





















Associate Professor of Internal Medicine
The University of Queensland Medical SchoolOchsner Health
New Orleans, Louisiana
Dr. Jonathan Hand specializes in infectious diseases at Ochsner Health. He earned his medical degree from Louisiana State University School of Medicine, New Orleans and completed his internship, residency and infectious diseases fellowship at the Icahn School of Medicine at Mount Sinai in New York. He is currently an associate professor of medicine at The University of Queensland School of Medicine Ochsner Clinical School. Dr. Hand specializes in the treatment of infections in immunocompromised patients who anticipate or undergo solid organ or hematopoietic stem cell transplantation as well as patients treated with other immunosuppressive therapies. He is a Fellow of the American Society of Transplantation and has served on the executive committee of the Infectious Diseases community of practice. He is also a member of The Transplantation Society, International Society for Heart and Lung Transplantation and Fellow of The Infectious Diseases Society of America. Dr. Hand is the Section Head of Transplant Infectious Diseases and the Medical Director of the Antimicrobial Stewardship Program at Ochsner Medical Center. He also serves as co-chair of the Louisiana Organ Procurement Agency’s Advisory Board and Associate Medical Director providing guidance on infectious diseases-related concerns in organ donors and recipients. Dr. Hand leads clinical trials as Associate Research Medical Director of Infectious Diseases for Ochsner Health. His practice and research interests include infectious complications of transplant donors and recipients, vaccine and antimicrobial clinical trials and antimicrobial stewardship.

Professor of Clinical Pharmacology and Respiratory Medicine, University of Manchester Medical Director, Medicines Evaluation Unit.
Honorary Respiratory Consultant, Manchester University Foundation Hospitals Trust London, United Kingdom
Dave Singh is professor of clinical pharmacology & respiratory medicine at the University of Manchester. He graduated in medicine from Cambridge University and then specialised in clinical pharmacology and respiratory medicine. His research interest is the development of new drugs for asthma and COPD. He is the medical director of the Medicines Evaluation Unit, where he has acted as principal investigator in over 300 clinical trials. He is a member of the Global initiative for the management of chronic Obstructive Lung Disease (GOLD) Science Committee, and currently is the chair of the European Respiratory Society airway pharmacology group. He has served as an editor of the British Journal of Clinical Pharmacology, and is currently an editor at the European Respiratory Journal. He has over 250 publications.

Professor of Respiratory Medicine
Associate Dean Faculty of Medicine University of Southampton Southampton, United Kingdom
Professor Tom Wilkinson is Professor of Respiratory Medicine at the University of Southampton, Faculty of Medicine, Associate Dean of the Faculty of Medicine and Honorary Consultant at University Hospitals Southampton. He trained at the University of Cambridge and Barts and the London School of Medicine and completed his PhD at UCL studying disease mechanisms driving Infective Exacerbations of COPD with Professor Wisia Wedzicha.
He is lead of the Southampton COPD research group, the Respiratory Theme of the NIHR Southampton Biomedical Research Centre and Senior Clinical Lead of the National Respiratory Audit Programme. His research seeks to improve understanding of the mechanisms which drive susceptibility to respiratory infections and exacerbations in patients with chronic lung disease, and to develop new vaccines and therapies to impact on these. Tom has taken these mechanistic discoveries through translation into new treatments in COPD and COVID-19.
He is co-founder and Chairman of the health technology company mymhealth which is the leading provider of digital therapeutics to the NHS in asthma and COPD. He has published over 200 peer reviewed papers and reviews on the topics of airways disease, infection and airway immunology. In 2023 he was appointed as an NIHR Senior Investigator in recognition of his contribution to respiratory research.
This activity is intended for pulmonology, critical care medicine, infectious disease, and clinical immunology/allergy specialists who manage patients with severe viral lower respiratory tract disease, COPD, or other respiratory disorders.
This live dinner symposium features expert panelists Dr. Jonathan M. Hand, Professor Dave Singh, and Professor Tom Wilkinson, who will explore the role of IL-33 in lower respiratory tract diseases and offer their insights on the latest advancements in treatment strategies that target its mechanisms. Agenda topics to be covered include the dual biologic function of IL-33 as both an inflammatory mediator and regulator of gene expression, IL-33 signaling pathways, implications of its dysregulation on the pathology of respiratory disease, rationale for targeting IL-33 for treatment development, and ongoing clinical investigations of biologic agents that inhibit IL-33. Interactive discussions and Q&A session will provide further opportunities for participant engagement and learning.
After completing this activity, the participant should be better able to:
• Describe IL-33 signaling and its pathophysiologic roles in COPD, severe viral lower respiratory tract disease, and other respiratory diseases
• Discuss the mechanisms of action, clinical profiles, and published evidence for emerging biologic therapies targeting IL-33 for respiratory diseases
• Incorporate emerging biologic therapies into practice for patients with COPD, severe viral lower respiratory tract disease, or other respiratory diseases as they become available
6:30 pm Registration and Dinner
7:00 pm Preactivity Questionnaire and Faculty Introductions
7:10 pm An Introduction to IL-33 Signaling
7:25 pm IL-33 and Respiratory Diseases
7:50 pm New Strategies to Target IL-33 Signaling in Respiratory Diseases
8:15 pm Postactivity Questionnaire and Q&A Session
Integritas Communications is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Integritas designates this live activity for a maximum of 1.5 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
For more information about the approval of this program, please contact Integritas at info@exchangecme.com.
In order to receive credit for this activity, the participant must attend the live symposium/live stream and complete the posttest and program evaluation.
There is no fee for this educational activity.
Integritas adheres to the policies and guidelines, including the Standards for Integrity and Independence in Accredited CE, set forth to providers by the Accreditation Council for Continuing Medical Education (ACCME) and all other professional organizations, as applicable, stating those activities where continuing education credits are awarded must be balanced, independent, objective, and scientifically rigorous. All persons in a position to control the content of an accredited continuing education program provided by Integritas are required to disclose all financial relationships with any ineligible company within the past 24 months to Integritas. All financial relationships reported are identified as relevant and mitigated by Integritas in accordance with the Standards for Integrity and Independence in Accredited CE in advance of delivery of the activity to learners. The content of this activity was vetted by Integritas to assure objectivity and that the activity is free of commercial bias.
All relevant financial relationships have been mitigated. The faculty have the following relevant financial relationships with ineligible companies:
Jonathan M. Hand, MD
Consulting Fees (e.g., advisory boards): AstraZeneca, Pfizer, Innoviva; Contracted Research: Pfizer, Janssen, Ferring, Scynexis, AstraZeneca
Professor Dave Singh MD, FERS, FBPhS
Consulting fees (e.g. advisory boards): Adovate, Aerogen, Almirall, Apogee, Arrowhead, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, CONNECT Biopharm, Covis, CSL Behring, DevPro Biopharma LCC, Elpen, Empirico, Epiendo, Genentech, Generate Biomedicines, GlaxoSmith Kline, Glenmark, Kamada, Kinaset Therapeutics, Kymera, Menarini, MicroA, OM Pharma, Orion, Pieris Pharmaceuticals, Pulmatrix, Revolvo, Roivant Sciences, Sanofi, Synairgen, Tetherex, Teva, theravance, Biopharma, Upstream and Verona Phar
Professor Tom Wilkinson MA Cantab MBBS PhD FRCP FERS
Consulting Fees (e.g., advisory boards): Fees from AZ, Synairgen, Enanta, Tidalsense, Jannssen, Sanofi, Biomerieux GSK; Contracted Research: Sanofi, Jannssen, Biomerieux, GSK, AZ, Syniairgen; Ownership Interest: Stock Options (or other ownership interest excluding diversified mutual funds): Tidalsense
The planners and managers have no relevant financial relationships with ineligible companies.
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the US Food and Drug Administration. Integritas does not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed
in this activity should not be used by clinicians without evaluation of patient conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Any activity registrant who feels s/he may need accommodation based on the impact of a disability should contact Integritas Communications at info@exchangecme.com to discuss their specific needs.
For information about this program, please contact us at info@exchangecme.com.





































» Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2025 report.
https://goldcopd.org/2025-gold-report/
» Global Strategy for Asthma Management and Prevention.
Global Initiative for Asthma, 2025. https://ginasthma.org/wp-content/uploads/2025/05/GINA-StrategyReport_2025-WEB-WMS.pdf
» Coronavirus Disease 2019 (COVID-19) Treatment Guidelines.
National Institute of Health (NIH).
https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_ NBK570371.pdf
» Interleukin-33 (IL-33): a critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine.
Cayrol C, Girard JP. Cytokine. 2022;156(155981):1-15. https://pubmed.ncbi.nlm.nih.gov/35640416/
» A large-scale, consortium-based genomewide association study of asthma.
Moffatt MF, et al. N Engl J Med. 2010;363(13):1211-1221. https://pubmed.ncbi.nlm.nih.gov/20860503/
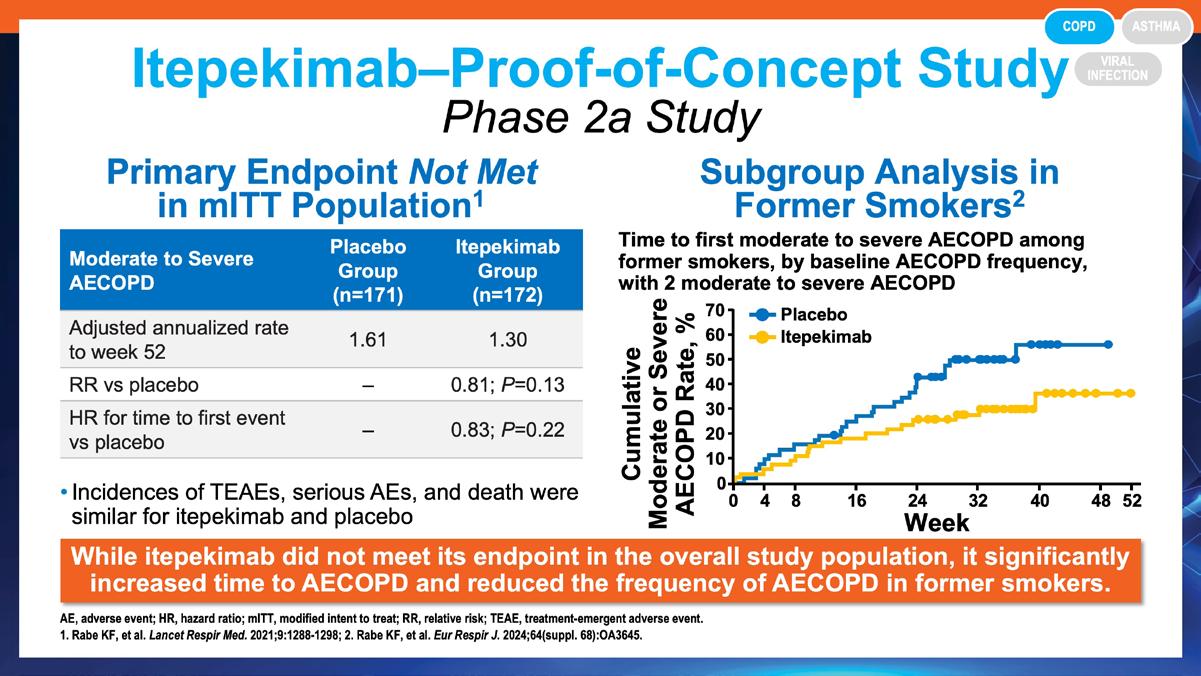
» Reduction in exacerbations with itepekimab in former smokers with chronic obstructive pulmonary disease (COPD) by prior exacerbation frequency.
Rabe KF, et al. Eur Respir J. 2024;64(suppl 68):OA3645. https://publications.ersnet.org/content/erj/64/suppl68/oa3645
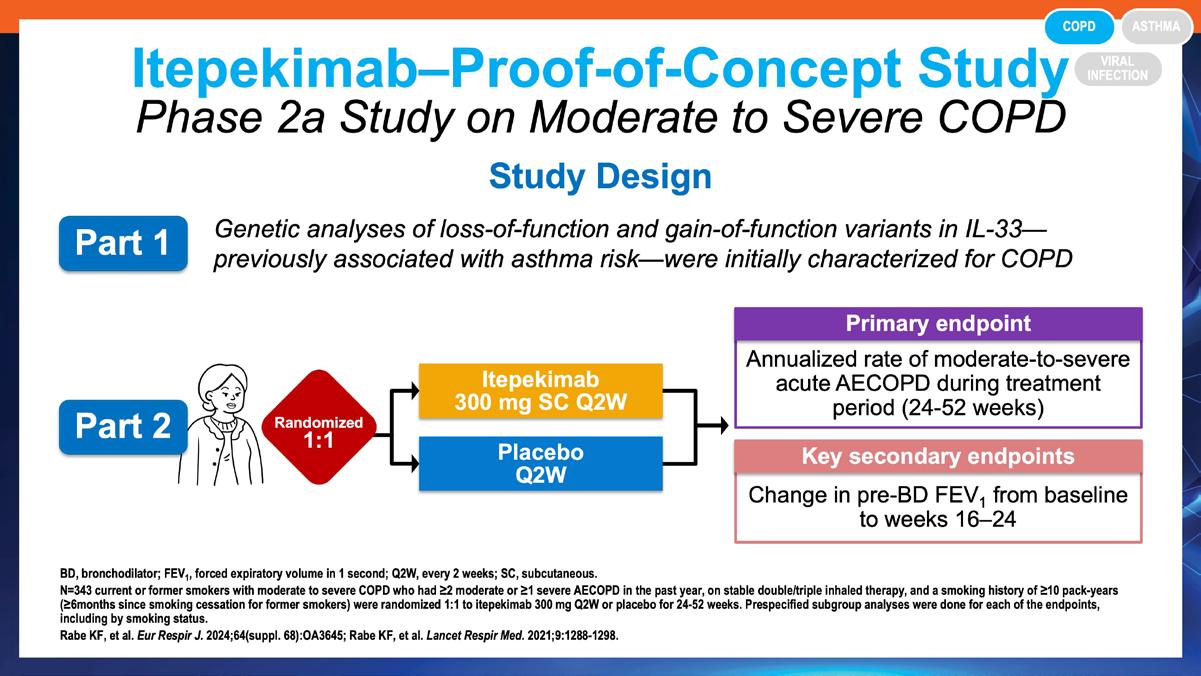
» Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial.
Rabe KF, et al. Lancet Respir Med. 2021;9(11):1288-1298. https://pubmed.ncbi.nlm.nih.gov/34302758/
» Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD
Calderon AA, et al. Eur Respir Rev. 2023;32(167):220144. https://pubmed.ncbi.nlm.nih.gov/36697211/
» Involvement of IL-33 in the pathogenesis and prognosis of major respiratory viral infections: future perspectives for personalized therapy.
Murdaca G, et al. Biomedicines. 2022;10(3):715. https://pmc.ncbi.nlm.nih.gov/articles/PMC8944994/
» IL-33 and the cytokine storm in COVID-19: from a potential immunological relationship towards precision medicine.
Furci F, et al. Int J Mol Sci. 2022;23(23):14532. https://pubmed.ncbi.nlm.nih.gov/36498859/
» The paradoxical effect of IL-6 and implications for the use of tocilizumab in COVID-19 patients.
Bruzzese V, Lazzarino AI. Med Hypotheses. 2020;144:110284.
https://pubmed.ncbi.nlm.nih.gov/33245278/
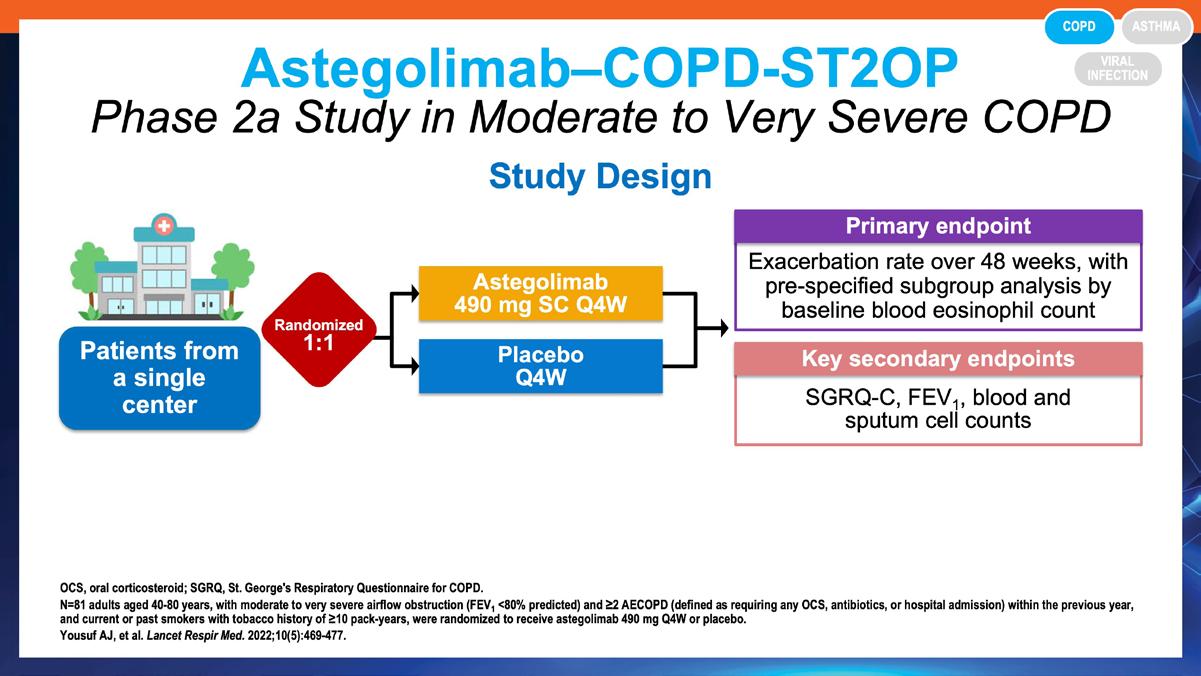
» Astegolimab, an anti-ST2, in chronic obstructive pulmonary disease (COPD-ST2OP): a phase 2a, placebo-controlled trial.
Yousuf AJ, et al. Lancet Respir Med. 2022;10(5):469-477.
https://pubmed.ncbi.nlm.nih.gov/35339234/
» A phase 2a trial of the IL-33 mAb tozorakimab in patients with COPD: FRONTIER-4.
Singh D, et al. Eur Respir J. 2025;2402231. Epub ahead of print. https://publications.ersnet.org/content/erj%3A%3A%3Aearly%3A%3A% 3A2025%3A%3A%3A03%3A%3A%3A28%3A%3A% 3A13993003.02231-2024.full.pdf
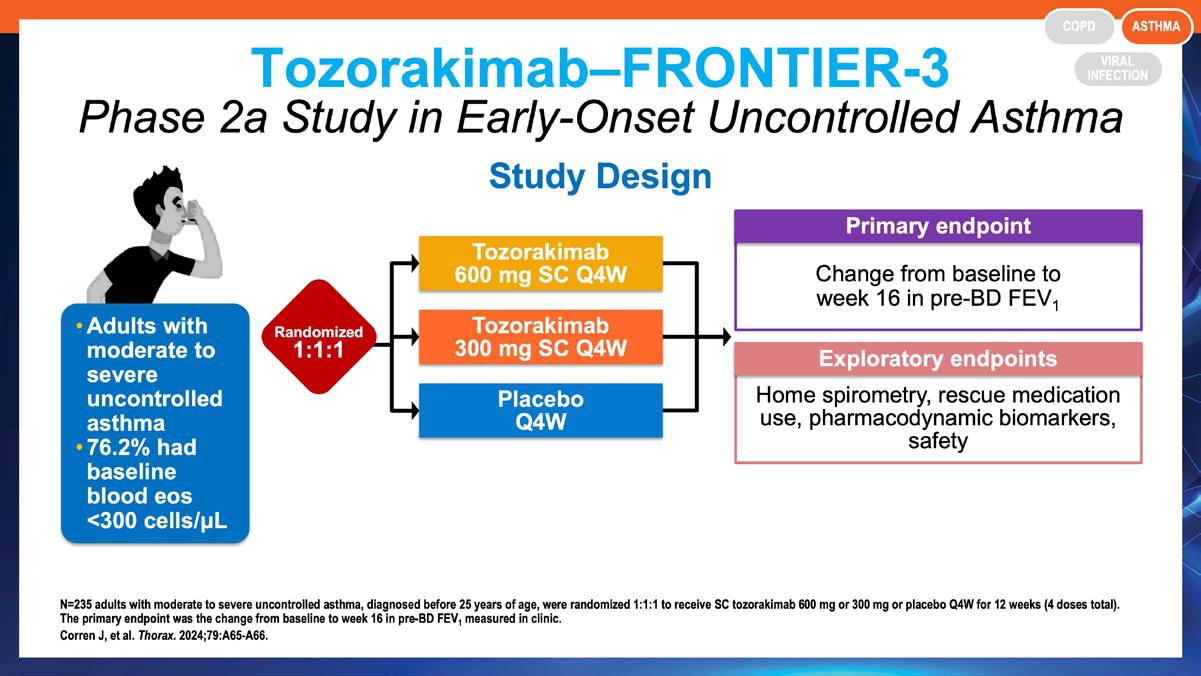
» S90 FRONTIER-3: a randomized, phase 2a study to evaluate the efficacy and safety of tozorakimab (an anti-interleukin-33 monoclonal antibody) in early-onset asthma.
Corren J, et al. Thorax. 2024;79:A65-A66. https://thorax.bmj.com/content/79/Suppl_2/A65.2
» A randomized phase 2a study to investigate the effects of blocking interleukin-33 with tozorakimab in patients hospitalized with COVID-19: ACCORD-2. Wilkinson T, et al. ERJ Open Res. 2023;9(5):00249-2023. https://pubmed.ncbi.nlm.nih.gov/37868151/







Clinical Resource Centers on various therapeutic areas


Live streaming of symposia at major medical conferences



