


E S O




E S O
With the book you are holding you will:
➜ Gain insights into Physics and Chemistry employing the 5-stage methodology, mirroring the Spanish version: Engage, Explore, Explain, Elaborate, and Evaluate.
➜ Enhance and expand your English skills through activities designed for practicing written and oral comprehension and expression.
A learning experience related to Andalusia is explored through the following steps: Engage. A motivational video related to the unit’s content.
Explore. Activities to investigate the situation. Elaborate. Activities to apply the concepts.


The left-hand pages focus on presenting content in the Explain phase. On the right-hand pages, you’ll find a variety of activities designed to reinforce the content.
The final step of the 5-stage model, Evaluate, is completed with an assessment at the end of each section.
The term closes with a double-page spread that includes texts and activities related to some of the topics covered during the term.






The scientific community today undeniably plays a crucial role in advancing sustainable development, with a notable emphasis on environmental conservation.
This commitment to the sustainable development goal (SDG) is particularly evident in Europe through the implementation of the 2030 Agenda, a framework embraced by each country based on its legislative autonomy. In Spain, including Andalusia, this dedication extends across institutions, businesses and citizens alike.
You will work in groups, following your teacher’s instructions, on initiatives aimed at environmental preservation in Andalusia and how they are related to the disciplines of physics and chemistry.
1 Considering our current lifestyle, what are the most environmentally polluting activities? Look for some data, especially related to Andalusia.
2 Focusing on the chemical industry, what initiatives are being undertaken to reduce its environmental impact? Investigate specific industries if possible near your place of residence.
3 In Andalusia, the agri-food sector, particularly olive oil production, is crucial. What impact does it have on the environment? Look for information and list some actions to reduce this impact, related to physics and chemistry.
4 Transportation is highly relevant in Andalusia, as in any other developed region. What initiatives have been taken in this area to reduce the negative impact on the environment? Explain with the help of information sources.
5 Concerning science in Andalusia, which public and private institutions or organisations primarily focus on reducing environmental pollution? Investigate and describe some of these lines of work.
1 Based on your findings, prepare a presentation about environmental initiatives in Andalusia and how they are related to physics and chemistry.
2 Make sure you include graphs, photos and interesting facts and you quote your sources.
3 On the last slide, present your conclusions as well as your personal opinion and the group’s opinion.
1 When you have finished your presentation, present it to the rest of the class.
2 You can finish with a discussion about the need for environmental preservation in Andalusia and how physics and chemistry can contribute to achieving this goal.

The scientific method, used in experimental science, consists of a series of systematic steps by which hypotheses are proposed to explain specific phenomena. These hypotheses must align with the observed facts. If you remember, there are four stages:
➜ Scientific work begins with observing unexplained phenomena and gathering detailed information.
➜ Next, a hypothesis is formed, providing a logical explanation based on the collected data.
➜ Key experiments follow, replicating the phenomenon to test the validity of the hypothesis.
➜ Conclusions are drawn, turning the validated hypothesis into a scientific law that predicts related phenomena.
Measurable properties are generally referred to as quantities. Common quantities include mass, length, volume, time and speed.
To determine the value of a quantity, a measuring process is carried out, involving a comparison with a known reference or standard, which we call a unit. A correct expression of a measurement result should include the quantity, its value, and the unit used as a reference.
One way to classify quantities is to differentiate between scalar and vector quantities.
Scalar quantities are those for which indicating the value of the measured property is sufficient for a clear definition.
Time, mass, and temperature are examples of scalar quantities.
However, there are other quantities for which, in addition to indicating their value, information about their direction is required. These quantities are called vector quantities.
Examples include the velocity and acceleration of a mobile object or the applied force.
Force is a vector quantity because the same force applied to different objects can have varied effects. For instance, applying the force horizontally forwards or vertically upwards can result in different outcomes depending on the direction of application.


1. Briefly answer the following questions:
a) What do we mean by science?
b) Why are physics and chemistry considered experimental science?
c) Why is it important to conduct a literature review before formulating a hypothesis?
d) Once the hypothesis is formulated, can we consider it valid and use it to generate a scientific law?
e) What are the primary sources of information that you are familiar with?
2. Science has made significant progress in the past four centuries, especially in the 20th century, thanks to the application of the scientific method.
Working in pairs, choose a notable scientific achievement in physics or chemistry. Research its background using books or the Internet. Identify the steps of the scientific method in your chosen example.
Use the information and conclusions to create a presentation for your class.
3. Some groundbreaking discoveries happened entirely by chance. This is known in the history of science as serendipity. Search for information on the Internet and choose one of these discoveries in the fields of physics or chemistry. Then, create a datasheet with the following information:
– Who made the discovery, and when did it take place?
– What exactly was the discovery and how did it happen?
– What significance has the discovery had for physics or chemistry?

Components such as circuits, chips, casings, batteries, etc., are developed through scientific research and are integral parts of mobile phones.
4. Briefly answer the following questions, giving an example:
a) What is a quantity?
b) How do we correctly express a measurement?
c) What is a unit of measurement? Can there be several units to measure the same quantity?
The unit helps us to correctly interpret measurement results. For instance, it tells us that the object moves at a rate of 25 m per second or covers 90 km per hour.
In this context, the measured quantity is velocity, which tells us the distance that an object travels in a given unit of time.
Velocity = 25 m/s
Velocity = 90 km/h
5. One classification of magnitudes divides them into scalar quantities and vector quantities.
a) When should we consider a quantity as vectorial? Give an example.
b) Would temperature be a scalar or vector quantity? Explain your answer.
6. It is a well-known fact that windows steam up after a cold night. Develop a hypothesis justifying this phenomenon and describe an experiment you could design to validate that hypothesis.
7. Listen and define or explain the following terms that are related to what you have learnt in this section: Scientific method. Hypothesis. Experimentation. Quantity. Vector quantity. Scalar quantity.
8. Draw a flow chart to summarise the contents of this section.
Remember that you can add the definitions or explanations from the previous exercise.
Currently, units for each quantity are established by agreement within the scientific community.
This is the International System of Units (SI). It defines base quantities, which are those most commonly used, and derived quantities.
Sometimes, measuring certain quantities results in a value that is either very large or very small in the chosen reference unit. To address this issue, the International System establishes a series of multiples and submultiples.
When we need to convert units, unit conversion is the process that allows us to express a measurement in a different unit using conversion factors and equivalence statements. Base quantities
Length (l)
Time (t)
Mass (m)
Temperature (T)
Current (I)
Luminous intensity (Iv)
Metre m
Second s
Kilogram kg
Kelvin K
Ampere A
Candela cd
Amount of substance (n) Mole mol
Quantities and base units in the International System.
P- peta- 1 000 000 000 000 000 (1015)
T- tera- 1 000 000 000 000 (1012)
G- giga- 1 000 000 000 (109)
M- mega- 1 000 000 (106)
k- kilo- 1 000 (103)
h- hecto- 100 (102)
da- deca- 10
d- deci- 0,1 (10–1)
c- centi- 0,01 (10–2)
m- milli- 0,001 (10–3)
µ- micro- 0,000001 (10–6)
n- nano- 0,000000001 (10–9)
p- pico- 0,000000000001 (10–12)
f- femto- 0,000000000000001 (10–15)
Read and learn
The result of measuring the reaction rate of a chemical process is 345 mmol/L · min. Perform the necessary conversion to express this reaction rate in International System units (mol/m3 · s).
• To begin, we will consider the necessary equivalences: 1 mol = 103 mmol 1 m3 = 103 L 1 min = 60 s
• Next, we set up the conversion factors: v = 345 mmol L · min · 1 mol 103 mmol · 103 L 1 m3 · 1 min 60 s = 5,75 mol m3 · s
In International System units, the rate of this chemical process is: v = 5.75 mol/m3 · s.

9. The average distance between the Earth and the Sun is approximately 150 million kilometres. The diameter of an atom is 0.1 nanometre. Express both quantities in metres using scientific notation.

Atoms have microscopic sizes.
10. In which unit would you measure the following quantities? Use the appropriate multiples or submultiples of the base unit in question:
a) The age of the Earth.
b) The diameter of an atom.
c) The mass of the Moon.
d) The mass of a virus.
e) The distance from the Sun to Pluto.
11. Fill in the missing gaps:
a) kg = 2 · 106 mg.
b) 6 pm = 0.000006
c) 70 s = fs.
d) 50 mm = km.
e) 830 mg = 8.3 · pg.
12. Write and apply the necessary conversion factors to convert the following units.
a) 36 pm → km.
b) 0.03 Ms → s.
c) 20 dg → µg.
d) 25 nmol → mol.
e) 0.5 mA → A.
13. Convert the following derived units:
a) 60 km2 → dm2.
b) 9 km/h → m/s.
c) 540 mg/dL → g/L.
d) 120 L/m2 → L/cm2
e) 50 m3/h → L/s.
e) 2.45 · 107 µN · mm → N · m.
14. Match each velocity to its means of transport, converting the units as necessary:
a) Bicycle • 0,08 km s
b) Racing car • 200 m s
c) High-speed train • 60 cm min
d) Supersonic aeroplane • 1,7 · 105 m h
15. Convert the following derived units and express the result as indicated in each case:
a) Molar mass = 40 mg/mmol → in g/mol.
b) Concentration = 1.5 · 104 mg/L → in mg/mL.
c) Electric field = 18 N/C → in mN/mC.
d) Power = 2.5 · 10–3 kJ/ms → in J/s.
e) Pressure = 2 · 106 N/m2 → in kN/cm2
16. Listen and define or explain the following terms that are related to what you have learnt in this section: International System. Base quantity. Multiples and submultiples. Unit conversion.
Equivalence statements. Conversion factors.

In the world of computing, the unit used to measure information is the bit.
17. Draw a flow chart to summarise the contents of this section.
Remember that you can add the definitions or explanations from the previous exercise.
In the scientific method, measurement plays a vital role, employing specialised instruments tailored for specific quantities. These instruments must be accurate, precise, and high-resolution.
It is crucial to recognise that a measured value is an approximation of the true value. The difference between the measured and actual values is the measurement error, often estimated through statistical methods due to the unknown true value. The best practice involves repeating measurements multiple times and calculating the average. To quantify the error, scientists use measurement uncertainty.
The mass of an object has been measured with the following results: 1.23 g; 1.18 g; 1.25 g; 1.20 g. Calculate the measurement uncertainty and express the result based on it.
Based on the results we have, the mass measurements have been conducted with a resolution of hundredths of a gram (0.01 g). Therefore, the result should be expressed considering this resolution. To calculate the measurement uncertainty, we follow these steps:
1.° We calculate the arithmetic mean of the measured values. We will consider this result as the true value, rounded to the nearest hundredth of a gram (1.22 g).
m –= 1.215 g
Average = 0.025 g
2.° The difference between each value and the measured mass is calculated, taking the absolute value of the difference in each case.
3.° The measurement uncertainty is the mean value of the calculated deviations for each measurement, and in this case, we will take it as 0.03 grams.
Given that the precision is 0.01 g and the calculated measurement uncertainty is higher, we will state the mass of the object as 1.22 ± 0.03 g.
Scientific progress comes from using the scientific method to solve problems and explain observations.
Scientific research systematically applies this method to make new discoveries, deepen our understanding of phenomena, and develop technology across various scientific fields.
Today, scientific research is a respected and professional activity. Science has become a collective effort, given its extensive body of knowledge. It is also shared extensively, reaching a broader audience, whose level of education has significantly risen over the years.


Researchers in a laboratory.
18. One of the following statements is incorrect. Find and correct the mistake.
a) An instrument is considered high-resolution when it consistently gives very close values in multiple measurements of the same quantity.
b) An exact instrument provides values very close to the real measurements of the quantity being measured.
19. Look at the following picture of a lab balance. What is its resolution? How do you know?

20. In an experiment, the same measurement has been repeated several times, and after calculating the measurement uncertainty, the final result is t = 3.89 ± 0.42 s. Do you think this result is reliable? Explain your answer.
21. A scientist is measuring the time it takes for a ball to fall from a certain height, with the following results: 34.56 ms; 34.32 ms; 34.45 ms; 34.52 ms; 34.48 ms; 34.54 ms.
a) What is the resolution of the measuring instrument?
b) Find the real value of the measured time and the measurement uncertainty. Express the measurement based on the calculated values.
c) Calculate the relative error. Is it a reliable measurement?
22. Several measurements of the current flowing through an electrical circuit have been taken using an ammeter, with the following results:
23. Explain what scientific research is and the main difference between contemporary research and research conducted more than a century ago.
24. As you already know, contemporary scientists work professionally and collectively under the guidelines of certain international organisations. Look for information about the following research organisations and briefly describe what they are, where they are based, and what their main line of work is:
a) NASA.
b) CSIC.
c) CERN.
d) IUPAC.
25. Scientific problems nowadays are approached from a multidisciplinary perspective. What do you think this means? Find a recent example in journals or on the Internet, and explain briefly why it is necessary to address it from a multidisciplinary perspective.

Scientific research is a multidisciplinary endeavour.
26. Listen and define or explain the following terms that are related to what you have learnt in this section:
Measuring apparatus or instrument.
Resolution of an instrument.
Measurement uncertainty.
Relative error.
Scientific research.
a) Calculate the true value for the current flowing through this circuit.
b) Find the measurement uncertainty and express the result based on it. Is it a reliable measurement? Explain your answer.
27. Draw a flow chart to summarise the contents of this section.
Remember that you can add the definitions or explanations from the previous exercise.



Andalusia boasts an undeniable cultural and monumental legacy, making it a prime attraction. Notably, the ‘white villages’ stand out as distinctive elements in this rich heritage, each with its unique identity.
The traditional whitewashing of houses in these villages carries more than meets the eye —hidden behind this practice are principles from the realms of physics and chemistry.
You will work in groups, following your teacher’s instructions, on the white villages of Andalusia and their connection with physics and chemistry.
1 The distinctive white colour of these towns is achieved by regularly whitewashing the outside walls of the houses. This is accomplished through the application of lime. What is lime, from a chemical standpoint? Look in books or on the Internet.
2 When whitewashing the walls, they take on the bright white hue that characterises this tradition. What are the benefits of this lime coating? Look for information in books or by asking people who live in one of these villages, if possible.
3 The lime used in the process is derived from a chemical procedure that uses limestone rocks as raw materials, which are plentiful in certain regions of Andalusia. Describe this procedure in simple terms. You can look for information in chemistry books or the Internet.
4 Quicklime (calcium oxide) has other uses, for example, it is a potent disinfectant and fungicide. Why does it have such properties? Look for the answer in chemistry books.
5 The white villages tradition is a compelling tourist attraction. Look for information about the white villages of Cádiz and the economic impact of tourism in this area of Andalusia. Could this tradition improve a village’s economy? Give reasons for your answer.
1 Based on your findings, prepare a presentation about the white villages of Andalusia and how they are related to physics and chemistry.
2 Make sure you include graphs, photos and interesting facts and you quote your sources.
3 On the last slide, present your conclusions.
1 When you have finished your presentation, present it to the rest of the class.
2 You can finish with a discussion about the importance of keeping certain traditions alive from both social and economic standpoints.

Since the 19th century, scientists have understood that matter on a microscopic scale is composed of atoms, particles with sizes around 10–10 m. Atoms consist of smaller particles known as subatomic particles.
➜ The electron (e–) is a negatively charged particle with a charge of 10–19 coulombs and a mass of 10–31 kg.
➜ The proton (p+) carries a positive charge of the same magnitude as the electron but has a mass approximately 1,840 times greater.
➜ The neutron (n) is a neutral particle with a mass similar to that of the proton.
The contemporary atomic model is quite intricate, relying on complex mathematical calculations. It suggests that electrons cannot be precisely pinpointed in elliptical orbits but are found in specific regions of space called atomic orbitals.
Atoms maintain an electrically neutral state, consisting of an equal number of protons and electrons, along with a certain number of neutrons.
➜ Protons and neutrons reside in the nucleus, which carries a positive charge, while electrons form the electron cloud.
➜ Electrons are organised in energy levels and, within these levels, in different orbitals. They can transition between energy levels by absorbing or emitting energy.
To describe an atom, we use two numbers associated with its chemical identity and mass, respectively.
➜ The atomic number (Z) represents the count of protons in an atom.
➜ The mass number (A) signifies the total particles in the atom’s nucleus, which is the sum of protons and neutrons.
➜ Isotopes are atoms sharing the same atomic number but varying mass numbers. In essence, they are atoms of the same chemical element differing in their mass.
An atom has atomic number Z = 19, mass number A = 39, and charge +1. How many particles does it have? What element it is? What is its mass?
It is a potassium atom (K), the element with atomic number 19.
Its atomic number is Z = 19 → It has 19 protons in its nucleus.
Its mass number is A = 39 → So its mass is 39 u.
The sum of protons and neutrons is 39. In this case: N.o of neutrons A – Z = 39 – 19 = 20 neutrons.
Its charge is +1. This means it has lost an electron. So, it has 18 electrons in its shell. This potassium cation has 19 protons and 20 neutrons in its nucleus and 18 electrons in its shell. Its atomic mass is 39 u.





















1. The electron was discovered in cathode ray tubes, leading to experiments to understand its properties. Look in books or the Internet to find out how scientists determined the electron’s charge, and create a short presentation on this chemistry breakthrough.
2. An atom with a charge of –1 has atomic number 35 and mass number 80. Based on this data, indicate what we can deduce about this atom.
3. Chlorine has two natural isotopes: chlorine-35, with an abundance of 75.53%, and chlorine-37, with an abundance of 24.47%.
a) How many protons and neutrons do each of these isotopes have?
b) What are the atomic masses of chlorine-35 and chlorine-37 atoms?
c) In the periodic table, chlorine is listed with an atomic mass of 35.5. How can you interpret this value considering the abundance of each isotope of this element?
4. According to the current conception of the atom, indicate whether the following statements are correct or incorrect, justifying your response in each case:
a) The atom has a central, electrically neutral nucleus where protons and neutrons are located.
b) Most of the atom’s mass is concentrated in the nucleus.
c) Electrons in the electron cloud move in elliptical orbits around the nucleus.
d) An electron in a specific energy level can shift to other levels under particular conditions.

5. Draw a table for the following atoms, indicating their atomic number, mass number, charge, type of ion and number of protons, neutrons and electrons.
a) 168O2–
b) 58 28Ni3+
c) 64 30 Zn2+
d) 75 33 As3–
e) 11448Cd+
f) 20280Hg2+
6. Correct the mistakes in the following statements:
a) An atom of scandium that has lost 3 electrons has become a Sc3– anion.
b) By gaining 2 electrons, an atom’s atomic number has changed from Z = 4 to Z = 6.
c) A magnesium atom with 12 protons, 12 neutrons, and 10 electrons is written as 12 24Mg2–.
7. The mass of an atom expressed in atomic mass units is always a whole number. How can you explain that most of the masses assigned to the chemical elements in the periodic table are decimal numbers?
8. Listen and define or explain the following terms that are related to what you have learnt in this section:
Atom.
Electron. Proton. Atomic number. Mass number.
Isotopes.

Radioactive isotopes, such as 13153I, are used in medicine.
9. Draw a flow chart to summarise the contents of this section.
Remember that you can add the definitions or explanations from the previous exercise.
Simulation of the rotation of electrons around the nucleus.In the current atomic model, electrons are arranged in different energy levels in the electron cloud, known as electronic configuration.
Read and learn
A strontium atom has atomic number Z = 38. How many electrons are in the atom’s shell? Write its electronic configuration.
➜ Since it is a neutral atom, the number of electrons is equal to the number of protons, 38.
➜ The 38 electrons are in their corresponding levels. Each orbital’s maximum number and the diagonal rule determine the order that the levels fill. According to this, orbital 1s has 2 e–; orbital 2s has 2 e–; orbital 2p has 6 e– and orbitals 3s and 3p have 8 e–
Orbital 4s is completed with 2 e–; then orbital 3d with 10 e–; then 4p with 6 e–; and lastly, orbital 5s has the last 2e– to complete the total of 38 electrons.
Orbital 4s fills before 3d.
Orbital 5s has 2 e–. In total, the atom has 38 e–
Strontium’s 38 electrons are in 5 energy levels, from orbital 1s to orbital 5s.
Today 118 chemical elements have been identified. They are arranged in rows and columns, in order of increasing atomic number. Elements with similar chemical properties are included in the same column. This arrangement is known as the periodic table and is constantly overseen and revised by the IUPAC (International Union of Pure and Applied Chemistry).
Most elements are metals. The elements on the top are abundant in nature.
The semimetals lie along a diagonal line separating the metals and nonmetals.
Atomic orbital filling order. There is one or more orbital for each energy level. The energy order for the orbitals is from top to bottom, as indicated by the diagonals.
10. What is an electronic configuration? Why do we need to know it? Describe the procedure to write the electronic configuration of an atom.
11. The order of orbital filling is complex. Using the atomic order filling diagram, answer the following questions:
a) Which orbital is filled before 4p?
b) Which orbital should we start filling after 2p?
c) In which orbital should we place the fifteenth electron?
d) Which orbital has intermediate energy between 4p and 4d?
12. Write the electronic configuration for the following chemical elements:
a) Beryllium → Be (Z = 4).
b) Fluorine → F (Z = 9).
c) Silicon → Si (Z = 14).
d) Chromium → Cr (Z = 24).
e) Rubidium → Rb (Z = 37).
f) Antimony → Sb (Z = 51).
13. Write the electronic configuration for these atoms:
a) Mg (Z = 12)
b) S (Z = 16)
c) V (Z = 23)
d) Zn (Z = 30)
e) Se (Z = 34)
f) Sr (Z = 38)
14. Using the periodic table, give three examples of chemical elements that belong to:
a) Transition metals.
b) Halogens.
c) Noble gases.
d) Alkaline earth metals.
e) Actinides.

table.
15. Answer these questions about the periodic table:
a) How many chemical elements have been identified and accepted by the IUPAC?
b) Where are nonmetals located?
c) Why are the lanthanides and actinides, among others, called ‘rare earth elements’?
d) What characteristic of an element’s atom defines its chemical properties and its ability to combine with other elements?
16. Copper, nickel, iron, chromium, aluminium, gold, silver, and tungsten are found in numerous everyday objects, either individually or in alloys. Match these metals to the specified utensils or objects (consult additional information sources as needed):
a) A spoon.
b) Some electrical wires.
c) A 5 cent coin.
d) A 2 euro coin.
e) Bath taps.
f) A bracelet.
g) A filament of an incandescent light bulb.
h) A window frame.

Metals.
17. Listen and define or explain the following terms that are related to what you have learnt in this section: Orbital.
Electronic configuration. Periodic table. Group.
Period.
18. Draw a flow chart to summarise the contents of this section.
Remember that you can add the definitions or explanations from the previous exercise.
PeriodicPure substances are those that do not break down into different ones when subjected to physical processes. These are further classified into simple substances (elements) and compound substances (compounds).
A compound is a pure substance in which its particles are formed by various types of different atoms, giving rise to different substances when subjected to chemical processes.
A compound is formed by the combination of different chemical elements. Although there are slightly more than a hundred chemical elements, their combination possibilities are virtually limitless, resulting in millions of known compounds, and new ones are discovered or synthesised every day.
To form a compound, elements combine in fixed proportions. The macroscopic proportion observed corresponds to the actual proportion of atoms of each type that bond to create the compound, represented by a formula.
MICROSCOPIC SCALE
Proportion of atoms
2 H atoms 1 O atom
mass
MACROSCOPIC SCALE
Proportion in mass

2 g of H and 16 g of O in 18 g (1 mole) of water
The connections between atoms are called bonds. They occur because, in this way, atoms gain stability. The octet rule, meaning the tendency of atoms to complete 8 electrons in their outermost layer, helps explain the formation of most bonds.
➜ Ionic bonding is the connection between ions of opposite charge, formed when atoms of a metallic element donate electrons to atoms of a non-metallic element.
➜ Covalent bonding is the connection between atoms that share one or more pairs of electrons to create a molecule. Different intensities of intermolecular forces arise between these molecules.
➜ Metallic bonding is the connection between atoms of a metal that, becoming cations, share the donated electrons.



19. The synthesis of new chemical compounds is a significant focus of chemical research, with many of these compounds being intended for medical use. Choose one of these compounds –you can check the Internet or look at medicines you have at home– and create a datasheet explaining its composition, uses, discovery, etc.
20. The formula for carbonic acid is H2CO3.
a) What elements form this compound, and in what proportion?
b) By consulting the periodic table, calculate the molecular mass of this acid.
c) What macroscopic proportion (in mass) will be observed for this compound? In your calculation bear in mind the atomic masses of the elements and the calculated molecular mass.
21. Write and interpret the formula for the following compounds based on the given data. Consult the periodic table when needed:
a) It is composed of carbon and hydrogen in a 1:2 ratio (in atoms), and its molecular mass is 28 u.
b) It contains hydrogen and oxygen in the same proportion (in atoms) and phosphorus. Its molecular mass is 82 u.
22. From the formula of the following compounds, calculate the percentage composition of the elements in each of them. Consult the atomic mass data from any periodic table:
a) Nitric acid, HNO3
b) Butane gas, C4H10
c) Oleic acid, C18H34O2
d) Sodium sulphate, Na2SO4.
e) Aluminium hydroxide, Al(OH)3

23. Indicate the type or types of bonds that exhibit the following characteristics:
a) Electron pairs are shared between bonded atoms.
b) Ions of opposite charge are formed.
c) Ions form a three-dimensional network.
d) Molecules are formed.
e) Electrons move freely.
24. Correct the mistakes in these statements about ionic bonding:
a) Ionic bonding results in a flat network of ions.
b) Ions that bond are of the same charge.
c) The ionic network has a positive or negative charge, depending on whether there are more cations or anions.

Salt crystals (sodium chloride).
25. How is covalent bonding formed? What type of atom grouping is formed through this bond? Explain with an example.
26. Explain what type of bonding we will find in the following cases:
a) The union between an alkali metal and a halogen.
b) The union between atoms of a gaseous element.
c) The union between atoms of a metallic element.
d) The union between atoms of a non-metallic element.
27. Listen and define or explain the following terms that are related to what you have learnt in this section:
Chemical compound.
Formula.
Ionic bonding.
Covalent bonding.
Metallic bonding.
Intermolecular forces.
28. Draw a flow chart to summarise the contents of this section.
Remember that you can add the definitions or explanations from the previous exercise.
Butane gas and natural gas are organic compounds.


In Andalusia, the rich culinary tradition is a vital part of our culture, deeply rooted in the healthy Mediterranean diet. The region’s mild climate, centuries of history, and the availability of top-quality natural ingredients contribute to a diverse and delicious gastronomy.
Surprisingly, the connection between gastronomy and chemistry is more profound than it seems, especially with the modern techniques used in cooking.
You will work in groups, following your teacher’s instructions, on the unique flavours of Andalusian cuisine and how it is related to the world of chemistry.
1 In ancient times, cooking played a vital role in expanding the human diet and facilitating nutrient assimilation. What kind of process is involved in cooking food? Give reasons for your answer.
2 Besides cooking, various techniques were employed in ancient times for food preservation, with salting being one of them. How does salting work from a chemical perspective? Use the information sources available to you.
3 Andalusian gastronomy places a strong focus on frying in olive oil. What chemical transformations take place when frying fish or eggs? Look for information in chemistry books or on the Internet.
4 Modern culinary innovations often involve scientific experimentation, such as the technique of spherification, which revolves around creating spheres with different ingredients. What chemical process underlies spherification? Investigate this in a recipe book or on the Internet.
5 Do you believe that chemistry can contribute to improving gastronomy? Discuss this.
1 Based on your findings, prepare a presentation about Andalusian cuisine and how it is related to chemistry.
2 Make sure you include photos and interesting facts and you quote your sources.
3 On the last slide, present your conclusions.
1 When you have finished your presentation, present it to the rest of the class.
2 You can finish with a discussion about how chemistry plays a vital role in our daily life, for example, in cooking.

When new substances are formed through a process, it is known as a chemical change or chemical reaction.
In a chemical reaction, initial substances ( reagents ) are transformed into different substances (products). If the reaction gives off heat, it is exothermic, and if it absorbs heat, it is endothermic.
At a microscopic level, a chemical reaction involves the rearrangement of atoms in the reagents to create the products. The speed of this process depends on the type of reagent and factors like temperature, concentration, pressure, surface area, agitation, and the presence of catalysts.
The collision theory explains that chemical reactions occur due to collisions between particles in the reagents. Not all collisions are effective; only those that have the right orientation and enough energy can break existing bonds between atoms and form new bonds, leading to the products of the reaction.
If the orientation is correct, there will be an effective collision (above). If not, there will be an ineffective collision (below).
The early laws regarding chemical reactions, known as gravimetric laws, were discovered between the 18th and 19th centuries through precise measurements of the masses of reagents and products in various chemical processes.
➜ Law of conservation of mass. In every chemical reaction, the total mass of the reagents is equal to the total mass of the products. In other words, mass is conserved.
➜ Law of definite proportions. The masses of the elements that combine to form a specific compound always have the same proportion.
All information, both qualitative and quantitative, about a chemical reaction is captured in a chemical equation. In the equation, reagents are represented on the left, products on the right, separated by an arrow indicating the direction of the process.
To ensure that the chemical equation reflects the actual process occurring at the atomic scale, each substance is preceded by a number, its stoichiometric coefficient. This ensures that the number of atoms of each element in the reagents and products is the same. When this is the case, we say the equation is balanced, and it can be interpreted both qualitatively and quantitatively.

A piece of wood burns, releasing gases and leaving behind ashes.
1. Why do food items stay fresh in the refrigerator or, even better, in the freezer? Write a brief explanation, considering the factors that influence reaction rates.
2. Among the various ways to increase the speed of a chemical reaction, what practical advantages do you think the use of a catalyst has? Argue your point, looking for additional information in books or on the Internet if needed.
3. Read the following statements and indicate whether they are correct or not:
a) In all chemical reactions, there are as many reagents as products.
b) There always have to be at least two reagents for a reaction to take place.
c) In a reaction, a single product can be obtained even if there are multiple reagents.
4. Answer the following questions, giving an explanation:
a) Does a chemical reaction always occur when there is a collision between the particles of the reagents?
b) Besides orientation, what other factor decisively influences the occurrence of an effective collision?
c) Why is it necessary to apply a match or a spark to a gas lighter for it to start burning?
5. True or false? Explain your answers:
a) The proportion between reagents and products in a chemical reaction is fixed because mass is conserved.
b) The law of conservation of mass is only valid for reactions where reagents and products are solids.
c) The law of definite proportions refers only to formation reactions.

In many chemical reactions, the reagents or products are gases.
6. In the following diagram, the energy involved in the process of forming 10 g of a substance C from 6 g of A and 4 g of B is represented:
Energy (kJ)
Breaking-formation of bonds A + B Reagents
a) Can we say that this diagram corresponds to an exothermic reaction? Why/why not?
b) How much energy is released in the process?
c) What activation energy does this reaction have?
d) What would this diagram look like if we add a catalyst that reduces the activation energy by half?
7. Balance the following chemical equations:
a) NO (g) + O2 (g) → NO2 (g)
b) N2O5 (g) → NO2 (g) + O2 (g)
c) C6H14 (l) + O2 (g) → CO2 (g) + H2O (g)
d) Al2O3 (s) + HCl (ac) → AlCl3 (ac) + H2O (l)
e) NO2 (g) + H2O (l) → HNO3 (ac) + NO (g)
8. Listen and define or explain the following terms that are related to what you have learnt in this section: Reagents.
Products.
Exothermic reaction.
Endothermic reaction.
Law of conservation of mass.
Law of definite proportion.

Laboratory scales are used to weigh amounts of substances.
9. Draw a flow chart to summarise the contents of this section.
Remember that you can add the definitions or explanations from the previous exercise.
In stoichiometric calculations, we work with the amount of substance, a SI base unit measured in moles, where one mole represents 6.022 · 10 23 particles (Avogadro’s number). This establishes a link between the number of particles and the mass of a substance, which is very useful for calculations involving chemical reactions.
The molar mass (M) of a substance is the mass of 1 mole (6.022 · 1023 particles) of that substance and is equivalent to its molecular mass. To find the number of moles (n), you can use the mass (m) and molar mass (M). n = m M
Starting with a balanced chemical equation, which provides a quantitative understanding of a chemical process involving atoms and molecules, we can derive stoichiometric relationships.
How is the stoichiometric relationship in moles obtained?
The stoichiometric relationship in moles is obtained by considering the molecular stoichiometric relationship and multiplying it by Avogadro’s number. For example:
2 H2 (g) + O2 (g) → 2 H2O (g)
2 molecules H2 + 1 molecule O2 → 2 molecules H2O
2 · NA 1 · NA 2 · NA
2 mol H2 + 1 mol O2 → 2 mol H2O
In this reaction, 2 moles of hydrogen (H2) reacting with 1 mole of oxygen (O2) produce 2 moles of water (H2O). Note that the molar stoichiometric relationship aligns with the proportion between molecules.
How is the stoichiometric relationship in mass obtained?
The stoichiometric relationship in mass is derived from the stoichiometric relationship in moles, considering the molar masses of reagents and products. Going back to the example of water formation:
2 H2 (g) + O2 (g) → 2 H2O (g)
2 mol H2 + 1 mol O2 → 2 mol H2O
2 · MH2 (2 g/mol) 1 · MO2 (32 g/mol) 2 · MH2O (18 g/mol)
4 g H2 + 32 g O2 → 36 g H2O

If 4 g of hydrogen (H 2) react with 32 g of oxygen (O 2), 36 g of water (H 2O) are formed. It is crucial to note that this stoichiometric relationship holds true for other quantities as long as they maintain the same proportion. For instance, 1 g of H 2 and 8 g of O 2 would still yield 9 g of H 2O. H2O
10. Calcium sulfide (CaS) is a binary salt obtained by heating gypsum with carbon.
a) Calculate the molecular mass and molar mass of calcium sulfide. Interpret the values.
b) How many molecules are there in a container containing 3.5 moles of this salt?
c) What is the mass of this substance in the previous container?
d) What mass is equivalent to 4 moles of calcium sulfide? How many molecules does it contain?
11. Considering the definition of a mole, answer the following questions:
a) How many moles correspond to a number of carbon dioxide molecules equal to 2.1077 · 1024?
b) How many molecules are there in a container that contains 1.2 moles of water?
c) In a piece of copper cable, there are 9.033 · 1022 atoms of this metallic element. How many moles does this number of atoms correspond to?

12. In a container, there are 72.5 g of substance A, with a molar mass of 29 g/mol. True or false? Explain your answer.
a) There are 3 moles of A in the container.
b) The mass of one mole of A is 72.5 g.
c) There are 1.5055 · 1024 particles of A in the container.
13. Calculate the molecular and molar mass of each of these substances, and the number of moles corresponding to the given quantities. Take the necessary data from the periodic table.
a) 55 g of beryllium dihydride, BeH2
b) 165 g of diphosphorus trioxide, P2O3
c) 119 g of sodium nitrate, NaNO3
d) 34.26 g of barium dihydroxide Ba(OH)2
14. Use stoichiometric relationships in moles and in mass for the water formation reaction to calculate:
a) The mass of oxygen required to produce 10 L (10 kg) of water.
b) The moles of water formed when 15 moles of oxygen react.
c) The mass of water formed when 13 moles of hydrogen react.
15. Given the following reaction:
SO2 (g) + O2 (g) → SO3 (g)
a) Balance the chemical equation and write the stoichiometric relationships in moles and in mass for this process. Consult the periodic table as needed.
b) Calculate the moles of O2 that will react and the moles of SO3 that will be produced when 3.75 mol of SO2 react completely.
16. The reaction between zinc (Zn) and hydrochloric acid (HCl) produces zinc dichloride (ZnCl) and releases hydrogen gas (H2), according to the following equation: Zn (s) + 2 HCl (ac) → ZnCl2 (ac) + H2 (g)
a) Find the stoichiometric relationship in mass.
b) What quantity of hydrogen will be produced if 438 g of HCl reacts?
c) If 98.1 g of zinc reacts completely, what quantity of ZnCl2 will be produced?

17. Listen and define or explain the following terms that are related to what you have learnt in this section: Mole.
Molar mass.
Stoichiometric relationship.
Stoichiometric relationship in moles.
Stoichiometric relationship in mass.
18. Draw a flow chart to summarise the contents of this section.
Remember that you can add the definitions or explanations from the previous exercise.
Copper cable. Chemical reaction.Acids and bases are substances widely encountered in everyday life, characterised by physical and chemical properties known since ancient times. Within this category, there are strong acids or bases with intense properties and weak acids or bases with more subdued characteristics. To quantify the strength of an acid or base, the Danish chemist Soren Peter Sorensen introduced the pH scale in 1909.
Properties
Acids
Bases
Sour taste.
Corrosive.
React with metals and attack marble, emitting gases.
Neutralise bases. Turn litmus dye red.
Bitter flavour.
Corrosive, soapy to the touch.
Neutralise acids. Turn litmus dye blue.



Fruit and vegetable juices. Vinegar.
Cola drinks.
Nitric acid. Gastric juice.
Bleach, cleaning products, drain cleaner.
Baking soda and other antacids. Blood.




The pH scale.
Neutralisation and redox reactions
In an acid-base neutralisation reaction, the reagents consist of an acid and a base, yielding a salt and water as products.
H2SO4 (ac) + 2 KOH (ac) → K2SO4 (ac) + 2 H2O (l)
Sulphuric acid Potassium hydroxide Potassium sulphate Water
In contrast, an oxidation-reduction or redox reaction is a chemical process where reagents exchange electrons to form the resulting products. The reagent losing electrons undergoes oxidation, called the reductant, while the one gaining electrons undergoes reduction, referred to as the oxidant.
Iron (Fe) loses electrons and oxidises. It is the reductant.
Oxygen (O2) gains electrons from iron and reduces. It is an oxidant. It oxidises the iron.
19. pH plays a crucial role in cosmetics and personal hygiene products. You will often see the term ‘pH neutral’ on product labels such as lotions or shampoos. What does this term mean in this context? Investigate from information sources and provide a brief explanation in a paragraph.

Cosmetics.
20. The following statements are incorrect. Identify and explain the mistakes, then rewrite them correctly:
a) Acids and bases do not react with each other.
b) The products of a neutralisation reaction are an oxide and water.
c) A base produces H+ ions in a solution, and an acid produces OH– ions.
21. 56 g of sodium hydroxide were needed to neutralise a certain quantity of nitric acid (HNO3). Write the neutralisation reaction and use the stoichiometric relationship in mass to calculate the quantity of acid neutralised.
22. Given the following substances, identify which is the acid and which is the base in each case, and write the neutralisation reaction that will take place between them.
a) HNO3 (ac) + KOH (ac)
b) HBr (ac) + Ca(OH)2 (ac)
c) Fe(OH)2 (ac) + HCl (ac)
d) H3PO4 (ac) + NaOH (ac)
23. Explain the difference between:
a) Oxidation and reduction.
b) Oxidant and reductant.
c) Oxidant and oxidation.
24. Acids and bases are substances widely encountered in everyday life, characterised by physical and chemical properties known since ancient times.
Working in pairs, choose four products –natural or synthetic– that are commonly found in the environment and are or contain acids or bases. You can consult information sources.
For each of the chosen products, you will need to establish, through experiments, whether it is an acid or a base. To do this, you can use the properties table you have studied, with the help of an acid-base indicator or your school laboratory’s pH meter.
Once you know what type of substances they are, you will need to create a datasheet for each one, classifying it as an acid or base, indicating whether it is strong or weak, and explaining the uses based on these acidic or basic properties.
25. The following chemical equations represent redox processes. Identify the oxidant and the reductant.
a) Zn (s) + CuCl2 (ac) → ZnCl2 (ac) + Cu (s)
b) I2O5 (s) + 5 CO (g) → I2 (s) + 5 CO2 (g)
c) 4 FeS (s) + 7 O2 (g) → 2 Fe2O3 (s) + 4 SO2 (g)
d) 2 Na (s) + 2 H2O (l) → H2 (g) + 2 NaOH (ac)
26. Listen and define or explain the following terms that are related to what you have learnt in this section: Acid.
Base.
Neutralisation reaction. Oxidation, Reduction. Redox reaction.

27. Draw a flow chart to summarise the contents of this section.
Remember that you can add the definitions or explanations from the previous exercise.
Quicklime is, chemically speaking, calcium oxide. Traditionally, it has been extracted from limestone. Arcos de la Frontera (Cádiz).1, 2, 3
Copy the following sentences in your notebook. Then write the missing words.
1. In this stage of the scientific method, we gather all data and information related to the observed phenomenon.
2. These quantities are established by agreement as they commonly play a role in most studied scientific phenomena.
3. This characteristic of scientific instruments implies that they should provide a measured value as close as possible to the true value.
4. It represents a numerical value derived from the protons of an atom and is distinctive for each chemical element.
5. The outermost layer of atoms, the electron cloud, is composed of electrons, which arrange themselves in different orbitals in ascending order of energy.
6. When two atoms with similar tendencies to gain or lose electrons come together, they form this type of bond.
7. Each of the rows in the periodic table, containing a distinct number of elements.
8. Substances added to reactants to expedite a chemical reaction, remaining unconsumed in the process.
9. Derived from the balanced chemical equation, it can be expressed in moles, mass, or volume.
10. A corrosive substance with an acidic taste, with a pH below 7 on the scale.

Write the correct answer in your notebook. There is only one possible answer!
1. The scientific method, with its phases, ensures that a proposed hypothesis:
A Is valid.
B Is the best possible explanation.
C Will be tested against observed facts.
2. Which density is less than that of water? Remember that 1 L has a mass of 1 kg.
A 1.4 g/cm 3
B 960 kg/cm 3
C 1,050 g/L.
3. The result of a time measurement is 16.5 ± 0.3 seconds. This means that:
A 16.5 s is taken as the true value.
B The relative error is higher than 5%.
C The measurement is valid.
4. Contemporary science possesses some significant traits, such as:
A Being a challenging endeavour.
B Being limited to a select few.
C Enjoying considerable prestige.
5. In the current model of the atom, electrons:
A Are located in fixed orbitals.
B Can occupy different orbitals based on their energy.
C Can be anywhere in the atom.
6. A neutral atom of Al has an atomic number of 13. What is its electronic configuration?
A 1s2 2s2 2p 6 3s2 3p 3
B 1s2 2s2 2p 6 3s2 3p1
C 1s2 2s2 2p 6 3s2 3p2
7. The octet rule tells us that:
A Atoms with 8 electrons are more stable.
B Atoms tend to gain electrons.
C Atoms with 8 electrons in their outermost shell are more stable.
8. A characteristic of ionic bonding is that:
A Highly stable molecules are formed.
B An electrically neutral network is formed by ions of opposite charges.
C A three-dimensional network of molecules is formed.
9. Metallic bonding differs from ionic bonding in that:
A It produces substances with a positive electrical charge.
B It is much less stable.
C The ion network is formed only by cations.
10. If two atoms have the same number of protons and a different number of neutrons:
A They belong to different chemical elements.
B They have the same atomic mass number.
C They are isotopes of the same chemical element.
11. Which would not be a correct statement for any chemical reaction?
A New substances appear.
B The mass remains constant.
C It is a very slow process.
12. What are the key features of an effective collision according to the collision theory?
A It is very unlikely to happen.
B It has the right orientation and enough energy.
C No specific conditions are necessary, as long as the product is produced.
13. The balancing of chemical equations is based on:
A The law of definite proportions.
B Stoichiometric relationships.
C The law of conservation of mass.
14. The molar stoichiometric relationship:
A Is based on the law of conservation of mass.
B Is equivalent to the stoichiometric mass relationship.
C Is given by the stoichiometric coefficients.
15. The neutralisation reaction between an acid and a base:
A Results in a salt and another weaker acid.
B Only occurs between strong acids and bases.
C Produces a salt and water.
16. A redox reaction is defined as one in which:
A Oxygen acts as a reactant.
B A metal oxide is generated.
C There is an exchange of electrons between the reactants.
The chemical industry produces goods that are essential for maintaining our quality of life. These range from basic necessities such as healthcare, food and hygiene, to those that contribute to our overall well-being. Without the advancements made possible by chemistry, our life expectancy would likely be no more than 40 years. It is this field of science that develops treatments for diseases, improves agricultural productivity and ensures access to clean drinking water.
The chemical industry in Spain plays a vital role in our economy, contributing substantially to wealth creation and job opportunities. With over 3,120 companies, it generated a revenue of €77.241 billion in 2021 and provided employment for 710,430 people directly and indirectly. This sector represents 5.4% of the GDP and stands out as a significant exporter, with exports exceeding €50 billion.
Innovation is crucial for Spanish chemical companies. They are known for continually developing new technologies and products. This innovative approach not only helps the chemical sector but also boosts competitiveness in key areas such as manufacturing, construction and agriculture. Committed to sustainability, Spanish chemical companies lead the way in adopting eco-friendly practices. Using renewable energies and recycled raw materials are just a few examples of how the chemical sector actively contributes to preserving the environment.
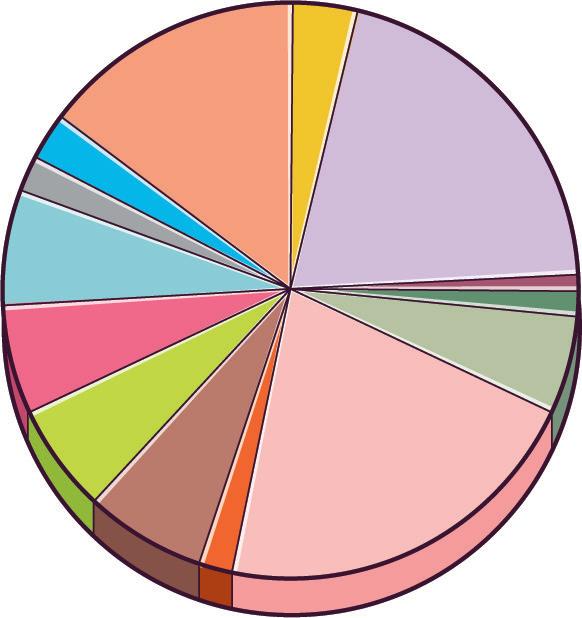
It also ranks as the second-largest export category, totalling €44.527 billion in exports in 2021. A significant portion, 57.6%, of Spanish chemical production is exported.
Breakdown of turnover by sector (%), 2023


Think about it
What percentage of the Spanish GDP does chemical production represent in Spain?
Approximately how much does the Spanish pharmaceutical industry generate in revenue?
Why is the chemical industry important in terms of its economic impact and what are its main characteristics?
Discuss your conclusions
The chemical industry sometimes faces social backlash because it is seen as harmful to the environment. Share your thoughts with your classmates.
➜ Keep track of how much waste is produced, how often, what type it is, where it goes and how much it costs. This helps set goals for reducing waste.
➜ Before buying or renting machinery, consider environmentally friendly options with similar performance (fewer emissions, discharges and waste).
➜ Make sure all workers are aware of the dangers of the chemicals used regularly. This cuts down on contamination risks and accidents at work.
➜ Recover particles from crushing processes to prevent inhalation.
➜ Crush, reuse or sell rejected quality control materials as by-products to reduce waste and save on raw materials.
➜ Avoid plastics containing heavy metals, as they are highly contaminating.
➜ Prevent untreated process water from being discharged into the sewage system.
➜ Collect solvent evaporation using extraction hoods.
➜ Extend the lifespan of baths by regularly removing sludge and maintaining low levels of grease and oil to reduce waste and discharge.
➜ Baths that have come to the end of their useful life must be managed as hazardous waste.

Inside a chemical plant.
What is the purpose of implementing good environmental practices in the chemical industry? How do they help minimise the environmental impact of this industrial activity?
Connect the good practices outlined in the text with ‘the three Rs’, a fundamental aspect of environmental conservation.
Current legislation requires the chemical industry to invest in clean processes as part of their research investment. What is your opinion on this? Explain your point of view.
What do you think about the environmental impact caused by the chemical industry? Think about the industry’s size and the volume and properties of the waste it produces.
Efforts are underway to mitigate the negative environmental effects of industrial activities. Do you believe these efforts are adequate, or should more decisive action be taken? Share your viewpoint.
© GRUPO EDITORIAL BRUÑO, S. L., 2024 - C/ Valentín Beato, 21 - 28037 Madrid.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the publishers.