





• Identify opportunities for panviral screening within addiction services
• Educate patients to support screening, diagnosis, and care navigation
• Review the importance of community partnerships to enhance care coordination and patient outcomes



• 7% of new HIV diagnoses are due to IDU1
• A person without HIV has a 1 in 160 chance of getting HIV every time they share a needle with a person with HIV2
• Sharing syringes in the second riskiest behavior for acquiring HIV3
• SUD may increase the risk of getting HIV through sex due to increased likelihood of higher-risk sexual behaviors3
• Although all-cause death rates have declined in PWH, deaths related to substance use in PWH have not declined, and, in fact, have increased 7% in women4
IDU, injection drug use; PWH, people with HIV.
1. Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/hiv/data-research/facts-stats/index.html; 2. Medical News Today. https://www.medicalnewstoday.com/articles/324052#survival -outside-the-body; 3. HIVinfo.nih.gov. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-and-substanceuse; 4. Trickey A, et al. Lancet HIV. 2024;11(3):e176-e185.

• Percutaneous or mucosal exposure to infected blood or body fluids via1:
1. IDU2,3
• Includes shared equipment and unsterilized syringes
• Leading cause of hepatitis transmission in the US4,5
2. Unprotected sex2-4
• 38.2% of acute HBV infections (2013-2018)
3. Mother-to-child, typically during delivery2
• HCV-positive pregnancies increased 16-fold (1998-2018)6

• HBV-positive pregnancies increased by 5.5% annually since 19987


1. World Health Organization (WHO). Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection. Geneva; March 2024. https://iris.who.int/server/api/core/bitstreams/34470cc8 -af90-4d7b-a949-ef27e5d0726f/content; 2. CDC. https://www.cdc.gov/hepatitis/php/surveillance-guidance/hepatitisb.html#:~:text=Background,least%207%20days%20(46); 3. CDC. https://www.cdc.gov/hepatitis -surveillance-2023/hepatitis-c/figure-3-7.html; 4. Perez-Molina JA, et al. Pathogens. 2023;12(9):1137; 5. CDC. https://www.cdc.gov/hepatitis-surveillance-2023/hepatitis-b/table-2-3.html; 6. Chen P, et al. JAMA Netw Open. 2023;6(7):e2324770; 7. US Preventive Service Task Force. JAMA. 2019;322(4):349-354.
• ≈70.5 million people (24.9%) used an illegal drug or misused prescription drugs within the last year1
• 51% of people aged ≥12 years have used illicit drugs at least once1

of PWID have chronic HBV4 1 in 2 PWID has acquired
PWID, people who inject drugs.
1. National Center for Drug Abuse Statistics. https://drugabusestatistics.org/; 2. Stone J, et al. Lancet Public Health. 2022;7(2):E136-E145; 3. Ko JY, et al. MMWR Morb Mortal Wkly Rep. 2019;68(39);833-838; 4. Hepatitis B Foundation. https://www.hepb.org/blog/hepatitis-b-injection-drug-use-risks-barriers-care-prevention-strategies/; 5. Fattovich G. J Hepatol
2003;39(1):50-58; 6. National HIV Curriculum. https://www.hiv.uw.edu/go/co-occurring-conditions/hepb-coinfection/core-concept/all. of pregnant females with HCV have opioid use disorder3
Who should be tested?1
• CDC: All adults and teens (range 13-64 years) should be tested at least once in routine health care
• All pregnant persons
• Tested at least once a year (some as often as every 3 months):
– MSM or transgender individuals
– PWID and their sex partners
– People engaging in survival sex
– Individuals with a current STI, hepatitis, or TB
– Partner with HIV or ≥1 partner since last HIV test
– People receiving treatment for hepatitis, tuberculosis, or a sexually transmitted infection
1,2
• Perform opt-out testing (testing is standard for all patients unless they decline it)
• Normalize testing for all patients
• The CDC recommends initial testing with an FDA-approved Ag/Ab assay; no oral tests

Ag/Ab, antigen/antibody; MSM, men who have sex with men; PWUD, people who use drugs; STI, sexually transmitted infection; TB, tuberculosis. 1. CDC. https://www.cdc.gov/hivnexus/hcp/diagnosis-testing/; 2. CDC. https://www.cdc.gov/hivnexus/hcp/diagnosis-testing/.

• HCV screening at least once in a lifetime for all adults aged ≥18 yearsa
• HCV screening for all pregnant people during each pregnancya
ü One-time HCV testing
• Persons who ever injected drugs and shared needles, syringes, or other equipment
• PWH
• Infants born to mothers with HCV infection
• People with selected medical conditionsb
• Prior recipients of transfusions or organ transplants
• Those exposed to HCV-positive blood via needle sticks, sharps, or mucosal exposures
ü Routine periodic testing
• PWID and share needles, syringes, or other equipment
• Persons with selected medical conditions, including persons who ever received maintenance hemodialysis
ü Any person who requests HCV testing should receive it, regardless of disclosed risk
aExcept in settings where the prevalence of HCV infection (HCV RNA-positivity) is <0.1%; bIncludes persons who have ever received maintenance hemodialysis, as well as those with persistently abnormal alanine transaminase levels.
CDC. https://www.cdc.gov/hepatitis-c/hcp/diagnosis-testing/index.html.

• HBV screening for all pregnant women during each pregnancy, preferably in the first trimester, regardless of vaccination status or history of testing
• Pregnant persons with a history of appropriately timed triple panel screening and without subsequent risk for exposure to HBV (ie, no new HBV exposures since triple panel screening) only need HBsAg screening
• Testing for all persons with a history of increased risk for HBV infection
• Periodic testing for susceptible persons with ongoing risk for exposure

EE, et al. MMWR Recomm Rep. 2023;72(1):1-25.
Organization Recommendations for Triple Testing in High-Risk
• Triple testing=testing for 3 viruses (HIV, HBV, and HCV) at once, with a single blood sample1
• Who?1
– PWUD, MSM, and prisoners
• Why?1,2
– Improved identification

…but if we extend to additional populations
Results of extending WHO’s triple testing campaign to additional populations. . .
• HBV and HCV are underdiagnosed compared to HIV
– Cost-effectiveness
– Alignment with elimination goals
– Can be combined with other public health efforts
• Vaccination, linkage to care, etc
– Improvement in health outcomes
WHO, World Health Organization.







1. WHO. Consolidated guidelines on HIV, viral hepatitis and STI prevention, diagnosis, treatment and care for key populations. Geneva, Switzerland: WHO; 2022; 2. Beard N, et al. Open Forum Infect Dis. 2024;11(2):ofad666.
Image adapted from https://www.healio.com/news/infectious-disease/20240123/triple-testing-for-hiv-hbv-hcv-at-once-would-help-identify-more-cases-across-populations#.

• Multiple tests available1
– Most detect only HIV Ab1
– Only 1 approved rapid POC test to detect both Ab and Ag2
• Many fingerstick test options and, though not recommended, there are oral tests available1
• Results within a few minutes1

• Most tests only detect HCV Ab, which only shows if there was past exposure to HCV3
• But…POC HCV RNA confirmatory tests are now available4
– Fingerstick3,4
– Dried blood spot testing5
• Results within a few minutes3
Next steps for a positive Ab test: send blood for confirmatory testing
Note: POC tests are not available for HBV.

POC, point of care.
1. CDC. https://www.cdc.gov/hivpartners/php/hiv-testing/index.html; 2. FDA. https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/determine-hiv-12-agab-combo; 3. CDC. https://www.cdc.gov/hepatitis-c/testing/index.html#cdc_testing_type_test-testing-methods; 4. FDA. https://www.fda.gov/news-events/press-announcements/fda-permitsmarketing-first-point-care-hepatitis-c-rna-test; 5. New York State Department of Health. https://www.health.ny.gov/publications/16129.pdf.

• Can help determine acute HIV infection
• Differentiate between HIV-1 and HIV-2
• Detect HIV RNA and viral load

• Reflex testing detects HCV RNA to establish if infection is active as opposed to previously resolved
• Eliminate any false-positive results
• The 2-step HCV testing process should be achieved in a single patient encounter

Same blood sample used for confirmatory HIV and HCV testing can be used for an HBV test.
1. New York State Department of Health AIDS Institute. https://www.hivguidelines.org/guideline/hiv-testing/; 2. Adapted from CDC. MMWR Morb Mortal Wkly Rep. 2013;62(18):362-365.

• No rapid POC test available in the US for HBV1
– Blood sample can be obtained on site but needs to be sent to a lab, as would any confirmatory HIV test (and most HCV tests)
• Laboratory-based screening for HBV2-4
1. Molecular Testing Labs. https://moleculartestinglabs.com/proper-dried-blood-spot-card-collection/; 2. Conners EE, et al. MMWR Recomm Rep. 2023;72(1):1-25; 3. State Laboratory of Public Health. https://slph.dph.ncdhhs.gov/doc/Hepatitis-A-and-B-Serology-Interpretation-Charts.pdf; 4. Hepatitis B Foundation. https://www.hepb.org/assets/Uploads/ProviderInfographic.pdf. HbsAg Anti-HBs Anti-HBc Interpretation
+ Currently infected with HBV
Never infected with HBV; never vaccinated against HBV
Vaccinated/Protected against HBV
Resolved HBV infection; no risk of transmission
Interpretation unclear; resolved infection, chronic infection, occult infection or false positive


Opt-out approach for testing is recommended by the CDC.1

Inform patients that panviral testing (bundling HIV, HBV, and HCV testing together) is part of routine care.1,2
This could reduce stigma associated with testing.1

Opt-out viral testing has been shown to increase:
• Testing rates at SUD centers and SSPs1
• Rates of linkage to care3
Opt-out panviral testing could occur during intake, while the patient is waiting for a dose of MOUD, after a group session if on-site support groups are offered, or during an initial or routine clinic visit4
SSP, syringe services program.
1. Bartholomew TS, et al. Int J Drug Policy. 2020;84:102875; 2. Westgard LK, et al. Open Forum Infect Dis. 2024;11(5):ofae204; 3. National Health Service (NHS).
https://www.england.nhs.uk/long-read/emergency-department-opt-out-testing-for-hiv-hepatitis-b-and-hepatitis-c-the-first-100-days/; 4. Conners EE, et al. Int J STD AIDS. 2012;23(11):799-805.


• Methadone clinics with a phlebotomist can draw blood for panviral testing1
• Helpful if bundled together, and is associated with higher rates of completion2
• Intake process can be modified to include panviral screening in addition to any other lab work3
– Funding may potentially come from grants or bundled patient intake payments
• Connect with county health department for reporting positive tests4
Existing protocols can be amended to include blood draws for panviral testing for locations with phlebotomists on staff.
1. Practical Nursing. https://www.practicalnursing.org/what-expect-working-methadone-clinic; 2. Frimpong JA, et al. Med Care. 2020;58(5):445-452; 3. Behrends CN, et al. J Public Health Manag Pract. 2021;27(4):393-402; 4. National Alliance of State and Territorial AIDS Directors (NASTAD). https://nastad.org/sites/default/files/2024-03/PDF-Reporting-Requirements-forNegative-HIV-and-Hepatitis-C-Test-Results.pdf.
• Rapid POC test only for HIV or HCV Abs or oral HIV rapid tests require confirmatory testing with phlebotomy
• An HBV test requires blood to be sent to a lab
• Partnership with community-based health centers, health departments, or FQHCs, where patients can go for same-day confirmatory blood draw
– May have a co-pay associated with a blood draw
– Medicaid may dictate cost to patient
• Location where blood is drawn will obtain results and be responsible for communicating results to the patient
FQHC, Federally Qualified Health Center.
Behrends CN, et al. J Public Health Manag Pract. 2021;27(4):393-402.


• EMR can be used to identify if a patient has been tested or needs to be tested for HIV, HCV, or HBV and test results1
• Identify locations in workflow where patients can have blood drawn for testing2
– Encourage checklists in intake process
• Incorporate pop-up reminders, order sets, and templates to facilitate testing

• Largest barriers to EMR adoption include cost of start-up, ongoing maintenance costs, and privacy concerns, but EMR can help streamline workflow3

All SUD center staff should be trained to utilize EMR tools.
EMR, electronic medical records.
1. Behrends CN, et al. J Public Health Manag Pract. 2021;27(4):393-402; 2. Fields AK, et al. J Healthc Sci Humanit. 2021;11(1):84-100; 3. Frimpong JA, et al. J Med Internet Res. 2023;25:e45238.

Develop and agree upon testing protocols




Appoint a testing “champion”
Create clinic reminder in EMR and include documentation template


Perform opt-out testing at agreed-upon point in intake process


If on-site blood draw, deliver test results once available
If off-site blood draw, coordinate with facility where blood was drawn to obtain test results


Refer patient for treatment or prevention services as necessary

• Site champion to oversee protocol development, implementation, and outcomes
– Include staff training on new protocols
• Phlebotomist or clinician to incorporate testing into regular blood draws, if applicable
• Train clinician or other staff member on rapid POC tests if no phlebotomy on site
• Peer navigator (if available) for patient education and linkage to care

• Site must determine whether behavioral health payers cover panviral testing
– May be able to expand coverage to support more testing
• Sites may need to identify and collaborate with physical health payers
– Highlight benefits of panviral testing with physical health payers
• Investigate whether state Medicaid programs cover testing and treatment
• 340B programs, if applicable to the site, can help fund panviral testing

• Key approaches to improving testing and treatment:
– Ensure testing is part of all MOUD intakes
– Have an automated process or checklist for ordering the tests
– Allow bundled orders for intake testing
– Ensure communication of the results back to patients
– Facilitate linkage to or on-site treatment
– Provide counseling for those who are negative to maintain the negative status



• Ensure the patient is in a private space
• If rapid test:
– Calmly explain that a rapid test is positive and a confirmatory test is needed
• If confirmatory test:
– Explain that it means the patient has the infectious disease
• Emphasize that medications are available
• If a test is negative, be prepared to discuss prevention techniques

There are numerous training modules or scripts for delivering test results:
https://www.nyc.gov/site/doh/providers/health-topics/aids-hiv-presenting-results.page#
https://findtbresources.cdc.gov/view?id=342385
https://www.hiv.va.gov/pdf/HIV-test-results-2021-508.pdf
https://stacks.cdc.gov/view/cdc/31565
NYC Health. https://www.nyc.gov/site/doh/providers/health-topics/aids-hiv-presenting-results.page#.
• Ask open-ended questions about current knowledge level of the disease and any factors that can affect adherence1
• Promote patient empowerment and use positive reinforcement1
• Consider putting patient in touch with people with lived experience1
• Provide educational materials at the appropriate literacy level1
• Acknowledge and address competing priorities1
• Combine education with other interventions, such as providing community resources2

1. US Department of Health and Human Services (DHHS). https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/adherence-continuumcare; 2. Pugh LE, et al. J Infect Public Health. 2022;15(10):1053-1060.

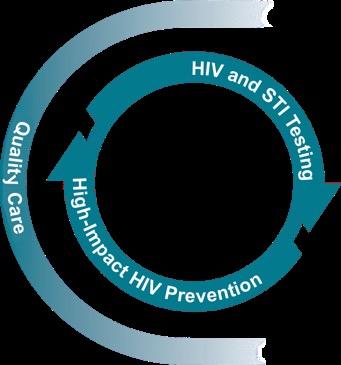
People whose tests are negative are offered powerful prevention tools such as PrEP, condoms, vaccines, harm reduction, and supportive services to stay negative.
The
PrEP, preexposure prophylaxis. CDC. https://stacks.cdc.gov/view/cdc/129024/cdc_129024_DS1.pdf.
Culturally Inclusive and Responsive Quality Care
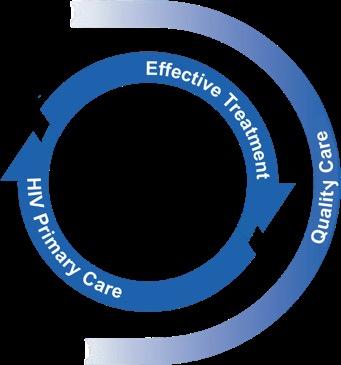
People whose tests are positive enter primary care and are offered effective treatment and supportive services for disease management or cure.

PrEP is a comprehensive set of services to reduce risk of HIV infection
• 4 FDA-approved medications for PrEP1,2
– May only be used in persons without HIV
• Comprehensive services include1:
– Regular HIV screening
– Regular STI screening
– Safer sex + risk-reduction counseling
• Available medication options for PrEP1,2:



Refer any patient interested in PrEP to a provider who can prescribe PrEP if your center cannot do so.


Oral daily tenofovir disoproxil fumarate/emtricitabine (TDF/FTC; Truvada® ); available in generic
Oral daily tenofovir alafenamide/emtricitabine (TAF/FTC; Descovy® ); not for use in people at risk of HIV through vaginal receptive sex
Cabotegravir long-acting injectable (CAB LAI; Apretude® ) every 2 months
Lenacapavir long-acting injectable (LEN LAI; Yeztugo® ) every 6 months
1. CDC. https://stacks.cdc.gov/view/cdc/129024/cdc_129024_DS1.pdf; 2. HIV.gov. https://www.hiv.gov/hiv-basics/hiv-prevention/using-hiv-medication-to-reduce-risk/pre-exposureprophylaxis.

• Nonoccupational postexposure prophylaxis (nPEP) is the use of antiretroviral medication after a very recent potential exposure to HIV, to prevent infection
– Medication must be started within 72 hours of possible exposure, ideally within 24 hours
– Test for HIV prior to initiating nPEP
– Patient should follow up with the prescribing clinician within 72 hours
– Medication is taken daily for 28 days, after which a discussion can be had about transition to PrEP
– Continue HIV/STI testing
– Usually covered by insurance (or patient assistance program on an urgent basis)
• Who should be vaccinated?
– Everyone from infants to those aged 59 years who have not been vaccinated; including pregnant women
– Adults aged ≥60 years at risk for HBV

Must be HBsAb negative, anti-HBs (−)
• How does it work?
– A series of 2 or 3 doses, depending on age, the vaccine, or medical conditions
– Boosters are often not necessary
CDC. https://www.cdc.gov/hepatitis-b/vaccination/index.html.


• Life expectancy nearly equal between people with and without HIV1
• People diagnosed today have longer life expectancies vs those diagnosed many years ago2
• More likely to die of nonHIV–related illnesses 2


• Ask open-ended questions about current knowledge level
• Consider putting patient in touch with adults with HIV to hear their experience
• Provide educational materials at the appropriate literacy level



• Initiating antiretroviral therapy (ART) as soon as patients are diagnosed helps them become virally suppressed more quickly, and it is impossible to transmit HIV to sexual partners when virally suppressed (undetectable= untransmittable, or U=U)
1. Marcus JL, et al. JAMA Netw Open. 2020;3(6):e207954; 2. Bosh KA, et al. MMWR Morb Mortal Wkly Rep. 2020;69(46):1717-1724; 3. DHHS. https://clinicalinfo.hiv.gov/en/guidelines/hivclinical-guidelines-adult-and-adolescent-arv/adherence-continuum-care; 4. DHHS. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/initiationantiretroviral-therapy.

ü HCV is curable with treatment
ü Treatment is both feasible and highly effective in PWUD
ü Treatment is as simple as 1 to 3 pills once per day, and is approximately 8 to 12 weeks in duration
ü Treatment is well tolerated
ü Posttreatment follow-up is essential to check for cure (SVR12)
ü Reinfection can occur and is treatable
ü HCV Ab will remain positive indefinitely
ü HCV RNA must be tested to assess for reinfection

http://www.hcvguidelines.org/evaluate/when-whom.
ü HBV is treatable, with the goal of viral suppression
ü Treatment is effective in PWUD
ü Treatment can be as simple as 1 tablet daily, typically of long duration that may vary by case, and can be lifelong
ü Treatment is well tolerated
ü Yearly labs/ultrasound are necessary to monitor for reactivation, cirrhosis, HCC, and kidney health
ü Vaccination can protect against infection and offers lifelong immunity; the entire vaccine series must be completed

HCC, hepatocellular carcinoma.
Dieterich D, et al. Gastro Hep Advances. 2023;2(2):209-218.


• Refer to a low barrier to care clinic, if nearby
• SUD centers can refer patients who require treatment to:
– FQHCs
– Community health clinics
– Ryan White centers for HIV care
– Local hospital systems with an infectious disease department
• Determine if there are any existing partnerships with health care facilities
• Look for nearby locations that patients can access easily



• Welcoming and nonstigmatizing1,2
• Participates in harm reduction, including providing MOUD3
• Uses a peer navigator or person with lived experience1
• Collaborates with external partners, including SUD centers, shelters, or mental health services1
• Has low barriers to care1,2:
– Flexible clinic hours, no penalty for missed appointments, colocated care
• Can provide incentives or necessities, such as showers and clothing1,2

1. Dombrowski JC, et al. Clin Infect Dis. 2023;77(2):252-257; 2.
MD, et al. J Infect Dis. 2022;226(suppl 3):S353-S362; 3. Korthuis PT, et al. Addiction. 2017;112(6):1036-1044.
Walk-in access

Removes central barrier
• All visits available without scheduled appointments
Integrated care team with case managers
Incentives
Low-barrier care philosophy
Multisector coordination
Addresses social needs
Commitment to rapid modification
Provides tangible reward and helps address immediate needs
Adapts care goals to reflect individual needs
Coordinates services with other agencies
• Case managers get to know patients as individuals
• Low caseload case management support
• Nonmedical case management
• Streamlined care and shortened visits
• Harm reduction approach to SUD
• Minimizing the number of medications where possible
• Minimizing specialty referrals as clinic takes a larger role
• Creating specialty network willing to see patients on a walk-in basis
• Housing agencies
• Jail release planners
• Adherence support programs
• SUD treatment programs
• Behavioral health programs
Requires continual improvements to optimize care
Dombrowski JC, et al. Clin Infect Dis. 2023;77(2):252-257.
• Iterative improvements based on experience
• Patient and staff feedback incorporated
• Able to abandon plans that don’t work
• Acceptance that policies require flexibility on case-by-case basis


• Assist patients with appointments for wraparound services1
• Build support networks through shared backgrounds and community knowledge1,2
• Encourage medication adherence1
• Provide support throughout treatment pathways2
• Make multiple peer navigator training modules available that incorporate perspectives from various initiatives3-5
– Implementation guidelines, SOPs, client flow algorithms, reference documents

SOP, standard operating procedure.



1. San Francisco Department of Public Health. https://www.sfcommunityhealth.org/program/hhome; 2. Jugnarain DV, et al. J Viral Hepat. 2022;29(1):43-51; 3. FHI360. https://www.fhi360.org/sites/default/files/media/documents/resource-linkages-peer-navigation-facilitators-guide.pdf; 4. AIDS Education & Training Center (AETC) Program. https://aidsetc.org/resource/community-health-worker-training-resources; 5. National Minority AIDS Council. https://www.nmac.org /capacity-building/hiv-prep-navigation-program/.
• Assess center’s readiness for a peer navigator program1
• Identify intended roles for the peer navigator1
• Decide where peer navigators fit in the clinic workflow1
• Look for a peer navigator who2:
– Can act as an educator, facilitator, coach, advocate, and community resource
– Is committed to meeting center goals, accountable to the community, tolerant of others’ ideas and behaviors
– Can maintain confidentiality, boundaries, and ethics

1. Health Resources and Services Administration Ryan White HIV/AIDS Program. https://targethiv.org/sites/default/files/media/documents/202303/CQII_Peer_Navigator_IG_final_Jan2023.pdf; 2. FHI360. https://www.fhi360.org/wp-content/uploads/drupal/documents/resource-linkages-peer-navigation-facilitators-guide.pdf.

• Peer navigators (anyone with a close understanding of the community served) interacted for an average of 11 days over 6 months per patient
• Peer navigators most often provided coaching and education, emotional support, and appointment reminders
• Most contact (63%) was virtual (phone, text, social media, or email) – If in person, 72% occurred at the program site
1 2 3 4 5
Establish a clinic definition of lost from care and ways to identify patients meeting this definition

Identify which method(s) should be used to try to contact patients
Identify staff who can make phone calls or try to contact patients in another way
Try to note if patient has moved or gone elsewhere so that outreach is not done
Contact patients by the established method and bring back into care

• Acknowledge and address competing priorities, such as mental health or social needs
• Obtain comprehensive picture of the patient’s complete health and needs
• Promote empowerment
• Provide information on medications, access to care, and treatment rights
• Build staff-patient relationships
• Build patient knowledge and skills
• Provide low-barrier options for care

Clinicians should incorporate trauma-informed care for every patient.




















• Using clinically accurate and medically appropriate language matters1,2
• Stigma and stereotypes can impede solutions that will reduce HIV and viral hepatitis transmission2
• Person-first language can reduce stigma, increase patient willingness to seek treatment, reduce negative provider perceptions of PWUD, PWH, or those seeking PrEP, and improve care3-5
– Use “a person experiencing homelessness” instead of “homeless person”
– Use “a person who uses drugs” instead of “addict”
– Use “a person with HCV” instead of “HCV infected”

1. Botticelli MP, Koh HK. JAMA. 2016;316(13):1361-1362; 2. United States Interagency Council on Homelessness. https://www.usich.gov/news-events/news/peopleexperience-homelessness-they-arent-defined-it; 3. Goddu AP, et al. J Gen Intern Med. 2018;33(5):685-691; 4. National Institute on Drug Abuse. https://nida.nih.gov/nidamedmedical-health-professionals/health-professions-education/words-matter-terms-to-use-avoid-when-talking-about-addiction#; 5. CDC. https://www.cdc.gov/healthcommunication/php/toolkit/preferred-terms.html?CDC_AAref_Val=https://www.cdc.gov/healthcommunication/Preferred_Terms.html.

Individual trauma results from an event that is experienced by an individual that leaves a long-term negative effect on the individual’s functioning and well-being. It can be experienced by a person or an entire population.

• Safety (physical and psychological)
• Trustworthiness and transparency
• Peer support (from individuals with lived experiences of trauma)
• Collaboration and mutuality
• Empowerment, voice, and choice
• Cultural, historical, and gender issues

One of the most important changes when incorporating TIC is shifting the question from “What’s wrong with you?” to “What happened to you?”
TIC, trauma-informed care. Substance Abuse and Mental Health Services Administration (SAMHSA). SAMHSA’s Concept of Trauma and Guidance for a Trauma-Informed Approach. HHS Publication No. (SMA) 14-4884. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
Organization that is unaware or ignorant of the impacts of trauma on clients and staff
Recognition and naming of trauma and toxic stress, and opportunities for resilience among clients and staff
Staff have foundational knowledge of NEAR science, trauma-informed principles, and recognize signs and symptoms of trauma
Organization fully integrates knowledge about trauma and resilience into trauma policies, procedures, and practices
Organization actively realizes, recognizes, responds to, and resists retraumatization of staff and clients

NEAR, neurobiology, epigenetics, adverse childhood experiences, and resilience. NASTAD. https://targethiv.org/sites/default/files/media/documents/2023 -01/Trauma-Informed-Approaches-Toolkit-2022_NASTAD.pdf.
Organization actively centers resilience and healing, for both clients and staff



• Adherence is improved when competing priorities are addressed1
• Community partners can help encourage retention in care and medication adherence2
• Utilize existing community partner relationships and collaborate with other clinicians to identify relationships2,3






1. Palacio-Vieira J, et al. BMC Public Health. 2021;21(1):1596; 2. Jones MD, et al. PLoS One. 2023;18(1):e0276852;
Broz D, et al. Am J Prev Med. 2021;61(5 suppl 1):S118-S129.
• SUD service centers can play a key role in the expansion of viral hepatitis prevention services, testing, vaccination, and linkage to care
• Collaboration between SUD service providers and treatment providers is critical to success
• Systems whereby the treatment provider notifies SUD service centers regarding patient engagement, treatment initiation, and treatment completion are needed
• Feedback from the treatment provider is essential for coordination of patients’ harm reduction services, DAA-adherence support, and postcure monitoring reminders
The reduction of viral hepatitis can best be achieved by supporting implementation of comprehensive community-level programs for PWUD (eg, access to SSP, linkage to MOUD programs, testing, treatment, and vaccination).2
DAA, direct- acting antiviral.
1. Mehta SH, Thomas DL. J Hepatol. 2016;65(1):5-6; 2. CDC. 2020 National Viral Hepatitis Progress Report.
https://archive.cdc.gov/www_cdc_gov/hepatitis/policy/npr/2020/NationalProgressReport-HepC-ReduceInfections.htm.


• Intervention involved admitted patients meeting with an infectious disease–trained nurse navigator
• Screening at the time of admission
• Patient counseled about test results, prevention, and treatment

An 18-bed inpatient SUD program implemented a novel approach to panviral screening and treatment from September 2022 to June 202 3 and compared data to usual care from September 2021 to June 2022. Dyer KE, et al. Open Forum Infect Dis. 2025;12(8):ofaf403.

N=27 patients received initial HIV care at the IDEA clinic, then after 6 months transitioned to HIV care at a traditional cli nic, including Ryan White–funded clinics. Patients had access to peer navigation and medication management for the 12 months of the study.
Plesons M, et al. Ann Med. 2025;57(1):2461670.

• Patients with SUD, particularly those who inject drugs, are at high risk of contracting HIV, HCV, and HBV
• Screening for all 3 infections should occur simultaneously
• A site champion can help develop and tailor protocols to implement testing into existing workflow
• Patients should be educated about each disease and prevention or treatment options
• Patients should be referred to treatment centers, preferably low barrier of care clinics
• Collaborating with external partners improves patient outcomes