

Your Editor of the
Future realism with a scientific flair and an entertaining twist -Independent and Sponsor free-
JUNE 2025 – Edition - 5th Year-
2-BIOELECTRONIC MEDECINE




Your Editor of the
Future realism with a scientific flair and an entertaining twist -Independent and Sponsor free-
JUNE 2025 – Edition - 5th Year-



Two pioneers, Sue Desmond-Hellmann and Charles Sawyers, laid the foundational stones for what we now celebrate as precision medicine, transforming healthcare from a generalized approach into highly individualized patient care.
Sue Desmond-Hellmann, an American oncologist and biotechnology executive, championed the concept of tailoring treatments based on a deep understanding of individual genetics.
Her groundbreaking contributions at Genentech during the development of targeted cancer therapies, particularly trastuzumab (Herceptin) for HER2-positive breast cancer, proved pivotal.
This revolutionary approach provided concrete proof that treatments designed to target specific genetic profiles could significantly improve patient outcomes, fundamentally changing oncology forever.
Similarly transformative was the work of Charles Sawyers, who stands alongside Desmond-Hellmann as a foundational figure.
A renowned physician-scientist, Sawyers pioneered the development of targeted drugs against chronic myeloid leukemia (CML).
His efforts led to the creation of imatinib (Gleevec), one of the first successful precision medicines, dramatically improving patient survival rates and setting new standards in oncology care.
Together, their insights reshaped modern medicine, laying essential groundwork upon which today's remarkable innovations stand. Their visionary leadership not only advanced cancer treatment but also laid the intellectual foundation for breakthroughs that are continually reshaping how we diagnose, treat, and understand human disease.


A groundbreaking 20-minute procedure could replace invasive surgery for millions with a hidden form of high blood pressure, according to research published in The Lancet.
The innovative treatment, called Triple T (Targeted Thermal Therapy), offers new hope for people with primary aldosteronism—a condition where tiny nodules in the adrenal glands produce excess hormones that raise blood pressure.
Though this condition affects one in twenty people with hypertension, fewer than one percent are ever diagnosed.
Until now, the only cure required surgical removal of an entire adrenal gland, involving general anesthesia, days in hospital, and weeks of recovery. Many patients simply went untreated, facing higher risks of heart attacks, strokes, and kidney failure.
Triple T changes everything by precisely targeting just the problematic nodule. Using advances in molecular imaging to locate the nodule, doctors insert a tiny camera through the mouth into the stomach. From there, they guide a fine needle to the adjacent adrenal gland and deliver short bursts of radiofrequency heat that destroy only the malfunctioning tissue.
"It's like performing surgery without making a single cut," explains Professor Morris Brown from Queen Mary University of London, cosenior author of the study that tested Triple T in 28 patients.
The results were remarkable—most participants had normal hormone levels six months later, with many stopping all blood pressure medications. Michelina Alfieri, a trial participant who suffered debilitating headaches for years, reported returning to her normal routine immediately after treatment.
This medical advance combines two established technologies: heat generation through radiofrequency waves and real-time visualization using ultrasound. It's a perfect example of how repurposing existing tools can create revolutionary treatments.
A larger trial comparing Triple T with traditional surgery is underway, with results expected in 2027. If successful, this wavebased therapy could soon be available worldwide, transforming treatment for a common yet overlooked cause of hypertension.


Scientists are beginning to create living therapies: cells that can sense trouble in the body and respond with a cure. These bioengineered “smart cells” are genetically modified or synthetic cells programmed to detect, respond to, and treat diseases dynamically.
In essence, they behave like tiny protein-based processors that “decide” how to act based on signals around them. For example, a smart cell might recognize an inflammatory or tumor signal and instantly release a tailored therapeutic molecule in response (Bioengineers develop construction kit for 'smart cell' design), delivering treatment at the perfect time and place.
How is this possible? Researchers equip cells with synthetic molecular circuits that kick into action when needed. One approach uses a lock-and-key protein switch that stays inactive until it encounters its specific molecular “key,” then unleashes a controlled response inside the cell
.With tools like this, scientists can endow cells with remarkable abilities — from releasing drugs on cue to communicating with other cells to coordinate healing. In fact, artificial cells have been designed that detect changes in their environment and send protein signals to influence neighboring cells, meeting the body’s needs without flooding the whole organism with drugs (Smart cells: Chemists develop tool with potential to treat illness at the cellular level | ScienceDaily).
The emergence of smart cells marks a new frontier in precision medicine. Because these cells can be custom-designed for each patient and condition, they enable highly personalized treatments that adapt in real time to the individual’s needs. This patient-specific approach promises to improve outcomes across a range of applications:
• Cancer Immunotherapy: A patient’s own immune cells can be reprogrammed to hunt and destroy their unique cancer cells. CAR-T cell therapy, for instance, harnesses the body’s T-cells to deliver targeted anticancer effects and has been called the ultimate personalized treatment (CAR-T-cell therapy, the ultimate personalised cancer treatment).
• Autoimmune Disease Control: Smart cells can sense inflammatory molecules and release soothing factors on site. One experimental circuit was shown to detect inflammatory signals and could help control autoimmune flare-ups while reducing toxic side effects of treatment (Revolutionary Advances in ‘Smart Cell’ Design Unveiled).
• Diabetes Management: Engineered cells may act as living insulin pumps, automatically secreting insulin when blood sugar levels rise (Bioengineers develop construction kit for 'smart cell' design). This could provide diabetics with precise, real-time glucose control without external insulin injections.
Bioengineered smart cells exemplify the promise of precision medicine. Researchers predict they will “usher in a new generation of smart, precise and robust live cell therapies” (Artificial proteins transform ordinary cells into smart cells) for diseases once deemed untreatable. By delivering the right intervention at exactly the right time for each patient, smart cells are transforming medicine from a one-size-fits-all endeavor into a truly personalized and dynamic healing process.


Metatranscriptomics stands as a cutting-edge sequencing approach – arguably the most advanced for studying microbial communities. It captures the RNA transcripts of all microbes in a sample, so researchers can see not just which microbes are present but also what genes they are actively expressing in real time (How to avoid wasting RNA-seq reads in metatranscriptomics).
In effect, it provides a live snapshot of microbial activity and functional pathways across an entire community. For example, the human gut contains on the order of 100 trillion microorganisms and metatranscriptomic analysis can rapidly sequence and analyze their collective gene expression.
The scale and speed of this approach are revolutionary: early genome sequencing projects like the Human Genome Project took 13 years to sequence one genome), whereas metatranscriptomics now allows profiling of whole microbiomes within days or even hours.
Precision Medicine Potential This real-time window into microbiome activity is driving a new era of personalized healthcare. Metatranscriptomics enables precision medicine by:
• Identifying microbial imbalances: Pinpointing shifts in a patient’s microbiome (dysbiosis) that are linked to disease, and revealing microbial biomarkers for diagnosis and risk prediction (Frontiers | Multi-omics approaches to studying gastrointestinal microbiome in the context of precision medicine and machine learning).
• Predicting treatment responses: Indicating how an individual might respond to specific drugs or dietary interventions based on their microbiome’s active metabolic pathways.
• Optimizing therapies: Guiding targeted treatments (like tailored probiotics or diet changes) to restore balance and beneficial functions, and even suggesting microbe-focused interventions for more effective outcomes (Beyond microbial identification: analyzing metatranscriptomics versus metagenomics).
This paradigm shift is helping to realize the promise of precision medicine.

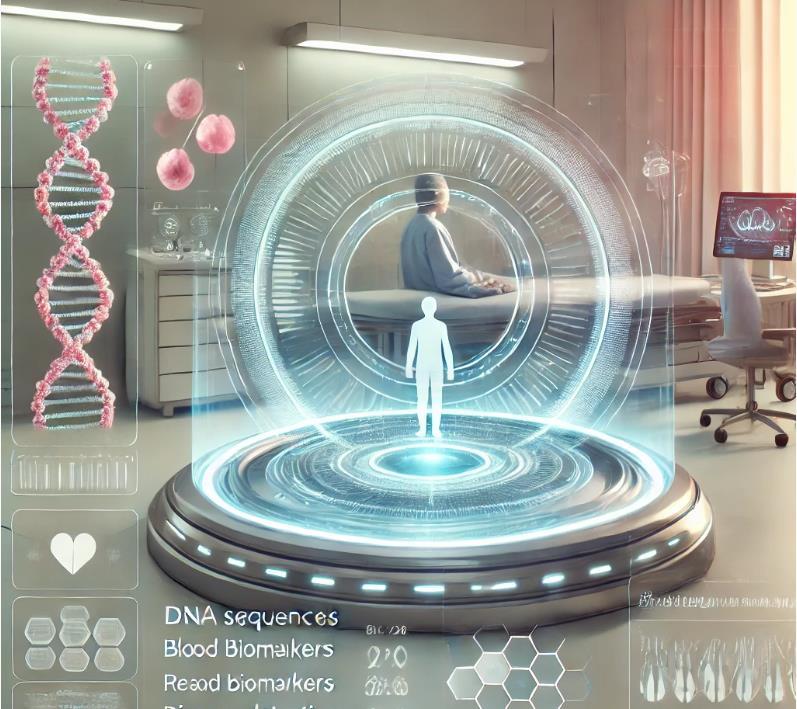
Molecular diagnostics is redefining disease detection and management by analyzing biological markers in DNA, RNA, and proteins. This technology enables noninvasive, highly accurate, and early diagnosis, making it a game-changer in oncology, neurology, and infectious diseases.
One of its most significant breakthroughs is blood-based biomarker testing, which can detect conditions like Alzheimer’s disease up to 15 years before symptoms appear. Researchers have identified key blood
proteins GFAP, NEFL, GDF15, and LTBP2 that, when combined with genetic risk factors, provide up to 90% accuracy in predicting dementia.
In cancer detection, liquid biopsies are revolutionizing early diagnosis. Tests like Galleri can identify over 50 types of cancer by analyzing circulating tumor DNA in the bloodstream. Early detection significantly increases survival rates by enabling treatment before metastasis occurs.
Molecular diagnostics is also transforming infectious disease management. CRISPR-based tools such as Sherlock Biosciences’ Inspect platform can detect viral RNA in under 30 minutes, allowing for rapid outbreak response. Oxford Nanopore’s portable MinION sequencer offers real-time pathogen sequencing outside traditional labs, improving accessibility in remote areas.
Beyond early detection, molecular diagnostics is a pillar of precision medicine. By identifying patient-specific genetic and proteomic profiles, this technology allows treatments to be tailored to individual needs. In neurodegenerative diseases like Alzheimer’s, biomarker detection can guide targeted therapies, while in cancer, molecular profiling enables personalized immunotherapy strategies.
With the global market expected to surpass $26 billion by 2032, molecular diagnostics is set to drive down healthcare costs, improve survival rates, and accelerate drug development. As testing becomes more accessible and refined, this field is ushering in a new era of data-driven, individualized healthcare solutions.


The future of medical research is being reshaped by silico simulations, virtual reality (VR), and decentralized clinical trials.
These technologies accelerate drug and device development by improving trial efficiency, reducing costs, and broadening accessibility. Unlike traditional methods, which rely on large patient cohorts and lengthy protocols, new trial models leverage digital tools to simulate biological responses, test interventions in controlled virtual environments, and gather real-world patient data remotely.
In silico trials use computational models to predict how drugs interact with human physiology. By simulating disease progression and treatment effects, researchers can optimize drug formulations and refine dosing strategies before human testing begins. This method, exemplified by the FDA’s VICTRE project, demonstrates how synthetic trial participants can generate regulatory-grade evidence.
VR-based trials introduce a new dimension to testing, allowing patients to engage with medical devices and therapies in immersive, controlled settings. These simulations provide researchers with deeper insights into patient behavior and treatment efficacy. Meanwhile, remote trials powered by AI-driven platforms and wearable technology enable participants to contribute data from home, minimizing geographic and logistical barriers.
These innovations directly enhance precision medicine, a field that tailors treatments to individual genetic, environmental, and lifestyle factors. In silico models allow for the rapid testing of therapies on virtual patients with diverse genetic profiles, accelerating the identification of optimal treatments for specific subpopulations.
VR environments help personalize rehabilitation strategies, mental health interventions, and patient adherence programs. Remote trials improve the granularity of patient monitoring, capturing continuous physiological data that refines precision treatment strategies.
By reducing trial costs, improving patient access, and enhancing data accuracy, these novel approaches are revolutionizing clinical research. Their integration into regulatory frameworks will be key to unlocking a new era of truly personalized, data-driven healthcare.


Transformation of Healthcare: The integration of genetic engineering is changing how precision medicine is approached, moving away from a "one-size-fits-all" model to more personalized treatments tailored to individual patients based on their unique genetic, environmental, and lifestyle factors .
Role of Genetic Engineering: Genetic engineering plays a crucial role in advancing precision medicine. It is applied in various areas such as drug discovery, pharmacogenomics (the study of how genes affect a person's response to drugs), and innovative therapies, including gene and cell treatments .
Technological Advancements: Tools like CRISPR-Cas9 and advancements in omics technologies (which study the roles, relationships, and actions of the various types of molecules that make up the cells of an organism) have significantly accelerated the development of personalized therapies. These technologies also enhance our understanding of disease mechanisms .
Challenges:Despite the advancements, there are still challenges to overcome. These include technical issues like off-target effects (unintended changes to the genome), ethical concerns regarding germline editing (changes made to the genes in sperm or eggs), and the high costs associated with these technologies .
Opportunities for Innovation: The paper highlights the potential for innovation through artificial intelligence and collaborative research initiatives, which could streamline the development of genetic engineering applications. This interdisciplinary approach is reshaping drug development and expanding treatment options for complex diseases.
Future of Precision Medicine is with continued investment, collaboration across disciplines, and a focus on equitable access, the vision of precision medicine—where treatments are customized to each individual's genetic makeup—will become a reality.



George Malliaras and Daniel Simon are widely regarded as pivotal figures in shaping the emerging discipline of modern bioelectronics, a field that bridges biology and electronics to unlock unprecedented opportunities for medical innovation.
George Malliaras, a Greek-born scientist and professor at the University of Cambridge, profoundly influenced the domain through his pioneering research on organic bioelectronic devices. He demonstrated how conductive polymers could effectively interface with biological tissues, opening entirely new pathways for treating neurological disorders.
Malliaras's groundbreaking work on organic electrochemical transistors (OECTs) provided the scientific foundation for modern bioelectronic medicine, enabling precise sensing and stimulation of neural activity at unprecedented sensitivity and specificity. His innovative contributions paved the way for revolutionary implantable devices capable of
restoring damaged neural functions and treating chronic illnesses through electricity rather than pharmaceuticals alone.
Similarly impactful, Daniel Simon's visionary research has advanced bioelectronics into transformative medical territory. Simon, a leading scientist in organic bioelectronics, has driven critical innovations that bridge electronics and living systems, particularly focusing on biosensing and real-time monitoring of biochemical signals.
His work enabled precise, minimally invasive diagnostics and treatments by leveraging bioelectronics to monitor cellular activities and disease markers continuously.
Simon's pioneering studies in bioelectronic interfaces significantly enhanced the field’s understanding of how electronic signals can be harnessed at the cellular level, transforming our approach to personalized medicine and laying vital groundwork for future therapeutic breakthroughs.


An Australian man in his 40s has made medical history by living 100 days with a titanium artificial heart before receiving a donor transplant. This milestone represents the longest anyone has survived with this revolutionary device, and he even became the first person ever discharged from hospital with it, continuing his life at home while awaiting a suitable donor.
The BiVACOR Total Artificial Heart defies conventional engineering with its elegant simplicity. Unlike complex mechanical hearts of the past, this device contains just a single moving part: a titanium rotor that floats in place using magnetic levitation. With no valves or mechanical bearings
to wear out, the device pumps blood to both the body and lungs without damaging blood cells.
For Australian bioengineer Daniel Timms, this breakthrough is deeply personal. After losing his father to heart disease, Timms dedicated his career to developing this lifesaving technology.
"The entire BiVACOR team is deeply grateful to the patient and his family for placing their trust in our Total Artificial Heart," Timms said. "Their bravery will pave the way for countless more patients to receive this lifesaving technology.

Professor Chris Hayward from the Victor Chang Cardiac Research Institute, who oversaw the patient's recovery, believes we're entering "a whole new ball game for heart transplants."
"Within the next decade we will see the artificial heart becoming the alternative for patients who are unable to wait for a donor heart or when a donor heart is simply not available," Hayward predicts.
This breakthrough offers new hope for addressing heart disease, the world's leading killer. Cardiovascular diseases claim approximately 18 million lives annually, and the mathematics of organ donation creates an inevitable shortage there simply aren't enough donor hearts for everyone who needs one.
While still undergoing clinical trials in both the US and Australia, this spinning titanium heart represents not just a temporary solution but a potential transformation in how we approach endstage heart failure. For millions worldwide waiting for a heart that may never come, this technology could replace the ticking clock of mortality with the steady hum of innovation—a bridge that carries patients safely from diagnosis to cure.


Digital biomarkers are redefining early disease detection by leveraging continuous, real-time health data from wearables, smartphones, and sensors.
Unlike traditional biomarkers, which depend on periodic clinical visits and invasive tests, digital biomarkers provide a non-invasive, dynamic, and objective way to track physiological and behavioral changes before diseases fully manifest.
In neurodegenerative disorders like Alzheimer’s, digital biomarkers detect subtle shifts in speech patterns, motor function, and sleep cycles, often years before clinical symptoms appear. AI-powered analytics can track cognitive decline through smartphone-based voice analysis, offering a window into disease progression and enabling earlier, more effective interventions.
Similarly, in cardiovascular health, wearable ECG monitors can detect irregular heart rhythms, heart rate variability, and subtle fluctuations in blood pressure, helping identify early signs of heart disease. These insights allow physicians to intervene before life-threatening events, significantly improving patient outcomes.
In neurodegenerative diseases like Alzheimer's and Parkinson's, digital biomarkers have demonstrated remarkable potential. Advanced algorithms can detect microscopic changes in motor control through smartphone touchscreen interactions, identifying Parkinson's symptoms years before clinical diagnosis. Similarly, voice analysis applications can identify subtle speech pattern alterations characteristic of cognitive decline, creating opportunities for early intervention in Alzheimer's disease.
These technologies monitor changes in speech patterns, sleep disturbances, and cognitive function long before clinical symptoms become apparent, providing a window for preventative measures that was previously unimaginable (Seyhan et al., 2019).
Cardiovascular health monitoring has been particularly transformed by digital biomarker technology. Modern wearable ECG monitors can detect atrial fibrillation with clinical-grade accuracy, while photoplethysmography sensors in common smartwatches measure
blood oxygen levels and heart rate variability. These capabilities enable continuous cardiovascular assessment outside clinical settings, detecting arrhythmias or subtle changes in heart function that might otherwise go unnoticed until a catastrophic event occurs. One study found that wearable-derived digital biomarkers identified heart failure exacerbation an average of 6.5 days before traditional clinical detection methods, potentially preventing hospitalizations and improving outcomes.

The true power of digital biomarkers emerges through their integration with artificial intelligence and machine learning systems. These
advanced analytical tools process the massive datasets generated by digital devices, identifying complex patterns invisible to human observation. AI algorithms can synthesize multiple biomarker inputs— from gait analysis to sleep patterns to social media usage—creating multi-dimensional health profiles that significantly outperform singlemetric approaches. For example, an AI system analyzing smartphone accelerometer data combined with app usage patterns achieved 87% accuracy in predicting depressive episodes, compared to just 63% accuracy using clinical questionnaires alone.
Beyond detection, digital biomarkers enable truly personalized treatment approaches. Continuous glucose monitors paired with AI can predict hypoglycemic events hours in advance for diabetic patients, while emotion recognition algorithms analyzing facial expressions can assess psychiatric medication efficacy more objectively than selfreporting. This real-time monitoring allows clinicians to adjust therapies dynamically, optimizing treatment based on individual patient responses rather than population averages.
Significant challenges remain before digital biomarkers can reach their full potential. Data privacy concerns are paramount, as these technologies collect intimate health information continuously. Algorithmic bias presents another obstacle, as most AI systems have been trained on non-representative population samples. Additionally, regulatory frameworks are still evolving to address these novel diagnostic approaches, creating uncertainty for developers and healthcare providers.
Despite these hurdles, the integration of AI-driven analysis and digital health monitoring is rapidly advancing. As technology becomes more accessible and algorithms more sophisticated, digital biomarkers promise to democratize advanced health monitoring, enabling early
intervention across socioeconomic boundaries and transforming our healthcare paradigm from reactive treatment to proactive prevention
Beyond detection, digital biomarkers play a crucial role in precision medicine by continuously monitoring patient responses to treatment. Machine learning (ML) models analyze vast amounts of patient data, allowing doctors to personalize therapies in real time. For example, in diabetes management, continuous glucose monitoring (CGM) devices help fine-tune insulin dosages based on real-time blood sugar fluctuations.
However, challenges persist. Data privacy, algorithmic biases, and regulatory gaps must be addressed to ensure equitable access and reliability. Despite these concerns, the integration of AI-driven digital biomarkers into routine healthcare is ushering in an era of proactive, personalized medicine, where disease prevention and intervention happen earlier and with greater precision than ever before.


Neurons communicate through tiny electrical impulses – the language electroceuticals use to heal.
Imagine a future where doctors treat illness not with a cocktail of pills, but with a tiny electronic implant that “tunes” your nerve signals. This is
the promise of electroceuticals, also known as bioelectronic medicine. These therapies use targeted electronic devices to modulate nerve activity and influence organ functions, essentially treating disease by altering the body’s electrical signals. In other words, electroceuticals speak the body’s neural language, adjusting the messages that organs receive to restore health. Unlike drugs that wash broadly through the body, an electroceutical can send a precise pulse to a specific nerve pathway – a direct line of communication with the body’s control circuits. This approach offers a highly localized treatment: by hacking the nervous system’s code, doctors can correct malfunctioning signals at the source and thereby address medical conditions in a novel way.
Some electroceutical devices are already saving lives and improving health. A prime example is the cardiac pacemaker, an implanted device that delivers rhythmic electrical pulses to keep a sluggish heart beating in perfect time. Pacemakers have been used for decades to correct irregular heartbeats, illustrating the power of electrical intervention in medicine. In neurology, deep brain stimulators (DBS) are implants that send tiny jolts into specific brain regions; they can quell the tremors of Parkinson’s disease or even alleviate severe depression when medications fall short. Another breakthrough is vagus nerve stimulators (VNS), which periodically zap the vagus nerve (a key highway between brain and organs) to prevent epileptic seizures and treat certain cases of depression. These three – pacemakers, DBS, and VNS – are flagship examples of bioelectronic medicine, using electronics to override or assist the body’s own nerve signals. Each illustrates how modulating neural circuits can effectively treat conditions that once relied solely on drugs or surgery, marking a significant shift in modern medicine’s toolkit.

Designing these futuristic therapies comes with hefty engineering challenges. First, any implant that lives inside the body must be biocompatible – built from materials that can safely coexist with living tissue for years. The metal electrodes and wires need to conduct electricity without corroding or triggering immune rejection, all while being sealed against bodily fluids. Second, achieving precision at the nerve-cell interface is crucial. Nerves are tiny and densely packed; electrodes must be small and exact enough to stimulate only the intended nerve fibers without disturbing neighboring signals.

This often means developing ultra‐fine, flexible electrode arrays that can wrap around or even penetrate nerve bundles with micrometer accuracy. Another challenge is power and communication:
How do you run and control an implanted device deep inside the body? Ideally, electroceuticals should be wireless – no bulky batteries or transdermal wires that risk infection.
Engineers are working on ways to beam power and data through the skin using radio waves or magnetic induction, but sending sufficient energy to a tiny implant is difficult when the device must remain very small.
The implants must operate on ultra–low power, sipping only a few microwatts, to prolong battery life or even to harvest energy from the body itself. Ensuring reliable wireless communication is equally important, so that doctors can adjust device settings or receive diagnostic feedback without surgery. Balancing all these requirements –
biocompatibility, precision, wireless connectivity, and low power – is a complex task, making electroceutical design a frontier at the intersection of bioengineering and medicine.
Getting Smaller and SmarterDespite the challenges, rapid advances are making electroceuticals smaller, smarter, and more selective every year. Early nerve stimulators were sometimes as big as a vitamin pill or a pen, but today they’ve shrunk to the size of a fingernail – and future versions may become microscopic.
This extreme miniaturization means implants can be placed with minimal invasiveness and positioned closer to target nerves, improving accuracy. At the same time, smarter electronics and software are boosting efficacy.
Modern implants often include sensors and microprocessors, enabling closed-loop control systems that can adjust stimulation on the fly.
For example, a next-generation vagus nerve stimulator might monitor your heart rate or inflammatory markers and automatically tweak its signals in real time, avoiding over-stimulation or under-stimulation of the nerve. Such an AI-driven closed-loop approach uses algorithms to interpret the body’s feedback and deliver just the right dose of electrical therapy at just the right moment.
The result is more precise therapy tailored to each patient’s immediate needs – essentially an implant that “learns” and responds, rather than just running pre-set pulses. Together, miniaturization and intelligent control are dramatically improving these devices’ selectivity (hitting only the intended targets) and efficacy (delivering better outcomes).
It’s now possible to imagine electroceutical implants that are unobtrusive and autonomous, finely tuning our internal signals as seamlessly as a thermostat regulates room temperature.
Electroceuticals are poised to transform personalized medicine by replacing many medications with targeted electronic interventions. Instead of systemic chemicals, these devices deliver precise electrical stimulation to specific nerves, addressing conditions from depression to diabetes at their source.
Early successes include vagus nerve stimulation for rheumatoid arthritis, which activates the body's anti-inflammatory responses without immunosuppressive drugs. This approach could soon extend to cardiology, neurology, immunology, and endocrinology.
By communicating in the body's electrical language, these technologies can regulate organ function with unprecedented precision and fewer side effects. This represents a paradigm shift toward truly personalized medicine—treatments continuously adjusted to your body's real-time signals.
Bioelectronic medicine stands to revolutionize healthcare, creating a future where physicians prescribe electrical pulses instead of pills, potentially improving countless lives.


3D bioprinting is revolutionizing regenerative medicine by constructing tissues layer by layer using bioactive materials, including living cells and biomaterials.
This cutting-edge technology enables the fabrication of complex biological structures, addressing organ shortages, customized implants, and precision medicine applications.
One of the most critical challenges in modern medicine is the lack of transplantable organs. Millions of patients suffer from organ failure, but donor availability remains severely limited. Bioprinting aims to engineer fully functional organs, reducing reliance on donors while minimizing transplant rejection through patient-derived cells.
Advancements in bioinks—composed of hydrogels, biomaterials, and live cells—allow for the printing of functional tissues such as skin, bone, and muscle. Scientists have already demonstrated the ability to bioprint bone scaffolds, cartilage replacements, and vascularized tissues. In space, microgravity research is further refining these techniques, with the International Space Station testing the fabrication of heart and muscle tissues without the constraints of gravity.
Beyond organ printing, 3D bioprinting is reshaping personalized medicine. By using a patient’s own cells, scientists can print tissue models to study individual drug responses, reducing reliance on generalized testing methods. This patient-specific approach enhances drug safety, minimizes adverse effects, and accelerates treatment development.
In regenerative medicine, bioprinting holds promise for customized implants that seamlessly integrate with a patient’s body, offering alternatives to invasive surgical interventions. For individuals with chronic wounds or degenerative diseases, tailored bioprinted tissues may accelerate healing and improve long-term outcomes.
As bioprinting technology advances, it brings us closer to a future where organ failure, tissue damage, and medical shortages are overcome with precision-engineered biological solutions.


Innovating Diagnostics with Bioelectronics. Imagine holding an entire laboratory in the palm of your hand—this is the promise of lab-on-a-chip (LoC) technology. These micro-sized diagnostic platforms integrate complex laboratory functions onto a single chip, using microfluidics to manipulate tiny liquid samples with extreme precision.
More than just a breakthrough in diagnostics, LoC technology is at the intersection of bioelectronics and precision medicine, bridging the gap between biological sensing and real-time electronic analysis.
LoC systems work by channeling microscopic amounts of fluid through networks of etched pathways on a microchip, performing tests that once required bulky lab equipment. These devices enable real-time chemical analysis, molecular diagnostics, and bio signal monitoring, making them invaluable for rapid medical testing, disease detection, and even neural and metabolic health monitoring.
Recent advancements have taken LoC technology to new heights. Researchers at the University of Bath developed LoCKAmp, an LoC system capable of detecting viruses within minutes using an advanced RNA sequencing method. By integrating artificial intelligence (AI) to optimize microfluidic control, the device self-regulates in real-time, increasing accuracy and efficiency.
Meanwhile, scientists at the University of Pittsburgh have engineered a self-powered LoC system that harvests energy from blood flow using a triboelectric nano-generator. This innovation has the potential to revolutionize bioelectronic medicine, particularly in diabetes monitoring and metabolic tracking, by continuously analyzing blood conductivity and biochemical changes without external power sources.
LoC devices are also being integrated into wearable and implantable bioelectronic systems, enhancing real-time patient monitoring. By combining biosensors, electrical stimulation, and microfluidic diagnostics, researchers are pushing LoC technology beyond traditional testing—toward continuous health management and neural interface applications.
Beyond healthcare, LoC devices offer a sustainable alternative to traditional lab tests, which generate large amounts of plastic waste. Miniaturizing diagnostic processes reduces material consumption and lowers the environmental footprint of medical research.
Moreover, LoC technology is democratizing diagnostics. In Bolivia, students were introduced to internet-connected LoC devices, allowing them to conduct hands-on research remotely. This educational initiative not only enhanced their technical skills but also sparked interest in STEM careers, shaping the next generation of scientists and engineers.
From bioelectronic disease detection to self-powered health monitoring, lab-on-a-chip technology is ushering in a future where high-precision diagnostics merge with electronic biointerfaces to reshape personalized medicine.


Biologist Michael Levin's groundbreaking research suggests bioelectricity could be the key to regenerating limbs, organs, and even brain tissue—potentially revolutionizing medicine as we know it.
The Body's Electrical Language Imagine regenerating lost limbs or damaged organs without transplants or prosthetics. This isn't science fiction it's the promise of bioelectricity research pioneered by Dr. Michael Levin at Tufts University.
"There is unbelievable medical suffering out there," Levin explains. "What keeps me up at night is not living up to the potential of this technology."
Levin has discovered that our cells communicate through electrical signals from the earliest stages of embryonic development. This forms a kind of collective intelligence that guides how our bodies form.
"All of the cells of your body are forming electrical networks that process information," says Levin. "The body, much like the brain, is a collective intelligence."
This explains remarkable phenomena like how a split embryo creates identical twins rather than two half-bodies. The cells somehow "know" what's missing and rebuild accordingly.
While modern medicine focuses on genetic editing—what Levin calls the "hardware" level his research explores the "software": bioelectrical patterns that guide cellular behavior. This approach offers the ability to reprogram cells without altering their DNA.
Levin's discoveries began with planarian flatworms, which can regenerate even when cut into hundreds of pieces. By manipulating their bioelectrical patterns, his team reprogrammed worms to grow two heads instead of one—with no genetic changes.
Building on this success, they regenerated tadpole tails and adult frog hind legs. Now they're working with mammals, using wearable bioreactors that create protective environments for regenerating tissue.
The applications extend beyond limb regeneration to preventing birth defects and treating cancer by correcting electrical signaling that normally keeps cells working toward common anatomical goals.
Levin envisions a transformative shift in medical practice from constantly chasing symptoms to harnessing the body's innate intelligence through bioelectricity.
"I firmly believe that ultimately we are going to have complete control overgrowth and form, including growing back brain tissue," he says. "I believe that I'm going to see this in my lifetime."
For millions suffering from injuries, birth defects, and degenerative diseases, bioelectricity represents not just a scientific breakthrough, but a revolution in healing one that works with the body's own intelligence rather than against it.


Bioelectronics stands at the fascinating intersection of biology and electronic engineering, representing one of the most promising frontiers in modern medicine and technology. This rapidly evolving field harnesses our understanding of the body's electrical signals to develop innovative therapeutic approaches and diagnostic tools that were once the stuff of science fiction.
At its core, bioelectronics explores how electronic devices can interface with biological systems to monitor, influence, or restore normal physiological functions. Unlike traditional pharmaceuticals that rely on chemical interactions, bioelectronic approaches leverage electrical impulses—the body's natural communication language—to achieve therapeutic effects with potentially greater precision and fewer side effects.
The human body is, in many ways, an electrical marvel. From the rhythmic firing of neurons in our brains to the coordinated contractions of our heart muscle, electrical signals govern countless biological processes. Bioelectronic medicine taps into these intrinsic mechanisms, using technologies ranging from implantable microdevices to wearable sensors to either read these signals or modulate them when they go awry.
Several breakthrough applications have already emerged from this field. Deep brain stimulation has transformed treatment for Parkinson's disease by delivering targeted electrical impulses to specific brain regions. Cochlear implants restore hearing by converting sound waves into electrical signals that directly stimulate the auditory nerve. Cardiac pacemakers perhaps the most established bioelectronic devices—have been regulating heartbeats for decades.
But today's innovations push far beyond these pioneering technologies. Researchers are developing miniaturized implants that can detect and halt epileptic seizures before they fully manifest. Others are creating neural interfaces that allow paralyzed individuals to control prosthetic limbs using only their thoughts. Some teams are even investigating how electrical stimulation of the vagus nerve—a key communication pathway between brain and body might treat inflammatory conditions like rheumatoid arthritis or inflammatory bowel disease.
What makes bioelectronics particularly promising is its potential for precision. While conventional drugs circulate throughout the entire body, often causing unwanted side effects, bioelectronic devices can target specific nerves or tissues with remarkable accuracy. This focused approach may eventually enable treatments for conditions that have resisted traditional pharmaceutical interventions.
The field also benefits from rapid advances in related technologies. Miniaturization allows for increasingly smaller and less invasive devices. Improved battery technologies and energy harvesting methods extend device lifespans. Wireless capabilities enable remote monitoring and adjustment without additional procedures.
Despite these exciting developments, significant challenges remain. The long-term biocompatibility of implanted materials, securing wireless communications from unauthorized access, and ensuring devices can function reliably for years within the body all present formidable engineering hurdles. Ethical considerations around device control, data privacy, and equitable access to these technologies also require careful attention.
As bioelectronics continues to mature, we can expect an expansion beyond purely therapeutic applications into preventative and enhancement domains. From early warning systems for impending medical events to interfaces that augment human capabilities, the boundaries between biology and technology grow increasingly blurred.
The bioelectronic revolution represents not just a new set of medical tools, but potentially a fundamental shift in how we conceptualize the relationship between technology and human physiology—a true marriage of silicon and cell that promises to transform healthcare in the decades ahead.


Lloyd Minor and Ralph Snyderman are widely recognized as the founding figures who shaped and advanced the concept of precision health—a proactive approach aiming to maintain and enhance individual well-being before diseases emerge.
Lloyd Minor, a prominent physician-scientist and Dean of Stanford University School of Medicine, fundamentally redefined healthcare by advocating for precision health as a proactive, preventive, and personalized model.
Minor's vision was transformative: he argued that rather than simply reacting to illness, healthcare should focus on predicting risks, preventing disease, and promoting lifelong wellness tailored to each person's genetic, environmental, and lifestyle factors. Under his leadership,
Stanford Medicine embraced digital tools, genomics, and big data analytics, driving groundbreaking efforts to integrate technology seamlessly into everyday health practices.
Similarly, Ralph Snyderman, often referred to as the "Father of Personalized Medicine," championed precision health decades earlier. As Chancellor Emeritus of Duke University, Snyderman redefined healthcare by emphasizing prevention and personalization rather than reactive treatments.
His pioneering vision led to the establishment of integrative medicine models that combined predictive analytics, personalized prevention, and patient empowerment. Snyderman argued compellingly that healthcare should focus on predicting risks and intervening early, reshaping medicine from treatment-oriented care to health preservation—truly laying the groundwork for today's precision health paradigm.


Precision health expands beyond treating illness—it emphasizes overall wellness by integrating personalized prevention and proactive care. It harnesses detailed information about an individual's lifestyle, environment, genetics, and behaviors to optimize long-term health.
Traditional healthcare often reacts to illnesses after symptoms appear. Precision health shifts this paradigm, emphasizing preventive strategies tailored to the individual's
unique health profile, significantly reducing the risk of disease and promoting sustained wellbeing.
The Power of Personalized Prevention By analyzing personal health data including genetic tests, family history, lifestyle patterns, and environmental factors—precision health provides individualized recommendations, such as personalized nutrition, targeted fitness programs, and lifestyle modifications to maintain optimal health.
Technology and Precision Health Emerging technologies like wearable devices, digital health apps, and advanced diagnostics play a critical role in precision health. These tools provide continuous realtime health monitoring, enabling early detection of health anomalies and empowering individuals to take informed action immediately.
Precision Health in Daily Life Practical examples of precision health include personalized dietary advice based on genetic testing, customized fitness routines designed around individual physiology, and precise supplementation to address specific nutrient needs.
Mental Health and Precision Approaches Precision health also addresses mental wellness by integrating personalized strategies for stress management, sleep optimization, and emotional resilience based on individual physiological and psychological profiles.
Challenges and Ethical Considerations Precision health raises vital considerations around data privacy, equity in healthcare access, and ethical use of personal health information. Addressing these issues responsibly is critical for widespread adoption and trust.
Precision health places you at the center of your wellness journey, enabling you to live a healthier, more vibrant life through personalized, informed choices.

Scientists at Virginia Tech have pioneered an ingenious approach to one of medicine's persistent challenges: delivering drugs to the lower intestine. Their solution? Turning your gut bacteria into tiny pharmaceutical factories.
The research team, led by biologist Bryan Hsu and immunologist Liwu Li, has engineered bacteriophages—viruses that naturally infect bacteria—to reprogram gut microbes. Instead of just producing more phages, these modified bacteria now manufacture therapeutic proteins directly where they're needed most.
How It Works
The process is both elegant and efficient: Engineered bacteriophages attach to bacteria in the gut and inject special genetic instructions into the bacterial cells. The bacteria begin producing therapeutic proteins, and when they eventually burst (lysis), they release both new phages and the medicinal proteins. This creates a continuous, targeted drug delivery system.
What makes this approach revolutionary is how it bypasses the stomach's harsh environment, which typically destroys most oral medications before they can reach the lower intestine.
Promising Results The research team has already demonstrated the technique's effectiveness in mice, showing it can reduce inflammation associated with inflammatory bowel disease and decrease obesity in mice fed high-fat diets by triggering feelings of fullness.
Future Potential This breakthrough could transform treatment approaches for various chronic gastrointestinal conditions. The researchers are currently exploring commercial applications through the National Science Foundation I-Corps program and the Fralin Commercialization Fellowship.
Their next challenge involves finding ways to get these locally-produced drugs absorbed into systemic circulation, potentially expanding the technique's applications beyond intestinal disorders.
This innovative approach effectively turns some of the hundreds of microbial species in your gut into "microscopic in-house pharmacists," offering new hope for precisely targeted and sustained therapies where traditional drug delivery methods fall short.


Medicine is entering an era where your unique genetic blueprint— not just your symptoms—guides your treatment. Welcome to the world of precision medicine, where healthcare becomes as individual as your fingerprint.
Reading Your Body's Source Code
Imagine if doctors could read the instruction manual for your body. That's essentially what's happening with technologies like CRISPR-Cas9, which allows scientists to edit genes with the precision of a word processor. These genetic editing tools can
correct faulty DNA instructions that cause disease, offering hope for conditions once considered untreatable.
"We're no longer just treating symptoms we're addressing the root cause written in our genetic code," explains Dr. Mina Hossen, whose 2025 research demonstrated how genetic engineering is transforming pharmacology.
Cancer treatment is witnessing perhaps the most dramatic transformation. Oncologists now routinely analyze tumor DNA through next-generation sequencing and "liquid biopsies" that detect cancer fragments in blood samples. These tests reveal the unique genetic mutations driving each person's cancer.
A patient with breast cancer might carry the HER2 mutation, requiring a completely different treatment approach than someone whose tumor has a BRCA mutation. This targeted approach means more effective treatments with fewer side effects.
"Every tumor has its own molecular fingerprint," notes oncologist Dr. Botwe. "By decoding this fingerprint, we can match patients with drugs designed specifically for their cancer type."
Artificial intelligence has become precision medicine's most powerful ally. Machine learning algorithms can analyze millions of data points— far more than any human could process to discover subtle patterns in disease progression and treatment response.
These AI systems help doctors predict which treatments will work best for individual patients by comparing their profiles against vast databases of similar cases. When a new patient arrives, AI can instantly identify the most successful treatments for people with matching characteristics.
Digital biomarkers tracked through smartphones and wearable devices further personalize care by monitoring real-time health metrics, from heart rhythms to sleep patterns, creating a continuous picture of your health rather than the snapshots provided by occasional doctor visits.
This transformation isn't just happening in research labs—it's reshaping clinics today. Patients with certain cancers, rare genetic disorders, and chronic conditions are already benefiting from treatments tailored to their specific biology.
Challenges remain, including ensuring equal access to these breakthrough technologies and navigating the ethical questions that arise when we can read and potentially rewrite our genetic code. Yet the promise is undeniable: a future where medical treatment is as unique as you are.
As we continue to merge our understanding of genetics with the computational power of AI, medicine is evolving from a one-size-fits-all approach to a precisely tailored experience. The result? Treatments that work better, cause fewer side effects, and address the underlying causes of disease rather than just managing symptoms.
Welcome to healthcare's most personalized transformation.


Remote patient monitoring (RPM) is transforming healthcare by enabling hospital-level care at home. Using wearable sensors, AIdriven analytics, and telehealth platforms, RPM allows continuous tracking of vital signs, chronic conditions, and post-surgical recovery—without requiring patients to stay in hospitals.
Healthcare systems are increasingly adopting RPM to reduce costs, prevent hospital overcrowding, and enhance patient outcomes. For instance, Mass General Brigham has transitioned 10% of its medical patients to home care, cutting hospital stays and lowering infection risks. Similarly, virtual palliative care programs, such as Island Health’s VPSC, support end-of-life care remotely through video check-ins and real-time health monitoring.
In critical care, RPM is revolutionizing tele-ICUs, where specialists monitor ICU patients from remote hubs, adjusting treatments based on AI-assisted physiological data. This market, valued at $3.5 billion in 2023, is projected to surpass $11.8 billion by 2032, highlighting its growing role in intensive care.
RPM is not just about convenience—it is an essential pillar of precision medicine. By collecting real-time patient data, RPM personalizes treatment plans for conditions like heart disease, diabetes, and respiratory disorders. AI algorithms analyze patterns to detect early warning signs, allowing proactive interventions that reduce emergency hospitalizations.
Moreover, RPM enhances healthcare equity by expanding access to rural and underserved communities, where specialized care is scarce. By reducing readmissions and hospital strain, RPM also plays a critical role during pandemics and public health crises.
As AI further refines RPM’s predictive capabilities, healthcare will shift towards more proactive, personalized, and cost-efficient care, ensuring better health outcomes with fewer resources. This digital evolution is not just modernizing healthcare—it’s reshaping the future of medicine.


Biological Intelligence + Human Intelligence × Artificial Intelligence = Precision Health
At the intersection of three powerful forces lies the future of healthcare. This isn't merely an equation, it's a vision for how we might transform medicine from reactive to proactive, from standardized to personalized, from approximate to precise.
Our bodies represent the original complex intelligence system. For billions of years, biological systems have evolved sophisticated mechanisms to maintain homeostasis, fight pathogens, and repair damage. This biological intelligence the innate wisdom of our cells, tissues, and microbiome forms the foundation of our health. The gut microbiome research at Virginia Tech exemplifies this understanding,
harnessing the natural intelligence of bacterial systems to create targeted therapeutic delivery.
Human intelligence brings something different to the equation: our capacity for pattern recognition, theoretical thinking, and empathic understanding. Healthcare practitioners have always relied on their trained intuition, accumulated knowledge, and compassionate insight to diagnose and treat.
The researchers who envisioned turning gut bacteria into pharmaceutical factories demonstrated this unique human capacity for creative problem-solving.
Artificial intelligence multiplies these capabilities exponentially. By processing vast datasets, identifying subtle correlations, and modeling complex biological systems, AI amplifies both biological and human intelligence. It doesn't replace the doctor's intuition or the body's wisdom—it extends them, revealing patterns too complex for the human mind alone to discern.
The product of this synergy is precision health: interventions tailored to individual biological profiles, delivered at exactly the right time, in exactly the right way. It means treatments designed for your specific genome, microbiome, and lifestyle.
It means predictive models that catch disease before symptoms appear. It means pharmaceutical factories living within your own body, producing personalized medicine continuously. This formula represents more than technological advancement—it embodies a philosophical shift. We're moving from a healthcare model that treats the average patient to one that recognizes there is no average patient.
Each person's biology tells a unique story, interpreted through human expertise, amplified by artificial intelligence.

The bacteriophage therapy developed at Virginia Tech offers a glimpse of this future. By combining our understanding of biological systems (microbiome), human ingenuity (engineered phages), and computational modeling (to optimize delivery), researchers have created a precision therapy that works with the body's own systems.
As we stand at the threshold of this new era, the most exciting realization is that we're just beginning to understand the potential of this formula. Each variable continues to evolve in understanding biological intelligence deepens, human expertise grows, and artificial intelligence becomes more sophisticated.
This is the promise encoded in that elegant formula: a healthcare revolution written in the language of intelligence—biological, human, and artificial—working in concert to create a future where medicine is as unique as each person it serves.


Imagine a future where vaccinations are as simple as applying cream to your skin—no needles, no discomfort. Stanford University scientists are turning this vision into reality by developing a painless, living vaccine that you can rub onto your skin.
Our skin naturally hosts a variety of harmless bacteria, including Staphylococcus epidermidis. The Stanford team engineered this common bacterium to carry antigens from pathogens like tetanus and diphtheria. When applied to the skin of mice, these modified bacteria prompted the immune system to produce specific antibodies, effectively vaccinating the animals without any injections.
The key lies in a surface protein called accumulation-associated protein (Aap) found on S. epidermidis. This protein protrudes from the bacterial cell wall, making it easily detectable by the immune system.
By attaching fragments of toxins from diseases like tetanus and diphtheria to Aap, the researchers created a scenario where the immune system recognizes these fragments as threats and generates antibodies against them.
In experiments, mice treated with the engineered bacteria developed high levels of antibodies against the tetanus toxin. When later exposed to lethal doses of the toxin, these mice remained healthy, while untreated mice succumbed. This demonstrates the vaccine's potential effectiveness. cite turn0news11
The next step for the researchers is to test this vaccine method in primates, with hopes to begin human clinical trials within two to three years. If successful, this approach could revolutionize how we administer vaccines, making the process more accessible and eliminating the discomfort associated with needles.
This innovative method not only simplifies vaccination but also opens the door to developing needle-free vaccines for various diseases, potentially improving global vaccination rates and public health outcomes.


In today’s healthcare landscape, the constant influx of data from wearable devices often leaves both patients and healthcare providers overwhelmed. Excessive metrics can cloud critical health insights, fueling anxiety rather than empowering informed decisions.
In the near future “Clear Pulse” will changes the game by transforming extensive health data into concise, meaningful summaries.
This compact, e-ink device provides a clear snapshot of daily health status, highlighting significant changes such as energy dips, sleep disruptions, or hydration needs. By intelligently filtering out redundant or minor fluctuations,
Clear Pulse will deliver only the most relevant and actionable insights in plain language, offering updates like "Increased hydration advised" or "Consistent sleep improvement noted."
For healthcare providers, Clear Pulse ensures consultations remain patient-centered, efficient, and informed. Quick, actionable summaries mean providers can rapidly interpret patient health trends without being overwhelmed by excess data.
The result is clearer communication, enhanced trust, and better overall care—benefiting both patients and providers.


The year is 2055. Elena wakes to the soft glow of her bedroom walls transitioning from dep indigo to morning amber. She doesn't need an alarm anymore—her circadian rhythm is perfectly synchronized through a combination of ambient light orchestration and the invisible web of quantum sensors embedded throughout her living space.
"Good morning, Elena," comes the warm voice of ARIA, her Augmented Responsive Intelligence Assistant. "Your sleep architecture was excellent last night 90 minutes of deep sleep, 110 minutes of REM. Your cortisol levels are optimal, though your magnesium showed a slight dip. I've adjusted your morning nutrient synthesis accordingly."
Elena stretches, feeling the microscopic neural mesh in her sleepwear gently detach from her skin. Throughout the night, these quantum biosensors had been monitoring everything from her neurotransmitter fluctuations to her microbiome composition, all without a single needle or blood draw.
As she enters her kitchen, the neural hub projects her Digital Twin a complete molecular simulation of her body that's been evolving alongside her since birth. This isn't just data visualization; it's a living, breathing quantum model of every cell, every gene, every neural pathway in her body.
"Your pancreatic function shows early signs of stress," ARIA notes as Elena examines the pulsing blue glow around her Digital Twin's abdomen. "Nothing concerning yet, but your genetic predisposition suggests monitoring. I've already informed your genetic counselor."
Elena nods, watching as the kitchen synthesizes her breakfast a precision-formulated meal that contains exactly what her body needs
this morning, including calibrated nutrients to support her pancreas. The food looks and tastes like a gourmet omelet, but its molecular structure has been customized down to the last protein, tailored to her epigenetic profile that morning.
As she eats, Elena feels a gentle warmth at her wrist—her bio interface band is administering a personalized micro cocktail of immunomodulators. She had been exposed to a novel respiratory virus yesterday, according to environmental sensors in her workplace. The prophylactic response had been automatically authorized by her health trust algorithm, which continuously balances immediate interventions against long-term immune resilience.
"Your 10 AM neural enhancement session is confirmed," ARIA reminds her. "Dr. Zhou's lab has updated your cognitive profile based on yesterday's creativity metrics."
Elena works as a bio architectural designer, creating living buildings that respond to their inhabitants' health needs. Her own neural patterns are enhanced through targeted transcranial stimulation sessions, calibrated weekly to optimize her creative output while preventing cognitive fatigue.
At work, her desk adjusts its position microscopically throughout the day, responding to subtle changes in her posture and muscle tension. The lighting shifts in harmony with her attention cycles, brightening during conceptual work and softening when she needs to access her intuitive thinking.
When her heart rate variability indicates mounting stress during a client meeting, her neural mesh triggers a subtle vagus nerve stimulation. The bioelectronic pulse is so gentle she barely notices, but within seconds her autonomic nervous system rebalances, allowing her to remain focused and present.

That afternoon, Elena receives a notification that her grandmother's neurological implant has detected early signs of protein misfolding a precursor to what was once called Alzheimer's disease. Before any cognitive symptoms appear, her grandmother's brain-computer interface has already initiated targeted clearance of the problematic proteins through acoustically controlled nanobots.
Elena remembers stories of how people once waited until diseases manifested symptoms before treating them. How primitive that seemed now, when molecular prevention had become the standard of care.
At her quarterly health optimization consultation, Elena meets with her integrative health team—not in a hospital or clinic, but in a biophilic garden space designed to enhance their connection. Her physician, nutritional geneticist, and quantum biologist appear as holograms, but the sensory feedback is so precise that she can feel the reassuring touch of her doctor's hand.
The team reviews her Digital Twin's predictive scenarios she has a 3.7% probability of developing autonomic dysregulation in the next decade, down from 15% thanks to her consistent use of neural harmonization techniques. Together, they refine her precision health protocol, enhancing the balance between technological intervention and her body's innate healing capabilities.
Later that evening, while having dinner with friends at a vertical farm restaurant, Elena witnesses a man at a nearby table suddenly clutch his chest. Before anyone can react, the ambient medical system has already detected his cardiac irregularity. Emergency protocols engage instantaneously:
Microscopic defibrillator particles, dormant in his bloodstream since his last cardiogenetic screening, activate precisely at his heart's vulnerable regions. Neural mesh in his clothing stabilizes his posture to prevent injury from falling. The restaurant's atmospheric composition adjusts, increasing oxygen flow to his table.
A medical response drone arrives within 30 seconds, extending a bio interface arm that delivers personalized treatment based on his medical genome and real-time physiological data.
The entire intervention occurs so smoothly that most diners barely notice, and the man is stabilized before the emergency health coordinator's hologram even arrives to oversee his care.
As Elena prepares for sleep, she reflects on the interconnected health ecosystem that surrounds her. Her own body's data contributes anonymously to the global health neural network, helping algorithms refine treatment protocols for millions worldwide.
The boundaries between prevention and treatment, between health and technology, have dissolved into a continuous flow of optimized wellbeing.
Her neural mesh detects her philosophical musings and subtly adjusts her bedroom lighting to enhance her introspective state. ARIA doesn't interrupt—the AI has learned when Elena values silent contemplation.
She thinks about historical accounts of medicine in the early 21st century: the reactive treatments, the side effects, the one-size-fits-all approaches. How strange that people once lived with such uncertainty about their own bodies.
As she drifts toward sleep, her neural interface synchronizes with her circadian rhythms, gently guiding her brain waves toward restorative delta patterns. Her final conscious thought is not about technology at all, but about connection—to her own cellular intelligence, to her community, and to the intricate biological symphony that technology now helps orchestrate rather than override.
In 2055, precision health isn't just about targeted treatments or personalized prevention. It's about living in conscious harmony with our most sophisticated technology: the human body itself.

interesting engineering popular mechanics
Inverse mckinsey technology review azoai scientific american the conversation neuroscience news medium live science eetimes investorplace spectrum scmp robb report science post serverobotics future sciences futurism spectra we forum gates notes azorobotics Business insider wired technology review arstechnica new atlas trust my science robotics and automation phonandroid silicon india freethin

