














Issue 315 | June 2024
Control & Therapy Series
PUBLISHER
Centre for Veterinary Education
Veterinary Science Conference Centre
Regimental Drive
The University of Sydney NSW 2006 + 61 2 9351 7979
cve.marketing@sydney.edu.au
cve.edu.au
Print Post Approval No. 10005007
EDITOR
Lis Churchward
elisabeth.churchward@sydney.edu.au
EDITORIAL ASSISTANT
Dr Jo Krockenberger joanne.krockenberger@sydney.edu.au
VETERINARY EDITOR
Dr Richard Malik
DESIGNER
Samin Mirgheshmi
ADVERTISING
Lis Churchward elisabeth.churchward@sydney.edu.au
To integrate your brand with C&T in print and digital and to discuss new business opportunities, please contact:
MARKETING & SALES MANAGER
Ines Borovic ines.borovic@sydney.edu.au
DISCLAIMER
All content made available in the Control & Therapy (including articles and videos) may be used by readers (You or Your) for educational purposes only.
Knowledge and best practice in this field are constantly changing. As new research and experience broadens our knowledge, changes in practice, treatment and drug therapy may become necessary or appropriate. You are advised to check the most current information provided (1) on procedures featured or (2) by the manufacturer of each product to be administered, to verify the recommended dose or formula, the method and duration of administration, and contraindications.
To the extent permitted by law You acknowledge and agree that:
I. Except for any non-excludable obligations, We give no warranty (express or implied) or guarantee that the content is current, or fit for any use whatsoever. All such information, services and materials are provided ‘as is’ and ‘as available’ without warranty of any kind.
II. All conditions, warranties, guarantees, rights, remedies, liabilities or other terms that may be implied or conferred by statute, custom or the general law that impose any liability or obligation on the University (We) in relation to the educational services We provide to You are expressly excluded; and
III. We have no liability to You or anyone else (including in negligence) for any type of loss, however incurred, in connection with Your use or reliance on the content, including (without limitation) loss of profits, loss of revenue, loss of goodwill, loss of customers, loss of or damage to reputation, loss of capital, downtime costs, loss under or in relation to any other contract, loss of data, loss of use of data or any direct, indirect, economic, special or consequential loss, harm, damage, cost or expense (including legal fees).
An Unexpected Diagnosis of Dirofilaria immitis (Heartworm Infection) in a Dog on Liver FNAs Yuqin
Katrina Cheng
What to Tube & When? Feeding Tube Management & Care Cecilia Villaverde & Sam Taylor ...........................................................20 Research Roundup
CANCV-4—Canine Autologous Nanoparticle Cancer Vaccine (V4) Immunotherapy for Dogs Chris Weir 27
Treatment of ‘Swamp Cancer’ (Oomycete Infections) In Horses With Metalaxyl-M Andrea Harvey, Sarah Townsend, Jamin Farebrother, Richard Olsen, SallyAnn Olson, Luisa Monteiro de Miranda, Mark Krockenberger, Cathy Shilton, Oliver Liyou & Richard Malik ............................................................................................ 39
A Canine Desexing Model Utilised by 3rd Year Students in a Tutorial Prior to Their First Live Animal Spey .................................................... 38 Transform Your Passion into Impact: Join Our PhD Program
The C&T forum gives a ‘voice’ to the profession and everyone interested in animal welfare. You don’t have to be a CVE Member to contribute an article—please send your submissions to Dr Jo Krockenberger. joanne.krockenberger@sydney.edu.au
Join In!
The C&T is not a peer-reviewed journal.
We are keen on publishing short, pithy, practical articles (a simple paragraph is fine) that our readers can immediately relate to and utilise. Our editors will assist with English and grammar as required.
I enjoy reading the C&T more than any other veterinary publication.
-Terry King, Veterinary Specialist Services, QLD
The C&T Series thrives due to your generosity.
Vouchers can be used towards membership fees or continuing education courses
Prize: A CVE$500 voucher
Bearded Dragons in Practice
Harry Sollom
Winners Prize: A CVE$100 voucher
An Unexpected Diagnosis of Dirofilaria immitis (Heartworm Infection) in a Dog on Liver FNAs
Yuqin Zhu & Lucy Barker
Heartworm Case in Southwest Sydney
Michael Yazbeck
Necrotising Pancreatitis with Concurrent Chronic Hepatitis in a Dog
Alexander Teh
Enhancing the Euthanasia Experience: An Overview of Advancements in End-of-Life Care and Best Practice
Euthanasia
Jessica Pockett

The C&T thrives due to its unique and altruistic nature: it’s a forum rather than an academic journal. Contributors share their experiences—warts and all—with colleagues to contribute to animal welfare everywhere.

On this special anniversary, the last word goes to its founder Tom Hungerford OBE BVSc FACVSc HAD who wanted a forum for uncensored and unedited material.
Tom wanted to get the clinicians writing:
not the academic correctitudes, not the theoretical niceties, not the super correct platitudes that have passed the panel of review… not what he/she should have done, BUT WHAT HE/ SHE DID, right or wrong, the full detail, revealing the actual ‘blood and dung and guts’ of real practice as it happened, when tired, at night, in the rain in the paddock, poor lighting, no other vet to help.
Now every one of you who has done anything practical that’s not easily obtainable in all the common textbooks, write it up. Being local Australian experience it’s invaluable.
We welcome articles from everyone involved in the profession, worldwide. You don’t have to be a CVE member to contribute.
Email: cve.marketing@sydney.edu.au
Here’s to the next 55 years!
The CVE Team cve.edu.au
Dip-ECVIM
Internal Medicine Specialist
Small Animal Specialist Hospital, Western Sydney
C&T No. 6019
Mojo is a 13-year-old male neutered Maltese Shih Tzu cross that first presented to Small Animal Specialist Hospital Western Sydney emergency service for a sudden collapse at home. The owner reported that Mojo was conscious but laterally recumbent. He recovered in 15 minutes.
The owner also reported that Mojo had a progressively worsening dry cough for over a year. In the last few days, Mojo seemed to have difficulty breathing.
On presentation, Mojo was bright, alert and responsive. His physical examination was unremarkable. General blood profile and thoracic radiographs were recommended for further assessment and investigation. The owner declined this option, and Mojo was discharged for monitoring.
Two weeks later, Mojo presented to the emergency service again for a different reason: acute vomiting and diarrhoea.
A complete history revealed that Mojo was not up to date with vaccines and worm preventatives. His overall energy level had decreased. He was lethargic and had reduced appetite. He had never travelled outside New South Wales.
Mojo was admitted for initial work-ups and supportive care overnight. He was referred to the medicine specialist service the next day for further management and investigation of vomiting and diarrhoea.
The emergency service performed thoracic and abdominal radiographs which were reviewed as unremarkable.
A general blood profile was performed, the blood test result revealed moderately elevated ALT 348 IU/L (10-125), ALKP 2711 U/L (23-212), moderate hypercholesterolaemia CHOL 12.29 mmo/L (2.84-8.26), and mild hyperproteinaemia TP 86 g/L (52-82) with mild hyperglobulinaemia GLOB 50 g/L (25-45).
Due to the elevated liver enzyme values, abdominal ultrasound was recommended.
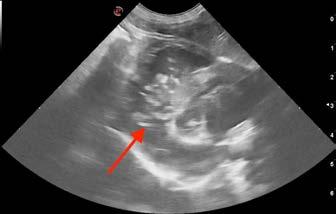
On ultrasound, the liver was enlarged with a diffuse patchy parenchyma (Figure 1). There was sonographic evidence of renal cysts, splenic nodules, and pancreatic changes consistent with chronic pancreatitis. The cause of vomiting and diarrhoea remained open, but due to hepatic changes, fine needle aspirates of liver were taken and submitted for cytology.



Mojo’s gastrointestinal signs improved with supportive care during hospitalisation (Hartmann’s solution at 1x maintenance rate 13mL/hr, and maropitant 1mg/kg IV q 24 hours), therefore suspected to be consistent with acute gastroenteritis or dietary indiscretion. He was discharged while pending liver aspirates result.
Surprisingly, the cytology results returned for Mojo revealed Dirofilaria immitis microfilariae within the hepatic parenchyma (Figure 2). Although an unexpected finding, these results were consistent with heartworm infestation. Based on these results, heartworm antigen test was added, and it came back positive. Due to the presence of circulating microfilariae in the liver, a microfilariae blood test was not performed.
The thoracic radiographs taken by the emergency service were reviewed by the radiology specialists but there was no evidence of significant heart disease, and changes in the pulmonary artery were not seen.
Though not evident on thoracic radiographs, heartworm infection is suspected to be the answer to Mojo’s chronic coughing and intermittent weakness. The concern is that his collapse event may also have reflected pulmonary thromboembolism. The owner declined performing echocardiogram to investigate the extent of cardiac changes further.
Mojo received American Heartworm Society’s recommended heartworm treatment protocol¹ (Table 1) The treatment was commenced two days after diagnosis.
Figure 2. Dirofilaria immitis microfilaria within the hepatic parenchyma (Photomicrographs courtesy of Dr George Reppas Vetnostics)

Mojo was last seen by the medicine specialist service on Days 61, 90 and 91 for melarsomine injections. Since the treatment started, Mojo had been doing well with improved energy level and appetite. No more collapse episodes were observed. Cough was ongoing but improved.
The owner reported that Mojo was lethargic and inappetent for approximately 12 hours after the second melarsomine injection, then spontaneously returned to normal. No other concerns were reported.
Overall, Mojo showed a positive response to treatment. His case has been lost to follow-up, so repeated microfilariae testing has not been performed.
This case implies that despite now being classed a rare diagnosis, heartworm infection still exists around the Greater Sydney Region (Mojo had never travelled outside of Western Sydney in his lifetime).
Therefore, veterinarians should continue to stay vigilant for this infectious disease and continue raising client awareness on the risks of this infection, especially in animals who are not up-to-date with heartworm preventatives. For dogs that present to clinic with clinical signs of cardiac or respiratory disease, with a lapse of heartworm prevention, heartworm antigen test is strongly recommended. Regular heartworm prevention should remain as an important part of all pets’ health care plan.
1. American Heartworm Society. (2018). Current Canine Guidelines for the Prevention, Diagnosis, and Management of Heartworm (Dirofilaria immitis) Infection in Dogs heartwormsociety.org/images/pdf/2018AHS-Canine-Guidelines.pdf
Day 0 In a dog diagnosed and verified as heartworm positive:
Positive antigen (Ag) test verified with microfilaria (MF) test
If no MF are detected, confirm with second Ag test from a different manufacturer
Apply an EPA-registered canine topical product labeled to repel and kill mosquitoes
Begin exercise restriction—the more pronounced the signs, the stricter the exercise restriction
If the dog is symptomatic:
Stabilize with appropriate therapy and nursing care
Prednisone prescribed at 0.5 mg/kg BID first week, 0.5 mg/kg SID second week, 0.5 mg/kg every other day (EOD) for the third and fourth weeks
Apply frontline plus (Fipronil 9.8%, (S)-methoprene 8.8%)
Begin exercise restriction
Start steroids Prednisolone 5mg twice daily for first week, 5mg once daily for second week, 5mg every other day for the third and fourth weeks
Chlorphenamine 4mg one
tablet orally twice daily

Day 1
Administer appropriate heartworm preventive
y If MF are detected, pre-treat with antihistamine and glucocorticosteroids, if not already on prednisone, to reduce risk of anaphylaxis
y Observe for at least 8 hours for signs of reaction
Administer milbemycin oxime
Days 1-28
Administer doxycycline 10 mg/kg BID for 4 weeks
Reduces pathology associated with dead heartworms
Disrupts heartworm transmission
Day 30 Administer appropriate heartworm preventive
Apply an EPA-registered canine topical product to repel and kill mosquitoes
Days 31-60 A one-month wait period following doxycycline before administering melarsomine is currently recommended as it is hypothesized to allow time for the Wolbachia surface proteins and other metabolites to dissipate before killing the adult worms. It also allows more time for the worms to wither as they become unthrifty after the Wolbachia endosymbionts are eliminated.
Day 61
Administer appropriate heartworm preventative
Administer first melarsomine injection, 2.5 mg/kg intramuscularly (IM)
Prescribe prednisone 0.5 mg/kg BID first week, 0.5 mg/kg SID second week, 0.5 mg/ kg EOD for the third and fourth weeks
Decrease activity level even further: cage restriction; on leash when using yard
Administer doxycycline 75mg PO BID for 4 weeks
Administer milbemycin oxime
Apply frontline plus
The owner rested Mojo for the next month with no medication to be given.
Administer milbemycin oxime
Apply frontline plus Administer first melarsomine injection (0.65mL) IM
Prednisolone 5mg twice daily for first week, 5mg once daily for second week, 5mg every other day for the third and fourth weeks
Table 1. American Heartworm Society’s recommended heartworm treatment protocol and Mojo’s treatment schedule
Day 90
Administer appropriate heartworm preventative
Administer second melarsomine injection, 2.5 mg/kg IM
Prescribe prednisone, 0.5 mg/kg BID first week, 0.5 mg/kg SID second week, 0.5 mg/ kg EOD for the third and fourth weeks
Day 91
Administer third melarsomine injection, 2.5 mg/kg IM
Continue exercise restriction for 6 to 8 weeks following last melarsomine injections
Day 120 Test for presence of MF
y If positive treat with a microfilaricide and retest in 4 weeks
Continue a year-round heartworm prevention program based on risk assessment
Day 365 Antigen test 9 months after last melarsomine injection; screen for MF
If still Ag positive, re-treat with doxycycline followed by two doses of melarsomine 24 hours apart
Administer milbemycin oxime
Apply frontline plus Administer second melarsomine injection (0.65mL) IM
Prednisolone 5mg twice daily for first week, 5mg once daily for second week, 5mg every other day for the third and fourth weeks
Continue to restrict exercise for 8 weeks
Administer third melarsomine injection (0.65mL) IM
Administer prednisolone as prescribed
Continue to restrict exercise for 8 weeks
A heartworm test is recommended. It is recommended to continue a heartworm preventative medication.

A repeat heartworm test is recommended. If this test remains positive, further/repeat treatment with injections would likely be recommended.
Table 1. American Heartworm Society’s recommended heartworm treatment protocol and Mojo’s treatment schedule


The second book will be a celebration of all things veterinary, the days when everything went well and the days when it didn’t. It’s going to be second collector’s item—a coffee table showpiece—so don’t miss the opportunity to be a part of it.
The CVE is again donating resources and expertise free of charge to design, deliver and market this second edition. Thanks to the contributors and sponsors who made the first Vet Cookbook
Or earlier—first in, first published
Upload your recipe: cve.edu.au/vet-cookbook
Winner
Entitled to a CVE$100 voucher
Greencross Vets Campbelltown
3 Blaxland Serviceway
Campbelltown NSW 2560
t. 02 9146 1163
e. michael.yazbeck@greencrossvet.com.au
C&T No. 6020
History
Sheba, an 11 1/2-year-old female speyed Chihuahua, presented for a 2-day history of hyporexia, lethargy and laboured breathing. Gradual weight loss was noted over the preceding 2 weeks and there was no coughing, sneezing, vomiting or diarrhoea. She was not on any medications or supplements, and her usual diet consisted of commercial wet and dry dog food and home cooked meals. She was on an isoxazoline drug for parasite prevention, which was last administered approximately 4 weeks prior and was believed to be upto-date with this regime for an unknown number of years, but prior to this her parasite prophylaxis was unknown.
She was acquired at 6 to 8 weeks old from a local market in Southwest Sydney and has lived locally ever since with no history of travel. She’s had no significant medical history, except for a broken right hindlimb at 8-weeks-old.
Examination
Sheba was quiet, alert and responsive with a 5/6 systolic heart murmur and normal pulses. She was mildly tachypnoeic with a mild to moderate increase in respiratory effort and crackles on auscultation. She had pale pink mucous membranes and periodontal disease. She was mildly hypothermic, had a lean body condition and her right hindlimb was non-weight bearing. The remainder of the examination was unremarkable.
Inhouse biochemistry and CBC were performed, which revealed a PCV of 19% and total protein of 68 g/L. There were mild elevations in glucose, BUN, ALP and AST. The CBC revealed a normocytic, normochromic moderate non/pre-regenerative anaemia (reticulocytes not included) and mild thrombocytopaenia. Polychromasia was noted on the blood smear.
Sheba was provided flow-by oxygen and a thoracic point of care ultrasound was performed. The LA:Ao ratio was 1.7 indicating mild left atrial enlargement; there were occasional B lines present, and pleural space disease was excluded based on the absence of pleural effusion and pneumothorax (glide sign present). Surprisingly, there were a number of segments of hyperechoic, parallel lines within the right ventricle, indicative of heartworm infection. The finding was supported by a positive heartworm antigen test.
Conscious thoracic radiographs were taken, which highlight the very dilated and tortuous pulmonary lobar arteries in the lateral and dorsoventral views (credit to Dr Catheryn Walsh), consistent with heartworm infection. Cardiomegaly was appreciated; however, the left atrium did not appear obviously enlarged on radiographs and there was no obvious interstitial to alveolar pattern consistent with left-sided congestive heart failure.
Although recently published data Australia-wide is lacking, Zoetis have been encouraging vets to report heartworm cases onto a company website and have advised that over 3,000 positive cases have been reported from practices Australia-wide since 2017, of which approximately 10% are external to Queensland.
Despite not being commonly encountered in Sydney, this case serves to remind vets to continue to inform pet owners on heartworm disease, to recommend diligent heartworm prevention and to maintain heartworm disease as a differential diagnosis for cardiorespiratory cases, even in the absence of a more typical locality or history of travel. Sheba represents a signalment where myxomatous mitral valve disease (MMVD) and leftsided congestive heart failure, or primary airway diseases, are highest on the differential diagnosis list for similar case presentations. Sadly, due to multiple patient factors, Sheba was euthanased. Her owners hope her case may raise awareness of the risk of heartworm disease in our local area and Australia-wide.

Heartworm is transmitted by mosquitoes, the obligate intermediate host. Heartworm infection causes a spectrum of disease, ranging from subclinical to severe pulmonary disease and secondary right-sided congestive heart failure due to the presence of worms in the pulmonary arteries and heart (and potentially vena cava), the host-parasite interaction and modulation by the intracellular bacterium, Wolbachia
Anaemia, as in this case, can be caused by inflammatory disease or the shearing of the red blood cells as they flow past the worms, causing intravascular haemolysis. The thrombocytopaenia was likely due to consumption in the pulmonary arterial system or artefact due to clumping. The mildly elevated BUN was likely pre-renal, or due to immune-complex glomerulonephritis. The mildly elevated ALP may have been due to passive congestion of

the liver or an unrelated process. The elevated AST could have been due to intravascular haemolysis, or concurrent hepatopathy or myopathy. Ideally, a urinalysis could have been performed to screen for haemoglobinuria (suggestive of intravascular haemolysis), proteinuria and to assess USG. If treatment was to be pursued, a proper echocardiogram would be recommended to detect concurrent abnormalities, such as MMVD, pulmonary hypertension, pericardial effusion and caval syndrome. 1 3 4 5 2

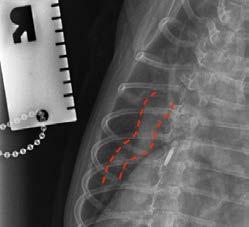

Figure 4. Tortuous pulmonary artery on lateral view
Figure 5. Visit the open access eBook to view the video
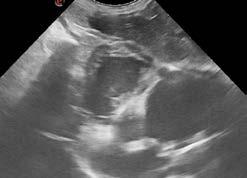 Figure 1. Heartworm evident on echo
Figure 2. Heartworm evident on echo
Figure 3. Tortuous pulmonary artery on dorsoventral view
Figure 1. Heartworm evident on echo
Figure 2. Heartworm evident on echo
Figure 3. Tortuous pulmonary artery on dorsoventral view
University of Sydney e. jan.slapeta@sydney.edu.au
These two intriguing cases of canine heartworm serve as compelling evidence that Dirofilaria immitis is not as ‘extinct’ in New South Wales as thought. The first case is reminiscent of a similar instance we encountered at the University Veterinary Teaching Hospital Sydney a couple of years ago.1 In smaller breeds, ultrasound proves invaluable, as the distinct ‘tram-tracks’ are an unmistakable telltale sign. Utilising X-rays to visualise the tortuous pulmonary artery is a crucial aspect of heartworm disease assessment. Despite these two cases, Sydney remains comparatively hypo-endemic to Far North Queensland for D. immitis.
In the McKeever et al 1 case report, we speculated that occurrences like these may stem from what could be termed ‘baggage heartworm’. This occurs when infected mosquitoes, vectors of D. immitis, are inadvertently transported in luggage from endemic areas such as Far North Queensland. It is analogous to ‘baggage malaria’ in humans in non-endemic regions.2
Though there is no recent epidemiological data for New South Wales, two recent studies are relevant. One involving around 400 dogs from New South Wales found none tested positive for D. immitis antigen.3 However, absence of evidence is not evidence of absence, given the irregular (not normal) distribution of parasites. Another study in Queensland shelters revealed a high prevalence of D. immitis (~20%) in Far North Queensland and a lower prevalence (~5%) in Southern Queensland pound dogs.4 Unfortunately, a similar study in New South Wales is not feasible due to legislation barring research on impounded dogs, and that includes collection of few millilitres of blood for such research purpose.
One can infer that compared to Queensland, the incidence of D. immitis infection in New South Wales is currently lower. However, the situation is dynamic, particularly with the weather patterns observed in recent years. Not too long ago (1980s to 1990s), D. immitis infection was a common diagnosis in Sydney, with figures similar to those now reported by colleagues in Far North Queensland.
The detection of microfilariae in liver biopsies is certainly feasible in dogs with patent D. immitis infection. Counts vary significantly in positive dogs; we have seen counts exceeding 50,000 microfilariae per mL. However, not all D. immitispositive dogs have circulating microfilariae. In Australia, 30-50% of D. immitis-positive dogs are amicrofilaremic, often with single-sex infections.
While I advocate for the use of rapid antigen tests (RAT), I still emphasise the necessity of checking suspect positive dogs for microfilariae. Performing tests like the Knott’s test in-clinic is essential. This simple yet effective test not only confirms diagnosis but also assesses the risk posed by the infected dog to others. Access to a microscope and centrifuge makes conducting the Knott’s test feasible for any practitioner.
1. McKeever B, Podadera JM, Beijerink NJ, Slapeta J. Suspect ‘baggage canine heartworm’ case: canine heartworm disease in a dog from Sydney, New South Wales. Aust Vet J 2021;99:359-362. https://doi.org/10.1111/avj.13074
2. Mantel CF, Klose C, Scheurer S, Vogel R, Wesirow AL, Bienzle U. Plasmodium falciparum malaria acquired in Berlin, Germany. Lancet 1995;346:320-321. https://doi.org/10.1016/s0140-6736(95)92212-1
3. Orr B, Ma G, Koh WL, Malik R, Norris JM, Westman ME, Wigney D, Brown G, Ward MP, Slapeta J. Pig-hunting dogs are an at-risk population for canine heartworm (Dirofilaria immitis) infection in eastern Australia. Parasit Vectors 2020;13:69. https://doi.org/10.1186/s13071-020-3943-4
4. Panetta JL, Calvani NED, Orr B, Nicoletti AG, Ward MP, Slapeta J. Multiple diagnostic tests demonstrate an increased risk of canine heartworm disease in northern Queensland, Australia. Parasit Vectors 2021;14:393. https://doi.org/10.1186/s13071-021-04896-y
“The American Heartworm Society and the European Society of Dirofilariosis and Angiostrongylosis currently suggest a monthly treatment based on macrocyclic lactones (ML)(e.g. moxidectin) along with doxycycline for a 4-week period as an alternative to melarsomine.”
Treatment with Moxi/doxy is a bit contested. No one actually argues that it works but there are 3 potential issues that have been flagged over the years:
1. Owner compliance is required for an extended period of time.
2. Resistance to DOX in Wolbachia or any other bacteria (theoretically human ones as well).
3. Resistance to MOX (=ML) in Dirofilaria.
Plus the requirement to use whatever is registered in US first makes MOXI-DOXY bit of a liability for the vets there.

When I tell students about it, I call it 'slow kill'compared to “fast kill” with Immiticide.
Fast kill is mostly under control of the vet (assuming the owners actually decide to come back after the first round of Immiticide) and is completed in 3-4 months. With slow kill, you treat for 2-3 years, assuming they are compliant, and vets need to justify that having few dying worms is kind of ok [it seems it is].
parasitesandvectors.biomedcentral.com/ articles/10.1186/s13071-023-05690-8
e. richard.malik@sydney.edu.au
I think the America HW Society treatment guidelines are far too influenced by vets in the USA. The treatment protocol is highly complex and VERY expensive. Many vets’ visits – lots of changes and different drugs.
The newer treatment using doxycycline and moxidectin is IN MY OPINION just as effective, just as safe and should be industry standard medicine.
In cats – we have been accidentally doing this for many years - as killing worms SUDDENLY in cats often kills the cats – so we just stuck them on monthly prevention. So, I was already thinking in this direction when the Italians dreamed up killing the endosymbiont Wolbachia, and then slowly killing the adults with moxidectin.
I don’t want to force my view on everyone – but that’s MY considered opinion. 4 weeks of VibraVet and a year of Advocate – is SO MUCH CHEAPER than following the US guidelines. Also easy to remember!
Christopher Simpson
Victoria Veterinary Clinics
Hong Kong
e. simpson_christo@icloud.com
The AHS guidelines protocol is absolutely not a benign process. Over the years I can recall multiple dogs which have dropped dead in the first or second day after one of the Immiticide injections.
So far, I have never seen that happen with Moxi/Doxy.
It is now close to ‘standard of care’ in our practice. We use it routinely in Class 1 and 2 disease.
If our regular clients come to us with a HW patient, we will explain that the American Heartworm Society ( AHS) Guidelines exist, and are probably the most formally recognised option, but that we have good recent experience with an “alternative” protocol which we consider has significant potential advantages. Our good clients will then usually ask us to choose what we think is best, and trust our judgement.
We choose to use the AHS protocol in three scenarios:
a. if the dog is in heart failure due to a very heavy intracardiac burden (causing severe tricuspid regurgitation)
b. if the client is not well known to us, and we want to do things “by the book” for medico-legal reasons
c. if we have tried the Moxi/doxy protocol and no evidence of efficacy at the 6-month recheck (this has happened once).
We still do both, as above, and in most cases Moxi/doxy is smoother, cheaper, strongly preferred by the clients, and appears highly effective in many cases in our anecdotal experience.
I would be interested to see an randomised controlled trial comparing the two, but of course I never will…
ncbi.nlm.nih.gov/pmc/articles/PMC8353148/

Queensland
C&T No. 6021
A 6-month-old female Australian Cattle Dog (recently desexed), weighing 15kg, presented one evening after the owners had arrived home to find her in a collapsed state. She had been observed as normal that morning and was locked up with no access to intoxications or envenomations that day. She was a compost eater and had been seen regularly eating the remnants of chicken feed (containing amprolium).
Initial clinical examination revealed temperature 38.8ºC, HR 170, panting, MM pink, CRT 1+ seconds with tachycardia the only recorded thoracic auscultation abnormality. Neurologically she was recumbent and unable to move, although she was hyperaesthetic to stimuli; pupils were dilated and unresponsive with mild bilateral third eyelid protrusion. CBC/MBA/ACT were essentially normal. The dog was treated with diazepam, vitamin B1 injection, and IV fluids. She was discharged to owner care the next morning, a mild ataxia the only residual symptom.
She was represented the following evening when the owners returned home to find her again (in her locked room) collapsed and unresponsive. Physical examination on representation showed a recumbent, seemingly comatose adolescent dog with temperature 37.8ºC, HR 160, RR 52, MM pink, CRT 1+ seconds. Her ocular palpebrae were shut and on examination, her pupils were pin-points, bilaterally. Some response was elicited with vigorous stimulation, e.g. placement of an intra-nasal oxygen line, urinary catheter placement; she displayed an exaggerated hyperaesthetic response to subcutaneous injections. Abdominal palpation indicated very firm, cranial abdominal organomegaly.
CBC/MBA were again normal. Abdominal ultrasound examination revealed a markedly distended stomach creating marked gaseous shadowing with the spleen displaced caudally. A right lateral abdominal radiograph revealed a distended food-filled stomach.
Methadone 0.25 mg/kg was given IM, she was intubated, and gastric lavage was performed without any other chemical restraint—this procedure returned copious amounts of sodden pellets of dry dog food (owners subsequently reported that she had eaten a huge amount of dry dog food after going home that morning).
Oxygen therapy ceased after extubation, IV fluids were continued, and Vitamin B1 was administered at 250mg IM.
Within 12 hours, the pup was assessed as being normal neurologically with all vital signs normal. She was again discharged to her owner’s care; the treatment prescribed was Vitamin B1 (thiamine) 100mg PO Daily for a minimum of 3 weeks. Stopping access to the household’s chicken feed was also prescribed. Subsequently, amprolium was detected in the gastric lavage contents by laboratory analysis. The dog was normal at follow-up examination a week later and was reported as being normal at 3 week and 3-month phone follow-up.
The coccidiostat Amprolium hydrochloride is 1-[(4-amino-2-propyl-5-pyrimidinyl) methyl]-2ethylpyridinum chloride which is used in the prevention and treatment of coccidiosis in chickens and turkeys. A structural analogue of Vitamin B1 (thiamine) it competitively inhibits thiamine utilisation by parasites (coccidia).
Amprolium is not considered to have antimicrobial activity other than its action on coccidia; it does not possess any significant antibacterial activity. Amprolium is authorised as a feed additive for poultry at concentrations in the range of 62.5-125 mg/kg of complete feed; the authorisation prohibits the use of the substance from laying age onwards and for at least 3 days before slaughter.
Amprolium is a thiamine (vitamin B1) analogue and is a competitive antagonist of thiamine transport mechanisms. The affinity for the blood-brain uptake system is similar to that of thiamine. At sub-optimal dietary intakes of thiamine, amprolium can cause decreases in body weight gain and tissue thiamine concentrations, suggesting a selective inhibition of thiamine uptake. With oral ingestion, peak plasma activity is reached within 4 hours after a meal containing amprolium and declines gradually over 24 hours. With continuous access to amprolium-containing feeds, peak concentrations are maintained at a steady state. Total excretion approximates 90%, with the faeces being the major route of excretion (~80%); urinary excretion accounts for some 9-10% of the total dose.


Parent amprolium—up to 50% of the amount ingested—is excreted unchanged in the faeces. Significant tissue concentrations are rapidly reached in the liver and gastrointestinal tract and up to 17 metabolites are detectable in the liver and muscles (within 4 hours), and urine (continuously for up to 7 days).
In experimental studies, acute toxicity causing death within 36 hours occurs in dogs receiving an oral dose of 500mg/kg amprolium; signs of toxicity included emesis, head drooping, ataxia, loss of righting reflex, tremors and dyspnoea. In comparison, a single oral dose of 300mg/kg amprolium produced no clinical signs.
The concurrent supplementation with thiamine in trials with lower doses of amprolium (up to 300 mg/kg) did not improve survival, but clinical signs (similar to the acute toxicity signs) resulting in death took up to 16 weeks to emerge, especially if amprolium-meals were limited to 5 days weekly. There were no substance-related effects on cardiovascular parameters or none could be detected on ophthalmoscopic examination; haematology, biochemistry and routine urinalysis values were normal. The major signs of toxicity included pupillary dilatation, paralysis and collapse. Supportive studies have established a NOEL (non-observed effect level) of 100mg/kg.
A degenerative encephalomyelopathy in seven out of ten Kuvasz puppies was suspected to be due to the effects of an amprolium-induced thiamine deficiency on the developing brains of those puppies. The litter was treated, beginning at 4.5 weeks of age, with amprolium 48mg/dog/day for 10 days for the prevention of coccidiosis. Dragging of the hindlimbs and lethargy was noticed in all the pups around the end of the treatment, and four of the pups showed progressive ataxia, weakness, and reluctance to rise over a 4-to-5-month period. Neurological examination indicated a diffuse or multifocal lesion involving the thalamocortex, the rostral medulla (with signs suggesting vestibular-cerebellar connection involvement) and possibly the spinal cord.
A second litter from the same parents had amprolium coccidiostat prevention stopped after 6 days (initiated at 4 weeks of age) because 3 of the 5 pups showed trembling that progressed rapidly to a crawling gait (inability to stand). Thiamine supplementation was instituted at 50 mg/pup/day for 5 months; improvement was slow, ending with mild weakness and ataxia with reduced exercise tolerance and reduced learning ability (subjective observation). On neurological examination, all pups showed cerebellar ataxia (with truncal ataxia and dysmetria), decreased proprioception (more pronounced in the hindlimbs), and variable degrees of ventral strabismus on elevation and extension of the head and neck. The signs these pups showed suggest diffuse
central nervous lesion involving the thalamocortex, cerebrum, vestibular (central) systems, and spinal cord.
Postmortems were performed in 3 of the pups from each of the litters, and microscopic lesions were seen in all 6, with focal necrosis of the caudate nucleus and gliosis with spongy change in the cerebellar nuclei the most prominent and consistent findings. Spongy change was seen in other areas, including the spinal cord (mostly the descending tracts), in all 6 pups necropsied.
2 subsequent litters that were not treated with amprolium produced 14 normal puppies; these were from the same bitch but a different sire.
It would seem that the amount given to each of these pups (50mg/pup/day) falls well within the recommended safety margin for amprolium (200mg/dog/day for no longer than 14 days), unless an accidental overdose was given, which seems unlikely. Plumb (5th Edition) states that prolonged high doses can cause thiamine deficiency in the host, and it is not recommended to be used for over 12 days in puppies.
Plumb also states that exogenously administered thiamine in high doses may reverse or reduce the efficacy of amprolium. The fact that the second litter improved with thiamine supplementation supports the implication of amprolium in the onset of signs, either as a direct toxicosis or its associated thiamine deficiency, especially as the lesions were bilateral and lacking in inflammation. The very young age of the puppies (immature metabolism neurologically and systemically, and undeveloped blood brain barrier integrity perhaps augmenting the neurotoxicity of the amprolium), as well as the possible predisposition of an exotic breed may help explain why this presentation hasn’t been described before.
No functional tests for vitamin B1 status in the blood were performed on this ACD adolescent (the case study above), and no urinary organic acid assays were done that may indicate a deficiency of at least one of the components of the dehydrogenase complex using thiamine pyrophosphate as a cofactor—blood levels of thiamine can be performed but the laboratory process is difficult (need to discuss with the lab). However, the detection of the thiamine inhibitor, amprolium, in the gastric contents of the dog showing bizarre acute neurological signs that resolved with thiamine supplementation, is compelling evidence of overdosage of this product inducing acute thiamine deficient neurotoxicity.
Reference
Murray J. Hazlett, Laura L. Smith-Maxie, Alexander de Lahunta (May 2005), Case Report: A degenerative encephalomyelopathy in 7 Kuvasz puppies, CanVetJ Volume 46.

The Cat Clinic, Hobart
t. +613 6227 8000
e. moira@catvethobart.com.au
C&T No. 6022
Bingo is a 3-year-old male neuter domestic long hair cat. He presented to our clinic for his first vaccination as an 850 g kitten that had been found as a stray with a suspected female litter mate. He was a healthy kitten. He received his full kitten vaccination course and had a routine castration at 11-weeks-of-age.
He had a dental procedure at 11 months-of-age where 106, 109, 206 and 209 were extracted with gingivoplasty performed on 309 due to marked gingival hyperplasia. He had an unremarkable recovery.
In August of this year, he presented for ear flicking and a black substance in his ears. His owners had done some googling and diagnosed ear mites. He lived with 2 other cats at home and 2 dogs; interestingly, no other animals within the household had similar clinical signs. Bingo is meant to be an inside only cat but did have a habit of escaping every now and then. On examination, only his ears were affected and he had no other skin issues. Bilaterally, his ears had a crusty brown discharge
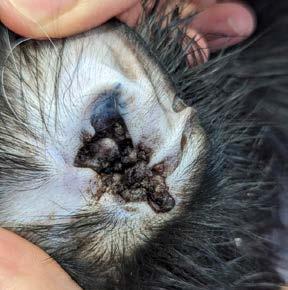

with friable tissue which bled after swabbing (Figure 1) On microscopic examination of the swabs taken from the ears there were no mites, Malassezia or bacteria detected. There were occasional neutrophils and many red blood cells.
Initial treatment included 5mg prednisolone PO SID and Dermotic topical ear ointment applied to the external cartilage and rubbed into the canal (taking care with application on the friable tissues). Bingo was very distressed about visiting the vets and PVP’s (pre visit pharmaceuticals- pregabalin) was also dispensed for future visits to the clinic.
Bingo revisited 7 days later. His ears were not much improved. He had a moist brown discharge within the canals, the external cartilage was thickened and inflamed. He resented examination of his ears, although he had had a positive response to the PVP’s. On cytology, there were minimal red blood cells but many squames and cocci were seen. Oral antibiotics were dispensed (amoxyclav 75 mg BID PO) and a revisit arranged for 14 days.
At his revisit 14 days later, both pinnae and canals were thickened, proliferative and hardened (see images). Repeated cytology again showed many squames and bacteria.
He was admitted to hospital for sedation and biopsies. Multiple biopsies were taken from both ears and samples submitted to VPDS, the University of Sydney, for histopathology. A tentative diagnosis of feline proliferative and necrotising otitis externa was made.
His antibiotics (due to diarrhoea with blood), prednisolone and Demotic were ceased. We applied a fentanyl (25 µg/hr) patch for analgesia (his ears looked very painful), started metacam oral (2.5 mg SID PO) and Pro-kolin (2mL BID PO) for his diarrhoea. Two days later,
 Figure 1. External ear canal of Bingo. Note the blackish proliferative lesions emanating from the external ear canal.
Figure 1. External ear canal of Bingo. Note the blackish proliferative lesions emanating from the external ear canal.

The findings are similar across the specimens with varying degrees of severity. There is marked hyperplastic thickening of the epidermis with marked orthokeratotic and parakeratotic hyperkeratosis. There is a moderate pallor of the stratum spinulosum, proliferation of the stratum basale, with variable degrees of epidermal spongiosis present. Scattered apoptotic cells are present. Underlying sebaceous glands are frequently hyperplastic and apocrines frequently dilated. There is a mild superficial dermal perivascular inflammatory infiltrate of small lymphocytes and plasma cells and occasionally neutrophils. In many of the specimens, there is increased density of the connective tissue of the superficial dermis with relative lack of hair follicles, consistent with scarring.
Marked multifocal epidermal hyperplasia, hyperkeratosis and surface necrosis
his owners called to say he smelt like ‘roadkill’. His stools were now normal but his owners were happy to start antibiotic cover again.
A diagnosis of feline proliferative and necrotising otitis externa was confirmed, see Uni Syd Report below. 0.03 % tacrolimus ointment was ordered from BOVA and started. Due to having never seen this condition prior, I was unsure as to how to apply the ointment to his ears. In the end his owner and I decided that she would apply the ointment to the areas that she could easily apply it to using her finger and hoped that it would ooze into his canals.
His owner recently reported that Bingo was responding really well to the 0.03% tacrolimus ointment. We are waiting for him to come back in for examination and photos.
The histopathology appears to be broadly consistent with the clinical suspicion of proliferative and necrotising otitis externa, although the feature of that condition is reported to have a severe acanthosis of the outer root sheath of hair follicles with increased apoptosis of epidermal cells. These were not prominent features in this case. Few of the samples had good numbers of hair follicles included in the specimens and the dermal scarring and relative lack of follicles may reflect past damage.
While viral cytopathic features are not evident in this case, a viral aetiology cannot be excluded as a possibility. Mauldlin et al Veterinary Dermatology 18(5):370-377
Figure 3. The external ear canal after a good clean-upResident in Anatomical Pathology
SSVS, The University of Sydney e. alexander.teh@sydney.edu.au
C&T No. 6023
Signalment, History, and Clinical Presentation
A 13-year-old female entire Staffordshire Bull Terrier cross presented to the University Veterinary Teaching Hospital Sydney for chronic weight loss and inappetence over a duration of approximately two to three months.
She later re-presented for worsening lethargy and developed acute respiratory distress and tachypnoea with a marked abdominal effort. Her oxygen saturation levels were reduced (SpO2 89-90%) and she became oxygen dependent.
She continued to deteriorate clinically and was ultimately euthanised and submitted for a post-mortem examination.
Bloodwork
Haematology revealed a moderate non-regenerative normocytic normochromic anaemia with mild hyperproteinaemia, thrombocytopaenia, and segmented neutrophilia with monocytosis. The plasma appearance was moderately lipaemic.
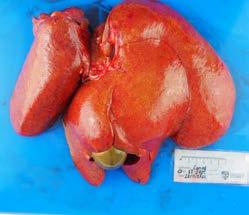
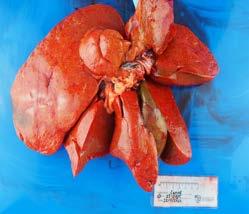

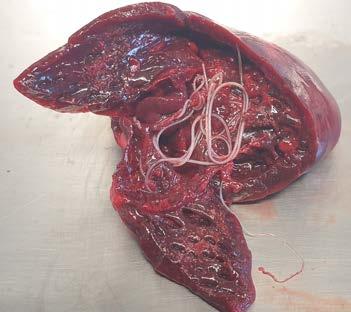
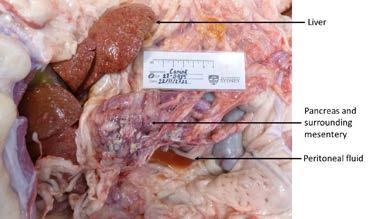 Figure 1. Abdominal organs at necropsy
Figure 1. Abdominal organs at necropsy
Biochemistry revealed mild elevations in amylase, ALP, globulins, urea, and C-reactive protein, and a slight hypoalbuminaemia.
At necropsy, a number of changes were identified primarily within the abdominal cavity. The liver was enlarged and firm with an irregular to slightly nodular capsular surface and an orange-yellow tinge. The common bile duct was patent, and bile was readily expressed from the gallbladder with mild pressure.
The pancreas was markedly enlarged and firm with an irregular surface and there were variable degrees of erythema and a few fibrin strands in the surrounding mesentery. Grossly, it appeared consistent with a severe necrotising pancreatitis with associated fibrinous peritonitis.
The abdomen also contained approximately 5mL of thin turbid dark orange fluid which was collected for fluid analysis and cytology.
Analysis of the abdominal fluid yielded the following information:
Protein: 47g/L (refractometer)
Nucleated cells: 8575 x 10⁶/L
Erythrocytes: 28,000 x 10⁶/L
Cytology of the fluid revealed a population of predominantly non-lytic neutrophils with a few reactive mesothelial cells and macrophages.
Overall, the abdominal fluid was consistent with a nonseptic exudate
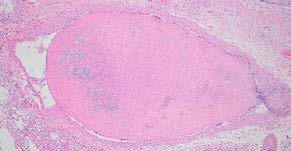
Histopathology of the pancreas confirmed necrotising pancreatitis with surrounding peritonitis and fat necrosis (Figure 3)

Additionally, pulmonary thromboembolism was identified in the lungs (Figure 4) which likely accounted for the patient’s acute respiratory distress.
The liver had evidence of widespread fibrosis with an interesting intra-sinusoidal pattern (Figure 5) Intrahepatic cholestasis was additionally observed. Chronic hepatitis likely accounted for the patient’s chronic weight loss and hyporexia, and the extensive fibrosis was the presumptive cause of the liver firmness at necropsy. In the liver, special stains for copper (Rubeanic acid) and iron (Perl’s Prussian Blue) were both negative.

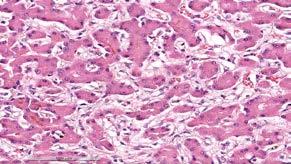
5. Hepatic sinusoidal fibrosis with bile plugs within bile canaliculi and hepatocytes indicative of intrahepatic cholestasis
In dogs, the cause of necrotising pancreatitis is often unknown or obscure, as in this case. Trauma, hypoperfusion, and/or nutritional factors (high fat diets) are possible contributing factors. Clinical signs usually associated with acute pancreatitis include vomiting, diarrhoea, and abdominal pain. Severe necrotising pancreatitis is associated with a number of serious lifethreatening complications including shock, SIRS, multiorgan dysfunction syndrome (MODS), sepsis, consumptive coagulopathies, and death.
Figure 3. Severe necrotising pancreatitis Figure 4. A massive pulmonary thrombusPulmonary thromboembolism (PTE) is a known but relatively uncommon complication of pancreatitis in both human and veterinary medicine. Presenting clinical signs can include an acute onset of dyspnoea/ respiratory distress and hypoxaemia. In this case, PTE was presumably due to a combination of blood hypercoagulability and endothelial injury related to the pancreatitis. Severe necrotising pancreatitis is often related to the systemic release of pro-inflammatory mediators such as TNF-a, IL-1 and IL-8 resulting in hypercytokinaemia/cytokine storms which altogether contribute to blood hypercoagulability (thrombophilia). Additionally, widespread tissue damage and necrosis inflict endothelial damage further exacerbating the patient’s thrombotic state.
In dogs, most cases of chronic hepatitis are idiopathic and presenting clinical signs can be non-specific and vague. However, known causes of canine chronic hepatitis include copper and iron-associated hepatopathies, metabolic disturbances, toxicity, infection, and possibly immune-mediated mechanisms. In this case, an overt inciting cause of the chronic hepatitis was difficult to identify. Copper and iron excess were not detected with special stains.
If the owners had unlimited emotional and financial commitment to the patient, how would have you investigated the case to arrive at a definitive diagnosis, and how would you have treated the case?
Email cve.marketing@sydney.edu.au Best complete answer will receive a CVE $100 voucher!
Dr Natalie Courtman
Associate Professor of Veterinary Clinical Pathology
Veterinary Pathology Diagnostic Services, Sydney School of Veterinary Science
t. +61 2 9351 3099
e. natalie.courtman@sydney.edu.au
C&T No. 6024
Thank you to all our readers who answered the What’s Your Diagnosis. The most common answer given was histiocytoma.
This case is a cutaneous poorly pigmented melanoma. The cytologic appearance of the cells is very similar to a histiocytoma but there are two key (but subtle) differences. Firstly, some of the cells contain a fine dusting of green pigment in the cytoplasm which is consistent with melanin. This is not expected with a histiocytoma but is expected in a melanoma, melanocytoma or pigmented epithelial cell tumour. The cells being round and not cohesive makes epithelial origin unlikely. Secondly, the nuceloli are prominent in these cells and sometimes quite large which is not expected with a histiocytoma nor a melanocytoma, but is a characteristic feature of melanoma (which is a malignant tumour).
of the cytology smear is shown below.



A 13-year-old neutered female Staffordshire bull terrier was referred for evaluation of a raised round 2cm pigmented haired left foreleg mass above the carpal pad. Fine needle aspirate smears were prepared from the mass and stained with Wrights Giemsa.
 Figure 3. FNA from leg mass (1000x magnification)
Figure 1: FNA from leg mass (200x magnification)
Figure 2: FNA from leg mass (1000x magnification)
Image
Figure 3. FNA from leg mass (1000x magnification)
Figure 1: FNA from leg mass (200x magnification)
Figure 2: FNA from leg mass (1000x magnification)
Image
What is your diagnosis based on the cytologic appearance?
Cytology description: The cytology is highly cellular with good cell preservation and staining, containing round cells amidst small amounts of blood in a light blue proteinaceous background. The round cells have round nuclei of granular chromatin with 1-3 prominent nucleoli and small to moderate amount of light blue cytoplasm often containing a few fine vacuoles and rarely containing a dusting of fine green pigment ( Figure 2 green arrows). They show marked anisokaryosis and anisocytosis (3-fold variation), variable nucleolar size and shape, frequent macronucleoli ( Figure 3 red arrow), variable N:C ratio, occasional binucleation and occasional mitoses are evident ( Figure 3 yellow arrow).
Interpretation: Melanocytic neoplasm, most likely malignant melanoma.
Differentials: Melanocytoma, less likely other round cell neoplasia e.g. histiocytic sarcoma, plasmacytoma, agranular mast cell tumour. A pigmented basilar cell tumour is considered unlikely as no cell cohesion is evident. Histiocytoma is considered unlikely based on the nuclear atypia and prominent nucleoli.
Melanocytic neoplasms are derived from neural crest and include benign melanocytoma, benign melanoacanthoma, and malignant melanoma. Most melanocytic neoplasms arising in the haired skin of dogs are benign melanocytomas whereas most melanocytic neoplasms arising in the nail bed, oral cavity and at mucocutaneous junctions are malignant melanomas (Bolon 1990, Smedley 2011). Cutaneous melanocytomas tend to be heavily pigmented, small, raised, nonulcerated and confined to the dermis. Cutaneous malignant melanomas tend to be poorly pigmented, larger, ulcerated, extend deeper than the dermis and show greater variation in nuclear morphology and more frequent mitoses (Smedley 2022).
Malignant melanomas usually affect dogs older than 6 years. Breeds reported to have increased risk include Schnauzer (miniature, standard and giant), Chow Chow, Shar Pei, Scottish terrier and Doberman Pinscher. Most cases of malignant melanoma involve the nasal bed, oral cavity and mucocutaneous junctions; however, they also occur in haired skin particularly of the head and scrotum (Goldschmidt 2017). Malignant melanomas can grow rapidly, are locally invasive, and can metastasise via lymphatics to regional lymph nodes. They can also spread to more distant sites such as brain, lung, heart, kidney and spleen (Bolon 1990).
Histologic criteria associated with a poorer prognosis in cutaneous melanocytic neoplasms include nuclear atypia (≥20% atypical nuclei e.g. large nuclei, large nucleoli, irregularly shaped nucleoli, multiple nucleoli, eccentric nucleoli), poor pigmentation (<50% of cells pigmented), mitotic count (≥3 mitoses per field area of 2.37mm²), proliferation marker Ki-67 index (≥15% positive cells), size (tumour thickness >0.95cm), extension beyond the dermis and vascular invasion. (Spangler 2006, Smedley 2011)
Of these criteria, only nuclear atypia and degree of pigmentation can be evaluated on cytology smears, along with a subjective assessment of frequency of mitoses. Nuclear atypia and poor pigmentation were evident in the smears from this case supporting malignant melanoma as the most likely diagnosis. Histologic evaluation of the excised mass confirmed malignant melanoma with a high mitotic count (>10 per high power field).
Histologic evaluation of sentinel lymph nodes is recommended even if the lymph nodes are not enlarged as metastatic disease can still be present (Grimes 2017). Cytologic evaluation of lymph node aspirates for metastatic melanoma is problematic as differentiating melanocytes from melanophages (macrophages that contain melanin pigment) and haemosiderophages (macrophages that contain haemosiderin) can be difficult leading to equivocal results. Agreement between routine cytology and histopathology for staging of lymph nodes in dogs with melanocytic neoplasms has been reported as slight to fair, with cytology showing lower sensitivity for detection of metastatic disease (Grimes 2017).
Bolon, B., Calderwood Mays, M. B., & Hall, B. J. (1990). Characteristics of canine melanomas and comparison of histology and DNA ploidy to their biologic behaviour. Veterinary pathology 27(2), 96–102. https://doi.org/10.1177/030098589002700204
Spangler, W. L., & Kass, P. H. (2006). The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Veterinary pathology, 43(2), 136–149. https://doi.org/10.1354/vp.43-2-136
Goldschmidt MH and Goldschmidt KH. Chapter 4 Epithelial and melanocytic tumors of the skin, in Tumors in Domestic Animals. Meuten DJ ed. Page 125-131. Wiley Blackwell 5th edition 2017.
Smedley, R. C., Sebastian, K., & Kiupel, M. (2022). Diagnosis and Prognosis of Canine Melanocytic Neoplasms. Veterinary sciences 9(4), 175. https://doi.org/10.3390/vetsci9040175
Grimes, J. A., Matz, B. M., Christopherson, P. W., Koehler, J. W., Cappelle, K. K., Hlusko, K. C., & Smith, A. (2017). Agreement Between Cytology and Histopathology for Regional Lymph Node Metastasis in Dogs With Melanocytic Neoplasms. Veterinary pathology 54(4), 579–587. https://doi.org/10.1177/0300985817698209
Smedley, R. C., Spangler, W. L., Esplin, D. G., Kitchell, B. E., Bergman, P. J., Ho, H. Y., Bergin, I. L., & Kiupel, M. (2011). Prognostic markers for canine melanocytic neoplasms: a comparative review of the literature and goals for future investigation. Veterinary pathology, 48(1), 54–72. https://doi. org/10.1177/0300985810390717

Specialist in veterinary oncology
One Cancer Care for Pets
e. Katrina.cheng@onepetcancercare.com
Cutaneous melanoma tends to have less aggressive behaviour compared to oral melanoma, although occasionally they can have aggressive behaviour. Although we know that stage is prognostic for oral melanoma, this is less defined in dogs with cutaneous melanoma. In a recent study looking at post-surgical outcome and prognostic factors in canine malignant melanomas of the haired skin ( Laver et al. 2018), the post-surgery median PFS and median OST of 87 cases were 1,282 days and 1,363 days, respectively. In the dogs that lymph node status was known, only 5 dogs had lymph node metastasis, compared to 37 without lymph node metastasis. The survival time between the two groups did not result in any statistical significance.
If this dog had lymph node metastasis (and confirmed by histopathology), especially if other negative prognostic factors (such as high mitotic count and nuclear atypia) were also present, I would consider the use of immunotherapy, such as the Oncept vaccine. However, as far as I know, there has not been any evidence on the survival benefit of using Oncept in cutaneous melanoma. In Laver et al’s study, the median OST of dogs receiving the Oncept vaccine was not statistically different from patients receiving no adjuvant therapy after surgery. Therefore, the use of Oncept vaccine in this scenario is based on the lack of other systemic treatment, and the evidence of efficacy in oral melanoma.
Oncept melanoma vaccine contains xenogeneic (human) DNA encoding tyrosinase. Tyrosinase is essential for melanin synthesis and overexpressed in tumour cells, but not normal melanocytes. Injection of xenogeneic tyrosinase DNA produces a human antigen that is homologous to canine tyrosinase, but recognised as foreign, therefore elicits an immune response against malignant melanoma cells. Oncept is licensed to use in dogs with stage II or III oral melanoma.
References
Laver T, Feldhaeusser BR, Robat CS, Baez JL, Cronin KL, Buracco P, Annoni M, Regan RC, McMillan SK, Curran KM, Selmic LE, Shiu KB, Clark K, Fagan E, Thamm DH. Post-surgical outcome and prognostic factors in canine malignant melanomas of the haired skin: 87 cases (2003-2015). Can Vet J. 2018 Sep;59(9):981-987. PMID: 30197441; PMCID: PMC6091115.

Professor Bruce Andrew Christie
By Wing Tip WongDr Bruce passed away peacefully surrounded by family on 19 February 2024. As an educator, Bruce was noted for his calm and non-judgemental approach to helping someone discover the gift of learning. His strength lay in supporting and guiding a learner to build a strong foundation in principles which can be utilised to understand why things happen, to maximise a favourable outcome, and to navigate out of a problem. The veterinary community has lost a passionate mentor and a selfless educator. His contributions will live on in those who have learned from and been inspired by him.

Read the full obituary here

Adj Professor Philip Moses BVSc MRCVS Cert SAO MANZCVS FANZCVS AM
By Mandy Burrows, Terry King & Richard MalikPhilip Moses died peacefully on 16 March 2024, surrounded by his family. The veterinary profession has lost an exceptional individual, a colourful, bigger than life personality with an authentic life story. Philip loved and served the veterinary profession. There are probably no continents on earth where his influence on the veterinary profession has not reached. We will remember Philip as a dedicated veterinarian and great friend. The world is a shade darker with his loss.

Read the full obituary here

The International Society of Feline Medicine is the veterinary division of the pioneering cat welfare charity International Cat Care. Trusted by vets and nurses, it provides a worldwide resource on feline health and wellbeing, via the Journal of Feline Medicine of Surgery, by fostering an international community of veterinary professionals with a shared vision of feline welfare, and supporting professional development with practical CPD. Additionally, International Cat Care’s website provides a valuable resource of accurate information delivering what both vets and cats would want owners to know.
Cecilia Villaverde BVSc PhD DACVIM (Nutrition) DECVCN
Sam Taylor BVetMed(Hons)
CertSAM DipECVIMCA MANZCVS FRCVS
C&T No. 6025
Feeding tubes are an excellent tool to manage cats that cannot or will not eat enough, and, depending on the case, can be used both in the hospital and at home. This article looks at important factors to consider when deciding in which cases feeding tubes should be used, when to place them and how to develop a feeding plan.
There is a close relationship between illness and malnutrition. An inadequate nutritional status can predispose to illness via several mechanisms, such as effects on the immune system and damage to the intestinal barrier function. On the other hand, ill animals are more prone to malnutrition due to either reduced food intake and/or increased nutrient and energy requirements. Several studies in veterinary medicine support that patients not meeting their energy requirements can have poorer outcomes,1–3 and that nutritional support can help in these situations. Therefore, efforts should be made to ensure that ill cats receive adequate nutritional support.
Performing a complete nutritional assessment⁴ is important for several reasons, such as deciding on the


best feeding plan (diet, allowance, feeding method) and best route of feeding, and also to identify the patients that will benefit from nutritional support and when to start it. For hospitalised patients, Table 1 shows some of the risk factors that will indicate that the patient requires nutritional support.
Age: kittens and older animals are more prone to malnutrition than young adults. Chronic losses: chronic losses (e.g. vomiting, diarrhoea, polyuria or hypermetabolic state) accelerate the rate of development of: Involuntary weight loss: this is one of the clearer markers of malnutrition, but also denotes that the process is quite advanced.
Days of decreased/absent food intake: the length of anorexia/ hyporexia correlates tightly with malnutrition risk and it precedes weight loss; therefore, this risk factor alone can be enough to start assisted feeding.
Body condition score (BCS): low BCS is related to inadequate energy intake of some duration, as loss of at least 10% of body weight is needed to note a low BCS. While patients that present with a low BCS require more urgent support, a high BCS is not a reason to not provide nutritional support if other risk factors are present. Overweight cats that do not eat are at high risk of hepatic lipidosis,⁵ and it is important to provide assisted feeding in a timely manner to prevent this serious condition.
Muscle condition score (MCS): a low MCS is a nutritional risk factor but is not specific, meaning that low protein and energy intake can contribute
to a low MCS, but many other factors do as well, such as sarcopenia, cachexia or intestinal disease.
Skin and coat: the skin is the largest organ in the body and has a high turnover rate; as such, nutritional deficiencies can show there.⁶
Some laboratory findings can be representative of malnutrition; however, these measurements are late (i.e. the malnutrition is already advanced) and are also not specific or sensitive, so they should be interpreted together with the other factors.
The sooner the better, as suggested by most studies from human and veterinary medicine.1,7,8 It is important to stabilise the patient, i.e. they are well hydrated, haemodynamically stable and there are no acid–base or electrolyte abnormalities.
Oral voluntary intake is the preferred feeding method and there are a variety of strategies that can be used to promote this. If oral intake is not sufficient, and the patient is still anorectic or hyporectic and losing weight, assisted feeding is required. This can be via the enteral or parenteral route; enteral is preferred9,10 as it is safer, cheaper, easier, and more adequate nutritionally. Parenteral feeding (central or peripheral) is not nutritionally complete, therefore it can be used only for the short term.11,12
Syringe/force feeding is not recommended, as it can result in food aversions, aspiration pneumonia or injuries, and is also stressful for the patient. It is also unlikely to meet feeding requirements.
Feeding tubes may be placed to provide fluids and nutrition but also to facilitate long-term medication (e.g. for mycobacteriosis). They can be placed quickly and should be considered proactively; for example, if a patient is being anaesthetised for diagnostic tests but is consuming less than the resting energy requirement (RER) or is predicted to be inappetent after surgery.
The type of feeding tube chosen will depend on the patient, its underlying illness, its temperament and the likely duration of the inappetence. The most commonly placed are naso-oesophageal (NO), nasogastric (NG) and oesophagostomy (O) tubes. Each has advantages and disadvantages.
NO or NG-Tubes (Figure 1), can be used to provide nutrition for up to 5 days and are also useful when a cat
is not a candidate for general anaesthesia. The narrow diameter reduces diet choice to liquid diets and the tube can become obstructed. Crushed medications may also obstruct the tube but liquid medications usually pass easily.
O-Tubes (Figure 2) are well tolerated and larger bore so facilitate the use of many diets and the administration of crushed or liquid medications. However, placement requires general anaesthesia. Common complications are dislodgement, obstruction and stoma site infection.
Other types of feeding tube, such as gastrostomy (G)-Tubes (Figure 3), are less commonly placed but are indicated in cases of oesophageal disease/dysmotility.


 Figure 1. Naso-oesophageal or nasogastric tubes are useful for short-term assisted feeding
Figure 1. Naso-oesophageal or nasogastric tubes are useful for short-term assisted feeding

Figure 3. Cat with gastrostomy tube in situ
Step-by-step guides to the placement of NO/NG and O-Tubes are available via the QR codes (below). Placement of an NO/NG-Tube can be facilitated by mild sedation/ anxiolysis (e.g., gabapentin, butorphanol) and placement should be confirmed with radiography and/or capnography and checking for a vacuum or aspiration of acidic stomach content in the case of an NG-Tube.
Placement of O-Tubes can be assessed by radiography, endoscopy or fluoroscopy, and should be checked before each use by checking for negative pressure and injection of 2 mL sterile saline to monitor for coughing or respiratory noise that could suggest endotracheal intubation. G-Tubes are placed surgically or with endoscopic guidance and can be used long-term.
Complications are unusual and generally minor with both NO/NG and O-Tubes. Patient interference can be minimised with cat friendly interactions and environment and using soft collars.
Serious complications include endotracheal intubation and, for O-Tubes, damage to neurovascular structures in the neck resulting in haemorrhage or transient Horner’s syndrome (both rare). Stoma site infection (Figure 4) can occur with O and G-Tubes and is usually managed with antimicrobial dressings and increased cleaning of the area, and avoided with aseptic placement. Cats on chemotherapy or corticosteroids may be more likely to suffer stoma site infections. Complications of G-Tubes can include injury to abdominal viscera, peritonitis, cellulitis, vomiting and metabolic derangements.
Click on the links below to see the videos: youtube.com/watch?v=MEge0fUqotY youtube.com/watch?v=w3K9KWJwsqc
Cats should be offered food under supervision, while the NO/NG feeding tube is in place. Removing Elizabethan collars, or using soft fabric versions, may help to encourage voluntary intake.
If cats are still not eating adequately voluntarily when the NO or NG-Tube has been in place for 5 days, consideration should be given to placing a more mediumterm tube such as an O-Tube. Some cats may be deterred from eating by the presence of the tube; hence, tube removal and ‘testing’ of appetite may be needed, with the tube replaced if voluntary intake remains inadequate. With O-Tubes, cats should be able to eat normally (fabric collars, rather than hard plastic Elizabethan collars can help) and appetite and food intake can be monitored. Sometimes, O-Tubes can be left in place to facilitate medicating the cat, even when its appetite has returned.
G-Tubes cannot be removed for 10–14 days postplacement to allow a seal to form at the gastrostomy site. NO/NG and O-Tubes can be removed with the cat conscious; G-Tube removal is dictated by the type, with some requiring endoscopic removal.
Every cat with a feeding tube should be given a customised feeding plan, which includes diet, allowance, and feeding method.
The diet choice must cover the nutritional requirements of the patient (which depend on its life stage), be well tolerated and include any necessary modifications if the cat has a nutrient-sensitive disease. Other factors that will affect diet choice include availability and cost, palatability (which does not matter with the feeding tube, but can help when stimulating food intake), energy density (the higher the better) and texture (some foods will not be able to fit through certain feeding tubes).
If possible, choosing a convalescence-type diet is indicated, as these have a high energy density, are palatable and are made specifically to be easy to fit through feeding tubes. Some are liquid and some have a slurry consistency. Moreover, these diets are highly digestible, high in good-quality protein, high in fat (which helps with texture, palatability, and energy density) and low in carbohydrates and fibre (as these cats can have insulin resistance and fibre can clog the feeding tube).
For hospitalised patients, the goal is to feed RER (70 × body weight [kg]0.75, kcal/day). Once RER has been reached, and well tolerated, the amount can be

increased if needed; for example, if the patient is losing weight, a kitten is not growing or an underweight patient is not gaining weight. The RER should be calculated using the current weight of the cat, to avoid both over, and underfeeding issues, and afterwards adjustments in 10% intervals can be made to achieve the weight goals of each case.
When the RER is calculated, the daily allowance can be calculated by dividing the RER (kcal/day) by the energy content of the diet (kcal/g or mL) to give the g or mL per day. Using mL is easier as the feedings through the tube are usually done with a syringe. The manufacturer of the diet might need to be contacted for the energy content, or this can be found in product guides in the case of veterinary diets.
Once the feeding tube is in place and the cat has recovered from the anaesthetic or sedation, the tube needs to be tested with a small amount of water (the authors use 3–5 mL/kg body weight, a couple of times) to test that water flows with no obstacles. After that, feedings can be started. It is recommended to start with one-quarter or one-third of the RER, divided over 3–6 meals per day. The longer the period of inappetence and the more severe the disease, the slower the transition should be to prevent refeeding issues.
The food should be warmed to body temperature and placed in the syringe. Before feeding, the tube should be flushed with 5 mL of warm water to ensure there are no obstacles. The meal should be given slowly (at least 10–15 mins per meal). Afterwards, the tube should be flushed with 5–10 mL of warm water to make sure all the food has gone into the cat and to prevent obstruction (Figure 5).
It is important to monitor body weight to adjust the food allowance and achieve weight goals: weight stability
in patients with good or a high BCS, and weight gain (after weight stability is achieved) in growing kittens, underweight cats, or in all cats losing weight with RER. Overweight cats should be kept well stable, and a weight loss plan should be developed and implemented once the patient has fully recovered from the present disease and is eating on its own.
Food can be offered orally once the cat is showing signs of improvement, and the feeding tube can be removed once the cat has been consuming its full RER voluntarily for 3–4 days. If the feeding tube has been in place for a few days (such as for NO/NG-Tubes) and the patient is still not eating enough, a more permanent tube (e.g. O-Tube) should be placed.


body temperature
1. Molina J, Hervera M, Manzanilla EG, et al. Evaluation of the prevalence and risk factors for undernutrition in hospitalized dogs. Front Vet Sci 2018; 5. DOI: 10.3389/fvets.2018.00205.
2. Brunetto MA, Gomes MOS, Andre MR, et al. Effects of nutritional support on hospital outcome in dogs and cats. J Vet Emerg Crit Care 2010; 20: 224–231.
3. Remillard RL, Darden DE, Michel KE, et al. An investigation of the relationship between caloric intake and outcome in hospitalized dogs. Vet Ther 2001; 2: 301–310.
4. Freeman L, Becvarova I, Cave N, et al. WSAVA nutritional assessment guidelines. J Small Anim Pract 2011; 52: 385–396.
5. Armstrong PJ and Blanchard G. Hepatic lipidosis in cats. Vet Clin North Am Small Anim Pract 2009; 39: 599–616.
6. Hensel P. Nutrition and skin diseases in veterinary medicine. Clin Dermatol 2010; 28: 686–693.
7. Will K, Nolte I and Zentek J. Early enteral nutrition in young dogs suffering from haemorrhagic gastroenteritis. J Vet Med A Physiol Pathol Clin 2005; 52: 371–376.
8. Mohr AJ, Leisewitz AL, Jacobson LS, et al. Effect of early enteral nutrition on intestinal permeability, intestinal protein loss, and outcome in dogs with severe parvoviral enteritis. J Vet Intern Med 2003; 17: 791–798.
9. Eirmann L and Michel KE. Enteral nutrition
10. In: Silverstein DC, Hopper K (eds). Small animal critical care medicine. 2nd ed. St. Louis: Elsevier Saunders, 2014, pp 687–690.
11. Chan DL. Nutritional support of the critically ill small animal patient. Vet Clin North Am Small Anim Pract 2020; 50: 1411–1422.
Figure 4: Oesophagostomy tube stoma site infection Figure 5. Prepare flushes for pre- and post-feeding and warm the food to12. Chan DL, Freeman LM. Parenteral nutrition in small animals. In: Chan D (ed). Nutritional management of hospitalized small animals. Chichester: Wiley, 2015, pp 100–116.
13. Pyle SC, Marks SL, Kass PH. Evaluation of complications and prognostic factors associated with administration of total 14. parenteral nutrition in cats: 75 cases (1994– 2001). J Am Vet Med Assoc 2004; 225: 242–250.
More details on feeding tubes/ placement/management and appetite stimulants can be found in the 2022 ISFM
Consensus Guidelines on Management of the Inappetent Hospitalised Cat here: journals.sagepub.com/doi/ full/10.1177/1098612X221106353, including videos of the techniques, nutritional history forms and tube feeding records to download.
Further reading

C&T No. 5723 Danielle's Top Tip for Feeding Tubes
C&T No. 5845 Horner's Syndrome as a complication of O-Tube Placement
Welcome to Research Roundup, where ISFM brings you summaries of the latest feline research. This issue features interesting articles covering different areas of feline health and welfare including obesity, chronic kidney disease, bicavitary effusions and hypertension. Additionally, the new ISFM/AAFP guidelines on the long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) in cats. There are also useful caregiver guides for owners.
1. Clinical Spotlight: 2024 ISFM and AAFP consensus guidelines on the long-term use of NSAIDs in cats
2. Blood pressure in hyperthyroid cats before and after radioiodine treatment
3. Bicavitary effusion in cats: retrospective analysis of signalment, clinical investigations, diagnosis and outcome
4. Information about life expectancy related to obesity is most important to cat owners when deciding whether to act on a veterinarian’s weight loss recommendation
5. Risk factors and implications associated with ultrasound-diagnosed nephrocalcinosis in cats with chronic kidney disease

Read the articles here


1 Feb 2025 - 30 Nov 2026
Enrol now at Super Early Bird rates before 30 Jun 2024 to save AU$827 and automatically be entered in the lucky draw to win AU$1,000 off your course registration fee. cve.edu.au/feline-medicine
Delivered in Partnership with:


Tutor for the Backyard Poultry TimeOnline course
e. r.doneley@uq.edu.au
C&T No. 6026
I was interviewed by the ABC on Friday 12 April regarding an article published in The Guardian: Australia’s back yard chicken owners urged to implement biosecurity measures in case of bird flu outbreak. Paraphrasing the old advertisement, ‘Flu ain’t flu’.
Many people think that Avian Influenza (AI) is just one disease and forget the myriad of strains that exist.
From the Qld Department of Agriculture and Fisheries website:
Highly pathogenic avian influenza - HPAI
8 outbreaks of HPAI have occurred in Australia since 1976. HPAI viruses caused clinical disease in commercial poultry in Victoria in 1976 (H7N7), 1985 (H7N7) 1992 (H7N3) and 2020 (H7N7), in Queensland in 1994 (H7N3), and in New South Wales in 1997 (H7N4), 2012 (H7N7) and 2013 (H7N2). Each time, there was severe disease in affected chicken flocks. All had obvious or circumstantial evidence of contact with wild waterfowl or surface water contaminated by wild waterfowl, or with free-range farmed ducks. There is some evidence that, initially, low pathogenicity avian influenza (LPAI) may have been involved in the outbreaks in 1976, 1992 and 1997.
There have also been about 10-20 detections of Low Pathogenicity AI (LPAI).
In other words, there’s nothing new about AI in Australia, but none of the outbreaks have involved the zoonotic strain, H5N1.
The prevailing thought has been that Australia’s good fortune in avoiding H5N1 has been the low number of migratory bird pathways crossing our shores, especially when compared to the northern hemisphere. However, there is a growing concern that, with climate change, these pathways may shift and put Australia in the firing line.
So, at the moment, vets and their clients should be aware that:
1. Our first line of defence against AI is our geographical isolation, although that may change one day.
2. Our second line of defence are Government surveillance programs in northern Australia, catching and testing wild birds for AI.
3. Our third line of defence is government and private veterinarians monitoring for unusual mortality events in chicken flocks (both commercial and backyard)
4. Our fourth and final line of defence is the experience gained through the outbreaks of AI in Australia over the last 50 years. The containment and eradication plans are not just theoretical, but have been tested in real outbreaks, and have been found to work well.
The other point to note in the QLD DAF statement is: All had obvious or circumstantial evidence of contact with wild waterfowl or surface water contaminated by wild waterfowl, or with free-range farmed ducks. This virus is spread by wild migrating waterfowl; domestic and commercial chickens are infected when they consume water contaminated by these wild birds. People are infected by infected chickens, through aerosol transmission in a poultry shed. Using only town or bore water minimises this risk. If this is not an option, in-line water chlorinators, adding 2ppm chlorine, can often prevent infection.
Water chlorination, good hygiene, and reporting unusual mortalities can make a significant contribution to keeping Australia and its people safe.





STOP PRESS! STOP PRESS!
Latest update from Bob Doneley as at 12 June 2024 after the issue was printed.
Read Update for the latest outbreak of HPAI in Victoria (22 May 2024)
Read Australia’s back yard chicken owners urged to implement biosecurity measures in case of bird flu outbreak
Principal Scientist | Dog Oncology Group Pty Ltd.
e. dr.c.w@outlook.com
C&T No. 6027
Some cancers produce unique proteins or antigens on their surface which differ from that of a normal cell. These proteins can provide a target for the immune system. CANCV-4 utilises the dog’s own cancer tissue to produce a vaccine which ‘re-educates’ and stimulates the immune system so it recognises and targets the cancer.
The Evolution: CACV-1—CANCV-4
Over a period of 7 years CANCV has been tested, refined, and improved to increase levels of immune stimulation and response:
CACV-1 Used a commercially available adjuvant— immunity waned over time and no apparent response in tumour types like Melanoma. Results of this initial cohort were published Vet. Sci. 2018, 5(4), 87; doi.org/10.3390/vetsci5040087
CANCV-2 Nanoparticle adjuvant was introduced to replace commercially available one after increased efficacy was seen in rodent cancer models.
CANCV-3 Higher dose nanoparticles and improved cancer protein isolation and extraction. Introduction of immune stimulant into the vaccine to draw more immune cells into the vaccination site.
CANCV-4 Further modified nanoparticles for increased immune stimulation with improved dendritic cell uptake and antigen presentation to T cells.
Incorporation of tumour antigens into or onto the nanoparticle for slower release and improved immune cell uptake.
Ability to use fresh, frozen, formalin fixed or paraffin embedded tumour tissue.
Fresh or frozen tissue is collected and sent to the lab for processing. Formalin fixed, paraffin embedded tissue can also be used to make a vaccine, but this takes longer to process (4 days). The process involves:
1. Processing the tissue to a singular protein level.
2. Treating the proteins to expose more potential antigens.
3. Combining with or incorporating the antigens into the nanoparticles with immune stimulant.
4. Bottling for administration.
Normal cell surface antigen
Cancer associated antigen

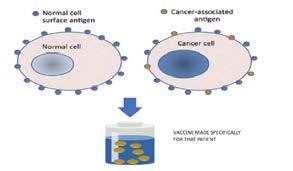
Vaccine made specifically for that patient
Figure 1. 0.2g of fresh, Paraffin embedded, or formalin fixed tissue is required for an initial eight dose course of vaccine
Initial doses (0.4 mL) are given intramuscularly 4 x weekly, 2 x weekly and 2 x fortnightly or 4 x fortnightly and then four monthly boosters are administered. Depending on your dog’s cancer scenario and sample size, more doses can be made and administered after initial course.
Various versions of CANCV have been used to treat over 700 dogs with different types of cancer. Minor, temporary side-effects, lasting 24-48 hours may occur, such as lumps at the vaccination site, malaise, fatigue, or loss of appetite. No major side effects or anaphylaxis seen.
Primary treatment—following tumour removal by surgery (clean/narrow/dirty margins)—to prevent or slow tumour regrowth.
Combined with standard of care treatments (e.g. chemotherapy)—particularly single agent chemotherapy.
As an accessory to debulking large tumours—to aid regression, ‘stabilise the tumour’ or slow tumour regrowth.
As part of palliative care—In dogs with advanced disease combining the vaccine with prednisone can in some cases improve expected survival.
The publication (see below) from 2018 reported initial data obtained from CACV-1 which incorporated a commercially available adjuvant. This study between 2016-2018 was designed to show safety and look for evidence of efficacy with the CACV-1 vaccine formulation. The study also compared the relative response rates of adding an extra stimulant (Rhizavidin) to the vaccine versus without. At the time of publication, dogs receiving Rhizavidin in their vaccine had lower survival rates (50%) compared to dogs receiving vaccine without it (75%). By 2019, all dogs with vaccine formulated with rhizavidin were deceased and approximately 20% of those not receiving it remained alive. One key observation from the study was that weekly vaccinations to start appeared to improve survival and this practice has been followed with CANCV-2 to 4 or with slight variations. The study highlighted also that the age of a dog effects efficacy of this autologous vaccine approach with dogs greater than >12 years of age less likely to gain benefit.
By the end of 2019, CACV-1 was being phased out after the remaining dogs relapsed. Many of these were switched to CANCV-2/3 and a proportion of these dogs remain alive today.
Disclaimer
While we have seen excellent results when treating dogs with CANCV2-4 we cannot guarantee that this treatment will be effective for all dogs. While every effort is made to obtain the best outcome, there can be many factors that have an impact on the effectiveness of the vaccine. Variables may include how advanced the cancer is, what type of cancer, age of the dog, the level of disease, prior treatments for the cancer, as well as the quality of the tumour sample used to create the vaccine. For best potential outcomes, the full course of recommended vaccinations must be completed.
Dr Michaela Swan BVSc Hons MANZCVS (Small Animal Medicine)
Oncology Registrar
I have used autologous vaccines created by Dr Weir since 2020. The process of submitting tumour samples, receiving the vaccines, and communicating with Dr Weir has always been easy. I have used the vaccines for a wide variety of cancers in both cats and dogs. The vaccines are well tolerated, and I have not seen any side effects of the vaccines. These personalised vaccines harness the patient’s immune system to attack their cancer.
Thus, this is another form of treatment in addition to more traditional therapies for cancers such as surgery, chemotherapy, and radiation. The lack of side effects and possibility of a benefit from these vaccines entices owners to elect this therapy, often in addition to other more traditional treatments. Anecdotally, I have seen improved survival times in dogs with osteosarcomas who have had amputations, chemotherapy, and ongoing autologous vaccine therapy.
Immunotherapy is rapidly growing field and it is becoming a huge component of therapy for a number of human cancers. With ongoing research like Dr Weir’s, hopefully it can have a similar role in veterinary medicine.

Dr Fiona Coghill BVSc MANZCVS MVM DECVS EBVS European Specialist in Small Animal Surgery
Brindabella Veterinary Referral Service, Canberra
Floki, an 8-year-old Dogue de Bordeaux cross who is full of life, 4 months after treatment with mandibulectomy and CANCV-4 for stage 3 oral malignant melanoma
I use Canine Autologous Nanoparticle Cancer Vaccine V4 ( CANCV-4) in my patients after oncological surgery. It is well tolerated by patients and is easily combined with conventional chemotherapy protocols. Having the vaccine made is as easy as posting a frozen sample of the tumour to Dr Chris Weir at Dog Oncology Group (DOG). The vaccine is made quickly, usually having it back in time for the first post-surgical recheck. In my experience, owners are excited to consider CANCV-4 due to the safety aspect of a vaccine created from their dog’s own tissue and the knowledge that immunotherapy is an additional weapon in the fight against their dog’s cancer. My current osteosarcoma and oral malignant melanoma cases are surviving beyond expected median survival times using the combination of surgery, chemotherapy and CANCV-4.

Cancer Types: Resources akcchf.org acfoundation.org/ Read the 2018 study here.


More case reports and testimonials.
Lymphoma In early 2020, a phase 2 placebo trial of CANCV-2 (version 2) combined with CHOP for Multicentric B and T-cell lymphoma was commenced just as the Covid-19 pandemic started which severely affected the study. The aim of the study was to see if adding vaccine to CHOP or palliative care extends survival. Of the 28 dogs recruited, 4 died prior to receiving the vaccine or placebo vaccine (adjuvant only); of the remaining 24, 13 received the vaccine and 11 the placebos. The results were inconclusive due to low recruitment with median survival times of 10-11 months in both groups. Despite this, the trial produced some important observations:
1. That vaccination with adjuvant only can have an immune boosting benefit on its own
2. Only 20-25% of dogs probably benefit from the addition of vaccine or adjuvant with CHOP
3. Single agent Dox + vaccine could be another cheaper treatment option
4. Boosting with adjuvant prior to surgery and treatment may be another option to improve survival times
5. While palliative, prednisone is immunosuppressive; approximately 10-15% dogs will have a significant extension of survival with the addition of vaccine over prednisone alone (4-6 weeks)
Haemangiosarcoma Dogs with haemangiosarcoma survive between 30-90 days if treated with surgery alone. Standard of care surgery and chemotherapy (Doxorubicin) improves average survival to 6 months. If the cancer has not spread to other parts of the body (metastasized), evidence indicates that treatment with CANCV-2/3 can improve survival times, with some dogs surviving for more than 2 years. No dogs have been treated with CANCV-4 yet.

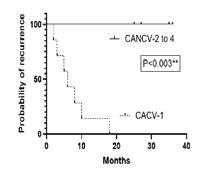
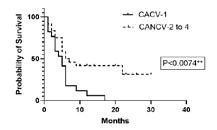
 Mast cell tumours (MCT)
A.
C.
Mast cell tumours (MCT)
A.
C.
Mast cell tumours (MCT) continued
Melanoma
Osteosarcoma
Nearly 200 dogs have been treated for both high and low grade MCT with vaccine alone or with chemotherapy (CCNU, Vincristine) or palladia. Figure A Shows the significant lower rate of recurrence in dogs with low grade treated with CANCV-2-4 compared to CACV-1 which vaccine formulation has now been abandoned. Figure B shows that no dogs with recurring low grade MCT have recurred again after CANCV-2-4 treatment compared to CACV1. Figure C compares the outcomes of high grade MCTs treated with CACV-1 v CANCV2-4 alone or with CCNU/Palladia/Vincristine—Demonstrating a significant extension of survival. (Data collected 2017-2023)
CACV-1 showed no efficacy against melanoma, and this was a driver to switch to a nanoparticle adjuvant. CANCV-2 to CANCV-4 have demonstrated they can slow progression, reduce metastatic disease, and extend survival in some dogs with melanoma.
Standard therapy of amputation and treatment with chemotherapy (Carbo/Cisplatin), gives a median survival time of 11 months with 20% of dogs surviving for over 2 years. Seventy dogs have been treated with a combination of carboplatin and CANCV-2 to-4. Dogs treated with CANCV-3 and carboplatin have a median survival of 14 months with 30% of dogs surviving to 31 months. Currently 19 dogs have been treated with CANCV-4 and carboplatin. These results will take another 18-24 months to fully obtain and see if there is an improvement on CANCV-3.

Soft tissue sarcoma
Carcinoma (various types and tumour locations)
Rare cancers
Other species
These tumours recur in approximately 20% of dogs after surgery alone, with metastatic spread to other tissues occurring in 25%. CANCV-4 has successfully stopped recurrence and slowed regrowth in dogs with high grade residual tumour.
CANCV-4. can potentially stabilise disease, prolong survival or prevent recurrence. Combined with chemotherapy, dogs with anal sac carcinoma benefit from the inclusion of CANCV-4 in the treatment regime. Several dogs with mammary carcinoma have extended survival expectation with some having no recurrence over a 12 to24 month period post-surgery + vaccination.
CANCV-2 to-4 have been used to treat rare cancers. Some have far exceeded expected survival/recurrence rates but with low case numbers this evidence is purely anecdotal.
Cats: Around 20 feline patients have been treated for cancer with CANCV-2 to-4. While small numbers, the vaccine appears to slow the grow and spread of melanomas and fibrosarcomas.
Horses: Most horses treated with CANCV-2 to-4 have been with Melanoma. The dose size for horses is 1mL and, while anecdotal, there is evidence the vaccine stops recurrence or slows regrowth. Two horses have been treated for sarcoid tumours one of which had no response to vaccine or other treatments; the other has just been treated so it is too early to see benefit.
Entitled to a CVE$500 voucher
North Richmond Vet Hospital
e. harry.s@northrichmondvet.com.au
C&T No. 6028

Harry pictured with a Central Bearded Dragon named Thor
Introduction
Central Bearded Dragons (Pogona vitticeps), sometimes affectionately known as ‘beardies’, are Australian native lizards that have taken the pet industry by storm in the past few decades. They are often regarded as low-maintenance, charismatic, sociable animals that make great pets for people who might be time-poor or not possess the space for a conventional companion. Whilst this might be true to an extent, caring for a central bearded dragon correctly does require a decent investment of time and money, as well as thorough research about their husbandry and nutritional requirements. Like most captive reptiles, the most common diseases seen in central bearded dragons are usually related to improper husbandry.
In March 2023, along with Dr Hamish Baron BVSc (Hons) FANZCVS (Avian Health), I published an article in the Australian Veterinary Journal, titled: Clinical presentation and disease prevalence of captive central bearded dragons (Pogona vitticeps) at veterinary clinics in Australia.¹ This paper aimed to identify the most common reasons for pet central bearded dragons to be presented to veterinary clinics and determine the most common diseases diagnosed in these lizards to provide a useful reference for veterinarians and central bearded dragon owners. This article will give an overview of the Top 10 most common diseases that were seen in captive central bearded dragons across three veterinary clinics in Australia, including their diagnosis, treatment principles, and prevention.
It is important to know what the normal behaviour, natural habitat and diet of these lizards are to understand how they should be cared for in captivity.
Central bearded dragons grow to between 40 and 55 centimetres in length and are a generalist species that inhabit a wide range of habitats, from dry woodland forests to sandy deserts in the middle of Australia.² They are semi-arboreal and climb on trees, fence posts and rocks to bask. Although in captivity they can live for 10-14 years, in the wild, they have an average life expectancy of less than 4 years.² They are naturally solitary, territorial animals.³ They are diurnal and heliothermic, sourcing heat by basking in sunlight for multiple hours a day to reach their preferred body temperature of about 36°C.² In their natural habitat, the relative humidity level fluctuates between 10% and 60%, spiking and dropping throughout the day, although they seem to be most active between 10% and 40% relative humidity.² Ultraviolet B (UVB) is essential for central bearded dragons to synthesise Vitamin D3, required to absorb calcium from the gastrointestinal tract. UVB radiation is correlated with UVI (ultraviolet index). Central bearded dragons have been recorded basking between 0 and 10.7 UVI, with an average of 4.1 UVI.² Central bearded dragons go into brumation during the winter months, this is a period of dormancy that is important for reproduction.⁴ During brumation, they shelter from the cold and will not seek food; however, they may still come out to bask on warmer days.
Central bearded dragons are opportunistic omnivores, eating both animal and plant matter. The majority of the animal matter consumed consists of invertebrates. One study found that there was a wide diversity of invertebrates eaten by wild central bearded dragons, but the most common were termites, ants, wood cockroaches, and wasps.⁵ Plant matter consumed was mostly flowering plants and accounted for more than 60% of the diet in adult animals.⁵
Our study found that the majority of these lizards were presented to veterinary clinics for non-specific signs of illness. These included lethargy, inappetence, weight/ condition loss, and constipation. These could all be signs or symptoms of multiple diseases, meaning that a good history, thorough physical examination, and a sound diagnostic plan are often required to reach a diagnosis. Other common reasons for presentation such as an attack from a cage mate, skin lump, or traumatic injury are more distinct and a diagnosis and treatment plan are easier to determine in these cases.
1. Significant Endoparasite Burden
The reason why this category was named ‘significant’ endoparasite burden was because there is such a thing as an insignificant parasite burden in the gastrointestinal

tract of central bearded dragons. Pinworms (Oxyurida) are part of the normal gastrointestinal microbiome, and improve the breakdown of ingested material.⁶ Detection of Isospora and Eimeria oocysts is a common finding that does not always indicate the presence of disease; in healthy animals it is indicative of a subclinical infection. Clinical signs of a significant parasite burden include reduced appetite, weight loss, regurgitation, abnormal faeces, and diarrhoea. The presence of parasites should be confirmed before commencing treatment.
Diagnostics: Microscopic examination of a faecal flotation and a direct faecal smear – look for eggs, oocysts, and flagellates and count how many per field of view. Cryptosporidium specific – PCR, ELISA.⁶
Treatment principles: If clinical signs are present and there is the presence of coccidian oocysts, or if Cryptosporidium is suspected, medical treatment is indicated along with an improvement in enclosure hygiene. If an animal is immunosuppressed (e.g., infected with agamid adenovirus-1, or has nutritional secondary hyperparathyroidism), treatment is indicated. Treatment is not required if the animal is healthy with no clinical signs and oxyurid eggs with or without low numbers of coccidian oocysts are present; however, monitoring the burden is essential.
Preventable with good husbandry and nutrition?
Yes, by removing faeces from the enclosure as soon as practicable, not cohabitating with other lizards, and keeping the lizard’s enclosure at the correct temperatures to support its immune system.
Nutritional secondary hyperparathyroidism (NSHP) is the most common metabolic bone disease that is seen in captive reptiles. Clinical signs include ill-thrift, weakness, musculoskeletal deformities, fractures, anorexia, paresis/paralysis, and tremors. Lizards with this disease are prone to acquiring secondary infections and pathological fractures.
Diagnostics: History (may include no access to UVB light, no dietary calcium). Physical examination. Radiographs (decreased bone density). Ionised calcium level.
Treatment principles: Need to correct the hypocalcaemia, correct the husbandry and nutrition, and manage pain. It can take a long time to recover if they do.
Preventable with good husbandry and nutrition?
Yes, nutritional secondary hyperparathyroidism is completely preventable with proper husbandry including access to UVB light, correct enclosure temperatures, and appropriate levels of calcium in the diet.
The majority of skin wounds seen in central bearded dragons in the study were due to bites from other lizards being housed in the same enclosure. Other common skin wounds seen are rostral abrasions from ‘glass surfing’.
Diagnostics: History (usually cohabitation with another lizard, and/or the owner saw the incident). Physical examination. Cytology. Bacterial culture and sensitivity.
Treatment principles: If it is a superficial injury, keep the wound clean and use a topical antibacterial. If it is a deeper injury or the risk of infection is suspected to be higher, a systemic antimicrobial may also be indicated.⁷
Preventable with good husbandry and nutrition?
Yes, central bearded dragons should not be cohabitated in the same enclosure unless it is short-term and for breeding purposes. Minimise stress if glass surfing is occurring.
Periodontal disease in central bearded dragons is initially seen as tooth staining, exposed mandibular and maxillary bone, gingivitis, and calculus accumulation.⁸ It then progresses further to include gingival recession and gingival pocket formation, with end-stage disease including osteomyelitis of the mandible and/or maxilla.⁸
Diagnostics: Physical examination. Radiographs (assess for bony involvement).
Treatment principles: Cleaning the teeth with a dental scaler and debriding necrotic gingiva and bone, with pain management and antimicrobial therapy if indicated.⁸
Preventable with good husbandry and nutrition?
Partially, older lizards are prone to the development of periodontal disease; however, it can be prevented in younger animals by excluding fruit from the diet.⁸
Similarly to skin wounds, tail necrosis is most often caused by bites from enclosure cohabitants. It can occur less commonly as a result of dysecdysis when an old shed is not removed from the tip of the tail and it restricts blood supply.⁶
Diagnostics: Physical examination. Radiographs (assess for bony involvement).
Treatment principles: Surgical amputation of the necrotic portion of the tail.
Preventable with good husbandry and nutrition?
Yes, central bearded dragons should not be cohabitated in the same enclosure unless it is short-term and for

breeding purposes. Dysecdysis should be managed appropriately.
In the study, respiratory infections were not classified as being of bacterial, fungal, viral, or parasitic origin. In central bearded dragons, bacterial infection is the most common cause, usually manifesting as a lower respiratory tract infection.⁶
Diagnostics: Physical examination (oral or nasal discharge, wheezing). Radiographs. Cytology. Bacterial culture and sensitivity. Haematology. Biochemistry.
Treatment principles: Correction of any husbandry deficits, antimicrobial therapy after culture and sensitivity if indicated.
Preventable with good husbandry and nutrition?
Yes, if the enclosure is kept hygienic, the lizard can be at its preferred body temperature, and the relative humidity is not kept consistently high or low, then the lizard’s immune system should be able to prevent an infection.
7. Agamid Adenovirus-1
Agamid adenovirus-1 is the most common genotype of adenovirus in central bearded dragons in Australia, affecting both wild and captive animals.⁹ Infected lizards can either be symptomatic or asymptomatic.⁹ Infected lizards can show clinical signs related to the gastrointestinal tract, liver, or central nervous system.⁹
Diagnostics: PCR – recommended to test if the result will have any implications on management, such as deciding whether to breed an animal or buy an animal.⁹
Treatment principles: Agamid adenovirus-1 is not a treatable disease, but infected animals can be managed appropriately if they are clinically well. Proper husbandry and nutrition are of paramount importance for infected lizards as they are immunocompromised, so secondary infections are much more likely. If the lizard’s quality of life is negatively affected, palliation or euthanasia may be an appropriate option.
Preventable with good husbandry and nutrition? May be prevented from entering collections with testing and quarantining of new animals; however, the effectiveness of this management plan is a debated topic.
In order of highest to lowest prevalence in the study, fibrosarcomas, squamous cell carcinomas, melanomas and myxomas are seen in central bearded dragons. They often present as nodular lesions with or without overlying
ulceration.⁶ Squamous cell carcinomas are often seen in the periocular region or near the mouth.10
Diagnostics: Physical examination. Cytology. Histopathology. Radiographs. Ultrasound. CT scan.
Treatment principles: Surgical excision may be indicated in some cases, in other cases, palliation or euthanasia may be a more appropriate option.
Preventable with good husbandry and nutrition? Not proven; however, it has been postulated that excessive ultraviolet light can lead to the formation of some skin neoplasms in central bearded dragons. It is good husbandry to have an area of the enclosure with a UVI of zero.
In female central bearded dragons, this condition occurs after vitellogenesis when there is a failure of ovulation or resorption of the ova.⁶ Clinical signs include coelomic distension, reduced appetite or anorexia, and lethargy. If left untreated, follicles can rupture and cause yolkassociated coelomitis.
Diagnostics: Physical examination (palpation of follicles). Ultrasound. CT scan. Haematology. Biochemistry.
Treatment principles: Waiting for resorption is often unrewarding, performing an ovariectomy or ovariosalpingectomy is required in most cases. These lizards must be stabilised with supportive care before surgery.
Preventable with good husbandry and nutrition?
Some risk factors can be prevented but the aetiology of preovulatory follicular stasis is not well understood in these lizards. Risk factors for this disease include obesity, suboptimal nutrition, nutritional secondary hyperparathyroidism, inappropriate enclosure temperatures, and inappropriate photoperiods. Surgical sterilisation of female central bearded dragons that are not required for breeding is an option for prevention.⁶
Also known commonly as ‘egg-binding’, or post-ovulatory egg stasis, this disease occurs when female lizards are not able to lay their eggs. This condition may occur due to environmental factors such as suboptimal husbandry or an inappropriate nesting site, or host factors such as concurrent disease. Clinical signs can be non-specific or similar to those seen in preovulatory follicular stasis.
Diagnostics: History (may be struggling to lay). Physical examination (palpation). Ultrasound. Radiographs. CT scan.

Treatment principles: Treatment depends on whether the dystocia is obstructive or non-obstructive. In nonobstructive dystocia, attempting correction of husbandry deficits and providing supportive care is the first option. Obstructive dystocia is often managed surgically to remove the eggs. These lizards must be stabilised with supportive care before surgery.
Preventable with good husbandry and nutrition?
Similar to preovulatory follicular stasis. Risk factors such as obesity, hypocalcaemia, dehydration, inappropriate husbandry, and inappropriate nesting sites can be avoided.
Central bearded dragons are popular unusual pets in Australia and across the world. Allowing them to have access to veterinarians who are confident in examining and treating them is important for their welfare. General health checks are recommended from a young age to avoid any deficits in husbandry and nutrition and they should be performed every 6 to 12 months, especially before brumation throughout winter. As the saying goes: ‘prevention is better than the cure’, so being able to give owners sound husbandry and nutrition advice will help to prevent the development of the most common diseases faced by these lizards in captivity.
For the full paper about the clinical presentation and disease prevalence of central bearded dragons in Australia, please visit doi.org/10.1111/avj.13234
1. Sollom H, Baron H. Clinical presentation and disease prevalence of captive central bearded dragons (Pogona vitticeps) at veterinary clinics in Australia. 2023;101:200-207. doi.org/10.1111/avj.13234
2. Howard J. Ecology of the Central Bearded Dragon (Pogona vitticeps) The Australian Veterinary Association Ltd, Melbourne, 2 December 2019.
3. Johnson R, Adwick S. Central Bearded Dragons (Pogona vitticeps) In: Yeates J, editor. Companion Animal Care and Welfare: The UFAW Companion Animal Handbook . First edn. Sussex: John Wiley & Sons Ltd; 2018:395-411.
4. Divers S, Stahl S. Mader’s Reptile and Amphibian Medicine and Surgery. Third edn . St. Louis: Elsevier; 2019.
5. Oonincx D, van Leeuwen J, Hendriks W, van der Poel A. The diet of free-roaming Australian Central Bearded Dragons (Pogona vitticeps) 2015;34:271-277. doi.org/10.1002/zoo.21209
6. Doneley B, Monks D, Johnson R, Carmel B. Reptile Medicine and Surgery in Clinical Practice. Newark: John Wiley & Sons, Incorporated; 2018.
7. Hedley J, Whitehead M, Munns C et al. Antibiotic stewardship for reptiles. 2021;62:829-839. doi.org/10.1111/jsap.13402
8. Mott R, Pellett S, Hedley J. Prevalence and Risk Factors for Dental Disease in Captive Central Bearded Dragons (Pogona vitticeps) in the United Kingdom. J Exot Pet Med 2021;36:1-7. doi.org/10.1053/j.jepm.2020.09.002
9. Hyndman T. An update on Australian reptile infectious diseases (2022). The Australian Veterinary Association Ltd, Darwin, 14 September 2022.
10. Hannon D, Garner M, Reavill D. Squamous Cell Carcinomas in Inland Bearded Dragons (Pogona vitticeps) . 2011;21:101-106, 106.





Figure 2. Fresh vegetables make up a large amount of an adult Central Bearded Dragon’s diet in captivity
Figure 3. Gross appearance of faeces from a bearded dragon with a high endoparasite burden
Figure 4. A direct prep of a faecal sample from a bearded dragon. Two Oxyurid eggs are seen along with several coccidian oocysts (Isospora)
Figure 5. Frequent exposure to natural sunlight is an effective way to combat the development of metabolic bone disease. It is also a great source of enrichment for captive reptiles.





6. Injuries inflicted by other lizards can cause the tail to become necrotic, often resulting in surgical amputation of part of the tail
Hatchling
(less than 12 weeks old) are most prone to developing serious illness due to Agamid Adenovirus-1 infection
Figure 8. A central bearded
with preovulatory follicular
that is having an ovariectomy
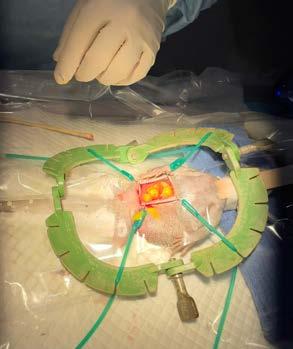

performed as treatment
Figure 9. An anaesthetised bearded dragon prior to surgery
Figure Figure 7. bearded dragons dragon stasis





Figure 10. A bearded dragon having a CT scan performed
Figure 11. Newly hatched central bearded dragons fresh out of the incubator
Figure 12. A central bearded dragon presenting for lethargy displaying a hunched posture, indicating pain. She ended up having preovulatory follicular stasis.
Figure 13. A bearded dragon on the x-ray table. This is a right lateral view to assess the internal organs.
Figure 14. A bearded dragon during the shedding process, known as ecdysis.

At the Sydney School of Veterinary Science, we’re not just about creating veterinarians; we’re about pioneering research and innovation that has a real-world impact. Our PhD program is designed for those who are driven to make a significant impact in the field of evidence-based veterinary practice through impactful research.
To apply or for more information vetsci.research@sydney.edu.au
Read the full article here

A canine desexing model utilised by 3rd year students in a tutorial prior to their first live animal spey
The Doug Anthony Veterinary Clinical Skills Space, a beacon of innovation at Sydney University dedicated to shaping the future of veterinary professionals while prioritising and continuing to advance animal welfare, utilises 3D printing technology, handcrafted silicone models, retrofitted stuffed toys and collaborations to enhance students’ learning experience in the Doctor of Veterinary Medicine degree.

Read the full article here
Andrea Harvey,1 Sarah Townsend, 2
Jamin Farebrother, 3 Richard Olsen,4
SallyAnn Olson,4 Luisa Monteiro de Miranda,1 MarkKrockenberger,1 Cathy Shilton, 5 Oliver Liyou,6 Richard Malik 1*
1Sydney School of Veterinary Science, 2 Equine Vet Services Pty Ltd Idalia, 3 Howard Spring Veterinary Hospital, 4 Capricorn Vet Surgery, 5 Berrimah Veterinary Laboratory, 6 Equine Veterinary Dental Services
*Author for Correspondence
C&T No. 6029
Thanks to the treating vet—Sarah Townsend—for spreading the word about this new treatment and providing the great images.
Introduction
Since early reports by Dave Hutchins, Phil Ladds and colleagues and the seminal PhD of Richard Miller, the clinical and laboratory features of oomycete infections in horses in northern Australia have been well described. Modern molecular studies have demonstrated that these infections in Australia can be caused by both Pythium and Lagenidium species (Halliday, Shilton, Krockenberger and Malik, unpublished observations). Numerous treatment regimens have been published utilising radical surgical excision, medical treatments including iodides, amphotericin B and autogenous vaccines which are no longer available (Mark White, personal communication). Some cases do well with therapy and others are a considerable challenge, often resulting in euthanasia. All treatment regimens are potentially expensive.
We wish to describe a much simpler treatment using an agricultural chemical called metalaxyl. It is available as a racemic mixture of two optical isomers (Zemil; 250g/L) which we use topically and a more expensive formulation containing just the active optical isomer (Solitaire Metalaxyl-M 240g/L; specialistsales.com.au/ shop/turf-domestic/fungicides-turf-domestic/solitaire240-me-fungicide-metalaxyl-m/ also called mefenoxam) which can be used both topically and orally (systemically). All treatments are off-label.
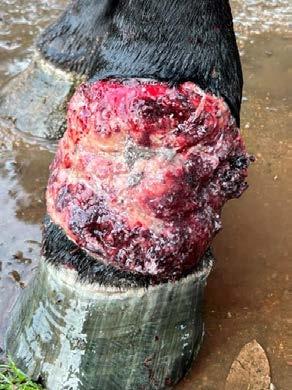




So far, 3 horses have been treated with metalaxyl. All accessible lesions are treated topically by spraying either Zemil neat or diluted with distilled water, using a spray bottle or syringe. As well, horses are given metalaxyl-M orally starting at 1 mg/kg orally twice a day for 7 days, and gradually building the dose up to 3 mg/kg twice daily. We mix metalaxyl-M in water and some raspberry jam to hide the taste and find that horses tolerate this method of administration. We are not sure whether this represents the optimal dose, and 5 mg/kg twice a day has been given to dogs. Treatment is continued until all lesions have resolved clinically. In cases with extensive disease, debulking surgery to remove ‘kunkers’ and obviously infected tissue is done after preliminary metalaxyl-M therapy has shrunk lesions to some extent. One case has completed therapy, two are ongoing, one of which has undergone surgical debulking after preliminary systemic therapy.


Metalaxyl-M costs $825 for 5 litres, and at a dose of 3 mg/kg orally BID, a 400 kg horse requires 5 mL BID, which costs $1.65 per day. Perceived high efficacy combined with low cost and acceptable toxicity makes this a promising treatment modality for swamp cancer in horses, and presumably this drug could be combined with other therapeutic modalities, as required.
Currently the investigators will pay for metalaxyl-M for any colleagues interested in trying this therapy in affected horses. It’s just a matter of e-mailing Richard Malik at richard.malik@sydney.edu.au
Hutchins, D.R. and Johnston, K.G. (1972) Phycomycosis in the horse. Australian Veterinary Journal, 48: 269-278. doi.org/10.1111/j.1751-0813.1972.tb05155.x
Miller, R.I. and Campbell, R.S.F. (1982), Clinical observation on equine phycomycosis. Australian Veterinary Journal 58: 221-226. doi.org/10.1111/j.1751-0813.1982.tb00681.x
Murray, D.R., Ladds, P.W. and Campbell, R.S.F. (1978) Granulomatous and neoplastic disease of the skin of horses. Australian Veterinary Journal 54: 338341. doi.org/10.1111/j.1751-0813.1978.tb02484.x
Billings P, Walton S, Shmalberg J, Santoro D. The Use of Mefenoxam to Treat Cutaneous and Gastrointestinal Pythiosis in Dogs: A Retrospective Study. Microorganisms. 2023; 11(7):1726. doi.org/10.3390/microorganisms11071726

Euthanasia
Dr Jessica Pockett DVM CAETARest Your Paws
In Home Palliative & End-of-Life Care
Located across New South Wales, Victoria, Adelaide & Perth
e. jessicamareepockett@gmail.com
Perspective No. 162
Introduction
The connection between humans and animals has transformed remarkably over the past two decades. The bond we share with our cherished pets is now stronger than ever, with research verifying the vital, enriching and mutually beneficial role pets play in our daily lives. The number of households in Australia that are home to pets has also drastically increased over the past few years, with Australia currently boasting one of the highest rates of pet ownership globally.1. Palliative care for pets is an emerging field of veterinary science within Australia. Its main focus is to provide comfort and supportive care to maintain a pet’s quality of life during their end-of-life stage, whilst providing support and guidance to their caregivers. When that inevitable time comes, palliative care veterinarians are also trained to provide a gentle and loving euthanasia experience, most commonly performed in the comfort of the family’s own home. Ensuring competency when performing euthanasia is not only vital for the comfort of the patient but is also essential for the well-being of veterinary clients, members of the veterinary team and the veterinarianclient relationship.2
Although euthanasia is considered to be a day-one competency in the veterinary industry, research has found remarkable variability in the teaching of end-oflife decision making and euthanasia techniques within Australasian veterinary schools.3 A survey conducted with veterinarians in New Zealand showed that one third of participants had received no formal training of the key aspects of euthanasia in veterinary school and over 84% of participants had not performed euthanasia of a dog during their training.4 In addition to this, studies have also revealed that veterinarians and veterinary
staff receive minimal training on how to deal with the emotional, verbal and non-verbal aspects of euthanasia.5 The emotional and social aspects of euthanasia are often considered the most challenging and, if not properly addressed, can quickly lead to compassion fatigue or an apprehension to perform this essential procedure.6 How we approach euthanasia consults also has a significant impact on our clients, with one study finding that the manner in which veterinarians approach companion animal euthanasia has a direct correlation with either mitigating or exacerbating a client’s grief.7 We therefore carry an immense responsibility to ensure that this procedure is conducted with humanity, respect and empathy and that our staff are educated about best practice techniques, in addition to the social and emotional requirements for euthanasia.
Not only has the role that pets play in our lives undergone a significant transformation, but the values and expectations of pet owners around their pets’ health and end-of-life care has also evolved. Below, we have outlined what our Rest Your Paws clients have valued most during the end-of-life journey with their pets.
Deciding to euthanise a pet is one of the toughest decisions that a pet owner will have to make. Providing sound professional guidance and client education plays a crucial role in assisting pet owners in making the decision about euthanasia for their beloved companions. The decision to euthanise should be made together, as a team, to help alleviate the guilt that comes with making that decision alone. As veterinarians, we possess a deep understanding of the pet’s medical condition, their prognosis, and quality of life considerations. We can, and should, be relaying this knowledge to the owners and providing owners with an evaluation of their pet’s health, the treatment or palliative options, and provide insights into the progression of the illness or injury ( Figure 1).
This should include informing owners, particularly those with pets that have several comorbidities, that a peaceful death without intervention is uncommon. This medical expertise helps pet owners make an informed decision based on the best interests of their pet. We should also be providing owners with resources so that they can continue to track their pet’s progression and identify any signs of decline. In particular, owners need to be educated and shown how to perform quality of life assessments and use pain-scoring tools. These quality of life assessments should take into consideration all aspects of the patient’s life, including their emotional and social wellbeing, not just their physical wellbeing. For

our library of resources, including printed and downloadable PDF’s, reach out to the Rest Your Paws team.
We recommend the following tools
The Ohio State University: ‘How do I know when it’s time?’ - Quality of life sliding scale. BEAP pain scale (chronic and acute) for cats and dogs
Feline grimace scale - available online or as a phone app.
2. Empathy, Support and help with Pre-Planning
Many pet owners have limited knowledge or misconceptions about the euthanasia process. Veterinary professionals should educate pet owners about what to expect during euthanasia, including the procedure itself, and what the options are for the pet’s remains. The more prepared the owner feels, the less apprehensive and fearful they will be moving forward. Beyond just the euthanasia process, pre-planning should also include giving the client additional resources or ideas on how to include children or other pets in the process, available grief support and ways to memorialise their pet’s life.
3. Time
Euthanasia appointments represent a time of grieving and mourning for the pet’s family and close friends. It is the final opportunity for owners to express their concerns and for us to then help and resolve any feelings of doubt or guilt. Most importantly, this is the final moment that families have to spend with their best friends. Euthanasia consults have transformed from a routine procedure into funeral-like services and celebrations of life, with owners providing memorabilia, such as toys and blankets, sharing stories of their favourite adventures together and wanting keepsakes such as locks of fur or paw prints. These consults should not be rushed and ideally, one hour should be put aside for each family. The use of pre-sedation can also help to slow down the procedure. Not only does this provide families with additional time, but it ensures the procedure itself is not so sudden and ‘clinical’. This is something that owners really cherish—our time, emotional connection and our care.
The last 30-60 minutes an owner has with their pet is very precious. At this point, we would never want our pet to leave our side and this courtesy should be extended to our clients. The pet should not be removed from the owner or from the room, even for IV catheterization or intraperitoneal or intraorgan injections if possible. More often than not,
clients want to be involved in these final moments and they want to be there for their pet. Adequate communication and signposting throughout the appointment is necessary to ensure that the owner is comfortable and prepared for each part of the procedure.
5. Pre-sedation
Physical restraint for IV catheterisation and administration of pentobarbitone often results in patient anxiety, distress, and discomfort. Extravasation of pentobarbitone can also result in pain for the pet. The administration of a sedative or anaesthetic provides chemical restraint and inhibits patient awareness, eliminating this stress and ensuring the remainder of the procedure is pain free. For some families, particularly those that have elected euthanasia due to behavioural issues (e.g. aggression) or conditions that are severely painful, pre-sedation enables physical touch and the chance for families to hold, comfort and cuddle their pet. In many cases, a physical connection with their pet had not been possible during those final few days, weeks, or months of the pet’s life. As a result, pre-sedation helps to reduce any feelings of anxiety, grief, and guilt for the family by making this a more pleasant experience for all of those present.
6. Smooth Procedure
Competence in performing euthanasias is important to ensure the experience is as good as it can possibly be. Training in best practice euthanasia techniques should be provided to staff. The use of sedatives to alleviate pain and distress, and reduce the risk of complications during the procedure, is considered an essential part of a smooth euthanasia. Reach out to our team if you’re interested in further training or access to our veterinary resource library.
The initial phone call or email to the vet clinic to discuss the possibility of euthanasia has been repeatedly reported, to the Rest Your Paws team, as being one of the hardest parts of the end-of-life process for pet owners. The veterinary team can make a profound difference at this point in the euthanasia experience by giving caregivers time, support and empathy.
Even for those owners that are certain in their decision that euthanasia is the best option, the use of the term ‘euthanasia’ is often avoided and can be associated with feelings of guilt, self-blame and the fear of being judged. It is therefore so important that we are the first to bring up the ‘E’ word to owners. Compassionately starting

this conversation will help alleviate those feelings of guilt by letting owners know that euthanasia is in fact an appropriate option and one they no longer have to make alone. From this point onwards, you and the owner are a team; decisions should be made together, and a profound impact can be made by consciously using the word ‘we’ instead of ‘you’.
Here are some of our suggestions on how to turn that ‘dreaded’ first phone call into a supportive and empathetic experience for the caregiver:
Once a member of your staff is aware that the conversation is in relation to a possible euthanasia, their entire focus should be given to that conversation. If possible, move to somewhere quiet where you can talk without interruption or disturbance. It is important for your staff to learn both the owner’s and pet’s name and that these are used often throughout the discussion.
Offer empathy, reassurance and compassion. It is important that owners know that we care and are here for them during this difficult time. Show empathy by saying ‘We are so sorry that you are facing this’ and acknowledging that ‘this is often one of the hardest decisions we have to make for our companions’. If you are familiar with the pet, share a loving memory or a feature of the pet that you love or that has always made you smile (e.g. Fluffy’s excitement for any and every treat in the clinic).
It is important we remain calm, confident and relaxed. We should reassure owners at this point that they are making the right decision, that you are in agreement that this is now the kindest option for their beloved pet. If the owner is unsure, then offer for them to come into the clinic or have a telehealth with a vet, so that we can continue this conversation and make the best decision for their pet as a team.
During this initial phone call or email it is important to gather further information about the pet and its condition.
Use the basics—ask open-ended questions and then move to yes/no questions as you get a better understanding of the pet’s clinical condition. Actively listen—listen to understand, not to respond. Give the owners time to answer and be comfortable with silence if the owner is struggling to tell their story—take a few breaths and inform the owner to take their time, you are here when they are ready. In some cases, the pet will need to be triaged to determine the right time for euthanasia. If the pet
is in pain or respiratory distress, advise that seeing a vet sooner rather than later would be in their best interest. If the dog is otherwise stable and, ensuring they are comfortable, allow the owners to elect their preferred time or day. If you do not have availability to see a pet in distress, then give the owners options—the contact details of emergency centres or in-home euthanasia services. This is also a good time to determine who will be present during the euthanasia consult, if there will be children present, the age of each child and if there are any other considerations required for the appointment. For example: if the dog is known to be nervous or aggressive, oral presedation should be organised ( Figures 3 and 4).
Being upfront and providing owners with an estimate of the costs for the euthanasia appointment, including any additional oral medication needed prior to appointment, is advised.
Discuss the aftercare options available (burial, general cremation or private cremation) and the estimated associated costs. For example: ‘If it helps with your decision, here are the associated costs for private cremation versus general cremation for our preferred providers’. We recommend that you also use this time to settle payment and finalise consent forms, both of which can be done digitally. Finalising the invoice and any required forms can help to streamline the euthanasia consultation by allowing both the family and your staff to focus solely on the family, the beloved pet and their needs.
A follow up email outlining your discussion and all of the required details, including costs, aftercare options and any additional resources, should be sent to the owner following your conversation. This is also a good time to advise owners that if their beloved pet has any favourite toys, blankets or other keepsakes that they would like to bring along with them for their pet’s aftercare.
If the pet is appetent, consider encouraging owners to bring in the pet’s favourite foods or something the pet has never really been able to have—like a cheeseburger, chocolate cake or ice cream. Seeing their beloved pet enjoying something delicious often brings a smile to their faces.
If the owners have a multi-pet household, allow them to bring a calm housemate with them to the appointment. We know that pets do experience grief, and this gives them a chance to understand the situation and to say goodbye.

Arrival at the clinic
Place a framed note or light a candle to signify to other clients that someone is saying goodbye to their pet and to speak softly and be respectful. Consider having a designated car park for owners who are arriving for a euthanasia appointment, that allows them easy access in and out of the clinic. An easily accessible car spot is ideal for owners with big dogs or for owners that would prefer their pet to stay in the car for the euthanasia (behavioural issues, mobility issues, or if the owners have elected home burial)
Greet the owners at their car and warmly welcome the family to the clinic. Ensure names are used and that eye contact is maintained.
Greet the pet and give them lots of praise and love.
Avoid weighing the pet on the day and move the family straight into a consultation room or designated visiting room where they can have privacy from other clients.
Be prepared!
y Create a cosy and warm environment within the consult room. We recommend that every vet hospital has a comfy bed, some blankets and cushions for the pet. If possible, dim the lights and turn off any bright, fluorescent lighting. Ensure there are seats for each member of the family. Ensure there are tissues in the room and provide water if possible.
y Consider having background music playing—owners may have a preference and may request silence or they may even have a particular song request to celebrate their pet’s life.
y Have everything you need for the euthanasia appointment in the room with you. Avoid leaving the room multiple times, it can be really disruptive to their overall experience.
Always prepare the client prior to moving forward—ask the family if they have been through euthanasia with a pet previously. The procedure should always be explained in as much or as little detail as the family needs, and outline what they are to expect at each stage of the procedure (e.g. eyes may stay open, he/she may lose control of their bladder as they relax following sedation). Advise that if anyone feels uncomfortable or overwhelmed, that it’s okay to leave the room, they do not need to stay the whole time.
If you were unable to complete consent forms or payment prior to the consultation, make sure that the paperwork is in the room and that this is addressed first. If you feel uneasy about asking for payment, you can have a few options on the
consent form so that owners can elect their payment preference (e.g. Payment can be completed at this point in time, following the consult or payment can be collected the following day over the phone), which you can then proceed with accordingly.
During the procedure
This is commonly a time where emotions and feelings of guilt can become quite overwhelming for the family. Start with a visual assessment of the pet and relay your findings back to the pet’s history and current condition. It is important to not pass judgement and to make sure that you are comfortable with the decision to euthanise. Provide reassurance that we are doing the right thing while acknowledging just how hard this decision is to have to make.
Remind owners that euthanasia quite literally translates to ‘good death’. For many animals that are facing several comorbidities, a peaceful death is a rare occurrence. Our decision to proceed with euthanasia is therefore an act of absolute love and kindness, as we can prevent suffering and provide a pain-free and dignified goodbye.
Signpost! Advise the family of each step before it is carried out. This gives family members the opportunity to look or move away if they are uncomfortable with watching. It also helps to eliminate any confusion as to what part of the procedure you are up to, to avoid confusion and anxiety.
Continue to check in with the owners—if they need more time, allow for this.
Avoid separating the pet from their family. These final moments with their pet are precious and owners will appreciate their pet remaining with them throughout the procedure, as will the pet! This includes placement of a catheter—perform this in the room with the owners present once the pet is sedated.


Why we need to do better
~84%
of vets graduate without ever administering euthanasia agents.4 ~80% of pets will have their life ended by euthanasia
(Survey conducted in NZ)
Leads to compassion fatigue, depression and burnout
(Survey conducted in the UK; Compassion Understood, 2015a).
If done right, euthanasia can have a positive impact on the team A significant portion of pets will require euthanasia.
Being a common procedure, competency is essential for the wellbeing of pets, clients and veterinary staff
~20-25%
of client don’t return to the same clinic after a pet has been euthanised
(Survey conducted in the UK; Compassion Understood, 2015a).
Clients will remember their pet’s goodbye forever and often find it hard to return to the same clinics for treatment of other pets.
Image 1: Statistics for surveys conducted on veterinary students and veterinarians in New Zealand and the United Kingdom.
The following graph shows how common diseases tend to progress in our pets

Cancer (n=5)
Organ failure (n=6)
Physical and cognitive frailty (n=7)
Other (n=2)
Source: Murray, SA, et al
Figure 1: Derived from The Royal Australian College of General Practitioners (RACGP) - Typical illness trajectories and palliative care phases towards the end of life
Resource: cve.edu.au/RACGP-Palliative-Care
Pre-Appointment
Gabapentin: 40-100mg/kg PO +/- Trazodone: 7-10mg/kg +/- ACP 2mg/kg

For best results, give the night before, in the morning AND 2 hours before the appointment
Support staff can organise this when appointment booked
Reduces stress for animal, owner and vet team
Reduces risks to vet team and owner
Figure 3. Oral sedatives for reactive, anxious or aggressive dogs that can be given to owners prior to the euthanasia appointment
Pre-Appointment
Gabapentin: 50-200mg per cat +/Trazodone: 50-100mg per cat

For best results, give the night before, in the morning AND 2 hours before the appointment
Support staff can organise this when appointment booked
Reduces stress for animal, owner and vet team
Reduces risks to vet team a nd owner
Figure 4. Oral sedatives for reactive, anxious or aggressive cats than can be given to owners prior to the euthanasia appointment.

As we discussed, all pets should receive sedation or an anaesthetic prior to euthanasia.
Give the sedation subcutaneously instead of intramuscularly. This is less painful, therefore reduces the incidence of vocalisation during administration.
Pinch the skin a few times before administration to help desensitise the area.
Whilst you are administering the sedation, get the owners to give the pet some food or lots of cuddles and pats as a distraction.
Use a small gauge needle (23 or 25G) and administer the sedation slowly.
If you are using a sedative with a low pH that is known to cause some irritation when administering (e.g., Zoletil), administer a neutral sedative first (e.g. Butorphanol and acepromazine) and wait 5 minutes before giving the second sedative.
Give the owners an idea of how long you expect it to take for the pet to become sedated—this allows them to maximise the time they have left and say their goodbyes while the pet is still conscious. Have a comfy bed for the pet to fall asleep into or help position the sleeping pet in the owner’s arms comfortably.
Once the pet is adequately sedated, you can place your IV catheter. We recommend placing a catheter in the hindlimb if possible to allow the family to remain at the pet’s head.
Keep the pet with the family at all times - place the catheter with the owners in the room. During this point, if deemed appropriate, engage the owners in conversation about their pet. Offer the family keepsakes —a paw print, a nose print, a lock of fur or some whiskers (Image 2 and 3). Some owners will want to do this whilst the animal is sedated, whilst others might opt to do this at the end of the appointment— give owners an option.
Just prior to giving the euthanasia agent, advise owners of what to expect (i.e., an increase in breathing rate, release of the bladder, possibly some skin twitching).
If a pet is sedated, the euthanasia solution should be given slowly, treated like an anaesthetic, so as to avoid triggering reflexes such as agonal breathing.
Once the pet’s breathing has stopped, listen to the heart with a stethoscope and, in the absence of a heartbeat, inform the owner when the pet has passed away

Following euthanasia, give the owners time to grieve. Encourage the owners to spend as much time in the consultation room as they require before they leave or before the pet is taken away.
If you feel comfortable, giving the owner a hug or placing your hand on their shoulder can help provide additional comfort during this difficult and surreal moment.
If a family or particular family member can be seen struggling emotionally, offer additional support and provide contact details of your preferred pet loss support counsellors.
Body handling after death should be soft, gentle and caring—treated the same as if the pet was still alive. Support the weight of the head while moving the animal.
If the pet is in the owner’s arms, when the owners are ready, help to gently place the pet into a bed. Tuck them in with a blanket and place their favourite toys around them (Image 4)
Burial:
Have biodegradable burial bags available to purchase in the clinic. Offer to help owners place the pet within these bags (or their own blankets). Owners often prefer the head to remain uncovered so that they can say their final goodbyes at home prior to burial.
Prepare the family for the timeline of rigor mortis and what to expect. We recommend placing a blanket over the pet at this point in time, so these changes are not observed by the family.
Recommend that your client understands council regulations regarding burial and warn of risks to other animals if uncovered and consumed.
Cremation:
Provide a rough idea of cremation turn-around time and reassure the owners of your faith in the cremation company’s professionalism and service. Do not show clients the body storage bags or do this in front of the family. Their last memory should be of their pet resting in a comfortable bed.
 Figure 2: Collage of Ohio, BEAP and Cta pain scale
Figure 2: Collage of Ohio, BEAP and Cta pain scale
There are 2 points during the consultation that we should be offering families a few moments of privacy:
1. Once you have placed the intravenous catheter and are ready to proceed with giving the euthanasia agent, ask the families if they would like one final, private moment to say goodbye to their beloved pet. Advise that they can take all the time that they need and to let you know once they are ready.
2. When the pet has passed away, gently tuck the pet into their bed and quietly retrieve your things. After a few minutes, advise the owner that you are going to step out of the room to give them some privacy. Again, advise them that they can take all the time they need and that you will be right outside when needed. Consider having a bell that they can ring so there is no confusion for when you should return into the room.
Taking these moments to provide families with some additional time and privacy with their beloved pet is important, as it allows the family to proceed in their own time and can help to provide some comfort and a level of closure.
Incorporating children into the appointment
It is important for children to feel part of the process, giving them a sense of control and allowing them to express their emotions. Children should always be given the option as to whether they want to be present or not during the entirety of the procedure. Describe the procedure clearly, adjusting your language as required (e.g., Using ‘died’ instead of ‘passed away’ in an effort to avoid euphemisms), and answer any questions or concerns they may have to reduce fear and confusion. Involving children in the making of keepsakes and asking them questions about their beloved pet throughout the procedure, is a great way to keep them involved to better process the finality of the situation.
In addition to above, the following can be provided in the consultation or suggested to the family during the initial phone call to prepare in advance at home.
Colouring Books
Involve children in making Paw Prints
Make the pet a Goodbye Card, write a letter or poem (Image 5)
Give them a toy to take to ‘on their next journey’
Plan a ceremony at Home
The euthanasia consultation should not represent the end of your relationship with the client. It is important that we continue to show love and support to our clients during this difficult time.
Have a staff member send an email or text to the family the following day
y In this short email or text, offer your condolences and your continued support by advising them that you and the team are here if they need anything or just want to talk. As some owners like to mourn privately, an email or text allows us to reach out without interrupting this grieving period. However, if you know that a particular client would prefer a phone call or face to face follow-up, then be brave and reach out—it’s okay if you don’t know what to say—just let them know that you’re thinking of them and offer support.
y If the owner is struggling to cope with the breadth of their emotions following the euthanasia appointment, linking them to external helplines or grief counsellors can be included.
Ask the family to send a photo (or multiple photos!) of their pet and celebrate the pet’s life on a ‘rainbow bridge memory wall’ within the clinic.
Similarly, these photos can be used to create a memorial page on your website or posted on social media to honour the pet. These are great ways to help the owners celebrate the wonderful life of their beloved pet.
Sympathy Cards (hand-written), flowers or candles can also be sent to the owner’s home or they can be handed to the client if they are returning to the clinic to collect ashes. If you are sending these cards in the mail, we recommend sending these no later than 5 days following the appointment.
1. Preparation and communication before the appointment
2. Providing sedation before euthanasia
3. Keep the pet and owner together throughout the entire process
4. Provide reassurance and help to alleviate guilt
5. Offer keepsakes to create a memorial - paw and nose prints, locks of fur, whiskers and candles

We are incredibly grateful for the unwavering dedication, passion, and commitment of Dr. Courtney Prue, the founder of Rest Your Paws. Without Courtney’s tireless efforts, this article would not have been possible.

Image 2: Memorial for ‘Banjo’ - A beautiful example of the impact providing keepsakes can have on owners, allowing them to celebrate their beloved pets' lives.



Image 4: Aftercare for Trixie - Trixie was gently placed in a soft bed, next to her favorite toy following her passing. She seems as though she is just sleeping peacefully and this is the everlasting memory we want to provide for our beautiful families.
1. Animal Medicines Australia. (2019, October 22). Pets in Australia: A national survey of pets and people AMAU008-Pet-Ownership22Report v1.6 WEB.pdf. Retrieved August 18, 2023, from animalmedicinesaustralia.org.au
2 Littlewood, K. E Beausoleil, N J., Stafford, K. J., Stephens, C., Collins, T., Fawcett, A., Hazel, S., Lloyd, J. K. F., Mallia, C., Richards, L., Wedler, N. K., & Zito, S. (2018). Exploring how end-of-life management is taught to Australasian veterinary students. Part 1: technical euthanasia. The Veterinary record , 183(22), 691. doi.org/10.1136/vr.104775
3 Veterinary Practitioners Board of New South Wales. (2013, September). Veterinary Practitioners Code of Professional Conduct. Retrieved from NSW Legislation: vpb.nsw.gov.au/sites/default/files/ images/GR01%20Veterinary%20Practitioners%20Code%20of%20 Professional%20Conduct.pdf
4 Gates, M C. Kells N J Kongara, K & Littlewood, K. E (2023). Euthanasia of dogs and cats by veterinarians in New Zealand: protocols, procedures and experiences. New Zealand Veterinary Journal , 71(4), 172–185. doi.org/10.1080/00480169.2023.219468
5 Pun J. K. H. (2020). An integrated review of the role of communication in veterinary clinical practice. BMC veterinary research , 16(1), 394. doi.org/10.1186/s12917-020-02558-2
6 Tran, L., Crane, M F., & Phillips, J. K. (2014). The distinct role of performing euthanasia on depression and suicide in veterinarians. Journal of Occupational Health Psychology , 19(2), 123–132. doi.org/10.1037/a0035837
7 Matte, A. R., Khosa, D. K., Coe, J. B., Meehan, M., & Niel, L. (2020). Exploring pet owners’ experiences and self-reported satisfaction and grief following companion animal euthanasia. The Veterinary Record 187(12), e122. doi.org/10.1136/vr.105734

Image 5: Children & Pet Loss: Encouraging children to make goodbye cards, letters, pictures or a poem during a euthanasia appointment can help include children in the process
Image 3: A Keepsake - Nose print
This course presented by Taronga will support veterinary professionals and veterinary nurses to develop knowledge and skills in native wildlife triage, including first aid, initial treatment and emergency care.
• Supported by the NSW Government Department of Planning and Environment
Email: tarongprofvet@zoo.nsw.gov.au Visit: https://taronga.org.au/vet-professional-training
• AVA and VNCA certified
• Online Course (20 CPD points)
• Hands-on Workshop (12 CPD Points) –available in NSW, QLD and Vic (see website for details)
• Subsidised positions available to eligible applicants nationally





