
Novel Imaging Technique Could Revolutionize Pancreatic Cancer Surgery
Novel Imaging Technique Could Revolutionize Pancreatic Cancer Surgery

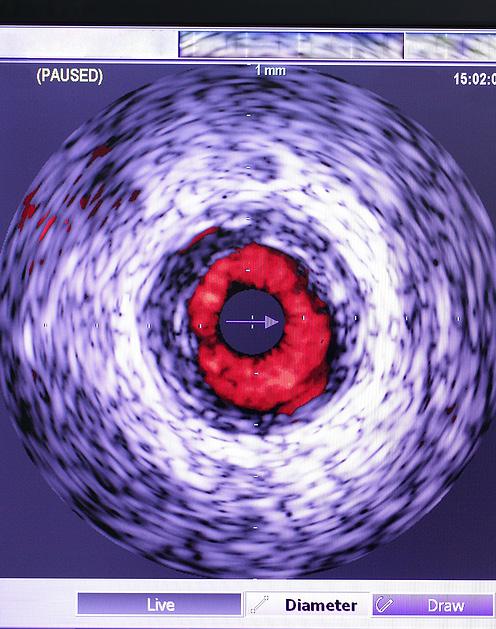
IVUS Improves Outcomes in Cardiovascular Stenting
Introduced over three decades ago, intravascular ultrasound (IVUS) offers a more precise and detailed view of the coronary arteries. Studies have demonstrated that IVUS-guided percutaneous coronary intervention (PCI) is more effective than angiography-guided PCI in reCont’d on page 4

TInternational Hospital Federation Announces 2023 IHF Award Winners
he International Hospital Federation (IHF; Geneva, Switzerland; www.ihf-fih.org) held its annual Award Ceremony and Gala Dinner on October 26, 2023, to celebrate excellence in hospital and healthcare leadership. The Awards Ceremony was the highlight of
the 46th World Hospital Congress, which saw 1500 attendees from over 80 countries and territories. The ceremony was held in Lisbon (Portugal) at the Lisbon Congress Centre in front of an audience comprising 500 international hospitals and healthcare executives.
Tuberculosis (TB) is a serious bacterial disease that primarily affects the lungs and can be deadly if left untreated. It spreads through the air when people with TB expel bacteria via droplets. While many individuals who contract TB remain asymptomatic, a small percentage do not control the infection, leading to active disease. Current TB testing methods, such as skin tests or blood tests like the interferon gamma



Identifying Higher Risk
TB Infection
of
Cont’d on page 21
Cont’d
12 INSIDE GLOBETECH MEDIA >>> <<< International Calendar 22 Industry News . . . . . . . . . 21 News Update 5 HospiMedica EXPO . . . 6-10 News Update . . . . . . . . . 17 HospiMedica EXPO 18-20 News Update 11 HospiMedica EXPO . . 12-16
Cont’d on page 9
on page
your subscription is not renewed every 12 months your Free Subscription may be automatically discontinued Renew / Start your Free Subscription
Interactive
Magazine
Online
Information: Identify LinkXpress ® codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress ® inquiry matrix 1 2 3 VISIT READER SERVICE PORTAL LINKXPRESS COM ® See article on Page 2
If
Access
Digital
Instant
Product
groundbreaking imag-
platform
outcomes and extending patient survival . INTERNATIONAL ® Vol.42 No.1 • 2-3/2024 ISSN 0898-7270
A
ing
called Multispectral Optoacoustic Tomography (MSOT) can now enhance the detection of pancreatic cancer cells at the microscopic level, potentially improving surgery
3 mm 200μm
Image courtesy of University of Oklahoma
Novel Imaging Technique Could Revolutionize Pancreatic Cancer Surgery
Pancreatic cancer, known for being particularly challenging to cure, often goes undetected at the microscopic level due to typically absent early symptoms. This often leads to late diagnosis when the cancer has already spread, resulting in dire survival rates — approximately a 9% overall chance. The best chances for patient survival currently hinge on surgery and chemotherapy, with the effectiveness of surgery dependent on the complete removal of the cancer, a task complicated by the limitations of current imaging techniques like CT scans, which only detect cancer at a more advanced stage. A groundbreaking imaging approach can now enhance the detection of pancreatic cancer cells at the microscopic level, around 200 microns in size, similar to the thickness of an eyelash, potentially improving surgery outcomes and extending patient survival.
At OU Health Stephenson Cancer Center at the University of Oklahoma (Norman, OK, USA; www.ou.edu), researchers have launched an innovative study combining a novel contrast agent, specifically designed to target pancreatic cancer cells, with Multispectral Optoacoustic Tomography (MSOT). This approach promises to detect cancer cells at a magnitude approximately 10 times smaller than current capabilities.





For small and medium labs, @SINGUWAY is your answer.





≤
30 minutes
≤
35 minutes
qPCR Detection Kits (PCR fluorescence method):


Respiratory Diseases: COVID-19, fluA, fluB, AdV, TB and multiplex test
Blood Diseases: HBV, HCV, HIV and multiplex test
Sexually Transmitted Diseases: HPV, CT, NG, UU and multiplex test
Viral Zoonotic Diseases: MPV
Vector-borne Diseases: PF, ZIKV
Genetic Diseases: MTHFR
Animal Diseases: Swine/Avian/Aquatic
Animal/Ruminant/Companion Animal Diseases






In the lab, the team developed a unique contrast agent that reacts specifically to the acidic environment of pancreatic cancer cells. Delivered intravenously, this agent can distinguish pancreatic cancer cells from others by activating its dye in the cancer's acidic environment.
The MSOT device complements this contrast agent by emitting infrared light into the body, which activates the dye. This interaction produces sound waves that the MSOT device captures and translates into a color-coded image. The resulting images are so detailed that they can reveal cancer cells usually undetectable by other means, potentially revolutionizing pancreatic cancer surgery. This is particularly vital for older patients, who are more commonly affected by pancreatic cancer and face higher risks from major operations, often unable to undergo a second surgery. In such cases, this new imaging technique could significantly inform surgical planning. For instance, if the MSOT detects cancer invasion in critical blood vessels near the pancreas, it would impact the surgical approach. It can also assess the effectiveness of pre-surgical chemotherapy, indicating whether cancer cells on blood vessels are alive or if microscopic cancer remains. Going forward, this innovative imaging method holds the potential to become a screening tool for those at heightened risk of developing pancreatic cancer, such as individuals with a family history or genetic predisposition to the disease.
“This is a hybrid approach that accomplishes what a CT cannot,” said Lacey McNally, Ph.D., professor of surgery at the OU College of Medicine. “Pancreatic cancer often creates tentacles that spread out beyond the primary tumor. Currently, there is no way for the surgeon to know where they are. But if the surgery team can use this MSOT approach in the operating room, it can tell them in real time where the cancer has metastasized so they can remove it.”
102 HMI-03-24 LINKXPRESS COM HospiMedica International To view this issue in interactive digital magazine format visit www.HospiMedica.com 2 HospiMedica International February-March/2024
Image: Using MSOT to image the carotid artery (Courtesy of OMIG).
Singu20 Nucleic Acid Extractor AccuRa-32 Real-Time PCR System (32 samples)
Singu20 Nucleic Acid Extractor AccuRa mini Real-Time PCR System (8-16 samples)
5-Part Hematology Analyzer Hematology + CRP +SAA Joint Analyzer (Auto Sampling)

COVID-19 Update

Cont’d from cover
ducing cardiovascular events. Despite this, IVUS is utilized in only about 15-20% of PCI cases in the United States, partly due to the complexity of interpreting the images and incomplete reimbursement for the procedure. Optical coherence tomography (OCT), another method that employs light rather than sound to create images, delivers higher resolution, more accurate, and detailed images than IVUS and is easier to interpret. However, OCT is only used in about 3% of PCI cases, largely due to limited research data.
Individuals with coronary artery disease, which involves plaque accumulation in the arteries leading to symptoms like chest pain, shortness of breath, and heart attacks, often undergo percutaneous coronary intervention (PCI). This non-surgical procedure, conducted by interventional cardiologists, involves using a catheter to place stents in the obstructed coronary arteries to improve blood flow. Typically, angiography guides PCI, using a special dye and X-rays to visualize blood flow and identify blockages in the heart arteries. However, angiography has its shortcomings, such as difficulty in accurately assessing the true size of the artery, the composition of the plaque, and issues in confirming whether a stent is properly expanded after PCI. It’s also less effective in spotting other conditions impacting the procedure’s early and late results.
Now, a new study by researchers at Mount Sinai (New York City, NY, USA; www.mountsinai.org) has found that using intravascular imaging to guide stent placement during PCI significantly enhances patient survival and reduces adverse cardiovascular events compared to relying on angiography-guided PCI alone. The study, published in The Lancet on February 21, 2024, is the largest and most comprehensive of its kind, comparing IVUS and OCT against angiography-guided PCI. It is the first study to demonstrate that these
two high-resolution imaging techniques can lower the rates of all-cause death, heart attacks, stent thrombosis, and the necessity for revascularization.
In this study, the researchers evaluated data from 15,964 patients undergoing PCI across 22 trials in various centers from the United States, Europe, Asia, and other regions between March 2010 and August 2023. Patients received either angiography-guided PCI or intravascular imaging-guided PCI using IVUS or OCT. During a follow-up period ranging from 6 to 60 months, averaging two years, those who received guidance via intravascular imaging saw a 25% reduction in all-cause death, 45% reduction in cardiac death, 17% reduction in all myocardial infarctions, and 48% reduction in stent thrombosis compared to those who had angiography guidance. The study also indicated that intravascular imaging reduced target vessel myocardial infarction by 18% and target lesion revascularization by 28%, with similar outcomes for both OCT-guided and IVUS-guided PCI.
“With these results, we now need to shift from performing more studies to determine whether intravascular imaging is beneficial, to increasing efforts to overcome the remaining impediments to the routine use of OCT and IVUS, including better training of physicians and staff and increasing reimbursement,” said Gregg W. Stone, MD, Director of Academic Affairs for the Mount Sinai Health System. “In this regard, we now have better ‘hard evidence’ that intravascular imaging guidance of PCI procedures makes a greater impact to improving our patients’ lives than other routine therapies which are more widely used and reimbursed.”
Image: Intravascular ultrasound provides a more accurate and specific picture of the coronary arteries (Photo courtesy of Adobe Stock)
www . hospimedica . com
Publishers of: HospiMedica International • LabMedica International LabMedica en Español • HospiMedicaExpo.com • LabMedicaExpo.com HospiMedica.com • HospiMedica.es • MedImaging.net LabMedica.com • LabMedica.es
Dan Gueron
David Gueron
Publisher
Managing Editor
Sanjit Dutt
Carolyn Moody, RN
Simone Ciolek
Parker Xu
Subscriptions:
Send Press Releases to:
Advertising & Ad Material:
Other Contacts:
www LinkXpress com HMNews@globetech net ads@globetech .net info@globetech net
USA, UK Miami, FL 33280, USA
Carolyn.Moody@globetech.net Tel: (1) 954-686-0838 Joffre.Lores@globetech.net Tel: (1) 954-686-0838
GERMANY, SWITZ , AUSTRIA Bad Neustadt, Germany
Simone.Ciolek@globetech.net Tel: (49) 9771-1779-007
BENELUX, FRANCE Hasselt, Belgium
Nadia.Liefsoens@globetech.net Tel: (32) 11-22-4397
JAPAN Tokyo, Japan
Katsuhiro.Ishii@globetech.net Tel: (81) 3-5691-3335
CHINA Shenzhen, Guangdong, China
Parker.Xu@globetech.net Tel: (86) 755-8375-3877
ALL OTHER COUNTRIES
Contact USA Office ads@globetech.net
Tel: (1) 954-686-0838
HospiMedica is published 4 times a year and is circuIated worldwide (outside the USA and Canada), without charge and by written request, to medical department chiefs and senior medical specialists related to critical care, surgical techniques and other hospital-based specialties; hospital directors/administrators; and major distributors/dealers or others allied to the field.
To all others: Paid Subscription is available for a twoyear subscription charge of US$ 100. Single copy price is US$ 20. Mail your paid subscription order accompanied with payment to Globetech Media, LLC, P.O.B. 800222, Miami, FL 33280-0222, USA.
For change of address or questions on your subscription, write to: HospiMedica lnternational, Circulation Services at above address or visit: www LinkXpress com Vol
42 No 1 • Published, under license, by Globetech Media LLC Copyright © 2024. All rights reserved. Reproduction in any form is forbidden without express permission. Teknopress Yayıncılık ve Ticaret Ltd. Şti adına İmtiyaz Sahibi: M. Geren • Yazı işleri Müdürü: Ersin Köklü Müşir Derviş İbrahim Sok. 5/4, Esentepe, 34394 Şişli, İstanbul P. K. 1, AVPIM, 34001 İstanbul • E-mail: Teknopress@yahoo.com Baskı: Postkom A.Ş. • İpkas Sanayi Sitesi 3. Etap C Blok • 34490 Başakşehir • İstanbul Yerel süreli yayındır. Yılda dört kere yayınlanır, ücretsiz dagıtılır. ISSN 0898-7270 SUBSCRIPTION INFORMATION ADVERTISING SALES OFFICES INTERNATIONAL HOW TO CONTACT US
A GLOBETECH PUBLICATION
News
Karina Tornatore 4 HospiMedica International February-March/2024
Editor Regional Director Regional Director Regional Director Reader Service Manager
HospiMedica International To view this issue in interactive digital magazine format visit www.HospiMedica.com
Marc Gueron Founder & Editorial Director
IVUS Improves Outcomes in Cardiovascular Stenting Procedures
Susceptibility Tensor Imaging (STI) Creates “Super-Scans” of the Brain
Susceptibility Tensor Imaging (STI) is a specialized MRI technique that can measure the magnetic susceptibility of various brain tissues. This process involves quantifying how these tissues become magnetized in an MRI scanner's magnetic field. Such detailed information is crucial in enhancing understanding, diagnosis, and monitoring of neurological diseases like multiple sclerosis (MS) and Alzheimer's disease. Researchers have now made a significant advancement by developing DeepSTI, a new algorithm that gathers data from multiple scans to produce a comprehensive "super-scan" of the brain. This scan offers precise information about brain tissue susceptibility. Remarkably, DeepSTI requires fewer images and head positions than conventional STI, thus streamlining the process for patients.
Developed by researchers at Johns Hopkins University (Baltimore, MD, USA; www.jhu.edu), this algorithm creates a detailed 3D map of the brain's magnetic susceptibility. Its primary breakthrough lies in its capacity to measure critical brain tissue components, such as myelin and iron, with fewer scans. Monitoring changes in these tissues is essential for characterizing the type, stage, or progression of neurological diseases. For instance, DeepSTI can visualize myelin changes in MS patients using data from a single head orientation scan.
DeepSTI leverages machine learning, particularly an approach known as regularization, which narrows the range of possible solutions to the most accurate ones. The model uses special regularizers, informed by previous scan data, to guide it towards optimal brain reconstructions. These data-driven regularizers lead the model to

the most plausible solution for each new scan set. This machine learning-enhanced algorithm is poised to make STI a more practical imaging choice for clinicians and radiologists by reducing scan duration and enhancing image quality.

"Usually, STI imaging requires at least six different scans at different head orientations to achieve a good reconstruction, and that's mainly why it's not currently broadly used despite its potential to understand the human brain," said senior author Jeremias Sulam, an assistant professor of biomedical engineering. "Our AI-assisted reconstructions greatly expand the amount of useful information that can be gleaned while requiring much less data, and we hope that will help move this imaging technique from lab to clinic."
Image: A new algorithm called DeepSTI takes data from multiple individual scans and provides a `super-scan` of the brain (Photo courtesy of Johns Hopkins University)
Pioneering Full-Body MRI Device Tracks Moving Tumors in Real-Time During Proton Therapy
For the first time globally, scientists have combined a full-body MRI device for real-time imaging with a proton therapy system in the form of a prototype. With this, experts from the fields of medicine, medical physics, biology, and engineering will now conduct scientific testing of a new form of radiotherapy for treating cancer.
Scientists at Helmholtz-Zentrum Dresden-Rossendorf (HZDR, Dresden, Germany; www.hzdr.de) and the Dresden University Medical Center (Dresden, Germany; www.tu-dresden.de) have ingeniously combined the capabilities of a full-body MRI machine, designed to rotate around the
duce high-contrast images of tumors, enabling more accurate differentiation of the tumor from adjacent healthy tissues. This precision allows for a more precise definition of the radiation target area. Moreover, MRI can track changes in the tumor’s size and shape across treatment sessions, facilitating the tailoring of the radiation beam to each patient’s unique needs. Significantly, this technology also enables the visualization of tumor movement during radiation sessions, allowing for synchronization between the tumor’s movement and the application of radiation.
Creating this novel system presented substantial technological chal

on page 6


For further details: Contact us at +1 (626) 357-7921 • sales@radcal.com or www.radcal.com Accu-Gold 3 New X-ray QA SOFTWARE vc HospiMedica_clr_Eng 7.625 x 3.5 due 2/8/2024 Visit us at MD Expo #3312, 4/7-4/9 • Optimize Work Time – Machine Specific Profiles – Favorites Folder – Advanced Mammography: Single Exposure Combo-mode – Advanced CBCT Dental: Simultaneous DAP, kV and Dose • More Values Displayed vc HospiMedica_clr_3.75x7.5_Eng24Feb08_12868.pdf 1 2/5/24 1:10 PM
Cont’d

RTo receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
CT BRAIN IMAGING SOFTWARE SHINA SYSTEMS

PaxeraView PRO is a powerful, stand-alone PACS workstation allowing users to securely archive and easily access patient images. Ideal for small facilities, it incorporates a complete diagnostic viewer and

New PET Tracer Detects Inflammatory Arthritis Before Symptoms Appear
heumatoid arthritis, the most common form of inflammatory arthritis, affects 18 million people globally. It is a complex autoimmune disease marked by chronic inflammation, leading to cartilage and bone destruction, functional limitations, disability, decreased quality of life, and potentially reduced life expectancy. A significant focus in rheumatology is the use of precision diagnostics to predict the onset of rheumatoid arthritis in individuals with risk factors. The goal is to identify such individuals before they exhibit symptoms, enabling early treatment to prevent the disease's development. Now, a novel PET imaging technique can noninvasively detect active inflammation in the body, even before clinical symptoms emerge.
CD69, an early marker of cell inflammation, is found in the tissue of patients with active rheumatoid arthritis. Using 68Ga-DOTA-ZCAM241, a CD69-targeting PET agent that binds to proteins present in activated immune cells, researchers at the Karolinska Institutet (Solna, Sweden; www. ki.se) showed that the technique produces images of ongoing inflammation throughout the body, such as rheumatoid arthritis. This makes it easier for physicians to correctly diagnose and treat patients. In their study, the researchers evaluated the performance of the 68Ga-DOTA-ZCAM241 for early disease detection in a mouse model of inflammatory arthritis.
In the study, which was published in the February issue of The Journal of Nuclear Medicine, mice underwent 68Ga-DOTA-ZCAM241 PET imaging before and at various intervals after arthritis induction. Disease progression was monitored through clinical parameters like body weight and paw swelling. Analysis of 68Ga-DOTA-ZCAM241 uptake in the paws was conducted, and CD69 expression was examined in tissue biopsies post the final PET scan. A control group of mice was scanned using a nonspecific control peptide. The results showed increased uptake of the CD69-specific tracer 68Ga-DOTA-ZCAM241 in the paws of mice with induced inflammatory arthritis as early as three days post-induction, preceding clinical symptom manifestation by five to seven days. The tracer uptake also corresponded with clinical scores and disease severity, while the nonspecific control peptide showed minimal binding.
“68Ga-DOTA-ZCAM241 is a potential candidate for PET imaging of activated immune cells during rheumatoid arthritis onset,” said Olof Eriksson, PhD, associate professor at Uppsala University. “We know that physicians are asking for better methods to image inflammation, for example in rheumatoid arthritis, and we hope this technology will be broadly used in many diseases that involve activated immune cells and inflammation.”

3Di Brain Perfusion is a powerful application for the evaluation of brain perfusion. It calculates and displays optimized color result maps for CBV, CBF, MTT, and TTP, mirroring ROIs about the midline of the brain.


Pioneering Full-Body MRI Device Tracks Moving Tumors in Real-Time During Proton Therapy
Cont’d from page 5
interactions can potentially affect both the quality of the imaging and the accuracy of the proton beam application. Building on the success of a previous prototype that demonstrated the technical feasibility of simultaneous radiation and imaging, this latest development marks the first-ever use of real-time MRI imaging in this context. The research team plans to use this prototype in future studies to assess its potential benefits, particularly for mobile tumors located in areas like the chest, abdomen, and pelvis.
“This new prototype with integrated full-body MRI makes it possible to visualize moving tumors using high-contrast real-time imaging. Our work aims to develop a technique to irradiate tumors only when they are hit reliably by the proton beam,” said Prof. Aswin Hoffman who developed the new system. “The MRI device, which can rotate around the patient, enables us to use innovative types of patient positioning for proton therapy in both lying or in upright positions.”
202
6 HospiMedica International February-March/2024 WORLD’S MEDICAL DEVICE MARKETPLACE
203 HMI-03-24 LINKXPRESS COM
Image: The PET imaging technique can noninvasively detect active inflammation before clinical symptoms arise (Photo courtesy of 123RF)
Ultrafast Ultrasound Imaging Technique Captures 1000 Images per Second
The kidney’s critical role in filtering waste and excess substances from the bloodstream can be severely impacted by conditions like hypertension and diabetes, potentially leading to kidney failure. This irreversible condition requires lifelong management through artificial hemodialysis or kidney transplantation. The direct connection between blood perfusion in the kidneys and their filtration function makes microvascular imaging a crucial tool for both the prevention and treatment of kidney failure.
Contemporary imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) face challenges in accurately capturing fine vascular structures. This is due to their inherent limitations in resolution and sensitivity. Additionally, in patients with kidney disease, the use of contrast agents in these methods is limited due to the risk of potentially fatal side effects. On the other hand, ultrasound imaging, known for its safety even in fetal monitoring, uses the Doppler effect to measure blood flow velocity and direction in real time without requiring contrast agents. However, traditional ultrasound imaging speeds are not sufficient to capture the fine blood vessels with the necessary sensitivity.
A research team at Pohang University of Science and Technology (POSTECH, Pohang, South Korea; www. postech.ac.kr) has realized significant advancements in microvascular sensitivity. They have achieved this by employing ultrafast ultrasound acquisition techniques that capture images at 1,000 frames per second, over 100 times faster than conventional ultrasound methods. This breakthrough allowed them to image the three-dimensional microvasculature of the kidneys without the need for any contrast agents. In a pioneering feat, they achieved imaging of the entire three-dimensional vascular network of the renal artery, vein, and the minute 167μm (micrometer) thick interlobular arteries and veins in the renal cortex.
Additionally, the team conducted a continuous observation of renal vascular changes in an animal model with induced renal failure. Through this, they performed a multivariate analysis using various hemodynamic and vascular morphological indicators. Their findings revealed a significant decrease in renal blood flow during acute renal failure. In cases of diabetic nephropathy, they observed chronic vascular degeneration in the kidneys, characterized by vascular distortion. This innovative imaging technique holds promise in revolutionizing the monitoring and treatment of kidney diseases.
“The system allows us to understand the pathophysiology of diseases leading to kidney failure, enabling the observation of vascular changes before

and after kidney transplantation,” said Professor Chulhong Kim. “It has significant potential to be used to study blood circulation and functional impairment across various organs including the digestive system, circulatory system, and cerebral nervous system.”



7 HospiMedica International February-March/2024 Medical Imaging To view this issue in interactive digital magazine format visit www.HospiMedica.com To view this issue in interactive digital magazine format visit www.HospiMedica.com
ANNUAL MEETING OF MYESR.ORG
THE
Image: Vascular changes in acute and diabetic renal failure (Photo courtesy of POSTECH)

To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
UNITED IMAGING HEALTHCARE
The uMR Jupiter 5T is the world’s first whole-body ultra-high field (UHF) MRI, representing a breakthrough in the evolution of MRI. The new 5.0T superconducting magnet and RF system are designed using state-

New Helmet with Tiny Sensors Could Conduct Brain Scans of People in Motion
Researchers have created a pioneering helmet equipped with miniature LEGO-sized sensors capable of scanning the brain while a person is in motion. This groundbreaking development, led by the University of Nottingham (Nottingham, UK; www.nottingham.ac.uk), marks a significant leap in brain scanning technology. Previously, capturing accurate magnetic fields generated by brain activity was only possible when a person remained stationary. This new lightweight helmet paves the way for easier brain scans in young children and those with neurological disorders who may find it challenging to stay still in traditional scanners. It's adaptable to various head sizes and shapes, opening new avenues for understanding brain development and the changes occurring in neurological conditions such as autism, epilepsy, stroke, concussion, and Parkinson's disease.
When neurons in the brain interact, they produce a tiny electric current that generates a magnetic field. This field is detectable and recordable through a process known as magnetoencephalography (MEG). MEG technology is capable of capturing both normal and abnormal brain signals with millisecond precision, and its results can be superimposed on an anatomical brain image to pinpoint the origins of specific brain activities. Traditional MEG systems, resembling old-fashioned hair dryers, require the subject's head to remain still and have sensors that need to be cooled to freezing temperatures or below, preventing direct contact with the scalp.
The research team at Nottingham used advanced optically pumped magnetometers (OPMs) for their helmet, which function at room temperature and can be placed close to the head, significantly enhancing data quality. The flexible design of the sensors allows for movement during scanning, addressing a major limitation of conventional MEG systems. However, OPMs require an environment free from background magnetic “noise” to ensure high-quality recordings. To tackle this, the team devised a magnetic shielding system capable of negating or compensating for these interfering magnetic fields.
They built a system with electromagnetic coils, arranged on two panels around the participant, to shield against background noise. Previous research utilized eight large coils that limited head movement due to their fixed position. The Nottingham team innovated a matrix coil system with 48 smaller coils on two panels, allowing individual control and continuous recalibration to counteract magnetic field fluctuations caused by sensor movement. This setup guarantees high-quality MEG data recording in any position, making
MAMMOGRAPHY SYSTEM SIEMENS HEALTHINEERS

The MAMMOMAT B.brilliant brings the next generation of 3D mammography, featuring PlatinumTomo, a completely new tomosynthesis image acquisition technology. It offers a 50° wide-angle tomosynthesis scan time of just five seconds.


OPM-MEG scans more comfortable and accommodating for individuals to move around.
The efficacy of this new matrix coil system was validated through four experiments. Initially, they confirmed that the helmet, when stationary and placed within the coil panels, effectively reduced background magnetic fields. A subsequent test with a healthy participant wearing the helmet demonstrated successful recording of brain function during head movement, with the coils effectively canceling out magnetic fields. Another experiment involved a wire coil attached to the helmet, which mimicked brain cell activity and confirmed the system's ability to compensate for motion-related changes. Finally, a second participant wearing the helmet illustrated the system's capability to produce high-quality brain activity recordings while walking around.
“Unconstrained movement during a scan opens a wealth of possibilities for clinical investigation and allows a fundamentally new range of neuroscientific experiments,” said Niall Holmes, Ph.D., a Mansfield Research Fellow in the School of Physics and Astronomy at the University of Nottingham, who led the research.
“By taking advantage of recent OPM-MEG technology and designing a new magnetic shielding system, this helmet represents a novel magnetoencephalography approach that could help reveal more about how the brain works,” said Shumin Wang, Ph.D., a program director in the NIBIB Division of Applied Science & Technology (Bioimaging).
205
8 HospiMedica International February-March/2024 WORLD’S MEDICAL DEVICE MARKETPLACE
206 HMI-03-24 LINKXPRESS COM
Image: The brain scanning helmet with sensors records brain function (Photo courtesy of Virginia Tech)
Identifying Higher Risk of TB Infection
release assay (IGRA), can detect an immune response to TB but fail to differentiate between those at high or low risk of disease progression. This limitation underscores the need for improved testing methods to identify individuals at higher risk of developing TB, allowing for more focused preventative treatment. Researchers have now introduced an innovative approach to studying the progression of TB from infection to disease, identifying and treating individuals at increased risk who might be missed by existing testing methods.
Researchers at the University of Leicester (Leicester, UK; hwww.le.ac.uk) employed PET-CT, an advanced imaging technique, to study how TB infection progresses and to identify individuals at higher risk of developing the disease. This method enabled the team to evaluate a potential new blood test to identify those at higher risk without the need to recruit a large and costly cohort. The study involved 20 adults linked to households of individuals being treated for TB. These participants underwent chest radiography and IGRA screening for TB infection. The research team then utilized two novel methods to monitor disease progression over the next year: PET-CT imaging and a unique bacteriophage-based assay called Actiphage, developed by PBD Biotech (Saskatoon, Canada; www.pbdbio.com). Actiphage utilizes bacteriophages, viruses that specifically infect bacterial cells, to target TB bacteria. When the bacteriophage infects TB bacteria, it releases bacterial DNA, which can then be detected, even at very low levels that other clinical tools cannot identify.
All participants in the study were asymptomatic with normal chest X-rays. They first underwent a baseline PETCT scan. If the scan showed metabolic activity indicative of TB that could be sampled, they underwent bronchoscopy and sampling. Participants without sampleable findings on the initial PETCT, or with negative sampling results, received a follow-up PET-CT scan after three to four months. Through PET-CT, the researchers identified four individuals from whom TB bacteria were isolated either from the lung airway or from PET-positive lymph nodes. Additionally, two more participants showed progressive changes on the second PETCT scan. All six individuals received TB treatment, and follow-up PET-CT scans three months post-treatment showed resolving or completely resolved changes, suggesting the PET-CT changes were due to active TB infection.
The Actiphage test results were also promising. The researchers observed a significant correlation between a pos-
itive baseline Actiphage test and subsequent treatment for high-risk TB infection features. Actiphage results were positive in 12 (60%) participants at baseline and in all six of the treated PET-CT-positive participants. The study’s findings led the researchers to propose that blood biomarkers aimed at detecting bacterial presence could complement existing biomarkers of the host immune response, thus improving the stratification of TB risk in individuals with TB infection.
“Our results are exciting for two reasons. Firstly, they show that PET-CT could be an effective tool for identifying people with higher risk TB infection. This can help us to perform studies to develop new tests and evaluate new treatments, including vaccines more efficiently and at lower cost,” said Dr. Pranabashis Haldar, Clinical Senior Lecturer
in Respiratory Medicine at the University of Leicester. “Secondly, our findings suggest that TB bacteria are found in blood more often than has previously been thought and importantly, the presence of the bacteria in blood may be an indicator of uncontrolled or progressive TB infection.”
Digital Microscopy and AI
Clinical and Research Applications


































Medical Imaging To view this issue in interactive digital magazine format visit www.HospiMedica.com 9 HospiMedica International February-March/2024
Cont’d from cover
Image: Novel approach identifies people at risk of developing TB (Photo courtesy of 123RF)
109 HMI-03-24 LINKXPRESS COM

To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
MAMMOGRAPHY FLAT PANEL DETECTOR CARERAY DIGITAL MEDICAL SYSTEM

The 750M Flat Panel Detector (FPD) is designed for low-dose breast screening and tomosynthesis, offering a 10″ x 12″ imaging area, and utilizing a perfect balance of HDR pixel design and direct-deposit CsI
208

Wearable Ultrasound Predicts Liver Failure
nique, akin to ultrasound imaging, involves a technician using a handheld probe over the skin to send sound waves into the body. These waves cause slight vibrations in internal organs, generating returning waves. The probe detects these vibrations, and their pattern is translated into a measure of the organ’s stiffness. Ultrasound elastography is mainly utilized in intensive care units to monitor post-transplant patients, allowing technicians to check the new organ for signs of stiffening, acute failure, or rejection. However, continuous long-term monitoring isn’t feasible with this method, creating a risk of missing critical changes. Now, an ultrasound sticker previously developed to image deep tissues and organs could offer a more continuous, wearable alternative.
Engineers at MIT (Cambridge, MA, USA; www.web.mit.edu) have developed a small ultrasound sticker, roughly the size of a postage stamp, capable of monitoring deep internal organ stiffness. When worn on the skin, this sticker can detect signs of diseases like liver and kidney failure or the progression of solid
tumors. The sticker emits sound waves that penetrate the skin and reflect off internal organs, with the returning wave patterns indicating organ rigidity. The team has successfully demonstrated that the sticker can continuously monitor organ stiffness for up to 48 hours, detecting subtle changes indicative of disease progression. In early experiments, the sensor identified signs of acute liver failure in rats.
Currently, the engineers are modifying the sticker’s design for human application. They are collaborating with clinicians to adapt the sticker for use in ICU patients recovering from organ transplants. The design is expected to remain largely unchanged for this application, with the sticker attaching to a patient’s skin. Sound waves sent and received by the sticker can be processed by connected electronics, similar to EKG machines. The team also aims to develop a more portable, self-contained version of the sticker, incorporating all necessary electronics and processing into a larger patch. This advancement could enable patients to wear the sticker at home for extended periods, continuously monitoring conditions like the progression of solid
COMPUTED TOMOGRAPHY (CT) SCANNER CANON

The Aquilion Serve SP computed tomography (CT) scanner offers high-quality, low-dose imaging while increasing efficiency, consistency, and throughput. It combines the power of AI-enabled technologies with a redesigned workflow.
209 HMI-03-24 LINKXPRESS COM


tumors, which tend to harden as they worsen.
“When some organs undergo disease, they can stiffen over time,” said Xuanhe Zhao, professor of mechanical engineering at MIT. “With this wearable sticker, we can continuously monitor changes in rigidity over long periods of time, which is crucially important for early diagnosis of internal organ failure.”
“We believe this is a life-saving technology platform,” Zhao added. “In the future, we think that people can adhere a few stickers to their body to measure many vital signals, and image and track the health of major organs in the body.”
Wearable Ultrasound Non-Invasively Treats Chronic Limb-Threatening Ischemia
Peripheral arterial disease (PAD) is a condition affecting millions globally, where peripheral arteries become narrowed, restricting blood flow from the heart to other body parts. PAD patients with extreme blockages can progress to Chronic Limb-Threatening Ischemia (CLTI), experiencing severe pain, non-healing sores, and wounds, often leading to limb amputation. Now, a novel, non-invasive, wearable therapeutic ultrasound device designed to treat CLTI and PAD can improve tissue perfusion, reduce symptoms, and save limbs.
Vibrato Medical (Newport Beach, CA, USA; www.vibratomedical.com) is developing the first wearable therapeutic ultrasound device designed to promote vasodilation and vessel
growth. Based on decades of therapeutic ultrasound research and the latest technological advancements, Vibrato’s technology is the first wearable therapeutic ultrasound device designed to promote vasodilation and vessel growth. Unlike endovascular and surgical revascularization, Vibrato’s technology can be applied without a single skin incision. Therapeutic ultrasound, the scientific basis of Vibrato’s approach, has been validated through animal and clinical studies that found it demonstrated vasodilation, collateral vessel growth, and angiogenesis.
Vibrato has now announced that data from an early feasibility study of non-invasive therapeutic ultrasound (TUS) to treat CLTI has successfully met its endpoint. The study evaluated patients with infrapopliteal PAD and measured
changes in foot perfusion and oxygenation as well as therapy tolerance, compliance, and perception. Participants, categorized as having Rutherford class 3, 4, or 5 PAD, underwent 3040 TUS treatment sessions over two months. Remarkably, each participant exhibited statistically significant improvements in perfusion post-treatment, demonstrating the potential of this device to revolutionize treatment for individuals suffering from severe PAD and CLTI.
“These early findings are promising for the future of non-invasive therapeutic options to treat chronic limb-threatening ischemia,” said Juliana Elstad, CEO at Vibrato. “We’re looking forward to building on these findings as we begin our next prospective multi-center randomized clinical trial.”
10 HospiMedica International February-March/2024 WORLD’S MEDICAL DEVICE MARKETPLACE
Cont’d from cover
Image: The sticky, wearable sensor senses changing stiffness of deep internal organs (Photo courtesy of MIT)
Tiny Ear Canal Device
Monitors Heart Health
Wearables such as smartwatches that are capable of monitoring functions can provide details of an individual’s heartbeat but cannot accurately and unobtrusively measure the electrical current of the heart, which can help diagnose an irregular heartbeat. Now, a study has shown for the first time that tiny devices, situated in a single ear, can effectively capture electrocardiogram (ECG) data in real time. This innovation marks a significant advancement toward monitoring heart health more precisely.
The research, conducted by a team at Imperial College London (London, UK; www.imperial.ac.uk), builds on their previous work where they identified the ear as a viable location for monitoring brain functions and vital signs through “hearable devices” – wearables that fit comfortably within the ear canal. The team also pioneered an earECG technology, where electrodes placed in both ears can generate valid electrocardiograms. This new study, however, explored the potential of using hearables for cardiac health monitoring from just one ear, a concept not yet thoroughly established. To validate their approach, the Imperial College team examined ECG signals and mapped the chest-ECG potential across the ear, neck, and scalp areas. They then tested the feasibility of single ear-ECG measurements under real-world recording conditions.
The study successfully measured cardiac cycles using electrodes placed around the ear region, confirming the accuracy of ECG signals obtained from a single ear-ECG in terms of their shape and timing. The researchers envision that this technology could eventually be used for continuous 24/7 monitoring of various groups such as patients and athletes. It could also be employed to assess the impact of physical strain and stress in different workplace environments, offering a more non-invasive and continuous method of monitoring heart health.
“The significance of our findings lies in the high practicality and usability of the single ear-ECG,” said Metin Yarici, lead author of the study published in the Royal Society Journal of Open Science in January 2024. “We believe that this method holds great promise in bringing continuous cardiac motoring out of a clinical setting and into society, and with it, new insights into heart functioning for healthy and patient populations alike. An important next step in this research is to test the feasibility of detecting specific abnormalities in heart function, such as atrial fibrillation or myocardial infarction, via the single ear-ECG.”
Image: Research shows how a tiny device in the ear canal can monitor heart health (Photo courtesy of Danilo Mandic)
Self-Propelling Nanorobots Reduce Bladder Tumors by 90%
Bladder cancer is one of the most common cancers worldwide, especially among men where it ranks fourth. It has a high recurrence rate, with about half of the cases recurring within five years, creating the need for continuous monitoring. This constant need for follow-up and repeated treatments makes bladder cancer treatment one of the costliest. While current treatments, which involve administering drugs directly into the bladder, offer favorable survival rates, their therapeutic effectiveness is still limited. An emerging and promising approach is the use of nanoparticles, particularly nanorobots, that can self-propel and deliver therapeutic agents directly to cancer cells.
A recent breakthrough by scientists at IRB Barcelona (Barcelona, Spain; www.irbbarcelona.org) has demonstrated the potential of urea-powered nanorobots in bladder cancer treatment. In their study, the team achieved a significant 90% reduction in bladder tumor size in mice using a single dose administered by these nanorobots. The nanorobots are essentially tiny machines, composed of porous silica spheres. Their surfaces are equipped with various components, each serving a specific purpose. One key component is the enzyme urease, which reacts with urea in urine, propelling the nanorobot forward. Another crucial element
Cont’d on page 12








11 HospiMedica International February-March/2024 111 HMI-03-24 LINKXPRESS COM
Human - Veterinary - Bio-Threat Diagnostics/ Research/ Forensic QuickProfile TM QuickStatus® Quicknostics® EnviroSafe TM SAFE TM Infectious Diseases Drugs of Abuse Cancer Markers Cardiac Markers Hormones Veterinary Tests Oxidative Stress Bio-Threat Agents ADLM 2024 BOLD MOVE. Visit us at the Clinical Lab Expo Booth 2160 JULY 28-AUGUST 1 CHICAGO, IL www.LumiQuick.com www.QuickProfileDOA.com sales@LumiQuick.com +1(408)855-0061


To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
TROLLEY LUNG VENTILATOR SIARE
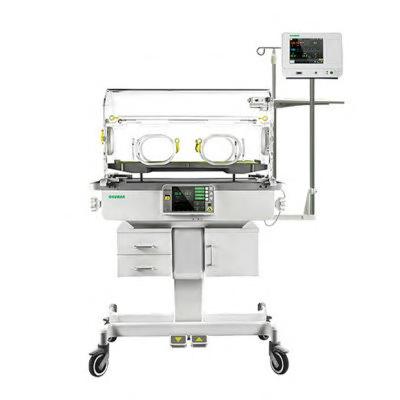
The OKM 801 infant incubator monitors skin temperatures and ambient temperatures, and provides access to the baby from four sides of the canopy. It features an 8″ LCD color screen and allows an optional
211
Catheter Prevents Bacterial Infections
searchers have developed a novel catheter tube that significantly hampers the ability of bacteria to move upstream, effectively reducing the potential for infections without relying on antibiotics or other chemical treatments. This new design, optimized through advanced artificial intelligence (AI), has shown a remarkable 100-fold reduction in the number of bacteria swimming upstream in laboratory experiments.
Fluid inside catheter tubes exhibits what’s known as Poiseuille flow, where the fluid moves faster in the center and slows near the walls. Bacteria exploit this by using a unique motion, moving forward along the walls and then back in the middle, to progress through the tube. Researchers at California Institute of Technology (Caltech, Pasadena, CA, USA; www.caltech.edu) decided to tackle this problem with simple geometries by designing tubes with triangular protrusions, similar to shark fins, lining the tube’s walls. Simulated models demonstrated that these structures effectively redirect bacteria towards the center of the tube where the faster flow sweeps them back downstream. Additionally, the triangles’ fin-like curvature creates vortices that disrupt the bacteria’s progress. The researchers then set out to verify the design experimentally with the help of additional biology expertise. The team was supported by their previous research into the navigation mechanisms of the nematode Caenorhabditis elegans, a rice grain–sized soil organism commonly studied in research labs, providing them with the tools needed to observe and analyze the movements of microscopic organisms. They utilized 3D printing to create these specially designed catheter tubes and employed high-speed cameras to track bacterial movements. The results were significant, showing a two-order magnitude decrease in the ability of bacteria to swim upstream.


ARIA 150 is the latest generation lung ventilator on a trolley with a turbine flow generator making it autonomous from compressed air. It offers a wide range of operating modes suitable for invasive and non-invasive ventilation.


Further simulations were conducted to identify the most effective shape for the triangular obstacles. The team created microfluidic channels, mimicking common catheter tubes, with these optimized triangular designs. Observations of E. coli bacteria moving through these channels closely matched their simulations. To enhance the design further, the team employed advanced AI techniques known as neural operators, drastically reducing the computation time from days to minutes. This AI-optimized model suggested slight modifications to the triangle shapes, boosting their efficacy by an additional 5% in preventing bacteria from swimming upstream. This groundbreaking design represents a significant stride in medical technology, offering a safer and more efficient way to prevent catheter-associated urinary tract infections without the need for antibiotics, marking a significant advancement in patient care and infection control.
“Our journey from theory to simulation, experiment, and, finally, to real-time monitoring within these microfluidic landscapes is a compelling demonstration of how theoretical concepts can be brought to life, offering tangible solutions to real-world challenges,” said Tingtao Edmond Zhou, a co-first author of the study, which was published in the journal Sciences Advances on January 3, 2024.
Self-Propelling Nanorobots Reduce Bladder Tumors by 90%
Cont’d from page 11
is radioactive iodine, widely used in localized tumor treatment.
Understanding how these nanorobots penetrate the tumor was challenging, as they do not possess specific antibodies for tumor recognition and because tumor tissue is generally stiffer than healthy tissue. However, the team discovered that the nanorobots could break down the tumor's extracellular matrix by locally increasing pH through their self-propelling action. This action enhances their penetration into and accumulation within the tumor. The researchers observed that while the nanorobots collide with the urothelium, acting as if they hit a wall, they effectively penetrate and accumulate inside the spongier tumor tissue.
The mobility of these nanobots significantly increases their chances of reaching and impacting the tumor. Additionally, the localized delivery
of these nanorobots, carrying the radioisotope, reduces potential side effects. The high accumulation of these nanorobots in tumor tissue also intensifies the radiotherapeutic impact. This research offers promising directions for bladder cancer treatment, potentially reducing hospital stays, lowering costs, and improving patient comfort. The next research phase is already in progress, focusing on whether tumors recur post-treatment with these nanorobots.
"With a single dose, we observed a 90% decrease in tumor volume. This is significantly more efficient given that patients with this type of tumor typically have 6 to 14 hospital appointments with current treatments," said Samuel Sánchez, ICREA research professor at IBEC and leader of the study. “Such a treatment approach would enhance efficiency, reducing the length of hospitalization and treatment costs.”
12 HospiMedica International February-March/2024 WORLD’S MEDICAL DEVICE MARKETPLACE
212 HMI-03-24 LINKXPRESS COM
Cont’d from cover
Image: Diagram of new catheter design (Photo courtesy of Caltech)
World’s First Safe Electric
Drug Infusion Pump to Prevent Medical Accidents
Medical mishaps caused by the over-administration of pain relief medication during or post-surgery can lead to fatalities, particularly in surgery and cancer treatment scenarios. These incidents often arise from issues with drug infusion pumps or errors in medical supplies. To prevent such accidents, researchers have developed the world’s first drug infusion pump with a safe medication administration detection technology.
A research team at the Korea Institute of Machinery and Materials (KIMM, Daejeon, South Korea; www.kimm.re.kr) has successfully created the technology for customized sensor modules. These modules are designed to measure the very low flow rates typical of analgesic drug infusion pumps, as well as to detect air bubbles within these pumps. To manage post-operative pain, narcotic analgesics are usually administered at flow rates as low as 1 to 2 mL/h. The team developed a novel thermal micro-flow sensor, which incorporates a micro-heater and multiple temperature sensors, to accurately gauge these minimal flow rates. This was achieved by balancing the cooling effect on the microheater due to heat loss and its heating effect on the fluid.
In line with updated FDA regulations requiring bubble sensors in drug infusion pumps, the new pumps are equipped with temperature sensors at both ends of the tube. These sensors can detect bubbles by observing the variation in heat diffusion between air and liquid in the tube. Notably, by attaching the sensor to the exterior of the drug injection tube, both the flow rate and bubble presence can be measured non-invasively. This design also allows for sensor reuse, addressing the cost issues related to medical disposables.
This technology ensures performance on par with high-cost MEMS sensors in terms of sensitivity, accuracy, measurement range, and bubble detection. The sensor has been developed as a customized module to replace the ultrasonic bubble sensor in existing drug infusion pumps. This sensor module is currently being prepared for large-scale production for use in new drug infusion pumps. The introduction of this technology is poised to play a significant role in preventing medical accidents caused by excessive analgesic administration post-surgery. It is also expected to facilitate speedy medical services by providing highly accurate data on medication speed and dosage and to reduce the medical staff’s workload in drug injection management.
“This is a technology for a sensor capable of simultaneously measuring extremely low flow rates and bubbles without coming into contact with the drug outside the tube and without having to apply the expensive MEMS sensor technology, simply by attaching the drug infusion tube to the sensor,” said senior researcher Dong-kyu Lee of the KIMM. “It is a technology that is customized for the injection of medications.”

Image: Integrated drug infusion pump with flow and bubble sensor modules (Photo courtesy of KIMM)
ESC Congress 2024 London
30 August –2 September Onsite & Online
Abstract submission: 12 December - 1 March
Clinical Case submission: 16 January - 8 March
Late-Breaking Science submission: 27 March - 6 June
Early registration deadline: 31 May
Late registration deadline: 31 July
Last-minute registration until: 2 September
Your journey to the heart of cardiology
Critical Care To view this issue in interactive digital magazine format visit www.HospiMedica.com
13 HospiMedica International February-March/2024
#ESCCongress

To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
CRITICAL CARE TRANSFER TROLLEY PARAID MEDICAL SYSTEMS

The Transporter Infinite Neo is a critical care transfer trolley designed specifically for neonates that provides the clinical team with a safe and secure method of transfer of neonates and medical equipment by
214
ECG Vest Allows For Non-Intrusive, Non-Invasive Heart Monitoring
An arrhythmia, an irregular heartbeat, can lead to serious health issues like blood clots, stroke, and heart failure. Traditional methods for monitoring such arrhythmia, like the Holter monitor, have limitations in terms of wearability and duration, typically offering only a 24- or 48-hour observation window. More longterm solutions tend to be invasive, requiring anesthesia for insertion. Now, an ECG monitoring system integrated into a T-shirt can accurately and non-invasively monitor the patient’s heart for weeks or months.
PA Consulting (London, UK; www.texasheart.org) has developed Viscero, a revolutionary approach to ECG monitoring that integrates sensory technology seamlessly into a vest, allowing for effortless capture and transmission of diagnostic-quality data. The system positions dry electrodes in peripheral locations away from the chest, ensuring constant compression points for accurate readings. The brain behind Viscero, similar in size to a matchbox, conveniently fits into a pocket on the T-shirt. This allows for easy removal for recharging purposes or when the T-shirt needs washing.

BLADDER SCANNER COMSSEN INDUSTRIAL CO.

The Z3 Bladder Scanner is a real-time ultrasound bladder scanning device that accurately measures bladder volume and PVR. Its great portability, small size, and ultra lightweight scanner console make it ideal for ‘use on the go’.
215 HMI-03-24 LINKXPRESS COM

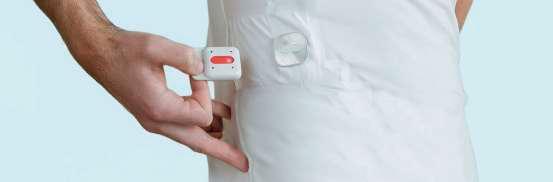
Additionally, Viscero is equipped with an onboard accelerometer and gyroscope, enriching the heart signal data with contextual information about the wearer’s physical activities preceding an arrhythmic event.
This comprehensive data is accessible through an AI-enhanced dashboard that categorizes recordings into potential arrhythmic episodes, facilitating efficient review by cardiologists and significantly reducing the time spent analyzing ECG recordings. Distinct from consumer devices that offer ECG recording, Viscero provides high-fidelity, continuous monitoring over extended periods, capable of spanning weeks or months. This innovation marks a significant leap in wearable healthcare technology, presenting a practical, non-invasive solution for long-term heart monitoring.
Minimally Invasive Injectable Electrode Could Revolutionize Neuromodulation Pain Treatment
The primary approach to pain management often includes corticosteroid drug injections, delivered using needles ranging from 18-23 gauge. These injections provide pain relief lasting from a few weeks to several months. However, their use is restricted to 2-4 times a year due to possible side effects like tissue weakening and hyperglycemia. If the intervals between treatments are surpassed, doctors may explore other options such as alternative medications, radiofrequency ablation, surgery, or neuromodulation. Now, a minimally invasive injectable electrode minimizes or eliminates the need for surgery, as well as more drug injections or oral drugs for pain management. Once implanted, this device establishes a low-impedance pathway for electrical signals to travel from just below the skin to the nerve responsible for pain transmission, all without requiring sutures or leaving any visible scars.
Neuronoff, Inc. (Cleveland, OH, USA; www.neuronoff.com) has developed the Injectrode, a groundbreaking device for peripheral nerve stimulation and a pioneer in minimally invasive transcutaneous stimulation. This technology offers a simple treatment alternative for chronic pain sufferers while avoiding the need for medications or invasive surgeries. The Injectrode is designed as an easy-to-place, long-term lead that remains virtually undetectable externally, maintaining patient privacy. The aesthetic results of a completely injected lead without any external wires or protrusions mark a significant improvement over current offerings.
Neuronoff has recently achieved a significant milestone in chronic
pain treatment by successfully conducting the first human implant of the Injectrode. This development is poised to transform chronic pain management by offering a less invasive, transcutaneously stimulated option compared to traditional neuromodulation therapies. In this landmark study, two participants underwent the minimally invasive procedure, with the injectable electrode targeting peripheral nerve branches in the lumbar lower back, an important site for novel lower back pain treatments. This accomplishment represents a major step forward in minimally invasive neuromodulation therapies. The initial human trial highlights the Injectrode's ability to effectively target nerve sites with minimal or no unintended muscle activation, offering a new option for treating chronic lower back pain.
"The ease of placement of the Injectrode in this initial study is incredibly promising," said principal investigator Dr. Amol Soin who led the study. "This simple needle-based approach is poised to give patients and physicians a viable early treatment option that isn't a steroid injection or pharmacological solution."
"This successful start of our lumbar peripheral nerve stimulation study marks a significant milestone for Neuronoff,” said Manfred Franke, CEO of Neuronoff. “It represents a major step toward our goal of reducing the barriers to entry for neuromodulation-based treatments by addressing the common patient concern about the surgery often required for chronic neuromodulation.”
14 HospiMedica International February-March/2024 WORLD’S MEDICAL DEVICE MARKETPLACE
AI Algorithm Monitors Vital Signs to Detect Sepsis Before Symptoms
Cont’d from cover
Algorithms designed to aid early sepsis recognition could potentially enhance patient outcomes, yet there is limited research on their real-world impact.
At UC San Diego Health (San Diego, CA, USA; www.health.ucsd. edu), researchers have developed an AI model named COMPOSER to rapidly identify patients at risk of sepsis. This model leverages real-time data to predict sepsis before clear clinical signs emerge. Operating discreetly, COMPOSER continually monitors each patient from the moment they enter the emergency department, analyzing over 150 variables linked to sepsis, including lab results, vital signs, medications, demographics, and medical history. The advanced AI algorithms in COMPOSER can detect subtle patterns not immediately apparent to clinicians. By evaluating these risk factors, the system generates highly accurate sepsis predictions.
Should a patient exhibit a combination of high-risk factors for sepsis, COMPOSER alerts the nursing staff through the hospital’s electronic health record. The nurses then collaborate with physicians to decide the best course of action. If the algorithm determines that the risk patterns are more likely attributed to other conditions, it does not send an alert. Since its activation in December 2022, COMPOSER has been implemented in various in-patient units at UC San Diego Health. A study involving over 6,000 patient admissions before and after deploying COMPOSER in UC San Diego Medical Center and Jacobs Medical Center emergency departments revealed a 17% reduction in mortality, marking the first reported instance of improved
Wearable Sensor Accurately Measures Biomarker Concentrations in Sweat Samples
Skin-applied sensors are emerging as a non-intrusive, affordable method for detecting vital biomarkers in sweat, aiding clinicians in making prompt and precise diagnoses. However, until now, these sensors could only identify the presence of biomarkers and struggled with accurately detecting their concentrations due to the sporadic and unpredictable nature of sweat production. To address this challenge, a team of scientists has introduced a sensor that precisely measures biomarker concentrations in sweat samples.
The research team from Penn State (University Park, PA, USA; www.psu.edu) designed a dual-channel sensor for capturing sweat. One channel is tasked with measuring the biomarker level, while the other assesses the sweat volume. This sensor employs a dye that reacts to the presence of the biomarker and produces a visible indication, allowing for a simple, equipment-free reading. This feature makes the sensor particularly beneficial in remote settings where advanced technological resources may be scarce. Detecting the concentration of a biomarker is critical for accurate diagnostics. For instance, the team has proposed their sensor's application in diagnosing conditions like cystic fibrosis, typically characterized by elevated chloride levels in the patient.
“The typical course of action to diagnose cystic fibrosis is to induce a local sweat through exercise, but with our sensor, we can detect the chloride concentration in sweat without requiring the patient to exercise, since we can use passive heat-induced sweating with our wearable form of the testing setup,” said Huanyu “Larry” Cheng, the James L. Henderson, Jr. Memorial Associate Professor of Engineering Science and Mechanics at Penn State.

Image: The AI surveillance tool successfully helps to predict sepsis (Photo courtesy of UC San Diego Health)
patient outcomes through an AI deep-learning model.
“It is because of this AI model that our teams can provide life-saving therapy for patients quicker,” said study co-author Gabriel Wardi, MD, chief of the Division of Critical Care in the Department of Emergency Medicine at UC San Diego School of Medicine. The study was published online in npj Digital Medicine on January 23, 2024.

Critical Care To view this issue in interactive digital magazine format visit www.HospiMedica.com
15 HospiMedica International February-March/2024
April 25-27, 2024 The Leading Medical Fair in Eurasia www.expomedistanbul.com @expomedeurasia Scan the QR code for more information!


RTo receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
INTRAVASCULAR TEMPERATURE MANAGEMENT SYSTEM ZOLL MEDICAL

The Thermogard HQ intravascular temperature management system streamlines the path to high-quality temperature management with enhanced data insights and improved

ISOLATION STRETCHER SAVION INDUSTRIES

The IS 736 isolation stretcher features a carbon filter and blower for full air circulation within the chamber. It comes with rechargeable batteries allowing full mobility of the units for up to 10 hours of continuous operation.

Fully Implantable, Wirelessly Charged Device Treats Recurrent or Refractory Ascites Due to Liver Cirrhosis
ecurrent and refractory ascites is a key complication of liver cirrhosis, characterized by the accumulation of fluid in the abdomen. These patients can have up to 15 liters of extra fluid in their bodies, causing many health issues and severely impacting their daily lives. Although diuretics are the standard of care, the problem is that in many patients they are no longer effective and/or tolerable, requiring patients to undergo regular paracentesis. Paracentesis is a painful and burdensome procedure that drains ascites from the abdomen using a large needle over an extended period, with only short-term benefits for the patients, requiring frequent hospitalizations and severely impacting their quality of life. Now, a fully implantable, wirelessly charged device for patients with recurrent or refractory ascites due to liver cirrhosis could transform the lives of these patients by virtually eliminating the need for paracentesis and delivering clinically important improvements in quality of life.
Sequana Medical’s (Ghent, Belgium; www.sequanamedical.com) alfapump automatically and continuously removes ascites from the abdomen into the bladder, where it is naturally eliminated through urination. Sequana has submitted a Premarket Approval (PMA) application to the US Food and Drug Administration (FDA) for alfapump which could become the first active implantable medical device in the US for treating liver ascites upon approval. The PMA filing is based on the successful execution of Sequana’s pivotal POSEIDON study, a landmark study across 18 centers in the US and Canada with a total of 69 patients implanted with the alfapump. The primary effectiveness endpoints at six months post-implantation in the Pivotal Cohort exceeded the predefined thresholds with statistical significance, and primary safety endpoint data was in line with expectations.
Data at 12 months post-implantation continued to show a strong and durable clinical profile, virtually eliminating the need for therapeutic paracentesis and delivering a clinically meaningful improvement in patients’ quality of life. Data from the patient preference study and a matched cohort analysis of the NACSELD registry with the POSEIDON Pivotal Cohort indicated that US patients have a strong preference for the alfapump vs. standard paracentesis procedures and that the safety profile of the alfapump is comparable to standard of care.
“The submission of our Premarket Approval application to the FDA is the result of an enormous team effort and a clear demonstration of our intensive preparation to fulfil US regulatory requirements,” said Timur Resch, Global Vice President QM/QA/RA at Sequana Medical. “We have great confidence in the strength of our PMA submission and look forward to work in close collaboration with the FDA to facilitate

Image: Pending FDA approval, alfapump could become the first active implantable medical device in the US for treating liver ascites (Photo courtesy of Sequana Medical)
a seamless and thorough review process intended to bring our breakthrough device to the US market as soon as possible.”
“This is a key milestone for the alfapump and underscores our commitment to improving treatment options for patients with recurrent or refractory liver ascites,” said Ian Crosbie, Chief Executive Officer of Sequana Medical. “This overlooked patient group is forecast to grow strongly due to NASH / MASH and today’s limited treatment options often lead to poor clinical outcomes, severely reduced quality of life, a substantial burden on their caregivers and high costs to payors. Data from our North American pivotal study demonstrate the potential for alfapump to transform the lives of these patients by virtually eliminating the need for paracentesis and delivering clinicially important improvements in quality of life. We anticipate FDA approval in the second half of 2024 and look forward to introducing the alfapump through our own specially sales force focused on US liver transplant centers.”
217
16 HospiMedica International February-March/2024 WORLD’S MEDICAL DEVICE MARKETPLACE
218 HMI-03-24 LINKXPRESS COM
Surgical Imaging Module Offers Enhanced
Real-Time Insights
During surgeries, identification of critical structures and assessment of tissue perfusion are vital for providing patients with the best possible chance of healing well without facing life-threatening or expensive complications. Now, an intraoperative imaging module provides enhanced visualization and real-time, on-demand surgical insights in the operating room while seamlessly attaching to today’s laparoscopic and robotic systems as well as integrating with standard monitors.
Activ Surgical’s (Boston, MA, USA; www.activsurgical.com) ActivSight Intelligent Light is an easy-to-adapt module that is transforming the operating room by seamlessly upgrading existing operating room equipment while serving as the “eyes” of their cutting-edge platform. The hardware-agnostic imaging module is designed to provide surgeons with real-time intraoperative visual data and imaging not presently available to surgeons through existing technologies. With ActivSight, surgeons can access critical intraoperative visual data as augmented reality overlays, helping to increase surgical outcomes and patient safety. More informed real-time decisions during surgery using intelligent visualization technology means potentially fewer complications and tissue injuries promising better clinical outcomes.
A recent clinical study demonstrated that ActivSight’s Laser Speckle Contrast Imaging (LSCI) provides real-time, repeatable, and on-demand perfusion assessment without dyes to help prevent esophageal anastomotic leaks. Attributed to inadequate tissue perfusion or excess tension, esophageal anastomotic leaks are among the most feared in gastrointestinal surgery. Real-time intraoperative perfusion assessment can identify perfusion deficits to help prevent leaks. The study saw the completion of the first international procedure using the 510(k)-cleared and CE-
Bioengineered Material Rapidly Stops Bleeding During Surgery in Patients on Blood Thinners
Anticoagulation and antiplatelet medications like heparin or aspirin, commonly taken by millions worldwide to treat heart attack and stroke, also elevate the risk of potentially fatal bleeding during injuries or surgeries. Globally, over five million people die annually from trauma, with more than a third of these deaths due to uncontrolled bleeding. Now, researchers have created a porous material that significantly absorbs blood and effectively initiates clotting, even in patients on these medications. Remarkably, this new hemostat managed to stop bleeding in an average of about five minutes in cardiac catheterization patients on anticoagulants, marking a substantial improvement from the lengthy traditional compression methods that can take more than two hours.
The research team, led by investigators from Brigham and Women’s Hospital (Boston, MA, USA; www.brighamandwomens.org), developed a more effective hemostat by employing a “rational engineering” approach. They simulated blood flow through various pore structures, drawing inspiration from the lung’s alveoli – spherical air sacs with a large surface area and a complex porous structure that enable efficient blood interaction. This guided them to design their material with a similar intricate, spherical microporous structure, optimizing blood absorption and the accumulation of vital clotting components like platelets.
Chitosan, a substance extracted from shellfish, forms the base of this alveoli-like structure. Already utilized in some hemostats, chitosan’s positive charge effectively attracts negatively charged platelets and fibrinogen, key blood clot components. The researchers discovered an additional benefit: chitosan activates the TLR-2 clotting pathway, directly stimulating clotting even in patients on anticoagulants. When tested on 70 patients undergoing cardiovascular catheterization and on heparin, the material reduced bleeding to an average stoppage time of about
Cont’d on page 18

marked ActivSight device’s LSCI.
“As we continue to achieve significant milestones such as this, our vision is to transform the collective surgical experience by leveraging emerging technologies and data into insights that make cutting-edge surgery accessible for all,” said Manisha ShahBugaj, Chief Executive Officer at Activ Surgical.

Image: The ActivSight device is 510(k)-cleared and CE-marked (Photo courtesy of Activ Surgical)

















117 HMI-03-24 LINKXPRESS COM
Size: 2400 x 1200 mm (3 mm thick) 100% Silicone YOUR GLOBAL SOURCE FOR STERILIZATION ACCESSORIES
(- 60 °C to 300 °C) Fully Washable & Flexible Suitable for central sterilization services Sterilizable STERILIZABLE INSTRUMENT & WORK-SURFACE MATS Front Back WASHING TRAYS MAT Heavy Silicone Cover & Transport Tablet TURBO WASHING MACHINES TRAYS SILICON INSTRUMENT MAT Front Back MICRO INSTRUMENT MAT Exchangable Net Exchangable Nets INVITEDTOAPPLY DISTRIBUTORS Up to 37 cm in length THERMO RESISTANT GLOVES Front Back WASHING TRAYS MAT NEW! VICOTEX Place de la Gare 1 • 1009 Pully • Switzerland Tel: (41) 21-728-4286 • Fax: (41) 21-729-6741 E-Mail: contact@vicotex.com www.vicolab.com S.A. SILICONE TABLET AND STEEL COVER NETS 17 HospiMedica International February-March/2024
Thermo-Resistant

To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
OPERATING CEILING LED LIGHT

The DL-66CM operating ceiling LED light has two light heads with 160,000 lux light power along with a 24” Active Matrix TFT LCD medical monitor with 1920 x 1200 resolution
AI Predicts Death and Complications in Angioplasty and Stent Patients
Percutaneous coronary intervention (PCI) is a minimally invasive procedure used to treat blocked heart arteries. Traditionally, during PCI, blocked arteries are cleared by inflating a balloon and potentially inserting a stent to enhance blood flow from the heart. Although this procedure is less risky than open-heart surgery, it can still lead to complications such as bleeding and kidney injury. Recognizing these risks, a team of researchers has developed a new AI-powered algorithm that can accurately predict mortality and complications following a PCI. This innovative tool holds promise for aiding clinicians in making more informed treatment decisions.
Several risk stratification tools have been developed to identify risk after PCI, although most are modestly accurate and were created without involving patients. The research team at Michigan Medicine (Ann Arbor, MI, USA; www.uofmhealth.org) set out to develop a more accurate risk stratification tool, incorporating patient data into the design process, unlike previous models. The research team gathered comprehensive data on all adult patients who underwent PCI from April 2018 to the end of 2021. This data was sourced from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) registry, a network of hospitals throughout Michigan that uses collective data to enhance care quality and patient outcomes.
Utilizing over 20 pre-procedural characteristics, including factors like age, blood pressure, and total cholesterol, the team employed the machine learning software "XGBoost" to construct a risk prediction model. This AI-driven algorithm demonstrated high accuracy in predicting deaths, major bleeding events, and the necessity for blood
220

MOBILE LITHOTRIPTER (SWL) STORZ MEDICAL

The MODULITH SLK intellect is a flexible and mobile lithotripter (SWL) that can be combined with various modular components such as Carcs and patient tables. It features the proven STORZ MEDICAL cylindrical shock wave source.
221 HMI-03-24 LINKXPRESS COM


transfusions, surpassing other models that used similar pre-procedural characteristics. To make this advanced technology widely accessible, it has been integrated into both computer and phone applications, available for free use. This development represents a significant step forward in improving clinical decision-making for patients undergoing PCI.
"Precise risk prediction is critical to treatment selection and the shared decision-making process,” said lead David E. Hamilton, M.D., a cardiology-critical care fellow at Michigan Medicine. “Our tool can recognize a wide array of outcomes after PCI and can be used by care providers and patients together to decide the best course of treatment."
"In the age of widespread smartphones and electronic medical records, this computerized risk score could be integrated into electronic health systems and made easy to use at the bedside,” added senior author Hitinder Gurm, MBBS, interim chief medical officer at U-M Health. “It would not only help relay complex information to the provider quickly, but it could also be used to enhance patient education on the risks related to PCI."
Bioengineered Material Rapidly Stops Bleeding During Surgery in Patients on Blood Thinners
Cont’d from page 17
five minutes for low-dose heparin patients, and under nine minutes for those on up to 12,500 IU heparin doses.
The chitosan pad simplifies application and removal compared to traditional gauze, which often requires long, strong compression and can be painful and risky to remove. The chitosan hemostat, being more absorptive, can be removed more cleanly and comfortably from wounds. The research team is now exploring further advancements, including studying the wound healing process post-application of the chitosan hemostat and developing next-generation wound dressings capable of drug delivery or enhancing wound cleanliness, potentially reducing the frequency of dressing changes.
“This is a next-generation hemostat that effectively stops bleeding, even in patients who take anticoagulation or antiplatelet medications,”
said corresponding author Hae Lin Jang, Ph.D., of the Center for Engineered Therapeutics. “We used an exciting, interdisciplinary approach that combines engineering principles, materials science, and understandings of molecular biology to overcome the limitations of existing therapies and address a real clinical need.”
“This hemostat can save valuable time in emergency situations,” added first author Vivian K. Lee, PhD, of the Center for Engineered Therapeutics. “In emergencies, it can be extremely challenging to screen the prescription information of a patient to provide appropriate anticoagulation reversal therapy to patients on anticoagulants. If a hemostat can bypass a medication’s anticoagulating mechanisms, it can be used in a wide range of patients, saving time, and potentially saving lives.” The research team’s findings were published in the journal PNAS on January 22, 2024.
18 HospiMedica International February-March/2024 WORLD’S MEDICAL DEVICE MARKETPLACE
Replacement Valve That Grows Inside Body to Revolutionize Heart Treatment
Heart valve replacement surgery, a life-saving procedure, has been available for over six decades. However, it comes with significant medical limitations, whether the valves used are mechanical or biological. Patients with mechanical heart valves need lifelong medication to prevent blood clotting. Biological valves, in contrast, have a lifespan of only 10 to 15 years. The situation is even more complex for children with congenital heart defects, as their growing bodies necessitate multiple valve replacements before they reach adulthood. Now, recent research suggests that the natural repair mechanisms in humans can be leveraged to build a living heart valve that grows inside the body along with the patient.
The new approach developed by researchers at Imperial College London (London, UK; www.imperial.ac.uk) involves a procedure that begins with a nanofibrous polymeric valve created from a biodegradable polymer scaffold, unlike the durable plastic that is typically used. Once implanted, this scaffold recruits cells and guides their development, turning the body into a bioreactor for new tissue growth. Over time, the scaffold is naturally replaced by the body’s own tissues. At the heart of this innovation is the scaffold material, designed to attract, house, and direct the patient’s cells, thereby encouraging tissue growth while preserving valve functionality.
The research team conducted laboratory validation studies and reported the initial results from animal tests. The valves, transplanted into sheep, were observed for up to six months. They functioned effectively throughout this period and demonstrated promising cellular regeneration. Notably, the study highlighted the scaffold’s ability to attract blood cells that transform into functional tissues through a process known as endothelial-to-mesenchymal transformation (EndMT). Additionally, nerve and fatty tissue growth within the scaffold was observed, mirroring what one would expect in a normal valve. Concurrently, the polymer scaffold underwent degradation, paving the way for new tissue growth. This degradation was monitored using gel permeability chromatography (GPC) at the Agilent Measurement Suite (AMS) in Imperial’s Molecular Sciences Research Hub in White City, which is equipped with sophisticated analytical tools.
Further research is needed to fully understand the mechanisms behind the polymer’s degradation and its correlation with tissue regeneration. The next phase involves extending animal studies to monitor tissue regeneration over longer periods. This data will be vital for obtaining regulatory approval for the first human clinical trials, expected within the next five years. Additionally, refining the manufacturing processes of the valves is necessary. As the project progresses, the team plans to seek commercial partners for later-stage clinical trials.

Although currently focused on heart valve replacement, this technology has potential applications in other areas, such as treating vascular conditions, repairing blood vessels damaged by dialysis, and creating cardiac patches for heart repair.
“The aim of the concept we’ve developed is to produce a living valve in the body, which would be able to grow with the patient,” said Dr. Yuan-Tsan Tseng, a biomaterials scientist. “Once you have the scaffold, it becomes a platform technology that you can use to engineer other tissues.”









19 HospiMedica International February-March/2024 To view this issue in interactive digital magazine format visit www.HospiMedica.com Surgical Techniques
PREMIER MULTIMEDIA PLATFORM SERVING THE WORLD’S HOSPITAL / MEDICAL COMMUNITY Anytime, Anywhere, On the Go... PRINT MAGAZINE INTERACTIVE DIGITAL EDITION WEB PORTAL ENGLISH • SPANISH MOBILE VERSION PRINT MAGAZINE • INTERACTIVE DIGITAL EDITION WEB PORTAL • MOBILE VERSION • MOBILE APPS HOSPIMEDICA EXPO • E-NEWSLETTER E-MAIL MARKETING • ONLINE SOLUTIONS DAILY CLINICAL NEWS .com Image: Inside the Agilent Measurement Suite at Imperial’s Molecular Sciences Research Hub (Photo courtesy of Imperial College London)

To receive prompt and free information on products, log on to www.linkXpress.com or scan the QR code on your mobile device
INTRAVENTRICULAR NEUROENDOSOPIC BRAUN, B.

MINOP is an intraventricular neuroendosopic system designed primarily for the treatment of intraventricular indications such as occlusive hydrocephalus and the removal of tumors
223

ELECTROSURGICAL UNIT BOWA

The ARC 250 electrosurgical unit offers all basic electrosurgical functions in combination with the latest control technology. It is designed for use in various specialty areas and wide range of applications.

Surgical Guidance System Targets Tumor Location in Multiple Dimensions for Precise Excision
Every 14 seconds, a woman receives a breast cancer diagnosis, making it the world’s most prevalent cancer. In 2020 alone, 2.3 million women were diagnosed, and 685,000 succumbed to the disease. Lumpectomy, a breast-conserving surgery, is a common treatment option. Yet, approximately 20-30% of women who undergo this procedure require a second surgery. Now, a new surgical guidance system targets tumor location in multiple dimensions for precise excision and successful surgeries, marking a significant advancement in breast cancer care.
The SCOUT MD Surgical Guidance System from Merit Medical Systems (South Jordan, UT, USA; www.merit.com) is a groundbreaking solution that allows for the implantation of up to four different reflector configurations in abnormal breast tissue or other soft tissues. This enables precise localization of tumors in multiple dimensions, aiding in more accurate surgical excision. Physicians and patients have the option to implant the reflector during a biopsy, and its long-term implant can be done any time before the surgery. The reflector, with a body size of just 4mm - smaller than a grain of rice – features antennas made of super-elastic nitinol alloy, a material frequently used in medical devices. It offers a real-time
distance measurement capability within a 60mm range and 360-degree detection with an accuracy of ± 1mm.

The system offers flexibility in placement methods, including ultrasound, radiography, and stereotactic guidance. The SCOUT Guide activates the previously passive reflector, providing instant feedback to guide the surgical dissection path and reduce guesswork. This precise method aims to minimize harm to surrounding healthy tissue, decrease the chances of needing a second operation, and prevent the emotional and physical distress often associated with repeat surgeries. The SCOUT MD Surgical Guidance System has received 510(k) clearance from the US Food and Drug Administration (FDA), expanding Merit’s oncology portfolio aimed at improving the diagnosis and treatment of breast cancer and other soft tissue cancers.
Image: Using the SCOUT system, surgeons can precisely target the affected tissue to pinpoint its location within 1mm (Photo courtesy of Merit Medical)
Blood-Brain-Barrier Opening Device Enhances Chemotherapy Drug Delivery to Brain
Tissue
The blood-brain barrier (BBB) presents a significant challenge in treating gliomas, a type of diffuse tumor that infiltrates the peri-tumoral normal brain. This barrier prevents over 95% of small-molecule drugs and all large-molecule drugs from entering the brain, thus reducing their therapeutic impact. Now, an implantable device utilizes the power of pulsed ultrasound to temporarily open the BBB. This provides a window period during which drug therapies can be administered and can reach the brain in higher and more effective concentrations when the BBB is disrupted.
Carthera’s (Lyon, France; carthera.eu) SonoCloud device is designed to be implanted in a skull window beneath the skin, remaining invisible from the outside. When activated for a few minutes via a transdermal needle connected to an external control unit, the device employs low intensity pulsed ultrasound (LIPU) to breach the BBB for several hours. This window of opportunity allows for the administration of drug therapies. The concept of using LIPU to disrupt the BBB has been under pre-clinical development for over two decades. The technology leverages low-intensity ultrasound, akin to levels used in diagnostic imaging, in conjunction with an intravenous microbubble agent to stimulate a therapeutic effect. The LIPU causes the injected microbubbles in brain microvessels to vibrate, mechanically disrupting the BBB’s tight junc-
tions, enhancing active transport across the BBB, and reducing active drug efflux transporters.
Carthera has developed multiple versions of the SonoCloud device, including SonoCloud-1 and SonoCloud-9. These MRI-compatible devices are engineered to disrupt large regions of the BBB, aiming to improve the therapeutic efficacy of drugs in specific brain areas. Currently, these devices are undergoing clinical trials for glioblastoma, brain metastases, and Alzheimer’s Disease. Carthera has initiated the SONOBIRD clinical trial, an open-label, comparative, randomized, multicenter study with a two-arm design and a 1:1 ratio. This trial will assess the overall survival of glioblastoma patients treated with carboplatin chemotherapy in conjunction with the SonoCloud-9 system to breach the BBB. Outcomes will be compared against standard regimens recommended by medical consensus, such as lomustine or temozolomide. Additionally, the trial aims to evaluate the effectiveness of the SonoCloud-9 and carboplatin treatment in delaying or reducing tumor growth.
“The launch of the SONOBIRD trial is a significant achievement in the clinical development of the SonoCloud-9 system. If the efficacy of carboplatin in combination with our device is proven, it will change the paradigm of how we treat glioblastoma,” said Carole Desseaux, chief clinical officer at Carthera.
20 HospiMedica International February-March/2024 WORLD’S MEDICAL DEVICE MARKETPLACE
224 HMI-03-24 LINKXPRESS COM
International Hospital Federation Announces 2023 IHF Award Winners
Cont’d from cover
The Awards Committee, comprising health leaders from across the world and chaired by Dr Lawrence Lai, IHF Honorary Member, received more than 500 entries from 43 countries and territories –marking a record since the establishment of the Awards in 2015.
The IHF Awards 2023 recipients in each category are as follows: Dr Kwang Tae Kim Grand Hospital Award:
.Gold: Sheikh Shakhbout Medical City (United Arab Emirates).
.Silver: King Faisal Medical Complex in Taif (Saudi Arabia).
.Bronze: Apollo Cancer Centres (India).
.Honorable mentions: Al Qassimi Women and Children Hospital (United Arab Emirates), Avenue Healthcare (Kenya), King Khalid Alkharj Hospital, Riyadh 1st Health Cluster (Saudi Arabia), French Medical Institute for Mothers and Children (Afghanistan), Prince Mohammed bin Abdulaziz Hospital (Saudi Arabia).
Seddiqi Holding Excellence Award for Corporate Social Responsibility:
.Gold: Myongji Hospital (Republic of Korea).
.Silver: The Nairobi Hospital (Kenya).
.Bronze: Al Amal Psychiatric Hospital (United Arab Emirates), and Yale New Haven Health System (United States of America).
.Honorable mentions: French Medical Institute for Mothers and Children (Afghanistan), Manila Doctors Hospital (Philippines), Lusiadas Saúde (Portugal), Amiri Medical Complex (Afghanistan), Mediker LLP (Kazakhstan).
Ashikaga-Nikken Excellence Award for Green Hospitals:
.Gold: Oulu University Hospital (Finland).
.Silver: Mediclinic Welcare Hospital (United Arab Emirates).
.Bronze: Dubai Health Authority – Dubai Academic Health Corporation (United Arab Emirates).
.Honorable mentions: Aga Khan University (Pakistan), Emirates Health Services (United Arab Emirates), Sharm El-Sheikh International Hospital (Egypt), Royal Hospital (Oman), Insel Gruppe (Switzerland), Cleveland Clinic Abu Dhabi (United Arab Emirates), Performance and Digital Health, EHS (United Arab Emirates). Sultanate of Oman Excellence Award for Health Services During Crisis:
.Gold: Tondo Medical Center (Philippines), and Hope Field Hospital for Women and Children of Bangladesh (Bangladesh).
.Silver: Sheba Medical Center (Israel).
.Bronze: LUX MED (Poland), Eka Kotebe General Hospital (Ethiopia), and Mariano Marcos Memorial Hospital and Medical Center (Philippines).
.Honorable mentions: People’s Hospital 115 (Vietnam), Apollo Health & Lifestyle Limited (India), Predisan Health Ministry (Honduras), Mariano Marcos Memorial Hospital and Medical Center (Philippines).
American Hospital Association Excellence Award for Healthcare Workers’ Wellbeing:
.Gold: Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (Taiwan), and Alder Hey Children’s NHS Foundation Trust (United Kingdom).

.Silver: Vicente Sotto Memorial Medical Center (Philippines), and Apollo Multispeciality Hospitals, Kolkata (India).
.Bronze: Saudi German Health (Egypt), Samsung Medical Center (Republic of Korea), and Emirates Health Services (United Arab Emirates).
.Honorable mentions: Emirates Health Services (United Arab Emirates) Intelligence program for healthcare decisions, Jubail General Hospital (Saudi Arabia), Children’s Wisconsin (United States of America), Apollo Proton Cancer Centre (India), Lusíadas Saúde (Portugal).
American College of Healthcare Executives
Excellence Award for Leadership and Management:
.Gold: Matosinhos Local Health Unit – ULSM (Portugal).
.Silver: Royal Hospital (Oman).
.Bronze: Dubai Health Authority (United Arab Emirates).
.Honorable mentions: Al-Kharj Maternity and Children Hospital (Saudi Arabia), Emirates Health Services (United Arab Emirates), SEHA Kidney Care (United Arab Emirates), Karolinska University Hospital (Sweden), Henry Ford Health (United States of America). Mastercard Excellence Award for Quality and Patient Safety:
.Gold: Fundació de Gestió Sanitària Hospital de la Santa Creu i Sant Pau (Spain)
.Silver: Hopitaux Universitaires de Genève (Switzerland)
.Bronze: Tan Tock Seng Hospital (Singapore).
.Honorable mentions: Health Information Systems – Emirates Health Services (United Arab Emirates), Fujairah Hospital (United Arab Emirates), Jubail General Hospital (Saudi Arabia), Al Qassimi Hospital (United Arab Emirates), Cleveland Clinic Abu Dhabi (United Arab Emirates), Tawam Hospital (United Arab Emirates), King Saud Medical City (Saudi Arabia), China Medical University Hospital (Taiwan), Emirates Health Services (United Arab Emirates).
The IHF Awards 2024 will be awarded at the 47th World Hospital Congress to be held in Rio de Janeiro (Brazil), and the call for entries will be announced in February 2024.
M&A Briefs
Mindray (Shenzhen, China; www.mindray.com) is to acquire control of APT Medical (Shenzhen, China; www.aptmed.com), a cardiovascular device company. The acquisition aims to merge the expertise of both Mindray and APT Medical in the field.
Olympus Corporation (Tokyo, Japan; www.olympus-global. com) acquired Taewoong Medical Co., Ltd. (Seoul, Korea; www. taewoongmedical.com). Taewoong will join Olympus’ Therapeutic Solutions Division, expanding Olympus's offerings in the GI market.
Karl Storz (Tuttlingen, Germany; www.karlstorz.com), acquired Innersight Labs Ltd. (ISL, London, UK; www.innersightlabs. com), a software manufacturer, continuing its expansion into inno-
vative software solutions.
Medical Illumination International (Chatsworth, CA, USA; www.medillum.com) acquired IsoLux (Naples, FL, USA; www. isoluxllc.com). The acquisition of IsoLux, known for its innovative surgical headlight products, is set to expand Medical Illumination's portfolio in surgical and medical lighting solutions.
Stryker (San Jose, CA, USA; www.stryker.com) is to acquire SERF SAS (Décines-Charpieu, France; www.serf.fr), a joint replacement company. The acquisition is expected to strengthen Stryker's presence in France and across Europe, as well as expand its global joint replacement portfolio.
21 HospiMedica International February-March/2024 To view this issue in interactive digital magazine format visit www.HospiMedica.com Industry News
Image: The IHF Awards promote exchange of good practices in areas of healthcare leadership, environmental sustainability, and innovation (Photo courtesy of IHF)
2024
MARCH
ICE 2024 – 21st International Congress of Endocrinology. Mar 1-3; Dubai, UAE; icecongress.com
CRITICARE 2024 – 30th Annual Conference of the Indian Society of Critical Care Medicine (ISCCM). Mar 1-3; Kolkata, India; criticare.isccm.org
WCA 2024 – 18th World Congress of Anaesthesiologists. Mar 3-7; Singapore; wca2024. org
5th MedExpo Ethiopia 2024. Mar 6-8; Addis Ababa, Ethiopia; expogr.com/ethiopia/ medexpo
ESGO 2024 – 25th European Congress on Gynaecological Oncology. Mar 7-10; Barcelona, Spain; congress.esgo.org
HIMSS24 - Healthcare Information and Management Systems Society. Mar 11-15; Orlando, FL, USA; himss.org
EMIM 2024 – 19th European Molecular Imaging Meeting. Mar 12-15; Porto, Portugal; e-smi.eu
Medical Fair India 2024. Mar 13-15; Mumbai, India; medicalfair-india.com
51st Annual Meeting of the Japanese Society of Intensive Care Medicine (JSICM). Mar 14-16; Sapporo, Japan; jsicm.org
KIMES 2024 – Korea International Medical & Hospital Equipment Show. Mar 14-17; Seoul, Korea; kimes.kr
SALMED International Medical Fair 2024. Mar 19-21; Poznan, Poland; salmed.pl
43rd ISICEM – International Symposium on Intensive Care & Emergency Medicine. Mar 19-22; Brussels, Belgium; isicem.org
AOCR 2024 – Asian Oceanian Congress of Radiology. Mar 22-25; Taipei, Taiwan; aocr2024.org
SIR 2024 – Annual Meeting of the Society of Interventional Radiology. Mar 23-28; Salt Lake City, UT, USA; sirmeeting.org
APRIL
144th Annual Meeting of the American Surgical Association (ASA). Apr 4-6; Washington, DC, USA; meeting.americansurgical.org
39th Annual EAU Congress – European Association of Urology. Apr 4-7; Paris, France; uroweb.org
UltraCon 2024 – Annual Conference of the American Institute of Ultrasound in Medicine (AIUM). Apr 6-10; Austin, TX, USA; aium.org
37th Medicall Expo. Apr 6-8; Hyderabad, India; medicall.in
ACC.24 – American College of Cardiology’s
73rd Annual Scientific Session & Expo. Apr 6-8; Atlanta, GA, USA; accscientificsession. acc.org
83rd Annual Meeting of Japan Radiological Society (JRS). Apr 11-14; Yokohama, Japan; radiology.jp
WCO-IOF-ESCEO 2024 - World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Apr 11-14; London, UK; wco-iof-esceo.org
CMEF Spring 2024 – 89th China Medical Equipment Fair. Apr 11-14; Shanghai, China; www.cmef.com.cn
WCN 2024 – World Congress of the International Society of Nephrology (ISN). Apr 1316; Buenos Aires, Argentina; theisn.org
AAN 2024 – 76th Annual Meeting of the American Academy of Neurology. Apr 13-18; Denver, CO, USA; aan.com
Medic West Africa 2024. Apr 17-19; Lagos, Nigeria; medicwestafrica.com
SEACare 2024 – 24th Southeast Asian Healthcare & Pharma Show. Apr 17-19; Kuala Lumpur; Malaysia; abcex.com
24th MEDEXPO Africa 2024. Apr 17-19; Nairobi, Kenya; expogr.com/kenyamed
SAGES 2024 – Annual Meeting of the Society of American Gastrointestinal and Endoscopic Surgeons. Apr 17-20; Cleveland, OH, USA; sages.org
124th Congress of the Japan Surgical Society
(JSS). Apr 18-20; Tokoname, Japan; jp.jssoc. or.jp
DCK 2024 – 141st Congress of the German Society for Surgery (DGCH). Apr 24-26; Leipzig; Germany; dgch.de
KSCCM-ACCC 2024 – 44th Annual Congress of the Korean Society of Critical Care Medicine. Apr 24-26; Seoul, Korea; accc.or.kr
Expomed Euroasia 2024. Apr 25-27; Istanbul, Turkey; expomedistanbul.com AAEM24 – 30th Annual Scientific Assembly of the American Academy of Emergency Medicine. Apr 27 - May 1; Austin, TX, USA; aaem.org
ECTES 2024 – 23rd Congress of the European Society for Trauma & Emergency Surgery (ESTES). Apr 28-30; Lisbon, Portugal; estes-congress.org
ECIO 2024 – European Conference on Interventional Oncology. Apr 28 - May 1; Palma de Mallorca, Spain; ecio.org
MAY
APSC-ECS 2024 - 28th Asian Pacific Society of Cardiology Congress & 15th Annual Emirates Cardiac Society Congress. May 2-4; Dubai, UAE; apsc-ecs2024.com
APSCVIR 2024 – 18th Annual Meeting of the Asia Pacific Society of Cardiovascular and Interventional Radiology. May 3-5; Bangkok, Thailand; apscvir2024.com
AUA 2024 – Annual Meeting of the American Urological Association. May 3-6; San Antonio, TX, USA; auanet.org
ESTRO 2024 – Annual Congress of the European Society of Radiology & Oncology. May 3-7; Glasgow, UK; estro.org
2024 ISMRM & ISMRT Annual Meeting & Exhibition – International Society for Magnetic Resonance in Medicine. May 4-9; Singapore; ismrm.org
ARRS 2024 Annual Meeting – American Roentgen Ray Society. May 5-9; Boston, MA, USA; arrs.org
Vietnam Medi-Pharm 2024 - The 31st Vietnam International Medical and Pharmaceutical Exhibition. May 9-12; Hanoi, Vietnam; vietnammedipharm.vn
ECCC Dubai 2024 - 20th Emirates Critical Care Conference. May 10-12; Dubai, UAE; eccc-dubai.com
ECE 2024 – 26th Congress of the European Society of Endocrinology. May 11-14; Stock-
holm, Sweden; ese-hormones.org
KIHE 2024 – 29th Kazakhstan International Healthcare Exhibition. May 15-17; Almaty, Kazakhstan; kihe.kz
ATS 2024 – International Conference of the American Thoracic Society. May 17-22; San Diego, CA, USA; conference.thoracic.org
Hospitalar 2024. May 21-24; Sao Paulo, Brazil; hospitalar.com
EFORT Congress 2024 – 25th Annual Congress of European Federation of National Associations of Orthopaedics and Traumatology. May 22-24; Hamburg, Germany; congress.efort.org
61st ERA Congress – European Renal Association. May 23-26; Stockholm, Sweden; era-online.org
ASCVTS – 32nd Annual Meeting of the Asian Society for Cardiovascular and Thoracic Surgery. May 23-26; Wuhan, China; ascvts.org EuroAnaesthesia 2024 – European Society of Anaesthesiology. May 25-27; Munich, Germany; euroanaesthesia.org
ESTS 2024 – 32nd Meeting of the European Society of Thoracic Surgeons. May 26-28; Barcelona, Spain; ests2024.com
92nd EAS Congress 2024 – European Atherosclerosis Society. May 26-29; Lyon, France; eas-society.org
ESGAR 2024 – Annual Meeting of the European Society of Gastrointestinal and Abdominal Radiology. May 28-31; Gothenburg, Sweden; esgar.org
JUNE
12th Congress of the World Federation of Pediatric Intensive and Critical Care Societies. Jun 1-5; Cancun, Mexico; wfpiccs.org
India Health 2024. Jun 13-15; New Delhi, India; indiahealth-exhibition.com
EHA 2024 - Annual Congress of the European Hematology Association. Jun 13-16; Madrid, Spain; ehaweb.org
MedtecLIVE 2024. Jun 18-20; Stuttgart, Germany; medteclive.com
CARS 2024 – Computer Assisted Radiology and Surgery. Jun 18-21; Barcelona, Spain; cars-int.org
FIME 2024 – Florida International Medical Expo. Jun 19-21; Miami, FL, USA; fimeshow.com
ICEM 2024 – 22nd International Conference on Emergency Medicine. Jun 19-23; Taipei. Taiwan; ifem.cc

International Calendar
For a free listing of your event, or a paid advertisement in this section, contact: International Calendar, HospiMedica International E-mail: info@globetech.net
Medical Taiwan 2024. Jun 20-22; Taipei, Taiwan; medicaltaiwan.com.tw
ESICM EuroAsia 2024 – European Society of Intensive Care Medicine (ESICM) & Indian Society of Critical Care Medicine (ISCCM). Jun 21-23; Bengaluru, India; esicm.org
SIIM 2024 – Annual Meeting of the Society for Imaging Informatics in Medicine. Jun 27-29; National Harbor, MD, USA; siim.org
JULY
ESHRE 2024 – 40th Annual Meeting of the European Society of Human Reproduction and Embryology. Jul 7-10; Amsterdam, Netherlands; eshre.eu
Meditech 2024 – 8th International Health Fair. Jul 9-12; Bogota, Colombia; feriameditech.com
Asia Health 2024. Jul 10-12; Bangkok, Thailand; medlabasia.com
SCCT 2024 – 19th Annual Scientific Meeting of the Society of Cardiovascular Computed Tomography. Jul 18-21; Washington, DC, USA; scct.org
IndoHealthCare 2024. Jul 31 - Aug 2; Jakarta, Indonesia; indohealthcareexpo.com
AUGUST
38th Medicall Expo. Aug 2-4; Chennai, India; medicall.in
APICS 2024 – Asia Pacific Intensive Care Symposium. Aug 17-19; Singapore; sg-apics.com
Expo Med – Hospitalar Mexico 2024. Aug 2022; Mexico City, Mexico; expomed.com.mx Medical Fair China 2024. Aug 21-23; Shanghai, China; medicalfair.cn
International Surgical Week 2024 – 50th World Congress of the International Society of Surgery ISS/SIC. Aug 25-29; Kuala Lumpur, Malaysia; isw2024.org
ESC Congress 2024 - European Society of Cardiology. Aug 30 - Sep 2; London, UK: escardio.org
SEPTEMBER
Medic East Africa 2024. Sep 4-6; Nairobi, Kenya; www.mediceastafrica.com
ESRA 2024 – 41st Annual Congress of the European Society of Regional Anaesthesia and Pain
Therapy. Sep 4-7; Prague, Czech Republic; esracongress.com
UAA 2024 – 21st Urological Association of Asia Congress. Sep 5-8; Bali, Indonesia; uaanet.org
ERS International Congress 2024 – European Respiratory Society. Sep 7-11; Vienna, Austria; erscongress.org
EASD 2024 – 60th Annual Meeting of the European Association for the Study of Diabetes. Sep 9-13; Madrid, Spain; easd.org
47 World Hospital Congress of the International Hospital Federation (IHF). Sep 10-12; Rio de Janeiro, Brazil; ihf-fih.org
Medical Fair Asia 2024. Sep 11-13; Singapore; medicalfair-asia.com
ASCI 2024 – The 17th Congress of Asian Society of Cardiovascular Imaging. Sep 12-14; Shanghai, China; asci-heart.org
ESMO Congress 2024 - European Society for Medical Oncology. Sep 13-17; Barcelona, Spain; esmo.org
CIRSE 2024 – Annual Congress of the Cardiovascular and Interventional Radiological Society of Europe. Sep 14-18; Lisbon, Portugal; cirse.org
ISUOG World Congress 2024 – 34th World Congress on Ultrasound in Obstetrics and Gynecology. Sep 15-18; Budapest, Hungary; isuog.org
ISS 2024 Annual Meeting – International Skeletal Society. Sep 15-20; Montreal, QC, Canada; internationalskeletalsociety.com
ExpoMedical 2024. Sep 18-20; Buenos Aires, Argentina; expomedical.com.ar
20th EuGMS Congress – European Geriatric Medicine Society. Sep 18-20; Valenica, Spain; eugms2024.com
CBR24 – 53rd Congress of the Brazilian College of Radiology and Diagnostic Imaging. Sep 1921 Salvador, Brazil; cbr.org.br
REHACARE 2024 – International Trade Fair for Rehabilitation and Care. Sep 25-28; Dusseldorf, Germany; rehacare.com
CADI 2024 – Argentinian Congress of Diagnostic Imaging. Sep 26-28; Buenos Aires, Argentina; sar.org.ar
ASTRO 2024 – 66th Annual Scientific Meeting of the American Society for Radiation Oncology. Sep 29 – Oct 2; Washington, DC, USA; astro.org
ACEP24 – Scientific Assembly of the American College of Emergency Physicians. Sep 29 - Oct 2; Las Vegas, NV, USA; acep.org
OCTOBER
ESSO 43 – 43rd Congress of the European Society of Surgical Oncology. Oct 2-4; Antwerp, Belgium; esso43.org
KCR 2024 – 80th Korean Congress of Radiology. Oct 2-5; Seoul, Korea; kcr4u.org
EUSOBI 2024 - Annual Scientific Meeting of the European Society of Breast Imaging. Oct 3-5; Lisbon, Portugal; eusobi.org
JFR 2024 - Journées Francophones de Radiologie. Oct 4-7; Paris, France; www.jfr.plus 39th Medicall Expo. Oct 5-7; New Delhi, India; medicall.in
ECISM LIVES 2024 – Annual Congress of European Society of Intensive Care Medicine. Oct 5-9; Barcelona, Spain; esicm.org
MICCAI 2024 – 27th International Conference on Medical Image Computing and Computer Assisted Intervention. Oct 6-10; Marrakesh, Morocco; miccai.org
Medical Japan 2024 Tokyo– International Medical and Elderly Care Expo. Oct 9-11; Tokyo, Japan; medical-jpn.jp
UEG Week 2024 – United European Gastroenterology. Oct 12-15; Vienna, Austria; ueg.eu
CMEF Autumn 2024 – 90th China Medical Equipment Fair. Oct 12-15; Shenzhen, China; www.cmef.com.cn
EUSEM 2024 – 18th European Emergency Medicine Congress. Oct 13-16; Copenhagen, Denmark; eusemcongress.org
RANZCR 2024 – 74th Annual Scientific Meeting of The Royal Australian and New Zealand College of Radiologists. Oct 17-19; Perth, Australia; ranzcrasm.com
ANESTHESIOLOGY 2024 – Annual Meeting of the American Society of Anesthesiologists. Oct
18-22; Philadelphia, PA, USA; asahq.org
ACS Clinical Congress 2024 – American College of Surgeons. Oct 19-22; San Francisco, CA, USA; facs.org
EANM 2024 – 37th Annual Congress of the European Association of Nuclear Medicine. Oct 19-23; Hamburg, Germany; eanm.org
Global Health Exhibition 2024. Oct 22-24; Riyadh, Saudi Arabia; globalhealthsaudi.com
Africa Health 2024. Oct 22-24; Johannesburg, South Africa; africahealthexhibition.com
16th World Stroke Congress - World Stroke Organization. Oct 23-26; Abu Dhabi, UAE; worldstrokecongress.org
NOVEMBER
APSR 2024 – 28th Congress of the Asian Pacific Society of Respirology. Nov 7-10; Singapore; apsr2024.hk
MEDICA 2024. Nov 11-14; Dusseldorf, Germany; medica-tradefair.com
CBMI 2024 – 29th Brazilian Congress of Intensive Care Medicine. Nov 14-16; Sao Paulo, Brazil; amib.org.br
ASUS 2024 – 7th Congress of Asian Surgical Ultrasound Society. Nov 16-17; Seoul, Korea, asus2024.org
DECEMBER
RSNA 2024 – Annual Meeting of the Radiological Society of North America. Dec 1-5; Chicago, IL, USA; rsna.org
66th ASH Annual Meeting and Exposition –American Society of Hematology. Dec 7-10; San Diego, CA, USA; hematology.org
2025
JANUARY
Arab Health 2025. Jan 27-30; Dubai, UAE; arabhealthonline.com
FEBRUARY
Critical Care Congress 2025 – 54th Annual Meeting of the Society of Critical Care Medicine (SCCM). Feb 23-25; Orlando, FL, USA; sccm.org
International Calendar Provided as a service to advertisers. Publisher cannot accept responsibility for any errors or omissions. Advertising Index Inq .No . Advertiser Page – ECR 2024 ............................ 7 – ESC Congress 2024 ................... 13 – Expomed Eurasia 15 – HospiMedica 19 – HospiMedica EXPO 3 – IHF Rio 2024 22 111 Lumiquick 11 105 Radcal ............................... 5 124 Sekisui .............................. 24 102 Singuway ............................. 2 117 Vicolab .............................. 17 109 West Medica .......................... 9 Vol. 42 Issue 1 3/2024 HospiMedica International
ATTENTION: IF YOUR APPLICATION IS NOT RECEIVED AT LEAST ONCE EVERY 12 MONTHS YOUR FREE SUBSCRIPTION MAY BE AUTOMATICALLY DISCONTINUED READER SERVICE PORTAL LINKXPRESS COM ® Every advertisement or product item in this issue contains a LinkXpress number as shown below: 999 HMI-03-24 LINKXPRESS COM Identify LinkXpress codes of interest as you read magazine Click on LinkXpress.com to reach reader service portal Mark code(s) of interest on LinkXpress inquiry matrix 1 2 3 Renew/ S tart Your Free Subscription Access Instant Online Product Information VISIT
50%
42%
40%
Are Vaginitis Disorders Underdiagnosed?
Beverly and Trixie can be very hard to diagnose which is why the OSOM® Trichomonas and OSOM® BVBLUE® tests are a valuable tool.
They offer non-invasive, point-of care testing with over 90% accuracy. Both products give accurate results within 10 minutes or less, with colour changing results allowing for quick, easy interpretation.



1.Carr, P. “Shotgun” Versus Sequential Testing. J Gen Intern Med. 2005. 2. Hiller, S. Diagnosis and Treatment of Vaginal Discharge Syndromes in Community Practice Settings. Clinical Infectious Disease. 2020. 3. Brown, H. Improving the Diagnosis of Vulvovaginitis. Population Health Management. Vol. 23, suppl 1, 2020. © 2024 SEKISUI Diagnostics, LLC. All rights reserved. OSOM® and QC Inside® are registered trademark of SEKISUI Diagnostics, LLC. Because every result matters™ is a trademark of SEKISUI Diagnostics, LLC. Click or scan the QR code to watch our Webinar on Vaginitis Click or scan the QR code to access our Vaginosis article
of women leave the medical visit undiagnosed1
treatment2
of women receive inappropriate
of time vaginitis symptoms are misdiagnosed3 Trixie Beverly Visit us at osomtests.com Speed, Accuracy and Value. Awesome........yes, OSOM. 124 HMI-03-24 LINKXPRESS COM






























































































































