I J S PT











LightForce® Therapy Lasers empower you to treat soft tissue with confidence. Harnessing photobiomodulation (PBM)—a powerful form of light therapy—our lasers stimulate cellular metabolism to help treat muscle and joint pain from acute and chronic conditions.
Equipped with smart features like dosing recommendations, real-time visual and haptic feedback, and convenient portability, our range of therapy lasers combines a fusion of power with intelligence to enhance the patient and user experience. With the ability to reach deep tissues, LightForce lasers can cut the time needed by clinicians to treat patients—making light work of pain.
More than 250 professional and collegiate sports teams around the world trust LightForce Therapy Lasers to provide rehabilitation and pain management.
Scan the QR code to request a demo, or visit https://learn.chattanoogarehab.com/ijspt-journal

Turner A Blackburn, APTA Life Member, AT-Ret, AOSSM-Ret President
Mary Wilkinson Executive Director
Michael Voight Executive Editor and Publisher
Joe Black, PT, DPT, SCS, ATC
Eric Fernandez
Jay Greenstein, DC
Skip Hunter, PT, ATC-Ret
Russ Paine, PT, DPT
Tim Tyler, PT, ATC
Sports Legacy Advisory Board
Turner A. Blackburn, PT, ATC
George Davies, PT, DPT, MEd, SCS, ATC, LAT, CSCS, PES, FAPTA
Terry Malone, PT, PhD
Bob Mangine, PT
Barb Sanders, PT, PhD
Tim Tyler, PT, ATC
Kevin Wilk, PT, DPT, FAPTA
Executive Editor/Publisher
Michael L. Voight, PT, DHSc, OCS, SCS, ATC, CSCS
Editor in Chief
Barbara Hoogenboom, PT, EdD, SCS, ATC
Managing Editor
Ashley Campbell, PT, DPT, SCS
Manuscript Coordinator
Casey Lewis, PTA, ATC
Publisher
Contact Information
International Journal of Sports Physical Therapy 6011 Hillsboro Pike Nashville, TN 37215, US, http://www.ijspt.org
IJSPT is a monthly publication, with release dates on the first of each month.
ISSN 2159-2896
Titling Sponsor ATI
Founding Sponsors Enovis Hyperice Woodway
Platinum Sponsor Elvation
Gold Sponsors Hawkgrips Kayezen
Structure + Function Education Winback Partners
Northeast Seminars Academy of Human Movement
American Academy of Sports Physical Therapy
IJSPT is an official journal of the International Federation of Sports Physical Therapy (IFSPT). Countries with access to IJSPT as a member benefit. Reach us at www.ifspt.org.

IJSPT is an official journal of the ICCUS Society for Sports Rehabilitation. www.iccus.org






Stand out in your community with a diversified patient experience. Designed to improve outcomes, attract new patients, and increase revenue through insurance, cash-based services, and retail sales.


Gain access to a robust library of research, clinical education, and marketing tools including:
• On-demand clinical education courses
• Written treatment protocols
• Over 50 research studies specific to Hyperice technology
• Marketing tips and best practices including social media content, videos, and more
• Live trainings
Executive Editor/Publisher
Michael L. Voight, PT, DHSc, OCS, SCS, ATC, CSCS
Belmont University
Nashville, Tennessee – USA
Editor in Chief
Barbara Hoogenboom, PT, EdD, SCS, ATC
Grand Valley State University Grand Rapids, Michigan - USA
Managing Editor
Ashley Campbell, PT, DPT, SCS
Nashville Sports Medicine and Orthopaedic Center Nashville, Tennessee – USA
Manuscript Coordinator
Casey Lewis, PTA, ATC
Nashville Sports Medicine and Orthopaedic Center
Nashville, Tennessee – USA
Editors
Robert Manske PT, DPT, Med, SCS, ATC, CSCS
University of Wichita Wichita, KS, USA
Terry Grindstaff, PT, PhD, ATC, SCS, CSCS
Creighton University Omaha, NE, USA
Phil Page PT, PhD, ATC, CSCS Franciscan University DPT Program Baton Rouge, LA, USA
Kevin Wilk PT, DPT, FAPTA
Clinical Viewpoint Editor Champion Sports Medicine Birmingham, AL, USA
International Editors
Luciana De Michelis Mendonça, PT, PhD UFVJM
Diamantina, Brazil
Colin Paterson PT, MSc PGCert(Ed), MCSP, RISPT, SFHEA
University of Brighton Brighton, England, UK
Chris Napier, PT, PhD Clinical Assistant Professor
University of British Coumbia, Vancouver, BC, Canada
Nicola Phillips, OBE, PT, PhD, FCSP Professor School of Healthcare Sciences Cardiff University, Cardiff, Wales, UK
Associate Editors
Eva Ageberg, PT, PhD Professor, Lund University Lund, Sweden
Lindsay Becker, PT, DPT, SCS, USAW Buckeye Performance Golf Dublin, Ohio, USA
Keelan Enseki, PT, MS, OCS, SCS, ATC University of Pittsburgh Pittsburgh, PA, USA
John Heick, PT, PhD, DPT, OCS, NCS, SCS
Northern Arizona University Flagstaff, AZ, USA
Julie Sandell Jacobsen, MHSc, PhD
VIA University
Aarhus, Denmark
RobRoy L. Martin, PhD, PT, CSCS Duquesne University Pittsburgh, PA, USA
Andrea Mosler, PhD, FACP, FASMF
La Trobe Sport and Exercise Medicine Research Centre, School of Allied Health, Human Services and Sport, La Trobe University Melbourne, Victoria, Australia
Brandon Schmitt, DPT, ATC
PRO Sports Physical Therapy Scarsdale, NY, USA
Barry Shafer, PT, DPT
Elite Motion Physical Therapy Arcadia, CA, USA
Laurie Stickler, PT, DHSc, OCS
Grand Valley State University
Grand Rapids, MI, USA
Editorial Board
James Andrews, MD
Andrews Institute & Sports Medicine Center
Gulf Breeze, AL, USA
Amelia (Amy) Arundale, PT, PhD, DPT, SCS
Red Bull/Ichan School of Medicine
Salzburg, Austria/New York, NY, USA
Gary Austin, PT PhD
Belmont University Nashville, TN, USA
Roald Bahr, MD
Oslo Sports Trauma Research Center Oslo, Norway
Lane Bailey, PT, PhD
Memorial Hermann IRONMAN Sports Medicine Institute
Houston, Texas, USA
Gül Baltaci, PT,Ph.D. Professor, CKTI, FACSM
Private Guven Hospital Ankara, Turkey
Asheesh Bedi, MD
University of Michigan
Ann Arbor, MI, USA
David Behm, PhD Memorial University of Newfoundland St. John's, Newfoundland, Canada
Barton N. Bishop, PT, DPT, SCS, CSCS Kaizo Clinical Research Institute Rockville, Maryland, USA
Mario Bizzini, PhD, PT Schulthess Clinic Human Performance Lab Zürich, Switzerland
Joe Black, PT, DPT, SCS, ATC Total Rehabilitation Maryville, Tennesse, USA
Turner A. "Tab" Blackburn, APTA Life Member, ATC-Ret, AOSSM-Ret NASMI Lanett, AL, USA
Lori Bolgla, PT, PhD, MAcc, ATC Augusta University Augusta, Georgia, USA
Matthew Briggs The Ohio State University Columbus, OH, USA
Tony Brosky, PT, DHSc, SCS Bellarmine University Louisville, KY, USA
Brian Busconi, MD UMass Memorial Hospital Boston, MA, USA
Robert J. Butler, PT, PhD St. Louis Cardinals St. Louis, MO, USA
Duane Button, PhD Memorial University St. Johns, Newfoundland, Canada
J. W. Thomas Byrd, MD Nashville Sports Medicine and Orthopaedic Center Nashville, TN, USA
Lyle Cain, MD Andrews Institute & Sports Medicine Center Birmingham, AL, USA
Gary Calabrese, PT, DPT Cleveland Clinic Cleveland, Ohio, USA
Meredith Chaput, PT, DPT, SCS Ohio University Athens, OH, USA
Rita Chorba, PT, DPT, MAT, SCS, ATC, CSCS United States Army Special Operations Command Fort Campbell, KY, USA
John Christoferreti, MD Texas Health Dallas, TX, USA
Richard Clark, PT, PhD Tennessee State University Nashville, TN, USA
Juan Colado, PT, PhD University of Valencia Valencia, Spain
Brian Cole, MD Midwest Orthopaedics at Rush Chicago, IL, USA
Ann Cools, PT, PhD
Ghent University Ghent, Belgium
Andrew Contreras, DPT, SCS Washington, DC, USA
George Davies, PT, DPT, MEd, SCS, ATC, LAT, CSCS, PES, FAPTA
Georgia Southern University Savannah, Georgia, USA
Pete Draovich, PT
Jacksonville Jaguars Footbal Jacksonvile, FL, USA
Jeffrey Dugas, MD Andrews Institute & Sports Medicine Center Birmingham, AL, USA
Jiri Dvorak, MD Schulthess Clinic Zurich, Switzerland
Todd Ellenbecker Rehab Plus Phoenix, AZ, USA
Carolyn Emery, PT, PhD University of Calgary Calgary, Alberta, Canada
Ernest Esteve Caupena, PT, PhD University of Girona Girona, Spain
Sue Falsone, PT, MS, SCS, ATC, CSCS, COMT Structure and Function Education and A.T. Still University Phoenix, Arizona, USA
J. Craig Garrison, PhD, PT, ATC, SCS Texas Health Sports Medicine Fort Worth, Texas, USA
Maggie Gebhardt, PT, DPT, OCS, FAAOMPT Fit Core Physical Therapy/Myopain Seminars Atlanta, GA and Bethesda, MD, USA
Lance Gill, ATC
LG Performance-TPI Oceanside, CA, USA
Phil Glasgow, PhD, MTh, MRes, MCSP Sports Institute of Northern Ireland Belfast, Northern Ireland, UK
Robert S. Gray, MS, AT Cleveland Clinic Sports Health Cleveland, Ohio, USA
Jay Greenstein, DC Kaizo Health Baltimore, MD, USA
Martin Hagglund, PT PhD
Linkoping University Linkoping, Sweden
Allen Hardin, PT, SCS, ATC, CSCS
University of Texas Austin, TX, USA
Richard Hawkins, MD
Professor of surgery, University of South Carolina
Adjunct Professor, Clemson University
Principal, Steadman Hawkins, Greenville and Denver (CU)
John D.Heick, PT, PhD, DPT, OCS, NCS, SCS
Northern Arizona University Flagstaff, AZ, USA
Tim Hewett, PhD
Hewett Consulting Minneapolis, Minnesota, USA
Per Hølmich, MD
Copenhagen University Hospital Copenhagen, Denmark
Kara Mae Hughes, PT, DPT, CSCS
Wolfe PT Nashville, TN, USA
Lasse Ishøi, PT, MSc
Sports Orthopedic Research Center
Copenhagen University Hospital Hvidovre, Denmark
Jon Karlsson, MD Sahlgrenska University Goteborg, Sweden
Brian Kelly, MD Hospital for Special Surgery New York, NY, USA
Benjamin R. Kivlan, PhD, PT, OCS, SCS Duquesne University Pittsburgh, PA, USA
Dave Kohlrieser, PT, DPT, SCS, OCS, CSCS
Ortho One Columbus, OH, USA
Andre Labbe PT, MOPT
Tulane Institute of Sports Medicine New Orleans, LA USA
Henning Langberg, PT, PhD University of Copenhagen Copenhagen, Denmark
Robert LaPrade, MD Twin Cities Orthopedics Edina, MN, USA
Lace Luedke, PT, DPT University of Wisconsin Oshkosh Oshkosh, WI, USA
Phillip Malloy, PT, PhD
Arcadia University/Rush University Medical Center Glenside, PA and Chicago, IL, USA
Terry Malone, PT, EdD, ATC, FAPTA University of Kentucky Lexington, KY, USA
Robert Mangine, PT University of Cincinnati Cincinnati, OH, USA
Eric McCarty, MD University of Colorado Boulder, CO, USA
Ryan P. McGovern, PhD, LAT, ATC Texas Health Sports Medicine Specialists Dallas/Fort Worth, Texas, USA
Mal McHugh, PhD
NISMAT
New York, NY, USA
Joseph Miller, PT, DSc, OCS, SCS, CSCS
Pikes Peak Community College Colorado Springs, CO, USA
Havard Moksnes, PT PhD
Oslo Sports Trauma Research Center Oslo, Norway
Andrew Murray, MD, PhD
European PGA Tour Edinburgh, Scotland, UK
Andrew Naylor, PT, DPT, SCS
Bellin Health
Green Bay, WI, USA
Stephen Nicholas, MD NISMAT New York New York, NY, USA
John O'Donnel, MD
Royal Melbourne Hospital Melbourne, Australia
Russ Paine, PT McGovern Medical School Houston, TX, USA
Snehal Patel, PT, MSPT, SCD
HSS Sports Rehabilitation Institute New York, NY, USA
Marc Philippon, MD
Steadman-Hawkins Clinic Vail, CO, USA
Kevin Plancher, MD, MPH, FAAOS
Plancher Orthopedics and Sports Medicine
New York, NY USA
Marisa Pontillo, PT, PhD, DPT, SCS
University of Pennsylvania Health System Philadelphia, PA, USA
Matthew Provencher, MD
Steadman Hawkins Clinic Vail, CO, USA
Charles E. Rainey, PT, DSc, DPT, MS, OCS, SCS, CSCS, FAAOMPT
United States Public Health Service Springfield, MO, USA
Alexandre Rambaud, PT PhD Saint-Etienne, France
Carlo Ramponi, PT Physiotherapist, Kinè Rehabilitation and Orthopaedic Center Treviso, Italy
Michael Reiman, PT, PhD Duke University Durham, NC, USA
Mark F. Reinking, PT, PhD, SCS, ATC Regis University Denver, CO, USA
Mark Ryan, ATC Steadman-Hawkins Clinic Vail, CO, USA
David Sachse, PT, DPT, OCS, SCS USAF San Antonio, TX, USA
Marc Safran, MD Stanford University Palo Alto, CA, USA
Alanna Salituro, PT, DPT, SCS, CSCS New York Mets Port Saint Lucie, FL, USA
Mina Samukawa, PT, PhD, AT (JSPO) Hokkaido University Sapporo, Japan
Barbara Sanders, PT, PhD, FAPTA, Board Certified Sports Physical Therapy Emeritus Professor and Chair, Department of Physical Therapy Texas State University Round Rock, TX, USA
Felix “Buddy” Savoie, MD, FAAOS Tulane Institute of Sport Medicine New Orleans, LA, USA
Teresa Schuemann, PT, DPT, ATC, CSCS, Board Certified Specialist in Sports Physical Therapy Evidence in Motion Fort Collins, CO, USA
Timothy Sell, PhD, PT, FACSM Atrium Health Musculoskeletal Institute Charlotte, NC, USA
Andreas Serner, PT PhD
Aspetar Orthopedic and Sports Medicine Hospital Doha, Qatar
Ellen Shanley, PT, PhD ATI Spartanburg, SC, USA
Karin Silbernagel, PT, PhD University of Delaware Newark, DE, USA
Holly Silvers, PT, PhD Velocity Physical Therapy Los Angeles, CA, USA
Lynn Snyder-Mackler, PT, ScD, FAPTA STAR University of Delaware Newark, DE, USA
Alston Stubbs, MD Wake Forest University Winston-Salem, NC, USA
Amir Takla, B.Phys, Mast.Physio (Manip), A/Prof
Australian Sports Physiotherapy The University of Melbourne Melbourne, Australia
Charles Thigpen, PhD, PT, ATC ATI
Spartanburg, SC, USA
Steven Tippett, PT, PhD, ATC, SCS Bradley University Peoria, IL, USA
Tim Tyler, PT, ATC NISMAT New York, NY, USA
Timothy Uhl, PT, PhD, ATC University of Kentucky Lexington, KY, USA
Bakare Ummukulthoum, PT University of the Witswatersrand Johannesburg, Gauteng, South Africa
Yuling Leo Wang, PT, PhD Sun Yat-sen University Guangzhou, China
Mark D. Weber, PT, PhD, SCS, ATC Texas Women’s University Dallas, TX, USA
Richard B. Westrick, PT, DPT, DSc, OCS, SCS US Army Research Institute Boston, MA, USA
Chris Wolfe, PT, DPT Belmont University Nashville, TN, USA
Tobias Wörner, PT, MSc Lund University Stockholm, Sweden
PAGE TITLE
SYSTEMATIC REVIEW
142 Effectiveness of Vestibular Rehabilitation in Children Post-Concussion: A Systematic Review. Tiwari D, Erdal M, Alonzo K, et al.
ORIGINAL RESEARCH
157 Assessing the Inter-Rater and Inter-Trial Reliability of the NeurOptics Pupillary Light Response-3000 Pupillometer.
Jehu DA, Bolgla LA, Armas S, et al.
168 The Impact of a Concomitant Meniscus Surgery on Hop Performance Symmetry in Patients Rehabilitating After Anterior Cruciate Ligament Reconstruction.
Malliah K, VanZile A, Walden M, et al.
176 Hamstrings and Quadriceps Weaknesses following Anterior Cruciate Ligament Reconstruction Persist up to Six Months after Return-to-sport: An Angle-Specific Strength Analysis.
Hagen M, Vanrenterghem J, Van den Borne Y, et al.
189 Are We Overlooking Anatomical Contributions to Dynamic Knee Valgus?
Dewald M, Andersen MP, Higgins LI, et al.
199 Patellofemoral Joint Loading During Bodyweight One-Legged and Two-Legged BOSU-Ball and Floor Squats.
Escamilla RF, Zheng N, MacLeod TD, et al.
210 The Effect of the Addition of Core Exercises to Supervised Physiotherapy in Patients With Subacromial Impingement Syndrome.
Gutiérrez-Espinoza H, Méndez-Rebolledo G, Zavala-González J, et al.
221 Preseason Workload in Collegiate Baseball Pitchers.
Tabaracci B, Sudhir S, Gauthier M, et. al.
231 The Effects of Noxious Electrical Stimulation and Eccentric Exercise on Mechanical and Thermal Pain Sensitivity in Recreational Runners with Achilles Tendinopathy.
Stackhouse SK, Madara KC, Eckenrode BJ.
243 Clinical Evolution and Safety of a Cryotherapy -based Medical Device for Mild to Moderate Joint and Muscle Pain: A Descriptive Observational Study.
Ballester Herrera MA, Muñoz Vives JM, Marti Gil A.
254 Reliability and Agreement of Hand-Held Dynamometry Using Three Standard Rater Test Positions. Aerts FE, Sheets HA, Anderson CS, et al.
264 Excellent Reliability for an Instrumented Test of Ankle Plantarflexion Force.
Glaied M, Whiteley R.
276 Reliability of Ultrasound Based Compressibility of the Lower Leg Anterior Tibial Muscle Compartment in Healthy Volunteers. van Heeswijk K, Spek D, Muijsenberg J, et al.
CLINICAL COMMENTARY
286 Scapular Stabilization for Shoulder Pain: Putting the Cart Before the Horse? Elder AM, Powers CM.
CLINICAL VIEWPOINT
294 Ulnar Collateral Ligament Hybrid Reconstruction Surgery in the Overhead Athlete. Meister KM, Evans D, Wilk KE.
MSK ULTRASOUND BITES: TIPS AND TRICKS
307 Diagnostic Musculoskeletal Ultrasound of the Achilles Tendon Page P, Manske RC, Voight M, Wolfe C.

2025 DATES: March 19 • April 16 • May 21
June 18 • July 16 • August 20 • September 17
October 15 • November 19 • December 17 ALL BEGIN AT 6:30 PM CST
February's Journal Club features Rachel Frank, MD, from the University of Colorado, who specializes in Cartilage Restoration and Shoulder surgery.
Articles
In elite athletes with meniscal injuries, always repair the lateral, think about the medial! A systematic review.
D’Ambrosi R, Meena A, Raj A, et al
Knee Surg Sports Traum Arthros. 2023;31:2500–2510
https://doi.org/10.1007/s00167-022-07208-8
High Rate of Return to Sport for Athletes Undergoing Articular Cartilage Restoration Procedures for the Knee: A Systematic Review of Contemporary Studies.
Kunze KN, Mazzucco M, Thomas Z, et al.
AJSM. 2025;1:1–12 DOI: 10.1177/03635465241280975
Greater rate of return to play and re�injury following all-inside meniscal repair compared to the inside�out technique: a systematic review.
Migliorini1 F, Asparago G, Oliva F, et al.
Arch Ortho Trauma Surg. 2023;143:6273–6282.
https://doi.org/10.1007/s00402-023-04933-8



Most Advanced Electrotherapy Device: Powerful, intuitive and user-friendly
Treat up to three body zones at once on all types of tissues
Effective in less than 10 minutes Enter A New Era of Therapy
TECAR
HIGH FREQUENCY
Metabolic Action at Cell Level
Hi-TENS
LOW FREQUENCY IN PULSED HIGH FREQUENCY
Ultimate Pain Management
Hi-EMS
MEDIUM FREQUENCY
Deep Muscle Contraction








Devashish Tiwari1a , Melisa Erdal1 , Kristyn Alonzo1 , Victoria Twombly2 , Paige Concannon2 , August West2 , Mairead O'Byrne2
1 Department of Physical Therapy, MGH Institute of Health Professions, 2 Department of Physical Therapy, Simmons University
Keywords: Concussion, vestibular rehabilitation, children, function, symptoms https://doi.org/10.26603/001c.128282
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background
Concussion in children is a significant public health burden in the United States with 2.3 million children under the age of 17 years sustaining a concussion in 2022 alone. Children post-concussion experience a wide range of symptoms of vestibular dysfunction. Vestibular rehabilitation therapy (VRT) has been shown to substantially decrease dizziness and improve gait and balance function in adults post-concussion, but limited information is available for children.
Purpose: The purpose of this systematic review was to determine the effectiveness of VRT on improving vestibular function, postural control, and gait in children post-concussion.
Study design
Systematic review.
Methods
An electronic search of MEDLINE and CINAHL was conducted in October 2022 and later updated in April 2024 using MeSH terms and keywords related to vestibular rehabilitation, concussion, and children. Quality appraisal was conducted independently by two reviewers using the Joanna Briggs Institute checklist, the Critical Appraisal Skills Programme checklist and Cochrane risk of bias assessment tool. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were utilized for screening and data extraction.
Results
Overall, twelve studies (three randomized controlled trials, five cohort studies, two case series and two case reports) were included in the review. The Dizziness Handicap Inventory (DHI) was the most frequently utilized measure (five studies). Three studies reported a statistically significant improvement in DHI (change scores = 19-25, p < 0.05), gait speed (F = 38.3, p < 0.001), Balance Error Scoring System (BESS) (change score percentage 12.1 – 52%, p < 0.01), and Activities-specific Balance Confidence (ABC) scale (change = 20-29 points, p <0.01).
Corresponding Author: Devashish Tiwari PhD, DPT, NCS Department of Physical Therapy
MGH Institute of Health Professions 36, 1st Ave Boston, MA-02129
Email: dtiwari@mghihp.edu
ORCID ID: 0000-0001-9588-6489
Twitter: @DevTiwari81
VRT shows promise and may result in symptom improvements in children post-concussion when used as part of a multimodal intervention plan. Further research with larger samples is recommended to make informed decisions about dosage and long-term functional outcomes in children post-concussion.
Concussion in children is a significant public health burden in the United States with an estimated rise in prevalence by 71% since 2010.1 In 2020, the CDC reported that 2.3 million children under the age of 17 years sustained a concussion in 2022 alone with the highest reported lifetime concussion symptoms in the 12-17 age group.2,3 Given the prominent anatomical and physiological differences in the nervous and musculoskeletal system from adults, children experience higher symptom severity and prolonged recovery times post-concussion.4‑6
Children may experience a wide range of symptoms of vestibular dysfunction including dizziness, vertigo, poor postural control, poor vision, oculomotor control, and cognitive dysfunction post-concussion.7 Among these, dizziness is the most disabling symptom. Children with dizziness post-concussion are at six times higher risk for delayed recovery as compared to children without dizziness.8 Studies have reported that dizziness post-concussion was strongly associated with learning disability (95% CI = 2.18-5.45), attention deficit disorder (95% CI = 1.06-2.81), and intellectual disability (95% CI = 2.6-16.79) when compared to no dizziness post-concussion.9 Additionally, children with vestibular dysfunction are 2.46 times (95% CI = 1.48-4.10) more likely to use special education services when compared to children without vestibular dysfunction.9,10
Vestibular rehabilitation therapy (VRT) is an exercise approach that has been shown to substantially decrease dizziness and improve gait and balance function in adults postconcussion.11,12 Recent reviews have highlighted preliminary evidence indicating a possibility of VRT as a treatment option for children post-concussion.13,14 Given the growing attention in this area, there is a need to systematically examine evidence to determine the effectiveness of VRT specifically for children post-concussion. Additionally, previous reviews contained a small number of studies and were limited to randomized controlled trials and retrospective studies. Given the limited availability of randomized controlled trials for children in this area, multiple study designs must be included. To the authors’ knowledge, this is the first systematic review which has focused specifically on VRT for children post-concussion. The purpose of this systematic review was to determine the effectiveness of VRT on improving vestibular function, postural control, and gait in children post-concussion.
This study was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement guidelines.15 A systematic literature search was conducted, where four study team members independently screened articles for inclusion using a screening form created by the study team. Titles and abstracts of retrieved studies were screened prior to obtaining full text articles for full text review
Studies were included if they: 1) involved children between the ages of 0 and 18, 2) examined the effectiveness of VRT for mild TBI or concussion, and 3) were peer-reviewed and published in the English language. Studies were excluded if participants sustained moderate to severe TBI. All types of study designs were included. Grey literature was excluded.
LITERATURE REVIEW AND SEARCH STRATEGY
A systematic electronic literature search was conducted in October 2022 and later updated in April 2024 to identify relevant published work from January 2009 to May 2023. Medline, CINAHL, and PubMed were searched using the following keywords and MeSH terms related to concussion and vestibular rehabilitation in children: cervical rehabilitation or vestibular rehabilitation or vestibular therapy or cervical therapy AND children or youth or child or teenager or kids or pediatric or paediatric AND concussion or mild Traumatic Brain Injury or mild TBI or mTBI. A research librarian was consulted while designing the literature search. The search was also supplemented by manual search to identify additional studies from the back references of published articles.
Four study team members (VT, PC, AW, and MO) independently extracted data using a data extraction sheet designed by the study team. Variables extracted included author and year, study design, average age of participants, total number of participants, sex, setting, details of experimental and control intervention, frequency and duration of intervention, scores on outcomes measures pre and postintervention, outcomes with statistically significant and Effectiveness
clinically meaningful improvements, attrition rate, and reasons for attrition.
Given multiple study designs included in this systematic reviews, risk of bias assessment was conducted using multiple tools. The Joanna Briggs institute (JBI) checklist16 was utilized for case reports and case series, Cochrane risk of bias assessment (ROB-2)17 was used for randomized controlled trials, and the Critical Appraisal Checklist Programme (CASP) checklist18 was used for the cross-sectional and cohort studies.
The JBI tool contains 10 questions for case series and 8 questions for case reports with each item being scored using four criteria (yes, no, unclear and not applicable).16 The CASP checklist is comprised of 12 items and each item is scored on a 3-point scale (yes, no, can’t tell) except items 7 and 8 (open-ended responses) that describe the key results and their precision.18 Two reviewers (one licensed physical therapist and one Doctor of Physical Therapy student) independently completed the appraisals. To ensure consistency in rating, the reviewers underwent training to use the critical appraisal tools. The first author (DT) trained for the two reviewers (ME and KA) to ensure consistency in the reviewing process. The training comprised of detailed discussion of each critical appraisal tool which was followed by independent appraisal of two articles by each reviewer The appraisals were then discussed with the first author to ensure uniformity of rating. Appraisal for the remaining articles was completed independently by each reviewer with periodic check ins by first author (DT). Any conflicts were resolved by mutual consensus. If the conflict was not resolved, a third reviewer was consulted.
The initial electronic search yielded 901 articles. As indicated in the PRISMA flow diagram, after removing duplicates and completed the title and abstract screening, 55 articles went through a full-text review for eligibility Finally, twelve articles met the inclusion criteria and were selected (Figure 1).
Included studies comprised three randomized controlled trials,19‑21 five retrospective cohort studies,11,22‑25 two case series26,27 and two case reports.28,29 A total of 585 participants between the age range of 8-37 years were found across studies. Four studies included children and adults11,21,24,27 while other studies focused primarily on children. Included studies utilized VRT comprising of vestibular-ocular reflex exercises, gaze control, habituation, postural stability training, balancing challenges, Canalith repositioning procedure, and convergence training. Three of the studies also included aerobic training as part of the intervention.23,26,28 The duration of rehabilitation programs varied among the studies, ranging from 72
hours to 266 days. Detailed characteristics of the included studies are presented in Table 1.
Risk of bias assessment results are reported in Appendix 1. The most identified factors for a potential bias in case series studies included 1) clear reporting of participant demographics, 2) clear reporting of the presenting site(s)/clinic(s) demographic information, 3) consecutive and complete inclusion of participants and 4) use of appropriate statistical analysis. In terms of case reports, no significant concerns related to risk of bias were observed. Two factors were identified in three studies23,25,30 in the cohort and cross-sectional segment included 1) identification of confounding factors and 2) accounting for the confounding factors in the design and/or statistical analysis. Finally, for the randomized controlled trials, only one19 of the three studies demonstrated concerns for bias in terms of measurement of outcomes. As acknowledged in the study, clinicians may have not been completely blinded to the participant group given the significant differences in nature of the interventions.19 (Appendix 1).
Five studies used the Dizziness Handicap Inventory (DHI) to report the effectiveness of VRT on dizziness.11,19,21,27,29 Three studies found significant improvements in DHI after VRT (Table 2).11,21,27 All three studies included both pediatric and adult populations. However, results were not reported separately for the pediatric age group.
Two studies determined improvements in VOMS following VRT Alsalaheen et al.19,23 found significant improvements in all subcategories involved in VOMS including smooth pursuit, horizontal saccades, near-point convergence distance (Effect size (ES) = 0.6, p < 0.001), vertical saccades, convergence, horizontal VOR, vertical VOR, visual motion sensitivity (ES = 0.7, p < 0.001). In contrast, Kontos et al19 found no significant improvements in the following subcategories: smooth pursuits (ES = 0.01, p = 0.41), horizontal saccades (ES = 0.01, p = 0.22), vertical saccades (ES = 0.06, p = 0.09), near-point convergence (ES = 0.01, p = 0.32), near-point convergence distance (ES = 0.06, p = 0.07), and VOMS total score (ES = 0.06, p = 0.17).19 The study did not discuss results for VOMS score improvements for horizontal VOR, vertical VOR, and visual motion sensitivity
The Activities Specific Balance Confidence (ABC) scale11, 21,27,29 and the Balance Error Scoring System (BESS) were used to evaluate postural control across studies.24‑26,28 Four studies investigated improvements in self-perceived balance confidence using the ABC Scale with two studies reporting statistically significant improvements.11,27 Schneider et al.21 reported improvements in the ABC scale of 8
Table 1. Treatment frequency and duration from included studies for the current systematic review.
Author Study
Ahluwalia 2021
Alsalaheen 2020
Alsalaheen 2010
(2.98)
Total PCSS scores, BESS, symptom free heart rate, mean duration of exercise.
2022
speed, FGA, ABC, DHI, VVAS, VRBQ QOL, VRBQ Total, VRBQ Symptoms, VRBQ MOTPROV Kontos 2021
2014
2019
BESS, tandem gait backwards with eyes closed
Note: ABC= Activities-Specific Balance Confidence Scale, BESS= Balance Error Scoring System, d= Day, DGI= Dynamic Gait Index, DHI= Dizziness Handicap Inventory, DVAT= Dynamic Visual Acuity Test, FGA= Functional Gait Assessment, FTSTS= Five Times Sit to Stand, hr.= Hour, JPE= Joint Position Error Test, min= Minute, MOTPROV= Motion provoking dizziness, NR = Not reported, NRS= Numeric Rating Scare, NSI= No significant improvements, PCSS= Post-Concussion Symptom Scale, QOL= Quality of Life, RCT= Randomized clinical trial, RTP= Return to play, RTS= Return to sport, SCAT5= Sport Concussion Assessment Tool 5, SD= Standard deviation, SOT= Sensory Organization Test, TUG= Timed Up and Go, VMS= Visual Motion Sensitivity, VOMS= Vestibular/ocular Motor Screening ,VOR= Vestibular-Ocular Reflex, VRBQ= Vestibular Rehabilitation Benefit Questionnaire, VVAS= Visual Vertigo Analog Scale, wk.= Week.

Figure 1. PRISMA flow diagram (N = 12 studies)
points for those cleared to return to sport and 19.5 points for those not cleared to return to sport. However, the improvements were not statistically significant.
The BESS was used in four studies to evaluate changes in scores after VRT24‑26,28 with two studies reporting statistically significant improvements (change score = 52%, p < 0.01, 35.8%, p < 0.001).24,25 An improvement of 68.42% and 12.1% was observed in the remaining two studies but it did not reach statistical significance (Table 2).26,28 However, improvements observed in two studies25,28 were more than the established minimal detectable change values for concussion (8.6-11.3 errors).31
Gait speed using 10-meter-walk test11,27 and Functional Gait Assessment (FGA)11,21,27 were utilized to examine gait. Both studies that included gait speed using the 10-meter walk test, reported statistically significant improvements.11,27 Alsalaheen et al. (change score = 6 points, p < 0.001) and Hurtado (multiple regression estimate = 0.12 (0.04), p = 0.01) found significant improvements on the FGA
scores,11,27 whereas Schneider et al.21 did not find a significant improvement.
POST-CONCUSSION SYMPTOM SCALE (PCSS)
Five studies assessed the effects of VRT on improvements in the Post-Concussion Symptom Scale (Table 2).19,20,24,26,29 Of those studies, only one study reported statistically significant improvement in the PCSS scores (change score = 9.1 points, p < 0.001).24 Similar results were reported by Renekar et al.20 where the pragmatic progressive group (including VRT and manual therapy) to recover faster than the control group on PCSS (Hazard ratio = 2.91; 95% CI = 1.01-8.43) but the difference between groups were not statistically significant. Although the PCSS scores showed variable reduction in studies by Hugentobler and, Zikas (11.8-36 points) tests of significance were not conducted.26, 29 Kontos and colleagues reported a non-significant small effect size for the total PCSS scores (ES = 0.01, p = 0.58) indicating no statistically significant difference between groups.19
Additional measures included the number of days for return to play or symptoms resolution, joint position error, visual motion sensitivity test, dynamic visual acuity test, brain injury vision symptom survey, and the convergence insufficiency symptom survey Ahluwalia et al.22 found significant improvements in symptom reduction (p = 0.02) and number of days for return to play (p = 0.03). The studies reporting on changes in joint position error, visual motion sensitivity test, dynamic visual acuity, brain injury vision symptom survey, and convergence insufficiency symptoms survey did not report significance or mean changes in scores.28,29
This systematic review included twelve articles to discernt the evidence for the effectiveness of VRT in improving dizziness, postural control, gait, and return to sport in children post-concussion. Previous systematic reviews conducted on the effectiveness of VRT in the adult population reported that VRT may reduce time to return to play in the acute phase and may result in improvements in dizziness, gait, and quality of life for patients with concussion.32,33 This systematic review showed that the dosage for VRT ranged from 30-60-minute sessions, one to two times per week for 4-10 weeks and it may result in improvements in dizziness, oculomotor control, postural control, and gait.
Although the DHI scores showed statistically significant improvement in only three out of the five studies, all five studies demonstrated clinically meaningful improvements in the DHI scores.11,19,21,27,29 However, it is important to consider that the DHI currently has not been validated for the population below 18 years34 and minimally clinically important difference values have not been established for children. Hence, the generalization of these findings is limited.
As children are in the developmental phase of both physical and cognitive systems, their perception of disability as well as contextual expectations are significantly different form adults warranting use of age-specific outcome measures.35 Therefore, it is questionable whether drawing inference from a measure designed for the adult population may accurately represent true perceived disability and warrants further investigation.
Conflicting results were obtained for the VOMS scores between Alsalaheen et al.23 and Kontos et al.19 with Alsalaheen et al. reporting significant improvements with moderate effect size in smooth pursuits, horizontal and vertical saccades, Vestibulo-ocular reflex, visual motion sensitivity and near point convergence distance whereas results from Kontos et al.19 indicated no improvements in either the total VOMS or in any individual category of the VOMS. Variability was observed in VOMS score reporting between the studies. Mean scores were reported by Kontos et.al 19 whereas Alsalaheen et. al.23 reported median scores. Additionally, it is possible that variability existed in assessing
VOMS given the retrospective study design of the study by Alsalaheen et al.23
The RCT by Kontos et al.19 provided 30-minute individualized vestibular rehabilitation exercises and instructed the patients to perform them at home for 30 minutes per day A retrospective chart review by Alsalaheen et al.23 indicated that the patients received a one-hour weekly VRT session (total sessions ranging from 2-4) in the clinic and VRT was further augmented by a 45-minute home exercise program that was modified and progressed each visit. In-clinic sessions augmented with a home exercise program may provide additional practice and may lead to larger improvements in vestibular-ocular function.
High variability in the conventional BESS scores across studies could be attributed to the developing sensorimotor system in children.36 Additionally, the reliability of the conventional BESS may vary greatly depending on the clinician’s experience with experienced clinicians demonstrating better reliability.37 It was also difficult to determine whether the scores demonstrated a clinically meaningful change, as currently there is still no consensus on the clinically meaningful change values of the BESS. Future studies using instrumented BESS (posturography) may provide objective data that can detect subtle meaningful clinical changes as it greatly reduces inter-rater bias which is more likely to be observed in conventional BESS.
Although the ABC scale showed improvements in balance confidence across studies with some studies potentially showing a tendency toward a ceiling effect,11,29 it is important to highlight that the ABC scale like the DHI was primarily developed for older adults and its measurement properties have not been established for the pediatric population.38,39 Additionally, some items of the ABC scale (sweep the floor, step on and off an escalator while you are holding onto a railing, step onto or off an escalator while holding onto parcels such that you cannot hold onto the railing) may not be contextually relevant for children. Finally, most of the items are focused on walking and do not involve age-appropriate activities like running, playing, and participating with peers. Hence, the results relevant to balance confidence must be interpreted with caution as there could be a ceiling effect for this population.39
Gait was one of the less examined constructs across studies with only three studies including gait assessment. Similar to the ABC scale, the FGA may demonstrate ceiling effect to a certain extent in children.39 Using more challenging measures like high level mobility assessment tool (HiMAT) may provide precise results in this population.40
PCSS scores showed improvements in four out of five studies. It is a possibility that early initiation of VRT may result in earlier symptom resolution on the PCSS and earlier re-
Measure Pre-intervention mean (SD)/ median (range)/percentage
Post-intervention mean (SD)/ median (range)/percentage.
scores mean (SD)/ median (range)/percentage.
Alsalaheen 2010
Hurtado 2022
Kontos 2021
Schneider 2014
(21)
= 35.9 (14.9)
= 30.1 (11.8)
= 46 (6-84)
= 42 (0-66)
Zikas 2019 78 14
Kontos 2021
saccades
(1.6)
(1.4)
= 1.8 (1.8)
= 1.2 (1.7)
Vestibular/ocular motor screening (VOMS)
= -24.94 (3.72)
= -17.89 (3.61)
(0.33)
= -1.65 (0.41)
= - 0.94(0.40)
= 0.01, p = 0.22 Vertical saccades
= 2.4 (2.3) Cont = 1.8 (1.9)
NPC Exp = 2.6 (2.4) Cont = 3.0 (2.8)
NPC distance
Horizontal VOR
Vertical VOR
VOMS total
= 4.7 (5.7) Cont = 4.4 (6.2)
= 4.0 (2.6) Cont = 4.1 (2.6)
= 4.5 (2.9)
= 4.3 (3.3)
= 5.0 (3.7)
= 4.9 (3.8)
= 56.6 (32.7) Cont = 54.3 (33.4)
= -2.41 (0.52) Cont = -1.17 (0.50)
= -2.77 (1.6)
= 0.8 (1.4)
= -3.46 (1.03)
= -0.76 (1.4)
= 0.06, p = 0.09
= 0.01, p = 0.32
= 0.06, p = 0.07
= -57.59 (8.36) Cont = -41.22 (8.12)
= 0.06, p = 0.17
Measure
Alsalaheen 2020 (Median)
Pre-intervention mean (SD)/ median (range)/percentage
Smooth pursuit
Horizontal saccades
saccades
Convergence
= 0 (0-6)
(0-2)
1 (0-11)
(0-3)
= 1 (0-12)
(0-3)
(0-11)
(0-4)
2 (0-10)
0 (0-3)
Post-intervention mean (SD)/ median (range)/percentage.
Change scores mean (SD)/ median (range)/percentage.
(0-3)
(0-4)
(0-6)
(2-39)
(0-52)
= 19.5 (-6 - 43.5)
(N = 1) = 30
= 12.75 (0-55)
Measure
Alsalaheen 2010
Hurtado 2022
Schneider 2014
Pre-intervention mean (SD)/ median (range)/percentage
(5)
Post-intervention mean (SD)/ median (range)/percentage.
(3)
2017
= 27 (17-30)
= 27 (24-30)
Balance Error Scoring System (BESS)
Change scores mean (SD)/ median (range)/percentage.
2017
2021
cleared (N = 1) = 3
not cleared = 1 (2-6)
Measure
scores mean (SD)/ median (range)/percentage.
turn to play 20‑22 Vestibular network contributes to modulate space, body, and self-awareness expanding into dimensions of emotion processing, mental health, and social cognition.41 VRT may result in potential habituation of vestibular system and responses similar to exercise training.19 VRT post-concussion requires the patient to be repeatedly exposed to the provocative stimuli to reduce symptom severity thereby improving recovery time.19
Ahluwalia and colleagues reported potential benefits of early VRT and showed that early initiation of VRT may result in faster symptom resolution and earlier return to play 22 This was supported by a recent systematic review which highlighted that early VRT post-concussion in athletes has been shown to reduce severity of symptoms and duration, therefore decreasing recovery time to less than 21 days.14 Early intervention timeline was reported to be within 30 days post-concussion in an adult population, but there is no consensus on the time frame for children.22 Hence, early initiation of VRT may allow for improved spatiotemporal adjustments limiting the duration of symptoms.
This systematic review included only the studies that were published in English which could have resulted in exclusion of research published in other languages. A second limitation was the specific age constraints which led to the exclusion of some articles due to a lack of separation between the children and adults as well as limiting the effectiveness of one study that did not report separation in results based on age group. Outcomes measures varied greatly across studies that studied the effectiveness of VRT. Although VRT shows promise in pediatric concussion, the evidence re-
mains limited. Given that most studies included in this review were non-experimental (case series, case report, and cohort studies), findings must be interpreted with caution when making informed clinical decisions.
Many of the studies included in this review used a multimodal approach examining vestibular therapy in conjunction with other methods of intervention. Future studies should assess the stand-alone effect of VRT compared to conventional therapy using rigorous experimental designs. Finally, due to substantial variability in study design, sample size and outcome measures used, a meta-analysis could not be performed to statistically support the effectiveness of VRT in children post-concussion.
In conclusion, this review suggests that VRT shows promise and may result in symptom improvements in children postconcussion when used as part of a multimodal intervention plan. However, it is important to establish the most effective intervention dose and training parameters. Additionally, it is important to utilize age-appropriate validated measures to ascertain the effectiveness of VRT in this population.
The authors report no conflicts of interest.
Submitted: July 28, 2024 CST Accepted: December 14, 2024 CST. Published: February 01, 2025 CST.
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Yaramothu C, Goodman AM, Alvarez TL. Epidemiology and incidence of pediatric concussions in general aspects of life. Brain Sci. 2019;9(10). doi:10.3390/brainsci9100257
2. Prevention CfDCa. Quickstats: Percentage of children and adolescents aged ≤17 years who had ever received a diagnosis of concussion or brain injury, by sex and age group national health interview survey, United States, 2022. 2023. Accessed April 10, 2024. https://www.cdc.gov/mmwr/volumes/72/wr/ mm7233a5.htm?s_cid=mm7233a5_w#suggestedcitati on
3. Black L, Zablotsky B. Concussions and brain injuries in children: United States, 2020. CDC.gov 2021. Accessed April 10, 2024. https://stacks.cdc.gov/ view/cdc/111174
4. Schilling S, Mansour A, Sullivan L, Ding K, Pommering T, Yang J. Symptom burden and profiles in concussed children with and without prolonged recovery Int J Environ Res Public Health 2020;17(1). doi:10.3390/ijerph17010351
5. Karlin AM. Concussion in the pediatric and adolescent population: Different population, different concerns. PM R. 2011;3(10 Suppl 2):S369-379. doi:10.1016/j.pmrj.2011.07.015
6. Zhang AL, Sing DC, Rugg CM, Feeley BT, Senter C. The rise of concussions in the adolescent population. Orthop J Sports Med. 2016;4(8):2325967116662458. doi:10.1177/2325967116662458
7 Corwin DJ, Wiebe DJ, Zonfrillo MR, et al. Vestibular deficits following youth concussion. J Pediatr. 2015;166(5):1221-1225. doi:10.1016/ j.jpeds.2015.01.039
8. Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311-2318. doi:10.1177/ 0363546511410655
9. Bigelow RT, Semenov YR, Hoffman HJ, Agrawal Y. Association between vertigo, cognitive and psychiatric conditions in us children: 2012 national health interview survey. Int J Pediatr Otorhinolaryngol 2020;130:109802. doi:10.1016/ j.ijporl.2019.109802
10. Master CL, Master SR, Wiebe DJ, et al. Vision and vestibular system dysfunction predicts prolonged concussion recovery in children. Clin J Sport Med. 2018;28(2):139-145. doi:10.1097/ JSM.0000000000000507
11. Alsalaheen BA, Mucha A, Morris LO, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther 2010;34(2):87-93. doi:10.1097/ NPT.0b013e3181dde568
12. Galeno E, Pullano E, Mourad F, Galeoto G, Frontani F. Effectiveness of vestibular rehabilitation after concussion: A systematic review of randomised controlled trial. Healthcare 2022;11(1). doi:10.3390/ healthcare11010090
13. Park K, Ksiazek T, Olson B. Effectiveness of vestibular rehabilitation therapy for treatment of concussed adolescents with persistent symptoms of dizziness and imbalance. J Sport Rehabil 2018;27(5):485-490. doi:10.1123/jsr.2016-0222
14. Babula G, Warunek E, Cure K, Nikolski G, Fritz H, Barker S. Vestibular rehabilitation as an early intervention in athletes who are post-concussion: A systematic review. Int J Sports Phys Ther. 2023;18(3):577 doi:10.26603/001c.75369
15. Page MJ, McKenzie JE, Bossuyt PM, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
16. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: An introduction to the jbi critical appraisal tool. JBI Evidence Synthesis. 2020;18(10):2127-2133. doi:10.11124/jbisrir-d-19-00099
17 Sterne JAC, Savović J, Page MJ, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
18. Critical apprisal skills programme. CASP (cohort studies). 2023. Accessed November 12, 2023. https:// casp-uk.net/checklists/casp-cohort-studieschecklist.pdf
19. Kontos AP, Eagle SR, Mucha A, et al. A randomized controlled trial of precision vestibular rehabilitation in adolescents following concussion: Preliminary findings. J Pediatr 2021;239:193-199. doi:10.1016/j.jpeds.2021.08.032
20. Reneker J, Hassen A, Phillips R, Moughiman M, Donaldson M, Moughiman J. Feasibility of early physical therapy for dizziness after a sports-related concussion: A randomized clinical trial. Scand J Med Sci Sports 2017;27(12):2009-2018. doi:10.1111/ sms.12827
21. Schneider KJ, Meeuwisse WH, Nettel-Aguirre A, et al. Cervicovestibular rehabilitation in sport-related concussion: A randomised controlled trial. Br J Sports Med. 2014;48(17):1294-1298. doi:10.1136/ bjsports-2013-093267
22. Ahluwalia R, Miller S, Dawoud FM, et al. A pilot study evaluating the timing of vestibular therapy after sport-related concussion: Is earlier better? Sports Health 2021;13(6):573-579. doi:10.1177/ 1941738121998687
23. Alsalaheen B, Carender W, Grzesiak M, et al. Changes in vestibular/ocular-motor screen scores in adolescents treated with vestibular therapy after concussion. Pediatr Phys Ther. 2020;32(4):331-337. doi:10.1097/pep.0000000000000729
24. Grabowski P, Wilson J, Walker A, Enz D, Wang S. Multimodal impairment-based physical therapy for the treatment of patients with post-concussion syndrome: A retrospective analysis on safety and feasibility. Phys Ther Sport. 2017;23:22-30. doi:10.1016/j.ptsp.2016.06.001
25. Storey EP, Wiebe DJ, DʼAlonzo BA, et al. Vestibular rehabilitation is associated with visuovestibular improvement in pediatric concussion. J Neurol Phys Ther 2018;42(3):134-141. doi:10.1097/ npt.0000000000000228
26. Hugentobler JA, Vegh M, Janiszewski B, Quatman-Yates C. Physical therapy intervention strategies for patients with prolonged mild traumatic brain injury symptoms: A case series. Int J Sports Phys Ther 2015;10(5):676-689.
27. Hurtado JE, Heusel-Gillig L, Risk BB, et al. Technology-enhanced visual desensitization home exercise program for post-concussive visually induced dizziness: A case series. Physiother Theory Pract. 2022;38(8):985-994. doi:10.1080/ 09593985.2020.1815259
28. Gunter KB, Shields CJ, Ott SD, Coronado RA. Rehabilitation of an adolescent equestrian athlete with a history of multiple concussions: A case report describing an adapted return-to-sport protocol. J Orthop Sports Phys Ther 2018;48(12):934-942. doi:10.2519/jospt.2018.8214
29. Ziaks L, Giardina R, Kloos A. Integration of vision and vestibular therapy for vestibulo-ocular postconcussion disorder–a case study Internet J Allied Health Sci Pract. 2019;17(3):11. doi:10.46743/ 1540-580X/2019.1754
30. Wong CK, Ziaks L, Vargas S, DeMattos T, Brown C. Sequencing and integration of cervical manual therapy and vestibulo-oculomotor therapy for concussion symptoms: Retrospective analysis. Int J Sports Phys Ther. 2021;16(1):12-20. doi:10.26603/ 001c.18825
31. Carlson CD, Langdon JL, Munkasy BA, Evans KM, Buckley TA. Minimal detectable change scores and reliability of the balance error scoring system in student-athletes with acute concussion. Athl Train Sports Health Care. 2020;12(2):67-73. doi:10.3928/ 19425864-20190401-02
32. Galeno E, Pullano E, Mourad F, Galeoto G, Frontani F. Effectiveness of vestibular rehabilitation after concussion: A systematic review of randomised controlled trial. doi:10.3390/healthcare11010090
33. Kinne BL, Bott JL, Cron NM, Iaquaniello RL. Effectiveness of vestibular rehabilitation on concussion-induced vertigo: A systematic review Phys Ther Rev 2018;23(6):338-347 doi:10.1080/ 10833196.2018.1517032
34. Tamber AL, Wilhelmsen KT, Strand LI. Measurement properties of the dizziness handicap inventory by cross-sectional and longitudinal designs. Health Rel Qual of Life 2009;7(1):101. doi:10.1186/1477-7525-7-101
35. Arbuckle R, Abetz-Webb L. “Not just little adults”: Qualitative methods to support the development of pediatric patient-reported outcomes. The Patient. 2013;6(3):143-159. doi:10.1007/ s40271-013-0022-3
36. Schönberg NKT, Poppel J, Howell D, et al. Instrumented balance error scoring system in children and adolescents—a cross sectional study Diagnostics 2024;14(5):513. doi:10.3390/ diagnostics14050513
37 Kuo KT, Hunter BC, Obayashi M, et al. Novice vs expert inter-rater reliability of the balance error scoring system in children between the ages of 5 and 14. Gait Posture 2021;86:13-16. doi:10.1016/ j.gaitpost.2021.02.026
38. Powell LE, Myers AM. The activities-specific balance confidence (abc) scale. J Gerontol A Biol Sci Med Sci 1995;50a(1):M28-34. doi:10.1093/gerona/ 50a.1.m28
39. Alsalaheen BA, Whitney SL, Marchetti GF, et al. Performance of high school adolescents on functional gait and balance measures. Pediatr Phys Ther 2014;26(2):191-199. doi:10.1097/ pep.0000000000000037
40. Williams GP, Greenwood KM, Robertson VJ, Goldie PA, Morris ME. High-level mobility assessment tool (himat): Interrater reliability, retest reliability, and internal consistency Phys Ther 2006;86(3):395-400. doi:10.1093/ptj/86.3.395
41. Lopez C. The vestibular system: Balancing more than just the body. Curr Opin Neurol. 2016;29(1):74-83. doi:10.1097/ wco.0000000000000286
Effectiveness of Vestibular Rehabilitation in Children Post-Concussion: A Systematic Review
Download: https://ijspt.scholasticahq.com/article/128282-effectiveness-of-vestibular-rehabilitation-in-children-postconcussion-a-systematic-review/attachment/261629.docx?auth_token=dXOuigeKOwC7xwof6AdX

Jehu DA, Bolgla LA, Armas S, Dutton F. Assessing the Inter-Rater and Inter-Trial Reliability of the NeurOptics Pupillary Light Response-3000 Pupillometer. IJSPT 2025;20(2):157-167. doi:10.26603/001c.128047
Deborah A Jehu1a , Lori A Bolgla2 , Samantha Armas1 , Forest Dutton2
1 Interdisciplinary Health Sciences , Augusta University, 2 Physical Therapy, Augusta University
Keywords: pupillometry, pupillary light reflex, pupillary light response, psychometrics https://doi.org/10.26603/001c.128047
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background
An automated pupillometer is a handheld device used to stimulate the pupillary light response (PLR) and track the entirety of the response from constriction to dilation. Pupillometers provide objective data that clinicians can use to identify and assess brain injury. The validity of these devices has been previously established; however, the inter-rater and inter-trial reliability are unknown.
The purpose of this study was to assess the inter-rater and inter-trial reliability of the NeurOptics PLR-3000 pupillometer device in measuring pupil size changes, constriction velocities, and dilation velocities. The authors hypothesized that inter-rater and inter-trial reliability would have intraclass correlation coefficients (ICC) greater than or equal to 0.70 for all PLR parameters.
Design: Observational, reliability study
Methods: Forty-eight healthy adults (age 18-40 years) without a history of neurological injury, optical surgery, or cognitive impairment participated. Two independent raters used the NeurOptics PLR-3000 to measure PLR parameters in the left and right eyes of each subject. Data for the average and individual trials of each PLR parameter were used to determine inter-rater and inter-trial reliability, respectively. Inter-rater and inter-trial reliability was evaluated using descriptive statistics, ICC, the standard error of measurement, Bland-Altman plots, and the minimal detectable change.
Seven out of eight NeurOptics 3000-PLR parameters demonstrated moderate-to-excellent inter-rater (ICC range 0.72-0.96) and good-to-excellent inter-trial reliability (ICC range 0.76-0.98). The 75% recovery time parameter exhibited moderate inter-rater (ICC range 0.64-0.67) and poor-to-moderate inter-trial (ICC range 0.41-0.65) reliability
The NeurOptics 3000-PLR demonstrated acceptable reliability in measuring initial and end pupil size, constriction and dilation velocity, and latency to change between different users and trials. However, the device exhibited unacceptable reliability when measuring
The Graduate School Augusta University Augusta, GA 30912 djehu@augusta.edu a
Corresponding Author:
Deborah A. Jehu, PhD Assistant Professor Department of Community & Behavioral Health Sciences
School of Public Health
the time to 75% pupil size recovery The device can be used in detecting and monitoring brain injury but should be limited to reliable measures only.
A concussion can provoke changes in pupil size and the pupillary light response (PLR). These subtle, yet significant, changes have led healthcare providers to measure neurological injury with a pupillometer 1‑4 Pupillometers can provide insight regarding the location of neurological lesions and predict recovery trajectory following traumatic brain injury 5 The PLR is both a response and a visual reflex to the level of light sensed in the environment and serves as an accessible marker of the autonomic nervous system.6, 7 The PLR provides a comprehensive manner to assess sympathetic and parasympathetic function.7 Specifically, the sympathetic pathway controls the eye muscles responsible for pupil dilation, while the parasympathetic pathway controls the eye muscles responsible for pupil constriction.8 The parasympathetic system causes eye constriction when a light stimulus is applied, and the sympathetic system causes eye dilation to the baseline state when the light stimulus is removed. Studying the static and dynamic properties of the PLR has emerged as an attractive field of interest given its ease of access, non-invasiveness, and insight into numerous neurological disorders and physiological states.6,9‑11
Historically, healthcare providers have used penlights to assess pupil symmetry and PLR. Concerns related to the use of penlights are low inter-rater reliability, higher error rates in prognosis, and the reduced ability to monitor the recovery of the PLR.2,5,6 Automated pupillometer systems have been developed and shown to be more accurate and reliable for examining the PLR.2,12 These devices are superior to manual observation because of their ability to monitor intracranial pressure, provide a prognosis following concussion, and assess cognitive load.1,6,11,13‑16 At a minimum, automated devices provide readouts on the static (e.g., minimum and maximum pupil diameter) and dynamic (pupil constriction and dilation velocity) parameters.15 More advanced devices, such as the NeurOptics Neurologic Pupil index (NPi)-200 and NPi-300, provide the NPi with a calculation that incorporates pupil size, constriction latency, constriction velocity, and dilation velocity. These devices are useful because they can compare scores to a normative database. More specifically, they provide a score range of 0-5 points, in which a score less than 3 points indicates abnormal pupil function.17 The NeurOptics PLR-3000 (NO3000) better characterizes the PLR response as it provides the time from peak pupil constriction size to 75% of its baseline size, commonly known as the T-75 recovery time parameter (T75). Users of earlier models like NPi-300 could not obtain this parameter and had to extrapolate the graphical data to calculate T75.10
The T75 represents the sympathetic drive behind the dilation phase and is influenced by the amplitude of the light reflex.18 The larger the percent change from baseline to maximum dilation size results in more time needed for the pupil to constrict and return to baseline. Researchers have
reported longer T75 times in children with mild concussions and athletes with sport-related concussions compared to controls.14,19 While the T75 can discriminate between concussed and healthy groups, its reliability has not been extensively examined.14,19
Establishing the psychometric properties of commonly used PLR systems is important to ensure they appropriately acquire meaningful information that aids in diagnosis, clinical prognosis, and research. In addition to concussions and traumatic brain injuries, pupillometers show promise in better understanding different neurological and chronic diseases such as Parkinson’s and Alzheimer’s.4,6,9 As these systems, particularly the hand-held automatic pupillometers, are increasingly integrated into common healthcare settings, the importance of verifying a device’s robustness is critical.4,9,17,20 Importantly, devices have inherent differences due to their design, which may introduce measurement variability 4 NO3000 has established inter-trial reliability, but inter-rater reliability has not been established.21 Therefore, the purpose of our study was to establish the inter-rater reliability and confirm the inter-trial reliability of the NO3000 pupillometer among healthy adults. The authors hypothesized that inter-rater and inter-trial reliability for all PLR measures would have intraclass correlation coefficients (ICC) greater than or equal to 0.70, which has been deemed as acceptable reliability 22
Before subject recruitment, we conducted an a priori power analysis. Based on a minimum acceptable ICC of 0.70 and expected reliability ICC of 0.86, a two-tailed significance of alpha=0.05, a power of 80%, and two raters, at least 39 subjects would be needed.22 Fourty-eight healthy adults were recruited from Augusta University via word of mouth and email advertisement (25 males, age = 25.0 + 4.7 y; 23 females, age = 25.3 + 6.4 y). Eligible participants were between the ages 18-40 and did not have a history of known neurological injury (including stroke, traumatic brain injury, concussion), cognitive impairment, neurodegenerative disorders, migraine headache diagnosis, seizure disorder, blindness, dysautonomia/postural orthostatic tachycardia syndrome, and history of eye surgery/amblyopia/strabismus or other congenital eye disorders that could alter pupil response before measurement. This age range of 18 to 40 years was chosen because differences in PLR differ between pediatric and adult cohorts.19,23 Also, pupil sizes tend to decrease after the fourth decade of life.19,23 Individuals who could not provide accurate measurements due to repeated blinking throughout data collection also were excluded. All subjects signed an institutional-approved informed consent form prior to participation.

Figure 1. Procedure for measuring the pupillary light reflex variables.
Procedures were developed in accordance with the Quality Appraisal of Diagnostic Reliability (QAREL) Checklist (Appendix A).24,25 Both the investigator and participant were seated in identical 18" tall chairs across from one another at a standardized table in an environment with fluorescent lighting. We asked subjects to focus their non-measured eye on a fixed point located 2 meters away from them to avoid accommodation of the eye being measured. The pupillometer (NeurOptics PLR-3000, Irvine, CA), which operates in a monocular manner, was then placed against the eye (Figure 1). We used settings that were identical to those described by Asakawa et al.21 Settings included a positive pulse stimulus, light stimulus pulse intensity of 10 uW, and background intensity of 0 uW The measurement duration was 5.01 s, the pulse duration was 0.80 s, and the pulse onset was immediate (0 s) to stimulate the PLR. Subjects remained as still as possible and refrained from blinking during the 5-s measurement period. The investigator recorded all PLR measurements (initial pupil diameter [INITIAL], end pupil diameter [END], % change [DELTA], constriction latency [LATENCY], average constriction velocity [ACV], maximum constriction velocity [MCV], average dilation velocity [ADV], and T75). The subjects rested between 30 seconds and one minute before the investigator measured the other eye. The investigators took three trials for the right eye and three trials for the left eye. Subjects rested one to two minutes before a second investigator repeated the same measurements. Raters examined subjects and recorded values independent of each other The average of the three trials for each eye was used to determine inter-rater reliability; individual trials were used to determine inter-trial reliability
All analyses were conducted using IBM SPSS Statistics for Windows, Version 28 (IBM Corp, Armonk, NY, USA) with the level of significance established at the 0.05 level. Means, standard deviations, and 95% confidence intervals were calculated for all dependent measures.
Separate independent t-tests were used to compare group differences between rater 1 and rater 2. Separate ICC [2,3] and standard error of measurement (SEM) were used to determine inter-rater reliability and measurement precision.26 The minimal detectable change (MDC) was also calculated for inter-rater reliability to determine each measure’s responsiveness.27 The MDC represents the minimal amount of change exceeding the SEM, and represents a change beyond measurement error.27 Separate ICC [3,1] and SEM were used to determine inter-trial reliability and measurement precision for Rater 1 and Rater 2. ICC values <0.5 were indicative of poor, between 0.5 and 0.75 were indicative of moderate, between 0.75 and 0.9 were indicative of good, and >0.90 were indicative of excellent reliability 28
Bland-Altman plots were used to determine the similarity between each measure. For this purpose, the difference (bias) between raters and the mean score (magnitude) for the raters were plotted to provide important information regarding bias.29 Between-rater score differences that were scattered (i.e., no tendency for a score to be higher or lower) were considered unbiased. The plots also assessed for bias associated with the magnitude of a score. Bias would occur when the between-rater score differences were associated with an increase in the score magnitude.29
No significant differences (p > 0.05) existed between any of the measures taken by Rater 1 and Rater 2 (Table 1). For inter-rater reliability, ICC [2,3] exceeded 0.70 for all measures except for T75 (Table 2). Four of the eight measures had excellent reliability for each eye as evidenced by ICCs exceeding 0.90 (INITIAL, END, ACV, and MCV). A similar pattern of values existed (Table 3) for inter-trial reliability.
Except for T75, the Bland-Altman plots showed a random pattern between the difference and mean for each measure (Figures 2 - 4). These plots also did not show a pattern of differences increasing or decreasing as the score magnitude (mean) increased. These factors taken together suggested no bias for these measures. For T75, the BlandAltman plots appeared less scattered, and many differences appeared to increase with greater score magnitude. This finding suggested bias for T75, especially for the right eye.
The current study was the first to examine inter-rater reliability for the eight PLR parameters obtained using the NO3000. Except for the T75, inter-trial and inter-trial reliability was moderate to excellent for all measures. ICCs for T75 were poor to moderate and did not meet the minimum
Table 1. Means + standard deviations and (95% confidence intervals) for all dependent measures (n=48).
Left Eye
Right Eye
Measure
* Means compared using an independent t-test
Table 2. Summary of intraclass correlation coefficients (ICC), standard error of measurement (SEM), and minimal detectable change (MDC) for inter-rater reliability (n=48).
acceptable ICC of 0.7, suggesting that T75 may not be a useful biomarker
INTER-RATER AND INTER-TRIAL RELIABILITY OF THE NO3000
Moderate to excellent inter-rater ICCs existed when measuring seven of the eight PLR measures using the NO3000, supporting its robustness as an automated pupillometer. Equally important was acceptable inter-trial reliability, the ability for a user to repeat measures and obtain consistent results12,21 Most inter-trial ICCs were good to excellent and agreed with Asakawa et al.,21 who used the NO3000 to examine inter-trial reliability. McKay et al.30 compared measures from the NO3000 to BrightLamp, a pupillometer app, and also found strong measurement reproducibility for the NO3000. Findings from the current study further support the reliability of the NO3000.21,30 Master et al.19 have used PLR as a biomarker for identifying sport-related concus-
sions in adolescents; having a device with acceptable reliability is critical for clinical decision-making.31
INCONSISTENCIES IN THE INTER-RATER AND INTERTRIAL RELIABILITY OF T75
The current findings suggest that both the inter-rater and inter-trial reliability of T75 were poor to moderate.32 Unacceptable T75 reliability may have resulted from measurement precision. To obtain T75, subjects must keep their eyes still throughout the entire measurement period. Researchers who have examined pediatric populations have reported sources of error from movement14 and shorter stimulus durations.33 In studies analyzing T75 using the PLR-2000 or PLR-3000 models, the duration of the stimulus was 154 ms or 800 ms.14,19,34‑37 This variation in stimulus duration across studies may represent a source of error contributing to unacceptable reliability.
Table 3. Summary of intraclass correlation coefficients (ICC) and standard error of measurement (SEM) for intertrial reliability (n=48).

Figure 2. Bland-Altman plots comparing differences in measures for the left eye between rater 1 and rater 2 for initial pupil size (A), end pupil size (B), pupil size change (C), and latency (D). The solid black line represents the mean of the difference between raters. The gray-dashed lines represent the mean difference + 2 standard deviations.
The current findings coincided with Asakawa et al.,21 who also used the NO3000. Asakawa et al. reported poor inter-trial T75 reliability and suggested that specified device settings be used to obtain this parameter 21 They used a 180-µwatt/cm2 stimulus with an 800-ms duration that was considerably higher compared to our 30-ms duration.21 Asakawa et al. concluded that poor reliability resulted from a lack of optimal settings to accommodate the time required for the eye to reach 75% of its baseline size. The time between trials also may need to be lengthened and standardized to give the eye adequate time to recover before be-
ing re-stimulated. Yoo et al.37 recorded for a 5 s duration after initiating a 180 µwatts/cm2 stimulus for 185 ms and found significant differences in T75 and pupil diameters between healthy individuals and those with Horner Syndrome. These settings differed from the settings used in the current research of 180 µwatts/cm2 for 30 ms and suggested the light stimulus settings, particularly the duration, be increased to obtain consistent T75 data. Others14,19 that used T75 analysis in populations with concussions used pupillometer settings with a 180-µwatt/cm2 but a 154 ms stimulus duration. Findings from these studies were more con-

Figure 3. Bland-Altman plots comparing differences in measures for the left eye between rater 1 and rater 2 for average constriction velocity (A), maximum constriction velocity (B), average dilation velocity (C), and 75% time to recovery (D). The solid black line represents the mean of the difference between raters. The gray-dashed lines represent the mean difference + 2 standard deviations.

Figure 4. Bland-Altman plots comparing differences in measures for the right eye between rater 1 and rater 2 for initial pupil size (A), end pupil size (B), pupil size change (C), and latency (D). The solid black line represents the mean of the difference between raters. The gray-dashed lines represent the mean difference + 2 standard deviations.
sistent, which suggests the importance of a longer duration range to obtain reliable T75 data.14,19 Future investigators should pay special attention to the light stimulus intensity and duration when obtaining PLR parameters and which testing conditions are needed to optimize data collection.

Figure 5. Bland-Altman plots comparing differences in measures for the right eye between rater 1 and rater 2 for average constriction velocity (A), maximum constriction velocity (B), average dilation velocity (C), and 75% time to recovery (D). The solid black line represents the mean of the difference between raters. The gray-dashed lines represent the mean difference + 2 standard deviations.
Measurement reliability is critical to enhance clinical decision-making.31 It supports that a change in a parameter represents a “true” change in the behavior. Clinicians also can use the MDC values (Table 2) to determine if changes in a measure exceed the inherent measurement variability, thus representing a “true” change.27 Other previous pupillometer models from NeurOptics have established intertrial and inter-device reliability, supporting their use in the critical care field for the evaluation of traumatic brain injury 17 Findings from the current study generally support the use of the NO3000 to assess PLR in screening settings and research applications. The NO3000 is useful in detecting concussions because there are known changes to the pupillary light response following trauma.4 Pupillometers can increase detection, especially when clinical symptoms may be lacking, and mitigate human error.3,4 With their user-friendly and portable designs, settings beyond research labs such as sports medicine or physical therapy clinics can use this device to monitor recovery progress.3, 4 However, caution is required when measuring T75 due to more sources of error. Future investigations should determine the optimal settings for this measure.
This study has limitations. Only one setting of the light stimulus and recording period was used during data collection. Having conducted trials using different light stimulus intensities and durations could have elicited ranges that
accommodate and improve the reproducibility of T75. Eye dominance in our participants was not determined, thus, the authors are unable to explain the discrepancies in T75 observed in the right eye only rather than both eyes. Only the most recent version of the model was used, making the current findings only generalizable to the NO3000. A final limitation was the use of healthy subjects, which has limited generalization to clinical populations.
The inter-rater and inter-trial reliability of the NO3000 was established. All parameters, except T75, exhibited good to excellent inter-rater and inter-trial reliability T75 had moderate inter-rater and inter-trial reliability, which likely reflected inherent challenges when obtaining this measure. The NO3000 can be used in future pupillometry studies focused on measuring static and dynamic PLR parameters, but attention and rationale regarding the stimulus settings and environment are needed to minimize measurement error Further investigation is needed to examine if other pupillometers can reliably measure T75 using different light stimulus intensities and durations.
The authors report no conflicts of interest.
Submitted: April 18, 2024 CST. Accepted: November 22, 2024
CST Published: February 01, 2025 CST
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Couret D, Boumaza D, Grisotto C, et al. Reliability of standard pupillometry practice in neurocritical care: an observational, double-blinded study. Crit Care 2016;20:99. doi:10.1186/s13054-016-1239-z
2. Taylor WR, Chen JW, Meltzer H, et al. Quantitative pupillometry, a new technology: normative data and preliminary observations in patients with acute head injury Technical note. J Neurosurg 2003;98(1):205-213. doi:10.3171/jns.2003.98.1.0205
3. Boulter JH, Shields MM, Meister MR, Murtha G, Curry BP, Dengler BA. The expanding role of quantitative pupillometry in the evaluation and management of traumatic brain injury Front Neurol 2021;12:685313. doi:10.3389/fneur.2021.685313
4. Vrettou CS, Fragkou PC, Mallios I, et al. The role of automated infrared pupillometry in traumatic brain injury: a narrative review J Clin Med 2024;13(2). doi:10.3390/jcm13020614
5. Olson DM, Stutzman S, Saju C, Wilson M, Zhao W, Aiyagari V Interrater reliability of pupillary assessments. Neurocrit Care. 2016;24(2):251-257. doi:10.1007/s12028-015-0182-1
6. Hall CA, Chilcott RP Eyeing up the future of the pupillary light reflex in neurodiagnostics. Diagnostics 2018;8(1). doi:10.3390/diagnostics8010019
7 Mathot S. Pupillometry: psychology, physiology, and function. J Cogn 2018;1(1):16. doi:10.5334/joc.18
8. Ciuffreda KJ, Joshi NR, Truong JQ. Understanding the effects of mild traumatic brain injury on the pupillary light reflex. Concussion 2017;2(3):CNC36. doi:10.2217/cnc-2016-0029
9. Troiani V. The future of quantitative pupillometry in health and disease. Clin Auton Res 2020;30(1):11-12. doi:10.1007/s10286-019-00655-3
10. Park JG, Moon CT, Park DS, Song SW. Clinical utility of an automated pupillometer in patients with acute brain lesion. J Korean Neurosurg Soc 2015;58(4):363-367. doi:10.3340/jkns.2015.58.4.363
11. Chen JW, Gombart ZJ, Rogers S, Gardiner SK, Cecil S, Bullock RM. Pupillary reactivity as an early indicator of increased intracranial pressure: The introduction of the Neurological Pupil index. Surg Neurol Int 2011;2:82. doi:10.4103/2152-7806.82248
12. Nyholm B, Obling L, Hassager C, et al. Superior reproducibility and repeatability in automated quantitative pupillometry compared to standard manual assessment, and quantitative pupillary response parameters present high reliability in critically ill cardiac patients. PLoS One. 2022;17(7):e0272303. doi:10.1371/ journal.pone.0272303
13. Hershaw JN, Ettenhofer ML. Insights into cognitive pupillometry: Evaluation of the utility of pupillary metrics for assessing cognitive load in normative and clinical samples. Int J Psychophysiol. 2018;134:62-78. doi:10.1016/j.ijpsycho.2018.10.008
14. Hsu J, Stec M, Ranaivo HR, Srdanovic N, Kurup SP Concussion alters dynamic pupillary light responses in children. J Child Neurol 2021;36(3):195-202. doi:10.1177/0883073820964040
15. Jahns FP, Miroz JP, Messerer M, et al. Quantitative pupillometry for the monitoring of intracranial hypertension in patients with severe traumatic brain injury. Crit Care. 2019;23(1):155. doi:10.1186/ s13054-019-2436-3
16. Meeker M, Du R, Bacchetti P, et al. Pupil examination: validity and clinical utility of an automated pupillometer J Neurosci Nurs 2005;37(1):34-40. doi:10.1097/ 01376517-200502000-00006
17. Stutzman S, Iype P, Marshall J, et al. Inter-device reliability of the NPi-200 and NPi-300 pupillometers. J Clin Neurosci. 2022;100:180-183. doi:10.1016/ j.jocn.2022.04.023
18. Patwari PP, Stewart TM, Rand CM, et al. Pupillometry in congenital central hypoventilation syndrome (CCHS): quantitative evidence of autonomic nervous system dysregulation. Pediatr Res 2012;71(3):280-285. doi:10.1038/pr.2011.38
19. Master CL, Podolak OE, Ciuffreda KJ, et al. Utility of pupillary light reflex metrics as a physiologic biomarker for adolescent sport-related concussion. JAMA Ophthalmol. 2020;138(11):1135-1141. doi:10.1001/jamaophthalmol.2020.3466
20. Hall CA, Chilcott RP Eyeing up the future of the pupillary light reflex in neurodiagnostics. Diagnostics (Basel) 2018;8(1):19. doi:10.3390/diagnostics8010019
21. Asakawa KI, H. Reproducibility and normative values of the parameters of a new hand-held digital pupillometer J Clin Exp Opthamol 2017;8:654.
22. Arifin WN. A web-based sample size calculator for reliability studies. Educ Med J. 2018;10(3):67-76. doi:10.21315/eimj2018.10.3.8
23. Rickmann A, Waizel M, Kazerounian S, Szurman P, Wilhelm H, Boden KT. Digital pupillometry in normal subjects. Neuroophthalmology 2017;41(1):12-18. doi:10.1080/ 01658107.2016.1226345
24. EQUATOR Network. Enhancing the QUAlity and Transparency Of health Research. Accessed August 15, 2023. https://www.equator-network.org/ reporting-guidelines/recommendations-forreporting-the-results-of-studies-of-instrument-andscale-development-and-testing/
25. Streiner DL, Kottner J. Recommendations for reporting the results of studies of instrument and scale development and testing. J Adv Nurs. 2014;70(9):1970-1979. doi:10.1111/jan.12402
26. Denegar C, Ball D Assessing reliability and precision of measurement: an introduction to intraclass correlation and standard error of measurement. J Sports Rehabil 1993;2:35-42. doi:10.1123/jsr.2.1.35
27 Jewell DV Appraising the evidence. In: Guide to Evidence-Based Physical Therapist Practice 5th ed. Jones & Bartlett Learning; 2023:277-290.
28. Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155-163. doi:10.1016/j.jcm.2016.02.012
29. Hergott CG, Bolgla LA, Waller JL, et al. TUG-10: a modification of the timed up and go test for aerobic assessment in older adults. Cardiopulm Phys Ther J. 2022;33(4):157-165. doi:10.1097/ CPT.0000000000000202
30. McKay RE, Kohn MA, Schwartz ES, Larson MD Evaluation of two portable pupillometers to assess clinical utility Concussion 2020;5(4):CNC82. doi:10.2217/cnc-2020-0016
31. Portney LG. Concepts of measurement reliability. In: Foundations of Clinical Research. Applications to Evidence-Based Practice 4th ed. FA Davis; 2020:115-125.
32. Portney LG, Gross KD Measurement revisted: reliability and validity statistics. In: Foundations of Clinical Research. Applications to Evidence-Based Practice 4th ed. FA Davis; 2020:486-508.
33. Winston M, Zhou A, Rand CM, et al. Pupillometry measures of autonomic nervous system regulation with advancing age in a healthy pediatric cohort. Clin Auton Res 2020;30(1):43-51. doi:10.1007/ s10286-019-00639-3
34. Bista Karki S, Coppell KJ, Mitchell LV, Ogbuehi KC. Dynamic pupillometry in type 2 diabetes: pupillary autonomic dysfunction and the severity of diabetic retinopathy Clin Ophthalmol 2020;14:3923-3930. doi:10.2147/OPTH.S279872
35. Lynch GTF, James SM, Cardon TA, McPherson SM. Sensitivity and specificity of pupillary light reflex measures for ASD using monocular pupillometry Neurol Sci. 2022;43(7):4537-4545. doi:10.1007/ s10072-022-05976-2
36. Papangelou A, Boorman DW, Sharifpour M, Patel HP, Cassim T, Garcia PS. Associations of an eyetracking task and pupillary metrics with age and ASA physical status score in a preoperative cohort. J Clin Monit Comput. 2023;37(3):795-803. doi:10.1007/ s10877-023-00974-x
37 Yoo YJ, Yang HK, Hwang JM. Efficacy of digital pupillometry for diagnosis of Horner syndrome. PLoS One 2017;12(6):e0178361. doi:10.1371/ journal.pone.0178361
Download: https://ijspt.scholasticahq.com/article/128047-assessing-the-inter-rater-and-inter-trial-reliability-of-theneuroptics-pupillary-light-response-3000-pupillometer/attachment/ 260638.docx?auth_token=kjTXfbs4DJ9joJUVMGZP Assessing

Krishna Malliah1 , Adam VanZile1 , Mark Walden1 , Matthew Pennucci1 , Adam Botts1 , Caitlyn Ailor1 , Scott Ruse1 , Michael Taylor1 , Ian Nelson2 , Matthew Snyder2 , Daniel Abreu3 , Emma Yeager4 , Sean McBride5 , Thomas G. Almonroeder4a
1 Optimum Performance Therapy, Lutheran Health Network, 2 Sports Medicine, Fort Wayne Orthopedics, 3 Fort Wayne Medical Education Program, 4 Brooks College of Health Professions, Trine University, 5 Doctor of Physical Therapy Program, Medical University of South Carolina
Keywords: ACL, meniscus repair, meniscectomy, return-to-sport testing, sports medicine https://doi.org/10.26603/001c.128153
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background
Meniscus injuries often accompany anterior cruciate ligament (ACL) tears. However, little is known about how different surgical approaches to treat concomitant meniscus injuries impact hop performance after ACL reconstruction.
Purpose: The purpose of this study was to compare hop test inter-limb symmetry for patients who had undergone ACL reconstruction without an associated meniscal surgery, patients who had undergone ACL reconstruction with a meniscus repair, and patients who had undergone ACL reconstruction with a partial meniscectomy
Study Design
Cross-sectional study
Methods
Hop test data collected at the time of return-to-sport testing (average of 6.4 ± 1.4 months after surgery) was extracted from electronic medical records for 192 patients who had undergone ACL reconstruction. Of these patients, 102 had undergone an isolated ACL reconstruction, 60 had undergone an ACL reconstruction along with a meniscus repair, and 30 had undergone an ACL reconstruction along with a partial meniscectomy Analysis of variance was used to compare limb symmetry indices for the single- and triple-hop tests. These limb symmetry indices reflected the ratio of the hop distance for the involved limb relative to the uninvolved limb, expressed as a percentage.
Results
The sample was comprised of 100 males and 92 females. Their average age was 20.6 ± 8.2 years. There were significant differences among the groups for the single-hop test (p = 0.031) and triple-hop test (p = 0.024) limb symmetry indices. For both tests, the patients who had undergone ACL reconstruction with a partial meniscectomy tended to exhibit greater deficits in hop performance for their involved limb (relative to their uninvolved
Corresponding author: Thomas Gus Almonroeder, DPT, PhD
Associate Professor
Brooks College of Health Professions, Doctor of Physical Therapy Program
Trine University
12817 Parkview Plaza Dr., Fort Wayne, IN, USA 46845
Personal email: almonroeder.thomas@gmail.com
University email: almonroedert@trine.edu
Phone: 608-738-6174
Fax: 260-702-8020
limb), compared to those without a meniscal injury and those who had undergone meniscus repair.
The results of this study suggest that patients who undergo ACL reconstruction along with a partial meniscectomy tend to experience less complete and/or delayed recovery of involved-limb hop performance, which could reflect more persistent deficits in lower body power
Level of Evidence 3
Anterior cruciate ligament (ACL) tears are common among athletes and other individuals who regularly engage in activities that involve landing, cutting, and pivoting.1‑3 ACL reconstruction is generally recommended for those who plan to resume participation in these types of highly demanding activities.4 Following ACL reconstruction, patients typically complete extensive post-operative rehabilitation.5 During the earlier stages of rehabilitation, the primary focus is on controlling swelling/effusion, restoring knee motion, facilitating quadriceps activation, and promoting weight-bearing. As individuals progress, the focus shifts towards restoring lower body strength and control, and gradually transitioning to more demanding activities, such as jogging, jumping, and landing. The final phases of rehabilitation typically focus on preparing individuals to return to full activity (e.g. resumption of sport-specific activities for athletes).
Clearance to return to full activity following ACL reconstruction is typically based on multiple factors, including time since surgery, the demands associated with an individual’s sport/activities, an individual’s psychological readiness, and return-to-sport testing performance.6 Return-tosport testing is typically conducted to gauge an individual’s physical readiness to resume their pre-injury activities by assessing lower body strength, power, and control.7 Hop tests are routinely conducted during return-to-sport testing to assess lower body power 8 For these tests, the individual is asked to perform single-leg hops for maximal distance with both their involved and uninvolved limbs, allowing clinicians to compare performance for the involved limb relative to the uninvolved limb. Although hop tests are just one component of a comprehensive return-to-sport testing battery, they can provide valuable insights regarding knee-related function.9,10
Despite advances in ACL reconstruction surgery and rehabilitation, long-term outcomes after ACL reconstruction remain suboptimal, as many individuals do not return to their pre-injury level,11,12 and those who do are at elevated risk of sustaining a second ACL injury.13‑15 Therefore, it is important to understand factors that may impact recovery after ACL reconstruction. One factor that has received little attention is the presence of a concomitant meniscus injury, which often accompany ACL tears.16,17 When a meniscus tear has occurred, the injured region will typically either be surgically removed (partial meniscectomy) or repaired
(meniscal repair), depending on the location, nature, and extent of the injury.17 Only one previously published study has compared hop test performance among individuals with isolated ACL tears vs. those with ACL tears and concomitant meniscal tears. This previous study by VanZile et al.18 found no difference in single- or triple-hop test symmetry among patients who had undergone ACL reconstruction without an associated meniscal surgery, patients who had undergone ACL reconstruction with a meniscus repair, and patients who had undergone ACL reconstruction with a partial meniscectomy However, the study by VanZile et al. was limited by a small sample size, with only 34 total subjects; 19 with isolated ACL tears and 15 with ACL tears and concomitant meniscus tears (6 who had undergone partial meniscectomy and 9 who had undergone meniscal repair). A larger sample size would increase statistical power, allowing for detection of more subtle differences in hop performance among the different subgroups who underwent ACL reconstruction.
The purpose of this study was to compare hop test interlimb symmetry for patients who had undergone ACL reconstruction without an associated meniscal surgery, patients who had undergone ACL reconstruction with a meniscus repair, and patients who had undergone ACL reconstruction with a partial meniscectomy. The results of this study may provide valuable insights for clinicians working with patients after ACL reconstruction, as it could help to inform their expectations for recovery of lower body power and knee function.
Hop test results and patient demographic information were extracted from 192 electronic medical records for patients who rehabilitated at two physical therapy clinics associated with Optimum Performance Therapy (Fort Wayne, IN, USA). Of these patients, 102 (53.1%) had undergone an isolated ACL reconstruction (ACLR group), 60 (31.3%) had undergone an ACL reconstruction along with a meniscus repair (ACLR+mnrepair group), and 30 (15.6%) had undergone an ACL reconstruction along with a partial meniscectomy (ACLR+mnectomy group). An a priori sample size estimate indicated that a minimum of 159 subjects were needed in order to be adequately powered to detect a medium-sized between-group difference (inputs: f = 0.25; alpha level =
0.05; power = 0.80; number of groups = 3). G*Power software was used for sample size estimation (G*Power 3.1.9.7).19 In order to be included in this study, a patient needed to have undergone a primary, unilateral ACL reconstruction (regardless of graft type) and completed rehabilitation and return-to-sport testing at one of the physical therapy clinics participating in this study Records were excluded for patients with a history of contralateral ACL injury or for those who experienced a concomitant injury involving a structure other than the meniscus, such as a collateral ligament. This project was reviewed and approved by the Institutional Review Board at Lutheran Hospital. The data used for this study was obtained through retrospective chart review; therefore, patient consent was not required. No information that could be used to identify specific patients was extracted (e.g. names, social security numbers) in order to maintain patient confidentiality
The patients who participated in this study received rehabilitation from one of eight physical therapists, who all followed a similar post-operative rehabilitation protocol, which progressed through four phases. Phase 1 focused on controlling knee swelling/edema, maintaining patellar mobility, regaining knee motion, facilitating quadriceps activation, and initiating/progressing weight-bearing. Phase 2 focused on restoring full knee range of motion, improving quadriceps and hamstring strength, minimizing gait deviations, promoting knee control during functional activities, general lower body strengthening, and aerobic conditioning. Phase 3 focused on more advanced strengthening and initiating/progressing general skills such as running, landing, jumping, and cutting. Phase 4 focused on more advanced strengthening and training for sport-/activity-specific skills. While rehabilitation followed this general protocol, patients progressed through the stages at different times, depending on their rate of recovery, surgical factors, etc. An initial return-to-sport testing session was conducted once patients exhibited full knee motion, minimal pain/effusion, symmetrical knee extension strength (within 90% of the uninvolved limb based on strength testing with a handheld dynamometer), and no major movement faults (e.g. excessive knee valgus) during dynamic activities such as landing and jumping.
The hop test data used for this study were recorded at the time of the patient’s return-to-sport testing session. On average, return-to-sport testing was completed 6.4 ± 1.4 months after surgery (5-12 month range), which is consistent with the typical return-to-sport testing timeline after ACL reconstruction.20 Patients completed the singlehop test and triple-hop test during return-to-sport testing. The single- and triple-hop tests are both commonly used to assess lower body power and knee-related function after ACL reconstruction.8 Previous studies indicate that these tests exhibit excellent test-retest reliability,21,22 are sensitive to changes in knee-related function throughout reha-
bilitation,21 and correlate with more direct assessments of lower body muscular power.23
Hop testing was conducted in a manner consistent with previous studies.21,24 For the single-hop test, patients started standing on one limb with their toes behind a marked line. They then hopped forward for maximal distance, landing on the same limb. The triple-hop test was performed in the same manner, except patients completed three consecutive hops with the same limb, instead of a single hop. A tape measure was used to record hop distances. For both tests, the uninvolved limb was tested first, followed by the involved limb. No restriction was placed on arm movements during hop testing. The hop tests were explained and demonstrated to patients and patients completed a practice trial with each limb prior to testing. For each test, two successful trials were completed with each limb, with the average of the two trials recorded for analysis. Successful trials were those where patients were able to maintain their balance on a single limb for at least two seconds after their final landing, reflecting adequate body control. Trials were repeated when patients were unable to maintain control after landing. Patients completed testing in their own shoes and athletic apparel.
The physical therapists who conducted the return-to-sport testing calculated a limb symmetry index (LSI) based on the hop distances recorded for the involved and uninvolved limbs using Equation 1.18,21 These LSIs reflected the ratio of the hop distance for the involved limb, relative to the uninvolved limb, expressed as a percentage. An LSI of 100% reflects perfect symmetry, while a value less than 100% reflects a deficit for the involved limb relative to the uninvolved limb.
Equation 1. LSI = 100 * (involved limb hop distance / uninvolved limb hop distance)
For this study, the primary dependent variables of interest were the LSIs for the single-hop test and the triplehop test. One-way analysis of variance (ANOVA) was used to compare LSIs across the three groups (ACLR, ACLR+mnrepair, ACLR+mnectomy). The factor “graft” (bone-patellar tendon-bone, hamstring, quadriceps tendon) was added to the statistical model to determine if the between-group differences were dependent on graft type. However, there was not a significant group-by-graft interaction effect for either the single-hop test (p = 0.808) or triple-hop test LSIs (p = 0.798). Therefore, the factor graft was removed from the model. One-way ANOVA was also conducted to compare patient age and time since surgery among the three groups. Tukey post hoc tests were conducted in the case of a significant omnibus test. Cohen’s d effect size (ES) statistics were calculated to describe the magnitude of the differences between the groups by dividing the mean difference by the pooled standard deviation.25,26 Effect sizes of 0.2, 0.5, and 0.8 were considered “small”, “moderate”, and “large” differences, respectively 26 The groups differed with respect to their ages. Therefore, age was considered as a potential covariate. However, the correlations between age and the LSI values were weak, with age explaining only 4%
Table 1. Patient characteristics
Graft type (BPTB, HS, QT)
Mean ± standard deviation or number of patients
BPTB bone-patellar tendon-bone; HS hamstring; QT quadriceps tendon
ACLR: had undergone isolated ACL reconstruction
ACLR+mnrepair: had undergone ACL reconstruction and a meniscus repair
ACLR+mnectomy: had undergone ACL reconstruction and a partial meniscectomy
of the variance in the single-hop test LSI (r2 = 0.04) and 2% of the variance in the triple-hop test LSI (r2 = 0.02). Consequently, age was not included as a covariate within the statistical model.
As a secondary analysis, the proportion of patients in each group who achieved at least a 90% LSI was examined, since a 90% threshold is often considered adequate (“passing”) when conducting hop testing as part of return-tosport testing.27,28 Chi-square tests were conducted to compare the proportions of patients who met the 90% LSI threshold across the three groups. An alpha level of 0.05 was used for all tests of statistical significance. jamovi software was used for statistical analysis.29
Table 1 describes the patient characteristics (sex, age, time since surgery, graft type) for the ACLR, ACLR+mnrepair, and ACLR+mnectomy groups. The groups differed with respect to their age (p = 0.010) (Table 1). Post hoc test results indicated that the ACLR+mnectomy group was older than the ACLR+mnrepair group (p = 0.010), while there was no difference in age between the ACLR group and the ACLR+mnectomy group (p = 0.178) or the ACLR group and the ACLR+mnrepair group (p = 0.173). There were no differences among the groups with respect to their time since surgery (p = 0.972) (Table 1).
There were significant differences among the groups for the single-hop test LSIs (p = 0.031) and the triple-hop test LSIs (p = 0.024) (Table 2; Figure 1). For the single-hop test, the ACLR+mnectomy group exhibited lower LSIs compared to the ACLR+mnrepair group (p = 0.013; ES = 0.64). The ACLR+mnectomy group also exhibited lower LSIs compared to the ACLR group, with a moderate effect size (ES = 0.47); however, this difference was not statistically significant (p = 0.063). There was no difference in the single-hop test LSIs for the ACLR and ACLR+mnrepair groups (p = 0.553; ES = 0.17). The results were similar for the triple-hop test, as the ACLR+mnectomy group exhibited lower LSIs compared to the ACLR (p = 0.048; ES = 0.49) and ACLR+mnrepair (p = 0.041; ES = 0.55) groups, with no difference between the ACLR and ACLR+mnrepair groups (p = 0.944; ES = 0.05). These results indicate that the ACLR+mnectomy group tended to exhibit greater deficits in hop performance
for their involved limb (relative to their uninvolved limb), for both the single-hop test and the triple-hop test, compared to the ACLR and ACLR+mnrepair groups.
For the single-hop test, 53.9% (55 of 102 patients), 56.7% (34 of 60 patients), and 43.3% (13 of 30 patients) of patients achieved the 90% LSI threshold for the ACLR, ACLR+mnrepair, and ACLR+mnectomy groups, respectively (Table 2). This difference in proportions among the groups was not statistically significant based on the results of the Chi-square test (p = 0.476). In contrast, for the triple-hop test, the differences in proportions were statistically significant (p = 0.005), as 56.9% (58 of 102 patients), 61.7% (37 of 60 patients), and 26.7% (8 of 30 patients) of patients achieved the 90% LSI threshold for the ACLR, ACLR+mnrepair, and ACLR+mnectomy groups, respectively (Table 2).
The purpose of this study was to compare hop test interlimb symmetry for patients who had undergone ACL reconstruction without an associated meniscal surgery, patients who had undergone ACL reconstruction with a meniscus repair, and patients who had undergone ACL reconstruction with a partial meniscectomy In general, the results of this study indicate that patients who underwent ACL reconstruction with a partial meniscectomy exhibited greater hop performance deficits for their involved limb (relative to their uninvolved limb), compared to those who underwent isolated ACL reconstruction and ACL reconstruction with a meniscus repair. This suggests that patients who undergo ACL reconstruction along with a partial meniscectomy tended to experience less complete and/or delayed recovery of involved limb hop performance, which may reflect more persistent deficits in lower body power.
The results of the current study conflict with findings from an earlier study by VanZile et al.18 which found no difference in single- or triple-hop test symmetry among patients who had undergone ACL reconstruction without an associated meniscal surgery, patients who had undergone ACL reconstruction with a meniscus repair, and patients who had undergone ACL reconstruction with a partial meniscectomy However, the study by VanZile et al. was limited by a relatively small sample size of only 34 total subjects. Therefore, their study may have been underpow-
Table 2. Hop test results
SHT = single-hop test; THT = triple hop test; LSI = limb symmetry index
Means ± standard deviations and lower and upper bounds of a 95% confidence interval (in parentheses) ≥90% reflects percentage of patients who met the 90% LSI threshold

Figure 1. Mean limb symmetry index (LSI) for the single-hop test (top) and triple-hop test (bottom) for the ACLR, ACLR+mnrepair, and ACLR+mnectomy groups. Error bars reflect the lower and upper bounds of a 95% confidence interval.
ered to detect relatively subtle between-group differences in hop performance symmetry In the current study, the ACLR+mnectomy group exhibited greater inter-limb asymmetry in hop test performance, compared to the ACLR and ACLR+mnrepair groups, with moderate between-group differences (effect sizes ranging from 0.47 to 0.64).
The menisci serve several important functions within the knee joint.30,31 From a biomechanical perspective, the menisci assist with load transmission by dispersing tibiofemoral joint contact forces and attenuating shock during weight-bearing activities, while also providing additional structural stability for the knee joint. The menisci also provide proprioceptive input regarding knee joint position and motion, as they are rich in mechanoreceptors. This proprioceptive information may support neuromuscular control of the knee. There is evidence to suggest that
partial meniscectomy may negatively impact these important knee-related functions32,33 and that longer-term patient-reported outcomes tend to be worse among patients who undergo partial meniscectomy vs. meniscal repair along with ACL reconstruction.32,34 The results of the current study appear to provide additional evidence that a patient’s prognosis after ACL reconstruction may be poorer if they have undergone a partial meniscectomy
Clinically, the data presented in this study seem to suggest that at the time of initial return-to-sport testing, the mean hop test scores did not meet or exceed the typical 90% LSI cut-off suggested in the literature.27,28 In fact, only the ACLR+mnrepair group demonstrated 95% confidence intervals around the mean that contained a 90% LSI score. Thus, at a mean of six months post operative, clinicians might not expect a 90% LSI for hop testing. In fact, the lower bounds of the 95% CIs for the ACLR+mnectomy group were as low as 72.9% and 75.0% for the single- and triple-hop tests, respectively Since this study represents the largest sample of hop test data for individuals undergoing these surgeries, there is the potential for the values in Table 2 to help guide clinical expectations and goal setting. Additionally, it appears that a gap exists between LSI values for muscle strength and hop testing, especially in the ACLR+mnectomy group. As the patients in the ACLR+mnectomy group achieved relatively symmetrical knee extension strength (as this was a requirement to be eligible for return-to-sport testing), but still exhibited notable deficits in hop performance. To address these persistent deficits in lower body power, it may be beneficial to factor a higher dosage of muscle power interventions into the plan of care for these patients, especially towards the later stages of rehabilitation. Future research is required to see whether this would help to bridge the gap observed in this study It may also be worth examining the extent to which other factors, such as kinesiophobia and fear of re-injury contribute to the observed deficits in hop performance, particularly for those who have undergone ACL reconstruction with a partial meniscectomy, as there is evidence to suggest that these types of psychological factors can influence movement performance after ACL reconstruction.35
While the results of the current study may be noteworthy, there are limitations that should be considered. First, the samples included in this study were somewhat heterogenous in terms of their ages and sport/activity back-
grounds. Although this is reflective of the types of patients who undergo ACL reconstruction, focusing on more homogenous sub-samples of patients (e.g. adolescent athletes) could have limited some potential confounding variables. In addition, the groups differed with respect to their age, as the patients who underwent partial meniscectomy tended to be older than those who underwent meniscal repair The authors considered including age as a covariate within the statistical model to account for this difference; however, it was thought to not be appropriate in this case, since the observed age difference likely reflects what occurs in normal clinical practice, where there is a tendency to repair the meniscus with younger patients.36,37 While the differences in age could be considered a confounding variable when comparing outcomes for the different surgeries, statistically eliminating the potential influence of the age difference would produce findings that are not consistent with the reality of clinical practice. Preliminary analyses also indicated that age explained minimal variance in hop test performance (≤4%), which suggests that age has a small impact on hop test symmetry after ACL reconstruction. Another limitation is that hop performance was only assessed once, with the timing of testing varying among patients depending on their rate of recovery Another option would have been to compare hop test performance at a consistent time point; however, this is not reflective of routine clinical practice, where the timing of return-to-sport testing is individualized for each patient. Also, patient data were extracted from two different physical therapy clinics, which introduces another potential source of variability within the data set. However, preliminary independent t-tests indicated that single-hop test (p = 0.848) and triple-hop test (p = 0.615) LSIs did not differ between patients from the two clinic sites. Finally, the nature, location, and extent of the meniscus injuries were not analyzed as part of this study Future studies should examine how these factors impact
clinical outcomes among patients with ACL tears and concomitant meniscal tears. Although not a limitation per se, it is also important to note that the results of this study do not provide insights into the underlying contributors to hop performance deficits (e.g. strength deficits, motor control adaptations, fear of re-injury).
The results of this study suggest that patients who have undergone ACL reconstruction with a partial meniscectomy tend to exhibit greater hop performance deficits for their involved limb (relative to their uninvolved limb), compared to those who have undergone isolated ACL reconstruction or ACL reconstruction with a meniscus repair Multiple factors may have contributed to the observed differences in hop performance, including persistent deficits in lower body power and age differences among the patient subgroups. Clinicians working with patients after ACL reconstruction should understand how meniscal injury may impact a patient’s recovery
The authors report no conflicts of interest.
The authors would like to thank Gordon Bokhart and the staff at Lutheran Hospital for supporting this project.
Submitted: June 11, 2024 CST Accepted: November 22, 2024 CST Published: February 01, 2025 CST
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Aguero AD, Irrgang JJ, MacGregor AJ, Rothenberger SD, Hart JM, Fraser JJ. Sex, military occupation and rank are associated with risk of anterior cruciate ligament injury in tactical-athletes. BMJ Mil Health 2023;169(6):535-541. doi:10.1136/ bmjmilitary-2021-002059
2. Chia L, De Oliveira Silva D, Whalan M, et al. Noncontact anterior cruciate ligament injury epidemiology in team-ball sports: a systematic review with meta-analysis by sex, age, sport, participation level, and exposure type. Sports Med 2022;52(10):2447-2467. doi:10.1007/ s40279-022-01697-w
3. Posch M, Schranz A, Lener M, Tecklenburg K, Burtscher M, Ruedl G. In recreational alpine skiing, the ACL is predominantly injured in all knee injuries needing hospitalization. Knee Surg Sports Traumatol Arthrosc. 2021;29(6):1790-1796. doi:10.1007/ s00167-020-06221-z
4. Shea KG, Carey JL. Management of anterior cruciate ligament injuries: evidence-based guideline. J Am Acad Orthop Surg 2015;23(5):e1-5. doi:10.5435/ JAAOS-D-15-00094
5. Greenberg EM, Greenberg ET, Albaugh J, Storey E, Ganley TJ. Anterior cruciate ligament reconstruction rehabilitation clinical practice patterns: a survey of the PRiSM Society. Orthop J Sports Med. 2019;7(4):2325967119839041. doi:10.1177/ 2325967119839041
6. Cheney S, Chiaia TA, de Mille P, Boyle C, Ling D. Readiness to return to sport after ACL reconstruction: a combination of physical and psychological factors. Sports Med Arthrosc Rev. 2020;28(2):66-70. doi:10.1097/JSA.0000000000000263
7 Gokeler A, Dingenen B, Hewett TE. Rehabilitation and return to sport testing after anterior cruciate ligament reconstruction: where are we in 2022? Arthrosc Sports Med Rehabil 2022;4(1):e77-e82. doi:10.1016/j.asmr.2021.10.025
8. Davies WT, Myer GD, Read PJ. Is it time we better understood the tests we are using for return to sport decision making following ACL reconstruction? a critical review of the hop tests. Sports Med 2020;50(3):485-495. doi:10.1007/s40279-019-01221-7
9. Kyritsis P, Bahr R, Landreau P, Miladi R, Witvrouw E. Likelihood of ACL graft rupture: not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med 2016;50(15):946-951. doi:10.1136/ bjsports-2015-095908
10. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules reduce reinjury risk after anterior cruciate ligament reconstruction. Br J Sports Med 2016;50(13):804-808. doi:10.1136/bjsports-2016-096031
11. Arden C, Taylor NF, Feller JA, Webster KE. Fiftyfive per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med 2014;48(21):1543-1552. doi:10.1136/ bjsports-2013-093398
12. Walden M, Hagglund M, Magnusson H, Ekstrand J. ACL injuries in men’s professional football: a 15-year prospective study on time trends and returnto-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med 2016;50(12):744-750. doi:10.1136/ bjsports-2015-095952
13. Barber-Westin S, Noyes FR. One in 5 athletes sustain reinjury upon return to high-risk sports after ACL reconstruction: a systematic review in 1239 athletes younger than 20 years. Sports Health. 2020;12(6):587-597 doi:10.1177/1941738120912846
14. Webster KE, Feller JA. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med 2016;44(11):2827-2832. doi:10.1177/ 0363546516651845
15. Webster K, Hewett TE, Feller JA. Anterior cruciate ligament injuries in Australian Rules football: incidence, prevention and return to play outcomes. Open Access J Sports Med 2021;12:33-41. doi:10.2147/OAJSM.S250414
16. Gupta R, Singhal A, Sharma AR, Shail S, Masih GD Strong association of meniscus tears with complete anterior cruciate ligament (ACL) injuries relative to partial ACL injuries. J Clin Orthop Trauma. 2021;23:101671. doi:10.1016/j.jcot.2021.101671
17 Keyhani S, Esmailiejah AA, Mirhoseini MS, Hosseininejad SM, Ghanbari N. The prevalence, zone, and type of the meniscus tear in patients with anterior cruciate ligament (ACL) injury; does delayed ACL reconstruction affects the meniscal injury? Arch Bone Jt Surg 2020;8(3):432-438.
18. VanZile A, Driessen M, Grabowski P, Cowley H, Almonroeder TG. Deficits in dynamic balance and hop performance following ACL reconstruction are not dependent on meniscal injury history. Int J Sports Phys Ther 2022;17(7):1298-1306. doi:10.26603/ 001c.55542
19. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149-1160. doi:10.3758/ BRM.41.4.1149
20. Webster KE, Hewett TE. What is the evidence for and validity of return-to-sport testing after anterior cruciate ligament reconstruction surgery? A systematic review and meta-analysis. Sports Med 2019;49(6):917-929. doi:10.1007/s40279-019-01093-x
21. Reid A, Birmingham TB, Stratford PW, Alcock GK, Giffin JR. Hop testing provides a reliable and valid outcome measure during rehabilitation after anterior cruciate ligament reconstruction. Phys Ther. 2007;87(3):337-349. doi:10.2522/ptj.20060143
22. Ross MD, Langford WPJ. Test-retest reliability of 4 single-leg horizontal hop tests. J Strength Cond Res. 2002;16(4):617-622.
23. Hamilton RT, Shultz SJ, Schmitz RJ, Perrin DH. Triple-hop distance as a valid predictor of lower limb strength and power J Athl Train 2008;43(2):144-151. doi:10.4085/1062-6050-43.2.144
24. Myers BA, Jenkins WL, Killian C, Rundquist P. Normative data for hop tests in high school and collegiate basketball and soccer players. Int J Sports Phys Ther. 2014;9(5):596-603.
25. Cohen J. Statistical Power Analysis for the Behavioral Sciences 2nd ed. Lawrence Erlbaum Associates; 1988.
26. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for ttests and ANOVAs. Front Psychol 2013;4(863):1-12. doi:10.3389/fpsyg.2013.00863
27 Markstrom JL, Naili JE, Hager CK. A Minority of athletes pass symmetry criteria in a series of hop and strength tests irrespective of having an ACL reconstructed knee or being noninjured. Sports Health 2023;15(1):45-51. doi:10.1177/ 19417381221097949
28. Thomee R, Kaplan Y, Kvist J, et al. Muscle strength and hop performance criteria prior to return to sports after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1798-1805. doi:10.1007/s00167-011-1669-8
29. The jamovi project. Jamovi Computer Software; 2024. https://www.jamovi.org
30. Aagaard H, Verdonk R. Function of the normal meniscus and consequences of meniscal resection. Scan J Med Sci Sports 1999;9(3):134-140. doi:10.1111/j.1600-0838.1999.tb00443.x
31. Fox AJS, Bedi A, Rodeo SA. The basic science of human knee menisci: structure, composition, and function. Sports Health. 2012;4(4):340-351. doi:10.1177/1941738111429419
32. Basar B, Basar G, Aybar A, Kurtan A, Basar H. The effects of partial meniscectomy and meniscal repair on the knee proprioception and function. J Orthop Surg 2020;28(1):2309499019894915. doi:10.1177/ 2309499019894915
33. Bedi A, Kelly NH, Baad M, et al. Dynamic contact mechanics of the medial meniscus as a function of radial tear, repair, and partial meniscectomy. J Bone Joint Surg Am 2010;92(6):1398-1408. doi:10.2106/ JBJS.I.00539
34. Sarraj M, Coughlin RP, Solow M, et al. Anterior cruciate ligament reconstruction with concomitant meniscal surgery: a systematic review and metaanalysis of outcomes. Knee Surg Sports Traumatol Arthrosc 2019;27(11):3441-3452. doi:10.1007/ s00167-019-05389-3
35. Norte GE, Solaas H, Saliba SA, Goetschius J, Slater LV, Hart JM. The relationships between kinesiophobia and clinical outcomes after ACL reconstruction differ by self-reported physical activity engagement. Phys Ther Sport 2019;40:1-9. doi:10.1016/ j.ptsp.2019.08.002
36. Everhart JS, Higgins JD, Poland SG, Abouljoud MM, Flanigan DC. Meniscal repair in patients age 40 years and older: A systematic review of 11 studies and 148 patients. Knee. 2018;25(6):1142-1150. doi:10.1016/j.knee.2018.09.009
37 Dadoo S, Keeling LE, Engler ID, et al. Higher odds of meniscectomy compared with meniscus repair in a young patient population with increased neighbourhood disadvantage. Br J Sports Med 2024;58(12):649-654. doi:10.1136/ bjsports-2023-107409

Hagen M, Vanrenterghem J, Van den Borne Y, et al. Hamstrings and Quadriceps Weaknesses Following Anterior Cruciate Ligament Reconstruction Persist Up to 6 Months After Return-to-Sport: An Angle-specific Strength Analysis. IJSPT 2025;20(2):176-188. doi:10.26603/001c.128505
Michiel Hagen1 , Jos Vanrenterghem1 , Yves Van den Borne1 , Maria A. Diaz2 , Sabine Verschueren1 , Mark A. Robinson3 , Annemie Smeets1a
1 Rehabilitation Sciences, KU Leuven, 2 Physical Education and Physiotherapy, VU Brussels, 3 School of Sport and Exercise Sciences, Liverpool John Moores University
Keywords: Anterior cruciate ligament reconstruction, return to sport, isokinetic strength test, quadriceps, hamstrings https://doi.org/10.26603/001c.128505
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
BACKGROUND
Hamstrings and quadriceps strength recovery and restoration of the hamstrings-to-quadriceps ratio (H/Q ratio) is a major concern after anterior cruciate ligament reconstruction (ACLR). Recently, moment-angle profiles and angle-specific H/Q ratios are receiving increasing interest.
The first objective of this study was to investigate moment-angle profiles and angle-specific H/Q ratio profiles in athletes with ACLR at the time of RTS. The second objective of this study was to assess whether strength asymmetries identified at the time of RTS, persist after six months.
STUDY DESIGN
Case-Control study
METHODS
Twenty athletes who had undergone ACLR performed isokinetic strength tests for concentric knee flexion and extension (60°/s) at RTS, and three and six months later Twenty controls were tested once. T-tests were used to compare strength differences between 1) ACLR athletes and controls and 2) the injured and uninjured leg of the ACLR athletes. Finally, to assess strength deficits over time, two-way ANOVAs were used.
Angle-specific analyses and peak moments showed lower hamstrings strength in the injured leg of ACLR athletes compared to their uninjured leg at RTS. Furthermore, angle-specific analyses showed a lower hamstrings strength and H/Q ratio in the injured leg compared to controls at larger knee flexion angles. The latter deficit was not identified with a peak-based analysis. The asymmetries identified at RTS did not change over the six months following RTS.
Athletes with ACLR show strength deficits and asymmetries that persist even six months after RTS. As some asymmetries may go undetected by peak-based analyses, angle-specific analyses are recommended.
a
Corresponding author:
Annemie Smeets
Tervuursevest 101 bus 1501 3001 Leuven, Belgium
e-mail: annemie.smeets@kuleuven.be
Level 3b
The use of objective functional measures in the return-tosport (RTS) decision making process is a big step forward in the rehabilitation of individuals who have sustained anterior cruciate ligament (ACL) injuries and subsequent ACL reconstruction (ACLR).1‑3 However, in the population of athletes with ACLR, 65% returns to their pre-injury sports level4 and there remains a 20% chance of sustaining a secondary ACL injury 5 These observations justify the continued search for more solid RTS criteria to capture remaining deficits and alterations associated with re-injury that are currently not identified.1,2,6,7 One commonly used RTS assessment is hamstrings and quadriceps strength and the hamstrings-to-quadriceps strength ratio (H/Q ratio).6,8, 9 The H/Q ratio is suggested to represent the muscular capacity to actively stabilize the knee joint since the hamstrings work as an agonist of the ACL and might reduce the anterior pull of the quadriceps on the tibia.10‑14 Remaining strength asymmetries6,15 and a reduced H/Q ratio6 at RTS have been associated with increased risk for sustaining re-injuries. However, up to 46% of the ACLR patients still return to sport with remaining hamstrings and quadriceps strength asymmetries.9,16 It remains questionable whether these strength asymmetries resolve in the months following RTS without further rehabilitation.9,17 For example, a systematic review by Tayfur et al.18 concluded that there was strong evidence for remaining quadriceps and hamstrings concentric strength deficits in ACLR athletes 24 months after surgery compared to uninjured controls.
Hamstrings and quadriceps muscle strength is frequently evaluated by an isokinetic dynamometer. This assessment provides moment-angle data over a pre-defined range of motion (ROM) from which peak values (e.g. peak moment and angle of peak moment) are typically extracted. Subsequent analyses such as the limb symmetry index and H/Q ratio are consequentially calculated based on these peak values. The reduction of the moment-angle data to peak values however, leads to the loss of potentially relevant clinical information. First, normal peak values could mask strength deficits or asymmetries in other knee flexion angles than the angle of peak moment. For example, Baumgart et al.19 showed that ACLR patients at an average of 6.6 months after surgery have larger between-leg hamstrings strength differences at larger knee flexion angles compared to more extended knee positions. Second, calculating the H/Q ratio by dividing the peak hamstrings moment by the peak quadriceps moment seems physiologically irrelevant as the quadriceps peak moment is achieved at a more flexed knee angle than the hamstrings peak moment, leaving them functionally unrelated.11 Angle-specific H/Q ratios, which are ratios between hamstrings and quadriceps moments reached at identical knee flexion angles, would appear to be more relevant to assess muscle imbalance throughout the entire ROM.11,14,20 Angle-specific analysis of the H/Q ratio and subsequent analysis of the hamstrings
and quadriceps moment-angle profile is increasingly used in both uninjured athletes11,20‑22 and athletes with ACLR.17,23 Considering that the moment arm of the hamstring muscles becomes suboptimal when the knee joint angle is not in mid-range (particularly in deeper knee flexion), and this in comparison to a relatively constant moment arm of the quadriceps muscles, calculating the H/Q ratio based on peak moments alone could conceal incomplete hamstring muscle strength recovery.24 However, the evolution of angle-specific strength analyses after RTS has not yet been investigated. In conclusion, a longitudinal observational study on angle-specific strength analyses is necessary to fully understand muscle adaptations in athletes with ACLR at RTS and later on. Such information is necessary to improve rehabilitation strategies and RTS decision criteria.
Therefore, the first objective of this study was to investigate moment-angle profiles and angle-specific H/Q ratio profiles in athletes with ACLR at the time of RTS. In order to evaluate the potential added value of angle-specific analyses over peak-based measures, hamstrings and quadriceps peak moments, and peak-based H/Q ratios were analyzed. The hypothesis was that angle-specific analyses will reveal more strength shortcomings compared to peakbased evaluations. The second objective of this study was to assess whether strength asymmetries identified at the time of RTS, persist after six months. It was hypothesized that part of the strength shortcomings persist despite RTS.
In this prospective observational study, 20 athletes who underwent ACLR (ipsilateral semitendinosus autograft) were recruited. They all completed rehabilitation with their own physiotherapist and had been cleared by the surgeon to restart training, based on subjective assessment (there were no strict criteria imposed by the study). If ACLR surgery concerned a re-injury, the athlete was excluded from this study. All participants practiced sport at competitive level (from lowest division to National division) before their injury and wished to return to sport. Isokinetic muscle strength tests were performed three times in the ACLR group: at the time of RTS (262 ± 60 days post-surgery and maximum two weeks between the test session and the first full training session), three months post RTS (96 ± 19 days after RTS) and 6 months post RTS (201 ± 20 days after RTS). At the three month post RTS test, four athletes with ACLR dropped out (one hamstrings injury, three lost interest in participation). Two additional athletes with ACLR dropped out at six months post RTS (one ACL re-injury, one lost interest in participation).
Twenty control athletes with no history of an ACL injury and no lower limb injury in the six months before the test session, were also recruited. These control athletes were
tested only once. In both groups only competitive athletes who complete at least one training and one match per week (before the ACL injury) were included. The ACLR group and control group were matched for sex and sports type. Two mismatches in sports type could not be avoided. For the identification of strength deficits and asymmetries at the moment of RTS, the data of all 20 athletes with ACLR and control athletes were included. For the follow-up of the strength asymmetries, only the data of the 16 ACLR athletes who were tested at RTS and at three months were included. The data of the two patients (one male and one female) who dropped out between three and six months after RTS were estimated through a data imputation technique (see statistics). All participants provided written informed consent prior to inclusion in the data collection procedure. The study was approved by the local ethical committee(Ethics Committee Research UZ/KU Leuven) and executed in accordance with the Declaration of Helsinki.
All athletes performed isokinetic muscle strength tests on a Biodex System 4 Pro (Biodex Medical Systems, Shirley, NY, USA). A five-minute general warm-up on a cycle ergometer was implemented prior to testing. For the isokinetic testing, the athletes were seated with a hip flexion angle of 95-100°. Straps were applied across the chest, pelvis and distal thigh. The rotational axis of the knee joint and the isokinetic dynamometer crank arm were aligned. The distal attachment pad of the crank arm was firmly attached to the distal part of the shank, two fingers proximal to the medial malleolus. This position was standardised over the different sessions in the ACLR athletes. Every athlete performed a continuous series of five maximal effort trials for concentric knee extensions and flexions at 60°/s with each leg. A practice series was allowed before each test series. Between series, a resting period of 60 seconds was provided.
Moment-angle profiles for hamstrings and quadriceps were calculated using the open source Matlab package IKD1D (version 0.02, http://ikd1d.org/). This program standardizes the generation of a representative moment-angle profile from several trials (Appendix A). After joint-angle based gravitational correction of the raw moment values, the selection of valid trials was standardized between participants through an automatic selection procedure. This selection procedure is based on three criteria: the acceptable variation from the target angular velocity, the minimally achieved ROM at the target angular velocity and the between trial variation in peak moment. The target angular velocity was set at 60°/s, the minimal ROM was set at 80° and the acceptable variation between trials in terms of angular velocity, ROM and peak moment were all set at 10%.
To avoid the automatic selection picking one outlier, a minimum of three trials for flexion and three trials for extension were included per participant. If less than three trials were automatically selected, moment tolerance was augmented until the minimal number of three trials was in-
cluded. Next, moment-angle profiles for each participant were averaged over the entire ROM using a moving average with 10 degrees window These average moment-angle profiles were used to calculate the angle-specific H/Q ratio profile. The average hamstrings and quadriceps moment-angle profiles were also used to determine the hamstrings and quadriceps peak moment values, from which the peakbased H/Q ratios were calculated.
In earlier studies20,22,25,26 angle-specific moment values and angle-specific H/Q ratios were extracted at a limited number of joint angles, omitting the fact that the ratio profile is a continual measure. Through the introduction of statistical parametric mapping (SPM) in biomechanical research,27 hypotheses on moment-angle profiles and angle-specific H/Q ratio profiles can be tested without neglecting the interdependence between measures across different joint angles. One-dimensional SPM analyses were performed using the open source package SPM1D (version M.0.4.5, http://www.spm1d.org/).28 For the hamstrings and quadriceps peak moments and the peak-based H/Q ratios, zero-dimensional analyses were performed using IBM SPSS Statistics (version 25, SPSS Inc., Chicago IL, United States https://www.ibm.com/analytics/spss-statistics-software).
Three statistical analyses were performed. First, to identify general strength deficits in the ACLR group at the time of RTS, the injured legs of the 20 athletes with ACLR were compared to the data of the 20 control athletes. For those athletes with ACLR who had their dominant leg injured (n=7), the dominant leg was also selected in their matched control athlete, and vice versa for non-dominant leg. Zeroand one-dimensional independent groups t-tests were used for the statistical analysis of these strength deficits. Second, the data of the injured and uninjured leg of all 20 athletes with ACLR at the time of RTS, were compared to assess strength asymmetries. For the statistical analysis of strength asymmetries, zero- and one-dimensional paired t-tests were used. Finally, to assess strength asymmetries over time, two-way repeated measures ANOVAs (leg time) were used. The data of the injured and uninjured leg of the 16 athletes with ACLR that were tested at the time of RTS and at three months post RTS, were implemented in this analysis. The data of the two participants that dropped out at six months follow-up, were estimated through data imputation (based on adding the average difference between three and six months to their value at three months post RTS). Alpha was set at 0.05 for all statistical analyses. Considering the explorative nature of the study, no correction for multiple testing (Bonferroni) was applied to avoid overly conservative statistical interpretations.
The data of both sexes was pooled, although differences in absolute strength might be expected between women and men. This was justified because the group of athletes with ACLR and control athletes were matched for sex and thus had the same proportion of men/women. Also, the longitudinal analysis is not affected by the pooling of both sexes since the same group of athletes is followed over time and the proportion of men and women is thus not different
Table 1. Demographic characteristics of the athletes with ACLR and control participants.
Age, body mass and body height are expressed as the mean value ± standard deviation (SD) and are compared through an unpaired t-test (* p<0.05). The baseline sample consisted of 20 athletes with ACLR that performed strength assessments at RTS. The follow-up sample consisted of 16 athletes with ACLR since four patients dropped-out between RTS and three months after RTS.
between conditions. Furthermore, the authors did not expect differences in strength improvements between sexes, based on the meta-analysis of Roberts et al.29
A detailed description of the included participants is provided in Table 1.
DEFICITS AT TIME OF RTS
The hamstrings moment-angle profile of the ACLR group was significantly lower between 65° and 95° of knee flexion (p=0.01) compared to the controls (Figure 1). The quadriceps moment-angle profile was not significantly different between groups across the entire ROM. The angle-specific H/Q ratio profile was significantly lower in the ACLR group compared to healthy control subjects between 70° and 95° of knee flexion (p<0.01). The peak hamstrings and quadriceps moment and peak-based H/Q ratio were not significantly different between the ACLR and control group (Table 2).
Within the ACLR athletes, the hamstrings moment-angle profile of the injured leg was significantly lower compared to the uninjured leg between 33° and 95° of knee flexion (p<0.001) at the time of RTS (Figure 2). This is most of the measured ROM. There was no significant difference between the legs in the quadriceps moment-angle profile. The angle-specific H/Q ratio profile was significantly lower for the injured leg compared to the uninjured leg, but only between 84° and 95° of knee flexion (p=0.039). Analyses of the peak values showed a significantly lower hamstrings peak moment for the injured leg compared to the uninjured leg (p<0.001) and no significant difference for the quadriceps peak moment or the peak-based H/Q ratio (Table 3).
OVER SIX MONTHS AFTER RTS
The one-dimensional repeated measures ANOVAs showed no main time effects or interaction effects (leg time) for any of the angle-specific profiles (Figure 3). A main leg effect was found for the hamstrings and quadriceps moment-angle profiles, but not for the angle-specific H/Q ratio profile. The hamstrings and quadriceps moments of the injured leg were lower compared to the uninjured leg, respectively during the entire ROM (p<0.001) and around peak moment (61°- 92°) (p=0.013). The absence of a main time effect or interaction effect means that the identified strength asymmetries persisted throughout the six-month follow-up period. The zero-dimensional repeated measures ANOVA showed similar results. No main time effects or interaction effects were found for any of the peak-based parameters (Table 4). A main leg effect was found for the hamstrings (p<0.001) and quadriceps (p=0.004) peak moments, with significantly lower peak moments on the injured side compared to the uninjured side. No main leg effect was found for the peak-based HQ ratio.
ADDED VALUE OF ANGLE-SPECIFIC STRENGTH PROFILES
The first objective of this study was to assess deficits and asymmetries in the hamstrings and quadriceps momentangle profiles and angle-specific H/Q ratio profiles in athletes with ACLR at time of RTS. Both the hamstrings moment-angle profile and the angle-specific H/Q ratio profile were significantly lower in the injured leg of athletes with ACLR compared to the control participants at larger knee flexion angles (>65° knee flexion for hamstrings strength and >75° knee flexion for H/Q ratio profile). Comparing the injured to the uninjured leg of the ACLR athletes at the

Figure 1. Upper row: angle-specific comparison of the (a) hamstrings and (b) quadriceps moment-angle profile and (c) angle-specific H/Q ratio profile between the injured legs of athletes with ACLR and the control participants at the time of RTS.
Mean values are indicated by the solid lines and the standard deviation clouds are represented by the shaded zones. Middle row: Statistical Parametric Mapping (SPM) output of the SPM t-tests that compare the patients with ACLR and controls. If the t-curve (black line) exceeds the critical threshold (horizontal dashed line), significant differences were found between legs. Lower row: colour plots that indicate the size of the t-value indicating the differences at each joint angle (dark blue or red represent lower or higher t-scores respectively and therefore larger differences – cf legend). The second – grey – bar indicates whether these differences were significant (dark grey) or not (light grey).
Table 2. Zero-dimensional analysis of the hamstrings and quadriceps peak moment and peak-based H/Q ratio between the injured legs of athletes with ACLR and the control participants at the time of RTS.
time of RTS, a hamstrings strength asymmetry was unveiled throughout almost the entire measured ROM (>33° knee flexion). The angle-specific H/Q ratio profile showed only significant between leg differences at larger knee flexion angles (>84° knee flexion). The peak-based analyses could only identify between-leg asymmetries for hamstrings peak moment and were not able to reveal the hamstrings strength deficit and H/Q ratio imbalances at larger knee flexion angles. This confirms the importance of analyzing angle-specific strength profiles in ACLR athletes instead of only evaluating restoration of peak strength.
The hamstrings strength deficits at larger knee flexion angles were also identified in other studies that assessed hamstrings strength in athletes that underwent ACLR
surgery with a hamstring tendon graft.30‑32 This hamstrings weakness in deep knee flexion has been suggested to be a possible consequence of two phenomena: 1) atrophy and shortening of the semitendinosus muscle after its tendon has been harvested for the ACL graft,23 and 2) a lack of compensation from the semimembranosus and biceps femoris.32 First, several studies showed that the musculotendinous junction of the regenerated semitendinosus is proximally shifted (i.e. tendon retraction). This retraction occurs because once the tendon is harvested there is nothing left that keeps the semitendinosus muscles fibres to length.31 Studies showed proximal shifts of the musculotendinous junction from 3.8cm33 up to even 7cm.34 Since the muscle will be of shorter length at a given flexion angle,

Figure 2. Upper row: angle-specific comparison of the (a) hamstrings and (b) quadriceps moment-angle profile and (c) angle-specific H/Q ratio profile between the injured and uninjured legs of athletes with ACLR at the time of RTS.
Mean values are indicated by the solid lines and the standard deviation clouds are represented by the shaded zones. Middle row: Statistical Parametric Mapping (SPM) output of the SPM t-tests that compare the injured and uninjured legs of the patients with ACLR. If the t-curve (black line) exceeds the critical threshold (horizontal dashed line), significant differences were found between legs. Lower row: colour plots that indicate the size of the t-values indicating the differences at each joint angle (dark blue or red represent lower or higher t-scores respectively and therefore larger differences – cf legend). The second – grey – bar indicates whether these differences were significant (dark grey) or not (light grey).
Table 3. Zero-dimensional analysis of the hamstrings and quadriceps peak moment and peak-based H/Q ratio between the injured and uninjured legs of athletes with ACLR at the time of RTS.
(*** p<0.001)
muscle contractile behaviour will alter. Namely the angle at which the semitendinosus exhibits maximum strength will be at a more extended position of the knee.30 Second, the different hamstring muscles all have a separate origin, insertion, and muscle architecture influencing their contribution to the overall flexion strength and the ability to compensate at a specific joint angle.30 The semitendinosus plays the most important role in larger knee flexion angles because of its fusiform architecture (75-120°) while the biceps femoris, which has a pennate architecture, is the primary flexor at angles between 15-45° of knee flexion.30,32 Semitendinosus harvesting, will thus mainly affect strength in the larger knee flexion angles. The clinical relevance of
these strength deficits at larger knee flexion angles during seated isokinetic dynamometry requires further investigation (Appendix B).
The second objective of this study was to assess if strength asymmetries resolve spontaneously over the first six months following RTS. Lower hamstrings and quadriceps strength was observed in the injured leg of ACLR athletes compared to their uninjured leg which remained unchanged over the six month follow-up period. The anglespecific analyses revealed a persisting hamstrings weakness

Figure 3. Upper row: follow-up of the strength asymmetries in (a) hamstrings and (b) quadriceps mean momentangle profile and (c) angle-specific mean H/Q ratio profile over 6 months after RTS. Lower 3 rows: Statistical Parametric Mapping (SPM) output of the repeated measures ANOVA: main effect for leg, main effect for time and interaction effect (leg x time). If the F-curve (black line) exceeds the critical threshold (horizontal dashed line), significant differences were found for the respective effect.
in the injured leg over the entire measured ROM and a quadriceps weakness in the injured leg around 60-90° of knee flexion, the range of the quadriceps peak moment. Several authors35‑37 attribute prolonged quadriceps weakness to arthrogenic muscle inhibition (AMI), a natural mechanism of reflex inhibition of muscles surrounding an injured joint to prevent potentially detrimental movements. Rice et al.38 state that AMI in ACLR often results in the inability to fully activate the quadriceps muscle, restricting peak quadriceps moment. AMI could thus explain the current findings of prolonged quadriceps weakness around peak moment angles observed in the injured leg compared to the uninjured leg of the ACLR athletes. Early interventions, that target AMI from the first day after injury are crucial. In the acute phase, it is essential to address inflammation, pain and effusion as soon as possible.39 Furthermore, there is growing evidence supporting the efficacy of neuromodulatory strategies such as cryotherapy, TENS, eccentric cross-exercise in treating AMI.39‑41
The longitudinal analyses showed that the quadriceps and hamstrings strength asymmetries, did not resolve over
time. As the athletes with ACLR returned to their competitive sports without receiving additional rehabilitation after the moment of RTS, this suggests that strength asymmetries do not resolve by sports participation alone. Next to the angle-specific analysis, scientific literature has frequently reported on prolonged hamstrings17,42‑44 and quadriceps9,16,17,43‑45 peak strength asymmetries and deficits in ACLR athletes.37 Therefore, additional rehabilitation after RTS in the form of strength training, might be required in athletes with ACLR.44 For example, to target the hamstrings weakness, Buckthorpe et al.46 advised to prioritize eccentric hamstring training. They state that eccentric exercises have the potential to shift the moment-angle profile of the knee flexors to more extended knee angles, probably because of the positive effect of eccentric training on fascicle length.47 Similarly, concentric exercises at long muscle length might increase fascicle length48 and as such shift the moment-angle profile of the knee flexors to more extended knee angles. However, the results of this study show hamstrings strength asymmetries over the entire ROM and hamstrings strength deficits particularly at more flexed knee angles. Therefore, future studies should
Table 4. Follow-up of strength asymmetries in hamstrings and quadriceps peak moment and peak-based H/Q ratio over 6 months after RTS.
Mean ± SD
investigate whether adding hamstrings strength exercises at shorter muscle lengths to the rehabilitation programs reduces these persistent flexion strength deficits in athletes with a semitendinosus autograft.
Some limitations of this study have to be noted. First of all, it is important to notify that only isokinetic concentric strength at an angular velocity of 60°/s was measured. Consequently, the absolute values of this study cannot be compared to strength values or H/Q ratios measured at different angular velocities or to functional H/Q ratios that divide the eccentric knee flexor moment by the concentric knee extensor moment.17,20‑22,49‑51 Second, the rehabilitation program of the ACLR patients up to RTS was performed at their home physiotherapy practices and thus not standardised. Therefore, it is not possible to make any conclusion about the influence of the type of rehabilitation program on strength deficits. Part of the variability in strength deficits and asymmetries might be attributed to the various exercise programs that the ACLR athletes were exposed to during their rehabilitation.52‑54 Furthermore, the patients were not all treated by the same surgeon, leading to heterogeneity in surgery technique and decision making on RTS clearance. Third, the ACLR athletes were significantly older than the controls (24.0 ± 4.3 vs. 21.8 ± 1.5 years, p=0.043). Although, strength declines with age, it is not expect that this small difference in age has a relevant impact on the results of this study as strong strength declines are only seen around 40 years of age.55 Finally, the small sample size and relatively short follow-up, did not allow to evaluate the predictive value of angle-specific strength measures or H/Q ratios for ACL re-injury
This study revealed clear angle-specific strength deficits and asymmetries in ACLR athletes at time of RTS that persisted for six months after athletes who had undergone ACLR returned to sport. More specifically, ACLR athletes have lower quadriceps strength in the injured leg compared to their uninjured leg around the angle of peak moment. Furthermore, their injured leg showed lower hamstrings strength compared to their uninjured leg throughout the entire measured ROM and compared to controls at larger knee flexion angles. The latter deficit was not identified with a traditional peak-based analysis, which stresses the need for angle-specific analyses. Since the strength asymmetries did not resolve naturally in the first six months after RTS, additional targeted interventions are needed to restore these shortcomings.
We wish to recognise Ian Poole, Raja Mohammed Firhad Raja Azidin, Anne Delextrat and Vasilios Baltzopoulos for their contribution to the development IKD1D and the drafting of supplementary materials.
The researchers received funding of FWO Flanders (G068221N) and internal funding of KU Leuven (C24M/21/ 042).
The authors have no conflicts of interest to report.
Submitted: June 17, 2024 CST. Accepted: December 14, 2024
CST Published: February 01, 2025 CST
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Ardern CL, Glasgow P, Schneiders A, et al. 2016 Consensus statement on return to sport from the First World Congress in Sports Physical Therapy, Bern. Br J Sports Med 2016;50(14):853-864. doi:10.1136/bjsports-2016-096278
2. Van Melick N, Van Cingel REH, Brooijmans F, et al. Evidence-based clinical practice update: Practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br J Sports Med 2016;50(24):1506-1515. doi:10.1136/ bjsports-2015-095898
3. Dingenen B, Gokeler A. Optimization of the return-to-sport paradigm after anterior cruciate ligament reconstruction: A critical step back to move forward. Sports Medicine 2017;47(8):1487-1500. doi:10.1007/s40279-017-0674-6
4. Ardern CL, Taylor NF, Feller JA, Webster KE. Fiftyfive per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: An updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med 2014;48(21):1543-1552. doi:10.1136/ bjsports-2013-093398
5. Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction. Am J Sports Med 2016;44(7):1861-1876. doi:10.1177/ 0363546515621554
6. Kyritsis P, Bahr R, Landreau P, Miladi R, Witvrouw E. Likelihood of ACL graft rupture: Not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med. 2016;50(15):946-951. doi:10.1136/ bjsports-2015-095908
7 Dingenen B, Gokeler A. Optimization of the returnto-sport paradigm after Anterior cruciate ligament reconstruction: A critical step back to move forward. Sports Medicine 2017;47(8):1487-1500. doi:10.1007/ s40279-017-0674-6
8. Gokeler A, Welling W, Zaffagnini S, Seil R, Padua D Development of a test battery to enhance safe return to sports after anterior cruciate ligament reconstruction. Knee Surg, Sports Traumatol, Arthrosc 2017;25(1):192-199. doi:10.1007/s00167-016-4246-3
9. Welling W, Benjaminse A, Seil R, Lemmink K, Zaffagnini S, Gokeler A. Low rates of patients meeting return to sport criteria 9 months after anterior cruciate ligament reconstruction: a prospective longitudinal study Knee Surg, Sports Traumatol, Arthrosc. 2018;26(12):3636-3644. doi:10.1007/s00167-018-4916-4
10. Aagaard P, Simonsen EB, Magnusson SP, Larsson B, Dyhre-Poulsen P. A new concept for isokinetic hamstring: quadriceps muscle strength ratio. Am J Sports Med 1998;26(2):231-237 doi:10.1177/ 03635465980260021201
11. Coombs R, Garbutt G. Developments in the use of the hamstring/quadriceps ratio for the assessment of muscle balance. J Sports Sci Med. 2002;1(3):56-62.
12. Cheung R, Smith A, Wong D H:Q ratios and bilateral leg strength in college field and court sports players. J Hum Kinet. 2012;33(1):63-71. doi:10.2478/ v10078-012-0045-1
13. de Lira CAB, Mascarin NC, Vargas VZ, Vancini RL, Andrade MS. Isokinetic knee muscle strength profile in Brazilian male soccer, futsal, and beach soccer players: a cross-sectional Study Int J Sports Phys Ther. 2017;12(7):1103-1110. doi:10.16603/ ijspt20171103
14. Ruas CV, Pinto RS, Haff GG, Lima CD, Pinto MD, Brown LE. Alternative methods of determining hamstrings-to-quadriceps ratios: a comprehensive review Sports Med Open 2019;5(11). doi:10.1186/ s40798-019-0185-0
15. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50(13):804-808. doi:10.1136/ bjsports-2016-096031
16. Gokeler A, Welling W, Zaffagnini S, Seil R, Padua D Development of a test battery to enhance safe return to sports after anterior cruciate ligament reconstruction. Knee Surg, Sports Traumatol, Arthrosc. 2017;25(1):192-199. doi:10.1007/s00167-016-4246-3
17 Baumgart C, Welling W, Hoppe MW, Freiwald J, Gokeler A. Angle-specific analysis of isokinetic quadriceps and hamstring torques and ratios in patients after ACL-reconstruction. BMC Sports Sci Med Rehabil. 2018;10(1):1-8. doi:10.1186/ s13102-018-0112-6
18. Tayfur B, Charuphongsa C, Morrissey D, Miller SC. Neuromuscular function of the knee Joint following knee injuries: Does it ever get back to normal? A systematic review with meta analyses. Sports Medicine 2020;(0123456789). doi:10.1007/ s40279-020-01386-6
19. Baumgart C, Welling W, Hoppe MW, Freiwald J, Gokeler A. Angle-specific analysis of isokinetic quadriceps and hamstring torques and ratios in patients after ACL-reconstruction. BMC Sports Sci Med Rehabil 2018;10(1):1-8. doi:10.1186/ s13102-018-0112-6
20. Aagaard P, Simonsen EB, Magnusson SP, Larsson B, Dyhre-Poulsen P. A new concept for isokinetic hamstring: Quadriceps muscle strength ratio. Am J Sports Med. 1998;26(2):231-237. doi:10.1177/ 03635465980260021201
21. Alhammoud M, Morel B, Hansen C, et al. Discipline and sex differences in angle-specific isokinetic analysis in elite skiers. Int J Sports Med. 2019;40(5):317-330. doi:10.1055/a-0850-0016
22. De Ste Croix M, ElNagar YO, Iga J, Ayala F, James D. The impact of joint angle and movement velocity on sex differences in the functional hamstring/ quadriceps ratio. Knee 2017;24(4):745-750. doi:10.1016/j.knee.2017.03.012
23. Rogowski I, Vigne G, Blache Y, et al. Does the graft used for acl reconstruction affect the knee muscular strength ratio at six months postoperatively? Int J Sports Phys Ther 2019;14(4):546-553. doi:10.26603/ijspt20190546
24. Herzog W, Read LJ. Lines of action and moment arms of the major force-carrying structures crossing the human knee joint. J Anat 1993;182(2):213-230.
25. Huang H, Guo J, Yang J, et al. Isokinetic anglespecific moments and ratios characterizing hamstring and quadriceps strength in anterior cruciate ligament deficient knees. Sci Rep. 2017;7(1):1-11. doi:10.1038/ s41598-017-06601-5
26. Coombs R, Garbutt G, Cramp M. Part I: Biomechanics. J Sports Sci. 2002;20(1):3-16. doi:10.1080/026404102317126137
27 Pataky TC. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J Biomech. 2010;43(10):1976-1982. doi:10.1016/ j.jbiomech.2010.03.008
28. Pataky TC. One-dimensional statistical parametric mapping in Python. Comput Methods Biomech Biomed Engin 2012;15(3):295-301. doi:10.1080/10255842.2010.527837
29. Roberts BM, Nuckols G, Krieger JW Sex differences in resistance training: A systematic review and meta-Analysis. J Strength Cond Res 2020;34(5):1448-1460. doi:10.1519/ JSC.0000000000003521
30. Tashiro T, Kurosawa H, Kawakami A, Hikita A, Fukui N. Influence of medial hamstring tendon harvest on knee flexor strength after anterior cruciate ligament reconstruction: A detailed evaluation with comparison of single- and double-tendon harvest. Am J Sports Med 2003;31(4):522-529. doi:10.1177/ 31.4.522
31. Carofino B, Fulkerson J. Medial hamstring tendon regeneration following harvest for anterior cruciate ligament reconstruction: Fact, myth, and clinical implication. Arthroscopy. 2005;21(10):1257-1265. doi:10.1016/j.arthro.2005.07.002
32. Makihara Y, Nishino A, Fukubayashi T, Kanamori A. Decrease of knee flexion torque in patients with ACL reconstruction: Combined analysis of the architecture and function of the knee flexor muscles. Knee Surg, Sports Traumatol, Arthrosc. 2006;14(4):310-317 doi:10.1007/s00167-005-0701-2
33. Lee DW, Shim JC, Yang SJ, Cho SI, Kim JG. Functional effects of single semitendinosus tendon harvesting in anatomic anterior cruciate ligament reconstruction: comparison of single versus dual hamstring harvesting. Clin Orthop Surg. 2019;11(1):60-72. doi:10.4055/cios.2019.11.1.60
34. Nakamae A, Deie M, Yasumoto M, et al. Threedimensional computed tomography imaging evidence of regeneration of the semitendinosus tendon harvested for anterior cruciate ligament reconstruction: A comparison with hamstring muscle strength. J Comput Assist Tomogr 2005;29(2):241-245. doi:10.1097/ 01.rct.0000153779.86663.92
35. Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. 2010;40(3):250-266. doi:10.1016/ j.semarthrit.2009.10.001
36. Pamukoff DN, Pietrosimone BG, Ryan ED, Lee DR, Blackburn TJ. Quadriceps function and hamstrings co-activation after anterior cruciate ligament reconstruction. J Athl Train. 2017;52(5). doi:10.4085/ 1062-6050-52.3.05
37 Tayfur B, Charuphongsa C, Morrissey D, Miller SC. Neuromuscular function of the knee joint following knee injuries: does it ever get back to normal? A systematic review with meta analyses. Sports Medicine. 2020;(0123456789). doi:10.1007/ s40279-020-01386-6
38. Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum 2010;40(3):250-266. doi:10.1016/ j.semarthrit.2009.10.001
39. Norte G, Rush J, Sherman D Arthrogenic muscle inhibition: Best evidence, mechanisms, and theory for treating the unseen in clinical rehabilitation. J Sport Rehabil Published online 2021:1-19. doi:10.1123/jsr.2021-0139
40. Sonnery-Cottet B, Saithna A, Quelard B, et al. Arthrogenic muscle inhibition after ACL reconstruction: A scoping review of the efficacy of interventions. Br J Sports Med. 2019;53(5):289-298. doi:10.1136/bjsports-2017-098401
41. Kotsifaki R, Korakakis V, King E, et al. Aspetar clinical practice guideline on rehabilitation after anterior cruciate ligament reconstruction. Br J Sports Med Published online 2023. doi:10.1136/ bjsports-2022-106158
42. Xergia SA, McClelland JA, Kvist J, Vasiliadis HS, Georgoulis AD The influence of graft choice on isokinetic muscle strength 4-24 months after anterior cruciate ligament reconstruction. Knee Surg, Sports Traumatol, Arthrosc 2011;19(5):768-780. doi:10.1007/s00167-010-1357-0
43. Chung KS, Ha JK, Yeom CH, et al. Are muscle strength and function of the uninjured lower limb weakened after anterior cruciate ligament injury? Am J Sports Med 2015;43(12):3013-3021. doi:10.1177/ 0363546515606126
44. Welling W, Benjaminse A, Lemmink K, Dingenen B, Gokeler A. Progressive strength training restores quadriceps and hamstring muscle strength within 7 months after ACL reconstruction in amateur male soccer players. Phys Ther Sport 2019;40:10-18. doi:10.1016/j.ptsp.2019.08.004
45. Nagelli CV, Hewett TE. Should return to sport be delayed until 2 years after anterior cruciate ligament reconstruction? Biological and functional considerations. Sports Medicine. 2017;47(2):221-232. doi:10.1007/s40279-016-0584-z
46. Buckthorpe M, Danelon F, La Rosa G, Nanni G, Stride M, Della Villa F. Recommendations for hamstring function recovery after ACL reconstruction. Sports Medicine Published online 2020. doi:10.1007/s40279-020-01400-x
47 Potier TG, Alexander CM, Seynnes OR. Effects of eccentric strength training on biceps femoris muscle architecture and knee joint range of movement. Eur J Appl Physiol. 2009;105(6):939-944. doi:10.1007/ s00421-008-0980-7
48. Blazevich AJ, Cannavan D, Coleman DR, Horne S. Influence of concentric and eccentric resistance training on architectural adaptation in human quadriceps muscles. J Appl Physiol 2007;103(5):1565-1575. doi:10.1152/ japplphysiol.00578.2007
49. Hewett TE, Myer GD, Zazulak BT Hamstrings to quadriceps peak torque ratios diverge between sexes with increasing isokinetic angular velocity. J Sci Med Sport 2008;11(5):452-459. doi:10.1016/ j.jsams.2007.04.009
50. Daneshjoo A, Rahnama N, Mokhtar AH, Yusof A. Bilateral and unilateral asymmetries of isokinetic strength and flexibility in male young professional soccer players. J Hum Kinet. 2013;36(1):45-53. doi:10.2478/hukin-2013-0005
51. Kabacinski J, Murawa M, Mackala K, Dworak LB. Knee strength ratios in competitive female athletes. PLoS One 2018;13(1):1-12. doi:10.1371/ journal.pone.0191077
52. Guex K, Degache F, Morisod C, Sailly M, Millet GP Hamstring architectural and functional adaptations following long vs. short muscle length eccentric training. Front Physiol. 2016;7(AUG):1-9. doi:10.3389/fphys.2016.00340
53. Delextrat A, Bateman J, Ross C, et al. Changes in torque-angle profiles of the hamstrings and hamstrings-to-quadriceps ratio after two hamstring strengthening exercise interventions in female hockey players. J Strength Cond Res. 2020;34(2):396-405. doi:10.1519/ JSC.0000000000003309
54. Monajati A, Larumbe-Zabala E, Goss-Sampson M, Naclerio Naclerio F. The effectiveness of injury prevention programs to modify risk factors for noncontact anterior cruciate ligament and hamstring injuries in uninjured team sports athletes: A systematic review PLoS One 2016;11(5):1-15. doi:10.1371/journal.pone.0155272
55. Haynes EMK, Neubauer NA, Cornett KMD, O’connor BP, Jones GR, Jakobi JM. Age and sexrelated decline of muscle strength across the adult lifespan: A scoping review of aggregated data. Appl Physiol, Nutr Metab 2020;45(11):1185-1196. doi:10.1139/apnm-2020-0081
Download: https://ijspt.scholasticahq.com/article/128505-hamstrings-and-quadriceps-weaknesses-followinganterior-cruciate-ligament-reconstruction-persist-up-to-6-months-after-return-to-sport-an-angle-specif/attachment/ 262301.docx?auth_token=TgUS4N3uIoXADV_EgnL7
Download: https://ijspt.scholasticahq.com/article/128505-hamstrings-and-quadriceps-weaknesses-followinganterior-cruciate-ligament-reconstruction-persist-up-to-6-months-after-return-to-sport-an-angle-specif/attachment/ 262300.docx?auth_token=TgUS4N3uIoXADV_EgnL7

Matt Dewald1a , Madison Andersen, Laura Higgins, Emma Porter, Alex Wickersham
1 Physical Therapy, University of South Dakota
Keywords: dynamic knee valgus, jump performance, kinematics, bony anatomy, 2-D analysis. https://doi.org/10.26603/001c.128587
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background
Dynamic knee valgus (DKV) is widely considered a risk factor for injuries, despite contradictory research. Consequently, athletic performance and injury rehabilitation cueing has primarily focused on keeping the “knees out”
Purpose
The purpose of this study was to assess jump performance measures and anatomical contributions.
Study Design
Cross-Sectional Study
Methods
Jump height, ground contact time, reactive strength index, and DKV was collected with the MyJump2 and Coach My Video apps. Static anatomical measurements were collected. Subjects completed nine jumps with each leg using the same set-up; performing three single leg six-inch depth jumps with their natural form, three depth jumps with external cuing towards increased DKV, and three depth jumps with cuing towards no DKV. ANOVA was used to compare jump data. Pearson Correlation Coefficients were used to assess relationships between DKV and anatomical measurements, jump height, ground contact time, and reactive strength index. Intraclass correlation coefficient (ICC) was used to assess inter-rater reliability of MyJump2 and Coach My Video measurements.
50 subjects (35 included) participated in this study. With a cued DKV jump, ground contact time had a moderate positive correlation with DKV measurements (r=.49, p<0.01), however, this was not the case with subjects’ natural jump and cued no DKV alignment jumps. Static anatomical measurements of static knee valgus and Q-angle had a weak positive correlation with DKV measurements for subjects’ natural jumps (r=.37, p<0.01 and r=.34, p=0.04, respectively). When DKV measurements were normalized to an anatomical measurement, no correlations existed with any of the performance measurements. There was very strong inter-rater reliability (ICC=.96-.99) of all the measurements.
Corresponding author: Matt Dewald, PT, DPT, OCS, SCS
University of South Dakota
Department of Physical Therapy
414 E. Clark St. Vermillion, SD 57069
Phone: 605-658-6359
Fax: 605-638-5637
Email: matt.dewald@usd.edu
Bony anatomical alignment should be considered alongside kinematics, as normalization controlled for the differences in DKV Future research should normalize DKV measurements by bony anatomy when addressing DKV and jump performance.
Dynamic knee valgus (DKV), also known as dynamic genu valgus is defined as a lower limb movement pattern that is a combination of internal rotation and adduction of the femur, knee abduction, anterior tibial translation, and external tibial rotation.1 The normal range of DKV of a singleleg landing task has been reported as 1-9 degrees for males and 5-12 degrees for females when measured from a digital video camera two-dimensionally 2 Anatomical characteristics contribute to DKV, with the Q-angle being the most significant.3‑5 Q-angles are measured with similar, although different anatomical landmarks than knee valgus. DKV and static knee valgus are measured from the Anterior Superior Iliac Spine (ASIS) to the mid-patella to a line along the tibia between the medial and lateral malleoli. Meanwhile, Q-angle is measured from the ASIS, through the mid-patella, and ending on a line through the middle of the tibial tubercle.
Health professionals and coaches traditionally have considered DKV as being associated with increased risk of injuries and decreased jump performance. This has commonly led to a “knees out” cueing to prevent DKV during athletic performance, to the point that some clinicians attempt to avoid any degree of DKV. For example, the International Olympic Committee has published a review on ACL injuries encouraging clinicians to avoid excessive dynamic knee valgus and focus on a knee over toe position.6 Additionally, static anatomical measurements, such as Q-angle and Craig’s test, are rarely considered when providing feedback to athletes regarding their DKV.
Presently, there is contradictory evidence on DKV and injury risk.7‑13 The 2005 prospective study by Hewett et al. identifying DKV as the primary culprit of ACL injury in female athletes has most commonly been referenced by clinicians as the reason to avoid DKV.10 This study reported those suffering an ACL injury (n=9) had 8 degrees more DKV than those who did not (n=196).10 Conversely with a similar set-up Nilstad et al. identified no association between DKV and ACL tears in female athletes who sustained an ACL injury (n=56) compared to uninjured athletes (n=722).7 When synthesizing the body of literature on DKV and ACL injuries, a 2020 meta-analysis of the nine highest quality articles with over 1300 total subjects found no association between DKV and ACL injuries.8
Additionally, many studies suggest a positive association between DKV and patellofemoral pain, stress fractures, iliotibial band syndrome, and Achille’s tendinopathy However, these studies have not considered the static bony anatomy of the subjects, overlooking the possible contributions of anatomical characteristics.3‑5 In parallel, bony
anatomy of static knee valgus was found to have a significant relationship with DKV during a drop-jump, while foot alignment, hamstring strength, quadriceps strength, and hip abductor strength had no association.3 Furthermore, anatomical measurements in both the frontal and transverse planes at the hips and knees have been shown to have an association with DKV during double-leg landing, further adding value to the idea that anatomical differences contribute to dynamic measures.14
Moreover, there is conflicting evidence on the relationship between DKV and jump performance.5,15‑17 Confounding variables such as specific training effects and bony anatomy was not considered with these reports. There is also dispute regarding the neuromuscular contributions to DKV, specifically, the association of hip abductor muscle weakness to DKV 5,18‑22 Finally, interventional studies for improving DKV have had mixed results, with some studies demonstrating improved DKV with training proximally at the hip and core, while others found no change.23,24 Seeing that there is conflicting evidence surrounding the current consensus on DKV, additional information is needed to establish a better understanding of these relationships. The purpose of this study was to assess jump performance measures and anatomical contributions.
This cross-sectional study recruited 50 subjects from the university wellness center between the ages of 18 and 30 years old who felt they could complete nine single leg depth jumps from a 6-inch box with each leg. A depth jump consists of dropping from a box of a predetermined height and immediately, upon landing a single maximal jump is performed.25 All subjects were recruited from a common area near the entrance of the wellness center All data were collected at this location as well. The subjects were incentivized with a sports drink for their participation. Fifteen subjects were excluded following poor execution of a cued jump for dynamic knee valgus, primarily due to turning their foot inward. After consenting to the research, background demographic information including sex, age, weight, height, injury history, and past sport participation was collected from subjects. BMI was later calculated based on the subjects’ self-reported height and weight.
Static anatomical measurements of transverse plane femoral anteversion and frontal plane genu valgus were completed by the same researcher Femoral anteversion was measured with the Craig’s test, while genu valgus was measured with Q-angle26 and static knee valgus. Craig’s test is

Figure 1. Subject performing the single-leg depth jump. A. The subject is demonstrating their natural form. B. The subject is demonstrating a DKV cued depth jump. C. The subject is demonstrating a no DKV cued depth jump.
the most commonly used clinical assessment of femoral anteversion, however, the validity of this test has been questioned.27 Souza and Powers reported good reliability with a strong positive correlation (r=.61, p<0.01) when compared with MRI measurements of femoral anteversion.28 This suggests that Craig’s test can be a useful clinical test for screening femoral anteversion without higher costs associated with imaging.
Static knee valgus and 2-Dimensional DKV utilize the same landmarks: from the subject’s ASIS, through the midpatella, and ending at the midline of the anterior tibia between the medial and lateral malleoli. Q-angle was measured from the ASIS, through the mid-patella, and ending on a line through the middle of the tibial tubercle. Both the Q-angle and static knee valgus were measured in supine. Landmarks were marked with either tape or marker to facilitate recording of DKV The sample size was determined using G*Power Version 3.1.9.7 with .05 alpha, .8 power, and a medium-large effect size of .5 resulting in 34 subjects needed, thus, 50 were recruited in anticipation of dropouts and excluded subjects.
The independent variables were the three single-leg depth jump modes: a natural jump form, jump form with cued DKV, and jump form cued for no DKV The natural depth jump (Figure 1a) consisted of the subjects jumping with no cue. The DKV cued depth jump (Figure 1b) consisted of a “knees-in” verbal cue with an external reference cone 30 degrees medial. The no DKV cued depth jump (Figure 1c) consisted of a verbal “knees straight forward” cue with an external reference cone directly in front of the landing zone. A single-leg depth jump was chosen over a doubleleg, due to the greater amount of knee valgus that often occurs with single-leg.29 The different jump forms were chosen to assess changes in jump performance based on DKV angles.
The dependent variables measured were jump height, ground contact time, reactive strength index (RSI), and DKV of the jump. Additionally, a true DKV measurement, normalized for frontal plane anatomical measurements, was collected consisting of subtracting the subject’s static knee valgus from their DKV measurements. True DKV was calculated as it represents the degree of frontal plane motion that was occurring, controlling for anatomical differences between subjects. True DKV provides insights into DKV motor control. Three trials were collected for each leg and each jump with means being recorded for each subject. Static anatomical measurements included Craig’s test for femoral anteversion, Q-angle for genu valgus, and static knee valgus for genu valgus.26 Static knee valgus was used in calculating true DKV measurement as it was the most ecologically similar to DKV and it had the highest and strongest correlation with DKV (r=.37, p=0.03).
The MyJump2 application was used to measure ground reaction time, RSI, and jump height. MyJump2 is a reliable and valid tool, frequently used in jump studies.30‑32 The Coach My Video application was used to measure DKV. DKV was measured at the most extreme point, often at the deepest point of the depth jump. 2-D phone-based apps have been found reliable and validated for assessing kinematics.33‑35 The apps were installed on a university issued iPad.
A 6-inch box was used for the depth jump with an iPad placed seven feet in front of the box with the camera mounted 15 inches from the ground to standardize recordings of the jumps. To ensure focus on jump performance, subjects were told to jump as high and as quickly as possible. Each jump was demonstrated by the same researcher for every subject. The subjects were cued with “Point your knee towards the cone.” Subjects were given the option to perform a slow, step-through practice trial as well as fullspeed practice trials. Subjects were given as many practice
trials as they felt necessary to gain full understanding of the test. All subjects began with their natural jump form (Figure 1a), completing three jumps with their right and then three on their left. Random numbers were used to determine whether the cued DKV (Figure 1b) or cued no DKV (Figure 1c) would be the second or third set of jumps. The same procedure of completing three jumps for the right leg was completed before switching to the left for the cued jumps.
To ensure reliability of anatomical characteristics, the same researcher placed markers on designated landmarks and measured Q-angle, static knee valgus, and Craig’s test for all sessions. Craig’s test validity is not that of CT or MRI, although it is regarded as an acceptable clinical exam to screen for femoral anteversion in the absence of imaging.27, 28 Therefore, transverse plane anatomical contributions to DKV should be interpreted cautiously Randomization of the DKV and no DKV jumps was used to reduce order effects. Performance bias was controlled with a standardized assessment preserving fidelity of the protocol. The high reliability of the measurement techniques used to assess jump performance and DKV ensures quality of the data. Two blinded researchers not associated with the study assessed the jump performances and kinematics at a different and neutral site, minimizing expectation bias. There was no missing data.
All statistical analysis was completed with SAS. All alphas were set at 0.05. Normality of the jump variables were assessed with Shapiro-Wilk, Histograms, and Q-Q plots.36 ANOVA was used to compare jump height, contact time, RSI, and DKV with each jump method. Pearson Correlation Coefficients were used to assess DKV and anatomical measurements, jump height, ground contact time, and RSI. Intraclass correlation coefficient (ICC) was used to assess inter-rater reliability of the measurement tools. Strength of correlations was described using the following scale outlined by the British Medical Journal: 0-0.19 is regarded as very weak, 0.2-0.39 as weak, 0.40-0.59 as moderate, 0.6-0.79 as strong, and 0.8-1 as very strong correlation.37
SUBJECTS
Fifty subjects were recruited and completed the study. Following analysis of the jump data, 15 subjects’ data were excluded as they did not complete the cued DKV jump correctly, pointing their feet too far towards midline. The remaining 35 subjects were included in final data analysis.
DESCRIPTIVE DATA
The average age of subjects was 21.91 years-old (95% CI 21.12-22.71). Their average height was 68.97 inches (95%
Table 1. Subject characteristics, reported as mean (95% CI), unless otherwise indicated
Variable Outcome
Number of Subjects, n
Females, n (%)
Age (years),
Weight (pounds)
Height (inches)
BMI (kg/m2)
Q-angle (degrees)
Static knee valgus (degrees) Craig’s Test (degrees) Significant Injury History, n (%) Past Physical Therapy, n (%) Jump Training History, n (%)
35
18 (51.4%)
21.91 (21.12-22.71)
165.94 (154.93-176.96) 68.97 (67.81-70.13) 24.38 (23.15-25.61) 16.26 (14.86-17.66) 9.90 (8.94-10.86) 9.40 (8.57-10.23) 15 (42.9%)
18 (51.4%) 27 (77.1%)
Abbreviations: n= number of subjects, CI= Confidence Interval, BMI= Body Mass Index, kg= Kilogram, m= Meter, Q-angle= Quadriceps Angle
CI 67.81-70.13). Their average weight was 165.94 pounds (95% CI 154.93-176.96). Their average BMI was 24.38 (95% CI 23.15-25.61). Their average Q-angle was 16.26 degrees (95% CI 14.86-17.66). Static knee valgus was 9.90 degrees (95% CI 8.94-10.86). Craig’s test was 9.40 degrees (95% CI 8.57-10.23). Over half (51.4%) of subjects had past physical therapy, 42.9% had a history of significant lower extremity injury, and 77.1% had past jump training. All subject characteristics can be found in Table 1.
When stratifying the study characteristics by sex, five variables were significantly different between females and males. Weight, height, BMI, Q-angle, and static knee valgus were all statistically different. See Table 2 for subject characteristics stratified by sex.
The average normal single leg depth jump height was 5.87 inches (95% CI 4.99-6.76). The average contact time was 419.72 ms (95% CI 388.71-450.73). The average RSI was .86 (95% CI .76-.97). The average DKV with the subjects’ natural jump was 13.12 degrees (95% CI 11.29-14.96). The true DKV was 3.22 degrees (95% CI 1.50-4.95). Table 3 contains the jump performance data of the included subjects.
Jump height and DKV were significantly different when comparing the jump performances based on sex. Interestingly, there were no statistically significant differences for DKV between sexes once the measure was normalized for static frontal plane anatomical measures, also known as the true DKV See all subject jump characteristics stratified by sex in Table 4.
Performance and DKV averages for each jump type can be found in Table 5 There was no difference in jump heights between jump types (p=0.59). There was a significant difference between contact times, with the cuing for DKV jumps being longer than the natural (p= 0.03) and cuing for no DKV jumps (p=0.04). RSI was significantly lower for the jump cued for DKV compared to the natural jump (p=0.03). Cuing for DKV was effective, as those jumps had a signifi-
Table 2. Subject characteristics stratified by sex, reported as mean (95% CI) Variable
Number of Subjects, n
Age (years)
Weight (pounds)
Height (inches)
BMI (kg/m2)
Q-angle (degrees)
Static knee valgus (degrees)
Craig’s Test (degrees)
(21.12-22.71)
(154.93-176.96)
(67.81-70.13)
(23.15-25.61)
(14.86-17.66)
(8.94-10.86)
(8.57-10.23)
(21.27-23.62)
(135.56-166.67)
(65.46-68.87)
(21.63-25.16)
(15.61-19.89)
(10.36-12.81) 9.67 (8.63-10.70)
(20.23-22.47)
(168.69-194.60)
(69.82-71.94)
(23.66-27.16)
(13.01-16.34)
(7.17-9.06)
Abbreviations: n= number of subjects, CI= Confidence Interval, BMI= Body Mass Index, kg= Kilogram, m= Meter, Q-angle= Quadriceps Angle *p values reference differences between female and male subjects
Table 3. Subject jump characteristics, reported as mean (95% CI)
Variable
Jump Height (inches)
Contact Time (ms)
RSI
DKV (degrees)
True DKV (degrees)
Abbreviations: CI= Confidence Interval, ms= milliseconds, RSI= Reactive Strength Index, DKV= Dynamic Knee Valgus
5.87 (4.99-6.76) 419.72 (388.71-450.73) .86 (.76-.97) 13.12 (11.29-14.95) 3.22 (1.50-4.95)
Table 4. Subject jump characteristics stratified by sex, reported as mean (95% CI)
Variable Included Subjects
Jump Height (inches)
Contact Time (ms)
RSI
DKV (degrees)
True DKV (degrees)
5.87 (4.99-6.76) 419.72 (388.71-450.73) .86 (.76-.97) 13.12 (11.29-14.95) 3.22 (1.50-4.95) 4.53 (3.74-5.32) 422.37 (376.11-468.63) .78 (.63-.92) 14.97 (12.53-17.41) 3.39 (1.07-5.71) 7.29 (5.90-8.69) 416.92 (370.63-463.20) .95 (.81-1.10) 11.16 (8.49-13.83) 3.04 (.21-5.88)
Abbreviations: CI= Confidence Interval, ms=
Table 5. Performance and Kinematic Measurements of Jump Types, reported as mean (95% CI)
Jump Height (inches)
Contact Time (ms)
RSI
DKV (Degrees)
True DKV (Degrees)
5.87 (4.99-6.76) 419.72 (388.71-450.73) .86 (.76-.97)
13.12 (11.29-14.95) 3.22 (1.50-4.95)
5.38 (4.52-6.23)
528.47 (467.93-589.02) 0.68 (.59-.77)
35.02 (31.52-38.52) 25.12 (21.71-28.53)
Abbreviations: CI= Confidence Interval, ms= milliseconds, RSI= Reactive Strength Index, DKV= Dynamic Knee Valgus
cantly higher DKV (p<0.01) than the natural jump and cuing for no DKV Interestingly, the jumps in which subjects were cued for no DKV did not lower the DKV below the subjects’ natural jumps.
Craig’s test was not correlated to DKV measurements of natural jumps (p=0.72). Both static knee valgus (r=.37, p=0.03) and Q-angle (r=.34, p=0.04) had positive, weak significant correlations with DKV measurements for the subjects’ natural jumps. Static knee valgus demonstrated a greater positive significant correlation with DKV than the traditional Q-angle and the adjusted R-square suggests that the static knee valgus accounts for 11% of the DKV (Figure 2).
The ICC for jump height, ground contact time, and RSI measurements taken with the MyJump2 app was near per-
5.98 (5.04-6.92)
448.63 (408.10-489.15) .82 (.72-.92)
13.79 (12.03-15.55)
3.89 (2.13-5.64)
fect at .99 for each. The ICC for DKV measured with the Coach My Video app was also very strong at .96.
This study highlights the need to consider static bony alignment when assessing DKV with jump performances, as the differences in DKV between sexes was accounted for by the frontal plane anatomy Poor clinical decision making can occur when making assumptions about movement faults without measuring anatomical contributions. Furthermore, static knee valgus could be considered over the use of Q-angles since using the same landmarks for static and dynamic tasks increases the ecological validity of applying static measurements. That being so, the static knee

Figure 2. Correlation of DKV with static knee valgus.
Table 6. Inter rater reliability of dependent variables
Abbreviations: ICC= Intraclass Correlation Coefficients
valgus measurements were more highly correlated to DKV in this study.
Since the no DKV cued jump did not lower the DKV of subjects, in fact, increased it, the researchers question the effectiveness of using an external visual cue. When cued for DKV, ground contact time had a significant correlation with true DKV measurements, suggesting that when a patient has high DKV, instead of cuing to keep the knee from caving in, clinicians could cue for less contact time to indirectly control DKV While external cuing has demonstrated effectiveness, it traditionally has not focused on contact time for improving DKV 38‑40 Motor learning studies have found that cuing for contact time has improved both ground contact time and RSI, however, these studies have not assessed the impact on DKV 41,42 RSI was significantly lower for the jump cued for DKV compared to the natural jump (p=0.03), likely related to the contribution of contact time on RSI. However, increased impulse and impact forces have been associated with cueing for less ground contact time, both of which have been linked to increased injury risk.41,43,44
When considering improving jump height, this study did not find any difference within subjects when considering DKV This is comparable to findings on squat jumps and
countermovement jumps in past research.16 This study’s findings were contrary to a 3-month interventional study that found improved jump height with an exercise program targeting less DKV 17 However, that interventional study only had eight subjects complete the intervention and did not control for confounding that the improvements in jump height could be linked to improved strength and power from the exercise program and not decreased DKV 17
The high reliability of the apps found in this study is encouraging for clinicians without an expensive performance laboratory These apps are readily accessible and do not require extensive time and monetary investments. When considering this along with the high reliability and validity of 2-D phone apps found in past studies, clinicians can be confident in the reliability of using app-based 2-D analysis tools for assessing jump performance and DKV.30‑35
It must be noted that the population used in this study was primarily college students, but that does not change the fact that their anatomical alignment contributes to their DKV These considerations are most dramatic for females, as females typically have increased frontal plane knee valgus and femoral anteversion, both of which can masquerade as DKV if not assessed.4 Additionally, the validity of craig’s test has been questioned, potentially over or underrepresenting transverse plane anatomical contributions in this study Thirty percent of subjects were excluded due to not being able to complete the cued DKV jump correctly, these subjects could have swayed the results or could have added statistical power if included. Additionally, the fact that 30 percent of the subjects struggled with the cued DKV jump suggests difficulty in completing the task and possibly less validity when comparing to natural jumping mechanics. Participants who had experienced past injuries, physical therapy, and jump training were not excluded from
this study The influence of previous rehab and jump training may have impacted performance throughout this study. Additionally, the external cuing may have caused subjects to focus more on the cone rather than their best jump performance.
Bony alignment should be considered with kinematics, as normalization of DKV measurements by static anatomical measurements controlled for the differences in DKV between men and women during subjects’ natural jumping performance. When jumping with cued DKV alignment, ground contact time had a significant positive correlation
with DKV measurements, therefore cueing for less ground contact time may be an external cue to use to control for excessive DKV, although prospective studies would be needed to test this in the future.
The authors declare no conflict of interest.
Submitted: July 05, 2024 CST Accepted: December 14, 2024
CST Published: February 01, 2025 CST
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Wilczyński B, Zorena K, Ślęzak D Dynamic knee valgus in single-leg movement tasks. Potentially modifiable factors and exercise training options. A literature review Int J Environ Res Public Health 2020;17(21):8208. doi:10.3390/ijerph17218208
2. Herrington L, Munro A. Drop jump landing knee valgus angle; normative data in a physically active population. Phys Ther Sport 2010;11(2):56-59. doi:10.1016/j.ptsp.2009.11.004
3. Nilstad A, Krosshaug T, Kam-Ming MOK, Bahr R, Andersen TE. Association between anatomical characteristics, knee laxity, muscle strength, and peak knee valgus during vertical drop-jump landings. J Ortho Sports Ther 2015;45(12):998-1005. doi:10.2519/jospt.2015.5612
4. Almeida GP, Silva AP, França FJ, Magalhães MO, Burke TN, Marques AP Relationship between frontal plane projection angle of the knee and hip and trunk strength in women with and without patellofemoral pain. J Back Musculoskelet Rehabil 2016;29(2):259-266. doi:10.3233/BMR-150622
5. Stickler L, Finley M, Gulgin H. Relationship between hip and core strength and frontal plane alignment during a single leg squat. Phys Ther Sport. 2015;16(1):66-71. doi:10.1016/j.ptsp.2014.05.002
6. Renstrom P, Ljungqvist A, Arendt E, et al. Noncontact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med 2008;42(6):394-412. doi:10.1136/bjsm.2008.048934
7. Nilstad A, Petushek E, Mok KM, Bahr R, Krosshaug T Kiss goodbye to the “kissing knees”: no association between frontal plane inward knee motion and risk of future non-contact ACL injury in elite female athletes. Sports Biomech 2023;22(1):65-79. doi:10.1080/14763141.2021.1903541
8. Cronström A, Creaby MW, Ageberg E. Do knee abduction kinematics and kinetics predict future anterior cruciate ligament injury risk? A systematic review and meta-analysis of prospective studies. BMC Musculoskelet Disord 2020;21(1):563. doi:10.1186/ s12891-020-03552-3
9. Goerger BM, Marshall SW, Beutler AI, Blackburn JT, Wilckens JH, Padua DA. Anterior cruciate ligament injury alters preinjury lower extremity biomechanics in the injured and uninjured leg: the JUMP-ACL study Br J Sports Med 2015;49(3):188-195. doi:10.1136/ bjsports-2013-092982
10. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study Am J Sports Med 2005;33(4):492-501. doi:10.1177/0363546504269591
11. Krosshaug T, Steffen K, Kristianslund E, et al. The vertical drop jump is a poor screening test for ACL injuries in female elite soccer and handball players: a prospective cohort study of 710 athletes. Am J Sports Med 2016;44(4):874-883. doi:10.1177/ 0363546515625048
12. Leppanen M, Pasanen K, Kujala UM, et al. Stiff landings are associated with increased ACL injury risk in young female basketball and floorball players. Am J Sports Med 2017;45(2):386-393. doi:10.1177/ 0363546516665810
13. Numata H, Nakase J, Kitaoka K, et al. Twodimensional motion analysis of dynamic knee valgus identifies female high school athletes at risk of noncontact anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc 2018;26(2):442-447 doi:10.1007/s00167-017-4681-9
14. Uota S, Nguyen AD, Aminaka N, Shimokochi Y. Relationship of knee motions with static leg alignments and hip motions in frontal and transverse planes during double-leg landing in healthy athletes. J Sport Rehabil 2017;26(5):396-405. doi:10.1123/ jsr.2016-0053
15. Arundale AJH, Kvist J, Hägglund M, et al. Jump performance in male and female football players. Knee Surg Sports Traumatol Arthrosc 2020;28:606-613. doi:10.1007/s00167-019-05747-1
16. Lagares LS, de Silva MS, de Macedo R, et al. Dynamic knee valgus and its relationship with performance in the countermovement jump and the squat jump. J Ex Phys 2021;24(6):1-10.
17 Giustino V, Messina G, Patti A, et al. Effects of a postural exercise program on vertical jump height in young female volleyball players with knee valgus. Int J Environ Res Public Health 2022;19(7):3953. doi:10.3390/ijerph19073953
18. Hollman JH, Ginos BE, Kozuchowski J, Vaughn AS, Krause DA, Youdas JW Relationships between knee valgus, hip-muscle strength, and hip-muscle recruitment during a single-limb step-down. J Sport Rehabil 2009;18(1):104-117 doi:10.1123/jsr.18.1.104
19. Baldon R de M, Piva SR, Scattone Silva R, Serrão FV. Evaluating eccentric hip torque and trunk endurance as mediators of changes in lower limb and trunk kinematics in response to functional stabilization training in women with patellofemoral pain. Am J Sports Med 2015;43(6):1485-1493. doi:10.1177/0363546515574690
20. Bredeweg S, Buist I. No relationship between running related injuries and kinetic variables. Br J Sports Med. 2011;45:328. doi:10.1136/ bjsm.2011.084038.52
21. Dicharry J. Kinematics and kinetics of gait: from lab to clinic. Clin Sports Med 2010;29(3):347-364. doi:10.1016/j.csm.2010.03.013
22. Llurda-Almuzara L, Pérez-Bellmunt A, López-deCelis C, et al. Normative data and correlation between dynamic knee valgus and neuromuscular response among healthy active males: a crosssectional study Sci Rep 2020;10:17206. doi:10.1038/ s41598-020-74177-8
23. Baldon R, Serrao FV, Silva RS, Piva SR. Effects of functional stabilization training on pain, function, and lower extremity biomechanics in women with patellofemoral pain: A randomized clinical trial. J Orthop Sports Phys Ther 2014;44(4):240-251. doi:10.2519/jospt.2014.4940
24. Mozafaripour E, Seidi F, Minoonejad H, Bayattork M, Khoshroo F. The effectiveness of the comprehensive corrective exercise program on kinematics and strength of lower extremities in males with dynamic knee valgus: a parallel-group randomized wait-list controlled trial. BMC Musculoskelet Disord 2022;23(1):700. doi:10.1186/ s12891-022-05652-8
25. Radcliffe J. Form and safety in plyometric training. Perf Train J 2003;2(2):21-25.
26. Horton MG, Hall TL. Quadriceps femoris muscle angle: normal values and relationships with gender and selected skeletal measures. Phys Ther 1989;69(11):897-901. doi:10.1093/ptj/69.11.897
27. Uding A, Bloom NJ, Commean PK, et al. Clinical tests to determine femoral version category in people with chronic hip joint pain and asymptomatic controls. Musculoskelet Sci Pract. 2019;39:115-122. doi:10.1016/j.msksp.2018.12.003
28. Souza RB, Powers CM. Concurrent criterionrelated validity and reliability of a clinical test to measure femoral anteversion. J Orthop Sports Phys Ther 2009;39(8):586-592. doi:10.2519/ jospt.2009.2996
29. Neal BS, Lack SD, Lankhorst NE, Raye A, Morrissey D, van Middelkoop M. Risk factors for patellofemoral pain: A systematic review and metaanalysis. Br J Sports Med. 2019;53(5):270-281. doi:10.1136/bjsports-2017-098890
30. Alias MF, Hasan H, Miswan MS, Man DD Interrater reliability and intra-rater reliability testing of my jump 2 mobile application in measuring countermovement jump. Int J Health Move Sci 2021;9(6):1319-1323. doi:10.13189/saj.2021.090628
31. Bogataj Š, Pajek M, Hadžić V, Andrašić S, Padulo J, Trajković N. Validity, reliability, and usefulness of My Jump 2 app for measuring vertical jump in primary school children. Int J Environ Res Public Health 2020;17(10):3708. doi:10.3390/ ijerph17103708
32. Haynes T, Bishop C, Antrobus M, Brazier J. The validity and reliability of the My Jump 2 app for measuring the reactive strength index and drop jump performance. J Sports Med Phys Fit. 2019;59(2):253-258. doi:10.23736/ S0022-4707.18.08195-1
33. McLean SG, Walker K, Ford KR, Myer GD, Hewett TE, van den Bogert AJ. Evaluation of a two dimensional analysis method as a screening and evaluation tool for anterior cruciate ligament injury. Br J Sports Med 2005;39(6):355-362. doi:10.1136/ bjsm.2005.018598
34. Mousavi SH, Hijmans JM, Moeini F, et al. Validity and reliability of a smartphone motion analysis app for lower limb kinematics during treadmill running. Phys Ther Sport. 2020;43:27-35. doi:10.1016/ j.ptsp.2020.02.003
35. Ortiz A, Rosario-Canales M, Rodriguez A, Seda A, Figueroa C, Venegas-Rios H. Reliability and concurrent validity between two-dimensional and three-dimensional evaluations of knee valgus during drop jumps. J Sports Med. 2016;7:65-73. doi:10.2147/ OAJSM.S100242
36. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch AE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models 2nd ed. Springer; 2012. doi:10.1007/ 978-1-4614-1353-0
37 BMJ. Statistics at square one: 11. Correlation and regression. BMJ. Accessed January 19, 2025. https:// www.bmj.com/about-bmj/resources-readers/ publications/statistics-square-one/11-correlationand-regression
38. Gokeler A, Benjaminse A, Hewett TE, et al. Feedback techniques to target functional deficits following anterior cruciate ligament reconstruction: implications for motor control and reduction of second injury risk. Sports Med 2013;43(11):1065-1074. doi:10.1007/ s40279-013-0095-0
39. Gokeler A, Benjaminse A, Welling W, Alferink M, Eppinga P, Otten B. The effects of attentional focus on jump performance and knee joint kinematics in patients after ACL reconstruction. Phys Ther Sport 2015;16(2):114-120. doi:10.1016/j.ptsp.2014.06.002
40. Myer GD, Ford KR, Brent JL, Hewett TE. An integrated approach to change the outcome part II: targeted neuromuscular training techniques to reduce identified ACL injury risk factors. J Strength Cond Res 2012;26(8):2272-2292. doi:10.1519/ JSC.0b013e31825c2c7d
41. Oliver JL, Barillas SR, Lloyd RS, Moore I, Pedley J. External cueing influences drop jump performance in trained young soccer players. J Strength Cond Res 2021;35(6):1700-1706. doi:10.1519/ JSC.0000000000002935
42. Barker L, Bankers S, Farmer B, Siedlik J, Harry JR, Grindstaff T The influence of verbal cues on drop jump landing strategies in NCAA Division I soccer players. Am J Sports Sci 2021;9(2):37-42. doi:10.11648/j.ajss.20210902.12
43. Bates NA, Ford KR, Myer GD, Hewett TE. Timing differences in the generation of ground reaction forces between the initial and secondary landing phases of the drop vertical jump. Clin Biomech (Bristol, Avon) 2013;28(7):796-799. doi:10.1016/ j.clinbiomech.2013.07.004
44. Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359-367. doi:10.1177/ 0363546506293899

2025;20(2):199-209. doi:10.26603/001c.128628
Rafael Escamilla1a , Naiquan Zheng2 , Toran D. MacLeod1 , Rodney Imamura3 , Kevin E Wilk4 , Shangcheng Wang5 , Robert Asuncion1 , Irwin S. Thompson1 , Arnel L. Aguinaldo6 , Glenn S Fleisig7
1 Physical Therapy, California State University, Sacramento, 2 The Center for Biomedical Engineering and Science, Department of Mechanical Engineering and Engineering Science, University of North Carolina at Charlotte, 3 3Kinesiology and Health Science Department, California State University, Sacramento, 4 Champion Sports Medicine, 5 The Center for Biomedical Engineering and Science, Department of Mechanical Engineering and Engineering Science, University of North Carolina, Charlotte, 6 Department of Kinesiology, Point Loma Nazarene University, 7 American Sports Medicine Institute
Keywords: Anterior knee pain, kinetics, squat, patella, rehabilitation https://doi.org/10.26603/001c.128628
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background
While one-legged and two-legged bodyweight squats on unstable and stable surfaces are commonly used during patellofemoral rehabilitation, patellofemoral loading during these exercises is unknown. Understanding how patellofemoral force and stress magnitudes affects different squat variations will aid clinicians in determining how and when to prescribe and progress these squatting types of exercises in patients with patellofemoral pain.
Hypothesis/Purpose
To quantify patellofemoral force and stress between two squat type variations (BOSU squat versus floor squat) and between two leg variations (one-legged squat versus two-legged squat). It was hypothesized that patellofemoral force and stress would be greater in BOSU squat than floor-squat, and greater in one-legged squat than two-legged squat.
Study Design
Controlled laboratory biomechanical, repeated-measures, counterbalanced design.
Methods
Sixteen healthy participants performed one-legged and two-legged BOSU and floor squats. Kinematic and ground-reaction force data were used to calculate resultant knee force and torque using inverse-dynamics, with electromyographic data employed in a knee muscle model to predict resultant knee force and torque at every 10° between 10°-100° knee-angles during the squat-descent and squat-ascent. Repeated-measures 2-way ANOVA (p < 0.01) was employed for statistical analyses.
Results
Collapsed across one-legged and two-legged conditions, patellofemoral joint force and stress were significantly greater during floor squats than BOSU squats at 40°, 50°, and 70° knee-angles during squat descent and 60° and 50° knee-angles during squat ascent. Collapsed across BOSU and floor squats, patellofemoral joint force and stress were
Corresponding Author: Rafael Escamilla, PhD, PT, CSCS, FACSM Professor California State University, Sacramento Department of Physical Therapy 6000 J Street; Sacramento, CA 95819-6020 916-278-6930 (office); 916-278-5053 (fax) rescamil@csus.edu (e-mail)
significantly greater for one-legged squats than two-legged squats at all knee-angles. Significant interactions between squat types and leg conditions were found at 30°, 40°, 50°, 60°, and 100° knee-angles during squat-descent, and 100°, 90°, 80°, and 70° knee-angles during squat-ascent, with patellofemoral joint force and stress significantly greater in two-legged floor-squat than two-legged BOSU squat, but no significant differences between one-legged floor-squat and one-legged BOSU squat.
Squatting progression employing lower to higher patellofemoral loading over time during PFP rehabilitation may be considered: 1) two-legged BOSU squats at lower knee angles (0° - 50°); 2) two-legged floor squats at lower knee angles (0° - 50°); 3) one-legged BOSU and floor squats at lower knee angles (0° - 50°); 4) two-legged BOSU squats at lower and higher knee angles (0° - 100°); 5) two-legged floor squats at lower and higher knee angles (0° - 100°); 6) one-legged BOSU and floor squats at lower and higher knee angles (0°100°).
Level of Evidence
2
Squatting exercises are described in clinical practice guidelines for patellofemoral pain (PFP) both for diagnosing PFP and to be used as an rehabilitation intervention.1 While several studies have quantified patellofemoral force and stress during squatting exercises,2‑10 it is unclear how different types of squat exercises load the patellofemoral. Moreover, it is not well understood what patellofemoral joint force or stress magnitudes, and over what time interval, can lead to PFP There are several factors that can contribute to PFP, including weakness in the quadriceps or hip external rotators, tight quadriceps, hamstrings, or iliotibial band, overuse or trauma, dysfunctional extensor mechanism, malalignment of the lower extremity, and excessive rear-foot pronation.1,11 Repetitive and high patellofemoral stress may result in or worsen PFP and adversely affect soft tissues surrounding the patellofemoral joint, including infrapatellar fat pad, synovial plicae, retinacula, joint capsule, and patellofemoral ligaments.11 High magnitude patellofemoral joint force can also increase subchondral bone stress in the patellofemoral joint.12 Because subchondral bone plates are abundant in pain receptors,13 high subchondral bone stress may result in or exacerbate PFP 11 High magnitude patellofemoral joint stress can result in a decreased ability of the cartilage to distribute and absorb patellofemoral force, resulting in cartilage degeneration.12 A better understanding of patellofemoral joint force and stress magnitudes among different squatting exercises, technique variations, and functional activities may facilitate rehabilitation of those with PFP
Repetitive and large patellofemoral force magnitudes that occur during sport can result in high patellofemoral joint stress (patellofemoral force/patella contact area), which over time can result in PFP Squatting exercises, such as those performed on an unstable BOSU ball (also referred to as BOSU Balance Trainer) versus a stable level ground, are often used for strengthening of thigh and hip musculature and are important training and rehabilitation exercises to enhance patellofemoral joint stability and im-
prove optimal articulation between the femur and patella during sport and activity.2‑10 Understanding the force and stress magnitudes at the patellofemoral joint and how they vary while performing one-legged and two-legged BOSU and floor squats may be helpful to clinicians when prescribing and progressing squatting exercises to individuals with PFP.
There are no known studies that have examined patellofemoral force and stress between the bodyweight one-legged and two-legged BOSU and floor squat exercises. In patellofemoral rehabilitation progression, squatting exercises are usually initially performed with no external resistance (bodyweight only) and progressed to employing external weights (dumbbells or barbells) or other external resistance, such as resistance bands.1 This progression increases both hip and thigh muscle recruitment and patellofemoral force and stress.2‑4 The floor squat, and presumably the BOSU squat also, are commonly progressed in knee rehabilitation starting with a two-legged squat and progressing to a one-legged squat.1 Although progressing the exercises may increase the loads on hip and thigh musculature and subsequently increase hip and thigh strengthening, these exercises may also increase PFP as patellofemoral joint loading increases, and this needs to be considered when progressing patients with PFP using squatting exercises. Through visual observation, squatting on BOSU versus squatting on a level ground produces differences in squat kinematics, such as a greater forward trunk tilt when squatting on a BOSU. It is plausible that these differences in squat kinematics may affect patellofemoral loading (which is directly proportional to quadriceps loading) given that squatting with a more forward trunk tilt likely increases hamstrings activity and may also decrease quadriceps activity, which would imply potentially less patellofemoral loading with the BOSU squat compared to the floor squat. Therefore, the purpose of this study was to quantify patellofemoral force and stress between two squat type variations (BOSU squat versus floor squat) and between two leg variations (one-legged squat versus two-legged squat). The hypotheses were that
patellofemoral force and stress would be greater performing the floor squat compared to the BOSU squat, and greater performing the one-legged squat compared to the twolegged squat.
Sixteen healthy participants without a history of patellofemoral pathology were recruited by bulletin board announcements, posters, flyers, brochures, and e-mail distributions within the California State University, Sacramento community. Inclusion criteria were being able to perform BOSU and floor squats pain-free with proper technique for 12 repetitions using bodyweight and having at least five years’ experience in performing squatting exercises, including previous experience squatting on a BOSU. Based on pilot work measuring forward trunk tilt in experienced squatters during one-legged and two-legged squats, inclusion criteria also included a forward trunk tilt from a vertical position at the lowest portion of the squat of approximately 30°-40° for the floor squat and approximately 40°-50° for the BOSU squat. Exclusion criteria were not being able to perform both one-legged and two-legged BOSU and floor squat exercises, not being able to achieve approximately 100°-100° of knee flexion at the lowest position of the squat, and any history of lower extremity surgery or injury All participants provided written informed consent in accordance with the Institutional Review Board at California State University, Sacramento.
Each participant attended a pre-test session one week prior to testing and practiced performing the one-legged and two-legged BOSU (Figures 1a and 1b) and one-legged and two-legged floor squats (Figures 1c and 1d). The position of the feet for both the two-legged BOSU and floor squats were the same for both exercises for each subject and in accordance with each subject’s preference. Both feet were positioned slightly wider than hip width and had a mean±SD inside heel to inside heel distance of 50.1±3.3 cm for males and 47.9±1.2 cm for females. Moreover, both feet were slightly turned outward from the direction the subject was facing, with a mean±SD foot angle of 20.8±4.3° cm for males and 23.1±5.2° for females. For both the one-legged and two-legged floor squat, the right foot was positioned on an AMTI force platform (Model OR6-6-2000, Advanced Mechanical Technologies, Inc.) which was flush with the floor For the two-legged floor squat the left foot was positioned on the floor. For the one-legged squat, the right foot was positioned on an AMTI force platform for the floor squat and on the flat platform side of the BOSU for the BOSU squat, with the dome side of the BOSU positioned on an AMTI force platform (Figures 1b and 1d). For the twolegged BOSU squat, each foot was placed on the flat platform side of the BOSU with the dome side of one BOSU po-

Figure 1. Unstable BOSU squat two-legged (a), Unstable BOSU squat one-legged (b), Stable floor squat two-legged (c), and Stable floor squat one-legged (d).
sitioned on an AMTI force platform and the dome side of a second BOSU positioned on the floor (Figure 1a).
The starting position for the two-legged squat exercises was with both knees fully extended, and the ending position was at maximum knee flexion at the lowest position of the squat, as shown in Figures 1a and 1c The starting position for the one-legged squat exercises was with the right knee fully extended and the left knee flexed, and the ending position was in a squat position with the right knee maximally flexed at the lowest position of the squat, as shown in Figures 1b and 1d A metronome was employed for all squat variations and set at 25 bpm to help ensure the knee(s) flexed and extended slowly at approximately 45°/s during both the squat descent and squat ascent. This resulted in a squat descent of approximately 2.33 sec to achieve approximately 100°-100° knee flexion at 45°/s, and a squat ascent of 2.33 sec to fully extend the knees at 45°/s. Therefore, for each squat repetition, a first metronome beat presented the start of the squat descent, the second metronome beat represented maximum squat descent, and the third metronome beat represented the end of the squat ascent.
Blue Sensor (Ambu Inc., Linthicum, MD) disposable surface electrodes (type M-00-S; 22 mm wide, 30 mm long) were used to collect EMG data and were positioned along the longitudinal axis of each muscle in a bipolar configuration, with a center-to-center distance of 3 cm between electrodes. Before applying the electrodes, the skin was shaved, abraded, and cleaned with isopropyl alcohol wipes in order to decrease skin impedance. Using locations previously described,3,4,8 electrode pairs were placed on the participant’s right side for the following muscles: a) rectus femoris; b) vastus lateralis; c) vastus medialis; d) medial hamstrings
(semimembranosus and semitendinosus); e) lateral hamstrings (biceps femoris); and f) gastrocnemius (middle portion between lateral and medial bellies).
For three-dimensional (3D) motion capture, spheres (3.8 cm in diameter) covered with reflective tape were attached to adhesives and positioned over the following bony landmarks as described previously3,4,8: a) medial and lateral malleoli of right leg; b) third metatarsal head of right foot ; c) upper edges of lateral and medial tibial plateaus of right knee; d) posterosuperior greater trochanters of left and right femurs; and e) lateral acromion of right shoulder.
After the spheres and electrodes were in place, the participant warmed-up and practiced all squat exercises until they felt warmed up and ready to be tested, and then data collection began. An eight-camera Vicon-Peak Performance motion analysis system (Vicon-Peak Performance Technologies, Inc., Englewood, CO) was employed for 60 Hz video data collection. Force data were collected at 960 Hz using an AMTI force platform (Model OR6-6-2000, Advanced Mechanical Technologies, Inc.). EMG data were collected at 960 Hz using a Noraxon Myosystem unit (Noraxon USA, Inc., Scottsdale, AZ). EMG amplifier bandwidth frequency was 10-500 Hz with an input impedance of 20,000 k. The common-mode rejection ratio was 130 dB. Video, force, and EMG data were all electronically synchronized and simultaneously collected employing a randomized order with each participant performing one set of three repetitions for one-legged and two-legged BOSU and floor squat exercises. A two minute rest period was given between performing each of the four exercise variations. The BOSU ball was filled with an amount of air which caused the dome to be approximately 21.6 cm high, as recommended by the manufacturer
Subsequent to completing the four squat exercises, EMG data were collected during maximum voluntary isometric contractions (MVIC) to normalize EMG data, as described previously 3,4,8 The MVIC for the rectus femoris, vastus medialis, and vastus lateralis were collected in a seated position at 90° knee and hip flexion during a maximum effort knee extension. The MVIC for the lateral and medial hamstrings were collected in a seated position at 90° knee and hip flexion during a maximum effort knee flexion. MVIC for the gastrocnemius was collected during maximum effort unilateral stance heel raise while standing, employing an ankle position halfway between neutral and full plantar flexion. Two trials (each five seconds in duration) were collected for each MVIC for each muscle in a randomized order for all three muscle groups, and a two minute rest interval was given between the two trials and between each muscle tested. The MVIC was calculated using the highest EMG signal over a one second time interval during the five-second MVIC trials, as described previously.3,4,8
Video images from the reflective markers were tracked and digitized in 3D space with Vicon-Peak Performance software, employing the direct linear transformation calibration method. The calibration system accuracy resulted in reflective markers that could be located in 3D space with an
error less than 0.3 cm. Raw position data were smoothed using a double-pass fourth-order Butterworth low-pass filter, using a cut-off frequency of 6 Hz.3,4,8 Joint angles, linear and angular velocities, and linear and angular accelerations were calculated employing appropriate kinematic equations, as described previously.3,4,8
The raw EMG signals were full-wave rectified, smoothed with a 10 ms moving average window, and linear enveloped throughout the knee flexion range of motion for all repetitions.3,4,8 EMG data were then normalized for each muscle and expressed as a percentage of each participant’s highest corresponding MVIC trial. Normalized EMG data for all three trials (repetitions) were averaged at corresponding knee angles between 0°-100° with 0° defining full knee extension, 0°-100° defining the squat descent, and 100-0° defining the squat ascent. EMG data were used to calculate patellofemoral force and stress in a knee biomechanical model (see Appendix) and were not analyzed separately.
A repeated-measures 2-way analysis of variance (ANOVA) was employed for each 10° knee angle (from 10° to 100°) during the squat descent and each 10° knee angle (from 100° to 10°) during the squat ascent in order to assess the effects of squat type (BOSU versus floor squat) and leg type (one-legged versus two-legged) on patellofemoral compressive force and stress. The level of significance employed was p < 0.01. Bonferroni t-tests were used to assess pairwise comparisons among the four squat conditions.
Sixteen healthy participants were studied, with a mean (±SD) age, mass, and height of 29.3±7.6 y, 76.9±6.5 kg, and 176.0±2.4 cm, respectively, for males, and 30.7±9.6 y, 61.4±6.6 kg, and 166.5±8.3 cm, respectively, for females. Tables 1 and 2 provide patellofemoral joint force and stress values between the two squat type conditions collapsed across both leg conditions. The p-values shown in Tables 1 and 2 for the squat type conditions represent the main effects of the ANOVA. When collapsed across the one-legged and two-legged squat type conditions, patellofemoral joint force and stress were significantly greater (p < 0.01) in the floor squat compared to the BOSU squat at 40°, 50°, and 70° knee angles during the squat descent and 60° and 50° knee angles during the squat ascent.
When collapsed across the BOSU squat and the floor squat, patellofemoral joint force and stress were significantly greater (p < 0.01) in the one-legged squat compared to the two-legged squat for all knee angles during the squat descent and the squat ascent.
Significant interactions (p < 0.01) between squat types and leg conditions were found at 30°, 40°, 50°, 60°, and 100° knee angles during the squat descent, and 100°, 90°, 80°, and 70° knee angles during the squat ascent. Patellofemoral joint force and stress were significantly greater (p < 0.01) in the two-legged floor squat compared to the two-legged BOSU squat, but no significant differ-
Table 1. Mean (± SD) patellofemoral joint force (all are reported in N) values during the bodyweight one-legged and two-legged unstable BOSU ball squat and the stable floor squat.
Squat Type Variations
Knee Angles for Squat Descent
Leg Variations
Knee Angles for Squat Ascent Phase
*Significant difference (p < 0.01) between squat type conditions or between leg conditions
Note: The mean values given for the two squat type conditions (BOSU squat and floor squat) were collapsed across both leg conditions (one-legged and two-legged), while the mean values given for both leg conditions were collapsed across the two squat type conditions. The p-values shown for squat type conditions and leg conditions represent the main effects from the repeated measure 2-way ANOVA.
ences were found between the one-legged floor squat and the one-legged BOSU squat (Figures 2a, 2b, 3a, and 3b).
Patellofemoral joint force and stress generally increased progressively as knee flexion increased during the descent phase and decreased progressively as knee flexion decreased during the ascent phase. Moreover, for a given knee angle, patellofemoral joint force and stress were generally slightly greater during the ascent phase compared to the descent phase. For the one-legged and two-legged floor squat exercises, at the lowest portion of the squat the trunk was tilted forward approximately 30°-40° from a vertical position. In contrast, for the one-legged and two-legged BOSU squat exercises, at the lowest portion of the squat the trunk was tilted forward approximately 40°-50° from a vertical position.
This is the first known study that examined the effects of performing one-legged and two-legged BOSU and floor squats on patellofemoral force and stress. As hypothesized, patellofemoral force and stress were greater performing the
floor squat compared to the BOSU squat, and greater performing the one-legged squat compared to the two-legged squat. The greater patellofemoral force and stress when performing the floor squat compared to the BOSU squat may in part be due to less forward trunk tilt in the floor squat (approximately 30°-40° from a vertical position) compared to the BOSU squat (approximately 40°-50° from a vertical position). One plausible explanation is that squatting with less forward trunk tilt (floor squat) moves the center of mass (COM) of trunk/arms/head posterior away from the knees, thus increasing the external knee flexor moment arm and torque produced by COM of trunk/arms/head. Conversely, squatting with greater forward trunk tilt (BOSU squat) moves the COM of the trunk/arms/head anterior towards the knees, thus decreasing the external knee flexor moment arm and torque. Thus, a greater knee extensor muscle torque and quadriceps force would be needed during the floor squat to overcome the greater external knee flexor torque, and less knee extensor muscle torque and quadriceps force is needed during the BOSU squat because of less external knee flexor torque. Since patellofemoral loading is directly proportional to quadriceps force (Figure
Patellofemoral Joint Loading During Bodyweight One-Legged and Two-Legged BOSU and Floor Squats
Table 2. Mean (± SD) patellofemoral joint stress (all are reported in MPa) values during the bodyweight onelegged and two-legged unstable BOSU ball squat and the stable floor squat. Squat Type Variations
Variations
Knee Angles for Squat Descent
Knee Angles for Squat Ascent Phase
*Significant difference (p < 0.01) between squat type conditions or between leg conditions
Note: The mean values given for the two squat type conditions (BOSU squat and floor squat) were collapsed across both leg conditions (one-legged and two-legged), while the mean values given for both leg conditions were collapsed across the two squat type conditions. The p-values shown for squat type conditions and leg conditions represent the main effects from the repeated measure 2-way ANOVA.
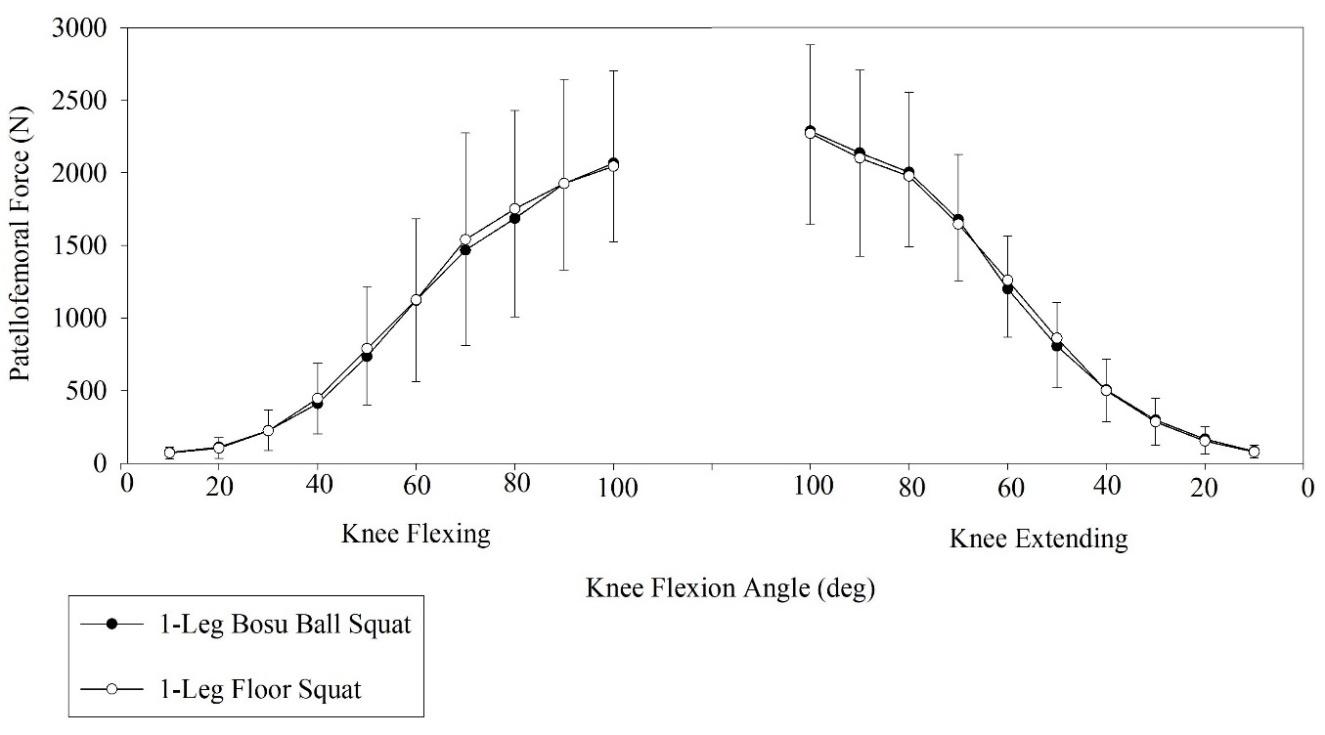
Figure 2a. Mean (SD) patellofemoral compressive force between one-legged BOSU squat and one-legged floor squat.
Note: There were no significant differences (p < 0.01) in patellofemoral compressive force at any knee angle during knee flexing (squat descent) and knee extending (squat ascent) between one-legged BOSU squat and one-legged floor squat.
A2 in Appendix), potentially greater quadriceps force in the floor squat may result in greater patellofemoral loading in the floor squat compared to the BOSU squat. However,

Figure 2b. Mean (SD) patellofemoral compressive force between two-legged BOSU squat and two-legged floor squat.
Note: There were significant differences* (p < 0.01) in patellofemoral compressive force at 30°, 40°, 50°, 60°, and 100° during the knee flexing squat descent and at 100°, 90°, 80°, and 70° during the knee extending squat ascent between two-legged BOSU squat and two-legged floor squat.
this simplistic explanation is actually more complex due to the biarticular hip and knee rectus femoris and ham-

Figure 3a. Mean (SD) patellofemoral compressive stress between one-legged BOSU squat and one-legged floor squat.
Note: There were no significant differences (p < 0.01) in patellofemoral compressive stress at any knee angle during knee flexing (squat descent) and knee extending (squat ascent) between one-legged BOSU squat and one-legged floor squat.

Figure 3b. Mean (SD) patellofemoral compressive stress between two-legged BOSU squat and two-legged floor squat.
Note: There were significant differences* (p < 0.01) in patellofemoral compressive stress at 30°, 40°, 50°, 60°, and 100° during the knee flexing squat descent and at 100°, 90°, 80°, and 70° during the knee extending squat ascent between two-legged BOSU squat and two-legged floor squat.
strings musculature, which simultaneously generates hip and knee torque during squatting. Although increasing forward trunk tilt decreases the external knee flexor moment arm and torque, it simultaneously increases the external hip flexor moment arm and torque. To overcome this external hip flexor torque, a hip extensor muscle torque from the hip extensors (hamstrings, gluteus maximus) is needed, which must be even greater given that rectus femoris force simultaneously produces hip flexor muscle torque and knee extensor muscle torque. Given that hamstrings force simultaneously generates hip extension muscle torque and knee flexor muscle torque, greater hamstrings force and knee flexor muscle torque results in greater quadriceps force and knee extensor muscle torque being needed, and less hamstrings force and knee flexor muscle torque results in less quadriceps force and knee extensor muscle torque being needed.
One of the more interesting findings were the interactions between squat types and leg conditions, which demonstrated that patellofemoral joint loading was similar
between the BOSU squat and floor squat when squatting on one leg, but significantly higher in the floor squat compared to the BOSU squat when squatting on two legs. Clinical applications are that early in the patellofemoral rehabilitation process when the goal is to minimize patellofemoral loading, two-legged squatting should precede one-legged squatting, and the BOSU squat should precede the floor squat. Subsequently, when the patient is able to sustain greater patellofemoral loading, one-legged squatting should be employed over two-legged squatting, and floor squatting should be employed over BOSU squatting. These findings may be due to differences in how the two squat type exercises were performed with varying amounts of stability With one-legged squatting, one leg was on a stable surface (floor squat) or one leg was on an unstable surface (BOSU squat), while with two-legged squatting, both legs were either on a stable surface (floor squat) or on an unstable surface (BOSU squat). Regardless of what influenced these differences or similarities in patellofemoral loading between one-legged and two-legged squatting, these findings provide insight to how the patellofemoral joint can be loaded and progressed in PFP rehabilitation using onelegged and two-legged BOSU and floor squats.
Escamilla et al.6 also quantified patellofemoral force and stress during the two-legged bodyweight squat, while their participants performed the ball and wall squats with their back against a swiss ball or against the wall. The ball and wall squats in that study were performed with the trunk in a vertical position instead of tilted forward 30° - 50° as in the current study, which is a functional squat position. Escamilla et al.6 reported a peak patellofemoral force and stress of 1223±348 N and 3.05±0.87 MPa, respectively, during the ball squat and 1385±393 N and 3.45±0.98 MPa, respectively, during the wall squat, at 80° knee angle during squat ascent. In contrast, peak patellofemoral force and stress in the current study were 970±264 N and 2.42±0.66 MPa, respectively, during the two-legged BOSU squat, and 1125±353 N and 2.80±0.88 MPa, respectively, during the two legged floor squat (18-22% less compared to the twolegged bodyweight ball and wall squats), also occurring at the 80° knee angle during squat ascent. Average patellofemoral force and stress throughout the squat descent (10° - 100°) and squat ascent (100° - 0°) was also 22% less in the BOSU squat compared to the ball squat, but was nearly identical between the wall squat, ball squat and floor squat. Thus, when the goal is to minimize patellofemoral force and stress and gradually progress patellofemoral loading, the BOSU squat should be performed first, followed by the floor squat, ball squat, and finally wall squat, which had the highest peak patellofemoral force and stress.
Kernozek et al.9 also examined the two-legged bodyweight squat using two techniques - one squatting involving the knees progressing past toes (SPT) and one squatting with knees positioned behind toes (SBT). These authors reported mean patellofemoral force and stress of approximately 1450 N and 3.4 MPa, respectively, for SBT, and approximately 1950 N and 4.2 MPa, respectively, for SPT, which were slightly greater than the force and stress values than those in the current study. Wallace et al.10 reported
peak patellofemoral joint force and stress magnitudes of approximately 1700 N and 9.3 MPa, respectively, during the bodyweight squat at 90° knee angle. Almonroeder et al.7 reported a mean patellofemoral joint force and stress magnitudes of approximately 2300 N and 11 MPa, respectively, during the bodyweight squat. Patellofemoral force and stress magnitudes from both Wallace et al.10 and Almonroeder et al.7 are considerably higher than those reported in the current study, which may be due to methodological differences among studies.
The results from the current study can be compared to patellofemoral joint loading during squatting with greater intensities to help clinicians progress a patient with PFP. Escamilla et al.2,3,8 reported peak patellofemoral joint force and stress magnitudes of 4500-4700 N and 11-12 MPa, respectively, at 90° knee angle during the 12 RM barbell squat, which is 4-5 times greater than peak magnitudes in the current study. Escamilla et al.4 also reported peak patellofemoral joint force and stress magnitudes of approximately 3500 N and 9 MPa, respectively, between 70°-80° knee angle during the 12 RM one leg squat and wall squat, 3-4 times greater than peak magnitudes in the current study Wallace et al.10 reported peak patellofemoral joint force and stress magnitudes of approximately 2400 N and 13 MPa, respectively, during the barbell squat with a 35% bodyweight external load, a little over twice the force magnitudes and five times the stress magnitudes from the current study
When considering patellofemoral loading from the aforementioned and current studies, squatting exercises during PFP rehabilitation can be progressed from lower to higher loads by initially performing two-legged and BOSU bodyweight squat exercises, then one-legged and floor bodyweight squat exercises, then lower intensity barbell squats (eg, 1/3 bodyweight external load), then higher intensity ball or wall squat (e.g., 12 RM external load), and then higher intensity barbell squats (e.g., 12 RM external load).
Peak patellofemoral joint force and stress magnitudes from the current study are similar, lower, or higher compared to many functional activities. Peak patellofemoral joint force and stress in healthy participants performing fast walking are approximately 900 N and 3.13 MPa, respectively,14 similar to peak magnitudes in the current study
Peak patellofemoral joint force and stress magnitudes in healthy participants ascending and descending stairs are approximately 2500 N and 7 MPa, respectively,14 2-3 times the peak magnitudes in the current study Based on these findings, when the goal is to minimize and later progress patellofemoral loading, the order of progression when integrating functional activities with one-legged and twolegged squatting could be to start with slow walking, progress to fast walking, progress to two-legged BOSU squat followed by two-legged floor squat, progress to onelegged BOSUs-ball and floor squats, and finally progress to ascending and descending stairs.
The injury risk to the patellofemoral joint may not increase with knee angles between 70°-100° or greater because of similar magnitudes seen in patellofemoral joint
stress during these knee angles. The benefits of exercising in deeper knee flexion angles include greater quadriceps, hamstrings, and gluteus maximus activity during training at 70°-100° knee angles or greater when compared to training at lesser knee angles between 0° - 60°. Exercising in smaller knee flexion angles between 0° - 60° becomes more quadriceps dominant with less hamstring and gluteus maximus involvement.3,4,8
All biomechanical models have modeling limitations (see Appendix for an overview of biomechanical model employed, and its potential limitations). Firstly, knee kinematic MRI data have shown that during the weight bearing squat the femur moves and rotates under a relatively stationary patella, and excessive femoral rotation may increase patellofemoral joint stress on the contralateral patellar facets.15 Unfortunately, knee kinematic MRI data do not currently exist for BOSU and floor squat exercises through functional ranges of motion. Therefore, it is unknown how much the femur rotates and how this rotation varies among healthy individuals when compared to those with pathologies. Secondly, patellofemoral joint stress magnitudes were calculated employing patellar contact area values from MRI data in the literature and were not measured directly for each subject. However, the contact areas employed from the literature were determined while performing loaded weight bearing exercise in healthy female and male participants, similar to the current study. Moreover, the near linear and direct relationship between knee angle and contact area has been shown to be similar among studies.12,16,17 Therefore, it is a fair assumption that patellofemoral joint stress curve patterns in Figures 3a and 3b using contact areas from the literature are similar to patellofemoral joint stress curve patterns for contact areas measured directly from MRI. Patellofemoral joint stress patterns are important for clinicians to understand to help determine what knee range of motions patellofemoral joint stress increases or decreases, which can exacerbate PFP Finally, the focus of the current study was to compare right knee patellofemoral force/stress between the BOSU squat and floor squat, so the authors arbitrarily chose the right leg to use in the knee model to assess this using only one force platform. The focus was not to compare patellofemoral force/stress between left and right knees and assess symmetry between left and right sides of the body during two-legged squatting, although symmetry was assumed in these healthy subjects.
Patellofemoral joint loading changed according to variations in both squat type and leg variations. Squatting progression employing lower to higher patellofemoral loading over time during PFP rehabilitation may be considered: 1) two-legged BOSU squats at lower knee angles (0° - 50°); 2) two-legged floor squats at lower knee angles (0° - 50°); 3) one-legged BOSU and floor squats at lower knee angles (0° - 50°); 4) two-legged BOSU squats at lower and higher knee angles (0° - 100°); 5) two-legged floor squats at lower and higher knee angles (0° - 100°); 6) one-legged BOSU and
floor squats at lower and higher knee angles (0° - 100°). Future research could focus on examining using external loads on patellofemoral force and stress while performing similar squatting exercises, and investigating the clinical relevance of performing these exercises in patients with PFP
Patellofemoral Joint Loading During Bodyweight One-Legged and Two-Legged BOSU and Floor Squats International Journal of Sports Physical Therapy
Submitted: June 13, 2024 CST. Accepted: November 22, 2024
CST Published: February 01, 2025 CST
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Willy RW, Hoglund LT, Barton CJ, et al. Patellofemoral Pain. J Orthop Sports Phys Ther 2019;49:CPG1-CPG95. doi:10.2519/jospt.2019.0302
2. Escamilla RF. Knee biomechanics of the dynamic squat exercise. Med Sci Sports Exerc 2001;33:127-141. doi:10.1097/00005768-200101000-00020
3. Escamilla RF, Fleisig GS, Zheng N, Barrentine SW, Wilk KE, Andrews JR. Biomechanics of the knee during closed kinetic chain and open kinetic chain exercises. Med Sci Sports Exerc 1998;30:556-569. doi:10.1097/00005768-199804000-00014
4. Escamilla RF, Zheng N, Macleod TD, et al. Patellofemoral joint force and stress during the wall squat and one-leg squat. Med Sci Sports Exerc 2009;41:879-888. doi:10.1249/ MSS.0b013e31818e7ead
5. Powers CM, Ho KY, Chen YJ, Souza RB, Farrokhi S. Patellofemoral joint stress during weight-bearing and non-weight-bearing quadriceps exercises. J Orthop Sports Phys Ther 2014;44:320-327 doi:10.2519/ jospt.2014.4936
6. Escamilla RF, Zheng N, Macleod TD, et al. Patellofemoral joint loading during the performance of the wall squat and ball squat with heel-to-walldistance variations. Med Sci Sports Exerc. 2023;55:1592-1600. doi:10.1249/ MSS.0000000000003185
7. Almonroeder TG, Watkins E, Widenhoefer T. Verbal instruction reduces patellofemoral joint loading during bodyweight squatting. J Sport Rehabil 2020;29:463-468. doi:10.1123/jsr.2018-0157
8. Escamilla RF, Fleisig GS, Zheng N, et al. Effects of technique variations on knee biomechanics during the squat and leg press. Med Sci Sports Exerc. 2001;33:1552-1566. doi:10.1097/ 00005768-200109000-00020
9. Kernozek TW, Gheidi N, Zellmer M, Hove J, Heinert BL, Torry MR. Effects of anterior knee displacement during squatting on patellofemoral joint stress. J Sport Rehabil. 2018;27:237-243. doi:10.1123/ jsr.2016-0197
10. Wallace DA, Salem GJ, Salinas R, Powers CM. Patellofemoral joint kinetics while squatting with and without an external load. J Orthop Sports Phys Ther. 2002;32:141-148. doi:10.2519/jospt.2002.32.4.141
11. Biedert RM, Sanchis-Alfonso V Sources of anterior knee pain. Clin Sports Med. 2002;21:335-347. doi:10.1016/S0278-5919(02)00026-1
12. Besier TF, Draper CE, Gold GE, Beaupre GS, Delp SL. Patellofemoral joint contact area increases with knee flexion and weight-bearing. J Orthop Res 2005;23:345-350. doi:10.1016/j.orthres.2004.08.003
13. Wojtys EM, Beaman DN, Glover RA, Janda D Innervation of the human knee joint by substance-P fibers. Arthroscopy 1990;6:254-263. doi:10.1016/ 0749-8063(90)90054-H
14. Heino Brechter J, Powers CM. Patellofemoral stress during walking in persons with and without patellofemoral pain. Med Sci Sports Exerc 2002;34:1582-1593. doi:10.1097/ 00005768-200210000-00009
15. Powers CM. The influence of altered lowerextremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther 2003;33:639-646. doi:10.2519/ jospt.2003.33.11.639
16. Patel VV, Hall K, Ries M, et al. Magnetic resonance imaging of patellofemoral kinematics with weight-bearing. J Bone Joint Surg Am. 2003;85-A:2419-2424. doi:10.2106/ 00004623-200312000-00021
17. Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res Published online 2003:277-284. doi:10.1097/ 01.blo.0000093024.56370.79
Download: https://ijspt.scholasticahq.com/article/128628-patellofemoral-joint-loading-during-bodyweight-onelegged-and-two-legged-bosu-and-floor-squats/attachment/262718.docx?auth_token=52LT7sq1mshMsnFAsRve

Gutiérrez-Espinoza H, Méndez-Rebolledo G, Zavala-González J, Torreblanca-Vargas S, Araya-Quintanilla F. The Effect of the Addition of Core Exercises to Supervised Physiotherapy in Patients With Subacromial Impingement Syndrome. IJSPT 2025;20(2):210-220. doi:10.26603/001c.128630
Héctor Gutiérrez-Espinoza1 , Guillermo Méndez-Rebolledo2 , Jonathan Zavala-González3 , Serghio Torreblanca-Vargas4 , Felipe Araya-Quintanilla5a
1 One Health Research Group, Universidad de las Americas, Quito, Ecuador, 2 Laboratorio de Investigación Somatosensorial y Motora, Escuela de Kinesiología, Facultad de Salud, Universidad Santo Tomás, 3 Kinesiology Service, Hospital San Borja Arriarán, 4 Service of Rehabilitation, Hospital Provincia Cordillera, 5 Escuela de Kinesiología, Facultad de Odontología y Ciencias de la Rehabilitación, Universidad San Sebastian
Keywords: subacromial impingement syndrome, muscular strength, exercise therapy, core exercise program, observational study https://doi.org/10.26603/001c.128630
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background
Weakness of the rotator cuff has been reported in patients with subacromial impingement syndrome (SIS). A novel therapeutic approach proposes adding exercises for the core musculature to aid in functional recovery in these patients.
Purpose
The aim of this study was to assess the short-term effects of adding a core exercise program to supervised physiotherapy on improve lateral rotator strength and functional outcomes in patients with SIS.
Study Design
A pre–post single-group study.
Methods
A total of 47 participants with SIS were recruited. All patients were treated with five weeks of supervised physiotherapy plus a core exercise program. The primary outcomes were isometric lateral rotator strength and grip strength, measured with a dynamometer Secondary outcomes included muscular endurance assessed with the Closed Kinetic Chain Upper Extremity Stability Test (CKCUEST), shoulder function with the Constant-Murley (CM) questionnaire, and pain intensity reported using the Visual Analog Scale (VAS). Need a brief statement of statistical approach.
Results
At end of the five week intervention, isometric lateral rotator strength showed an increase of 9.2 kg (d = 2.1; p < 0.001) and grip strength an increase of 10.6 kg (d = 2.4; p < 0.001). The CKCUEST showed an increase of 5.6 repetitions (d = 3.7; p < 0.001), the CM questionnaire showed an increase of 30.3 points (d = 4.9; p < 0.001) and the VAS showed a decrease of 3.9 cm (d = 6.0; p < 0.001). All outcomes showed large effect sizes and statistically significant differences.
Corresponding author:
Felipe Araya-Quintanilla
E-mail address: Felipe.arayaq@uss.cl a
Escuela de Kinesiología, Facultad de Odontología y Ciencias de la Rehabilitación, Universidad San Sebastián, Santiago, Chile Lota 2465 Zip code 7510157
Phone +56997122483
Conclusion
In the short term, adding a core exercise program to supervised physiotherapy showed statistically and clinically significant differences in lateral rotator strength and functional outcomes in patients with SIS.
Level 3
Subacromial impingement syndrome (SIS), also called external impingement, is the most common diagnostic label for shoulder pain.1 Neer defined SIS as a mechanical compression or abrasion of the supraspinatus tendon, subacromial bursa or long head of the biceps tendon beneath the anterior undersurface of the acromion, coracoacromial ligament or acromioclavicular joint during elevation of the arm.2 Currently, this pathoanatomic model is controversial because recent evidence suggests that it does not fully explain the mechanisms related to SIS.3,4
Biomechanical factors such as alterations in glenohumeral and scapulohumeral kinematics and impairment of the rotator cuff and scapular muscles are commonly associated with SIS.5,6 Although there is no consensus in the systematic reviews of electromyographic studies examining the scapulohumeral musculature, the most significant findings were increased upper trapezius activity with reduced serratus anterior and inferior trapezius activity, associated with a delay in the activation time of the latter two muscles.7,8 Regarding the glenohumeral musculature, the activity and coactivation of the rotator cuff muscles, especially the subscapularis with the infraspinatus or supraspinatus, was found to be reduced during the first phase of arm elevation.9
Based on the above, weakness of the scapular and glenohumeral muscles is one of the main impairments in patients with SIS.10‑13 Indeed, in these patients, the greatest impairment in muscle strength has been reported in the lateral rotator muscles of the shoulder (i.e., infraspinatus and teres minor, supraspinatus),11,12 leading to imbalance in the function of the rotator cuff muscles and alterations in the glenohumeral kinematics.11 Additionally, positive correlations between shoulder muscle strength, grip strength and upper limb function have been reported in previous studies.10,13 Due to the shoulder muscle imbalance that commonly exists in patients with SIS, the entire upper limb kinetic chain is affected, negatively impacting energy transfer through the upper limb as well as grip function.10 Therefore, shoulder rehabilitation programs should focus on the prescription of exercises to improve lateral rotation and handgrip strength deficits in these patients. Although therapeutic exercise has been described as an important component for the treatment of SIS, no specific type of exercise has been described as a reference standard for the non-surgical treatment of SIS.14,15 Currently, musculoskeletal approaches focus on strengthening the shoulder joint complex and achieving dynamic stabilization.16 A novel therapeutic approach proposes adding lumbar stability training in individual athletes through the core mus-
culature.16,17 Despite some controversies, it has been observed that this approach has general benefits in the functional recovery of the shoulder 16‑18 These benefits include an increase in electromyographic activity of the middle trapezius and serratus anterior muscles, in addition to an increases in the power of throws and strength in the punches of athletes.17,19 Based on biomechanical foundation as energy transfer through kinetic chains, the authors hypothesized that, in addition to supervised physiotherapy, the use of core-focused exercises could be an effective approach for improving lateral rotator strength in the early stages of rehabilitation in patients with SIS.
To the authors’ knowledge, there are no published studies that have analyzed the effect of adding a core exercise program to supervised physiotherapy in order to improve lateral rotator strength in patients with SIS. Thus, the aim of this study was to assess the short-term effects of adding a core exercise program to supervised physiotherapy on improve lateral rotator strength and functional outcomes in patients with SIS. The authors hypothesized that, in addition to supervised physiotherapy, core-focused exercises could be an effective approach for improving lateral rotator and grip strength in the early stages of rehabilitation in patients with SIS.
A single-group pre- and post-test study was performed and reported according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.20 The study was approved by the ethics committee of the Central Metropolitan Health Service of Chile on 7 October 2019 (ID: 048975). Between February 2020 and February 2021, a total of 47 patients with SIS were prospectively recruited. All patients signed an informed consent form approved by the ethics committee.
Participants were recruited from the Physical Therapy Department of Clinical Hospital San Borja Arriaran in Santiago, Chile. To be eligible for participation, patients with SIS had to meet the following conditions: age ≥ 18 years; pain located on the anterolateral side of the shoulder for ≥ 3 months; and three or more positive clinical signs of SIS, such as the Neer or Hawkins-Kennedy test, a painful arc, pain on resisted external rotation or the Empty Can test. The sensitivity and specificity of these combined clinical signs is >74% for a diagnosis of SIS.21 Exclusion criteria
were a diagnosis of cervical radiculopathy, osteoarthritis in the acromioclavicular or glenohumeral joint, calcific tendinitis, adhesive capsulitis, glenohumeral instability or a partial or full-thickness rotator cuff tear; radiographs and magnetic resonance imaging were performed to confirm the absence of these pathologies. Other exclusion criteria were athlete’s subjects or participate in a structured sport, a clinical history of acute trauma, previous surgery, previous fracture in the affected shoulder or corticosteroid injection into the shoulder joint in the prior 12 months.
Physiotherapy treatment consisted of a five-week, twice a week, supervised, specific exercise program based on the clinical decision algorithm proposed by a panel of experts.22 In the initial stage, ‘scapular orientation’ was trained to improve proprioception and normalize the resting position of the scapula. Then, three scapular control exercises were performed: bilateral shoulder flexion up to 60°; a closed kinetic chain exercise (the ‘unilateral bench press’); and a scapular control exercise with bilateral shoulder retraction and extension in the prone position. The final stage included two glenohumeral control exercises to restore centralization and prevent superior translation of the humeral head: isometric lateral rotation performed with shoulder adduction; and isometric adduction of the shoulder in the scapular plane at 30° and 60° of elevation. The exercises were performed without or with minimal pain (< 3 cm on the Visual Analog Scale [VAS]), and a maximum of four exercises per session. The dose and progressions were related to the goal of each exercise: 8–10 repetitions of each exercise, with a 5–10-s duration and 30s to 1 min of rest between each repetition.23 (see Supplementary Table S1).
The ‘core’ is a functional term that represents the trunk musculature (back, abdominal, pelvic floor, diaphragm, hip and gluteus) and connects the upper and lower extremities.18,24 Alongside conventional physiotherapy treatment, a Level 1 core exercise program (patient in a stationary position with static contraction) was performed; following the levels of as suggested by Jeffreys et al.25 Four core exercises were performed: ‘knee push-up’, ‘isometric forearm plank’, ‘gluteal bridge’ and ‘dead bugs’ Ten repetitions of each exercise with a 10–15-s duration and 1 min of rest between each repetition were performed (see Supplementary Table S2).
Two physiotherapists external to the research team performed the assessments at baseline and at the end of the five-week treatment. Both physiotherapists assessed the same number of patients.
The primary outcome measure was isometric lateral rotator strength and grip strength assessed using a dynamometer Lateral rotator muscle isometric strength was measured using a handheld dynamometer (Hoggan MicroFET2; Scientific LLC, Salt Lake City, UT, USA). Participants completed 5-s maximal contractions. Participants were in the standing position with their feet approximately shoulder width apart, the arm and wrist in neutral position, and the elbow at 90° flexion.10 Three measurements were performed with the dynamometer against a wall for stability and to give resistance to counter the maximal contraction.10 The affected shoulder was tested, and verbal encouragement was used to ensure maximal contractions. Additionally, a physiotherapist observed whether the participant compensated for lateral rotation with shoulder abduction; if this occurred, the attempt was discarded and the assessment was repeated. A rest time of one minute between each contraction ensured sufficient recovery The average value obtained from the three attempts was used for analysis.
A Jamar™ dynamometer (Model FS360, Performance health; Illinois, United States) was used to assess handgrip strength in the affected side. Participants completed 5-s maximal contractions. Participants were in the standing position with their feet approximately shoulder width apart, a wall behind them to add stability, prevent trunk rotation, and help maintain arm position. Three measurements were performed with the arm and wrist in neutral position and the elbow at 90° flexion. The affected side was tested, and verbal encouragement was used to ensure maximal contractions. A rest time of one minute between each contraction ensured sufficient recovery An adjustment of 10% between the force of the dominant and non-dominant side was made. The average value obtained from the three attempts was used for analysis.
The main secondary outcome measure was muscular endurance, assessed using the Closed Kinetic Chain Upper Extremity Stability Test (CKCUEST).26 Participants assume a push-up position, with the spine and lower limbs aligned and feet separated shoulder-width apart. During the 15 s of testing, participants moved one hand to touch the dorsum of the opposite hand and then returned the moving hand back to the starting position. Subsequently, participants performed the same movement with the other hand. If one of the touches did not reach the required distance of the test, the testing continued but the attempt was not counted.26 The CKCUEST is considered easy for clinicians to apply, and also easy for patients to understand.26 Additionally, shoulder function was assessed with the ConstantMurley questionnaire.27 Scores range from 0 to 100 points, with lower scores indicating a worse condition.27 Finally, pain intensity during isometric strength testing of the lateral rotator muscles was assessed with the VAS. This is a valid scale, with scores ranging from 0 to 10 cm and higher scores indicating greater pain intensity 28
Table 1. Baseline characteristics of patients with SIS.
• Male
• Female
• Primary • Middle
• University
SIS: Subacromial impingement syndrome; SD: Standard deviation; BMI: Body mass index.
Descriptive statistics were used to describe the demographic and clinical characteristics of the patients. For continuous outcomes, data are presented as the mean and standard deviation (SD); for categorical outcomes, data are presented as the number and percentage (%). The normality distribution was evaluated using both statistical (Shapiro-Wilk test) and graphical (normal probability Q–Q plot) methods. To assess the differences between baseline and post-treatment, the paired t-test or Wilcoxon test was used. Additionally, the Cohen’s d (d) was reported to provide the effect size of adding the core exercise program to the supervised physiotherapy treatment, considering the effect as trivial (< 0.2), small (0.2–0.5), medium (0.5–0.8) or large (> 0.8).29 The significance level was set at p < 0.05 and the confidence interval (CI) at 95%. Data were analyzed using the Statistical Package for the Social Sciences (SPSS), Version 27 (SPSS Inc., Chicago, IL, USA).
Thirty-four subjects with SIS were included in the study (43.9 +/-5.4 years; 34 male and 17 female). All baseline characteristics for the patients are presented in Table 1.At the end of the five week intervention period, there were no complications associated with the treatment received and no dropouts or withdrawals.
Table 2 shows the baseline, post-treatment and a comparison of results for all the functional outcomes. At the end of the five weeks, the isometric lateral rotator strength showed an increase of 9.2 kg (95% CI = 7.9–10.4; Cohen’s d = 2.1; p < 0.001) and grip strength showed an increase of 10.6 kg (95% CI = 9.3–11.9; Cohen’s d = 2.4; p < 0.001). For secondary outcomes, the CKCUEST showed an increase of 5.6 repetitions (95% CI = 5.1–6; Cohen’s d = 3.7; p < 0.001), the Constant-Murley questionnaire showed an increase of 30.3 points (95% CI = 28.5–32.1; Cohen’s d = 4.9; p < 0.001) and the VAS showed a decrease of 3.9 cm (95% CI = −3.7 to
−4.1; Cohen’s d = 6; p < 0.001) (Figures 1-5). For all the outcomes assessed, the effect sizes were large (> 0.8) and the differences were statistically significant (p < 0.05).
Weakness of the rotator cuff musculature has been reported in patients with SIS. A novel therapeutic approach adding exercises of core musculature for functional recovery in these patients was examined. The main findings were that this exercise program showed statistically and clinically significant differences for improving the isometric strength of shoulder lateral rotator muscles, grip strength, muscular endurance
While shoulder strength has been studied extensively in the rehabilitation, reintegration, and sports training of throwers and healthy population, there is a notable lack of research focusing on the recovery of lateral rotator muscle strength in adult non-athletes with SIS.18,30 This study is the first to report improvement in the isometric strength of the lateral rotator muscles and grip strength in the early stages of rehabilitation with a supervised physiotherapy treatment that included a core exercises program. Based on biomechanical foundations, all upper limb tasks are performed through the integration of multiple body segments and sequential activation of different muscle groups.31 In this sense, the greater proportion of kinetic energy and force in this sequencing is derived from the larger proximal body segments.31,32 For instance, 51% of the total kinetic energy and 54% of the total force generated in a tennis service are generated by the lower legs, hip and trunk.32
Supporting this notion, dysfunction within a motion sequence from the pelvis to the shoulder girdle may cause an alteration in energy transfer through kinetic chains, resulting in significantly greater shoulder distraction forces.33 On the other hand, previous studies have shown that the muscles of the anterolateral and posterolateral kinetic chain present greater myoelectric activation during the execution of core exercises.18,25 Thus, the increase in grip and shoul-
Table 2. Comparison of results at baseline and post-treatment for lateral rotator and grip strength and functional outcomes in patients with SIS.
Outcome
RC: Rotator cuff; CKCUES: Closed Kinetic Chain Upper Extremity Stability Test; VAS: Visual Analog Scale; SD: Standard Deviation; *: Statistically significant difference between baseline and post-treatment with t-test; ǂ: Statistically significant difference between

Differences at baseline and post treatment for lateral rotator (RC) strength
der lateral rotation strength observed in the current study could be a result of improvement in energy transfer from the trunk, shoulder complex and remaining links of the kinetic chain of the upper limb.17,19 Furthermore, participants with SIS showed an improvement in the CKCUEST performance, which may also be related to optimization of energy transfer along the links of the closed kinetic chain that occur in a plank position and because the xercise program is performed in this position.34 Therefore, the use of core-focused exercises seems to be an effective and safe approach, without the prescription of the dynamic exercises specifically targeting to lateral rotator cuff muscles, for improving shoulder muscle strength in patients with SIS in early stages of treatment.
Usually, core-strengthening exercises are recommended to improve muscle activity in athletes with musculoskeletal injuries.18,24 However, this recommendation lacks strong evidential support when the treatment is applied to nonathletes with SIS.16,17 In accordance with the current find-

2. Differences at baseline
ings, some studies on throwing athletes and healthy nonathletes showed improvement of isometric rotator cuff strength.16,19,30 Indeed, it has been reported that isometric core exercises involving the upper limb can improve shoulder strength, while dynamic exercises improve the throwing speed.13,35 In addition, other studies showed that lumbopelvic stabilization exercises improve the activity of the shoulder muscles.16,30 However, additional studies are needed to analyze the effects of these exercises in the rehabilitation of non-athletes with shoulder injuries.
The current findings could be explained from different viewpoints. Some authors have shown a relationship between the scapular and shoulder muscles and the central stabilizer muscles of the spine during movement of the upper limbs.35,36 A proposed mechanism to explain this association is that core stabilization provides stability and support for load transfer to the limbs, suggesting that core muscles provide proximal stability for distal mobility.37 De-

Figure 3. Differences at baseline and post treatment for CKCUEST

Figure 4. Differences at baseline and post treatment for Constant-Murley

Figure 5. Differences at baseline and post treatment for VAS during strength testing.
spite this, there is still a lack of high-quality evidence showing the direct effects of central stabilization exercises on the strength of rotator cuff muscles. Supporting the current findings, some authors have reported a positive correlation between grip strength and rotator cuff muscle activity.10,12,38 One study found that a neutral grip task increased the electromyographic activity in supraspinatus and infraspinatus muscles.38 Another study showed that patients with SIS who participated in handgrip exercises had reduced pain intensity and improved shoulder function.12 Finally, a strong correlation has been demonstrated between handgrip strength and lateral rotator strength in healthy populations.10 These findings could be explained by neural inputs in the corticospinal pathways during handgrip strength exercises.12 This mechanism is mediated by a neural network in the cortical and subcortical areas of the brain that increases the motor variability of the rotator cuff muscles when performing a handgrip exercise.12,39,40
The safety and clinical applicability of the core exercise program in conjunction with supervised physiotherapy treatment are the main strengths of this study However, this study has several limitations as it is a pre–post singlegroup study without a control group, the clinical effectiveness of the core exercise program cannot be established in these patients. The lack of control of confounding factors inherent in observational studies may have caused overestimation of the treatment effects, although the effect sizes indicate strong effects of the intervention. Changes in the glenohumeral or scapular kinematics were not assessed so any kinematic reasons for improvements cannot be assumed. Self-reported questionnaires were used for assessment, which are prone to subjectivity and recall bias, which may have affected CM outcomes. Finally, the method used to assess lateral rotator strength is one of the most recommended for clinical use but is not the ‘gold standard’. These limitations should be considered when attempting to extrapolate the findings of this study to all patients with SIS.
Despite the limitations, the results of this study provide an important clinical implication for the physiotherapy treatment of patients with SIS. Although physiotherapy is considered a first-line treatment for SIS, the effectiveness of therapeutic interventions to improve lateral rotator strength in the early stages of rehabilitation is unclear Therefore, this study’s results could guide physiotherapists to consider these exercises in patients with SIS. Further studies are needed to determine the specific stabilization exercises that are most effective in this clinical population.
In the short term, adding a core exercise program to supervised physiotherapy showed statistically and clinically significant differences improvements in isometric lateral rotator and grip strength, as well as improvements in functional outcomes in patients with SIS. However, these findings must be interpreted in the context of the studied pop-
ulation and further high-quality research is needed to further explore this approach.
The authors report no conflicts of interest.
Submitted: May 22, 2024 CST. Accepted: November 22, 2024
CST Published: February 01, 2025 CST
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Ostör AJ, Richards CA, Prevost AT, Speed CA, Hazleman BL. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology 2005;44(6):800-805. doi:10.1093/ rheumatology/keh598
2. Neer CS. Impingement lesions. Clin Orthop Relat Res 1983;173:70-77 doi:10.1097/ 00003086-198303000-00010
3. Cools AM, Michener LA. Shoulder pain: can one label satisfy everyone and everything? Br J Sports Med 2017;51(5):416-417 doi:10.1136/ bjsports-2016-096772
4. Ludewig PM, Kamonseki DH, Staker JL, Lawrence RL, Camargo PR, Braman JP Changing our diagnostic paradigm: movement system diagnostic classification. Int J Sports Phys Ther 2017;12(6):884-893. doi:10.26603/ijspt20170884
5. Timmons MK, Thigpen CA, Seitz AL, Karduna AR, Arnold BL, Michener LA. Scapular kinematics and subacromial-impingement syndrome: a metaanalysis. J Sport Rehabil. 2012;21(4):354-370. doi:10.1123/jsr.21.4.354
6. Keshavarz R, Bashardoust Tajali S, Mir SM, Ashrafi H. The role of scapular kinematics in patients with different shoulder musculoskeletal disorders: A systematic review approach. J Bodyw Mov Ther 2017;21(2):386-400. doi:10.1016/j.jbmt.2016.09.002
7. Chester R, Smith TO, Hooper L, Dixon J. The impact of subacromial impingement syndrome on muscle activity patterns of the shoulder complex: a systematic review of electromyographic studies. BMC Musculoskelet Disord 2010;11:45. doi:10.1186/ 1471-2474-11-45
8. Struyf F, Cagnie B, Cools A, et al. Scapulothoracic muscle activity and recruitment timing in patients with shoulder impingement symptoms and glenohumeral instability. J Electromyogr Kinesiol. 2014;24(2):277-284. doi:10.1016/j.jelekin.2013.12.002
9. de Oliveira FCL, Bouyer LJ, Ager AL, Roy JS. Electromyographic analysis of rotator cuff muscles in patients with rotator cuff tendinopathy: A systematic review J Electromyogr Kinesiol 2017;35:100-114. doi:10.1016/j.jelekin.2017.06.002
10. Horsley I, Herrington L, Hoyle R, Prescott E, Bellamy N. Do changes in hand grip strength correlate with shoulder rotator cuff function? Shoulder Elbow 2016;8(2):124-129. doi:10.1177/ 1758573215626103
11. Land H, Gordon S, Watt K. Isokinetic clinical assessment of rotator cuff strength in subacromial shoulder impingement. Musculoskelet Sci Pract. 2017;27:32-39. doi:10.1016/j.msksp.2016.11.012
12. AlAnazi A, Alghadir AH, Gabr SA. Handgrip strength exercises modulate shoulder pain, function, and strength of rotator cuff muscles of patients with primary subacromial impingement syndrome. Biomed Res Int. 2022;30:9151831. doi:10.1155/2022/9151831
13. Lee B, McGill S. The effect of core training on distal limb performance during ballistic strike manoeuvres. J Sports Sci. 2017;35(18):1-13. doi:10.1080/02640414.2017.1313441
14. Dong W, Goost H, Lin XB, et al. Treatments for shoulder impingement syndrome: a PRISMA systematic review and network meta-analysis. Medicine 2015;94(10):e510. doi:10.1097/ MD.0000000000000510
15. Gutiérrez-Espinoza H, Araya-Quintanilla F, Cereceda-Muriel C, Álvarez-Bueno C, MartínezVizcaíno V, Cavero-Redondo I. Effect of supervised physiotherapy versus home exercise program in patients with subacromial impingement syndrome: A systematic review and meta-analysis. Phys Ther Sport. 2020;41:34-42. doi:10.1016/j.ptsp.2019.11.003
16. Briggs MS, Givens DL, Best TM, Chaudhari AM. Lumbopelvic neuromuscular training and injury rehabilitation: a systematic review. Clin J Sport Med. 2013;23(3):160-171. doi:10.1097/ JSM.0b013e318280aabb
17. Jha P, Nuhmani S, Kapoor G, et al. Efficacy of core stability training on upper extremity performance in collegiate athletes. J Musculoskelet Neuronal Interact. 2022;22(4):498-503.
18. Lupowitz LG. Comprehensive approach to core training in sports physical therapy: Optimizing performance and minimizing injuries. Int J Sports Phys Ther 2023;18(4):800-806. doi:10.26603/ 001c.84525
19. Silfies SP, Ebaugh D, Pontillo M, Butowicz CM. Critical review of the impact of core stability on upper extremity athletic injury and performance. Braz J Phys Ther. 2015;19(5):360-368. doi:10.1590/ bjpt-rbf.2014.0108
20. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. doi:10.1016/ j.ijsu.2014.07.013
21. Michener LA, Walsworth MK, Doukas WC, Murphy KP. Reliability and diagnostic accuracy of 5 physical examination tests and combination of tests for subacromial impingement. Arch Phys Med Rehabil. 2009;90(11):1898-1903. doi:10.1016/ j.apmr.2009.05.015
22. Klintberg IH, Cools AM, Holmgren TM, et al. Consensus for physiotherapy for shoulder pain. Int Orthop 2015;39(4):715-720. doi:10.1007/ s00264-014-2639-9
23. Gutiérrez Espinoza H, Araya-Quintanilla F, PintoConcha S, Valenzuela-Fuenzalida J, López-Gil JF, Ramírez-Velez R. Specific versus general exercise programme in adults with subacromial impingement syndrome: a randomised controlled trial. BMJ Open Sport Exerc Med. 2023;9(3):e001646. doi:10.1136/ bmjsem-2023-001646
24. Rodríguez-Perea Á, Reyes-Ferrada W, JerezMayorga D, et al. Core training and performance: a systematic review with meta-analysis. Biol Sport. 2023;40(4):975-992. doi:10.5114/ biolsport.2023.123319
25. Jeffreys I. Developing a progressive core stability program. Strength Condit J 2002;24(5):65-66. doi:10.1519/00126548-200210000-00017
26. Tucci HT, Martins J, Sposito G de C, Camarini PM, de Oliveira AS. Closed Kinetic Chain Upper Extremity Stability test (CKCUES test): a reliability study in persons with and without shoulder impingement syndrome. BMC Musculoskelet Disord 2014;15:1. doi:10.1186/1471-2474-15-1
27. Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder Clin Orthop 1987;214:160-164.
28. McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review Psychol Med 1988;18(4):1007-1019. doi:10.1017/S0033291700009934
29. Fritz CO, Morris PE, Richler JJ. Effect size estimates: Current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2-18. doi:10.1037/a0024338
30. Mısırlıoğlu TÖ, Eren İ, Canbulat N, Çobanoğlu E, Günerbüyük C, Demirhan M. Does a core stabilization exercise program have a role on shoulder rehabilitation? A comparative study in young females. Turk J Phys Med Rehabil 2018;64(4):328-336. doi:10.5606/tftrd.2018.1418
31. Kibler WB, Ludewig PM, McClure PW, Michener LA, Bak K, Sciascia AD. Clinical implications of scapular dyskinesis in shoulder injury: the 2013 consensus statement from the “Scapular Summit.” Br J Sports Med 2013;47(14):877-885. doi:10.1136/ bjsports-2013-092425
32. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337 doi:10.1177/ 03635465980260022801
33. Manzi JE, Dowling B, Dines JS, Richardson A, McElheny KL, Carr JB 2nd. Increased shoulder distraction force and shoulder horizontal abduction in professional baseball pitchers with discordant torso rotation Order Am J Sports Med 2021;49(13):3638-3646. doi:10.1177/ 03635465211041381
34. Méndez-Rebolledo G, Cools AM, RamírezCampillo R, Quiroz-Aldea E, Habechian FAP Association between lower trapezius isometric strength and Y-balance test upper quarter performance in college volleyball players. J Sport Rehabil 2022;31(2):140-145. doi:10.1123/ jsr.2021-0048
35. Vega Toro AS, Cools AM, de Oliveira AS. Instruction and feedback for conscious contraction of the abdominal muscles increases the scapular muscles activation during shoulder exercises. Man Ther 2016;25:11-18. doi:10.1016/j.math.2016.05.331
36. Hodges PW, Richardson CA. Feedforward contraction of transversus abdominis is not influenced by the direction of arm movement. Exp Brain Res 1997;114(2):362-370. doi:10.1007/ PL00005644
37. Huxel Bliven KC, Anderson BE. Core stability training for injury prevention. Sports Health 2013;5(6):514-522. doi:10.1177/1941738113481200
38. Alizadehkhaiyat O, Fisher AC, Kemp GJ, Vishwanathan K, Frostick SP Shoulder muscle activation and fatigue during a controlled forceful hand grip task. J Electromyogr Kinesiol. 2011;21(3):478-482. doi:10.1016/j.jelekin.2011.03.002
39. Hodges PW, Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain. 2011;152(3 Suppl):S90-S98. doi:10.1016/ j.pain.2010.10.020
40. Bachasson D, Singh A, Shah SB, Lane JG, Ward SR. The role of the peripheral and central nervous systems in rotator cuff disease. J Shoulder Elbow Surg 2015;24(8):1322-1335. doi:10.1016/j.jse.2015.04.004
Download: https://ijspt.scholasticahq.com/article/128630-the-effect-of-the-addition-of-core-exercises-to-supervisedphysiotherapy-in-patients-with-subacromial-impingement-syndrome/attachment/ 262721.docx?auth_token=UDp1dah3W8KmADWG23n0

Bennett Tabaracci1 , Shraddha Sudhir2 , Matthew Gauthier2 , Lindsay Hannigan2a
1 Physical Therapy, University of Southern California, 2 Physical Therapy, University of Illinois at Chicago
Keywords: pitch count, wearables, elbow, injury https://doi.org/10.26603/001c.128051
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background
Upper extremity injuries are common in baseball spanning from youth through professional leagues, especially in preseason. Although there are some arbitrary guidelines for number of throws during practices and games, there is no current information on workload during preseason in baseball pitchers.
Hypothesis/Purpose
The purpose of this study was to quantify the number of throws and workload, as defined by angular velocity, during preseason training in a collegiate baseball season.
Study Design
Descriptive Epidemiology Study
Methods
Nine baseball pitchers wore an inertial measurement unit on the forearm during all preseason training. Movements were captured at 100Hz and classified as a throw when the forearm velocity was greater than 800°/second. Peak angular velocity was exported for each throw and total workload was calculated as the median angular velocity multiplied by total throws for each day Chronic workload was calculated as the rolling 28 days average workload and acute workload was calculated as the average seven-day workload. Acute to chronic workload ratio (ACWR) was calculated for each week. A repeated measures ANOVA with pairwise comparisons was used to compare throws, acute workload, and ACWR between weeks. Cohen’s d effect sizes were calculated for all significant differences.
Results
The pitchers averaged 1990.6 ± 881.7 throws throughout preseason at an average angular velocity of 1686.2 ± 334.9 m/s. Acute workload was reduced in Week 4 compared to Week 2 (p=0.018, d=1.73) and week 3 (p=0.007, d =2.30). ACWR was above 1.27 on weeks 1,2,3, and 5. ACWR was significantly reduced in week 4 (0.79) compared to week 3 (1.50; p=0.021, d =0.71).
Conclusion
ACWR was above 1.27 for four of the six weeks of preseason, suggesting that there may be a need to reduce workload and progressively build during the preseason. Clinicians should consider monitoring workload during preseason throwing to decrease risk of chronic overuse injuries.
a
Corresponding Address:
Lindsay Hannigan
1640 W. Roosevelt Road Chicago, IL 60608
Email: slaterlv@uic.edu
Office Phone: (312) 355-8965
Upper extremity injuries are common in competitive baseball and account for up to 49-58% of all reported injuries in collegiate baseball and Major League Baseball (MLB) with upper extremity injuries accounting for 75% of total time lost from the sport.1,2 Injury rates are not uniform across the baseball season with the highest number of injuries occurring during the start of season.3,4 In the MLB, the highest injury rate was during April (the first month of seasonal play) and declined each month with the lowest injury rate in September (the final month of play).3 High rate of preseason injuries are not unique to baseball, with many other sports, such as soccer and football, demonstrating increased risk for overuse injuries during the beginning of season compared to the remainder of the season.5,6 Most research in the preseason focuses on the use of assessments to identify those who are most likely to become injured, however there is little information regarding training loads during the preseason that may lead to overuse injuries early in the season. Increased preseason training load is particularly important for baseball pitchers, who experience the highest rate of injuries compared to other position players.7,8 Pitchers are uniquely at risk for elbow and shoulder injuries due to the high demand of the position, requiring angular velocities at the elbow greater than 2000 degrees/ second and maximum shoulder external rotations over 150 degrees.9
A main theory associated with the high rate of injuries in pitchers is the high number of throws during training throughout their career As an early specialization sport, many youth baseball pitchers solely train for baseball yearround, increasing the stress at the upper extremity without proper recovery.10 The relationship between increased number of throws and injury risk in youth and high school players has led to some preliminary guidelines regarding the number of throws a player should complete over the course of a game and season. The MLB published guidelines for daily maximums for pitch counts in youth to collegiate baseball, suggesting that collegiate pitchers should not throw more than 120 daily pitches and require five days of recovery when throwing more than 106 pitches.11 These data are the aggregate of pitch counts; however pitch counts often underestimate the total number of throws.12 Pitchers are likely surpassing these thresholds regularly, especially during times when exact pitch counts are not monitored, such as preseason training.
The most common method of measuring workload is through the pen and paper method and counting each individual throw which is cumbersome and requires either accurate recall or immediate attention to tally the throw 13‑15 Another method that is often used to quantify workload is the use of ball velocity 16,17 Ball velocity, particularly during fastballs, increases over consecutive games17 and players with higher ball velocity have increased risk of elbow injury 16 Despite these relationships between increased ball
velocity and injury, decreasing ball velocity would be counterproductive to performance and therefore it is not feasible to reduce ball velocity as a method of decreasing training load. In contrast to objective measures, rating of perceived exertion has also been suggested as a proxy for workload,18 however, rating of perceived exertion measures more internal workload rather than an external workload and may not accurately represent effort.19
Wearables are becoming the most popular method of measuring workload in many sports, especially in traditional running sports such as football and soccer.2,20,21 Football and soccer often use global positioning systems to quantify distance covered during training and games as a proxy for workload.22,23 More recently, inertial measurement units (IMUs) have been used to identify kinematic patterns in baseball to quantify throwing workload.24,25 Kinematics include a variety of measures including arm slot, angular velocity, arm stress, and shoulder rotation, however the most accurate measure is angular velocity 26, 27 Furthermore, the forearm is the most accurate place for sensors in baseball, rather than the proximal arm closer to the shoulder 28 Therefore, the authors quantified workload as angular velocity from throws through a single IMU on the forearm. Using forearm angular velocity, the purpose of this study was to quantify the number of throws and workload, as defined by angular velocity, during preseason training in a collegiate baseball season.
Nine NCAA Division I baseball pitchers from the same college program volunteered to participate in this study The recruited participants were from a sample of convenience from the same college program in the Chicagoland area. All participants were free of injury during study participation which limited the number of included participants. The University’s Institutional Review Board for biological research approved all procedures and participants provided written informed consent prior to participation.
An observational study was conducted to collect all data during practices in real time on a baseball field during preseason over six weeks. Pitching data were captured using a single IMU (MBIENTLAB, MetaMotionRL) placed approximately 1-3 inches proximal to the throwing wrist of the pitcher. All movements were captured at 100Hz and the forearm detection threshold was set at 800°/second, meaning that any movement was classified as a throw if the forearm velocity was greater than 800°/second.29 This forearm velocity threshold was set to include all throws during practice including low intensity throws (classified as a toss with lower angular velocity) while excluding warmup plyomet-
rics and other exercises when the arm movement was below the threshold.29 The angular velocity threshold of 800°/second was confirmed as an appropriate threshold during pilot testing in the fall season to identify all throwing behaviors during training, including short distance tosses and pitches from the mound.
All sensors along with their charging stations were distributed to the pitchers. All participants were instructed on sensor placement, sensor calibration, and sensor charging. At the beginning of practice, participants connected their sensor to their personal profile in the 4D Motion Sports application on an iOS device. After calibrating the sensor, they placed it in the sleeve on an arm band where it remained secured throughout the session. At the end of each practice session, the sensors were returned to their charging docks after ending the data collection module. The participants were responsible for connecting and wearing the device during practice. During preseason training, there were no instances of participants throwing outside of scheduled practice. Raw data were exported through the commercially available mobile application (4D Motion Sports, Inc.) and analyzed using MATLAB.
Total number of throws for each pitcher for each day pitched was recorded as the number of times the angular velocity threshold (800°/second) was met. Peak angular velocity was extracted for each throw because it is the most consistent variable using the IMU.27 Total workload for each day was calculated as the median peak angular velocity for each day multiplied by the total number of throws for the day and divided by 100,000 so that the acute workloads ranged from 0-5, similar to previous measures of workload.30‑32 Each player’s acute workload was calculated as the average workload for each seven-day period and chronic workload was calculated as the rolling workload across the previous 28 days.33 An acute to chronic workload ratio (ACWR) was calculated for each week and each player for an overall ACWR during each week of preseason.
Average of the peak angular velocity for each week, total number of throws pitched during each week, and ACWR for each week were summarized as means and standard deviations for each pitcher The group average of acute workload and ACWR for each week of preseason was calculated to compare weekly workload progression. A repeated measures ANOVA with pairwise comparisons was used to compare workload between weeks. As an observational study with a sample of convenience, power was not calculated, however, the number of participants is similar to previous studies.27,34 Cohen’s d effect sizes with 95% confidence intervals were calculated for all significant differences and were interpreted as weak (<0.21), small (0.21-0.39), medium (0.4-0.7), or large (>0.7).35 Statistical significance was set a priori at p < 0.05. All statistical analyses were performed in SPSS (version 29, IBM, Armonk, NY).
The nine included participants were 20.89±1.69 years of age, 1.88±4.66 meters tall, weighed 91.41±8.56 kg, and had 2.44±1.59 years of collegiate experience. The pitchers in the current study averaged 1990.6±881.7 throws throughout preseason at an average angular velocity of 1686.2±334.9 m/s (Table 1). The average number of throws each week ranged from 216-487 during preseason, with the greatest number of throws occurring during week 5 (Figure 1). Acute workload was reduced in Week 4 compared to Week 2 (p=0.018, d=1.73) and Week 3 (p=0.007, d =2.30) (Figure 2). Week 4 ACWR was also reduced in compared to Week 3 (p=0.021, d =0.71). Weeks 1, 2, 3, and 5 of preseason had an ACWR above 1.27 (Figure 3).
The purpose of the current study was to quantify workload in collegiate baseball pitchers during preseason training. The pitchers in this study had a variety of weekly throws, ranging from as few as 51 throws in a week to more than 700 weekly throws. ACWR was significantly greater on Week 3 of preseason in comparison to Week 4, with a large effect size. Acute workload was significantly greater on Weeks 2 and 3 compared to Week 4 with a very large effect size. This suggests that workload was high throughout the sixweek preseason with only one deload week (Week 4). The decreased ACWR in Week 4 was not due to decreased number of throws (Figure 1), however was likely the result of a decreased angular velocities during Week 4 (Figure 2). Participation in preseason training is associated with decreased injury risk36 and increased in-season aerobic performance37 and coaches consider preseason as an avenue to increase fitness as quickly as possible to prepare athletes for in-season performance, however increases during preseason need to be progressive to improve physical abilities, technical skills, and endurance for seasonal play In baseball, the seasonal demand for pitchers largely depends on pitching role, with starting pitchers throwing more than relievers.38 Pitchers who throw more during season have higher chronic workloads and need to be exposed to increasing workloads, especially during preseason, to allow for physiologic adaptations to the high workload demands of the position and reduce injury risk. When the acute workload increases too much (over a seven-day period) in comparison to chronic workload, injury risk increases.32 This is particularly evident for pitchers with an ACWR greater than 1.27, which the pitchers in this study surpassed during four of the six weeks in preseason.39 Further, significant changes in ACWR is associated with risk of upper extremity injury in collegiate baseball pitchers.40 This supports that many of the pitchers were at increased risk for injury during the end of preseason and the beginning of season play
Preseason injuries during practice across all collegiate sports are higher than during in-season practices.41 This has led to more research regarding workload monitoring during preseason training to better understand how to
Table 1. Total number of throws and mean angular velocity of all throws for each participant (ID) during each week of preseason.
-Indicates that a pitcher did not log any throws in that week.

Figure 1. Average total throws with standard deviations for all nine players during each week of preseason training. There were no significant differences in total throws between weeks.
progress training to best prepare for the season. Researchers in soccer have consistently reported increased training intensity during preseason compared to in-season.42,43 Greater internal and external loading during preseason has led to larger conversations regarding designing training programs during preseason through progressive overload.33 Progressive overload principles in training suggests a gradual and systematic increase in the workload over time exposes the muscles and tissues to increasingly greater loads without surpassing load capacity. Progressive overload requires three concepts: the floor, ceiling, and time33 and coaches may need to consider all three when designing the preseason training plans for individual pitchers. The floor represents an athlete’s current capacity, and the ceiling is the required capacity for the position. Ideally, there is little difference between the two, however, when there are larger differences, the athlete needs time to progressively increase workload to reach and maintain the ceiling. These concepts have successfully been applied in other sports, such as rugby, using technology to monitor workload and progressively increase the workload over the preseason period.44 Current baseball progressions include a variety of throwing quantities and distances and often have ACWR spikes above the safe range.45 Recently, there has been some evidence to suggest that IMUs can assist with baseball throwing progressions during season,25 however more research is needed to define successful guidelines for preseason and in-season training in pitchers and maintain safe workload demands.
The calculation of ACWR in the current study followed previously described concepts and calculations of workload, however utilized angular velocity to define throwing intensity rather than ball velocity or valgus load.16,39 Angular velocity is the most consistent variable in the 4D Motion
sensors27 and provides a unique measure of upper extremity demand during throwing without estimating the exact valgus at the elbow through kinematic measures.26 Larger angular velocities at the elbow are associated with higher intensity throws and lead to increased stress.46 Through calculating workload as the median angular velocity for the training day multiplied by the total number of throws, we were able to account for both the volume and intensity of throws for the day Utilizing angular velocity may provide a framework for future preseason training protocols to optimize training without surpassing the ACWR threshold for safe workload.
There were some limitations in the current study The IMU captured most throws, however, there were instances when participants completed a throw that was not recorded through the capture algorithm. Although these instances were rare, there were still some throws that may not have been accounted for in the overall workload. The authors took this into account in the calculation of ACWR by utilizing the median angular velocity for daily intensity rather than the mean, which could be skewed based on outliers. Therefore, the authors do not expect the results to shift significantly from the few dropped throws in the dataset. There was not complete adherence from all participants in the study, with a few not wearing their sensors during all sessions in preseason training. Despite these missing data, the authors were still able to capture thousands of throws during the six-week period. Rest days were not monitored; however, this would be taken into account in the acute workload calculation. The study utilized a small sample of participants, which may impact generalizability of these data. In the future, the authors plan to include more participants to account for missing data from lack of adherence. Lastly, the results from this small sample of collegiate
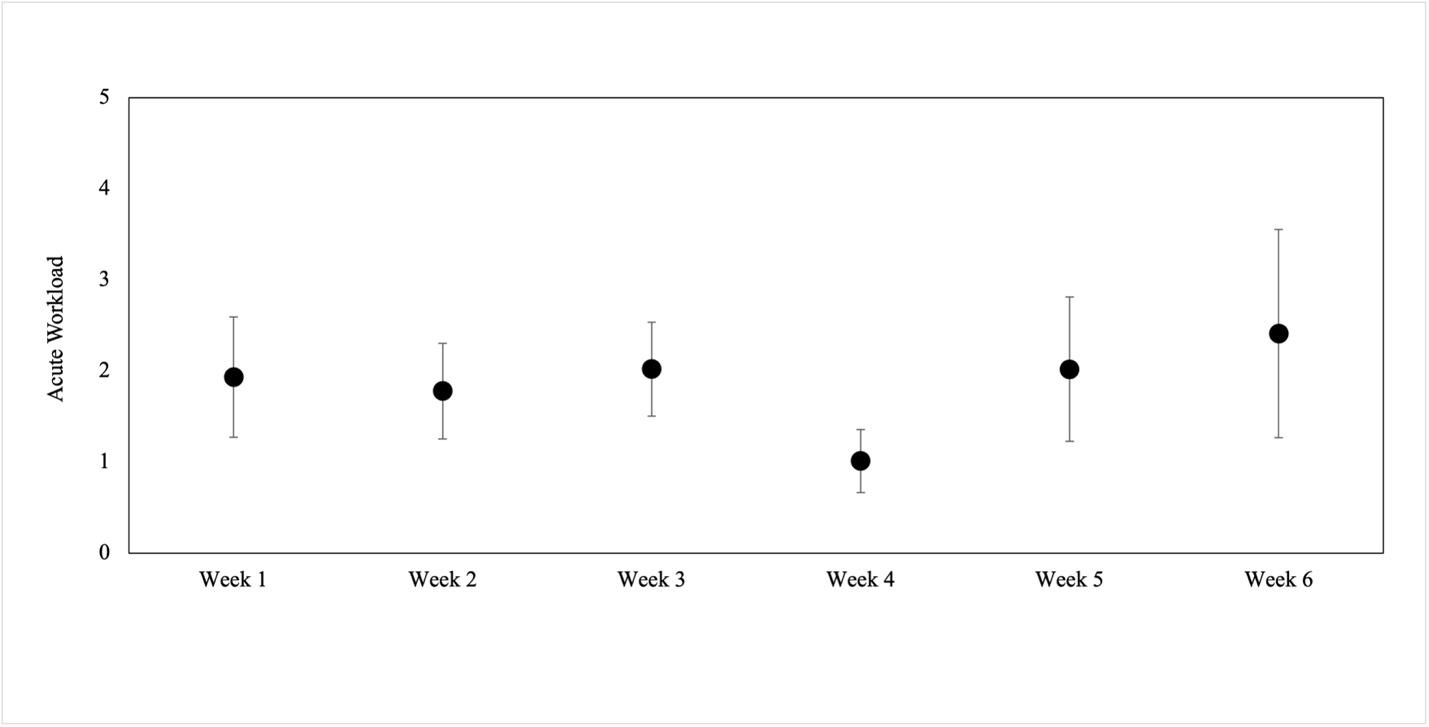
Figure 2. Acute workload, defined as the average workload for the 7-day period, for all nine players during each week of preseason. Acute workload was calculated as the average workload (median angular velocity multiplied by total daily throws) for the 7-day period. Acute workload was reduced in week 4 compared to week 2 (p=0.018, d=1.73) and week 3 (p=0.007, d =2.30).

Figure 3. Acute to chronic workload ratio (ACWR) mean and standard deviation for each week of preseason. Acute workload was calculated as the average workload for the 7-day period and chronic workload was calculated as the average workload for the entire 6-week preseason. ACWR greater than 1.0 indicates that ACWR was greater during the week in comparison to the entire preseason while ACWR less than 1.0 indicates that workload was reduced during the week. There was a significant difference between week 3 and week 4 (p=0.021, d =0.71).
baseball pitchers may not be applicable to other baseball levels (e.g., high school and professional). Future studies should explore changes in angular velocities in other baseball levels and larger, multiteam studies.
ACWR was above the 1.27 threshold during four of the six weeks of preseason, suggesting that there may be a need to
reduce overload over the course of the collegiate preseason in baseball. Increased workload over a short time may be associated with greater upper extremity injuries during preseason in collegiate baseball. Monitoring throwing workload using peak angular velocity through a single IMU may provide valuable insight to the sports performance team to prevent chronic overuse injury Reducing ACWR below 1.27 throughout preseason and season play in throwing sports may reduce injuries. Additionally, this methodology can be used to design progressive overload training during the preseason.
The authors have no conflict of interests to disclose.
Submitted: June 12, 2024 CST Accepted: November 22, 2024 CST. Published: February 01, 2025 CST.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Wasserman EB, Sauers EL, Register-Mihalik JK, et al. The first decade of web-based sports injury surveillance: Descriptive epidemiology of injuries in US high school boys’ baseball (2005-2006 Through 2013-2014) and National Collegiate Athletic Association men’s baseball (2004-2005 Through 2013-2014). J Athl Train 2019;54(2):198-211. doi:10.4085/1062-6050-239-17
2. Benson LC, Raisanen AM, Volkova VG, Pasanen K, Emery CA. Workload a-WEAR-ness: Monitoring workload in team sports with wearable technology A scoping review. J Orthop Sports Phys Ther. 2020;50(10):549-563. doi:10.2519/jospt.2020.9753
3. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med 2011;39(8):1676-1680. doi:10.1177/0363546511411700
4. Dick R, Sauers EL, Agel J, et al. Descriptive epidemiology of collegiate men’s baseball injuries: National Collegiate Athletic Association injury surveillance system, 1988-1989 through 2003-2004. J Athl Train 2007;42(2):183-193.
5. Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football-analysis of preseason injuries. Br J Sports Med 2002;36(6):436-441. doi:10.1136/bjsm.36.6.436
6. Ready LV, Li NY, Worobey S, et al. Influence of preseason versus in-season play on Achilles tendon injuries in the National Football League. Orthop J Sports Med 2021;9(12):23259671211056083. doi:10.1177/23259671211056083
7. Carr JB 2nd, McElheny KD, Corrigan A, Rowe D, Ma K, Curriero FC. The most common type, severity, and expected frequency of injuries vary by defensive position in professional baseball players. Am J Sports Med 2022;50(9):2534-2541. doi:10.1177/ 03635465221104490
8. Shanley E, Rauh MJ, Michener LA, Ellenbecker TS. Incidence of injuries in high school softball and baseball players. J Athl Train 2011;46(6):648-654. doi:10.4085/1062-6050-46.6.648
9. Werner SL, Suri M, Guido JA Jr, Meister K, Jones DG. Relationships between ball velocity and throwing mechanics in collegiate baseball pitchers. J Shoulder Elbow Surg. 2008;17(6):905-908. doi:10.1016/ j.jse.2008.04.002
10. Fleisig GS, Andrews JR, Cutter GR, et al. Risk of serious injury for young baseball pitchers: a 10-year prospective study. Am J Sports Med. 2011;39(2):253-257 doi:10.1177/0363546510384224
11. Guidelines for youth and adolescent pitchers. Major League Baseball. https://www.mlb.com/pitchsmart/pitching-guidelines
12. Wahl EP, Pidgeon TS, Richard MJ. Youth baseball pitch counts vastly underestimate high-effort throws throughout a season. J Pediatr Orthop 2020;40(7):e609-e615. doi:10.1097/ BPO.0000000000001520
13. Karakolis T, Bhan S, Crotin RL. An inferential and descriptive statistical examination of the relationship between cumulative work metrics and injury in Major League Baseball pitchers. J Strength Cond Res 2013;27(8):2113-2118. doi:10.1519/ JSC.0b013e3182785059
14. Lyman S, Fleisig GS, Andrews JR, Osinski ED. Effect of pitch type, pitch count, and pitching mechanics on risk of elbow and shoulder pain in youth baseball pitchers. Am J Sports Med 2002;30(4):463-468. doi:10.1177/ 03635465020300040201
15. Olsen SJ 2nd, Fleisig GS, Dun S, Loftice J, Andrews JR. Risk factors for shoulder and elbow injuries in adolescent baseball pitchers. Am J Sports Med. 2006;34(6):905-912. doi:10.1177/ 0363546505284188
16. Bushnell BD, Anz AW, Noonan TJ, Torry MR, Hawkins RJ. Association of maximum pitch velocity and elbow injury in professional baseball pitchers. Am J Sports Med. 2010;38(4):728-732. doi:10.1177/ 0363546509350067
17 Crotin RL, Bhan S, Karakolis T, Ramsey DK. Fastball velocity trends in short-season minor league baseball. J Strength Cond Res. 2013;27(8):2206-2212. doi:10.1519/JSC.0b013e31827e1509
18. Pexa B, Ryan ED, Blackburn JT, Padua DA, Garrison JC, Myers JB. Influence of baseball training load on clinical reach tests and grip strength in collegiate baseball players. J Athl Train 2020;55(9):984-993. doi:10.4085/1062-6050-0456.19
19. Melugin HP, Larson DR, Fleisig GS, et al. Baseball pitchers’ perceived effort does not match actual measured effort during a structured long-toss throwing program. Am J Sports Med. 2019;47(8):1949-1954. doi:10.1177/ 0363546519850560
20. Jennings D, Cormack S, Coutts AJ, Boyd L, Aughey RJ. The validity and reliability of GPS units for measuring distance in team sport specific running patterns. Int J Sports Physiol Perform. 2010;5(3):328-341. doi:10.1123/ijspp.5.3.328
21. Coutts AJ, Duffield R. Validity and reliability of GPS devices for measuring movement demands of team sports. J Sci Med Sport. 2010;13(1):133-135. doi:10.1016/j.jsams.2008.09.015
22. Di Salvo V, Baron R, Gonzalez-Haro C, Gormasz C, Pigozzi F, Bachi N. Sprinting analysis of elite soccer players during Eurpoean Champions League and UEFA Cup matches. J Sports Sci 2010;28(14):1489-1494. doi:10.1080/ 02640414.2010.521166
23. Slater LV, Baker R, Weltman AL, Hertel J, Saliba SA, Hart JM. Activity monitoring in men’s college soccer: a single season longitudinal study Res Sports Med 2018;26(2):178-190. doi:10.1080/ 15438627.2018.1431535
24. Freehill MT, Rose MJ, McCollum KA, Agresta C, Cain SM. Game-day pitch and throw count feasibility using a single sensor to quantify workload in youth baseball players. Orthop J Sports Med 2023;11(3):23259671231151450. doi:10.1177/ 23259671231151450
25. Reinold MM, Dowling B, Fleisig GS, et al. An interval throwing program for baseball pitchers based upon workload data. Int J Sports Phys Ther. 2024;19(3):326-336. doi:10.26603/001c.94146
26. Camp CL, Loushin S, Nezlek S, Fiegen AP, Christoffer D, Kaufman K. Are wearable sensors valid and reliable for studying the baseball pitching motion? An independent comparison with markerbased motion capture. Am J Sports Med. 2021;49(11):3094-3101. doi:10.1177/ 03635465211029017
27. Loushin SR, Verhoeven M, Christoffer DJ, Camp CL, Kaufman KR. Are 4D motion sensors valid and reliable for studying baseball pitching? Am J Sports Med. 2023;51(6):1608-1614. doi:10.1177/ 03635465231166423
28. Agresta C, Freehill MT, Zendler J, Giblin G, Cain S. Sensor location matters when estimating player workload for baseball pitching. Sensors 2022;22(22):9008. doi:10.3390/s22229008
29. Rose MJ, McCollum KA, Freehill MT, Cain SM. Quantifying throw counts and intensities throughout a season in youth baseball players: A pilot study J Biomech Eng. 2021;143(3). doi:10.1115/1.4049025
30. Malone S, Owen A, Newton M, Mendes B, Collins KD, Gabbett TJ. The acute:chronic workload ratio in relation to injury risk in professional soccer J Sci Med Sport. 2017;20(6):561-565. doi:10.1016/ j.jsams.2016.10.014
31. Schumann C, Wojciechowski M, Bunn JA. Comparing Two Methods of Acute: Chronic Workload Calculations in Girls’ Youth Volleyball. Sports 2023;11(3). doi:10.3390/sports11030051
32. Mehta S, Tang S, Rajapakse C, Juzwak S, Dowling B. Chronic workload, subjective arm health, and throwing injury in high school baseball players: 3-year retrospective pilot study. Sports Health. 2022;14(1):119-126. doi:10.1177/19417381211055142
33. Gabbett TJ. How much? How fast? How soon? Three simple concepts for progressing training loads to minimize injury risk and enhance performance. J Orthop Sports Phys Ther 2020;50(10):570-573. doi:10.2519/jospt.2020.9256
34. Boddy KJ, Marsh JA, Caravan A, Lindley KE, Scheffey JO, O’Connell ME. Exploring wearable sensors as an alternative to marker-based motion capture in the pitching delivery PeerJ 2019;7:e6365. doi:10.7717/peerj.6365
35. Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 2009;41(1):3-13. doi:10.1249/MSS.0b013e31818cb278
36. Windt J, Gabbett TJ, Ferris D, Khan KM. Training load--injury paradox: is greater preseason participation associated with lower in-season injury risk in elite rugby league players? Br J Sports Med 2017;51(8):645-650. doi:10.1136/ bjsports-2016-095973
37. Jaspers A, Brink MS, Probst SGM, Frencken WGP, Helsen WF. Relationships between training load indicators and training outcomes in professional soccer Sports Medicine 2017;47(3):533-544. doi:10.1007/s40279-016-0591-0
38. Love S, Aytar A, Bush H, Uhl TL. Descriptive analysis of pitch volume in southeastern conference baseball pitchers. N Am J Sports Phys Ther 2010;5(4):194-200.
39. Mehta S. Relationship between workload and throwing injury in varsity baseball players. Phys Ther Sport 2019;40:66-70. doi:10.1016/j.ptsp.2019.08.001 Preseason
40. Slowik R, Morris C, Hoch M, Uhl T Identifying risk factors of upper extremity injuries in collegiate baseball players: A pilot study Int J Sports Phys Ther 2021;16(3):797-806. doi:10.26603/001c.24146
41. Agel J, Schisel J. Practice injury rates in collegiate sports. Clin J Sport Med 2013;23(1):33-38. doi:10.1097/JSM.0b013e3182717983
42. Lago-Fuentes C, Jimenez-Loaisa A, Padron-Cabo A, et al. Monitoring workloads of a professional female futsal team over a season: A case study Sports. 2020;8(5). doi:10.3390/sports8050069
43. Nobari H, Ramachandran AK, Brito JP, Oliveira R. Quantification of pre-season and in-season training intensity across an entire competitive season of Asian professional soccer players. Healthcare 2022;10(8). doi:10.3390/healthcare10081367
44. Fairbank M, Highton J, Daniels M, Twist C. The content and load of preseason field-based training in a championship-winning professional rugby league team: A case study. Int J Sports Sci Coaching. 2022;17(6):1445-1454. doi:10.1177/ 17479541211064872
45. Dowling B, Brusalis CM, Streepy JT, et al. Workload comparison of contemporary interval throwing programs and a novel optimized program for baseball pitchers. Int J Sports Phys Ther. 2024;19(2):176-188. doi:10.26603/001c.92016
46. Urbin MA, Fleisig GS, Abebe A, Andrews JR. Associations between timing in the baseball pitch and shoulder kinetics, elbow kinetics, and ball speed. Am J Sports Med 2013;41(2):336-342. doi:10.1177/ 0363546512467952

Stackhouse SK, Eckenrode BJ, Madara KC. The Effects of Noxious Electrical Stimulation and Eccentric Exercise on Mechanical and Thermal Pain Sensitivity in Recreational Runners with Achilles Tendinopathy. IJSPT. 2025;20(2):231-242.
doi:10.26603/001c.128155
1a
Scott K. Stackhouse
, Brian J. Eckenrode
2
, Kathleen C. Madara3
1 Physical Therapy, University of New England, 2 Physical Therapy, Arcadia University, 3 Department of Rehabilitation Sciences, Moravian University
Keywords: Achilles tendinopathy, eccentric exercise, noxious electrical stimulation, quantitative sensory testing, chronic pain https://doi.org/10.26603/001c.128155
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background
Achilles tendinopathy is a common overuse condition that can become persistent despite conservative treatment. Sensitization of both the peripheral and central nervous systems may contribute to the persistent pain. Both exercise and electrical stimulation have the potential to modulate the nervous system’s sensitivity to painful stimuli.
Hypothesis/Purpose
The purpose of this study was to describe the changes in pain sensitivity and self-reported function in runners with chronic Achilles tendon pain following sequential treatment with noxious electrical stimulation (NxES) and eccentric plantarflexion exercise.
Study Design
Single group, repeated measures design.
Methods
Sixteen participants with chronic Achilles tendinopathy completed the Lower Extremity Functional Scale (LEFS) and the Victorian Institute of Sport Assessment-Achilles scale (VISA-A) and quantitative sensory tests (pressure pain threshold, heat temporal summation, and heat pain threshold) at baseline, one week, seven weeks, and then at a one month post intervention follow-up. The NxES was applied for one week, then followed by plantarflexion eccentric exercise for six weeks. Changes across timepoints were assessed using repeated measures ANOVA and post hoc analysis to describe differences. Hedges g effect sizes were also calculated.
Results
There was a significant improvement in LEFS (p < 0.001) and VISA-A (p < 0.001) from baseline to one month follow-up, with a mean change of 9.6 ± 7.7 and 19.4 ± 17.7 points respectively Pressure pain threshold of the involved Achilles tendon increased over time (p < 0.001) with significant improvements after NxES application (p = 0.002) and after six weeks of eccentric exercise (p < 0.001). There were significant improvements from baseline to one month follow-up for heat temporal summation (p = 0.001) and heat pain threshold ( p < 0.001).
Corresponding Author: Scott Stackhouse PT, PhD University of New England, Department of Physical Therapy 716 Stevens Avenue Portland, ME 04103 sstackhouse@une.edu (207) 221-4581 a
For individuals with chronic Achilles tendinopathy, a sequential treatment of NxES followed by eccentric exercise resulted in a clinically significant improvement in self-reported pain and function. During the first week of treatment there was a reduction in mechanical hyperalgesia during the NxES-only phase, while a large reduction in primary heat hyperalgesia and additional desensitization to mechanical pain occurred during the eccentric training phase of treatment.
Achilles tendinopathy is the most common overuse-related injury in runners,1,2 with an approximate 52% lifetime incidence in recreational runners.3 In a retrospective study, development of pain in the mid-portion (2-6 cm proximal to the insertion) occurred in 66% of cases, while Achilles insertion pain occurred in 23% of cases.4 Pain is particularly pronounced during activities that load the Achilles such as running, jumping, walking uphill, or going up and down stairs.5,6 The etiology of Achilles tendinopathy is not agreed upon, but is believed to result from mechanical overuse and likely involves inflammation in the acute stage6‑8 and is accompanied by tendon degeneration in the chronic stage.4,6 Once tendinopathy has progressed to the chronic stage, continual peripheral nociceptive input may cause changes in central sensory processing and modulation.9‑11 Chronic upper and lower extremity tendinopathies have shown changes in sensory processing: shoulder impingement syndrome,12 lateral epicondylalgia,9,13 and Achilles tendinopathy 10,11 Some trials, however, have not found any sensory processing differences compared to controls in Achilles tendinopathy 14 These adaptive changes in the nociceptive system may contribute to the persistence of pain observed in trials of conservative therapy for chronic Achilles tendinopathies.7,15‑18
Exercise, to reload the plantarflexion musculature through eccentric contractions or using heavy-load, slow speed concentric and eccentric contractions, can make clinically important improvements in pain and function, and has been given the highest clinical practice guidelines rating of evidence (Grade A).19 Despite this high recommendation, approximately 20-50% of those affected by Achilles tendinopathy may have continued pain symptoms at longterm follow-up after a three-month intervention.15‑18
A common rehabilitation intervention that has been used to reduce acute and chronic pain conditions is transcutaneous electrical nerve stimulation (TENS).20 Technically, the term TENS refers to any type of electrical current that crosses the skin, however, to many clinicians and patients the term TENS has become synonymous with electrical stimulation that is delivered at a comfortable intensity The mechanisms of comfortable sensory-level TENS include pain inhibitory pathways comprising the periaqueductal gray area of the brainstem and the rostral ventromedial medulla that inhibit central sensitization of dorsal horn neurons in the spinal cord.21 Evidence of the effec-
tiveness of TENS to reduce pain has been mixed, very likely because of differences in how it has been dosed.22‑25
One type of less commonly used electrical stimulation, noxious electrical stimulation (NxES), involves the delivery of electrical stimulation at much higher intensities that are painful but tolerable.26 The mechanisms of NxES would be similar to that of TENS; however, NxES also activates the well-known pain modulatory phenomenon called diffuse noxious inhibitory control (DNIC) in animals or conditioned pain modulation (CPM) in humans, which is the inhibition of pain in one area due to pain elicited in another part of the body.27,28 The areas of the central nervous system that are activated include the locus coeruleus in the dorsal pons; a major source of noradrenaline in the brain.29, 30 The locus coeruleus sends projections to the subnucleus reticularis dorsalis in the medulla and the resultant pain modulation is thought to involve the arousal and stress responses as well as inhibition of sensitized neurons in dorsal horn.29‑33 Pain modulation due to DNIC has been studied extensively in animal models and advances in MRI technology have resulted in fMRI studies that have shown that acute noxious conditioning stimuli in humans is also associated with changes in activity in similar areas of the central nervous system as the animal models,32 thus illustrating that the mechanisms underlying CPM appear similar to those of DNIC.
Recently, a published case report demonstrated a successful recovery after a 20-minute NxES intervention of a runner with chronic bilateral Achilles tendinopathy, after the patient had failed to progress with an eccentric loading program.34 Therefore, the purpose of this study was to describe the changes in pain sensitivity and self-reported function in runners with chronic Achilles tendon pain following sequential treatment with NxES followed by eccentric plantarflexion exercise.
Participants were recruited through flyers posted around the campus community, at local running shoe stores, and at trailheads of running/walking trails. The inclusion criteria were individuals with Achilles tendon pain (mid-portion or insertional) for at least three months duration, who selfidentified as actively running at least three days per week for at least the prior month. Subjects with unilateral or bilateral Achilles tendon pain were included. Participants
were excluded if they had any significant medical issues such as cardiac history, neurological disorder, psychological disorder affecting ability to perform interventions safely, and/or any additional chronic injury outside of the Achilles. Each participant went through an informed consent process and physical examination to confirm the diagnosis by two physical therapists (BJE and SKS). The physical exam included palpation of the Achilles tendon to confirm pain and to examine for the presence of swelling as well as the performance of single leg calf raise or hopping to confirm pain with loading of the Achilles tendon. Additional screening was performed to rule out any potential referral from the lumbar spine and lower quarter, including adverse neural tension. This study protocol was approved by the IRB at Arcadia University Study enrollment and participation took place between December 2012 through November 2013.
The intervention was split into two treatment phases (see below for details). The first phase lasted one week during which the subject utilized NxES surrounding the painful portion of the Achilles tendon. Subjects with bilateral symptoms were treated with NxES bilaterally. The second phase totaled ten to eleven weeks (six weekly visits and a one-month follow-up) and included eccentric exercises for the plantarflexors/Achilles, core strengthening exercises, and quadriceps and hamstring stretches. All subjects were also educated by two physical therapists (BJE and SKS) to continue with their running routine and follow the painmonitoring model for progression and regression of running volume. The pain-monitoring model has been proposed as a means to adequately dose the mechanical loads on the Achilles tendon using subjective pain reports.35
Participants performed NxES on their first day in the lab, and were given verbal and written instruction of how to set up and perform the NxES at home. Subjects kept a log of their NxES use and current intensities reached during the treatments. NxES was delivered using an Empi 300PV (DJO, LLC, Vista, CA, USA) portable electrical stimulation device and two self-adhesive electrodes (Supertrodes, 2x2 inch, SME, Inc, Wilmington, NC, USA) that were cut down to 1cm x 5cm and placed on the medial and lateral borders of the affected Achilles tendon to bracket the painful area (Figure 1). Noxious electrical stimulation was performed only during the first week of treatment, where participants were instructed to use NxES at home on any days that they experienced Achilles pain symptoms upon loading the limb when getting out of bed in the morning. Parameters were set and locked on the device to be delivered for 20 minutes with a cycle timing of 10 seconds on (including a 2-second rampup time) and 10 seconds off, at 150 pulses per second, and a phase duration of 400µs. The intensity of the stimulus was dosed to reach a sharp, prickly, buzzing/vibration sensation that was rated as 5-7/10 on a 0 to 10 numeric pain rating scale. The current amplitude (in mA) was recorded by the participant for sensory threshold, as was the initial stim-

Figure 1. Electrode placement for application of noxious electrical stimulation treatment on the midportion of the Achilles tendon.
ulation intensity that produced the 5-7/10 pain rating on each day of use. Current was allowed to be adjusted by participants to maintain a 5-7/10 pain rating throughout the 20-minute NxES treatment.
After one week of treatment, NxES was discontinued. After reassessment, a plantarflexion eccentric exercise program was taught, performed, and written instructions with illustrations were provided to start the second phase of treatment. Participants were instructed to complete 3 sets of 15 eccentric plantarflexion contractions (knee extended) two times a day, seven days per week (Table 1). The load was adjusted to reproduce a mild symptom in the Achilles with pain less than or equal to 3/10 on a verbal pain rating scale. Load adjustment occurred as follows: bilateral to unilateral; unilateral to unilateral plus weight in a backpack; addition of weight to backpack as needed. Exercise progression and load adjustments were made by the physical therapist during weekly visits made throughout Phase 2. In addition, participants also were given written instructions with illustrations to perform core strengthening exercises three days per week (side plank, 2 reps with 30-90 second holds; sidelying hip abduction 3 sets of 8-15 reps), and daily quadriceps and hamstring stretches (2 reps held for 30 seconds).
Data were collected at baseline, Week 1 (end of Phase 1), Week 7 (end of Phase 2), and at a one-month follow-up (approximately 11-weeks after the start of the study). Subjects completed self-report outcome measures, and measures of quantitative sensory testing were conducted at each time point. Quantitative sensory testing was performed to assess
Table 1. Phase 2 Exercise Program
Achilles Loading Program Progression
3 sets of 15 repetitions eccentric plantarflexion (performed twice daily) based on pain provocation and reported fatigue
Bilateral to unilateral at bodyweight
Unilateral to unilateral plus weight in backpack
Additional weight in backpack as needed
the pain sensitivity to pressure and heat with threshold testing, and to evaluate the central nervous system’s reactiveness to repeated stimuli using a temporal summation test (sometimes referred to as “wind-up” or “facilitation”). Testing was performed in a standardized order: Pressure Pain Threshold (PPT), Heat Pain Threshold (HPT), and Heat Temporal Summation (HTS).
Participants completed the Lower Extremity Functional Scale (LEFS), which is a region-specific self-report scale of physical function and the Victorian Institute of Sports Assessment – Achilles (VISA-A) questionnaire that includes questions that are pathology-specific to Achilles tendinopathy in regard to stiffness, pain, and function in ADLs and sporting activities. The LEFS has a high degree of test-retest reliability and the minimal detectable change (MDC[90% CI]) for general musculoskeletal disorders was reported as 6 points, and the minimal clinically important difference (MCID) as 9 points.36 The VISA-A however, has low certainty evidence for being reliable due to high heterogeneity across studies and a large range for the MDC exists (7-19 points across four studies).37 The MCID for active people with mid-portion Achilles tendinopathy was reported in a recent study to be 14 points.38
Additionally, the Pain Catastrophizing Scale (PCS) was collected only at baseline as a means to report on the degree of pain catastrophizing in the sample. The PCS is a reliable and valid 13-item self-report measure designed to quantify an individual’s negative thoughts and behaviors in response to actual or potential pain. A score of 30 or more represents clinically relevant catastrophizing in populations with chronic musculoskeletal pain.39,40
The PPT test sites occurred bilaterally over the Achilles tendons (dermatome S1) and tibialis anterior muscle bellies (dermatome L4) using techniques previously described.11, 41 The PPT assessments were conducted with a pressure algometer (FDIX25, Wagner Instruments, Greenwich, CT, USA) with a 1 cm2 rubber tip, with pressure was applied at a rate of 1 kg/s. Standardized instructions were read to the participants prior to each PPT test session and were instructed to indicate the first instance that they felt the sensation of pressure change to that of pain. When the
Side plank (three days/week, 2 reps with 30-90 second holds)
Sidelying hip abduction (three days/week, 3 sets of 8-15 reps)
Quadriceps and hamstring stretching (daily, 2 reps held for 30 seconds)
participant indicated pain, the pressure was immediately removed and the peak force in kilograms was recorded. The Achilles tendon was tested in prone with the ankle joint fixed at neutral dorsiflexion/plantarflexion with an inelastic strap.11,14,34 The site of maximum tenderness was determined by manual palpation and this point was recorded as the distance from the calcaneal insertion (mean = 2.17 ± 1.72 cm, range = 0 to 5 cm proximal) and used as the PPT test point. The tibialis anterior testing site was determined as the midpoint from the fibular head to the medial malleolus. Each site was tested twice with a minimum of approximately one minute between repetitions and the two trials were averaged at each site. A standardized order of testing with the following sequence: right Achilles tendon, left Achilles tendon, right anterior tibialis, and left anterior tibialis. PPTs were tested at baseline, after the first week of treatment with NxES only, after six weeks of eccentric exercise, and finally at the approximate one month follow-up. In a prior study, test-retest for Achilles tendon PPT of healthy individuals was high (ICC = 0.91, MDC(90) = 2.05 kg/cm2).41 Participants with bilateral symptoms were excluded from the Achilles PPT data analysis of the unaffected side, but both sides were included in the analysis of the affected side.
After a two-minute washout period, additional quantitative sensory tests were performed. The heat pain threshold (HPT) assessed heat pain sensitivity and heat temporal summation (HTS) assessed the degree of pain facilitation (wind-up) and were performed as described previously 11, 41 For both tests, a computer-controlled thermode with a 3-cm2 contact area (TSA-II Neurosensory Analyzer, Medoc, Ramat Yishai, Israel) was secured to the subject’s most painful aspect of the Achilles tendon with the probe lying as evenly as possible along the tendon and secured with a Velcro strap. For subjects with bilateral symptoms, the most painful side was utilized for HTS and HPT During the HPT test, the probe’s temperature increased from 35°C at a rate of 0.5°C per second. Participants clicked on a computer mouse when they first felt the sensation of warmth change to that of pain, which would terminate the test. The test was terminated if the probe temperature reached a maximum of 51°C. The HPT was repeated after a one minute rest period and the two repetitions were averaged. A prior study
showed moderate test-retest reliability in healthy individuals (ICC = 0.78, MDC(90) = 1.35°C).41
After an additional two-minute washout period, HTS was assessed with a program that applied 10 consecutive heat pulses to the Achilles tendon at a rate of one pulse every 2.5 seconds using the same computer-controlled thermode. Each pulse of heat climbed from 42 to 50.5°C at a rate of 10°C per second and then returned to the starting temperature of 42°C. Participants were asked to rate their perception of the intensity of the pain for each pulse on a standardized visual analog scale from 0 to 100mm. The scale contained the following written descriptor anchors: 0 mm = “ no sensation”, 10 mm = “warm”, 20 mm = “pain threshold”, and 100 mm = “worst pain imaginable” Temporal summation was calculated at the first rating (measured in mm) subtracted from the maximum rating of the series. Prior research has indicated a high test-retest reliability in healthy individuals (ICC = 0.89; MDC(90) = 10 mm).41
Statistical analysis was completed using SPSS Version 29 (IBM Corporation). Repeated measures ANOVA was performed to compare the change across variables over four time points (baseline, Week 1, Week 7, and the one month follow-up). Bonferroni post hoc testing was then calculated to see if there were significant differences between the four time points. Two-sided tests for significance were reported. A priori ɑ < 0.05 was used to determine statistical significance. Effect sizes were calculated with Hedge’s g for mean differences between the time points (trivial <0.2, small ≥0.2 to <0.5, moderate ≥0.5 to <0.8, large ≥0.8 to <1.20, and very large ≥1.2.).42
Seventeen recreational runners with chronic Achilles tendinopathy met inclusion criteria and were enrolled, however one subject withdrew due to an undisclosed cardiac history that was revealed during their first week of treatment. Therefore, a total of 16 participants (eight males/eight females; mean age = 39.8 ± 10.3 years, range 24-55 years) successfully completed all testing sessions of the study Table 2 provides the characteristics of the subjects, including duration of symptoms (mean 19.76 ± 30.28 months, range 3 months to 8 years), as well as running history. Of the 16 subjects, three presented with bilateral Achilles tendinopathy and five had symptoms located at the Achilles insertion on the posterior calcaneus. The subjects exhibited non-clinically-relevant levels of catastrophizing at baseline (mean score 10.00 ± 8.68, range 0-28).
Regarding the self-application of NxES, subjects tracked their usage, which was an average of 5.5 days (range 3-7 days) over week one of the study protocol. During the initial application of NxES, the average maximum amplitude each subject reached ranged from 6-32mA (mean = 20.51 mA), which equated to an average of 3.9 times above sensory perception threshold. For the final application of NxES, subjects recorded an average maximum intensity ranging
from 7.5-36 mA (mean = 20.97mA). There were no adverse events reported by the subjects with any of the treatment interventions over the course of the study
There was a significant effect of time in the mean LEFS score (F(3) = 16.90, p < 0.001; Table 3). Post hoc testing (Table 4) indicated a significant difference between baseline and week one (p =0 .011, 95% CI [1.1, 6.9]), week one and week seven (p = .008, 95% CI [1.7, 9.4]), and baseline and one month post (p < 0.001, 95% CI [5.5, 13.7]), but not between week seven and the one month follow-up (p = 0.969). The reported MCID of the LEFS is 9 points,35 which was exceeded between baseline and the one month follow-up (9.6 ± 7.7; Table 4).
For the total VISA-A score, there was a significant effect of time in the mean score (F(3) = 14.57, p < .001; Table 3). Post hoc testing indicated a significant difference between baseline and week one (p =0 .015, 95% CI = [1.7, 13.5]), week one and week seven (p = 0.005, 95% CI = [3.4, 16.0]), and baseline and the one month follow-up (p <0 .001, 95% CI = [9.9, 28.8]). For the VISA-A, the lower end of the MDC range (range 7-19 points)36 was exceeded between baseline and week 1 (7.6 ± 11.0) and week one and week seven (9.7 ± 11.8). The MCID of 14 points was exceeded between baseline and one month follow-up (19.4 ± 17.7), which was approximately 11-12 weeks from baseline.
Specific to the VISA-A, two questions were examined from this outcome measure due to their relationship to running. Question 5 of the VISA-A asks about Achilles pain during or immediately after doing 10 (single leg) heel raises from a flat surface, with 0 representing “strong severe pain” and 10 indicating no pain.43 For the responses to this question, there was a significant effect of time in mean response (F(3) = 8.56, p <0.001; Table 3). Post hoc testing (Table 4) indicated a significant improvement between week one and week seven (p = 0.006, 95% CI = [0.6, 3.0]) and for baseline and the one month follow-up (p = 0.004. 95% CI = [1.0, 4.3]), but not from baseline to week one (p =0 .296). Question 6 of the VISA-A asks about the number of single leg hops that can be performed without Achilles pain with a range of 0-10.43 For VISA-A question 6, there was a significant effect of time in mean question score (F(3) = 6.93, p <0 .001; Table 3). Post hoc testing (Table 4) indicated a significant improvement between week one and week seven (p =0 .028, 95% CI = [0.2, 3.4]) and for baseline and after one month follow-up (p = 0.009, 95% CI = [1.0, 6.1]), but not for baseline to week one (p =0 .397).
For PPT of the affected Achilles tendons (n=19), there was a significant effect of time in the mean PPT (F(3) = 26.82, p <0 .001; Table 5). Post hoc testing (Table 6) indicated a significant difference between baseline and week one (p = 0.002, 95% CI = [1.1, 3.9]), week one and week seven (p < 0.001, 95% CI = [2.7, 6.0]), and baseline and one month follow-up (p <0 .001, 95% CI [4.5, 10.6]). Following the NxES treatment, there was a 2.5 ± 2.9kg increase in the involved
Table 2. Participant Demographics
Participants
Tendon Involvement
16 (8 male, 8 female)
13 unilateral, 3 bilateral
Achilles Tendon Pain Location 11 mid-portion, 5 insertional
Subject Age (years)
39.8 ± 10.3, range 24-55
Symptom Duration (months) 19.76 ± 30.28, range 3 months - 8 years
PCS Total Score at Baseline (scale points)
10.00 ± 8.68, range 0-28
Weekly Running Mileage 26.33 ± 23.13 miles/week; range 8-50
Average Running Pace 8:49 ± 1:30 minutes/mile; range 6:45-11:00
PCS= Pain Catastrophizing Scale
Table 3. Mean scores of subject self-reported function over time. All outcomes are reported as Mean ± Standard Deviation. Baseline
ANOVA, analysis of variance repeated measures; LEFS, Lower Extremity Functional Scale; VISA-A Victorian Institute of Sports Assessment – Achilles; Q5, question number 5 from VISA-A; Q6, VISA-A, question number 6 from VISA-A
* “Do you have pain during or immediately after doing 10 (single leg) heel raises from a flat surface?”
** “How many single leg hops can you do without pain?”
Table 4. Difference in self-reported outcome scores between time points.
±
A Q6**
.397 (0.8, 1.9)
SD, standard deviation; CI, confidence intervals; LEFS, Lower Extremity Functional Scale; VISA-A Victorian Institute of Sports Assessment – Achilles;Q5, question number 5 from VISA-A; Q6, VISA-A, question number 6 from VISA-A * “Do you have pain during or immediately after doing 10 (single leg) heel raises from a flat surface?”,** "How many single leg hops can you do without pain?
Achilles PPT from baseline to week one which surpasses the MDC(90) of 2.05 kg.41 Following the eccentric exercise phase (week one through week seven), there was an increase in PPT values of 4.3 ± 3.4 kg, again exceeding the MDC(90).
For PPT of the unaffected Achilles tendon (n=13), there was a significant effect of time in the mean PPT (F(3) =
12.60, p < 0.001; Table 5). Post hoc testing (Table 6) indicated a significant difference between week one and week seven (p < 0.001, 95% CI = [3.0, 7.1]) and baseline and one month follow-up (p = 0.006, 95% CI [2.3, 10.9]), but not for baseline to week one (p = 0.232). For PPT of the tibialis anterior location across both extremities, there was a significant effect of time in the mean PPT (F(3) = 45.50, p <0
Table 5. Mean values of quantitative sensory testing variables over time. All results are presented as Mean ± Standard Deviation. Baseline Week 1 Week 7 1 Month FollowUp
Table 6. Difference in quantitative sensory testing variables between time points.
Baseline to Week 1
1 to Week 7
CI, confidence intervals; HPT, heat pain threshold; HTS, heat temporal summation; PPT, pressure pain threshold; SD, standard deviation
.001; Table 5). Post hoc testing (Table 6) found a significant difference between baseline and week one (p = 0.005, 95%
CI = [0.5, 2.5]), week one and week seven (p < 0.001, 95%
CI = [2.8, 5.1]), and baseline and one month follow-up (p < 0.001, 95% CI = [3.7, 6.5]).
HEAT TEMPORAL SUMMATION AND HEAT PAIN THRESHOLD
For HPT, there was a significant effect of time in the mean temperature (F(3) = 25.71, p < 0.001; Table 5). Post hoc testing (Table 6) found a significant difference between week one and week seven (p < 0.001, 95% CI = [2.1, 5.5]) during
the eccentric exercise phase and baseline and one month follow-up (p < 0.001, 95% CI [2.9, 5.3]), but not between baseline and week one during the NxES phase (p = 0.376). The increase in HPT during eccentric exercise (3.8 ± 3.1℃) surpassed the MDC(90) of 1.35℃ 41
For HTS, there was a significant effect of time in the mean amount of pain facilitation (F(3) = 6.05, p = 0.001; Table 5). Post hoc testing (Table 6) found a significant difference between baseline and one month follow-up (p = 0.002, 95% CI = [-37.0, -10.5]), but not for baseline and week one (p = 0.130) and week one and week seven (p = 0.145). Neither the application of NxES (baseline to week one) or eccentric loading (week one to week seven) reached signifi-
cance for change in HTS, but the MDC(90) of 10 points was exceeded between baseline and one month follow-up (mean change = -23.7 ± 24.8).41
In this group of recreational runners with chronic Achilles tendinopathy, a staged intervention of NxES followed by eccentric-focused plantarflexor exercise had a progressive effect on decreasing mechanical and thermal sensitivity of the affected Achilles tendon (reduced primary hyperalgesia), and mechanical sensitivity of the unaffected Achilles and the tibialis anterior, bilaterally (reduced secondary hyperalgesia). Thus, treatment with NxES followed by eccentric exercise provides evidence for the reduction in both peripheral and central sensitization when compared to baseline measures in a pain-free group of runners.11 The study participants, on average, also improved their selfreported physical functioning by meaningful degrees on both the LEFS and VISA-A questionnaires. From baseline through the follow-up time period, 62% of participants exceeded the MCID for the LEFS (≥ 9 points) and 68% for the VISA-A (≥ 14 points). The magnitude of change observed in the current study for the VISA-A scale is comparable to other studies of eccentric training or heavy slow resistance training of the plantarflexors over similar time periods.18, 44‑52
From the studies of resistance training for Achilles pathology, only four45‑48 examined PPT of the Achilles tendon, and only the study by Rompe and colleagues48 found a significant increase in PPT (decreased sensitivity) due to eccentric training. The PPT baseline values and change in PPT from the current study were much larger than reported by other authors.45‑48 In the current study, baseline PPT of the affected Achilles in the included subjects was 5.8 ± 3.1 kg, where the other studies participants ranged from (1.5 ± 0.6 kg to 4.23 ± 1.7 kg). This difference may be due to contrasts in PPT methodology, as the current study fixed the ankle in neutral dorsiflexion/plantarflexion to put some tension on the muscle-tendon unit. The observed increase in the involved Achilles PPT of 2.5 ± 2.9 kg after one week of treatment with NxES, and an increase of 7.5 ± 6.3 kg across the study period, brought the tendinopathy group to a similar Achilles PPT value (Achilles PPT post-treatment follow-up = 13.4 ± 6.9 kg) to a prior study involving control runners using the same PPT methodology (Control Runners Achilles PPT = 12.7 ± 5.4 kg).11 Additionally, no other studies have reported on changes in HPT over the course of treatment for Achilles tendinopathy Eckenrode and colleagues11 showed that runners with Achilles tendinopathy had a lower HPT at the site of the tendinopathy compared to runners without Achilles tendinopathy (44.7°C vs 46.7°C). Noxious electrical stimulation did not change the HPT, but eccentric training of the plantarflexors may have contributed to a large increase of 3.8°C which was seen in this study (large effect size). The observed changes in PPT and HPT could represent a reduction in primary hyperalgesia of the affected tissues over the course of treatment, potentially leading to increased tolerance to loading of the
Achilles tendon during running and other functional activities.
Pain facilitation, as measured by the heat temporal summation test, has not been well-studied in response to Achilles tendinopathy treatments. Only Chimenti et al45,53 have previously reported on pain facilitation but they utilized different assessments than used in the current study They used a constant cold pain stimulus delivered to the hand and examined the pain ratings at five and 20 seconds of immersion of the hand in cold water, and found no significant difference in temporal summation between before and after a local anesthetic injection53 or after eight weeks of exercise-based treatment in people with Achilles tendinopathy 45 In the present study, the delivery of 10 rapid heat pulses (50.5°C pulses delivered at 2.5 second intervals) as a pain facilitation assessment found a depression in the amount of Achilles heat temporal summation that was not statistically significant (-11 mm; small effect size) after one week of NxES, but reduced significantly over the treatment period and maintained the one month follow-up (-23.7 mm; large effect size). This finding is in agreement with a prior study that showed significant HTS suppression 24hrs after a single session of NxES and eccentric plantarflexor exercise, but not after low intensity cycling.41 A similar suppression was recently observed in HTS at the knee in a small group of people with knee osteoarthritis after NxES.54 Over the course of treatment and follow-up in the present study, the reduction in HTS supports the concept that the treatments served to reduce the pain facilitation response despite not being elevated prior to treatment when compared to those reported in prior study of healthy runners.11
In addition to reductions in primary mechanical and heat hyperalgesia and pain facilitation over the course of treatment, the participants also showed desensitization (increased) in pressure pain thresholds in the uninvolved Achilles and at the tibialis anterior bilaterally Increased mechanical sensitivity in areas outside of the involved tissues has not regularly been observed in lower extremity tendinopathies, but have been more widely observed in upper extremity tendinopathies.55,56 The group of runners with Achilles tendinopathy in the current study did show some signs of mild central sensitization with the presence of secondary hyperalgesia seen in lower PPTs in the uninvolved Achilles and bilateral tibialis anterior at baseline when compared to those reported in another study of control runners.11 The PPT’s increased (became less sensitive) in the uninvolved Achilles (by 6.6 kg, large effect size) and the tibialis anterior (by 5.1 kg, large effect size) over the entire treatment and follow-up period.
Limitations of this study include the small sample size and no control group; therefore, cause and effect inferences cannot be made, as the improvements seen could have been a result of the natural course of injury recovery or due to repeated testing. However, the changes in PPT, HPT, and HTS observed across the duration of the study are beyond error as indicated by exceeding the minimal detectable change scores.41 Thus, the changes observed in this study may not be due to a repeated testing effect; however, there is no
data establishing the reliability of PPT (over the Achilles), HPT, and HTS in people with Achilles tendinopathy. The current study used the pain-monitoring model35 to educate subjects on how to manage running volume; therefore, the education could have influenced symptoms and pain sensitivity. It is unknown how variations in running activity could have influenced findings, either favorably or unfavorably Lastly, the study participants investigated consisted of active runners with chronic Achilles tendinopathy; therefore, the findings should be interpreted with caution when applied to a less active group or those with acute and subacute Achilles tendon pain.
Future studies on individuals with Achilles tendinopathy should look to compare the effects of NxES followed by eccentric exercise versus exercise-only, and additionally investigate if a dose-response relationship exists for NxES. While this study focused on painful eccentric exercise of the plantarflexor muscle groups, the response from other contraction types or tendon loading protocols should also be investigated.
A sequential treatment of NxES with eccentric Achilles strengthening may prove to be a beneficial intervention for individuals who present with chronic Achilles tendinopa-
thy Primary mechanical hyperalgesia was reduced with NxES intervention after one week of treatment using NxES. Over the entire course of treatment, secondary mechanical hyperalgesia, sensitivity to heat pain, and pain facilitation all decreased. Clinically meaningful changes in LEFS and VISA-A were achieved through this intervention approach.
The authors have no disclosures.
We would like to thank Evelyn Brown, DPT, Brooke Greer, DPT, and Ashley O’Brien, DPT, for their assistance with elements of this project.
Submitted: May 29, 2024 CST Accepted: November 18, 2024
CST. Published: February 01, 2025 CST.
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Knobloch K, Yoon U, Vogt PM. Acute and overuse injuries correlated to hours of training in master running athletes. Foot Ankle Int. 2008;29(7):671-676. doi:10.3113/FAI.2008.0671
2. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of Achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med 2005;15(3):133-135. doi:10.1097/ 01.jsm.0000165347.55638.23
3. Scott A, Huisman E, Khan K. Conservative treatment of chronic Achilles tendinopathy Can Med Assoc J. 2011;183(10):1159-1165. doi:10.1503/ cmaj.101680
4. Paavola M, Kannus P, Jarvinen TA, Khan K, Jozsa L, Jarvinen M. Achilles tendinopathy. J Bone Joint Surg Am 2002;84-A(11):2062-2076. doi:10.2106/ 00004623-200211000-00024
5. Paavola M, Kannus P, Paakkala T, Pasanen M, Jarvinen M. Long-term prognosis of patients with Achilles tendinopathy An observational 8-year follow-up study. Am J Sports Med. 2000;28(5):634-642. doi:10.1177/ 03635465000280050301
6. Abate M, Silbernagel KG, Siljeholm C, et al. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther 2009;11(3):235. doi:10.1186/ar2723
7. Barbe MF, Gallagher S, Massicotte VS, Tytell M, Popoff SN, Barr-Gillespie AE. The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet Disord. 2013;14:303. doi:10.1186/ 1471-2474-14-303
8. Gao H, Fisher P, Lambi A, Wade C, Barr-Gillespie A. Increased serum and musculotendinous fibrogenic proteins following persistent low-grade inflammation in a rat model of long-term upper extremity overuse. PLOS One. 2013;8(8):e71875. doi:10.1371/ journal.pone.0071875
9. Fernandez-Carnero J, Fernandez-de-Las-Penas C, de la Llave-Rincon AI, Ge HY, Arendt-Nielsen L. Widespread mechanical pain hypersensitivity as sign of central sensitization in unilateral epicondylalgia: a blinded, controlled study. Clin J Pain. 2009;25(7):555-561. doi:10.1097/ AJP.0b013e3181a68a040
10. Tompra N, van Dieën JH, Coppieters MW Central pain processing is altered in people with Achilles tendinopathy. Br J Sports Med. 2016;50(16):1004-1007 doi:10.1136/ bjsports-2015-095476
11. Eckenrode BJ, Kietrys DM, Stackhouse SK. Pain sensitivity in chronic Achilles tendinopathy Int J Sports Phys Ther 2019;14(6):945-956. doi:10.26603/ ijspt20190945
12. Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery J Bone Joint Surg Br 2011;93(4):498-502. doi:10.1302/ 0301-620X.93B4.25054
13. Slater H, Arendt-Nielsen L, Wright A, GravenNielsen T Sensory and motor effects of experimental muscle pain in patients with lateral epicondylalgia and controls with delayed onset muscle soreness. Pain 2005;114(1-2):118-130. doi:10.1016/ j.pain.2004.12.003
14. Plinsinga ML, van Wilgen CP, Brink MS, et al. Patellar and Achilles tendinopathies are predominantly peripheral pain states: a blinded case control study of somatosensory and psychological profiles. Br J Sports Med 2018;52(5):284-291. doi:10.1136/bjsports-2016-097163
15. Silbernagel KG, Brorsson A, Lundberg M. The majority of patients with Achilles tendinopathy recover fully when treated with exercise alone: a 5-year follow-up. Am J Sports Med 2011;39(3):607-613. doi:10.1177/0363546510384789
16. van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46(3):214-218. doi:10.1136/bjsports-2011-090035
17 Nicholson CW, Berlet GC, Lee TH. Prediction of the success of nonoperative treatment of insertional Achilles tendinosis based on MRI. Foot Ankle Int. 2007;28(4):472-477 doi:10.3113/FAI.2007.0472
18. Sayana MK, Maffulli N. Eccentric calf muscle training in non-athletic patients with Achilles tendinopathy J Sci Med Sport 2007;10(1):52-58. doi:10.1016/j.jsams.2006.05.008
19. Martin RL, Chimenti R, Cuddeford T, et al. Achilles pain, stiffness, and muscle power deficits: midportion Achilles tendinopathy revision 2018. J Orthop Sports Phys Ther. 2018;48(5):A1-A38. doi:10.2519/jospt.2018.0302
20. Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. 2014;4(3):197-209. doi:10.2217/pmt.14.13
21. Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci 2018;19(8). doi:10.3390/ijms19082164
22. Dailey DL, Rakel BA, Vance CG, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154(11):2554-2562. doi:10.1016/ j.pain.2013.07.043
23. Santos CM, Francischi JN, Lima-Paiva P, Sluka KA, Resende MA. Effect of transcutaneous electrical stimulation on nociception and edema induced by peripheral serotonin. Int J Neurosci. 2013;123(7):507-515. doi:10.3109/ 00207454.2013.768244
24. Rakel BA, Zimmerman BM, Geasland K, et al. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: A randomized, blinded, placebocontrolled trial. Pain 2014;155(12):2599-2611. doi:10.1016/j.pain.2014.09.025
25. Zeng C, Li H, Yang T, et al. Electrical stimulation for pain relief in knee osteoarthritis: systematic review and network meta-analysis. Osteoarthritis Cartilage. 2015;23(2):189-202. doi:10.1016/ j.joca.2014.11.014
26. Defrin R, Ariel E, Peretz C. Segmental noxious versus innocuous electrical stimulation for chronic pain relief and the effect of fading sensation during treatment. Pain 2005;115(1-2):152-160. doi:10.1016/ j.pain.2005.02.018
27 Yarnitsky D Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23(5):611-615. doi:10.1097/ ACO.0b013e32833c348b
28. van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain. 2010;11(5):408-419. doi:10.1016/ j.jpain.2009.10.009
29. de Resende MA, Silva LF, Sato K, Arendt-Nielsen L, Sluka KA. Blockade of opioid receptors in the medullary reticularis nucleus dorsalis, but not the rostral ventromedial medulla, prevents analgesia produced by diffuse noxious inhibitory control in rats with muscle inflammation. J Pain 2011;12(6):687-697. doi:10.1016/j.jpain.2010.12.009
30. Mills EP, Keay KA, Henderson LA. Brainstem painmodulation circuitry and its plasticity in neuropathic pain: insights from human brain imaging investigations. Front Pain Res (Lausanne) 2021;2:705345. doi:10.3389/fpain.2021.705345
31. Wen YR, Wang CC, Yeh GC, et al. DNIC-mediated analgesia produced by a supramaximal electrical or a high-dose formalin conditioning stimulus: roles of opioid and alpha2-adrenergic receptors. J Biomed Sci. 2010;17(1):19. doi:10.1186/1423-0127-17-19
32. Khan HS, Stroman PW Inter-individual differences in pain processing investigated by functional magnetic resonance imaging of the brainstem and spinal cord. Neuroscience 2015;307:231-241. doi:10.1016/ j.neuroscience.2015.08.059
33. Bannister K, Dickenson AH. The plasticity of descending controls in pain: translational probing. J Physiol. 2017;595(13):4159-4166. doi:10.1113/ JP274165
34. Eckenrode BJ, Stackhouse SK. Improved pressure pain thresholds and function following noxious electrical stimulation on a runner with chronic Achilles tendinopathy: a case report. Int J Sports Phys Ther. 2015;10(3):354-362.
35. Silbernagel KG, Crossley KM. A proposed returnto-sport program for patients with midportion Achilles tendinopathy: rationale and implementation. J Orthop Sports Phys Ther 2015;45(11):876-886. doi:10.2519/jospt.2015.5885
36. Mehta SP, Fulton A, Quach C, Thistle M, Toledo C, Evans NA. Measurement properties of the lower extremity functional scale: a systematic review J Orthop Sports Phys Ther. 2016;46(3):200-216. doi:10.2519/jospt.2016.6165
37 Korakakis V, Whiteley R, Kotsifaki A, Stefanakis M, Sotiralis Y, Thorborg K. A systematic review evaluating the clinimetric properties of the Victorian Institute of Sport assessment (VISA) questionnaires for lower limb tendinopathy shows moderate to highquality evidence for sufficient reliability, validity and responsiveness-part II. Knee Surg Sports Traumatol Arthrosc. 2021;29(9):2765-2788. doi:10.1007/ s00167-021-06557-0
38. Lagas IF, van der Vlist AC, van Oosterom RF, et al. Victorian Institute of Sport assessment-Achilles (VISA-A) questionnaire-minimal clinically important difference for active people with midportion achilles tendinopathy: a prospective cohort study J Orthop Sports Phys Ther 2021;51(10):510-516. doi:10.2519/ jospt.2021.10040
39. Sullivan MGL, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;(7):524-532. doi:10.1037// 1040-3590.7.4.524
40. Sullivan MJ, Stanish W, Waite H, Sullivan M, Tripp DA. Catastrophizing, pain, and disability in patients with soft-tissue injuries. Pain. 1998;77(3):253-260. doi:10.1016/ S0304-3959(98)00097-9
41. Stackhouse SK, Taylor CM, Eckenrode BJ, Stuck E, Davey H. Effects of noxious electrical stimulation and eccentric exercise on pain sensitivity in asymptomatic individuals. Phys Med Rehabil. 2016;8(5):415-424. doi:10.1016/j.pmrj.2015.07.009
42. Sullivan GM, Feinn R. Using effect size—Or why the P value is not enough. J Grad Med Educ. 2012;4:279-282. doi:10.4300/JGME-D-12-00156.1
43. Robinson JM, Cook JL, Purdam C, et al. The VISAA questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy Br J Sports Med 2001;35(5):335-341. doi:10.1136/bjsm.35.5.335
44. Silbernagel KG, Thomee R, Eriksson BI, Karlsson J. Full symptomatic recovery does not ensure full recovery of muscle-tendon function in patients with Achilles tendinopathy. Br J Sports Med. 2007;41(4):276-280. doi:10.1136/bjsm.2006.033464
45. Chimenti RL, Post AA, Rio EK, et al. The effects of pain science education plus exercise on pain and function in chronic Achilles tendinopathy: a blinded, placebo-controlled, explanatory, randomized trial. Pain. 2023;164(1):e47-e65. doi:10.1097/ j.pain.0000000000002720
46. Stefansson SH, Brandsson S, Langberg H, Arnason A. Using pressure massage for Achilles tendinopathy: a single-blind, randomized controlled trial comparing a novel treatment versus an eccentric exercise protocol. Orthop J Sports Med. 2019;7(3):2325967119834284. doi:10.1177/ 2325967119834284
47. Mansur NSB, Matsunaga FT, Carrazzone OL, et al. Shockwave therapy plus eccentric exercises versus isolated eccentric exercises for Achilles insertional tendinopathy: a double-blinded randomized clinical trial. J Bone Joint Surg Am 2021;103(14):1295-1302. doi:10.2106/JBJS.20.01826
48. Rompe JD, Nafe B, Furia JP, Maffulli N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med 2007;35(3):374-383. doi:10.1177/ 0363546506295940
49. Beyer R, Kongsgaard M, Hougs Kjaer B, Ohlenschlaeger T, Kjaer M, Magnusson SP. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: a randomized controlled trial. Am J Sports Med 2015;43(7):1704-1711. doi:10.1177/0363546515584760
50. Johannsen F, Olesen JL, Ohlenschlager TF, et al. Effect of ultrasonography-guided corticosteroid injection vs placebo added to exercise therapy for Achilles tendinopathy: a randomized clinical trial. JAMA Netw Open 2022;5(7):e2219661. doi:10.1001/ jamanetworkopen.2022.19661
51. Rabusin CL, Menz HB, McClelland JA, et al. Efficacy of heel lifts versus calf muscle eccentric exercise for mid-portion Achilles tendinopathy (HEALTHY): a randomised trial. Br J Sports Med. 2021;55(9):486-492. doi:10.1136/ bjsports-2019-101776
52. Stevens M, Tan CW Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther 2014;44(2):59-67 doi:10.2519/jospt.2014.4720
53. Chimenti RL, Hall MM, Dilger CP, Merriwether EN, Wilken JM, Sluka KA. Local anesthetic injection resolves movement pain, motor dysfunction, and pain catastrophizing in individuals with chronic Achilles tendinopathy: a nonrandomized clinical trial. J Orthop Sports Phys Ther 2020;50(6):334-343. doi:10.2519/jospt.2020.9242
54. Stackhouse SK, Rudolph KS. Harnessing the condition pain modulation effect for therapeutic use in knee osteoarthritis. J Orthop Sports Phys Ther 2023;53(2):CSM99.
55. Rio E, Sandler J, Cheng K, Moseley GL, Cook J, Girdwood M. Sensory processing in people with and without tendinopathy: a systematic review with meta-analysis of local, regional, and remote sites in upper- and lower-limb conditions. J Orthop Sports Phys Ther. 2021;51(1):12-26. doi:10.2519/ jospt.2021.9417
56. Alsulaimani B, Perraton L, Stasinopoulos D, Tavakkoli S, Malliaras P Evidence for improvement in local but not diffuse pressure pain thresholds following physical therapist interventions for tendinopathy: a systematic review. Phys Ther. 2023;103(2). doi:10.1093/ptj/pzac159

Frank Aerts1 , Holly Sheets1a , Chance Anderson1 , Natalie Bussie1 , Rose Hoskins1 , Amanda Maninga1 , Emily Novak1 1 Doctor of Physical Therapy Program, Manchester University
Keywords: dynamometry, hand-held, mechanical force, reliability, test position, agreement https://doi.org/10.26603/001c.128286
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background
The use of portable hand-held dynamometers is increasing in popularity due to their ease of use in different clinical settings, convenient size, portability, and overall affordability. Reported reliability for external fixation and rater-stabilized hand-held dynamometry (HHD) strength measurements have been found to be ‘good’ to ‘excellent’. Inconsistent agreement has been found between the two stabilization methods and isokinetic HHD testing.
Determine the reliability and agreement of HHD measurements in three different rater test positions against three different mechanically produced force magnitudes. The study compared measurements obtained by rater-stabilization to external fixation methods.
Intra-rater and inter-rater reliability study
Methods
Ten raters took measurements in three different rater test positions against three different force magnitudes created by an external force. Raters were blinded to the randomized force magnitudes. The rater’s measurements were compared to measurements taken against an external fixation stabilization device. To establish reliability, Intraclass Correlation Coefficient (ICC), and Standard Error of Measurement (SEM), and Minimal Detectable Change (MDC) were used. To establish agreement, error rates between the rater-stabilized and external fixation stabilization measurements were calculated.
Results
ICC’s were found to be ‘excellent’ at .97 and above. The relative SEM ranged from 0.2% to 0.9 % and the relative MDC ranged from 0.7% to 2.8%. The overall error rate was 15.5% and was influenced by force magnitude.
Conclusion
The use of standardized rater test positions resulted in ‘excellent’ intra-rater, inter-rater reliability, low SEM, and low MDC for rater-stabilized HHD measurements. A systematic error was observed, with rater-stabilized measurements resulting in higher values compared with values obtained with the external fixation method.
Corresponding Author:
Holly Sheets
10627 Diebold Road, Fort Wayne, IN, 46845
Phone: 260-470-4069
Fax: 260-470-4404
Email: hasheets@manchester.edu
Measuring and restoring strength to aid in rehabilitating a patient’s function is considered a key aspect of physical therapist practice.1‑3 The demand for objective quantitative measurements has facilitated the increased utilization of dynamometry in clinical practice.4 Isokinetic dynamometry provides highly reproducible results but is limited in clinical practice.5 The use of portable or hand-held dynamometers (HHDs) is increasing in popularity due to their affordability, operational and clinical efficiency Hand-held dynamometry (HHD) is easy to use in different clinical settings with different populations.5,6 HHDs are commonly used with rater stabilization or with external fixation stabilization.
Reported psychometric properties for HHD measurements have been reported to be good to excellent.3,5,7 A recent systematic review with meta-analysis for hip muscle strength measures with portable dynamometers found moderate to high-quality evidence for sufficient intra-rater and inter-rater reliability for some positions regardless of fixation method.7 Florencio et al. report that studies show HHD reliability with rater-stabilized measurements with ICC between 0.70 and 0.98, and measurements with external fixation stabilization with ICC between 0.49 and 0.99 for measurements related to hip and knee.2 Maximal isometric force values obtained by HHD are comparable to values obtained with isokinetic dynamometry, but inconsistent validity has been found with both rater-stabilized and external fixation methods.3,7
HHD is susceptible to many sources of error and lacks standardization. Sources of error may be grouped into subject attributes, testing procedures, instrument characteristics, and rater attributes. Studies found the reliability of HHD measurements to be reliable in various patient populations.8 For example, Vaz et al. found excellent (≥0.90) intra-rater and inter-rater intraclass correlation coefficient (ICC) for hip measurements in individuals with symptomatic hip osteoarthritis.9 Koblauer et al. found similar excellent intra-rater and inter-rater ICC for knee extensor strength for patients awaiting total knee replacement.8 However, they did find a high error rate, expressed in the smallest detectable difference (SDD), ranging from 19.0% to 57.5%, concluding that the use of HHD is not advised for clinical practice. The lack of standardized testing protocols and testing positions for different muscle groups is another source of error 3,5,7 Instruments characteristics might be another source. Du et al. examined the variability between different HHD instruments for measuring muscle strength and found differences between 0.2% and 16% between dynamometers.10 Errors associated with the rater include experience with HDD and rater strength.3,5,11,12 The rater’s inability to maintain a stable base against higher torque outputs, has led to the recommendation to use external fixation methods.7
Operational considerations might not make it feasible to use external fixation methods, such as in inpatient acute care setting. The lack of consensus on using external fixation stabilization versus rater-stabilized methods for stabilization warrants further investigation. In addition, there is no consensus on the most appropriate rater testing positions to enable proper stabilization against an external force.13 A wide variety of methods have been described in research and clinical practice.7
The primary aim of this study was to assess the interrater and intra-rater reliability of rater-stabilized HHD measurements from a mechanically produced force in three different standardized rater test positions. Reliability is defined as the degree of consistency with which a rater measures a variable.14 A second aim of the study was to assess the agreement between external fixation stabilization and rater-stabilized measurements.
This is an intra-rater and inter-rater reliability study approved by the Manchester University Institutional Review Board.
AND RATER TEST POSITIONS
A novel device was designed to provide a mechanical force. A mannequin leg was securely attached to the base of a height-adjustable plinth. Through a rope, the mannequin leg was attached to a pulley with a 60 kg weight stack (Speed Pulley 702600, STEENS, Norway). The external leverage arm, the point of rotation to the location of the instrument placement, was constant at 43.5 cm. The setup was configured to allow for three standardized rater test positions commonly used in clinical practice. The positions were selected to allow for optimal rater stabilization and are commonly used to measure hip flexion, hip abduction and knee extension. The selected positions are described by Aerts and Alwood15 and in Figure 1.
A newly obtained Lafayette Hand-Held Dynamometer (Lafayette Instrument Company, Lafayette IN, USA, Model 01165A) was used to obtain and record the measurements. The hand-held dynamometer was not altered in any way. The device was accompanied by a certificate of calibration with absolute errors ranging from -3.1 newton (N) to +0.6 N, and relative errors ranging from -0.25% to + 0.65% between the HHD measurement and actual value. An external fixation stabilization device (Hand-Held Dynamometer Support Stand Model 01166, Lafayette Instrument Company, Lafayette IN, USA) was used to stabilize the HHD measurements obtained by external fixation (Figure 2).

A convenience sample of raters was recruited by the Manchester University faculty from the local geographical area (Fort Wayne, Indiana – USA). After signing a consent form to participate, raters were asked to complete a survey inquiring about their practice experience and familiarity with using HHD. Age, sex, anthropometric information, and hand grip strength were collected. Each rater was assigned a random number between 1 through 10. The raters underwent a thirty-minute training session and performed several practice measurements until they reported being comfortable with the measurement techniques and rater test positions.
The mechanical set-up was calibrated to produce predetermined external forces. The predetermined external forces were based on forces that a rater may encounter during clinical practice when measuring hip abduction, hip flexion, and knee extension using the selected test positions. The force values were based on a data set obtained in clinical practice (n=800) by the main investigator (unpublished clinical data). The force values were categorized into three groups: 1. low (-1SD), 2. medium (mean), and 3. high (+1SD). The testing was performed over three days. Before each testing session, the investigators performed three measurements against each external force magnitude, by using the external fixation method to ensure that the me-

chanical setup produced the expected predetermined forces. The force values are presented in Table 2
Each rater was then asked to complete nine measurements in each of the three different test positions, i.e. hip abduction, hip flexion, and knee extension. Three different force magnitudes (i.e. low, medium, high) were randomized across the nine measurements so that each rater performed three measurements against each force magnitude in random order To mimic the clinic situation where therapists do not exactly know how much force the patient or client will produce, the raters were blinded to the preset external forces. The investigators, recording the rater-stabilized HHD measurements, were also blinded to the preset external forces. The reading of the peak force (N) of each measurement was recorded. The raters used a “make” technique where the raters matched the external mechanical force for a duration of five seconds. With a “make” technique the rater holds the hand-held dynamometer stationary matching the external torque. This would mimic the measurements taken when the patient / client produces force through an isometric muscle contraction. In contrast with a “break” technique, the rater must create enough force to break the isometric muscle contraction. This would mimic measurements taken when the patient / client produces force through an eccentric muscle contraction. Both techniques have been used in clinical practice and research. A “make” technique might be favored during early rehabilitation as a “break” technique may increase patients / client risk for injury 6 Additionally, a “make” technique may have better reliability and provide more accurate measurements.3,8
Statistical analysis was performed using the Statistical program for the Social Sciences version 28 (SPSS, IBM corp., Armonk, NY) and significance was set at α<0.05. Raters’ demographics were analyzed and reported using descriptive statistics. Quantitative variables are expressed in mean (N) and standard deviation (SD). Based on the sam-
ple calculator presented by Bonett, using 10 raters, sample size calculation was conducted for reliability data with an ICC estimated at 0.80, an amplitude-based confidence interval of 0.3, that is, 0.5 < ICC> 1.0, and a confidence coefficient α<0.05, resulted in 11 different tests (subjects).2,16
The intraclass correlation coefficient (ICC) is the most common statistic used to assess intra-rater and inter-rater reliability 8 Intra-rater reliability for each rater was assessed by using the three measurements of each force magnitude obtained in each test position. The two-way mixed intraclass correlation coefficient model 3 (ICC3,k)14 and the 95% confidence intervals were calculated. Inter-rater reliability was assessed using the average of the three trials for each measurement. The random effects absolute agreement inter-class correlation coefficient model 2 (ICC2,k) and the 95% confidence intervals were calculated.14 The following guidelines were used to interpret the ICC: below 0.75 as poor to moderate, above 0.75 as ‘good’, and above 0.90 as ‘excellent’ which ensures reasonable reliability 14
The clinical utility of the ICC is minimal as it provides a measure of reliability within a study group and not individual measures. Therefore, the Standard Error of Measurement (SEM) and Minimal Detectable Change (MDC) are used as additional measures of reliability
The (SEM) and (MDC) were calculated as an additional assessment of inter-rater reliability Lower values of SEM and MDC values are indicative of lower measurement error and better reliability 8 SEM was calculated using the following formula: . MDC was calculated as follows: All SEM and MDC values are presented in absolute values and as a percentage of the mean maximal strength. For each raterstabilized test, the Mean and SD were calculated using the average of the three measurements from each rater For the SEM and MDC, the inter-rater ICC for each test position was used.
The agreement refers to the ability of a measurement tool to produce the same exact values.17 Agreement was assessed by calculating the error rate between external fixation stabilization and rated-stabilized measurements. Both external fixation stabilization and rater-stabilized HHD measurements were obtained from a mechanical force therefore controlling for patient / client variability By controlling errors associated with subjects, testing procedures, and instrument variability, the researchers aimed to obtain an estimate of error associated solely with the rater
The agreement was estimated by calculating the error rate as follows18:
The error rates of the average measurements of each rater’s tests were used to estimate overall accuracy A one-way ANOVA was conducted to determine if the error rate (%) between the rater-stabilization and external fixation stabilization measurements were different by rater, test position, or by force magnitude.
Table 1. Rater Characteristics
Sex (Female / Male)
Age (years)
4 / 6
33.7 (7.6)
Height (m) 1.77 (0.9)
Weight (Kg) 91.7 (23.2)
Handedness (Right / Left)
Maximum Grip Strength (N)
Years of Practice
Practice Setting
Specialization
Practical HHD Experience
(Estimated by the number of patients / clients a rater uses HHD)
*Continuous variables presented in mean (SD)
Ten participants consented to be a part of the study All raters were licensed physical therapists and worked in various clinical settings. Raters’ characteristics are presented in Table 1 Six raters reported using HHD with 50 to 90% of their patient / clients, two raters with 10 to 50% of their patient / clients, and two raters stated they never used HHD The overall strength of the raters was assessed through grip strength. The mean grip strength for all raters was 456N ± 140. All 10 raters performed all measurements in Test Position 1 - Hip Abduction and Test Position 2 - Hip Flexion. Two raters did not complete measurements in Test Position 3 - Knee Extension against the highest external force magnitude. The measurement attempts were halted as the raters were unsure if they could maintain a stable base. The raters who were unable to maintain a stable base were both females who never or rarely uses HHD and had the lowest mean dominant grip strength measurements of 275N and 314N.
Intra-rater reliability for each rater was assessed using the three trials for each measurement. Two providers did not perform measurements in Test Position 3 - Knee Extension against the highest resistance. The intra-rater reliability (ICC3,k) for the different raters across the measurements ranged from 0.97 to 1.00. Inter-rater reliability was assessed using the means of the three trials from each rater for each measurement. After examining box plots, one extreme outlier (three box lengths away from the edge of the box), classified as (Rater 3, Test Position 2 - Hip Flexion, low force magnitude) was identified but was not removed before further analysis. The inter-rater reliability (ICC2,k) between the different raters in the three test positions was 0.99 (CI 95% 0.93; 1.00). The absolute SEM ranged from 0.5
9 / 1
456 (140)
Less than 10 years: 6 raters
Between 10 and 19 years: 4 raters
Collegiate Sport Facility: 1 rater
Hospital Based Outpatient: 3 raters
Private Practice: 6 raters
Board-Certified Orthopaedic Clinical Specialist: 3 raters
50-90%: 6 raters
10-50%: 2 raters
Never used: 2 raters
to 3.0 N and the relative SEM from 0.2% to 0.9 % respectively The absolute MDC ranged from 1.4 to 8.3N and the relative MDC from 0.7% to 2.8%. Table 2 provides a summary of the measurements by test position and force magnitude (nine tests) for the external fixation and rater-stabilized measurements.
After examining boxplots, two outliers were identified. The authors did not remove this data from the analysis as it did not impact the analysis outcome. The means obtained by rater-stabilized measurements compared to the means obtained by external fixation measurements were higher in all nine tests. The error rate between the external fixation and rater-stabilized measurements ranged from 6.9% in Test Position 3 – Knee Extension, low force magnitude to 31.2% in Test Position 3 – Knee Extension, high force magnitude. Data was normally distributed for all groups, as assessed by Shapiro-Wilk’s test (p > 0.05), except for one group i.e. “Test Position 2 - Hip Flexion”. After reviewing the “normal Q-Q plot” and considering the robustness of one-way ANOVA against Type I error with equal group sizes, the analysis proceeded.
For “Rater”, variances were homogeneous by Levene’s for equality of variances (p = 0.443). There was no statistically significant difference in error rate (%) between the different raters (F (9,77) = 1.358, p = 0.222). Error rates by rater are presented in Table 3
For “Test Position”, there was no homogeneity of variances by Levene’s for equality of variances (p < 0.001). There was no statistically significant difference in error rate (%) between the different test positions (Welch’s F (2,47.691) = 1.583, p = .216). Error rates by test position are presented in Table 4
For “Force Magnitude”, there was no homogeneity of variances by Levene’s for equality of variances (p <0.001). There was a statistically significant difference in error rate (%) between the different force magnitudes (Welch’s F
Table 3. Error rate by rater
Rater 1 (n=9)
± 9.6 Rater 2 (n=9)
± 7.6 Rater 3 (n=9)
± 15.4 Rater 4 (n=9)
Rater 5 (n=9) 13.5 ± 9.0
Rater 6 (n=9)
± 8.4 Rater 7 (n=8)
± 9.6 Rater 9 (n=8)
The values of the current study compare with the values reported by Morin et al. who reports relative inter-rater SEM values between 1.1% and 3.0% and relative MDC values between 3.1% and 8.3% for hip and knee measurements obtained by using a semi-fixed and pull dynamometer 5 The values found in this study for relative SEM were lower than the values reported by other studies.2,20 Florencio et al. reported intra-session relative SEM values for HHD hip and knee, using examiner and belt stabilized measurements, between 6% and 15% using a similar instrument.2
Error rates were calculated to assess agreement. The error rates between the external fixation stabilization and rater-stabilized measurements ranged from 6.9% to 31.2% comparable to –4.9% to 27.1% calculated values based on the data provided by Florencia et al.2
± 9.9
± 9.6 Rater 10 (n=9)
n, number; SD, Standard Deviation
Table 4. Error rate by test position
n, number; SD, Standard Deviation
Table 5. Error rate by force magnitude
n, number; SD, Standard Deviation
(2,50.798) = 42.938, p <0 .001). Error rates by force magnitude are presented in Table 5.
This study investigated the variability associated with the rater by assessing the reliability and agreement of HHD measurements. External fixation was compared to raterstabilized measurements using the same instrument, taken in three standardized rater test positions against three different force magnitudes. The external force was created by a mechanical device eliminating the variability associated with patients or clients.
Both intra-rater and inter-rater reliability were excellent in this study with intra-rater and interclass correlation coefficients of 0.97 and above. These results are consistent with previous studies reporting reliability for HHD measurements for hip measurements.5,7,19 The inter-rater relative SEM values obtained in this study ranged from 0.2% to 1.0% and relative MDC values ranged from 0.7% to 2.8%.
The raters consistently, regardless of the force magnitude and test position, measured higher values compared to the external fixation stabilization measurement. This finding is consistent with Florencio et al. who reported that measurements obtained with examiner stabilization were generally greater than those observed for belt-stabilization in 13 of 16 measurement positions.2 This systematic error might be related to the raters trying to adjust to the direction and magnitude of the force. Providers were instructed to ensure that the pressure pad stayed perpendicular to the movement arm (artificial limb), which was not assured by the mechanical stabilization. This finding is similar to Florencia et al. who suggested that better control of the dynamometer position is guaranteed when the stabilization is provided by the rater 2 Another reason can be related to the increased impulse force created when raters are trying to adjust to the force magnitude. The raters were instructed to maintain the starting position and not to push back. The external force was released in a controlled two-second manner By adjusting to the unknown force, the rater may create a higher impulse force increasing the force output registered by the hand-held dynamometer In addition, the results of this study suggest that this systematic relative error is more pronounced with lower force magnitudes.
It has been recognized that assessor strength impacts the ability to provide proper stabilization when confronted with higher forces exceeding the accessor’s strength.6 In this study, two raters were unable to provide a stable base when confronted with a higher force magnitude in Test Position 3 - Knee Extension.
The major strength of this study is the exclusion of variability related to patients or clients. The investigators could only identify one other study investigating HHD accuracy using a mechanically produced force by a spring-loaded device.18 Overall accuracy was estimated at 3%. The main difference was the raters used a “break technique” compared to a “make technique” in this study. Another strength of the study was the use of the same instrument for both stabilization methods, eliminating the error associated with the use of different instruments. A third strength of this study was the randomization and blinding of recording investigators and raters to the forces applied by the mechanical device. This mimics the clinical situation where raters do
not know how much resistance to provide to counteract the forces produced by the patient / client.
This study has several limitations. The limited sample size (n = 9 tests) was smaller than the calculated sample size required (n=11), which may have resulted in an under powered study. The mechanical setup did not accommodate an additional test position. In retrospect, an additional force magnitude could have been included. The limited sample size precluded the investigation of interaction effects between rater, test position, and force magnitude. Further research should explore these possible interaction effects. A second limitation was the minimal training provided to the participating raters, approximately 30 minutes of instruction and practice. In the study of Morin et al., trainers received three[ days of training followed by 20 hours of practice.5 This limited training may have an impact on the raters’ ability to provide the proper stabilization. A third limitation might be related to the external fixation stabilization device. The relative SD and relative SEM for our external fixation stabilization device measurements ranged from 2.7 % to 16.8% and from 0.1% to 0.9% respectively. Florencio et al. reported data for knee and hip strength measurements from healthy young individuals using a similar HHD LaFayette instrument.2 Based on the data from Florencio et al., the relative SD which ranges from 31.6% to 37.3%. were calculated2 Florencio et al. reported relative SEM from 7% to 15%.2 The relative SEM includes the error associated with the instrument itself (-0.25%0.65%).2 Both studies used fixed stabilization technique, but the external mechanical produced torque in our study resulted in lower relative SD and relative SEM compared to the subject produced torque in the study from Florencio et al.2
This research study provides evidence that rater-stabilized HHD measurements have excellent intra-rater and interrater reliability, with low SEM and MDC. The maximal ex-
ternal force should not exceed the rater capability and raters should use standardized test positions. In clinical practice, providers should be aware that HHD may have a degree of inaccuracy. The values obtained with rater-stabilized HHD were consistently higher compared to the values obtained by external fixation HHD. Further research should investigate if this overestimation is influenced by the force magnitude.
The authors would like to thank all raters for their contributions. We would like to thank Adam Fischer for constructing the mechanical setup and Ed Ball for soliciting the participating raters.
This work was supported in part by the Manchester University Health Sciences and Pharmacy Programs Internal Research Grant. The funding source had no involvement in study design, data collection, analysis, interpretation, manuscript writing, or decision to submit the article for publication.
The authors report no conflicts of interest.
Submitted: June 25, 2024 CST. Accepted: December 14, 2024 CST Published: February 01, 2025 CST
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. APTA Guide to Physical Therapist Practice 4.0. American Physical Therapy Association. 2023. Accessed April 19, 2024. https://guide.apta.org
2. Florencio LL, Martins J, da Silva MRB, da Silva JR, Bellizzi GL, Bevilaqua-Grossi D Knee and hip strength measurements obtained by a hand-held dynamometer stabilized by a belt and an examiner demonstrate parallel reliability but not agreement. Phys Ther Sport. 2019;38:115-122. doi:10.1016/ j.ptsp.2019.04.011
3. Morin M, Duchesne E, Bernier J, Blanchette P, Langlois D, Hébert LJ. What is known about muscle strength reference values for adults measured by hand-held dynamometry: A scoping review Arch Rehabil Res Clin Transl. 2021;4(1):100172. doi:10.1016/j.arrct.2021.100172
4. Loria K. A look ahead: Technology APTA Magazine 2021;13(8).
5. Morin M, Hébert LJ, Perron M, Petitclerc É, Lake SR, Duchesne E. Psychometric properties of a standardized protocol of muscle strength assessment by hand-held dynamometry in healthy adults: a reliability study BMC Musculoskelet Disord 2023;24(1):294. doi:10.1186/s12891-023-06400-2
6. Garcia MAC, Souza VH. The (un)standardized use of handheld dynamometers on the evaluation of muscle force output. Braz J Phys Ther. 2020;24(1):88-89. doi:10.1016/j.bjpt.2019.10.004
7 Waiteman MC, Garcia MC, Briani RV, et al. Can clinicians trust objective measures of hip muscle strength from portable dynamometers? A systematic review with meta-analysis and evidence gap map of 107 studies of reliability and criterion validity using the COSMIN methodology. J Orthop Sports Phys Ther. 2023;53(11):655-672. doi:10.2519/jospt.2023.12045
8. Koblbauer IF, Lambrecht Y, van der Hulst ML, et al. Reliability of maximal isometric knee strength testing with modified hand-held dynamometry in patients awaiting total knee arthroplasty: useful in research and individual patient settings? A reliability study BMC Musculoskelet Disord 2011;12:249. doi:10.1186/1471-2474-12-249
9. Vaz GF, Freire FF, Gonçalves HM, de Aviz MAB, Martins WR, Durigan JLQ Intra- and inter-rater reliability, agreement, and minimal detectable change of the handheld dynamometer in individuals with symptomatic hip osteoarthritis. PLoS One 2023;18(6):e0278086. doi:10.1371/ journal.pone.0278086
10. Du W, Cornett KMD, Donlevy GA, Burns J, McKay MJ. Variability between different hand-held dynamometers for measuring muscle strength. Sensors 2024;24(6):1861. doi:10.3390/s24061861
11. Ieiri A, Tushima E, Ishida K, Inoue M, Kanno T, Masuda T. Reliability of measurements of hip abduction strength obtained with a hand-held dynamometer Physiother Theory Pract 2015;31(2):146-152. doi:10.3109/ 09593985.2014.960539
12. Krause DA, Neuger MD, Lambert KA, Johnson AE, DeVinny HA, Hollman JH. Effects of examiner strength on reliability of hip-strength testing using a handheld dynamometer J Sport Rehabil 2014;23(1):56-64. doi:10.1123/jsr.2012-0070
13. Mentiplay BF, Perraton LG, Bower KJ, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: A reliability and validity study PLoS One 2015;10(10):e0140822. doi:10.1371/journal.pone.0140822
14. Portney L, Watkins M. Foundations of Clinical Research: Applications to Practice 3rd ed. F.A. Davis Company; 2015.
15. Aerts F, Alwood B. Hand-Held Dynamometry: Guidelines for Daily Clinical Practice. 1st ed. MET Seminars; 2018.
16. Bonett DG. Sample size requirements for estimating intraclass correlations with desired precision. Statistics Med 2002;21(9):1331-1335. doi:10.1002/sim.1108
17. Berchtold A. Test–retest: Agreement or reliability? Methodological Innovations 2016;9. doi:10.1177/2059799116672875
18. Goonetilleke A, Modarres-Sadeghi H, Guiloff RJ. Accuracy, reproducibility, and variability of handheld dynamometry in motor neuron disease. J Neurol Neurosurg Psychiatry. 1994;57(3):326-332. doi:10.1136/jnnp.57.3.326
19. González-Rosalén J, Benítez-Martínez JC, Medina-Mirapeix F, Cuerda-Del Pino A, Cervelló A, Martín-San Agustín R. Intra- and inter-rater reliability of strength measurements using a pull hand-held dynamometer fixed to the examiner’s body and comparison with push dynamometry Diagnostics 2021;11(7):1230. doi:10.3390/diagnostics11071230
20. Ishøi L, Hölmich P, Thorborg K. Measures of hip muscle strength and rate of force development using a fixated handheld dynamometer: Intra-tester intraday reliability of a clinical set-up. Int J Sports Phys Ther 2019;14(5):715-723. doi:10.26603/ ijspt20190715

Moez Glaied1a , Rodney Whiteley1,2
1 Aspetar Orthopaedic and Sports Medicine Hospital, Doha, Qatar, 2 Curtin University, Perth, Australia
Keywords: soleus, plantar-flexion, force, instrumented test, reliability https://doi.org/10.26603/001c.128591
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background/Purpose
The assessment of ankle plantarflexion force is commonly required in athletic performance and clinical rehabilitation settings to assess the integrity of the calf and lower limb musculature. The force generating capacity of the soleus muscle is thought to be important in many aspects of sporting and everyday function. Unfortunately, there are only a few reliable tests describing the assessment of the strength of the soleus muscle, especially in dorsiflexion greater than plantar grade/neutral which mimics ankle joint positions associated with higher ground contact forces. Accordingly, the purpose of this study is to describe the reliability, feasibility, and clinimetrics of a novel test of plantarflexion force in a clinical setting
Methods
Test-retest reliability of a seated isometric plantar flexion strength test performed a minimum of one day apart (maximum of six) using the maximum value of four trials was investigated using a force plate and custom apparatus in 61 volunteer adults (of varying activity levels (Tegner one to ten). Inter-rater reliability (ICC2,1), Bland-Altman, and minimal detectable change values were estimated.
Results
Sixty-one subjects were tested (49 male, 12 female, 39.6±12.6 years, 81.1±13.8kg).
Excellent test-retest reliability was demonstrated (ICC2,1)=0.976 [0.97 to 0.98], p<0.001; and minimal detectable change (MDC) was found to be 118N.
Conclusion
Excellent test-retest reliability and a minimal detectable change of 118N (14.8% bodyweight) were demonstrated for this measure of plantar flexion force. MDC data can inform clinical progression and between-limb differences in healthy and injured individuals. Further, these results can be used to explore the clinical importance of the measurement using the instrumentation.
Level of Evidence
3b
Corresponding Author: Moez Glaied
Email: moez.glaied@aspetar.com a
Orthopedic and Sports Medicine Hospital
Al Buwairda Street - Inside Aspire Zone. P.O. Box 29222
Doha | Qatar www.aspetar.com
Mobile phone: +97477508333
The soleus muscle is an important contributor to performance in daily activities, and sports where fast running, jumping, acceleration, or direction change is required.1 The soleus is considered a primary muscle involved in forward propulsion during gait.2,3 In addition, the soleus acts as an agonist for the anterior cruciate ligament (ACL) during cutting and other “at-risk” movements.3
Performance and strength tests that quantify components of physical fitness and describe key performance indicators allow for measurable clinical goals and objectives.4 In athlete screening, measuring the soleus’ isometric muscle strength provides a quantifiable baseline.5‑9 Patients or athletes may display strength deficits altering either their muscle performance1 or knee stability.3 Accurate assessment of soleus strength is crucial in the rehabilitation and physical preparation phases of lower-limb injured patients and those participating in “on-legs” sports.
Documenting the strength of the ankle plantar flexors, especially the soleus muscle, has been identified as an important gap in clinical and performance practice,10 and necessary reliability data11 for tests allowing detection of soleus muscle strength measures are rare.
In one study, Rhodes et al10 investigated the test-retest reliability of an isometric strength test of the soleus in academy football players. They found high reliability but a slight bias (increased second test score) for their test. Their test placed the participants’ hip, knee, and ankle at 90° of flexion, and they used a soft (Airex®) pad between the thigh and a fixed bar above the point of contact. This testing protocol likely produces lower values12,13 as it allows thigh movement towards hip flexion against the soft pad. Starting the test at 90 degrees, closer to the ankle’s inner range of the plantar flexors – i.e. a more plantarflexed position –also contributes to lower force generation due to active insufficiency 14
In an effort to address the clinical gap of measuring plantar flexion force in a mixed population, an instrumented test of plantar flexion strength was developed at the authors’ facility that underwent an extensive period of pilot testing and refinement. Briefly the device comprises an externally adjustable frame using commercially available clamps mounted to a steel plate upon which a force place is positioned. For participant comfort, the horizontal bar is padded and an additional firm polyurethane pad is placed between this bar and the participant’s knee during the test. In contrast to the method of Rhodes,10 the test position is in greater ankle dorsiflexion, and modifications have been made to the thigh bar padding of the device resulting in better reported participant comfort during our pilot testing. To minimize ankle movement during the test a pre-load of 200N is applied prior to each test, and verified after the patient reports they are relaxed, not pushing, prior to each repetition through inspection of the force trace output from the force plate software. Note that ankle plantarflexion torque while the knee is flexed is not solely created by the soleus muscle as the remainders of the calf musculature have the capacity to generate this torque in
addition to the soleus.15 For convenience, in this investigation this measure will be termed a “soleus strength test”. Despite good reports of feasibility and patient acceptance, before recommending routine clinical implementation, reliability and minimal detectable change data are required to allow accurate interpretation of these scores.9 Hence, the purpose of this study was to investigate the testretest reliability, bias, and describe the minimal detectable changes for the measures obtained during the test of seated plantarflexion strength.
A pilot study suggested that test-retest intra-class correlation (ICC) would be approximately 0.85. A more conservative estimate of 0.45 for the final correlation in planning the sample size was used, and therefore, to achieve the power of 0.95 (alpha of 0.05, two-tailed) a minimum sample size of 54 participants was required. Accordingly, a sample size of 61 was planned to allow for sufficient power despite a 10% dropout rate.16 Inclusion criteria were any consenting healthy volunteer adults at any level of physical activity from a convenience sample. Exclusion criteria were any current or recent injury which would prevent the participants from safely performing the test.
Prior to testing, participants’ gender, age, body weight, physical activity level (Tegner), and medical history were recorded, and subsequently, the estimates of soleus muscle maximal voluntary strength were collected. Note that the term ‘soleus strength test’ is one of convenience and does not reflect the fact that plantarflexion force is likely a combination of a number of muscles in the leg. Ethical approval, including provision for participant safety, confidentiality, and personal data storage, was obtained from both the Research Ethics Approval Committee (University of Bath-Application Number: 2023-021) and the Aspire Zone Foundation Institutional Review Board (Qatar, Application Number: E202301055).
The test setting used in the study comprises a force plate, an adjustable height treatment table and an adjustable bar with padding below the individual’s foot and above the thigh (Figure 1).
Initially, the participants had the procedure explained and demonstrated to them by the investigator, and informed consent was sought and obtained. The participants then performed a minimum of six minutes of stationary cycling, walking, and/or running, along with any exercises they preferred until they felt ready to perform the test. The left limb was arbitrarily tested first. All participants were positioned to favor soleus muscle activation and optimize high plantar flexion force production. Individuals were seated at the end of an adjustable height treatment table with horizon-

Figure 1. The test setting: force plate, adjustable bar, and appropriate padding.
The leading edge of the tape where the participant placed their big toe is 5cm anterior to a vertical (plumb) line from the front-most aspect of the (white) horizontal bar, and this point was colinear with the most anterior aspect of the participant’s knee during testing. The treatment table height is adjusted so that the participant’s thigh is horizontal, and the participant is asked to “relax” their ankle into dorsiflexion before applying 200N of vertical preload (confirmed using the force plate software).
tal thighs and their foot and ankle at rest in dorsiflexion exceeding 90°. The foot was positioned such that the anterior tip of the big toe was placed at the edge of taped mark on the surface of the force plate 5 cm anterior to a vertical (plumb) line from the front-most aspect of the horizontal bar. The anterior aspect of the knee was then vertically aligned to this point (Figure 1).
In this position, after ensuring the participants had relaxed their foot and ankle, and they were comfortable, a preload of 200 Newtons was applied to the lower thigh using an external clamp and an intervening custom polyurethane pad (confirmed from the output of the force plate software). The participants then performed several submaximal contractions to confirm comfort, familiarity, and correct performance of the test. This was repeated until both the examiner and the participants felt ready to perform the test. The participants were then asked to provide a forceful, rapid contraction, which was encouraged with the verbal instructions: “3, 2, 1 Yalla! – PUSH!!, PUSH!!, PUSH!!, PUSH!!”. The test continued for at least three seconds, then after a short rest period where resting pressure of 200N was reaffirmed, the test was repeated until four satisfactory trials were performed on the limb. The test was
then repeated on the opposite limb. A total of four trials per leg, per participant were performed. The entire test was repeated a second time by the same physiotherapist, not less than one day and not more than seven days later. During the second test, the same physiotherapist was blinded to the initial test results to minimize bias in strength measure estimation and recording.17
The pilot study, which informed the sample size calculations, involved five repetitions for each participant. Subsequent analyses, considering maximum value, average value, and varying numbers of repetitions, demonstrated acceptable reliability when using the maximum value observed in the first four trials. Accordingly, the maximum value observed in four trials during the test and retest was utilized in this study in the final analyses.
Initially, descriptive data were calculated, and exploratory analyses were conducted to ensure data veracity Subsequently, ICC(2,1) test-retest reliability (absolute agreement) was calculated, and Bland-Altman analyses were created. Note that Intraclass Correlation Coefficient (ICC2,1) is commonly used to assess test-retest reliability as it evaluates the consistency of measurements across repeated tests when there are multiple raters or assessments under the same conditions. Specifically, it is ideal when each participant is measured by the same raters (or under the same conditions) in both tests, and when the raters or conditions are considered to be a random effect, that is representative of a larger population of potential raters or test conditions. This model also considers both between-participant and within-participant variability, making it a good choice for assessing the stability of measurements over time in testretest studies.18 Reliability descriptors were reported as “excellent” for ICC ≥0.90, “good” for 0.75-0.89, “moderate” between 0.50 and 0.74, and “poor” as <0.50.18 The minimal detectable change19 was calculated as 1.96 * √2 * Standard Error of the Measure,20 and the bias along its 95% confidence interval was described using Bland-Altman analysis.21
Valid data were obtained from all 61 participants. Descriptive values for Age were average = 39.6 years (SD=12.6); median=40, (IQR: 32 to 48), [range: 18 to 61]; and weight = 81.1kg (13.8); 83(71.4 to 89) (Figure 2).
The mean (SD) of force was 1254.1N (276.1N) which equated to a mean (SD) of 1.58 bodyweights (0.29BW) and 15.54 (2.83) N/kg. Excellent test-retest reliability (ICC(2,1) = 0.976 [0.97 to 0.98], p<0.001) was found for the soleus strength test (Figure 3).
Bland-Altman analyses (Figure 4) showed no systematic bias between tests (mean difference= -6.30N [95%CI -21.44 to 8.85N) or across the ranges of observed strengths. The minimal detectable change (MDC) was 118N (0.148 bodyweights). Due to the uneven distribution of the participants’ Tegner scale no meaningful analysis was possible

Figure 2. Participant demographics. Data bars for each category represent relative distributions for that category with respect to their activity level. Supplemental Figures 1-3 present bodyweight-adjusted force values with Tegner scores as covariates.
The results of this study indicate excellent test-retest reliability (ICC2,1=0.976 [0.97 to 0.98], p<0.001) of an instrumented isometric soleus strength test with a minimal detectable change of 118N (14.8% of body weight).11 These findings, along with the patient and therapist-reported clinical acceptance, help fill the gap in clinical and performance practice,10 where the documentation of plantarflex-
ion strength, especially soleus muscle strength, is required. These data help inform the findings from baseline/pre-season strength testing in athletes who may suffer calf muscle, ankle joint, or ACL injuries during the season.
The test-retest reliability calculation (ICC2,1 = 0.976 [0.97 to 0.98], p<0.001) and the Bland-Altman analyses in this study appear slightly better than previously reported methods.10,12 Rhodes et al.10 reported high test-retest correlation (Right: 0.89; Left: 0.79, p<0.05) with no significant difference detected between legs. In addition, McMahon et al.12 identified good to excellent absolute reliability for the peak force for both limbs with no meaningful differences between peak force values at either time point (p=0.306–0.808; g=0.05–0.22).12 The absence of reported minimal detectable changes nor Bland-Altman analysis in the study by McMahon et al. preclude comparison of these aspects to the current research. However, calculating the MDC from the Rhodes et al. data using the same methodology used in the current study, provides an MDC of 531N, which was higher than the calculation reported here of 118N.
This study’s findings concur with the previous researchers10,12 and extend them to a wider population in terms of activity level (Tegner), resistance training experience, and to both genders. In contrast to the protocol of Rhodes et al.10 the participants were placed in greater dorsiflexion which is speculated to have several advantages in terms of ecology and feasibility 22 Firstly, this position more closely mimics the ankle at mid-stance during gait23 and allows for more force generation than in a less dorsiflexed position.14 Secondly, during pilot testing, where a less dorsiflexed position was initially employed, occasionally, individuals experienced painful cramping of the calf muscles during the test.24 In this study, and in all clinical tests subsequently performed in this position, no reports of any adverse effects have been noted at the time of the test or in the ensuing days. Some authors have carefully controlled thigh position,12 but not others.13 In practice, the use of an adjustable height treatment table greatly simplified the positioning of the thigh to horizontal, and due to the standardization of the knee and foot position, the authors are confident the knee position was also consistent throughout tests; however, these angles and heights were not recorded.
The data presented here contrasts with that of the professional rugby players examined by Lee et al.13 and youth football players12 showing a greater range, but approximately similar maximum values: 6.32 to 22.89N/kg; 0.64 to 2.33BW
The narrow confidence intervals for the reliability estimate (0.97 to 0.98) suggest further data collection will not meaningfully increase precision. However, the clinical utility of these findings still needs to be demonstrated despite the excellent reliability found here. One method of doing this would be to document clinical improvement on some metric thought to be associated with plantar flexor strength (e.g., short sprint times or jump height)25 or differences between populations (e.g., healthy compared to injured calf), and then see if there is any association with changes in the

Figure 3. Joint plot of the values for the two tests of soleus muscle strength.
Horizontal and vertical axes represent strength values (N) at the first and second test times respectively with each dot representing an individual’s measurements at these times. The histograms and contours on the secondary axes represent the relative distributions of these values across the ranges of strengths observed. Regression line and its 95% confidence interval overlaid.
strength test values which exceed the minimum detectable change values documented in this study
It is noted that this reliability study was conducted with tests a minimum of 24 hours (up to one week) apart, a condition put in place by the local scientific committee. Several participants reported difficulty performing a maximal test as they were tired from a recent bout of recreational exercise (e.g., a football match that morning). This situation reflects usual clinical practice of re-test some days after an initial test. However, it’s speculated that higher reliability (and therefore lower minimal detectable change values) would have been demonstrated if testing was performed a short period (e.g., an hour) apart where there would be no meaningful change in the individual’s force-generating capacity due to activity 26 Similarly, it is expected there could be better reliability demonstrated if it were possible to control of the individuals’ activity level in the preceding 48 hours.12
The population in this study was a convenience sample. Even though it displayed good representativeness in terms of activity level, age, and body weight, different results may be seen in other populations encountered in clinical practice with varying requirements of soleus muscle strength. The relatively low proportion of female participants (20%, 12 women) suggests some caution should be applied in ex-
trapolating these results to women. Future research may refine and extend these data to other populations. Finally, the test examined is termed, for convenience, a “soleus strength test” however the plantarflexion force generated by the participant will not be limited to activity of the soleus muscle.15 Future research could examine the relative contribution of the soleus muscle, along with the other plantar flexors to determine the relative contributions of each and better allow interpretation of these values in a clinical context.
Excellent test-retest reliability (ICC2,1=0.976) and a minimal detectable change of 118N (14.8% bodyweight) were demonstrated for this measure of plantar flexion force in healthy adults of varying physical activity levels in both genders.
The authors note that while they developed the custom strength testing device, they have no financial links, nor any conflicts of interest perceived or actual regarding this

Figure 4. Bland-Altman analysis for the isometric soleus strength test– vertical axis is the difference for the strength test of an individual at the two time points, and the horizontal axis is the mean of these two measures.
The mean difference and it’s 95% confidence interval (-6.30N [95%CI -21.44 to 8.85N]) is shown the horizontal solid and dashed lines. No apparent bias is evident in the distribution across the range of strengths observed (95% confidence interval of the mean difference includes 0).
device. Specifically, the device is not for sale by the authors or any of their affiliates, and we have provided all information for readers to manufacture their own versions of the same herein or upon reasonable request.
ACKNOWLEDGMENTS
The Rehabilitation Department in Aspetar
Dr Ezio Preatoni; Internal supervisor for the study
The participants who volunteered for the study.
Submitted: June 27, 2024 CST Accepted: December 14, 2024 CST. Published: February 01, 2025 CST.
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Gupta S, Sen ED, Gupta D, Kumar D Surface Electromyographic analysis of soleus muscle activity in different shod conditions in healthy individuals–Systematic review J Arthros Joint Surg 2021;8(4):333-339. doi:10.1016/j.jajs.2021.09.001
2. Dorn TW, Schache AG, Pandy MG. Muscular strategy shift in human running: dependence of running speed on hip and ankle muscle performance. J Exp Biol. 2012;215(Pt 11):1944-1956. doi:10.1242/ jeb.064527
3. Elias JJ, Faust AF, Chu YH, Chao EY, Cosgarea AJ. The soleus muscle acts as an agonist for the anterior cruciate ligament. An in vitro experimental study Am J Sports Med 2003;31(2):241-246. doi:10.1177/ 03635465030310021401
4. O’Donoghue P Principal components analysis in the selection of key performance indicators in sport. Int J Perform Anal Spor. 2008;8(3):145-155. doi:10.1080/24748668.2008.11868456
5. Green B, Lin M, McClelland JA, et al. Return to play and recurrence after calf muscle strain injuries in elite Australian football players. Am J Sports Med 2020;48(13):3306-3315. doi:10.1177/ 0363546520959327
6. Green B, Lin M, Schache A, et al. 035 Can we predict recovery and re-injury following calf muscle strain injury? Br J Sports Med. 2021;55(Suppl 1):A14-A15. doi:10.1136/bjsports-2021-IOC.33
7 Green B, Lin M, Schache AG, et al. Calf muscle strain injuries in elite Australian Football players: A descriptive epidemiological evaluation. Scand J Med Sci Sports 2020;30(1):174-184. doi:10.1111/ sms.13552
8. Green B, McClelland JA, Semciw AI, Schache AG, McCall A, Pizzari T The assessment, management and prevention of calf muscle strain injuries: A qualitative study of the practices and perspectives of 20 expert sports clinicians. Sports Med Open 2022;8(1):10. doi:10.1186/s40798-021-00364-0
9. Stotz A, Maghames E, Mason J, Groll A, Zech A. Maximum isometric torque at individually-adjusted joint angles exceeds eccentric and concentric torque in lower extremity joint actions. BMC Sports Sci Med Rehabil 2022;14(1):13. doi:10.1186/ s13102-022-00401-9
10. Rhodes D, Jeffery J, Brook-Sutton D, Alexander J. Test-retest reliability of the isometric soleus strength test in elite male academy footballers. Int J Sports Phys Ther 2022;17(2):286-292. doi:10.26603/ 001c.31047
11. Bohannon RW. Minimal clinically important difference for grip strength: a systematic review J Phys Ther Sci 2019;31(1):75-78. doi:10.1589/ jpts.31.75
12. McMahon JJ, Ripley NJ, Comfort P, et al. The kneeling isometric plantar flexor test: preliminary reliability and feasibility in professional youth football. J Funct Morphol Kinesiol 2023;8(4):164. doi:10.3390/jfmk8040164
13. Lee M, Lancaster M, Tulloch L, et al. Normative isometric plantarflexion strength values for professional level, male rugby union athletes. Phys Ther Sport. 2023;61:114-121. doi:10.1016/ j.ptsp.2023.03.007
14. Kawakami Y, Ichinose Y, Fukunaga T Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol (1985) 1998;85(2):398-404. doi:10.1152/ jappl.1998.85.2.398
15. Kunugi S, Holobar A, Kodera T, Toyoda H, Watanabe K. Motor unit firing patterns of lower leg muscles during isometric plantar flexion with flexed knee joint position. J Electromyogr Kinesiol. 2022;67:102720. doi:10.1016/j.jelekin.2022.102720
16. Zou GY Sample size formulas for estimating intraclass correlation coefficients with precision and assurance. Stat Med 2012;31(29):3972-3981. doi:10.1002/sim.5466
17. Karanicolas PJ, Bhandari M, Kreder H, et al. Evaluating agreement: conducting a reliability study J Bone Joint Surg Am 2009;91(Suppl 3):99-106. doi:10.2106/JBJS.H.01624
18. Portney LG, Watkins MP Foundations of Clinical Research: Applications to Practice Vol 892. Pearson/ Prentice Hall; 2009.
19. Gatchel RJ, Mayer TG. Testing minimal clinically important difference: consensus or conundrum? Spine J. 2010;10(4):321-327. doi:10.1016/ j.spinee.2009.10.015
20. Koumantakis GA, Winstanley J, Oldham JA. Thoracolumbar proprioception in individuals with and without low back pain: intratester reliability, clinical applicability, and validity. J Orthop Sports Phys Ther 2002;32(7):327-335. doi:10.2519/ jospt.2002.32.7.327
21. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1(8476):307-310.
22. Hebert-Losier K, Schneiders AG, Newsham-West RJ, Sullivan SJ. Scientific bases and clinical utilisation of the calf-raise test. Phys Ther Sport 2009;10(4):142-149. doi:10.1016/j.ptsp.2009.07.001
23. Kang MH, Oh JS. Relationship between weightbearing ankle dorsiflexion passive range of motion and ankle kinematics during gait. J Am Podiatr Med Assoc. 2017;107(1):39-45. doi:10.7547/ 14-112
24. Schwellnus MP Skeletal muscle cramps during exercise. Phys Sportsmed. 1999;27(12):109-115. doi:10.3810/psm.1999.11.1116
25. Comfort P, Dos’ Santos T, Beckham GK, Stone MH, Guppy SN, Haff GG. Standardization and methodological considerations for the isometric midthigh pull. Strength Cond J 2019;41(2):57-79.
26. Parcell AC, Sawyer RD, Tricoli VA, Chinevere TD. Minimum rest period for strength recovery during a common isokinetic testing protocol. Med Sci Sports Exerc. 2002;34(6):1018-1022. doi:10.1097/ 00005768-200206000-00018

Supplementary Figure 1. Bland-Altman analysis for the isometric soleus strength test– vertical axis is the difference for the strength test of an individual at the two time points, and the horizontal axis is the mean of these two measures.
The mean difference and it’s 95% confidence interval (-6.30N [95%CI -21.44 to 8.85N]) is shown the horizontal solid and dashed lines. Shading of the individual points represents the Tegner activity level for the individual. No apparent bias is evident in the distribution across the range of strengths observed (95% confidence interval of the mean difference includes 0).

Supplementary Figure 2. Joint plot of the values for the two tests of soleus muscle strength.
Horizontal and vertical axes represent strength values (percent bodyweight) at the first and second test times respectively with each dot representing an individual’s measurements at these times. The individual dots are shaded with that individual’s Tegner value. The histograms and contours on the secondary axes represent the relative distributions of these values across the ranges of strengths observed. Regression line and its 95% confidence interval overlaid.

Supplementary Figure 3. Joint plot of the values for the two tests of soleus muscle strength.
Horizontal and vertical axes represent strength values (percent bodyweight) at the first and second test times respectively with each dot representing an individual’s measurements at these times. The point colours are shaded to represent the Tegner value for the individual as are the marginal contour on the secondary axes representing the relative distributions of these values across the ranges of strengths observed. Regression line and its 95% confidence interval overlaid.
Supplemental Figure 1
Download: https://ijspt.scholasticahq.com/article/128591-excellent-reliability-for-an-instrumented-test-of-ankleplantarflexion-force/attachment/262588.docx?auth_token=JC8rif09Hk61TfCbTOO5
Supplemental Figure 2
Download: https://ijspt.scholasticahq.com/article/128591-excellent-reliability-for-an-instrumented-test-of-ankleplantarflexion-force/attachment/262587.docx?auth_token=JC8rif09Hk61TfCbTOO5
Supplemental Figure 3
Download: https://ijspt.scholasticahq.com/article/128591-excellent-reliability-for-an-instrumented-test-of-ankleplantarflexion-force/attachment/262589.docx?auth_token=JC8rif09Hk61TfCbTOO5

van Heeswijk K, Spek D, Muijsenberg J, et al. Reliability of Ultrasound Based Compressibility of the Lower Leg Anterior Tibial Muscle Compartment in Healthy Volunteers. IJSPT. 2025;20(2):276-285. doi:10.26603/001c.128284
Kay van Heeswijk1a , Daniëlle Spek1 , Jesse Muijsenberg1 , Loes Janssen1 , Michiel Winkes2 , Adwin R. Hoogeveen3 , Marc Scheltinga1
1 Surgery, Máxima Medisch Centrum, 2 Surgery, VieCuri Medisch Centrum, 3 Sports Medicine, Máxima Medisch Centrum
Keywords: cecs, non-invasive, repeatability, intra-observer, inter-observer https://doi.org/10.26603/001c.128284
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Background
Some individuals have exercise-induced lower leg pain (ELP) caused by a chronic exertional compartment syndrome (CECS). As intracompartmental muscle pressure measurements are invasive with suboptimal test characteristics, other diagnostic tools are needed. Recently, ultrasound-based muscle compartment thickness analysis at 10mmHg (d10) and 80mmHg (d80) external pressure was introduced for this purpose. The difference in compartment thickness at these two external pressures induced by the study device is used to calculate muscle compressibility, a possible marker for CECS.
Purpose
The purpose of this study was to investigate the reliability of a novel ultrasound compressibility technique using two distinct internal landmarks at the lower leg in a diverse group of asymptomatic adults.
Study design
Cross-sectional study
Methods
Healthy volunteers (n=35; 21 female; median age 40 years, range 19-72; BMI 24.1 kg/m2, range 18.3-31.6) not having ELP underwent serial compressibility measurements (n=1678) of both legs by three observers at the tibialis anterior (TA) using the interosseous membrane (IM) and transition zone IM to tibial bone (TZIT) as internal landmarks. Inter- and intra-observer reliability was calculated for values of d10, d80 and compressibility using intraclass correlations (ICC).
Results
TA compartments are less compressible using the IM landmark compared to the TZIT landmark (10.5% vs 12.5%; p<0.001). Inter-observer ICC for IM was always higher (d10 0.85; d80 0.82; compressibility 0.51) than for TZIT (d10 0.65; d80 0.53; compressibility 0.20). The intra-observer reliability for d10 and d80 was excellent (ICC>0.90) for all three observers. ICC of compressibility varied among observers and ranged from 0.76 to 0.48, with higher ICCs demonstrated for IM compared to TZIT
Conclusion
Ultrasound based anterior tibial muscle compressibility measurements have moderate inter-observer reliability and excellent intra-observer reliability if the interosseous
Corresponding author: Kay van Heeswijk
Telephone number: +31642095633 a
Address: Department of Surgery, Máxima Medical Center, P.O. Box 7777, 5500 MB Veldhoven, the Netherlands
E-mail: k.vanheeswijk@mmc.nl / kayvanheeswijk@hotmail.com
membrane is used as internal landmark. Future studies are aimed to test muscle compressibility after exercise and in CECS.
Level of Evidence
Level 3
Some individuals have exercise-induced lower leg pain (ELP) that is occasionally caused by a chronic exertional compartment syndrome (CECS).1 The pathophysiology of CECS is likely associated with an abnormal muscle pressure build-up. Most often, CECS occurs in the anterior tibial compartment (ant-CECS). Symptoms are anterior lower leg pain and tightness occurring during and after exertion.2 If three to six months of conservative measures including gait retraining are to no avail, a diagnostic intracompartmental pressure measurement (ICPM) is advised.3 However, this invasive technique is not patient friendly, and the execution is operator dependent with low diagnostic specificity 4, 5
A range of non-invasive tools are currently being tested for diagnosis of ant-CECS.6,7 An ultrasound (US) technique combined with tissue pressure analysis has been used to diagnose acute compartment syndrome (ACS).8‑10 However, methodologies of US studies for detection of CECS are not standardized and reports lack detail on external force applied.11‑13 Standardization of methodology is crucial for interpreting compartment cross sectional diameter (thickness) or compressibility 14,15
Recent researchers have investigated tibialis anterior (TA) compartment compressibility with a pressure manometer fixed to the US probe demonstrating the importance of standardizing externally applied pressure.14‑16 It was concluded that this method is reliable with very high intra- and inter-observer correlations. These findings suggest that US with tissue pressure analysis may play a diagnostic role in suspected CECS. The purpose of the present study was to investigate the reliability of a novel US compressibility technique using two distinct internal landmarks on the lower leg in a diverse group of asymptomatic adults.
The study was performed at Máxima Medical Center, Veldhoven between February and June 2023. The departments of sports medicine and surgery are having a long-time collaborative interest in the management of patients with ELP Yearly approximately 300 patients with suspected CECS undergo analysis including ICPM and treatment at this institution. Procedures of the study complied with the Declaration of Helsinki (1964) and its amendments. The study protocol was approved by MMC’s medical ethical testing committee (NL82601.015.22). In addition, safety of the tool has been confirmed in recent studies.8,9,14‑16
Study participants were locally recruited among relatives and colleagues via public talks and advertisements. They were eligible if >18 years of age without a history of muscle disorders or arteriovenous diseases. The absence of previous lower leg traumas or ELP related complaints (pain, tightness, cramps etc.) were confirmed by completion of a modified Netwerk Inspannings Afhankelijke PijnSyndromen (NIAPS) questionnaire. This questionnaire has been standardly used in a number of Dutch hospitals at the intake of ELP patients since 2013.17 Potential participants were excluded if they judged that they were not able to complete two five-minute walks at 5.5km/hour with a 15% slope (n=1).
Three observers participated in this study They had no earlier professional experience with US imaging techniques. Prior to study initiation, they were informed on specifics of the device and the measurement protocol by the manufacturing company, including subject positioning. Their protocol indicates that measurements should be performed while individuals are positioned on the side with full medial leg support with the transition zone interosseous membrane to tibial bone (TZIT) as the internal landmark. Additionally, three one-hour sessions were used for training in the handling and positioning of the study device, operating of the equipment and interpretation of the data in accordance to the study protocol. Goals of attaining a SD <0.5 for d10 and d80, and a SD <1.5 for compressibility after four measurements were achieved.
The study device (CPMX1, Compremium AG, Switzerland) has previously been described in detail.15 In short, the tool consists of an US probe with an integrated pressure sensor connected to a tablet. The probe is manually placed on an external landmark of the lower leg and is directed towards an internal landmark. As the device is time-synchronized, images are automatically obtained at 10mmHg and 80mmHg external US probe pressure. After manually marking the superficial and deep muscle landmark on the screen, the integrated software displays compartment thickness at both pressures (d10, d80; millimeters) and calculates muscle compressibility using a formula (d10 – d80) / d10 * 100%). A single measurement takes approximately 20-30 seconds.

Figure 1. Observer and participant set-up for right hand dominant observers. The left panel shows a measurement of the left leg. On the right panel, a measurement of the right leg is depicted. Participants rested in supine position with a triangular pillow at their knee and heels on the bench. The probe was held in the right hand of the observer and the US screen was in front of the observer.
The study participants were dressed in shorts wearing sport or comfortable shoes and rested for five minutes on an examination bench in supine position in a dedicated examination room with a stable 20-degree centigrade temperature prior to a measurement. Both legs were supported at the knee with a triangular pillow and supported on the ankle joints as recently suggested by van Heeswijk et al. (Figure 1).15 The distance between fibular head at the knee and lateral malleolus at the ankle was measured. At 2/5th of this distance, some 2 centimeters lateral to the anterior tibial crest, the US probe position was provisionally marked with a skin marker Both earlier and recent studies suggested that, apart from the TZIT, the interosseous membrane (IM) may also serve well as internal landmark.10,12,13, 15 At this provisional mark, the probe was oriented perpendicularly towards the internal landmarks (Figure 2). When these were clearly visible, they received their respective definitive skin mark that was used by all three observers. The first observer (OB1) completed the measurement protocol, followed by the second (OB2) and third observer (OB3). Order of observers, leg of measurement (left or right), and internal landmark (IM or TZIT) were randomized.
Intra-observer and inter-observer reliability were obtained by having the observers execute a compressibility measurement four times in each configuration (right legIM, right leg-TZIT, left leg-IM, left leg-TZIT) without excluding potential false or wrong measurements. By doing so, a total of 4x4=16 compressibility measurements per observer per study participant were performed. Observers were blinded for each other’s measurements.
The primary outcome variable was inter-observer reliability expressed as intraclass correlation (ICC) regarding values of compartment thickness at two different pressures (10 and 80 mmHg) and compressibility. A secondary outcome variable was intra-observer reliability regarding these values,
expressed as ICC as well. Additional possible confounding variables of interest were age (<30 years, 30-49 years, ≥50 years), gender, and BMI (<25kg/m2 , ≥25kg/m2).
The sample size was determined using G*Power 3.1.18 Calculations were based on published data.14,15,19 A one sided 95% target confidence interval width of 0.05 aiming for an ICC≥0.75 based on three observers resulted in a sample size of n=140. As two different internal landmarks per leg per participant were observed, 35 participants were required.
Data were analyzed using SPSS IBM 22. Data variables were checked for normality using kurtosis, skewness and histograms. If normal, they were depicted as mean (± standard deviation; SD). If the distribution was non-normal, data were depicted as median (interquartile range; IQR).
First, mean (or median) compartment thickness at 10 mmHg (d10), compartment thickness at 80 mmHg (d80) and compressibility were averaged across participants and observers. It was tested whether these variables differed between the two landmarks (TZIT and IM), or between left and right leg measurements using student t-tests for normal distributed data and Mann-Whitney U for non-normal distributed data. The literature suggested the possible presence of differences between the internal landmarks or side of measurement.14,15 Similarly, these variables were compared between groups based on age, gender or BMI category
Second, inter-observer reliability based on d10, d80 and compressibility was presented as ICC with 95%-confidence interval. The data were based on the averages of the four repeat measurements per observer An absolute agreement with two-way random single observer model was used. In addition, ICCs were presented separately for the two landmarks (TZIT and IM) or for the left and right leg measurements. Data were considered significant for an α<0.05.

Figure 2. On the left, an US image of the anterior tibial compartment at 10mmHg of external pressure using the transition zone interosseous membrane to tibial bone (TZIT) as internal landmark is depicted. Interrupted orange line shows orientation of probe towards internal land mark. On the right, a schematic representation is depicted. From top to bottom: subcutaneous tissue (blue), superficial muscle sheath (red), anterior compartment (salmon), interosseous membrane (red), deep compartment (orange), bones (white).
An ICC <0.5 was considered as poor, 0.5-0.7 as moderate, 0.7-0.9 as good and >0.9 as excellent according to Koo et al.20
Intra-observer reliability of d10, d80 and compressibility were presented for each observer separately as ICCs with 95%-CI. Again, ICCs were based on the averages of the four repetitions and as an absolute agreement with a two-way random single measure model. In addition, ICCs were presented separately for the two landmarks (TZIT and IM) or for the left and right leg measurements.
To investigate the influence of number of measurements on the primary outcome (inter-observer reliability), ICCs were calculated based on one, two, three and four repetitions per measurement site. Absolute agreement was calculated using a two-way random average measure model for the two landmarks (TZIT and IM) separately
All 35 study participants provided verbal and written consent and completed the measurements without reporting any discomfort. The study population consisted of 21 females and 14 males with a median age of 40 years (range, 19-72 years) and an average BMI of 24.1 kg/m2 (range, 18.3-31.6 kg/m2). Two of a total of 1680 measurements resulted in a negative compressibility value and were excluded as per protocol.
Overall, anterior lower leg compartments were thicker and less compressible using the IM landmark compared to the TZIT landmark. No differences in thickness and com-
pressibility were observed regarding left and right leg. As was expected, male compartments were thicker than female compartments. Interestingly, age did not influence thickness. Details of thickness of the TA using the two different internal landmarks for males and females, age groups, and BMI groups are listed in Table 1
The overall ICC of d10 and d80 measurements combined for left leg, right leg, IM and TZIT based on the average of four repetitions was considered good, but poor for compressibility
Figure 3 depicts ICC subdivided regarding internal landmark and leg side. The ICCTZIT for d10 was 0.65 (0.470.77), for d80 was 0.53 (0.29 - 0.70), and for compressibility was 0.20 (0.05 - 0.37). Conversely, the ICCIM was always higher (d10 0.85 (0.78 - 0.90), d80 0.82 (0.73 - 0.88), as was compressibility 0.51 (0.37 - 0.64)). These data indicate that measurements using the IM internal landmark were more reliable than the TZIT as internal landmark.
Repeatability was different between the two legs. For instance, the ICCleft for d10 was 0.78 (0.67 - 0.86), for d80 was 0.70 (0.54 - 0.81), and for compressibility was 0.24 (0.100.40). The ICCright was 0.76 (0.65 - 0.85) for d10 and 0.70 (0.53 - 0.81) for d80 but higher for compressibility 0.43 (0.25 - 0.58). These data indicate that compressibility measurements on the right leg were more reliable.
Table 1. Anterior tibial muscle compartment thickness (in mm) using two different internal landmarks (TZIT, IM) with 10 and 80 mmHg external US probe pressure in healthy volunteers.
d10 compartment thickness at 10 mmHg external pressure; d80 compartment thickness at 80 mmHg external pressure; Compressibility (d10-d80)/d10 x 100%. SD Standard Deviation; IQR Inter Quartile Range; BMI Body Mass Index *T-test p<0.05 for comparison with Male; † Mann-Whitney U p<0.05 for comparison with 30-50 years and ≥50 years; ‡T-test p<0.05 for comparison with BMI<25
INTRA-OBSERVER RELIABILITY
The ICC for compartment thickness measurements using either 10 or 80 mmHg external probe pressure (d10, d80) were excellent for all three observers if TZIT, IM, left and right leg measurements were combined.
Focusing on the individual observers (OB1, OB2 and OB3), ICCOB1 for d10 was 0.97 (0.96 - 0.98), 0.96 (0.950.97) for ICCOB2, and 0.91 (0.89 - 0.93) for ICCOB3 For d80 the ICCOB1 was 0.97 (0.96 - 0.97), ICCOB2 was 0.95 (0.93 - 0.96), and ICCOB3 was 0.90 (0.87 - 0.92). If outcome was subdivided for TZIT, IM, left and right leg, ICC remained excellent for two of the three observers. However, OB3 scored some ICCs as ‘good’ instead of excellent. ICCs for compressibility differed among the three observers. Combined for both the TZIT, IM, left and right leg. ICCOB1 was 0.76 (0.70 - 0.81), ICCOB2 0.65 (0.58 - 0.72), and ICCOB3 0.48 (0.39 - 0.56). Figure 4 depicts ICCs for three observers in relation to internal landmarks and leg side.
Considering all measurements combined, the inter-observer ICC for d10 and d80 for the first measurement were 0.90 (0.85 - 0.93) and 0.87 (0.78 - 0.92), respectively Performing additional measurements did not further improve their ICCs.
The ICC for both landmarks combined were for one, two, three and four repetitive compressibility measurements 0.53 (0.36 - 0.65), 0.58 (0.42 - 0.70), 0.60 (0.43 - 0.71), and 0.61 (0.44 - 0.73), respectively (Figure 5, lower panel). However, the IM landmark repeatability was higher compared to
the TZIT (Figure 5, upper and middle panel). Generally, a stable ICC was achieved after two repetitive measurements.
The diagnosis chronic exertional compartment syndrome (CECS) causing exercise-induced leg pain (ELP) is in part based on a suggestive history and physical examination. The current gold standard is provided by an intracompartmental pressure measurement (ICPM), but this tool is associated with several disadvantages. Besides its invasive character, results may be suboptimal as it lacks a potential to predict fasciotomy outcome. Recently, researchers have suggested that ultrasound (US) based techniques may possibly be used to diagnose suspected CECS of the TA.2,6,7,19 The aim of the present study was to investigate the reliability of a new US compressibility technique using two distinct lower leg internal landmarks in a heterogenous group of asymptomatic individuals. Findings of the present study demonstrate moderate to good reliability for the proposed technique. In addition, measurements at proximal portions of the TA using the interosseous membrane (IM) as internal landmark are more reliable compared to the transition zone IM to tibial bone (TZIT).
A small number of studies have reported on the validity of US in possible compartment syndromes.6,11,13,21 Birtles et al. found differences in TA cross-sectional area in CECS patients compared to healthy controls.21 Wasserman and Oschman claimed that a preoperative change of compartment thickness during exercise in CECS was associated with a successful treatment outcome.11 However, muscle compartment thickness is often assessed using a random and

Figure 3. Inter-observer reliability (n=3) expressed as intraclass correlation (two-way random, absolute agreement, assuming single measures) for values of compartment thickness at 10mmHg external pressure (d10; blue), 80mmHg external pressure (d80; orange) and compressibility (grey). Values are mean of four measurements in 35 volunteers. Measurements are specified for internal landmark (IM, interosseous membrane; TZIT, transition zone interosseous membrane to tibia) and leg (left or right).
not literature supported US probe position. Moreover, the force applied by the US probe is frequently not monitored. The present study demonstrates that compressibility values and compartment thickness vary depending on the internal landmarks. This finding highlights the importance of standardizing probe placement and the pressure applied during measurements to ensure reliable results.
Recent studies focused on optimizing methodology of US techniques by varying externally exerted US probe pressure. The technique was recently found to accurately detect acute compartment syndromes.8,16,22 A study in ten human volunteers measured TA compartment compressibility at two different US probe induced external pressures.14 It was concluded that the method had potential with high intra- and inter-observer correlations. Sellei et al. found a 0.93 ICC between TA compressibility using an US technique and ICPM in six trauma patients with possible ACS.8 Another feasibility study testing a next generation US probe with an integrated pressure sensor found that compressibility measurements at the lower leg with knee and heel support using the IM as an internal landmark yielded the lowest variability (ICC 0.98).15 These studies indicate that US imag-
ing with an integrated tissue pressure analysis mode can determine TA compartment compressibility and may possibly play a diagnostic role in ant-CECS. However, these results required confirmation in larger group of humans of different ages, BMI and sex. The present study in 35 resting healthy individuals demonstrates that the internal landmark IM yields more reliable test results compared to the TZIT
The current study also tested the potential influence of ultrasonographist position relative to the study subject. As a rule, an observer sits at the right side of the patient during examination. However, this preferential position may possibly introduce bias such as different angles towards internal landmark and altered pressure distribution of left and right leg measurements. For instance, Anwander et al. found a significant 1.8% compressibility difference between the right and left leg, possibly explained by position of the observer or anatomical difference.14 In that study, the observer was seated at the right side of the participant at all times. In the present study, the dominant hand of observers (all three right-handed) was used for both legs. As a consequence, the observer faced the participant during a

Figure 4. Intraclass correlation of compressibility values based on three observers each executing a series of four measurements in four different configurations in 35 volunteers (two-way random, absolute agreement, assuming single measures). The measurements (n=560 per observer) are depicted as added altogether (overall) and specified for internal landmark (IM, interosseous membrane; TZIT, transition zone interosseous membrane to tibia)) and leg (left or right).
right leg measurement but was oriented towards the participant’s feet during a left leg measurement (Figure 1). Significant compressibility left-right differences using either landmark were not observed as expected as healthy person’s legs are identical. However, the intraclass correlation of left leg measurements were overall lower compared to the right side suggesting that US measurements for bilateral CECS should be executed with the observer facing the patient.
Interclass correlations in the current study are lower compared to previous studies.8,14,15 One of the contributing factors could be the lack of observer experience. The study aimed to test the technique in a real-world situation reflecting the performance of average young doctors instead of highly experienced ultrasonograpers. The combination of limited US experience, a different observer positioning depending on measurement of left and right leg, and the spread of measurements over consecutive months may have contributed to this moderate ICC. In addition, earlier data were obtained from populations who were small or having extreme values leading to relatively high ICC values.8,14,15 It is expected that compressibility in
CECS patients will deviate from healthy volunteers leading to higher values of ICC.
A possible study limitation is a minimal number of internal landmarks. However, it should be appreciated that earlier studies focused on just the TZIT. As previous literature showed that the IM was also an option, it was decided to compare these two.13,15 During this study, the observers experienced that the TZIT was more difficult to identify compared to the IM, likely influenced by lack of ultrasound experience. An additional limitation is generalizability The volunteers had no ELP and were in a resting state. The present study should thus be regarded as generating normal lower leg muscle compressibility values associated with sex, BMI, and age in normals. TAs thicknesses was not related to age. This may be due to the active lifestyle of our healthy volunteers. Depending on type of internal landmark, compressibility differed among age groups. Small sample sizes and varying subcutis thickness are potential causes. The latter has not been analyzed in this study. Future studies in larger populations following exertion and in patients possibly having CECS should include subcutis thickness measurements.

Figure 5. Inter-observer reliability for compressibility of three observers for both the left and right leg’s measurements combined for n=35 participants using interclass correlation (ICC) with 95% confidence interval as error bars (two-way random, absolute agreement, assuming average measures). A darker color indicates the ICC after addition of one consecutive measurement (e.g. second, third or fourth measurement). IM = interosseous membrane, TZIT = transition zone interosseous membrane to tibal bone.
This study reports on specifics of a novel US technique for non-invasively determining lower leg anterior tibial muscle compressibility The use of the interosseous membrane as internal reference point resulted in most optimal reliability test results. Additionally, observer positioning is of importance as reliability improved when an operator was oriented towards the participant. Future studies should aim to test muscle compressibility after exercise and in CECS patients.
The study devices were provided by the manufacturer free of charge. Apart from instructions on use and maintenance of the study devices, the manufacturer was not involved in
any way in the conduct, analysis, or reporting of the results of this study
The support of Stichting Stimuleren Sportgeneeskunde Zuidoost-Brabant is greatly acknowledged. The study devices (CPMX1) were provided by Compremium AG, Switzerland.
Submitted: July 16, 2024 CST Accepted: November 22, 2024
CST. Published: February 01, 2025 CST.
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Chandwani D, Varacallo M. Exertional compartment syndrome. In: StatPearls StatPearls Publishing LLC; 2023.
2. Tarabishi MM, Almigdad A, Almonaie S, Farr S, Mansfield C. Chronic exertional compartment syndrome in athletes: An overview of the current literature. Cureus 2023;15(10):e47797 doi:10.7759/ cureus.47797
3. Dean RS, Farley KX, Waterman BR, Guettler J, Bicos J. Chronic exertional compartment syndrome is frequently diagnosed through static compartment pressure measurements and managed with fasciotomy: A systematic review J ISAKOS 2024;9(1):71-78. doi:10.1016/j.jisako.2023.09.005
4. Large TM, Agel J, Holtzman DJ, Benirschke SK, Krieg JC. Interobserver variability in the measurement of lower leg compartment pressures. J Orthop Trauma. 2015;29(7):316-321. doi:10.1097/ bot.0000000000000317
5. Pasic N, Bryant D, Willits K, Whitehead D Assessing outcomes in individuals undergoing fasciotomy for chronic exertional compartment syndrome of the leg. Arthroscopy: J Arthrosc; Rel Surg 2015;31(4):707. doi:10.1016/j.arthro.2014.10.018
6. van der Kraats AM, Winkes M, Janzing HMJ, Eijkelenboom RPR, de Koning MTG. Review of reliable and valid noninvasive tools for the diagnosis of chronic exertional compartment syndrome. Orthop J Sports Med 2023;11(1):23259671221145151. doi:10.1177/23259671221145151
7. Ritchie ED, Vogels S, Van Dongen TCF, et al. Systematic review of innovative diagnostic tests for chronic exertional compartment syndrome. Int J Sports Med. 2022;44(01):20. doi:10.1055/a-1866-5957
8. Sellei RM, Wollnitz J, Reinhardt N, et al. Noninvasive measurement of muscle compartment elasticity in lower limbs to determine acute compartment syndrome: Clinical results with pressure related ultrasound. Injury. 2020;51(2):301-306. doi:10.1016/j.injury.2019.11.027
9. Sellei RM, Beckers A, Kobbe P, et al. Non-invasive assessment of muscle compartment elasticity by pressure-related ultrasound in pediatric trauma: A prospective clinical study in 25 cases of forearm shaft fractures. Eur J Med Res. 2023;28(1):296. doi:10.1186/ s40001-023-01232-1
10. Herring MJ, Donohoe E, Marmor MT A novel non-invasive method for the detection of elevated intra-compartmental pressures of the leg. J Visualized Exp 2019(147):e59887 doi:10.3791/59887
11. Wassermann D, Oschman Z. Role of ultrasound as a non-invasive method of diagnosis of chronic exertional compartment syndrome. SA Orthopaedic Journal 2011;10(4):59-65.
12. Brahim F, Zaccardelli W. Ultrasound measurement of the anterior leg compartment. Am J Sports Med 1986;14(4):300-302. doi:10.1177/ 036354658601400410
13. Rajasekaran S, Beavis C, Aly A, Leswick D. The utility of ultrasound in detecting anterior compartment thickness changes in chronic exertional compartment syndrome: A pilot study Clin J Sport Med 2013;23(4):305-311. doi:10.1097/ JSM.0b013e3182856046
14. Anwander H, Büchel L, Krause F, Siebenrock K, Schmid T Tibial anterior compartment compressibility in healthy subject, measured using compression sonography Injury 2022;53(2):719-723. doi:10.1016/j.injury.2021.12.014
15. van Heeswijk K, Janssen L, Heijmans M, Scheltinga M. Ultrasound guided compressibility of the lower leg anterior tibial muscle compartment: A feasibility study. The Phys Sportsmed. 2024:1. doi:10.1080/00913847.2024.2340421
16. Marmor MT, Barker JP, Matz J, Donohoe E, Herring MJ. A dual-sensor ultrasound based method for detecting elevated muscle compartment pressures: A prospective clinical pilot study Injury 2021;52(8):2166-2172. doi:10.1016/ j.injury.2021.02.054
17 de Bruijn JA, van Zantvoort APM, van Klaveren D, et al. Factors predicting lower leg chronic exertional compartment syndrome in a large population. Int J Sports Med 2018;40(01):58-66. doi:10.1055/ s-0043-119225
18. Faul F, Erdfelder E, Buchner A, Lang A. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Meth 2009;41(4):1149. doi:10.3758/brm.41.4.1149
19. Sellei RM, Kobbe P, Hildebrand F. Non-invasive diagnostics in acute compartment syndrome. In: A Comprehensive Review of Compartment Syndrome. ; 2021:14. doi:10.5772/intechopen.97874
20. Koo TK, Li MY A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiro Med 2016;15(2):155-163. doi:10.1016/j.jcm.2016.02.012
21. Birtles DB, Rayson MP, Jones DA, Newham DJ, et al. Effect of eccentric exercise on patients with chronic exertional compartment syndrome. Eur J Appl Physiol. 2003;88(6):565-571. doi:10.1007/ s00421-002-0740-z
22. Mühlbacher J, Pauzenberger R, Asenbaum U, et al. Feasibility of ultrasound measurement in a human model of acute compartment syndrome. World J Emerg Surg. 2019;14(1). doi:10.1186/ s13017-019-0222-9

Alyssa Elder1a , Christopher M. Powers1
1 Biokinesiology, University of Southern California
Keywords: shoulder, shoulder pain, physical examination, biomechanics https://doi.org/10.26603/001c.128049
International Journal of Sports Physical Therapy Vol. 20, Issue 2, 2025
Observational evaluation of arm elevation is a routine part of the examination of patients with shoulder pain and dysfunction. However, the interdependency of the glenohumeral and scapulothoracic joints during arm elevation presents a challenge for clinicians when attempting to characterize movement impairments and underlying causes. Given that identification of movement impairments related to the scapulothoracic joint (i.e. scapular winging or excessive scapular elevation) are more easily observed compared to movement faults at the glenohumeral joint (i.e. superior or anterior translation of the humeral head) an inherent bias may exist in which clinicians prioritize movement impairments and associated physical impairments at the scapulothoracic joint in developing a treatment plan. Interpreting the cause(s) of abnormal scapulothoracic motion without considering the potential influence of the glenohumeral joint (and vice-versa) may lead to faulty clinical reasoning when developing a plan of care.
The purpose of this clinical commentary is to highlight the potential impact of faulty glenohumeral joint mechanics as being contributory to scapulothoracic joint kinematics. We first review the normal kinematics and muscular actions associated with typical arm elevation and then discuss how impairments at the glenohumeral joint may be contributory to faulty scapulothoracic motion. Specifically, we address movement faults characterized by excessive motion of the scapula.
Level of Evidence 5
The shoulder is one of the most intricate joints in the human body owing to the interdependency of multiple bony segments and muscle groups. Functional tasks such as overhead reaching require adequate mobility and coordinated muscular control of the scapulothoracic, glenohumeral, acromioclavicular, and sternoclavicular joints to achieve full, functional range of motion.1,2 Disruptions in any aspect of the shoulder complex may interrupt the normal inter-joint coordination that is characteristic of normal upper limb function.
Observational evaluation of arm elevation is a routine part of the examination of patients with shoulder pain and dysfunction.3 Assessing arm elevation affords clinicians insight into an individual’s functional range of motion and movement quality Importantly, observational assessment of arm elevation is necessary to identify movement impairments that should be considered when developing a treatment plan.
The interdependency of the glenohumeral and scapulothoracic joints during arm elevation presents a challenge for clinicians when attempting to characterize movement impairments and underlying causes. The assessment of arm elevation is further complicated by the fact that identifica-
Corresponding Author:
Alyssa M. Elder PT, DPT
Email: ali.elder@pt.usc.edu a
USC Division of Biokinesiology & Physical Therapy
1540 E. Alcazar St. CHP-155
Los Angeles, CA 90089-9006
Phone: 323.442.1928
Fax: 323.442.1515
tion of movement impairments related to the scapulothoracic joint are easily observed (i.e. scapular winging or excessive scapular elevation) while movement faults at the glenohumeral joint (i.e. superior or anterior translation of the humeral head relative to the glenoid fossa) are more difficult to detect. As such, an inherent bias may exist in which clinicians prioritize movement impairments and associated physical impairments at the scapulothoracic joint in developing a treatment plan.
Because of the inherent interdependency of the glenohumeral and scapulothoracic joints, it is plausible that impairments at one joint could contribute to abnormal motion at the other. For example, abnormal scapular motion during arm elevation may be compensatory in nature owing to mobility or muscle performance impairments at the glenohumeral joint.4 This viewpoint differs from perspectives in which altered scapular kinematics are thought to be the primary cause of shoulder pain and disability.5,6 It is the authors’ contention that interpreting the cause(s) of abnormal scapulothoracic motion without considering the potential influence of the glenohumeral joint (and viceversa) may lead to faulty clinical reasoning when developing a plan of care.
Given the complexity of the shoulder and the inherent challenges in the interpretation of the functional assessment of arm elevation, the purpose of this clinical commentary is to highlight the potential impact of faulty glenohumeral joint mechanics as being contributory to scapulothoracic joint kinematics. First the normal kinematics and muscular actions associated with typical arm elevation are reviewed, and then how impairments at the glenohumeral joint may be contributory to faulty scapulothoracic motion is discussed. Specifically, the authors address movement faults characterized by excessive motion of the scapula. It is hoped that this perspective will provide clinicians and researchers with a framework that can better inform the examination and treatment of various shoulder conditions.
For the purposes of this commentary, arm elevation will be discussed as shoulder motion in the scapular plane. Arm elevation occurs primarily through motions at the glenohumeral and scapulothoracic joints with accessory motions occurring at the acromioclavicular and sternoclavicular joints.5,7,8 While motions at the acromioclavicular and sternoclavicular joints during arm elevation are passive in nature, the glenohumeral and scapulothoracic joints are controlled by separate but interdependent muscular force couples. The muscular force couples at the glenohumeral and scapulothoracic joints produce coordinated motion between the scapula and humerus commonly referred to as scapulohumeral rhythm. A brief overview of the kinematics and muscular actions of the glenohumeral and scapulothoracic joint follows.

Figure 1. The glenohumeral joint is the largest contributor to arm motion during elevation. Shoulder elevation in the scapular plane requires the head of the humerus to glide inferiorly and roll superiorly Figure reproduced with permission from Neumann DA, Kinesiology of the Musculoskeletal System: Foundation for Rehabilitation, 3rd ed., Elsevier, 2017.
GLENOHUMERAL JOINT KINEMATICS & MUSCULAR ACTIONS
The glenohumeral joint is the largest contributor to arm motion during elevation. Shoulder elevation in the scapular plane requires the head of the humerus to glide inferiorly and roll superiorly (Figure 1). These motions prevent the humeral head from impinging on the coracoacromial arch and assist in maintaining an instant center of rotation throughout movement.9‑11 Additionally, the humerus externally rotates within the glenoid fossa during arm elevation to avoid abutment of the greater tubercle and the acromion.12
The muscles responsible for typical glenohumeral joint motion include the four rotator cuff muscles and the deltoid complex. While the deltoid is the prime mover of the humerus, the muscular actions of the rotator cuff provide the foundation by which the arm can elevate. The supraspinatus is particularly important for initiating the spinning action of the humeral head owing to its horizontal line of pull, while the infraspinatus and teres minor provide the external rotation motion of the humerus during arm elevation.13 The combined actions of the subscapularis, infraspinatus and teres minor counteract the superior translation of humeral head resulting from contraction of the deltoid owing to their downward lines of pull (Figure 2). Importantly, the lines of action of all the rotator cuff muscles stabilize the glenohumeral joint through medially di-

Figure 2. The combined actions of the subscapularis, infraspinatus and teres minor counteract the superior translation of humeral head resulting from contraction of the deltoid owing to their downward lines of pull. Figure reproduced with permission from Neumann DA, Kinesiology of the Musculoskeletal System: Foundation for Rehabilitation, 3rd ed., Elsevier, 2017.
rected compressive forces. Apart from the muscular forces, passive structures such as the joint capsule, ligaments, and labrum assist in guiding the intricate arthrokinematics of the glenohumeral joint.14,15
SCAPULOTHORACIC JOINT KINEMATICS & MUSCULAR ACTIONS
In general, the scapula upwardly rotates and tilts posteriorly along the convex surface of the thorax as the arm elevates.16 These motions maintain broad surface contact with the rib cage to allow for force transfer from the upper extremity to the trunk.6,17 Scapular motions provide proper orientation of the glenoid fossa to ensure normal glenohumeral joint arthrokinematics as the arm moves overhead.18
Motion of the scapula along the thorax during arm elevation is the result of a muscular force couple consisting of the upper and lower portions of the trapezius and the serratus anterior (Figure 3).8 Given its origin on the superolateral surfaces of the upper ribs, action of the serratus anterior maintains contact of the scapula against the thorax, thereby facilitating the motions of posterior tilt and rotation as the arm elevates.19 This force couple allows the scapula to upwardly rotate with overhead movement and functions to eccentrically control the scapula during arm lowering.

Figure 3. Motion of the scapula along the thorax during arm elevation is the result of a muscular force couple consisting of the upper and lower portions of the trapezius and the serratus anterior Figure reproduced with permission from Neumann DA, Kinesiology of the Musculoskeletal System: Foundation for Rehabilitation, 3rd ed., Elsevier, 2017.
SCAPULOHUMERAL RHYTHM
During the initiation of arm elevation (0-30°) motion primarily occurs at the glenohumeral joint.20 Little to no scapular motion is observed during this phase. Beyond 30° of arm elevation, the scapula upwardly rotates approximately 1° for every 2° of humeral elevation.21 The coordinated movement of the scapula and the humerus ensures that the length-tension relationship of the rotator cuff muscles is maintained throughout arm elevation.6 Importantly, scapulohumeral rhythm allows for the scapula to function as a moving base for the humerus thereby providing maximum stability as the arm moves overhead.
GLENOHUMERAL JOINT INFLUENCE ON ALTERED SCAPULOTHORACIC JOINT MOTION
There is evidence that altered scapular kinematics are present in individuals with a wide range of shoulder pathologies; however, underlying causes remain speculative and a source of debate.4,22 Specifically, authors have reported that individuals with various shoulder diagnoses exhibit excessive elevation, upward rotation, internal rotation, and anterior tilt.3,23 In the sections below, excessive scapular motions will be discussed in terms of possible glenohumeral impairments in which there is no apparent nerve involvement (ie. neuritis, neuropathy, or peripheral nerve injury).

Figure 4. Example of compensatory scapular elevation and upward rotation owing to inadequacy of the downward spinning action of supraspinatus.
Inadequacy of the rotator cuff force couple has been hypothesized as being contributory to compensatory elevation and/or upward rotation of the scapula.4 Failure of the supraspinatus to provide the downward spinning action of the humeral head during the initiation of arm elevation owing to pain, injury and/or pathology could result in premature scapular elevation and upward rotation. These compensatory motions would serve to initiate arm elevation while preserving the subacromial space. (Figure 4)
The notion that excessive scapular motions during arm elevation could be the result of rotator cuff pathology was highlighted by a recent systematic review that examined the relationship between rotator cuff pathology and scapular dyskinesis.24 The summary of research in this area indicated that persons with rotator cuff tears (specifically supraspinatus) exhibited excessive scapular upward rotation, with greater motion impairments being present in persons with larger tears and those who were symptomatic. The presence of excessive scapular motions in persons with rotator cuff tears is consistent with the fact that superior humeral head migration is common in these individuals.3 Of note, several studies within this systematic review reported that abnormal scapular kinematics normalized following rotator cuff repair surgery, highlighting the fact that the excessive scapular motions observed pre-surgery may have been compensatory in nature to maximize arm function.27,28,29
As mentioned previously, the rolling and spinning action of the humeral head within the glenoid fossa is important to minimize abutment of the humeral head against the coracoacromial arch during arm elevation. In the presence of soft tissue restrictions or capsular tightness, the normal arthrokinematics of the glenohumeral joint can be impacted. In particular, the inability of the humeral head to translate inferiorly could result in the humeral head being positioned superiorly relative to the glenoid fossa. To maintain joint congruency and avoid abutment of the humerus against the coracoacromial arch, the scapula may be obligated to translate superiorly. Evidence in support of this premise is provided by studies that have reported persons

Figure 5. Excessive amounts of scapula internal rotation and anterior tilt could cause the medial border of the scapula to come away from the thorax, giving the appearance of scapular winging. This is often observed in the mid-ranges of arm elevation when the torque on the scapula is greatest.
with adhesive capsulitis exhibit significantly greater scapular elevation and upward rotation on the affected side when compared to the unaffected side.25,26 Additionally, Vermeulen et al. demonstrated that following the utilization of mobilization techniques to treat adhesive capsulitis, patients demonstrated a pattern of scapular motion that was closer to that of their unaffected side.26
EXCESSIVE SCAPULAR INTERNAL ROTATION AND ANTERIOR TILT
As noted above, external rotation of the humerus is required during arm elevation to prevent abutment of the greater tubercle against the acromion.12 Glenohumeral joint impairments that may limit external rotation of the humerus include anterior capsular tightness/stiffness, shortness of the internal rotators of the humerus (ie. subscapularis, latissiumus dorsi, pectoralis muscles) and/or weakness of the posterior rotator cuff musculature (infraspinatus, teres minor). In the presence of limited humeral external rotation, the scapula may compensate through the combined motions of internal rotation and anterior tilt.27, 28 These motions would result in relative external rotation of the humerus with respect to the scapula and allow for greater surface area contact between the glenoid fossa and head of the humerus. Excessive amounts of scapular internal rotation and anterior tilt could cause the medial border of the scapula to come away from the thorax, giving the appearance of scapular winging. (Figure 5)
Glenohumeral stability is necessary during arm elevation to keep the humeral head centered within the glenoid fossa. Asymmetric tension resulting from either hypermobility or hypomobility of the active and passive structures surrounding the joint can lead to excessive translations of the humeral head during arm elevation.29 Glenohumeral joint impairments that may impair stability include hypermobility of the joint capsule, impaired ligamentous or labral integrity, asymmetric anterior or posterior rotator cuff stiffness/tightness and/or disruption of the transverse plane force couple (ie. dynamic imbalance between the subscapu-
laris and infraspinatus/teres minor). In the presence of anterior instability, the humeral head could be positioned anteriorly within the glenoid fossa. To maintain joint stability and congruency, the scapula may compensate through the combined motions of internal rotation and anterior tilt. This premise is supported by several studies that have reported an association between anterior glenohumeral joint instability and excessive scapular internal rotation.15,30‑32 Regardless of the direction of instability, an argument could be made that compensatory motions of the scapula would be necessary to keep the humeral head centered within the glenoid fossa as the arm moves overhead.
As presented above, it is plausible that abnormal scapular motions could be the result of glenohumeral joint impairments. This is not to discount the possibility however that scapulothoracic joint impairments (i.e. impaired muscular control of the scapula) could be contributory As in the case with any form of movement analysis, the question as to whether the observed movement impairment is primary or compensatory needs to be considered.
Since the introduction of the concept of scapular dyskinesis in 1995, scapular stabilization exercises have been a mainstay of shoulder rehabilitation protocols.33‑35 There have been several systematic reviews that have summarized the existing literature related to the efficacy of scapular stabilization exercises in reducing pain and disability in persons with various shoulder diagnoses.36‑38 A consistent theme across these systematic reviews is that scapular stabilization exercises are effective in reducing shoulder pain and disability. Paradoxically, scapular stabilization exercises do not appear to result in a change in scapular kinematics, despite this being the intent.36,39‑41
The disconnect between improvements in shoulder symptoms and the lack of corresponding changes in scapular kinematics is difficult to rectify Exercises commonly prescribed to improve scapular stability (i.e. Y’s and T’s) require significant transfer of forces through the glenohumeral joint, owing to the long lever arm of the upper extremity.11 It is possible that the biomechanical demands of scapular exercises have an unintended positive effect on the glenohumeral joint in that they may provide a strengthening stimulus for the rotator cuff muscles, owing to the need for glenohumeral joint stability In support of this premise, a systemic review by Edwards and colleagues reported that the supraspinatus and infraspinatus muscles exhibit a high-level of activation (75% and 64% of maximal
voluntary isometric contraction respectively) during prone horizontal abduction.42
The high recurrence of shoulder pain in the general population highlights the fact that current treatment approaches for shoulder pain do not provide long-term benefits for many patients.43 Given as such, the underlying principles of shoulder rehabilitation may have to be re-examined. For example, the phrase “distal mobility requires proximal stability” is often used when devising an exercise program for patients with shoulder pain.44 While this statement may be suitable to describe functional movements of the arm, it may not be appropriate when designing an exercise program for shoulder pain.
Regardless of the cause-and-effect relationships between motions of the glenohumeral and scapulothoracic joints, a thorough assessment of the glenohumeral joint should be performed when faulty scapular kinematics are observed during arm elevation. Based on the arguments presented above, such an assessment should include an evaluation of rotator cuff strength and the presence of glenohumeral joint instability or hypomobility Information obtained by the assessment of the glenohumeral joint should be considered when interpreting potential causes of abnormal scapular kinematics (if present).
Observational evaluation of arm elevation is an important element of the examination of the patient with shoulder pain. Due to the complexity of the shoulder owing to the intricate interaction of multiple joints and muscle systems required to achieve normal motion, teasing out cause and effect of the abnormal arm elevation is difficult. The purpose of this clinical perspective was to highlight the possibility that faulty scapular kinematics may be result of glenohumeral impairments. Clinicians should consider this possibility when prioritizing exercises for the patient who presents with shoulder pain and abnormal scapula kinematics. Although evidence exists to support the concepts presented in this clinical perspective, further research in this area is needed.
The authors report no conflicts of interest.
Submitted: May 22, 2024 CST. Accepted: November 22, 2024
CST Published: February 01, 2025 CST
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Ebaugh DD, Spinelli BA. Scapulothoracic motion and muscle activity during the raising and lowering phases of an overhead reaching task. J Electromyog Kinesiol 2010;20(2):199-205. doi:10.1016/ j.jelekin.2009.04.001
2. Turgut E, Pedersen Ø, Duzgun I, Baltaci G. Threedimensional scapular kinematics during open and closed kinetic chain movements in asymptomatic and symptomatic subjects. J Biomech. 2016;49(13):2770-2777 doi:10.1016/ j.jbiomech.2016.06.015
3. Fruth SJ. Fundamentals of the Physical Therapy Examination : Patient Interview and Tests & Measures 2nd ed.; 2018.
4. Ludewig PM, Reynolds JF. The association of scapular kinematics and glenohumeral joint pathologies. J Orthop Sports Phys Ther 2009;39(2):90-104. doi:10.2519/JOSPT.2009
5. Braman L. Shoulder impingement: Biomechanical considerations in rehabilitation. Bone 2011;23(1):1-7. doi:10.1016/ j.math.2010.08.004.Shoulder
6. Kibler WB, Sciascia A. Evaluation and management of scapular dyskinesis in overhead athletes. Curr Rev Musculoskelet Med. 2019;12(4):515-526. doi:10.1007/ s12178-019-09591-1
7 Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: Spectrum of pathology part III: The SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy:J Arthrosc Rel Surg. 2003;19(6):641-661. doi:10.1016/ S0749-8063(03)00389-X
8. Karduna AR, McClure PW, Michener LA, Sennett B. Dynamic measurements of three-dimensional scapular kinematics: A validation study J Biomech Eng 2001;123(2):184-190. doi:10.1115/1.1351892
9. Yamaguchi K, Sher JS, Andersen WK, et al. Glenohumeral motion in patients with rotator cuff tears: a comparison of asymptomatic and symptomatic shoulders. J Shoulder Elbow Surg. 2000;9(1):6-11. doi:10.1016/s1058-2746(00)90002-8
10. Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clinical results and biomechanical rationale. Clin Orthop Relat Res 1991;(267):45-56. doi:10.1097/00003086-199106000-00006
11. Poppen NK, Walker PS. Forces at the glenohumeral joint in abduction. Clin Orthop Relat Res. 1978;(135):165-170. doi:10.1097/ 00003086-197809000-00035
12. Kozono N, Okada T, Takeuchi N, et al. In vivo kinematic analysis of the glenohumeral joint during dynamic full axial rotation and scapular plane full abduction in healthy shoulders. Knee Surg Sports Traumatol Arthrosc. 2017;25(7):2032-2040. doi:10.1007/s00167-016-4263-2
13. Khandare S, Arce RA, Vidt ME. Muscle compensation strategies to maintain glenohumeral joint stability with increased rotator cuff tear severity: A simulation study J Electromyogr Kinesiol 2022;62. doi:10.1016/j.jelekin.2019.07.005
14. Myers JB, Ju YY, Hwang JH, McMahon PJ, Rodosky MW, Lephart SM. Reflexive muscle activation alterations in shoulders with anterior glenohumeral instability Am J Sports Med 2004;32(4):1013-1021. doi:10.1177/0363546503262190
15. Warner JJ, Micheli LJ, Arslanian LE, Kennedy J, Kennedy R. Patterns of flexibility, laxity, and strength in normal shoulders and shoulders with instability and impingement. Am J Sports Med. 1990;18(4):366-375. doi:10.1177/ 036354659001800406
16. Borstad JD, Ludewig PM. Comparison of scapular kinematics between elevation and lowering of the arm in the scapular plane. Clin Biomech 2002;17(9-10):650-659. doi:10.1016/ s0268-0033(02)00136-5
17 Neumann DA, Camargo PR. Kinesiologic considerations for targeting activation of scapulothoracic muscles - part 1: serratus anterior. Braz J Phys Ther 2019;23(6):459-466. doi:10.1016/ j.bjpt.2019.01.008
18. Kibler WB, Sciascia A. The role of the scapula in preventing and treating shoulder instability Knee Surg Sports Traumatol Arthrosc. 2016;24(2):390-397. doi:10.1007/s00167-015-3736-z
19. Camargo PR, Neumann DA. Kinesiologic considerations for targeting activation of scapulothoracic muscles – part 2: trapezius. Braz J Phys Ther 2019;23(6):467-475. doi:10.1016/ j.bjpt.2019.01.011
20. Kijima T, Matsuki K, Ochiai N, et al. In vivo 3-dimensional analysis of scapular and glenohumeral kinematics: Comparison of symptomatic or asymptomatic shoulders with rotator cuff tears and healthy shoulders. J Shoulder Elbow Surg 2015;24(11):1817-1826. doi:10.1016/j.jse.2015.06.003
21. Scibek JS, Carcia CR. Assessment of scapulohumeral rhythm for scapular plane shoulder elevation using a modified digital inclinometer World J Orthop. 2012;3(6):87-94. doi:10.5312/wjo.v3.i6.87
22. Hickey BW, Milosavljevic S, Bell ML, Milburn PD. Accuracy and reliability of observational motion analysis in identifying shoulder symptoms. Man Ther 2007;12(3):263-270. doi:10.1016/j.math.2006.05.005
23. Phadke V, Camargo PR, Ludewig PM. Scapular and rotator cuff muscle activity during arm elevation: A review of normal function and alterations with shoulder impingement. Revista Brasileira de Fisioterapia 2009;13(1):1-9. doi:10.1590/ S1413-35552009005000012
24. Barcia AM, Makovicka JL, Spenciner DB, et al. Scapular motion in the presence of rotator cuff tears: a systematic review. J Shoulder Elbow Surg. 2021;30(7):1679-1692. doi:10.1016/ j.jse.2020.12.01226
25. Fayad F, Roby-Brami A, Yazbeck C, et al. Threedimensional scapular kinematics and scapulohumeral rhythm in patients with glenohumeral osteoarthritis or frozen shoulder. J Biomech. 2008;41(2):326-332. doi:10.1016/j.jbiomech.2007.09.004
26. Vermeulen HM, Stokdijk M, Eilers PHC, Meskers CGM, Rozing PM, Vliet Vlieland TPM. Measurement of three dimensional shoulder movement patterns with an electromagnetic tracking device in patients with a frozen shoulder. Ann Rheum Dis. 2002;61(2):115-120. doi:10.1136/ard.61.2.115
27 Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337
28. Ludewig PM, Reynolds JF. The association of scapular kinematics and glenohumeral joint pathologies. J Orthop Sports Phys Ther 2009;39(2):90-104. doi:10.2519/jospt.2009.2808
29. Harryman BT, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343. doi:10.2106/00004623-199072090-00009
30. Lefèvre-Colau MM, Nguyen C, Palazzo C, et al. Kinematic patterns in normal and degenerative shoulders. Part II: Review of 3-D scapular kinematic patterns in patients with shoulder pain, and clinical implications. Ann Phys Rehabil Med 2018;61(1):46-53. doi:10.1016/j.rehab.2017.09.002
31. Ogston JB, Ludewig PM. Differences in 3-dimensional shoulder kinematics between persons with multidirectional instability and asymptomatic controls. Am J Sports Med. 2007;35(8):1361-1370. doi:10.1177/0363546507300820
32. Eshoj H, Ingwersen KG, Larsen CM, Kjaer BH, Juul-Kristensen B. Intertester reliability and laxity tests in subjects with and without self-reported shoulder problems. BMJ Open 2018;8(2):e018472. doi:10.1136/bmjopen-2017-018472
33. Bury J, West M, Chamorro-Moriana G, Littlewood C. Effectiveness of scapula-focused approaches in patients with rotator cuff related shoulder pain: A systematic review and meta-analysis. Man Ther. 2016;25:35-42. doi:10.1016/j.math.2016.05.337
34. Diercks R, Bron C, Dorrestijn O, et al. Guideline for diagnosis and treatment of subacromial pain syndrome. Acta Orthop 2014;85(3):314-322. doi:10.3109/17453674.2014.920991
35. Escamilla RF, Yamashiro K, Paulos L, Andrews JR. Shoulder muscle activity and function in common shoulder rehabilitation exercises. Sports Med 2009;39(8):663-685. doi:10.2165/ 00007256-200939080-00004
36. Takeno K, Glaviano NR, Norte GE, Ingersoll CD Therapeutic interventions for scapular kinematics and disability in patients with subacromial impingement: A systematic review J Athl Train 2019;54(3):283-295. doi:10.4085/1062-6050-309-17
37 Ravichandran H, Janakiraman B, Gelaw AY, Fisseha B, Sundaram S, Sharma HR. Effect of scapular stabilization exercise program in patients with subacromial impingement syndrome: a systematic review J Exerc Rehabil 2020;16(3):216-226. doi:10.12965/jer.2040256.128
38. Nodehi Moghadam A, Rahnama L, Noorizadeh Dehkordi S, Abdollahi S. Exercise therapy may affect scapular position and motion in individuals with scapular dyskinesis: a systematic review of clinical trials. J Shoulder Elbow Surg 2020;29(1):e29-e36. doi:10.1016/j.jse.2019.05.037
39. Struyf F, Cagnie B, Cools A, et al. Scapulothoracic muscle activity and recruitment timing in patients with shoulder impingement symptoms and glenohumeral instability J Electromyogr Kinesiol 2014;24(2):277-284. doi:10.1016/j.jelekin.2013.12.002
40. Turgut E, Duzgun I, Baltaci G. Effects of scapular stabilization exercise training on scapular kinematics, disability, and pain in subacromial impingement: A randomized controlled trial. Arch Phys Med Rehabil 2017;98(10):1915-1923.e3. doi:10.1016/j.apmr.2017.05.023
41. Jafarian Tangrood Z, Sole G, Ribeiro DC. Is there an association between changes in pain or function with changes in scapular dyskinesis: A prospective cohort study. Musculoskelet Sci Pract. 2020;48. doi:10.1016/j.msksp.2020.102172
42. Edwards PK, Ebert JR, Littlewood C, Ackland T, Wang A. A systematic review of electromyography studies in normal shoulders to inform postoperative rehabilitation following rotator cuff repair. J Orthop Sports Phys Ther 2017;47(12):931-944. doi:10.2519/ jospt.2017.7271
43. Hogan C, Corbett JA, Ashton S, Perraton L, Frame R, Dakic J. Scapular dyskinesis is not an isolated risk factor for shoulder injury in athletes: A systematic review and meta-analysis. Am J Sports Med. 2021;49(10):2843-2853. doi:10.1177/ 0363546520968508
44. Roche SJ, Funk L, Sciascia A, Kibler WB. Scapular dyskinesis: the surgeon’s perspective. Shoulder Elbow. 2015;7(4):289-297 doi:10.1177/1758573215595949

Meister KM, Evans D, Wilk KE, Arrigo CA. Ulnar Collateral Ligament Hybrid Reconstruction Surgery & Rehabilitation in the Overhead Athlete. IJSPT 2025;20(2):294-306. doi:10.26603/001c.128512
Keith M Meister, MD1 , Daniel Evans, MS, PT, OCS, CSCS2,3a , Kevin E Wilk, PT, DPT, FAPTA4,5 , Christopher A Arrigo, MS, PT, ATC6
1 TMI Sports Medicine, 2 Director of Rehabilitation, TMI Sports Medicine, 3 Rehabilitation Consultant, Texas Rangers Baseball Club, 4 Associate Clinical & Co-Founder, Champion Sports Medicine, 5 Director Rehabilitative Research, American Sports Medicine Institute, 6 Clinical Manager & Founder, Advanced Rehabilitation
Keywords: Elbow, Baseball, Ligament, Rehabilitation https://doi.org/10.26603/001c.128512
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Injuries to the ulnar collateral ligament (UCL), have become increasingly prevalent among overhead-throwing athletes, especially baseball pitchers. From 2011 to 2023, UCL injuries were the most common injury in Major League Baseball (MLB). Contributing factors include high pitching velocity, fatigue, overuse, and year-round pitching. Research indicates that 25% of MLB pitchers and 14% of Minor League pitchers have undergone UCL surgery, with these numbers steadily rising. After traditional UCL reconstruction, 83% of athletes return to the same or higher levels of play. While the success rate for UCL surgery is high, revision surgeries are becoming more frequent, with mixed outcomes. This underscores the need for improved surgical techniques and rehabilitation strategies. The hybrid UCL reconstruction technique presents a reliable and effective solution for treating UCL injuries, combining the benefits of autogenous grafting with internal brace augmentation. Current research, however, lacks focus on the surgical technique and rehabilitation following UCL hybrid surgery. Achieving successful outcomes with this procedure relies on a collaborative approach, from surgery to rehabilitation with adherence to the rehabilitation protocol and throwing program. Full recovery typically requires 12-14 months, depending on the athlete’s level of play With over 400 successful surgeries to date, this technique has proven to enhance stability and facilitate recovery, particularly in elite-level throwing athletes. The purpose of this paper is to describe this new surgical technique and its associated rehabilitation programs, emphasizing the importance of rehabilitation under the guidance of a rehabilitation professional experienced with overhead athletes.
Level of Evidence: 5
Elbow injuries in overhead throwing athletes continue to increase at all levels and ages, particularly among baseball pitchers. Between 2011 and 2023, elbow injuries were the most common injury reported by Major League Baseball (MLB).1 In particular, injuries to the ulnar collateral ligament (UCL) have seen an early rise in professional baseball, with pitching velocity, fatigue/overuse, shoulder loss of motion, and year-round play all contributing to this trend.2‑5 Conte et al6 reported that 25% of major league and 14% of minor league pitchers had undergone UCL surgery. In 2019, Leland et al7 reported an increase in those percentages to 26% and 19%, respectively Following UCL
reconstruction, previous reports have cited that 83% of athletes were able to return to the same level of play or higher following surgery 8 Recently, Cain et al9 have reported a 98% success rate with UCL reconstructions and a 99% success rate with UCL repair utilizing the internal brace.
On the other hand, UCL revision surgeries are also increasing in frequency Keyt et al10 reported a 1 to 15% revision rate in the past several years. The success rates following revision reconstruction of the UCL have been less than favorable. Camp et al11 reported a 77% rate of return to play but only 55% returned to same level of play Andrews et al12 reported only 47% of pitchers were able to return to their pre-injury level of participation after revision. Giogavac et al13 in a systematic review of five articles on UCL revision
Corresponding Author: Daniel Evans, DPT, TMI Sports Medicine, 3533 Matlock Rd, Arlington, TX 76015. devans@tmisportsmed.com a

Figure 1. Palmaris harvest / Graft
surgery, reported a 78% return to pre-injury level in pitchers at the major league, minor league and collegiate levels. The increase in revision surgeries and the variability in outcomes highlight the need for improved surgical techniques and individualized rehabilitation approaches.
There have been numerous articles written describing the rehabilitation following UCL reconstruction and UCL repair with internal brace but no articles to date have discussed the rehabilitation following the UCL hybrid surgery procedure.14‑16 The purpose of this paper is to describe this new surgical technique and its associated rehabilitation programs, emphasizing the importance of rehabilitation under the guidance of a rehabilitation professional experienced with overhead athletes.
Since 2008, the senior author (KM) has employed a novel technique for reconstructing the UCL of the medial elbow. Today, over 400 cases have been reconstructed using this technique. The method involves using a standard autogenous graft, either palmaris longus or gracilis tendon, and internal brace augmentation.
SURGICAL TECHNIQUE - OVERVIEW SETUP
A standard set-up is utilized, and a tourniquet is applied for hemostasis. The affected upper extremity is prepped and draped in a standard fashion.
PALMARIS LONGUS HARVEST
A 1 cm transverse incision is made at the flexion crease of the wrist. The palmaris longus (PL) tendon is isolated. A 2-0 vicryl suture is weaved through the tendon with a baseball stitch, and the tendon is released distally to this. The tendon is then harvested from the forearm with a tendon stripper Subcuticular closure of the wound follows.
The graft is prepared by removing the muscle belly and tubing the opposite tail with a second 2-0 vicryl stitch. The


graft is removed from the operative field and placed safely on the back table.
If a gracilis tendon graft is required, the senior author prefers using the contralateral lower extremity
The surgical approach is made through a skin incision beginning at the upper margin of the medial epicondyle and carried distally for a typical length of 6 cm.
The superficial nerve branches are isolated and preserved.
The ulnar nerve is elevated off the proximal aspect of the medial epicondyle facilitating exposure for tunnel placement. A partial release of the medial intermuscular septum

Figure 4. Ulnar nerve elevation at posterior edge of medial epicondyle with partial release of the medial intermuscular septum
5. Fascial split / elevation of the FDS muscle
is often employed as well providing decompression of the nerve.
The posterior raphe of the flexor mass is split longitudinally along the length of the skin incision. The split utilizes the interval between the flexor digitorum superficialis (FDS) and flexor carpi ulnaris (FCU). During exposure, care is taken to preserve the anterior fascial layer of the FCU to protect both the muscle and the ulnar nerve.
After exposing the anterior bundle of the medial UCL, the ulnar nerve is internally decompressed by incising the overlying fascia of the FCU along its course down to the concavity of the proximal ulna.
Beginning distally at the sublime tubercle, the ligament is split longitudinally proximally to the ligament footprint origin at the base of the medial epicondyle. The ligament is explored for any acute injury pattern. Additional suturing may be employed for distinct proximal and distal tears.
At the anterior margin of the proximal ulna, a small fascial window is made to facilitate placement of the ulnar tunnels for tendon passage.
On either side of the concavity of the sublime tubercle, 2 converging 3.5 mm drill holes are made. These tunnels are transitioned with a curved curette and a doubled vicryl suture is passed with a curved needle.



A 2.7 mm drill hole is made at the apex of the sublime tubercle 4 to 5 mm distal to the joint line but proximal to the ulnar tunnels. A 3.5 mm swivel lock anchor loaded with collagen coated fiber tape is placed in the tapped drill hole.
At the base of the medial epicondyle, with caretaken to lateralize the proximal tunnel, a 2.5 mm drill bit is used to make a starter hole for the larger drill bit to follow.
This initial hole is then over drilled with either a 4.0- or 4.5-mm drill bit to a depth of 15 to 17 mm.





A 2.5 mm drill bit is is used to create two divergent tunnels through the proximal cortex of the medial epicondyle. Utilizing a Hewson suture passer, doubled over 2-0 vicryl sutures are passed.
With the arm held in full supination and 30° of flexion primary repair of the distal footprint tear, if present, is performed.
One arm of the tendon graft is docked, with a #2 ortho cord suture with a baseball stitch with one arm of the internal brace. The second arm of the tendon graft is cut to an appropriate length, and the second #2 Ortho cord su-
ture is placed. The second arm of the tendon graft is then docked proximally, followed by the second arm of the internal brace.
The internal brace suture arms are first tensioned and tied using a knot tier Five throws are placed in the suture arms. The tendon graft sutures are then tensioned and tied as well. *Note that the internal brace is tensioned and tied first to preset the tension on the joint. (Figure 17)

15. Distal repair: The tendon graft is passed through the distal drill holes.

16. Tendon graft passage thru the distal drill holes

The proximal tunnel lateralized typically leaves a good cuff of the native ligament to sew over the top of the brace/ graft. A 2-0 vicryl suture is utilized here in a figure-of-eight fashion. A 2-0 fiber wire suture is then used more distally, bringing together the arms of the tendon graft, native ligament, and internal brace.
The wound is copiously irrigated. The posterior raphe is closed with a running 2-0 vicryl suture.


Figure 19. Native ligament closure over proximal graft and suturing of tendon graft to native ligament and brace

The skin is closed with a 3-0 monocryl suture in the subcutaneous layer, followed by a subcuticular 4-0 monocryl in the skin.
Steri-strips are applied to the wound, which is infiltrated with several cc’s of local anesthetic. A dry sterile dressing is then applied, and the arm is placed into a postop-hinged elbow brace locked at 90° of flexion and neutral forearm rotation. The patient is awakened and the tourniquet is released following dressing application.

Figure 21. Subcuticular skin closure
For a step-by-step description of the surgical technique, please review Table 1 and/or Video 1
Table 1. Surgical Technique Overview
Stage of Procedure
Preoperative Preparation
Graft Harvesting
Surgical Approach
Description/Steps of Stage
URL: https://www youtube.com/embed/8yvYU3YuljA
Rehabilitation following UCL hybrid surgery plays a vital role in the successful outcome following this procedure. The specific rehabilitation program employed following the UCL hybrid procedure presented can be found in Table 2
Adherence to the post-operatve timeframes, surgical healing constraints, rehabilitation guidelines, goals, and specific exercises prescribed is crucial to prevent complications such as elbow stiffness, graft stretch, or graft failure. The rehabilitation program is designed to be progressive and sequential with each successive phase building upon
The affected upper extremity is prepped and draped in a standard surgical fashion to maintain a sterile field.
Palmaris Longus Harvest:
1. Incision and Isolation: A 1 cm transverse incision is made at the flexion crease of the wrist. The palmaris longus tendon is identified and isolated.
2. Suture Weaving: A 2-0 vicryl suture is woven through the tendon using a baseball stitch. The tendon is then released distally
3. Tendon Harvesting: Using a tendon stripper, the palmaris longus is harvested from the forearm. The wound is closed appropriately
4. Graft Preparation: Muscle belly is removed, and the opposite tail is tubed with a second 2-0 Vicryl suture. The graft is placed on the back table for later use.
*If a gracilis tendon is required, the senior author prefers harvesting it from the contralateral lower extremity.
1. Incision: A skin incision is made starting at the upper margin of the medial epicondyle and extended distally for about 6 cm.
2. Nerve Preservation: Superficial nerve branches are preserved, and the ulnar nerve is elevated from the proximal aspect of the medial epicondyle to facilitate tunnel placement. A partial release of the medial intermuscular septum may also be performed.
3. Exposure of UCL: The posterior fascia of the flexor mass is split, preserving the anterior fascia of the flexor carpi ulnaris (FCU) to protect the muscle and ulnar nerve.
4. Ligament Exposure: The anterior bundle of the medial UCL is exposed. The ulnar nerve is identified along it’s on the proximal ulna, where small fascial windows are created for the placement of ulnar tunnels.
Tunnel Creation
1. Drill Holes: Two converging 3.5 mm drill holes are created on either side of the concavity of the sublime tubercle. These tunnels are transitioned with a curved needle and a doubled over 2-0 Vicryl suture is passed.
2. Proximal Tunnel: A 2.7 mm drill hole is made at the apex of the sublime tubercle, approximately 4-5 mm distal to the joint line, but proximal to the ulnar tunnels. A 3.5 mm swivel-lock anchor loaded with collagen-coated fiber tape is inserted into the tapped hole.
3. Medial Epicondyle Tunnel: A 2.5 mm drill bit creates a starter hole for the proximal tunnel, which is then overdrilled to 4-4.5 mm to create a tunnel approximately 15-17 mm deep. Two 2.5 mm divergent tunnels are made through the proximal cortex of the medial epicondyle.
Graft and Internal Brace Placement
Final Steps
Postoperative Care
1. Graft Docking: The tendon graft is passed through the distal drill holes. One arm of the tendon graft is docked with a #2 ortho cord suture and a baseball stitch, along with one arm of the internal brace. The second arm of the graft is cut to appropriate length and secured with a second #2 ortho cord suture.
2. Tensioning: The internal brace suture arms are tensioned and tied first, ensuring optimal tension on the joint. Five throws are placed in the suture arms before the tendon graft sutures are tensioned and tied.
1. Overlay Native Ligament: The proximal tunnel is lateralized, preserving a cuff of native ligament over the graft/ internal brace. A 2-0 Vicryl suture is utilized in a figure-of-eight fashion.
2. Additional Suturing: 2-0 fiber wire sutures bring together the arms of the tendon graft, native ligament, and internal brace.
3. Wound Closure: The wound is irrigated thoroughly. The flexor fascial split is closed with a running 2-0 Vicryl suture, followed by a two-layer closure of the skin. The wound is dressed and infiltrated with local anesthetic.
The arm is placed in a postoperative hinged elbow brace, locked at 90° of flexion and neutral forearm rotation. The patient is awakened, and the tourniquet is released after post-op dressing application.
Phase Timeline Details
Protective Phase 0-3 weeks - Protect healing tissues and allow gradual return of elbow motion.
- Reduce post-operative inflammation and swelling.
- Brace locked at 90° for the 1st week, then opened to 30-90° at week 1 post-op.
- Progressively open 10° into extension and flexion weekly. (See brace settings)
- Suture removal at 2 weeks post-op.
- After suture removal, initiate cardiovascular work and lower extremity exercises.
- Begin IR (Internal Rotation) and ER (External Rotation) manual resisted isometrics at 3 weeks.
- Continue protective scapular, hand, and forearm exercises to promote circulation and gradual return of ROM.
Intermediate Phase 3-12 weeks
- Continue progressive opening of the brace to allow gradual return of elbow ROM. Avoid overly aggressive manual therapy
- Contact surgeon if return of elbow ROM stalls or there is loss of ROM.
- Continue with protective isometrics, scapular, forearm, and hand exercises to prevent muscle atrophy.
- Discontinue brace at week 6 post-op and begin PRE (Progressive Resistance Exercises) for scapula, rotator cuff, elbow, forearm, and wrist.
- At 10 weeks post-op, introduce light compound lifting to prepare for unrestricted weight room.
Advanced Phase 12 weeks post-op
- At 12 weeks post-op, unrestricted weight room with elbow valgus precautions. Progress as tolerated to prepare for a plyometric program.
- College/High School: 15 weeks post-op begin 2-handed plyometrics, progress to 1-handed plyometrics at 17 weeks post-op.
- Professional level athletes: 17 weeks post-op begin 2-handed plyometrics, progress to 1-handed plyometrics at 19 weeks post-op.
Return to Sport Phase 5-6 months+
College/High School:
- 5 months post-op: Begin long toss ITP.
- ~8 months post-op: Begin mound progression.
- ~12 months post-op: Return to full sport.
Professional level athletes:
- 6 months post-op: Begin long toss ITP
- ~9 months post-op: Begin mound progression.
- ~14 months post-op: Return to full sport.
the previous milestones. Facilitating this progression are specific goals, exercises, and required criteria within each phase that must be met for advancement. To ensure success, a team approach to athlete management is imperative. A collaborative approach involving the athlete/patient, their family, the surgeon, athletic trainer, and the physical therapist is essential. The ultimate goal of the rehabilitation program is to return the athlete to their preinjury level of function and sports performance in a safe an expeditious manner.
This section will outline the specific timelines, rehabilitation techniques, exercise activities, and throwing progression for athletes following UCL hybrid reconstruction. The program provides a general framework designed to guide individualized care to ensure that personal differences and circumstances are taken into consideration and accounted for. Close communication between the athlete,
rehabilitation team, and surgeon is essential to ensure any appropriate adjustments can be made quickly keeping the recovery process on track.
PROTECTIVE PHASE (0-3 WEEKS POST-OPERATIVELY)
Immediately following surgery, the athlete is placed in an adjustable range of motion (ROM) elbow brace (Fig 22), locked at 90° of flexion to protect the hybrid graft and healing incision. Active range of motion (AROM) of the shoulder,wrist, and hand along with gripping exercises are performed to foster normal upper extremity, hand and finger mobility Sub-maximal isometric exercises for shoulder flexion, extension, hand/wrist motions are initiated to minimize muscle atrophy and promote blood flow Manually resisted rhythmic stabilization exercises may also be introduced to establish proprioception and neuromuscular control along with manual scapular neuromuscular control

exercises. It is recommended that these exercises are performed in the hinged brace locked at 90 degrees to protect from potential deleterious valgus forces at the elbow.
At one week post-surgery, the brace is unlocked to allow ROM from 30 degrees to 90 degrees flexion to begin gradually restoring full elbow and forearm motion. Every week following the end of week two, the brace will be opened another 10 degrees into both flexion and extension, allowing a progressive increase in motion (Table 3). At 2 weeks post-surgery, the sutures are removed, and cardiovascular exercises such as recumbent biking and walking may be initiated to resume some level of cardiovascular conditioning. Lower extremity strengthening can be initiated utilizing weighted vest exercises, leg press, single leg squats, clamshells, bridging, hip sled, or other interventions that eliminate or minimize the use of the upper extremities. Core stability exercises are also encouraged at this time. These exercises are progressed through the end of week 6 after surgery During this time, it is imperative to allow a gradual return of elbow motion and to avoid excessive overpressure, stretching, or overly aggressive manual therapies to gain ROM as these may lead to inflammation in the elbow and/or ulnar nerve irritation (Fig 23) This type of early aggressive motion may present as a generalized soreness and elbow pain inhibiting the return of full unrestricted elbow ROM. If a loss of elbow motion occurs or motion ceases to improve steadily during this phase, it is imperative to notify the surgeon and develop a modified plan of treatment, such as early brace adjustments or anti-inflammatory medication, to address this issue.

INTERMEDIATE PHASE (3-12 WEEKS POSTOPERATIVELY)
During this phase of the rehabilitation program, elbow ROM is gradually progressed while also restoring muscular endurance, strength, and neuromuscular control of the upper quarter. Controlled rhythmic stabilizations to resist shoulder internal and external rotation may be introduced to initiate valgus loading of the healing hybrid graft. This should be carried out carefully by the rehabilitation specialist and the manual resistance should be pain-free and submaximal in effort. Gentle manual therapy should be continued to assist in the normalization of elbow motion. Caution should still be taken to avoid aggressive PROM and/or manual techniques as the elbow and ulnar nerve may still become inflamed at this point in the recovery. Isotonic shoulder, forearm, and wrist/hand strengthening should all be continued.
At the end of week 6, the post-operative brace is discharged, as the elbow should be near full ROM. While substantial progress in motion is expected by this stage, slight improvements will continue to be seen over the next 1-2 weeks. Characteristically, hybrid reconstructions often have a slower return of full elbow flexion when compared to traditional UCL reconstruction that use a tendon graft alone. Aggressive intervention to achieve full flexion should always be avoided. Again, if there is any concern about the ROM progression, the surgeon should be immediately notified.
During this phase, progressive resistance exercises are introduced and should be advanced weekly The focus of the exercise program during this phase is to strengthen the
Post-op Timeframe
0-7 days
7-14 days
14-21 day
21-28 days
28-35 days
Brace Setting
Functional brace locked at 90°
Functional brace 30°-90°
Functional brace 20°-100°
Functional brace 10°-110°
Functional brace 0°-120°
muscles of the scapula, rotator cuff, upper arm, wrist and forearm, as well as improve the athlete’s overall posture.17 Examples of the exercises typically incorporated include prone shoulder horizontal abduction (T’s), prone shoulder extension (I’s), prone horizontal abduction at 105 deg with external rotation (Y’s), prone rowing into external rotation (W’s), side lying shoulder external rotation, standing shoulder scaption and abduction, and shoulder internal rotation with resistance bands. Weight room exercise activities for the lower extremities are generally unrestricted, although it is important to continue to avoid any exercise that may impart excessive valgus loads to the elbow Cardiovascular and core exercises may also be progressed with the same consideration.
At week 10 following surgery, upper body compound lifting may be gradually introduced to prepare the elbow joint for the demands of unrestricted weight training. Initial exercises may include light dumbbell chest press from the floor, bent over rowing with dumbbells, and high rowing on a cable column machine. It is essential to integrate exercises that strengthen the rotator cuff and scapular stabilizers in these movements to maintain optimal mechanics during compound lifting which will serve to support longterm recovery
STRENGTHENING PHASE (12 WEEKS TO 6 MONTHS POST-OPERATIVELY)
Beginning 12 weeks after surgery, the rehabilitation process focuses on the restoration of strength and power, as well as preparing the athlete for clearance to perform unrestricted weight room training. The athlete must demonstrate full, pain-free ROM of the elbow and no pain with any previous isotonic exercises to advance further (Fig 24) It is imperative that athletes coordinate their weight room training with a strength and conditioning coach and/or rehabilitation professional who is experienced in developing safe, effective training regimens for overhead athletes. Proper technique and exercise selection are critical to avoid high valgus loads on the elbow, which can compromise the graft and delay recovery
From 15 weeks to 6 months following surgery, there is a continued focus on strength and power with advancing weight room training and continued arm care exercises for endurance and neuromuscular control of the entire throwing extremity. 2-handed plyometrics with a medicine ball, including chest pass, chop pass, overhead side-to-side

throws, unilateral push throws, and two hand chest passes are initiated (Fig 25). After 3-4 weeks of successful 2-handed plyometrics, 1-handed plyometrics, such as 90/ 90 rebounder toss, overhead wall dribbles (Fig 26), shoulder decelerations, and prone flips with a weighted ball, can all be added. Plyometric volume should start low with 2-3 sets of 12-15 repetitions of each exercise and gradually build in additional volume, intensity, and complexity mimicking the throwing demands of the athlete. During this phase, the athlete should also continue to improve strength, endurance, and dynamic stabilization of the shoulder continuing with full weight room training and arm care exercises. Due to the higher demands of pitching in professional athletes, such as higher pitch velocities, spin rates, and pitch volumes, 2-handed plyometrics are initiated at 17-weeks post-surgery and 1-handed plyometrics at 20-weeks postsurgery In contrast, collegiate and high school level overhead athletes will generally start 2-handed plyometrics at 15-weeks and 1-handed plyometrics at 17-weeks after surgery.


The interval throwing progression (ITP) is a crucial phase of the rehabilitation process assisting the athlete to transition from rehabilitation to training and toward a return to unrestricted competion. It is utilized to condition the shoulder, arm, and body for return to sport and to gradually impose the valgus loads of throwing to the healing graft. During the ITP, the graft is exposed to unique valgus loads that vary for each individual. Characteristics such as throwing mechanics, throwing intensity, volume, and distance determine the load on the graft. As the intensity of the throwing program progresses via distance, volume, and velocity, so does the resilience of the hybrid graft, reading it for full unrestricted return to play
Although multiple interval throwing programs are used for rehabilitation, we have found that a velocity-based throwing progression leads to the fewest complications and setbacks during this process. The ITP utilized by this author group is outlined in Appendix A It includes progressive distances and velocities based on the individuals’ abilities when they were healthy, as well as de-load periods interspersed during the ITP The purpose of the de-load periods is to allow tissue recovery and reduce progressive load, to aid in the prevention of overtraining and reduce risk of injury. The timing of the implementation of an ITP also varies based on the athlete’s level of participation.
Due to higher demands of professional pitching described previously the throwing program is started 6 months following surgery In contrast, high school and collegiate athletes start their ITP at 5 months post-op.
Certain criteria must be met prior to starting an ITP It is recommended that the athlete complete all previous phases of the process without issues or setbacks, have full painfree ROM of the shoulder and elbow, exhibit full strength of the shoulder and elbow, have a satisfactory clinical examination of both upper extremities, and have clearance to begin throwing from their surgeon.
The throwing program begins with a long-toss progression starting at 45 feet and progressing to 60 ft, 75 ft, 90 ft, 105 ft, and 120 ft. During this time, volume and velocity are closely monitored, with recommended velocity ranges based on data from healthy throwers (Table 4a-4c). Frequent clinician-athlete communication allows for personalized adjustments, such as when to implement recovery periods, or the reduction of volume on days when soreness or mechanical inefficiencies are observed. If athletes experience persistent tightness or soreness following throwing sessions, additional rest is recommended, and if symptoms do not resolve within a week, their surgeon should be consulted. This long-toss portion of the program typically last 3 months.
Once the long-toss phase is completed, pitchers begin a mound progression, starting with half-mound sessions and gradually increasing to full-mound, full-distance sessions. Initial mound work focuses on fastballs, with off speed pitches such as change-ups and sliders introduced gradually over the following weeks. Once the mound progression has been completed, facing live batters in a practice setting is recommended. The number of live hitter sessions will vary based on the level of the athlete but should be at least 2 weeks prior to starting a rehab outing. Completion of the entire ITP should take roughly 6-8 months depending on de-load periods, build ups, and timing with the respective competitive season in mind. Athletes will be returned to full unrestricted participation once the throwing/ mound progressions have been completed without issues and the medical team and surgeon have cleared the player for unrestricted participation.
The hybrid UCL reconstruction technique offers a reliable and effective approach to addressing UCL injuries, combin-
Table 4a. Radar Gun Velocity Ranges for Pitchers with Average FB velo between 90-95 mph
Start at low end of Range and progress to top end of range
Table 4b. Radar Gun Velocity Ranges for Pitchers with Average FB velo between 85-90 mph Distance
Table 4c. Radar Gun Velocity Ranges for Pitchers with Average FB velo between 80-85 mph
Distance
ing the benefits of autogenous grafting with internal brace augmentation. Successful outcomes following this procedure are accomplished through a team approach from surgery to rehabilitation. Patient compliance with the program and adherence to progression timeframes is imperative to ensure a successful outcome. With over 400 successful applications of this hybrid technique, this method has proven to enhance stability and facilitate recovery in the
Start at low end of Range and progress to top end of range
elite-level throwing population. Further studies are needed to determine long-term success rates, possibly refine this surgical and/or rehabilitation technique, and optimize outcomes.
Published: February 01, 2025 CST.
© The Author(s)
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Conte S. The Injury Rates in Professional Baseball Players. Presented at: The 37th Annual Injuries in Baseball Course; January 26, 2024; Birmingham, AL.
2. Fleisig GF, Andrews JR, Cutter GR, et al. Risk of serious injury for young baseball pitchers: a 10-year prospective study. Am J Sports Med. 2011;39(2):253-257 doi:10.1177/0363546510384224
3. Erickson BJ, Harris JD, Tetreault M, Bush-Joseph C, Cohen M, Romeo AA. Is Tommy John surgery performed more frequently in Major League Baseball pitchers from warm weather areas? Orthop J Sports Med. 2014;2(10):2325967114553916.
4. Wilk KE, Macrina LC, Fleisig GS. Deficits in glenohumeral passive range of motion increase risk of elbow injury in professional baseball pitchers: a prospective study Am J Sports Med 2014;42:2075-2208. doi:10.1177/0363546514538391
5. Erickson BJ, Chalmers PN, Axe MJ. Exceeding Pitch Count Recommendations in Little League Baseball Increases the Risk of Tommy John Surgery as a Professional Baseball Pitcher. Orthop J Spts Med. 2017;5(3):2325967117695085. doi:10.1177/ 2325967117695085
6. Conte SA, Fleisig GS, Dines JS, Wilk KE, et al. Prevalence of Ulnar Collateral Ligament Surgeries in Professional Baseball Players. Am J Sports Med 2015;43(7):1764-1769. doi:10.1177/ 0363546515580792
7 Leland DP, Conte S, Flynn N, et al. Prevalence of Medial Collateral Ligament Surgeries in 6135 Current Professional Baseball Players; A 2018 Update. Orthop J Sports Med 2019;7(9):2325967119871442. doi:10.1177/2325967119871442
8. Cain EL Jr, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38:2426-2434. doi:10.1177/0363546510378100
9. Dugas JR, Froom R, Mussell EA, et al. Clinical Outcomes of Ulnar Collateral Ligament Repair with Internal Brace Versus Ulnar Collateral Ligament Reconstruction in Competitive Athletes. Am J Sports Med. Published online 2024. doi:10.1177/ 2325967124S00033
10. Keyt LK, Tangtiphaiboontana A, Turner TW Revision Medial Collateral Ligament Reconstruction in Baseball Pitchers; Surgical Techniques and Outcomes. Curr Rev Musculoskelet Med 2020;13(3):361-368. doi:10.1007/s12178-020-09619-x
11. Camp CL, Devi V, Conte S, et al. Revision Ulnar Collateral Ligament in Professional baseball Players; Current Trends, Surgical Techniques and Outcomes. Orthop J Sports Med. 2019;7(8):2325967119864104. doi:10.1177/2325967119864104
12. Andrews JR, Venkateswaran V, Christensen KD, et al. Outcomes After Ulnar Collateral Ligament Revision Reconstruction Surgery in Baseball Players. Am J Sports Med 2020;48(13):3359-3364. doi:10.1177/0363546520951529
13. Giogavac G, Grawe BM. Outcomes With a Focus on Return to Play for Revision Ulnar Collateral Ligament Surgery Among Elite-Level Baseball Players: A Systematic Review Am J Sports Med 2019;47(11):2759-2763. doi:10.1177/ 0363546518816960
14. Wilk KE, Thomas ZM, Arrigo CA, Campbell AM, Shahien A, Dugas J. The Use of the Internal Brace to Repair the UCL IInjury of the Elbow in Athletes. Int J Sports Phys Ther 2022;17(7):1208-1218. doi:10.26603/001c.39614
15. Wilk KE, Arrigo CA, Bagwell MS, Rothermich MA, Dugas JR. Repair of the Ulnar collateral ligament of the elbow: Rehabilitation following internal brace surgery. J Orthop Sports Phys Ther. 2019;49(4):253-26. doi:10.2519/jospt.2019.8215
16. Wilk KE, Marcina LC, Cain EL, Dugas JR, Andrews JR. Rehabilitation of the overhead athlete’s elbow. J Sports Health. 2012;4(5):404-414. doi:10.1177/ 1941738112455006
17 Wilk KE, Arrigo CA, Hooks TR, Andrews JR. Rehabilitation of the Overhead Throwing Athlete: There Is More to It Than Just External Rotation/ Internal Rotation Strengthening. PMR. 2016;8(3 Suppl):S78-90. doi:10.1016/j.pmrj.2015.12.005
Download: https://ijspt.scholasticahq.com/article/128512-ulnar-collateral-ligament-hybrid-reconstruction-surgeryrehabilitation-in-the-overhead-athlete/attachment/263271.docx?auth_token=gMioJtHKj9aAFG9XqHxv

Phil Page, PhD, ATC, CSCS, FACSM, Robert C. Manske, PT, DPT, Med, SCS, ATC, CSCS, Michael Voight, PT, DHSC, SCS, OCS, ATC, CSCS, FAPTA, Chris Wolfe, PT, DPT, OCS, Cert MDTa
Keywords: MSK ultrasound, Achilles Tendon, Tendinopathy
https://doi.org/10.26603/001c.129050
International Journal of Sports Physical Therapy
Vol. 20, Issue 2, 2025
Musculoskeletal ultrasound (MSKUS) is a pivotal imaging modality for the evaluation and management of Achilles tendon pathologies. Its ability to provide real-time, high-resolution imaging facilitates accurate diagnosis, dynamic assessment, and precise therapeutic interventions. The Achilles tendon, the largest and strongest tendon in the body, is critical for lower limb function and prone to a variety of pathologies, particularly in athletes and active individuals. This paper explores the normal sonographic anatomy of the Achilles tendon, common pathological findings—including tendinopathy, tears, insertional disorders, and retrocalcaneal bursitis—and ultrasound techniques to optimize diagnostic accuracy The integration of MSKUS into clinical practice has revolutionized the assessment and treatment of Achilles tendon injuries, offering a cost-effective, radiation-free alternative to other imaging modalities such as magnetic resonance imaging (MRI).
Musculoskeletal ultrasound (MSKUS) has become an essential tool for diagnosing and managing a wide range of musculoskeletal conditions. The Achilles tendon, which connects the gastrocnemius and soleus muscles to the calcaneus, is a critical structure that facilitates walking, running, and jumping. Given its high mechanical load, the tendon is vulnerable to acute injuries and chronic degenerative conditions, particularly in physically active individuals.1,2
MSKUS provides high-resolution, real-time imaging of the Achilles tendon, allowing for the evaluation of its structural integrity and viewing the surrounding tissues.3,4 Unlike MRI, MSKUS is dynamic, enabling functional assessment during movement, while being more accessible and cost-effective. This paper reviews the sonographic anatomy of the Achilles tendon, the appearance of common pathologies, and the role of MSKUS in clinical evaluation and rehabilitation.
The Achilles tendon, or tendo calcaneus, is the confluence of the gastrocnemius and soleus muscles, inserting onto the posterior aspect of the calcaneus. It spans approximately 15 cm and tapers distally, with its musculotendinous junction varying in height among individuals. The
tendon is enveloped by a vascularized paratenon, which aids in reducing friction during movement.
On ultrasound, the normal Achilles tendon in the long axis (LAX) view demonstrates a fibrillar echotexture with parallel hyperechoic lines. In the short axis (SAX) view, the tendon exhibits a reniform (kidney-shaped) contour and a homogenous hyperechoic appearance. The paratenon appears as a thin, hypoechoic layer, and Kager’s fat pad, situated deep to the tendon, serves as a hypoechoic reference landmark. Krager’s fat pad is the triangular region made up of the Achilles tendon, the flexor hallucis tendon, and a wedge of fat adjacent to the calcaneus.
Achilles tendinopathy is a degenerative condition characterized by tendon thickening, hypoechoic areas, and disrupted fibrillar architecture on ultrasound.5‑7 Doppler imaging often reveals neovascularization in chronic cases, particularly in the mid-portion of the tendon.8,9 Symptomatic tendons frequently measure >7 mm in thickness, compared to the normal range of 4–6 mm.10
Corresponding Author: chris.wolfe@belmont.edu
PARTIAL AND FULL-THICKNESS TEARS
Tears typically occur in the hypovascular “watershed area” 2–6 cm proximal to the calcaneal insertion.4,8,11 Partial tears appear as focal hypoechoic defects with preserved tendon continuity, while full-thickness tears demonstrate complete fiber disruption, visible as a hypoechoic or anechoic gap. Retraction of tendon ends is commonly observed in complete ruptures. The Kuwada classification system grades tears based on severity, ranging from partial (Grade I) to complete ruptures with a gap >6 cm (Grade IV).
INSERTIONAL TENDINOPATHY
Insertional tendinopathy affects the distal 2 cm of the tendon. Sonographic findings include hypoechoic and thickened tendon fibers, calcifications or enthesophytes at the calcaneal insertion, and fluid-filled retrocalcaneal bursitis.8,12 Chronic cases may also show hypervascularity on Doppler imaging. It is important to note that the tendon should be kept in a relaxed state when using Doppler as tension may obliterate any new vessels.13
HAGLUND’S DEFORMITY
This bony prominence at the posterosuperior calcaneus causes mechanical irritation of the Achilles tendon. Ultrasound reveals a prominent osseous contour, thickened tendon fibers, and retrocalcaneal bursitis with fluid accumulation.
TENDINOSIS
Tendinosis reflects chronic degenerative changes without inflammation, appearing as hypoechoic regions with tendon thickening and disorganized fibrillar architecture.2,9 Chronic tendinosis may also exhibit calcifications or fatty degeneration.
RETROCALCANEAL BURSITIS
Retrocalcaneal bursitis is associated with overuse and manifests as anechoic or hypoechoic fluid distension of the bursa anterior to the Achilles tendon. This entity can coexist with tendinopathy or inflammatory conditions.14 Chronic inflammation may cause synovial hypertrophy and calcifications.4,8
ADVANTAGES OF MSK ULTRASOUND FOR ACHILLES TENDON EVALUATION
There are several advantages in using MSK-US to evaluate the Achilles Tendon in patients with posterior ankle pain.
• Dynamic Imaging: Allows visualization of the Achilles during active movement, aiding in the diagnosis of Achilles tendon abnormalities.3
• Real-Time Guidance: Facilitates guided interventions such as injections or dry needling.12
• Soft-Tissue Differentiation: Provides high-resolution imaging of tendons, muscles, and bursae.4,8
• Cost-Effectiveness and Accessibility: Offers a less expensive and more readily available alternative to MRI.1,12
DIAGNOSTIC MDK ULTRASOUND TECHNIQUES FOR THE ACHILLES TENDON
Proper imaging technique is vital for accurate diagnosis. The patient is positioned prone, with the foot hanging freely or supported by a bolster, allowing dynamic assessment. A high-frequency (10–15 MHz) linear transducer is used.
The scanning protocol for the Achilles tendon includes 2 planes of transducer placement.
• Transverse Plane: The transducer is placed perpendicular to the Achilles to identify tendon abnormalities including thickening, partial tears or ruptures.
• Longitudinal Plane: The transducer is aligned along the muscle fibers to assess the muscle and tendon continuity.
Dynamic maneuvers, such as passive dorsiflexion and plantarflexion, or gentle squeezing (Thompson test) assess tendon glide and continuity Adjacent structures, including the retrocalcaneal bursa and Kager’s fat pad, are evaluated for associated pathology During movements of plantar flexion, Krager’s fat pad extends into the retrocalcaneal bursa as far as the enthesis. While in neutral, Kager’s fat pad is retracted so that the tendon is against the bone, the superior tuberosity of the calcaneus.15 Common artifacts, such as anisotropy, are mitigated by adjusting the transducer angle.3,8
CLINICAL APPLICATIONS
GUIDED INTERVENTIONS
MSKUS enhances precision in dry needling, corticosteroid injections, and platelet-rich plasma (PRP) therapy by confirming the target site and avoiding inadvertent tendon injury.3,12
Serial imaging tracks resolution of hypoechoic areas, restoration of fibrillar structure, and reduction of hypervascularity, guiding progression in rehabilitation.6
OPTIMIZING REHABILITATION
Sonographic findings guide individualized treatment plans, such as eccentric loading exercises for tendinopathy or range-of-motion protocols for post-rupture recovery.1,2,7,16
MSKUS is an invaluable tool for diagnosing and managing Achilles tendon pathologies. Its dynamic capabilities, costeffectiveness, and high-resolution imaging make it superior
Diagnostic Musculoskeletal Ultrasound of the Achilles Tendon
for assessing soft tissue conditions. By enabling accurate diagnosis and real-time intervention guidance, MSKUS enhances clinical outcomes and facilitates effective rehabilitation.1,2,7,16 Continued research and protocol standard-
ization will further integrate MSKUS into routine musculoskeletal care.

Patient Positioning
The patient should be positioned prone on the examination table, with the foot resting on a bolster or hanging over the edge of the table (shown in Figure 1A). This positioning facilitates dynamic assessment of the Achilles tendon by allowing it to be plantar flexed and dorsiflexed during the examination. Alternatively, the patient may be placed in a kneeling position in a stable armchair, providing support and allowing the transducer to be applied to the posterior aspect of the Achilles tendon. The examination typically begins with the foot in a dorsiflexed position to create tension across the Achilles tendon.
Figure 1A: Transducer Placement on Achilles Insertion in Long Axis View (LAX)
For the long axis view, the transducer is placed in a longitudinal (LAX) orientation, with the reference end positioned proximally on the Achilles tendon, directly over the calcaneal tuberosity at the Achilles insertion. The transducer should be angled slightly from posteromedial to anterolateral. A heel-to-toe rocking motion allows the examiner to scan the entire Achilles tendon at its insertion on the calcaneus, ensuring the tendon remains hyperechoic throughout the assessment.
Figure 1B: Transducer Placement on Achilles Tendon Mid-Portion in Long Axis View (LAX)
To visualize the entire Achilles tendon, maintain the transducer in the longitudinal (LAX) orientation and slide it proximally from the distal insertion toward the mid-portion of the tendon. Continue this approach up to the posterior knee to examine the gastrocnemius and soleus musculature. Scanning the tendon from medial to lateral enables a comprehensive evaluation of the tendon and associated musculature.
Figure 1C: Transducer Placement on Achilles Insertion in Short Axis View (SAX)
For the short axis view, position the transducer in a transverse (SAX) plane over the calcaneal tuberosity at the Achilles insertion. The transducer should be angled slightly from posteromedial to anterolateral. Using a heel-to-toe rocking motion, the examiner can scan the entire Achilles tendon at its insertion on the calcaneus, ensuring a hyperechoic appearance. To achieve a more thorough examination, slide the transducer proximally from the distal insertion toward the more proximal tissues.

Figures 2A and 2B: LAX View
The objective of this image is to capture the tapering, arrow-like contour of the Achilles insertion onto the calcaneus. Refer to Figure 1A for the transducer position for this image. The retro-calcaneal bursa, visible as mixed echoes, separates the tendon from the calcaneus. The Kager’s fat pad, a triangular region located deep to the Achilles tendon and proximal to the calcaneus, appears as mixed echoes typical of fat on ultrasound. Abnormalities in the fat pad, often seen as ossification, are commonly associated with Achilles injuries. The Achilles insertion may appear hypoechoic due to anisotropy, which should not be mistaken for pathology To ensure accurate visualization of all tendon fibers, employ a heel-to-toe toggling motion with the transducer to maintain a perpendicular orientation to the tendon as it curves at the insertion. Normative value for the Achilles tendon thickness is 4.94 + 1.24 mm.17
Published: February 01, 2025 CST.
© The Author(s)

Figures 3A and 3B: LAX View
When scanning proximally from the calcaneal insertion in a long axis (LAX) orientation, position the transducer approximately 5-7 cm (2-3 inches) from the insertion point. Refer to Figure 1B for the transducer position for this image. Dynamic evaluation of the Achilles integrity can be facilitated by plantarflexion and dorsiflexion of the foot. The height of the musculotendinous junction between the soleus muscle and the Achilles tendon varies among individuals, and in some cases, the plantaris tendon may also be visualized. As the transducer is moved proximally, no distinct bony landmarks will be encountered. The objective is to capture the bright, hyperechoic, intact fibrillar pattern of the most superficial fibers of the Achilles tendon.

Figures 4A and 4B: SAX View
Position the transducer slightly proximal to the true tendon insertion at the calcaneus to visualize the Achilles tendon in a transverse cross-sectional plane as it approaches the calcaneal insertion. Refer to Figure 1C for the transducer position for this image. This image does not include a bony reference point, and the objective is to capture the dense, “bristlelike” pattern characteristic of the Achilles tendon. In a normal short axis (SAX) view, the tendon exhibits a reniform or kidney-shaped contour However, with advancing tendinosis and increased intra-tendinous pressure, the Achilles tendon often takes on a round or ball-like shape. The Achilles insertion may appear hypoechoic due to anisotropy, which should not be mistaken for pathology Kager’s fat pad (KFP), situated deep to the Achilles tendon, should also be noted. Normative value for the Achilles tendon thickness in the SAX view are 14.43 + 4.07 16
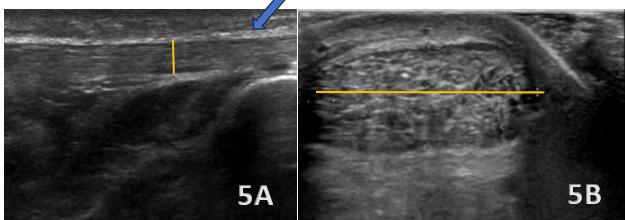
Figures 5A (LAX View) and 5B (SAX View)
In the LAX view, the Achilles tendon is significantly thickened (highlighted with blue arrow in figure 5A), measuring 10 mm in thickness (highlighted in figure 5A with yellow line) and 24 mm in width (highlighted in figure 5B with yellow line), with hypoechoic areas distributed throughout, indicating degenerative changes. This contrasts with the normal mean values for the Achilles tendon, which are typically 4.94 ± 1.24 mm. The SAX view shows an enlarged cross-sectional area with a rounded or irregular contour, heterogeneous echotexture, and hypoechoic regions, further highlighting the tendinopathic changes.

Figure 6A (LAX View):
A Haglund’s deformity is shown as cortical irregularities and a bony prominence on the posterior aspect of the calcaneus near the Achilles tendon insertion. The overlying soft tissues, including the retrocalcaneal bursa can show signs of inflammation, appearing hypoechoic or swollen. The Achilles tendon itself demonstrates thickening and increased echogenicity at its insertion due to chronic irritation. Additionally, there may be evidence of bursitis, with fluid accumulation seen as an anechoic or hypoechoic area within the retrocalcaneal bursa.

Figure 7A (LAX View):
Insertional Achilles tendinopathy (blue arrow highlighting) is characterized by pathological changes in the distal 2 cm of the Achilles tendon at its attachment to the calcaneus. On diagnostic ultrasound, hallmark findings include tendon thickening and hypoechoic areas indicative of collagen degeneration and disorganization. Calcifications or enthesophytes at the insertion site are common and appear as hyperechoic foci with posterior acoustic shadowing. Associated retrocalcaneal bursitis may be visualized as an anechoic or hypoechoic fluid collection between the anterior Achilles tendon and the calcaneus, with possible synovial hypertrophy in chronic cases. Power Doppler imaging may reveal hypervascularity at the tendon insertion, reflecting a chronic inflammatory response.

Figure 8A (LAX View):
Achilles tendon tears most commonly occur 2–6 cm proximal to the calcaneal insertion, a region of reduced vascularity On diagnostic ultrasound, full-thickness tears (highlighted with the blue arrows) appear as discontinuities in tendon fibers with a hypoechoic or anechoic gap, while partial tears (<50% disruption) demonstrate focal hypoechoic areas with some intact fibers. Fluid and debris are often visible in Kager’s fat pad, accompanied by mild hyperemia on Doppler imaging. Bone fragments may be present near the insertion in cases of avulsion injuries, and portions of the tendon may remain intact even with significant disruption. The Kuwada classification system categorizes tears: Grade I (partial rupture, <50%), Grade II (complete rupture, gap <3 cm), Grade III (gap 3–6 cm), and Grade IV (gap >6 cm). Dynamic imaging during dorsiflexion and plantarflexion confirms tendon discontinuity and retraction, guiding diagnosis and management.

Figures 8A (LAX View) and 8B (SAX View): Ultrasound findings of Achilles peritendonitis include a hypoechoic crescent (blue arrows) abutting the posterior surface of the distal Achilles tendon, with increased vascularity on Doppler imaging in the peritendinous region and Kager’s fat pad, without increased echogenicity The Achilles tendon itself appears intact, with no signs of tendinosis. These findings differentiate peritendonitis, which affects the tissues surrounding the tendon, from tendonitis, which involves intrinsic changes within the tendon structure.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CCBY-NC-4.0). View this license’s legal deed at https://creativecommons.org/licenses/by-nc/4.0 and legal code at https://creativecommons.org/licenses/by-nc/4.0/legalcode for more information.
1. Hopkins C, Fu SC, Chua E, et al. Critical review on the socio-economic impact of tendinopathy Asia Pac J Sports Med Arthrosc Rehabil Technol. 2016;4:9-20. doi:10.1016/j.asmart.2016.01.002
2. Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the understimulation of tendon cells?: Mechanobiological aetiology of tendinopathy. Int J Exp Pathol. 2007;88(4):217-226. doi:10.1111/ j.1365-2613.2007.00548.x
3. Pass B, Robinson P, Ha A, Levine B, Yablon CM, Rowbotham E. The Achilles tendon: Imaging diagnoses and image-guided interventions-AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2022;219(3):355-368. doi:10.2214/AJR.22.27632
4. Kainberger FM, Engel A, Barton P, Huebsch P, Neuhold A, Salomonowitz E. Injury of the Achilles tendon: diagnosis with sonography AJR Am J Roentgenol 1990;155(5):1031-1036. doi:10.2214/ ajr.155.5.2120931
5. McCreesh K, Lewis J. Continuum model of tendon pathology - where are we now? Int J Exp Pathol 2013;94(4):242-247. doi:10.1111/iep.12029
6. Matthews W, Ellis R, Furness JW, Rathbone E, Hing W Staging achilles tendinopathy using ultrasound imaging: the development and investigation of a new ultrasound imaging criteria based on the continuum model of tendon pathology BMJ Open Sport Exerc Med. 2020;6(1):e000699. doi:10.1136/ bmjsem-2019-000699
7 Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med 2009;43(6):409-416. doi:10.1136/ bjsm.2008.051193
8. Grechenig W, Clement H, Bratschitsch G, Fankhauser F, Peicha G. Ultrasound diagnosis of the Achilles tendon. Orthopade. 2002;31(3):319-325. doi:10.1007/s00132-001-0272-y
9. Abate M, Silbernagel KG, Siljeholm C, et al. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther. 2009;11(3):235. doi:10.1186/ar2723
10. Pang BSF, Ying M. Sonographic measurement of achilles tendons in asymptomatic subjects: variation with age, body height, and dominance of ankle. J Ultrasound Med 2006;25(10):1291-1296. doi:10.7863/ jum.2006.25.10.1291
11. Hartgerink P, Fessell DP, Jacobson JA, van Holsbeeck MT Full- versus partial-thickness Achilles tendon tears: sonographic accuracy and characterization in 26 cases with surgical correlation. Radiology 2001;220(2):406-412. doi:10.1148/ radiology.220.2.r01au41406
12. Scott A, Ashe MC. Common tendinopathies in the upper and lower extremities. Curr Sports Med Rep 2006;5(5):233-241. doi:10.1097/ 01.csmr.0000306421.85919.9c
13. Waring L, Hall A, Riley S. Musculoskeletal Ultrasound. How, Why and When. Elsevier; 2022.
14. Balint PV, Sturrock RD. Inflamed retrocalcaneal bursa and Achilles tendonitis in psoriatic arthritis demonstrated by ultrasonography Ann Rheum Dis 2000;59(12):931-933. doi:10.1136/ard.59.12.931
15. Theobald P, Bydder G, Dent C, Nokes L, Pugh N, Benjamin M. The functional anatomy of Kager’s fat pad in relation to retrocalcaneal problems and other hindfoot disorders. J Anat 2006;208(1):91-97 doi:10.1111/j.1469-7580.2006.00510.x
16. Fu SC, Rolf C, Cheuk YC, Lui PP, Chan KM. Deciphering the pathogenesis of tendinopathy: a three-stages process. Sports Med Arthrosc Rehabil Ther Technol. 2010;2(1):30. doi:10.1186/ 1758-2555-2-30
17 Wearing SC, Grigg NL, Hooper SL, Smeathers JE. Conditioning of the Achilles tendon via ankle exercise improves correla.