









































































• Resprep SPE & Q-sep QuEChERS
• PFAS Delay Columns
• Raptor & Force LC Columns
• PFAS Reference Materials
• Ultra-Clean Resin for Air Analyses
• ASE Cells & Parts
• Filters and vials
Access expert support and tailored PFAS testing solutions for U.S. EPA, ISO, ASTM methods, and more, at www.restek.com/PFAS

We have a bumper issue for you this December with the majority of articles focusing on new techniques and technologies within the sector. The cover story, Designer biocatalysts, looks at an interesting collaboration between the Manchester Institute of Biotechnology and a leading computational biologist that will see traditional ways of developing enzymes for industry transformed with the help of computational methods and big data. A piece from CN Bio (page 28) titled 'How OOC technology can reduce DILI in drug development’ explores organ-on-achip technology’s role in reducing drug-induced liver damage during trials. Another blue skies piece written by Rory O’Keefe from Europlaz (page 32) provides insight into global trends and innovations in point-of-care equipment.
Cloud computing plays a key role in data sharing and increasing speed to market for pharmaceutical companies, 'Cloud cover' on page 26 illustrates how cloud technologies can be used to ensure quality in pharma medicine. Readers who are interested in data management might also be interested in 'Too much data' (page 33) based on a report from data analytics company Phesi claiming that many trials are not discriminate enough with the data they're using.
Regular readers will be interested to read the third and final instalment in the 'Understanding the nip angle' series from Gerteis Maschinen on page 42.
A bumper piece from Restek on page 36 provides in-depth insight into ultra-short chain PFAS and their impact on human health.
Nicola Brittain Editor
Protecting sensitive cleanroom devices from ESD
Small-scale sampling Effective sampling techniques in cleanrooms
microplate market examined A deep dive on the reasons for a recent surge in demand
Innovations in cannabinoid research
Accelerating drug discovery with microplate readers
OOC and reducing DILI in drug development
OOC’s role in streamlining drug research and manufacturing to reduce costs
Designer biocatalysts
An innovative collaboration that promises to change the biocatalyst landscape
POC diagnostics
– an overview
Insight into global trends in this fast-moving market
Too much data
How the overcollection of data adds months to clinical trials
Advances in 3D imaging
A recent collaboration will pave the way for 3D imaging
C1-C10 PFAS into biomonitoring
How ultra-short chain
PFAS can be integrated into biomonitoring methods
PUBLISHER
Jerry Ramsdale
EDITOR
Nicola Brittain nbrittain@setform.com
STAFF WRITER
Saskia Henn shenn@setform.com
DESIGN
Dan Bennett, Jill Harris
HEAD OF MARKETING
Shona Hayes shayes@setform.com
HEAD OF PRODUCTION
Luke Wikner production@setform.com
BUSINESS MANAGER
John Abey +44 (0)207 062 2559
SALES MANAGER
Darren Ringer +44 (0)207 062 2566
ADVERTISEMENT EXECUTIVES
John Davis, Iain Fletcher, Paul Maher
Remarkable innovations
Cybersecurity threats and solutions facing the biotech industry
Dry granulation: understanding the nip angle
The third part in this series on the nip angle
The right tools
The role of tooling specifications in tablet manufacturing
CHROMATOGRAPHY
Optical solution for hydrogen testing
High brightness technology that can help test hydrogen for contaminants

Under the microscope
Exploring microscopy and current trends in battery technology
Filtech
A ‘must visit’ show reviewed


Setform’s international magazine for scientists is published twice annually and distributed to senior professionals throughout the world. Other titles in the company portfolio focus on Process, Design, Transport, Oil & Gas, Energy and Mining engineering.
The publishers do not sponsor or otherwise support any substance or service advertised or mentioned in this book; nor is the publisher responsible for the accuracy of any statement in this publication. ©2024. The entire content of this publication is protected by copyright, full details of which are available from the publishers. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner.
Setform Limited, 6, Brownlow Mews, London, WC1N 2LD, United Kingdom
+44 (0)207 253 2545


A collaboration that will transform the sector








































































Linkam Scientific Instruments has launched updates to its specialist CMS196 cryo-stage product. The CMS196V now supports cryocorrelative light and electron microscopy (cryo-CLEM) and allows researchers to investigate samples at cryogenic temperatures to < -195 °C. The latest updates include an improved user interface featuring a touch panel and joystick, and an encoded and motorised XY stage allowing highly precise automated mapping of sample grids. The new product also features different sample holders and interface options for the newly-interchangeable optical bridge for imaging.
For more information visit: www.linkam.co.uk/cms196






Lab informatics platform Sapio Sciences has signed a comarketing agreement with Waters Corporation to integrate with its Waters Connect software. The companies claim this will improve data integrity, workflow efficiency, and laboratory productivity. As laboratory-based organisations expand and handle increasing numbers of samples, integrating liquid chromatography mass spectrometry (LC-MS) software with a Laboratory Information Management System (LIMS) will mean laboratory operations are more efficient with more streamlined workflows, reduced data silos, enhanced compliance with regulatory standards, and the elimination of manual data entry and transcription errors.
For more information visit: www.sapiosciences.com



Introducing Unity, the world’s first combined Backscattered Electron and X-ray (BEX) imaging detector.
Accelerate your journey to scientific discovery with instant microstructural and chemical images, acquired simultaneously with the Unity detector.





out how we’re making sophisticated sample analyses simpler and faster than ever before.



In the dynamic environment of modern laboratories, the quality of water used is a fundamental factor that can significantly influence the accuracy and reliability of experimental results. Whether it’s for sensitive assays, reagent preparation, or equipment sterilisation, laboratories require water that meets specific purity standards. Laboratory technicians and managers are continually seeking solutions that not only deliver high-quality water but also integrate seamlessly into their daily workflow.
One common challenge laboratories face is the need for both Type 1 ultrapure water for critical applications and Type 2 water for general laboratory tasks. Managing multiple water purification systems to meet these requirements can be cumbersome, expensive, and space-consuming. The Purite Fusion benchtop water purification unit addresses this issue by providing dual-quality water from a single, compact system, integrating smoothly into laboratory operations and instilling confidence in its users.
Laboratory technicians and managers are continually seeking solutions that not only deliver high-quality water but also integrate seamlessly into their daily workflow
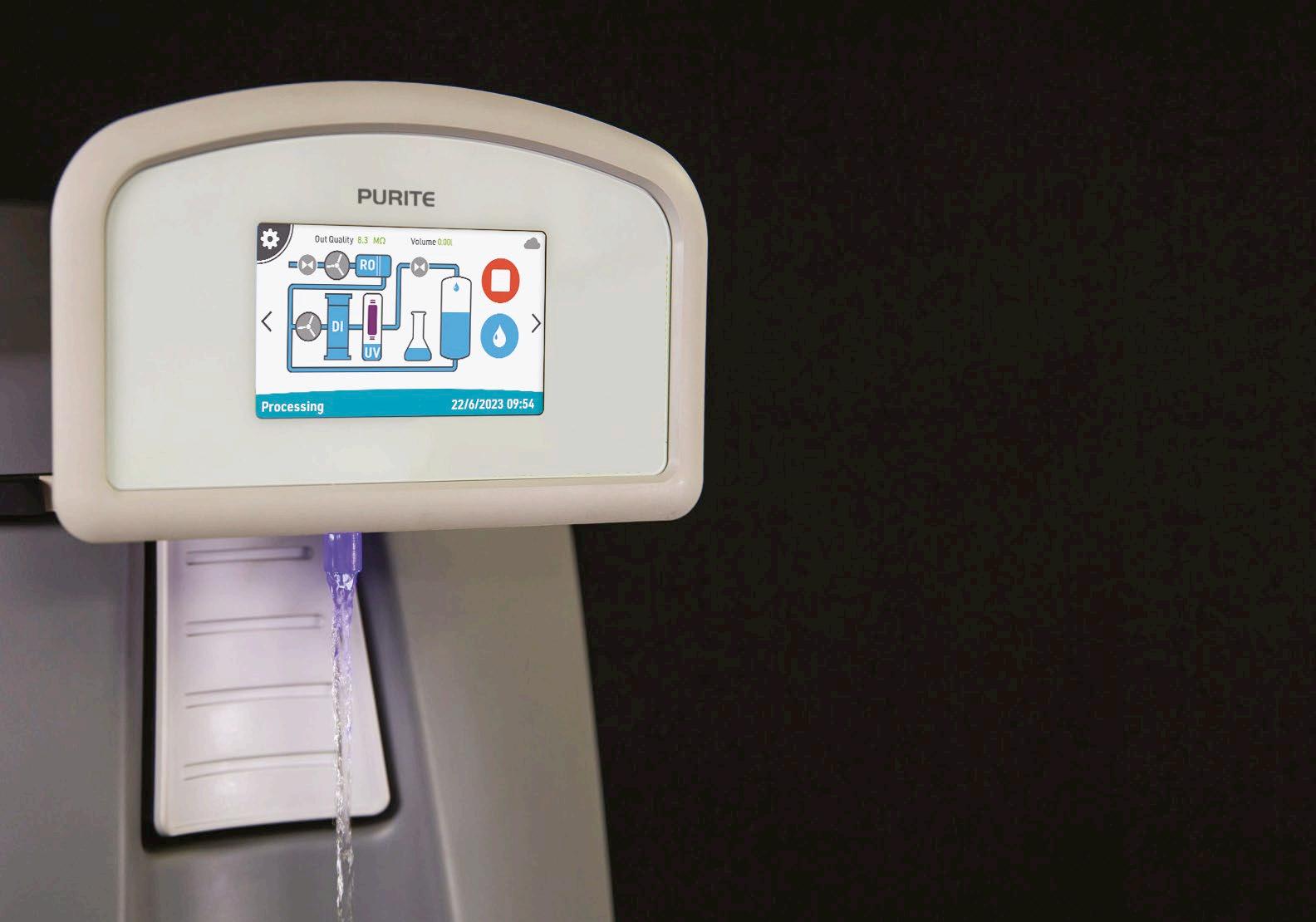
Laboratory workflows are intricate and demand precision. On any given day, a technician might perform high-sensitivity techniques like highperformance liquid chromatography (HPLC), polymerase chain reactions (PCR), or cell culture experiments, all of which require Type 1 ultrapure water. Simultaneously, they may need Type 2 water for less critical tasks such as preparing buffers and reagents, sample dilution or rinsing glassware.
The Purite Fusion unit is engineered to meet these diverse needs without disrupting the laboratory’s workflow. It delivers up to 2lpm of Type 1 ASTM 18.2MΩ.cm ultrapure water from the point of use (POU), ensuring immediate and volumetric access when precision is paramount. For general laboratory applications, Type 2 water is readily available from the integrated 20 tank or optional external 50 or 100 litre storage tank’s bib tap. This dual availability from a single unit means valuable bench

space is released and technicians no longer need to manage consumables and maintenance contracts for different systems, thereby reducing OPEX and the use of higher cost water for less critical applications.
Laboratories depend on consistent water quality. High maintenance and consumable costs can impact the ability of a lab to properly support the needs of their water purification system which in turn can impact the efficacy of the unit and the reliability of results, The Purite Fusion unit is designed to tackle these challenges more effectively:
1. Consistent high-quality water supply: The Fusion unit’s comprehensive purification process ensures a reliable supply of both Type 1 and Type 2 water direct from a potable water supply using treatment methods including:
• Carbon pre-treatment to remove
The Purite Fusion unit is a comprehensive solution to the water purification challenges faced by modern laboratories
chlorine and organic compounds
• Reverse osmosis (RO) membranes to eliminate particulates and ionic contaminants
• Primary deionisation
• Polishing deionisation with highgrade nuclear resin
• Ultrafiltration to remove any remaining particles, microorganisms, endotoxins, RNase and DNase
• UV photo-oxidation at 185nm for total organic carbon (TOC) reduction
• LED UV disinfection at the dispense point to ensure water remains contaminant-free.
This meticulous process guarantees that the water meets the stringent purity requirements necessary for accurate and reproducible results, giving laboratory personnel the confidence they need in their daily tasks.
2. Reduced maintenance and operational costs: Choosing a water purification system extends beyond its immediate functionality; it encompasses the assurance of consistent performance and dependable support. The Purite Fusion unit is constructed with an emphasis on reliability, durability,
and longevity, ensuring it stands up to the rigorous demands of daily laboratory use.
3. Space optimisation and workflow efficiency: Space is a valuable commodity in any laboratory. The compact benchtop design of the Fusion ‘IT’ saves precious workspace by combining dualquality water production and a 20 litre Integrated Tank (IT) in one unit with a footprint of only 440mm x 560mm. This consolidation simplifies laboratory layouts and streamlines workflows, as all water purity needs are met through a single system. Furthermore, the system can be expanded with the addition of an optional remote dispense point (Type I) which can be located up to five metres away from the unit to serve a second location from the main unit. The footprint of the remote dispenser is just 228mm x 305mm.
The Fusion unit’s design and performance directly contribute to building confidence among laboratory technicians and managers by:
• Ensuring repeatability: consistent water quality leads to reproducible
results, a cornerstone of credible scientific work.
• Simplifying operations: With fewer consumables and maintenance requirements, the unit reduces the complexity of laboratory operations.
• Providing robustness: The system’s durable construction minimises the risk of unexpected failures, reducing downtime and maintaining productivity.
• Satisfying various usage demands: The Purite Fusion 160 and 320 models produce RO permeate at a flow of 17.6 and 36 l/h respectively to the storage tank at a feed water temp of 15ºC offering performance options to meet the lab’s usage needs.
In an era where precision and reliability are paramount, the Purite Fusion unit is a comprehensive solution to the water purification challenges faced by modern laboratories. By delivering dualquality water from a single, spacesaving unit, it integrates well into daily workflows, enhancing efficiency and simplifying operations, according to the company.


• Manufactured from non-toxic high grade LDPE
` Shatterproof and safe to use
` Inert to prevent sample contamination



• Responsive control for ease-of-use & reliable performance
• Low-affinity surface for complete sample delivery
• Available sterile or non sterile to suit your application
• No mould release agents used during manufacturing








Inline degassing is the silent guardian of precision and reliability in fluidic systems. Installation of an inline degasser (see Figure 1) will drastically diminish the concentration of dissolved gases in the liquids passing through. This reduces variations, improves baseline stability, shortens startup times, and ensures more consistent results. By reducing the concentration of dissolved gases beyond the level where outgassing can occur, bubble formation will not be an issue despite changes in temperature, pressure, or compositions of the liquid managed throughout the flow path.
Degassers are important equipment components in laboratory analysis equipment such as liquid chromatography, HPLC, UHPLC, ion chromatography and mass spectrometry. Machines for semiconductor manufacturing or assembly will also typically deliver more consistent results with a degasser included in the fluid path. The same is true for instruments in immunology, haematology, and in vitro diagnostics.
An inline degasser assembly contains a purposedly designed vacuum pump connected to one or several degassing chambers through which the liquid with dissolved gases flows. Inside the degassing chamber (see Figure 2) there is an inert gas-permeable membrane that must be compatible with the liquid to be degassed. A control board with a vacuum sensor ensures that the vacuum level is kept at a constant level to minimise fluctuations in degassing performance and wear on the vacuum pump.
Figure 1. Example of a stand-alone inline degasser from the DEGASi family, suitable for aqueous liquids and organic solvents at flow rates from 25 µL/min up to 1000 mL/min, depending on model and configuration
Modern inline degassers incorporated into instruments handling liquids with high precision and accuracy, are fortunately essentially maintenancefree. However, there are a few things to keep in mind not to shorten the lifetime of your inline degasser. Firstly, one should use a degassing chamber compatible with the solvents that will be used. Organic solvents such as hexane, heptane, toluene, tetrahydrofurane (THF), and dichloromethane (DCM), typically demand specially designed degassing chambers. These degassing chambers are often labelled GPC to highlight their suitability in gel permeation chromatography, but they are equally suitable for normal phase and flash chromatography applications where such organic solvents are also employed. In addition, there are degassing chambers explicitly designed for hexafluoro isopropanol (HFIP) that are required when this aggressive organic solvent is used. As with any fluidic component, it is advised not to leave your degassing chamber with liquid inside when disconnected from use. This is especially important when there are salts or buffer components dissolved into the liquid since they may precipitate. Precipitates will block the flow path and are notoriously problematic to wash out again. In

addition, buffered aqueous solutions exposed to open air may constitute an attractive environment for microbial growth, which can constrict the flow path.
Finally, it is strongly recommended to always suck solutions through the degassing chambers, rather than pumping or pushing liquids through them. The internal gas-permeable membrane material that allows gases to penetrate while blocking liquids, is designed to withstand a certain pressure difference. That limit may accidentally be exceeded if liquids are pumped too fast into the degassing chamber, which may cause irreparable damage.
Should a degasser chamber malfunction, it is most often either related to use with incompatible organic solvents, or a blocked flow path. If this happens, the chamber needs to be replaced. Fortunately, this is a straightforward and quick procedure in most systems. Note however, that in cases where there has been a leakage from the fluidic line into the vacuum line, there is a high risk that the vacuum sensor on the control board has been impaired. In these situations, there are often so many damaged components that one should consider exchanging the entire degasser.

Since modern inline degassers are highly robust equipment with a long lifetime, it may well be that if their internal vacuum pumps eventually fail, there is a risk the original part is no longer available from the manufacturer. The stepper motor driven vacuum pumps, with sensor and control boards to ensure constant vacuum, come in a range of variants for different degasser
Figure 2. Schematic sketch of a degassing chamber containing a gas permeable Systec AF tubular membrane, connected to a vacuum pump with constant vacuum controller
Inline degassers are robust pieces of equipment that require minimal maintenance to continuously safeguard precision in fluidic systems by reducing dissolved gases and eliminating troubles from bubbles. With proper handling, the user can ensure that their degasser will keep performing for many years, and if it eventually fails, replacements of parts will be a simple procedure.
[1] https://biotechfluidics.com/ products/degassing-debubbling/ degasi-inline-degassers/degasserspare-parts/
assemblies. These pumps may differ in capacity for various fl ow rate ranges, or in the vacuum setpoint, or in the control board design, or in the mounting orientation. Conveniently, Biotech Fluidics upholds an online vacuum pump replacement fi nder [1] that facilitates exchange of obsolete pumps and control boards in inline degassers from many different brands.
Anders Grahn, Chairman & Founder, Biotech Fluidics AB, Onsala, Sweden Dr. Fritiof Pontén, CEO, Biotech Fluidics AB, Onsala, Sweden

systems for Type I, II and III water Suitable for applications from glasswashing and reagent makeup through to UPW for histology diagnostics and DNA Sequencing. Bench top units through to bespoke system design for centralised systems.







Water activity is a critical parameter in product development across various industries, particularly in food, pharmaceuticals, and cosmetics. It refers to the energy status of water in a product, which can impact chemical reactions, physical properties, and the growth of microorganisms. Unlike moisture content, which measures the total water in a product, water activity focuses on the energy of water, which influences the product’s stability and overall quality. Understanding and controlling water activity is essential for creating products that are safe, durable, and appealing to consumers.
One of the most significant roles of water activity in product development is its impact on microbial growth. Microorganisms require a certain energy of water to maintain their internal turgor pressure, which is necessary for normal functioning. By controlling water activity, manufacturers can
inhibit microbial growth, thereby enhancing product safety.
Water activity also plays a crucial role in the chemical stability of products. Many chemical reactions are influenced by the energy of water. By adjusting water activity, developers can either slow down or accelerate these reactions, depending on the desired product characteristics. A well-balanced water activity level can ensure that a product retains its intended flavour, appearance, and nutritional profile throughout its shelf life. Similarly, in pharmaceuticals, maintaining optimal water activity can prevent the degradation of active ingredients, ensuring the product remains effective until its expiration date.
Water activity affects not only the safety and stability of products but also their sensory attributes, particularly texture. In food products, for instance, water activity influences whether a product is perceived as crunchy, chewy, soft, or dry. A slight change in water activity can alter the texture significantly, impacting the consumer’s sensory experience and satisfaction.

There may be a tendency to think of water activity as only a QA test to be done on a finished product. However, incorporating water activity testing into product development can improve efficiency and help avoid costly mistakes. For example, if the impact of water activity is considered from the onset of product development, an ideal water activity can be identified and then passed on to production as the release specification for the product. If the product is made to that specification, there will be a significant reduction in waste or failed product. In addition, wasteful reformulation of an already released product to meet a necessary water activity is avoided.
Water activity should also be considered when making decisions about product packaging. It is important that packaging acts as a moisture barrier that prevents changes in product water activity owing to exposure to abuse storage conditions. Modeling can be used to determine the appropriate packaging that will sufficiently restrict water activity changes while avoiding costly over-packaging.
In summary, tracking water activity during product development helps to set release specifications, identify external factors that could alter the water activity, and choose packaging that will ensure the product is safely delivered to the consumer. Thus, water activity remains a cornerstone of successful product development, shaping the characteristics and longevity of countless products in the market.
Mockups, often made from wood or cardboard, can help mitigate installation problems further down the line. Saskia Henn reports
During the implementation of a new aseptic lling line, adequate preparation is necessary to prevent




Implementing a new aseptic filling line can be daunting, since everything from the layout of the cleanroom to the height of the operators can influence how well your contamination control management system works.
A recent webinar titled ‘The Successful Implementation of a New Aseptic Filling Line,’ outlined how difficult it can be, as well as how technicians can prevent issues further down the line.
The webinar was hosted by Mark Hallworth, the pharmaceutical manager for PMS, and Marc Machauer, global OEM coordinator for the company. As Hallworth explained, although risk assessment can ensure that regulatory requirements are met, this process often occurs at the end of filling line implementation once it is hard to change anything – such an assessment is therefore not enough to mitigate operational problems.
Rather than relying on risk assessment, operators should ensure they prepare adequately for the build before it goes ahead because once a design is complete, it is often replicated and sold to pharma companies that must adapt their own process to the machine. It can be difficult to make a specialised product work for someone else.
Hallworth recommends that lab operators invest time and resources into a facility right from the beginning and that they create an adequate mock up. This will make it less likely that something will need to be retrofitted.
Machauer explained that he often sees filling lines that have amendments once they are installed as well as the problems that occur.
“Many times, we get pulled in after a machine is installed and the customer has come to us, and now we have to make it work somehow,” he said.
“It is expensive and time consuming to resolve the issue from this standpoint.”
This is why the mock-up phase is so important. The physical representation of a machine provides a realistic idea of what the final product will look like. Mock-ups are commonly made virtually or from wood, cardboard and foam core, allowing designers to quickly make changes as needed.
This type of testing can help operators understand what the risks are, where the airflow patterns are and what changes need to occur prior to installation.
Hallworth said: “A process study for each phase of the machine will give us many sample points that reflect the best risk within that environment.”
During the mock-up phase, operators of different heights can reach into the machine to pick up microbial plates and test if they work smoothly. This simulation testing can also be applied to automated equipment, such as robotic arms.
“It’s always better to do a wood model where you can put in some real components and you have a real reach,” said Machauer.
An accurate mock-up can prevent problems leading to operational shutdown later on, such as on-site modifications, measurements not adding up or problems with the physical space where the filling line sits. www.pmeasuring.com
“We’ve got this whole quality by design strategy. Rather than trying to measure for quality at the end, we’ve got to push that quality forward into the design and mockup phase,” said Hallworth.






Cleanrooms present unique challenges for ESD control



Elizabeth Norwood, a senior chemist from MicroCare, explores how to protect sensitive cleanroom devices while ensuring they maintain quality and functionality
Electronic medical device manufacturers face the ongoing challenge of maintaining product reliability and performance. Electrostatic discharge (ESD) poses a serious threat across all production settings, particularly in cleanrooms. Sensitive devices like pacemakers, insulin pumps, imaging systems, and patient monitors are especially at risk owing to their intricate electronics. As these technologies become more compact and sophisticated, the potential for ESD-related damage increases. To address this, manufacturers must implement robust protection measures while adhering to strict regulatory requirements to ensure product quality and functionality.
Electrostatic discharge occurs when two objects with differing electrostatic potentials come into proximity or contact, resulting in a rapid transfer of electrons. Even minor ESD events can have severe consequences in manufacturing, particularly for sensitive electronic components such
as Printed Circuit Board Assemblies (PCBAs), which are integral to many modern medical devices.
ESD-related failures in medical devices typically take two forms: catastrophic failure and latent damage. Catastrophic failure results in immediate and detectable malfunction, often found during manufacturing or initial quality control procedures. Latent damage, however, poses a more complex challenge. Devices affected by latent ESD damage may pass initial tests but experience premature failure in the field, potentially compromising patient safety and the manufacturer’s reputation.
While essential for keeping sterility and precision in medical device production, cleanrooms present unique challenges for ESD control. Low humidity levels, necessary to inhibit microbial growth, can increase the risk of static build-up. Even though cleanroom garments are designed to prevent contamination, friction from personnel movement can still generate static charges. Routine activities like walking across the cleanroom floor can create static potentials,
sometimes reaching thousands of volts if ESD control measures are not effectively managed.
Furthermore, many cleanroom surfaces and equipment are constructed from insulating materials that can accumulate static charges.
To address these challenges, medical device manufacturers should implement a comprehensive, multifaceted approach to ESD control. This strategy begins with personnel management. All staff working in the cleanroom should use anti-static wrist straps or heel grounders connected to verified ground points. These devices continuously dissipate static charges that accumulate on the human body. ESD-safe footwear and clothing, including cleanroom-compatible garments manufactured from staticdissipative materials, further mitigate the risk of charge generation and accumulation.
Environmental modifications play a crucial role in ESD mitigation. Conductive flooring or floor mats
The
should be installed to dissipate static charges effectively. Humidity levels should be precisely regulated, typically kept between 40% and 70%, while temperatures should be controlled between 18-22°C (64-70°F) to balance static reduction with other cleanroom requirements. All work surfaces, equipment, and tools should be properly grounded, creating a network that safely channels static charges away from sensitive components. Material handling procedures are equally important in ESD prevention. PCBAs and components should be transported and stored in ESD-safe containers and packaging. In areas where non-conductive materials that cannot be grounded must be used, ionisation systems can help to neutralise static charges.
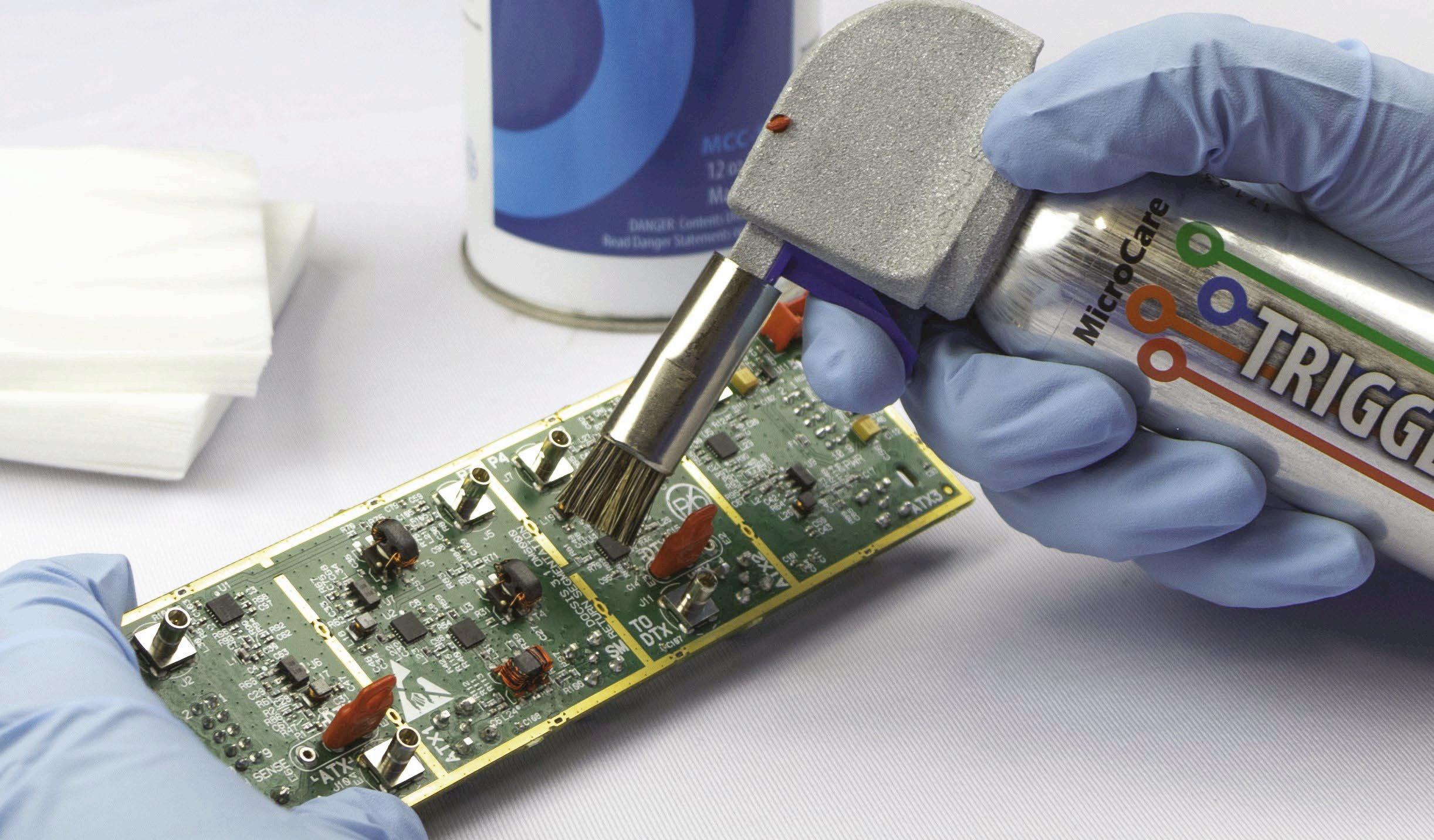
Cleaning and maintenance protocols are integral to ongoing ESD control. Effective cleaning in PCBA assembly is not just about removing contaminants but also about preventing ESD. Some cleaning fluids can generate significant static charges, worsening the ESD risk rather than mitigating it. For example, certain flux removers can
produce static as high as 12,000 volts owing to friction when dispensed from aerosol cans. To address this, manufacturers should use specially engineered static-dissipating tools that attach to the aerosol can to minimise the generation of static charges to just over 50 volts.
In addition, regular use of presaturated ESD-dissipating cleaning wipes can significantly reduce static build-up. These wipes effectively remove contaminants like fingerprints and grease without leaving lint, debris or static charges. They should be used in straight lines with overlapping strokes to ensure thorough coverage. It’s advisable to choose wipes with low/no alcohol content to prevent drying out surfaces and causing damage.
An ESD-safe controlled flux remover dispensing system is another tool that helps to manage ESD and improve cleaning outcomes. This system allows technicians to apply flux remover in a controlled manner, reducing waste and ensuring that the cleaning process does not introduce further static. Brushes attached to these systems can help clean under surface-mounted components more effectively while also grounding the cleaning tool to end any charge build-up.
Cleaning and maintenance protocols are integral
The effectiveness of ESD control measures relies on proper implementation by personnel meaning comprehensive training programs should be set up. All staff working in or entering the cleanroom should undergo regular ESD awareness training.
Manufacturers should conduct periodic audits of ESD control practices and equipment to ensure ongoing effectiveness and verify compliance with regulations.
Effective ESD control in cleanroom manufacturing medical devices is critical to the ongoing battle against electrostatic discharge. By minimising ESD-related damage, manufacturers significantly enhance the long-term reliability of their devices. This reduces costs associated with warranty claims, rework, and product recalls.
Microcare is a precision cleaning solutions company based in the US.
A paper written by Mark Hallworth from PMS explores sampling techniques in cleanrooms and associated controlled environments
The following article is an excerpt from the paper:
ISO/TR 14644-21:2023
Cleanrooms and Associated Controlled Environments: Airborne Particle Sampling Techniques, written by industry expert and ISO 14644 Technical Committee member Mark Hallworth of Particle Measuring Systems. It is an essential guide designed to help cleanroom operators and technicians refine their airborne particle sampling methods. The full paper is complete with specific requirements, explanatory diagrams and a decision tree.
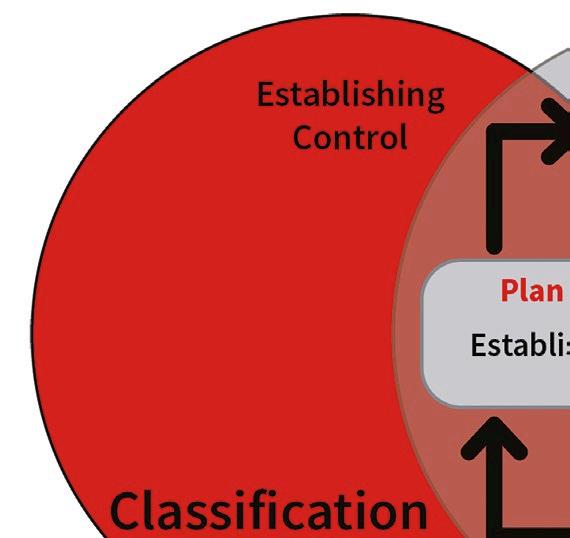
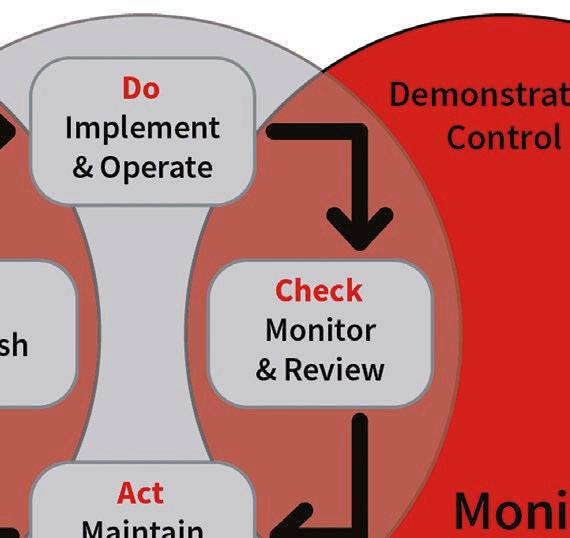
This technical report (TR21) was developed in response to updates in the EU GMP Annex 1 regulation regarding sterile medicinal product manufacturing. It provides a comprehensive discussion on classification and monitoring strategies for maintaining cleanroom
air quality and minimising particle contamination.
Cleanrooms are controlled environments where airborne particle concentration must be continuously monitored to meet standards, such as ISO 14644, required by regulatory bodies. Importantly, ISO/TR 1464421 discusses the importance of accurately detecting and measuring smaller particles, which also pose significant contamination risks.
ISO/TR 14644-21 identifies three primary areas of concern when sampling airborne particles:
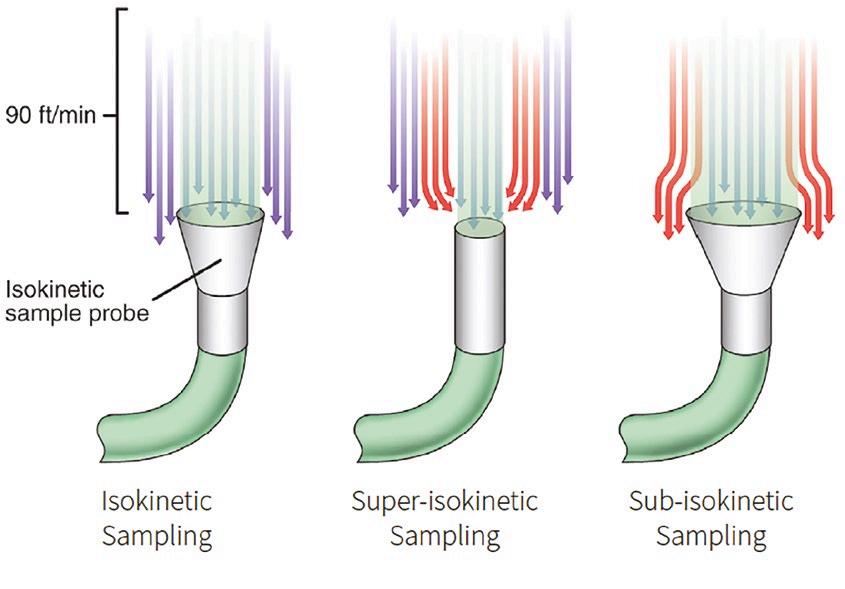
1. Sampling errors: These errors occur when the sample taken does not accurately represent the total air volume in the cleanroom. ISO/TR 1464421 stresses the importance of isokinetic sampling, where the air velocity at the sample inlet matches the room’s airflow. If this balance is not achieved, particles might be under or over-sampled, leading to inaccurate results.
2. Sample measurement errors: These errors stem
from the equipment used in particle counting. Light Scattering Aerosol Particle Counters (LSAPCs) are the most commonly used instruments. Particle coincidence, impact of optical contamination and accurate calibration of LSAPCs, based on ISO 21501-4, are crucial for reliable results.
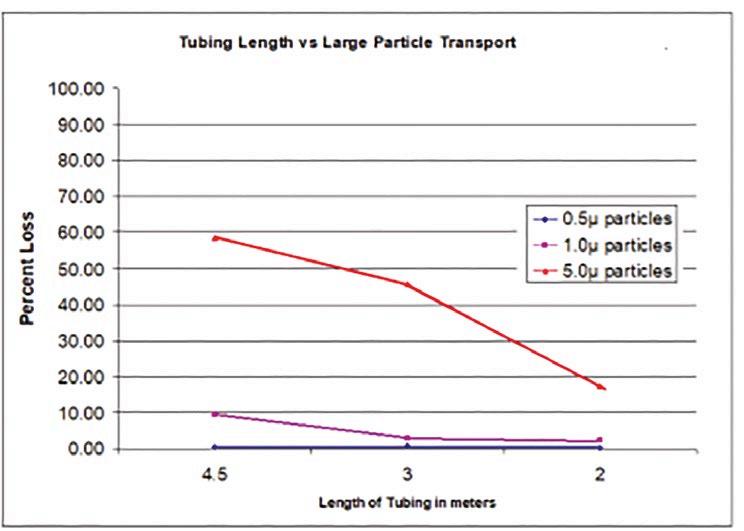
3. Sample transportation errors: When using tubing to transport air samples to a particle counter, particle losses can occur due to tubing bend sedimentation, electrostatic attraction, and Brownian motion. ISO/TR 1464421 gives specific advice for keeping tubing lengths short and using smooth-bore materials to minimize these losses.
ISO/TR 14644-21:2023 offers valuable guidance for both cleanroom classification and monitoring, with a focus on minimising errors in airborne particle sampling. By understanding and addressing the potential sources of error in particle sampling and transportation, cleanroom operators can better maintain control over their environments, ensuring compliance with regulatory standards and minimising contamination risk.
Microplate readers have evolved because of technological breakthroughs that have improved their features and broadened their range of applications





The recent market expansion has been driven by a need for high throughput screening in genomics and drug discovery, according to a recent report
Microplate readers are essential laboratory instruments used to analyse one or more parameters within microplates, such as absorbance, fluorescence, or luminescence signals. They are used for a variety of laboratory tasks, including quantifying protein, gene expression or metabolic processes; evaluating enzyme reactions; detecting diseases in cells; performing end point tasks; performing kinetic analyses; and detecting and measuring antibodies, proteins or antigens in biological samples.
Microplate readers work by detecting and quantifying light signals
produced by liquid samples; they use different detection modes and a standard curve to determine the experimental values as well as multiwell plates which are integral to the microplate reader and allow for many experiments to be performed at once.
A report released in November 2024 by research company Transparency Market Research predicts that the global microplate reader market will see compound annual growth rate of 7.0% to reach US$834.4m by 2031 (the market was valued at US$461.3 in 2022).
This surge will be driven by the requirement for high-throughput
screening in genomics and drug discovery, as well as increased demand in life sciences research and diagnostics.
Microplate readers have evolved because of technological breakthroughs that have improved their features and broadened their range of applications. Modern technology integration has helped to increase the versatility and effectiveness of these instruments. Advancements currently include multi-mode detection capabilities. Furthermore, increased sensitivity and signal-to-noise ratios have been made possible by sophisticated detectors, better light sources, and precise
optics, all of these present profitable prospects for market growth.
According to the report, the market for clinical trials is likely to grow at around 10% annually, creating a substantial need for advanced laboratory equipment. In 2022, the single-mode readers segment dominated the market. Dedicated instruments are increasingly needed for applications requiring specialised measurements, including enzyme assays, cell viability tests, or the detection of certain biomolecules, owing to their precision and accuracy. Measurements with higher sensitivity and specificity are possible thanks to single-mode readers, which are frequently tailored for a specific detection technique.
Single-mode microplate readers measure only one detection technology (for example absorbance or nucleic acid detection) and they are the best choice when it is clear that the instrument will be used for just one application. Such tasks are typically fairly long-term and they block the microplate reader for other measurements meaning one mode is sufficient. Examples of such studies include microbial growth monitoring which takes one or more days, or fluorescence detection of thioflavin T experiments which take up to a week.
Multi-mode readers are needed when a laboratory wants to measure several properties such as enzyme activity in combination with the quantity of DNA and proteins in a specimen for example. Lab technicians that currently plan applications based on one detection mode but think they may want to change in the future may want to consider a model that can be upgraded to a multi-mode machine.
All the major companies in the field are incorporating the aforementioned developments in the microplate

Multi-mode instruments are needed when a laboratory wants to measure enzyme activities and quantity of DNA and proteins
reader industry into their products in order to remain competitive. The following companies are well-known participants in the global microplate reader market:
- BMG LabTech (for more insight into the company’s work see the ‘Innovations in cannabis research’ on page 22).
• Bio-Rad Laboratories
• Merck KgaA
• Thermo Fisher Scientific
• Dynex Technologies Inc
• Grifols, S.A
• Tecan Group Ltd
• Monobind
The report from Transparency Market Research found that North America leads the global industry. The region’s market dynamics are being driven by
the presence of major companies and improved infrastructure in research laboratories. Two key drivers of the increase in demand are the rise in the number of clinical trials and the sizable government funding of current R&D projects.
The pharmaceutical and biotechnology industries using the technology are also located mostly in the US, according to the report, and the need for microplate readers is driven by ongoing innovation and expansion in drug research and screening.
In order to bolster their positions, major companies in the industry are increasingly focused on partnerships, collaborations, mergers, and the introduction of new products; and those observing the industry can expect to see an uptick in activity of this sort in the years to come.
For more information visit: www.transparencymarketresearch.com/microplate-readers-market.html

The PHERAstar FSX with its advanced assay stability function enables temperature control between 18oC and 45oC ensuring stable measuring conditions

Barry Whyte from BMG Labtech discusses how microplate readers could help accelerate drug discovery
Interest in cannabinoid research is growing as scientists look for new ways to advance drug discovery and development for different diseases. This field of study holds promise for new treatments for cancer, neurological disorders, pain management and other conditions. In this context, researchers need new approaches to investigate modes of action, ensure the safety of cannabinoid substances, and help develop novel drugs with reduced side effects.
Microplate readers can support a wide variety of analyses associated with cannabinoid research. The use of high-throughput methods offers time savings, reduced costs, and can help meet the growing demand for scientific analyses. Here, we look at some of the ways microplate readers can provide added value in cannabinoid research.
Cannabinoids are a diverse group of naturally occurring or
synthetic chemical compounds that fall into three main categories: Endocannabinoids are produced in the human body and are responsible for the regulation and control of functions like learning and memory; phytocannabinoids are plant-derived compounds but can partly bind to the same receptors; and synthetic cannabinoid receptor agonists (SCRAs) are made in the laboratory.
Microplate readers are often used to detect phytocannabinoids or their metabolites in medical samples or in forensics. For this purpose, immunoassays such as ELISAs serve as methods for quantification. Chemotyping of plants may also help identify the preferred botanical extracts for use or identify cannabis brought across geographic regions illegally. [1,2]
Researchers want to understand the pharmacology of naturally occurring and synthetic cannabinoids at the receptor level to aid drug discovery. Microplate-based methods using fluorescence polarisation, bioluminescence resonance energy transfer (BRET) or time-resolved
fluorescence resonance energy transfer (TR-FRET) enable the sensitive and reliable detection of receptor binding as well as the identification of downstream signal cascades.
In the following example, TR-FRET was used to study the differential binding of natural cannabinoids to the cannabinoid receptor 1 (CB1). The fluorescent molecule CELT-335 acts as a ligand for CB1. CB1 was additionally labelled with terbium to detect the interaction. When the fluorescent agonist binds to the receptor, energy is transferred from terbium to CELT-335 which consequently emits light at 665 nm in addition to 620 nm derived from the terbium (figure 1A). In competition with 100 nM CELT-335, the binding affinity of 0-10 µM of the phytocannabinoids Δ9-THC, Δ9-THCA and Δ9-THCV was monitored. The TR-FRET ratio was determined using BMG LABTECH’s PHERAstar FSX
The binding affinities were similar for ∆9-THC and ∆9-THCV with pKi values of 7.2 ± 0.6 and 7.0 ± 0.3,
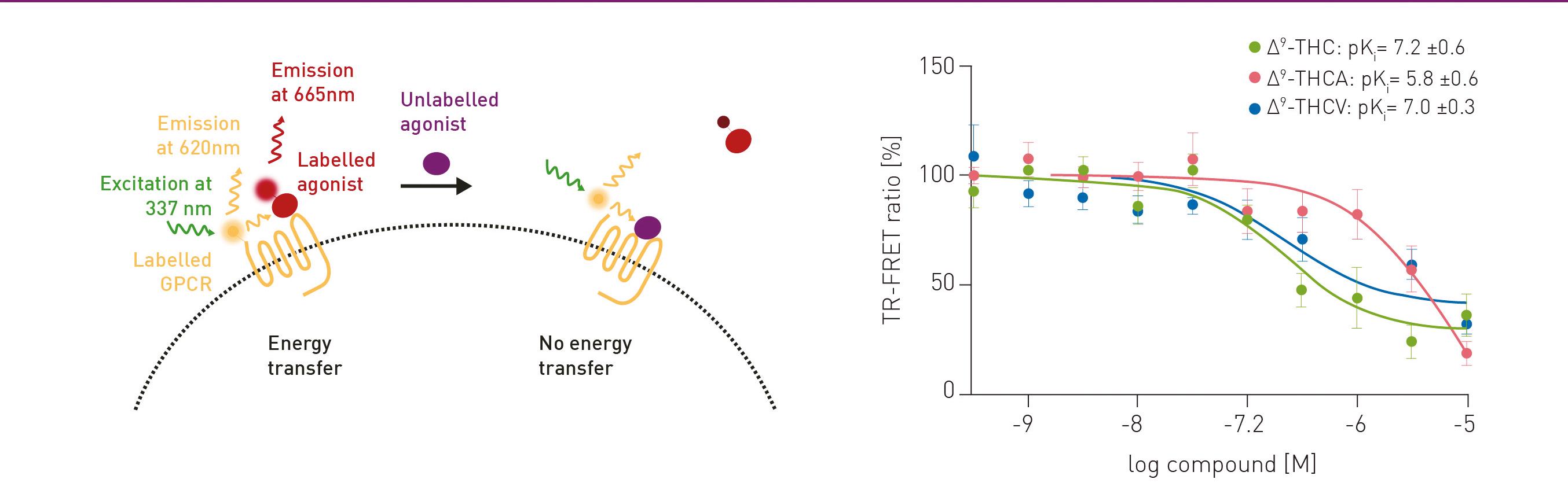
respectively (figure 1B). The affinity for ∆9-THCA was considerably lower with a pKi of 5.8 ± 0.6.
The Simultaneous Dual Emission (SDE) feature on the PHERAstar FSX allows both emission signals (from donor and acceptor) to be measured simultaneously. In addition to its high sensitivity and reliability, where even the smallest changes in receptorligand interactions can be measured, SDE results in further time savings and more robust data.
SCRAs were originally developed for scientific research or as therapeutic agents. Unfortunately, they have emerged as drugs of abuse and are associated with serious health risks including death.[3] Little is known about the detailed toxicology and pharmacology of SCRAs.[4] However, details of their mode of action are needed to reduce the risks they pose and pave the way for their use as therapeutic agents.
A B B
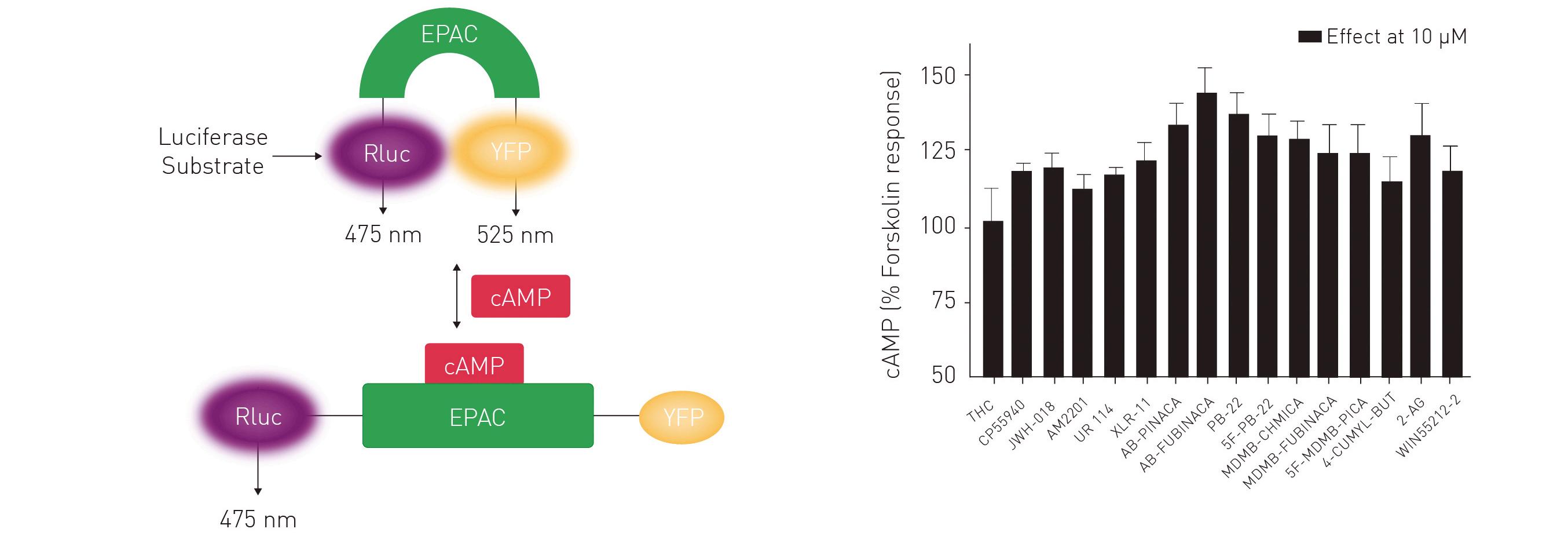
To study whether a selection of cannabinoids signal via the Gαi- or Gαs- subunit of the CB1 receptor, cAMP, a downstream second messenger of the Gαs-stimulated cascade was analysed in the following study. Binding of a receptor agonist to CB1 (a G-protein coupled receptor) may lead to dissociation of the trimeric G-protein complex and the release of the subunits into the cytoplasm, initiating various signalling pathways and cellular responses. cAMP levels were quantified with a BRET-based CAYMEL biosensor. The sensor is composed of an EPAC protein, a Renilla luciferase (Rluc) and a yellow fluorescent protein (YFP). Upon binding of cAMP by EPAC, Rluc and YFP are spatially separated owing to a conformational change. This leads to a reduction of the BRET signal which is generated in the absence of cAMP owing to proximity of Rluc and YFP (figure 2A). The overall levels of cAMP generated confirm that cannabinoid receptor agonists have significantly
different efficacies in the activation of the Gαs pathway (figure 2B).
Again, the high detection speed enabled the study of several potential compounds in parallel in a kinetic approach. The PHERAstar FSX also offers compatibility with other automation solutions which make it an ideal choice for high-throughput applications in all reading modes.
More information on cannabinoid research is available in this blog post or you can email applications@ bmglabtech.com with questions.
1. DOI: 10.1093/jat/bkab107
2. DOI: 10.1007/s10681-015-1585-y
3. DOI: 10.1056/NEJMp1505328
4. DOI: 10.3389/fpsyt.2020.00464
For more information visit: www.bmglabtech.com



The most common modality used in scanning electron microscopy (SEM) is secondary electron (SE) imaging, which detects lower energy electrons emitted from the sample surface owing to interactions with the SEM electron beam. SE imaging provides information about sample topography.
Backscattered electron (BSE) imaging, on the other hand, detects higher energy electrons scattered back from deeper within the sample. BSE imaging reveals sample composition by atomic number contrast with heavier elements appearing brighter.
BEX imaging combines BSE sensors with X-ray sensors placed below the objective lens. The X-ray detectors collect characteristic X-ray emissions that are generated by the sample when irradiated by the SEM electron beam. The X-ray signal is processed to identify and assign colours to the detected elements, which are then combined with the BSE signal to create a final image. BEX imaging provides more information about sample composition and elemental distribution in the same acquisition time as SE or BSE imaging.
Compared with an Energy Dispersive Spectroscopy (EDS)

detector, a BEX imaging system offers a higher solid angle, enabling X-ray information collection at normal imaging speeds. The position of the X-ray sensors near the objective lens eliminates shadowing effects and allows for sample investigation at various working distances.
The Unity detector, recognised as one of the top microscopy innovations of 2024 by the Microscopy Today Innovation Awards, combines two types of sensors within a single detector head. It integrates
backscattered electron (BSE) sensors and X-ray sensors to provide comprehensive imaging with atomic number and elemental data. The detector is designed for daily imaging, offering flexibility in working distance, the ability to capture data from challenging sample topography, a wide field of view, and compatibility with variable pressure mode for non-conductive samples. The Unity detector delivers reliable and instant imaging results supported by advanced technology and software integration.

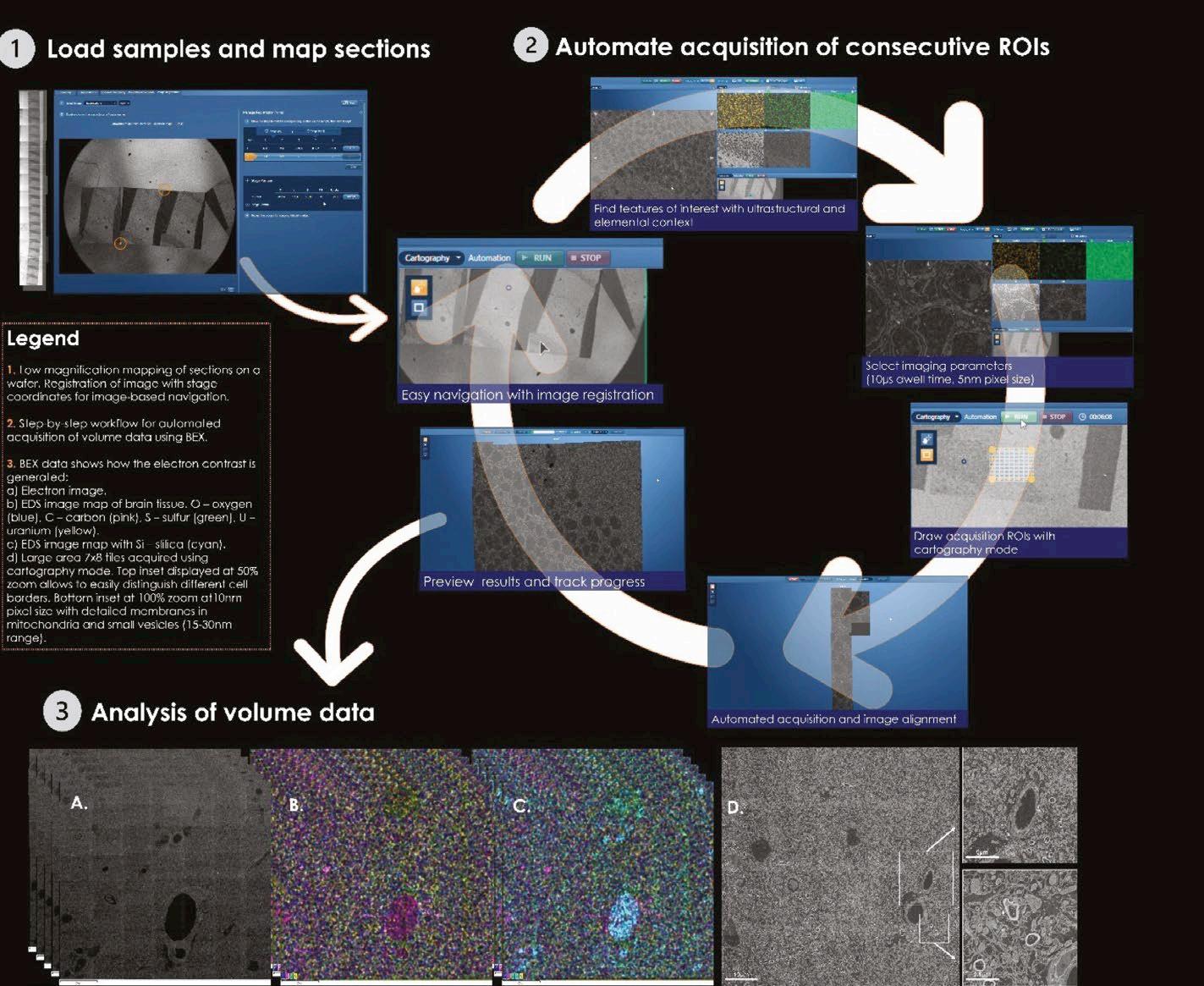
The Volume EM Technology Forum is a great opportunity for researchers and scientists to learn about the latest advances in volume electron microscopy (vEM). This year, the conference featured presentations from leading experts in the field, as well as technical presentations, posters, panel discussions, and hardware and software demos. Attendees also had the opportunity to network with other researchers and scientists, and to learn about new products and services from exhibitors.
In this year’s event (21st October 2024 – 24th October 2024), the Oxford Instruments team presented a demo of our BEX detector, Unity, where we connected remotely to the Oxford Instruments Innovation Centre, a state-of-the-art facility at the High Wycombe site. We showcased the advantages of BEX imaging for biological and nonbiological samples, especially for vEM applications, where the speed and flexibility of BEX for different specimens is key (Figure 1).
With Unity Cartography mode, Oxford Instruments achieved highresolution chemical mapping of entire
biological samples. This workflow would previously have taken several hours to complete (even overnight) and can now be achieved within minutes with BEX imaging, enabling higher throughput and less beam damage for sensitive samples such as cells and tissues (Figure 2).
With BEX, new possibilities for vEM are open, for collecting elemental and ultrastructural information simultaneously, without added imaging time. In the example in Figure 3, BEX cartography was used to achieve high resolution images and fast mapping of array tomography brain slices. Large areas were imaged with a relatively short beam dwell time (10µs), which reduces beam damage, drift and resin charging. Elemental information was acquired simultaneously and provided chemical differentiation that can be used to further distinguish between sample features, opening the possibility of improving subsequent segmentation of data. BEX also provided information about strain distribution, which was quantified by the EDS detector. Being able to measure the amount of stain taken up by the sample improves the team’s ability to make direct comparisons between samples enabling them to optimise sample preparation techniques.
In this Q&A Robert Gaertner from Veeva Systems explores how cloud-based platforms are helping to both ensure quality in pharma manufacturing and support the shift to personalised medicine
GIVEN THAT QUALITY IS SO IMPORTANT IN HEALTHCARE. WHAT CHALLENGES DO BIOPHARMAS CURRENTLY FACE IN MAINTAINING THE NECESSARY STANDARDS?
The pharmaceutical industry faces many challenges and maintaining quality is one of them. Patients must trust that medicines are safe, meet high standards, and comply with regulations. With personalised medicine and other modern therapies, this becomes even more challenging. Unlike traditional medicines distributed through pharmacies, personalised therapies require extra layers of quality control. A good example is the public discussion around vaccines during the COVID-19 pandemic. People knew some vaccines had to be refrigerated, but they likely didn’t understand the extensive effort required. You can produce a high-quality medicine, but for it to remain safe and effective, you must monitor it until it reaches the patient.
HOW DOES VEEVA HELP BIOPHARMACEUTICAL COMPANIES MAINTAIN QUALITY?
There has been a significant shift in recent years. In the past, companies manufactured and tested medicines in-house. Now, manufacturers must pay more attention to the quality of their suppliers, and logistics play a growing role as more of the work is outsourced. While some companies specialise in logistics, the manufacturer remains responsible. This shift requires solutions that not only manage internal business processes but also integrate the entire supply chain, including partners. That’s why cloud-based, industry-specific solutions like Veeva Vault Quality have become critical. Cloud technologies play a key role in maintaining quality because companies need to communicate with suppliers, partners, and even patients, in real-time.
This requires easily accessible, secure, and traceable data, all while staying compliant with regulations like GDPR.
WHERE DO CONNECTED
CLOUD SOLUTIONS HELP THE MOST, PARTICULARLY WITH TRANSPARENCY?
Here’s an example: when a raw material is produced in another country, the quality decision requires approval based on the data the company receives. If this data is handled on paper, it causes delays. Cloud platforms provide real-time insights, and ensure data integrity. With cloud solutions, everyone in the supply chain can access the same data simultaneously, reducing redundancies and improving decision making.
HOW DOES VEEVA HELP KEEP DATA ENTRY CLEAN AND SECURE?
You have to look at the entire supply chain. In production, for example, some machines have sensors that provide data relevant for quality decisions. Another example is patient complaint management. If a patient experiences side effects, a doctor or healthcare professional enters this data manually. Ideally, they only need to enter it once, and the system ensures secure entry. Artificial intelligence can help ensure data is entered correctly, and quality checks can occur as soon as data is available. Connected cloud technologies, like those from Veeva, also bring together different data sources to maintain quality control.
HAS VEEVA DEVELOPED A STREAMLINED WAY TO ACHIEVE THIS?
Yes. Veeva’s Quality applications are scalable and easy to access online. Companies can define which groups of people need convenient access to
the database, and we can configure the system accordingly.
LOOKING TO THE FUTURE, WHAT CHALLENGES COULD THE INDUSTRY BETTER ADDRESS IN THE NEXT THREE TO FIVE YEARS, POSSIBLY WITH AI?
There’s a constant tension between efficiency and compliance. Regulations must be met, but at what cost? The key for the pharmaceutical industry is balancing GxP compliance with streamlining quality systems to improve both cost-efficiency and quality outcomes. The focus should shift from managing quality issues to preventing them. AI can help predict potential risks and prevent them before they occur. Additionally, the definition of a pharmaceutical product will evolve. We often think of medicine as a pill or physical product, but future therapies will be more complex and personalised. Homecare and clinical trials are moving from hospitals to patients’ homes, where wearables will report data. This brings new quality challenges, such as qualifying devices and validating processes, which are easier to manage with cloud solutions.
WHAT DOES THE INDUSTRY EXPECT FROM VEEVA IN THIS REGARD?
We develop our solutions in collaboration with our customers. We aim to understand their challenges and work with our experts to develop innovative solutions that align with their needs. It’s a combination of customer input and our strategic vision, alongside regulatory requirements, particularly in the area of quality.








Drug-induced liver injury (DILI) is a common cause of drug withdrawal during late drug development as well as post-approval. The liver, a primary site of drug metabolism, is particularly susceptible to drug-induced injury. However, DILI can occur through several pathways, each involving different mechanisms.
Intrinsic DILI is generally caused when a drug, or its metabolites, cause damage at high doses. It poses the least risk and is the easiest to predict using traditional approaches owing to its dose-dependency and early onset. Traditional in vitro and in vivo animal studies are less able to predict more complex indirect or idiosyncratic effects that are latent in onset. The former is generally mechanistically related to the pharmacodynamics of the drug causing immune system, or metabolic effects, with some
dose-dependency. The latter is more unpredictable and is often driven by genetic predisposition or underlying disease. These risks can pass undetected through development and early clinical trials, causing financial and reputation losses upon attrition. This poses the question - how can preclinical workflows be modernised to reduce DILI risk?
The safety toxicology toolbox requires modernisation through humanisation to understand how DILI is induced. Identifying potential issues earlier provides an opportunity to perform exploratory investigations to unlock the mechanism behind the cause. Previously, unlocking the cause has proven difficult as maintaining the key
phenotypic and functional attributes of primary human hepatocytes is notoriously challenging. Now there is a path forward that offers the potential to recover good drugs by engineering out the flaws, terminating programs before the clinic, or delivering the foresight to manage liabilities and proceed with caution.
Liver-on-a-chip technology, or liver microphysiological systems, offer a sophisticated platform for studying DILI with greater accuracy and relevance to human physiology. These advanced in vitro NAMs replicate the complex 3D structure of the liver, including the arrangement of primary human hepatocytes and non-parenchymal cells, which are
❝ CN Bio’s approach has been recognised by the US FDA CDER (Centre for Drug
The company has created a webinar that aims to help people reduce their DILI risk
crucial for maintaining liver function and tissue-specific inflammatory responses. They are cultured under perfusion to simulate the liver’s microenvironment, including blood flow and shear stress, which are essential for promoting high metabolic activity and prolonged culture longevity to discover latent effects.
Liver-on-a-chip technology delivers deep mechanistic insights to elucidate the pathways through which drugs induce liver injury, such as oxidative stress, mitochondrial dysfunction, steatosis, dysregulation of bile acid synthesis or transport and importantly, immune-mediated damage to identify more indirect or idiosyncratic DILI events[1,2,3,4]. Additionally, by incorporating genetic variations and the presence or absence of common diseases such as metabolic disorders, these models can help to identify factors that increase susceptibility.
CN Bio’s PhysioMimix DILI assay utilises liver-on-a-chip technology to deliver exceptional performance, according to the company, as exemplified in a study using reference compounds from the IQ MPS Consortium DILI validation set and human-specific gene-therapies (antisense oligonucleotides), which are less-suited to animal testing. The
assay, cultured using PhysioMimix OOC Systems, delivered 100% sensitivity, 85% accuracy, and 100% precision, measuring six different biomarkers to produce a ‘signature of hepatotoxicity’, identifying hepatotoxicants that passed traditional in vitro tests [5].
CN Bio’s approach has been recognised by the US FDA CDER (Centre for Drug Evaluation and Research) group, which cited superior performance versus standard approaches in the first publication between the organ-on-a-chip (OOC) provider and the regulator [1]. Importantly, the utility of both the highly metabolically active hepatocytes and immune-competent Kupffer cells in the PhysioMimix assay were shown to be crucial in identifying hepatotoxic risk.
Recent technological advances have increased the assay’s throughput, enabling its use within lead optimisation, in addition to investigative toxicology, to justify the progression of promising drugs into in vivo studies by providing go/ no go or reengineer decisions at the tipping point between discovery and development.
The process of discovering and developing drugs is inefficient and costly. Change is required to reduce attrition rates and improve return on investment. OOC technology is becoming a lab essential because it provides the capacity to flag more liabilities than before and better inform next-step decisions [6]. CN Bio aims to help clients future proof their safety toxicology workflows, starting with DILI research, to help them reduce cost and time spent on projects.
1. https://doi.org/10.1111/cts.12969
2. https://doi.org/10.1016/j. tiv.2022.105540
3. https://doi.org/10.1016/j. tiv.2017.09.012
4. https://doi.org/10.1124/ dmd.116.074005
5. https://cn-bio.com/resource/ human-liver-microphysiologicalsystem-for-predicting-the-druginduced-liver-toxicity-of-differingdrug-modalities/
6. https://doi.org/10.1038/s41573-02200633-x
A collaboration between a team of enzyme engineers and a leading computational biologist promises to transform the industrial biocatalysis landscape, Nicola Brittain reports
Researchers at the Manchester Institute of Biotechnology (MIB) and the Institute for Protein Design at the University of Washington, recently received funding of £1.2m to set up an initiative called The International Centre For Enzyme Design which looks set to transform the design and engineering of enzymes for industrial applications.
The project, launched in November and to run for an initial four years, is a partnership between a group of University of Manchester UK academics and an international overseas partner, Professor David Baker, a renowned computational biologist based at the University of Washington. The consortium will use deep learning and computational techniques to create sustainable, industrial biocatalysts in a timely and cost-effective way.
Eurolab caught up with Anthony Green, director of the MIB, and Sarah Lovelock, a senior lecturer in the MIB, two of the lead researchers with the partnership to find out what the project will mean for business, the environment, and enzyme scientists more widely.
Green explained that the programme brings together leading computational and experimental teams to create a step-change in the speed of biocatalyst development. The methods developed will also allow






the development of new families of enzymes with valuable activities that are unknown in nature.
The Manchester-based team of scientists currently make use of experimental enzyme engineering techniques such as ‘directed evolution’ to create new biocatalysts for use in the chemical industry. This process aims to expedite the natural evolution of biological molecules and systems through iterative rounds of gene diversification and library screening. Green said: “The process is extremely powerful, but it’s also expensive, time consuming and requires specialist infrastructure.”
The new collaboration, and the resulting incorporation of advanced deep-learning methods for protein design pioneered by the Baker lab, will accelerate the enzyme development pipeline and potentially provide access to enzyme functions that are currently unavailable.
The enzymes developed will be
used for the sustainable manufacture of pharmaceuticals, including new therapeutic modalities, alongside other applications such as bioremediation (to help break down environmental pollutants), recycling of plastics, synthesis of flavours and fragrances, and production of bio-based fuels and commodity chemicals from renewable feedstocks.
The idea of designing new enzymes computationally has been around for decades, but the deep learning tools that have been developed recently offer a sufficiently high degree of accuracy, predictability of output and speed to make the researchers optimistic that the reliable design of efficient enzymes is within reach. These outcomes are likely to have wide-ranging impacts across the chemical industry, especially in the pharmaceutical sector for which time to market is extremely important.

Similarly, the process is much less computationally resource heavy than earlier methods of computational protein design making it less costly and time consuming.
Lovelock says: ‘’Although the activity of enzymes generated by computational methods may initially be lower than with natural enzymes, we can take advantage of the data generated to iteratively refine our design protocols, leading to improved success rates and higher catalytic efficiencies straight from the computer.
Experimental engineering will remain an important aspect of the process in the short-medium term, but over time we hope to reduce the need for extensive experimental engineering campaigns.’’
The enzymes developed can be used for the sustainable manufacture of pharmaceuticals, including new therapeutic modalities
As stated, the move from highthroughput experimental engineering of enzymes to a computational design process based on deep learning will save time and money. However, there are other key benefits according to Green. He explains that the findings will be shared widely via academic journals allowing more people in the field to carry out their own enzyme design campaigns. Over time the methods are likely to become more user friendly allowing non-specialists to work in the field and bring in new ideas. Green also expects that this will allow scientists like himself to devote their time to other aspects of their work.
Designing enzymes from scratch could also lead to an increase in the number of reactions that are accessible to the field of biocatalysis. “Designing enzymes computationally will allow access to new types of chemistry that we wouldn’t be able to access otherwise,” Lovelock says. “In this way we will maximize the impact that biocatalysis can have on the development of a greener and more sustainable chemical industry.”



Rory O’Keefe from Europlaz provides insight into global trends and innovations in this fast-moving market



As a specialist in medical device manufacturing, Europlaz is often at the front end of the latest product developments to hit the UK and global diagnostics marketplace. Commercial director at the company, Rory O’Keefe, believes the following changes in the point-of-care sector are destined to transform future clinical pathways.
Research recently published by Nova One Advisor indicates that the global point-of-care (PoC) diagnostics market reached US$44.25bn in 2023. In ten years it is expected to virtually double to US$80.75bn[1]. Much of this growth is being driven by rapid technological advances in non-invasive products, self-testing and digitally connected devices, coupled with an aging global population and the rising prevalence of chronic diseases.
There are some very established practices in this field. For example, PoC tests being done at bedsides, in wards, in community settings or at home. These are often done via portable devices that provide timely clinical decisions.
“PoC is far from new. Pregnancy and glucose over-the-counter self-tests have been around for decades. However, the pandemic demonstrated just how convenient PoC tests were for rapid results and population-scale testing.
“For remote communities where medical infrastructures are limited,

PoC tests are invaluable. We are now seeing the development of digital PoC devices which can provide contextual information on results to patients. This information can then be shared with healthcare professionals for further analysis, without them being physically present,” notes Rory. This reduces the burden on healthcare systems, while simultaneously speeding up the time it takes to get a result and treat conditions appropriately.
This transition from acute intervention to diagnostic technologies centered on wellness and prevention is being replicated across the world.
The three technologies that clinicians anticipate will most improve the efficiency and effectiveness of diagnostics in the next three to five years are Telehealth (83%), AI (75%) and Biosensors (74 %), according to one Deloitte survey.[2] Two-thirds of clinicians believe that these disruptive technologies will mean diagnostics will look ‘a great deal’ or ‘totally’ different in 6-10 years’ time.
Previous deterrents to PoC diagnostics, for instance expensive hardware, are no longer such big barriers to market access.
One company revolutionising the way people access diagnosis and treatment for major diseases, according to O’Keefe is Cambridgebased digital diagnostics platform
PocDoc. The company offers an appbased technology platform combining proprietary microfluidic assay tests, powered by its cloud-based applied AI diagnostics (HUESnap).
PocDoc’s Healthy Heart Check has been rolled out across the UK from Edinburgh down to Cornwall via pharmacies, the NHS and other healthcare providers. Healthy Heart Check screenings have also been run in underserved UK healthcare communities and at high footfall locations, including football stadiums.
PocDoc’s technology provides quantitative, multi-marker, clinical grade diagnostics using a bio-sensing approach that can be conducted across multiple settings, including at home. The company holds multiple patents, with a number pending.
PocDoc’s product development director Lucia Curson says “compared with conventional lateral flow tests, the PocDoc technology stack represents a huge leap forward in the field. It combines microfluidics, biochemistry, engineering, applied AI and integration with the healthcare system’s digital infrastructure, all through the same product experience.”
[1] https://www.novaoneadvisor.com/ report/point-of-care-diagnostics-market [2] https://www2.deloitte.com/uk/en/ pages/life-sciences-and-healthcare/ articles/future-of-diagnostics.html


Fuel therapeutic innovation with TriLink’s industry-leading nucleic acid products, available in Europe now through VWR




Through a new partnership with VWR, the delivery channel of Avantor, TriLink Biotechnologies catalog of nucleic acid technologies and capping reagents are now more accessible to customers in Europe. Explore a comprehensive series of products, including:
CleanCap® cap analogs – Achieve over 95% capping efficiency and high protein expression using a streamlined manufacturing process
Catalog mRNAs – Choose from three unique designs over 10 different genes, all capped with a CleanCap® cap analog
IVT enzymes – Discover IVT enzymes, including CleanScribe™ RNA Polymerase for up to 85% dsRNA reduction without compromising yield, quality, or purity

Modified and unmodified NTPs – Over 150 modified and unmodified nucleotides offered as catalog and custom options
See how TriLink’s cutting-edge offerings can advance your work, from discovery-stage research to commercial manufacturing.






A partnership between leading technology companies promises to provide a next generation multimodal imaging platform

The HT-X1 Plus offers high resolution 3D imaging and stability







Manufacturer of highend microscopy solutions CrestOptics has partnered with a leader in holotomography (HT) technology Tomocube to develop a next-generation multimodal imaging platform, the HT-X1 Plus.
By combining the expertise of both companies, this platform integrates CrestOptics’ spinning disk confocal technology with Tomocube’s latest HT innovation allowing users to leverage high-resolution, 3D holotomographic imaging alongside fluorescence-based detection. This enables advanced and precise label-free biophysical imaging, according to a spokesperson for CrestOptics. The technique uses physics-based methods to study biological systems and the interactions between cells and molecules. It’s used to provide insight into living matter,
which can help with disease treatment. It is therefore a powerful tool in life scientific research.
The HT-X1 Plus offers high-resolution, 3D imaging and stability, according to CrestOptics. The platform is compatible with various imaging plates and powered by the TomoAnalysis software, which provides researchers with tools for detailed quantitative analysis and imaging across a wide range of applications.
The product provides imaging of multi-layered specimens with improved illumination optics and advanced image reconstruction algorithms. It uses a high-spec camera featuring a 4x larger field of view and











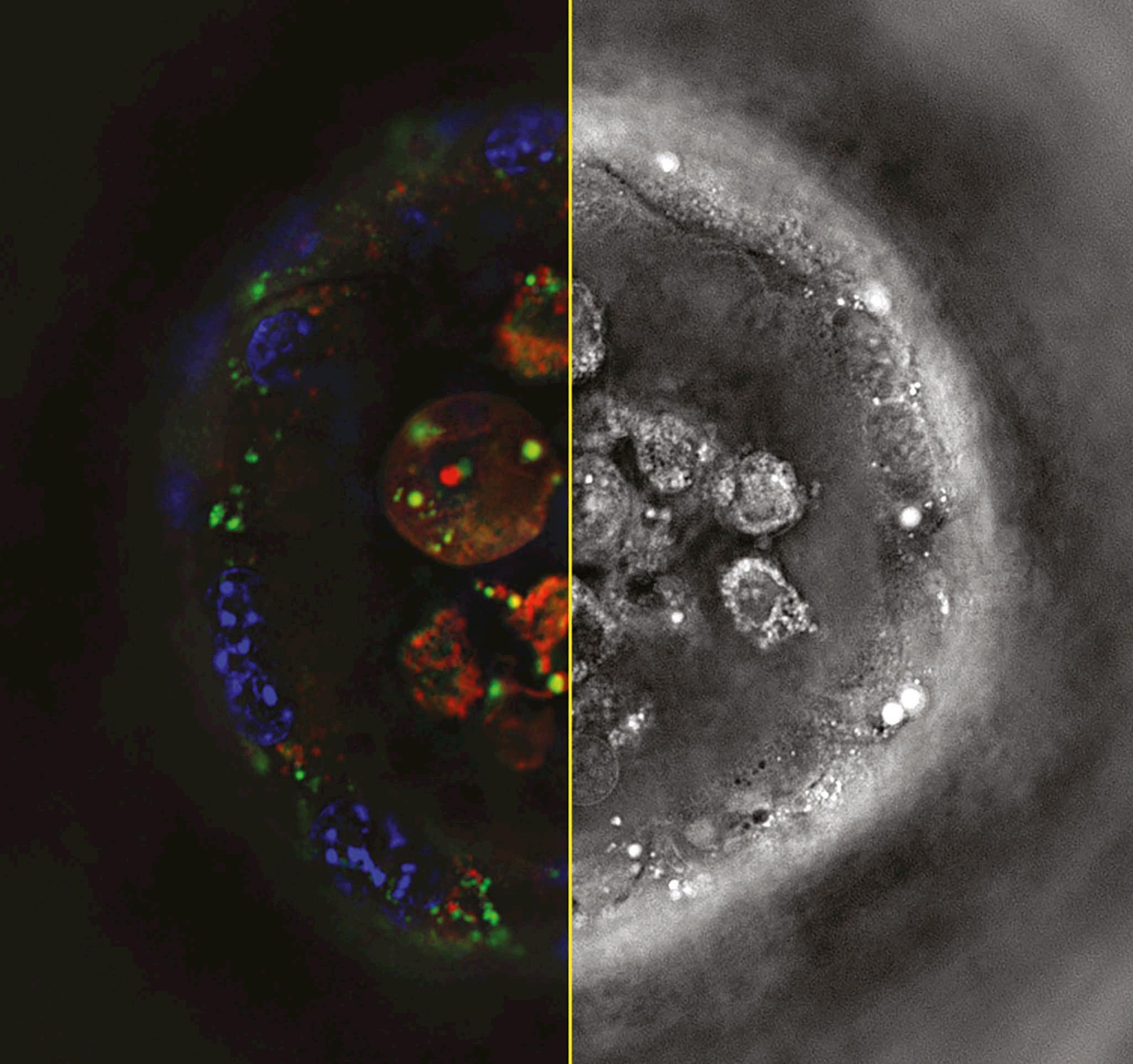
significantly reduced acquisition time than comparable products. It has been designed for use with highthroughput phenotypic screening of cells and organoids. The correlative imaging capabilities, incorporating an sCMOS-based fluorescence module, enable integration of molecular studies with single-cellresolution 3D images.
The product extends the reach of holotomography to a broad array of specimens, including dense organoids, tissue sections, and fastmoving microorganisms. The product enables acquisition of label-free, high-resolution 3D holotomography data and highly-sensitive, precise 3D fluorescence data.
Crest Optics’ product is the first commercially available spinning disc confocal holotomographic instrument enabling advanced 3D imaging with fluorescent-based detection according to the company.
CrestOptics is a specialist in spinning disc confocal technology (SDCM). This solution represents an alternative to laser scanning confocal technology (LSCM) and typically affords more detailed images. Rather than a single pinhole, a SDCM has hundreds of pinholes arranged in spirals on an opaque disk which rotates at high speeds. When spun, the pinholes scan across the sample

in rows, building up an image. The product will help researchers routinely perform challenging live-imaging experiments for extended periods of time. The multi-beam spinning method offers not only high-speed imaging but significantly reduced photo bleaching and phototoxicity. This gentle illumination combined with advanced optical sectioning makes the spinning disc confocal tool a standard for 3D live cell imaging, according to the company.

The product also uses Tomocube’s holotomographic imaging solution involving the illumination of a live cell with low power visible light from multiple illumination angles, this is followed by measurement of the phase delay of the transmitted light. The technique uses the measured refractive index as imaging contrast, allowing visualisation of 3D living cells and tissues without labelling or staining. With its high spatial resolution capable of capturing subcellular organelles, users have increasingly sought confocal fluorescence capabilities to design multiplex imaging workflows.
By combining these two non-invasive imaging modalities, the HT-X1 Plus affords more complete insights into molecular distribution within samples. Additionally, the integrated platform provides precision alignment of HT and fluorescence-based imaging datasets, allowing researchers to easily study molecular and phenotypic data in tandem and generate more powerful insights across biophysical imaging applications.
Dr Alessandra Scarpellini, chief commercial officer, CrestOptics, says “The integrated platform offers more complete insights into the molecular and phenotypic data from 3D cells and tissues, without requiring invasive methods, labelling or staining.”
Dr Sumin Lee, VP of customer development at Tomocube, said: “Collaborating with CrestOptics marks a major milestone in our commitment to meeting the needs of our customers. We’ve seen a growing demand for a solution that combines the label-free imaging of holotomography with the precision of fluorescence-based microscopy. The HT-X1 Plus provides unprecedented resolution and sensitivity for 3D biophysical imaging applications.”
Restek’s Shun-Hsin Liang (PhD) provides a more comprehensive understanding of how ultra-short chain PFAS can be integrated into biomonitoring methods

While concerns about adverse human health effects from long-chain perand polyfluoroalkyl substances (PFAS) have led to increased biomonitoring, less is known about the potential toxicity of ultrashort-chain PFAS, which are ubiquitous and can occur at very high levels. Since both human plasma and serum are widely used for biomonitoring longer-chain PFAS, developing methods that include shorter chain PFAS are critical to gaining a more comprehensive understanding of PFAS exposure and human health. The workflow presented here was developed for the simultaneous analysis of C1 to C10 perfluoroalkyl carboxylic and sulfonic acids, along with four alternative PFAS, in human plasma and serum.
PFAS-free human plasma and serum were not found during screening tests, so charcoal-stripped fetal bovine serum (FBS) was used as the blank matrix for method verification because it did not contain any of
the target analytes, except TFA. To confirm the method’s suitability for real-world samples, NIST SRM 1950 (human plasma) and NIST SRM 1957 (human serum), which contain known concentrations of short-chain and long-chain PFAS, were also tested. Both SRMs contained PFOA, PFNA, PFDA, PFHxS, and PFOS; and NIST SRM 1957 also contained PFHpA. Calibration standards were prepared in reverse osmosis (RO) purified water with 1x phosphate-buffered saline (PBS) added to better match the sample matrices. RO water (generated at Restek) was used to prepare the standards and mobile phases because our testing revealed that it was devoid of PFAS contamination, except for barely detectable TFA.
To assess accuracy and precision, FBS was fortified at 0.4, 2, 10, and 30 ppb with non-labeled PFAS and isotopically labeled 13C-TFA, which served as a surrogate for the determination of TFA recovery. The fortified FBS samples were mixed with a quantitative internal standard (QIS) working solution containing
five isotopically labeled PFAS and an extracted internal standard (EIS) working solution. A single-step protein precipitation was performed in clean polypropylene HPLC vials to minimise background PFAS contamination. The NIST SRMs were prepared using the same procedure.
Gradient LC-MS/MS analysis was performed using an Ultra IBD analytical column (3 µm; 100 mm x 2.1 mm; cat.# 9175312) because its polarembedded stationary phase provides strong retention of polar compounds, even in the presence of the various salts, electrolytes, and buffers that are inherently found in plasma and serum. A PFAS delay column (cat.# 27854) was installed between the pump mixer and the injector to prevent coelution of any instrument-related PFAS with target analytes in the sample. Complete sample preparation and analytical method details, as well as additional discussion, are available in the full application note, which can be accessed at www.restek.com by entering ‘CFAN4273-UNV’ in the Resources Hub search.
Figure 1: Analysis of a 10 ppb PFAS Standard
All analytes exhibited recovery values within the range of 82.3 – 115% across the three fortification levels
A ADONA 9Cl-PF3ONS 11Cl-PF3OUdS
TFA
TFMS
PFPrA (C2) PFEtS (C4) PFBA (C3) PFPrS (C5) PFPe A (C4) PFBS (C5) PFPe S (C6) PFHxA (C6) PFHx S (C7) PFHp S (C7) PFHpA (C8) PFOS (C8) PFOA (C9) PFNS (C10) PFDS (C9) PFNA (C10) PFDA
RESULTS AND DISCUSSION
As shown in Figure 1, strong retention and good separation of early eluting, ultrashort-chain PFAS were obtained under reversed-phase conditions using the polar embedded Ultra IBD column. More important, the extensive retention of the ultrashort-chain PFAS, especially for the first-eluted analyte (TFA), led to reduced matrix interferences.
Table I shows the specific linear range for each analyte, and all analytes had acceptable linearities (r2 >0.995) and deviations (<20%). Accuracy and precision for most analytes were assessed at 0.4, 2, and 10 ppb. However, HFPO-DA and TFA were evaluated at 2, 10, and 30 ppb because 0.4 ppb was below their linear range. All analytes exhibited recovery values within the range of 82.3–115%
across the three fortification levels. Satisfactory method precision was demonstrated with %RSD values of 0.965–11.3%. The LOQ was set as the lowest calibration concentration for each analyte, and the LOD was defined by a peak signal-to-noise ratio of 3:1 in fortified FBS.
Six preparations of each SRM were analysed and all measured EIS concentrations fell within 20% of the
Table I: Linearity, LOD, accuracy, and precision for fortified FBS samples (n = 9, interday) *for non-labelled TFA
nominal value. The average measured concentrations of most PFAS in the SRMs closely matched the reference concentrations and, when calculated as relative values, were within 20%. While the measured relative concentration of PFDA in NIST SRM 1957 had a slightly higher deviation (26%), it remained within the SRM’s reference concentration deviation range. These results demonstrated that the method is suitable for accurate measurement of PFAS in both human plasma and serum samples.
The simple, reliable workflow established here incorporates ultrashort-chain PFAS into a method
It uses a polarembedded Ultra IBD column to increase retention of these small polar analytes, and the results demonstrate that the method is accurate and precise
that also includes longer-chain PFAS that are commonly monitored in human plasma and serum. It uses a polar-embedded Ultra IBD column to increase chromatographic retention of these small polar analytes, and the results demonstrate that the method is accurate and precise. Most important, it can be a valuable tool for studying human exposure to emergent ultrashort-chain PFAS.
Shun-Hsin Liang PhD is a senior principal scientist for Restek Corporation


Siân
Group outlines some of the key cyber security threats and solutions faced by the biotech industry

The biotech industry is on the cusp of many remarkable innovations, driven by advanced robotics, sophisticated data analytics, and cutting-edge artificial intelligence (AI). From fully robotic genome foundries, like the one I recently visited through EPSRC at the University of Edinburgh, to the myriads of interconnected devices collecting and processing samples, the sector is rapidly evolving.
However, as we embrace these technological advancements, it is essential that labs implement robust cybersecurity measures. Protecting sensitive data, ranging from intellectual property (IP) to personal health information (PHI), is critical to maintaining the integrity and trust in the biotech industry.
Biotechnology today relies heavily on advanced robotics and computing power. These technologies facilitate the high-throughput collection and processing of biological samples and enable intensive data analysis, which is crucial for running complex models and deriving detailed results. For instance, the Edinburgh Genome Foundry exemplifies how robotic

systems can revolutionise genomics by automating the synthesis and assembly of DNA sequences. Such advancements not only accelerate research but also reduce human error, enhancing the reliability of experimental outcomes.
However, this heavy reliance on technology also introduces significant cyber-security challenges. The data processed in these labs is highly sensitive. It includes proprietary research data that represents significant IP, as well as personal data from individuals who provide biological samples. The potential for data breaches in this context is high, and the consequences can be severe, ranging from the theft of valuable IP to the exposure of personal health information.
In the biotech industry, data is gold. Research data underpins the development of new therapies, drugs, and biotechnologies. This data, often generated through years of meticulous research, holds immense IP value. Cyberattacks targeting this data can lead to significant financial losses, damage to reputation, and setbacks in scientific progress.
Equally important is the privacy of individuals who contribute biological samples for research. These samples
can reveal a wealth of PHI. In the wrong hands, such data can be misused for identity theft, insurance fraud, or other malicious activities.
The biotech industry must, therefore, ensure that data protection is a top priority, safeguarding both the interests of researchers and the privacy of individuals.
The cyber security landscape is continuously evolving, as are the threats – these can take the form of:
• Targeted attacks: Hackers may specifically target biotech companies to steal valuable IP or disrupt operations.
• Insider threats: Employees with access to sensitive data might intentionally or unintentionally compromise security.
• Supply-chain vulnerabilities: The integration of multiple technologies and third-party services can introduce weaknesses that attackers can exploit.
• Advanced persistent threats (APTs): Prolonged and targeted cyberattacks that aim to infiltrate and remain within systems to steal data over an extended period, without detection.


AI and machine learning are not only transforming biotech research but also revolutionising cyber security
To protect the sensitive data processed by modern robotics, big data analytics, and AI systems, biotech companies must implement multi-layered security measures:
• End-to-end encryption: Ensuring that data is encrypted both in transit and at rest can significantly reduce the risk of data breaches.
• Advanced authentication mechanisms: Multi-factor authentication (MFA) and biometric verification can prevent unauthorised access to sensitive data and systems.
• Regular security audits and penetration testing: These practices help identify and address vulnerabilities before they can be exploited by attackers.
• Employee training and awareness: Educating staff about cyber security best practices and the latest risks, to minimise the risk of them occurring or of insider threats, where deliberate attacks originate from within an organisation.
• Secure software development lifecycle (SDLC): Incorporating security at every stage of software development, ensuring applications are robust against attacks.
AI and machine learning are not only transforming biotech research but also revolutionising cyber security. These technologies can enhance threat detection and response by analysing vast amounts of data to identify anomalies and potential threats. For instance, AI-powered systems can detect unusual patterns of behaviour that may indicate a cyberattack, enabling quicker and better responses. However, the integration of AI in cyber security also presents challenges. AI systems themselves can be targeted by attackers, who may attempt to manipulate algorithms or feed them malicious data.
Addressing cyber security in the biotech industry requires collaborative
effort. Stakeholders, including researchers, technology providers, and policymakers, must work together to develop and implement industrywide standards and best practices. Organisations such as the National Cyber Security Centre (NCSC) and industry bodies can play a pivotal role in fostering collaboration and providing guidance on emerging threats and effective countermeasures.
The biotech industry stands at the forefront of scientific innovation, however, the increasing complexity and value of the data processed by these technologies makes the sector a prime target for cyberattacks. By prioritising cyber security, adopting advanced security measures, and fostering collaborative efforts, the biotech industry can safeguard its valuable data and maintain the trust of researchers and the public.

Creative Biolabs
A leading global supplier, offers highquality biological products like recombinant antibodies, membrane proteins, and cell lines, and custom services in therapeutics discovery to support your research needs.
T 1-631-416-1478
E info@creative-biolabs.com
W www.creative-biolabs.com

L.B.Bohle
Headquartered in Germany, a leading system supplier for the pharmaceutical industry. We provide sustainable machines and processes for efficient batch and continuous manufacturing, ensuring high-quality solutions for demanding production needs.
T +492524 – 93 23 0
E info@lbbohle.de
W www.lbbohle.com

Gerteis
A Swiss manufacturer of advanced highquality pharmaceutical roller compactors. Our understanding of the dry granulation process combined with patented roller compaction systems make Gerteis® the technology leaders in the field of dry granulation.
T +41 (0)55 222 55 22
E sales@gerteis.com
W www.gerteis.com

As part of PHC Group, we provide high-quality laboratory equipment for biomedical and diagnostic applications, ensuring precision, reliability and performance. We offer expert service, certification and innovative solutions for life science, healthcare and industrial sectors.
T +31 76 5433833
E marketing@eu.phchd.com
W www.phchd.com/eu/phceu

Hamamatsu Photon
Manufacturer of optoelectronic components and systems. Including sensors and systems for spectroscopy (inc. ultrafast), scientificgrade cameras, beam monitoring systems, photon-counting detectors and systems, photomultipliers, photodiodes and IR detectors.
T (49)8152-375-0
E info@hamamatsu.eu
W www.hamamatsu.com

A global leader in manufacturing affordable, high-quality reagents, including recombinant proteins, antibodies and cDNA clones in-house.
T +49 (0)6196 9678656
E marketing@sinobiological.com
W www.sinobiological.com



+44 (0)207 062 2566







This third and final part of the article series about understanding the nip angle by the thin layer model deals with the correlation of nip angle and powder draw-in
In Part 1[1] the model and the possibility to estimate nip angles easily is described. It also outlines that a stronger densification results inevitably in larger nip angles. Part 2[2] focuses on the interaction between nip angle and gap, and that a larger gap mandatory requires a larger specific roll force to achieve the same granule properties.
In addition to its influence on densification, the nip angle also plays an important role for the draw-in of powder by the rolls. The main driver for draw-in is the frictional force between powder and roll surface. This friction is responsible for conveying the powder towards the gap. Figure 1 visualises schematically the forces present during the roller compaction process based on the thin layer model for one roll.
Looking at the forces within the gliding area (Figure 1a): a loosely packed powder layer with a certain density applies a small force Fi onto the roll. By using the parallelogram of forces, Fi can be split into two components, one normal to the roll surface FN and the other one tangential to the roll surface Ft In principle, this tangential force counteracts the draw-in which can be expressed by the frictional force Ffr. If the tangential force Ft is larger than the frictional force Ffr, the powder
Gerteis
Mini-Pactor- an advanced R&D roller compactor

is not conveyed by the rolls. This is the case within the gliding area, in which the rolls glide under the powder without any transportation. In the right part of Figure 1 the same powder layer is shown at angle α’ located in the nip area. The layer still has the same density and therefore, the same small force Fi acts onto the roll as well as the same frictional force Ffr. But dividing the forces in its normal and tangential component result is a decrease of the tangential component Ft. In general, the smaller
the angle α, the smaller the tangential component becomes. As soon as Ft is smaller than the frictional force Ffr, the powder is drawn in by the rolls. Once the powder is drawn in, the powder layer is densified, and all forces change. The force Fi applied onto the roll increases and therefore also its tangential component. Even, if draw-in conditions for a densified layer would not be fulfilled anymore, it will still move towards the gap, since the layers on top will enforce its downward movement.

The maximum possible angle α at which powder will be drawn in depends on the extent of the frictional force between powder and roll surface
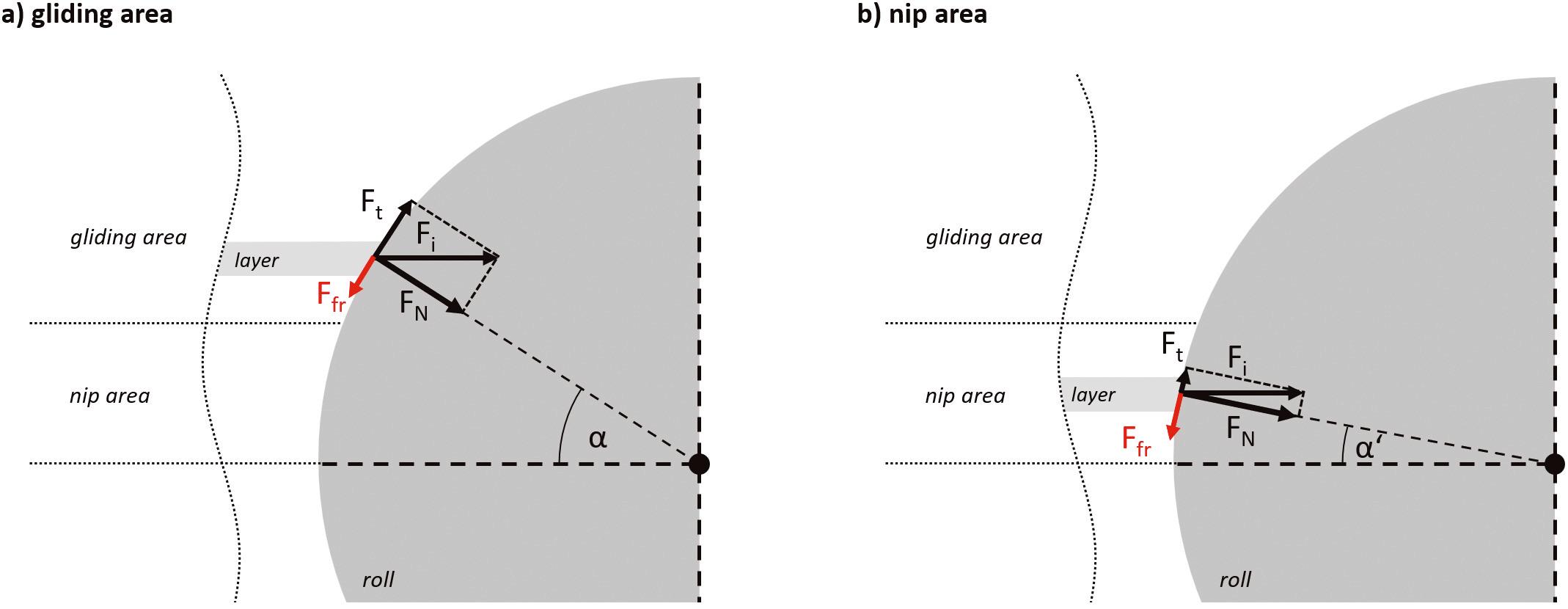
The maximum possible angle α at which powder will be drawn in depends on the extent of the frictional force between powder and roll surface. Adding lubricants like magnesium stearate is often accompanied by draw-in problems because they reduce the friction and in consequence, the maximum possible nip angle. This has also been found experimentally [3]. This means, to prevent draw-in problems, lubricants should be avoided or at least minimised in roller compaction formulations. Another possibility for improving draw-in is to use rollers with rough surfaces or indentations to change the powder-to-metal friction to the larger powder-to-powder friction. More details can be found here [4]
It is possible to select roller compactor settings that would require larger nip angles than is actually feasible. But if the theoretically required nip angle cannot be achieved, the applied specific roll force will cause a smaller gap than the set one, because the forces within the roller compactor must be balanced. When running with gap control, the feed system will try to provide more material into the area between the rolls to open the gap. But the rolls are already conveying the maximum possible amount of powder at the maximum possible nip angle. Then, the powder will be accumulated in the gliding area. This will cause an
increase of the tamp auger torque and finally, an overload of the tamp auger drive. To counteract draw-in problems, smaller gaps and lower specific roll forces can be used. But be aware that changing gap or specific roll force result in different ribbon densities.
By visualising the forces acting on the rolls, draw-in and its associated problems can be explained. Larger gaps and higher specific roll forces increase the risk of draw-in problems, because the necessary nip angle might exceed the maximum possible nip angle. The limitation of the nip angle depends on the frictional forces between the powder and the roll surface. Increasing these frictional forces, by, for example, choosing different roll surfaces, helps to avoid draw-in problems. Reducing them, for example, by lubricating the powder to avoid sticking, may enforce draw-in problems considerably.
Authors:
- Barbara Fretter, Managing Partner, Solids Development Consult GmbH
- Dr. Robert Frank Lammens, Managing Partner, Solids Development Consult GmbH
- Michael Schupp, Head of Process Engineering, Gerteis Maschinen + Processengineering AG
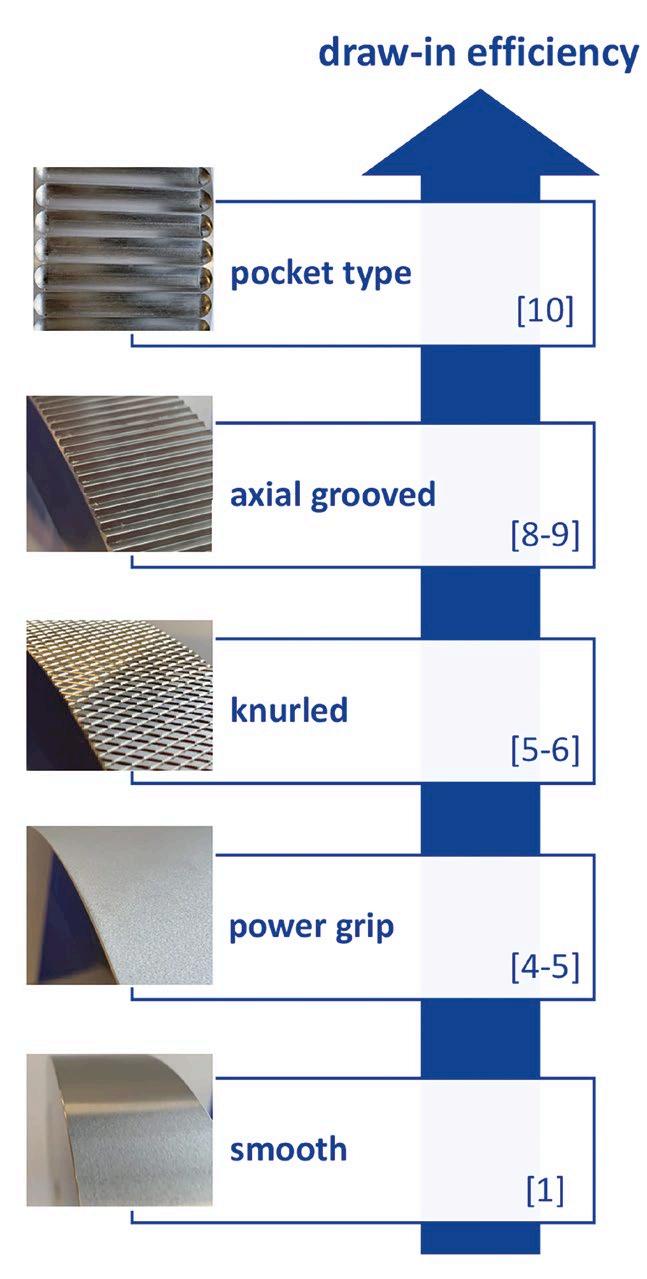
Press roller draw-in efficiency
Literature:
[1] Barbara Fretter, Michael Schupp, Understanding the Nip Angle, Eurolab (Dec 2023), https://content.yudu.com/ web/15ex3/0A2nilh/EurolabDec2023/html/ index.html?page=52&origin=reader
[2] Barbara Fretter, Michael Schupp, Understanding the Nip Angle, Eurolab (June 2024), https://content.yudu.com/ web/15ex3/0A2nilh/EurolabJune2024/html/ index.html?page=54&origin=reader
[3] A.M. Miguélez-Morán, C.-Y. Wu, J. P. K. Seville, The effect of lubrication on density distributions of roller compacted ribbons, Int. J. Pharm (2008) 362(1-2):52-9
[4] Barbara Fretter, Hartmut vom Bey, Press Roller Properties, Eurolab (June 2022), https:// content.yudu.com/web/15ex3/0A2nilh/ EurolabJune2022/html/index. html?page=50&origin=reader
Here tableting tool specialist
Natoli takes a deep dive into the role of tooling specifications in tablet manufacturing
Quality tablet compression tooling is critical for tablet quality and the optimisation of the tableting process. If substandard or tooling out of specification is used you can expect issues such as poor tablet quality, low yields, slower press speed and excessive cam wear to name only a few. Troubleshooting quality and performance involves the interplay between process parameters, including formulation characteristics, press condition, setup, operation, tablet design and tooling.
In many cases the value of compression tooling is not respected for what it is. Tooling is responsible for the last step of the Oral Solid Dosage (OSD) process, a compressing formulation that has taken years to develop. The inception of a new product, as well as pre-formulation, research and development, product development, granulating and mixing, all the way to clinical trials and scaleup requires a monumental investment. Once all the boxes are ticked and the product is approved, it is finally on its way to production. In production, tablet tooling is the last step in this daunting process of compressing powder into a usable form. When an issue arises and hinders production,

many tablet manufacturers reach out to the tooling supplier first since tooling optimisation can be an easy fix. Suggesting an alternate steel type, a specific tooling option, or even the addition of a punch coating is a common scenario.
Broader support from highquality tooling suppliers includes assessments of process parameters. Examples include assessing the formula, excipient selection, powder processing, moisture, particle size, and segregation. Segregation is often caused by powder transfer systems within the process, especially the tablet press feeder as well as the improper selection of a fill cam which can over work the excessive powder as it is recirculated. Excessive powder reintroduced into the feeder can result in packing or overfilling the feeder with powder, this will damage the feeder paddles and drive gears.
SUPAC guidelines describe and offer deviation when issues arise without regulatory resubmittal. However, when the formula and critical process attributes have already been approved, overcoming challenges such as poor flowability, sticking, and
capping may likely be compensated for with adjustments to the tooling specifications. To effectively mitigate tableting issues it is essential to have a good understanding of the compression cycle, tooling and tooling wear.
Three key tooling specifications to consider are the working length, cup depth, and land width. Variation in lower punch working length within a set directly affects tablet weight. Cup depth affects tool strength, friability, tablet density uniformity, and propensity for capping. Land is an incredibly important feature that impacts tool life and strength.
The two most common tool configurations are the ‘B’ and ‘D’ type tool. There are established and published standards related to the ‘B’ and ‘D’ type tools, and these are available in two formats, the American (TSM) and the European (EU). The TSM Standard (Tablet Specification Manual) is published by the American Pharmacist Association and is in its 7th edition with the enhanced 8th edition under review and available in the first quarter of 2025. The TSM Manual is the most complete and comprehensive manual related to tablet compression tools. The EU standard is published by the International Organisation for
Standardisation and is referred to as the ISO 18084. ISO 18084 covers the standard ‘B’ and ‘D’ tool configurations, but it is not as complete or comprehensive as the TSM.
The original standard was the IPT (now known as the TSM) first developed by multinational pharmaceutical companies in the 1960s. The purpose was to establish tool drawings that could be shared with local machine shops to ensure
that the tooling would properly fit and perform in the most common tablet presses, assuring consistency with tablet quality attributes such as tablet hardness, weight and thickness.
The engineered tooling drawings available with both standards can be misunderstood as wear limits to identify when the tools are unusable.

The ISO 18084 covers the standard B and D tool configurations
This is the furthest from the truth whereas the drawings are created for the tool manufacturer as a guide to manufacture and for tablet press manufacturers looking for help with cam configuration, turret guide and die pocket dimensions. The tool drawings published by these standards should be used for an incoming quality inspection process to ensure the tools were manufactured to the prescribed standard and will perform as intended.
Once the new tools pass acceptance inspection, they should be updated to an in-process status. When the tools have been reclassified then it is up to the tool user to establish a new set of tolerances that are an acceptable deviation related to the product or application. The tooling supplier will be able to help establish in-process tool deviations to assure the tool user that their tooling will continue to produce tablets that meet quality parameters. In most cases this will greatly reduce the annual tooling spend.
Tablet manufacturing involves the interplay of many factors. The role of the tooling supplier is crucial for the optimisation as well as troubleshooting the user’s tableting process.



Chromacity’s new solution is based on a single light source




A new solution combining high brightness technology offers advances in testing hydrogen for contaminants
Chromacity has developed a next-generation optical chromatography solution for the detection of contaminants in renewable hydrogen.
While renewable hydrogen is widely acknowledged to play a growing role in decarbonising the economy, there are still challenges around controlling its purity. With supply from diverse sources including green, blue and regasified hydrogen from storage media, users need confidence that the gas they use is of sufficient quality that it will not damage key components such as fuel cells or infrastructure.
Working in partnership with the Herriot Watt University and Frauhofer UK, the new, yet to be named, solution combines high brightness, coherent Optical Parametric Oscillator (OPO) laser technology with advanced FTIR spectroscopy techniques. This has been shown to offer advantages over current
technologies. The ISO 14687:2019 standard which defines thresholds for a wide range of contaminants in hydrogen for fuel cells, is being used to benchmark this development.
Julian Hayes, CEO of Chromacity says: “Existing optical solutions for determining the purity of renewable hydrogen either compromise on spectral resolution and detection sensitivity or are overly complex making them expensive, thereby limiting deployment. Likewise, the implementation of sensitive gas chromatography techniques is limited because the instrumentation is costly and bulky.”
He adds: “Based on a single light source, our solution removes the complexities of multi-source optical techniques and so lowers the cost of ownership. The broad, tuneable bandwidth of the OPO laser allows many contaminants to be detected,
including broad or complex chemical signatures. Our instrument is designed to be used in-line and has been shown to monitor the five key contaminants in the renewable hydrogen production process (as detailed in ISO 14687) in real time.”
Mr Hayes concludes: “Having been successfully tested in the lab on representative gas samples, the next stage of developing the system is to enable users to use live real-time data within their production process.”
*This development received investment from Scottish Government Emerging Energy Technologies Fund (EETF) –see https://www.gov.scot/publications/ emerging-energy-technologiesfund-hydrogen-innovation-schemesuccessful-projects/

Every year, the world becomes increasingly reliant on batteries. Ongoing advancements in battery technology are critical to the progress and transformation of these key industries. Here, Dr. Zhao Liu, senior market development manager at the company, focuses on clean energy application materials and explores the current trends in battery technology and how various analytical characterisation techniques play a crucial role.
A battery’s materials are critical to its performance, as they directly affect factors such as energy density, efficiency, safety and cycle life. For example, the choice of anode and cathode materials determines how much energy a battery can store, how quickly it charges and how long it lasts before degrading. The thermal and chemical stability of materials also influences safety, making material selection vital for developing efficient, durable and safe batteries.
In-depth materials analysis, such as Electron Microscopy (EM), is essential to understanding a material’s microstructure and how it behaves
Dr Zhao from Thermo Fisher Scientific explores the current trends in battery technology and how electron microscopy can ensure they are efficient and sustainable
during electrochemical cycling. This helps researchers detect failures such as dendrite formation or cracking that can lead to battery failure. Microscopy also allows for the optimisation of the interfaces between battery components, improving ion transport and performance. Ultimately, by analysing materials at the atomic level, scientists can develop batteries that are safer, more efficient and longer-lasting.
Materials characterisation and analysis play a vital role in every phase of battery development, from the initial research and design of new materials to manufacturing, quality control, failure
analysis and, ultimately, recycling. Emerging technologies, such as lithium-metal and solid-state batteries, offer substantial potential for transforming the energy storage landscape. These innovations promise higher energy density, extended cycle life and enhanced safety over traditional lithium-ion batteries. However, to fully realise these advantages, significant research is required to overcome persistent technical challenges. For instance, lithium-metal batteries, while capable of achieving high energy densities, face issues like dendrite formation - a growth of needle-like lithium structures that can pierce the separator, potentially leading to short circuits and safety hazards. Similarly, solid-state batteries offer enhanced safety by replacing flammable liquid electrolytes with solid ones, but they introduce complex interfacial challenges. Effective performance demands precise alignment between the solid electrolyte and electrode materials, and controlling degradation at this interface is essential to prevent premature failure.
Characterising these advanced materials is challenging owing to the highly reactive nature of key components, such as lithium metal anodes, solid electrolytes and the










solid electrolyte interface. These materials can degrade or alter upon exposure to air and moisture, making it difficult to preserve their native state for accurate analysis.
Solutions such as the Thermo Scientific Inert Gas Sample Transfer (IGST) workflow can help with these issues. This workflow combines multiple electron microscopy



techniques in a controlled environment, enabling researchers to examine battery materials in their native state down to the nanometre scale. By transferring samples in an inert gas atmosphere, IGST effectively preserves the integrity of reactive materials, allowing for more reliable and repeatable characterisations. These detailed analyses help researchers gain insights into degradation mechanisms, improving material design, and ultimately accelerating the commercialisation of next-generation battery technologies.
The analytical solution overall is central to ensuring compliance with new regulations and supporting the sustainability of the market. From January 2026, EV and industrial batteries in the EU will have to comply with the EU Battery Regulation Amendment. Regardless of the battery’s origin, a digital ‘passport’ will be required for it to be listed in the European market. The
regulation aims to help protect the environment, ensure a reduction in the amount of hazardous waste and drive circular growth in the industry. But its arrival lands responsibility directly on the shoulders of the party placing the battery on the market, to ensure that all required data is entered in the digital record and that this information is correct and up to date.
Battery manufacturers must therefore demonstrate that all contaminates and trace elements used across the production value chain align with the specifications and quality standards outlined in the battery passport. Achieving this will require not only sophisticated microscopy, spectroscopy, and spectrometry technology for material verification but also an integrated, comprehensive analysis strategy to authenticate material quality and origin throughout the supply chain.













Filtration, where solid particles in a liquid or gaseous fluid are removed by the use of a filter medium, is key work for many laboratory professionals. It can be that the clarified fluid or the solid particles removed from the fluid may be the desired product.
The Filtech trade show, a show dedicated to innovations in filtration and separation of all types of media, took place on the 12–14 November 2024 in Cologne, Germany. The annual show is one of the biggest of its type and aimed at filtration engineers from a variety of sectors.
Filtech 2024 provided an opportunity for professionals to expand their knowledge, discover new trends and gain valuable insights into the future of filtration and separation technology.
The show bridged scientific research with industrial applications and
covered a broad range of topics. These included liquid/solid and gas/ particle separation, advancements in filter media and membrane processes, as well as technologies for emission control and product recovery. Special emphasis was placed on the development of sustainable and energy-efficient filtration solutions.
Exhibitors showcased a wide variety of products and services, including filtration systems, laboratory equipment, and solutions for air and water treatment. The fair attracts representatives from diverse sectors, including chemicals, pharmaceuticals, food and beverages, automotive, aerospace, and water management.
“More than 460 exhibitors attended the show,” according to the organiser. “We received an exceptionally high level of interest from attendees

and exhibitors. The exhibitor space was fully booked, which proves the high demand and the confidence of the companies in the importance of Filtech as a leading industry platform.”
The associated conference, featuring more than 200 presentations, also provided a diverse program of technical presentations, panel discussions and poster sessions.
Keynotes included ‘membrane technology – new developments, challenges, markets and applications’; ‘how filtration and separation impact sustainability’; and ‘from process to operation: digital twins and filtration’.
The shows increased in size on the previous year (2023) which saw 438 exhibitors and 160 speakers.

Learn about the latest dry granulation innovations available only on GERTEIS® roller compactors. Introducing our cutting-edge In-Line Density Measurement Solution, the new Ultra Small Amount Funnel and the Design of Experiments Software Feature!
PAT: In-Line Density Measurement
Real-Time Insights: Say goodbye to off-line testing delays. Our system provides instant density measurements during production, allowing timely adjustments and informed decision-making.

High Precision: Accurate density data is key to robust process control. Whether you’re scaling up or down, our system ensures reliable results.




























Closed-Loop Feedback: Linking density measurements to process parameters enables dynamic adjustments. Stay ahead of variations and maintain product consistency.
Design: Ultra Small Amount Funnel





Ideal for Small Batches: Process materials with as little as 10 grams of product — perfect for laboratory development and pilot projects.
No Residuals: Almost no product remains in the equipment, making it an excellent high yield small amount option.

Software: Design of Experiments (DoE)
Ease of Use: The funnel seamlessly integrates with the compaction unit of the Mini-Pactor, providing the same superior performance you would find in a full scale production machine.


Efficient DoE Implementation: Design of Experiments is the fastest and most cost-efficient way to create effective experiments. Tap into the power of DoE without the long learning curve.
Built-in Process Support: This goes beyond ordinary DoE software. It guides you on how to make optimal experimental choices, skipping impossible settings to save product and time.


Complete Guidance: From parameterization to execution, the software provides step-by-step instruction and support.



Continuous Flow Measurement 10 nl – 80 µl/min
Software compatibility with leading CDS



Efficient degassing at µl/min flow rates
Widest Chemical Compatibility
