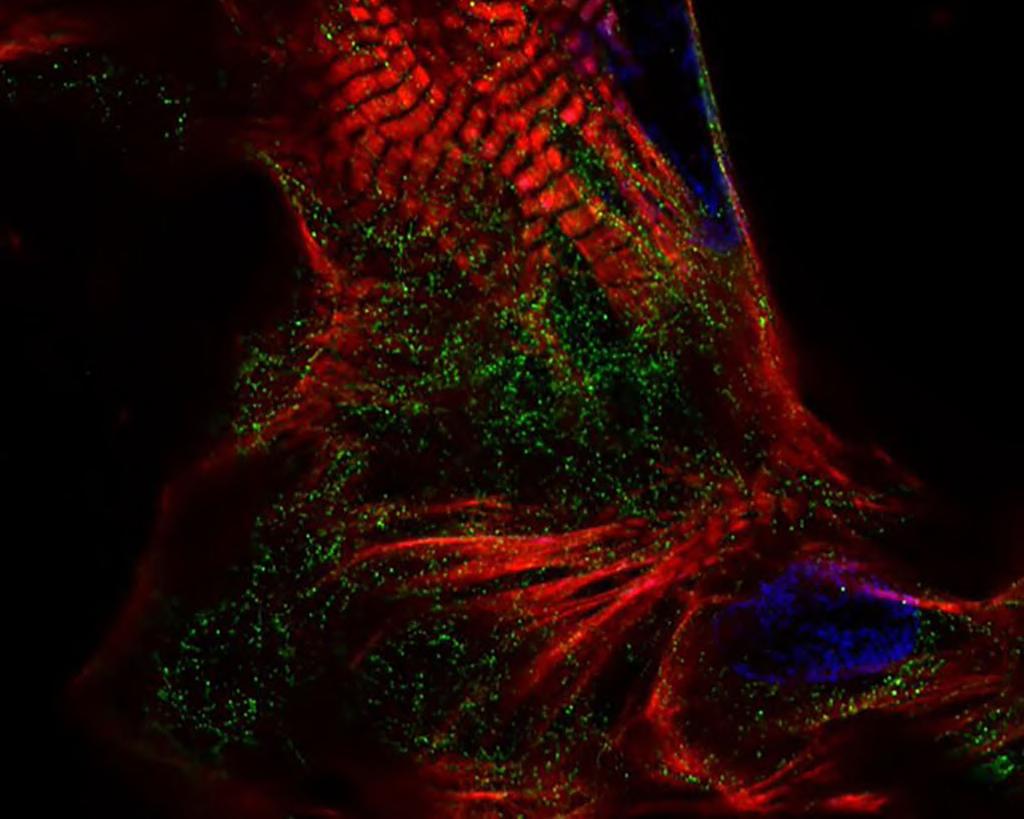
DNA Nanobait Test for Respiratory Viruses
Following the arrival of the winter cold, flu and RSV season in the northern hemisphere, healthcare workers are required to take quick decisions about the treatment to be given to patients presenting themselves at hospitals or clinics. PCR (polymerase
Cont’d on page 20
New Platform Aims to Accelerate Diagnostic Tests for Future Pandemics
The COVID-19 pandemic brought about a health crisis that highlighted severe fragilities within the healthcare sector, including lack of preparedness. In order to reduce vulnerability to future pandemics, there is a need for a large-scale, coordinated and
comprehensive health response. Now, a new project aims to establish a diagnostic assay platform that can enable a faster response time during emerging pandemics. A collaboration project, InfektoFlex, launched by Clickmer Systems (Manchester, UK; www.
Cont’d on page 13
Test Detects Predictive Biomarker for
Ergothioneine (ET) is a unique diet-derived compound discovered more than 100 years ago by Charles Tanret. However, it was only in 2005 when scientists discovered a transporter specific for ET that facilitates the uptake and accumulation of ET in the body. Now, a new study has revealed that low levels of ET in blood plasma may predict an increased risk of cognitive
Cont’d on page 14
First POC Test for ‘Insidious’ Malaria

Each year, there are over 200 million cases of malaria and about 620,000 deaths across the world. Malaria is caused by the Plasmodium vivax parasite, which is transmitted to humans via the bite of an infected mosquito. The Plasmodium vivax parasite poses a challenge as it can remain dormant in the liver for years, but reawaken later to continue spreading the disease.
See article on page 7
AI Combined with Infrared Imaging Automatically Classifies Tumors
In recent years, there has been a massive advancement in available treatments for colon cancer. To ensure treatments such as immunotherapies are effective, it is important to accurately diagnose the individual patient to provide specifically tailored treatments.

Cont’d
Thermo Fisher Completes Acquisition of UK’s Binding Site
Thermo Fisher Scientific Inc. (Waltham, MA; USA; www. thermofisher. com) has completed its acquisition of The Binding Site Group (Birmingham, UK; www.bindingsite.com) in an


all-cash transaction valued at GBP 2.3 billion, or USD 2.8 billion at the time of the transaction.
Thermo Fisher had announced the agreement to acquire The Binding Site on October 31, 2022.
Cont’d on page 18

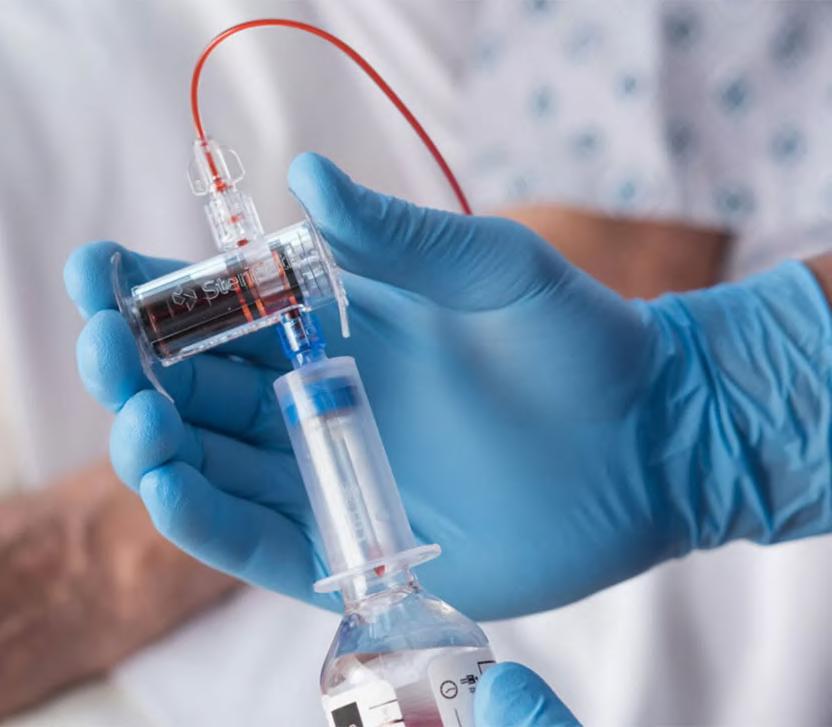
A novel technology can drastically reduce blood culture contamination, thereby largely eliminating sepsis misdiagnosis, and hence preventing harmful antibiotic treatments as well as extended hospital stays. ®
on page 25
Cont’d
on
If your subscription is not renewed every 12 months your Free Subscription may be automatically discontinued Renew / Start your Free Subscription Access Interactive Digital Magazine Instant Online Product Information: Identify LinkXpress ® codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress ® inquiry matrix 1 2 3 VISIT READER SERVICE PORTAL LINKXPRESS COM ®
page 4
All-in-One System Cleans Up Blood Culture Contamination, Cuts Sepsis False Positives All-in-One System Cleans Up Blood Culture Contamination, Cuts Sepsis False Positives GLOBETECH MEDIA >>> <<< PUBLISHED IN COOPERATION WITH International Federation of Clinical Chemistry and Laboratory Medicine INSIDE Clinical News ..... 4 LabMedica EXPO . 6-20 IFCC News ....... 21 Industry News . ... 25 Events Calendar . . 26
Dementia
®
Vol.40 No.1 • 2-3/2023 ISSN 1068-1760 VISIT DAILY CLINICAL LAB NEWS 4 OUR YEAR 0 th YEAR OUR WORLD’ S CLINICA L LABORATOR Y NEW S LEADER
INTERNATIONAL
CHEMISTRY ANALYSERS
Versatile biochemistry analysers, offering the world’s largest test menu to suit all laboratory sizes.
QUALITY CONTROL
Leading provider of complete quality control solutions including; daily quality control, calibration verification and proficiency testing for results you can trust.

MOLECULAR DIAGNOSTICS
Comprehensive range of infectious disease testing solutions for inherited diseases, mutation analysis and SNP genotyping.
POINT OF CARE
Near patient care solutions delivering fast results and workflow efficiencies.
THIRD PARTY REAGENTS
Extensive range of open channel biochemistry reagents facilitating routine and niche diagnostic testing.

DEDICATED TO IMPROVING HEALTH WORLDWIDE Visit store.randox.com to buy directly from Randox today randox.com
Product availability may vary from country to country. Some products may be for Research use Only. For more information on product application and availability, please contact your local Randox representative.
marketing@randox.com
102 LMI-3-23 LINKXPRESS COM

103 LMI-3-23 LINKXPRESS COM
AI Combined with Infrared Imaging Automatically Classifies Tumors
Now, researchers have paired artificial intelligence (AI) with infrared (IR) imaging to develop an automated and precise method for diagnosing colon cancer and tailoring treatments to the patient. This label-free and automated technique complements existing methods for analyzing tissue samples.

Over the course of the past several years, a research team at the Centre for Protein Diagnostics (PRODI) at Ruhr University Bochum (Bochum, Germany; www.ruhr-unibochum.de) has been working on creating a new digital imaging method known as label-free IR imaging. This method measures the genomic and proteomic composition of the examined tissue, providing molecular information based on the infrared spectra. The information is then decoded using AI and displayed as falsecolor images utilizing image analysis methods from the field of deep learning.
The PRODI team successfully demonstrated that using deep neural networks, it was possible to effectively determine the microsatellite status, a prognostically and therapeutically relevant parameter, in colon cancer. In this process, the tissue sample passes through a standardized, user-independent, automated process and allows for spatially resolved differential classification of the tumor within an hour. On the other hand, classical diagnostics is used to determine the microsatellite status either
through complex immunostaining of various proteins or via DNA analysis.
The ever-improving therapy options have made fast and uncomplicated determination of such biomarkers extremely important. Based on IR microscopic data, the researchers modified, optimized, and trained neuronal networks to establish label-free diagnostics. In contrast to immunostaining, the new approach does not need dyes and is much faster than DNA analysis.
“We were able to show that the accuracy of IR imaging for determining microsatellite status comes close to the most common method used in the clinic, immunostaining,” said PhD student Stephanie Schörner
Early Alzheimer’s Detection Sensor Could Also Diagnose Other Diseases at POC
Tumor Necrosis Factor alpha (TNF alpha), is a cytokine, a particular type of small protein, that is involved with inflammation in the body. Abnormal cytokine levels have been linked to various diseases including Alzheimer’s disease, cancers, autoimmune and heart disease. TNF alpha is capable of acting as a biomarker, a measurable characteristic indicating health status. Currently, screening tests for Alzheimer’s disease involve a questionnaire to determine the individual’s symptoms, brain imaging, or a spinal tap process to test for biomarker proteins in the cerebral spinal fluid. Now, researchers are developing a new biosensor for detecting TNF alpha that can be used to screen for Alzheimer’s disease and other diseases.
COVID-19 is also capable of causing inflammatory reactions known as ‘cytokine storms.’ Research has demonstrated that cytokine inhibitors can be an effective treatment for improving chances of survival. There are several established methods for detecting biomarker proteins such as enzyme-linked immunosorbent assay (ELISA) and mass spectrometry, although they have some limitations such as high cost, the need for samples to be sent to a lab for testing, and results being available after a day or more.
The sensor developed by researchers at
Simon Fraser University (Burnaby, B.C., Canada; www.sfu.ca) is capable of detecting TNF alpha in very low concentrations (10 fM) – much below the concentrations generally found in healthy blood samples (200–300 fM). The researchers have successfully completed the proof-of-concept stage by proving that the two-electrode diode sensor can effectively detect TNF alpha in a laboratory setting. The team now plans to conduct clinical trials to test if the biosensor can effectively detect biomarker proteins within a blood sample that contains several different interfering proteins and other substances.
“Our goal is to develop a sensor that’s less invasive, less expensive and simpler to use than existing methods,” said Engineering Science Assistant Prof. Michael Adachi, the project’s co-lead. “These sensors are also small and have potential to be placed in doctor’s offices to help diagnose different diseases, including Alzheimer’s disease.”
“We will continue testing the device’s ability to detect the same proteins using body fluid like blood samples,” added engineering science PhD student Hamidreza Ghanbari. “The other objective is to use the same device but a different receptor to detect proteins that are more specific to Alzheimer’s disease.”

INTERNATIONAL
labmedica.com
EDITORIAL BOARD
Graham Beastall United Kingdom
Hernán Fares Taie Argentina
Bernard Gouget France
Maurizio Ferrari Italy
Jocelyn M. Hicks United States
Tahir S. Pillay South Africa
Andreas Rothstein Colombia
Praveen Sharma India
Rosa I. Sierra-Amor Mexico
Peter Wilding United States
Andrew Wootton United Kingdom
A GLOBETECH PUBLICATION
Published in cooperation with the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC).
LabMedica International • LabMedica en Español • LabMedica.com HospiMedica International • HospiMedica.com • MedImaging.net HospiMedicaExpo.com • LabMedicaExpo.com • LinkXpress.com
Dan Gueron
Sanjit Dutt
David Gueron
Carolyn Moody, RN
Simone Ciolek
Parker Xu
Karina Tornatore
Publisher
News Editor New Products Editor
Regional Director
Regional Director Regional Director
HOW TO CONTACT US
Subscriptions:
Send Press Releases to:
Advertising & Ad Material: Other Contacts:
www.LinkXpress.com LMNews@globetech.net ads@globetech.net info@globetech.net
ADVERTISING SALES OFFICES
Reader Service Manager USA Miami, FL 33280, USA


Carolyn.Moody@globetech.net Tel: (1) 954-686-0838
GERMANY, SWITZ., AUSTRIA Bad Neustadt, Germany
Simone.Ciolek@globetech.net Tel: (49) 9771-1779-007
OTHER EUROPE & UK Miami, FL 33280, USA
Carolyn.Moody@globetech.net Tel: (1) 954-686-0838
SUBSCRIPTION INFORMATION
LabMedica lnternational is published eight times a year and is circuIated worldwide (outside the USA and Canada) without charge and by written request, to clinical laboratory specialists and administrators, and other qualified professionals allied to the field.
To all others: Paid Subscription is available for a two-year subscription charge of US$120. Single copy price is US$20. Mail your paid subscription order accompanied with payment to Globetech Media, P.O.B. 800222, Miami, FL 33280-0222.
For change of address or questions on your subscription, write to: LabMedica lnternational, Circulation Services at above address; or visit: www.LinkXpress.com
ISSN 1068-1760
Vol.40 No.1. Published, under license, by Globetech Media LLC; Copyright © 2023. All rights reserved. Reproduction in any form is forbidden without express permission. Opinions expressed are solely those of the authors, and do not represent an endorsement, or lack thereof, by the Publisher of any products or services.
Teknopress Yayıncılık ve Ticaret Ltd. Şti. adına İmtiyaz Sahibi: M. Geren • Yazı işleri Müdürü: Ersin Köklü Müşir Derviş İbrahim Sok. 5/4, Esentepe, 34394 Şişli, İstanbul P. K. 1, AVPIM, 34001 İstanbul • E-mail: Teknopress@yahoo.com
Baskı: Postkom A.Ş. • İpkas Sanayi Sitesi
3. Etap C Blok • 34490 Başakşehir • İstanbul Yerel süreli yayındır. Yılda sekiz kere yayınlanır, ücretsiz dagıtılır.
4 LabMedica International February-March/2023
JAPAN Tokyo, Japan Katsuhiro.Ishii@globetech.net Tel: (81) 3-5691-3335 CHINA Shenzen, Guangdong, China Parker.Xu@globetech.net Tel: (86) 755-8375-3877 OTHER COUNTRIES Contact USA Office ads@globetech.net Tel: (1) 954-686-0838 Founder & Editorial Director Marc Gueron LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Cont’d from cover
Image: AI with infrared imaging enables precise colon cancer diagnostics (Photo courtesy of Pexels)
New Whole Blood Glucose Reference Analyzer


Nova Primary addresses the needs of glucose device manufacturers and researchers for an accurate, easy-touse, blood glucose reference analyzer to replace the discontinued YSI STAT PLUS 2300. The Nova Primary glucose reference analyzer state-of-the-art features include:
An accurate Nova glucose sensor

25 µL whole blood sample
Automatic hematocrit measurement and correction for plasma equivalent glucose results
Simple, color touchscreen operation
Comprehensive data storage and connectivity
FDA cleared
TM
105 LMI-3-23 LINKXPRESS COM
New DNA Biosensor Could Make High-Quality Clinical Diagnostics More Accessible
DNA can indicate the presence of or predisposition to several diseases, including cancer. By flagging down these signals, known as biomarkers, medical professionals are able to arrive at critical early diagnoses and offer personalized treatments. However, the typical screening methods are often laborious, expensive or uncover limited information. Now, a new biosensor chip featuring an accurate and inexpensive design has the potential to improve accessibility to high-quality diagnostics.
The biosensor, developed by a team of researchers, including from the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA; www.nist.gov), identifies biomarkers by measuring how binding occurs between DNA strands and the device. The biosensor differs from other similar sensors mainly due to its modular design, which reduces costs by enabling mass production and reuse of the costliest components. In a study, the team demonstrated the device’s high sensitivity and precision despite its modularity, which is usually associated with diminished performance.


Similar to other DNA biosensors, the new device takes advantage of the fact that a single DNA strand, when not paired with another within the familiar double helix, is primed for chemical bonding. Part of the device is coated with single strands of DNA. When these “probes” encounter DNA biomarkers having a corresponding, or complementary, genetic sequence, the two strands bind, sending a signal that is picked up by the device. When a strand of target DNA binds to a probe, it induces a voltage shift that a semiconductor device, called a field-effect transistor (FET), can measure. Such voltage shifts can happen hundreds of times per second as the molecules pop on and off the sensor. As a result of its high
time resolution, the approach can tell whether a DNA strand is bound to a probe, as well as how long it takes to connect and disconnect –a factor called binding kinetics that is vital for discerning various markers that could bind to the same probe to varying degrees. The method also does not need much space to measure a lot.
However, FET-based methods are yet to become mainstream, mainly due to their single-use nature, which until now was viewed as a necessity but pushes up their cost. Similar to how the radio becomes noisier as one drives away from a radio station, electrical signals also become increasingly noisy the longer they travel within electronics. This unwanted random noise that is picked up along the way makes it harder to measure the signal. In order to limit noise, DNA probes in FETbased sensors are usually attached directly to the transistor, which converts the signal into readable data. However, this has a drawback as the probes are spent after being exposed to a sample, along with the entire device. In the new study, the researchers increased the distance between the probes and the transistor to allow for the more expensive elements of the circuitry to be reused. The researchers found that the distance could increase the amount of noise, although they gained a lot from the design choice, in addition of the cost savings.


The researchers had anticipated that the modular design would diminish the biosensor’s sensitivity and took a page out of the Internet of Things (IoT) playbook, which accommodates the losses associated with wireless devices. The team paired the circuitry with a specific type of extremely low-power FET developed at CEA-LETI used in smartwatches, personal assistants and other devices to amplify signals and compensate for the lost sensitivity. The researchers tested the device’s performance by placing it in liquid samples containing DNA
strands associated with exposure to harmful ionizing radiation. Complementary DNA probes adorned electrodes wired to the FET. The researchers varied the amount of target DNA across several samples and found that the binding kinetics were sensitive enough to enable accurate measurements even at low concentrations. They found that the performance of the modular design was as good as that of integrated, non-modular FET-based biosensors. The researchers now plan to examine if the sensor can perform similarly with varying DNA sequences due to mutations. Given that several diseases are caused by or associated with mutated DNA, this capability is essential for clinical diagnostics. They also plan to conduct other studies to examine the sensor’s ability to detect genetic material associated with viruses, such as SARS-CoV-2 that causes COVID-19, and could indicate infection.
“There’s an opportunity to develop more sophisticated modular sensors that are much more accessible without sacrificing high quality measurements,” said NIST researcher Arvind Balijepalli, a co-author of the new study.


6 LabMedica International February-March/2023
HB-TOTAL TEST
The Hb-Total quantitative test is an immunochromatographic rapid test for the quantitative determination of hemoglobin total in whole blood samples with a measuring range of
CKD TEST RANDOX LABORATORIES
202
203 LMI-3-23
COM
The Randox Chronic Kidney Disease (CKD) arrays utilize patented Biochip Technology, enabling early multiplex detection of CKD from a single sample for earlier intervention through treatment and preventing further kidney damage.
LMI-3-23 COM
LINKXPRESS
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Image: A new DNA biosensor could unlock powerful, low-cost clinical diagnostics (Photo courtesy of NIST)
All-in-One System Cleans Up Blood Culture Contamination, Cuts Sepsis False Positives
Blood cultures are considered the gold standard diagnostic test for the detection of blood stream infections, such as sepsis. However, positive blood culture results can be frequently wrong, and about 40% of positive results return a false-positive result owing to contamination. Such false-positive results can cause misdiagnosis of sepsis and expose the patient to unnecessary, prolonged, and harmful broad-spectrum antibiotic treatment and extended length of in-patient hospital stay. This may put the patent at a higher risk for acute kidney injury, Clostridioides difficile infection (CDI), Multidrug-Resistant Organism (MDRO) infections, other hospital-acquired infections (HAI), and significantly high hospital costs. Preventing false-positive results and sepsis misdiagnosis has to begin with reducing blood culture contaminations. Now, a new technology can help reduce blood culture contamination and false-positive test results, thereby preventing the patient from being harmed, and reducing unnecessary and prolonged usage of antibiotics, duration of stay, and hospital expenses.
Magnolia Medical Technologies’ (Seattle, WA, USA; medical.com

FDA 510(k)-cleared device that is specifically indicated to reduce blood culture contamination with an FDAcleared labeling claim for an 83% and 88% reduction in contamination rates. Optimally designed for blood culture contamination prevention, Steripath comes pre-assembled and sterile to actively divert and sequester the initial 1.5-2.0mL of blood, the volume that is known to contain contaminants. Blood cultures are collected through an independent, second flow path, creating a closed vein-to-bottle collection system meant to avoid bypassing diversion.
Magnolia is entering into partner ships with hospitals and healthcare providers in the U.S. to prevent sepsis misdiagnosis by improving the accu racy of blood culture results using Steripath. A recent peer-reviewed study published in a leading med ical journal constituted the largest controlled clinical dataset ever docu mented with zero blood culture con tamination events. Importantly, the study demonstrated that it was pos sible to eliminate blood culture contamination and “get to zero” with the use of Steripath. Another large-scale, peer-reviewed study quantified multiple devastating patient harms associated with blood culture contamination and most significantly, a 74% higher risk of in-hospital patient mortality.
“This study includes one of the largest data sets to date examining and directly quantifying the preventable consequences of blood culture contamination on patient safety and clinical outcomes,” said Greg Bullington, CEO and Co-Founder of Magnolia Medical. “This definitive data adds to the substantial body of evidence

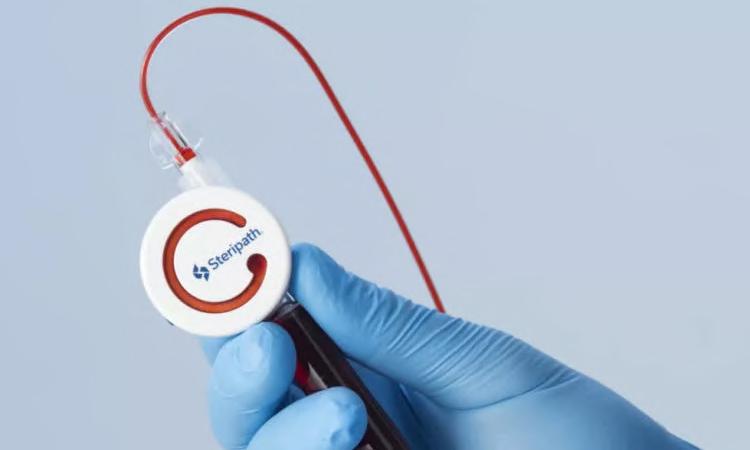
that already exists demonstrating that contaminated blood culture results drive avoidable, severely negative outcomes for patients and the healthcare system, including increased costs, inappropriate antibiotic usage that contributes to the rise of multidrug-resistant organisms, increased length of stay, and other preventable adverse events.”
Convenient cycling method
Wide measuring range for reliable detection of diagnostically relevant results


Outstanding onboard and calibration stability
Faster determination than current gold standard Applicable on various common clinical chemistry analyzers
7 LabMedica International February-March/2023 LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
DiaSys. Total confidence in patient results. www.diasys-diagnostics.com
T o t a l b i l e a c i d s 2 1 F S
107 LMI-3-23 LINKXPRESS COM
High quality solution for reliable determination of total bile acids in human stool
Breakthrough Test Enables Targeted Antibiotic Therapy for Various Enterobacter Species
Bacteria of the Enterobacter genus are considered to be the most dangerous bacteria linked to hospital infections across the world. Some of their representatives demonstrate high resistance to commonly-used antibiotics, as a result of which the reserve antibiotic colistin is used as the last resort therapy option. In order to avoid unnecessary reliance on colistin and reduce the risk of resistance, bacteria are tested for sensitivity or resistance to colistin before commencing treatment. However, commonly used tests for Enterobacter are unreliable. Scientists have resolved this problem by developing a simple, sensitive and robust test for the genus Enterobacter, which now enables targeted antibiotic therapy for the different Enterobacter species.
The microbiological tests currently used for colistin resistance and other antibiotic resistances do not enable accurate conclusions about the spread of resistance in various Enterobacter species. This can be partly attributed to the imprecise taxonomic classification of clinical Enterobacter isolates as well as high error rate in determining resistance. In a comprehensive study with broad participation within the German Center for Infection Research (DZIF, Brunswick, Germany; www. dzif.de), a team of scientists has now achieved a breakthrough and clarified the relationships between the numerous Enterobacter species as well as optimized resistance testing.
For their study, the researchers analyzed Enterobacter isolates collected at German university hospitals over a period of three years. Using genome-based taxonomic studies, they found Enterobacter xiangfangensis was the most frequently occurring species in German hospitals: An analysis of a data pool of more


than 3246 isolates worldwide – representing a collection from over 20 years – found that this species accounted for 68.7% of all Enterobacter detected. The determination of antibiotic resistance profiles using phenotypic assays recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) provided mixed results in terms of colistin resistance.
“It turned out that many isolates were either not or barely resistant in these tests, even though the bacteria carried all the genes necessary for the expression of colistin resistance,” explained Dr. Swapnil Doijad, the study’s first author. The result raised questions for which the researchers obtained an initial answer by further investigating the isolates in which resistance was not clearly detectable using mass spectrometry.

“Depending on the particular Enterobacter species, we detected low levels of modified lipid A, the anchor structure of lipopolysaccharides (LPS) – a crucial component of the bacterial membrane and required for colistin resistance – even from bacteria grown in colistin-free medium,” commented Dr. Nicolas Gisch, the study’s co-first author. “These modifications of lipid A appear to be dependent on the bacterial species and are inherent, meaning their expression is embedded in a more complex regulation and not alone triggered by colistin.”
“The result suggests that there is species-dependent variation in the heteroresistance seen in Enterobacter: In routine test systems, the bacteria are sometimes resistant, sometimes not,” explained Dr. Can Imirzalioglu, the study’s co-author.

Using more sophisticated methods, the researchers were able to elucidate the phe-


nomenon of heteroresistance in the genus Enterobacter. “Our analyses revealed that these bacteria have a sensor on their surface that responds to the pH value, i.e., the acidity in the environment, and regulates accordingly, either up or down, the genes required for the expression of colistin resistance,” explained Prof. Trinad Chakraborty, senior author of the study.
Genetic variations and interactions in this sensing pathway for environmental pH led to species-dependent differences regarding the extent of colistin resistance in the various Enterobacter species in conventional test systems. On the basis of their findings, the researchers have developed a simple new assay that eliminates heteroresistance effects and enables unambiguous and reliable determination of the true levels of colistin resistance for any isolate. The assay can prevent therapeutic failures when recommending the antibiotic, thereby paving the way for a targeted and economical treatment of Enterobacter species with the reserve antibiotic across the world.
8 LabMedica International February-March/2023
VAGINAL YEAST TEST SAVYON DIAGNOSTICS
The Vaginal Yeast Test is a rapid lateral flow-based assay for qualitative detection of Candida antigens in cervical secretion sampled by a swab. The test ensures fast diagnosis to provide the most appropriate
RSV/ADENOVIRUS TEST SEKISUI DIAGNOSTICS
The OSOM RSV/Adeno test is a rapid chromatographic immunoassay for the qualitative detection of Respiratory Syncytial Virus (RSV) and/or Adenovirus antigens directly from nasal swabs or nasal suction fluid.
205 LMI-3-23 COM
206 LMI-3-23 LINKXPRESS COM
Image: Medical illustration of Carbapenem-resistant Enterobacteriacea (Photo courtesy of CDC, Stephanie Rossow)
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
New Device




Detects Brain Tumors Using Urine




There has recently been an improvement in cancer survival rates due to early detection of the disease, although the survival rate for brain tumors has remained almost the same for the last 20 years, partly due to their late detection. Brain tumors are often discovered only after the onset of neurological symptoms, such as loss of movement or speech, by when the tumor achieves a considerable size. Detecting the tumor when it is still small and commencing treatment as soon as possible can help save lives. Now, researchers have used a new device to identify a key membrane protein in urine that indicates whether a person has a brain tumor. By using the protein to detect brain cancer, it will be possible to avoid invasive tests and increase the chances of the tumor being detected at an early enough stage for surgery. The finding could also have potential implications for detecting other types of cancer.



The presence of tumor-related extracellular vesicles (EVs) in urine can indicate that a person has a brain tumor. EVs are nano-sized vesicles that perform various functions, including cell-to-cell communication. The EVs found in brain cancer patients have specific types of RNA and membrane proteins, allowing them to be used for detecting the presence of cancer and its progression. EVs are excreted far from the brain, but many EVs from cancer cells still exist stably and are excreted in the urine without breaking down.


Researchers at Nagoya University (Nagoya, Japan; www.nagoya-u. ac.jp) have developed a new analysis platform for brain tumor EVs using nanowires at the bottom of a well plate. They used the device to identify two specific types of EV membrane proteins, known as CD31/CD63, from the urine samples of brain tumor patients. By looking for these telltale proteins, doctors can identify tumor patients before they develop symptoms. Additionally, urine testing offers several advantages and is an effective, simple, and non-invasive method because urine contains many informative biomolecules that can be traced back to identify the disease.
“Currently, EV isolation and detection methods require more than two instruments and an assay to isolate and then detect EVs,” said Assoc. Prof. Takao Yasui of Nagoya University Graduate School of Engineering. “The all-in-one nanowire assay can isolate and detect EVs using one simple procedure. In the future, users can run samples through our assay and change the detection part, by selectively modifying it to detect specific membrane proteins or miRNAs inside EVs to detect other types of cancer. Using this platform, we expect to advance the analysis of the expression levels of specific membrane proteins in patients’ urinary EVs, which will enable the early detection of different types of cancer.”
and AI Clinical and Research Applications






















109 LMI-3-23 LINKXPRESS COM LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Digital Microscopy
9 LabMedica International February-March/2023
Image: Microscopic image of the nanowires (Photo courtesy of Nagoya University)
REAL-TIME DIAGNOSTICS ONSCREEN VIEWER

Voice-Activated Sample Pre-Treatment Device Enables Hands-Free, Safer DNA Handling
Scientists using samples containing pathogens work with the smallest amounts possible in order to avoid accidental infection. In the case of highly contagious bacterial diseases, on-site sample analysis is suitable for rapid diagnoses. Additionally, scientists with visual or other physical impairments can find it difficult to operate complex instruments, particularly those designed for tiny volumes. Now, the same technology used by smart voice assistants could also make the laboratory a safer place for scientists and technicians handling infectious samples.


Hands-free devices that can be operated quickly using voice commands could make the laboratory safer for scientists and technicians. Researchers at Kyung Hee University (Yongin, South Korea; www. khu.ac.kr) set out to combine a speech recognition app with a miniaturized extraction system to do just that. The researchers first built a microfluidic chip with multiple chambers linked together by six 3-way solenoid valves, which were operated by a micro-controller connected to a Bluetooth module. The palm-sized device weighed only 11 ounces and was powered by a portable battery or a 5V smartphone charger. Using existing speech recognition software, the team went on to customize a smartphone app to listen for specific voice commands.
The voice-activated system is simple to operate. As soon as the user says one of the operation commands out loud, the app wirelessly sends an initiation signal to the micro-controller. After receiving the signal, the micro-controller automatically begins a series of steps, including sample loading, washing and releasing the purified DNA into a collection chamber. Currently, the system requires the user to touch the smartphone to start the speech recognition software, although the entire operation could soon become completely hands-free with the
addition of virtual assistant software.



The researchers conducted tests of the system in which the voice-controlled device extracted DNA from Salmonella Typhimurium, purifying a 10-µL sample with an efficiency of 70% in less than a minute. The system’s performance was lower as compared to a traditional DNA extraction kit, although its voice control, portability and quick automation lend it an advantage for convenient and safe bacterial DNA testing, according to the researchers.

10 LabMedica International February-March/2023
GEMweb Live is a real-time onscreen viewer that consolidates results from four Werfen systems in the cardiovascular operating room. These comprehensive test results, all on one screen, enable faster clinical decision-making.
PORTABLE ELECTROLYTE ANALYZER CARETIUM
The XI-951 is a portable electrolyte analyzer for indoor and field testing with remote access through Wi-Fi. It features a quick responsive CPU, RAM with storage capacity of 100,000+ results, and maintenance-free electrodes.
208 LMI-3-23 COM
209 LMI-3-23 LINKXPRESS COM
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Image: The small, voice-activated device extracts and pretreats bacterial DNA (Photo courtesy of ACS Sensors)
www.apccmi2023.com
Portable Real-Time PCR System Resolves Problems of Limited Lab Space and Fragmented Samples


Today, laboratories that are inside as well as outside of the hospital environment face the challenge of functioning in a limited space while ensuring that the quality of work being carried out is not impacted by the lack of room for equipment. Now, a new portable real-time PCR system designed for mobile or small laboratories or on-site testing solves the problem of limited space and fragmented samples in laboratories.
Tianlong Science and Technology (Shaanxi, China; www.tlgenetech.cn) has launched the new Gentier Mini+ real-time PCR system for mobile or small laboratories or on-site testing that is portable, fast, and always online. Designed for excellent performance and portability, the revolutionary Gentier

Mini+ solves the problem of limited space and fragmented samples in laboratories to make workflow easier, more accurate, and more efficient.
The Gentier Mini+ is an updated model of the Gentier Mini with two fluorescence channels that had proved popular among Tianlong’s clients. In order to fully meet the needs of its clients, Tianlong has launched the Gentier Mini+ which now comes with four channels, enabling a 16 sample throughput that makes it suitable for more applications. The Gentier Mini+ can be utilized for a variety of applications in the fields of animal and human infectious disease prevention and control, food safety and scientific research, among others.
Nanotechnology-Based

Blood Test Could Revolutionize Prostate Cancer Diagnosis
Cancer of the prostate is the most common cancer and second-leading cause of cancer death among men in the U.S. About 30% of post-surgical patients who get their prostate, a walnut-sized gland just below the bladder, removed can see an increase in prostate-specific antigen (PSA) levels in their blood that can also indicate cancer recurrence. In case a remnant of the cancer is left behind in the prostate bed, where the prostate gland once was, focused radiation therapy can be used to cure the disease or delay progression, although that treatment comes with its own risks. In patients with microscopic cancer deposits spread outside their prostate area, focused radiation treatment cannot prevent disease progression. Even the most advanced imaging cannot detect these deposits, called micro-metastases. Now, researchers have developed a new nanotechnology-based test that can detect and profile prostate cancers - even in microscopic amounts. The “liquid biopsy” test can help patients avoid unnecessary treatment-related side effects and direct them instead to effective therapies that could extend their life span.
The test developed by investigators at Cedars-Sinai (Los Angeles, CA, USA; www. cedars-sinai.org) isolates and characterizes extracellular vesicles, also called EVs, from blood samples. EVs are microscopic packets of protein and genetic material that are shed by cells. The EV Digital Scoring Assay can pull these EV packets from the blood with unprecedented efficiency and analyze them faster than any available test. For the study, the investigators tested blood samples from 40 patients with prostate cancer and found that the test could distinguish cancer localized to the prostate
from cancer that had spread to other parts of the body. The investigators were also able to detect microscopic cancer deposits, or micro-metastases, using the EV test.
The test can be used to help patients who have their prostate gland removed and later see increased PSA levels in their blood, according to the investigators. The test could also be adapted to guide treatment as prostate
cancer therapies become more targeted at the molecular level, ultimately extending patients’ lives. The investigators are now working to further refine the test so that it can be studied in greater detail.
“This research will revolutionize the liquid biopsy in prostate cancer,” said Edwin Posadas, MD, medical director of the Urologic Oncology Program and co-director of the Experimental Therapeutics Program in Cedars-Sinai Cancer.
“The test is fast, minimally invasive and cost-effective, and opens up a new suite of tools that will help us optimize treatment and quality of life for prostate cancer patients.”
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 11 LabMedica International February-March/2023
111 LMI-3-23 LINKXPRESS COM
GOLD STANDARD HPLC HbA1c Analyzer BORONATE AFFINITY POCT HbA1c Analyzer JULY 23-27 ANAHEIM, CA Goldsite Diagnostics Inc. en.goldsite.com.cn GSH-60 A1c Go Visit us at Booth number: 4478
Image: The new Gentier Mini+ portable real-time PCR system (Photo courtesy of Tianlong)
Antiphospholipid Antibodies in Patients With Unexplained Articular Manifestations

Joint pain is the most common chronic pain making it one of the largest causes of disabilities in the world. Arthritis is a frequent condition that causes edema, redness, heat, loss of function and pain. It can affect one or more joints and there exist more than 100 different types.
Antiphospholipid antibodies (aPL) represent a complex and heterogeneous group of antibodies directed against anionic phospholipids or protein-phospholipid complexes. Persistent aPL have been associated with antiphospholipid syndrome (APS), which is defined by the presence of recurrent venous and/or arterial thrombosis and often pregnancy morbidity.
Immunologists at the Farhat Hached Hospital (Sousse, Tunisia; www.tunisiemedicale.com) and their colleagues conducted a retrospective study including 313 patients suffering from arthritis or arthralgia without evident cause. Serum samples were collected from January 2017 to December 2019. Antinuclear antibodies (ANA), rheumatoid factors (RF) and anti-cyclic citrullinated peptides antibodies (CCP-Ab) were negative for all patients. Sera of 266 healthy blood donors (HBD) were included as normal controls. The aPL measured were anticardiolipin antibodies (aCL) and anti-beta 2-glycoprotein I antibodies (aβ2GPI).
The team detected aCL-IgG, IgM, and IgA using an enzyme-linked immunosorbent assay (ELISA) kit (Orgentec Diagnostika, Mainz, Germany; www.orgentec.com).Results were detected with an IRE 96 microtiter plate reader (SFRI Medical Diagnostics, Saint Jean d’Illac, France; www. sfri.fr). The scientists evaluated aβ2GPI-IgG and IgM by an ELISA kit (Orgentec Diagnostika) using a purified human β2GPI. aβ2GPI-IgA were also assessed by ELISA kit. RF IgG, IgM and IgA were assessed using three commercial ELISA kits (Orgentec Diagnostika). CCP-Ab were measured by an available second-generation ELISA and ANA were detected by indirect immunofluorescence on HEp-2 cells (Euroimmun, Luebeck, Germany; www.euroimmun.com).
The investigators reported that out of the 313 patients, 250 were females and 63 were males. The mean age of patients was 49 ± 14 years (17–87 years). One hundred eleven patients have arthralgia and 202 have arthritis. The frequency of aCL and/or aβ2GPI (24.9%) was significantly higher in patients than in HBD (10.9%). The frequency of aβ2GPI was 23.6% in patients and 9.4% in the control group. aβ2GPI-IgA was
significantly more frequent in patients than in the control group (20.4% versus 7.5%).
aβ2GPI was more commonly observed than aCL in patients (23.6% versus 6.4%). IgA isotype of aβ2GPI was the most frequent in 20.4% of patients while IgG and IgM were detected in 5.4% and 2.9% respectively.
The authors concluded that they had demonstrated an elevated frequency of aβ2GPI-IgA in patients with unexplained arthralgia or arthritis. The possible pathogenic mechanism of aβ2GPI remained to be demonstrated and a prospective study is necessary to known if aβ2GPI-IgA will persist over time. The study was published on December 13, 2022 in the Journal of Clinical Laboratory Analysis
Image: The SFRI IRE 96 is a simple and robust ELISA Absorbance Microplate Reader finds application in ELISA assays (Photo courtesy of HealthManagement.org)


New Immunosensor Paves Way to Rapid POC Testing for COVID-19 and Emerging Infectious Diseases

The incredibly fast spread of COVID-19 throughout the world brought to light a very important fact: we need better methods to diagnose infectious diseases quickly and efficiently. During the early months of the pandemic, polymerase chain reaction (PCR) tests were one of the most widely used techniques to detect COVID-19. However, these viral RNA-based techniques require expensive equipment and reaction times longer than an hour, which renders them less than ideal for point-of-care testing. The limitations of PCR fueled the development of various immunoassay methods, which use specially engineered antibodies to detect SARS-CoV-2 antigens with high sensitivity in little time. Today, scientists are still improving immunoassay technology to make available tools more convenient, sensitive, and cost-effective. Against this backdrop, a team of researchers has developed a new immunosensor based on Quenchbody technology that shows great potential as a fast, inexpensive, and convenient tool to detect SARS-CoV-2. This
Cont’d on page 13


12 LabMedica International February-March/2023
RAPID IMMUNOLOGICAL FOB TEST DIASOURCE
The Fecal Occult Blood (FOB) Test device is a rapid immunological test intended for the qualitative detection of fecal occult blood in feces by professional laboratories and physician office laboratories.
AUTO HEMATOLOGY ANALYSIS SYSTEM DIRUI INDUSTRIAL
The BF-7200 automatic hematology analysis system offers 29 blood test parameters, 2 scattergrams and 2 histograms as well as 7 body fluid test parameters, 1 scattergram and 1 histogram. It has a maximum throughput of 480T/H.
211 LMI-3-23 COM
212 LMI-3-23 LINKXPRESS COM
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
New Platform Aims to Accelerate Diagnostic Tests for Future Pandemics
apisassay.com) and Fraunhofer IMS (Duisburg, Germany; www.ims.fraunhofer.de) has received a EURO 1.1 million grant to develop an innovative diagnostic platform for fast pandemic responsiveness. The project which is due to commence in spring 2023 aims to establish a novel diagnostic assay platform that can enable a fast response time towards emerging pandemic situations. In the collaboration, Clickmers will be being combined with optical nanosensors, to develop a diagnostic assay platform with superior sensitivity and specificity having a faster response time for the detection of societal relevant emerging pathogens.
Clickmers are synthetic antibody analogues that can bind to targets with high specificity and affinity. Optical nanosensors, which are fluorescent in near infrared light, qualify for low signal-to-noise ratio. Upon binding of a pathogen to the detection structure, the fluorescence of the nanosensor changes, thereby enabling measurement of the binding events.
antibodies),” said Dr. Ian Kavanagh, COO of APIS Assay Technologies. “Clickmer Systems will provide an adaptive and innovative diagnostic platform for pandemic responsiveness with higher reproducibility.”
New Immunosensor Paves Way to Rapid POC Testing for COVID-19 and Emerging Infectious Diseases

Cont’d from page 12
highly efficient diagnostic approach will be useful not only for pointof-care testing, but also for high-throughput epidemiological studies of COVID-19 and other emerging infectious diseases.
The team of researchers at Tokyo Institute of Technology (Tokyo Tech, Tokyo, Japan; www.titech.ac.jp) has not only developed a new Quenchbody fluorescent immunosensor that can detect SARS-CoV-2 with exceptional speed and sensitivity, but also created a simple way to greatly enhance the immunosensor’s performance using a crowding agent. A Quenchbody is a molecular sensor originally developed by Prof. Ueda and colleagues using antibody fragments and fluorescent tags. The antibody fragment, which can be an antigen-binding region (or ‘Fab’), targets a specific viral molecule (antigen). Meanwhile, the fluorescent tags are small fluorescent dye molecules attached by a peptide linker to the Quenchbody, near the antigen-binding region. When the antigen is absent, the fluorescent tags are attracted to the Fab and intrinsic amino acids (mainly tryptophan) interact with the dyes and quench the fluorescence. However, when the antigen appears, it replaces the fluorescent tag at the Fab, causing it to move away and recover its fluorescence.
SWAB.
Thus, in a Quenchbody test, an increase in fluorescence indicates the detection of the target antigen.
In this study, the research team developed a double-tagged Quenchbody targeting the nucleocapsid protein (N protein) of SARS-CoV-2. To take things one step further, they also tested whether various commercially available compounds could improve the immunosensor’s sensitivity and detection time. In particular, adding polyethylene glycol 6000 (PEG6000) at the right concentration as a crowding agent increased performance quite significantly. To further validate their approach, the team tested their immunosensor on leftover clinical samples from COVID-19 positive patients. After careful analysis of the results, they concluded that their newly developed Quenchbody could measure N protein more easily and quantitatively than a commercial lateral flow antigen test.
“Our work shows the feasibility of using Quenchbody immunosensors as rapid and cost-efficient tools for the diagnosis and high-throughput analysis of swab samples in large-scale monitoring and epidemiological studies of COVID-19, as well as other emerging infectious diseases,” said Prof. Hiroshi Ueda at Tokyo Tech who led the research.
TRANSPORT.
We make it easy to find answers. Puritan® media transport kits make collection and transport to the lab quick and easy UniTranz-RT® (for virus) and Opti-Swab® (for bacteria), together with our patented flocked swabs, assure you get a quality sample with results you can trust.

LEARN MORE ONLINE
info.puritanmedproducts.com/media-transport

MADE IN THE USA
In stock and ready to ship!
VISIT US AT ECCMID BOOTH #C4-28!
DONE.
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 13 LabMedica International February-March/2023 113 LMI-3-23 LINKXPRESS COM
“We are excited to launch the project InfektoFlex with Fraunhofer IMS. The platform will provide a more cost-efficient and time-saving solution, with a reduction in development costs through a more sustainable process compared to the commonly used ELISA assay systems (which require the usage of animal-derived Cont’d from cover
AUTOMATIC COAGULATION ANALYZER DYMIND BIOTECHNOLOGY


Test Detects Predictive Biomarker for Dementia


impairment and dementia, suggesting possible therapeutic or early screening measures for cognitive impairment and dementia in the elderly.
A team of researchers from the National University of Singapore (NUS, Singapore; www.nus.edu.sg) demonstrated that ET is avidly retained in the human body following oral supplementation, and in preclinical models, ET is transported to almost all organs, although higher levels can be found in specific cells and tissues such as the blood cells, eyes, liver, lungs, and even the brain. Their earlier work demonstrated the potent antioxidant properties of ET and later its ability to protect cells from a range of different forms of stress and toxins. As its main dietary source is in mushrooms, it was found that increasing consumption of mushrooms such as golden, oyster, shiitake and white button mushrooms is associated with a reduced risk of mild cognitive impairment in elderly Singaporeans. Low ET levels are also associated with a number of other age-related diseases such as frailty, cardiovascular disease and macular degeneration, so ET may have a more general role in maintaining health.

An earlier study by the research team in 2016 showed lower ET levels in blood plasma among participants with mild cognitive impairment. This was verified in a much larger group of cognitively impaired participants with and without dementia. However, evidence of whether a low level of ET in blood plasma can predict the progression of cognitive impairment and dementia was unknown. The most recent study by the NUS research team addresses these gaps in ET research by demonstrating the potential of ET as a predictive biomarker for cognitive impairment and dementia in elderly Singaporeans.
In the latest study, the research team recruited 470 elderly patients and followed them for up to five years. The researchers measured ET levels in the blood plasma of the participants and followed their cognitive and functional abilities at different time points. They then examined the link between low ET levels and the risk of cognitive and functional decline over time. The researchers showed that participants with lower levels of ET displayed poorer cognitive performance at the start of the study and an accelerated rate of decline in cognitive and functional abilities over the follow-up period.
The team also observed structural changes in the brain seen from MRI (magnetic resonance imaging) scans of the participants, which suggested that the association between a low ET level in blood and
cognitive decline was due to underlying disease pathology. These structural changes, including reduced cortical thickness, lower hippocampus volume, and white matter hyperintensities, are characteristic of neurodegenerative disease. Based on this study, which showed that plasma ET levels in the blood can be a predictive biomarker for the risk of cognitive and functional decline, the research team hopes to gather further evidence of ET’s preventive and therapeutic potential through a double-blinded placebo-controlled clinical trial.
“Before this study, there was little evidence that ET levels in the blood can predict the risk of developing cognitive issues. The current study is significant because it measured the ET levels of elderly participants before developing dementia. Our findings demonstrate that if your ET levels are low, your risk of developing cognitive problems increases,” said Prof. Barry Halliwell from the Department of Biochemistry under the NUS Yong Loo Lin School of Medicine who led the research team. “This points to the possibility of using a simple blood test to detect ET levels for early screening in the elderly to identify those who may have higher risk of cognitive decline.”
14 LabMedica International February-March/2023
The CA1200 automatic coagulation analyzer uses the dual-method for economical and accurate measurement along with single-use reagent to fulfill the requirement of small quantity tests. Its internal PC saves both cost and space.
CARDIAC TROPONIN I TEST GUANGDONG WESAIL BIOTECH
Guangdong’s High Sensitivity Cardiac Troponin I Test (Fluorescence Immunoassay) detects cTnI which is a highly sensitive and specific marker for the diagnosis of myocardial injury/necrosis.
214 LMI-3-23 COM
215 LMI-3-23 LINKXPRESS COM
Cont’d from cover
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Image: Low levels of ergothioneine in blood plasma may predict increased risk of cognitive impairment and dementia (Photo courtesy of NUS)
Focused Ultrasound-Mediated Liquid Biopsy to Facilitate Diagnosis of Neurodegenerative Disorders

Anumber of progressive neurodegenerative disorders, including Alzheimer’s disease, are defined by having tau proteins in the brain. Researchers are trying to identify the mechanisms behind these tau proteins for developing treatments, although their attempt to detect biomarkers in blood has been hindered by the protective blood-brain barrier. Now, new research has found that using focused ultrasound-mediated liquid biopsy in a mouse model released more tau proteins and another biomarker into the blood than without the intervention. This noninvasive method could aid the diagnosis of neurodegenerative disorders, according to the researchers.

The results of the new research by investigators at Washington University in St. Louis (St. Louis, MO, USA; www.wustl.edu) are the first to pave the way for noninvasive and targeted diagnosis and monitoring of neurodegenerative disorders using focused ultrasound-mediated liquid biopsy. The method, known as sonobiopsy, targets a precise location in the brain using focus ultrasound. Once located, the researchers inject microbubbles into the blood that travel to the ultrasound-targeted tissue and pulsate, which opens the blood-brain barrier safely. The temporary openings enable biomarkers like tau proteins and neurofilament light chain protein (NfL), both of which are indicative of neurodegenerative disorders, to pass through the blood-brain barrier and release into the blood.
In the new research, the team obtained blood samples from young mice with abnormal tau proteins in the brain, or tauopathy, receiving either sonobiopsy or sham treatment. They found that sonobiopsy led to a 1.7-fold-increase in the normalized phosphorylated pTau-181 tau protein levels and a 1.4-fold increase in normalized pTau231 as compared with the control mouse group that had not had sonobiopsy. In a follow-up study, the researchers performed targeted sonobiopsy by targeting either the hippocampus or cerebral cortex in the early neurodegenerative stages of the tauopathy model and obtained blood samples before and after performing sonobiopsy. The targeted sonobiopsy led to a 2.3-fold increase in NfL protein, a secondary biomarker for neurodegenerative diseases, in the treated mice as compared with the control group.
Other liquid biopsy methods used to detect biomarkers for neurodegenerative disorders pose several challenges, such as lack of anatomical information about the location of the protein release, rapid clearance from the fluids and a filtering process by the blood-brain barrier. Sonobiopsy is an emerging technique that promises to address these and other challenges. Going forward, the researchers plan to examine the qualitative effects of sonobiopsy on plasma biomarkers and characterize the effects of focused ultrasound parameters. The will also determine an optimal blood collection time and how sonobiopsy can be applied to release larger brain-derived protein biomarkers.
“In our proof-of-concept study, we sought to determine whether sonobiopsy is able to release phosphorylated tau species and NfL into the bloodstream by opening the blood-brain barrier,” said Hong Chen, associate professor of biomedical engineering in the McKelvey School of Engineering and of radiation oncology in the School of Medicine at Washington University in St. Louis. “This demonstration showed that sonobiopsy significantly enhanced the release of pTau proteins and a secondary marker of neurodegeneration into the bloodstream for noninvasive diagnosis for neurodegenerative diseases.”
“While brain tumor behavior and treatment response are dictated by the specific mutations they harbor, the tau protein shows great heterogeneity in the pattern of phosphorylation as well as other post-translational modifications,” added co-senior author Arash Nazeri, MD, an assistant professor of radiology at the School of Medicine’s Mallinckrodt Institute of Radiology (MIR). “Current PET imaging and recently developed plasma biomarkers are sensitive to detect tauopathies even in early stages. Sonobiopsy could potentially play a role to further characterize the specific strains of tau protein present in the brain for personalized treatment of people with Alzheimer’s disease and other tauopathies.”
15 LabMedica International February-March/2023 LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Image: Focused ultrasound technique has led to release of neurodegenerative disorders biomarkers (Photo courtesy of Washington University in St. Louis)
Kenneth Timmis, Switzerland Jorge Galan, USA
Rita Colwell, USA
Julia Vorholt, Switzerland
Carmen Buchrieser, France Paul Lehner, UK
Revolutionary Transistor Could Allow Wearable Devices to Measure Sodium and Potassium in Blood






Researchers have developed a revolutionary transistor that could be suitable for lightweight, flexible, high-performance bioelectronics. The electrochemical transistor is compatible with blood and water and can amplify important signals, paving the way for its application in biomedical sensing. The transistor could allow for the use of wearable devices for onsite signal processing, right at the biology-device interface. Some of its likely applications could be for measuring heartbeat and the levels of sodium and potassium in blood, as well as eye motion in studies of sleep disorders.






The vertical electrochemical transistor developed by a transdisciplinary research team at Northwestern University (Evanston, IL, USA; www.northwestern.edu) is based on a new kind of electronic polymer and a vertical, instead of planar, architecture. The transistor conducts electricity as well as ions, and is stable in air. The design and synthesis of the new materials, and the fabrication and characterization of the
transistor was made possible by the collaborative expertise of chemists, materials scientists and biomedical engineers in the research team.
In order to make electronic circuits more reliable and powerful, there is a need for two types of transistors: p-type transistors that carry positive charges and n-type transistors that carry negative charges. These types of circuits are called complementary circuits. In the past, researchers have faced a challenge in building n-type transistors which are also typically unstable. The work by the transdisciplinary research team is the first to demonstrate electrochemical transistors with similar and very high performance for both types (p+n) of electrochemical transistors. This helped the researchers fabricate highly efficient electrochemical complementary circuits.
“All modern electronics use transistors, which rapidly turn current on and off,” said Tobin J. Marks, a co-corresponding author of the study. “Here we use chemistry to enhance the switching. Our electrochemical transistor takes performance to a totally new level. You have all the properties of a conventional transistor but far higher transconductance (a measure of the amplification it can deliver), ultra-stable cycling of the switching properties, a small footprint that can enable high density integration, and easy, low-cost fabrication.”
“This exciting new type of transistor allows us to speak the language of both biological systems, which often communicate via ionic signaling, and electronic systems, which communicate with electrons,” said Jonathan Rivnay, professor of biomedical engineering at the McCormick School. “The ability of the transistors to work very efficiently as ‘mixed conductors’ makes them attractive for bioelectronic diagnostics and therapies.”

HUMAN
CHORIONIC GONADOTROPIN NG BIOTECH
The NG-Test hCG Whole Blood is a qualitative test for the rapid detection of human chorionic gonadotropin (hCG) in human whole blood and serum. It delivers results in five minutes from a single drop of blood
LABORATORY QUALITY CONTROL RANDOX LABORATORIES
Acusera laboratory quality control is designed to monitor an IVD instruments response in the detection of HIL samples. The control can be used in laboratory interference testing to improve detection of pre-analytical errors.
217 LMI-3-23 COM
16 LabMedica International February-March/2023 116 LMI-3-23 LINKXPRESS COM
218 LMI-3-23 LINKXPRESS COM
Rapid, simple, cost effective carbapenemase detection Contact us for more information sales@mast-group.com www.mast-group.com MAST CARBA PAcE ® Results in <10 minutes
Image: The vertical electrochemical transistor is based on a new kind of electronic polymer and a vertical, instead of planar architecture (Photo courtesy of Northwestern University)
WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
Study of Emerging Pathogens to Better Understand Influenza-Antibody Interactions Could Improve Diagnostics


Outbreaks of Avian influenza have occurred around the world for over a century. The highly pathogenic H5N1 virus which was first identified in 1996 can lead to severe disease and has a high fatality rate among humans. If the H5N1 virus mutates and becomes easily transmissible from person-to-person while simultaneously maintaining its capability to cause severe disease, there could be serious consequences for public health. For long, scientists have wondered why aquatic birds, particularly ducks, which are carriers of influenza viruses rarely become severely ill themselves. They are yet to find the answer to how their immune systems can be a reservoir for such a highly infectious and pathogenic virus, but still remain mostly unharmed. Also, scientists are yet to find out of the immune system can be engineered to stop transmission of viruses to other animals and humans, thereby preventing future pandemics. Now, a team of investigators will attempt to answer these questions as part of an ambitious, three-year project.
The project involving four faculty members at the University of Illinois Urbana-Champaign (UIUC, Urbana, IL, USA; www.illinois. edu) was one of 13 selected by the Howard Hughes Medical Institute (HHMI, Chevy Chase, MD, USA; www.hhmi.org) as part of its USD 100 million Emerging Pathogens Initiative that will provide USD 9.5 million over three years to the project. The work and the platforms developed by the group over the coming years will help scientists better understand other avian viruses or other host-virus relationships and the steps needed to prevent them from spreading.
The investigators will first attempt to develop ways to purify antibody-producing cells from ducks to better understand their antibody repertoire. They plan to extract immune cells from the blood of ducks, sequence the antibodies and characterize them, to finally assemble a pool of antibodies for further investigations. For instance, the investigators will determine the different strains of influenza an antibody that could be neutralized and how effective those antibodies could be in neutralizing the virus. Based on observations from their sequencing work, the team will translate them into human systems.
The influenza virus enters its host through mucosal routes such as the nasopharynx and lungs and gut. The investigators will adopt various engineering approaches to address the different ways in which the influenza virus invades its hosts. The business part of antibodies is a series of loops that are hypervariable. The immune system selects those loops that bind tightly to a molecule of the pathogen. These loops can be mimicked with circular peptides to create antibody-like molecules. The information derived about the antibodies from ducks and human cells can be used to design the cyclic peptides against avian influenza. Ultimately, their work could have implications beyond influenza. The researchers expect to develop “modular” antibody evolution and engineering platforms that can be easily repurposed for targeting other emerging pathogens. Additionally, the platforms could be adapted for developing biologics to treat other diseases such as cancer.
“We will use the information gathered and combine it with evolution platforms in human cells to study how the duck antibody sequences evolve,” said UIUC chemistry Prof. Dr. Angad Mehta. “We’ll also engineer and evolve these antibody sequences to make human antibodies. Overall, our efforts could inform biologics development, diagnostics, and vaccine design.”
“We are optimistic that this initiative will help these scientists develop new, untested approaches that can reveal how pathogens work and how the human immune system responds to pathogen infection,” said HHMI Vice President and Chief Scientific Officer Leslie Vosshall. “With this program, we hope to gain some of the knowledge and tools we need to get a scientific head start on future epidemics.”
LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com 17 LabMedica International February-March/2023
Image: Scientists have won USD 9.5 million to study emerging pathogens (Photo courtesy of Pexels)
HEMATOLOGY CONTROL STRECK LABORATORIES


First POC Test for ‘Insidious’ Malaria



Now, new research aims to develop and deploy the world’s first diagnostic test for detecting ‘insidious’ malaria infections and accelerating malaria eradication.
Two projects by The Walter and Eliza Hall Institute of Medical Research (WEHI, Melbourne, Australia; www.wehi.edu.au) have received new funding from the National Health and Medical Research Council (NHMRC, Canberra, Australia; www.nhmrc.gov.au) to develop and deploy a test that is capable of detecting people with ‘hidden’ Plasmodium vivax. This malaria parasite is still the most widespread and resilient due to its ability to stay dormant in the liver for years. The funding will aid the development of the first such point-of-care rapid diagnostic test and deployment of its laboratory version in the Philippines.
The WEHI researchers had earlier developed a test that can accurately tell when an individual has been infected with the parasite and if there is a risk of relapse. The team will now develop a rapid point-of-care diagnostic test based on this earlier research. The researchers will first deploy a high throughput, laboratory version of the diagnostic test in the Philippines. They will leverage the expertise of research teams in the U.S. and Philippines to adopt a multi-disciplinary approach in order to focus their efforts on the Sultan Kudarat province where there have been Plasmodium vivax outbreaks recently.
In order to efficiently eliminate the Plasmodium vivax parasite, malaria control programs need tools to detect areas where there is ongoing transmission, and identify and treat people with dormant infections. The new test could be a game-changer for malaria control programs which have been struggling for decades to eliminate this relapsing parasite, according to the researchers. The team hopes that their research could assist in malaria eradication in the Philippines, as well as across East Asia. The generated data could be used to shape locally suitable and evidence-based interventions for improving the Philippines’ health policies.
“Being able directly target hidden liver-stage parasites is crucial for successful vivax elimination because they can be responsible for over 80% of all blood-stage infections. Unfortunately, there are currently no tests that can accurately detect who is carrying this insidious parasite in their bodies,” said Prof. Ivo Mueller, a world expert in the biology, epidemiology and control of P. vivax malaria. “Our test is the closest the scientific world has come to tracking and predicting these ‘hidden’ infections and we are thrilled to start translating these findings to a rapid diagnostic test to improve the lives of those affected by the malarial health burden.”

Image: The first point-of-care rapid diagnostic test can detect people at risk of malaria reinfection (Photo courtesy of WEHI)


New Blood Test Could Ensure Timely Life-Saving Treatment of Heart Attack Victims
Cardiovascular disease, one of the leading causes of death, generally manifests itself through heart attacks. Most patients with very large heart attacks are treated using an emergency procedure called primary percutaneous coronary intervention (PCI). Some of these patients do very well after the procedure but about a third do not. Now, new research shows that routine testing for a stress hormone Neuropeptide Y (NPY) in the hours following a heart attack could save thousands of lives.
In the new study, researchers at University of Oxford (Oxford, UK; www.ox.ac.uk) investigated NPY levels in the blood of 163 heart attack patients who had been given emergency treatment to open up a blocked blood vessel. Once NPY is released into the heart, it causes the smallest blood vessels to narrow. The researchers found that in patients with the highest NPY levels, the smallest blood vessels in their heart remained narrowed two days after a heart attack. MRI scans performed after six months showed more scarring in the hearts of these patients, resulting in the inability to pump blood efficiently.
The researchers also measured NPY levels in standard blood samples obtained from the veins of patients when they were given PCI treatment. The researchers found more heart and lung damage in patients having the highest NPY levels and their hearts were much more likely to fail irrespective of other risk factors over the subsequent six years. During follow-ups, 34 patients died or suffered from heart failure. Based on these
18 LabMedica International February-March/2023
Para 12 Plus is a whole blood assayed hematology control which offers complete assay values for the five-part white blood cell differential. The control features 3 mL vials with pierceable caps for autosampling.
POCT IMMUNOASSAY ANALYZER SUGENTECH
The INCLIX F-100 is a time-resolved fluorescence Immunoassay Analyzer designed for quantitative and qualitative measurement of various biomarkers for cardiovascular disease, infectious disease, cancer, diabetes, allergy, etc. 220 LMI-3-23 COM 221 LMI-3-23 LINKXPRESS COM
Cont’d from cover
Cont’d
page
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
on
19
New Blood Test Could Ensure Timely Life-Saving Treatment of Heart Attack Victims
“Our previous research has shown that NPY is raised during a heart attack and local levels within the heart correlate with how well it recovers,” said Neil Herring, Assoc. Prof. and lead researcher. “What this new study adds is that high NPY levels, even when measured through a standard blood test from a vein, predict which patients go on to develop heart failure or die. This provides extremely useful information for doctors and we hope that developing drugs that target the receptors NPY acts on may really be game changing for this cohort of patients and the blood test could help spot those patients who may need it right from the start.”
“This study identifies a ‘cut off’ value for the blood test which helps identify those patients that do badly after their large heart attack. Ideally, further studies should then test this cut off level in a different group of patients to ensure that it is robust in predicting heart failure and death. However, if successful, then it could be offered to all patients with large heart attacks undergoing emergency treatment,” added Prof. Herring. “We’re confident that, in time, this stress hormone will become an effective target for future treatments to reduce the life-limiting effects of a heart attack.”
Impact of Preanalytical Factors on Calprotectin Concentration in Stool



Image: The new blood test measures stress hormone levels after heart attacks (Photo courtesy of University of Oxford)

YOUR
GLOBAL
I



n inflammatory bowel disease (IBD), fecal calprotectin measurement is increasingly important in selecting patients for diagnostic endoscopy, monitoring of disease activity, and evaluation of treatment response.


Calprotectin is a calcium-binding protein mainly produced in neutrophils. It is said to be resistant to bacterial degradation in the colon, and the literature almost unanimously states that calprotectin is stable up to seven days at room temperature without preservation buffer.
Clinical Chemists at the University Medical Center Groningen (Groningen, The Netherlands; www.umcg.nl) and their colleagues from other institutions evaluated the impact of pre-analytical storage conditions on reliability of calprotectin testing using five different calprotectin immunoassays. The scientists distributed 45 frozen anonymized feces aliquots among the three participating centers. They assessed the calprotectin concentration over time under four conditions, including (a) untreated native stool stored at room temperature (NRT), (b), stool extract stored at room temperature, (c), untreated native stool stored at 4 °C, and (d), stool extract stored at 4 °C.
The five assays were: the Bühlmann fCAL turbo test (Bühlmann Laboratories AG, Schönenbuch Switzerland; www.buhlmannlabs.ch) is a particle-enhanced turbidimetric immunoassay performed on a COBAS 6000 e501 (Roche Diagnostics, Rotkreuz. Switzerland; diagnostics. roche.com); the Bühlmann fCAL enzyme-linked immunosorbent assay (ELISA; Bühlmann Laboratories AG) is a sandwich-based ELISA performed and analyzed using a DS2 Dynex ELISA robot (Dynex, Chantilly, VA, USA; www.dynextechnologies.com); the CALliaGold test (Sentinel CH, Milan Italy; www.sentineldiagnostics.com) is a particle-enhanced turbidimetric immunoassay (PETIA) and analysis was performed using a SENTiFIT 270 Analyzer (Sysmex Europe SE, Norderstedt, Germany; www.sysmex-europe.com).




The other two assays were the EliA Calprotectin test which is a fluorescence enzyme immunoassay and the analysis was performed using the Phadia 250 (Thermo Fisher Scientific, Waltham, MA, USA; www.thermofisher.com); and the QUANTA Flash Calprotectin (Inova Diagnostics, San Diego, CA, USA; www.inovadiag.com) a chemiluminescent immunoassay and the analysis was performed
Cont’d on page 20119 LMI-3-23 LINKXPRESS COM



19 LabMedica International February-March/2023 LabMedica International To view this issue in interactive digital magazine format visit www.LabMedica.com
Size: 2400 x 1200 mm (3 mm thick) 100% Silicone SOURCE
FOR STERILIZATION ACCESSORIES
STERILIZABLE INSTRUMENT & WORK-SURFACE MATS Front Back WASHING TRAYS MAT Heavy Silicone Cover & Transport Tablet TURBO WASHING MACHINES TRAYS SILICON INSTRUMENT MAT Front Back MICRO INSTRUMENT MAT Exchangable Net Exchangable Nets INVITEDTOAPPLY DISTRIBUTORS Up to 37 cm in length THERMO RESISTANT GLOVES Front Back WASHING TRAYS MAT NEW! VICOTEX Place de la Gare 1 • 1009 Pully • Switzerland Tel: (41) 21-728-4286 • Fax: (41) 21-729-6741 E-Mail: contact@vicotex.com www.vicolab.com S.A. SILICONE TABLET AND STEEL COVER NETS
Thermo-Resistant (- 60 °C to 300 °C) Fully Washable & Flexible Suitable for central sterilization services Sterilizable
findings, the researchers concluded that routine tests conducted in the hours following a heart attack could more quickly identify patients facing the highest risk and prioritize them for treatment.
Cont’d from page 18
DNA Nanobait Test for Respiratory Viruses
chain reaction) tests are highly specific and accurate, but are capable of testing for only a single virus at a time and return results after several hours. Now, a new test uses single strands of DNA as ‘bait’ to ‘fish’ for multiple respiratory viruses at once and delivers accurate results in less than an hour.
The new test developed by researchers at University of Cambridge (Cambridge, UK) uses DNA ‘nanobait’ to detect common respiratory viruses – including influenza, rhinovirus, RSV and COVID-19 – simultaneously. Many of the common respiratory viruses have similar symptoms, but need different treatments. The new approach tests for multiple viruses at once, allowing the right treatment to be administered quickly to the patients and minimizing the unwarranted use of antibiotics. Additionally, it is possible for healthcare workers to use the test in any setting, and it can also be easily modified to detect different bacteria and viruses, including future SARS-CoV-2 variants.
While PCR tests are powerful, sensitive and accurate, they require a piece of genome to be copied millions of times – a process that can take several hours. The researchers set
out to develop a test that uses RNA to detect viruses directly without the need for copying the genome, but had sufficiently high sensitivity for use in a healthcare setting. The team developed the test based on structures built from double strands of DNA with overhanging single strands. These single strands act as the ‘bait’ meaning they are programmed to ‘fish’ for specific regions in the RNA of target viruses. The nanobaits are then passed through very tiny holes called nanopores. Nanopore sensing is similar to a ticker tape reader that transforms molecular structures into digital information within milliseconds. Each nanobait’s structure reveals the target virus or its variant. The researchers demonstrated that it is possible to easily reprogram the test to allow it to discriminate between viral variants, including SARS-CoV-2 variants. The precision of the programmable nanobait structures allows the approach to offer almost 100% specificity.
“Good diagnostics are the key to good treatments,” said Filip Boškovic from Cambridge’s Cavendish Laboratory. “People show up at hospital in need of treatment and they might be carrying multiple different viruses, but unless you can discriminate between different viruses, there is a
risk patients could receive incorrect treatment.”
“For patients, we know that rapid diagnosis improves their outcome, so being able to detect the infectious agent quickly could save their life,” said co-author Prof. Stephen Baker, from the Cambridge Institute of Therapeutic Immunology and Infectious Disease. “For healthcare workers, such a test could be used anywhere, in the UK or in any low- or middle-income setting, which helps ensure patients get the correct treatment quickly and reduce the use of unwarranted antibiotics.”


Impact of Preanalytical Factors on Calprotectin Concentration in Stool
Cont’d from 19
on BIO FLASH (Werfen, Bedford, MA, USA; www.werfen.com).



The investigators reported that Calprotectin concentrations declined over time under all pre-analytical conditions with all assays, except for extracted feces stored at 4 °C. The rate of decline was greatest in untreated stool kept at room temperature, reaching significant difference from baseline already after one day. In extracted
feces kept at room temperature, significant difference from baseline was reached after two days, and in untreated feces at 4 °C, after four days. However, the results differed significantly between assays. After four days of storage at room temperature, the mean calprotectin decline from baseline differed between 30% and 60%, dependent on the assay used. In most, but not for all samples, the CALiaGold assay produced the highest calprotectin levels, and the QUANTA flash
assay, the lowest calprotectin levels.
The authors concluded that fecal calprotectin concentration in stool samples declines over time, and the rate of decline is greater at higher temperatures. In extracted feces stored at 4 °C, calprotectin is the most stable. It is assay-dependent how long extracted feces stored at 4 °C give reliable test results. The study was published in the November 2022 issue of The Journal of Applied Laboratory Medicine.

20 LabMedica International February-March/2023
PIPETTE CONTROLLER
223 LMI-3-23 COM
The Accumax Pipette Controller offers exceptional performance through unique features like the double safety valves which provide added protection against fluid penetration, dual speed modes, etc.
224 LMI-3-23 LINKXPRESS COM
RUBELLA VIRUS IGG IMMUNOASSAY AESKU.DIAGNOSTICS
The AESKULISA Rubella Virus IgG is a qualitative and quantitative immunoassay for the demonstration of human IgG antibodies in serum or plasma directed against Rubella Virus.
Image: A new test uses DNA ‘nanobait’ to detect common respiratory viruses (Photo courtesy of Pexels)
Cont’d from cover
To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
Edited by Katherina Psarra MSc, PhD
MESSAGE FROM THE PRESIDENT
 By Khosrow Adeli • President, IFCC
By Khosrow Adeli • President, IFCC

HappyNew Year to everyone in the IFCC family! We have an exciting year ahead, full of new priorities to further our mission of “advancing excellence in laboratory medicine for better healthcare worldwide” as well as the IFCC major scientific event, the XXV IFCC-EFLM WORLDLAB-EUROMEDLAB ROME 2023.
As our first new priority this year, IFCC will focus on breaking silos by enhancing collaboration with all major bodies involved in laboratory medicine and in vitro diagnostics. We have already started discussions with several organizations to build stronger relationships that will ultimately foster innovation and minimize overlap/duplication in scientific and educational activities. New memorandums of understanding (MOU) are being signed with EQALM, BCLF, WASPALM, and FIFBCLM.
Another key priority is promoting the web presence of the IFCC organization and its affiliates, which will increase the visibility of IFCC within the clinical chemistry and laboratory medicine community as well as outside the field in the broader medical and healthcare community. Work involved in this priority includes development of a new IFCC website with a modernized look and feel as well as improved navigation and functionality, which is already underway. Other improvements are also in the works, such as an update of the IFCC eAcademy website, redevelopment of the eNews and eJournal, and creation of new databases. The new IFCC website is almost ready and will be officially launched shortly.
Third, IFCC will be developing and disseminating evidence based IFCC Clinical Laboratory Practice Guidelines to support clinical laboratories around the world. These guidelines will provide practical recommendations to laboratory professionals based on evidence from a wide range of existing reputable guidelines, peer-reviewed publications, and expert consensus. All guidelines will provide specific implementation resources to ensure utility in clinical practice. Given that IFCC is home to leading experts in laboratory medicine, we will call upon our Committees, Task Forces, and Working Groups to help develop and disseminate these guidelines. A special meeting of the IFCC Executive Board with chairs of all divisions, taskforces and working groups is planned in early March to develop a roadmap for this important new program.
The fourth priority is ensuring accreditation for all IFCC educational programs including all IFCC Live Webinars and regional/international conferences. Continuing Education (CE) credits will be provided for the upcoming EuroMedLab/WorldLab 2023 with the support of EFLM and plans are underway to ensure accreditation of all future IFCC conferences.
On that note, we have a very exciting event planned later this year. The IFCC-EFLM EuroMedLab/WorldLab 2023 Congress will take place from May 21–25 in the historic and beautiful city of Rome. An excellent scientific program is planned for this biannual congress, filled with innovative and diverse education opportunities, including lectures, symposia, recent advancements in clinical practice and science, poster presentations, and industry exhibits. There will also be many social and networking opportunities, as this leading forum brings together scientists, lab-

oratory specialists, clinicians, industry colleagues, and other experts from around the world. In fact, we have already received over 2000 abstract submissions, indicating this is set out to be one of the most widely attended congresses in the IFCC history.
Ahead of EuroMedLab 2023, we have several important satellite meetings planned on May 20 and Mary 21 as well as a special IFCC Young Scientists Forum on Sunday May 21st. Stay tuned for more information on both the main congress as well as the satellite meetings and special forum.
As always, please feel free to email me at president@ifcc. org with any feedback, questions, or concerns you may have.
Cheers, Khosrow
www.2023roma.org
21 LabMedica International February-March/2023 NEWS
IFCC members may send news to: Email: enews@ifcc.org
EDITORIAL
By Katherina Psarra, MSc, PhD

Dear Colleagues,
I hope that among your decisions for the New Year is that you will read a lot more, a lot more books in fact. Because the first issue of the eNews for 2023 is a book. I invite you to read it.
First in the President Prof Khosrow Adeli’s message you will learn a lot about the ambitious and interesting IFCC plan. And then if you go through the articles, as I did, you will learn so much about AI in general, AI from the beginning till now, and in particular its applications in Laboratory
Medicine. You will find out that there is a software that can write even papers instead of you!!!
You will learn about evidence-based laboratory medicine as well and you will meet more wonderful scientific groups, UNIVANTS winners.
A lot of information about how several countries are dealing with the refugee crisis regarding their health issues can be found in this issue. The role of Laboratory is stressed in this important matter, concerning human lives.
It is really like reading a book, a very interesting book where our job plays a fundamental role.
ChatGPT – Timely Tool or Terrifying Threat?
By Jason Park, Paolo Fortina,
Kricka;
Avery recent and highly controversial development in Artificial Intelligence (AI) that has far reaching implications is ChatGPT from OpenAI (openai.com) (https://research.aimultiple.com/chatgpt/). Recently, the Working Group for Artificial Intelligence and Genomic Diagnostics (WG-AIGD) used this human-like text generator to prepare essay-format text for the WG-AIGD web page.
Firstly, to define ChatGPT we asked ChatGPT the simple question - “What is ChatGPT?” In a matter of seconds, the app responded with the following answer:

“ChatGPT is a chatbot based on the GPT-3 language model, developed by OpenAI. GPT-3 (short for “Generative Pre-training Transformer 3”) is a stateof-the-art natural language processing (NLP) model that has been trained on a vast amount of data and can generate
human-like text when given a prompt. ChatGPT is designed to be able to hold a conversation with a human user in real-time, responding to user input with appropriate and coherent responses.
To use ChatGPT, a user can type a message into a chat window and ChatGPT will generate a response based on the message it was given. The user can then continue the conversation by typing a follow-up message, and ChatGPT will generate a response to that as well. This process can continue for multiple turns, allowing for a back-and-forth conversation between the user and the chatbot.
ChatGPT is an example of how AI and NLP technology can be used to build chatbots that are able to hold natural and engaging conversations with humans. These chatbots can be used in a variety
János Kappelmayer, Editor-in-Chief, eJIFCC, and Harjit Pal Bhattoa, Assistant Editor, eJIFCC are glad to announce that the eJIFCC is adopting “Editorial Manager” for manuscript submission.
In the past years, the eJIFCC has become increasingly visible among laboratory specialists worldwide. This, on the one hand resulted in the increase of the number of downloads of papers published since the eJIFCC is a platinum open access publication, and on the other hand, it resulted in a considerable increase in the number of submissions. We detailed the progress in the advancement of the scientometric parameters of the journal in

of applications, such as customer service, online tutoring, and entertainment.”
Next, we asked ChatGPT a question relevant to the topic of our Working Group: “What are the applications of AI in genomic diagnostics?” Again, within seconds, back came the following summary of the specific application area of interest to our WG:
“Artificial intelligence (AI) has many potential applications in genomic diagnostics, which involve the use of genomic information to identify genetic conditions and guide treatment decisions. Some examples of how AI might be used in this field include:
Analyzing large amounts of genomic data: AI algorithms can be used to analyze large amounts of genomic data quickly and accurately, helping to identify genetic variations and patterns that
Cont’d on page 24
a review in the eJIFCC in Issue 4 of 2021. In order to cope with the increase in submissions we decided to advance our manuscript handling process, and recently subscribed for the Editorial Manager (EM) manuscript handling system. Editorial Manager is long-established and one of the best-known editorial submission and manuscript processing programs. It belongs to Aries, which was founded in 1986 and is headquartered in North Andover, MA
The introduction of Editorial Manager, along with the use of Ithenticate, the most trusted plagiarism checker by the world’s top researchers, publishers, and scholars, will increase eJIFCC reputation among the Scientific journals.
22 LabMedica International February-March/2023 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
The eJIFCC Adopts Editorial Manager (EM) System for Manuscripts
Larry
IFCC ETD Artificial Intelligence in Genomic Diagnostics Working Group (WG-AIGD)
News from the IFCC Working Group for Artificial Intelligence and Genomic Diagnostics (WG-AIGD)
Two Best Practices for Cardiac Health Excellence Recognized in Celebration of USA Heart Month


February is American heart month, an opportunity to celebrate and focus on heart health. It is with pleasure that we consequently recognize two integrated clinical care teams who have and continue to make monumental efforts in improved patient outcomes for one of the deadliest diseases of our time: cardiovascular disease.
At Medcan Health Management Inc in Ontario, Canada, an integrated care team has been focused on preventative cardiovascular care for the past 25 years, through their comprehensive annual health assessments (AHA). This assessment has previously included a stress test, lipid analysis and Framingham risk score, however, stress testing was not possible during COVID-19. Thus, this AHA evolved with time and now includes high sensitivity cardiac troponin I (hs-cTnI) testing, in place of stress testing. This new approach enabled enhanced capabilities for identifying patients at future risk for cardiovascular disease, while also improving patient experiences. Clients undergoing the annual evaluation were saving 38 minutes relative to previous years, whereas physicians also maximized their time, freeing up 45 additional minutes per day due to associated AHA efficiencies. A reduction in false positivity was also realized, saving $284,000 CDN per year for Medcan and $357,500 CDN for the Canadian healthcare System. To date, over 48 seemingly healthy individuals who underwent the AHA were identified as requiring urgent medical care, triggering a cascade of events that protecting each with life-saving measures.



Just south of the border, at Prisma Health Greenville Memorial Hospital in Greenville, South Carolina, a team led by Dr. Jason Guichard began revolutionizing their care delivery for patients with heart failure. Recognizing that the burden of heart failure is increasing, while also putting a strain on already taxed healthcare systems, this team sought to improve acute and post-acute patient management pathways to improve outcomes and reduce readmissions. This team focused on identifying patients with heart failure earlier to enable improved access to care and more efficient use of hospital resources. To achieve this, they used the electronic medical records and IT systems to identify patients at increased risk for hospitalization and decompensation, and subsequently enhanced their care coordination to improve their overall outcomes. This change in patient identification and management enabled a 48% increase in the number

23 LabMedica International February-March/2023 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
ONE EXPO YOU WON’T WANT TO MISS! For 75 years, professionals from a diverse range of specialties have gathered to discover the latest product innovations, emerging technologies and research. Whether you work in the lab or with lab solutions, this is ONE EXPO you won’t want to miss! Join us in Anaheim, CA, July 25-27, 2023 to experience: Register today for just $25. Scan the code with your smartphone or visit meeting.aacc.org/expo JULY 23-27 I ANAHEIM, CA Image: Human DNA Sequence for the Human Genome Project. James King-Holmes/Science Photo Library • 800+ exhibitors, 200+ product categories and live demos • Product showcase with interactive digital exhibits • Lecture Series Presentations and Industry Workshops exploring diagnostic technologies and breakthroughs • Networking opportunities to connect with industry peers and leaders • Disruptive Technology Award Semifinalists representing this year’s stand out startups • And so much more! GLOBAL LAB MEDICINE COMMUNITY ONE WE ARE The views and positions expressed in the IFCC News section are those of the IFCC or the individual authors, and do not necessarily represent the views or positions of LabMedica magazine or its publishers. IFCC OFFICE Via Carlo Farini 81, 20159 Milan, ITALY Tel: (39) 02-6680-9912 E-mail: ifcc@ifcc.org • Web: www.ifcc.org Staff Members: Paola Bramati, Silvia Cardinale, Silvia Colli-Lanzi,
Skenderaj
Smeralda
Cont’d on page 24
Picture (from left to right): Beth Wehlitz, Beverly Jameson, Jason Guichard, Stephanie Flippin, Sandi Stoudenmire
Two Best Practices for Cardiac Health Excellence Recognized in Celebration of USA Heart Month
Cont’d from page 23
of heart failure patients who were at high risk for decompensation to actively receiving care through an advanced heart failure specialist. Their efforts corresponded to a 12% improvement in mortality for all patients in their system, while also enabling a 67% improvement in heart failure clinic volume and subsequent 59% reduction in emergency and urgent care visits for patients with heart failure.
Both of these impressive integrated care teams utilized insights associated with laboratory medicine to provide proactive and preventative care to patients to enable improved patient outcomes, healthcare system efficiencies and saved healthcare dollars. For their efforts and outcomes, both were recognized by the 2021 UNIVANTS of Healthcare Excellence award program. Congratulations to both teams!
For more information, please visit www.UnivantsHCE.com
News From the WG-AIGD: ChatGPT – Timely Tool or Terrifying Threat?

may be relevant to a particular condition or disease.
Predicting disease risk: AI can be used to predict an individual’s risk of developing a particular disease based on their genomic information, allowing for earlier diagnosis and treatment.
Personalized medicine: AI can be used to tailor treatment plans to an individual’s specific genomic profile, helping to optimize treatment effectiveness and minimize side effects.
Drug discovery: AI can be used to identify potential new drugs and evaluate their effectiveness based on an individual’s genomic information, helping to speed up the drug development process.

Clinical decision support: AI can be used to provide clinicians with recommendations for diagnosis and treatment based on an individual’s genomic information, helping to improve the accuracy and efficiency of healthcare delivery.”
ChatGPT-3 has 175 billion parameters and was trained on 570 gigabytes of text and 300 billion words (https://analyticsindiamag.com/behind-chatgpts-wisdom-300-bn-words570-gb-data/). It is not connected to the internet, and has limited information post-2021 (https://help.openai.com/en/ articles/6783457-chatgpt-faq), so we are only seeing the very beginning of the utility and capabilities of this AI-based technology (https://www.nytimes.com/2022/12/05/technology/chatgpt-ai-twitter.html, https://www.medrxiv.org/content/1
0.1101/2022.12.19.22283643v1.full.pdf, https://www.nature. com/articles/d41586-022-04437-2, https://www.medrxiv.org/ content/10.1101/2022.12.16.22283512v2).
Already, at least four articles have been published that list ChatGPT as an author, and this has raised the question “Is it appropriate to cite software as an author?”.Early indications are that scientific journals will not allow bots to be authors and AI-generated text without proper citation could be considered plagiarism (https://www.nature.com/articles/ d41586-023-00107-z). An important tool has been the recently developed app, GPTZero, designed to detect Chatbot generated text (https://www.analyticsinsight.net/gptzero-anapp-to-detect-whether-text-is-written-by-chatgpt/, https://etedward-gptzero-main-zqgfwb.streamlit.app/).
An indication of the impact of ChatGPT is that “it was released in November 2022 and hit more than one million users within a week (https://www.demandsage.com/chatgpt-statistics/).
ChatGPT is one of many chatbots powered by AI. Earlier demonstrations of chatbots have been made by companies such as Google. The compelling aspect of ChatGPT is the ease of use; it is a consumer product that has immediate applications and no learning curve for use. We are likely to see a wave of companies emerge that create chatbots and uses for chatbots. Immediately, chatbots are being used by students to write essays and scientists to write research manuscripts. In the very near future we are likely to see chatbots replace our current modern habits of using search engines for quick access to information. We can now see a future where we no longer have to ‘Google’ information and find and read websites. Instead, we will query a chatbot by written or verbal interface and receive a direct answer to our question.
Like many foundational technologies, chatbots will present unique challenges to society. In the context of this present report, what is the value of academic writing and journals if the information is synthesized without human intervention? More fundamentally, how will we as a society and as a profession teach writing and scientific inquiry with the rapid and easy access to automated responses?

24 LabMedica International February-March/2023 News from the World of the International Federation of Clinical Chemistry and Laboratory Medicine Visit www.ifcc.org for more information NEWS
Picture (from left to right): Peter Baxter, Neil Mahon, Shaun Francis, Yogini Walli, Peter Nord
from page 22
Cont’d
Next-Generation Sequencing (NGS) Market Driven by Launch of Technologically Advanced Systems
ext generation sequencing (NGS) is a massively parallel sequencing technology that offers scalability, ultra-high throughput, and high speed to determine the order of nucleotides in the entire genome. DNA pre-sequencing is among the most significant steps in the overall sequencing protocol as it involves preparation of sample for the subsequent sequencing reaction. NGS is being gradually integrated into clinical laboratory analysis, testing, and diseases diagnostics in healthcare sectors across the world. The global NGS market is projected to grow at a CAGR of 15.7% from USD 13 billion in 2022 to USD 27 billion by 2027, driven by the launch of technologically advanced NGS systems and improving regulatory and reimbursement scenarios for NGS-based diagnostic tests. However, the end-user budget constraints in developing countries are expected to restrain market growth.
These are the latest findings of Markets and Markets (Northbrook, IL, USA; www.marketsandmarkets.com), a growth consulting and program management firm.
Based on product & service, the consumables segment dominated the global NGS market in 2021 with the largest share due to the launch of new NGS consumables and demand for reagents and kits. Based on technology, the sequencing by synthesis segment accounted for the largest share of the global NGS market in 2021 due to the launch of new NGS platforms based on sequencing by synthesis technology. Based on application, the diagnostics segment accounted for the largest share of the global NGS market in 2021 due to the increasing demand

Thermo Fisher Completes Acquisition of UK’s Binding Site
Cont’d from cover
Serving clinicians and laboratory professionals worldwide, The Binding Site provides specialty diagnostic assays and instruments to improve the diagnosis and management of blood cancers and immune system disorders. The Binding Site’s Freelite offering is widely recommended for multiple myeloma diagnosis and monitoring across all stages of the disease by major clinical guideline publications. The Binding Site has more than 1,200 employees globally and will become part of Thermo Fisher’s Specialty Diagnostics segment.
“We are very excited to welcome The Binding Site colleagues to Thermo Fisher Scientific,” said Marc N. Casper, chairman, president and chief executive officer of Thermo Fisher. “The Binding Site expands our existing specialty diagnostics portfolio with the addition of pioneering innovation in diagnostics and monitoring for multiple myeloma. Early diagnosis and well-informed treatment decisions can make a significant difference in patient outcomes, and we are excited by the opportunity to enable further advancements in this area for the benefit of patients.”
for NGS based diagnostic products for cancer and noninvasive prenatal testing (NIPT).
Based on end users, the academic institutes & research centers segment dominated the global NGS market in 2021 with the largest share due to a growing number of collaborations between NGS market players and academic & research institutes. Geographically, North America accounted for the largest share of the global NGS market in 2021, driven by the increasing demand for new NGS based diagnostic tools, availability of research funding, and presence of prominent market players in the region.
Chembio and Biosynex to Merge, Forging US-French Point-of-Care Testing Powerhouse
Biosynex SA (Alsace, France; www.biosynex.com), a company specializing in the design and distribution of rapid tests, and Chembio Diagnostics, Inc. (Hauppauge, NY, USA; www.chembio.com), a leading point-of-care diagnostics company focused on infectious diseases, have entered into a definitive merger agreement under which Biosynex, through a subsidiary, will acquire Chembio in an all-cash transaction valued at USD 17.2 million.
The acquisition combines two leading rapid diagnostic test companies that specialize in the development, manufacturing and marketing of point-of-care diagnostic tests for the professional and at home markets. Chembio focuses on infectious disease assays covering sexually transmitted infections, respiratory viruses and fever and tropical disease, built on the DPP, SURE CHECK and STAT-PAK proprietary, accurate and easy-to-use technology platforms. Biosynex provides pharmacies and professional healthcare settings with a diversified portfolio of rapid tests covering different market segments, including infectious disease and women’s health tests, point of care devices and molecular diagnostics systems.
DiaSorin Subsidiary to Divest Flow Cytometry Business to Cell Analysis Leader
DiaSorin S.p.A. (Saluggia, Italy; www.diasorin.com) and Cytek Biosciences, Inc. (Fremont, CA, USA; cytekbio.com) have announced that Luminex Corporation (Austin, TX, USA; cytekbio. com), a wholly owned subsidiary of DiaSorin, has signed an agreement with Cytek to sell substantially all of its assets related to the Flow Cytometry & Imaging (FCI) business unit.
The FCI business unit, acquired by Luminex in October 2018, is
based on both conventional flow cytometry and image-based flow cytometry instrumentation, which provide insights into all facets of cellular phenotypes and morphology. The FCI business unit includes dedicated commercial, operations, R&D, and supporting personnel. Following the acquisition, the Cytek umbrella will have an existing installed base of over 7,000 instruments, expanding its global commercial footprint.
25 LabMedica International February-March/2023 Industry News To view this issue in interactive digital magazine format visit www.LabMedica.com
N
For a free listing of your event or a paid advertisement contact: LabMedica International Calendar E-mail: info@globetech.net
2023 MARCH
CHINA LAB 2023. Mar 9-11; Guangzhou, China; chinalabexpo.com
USCAP 112th Annual Meeting - United States & Canadian Academy of Pathology. Mar 11-16; New Orleans, LA, USA; uscap.org
ExpoMED Eurasia 2023. Mar 16-18; Istanbul, Turkey; expomedistanbul.com
23èmes Journées Marocaines de Biologie Clinique. Mar 16-18; Marrakech, Morocco; smccbm.org.ma
Pittcon 2023. Mar 18-22; Philadelphia, PA, USA; pittcon.org
APRIL
MSACL 2023 – Congress of the Association for Mass Spectrometry & Advances in Clinical Lab. Apr 3-6; Monterey, CA, USA; msacl.org
AACR 2023 – Annual Meeting of the American Association for Cancer Research. Apr 14-19; Orlando, FL, USA; www.aacr.org
ECCMID 2023 – 33rd European Congress of Clinical Microbiology and Infectious Diseases. Apr 15-18; Copenhagen, Denmark; eccmid.org

Korea Lab 2023. Apr 18-21; Seoul, Korea; korealab.org
Analytica Vietnam 2023. Apr 19-21; Ho Chi Minh City, Vietnam; analyticavietnam.com
India Lab Expo & Analytica Anacon India. Apr 27-28; Mumbai, India; analyticaindia.com
ExpoLab 2023 – 23rd Mexican National Congress of Clinical Chemistry and Laboratory Medicine. Apr 28-30; Veracruz, Mexico; fenacqc.org.mx
MAY
AACE Annual Meeting 2023 – American Association of Clinical Endocrinology. May 4-6; Seattle, WA, USA; pro.aace.com
ECV 2023 – 8th European Congress of Virology. May 4-7; Gdansk, Poland; eusv-congress.eu
ESPID 2023 – 41st Annual Meeting of the European Society for Paediatric Infectious Disease. May 8-12; Lisbon, Portugal; espidmeeting.org
ISLH 2023 – International Society for Laboratory Hematology. May 11-13; New Orleans, LA, USA; islh.org
Immunology 2023 – Annual Meeting of the American Association of Immunologists (AAI). May 11-15; Washington, DC, USA; immunology2023.org
14th Uruguayan Congress of Clinical Biochemistry. May 11-13; Montevideo, Uruguay; congresoabu2023.org
ECE 2023 – 25th European Congress of Endocrinology. May 13-16; Istanbul, Turkey; ese-hormones.org
QICL 2023 - 14th International & 20th National Congress on Quality Improvement in Clinical Laboratories. May 16-19; Tehran, Iran; iqctehran.ir
IFCC-EFLM WorldLab & EuroMedLab 2023 - 25th International Congress of Clinical Chemistry and Laboratory Medicine. May 21-25; Rome, Italy; 2023roma.org
SLAS Europe 2023 Conference and Exhibition. May 22-26; Brussels, Belgium; slas.org/europe2023
Hospitalar 2023. May 23-26; Sao Paulo, Brazil; hospitalar.com
JUNE
106th Annual Meeting of the German Society for Pathology. Jun 1-3; Leipzig, Germany; pathologie-dgp.de
ASCO 2023 – Annual Meeting of the American Society of Clinical Oncology. Jun 2-6; Chicago, IL, USA; conferences.asco.org
66th Congress of the German Society for Endocrinology. Jun 5-7; BadenBaden, Germany; endokrinologie.net
EHA 2023 - Annual Congress of the European Hematology Association. Jun 8-11; Frankfurt, Germany; ehaweb.org
EAACI 2023 – Annual Congress of the European Academy of Allergy & Clinical Immunology. Jun 9-11; Hamburg, Germany; eaaci.org
ESHG 2023 – European Human Genetics Conference. Jun 10-13; Glasgow, UK; 2023.eshg.org
ENDO 2023 – Annual Meeting of the Endocrine Society. Jun 15-18; Chicago, IL, USA; endocrine.org
ASM Microbe 2023 – American Society for Microbiology. Jun 15-19; Houston, TX, USA; asm.org
33rd Regional Congress of the International Society of Blood Transfusion (ISBT). Jun 17-21; Gothenburg, Sweden; isbtweb.org
48th CBAC – Congress of the Brazilian Society of Clinical Analysis. Jun 18-21; Florianopolis, Brazil; sbac.org.br/cbac
FOCIS 2023 – Annual Meeting of the Federation of Clinical Immunology Societies. Jun 20-23; Boston, MA, USA; focisnet.org
FIME 2023 – Florida International Medical Expo. Jun 21-23; Miami, FL, USA; fimeshow.com
83rd Scientific Sessions of the American Diabetes Association. Jun 23-26; San Diego, CA, USA; professional.diabetes.org
ESHRE 2023 – 39th Meeting of the European Society of Human Reproduction and Embryology. Jun 25-28; Copenhagen, Denmark; eshre.eu
JULY
Analytica Lab Africa 2023. Jul 5-7; Johannesburg, South Africa; analyticaafrica.com
APCCMI 2023 – 19th Asia Pacific Congress of Clinical Microbiology and Infection. Jul 6-8; Seoul, Korea; apccmi2023.com
FEMS 2023 - 10th Congress of European Microbiologists. Jul 9-13; Hamburg, Germany; fems2023.org
Analytica China. Jul 11-13; Shanghai, China; analyticachina.com
2023 AACC Annual Scientific Meeting & Clinical Lab Expo. Jul 23-27; Anaheim, CA, USA; meeting.aacc.org
AUGUST
MedLab Asia 2023. Aug 16-18; Bangkok, Thailand; medlabasia.com
SEPTEMBER
Thailand LAB International 2023. Sep 6-8; Bangkok, Thailand; thailandlab.com
ECP 2023 - 35th Congress of the European Society of Pathology. Sep 9-13; Dublin, Ireland; esp-congress.org
EUROTOX 2023 – 57th Congress of the European Societies of Toxicology. Sep 10-13; Ljubljana, Slovenia; eurotox2023.com
India Lab Expo & Analytica Anacon India. Sep 14-16; Hyderabad, India; analyticaindia.com
Arab Lab 2023. Sep 19-21; Dubai, UAE; arablab.com
ESPE 2023 – 61st Annual Meeting of the European Society for Paediatric Endocrinology. Sep 21-23; The Hague Netherlands; eurospe.org
Analitica Latin America 2023. Sep 26-28; Sao Paulo, Brazil; analiticanet.com.br
92nd Annual Meeting of the American Thyroid Association (ATA). Oct 29 - Sep 1; Washington, DC, USA; www.thyroid.org
OCTOBER
ECC 2023 – 44th European Congress of Cytology. Oct 1-4; Budapest, Hungary; cytology2023.eu
EASD 2023 – 59th Annual Meeting of the European Association for the Study of Diabetes. Oct 3-6; Hamburg, Germany; easd.org
CAP23 – Annual Meeting of the College of American Pathologists. Chicago, IL, USA; cap.org
JFBM 2023 - Journées Francophones de Biologie Médicale. Oct 11-13; France; jfbm.fr
85th Annual Meeting of the Japanese Society of Hematology. Oct 13-15; Tokyo, Japan; www.jshem.org.jp
60th Annual Scientific Conference of the Australasian Association Clinical Biochemistry and Laboratory Medicine (AACB). Oct 16-19; Brisbane, Australia; aacb.asn.au
ASHI 2023 – 49th Annual Meeting of the American Society for Histocompatibility and Immunogenetics. Oct 16-20; San Antonio, TX, USA; ashi-hla.org
ASCP 2023 – Annual Meeting of the American Society for Clinical Pathology. Oct 18-20; Long Beach, CA, USA; ascp.org
LABCLIN 2023 – 17th National Congress of the Spanish Societies for Clinical Laboratory (AEBM-ML, AEFA & SEQCML). Oct 18-20; Zaragoza, Spain; seqc.es EndoBridge 2023. Oct 19-22; Antalya, Turkey; endobridge.org MedLab Africa 2023. Oct 25-27; Johannesburg, South Africa; africahealthexhibition.com
63rd Annual Academic Assembly of the Japan Society of Clinical Chemistry (JSCC). Oct 27-29; Toyama, Japan; jscc-jp.gr.jp
NOVEMBER
ASHG 2023 – Annual Meeting of the American Society of Human Gene Nov 1-5; Washington, DC, USA; ashg.org
MEDICA 2023. Nov 13-16; Dusseldorf, Germany; medica-tradefair.com
44th Annual Meeting of the American College of Toxicology (ACT). 15; Orlando, FL, USA; www.actox.org
AMP 2023–Annual Meeting & Expo of the Association for Molecular Pathology. Nov 16-18; Salt Lake City, UT, USA; amp.org
71st Annual Scientific Meeting of the American Society of Cytopath (ASC). Nov 16-19; Austin, TX, USA; cytopathology.org
IUIS 2023 – International Union of Immunological Societies. Nov 27 - Dec 2; Cape Town, South Africa; iuis2023.org
DECEMBER
65th Annual Meeting & Exposition of the American Society of Hematology (ASH). Dec 9-12; San Diego, CA, USA; hematology.org
2024
FEBRUARY
SLAS 2024 – International Conference & Exhibition of the Society Laboratory Automation and Screening. Feb 3-7; Boston, MA, USA; slas.org Medlab Middle East 2024. Feb 5-8; Dubai, UAE; medlabme.com
Labquality Days 2024 – International Congress on Quality in Labo Medicine. Feb 8-9; Helsinki, Finland; labqualitydays.fi
Events Calendar
Provided as a service to advertisers. Publisher cannot accept responsibility for any errors or omissions. Advertising Index Inq.No. Advertiser Page Inq.No. Advertiser Page Vol. 40 No. 1 3/2023 LabMedica International – AACC .............................. 23 – APCCMI 10 107 DiaSys Diagnostic Systems 7 – EuroMedLab 2023 ................... 21 – European Congress of Pathology 17 – FEMS 2023 ......................... 15 111 Goldsite Diagnostics .................. 11 – LabMedica EXPO 27 116 Mast Group ......................... 16 105 Nova Biomedical ...................... 5 113 Puritan 13 102 Randox .............................. 2 103 Snibe 3 119 Vicotex 19 128 Werfen ............................. 28 109 West Medica 9 ATTENTION: IF YOUR APPLICATION IS NOT RECEIVED AT LEAST ONCE EVERY 12 MONTHS YOUR FREE SUBSCRIPTION MAY BE AUTOMATICALLY DISCONTINUED READER SERVICE PORTAL LINKXPRESS COM VISIT Every advertisement or product item contains a LinkXpress number as below: 999 LMI-03-23 LINKXPRESS COM Identify LinkXpress codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress inquiry matrix 1 2 3 Renew/ S tart Your Free Subscription Access Instant Online Product Information
Send inquiries directly to suppliers... Receive latest product alerts... Chat live with suppliers, and more...

LabMedica EXPO provides a sophisticated yet easy-to-use global B2B platform for sourcing clinical laboratory equipment. LabMedica EXPO connects buyers and sellers worldwide through a safe, secure, and dynamic network, regardless of size or budget.



WORLD’S CLINICAL DIAGNOSTICS MARKETPLACE
The Intelligent Analyzer
GEM Premier 5000 blood gas testing system provides automated quality assurance with every whole-blood* sample. Now with next-generation Intelligent Quality Management (iQM2), featuring IntraSpect™ technology, potential errors are detected not only before and after, but also during sample analysis, along with real-time correction and documentation. Plus, it’s simple—just change the all-in-one GEM PAK once a month. So regardless of testing location or point-of-care operator, quality results and compliance are assured with every sample.
Real-time
assurance and advanced
simplicity. Now that’s intelligent.
For more information, contact your local Werfen representative.
werfen.com
*Heparinized.
128 LMI-09-22 LINKXPRESS COM
Acute Care
Diagnostics
GEM, Premier and iQM are trademarks of Instrumentation Laboratory Company and/or one of its subsidiaries or parent companies and may be registered in the United States Patent and Trademark Office and in other jurisdictions. The Werfen logo is a trademark of Werfen and may be registered in the Patent and Trademark Offices of jurisdictions throughout the world. ©2021 Instrumentation Laboratory. All rights reserved. 128 LMI-3-23 LINKXPRESS COM












































































































 By Khosrow Adeli • President, IFCC
By Khosrow Adeli • President, IFCC

















