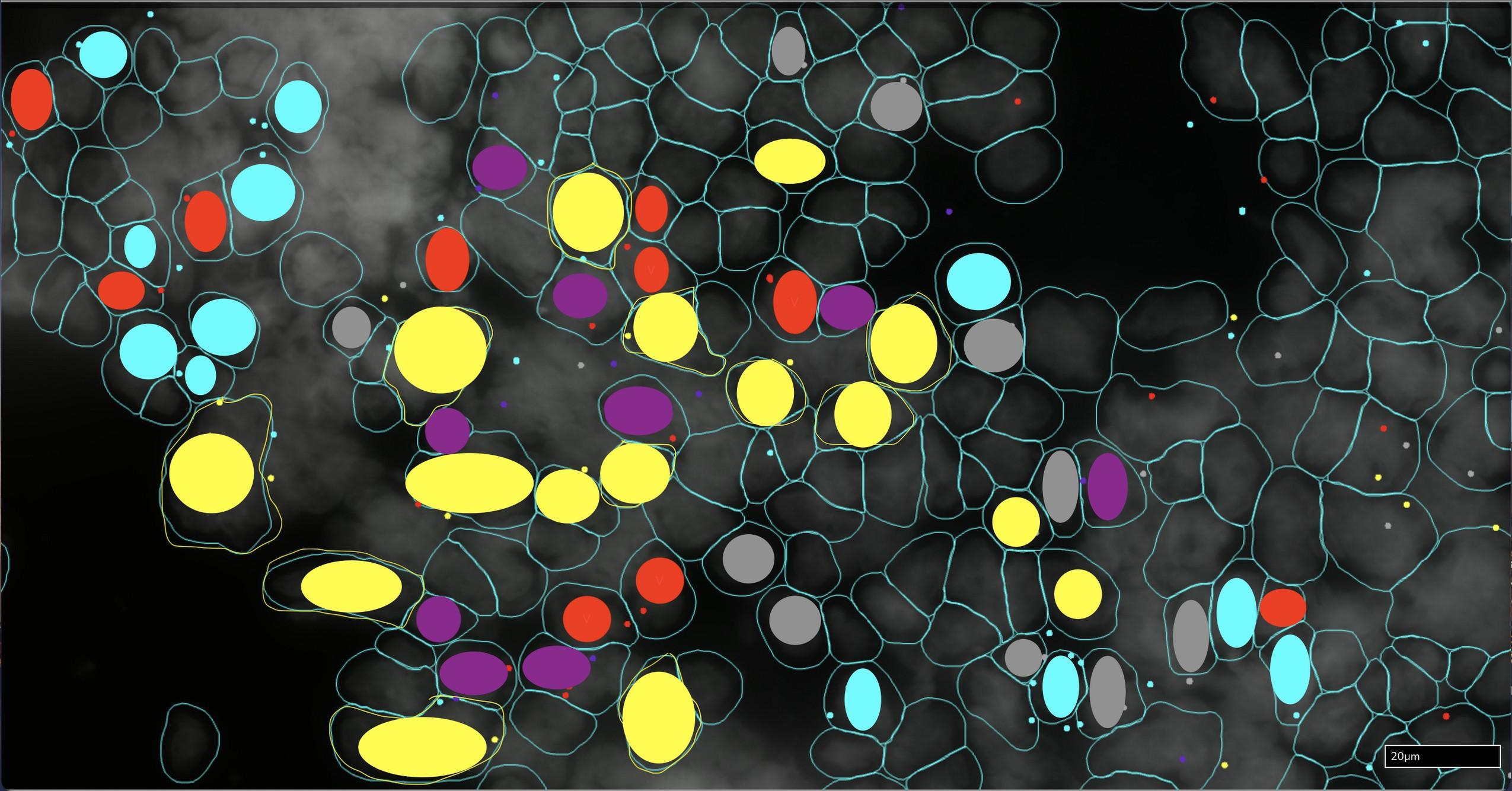

CD3+ TOX negative T cells
CD3+ TOX+ T cells

CD14+ S100A8/A9 negative cells
CD14+ S100A8+ cells
CD14+ S100A9+ cells
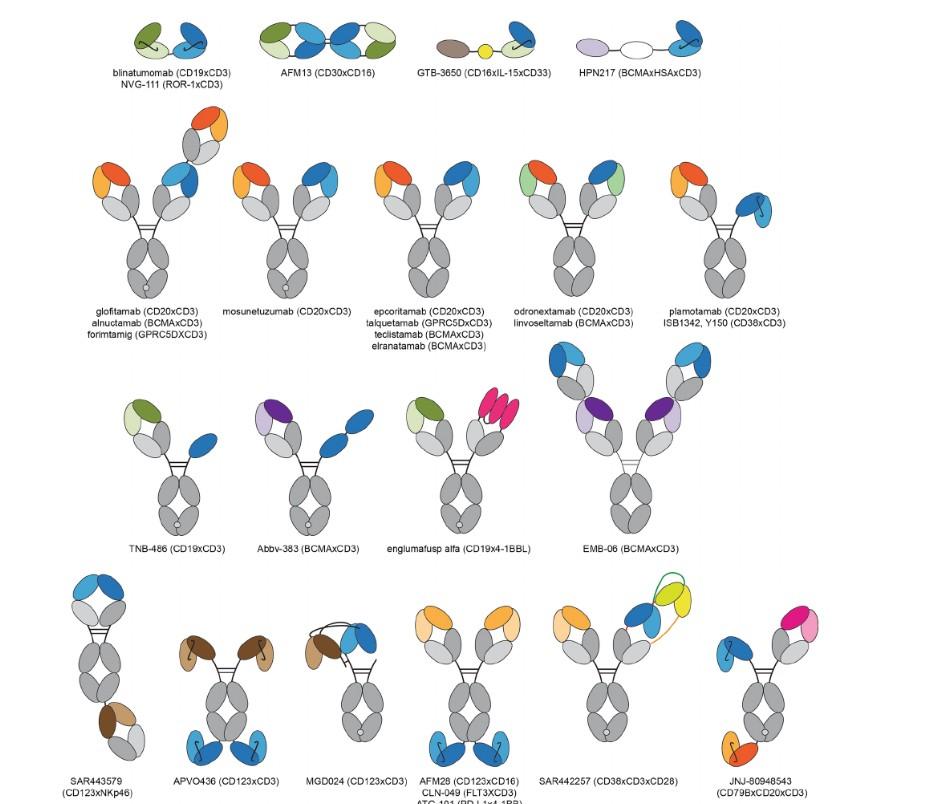




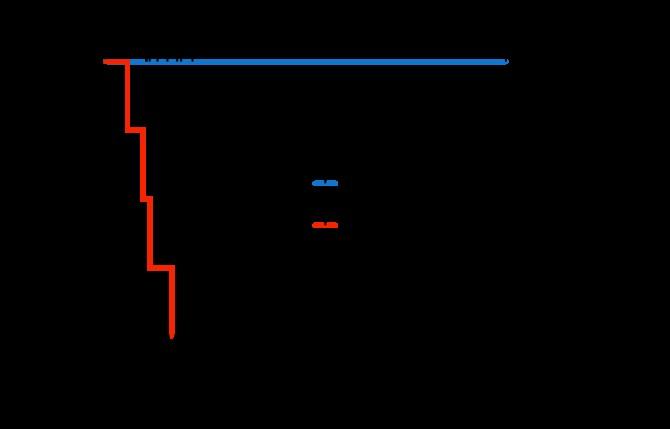
Alnuctumab (BCMA) Forimtamig (GPRC) Abbv-383 (BCMA) EMB06(BCMA) JNJ-79635322 (BCMA* GPRC)
extender 2:1 tumor:CD3 binding domain low CD3 affinity trispecific tetravalent


PFS 30 mos
Phase 1 (n=38)
1. Anderson L et al. ASCO 2021;abstract;8016 (poster presentation); 2. Berdeja J et al. Lancet 2021;398;314-24; 3. Lin Y et al. EHA 2022;abstract P961 (poster presentation);4 Freeman C et al ASH 2024. 5. Costello C, et al. ASH 2020;abstract 134; 6. Mohyuddin GR et al. Blood Adv 2021;5(4):1097-1101; 7. Oliver-Caldés A et al. ASH 2023
8. Li C et al. EHA 2022;abstract S187 (oral presentation); 9. Li C, et al. ASCO 2023; 8025 10. Mailankody S, et al. ASH 2021;abstract 651; 11. Dohlaria et al ASH 2023; abstract 3479.
Novel manufacturing
• Dd domain, piggy Bac, human scFV, allo
• Faster production
• In-vivo production (eg viral injection)
• CRISPR (non viral) CART
• Lower cost – academic
• Non-BCMA targets eg GPRC5d, CD70
• Dual targets eg. CD19 + BCMA, GPRC5d+ BCMA
Tumor Intrinsic
• Extramedullary Disease
• ISS3 –B2M
• High risk genomics
• High disease bulk/soluble blood Ag
• High soluble antigen in blood
• Functional ag loss
• better health in NDMM pts
• At apheresis - more naïve CD4+ T cells & less Tem
• higher CAR+ CD8+ stem cell like & lower CAR+ CD4r+ treg
• more T cell checkpoint expression after bsab
• Higher inflammation/cytopenias correlate with worse CART outcomes
• Higher S100 (associated with MDSC) correlate with worse outcomes with CARTs and bispecifics
• S100 promotes T cell exhuaustion, T reg differentiation
Patients and Caregivers
MDs
• Shagun Arora
• Ajai Chari
• Alfred Chung
• Anupama Kumar
• Tom Martin
• Nina Shah
• Peter Sayre
• Jeff Wolf
NPs/PAs
• Helen Diiulio
• Grace Sevilla
• Sam Shenoy (R)
• Nancy Wong
• Laura Zitella
Cellular Therapy Staff
• Jennifer Knoche
• Gabbi Perez
• Cheryl Slagle
RNs
• Jennifer Brustein
• Lisa Dunn
• Katharina Ganapathi
• Julie Mccluggage (R)
• Jenner Wells
• Jeani Wilmoth (R)
• Samanha Zylberman (R)
Social Workers
• Nina Balsamo
• Isabel Curtin
• Rachel Dornhelm
• Judy Smoker
• Ana Zermeño
Dieticians
Medical Assistants
• Nashia Raley
• Joan Viyela
Pharmacists
• Richard Fong
• Mimi Lo Administrative Staff
• Shelby Abuhatzira
• Deza Lynn Villanueva
Data Analyst
• Vishakha Malhotra
Clinical Research Staff (R)
• Jungeun Kim
• Alicia Aschauer
• Mrugakshi Dave
• Edlimar Delgado
• Liam Gima-Lange
• Kenya Gomez
• Jenny Nguyen
• Lauren Nguyen
• Ruixin Sun
• Lena Truong
Translational Research Team
Julia Carnevale
• Nupura Kale
• Stefanie Bachl
• Carter Ching
Justin Eyquem
Chang Liu
Joseph Muldoon
Alexis Talbot
David Oh
• Kai Wu Arun Wiita
Nikhil Chilakapati
Szu-Ying Chen
Bonell Patiño Escobar
Haley Johnson
Corynn Kasap,
Adila Izgutdina



Smoldering Multiple Myeloma – Dorado Room, Level -1 West Wing

Immune Therapy – Stay in this room
Quality of Life – Polar Room, -2 West Wing
Bone Disease – Alfa Room, - 2 North Wing
Combined Mass Spectrometry and MRD – Orione Room, - 2 West Wing


