

My Blood My Health


Welcome to My Blood, My Health Digital Magazine
In this fifth issue, we will focus on ASH 2024, the American Society of Hematology annual meeting. Why ASH? This conference, created in 1958, has become, over time, the world's most comprehensive hematology event. The data presented at this conference influences how hematologists and oncologists will manage blood diseases. Around 8000 presentations have been given to more than 30,000 hematologists and hematology-oriented investigators this year.
For this issue, we will focus on myeloid cancers, such as myeloproliferative neoplasia and chronic myeloid leukemia. Research and clinical development are essential for patients. However, our mission is to discuss what you can do today to improve your life. So, on these pages, you will find some pearls we found at ASH that you can apply now to your life and take control of your journey. Stay tuned for additional exceptional ASH 2024 issues on other blood disorders soon.
With this magazine, we aim to pursue Heal Canada's mission to empower patients to access better and equitable services. We're deeply grateful to our readers for their continued interest and support. Your engagement drives us to produce insightful and valuable content that encourages patient-centricity in healthcare.
Thank you for participating in our journey, and welcome to this enlightening issue!
From your dedicated team, Brigitte and Cheryl.


Petruk, MBA B.Mgt. CEO & Founder of Heal Canada

Ph.D Chief Scientific Officer
My Blood, My Health Director
Heal Canada is a registered not-for-profit organization in Canada. healcanada.org
Disclaimer: The Patient Advocacy Digital Magazine provides general information and resources to promote patient empowerment and awareness The content is not a substitute for professional medical advice or treatment Always consult with qualified healthcare professionals for personalized guidance regarding your specific medical condition or situation

Cheryl
Brigitte Leonard,

The Power of Presence: Why Patient Advocates Must Attend Conferences Like the AmericanSocietyofHematology
CherylPetruk,MBA
Conferences like the American Society of Hematology (ASH) Annual Meeting stand as monumental gatherings where the latest advancements in hematology are unveiled. For many, these events are synonymous with cutting-edge research, groundbreaking therapies, and the convergence of the best minds in the field. However, amid the dense academic and clinical focus lies an often underappreciated yet equally vital attendee: the patient advocate. The importance of patient advocates attending such conferences extends beyond token representation; it is about fostering a partnership that elevates the impact of hematological research and ensures patient-centric outcomes.
This article explores why patient advocates must be active participants in conferences like ASH, emphasizing their role in understanding clinical trials, engaging in research initiatives, identifying research gaps, and shaping the future of hematology.
Demystifying Clinical Trials
Patient advocates serve as interpreters of the clinical trial ecosystem. Their unique position armed with a blend of lived experience and knowledge allows them to distill intricate medical jargon into relatable narratives. At conferences like “ASH”, patient advocates gain firsthand access to the latest developments in clinical trials, and current and ongoing research for hematological malignancies and disorders, enabling them to:

Educate their communities about new clinical trials, research and emerging therapies
Alleviate fears and misconceptions surrounding participation in trials.
Promote equitable access by addressing barriers specific to underserved populations.
Amplifying Patient Voices in Trial Design
Clinical trials often falter due to low enrollment or retention rates, issues rooted in a disconnect between trial designs and real-world patient needs. Patient advocates attending conferences can provide critical feedback to researchers, ensuring trials are designed with patient-centric considerations, such as:

Convenience of trial locations
Managing side effects in daily life.
Clear communication of potential benefits and risks.
By fostering this dialogue, patient advocates help create trials that are not only scientifically robust but also patient-friendly, thus enhancing participation and success rates.
Patient Engagement in Hematology Research Initiatives: A Collaborative Necessity
Including patient advocates in hematology research initiatives is no longer optional it is a necessity. Conferences like ASH provide a platform for patient advocates to engage directly with researchers, clinicians, and industry leaders, conveying that patients are partners, not passive subjects, in the research journey.


Advocates as Bridges Between Stakeholders
Patient advocates excel in bridging the gap between researchers and the patient community. Their involvement ensures that the patient perspective is woven into the fabric of research initiatives, influencing:
Research priorities: Advocates can highlight areas that matter most to patients, such as quality of life and symptom management.
Communication strategies: Advocates can guide researchers in effectively communicating findings to lay audiences.
By attending conferences, advocates can connect with researchers and policymakers, establishing collaborative networks that prioritize patient-centric goals.
Building Trust in the Research Process
Trust is a foundational element in advancing hematology research. Patient advocates are instrumental in building trust with their deeprooted ties to their communities Their presence at conferences like ASH symbolizes transparency and commitment, reassuring patients that their voices are heard and valued in the research process.
Identifying Gaps in Hematology Research: The Advocate’s Perspective
Despite significant advancements in hematology, gaps persist especially in addressing the needs of underserved populations, rare disease communities, and non-traditional therapeutic outcomes. Patient advocates are uniquely positioned to identify these gaps and advocate for inclusive and comprehensive research
Highlighting the Unmet Needs
Patient advocates bring a fresh perspective to the research conversation. They can shed light on areas that may not be immediately apparent to researchers, such as:
The psychosocial impact of living with hematological conditions.
The financial toxicity of treatments and long-term care.
The lack of data on rare hematological disorders. Advocates can help shape a research agenda that aligns with patients' realities by raising awareness of these unmet needs.

Advocating for Diversity in Research
Diversity in research is critical for generating findings that apply to all patient populations. However, many clinical studies in hematology suffer from a lack of representation. Patient advocates can push for greater inclusion of:

Ethnically diverse participants Elderly patients and those with comorbidities. Participants from rural or low-income backgrounds.
Their advocacy ensures that research findings are equitable and reflect the broader patient population.
Patient Advocacy as a Valued Partner in Research
To truly advance hematology, the role of patient advocates must evolve from observers to active partners. Conferences like ASH provide the perfect environment to showcase the value of this partnership.
Driving Policy and Funding Decisions
Patient advocates can influence funding and policy decisions by articulating the human impact of hematological research. Their stories and insights provide a compelling case for prioritizing research initiatives that deliver tangible benefits to patients
Co-Authoring Research Outcomes
Collaborative models where patients advocate coauthoring research papers and contributing to conference presentations are gaining traction This practice acknowledges their contributions and ensures that the patient's perspective is integrated into academic discourse.


Creating a Culture of Collaboration
Patient advocates' presence at conferences fosters a culture of collaboration. Their involvement encourages researchers, clinicians, and industry leaders to view patients as allies, paving the way for scientifically and socially impactful innovations.
Breaking Barriers: Overcoming Challenges to Advocate Participation
While the importance of patient advocates at conferences is undeniable, barriers to their participation remain Financial constraints, lack of institutional support, and limited accessibility are a few challenges that must be addressed.



Building Capacity
Providing Financial Support
Conference organizers and sponsors must recognize the value of patient advocates and provide financial support for their attendance. Travel grants, reduced fees, and stipends can make a significant difference.
Enhancing Accessibility
Efforts to enhance accessibility such as providing virtual attendance options, offering plain-language summaries of presentations, and ensuring physical accommodations are essential to making conferences inclusive for all advocates
Investing in training programs for patient advocates ensures they are equipped to contribute effectively. Workshops on scientific literacy, public speaking, and advocacy skills can empower them to maximize their impact.
Patient advocates attending conferences like the American Society of Hematology are not merely symbolic but transformative. Their involvement enriches the research process, bridges communication gaps, identifies critical needs, and ensures that hematology advancements resonate with the patients who matter most.
As we continue to push the boundaries of what is possible in hematology, let us remember that true progress is only achieved when patients are not just participants but partners Together, researchers and advocates can build a future where hematological breakthroughs improve lives most meaningfully. Conferences like ASH are the stages where this collaboration begins and thrives.
For patient advocates, attending these conferences is not just an opportunity but a responsibility to their communities and a testament to the power of shared voices in driving change. For the scientific community, welcoming and empowering these advocates is a step towards a more inclusive and impactful future in hematology



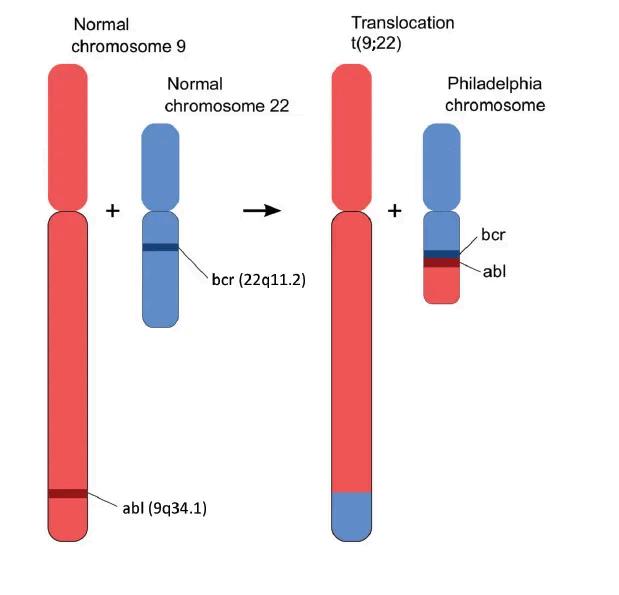
What are the best ways to manage CML in 2025?
Brigitte Leonard, Ph.D
Since the introduction of Gleevec (imatinib), a BCR-ABL targeted treatment (TKITyrosine kinase inhibitor), in 2000, the landscape of CML (Chronic myeloid leukemia) treatment has significantly evolved. Patients with this cancer now have a life expectancy almost on par with the general population, a stark contrast to the 5-year life expectancy before.
This remarkable progress instills hope and optimism for further advancements in CML treatment. Most regulatory agencies worldwide have approved six BCRABL TKIs for treating CML. The development of additional TKIs was initially encouraged because not all patients respond adequately to imatinib.
In 2025, CML experts are still facing some challenges. One is how to select the best TKI in a front-line setting. The second one is maximizing the patient's chance to reach a deep molecular response to attempt treatment-free remission. The ultimate goal for most patients.
See what we learned at ASH regarding these remaining challenges.
Treatment-free remission is the ultimate treatment goal
Initially, physicians were prescribing TKI to CML patients until the patient ceased to respond or died. The medical community thought that TKIs couldn't cure CML patients like transplantation.
Less than a decade after the approval of the 1st TKI (imatinib) and with the sophistication of the monitoring technique, Dr Mahon's team
demonstratedthatsomepatientswhoreachedundetectablelevelsofcancerousBCR-ABL cells could stop their treatment and remain in remission. The phenomenon was called treatment-free remission (TFR). The term cure was not acceptable to the CML expert communitybecauseroughlyhalfofthepatientsneededtoreinitiatetheirtreatmentdueto areturnofBCR-ABLcellstodetectablelevels.

The undetectable levels of cancerous BCR-ABL cells are often called deep molecular response (DMR). The depth of response is the principal factor in eligibility to attempt a supervised treatment discontinuation and remain in remission. Initially, the medical community thought that only a minority of patients could attempt TFR. These patients neededtorespondwellintheirfirstline,reachDMRandmaintaintheirtreatmentforan extendedperiod(5to10years).Severalclinicalstudieshavedemonstratedthathealthcare professionals(HCPs)cansafelyattemptTFRinpatientswhoreceiveseverallinesoftherapy aslongastheyachieveDMR.
At ASH 2024, additional real-world studies add to the evidence that patients can safely attemptTFRinvariouscontexts:
Patientswhofailedseverallinesoftreatment(1778)
PatientswithadditionalmutationssuchasASXL1(3158)
Patientsinlower-incomecountriesorcities(3162)
Stoppingthetreatmentmustbetakencautiouslyandundertightmonitoringbecausehalf ofthepatientsneedtoreinitiatetheirtreatmentuntiltheycantryadditionalTFRattempts.
ImatinibisnotthebestTKIforpatientswhowanttoattemptTFR.Thechoiceofthe1stline cansignificantlyimpactpatients'chancesofreachingTFRandthedurationoftreatments.
Fewer patients reached DMR when treated with imatinib versus second-generation TKIs (30%vs50%).Also,patientstreatedwithsecond-generationTKIscanstopearlierbecause they achieve DMR faster. Some early responders can stop after only three years of treatment.Asciminib,themostrecentTKI,providesaquickerandmoreprofoundresponse thansecond-generationTKIs.

Best front-line treatment
Regardless of the possibility of TFR, the choice of front-line TKI is crucial for patient outcomes. It impacts the patient's odds of acquiring additional mutations and progressing to more aggressive forms Progression is a death sentence for most patients with a survival rate inferior to a year.
Most patients respond well to all TKIs; less than 5% will progress to a more aggressive phase if they take their medication as prescribed. So, choosing one TKI over another does not impact survival curves. However, progressions tend to occur quickly in the first months of treatment. The level of response to a TKI at 3 months predicts patients' survival chances, and even if a switch of TKIs is done, it cannot always change the patient's fate.
When we analyzed phase III trials conducted in a front-line setting, we saw a clear trend: at least two times more patients in the imatinib group (2-5%) progressed during the first year compared to the second-generation TKIs and asciminib (1-2.3%).
The front-line TKI can also impact the long-term response. Phase III trials in front-line settings showed that patients treated with imatinib developed more mutations than those treated with second-generation TKIs. These additional mutations can impact the success rate of the following lines of treatment
Based on several registries and real-world studies at ASH, imatinib remains the most prescribed TKI in the front-line setting. Why do patients continue to receive an inferior TKI? Imatinib has a perceived better cardiovascular (CV) safety profile. The medical community recommends avoiding second-generation TKIs in patients with CV risk factors regardless of the severity of CML.
To assess the impact of these recommendations, Dr. Kim's team at Princess Margaret Toronto analyzed CML outcomes in line with a validated CV risk score (Framingham Risk Score) (1768). At diagnosis, 40 to 60% of CML patients have at least one comorbid condition, and 30% present with CV risk factors. Patients with CV risk factors tend to receive imatinib more than patients without risk (69 2% vs 14 9%) In this analysis, the type of front-line TKI is the most critical factor influencing treatment outcomes, with second-generation TKIs showing superior results to imatinib. These results highlight the need for alternative treatments with better efficacy and tolerability.
Is asciminib the one?
Asciminib emerges as a promising 1st line option for CML patients, potentially reshaping the treatment landscape. The ASC4FIRST Study's 96-week follow-up has revealed that asciminib provides a superior response rate and boasts a better-tolerated profile in the front line compared to all other TKIs (imatinib and second-generation TKIs). This exciting development paves the way for a more effective and tolerable CML treatment

Asciminibprovidesthebestmolecularresponseandtolerability:
Deepmolecularresponse:Basedonthestudy'sresults,after3to4yearsoftreatment,3 outof10patientswillbeeligibletoattemptTFRversusoneifinitiatedonimatiniband twoifinitiatedonasecond-generationTKI.
Tolerability:Patientstreatedwithasciminibhadtwotimeslowerchancesofbeing discontinuedfromthestudybecauseofsideeffectsthanpatientstreatedwithimatinib orasecond-generationTKI
Bestsecond-lineoptions
Hoperemainsforpatientswhoarefailingtheirfront-linetreatment.Physicianscanprescribe asecond-generationTKIafterimatinibfailureoranothersecond-generationTKIfailure. However,inthelattercase,theirefficacyislessimpressive.Ponatinib,athird-generationTKI, has provided the best response in the post-second-generation setting. Ponatinib is associatedwithseverecardiovascularsideeffects,andphysiciansarelookingforasafer alternative.AsciminibdeliverspromisingresultsintheASC2ESCALATEStudy,with96%of patientsachievingtheirearlyresponsemilestonesat3monthsand11%achievingDMRat6 months.Thetolerabilitywasconsistentwithprevioustrialsinfront-lineandlater-line(3+) settings.
Theadditionofasciminibtothetreatmentarsenalsecuresbettercoverageregarding mutations.PatientswhodevelopedmutationsintheATPbindingsiteofBCR-ABLshould respondverywelltoasciminib.Incontrast,patientswhodevelopamutationinthemyristoyl pocketorharbourASXL1shouldrespondverywelltotypicalTKIs.CombiningbothATPbindingsiteinhibitorswithasciminibcanmaximizeresponseforpatientswhodon'trespond adequately.
ThefutureofCML
AnotherATP-bindingsiteofBCR-ABLinhibitor,Olverembatinib,isinclinicaldevelopment, andithasanadequateresponserateandsafetyprofile.Asecondmyristoylpocket inhibitor,Tgrx,isalsoinclinicaldevelopmentandhasanadequateresponserateand safetyprofile
PatientswithCMLcantakecontroloftheirjourneybytrackingtheirPCRresults,reachingfor DMR,andtakingcareoftheirgeneralhealthbyreducingtheircardiovascularrisks, exercising and eating healthy because, after all, they have almost a normal life expectancy.
References:ASH24AbstractNumbers@https://ash.confex.com/ash/2024/webprogram

Is Myelofibrosis Better Treated In 2025?
Brigitte Leonard, Ph.D
Reducing spleen size and symptoms is critical for patients because both impact their quality of life (QoL) and survival. Since 2010, patients have benefited from a targeted treatment, ruxolitinib, a JAK inhibitor (JAKi), to manage these two hallmarks of myelofibrosis (MF).
Since the introduction of ruxolitinib (also known as JAKAFI or JAKAVI), a type of drug called a Janus kinase inhibitor (JAKi), three additional JAKi have been approved: fedratinib (Inrebic), momelotinib (Ojjaara) and pacritinib (Vonjo). Ruxolitinib remains the most frequent first-line JAKi therapy used.
While the myeloproliferative neoplasm (MPN) experts community is still debating the risk categories and the best clinical trial endpoint to assess new treatment in myelofibrosis, do patients receive the best care daily? Are there any clinical practice gaps that can be easily fixed?
Let's see what we learned this year at ASH! 3:45min.
Unmet medical needs with ruxolitinib
Ruxolitinib remains the most frequent first-line JAKi therapy used. It is known that half the patients must stop ruxolitinib within 3 years because of cytopenia (anemia) or lack of response. Before the approval of the newest JAKi, when patients failed ruxolitinib, the clinical options were limited, and their life expectancy was less than 1 year.
Anemia remains one of the major limiting factors in treating patients with ruxolitinib. Alternative approaches to anemia management involving supportive therapies are of limited efficacy.
Therefore, it is reassuring for the community to have access to momelotinib. Why momelotinib in this setting? Momelotinib uniquely can restore red blood cell formation by inhibiting an iron management molecule called ACVR1 At ASH, several real-life treatment analyses with momelotinib confirmed its marked benefit in improving anemia, with high rates of patients achieving transfusion independence (1790). The hemoglobin levels and platelet counts increase rapidly within 3 months of treatment (2430). More importantly, momelotinib reduces mortality by ~50% compared to the previous best available therapies in JAKi-exposed patients while reducing spleen volume and improving symptoms with an acceptable toxicity profile (3187). This significant improvement in survival rates should instill a sense of hope and optimism in the medical community.
Earlier is better
JAKi is typically reserved only for patients with symptoms and a more aggressive form of MF (Intermediate or High risk). This is regrettable because clinical benefits are superior in the early stage of the disease, with better spleen and symptom responses and fewer hematological and non-hematological side effects (1786). This underscores the importance of early intervention, making the medical community feel proactive and responsible in their approach to patient care
Additionally, treatment with a JAKi such as ruxolitinib significantly reduces the risk of thrombosis in patients with MF, which remains a significant cause of mortality in MPNs (3168).
Where are the gaps in current clinical practices?
Sadly, physicians still poorly assess their patients' real symptom burden. A robust analysis has demonstrated that physicians reported a much lower symptom presence than patients, even in cases with severe symptom burden (485). This observation is dramatic for patients because:
Symptoms are the main trigger for initiating the treatment with a JAK inhibitor. Some symptoms, such as fever, weight loss, and night sweats, impact survival. So, managing symptoms is crucial not only for the quality of life but also for survival.

These findings highlight the need to include patient-reported outcome tools, such as validated symptom questionnaires, in the routine clinical assessment of MPN patients. This is a quick fix because a validated symptom questionnaire already exists. The questionnaire is named MPN-SAF (MPN-Symptom assessment form) or MPN-10. Patients can easily access this form (click on the link: MPN Symptoms Assessment Form (MPN-SAF) | Jakafi.com).
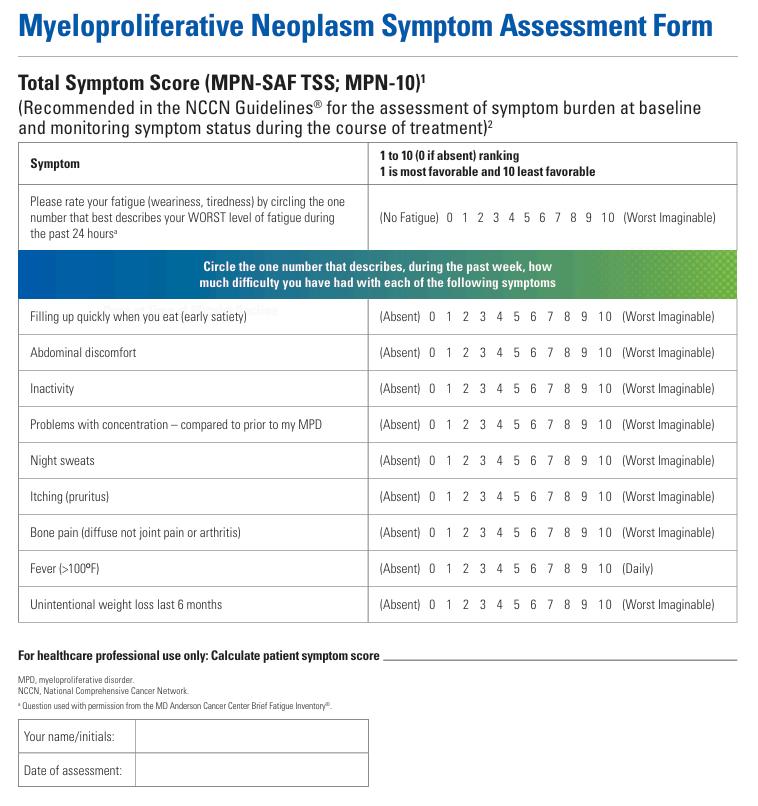
At ASH, a study addressing the impact of continuing education and quality improvement (QI) programs in myelofibrosis can influence physicians' behaviours (3673). The findings from these continuing education and QI programs demonstrate that cancer clinicians can improve care for patients with myelofibrosis by incorporating appropriate testing and tailoring therapy based on prognostic scores and patient symptoms After the intervention, the assessment of the symptoms with the MPN-SAF Form went from 0% to 80%. The prognostic score was assessed at 60% vs 25% at baseline. The proportion of patients treated with a JAKi improved.

MPN patient advocacy groups encourage patients to track their symptoms with the form and use the results during consultations with physicians It can positively impact and improve their care management.
The future of myelofibrosis
Several new agents with mechanisms of action different from JAKi are in clinical development. Some promising agents could be given as a single agent or in combination with a JAKi to maximize clinical response. The front runners are Navtemadlin and Selinexor in Phase III clinical trials, which have encouraging preliminary results This should spark excitement and intrigue in the medical community about the potential of these new agents.
Is myelofibrosis better treated in 2025?
The answer is yes.
JAKi are efficacious, well-tolerated, and safe. Patients on JAKi have a better quality of life, better disease control, and survival benefits
For patients with anemia and low platelet levels, momelotinib and pacritinib allow better disease control versus ruxolitinib.
We know that sooner is better. If JAKi is taken earlier in the patient journey, the clinical benefit is amplified, and the side effects are reduced.
Not all physicians use the symptoms assessment form and prognostic score to assess their patients, which delays the initiation of JAKi treatment.
As a patient or a caregiver, you have all the tools you need to improve your life Take control of your journey. Assess your symptoms, ask your physician about your risk category, and request a JAKi that is most appropriate for your situation. You can also join a patient group like the Canadian MPN network to support you in your journey.
References:ASH24AbstractNumbers@https://ashconfexcom/ash/2024/webprogram
Canadian MPN Network
Patient Advocacy and Education Group


Polycythemia Vera: Let’s surf the wave!
Brigitte Leonard, Ph.D
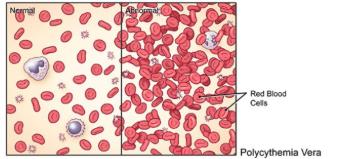
Polycythemia Vera (PV) is a blood cancer characterized by excessive production of red blood cells reflected by elevated hematocrit (Hct) levels. The excess of red blood cells increases the risk of developing blood clots in veins or arteries (thrombosis). Thrombosis is closely linked to elevated Hct levels (>45%). The most pressing medical intervention is to avoid coagulation with aspirin and control Hct levels by extracting a large amount of blood (phlebotomies) because half of the patients will die from cardiovascular complications (CV). What about PV patient's symptoms and risk of progression to more aggressive forms of cancer?
A recent analysis estimated that PV patients treated in specialty care centers have a 65% lower mortality risk (Kuykendall, 2024). Why? Some explanations have emerged from ASH 2024 presentations. PV experts discuss modifying risk assessment and adding new criteria to influence treatment decisions.
This is good news for PV patients because some are easy and quick fixes to optimize current treatments. Additionally, new treatments are underway to manage patients who run out of options.
Are you ready for a revolution? Because we are.
While myelofibrosis patients benefit from many risk classification updates, including mutations, PVisstillassessedwithamoresimplisticapproachbasedonageandhistoryof thrombosis.PVpatientsaredesignatedashigh-riskiftheyhavepreviousthrombosisorif theyare60yearsoldorolder.However,howarelow-riskpatientscomparedtothegeneral population?Theanswerisnotpretty.Low-riskpatientshave2.5to3.7timesmorethrombosis than the general population In comparison, high-risk patients have 35 to 5 times more thrombosisthanthegeneralpopulation.
Basedonachartreviewconductedin2020-2021,thepooreroutcomeinnon-specialized PV centers can be explained by the poor adherence to the current treatment recommendations(Crodel,2021):
In some cancer centers, less than 10% of high-risk patients are treated with cytoreductivetreatment
Often,low-riskpatientsstayinthelow-riskcategorywhentheyreach60yearsold,and theirtreatmentdoesn'tchange.
Whentreated,patientscanbemaintainedonHydroxyurea(HU)despiteclearsignsof insufficientdiseasecontrol.
For low-risk patients, treatment recommendations include aspirin and phlebotomies to maintainHctbelow45% Theissuewiththistreatmentplanisthatlessthan10%ofpatients successfullymaintainedtheirHctbelow45%withonlyphlebotomies(Verstovsek,2023).Let's befrank:frequentphlebotomiescanbeinconvenient.Also,itisassociatedwithsideeffects and complications due to iron depletion. PV experts dressed a list of clinical signs and symptoms,identifyinglow-riskpatientswhoshouldbeconsideredathighriskandreceive cytoreductiontherapy Thelistincludes:
Hcthigherthan53%atdiagnosis
DifficultyincontrollingHctorneedfrequentphlebotomies(>6peryear)
Phlebotomyintolerance
Signs of disease progression include high levels of white blood cells or high levels of plateletorspleenenlargement.
Significantsymptomsburdenlikefatigueanditching
Uncontrolledcardiovascularrisk(CV)factors
Aretrospectiveanalysisdemonstratesthattheseclinicalsignsaremorepredictivethanage toidentifyPVpatientsatincreasedthromboticrisk,confirmingthattheyarehelpfuland improveriskstratification.Additionally,basedontheconventionalclassification,mostlowriskpatientsrequirecytoreductivetherapy(242).

What about mutations other than JAK2?
JAK2 mutations are invariably associated with PV. However, more than 50% of affected patients also harbour non-JAK2 mutations, some considered prognostically relevant. These additional mutations can be linked with myelofibrosis and/or leukemia progression. Three mutations are significantly associated with inferior survival: SRSF2, ASXL1, and IDH2. SRSF2 and AXL1 mutations are associated with an increased risk of myelofibrosis and/or leukemia progression (1797). Mutation testing can assess the risk of progression and eventually facilitate the personalization of treatment. Can IDH2 inhibitors be used for patients harbouring IDH2 mutations? Only the future knows!
Let's talk about cytoreduction therapy!
Despite several issues, the first and most commonly used agent is hydroxyurea (HU). With HU, only 24% of patients achieved a complete response. The complete response rate is higher in patients tolerating a dose of 1g or more daily. However, this daily dose is associated with increased anemia and skin lesions. In fact, 10-15% of patients are intolerant to HU. Several physicians are reluctant to use HU in younger patients So, HU is reserved for high-risk patients, and the data on low-risk patients is limited.
In 2025, new therapies such as interferon and JAKi can replace HU. Interferon (IFN) has been known for decades to provide interesting results in PV However, the poor tolerability of the old formulation impacted its adoption in clinical practices. RopegIFN (BESREMI) has been studied in low-risk and high-risk patient populations. RopegIFN reduces the need for phlebotomy and normalizes leucocyte and platelet counts, resulting in no thrombotic events. Considered as a disease modifier. RopegIFN reduces the JAK2 mutation burden. It also improved the quality of life for PV patients, addressing concerns about drug tolerability and toxicity. In low-risk patients, the dose can be increased from 100 µg every 2 weeks to 250 → 350 → 500 µg every two weeks if the clinical benefits are not observed (1799). RopegIFN provides superior results in hydroxyurea-naive patients. Avoiding HU and initiating patients with RopegIFN could improve long-term care. Based on these findings, the National Cancer Network (NCCN) recommended RopegIFN as the preferred cytoreductive regimen in the first-line setting for patients with low-risk PV and a preferred interferon regimen in highrisk PV.
What about symptoms?
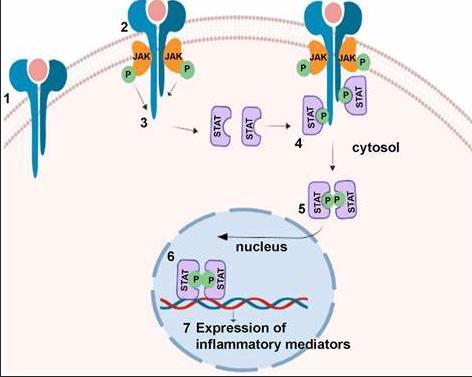
Every PV patient and their caregiver knows how the burden of symptoms can be heavy over time and how it impacts the quality of life. Symptoms such as fatigue, itching, concentration difficulty, insomnia, sexual dysfunction, night sweats, and early satiety are mainly caused by inflammatory dysregulation and vascular disturbances. Sadly, physicians still poorly assess their patients' real symptom burden. A robust analysis has demonstrated that physicians reported a much lower symptom presence than patients, even in cases with severe symptom burden (485).

This observation is dramatic for patients because symptoms are the main trigger for initiating more efficacious treatment like interferon or a JAK inhibitor. It is a quick fix because a validated symptom questionnaire already exists. The questionnaire is named MPN-SAF (MPNSymptom assessment form) or MPN-10. Patients can easily access this form (click the link: MPN Symptoms Assessment Form (MPN-SAF) | Jakafi.com). Patients and caregivers can fill it out and use it when discussing treatment choices with their physicians.
The future
Several new molecules introduced early in th

The front runners will be: Rusfertide completed a phase 2 trial, where patients achieved better long term Hct control and decreased the need for phlebotomy. Patients also observed symptom improvement when treated with Rusfertide. The randomized phase 3 VERIFY study (NCT05210790) is ongoing and will evaluate rusfertide vs placebo in PV patients. In a phase 2 trial, OB756, a JAK2 inhibitor, has shown meaningful clinical benefits in patients with PV, especially for quick spleen reduction and high JAK2 mutation burden reduction. OB756 also has shown a durable, complete hematological response and few side effects. Phase 3 is ongoing, comparing OB756 to HU in PV (3180).
What can PV patients do to improve their odds today?
Track your symptoms with the MPN assessment form. Track your Hct and blood count. Discuss with your physician about treatment options if your disease is not adequately controlled Even if you are a low-risk patient, you might need cytoreductive therapy RopegIFN provides superior control than HU. Is RopegIFN a good option for you? Also, PV patients should be encouraged to manage their cardiovascular risk factors: Stop smoking
Exercising more
Eating healthy and targeting healthy weight
Getting enough sleep
Controlling the blood tension with medication if hypertension
Controlling blood sugar with medication if diabetes
It is a lot of work but you can do it!











References:ASH24AbstractNumbers@https://ashconfexcom/ash/2024/webprogram

Has anyone heard about ET? ET for Essential thrombocythemia.
Brigitte Leonard, Ph.D
Essential thrombocythemia (ET) is a blood cancer characterized by high levels of platelets As for Polycythemia Vera (PV), life expectancy is shortened compared to the general population, and the leading causes of death are vascular complications and progression to more aggressive cancers.
Unlike other myeloproliferative neoplasia (MPN), such as myelofibrosis (MF) and PV, essential thrombocythemia (ET) patients didn't benefit much from recent scientific advancements. Why? ET is the most frequent MPN. So, it should retain the most attention, right? Not really, because ET has the best prognosis and a survival expectancy of nearly 20 years. A study assessing the efficacy of a novel agent will take at least 5 years to demonstrate substantial clinical benefits against thrombosis and 10 years against progression to myelofibrosis and other leukemia
So, most treatment recommendations are based on old risk classification scores and focused on baby aspirin and managing cardiovascular (CV) risk factors. Cytoreductive therapy, mostly Hydroxyurea (HU), is recommended only for high-risk patients or patients who demonstrate signs of progression. Even the criteria for treatment change are not well-established.
Is there hope for ET patients to gain access to a more comprehensive care plan?
We noticed some interesting trends at ASH that can be encouraging for ET patients. There are also some easy fixes to improve ET patient's journey today.
ET's current treatment goals
In 2017, a survey revealed that physicians' treatment goals focused on preventing vascular complications, while patients were also preoccupied with slowing progression and getting healthy blood counts. This discrepancy can explain why, even at ASH in 2024, most studies focused on cardiovascular risk and anticoagulation treatments
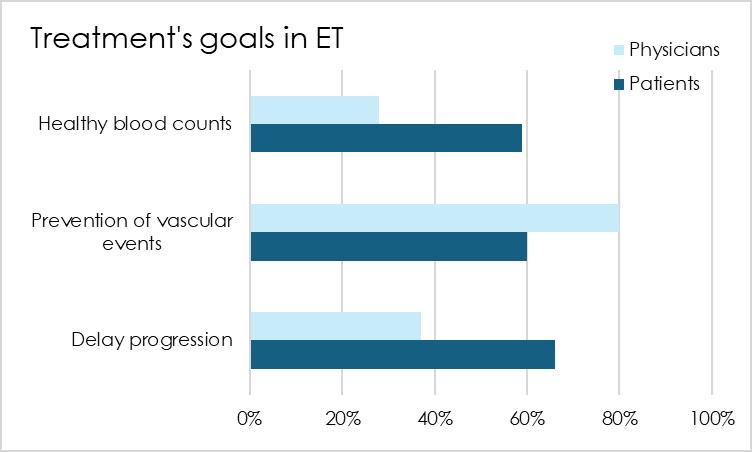
Physicians focus on the most pressing matter in ET: cardiovascular complications, which is perfectly logical However, it is fair that patients want a more holistic approach that addresses all the risks associated with the disease
The impact of driver mutation
ET is a complex cancer. The driver mutations can be either JAK2, CALR, MPL or triplenegative (TN) in a similar proportion to myelofibrosis. It might not be current practice to test mutations yet because it is not mandatory to confirm the diagnosis. However, recent findings demonstrated that the type of mutation impacts the clinical journey (Abu-Zeinah, 2024). Patients who harbour JAK2 mutation or TN are more at risk of having thrombotic events In contrast, patients who harbour CALR or MPL mutation are more at risk of having a disease progression to myelofibrosis (MF).
How does Hydroxyurea (HU) perform in ET?
HU will be the principal front-line treatment offered to ET patients. HU induces 67% of complete hematological response (CHR), and it takes a full year to achieve this response. The CHR means the normalization of platelet count, a reduction of the white blood cell count below 10, absence of disease symptoms and a normal spleen. From initial responders, 20% of patients developed a resistance or intolerance to HU. Patients who achieved CHR have better survival rates, lower risk of thrombosis and a lower rate of disease progression Failure to achieve CHR on HU could be considered an eligible criterion in second-line therapy (241). Peg-interferon (PegIFN) or anagrelide is also proposed for the 1st line. Based on this analysis, 53% of patients should be in second-line treatment in the second year of their treatments. The proposed therapies in 2nd line post-HU are PegIFN, anagrelide or ruxolitinib (JAK2 inhibitor used in MF and PV). In a late-breaking abstract at ASH24, RopegINF, a better-tolerated IFN formulation approved in PV, demonstrated interesting CHR results in ET patients (LBA-4). RopegINF could become a promising treatment alternative. A Phase 3 trial is ongoing to evaluate RopegINF in ET patients who are intolerant or refractory to current cytoreductive treatments

Is anagrelide still a good
treatment option?
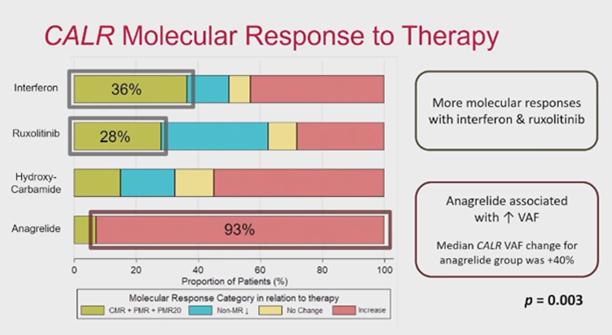
At ASH24, a recent analysis of the CALR mutation burden during treatment demonstrated significant differences among proposed therapies. This analysis combined 60 MF and 82 ET CALR-positive patients(485). Interferon (IFN), ruxolitinib, HU and anagrelide were tested.
Almost all patients treated with anagrelide see their CALR frequency increase during the treatment. In contrast, around 30% of patients treated with IFN and ruxolitinib experienced a decrease in their CALR frequency. An increase in CALR frequency correlates with a rise in BM fibrosis. Anagrelide requires caution, and INF or ruxolitinib may be used instead until more data is generated.
What about symptoms?
Every ET patient and their caregiver knows how the burden of symptoms can be heavy over time and how it impacts the quality of life. Symptoms such as fatigue, night sweats, dizziness, depression, numbness, headaches, vision changes, and insomnia are mainly caused by inflammatory dysregulation and vascular disturbances.
Sadly, physicians still poorly assess their patients' real symptom burden. A robust analysis has demonstrated that physicians reported a much lower symptom presence than patients, even in cases with severe symptom burden (485). This observation is dramatic for patients because symptoms are the main trigger for initiating more efficacious treatment like interferon or a JAK inhibitor It is a quick fix because a validated symptom questionnaire already exists. The questionnaire is named MPN-SAF (MPNSymptom assessment form) or MPN-10. Patients can easily access this form (click the link: MPN Symptoms Assessment Form (MPN-SAF) | Jakafi.com). So, patients and caregivers can fill it out and use it when discussing treatment choices with their physicians.


What can patients or caregivers do today to improve their journey?
As a patient or a caregiver, you have all the tools you need to improve your life. Take control of your journey.
1) Are you sure you have ET? Enlarged spleen or high LDL (cancer marker) are rare in ET. In that case, you might have myelofibrosis in the pre-fibrotic stage instead of ET. These two blood cancers can easily be confused. However, risks and treatments are different. So, it is better to double-check
2) Know which mutation drives your cancer and discuss how to manage your personalized risks with your physician.
3) Assess your symptoms with the MPN symptoms assessment form, and use the results during your discussion with your physician A frank conversation about your symptoms with your treating physician is essential because they are linked to disease progression and poorer survival. It is not only a question of quality of life.
4) Track your blood counts and discuss treatment changes if the results are suboptimal. Can you have access to RopegIFN, PegINF or a JAKi?
5) Assess your cardiovascular risk factors with your physicians and reduce them as much as possible:
Stop smoking
Exercising more
Eating healthy and targeting healthy weight
Getting enough sleep
Controlling the blood tension with medication if hypertension
Controlling blood sugar with medication if diabetes
You can also join a patient group like the Canadian MPN network to support you in your journey. We sincerely wish you the best and hope the information helps you.
References:ASH24AbstractNumbers@https://ashconfexcom/ash/2024/webprogram















C l i n i c a l T r i a l s a n d S u r v e y s




Alliances























Partners







My Blood, My Health Team
Brigitte Leonard, Ph.D

Brigitte Léonard is the Chief Scientific Officer at My Blood, My Health, a registered not-for-profit organization advocating equitable access to quality healthcare across Canada. She had the privilege of working in Pharma for over 20 years, contributing to bringing lifechanging treatments to patients with the highest ethical standards. Now, she wants to share her knowledge and utilize her scientific, strategic, and communication skills to help the patients' community.
She obtained her Ph.D. in Biomedical Sciences from Université de Montréal in 2003. Her doctoral research was conducted under the supervision of Dr. Denis-Claude Roy at Guy-Bernier, MaisonneuveRosemont Hospital Research Center. She developed a quantitative diagnostic assay in non-Hodgkin's lymphoma and evaluated the relevance of this marker in the patient's outcome.
Cheryl Petruk, MBA B.Mgt.

Cheryl A. Petruk is a multifaceted professional whose career spans across patient advocacy, business, and post-secondary education, showcasing her dedication to making a significant impact in each of these areas.
Cheryl's transition into patient advocacy was driven by a passion from her family circumstances and a deep commitment to ensuring patients' rights and access to care Cheryl has worked tirelessly to bridge the gap between the healthcare system, patients, and pharma stakeholders, ensuring that patients' voices are heard and their needs are met. Her work involves collaborating with Stakeholders and Patient Advocacy Organizations, lobbying for patient centricity, and providing patient support and guidance. Cheryl's empathetic approach and dedication to advocacy have made her a respected figure in this field, admired by patients, healthcare professionals, and fellow advocates.
Cheryl also leads and is the lead faculty member at CACHEducation, Patient Advocacy Training.
