















Professor of Neurology, Columbia University Irving Medical Center
Director, NYS Center of Excellence for Alzheimer’s Disease
Taub Institute for Research on Alzheimer’s Disease and the Aging Brain
Gertrude H. Sergievsky Center (PH19)
Department of Neurology
Columbia University Vagelos College of Physicians & Surgeons
New York, New York
Dr. Lawrence Honig is a Professor of Neurology at Columbia University Irving Medical Center, at the Vagelos College of Physicians and Surgeons in the Department of Neurology, Division of Aging and Dementia, the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, the Gertrude H. Sergievsky Center, and at New York Presbyterian Hospital. He directs the New York State Center of Excellence for Alzheimer’s Disease, codirects the Lewy Body Disease Association Research Center of Excellence as well as the Progressive Supranuclear Palsy Center of Care, and is Deputy Director of the Alzheimer’s Disease Research Center.
Dr. Honig obtained his MD from the University of Miami in Florida, and his PhD from the University of California at Berkeley. He completed his postgraduate internship in Medicine and residency in Neurology, training at Stanford University Medical Center in California. He served on the faculty of the Neurology departments at Stanford University Medical Center and then at the University of Texas Southwestern Medical Center in Dallas, prior to his arrival at Columbia University, where he has been on the faculty since 2000. He is a neuroscientist and board-certified clinical neurologist, with United Council for Neurologic Subspecialties (UCNS) subspecialty certifications in Behavioral Neurology and Neuropsychiatry, and Geriatric Neurology. His clinical specialization focuses on Alzheimer’s Disease, Lewy Body Dementia, Frontotemporal Dementias, Progressive Supranuclear Palsy, Creutzfeldt-Jakob disease, immune-mediated encephalitides, and other disorders of nervous system aging and degeneration. He is a principal investigator on both observational and clinical drug trials for neurodegenerative conditions including Alzheimer’s Disease, Frontotemporal Degeneration, Lewy Body Disease, and Progressive Supranuclear Palsy.

Director, ANA Clinical Trial Center
Abington Neurological Associates
Abington, Pennsylvania
Dr. David Weisman received his BA in philosophy from Franklin and Marshall College in Lancaster, Pennsylvania, then his MD from Penn State College of Medicine, in Hershey. After an internship at St. Mary’s Hospital in San Francisco, California, he completed his neurology residency at Yale School of Medicine in New Haven, Connecticut, where he served as Chief Resident. He then received his fellowship training in Alzheimer’s disease and other dementias at University of California, San Diego. In 2008, Dr. Weisman founded the Clinical Trial Center at Abington Neurological Associates. As the site’s director, Dr. Weisman has conducted numerous clinical trials in mild cognitive impairment and Alzheimer’s disease. The site participated in the first positive later-stage trial supporting a disease-modifying signal in early Alzheimer’s disease. His Alzheimer’s disease trial collaborations have been published in Neurology, New England Journal of Medicine, and Archives of Neurology. Clinically he is a proponent of early diagnosis to take advantage of more effective therapies for Alzheimer’s disease.
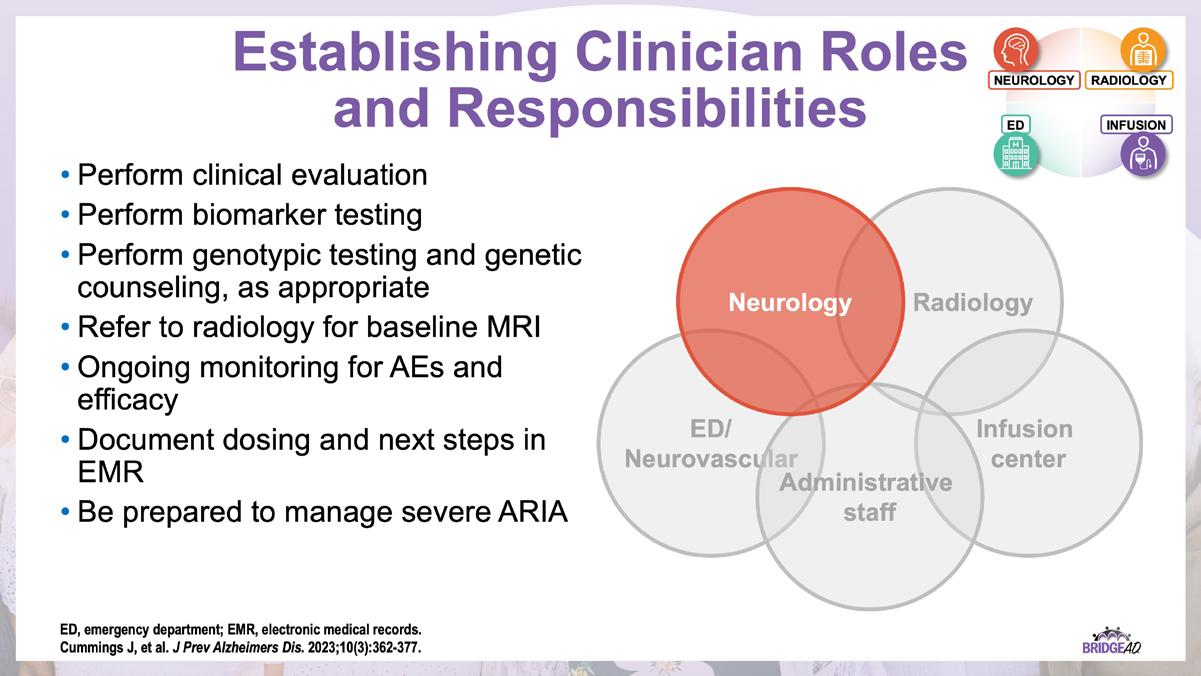
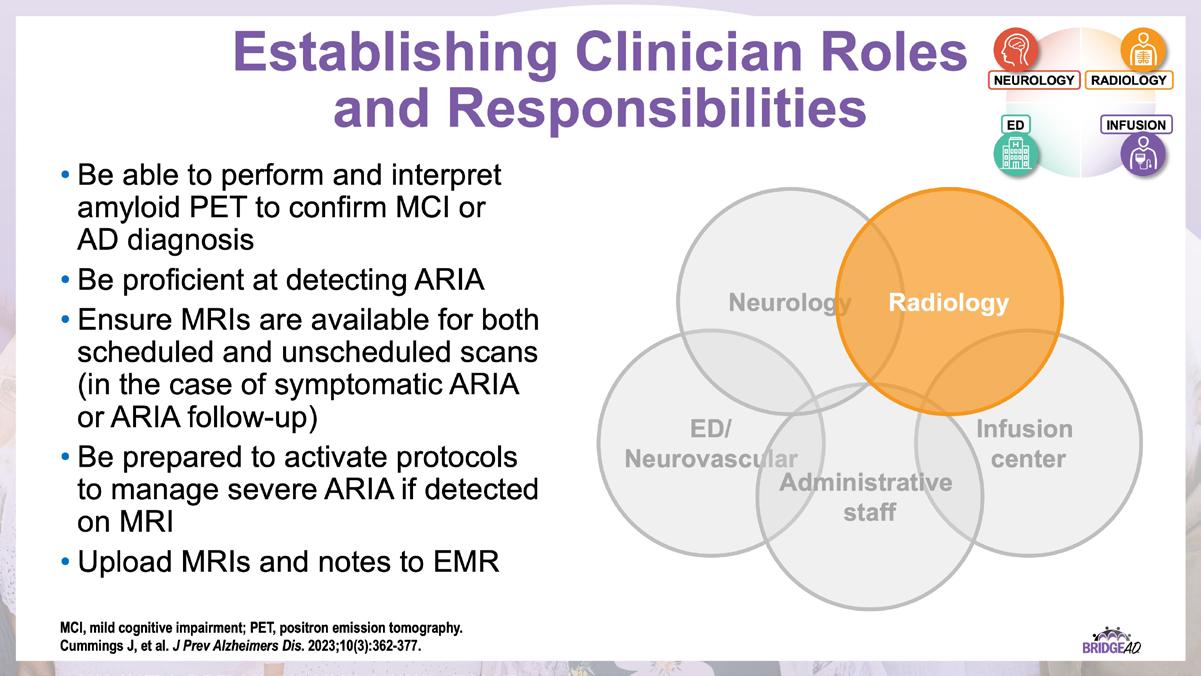
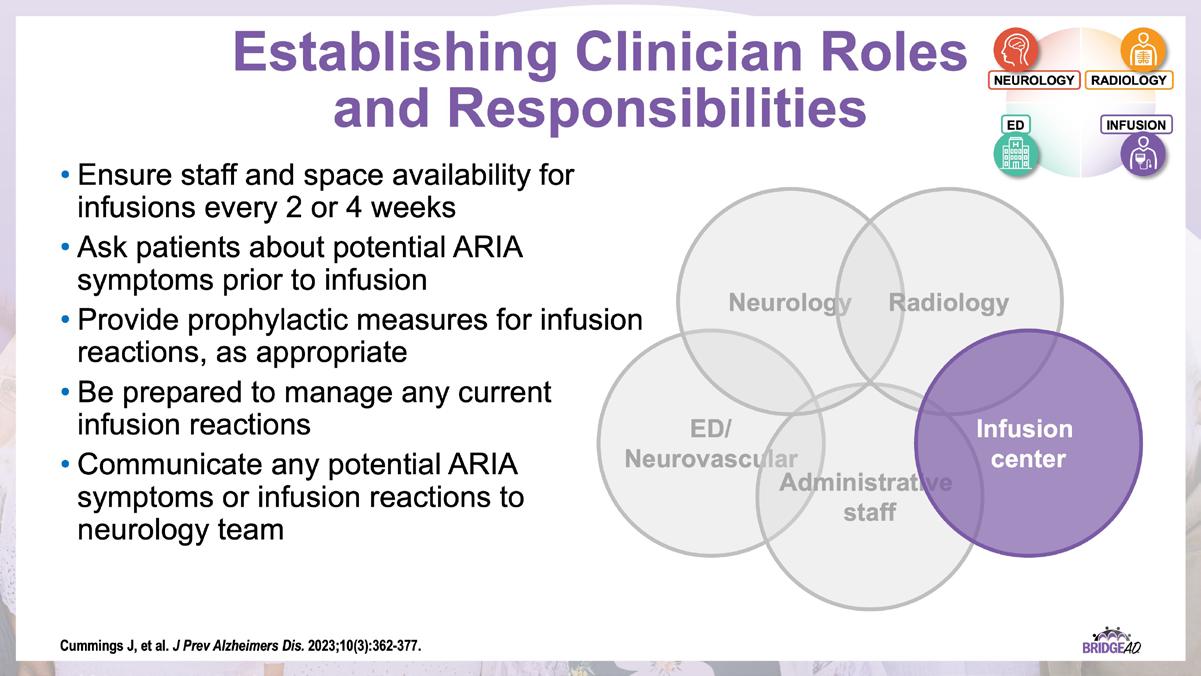
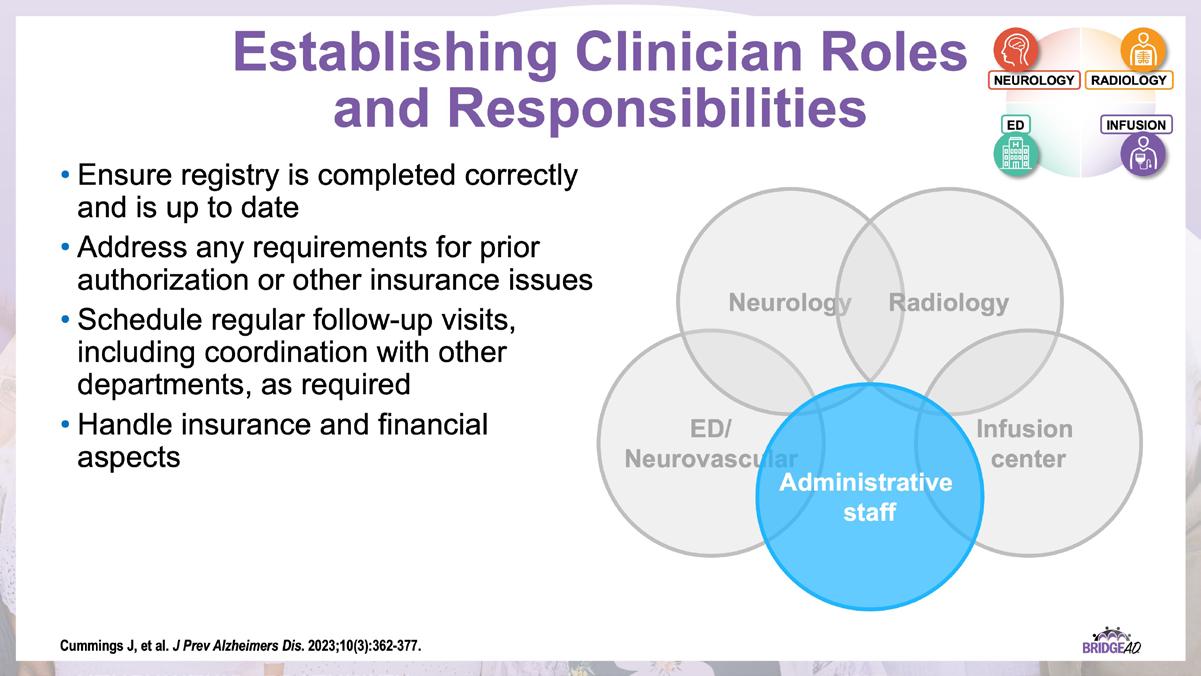
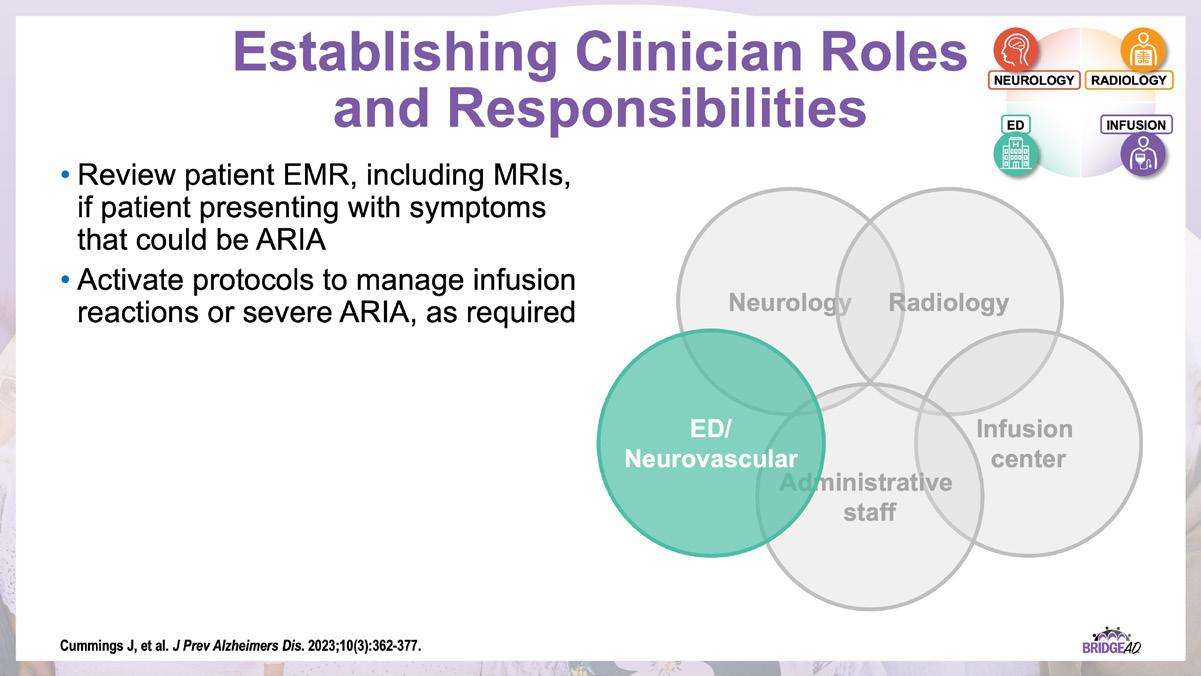
The educational design of this activity addresses the needs of neurology, radiology, emergency, geriatric, and primary care clinicians, and infusion center staff (physicians, nurse practitioners, physician assistants, nurses, and administrative staff) who are involved in the management of patients with Alzheimer’s Disease (AD).
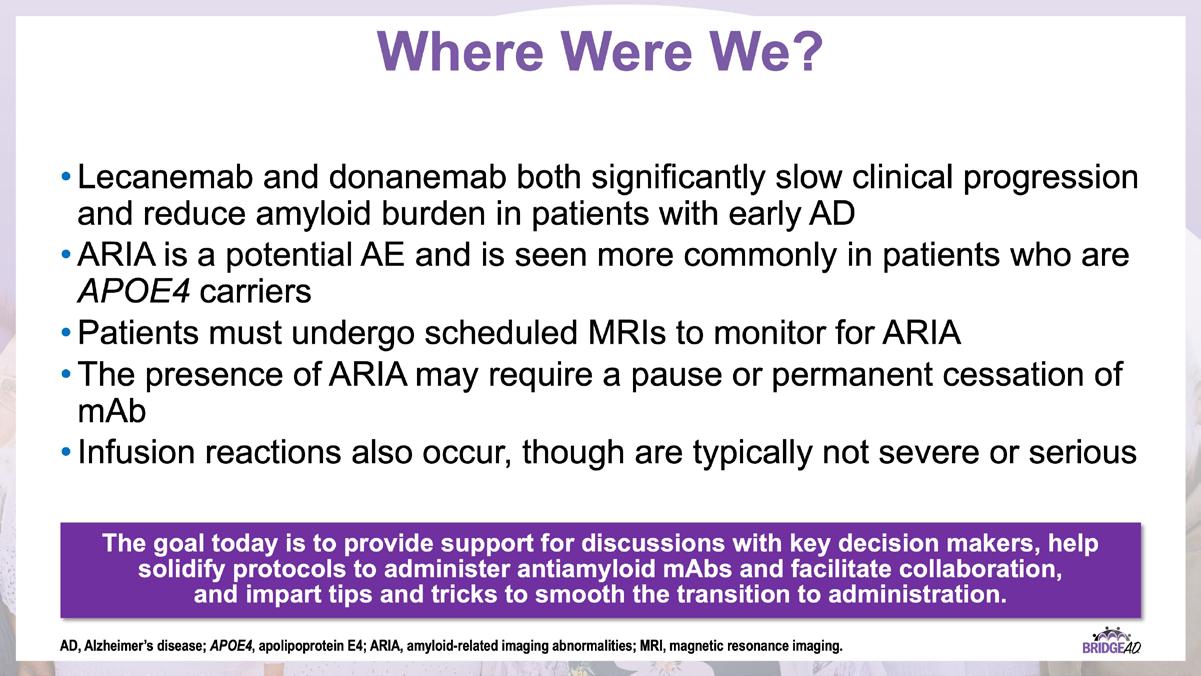
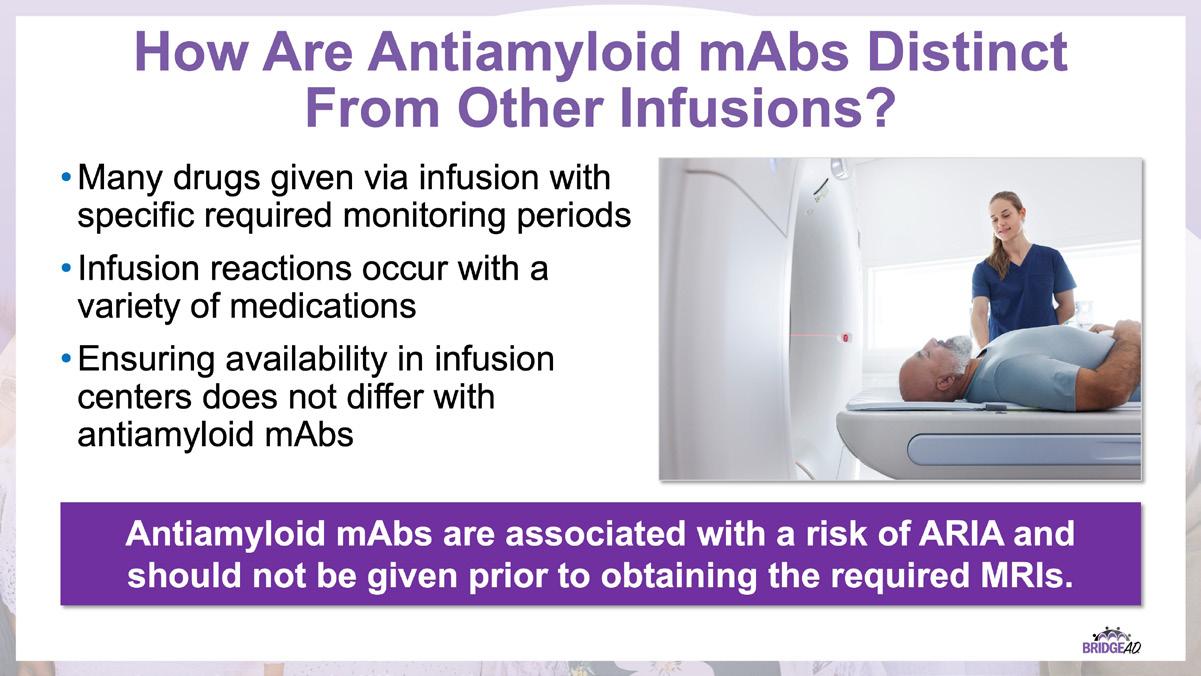
With recent approvals of antiamyloid monoclonal antibodies (mAbs), the field of AD is rapidly changing. Implementation of these therapies requires new protocols and workflows in clinics, including collaboration among multiple departments. This grassroots initiative consists of a 2-part series of live, on-site meetings targeted toward neurology clinicians, radiology clinicians, emergency department staff, and infusion center staff. Drs. Lawrence Honig and David Weisman will discuss details of antiamyloid mAbs, collaboration and communication tips, and best practices for developing implementation protocols. They will also help troubleshoot each location’s protocol development. Participating locations will receive numerous handouts and checklists that will aid in development and implementation of these novel protocols and new workflow procedures.
After completing this activity, the participant should be better able to:
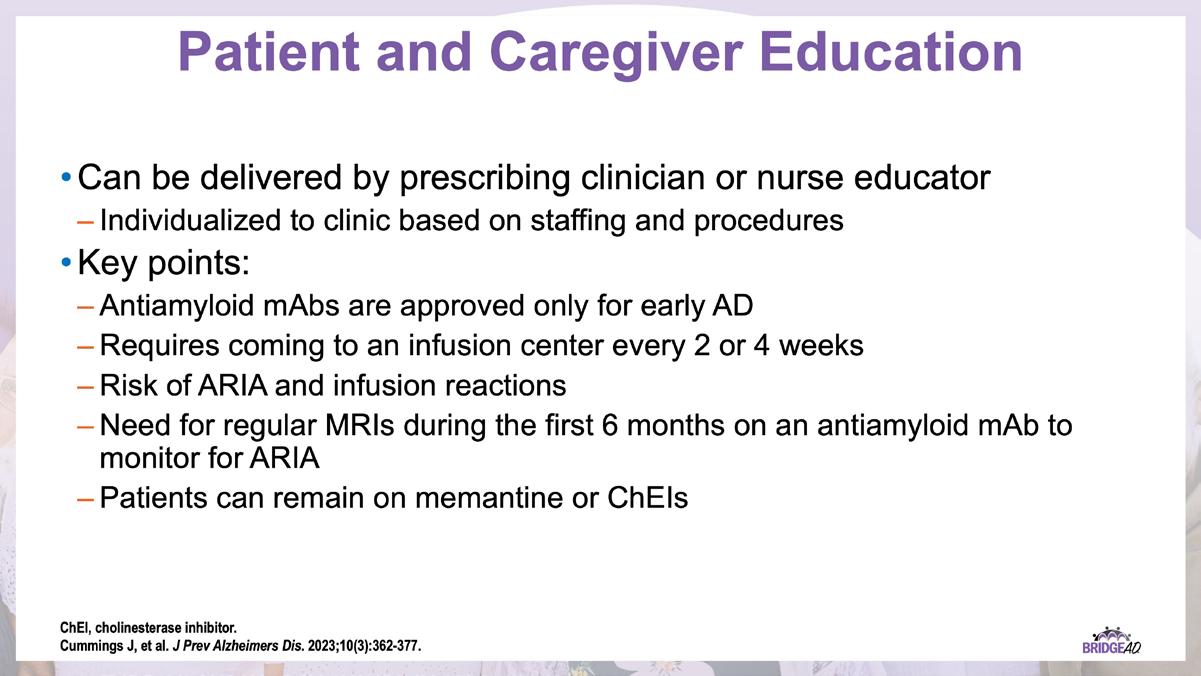
» Identify patients eligible for treatment with antiamyloid mAbs
» Review strategies to identify and address adverse events associated with antiamyloid mAbs
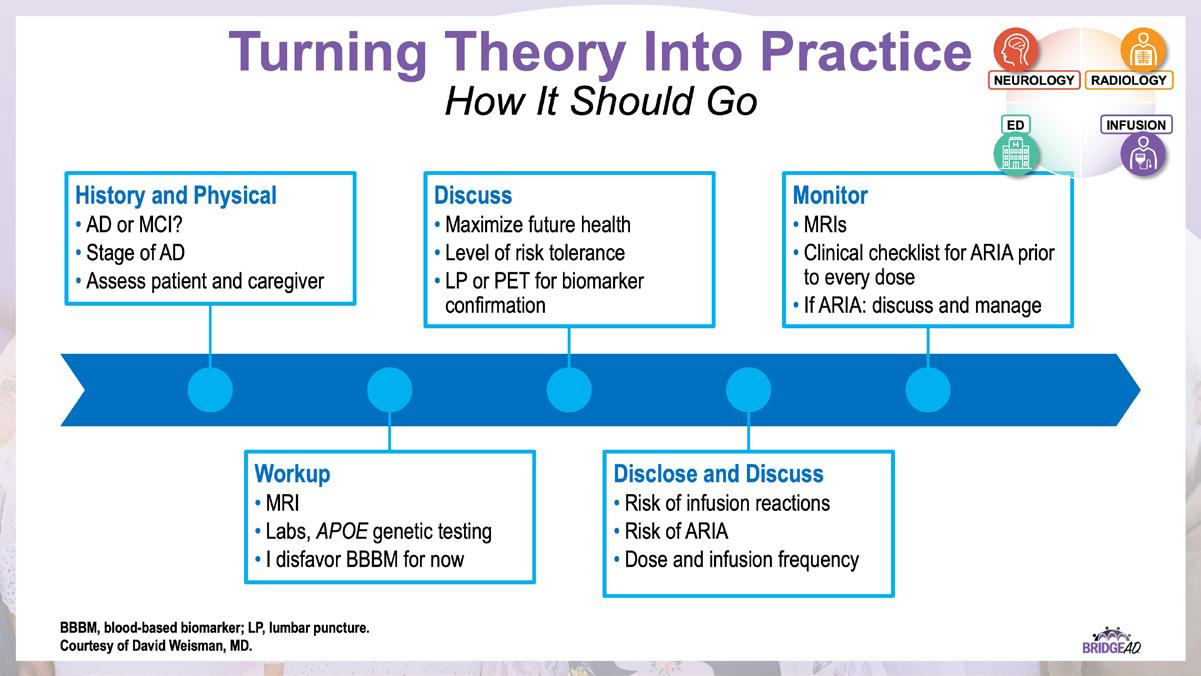
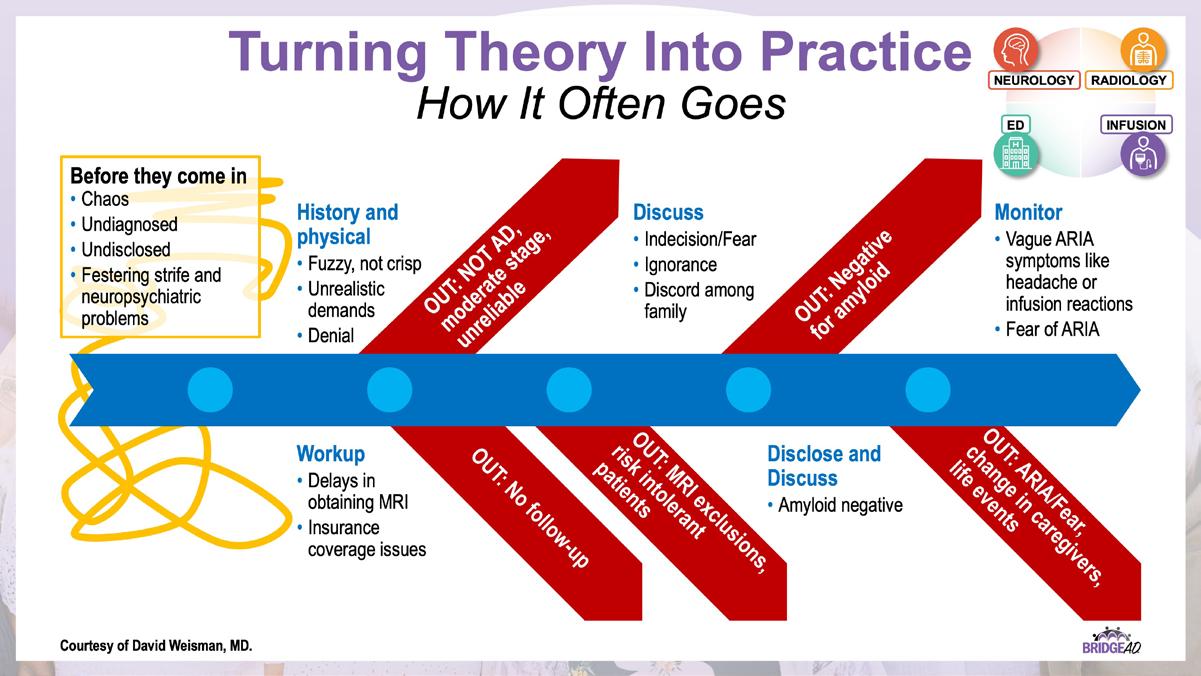
» Describe necessary components of clinic protocols for administering antiamyloid mAbs
» Establish interdepartmental communication regarding administration of antiamyloid mAbs, routine monitoring, and management of adverse events
Integritas Communications is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Integritas designates this live activity for a maximum of 1.0 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
The ANCC’s Commission on Accreditation recognizes educational activities that are approved for AMA PRA Category 1 Credits™ by providers who are accredited by the ACCME to award credit to learners. This reciprocity agreement allows nurses and nurse practitioners to use AMA PRA Category 1 Credits™ as approved ANCC contact hours for license renewal.
For more information about the approval of this program, please contact Integritas at info@exchangecme.com.
In order to receive credit for this activity, the participant must attend the live meeting and complete the posttest and program evaluation.
There is no fee for this educational activity.
Integritas adheres to the policies and guidelines, including the Standards for Integrity and Independence in Accredited CE, set forth to providers by the Accreditation Council for Continuing Medical Education (ACCME) and all other professional organizations, as applicable, stating those activities where continuing education credits are awarded must be balanced, independent, objective, and scientifically rigorous. All persons in a position to control the content of an accredited continuing education program provided by Integritas are required to disclose all financial relationships with any ineligible company within the past 24 months to Integritas. All financial relationships reported are identified as relevant and mitigated by Integritas in accordance with the Standards for Integrity and Independence in Accredited CE in advance of delivery of the activity to learners. The content of this activity was vetted by Integritas to assure objectivity and that the activity is free of commercial bias. All relevant financial relationships have been mitigated. The faculty have the following relevant financial relationships with ineligible companies: Lawrence S. Honig, MD, PhD : Consulting Fees: Biogen Inc., Eisai Inc., NewAmsterdam Pharma, F. Hoffmann-La Roche Ltd, Vigil Neuroscience, PPD, Inc.; Contracted Research: Acumen Pharmaceuticals, Alector, Inc., Biogen Inc., CervoMed, Inc./EIP Pharma Inc., Cognition Therapeutics, Inc., Eisai Inc., Grupo Ferrer Internacional, S.A., GSK plc, Transposon Therapeutics, Inc., UCB, Vaccinex Inc.
David Weisman, MD : Consulting Fees: Biogen Inc., Eisai Inc., Lantheus Holdings, Inc., MapLight Therapeutics, Inc., Novartis Pharmaceuticals Corporation, Viz.ai, Inc.; Fees for Non-CE Services Received Directly From an Ineligible Entity or Their Agents (eg, speakers’ bureaus): Eisai Inc., Eli Lilly and Company; Contracted Research: Acadia Pharmaceuticals Inc., Acumen Pharmaceuticals, Inc., Alnylam Pharmaceuticals, Inc., Alzheimer’s Association, Cerevel Therapeutics, Cognition Therapeutics, Inc., Eisai Inc., Eli Lilly and Company, EMD Serono, Inc., F. Hoffmann-La Roche Ltd, Sage Therapeutics, Inc., Sanofi, Ventus Therapeutics; Other (Regulatory): US Food and Drug Administration (FDA) Peripheral and Central Nervous System Drugs (PCNS) advisory committee.
The planners and managers have no relevant financial relationships with ineligible companies.
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. Integritas does not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed in this activity should not be used by clinicians without evaluation of patient conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.

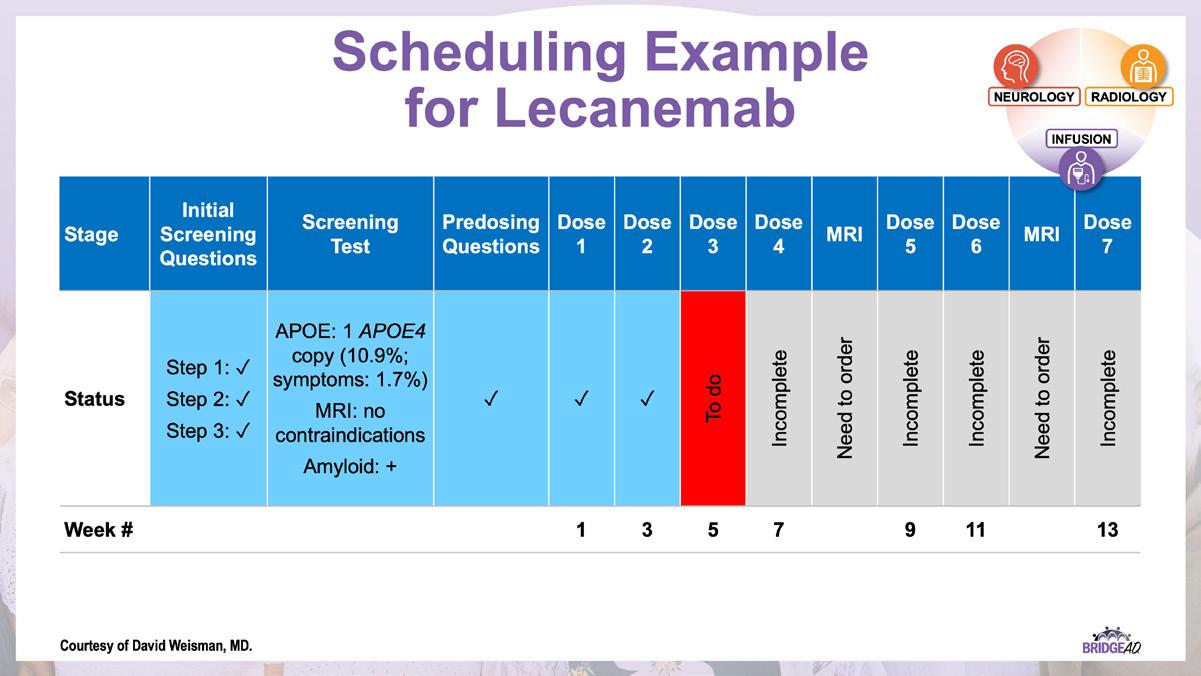

» Lecanemab: appropriate use recommendations.
Cummings J, Apostolova L, Rabinovici GD, et al. J Prev Alzheimers Dis. 2023;10(3):362-377.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10313141/
» Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup
Jack CR, Jr., Andrews JS, Beach TG, et al. Alzheimers Dement. 2024;20(8):5143-5169.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11350039/
» International Nuclear Medicine consensus on the clinical use of amyloid positron emission tomography in Alzheimer’s disease.
Tian M, Zuo C, Civelek AC, et al. Phenomics. 2023;3(4):375-389.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10425321/
» AD8 dementia screening interview
https://www.alz.org/getmedia/6e7291bf-4ac8-40ed-a148-824d4591ed7e/ad8-dementia-screening.pdf
» Alzheimer’s Association https://www.alz.org/
» The Alzheimer’s Questionnaire
https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/bc-guidelines/cogimp-alzheimer-questionnaire.pdf
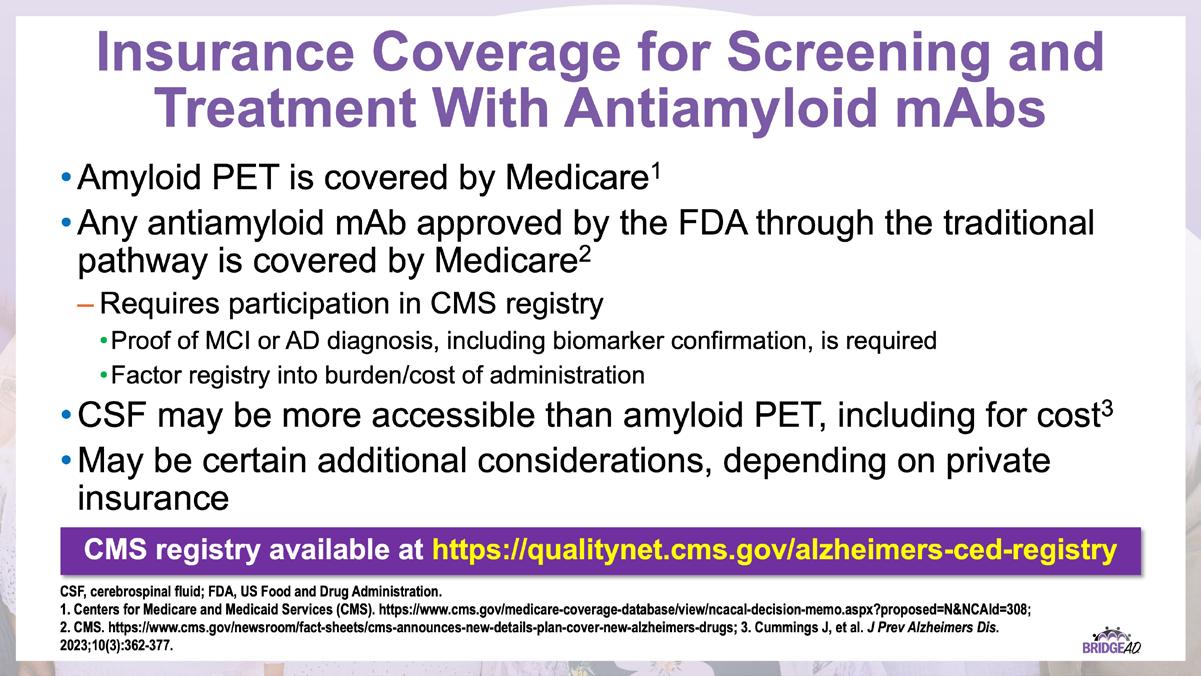
» Monoclonal antibodies directed against amyloid for the treatment of Alzheimer’s disease CED study registry
Center for Medicare and Medicaid Services. https://qualitynet.cms.gov/alzheimers-ced-registry
» LEQEMBI® (lecanemab) prescribing information.
US Food and Drug Administration (FDA).
https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761269s000lbl.pdf
» KISUNLA (donanemab) prescribing information. FDA.
https://www.fda.gov/media/180803/download
» Mini-Mental State Exam (MMSE) Alzheimer’s / dementia test: administration, accuracy and scoring
https://www.dementiacarecentral.com/mini-mental-state-exam/
» Montreal Cognitive Assessment Test (MoCA) for dementia & Alzheimer’s
https://www.dementiacarecentral.com/montreal-cognitive-assessment-test/
» Standardized Mini-Cog© Instrument
https://mini-cog.com/download-the-mini-cog-instrument/
» Diagnostic performance of plasma pTau217, pTau 181, Aβ1-42 and Aβ1-40 in the LUMIPULSE automated platform for the detection of Alzheimer disease.
Arranz J, Zhu N, Rubio-Guerra S, et al. Alz Res Therapy. 2024;16(1)139.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11200993/
» Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with anti-amyloid beta therapy.
Barakos J, Purcell D, Suhy J, et al. J Prev Alzheimers Dis. 2022;9(2):211-220.
https://www.sciencedirect.com/science/article/pii/S2274580724003339
» Multiple cerebral hemorrhages in a patient receiving lecanemab and treated with t-PA for stroke.
Reish NJ, Jamshidi P, Stamm B, et al. N Engl J Med. 2023;388(5):478-479.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10228637/
» Donanemab in early symptomatic Alzheimer disease: the
randomized clinical trial.
Sims JR, Zimmer JA, Evans CD, et al. JAMA. 2023;330(6):512-527. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10352931/
» Lecanemab in early Alzheimer’s disease.
van Dyck CH, Swanson CJ, Aisen P, et al. N Engl J Med. 2023;388(1):9-21.
https://www.nejm.org/doi/10.1056/NEJMoa2212948
» Clinical impact of microbleeds in patients with Alzheimer’s disease.
Vázquez-Justes D, Aguirregoicoa I, Fernandez L, et al. BMC Geriatrics. 2022;22(1):774. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9520821/

ExchangeCME.com is the fastest-growing digital platform for accredited CME/CE and MOC programming. Join the movement of thousands of health care professionals and make sure you stay up to date on best practices to manage your patients!

Free accredited online activities with immediate access to your certificates
Live in-person and online events


Clinical Resource Centers on various therapeutic areas

Live streaming of symposia at major medical conferences
Tailored e-newsletters based on your interest




