







• There are 1.2 million PLWH in the US1
• 1 in 8 people with HIV in the US do not know they have it1
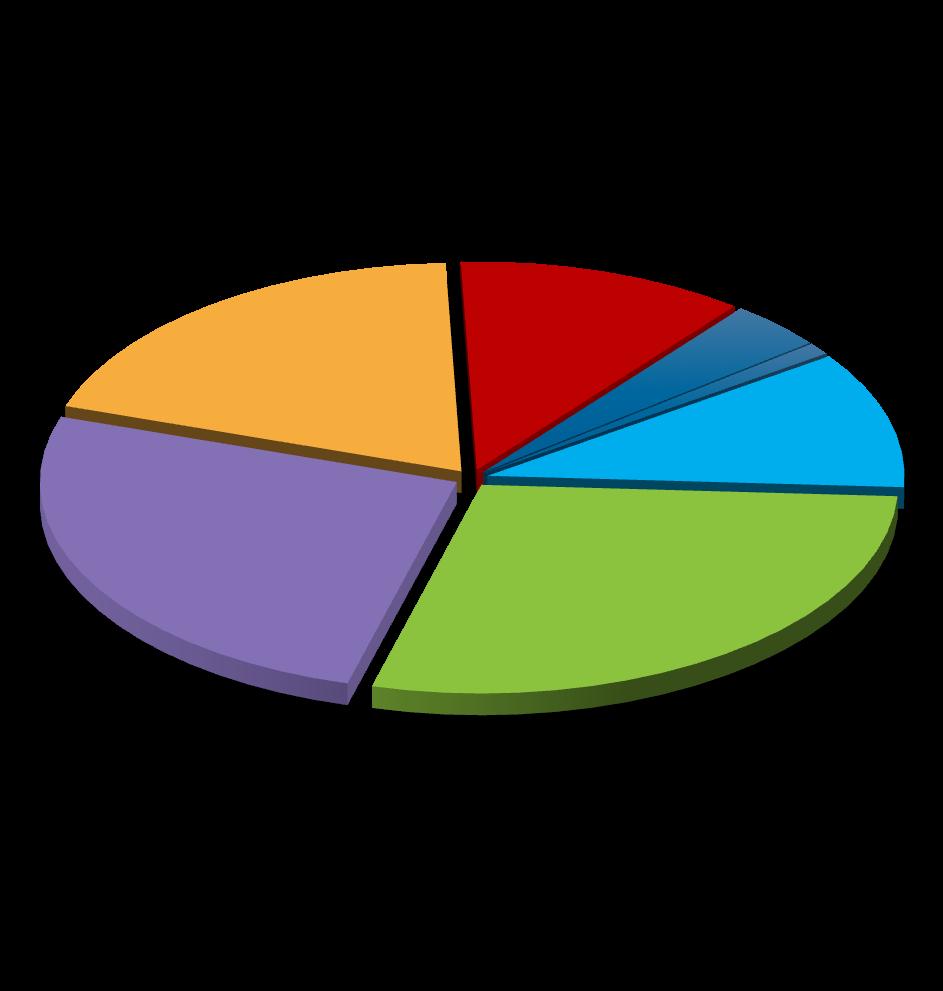
• Of the approximately 39,000 new HIV diagnoses in 20232: – 80% were among men
66% were among MSM, the population most affected by HIV
60% were among people aged 25 to 44 years
6% were among PWID

Areas: New Diagnoses Rate per 100K, 20223
diagnoses-deaths-and-prevalence-2025.html. Accessed July 4, 2025; 3. AIDSVu. https://map.aidsvu.org/prev/state/rate/none/none/usa?geoContext=national. Accessed July 4, 2025. Some data on HIV incidence and PrEP provision are no longer being updated in the US.

• Short-acting fentanyl leads to quicker onset of withdrawal symptoms and, thus, more injection events/day2
• This means higher probability of exposure to HIV2
• Greater need for sterile injection equipment2,b

IDU, injection drug use.
aUnited States and 6 territories and freely associated states; data have been statistically adjusted to account for missing tr ansmission category. Male-to-male sexual contact includes persons assigned male sex at birth, regardless of current gender identity, who have had sexual contact with














• New HIV diagnoses among women
– 18% of new HIV diagnoses were among women in 20221
– 84% acquired from heterosexual transmission; 16% acquired from IDU1
• Black women in the US
– Have 18 times the AIDS rate of White women2
– Are nearly 7 times more likely to die from HIV infection complications as are White women2
– Are less likely to have been infected through IDU than White women3
1. CDC. https://stacks.cdc.gov/view/cdc/156509. Accessed July 4, 2025; 2. US Department of Health and Human Services Office of Minority Health. https://minorityhealth.hhs.gov/hivaids-and-blackafrican-americans. Accessed July 4, 2025; 3. Kaiser Family Foundation. https://www.kff.org/hivaids/fact-sheet/blackamericans-and-hivaids-the-basics/. Accessed July 4, 2025.

A person without HIV has a
for acquiring HIV (receptive anal sex is #1) every time they use a needle that has been used by someone with HIV
SUD may increase the risk of getting HIV through sex as well
• Clients under the influence are more likely to engage in higher-risk behaviors for acquiring HIV such as:
– Anal or vaginal sex without a condom
– Having sex with multiple partners
– Trading sex for money or drugs
SUD, substance use disorder.
CDC. Injection drug use and HIV risk. https://iwantthekit.org/wp -content/uploads/2021/03/hiv-pdf.pdf. Accessed August 27, 2025.
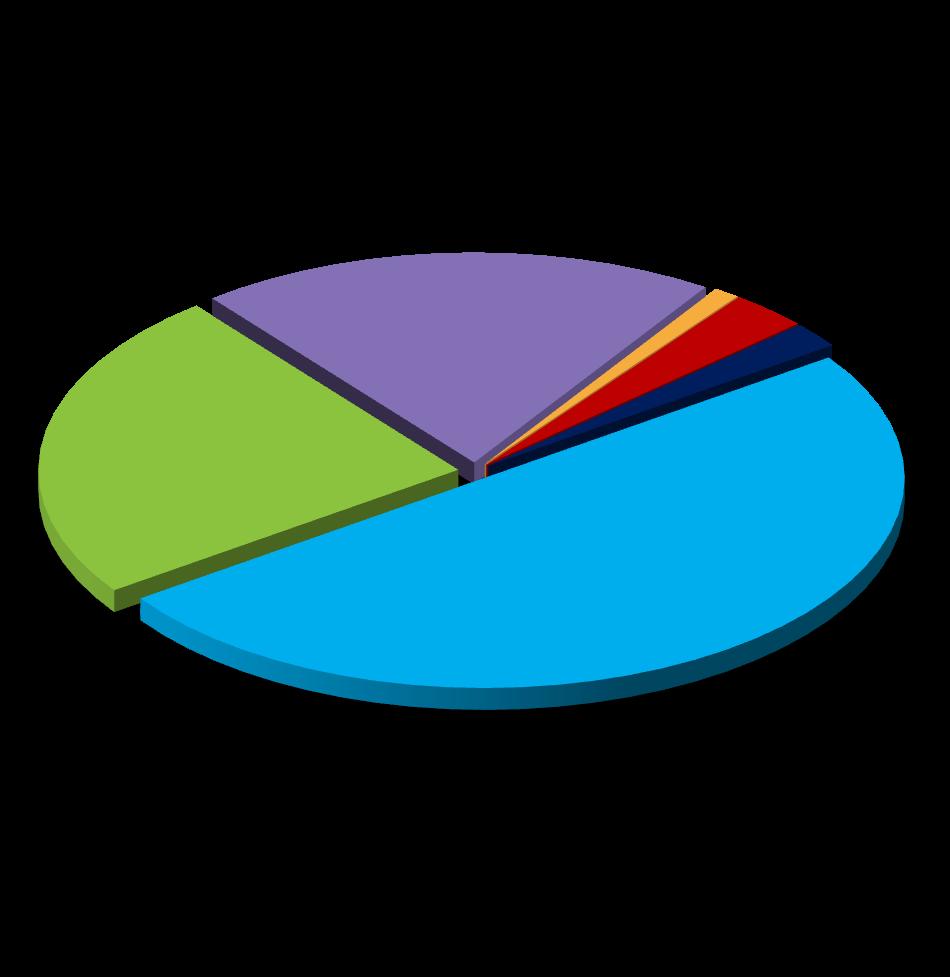
Chlamydia, gonorrhea, and syphilis cases have been increasing for years1

Syphilis, 290,253 cases Gonorrhea, 601,319 cases Chlamydia, 1.6 million cases
• Having an STI such as chlamydia, gonorrhea, and/or syphilis places people at higher risk for acquiring HIV
• 6% of sexually acquired HIV infections are attributed to chlamydia, gonorrhea, and syphilis
• HIV, substance use, and viral hepatitis affect similar populations as STIs
• Opioids and other substance use is linked to increasing STIs and outbreaks of infectious diseases1
aNote: 2021 and 2022 data reflect the effect of COVID-19 on STD surveillance trends. 1. CDC. STI fact sheet. https://www.cdc.gov/sti/media/pdfs/2024/11/syndemic-infographic-11-08-2024.pdf. Accessed July 10, 2025; 2. CDC. https://www.cdc.gov/stistatistics/annual/summary.html. Accessed July 14, 2025.

People whose HIV tests are negative are offered powerful prevention tools such as PrEP, condoms, harm reduction (eg, SSPs), and supportive services to stay HIV negative.

People whose HIV tests are positive enter primary care and are offered effective treatment and supportive services to achieve and maintain viral suppression.
The
SSP, syringe services program.
CDChttps://stacks.cdc.gov/view/cdc/129024/cdc_129024_DS1.pdf. Accessed July 10, 2025.

• SUD treatment centers are a primary access point for testing and receiving HIV-related services1
• A study of SUD centers showed that they were the most frequent location of participants’ last HIV test2 1. Aletraris L, Roman PM. J Subst Abuse Treat. 2015;57:1-8; 2. Kyle TL, et al. AIDS Behav. 2015;19(3):536-542.


Decrease in HIV transmission among PWID participating in needle and syringe programs
% Of PWID are less likely to engage in injection behavior exposing them to HIV if involved in HIV prevention programs that include motivational interviewing

Reduction in HIV acquisition among participants in MOUD
Decrease in equipment sharing among PWID participating in peer-based interventions


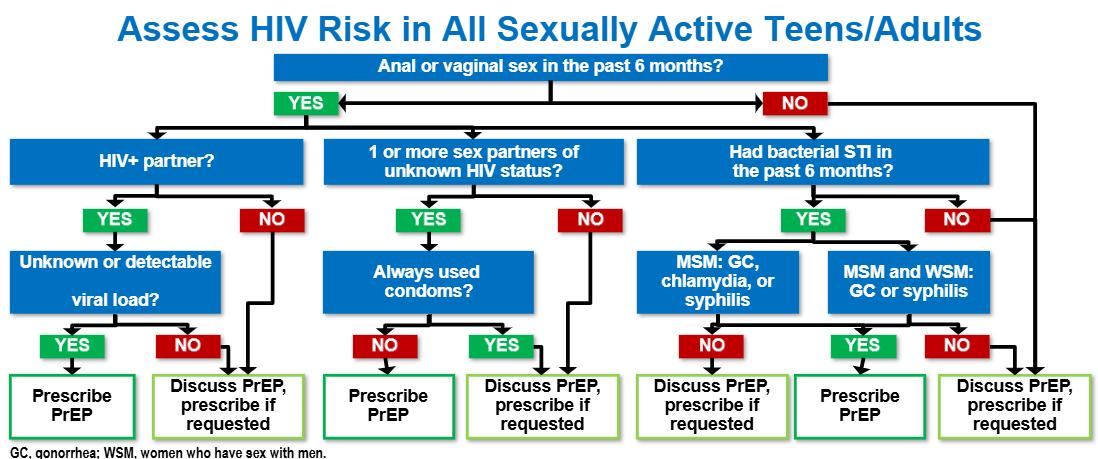
Who should be tested?1
• CDC: All adults and teens aged 13 to 64 years should be tested at least once in routine health care
• All pregnant persons
• Tested once a year (some up to every 3 months):
– MSM or transgender clients
– PWID who share equipment
• Not all injected drug use is illegal; may be a regular medication, like hormone injections
– Those performing “survival sex” for drugs or money
– Incarcerated
– Current STI, hepatitis, or TB
– Partner with HIV or ≥1 partner since last HIV test
– Those having sex without condoms

How should testing occur?1,2
• Opt-out testing
• Normalize testing
• The CDC recommends initial testing with an FDA-approved Ag/Ab assay; no oral tests
Ab, antibody; Ag, antigen; FDA, US Food and Drug Administration; TB, tuberculosis. 1. CDC. https://www.cdc.gov/hiv/testing/. Accessed July 10, 2025; 2. CDC. https://www.cdc.gov/hivnexus/hcp/diagnosis-testing/. Accessed July 10, 2025.


• Discuss PrEP and offer it to:
– All sexually active persons
– All persons requesting PrEP, and
– Anyone who injects nonprescription drugs, uses substances (alcohol, stimulants, opioids), or who has a SUD

Toss the old algorithms!
Screening tools and criteria for sexual or drug use behavior are not required to offer PrEP.
Gandhi RT, et al. JAMA. 2025;333(7):609-628.

• Engage in substance use
• Are pregnant or planning to conceive
• Are breast/chest feeding
• Inconsistently use condoms or other risk-reduction methods
• Have mental health disorders of any severity
• Experience intimate partner violence
• Have unstable housing or limited social support
• Have recently had an STI
• Have a partner with HIV who has an undetectable viral load
• Any patient requiring HIV/STI screening should be offered 3-site testing1
• 3-site STI screening for chlamydia and gonorrhea (GC)
– Swab oropharynx and rectum and test urine:
• Genital testing with a swab is preferred for patients with a vagina, but urine is acceptable
• Patients can self-swab all sites
• GC/Chlamydia often missed with urine/genital testing only
• Blood tests:
– HIV-1/2 Ag/Ab blood test (preferred) or a rapid, point-of care, FDA-approved, fingerstick Ag/Ab blood test2
– HIV RNA (viral load) test with a lower limit of quantification of 50 copies/mL or lower3
– Syphilis serology1

1. Workowski KA, Bolan GA. MMWR Recomm Rep. 2015;64(RR-03):1-137; 2. CDC. https://stacks.cdc.gov/view/cdc/112360. Accessed July 10, 2025; 3. Gandhi RT, et al. JAMA. 2025;333(7):609-628.

• Providers should counsel all gay, bisexual, and other MSM and transgender women (TGW) with a history of at least 1 bacterial STI (syphilis, chlamydia, or gonorrhea) during the past 12 months about the benefits and harms of using doxy PEP and should offer it through shared decision-making
– Doxycycline (any formulation) 200 mg once within 72 hours (not to exceed 200 mg per 24 hours) of oral, vaginal, or anal sex
– Ongoing need for doxy PEP should be assessed every 3 to 6 months
• No recommendation on the use of doxy PEP for cisgender women, cisgender heterosexual men, transgender men, and other queer and nonbinary persons

Bachmann LH, et al. MMWR Recomm Rep. 2024;73(2):1-8.

aDoxy PEP vs standard of care; all P<0.0001. In this open-label randomized study, N=501 MSM and transgender women aged ≥18 years who were taking PrEP for HIV prevention (PrEP cohort) or living with HIV infection (PLWH cohort) and who had had Neisseria gonorrhoeae (gonorrhea), Chlamydia trachomatis (chlamydia), or syphilis in the past year were randomly assigned 2:1 to take 200 mg of doxycycline within 72 hours after condomless sex (doxy PEP) or receive standard care without doxycycline. STI testing was performed quarterly. The primary end point was the incidence of at least 1 STI per follow-up quarter. Luetkemeyer AF, et al. N Engl J Med. 2023;388(14):1296-1306.

• Partner services programs1,2
– Help newly diagnosed clients notify their sexual and drug injection partners of their potential exposure and provide counseling, testing, and referral to treatment and other services
– Referral methods1,2








• Expedited partner therapy3
– Providing prescriptions or medications to a patient diagnosed with chlamydia or gonorrhea to take to their sex partner (without a health care provider first examining the sex partner)
• If a patient has a positive HIV test1,2
– Refer them immediately to an HIV treatment provider to start antiretroviral therapy (ART)
CDC. https://www.cdc.gov/hivnexus/hcp/partner-services/. Accessed July 12, 2025; 2. Task Force on Community Prevention Services. Am J Prev Med. 2007;33(2 suppl):S88; 3. CDC. https://www.cdc.gov/sti/hcp/clinical-guidance/expedited-partner-therapy.html. Accessed July 12, 2025. 15% of partners tested by Partner Services were positive for HIV and previously undiagnosed. Health department tells partners Patient tells partners Health department and patient tell partners


PrEP is a comprehensive set of services to reduce risk of HIV infection1
• 4 FDA-approved medications for PrEP1,2 – May only be used in persons without HIV
• Comprehensive services include – Regular HIV screening – Regular STI screening




Oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC; Truvada®); available in generic
1 pill taken daily

Oral tenofovir alafenamide/emtricitabine (TAF/FTC; Descovy®); may be used in people at risk of HIV through vaginal receptive sex if TDF/FTC is contraindicated or undesirable2
1 pill taken daily
(Truvada)

TDF/FTC Efficacy in PWID1 • When compared to TDF/FTC, studies showed: – TAF/FTC was not worse than TDF/FTC2 – CAB was statistically superior to TDF/FTC3,4 CAB, cabotegravir. 1. Choopanya K, et al. Lancet. 2013;381(9883):2083-2090; 2. Mayer KH, et al. Lancet. 2020;396(10246):239-254; 3. Landovitz RJ, et al. N Engl J Med. 2021;385(7):595-608;


• Recent recommendations by IAS-USA for LAI PrEP use in PWID who have sexual exposures1,2
– CAB LAI (Apretude®)3
• 1 IM gluteal injection every 2 months
– LEN LAI (Yeztugo®)4
• 2 SQ injections in abdomen or upper thigh every 6 months
• Nodules (also called drug depots) form under the skin and slowly elute the drug over time

1
initiation dose
initiation dose
DAY 1
Initiation injections 927 mg as 2×1.5-mL SQ injections and 600 mg orally as 2×300-mg tablets
IAS, International Antiviral Society; LEN, lenacapavir; IM, intramuscular; SQ, subcutaneous. 1. Gandhi Rt, et al. JAMA. 2025;333(7):609-628; 2. Landovitz RJ, et al. JAMA. 2025. Epub ahead of print; 3. Drugs@FDA. www.accessdata.fda.gov/drugsatfda_docs/label/2025/215499s009lbl.pdf. Accessed July 2, 2025; 4. Drugs@FDA. www.accessdata.fda.gov/drugsatfda_docs/label/2025/220018s000lbl.pdf. Accessed July 4, 2025.
DAY 2
600 mg orally as 2×300-mg tablets
927 mg as 2×1.5-mL SQ injections from the date of the last injection ± 2 weeks EVERY 6 MONTHS
Continuation injections




CAB Shows Higher Efficacy in Prevention of HIV vs TDF/FTC

CAB vs TDF/FTC Efficacy by Patient Subgroup




Participants in the CAB group had an 88% lower risk of HIV infection than those in the TDF/FTC group.
N=3224 patients in sub-Saharan Africa aged 18 to 45 years who were assigned female at birth and reported 2 episodes of vaginal intercourse in the previous 30 days, were at risk of HIV infection based on an HIV risk score, and agreed to use a long -acting reversible contraceptive method were randomly assigned 1:1 to CAB LAI or TDF/FTC. Delaney-Moretlwe S, et al. Lancet. 2022;399:1779-1789.
Incidence Rate Ratio Comparing HIV Incidence in LEN LAI and TAF/FTC Groups With Background HIV Incidence
Background HIV Incidence and HIV Incidence in LEN LAI, TAF/FTC, and TDF/FTC Groups




LEN LAI reduced HIV incidence by 100% and was statistically superior to TAF/FTC in preventing HIV infection among cisgender women.
P<0.001. N=5338; double-blind controlled trial randomly assigned 2:2:1 to LEN LAI at 927 mg in two 1.5 -mL injections SQ every 26 weeks, or daily oral TAF/FTC, or daily oral TDF/FTC and matching placebo in cisgender girls and young women ages 16 to 25 years, for up to 104 weeks. Bekker LG, et al. N Engl J Med. 2024;391(13):1179-1192.



oral TDF/FTC and matching placebo for up to 104 weeks. Kelley CF, et al. N Engl J Med. 2025;392(13):1261-1276. Incidence
aP=0.002; bincidence rate ratio, 0.04, (95%CI, 0.01 to 0.18, P<0.001); cincidence rate ratio, 0.11, (95% CI, 0.02 to 0.51, P=0.002). N=3265; double-blind active-controlled trial randomly assigned cisgender gay, bisexual, and other men, transgender women, transgender men, and gender nonbinary persons at least 16 years of age 2:1 to LEN LAI at 927 mg in two 1.5 -mL injections SQ every 26 weeks or

Other Considerations
Side Effects5,9 Diarrhea (6%); nausea (5%) Diarrhea (5%); nausea (4%)
Injection-site reaction (32%-81%); mostly mild and greatest initially
Injection site reaction (69%-83%); mostly mild (grade 1, 50%-66%) or moderate and greatest initially; injection site nodule (63%-64%)
aAlthough CAB LAI and LEN LAI are not indicated to reduce the risk of HIV acquisition in persons at risk through IDU, per the IAS-USA, they are recommended as PrEP options for PWID who have sexual exposures; bPer recommendations from the IAS-USA, TAF/FTC may be used for prevention of HIV acquisition from vaginal exposures for those in whom TDF/FTC is contraindicated or undesirable, but TAF/FTC does not have this indication in the prescribing information. 1. Drugs@FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/021752s064lbl.pdf. Accessed July 10, 2025; 2. Gandhi M, et al. Lancet HIV. 2016;3(11):e521-e528; 3. Drugs@FDA. www.accessdata.fda.gov/drugsatfda_docs/label/2025/215499s009lbl.pdf. Accessed July 10, 2025; 4. Drugs@FDA. www.accessdata.fda.gov/drugsatfda_docs/label/2025/208215s023s025lbl.pdf. Accessed July 10, 2025; 5. NYSDOH AIDS Institute. https://www.hivguidelines.org/guideline/hiv-prep/. Accessed July 10, 2025; 6. CDC. https://stacks.cdc.gov/view/cdc/112360. Accessed June 4, 2024; 7. Shah S, et al. AIDS. 2021;35(suppl 2):S189-S195; 8. Wood BR, Huhn GD. Open Forum Infect Dis. 2021;8(12):ofab542; 9. Drugs@FDA. www.accessdata.fda.gov/drugsatfda_docs/label/2025/220018s000lbl.pdf. Accessed July 10, 2025; 10. Landovitz RJ, et al. JAMA. 2025. Epub ahead of print; 11. Gandhi RT, et al. JAMA. 2025;333(7):609-628.

1. CDC. https://stacks.cdc.gov/view/cdc/112360. Accessed July 4, 2025; 2. Shaik JS, et al. Br J Clin Pharmacol. 2022;88(4):1667-1678; 3. CDC. https://www.cdc.gov/hiv/prevention/prep.html. Accessed July 4, 2025.
• MUST meet all the following criteria:
q Negative rapid whole blood HIV point-of-care test on day of assessment
q Agrees to have all lab tests collected the day of assessment per PrEP protocol
q No self-reported signs or symptoms of acute HIV
q No self-reported history of chronic liver disease (eg, chronic HBV)
q No self-reported history of chronic kidney disease
q No findings suggestive of acute HIV on a pointed physical examination
q Confirmed review of prescribed, over-thecounter, and herbal medications for significant drug interactions prior to dispensing PrEP
• From here:
1. Standard that all labs be reviewed and acted upon within 72 hours of being reported by the lab
2. Appropriate full lab evaluation is done (HBV sAg/HBV sAb/HBV Core Ab, HIV, CMP, CBC, etc)
3. If criteria met, a 30-day supply of TDF/FTC (M or F) or TAF/FTC (M) is prescribed, with NO refills
4. MUST be able to contact the client
5. No LAI is offered for rapid PrEP

CBC, complete blood count; CMP, comprehensive metabolic panel; F, female; HBV, hepatitis B virus; M, male; sAb, surface antib ody; sAg, surface antigen. Gandhi RT, et al. JAMA. 2025;333(7):609-628.
• On-demand (also called event-driven or 2:1:1) dosing1
– Taken at specific time points only around times of anal intercourse
– For adult MSM only! [86% efficacy]
– Not for PWID or those having vaginal sex
• Effective HIV prevention for MSM with infrequent sexual encounters and an alternative to daily Truvada1
• Not FDA approved, included in the CDC and other US and WHO guidelines1,2
• There may be some clients who use substances to enhance their sexual experiences and use clean equipment; on-demand dosing is a viable option for these clients





Test/Prescription TDF/FTC (Truvada® ) and TAF/FTC (Descovy® )
HIV Status
• HIV testing
• Discuss whether continued need for PrEP; adherence, side effects, etc
Kidney Status

At start and every 3 months
At start and every 12 months
STI Screening At start and every 3-6 months
Cholesterol/Triglycerides Screen
Only for persons prescribed Descovy; at start and every 12 months
Hepatitis Screen (HBV) At start
Pregnancy Test For women of childbearing potential (suggested but no longer in guidelines)
• 30- or 90-day supply TDF/FTC OR
Prescription
CDC. https://stacks.cdc.gov/view/cdc/112360. Accessed July 10, 2025.
• 30- or 90-day supply TAF/FTC (for MSM and TGW only) OR
• 30-day supply TDF/FTC for on-demand regimen (for MSM and TGW only)
HIV Status
• HIV testing
• Discuss whether continued need for PrEP; adherence, side effects, etc
Kidney Status

At start, at 1 month, then every 2 months At start, then every 3 months
STI Screening3,4 At start and every 2-6 months At start and every 3 months
Cholesterol/Triglycerides Screen
Hepatitis Screen (HBV)
Pregnancy Test
Prescription
For women of childbearing potential (suggested but no longer in guidelines)
• CAB LAI injection at start and again 1 month later OR
• CAB daily oral pill for 4 weeks at start, CAB LAI injection 1 month later, another CAB LAI injection 1 month later
• Thereafter, CAB LAI is given every 2 months
• LEN LAI injections on day 1 plus oral tablets; second dose of oral tablets on day 2
• Thereafter, LEN LAI is given every 6 months
1. Drugs@FDA. www.accessdata.fda.gov/drugsatfda_docs/label/2025/215499s009lbl.pdf. Accessed July 2, 2025; 2. Drugs@FDA. www.accessdata.fda.gov/drugsatfda_docs/label/2025/220018s000lbl.pdf. Accessed July 4, 2025; 3. CDC. USPHS. Update. https://stacks.cdc.gov/view/cdc/112360. Accessed July 10, 2025; 4. Landovitz RJ, et al. JAMA. 2025. Epub ahead of print.

• Contraindicated in individuals receiving:
– The anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, and phenytoin
– The antimycobacterials rifampin and rifapentine
• Dosage adjustment is needed for those taking the antimycobacterial rifabutin


• Not recommended for individuals receiving combined P-gp, UGT1A1, and strong CYP3A inhibitors
• Dosage adjustment is needed for those initiating strong and moderate CYP3A inducers, such as carbamazepine, dexamethasone, phenobarbital, phenytoin, rifamycins, and St. John’s wort CYP, cytochrome P450; P-gp, permeability glycoprotein; UGT, uridine diphosphate glucuronosyltransferase. 1. Drugs@FDA. www.accessdata.fda.gov/drugsatfda_docs/label/2025/215499s009lbl.pdf. Accessed July 2, 2025; 2. Drugs@FDA. www.accessdata.fda.gov/drugsatfda_docs/label/2025/220018s000lbl.pdf. Accessed July 4, 2025; 3. University of Liverpool. https://www.hiv-druginteractions.org/checker. Accessed August 20, 2025. The University
Study of Awareness of and Willingness to Take PrEP Among PWID in Los Angeles and San Francisco, CA, 2016-20181,a


• Only 40% were aware of PrEP

– PrEP awareness was associated with study site, sexual minority status, higher educational attainment, and HIV testing in the last 6 months
• 59% were willing to take PrEP
– Willingness to take PrEP was associated with self-reported risk (sharing drug paraphernalia and being injected by another PWID) and perceived HIV risk
• Only 2% were currently taking PrEP
• Most commonly perceived barriers:
– Co-pays
– Concerns about increase in HIV or sexually transmitted risk with PrEP
– Concerns about reduction of medication efficacy without daily use

Study of Awareness of and Willingness to Use LAI PrEP Among People Who Use Drugs (PWUD) in Connecticut, 2018-20192,b
40% were aware of PrEP, but upon learning about it, 59% expressed willingness to use it.

• Only 26% were aware of LAI PrEP

• 74% were willing to use LAI PrEP
– Willingness to take LAI PrEP was independently correlated with female sex, recent visit to health care provider, high self-perceived risk of acquiring HIV, and having previously taken oral PrEP
• Most common concerns related to LAI PrEP:
– Long-term side effects
– Efficacy over time
– Incomplete protection
– Cost
– Fear/Dislike of needles
– Having to return to clinic for injection of LAI-PrEP

26% were aware of LAI PrEP, but upon learning about it, 74% expressed willingness to use it. Female participants were significantly more willing to use LAI PrEP than were male participants.
aN=469 self-reported HIV-negative participants who had injected drugs within the past 6 months were interviewed; N=234 adults with self-reported HIV-negative or HIV status unknown who had reported drug- or sex-related potential exposure to HIV within ≤6 months, met DSM-V criteria for opioid use disorder, were on methadone maintenance treatment, and able to understand, speak, and read English were interviewed.
1. Walters SM, et al. Subst Use Misuse. 2020;55(14):2409-2419; 2. Shrestha R, et al. J Subst Abuse Treat. 2020;117:108058.
• A study of 100 opioid-dependent PWID between December 2020 and July 2022 evaluated persistence on PrEP
– Participants received a free 90-day oral PrEP prescription
– Followed up at 3 and 6 months
– Assessed sociodemographic characteristics, PrEP use, and behaviors related to sex and drug use
– PrEP use evaluated by how many times each participant picked up their PrEP prescription
Number of Participants Picking Up Their Prescription During the 6-Month Follow-up

Times Picked Up At least Once Only Once Twice Three Times
Number of Participants 60 42 16 2
Despite a high willingness to use PrEP among opioid-dependent PWID, adoption and persistence of PrEP were low.
• SUD center goals:
– Equip all staff with information about HIV prevention and resources for referral
– Educate staff regarding PrEP/nPEP indications and requirements for initiation, adherence, and monitoring
– Engage all relevant SUD center staff and stakeholders to increase utilization of PrEP and decrease new HIV infections
– Advocate for PWID regarding referrals to external sites
• Emergency departments, and medical specialist offices may not embrace harm-reduction principles
• May further traumatize clients

Talk to all clients about how PWID are at increased risk for HIV from sharing needles/works
Conduct opt-out HIV testing
Educate all clients about PrEP/nPEP to reduce the risk of acquiring HIV
Refer all clients who are clinically eligible for PrEP and those interested in PrEP to local health care providers to initiate PrEP
Discuss the importance of adherence to all clients taking PrEP

• Use individualized strategies to support PrEP continuity of care and monitoring1
• Promote PrEP as a tool for empowerment; continuity of medical care may lead to a sense of bodily autonomy1
• PrEP use may be episodic, as individuals can start and stop PrEP based on fluctuations in risk1
• Providing gender-affirming care to transgender individuals can increase their engagement in PrEP care1
– TDF/FTC does not lower estrogen levels1
• Addressing this directly with TGW may improve willingness to take/adhere to PrEP
• Available data suggest that TAF/FTC, CAB, and LEN do not reduce estrogen levels

• Normalize discussions on sexual health
• Open, nonjudgmental discussions about risk behaviors will:
– Reduce stigma and mistrust
• Become the client’s ally
• End silence about risky behaviors
• Acknowledge vaccines/daily medication/injections may bring on feelings of governmental mistrust in some clients
– Reveal HIV risk factors during counseling or interviewing
• Inaccurate perception of personal risk
• Mistakenly think PrEP is for MSM only CDC. https://stacks.cdc.gov/view/cdc/112360. Accessed July 10, 2025.

Ask all of your clients this 1 assessment question: What are you doing to protect yourself from HIV?


• WWID were offered 24 weeks of oral daily PrEP
• Starting PrEP associated with:
– Access to SSP (aOR=1.85)
– Inconsistent condom use (aOR=3.38)
– Experiencing sexual assault (aOR=5.89)
• At 24 weeks 44% were still in care
– Of those, 88% continued PrEP after the study
– Most preferred getting their PrEP care at the SSP

• Lessons learned
1. Daily PrEP is a good prevention tool for PWID
2. Behavioral interventions important for adherence
3. Helpful that this SSP already had harm reduction services in place (including clinical services)
4. Blood drawing was challenging due to collapsed veins and scarring from drug use
• Focus groups in King County, WA, found:
– PrEP should be part of a menu of HIV prevention options
– Education needed among PWID and providers
– Prescribing PrEP through tele-PrEP, community pharmacy PrEP, and on-demand PrEP may not work for PWID
• 3 potential models:
– SUD center—fixed site addressing a range of services on a walk-in basis
– Mobile outreach—outreach worker-led program engaging with clients in the field
– Add-on to existing service provider, such as SSPs

Corcorran MA. https://aidsetc.org/resource/prep-pwid-challenges-and-opportunities. Accessed July 10, 2025.
Awareness of PrEP Among PWID, %
Only ≈one-third of PWID were aware of PrEP in 2022
Use of PrEP Among PWID, %

• Increase in PrEP awareness was consistent across demographic and behavioral subgroups
• PrEP use was highest among those reporting past-year male-male sex and bacterial STI
• “Efforts to improve PrEP messaging, provider training, and access specifically for PWID may serve to further increase in PrEP awareness and use”
In 2022, only 36% of all patients eligible for PrEP in the US were prescribed it. 2


• Patient and counselor/Provider assistance:
PleasePrEPMe.org
– https://pleaseprepme.org/
– Information for clients and counselors about PrEP, nPEP, insurance and insurance rights, finding a PrEP/nPEP provider, and a list of PrEP resources by state
• Certificate programs and training
– HIV Prevention Certified Provider™ Certification Program
– HIV PrEP Navigation Certification Program
– https://healthhiv.org/

• Patient assistance (without insurance)
– The PrEP patient assistance program will provide medication at no cost for those who meet income requirements
– https://www.gileadadvancingaccess.com/hcp/faq#mail-order
• Uninsured 24/7 support online, by phone during business hours, and fax enrollment:
– https://www.gileadadvancingaccess.com/financial-support/uninsured
• Co-pay assistance/Out-of-pocket costs
– https://www.gileadadvancingaccess.com/copay-coupon-card
– Phone number: 1-877-505-6986
• ViiV Connect
– https://www.viivconnect.com/#hcp


• HIV and other STIs are parts of a syndemic that includes SUD.
• Use a status neutral approach to address HIV testing, prevention, and treatment without stigma.
• SUD centers are uniquely positioned to offer HIV testing and prevention referrals and services.
• Newer PrEP medications offer people more choices for HIV prevention options.