CPHI SEA: Accelerator of Pharmaceutical Industry
The Utility and Applicability of PROCALCITONIN in Clinical Practices
Thailand Industry Outlook: PHARMACEUTICALS

SUN PROTECTION
Test Methods
Introduction to Reference CULTURES







CPHI SEA: Accelerator of Pharmaceutical Industry
The Utility and Applicability of PROCALCITONIN in Clinical Practices
Thailand Industry Outlook: PHARMACEUTICALS

SUN PROTECTION
Test Methods
Introduction to Reference CULTURES






ด้วยเป้าหมายในการสร้างคอมมิวนิตีของกลุ่มผู้ผลิตยาและ ธุรกิจที่เกี่ยวเนื่อง อันคาดหวังจะช่วยเร่งการเติบโตและการพัฒนา อุตสาหกรรมยาในภูมิภาคเอเชียตะวันออกเฉียงใต้
With the goals to create ASEAN pharmaceutical community which will drive growth and development of South East Asian pharmaceutical industry by providing opportunity for players to sources products, learning opportunity with knowledge and expert sharing, and networking. Informa Markets, the no. 1 trade exhibition organizer in Asia, has brought CPHI (the well-known trade exhibition on pharmaceutical products and pharma supply chain) into Thailand under branding “CPHI South East Asia”.
Ms.Rungphech Chitanuwat, Regional Portfolio DirectorASEAN said that CPHI South East Asia 2023 has returned back with strong presence of major API suppliers from China, India, European countries. There are now more than 340 exhibitors confirmed to join CPHI South East Asia. As organizer we expected this year to be another buzz for more than 8000 trade visitors from around South East Asian countries to join the exhibition.
การจดทะเบียน และข้อบังคับของ FDA จาก ประเทศต่างๆ
ภาพรวมอุตสาหกรรมยาในแต่ละประเทศ
แนวโน้มอุตสาหกรรมสุขภาพสัตว์ในภูมิภาคเอเชียตะวัน
In 2023 , CPHI South East Asia will be organized at Queen Sirikit National Convention Center during 12th-14th July 2023. Exhibitions will covered the different profiles which are API and Excipients, Pharma processing machinery, Pharma packaging, Finished Dosage form, Natural Extract for herbal medicine, and specially this year we add “animal health” profile in to the exhibition. During the 3 days of the event, there will be series of seminar, and conference such as

Regulatory Framework sharing by FDA authorities
Outlook of Pharmaceutical industry in the region
Animal Health Outlook in South East Asia
How to build Drug Security in our region
Biopharmaceutical Products: Past, Present and Future and many more.
การจัดงานซีพีเอชไอ ในครั้งนี้ก็เป็นอีกงานหนึ่งที่ทางผู้จัดจะมีการจัดเนื้อหาสัมมนาและ การประชุมที่เกี่ยวกับการสร้างความมั่นคงทางการยาให้เกิดขึ้น ไม่ว่าจะเป็นทางด้านชีวภาพหรือการใช้ยาสมุนไพรที่เราสามารถ ผลิตได้ และในเรื่องที่สำาคัญอีกเรื่องหนึ่งคือการลดปัญหาขยะจาก บรรจุภัณฑ์สำาหรับยา ทางผู้จัดจะมีการจัดกิจกรรมที่ส่งเสริมความรู้ ทางด้านการใช้บรรจุภัณฑ์ในธุรกิจยาที่จะส่งเสริมและสร้างความยั่งยืน ที่ร่วมมือกับทางองค์กรด้านบรรจุภัณฑ์ระดับโลก
ด้านวิชาชีพเภสัชกรรม ภายในงานจะมีการจัดงานเพื่อส่งเสริมการ สร้างเครือข่ายเพื่อขยายไปยังกลุ่มนักศึกษาภาควิชาเภสัชศาสตร์
นางสาวรุ้งเพชรได้ระบความคืบหน้าในการเตรียมงานปีนี้ว่า “ทางทีมงานได้รับการตอบรับการสนับสนุนการร่วมจัดกิจกรรม และ การเข้าร่วมงานจากหน่วยงานที่เป็นที่รู้จักในภูมิภาค
At Informa Markets, we address sustainability insight in our exhibition, so we have developed our conference content, features inside the event embedded with sustainability, for example we will discuss on the new development to create drug security through biotechnology and herbal medicine. At CPHI South East Asia, we will have “innovation stage” which will feature the importance of packaging and how to drive sustainable packaging in pharma business. Innovation stage is a feature at CPHI South East Asia that co-organized by World Packaging Organization (WPO). One of the new activities at CPHI South East Asia this year, we engage the various academic, institute, associations to do university student program called “Pharma Quest”. It is aiming to connect the upcoming pharmacist to the industry. This provides them the opportunity to learn and understand what is happening in their industry they will be entering in few years. We hope with this local community engagement and connect with university students, it will help to create multiplier impact to the industry.


Ms.Rungphech shared the progress of the event that Pharmaceutical Association around ASEAN now has confirmed their participation to bring in their members who are pharmaceutical producer visiting the show. There are supporting organizations has confirmed the participation such as Thailand Pharmaceutical Manufacturers Association, Thailand Institute of Science Research and Technology, Thai-FDA, Thailand Industrial Pharmacist Associations, Philippines-FDA, NPRA (Malaysia-FDA), LPPOM MUI (Indonesia), GP-Famasi (Indonesia), Malaysian organization of Pharmaceutical Industry, Vietnam Pharmaceutical industry Association, Cambodian Pharmaceutical Traders Association, Philippines Chamber of Pharmaceutical Industries, KHIDI (Korea), CCMPHIE (China) and many more.
More information and register for visiting , you may follow us at www.cphi.com/sea or facebook CPHI South East Asia.
Can’t miss exhibitors !
OLIC (THAILAND) LIMITED - THAILAND
APTAR PHARMA INDIA PVT.LTD - INDIA
CAREPLUSONE CO., LTD. - KOREA
BCN PEPTIDES SA - SPAIN
BANGKOK LAB AND COSMETIC CO.,LTD. - THAILAND
GREATER PHARMA CO.,LTD. - THAILAND
EVERZINC BELGIUM SA A.H.A INTERNATIONAL CO.,LTD. - BELGIUM
A.H.A INTERNATIONAL CO.,LTD.- CHINA
ZHEJIANG HUAHAI PHARMACEUTICAL CO., LTD. - CHINA
DERMIFANT GMBH - GERMANY
CENTRIENT PHARMACEUTICALS INDIA PRIVATE LIMITED - INDIA
CAREGEN CO.,LTD. - KOREA
DUPONT - THAILAND
LINARIA CHEMICAL (THAILAND) LIMITED - THAILAND
NATURAL EXTRACTS
NANTONG JINGHUA PHARMACEUTICAL CO., LTD. - CHINA
NIPRO PHARMAPACKAGING INTERNATIONAL N.V. - BELGIUM

HEBEI YIYANG JINGCHENG TECHNOLOGY CO.,LTD. - CHINA
HARRO HOFLIGER VERPACKUNGSMASCHINE GMBH- GERMANY
STERIMAX INDIA PVT.LTD.- INDIA
SANNER PHARMACEUTICAL & MEDICAL PACKAGING MATERIALS (KUNSHAN) CO., LTD.- INDONESIA
TOYO MACHINE MANUFACTURING CO.,LTD. - JAPAN
BECTON DICKINSON HOLDINGS PTE., LTD. - SINGAPORE
GERRESHEIMER SINGAPORE PTE., LTD. - SINGAPORE
PERLEN PACKAGING - SWITZERLAND
CVC TECHNOLOGIES, INC. - TAIWAN
IMA PACIFIC CO., LTD. - THAILAND
KLOECKNER PENTAPLAST - THAILAND
LABWARE (THAILAND) CO., LTD. - THAILAND
REPASSA (THAILAND) LTD. - THAILAND
H&T PRESSPART - UK


The development of pharmaceutical products and self-care trend escalated by the pandemic of COVID-19 since 2019 including the rising number of NCD patients and elderly encourage the demand on pharmaceutical products and also medical supplies substantially. This concludes the industry is growing.
Let’s see how the industry grows via the leading pharmaceutical exhibition, CPHI South East Asia, taking place on July 12-14, 2023 at QSNCC, Bangkok. See
Officer
SUPAWAT SUKMARK ss@media-matter.com
KARUNA CHINTHANOM kc@media-matter.com
APAPAN CHATPAISARN editor@media-matter.com
SITTHIPORN CHOMPURAT journalist@media-matter.com
BENJAMAS SRISUK innolab@media-matter.com
จีนถนอม WANASANAN CHINTHANOM agency@media-matter.com THU DO innolab.vn@media-matter.com

43/308
10150
43/308 Moo 1, Jomthong Road, Jomthong, Bangkok 10150 Thailand TaxID 0105552007301
+66 875171651 F +66 2045 5358 innolab@media-matter.com
http://www.innolabmagazine.com innolabmagazine
The publisher endeavors to collect and include complete, correct and current information in INNOLAB but does not warrant that any or all such information is complete, correct or current. The publisher does not assume, and hereby disclaims, and liability to any person or entity for any loss or damage caused by errors or omissions of any kind, whether resulting from negligence, accident or any other cause. INNOLAB does not verify any claims or other information appearing in any of the advertisements contained in the magazine, and cannot take any responsibility for any losses or other damages incurred by readers in reliance on such content.











Sunscreen products are photoprotective topical products for the skin that help protect against sunburn and most importantly prevent skin cancer. Sunscreens come as lotions, sprays, gels, foams, sticks, powders, and other topical products. Sunscreens are classified into two types: mineral (physical) sunscreens (i.e., zinc oxide and titanium dioxide) and chemical (or petrochemical) sunscreens.
Sunlight generates visible light and ultraviolet light (UVL) which can penetrate human skin. The UV light is grouped as UVA, UVB and UVC. The shorter wavelength UVC rays are absorbed by the ozone layer and do not reach earth. However, both UVA and UVB penetrate the atmosphere and play a major role in causing conditions such as sunburn, premature skin ageing, and skin cancers. Many sunscreens do not block ultraviolet A (UVA) radiation,
yet protection from UVA is important for the prevention of skin cancer. To provide a better indication of their ability to protect against skin cancer and other diseases associated with UVA radiation (such as phytophotodermatitis), the use of broad-spectrum (UVA/UVB) sunscreens has been recommended.
What does SPF mean?
Sun protection factor or SPF is calculated by dividing the amount of time it takes to redden protected skin, by the amount of time it takes to redden unprotected skin. So, an SPF of 30+ means that it takes at least 30 times longer to get sunburn. These calculations are nearly always under-predictions.

To claim an SPF of 50+, for instance, manufacturers need to show that at least 60 times as much UV needs to get through the skin.
SPF, UVAPF Protection, and Broadspectrum Claims
Sunscreen products are regulated in many markets. The international standards are used to demonstrate the efficacy of sunscreen products. ISO has published so many standards and related documents covering almost every industry, from technology and food safety to agriculture and health care. ISO Working Group 7 (WG7), "Sun Protection Test Methods," was established in 2006.
• Wavelength 290–320 nm
• Most are intercepted by the ozone layer but with the depletion of the ozone layer more UVB rays are now reaching the earth’s surface
• Its intensity varies by season, location, and time of day but in the summer months it is most intense between 10 am and 4 pm
• At high altitudes and surfaces such as snow and ice, up to 80% of UVB rays are reflected so they hit the skin twice
• The main cause of skin reddening and sunburn and damages the upper epidermal layers of skin
• 20-30 minutes of UVB exposure a day helps the skin to produce bonebuilding vitamin D3
• Suppresses skin immune function
• Wavelength 320–400nm
• UVA I 340–400nm
• UVA II 320-340nm
• Accounts for up to 95% of UVL reaching the earth’s surface
• Present with relatively equal intensity throughout the year
• Can penetrate clouds and glass
• Penetrates the skin more deeply than UVB rays and damages skin cells in the basal layer of the epidermis
• Responsible for causing a deep tan which is an injury to the skin’s DNA
• Contributes to and may even start the development of skin cancers
• Suppresses skin immune function
SPF In vivo, ISO 24444: In 2010, the first ISO norm, “SPF in vivo” (ISO 24444), was published by WG7. This norm is accepted today as a gold standard around the world (except in the United States) to claim SPF. It was underwent a systematic review process to improve the method and reduce the inter-lab variability of results. Therefore, if the test is repeated with the updated version, the mean value will not change. The main updates concern the application techniques, addition of new standards with higher SPF values, control of the lamp beam uniformity, and better precision of the rejected cases.
UVA In vivo, ISO 24442: in 2011, ISO published the “UVA in vivo” method ISO 24442. Like the ISO 24444. This method being technically close to the SPF In vivo, the improvements are similar and will be harmonized with the ISO 24444.
UVA In vitro, ISO 24443: This method was published in 2012 and was optimized thanks to different modifications, such as the addition of a high UVA protection factor standard value; a more precise application protocol etc.
In conclusion, several methods are already available, such as:
ISO 24444 Cosmetics - Sun protection test methods - In vivo determination of the sun protection factor (SPF)
ISO 24442 Cosmetics - Sun protection test methods - In vivo determination of sunscreen UVA protection
ISO 24443 CosmeticsDetermination of sunscreen UVA photoprotection in vitro
ISO 16217 Cosmetics - Sun protection test methods - Water immersion procedure for determining water resistance
ISO 18861 Cosmetics - Sun protection test methods - Percentage of water resistance
ISO 23675 Cosmetics - Sun protection test Methods - In Vitro determination of Sun Protection Factor
ISO/CD 23698 Cosmetics - Sun protection test methods - Measurement of the Sunscreen Efficacy by Diffuse Reflectance Spectroscopy (In-Vivo/ In-Vitro SPF - UVAPF)
In-Vivo SPF according to ISO 24444 Product Application

A very accurately measured and controlled amount (2 mg/sq cm) of product is applied to a marked-out area of skin. The product is evenly spread, using a standardized technique. The product is then allowed to dry for 15 to 30 minutes.
Water Resistance
For most countries, 40 minutes, or 80 minutes. Up to 4 hrs is permitted in Australia.
A solar simulator, which has been designed and calibrated to imitate the spectrum of sunlight, is used to apply small incremental doses of light to the protected area. An unprotected area and an area with a Standard Sunscreen applied, are also exposed. Overnight, a mild erythema (slight sunburn) develops at test sites where the SPF has been exceeded.
In-Vivo UVAPF according to ISO 24442
UVAPF measurement is performed on a panel of at least 10 human adult volunteer test subjects with selected skin types who only tan and are usually darker skin. The test panel is selected from volunteers who do not have any history of sensitivity to skin product ingredients and who have an appropriate health history. The requirements of the test are now the same for all regulated markets.
A very accurately measured and controlled amount (2 mg/sq cm) of product is applied to a marked-out area of skin. The product is evenly spread, using a standardized technique. The product is then allowed to dry for 15 - 30 minutes.
An unprotected area and an area with a Standard Sunscreen applied, are exposed and in 3 - 6 hours, a tanning reaction known as persistent pigment darkening develops at test sites where the UVA Protection has been exceeded.
Article info
The results are read around 3 - 6 hours after the exposures are made. The test value taken is the point where there is a slight but clearly visible tanning of the skin. The UVAPF is a simple ratio of the number of seconds or accumulated J/sq m of light exposure, divided by the value for the unprotected exposure seconds.
In-Vitro UVAPF – critical wavelengths (CW) according to ISO 24443
The steps of the human UVA tanning test (persistent pigment darkening) can be imitated without the use of test volunteers.
Steps of the Test
A film of sunscreen is applied onto polymethacrylate (PMMA) plates which imitates skin roughness. After drying is completed. The UV Spectrum is measured. Then the plates are subjected to UV light equivalent to the expected in use performance of the sunscreen. The UV Spectrum is then re-measured. The UVAPF, as well as the Critical Wavelength, can be determined and both broad spectrum and UVA ratio calculated from that data.
Non-ISO method: Boots Star Rating
This is a proprietary method used to describe the protection offered by sunscreen products. Clearance is required from the trademark owners before the logo can be used. UVA/ UVB Ratio is calculated from this in vitro measurement technique.
The substrate for measurement is abraded Polymethylmethacrylate (PMMA) Plates. A thin film of the test product, at a thickness of 1.3 mg/sq cm, is applied, by a standard application technique. This involves applying a series of around 30 dots over the area of the plate and then rubbing out evenly with the finger which has been pre-impregnated to saturation with the same product. The prepared plates are left to air dry for 15 min.
After initial pre-irradiation measurements, a fixed dose of UV light of 17.5 J/sq cm is applied to the plates in a solar simulator, filtered to comply with the requirements of the Boots Protocol. Post irradiation measurements are then made. An SPF Analyzer is utilized for measurement. Measurements are taken against a matching blank glycerin loaded PMMA plate, at increments of 1 nm between the range of 290 and 400 nm. A minimum of 5 measurements are taken pre-irradiation and a corresponding 5 are made post irradiation, on non-overlaying areas of each of the plates, such that the accumulated measured area of 2.0 cm has been sampled.
Reporting
UVA/UVB Ratios are calculated for both before and after irradiation. Star rating is allocated according to the results of the two measurements. The original ‘one star’ and ‘two stars’ categories are obsolete in the new system due to the requirements of the EC recommendations.
Ellen Phiddian (2022). Explainer: how is sunscreen made and tested? COSMOS magazine: December 9, 2022. https://cosmosmagazine. com/health/medicine/sunscreen-tested-development-explainer-ingredients/
Marc Pissavini (2020). Standardizing Safety ISO Validation and Sun Protection Tests. Testing | C&T Peer-Reviewed. Cosmetics & Toiletries, April 2020.
Vanessa Ngan. (2012). Sunscreen testing and classification. DermNet. https://dermnetnz.org/topics/sunscreen-testing-and-classification

Prof. Anucha Apisarnthanarak, MD
Chief of Division of Infectious Diseases, Thammasart University Hospital

โปรแคลซิโทนิน (PCT) เป็นโปรตีนตั้งต้นที่ผลิตม�จ�กทีเซลล์ในกระบวนก�รสังเคร�ะห์ฮอร์โมนแคลซิโทนิน ถูกสร้�งขึ้นเพื่อตอบ สนองต่อก�รติดเชื้อแบคทีเรียและภ�วะก�รอักเสบอื่นๆ ในสภ�วะปกติ PCT
เกี่ยวข้องกับก�รควบคุมระดับของแคลเซียมในร่�งก�ย โดยปกติจะพบค่� PCT ในเลือดของกลุ่มคนที่มีสุขภ�พดีในปริม�ณเพียง
ประโยชน์ของโปรแคลซิโทนินในภาวะ Sepsis การติดเชื้อทางเดินหายใจ
จัดการภาวะติดเชื้อในกระแสเลือด (sepsis/ septicemia) การติดเชื้อทางเดินหายใจ ส่วนล่าง (LRTIs) และการควบคุมดูแลการ ใช้ยาต้านจุลชีพ (AMS) ในภาวะ Sepsis ระดับ PCT สามารถ ช่วยระบุผู้ป่วยที่มีความเสี่ยงสูงต่อการเกิด
(sepsis shock) ระดับ PCT ยังสามารถช่วยแนะนำา การรักษาด้วยยาปฏิชีวนะในผู้ป่วยติดเชื้อ
เชื้อแบคทีเรีย ค่า PCT จะมีปริมาณสูงขึ้น
3-6 ชั่วโมงแรกหลังการติดเชื้อ และ
เมื่อมีการติดเชื้อไวรัส ทำาให้การใช้ PCT สามารถช่วยลดการใช้ยาปฏิชีวนะโดยไม่ จำาเป็นในผู้ป่วยที่ติดเชื้อไวรัส
ค่าใช้จ่ายในโรงพยาบาล: ค่าใช้จ่ายเพิ่ม เติมสำาหรับการส่งตรวจ PCT มักจะถูก เปรียบเทียบกับค่าใช้จ่ายของยาปฏิชีวนะ ที่มีราคาต่ำามากในบางประเทศเท่านั้น แต่ ไม่คำานึงถึงการประหยัดค่าใช้จ่ายในส่วน อื่นๆ เช่น ระยะเวลาการพักรักษาตัวที่ สั้นลง (LOS) ผลข้างเคียงที่ลดลงจาก C. difficile เป็นต้น นอกจากนี้ งบประมาณที่ จำากัดสำาหรับห้องปฏิบัติการในโรงพยาบาล หลายแห่งจำากัดการใช้งาน PCT ที่มาก
การเบิกจ่ายค่าตรวจ PCT นั้นอนุญาตไว้สำาหรับลักษณะการใช้งานและ ช่วงเวลาที่ต้องใช้งานแค่บางอย่างเท่านั้น ขาดเทคโนโลยี Point-of-care (POC) คุณภาพสูง (ที่มีความไว): สำาหรับสถาน พยาบาลระดับปฐมภูมิและสถานพยาบาล ฉุกเฉินขนาดเล็ก
Procalcitonin (PCT) is a protein that is produced in response to bacterial infections and other inflammatory conditions. It is a precursor molecule to calcitonin, a hormone that is involved in the regulation of calcium levels in the body. PCT is normally and intermediate product from T-cells in the synthesis of calcitonin. Its concentration is normally low. Low PCT values in the blood of healthy persons: 46.7 pg/ mL, median 12.7 pg/mL.
Presence of bacterial infection stimulates PCT production. Lipopolysaccharide (LPS) of bacteria triggers releasing cytokine IL-1β and TNF-α that increases PCT excretion in organs. Blood PCT is then increased. During virus infection, the process can be blocked because virus contains no LPS. Sources of production in healthy people are thyroid gland and lung. In bacterial infection PCT is produced and released into circulation from the entire body.
PCT is a sensitive and specific marker of the inflammatory response to bacterial infection. It rises during 3-6 h after bacterial infection and stay at the peak for 12-24 hours. Effective treatment reduces PCT concentration to normal within 2-3 days. Inadequate infection control without source control, such as pus control, leads maintaining high concentration of PCT. PCT concentration correlates with bacterial burden and severity of disease, for examples, septic shock, systemic infection, PCT concentration is higher that local infection.
Available evidence concerning PCT is mostly in biomarkers. Intervention studies focused on three indications: upper respiratory tract infection, pneumonia, chronic obstructive pulmonary disease (COPD) exacerbation, acute bronchitis, severe sepsis, and septic shock. Other indications are not sufficiently investigated. Observation studies are not sufficient as well. Applications are mostly in these three indications.
Utility of procalcitonin in sepsis, lower respiratory tract infection (LRTI), and antimicrobial stewardship (AMS)
Procalcitonin (PCT) is a useful biomarker in the diagnosis and management of sepsis, lower respiratory tract infections (LRTIs), and antimicrobial stewardship (AMS).
In sepsis, PCT levels can help identify patients who are at high risk of developing severe sepsis or septic shock. PCT levels can also help guide antibiotic therapy in sepsis patients, as PCT levels tend to be elevated in bacterial infections and lower in viral infections. This can help reduce unnecessary use of antibiotics in patients with viral infections, which can help decrease the risk of developing antibiotic resistance.
In LRTIs, PCT levels can help distinguish between bacterial and viral infections, and can guide the use of antibiotics in patients with suspected bacterial infections. Studies have shown that using PCT levels to guide antibiotic therapy in LRTIs can help reduce the duration of antibiotic treatment and the overall use of antibiotics, without compromising patient outcomes.
In antimicrobial stewardship (AMS), PCT can help guide decisions about the appropriate use of antibiotics in a variety of clinical settings. By providing clinicians with objective data on the likelihood of a bacterial infection, PCT levels can help reduce the unnecessary use of antibiotics,
which can help reduce the risk of developing antibiotic resistance. Overall, the use of PCT in the diagnosis and management of sepsis, LRTIs, and antimicrobial stewardship can help improve patient outcomes and reduce the risk of antibiotic resistance. However, it is important to note that PCT levels should always be used in conjunction with clinical judgement and other diagnostic tests, and should not be used as the sole criterion for initiating or discontinuing antibiotic therapy.
PCT helps us to improve management of sepsis patients
Does PCT provide prognosis information?
Rapid induction of PCT after bacterial challenges. PCT induction time at 4-12 h is 1000x increased is induced by bacterial infection. It helps early diagnosis; monitoring is possible to mirror patient response. Serial PCT was used to predict mortality in severe sepsis patients. it was found that when PCT declines ≤80%, in Day 3, mortality rate is 20%., when PCT declined >80%, the rate is 10%, or 2-folds difference. PCT can improve antibiotic decision. It rules out bacterial infection in lowrisk patients and monitors patients for early stop of antibiotic therapy. For example, a 55-year-old patient; female, never-smoker, non-productive cough, chills, with sepsis shock still risk high mortality. PCT is used for prognosis and careful monitoring. The patient was daily reassessed for antibiotic de-escalation.
Why does PCT reduce mortality?
PCT can improve physical outcomes in the multicenter study conducted in 15 hospitals in the Netherlands. PCT-guided therapy vs standard cares found that 30-days mortality rate and treatment failure were decreased significantly.
Low PCT might prompt clinicians to seek alternative cause of their symptoms (such as heart failure or pulmonary embolism). Lack of reduction of PCT levels might identity earlier non-responders to empirical treatments, or the reduction side-effects and antibiotics is related to better outcomes. Reducing antimicrobial prescription to limit bacterial resistance is challenged by clinician’s concern about "withholding" antibiotics. This study reassures us that this strategy is safe and better.
PCR-guided antibiotic therapy in LRTI in "real life"
Result of the study demonstrated that antibiotic duration was significantly shorter (-1.52 days) if the PCT algorithm was followed compared with when it was overruled (5.9 vs 7.4 days). PCT guided therapy lowers antibiotic exposure in LRTI treatment.

PCT for risk assessment and rule-out of bacteria coinfection in COVID-19
Test PCT as an aid for early risk assessment and prioritize of highrisk patients on admission. If PCT concentration is <50 µg/L, there is low risk of bacterial infection and adverse outcome. Antibiotics may not be needed. On the other hands, if conc ≥50 µg /L, it is high risk of bacterial infection.
PCT uses during hospital stay helps monitoring and detect secondary bacterial infection and inform prognosis of disease.
PCT and clinical pulmonary infectious score (CPIS) reduce inappropriate antibiotics in COVID-19 among severely-to-critically ill COVID-19 patients, multidrugresistant organisms, and invasive fungal infections during intervention period. Antibiotics are not prescribed when PCT <0.5 and CPIS <6.
Antimicrobial Stewardship (AMS): Role of rapid diagnosis and PCT PCT kinetics - 4 points for antibiotic usage guidance
1. Confirm diagnosis
2. Antibiotic monitoring
3. Stop antibiotic treatment
4. Reduce antibiotic resistance risk
- Rapid diagnostic tests (RDTs) should provide results to clinicians within 4-6 hrs
- The role of RDTs must incorporate to identification of bacterial and nobacterial pathogens
- The RDTs should have a key substantial impact on AMS in 3 key decision nodes
- An AMS team should be put in place to interpret RDTs to guide antimicrobial use
- Key performance indicators should be put in place to monitor effectiveness of RDTs in AMS RTSs can provide prompt, accurate identification of infectious organisms and be key component of AMS programs.
1. Initiation stage (RDT is essential at this stage); within the first 4-6 hrs. The objective is to determine whether an antimicrobial required and to determine antibacterial or antiviral. Examples RDTs are PCT and rapid influenza test.
2. On-treatment stage (RDT desirable but not essential); within the first 24 hrs. The objective is used to facilitate targeting or broadening of therapy. Examples RDTs are molecular testing and drug resistant testing (e.g., for carbapenem-resistant Enterobacteriaceae (CRE)), respiratory or GI panels, PCT to inform patient prognosis.
3. De-escalation/cessation stage
(RDT desirable but not essential); 24 hrs onward. The objective is to facilitate early IV to oral switch and to facilitate de-escalation or cessation of antimicrobials. Examples RDTs are de-escalation - MALDI-TOF, liquid culture, lateral flow assay, and cessation-PCT.
Inventory of key infection related RTDs in Asia Pacific
Advantages: Fast discrimination of bacterial and non-bacterial infection
Disadvantage: More expensive than biomarkers
Use cases: Antimicrobial initiation/ cessation, centralized laboratories/ point of care.
PCT vs inflammatory testing
C-reactive protein (CRP) is acute phase reactant synthesized by the liver. Secretion is triggered by cytokine (IL-6, IL-1, TNF- α). It is produced in response to acute and chronic inflammation, bacterial and viral infection, rheumatic, inflammatory diseases, malignancy, surgery, trauma, tissue injury, necrosis, liver failure, and obesity.
Strength
- Highly sensitive for inflammatory and infectious states
- Inexpensive
- Broad availability
Limitations
- Rise in any inflammation condition (limited specificity for bacterial infection)
- Slow induction kinetics (peak 36-50 h)
- Suppressed by corticosteroid treatment
PCT vs CRP
Induction kinetics play important role for diagnostic performance; increase of PCT after 3-4 h versus >12
h for CRP, thus PCT is superior for early sepsis diagnosis. Slow kinetic further limits diagnosis performance of CRP. Moreover, PCT shows higher sensitivity and specificity for bacterial infection than CRP. Assessment of severity of disease (increasing organ dysfunction) by PCT and CRP suggested that PCT correlates with disease severity while CRP does not.
CRP utility may be limited due to delayed in induction (>12 h), impact by corticosteroids which suppress CRP response, and impact by inflammation which prolonged unspecific increase.
Barriers to the implementation of PCT in Asia Pacific countries
Lack of evidence for some patient populations
Tuberculosis: Few studies on tuberculosis, PCT remains low in patients with tuberculosis limited to the lung but increases in severe cases with systemic infection.
Hemorrhagic fever syndrome: Few studies have looked at hemorrhagic fever syndromes, PCT has been reported to be increased in severe cases despite lack of bacterial infection, PCT seems to provide prognostic information.
Resource
Cost to the hospital: Added costs for PCT testing are often only compared to the very low antibiotic costs in some countries, without considering other potential cost savings like from shorter length of stay (LOS), reduced adverse effects like C. difficile. etc. Also, fixed budget for the lab in some hospitals limit broader PCT implementation as costs and savings occur in different departments. PCT testing often limited to —one to three tests per patient.
Limitation for measurement by government: Some countries restrict PCT reimbursement to certain indications and time points.
Lack of high-quality (sensitive) point-of-care (POC) technology: For primary care and smaller emergency care institutions, POC devices may help to implement a PCT strategy. Currently available POC tests often have insufficient technical performance and are not validated to be used for antibiotic stewardship.
Educational support
Educational material on PCT: Many clinicians have no formal education for the use of PCT and educational material is scarce.
General antimicrobial stewardship program (ASP) education and resources: Lack of well-established infectious disease clinical training for hospital pharmacists, and the paucity of infectious disease specialists to oversee ASPs.
Cultural differences
Self-medication of patients with antibiotics: In some countries, overthe-counter use of antibiotics may overrule clinicians.
Patient expectation for antibiotics: Patients are demanding antibiotics for some conditions even if no bacterial infection is evident. Education of patients and relatives may be needed to make them understand the problems of antibiotic overuse.
Falsely elevated or falsely decreased in PCT results.
Falsely elevated: severe trauma, surgery, cardiac shock, burns, systemic vasculitis (granulomatosis, Kawasaki disease, adult-onset Still’s disease and Goodpasture's syndrome), end-stage renal disease, post-antithymocyte globulin (ATG), alemtuzumab.
Falsely decreased: early course of infection > re-measuring, localized infections (osteomyelitis, abscess, subacute endocarditis), atypical infection (Chlamydia, Mycoplasma, Legionella, Salmonella)
PCT has pitfalls... Cut-off range depends on clinical setting.
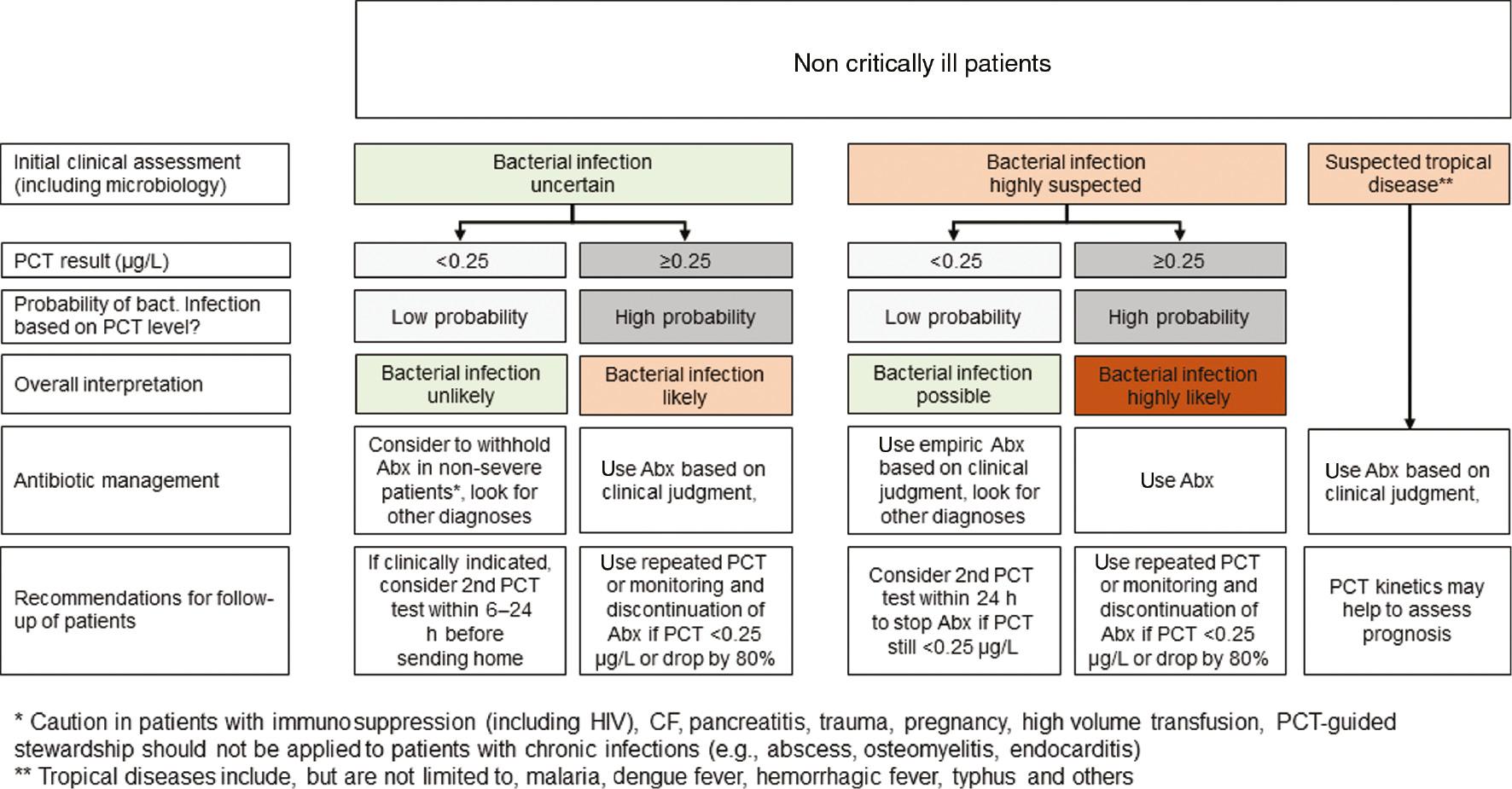
- PCT does not replace clinicians (pretest-probability)
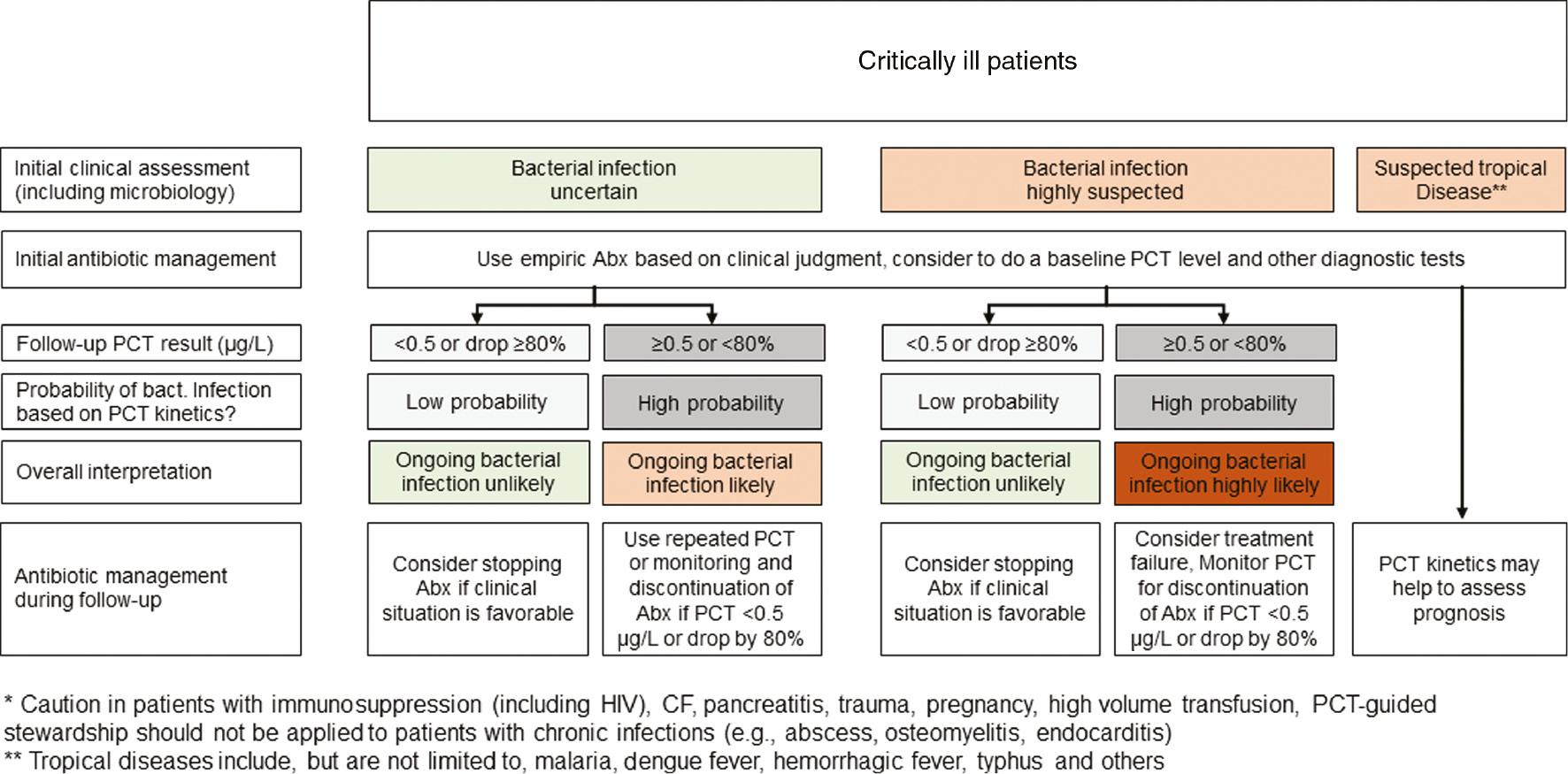
- Co-morbidity? setting? site & extent of infection? assay? False positive & negative values occur.
Pos: surgery, cardiac shock, cytokine storm
Neg: early, localized, subacute Single PCT measurement is of limited value.
- Course & progress if disease?
- Withhold antibiotic therapy? PCT cannot identify the bug.
- Should be used in additional to microbiology!
PCT-guided antibiotic stewardship in Asia Pacific countries
For most industries where microbes are used, it is important that they are properly preserved to ensure their viability, and beneficial properties are not altered. Many food and beverage companies have their own microbe banks. Such microbes usually consist of starter cultures, including genetically modified strains which offer better efficiency. They may also offer special properties, such as unique taste or resistance to contamination. These pure cultures are commonly called reference cultures.
Reference strains or reference cultures
They are pure microbes of known strains, biochemical and serological properties, and genetics. Sources of origins and storage facilities are clearly presented. So, users can employ them based on their scopes or purposes of uses.
Why is preservation of microbes required?
Microbiology laboratories need works of preserving microbes as pure reference cultures which are highly viable, and characteristics are completely maintained. It is due to the following reasons.
• Microbial isolation and purification are time-consuming and costly. Therefore, microbes shall be preserved appropriately for applications in various fields.
• Mutation occurs after continuous cultivation for a long time. It impacts on research or industrial production.
• Laboratories require viable, consistent, genetically intact, uncontaminated and easily accessible cultures.
• Genuine reference cultures are essential daily for quality control, comparison test with laboratory isolates, biological analysis, product standard analysis as control organisms, research and teaching purposes. For examples:
Product characterization testing reference such as:
• Efficiency testing of culture medium and disinfectants,
• Microbial tolerance test of products, such as walls, house paints, leather, for examples.
Contaminant indication of products such as antibiotics in animal feed, food, food contact materials, drugs, cosmetics, etc.
Classification of microorganisms such as API Kits, biochemical and biomolecular testing.
Microbiology laboratories where viable microorganism preservation is required, these may be employed in following applications:
• Quality control of culture media and testing methods,
• Sample preparation for quality control and training,
• Development and validation of new testing methods,
• Pathogens and spoilage microorganisms for routine tests or investigation of contamination,
• Microbiological analysis such as antibiotics and vitamin B12
• Isolates required for research purposes.
Should we preserve the reference microorganisms ourselves?
Reference microbes are essential, but there are many issues that laboratories should concern before collecting microorganisms in-house. For examples: what microorganisms should be stored, where the microorganisms are obtained, how long microorganisms to be kept, what
You can read full version of “Reference Cultures: Preservation and Application”. Scan to get a copy!

are storage techniques, what materials and equipment are required, is there any qualified person to operate and maintain the system, relevant regulations or laws, documents to be prepared for traceability and quality control, as well as operating cost. These are factors should be considered, conducting laboratory storage or rely on external reference microorganism service, in terms of suitability and economical aspects. Therefore, there are following options to preserve reference microorganisms:
• Laboratory storage of microorganisms, (availability of location, tools, responsible personnel and inspectors, including long-term storage cost),
• Deposit to the national microorganism preservation institute, (trustworthy agencies, expertise in such deposited microorganism),

• Employ a microorganism preservation institute for technical operations and store in a laboratory facility.
or click this link, https://forms.gle/2rByaVkbEWeQH4NZ9
2.5% ต่อปีในช่วงปี 2559-2563
(active ingredients) ปรับเพิ่มขึ้นมาก ผนวกกับ
(สัดส่วน 32.5% ของ
เพิ่มขึ้นถึง 862% ที่ระดับสูงสุดเป็นประวัติการณ์ 3.8 หมื่นล้าน
บาท (ปี 2559-2563 ขยายตัวเฉลี่ย 7.9%
ต่อปี) อาทิ Sinovac และ AstraZeneca
โดยไทยนำาเข้ายา (รวมวัคซีน) จากจีน เพิ่มขึ้นมากกว่า 900% จากปี 2563 อยู่
ที่ 2.2 หมื่นล้านบาท หากพิจารณาเฉพาะ การนำาเข้าวัคซีนจากจีน (สัดส่วน 50.0%
เพิ่มขึ้น
มากกว่า 3,000% จากปี 2563 ด้าน
เบลเยียม ไทยนำาเข้ายา +263.1% จาก การที่เบลเยียมเป็นแหล่งผลิตวัคซีนป้องกัน
COVID-19 ของ 3 บริษัท ได้แก่ บริษัท
Pfizer/BioNTech บริษัท AstraZeneca
และบริษัท Curevac ส่วนการนำาเข้ายา
2.3 แสน ล้านบาท (2.8% ของ GDP ปี 2565) เพิ่มขึ้นจาก 2.1% ของ GDP ปี 2553 (ที่มา: แผนพัฒนาสุขภาพแห่งชาติฉบับที่ 12) และ (2) การเข้าสู่สังคมเมือง ซึ่งวิถี ชีวิตต้องแข่งขันกับเวลา
อันดับ 1 ของประเทศ และมีอัตราความ
5% สูงกว่าค่าเฉลี่ย ประเทศ 2.7%) ด้านองค์การอนามัยโลก (WHO) รายงานว่าคนไทยเสียชีวิตด้วย กลุ่มโรค NCDs สูงถึง 76.6% (ปี 2562) สะท้อนความต้องการบริโภคยาในประเทศ

โดยเฉพาะยาจดสิทธิบัตร/ ยาต้นตำารับที่ใช้รักษาโรคซับซ้อน การเข้าถึงสิทธิ์การรักษาภายใต้ ระบบหลักประกันสุขภาพถ้วนหน้า ผ่าน บัตรประกันสุขภาพหรือบัตรทอง (71% ของประชากร) ประกันสังคม (18%) และ สวัสดิการรักษาพยาบาลข้าราชการ (8%) ซึ่งภายใต้ระบบฯ ดังกล่าว ได้เพิ่มช่อง ทางการเข้าถึงยาผ่านร้านขายยา อาทิ โครงการรับยาที่ร้านยาเพื่อลดความแออัด
ผลิตภัณฑ์อาหาร เสริม และเครื่องดื่มเสริมโภชนาการ ส่งผล ให้ผู้ประกอบการในอุตสาหกรรมยามีแนวโน้ม
เพื่อสุขภาพเพื่อเจาะตลาดผู้บริโภคกลุ่มนี้ มากขึ้น และสอดคล้องกับ
The value of the domestic market for pharmaceuticals is expected to grow by 4.5-5.0% in 2022 relative to the 2021 figure, thanks to the easing of fears about COVID-19 and the return of the Thai economy to somewhere close to its normal level, with the subsequent rebound in growth then feeding into stronger spending power. This has helped to encourage patients to return to hospitals and clinics as they seek treatment for health issues, resulting in greater overall demand for medicines and medical supplies. Beyond this, the reopening of the country to foreign arrivals has further boosted demand for pharmaceutical products, especially within tourist areas. Over 2023 to 2025, the market is expected to continue to grow, thanks to the likely ongoing rise in incidences of chronic non-communicable diseases (NCDs), the extension of universal health coverage programs to cover the whole of the Thai population, the increasing number of foreign patients seeking treatment in Thai hospitals, and the rising global concerns with wellness and preventative healthcare that are influencing Thai consumers.
However, the industry will also face challenges in the form of: (i) the country’s lack of manufacturing capacity, which means that the Thai pharmaceutical industry is highly dependent on imports; (ii) intensifying competition from new players, both Thai and international; and (iii) rising overheads, which are being driven up both by the need to overhaul and upgrade production lines to comply with the GMP-PIC/S standard, and by the greater cost of inputs. The net effect of these pressures will then be to place a limit on players’ ability to generate profits.
Over 2023 to 2025, the domestic market for pharmaceuticalsis expected to grow on increasing rates of NCDs among the general population, the broader access to healthcare provided for by the Universal Coverage Scheme, the greater number of overseas patients seeking treatment in Thai hospitals, and global trends that are encouraging greater consumer interest in wellness and preventative healthcare. However, stiffening competition will limit how far operators’ profits may grow.
Manufacturers of pharmaceuticals: Income will tend to strengthen on:
(i) continuing demand for COVID-19 vaccines and medicines to treat the illness once contracted,
while the relaxation of pandemic controls means that social and economic activity has returned to normal and with this, individuals are now increasingly seeking treatment in hospitals;
(ii) the steady rise in rates of NCDs;
(iii) the greater access to medical services provided by government welfare programs, which is underpinning growth in the volume of medicines distributed through hospitals, especially for treatments that are under patent; and
(iv) the ability of manufacturers to increase distribution through pharmacies and expand export markets in the ASEAN region, where demand for medicines and vaccines remains strong.
There are some challenges in this business as follows: (i) The entrance of new players which will intensify market competition; (ii) the steadily rising cost of imported precursors; (iii) moves by the government to control the prices of medicines used in private hospitals and business, which may make it difficult for manufacturers to raise their prices; and (iv) the additional costs imposed by the need to upgrade facilities to meet the GMP-PIC/S standards, which would affect business margin.
Distributors of pharmaceuticals (retailers and wholesalers): Income growth will be steady in the coming years, helped by: (i) increased interest in personal healthcare and preventative medicine; (ii) recovery in the tourism sector, which will bring increased footfall in pharmacies, especially in the major tourist areas; and (iii) the broadening of distribution channels to include online sales, advertising on digital media, and the development of mobile phone applications that offer health advice. However, it is also likely that players will have to contend with greater competition within their particular segment.
- Stand-alone pharmacies will likely have to contend with a worsening threat from large chain stores. For example, Fascino (a chain of pharmacies) plans to extend its network from its current total of 105 branches (as of 2020) by opening another 200 franchises by 2022, Save Drug (part of the Bangkok Hospital group) also hopes to expand its network (from current 80 stores nationwide). Pure Pharmacy (by BIG C) aims to expand additional 7 branches in 2022 from 146 branches in 2021. In addition, the range of retail sites from which pharmaceuticals are sold is broadening to include discount stores, supermarkets (of which at least 50 new branches open every year) and convenience
stores (7/11 plans to open 700 new branches a year).
- Wholesalers are increasingly moving into the market space currently occupied by retail operations. They expand new distribution channels, provide promotion campaign and advertise via online media so that they can respond to customers’ needs, with the lower cost of medicines purchased as their main advantage.
The pharmaceutical sector includes conventional medicines and medical supplies which are used in the diagnosis and treatment of illnesses. Conventional pharmaceuticals can be split into two groups:

1) Original drugs or patented drugs are medicines that have gone through the lengthy research & development (R&D) process, and therefore would involve significant investment. Manufacturers of original drugs are normally given a 20-year patent protection and when the patent expires, other manufacturers are then allowed to produce those medicines.
2) Generic drugs: These are drugs that are manufactured by a company that was not responsible for their development, but which begins to produce them once the patent for the original drug expires. Although they may be produced under a trademark, this will not be that of the primary manufacturer. Nevertheless, generic drugs are identical to their original counterpart in terms of active ingredients, and so their therapeutic value is likewise indistinguishable. However, because manufacturers of generics may be able to source cheaper inputs and are freed from the burden of research and development costs, generic manufacturing costs may be significantly lower than those of original drugs.
The continual and costly R&D required in the development of new medicines and medical supplies have prompted many global producers of pharmaceutical and medical supplies, especially original drugs, to cluster in developed economies such as the United States, Europe, and Japan, because of easy access to skilled professionals, expertise and manufacturing technology. These countries then export to meet global demand, while developing countries are left to play the role of importers of expensive patented medicines. The conventional medicines production chain is split into three stages.
1) Primary: This involves R&D of new medicines.
2) Intermediate: This involves the production of ingredients to be combined to make the final product.
These are either active pharmaceutical ingredients (APIs), or inactive pharmaceutical ingredients (or excipients) that are normally added to speed up chemical reaction. The ingredients manufactured in this stage are normally already available in the market but required special techniques to achieve the desired chemical reaction or to change the molecular structure of existing chemicals, which normally require advanced technology and a large investment.
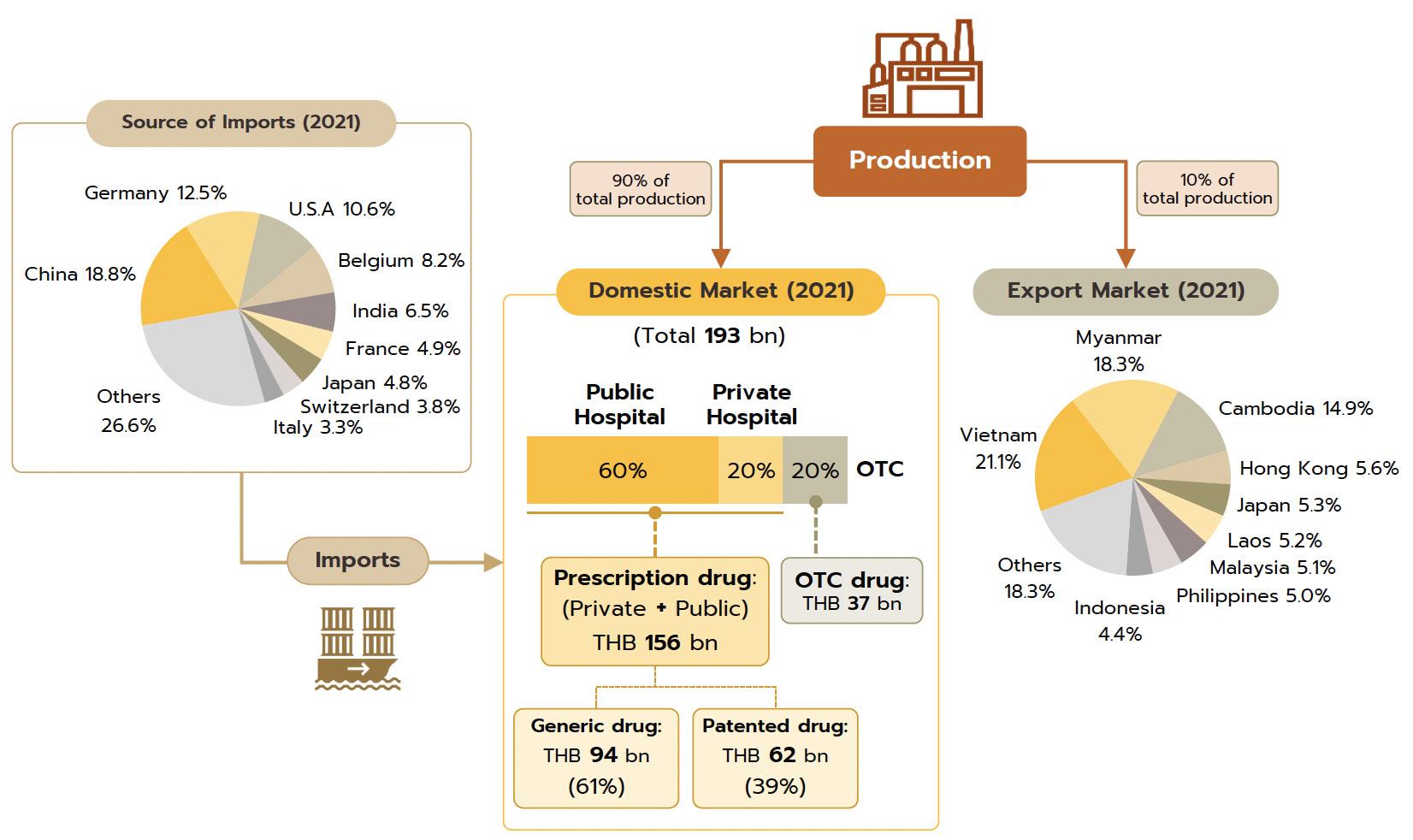
3) Manufacture of finished products: This is usually a manufacturing of generic drug, and generally depends on imports since Thailand in fact sources some 90% of the inputs used in the manufacture of pharmaceuticals from abroad. These imports are then used to produce generics in the form of pills, liquids, capsules, creams,
powders, and injectables. Product group with the highest value is pain medication. Most Thai players in the pharmaceuticals industry fall into this group.
Figures from the Thai Food and Drug Administration (FDA) indicate that as of December 2021, Thailand was home to 151 pharmaceutical production facilities that met the Good Manufacturing Practice (GMP) standards, though only 8% of these were able to manufacture active pharmaceutical ingredients. Output from the latter includes products such as aluminum hydroxide, aspirin, sodium bicarbonate, and deferiprone, most of which is used as inputs into manufacturer’s own production processes. Research and development also tends to be restricted to work on vaccines (e.g., for HIV, influenza/bird flu, and most recently, for COVID-19). Thailand has in fact had a strategic national plan for vaccine development since 2005, and at present, stage two of this, which covers 2023-2027 (stage one covered 2020-2022) is being drawn up. This lays out the goal of providing for the full vaccination of the population in both normal and emergency situations, as well as expanding domestic vaccine production to a level where this can replace imports.
Players in the medicines sector can be split into two groups
Group 1 comprises government agencies: (i) the Government Pharmaceutical Organization (GPO) as a manufacturer of original drugs and importer of high-value products, especially treatments for non-communicable diseases (e.g. cholesterol-lowering medications, diabetes); to be sold at low prices; and
(ii) the Defense Pharmaceutical Factory, which emphasize the production of generic drugs as alternatives to imported drugs.
The Government Procurement and Supplies Management Act B.E. 2560 has specified GPO as an entrepreneur similar to the private producer in the sector. This created a level playing field for private enterprises and increased competition between the GPO and private sector players, including suppliers in India and China which export low-cost products.
Group 2 comprises private sector producers. This can be divided into two sub-groups: (i) local manufacturers with Thai shareholders, which typically produce general-purpose low-cost generic drugs. Examples include Siam Pharmaceuticals, Berlin Pharmaceutical Industry, Thai Nakorn Patana, Biopharm Chemicals and Siam Pharmacy. Contract manufacturers such as Biolab, Mega Lifesciences and Olic (Thailand) also belong in this group; and (ii) multinational corporations (or MNCs) with foreign shareholders, which focus on original drugs and operate as agents to import pricier drugs for distribution in
Thailand, though some have also established production facilities in the country. Operators in this group include Pfizer, Novartis, Roche, and Sanofi-Aventis. In 2021, AstraZeneca (Thailand) had the largest share of revenues by providing COVID-19 vaccines via domestic production and imports.
Currently, two laws govern the manufacture of pharmaceuticals in Thailand. They are: (i) Patent Act B.E. 2522 (1979) and amendments, which grant patent-rights to discoverers and inventors (i.e. protects intellectual property rights), supervised by the Department of Intellectual Property; and (ii) Drug Act B.E. 2510 (1967) and amendments, which regulate the manufacture, import, sale and marketing of drugs in Thailand. In terms of regulatory bodies, the Food & Drug Administration (FDA) is responsible for overseeing compliance in the sector. Its tasks include licensing operators and registering drugs for domestic distribution.
In distribution, approximately 90% of Thailand’s pharmaceuticals output is consumed by the domestic market. Drugs and medicines now
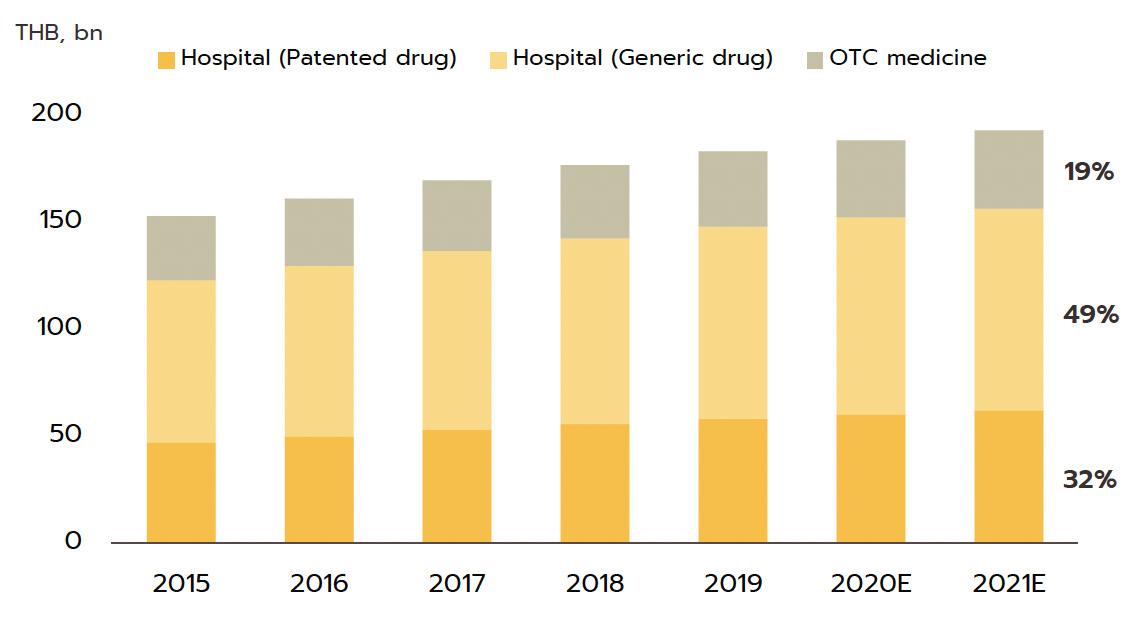
account for a quarter of all domestic medical expenses. This was largely triggered by the expansion of national universal healthcare coverage (UHC), specifically the Universal Coverage Scheme (UCS) which now covers 99.61% of total eligible insured persons population. This has increased access to healthcare for most of the population nationwide, which naturally caused the consumption of medicines to jump (The Fiscal year 2021 budget of the Universal Coverage Scheme (UCS) rose to THB 190bn, up 2.2% from the FY2020 budget).
Domestic market: Medicines and pharmaceuticals are distributed through two main channels.
Hospitals : Thailand’s public healthcare system is extensive, covering both civil servants and the majority of scheme claimants. By value, 80% of the total domestic market for medicines is distributed through hospitals, comprising 60% government hospitals and 20% private-sector operations. Medicines distributed through hospitals are generally prescription drugs, which can be further sub-divided into (i) generic drugs, which account for 61% of the value of medicines distributed via hospitals, and (ii) patented drugs, which make up the remaining 39%. But although this latter group has a smaller share, consumption of patented drugs is growing faster than the consumption of generic drugs, because they are mostly used to treat common chronic non-communicable conditions such as high blood pressure, diabetes, and heart disease.
Over-the-Counter (OTC) medicines: Although the government health insurance scheme encourages individuals to increasingly seek medical care in hospitals instead
of buying OTC medicines from pharmacies, the latter remain an important distribution channel for those with common minor ailments which can be treated with a quick trip to a pharmacy. Hence, the value of the OTC drugs market has been stable at 20% share of the total market for medicines. There are 22,205 registered pharmacies in Thailand (source: FDA, August 2022), 18,551 are modern pharmacies (83.5%), 19.8% of which are in Bangkok and 80.2% in the provinces. Pharmacies can be split into the two major groups: (i) stand-alone stores, mostly SMEs, which account for around 75% of modern pharmacy outlets in the country, and (ii) chain stores, which may either be run, centrally-funded, or organized for expansion through franchising, such as Fascino and Save Drug (a member of BDMS). Beyond this, modern trade outlets (including discount stores, supermarkets, convenience stores, and specialist health stores) are turning over a part of their floor space to medicines and pharmaceuticals, and so are able to reach a wide range of customers.
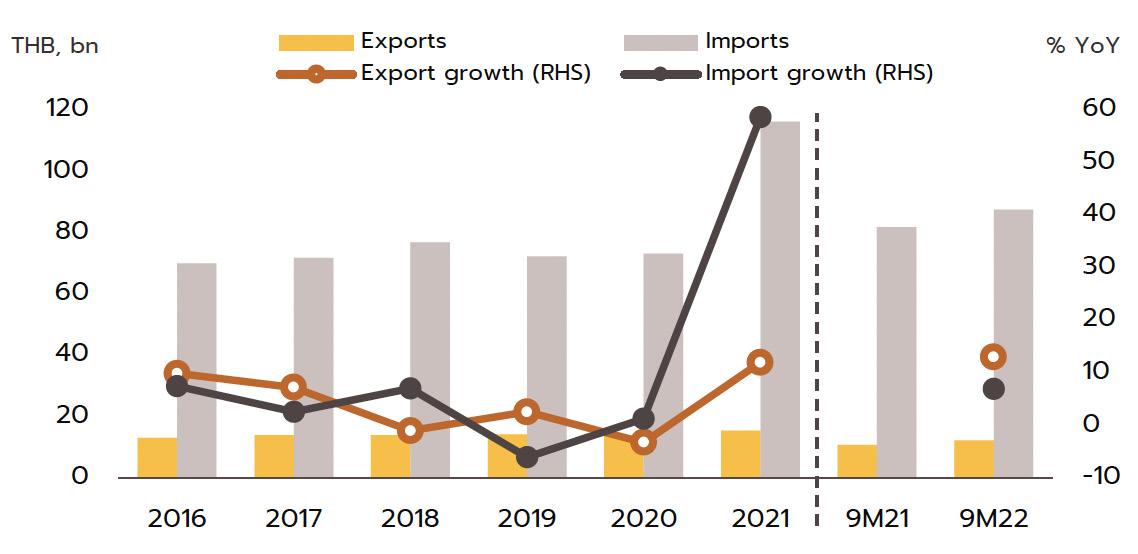
Export market: The value of Thai pharmaceutical exports increased at an average annual rate of 5.1% over the years 2014 to 2020, though because these are generally of lowvalue generics, this represented just 0.2% of the value of all national exports. Overseas sales are largely to countries in Southeast Asia, and so almost 60% of overseas sales are to Myanmar, Vietnam, Cambodia, and Lao PDR. The extended COVID-19 pandemic benefited Thai exports of pharmaceuticals, especially of vaccines, which in 2021 accounted for 12.9% of all pharmaceutical exports by value, up around 2.0% annually. In contrast, imports are
mostly of high-cost goods that cannot be manufactured domestically, such as anemia treatments, antibiotics, and cholesterol-lowering drugs, and pharmaceutical imports thus account for 1.0% of all imports to Thailand. These most often come from Germany, the US, and India, from which Thailand is importing an increasing volume of cheap generics (Share of drug imports from India has risen to 8.4% of the total by value, from 5.9% in 2013); India benefits from advantageous patent licensing laws that allow it to produce low-cost generic versions of original drugs, and the Thai pharmaceutical industry thus runs a permanent balance of trade deficit. However, the shape of the market changed markedly with the outbreak of COVID-19 since this led Thailand to ramp up imports of vaccines from China, Belgium, Germany, the US, and France and so in 2021, vaccines accounted for a full 32.5% of the value of all pharmaceutical imports, up from just 5.4% in 2020.
Private sector pharmaceuticals manufacturers typically face pressure from (i) Imports of cheap drugs from India and China, whose production costs are lower than Thailand’s; (ii) Disadvantages of private manufacturers relative to the GPO in terms of manufacturing and distribution; (iii) The Ministry of Public Health and the Comptroller General Department’s list of reference prices for approved drugs, which is used as a tool to control expenses and set appropriate costs for the purchase of pharmaceuticals by public healthcare providers;
(iv) rising manufacturing costs on production in private sector following the implementation of the GMP-PIC/S standards; and (v) appropriate storage and sufficient distribution according
to Public Health Ministry’s rules on conventional medicine distribution (effective January 1, 2022).
The worsening state of the pandemic and the steady rise in caseloads through 2021 stoked much stronger demand for products used in the prevention and treatment of COVID-19, including personal protective equipment, vaccines, analgesics and antipyretics, treatments for the respiratory system, vitamins, and traditional herbal cures. One outcome of this was that officials reported a significant jump in the number of businesses connected to the sale of medicines and of medical supplies and equipment, which went from 994 in 2019 to 1,792 in 2021. However, the number of patients seeking treatment in hospitals for non-urgent or non-critical conditions dropped and so demand for many medicines softened, a situation that was amplified by the difficulty Thais and non-Thais had traveling. However, at the same time, pharmacies took on a much more central role linking
state services, hospitals, and the public and so although trade came under pressure from the overall weakness in consumer spending power, pharmacies benefited from increased sales to some consumer groups. This trend was particularly noticeable for those with chronic non-communicable diseases, such as diabetes, hypertension, asthma, and mental health conditions, who tended to avoid hospitals for fear of contracting COVID-19 and instead made purchases of medications directly from pharmacies. The overall situation with the pharmaceutical industry through 2021 is summarized below.
The value of medicines distributed to the domestic market rose by 2.5% in 2021, and although these generated receipts of THB 193 billion, the rate of growth was down slightly from 2020’s 2.8%. Income was boosted by sales of drugs for the treatment of COVID-19 and of medical equipment such as disposable gloves, blood pressure monitors, and pulse oximeters that were often bought for storage at home, while treatments for non-communicable
chronic conditions remained largely unchanged, regardless of the fluctuating outlook for the pandemic. However, sales of medicines to treat one-off or emergency conditions such as antibiotics or medicines for diarrhea or conjunctivitis fell. This was because through the year, people spent more time at home, they wore a mask when in public, and they spent less time in the company of others and so overall rates of infection for these types of condition tended to drop.
1) Prescription drugs and medicines distributed through hospitals, the main domestic market for pharmaceuticals, grew 2.6% YoY. Receipts were split between THB 94 billion from generics (up 2.3%) and THB 62 billion from patented drugs (up 3.2%).
2) ‘Over the counter’ (OTC) distribution through pharmacies rose 1.7% to a value of THB 37 billion. Sales were lifted by changes in government policy that gave pharmacies a more central role in the distribution of medicines, for example by offering incentives to government welfare card holders to buy medicines from pharmacies
participating in the ‘Pracharat Blue Flag’ project and by encouraging members of the public treated under the Universal Coverage Scheme to help reduce crowding in hospitals by making purchases in pharmacies instead. Pharmacies have further expanded their role by becoming centers for the provision of services to high-risk groups (with regard to COVID-19 and other diseases), by distributing medicines and advice, and by providing access to services via telephone and mobile applications.
Domestic output of pharmaceuticals contracted -4.0% YoY to 42,000 tonnes, having already fallen -6.1% in 2020. This decline was driven by the imposition of strict COVID-19 controls that included closing high-risk environments, restricting travel across provincial borders, and preventing large numbers of people gathering together. In addition to this, demand for medicines to treat general illnesses also softened. Thus, production of solutions and liquids, which with a 47.2% share of output was the largest single product group, slipped -2.9%, while output of pills and tablets (31.5% of output), injectables (4.2%) and capsules (3.4%) fell by respectively -6.8%, -22.9% and -2.5%. On the other hand, output of powders (8.2% of output) and creams (5.0%) edged up by respectively 1.2% and 1.0%. Average capacity utilization across the industry also worsened in the year, falling from 56.9% in 2020 to 54.5% a year later. However, as the year closed, output picked up again, and for the last quarter of 2021, production reached 24-quarter high. This turnaround was benefited by the request by the Government Pharmaceutical Office (GPO) that private sector companies licensed for the production of treatments for
chronic non-communicable diseases should increase output so that the GPO could then switch its production away from these products and instead start to manufacture the COVID-19 treatment Favipiravir, which began to be distributed domestically at the start of August 2021.
Export value strengthened 12.0% in 2021 to a total of THB 16 billion on a surge in demand for vaccines against and treatments for COVID-19, which spread across the region in the year. Exports to the CLMV countries (responsible for a combined total of 56.2% of all export value) rose 8.0% overall, with those to Malaysia (5.2% of exports) +41.6%, the Philippines (5.0%) +8.6%, and Indonesia (4.4%) +156.3%. Given the effects of the pandemic, it is not surprising that exports of vaccines jumped 590% YoY to generate THB 2.0 billion in receipts. This stood against average annual growth of 21.3% over the period from 2016 to 2020. Growth was strongest in markets in Indonesia (up +3,635%, from a background rate of +5.0%), which absorbed 21.4% of vaccine exports by value, followed in importance by the Philippines (up +1,388%) and Vietnam (up +731%). Imports were also increasing strongly in the year, and compared to average growth of 2.5% per year between 2016-2020, the 58.7% jump brought the value of imports to a historic high of THB 116 billion. This sudden increase resulted from a combination of the rising cost of active ingredients and much higher imports of vaccines (e.g., from Sinovac and AstraZeneca), which surged 862% to another historic high of THB 38 billion. Vaccines thus had a 32.5% share of all imports of pharmaceutical goods, having grown at the much more sedate rate of 7.9% over the
five years prior to 2020. Including vaccines, imports from China also strengthened by more than 900% to THB 22 billion, though considering only Chinese vaccines (50.0% of all vaccine imports by value), the year-on-year increase climbs to over 3,000%. Imports from Belgium were also up 263.1% since this is the home of three manufacturers of COVID-19 vaccines, namely Pfizer/BioNTech, AstraZeneca, and Curevac. Imports from Japan rose 47.8% on strong demand for Favipiravir, while those from the US and Germany were up by 42.3% and 35.4%, respectively.
The outbreak of COVID-19 acted as a spur to investment in the pharmaceuticals industry, as reflected in the rise in applications for investment support as per the government’s 2015-2022 policy for investment promotion. Thus, in 2021, applications were received for 10 projects with a combined value of THB 1.87 billion, a rise of 238.3% relative to 2020. In the year, applications from 9 projects were approved, the value of these climbing 201.6% YoY to THB 1.6 billion, and almost 90% of these were for the production of medicines.
Over the first 9 months of 2022, the domestic market strengthened on a combination of factors. (i) COVID-19 continued to spread and from January 1 to September 30, an estimated 2.45 million new cases were seen. Some of these required hospital treatment, but many made do with home isolation, and so demand for medicines including antipyretics, analgesics, cough medicines, medications for sore throats, vitamins, and herbal medicines remained solid. (ii) The relaxation of pandemic controls allowed economic and social life to return to normal, and with this,
patients began to return to hospitals. (iii) With the reopening of the country, tourist numbers have started to rise again, and so for the first time in two years, health tourists and those seeking treatment in Thai hospitals began returning to the country. The consequences of this strengthening of demand are described below.
Pharmaceutical sales to the domestic market increased for almost all categories. Thus, sales were up 18.1% YoY for tablets and pills (49.5% of the value of the domestic market for pharmaceuticals), 29.2% YoY for liquids and solutions (22.9% of the market), 5.2% YoY for injectables (8.0%), 4.5% YoY for capsules (7.6%), 13.0% YoY for creams (6.8%), and 30.7% YoY for powders (5.3%). Naturally, this also improved the situation with capacity utilization, which climbed from an average of 54.9% in 2021 to 60.0% for 9M22.
The value of exports also climbed in the period, rising 13.2% YoY to THB 12 billion. Sales from the category of ‘medical treatments
and others’ comprised a full 95.6% of the total, and these were up 23.3% YoY. The remaining 4.4% of exports were of vaccines, and over 9M22, sales of these slipped -59.3% YoY. Sales to the CLMV zone (55.7% of total export value) were up 13.1% YoY overall, with those to Malaysia (7.2% of the total), Japan (5.7%), and the Philippines (5.2%) rising by respectively 59.4% YoY, 14.4% YoY, and 32.2% YoY. Alongside this, sales of vaccines to Myanmar and Cambodia rose by 800% YoY and 272% YoY, respectively. Imports increased by 7.0% YoY to THB 88 billion over 9M22, this being partly driven by the decision by the Food and Drug Administration to allow licensed private-sector players to import and distribute COVID-19 treatments (including molnupiravir, favipiravir, and remdesivir) to hospitals. Imports of goods in the category ‘medical treatments and others’ (68.4% of the total) edged up just 0.3% YoY, while those of vaccines (31.6% of the total) climbed 25.0% YoY. Overall imports
from the main suppliers of Germany, the US, India, and France (together 48.6% of the total) rose 53.2% YoY. Given the high baseline set a year earlier, imports from China slumped -87.0% YoY.
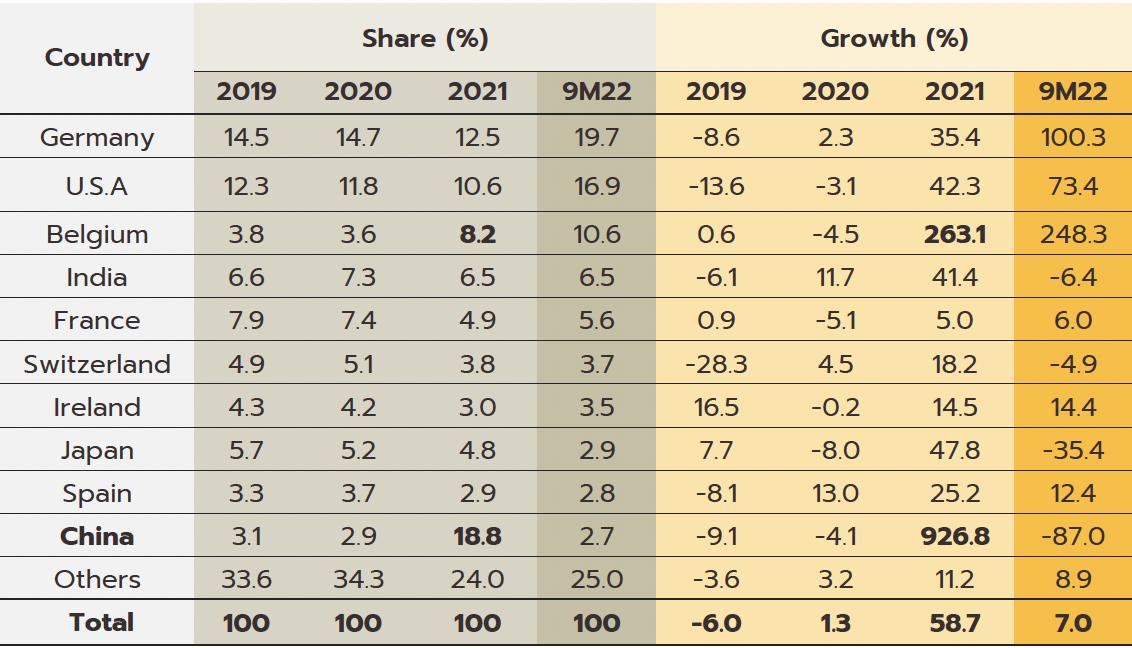
Looking just at vaccines, imports of these have exploded under the continuing pressure of the COVID-19 pandemic. By value, imports from Germany (29.4% of all vaccine imports) were up 3,631.3% YoY, while those from the US (28.3%) jumped 359.1% YoY thanks to ongoing demand for the Moderna and Pfizer vaccines. However, the abating of the earlier push to source COVID-19 vaccines from China means that the country accounted for just 1.1% of the segment, down from 50.0% in 2021, and these declines have been almost equally strong in terms of volume (-97.6% YoY) and value (-98.1% YoY).
The pharmaceuticals industry should see ongoing growth through
2022, helped by demand for medicines and medical supplies that will be boosted by several factors; (i) The effects of the COVID-19 outbreak are lessening and both society and the economy have now largely adjusted to its impacts. As such, life (including the level of economic activity) has mostly returned to its pre-pandemic state. (ii) Spending power will tend to strengthen in line with growth in the economy (Krungsri Research sees GDP growth reaching 3.2% this year, up from 1.6% in 2021) and this will help to bring patients back to hospitals for treatment. However, the effects of this may be limited by the continuing high cost of energy, which has pushed up the prices for goods and services and hence inflation. This will drag on spending power in some consumer groups. (iii) The full reopening of the country from July 1, 2022, will add substantially to tourist arrivals, including those traveling for health reasons. For all of 2022, Thailand expects to welcome 10.4 million visitors, up from just 0.43 million a year earlier. This will then boost demand for medicines, especially in the main tourist areas. In addition to these factors, COVID-19 is now an endemic disease and so large numbers of people will continue to be infected with it, strengthening overall demand for medicines, medical supplies, and vaccines. Krungsri Research thus expects that the value of the domestic market for these goods will grow by some 4.5-5.0% relative to a year earlier. Distribution through hospitals is benefitting from the government’s decision to allow hospitals to secure their own supplies of certain medications (from September 1, this has included favipiravir, molnupiravir and paxlovid). Likewise, pharmacies have also increased their
role in the market by becoming providers of healthcare services and by completing doctors’ prescriptions for COVID-19 related medications (over 800 businesses have joined the government’s self-isolation program for non-severe COVID-19 cases, and as of August 29, 55,482 patients with mild symptoms had been treated under this.) Pharmacies are also completing prescriptions for some treatments for non-communicable diseases, including diabetes and hypertension, and over 1,000 companies have joined a scheme to promote the distribution of medicines through pharmacies. Overall, the value of medicines distributed through hospitals and over the counter is therefore forecast to increase by respectively 5.2% and 4.5% YoY.
Growth will extend through the period 2023 to 2025 and the domestic market should increase in value by an average of 5.0-6.0% per year. By segment, distribution through hospitals will perform slightly better, growing at the average annual rate of 6.3%, compared to the expected 5.0% growth for OTC distribution. Factors supporting this growth are outlined below.
Rates of illness can be expected to increase for both communicable and non-communicable diseases (NCDs). Among the former, the most common are, in order, diarrhea, pneumonia, and dengue fever, while newly emerging diseases will also likely become more common, both at home and abroad. Recent examples of these have included SARS, bird flu, H1N1 influenza (2009), Ebola, Zika, monkeypox/Mpox (cases of which have recently broken out), and of course, COVID-19. In the case of the last of these, Pfizer expects that this will become endemic worldwide
by 2024, but with more than 50 major mutations recorded since the disease was first identified, the effectiveness of a double dose of vaccinations has declined significantly. With regard to NCDs, the most common are hypertension, diabetes, chronic obstructive pulmonary disease, heart disease, and strokes. Two principal factors have combined to increase rates of these. (i) Thailand is already an aging society, that is, more than 10% of the population are over 60. This has increased the incidence of conditions such as hypertension (almost half of the elderly in Thailand have elevated blood pressure), diabetes, heart disease, strokes, and cancers. Thailand is now expected to become an aged society (i.e., 20% of the population will be over 60) in 2023 and a super-aged society (i.e., more than 28% of the population will be over 60) by 2033. This will then add to medical bills, and as of 2022, the cost of providing medical care to the elderly has risen to THB 230 billion, or 2.8% of GDP and up from 2.1% in 2010 (source: 12th National Health Action Plan). (ii) Increasing urbanization will mean that an ever-greater share of the population will have to endure a rushed, high-pressure lifestyle lived out in a polluted environment and with only limited opportunities for exercise. This will add to rates of physical and mental ill-health. Indeed, Bangkok has the highest rates of cancer in the country, and at 5%, rates of depression are almost double the national average of 2.7%. Data from the World Health Organization for 2019 indicates that 76.6% of Thai deaths are attributable to NCDs, and this underscores the high and rising demand for medicines, especially for the patented drugs needed to treat more complex conditions.
For the Thai population, access to healthcare services has expanded substantially, and this may now be accessed through the Universal Coverage Scheme/Gold Card (which now covers 71% of the population), social security (18% of the population), and the civil service scheme (8% of the population). Recently, the government has extended the reach of these by including pharmacies among the channels through which healthcare is delivered to the public. This has been achieved through the ‘Pracharat Blue Flag’ project, the program to reduce crowding in hospitals by transferring demand to pharmacies, and an increase in the use of technology to dispense advice on health and medications, the latter including a greater reliance on telemedicine and the deployment of phone apps such as Mor Dee/Good Doctor and Clicknic (for which the userbase rose from 3 million in 2020 to 4 million a year later). Thanks to a combination of the greater convenience that these changes will bring and a wish to reduce the risk of infection, in the future the public are expected to show a much greater tendency to access health services through pharmacies, rather than by visiting a hospital. This will likely be most noticeable for those looking for treatments for NCDs such as diabetes, hypertension, asthma, and some mental health problems. Furthermore, private health insurance take-up is also expanding (coverage rose 10.6% in 2021), and this will add further to demand for products from the pharmaceuticals industry. Given this, for the period 2023-2025, spending on healthcare (medicines and treatments) will expand by an average of 7.6% annually, compared to a rise of 7.0% in 2022. This will
be split between increases of 8.1% in the public sector and of 5.7% in the private sector, which compares to rises of respectively 7.6% and 5.3% in 2022.
Foreign patients will return to Thai hospitals in larger numbers over the next few years, and Krungsri Research predicts that foreign arrivals (both regular and medical tourists) will number 22.7 million in 2023, 35.3 million in 2024, and 40.4 million in 2025. Medical tourists mostly come from East Asia, Europe, and the Middle East, as they are attracted by Thailand’s status as one of the world’s leading providers of medical tourism services. Within this market, Thailand draws particular strengths from the quality of its services, the high standard of the care that is provided, and the lower costs compared to competitors in Singapore and Malaysia. Growth in this market will then add further to demand for pharmaceuticals.
Demand for wellness and preventive healthcare services and products is also on the rise, though growth in this market was accelerated greatly by the outbreak of COVID-19 in 2020. Consumers are thus increasingly changing their regular day-to-day behaviors as they try to avoid ill health, while hospitals and other organizations have also adapted their business strategies to improve health outcomes. This is then likely to support stronger demand for pharmaceuticals and other medical goods, especially for vaccines or products that are believed to boost the immune system such as vitamins, traditional Thai herbal preparations, food supplements, and health drinks. Players already active in the pharmaceutical industry will likely respond to these changes in
consumer behavior by introducing or distributing new health products as they look to stake a claim to this rapidly growing market. This outlook is in keeping with work by Euromonitor, which estimates that the global market for health products will expand at an average annual rate of 5.7% over 2021-2025, up from the 3.4% growth maintained over the previous 5 years.
Continuing technological progress will help to make manufacturing processes more efficient and to expand the public’s access to medical treatments. Thus artificial intelligence and machine learning will help to increase the speed and efficiency of research and development programs; use of big data analytics will help manufacturers rapidly access in-depth information at all stages of the manufacturing process; use of bioreactor systems and lowenergy continuous manufacturing processes will maximize output while minimizing wastes; 3D printing will allow players to manufacture human cells or tissue to use in drug development; and 5G technologies will facilitate the development of online platforms and the provision of telepharmacy services, and help to connect pharmaceutical supply chains from upstream manufacturers to downstream consumers (e.g., Medcare). Taken together, this will help to increase the overall ease and convenience of the system, reduce travel and waiting times, and provide easier access to healthcare services, hopefully before any conditions become too serious.
The industry will in addition benefit from government policy that aims to increase investment inflows into the production of pharmaceuticals. (i) BOI investment promotion schemes
ฺBox: BOI Incentives Offered to Investors in Pharmaceuticals and Biotech Industries
ฺBox: Development and Production of Medicines, Vaccines, and Bio-pharmaceuticals under BGC Action Plan
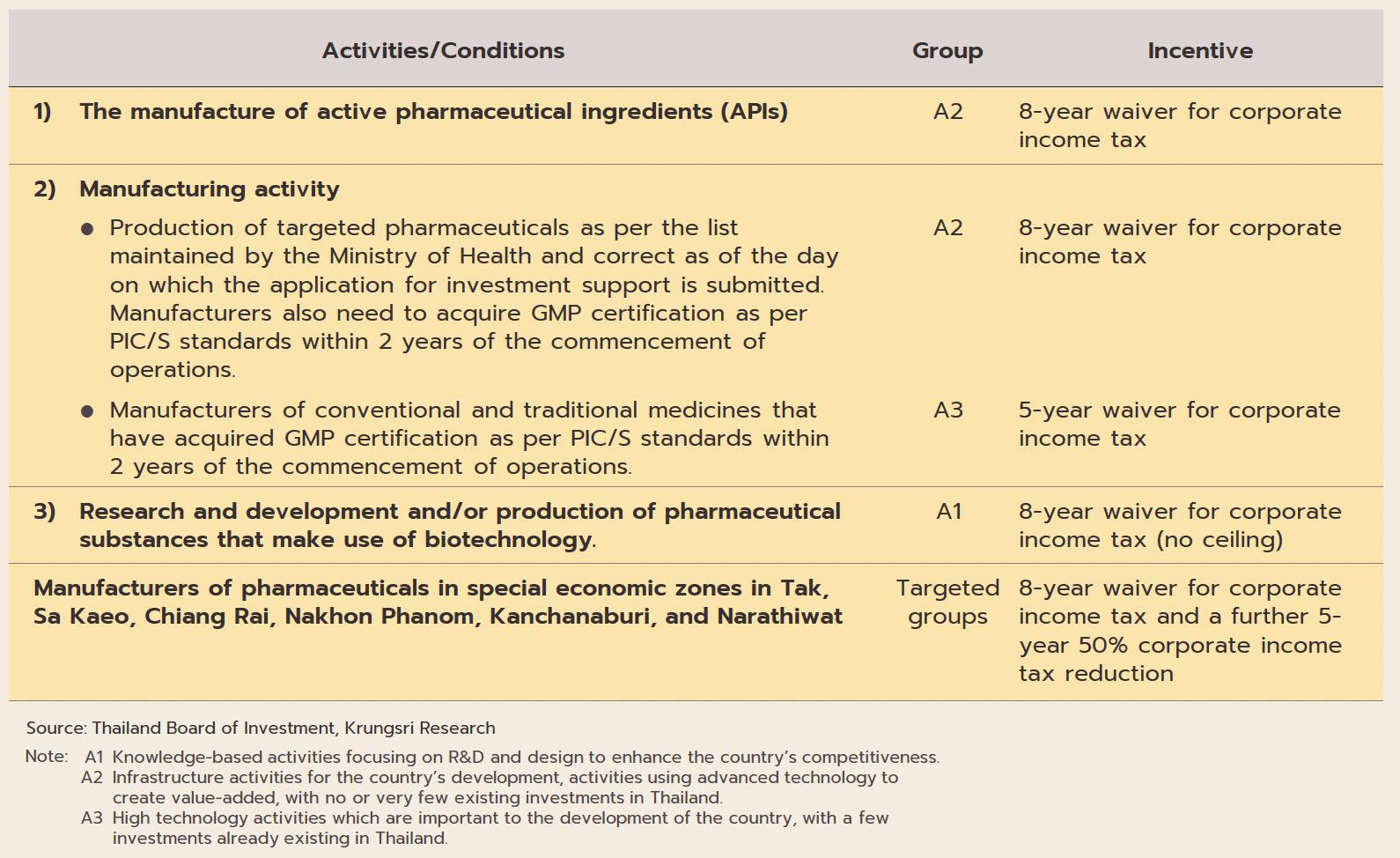
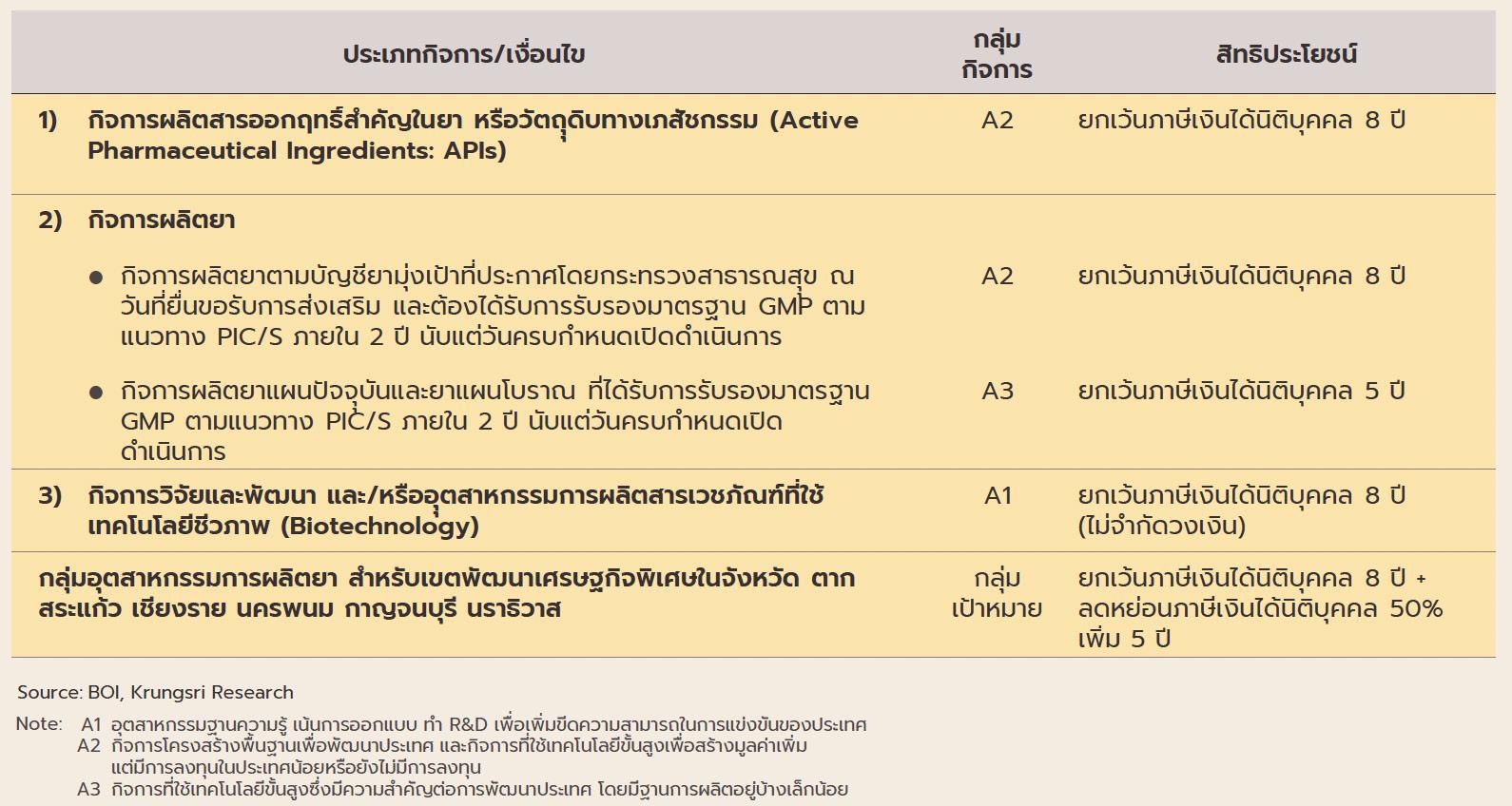
aim to stimulate greater investment in the medical and related industries by offering 8-year corporate tax waivers for manufacturers of active pharmaceutical ingredients (APIs) and 5-year waivers for general manufacturers of pharmaceuticals.
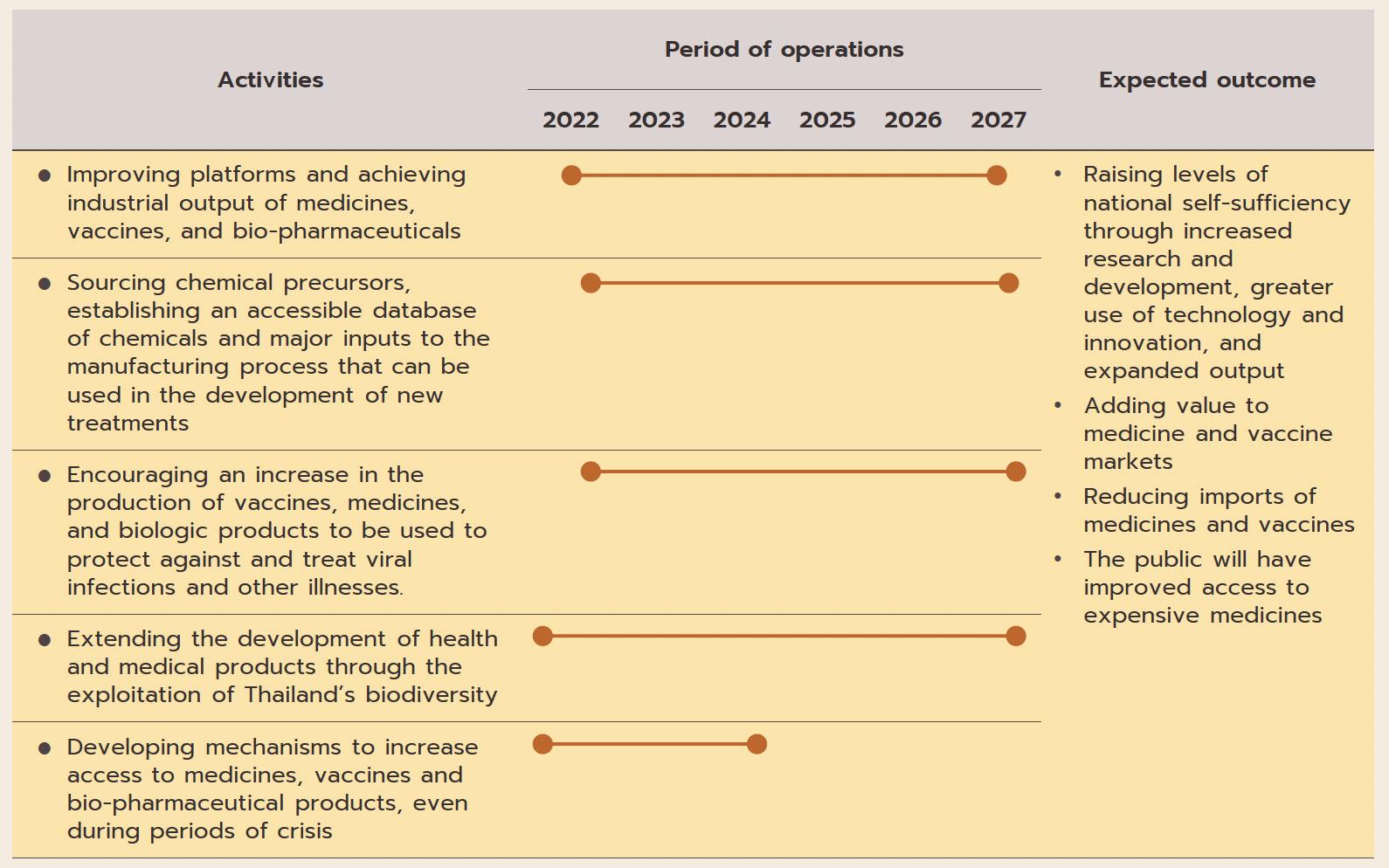
(ii) The pharmaceuticals industry is one of the government’s new S-curve industries in the EEC area, and promotion of the industry should lead to an increase in research and development, which will then help to lower production costs below those of imports, especially for medicines that require high-tech production processes. As part of this, the government is providing funding for R&D and offering further tax breaks (in the first half of 2022, one application was made for investment support for biotechnology-related R&D totaling THB 3.7 billion). (iii) Measures to support the domestic production of pharmaceuticals over 2023-2027 aim at increasing the value of national pharmaceuticals output by THB 100 billion and to grow export markets to a value of THB 13 billion. The motivation behind this is the wish to increase the security of the domestic supply of medicines.
(iv) The 2021-2027 strategic plan to shift the economy to the BCG (bio, circular, and green) model will help to increase the output of medical and health goods drawn from Thailand’s natural biodiversity, to increase the added-value in the markets for vaccines and medicines, and to cut imports, thus providing the general public with greater access to highcost medicines.
The government is also supporting an expansion in the domestic production of both high-value original drugs and those that have come out of patent, such as antibiotics and
treatments for high blood pressure and diabetes, as well as medicines developed from biological products (e.g., cancer treatments and vaccines), for which demand is rising. This will help to bolster the strength of domestic pharmaceutical production and reduce reliance on imports. In light of these changes, major players are now increasingly attracted to the industry and numerous plans are underway to build factories for the synthesis of active pharmaceutical inputs and other important materials used in the manufacturing process. The output from these will then be used to produce treatments for major illnesses and diseases. Examples of these developments include the joint venture between PTT and the GPO to build a facility for the production of three types of advanced cancer treatments: (i) chemotherapy drugs (one of the mainstays of cancer treatments); (ii) biosimilar monoclonal antibodies that will be manufactured in the form of tablets and injectables; and (iii) Targeted cancer therapies. This facility is being built in the PTT Wanarom Industrial Estate, also called the PTT WEcoZi Estate, in Rayong. Ground was broken in 2022 and the first treatments produced onsite should reach patients by 2027. SCG Chemicals is also investing in the manufacture of biologic medicals and advanced vaccines by Siam Bioscience, which is developing active ingredients for use in cancer treatments as well as for medicines targeting arthritis, psoriasis, and autoimmune conditions. (Recently, SCG Chemicals partnered with Siam Bioscience and AstraZeneca to produce the AZD1222 COVID-19 vaccine). In addition, the National Center for Genetic Engineering and Biotechnology (BIOTEC), the
National Science and Technology Development Agency (NSTDA), and the Government Pharmaceutical Office (GPO) are cooperating to develop the active ingredients needed for the production of Favipiravir, a chemical used in the treatment of SAR-CoV-2. This chemical is also attracting attention from PTT; the company is currently looking into the possibility of constructing a facility to synthesize the chemicals needed to manufacture this and other in-demand pharmaceuticals, which would then cut dependency on imports.
The overall assessment of the state of Thailand’s pharmaceutical industry and related areas is broadly positive.
(i) Thai doctors and biomedical engineers are skilled in medical research, especially with regard to vaccines. (ii) The wide range of Thai herbal treatments means that the country is well placed to use extracts from these as the basis for new biomedical/biopharma products.
(iii) Following recent outbreaks of Zika, MERS, Ebola and COVID-19, progress on bioinformatics has significantly improved research on drug development. (iv) The transfer of technology from AstraZeneca relating to the production of the COVID-19 vaccine (Thailand has been manufacturing this on a commercial basis since June 2021) means that country was home to the first center for the production of COVID-19 vaccines in the ASEAN zone. Thanks to the combination of these factors, Thailand now has a domestic industry capable of manufacturing high-quality but lower-cost vaccines and bio-pharmaceuticals. This will then help to undercut the country’s future dependency on imports of expensive patented medicines and to further establish the country as
the ASEAN hub for the provision of comprehensive medical services.

The Thai pharmaceuticals industry will also face a number of challenges over the coming years. (i) Thailand still lacks security of intermediate supply since the country does not have the domestic ability to manufacture many medicines. Domestic production is thus still widely dependent on imports of (and is largely focused on) the output of generics, while products that depend on high-tech manufacturing processes are almost entirely sourced abroad. (ii) Competition is tending to intensify. (a) Large foreign players have entered the Thai market, though this is generally to use the country as a manufacturing base for the export of finished goods back to their home country or on to
other overseas markets. For example, over 2019-2021, 14 applications for investment support schemes worth a total of THB 813.1 million were received from Japanese producers of medical/pharmaceutical goods, up from 2 projects worth THB 347.8 million in 2018. One application was also received in 2018 from a South Korean company for investments worth THB 10 billion. (b) Thai companies that are focused on other parts of the economy (e.g., chemicals, petrochemicals, and energy) are also expanding into the pharmaceuticals industry. (iii) Thai manufacturers’ production costs are tending to climb on: (a) the need to overhaul production processes to meet the GMP-PIC/S standards; (b) the requirement that manufacturers, importers, and
retailers of pharmaceuticals need to meet certain standards regarding the storage of medicines and medical equipment; and (c) the rising cost of imports and raw materials. (iv) The possibility of joining the CPTPP (currently under discussion) may have consequences for drug patents since the length of patent protection may be extended to 20 years, which would then add to uncertainty about the price of some medicines.
Nicharee Arun Rasameesopha, Marketing Executive, Informa Markets Thailand Nicharee.a@informa.com 099 0572244
ด้�นอ�ห�รให้เวทีอ�ห�รไทย
ของ 4S อาทิ Supple chain, Safety, Security และ Sustainability
1) Processing Tech Asia
2) Packaging Tech Asia 3) Drink Tech Asia 4) Pharma Tech Asia 5) Lab&Test Asia 6) Packaging Solution Asia 7) Coding, Marking&Labelling
Asia และ 8) Coldchain, Logistics, Warehousing&Factory Asia
สนับสนุนการจัดงาน ProPak Asia มาอย่าง ต่อเนื่อง โดยในปีนี้ได้ร่วมนำาผลงานวิจัย
า ที่เป็นผู้
และนวัตกรรม อย่างครบวงจร ก้าวเดินในอุตสาหกรรมได้ อย่างเข้มแข็ง มีมาตรฐานสากล แข่งขันได้ ในตลาดทั้งในและต่างประเทศ รวมทั้งโชว์ ผลงานวิจัยและบริการอย่างครบวงจร ไม่ ว่าจะเป็นด้านเกษตร อาหาร สุขภาพและ การแพทย์ บรรจุภัณฑ์ และวิจัยและพัฒนา เพื่อนำาวัสดุแปรกลับมาใช้ใหม่
Ms. Rungphech Chitanuwat, Regional Portfolio Director - ASEAN, Informa Markets Thailand, the organizer of ProPak Asia 2023, said: “Marking the 30th anniversary this year, ProPak has experienced challenges and triggers for improvements before it achieves this milestone. At ProPak, we aim beyond an exhibition platform, thus being a connector that connects upstream business (processing, packaging) to the downstream business (entrepreneurs). What’s special this year is that we noticed changes in markets as cutting-edge technology emerged. This emergence indeed is a way to add value to products of M-SMEs and farmers who could improve their packaging and strengthen competitiveness of their creation. With this success, it contributes to steering the community to feed products to the industrial market. This year, we have collaborated with the Food Innovation and Packaging Center, Chiang Mai University, in a pilot project to acquire and introduce community products to the global arena. Not only that, L-SMEs will
Informa Markets Thailand gears forward to host ProPak Asia 2023 on June 14-17, 2023, at BITEC, Bangna, reaffirming its readiness to welcome over 40,000 entrepreneurs. The event this time has been revamped with 8 zones to be exhibited and celebrate the 30th anniversary, spotlighting the status of upstream soft power in part of food to introduce Thai food to the global arena through innovative processing and packaging technology while driving business on the basis of the BCG Model for sustainability.









also experience Tech Solutions that will fulfill their business needs. The event is hence truly a one-stop place to visit.”
Ms. Rungphech added that: “At this year’s event, we are focusing on the theme of 4S (Supply Chain, Safety, Security, and Sustainability) in driving the economy of food industry, both directly and indirectly, through our 8 unmissable zones, including Processing Tech Asia, Packaging Tech Asia, Drink Tech Asia, Pharma Tech Asia, Lab&Test Asia, Packaging Solution Asia, Coding, Marking & Labeling Asia, and Coldchain, Logistics, Warehousing & Factory Asia. Highlights of this year are:

Investment Clinic: As a collaboration with the BOI, entrepreneurs, SMEs, startups can seize an opportunity to consult with financial experts about their investment, business planning, business scale-up and development.
Post-Harvest Tech Zone: In collaboration with the Food Innovation and Packaging Center, Chiang Mai University, this zone is to showcase
techniques for agricultural product storage and manufacturing process, e.g. storing, packaging, distributing.





Lab & Test Theater: In collaboration with INNOLAB, offers the neccessary content and the impartance of lab and test for food and feed safety.
As for the public sector, the event is honored by Dr.Pattra Maneesin, Deputy Governor Industrial Services, Thailand Institute of Scientific and Technological Research (TISTR), who said: “The Thailand Institute of Scientific and Technological Research (TISTR) has been an active partner with ProPak Asia. This year, the Institute is presenting research, scientific, technological and innovative services in the TISTR Total Solutions theme, comprehensively empowering entrepreneurs through science, technology, and innovation so that they can take bold steps in the industry, work against international standards, and be competitive in both domestic and international markets. Besides, it will showcase end-toend research and service findings, covering agriculture, food, health and
medicine, packaging while research and developments for recycling are also a focus. The TISTR provides Packaging Testing Services, e.g., for packaging materials (paper, plastic, metal); Biodegradable Material Test for transport packaging, retail packaging, dangerous goods packaging; Performance Test; as well as packaging design and development, e.g., labels and packages.
A representing entrepreneur to attend ProPak Asia 2023, Dr.Ongart Kittikhunchai Chief Executive Officer, Sunsweet Public Company Limited, mentioned future food trends during the press conference in an interesting way. “Food of the future will be subjected to significant changes. Considering the environment and the increasing number of populations, food must be supplied sufficiently, not only in terms of amount, but also its nutritious contents. This thus can be a key turning point. Apart from that, food security is essential in all food businesses, regardless of locations. If we cannot catch up with the trend, we can be disrupted in the future. With respect to this, Sunsweet has engaged in product research and development (R&D), seeking new opportunities by leveraging knowledge, technology and transformation approaches. It has aligned innovative food products with consumer needs. Product demands in the global market become more intense after security events set in, which can actually be regarded as a market opportunity for exportation. On the part of the company itself, it achieved a 30% growth. Products that offer a longer shelf life in form of cans obtained a greater demand.
Ms.Kanoknaphat Pachreang, General Manager, Strategy and Business Development, Kasetphand Industry Co., Ltd., mentioned adoption
of the BCG model in boosting 3 business dimensions. “The BCG model is not something new, as it is a principle further developed from a strong foundation rooted in Thailand, integrated and extended to the chain of goods and service production, especially in 4 s-curve industries. From an entrepreneur’s perspective, this is about self-reliance and it is forming the basis of sustainable development, as it harnesses the strengths of our country, including biodiversity and cultural diversity. The two are combined with knowledge and innovation to enhance products even better. The first return as a result of this is greater competitiveness, especially when it comes to innovation. Innovation brings changes as competition becomes intense and potential increases, and that means our income and environmental consciousness will be positively influenced. This is in line with the United Nations’ Sustainable Development Policies and Goals. If we can achieve this, we shall be able to pave the way for the country to the leading position in the 4 s-curve industries. Regardless of external factors, changes in climate, energy or food shortage, if the BCG model can be applied effectively, I believe all problems can be addressed.
Mr.Karan Tejasen, Chairman of the Board of VexCel Pack Co., Ltd., and Chief Operating Officer, SCG Packaging Public Company Limited, discussed the trend of packaging business. “Changes in demographics and lifestyles are conducive to the increasing use of packaging while packaging tends to be more functional and enhances convenience for users. As consumers are turning to greener alternatives, the choice becomes a factor that plays a key role in fueling growth
of paper and alternative packaging solutions, e.g., packaging designed to use fewer materials, reusable and effortlessly recyclable, green and resource efficient. Moreover, the rising trend of e-commerce and omnichannel retail models are major growth factors of the packaging market in the region.




Correspondingly, Mr.Theeraphon Nimitvanich, Chief Operating Officer, Eka Global Company Limited, commented: “Packaging contributes to a considerable influence on consumers, consumption behavior, purchase decisions, as, for example, it attracts consumers, being attractive and appealing while also engaging. Packaging can strengthen bonds between consumers and the product. Moreover, it can carry product information and benefits; for instance, properties and usage. It adds brand values, protects the content within and enhances durability – a way to confirm that a specific product remains intact and in a condition expected.
The 30th trade and innovation exhibition for manufacturing, processing, and packaging industries ProPak Asia 2023 will be held between June 14 – 17, 2023, at BITEC Exhibition and Convention Center, with pre-registration available at www.propakasia.com.


Global healthcare sector leaders streamed into Hong Kong for the second International Healthcare Week, organised by the Hong Kong Trade Development Council (HKTDC). As the flagship Healthcare Week events, the third Asia Summit on Global Health (ASGH) and the 14th Hong Kong International Medical and Healthcare Fair (Medical Fair) featured fruitful discussions on a wide range of topics including the Guangdong-Hong Kong-Macao Greater Bay Area as an innovation powerhouse, complemented by exchanges on Chinese-Western medicine, aging and women innovators. This flagship event has confirmed Hong Kong’s status as an international R&D and business healthcare platform, where university research projects have been linked with potential investors and innovative medical devices promoted to international markets.
The ASGH attracted over 2,500 participants from 40 countries and regions. The Medical Fair welcomed over 10,000 visitors from 56 countries and regions. More than 300 one-onone deal-making sessions and over 600 business-matching meetings were also arranged to help participants seize business opportunities.
Dr Peter K N Lam, Chairman of the HKTDC, said: “Drawing on its strengths in scientific research, a world-class medical system, a highly skilled workforce, strong financing and fundraising capabilities and advanced logistics and transportation infrastructure, and backed by the huge market of the Greater Bay Area, Hong Kong is the preferred hub and perfect springboard into Asia for the growing healthcare market.
“The strong support from the healthcare industry for the Healthcare Week is overwhelming. It has created synergies for medical and healthcare industry professionals and unleashed Hong Kong’s strengths in upstream and downstream healthcare industries, Hong Kong is ready to play a bigger role in the medical and healthcare fields in the region,” Dr Lam added.
ASGH, co-organised by the Government of the Hong Kong Special Administrative Region (HKSAR) and the HKTDC, continued to bring together healthcare leaders,
innovators and investors. The firstday Plenary Session: Reimagining the Future of Healthcare invited Sir Jonathan Symonds, CBE, Chair, GSK as one of the speakers. He said: “When COVID struck, the world didn’t collaborate in the way that it should have. There will be another COVID. We cannot operate in the same way as we did. We must tackle global problems globally.” Another speaker, Mr Tatsuo Yasunaga, Representative Director, Chair of the Board of Directors, Mitsui & Co., Ltd, noted: “In implementing value-based healthcare, we try to achieve both clinical outcome improvement and cost savings. We expect to achieve the first goal through new drug and medical device developments and health risk management. We are also moving towards personalised healthcare to bridge the gap between medical care and prevention.”
The Unleashing Opportunities in Integrative Chinese-Western Medicine session today examined research, treatment and medicine developments that transformed markets in Asia and abroad. The session showcased the potential for complementary, integrative healthcare and highlighted the business opportunities that collaboration between Chinese and Western medicine presented.
Mr Abraham Chan, Founder, Chairman, CEO and Executive Director at PuraPharm said modernising Chinese medicine included standardising
products by creating standardised granules from herbs. Computer programmes let practitioners diagnose conditions and dispense treatments. Big data collected by practitioners helped improve products and perform drug discovery.
Noting Hong Kong's high life expectancy and increase in the elderly population, panelists at the Ageing Well: Breakthroughs and Investment Landscape in Longevity session said this presented both challenges and opportunities, such as drug discovery, research financing and monitoring gadgets.
Dr Donald Li, Chairman of the Elderly Commission, said higher demand for a wide range of healthcare products and services generated opportunities. This was especially true of monitoring devices allowing the elderly to live at home safely.
The Medical Fair, organised by the HKTDC and co-organised by the Hong Kong Medical and Healthcare Device Industries Association (HKMHDIA) ran under the HKTDC’s EXHIBITION+ hybrid model. The physical fair at the Hong Kong Convention and Exhibition Centre (HKCEC) successfully closed today, while exhibitors and buyers from around the world can continue conducting business online via the Click2Match smart business-matching platform until 25 May.
In recent years, the Hong Kong SAR Government has actively promoted collaboration between the government, industry, academia and researchers by bringing together enterprises, universities and research institutes to cooperate and promote technological innovation.

At ASGH, the hybrid InnoHealth Showcase and exhibition zone featured projects and solutions from more than 140 healthcare-related innovative technology enterprises, from which 50 of the enterprises and projects are presented by five local major universities, namely CUHK, HKBU, HKU, HKUST and PolyU. The Co-founder, Director & Chief Scientific Advisor of exhibitor Gut Rhythm R&D (Hong Kong) Limited Prof John Anthony Rudd said: “The event was highly successful for us. We gained a lot of exposure and were able to attract several potential investors. We also learned a lot and are very confident we could move forward.”
Innovation and Technology Commission (ITC) and Cyberport joined ASGH as Health Innovation Partners from which ITC showcased 16 health-related InnoHK R&D centres to demonstrate Hong Kong’s strength as a global innovation powerhouse. Numerous companies in the healthcare industry as well as six local universities and various enterprises supported by the universities, which
are commercialising their research and development accomplishments, joined the Medical Fair to showcase cutting-edge medical products and technologies. Among the universityaffiliated companies are Sramek Insight Limited, Herbap Biotech, Agilis Robotics, Metasurgical Technology, Cellomics International and Greater Bay Biotechnology.
This year’s fair, the largest in its history, assembled more than 300 exhibitors to feature the latest medical technologies and equipment, healthcare products and solutions and related services, drawing many buyers and industry professionals to source both onsite and online.
“I am glad that I met nearly 100 representatives from various medical organisations, our main target customers. I estimate that this fair will make over 60% of our yearly sales,” said Mr King-Wah Tai, a sales representative for medical facility manufacturer Janley Limited (HK). The company has participated in the Medical Fair since 2014 and has benefited greatly from this year’s fair. “Taking this chance to interact with people from the sector, understand what they need and how they think is very important to our business. The fair is not just for generating sales but also the perfect place to gain insight," Mr Tai added.
Mr Valentin Cipian Pantea, Manager of Neomed S.R.L. from Romania, visited the Medical Fair to look for
new medical-uniform manufacturers. “I plan to order about US$200,000 worth of products and hope to build a long-term relationship with them. Besides, using the Click2Match platform, I made a valuable business connection with a manufacturer from Mainland China. The platform is very helpful in getting to know people entirely out of our usual circle.”
A number of content-rich events, including the highly anticipated HKMHDIA Medical Fair Forum, were held during the Medical Fair with keen participation from industry professionals. Those who missed the seminars can watch replay videos on the fair's website at https://bit. ly/3DNBBXy.

• International Healthcare Week: https://internationalhealthcareweek. hktdc.com/en
• Asia Summit On Global Health: https://www.asiasummitglobalhealth. com/conference/asgh/en
• Programme: https://www. asiasummitglobalhealth.com/ conference/asgh/en/programme
• Speakers: https://www. asiasummitglobalhealth.com/ conference/asgh/en/speaker
• Hong Kong International Medical and Healthcare Fair: https://www. hktdc.com/event/hkmedicalfair/en
• Interesting product list and Activity schedule: https://bit.ly/3V9mv6S


เห็นว่าความร่วมมือด้านวิจัยและถ่ายทอด เทคโนโลยีจากประเทศที่พัฒนาแล้ว เช่น สหราชอาณาจักร มีความสำาคัญอย่างยิ่ง เพื่อปิดช่องว่างทางเทคโนโลยีและสร้างขีด ความสามารถของประเทศกำาลังพัฒนาใน ด้านสุขภาพสัตว์ โดยเฉพาะการผลิตวัคซีน
สวทช. และ มหาวิทยาลัยเทคโนโลยีพระจอมเกล้าธนบุรี (มจธ.)
(UK) โดย
มหาวิทยาลัยเคนท์ (University of Kent)
ลอนดอน (University College London: UCL) แถลงข่าวความร่วมมือวิจัยไทย-UK

ใน 3 โครงการวิจัย ทุน
ร่วมกว่า 170 ล้านบาทจากรัฐบาล UK
และไทย เพื่อดำาเนินงานใน 2 เรื่องใหญ่ ได้แก่ ชีวเวชภัณฑ์และวัคซีนสำาหรับสัตว์
ที่มี ผลกระทบต่ออุตสาหกรรมชีวเวชภัณฑ์ และ อุตสาหกรรมเพาะเลี้ยงสัตว์น้ำาและปศุสัตว์
เทคโนโลยีสาหร่ายเซลล์เดียวเพื่อการ ป้องกันโรคในอุตสาหกรรมการเลี้ยงกุ้ง โดยความร่วมมือในการวิจัยเรื่องแรก เป็นผลงานที่พร้อมถ่ายทอดเทคโนโลยีสู่เชิง พาณิชย์แล้ว คือ เทคโนโลยีสาหร่ายเซลล์ เดียวเพื่อการป้องกันโรคในอุตสาหกรรม การเลี้ยงกุ้ง ซึ่งในช่วง 10 ปีที่ผ่านมา
2 (PCV2d) ซึ่งเป็นเชื้อ
นอกจากนี้ยังได้เริ่มงานวิจัย
Establishing a bioprocess workforce in Thailand to deliver affordable biological medicines through biomanufacturing education and industrial-research integration
UCL ซึ่งหลักสูตร อบรมนี้ถือเป็นครั้งแรกที่ออกแบบให้สอดคล้อง กับความต้องการของอุตสาหกรรมชีววัตถุ เป็นการอบรมแบบภาคปฏิบัติพร้อมกรณี

In a joint press conference, Thailand’s National Center for Genetic Engineering and Biotechnology (BIOTEC), King Mongkut’s University of Technology Thonburi (KMUTT), and the UK’s University of Kent and University College London (UCL) unveiled the remarkable achievements of three collaborative projects. These initiatives, backed by funding exceeding 170 million baht, were announced to the public, highlighting the fruitful partnership between the institutions.
ASEAN countries, including Thailand, play a significant role in global food production, specifically in aquaculture and livestock. As the demand for food from these industries rises, there is a need for efficient veterinary health management technology to prevent and manage emerging and re-emerging diseases, while maintaining the wellbeing of the animals.
Acknowledging the criticality of collaboration and technology transfer from advanced countries like the United Kingdom to Thailand, Professor Colin Robinson of University of Kent and his team has embarked on a transformative mission. Their efforts aim to enhance the country’s capabilities in animal health, particularly in the crucial domains of veterinary vaccine production and therapeutic development.
ไวรัสก่อโรค และเป็นสาเหตุหลักของกลุ่ม
BIOTEC, KMUTT, University of Kent and UCL have embarked on long-term research collaboration to enhance scientific advancement and to help increase the availability and affordability of veterinary vaccines in Thailand. Funded by the governments of UK and Thailand, several collaborative activities have successfully contributed to the overall improvement of animal health and welfare, food security, public health and human resource development.
Shrimp aquaculture has been threatened by significant losses due to viral pathogens such as white spot syndrome virus (WSSV).
Since 2018, University of Kent and BIOTEC have been actively engaged in the use of antiviral microalgae in aquafeed for disease control through collaborative projects on ‘Establishment of RNAibased algal technology for sustainable disease control in shrimp cultivation (funded by Thailand Research Fund and UK’s Royal Society)’ and ‘Development of novel microalgal-based systems for shrimp disease control in South East Asia (funded by UK’s Royal Society)’.
Dr. Vanvimon Saksmerprome, a principal researcher of BIOTEC, revealed that the team has made significant progress in the development of novel dsRNA production platforms in microalgae. The technology has been successfully implemented in shrimp farms, where it has proven highly effective in combatting viruses by knocking down viral genes. This breakthrough represents a significant step forward in sustainable practices to combat viral infections in the aquaculture industry.
Additionally, Professor Robinson of the University of Kent explained that their team has created a simple and affordable technology for precise engineering of the
chloroplast of microalgae, allowing for the production of biomolecules.
Prof. Saul Purton of UCL and Director of Algae-UK, working together with Professor Robinson, has created a simple and affordable technology for precise engineering of the chloroplast of microalgae, allowing the production of biomolecules such as dsRNA and protein vaccines. Both groups are building on their collaborative successes with Thai researchers in development of low-cost animal vaccines using algal platforms and to move these technologies towards commercialization.
With combined expertise, the use of antiviral microalgae in aquafeed for disease control has established a new paradigm for shrimp cultivation for future sustainable food production.
Swine industry is massive in Thailand, with close to 150,000 households maintaining pigs. However, the industry continues to be plagued by viral epidemics. Thailand relies on imported vaccines but they are often expensive and less effective as local viral strains are different.
Leading institutes in the UK (Kent, UCL, Imperial College, London School of Hygiene & Tropical Medicine) and Thailand (BIOTEC and KMUTT) have been working to develop the country’s own veterinary vaccine platforms in Thailand as part of a GBP 3.93 million (THB 160,000,000 million) joint project titled “Establishment of Biopharmaceutical and Animal Vaccine Production Capacity in Thailand and Neighbouring South East Asian Countries”. The project was funded by UK Research and Innovation’s Global Challenges Research Fund (GCRF) for the period of 2018-2022.
Prof. Robinson revealed that the project proposed two bold objectives based on the recombinant protein production platforms: affordable human biosimilars
and tailor-made swine vaccines and biotherapeutics. For the veterinary side, Dr. Peera Jaru-Ampornpan of BIOTEC, explained that the team has successfully developed a potential subunit vaccine prototype against PCV2d, a major subtype of a swine pathogen associated with postweaning multisystemic wasting syndrome. The team also developed a production platform for recombinant interferon to be used as future broadspectrum veterinary therapeutics.
Additionally, Dr. Lalintip Hocharoen of KMUTT explained that the project also includes building technical capacity in Thailand to produce recombinant proteins at large scale at the National Biopharmaceutical Facility (NBF), a state-of-the-art facility established by BIOTEC and KMUTT for large-scale production of biopharmaceuticals and animal vaccines. The team has successfully developed the PCV2d vaccine candidate at 30-L scale and the platform has shown reproducibility by technology transfer to another production site at UCL. Nonetheless, bioprocess workforce development has also been carried out via industrial-oriented research and codesign training platforms with the UK teams. Prof. Robinson added that the UK teams have provided support in protein production, purification, analytics, and technical training to ensure compliance with regulations.
Through the collaboration and expertise of both teams, improvements have been made at every stage of the animal vaccine production value chain.
Your subscription
Fill out your detail or attach your business card
• Source ingredients from 500+ suppliers


• Touch and try the latest innovations
• Meet R&D experts and perfect your formulation skills



• Learn about Asia Pacific’s future personal care trends
• Establish profitable business networks
Bangkok • 7-9 November 2023

Where personal care ingredients and creators come together