Control &









Discover clever ways to






































‘

























- Dr Phil Tucak Veterinarian and AVA Veterinary Business Professional of the Year 2020


... automating routine tasks to spend more quality time with clients is fundamental for veterinary business’











The Vetplus business efficiency program offers a number of time-saving opportunities for your staff, using automation to handle routine bookings and reminders, digital solutions like the new Pet Health Passport app, plus a suite of ready-to-go marketing campaigns where all the hard work has already been done. Do more with less! To discover what Vetplus can offer your practice, scan the QR code or talk to your Boehringer Ingelheim Territory Manager for details.





316 | September 2024
Control & Therapy Series
PUBLISHER
Centre for Veterinary Education
Veterinary Science Conference Centre
Regimental Drive
The University of Sydney NSW 2006 + 61 2 9351 7979
cve.marketing@sydney.edu.au cve.edu.au
Print Post Approval No. 10005007
EDITOR
Lis Churchward elisabeth.churchward@sydney.edu.au
EDITORIAL ASSISTANT
Dr Jo Krockenberger joanne.krockenberger@sydney.edu.au
VETERINARY EDITOR
Dr Richard Malik
DESIGNER
Samin Mirgheshmi
ADVERTISING
Lis Churchward elisabeth.churchward@sydney.edu.au
To integrate your brand with C&T in print and digital and to discuss new business opportunities, please contact:
MARKETING & SALES MANAGER
Ines Borovic ines.borovic@sydney.edu.au
DISCLAIMER
All content made available in the Control & Therapy (including articles and videos) may be used by readers (You or Your) for educational purposes only.
Knowledge and best practice in this field are constantly changing. As new research and experience broadens our knowledge, changes in practice, treatment and drug therapy may become necessary or appropriate. You are advised to check the most current information provided (1) on procedures featured or (2) by the manufacturer of each product to be administered, to verify the recommended dose or formula, the method and duration of administration, and contraindications.
To the extent permitted by law You acknowledge and agree that:
I. Except for any non-excludable obligations, We give no warranty (express or implied) or guarantee that the content is current, or fit for any use whatsoever. All such information, services and materials are provided ‘as is’ and ‘as available’ without warranty of any kind.
II. All conditions, warranties, guarantees, rights, remedies, liabilities or other terms that may be implied or conferred by statute, custom or the general law that impose any liability or obligation on the University (We) in relation to the educational services We provide to You are expressly excluded; and
III. We have no liability to You or anyone else (including in negligence) for any type of loss, however incurred, in connection with Your use or reliance on the content, including (without limitation) loss of profits, loss of revenue, loss of goodwill, loss of customers, loss of or damage to reputation, loss of capital, downtime costs, loss under or in relation to any other contract, loss of data, loss of use of data or any direct, indirect, economic, special or consequential loss, harm, damage, cost or expense (including legal fees).
The C&T forum gives a ‘voice’ to the profession and everyone interested in animal welfare. You don’t have to be a CVE Member to contribute an article—please send your submissions to Dr Jo Krockenberger. joanne.krockenberger@sydney.edu.au
Join In!
The C&T is not a peer-reviewed journal.
We are keen on publishing short, pithy, practical articles (a simple paragraph is fine) that our readers can immediately relate to and utilise. Our editors will assist with English and grammar as required.
I enjoy reading the C&T more than any other veterinary publication.
-Terry King, Veterinary Specialist Services, QLD
The C&T Series thrives due to your generosity.
Vouchers can be used towards membership fees or continuing education courses
Prize: A CVE$500 voucher
Nasal Aspergillus fumigatus Infection in a Dachshund (Sinonasal aspergillosis)
Georgina Milne
Prize: A CVE$100 voucher
Deaths in Lambs Drinking Wastewater From a Winery
Jeremy Rogers
The Story of ‘Ed’ The Horse Who Couldn’t Talk!
Stephen McClintock
Prize: A CVE$300 voucher
Osseointegrated Transcutaneous Amputation Prostheses in Veterinary Medicine
Daniel R James
cve.edu.au

We’re delighted to inform you that—despite the current cost of living crisis—CVE membership increased for the July 2024-June 2025 period to7,868 members. Your financial support means that the CVE can continue to fulfill its mission to provide accessible, unbiased, quality continuing education for the profession.
Director of The Centre for Veterinary Education (Associate Professor/Professor of Practice)
The only constant is change. Over the last 59 years and seven Directors, the role adapted and evolved. Accordingly, the position description for the eighth Director of the CVE has been revised to suit the challenge of the times.
The appointee will manage the CVE's strategic, financial, and operational plans to ensure the ongoing growth and development of the CVE as a world leader in veterinary continuing education in a financially sustainable manner. The role requires a person with strong strategic leadership and collaborative skills. The role is currently being advertised and can be accessed here.
If this sounds like you or someone you know, please read the job advertisement and reach out to us.
bit.ly/cve-director
One of the major ways we reward our loyal membership is exclusive access to very generous Early Bird rates. Our members certainly took advantage of them for the October Paws Under Pressure: An Emergency Medicine Conference which sold out in record time. Co-presenter Dom Barfield’s 10 Tips for Critical Cases WebinarLIVE and other courses are available to members in the Webinar Library.
Exciting times are ahead for the CVE as we celebrate our 60th anniversary as the world’s first and leading veterinary membership organisation next year. As well as the new scholarship, other celebrations are planned so watch this space!
Thank you.
The CVE Team
Dr Christopher Simpson BVSc MANZCVSc
Victoria Veterinary Clinics
Hong
Kong
e. simpson_christo@icloud.com
C&T No. 6030

A cervical and thoracic CT scan (see figure 5 ) was performed to assess for evidence of local invasion or metastatic spread, and none was evident, so Taco was taken to surgery.
Surgery was uneventful and the nodule was grossly completely excised, with histopathology indicating good clear margins.
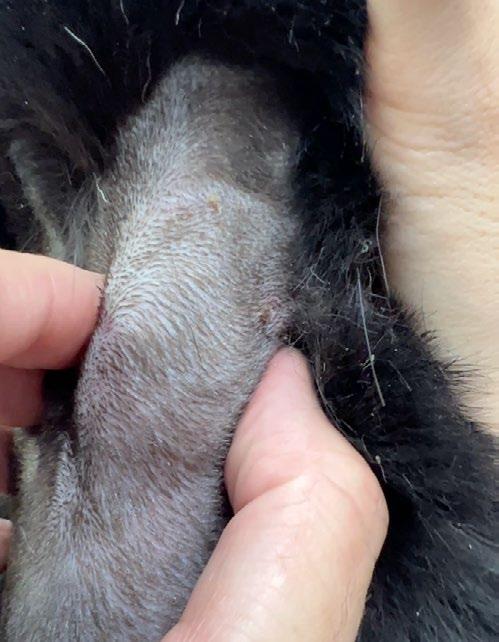
Taco is an 11-year-old male neutered mixed-breed dog who presented to our clinic with a history of weight loss despite a normal appetite and no gastrointestinal signs. Earlier this year he been treated for tick fever (Babesiosis, common in Hong Kong), and we initially suspected that the weight loss was an after-effect of the disease.
When he just kept on losing weight despite being apparently normal in all other respects, we decided to draw blood to recheck that the Babesia was definitely cleared. When we clipped the jugular vein for venepuncture, we discovered a cervical nodule...
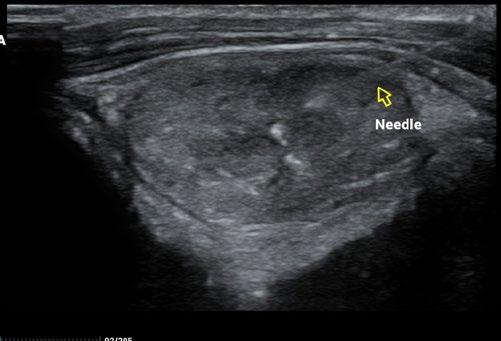
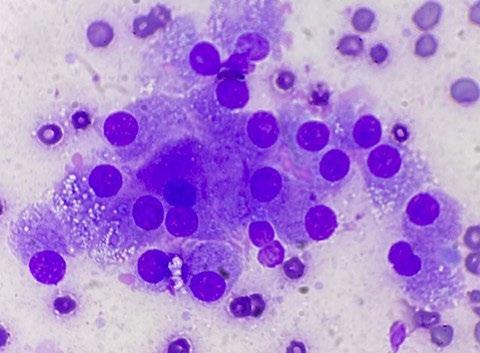
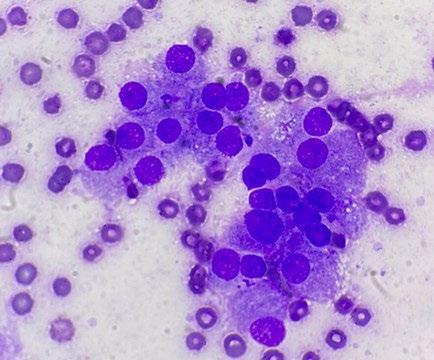
As a result, we ran full bloods, including a total T4, and confirmed hyperthyroidism. A fine needle aspirate of the nodule was consistent with a functional thyroid carcinoma.
Microscopic findings: Epithelial neoplasia, likely of endocrine derivation
Comments:
The tumor consists of a uniform population of fragile, bland, cytologically benign columnar epithelial cells, which are features that are typical for endocrine derivation. Location as described, coupled with the increased total T4 reported (and assuming no thyroid supplementation is being administered to the patient) is supportive of thyroid origin. Interestingly, however, the majority (greater than 90%) of thyroid tumours are nonfunctional. The differential for endocrine tumours in the thyroid region includes: follicular thyroid carcinomas (most common) medullary thyroid carcinoma of C cell (parafollicular origin), and parathyroid tumors (uncommon, often functional).
Thyroid carcinomas are frequently cytologically bland in appearance, but typically behave as malignant tumors. Prognosis is more favourable in those cases in which complete excision is achieved and there is no invasion of surrounding structures. Excisional biopsy is suggested (if possible). Prior to surgery, staging is recommended, including aspiration of any enlarged lymph nodes and chest radiographs.
Careful consideration was given to perioperative management, particularly with regard to the risk of endocrine derangements after removal of a functional nodule.
This patient would be expected to develop HYPOthyroidism after removal of a functional unilateral thyroid nodule. The excessive T3 and T4 released from the nodule over the previous several months is likely

to suppress TSH release from the anterior pituitary via negative feedback. As a result, the opposite thyroid gland would have received no TSH stimulation and atrophied. The parathyroid/Ca++ axis has remained unaffected, as all the parathyroid tissue is functioning normally, so there is no reason for this homeostatic system to be dysregulated.
The expanded thyroid panel ( figure 7 ) confirmed the exact derangements anticipated. Note the TSH result is technically within the normal reference interval, but inappropriately low for a dog with profound hypothyroidism.
We want to supplement enough L-thyroxine to avoid the kidneys flaming out, but not so much that we suppress TSH production by negative feedback. How do we know how much is enough? By repeat testing. The full thyroid panel is expensive, but benchtop total T4’s are perfectly OK for monitoring L-thyroxine supplementation. There is a giant difference between zero thyroxine and ‘some’
thyroxine, so we will aim to have a level more than zero but less than the lower end of the reference interval, so that the anterior pituitary gets interested and pumps out some TSH...that way we hope that a few months later, we can try a carefully staged and monitored withdrawal of the L-thyroxine supplementation.
L-Thyroxine supplementation was commenced after surgery at a deliberately low level. Our strategy was to prevent an endocrine ‘hard landing’, where the dog went from hyper, to hypothyroidism in a short space of time.
A repeat blood test 6-weeks after surgery confirmed we had achieved exactly what we were after: a permissive mildly low euthyroid state. The next step will be to taper down the dose of L-thyroxine slowly over time to get the pituitary interested again...
Thyroglobulin Autoantibody (ELISA):
< 20% Negative
20 - 35% Inconclusive
>35% Positive

Georgina Milne
Young Veterinary Clinic
341 Boorowa Street
Young NSW 2594
e. admin@youngvetclinic.com.au
t. 02 6382 5155
C&T No. 6031
Winnie is a 6-month-old female entire Dachshund cross dog who presented to our clinic for a chronic history (weeks) of ‘rapid fire’ sneezing and nasal discharge. On initial physical examination there was a purulent mucoid nasal discharge from the left nostril, and she sneezed a few times in consult. General anaesthetic (GA) and scoping of the nose was recommended. At the time, the owner opted for a medical treatment trial with amoxicillin clavulanic acid and a non-steroidal antiinflammatory (NSAID) for 5 days and re-check in a week.
At the re-check, the owner reported no clinical response to the treatment trial so GA and rhinoscopy were planned as we are lucky enough to have a video endoscope at our clinic. Winnie was pre-medicated with methadone (0.3mg/kg) and medetomidine (0.005mg/ kg) intramuscularly. An intravenous catheter was placed, and anaesthetic was induced with 10 mg alfaxalone slowly intravenously to effect. A cuffed endotracheal tube was placed, and anaesthetic was maintained on isoflurane in 100% oxygen.
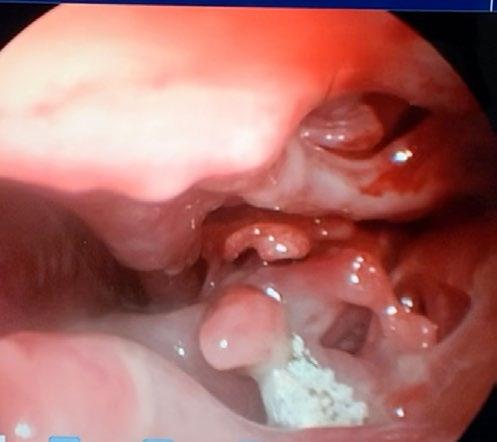
The most rostral aspect of the left nostril was ulcerated, and inflamed with purulent mucoid discharge which was difficult to direct the scope through. Once past it, there was extensive destruction of the nasal turbinates with a prominent fungal plaque (see Figure 1 and video). The right nostril was mildly inflamed; however, no plaques or destruction of turbinates was observed. We removed the plaque from the left nostril with forceps and placed it in a sterile container. There was also some tissue removed at the same time, half of which we placed in the sterile jar and half we placed in buffered neutral formalin on the off chance that if the culture of the plaque was
non-diagnostic, we could use the fixed sample to look for the presence of fungal hyphae, which, if present, can be seen on histopathology especially with special stains like PAS or Gomori silver.
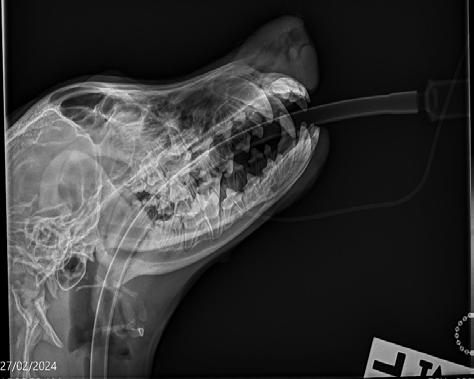
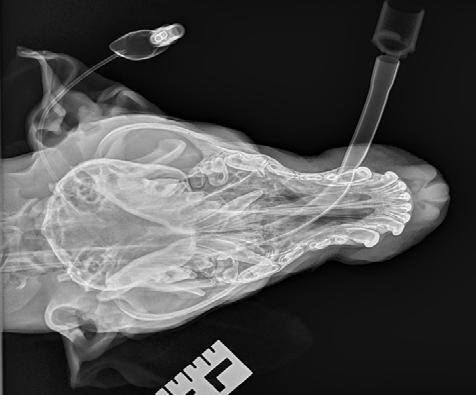
Radiographs (Figure 2) were performed of the nose and possibly some atrophy of the nasal turbinates could be appreciated; however, it was not extensive. Richard Malik noted for ‘next time’ the best radiographic view to perform is an open mouth with the X-ray plate placed into the mouth as far as it will go. Alternatively, CT would offer superior imaging and could be considered if wanting to explore the extent of the destruction, for example to determine if the cribriform plate was breached or the sphenoid sinus was infected, although it was not an option in this case due to cost considerations.
Surgical swabs were placed at the back of the throat and approximately 30 mL of 0.9% NaCl solution was drawn up in a syringe and a plastic ‘tom cat’ catheter was placed on the end. This was instilled into one nostril at a time while holding both the nostrils closed and repeated 3 times in each nostril. Once completed the swabs were removed and Winnie recovered uneventfully from surgery. NSAIDs were continued but no other medications were started while we waited for the results.
The samples were sent to Vetnostics and a bacterial and fungal culture was performed on the plaque sample as well as bacterial antibiotic susceptibility testing. The results (Table 1) returned with a heavy growth of two organisms:
Org 1: Pseudomonas aeruginosa
Org 2: Aspergillus fumigatus complex
Aspergillus fumigatus is a normal inhabitant of the nasal cavity in many animals usually in the form of spores that have been trapped by mucus lining the turbinates); however, in the rare dog or cat it can become a pathogen.2 It occurs most commonly in young male dogs of the large breeds; however, it can occur in any age or breed.2 Commonly, no underlying disease or other nasal condition is identified, as was the case in Winnie.2 Watch


Enrofloxacin 15-20mg/kg SID
y Prescribed three quarters (0.75) of a 150mg tablet SID
à 17mg/kg/day
y Chosen based on C&S results for the secondary bacterial infection of Pseudomonas aeruginosa
Posaconazole 5mg/kg SID with food.
y Prescribed one quarter (0.25) of a 100mg tablet every second day and half (0.5) of a 100mg tablet every other day for ease of dosing
à Averaged 5.2mg/kg/day
y There is a liquid formulation of posaconazole available; however, it is approximately 4 times the cost of the tablets for the equivalent amount.
y There is a paper ‘Therapeutic drug monitoring following crushed administration of delayed-release posaconazole tablets via enteral feeding tubes on the therapeutic drug monitoring of the tablets when crushed’.1 This was a game changer as, before then,
Occasionally a foreign body such as a grass seed is observed, and this might be a predisposing co-morbidity.
In literature, the two treatment options most commonly suggested are topical treatments or oral therapy. Topical treatments are generally performed under a GA and consist of either a non-invasive ‘soaking’ of the nose with a topical antifungal for an hour total or by surgically placing tubes into the frontal sinuses to instil the topical treatment twice daily for 7-10 days.2 These treatments are commonly repeated weeks later and in some cases oral therapy is also needed as an adjunct.2
Oral therapy consists of a prolonged treatment plan with one or more oral medications.2 At this point I was fortunate enough to be able to contact Dr Richard Malik who formulated an oral therapy plan for Winnie. This involved treatment for approximately 6 months’ duration pending response to treatment and is listed below.
MICROBIOLOGY REPORT - VETERINARY CULTURE
SITE: Nose
SPECIMEN: Tissue
MICROSCOPY COMMENTS :
Few leucocytes
Moderate gram negative bacilli
Scant yeast
CULTURE Heavy growth of
Org 1: Pseudomonas aeruginosa
Org 2: Aspergillus fumigatus complex
SUSCEPTIBILITY -1--2- -1--2-
Amikacin S Tobramycin S
Ampi/Amoxycillin R Marbofloxacin S
Chloramphenicol R Cefovecin R
Ceftazidime S Enrofloxacin S
Cephalexin R Sulpha/Trimeth R
Gentamicin S Clavulan/Amox R
Doxycycline R
No anaerobic bacterial pathogens isolated.
Table 1. Culture results and Susceptibility testing.
the recommendation was that the 100mg tablet was not to be crushed; but it appears the medication works as intended when divided, or even if crushed and given via a feeding tube.
Re-examination was performed and Winnie was clinically improved at home. Sneezing was reduced (but still present) and no nasal discharge was noted. There were no side effects to the medication noted.
Stop Enrofloxacin
Continue Posaconazole at current dose
Terbinafine 20-30 mg/kg BID added in
y It is recommended to start at the low end of dose range
y Prescribed half (0.5) a 250mg tablet twice a day
Continue Posaconazole at current dose
At the time of writing, we are at Day 20 of treatment and the patient is responding well. The plan is to continue these medications at this dose for 6 months total and/ or 2 months post complete resolution of clinical signs. At this time, we plan to perform another GA and rhinoscope and if this is within normal limits then we will stop treatment and monitor. Unfortunately, the damage to the nasal turbinates is permanent and Winnie will be prone to secondary infections lifelong and so we will continue to monitor for these and treat accordingly.
References
1. Ryan W Stevens, Casey O’Connell, Angie Huang, Kevin L Epps, Dan Ilges, Therapeutic drug monitoring following crushed administration of delayed-release posaconazole tablets via enteral feeding tubes, Journal of Antimicrobial Chemotherapy Volume 78, Issue 2, February 2023, Pages 553–555, https://doi.org/10.1093/jac/dkac427
2. Nelson, R. W., & Couto, C. G. (2019). Small Animal Internal Medicine (6th ed.). Elsevier - Health Sciences Division.
I saw Winnie on the 6/5/24. All clinical signs had subsided, and she was back to her normal self at home— no nasal discharge and markedly reduced sneezing. Clinically her physical examination was within normal limits. I spoke with her owner on the 8/8/24 and she is clinically still going well at home. Winnie rarely sneezes (I suspect she still does occasionally due to damage to turbinates) and has no nasal discharge. We will recheck next month for the follow up GA and scope and hopefully finish the course of medication

Karina Graham BVSc (hons) FANZCVS
Laurencie Brunel DVM MVSc CEAV DECVS
Veterinary Specialists of Sydney Miranda NSW 2228
e. heart@vsos.com.au
t. 02 8376 8767
C&T No. 6032
Mitral valve repair (MVR) is the gold standard treatment for mitral valve disease in human medicine. The MVR surgery is considered the preferred option for long- term survival. The aim of the surgery is to restore the mitral valve leaflets coaptation and reduce the regurgitation of blood, thus reducing the stress on the heart.
The surgery, performed by a team of highly experienced Japanese veterinarians, includes a mitral annuloplasty and artificial chordal replacement. MVR surgery requires stopping the heart contractions to allow its repair. To maintain the circulation and oxygenation of the other organs, a cardiopulmonary bypass (CPB) machine is used. The CPB machine circulates and oxygenates blood for the body while bypassing the heart and lungs.
The eligibility for MVR in dogs is determined based on several factors, (as in humans) but with considerations specific to veterinary medicine.
Severity of Disease: The severity of mitral valve disease is assessed through diagnostic tests such as echocardiography, comprehensive blood analysis, chest radiographs and a thorough physical examination. The degree of mitral regurgitation (MR), the presence of congestive heart failure, and the overall health of the dog are crucial factors in determining eligibility.
1. Anatomy of the Heart: The anatomy of the mitral valve and the overall structure of the heart are evaluated to determine if the valve is amenable to repair rather than replacement.
2. Overall Health: The general health and age of the dog are important considerations. MVR surgery is typically recommended for dogs that are otherwise in good health and have a good prognosis for recovery.
3. Owner Commitment: MVR often requires significant financial commitment, post-operative hospitalisation and ongoing management of heart disease including temporary medication for three months and follow-up visits. The commitment of the dog’s owner to provide necessary care and follow veterinary recommendations is essential.
In veterinary medicine, specialists such as veterinary cardiologists and internists play a critical role in evaluating dogs for MVR surgery. They assess each case individually to determine the most appropriate treatment plan based on the dog’s specific condition and needs.
The table below illustrates whether a patient’s staging is appropriate for this surgery.
Based on promising early clinical results from Drs Chris Orton and Brianna Potter at Colorado State University (CSU), Veterinary Specialists of Sydney are offering
Stage A Dogs at higher-than-average risk for developing heart failure but without any structural abnormality at the time of examination (e.g. no heart murmur). No
Stage B Dogs with structural abnormality who never had clinical signs of heart failure. No
Stage B1 Progression to heart failure is still uncertain. Monitoring is recommended. No
Stage B2 The asymptomatic MVD causes enough mitral regurgitation to cause enlargement to the left heart. Yes
Stage C Dogs have clinical signs of heart failure. Yes
Stage D Dogs have clinical signs of heart failure which are refractory to medical treatment. Yes
another option: a minimally invasive beating-heart MVR for dogs with severe MR.
Transcatheter edge-to-edge mitral valve repair (TEER) is a minimally invasive ‘beating-heart’ intervention for treatment of severe MR secondary to degenerative mitral valve disease. Worldwide, the TEER procedure (MitraClip) has been performed in more than 100,000 human patients and is now considered a viable alternative to surgical MVR.
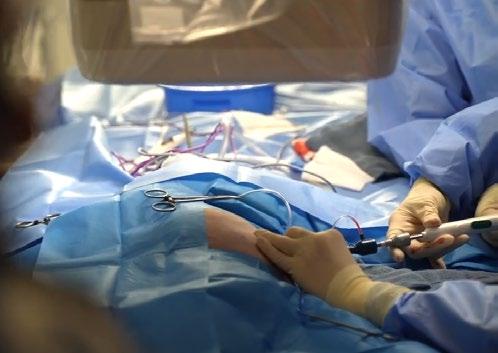
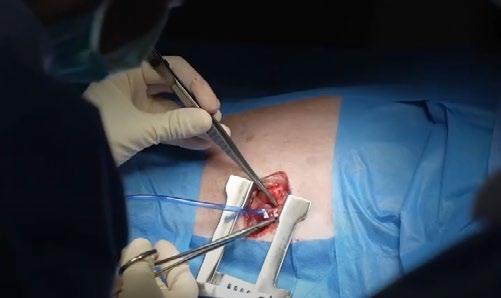
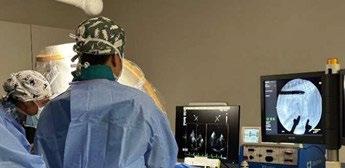
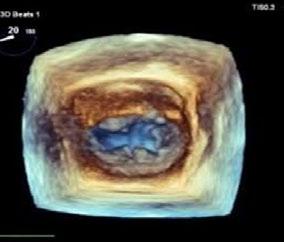
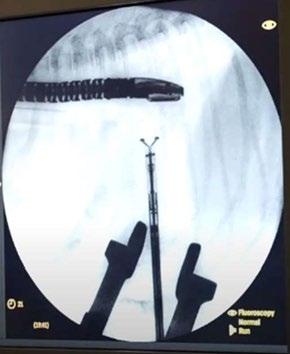
TEER procedure in dogs is performed under general anaesthesia through a small incision in the chest wall (35cm). A device called a V-Clamp is introduced via transapical cardiac approach in the beating heart under fluoroscopic and transesophageal echocardiography guidance.
Early clinical results of the TEER procedure from CSU in 50 dogs over the last 2.5 years have been associated with low-risk, meaningful decreases in MR severity, and rapid recovery. VSOS is in a very early stage of this procedure, and the only trained facility in Sydney.
Further stats from cases at CSU showed no procedural deaths and 95% of dogs were discharged from the hospital. Patients typically walked outside the day after the procedure and were released from the hospital within 2 days. Current expectations for decrease in MR severity range from 25% to 90%, depending on disease stage and severity. The best results are achieved in dogs earlier in the course of their disease.
Transapical Edge-to-Edge Mitral Valve Repair (TEER) | Veterinary Specialists of Sydney—YouTube
Patients should be referred to a veterinary cardiologist or internal medicine specialist with special interest in cardiology for a specific TEER echocardiogram. Eligibility will be determined thereafter by collaboration with the Chinese team at HongYu. Here are some general criteria that veterinary specialists consider when evaluating candidates for TEER in dogs:
1. Severity of MR: Dogs with moderate to severe mitral regurgitation are potential candidates for TEER. The severity of MR is often assessed using diagnostic tools such as echocardiography.
2. Suitability of Mitral Valve Anatomy: The anatomy of the mitral valve must be suitable for the placement
of the TEER device. This involves assessing the shape and condition of the valve leaflets to ensure they can be properly grasped and approximated.
3. Overall Health and Stability: Candidates for TEER should be in stable condition overall and able to tolerate anaesthesia. Recovery is often rapid and excellent.
4. Owner Commitment: The owner’s commitment to post-procedural care, including follow-up appointments and medication management, is crucial for successful outcomes.
5. Risk Assessment: The risks and potential benefits of TEER relative to other treatment options (such as medical management or surgery) are carefully considered for each individual case.


It’s important to note that the decision to proceed with TEER in dogs is made on a case-by-case basis, considering the specific characteristics of the dog’s mitral valve disease and overall health status. Veterinary cardiologists play a critical role in evaluating candidates and discussing the best treatment options with pet owners.
At VSOS, we provide a comprehensive range of cardiac surgical interventions, including MVR surgery, TEER procedure and for advanced refractory cases, Left Atrial Decompression (LAD). These advanced treatments represent our commitment to delivering state-of-theart medical care, ensuring that our patients receive the most effective and innovative solutions available in cardiac surgery today.
The availability of advanced cardiac procedures represents a significant advancement in veterinary
Figure 4 and 5. (Left ) Illustration of how the V-Clamp device is positioned; (Right) corresponding fluoroscopic image during placement. (Illustration Credit: www. fron ersin.org/ar cles/10.3389/ fvets.2020.597879/full;

medicine. These procedures, such as MVR, TEER and LAD, offer new hope and improved quality of life for pets suffering from cardiac conditions. By leveraging cuttingedge techniques and the expertise of specialists, we can provide tailored treatment plans that address the unique needs of each patient. This capability not only enhances the scope of care available to pets but also underscores the commitment of veterinary professionals to push the boundaries of medical care for companion animals. As research and technology continue to evolve, the future holds promise for further advancements in cardiac care, ensuring that veterinary hospitals remain at the forefront of providing comprehensive and compassionate treatment options for cardiac diseases in pets. If you would like to discuss this further, please call or email VSOS and speak to Drs Karina Graham or Laurence Brunel
Madeleine Reichstein
Southern Tablelands Veterinary Hospital
105 Robinson St, Goulbourn NSW 2580
e. madeleinereichstein@gmail.com
C&T No. 6033
A 1-year-old male neutered cat presented to our clinic on Friday afternoon, recumbent, tachycardic and tachypnoeic with fixed dilated pupils. The owner reported that he had been bitten by a snake on Wednesday morning (2 days previously) and she had been caring for him at home but was concerned when he wasn’t improving.
He was placed on flow-by oxygen and fluids immediately with blood work showing a creatinine kinase (CK) >7000U/L. We discussed with the owner whether to give the anti-venom 2 days post-bite and ultimately decided to administer Padula Serums Tiger/Brown Snake Antivenom 8000U, given slow IV over 20 minutes. The cat remained in hospital on fluid therapy and buprenorphine q8-12hrs as required for 3 days while making progressive improvement. He was able to sit up within the first 24 hours and by the third day was eating on his own and able to walk for short periods of time, though would tire easily.
He was discharged to owner care on Tuesday morning as he was eating and toileting normally, with instructions to keep him quiet and confined for the next 4-5 days. The owner reports he is doing well.

Dr Andrew Padula | Managing Director
BVSc(Hons) PhD MACVSc DipECAR e info@padulaserums.com.au w. padulaserums.com.au

This is a common question—it has not been studied in any clinical trials that I am aware of, but I have been involved with a couple of human cases where this issue has arisen.
We cannot assume there is no circulating venom in these cats. If that’s the case—then binding it with antibodies may be useful.
In the attached human case report, we were able to measure venom at 48 hours in this child in which antivenom was withheld. The child spent 9 days on mechanical ventilation and 14 days in hospital with only 66% respiratory capacity one month later.
I believe that administering antivenom up to 72 h post-snakebite is still worthwhile, although it is likely a diminishing returns scenario.
If the cat was your only child, would you withhold a potential therapy that could shorten morbidity and decrease mortality risk?
Delayed antivenom for life-threatening tiger snake bite: Lessons learnt James Tibballs, Andrew M Padula & Hamish D Jackson Volume 48, Issue 5
Abstract

An adolescent victim of an urban snakebite developed respiratory failure, rhabdomyolysis and consumption procoagulopathy but recovered with two vials of tiger snake antivenom administered after a delay of 48 hours. The clinical significance of a post-bite collapse was not initially appreciated. Tiger snake (Notechis spp.) venom antigen was measurable in blood before antivenom but not after whereas antivenom was measurable in blood for nine ensuing days. This case adds to growing evidence that further pharmacokinetic research of venom-antivenom interaction is required to establish the correct dose and timing of tiger snake antivenom. Antivenom therapy, even when delayed, facilitates recovery from snake envenomation
Meg Brownlow1 & Emily Streckfuss2
Greyhound Welfare Integrity Commission, Bathurst NSW
e. 1 mbro8605@uni.sydney.edu.au
e. 2 Emily.Streckfuss@gwic.nsw.gov.au
C&T No. 6034
The Concept of Thermal Windows & Their Role in Heat Exchange
Introduction
The greatest physiological challenge to any animal is strenuous exercise in hot/humid conditions. Thermoregulatory mechanisms are usually speciesspecific, but basically involve dissipation to the environment of the metabolic heat generated by exercise. Unless this is achieved, dangerously high levels of hyperthermia can occur with deleterious consequences. Veterinarians who work at racing and sporting venues must manage a variety of animals in athletic pursuits, often during adverse heat stress conditions. To perform that work, a thorough knowledge of species-specific thermoregulation is required, so that cooling strategies can be carried out most effectively. The welfare of the animal is the highest priority.
It has long been assumed that thermoregulatory function, whether it be by ‘dry’ heat loss or sweating, involves the dissipation of heat uniformly from an animal’s skin surface, and that different body segments will be homogeneous with respect to temperature. Recently, however, the concept of ‘thermal windows’ has emerged (Jessen, 2000), the major point of difference being that blood heated by strenuous exercise is not delivered uniformly to surface tissues, but to discrete anatomical areas that function as hot spots for heat exchange. Consequently, when managing hot animal athletes, (including humans) it is on these thermal windows that we should concentrate cooling strategies to achieve best outcomes.
Both authors have dealt with heat-stressed animals for some years, yet it was only a recent move from the care of sport horses to that of Greyhounds which brought to light the importance of species-specific thermal windows in cooling the heat-stressed animal. The horse has a body surface which could be considered a thermal window in its entirety, so that there is no strict requirement
to concentrate on particular areas when cooling that animal. Cooling dogs, however, is another matter. Thermal windows in dogs are anatomically distinct and their distribution was to some extent unexpected. Knowing their location and concentrating on those areas for cooling may reduce the morbidity and possible mortality associated with heat illness. We have found the whole concept of thermal windows intriguing, and for that reason the first part of the unabridged article deals with examples in different species, whilst the latter part deals with thermal window recognition and makes recommendations for cooling the hot Greyhound, which may be extrapolated to all dogs.
In thermoneutral conditions and with light exercise, heat is dissipated from the body surface to the environment by a process referred to as ‘dry or sensible heat loss’ which is largely dependent upon environmental conditions, chiefly the temperature difference between the skin surface and the surrounding air. As that difference decreases, heat loss by this route diminishes, and if the skin surface temperature becomes equal to or less than that of the ambient air, heat loss will cease or be reversed, so that heat can be gained by the body. At this point, depending on the species, other heat loss mechanisms take over, chiefly evaporative heat loss by sweating or panting. This type of heat loss requires a source of water, which in the skin of humans and horses is provided by specialised sweat glands. The dog, however, has no functional sweat glands and must rely on panting for evaporative heat loss. Water for that process is provided by the paired lateral nasal glands and to a minor extent salivary secretions, and the tongue assumes the major role of evaporative heat loss organ.
Thermal windows are species-specific in their anatomical location and have special characteristics. These include a relatively large non-insulated surface area and the presence of a rich sub-cutaneous vascular bed, enabling the movement of large volumes of blood through vessels which lie just under the skin. These specialised heat transfer units consist of venous plexuses and arteriovenous anastomoses (AVAs). The latter are precapillary structures which connect the arterial and venous sides of the circuit without blood having to pass through capillary beds, and represent a lowresistance pathway for high rates of blood flow. They are richly innervated by the autonomic nervous system, which controls the volume of flow by vasodilation and vasoconstriction. This mechanism increases the capacity for heat dissipation during strenuous exercise and/or heat stress but mainly occurs in those areas designated as thermal windows.
The use of infrared thermometry (IRT) to recognise the anatomical location of thermal windows
IRT has been used in a wide variety of practical applications for human and veterinary medicine. The measurement of infrared radiation emitted as heat from the skin surface reflects changes in the underlying microvasculature. Depending upon the degree of sophistication of the IRT device, it can provide a single temperature measurement at one point on the skin surface or a pictorial representation (thermogram) of surface temperature across the entire body. It is extremely useful for veterinary purposes because it provides a non-contact, non-invasive, safe method to detect changes in blood flow associated with a variety of clinical conditions. In this article, the relevance of IRT is in its provision of a non-invasive window into the thermal physiology of the racing greyhound, that is, to recognise the thermal window concept in that breed. Examples of the role of thermal windows in a variety of different species, such as the elephant, the toucan, the rat, the jackrabbit, the horse and the human can be found in the digital version of this article.
Recognition of Thermal Windows in the Canine & Recommendations for Cooling Strategies in That Species
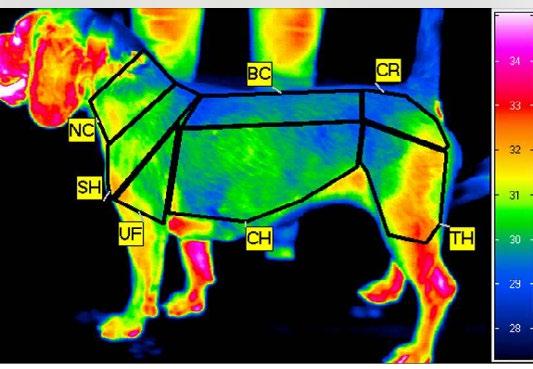
An IRT study investigated body surface temperature in Beagles immediately after high-speed physical exercise on a treadmill (Soroko et al., 2021). The aim of the study was to ensure that cardiac output increased sufficiently during the exercise activity to result in significant and optimised levels of blood flow to regions of maximal heat exchange, thus identifying thermal windows in that species. A thermogram was generated after the exercise activity was completed (see Figure 1) The areas
coloured red are those of increased blood flow, while areas that are blue or green have decreased blood flow and therefore participate only at very low levels of heat exchange.
In a seminal study using the injection of radioactive microspheres, thermal physiologists Hales and Dampney (1975) studied the redistribution of cardiac output in response to extreme levels of passive heat stress in conscious Greyhounds. They compared results obtained in a thermoneutral environment to one that caused severe heat stress. It was found that there was a 74% increase in cardiac output, with 4-6% of blood traversing AVAs compared to only 1% under thermoneutral conditions. There was increased blood flow to the skin of the lower legs and ears, the naso-buccal tissues of the tongue and mouth, nasal mucosa, turbinates of the respiratory tract, respiratory muscles, and spleen. This work correlated with the evidence from Soroko’s thermogram and supported the concept that the physiological responses to acute heat stress increased flow rates through certain tissues to meet heat loss requirements. The pattern of cardiac redistribution documented in this study validated the concept of thermal windows for heat exchange in the greyhound.
For the past four years, the first author has been working at Greyhound race meetings and observing

Figure 2 produced by the authors shows in solid red the identified thermal windows on the body surface of the racing Greyhound. They include the head and ears, the paw-pads extending upwards, the chest area on both sides just behind the elbows, and the inguinal area surrounding the femoral arteries between the hind legs which is hidden from view. The extent of the transition area (represented by red dots) will depend upon other factors such as intrinsic differences between individual dogs, the distance raced, the intensity of the exercise (effort) and the degree of adversity dictated by environmental conditions, particularly radiant heat and humidity. If the latter are substantially elevated or if the dog is suffering from heat stress, the transition areas will have greater colour and be more extensive.
the dogs’ capacity to cool after racing in a variety of environmental conditions. When it is hot and/or humid, white dogs are particularly useful because the thermal window areas become hyperaemic and quite obvious (see figures in digital version) which corroborates not only the thermogram of Soroko’s beagle but also the areas that Hales and Dampney (1975) associated with the redistribution of cardiac output due to acute heat stress. It is also apparent that professional trainers who are experts in the care of Greyhounds have learned exactly where to concentrate their cooling efforts, even though they may not know their physiological underpinnings. From this information the authors have recommended a cooling strategy based on the location of thermal windows, which can be extrapolated to all canines (see digital version).
The amount of heat lost through thermal windows located on the body surface of the dog is determined by temperature difference. Provided that the air is cooler than the skin, or a cool medium such as cold water is applied, heat will be ‘dumped’. Increasing the temperature difference by using colder water will increase the efficiency of that process. Using warm water (country areas) may be totally counterproductive and result in actual heat gain.
Although the tongue and mucous membranes of the respiratory tract (coloured blue in the diagram) must be regarded as a thermal window for heat exchange, the process by which this is achieved is totally different from that which occurs on the body surface. The respiratory system participates in an evaporative heat loss process whereby heat-laden secretions are produced, vaporised, and dissipated into the environment, thereby cooling the animal. Although the process is extremely efficient and there is no doubt that dogs can cool entirely by this mechanism, proactive cooling of skin surface thermal windows will relieve respiratory effort and reduce signs of thermal stress.

Read the booklet for dog owners here: cve.edu.au/Thermoregulation

Read a more detailed version of this article, along with many illustrations of the cooling process, discussion and conclusions here: cve.edu.au/Thermoregulation
References
Jessen C (2000) Temperature Regulation in Humans and Other Mammals Springer-Verlag New York.
Hales, J.R.S.; Dampney, R.A.L. (1875) The redistribution of cardiac oitput in the dog during heat stress. J. Thermal Biology 1, 29-34.
Soroko, M.; Gorniak, W.; Howell, K.; Zielinska, P.; Dudek, K. (2021) Changes in body surface temperature associated with high-speed treadmill exercise in beagle dogs measured by infrared thermography. Animals, 2021, 11,2982.



Dr Chloe Cheung
Resident in Small Animal Medicine
University Veterinary Teaching Hospital Sydney
Associate Professor
e. chloe.cheung@sydney.edu.au
Natalie Courtman
Specialist in Veterinary Clinical Pathology
Veterinary Pathology Diagnostic Services (VPDS)
Sydney School of Veterinary Science.
e. natalie.courtman@sydney.edu.au
t. VPDS +61 2 9351 3099
C&T No.6035
An 8-year-old male neutered beagle cross cavalier King Charles spaniel was presented for a 4-day history of inappetence, lethargy and melaena. The dog also suffered from intermittent mixed bowel diarrhoea, which was not previously investigated. The dog was a known scavenger, and rat bait toxicity was the initial suspicion.
At the initial visit, he had normocytic hypochromic non-regenerative or pre-regenerative anaemia on presentation with a haematocrit of 0.22 L/L (RI 0.350.58) and reticulocyte count 12 x 10⁹/L (RI 20-150), and severe thrombocytopenia with platelet estimate from the smear 5 x 10⁹/L (RI 111-379). The initial veterinarian documented the presence of red cell agglutination and spherocytes on in-house blood smear assessment. Both APTT and PT were within normal limits and Direct Coombs test was negative. His biochemistry and urinalysis were unremarkable.
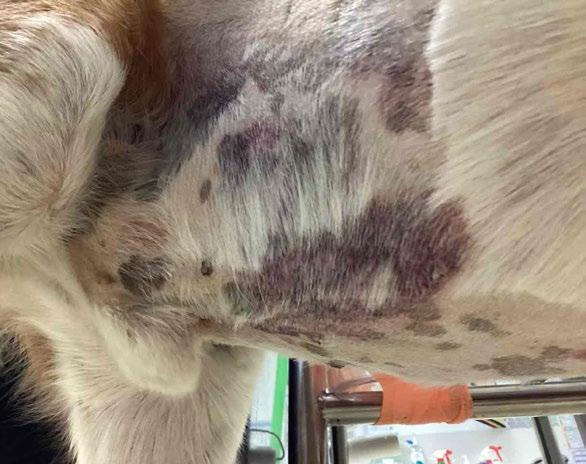
He was referred to the University Veterinary Teaching Hospital Sydney (UVTHS). On presentation, there were ecchymoses noted on the ventral abdomen (Figure 1) and multiple petechiae on the mucous membranes. The rest of the vitals were unremarkable. A whole blood transfusion was administered overnight as his PCV dropped from 0.22L/L to 0.17L/L, following
blood-typing as DEA 1.1 negative. Rat bait toxicity was considered unlikely, given the previous normal coagulation profile. Non-associative Evan’s syndrome was the leading differential at the time, given the severe thrombocytopenia and suspected concurrent immune-mediated haemolytic anaemia based on initial bloodwork findings. The patient was managed with an immunosuppressive dose of corticosteroids at 2mg/ kg/day until his platelet counts returned close to normal limits at 110x10⁹/L and was then managed as an outpatient.
Below are the haematology results from the subsequent visits.
1. Given the lack of improvement in haematocrit despite treatment with immunosuppressives, what are your other differentials?
2. What additional diagnostics would you consider at this point?
Email cve.marketing@sydney.edu.au
Best complete answer will receive a CVE $100 voucher!
A shortage of intravenous (IV) fluids is currently affecting healthcare and veterinary services in Australia. This shortage is attributed to manufacturing issues and unexpected increases in demand, as noted by the Therapeutic Goods Administration (TGA).
Access the following resources for alternative protocols:
Intravenous Fluid Shortages – Suggestions for Management & Conservation 2024 Executive of Veterinary Anaesthesia and Analgesia Chapter, ANZCVS.
Kelly Huitson
RVN, BSc (Hons) APVN (Herpetology, Small Mammal)
C&T No. 6036

Intravenous fluid administration is recommended for anaesthetised patients and those with fluid disturbances such as hypovolaemia or dehydration, which can increase the risk of organ dysfunction and failure if not corrected swiftly. This article discusses the types of fluids available and their uses in specific conditions, as well as how to correct electrolyte imbalances in conjunction with fluid therapy.
The fundamental aim of intravenous fluid therapy (IVFT) is to correct fluid deficits that may lead to organ dysfunction and failure if not addressed swiftly.¹
Hypovolaemia causes a reduction in intravascular volume, while dehydration (Figure 1) causes a water deficit across all fluid compartments, creating an increase in electrolyte concentrations.² In 2013, the American Animal Hospital Association (AAHA) and the American Association of Feline Practitioners (AAFP) created guidelines detailing fluid choice, rates and route of administration for cats and dogs,³ with additional allowances for deficits to be calculated on a case-bycase basis.⁴
A common formula used for calculating a maintenance fluid rate is 3 mL/kg/h; however, this underestimates the amount required for small patients.⁵ More accurate formulae for calculating maintenance fluids in cats are:
mL/day = 80 × body weight (kg)0.75 or mL/day = 30 × body weight (kg) + 70.
Dehydration deficits should be corrected over the initial 4–12 h period depending on cardiac function and the presence of an underlying disease process⁶ and is calculated using the formula:⁷
Dehydration deficit (mL) = body weight (kg) × estimated dehydration (%) × 10.
Isotonic solutions contain more sodium than plasma and can create electrolyte imbalances in patients with renal disease or cause hypernatraemia and hypokalaemia when used in the long term
Fluid choice is deemed by the function required and the fluid compartment requiring resuscitation.
Crystalloids
Crystalloids contain solutes such as electrolytes, which either cross semipermeable membranes or are metabolised by the patient. This fluid is rapidly distributed into the interstitial and intracellular compartments within 20–30 mins.⁸
Isotonic solutions
Isotonic solutions (such as compound sodium lactate [Hartmann’s] or lactated Ringer’s) are the most commonly utilised, particularly for maintenance of hydration status, hospitalised patients requiring hydration and for the replacement of extracellular fluids.⁹ These have a similar electrolyte level to plasma and are often described as balanced, with only 25% of the fluid remaining intravascularly after 30 mins.⁸ These solutions contain more sodium than plasma and can create electrolyte imbalances in patients with renal disease or cause hypernatraemia and hypokalaemia when used in the long term. These balanced solutions contain buffers (such as lactate) which can be alkalinising, whereas other isotonic solutions, such as 0.9% sodium chloride (NaCl), can be acidifying.⁸ Another option for a maintenance fluid solution is to use 0.45% NaCl with 13–20 mmol/L potassium chloride added.³
Hypotonic solutions
Hypotonic solutions contain less sodium and chloride ions than plasma, so have a lower osmolarity. This results in the redistribution of fluid across all cellular compartments with the majority moving into the intracellular space and, therefore, not expanding intravascular volume. This makes them ineffective for bolus therapy and they can cause cerebral oedema.⁸
Hypertonic solutions
Hypertonic solutions contain more sodium and chloride than plasma, so have a higher osmolarity. This leads to rapid intravascular expansion10 and is often used to treat hypovolaemia or to reduce intracranial pressure. This effect is transient, lasting 30 mins, as a result, administration of these solutions should be followed by isotonic solutions for a longer- term hydration benefit and to revive the extravascular fluid shifted into the vascular space.⁸
Colloids contain large molecules that cannot pass through the capillary poles, so will increase intravascular pressure, remain in the intravascular space longer,
increase oncotic pressure and increase vascular volume by retaining fluid within the vascular space.10
Maintenance colloids are designed for replacing ongoing fluid losses from normal metabolic functions and have a sodium concentration less than plasma, potassium concentrations higher than plasma and sometimes glucose added to bring solute concentrations closer to that of true extracellular fluid. Replacement crystalloids may have higher sodium than plasma, and most patients can manage this, although cats may benefit from supplemented potassium.⁵
The use of colloids, particularly starches, has come under scrutiny due to their potential side effects. In human studies, crystalloid starches were found to increase renal failure in septic patients and little significant benefit in their use over crystalloids has been shown.⁸
For healthy cats undergoing surgery, a maintenance rate, as discussed above, should be provided using a balanced crystalloid solution.11
Acute kidney injury treatment relies on replacement solutions such as lactated Ringer’s or 0.9% NaCl in severely hyperkalaemic patients, for fluid balance correction. Sodium chloride 0.9% solution may be less suitable for patients with pre-existing metabolic acidosis as this can be exacerbated with this treatment method. Exceeding the patient’s normal fluid maintenance levels puts the patient at risk of fluid overload with forced diuresis, which in turn increases the risk of morbidity⁶ and may not promote more uraemic solute excretion than non-aggressive fluid therapy.12 Diuresis should correct any hyperkalaemia present; if required, sodium bicarbonate may be considered if the patient persists with a pH <7.1 or TCO₂ <12 mEq/l after fluid intervention,

although this is associated with significant complications, including iatrogenic alkalosis, paradoxical central nervous system acidosis, hypokalaemia and hypernatraemia.⁶
Chronic kidney disease is more multifactorial than acute kidney injury and leads to several systemic effects, including reduced renal perfusion leading to dehydration, which exacerbates the poor renal function. Acutely affected cats or severely dehydrated patients need IVFT, as increased fluid hydration status can dilute uraemic toxins13 and extend quality and quantity of life by affecting the rate of disease progression.⁷ The fluid of choice is usually lactated Ringer’s or compound sodium lactate (Hartmann’s) and the rate should include a deficit calculation, as well as ongoing maintenance. Once deficits have been corrected, cats should be monitored carefully for fluid overload and once azotaemia has been corrected, the rate should be tapered daily until discontinuation.⁷
For non-acute cases or early International Renal Interest Society (IRIS) stage patients, adequate fluid maintenance at home can be encouraged by providing a wet diet, offering water fountains or flavoured water, or through subcutaneous administration by the caregiver at home (Figure 3). 14 In the majority of cases, subcutaneous fluid administration is well tolerated by cats and is easily learnt by the caregiver. In one study, the most common fluids given in this way were lactated Ringer’s, followed by 0.9% NaCl,15 although recommendations advise the preferable use of 0.45% NaCl as the first choice, with supplementation of potassium if required, and
lactated Ringer’s as a secondary choice, with a volume of approximately 75–150 mL every 1–3 days for cats at IRIS stage 3–4.⁷
Hyperkalaemia can be deemed mild to moderate with serum concentrations of 5.5–6.5 mmol/L. It can be treated with crystalloids to correct volume and dehydration deficits, dilute potassium and shift potassium by alkalinisation.
Traditionally, 0.9% NaCl has been administered to encourage this dilution but a buffered isotonic crystalloid (such as compound sodium lactate/ Hartmann’s or lactated Ringer’s) is sufficient to encourage intracellular potassium shift thanks to the presence of buffers.16 This is the same consideration for cats with a urinary blockage and the recommendation is to consider all options with the ability to correct metabolic acidosis.17 Urethral blockage in-hospital morbidity remains high due to electrolyte imbalances, including arrhythmias caused by extreme hyperkalaemia or reduced glomerular filtration and uraemic toxicosis.18
Moderate elevations of serum potassium exceed 6.5 mmol/L, with myocardial toxicity at levels over 7.5 mmol/L and is deemed severe over 9 mmol/l. At these higher levels, it can be treated differently; with crystalloids such as 0.9% NaCl or lactated Ringer’s at a bolus of 5 mL/kg over 10 mins, repeating according to response, with the addition of calcium gluconate 10% at 0.5–1.5 mL/kg slowly via the intravenous (IV) route over 10 mins to protect against cardiotoxic effect; as the heart rate slows, the rate of calcium gluconate should be reduced.

This is only effective for 20 mins and does not inherently alter serum potassium concentrations.16,17 Rarely, sodium bicarbonate is used for persistent ECG abnormalities at a dose of 1–3 mmol/kg IV over 20–30 mins (1 mmol/mL in an 8.4% sodium bicarbonate solution), ensuring that a total dose of 4 mmol/kg is not exceeded.17
Hypokalaemia correction is rarely required rapidly; however, potassium chloride can be supplemented following fluid resuscitation ensuring the rate does not exceed 0.5 mEq/kg/h.17
Vomiting causes metabolic alkalosis through the loss of stomach hydrochloric acid and potassium, and reduced water intake. For this reason, 0.9% NaCl supplemented with potassium is recommended in these patients rather than a balanced isotonic crystalloid such as compound sodium lactate/Hartmann’s.²
In contrast, diarrhoea causes metabolic acidosis and can be treated with 0.9% NaCl to utilise its mild acidifying effect² to correct the electrolyte imbalance created.19 In both vomiting and diarrhoea, the treatment should prioritise correcting fluid deficits with crystalloid administration as well as replacing ongoing losses.10
Hypoglycaemia is treated with an initial IV bolus of 0.5 g/ kg glucose administered slowly over 5–10 mins.
This can be achieved by diluting 50% dextrose in an equal volume of saline to create a 25% solution and administering 2 mL/kg. This is followed by a 2.5% glucose constant rate infusion (CRI), adjusting the level of infusion to achieve normoglycaemia. Peripheral administration of concentrates over 5% for CRI should be avoided owing to the risk of thrombophlebitis.17
Hyperglycaemic conditions should prioritise fluid resuscitation before administering insulin. Crystalloid therapy dilutes the glucose and increases renal excretion.17
Hypocalcaemia should be corrected with a CRI of 60–90 mg/kg/day.
Calcium salts should not be added to fluids containing lactate, acetate, bicarbonate, or phosphate due to the potential for precipitation of calcium. Calcium chloride is an irritant to the veins; therefore, calcium gluconate is preferred. The infusion rate should be altered until normocalcaemia is reached.17
Hypercalcaemia can be corrected using 0.9% NaCl, including calculations for both deficits and maintenance of ongoing losses.20
Hyponatraemia can be treated using the calculation:
Sodium deficit = 0.6 × body weight (kg) × (normal sodium [mEq/l] – patient sodium [mEq/l]).
Sodium should be replaced via an infusion at an average rate of 0.5 Eq/l/h,21 aiming to change the concentration at a rate of 0.5 mmol/L/h. A more rapid correction may cause cerebral dehydration, haemorrhage and demyelination.17 Where hypovolaemia is also present, the fluid selected should be within 5 mEq/L to the patient’s plasma. If a suitable solution is not commercially available, then a substitute can be made using 5% dextrose in water using the formula:21
Volume of 5% dextrose in water (mL) = ([current IV fluid sodium] − [desired IV fluid sodium]) x 1000mL/ ([desired IV fluid sodium] - [supplemental IV fluid sodium]).
Hyponatraemia in the presence of hyperglycaemia does not require specific treatment, as therapy should primarily be targeted to the management of hyperglycaemia.17
Hypernatraemia should be treated with an initial volume expansion using 0.9% NaCl to prevent an excessively rapid drop in sodium, followed by maintenance with 0.45% NaCl or 5% dextrose to correct the free water deficit over 2–3 days.
Assessment of sodium levels should be completed every 1–2 hours initially during treatment.17
In anaemic patients, crystalloid fluids can be given while a patient waits for a blood product transfusion to support perfusion deficits.17
Sepsis cases with hypoperfusion present and without cardiac disease, benefit from incremental isotonic crystalloid boluses of 10–15 mL/kg, with regular reassessments for fluid response. Renal vasoconstriction and acute kidney injury can be caused by high chloride fluids such as 0.9% NaCl, and synthetic colloids are also contraindicated owing to the potential for renal damage. Fresh frozen plasma products are only indicated in patients with concurrent coagulopathy and bleeding.22
Hypovolaemic shock cases require crystalloids, although the volume to be administered should be divided into ¼ or ⅛ of the total volume and administered in boluses of 5–10 mL/kg with frequent re-evaluation. Lactated Ringer’s or 0.9% NaCl are both suitable for this resuscitation.
If little or no improvement is seen, then two further crystalloid boluses can be given, followed by a colloid bolus of 5 mL/kg over 5–10 mins if considered appropriate, with tetra or pentastarch preferred to gelatins.23
The primary consideration for cats when considering fluid therapy is their reduced tolerance of high fluid loads, with a tendency to develop fluid overload, respiratory distress,10 pulmonary oedema and pleural effusion with excessive fluid rates.23 Occult or asymptomatic cardiac disease is common in cats either owing to primary cardiomyopathy or secondary to other disease processes, with an estimated 7–28% of apparently healthy cats suffering from hypertrophic cardiomyopathy, and 18–43% of cats with a heart murmur present experiencing structural heart disease of some kind.18 For this reason, auscultation before, during and following fluid therapy is essential to aid in the prevention and early identification of fluid overload before the patient decompensates into congestive heart failure23 although even in the absence of myocardial disease, some cats are more prone to fluid overload than dogs.24 In patients with cardiac insufficiency, the provision of isotonic crystalloids may predispose them to interstitial oedema; if 0.45% NaCl is utilised for maintenance, potassium and sodium levels must be monitored regularly as a precaution to prevent hypokalaemia or hyponatraemia.18

Figure 5. Auscultation prior, during and following fluid therapy is important to prevent/identify fluid overload
Fluid overload can also occur, owing to the lungs being considered the ‘shock’ organ in cats, leaving them susceptible to injury during systemic injury such as sepsis. As the blood volume of a cat is estimated at 66 mL/kg, rather than 90 mL/kg in the dog, this leads to lower doses of bolus fluid administration of 10–20 mL/kg rather than 40–60 mL/kg.23
Fluid overload clinical signs include hypervolaemia, pulmonary oedema, peripheral oedema, body cavity effusion and death. This can be secondary to impaired excretion of fluid in patients with kidney or liver disease. However, the lungs and organs confined in capsules such as the kidney, or rigid structures such as the brain, are particularly vulnerable to the damage caused.25
References
1. Poli G. Perfusion deficits and fluid resuscitation: a more indepth look. https://www.vettimes.co.uk/perfusiondeficitsand-fluid-resuscitation-a-more-in-depth-look (2021, accessed 15 November 2023).
2. Robinson R. Companion animal fluid therapy part 2: planning and monitoring. https://www.vettimes.co.uk/article/ companion- animal-fluid-therapy-part-2-planning-and-monit oring/ (2016, accessed 11 November 2023).
3. American Animal Hospital Association. AAHA/AAFP fluid therapy guidelines for dogs and cats. https://www.aaha.org/ aaha- guidelines/fluid-therapy/fluid-therapy-guidelines/ (2013, accessed 2 December 2023).
4 O’Dwyer L. Nursing notes: fluid therapy in anaesthetised patients. https://www.vettimes.co.uk/article/nursingnotesfluid-therapy-in-anaesthetised-patients/ (2016, accessed 13 November 2023).
5. Poli G. Maintenance fluids. https://www.vettimes.co.uk/ maintenance-fluids/ (2016, accessed 13 November 2023).
6. Monaghan K, Nolan B and Labato M. Feline acute kidney injury: 2. Approach to diagnosis, treatment and prognosis. J Feline Med Surg 2012; 14: 785–793.
7. Sparkes AH, Caney S, Chalhoub S, et al. ISFM Consensus Guidelines on the Diagnosis and Management of Feline Chronic Kidney Disease. J Feline Med Surg 2016; 18: 219–239.
8. Robinson R. Practical fluid therapy in companion animals –part 1. https://www.vettimes.co.uk/ article/practical-fluidtherapyin-companion- animals-part-1 (2016, accessed 2 December 2023).
9. Birkbeck R, Donaldson R, Chan D L. Nutritional management of a kitten with thermal burns and septicaemia. JFMS Open Rep 2020; 6. DOI: 10.1177/2055116920930486.
10. Pachtinger G. Fluid therapy. In: Drobatz KJ, Reineke E, Costello MF, et al (eds). Feline emergency and critical care. 2022.
11. Robertson SA, Gogolski SM, Pascoe P, et al. AAFP Feline Anesthesia Guidelines. J Feline Med Surg 2018; 20: 602–634.
12. Langston C and Eatroff A. Less is more – fluid therapy for kidney disease. J Feline Med Surg 2012; 14: 773–773.
13. Collin S. Nutritional support of cats and dogs with renal disease. Vet Nurse J 2017; 32.
14. Caney SMA. Long-term care of cats with renal disease. Vet Nurse J 2010; 25.
15. Cooley C M, Quimby J M, Caney S M, et al. Survey of owner subcutaneous fluid practices in cats with chronic kidney disease. J Feline Med Surg 2018; 20: 884–890.
16. Poli G. Hyperkalaemia, pt 2: treatment. https://www.vettimes. co.uk/hyperkalaemia-pt- 2-treatment-cpdurology (2022, accessed 14 November 2023).
17. Murphy K and Hibbert A. The flat cat: 2. The emergency database and management of common metabolic abnormalities. J Feline Med Surg 2013; 15: 189–199.
18. Cooper E, Guillaumin J, Her J, et al. Small animal fluid therapy. United Kingdom: CABI, 2023.
19. Thompson A. Nursing small animal patients with diarrhoea. Vet Nurse J 2010; 25.\
20. Maltman M. Disorders of calcium and the parathyroid glands Vet Nurse J 2022; 26: 229–232.
21. Poli G. Hyponatraemia, pt 3: correcting a sodium concentration of 110mEq/L. https://www.vettimes.co.uk/hyponatraemiapt3-correcting-a-sodium-concentration-of-110meql (2023, accessed 15 November 2023).
22. Montealegre F, Lyons B M. Fluid therapy in dogs and cats with sepsis. Front Vet Sci 2021; 8. DOI: 10.3389/fvets.2021.622127.
23. Murphy K and Hibbert A. The flat cat: 1. A logical and practical approach to management of this challenging presentation. J Feline Med Surg 2013; 1: 175–188.
24. Adamantos S. Fluid therapy in pulmonary disease: how careful do we need to be? Front Vet Sci 2021; 8. DOI: 10.3389/ fvets.2021.624833.
25. Hansen B. Fluid overload. Front Vet Sci 2021; 8. DOI 10.3389/ fvets.2021.668688.
Welcome to Research Roundup where we bring you summaries of the latest feline research. Included are articles on the practical use of POCUS in cats with ureteral obstruction, the significance of thrombocytosis and barriers to the measurement of blood pressure.
The International Society of Feline Medicine is the veterinary division of the pioneering cat welfare charity International Cat Care. Trusted by vets and nurses, it provides a worldwide resource on feline health and wellbeing, via the Journal of Feline Medicine of Surgery, by fostering an international community of veterinary professionals with a shared vision of feline welfare, and supporting professional development with practical CPD. Additionally, International Cat Care’s website provides a valuable resource of accurate information delivering what both vets and cats would want owners to know

-Mireia Balliu Cristina, Spain, 2021 Participant


Jen Nesbitt-Hawes BVSc MVSt MANZCVS (Veterinary Behaviour)
e. jen@petperspective.com.au
C&T No. 6037

Veterinarians currently give conflicting advice on what is considered ‘best practice.’ The common goal of all pet professionals is to end up with healthy, welladjusted dogs. This article will give practical advice on appropriately balancing socialisation and vaccination of puppies.
The core canine vaccine is a DHP (Distemper, Hepatitis, Parvovirus).
Canine distemper is uncommon in Australia, but outbreaks do occur intermittently, causing severe disease, with confirmed Australian cases occurring exclusively in young, unvaccinated dogs.² Clinical signs include fevers, rashes, discharge from the eyes, laboured breathing and coughing. Mortality rate has been estimated to be up to 77% in Australia.²
Infectious canine hepatitis is caused by an adenovirus. Clinical cases in Australia are very rare, thanks largely to ongoing vaccination programs worldwide. Clinical signs include vomiting, diarrhoea, abdominal pain, blue eyes (due to corneal oedema), or neurological signs.³ Canine hepatitis is potentially fatal.⁴
Canine parvovirus (CPV) poses the greatest threat to puppies in Australia because it is so much more common. Whilst one study documented 48 confirmed cases of canine distemper over an 8-year period in Australia,² there have been reported to be around 20,000 cases annually of parvovirus, at a case rate of 4 in every 1,000 dogs.⁵ 41% of the dogs in this particular study were euthanased, with costs of treatment being a significant factor. If untreated, the mortality rate of CPV is around 90%. In treated dogs, however, mortality rate is reported at 18.2%, which is much lower than most people, including veterinarians, estimate.⁷ Dogs with CPV have severe haemorrhagic diarrhoea, vomiting, dehydration and abdominal pain.
Vaccinating puppies is extremely important because it is protective against these diseases and the benefits are clear on both an individual and population level. However, the solution isn’t quite as simple as ‘go forth and vaccinate,’ largely due to the complication of Maternally Derived Antibodies (MDA).
If a bitch has been vaccinated or previously survived parvovirus infection, she’ll produce antibodies. These will be passed through to her puppies in the colostrum, so they will have early immunity protecting them against disease as neonates. The problem is that MDA can interfere with vaccination.
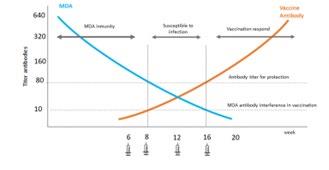
Figure 1. Graph demonstrating the possible window of susceptibility to infection, and the role of MDA 19 Source: Prof. Mary Marcondes- Vaccination Guidelines Group (WSAVA)
The y axis on Figure 1 is a measure of antibody levels. Along the x axis is the age of the puppy in weeks. The maternal antibody levels are depicted by the blue line which starts at a very high level when the puppy receives colostrum, but then gradually curves down, as the levels of MDA within the puppy reduce over time. In reality, how much time it takes to reduce varies between individuals, even within a single litter. It depends on the mother’s level of immunity, how much colostrum the puppy gets
and most importantly, whether it gets that colostrum within about 12 hours of birth.18 At some point in time, the MDA titre drops below the level needed to provide protection against disease, but remains above the level where it interferes with vaccination. Puppies should have their first vaccination at 6-8 weeks-of-age, but some of them still have a certain amount of MDA, which fight the vaccination as they would an infection. So, there is a window of time between around 8 and 16 weeks where a puppy may be vulnerable to infection, even though it has been vaccinated. For some puppies, MDA may interfere with vaccination until around 16 weeks-of-age.
Maternally derived antibody interference is the leading cause of vaccination failure and age at vaccine administration is a significant risk factor for failure.⁷ The potential for maternally derived antibody interference is the reason the final puppy vaccination should be given at 16-weeks-of-age. Puppies are not considered to have full immunity until 1-2 weeks after this final vaccination.
There are vaccines available that have licensed claims for an earlier finish (final vaccine to be given at 10-weeks-of-age). It should be kept in mind that the guarantee given by the manufacturer is NOT that no puppies will get parvovirus, but that if vaccination failure does occur (and if the vaccine doses given have been correctly documented), the manufacturer will pay for treatment. The puppies still experience the disease and those affected by parvovirus would have a mortality rate around 18%. A 2017 study17 showed that ‘the age at administration of the last CPV vaccination a puppy received prior to presenting with disease was a significant (P=0.0334) risk factor for vaccination failure, irrespective of whether the vaccine was marketed for a 10-week or 12-week or greater vaccination finish

protocol.’ The WSAVA Guidelines for the vaccination of dogs and cats14 continue to recommend a 16-week plus finish for the primary vaccination course in puppies.
Can we measure titres to determine level of immunity in puppies?
It is theoretically possible to take serial titre measurements to determine the level of MDA in a puppy and thus work out when first vaccination is most likely to be effective.14 However, the amount of time, effort, and expense involved make this impractical. It would also increase the number of potentially aversive vet experiences during a puppy’s socialisation period.
Titre levels can be used to determine if a puppy is properly vaccinated; WSAVA guidelines14 state that if a puppy had its last vaccine at 16-weeks-of-age, the titre level could be measured from 20 weeks-of-age, as any antibody measured at this stage could not be MDA. This could be useful if it were suspected that the puppy was a genetic non-responder but will not be useful as a test to determine whether it is safe for a puppy to socialise, as it is well past the end of the socialisation period.
The aim of socialisation is to develop confidence and optimism by positive experiences with novelty. We achieve this with a process of gently introducing puppies to other living beings, ensuring they are comfortable and in a positive emotional state. We tend to clump socialisation with habituation, which is introducing puppies and dogs to different objects and environmental stimuli like vehicles, vacuum cleaners, the feeling of walking across sand, the noise of storms and so on.
There is a sensitive period for socialisation, during which puppies are more likely to investigate or interact with new stimuli and, if paired with a positive emotional state, to accept the new things as part of life. The socialisation period lasts from about 3-14 weeks-of-age (as little as 12 weeks, or as much as 16 weeks, depending on the individual). Socialisation needs to be maintained throughout life to maintain confidence and resilience, but puppies’ brains are wired for most effective socialization during the socialisation period.
It is important to note that if puppies are introduced to new stimuli and the overall experience makes them feel anxious, afraid or overwhelmed, then sensitisation (not socialisation) is the more likely outcome. For this reason, arguably the most important thing to teach new puppy clients is how to accurately read their dog’s body language. Without this essential skill, caregivers cannot effectively socialise, train, supervise children and dogs, or advocate for their pet.
Socialisation is not something that people actively ‘do’ to a puppy. What people need to do is support the natural process by making sure the puppy has time and space to feel comfortable. When socialising, the puppy’s initial reaction may be excitement and a desire to approach, or it may be uncertain about the new thing; both can be a normal response. The key is that if the puppy is unsure, the caregiver should watch carefully and wait, giving the puppy a little time and space to decide what to do independently. The puppy should have the freedom to move away, or forwards to investigate at their own pace. It should then move into a relaxed/interested emotional state. If the puppy continues to look worried or concerned, the person should stop the interaction before the puppy becomes overwhelmed.
What happens if we don’t socialise appropriately or adequately during the socialisation period?
If they haven’t had positive experiences dealing with novel stimuli, then dogs are likely to be fearful of things they haven’t previously encountered. This may be displayed in various ways (separation distress, reactivity, fear of strangers, fear of car travel, etc). We know that if dogs are not adequately socialised, they are more likely to have behaviour problems. We know that behaviour problems are ongoing, chronic issues for clients, result in higher surrender rates, and are a leading cause of euthanasia for young dogs.13 It is not just parvovirus that has fatal outcomes for young dogs. We should not focus solely on protecting puppies against parvovirus at the cost of behavioural health.
The difficulty is in the overlap of time for the two essential processes of socialisation and vaccination. The socialisation period, this sensitive period during which we can best help puppies gain experience with novelty and set them up to be confident, optimistic and resilient dogs, lasts until the puppy is around 14-weeks-of-age. This period is over by the time that the puppy vaccination protocol is finished and immunity considered complete, at 18-weeks-of-age.
The following governing bodies and special interest groups have released position statements and guidelines regarding vaccination and socialisation.
The American Veterinary Society of Animal Behavior position statements says that ‘it should be the standard of care for puppies to receive such socialization before they are fully vaccinated…… Behavioral issues, not infectious diseases, are the number one cause of death for dogs under 3 year- of-age. ’15
The Pet Professionals Guild state: ‘PPG advocates for socialization to accompany puppy vaccinations rather than waiting to socialize a puppy until after the vaccinations are complete by which time the critical socialization period will have been missed.’
American Veterinary Medical Association: ‘Socialization should begin during the puppy’s ‘sensitive period’ which is between 3 and 14 weeks-of-age.”
World Small Animal Veterinary Association: ‘The WSAVA supports puppy socialisation and recommends that where puppies are exposed to other dogs (i.e., puppy parties) that all of those dogs (puppies and adults) are vaccinated and that the event occurs in an environment that is least likely to be contaminated with virus (particularly CPV2)’11
There is global recognition that socialisation is so important that we can’t simply advise clients not to take their puppies out until after they are fully vaccinated.
Fortunately, socialisation doesn’t have to be ‘all or nothing.’ Here are some practical suggestions for gaining that all-important socialisation, whilst minimising risk of disease. Keep in mind that although the list is extensive, puppies benefit most from a variety of quality socialisation experiences, not necessarily quantity of experiences. Puppies require around 20 hours of sleep daily, so caregivers need to allow for this too.
1. Socialisation within the home
Socialisation can (and does) occur within the home whether we are actively encouraging it or not. Caregivers should take advantage of this and be mindful of opportunities, for example:
y Novel items: Encourage exploration on different surfaces like tiles, yoga mats, wet grass, or through puddles. Set up obstacle courses with kids’ tunnels, hula hoops, or flapping materials. We know that dogs may have difficulty coping with things like moving wheelie bins, sweeping or vacuuming, so ensuring they are socialised with stimuli related to noise and movement from an early age is beneficial. Include seasonal stimuli too, e.g. the noise of thunderstorms or people swimming in a pool.
y Dressing up or having people over wearing different accessories (e.g. fluoro vests and hats, wetsuits like surfers, people with glasses, beards, facemasks and other common things to encounter in the community)
y Handling, grooming and cooperative care.
y Play dates with well mannered, vaccinated dogs, either at the puppy’s home or homes of friends or family members.
y Alone time (the opposite of socialisation). Habituating a puppy to being comfortable being alone should be facilitated in the home environment during the socialisation period.
2. Relative risk
Before people go out and about with their puppies, they should be made aware of which local areas are higher or lower risk. Veterinary staff have this information available and should advise clients (and other local pet professionals, like trainers) how many parvo cases you see each year, which areas to avoid, what time of year you see cases and where those cases are coming from.
People should be advised to avoid homes or backyards where parvovirus is known to have previously been present (the virus can survive several months to over a year). They should also avoid areas with large numbers of dogs (shelters/rescue centres/ puppy farms/ dog parks).
Clients should be counselled that even if they kept their puppy at home until it is 18-weeksof-age, they cannot totally eliminate the risk of parvovirus, as it can be tracked in on shoes or even carried by flies. This isolation will however set the dog up for poorer behavioural outcomes and an increased risk of anxiety and aggression later on. The possibility of picking up disease needs to be balanced against the risk of under-socialisation and subsequent long term behaviour issues. Confinement to the home is not an appropriate solution.
3. Car travel
y Travelling in a car is important to get used to; however clients should take care as many puppies get carsick, so short trips to fun places are best.
y Clients can have a ‘car boot party’ with their puppy. This involves creating a comfortable area in the boot of the car and hanging out there with the puppy, just watching the world go by. People can park near cafés, playgrounds or schools, and acclimatise to the sights and sounds of these places while still maintaining distance. An additional benefit is that while strangers may stop to say hello, in most cases they won’t climb into the boot with the puppy, so the

puppy is less likely to be forced into uncomfortable interactions.
4. Puppy schools
Well run puppy schools can be a wonderful place to socialise and inform new puppy families about behaviour. A 2013 study10 demonstrated that vaccinated puppies attending socialisation classes were at no greater risk of CPV infection than vaccinated puppies that did not attend those classes. Obviously, it’s particularly important to be aware of hygiene, especially when puppy classes are run in a veterinary clinic, and properly disinfect the area prior to running the classes. Puppies attending puppy classes should be between 8 and 12 weeks on the first day that they attend class, to ensure they are within their socialisation period.
If the risk of parvovirus is low, clients can bring puppies into the vet clinic to say hi, have a few treats and leave. Vet clinics’ waiting areas are regularly disinfected, and clients can be advised to carry puppies in if there is any concern about infectious diseases.
6. Off the ground
y Being carried: People sometimes carry puppies around in public so that they can see the sights without touching the ground. However, it is better to do this only when needed (for example from the car to vet clinic). Carrying is not an ideal way to facilitate socialisation, because it can cause frustration when the puppy can’t get down and explore, or fear when they can’t avoid something which they perceive is scary (like someone wanting to approach and pat them).
y Puppies can also travel in carry bags, or trolleys (in places like Bunnings), so that they can gain experiences but are off the ground. This has similar limitations as being carried in terms of the potential for frustration or fear, so caregivers must watch the puppy’s body language carefully and be prepared to actively change the environment (e.g. ask a stranger to keep their distance because the puppy is in training) if there is any concern.
y Prams or bike trailers are other options, but people must take the time to get the puppy comfortable with them first.
y People can put down a washable picnic rug and bring the puppy along to local sporting events, or parks. They could even set up an exercise pen with tarps on the ground if staying for a longer time.

1. George, A. M., Wille, M., Wang, J., Anderson, K., Cohen, S., Moselen, J., ... Barr, I. G. (2022). A novel and highly divergent Canine Distemper Virus lineage causing distemper in ferrets in Australia. Virology , 576, 117-126.
2. Wyllie, S. E., Kelman, M., & Ward, M. P. (2016). Epidemiology and clinical presentation of canine distemper disease in dogs and ferrets in Australia, 2006-2014. Australian Veterinary Journal , 94(7), 215-222. doi: 10.1111/avj.12457.
3. Decaro, N. (2021). Infectious Canine Hepatitis and Feline Adenovirus Infection. In Greene’s Infectious Diseases of the Dog and Cat (Fifth Edition).
4. Hornsey, S.J., Philibert, H., Godson, D.L. et al. Canine adenovirus type 1 causing neurological signs in a 5-week-old puppy. BMC Vet Res 15, 418 (2019).
5. Kelman, M., Ward, M. P., Barrs, V. R., & Norris, J. M. (2018). The geographic distribution and financial impact of canine parvovirus in Australia. Transboundary and Emerging Diseases , 65(1), e22-e31. doi: 10.1111/tbed.13022.
6. Kelman, M., Barrs, V. R., Norris, J. M., & Ward, M. P. (2020). Canine parvovirus prevention and prevalence: Veterinarian perceptions and behaviours. Preventive Veterinary Medicine , 174.
7. Horecka, K., Porter, S., Amirian, E. S., & Jefferson, E. (2020). A Decade of Treatment of Canine Parvovirus in an Animal Shelter: A Retrospective Study. Animals (Basel), 10(6), 939. doi: 10.3390/ ani10060939. PMCID: PMC7341501. PMID: 32485882.
8. Korbelik, J., Rand, J. S., & Morton, J. M. (2011). Comparison of early socialization practices used for litters of small-scale registered dog breeders and nonregistered dog breeders. Journal of the American Veterinary Medical Association , 239(8), 1090. doi: https://doi.org/10.2460/javma.239.8.1090.
7. Walking out and about Getting puppies used to walking on their own four paws in new environments is also important. Clients may have to drive to a low parvo risk area. They should walk puppies in areas where parvovirus will find it more difficult to survive. Roads and pavements are baked by the sun, don’t have as much organic material present, and it’s easier to visualise and avoid areas with obvious faecal contamination. Beaches below the tideline are another good example. If there are absolutely no low risk public areas, then accessing private areas (homes, properties, indoors and outdoors venues) with permission will at least provide some experience of novelty while walking on a leash (or exploring off-leash where this is safe and appropriate).
It is recommended that clients begin to socialise their puppies as soon as possible. Whilst some socialisation can take place within the home environment, puppies should also be given opportunities to learn about the outside world. This can be done in a way that minimises risk of disease, even before the primary vaccination series is complete.
9. Stepita, M. E., Bain, M. J., & Kass, P. H. (2013). Frequency of CPV infection in vaccinated puppies that attended puppy socialization classes. Journal of the American Animal Hospital Association, 49(2), 95-100. doi: 10.5326/JAAHA-MS-5825. Epub 2013 Jan 16. PMID: 23325595.
10. Day, M. J. (2017). Small animal vaccination: a practical guide for vets in the UK. In Practice - Clinical Practice. First published: 02 March 2017. https://doi.org/10.1136/inp.j615.
11. Howell, T. J., King, T., & Bennett, P. C. (2015). Puppy parties and beyond: the role of early age socialization practices on adult dog behavior. Veterinary Medicine : Research and Reports, 6, 143–153. doi: 10.2147/VMRR.S62081. PMCID: PMC6067676. PMID: 30101101.
12. Yu, Y., Wilson, B., Masters, S., van Rooy, D., & McGreevy, P. D. (2021). Mortality Resulting from Undesirable Behaviours in Dogs Aged Three Years and under Attending Primary-Care Veterinary Practices in Australia. Animals (Basel), 11(2), 493. doi: 10.3390/ ani11020493. PMCID: PMC7918417. PMID: 33668532.
13. Day, M. J., Horzinek, M. C., Schultz, R. D., Squires, R. A., & Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA). (2015). WSAVA Guidelines for the vaccination of dogs and cats. Veterinary Medicine: Research and Reports , 6, 143–153. doi: 10.2147/VMRR.S62081. PMCID: PMC6067676. PMID: 30101101.
14. AVSAB Position Statement on Puppy Socialization; https:// avsab.org/wp-content/uploads/2018/03/Puppy_Socialization_ Position_Statement_Download_-_10-3-14.pdf
15. APDTNZ Position statement Puppy Socialisation: Accessed September 2023 https://www.apdtnz.org.nz/positionstatement-puppy-socialisation-text
16. Altman KD, Kelman M, Ward MP. Are vaccine strain, type or administration protocol risk factors for canine parvovirus vaccine failure? Vet Microbiol. 2017 Oct;210:8-16. doi: 10.1016/j. vetmic.2017.08.019. Epub 2017 Aug 30. PMID: 29103701.
17. Chastant S, Mila H. Passive immune transfer in puppies. Anim Reprod Sci. 2019 Aug;207:162-170. doi: 10.1016/j. anireprosci.2019.06.012. Epub 2019 Jun 13. PMID: 31255495; PMCID: PMC7125514.
18. Graph: Accessed https://www.biogal.com/sin-categoria/pupprotection-the-effects-of-maternally-derived-antibody-mda/ 2/2/2024
Fergus Allerton, Koen Pouwels and John Buckell detail the use of discrete choice experiments for working out inferiority margins to guide antibiotic use decisions.
The One Health dangers from antimicrobial resistance (AMR) have been repeatedly highlighted amid calls for greater veterinary engagement with a wide variety of stewardship initiatives. Antibiotic over-prescribing is common in both human and veterinary medicine potentially driving antibiotic resistance without any benefit for the patient. The BSAVA/SAMSoc PROTECT ME poster (https://www.bsava.com/resources/veterinaryresources/protect-me/) was developed to try to guide prescribers to optimize their antimicrobial use. The recommendations within these guidelines are derived from the limited evidence available in the literature and consensus statements.
Robust clinical research is urgently required to provide support for this guidance. Ideally, randomized-controlled trials (the pinnacle of the evidence pyramid) should be pursued to establish the need (or lack thereof) for antibiotics in situations that are frequently encountered in veterinary practice (e.g., pets with acute diarrhoea or vomiting, prophylactic antibiotic use around surgical procedures) and to determine the optimal duration of antibiotic use (e.g., for sporadic cystitis). A potentially important route to reducing antibiotic use could be by shortening antibiotic course duration but any recommendation to do this must take into account any anticipated detrimental effects (e.g., under treatment, increased recurrence of infection).
In any study evaluating the impact of management with and without a treatment or with different treatment durations, a difference in outcome may be seen; however, when does a difference in outcome actually matter and justify the proposed treatment?
These differences are called non-inferiority margins and these help to assess whether and how treatment can be safely reduced. Until now, decisions about non-inferiority margins have been made by a small group of experts designing the trials, which is a pragmatic but suboptimal solution. It would be preferred to involve the end users when choosing the non-inferiority margin since ultimately it is their opinion that matters!
Instead of directly asking survey participants about their preferred non-inferiority margin, there is a large body of evidence that shows that discrete choice experiments (DCEs) better approximate true preferences
of respondents. Using DCEs we can estimate acceptable non-inferiority margins as well as the relative importance of (modifiable) facilitators and barriers of choice that are in line with best antibiotic stewardship. By running these DCEs in multiple countries we hope to identify the preferences of small animal veterinarians across the world. We use multiple DCEs in one survey to maximize the information gained from one study.
Discrete choice experiments (DCEs) are a research method for investigating the potential effects of policies prior to their implementation. Based on consumer choice theory, DCEs can be used to evaluate consumers’ stated preference for goods or services, for example a tobacco product, as a function of a set of different attributes. E-cigarette attributes might include flavours, nicotine concentrations, device characteristics, and warning labels.
Using an experimental design, consumers are asked to choose between two or more products, with the levels of the different attributes varying across several choice trials. How different levels of the attributes affect the choices is then analysed to determine preferences. This information can then be used to estimate the effects of different policies on tobacco product choices, such as banning menthol from all tobacco products versus only from cigarettes
Please help with a DCE that is integral to the study: Estimating acceptable Non-InFerioriTY margins for antibiotic stewardship interventions using discrete Choice exPErimenTS (NIFTY PETS).
Open to small animal veterinary practitioners worldwide
Translations are available for Portuguese and Spanish with more to come. Your answers will help researchers to interpret their results and guide developers to make recommendations that are acceptable to vets in practice (and thus hopefully adhered to!).
Thank You for your input
The more responses obtained, the greater the confidence in the study findings. This is your chance to dictate what you consider an acceptable cost.
Please also share the link with your colleagues around the world.
Sally Upham
Aberdeen Vet Clinic
Geelong
e. abervet174@gmail.com
C&T No. 6038
Clyde is an 8-year-old Male neutered British Shorthaired cat. He was treated for ‘ringworm’ as a 3-month-old kitten with 6 weeks of griseofulvin PO and Malaseb washes. He also had multiple episodes of conjunctivitis that resolved with Tricin ointment. He had been wormed and vaccinated regularly as per usual protocols.
When he was 6 years and 8-months-old, he presented with moderate gingivitis and had his upper and lower right carnassial teeth removed and right upper second molar removed. He was given post-op meloxicam and Betamox injections.

Three months later, he was seen at another vet clinic for weight loss. Bloods were taken and revealed normal biochemistry with band neutrophilia (left shift) and monocytosis. Urine was normal. He had lost 1.5kg since the dental. He was given a Convenia injection.
Two weeks later, his weight had increased by 700 grams, but he was now PU/PD and polyphagic. A grade 2/5 heart murmur was audible. Respiratory rate was normal, lung auscultation was clear, mucous membranes were pink and CRT <2. Another blood test was performed, and results were all within normal limits.
Three weeks later, the owner reported that Clyde was still polyphagic. He was being fed kitten food ad lib but was not gaining weight. His faeces were reported to be solid. His weight had decreased by 300gms. His coat was dry and in poor condition (Figure 1). Rectal temperature was normal at 38.4°C. FIV and FeLV snap blood tests were performed, and both were negative. Lateral and VD truncal radiographs were taken, and the chest appeared normal; however, peritoneal effusion was evident on both radiographs. A presumptive diagnosis of FIP was made.
Clyde was referred to a referral specialist clinic. It was decided to cease vaccinations until further notice. Clyde’s blood test revealed an elevated white cell count with neutrophilia, monocytosis and suspected bands.
Clyde was immediately started on remdesivir 90mg diluted in 100mL saline which was given SC daily for 7 days. After the week, he was given a GS-441454 50mg tablet once daily at least 1 hour before food for 84 days. His weight at the start of treatment was 4.66kg. The day of the last remdesivir injection, Clyde’s weight had increased slightly to 4.77kg. Eight days after starting the GS-441524 tablets, Clyde’s weight had increased to 5.16 kg. He was clinically very happy and gaining weight. His blood profile was within normal limits. Four days later, his weight had increased again to 5.4kg.
A week later, Clyde was presented for follow up bloods and radiographs. The blood profile was essentially normal.
Lateral and VD radiographs showed a clear chest as previously observed. There was much less abdominal fluid, although a granular pattern was still evident. We increased the GS-441524 medication to 10mg/kg twice daily.
Two weeks later, repeat radiographs showed a slight granular pattern in the abdomen. The GS-441524 was continued for another 4 weeks until it was discontinued.
Clyde was doing well at home. However, 6-months later he presented for lethargy. His weight was 5.23kg and rectal temperature was 39.5oC. No other significant clinical signs were evident. Blood was taken and the haematology showed neutrophilia with a left shift and some bands. Monocytes were within normal limits. Differential diagnoses were an acute infection or recurrent FIP. Noroclav was given for 6 days. When Clyde was presented 6 days later, his rectal temperature was 37.7 oC and he was much happier in himself. Repeat bloods showed a mature neutrophilia and a slight elevation of SDMA. He was given another 10 days of Noroclav PO.
Two weeks later, repeat bloods showed a normal WCC; however, a mild neutrophilia was present. His upper left canine and carnassial teeth had moderate-severe periodontitis. Noroclav was given again, and a dental procedure was performed a week later. Both the UL carnassial and UL canine teeth were removed. Loxicom and Noroclav injections were given post-op and another 5 days of Noroclav tablets was continued at home. As a consequence of upper canine removal, the lower canine caused an ulcer to develop in the opposing upper left lip region. Two weeks of Norclav tablets and oral Loxicom were given. The ulcer healed well.

capsule on each meal.
Ten days later, Clyde presented for weight loss despite a ravenous appetite. He was otherwise well in himself with only occasional vomiting. He was reported to have no diarrhoea and the owner was not sure when he was last wormed. His weight had plummeted to 4.16kg. His rectal temperature was 38.3oC. A grade 2-3 heart murmur was audible. No thyroid goitre was palpable. Blood was taken and was within normal limits.
Clyde was wormed with Milbemax and was given a Convenia injection. The owner was instructed to feed a high calorie kitten food. An ultrasound was performed to investigate Clyde’s heart murmur. The cause of the heart murmur was not confidently identified. It was possibly due to mild/minimal mitral regurgitation. He was not in congestive heart failure and hence the weight loss is not of cardiac origin. Abdominal ultrasound was unremarkable.
Two weeks later, Clyde’s weight had continued to decrease and was now 4.06kg. Again, he was clinically happy with no concerns other than a ravenous appetite and continued weight loss.
At this point our differential diagnoses were not a FIPrelated illness as the blood tests were not indicative of this. Also, hyperthyroidism was ruled out as the TT4 was low normal and no goitre was palpable. Diabetes was also ruled out based on blood and urine tests.
A week later, his weight had only increased by 70gms. Another week later, his weight had increased by 500gms. His coat was shinier, and he had started playing with his toys again. At this consult, the owner informed me that Clyde s faeces had improved in its bulk and consistency.
We decided to cease the Creon for up to a week and to monitor Clyde’s faeces. Within a few days, the faeces had become sloppy, pale yellow and was malodorous.
A diagnosis of EPI was made based on medical trial. Clyde was given a final vitamin B12 injection and was then started on oral vitamin B12 in conjunction with Creon. The Synbiotic D-C capsules were discontinued.
One month later, Clyde has continued to thrive. His weight is stable, and his faeces are more often than not solid and normal. He will be changing from Hills z/d food onto low fat food. Some cats concurrently have EPI and IBD so Clyde will be put back onto Hills z/d if his stools and weight reflect this.
This cat has caused significant scratching of our heads, and we are relieved to have discovered the cause of his recent weight loss. Having had FIP in the past, we were concerned that his recent illness was an underlying long-term complication of FIP. However, the EPI diagnosis appears to be unrelated and just a case of bad luck for Clyde. To definitively diagnose EPI, a trypsin-like immunoreactivity test can be performed; however, common sense can sometimes prevail and make a diagnosis obvious without the need for expensive and delayed laboratory testing such as in this case
C&T No. 6039
This contributer has kindly donated the prize voucher. See page 35.
Here is a small selection of my cautionary tales with a few images sourced from the web to show us how the internet thinks things should be done.
1. Injecting tiny kittens
Get that angle right. Microchips going missing in fur and vaccinations squirting out the other side— embarrassing. I still do this one from time to time.
1. Kitten vaccinations—I reckon this one’s going out the other side. Image source https://www.whiskas. com.ph/care/kitten/health/cat-vaccination-benefits-andschedule
2. Anal sac expression (Figure 2a-c)
Another case of getting the angle right. I have had multiple cases of anal sac contents all over my face and in my hair in spite of having a tissue or swabs in hand to catch it. Hilarious for colleagues but not nice to drive home with.
3. Removing IV catheter dressings
I can’t explain how this happened, but I must have been rushing, and probably chatting. Using bandage scissors to remove Elastoplast I neatly snipped the joint between the catheter and its hub. I don’t know where the catheter went and this was before we had advanced imaging, but thankfully the dog lived many years without any complications before dying from natural causes.
4. Clipping cats
If possible, leave it to an expert (usually a nurse). Even with the utmost care, it’s easy to nick the skin in the axillary area and anywhere really.
5. Check the use-by-date on vaccines and medications even if its someone else’s job
In spite of all the debacles listed and no adverse effect on the patient, this is the one that had the biggest repercussions in terms of owner outrage.



6. Double check cages are shut properly at top and bottom
I haven’t personally seen this one but I have heard some terrible stories about dogs getting stuck halfway out of cages.
7. IV sedation for large animals
A self-preserving section of my brain is preventing me from remembering this clearly, but I guess the message is that when giving IV sedation to large animals sometimes they get excited before they get sedated and run around where you would prefer they didn’t

The Vet Hub 104 Warrandyte Road
Ringwood Vic 3134 e. clinic@vethub3134.com.au
C&T No. 6040
2-year-old male neutered Siberian Forest cat.
8. Communication with various family members
Never assume they actually talk to each other. We had an incident where a dog was euthanised in hospital and the teenaged daughters turned up soon after to see the dog. While the (deceased) dog was being stretchered out to them, we suddenly twigged that the kids might not know their pet was dead—they didn’t.
9. Pugs and nails = bad
Advise the nurse holding them to be careful—it doesn’t take much restraint for some of them to turn a worrying shade of hypoxia. Another adverse pug event I experienced was trying to get a fine needle aspirate from a lump on top of the head but upon light restraint, the pug lurched upward, and the needle went straight into its bulging globe. Maybe the lesson for me is to sedate most pugs for most things. Fortunately, the eye was fine—the needle must have just gone very cleanly and briefly straight in and out and luckily it was a very fine needle!

New client and patient, history of ongoing pasty diarrhoea and flatulence for up to 2 weeks. Initially one day of lethargy, then he became bright in himself with normal appetite and no vomiting. No straining was seen when passing diarrhoea, and no blood was present in stools.
The patient had presented for a similar episode at another GP veterinary clinic 6-months prior, where he was treated with Hill’s prescription diet food sensitivities z/d and probiotics that the owner kept at home. Diarrhoea resolved.
Regular diet: mix of raw and cooked foods, and supermarket wet food.
Outdoor access: indoors and outdoors cat, outdoors access limited to garden. Weedkiller was used in the garden recently, but the owners cleaned the cat before he could groom himself and ingest poison.
Anthelmintics: up to date, wormed recently.
Vaccinations: up to date on F3.
Companion animals: no other pets.
Bright and alert, nervous. Mucous membranes pink, moist, CRT<2s. Heart and lung sounds normal with no audible murmur, HR 160bpm and RR 48bpm. Bodyweight 4.4kg, scored as 4/9. Dental condition great, grade 0/4. Abdominal palpation showed mild intestinal thickening and gas distension, but overall, he was comfortable. Rectal exam was not performed.
Figure 1. Tritrichomonas foetus, stained with Lugol’s iodine. Three anterior flagellae (‘tri’) can be seen, and an undulating membrane runs the length of the body; the trophozoites of Giardia spp. do not have the undulating membrane (Image courtesy https://www.abcdcatsvets.org/guidelinefor-tritrichomoniasis)
Given the young age of the cat, presentation and recurrent diarrhoea, a feline faecal multiplex PCR panel was recommended.
Results were positive for Tritrichomonas foetus, Giardia spp and Feline Coronavirus RNA.
We initially prescribed Metronidazole 25mg PO BID for 5 days while awaiting results. Faecal quality improved with metronidazole treatment, and then diarrhoea recurred when the antibiotics were finished. The owners were instructed to also give Hill’s gastrointestinal care diet i/d throughout treatment period. The patient did not find this formulation very palatable, so the owners ended up giving a mix of i/d diet and supermarket wet food. Raw food diet was ceased during the treatment period and pending results.
Once the test results were received, he was started on ronidazole 30mg/kg PO SID for 14 days to treat Tritrichomoniasis

The owners were advised that this is an off-label use for this medication for cats, and they will need to monitor closely for any side-effects including neurological symptoms. Concurrently to Ronidazole, the owners were instructed to continue i/d diet and probiotics. They were also educated on hygiene and cleaning litter trays thoroughly and keeping the patient clean and avoiding interaction with other cats (e.g. catteries, cat shows) due to the infectious nature of condition.
Diarrhoea improved with Ronidazole treatment over the 14-day period, and then recurred. Assuming that the Giardia had not yet resolved, Drontal® (praziquantel and pyrantel) oral tablets were given PO SID for 4 days. A repeat faecal multiplex PCR was also performed.
The second PCR results showed negative detection for both Tritrichomonas foetus and Giardia spp. Feline coronavirus RNA was still detected, although it was determined to be not significant.
Currently, the patient has intermittent softer quality stools but overall improved faecal quality and no diarrhoea. He continues to have probiotics and digestive care food mixed in with regular food. The risk of raw food and bacterial loads was discussed with owners as a poor gastrointestinal condition may predispose this patient to further episodes of diarrhoea, and they will reconsider whether they will continue a raw food diet for their feline companion. Faecal quality will be monitored over time to ensure any lasting effects from the extended period of gastroenteritis is well monitored and controlled.
Unpublished information suggests some (by no mean all) cats with positive PCR for feline enteric Coronavirus can benefit from a 5-day course of GS-441524 at 8 mg/kg once daily for 4 days
We offered a $200 CVE voucher to the first person who contributed their 'Cautionery Tail' for the March 2024 issue as we hope to make it a regular column. Terry King generously donated the prize to the next contributer, as did the anonymous auther in this issue. So, we've upped the ante and increased the voucher to $500!
cve.edu.au +61 2 9351 7979
With our compliments CVE$500
Use the voucher towards memberships (Individual $420, Part-time and Recent graduates $210, Vet Nurse $65, eMember $95. Practice Membership: Small $665, Medium $815 and Large $1,360) or enrol in a CVE course.
Jeremy Rogers
Strathalbyn, SA.
e. jhrog@optusnet.com.au
C&T No. 6041
Introduction
Waste or by-products from manufacturing are often very useful in farming systems, but producers should be aware of potential risks. Some lambs died on irrigated pasture apparently after drinking water from the irrigation system that contained acids used for cleaning vats and pipework. Denying access to the water directly with some electric fencing seemed to have fixed the problem, and both the source of the water and the producer are now better informed of the risks.
History
Seven lambs were found dead in an irrigated paddock adjacent to a winery. When a moribund lamb was found, an autopsy was performed in order to determine causes of morbidity and mortality. These 6–8-week-old lambs had been mulesed and marked about 5 days previously, but appeared to have recovered well, then were found dead in the irrigated paddock.
The main postmortem finding was significant haemorrhage and damage to abomasum, small intestine and caecum, with frank blood in abomasum, digested blood in intestines, indicative of a toxic insult to these tissues. Other organs appeared relatively normal.
Liver: Necrosis and degeneration, centrilobular and paracentral, multifocal, moderate to severe with cholestasis
Kidney: Moderate acute tubular degeneration and proteinosis
Gastrointestinal tract: Minimal acute erosive and ulcerative gastroenteritis
Comments
Liver and kidney findings are suggestive of toxic pathology. Certain chemicals/toxins (e.g. Fumonisin mycotoxin produced by Fusarium, Amanita mushrooms,
Averrhoea carambola, kerosene/petrol) which are both hepatotoxic and nephrotoxic can potentially cause GI irritation.
Discussion
Although a specific toxin was not identified in this case, the evidence is that the damage occurred only in lambs that had access to contaminated water from a winery. This water is used by the producer to irrigate and grow forage crops.
Immediately on learning the result of the necropsy, the producer fenced off an area where lambs could access water from this source, and no further deaths occurred. In checking with the winery, it was confirmed that vats are washed with citric acid, and sometimes phosphoric acid compounds, and it is likely that this wastewater was consumed by affected lambs.
Wastewater from processing is a valuable resource in many SA properties but producers should be aware of risks here, such as occurred in this case. Simply ensuring that livestock do not have direct access is often sufficient.
Other cases like this in the past involving waste milk and water and deaths in calves, cows and lambs have been documented in similar circumstances.
In situations like this, there is a temptation to blame common causes of sudden death in lambs post marking, (for example ‘pulpy kidney’) without a proper investigation. However, in this case, there was rapid identification of the cause, and a solution, that prevented further deaths.
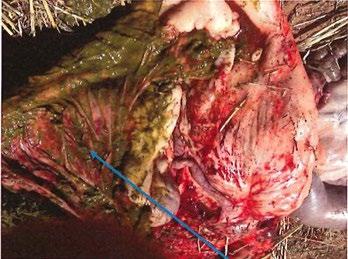




Stephen McClintock Retired
e. samcclintock@bigpond.com
C&T No. 6042
Since I stopped doing heroic things like colic surgery 20 years ago, it is not often in my job that I get to say that I have saved a horse’s life in a matter of minutes.
Maybe getting a live foal out of a mare in trouble is the most common scenario and that doesn’t happen all that often nowadays. But.........
Monday after dinner I went to see ‘Ed’, a pet pony belonging to a lovely Mt Hunter family. My wife had taken the phone call and it didn’t sound like a desperate emergency, so I really didn’t rush but scoffed down dinner quickly and took off.
Imagine my surprise when I got there and immediately realised that he could not breathe and was literally dying before our eyes. I told the owners that I had to do a tracheotomy or he was going to die very soon. They had that bewildered ‘deer in the headlights’ look on their faces but agreed. A quick sedation, prep, local anaesthetic and a few minutes later I had the tube in. One owner squatting holding a torch and the other holding his head straight and still.
You cannot imagine how rewarding it was to see Ed take those first few grateful big breaths. It must be a terrible feeling to be dying of suffocation and it surely must be the most amazing feeling to suddenly breathe again!
So now what was causing the obstruction to his airway?
Clearly, he had a blockage at his larynx but with such an acute onset it was hard to imagine what it would be.
I have a scope in the truck but I thought he would need hospital care anyway, so we loaded him up and choofed him off to Agnes Banks Equine. I followed the float to The Oaks and I could see his little ears pricked above the top of the back door so I was pretty sure he would make it there ok. The vet on duty at the hospital scoped him and found—wait for it—you will never guess—a mango seed perfectly and firmly lodged in his larynx!!!!!!!
Miraculously, she was able to grip it with some sharp forceps and pull it back out his nose. I couldn’t believe it fit. Fantastic job by the vet and staff. I caught up with the owner yesterday and she told me that driving back from the hospital late that night she and her partner had that ‘what just happened feeling’. I am sure they did.
Ed (his owners have unkindly nick-named him ‘Mango’—I told her not to call him that to his face—too soon!) will stay in hospital a few days but should make a full recovery.
Stick to grass, pellets and hay mate

Daniel R James BVSc(Hons) GCertBiofab
MANZCVS(Radiology) FANZCVS(Surgery)
Specialist in Small Animal Surgery
Sydney Veterinary Emergency & Specialists 675 Botany Rd, Rosebery NSW 2018 t. 9197 5800 e. djam3485@gmail.com
w. sydneyvetspecialists.com.au
Perspective No. 163
There is an old adage amongst veterinarians that our small animal patients have ‘three legs and a spare’ and most of us will have been impressed by a kelpie herding sheep minus a limb or similar remarkable feats at some stage in our career.
Amputations are frequently performed not only for the appropriate management of neoplasia but also as a financially appealing alternative to repair of traumatic injuries. This has resulted in many vets becoming quite blasé about the procedure.
Although it seems intuitive to owners that avoiding full limb amputation would be considered desirable, psychological concerns and aesthetics are not a consideration in pet patients as they are in humans, and dogs do actually have four limbs after all… There are, however, several evidence-based reasons why it is advantageous to maintain or re-establish the normal quadruped structure rather than amputating whenever possible.1
Major complications of amputation include wound dehiscence, pneumonia, contralateral limb arthrosis, haemorrhage, dehiscence, and surgical site infection.2 In a study of 67 patients (39 dogs and 28 cats), wound inflammation complications occurred in 20.9% of cases and wound infection complications in 9% (12.8% in dogs and 3.6% in cats).3
Kinetic and kinematic analyses of dogs who received total limb amputations reveal significant alterations to locomotive biomechanics.4-6 Such gait alterations can have deleterious effects on joints, muscles and vertebral columns leading to other quality of life issues associated
with chronic pain.1 Interestingly, while owner surveys published in the veterinary literature often indicate owner satisfaction with total limb amputation, owners still report negative changes to their dog’s mobility, attitude, and quality of life.7, 8 In one study, 27% of owners reported a change in their dog’s recreational activities, 42% reported challenges negotiating stairs and 23% could not return to pre-amputation walking routines among other decreases in wellbeing.7 Clearly, when more than one limb requires amputation, the consequences are exponentially higher.
Humans suffer from an incompletely understood array of (frequently debilitating) amputation-associated pain syndromes involving central and peripheral nervous systems as well as psychological components. While these phenomena were once considered rare in veterinary patients, recently published studies from The Canine Postamputation Pain (CAMPPAIN) initiative suggest that greater than one third of dogs may be showing specific behaviours suggestive of postamputation pain.9
To address the potential concerns associated with full limb amputation, some groups have been working on the application of socket prostheses to animals.10, 11 Developments in materials and design capabilities will continue to improve the efficacy of such devices; however, it is apparent (having been a member of social media amputee support groups for people over the last year) that they are often associated with significant morbidity in humans. Our patients have greater limitations on fit due to anatomy, and particularly due to hair growth as a suction effect is important for maintaining attachment in many devices. This makes them inherently less suited to socket usage.
Recent studies on socket prostheses use in companion animals report high complication rates: 9 complications, predominantly pressure sores and wound complications, were seen in 12 patients in one study.10 Another identified 62% short-term and a 19% long-term complication rate, predominantly skin sores, from 47 cases.11


In the absence of a functional prosthetic device, once the weight-bearing axis is lost, amputation of the whole limb by disarticulation at the hip or removal of the scapula is typically found to be the preferred management strategy. This is generally the most aesthetic and functional approach as it minimises bony prominences and eccentrically placed body mass.
In humans, the complications associated with socket prosthetic use are now increasingly being managed by the implantation of osseointegrated transcutaneous amputation prostheses (OTAP).
Many anecdotes of return to independence and activity from OTAP patients have made mainstream and social media but these outcomes are also supported in individual studies12 and confirmed in metanalyses. 13 The largest group operating in this field world-wide is Osseointegration International (OI), based in Sydney; however, cases only passed 1,000 in number a couple of years ago which is substantially below the potential population who could benefit. It’s not currently a firstline treatment for human amputees in most situations and the inherent lifelong infection risk associated with a trans-cutaneous implant will remain an important consideration for candidate patients.
While osseointegration of titanium implants into bone is well established and achieved with acceptable complication rates in human and veterinary medicine for joint replacement, the ‘transcutaneous’ component of OTAP is somewhat of a holy grail to master and something quite at odds with the experience of most veterinarians familiar with external fixation surgery. While a vetclient conversation ahead of placement of an external fixator frame is likely to discuss an inevitable endpoint where patient tissue reactions necessitate removal of transcutaneous implants, in the case of OTAP the expectation is of ‘managing’ a transcutaneous implant through the remainder of the patient’s life.

Figure 2. Osseointegrated transcutaneous amputation prosthesis
From Drygas, K.A., et al., Transcutaneous tibial implants: a surgical procedure for restoring ambulation after amputation of the distal aspect of the tibia in a dog. Vet Surg, 2008. 37(4): p. 322-7. Note porous tantalum skin on-growth surface.
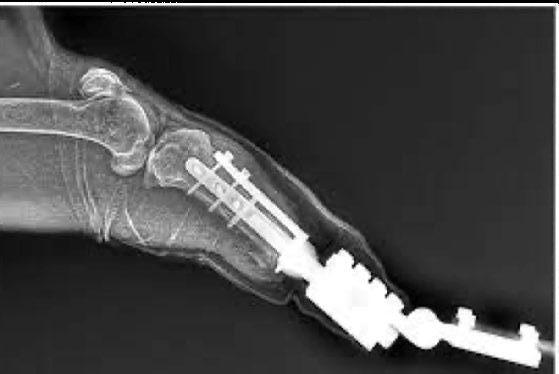
3. Radiographs of osseointegrated transcutaneous amputation prostheses from an Arabian Tahr (above)
From Golachowski, A., et al., Implantation of an Intraosseous Transcutaneous Amputation Prosthesis
Restoring Ambulation After Amputation of the Distal Aspect of the Left Tibia in an Arabian Tahr (Arabitragus jayakari). Front Vet Sci, 2019. 6: p. 182.
It is important to mention dental implants at this time: Seemingly because of the unique nature of gingival tissue, osseointegrated transgingival dental prostheses are a well-established and widely practiced treatment. The reality with transcutaneous implants, however, is that there remains little consensus even on the foundation ‘ethos’ of the desired nature of this interface.
Professor Noel Fitzpatrick is the leader in veterinary osseointegration surgery, having first published a small case series over a decade ago.14 The Fitzpatrick approach to managing the transcutaneous component mirrors the traditional status quo in humans and is to use a highly porous implant with the aim of achieving adherence of skin and a ‘seal’. Research continues in experimental animals looking to achieve such a barrier to micro-organisms through adhesion as a model for humans.15-17 Interestingly, the OI group approach is markedly different. The transcutaneous component of the OI implant is smooth and does not attempt to achieve any degree of adherence to the skin. Conversely, the aim is to achieve a healthy stoma as with other long term ‘ostomies’ such as gastrostomy tubes. A stoma is inhabited by commensal flora which provide resistance to infection with pathologic bacteria.
Humans with a ‘stoma type’ OTAP frequently have a persistent serous discharge in association with a healthy, functional stoma. While this appears to be well tolerated and easily managed in the human scenario, it is of much bigger concern in dogs and cats with their inclination for self-trauma through licking.
OTAP utilising the resources of Osseointegration International has now been performed on a small number of veterinary patients in Sydney with very pleasing early follow-up. Improvements in implants and technique
are ongoing; however, initial patients have shown exceptionally good performance and stoma management has been fairly low maintenance in those operated on so far. We are obtaining objective data including force plate analysis that will deliver measurable outcomes in time. The prolonged bandaging required to prevent licking in veterinary patients creates an imperfect environment for stoma health. This is being addressed using a tongueproof 3D printed ‘basket’ for use in the perioperative period with good effect. There is a fundamental fact that, unlike most veterinary orthopaedic procedures that have a perioperative complication risk, the presence of a stoma means that some attention will likely be required forever. We are hopeful that this will be routine observation and occasional superficial infection responsive to antibiotics as is the commonest scenario in humans;18 however, the potential for deep infection necessitating explanation long term must be considered. Experimental studies have typically measured only short-term outcomes.
In a sheep study19 with sacrifice at 16 weeks, there was no histological evidence of infection and none of the transcutaneous adapters failed.19 Bone-implant osseointegration was demonstrated in 2 of 3 limbs that underwent histological analysis and all animals immediately ambulated after the initial surgery.19 A subsequent sheep study showed 75-80% weight bearing (relative to contralateral) through 12 months of observations.20 In another experimental study of OTAP using 2 cats, both implanted animals demonstrated stable prosthetic walking with normal speeds until sacrifice at 21 weeks.15 The cats did not have clinical signs of skin infection, distress or pain, had normal temperature, white blood cell count and appetite. No indications of bone lysis, fracture or implant loosening could be found in X-ray images of the residual limb for the duration of the study.15 Another study of 4 cats by the same group found pain-free limbs with pleasing limb use at 4 to 6 months post OTAP placement.21
Clinical studies are similarly short-term. Fitzpatrick’s paper followed 4 patients until death at up to 17 months post-op with favourable performance until that time.14 One dog treated with bilateral tibial OTAP and followed for greater than 2 years in an older case report22 required revision of one side due to aseptic loosening at 14 months but recovered well. A single Arabian Tahr was reported to have low grade lameness and to have returned to a breeding program in another case report at 6 months post OTAP..23
With positive outcomes approaching 3 years postsurgery in the early patients, we are now able to provide

From Golachowski, A., et al., implantation of an intraosseous transcutaneous amputation prosthesis restoring ambulation after amputation of the distal aspect of the left tibia in an Arabian Tahr (Arabitragus jayakari). Front Vet Sci, 2019. 6: p. 182.
some veterinary-derived prognostic information for clients seeking OTAP for their pets and can more comfortably offer this procedure for clinical use. Scale will bring important benefits in terms of implant cost and particularly availability as a lack of off-the-shelf options had prevented OTAP usage in what will likely be its biggest role (long term), amputation for neoplasia. Distal radial osteosarcoma, in particular, is a common presentation for amputation but neither a two-stage procedure or delayed surgery as required for bespoke implant design and manufacture are considered appropriate for these patients. Multiple implants in a range of sizes are now available for immediate implantation.
Refinement of implant design and surgical technique, especially regarding stoma fashioning will likely be ongoing for some time. With some commitment to stoma care, though, we now have the ability to keep patients walking on four limbs when they’d historically be left on three or keep them alive when two or more limbs have been compromised and they’d normally be euthanized, and that is exciting.
The cases managed by the author have been done with ethics approval from the Secretary’s Animal Care & Ethics Committee Department of Regional NSW, NSW Department of Primary Industries Chief Scientist Branch, Locked Bag 21, ORANGE NSW 2800.
Sunday was a 2-year-old Rottweiler who lost her left pelvic limb distal to mid tarsus following maternal trauma as a neonate. She was taken on by a canine rehab professional who experimented with an array of different custom socket prostheses over her early life hoping to provide some function and avoid overloading other limbs. The limited function afforded by the prosthetics and her apparent discomfort (she eventually refused to allow them to be attached) meant that prosthetic use was abandoned by the time she was a year old.

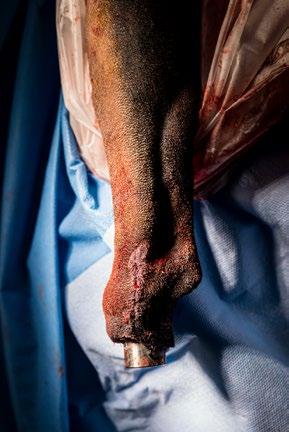
Sunday’s owner was very aware of OTAP in humans and, after long and deep discussions and much consideration about the many unknowns in dogs, decided to proceed with surgery.
Sunday’s exercise was limited to swimming performed at her owner’s rehabilitation centre and brief walks on soft surfaces. She still swung her limb when walking and it would touch the ground when playing on the grass, so she appeared likely to use it if a functional ‘foot’ could be attached.

A CT was performed for templating and revealed the remarkable difference in tibial growth pattern between the weight bearing and non-weight bearing sides. The tibia grew significantly longer, narrower and with thinner cortices versus the contralateral limb, providing challenges for implant design (See Figure 7).
Two orthopods and a biomechanical engineer were present along with a big vet team to assist in Sunday’s surgery. In many ways the technique is familiar to surgeons performing regular hip replacement, but alignment is much simpler (no joint to luxate) and the implant is placed much tighter to grip the cortical endosteum without the natural flare of the proximal
Threedimensional reconstruction of a computed tomography scan obtained for planning of osseointegrated transcutaneous amputation prosthesis surgery.
Note the marked difference in tibial development between limbs.



femur to hold the implant as per a hip prosthesis. Fashioning the stoma from the soft tissue closure is very important also and a less familiar process.
Function was remarkably good from early on, starting with a small rubber stopper placed on the protruding interface Day 2 after surgery. Limb use went from strength to strength with subsequent iterations of the external attachments. The fate of the soft tissues of the stoma was varied with moderate skin necrosis in areas. The skin margin retracted back from the smooth surface of the planned trans-cutaneous component over time which will be a reason for re-evaluating design and technique in the future.
Sunday’s stoma health is monitored closely and she’s had one important infection that resolved with culture based antimicrobial therapy. A lot of different custom designed, 3D printed limb designs have been trialled with the most important feature seemingly to have significant padding on the contact surface—she doesn’t like the jarring going straight into her bone.




Sunday walks regularly and has great quality of life almost 3 years post-surgery, but the presence of a stoma means this will never be a set-and-forget procedure.
Holly is a patient that would traditionally have been euthanised. Following motor vehicle trauma (and intensive care) debridement of necrotic, avascular tissue from both pelvic limbs left her with amputation at distal tibial diaphysis.
Cats ask a lot of an implant with their ‘untamed’ behaviour and three-dimensional lifestyle, and their tibial canal size is small. For these reasons, Holly’s implants were manufactured in chrome cobalt, a stronger material than titanium. Titanium was then ‘plasma sprayed’ on the outer surface to facilitate osseointegration.
Holly’s surgery went very well. The prosthesis had a scarily tight fit as the feline tibia widens from distal to proximal which is undesirable for holding the implant and facilitating osseointegration. Cortical fissuring was observed during impaction but, unlike in hip replacement, cerclage wires were not placed to protect from fissure propagation because maintaining the integrity of the soft tissue envelope around the bone is considered paramount to success. Her limb use has been remarkable from the beginning and has become increasingly normal looking as the external prosthetic length has been increased.


14. Holly, a domestic shorthair cat and osseointegrated transcutaneous amputation prosthesis recipient, and friend.
After photos, at 26 seconds into the video, you can watch Holly’s remarkable progress after the surgery at Days 5, 23, 33 & 70.
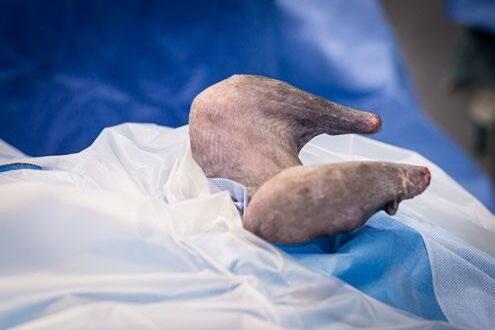
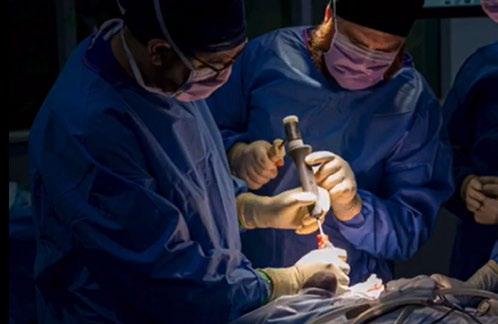

15 A,
photographs
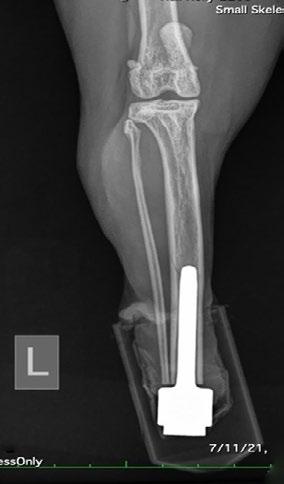

Figure 17 A & B. Examples of custom 3D printed foot attachments used on Holly, a domestic shorthair cat and osseointegrated transcutaneous amputation prosthesis recipient. Note the ‘basket’ component to facilitate airflow while preventing self-trauma and the rubberised contact points to minimise percussive forces travelling up the limb.


Note retraction of the skin from the planned polished transcutaneous component.
Figure 16. Post-operative radiograph from osseointegrated transcutaneous amputation prosthesis surgery on Holly, a domestic shorthair cat
She runs, hunt bugs, climbs stairs easily and can even scale her cat tree so life is good at more than nearly 3 years post-op.
Serenade is a 5-year-old, retired show star who had partial amputation of the pes to manage marked necrosis blamed on white-tailed spider envenomation. Unfortunately, her tiny Dachshund limb proved difficult to attach a socket prosthetic to, and her extreme sausage-like conformation made effective ambulation difficult so her owner (along with a big support group from the Dachshund community) sought OTAP surgery for her.
The major limitation in Serenade’s case was very small bone size and marked breed-associated recurvatum of the tibia. We had to first straighten the bone to have any chance of placing an OTAP stem.
There are many ways described for straightening bones in small animals including external linear and circular fixation and internal fixation with plates and screws or interlocking nails (ILN). With our priorities being to create a straight canal, and to minimise trauma to the periosseous soft tissues, ILN was the approach of choice. Serenade had an opening wedge osteotomy performed and stabilised with a 3mm Biomedtrix I-Loc and the deficit was packed with demineralised bone matrix. Healing was unremarkable.
Upon eventual arrival of a new generation titanium stem, just 4mm in diameter, Serenade underwent successful implantation into her now straight tibial medulla.
Post-surgery, Serenade has shown good skin healing and we’ve seen symmetry of weight bearing on force plate treadmill analysis even with quite unsophisticated prosthetic feet. Her first stoma infection was identified eleven months post-surgery and has responded well to appropriate culture-directed antibiotics.

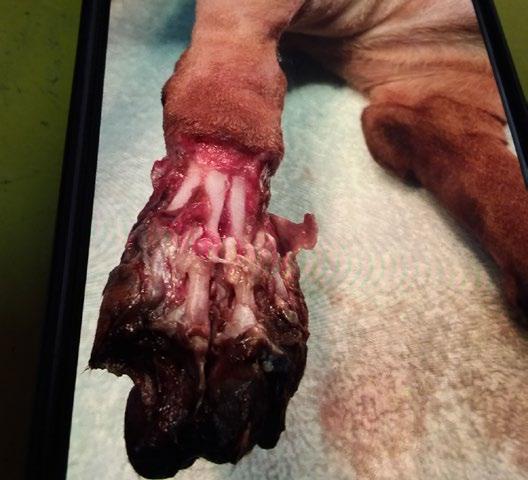

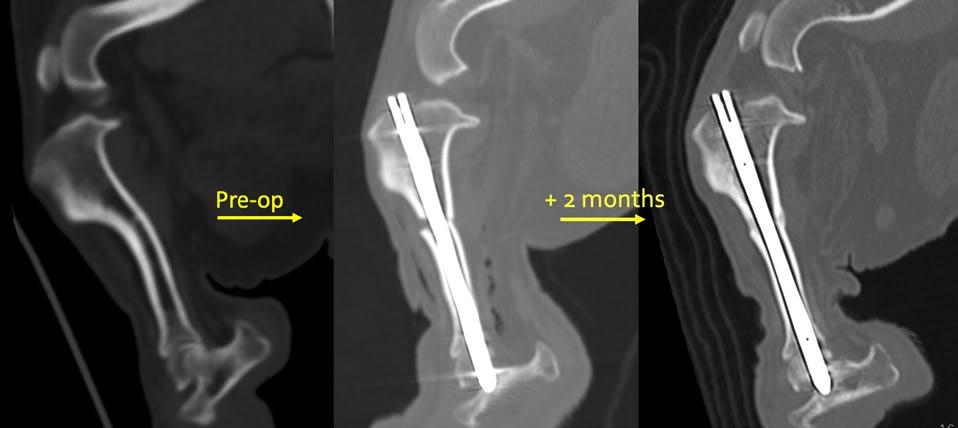




1. Borghese, I.F., L; Kaufmann, M; Mich, P, Assistive Devices, Orthotics, Prosthetics and Bandaging, in Canine Sports Medicine and Rehabilitation, J.B.V.D. N C Zink, Editor. 2015, John Wiley and Sons: Iowa. p. 201-222.
2. Seguin B, W.J., Amputations., in Veterinary Surgery: Small Animal . , J.A. Tobias KM, Editor. 2012, Elsevier: St. Louis. p. 1029-1036.
3. Raske, M., J.K. McClaran, and A. Mariano, Short-term wound complications and predictive variables for complication after limb amputation in dogs and cats. J Small Anim Pract 2015 56(4): p. 247-52.
4. Cole, G.L. and D. Millis, The effect of limb amputation on standing weight distribution in the remaining three limbs in dogs. Vet Comp Orthop Traumatol , 2017. 30(1): p. 59-61.
5. Hogy, S.M., et al., Kinematic and kinetic analysis of dogs during trotting after amputation of a pelvic limb. Am J Vet Res, 2013. 74(9): p. 1164-71.
6. Jarvis, S.L., et al., Kinematic and kinetic analysis of dogs during trotting after amputation of a thoracic limb. Am J Vet Res, 2013. 74(9): p. 1155-63.
7. Dickerson, V.M., et al ., Outcomes of dogs undergoing limb amputation, owner satisfaction with limb amputation procedures, and owner perceptions regarding postsurgical adaptation: 64 cases (2005-2012). J Am Vet Med Assoc , 2015 247(7): p. 786-92.
8. Kirpensteijn, J., R. van den Bos, and N. Endenburg, Adaptation of dogs to the amputation of a limb and their owners’ satisfaction with the procedure. Vet Rec , 1999. 144(5): p. 115-8.
9. Boesch, J.M., et al ., The Canine Postamputation Pain (CAMPPAIN) initiative: a retrospective study and development of a diagnostic scale. Vet Anaesth Analg , 2021. 48(6): p. 861-870.
10. Phillips, A., et al. , Clinical outcome and complications of thoracic and pelvic limb stump and socket prostheses. Vet Comp Orthop Traumatol , 2017. 30(4): p. 265-271.
11. Wendland, T.M., B. Seguin, and F.M. Duerr, Retrospective MultiCenter Analysis of Canine Socket Prostheses for Partial Limbs Front Vet Sci , 2019. 6: p. 100.
12. Van de Meent, H., M.T. Hopman, and J.P. Frolke, Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil , 2013. 94(11): p. 2174-8.
13. Ontario, H., Osseointegrated Prosthetic Implants for People With Lower-Limb Amputation: A Health Technology Assessment. Ont Health Technol Assess Ser , 2019. 19(7): p. 1-126.
14. Fitzpatrick, N., et al ., Intraosseous transcutaneous amputation prosthesis (ITAP) for limb salvage in 4 dogs. Vet Surg , 2011. 40(8): p. 909-25.
15. Farrell, B.J., et al. , An animal model to evaluate skin-implantbone integration and gait with a prosthesis directly attached to the residual limb. Clin Biomech (Bristol, Avon), 2014. 29(3): p. 336-49.
16. Farrell, B.J., et al. , Effects of pore size, implantation time, and nano-surface properties on rat skin ingrowth into percutaneous porous titanium implants. J Biomed Mater Res A , 2014. 102(5): p. 1305-15.
17. Shevtsov, M., et al. , Protecting the skin-implant interface with transcutaneous silver-coated skin-and-bone-integrated pylon in pig and rabbit dorsum models. J Biomed Mater Res B Appl Biomater , 2021. 109(4): p. 584-595.
18. Al Muderis, M., W. Lu, and J.J. Li, Osseointegrated Prosthetic Limb for the treatment of lower limb amputations : Experience and outcomes. Unfallchirurg , 2017. 120(4): p. 306-311.
19. Grisez, B.T. , et al. , Osseointegrated Transcutaneous Device for Amputees: A Pilot Large Animal Model. Adv Orthop 2018. 2018: p. 4625967.
20. Shelton, T.J., et al. , Percutaneous osseointegrated prostheses for amputees: Limb compensation in a 12-month ovine model. J Biomech , 2011. 44(15): p. 2601-6.
21. Jarrell, J.R., et al , Kinetics of individual limbs during level and slope walking with a unilateral transtibial bone-anchored prosthesis in the cat. J Biomech , 2018. 76: p. 74-83.
22. Drygas, K.A., et al ., Transcutaneous tibial implants: a surgical procedure for restoring ambulation after amputation of the distal aspect of the tibia in a dog. Vet Surg , 2008. 37(4): p. 322-7.
23. Golachowski, A., et al., Implantation of an Intraosseous Transcutaneous Amputation Prosthesis Restoring Ambulation After Amputation of the Distal Aspect of the Left Tibia in an Arabian Tahr (Arabitragus jayakari). Front Vet Sci , 2019. 6: p. 182
You are invited to contribute radiographic examples of proximal intertarsal joint luxation for a retrospective study conducted by a collaboration of international veterinarians. The aim of the study is to advance the strategic role that radiography may have in injury minimisation.
More information cve.edu.au/316-study
Or contact
David Larratt at dmpurplepalace@gmail.com


This course presented by Taronga will support veterinary professionals and veterinary nurses to develop knowledge and skills in native wildlife triage, including first aid, initial treatment and emergency care.
• Supported by the NSW Government Department of Planning and Environment
Applications for Semester 1, 2025 Open 27 September 2024
Email: tarongprofvet@zoo.nsw.gov.au
Visit: https://taronga.org.au/vet-professional-training
• AVA and VNCA certified
• Online Course (20 CPD points)
• Hands-on Workshop (12 CPD Points) –available in NSW, QLD and Vic (see website for details)
• Subsidised positions available to eligible applicants nationally




Workshops available at:

